Abstract
We recently reported that anti-CD13 mAbs induce homotypic aggregation of monocytic cells. This phenomenon is signal transduction dependent and does not require CD13 aminopeptidase activity. Since CD13 is heavily glycosylated and a member of the galectin family (galectin-4) has been shown to associate with CD13 in the intestinal epithelium, we hypothesized that CD13-mediated aggregation might proceed through a carbohydrate-dependent mechanism involving galectin-3, the most highly expressed galectin on monocytes. We report here that lactose and anti-galectin-3 antibodies completely abrogate homotypic aggregation induced by anti-CD13 antibodies. Furthermore, galectin-3 co-immunoprecipitates with CD13 from resting U-937 cells and this association decreases during the aggregation process, a phenomenon that may have functional implications. Together, the results presented here point to a key role for galectin-3 in CD13-mediated homotypic aggregation of monocytic cells.
Keywords: Galectin-3, CD13, Monocytes, Homotypic aggregation, Cell adhesion
Aminopeptidase N (CD13) is a metalloproteinase expressed in many tissues [1]. Numerous reports have demonstrated its key participation in cell migration/invasion and metastasis [2], [3], functions that may be related not only to its well known aminopeptidase activity but also to its less characterized role in cellular adhesion [4], [5]. Recently, we reported that anti-CD13 mAbs induce homotypic aggregation (HA) of the human monocytic cell line U-937. HA is involved in many cellular activation and maturation processes and in tumor cell invasion and metastasis [6], [7], [8]. Most of the leukocyte molecules that induce HA are integrins or depend on integrins to induce HA. In contrast, initiation of CD13-mediated HA is apparently independent of integrins [9]. This observation and the fact that the phenomenon occurs independently of CD13 enzymatic activity, led us to hypothesize a carbohydrate-dependent pro-adhesive mechanism for CD13-mediated HA.
CD13 is heavily glycosylated (∼40% of its mass) [10]. Its extracellular domain comprises a globular head (containing the enzymatic active site) connected to the cell membrane through a proline-rich stalk. The protein has “mucin-type”O-linked glycosylation sites located mainly in the stalk and at least ten predicted N-linked glycosylation sites [11], [12].
CD13 is a receptor for coronaviruses and for the bacterial CryAc toxin in insects. In both cases, the receptor function is highly dependent on its carbohydrate moieties. [13], [14].
Galectins are a family of lectins that recognize β-galactosides. Galectin-4 binds to CD13 in the intestinal brush border [15]. In monocytes, the most highly expressed galectin is galectin-3 [16], [17]. Galectin-3 has been extensively implicated in homotypic and heterotypic cell adhesion [18], [19], [20] and, as we have observed for CD13-mediated HA, galectin-3-mediated adhesion is independent of metal ions. Thus, we hypothesized that galectin-3 could have a role in CD13-mediated HA in monocytes.
Galectin-3 is expressed on the membrane associated with glycoproteins through its carbohydrate recognition domain (CRD). Among all members of the galectin family, galectin-3 is structurally unique as it has one CRD (highly conserved among all galectins) connected to a long N-terminal domain containing glycine, proline and tyrosine repeats [21]. Both the CRD and the N-terminal domain seem to participate in oligosaccharide binding and in multimerization of galectin-3 [22], [23], [24]. Based on its multivalency and its capacity to bind to several different membrane glycoproteins, galectin-3-mediated cell adhesion may involve self-association, mechanical bridging via soluble or immobilized glycoconjugates, activation of integrins, or a combination of all.
In this work we show that CD13-mediated HA involves a carbohydrate-dependent pro-adhesive mechanism and demonstrate that galectin-3 is essential for the phenomenon. Our results show for the first time a physical and functional association of CD13 with galectin-3.
Materials and methods
Cells and antibodies. The cell line U-937 (ATCC, Rockville, MD) was cultured as previously described [9]. Anti-human CD13 mAb (clone 452) was purified from culture supernatants of the hybridoma kindly provided by Dr. Meenhard Herlyn, The Wistar Institute of Anatomy and Biology, Philadelphia, PA. F(ab)’2 fragments of this antibody were prepared with immobilized Ficin (Pierce, Rockford, IL). Polyclonal antibodies anti-galectin-3 (H-160) and anti-galectin-1 (S-14) were from Santa Cruz Biotechnology, Santa Cruz, CA.
Homotypic aggregation assays. U-937 cells in 96-well culture plates (105/well) in RPMI-1640 were incubated with or without the indicated sugar or antibody. HA was induced with F(ab)’2 fragments of the anti-CD13 mAb (0.3 μg/ml) at 37 °C for the indicated times. Each experimental condition was assayed in triplicates. Images were captured with a camera attached to a Zeiss Axiovert microscope. Aggregation indexes (A.I.) were determined as previously described [9]. For experiments with fluorescent cells, they were labeled for 30 min with calcein-AM (Invitrogen) in PBS. Calcein-labeling did not change the adhesive properties of the cells.
Immunoprecipitation and immunoblot. Aggregated and non-aggregated U-937 cells (2 × 107) were lysed in ice-cold lysis buffer (150 mM NaCl, 10 mM Tris–HCl, 5% Glycerol, 1% Brij-97, pH 7.5) with 1 mM PMSF, 10 mM NaF, and 1 μg/ml each of Aprotinin, Leupeptin, Pepstatin A and Na3VO4. Lysates were centrifuged at 14,000 rpm for 15 min. Supernatants were incubated overnight at 4 °C with the anti-CD13 mAb or anti-galectin-3 antibodies (2 μg/ml). Immunocomplexes were precipitated with Protein G–agarose beads. Immunoprecipitated proteins were separated on 10% SDS–PAGE and transferred onto nitrocellulose membranes (Bio-Rad). CD13 or galectin-3 were detected with the corresponding antibody (2 μg/ml or 1:200 dilution respectively) followed by a secondary HRP-conjugated antibody. Chemiluminiscent signals were detected using Super Signal ECL Kit (Pierce). After stripping with 0.1 M glycine (pH 2.5) and blocking, the same membranes were blotted with the anti-CD13 or anti-galectin-3 antibody and HRP-conjugated secondary antibody.
Flow cytometry. U-937 cells were incubated with an isotype-matched IgG, or with anti-galectin-3 or anti-CD13 antibodies (8 and 5 μg/ml, respectively). Fluorescence of a secondary species-specific FITC-labeled antibody was quantified in a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA).
Results and discussion
Lactose but not sucrose inhibits CD13-mediated HA
Lectin-dependent functions of galectins are inhibited by their specific saccharide ligand, lactose. Therefore, we tested the effect of lactose on CD13-mediated homotypic aggregation. Lactose inhibited HA by 81.04% at a concentration of 0.2 M, whereas sucrose had no significant effect at the same concentration (Fig. 1 a and b). The inhibition of CD13-mediated HA by lactose was dose-dependent (Fig. 1c).
Fig. 1.
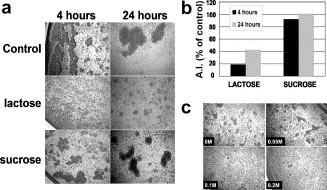
Lactose but not sucrose, inhibits CD13-mediated homotypic aggregation. (a) U-937 cells (5 × 104) in 100 μl of medium without (control) or with lactose or sucrose (0.2 M) were incubated at 37 °C for 30 min before addition of 100 μl of medium containing the anti-CD13 mAb (0.3 μg/ml). Images were taken after 4 or 24 h. (b) Aggregation indexes (A.I.s = number of aggregated cells × 100/total number of cells detected) were calculated from images such as those shown in (a). The effect of inhibitors is expressed as A.I. (percentage of control) = A.I. induced by the anti-CD13 mAb in the presence of the inhibitor × 100/A.I. obtained with the anti-CD13 mAb alone (control). (c) Cells were incubated with the indicated concentrations of lactose for 30 min at 37 °C and subsequently for 2 h after addition of the anti-CD13 mAb.
Pre-incubation of U-937 cells with anti-galectin-3 antibodies inhibits HA
As expected, resting U-937 cells express high membrane levels of galectin-3 (Fig. 2 A). Incubation of these cells with anti-galectin-3 antibodies raised against the N-terminus of the molecule before the addition of the HA-inducing anti-CD13 mAb resulted in an almost complete inhibition of HA at two hours of incubation (98.37% inhibition), whereas pre-incubation with an antibody against galectin-1, another galectin expressed in monocytes [25], did not inhibit CD13-induced HA at any time of incubation (Fig. 2B). The inhibitory effect of anti-galectin-3 antibodies was still evident after 24 h of incubation although the percentage of inhibition decreased progressively (Fig. 2B and D). This suggests that although galectin-3 is essential at the initial phases of CD13-mediated HA, a galectin-3-independent mechanism becomes involved at later phases. Because the anti-galectin-3 antibody used induces some HA (Fig. 2D, gal-3), residual HA could be due to galectin-3-induced HA and/or to an incomplete dependence of CD13 on galectin to mediate HA. Pre-incubation with anti-galectin-3 antibodies at 4 or 37 °C had the same inhibitory effect on HA, suggesting that the effect of anti-galectin-3 antibodies is not an energy-requiring process. Addition of the anti-galectin-3 antibody simultaneously with the anti-CD13 mAb resulted in a less significant inhibitory effect (Fig. 2C, co-incubation) suggesting that the participation of galectin-3 in CD13-mediated cell aggregation occurs at the initial steps following anti-CD13 mAb binding. In summary, these results demonstrate that blocking galectin-3 with antibodies significantly hampers the early steps of CD13-mediated HA.
Fig. 2.
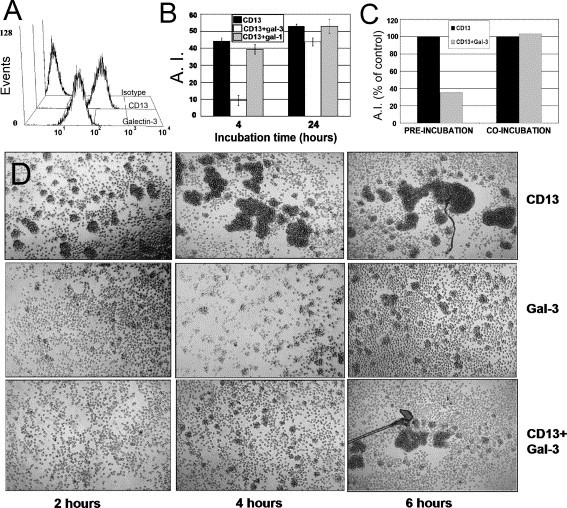
Anti-Galectin-3 antibodies bind to U-937 cells and block CD13-mediated homotypic aggregation. (A) Cells were incubated with anti-galectin-3, anti-CD13 or isotype matched IgG antibodies. Fluorescence of a secondary FITC-labeled antibody was detected by flow cytometry. (B) Cells were pre-incubated with anti-galectin-3 or anti-galectin-1 antibodies (8 μg/ml) for 1 h t 37 °C. Subsequently, HA was induced with the anti-CD13 mAb without washing out the anti-galectin antibody. The average AI from two independent experiments at the indicated incubation times is shown. (C) Anti-galectin-3 antibodies were added before (pre-incubation) or along with (co-incubation) anti-CD13 antibodies. Normalized data from two independent experiments is shown. (D) Kinetics of the effect of anti-galectin-3 antibodies on HA. At time 0, the indicated antibodies were added: anti-CD13 antibody alone (CD13), anti-galectin-3 antibody alone (Gal-3) or anti-CD13 antibody after a pre-incubation with the anti-galectin-3 antibody for 1 h (CD13+gal-3). Images were acquired at the indicated incubation times.
Galectin-3 co-immunoprecipitates with CD13 in U-937 cells and this association decreases during HA
A steric hindrance effect of the anti-galectin-3 antibody for binding of the anti-CD13 mAb was ruled out because pre-incubation of cells with the anti-galectin antibody at 37 or 4 °C did not block binding of the anti-CD13 mAb (Fig. 3 a). Consequently, a direct participation of galectin-3 in the mechanism of HA was hypothesized. An association between galectin-3 and CD13 was likely considering the predicted glycosylation characteristics of CD13 and the previously described interaction between galectin-4 and CD13 in the intestine. As shown in Fig. 3b, galectin-3 and CD13 co-immunoprecipitate from resting U-937 cells. Unexpectedly, induction of HA consistently diminished the amount of galectin-3 co-immunoprecipitating with CD13. These results show that galectin-3 is constitutively associated with CD13 and that induction of HA by the anti-CD13 mAb decreases this association. We have reported that during anti-CD13 mediated HA, the membrane levels of CD13 decrease to some extent due to its internalization [9]. Although the relevance of the CD13 internalization during HA is presently unknown, we tested the effect of anti-galectin-3 antibodies on this phenomenon. Galectin-3 antibodies did not inhibit CD13 internalization (Fig. 3c) suggesting that their inhibitory effect does not depend on disturbing this event.
Fig. 3.
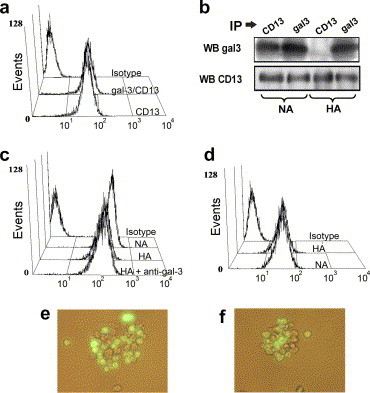
Galectin-3 is associated with CD13 in U-937 cells. (a) Binding of the anti-CD13 mAb 452 to U-937 cells pre-incubated or not with the anti-galectin-3 antibody for 1 h at 37 °C. (b) HA was induced with the anti-CD13 mAb for 2 h using 1x107 cells/ml. Antibody treated (HA) or untreated, non-aggregated cells (NA) were lysed and CD13 (IP CD13) or galectin-3 (IP gal-3) was immunoprecipitated as described in Materials and methods. A Western-blot was performed using anti-CD13 (WB CD13) and subsequently, the same membrane was re-probed for galectin-3 (WB gal-3). Immunoprecipitation of CD13 resulted in co-immunoprecipitation of galectin-3 but in lower levels in aggregated (lane 3) than non-aggregated cells (lane 1). Immunoprecipitation of galectin-3 also resulted in co-immunoprecipitation of CD13 (lane 2). Anti galectin-3 immunoprecipitates from HA cells contain CD13 because of the anti-CD13 antibody used to induce HA (lane 4). (c) Membrane expression of CD13 in NA and in HA cells that had been pre-incubated in the presence (HA+anti-gal-3) or absence (HA) of anti-galectin-3 antibodies. (d) Membrane expression of galectin-3 was determined by flow cytometry in HA and NA cells. (e) Cells were incubated with anti-CD13 mAb at 4 °C. After washing, they were co-incubated at 37 °C with calcein-labeled cells which had not been pre-incubated with anti-CD13 mAb. Images were taken after 4 h. (f) Same experiment as in (e) but the calcein-labeled population was pre-incubated with anti-galectin-3 antibodies before HA.
Membrane expression of galectin-3 is not altered in aggregated cells
After dissociating from CD13, galectin-3 could be released to the medium or become associated to a different membrane receptor. We found identical membrane levels of galectin-3 in aggregated and non-aggregated cells as determined by flow cytometry (Fig. 3d), suggesting that the galectin-3 that dissociates from CD13 either binds to a different membrane receptor or oligomerizes with other membrane-associated galectin molecules. In this regard, it has been reported that monomeric galectin-3 has a tendency to oligomerize at high concentrations, specially with galectins already attached to an immobilized ligand (e.g. laminin or a membrane receptor) [24], [26]. If oligomerization of galectin-3 is important for CD13-mediated HA, this could explain why anti-galectin-3 antibodies are capable of inhibiting HA.
Ligation of CD13 in one population of cells induces their aggregation with untreated cells
The results presented above show that during anti-CD13-mediated HA, the association of CD13 with galectin-3 decreases, the amount of CD13 on the membrane decreases, but the membrane levels of galectin-3 remain unaltered. Galectin-3 molecules that have dissociated from CD13 could participate in HA by interacting with other galectin-3 molecules or other galectin-3 receptors. Interaction of galectin-3 with molecules on neighboring cells may result in a “bridging” phenomenon as has been suggested for galectin-3 by other authors [18]. However, since CD13-mediated HA does not occur at 4 °C and is sensitive to inhibitors of protein kinases, it is likely that dissociated galectin-3 mediates cross-linking of CD13 and/or other membrane receptors thereby triggering transmembrane signals responsible for a pro-adhesive effect in the cell in which CD13 has been ligated. If this is true, incubation of one population of cells with the anti-CD13 mAb would induce their aggregation with calcein-labeled cells that had not been incubated with the mAb. As shown in Fig. 3e, after combining these two populations of cells, aggregates formed by both of them appeared. This result suggests that changes in the association of membrane proteins and signal transduction events induced through CD13 in one cell are sufficient to induce its adhesion to another cell in which no signaling has occurred. Next, to determine if the galectin-3 of the cells that were not incubated with anti-CD13 is involved in the aggregation observed in the previous experiment, the calcein-labeled cells were pre-incubated with the anti-galectin-3 antibodies at 4 or 37 °C before the assay. Aggregates appeared again formed by labeled and unlabeled cells (Fig. 3f) suggesting that the galectin-3 molecules involved in HA are mainly the ones on the cell that has been “activated” through CD13.
A model for the role of galectin-3 in CD13-mediated HA
Based in our observations, a model for the role of galectin-3 in CD13-induced HA can be proposed (Fig. 4 ). Galectin-3 is constitutively associated with CD13 in resting U-937 cells (Fig. 4A). Although a direct physical association is likely via highly glycosylated domains of CD13 such as the stalk or other domain, an indirect association through another receptor of galectin-3 complexed with CD13 can not be ruled out. Antibody binding to CD13 induces the release of galectin-3 from the targeted molecules and triggers a signal transduction event that results in changes in the adhesion properties of the cell. Conformational changes have been demonstrated after binding of substrates, inhibitors and antibodies to CD13 [27], [28]. Galectin-3 released from CD13 might participate in HA by different mechanisms. First, since membrane levels of galectin-3 do not change during HA and because of the results presented in Fig. 3f, dissociated galectin-3 appears to establish interactions with other molecules on the same cell (e.g. one of the known receptors for galectin-3 (integrins), a chemokine receptor, or other galectin-3 molecules). Galectin-3 has been shown to participate in integrin activation [29] and to be a chemoattractant for human monocytes/macrophages [30]. We have found that cells treated with the anti-CD13 mAb show a chemokinetic and later chemotactic behavior migrating to the zones where aggregates are starting to form [9]. Finally, it is known that galectin-3 can form homo-oligomers [24], [26] raising the possibility that galectin-3 molecules that have dissociated from CD13 participate in HA by a mechanism involving homo-oligomerization and/or bridging. The presence of excess lactose or anti-galectin-3 antibodies might inhibit galectin-3 oligomerization and/or the subsequent cross-linking of other receptors thus inhibiting HA.
Fig. 4.
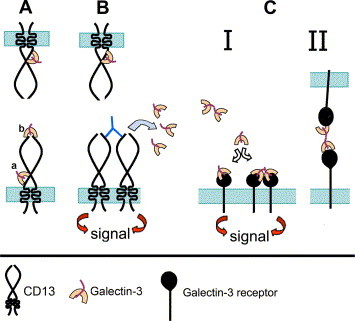
Hypothetical model of the role of galectin-3 in CD13-mediated HA. CD13 is depicted according to the “roll-over” model proposed by Lendeckel et al. [28]. (A) Galectin-3 is constitutively associated with CD13 (Fig. 3B) probably via the highly glycosylated stalk domain (a) or via another glycosylation site on CD13 (b). (B) Antibody binding and cross-linking of CD13 triggers a signal transduction cascade along with the release of the associated galectin-3 (Fig 3B) and [9]). (C) The displaced galectin-3 stays on the cell membrane (Fig. 3D) suggesting that it then associates with other receptor (either in monomeric or oligomeric form) (I), a phenomenon that might trigger transmembrane signals related to adhesion phenomena. Alternatively, oligomers of galectin-3 could bind to glycoconjugates on neighboring cells thus physically mediating aggregation through bridging (II).
Another possibility is the exposure of cryptic, adhesion-related epitopes in CD13. Although saturating concentrations of the anti-CD13 mAb do not induce HA when left in the medium during HA [9], they induce optimal aggregation levels when pre-incubated at 4 °C and washed to eliminate the excess of antibody (not shown). This suggests that an excess of antibody in the medium blocks interactions mediated by newly exposed epitopes. Cryptic epitopes have been shown to double after certain antibodies and substrates bind to the molecule [27]. In this scenario, CD13-bound galectin-3 could function as an epitope masker in resting cells.
Together, the results presented here suggest for the first time a physical and functional interaction between CD13 and galectin-3. A ligand for CD13 in humans has not been described and thus, the possible role of galectin-3 as an in vivo ligand for CD13 needs to be determined and the structural characteristics of this interaction must be studied.
Several functions of CD13 could be explained by its interaction with galectin-3 which has been implicated in many of them (e.g. FcγR-mediated phagocytosis, angiogenesis, invasion and metastasis) [31], [32]. CD13 is upregulated in many types of tumors and therefore it could replace or cooperate with other galectin-3 partners for the induction of metastasis-related homotypic aggregation [20].
Acknowledgments
We thank Dr. Meenhard Herlyn (The Wistar Institute of Anatomy and Biology), for his kind donation of the 452 mAb-producing hybridoma. P.M.O. was supported by a fellowship from Dirección General de Estudios de Posgrado, UNAM. This work was supported by grants from PAPIIT-UNAM (IN220705) and CONACYT (45092).
Contributor Information
Paola Mina-Osorio, Email: pmina@uchc.edu.
Enrique Ortega, Email: ortsoto@servidor.unam.mx.
References
- 1.Olsen J., Kokholm K., Noren O., Sjostrom H. Structure and expression of aminopeptidase N. Adv. Exp. Med. Biol. 1997;421:47–57. doi: 10.1007/978-1-4757-9613-1_7. [DOI] [PubMed] [Google Scholar]
- 2.Saiki I., Fujii H., Yoneda J., Abe F., Nakajima M., Tsuruo T., Azuma I. Role of aminopeptidase N (CD13) in tumor-cell invasion and extracellular matrix degradation. Int. J. Cancer. 1993;54:137–143. doi: 10.1002/ijc.2910540122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukasawa K., Fujii H., Saitoh Y., Koizumi K., Aozuka Y., Sekine K., Yamada M., Saiki I., Nishikawa K. Aminopeptidase N (APN/CD13) is selectively expressed in vascular endothelial cells and plays multiple roles in angiogenesis. Cancer Lett. 2006 doi: 10.1016/j.canlet.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 4.Chang Y.W., Chen S.C., Cheng E.C., Ko Y.P., Lin Y.C., Kao Y.R., Tsay Y.G., Yang P.C., Wu C.W., Roffler S.R. CD13 (aminopeptidase N) can associate with tumor-associated antigen L6 and enhance the motility of human lung cancer cells. Int. J. Cancer. 2005;116:243–252. doi: 10.1002/ijc.21089. [DOI] [PubMed] [Google Scholar]
- 5.van Hensbergen Y., Broxterman H.J., Rana S., van Diest P.J., Duyndam M.C., Hoekman K., Pinedo H.M., Boven E. Reduced growth, increased vascular area, and reduced response to cisplatin in CD13-overexpressing human ovarian cancer xenografts. Clin. Cancer Res. 2004;10:1180–1191. doi: 10.1158/1078-0432.ccr-0482-3. [DOI] [PubMed] [Google Scholar]
- 6.Rainger G.E., Buckley C., Simmons D.L., Nash G.B. Neutrophils rolling on immobilised platelets migrate into homotypic aggregates after activation. Thromb. Haemost. 1998;79:1177–1183. [PubMed] [Google Scholar]
- 7.Delemarre F.G., Hoogeveen P.G., De Haan-Meulman M., Simons P.J., Drexhage H.A. Homotypic cluster formation of dendritic cells, a close correlate of their state of maturation. Defects in the biobreeding diabetes-prone rat. J. Leukoc. Biol. 2001;69:373–380. [PubMed] [Google Scholar]
- 8.Glinsky V.V., Glinsky G.V., Glinskii O.V., Huxley V.H., Turk J.R., Mossine V.V., Deutscher S.L., Pienta K.J., Quinn T.P. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003;63:3805–3811. [PubMed] [Google Scholar]
- 9.Mina-Osorio P., Shapiro L.H., Ortega E. CD13 in cell adhesion: aminopeptidase N (CD13) mediates homotypic aggregation of monocytic cells. J. Leukoc. Biol. 2006;79:719–730. doi: 10.1189/jlb.0705425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riemann D., Kehlen A., Langner J. CD13--not just a marker in leukemia typing. Immunol. Today. 1999;20:83–88. doi: 10.1016/S0167-5699(98)01398-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sjostrom H., Noren O., Olsen J. Structure and function of aminopeptidase N. Adv. Exp. Med. Biol. 2000;477:25–34. doi: 10.1007/0-306-46826-3_2. [DOI] [PubMed] [Google Scholar]
- 12.Noren K., Hansen G.H., Clausen H., Noren O., Sjostrom H., Vogel L.K. Defectively N-glycosylated and non-O-glycosylated aminopeptidase N (CD13) is normally expressed at the cell surface and has full enzymatic activity. Exp. Cell Res. 1997;231:112–118. doi: 10.1006/excr.1996.3455. [DOI] [PubMed] [Google Scholar]
- 13.Wentworth D.E., Holmes K.V. Molecular determinants of species specificity in the coronavirus receptor aminopeptidase N (CD13): influence of N-linked glycosylation. J. Virol. 2001;75:9741–9752. doi: 10.1128/JVI.75.20.9741-9752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derbyshire D.J., Ellar D.J., Li J. Crystallization of the Bacillus thuringiensis toxin Cry1Ac and its complex with the receptor ligand N-acetyl-d-galactosamine. Acta. Crystallogr. D. Biol. Crystallogr. 2001;57:1938–1944. doi: 10.1107/s090744490101040x. [DOI] [PubMed] [Google Scholar]
- 15.Danielsen E.M., van Deurs B. Galectin-4 and small intestinal brush border enzymes form clusters. Mol. Biol. Cell. 1997;8:2241–2251. doi: 10.1091/mbc.8.11.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato S., Hughes R.C. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J. Biol. Chem. 1994;269:4424–4430. [PubMed] [Google Scholar]
- 17.Liu F.T., Hsu D.K., Zuberi R.I., Kuwabara I., Chi E.Y., Henderson W.R., Jr. Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am. J. Pathol. 1995;147:1016–1028. [PMC free article] [PubMed] [Google Scholar]
- 18.Inohara H., Raz A. Functional evidence that cell surface galectin-3 mediates homotypic cell adhesion. Cancer Res. 1995;55:3267–3271. [PubMed] [Google Scholar]
- 19.Pesheva P., Kuklinski S., Schmitz B., Probstmeier R. Galectin-3 promotes neural cell adhesion and neurite growth. J. Neurosci. Res. 1998;54:639–654. doi: 10.1002/(SICI)1097-4547(19981201)54:5<639::AID-JNR9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Khaldoyanidi S.K., Glinsky V.V., Sikora L., Glinskii A.B., Mossine V.V., Quinn T.P., Glinsky G.V., Sriramarao P. MDA-MB-435 human breast carcinoma cell homo- and heterotypic adhesion under flow conditions is mediated in part by Thomsen--Friedenreich antigen-galectin-3 interactions. J. Biol. Chem. 2003;278:4127–4134. doi: 10.1074/jbc.M209590200. [DOI] [PubMed] [Google Scholar]
- 21.Herrmann J., Turck C.W., Atchison R.E., Huflejt M.E., Poulter L., Gitt M.A., Burlingame A.L., Barondes S.H., Leffler H. Primary structure of the soluble lactose binding lectin L-29 from rat and dog and interaction of its non-collagenous proline-, glycine-, tyrosine-rich sequence with bacterial and tissue collagenase. J. Biol. Chem. 1993;268:26704–26711. [PubMed] [Google Scholar]
- 22.Barboni E.A., Bawumia S., Henrick K., Hughes R.C. Molecular modeling and mutagenesis studies of the N-terminal domains of galectin-3: evidence for participation with the C-terminal carbohydrate recognition domain in oligosaccharide binding. Glycobiology. 2000;10:1201–1208. doi: 10.1093/glycob/10.11.1201. [DOI] [PubMed] [Google Scholar]
- 23.Birdsall B., Feeney J., Burdett I.D., Bawumia S., Barboni E.A., Hughes R.C. NMR solution studies of hamster galectin-3 and electron microscopic visualization of surface-adsorbed complexes: evidence for interactions between the N- and C-terminal domains. Biochemistry. 2001;40:4859–4866. doi: 10.1021/bi002907f. [DOI] [PubMed] [Google Scholar]
- 24.Yang R.Y., Hill P.N., Hsu D.K., Liu F.T. Role of the carboxyl-terminal lectin domain in self-association of galectin-3. Biochemistry. 1998;37:4086–4092. doi: 10.1021/bi971409c. [DOI] [PubMed] [Google Scholar]
- 25.Cortez K.J., Lyman C.A., Kottilil S., Kim H.S., Roilides E., Yang J., Fullmer B., Lempicki R., Walsh T.J. Functional genomics of innate host defense molecules in normal human monocytes in response to Aspergillus fumigatus. Infect. Immun. 2006;74:2353–2365. doi: 10.1128/IAI.74.4.2353-2365.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu D.K., Zuberi R.I., Liu F.T. Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J. Biol. Chem. 1992;267:14167–14174. [PubMed] [Google Scholar]
- 27.Xu Y., Wellner D., Scheinberg D.A. Cryptic and regulatory epitopes in CD13/aminopeptidase N. Exp. Hematol. 1997;25:521–529. [PubMed] [Google Scholar]
- 28.Lendeckel U., Bukowska A., Lattig J., Brandt W. Alanyl-aminopeptidases in human T cells. In: Hooper N.M., Lendeckel U., editors. Aminopeptidases in Biology and disease. Kluwer Academic/Plenum Publishers; New York: 2004. pp. 201–227. [Google Scholar]
- 29.Furtak V., Hatcher F., Ochieng J. Galectin-3 mediates the endocytosis of beta-1 integrins by breast carcinoma cells. Biochem. Biophys. Res. Commun. 2001;289:845–850. doi: 10.1006/bbrc.2001.6064. [DOI] [PubMed] [Google Scholar]
- 30.Sano H., Hsu D.K., Yu L., Apgar J.R., Kuwabara I., Yamanaka T., Hirashima M., Liu F.T. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J. Immunol. 2000;165:2156–2164. doi: 10.4049/jimmunol.165.4.2156. [DOI] [PubMed] [Google Scholar]
- 31.Califice S., Castronovo V., Van Den Brule F. Galectin-3 and cancer (Review) Int. J. Oncol. 2004;25:983–992. [PubMed] [Google Scholar]
- 32.Sano H., Hsu D.K., Apgar J.R., Yu L., Sharma B.B., Kuwabara I., Izui S., Liu F.T. Critical role of galectin-3 in phagocytosis by macrophages. J. Clin. Invest. 2003;112:389–397. doi: 10.1172/JCI17592. [DOI] [PMC free article] [PubMed] [Google Scholar]


