Abstract
Enhanced infection and replication of porcine reproductive and respiratory syndrome (PRRS) virus in the presence of specific antibody has been demonstrated in vitro and in vivo, a phenomenon known as antibody-dependent enhancement (ADE). ADE is considered to be a significant obstacle to developing effective vaccines for many viruses for which ADE has been reported, since virus-specific antibodies of maternal origin or those conferred by vaccination can facilitate the entry of the virus into target cells, sometimes resulting in increased severity of the disease. In this study, the role of specific PRRS viral epitopes in ADE and/or virus neutralization (VN) was assessed in vitro using 14 monoclonal antibodies (mAbs) to 4 PRRS viral proteins: nucleocapsid (N), matrix (M), glycoprotein (GP) 5, and GP3. Each mAb recongnized a distinct epitope on one of these proteins. One-way ADE and VN assays were performed in vitro using homologous PRRS virus isolates in the presence or absence of each mAb. ADE activity was determined by detecting a significant increase of progeny virus yield in porcine alveolar macrophage cultures in the presence of individual mAbs. Neutralizing activity was determined by detecting a significant reduction or complete blocking of virus replication in MARC-145 cells in the presence of individual mAbs. mAbs could be categorized into 3 groups: enhancing, neutralizing and neither. Viral epitopes which are capable of inducing neutralizing antibodies appeared to reside on the M, GP3 and GP5 proteins, while epitopes that may induce ADE-mediating antibody were associated with the N and GP5 proteins. Identification of the viral proteins and antigens and epitopes responsible for ADE- and VN-mediating antibodies may provide the basis for developing efficacious second-generation vaccines for the control of PRRS virus; yet, further epitope mapping remains to be done.
Keywords: PRRSV, ADE, VN, Monoclonal antibodies
1. Introduction
For over a decade, porcine reproductive and respiratory syndrome (PRRS) has been a disease of great significance to the swine industry since it first appeared as catastrophic clinical outbreaks in swine herds in North America and Europe in the late 1980s (Collins et al., 1992, Wensvoort et al., 1991). Despite the efforts to control the syndrome, this disease is still responsible for great economic losses for pig producers throughout the world.
Porcine reproductive and respiratory syndrome is caused by PRRS virus (PRRSV), which is a small, enveloped RNA virus that belongs to the family Arteriviridae with equine arteritis virus (EAV), lactate dehydrogenase-elevating virus (LDV) of mice, and simian hemorrhagic fever virus (SHFV) (Cavanagh, 1997, Conzelmann et al., 1993, Meulenberg et al., 1994). Although smaller in size and lacking the surface projections characteristic of coronaviruses, the arteriviruses are classified in order Nidovirales with family Coronaviridae because of common traits in genomic organization and replication strategy (Cavanagh, 1997).
The PRRSV has a polyadenylated, single-stranded, non-segmented, positive-sense RNA genome of 15.1 kbs in size (Benfield et al., 1992, Conzelmann et al., 1993, Meulenberg et al., 1993). The genome consists of 8 open reading frames (ORFs) that are expressed through the production of a nested set of 7 subgenomic 3′ co-terminal mRNAs. ORF 1 encodes for the viral RNA-dependent RNA polymerase. ORFs 2–7 are postulated to encode for structural proteins, but only 3 proteins have been consistently identified in virions and/or lysates of virus-infected cells. These are the 15 kD nucleocapsid (N), 19 kD matrix (M), and 25 kD envelope (E or GP5) proteins that are encoded by ORFs 7, 6, and 5, respectively (Bautista et al., 1996, Conzelmann et al., 1993, Meulenberg et al., 1993, Meulenberg et al., 1995, Nelson et al., 1994, Yoon et al., 1995). Proteins encoded by ORFs 2–4 are designated GP2, GP3, and GP4, where ‘GP’ indicates ‘glycoprotein’ and the number designates the ORF from which it is derived (Meulenberg and Petersen-den Besten, 1996, van Nieuwstadt et al., 1996). They are postulated to be associated with the viral membrane.
The PRRSV possesses four characteristics that may contribute to difficulties in diagnosis and control of the disease, including production of effective vaccines (Yoon, 2003). These are: (1) tropism for macrophage or macrophage-lineage cells; (2) remarkable antigenic variation among PRRSV field isolates; (3) enhancement of virus infection by the presence of antibody, known as antibody-dependent enhancement (ADE); and (4) ability to establish persistent infection. Tropism for macrophages is a significant impediment for exposed animals to develop effective local and systemic immunity (Van Reeth and Adair, 1997). The antigenic variability has the potential of rendering useless any preexisting antibody that once was capable of neutralizing the virus and permits the development of new strains that can evade the immune system or revert to virulence (Poland et al., 1996, Vennema et al., 1998). ADE can facilitate the attachment and internalization of the virus into its host cells, such as macrophages and monocytes, through Fc receptor-mediated endocytosis using antibody present at subneutralizing levels (Cancel-Tirado and Yoon, 2003). Persistence has significant epidemiological implications related to virus perpetuation in a herd and transmission to naïve animals (Zimmerman et al., 1992).
ADE of virus infection has been described for various viruses belonging to 12 different families of various taxa and host (Cancel-Tirado and Yoon, 2003, Kurane et al., 1991). Among them are the human immunodeficiency virus type 1 (HIV-1) (Mascola et al., 1993, Prohaszka et al., 1997, Robinson et al., 1989), Ebola virus (Takada et al., 2001), West Nile virus (Fagbami et al., 1987), influenza virus (Ochiai et al., 1992), dengue virus (DV) (Burke et al., 1988, Halstead et al., 1984), feline infectious peritonitis virus (FIPV) (Corapi et al., 1992), equine infectious anemia virus (Wang et al., 1994), Aleutian disease virus of mink (Porter et al., 1980) and, foot and mouth disease virus (Mason et al., 1994), to name a few. ADE plays a role in enhancing the opportunity for viruses to infect target cells, and exacerbation of disease due to ADE has also been well documented for DV and FIPV infections (Burke et al., 1988, Kliks, 1990, Kliks et al., 1988, Sangkawibha et al., 1984, Szabo et al., 1999). The mechanism and biological significance of ADE are well described in a recent review article (Cancel-Tirado and Yoon, 2003).
ADE has been considered one of the major impediments to the development of efficacious vaccines for certain viruses such as FIPV, DV, and HIV-1 (Mascola et al., 1993, Morens, 1994, Olsen, 1993). To minimize the risk associated with ADE in controlling disease by vaccination, efforts have been made to formulate vaccines inducing a balanced immune response and to identify viral component(s) associated with ADE or neutralization of virus infection (Mascola et al., 1993, Morens, 1994). In addition, numerous researchers have studied the mechanisms of ADE, particularly cellular events besides simple increased uptake of the virus coupled with antibody (Ochiai et al., 1992, Prohaszka et al., 1997). The following study was conducted to characterize the role of PRRS viral proteins in ADE and virus neutralization and identify responsible epitope(s) recognized by monoclonal antibodies, working toward the development of a subunit vaccine that would offer protection against PRRSV.
2. Materials and methods
2.1. Experimental design
This study attempted to identify epitope(s) associated with neutralization and/or ADE of infection. The role of individual PRRS viral epitopes in ADE and virus neutralization was determined using a panel of 14 murine monoclonal antibodies (mAbs) specific for various PRRSV proteins. Prior to conducting ADE or VN tests, the compatibility between the Fc portion of murine mAb and Fc receptors (FcR) on porcine alveolar macrophages (PAM) was tested by a rosette formation assay. The amount of PRRSV-specific antibody in each ascitic fluid was measured by indirect fluorescent antibody (IFA) test, and adjusted to the same level. Enhancing or neutralizing activity of each mAb was then assessed utilizing an in vitro ADE assay and one-way virus neutralization (VN) test, respectively. Differences in virus progeny production between treated and non-treated groups were calculated and statistically compared.
2.2. PRRSV strains
Two PRRS viruses designated ISU-P and KY-35 were used in ADE and VN assays. Both isolates were collected from diseased pigs in swine operations in Iowa and Kentucky, respectively, and were used as antigens for producing the panel of mAbs used in this study (Table 1 ). These viruses shared most of the epitopes studied except those recognized by mAbs ISU19C, ISU45Ad and ISU45B. Both viruses were propagated in MARC-145 cells, a highly permissive clone of the African Monkey kidney cell line MA104 (Kim et al., 1993), and, after titration, aliquoted and kept frozen in minus 80 °C until used.
Table 1.
Characteristics of PRRSV monoclonal antibodies (mAb) used
| mAb | Specificity | IFA titer (log 10) | Homologous virus | Reactivity to mAbb |
|
|---|---|---|---|---|---|
| KY35 | ISU-P | ||||
| ISU15A | Nucleocapsid (15 kD) | 7 | ISU-P | + | + |
| ISU15B | 7 | ISU-P | + | + | |
| ISU15C | 7 | ISU-P | + | + | |
| ISU15D | 6 | ISU-P | + | + | |
| ISU15E | 6 | ISU-P | + | + | |
| ISU15Hda | 6 | KY-35 | + | + | |
| ISU19Ada | Matrix (19 kD) | 4 | KY-35 | + | + |
| ISU19Bda | 6 | KY-35 | + | + | |
| ISU19Cda | 4 | KY-35 | + | − | |
| ISU25A | GP5 (25 kD) | 5 | KY-35 | + | + |
| ISU25B | 5 | KY-35 | + | + | |
| ISU25C | 5 | KY-35 | + | + | |
| ISU45Ad | GP3 (43 kD) | 4 | KY-35 | + | − |
| ISU45B | 4 | KY-35 | + | − | |
Subfix “d” indicates that given mAb is specific for a discontinuous epitope.
Adapted from Yang et al. (2000). Both strains are considered homologous regarding all epitopes except ISU19Cd, ISU45Ad and ISU45B mAbs, which are only found in KY-35.
2.3. Cells
Porcine alveolar macrophages (PAM) and MARC-145 cell lines were used in ADE assays. The PAM were collected from 5- to 6-week-old piglets free of PRRSV through lung lavage as previously described (Yoon et al., 1996) and used for ADE assay. PAM cultures were prepared by quickly thawing frozen cells, suspending them in RPMI-1640 (Sigma Chemical Co., St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone Laboratories, Logan, UT, USA), 50 μg/ml gentamicin (Sigma Chemical Co.) and an antibiotic–antimycotic mixture composed of 100 μg/ml streptomycin, 100 IU/ml penicillin and 25 μg/ml amphotericin B (Sigma Chemical Co.), and seeding in multi-well-plates. The cells were used after 24 h incubation at 37 °C in a humid 5% CO2 incubator.
MARC-145 cells were used for virus titrations and VN assays. For use, the cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Sigma Chemical Co.) supplemented with 200 mM l-glutamine (Gibco/BRL Life Science, Grand Island, NY, USA), 10% FCS, 50 μg/ml gentamicin (Sigma Chemical Co.), and the antibiotic–antimycotic mixture. The cells were used for the assays after 24–48 h incubation at 37 °C in a humidified 5% CO2 incubator.
2.4. PRRSV-specific monoclonal antibodies
A total of 14 mAbs specific for PRRSV were obtained as mouse ascites and used in the study. Each mAb was specific for distinct epitopes on N, M, GP5 or GP3 proteins. Protein specificity and antibody level of each mAb and PRRSV strains used for production of mAbs are summarized in Table 1. Production, protein specificity and other characteristics of these mAbs have been previously described (Yang et al., 1999, Yang et al., 2000). All mAbs were of IgG1 isotype as determined by a commercial mouse mAb isotyping kit (IsoStrip® Mouse Monoclonal Antibody Isotyping Kit, Roche Diagnostic Corporation, Indianapolis, IN, USA). The level of PRRSV-specific antibody in each mAb was determined by a commercial ELISA kit (HerdCheck® PRRS, IDEXX Laboratory, Westbrook ME, USA) and by an indirect fluorescent antibody test. The level of antibody in each mAb was then adjusted to the same titer (approximately 1:10,000) according to IFA test results.
2.5. Indirect fluorescent antibody test
For the preparation of viral antigen, 24-day-old MARC145 monolayers were infected with 200 μl of a 104 TCID50 viral suspension of ISU-P strain of PRRSV and incubated at 37 °C. Normal cell controls were prepared in the identical manner with cell culture medium instead of the virus. After 48 h of incubation the cells were washed twice, with 0.01 M phosphate buffered saline (PBS), pH 7.0 and fixed with 80% acetone aqueous solution. A series of 10-fold dilution (101 to 109) was made with each mAb in PBS. Fifty microliters of each mAb dilution were added in triplicate wells and incubated at 37 °C for 30 min in a humidified environment. Unbound mAbs were washed off from wells as noted above. The presence of antigen–antibody reaction was visualized by adding 50 μl of goat anti-murine IgG conjugated to fluorescein isothiocyanate (FITC, Kirkegaard and Perry Laboratories Inc., Gaithesburg, MD, USA) to each well and performing immunofluorescence microscopy. The PRRSV-specific antibody titer of each mAb was expressed as reciprocal of the highest dilution in which specific fluorescence was observed in all 3 wells.
2.6. Enzyme-linked immunosorbent assay
A commercial ELISA kit (HerdCheck PRRS, IDEXX Laboratories Inc.) was used to determine PRRSV-specific antibody in each mAb as directed by the manufacturer with two exceptions. Instead of using the anti-porcine conjugate provided with the kit, goat anti-mouse IgG labeled with peroxidase (Kirkegaard and Perry Laboratories Inc.) was substituted. The presence or absence of specific antibody at a given dilution was determined using optical density (OD) rather than sample-to-positive (S/P) ratio because the kit is designed and adjusted to detect swine IgG in sera. Samples with OD values higher than the upper limit of 95% confidence interval of mean OD from normal mouse ascites were considered to be positive for antibody to PRRSV.
2.7. Rosette assay
The compatibility between murine antibody and Fc receptors on porcine macrophages was determined by a rosette assay utilizing sheep red blood cells (sRBC) coated with murine anti-sRBC polyclonal antibody. Murine anti-sRBC serum was obtained from BALB/c mice following a series of intraperitoneal inoculations of 0.5 ml of a 50% suspension of freshly collected, washed sRBC at a 2-week interval, and the level of anti-sRBC antibody was endpoint titrated by a hemagglutination assay. Antibody-coated sRBC were prepared by mixing 1% (w/v) sRBC suspension with an equal volume of anti-sRBC serum at a subagglutinating level and incubating the mixtures for 30 min at 37 °C, then an additional 30 min at 4 °C.
For the assays, porcine alveolar macrophages (PAM) were prepared in polystyrene 6 cm cell culture Petri dishes (Corning Inc., Corning, NY, USA) at a concentration of 106 cells per ml of media and incubated in RPMI 1640 medium supplemented with 10% FCS, 10 mM HEPES (Sigma Chemical Co.) and the antibiotic–antimycotic mixture for 24 h at 37 °C in 5% CO2 atmosphere. The cells were gently washed once with PBS and then 2 ml of 1% antibody-coated sRBC suspension was added. The control cells received the same amount of uncoated sRBC. The mixture was incubated for 60 min at 37 °C and unbound sRBCs were removed by rinsing with PBS. The cells were fixed and stained with Diff-Quick staining (Difco Laboratories, Detroit, MI, USA) as directed by the manufacturer, and observed under 100–200× magnification. A total of 200 cells in randomly selected microscopic fields were counted and the proportion of rosette forming cells was calculated. A rosette was defined as a macrophage with at least three sRBC associated with its membrane. The assay was independently repeated three times, and a mean value between treated and control groups was calculated and analyzed by Student's t-test.
2.8. ADE assay
Antibody-dependent enhancement of PRRSV infection was assessed by measuring increases in progeny virus yield as previously described (Yoon et al., 1996, Yoon et al., 1997). Briefly, PRRSV ISU-P (or KY-35 for mAbs isu19c, ISU45Ad and B) was diluted to a 104 TCID50/ml in FCS-free RPMI 1640 medium. For test groups, each virus suspension was mixed with an equal volume of each of the pre-standardized, serially diluted mAbs (2−1 to 2−8 after diluted 1:10) to be tested. Control groups were prepared by mixing each of the viruses with an equal volume of PRRSV-ADE positive serum collected from experimentally infected pigs or no antibodies at all in the same manner described above. The virus-mAb mixtures were incubated at 37 °C for an h and then 200 μl aliquots of each mixture was inoculated in triplicates onto PAM prepared in 96-well-plates at a ratio of 1 × 104 PAM/well 24 h earlier, resulting in 0.1 multiplicity of infection (MOI). After incubation for 1 h at 37 °C, the inoculum was replaced with RPMI 1640 medium with 10% FCS, followed by an incubation period of 2 days at 37 °C. At the end of the incubation period, the cells were subjected to 2 cycles of freeze-thawing at minus 80 °C and 37 °C, respectively, to disrupt any cells that may still contain the virus. The amount of PRRSV in each cell lysate was quantitated by virus titration as described below.
2.9. Virus titration
Each sample containing PRRSV was serially diluted and 100 μl of each diluted sample was inoculated in triplicates onto confluent MARC-145 monolayers prepared in 96-well-plates 24–48 h earlier. Plates were incubated for 2 days at 37 °C in a humid 5% CO2 incubator, and then the cells were fixed with 80% acetone aqueous solution. The presence of PRRSV was determined by staining fixed cells with a cocktail of PRRSV-specific mAbs SDOW17 and SR30 (Rural Technologies Inc., Brookings, SD, USA). The virus titer in each sample was then determined by counting fluorescent foci in wells showing between 50 and 100 foci and expressed as fluorescent foci units (FFU) per milliliter.
2.10. Virus neutralization assay
Neutralizing activity of individual mAbs was assessed using a fluorescent foci reduction assay with the KY-35 PRRSV strain. Briefly, a virus suspension containing approximately 100 FFU/ml based on pre-determined titer was mixed with an equal volume of each standardized mAb. Virus controls were prepared in an identical manner except for mixing with PRRSV-VN positive serum from an experimentally infected pig or FCS-free minimal essential medium (MEM, Sigma Chemical Co.) instead of mAb. Virus-mAb, virus-serum or virus-medium mixtures were incubated for 1 h at 37 °C. After that, 200 μl of each mixture were added to triplicate wells containing 24- to 48 h-old confluent MARC-145 cells monolayers in 96-well-plates. After incubation for 1 h at 37 °C, the inoculum was replaced with fresh MEM medium supplemented with 10% FCS and the antibiotic–antimycotic mixture. Then, cells were further incubated for 2–3 days at 37 °C in a humid 5% CO2 incubator. At the end of the incubation period, the monolayers were fixed with 80% acetone aqueous solution and stained with the anti-PRRSV mAb cocktail. Virus titers in samples treated with individual mAbs were expressed as FFU/ml as described above and compared to those in the virus control.
2.11. Data analysis
Results of neutralization assays were analyzed by calculating the percent reduction of progeny virus yields (FFUs) in the presence of mAb compared to those in the absence of mAb. When reduction of ≥60% in the yield was observed, mAbs were considered to possess neutralizing activity. The cut-off % reduction was set based on procedures used in previous studies by other investigators (Corapi et al., 1992, Yang et al., 2000, Yoon et al., 1997).
Progeny virus yields from ADE assays were determined by calculating FFU/ml for all dilutions of each mAb in the assay as well as in the virus only controls. Due to the work volume, progeny virus yields (FFU/ml) were assessed for a set of two or three mAb-treated virus groups and one untreated virus control at a time. At a given dilution, geometric means of progeny virus yields between mAb-treated and untreated groups were statistically compared using Wilcoxon–Kruskal–Wallis (rank sum) tests. Pair-wise comparisons were performed using both Dunnette's method and Student's t-test using the Bonferroni correction to guard against type I error inflation. For all dilutions, data between treated and untreated groups was statistically compared by the general regression model (Petrie and Watson, 1999). Significance of slopes and intercepts was determined at P ≤ 0.01.
3. Results
In the rosette assay, approximately 80% of the PAM counted formed rosettes when they were incubated with sRBC coated with murine anti-sRBC antibodies, while less than 7% of PAM formed rosettes with sRBC pre-treated with normal mouse serum, demonstrating that the Fc receptors on PAM can react with the Fc portion of murine immunoglobulins (Fig. 1 ).
Fig. 1.
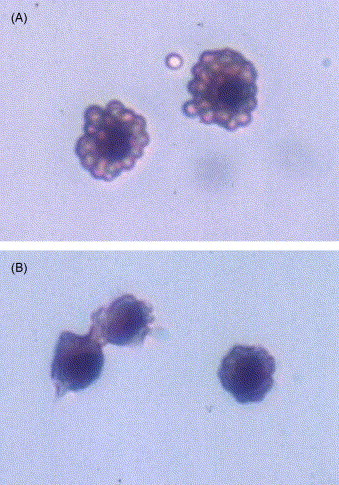
Photomicroscopy of H&E stained porcine alveolar macrophages (PAM) exposing to sheep red blood cells (sRBC) pre-treated with murine anti-sRBC serum (A) and untreated sRBC (B). Panel A shows rosette formation indicating the compatibility between porcine Fc receptor and murine immunoglobulin. Observed under light microscope at 200× magnification.
Each mAb was further characterized for protein concerntration and antibody titer through ELISA and IFA, which were needed for standardization among the mAbs to be used. IFA antibody titers of individual mAbs ranged from 104 to 107 (Table 1). On ELISA, mAbs specific for the N protein (i.e., ISU15A to Hd) showed detectable antigen-specific response; however, no linear relationship could be obtained between optical densities and dilutions (data not shown). It is believed that a sigmoidal response in ELISA could be avoided if OD values had been converted to S/P ratio as the ELISA kit was intended for swine sera. In addition to the non-linear plot of read-out, antigen-specific reactivity could not be demonstrated on the commercial ELISA with some mAbs, particularly those specific for M and GP5 proteins, which may be attributed to the lack of particular mAb epitopes in the ELISA kit or the difference in antigenic format (i.e., linear versus conformational). Thus, mAbs were standardized so that all mAbs would have a titer similar to the mAb with the lowest titer according to IFA test results, i.e., 104 IFA titer.
Neutralizing activity of mAbs against PRRSV isolate KY-35 is summarized in Fig. 2 . Monoclonal antibodies specific for the N protein (ISU15A to Hd) did not neutralize infection of MARC-145 cells by PRRSV. Using 60% reduction in progeny virus production as a cut-off, mAbs ISU19A, ISU25C and ISU45B were determined to have neutralizing activity to KY-35. Replication of KY-35 in MARC-145 was not inhibited when the viruses were treated with the remaining mAbs.
Fig. 2.
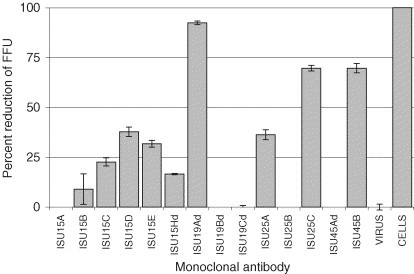
Relative susceptibility of PRRSV (isolate KY-35) to neutralization by monoclonal antibodies (mAbs) specific for distinct epitopes on various PRRSV proteins. Numbers in designation represent molecular masses of PRRS viral protein for which individual mAbs are specific. FFU stands for foci forming units. Each data point represents the mean value of triplicate measurements of each treatment.
In vitro ADE assays using serially diluted mAbs revealed that mAbs tested at the lowest dilution (approximately 103 IFA units) could be categorized into 3 groups: enhancing, suppressing and neither (Fig. 3 ). Based on progeny virus yield, significant enhancement of PRRSV infection (P ≤ 0.01) was observed only with ISU25B, suggesting that the epitope represented by this antibody is associated with ADE of PRRSV infection. Mean virus yield from PAM infected with PRRSV in the presence of mAb ISU25B was of 1.5 log10 higher than that from PAM exposed to the virus only. On the other hand, infectivity and/or replication of PRRSV in PAMs was significantly suppressed (P ≤ 0.01) after treatment with mAbs ISU15B, ISU15Hd, and ISU45B. Mean progeny virus yields were reduced by 0.6 log10 to 5 log10 relative to those of their respective untreated control group. Neither significant enhancement nor suppression of progeny virus production in PAMs was observed when PRRSV was pre-treated with the remaining mAbs.
Fig. 3.
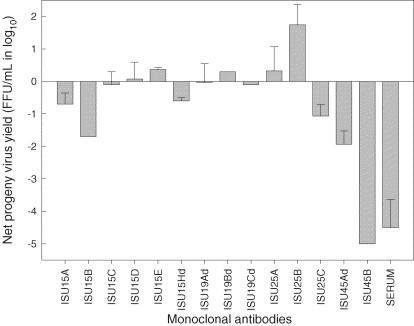
Production of infectious progeny PRRSV in porcine alveolar macrophages. Cells were infected with 0.1 MOI virus in the presence of monoclonal antibodies specific for distinct epitopes at a rate of approximately 1:1000 IFA antibodies. Each bar represents the geometric mean of net progeny virus yield (foci forming units per milliliter) relative to untreated control group. Error bars are the standard deviation of the mean.
Significant enhancing activity (P < 0.001) of mAb ISU25B for PRRSV replication continued to be present over a series of dilutions, i.e., lower antibody concentration (Fig. 4 ). In addition, progeny virus yield in PAMs appeared to increase when PRRSV (ISU-P) was pre-treated with mAb ISU15E (P = 0.04) at higher dilutions (Fig. 5 ).
Fig. 4.
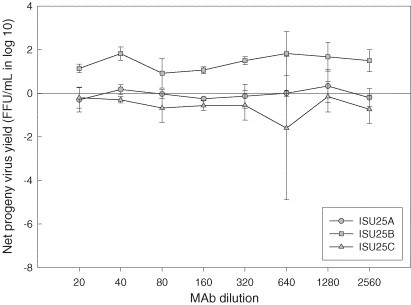
Production of infectious progeny PRRSV in porcine alveolar macrophages. Cells were infected with ISU-P treated with monoclonal antibodies specific for distinct epitopes of PRRSV major envelope protein. Values represent mean difference in progeny virus yields between treated and untreated groups at each dilution. Error bars are the standard deviation of the mean.
Fig. 5.
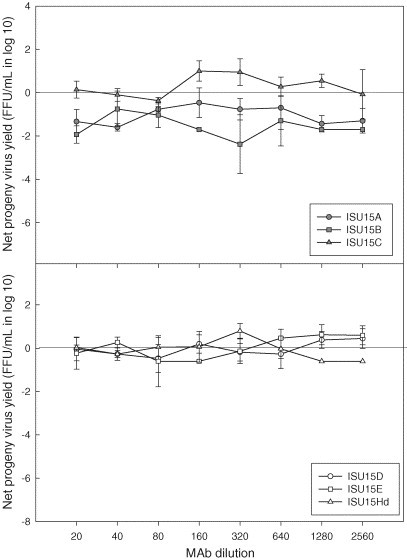
Production of infectious progeny PRRSV in porcine alveolar macrophages. Cells were infected with ISU-P treated with monoclonal antibodies specific for distinct epitopes of PRRSV nucleocapsid protein. Values represent mean difference in progeny virus yields between treated and untreated groups at each dilution. Error bars are the standard deviation of the mean.
Some of the mAbs that significantly suppressed progeny virus production in PAMs at the lowest dilution (i.e., ISU15B and ISU45B) retained the same effect on PRRSV replication even after their concentration was diluted (Fig. 5, Fig. 6 ). Although ISU19Cd showed a similar effect (Fig. 7 ), statistically this mAb along with other mAbs specific for epitopes on M protein (ISU19Ad and ISU19Bd) was not considered to be a suppressing mAb over a series of dilutions. In addition to these mAbs, mAbs ISU15A and ISU25C were determined to significantly suppress the production of PRRSV progeny virus in PAMs over a series of dilutions at P ≤ 0.01 (Fig. 4, Fig. 5). In comparison, mAb ISU15Hd did not show significant suppressing activity at any dilution except the lowest (Fig. 5).
Fig. 6.
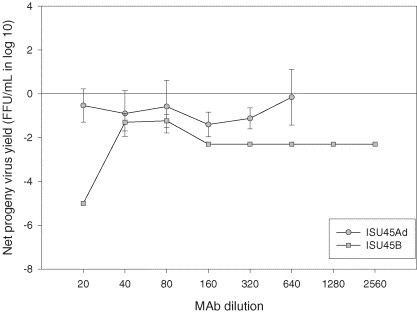
Production of infectious progeny PRRSV in porcine alveolar macrophages. Cells were infected with KY-35 (ISU45Ad) or ISU-P (SIU45B) treated with monoclonal antibodies specific for distinct epitopes of PRRSV GP3. Values represent mean difference in progeny virus yields between treated and untreated groups at each dilution. Error bars are the standard deviation of the mean.
Fig. 7.
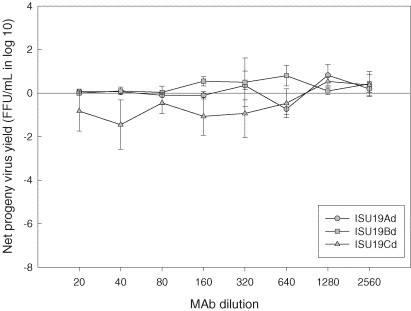
Production of infectious progeny PRRSV in porcine alveolar macrophages. Cells were infected with KY-35 (ISU19Cd) or ISU-P (ISU19Ad and Bd) treated with monoclonal antibodies specific for distinct epitopes of PRRSV matrix protein. Values represent mean difference in progeny virus yields between treated and untreated groups at each dilution. Error bars are the standard deviation of the mean.
4. Discussion
The present study attempted to identify antigens and epitopes associated with ADE of PRRSV and those responsible for virus neutralization using a panel of well-characterized mAbs against epitopes on various PRRSV proteins. Monoclonal antibodies have been commonly used for this purpose since each mAb recognizes a distinct epitope. Furthermore, the usefulness of mAbs for this purpose has been proven by other investigators (Corapi et al., 1992, Halstead et al., 1984, Hohdatsu et al., 1991, Morens et al., 1987a, Prohaszka et al., 1997).
In general, ADE of infection by enveloped viruses is believed to be mediated by epitope(s) associated with envelope protein or membrane-associated protein(s) (Cancel-Tirado and Yoon, 2003). Our observations with PRRSV mAbs were in agreement with other investigators’ observations. Epitopes associated with ADE are determined to reside on the 15 kD nucleocapsid and 25 kD envelope (GP5) proteins, whereas epitopes of the 19 kD matrix, GP5 and 45 kD GP3 proteins were mainly associated with virus neutralization based on the panel of mAbs used for the study. It is worthwhile to note that mAbs ISU25C and ISU45B suppressed PRRSV replication in both the MARC-145 cell line and PAMs. Involvement of GP5 protein in inducing neutralizing or enhancing antibodies has been demonstrated by our laboratory and others (Gonin et al., 1999, Kwang et al., 1999, Weiland et al., 1999, Yoon, 2003, Yang et al., 2000, Yoon et al., 1996). It was, however, unexpected that an antigenic determinant of the GP3 protein, recognized by mAb ISU45B, would be associated with inhibition of PRRSV replication. Statistically, mAb ISU45B showed much stronger inhibition of PRRSV replication in porcine alveolar macrophages (PRRSV natural target cells) than mAb ISU25C that recognizes an epitope on the GP5 protein (P < 0.01). For some North American PRRSV isolates, GP3 protein has been reported as a non-structural protein. In contrast, it has been described as a structural protein for some European PRRSV isolates (Meulenberg and Petersen-den Besten, 1996). A recent study reported that ORF3 product of a Spanish PRRSV strain from recombinant Baculoviruses provided pigs with protective immunity against PRRSV challenge (Plana Duran et al., 1997). Further investigation remains to determine the role of GP3 in PRRSV pathogenesis or immunity.
PRRSV infection was also enhanced or suppressed when treated with mAbs specific to epitopes on the nucleocapsid (N) protein (i.e. ISU15E enhanced, ISU15A and B suppressed). These were unexpected observations since no part of the N protein has been demonstrated to be exposed to the outside of intact virions. Theoretically, such observations are possible if naked or partially stripped viruses containing intact infectious RNA were in the virus preparation used for the assay, which is likely since the virus material used in our study was not purified through zonal centrifugation. However, it also has been demonstrated that the requirement of intact infectious virions for infection in permissive cells can be bypassed through ADE pathway for virus entry (Mason et al., 1994). Consequently, our observation on ISU15E may have a significant implication in the pathogenesis of PRRSV. The 15 kD N protein is known to be highly immunogenic, and abundant expression of ORF 7 (the gene encoding for the N protein) in the early stage of infection has been demonstrated in vitro and in vivo (Bautista et al., 1996, Loemba et al., 1996, Mardassi et al., 1994, Yoon et al., 1996). Likewise, it has also been reported that high levels of non-neutralizing antibody, most of which is specific for the N protein, are produced in pigs following exposure (Nelson et al., 1994, Yoon et al., 1996). Subsequently, PRRSV could take advantage of ADE for entry to target cells (i.e., macrophages or macrophage-lineage cells). Nevertheless, as demonstrated in our results, some of the anti N antibodies may have a suppressive effect, but as stated above this could be related to the presence of naked virus or virions partially stripped from their envelope in the virus suspension used in the assay.
In vitro assessment of ADE activity by antibody can be affected by various factors. These include isotype or subtype of immunoglobulin (Hohdatsu et al., 1994) and assay conditions such as virus strain (e.g., homologous versus heterologous), antibody titer, multiplicity of infection and parameter(s) for measuring enhancement (Morens et al., 1987a, Morens et al., 1987b, Ochiai et al., 1992, Robinson et al., 1989, Szabo et al., 1999). All of these factors were taken into consideration for the study, including isotype determination. All mAbs used were of the IgG1 subtype, thus, any results from ADE or neutralization assays described in the present study are independent of the immunoglobulin isotype/subtype and a property intrinsic to the epitope bound. Although all isotypes of murine IgG were shown to be capable of enhancing virus infection to macrophages of murine origin (Morens et al., 1987a), our observations of ADE of PRRSV infection to PAM by mAbs of the IgG1 subtype are distinct from results of previous studies on ADE of FIPV infection to macrophages of feline origin by mAbs. Corapi et al. evaluated the ability of 19 mAbs specific for the S envelope protein of FIPV to induce ADE of infection in feline peritoneal macrophages (Corapi et al., 1992). All mAbs were capable of neutralizing the ability of the virus to infect a permissive cell line. Fifteen of 19 mAbs induced ADE of infection in macrophages and all but one were of the IgG2a subclass. The remaining four mAbs that did not induce ADE of infection were IgG1. The difference in the isotypes between neutralizing mAbs that induced ADE and those that did not induce ADE suggests that there might be a restriction in the subclasses capable of mediating ADE of virus infection. It is also possible that the difference in the ability of FIPV-specific murine IgG isotypes to mediate ADE of infection is due to differences in the binding affinity of murine isotypes to FcR on feline macrophages (Hohdatsu et al., 1994). It remains to be further studied whether similar isotype restriction exists in murine mAbs for their capability of enhancing or suppressing PRRSV infection to PAM.
It should be noted that specific classification of PRRSV mAbs as to their role in VN and ADE may not be conclusive for some mAbs, since antibody concentration of individual mAbs were adjusted to the same or similar antibody level for quality control of the in vitro assays. Previous studies have suggested that PRRSV infection can be inhibited or enhanced in the presence of antibody, depending upon the ratio between immunoglobulins and available specific epitopes or between antibody molecules and virion numbers (Hawkes and Lafferty, 1967, Porterfield, 1986, Yoon et al., 1996, Yoon et al., 1997). The same observation was also made in this study with polyclonal anti-PRRSV swine serum and may be attributed to the fact that an antiserum consists of various subpopulations of antibodies, each of which represents different specificity, affinity, and avidity. Since a mAb recognizes a single epitope, such a phenomenon was not expected to occur. As anticipated, the role of individual mAbs in ADE and VN was distinct as shown by the general regression model, except to ISU19C. This mAb suppressed PRRSV replication when the virus was treated with it at higher concentration (P ≤ 0.01). As antibody concentration was lowered by serial dilution the suppressive effect of this mAb disappeared and became enhancing; however, such a trend was not considered statistically significant at P ≤ 0.01.
Antibody-dependent enhancement of virus infection is a significant obstacle to the development of effective vaccines to control certain viral diseases of public health and veterinary importance. The ADE of PRRSV infection has been suspected as one of the possible reasons for the relative ineffectiveness of vaccination in controlling PRRS. Our present study provides the antigenic basis for developing subunit vaccines that would induce protective immunity with minimal or no risk for ADE. However, molecular characterization of epitopes that were postulated to induce enhancing and neutralizing antibodies in this study remains to be further explored. The biological significance of our findings in pigs also remains to be determined as the work using murine monoclonal antibodies represents only a model for solving the problem of ADE. Practical application of our findings remains to be seen in an animal study. Furthermore, the presence and absence of epitopes associated with ADE and/or VN should be further assessed using expanded mAbs.
Acknowledgement
We thank Dr. Kenneth Platt in the Department of Veterinary Microbiology and Preventive Medicine at the Iowa State University for kindly providing the valuable monoclonal antibodies for this study. The study was supported in part by funding from the Iowa Livestock Health Advisory Council and National Pork Producers Council on behalf of the National Pork Board.
References
- Bautista E.M., Meulenberg J.J., Choi C.S., Molitor T.W. Structural polypeptides of the American (VR-2332) strain of porcine reproductive and respiratory syndrome virus. Arch. Virol. 1996;141:1357–1365. doi: 10.1007/BF01718837. [DOI] [PubMed] [Google Scholar]
- Benfield D.A., Nelson E., Collins J.E., Harris L. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332) J. Vet. Diagn. Invest. 1992;4:127–133. doi: 10.1177/104063879200400202. [DOI] [PubMed] [Google Scholar]
- Burke D.S., Nisalak A., Johnson D.E., Scott R.M. A prospective study of dengue infections in Bangkok. Am. J. Trop. Med. Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- Cancel-Tirado S.M., Yoon K.-J. Antibody dependent enhancement of virus infection and disease. Viral Immunol. 2003;16:69–86. doi: 10.1089/088282403763635465. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 1997;142:629–633. [PubMed] [Google Scholar]
- Collins J.E., Benfield D.A., Christianson W.T., Harris L. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J. Vet. Diagn. Invest. 1992;4:117–126. doi: 10.1177/104063879200400201. [DOI] [PubMed] [Google Scholar]
- Conzelmann K.K., Visser N., Van Woensel P., Thiel H.J. Molecular characterization of porcine reproductive and respiratory syndrome virus, a member of the arterivirus group. Virology. 1993;193:329–339. doi: 10.1006/viro.1993.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corapi W.V., Olsen C.W., Scott F.W. Monoclonal antibody analysis of neutralization and antibody-dependent enhancement of feline infectious peritonitis virus. J. Virol. 1992;66:6695–6705. doi: 10.1128/jvi.66.11.6695-6705.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagbami A.H., Halstead S.B., Marchette N.J., Larsen K. Cross-infection enhancement among African flaviviruses by immune mouse ascitic fluids. Cytobios. 1987;49:49–55. [PubMed] [Google Scholar]
- Gonin P., Pirzadeh B., Gagnon C.A., Dea S. Seroneutralization of porcine reproductive and respiratory syndrome virus correlates with antibody response to the GP5 major envelope glycoprotein. J. Vet. Diagn. Invest. 1999;11:20–26. doi: 10.1177/104063879901100103. [DOI] [PubMed] [Google Scholar]
- Halstead S.B., Venkateshan C.N., Gentry M.K., Larsen L.K. Heterogeneity of infection enhancement of dengue 2 strains by monoclonal antibodies. J. Immunol. 1984;132:1529–1532. [PubMed] [Google Scholar]
- Hawkes R.A., Lafferty K.J. The enchancement of virus infectivity by antibody. Virology. 1967;33:250–261. doi: 10.1016/0042-6822(67)90144-4. [DOI] [PubMed] [Google Scholar]
- Hohdatsu T., Nakamura M., Ishizuka Y., Yamada H. A study on the mechanism of antibody-dependent enhancement of feline infectious peritonitis virus infection in feline macrophages by monoclonal antibodies. Arch. Virol. 1991;120:207–217. doi: 10.1007/BF01310476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Tokunaga J., Koyama H. The role of IgG subclass of mouse monoclonal antibodies in antibody-dependent enhancement of feline infectious peritonitis virus infection of feline macrophages. Arch. Virol. 1994;139:273–285. doi: 10.1007/BF01310791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.S., Kwang J., Yoon I.J., Joo H.S. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in a homogeneous subpopulation of MA-104 cell line. Arch. Virol. 1993;133:477–483. doi: 10.1007/BF01313785. [DOI] [PubMed] [Google Scholar]
- Kliks S. Antibody-enhanced infection of monocytes as the pathogenetic mechanism for severe dengue illness. AIDS Res. Hum. Retroviruses. 1990;6:993–998. doi: 10.1089/aid.1990.6.993. [DOI] [PubMed] [Google Scholar]
- Kliks S.C., Nimmanitya S., Nisalak A., Burke D.S. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am. J. Trop. Med. Hyg. 1988;38:411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- Kurane I., Mady B.J., Ennis F.A. Antibody-dependent enhancement of dengue virus infection. Rev. Med. Virol. 1991;1:211–221. [Google Scholar]
- Kwang J., Zuckermann F., Ross G., Yang S. Antibody and cellular immune responses of swine following immunisation with plasmid DNA encoding the PRRS virus ORF's 4, 5, 6 and 7. Res. Vet. Sci. 1999;67:199–201. doi: 10.1053/rvsc.1998.0291. [DOI] [PubMed] [Google Scholar]
- Loemba H.D., Mounir S., Mardassi H., Archambault D. Kinetics of humoral immune response to the major structural proteins of the porcine reproductive and respiratory syndrome virus. Arch. Virol. 1996;141:751–761. doi: 10.1007/BF01718333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardassi H., Athanassious R., Mounir S., Dea S. Porcine reproductive and respiratory syndrome virus: morphological, biochemical and serological characteristics of Quebec isolates associated with acute and chronic outbreaks of porcine reproductive and respiratory syndrome. Can. J. Vet. Res. 1994;58:55–64. [PMC free article] [PubMed] [Google Scholar]
- Mascola J.R., Mathieson B.J., Zack P.M., Walker M.C. Summary report: workshop on the potential risks of antibody-dependent enhancement in human HIV vaccine trials. AIDS Res. Hum. Retroviruses. 1993;9:1175–1184. doi: 10.1089/aid.1993.9.1175. [DOI] [PubMed] [Google Scholar]
- Mason P.W., Rieder E., Baxt B. RGD sequence of foot-and-mouth disease virus is essential for infecting cells via the natural receptor but can be bypassed by an antibody- dependent enhancement pathway. Proc. Natl. Acad. Sci. U.S.A. 1994;91:1932–1936. doi: 10.1073/pnas.91.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenberg J.J., Hulst M.M., de Meijer E.J., Moonen P.L.M.J. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 1993;192:62–72. doi: 10.1006/viro.1993.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenberg J.J., Hulst M.M., de Meijer E.J., Moonen P.L.M.J. Lelystad virus belongs to a new virus family, comprising lactate dehydrogenase-elevating virus, equine arteritis virus, and simian hemorrhagic fever virus. Arch. Virol. Suppl. 1994;9:441–448. doi: 10.1007/978-3-7091-9326-6_43. [DOI] [PubMed] [Google Scholar]
- Meulenberg J.J., Petersen-den Besten A., De Kluyver E.P., Moormann R.J.M. Characterization of proteins encoded by ORFs 2 to 7 of Lelystad virus. Virology. 1995;206:155–163. doi: 10.1016/S0042-6822(95)80030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenberg J.J., Petersen-den Besten A. Identification and characterization of a sixth structural protein of Lelystad virus: the glycoprotein GP2 encoded by ORF2 is incorporated in virus particles. Virology. 1996;225:44–51. doi: 10.1006/viro.1996.0573. [DOI] [PubMed] [Google Scholar]
- Morens D.M., Venkateshan C.N., Halstead S.B. Dengue 4 virus monoclonal antibodies identify epitopes that mediate immune infection enhancement of dengue 2 viruses. J. Gen. Virol. 1987;68:91–98. doi: 10.1099/0022-1317-68-1-91. [DOI] [PubMed] [Google Scholar]
- Morens D.M., Halstead S.B., Marchette N.J. Profiles of antibody-dependent enhancement of dengue virus type 2 infection. Microb. Pathog. 1987;3:231–237. doi: 10.1016/0882-4010(87)90056-8. [DOI] [PubMed] [Google Scholar]
- Morens D.M. Antibody-dependent enhancement of infection and the pathogenesis of viral disease. Clin. Infect. Dis. 1994;19:500–512. doi: 10.1093/clinids/19.3.500. [DOI] [PubMed] [Google Scholar]
- Nelson E.A., Christopher-Hennings J., Benfield D.A. Serum immune responses to the proteins of porcine reproductive and respiratory syndrome (PRRS) virus. J. Vet. Diagn. Invest. 1994;6:410–415. doi: 10.1177/104063879400600402. [DOI] [PubMed] [Google Scholar]
- Ochiai H., Kurokawa M., Matsui S., Yamamoto T. Infection enhancement of influenza A NWS virus in primary murine macrophages by anti-hemagglutinin monoclonal antibody. J. Med. Virol. 1992;36:217–221. doi: 10.1002/jmv.1890360312. [DOI] [PubMed] [Google Scholar]
- Olsen C.W. A review of feline infectious peritonitis virus: molecular biology, immunopathogenesis, clinical aspects, and vaccination. Vet. Microbiol. 1993;36:1–37. doi: 10.1016/0378-1135(93)90126-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie A., Watson P. Blackwell Science; MA: 1999. Statistics for Veterinary and Animal Sciences. [Google Scholar]
- Plana Duran J., Climent I., Sarraseca J., Urniza A. Baculovirus expression of proteins of porcine reproductive and respiratory syndrome virus strain Olot/91. Involvement of ORF3 and ORF5 proteins in protection. Virus Genes. 1997;14:19–29. doi: 10.1023/a:1007931322271. [DOI] [PubMed] [Google Scholar]
- Poland A.M., Vennema H., Foley J.E., Pedersen N.C. Two related strains of feline infectious peritonitis virus isolated from immunocompromised cats infected with a feline enteric coronavirus. J. Clin. Microbiol. 1996;34:3180–3184. doi: 10.1128/jcm.34.12.3180-3184.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D.D., Larsen A.E., Porter H.G. Aleutian disease of mink. Adv. Immunol. 1980;29:261–286. doi: 10.1016/s0065-2776(08)60046-2. [DOI] [PubMed] [Google Scholar]
- Porterfield J.S. Antibody-dependent enhancement of viral infectivity. Adv. Virus Res. 1986;31:335–355. doi: 10.1016/s0065-3527(08)60268-7. [DOI] [PubMed] [Google Scholar]
- Prohaszka Z., Nemes J., Hidvegi T., Toth F.D. Two parallel routes of the complement-mediated antibody-dependent enhancement of HIV-1 infection. AIDS. 1997;11:949–958. doi: 10.1097/00002030-199708000-00002. [DOI] [PubMed] [Google Scholar]
- Robinson W.E., Jr., Montefiori D.C., Gillespie D.H., Mitchell W.M. Complement-mediated, antibody-dependent enhancement of HIV-1 infection in vitro is characterized by increased protein and RNA syntheses and infectious virus release. J. Acq. Immune Def. Syndr. 1989;2:33–42. [PubMed] [Google Scholar]
- Sangkawibha N., Rojanasuphot S., Ahandrik S., Viriyapongse S. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong. Thailand. I. The 1980 outbreak. Am. J. Epidemiol. 1984;120:653–669. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- Szabo J., Prohaszka Z., Toth F.D., Gyuris A. Strong correlation between the complement-mediated antibody-dependent enhancement of HIV-1 infection and plasma viral load. AIDS. 1999;13:1841–1849. doi: 10.1097/00002030-199910010-00005. [DOI] [PubMed] [Google Scholar]
- Takada A., Watanabe S., Okazaki K., Kida H. Infectivity-enhancing antibodies to Ebola virus glycoprotein. J. Virol. 2001;75:2324–2330. doi: 10.1128/JVI.75.5.2324-2330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nieuwstadt A.P., Meulenberg J.J., van Essen-Zanbergen A., Petersen den Besten A. Proteins encoded by open reading frames 3 and 4 of the genome of Lelystad virus (Arteriviridae) are structural proteins of the virion. J. Virol. 1996;70:4767–4772. doi: 10.1128/jvi.70.7.4767-4772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeth K., Adair B. Macrophages and respiratory viruses. Pathol. Biol. (Paris) 1997;45:184–192. [PubMed] [Google Scholar]
- Vennema H., Poland A., Foley J., Pedersen N.C. Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. Virology. 1998;243:150–157. doi: 10.1006/viro.1998.9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.Z., Rushlow K.E., Issel C.J., Cook R.F. Enhancement of EIAV replication and disease by immunization with a baculovirus-expressed recombinant envelope surface glycoprotein. Virology. 1994;199:247–251. doi: 10.1006/viro.1994.1120. [DOI] [PubMed] [Google Scholar]
- Weiland E., Wieczorek-Krohmer M., Kohl D., Conzelmann K.K. Monoclonal antibodies to the GP5 of porcine reproductive and respiratory syndrome virus are more effective in virus neutralization than monoclonal antibodies to the GP4. Vet. Microbiol. 1999;66:171–186. doi: 10.1016/s0378-1135(99)00006-1. [DOI] [PubMed] [Google Scholar]
- Wensvoort G., Terpstra C., Pol J.M., ter Laak E.A. Mystery swine disease in The Netherlands: the isolation of Lelystad virus. Vet. Q. 1991;13:121–130. doi: 10.1080/01652176.1991.9694296. [DOI] [PubMed] [Google Scholar]
- Yang L., Yoon K.-J., Li Y., Lee J.H. Antigenic and genetic variations of the 15 kD nucleocapsid protein of porcine reproductive and respiratory syndrome virus isolates. Arch. Virol. 1999;144:525–546. doi: 10.1007/s007050050523. [DOI] [PubMed] [Google Scholar]
- Yang L., Frey M.L., Yoon K.-J., Zimmerman J.J. Categorization of North American porcine reproductive and respiratory syndrome viruses: epitopic profiles of the N, M. GP5 and GP3 proteins and susceptibility to neutralization. Arch. Virol. 2000;145:1599–1619. doi: 10.1007/s007050070079. [DOI] [PubMed] [Google Scholar]
- Yoon K.-J. Virology. In: Zimmerman J.J., Yoon K.-J., editors. 2003 PRRS Compendium: A comprehensive reference on PRRS for pork producers, veterinary practitioners, and researchers. 2nd ed. National Pork Board; Des Moines, IA: 2003. pp. 163–184. [Google Scholar]
- Yoon K.-J., Zimmerman J.J., Swenson S.L., McGinley M.J. Characterization of the humoral immune response to porcine reproductive and respiratory syndrome (PRRS) virus infection. J. Vet. Diagn. Invest. 1995;7:305–312. doi: 10.1177/104063879500700302. [DOI] [PubMed] [Google Scholar]
- Yoon K.-J., Wu L.L., Zimmerman J.J., Hill H.T. Antibody-dependent enhancement (ADE) of porcine reproductive and respiratory syndrome virus (PRRSV) infection in pigs. Viral Immunol. 1996;9:51–63. doi: 10.1089/vim.1996.9.51. [DOI] [PubMed] [Google Scholar]
- Yoon K.-J., Wu L.L., Zimmerman J.J., Platt K.B. Field isolates of porcine reproductive and respiratory syndrome virus (PRRSV) vary in their susceptibility to antibody dependent enhancement (ADE) of infection. Vet. Microbiol. 1997;55:277–287. doi: 10.1016/s0378-1135(96)01338-7. [DOI] [PubMed] [Google Scholar]
- Zimmerman J.J., Sanderson T., Eernisse K.A., Hill H.T. Transmission of SIRS virus in convalescent animals to comingled penmates under experimental conditions. Am. Assoc. Swine Pract. Newslett. 1992;4:25. [Google Scholar]


