To the Editor:
Rhinovirus (RV) and human bocavirus 1 (HBoV1) are common causes of respiratory tract infections in early childhood.1 While RV is an RNA virus and causes recurrent infections with new strains, HBoV1 is a DNA virus that may cause prolonged shedding and is mostly found simultaneously with other respiratory viruses.1 Both viruses are associated with early wheezing: RV has been detected in approximately 20% to 40% of the cases, acute HBoV1 infection has been serodiagnosed in 19% of the cases, and RV-HBoV1 coinfection has been detected in 6% of the cases.1 Unlike RV wheeze,2 HBoV1 wheeze has not been linked with an increased risk of asthma in early childhood. The asthma and atopy in children are closely interrelated with increased TH2-type cells, and decreased type I/II/III interferon (IFN) responses and susceptibility to RV infections.3 Less is known about HBoV1, but HBoV1 bronchiolitis has been associated with balanced TH1/TH2-type response in nasopharyngeal mucosa.4 Whether these cytokine responses reflect the immunity of the host or the species of the virus is unclear. Interestingly, cloned HBoV1 has inhibited Sendai virus–induced IFN production in vitro, suggesting that it may also affect the host responses against other viruses in vivo.5 However, in vivo data of virus interference by respiratory virus in humans are lacking.
The objective of this study was to compare the systemic TH1-type, TH2-type, IL-10, and proinflammatory cytokine profiles in young children with sole RV- or sole HBoV1-associated wheezing. Moreover, we wanted to investigate whether codetection of RV and HBoV1 would be associated with a different cytokine response than would detection of either virus alone.
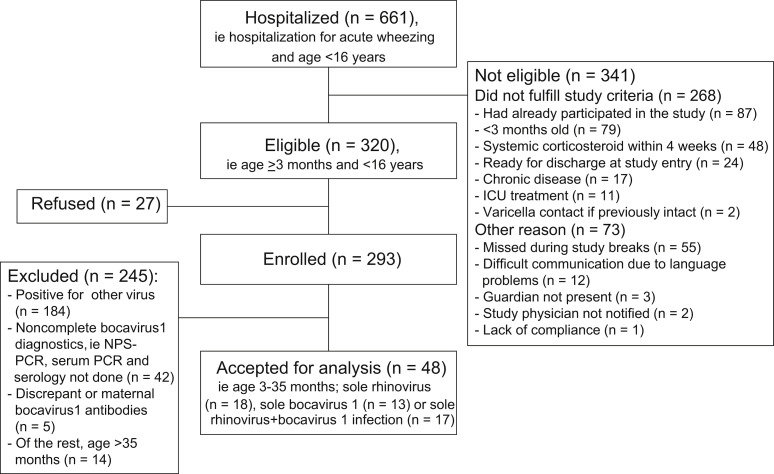
This study is a substudy of the larger Vinku study including children hospitalized for acute wheezing in the Department of Pediatrics, Turku University Hospital, Turku, Finland, during the period from September 2000 through May 2002. (For study flow chart, see Fig E1 in this article's Online Repository at www.jacionline.org.)2, 6 The present study included all 3- to 35-month-old children of the cohort who had their first or second wheezy episode and had sole RV (n = 18), sole HBoV1 (n = 13), or combined RV-HBoV1 infection without evidence of other viruses (n = 17). The study protocol was approved by the Ethics Committee of the Turku University Hospital, and informed consent was obtained from the guardian before commencing the study.
At hospital admission, 16 respiratory viruses were tested. Virus culture was done for adenovirus, enteroviruses, RV, influenza A and B viruses, human metapneumovirus, parainfluenza virus types 1 to 3, and respiratory syncytial virus. Viral antigens were detected for adenovirus, influenza A and B viruses, and respiratory syncytial virus. Levels of specific IgG antibodies were measured from paired serum samples for adenovirus, enteroviruses, influenza A and B viruses, and parainfluenza virus 1 to 4. PCR was used for the detection of adenovirus, coronaviruses (229E, OC43, NL63, and HKU1), enteroviruses, HBoV1, RV (including RV-C species), influenza A and B viruses, metapneumovirus, respiratory syncytial virus, and parainfluenza virus 1 to 4. All acute HBoV1 infections were serologically confirmed.6 Serum taken at study entry was analyzed for the following cytokines by using the Human Cytokine LINCO plex Kit (Millipore Corporation, Billerica, Mass; sensitivities for each cytokine are shown in Table E1 in this article's Online Repository at www.jacionline.org): GM-CSF, IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 (p70), IL-13, and TNF-α.7 Children were followed up for 7 years, and the time to recurrent wheezing was assessed as previously defined.2
The normality of data distribution was tested by using the Kolmogorov-Smirnov test. Because of the skewness of the data, cytokine levels were log10 transformed. For other statistics, t test, Mann-Whitney U test, χ2 test, 1-way ANOVA, Kruskal-Wallis test, Log-rank test, and Cox proportional hazard ratio test were used when appropriate (PASW 18.0 software; SPSS, Inc, Chicago, Ill).
The mean age of the whole cohort was 1.6 years with slight male predominance (69%). Sensitization was detected in 35% of the patients (allergen-specific IgE > 0.35 kU/L for common allergens, Phadiatop Combi; Phadia, Uppsala, Sweden), 67% of the children had their first wheezy episode, and parental asthma was reported in 17% of the children. Long-term controller medication for recurrent wheezing was initiated for 61% of the children during the follow-up, all within the first 2 years. Patient characteristics did not differ between the virus groups (see Table E2 in this article's Online Repository at www.jacionline.org).
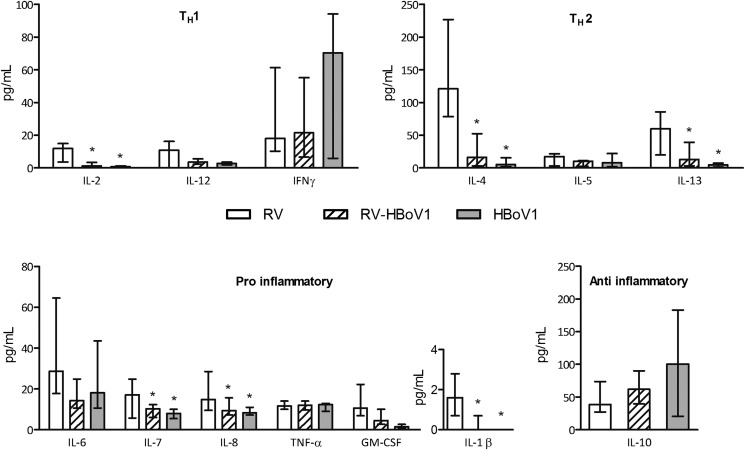
Wheezing children with RV had higher proinflammatory (IL-1β, IL-7, and IL-8), TH 1-type (IL-2), and TH 2-type (IL-4 and IL-13) responses than did those with HBoV1 (Fig 1 ). Interestingly, no differences in the cytokine levels were found between the HBoV1 and RV-HBoV1 coinfection groups, and the cytokine responses seen in the RV group were generally lower in the RV-HBoV1 group (Fig 1). While IFN-γ or IL-10 levels did not differ between the study groups, the IL-4/IFN-γ ratio was higher (median [interquartile range], RV 4.8 [1.7-16.0]; RV-HBoV1 1.4 [0.1-4.2]; HBoV1 0.2 [0.1-0.4]; overall P = .001) and the IL-10/IL-12 ratio was lower (RV 4.1 [2.3-6.4]; RV-HBoV1 17.3 [7.1-29.0]; HBoV1 30.5 [6.5-52.7], respectively; P = .007) in the RV group than in the HBoV1 or RV-HBoV1 groups. Otherwise, no differences in cytokine responses were detectable between the virus groups.
Fig 1.
Cytokine responses in wheezing associated with RV, HBoV1, and combined RV-HBoV1 in young children. The data are expressed as median (interquartile range) and analyzed by 1-way ANOVA and Tukey post hoc comparison. *P < .05 vs the RV group.
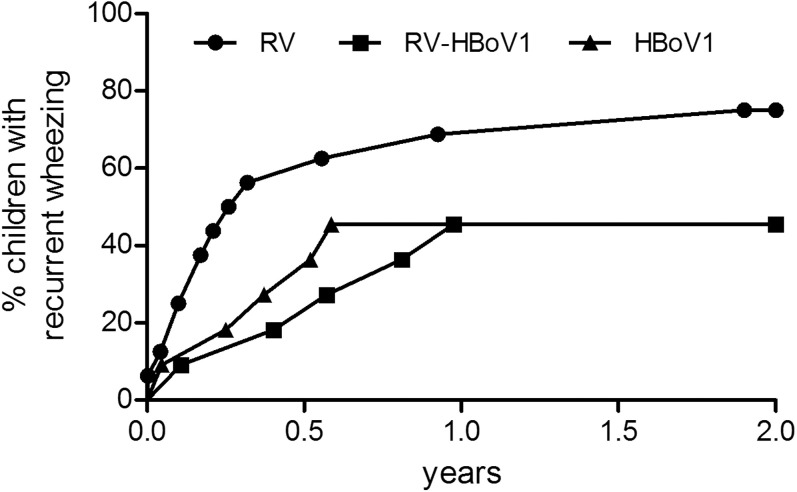
Sensitized children had lower IL-10 and IFN-γ levels and a lower IL-10/IL-12 ratio but a higher IL-4/IFN-γ ratio than did nonsensitized children (P < .05 for both; see Table E3 in this article's Online Repository at www.jacionline.org). On repeating virus group comparisons without sensitized children, all significant differences persisted except for IL-10/IL-12 and IL-4/IFN-γ ratios (data not shown). Wheezing children with RV had a trend to develop recurrent wheezing more often and sooner than did those with RV-HBoV1 or HBoV infection (P = .10, Fig 2 ; see also Table E2).
Fig 2.
Incidence of recurrent wheezing in young children with RV (n = 16), HBoV1 (n = 11) and combined RV-HBoV1 (n = 11)-associated wheezing. The data are expressed as percentage of children with recurrent wheezing after the study entry. Data were analyzed by using the Log-rank test. Overall comparison, P = .10; RV vs RV-HBoV1, P = .07; RV vs HBoV1, P = .11.
Our study shows 2 important findings. First, unlike RV, HBoV1 is not associated with systemic proinflammatory or TH 2-type cytokine responses during acute wheezing. Interestingly, coinfection with RV and HBoV1 resulted in a modified, non–TH 2-type cytokine response. This finding together with previous in vitro data5 suggests that HBoV1 may interfere with RV-induced immune responses. The immunological responses were accompanied by the clinical finding that children with HBoV1 or combined RV-HBoV1 wheeze tended to develop recurrent wheezing later and less often than did those with RV wheeze. Because the severity of RV infections is known to be related to atopic status,8 understanding the mechanism of HBoV1 infection may open new targets to tackle RV-associated undesired events such as recurrent wheeze and asthma development.2, 9
Second, sensitized children had higher IL-4/IFN-γ and lower IL-10/IL-12 ratios in consistence with atopic inflammation. These changes were similar to those reported in RV infection,3 although we were not able to show a significant link between RV and atopy. These findings fit in a hypothesis that early TH2-skewed inflammation exists in RV wheeze before sensitization can be detected.2 Altogether, our findings suggest that immunological responses in acute wheezing are dependent on both host (atopy-related inflammation) and virus-specific factors and that virus-virus interaction may be of significance in modulating these responses.
Footnotes
This study was supported by the European Commission's Seventh Framework program (grant no. 260895 [PreDicta] to H.L., T.V., and T.J.); Academy of Finland, Helsinki (grant no. 1257964 to K.H. and grant numbers 114034 and 132595 to T.J.); the Finnish Medical Foundation (to T.J.); the Sigrid Juselius Foundation, Helsinki (to M.S.-V., K.H., and T.J.); the Foundation for Pediatric Research, Helsinki (to T.J.); the TYKS Foundation, Turku (to H.L.); research funds from specified government transfers, Turku (grant no. 13032 to T.J. and grant no. 13933 to H.L.); the Allergy Research Foundation, Helsinki (to T.J. and H.L.); and the Helsinki University Research Fund (to M.S.-V.), all in Finland.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Appendix
Fig E1.
Study flow chart. ICU, Intensive care unit.
Table E1.
Sensitivities of the assay (in pg/mL) (www.millipore.com)
| Cytokine | Lowest detectable concentration | No. of samples below the detection limit |
|---|---|---|
| IL-1β | 0.19 | 22 of 47 |
| IL-2 | 0.38 | 8 of 48 |
| IL-4 | 2.87 | 7 of 48 |
| IL-5 | 0.12 | 3 of 47 |
| IL-6 | 0.79 | 0 of 47 |
| IL-7 | 0.42 | 4 of 48 |
| IL-8 | 0.32 | 0 of 48 |
| IL-10 | 0.41 | 0 of 48 |
| IL-12 | 0.23 | 6 of 48 |
| IL-13 | 4.06 | 12 of 48 |
| IFN-γ | 0.55 | 6 of 48 |
| GM-CSF | 0.23 | 3 of 48 |
| TNF-α | 0.22 | 0 of 48 |
Table E2.
Patient characteristics
| RV (n = 18) | RV-HBoV1 (n = 17) | HBoV1 (n = 13) | |
|---|---|---|---|
| Age (y), mean ± SD | 1.4 ± 0.6 | 1.6 ± 0.9 | 1.6 ± 0.7 |
| Male | 13 (72%) | 11 (65%) | 9 (64%) |
| Sensitized∗ | 8 (44%) | 3 (18%) | 7 (54%) |
| Aeroallergen | 5 (28%) | 2 (12%) | 0 (0%) |
| Food allergen | 8 (44%) | 3 (18%) | 7 (54%) |
| Eczema | 7 (39%) | 5 (29%) | 5 (36%) |
| Parental | |||
| Asthma | 2 (11%) | 2 (12%) | 3 (21%) |
| Allergy | 10 (56%) | 10 (59%) | 11 (79%) |
| Smoking | 10 (56%) | 3 (18%) | 7 (50%) |
| First wheeze | 10 (56%) | 12 (71%) | 10 (77%) |
| Recurrent wheezing† | 12 of 16 (75%) | 5 of 11 (46%) | 5 of 11 (46%) |
Values are expressed as mean ± SD or n (%). The overall differences between virus groups were tested by using the chi-square test: all P > .10.
Allergen-specific IgE > 0.35 kU/L.
After study entry, P = .19 for overall group comparisons.
Table E3.
Cytokine levels in sensitized vs nonsensitized children
| Sensitized∗ | IQR | Nonsensitized | IQR | P value | |
|---|---|---|---|---|---|
| IL-1β | 0.6 | 0.0-1.7 | 0.0 | 0.0-0.9 | .29 |
| IL-2 | 1.2 | 0.1-9.5 | 1.2 | 0.3-7.7 | .93 |
| IL-4 | 111.9 | 9.8-211.0 | 22.5 | 3.9-107.2 | .62 |
| IL-5 | 4.0 | 1.7-13.8 | 5.8 | 2.1-15.6 | .53 |
| IL-6 | 24.9 | 9.5-53.3 | 18.0 | 10.6-32.7 | .54 |
| IL-7 | 10.9 | 6.6-20.9 | 10.3 | 6.4-16.4 | .99 |
| IL-8 | 12.5 | 8.9-19.6 | 10.0 | 8.1-15.1 | .93 |
| IL-10 | 28.6 | 21.8-46.9 | 69.3 | 29.7-108.3 | .004 |
| IL-12 | 2.6 | 0.0-11.7 | 3.2 | 0.9-7.9 | .80 |
| IL-13 | 38.6 | 9.5-92.0 | 8 | 3.0-54.8 | .25 |
| IFN-γ | 9.1 | 0.0-18.0 | 24.2 | 5.6-79.1 | .02 |
| GM-CSF | 6.5 | 2.5-15.2 | 3.5 | 2.1-9.6 | .28 |
| TNF-α | 13.4 | 10.6-16.6 | 12.1 | 10.2-16.3 | .85 |
| IL-4/IFN-γ | 3.2 | 0.8-12.3 | 0.5 | 0.1-3.4 | .01 |
| IL-10/IL-12 | 5.4 | 2.4-8.1 | 24.1 | 6.0-45.7 | .03 |
All values are in pg/mL and expressed as median (interquartile range, IQR).
Significant differences are shown in italics.
Allergen-specific IgE > 0.35 kU/L for common allergens (Phadiatop Combi; Phadia, Uppsala, Sweden).
References
- 1.Jartti T., Hedman K., Jartti L., Ruuskanen O., Allander T., Soderlund-Venermo M. Human bocavirus—the first 5 years. Rev Med Virol. 2012;22:46–64. doi: 10.1002/rmv.720. [DOI] [PubMed] [Google Scholar]
- 2.Lukkarinen M., Lukkarinen H., Lehtinen P., Vuorinen T., Ruuskanen O., Jartti T. Prednisolone reduces recurrent wheezing after first rhinovirus wheeze: a 7-year follow-up. Pediatr Allergy Immunol. 2013;24:237–243. doi: 10.1111/pai.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baraldo S., Contoli M., Bazzan E., Turato G., Padovani A., Marku B. Deficient antiviral immune responses in childhood: distinct roles of atopy and asthma. J Allergy Clin Immunol. 2012;130:1307–1314. doi: 10.1016/j.jaci.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Chung J.Y., Han T.H., Kim J.S., Kim S.W., Park C.G., Hwang E.S. Th1 and Th2 cytokine levels in nasopharyngeal aspirates from children with human bocavirus bronchiolitis. J Clin Virol. 2008;43:223–225. doi: 10.1016/j.jcv.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Z., Zheng Z., Luo H., Meng J., Li H., Li Q. Human bocavirus NP1 inhibits IFN-beta production by blocking association of IFN regulatory factor 3 with IFNB promoter. J Immunol. 2012;189:1144–1153. doi: 10.4049/jimmunol.1200096. [DOI] [PubMed] [Google Scholar]
- 6.Soderlund-Venermo M., Lahtinen A., Jartti T., Hedman L., Kemppainen K., Lehtinen P. Clinical assessment and improved diagnosis of bocavirus-induced wheezing in children, Finland. Emerg Infect Dis. 2009;15:1423–1430. doi: 10.3201/eid1509.090204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jartti T., Paul-Anttila M., Lehtinen P., Parikka V., Vuorinen T., Simell O. Systemic T-helper and T-regulatory cell type cytokine responses in rhinovirus vs. respiratory syncytial virus induced early wheezing: an observational study. Respir Res. 2009;10:85. doi: 10.1186/1465-9921-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James K.M., Gebretsadik T., Escobar G.J., Wu P., Carroll K.N., Li S.X. Risk of childhood asthma following infant bronchiolitis during the respiratory syncytial virus season. J Allergy Clin Immunol. 2013;132:227–229. doi: 10.1016/j.jaci.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guilbert T.W., Singh A.M., Danov Z., Evans M.D., Jackson D.J., Burton R. Decreased lung function after preschool wheezing rhinovirus illnesses in children at risk to develop asthma. J Allergy Clin Immunol. 2011;128:532–538. doi: 10.1016/j.jaci.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]