Abstract
Background
Children and adults with asthma and impaired lung function have been reported to have low-grade systemic inflammation, but it is unknown whether this inflammation starts before symptoms and in particular whether low-grade inflammation is present in asymptomatic neonates with reduced lung function.
Objective
We sought to investigate the possible association between neonatal lung function and biomarkers of systemic inflammation.
Methods
Plasma levels of high-sensitivity C-reactive protein (hs-CRP), IL-1β, IL-6, TNF-α, and CXCL8 (IL-8) were measured at age 6 months in 300 children of the Copenhagen Prospective Study on Asthma in Childhood2000 birth cohort who had completed neonatal lung function testing at age 4 weeks. Associations between neonatal lung function indices and inflammatory biomarkers were investigated by conventional statistics and unsupervised principal component analysis.
Results
The neonatal forced expiratory volume at 0.5 seconds was inversely associated with hs-CRP (β-coefficient, −0.12; 95% CI, −0.21 to −0.04; P < .01) and IL-6 (β-coefficient, −0.10; 95% CI, −0.18 to −0.01; P = .03) levels. The multivariate principal component analysis approach, including hs-CRP, IL-6, TNF-α, and CXCL8, confirmed a uniform upregulated inflammatory profile in children with reduced forced expiratory volume at 0.5 seconds (P = .02). Adjusting for body mass index at birth, maternal smoking, older children in the home, neonatal bacterial airway colonization, infections 14 days before, and asthmatic symptoms, as well as virus-induced wheezing, at any time before biomarker assessment at age 6 months did not affect the associations.
Conclusion
Diminished neonatal lung function is associated with upregulated systemic inflammatory markers, such as hs-CRP.
Key words: Asthma, children, high-sensitivity C-reactive protein, proinflammatory cytokines, spirometry
Abbreviations used: BMI, Body mass index; COPSAC2000, Copenhagen Prospective Study on Asthma in Childhood; CRP, C-reactive protein; FEF50, Forced expiratory flow at 50% of the forced vital capacity; FEV0.5, Forced expiratory volume at 0.5 seconds; FVC, Forced vital capacity; hs-CRP, High-sensitivity C-reactive protein; IQR, Interquartile range; PC1, First principal component; PCA, Principal component analysis; TROLS, Troublesome lung symptoms
C-reactive protein (CRP) is an acute-phase reactant found in the blood in response to acute and chronic inflammatory conditions and has a broad clinical application in screening for infectious and immune-mediated diseases.1 CRP has important innate immunity properties and is released from the liver after triggering by proinflammatory cytokines, such as IL-6, IL-1β, and TNF-α.2
CRP assays3 with increased sensitivity (high-sensitivity C-reactive protein [hs-CRP]) have demonstrated low-grade inflammation in patients with disorders such as cardiovascular disease,4 obesity,5 and diabetes mellitus.6 Increased hs-CRP levels have also been demonstrated during and shortly after viral respiratory tract infections7 and in patients with symptomatic airway diseases, such as asthma8 and chronic obstructive pulmonary disease.9 In addition, impaired lung function in asthmatic children and adults has been associated with the presence of systemic low-grade inflammation.10, 11
We hypothesized that impaired lung function would be associated with the systemic inflammatory process, even before development of any respiratory symptoms. Therefore we measured plasma hs-CRP, IL-1β, IL-6, TNF-α, and CXCL8 (formerly IL-8) levels at the early age of 6 months and related these to neonatal lung function assessed at age 4 weeks in the Copenhagen Prospective Study on Asthma in Childhood2000 (COPSAC2000) birth cohort.
Methods
Study cohort
The study participants were 411 neonates born of mothers with a history of asthma and enrolled at 4 weeks of age in the COPSAC2000 prospective birth cohort study.12, 13, 14 Exclusion criteria were any respiratory symptoms or respiratory support before inclusion, gestational age of less than 36 weeks, and any congenital abnormality or systemic illness, such as severe neonatal sepsis. The children attended the COPSAC research clinic at age 4 weeks for assessment of neonatal lung function and subsequently at 6-month intervals, as previously detailed.12, 13, 14
Ethics
The study was conducted in accordance with the guiding principles of the Declaration of Helsinki and was approved by the local ethics committee (KF 01-289/96) and the Danish Data Protection Agency (2008-41-1754). Both parents provided oral and written informed consent before enrollment.
Inflammatory biomarkers
Blood was drawn in an EDTA tube from a cubital vein at the age of 6 months, centrifuged to separate plasma and plasma cells, and immediately stored at −80°C until analysis. The samples were transported on dry ice to the laboratory, where levels of the a priori selected biomarkers were determined by using high-sensitivity ELISAs based on electrochemiluminescence in a 4-plex setting for IL-1β, IL-6, CXCL8, and TNF-α and as a single assay for hs-CRP. Samples were read in duplicates by using the Sector Imager 6000 (Meso Scale Discovery, Gaithersburg, Md). The limit of detection (mean signal from blanks + 3SD) was 9.54 pg/mL for hs-CRP, 0.15 pg/mL for IL-1β, 0.17 pg/mL for IL-6, 0.09 pg/mL for CXCL8, and 0.08 pg/mL for TNF-α.
Neonatal lung function
Neonatal spirometric results were measured at age 4 weeks, applying the raised-volume rapid thoracoabdominal “squeeze” jacket compression technique.15 Repeated ventilations to predefined mouth pressures ensured expansion of the lung volume before an instant inflation of the jacket caused a full exhalation during which the flow was measured by using a pneumotachograph with an air-cushion facemask.16, 17 The software identified forced vital capacity (FVC) as the first plateau on the volume-time curve, and measurements with FVC appearing after 0.5 seconds and the forced expiratory volume at 0.5 seconds (FEV0.5) being less than or equal to FVC were accepted. Three to 5 acceptable curves were obtained for each measurement, and the curve containing the median value of FEV0.5 was used for analysis of FEV0.5 and forced expiratory flow at 50% of forced vital capacity (FEF50).
For neonatal bronchial responsiveness, after an initial saline inhalation, methacholine was administered in quadrupling dose steps with a dosimeter attached to a nebulizer (SPIRA 08 TSM 133; Respiratory Care Center, Hämeenlinna, Finland).17 Bronchial responsiveness was determined by means of continuous assessment of transcutaneous oxygen saturation (TCM3; Radiometer, Copenhagen, Denmark). The provocative dose of methacholine causing a 15% decrease in transcutaneous oxygen saturation was estimated from the dose-response curves fitted with a logistic function.
Troublesome lung symptoms
Troublesome lung symptoms (TROLS) were defined as significant cough or wheeze or dyspnea severely affecting the well-being of the child and recorded by the parents in a daily diary chart as a dichotomized score (yes/no) from birth.18, 19, 20 At acute episodes of TROLS (≥3 consecutive days with TROLS), the children were seen at the COPSAC clinic for a clinical examination, including a rhinopharyngeal aspirate for viral detection (picornaviruses, respiratory syncytial virus, coronaviruses, parainfluenza viruses, influenza viruses, human metapneumoviruses, adenoviruses, and bocavirus).21
Covariates
Covariates included heredity (father's history of asthma, eczema, or allergy [yes/no]); anthropometrics (birth body mass index [BMI; 7-12, 12-13, 13-14, and 14-17 m/kg2]); demographics (sex, older children in the home at birth [yes/no], and yearly household income [low at <€53,000, medium at €53,000-€80,000, and high at >€80,000]); prenatal and antenatal exposures (maternal smoking during the third trimester of pregnancy [yes/no] and cesarean section [yes/no]); postnatal exposures (bacterial airway colonization with Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis at age 4 weeks [yes/no],14 length of sole breast-feeding [0-3, 3-6, and >6 mo], age at start in day care [0-9, 9-12, and >12 mo], and pets in the home in the first year of life: cat [yes/no] or dog [yes/no]); any TROLS (yes/no) and any episodes of TROLS with virus detected before biomarker assessment (yes/no); and any infection 14 days before biomarker assessment (upper and lower respiratory tract infections, gastroenteritis, or fever with unknown cause [yes/no]).
Statistics
Biomarker null values were set to half of the lowest detected value for the specific biomarker, values were log-transformed, and the mean of the duplicate measurements were used for association analyses. z Scores were calculated for FEV0.5 and FEF50, and the provocative dose of methacholine causing a 15% decrease in transcutaneous oxygen saturation was log-transformed to obtain normality.
The associations between neonatal lung function indices and inflammatory biomarkers were tested by using conventional statistics with general linear models and by using unsupervised pattern recognition with principal component analysis (PCA). In the PCA analyses we extracted underlying orthogonal components that described the systematic part of the variation across the biomarkers using log-transformed and z score mediator levels.
All results are presented as raw estimates with 95% CIs and as estimates obtained from partial regression analyses, adjusting for covariates associated with levels of hs-CRP by using a cutoff P value of .10 or less. Birth BMI and maternal smoking during the third trimester were retained in the multivariable models independently of their association with hs-CRP because these are important determinants of neonatal lung function.22 Interaction with bacterial airway colonization, any TROLS, and acute episodes of TROLS with virus detected was tested by adding cross-products to the models. A P value of .05 or less was considered significant. All analyses were done with SAS software, version 9.3 (SAS Institute, Cary, NC).
Results
Inflammatory biomarker assessments
Measurements of IL-1β, IL-6, TNF-α, and CXCL8 levels were performed on 309 plasma samples collected at age 6 months, and measurements of hs-CRP levels were performed on 301 plasma samples collected at age 6 months. One sample was lost for technical reasons while performing the 4-plex assay, resulting in 300 children (73% of the original 411 cohort children) with available measurements for all 5 biomarkers. We found no significant differences in baseline characteristics between children with and without available biomarker assessments (see Table E1 in this article's Online Repository at www.jacionline.org).
Median levels were as follows: hs-CRP, 1.39 mg/L (interquartile range [IQR], 0.46-4.61 mg/L); IL-1β, 0.01 ng/L (IQR, 0.001-0.04 ng/L); IL-6, 0.20 ng/L (IQR, 0.11-0.31 ng/L); TNF-α, 2.34 ng/L (IQR, 1.92-2.88 ng/L); and CXCL8, 3.04 ng/L (IQR, 2.19-4.37 ng/L). IL-6 and TNF-α levels were strongly positively correlated with hs-CRP levels (P < .001 for both), whereas IL-1β and CXCL8 levels were not correlated with hs-CRP levels (P ≥ .62). The measured values of hs-CRP, IL-6, TNF-α, and CXCL8 were within the expected range,23 with very few null values, whereas IL-1β levels were much lower than expected,23 with null values for 72 (23%) of 308 children. Because of this and the fact that IL-1β has been shown to significantly degrade over time, even at −80°C,24 IL-1β was not included in further analyses.
Determinants of hs-CRP
Children with older children in the home at birth had significantly higher hs-CRP levels at age 6 months compared with children without older children in the home (median hs-CRP level, 2.20 mg/L [IQR, 0.63-5.05 mg/L] vs 1.16 mg/L [IQR, 0.41-3.40 mg/L], P = .005). In addition, hs-CRP levels were increased in children who experienced an infectious episode within 14 days before biomarker assessment compared with children without apparent infections (4.29 mg/L [IQR, 1.71-5.34 mg/L] vs 0.84 mg/L [IQR, 0.36-2.67 mg/L], P < .0001); in children experiencing TROLS at any time point before biomarker assessment compared with children without TROLS (1.79 mg/L [IQR, 0.50-4.72 mg/L] vs 1.19 mg/L [IQR, 0.46-4.14 mg/L], P = .05); and in children with acute episodes of TROLS with an airway virus detected (3.78 mg/L [IQR, 1.00-5.42 mg/L] vs 1.16 mg/L [IQR, 0.41-3.85 mg/L], P < .0001). Children with bacterial airway colonization at age 4 weeks compared with noncolonized children showed a trend of increased hs-CRP levels (2.68 mg/L [IQR, 0.84-5.17 mg/L] vs 1.31 mg/L [IQR, 0.49-4.64 mg/L], P = .08). We did not detect associations between hs-CRP levels and paternal history of asthma, eczema, or allergy; child's sex; birth BMI; household income; maternal smoking during the third trimester of pregnancy; birth by means of cesarean section; breast-feeding; day care attendance; and pets in the home (Table I ).
Table I.
Heredity; anthropometrics; demographics; prenatal, perinatal, and postnatal exposures; TROLS; airway microbiology; and infections before assessment of low-grade inflammation in relation to hs-CRP levels at age 6 months
| Characteristic | No. | hs-CRP (mg/L) at age 6 mo |
|
|---|---|---|---|
| Median (IQR) | P value | ||
| Paternal asthma, allergy, or eczema | .54 | ||
| Yes | 135 | 1.52 (0.46-4.61) | |
| No | 153 | 1.31 (0.46-4.44) | |
| Sex | .62 | ||
| Male | 155 | 1.37 (0.37-4.44) | |
| Female | 146 | 1.51 (0.49-4.81) | |
| BMI at birth | .56 | ||
| 7-12 m/kg2 | 78 | 1.06 (0.45-4.46) | |
| 12-13 m/kg2 | 73 | 1.80 (0.56-4.69) | |
| 13-14 m/kg2 | 74 | 1.41 (0.48-4.98) | |
| 14-17 m/kg2 | 76 | 1.25 (0.39-3.75) | |
| Older children in home at birth | .005 | ||
| Yes | 114 | 2.20 (0.63-5.05) | |
| No | 177 | 1.16 (0.41-3.40) | |
| Household income at birth (yearly)∗ | .17 | ||
| Low | 77 | 0.83 (0.38-3.57) | |
| Average | 144 | 1.31 (0.46-4.63) | |
| High | 70 | 2.27 (0.67-4.92) | |
| Maternal smoking during third trimester | .33 | ||
| Yes | 51 | 1.40 (0.41-4.46) | |
| No | 250 | 1.22 (0.64-5.02) | |
| Cesarean section | .20 | ||
| Yes | 60 | 1.81 (0.52-5.13) | |
| No | 205 | 1.31 (0.46-3-93) | |
| Solely breast-feeding period | .43 | ||
| 0-3 mo | 64 | 2.16 (0.49-5.35) | |
| 3-6 mo | 160 | 1.51 (0.46-4.31) | |
| >6 mo | 40 | 1.02 (0.55-3.87) | |
| Age at start in day care | .26 | ||
| 0-9 mo | 89 | 1.80 (0.50-5.13) | |
| 9-12 mo | 77 | 1.13 (0.36-3.40) | |
| >12 mo | 123 | 1.59 (0.50-4.82) | |
| Cat in home in first year of life | .42 | ||
| Yes | 46 | 1.74 (0.67-3.87) | |
| No | 248 | 1.41 (0.44-4.67) | |
| Dog in home in first year of life | .56 | ||
| Yes | 44 | 1.15 (0.50-3.65) | |
| No | 249 | 1.52 (0.49-4.69) | |
| Bacterial airway colonization at age 4 wk† | .08 | ||
| Yes | 51 | 2.68 (0.84-5.17) | |
| No | 189 | 1.31 (0.49-4.64) | |
| Any TROLS at age 1-6 mo | .05 | ||
| Yes | 141 | 1.79 (0.50-4.72) | |
| No | 160 | 1.19 (0.46-4.14) | |
| Episodes of virus-induced TROLS at age 1-6 mo‡ | <.0001 | ||
| Yes | 57 | 3.78 (1.00-5.42) | |
| No | 244 | 1.16 (0.41-3.85) | |
| Any infection 14 d before hs-CRP assessment§ | <.0001 | ||
| Yes | 95 | 4.29 (1.71-5.34) | |
| No | 206 | 0.84 (0.36-2.67) | |
Values in boldface are P < .10.
Yearly household income at birth of neonate: low (<€53,000), medium (€53,000-€80,000), and high (>€80,000).
Bacterial airway colonization with S pneumoniae, H influenzae, or M catarrhalis at the time of neonatal lung function testing.
Picornaviruses, respiratory syncytial virus, coronaviruses, parainfluenza viruses, influenza viruses, human metapneumoviruses, adenoviruses, or bocavirus.
Any infection includes any upper or lower respiratory tract infection, gastroenteritis, or fever with unknown cause.
Neonatal lung function and systemic low-grade inflammation
hs-CRP
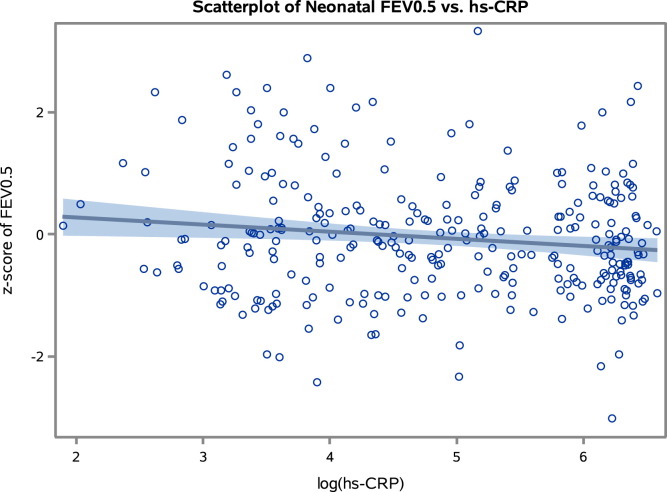
The conventional statistical approach showed a strong linear inverse association between FEV0.5 values at age 4 weeks and hs-CRP levels at age 6 months (β-coefficient, −0.12; 95% CI, −0.21 to −0.04; P = .004), suggesting increasing grade of inflammation by diminished neonatal lung function (Fig 1 ). The association was unchanged by adjustment for older children in the home, bacterial airway colonization at age 4 weeks, infections 14 days before, and any TROLS, as well as acute virus-related episodes of TROLS at any time before biomarker assessment, birth BMI, and maternal smoking in the third trimester (β-coefficient, −0.12; 95% CI, −0.22 to −0.02; P = .02). Furthermore, we found no interaction with bacterial airway colonization (P = .21), any TROLS (P = .76), or any acute episodes of TROLS with a virus detected (P = .20).
Fig 1.
Scatter plot illustrating the relationship between neonatal lung function (z score of FEV0.5) and hs-CRP level at age 6 months (log-transformed values). Solid line, Regression line; shaded area, 95% confidence limits.
FEF50 values also seemed inversely associated with hs-CRP levels but was not significant (β-coefficient, −0.06; 95% CI, −0.15 to 0.02; P = .14).
IL-6
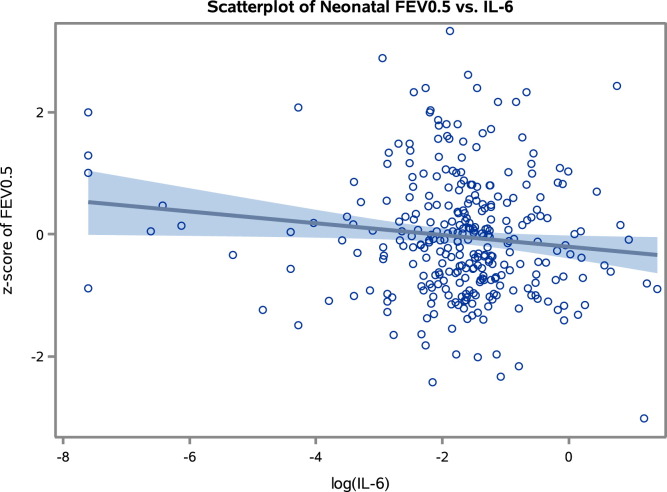
Increasing FEV0.5 values were also significantly associated with decreasing IL-6 levels (β-coefficient, −0.10; 95% CI, −0.18 to −0.01; P = .03; Fig 2 ). Confounder adjustment did not substantially change the association (β-coefficient, −0.11; 95% CI, −0.22 to 0.01; P = .07). We did not detect a significant association between FEF50 values and IL-6 levels.
Fig 2.
Scatter plot illustrating the relationship between neonatal lung function (z score of FEV0.5) and IL-6 levels at age 6 months (log-transformed values). Solid line, Regression line; shaded area, 95% confidence limits.
TNF-α and CXCL8
FEV0.5 and FEF50 measurements were not associated with CXCL8 or TNF-α levels, although the β-coefficients suggested an inverse association between lung function indices and TNF-α levels (Table II ).
Table II.
Association between neonatal lung function and inflammatory biomarkers at age 6 months: conventional and PCA approach
| Log hs-CRP |
Log IL-6 |
Log TNF-α |
Log CXCL8 |
PC1 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β-Coefficient (95% CI) | P value | β-Coefficient (95% CI) | P value | β-Coefficient (95% CI) | P value | β-Coefficient (95% CI) | P value | β-Coefficient (95% CI) | P value | |
| Unadjusted analysis | ||||||||||
| z-FEV0.5 | −0.12 (−0.21 to −0.04) | .004 | −0.10 (−0.18 to −0.01) | .03 | −0.11 (−0.38 to 0.17) | .44 | 0.02 (−0.15 to 0.19) | .83 | −0.10 (−0.19 to −0.01) | .02 |
| z-FEF50 | −0.06 (−0.15 to 0.02) | .14 | −0.02 (−0.11 to 0.06) | .61 | −0.09 (−0.37 to 0.18) | .52 | −0.06 (−0.22 to 0.11) | .49 | −0.06 (−0.14 to 0.03) | .17 |
| Log PD15 | 0.04 (−0.12 to 0.21) | .60 | −0.03 (−0.21 to 0.15) | .75 | −0.02 (−0.56 to 0.52) | .94 | 0.15 (−0.17 to 0.46) | .36 | 0.03 (−0.14 to 0.19) | .76 |
| Adjusted analysis∗ | ||||||||||
| z-FEV0.5 | −0.12 (−0.22 to −0.02) | .02 | −0.11 (−0.23 to 0.01) | .07 | −0.21 (−0.58 to 0.17) | .27 | 0.01 (−0.23 to 0.26) | .91 | −0.12 (−0.23 to 0.00) | .03 |
| z-FEF50 | −0.07 (−0.18 to 0.03) | .16 | −0.05 (−0.17 to 0.07) | .42 | −0.16 (−0.54 to 0.21) | .40 | −0.07 (−0.32 to 0.17) | .56 | −0.08 (−0.19 to 0.03) | .16 |
| Log PD15 | 0.05 (−0.13 to 0.23) | .60 | 0.00 (−0.20 to 0.21) | .97 | −0.07 (−0.73 to 0.59) | .84 | 0.15 (−0.29 to 0.59) | .49 | 0.03 (−0.17 to 0.22) | .78 |
Values in boldface are P < .10.
PD15, Provocative dose of methacholine causing a 15% decrease in transcutaneous oxygen saturation.
Partial linear regression analyses adjusted for birth BMI, maternal smoking during the third trimester of pregnancy, older children in the home at birth, bacterial airway colonization at age 4 weeks, infections 14 days before, and any TROLS, as well as episodes of virus-induced TROLS at any time point before blood sampling for inflammatory biomarker assessment.
PCA
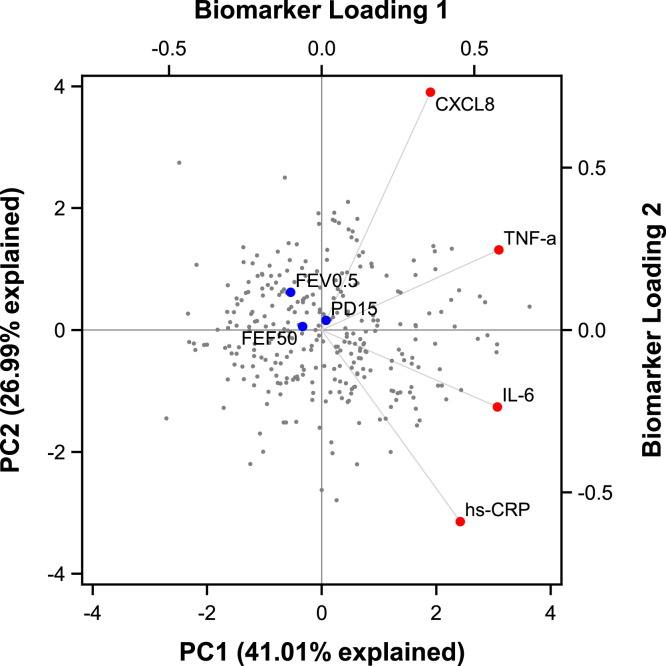
Unsupervised PCA showed that hs-CRP, IL-6, TNF-α, and CXCL8 levels were positively correlated in the first principal component (PC1), which explained 41% of the total variation in the data. The PCA approach is illustrated in the biplot (Fig 3 ), showing scores for PC1 and PC2 and loadings for the biomarkers. Because of the univocal pattern in PC1, we focused on PC1 in the further analyses. Confirming the findings from conventional statistics, we found that FEV0.5 values were inversely associated with PC1 values (P = .02) and remained significant after confounder adjustments (P = .03). There was no interaction with bacterial airway colonization, any TROLS, or any acute episodes of TROLS with a virus detected (all interaction P ≥ .20). The β-coefficients also suggested an inverse association between FEF50 and PC1 values, but the model was not significant (Table II).
Fig 3.
PCA biplot showing the children's individual scores (gray dots) in the first principal component (PC1) and second principal component (PC2), as well as biomarker loadings for hs-CRP, IL-6, TNF-α, and CXCL8 (red dots) and correlation with lung function indices (blue dots). Percentages in parentheses are the part of the total variation in the data set explained by the components.
Neonatal bronchial responsiveness and systemic low-grade inflammation
Bronchial responsiveness to methacholine in neonatal life was not associated with biomarkers of low-grade inflammation at age 6 months (Table II).
Discussion
Key findings
Infants with reduced pulmonary capacity as neonates are characterized by systemic low-grade inflammation with an upregulated blood inflammatory response, including increased hs-CRP levels. This association suggests that reduced neonatal lung function is part of a condition with an ongoing asymptomatic airway inflammation and a measurable systemic component from the beginning of life.
Strengths and limitations of the study
A major strength of the study is the unique assessment of neonatal lung function with the state-of-the-art raised-volume rapid thoracoabdominal compression technique performed strictly in adherence with recognized guidelines15 in the full mother-child birth cohort. The neonatal spirometric measurements were obtained in this cohort of asymptomatic children before any respiratory symptoms and are thus unbiased from previous or concurrent airway symptoms.
Another significant strength of the study is the availability of a range of environmental exposure assessments, including bacterial airway colonization and the presence of a virus, enabling robust confounder adjustment for factors with a possible influence on neonatal lung function and low-grade inflammation. However, it is a limitation that we did not assess the presence of bacteria and viruses at both lung function and inflammatory biomarker testing.
There were strong linear correlations between IL-6 and TNF-α levels and hs-CRP levels. Because IL-6 and TNF-α are the main triggers of CRP release from the liver,2 these expected correlations serve as a biological validation of the data. The lack of correlation between CXCL8 and hs-CRP levels was not surprising because CXCL8 primarily has a neutrophilic chemotactic function in the innate immune system and does not directly induce CRP release.25 The finding of significantly increased hs-CRP levels in children experiencing an infectious episode within 14 days before biomarker assessment further validates the data because CRP is a reliable biomarker of airway inflammation.1 Even after adjusting for this confounder, the association between neonatal lung function and hs-CRP levels remained, with largely unchanged effect estimates.
Both the standard statistical approach and the unsupervised data-driven PCA approach showed similar associations, which strengthened confidence in our findings. Still, we did not detect association between neonatal bronchial hyperresponsiveness and low-grade inflammation, which we would have expected given our previous finding of association between methacholine challenge results and subsequent asthma development.26
It is a limitation of the study that we were unable to detect a biologically meaningful signal from IL-1β, which is presumably caused in part by the plasma storage time of up to 13 years before analysis, during which samples had been thawed and frozen on several occasions. IL-1β is particularly sensitive to freeze-thaw cycles and degrades by greater than 50% over time, even when samples are stored at −80°C.24 It is well known that circulating IL-1β levels are approximately 5 times less than TNF-α levels in healthy adults,23 but in our case the median IL-1β level was 200 times less than the median TNF-α level (0.01 vs 2.34 ng/L), and we were unable to detect an association between IL-1β and hs-CRP levels.
Another limitation of the study is the at-risk nature of the cohort because all children were born to mothers with a history of asthma. We demonstrated recently that the offspring of mothers with a history of asthma, allergy, or eczema in an unselected mother-child cohort have a topical downregulated immune signature in the airway mucosa compared with children of mothers without such disorders.27 Yet even though the at-risk nature of the cohort might have affected the absolute biomarker levels, this should not influence our ability to explore the association between neonatal spirometry and markers of systemic low-grade inflammation within the cohort.
It is another limitation of our study that biomarkers were assessed at 6 months while neonatal lung function was tested at 4 weeks. However, we adjusted for any lung symptoms in the period between based on the daily diary cards filled out by the parents.
Study implications
We show that impaired lung function in neonates is associated with a systemic inflammatory process, even before the development of any respiratory symptoms. This suggests a link between reduced neonatal lung function and a disorder characterized by a systemic inflammatory component.
A number of recent larger cross-sectional analyses in adults and adolescents have shown that increased hs-CRP levels are associated with respiratory impairment in both population-based settings and in asthmatic and nonasthmatic strata.11, 28, 29 hs-CRP levels have also been reported in relation to pulmonary function outcomes in studies of children with established asthma.10, 30, 31 A study of 63 asthmatic children aged 2 to 12 years with and without acute exacerbations31 and a study of 60 school-aged children treated with inhaled corticosteroids, as well as steroid-naive children,10 showed a reciprocal relationship between FEV1 values and hs-CRP levels. In contrast, another study of 62 school-aged children with controlled and uncontrolled asthma30 did not detect an association between hs-CRP levels and FEV1 values but found that hs-CRP levels were greater in patients with uncontrolled versus those with controlled asthma, which might reflect the degree of airway inflammation. These studies might be underpowered and are hampered by the wide age ranges and lack of control groups. Importantly, it is a different research question whether established asthma is associated with detectable systemic inflammation, unlike our aim to study whether systemic inflammation in very early life is associated with neonatal lung function before symptom debut.
A possible explanation of the identified association between reduced neonatal lung function and increased hs-CRP levels is that diminished forced volume is caused by airway inflammation. In vitro murine and human lung cell studies have established a possible role of the proinflammatory cytokines stimulating CRP release, such as IL-6, TNF-α, and IL-1β, in the pathophysiology of obstructive airway inflammation.32, 33 Persistently increased CRP levels might induce an increased vulnerability to changes in the early-life environment through its actions as a general scavenger protein with important innate immune functions in the recognition and elimination of bacteria and damaged human cells through opsonization, phagocytosis, and cell-mediated cytotoxicity.1 Therefore reduced neonatal lung function might reflect subclinical bacterial airway colonization and airway inflammation predating detection of clinical symptoms and systemic low-grade inflammation. Such a disease trajectory is well known in patients with cystic fibrosis, for example, in whom reduced lung function has been shown to precede clinical disease penetrance34 and correlates with Pseudomonas aeruginosa airway colonization before exacerbations.35
Alternatively, reduced neonatal lung function does not lead to systemic inflammation but is rather an independent characteristic of neonates with sustained low-grade inflammation in early life. Such inefficient immune regulation might be driven by the newborn's genotype interacting with the intrauterine and early-life environment, thereby affecting the plasticity of the developing immune system. In support of the latter theory, higher baseline CRP levels have been demonstrated in westernized populations, where obstructive airway disorders are more prevalent compared with rural societies.36
We assessed lung function in the 4-week-old asymptomatic neonates and the inflammatory biomarkers at 6 months. We can only speculate whether this concurs with the onset of an underlying disorder or whether this possibly reflects a disorder beginning even earlier in life, perhaps during pregnancy.
Children of the Danish COPSAC2000 at-risk cohort exhibited an association between reduced neonatal lung function and upregulated systemic inflammatory biomarkers before symptom onset, suggesting that reduced lung function reflects an ongoing inflammatory disorder with a measurable systemic component early in life.
Key messages.
-
•
Asthmatic children and adults with diminished pulmonary function have increased levels of hs-CRP, a marker of systemic low-grade inflammation. However, it is unknown whether asymptomatic neonates with reduced lung function have signs of systemic inflammation.
-
•
Neonates with impaired respiratory capacity are characterized by an upregulated blood inflammatory profile, including hs-CRP, suggesting the presence of systemic low-grade inflammation in early life before symptom onset.
Footnotes
COPSAC is funded by private and public research funds listed on www.copsac.com. The Lundbeck Foundation (R16-A1694), the Danish State Budget (0903516), the Danish Strategic Research Council (0603-00280B), and the Danish Medical Research Council (10-082884 & 271-08-0815) provided the core support for COPSAC research center. The funding agencies did not have any role in design and conduct of the study; collection, management, and interpretation of the data; or preparation, review, or approval of the manuscript.
Disclosure of potential conflict of interest: B. L. K. Chawes has received research support from A.P. Møller og Hustru Chastine Mc-Kinney Møllers Fond til almene Formaal (13-41). H. Bisgaard has received research support from the Danish Council for Strategic Research (0603-00280B) and the Lundbeck Foundation (R16-A1694) and has grants pending from the European Commission. The rest of the authors declare that they have no relevant conflicts of interest.
Appendix
Table E1.
Comparison of baseline characteristics between children with and without complete assessment of early-life low-grade inflammation
| Baseline characteristic | Children with biomarker assessment (n = 300) | Children without biomarker assessment (n = 111) | P value |
|---|---|---|---|
| Paternal asthma, allergy, or eczema (no.) | 47% (135) | 46% (50) | .84† |
| Male sex (no.) | 51% (154) | 44% (49) | .20† |
| BMI at birth (m/kg2), mean (SD) | 12.79 (1.34) | 12.84 (1.22) | .63‡ |
| Older children in home at birth (no.) | 39% (114) | 40% (38) | .91† |
| Household income at birth∗ (no.) | .12† | ||
| Low | 27% (77) | 38% (35) | |
| Average | 49% (143) | 41% (39) | |
| High | 24% (70) | 21% (20) | |
| Maternal smoking during third trimester (no.) | 17% (51) | 11% (12) | .12† |
| Cesarean section (no.) | 23% (60) | 27% (25) | .45† |
| Solely breast-feeding length (d), median (IQR) | 122 (90-155) | 122 (74-164) | .90§ |
| Age at start in day care (d), median (IQR) | 345 (240-415) | 307 (216-412) | .27§ |
| Cat in home in first year of life (no.) | 16% (46) | 14% (14) | .61† |
| Dog in home in first year of life (no.) | 15% (44) | 10% (10) | .16† |
Yearly household income at birth of neonate: low (<€53,000), medium (€53,000-€80,000), and high (>€80,000).
χ2 Test.
t Test.
Wilcoxon rank sum test.
References
- 1.Pepys M.B., Hirschfield G.M. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voleti B., Agrawal A. Regulation of basal and induced expression of C-reactive protein through an overlapping element for OCT-1 and NF-kappaB on the proximal promoter. J Immunol. 2005;175:3386–3390. doi: 10.4049/jimmunol.175.5.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rifai N., Tracy R.P., Ridker P.M. Clinical efficacy of an automated high-sensitivity C-reactive protein assay. Clin Chem. 1999;45:2136–2141. [PubMed] [Google Scholar]
- 4.Sabatine M.S., Morrow D.A., Jablonski K.A., Rice M.M., Warnica J.W., Domanski M.J. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007;115:1528–1536. doi: 10.1161/CIRCULATIONAHA.106.649939. [DOI] [PubMed] [Google Scholar]
- 5.Nappo A., Iacoviello L., Fraterman A., Gonzalez-Gil E.M., Hadjigeorgiou C., Marild S. High-sensitivity C-reactive protein is a predictive factor of adiposity in children: results of the identification and prevention of dietary- and lifestyle-induced health effects in children and infants (IDEFICS) study. J Am Heart Assoc. 2013;2:e000101. doi: 10.1161/JAHA.113.000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hung M.J., Hsu K.H., Hu W.S., Chang N.C., Hung M.Y. C-reactive protein for predicting prognosis and its gender-specific associations with diabetes mellitus and hypertension in the development of coronary artery spasm. PLoS One. 2013;8:e77655. doi: 10.1371/journal.pone.0077655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melbye H., Hvidsten D., Holm A., Nordbo S.A., Brox J. The course of C-reactive protein response in untreated upper respiratory tract infection. Br J Gen Pract. 2004;54:653–658. [PMC free article] [PubMed] [Google Scholar]
- 8.Takemura M., Matsumoto H., Niimi A., Ueda T., Matsuoka H., Yamaguchi M. High sensitivity C-reactive protein in asthma. Eur Respir J. 2006;27:908–912. doi: 10.1183/09031936.06.00114405. [DOI] [PubMed] [Google Scholar]
- 9.Thomsen M., Ingebrigtsen T.S., Marott J.L., Dahl M., Lange P., Vestbo J. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA. 2013;309:2353–2361. doi: 10.1001/jama.2013.5732. [DOI] [PubMed] [Google Scholar]
- 10.Deraz T.E., Kamel T.B., El-Kerdany T.A., El-Ghazoly H.M. High-sensitivity C reactive protein as a biomarker for grading of childhood asthma in relation to clinical classification, induced sputum cellularity, and spirometry. Pediatr Pulmonol. 2012;47:220–225. doi: 10.1002/ppul.21539. [DOI] [PubMed] [Google Scholar]
- 11.Kony S., Zureik M., Driss F., Neukirch C., Leynaert B., Neukirch F. Association of bronchial hyperresponsiveness and lung function with C-reactive protein (CRP): a population based study. Thorax. 2004;59:892–896. doi: 10.1136/thx.2003.015768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bisgaard H. The Copenhagen Prospective Study on Asthma in Childhood (COPSAC): design, rationale, and baseline data from a longitudinal birth cohort study. Ann Allergy Asthma Immunol. 2004;93:381–389. doi: 10.1016/S1081-1206(10)61398-1. [DOI] [PubMed] [Google Scholar]
- 13.Bisgaard H., Hermansen M.N., Loland L., Halkjaer L.B., Buchvald F. Intermittent inhaled corticosteroids in infants with episodic wheezing. N Engl J Med. 2006;354:1998–2005. doi: 10.1056/NEJMoa054692. [DOI] [PubMed] [Google Scholar]
- 14.Bisgaard H., Hermansen M.N., Buchvald F., Loland L., Halkjaer L.B., Bonnelykke K. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 15.ATS/ERS statement: raised volume forced expirations in infants: guidelines for current practice. Am J Respir Crit Care Med. 2005;172:1463–1471. doi: 10.1164/rccm.200408-1141ST. [DOI] [PubMed] [Google Scholar]
- 16.Loland L., Bisgaard H. Feasibility of repetitive lung function measurements by raised volume rapid thoracoabdominal compression during methacholine challenge in young infants. Chest. 2008;133:115–122. doi: 10.1378/chest.07-1328. [DOI] [PubMed] [Google Scholar]
- 17.Loland L., Buchvald F.F., Halkjaer L.B., Anhoj J., Hall G.L., Persson T. Sensitivity of bronchial responsiveness measurements in young infants. Chest. 2006;129:669–675. doi: 10.1378/chest.129.3.669. [DOI] [PubMed] [Google Scholar]
- 18.Bisgaard H., Bonnelykke K., Sleiman P.M., Brasholt M., Chawes B., Kreiner-Moller E. Chromosome 17q21 gene variants are associated with asthma and exacerbations but not atopy in early childhood. Am J Respir Crit Care Med. 2009;179:179–185. doi: 10.1164/rccm.200809-1436OC. [DOI] [PubMed] [Google Scholar]
- 19.Bisgaard H., Pipper C.B., Bonnelykke K. Endotyping early childhood asthma by quantitative symptom assessment. J Allergy Clin Immunol. 2011;127:1155–1164.e2. doi: 10.1016/j.jaci.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Chawes B.L., Buchvald F., Bischoff A.L., Loland L., Hermansen M., Halkjaer L.B. Elevated exhaled nitric oxide in high-risk neonates precedes transient early but not persistent wheeze. Am J Respir Crit Care Med. 2010;182:138–142. doi: 10.1164/rccm.200909-1377OC. [DOI] [PubMed] [Google Scholar]
- 21.Bisgaard H., Hermansen M.N., Bonnelykke K., Stokholm J., Baty F., Skytt N.L. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978. doi: 10.1136/bmj.c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bisgaard H., Loland L., Holst K.K., Pipper C.B. Prenatal determinants of neonatal lung function in high-risk newborns. J Allergy Clin Immunol. 2009;123:651–657. doi: 10.1016/j.jaci.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 23.Jackman R.P., Utter G.H., Heitman J.W., Hirschkorn D.F., Law J.P., Gefter N. Effects of blood sample age at time of separation on measured cytokine concentrations in human plasma. Clin Vaccine Immunol. 2011;18:318–326. doi: 10.1128/CVI.00465-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Jager W., Bourcier K., Rijkers G.T., Prakken B.J., Seyfert-Margolis V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol. 2009;10:52. doi: 10.1186/1471-2172-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohidai L., Csaba G. Chemotaxis and chemotactic selection induced with cytokines (IL-8, RANTES and TNF-alpha) in the unicellular Tetrahymena pyriformis. Cytokine. 1998;10:481–486. doi: 10.1006/cyto.1997.0328. [DOI] [PubMed] [Google Scholar]
- 26.Bisgaard H., Jensen S.M., Bonnelykke K. Interaction between asthma and lung function growth in early life. Am J Respir Crit Care Med. 2012;185:1183–1189. doi: 10.1164/rccm.201110-1922OC. [DOI] [PubMed] [Google Scholar]
- 27.Folsgaard N.V., Chawes B.L., Rasmussen M.A., Bischoff A.L., Carson C.G., Stokholm J. Neonatal cytokine profile in the airway mucosal lining fluid is skewed by maternal atopy. Am J Respir Crit Care Med. 2012;185:275–280. doi: 10.1164/rccm.201108-1471OC. [DOI] [PubMed] [Google Scholar]
- 28.Fogarty A.W., Jones S., Britton J.R., Lewis S.A., McKeever T.M. Systemic inflammation and decline in lung function in a general population: a prospective study. Thorax. 2007;62:515–520. doi: 10.1136/thx.2006.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaaban R., Kony S., Driss F., Leynaert B., Soussan D., Pin I. Change in C-reactive protein levels and FEV1 decline: a longitudinal population-based study. Respir Med. 2006;100:2112–2120. doi: 10.1016/j.rmed.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Navratil M., Plavec D., Dodig S., Jelcic Z., Nogalo B., Erceg D. Markers of systemic and lung inflammation in childhood asthma. J Asthma. 2009;46:822–828. [PubMed] [Google Scholar]
- 31.Soferman R., Glatstein M., Sivan Y., Weisman Y. HsCRP levels: measurement of airway inflammation in asthmatic children. Pediatr Int. 2008;50:12–16. doi: 10.1111/j.1442-200X.2007.02517.x. [DOI] [PubMed] [Google Scholar]
- 32.Doganci A., Sauer K., Karwot R., Finotto S. Pathological role of IL-6 in the experimental allergic bronchial asthma in mice. Clin Rev Allergy Immunol. 2005;28:257–270. doi: 10.1385/CRIAI:28:3:257. [DOI] [PubMed] [Google Scholar]
- 33.Hardyman M.A., Wilkinson E., Martin E., Jayasekera N.P., Blume C., Swindle E.J. TNF-α–mediated bronchial barrier disruption and regulation by src-family kinase activation. J Allergy Clin Immunol. 2013;132:665–675. doi: 10.1016/j.jaci.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Ranganathan S.C., Stocks J., Dezateux C., Bush A., Wade A., Carr S. The evolution of airway function in early childhood following clinical diagnosis of cystic fibrosis. Am J Respir Crit Care Med. 2004;169:928–933. doi: 10.1164/rccm.200309-1344OC. [DOI] [PubMed] [Google Scholar]
- 35.Pillarisetti N., Williamson E., Linnane B., Skoric B., Robertson C.F., Robinson P. Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am J Respir Crit Care Med. 2011;184:75–81. doi: 10.1164/rccm.201011-1892OC. [DOI] [PubMed] [Google Scholar]
- 36.McDade T.W. Early environments and the ecology of inflammation. Proc Natl Acad Sci U S A. 2012;109(suppl 2):17281–17288. doi: 10.1073/pnas.1202244109. [DOI] [PMC free article] [PubMed] [Google Scholar]