Abstract
Blood was collected from 55 cats with feline infectious peritonitis (FIP) and from 50 control cats in order to define whether differences in pathological findings and in distribution of feline coronaviruses (FCoV) can be associated with changes in haemograms, serum protein electrophoresis, and antibody titres. Compared to controls, the whole group of FIP-affected cats had blood changes consistent with FIP. Based on the pathological findings or on the immunohistochemical distribution of viral antigen, FIP-affected cats were divided in the following groups: subacute against acute lesions; low against strong intensity of positivity; intracellular against extracellular positivities; positive against negative lymph nodes. Lymphopenia was more evident in cats with acute forms, strong intensity of positivity, extracellular antigen and negative lymph nodes. Cats with positive lymph nodes had the most evident changes in the protein estimations. These results suggest that differences in pathological findings might depend on different reactive patterns to the FCoVs.
Feline infectious peritonitis (FIP) is a fatal disease for wild and domestic felidae, caused by a Feline Coronavirus (FCoV). The feline infectious peritonitis virus (FIPV) is a mutated form of the enteric one (FECV) (Vennema et al 1995, Poland et al 1996). Both these FCoVs are able to pass from the intestine to the blood (Herrewegh et al 1995); unlike FECV, FIPV is able to replicate within the macrophages that phagocytose the virus in the lymph nodes and diffuse it through the body (Pedersen 1995a). The development of the disease depends on the balance between humoral and cellular immunity: antibodies could facilitate the viral uptake by macrophages (Hodatsu et al 1993, Hodatsu et al 1994) and in the absence of cell-mediated immunity, antigen-antibody complexes are responsible for a type III hypersensitivity reaction that leads to vasculitis and effusion (Hayashi et al 1977, Pedersen, 1995a). Weak cellular immunity leads to dry forms, characterised by type IV hypersensitivity reactions (Pedersen 1987, Paltrinieri et al 1998a). In vivo the two types of immune reactions probably coexist (Paltrinieri et al 1998b). In experimental and in spontaneously occurring FIP, fluctuating levels of circulating antibodies and immune complexes as well as discrepancies between γ-globulin levels and antibody titre have been reported (Jacobse-Geels et al 1982, Pedersen 1995a, Paltrinieri et al 1998b). The severity of the disease has been associated with a decrease of circulating lymphocytes (Ward et al 1974, Pedersen 1995a, Paltrinieri et al 1998b). Furthermore, the distribution and the histological pattern of the lesions, as well as the distribution of viral antigens within the lesions, show great variations among the cats and often among different organs of the same cat (Weiss & Scott 1981, Walter et al 1989, Tammer et al 1995, Kipar et al 1997, Kipar et al 1998, Paltrinieri et al 1998a).
In order to define whether this variability is a casual finding or if it could be associated with different systemic reactive patterns, the relationship between the histological findings and the distribution of the virus on one hand, and haemograms, protein levels and antibody titres on the other were analysed in this study.
Materials and methods
Fifty-five cats presenting clinical signs of FIP were referred from private clinicians of the area of Milan (Italy). The characteristics of these cats, their pathological findings and the sampled organs are reported in Table 1.
Table 1.
Characteristics of the examined cats with FIP and samples taken for histological and immunohistochemical studies
| no. | Breed | Sex | Age | Pathological findings | Sampled organs |
|---|---|---|---|---|---|
| 1 | DSH | M | 8 mo | be | L,K,ML,LU,TL |
| 2 | DSH | M | 4 mo | be | I,K,ML,LU |
| 3 | DSH | M | 1 y | pe+abdominal mass | I,K,ML |
| 4 | DSH | F | 4 mo | pe | I,K,ML |
| 5 | DSH | M | 4 mo | te+subserosal abdominal foci | I,LU,TL |
| 6 | P | F | 2 mo | pe | L,I,S,K,ML |
| 7 | DSH | Mc | 2 y | pe | L,I,S,K,ML |
| 8 | DSH | M | 8 y | pe | L,K,ML |
| 9 | BU | M | 8 mo | pe | K,ML |
| 10 | DSH | M | 4 mo | be | L,I,S,K,ML,LU,TL |
| 11 | DSH | F | 1 y | pe | L,I,S,K,ML |
| 12 | P | M | 3 mo | te | LU,TL |
| 13 | DSH | M | 8 mo | pe | L,I,K,ML |
| 14 | DSH | M | 1 y | pe | L,I,S,K,ML |
| 15 | DSH | M | 4 mo | pe | L,I,S,K,ML |
| 16 | DSH | F | 6 mo | pe | L,I,S,K,ML |
| 17 | DSH | F | 6 mo | pe | L,I,S,K,ML |
| 18 | DSH | M | 7 mo | pe | L,I,S,K,ML |
| 19 | P | M | 2 y | be | L,I,S,K,ML,LU |
| 20 | DSH | M | 5 y | te+renal foci | K,ML,LU,TL |
| 21 | DSH | M | 5 mo | pe | L,I,S,K,ML |
| 22 | DSH | M | nd | be | L,I,S,ML,LU,TL |
| 23 | DSH | F | 11 mo | pe | L,ML |
| 24 | DSH | M | 4 y | pe | L,I,S,ML |
| 25 | P | F | 1 y | be | L,S,K,ML,LU,TL |
| 26 | DSH | M | 4 y | pe | L,I,S,K,ML |
| 27 | P | F | 1 y | pe | L,I,S,K,ML |
| 28 | DSH | M | 4 y | be | I,K,ML,LU,TL |
| 29 | P | F | 4 mo | pe | L,I,S,K,ML |
| 30 | P | F | 6 m | pe | L,I,K,ML |
| 31 | P | F | 1 y | be | L,K,ML,LU,TL |
| 32 | DSH | M | nd | pe | L,I,S,ML |
| 33 | DSH | M | 3 y | pe | L,K,ML |
| 34 | P | F | 8 mo | pe | L,I,S,ML |
| 35 | P | F | 1 y | pe | I,ML |
| 36 | DSH | M | 10 y | te | LU,TL |
| 37 | DSH | M | nd | pe | L,ML |
| 38 | DSH | F | 3 mo | pe | L,I,S,K,ML |
| 39 | P | F | nd | pe | L,K,ML |
| 40 | DSH | F | nd | pe | L,I,S,ML |
| 41 | DSH | F | nd | be | L,I,K,ML,LU,TL |
| 42 | DSH | F | nd | pe | I,ML |
| 43 | DSH | F | 12 y | pe | L,I,ML |
| 44 | DSH | F | 7 mo | pe | I,S,ML |
| 45 | DSH | M | 1 y | be | L,I,ML,LU,TL |
| 46 | S | M | 3 mo | pe | L,I,ML |
| 47 | P | M | nd | pe | L,I,K,ML |
| 48 | DSH | F | 1 y | pe | L,I,K,ML |
| 49 | DSH | F | nd | te | L,LU,TL |
| 50 | DSH | M | nd | te | L,LU,TL |
| 51 | P | F | 1 y | pe | L,I,S,ML |
| 52 | BA | M | 4 mo | be | L,I,K,ML,LU,TL |
| 53 | DSH | F | 1 y | pe | I,K,ML |
| 54 | DSH | F | nd | pe | L,I,ML |
| 55 | DSH | M | 7 y | pe | I,S,K,ML |
DSH=domestic shorthair; P=Persian; BU=Burmese; S=Siamese; BA=Balinese; M=male; F=female; mo=months; y=year; be=bicavitary effusion; pe=peritoneal effusion; te=thoracic effusion; L=liver; I=intestine; K=kidney; S=spleen; ML=mesentheric lymph node; LU=lung; TL=thoracic lymph node; nd=not determined.
As a negative control, blood was taken from a group of 50 healthy cats, comparable with the group of cats with FIP in terms of age, breed, sex and mode of life (pets, free roaming, and breeding cats).
Blood tests
Blood (3 ml) was withdrawn from the cephalic or from the jugular vein of each cat. One millilitre of blood was collected in EDTA-coated tubes and the remaining amount was put in tubes without anticoagulant.
By mean of an automatic cell counter (Hemat 8, SEAC, Firenze, Italy) leukocyte, platelet and erythrocyte numbers as well as haemoglobin (Hb) concentration, hematocrit (Ht) percentage, mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC) were evaluated. The differential leukocyte count was microscopically evaluated on May Grünwald–Giemsa (MGG) stained smears and the reticulocyte percentage on brillant cresyl blue stained smears as described by Pasquinelli (1984). The reticulocyte production index (RPI) was evaluated as suggested by Jain (1993).
Total proteins in serum were measured by a discrete analyser (Abbott VP, Abbott Lab., Irving, TX, USA) using the Biuret method (Abbott Lab., Abbott Park, IL, USA). Serum protein electrophoresis was performed using the semimicromethod, with cellulose polyacetate strips (SEAC, Firenze, Italy) in a barbitone and tris buffer (Helena Lab. Italia Spa, Assago, MI, Italy). The strips were run for 40 min, 150 V and then stained for 15 min in Red Ponceau (0.5 g in 100 ml of 5% trichloroacetic acid), destained in 5% acetic acid and put in a diaphanising solution (Helena Lab. Italia Spa, Assago, MI, Italy). The gels were scanned in a densitometer (BT512, Biotecnica Instruments, Roma, Italy).
Antibody titres against FCoVs were evaluated using a commercially available ELISA kit (Dyaset, Portomaggiore, FE, Italy) in 96 wells microtitre plates coated with FIPV proteins and using anti-feline horseradish peroxidase-conjugated antibodies. The plates were read at 450 nm in an automatic ELISA analyser (Dasit Multiskan, Dasit Spa, Cornaredo, Mi, Italy).
Serology for feline immunodeficiency virus (FIV) and for feline leukaemia virus (FELV) was performed using commercially available ELISA kits (SNAP, IDEXX Lab, Westbrook, MA, USA), according the procedure suggested by the manufacturer.
Post-mortem examinations
A sample (approximately 1 cm3) of the affected organs (see Table 1) was taken from each dead cat, fixed in buffered 10% iso-osmotic formalin and embedded in paraffin. Microtome sections (5 μm) were used to confirm the diagnosis by hematoxylin-eosin stain and by immuno-histochemistry using a monoclonal antibody against the FCoV (kindly provided by Prof N.C. Pedersen, Davis, USA). Two to five sections of each sample and 5 to 10 serial sections from lymph nodes draining the lesions were immuno-histochemically analysed. The Avidin Biotin Complex (ABC) method with a commercially available kit (Vectastain Elite, Vector Laboratories Inc., Burlingame, CA, USA) was used to detect the positive reaction, as described by Hsu et al (1981) after inhibition of the endogenous peroxidase (H2O2 1% in methanol) and antigen unmasking using microwave pretreatment (2 cycles of 5 min in citrate-buffered solution, 0.01 M, pH 6) (Cattoretti et al 1993) and 3-amino-9-ethyl-carbazole as chromogen. Some sections of each sample were used as negative controls, with the primary antibody substituted by an equal amount of normal mouse serum (DAKO A/S, Glostrup, Denmark). In each session of test a section of liver with a fibrinous perihepatitis and with intraparenchymatous pyogranulomatous foci from a cat with FIP was used as positive control.
Formation of groups
Histological and immunohistochemical sections were read by two different readers. Since different histopathological patterns as well as different immunohistochemical characteristics were present in different lesions of the same animal and often in different foci of the same organ, for each cat the histological and immunohistochemical characteristics of each sample were recorded and groups were formed on the basis of the most prevalent finding in the same cat.
The following groups were analysed and compared each other:
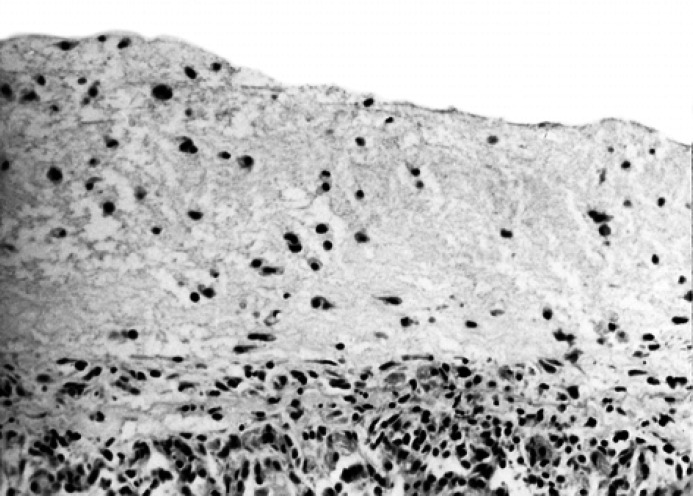
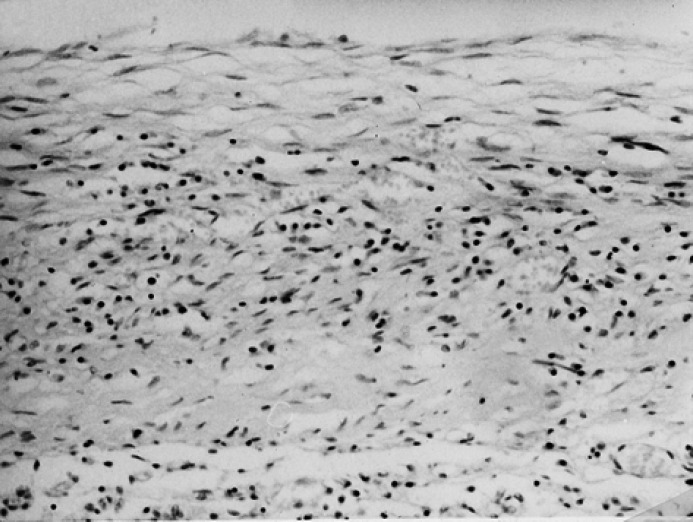
Acute vs subacute forms. The cases of FIP on which the main histological finding was the presence of perivisceral fibrin and necrosis with scattered leucocytes were classified as acute (Fig. 1); the cases of FIP with histological signs of organisation of the perivisceral fibrin, with larger pyogranulomatous foci and/or with presence of intraparenchymatous pyogranulomatous foci were classified as subacute (Fig. 2).
Fig 1.
Lung; cat, domestic shorthair, male, 8 months. Severe, acute, diffuse fibrinous pleuritis with scattered inflammatory cells. HE stain. Original magnification, 200 ×.
Fig 2.
Lung; cat, domestic shorthair, male, 1 year. Severe, subacute, diffuse fibrinous pleuritis with organised fibrin and large lymphoplasmocytic foci. HE stain. Original magnification, 200 ×.
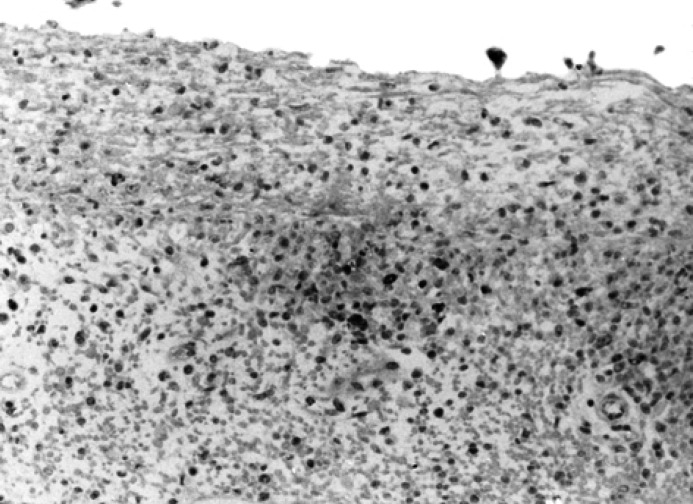
Intensity of the positivity. On the basis of the number of positive cells within the foci two groups were formed: low intensity when less than 50% of the cells were positive (Fig. 3), and strong intensity when more than 50% of the cells were positive (Fig. 4).
Fig 3.
Spleen; cat, domestic shorthair, female, 3 months. Severe, subacute, diffuse fibrinous perisplenitis with rare FCoV-positive cells. Avidin biotin peroxidase complex method, Mayer's hematoxylin counterstain. Original magnification, 200 ×.
Fig 4.
Intestine; cat, persian, female, 8 months. Severe, subacute, diffuse fibrinous peritonitis with many FCoV-positivities. Avidin biotin peroxidase complex method, Mayer's hematoxylin counterstain. Original magnification, 200 ×.
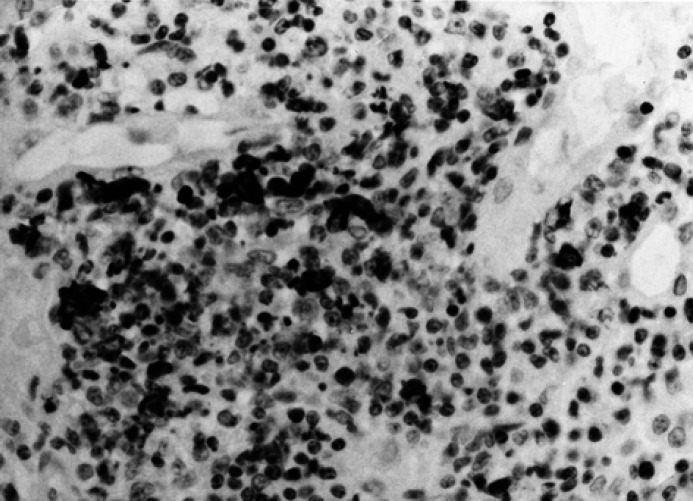
Type of the positivity. Two groups were formed according to the presence of intracellular (Fig. 5) or extracellular (Fig. 6) positivity: in the latter group, viral antigen was detectable both in the cells and in the extracellular spaces, where granular positivities were present.
Fig 5.
Liver; cat, domestic shorthair, female, 1 year. Pyogranulomatous hepatitis with prevalence of intracellular positivities. Avidin biotin peroxidase complex method, Mayer's hematoxylin counterstain. Original magnification, 400 ×.
Fig 6.
Kidney; cat, domestic shorthair, male, 7 years. Pyogranulomatous nephritis with presence of intracellular and extracellular granular positivities. Avidin biotin peroxidase complex method, Mayer's hematoxylin counterstain. Original magnification, 400 ×.
Presence or absence of positivity in the lymph nodes draining the lesions. These groups were formed based on the presence or absence of positive cells that appeared as single cells with dendritic projections or large vescicular cells in germinal centres (Fig. 7), in absence of FIP lesions such as pyogranulomatous lymphadenitis or of lymphoid depletion.
Fig 7.
Lymph node; cat, persian, female, 8 months. Presence of viral antigen in the germinal centres of the lymph node. Avidin biotin peroxidase complex method, Mayer's hematoxylin counterstain. Original magnification, 100 ×.
Statistical analysis
Haematological, serological and electrophoretic data of FIP-affected cats were compared with those of controls using specific software (Statsoft. Inc, Tulsa, OK, USA), by Student's t-test. When the data did not have a normal distribution, the corresponding non-parametric test Mann–Whitney U test was used.
The same tests were used to compare each of the results obtained in the different groups and to compare the different groups with controls.
Results
Results from control cats (Table 2) were in agreement with those reported in literature (Jain 1993, Kaneko et al 1997). Furthermore all the cats from this group were FIV and FeLV negative. In contrast variable anti-FCoV antibody titres were detected in this group.
Table 2.
Results (mean ± SD) from controls and from FIP-affected cats
| controls | FIP | ||
|---|---|---|---|
| Erythrocytes× 106/μl | 7.43 ± 1.79 | 5.81 ± 1.73 | *** |
| Reticulocytes× 103/μl | 0.12 ± 0.15 | 0.33 ± 0.77 | ns |
| RPI | 0.03 ± 0.04 | 0.07 ± 0.15 | ns |
| Hb (g/dl) | 11.13 ± 2.69 | 8.72 ± 2.52 | *** |
| Ht (%) | 31.66 ± 7.18 | 25.36 ± 7.58 | *** |
| MCV (fl) | 42.92 ± 3.97 | 44.26 ± 7.52 | ns |
| MCHC (%) | 35.48 ± 4.72 | 34.75 ± 4.98 | ns |
| MCH (pg) | 15.12 ± 1.69 | 15.28 ± 2.56 | ns |
| Leucocytes × 103/μl | 9.54 ± 3.68 | 15.86 ± 9.14 | *** |
| Neutrophils× 103/μl | 6.19 ± 2.94 | 13.10 ± 8.19 | *** |
| Band neutrophils × 103/μl | 0.12 ± 0.18 | 0.97 ± 1.39 | *** |
| Eosinophils×103/μl | 0.71 ± 0.70 | 0.06 ± 0.11 | *** |
| Lymphocytes × 103/μl | 2.47 ± 1.14 | 1.36 ± 1.30 | *** |
| Monocytes × 103/μl | 0.10 ± 0.14 | 0.34 ± 0.38 | *** |
| Total proteins (g/dl) | 7.59 ± 0.77 | 7.78 ± 1.72 | ns |
| A: G ratio | 0.94 ± 0.29 | 0.40 ± 0.22 | *** |
| Albumin (g/dl) | 3.61 ± 0.76 | 2.09 ± 0.57 | *** |
| Globulins (g/dl) | 4.05 ± 0.83 | 5.70 ± 1.63 | *** |
| α1 globulins (g/dl) | 0.48 ± 0.27 | 0.56 ± 0.41 | ns |
| α2 globulins (g/dl) | 0.64 ± 0.28 | 1.15 ± 0.47 | *** |
| β globulins (g/dl) | 0.79 ± 0.29 | 1.23 ± 1.01 | ** |
| γ globulins (g/dl) | 2.10 ± 0.68 | 2.73 ± 1.49 | ** |
| Antibody titre | 1:165 ± 1:218 | 1:283 ± 1:332 | ns |
P < 0.01;
P < 0.001; ns=not significant.
When considered as a whole and compared against controls (Table 2) cats with FIP had a normocytic, normochromic non-regenerative anaemia, neutrophilic leucocytosis with lymphopenia, eosinopenia and monocytosis, hypoalbuminaemia and hyperglobulinaemia with a decreased albumin/globulin (A: G) ratio, and increased α2- β- and γ-globulin concentrations. Also in this group no FIV or FeLV positives were present.
The cats were then divided into groups according to their pathological and immunohistochemical findings. Values related to erythrograms never exhibited any significant or evident difference among the groups. For this reason that these data are not reported in the following tables.
Acute against subacute forms (Table 3) . Thirty-five cats (63.6%) had acute forms, while in 20 cases (36.4%) histological findings were consistent with subacute forms. Compared to controls, both groups had leucocytosis, neutrophilia with left shift, eosinopenia, monocytosis, hypoalbuminaemia, hyperglobulinaemia with inverted A: G ratio and increased α2-, β- and γ-globulins. In contrast lymphopenia was present only in acute forms and hyperproteinaemia in the subacute ones. Lymphocyte numbers and β-globulins were significantly lower in cats with acute forms than in those with subacute forms.
Table 3.
Results (mean ± S.D.) from cats with acute and subacute forms
| Acute | Subacute | ||||
|---|---|---|---|---|---|
| Leucocytes × 103/μl | 16.5 ± 9.5 | *** | 14.8 ± 8.7 | *** | ns |
| Neutrophils × 103/μl | 14.2 ± 8.7 | *** | 11.1 ± 7.0 | *** | ns |
| Band neutrophils × 103/μl | 0.7 ± 0.6 | *** | 1.4 ± 2.1 | *** | ns |
| Eosinophils × 103/μl | 0.1 ± 0.1 | *** | 0.1 ± 0.1 | *** | ns |
| Lymphocytes × 103/μl | 1.1 ± 0.7 | *** | 1.8 ± 1.9 | nsc | # |
| Monocytes × 103/μl | 0.3 ± 0.4 | *** | 0.3 ± 0.3 | *** | ns |
| Total proteins (g/dl) | 7.5 ± 1.8 | nsc | 8.2 ± 1.5 | * | ns |
| A: G ratio | 0.4 ± 1.3 | *** | 0.4 ± 0.1 | *** | ns |
| Albumin (g/dl) | 2.0 ± 0.6 | *** | 2.2 ± 0.5 | *** | ns |
| Globulins (g/dl) | 5.5 ± 1.7 | *** | 6.0 ± 1.4 | *** | ns |
| α1 globulins (g/dl) | 0.5 ± 0.3 | nsc | 0.7 ± 0.5 | nsc | ns |
| α2 globulins (g/dl) | 1.1 ± 0.5 | *** | 1.1 ± 0.5 | *** | ns |
| β globulins (g/dl) | 1.0 ± 0.6 | * | 1.6 ± 1.4 | *** | # |
| γ globulins (g/dl) | 2.8 ± 1.6 | * | 2.6 ± 1.3 | * | ns |
| Antibody titre | 1:206 ± 1:209 | nsc | 1:400 ± 1:445 | nsc | ns |
P < 0.05 vs controls;
P < 0.001 vs controls; nsc=not significant vs controls;
P < 0.05; ns=not significant.
Before analysing the results of groups formed according their immunohistochemical findings, it must be underlined that often foci with different characteristics of positivity were detectable in the same cat. Nevertheless, the characteristics of positivity were similar in contiguous organs (eg intestine-omentum, liver-diaphragm, lung-pericardium, etc.). The immunohistochemical findings that were more represented in the cats with FIP were low intensity (31/55=56.4%), extracellular positivities (38/55=69.1%) and negative lymph nodes (30/55=54.5%) (Table 4). However immunohistochemical findings were not uniformly distributed among the different groups of cats: the main immunohistochemical finding in cats with acute lesions were negative lymph nodes and extracellular positivities, while cats with subacute lesions more frequently had low intensity of positivity; cats with intracellular positivities more often had positive lymph nodes, while those with extracellular positivities were mainly characterised by negative lymph nodes; finally, cats with negative lymph nodes showed a prevalence of low intensity of positivity.
Table 4.
Relationship among the different pathological and immunohistochemical findings in FIP cats
| AF (n=35) | SF (N=20) | LI (n=31) | SI (n=24) | CP (n=17) | EP (N=38) | NL (n=30) | PL (n=25) | |
|---|---|---|---|---|---|---|---|---|
| AF | — | — | 18 | 17 | 7 | 28 | 20 | 15 |
| (n=35) | (51%) | (49%) | (20%) | (80%) | (57%) | (43%) | ||
| SF | — | — | 13 | 7 | 10 | 10 | 10 | 10 |
| (n=20) | (65%) | (35%) | (50%) | (50%) | (50%) | (50%) | ||
| LI | 18 | 13 | — | — | 11 | 20 | 18 | 13 |
| (n=31) | (58%) | (42%) | (35%) | (65%) | (58%) | (42%) | ||
| SI | 17 | 7 | — | — | 6 | 18 | 12 | 12 |
| (n=24) | (71%) | (29%) | (25%) | (75%) | (50%) | (50%) | ||
| CP | 7 | 10 | 11 | 6 | — | — | 6 | 11 |
| (n=17) | (41%) | (59%) | (65%) | (35%) | (35%) | (65%) | ||
| EP | 28 | 10 | 20 | 18 | — | — | 24 | 14 |
| (n=38) | (74%) | (26%) | (53%) | (47%) | (63%) | (37%) | ||
| NL | 20 | 10 | 18 | 12 | 6 | 24 | — | — |
| (n=30) | (67%) | (33%) | (60%) | (40%) | (20%) | (80%) | ||
| PL | 15 | 10 | 13 | 12 | 11 | 14 | — | — |
| (n=25) | (60%) | (40%) | (52%) | (48%) | (44%) | (56%) |
AF=Acute forms; SF=subacute forms; LI=low intensity of positivity; SI=strong intensity of positivity; CP=cellular positivity; EP=extracellular positivity; NL=negative lymph node; PL=positive lymph node.
Blood parameters were then analysed in the different groups of cats with the following results (Table 5):
Table 5.
Results (mean ± SD) from cats with different immunohistochemical findings
| LI | SI | CP | EP | NL | PL | ||||
|---|---|---|---|---|---|---|---|---|---|
| Leucocytes × 103/μl | 18.2 ± 9.9*** | 12.8 ± 7.2** | # | 14.1 ± 7.8** | 16.7 ± 9.7*** | ns | 14.9 ± 8.0*** | 17.1 ± 10.4*** | ns |
| Neutrophils× 103/μl | 14.9 ± 9.0*** | 10.8 ± 6.5*** | ns | 10.3 ± 5.6*** | 14.3 ± 8.9*** | ns | 12.6 ± 7.5*** | 13.7 ± 9.0*** | ns |
| Band neutrophils× 103/μl | 1.1 ± 1.7*** | 0.7 ± 0.8*** | ns | 1.2 ± 2.3** | 0.9 ± 0.7*** | ns | 1.0 ± 0.9*** | 1.0 ± 1.8** | ns |
| Eosinophils×103/μl | 0.1 ± 0.1*** | 0.0 ± 0.1*** | ns | 0.1 ± 0.1*** | 0.0 ± 0.1*** | ## | 0.1 ± 0.1*** | 0.1 ± 0.1*** | ns |
| Lymphocytes × 103/μl | 1.7 ± 1.5* | 0.9 ± 0.7*** | # | 2.0 ± 1.9 nsc | 1.1 ± 0.7*** | # | 0.9 ± 0.7*** | 1.9 ± 1.7 nsc | ## |
| Monocytes × 103/μl | 0.3 ± 0.4*** | 0.3 ± 0.3*** | ns | 0.4 ± 0.4*** | 0.3 ± 0.4*** | ns | 0.3 ± 0.3*** | 0.4 ± 0.5*** | ns |
| Total proteins (g/dl) | 8.0 ± 1.7* | 7.5 ± 1.8 nsc | ns | 7.7 ± 1.3 nsc | 7.8 ± 1.9 nsc | ns | 7.4 ± 1.5 nsc | 8.2 ± 1.8* | ns |
| A: G ratio | 0.4 ± 0.3*** | 0.4 ± 0.1*** | ns | 0.3 ± 0.1*** | 0.4 ± 0.2*** | ns | 0.4 ± 0.3*** | 0.3 ± 0.1*** | ns |
| Albumin (g/dl) | 2.2 ± 0.6*** | 2.0 ± 0.5*** | ns | 2.00 ± 0.6*** | 2.1 ± 0.6*** | ns | 2.1 ± 0.7*** | 2.0 ± 0.4*** | ns |
| Globulins (g/dl) | 5.9 ± 1.7*** | 5.5 ± 1.6*** | ns | 5.7 ± 1.1*** | 5.7 ± 1.8*** | ns | 5.3 ± 1.5*** | 6.2 ± 1.7*** | # |
| α1 globulins (g/dl) | 0.6 ± 0.5 nsc | 0.5 ± 0.2 nsc | ns | 0.6 ± 0.5 nsc | 0.5 ± 0.4 nsc | ns | 0.6 ± 0.5 nsc | 0.5 ± 0.3 nsc | ns |
| α2 globulins (g/dl) | 1.2 ± 0.5*** | 1.1 ± 0.4*** | ns | 1.1 ± 0.5*** | 1.1 ± 0.4*** | ns | 1.2 ± 0.5*** | 1.1 ± 0.4*** | ns |
| β globulins (g/dl) | 1.3 ± 1.0*** | 1.1 ± 1.0** | ns | 1.2 ± 0.9** | 1.2 ± 1.0** | ns | 1.3 ± 1.2** | 1.2 ± 0.8** | ns |
| γ globulins (g/dl) | 2.7 ± 1.6** | 2.8 ± 1.4* | ns | 2.7 ± 1.4* | 2.7 ± 1.5** | ns | 2.2 ± 1.1 nsc | 3.3 ± 1.7*** | ## |
| Antibody titre | 1:214 ± 1:252 nsc | 1:388 ± 1:414 nsc | ns | 1:302 ± 1:505 nsc | 1:274 ± 1:225 nsc | ns | 1:296 ± 1:258 nsc | 1:262 ± 1:431 nsc | ns |
P < 0.05 vs controls;
P < 0.01 vs controls;
P < 0.001 vs controls;
nsc=not significant vs controls;
P < 0.05;
P < 0.01;
ns=not significant.
LI, low intensity of positivity; SI, strong intensity of positivity; CP, cellular positivity; EP, extracellular positivity; NL, negative lymph node; PL, positive lymph node.
Low against strong intensity of positivity . Compared to controls, both groups had the above mentioned changes consistent with FIP, except for hyperproteinemia that was present only in cats with low intensity of positivity. Leucocytosis was significantly higher in cats with low intensity of positivity and lymphocyte counts were significantly lower in cats with strong intensity of positivity.
Intracellular against extracellular positivity . Compared to controls, both the groups showed the changes consistent with FIP, except for lymphopenia, that was present only in cats with extracellular positivity. These cats also had significantly lower eosinophil and lymphocyte counts than those with intracellular positivities.
Presence against absence of viral antigen in the lymph nodes . Compared to controls, all the changes consistent with FIP were detectable in both the groups, except for lymphopenia, that was present only in cats with negative lymph nodes, and hyperproteinaemia and hyper-γ-globulinaemia, that were present only in cats with positive lymph nodes. Lymphocyte counts and total and γ-globulin concentrations were significantly lower in absence than in presence of lymph node positivities.
Discussion
All the parameters in control cats were within the normal range (Jain 1993, Kaneko et al 1997). However, it must be underlined that the total proteins and protein fractions were characterised by marked individual variations, most likely due to the composition of the control group. In order to be comparable with cats with FIP, in fact, the control group was composed of cats of different age, sex, and breed, as well as of cats living in different environments and it is well known that these variables can influence the protein levels (Kristensen & Barsanti 1977). The finding of variable antibody titres in control cats is not surprising: anti-FCoV positivities have already been reported in healthy cats (Pedersen 1976, Barlough & Stoddart 1990, Pedersen 1995a, Paltrinieri et al 1998b). In the present sample cats from catteries were included. This increased further the possibility to have high anti-FCoV antibody titres, since in multiple-cat environments the FCoVs are often endemic, and seropositives are frequent (Pedersen 1995b, Richards 1995).
The haematological and serum protein profiles of cats with feline infectious peritonitis (FIP) were in agreement with those reported in previous works (Sparkes et al 1991, Pedersen 1995a, Paltrinieri et al 1998b).
After forming the groups and subsequently reducing the number of cats per group, variability among the cats increased further. By consequence, for some parameters statistical significance was rarely reached, despite strong differences in the mean values among the different groups.
Nevertheless, cats with acute lesions showed a significant decrease of lymphocytes compared to controls and to animals with subacute lesions. This suggests that the decrease of lymphocytes might exacerbate the disease, in agreement with the previous hypothesis on the role of lymphocytes in modulating the development of the disease (Ward et al 1974, Pedersen 1995a, Haagmans et al 1996). β-globulins and antibody titres were higher in subacute than in acute FIP, suggesting an early involvement of immune system, although it cannot be excluded that the low antibody titre in acute FIP depends on immune-complexes formation.
In experimental infections, an early increase of α2-globulins was reported (Stoddart et al 1988), while γ-globulins and antibody titres increase just before the appearance of the symptoms either in effusive (Stoddart et al 1988, Pedersen 1995b, Gunn-Moore et al 1998) or in non-effusive forms (Pedersen, 1976). In the present study it is not possible to find this correlation: in spontaneously occurring FIP, in fact, it is not possible to assess the moment of infection nor to have an exhaustive follow-up, because the cats are usually euthanatised just after blood sampling. Furthermore the development of the disease is heavily influenced by the serological status before infection (Pedersen 1976). Pedersen (1995b) suggested that cats that develop FIP are almost always FCoV seropositive, while other authors found that seropositive cats are less susceptible to the disease (Addie et al 1995, Herrewegh et al 1997). Although it is not possible to assess whether our cats were seropositive or not, it cannot be excluded that the above-mentioned increase of antibody titres and γ-globulins could have been present before the samplings.
The distribution of the viral antigen and the characteristics of positivity in the different animals, as already reported (Cammarata Parodi et al 1993, Tammer et al 1995, Kipar et al 1998), were very variable. For these reasons the prevalence of the different characteristics of positivity were recorded and used to form the groups. The finding of similar characteristics of positivity in contiguous organs, however, suggests the possibility of an early diffusion of the virus from the site of the first appearance of the lesions to the nearest tissues, as previously demonstrated in experimentally induced diseases (Weiss & Scott 1981).
In most of the cats, viral antigen was detectable in less than 50% of the cells within the lesions and it was also found as granular positivity in extracellular spaces. Although this might be an artifact due to a different distribution of the virus within each lesion, it is most likely due to a different reactivity against the virus, probably related to a different immune status of the cats. Cats with different susceptibility to FCoV infections, due to a breed predisposition, have been already reported (Foley & Pedersen 1996). In the present sample the distribution of viral antigen in Persian cats was similar to that of cats from other breeds; however, the hypothesis of an individual predisposition is supported by the finding that cats with more acute lesions and with extracellular antigen, that has been interpreted as the release of virus from lysed cells (Weiss & Scott 1981, Paltrinieri et al 1998a) mainly had negative lymph nodes.
A further support on the hypothesis of a different reactivity in cats with different immunohistochemical findings, came from the observation that some haematological changes were associated with the increasing spread of viral antigen. In particular, lymphopenia was present in cats with stronger positivity and with extracellular antigen. The relationship between lymphopenia and the severity of the clinical symptoms has been already underlined (Weiss & Scott 1981) and the lack of protective cellular immunity is considered to play a central role in the development of effusive FIP (Pedersen 1987). The association detected in this study between lymphopenia and the spread of the virus supports this hypothesis. However, the data presented here do not allow determination if viral spread can exacerbate the lymphopenia, as previously suggested by the finding of apoptotic lymphocytes close to FIPV-infected macrophages (Haagmans et al 1996).
The finding of viral antigen in the germinal centres of lymph nodes in the absence of typical FIP lesions must be considered with particular attention. The positive cells were morphologically identifiable as interfollicular dendritic cells, that work as antigen presenting cells (Steinmann 1991) and are thus involved in the activation of the immune system. Cats with positive lymph nodes had γ-globulin levels significantly higher than those of the other group. This suggests that they have already mounted an humoral reaction against the virus. This is consistent with the lower amount of viral antigen found in these cats and it is in agreement with previous reports in which the ability to resist the infection has been associated with a follicular hyperplasia in the lymph nodes (Kipar et al 1999). Lymphopenic cats, in contrast, are most likely unable to mount this response: their lymph nodes were negative and γ-globulins were lower than in the other cats.
In conclusion the simultaneous analysis of pathological, immunohistochemical and blood findings in cats affected by FIP allowed us to define different reactive patterns against the virus. Ultimately, the results presented here further support the hypothesis of an association between lymphopenia and the severity of FIPV infection. Further efforts to investigate the relationship between FIPV and the immune system are needed to help us understand the histopathogenesis of the lesions.
Acknowledgements
This work was supported by the grant ex-M.U.R.S.T. 60% (Italy). The authors are grateful to Dr Giuseppe Sironi and Manuela Teti for their technical assistance and to Dr Cristina Crosta, Emiliana Monzani and to the other practicians that submitted their cases.
References
- Addie DD, Toth S, Murray GD, Jarrett O. (1995) The risk of feline infectious peritonitis in cats naturally infected with feline coronavirus. American Journal of Veterinary Research 56, 429–434. [PubMed] [Google Scholar]
- Barlough JE, Stoddart CA. (1990) Feline Infectious Peritonitis. Veterinary Reports 1, 13–17. [Google Scholar]
- Cammarata Parodi M, Cammarata G, Paltrinieri S, Ape F. (1993) Using direct immunofluorescence to detect corona-viruses in peritoneal and pleural effusion. Journal of Small Animal Practice 34, 609–613. [Google Scholar]
- Cattoretti G, Pileri S, Parravicini C, Becker MHG, Poggi S, Bifulco C, Key G, D'Amato L, Sabattini E, Feudale E, Reynolds F, Gerdes J, Rilke F. (1993) Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. Journal of Pathology 171, 83–98. [DOI] [PubMed] [Google Scholar]
- Foley JE, Pedersen NC. (1996): The inheritance of susceptibility to feline infectious peritonitis in purebreed cats. Feline Practice 24, 14–22. [Google Scholar]
- Gunn-Moore DA, Caney SMA, Gruffyd-Jones TJ, Helps CR, Harbour DA. (1998) Antibody and cytokine responses in kittens during the development of feline infectious peritonitis (FIP). Veterinary Immunology and Immunopathology 65, 221–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagmans BL, Egbernik HF, Horzinek MC. (1996) Apoptosis and T-cell depletion during feline infectious peritonitis. Journal of Virology 70, 8977–8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Goto N, Takahashi R, Fujiwara K. (1977) Systemic vascular lesions in feline infectious peritonitis. Japanese Journal of Veterinary Science 39, 365–377. [DOI] [PubMed] [Google Scholar]
- Herrewegh AAPM, de Groot RJ, Cepica A, Egbernik HF, Horzinek MC, Rottier PJM. (1995) Detection of feline coronavirus RNA in feces, tissue and body fluids of naturally infected cats by reverse transcriptase PCR. Journal of Clincal Microbiology 33, 684–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrewegh AAPM, Mahler M, Hedrich HJ, Haagmans BL, Egbernik HF, Horzinek MC, Rottier PJM, de Groot RJ. (1997) Persistence and evolution of feline coronavirus in a closed cat-breeding colony. Virology 234, 349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodatsu T, Yamada H, Ishizuka Y, Koyama H. (1993) Enhancement and neutralization of feline inectious peritonitis infection in feline macrophages by neutralizing monoclonal antibodies recognizing different epitopes. Microbiology and Immunology 37, 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodatsu T, Tokunaga J, Koyama H. (1994) The role of IgG subclass of mouse monoclonal antibodies in antibody-dependent enhancement of feline infectious peritonitis virus infection of feline macrophages. Archives of Virology 139, 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Farger H. (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: A comparison between ABC and unlabeled antibody (PAP) procedures. Journal of Histochemistry and Cytochemistry 29, 577–580. [DOI] [PubMed] [Google Scholar]
- Jacobse-Geels HEL, Daha MR, Horzinek MC. (1982) Antibody, immune complexes, and complement activity fluctuations in kittens with experimentally induced feline infectious peritonitis. American Journal of Veterinary Research 43, 666–670. [PubMed] [Google Scholar]
- Jain NC. (1993) Essential of veterinary hematology (1st edn.). Lea & Febiger, Philadelphia. [Google Scholar]
- Kaneko JJ, Harvey JW, Bruss ML. (1997) Blood analyte reference values in small and some laboratory animals. In: Clinical Biochemistry of Domestic Animals (5th edn.). Kaneko JJ, Harvey JM, Bruss ML. (eds). Academic Press, San Diego, pp 895–899. [Google Scholar]
- Kipar A, Bellmann S, Kremendhal J, Reinacher M. (1997) Antibody production in situ in cats with feline infectious peritonitis. Proceedings of the 15th meeting of the European Society of Veterinary Pathology, page 94.
- Kipar A, Bellmann S, Kremendhal J, Kohler K, Reinacher M. (1998) Cellular composition, coronavirus antigen expression and production of specific antibodies in lesions in feline infectious peritonitis. Veterinary Immunology and Immunopathology 65, 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipar A, Bellmann S, Gunn-Moore DA, Leucert W, Kohler K, Menger S, Reinacher M. (1999) Histopathological alterations of lymphatic tissues in cats without feline infectious peritonitis after long term exposure to FIP virus. Veterinary Microbiology 69, 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen F, Barsanti J. (1977) Analysis of serum proteins in clinically normal pet and colony cats, using agarose electrophoresis. American Journal of Veterinary Research 38, 399–402. [PubMed] [Google Scholar]
- Paltrinieri S, Parodi Cammarata M, Cammarata G, Mambretti M. (1998a) Type IV hypersensitivity in the pathogenesis of FIPV induced lesions. Journal of Veterinary Medicine B 45, 151–159. [DOI] [PubMed] [Google Scholar]
- Paltrinieri S, Cammarata Parodi M, Cammarata G, Comazzi S. (1998b) Some aspects of humoral and cellular immunity in spontaneously occuring feline infectious peritonitis. Veterinary Immunology and Immunopathology 65, 205–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquinelli F. (1984) Tecniche ematologiche. In: Diagnostica e tecniche di laboratorio, Vol. 2 (1st edn.). Pasquinelli F. (ed). Rosini editrice s.r.l., Firenze, pp 835–854. [Google Scholar]
- Pedersen NC. (1976) Serologic studies of naturally occurring feline infectious peritonitis. American Journal of Veterinary Research 37, 1449–1453. [PubMed] [Google Scholar]
- Pedersen NC. (1987) Virologic and immunologic aspects of feline infectious peritonitis virus infection. Advances in Experimental Medicine and Biology 218, 529–550. [DOI] [PubMed] [Google Scholar]
- Pedersen NC. (1995a) An overview of feline enteric coronavirus and infectious peritonitis virus infections. Feline Practice 23, 7–20. [Google Scholar]
- Pedersen NC. (1995b) The history and interpretation of feline coronavirus serology Feline Practice 23, 46–51. [Google Scholar]
- Poland AM, Vennema H, Foley JE, Pedersen NC. (1996) Two related strains of feline infectious peritonitis virus isolated from immunocompromised cats infected with a feline enteric coronavirus. Journal of Clinical Microbiology 34, 3180–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JR. (1995) Problems in the interpretation of feline coronavirus serology (specificity vs. sensitivity of test procedures). Feline Practice 23, 52–55. [Google Scholar]
- Sparkes AH, Gruffydd-Jones TJ, Harbour DA. (1991) Feline infectious peritonitis: A review of clinicopathological changes in 65 cases, and a critical assessment of their diagnostic value. Veterinary Record 129, 209–212. [DOI] [PubMed] [Google Scholar]
- Steinman RM. (1991) The dendritic cell system and its role in immunogenicity Annual Review of Immunology 9, 271–296. [DOI] [PubMed] [Google Scholar]
- Stoddart ME, Whicher JT, Harbour DA. (1988) Cats inoculated with feline infectious peritonitis virus exhibit a biphasic acute phase plasma protein response. Veterinary Record 123, 621–624. [PubMed] [Google Scholar]
- Tammer R, Evensen O, Lutz H, Reinacher M. (1995) Immunological demonstration of feline infectious peritonitis virus antigen in paraffin-embedded tissues using feline ascites or murine monoclonal antibodies. Veterinary Immunology and Immunopathology 49, 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema H, Poland A, Floyd Hawkins K, Pedersen NC. (1995) A comparison of the genomes of FECVs and FIPVs: What they tell us about relationship between feline coronaviruses and their evolution. Feline Practice 23, 40–44. [Google Scholar]
- Walter J, Dohse K, Rudolph R. (1989) Eine modification der ABC-methode (avidin-biotin-peroxidase-complex) für den nachweis von viralen antigenen bei der infektion der katze durch ein coronavirus (FIP) und der infektion des hundes durch das parvovirus-typ 2. Journal of Veterinary Medicine B 36, 321–332. [PubMed] [Google Scholar]
- Ward JM, Gribble DH, Dungworth DL. (1974) Feline infectious peritonitis: Experimental evidence for its multiphasic nature. American Journal of Veterinary Research 35, 1271–1275. [PubMed] [Google Scholar]
- Weiss RC, Scott FW. (1981) Pathogenesis of feline infectious peritonitis: Pathologic changes and immunofluorescence. American Journal of Veterinary Research 42, 2036–2048. [PubMed] [Google Scholar]