Abstract
Iron deficiency is the most common micronutrient deficiency in the world and disproportionately affects pregnant women and young children. Iron deficiency has negative effects on pregnancy outcomes in women and on immune function and neurodevelopment in children. Iron supplementation programs have been successful in reducing this health burden. However, iron supplementation of iron-sufficient individuals is likely not necessary and may carry health risks for iron-sufficient and potentially some iron-deficient populations. This review considers the physiology of iron as a nutrient and how this physiology informs decision-making about weighing the benefits and risks of iron supplementation in iron-deficient, iron-sufficient, and iron-overloaded pregnant women and children.
Keywords: iron, pregnancy, infancy, supplementation, neurodevelopment, malaria
IRON AS A NUTRIENT
Iron is the most abundant element, comprising 35% of the earth’s physical mass. While most of iron’s interactions with the environment are inorganic, it nevertheless plays a critical role in organic biological processes in virtually all living organisms. Iron accretion by cells and its utilization in biological processes within cells are remarkably conserved across the taxonomic hierarchy. This speaks to its fundamental importance in sustaining the metabolic processes essential to the survival and function of living organisms.
Iron is classified as a micronutrient. A primary nutritional role of iron is to support erythropoiesis. Iron is prioritized to red blood cells over all other organ systems, including the brain, in the developing fetus and young child to support hemoglobin synthesis (46, 48, 104, 161). Every cell and organ system in the body requires iron for proper development and subsequent metabolic function. Its physiologic role in iron cluster proteins and hemoproteins is to facilitate enzymatic processes that are essential to cellular metabolic function, including those essential for oxygen delivery and cellular adenosine triphosphate generation. While iron-deficiency anemia is associated with clinical symptoms, tissue-level iron deficiency and not necessarily anemia is responsible for organ dysfunction in the iron-deficient state. Symptoms of poor organ performance are present prior to the onset of anemia and persist after the anemia resolves following iron treatment (8).
A precise estimate of the burden of iron deficiency is difficult to obtain because of the various definitions of iron deficiency used worldwide. Approximately 2 billion of the earth’s 7.2 billion people are anemic, with iron deficiency as the root cause in an estimated 25% to 50% (104, 134). However, the prevalence of nonanemic iron deficiency, which is associated with neurodevelopmental consequences, is estimated to be threefold greater than the prevalence of iron-deficiency anemia. Conservative projections indicate that between 2 and 4 billion people, or approximately one-quarter to one-half of the world’s population, may be iron deficient (104).
Iron-deficiency effects are particularly profound on cells with the highest metabolic rates, presumably because iron deficiency compromises mitochondrial and cellular energetics (7). Thus, the consequences of iron deficiency are more profound during development, when the oxygen consumption rates of cells are highest, driven by the energy demands of growth and differentiation. On a per kilogram of weight basis, the total-body oxygen consumption of a neonate is three to four times greater than that of an adult. Moreover, the neonatal brain alone utilizes 60% of that oxygen consumption, compared with 20% in the adult brain (72). Given this large iron-dependent energy demand, the prevalence of iron deficiency is greatest in pregnant women and young children.
The main reason to maintain iron sufficiency in these vulnerable populations is to optimize organ development and function, including immune function and brain development (30, 46). Within the brain, iron deficiency negatively affects iron-dependent myelination, monoamine metabolism, and cellular energetics (80, 87). Early-life iron deficiency patterns gene expression in the brain across the lifetime via histone-mediated stable epigenetic modifications (137, 138). These findings may explain why certain neurobehavioral effects of early-life iron deficiency appear to be permanent (80, 86). Whether early-life iron deficiency affects the regulation of other organ systems, including the production of red blood cells, across the life span is being investigated (51).
Like most other nutrients, iron exhibits a U-shaped risk curve (Figure 1). The risks of iron deficiency have been well described (80). However, as a divalent metal, iron has the potential to react in inorganic reactions that can damage organic components of the cell. Specifically, nonprotein-bound iron (NPBI) can react with oxygen to form reactive oxygen species, which if unquenched, can damage lipid membranes and organelles. These are not typically thought of as consequences of nutritional iron intake, but rather as pathologic conditions in which iron is suddenly released in large quantities from damaged or dead cells. The exquisitely tight, coordinated regulation of iron uptake, storage, and trafficking, combined with the effectiveness of iron-transport proteins (e.g., transferrin) in hiding iron within their structures, generally prevent NPBI from being present in circulation following conventional dietary intake of iron-containing foods (44).
Figure 1.
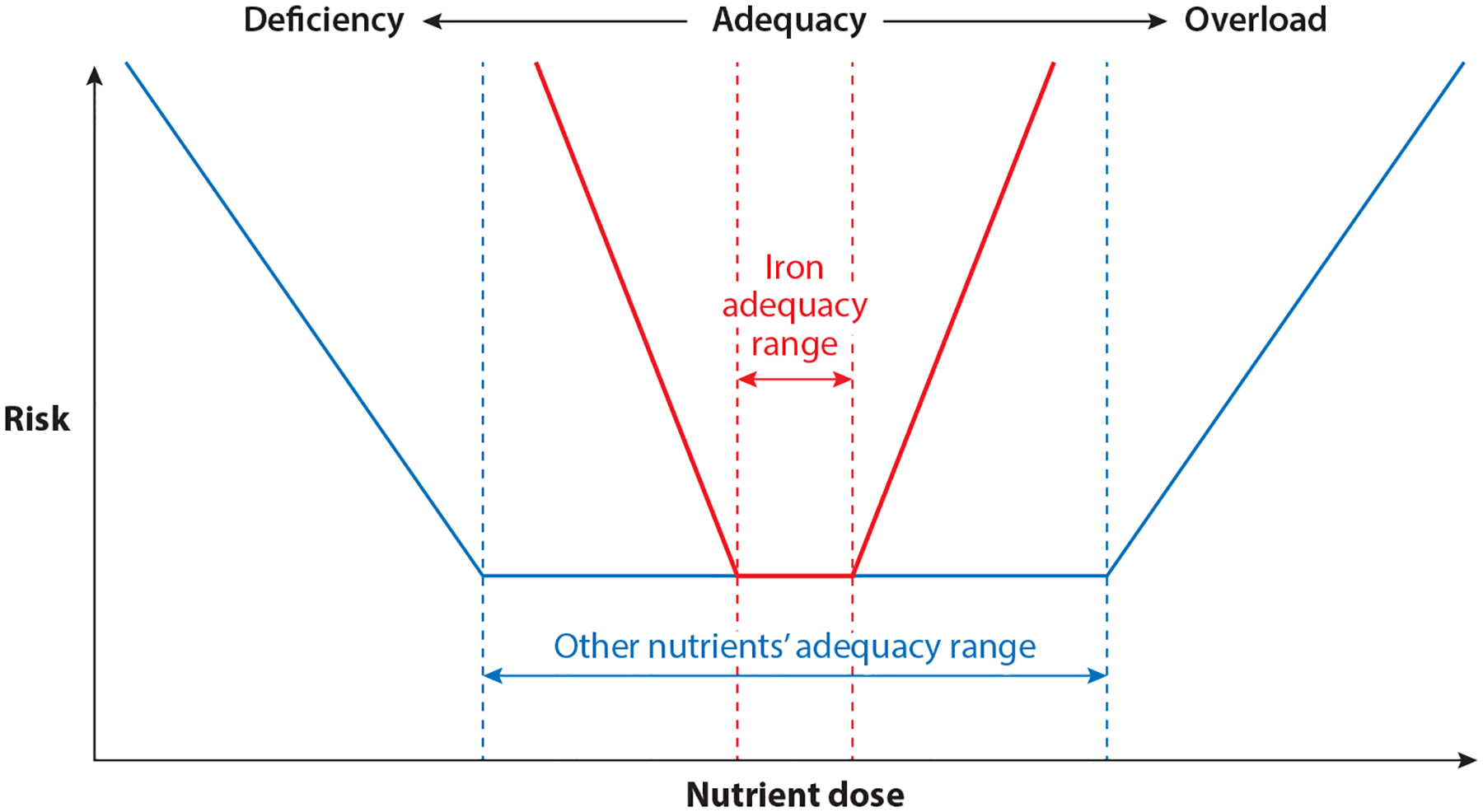
The U-shaped risk curve exhibited by most nutrients. Iron has a narrower adequacy range (red dashed lines), and risks from deficiency or overload states are greater compared with those from most other nutrients.
Nevertheless, conditions may be present in which regulation breaks down (e.g., genetic conditions, such as hereditary hemochromatosis) or is overwhelmed by excessively rapid iron delivery, following which NPBI is generated and is potentially able to react with oxygen. Excessive iron delivery could occur through the lysis of red blood cells after transfusion or as a consequence of rapid infusion of intravenous iron. Concerns have been raised as to whether enteral iron supplementation can result in NPBI, particularly under conditions of high oral dosages or low binding protein concentrations. The former may occur with aggressive enteral iron supplementation. Brittenham et al. (15) recently demonstrated that a supplemental dose of iron sulfate on an empty stomach in healthy, iron-replete women resulted in the appearance of NPBI but not reactive oxygen species. Premature infants, who typically have low serum protein concentrations, including low transferrin, and immature antioxidant systems would theoretically be at increased risk for the presence of NPBI. Yet enteral iron doses of up to 12 mg/kg body weight in preterm infants have not been shown to generate NPBI or evidence of reactive oxygen species (14).
Beyond the possibility of generating reactive oxygen species, competition for nutritional iron by other highly metabolic cells, such as pathogenic organisms, must be considered as a potential risk of nutritional iron supplementation. Enteral nonheme iron absorption is tightly regulated by hepcidin, and the absorption rate rarely exceeds 30% (44). Thus, a large portion of enteral iron, especially in fortified foods, is unabsorbed and is potentially available as an essential nutrient for bacteria residing in the colon. Bacteria species have variable iron needs, and some of the most siderophilic species include Escherichia coli and Salmonella, both potential pathogens (99). Bifidobacteria, in contrast, have low iron requirements (70). In addition, pathogenic organisms dwelling within the body (e.g., the malaria protozoa) also proliferate more aggressively in iron-rich environments compared with iron-poor environments (49). Taken together, there are good theoretical reasons to propose that while iron shows the same general U-shaped risk curve as all nutrients, the stakes are greater from both under- and overnutrition than for most nutrients (Figure 1).
It is against this background that the following sections discuss the relative benefits and risks of iron supplementation in the two populations at greatest risk for the consequences of iron deficiency: pregnant women and young children. Consideration is given for both populations as to how the benefit–risk balance of enteral iron supplementation is affected by iron status.
BENEFITS AND RISKS OF IRON SUPPLEMENTATION DURING PREGNANCY
Physiologic Considerations
Pregnancy poses a large risk of negative iron balance to a woman (39). Compared with the nonpregnant state, iron demands are greatly amplified for two reasons. First, the fetoplacental unit requires a large amount of iron for its own growth and development during gestation. One gram of iron needs to be accreted by the mother during pregnancy—of which 360 mg is transferred from the mother to the fetus, particularly during the third trimester when growth is most rapid—in order to maintain a content of 75 mg of iron per kg body weight of the fetus (39, 169). The pregnant woman expands her own plasma and blood volumes to maintain proper circulation and oxygen delivery to her own organs as well as to the placenta. The blood volume expansion consumes 450 mg of the 1 g of additional iron required during pregnancy (39). Decreasing hepcidin concentrations during pregnancy indicate the pregnant woman’s need to absorb more iron for both her own hemoglobin synthesis as well as for transport across the placenta to the highly metabolic and growing fetus (5, 142). Iron deficiency is generally acknowledged as a greater risk than iron overload during human pregnancy (39).
The goals of maintaining iron sufficiency during pregnancy are to reduce maternal morbidity, promote fetal health, and to set up the newborn with adequate nutrient stores for early postnatal life. Increasing evidence supports the concept that postnatal iron status at 9 months of age depends on proper fetal iron loading during pregnancy (164). The risk of postnatal iron deficiency in infants is reduced when neonatal iron stores are normal following gestation, delayed cord clamping is practiced, and postnatal growth rate is not excessive (30). It is also likely that proper loading of the newborn via the maternal–fetal route reduces the need for excessive early iron supplementation of the infant postnatally in certain iron-sufficient populations.
Pregnancies in Iron-Deficient Populations
There is little debate that iron-deficient women have an increased risk of adverse pregnancy outcomes, that is, those that affect the woman, her fetus, or, consequently, her offspring. Most studies utilize hemoglobin as the biomarker for iron status because of the ubiquitous availability of this measurement and because iron deficiency is the most common cause of anemia in most populations. However, anemia and iron deficiency are not synonymous, which makes interpreting the outcomes of these studies problematic. Anemia at various time points in pregnancy is associated with an increased risk of preterm birth (18, 52, 61, 91, 107, 113, 159, 163, 165), birth weight <2,500 grams (56, 91, 107, 165), and low weight for gestational age (28, 52, 61, 107, 144) (all summarized in Reference 30). In most studies, supplementation of anemic women with iron during pregnancy reduces the rate of iron-deficiency anemia and nonanemic iron deficiency at term, and in some studies, it reduces the risk of adverse outcomes, suggesting that supplementation in this population is beneficial (101, 102) (Table 1).
Table 1.
Interpretation and risk–benefit analysis of maternal iron supplementation during pregnancy based on whether hemoglobin or ferritin was used as the primary biomarker to assess iron status and the information added by a second biomarker
| Primarymarker | Literatureinterpretation | Biochemical interpretation with second biomarker | Agreement between literature and biochemical finding? | Low hepcidin (likely response to therapy) | Estimate of risk-benefit of routine supplementationa |
|---|---|---|---|---|---|
| Hemoglobin | |||||
| Low | Iron-deficiency anemia | Low ferritin: iron-deficiency anemia | Low ferritin: yes | Low ferritin: yes | B ≫ R |
| Normal ferritin: anemia of inflammation | Normal ferritin: unknown because total-body iron is unmeasurable | Normal ferritin: no | Unknown, but R > B because iron will not be absorbed in high hepcidin state | ||
| High ferritin: anemia of inflammation | High ferritin: unknown because total-body iron is unmeasurable | High ferritin: no | |||
| Normal | Iron sufficient | Low ferritin: low-iron state | Low ferritin: no | Low ferritin: yes | B > R |
| Normal ferritin: iron sufficient | Normal ferritin: yes | Normal ferritin: no | Unknown | ||
| High ferritin: iron overload | High ferritin: no | High ferritin: no | R > B | ||
| High | Iron sufficient | Low ferritin: polycythemia by volume contraction or other non-iron-related condition | Low ferritin: nonanemia iron deficiency | Low ferritin: yes | R > B |
| Normal ferritin: polycythemia by volume contraction or other non-iron-related condition | Normal ferritin: iron overload | Normal ferritin: no | R > B | ||
| High ferritin: iron overload | High ferritin: iron overload | High ferritin: no | R > B | ||
| Ferritin | |||||
| Low | Low-iron state | Low hemoglobin: iron-deficiency anemia | Low hemoglobin: partly | Low hemoglobin: yes | B ≫ R |
| Normal hemoglobin: nonanemia iron deficiency | Normal hemoglobin: yes | Normal hemoglobin: yes | B > R | ||
| High hemoglobin: polycythemia by volume contraction or other non-iron-related condition | High hemoglobin: no | High hemoglobin: no | R > B | ||
| Normal | Iron sufficient | Low hemoglobin: anemia of inflammation | Low hemoglobin: no | Low hemoglobin: no | R > B |
| Normal hemoglobin: iron sufficient | Normal hemoglobin: yes | Normal hemoglobin: no | Unknown | ||
| High hemoglobin: polycythemia by volume contraction or other non-iron-related condition | High hemoglobin: no | High hemoglobin: no | R > B | ||
| High | Iron sufficient | Low hemoglobin: anemia of inflammation | Low hemoglobin: no | Low hemoglobin: no | R > B |
| Normal hemoglobin: iron overload | Normal hemoglobin: no | Normal hemoglobin: no | R > B | ||
| High hemoglobin: iron overload | High hemoglobin: no | High hemoglobin: no | R > B | ||
Abbreviations: B, benefit; R, risk.
Estimate of whether the risk (R) of adverse outcomes or the benefit (B) associated with iron supplementation is greater, given each combination of iron markers.
A minority of clinical studies that assessed outcomes as a function of iron status used iron-specific biomarkers as opposed to or in conjunction with hemoglobin concentrations (30, 87). Iron-specific markers can be problematic for routine screening for analytic and interpretative reasons. The limited availability of the analytic equipment needed to measure specific iron parameters—especially serum ferritin, percent total iron-binding capacity saturation, soluble transferrin receptor, or hepcidin—is a major hurdle, particularly in low-resource countries. An ideal biomarker would index the risks of negative iron balance before physiologic consequences are present (24). Serum ferritin, which typically indexes iron stores, could theoretically serve this purpose since there are no known consequences of low iron stores, per se, as long as adequate iron is available to support hematopoiesis, tissue-level iron proteins (e.g., cytochromes), and iron transport to the fetus. However, ferritin acts as an acute-phase reactant to infection and inflammation, which undermines its effectiveness as a screening tool (128). Nevertheless, a meta-analysis of pregnancy outcomes as a function of iron stores demonstrated that low iron stores, particularly during the first trimester, are associated with a greater risk of low birth weight, prematurity, and small size for dates (101, 102).
Clinical studies have also assessed maternal iron status exclusively as a function of dietary iron intake during pregnancy (30). This approach has potential drawbacks, including the inherent variability of dietary recall and, more importantly, the question of whether dietary intake is tightly linked to actual iron accretion. This linkage can be tenuous because multiple inflammatory events during pregnancy could result in relatively less absorption of dietary iron due to activation of hepcidin by proinflammatory cytokines, including interleukin-6 (149). Furthermore, dietary iron intake gives no information about the distribution of iron between mother and fetus. Nevertheless, a meta-analysis of studies of maternal iron intake in iron-deficient populations shows that iron supplementation of iron-deficient populations is beneficial (101, 102). Neurobehavioral pathologies in offspring related to low maternal iron intake during critical periods of pregnancy include increased risks of schizophrenia (64) and autism (115).
Overall, clinical studies support iron supplementation of pregnant women with iron deficiency defined by any of the three biomarker approaches (i.e., hemoglobin, serum ferritin, or dietary intake). Little discordance exists among the three biomarkers, except in the case of active inflammatory processes. Table 1 presents the various potential interpretations and misinterpretations of utilizing hemoglobin or ferritin as the primary biomarker to assess iron status.
The role of inflammation in confounding iron status assessments is important. Inflammation activates hepcidin and thereby countermands the normal increase in iron accretion mediated by low hepcidin concentrations during pregnancy (5, 39, 142). Hepcidin increases iron in the storage pool, as evidenced by high serum ferritin concentrations, while shortchanging iron availability for hemoglobin synthesis by reducing intestinal iron absorption. Chronic inflammation results in reduced total-body iron during pregnancy and less iron availability for the fetus, yet the condition may be interpreted as iron overload or iron sufficiency if ferritin is the only biomarker used by the clinician to assess iron status (Table 1).
Although chronic low-grade inflammation may be relatively common in austere settings (4), the most common inflammatory condition worldwide during pregnancy is malaria. Worldwide, approximately 35 million pregnant women are at risk of Plasmodium falciparum malaria each year (154). The vast majority of populations at risk for malaria live in regions where iron deficiency is endemic. Iron supplementation in areas where both iron deficiency and malaria are endemic must be viewed within in the context of the 2006 landmark study on Pemba Island, Tanzania, that found that universal, daily supplementation with iron and folic acid increased the risk of hospitalization and death in young children (111). Subsequent cross-sectional studies seemed to support this association in pregnant women, finding a lower prevalence of placental malaria among women with iron-deficiency anemia (66, 118). In vitro studies have provided apparent mechanistic support of these findings, demonstrating that red blood cells taken from both anemic children and anemic pregnant women support a lower rate of Plasmodium growth than nonanemic red blood cells. Iron supplementation without treatment of malaria leads to increased parasite growth in red blood cells from both children and women (22, 49).
However, two recent randomized placebo-controlled trials have not supported a harmful link between healthy iron status and the risk of malaria in pregnant women. The trials demonstrate the importance of treating the malaria first and then addressing iron status (37, 94). In the first study, 1,500 HIV-uninfected pregnant Tanzanian women with serum ferritin concentrations >12 μg/L and hemoglobin concentrations >85 g/L were enrolled before 27 weeks’ gestation and were randomized to 60 mg of daily iron as ferrous sulfate or to placebo (37). All women received monthly prenatal health checks that included malaria screening, intermittent presumptive treatment for malaria, and antimalarial treatment if needed. Iron supplementation was not associated with an increased risk of placental malaria or other adverse events, and while iron did not increase birth weight, hemoglobin concentration and iron status as measured by serum ferritin concentration were greater among women in the iron-supplemented group. Although this study was conducted in an urban setting with a relatively low risk of malaria, a subsequent study in a malaria-endemic area of rural Kenya had similar findings (94). In that randomized placebo-controlled trial, daily supplements of 60 mg iron as ferrous fumarate given to pregnant women between the ages of 15 and 46 years, with no other inclusion criteria, did not increase the risk of maternal malaria and were associated with greater birth weight, lower risk of premature birth, longer length of gestation, and higher maternal and infant iron stores 1 month after birth when compared with placebo.
After the Pemba study, all iron supplementation trials in malaria-endemic areas have included a malaria control component for ethical reasons. These findings support the current World Health Organization (WHO) recommendation for universal daily supplementation with 30 to 60 mg elemental iron during pregnancy in regions where the prevalence of anemia is 20% or higher, with a stipulation in malaria-endemic areas that supplementation should be given in conjunction with “adequate measures to prevent, diagnose and treat malaria” (157).
Pregnancies in Iron-Sufficient Populations
Further controversy with respect to the accurate diagnosis of iron status and subsequent iron supplementation surrounds the routine iron supplementation of apparently iron-sufficient (i.e., non-iron-deficient) women during pregnancy. The US Preventive Services Task Force stated that there was insufficient evidence to advocate routine iron supplementation during pregnancy (140). A similar statement from the European Food Safety Authority concluded that iron supplementation during pregnancy should be reserved for those at risk for or with documented iron deficiency (34). The controversy stems from the difficulty in demonstrating any added benefit versus any potential risks of iron supplementation.
The majority of this literature defines iron status by measuring maternal hemoglobin concentration (30). However, precisely defining iron status in the context of what is termed the physiologic anemia of pregnancy is problematic. The physiologic anemia of pregnancy occurs because of a disproportionately greater expansion of the plasma volume (+50%) than of the red cell mass (+25%), leading to a dilutional reduction in hemoglobin concentration (146). Maternal hemoglobin concentrations between 95 and 110 g/dL have been associated with the best pregnancy outcomes and, thus, would be considered normal (17). Hemoglobin concentrations higher than this range have been associated with higher rates of preeclampsia, prematurity, and fetal growth restriction (18, 52, 113, 116, 136, 166). A smaller number of trials have assessed the effect of iron supplementation on women with high hemoglobin concentrations (i.e., >132 g/L) and found an increased rate of maternal preeclampsia and fetal growth restriction (166).
A nonanemic hemoglobin concentration would typically be considered a biomarker of iron sufficiency in nonpregnant women, whereas excessively high hemoglobin would be consistent with total-body iron overload, given that iron is predominantly found in red cells. Alternatively, elevated hemoglobin concentrations during pregnancy may not indicate iron overload but instead reflect low plasma volume expansion, that is, cases in which the 2:1 ratio of plasma volume to red cell mass expansion is not achieved (146). In that circumstance, serum ferritin may be normal or even low (Table 1). Thus, an elevated hemoglobin concentration may be misinterpreted as iron overload. Studies that associate high hemoglobin concentrations during pregnancy with poorer outcomes should be interpreted cautiously as to whether the mechanistic causes of the poorer outcomes are a fundamental gestational pathology that leads to low plasma volume expansion, true iron overload, or both (162). The possibility that iron plays a primary role in plasma volume dysregulation must be considered because such information would have a direct impact on the decision to offer iron supplementation to women with normal or high hemoglobin concentrations (162). The lack of adequate studies hinders the ability to provide guidelines regarding universal iron supplementation in nonanemic pregnant women (146).
Future studies could avoid dividing pregnant women into the two traditional hemoglobin categories, anemic or nonanemic, and instead consider a three-group model: anemic, normal, and polycythemic. Data support this approach since a U-shaped risk curve of pregnancy complications as a function of hemoglobin concentration has been described (146). Moreover, women with normal hemoglobin concentrations may have preanemic iron deficiency. Women with high hemoglobin concentrations may be iron-sufficient, but not iron overloaded, if the high hemoglobin concentration is due solely to the failure of plasma volume expansion (Table 1).
Assessing pregnancy outcomes as a function of iron-specific biomarkers could potentially provide more direct insight than measuring hemoglobin alone. In turn, these markers could be used to identify candidates for supplementation during pregnancy. Among these markers, serum ferritin has been most often utilized in outcome studies. WHO recently assessed its usefulness as a screening tool (156). Clinical interpretation of ferritin concentrations relies on the understanding that if iron stores are replete, sufficient iron is present to support iron-dependent cellular processes at the tissue level. Serum ferritin is an excellent specific metric for low-iron states because no condition other than iron deficiency results in low serum ferritin concentrations. Interpreting high serum ferritin concentrations is more problematic with respect to understanding serum ferritin’s relationship to tissue iron status. High ferritin concentrations could indicate iron overload or, alternatively, a shift of iron into reticuloendothelial cell storage as part of a response to inflammation (128). Mathematical correction of ferritin concentrations for the degree of inflammation as indexed by an inflammatory biomarker has been proposed, but has not been widely implemented (128).
The majority of published studies on pregnancy outcomes as a function of maternal iron status utilized serum ferritin as the biomarker (30). They reported that higher ferritin concentrations early in pregnancy were associated with more positive pregnancy outcomes, whereas higher ferritin concentrations in the third trimester were associated with poorer outcomes, including premature delivery and low birth weight (50, 73, 116, 131). Interpreting these studies is difficult because it is unclear whether the high ferritin concentrations during the third trimester indexed increased total-body iron or a shift of iron into the storage pool due to inflammation. There is a great need for a sensitive and specific biomarker that indexes tissue iron status and is not influenced by inflammation.
Assessing iron status by quantifying iron intake has yielded mixed results with respect to pregnancy and offspring outcomes. On one hand, iron intake in non-iron-deficient mothers early in pregnancy appears to protect against autism in the offspring (115), and iron intake during the third trimester induces a more mature gray matter pattern on diffusion tensor imaging in term infants (92). Conversely, iron supplementation in women with high hemoglobin concentrations (i.e., >132 g/L) during the second trimester leads to even higher hemoglobin concentrations in the mother, but a greater risk of fetal growth restriction, most likely due to maternal hypertension (166). Iron supplementation in pregnant women has also been linked in observational studies to a greater risk of gestational diabetes mellitus (162).
Conclusions Regarding Iron Supplementation in Pregnant Women
Iron sufficiency during pregnancy results in better pregnancy outcomes for the mother and the child. The benefits of iron supplementation outweigh the risks in women about to become pregnant and in pregnant women with evidence of iron deficiency. Women living in areas where iron deficiency is prevalent also benefit from routine iron supplementation during pregnancy, even in malaria-endemic areas. In these areas, iron should be given along with implementing malaria-control efforts, for example, insecticide-treated nets, and with malaria diagnosis and treatment services readily available.
Typically, there is little additional benefit to supplementing a nutrient that is already replete in an individual. This approach may also apply to iron supplementation of iron-sufficient or iron-overloaded pregnant women, for whom little additional benefit would be expected and potential risks exist. However, the inability to reliably distinguish total-body iron status from three iron-replete states of hemoglobin in nonanemic women—nonanemic iron deficiency, optimal iron status, and iron overload—presents a major problem in terms of benefit–risk analysis since the effects of iron supplementation on these three states likely differ. A more complete assessment of iron status, made by measuring both hemoglobin and ferritin concentrations simultaneously, would resolve certain, but not all, ambiguities. The addition of hepcidin concentration to the arma-mentarium of iron screening tools would greatly enhance the clarification of who would benefit from enteral iron supplementation. Because hepcidin is upregulated by high iron status and by inflammation, elevated concentrations in iron-sufficient, iron-overloaded, and inflamed individuals would reduce or abolish absorption. In those cases, enteral iron supplementation would be pointless (i.e., nonabsorbed) or risky (i.e., nonabsorbed plus adversely altering the microbiome). Conversely, low hepcidin concentrations would indicate individuals for whom the benefits of iron supplementation would likely outweigh risks.
BENEFITS AND RISKS OF IRON SUPPLEMENTATION IN EARLY CHILDHOOD
Physiologic Considerations
Early childhood, especially the first 3 years, is a period of rapid growth and development for all organ systems, particularly the brain. Newborns have higher hemoglobin concentrations (79) and iron stores, as reflected in their serum ferritin concentrations (121), than older infants. Both decline with age (54, 167), and the iron that is liberated is utilized for expansion of the red cell mass that occurs with the physical growth of the infant and for biochemical reactions to catalyze tissue development and function.
Evidence from humans (47, 103), monkeys (106), sheep (161), rats, and mice (150) demonstrates that the brain’s iron status is compromised before the iron status of the red cells. This prioritization explains why anemia is the end-stage of iron deficiency and why monitoring hemoglobin concentration is a poor (and late) measure of iron status in children. Studies in humans (83, 86) and preclinical models (41, 114, 139) demonstrate that brain iron deficiency early in life confers a risk for poorer brain function in adulthood. The clinical consequences of early-life iron deficiency include poorer school achievement, lower job potential, and increased risks of psychopathology (86). Recent preclinical studies suggest that the failure of brain systems to be properly constructed during this critical period (41) is responsible for behavioral abnormalities in adulthood (67, 138).
The brain systems that are rapidly developing prior to birth and during the first year after birth include the monoaminergic neurotransmitter systems (9, 160) and the hippocampus (41), and the process of myelination (25). Early-life iron deficiency results in abnormal neuronal structure (16, 41) and function (105), metabolism (106), gene expression (138), and neurotransmitter concentrations (139) in adulthood. The areas of the brain that have been identified as affected in preclinical models are concordant with the abnormal behaviors documented in humans (80, 87), indicating a high biological plausibility for the long-lasting effects of early-life iron deficiency on the human brain (87).
Whereas brain and behavior are major outcome variables in many studies of childhood iron deficiency, it is clear that the function of other systems, especially innate immunity, also depends on iron sufficiency (30). Thus, preventing iron deficiency is important because of its long-term cost to society, and it is a far better strategy than treating iron-deficiency anemia once it is present.
The appropriate-for-gestational-age term neonate who received the benefits of delayed cord clamping has enough iron in red cells and storage pools to sustain tissue iron sufficiency for at least 4 months and, perhaps, for as long as 6 months, even on a diet with as low an iron content as human breast milk (30, 77). This sustainability assumes that the infant’s postnatal growth rate (and hence expansion of the red cell volume) is appropriate as defined by WHO’s growth curves; rapid infant weight gain is associated with earlier depletion of infant stores.
The exceptions to this trifecta of term infants with normal iron stores, delayed cord clamping, and reasonable postnatal growth velocity are common, not just in low-resource countries but also in high-resource ones. Reduced fetal iron accretion can occur as a function of maternal iron deficiency, defined as a hemoglobin concentration of <85 mg/L or a serum ferritin concentration of <13 μg/L (119), or both. Other gestational conditions that reduce neonatal supply include premature delivery and fetal growth restriction due to maternal hypertension (19). The former complicates >10% of pregnancies and is important because the majority of fetal iron is accreted during the last trimester (169). The earlier the preterm delivery, the greater the risk for subsequent iron deficiency (32). The postnatal iron requirements of the preterm infant are two- to threefold greater than those of the term infant (32). Approximately 50% of newborns with fetal growth restriction have ferritin concentrations below the fifth percentile, indicative of compromised iron stores at birth and greater risk for postnatal iron deficiency at an earlier age (19). Worldwide, the most common cause of fetal growth restriction is maternal undernutrition (which includes iron deficiency), whereas in the United States it is maternal hypertension, including preeclampsia. Maternal smoking during pregnancy and pregestational or gestational diabetes mellitus are significant risk factors for low fetal iron status (121, 129). These two common gestational conditions complicate >10% of pregnancies.
Given how common these potential risks to fetal and neonatal iron status are, measuring iron status at birth would be useful to provide information about which babies are already iron deficient at birth or at an increased risk for earlier postnatal iron deficiency. The current American Academy of Pediatrics (AAP) policy of screening for iron deficiency via assessment of hemoglobin concentration at 1 year of age may be ineffective because it fails to consider that all other organ systems are affected prior to the onset of anemia (6). A biomarker that indexes the risk to organ systems other than the erythron is urgently needed to inform decisions about iron supplementation (46), particularly because nonanemic iron deficiency is threefold more common than iron-deficiency anemia and is also associated with altered neurobehavioral development (83). Recent changes by WHO to utilize serum ferritin as opposed to or in addition to hemoglobin and by the AAP to add reticulocyte hemoglobin content are steps in the right direction. However, all of the commonly used iron-specific biomarkers (e.g., serum ferritin, soluble transferrin receptor, percentage total iron-binding capacity saturation) are influenced by inflammation. Unlike the case in adults (44), the regulation of hepcidin in very young infants is not well understood or described.
The following sections review the benefits and risks of iron supplementation in populations with or at high risk for iron deficiency and those that are iron sufficient or at low risk for iron deficiency. As with the literature on iron supplementation during pregnancy, the lack of adequate biomarkers of iron status and bioindicators of the physiologic consequences of alterations in iron status causes significant problems for reaching universal conclusions regarding supplementation in young infants (87).
Iron-Deficient Populations of Children
Iron-deficient pediatric populations are found predominantly, but not exclusively, in low-resource settings. Rates upward of 80% have been reported in certain low-resource settings, while Europe reports rates ranging from 4% to 50% in 6- to 36-month-olds, with a greater predominance in Eastern Europe compared with Western Europe (141). The current rate of iron deficiency in 1-to 2-year-olds in the United States is 13.5% (55). The benefits and risks of iron supplementation in iron-deficient populations can be thought of as striking a balance between the individual and societal downsides of iron deficiency as it relates to lost intellectual potential and poorer immune capacity versus any potential risks of infection posed to individuals by iron supplementation.
There is little disagreement that most term neonates are born with iron stores adequate to last them for up to 6 months, but that after that time, a source of dietary iron is required to prevent iron deficiency and anemia (30, 170). Thus, the question of whether to provide iron supplementation at some point during the first few months of life at a dose that supports optimal growth and development is not in debate. By 6 months of age, all infants who were born iron sufficient need a dietary source of iron because fetal stores will have been exhausted and human breast milk does not contain adequate iron to support the erythropoietic and tissue-driven iron needs of the infant.
Multiple common gestational conditions place the newborn at risk for lower than normal iron stores. These conditions (e.g., fetal growth restriction, severe maternal iron-deficiency anemia) are more common in low-resource countries and set up the newborn for an earlier onset of postnatal iron deficiency. Exacerbating postnatal factors include rapid postnatal growth rate, earlier introduction of complementary foods with poor iron content and/or bioavailability, and frequent illnesses, including infections that limit food intake and activate hepcidin-driven reductions in iron absorption (23, 170).
Iron supplementation is beneficial if the goal is to alleviate anemia. Clinical studies, both observational and randomized controlled trials (RCTs), demonstrate that iron and multimicronutrient supplementation are effective in improving iron status and reducing the rate of iron-deficiency anemia in iron-deficient populations of infants, whether the supplementation is through the iron-fortification of formula or milk, or administration of medicinal iron (108, 112, 125, 145, 152, 168, 170). Hematologic recovery from iron-deficiency anemia through iron supplementation occurs at all ages, whereas neurobehavioral recovery is not always complete (86, 87). There is minimal literature on the recovery of immune health following iron supplementation in iron-deficient populations.
The literature is robust with respect to the effect of iron status on neurodevelopment. Iron deficiency is associated with neurobehavioral abnormalities while the infant is deficient (3, 83, 109, 110, 120). Some abnormalities continue long after treatment of iron deficiency with iron supplements (80, 86). A series of reports in the Lancet and by the BOND (Biomarkers of Nutrition for Development) iron work group identified 20 studies that showed poorer functioning in multiple neurodevelopmental domains in iron-deficient children or children with iron-deficiency anemia (87, 147, 148). Findings of particular concern include lower general mental functioning (1, 57, 62, 81, 85, 151), poorer motor performance (1, 62, 85), and a general loss of capacity relative to an iron-sufficient population across childhood (80).
Whether iron supplementation as a preventative or a treatment strategy is effective against these neurobehavioral deficits in populations at risk for iron deficiency depends on the age range of the children, the timing of the intervention, the baseline rate and the degree of iron deficiency in the population, and the selection of appropriately sensitive and specific neurodevelopmental outcome indicators. Interpreting the specific role of iron is made more complicated when iron is provided as part of a generalized nutritional plan (e.g., fortified formula or milk) or as a multimicronutrient supplement.
Overall, iron supplementation in populations at increased risk for iron-deficiency anemia improves motor outcomes (12, 74, 84, 127), neurocognitive and language outcomes (84, 127), and social development (12, 74, 84). The effects can be long-lasting. Infants with hemoglobin concentrations <105 g/L who were randomized to iron-supplemented infant formula (12.7 mg/L) at 6 months of age had better 10-year outcomes than those randomized to a low-iron formula (2.3 mg/L) (84). A greater impact on neurodevelopment has been found with earlier supplementation, including starting during the fetal period via maternal supplementation (20, 21, 26, 93). Later supplementation (e.g., after 1 year of age) was not beneficial from a neurodevelopmental stand-point, suggesting that the critical period for providing supplementation to ensure iron sufficiency to protect neurodevelopment is earlier in life and includes the late fetal period (20, 21, 26, 93).
Iron status can be defined using various biomarkers. These include assessing anemia (with supporting evidence that the anemia is due to iron deficiency), iron-specific markers, and iron intake. The bulk of the literature has focused on preventing, detecting, and treating iron-deficiency anemia. However, nonanemic iron deficiency, as indexed by altered iron-specific markers, is associated with worse neurobehavioral function, including poorer motor function (83), social function (83), speed of processing (3), and recognition memory (45, 120). A recent RCT of mainly nonanemic iron-deficient 9- to 24-month-old infants with 6 mg iron/kg body weight demonstrated recovery of serum ferritin concentrations, although neurodevelopmental outcomes were not measured (133). Specific cutoffs for serum ferritin concentration to detect neurodevelopmental deficits are present for neonates (3, 45, 120) but not for other ages.
Studies of populations with increased early-life iron requirements—for example, those born following gestational conditions that resulted in inadequate fetal iron loading, such as maternal hypertension or premature delivery—indicate a positive role for iron supplementation in preserving neurobehavioral development. Breastfed Swedish infants with birth weights between 2,000 and 2,500 g as a result of either late preterm birth or intrauterine growth restriction were randomized to iron supplementation of 0, 1, or 2 mg/kg body weight (11). The group randomized to no supplementation had a higher rate of iron deficiency and iron-deficiency anemia in the first year of life and an increased rate of mild neurodevelopmental abnormalities at 8 years of age compared with the group that received either of the two supplemental doses (10, 11). Preterm infants who received iron supplementation earlier in their neonatal course have higher mental processing composite scores at 5 years of age (40, 124).
Given the beneficial effects of supplementing iron-deficient infants, consideration must still be given as to whether there are any contraindications to iron supplementation in this population. In 1998, in response to the mounting evidence of the potentially permanent neurobehavioral consequences of iron deficiency in young children, WHO issued a recommendation for daily, universal iron supplementation of all children aged 6 to 59 months living in areas where the prevalence of anemia was 40% or greater (126). The Pemba trial (described in the section Pregnancies in Iron-Deficient Populations) first tested this recommendation, enrolling >30,000 children between the ages of 4 and 48 months and randomizing them to daily supplementation with 12.5 mg iron and folic acid with or without zinc or to placebo (111).
The iron-containing arms of the Pemba trial were halted by the study’s data and safety monitoring board after only 18 months because of a 12% increased risk of serious adverse events (i.e., hospitalizations and deaths) among children who received iron compared with those who did not. A concurrent study of identical design and equivalent size conducted by the same researchers in Nepal (135), where there is no malaria, found no harmful effect of iron supplementation, leaving researchers to conclude that iron supplementation in malaria-endemic areas may be risky.
A Pemba substudy assessed roughly 3,000 children who had better access to health care and also measurements of hemoglobin and zinc protoporphyrin (ZPP) and found that the harmful effect of iron appeared to be confined only to those children who were iron replete (ZPP < 80 μmol/mol heme) (111). In contrast, iron-deficient children (ZPP ≥ 80 μmol/mol heme) benefited from iron, experiencing significantly fewer serious adverse events compared with iron-deficient children who received placebo. The resulting recommendation from WHO was to halt universal supplementation in malaria-endemic areas and to screen for iron deficiency before giving the supplement (158). However, this screen-and-treat approach failed to gain momentum in the field because of the expense of screening in the low-resource settings where malaria is endemic and the impossibility of conventional interpretation of iron biomarkers in areas where infection and resulting inflammation are commonplace. Although three Cochrane Reviews of iron supplementation in children in malaria-endemic areas conducted after the Pemba study found no harmful effects provided that malaria control and treatment resources were in place (95–97), iron supplementation programs in these areas where the public health need is potentially the greatest were drastically cut back or eliminated altogether.
Red blood cells from anemic children in malaria-endemic areas are more resistant to invasion by the malaria parasite, and this resistance is reversed with iron supplementation (22). Because the malaria parasite preferentially invades reticulocytes, the transient reticulocytosis that follows iron supplementation could explain the increased frequency of clinical malaria that occurs with the provision of supplemental iron (49). This mechanism would not explain the increased frequency of nonmalarial infections, including diarrhea and respiratory infections, reported in children who received supplemental iron, either in the form of a supplement or micronutrient powder, in areas where malaria or other infections are endemic (65, 123, 143). The explanation may reside in the gut bacterial microbiome. In two separate studies of African infants, the regular consumption of iron-fortified biscuits and iron-fortified food powders shifted the profile of the gut microbiota from one in which more beneficial bifidobacteria barrier strains presided to one where pathogenic enterobacteria strains prevailed (132). Recent preclinical models also suggest that pathogenic shifts in the gut microbiome are associated not only with diarrheal illness but also with an increased risk of a range of respiratory viruses, including respiratory syncytial virus and pneumococcal pneumonia (117). Recent work suggests that these effects of iron on the microbiome and the resulting morbidity may be mitigated by lowering the dose of iron to approximately 5 mg and giving it in conjunction with a prebiotic, such as galacto-oligosaccharide (98). This combination reduced anemia in Kenyan children with the same efficacy as a 12.5 mg dose of iron and was also associated with a significantly lower frequency of respiratory infections (99).
The absorbability of iron and the corollary question about the amount of residual iron delivered to the gut microbiotic community is particularly pertinent for children living in low-resource malaria-endemic areas, as these children typically have a higher baseline level of inflammation due to poor sanitation, unclean water, and crowded living conditions. The resultant inflammation and accompanying high hepcidin concentration likely limit the absorbability of supplemental iron, leaving more iron in the gut lumen to the potential benefit of pathogenic bacteria.
Delaying the provision of iron until the resolution of inflammation and the nadir of hepcidin is a key element of a successful and safe pediatric iron program in areas endemic for malaria and other infections. This concept has recently been demonstrated in a series of studies examining the timing of iron therapy relative to antimalarial treatment in Ugandan children with iron deficiency and malaria (26, 27, 65). The standard of care for co-occurring iron deficiency and malaria is to treat both simultaneously, a practice supported by three Cochrane Reviews and WHO guidelines (153). Recent research shows that staging the interventions, by treating children with either severe or uncomplicated malaria first and delaying the start of iron until 28 days after the onset of antimalarial treatment, leads to a greater reduction in hepcidin, greater incorporation of iron into red blood cells, and fewer infectious illnesses compared with children started on iron concurrently with antimalarial treatment. Whether the initial better incorporation of iron with delayed provision results in better long-term iron status and neurodevelopment is currently being tested (26, 27, 65).
In the absence of the eradication of malaria, the optimal method for safe maintenance of healthy iron status in children living in these areas remains unclear. However, providing smaller amounts of iron at times when hepcidin is lowest appears to be part of an effective solution. These components are reflected in WHO’s current recommendations, which again stipulate delivering universal iron supplementation to children between the ages of 6 and 59 months in regions where the prevalence of anemia is 40% or greater, but delivering it intermittently (i.e., once, twice, or three times per week) where anemia prevalence is 20% to 40% (155). In areas of where malaria is endemic, this supplementation should be given concomitantly with efforts to control, diagnose, and treat malaria (155).
In addition to any potential risk of iron supplementation on infection rates and microbiome alterations, investigators have been concerned about iron’s ability to induce reactive oxygen species. This reaction occurs in the setting of NPBI, which would be most likely to occur in settings of large iron doses in patients with low iron-binding protein concentrations. The generation of reactive oxygen species, as indexed by biomarkers of the process, has failed to be found in multiple studies of iron supplementation in premature infants (121). Premature infants receiving doses as high as 18 mg of iron/day fail to show evidence of such a response (14). Nevertheless, it remains prudent to assess this risk as a safety measure in future trials because high-dose iron will be required to support the erythropoietic benefits of recombinant erythropoietin therapy in preterm infants (121).
Iron-Sufficient Populations of Children
Although iron deficiency is the most common nutrient deficiency worldwide, the reality is that the majority of newborn infants are likely to be iron sufficient, with normal iron storage pools. In this context, a policy of universal dietary iron supplementation of all newborn infants, both those who are iron sufficient and those at risk for iron deficiency, is questionable unless there are absolutely no risks to iron supplementation, an assumption that is not supported by the discussion in the preceding sections.
Any potential negative effects of iron supplementation should be understood within the context of the iron status of the infant. It is worth considering a distinction between the labels iron replete and iron overload as they relate to biomarkers of iron status in infants. True iron overload is rare in neonates and infants. The genetic syndromes responsible for neonatal iron overload are often fatal. Infants with neonatal polycythemia (e.g., infants of diabetic mothers), who appear to be iron overloaded, as defined by an elevated hemoglobin concentration, actually have lower storage and tissue iron contents (47, 103). This combination of high hemoglobin and low serum ferritin concentrations indicates a shift of iron—that is, iron has been prioritized for erythropoiesis through chronic fetal hypoxemia—rather than iron overload. Multiple red cell transfusions could cause iron overload, but infants who receive such therapy are relatively rare and would not drive universal policies. Nevertheless, two interesting studies that demonstrate the potential neurodevelopmental risk of iron overload illustrate the need for better differentiation between iron-replete and iron-overload states (82, 130).
Tamura et al. (130) demonstrated that the school-age outcomes of newborns who were divided into quartiles of cord blood serum ferritin concentrations were poorer for the children in the lowest (i.e., iron-deficient children) and highest quartiles. While the former finding is not surprising and is consistent with other neurodevelopmental studies of newborns with low ferritin concentrations (3, 45, 120), the latter finding was unexpected. The etiology of the high ferritin concentrations in the upper-quartile newborns is not known and may represent inflammation (with its own risks to neurodevelopment), iron overload (by unknown mechanisms), or both. Postnatal iron intake was not assessed in this cohort and thus not factored into the assessment of the school-age outcomes.
The RCT of iron supplementation of infant formula performed in Chile that demonstrated a benefit of iron-fortified infant formula (12.7 mg/L) on hematologic and neurodevelopmental outcomes (84) also demonstrated that infants with excessively high hemoglobin concentrations (i.e., >125 g/L) who were randomized to iron-fortified formula at 6 months had poorer neurodevelopment at 10 years of age (82). The number of infants in this group was small (n = 26), the etiology of their relative polycythemia/iron overload at 6 months was unclear, and no mechanistic studies were performed because the finding was a secondary analysis of neurodevelopmental data collected 10 years later. The negative effect of higher levels of iron fortification in infant formula on neurodevelopment was not seen in children with normal hemoglobin concentrations (i.e., between 105 and 125 g/L), and the same degree of fortification improved neurodevelopment in children with hemoglobin concentrations of <105 g/L (82, 84). These two studies raise the possibility that some infants who would normally have been assigned to an iron-replete or iron-sufficient category by ferritin (130) or hemoglobin (82) concentration may instead have had underlying abnormalities that led to an iron-overload state and that neurologic morbidities that may be associated with an iron-overload state may be further exacerbated by high-iron diets.
The risks and benefits of dietary iron delivery in presumably iron-sufficient, but not iron-overloaded, infants and young children have been the focus of recent reviews and were a major influence on the decision by the US National Institute of Health’s Office of Dietary Supplements to convene a workshop that took place in 2017 (134) as well as the work of an expert panel that occurred in 2015 (68).
The iron status of infants as a function of iron in the diet has been extensively studied. Human breast milk contains approximately 0.5 mg/L of iron. Irrespective of whether this iron is more absorbable or not, this amount is not sufficient to supply the iron needs of the growing infant without concomitant mobilization of stored iron (30, 70). Ferritin concentrations decrease during the first 6 postnatal months in breastfed infants (167). Breastfed infants not supplemented with iron or iron-containing foods will typically exhaust iron stores by 6 months of age, and, thus, the rate of iron deficiency in exclusively breastfed infants at 7 months of age is greater than 30% (71). Iron supplementation slows this decline (167). Thus, the issue is not whether breastfed infants need iron supplementation, but when to introduce a source of iron. To answer this question, consideration should be given to factors that affect the benefit–risk balance. The benefits of maintaining iron sufficiency—whether through endogenous stores accrued prenatally or through postnatal supplementation—have been discussed.
The iron content of the most highly fortified formulas is as high as 12–14 mg/L, thus far exceeding the amount in human breast milk. The fortification of infant formula at 12 mg/L began more than 50 years ago when the prevalence of iron deficiency in infants in the United States approached 40% and was a major public health risk. Fortification at this level was successful in reducing the prevalence of iron deficiency to much lower levels (55). Studies assessed whether there were increased rates of diarrheal illness, gastrointestinal distress, or changes to stool bacterial content. Although the stool of infants receiving highly fortified formula had a greater content of siderophilic bacteria that are potential pathogens (e.g., E. coli), no increases in diarrheal illness or gastrointestinal symptoms have been found (70, 122). Interestingly, the intake of medicinal iron results in a different microbiome profile than intake of highly fortified formula (122). It should be noted that this recent study was performed in Sweden, where a lower infection burden and low rate of infant mortality from infectious diseases are present (122).
The original assumptions behind delivering fortification at such a high level from birth were likely misguided because of the lack of knowledge of the regulation of iron absorption by hepcidin. At that time, breastfeeding rates in the United States were at their lowest point. Formulas contained only the amount of iron inherent to breast milk (i.e., approximately 1.15 mg/L), and infants were often switched to cow’s milk, another low-iron beverage, at 6 months of age. It was thought that intensely fortified infant formula delivered from birth would result in infants storing additional iron beyond their immediate needs in order to have a reserve for what was inevitably a negative iron balance state in the second 6 months of the first year.
The discovery of hepcidin as a master regulator of gut absorption provided evidence that the goal of storing extra iron was not likely to be achieved. Intestinal iron absorption ranges from 4% to 40% based on hepcidin activity (44). Hepcidin is secreted by the liver in response to sensors of iron status (44). As a negative regulator of intestinal absorption, hepcidin levels are high when the individual is iron sufficient, thereby limiting iron absorption, and low when an individual is iron deficient, thereby promoting absorption (44). It is likely that young infants receiving formula fortified to 12 mg/L were iron replete with high hepcidin levels, effectively reducing iron absorption to a low percentage and preventing the intended storage of excess iron. The developmental ontogeny of effective hepcidin regulation has not yet been completely defined, although evidence suggests that it is intact by 6 weeks post-term gestational age (38). Hepcidin has been detected in preterm infants, but the fact that isotope studies of iron absorption in preterm infants consistently demonstrate an absorption rate of 35% to 40% (35, 53, 89) in spite of a wide range of iron statuses, (79, 121) suggests that it may not be functional in that population.
A study in Sweden, a country with a low prevalence of iron deficiency, compared providing formula fortified with 4 mg/L versus 7 mg/L starting at birth and revealed no difference in hemoglobin concentrations (78). Infants fed the 4 mg/L formula had higher soluble transferrin receptor than those fed the 7 mg/L formula, indicating a more iron-deficient state. While there is no physiologic consequence of these changes, it does suggest that 4 mg/L places the infant in a slightly negative iron balance. This evidence of negative iron balance provided the rationale for the AAP’s statement advocating for at least 4.5 mg/L of iron in formula (2).
The timing of iron exposure in iron-sufficient populations is key. The RCT in a high-risk Chilean population began the iron intervention at 6 months of age and showed that 2.4 mg/L was not sufficient to support iron status, albeit one could argue that many of the infants were already total-body iron compromised at the time of randomization (84). In contrast, the Swedish study began delivering iron-supplemented formula at birth in a population with a very high likelihood of iron sufficiency at birth (78).
Dose consistency is also an important factor. The United States utilizes a one-size-fits-all-ages approach, with infant formulas designed to be utilized from day 1 to 12 months of age (59). Europe utilizes formulas that gradually step up the iron concentration as babies age. This approach makes sense since there appears to be little physiologic rationale to supplement iron starting at birth in iron-sufficient populations with adequate neonatal hemoglobin and ferritin concentrations (31, 59).
If there were no risks to iron supplementation, management would be relatively straightforward, and universal supplementation, medicinally or through the diet, would be acceptable. However, the potential risks of infant iron supplementation have been identified (59, 70, 77) and include (a) compromise of the status of other divalent metals, especially zinc; (b) an altered gut microbiome, with a potentially increased infection risk; and (c) growth restriction. These risks, especially the effects on the microbiome, have not been defined by rigorous trials (70).
Iron in doses typically prescribed to nonanemic healthy infants does not appear to have an adverse effect on either copper or zinc absorption, but it does affect plasma and serum zinc concentrations (33, 36, 58, 70, 75). This range of iron delivery is well beyond typical iron supplementation or fortification regimens. Thus, negative effects on other divalent metals are avoidable with low or moderate iron doses (70).
The intestinal intraluminal iron environment influences the microbiome, which is highly mutable and is establishing itself in the first months after birth. A low intraluminal iron concentration favors the growth of the preferred Lactobacillus organisms, while high iron fosters a shift in the microbiome toward potentially pathogenic Bacteroides and E. coli as early as 1 week of postnatal age (69, 90, 99). High intraluminal iron most likely occurs in the setting of high dietary iron intake or inflammation. Both are high hepcidin states in which iron absorption is low and unabsorbed iron is more likely to be retained intraluminally. Whether the shift in microbiome character increases the incidence of clinical infectious diseases or alters the developing innate immune system in iron-sufficient populations remains an area of intense study, the results of which will likely inform policy in the future.
A long-standing concern has been whether early-life iron supplementation reduces growth velocity (77). Studies have had equivocal results from which it is difficult to draw firm conclusions. A meta-analysis of 35 studies of iron supplementation in children from a wide age range and in whom initial iron status was usually not known showed an overall effect of linear growth suppression (100), but results of individual studies vary widely. The lack of consistent findings may reflect which infants were supplemented (iron-deficient versus iron-sufficient), their diets (human breast milk versus infant formula), the amount of iron provided, and the method of supplementation (medicinal iron versus fortified formula).
Medicinal iron supplementation dosed at 1 mg/kg body weight daily in 4- to 6-month-old term breastfed infants in Sweden and in Honduras yielded different results. The Honduran infants, who were likely at high risk for negative iron balance or frank iron deficiency, improved their iron status and did not have growth suppression (29). In contrast, the iron-sufficient Swedish infants did not increase their iron status, as would have been expected, but did show linear growth suppression. The Honduran infants showed growth suppression only if they had hemoglobin concentrations >110 g/L, likely indicating an iron-replete state. Several other studies of iron supplementation starting in late infancy and ranging up to 2 years of age showed negative effects on growth if the infants were iron sufficient (63, 76, 88) but beneficial effects on iron and hematologic statuses without growth restriction if the infants were iron deficient at the start of treatment. Interestingly, other studies, including the RCT from Chile that identified a neurodevelopmental risk with iron supplementation of 6-month-olds who had high hemoglobin concentrations (82), did not show an adverse effect of iron on growth (42, 43).
As noted, the studies that demonstrated growth-suppression effects were conducted in older infants. No growth differences were seen in 1- to 6-month-old infants receiving formula containing 4 versus 2 mg/L of iron (60). Infants randomized to iron-fortified formula containing either 7.4 or 12.7 mg/L also showed no difference in growth and no evidence of growth suppression (13). These sets of studies suggest that older age and a medicinal versus an infant formula–based iron source may be important factors to consider in iron supplementation of already iron-sufficient infants.
CONCLUSIONS
In the clearly iron-sufficient newborn, there is little if any benefit to beginning iron supplementation at birth. However, allowing iron stores to decrease to levels that threaten availability for developing tissues (without a source of dietary iron) is also potentially risky, particularly as the infant approaches and exceeds 6 months of age. A gradual introduction of iron into the diet between months 3 and 5 appears to maximize the benefit–risk ratio, as the risk of infection declines after 3 months of age as immune competence builds.
Close monitoring of iron status and iron homeostasis is key. Management of all populations would be easier if total-body iron status were estimated at birth via hemoglobin and ferritin concentrations (47) and at mid-year, depending on the risk factors for poor iron status. Waiting until 12 months and measuring only the hemoglobin concentration is simply not defensible policy with what is now known about risks to iron status and the negative effects of preanemic iron deficiency on the brain (83). In the future, the measurement of hepcidin will likely be used to guide iron therapy. Infants with low hepcidin levels likely require iron, while those with high levels are not likely to benefit from iron supplementation and may well be at risk for adverse effects.
NPBI: nonprotein-bound iron
WHO: World Health Organization
AAP: American Academy of Pediatrics
RCT: randomized controlled trial
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health to M.K.G. (R01-HD089989, HD29421-20, R01-NS099178, R01-HD094809), N.F.K. (2UG1HD076474-06), and S.E.C. (R01 HD092391-01, R03 HD074262) and from the Bill & Melinda Gates Foundation (OPP1055867).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Akman M, Cebeci D, Okur V, Angin H, Abali O, Akman AC. 2004. The effects of iron deficiency on infants’ developmental test performance. Acta Paediatr 93:1391–96 [PubMed] [Google Scholar]
- 2.Am. Acad. Pediatr. 1999. Iron fortification of infant formulas. Pediatrics 104:119–23 [PubMed] [Google Scholar]
- 3.Amin SB, Orlando M, Eddins A, MacDonald M, Monczynski C, Wang H. 2010. In utero iron status and auditory neural maturation in premature infants as evaluated by auditory brainstem response. J. Pediatr 156:377–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashorn P, Hallamaa L, Allen LH, Ashorn U, Chandrasiri U, et al. 2018. Co-causation of reduced newborn size by maternal undernutrition, infections, and inflammation. Matern. Child Nutr 14(3):e12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bah A, Pasricha SR, Jallow MW, Sise EA, Wegmuller R, et al. 2017. Serum hepcidin concentrations decline during pregnancy and may identify iron deficiency: analysis of a longitudinal pregnancy cohort in the Gambia. J. Nutr 147:1131–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker RD, Greer FR, Comm. Nutr. Am. Acad. Pediatr 2010. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age). 126:1040–50 [DOI] [PubMed] [Google Scholar]
- 7.Bastian TW, von Hohenberg WC, Mickelson DJ, Lanier LM, Georgieff MK. 2016. Iron deficiency impairs developing hippocampal neuron gene expression, energy metabolism and dendrite complexity. Dev. Neurosci 38:264–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beard JL. 2001. Iron biology in immune function, muscle metabolism and neuronal functioning. J. Nutr 131:568S–79S [DOI] [PubMed] [Google Scholar]
- 9.Beard JL, Erikson KM, Jones BC. 2003. Neonatal iron deficiency results in irreversible changes in dopamine function in rats. J. Nutr 133:1174–79 [DOI] [PubMed] [Google Scholar]
- 10.Berglund SK, Chmielewska A, Starnberg J, Westrup B, Hägglöf B, et al. 2018. Effects of iron supplementation of low-birth-weight infants on cognition and behavior at 7 years: a randomized controlled trial. Pediatr. Res 83:111–18 [DOI] [PubMed] [Google Scholar]
- 11.Berglund SK, Westrup B, Hägglöf B, Hernell O, Domellöf M. 2013. Effects of iron supplementation of LBW infants on cognition and behavior at 3 years. Pediatrics 131:47–55 [DOI] [PubMed] [Google Scholar]
- 12.Black MM, Baqui AH, Zaman K, Ake Persson L, El Arifeen S, et al. 2004. Iron and zinc supplementation promote motor development and exploratory behavior among Bangladeshi infants. Am. J. Clin. Nutr 80:903–10 [DOI] [PubMed] [Google Scholar]
- 13.Bradley CK, Hillman L, Sherman AR, Leedy D, Cordano A. 1993. Evaluation of two iron-fortified, milk-based formulas during infancy. Pediatrics 91:908–14 [PubMed] [Google Scholar]
- 14.Braekke K, Bechensteen AG, Halvorsen BL, Blomhoff R, Haaland K, Staff AC. 2007. Oxidative stress markers and antioxidant status after oral iron supplementation to very low birth weight infants. J. Pediatr 151:23–28 [DOI] [PubMed] [Google Scholar]
- 15.Brittenham GM, Andersson M, Egli I, Foman JT, Zeder C, et al. 2014. Circulating non-transferrin-bound iron after oral administration of supplemental and fortification doses of iron to healthy women: a randomized study. Am. J. Clin. Nutr 100:813–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunette KE, Tran PV, Wobken JD, Carlson ES, Georgieff MK. 2010. Gestational and neonatal iron deficiency alters apical dendrite structure of CA1 pyramidal neurons in adult rat hippocampus. Dev. Neurosci 32:238–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao C, O’Brien KO. 2013. Pregnancy and iron homeostasis: an update. Nutr. Rev 71:35–51 [DOI] [PubMed] [Google Scholar]
- 18.Chang SC, O’Brien KO, Nathanson MS, Mancini J, Witter FR. 2003. Hemoglobin concentrations influence birth outcomes in pregnant African-American adolescents. J. Nutr 133:2348–55 [DOI] [PubMed] [Google Scholar]
- 19.Chockalingam UM, Murphy E, Ophoven JC, Weisdorf SA, Georgieff MK. 1987. Cord transferrin and ferritin levels in newborn infants at risk for prenatal uteroplacental insufficiency and chronic hypoxia. J. Pediatr 111:283–86 [DOI] [PubMed] [Google Scholar]
- 20.Christian P, Morgan ME, Murray-Kolb L, LeClerq SE, Khatry SK, et al. 2011. Preschool iron–folic acid and zinc supplementation in children exposed to iron–folic acid in utero confers no added cognitive benefit in early school-age. J. Nutr 141:2042–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christian P, Murray-Kob LE, Khatry SK, Katz J, Schaefer BA, et al. 2010. Prenatal micronutrient supplementation and intellectual and motor function in early school-aged children in Nepal. JAMA 304:2716–23 [DOI] [PubMed] [Google Scholar]
- 22.Clark MA, Goheen MM, Fulford A, Prentice AM, Elnagheeb MA, et al. 2014. Host iron status and iron supplementation mediate susceptibility to erythrocytic stage Plasmodium falciparum. Nat. Commun 5:4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark KM, Li M, Zhu B, Liang F, Shao J, et al. 2017. Breastfeeding, mixed, or formula feeding at 9 months of age and the prevalence of iron deficiency and iron deficiency anemia in two cohorts in China. J. Pediatr 181:56–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Combs GF, Trumbo PR, McKinley MC, Milner J, Studenski S, et al. 2013. Biomarkers in nutrition: new frontiers in research and application. Ann. N. Y. Acad. Sci 1278:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connor JR, Menzies SL. 1996. Relationship of iron to oligodendrocytes and myelination. Glia 17:83–93 [DOI] [PubMed] [Google Scholar]
- 26.Cusick SE, Georgieff MK. 2016. The role of nutrition in brain development: the golden opportunity of the “first 1000 days.” J. Pediatr 175:16–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cusick SE, Opoka RO, Abrams SA, John CC, Georgieff MK, Mupere E. 2016. Delaying iron therapy until 28 days after antimalarial treatment is associated with greater iron incorporation and equivalent hematologic recovery after 56 days in children: a randomized controlled trial. J. Nutr 146:1769–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delpisheh A, Brabin L, Drummond S, Brabin BJ. 2008. Prenatal smoking exposure and asymmetric fetal growth restriction. Ann. Hum. Biol 35:573–83 [DOI] [PubMed] [Google Scholar]
- 29.Dewey KG, Domellöf M, Cohen RJ, Landa Rivera L, Hernell O, Lönnerdal B. 2002. Iron supplementation affects growth and morbidity of breast-fed infants: results of a randomized trial in Sweden and Honduras. J. Nutr 132:3249–55 [DOI] [PubMed] [Google Scholar]
- 30.Dewey KG, Oaks BM. 2017. U-shaped curve for risk associated with maternal hemoglobin, iron status, or iron supplementation. Am. J. Clin. Nutr 106(Suppl.):1694S–702S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Domellöf M, Cohen RJ, Dewey KG, Hernell O, Landa Rivera L, Lönnerdal B. 2001. Iron supplementation of breast-fed Honduran and Swedish infants from 4 to 9 months of age. J. Pediatr 138:679–87 [DOI] [PubMed] [Google Scholar]
- 32.Domellöf M, Georgieff MK. 2015. Postdischarge iron requirements of the preterm infant. J. Pediatr 167(Suppl.):S31–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Domellöf M, Hernell O, Abrams SA, Chen Z, Lönnerdal B. 2009. Iron supplementation does not affect copper and zinc absorption in breastfed infants. Am. J. Clin. Nutr 89:185–90 [DOI] [PubMed] [Google Scholar]
- 34.EFSA Panel Diet. Prod. Nutr. Allerg. 2015. Scientific opinion on dietary reference values for iron. EFSA J 13:4254 [Google Scholar]
- 35.Ehrenkranz RA, Gettner PA, Nelli CM, Sherwonit EA, Williams JE, et al. 1992. Iron absorption and incorporation into red blood cells by very low birth weight infants: studies with the stable isotope 58Fe. J. Pediatr. Gastroenterol. Nutr 15:270–78 [DOI] [PubMed] [Google Scholar]
- 36.Esamai F, Liechty E, Ikemeri J, Westcott J, Kemp J, et al. 2014. Zinc absorption from micronutrient powder is low but is not affected by iron in Kenyan infants. Nutrients 6:5636–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Etheredge AJ, Premji Z, Gunaratna NS, Abioye AI, Aboud S, et al. 2015. Iron supplementation in iron-replete and nonanemic pregnant women in Tanzania: a randomized clinical trial. JAMA Pediatr 169:947–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finkelstein JL, O’Brien KO, Abrams SA, Zavaleta N. 2013. Infant iron status affects iron absorption in Peruvian breastfed infants at 2 and 5 months of age. Am. J. Clin. Nutr 98:1475–84 [DOI] [PubMed] [Google Scholar]
- 39.Fisher A, Nemeth A. 2017. Iron homeostasis during pregnancy. Am. J. Clin. Nutr 106(Suppl.):1567S–74S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franz AR, Mihatsch WA, Sander S, Kron M, Pohlandt F. 2000. Prospective randomized trial of early versus late enteral iron supplementation in infants with a birth weight of less than 1301 grams. Pediatrics 106:700–6 [DOI] [PubMed] [Google Scholar]
- 41.Fretham SJB, Carlson ES, Wobken J, Tran PV, Petryk A, Georgieff MK. 2012. Temporal manipulation of transferrin-receptor-1-dependent iron uptake identifies a sensitive period in mouse hippocampal neurodevelopment. Hippocampus 22:1691–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friel JK, Aziz K, Andrews WL, Harding SV, Courage ML, Adams RJ. 2003. A double-masked, randomized control trial of iron supplementation in early infancy in healthy term breast-fed infants. J. Pediatr 143:582–86 [DOI] [PubMed] [Google Scholar]
- 43.Gahagan S, Yu S, Kaciroti N, Castillo M, Lozoff B. 2009. Linear and ponderal growth trajectories in well-nourished, iron-sufficient infants are unimpaired by iron supplementation. J. Nutr 139:2106–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ganz T, Nemeth E. 2012. Hepcidin and iron homeostasis. Biochim. Biophys. Acta 1823:1434–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geng F, Mai X, Zhan J, Xu L, Zhao Z, et al. 2015. Impact of fetal–neonatal iron deficiency on recognition memory at 2 months of age. J. Pediatr 167:1226–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Georgieff MK. 2017. Iron assessment to protect the developing brain. Am. J. Clin. Nutr 106(Suppl. 6):1588S–93S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Georgieff MK, Landon MB, Mills MM, Hedlund BE, Faassen AE, et al. 1990. Abnormal iron distribution in infants of diabetic mothers: spectrum and maternal antecedents. J. Pediatr 117:455–61 [DOI] [PubMed] [Google Scholar]
- 48.Georgieff MK, Schmidt RL, Mills MM, Radmer WJ, Widness JA. 1992. Fetal iron and cytochrome c status after intrauterine hypoxemia and erythropoietin administration. Am. J. Physiol 262:R485–91 [DOI] [PubMed] [Google Scholar]
- 49.Goheen MM, Bah A, Wegmüller R, Verhoef H, Darboe B, et al. 2017. Host iron status and erythropoietic response to iron supplementation determines susceptibility to the RBC stage of falciparum malaria during pregnancy. Sci. Rep 7:17674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldenberg RL, Tamura T, DuBard M, Johnston KE, Copper RL, Neggers Y. 1996. Plasma ferritin and pregnancy outcome. Am. J. Obstet. Gynecol 175:1356–59 [DOI] [PubMed] [Google Scholar]
- 51.Golub MS, Hogrefe CE, Malka R, Higgins JM. 2014. Developmental plasticity of red blood cell homeostasis. Am. J. Hematol 89:459–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonzales GF, Steenland K, Tapia V. 2009. Maternal hemoglobin level and fetal outcome at low and high altitudes. Am. J. Physiol 297:R1477–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Griffin I, Cooke RJ. 2010. Iron retention in preterm infants fed low iron intakes: a metabolic balance study. Early Hum. Dev 86(Suppl. 1):49–53 [DOI] [PubMed] [Google Scholar]
- 54.Gupta PM, Hamner HC, Suchdev PS, Flores-Ayala R, Mei Z. 2017. Iron status of toddlers, non-pregnant females and pregnant females in the United States. Am. J. Clin. Nutr 106(Suppl. 6):1640S–46S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta PM, Perrine CG, Mei Z, Scanlon KS. 2016. Iron, anemia and iron deficiency anemia among young children in the United States. Nutrients 8:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hämäläinen H, Hakkarainen K, Heinonen S. 2003. Anaemia in the first but not in the second or third trimester is a risk factor for low birth weight. Clin. Nutr 22:271–75 [DOI] [PubMed] [Google Scholar]
- 57.Hasanbegović E, Sabanović S. 2004. [Effects of iron therapy on motor and mental development of infants and small children suffering from iron deficiency anaemia]. Med. Arh 58:227–29 (In Bosnian) [PubMed] [Google Scholar]
- 58.Haschke F, Ziegler EE, Edwards BB, Fomon SJ. 1986. Effect of iron fortification of infant formula on trace mineral absorption. J. Pediatr. Gastroenterol. Nutr 5:768–73 [DOI] [PubMed] [Google Scholar]
- 59.Hernell O, Fewtrell MS, Georgieff MK, Krebs NF, Lönnerdal B. 2015. Summary of current recommendations on iron provision and monitoring of iron status for breastfed and formula-fed infants in resource-rich and resource-constrained countries. J. Pediatr 167(Suppl.):S40–47 [DOI] [PubMed] [Google Scholar]
- 60.Hernell O, Lönnerdal B. 2002. Iron status of infants fed low-iron formula: no effect of added bovine lactoferrin or nucleotides. Am. J. Clin. Nutr 76:858–64 [DOI] [PubMed] [Google Scholar]
- 61.Hwang HS, Kim YH, Kwon JY, Park YW. 2010. Uterine and umbilical artery Doppler velocimetry as a predictor for adverse pregnancy outcomes in pregnant women with anemia. J. Perinat. Med 38:467–71 [DOI] [PubMed] [Google Scholar]
- 62.Idjradinata P, Pollitt E. 1993. Reversal of developmental delays in iron-deficient anaemic infants treated with iron. Lancet 341:1–4 [DOI] [PubMed] [Google Scholar]
- 63.Idjradinata P, Watkins WE, Pollitt E. 1994. Adverse effect of iron supplementation on weight gain of iron-replete young children. Lancet 343:1252–54 [DOI] [PubMed] [Google Scholar]
- 64.Insel BJ, Schaefer CA, McKeague IW, Susser ES, Brown AS. 2008. Maternal iron deficiency and the risk of schizophrenia in offspring. Arch. Gen. Psychiatry 65:1136–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jaramillo EG, Mupere E, Opoka RO, Hodges JS, Lund TC, et al. 2017. Delaying the start of iron until 28 days after antimalarial treatment is associated with lower incidence of subsequent illness in children with malaria and iron deficiency. PLOS ONE 12:e0183977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kabyemela ER, Fried M, Kurtis JD, Mutabingwa TK, Duffy PE. 2008. Decreased susceptibility to Plasmodium falciparum infection in pregnant women with iron deficiency. J. Infect. Dis 198:163–66 [DOI] [PubMed] [Google Scholar]
- 67.Kennedy BC, Dimova JG, Siddappa AJM, Tran PV, Gewirtz JC, Georgieff MK. 2014. Prenatal choline supplementation ameliorates the long-term neurobehavioral effects of fetal–neonatal iron deficiency in rats. J. Nutr 144:1858–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kleinman RE. 2015. Expert recommendations on iron fortification in infants. J. Pediatr 167(Suppl.):S48–49 [DOI] [PubMed] [Google Scholar]
- 69.Kortman GA, Raffatellu M, Swinkels DW, Tjalsma H. 2014. Nutritional iron turned inside out: intestinal stress from a gut microbial perspective. FEMS Microbiol. Rev 38:1202–1234 [DOI] [PubMed] [Google Scholar]
- 70.Krebs NF, Domellöf M, Ziegler E. 2015. Balancing benefits and risks of iron supplementation in resource-rich countries. J. Pediatr 167(Suppl.):S20–25 [DOI] [PubMed] [Google Scholar]
- 71.Krebs NF, Westcott J, Butler N, Robinson C, Bell M, Hambidge KM. 2006. Meat as a first complementary food for breastfed infants: feasibility and impact on zinc intake and status. J. Pediatr. Gastroenterol. Nutr 42:207–14 [DOI] [PubMed] [Google Scholar]
- 72.Kuzawa CW. 1998. Adipose tissue in human infancy and childhood: an evolutionary perspective. Am. J. Phys. Anthropol 107(Suppl. 27):177–209 [DOI] [PubMed] [Google Scholar]
- 73.Lao TT, Tam KF, Chan LY. 2000. Third trimester iron status and pregnancy outcome in non-anaemic women: pregnancy unfavourably affected by maternal iron excess. Hum. Reprod 15:1843–48 [DOI] [PubMed] [Google Scholar]
- 74.Lind T, Lönnerdal B, Stenlund H, Gamayanti IL, Ismail D, et al. 2004. A community-based randomized controlled trial of iron and zinc supplementation in Indonesian infants: effects on growth and development. Am. J. Clin. Nutr 80:729–36 [DOI] [PubMed] [Google Scholar]
- 75.Lind T, Lönnerdal B, Stenlund H, Ismail D, Seswandhana R, et al. 2003. A community-based randomized controlled trial of iron and zinc supplementation in Indonesian infants: interactions between iron and zinc. Am. J. Clin. Nutr 77:883–90 [DOI] [PubMed] [Google Scholar]
- 76.Lind T, Seswandhana R, Persson LA, Lönnerdal B. 2008. Iron supplementation of iron-replete Indonesian infants is associated with reduced weight-for-age. Acta Paediatr 97:770–75 [DOI] [PubMed] [Google Scholar]
- 77.Lönnerdal B. 2017. Excess iron intake as a factor in growth, infections, and development of infants and young children. Am. J. Clin. Nutr 106(Suppl. 6):1681S–87S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lönnerdal B, Hernell O. 1994. Iron, zinc, copper and selenium status of breast-fed infants and infants fed trace element fortified milk-based infant formula. Acta Paediatr 83:367–73 [DOI] [PubMed] [Google Scholar]
- 79.Lorenz L, Peter A, Poets CF, Franz AR. 2013. A review of cord blood concentrations of iron status parameters to define reference ranges for preterm infants. Neonatology 104:194–202 [DOI] [PubMed] [Google Scholar]
- 80.Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. 2006. Long-lasting neural and behavioral effects of early iron deficiency in infancy. Nutr. Rev 64(Suppl. 2):S34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lozoff B, Brittenham GM, Wolf AW, McClish DK, Kuhnert PM, et al. 1987. Iron deficiency anemia and iron therapy effects on infant developmental test performance. Pediatrics 79:981–95 [PubMed] [Google Scholar]
- 82.Lozoff B, Castillo M, Clark KM, Smith JB. 2012. Iron-fortified versus low-iron infant formula: developmental outcome at 10 years. Arch. Pediatr. Adolesc. Med 166:208–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lozoff B, Clark KM, Jing Y, Armony-Sivan R, Angelilli ML, Jacobson SW. 2008. Dose–response relationships between iron deficiency with or without anemia and infant social–emotional behavior. J. Pediatr 152:696–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, Pino P. 2003. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics 112:846–54 [PubMed] [Google Scholar]
- 85.Lozoff B, Wolf A, Jimenez E. 1996. Iron-deficiency anemia and infant development: effects of extended oral iron therapy. J. Pediatr 129:382–89 [DOI] [PubMed] [Google Scholar]
- 86.Lukowski AF, Koss M, Burden MJ, Jonides J, Nelson CA, et al. 2010. Iron deficiency in infancy and neurocognitive functioning at 19 years: evidence of long-term deficits in executive function and recognition memory. Nutr. Neurosci 13:54–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lynch S, Pfeiffer CM, Georgieff MK, Brittenham G, Fairweather-Tait S, et al. 2018. Biomarkers of Nutrition for Development (BOND)—iron review. J. Nutr 48(Suppl. 1):1001S–67S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Majumdar I, Paul P, Talib VH, Ranga S. 2003. The effect of iron therapy on the growth of iron-replete and iron-deplete children. J. Trop. Pediatr 49:84–88 [DOI] [PubMed] [Google Scholar]
- 89.McDonald MC, Abrams SA, Schanler RJ. 1998. Iron absorption and red blood cell incorporation in premature infants fed an iron-fortified infant formula. Pediatr. Res 44:507–11 [DOI] [PubMed] [Google Scholar]
- 90.Mevissen-Verhage EAE, Marcelis JH, Harmsen-Van Amerongen WCM, de Vos NM, Verhoef J. 1985. Effect of iron on neonatal gut flora during the first three months of life. Eur. J. Clin. Microbiol 4:273–78 [DOI] [PubMed] [Google Scholar]
- 91.Mohamed MA, Ahmad T, Macri C, Aly H. 2012. Racial disparities in maternal hemoglobin concentrations and pregnancy outcomes. J. Perinat. Med 40:141–49 [DOI] [PubMed] [Google Scholar]
- 92.Monk C, Georgieff MK, Xu D, Hao X, Bansal R, et al. 2015. Maternal prenatal iron status and tissue organization in the neonatal brain. Pediatr. Res 79:482–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murray-Kolb LE, Khatry SK, Katz J, Schaefer BA, Cole PM, LeClerq SC. 2012. Preschool micronutrient supplementation effects on intellectual and motor function in school-aged Nepalese children. Arch. Pediatr. Adolesc. Med 166:404–10 [DOI] [PubMed] [Google Scholar]
- 94.Mwangi MN, Roth JM, Smit MR, Trijsburg L, Mwangi AM, et al. 2015. Effect of daily antenatal iron supplementation on Plasmodium infection in Kenyan women: a randomized clinical trial. JAMA 314:1009–20 [DOI] [PubMed] [Google Scholar]
- 95.Neuberger A, Okebe J, Yahav D, Paul M. 2016. Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst. Rev 2:CD006589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ojukwu JU, Okebe JU, Yahav D, Paul M. 2009. Oral iron supplementation for preventing or treating anaemia among children in malaria-endemic areas. Cochrane Database Syst. Rev 3:CD006589. [DOI] [PubMed] [Google Scholar]
- 97.Okebe JU, Yahav D, Shbita R, Paul M. 2011. Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst. Rev 10:CD006589. [DOI] [PubMed] [Google Scholar]
- 98.Paganini D, Uyoga MA, Cercamondi CI, Moretti D, Mwasi E, et al. 2017. Consumption of galacto-oligosaccharides increases iron absorption from a micronutrient powder containing ferrous fumarate and sodium iron EDTA: a stable-isotope study in Kenyan infants. Am. J. Clin. Nutr 106:1020–31 [DOI] [PubMed] [Google Scholar]
- 99.Paganini D, Zimmermann MB. 2017. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: a review. Am. J. Clin. Nutr 106(Suppl.):1688S–93S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pasricha SR, Hayes E, Kaumba K, Biggs BA. 2013. Effect of daily iron supplementation on health in children aged 4–23 months: a systematic review and meta-analysis of randomized controlled trials. Lancet Glob. Health 1:e77–86 [DOI] [PubMed] [Google Scholar]
- 101.Pena-Rosas JP, De-Regil LM, Dowswell T, Viteri FE. 2012. Daily oral iron supplementation during pregnancy. Cochrane Database Syst. Rev 12:CD004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pena-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T. 2015. Daily oral iron supplementation during pregnancy. Cochrane Database Syst. Rev CD004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Petry CD, Eaton MA, Wobken JD, Mills MM, Johnson DE, Georgieff MK. 1992. Iron deficiency of liver, heart, and brain in newborn infants of diabetic mothers. J. Pediatr 121:109–14 [DOI] [PubMed] [Google Scholar]
- 104.Petry N, Olofin I, Hurrell RF, Boy E, Wirth JP, et al. 2016. The proportion of anemia associated with iron deficiency in low, medium, and high human development index countries: a systematic analysis of national surveys. Nutrients 8:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pisansky MT, Wickham RJ, Su J, Fretham S, Yuan L-L, et al. 2013. Iron deficiency with or without anemia impairs prepulse inhibition of the startle reflex. Hippocampus 23:952–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rao R, Ennis K, Lubach G, Lock E, Georgieff MK, Coe C. 2016. Metabolomic analysis of CSF indicates brain metabolic impairment precedes hematological indices of anemia in the iron-deficient infant monkey. Nutr. Neurosci 21:40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ren A, Wang J, Ye RW, Li S, Liu JM, Li Z. 2007. Low first-trimester hemoglobin and low birth weight, preterm birth and small for gestational age newborns. Int. J. Gynaecol. Obstet 98:124–28 [DOI] [PubMed] [Google Scholar]
- 108.Rivera JA, Shamah T, Villalpando S, Monterrubio E. 2010. Effectiveness of a large-scale iron-fortified milk distribution program on anemia and iron deficiency in low-income young children in Mexico. Am. J. Clin. Nutr 91:431–39 [DOI] [PubMed] [Google Scholar]
- 109.Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B. 1998. Interaction of iron deficiency anemia and neurofunctions in cognitive development. Am. J. Clin. Nutr 68:683–90 [DOI] [PubMed] [Google Scholar]
- 110.Santos DCC, Angulo-Barroso RM, Li M, Bian Y, Sturza J, et al. 2018. Timing, duration and severity of iron deficiency in early development and motor outcomes at 9 months. Eur. J. Clin. Nutr 72:332–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ. 2006. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet 367:133–43 [DOI] [PubMed] [Google Scholar]
- 112.Sazawal S, Dhingra U, Dhingra P, Hiremath G, Sarkar A, Dutta A. 2010. Micronutrient fortified milk improves iron status, anemia and growth among children 1–4 years: a double masked, randomized, controlled trial. PLOS ONE 5:e12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scanlon KS, Yip R, Schieve LA, Cogswell ME. 2000. High and low hemoglobin levels during pregnancy: differential risks for preterm birth and small for gestational age. Obstet. Gynecol 96:741–48 [DOI] [PubMed] [Google Scholar]
- 114.Schmidt AT, Waldow KJ, Grove WM, Salinas JA, Georgieff MK. 2007. Dissociating the long-term effects of fetal/neonatal iron deficiency on three types of learning in the rat. Behav. Neurosci 121:475–82 [DOI] [PubMed] [Google Scholar]
- 115.Schmidt RJ, Tancredi DJ, Krakowiak P, Hansen RL, Ozonoff S. 2014. Maternal intake of supplemental iron and risk of autism spectrum disorder. Am. J. Epidemiol 180:890–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scholl TO. 1998. High third-trimester ferritin concentration: associations with very preterm delivery, infection, and maternal nutritional status. Obstet. Gynecol 92:161–66 [DOI] [PubMed] [Google Scholar]
- 117.Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJ, et al. 2016. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 65:575–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Senga EL, Harper G, Koshy G, Kazembe PN, Brabin BJ. 2011. Reduced risk for placental malaria in iron deficient women. Malar. J 10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shao J, Lou J, Rao R, Georgieff MK, Kaciroti N, et al. 2012. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J. Nutr 142:2004–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Siddappa AM, Georgieff MK, Wewerka S, Worwa C, Nelson CA, deRegnier R-A. 2004. Auditory recognition memory in iron-deficient infants of diabetic mothers. Pediatr. Res 55:1034–41 [DOI] [PubMed] [Google Scholar]
- 121.Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK. 2007. The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatology 92:73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Simonyté Sjodin K, Domellöf M, Lagerqvist C, Hernell O, Lönnerdal B, et al. 2018. Administration of ferrous sulfate drops has significant effects on the gut microbiota of iron-sufficient infants: a randomized controlled study. Gut. In press 10.1136/gutjnl-2018-316988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Soofi S, Cousens S, Iqbal SP, Akhund T, Khan J, et al. 2013. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomised trial. Lancet 382:29–40 [DOI] [PubMed] [Google Scholar]
- 124.Steinmacher J, Pohlandt F, Bode H, Sander S, Kron M, Franz AR. 2007. Randomized trial of early versus late enteral iron supplementation in infants with a birth weight of less than 1301 grams: neurocognitive development at 5.3 years’ corrected age. Pediatrics 120:538–46 [DOI] [PubMed] [Google Scholar]
- 125.Stekel A, Olivares M, Pizarro F, Chadud P, Cayazzo M, et al. 1986. [Prevention of iron deficiency in infants by fortified milk: field study of a low-fat milk]. Arch. Latinoam. Nutr 36:654–61 (In Spanish) [PubMed] [Google Scholar]
- 126.Stoltzfus RJ, Dreyfuss ML, eds. 1998. Guidelines for the Use of Iron Supplements to Prevent and Treat Iron Deficiency Anemia. Washington, DC: ILSI Press [Google Scholar]
- 127.Stoltzfus RJ, Kvalsvig JD, Chwaya HM, Montresor A, Albonico M, et al. 2001. Effects of iron supplementation and anthelmintic treatment on motor and language development of preschool children in Zanzibar: double blind, placebo controlled study. BMJ 323:1389–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Suchdev PS, Williams AM, Mei Z, Flores-Ayala R, Pasricha S-R, et al. 2017. Assessment of iron status in settings of inflammation: challenges and potential approaches. Am. J. Clin. Nutr 106(Suppl. 6):1626S–33S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sweet DG, Savage G, Tubman TR, Lappin TR, Halliday HL. 2001. Study of maternal influences on fetal iron status at term using cord blood transferrin receptors. Arch. Dis. Child 84:F40–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, et al. 2002. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J. Pediatr 140:165–70 [DOI] [PubMed] [Google Scholar]
- 131.Tamura T, Goldenberg RL, Johnston KE, Cliver SP, Hickey CA. 1996. Serum ferritin: a predictor of early spontaneous preterm delivery. Obstet. Gynecol 87:360–65 [DOI] [PubMed] [Google Scholar]
- 132.Tang M, Frank DN, Hendricks E, Ir D, Esamai F, et al. 2017. Iron in micronutrient powder promotes an unfavorable gut microbiota in Kenyan infants. Nutrients 9:776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tang M, Frank DN, Sherlock L, Ir D, Robertson CE, Krebs NF. 2016. Effect of vitamin E with therapeutic iron supplementation on iron repletion and gut microbiome in US iron deficient infants and toddlers. J. Pediatr. Gastroenterol. Nutr 63:379–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Taylor CL, Brannon PM. 2017. Introduction to workshop on iron screening and supplementation in iron-replete pregnant women and young children. Am. J. Clin. Nutr 106(Suppl. 6):1547S–54S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tielsch JM, Khatry SK, Stoltzfus RJ, Katz J, LeClerq SC, et al. 2007. Effect of daily zinc supplementation on child mortality in southern Nepal: a community-based, cluster randomised, placebo-controlled trial. Lancet 370:1230–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Toldi G, Stenczer B, Molvarec A, Takats Z, Beko G, et al. 2010. Hepcidin concentrations and iron homeostasis in preeclampsia. Clin. Chem. Lab. Med 48:1423–26 [DOI] [PubMed] [Google Scholar]
- 137.Tran PV, Kennedy BC, Lien YC, Simmons RA, Georgieff MK. 2015. Fetal iron deficiency induces chromatin remodeling at the Bdnf locus in adult rat hippocampus. Am. J. Physiol 308:R276–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tran PV, Kennedy BC, Pisansky MT, Won KJ, Gewirtz JC, et al. 2016. Prenatal choline supplementation diminishes early-life iron deficiency–induced preprogramming of networks associated with behavioral abnormalities in the adult rat hippocampus. J. Nutr 146:484–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Unger EL, Hurst AR, Georgieff MK, Schallert T, Rao R, et al. 2012. Behavior and monoamine deficits in prenatal and perinatal iron deficiency are not corrected by early postnatal moderate-iron or high-iron diets in rats. J. Nutr 142:2040–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.US Prevent. Serv. Task Force. 2015. Iron deficiency anemia in pregnant women: screening and supplementation. US Prevent. Serv. Task Force https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/iron-deficiency-anemia-in-pregnant-women-screening-and-supplementation [Google Scholar]
- 141.van der Merwe LF, Eussun SR. 2017. Iron status of young children in Europe. Am. J. Clin. Nutr 106(Suppl. 6.):1663S–71S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.van Santen S, Kroot JJ, Zijderveld G, Wiegerinck ET, Spaanderman ME, Swinkels DW. 2013. The iron regulatory hormone hepcidin is decreased in pregnancy: a prospective longitudinal study. Clin. Chem. Lab. Med 51:1395–401 [DOI] [PubMed] [Google Scholar]
- 143.Veenemans J, Schouten LR, Ottenhof MJ, Mank TG, Uges DR, et al. 2012. Effect of preventive supplementation with zinc and other micronutrients on non-malarial morbidity in Tanzanian pre-school children: a randomized trial. PLOS ONE 7:e41630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Verhoeff FH, Brabin BJ, van Buuren S, Chimsuku L, Kazembe P, et al. 2001. An analysis of intra-uterine growth retardation in rural Malawi. Eur. J. Clin. Nutr 55:682–89 [DOI] [PubMed] [Google Scholar]
- 145.Villalpando S, Shamah T, Rivera JA, Lara Y, Monterrubio E. 2006. Fortifying milk with ferrous gluconate and zinc oxide in a public nutrition program reduced the prevalence of anemia in toddlers. J. Nutr 136:2633–37 [DOI] [PubMed] [Google Scholar]
- 146.Vricella LK. 2017. Emerging understanding and measurement of plasma volume expansion in pregnancy. Am. J. Clin. Nutr 106(Suppl. 6):1620S–25S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Walker S, Wachs T, Gardner J, Lozoff B, Wasserman G, et al. 2007. Child development: risk factors for adverse outcomes in developing countries. Lancet 369:145–57 [DOI] [PubMed] [Google Scholar]
- 148.Walker S, Wachs T, Grantham-McGregor S, Black M, Nelson C, et al. 2011. Inequality in early childhood: risk and protective factors for early child development. Lancet 378:1325–38 [DOI] [PubMed] [Google Scholar]
- 149.Wallace DF. 2016. The regulation of iron absorption and homeostasis. Clin. Biochem. Rev 37:51–62 [PMC free article] [PubMed] [Google Scholar]
- 150.Wallin DJ, Tkac I, Stucker S, Ennis KM, Sola-Visner M, et al. 2015. Phlebotomy-induced anemia alters hippocampal neurochemistry in neonatal mice. Pediatr. Res 77:765–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Walter T, De Andraca I, Chadud P, Perales CG. 1989. Iron deficiency anemia: adverse effects on infant psychomotor development. Pediatrics 84:7–17 [PubMed] [Google Scholar]
- 152.Walter T, Pino P, Pizarro F, Lozoff B. 1998. Prevention of iron-deficiency anemia: comparison of high- and low-iron formulas in term healthy infants after six months of life. J. Pediatr 132:635–40 [DOI] [PubMed] [Google Scholar]
- 153.WHO (World Health Organ.). 2007. Conclusions and recommendations of the WHO consultation on prevention and control of iron deficiency in infants and young children in malaria-endemic areas. Food Nutr. Bull 28(Suppl. 4):S621–27 [DOI] [PubMed] [Google Scholar]
- 154.WHO (World Health Organ.). 2014. World Malaria Report 2014. Geneva: WHO [Google Scholar]
- 155.WHO (World Health Organ.). 2016. Guideline: Daily Iron Supplementation in Infants and Children. Geneva: WHO; [PubMed] [Google Scholar]
- 156.WHO (World Health Organ.). 2016. WHO guideline development group—the use of ferritin concentrations to assess iron status in populations. WHO. http://www.who.int/nutrition/events/2016_meeting_guidelinedevelopmentgroup_ferritin_2to4march/en/ [PubMed] [Google Scholar]
- 157.WHO (World Health Organ.). 2019. Iron with or without folic acid supplementation in women in malaria-endemic areas: full set of recommendations. WHO. http://www.who.int/elena/titles/full_recommendations/ifa_supplementation_malaria/en/ [Google Scholar]
- 158.WHO (World Health Organ.), UNICEF. 2006. Iron Supplementation of Young Children in Regions Where Malaria Transmission Is Intense and Infectious Disease Highly Prevalent. Geneva: WHO, UNICEF [Google Scholar]
- 159.Xiong X, Buekens P, Alexander S, Demianczuk N, Wollast E. 2000. Anemia during pregnancy and birth outcome: a meta-analysis. Am. J. Perinatol 17:137–46 [DOI] [PubMed] [Google Scholar]
- 160.Youdim MB, Sills MA, Heydorn WE, Creed GJ, Jacobowitz DM. 1986. Iron deficiency alters discrete proteins in rat caudate nucleus and nucleus accumbens. J. Neurochem 47:794–99 [DOI] [PubMed] [Google Scholar]
- 161.Zamora TG, Guiang SF, Georgieff MK, Widness JA. 2016. Iron is prioritized to red blood cells over the brain in phlebotomized anemic newborn lambs. Pediatr. Res 79:922–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang C, Rawal S. 2017. Dietary iron intake, iron status and gestational diabetes. Am. J. Clin. Nutr 106(Suppl. 6.):1672S–80S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang Q, Ananth CV, Li Z, Smulian JC. 2009. Maternal anaemia and preterm birth: a prospective cohort study. Int. J. Epidemiol 38:1380–89 [DOI] [PubMed] [Google Scholar]
- 164.Zhao G, Guobin X, Zhou M, Jiang Y, Richards B, et al. 2015. Prenatal iron supplementation reduces maternal anemia, iron deficiency, iron deficiency anemia in a randomized clinical trial in rural China, but iron deficiency remains widespread in mothers and neonates. J. Nutr 145:1916–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhou LM, Yang WW, Hua JZ, Deng CQ, Tao X, Stoltzfus RJ. 1998. Relation of hemoglobin measured at different times in pregnancy to preterm birth and low birth weight in Shanghai, China. Am. J. Epidemiol 148:998–1006 [DOI] [PubMed] [Google Scholar]
- 166.Ziaei S, Norrozi M, Faghihzadeh S, Jafarbegloo E. 2007. A randomized placebo-controlled trial to determine the effect of iron supplementation on pregnancy outcome in pregnant women with haemoglobin ≥13.2 g/dl. BJOG 114:684–88. Corrigendum. 2007. BJOG. 114:1311 [DOI] [PubMed] [Google Scholar]
- 167.Ziegler EE, Nelson SE, Jeter JM. 2009. Iron supplementation of breastfed infants from an early age. Am. J. Clin. Nutr 89:525–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ziegler EE, Nelson SE, Jeter JM. 2009. Iron status of breastfed infants is improved equally by medicinal iron and iron-fortified cereal. Am. J. Clin. Nutr 90:76–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ziegler EE, O’Donnell AM, Nelson SE, Fomon SJ. 1976. Body composition of the reference fetus. Growth 40:329–41 [PubMed] [Google Scholar]
- 170.Zlotkin SH, Davidsson L, Lozoff B. 2015. Balancing the benefits and risks of iron fortification in resource-constrained settings. J. Pediatr 167(Suppl.):S26–30 [DOI] [PubMed] [Google Scholar]


