Abstract
Animal experimentation has a long tradition for risk assessment of new drugs before they reach the clinic. To reduce expensive animal experimentation, attempts have been made to build inexpensive and convenient intestinal functional cell models to study toxicity and bioavailability of new substances along with providing relevant models to study interactions between the host, pathogens and intestinal microflora. We review the available cell lines and models of the intestine and their potential uses. Tumor derived cell lines such as Caco-2, T84 and HT-29 are widely used despite many drawbacks, which are discussed with respect to complexity of the gut, where various cell types interact with commensal microbiota and gut-associated lymphoid tissue. To address this complexity, 3D models of human and animal gut represent a promising in vitro system to mimic in vivo situation without the use of transformed cell lines.
Keywords: Cell model, Gut, Probiotics, Intestine, Pathogens, Risk assessment
1. Introduction
The need for appropriate intestinal cell lines has been recognised in the past decade to find a good in vitro model of the intestine for studying gut interactions, nutrition, toxicology and food microbiology. There are few intestinal models available, although their quality and reliability are questionable because of the inappropriate experimental layout and mainly tumorigenic cell lines, which are usually used to build the models.
Intestinal models are of great interest to food and pharmaceutical industry, principally are toxicological and bioavailability tests of newly developed food ingredients and drugs inevitable for bringing products to the market. In food industry, risk assessment regarding the safety and efficacy of probiotics and new functional foods is an open issue, since food safety is of utmost concern in the western world.
Models of intestine are essential for research into enteric pathogens. The mechanisms of interactions between foodborne pathogens, mammalian host and intestinal microflora including mechanisms of microbial attachment and cross-talk with host epithelium and preventive and curative effects of probiotic bacteria remain largely unknown. The present review summarises available models of intestine. First we describe general functions and histology of the intestine. This information is necessary to understand drawbacks and advantages of the models, which are discussed with respect to the complexity of the gut and the cell lines used in vitro. Special focus is on our own studies on functional cell models of the human and animal gut.
2. The intestine
The intestine is an important internal environment where a number of processes occur in order to nourish the body and protect it against the enteropathogens or harmful substances entering the gut. The small intestine is the longest section of the digestive tube and consists of three segments forming a passage from the pylorus to the large intestine: Duodenum, Jejunum (considered to be roughly 40% of the small gut in human, but closer to 90% in animals) and Ileum. The total length of the small intestine is roughly 6 m in humans. Although precise boundaries between these three segments of bowel are not observed grossly or microscopically, there are histological differences among them (Tortora and Derrickson, 2008).
3. Histology of the intestine
The luminal face of the gastrointestinal tract comprises several layers which interact with the luminal contents. The mucosal epithelium, lamina propria, glycocalyx and secreted mucus each make a contribution to a “barrier function” (Patsos and Corfield, 2009).
The gastrointestinal mucosa forms an interface between the body and the lumenal environment which not only contains nutrients, but potentially damaging microorganisms and toxins. The challenge is to allow efficient transport of nutrients across the epithelium while rigorously excluding passage of harmful molecules and pathogens. This is a complex and dynamic process including both transcellular and paracellular pathways (Balimane et al., 2000). The structure of the mucosa is defined by sheets of epithelial cells (Fig. 1 ) (Cheng and Leblond, 1974), connected laterally by tight junctions, which regulate the paracellular spaces and thereby establish the tight epithelia (Yu and Yang, 2009). Integrity of epithelia is critical since toxins and microorganisms that are able to breach the single layer of epithelial cells have unimpeded access to the systemic circulation (Schierack et al., 2006).
Fig. 1.
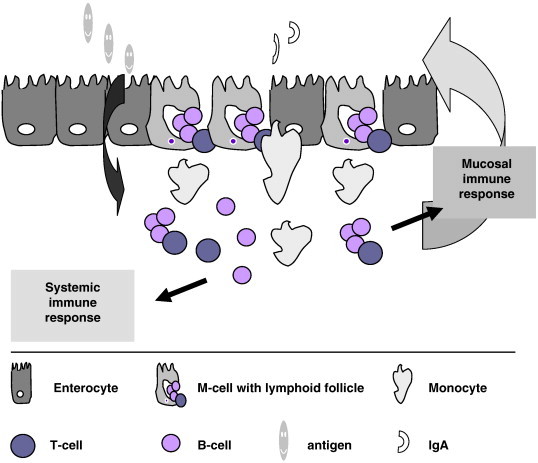
Schematic presentation of interactions in the small intestine. Intestinal epithelial cells are polarised cells with distinct apical (towards the lumen) and basolateral sides (towards the body). Tight junctions between the cells keep integrity of the epithelia. Underneath the epithelial barrier lay the mucosal lymphatic tissues, responsible for induction and regulation of the immune system. Nutrients and antigens are selectively sampled in the lumen and transported through the barrier. Besides enterocytes other cells populate the epithelial barrier (M, Goblet, Paneth, endocrine cells) with distinct specialised functions.
The predominant common type in the epithelial layer is the enterocyte, the mature absorptive unit that cover the villi (Fig. 2 ) and crypts. Enterocytes are polarised cells with a distinct apical and basolateral cytoplasmatic membrane. However, other cell types can be found in the epithelia, including enteroendocrine, goblet, Paneth, M and cup cells (Cheng and Leblond, 1974). Unique oligosaccharides that form the glycocalyx on the surface of epithelial cells possess high specificity and affinity for lectins, viruses, bacterial toxins, bacteria and immune cells (Falk et al., 1994, Sharma et al., 1998).
Fig. 2.
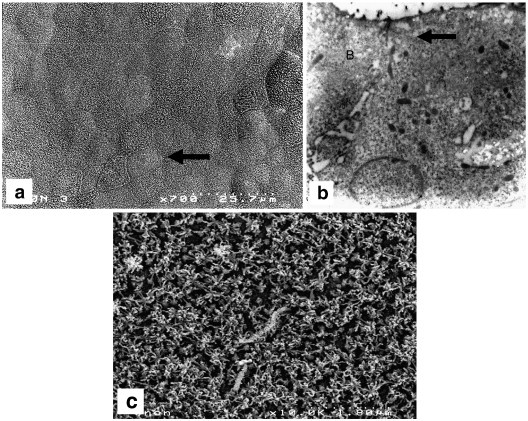
Morphology of the epithelial cells: (a) SEM micrograph of a typical morphology of the cell with established epithelial character; (b) TEM micrograph of the epithelial cells: arrow indicates proper tight junctions formation between the adjacent epithelial cells; (c) SEM micrograph of apical surface microvilli of the fully differentiated epithelial cell. Establishment of densely packed epithelial layer is crucial for the barrier between the lumen and the body; layer morphology depends on the matrix, which is used to grow the cells.
Underneath the epithelial barrier lay the mucosal lymphatic tissues in the form of nonencapsulated submucosal lymphoid nodules which diffuse lymphocytic infiltrates in the submucosa of intestinal and respiratory tracts. Specialised lymphoid follicles of the gut mucosa are the major sites for induction and regulation of the immune system (Guarner, 2006) and imbalance can lead to intestinal inflammation. A key role in the maintenance of an adequate balance between antigenic stimulation and host immune response is played by the immunoregulatory molecules released by activated immunocytes in the human gut but the influence of intestinal epithelial cells in the regulation of the associated mucosal immune system still remains unclear (Biancone et al., 2002, Zoumpopoulou et al., 2009).
4. Microbial gut ecosystem
The human gut is home to a complex community of microbes (the microbiota) that plays a critical role in host nutrient acquisition and metabolism, development of intestinal epithelial cells and host immune system. Recent reports show a systematic environment of the microbial diversity in the human gut of more than 1000 different species-level phylogenetic types (Andoh et al., 2009, Hart et al., 2002). Genetic background, nutritional status, and environmental factors influence the structure and function of the gut microbiota. Microbes actively communicate between themselves and with the host by releasing peptides that resemble peptide hormones of vertebrates, possibly influencing host cell function (Hsiao et al., 2008). Commensal microbes influence the epithelial differentiation and affect the underlying mucous immune system (Falk et al., 1998). Gut bacteria play an essential role in the development and homeostasis of the immune system. Moreover, colonisation by the microbiota plays an important role in intestinal tract maturation of newborn, it protects against infectious aggression and provides the body with essential nutrients (Gupta and Garg, 2009). Microbiota colonisation can be modulated by probiotics (live microorganisms that when administered in adequate amount confer a health benefit on the host (FAO/WHO, 2002)), prebiotics (non-digestible food ingredients that stimulate the growth and/or activity of bacteria) or synbiotics (combination of probiotics and prebiotics). However, human data are limited and more experimental and controlled clinical trials must be carried out for a more precise understanding of the mechanisms underlying the probiotic action and the balance of the complex gastrointestinal ecosystem with which probiotics are expected to interact (Penna et al., 2008).
5. Experimental models
Being aware of the complex interactions in the subtle environment of the gut it is obvious that to find an appropriate experimental model is not an easy accomplishment. Several experimental (mainly animal) models have been employed and subsequent clinical studies were performed when applicable. Germ-free mice were widely used as in vivo experimental system to study the colonisation and defining its underlying mechanisms (Falk et al., 1998). Although this system offers a good model for such studies, it has major disadvantages: 1) it is not in accordance with the spirit of reduced animal use for research purposes and animal welfare, 2) it is not suitable for wide laboratory testing, as special facilities and special trained personnel are needed, 3) it is expensive and time consuming to obtain results in comparison with good in vitro studies and 4) it is not always possible to create a good model for human enteric pathogens as some of them cannot infect the rodents gut epithelia (e.g. Listeria monocytogenes). There is also scientific criticism as animal and human models failed to develop a mechanistic approach. Indeed, in the study of Cao et al., it was found that a rat model could not be used to predict drug metabolism and oral bioavailability in humans based on the underlying molecular mechanisms (Cao et al., 2006). Moreover, results obtained in such models were difficult to reproduce, were not clearly linked to clinical outcomes and were outside any physiology of host (Clancy, 2003, Suntharalingam et al., 2006). Pigs are widely used due to their close intestinal resemblance to humans and good likelihood of applicability of results to human (Lunney, 2007). However, animal experiments are not favoured by the EU that is eager to reduce animal experimentation.
Although technological innovations in the fields of genomics and proteomics in recent years are providing new data, in vitro models are still essential tools in biological mechanistic studies. For example in cytokine production, it is necessary to localise the protein and mRNA. Localisation of protein alone can identify cells producing cytokines as well as the target cells and cells which have taken up the protein by endocytosis. mRNA detection only may mislead as some cytokines (like tumor necrosis factor) are regulated posttranscriptionally (Cappello et al., 1992). Primary cells, isolated from human or animal tissue, retain majority of the in vivo functionality, but they survive only few days in cell culture (Marian, 2002, Moue et al., 2008, Niku et al., 2006, Panja, 2000, Quaroni and Beaulieu, 1997, Whitehead et al., 1993). Moreover, the primary cells usually derive from different individuals in each test, consequently the reproducibility of results may significantly differ from one test to another.
In vitro cell models have to satisfy two basic requirements: (1) availability and easy handling for high-throughput testing and (2) good human predictive power , i.e. yield data that support interpretation of results for the in vivo situation (Cencic et al., 2008a, Cencic et al., 2008b).
6. Cell models of the gut
As described above, in vitro cell models of the gut should functionally resemble the in vivo situation. Since the gut is a complex system with many interacting cell types and the microbiota, models should take into consideration as many of these factors as possible.
Expression of tight junction proteins is necessary for the formation of epithelial barrier, integrity and polarity (Shin et al., 2006). Primary epithelial cells in vivo develop a tightly packed selectively permeable membrane with measurable transepithelial resistance (R T, also abbreviated as TER) and V T (transepithelial potential difference); in vitro epithelial cells that polarise should develop these features when grown on microporous membrane. Epithelial cells in combination with other cell lines (if applied in the model) should respond to environmental factors like cytokines and inflammatory molecules. Moreover, the origin of the cell lines is important since cancerogenic cells have different glycosylation (Brooks et al., 2008), their proliferation and behaviour under environmental stimuli can be significantly altered. Characterization of cell markers, receptors and expression of functionally important proteins is also important for the elucidation of cell line functionality and determination of the differentiation or activation status. Based upon this information, models can be built to study particular situations. Secretion of mucins from epithelial cells helps to distinguish mucin producing cells from normal enterocytes. Cytokeratins are characteristic epithelial cytoskeletel proteins and are involved in infections by pathogens (Carlson et al., 2002). Alkaline phosphatase is involved in epithelial cell differentiation (Wood et al., 2003) and maintanance of gut barrier (Geddes and Philpott, 2008).
When immune cells are used in the models, activation status is determined by the level of endocytosis, expression of MHC II, coactivator (CD80/86) and other molecules (Nurieva et al., 2009). Immature non-activated cells are useful in determination of the effects microbes could have when applied to the system; activated cells should be used to study inflammatory disorders in the intestine.
In most of the in vitro studies of the gut, human colon tumorigenic cell lines Caco-2, T84 and HT-29 have been widely used for attachment assays and mechanistic studies. The brief description of available cell lines of the intestinal epithelial cells as stated below and in Table 1 can help food microbiologists to choose the ones that are of most relevance to their studies.
Table 1.
Available cell lines and cell models of the animal and human gut.
Among commercially available cell lines (ATCC and ECACC), most widely used are definitely human cancer Caco-2 cells. Caco-2 (Fig. 3a) (colon adenocarcinoma) were isolated from a 72-year old Caucasian male. Upon reaching confluence, the cells were found to express characteristics of enterocytic differentiation and functionality (Cui et al., 2009, Jalal et al., 1992, Lee et al., 2009, Schnabl et al., 2009, Uchida et al., 2009, Visco et al., 2009). Another human colon adenocarcinoma cell line HT-29 was isolated in 1964 from the colon tumor of a 44-year old Caucasian female. Ultrastructural features reported for HT-29 cells include microvilli, microfilaments, large vacuolated mitochondria with dark granules, smooth and rough endoplasmic reticulum with free ribosomes, lipid droplets, few primary and many secondary lysosomes. The cells express urokinase receptors, but do not have detectable plasminogen activator activity (Chen et al., 1987, Didier et al., 1996, Takahashi et al., 1996). The human cell line: Intestine 407 was originally thought to be derived from normal female embryonic intestinal tissue, but was subsequently found, based on isoenzyme analysis, HeLa marker chromosomes and DNA fingerprinting to have been established via HeLa cell contamination. The cells are positive for keratin by immunoperoxidase staining and cells contain human papilloma virus (HPV-18) (Henle and Deinhardt, 1957, Lavappa et al., 1976). Then, from a 40-year old woman with epithelioid carcinoma of metastatic cervix cancer, CaSki cells were isolated from a metastasis in the small intestine. The cells secrete beta subunit of human chorionic gonadotropin (hCG); tumor associated antigen (Baker et al., 1987, Hussa et al., 1986, Pattillo et al., 1977). Although the cell line has been widely used in experiments, it does not derive from the small intestine, it has a tumorigenic phenotype distinguished from the normal gut epithelia and it expresses modified surface glycoconjugates (Tremblay et al., 2006). The most widely used rodent lines are the commercially available rat IEC-6 and IEC-18 cell lines, derived from the rat small intestine (Quaroni and Isselbacher, 1981, Quaroni et al., 1978). The normal human crypt small intestinal cells HIEC-6 (Fig. 3b) were isolated in the group of Beaulieu at the University of Sherbrooke, Canada (Beaulieu, 1997, Beaulieu, 1999, Belanger and Beaulieu, 2000, Lussier et al., 2000, Pageot et al., 2000). These cells have been widely used in integrin and extracellular matrix research, but are not fully characterised (Benoit et al., 2009, Dydensborg et al., 2009, Levy et al., 2009). Human foetal small intestinal cell line H4 (Fig. 3c) was established by Walker and colleagues (Nanthakumar et al., 2000) and represents the normal enterocytes of immature human intestine (Claud et al., 2003, Lu et al., 2008, Lu et al., 2009). H4 cell line was subsequently subcloned in our laboratory to obtain pure epithelial cells capable of transepithelial resistance and voltage formation. The IPEC-J2 cell line (Fig. 3d) is a non-transformed intestinal cell line originally derived from jejunal epithelia isolated from a neonatal, unsuckled piglet, maintained as a continuous culture (Berschneider, 1989) and characterised (Schierack et al., 2006). The IPEC-J2 cell line was subcloned in our laboratory to obtain cultures of enterocyte like (Fig. 3e) and mucin producing cell types (Fig. 3f).
Fig. 3.
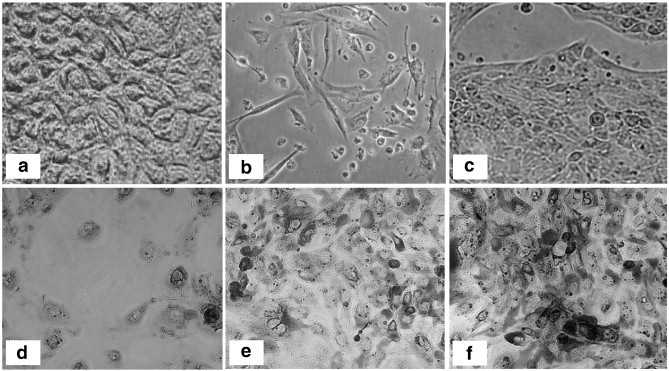
Intestinal epithelial cell lines: (a) Caco-2; (b) HIEC; (c) H4; (d) IPEC-J2; (e) IPEC-J2-3; (f) IPEC-J2-9. Magnification 200×. Caco-2, HIEC and H4 are human-derived cell lines while IPEC-J2 was isolated from a pig. Although all cells are classified as epithelial, there are morphological differences between them. Except for Caco-2, all the cell lines have a non-tumorigenic origin.
In most of the in vitro experimental models, the epithelial cells are mostly cultivated as monolayers on plastic surfaces, where the establishment of functional, epithelial character is not achieved, leading to potential misinterpretation of results, especially in adhesion assays (Fig. 4 ). Caco-2 cells grown on the microporous membranes for studies of pathogen translocation are widely described (see Klingberg et al., 2005, McCormick, 2003).
Fig. 4.

Differences in growth morphology between normal intestinal epithelial (IPEC-J2) (a, c) and tumorigenic cells (Caco-2) (b, d) and growth type (a,b — simple monolayers on plastic; c,d — 3D growth). Magnification 100×. Both cell lines develop different surface morphology depending on the growth matrix (monolayer — 3D). 3D growth allows the cells to polarise with distinct apical and basolateral parts, mimicking the in vivo situation.
In the frame of the EU funded ‘PathogenCombat’ project 3D intestinal epithelial models from various species were developed in our laboratory and are described below (Fig. 5 ; Table 1) (Cencic et al., 2006, Cencic et al., 2007a, Cencic et al., 2007b, Cencic et al., 2008a, Cencic et al., 2008b, Cencic et al., 2009). 3D models are built from intestinal epithelial cell line of non-cancerogenic origin, growing on a microporous membrane, enabling polarisation of the cells and development of TER/TEP. Below the microporous membrane (basolateral side) epithelial cells are underlaid with immune cells (macrophages, dendritic cells), mimicking mucosal lymphoid tissue. Intestinal microbiota can be added to the apical side of the membrane to study effects of microbiota. These three components (epithelia, immune cells and microbiota) are the most important factors in the gut, which makes these models close to the in vivo situation.
Fig. 5.
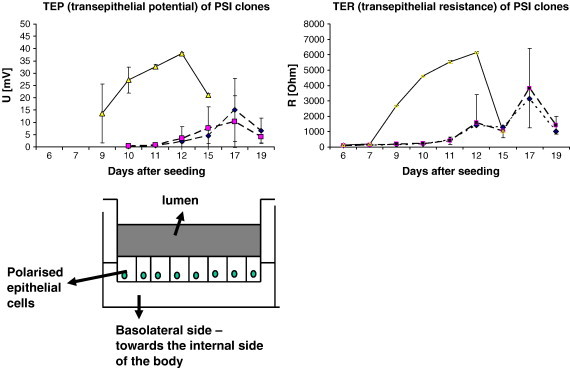
Schematic presentation of the functional (3D) model of the gut and functional polarity of the intestinal epithelial cells growing in it. Cells growing on a microporous membrane develop transepithelial resistance (TER) and potential (TEP), measured between apical and basolateral compartments. Graphs represent time dependent development of TER/TEP after seeding on the membrane for three porcine intestinal epithelial cell line clones (PSI cl.1 (yellow); PSI cl. 3 (blue); PSI cl. 9 (magenta)).
7. A 3D intestinal cell model of mature pig intestine
CLAB: This model comprises enterocytes, obtained from the adult pig (ileum???) at slaughter in Slovenia (Fig. 6a). Cells are positive for epithelial markers cytokeratins (CK) 5–18 and CK-19 (a specific marker for porcine enterocytes) and for alkaline phosphatase, and essentially negative for CK-18 (a marker for pig M-cells) and actin (marker for myofibroblasts). Some of the cells are positive for desmin (marker for cells derived from mesenchyme). Cells are strongly positive in PAS staining, indicating the presence of mucins at the surface of the cells that is one of the significant features of differentiated enterocytes and goblet cells. While epithelial in origin, these cells do not polarise in vitro (i.e. fail to generate a TER and V T). CLAB cells are weakly positive for non-specific esterases, amido-black staining and acid-phosphatase but essentially negative for markers indicating cells of myeloid origin. Upon treatment with proinflammatory cytokines the cells respond significantly in secretion of nitric oxide (NO), reactive oxygen species (ROS) production and mitochondrial dehydrogenases activity (Cencic et al., 2008a, Cencic et al., 2008b, Gradisnik et al., 2006).
Fig. 6.
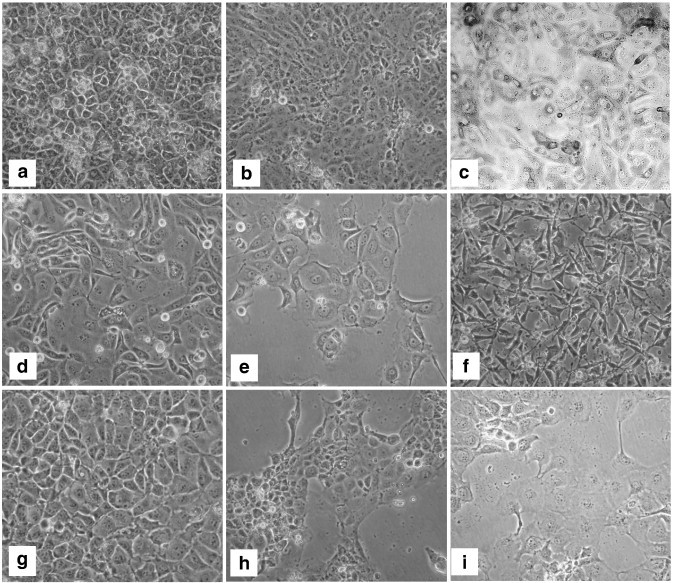
Cell lines (epithelial and monocytes/macrophages) developed in our laboratory to build 3D functional cell models of intestine: porcine (a) CLAB; (b) PSI; (c) PoM; ovine (d) OSI; goat (e) GIE; (f) GOMA; chicken (g) B1OXI; (h) COMA; human (i) TLT. Magnification 200×. PoM, GOMA and COMA are macrophages, while others are epithelial cells. All the cell lines are of non-tumorigenic origin, isolated from dissected animal tissue using limiting dilution technique.
PSI: Pig Small Intestine from the adult pig (Fig. 6b). Cells were classified in our laboratory, as cryptic, not fully differentiated intestinal epithelial cells. Cells are positive for epithelial markers cytokeratins (CK) 5–18 and weakly positive for alkaline phosphatase and PAS, but negative for CK-19 and all markers characteristic for myeloid cells, myofibroblasts or mesenchyme. PSI cells were able to form tightly packed epithelial barrier when grown on the microporous inserts with or without collagen. PSI clones give very high TER (up to 7000 Ω) and high V T (up to 40 mV) indicating a highly differentiated epithelial character with high trans-membrane transport activity. Upon treatment, cells responded in mitochondrial dehydrogenases activity and weakly for ROS and NO production (Cencic et al., 2008a, Cencic et al., 2008b, Gradisnik et al., 2006).
Among cells of immune system, PoM — pig monocytes/macrophage cell line (Fig. 6c) were established from the peripheral blood. Cells are positive for markers of myeloid cells, and functional characteristics (positive for MHC class II, membrane glycosylation patterns, presence of non-specific esterases, acid-phosphatase, amido-black staining, activation ability (phagocytosis, NO and ROS production (Fig. 6c).
Dendritic cells (DCs) were developed by an in vitro differentiation system to obtain porcine immature DCs from purified blood monocytes. Flow cytometry analysis indicated that these cells expressed the CD1 marker, major histocompatibility class (MHC) II molecules and CD80/CD86 coactivator molecules. These markers are characteristics of immature DCs (Lefevre et al., 2006).
8. Ruminant functional (3D) intestinal cell models
Among ruminants, bovine, ovine and goat functional intestinal cell models were developed. Cell lines used to build them were characterised in the same way as in the porcine model. All models consist of intestinal epithelial and macrophage cell line: bovine (CIEB — calf small intestinal epithelial cells B, obtained from a 450 kg Limousine calf and BOMA — bovine macrophages); ovine (OSI — ovine small intestinal cells (Fig. 6d); obtained from sheep, Solcavska breed and MOLT-4 — monocyte/macrophage cell line, identified at INRA, Tours (Olivier et al., 2001)); goat (GIE — goat intestinal epithelial cells (Fig. 6e) and GOMA — goat macrophages (Fig. 6f)).
In addition, we have established chicken (Fig. 6g,h), human (Fig. 6i) and horse functional (3D) model of the gut that all consist of intestinal epithelial cell line as well as the corresponding monocyte/macrophage cell line.
A few of the potential application of these cell models are outlined below.
8.1. Adhesion assays for potential probiotic/protective cultures
Industry-based consensus workshops have agreed criteria for the selection and assessment of probiotic lactic bacteria without any defined mechanistic framework (Clancy, 2003). Nevertheless, one of the most important criteria that a probiotic candidate should fulfil is to colonise the host gut enabling biological effects (Dunne et al., 1999, Edwards and Parrett, 2002, Falagas et al., 2008, Gibson et al., 2005, Myllyluoma et al., 2008, Parkes et al., 2009). Initial binding of bacteria is often mediated through the recognition of sugar moieties of the intestinal cell surface, including glycolipids and glycoproteins, as shown for Lactobacilli (Cencic; article in preparation; Cencic and Jakobsen, 2002) and for Bifidobacteria (He et al., 2001). Despite widespread use, Caco-2 and HT-29 are tumorigenic cell lines and are far from being a perfect in vitro model for studying the colonisation ability and mechanisms of probiotics–host interactions since they possess mixed large and small bowel phenotype. An important characteristic of epithelial cells is the development of apical and basolateral membrane with different structural and functional characteristics. It became clear 25 years ago that cancer cells have different sugar composition on the cell surface than do normal cells (Hakomori, 1996) thus, intestinal cancerogenic cells, express higher amount of sialic-reach carbohydrates (like sialyl Lewis X carbohydrate) than do normal cells (Alper, 2003). Therefore, it is important to note that results of attachment ability of probiotic bacteria to epithelial cells cultivated as monolayers on plastic surfaces do not give relevant data on adhesion ability in vivo. For example, in Caco-2 model where cells were grown on plastic surfaces, the Lactobacillus rhamnosus GG (LGG) or Lactobacillus casei strain Shirota was found to adhere with low capacity while for example in the normal intestinal functional cell model the same strain was found to bind almost to 50% (Jacobsen et al., 1999, Nissen et al., 2009, Roselli et al., 2006). The significant difference is found also where the adhesion assay was performed in the one dimension monolayer and functional cell model of IPEC-J2 cells (Fig. 4). The knowledge of adhesion ability and relevant receptors for probiotic/protective cultures is of utmost importance especially for industrial applications where combination of strains was used, as individual strains may compete for the same receptor and the activity would be diminished rather than enhanced and vice versa (Botic et al., 2007, Cencic et al., 2007b, Collado et al., 2005, Collado et al., 2008, Collado et al., 2006, Corr et al., 2009, Gueimonde et al., 2007, Ivec et al., 2007, Li et al., 2008, Mountzouris et al., 2009, Wine et al., 2009).
8.2. Host–beneficial bacteria–pathogen interactions
Upon reaching the intestinal tract, foodborne pathogens not only interact with the host intestinal barrier but also with commensal microorganisms that colonise intestinal epithelia. Most foodborne pathogens must cross intestinal epithelial barrier to exert their physiopathological effects and to interact with mucosa-associated lymphoid tissue (MALT) (Kerneis et al., 1997). For this reason good models of the gut should involve all these components to elucidate mechanisms of pathogen invasion and colonisation.
It was suggested that probiotic (beneficial bacteria) may alter the gut environment by downregulating the mucosal secretory response to pathogens and activate a local immune response (Clancy, 2003, Hart et al., 2002). Immune effects of probiotics in humans include the stimulation of cell-mediated immune effector functions with enhanced secretion of IFN-gamma by blood cells, enhanced phagocytosis and cell activation (Clancy, 2003). A relationship between the normal flora and the host immune system exists with a mutual dependency between the two. Successful coexistence with a complex microflora presents a particular challenge to the immune system of the host (Hart et al., 2002). It is important to elucidate the actions of probiotics in their hosts as it was shown that a combination therapy with mixture of probiotics led to inhibition of stimulatory effects exerted by individual probiotic strains (Clancy, 2003).
Many enteric pathogens translocate through M-cells or disrupted tight epithelia and use host inflammatory response to reorganise enterocytic cytoskeleton and open the paracellular pathway to enhance entrance to the MALT. Recently it was described that dendritic cells open the tight junctions between epithelial cells permitting dendrites to pass outside the epithelium to directly sample bacteria (McCormick, 2003). Enteropathogenic E. coli infection leads to significant decrease in the barrier function, loss of microvilli and altered distribution of tight junctional protein ZO-1 with accompanying increase in flux of paracellular fluid markers (Canil et al., 1993).
Viruses which enter the host via the alimentary tract (oral infection) either proliferate locally within the epithelial cells (coronaviruses, rotaviruses, Norwalk agent) or cross the mucosal layer and cause systemic infection, which may involve the CNS as in case of poliovirus (Iwasaki et al., 2002).
The pathogen-induced intracellular signalling pathways (protein kinases and their targets) are still largely undefined. In the same way, many pathogen-regulated genes of interest remain to be identified in the genome of mammals. Identification and characterisation of pathogen-regulated genes represent a considerable task to understand the evolution of the infection at the local level and to develop new ways to control these phenomena.
The mechanisms of interactions between foodborne pathogens, mammalian host and intestinal microflora, including mechanisms of microbial attachment and cross-talk with host epithelium, preventive and curative effects of probiotic bacteria still remain obscure, mainly due to restricted experimental systems and in vitro cell models. It has been previously shown that established cell lines provide a relevant in vitro model system to study intestinal pathogen–host cell interactions (Botic et al., 2007, Filipic et al., 2009, Filipic et al., 2006, Ivec et al., 2007, Nissen et al., 2009, Pipenbaher et al., 2009, Schierack et al., 2006). Epithelial cells in pig intestinal cell model were tested for susceptibility to virus infection using rotavirus, transmissible gastroenteritis virus (TGEV) and hepatitis E virus (HEV). PSI cell line was susceptible to infection with rotavirus, TGEV and in a weaker extent to the HEV. CLAB was susceptible to all porcine viruses used in the experiments. To our knowledge, we were the first to succeed in efficient propagation of HEV in intestinal cell cultures (Fig. 7 ). Preliminary tests are in progress to define the conditions that will allow high rate propagation of several strains of HEV in pig cell cultures. Tick-borne encephalitis virus (TBEV), strain Ljubljana was also shown to efficiently propagate in the pig intestinal and macrophage cells (Fig. 8 ). By using anti TBEV hyperimmune serum we were able to detect the virus infected cells 3–5 days post inoculation (Cencic and Avsic-Zupanc, 2009, Cencic et al., 2008a, Cencic et al., 2008b).
Fig. 7.
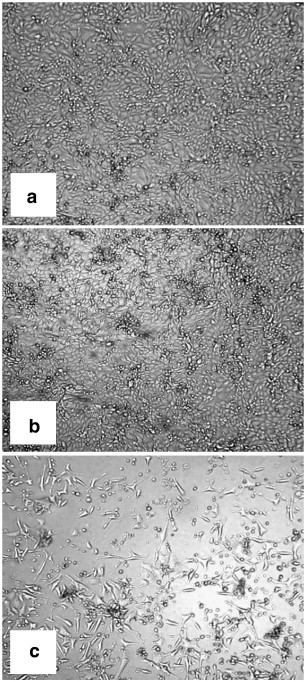
Infection of porcine monocyte/macrophage cell line PoM with hepatitis E virus (HEV): (a) PoM before inoculation; (b) control after 48 h; (c) infection with HEV after 48 h. Magnification 40×. In our laboratory developed intestinal/macrophage cell lines and 3D models of animal and human intestines can be used to study host–pathogen interactions, isolation of probiotics and for other studies in the intestine.
Fig. 8.

Infection of porcine intestinal epithelial cell line PSI with tick-borne encephalitis virus (TBEV): (a) PSI before inoculation; (b) control after 5 days; (c) infection with TBEV after 5 days. Magnification 40×. 3D models of intestine can be applied in the research of new viruses and zoonotic diseases.
9. Risk assessment of potential probiotic/protective cultures
In risk assessment the approval of strain to be probiotic requires a suitable animal model for assessing the potential enterotoxigenicity. For example: animal toxicity studies of Bacillus probiotic strains to date have found no evident toxicity in lower animals such as mice, guinea pigs and occasionally piglets (Duc le et al., 2004, Hong et al., 2005, Sorokulova et al., 2008). However, as stated above the need for testing in a more biologically relevant intestinal model remains since information on whether these animal species are able to develop diarrhea in response to ingestion of a known enterotoxic B. cereus remains unknown. Currently, the safety of probiotics containing spores of Bacillus species to date relies on tests to exclude the presence of enterotoxin genes or to demonstrate low titre production in laboratory cultures of the vegetative organisms using cell cytotoxicity or ELISA kits against particular enterotoxin proteins, combined with an absence of significant problems reported in the scientific literature. The need for an appropriate cell culture model for assessing the enterotoxicity of these organisms which has been validated in animal tests remains paramount. A reliable and validated intestinal cell model for testing the potential toxicity of the probiotic/protective strains has yet to be described. In vitro testing for cytotoxicity and adherence to the pig and human intestinal epithelia is currently carried out in our laboratory under the umbrella of SAFEFOODERA — Probiosafe project to determine if such assays correlate with the clinical findings in the pig model.
To conclude, to avoid the use of large numbers of animals, the Registration, Evaluation and Authorization of Chemicals (REACH) regulation has mandated that in vitro alternatives should be used to replace animal testing whenever possible. While the list of currently validated in vitro alternative methods is alarmingly short, there are many new methods in the process of validation that could be introduced in the very near future. Therefore, the functional intestinal cell models that have been developed for studying probiotic–pathogen–gut epithelial interactions and for the validation of the new potential drugs aims to employ these new tools to study the gut interactions and risk assessment in the future research in food microbiology as well.
Acknowledgements
This work was supported by grant from the EU-FP6 project PathogenCombat-FOOD-CT-2005-007081. The authors would like to thank Dr. Simon Hardy, from the Norwegian Veterinary School in Oslo, for the English revision of the manuscript.
References
- Alper J. Glycobiology. Turning sweet on cancer. Science. 2003;301:159–160. doi: 10.1126/science.301.5630.159. [DOI] [PubMed] [Google Scholar]
- Andoh A., Benno Y., Kanauchi O., Fujiyama Y. Recent advances in molecular approaches to gut microbiota in inflammatory bowel disease. Current Pharmaceutical Design. 2009;15:2066–2073. doi: 10.2174/138161209788489186. [DOI] [PubMed] [Google Scholar]
- Baker C.C., Phelps W.C., Lindgren V., Braun M.J., Gonda M.A., Howley P.M. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J. Virol. 1987;61:962–971. doi: 10.1128/jvi.61.4.962-971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balimane P.V., Chong S., Morrison R.A. Current methodologies used for evaluation of intestinal permeability and absorption. J. Pharmacol. Toxicol. Methods. 2000;44:301–312. doi: 10.1016/s1056-8719(00)00113-1. [DOI] [PubMed] [Google Scholar]
- Beaulieu J.F. Extracellular matrix components and integrins in relationship to human intestinal epithelial cell differentiation. Prog. Histochem. Cytochem. 1997;31:1–78. doi: 10.1016/s0079-6336(97)80001-0. [DOI] [PubMed] [Google Scholar]
- Beaulieu J.F. Integrins and human intestinal cell functions. Frontiers in Bioscience. 1999;4:D310–D321. doi: 10.2741/beaulieu. [DOI] [PubMed] [Google Scholar]
- Belanger I., Beaulieu J.F. Tenascin in the developing and adult human intestine. Histol. Histopathol. 2000;15:577–585. doi: 10.14670/HH-15.577. [DOI] [PubMed] [Google Scholar]
- Benoit Y.D., Lussier C., Ducharme P.A., Sivret S., Schnapp L.M., Basora N., Beaulieu J.F. Integrin alpha8beta1 regulates adhesion, migration and proliferation of human intestinal crypt cells via a predominant RhoA/ROCK-dependent mechanism. Biol. Cell. 2009;101:695–708. doi: 10.1042/BC20090060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berschneider H.M. 1989. Abstract of the Annual Meeting of the American Gastroenterological Association. [Google Scholar]
- Biancone L., Monteleone I., Del Vecchio Blanco G., Vavassori P., Pallone F. Resident bacterial flora and immune system. Digestive and Liver Disease. 2002;34(Suppl 2):S37–S43. doi: 10.1016/s1590-8658(02)80162-1. [DOI] [PubMed] [Google Scholar]
- Botic T., Klingberg T.D., Weingartl H., Cencic A. A novel eukaryotic cell culture model to study antiviral activity of potential probiotic bacteria. Int. J. Food Microbiol. 2007;115:227–234. doi: 10.1016/j.ijfoodmicro.2006.10.044. [DOI] [PubMed] [Google Scholar]
- Brooks S.A., Carter T.M., Royle L., Harvey D.J., Fry S.A., Kinch C., Dwek R.A., Rudd P.M. Altered glycosylation of proteins in cancer: what is the potential for new anti-tumour strategies. Anticancer Agents in Medicinal Chemistry. 2008;8:2–21. doi: 10.2174/187152008783330860. [DOI] [PubMed] [Google Scholar]
- Canil C., Rosenshine I., Ruschkowski S., Donnenberg M.S., Kaper J.B., Finlay B.B. Enteropathogenic Escherichia coli decreases the transepithelial electrical resistance of polarized epithelial monolayers. Infect. Immun. 1993;61:2755–2762. doi: 10.1128/iai.61.7.2755-2762.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Gibbs S.T., Fang L., Miller H.A., Landowski C.P., Shin H.C., Lennernas H., Zhong Y., Amidon G.L., Yu L.X., Sun D. Why is it challenging to predict intestinal drug absorption and oral bioavailability in human using rat model. Pharm. Res. 2006;23:1675–1686. doi: 10.1007/s11095-006-9041-2. [DOI] [PubMed] [Google Scholar]
- Cappello M., Keshav S., Prince C., Jewell D.P., Gordon S. Detection of mRNAs for macrophage products in inflammatory bowel disease by in situ hybridisation. Gut. 1992;33:1214–1219. doi: 10.1136/gut.33.9.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson S.A., Omary M.B., Jones B.D. Identification of cytokeratins as accessory mediators of Salmonella entry into eukaryotic cells. Life Sci. 2002;70:1415–1426. doi: 10.1016/s0024-3205(01)01512-0. [DOI] [PubMed] [Google Scholar]
- Cencic A., Avsic-Zupanc T. PathogenCombat for Safe Food: New Trends and Tools in Food Safety [Workshop]. University of Copenhagen 21st August 2009. Copenhagen; Denmark: 2009. Tick borne encephalitis virus (TBEV) [Google Scholar]
- Cencic A., Jakobsen M. vol. 42. EDP Sciences, Jouy-en-Josas; France: 2002. Functional cell model of non-tumorigenic intestinal epithelial cells IPEC-J2 to study probiotic–pathogen–gut epithelium interactions; p. 16. (Beyond Antimicrobials — the Future of Gut Microbiology (Reproduction Nutrition Development)). supl. 1. [Google Scholar]
- Cencic A. EU-WP6 Programme PathogenCombat Annual Report. 2006. www.pathogencombat.com. [Google Scholar]
- Cencic A. EU-WP6 Programme PathogenCombat Annual Report. 2007. www.pathogencombat.com. [Google Scholar]
- Cencic A. vol. 1(1) Faro; Portugal: 2007. Intestinal cell models as alternative to experimental animals in research of food and water born viruses interactions with the host: oral presentation, 8th International symposium on experimental techniques; p. 57. (Experimental Pathology and Health Sciences). [Google Scholar]
- Cencic A. EU-WP6 Programme PathogenCombat Annual Report. 2008. www.pathogencombat.com. [Google Scholar]
- Cencic A., Gradisnik L., Vaukner M., Avsic-Zupanc T., Filipic B., Rannou O., Chingwaru W., Botic T., Stopinsek N., Lefevre F. International conference of biotechnology — INCOB 2008: Programme and Abstracts. Vellore (India): School of Biotechnology, Chemical and Biomedical Engineering: Vit University, Vellore. Tamilnadu; India: 2008. Alternative to experimental animals — functional cell models with the application in the research of foodborne pathogens in the gut: plenary lecture; pp. 5–6. [Google Scholar]
- Cencic A. EU-WP6 Programme PathogenCombat Annual report. 2009. www.pathogencombat.com. [Google Scholar]
- Chen T.R., Drabkowski D., Hay R.J., Macy M., Peterson W., Jr. WiDr is a derivative of another colon adenocarcinoma cell line, HT-29. Cancer Genet. Cytogenet. 1987;27:125–134. doi: 10.1016/0165-4608(87)90267-6. [DOI] [PubMed] [Google Scholar]
- Cheng H., Leblond C.P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. American Journal of Anatomy. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- Clancy R. Immunobiotics and the probiotic evolution. FEMS Immunol. Med. Microbiol. 2003;38:9–12. doi: 10.1016/S0928-8244(03)00147-0. [DOI] [PubMed] [Google Scholar]
- Claud E.C., Savidge T., Walker W.A. Modulation of human intestinal epithelial cell IL-8 secretion by human milk factors. Pediatr. Res. 2003;53:419–425. doi: 10.1203/01.PDR.0000050141.73528.AD. [DOI] [PubMed] [Google Scholar]
- Collado M.C., Gueimonde M., Hernandez M., Sanz Y., Salminen S. Adhesion of selected Bifidobacterium strains to human intestinal mucus and the role of adhesion in enteropathogen exclusion. Journal of Food Protection. 2005;68:2672–2678. doi: 10.4315/0362-028x-68.12.2672. [DOI] [PubMed] [Google Scholar]
- Collado M.C., Jalonen L., Meriluoto J., Salminen S. Protection mechanism of probiotic combination against human pathogens: in vitro adhesion to human intestinal mucus. Asia Pacific Journal of Clinical Nutrition. 2006;15:570–575. [PubMed] [Google Scholar]
- Collado M.C., Isolauri E., Salminen S. Specific probiotic strains and their combinations counteract adhesion of Enterobacter sakazakii to intestinal mucus. FEMS Microbiol. Lett. 2008;285:58–64. doi: 10.1111/j.1574-6968.2008.01211.x. [DOI] [PubMed] [Google Scholar]
- Corr S.C., Hill C., Gahan C.G. Chapter 1 understanding the mechanisms by which probiotics inhibit gastrointestinal pathogens. Advances in Food & Nutrition Research. 2009;56:1–15. doi: 10.1016/S1043-4526(08)00601-3. [DOI] [PubMed] [Google Scholar]
- Cui M., Klopot A., Jiang Y., Fleet J.C. The effect of differentiation on 1, 25 dihydroxyvitamin D-mediated gene expression in the enterocyte-like cell line, Caco-2. J. Cell. Physiol. 2009;218:113–121. doi: 10.1002/jcp.21574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier E.S., Rogers L.B., Orenstein J.M., Baker M.D., Vossbrinck C.R., Van Gool T., Hartskeerl R., Soave R., Beaudet L.M. Characterization of Encephalitozoon (Septata) intestinalis isolates cultured from nasal mucosa and bronchoalveolar lavage fluids of two AIDS patients. J. Eukaryot. Microbiol. 1996;43:34–43. doi: 10.1111/j.1550-7408.1996.tb02470.x. [DOI] [PubMed] [Google Scholar]
- Duc le H., Hong H.A., Barbosa T.M., Henriques A.O., Cutting S.M. Characterization of Bacillus probiotics available for human use. Appl. Environ. Microbiol. 2004;70:2161–2171. doi: 10.1128/AEM.70.4.2161-2171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne C., Murphy L., Flynn S., O'Mahony L., O'Halloran S., Feeney M., Morrissey D., Thornton G., Fitzgerald G., Daly C., Kiely B., Quigley E.M., O'Sullivan G.C., Shanahan F., Collins J.K. Probiotics: from myth to reality. Demonstration of functionality in animal models of disease and in human clinical trials. Antonie Van Leeuwenhoek. 1999;76:279–292. [PubMed] [Google Scholar]
- Dydensborg A.B., Teller I.C., Basora N., Groulx J.F., Auclair J., Francoeur C., Escaffit F., Pare F., Herring E., Menard D., Beaulieu J.F. Differential expression of the integrins alpha6Abeta4 and alpha6Bbeta4 along the crypt-villus axis in the human small intestine. Histochem. Cell Biol. 2009;131:531–536. doi: 10.1007/s00418-008-0547-z. [DOI] [PubMed] [Google Scholar]
- Edwards C.A., Parrett A.M. Intestinal flora during the first months of life: new perspectives. Br. J. Nutr. 2002;88(Suppl 1):S11–S18. doi: 10.1079/BJN2002625. [DOI] [PubMed] [Google Scholar]
- Falagas M.E., Rafailidis P.I., Makris G.C. Bacterial interference for the prevention and treatment of infections. International Journal of Antimicrobial Agents. 2008;31:518–522. doi: 10.1016/j.ijantimicag.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Falk P., Boren T., Normark S. Characterization of microbial host receptors. Methods Enzymol. 1994;236:353–374. doi: 10.1016/0076-6879(94)36027-8. [DOI] [PubMed] [Google Scholar]
- Falk P.G., Hooper L.V., Midtvedt T., Gordon J.I. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol. Mol. Biol. Rev. 1998;62:1157–1170. doi: 10.1128/mmbr.62.4.1157-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipic B., Gradisnik L., Botic T., Sladoljev S., Toth S., Somogyvari F., Pipenbaher N., Cencic A., Koren S. Use of calf intestinal epithelial (CIEB) cells to measure the biological activity of human interferons. In: Schwarzmeier J.D., editor. 6th International Cytokine Conference. Bologna: Medimond International Proceedings. Vienna; Austria: 2006. pp. 41–45. [Google Scholar]
- Filipic B., Gottstein Z., Sladoljev S., Cencic A., Koren S., Mazija H. Multiplication of HVT FC-126 [cherpesvirus turkey] Marek's disease virus in cells of nonavian origin. In: Balenovic M., editor. Peradarski dani 2009. Zbornik. Centar za peradarstvo; Zagreb: 2009. pp. 64–69. [Google Scholar]
- Geddes K., Philpott D.J. A new role for intestinal alkaline phosphatase in gut barrier maintenance. Gastroenterology. 2008;135:8–12. doi: 10.1053/j.gastro.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Gibson G.R., McCartney A.L., Rastall R.A. Prebiotics and resistance to gastrointestinal infections. Br. J. Nutr. 2005;93(Suppl 1):S31–S34. doi: 10.1079/bjn20041343. [DOI] [PubMed] [Google Scholar]
- Gradisnik L., Filipic B., Vaureix C., Lefevre F., La Bonnardiere C., Cencic A. Establishment of a functional cell culture model of the pig small intestine. ALTEX, Alternativen zu Tierexperimenten. 2006;23(2):94. [Google Scholar]
- Guarner F. Enteric flora in health and disease. Digestion. 2006;73(Suppl 1):5–12. doi: 10.1159/000089775. [DOI] [PubMed] [Google Scholar]
- Gueimonde M., Margolles A., de los Reyes-Gavilan C.G., Salminen S. Competitive exclusion of enteropathogens from human intestinal mucus by Bifidobacterium strains with acquired resistance to bilea preliminary study. Int. J. Food Microbiol. 2007;113:228–232. doi: 10.1016/j.ijfoodmicro.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Gupta V., Garg R. Probiotics. Indian Journal of Medicinal Microbiology. 2009;27:202–209. doi: 10.4103/0255-0857.53201. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- Hart A.L., Stagg A.J., Frame M., Graffner H., Glise H., Falk P., Kamm M.A. The role of the gut flora in health and disease, and its modification as therapy. Aliment. Pharmacol. Ther. 2002;16:1383–1393. doi: 10.1046/j.1365-2036.2002.01310.x. [DOI] [PubMed] [Google Scholar]
- He F., Ouwehan A.C., Hashimoto H., Isolauri E., Benno Y., Salminen S. Adhesion of Bifidobacterium spp. to human intestinal mucus. Microbiol. Immunol. 2001;45:259–262. doi: 10.1111/j.1348-0421.2001.tb02615.x. [DOI] [PubMed] [Google Scholar]
- Henle G., Deinhardt F. The establishment of strains of human cells in tissue culture. J. Immunol. 1957;79:54–59. [PubMed] [Google Scholar]
- Hong H.A., Duc le H., Cutting S.M. The use of bacterial spore formers as probiotics. FEMS Microbiol. Rev. 2005;29:813–835. doi: 10.1016/j.femsre.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Hsiao W.W., Metz C., Singh D.P., Roth J. The microbes of the intestine: an introduction to their metabolic and signaling capabilities. Endocrinology Metabolism Clinics of North America. 2008;37:857–871. doi: 10.1016/j.ecl.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussa R.O., Fein H.G., Pattillo R.A., Nagelberg S.B., Rosen S.W., Weintraub B.D., Perini F., Ruddon R.W., Cole L.A. A distinctive form of human chorionic gonadotropin beta-subunit-like material produced by cervical carcinoma cells. Cancer Res. 1986;46:1948–1954. [PubMed] [Google Scholar]
- Ivec M., Botic T., Koren S., Jakobsen M., Weingartl H., Cencic A. Interactions of macrophages with probiotic bacteria lead to increased antiviral response against vesicular stomatitis virus. Antiviral Res. 2007;75:266–274. doi: 10.1016/j.antiviral.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Iwasaki A., Welker R., Mueller S., Linehan M., Nomoto A., Wimmer E. Immunofluorescence analysis of poliovirus receptor expression in Peyer's patches of humans, primates, and CD155 transgenic mice: implications for poliovirus infection. J. Infect. Dis. 2002;186:585–592. doi: 10.1086/342682. [DOI] [PubMed] [Google Scholar]
- Jacobsen C.N., Rosenfeldt Nielsen V., Hayford A.E., Moller P.L., Michaelsen K.F., Paerregaard A., Sandstrom B., Tvede M., Jakobsen M. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 1999;65:4949–4956. doi: 10.1128/aem.65.11.4949-4956.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal F., Jumarie C., Bawab W., Corbeil D., Malo C., Berteloot A., Crine P. Polarized distribution of neutral endopeptidase 24.11 at the cell surface of cultured human intestinal epithelial Caco-2 cells. Biochem. J. 1992;288(Pt 3):945–951. doi: 10.1042/bj2880945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerneis S., Bogdanova A., Kraehenbuhl J.P., Pringault E. Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:949–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- Klingberg T.D., Pedersen M.H., Cencic A., Budde B.B. Application of measurements of transepithelial electrical resistance of intestinal epithelial cell monolayers to evaluate probiotic activity. Appl. Environ. Microbiol. 2005;71:7528–7530. doi: 10.1128/AEM.71.11.7528-7530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavappa K.S., Macy M.L., Shannon J.E. Examination of ATCC stocks for HeLa marker chromosomes in human cell lines. Nature. 1976;259:211–213. doi: 10.1038/259211a0. [DOI] [PubMed] [Google Scholar]
- Lee I.J., Hom K., Bai G., Shapiro M. NMR metabolomic analysis of caco-2 cell differentiation. Journal of Proteome Research. 2009;8:4104–4108. doi: 10.1021/pr8010759. [DOI] [PubMed] [Google Scholar]
- Lefevre F., editor. EU-WP6 Programme PathogenCombat Annual Report. 2006. www.pathogencombat.com. [Google Scholar]
- Levy E., Menard D., Delvin E., Montoudis A., Beaulieu J.F., Mailhot G., Dube N., Sinnett D., Seidman E., Bendayan M. Localization, function and regulation of the two intestinal fatty acid-binding protein types. Histochem. Cell Biol. 2009;132:351–367. doi: 10.1007/s00418-009-0608-y. [DOI] [PubMed] [Google Scholar]
- Li X.J., Yue L.Y., Guan X.F., Qiao S.Y. The adhesion of putative probiotic lactobacilli to cultured epithelial cells and porcine intestinal mucus. J. Appl. Microbiol. 2008;104:1082–1091. doi: 10.1111/j.1365-2672.2007.03636.x. [DOI] [PubMed] [Google Scholar]
- Lu L., Bao Y., Khan A., Goldstein A.M., Newburg D.S., Quaroni A., Brown D., Walker W.A. Hydrocortisone modulates cholera toxin endocytosis by regulating immature enterocyte plasma membrane phospholipids. Gastroenterology. 2008;135(185–193):e181. doi: 10.1053/j.gastro.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Khan A., Walker W.A. ADP-ribosylation factors regulate the development of CT signaling in immature human enterocytes. American Journal of Physiology — Gastrointestinal and Liver Physiology. 2009;296:G1221–G1229. doi: 10.1152/ajpgi.90686.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunney J.K. Advances in swine biomedical model genomics. International Journal of Biological Sciences. 2007;3:179–184. doi: 10.7150/ijbs.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier C., Basora N., Bouatrouss Y., Beaulieu J.F. Integrins as mediators of epithelial cell–matrix interactions in the human small intestinal mucosa. Microsc. Res. Tech. 2000;51:169–178. doi: 10.1002/1097-0029(20001015)51:2<169::AID-JEMT8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Marian B. In vitro models for the identification and characterization of tumor-promoting and protective factors for colon carcinogenesis. Food Chem. Toxicol. 2002;40:1099–1104. doi: 10.1016/s0278-6915(02)00061-3. [DOI] [PubMed] [Google Scholar]
- McCormick B.A. The use of transepithelial models to examine host–pathogen interactions. Current Opinion in Microbiology. 2003;6:77–81. doi: 10.1016/s1369-5274(02)00003-6. [DOI] [PubMed] [Google Scholar]
- Moue M., Tohno M., Shimazu T., Kido T., Aso H., Saito T., Kitazawa H. Toll-like receptor 4 and cytokine expression involved in functional immune response in an originally established porcine intestinal epitheliocyte cell line. Biochim. Biophys. Acta. 2008;1780:134–144. doi: 10.1016/j.bbagen.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Mountzouris K.C., Balaskas C., Xanthakos I., Tzivinikou A., Fegeros K. Effects of a multi-species probiotic on biomarkers of competitive exclusion efficacy in broilers challenged with Salmonella enteritidis. Br. Poult. Sci. 2009;50:467–478. doi: 10.1080/00071660903110935. [DOI] [PubMed] [Google Scholar]
- Myllyluoma E., Ahonen A.M., Korpela R., Vapaatalo H., Kankuri E. Effects of multispecies probiotic combination on Helicobacter pylori infection in vitro. Clinical and Vaccine Immunology. 2008;15:1472–1482. doi: 10.1128/CVI.00080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanthakumar N.N., Fusunyan R.D., Sanderson I., Walker W.A. Inflammation in the developing human intestine: a possible pathophysiologic contribution to necrotizing enterocolitis. Proceedings of the National Academy of Sciences U S A. 2000;97:6043–6048. doi: 10.1073/pnas.97.11.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niku M., Ekman A., Pessa-Morikawa T., Iivanainen A. Identification of major cell types in paraffin sections of bovine tissues. BMC Veterinary Research. 2006;2:5. doi: 10.1186/1746-6148-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen L., Chingwaru W., Sgorbati B., Biavati B., Cencic A. Gut health promoting activity of new putative probiotic/protective Lactobacillus spp. strains: a functional study in the small intestinal cell model. Int. J. Food Microbiol. 2009;135:288–294. doi: 10.1016/j.ijfoodmicro.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Nurieva R.I., Liu X., Dong C. Yin-Yang of costimulation: crucial controls of immune tolerance and function. Immunol. Rev. 2009;229:88–100. doi: 10.1111/j.1600-065X.2009.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier M., Berthon P., Chastang J., Cordier G., Lantier F. Establishment and characterisation of ovine blood monocyte-derived cell lines. Vet. Immunol. Immunopathol. 2001;82:139–151. doi: 10.1016/s0165-2427(01)00330-0. [DOI] [PubMed] [Google Scholar]
- Pageot L.P., Perreault N., Basora N., Francoeur C., Magny P., Beaulieu J.F. Human cell models to study small intestinal functions: recapitulation of the crypt-villus axis. Microsc. Res. Tech. 2000;49:394–406. doi: 10.1002/(SICI)1097-0029(20000515)49:4<394::AID-JEMT8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Panja A. A novel method for the establishment of a pure population of nontransformed human intestinal primary epithelial cell (HIPEC) lines in long term culture. Lab. Invest. 2000;80:1473–1475. doi: 10.1038/labinvest.3780154. [DOI] [PubMed] [Google Scholar]
- Parkes G.C., Sanderson J.D., Whelan K. The mechanisms and efficacy of probiotics in the prevention of Clostridium difficile-associated diarrhoea. The Lancet Infectious Diseases. 2009;9:237–244. doi: 10.1016/S1473-3099(09)70059-3. [DOI] [PubMed] [Google Scholar]
- Patsos G., Corfield A. Management of the human mucosal defensive barrier: evidence for glycan legislation. Biological Chemistry. 2009;390:581–590. doi: 10.1515/BC.2009.052. [DOI] [PubMed] [Google Scholar]
- Pattillo R.A., Hussa R.O., Story M.T., Ruckert A.C., Shalaby M.R., Mattingly R.F. Tumor antigen and human chorionic gonadotropin in CaSki cells: a new epidermoid cervical cancer cell line. Science. 1977;196:1456–1458. doi: 10.1126/science.867042. [DOI] [PubMed] [Google Scholar]
- Penna F.J., Peret L.A., Vieira L.Q., Nicoli J.R. Probiotics and mucosal barrier in children. Current Opinion in Clinical Nutrition & Metabolic Care. 2008;11:640–644. doi: 10.1097/MCO.0b013e32830a70ab. [DOI] [PubMed] [Google Scholar]
- Pipenbaher N., Moeller P.L., Dolinsek J., Jakobsen M., Weingartl H., Cencic A. Nitric oxide (NO) production in mammalian non-tumorigenic epithelial cells of the small intestine and macrophages induced by individual strains of lactobacilli and bifidobacteria. International Dairy Journal. 2009;19:166–171. [Google Scholar]
- Quaroni A., Beaulieu J.F. Cell dynamics and differentiation of conditionally immortalized human intestinal epithelial cells. Gastroenterology. 1997;113:1198–1213. doi: 10.1053/gast.1997.v113.pm9322515. [DOI] [PubMed] [Google Scholar]
- Quaroni A., Isselbacher K.J. Cytotoxic effects and metabolism of benzo[a]pyrene and 7, 12-dimethylbenz[a]anthracene in duodenal and ileal epithelial cell cultures. J. Natl Cancer Inst. 1981;67:1353–1362. [PubMed] [Google Scholar]
- Quaroni A., Isselbacher K.J., Ruoslahti E. Fibronectin synthesis by epithelial crypt cells of rat small intestine. Proceedings of the National Academy of Sciences U S A. 1978;75:5548–5552. doi: 10.1073/pnas.75.11.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli M., Finamore A., Britti M.S., Mengheri E. Probiotic bacteria Bifidobacterium animalis MB5 and Lactobacillus rhamnosus GG protect intestinal Caco-2 cells from the inflammation-associated response induced by enterotoxigenic Escherichia coli K88. Br. J. Nutr. 2006;95:1177–1184. doi: 10.1079/bjn20051681. [DOI] [PubMed] [Google Scholar]
- Schierack P., Nordhoff M., Pollmann M., Weyrauch K.D., Amasheh S., Lodemann U., Jores J., Tachu B., Kleta S., Blikslager A., Tedin K., Wieler L.H. Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochem. Cell Biol. 2006;125:293–305. doi: 10.1007/s00418-005-0067-z. [DOI] [PubMed] [Google Scholar]
- Schnabl K.L., Field C., Clandinin M.T. Ganglioside composition of differentiated Caco-2 cells resembles human colostrum and neonatal rat intestine. Br. J. Nutr. 2009;101:694–700. doi: 10.1017/S0007114508048289. [DOI] [PubMed] [Google Scholar]
- Sharma M.D., Leite de Moraes M., Zavala F., Pontoux C., Papiernik M. Induction and inhibition of CD40–CD40 ligand interactions: a new strategy underlying host–virus relationships. J. Immunol. 1998;161:5357–5365. [PubMed] [Google Scholar]
- Shin K., Fogg V.C., Margolis B. Tight junctions and cell polarity. Annual Review of Cell and Developmental Biology. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- Sorokulova I.B., Pinchuk I.V., Denayrolles M., Osipova I.G., Huang J.M., Cutting S.M., Urdaci M.C. The safety of two Bacillus probiotic strains for human use. Dig. Dis. Sci. 2008;53:954–963. doi: 10.1007/s10620-007-9959-1. [DOI] [PubMed] [Google Scholar]
- Suntharalingam G., Perry M.R., Ward S., Brett S.J., Castello-Cortes A., Brunner M.D., Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Bucana C.D., Liu W., Yoneda J., Kitadai Y., Cleary K.R., Ellis L.M. Platelet-derived endothelial cell growth factor in human colon cancer angiogenesis: role of infiltrating cells. J. Natl Cancer Inst. 1996;88:1146–1151. doi: 10.1093/jnci/88.16.1146. [DOI] [PubMed] [Google Scholar]
- Tremblay E., Auclair J., Delvin E., Levy E., Menard D., Pshezhetsky A.V., Rivard N., Seidman E.G., Sinnett D., Vachon P.H., Beaulieu J.F. Gene expression profiles of normal proliferating and differentiating human intestinal epithelial cells: a comparison with the Caco-2 cell model. J. Cell. Biochem. 2006;99:1175–1186. doi: 10.1002/jcb.21015. [DOI] [PubMed] [Google Scholar]
- Tortora G., Derrickson B. Principles of Anatomy and Physiology. 12th ed. Wiley; 2008. [Google Scholar]
- Uchida M., Fukazawa T., Yamazaki Y., Hashimoto H., Miyamoto Y. A modified fast (4 day) 96-well plate Caco-2 permeability assay. J. Pharmacol. Toxicol. Methods. 2009;59:39–43. doi: 10.1016/j.vascn.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Visco V., Bava F.A., d'Alessandro F., Cavallini M., Ziparo V., Torrisi M.R. Human colon fibroblasts induce differentiation and proliferation of intestinal epithelial cells through the direct paracrine action of keratinocyte growth factor. J. Cell. Physiol. 2009;220:204–213. doi: 10.1002/jcp.21752. [DOI] [PubMed] [Google Scholar]
- Whitehead R.H., VanEeden P.E., Noble M.D., Ataliotis P., Jat P.S. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proceedings of the National Academy of Sciences U S A. 1993;90:587–591. doi: 10.1073/pnas.90.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wine E., Gareau M.G., Johnson-Henry K., Sherman P.M. Strain-specific probiotic (Lactobacillus helveticus) inhibition of Campylobacter jejuni invasion of human intestinal epithelial cells. FEMS Microbiol. Lett. 2009;300:146–152. doi: 10.1111/j.1574-6968.2009.01781.x. [DOI] [PubMed] [Google Scholar]
- Wood S.R., Zhao Q., Smith L.H., Daniels C.K. Altered morphology in cultured rat intestinal epithelial IEC-6 cells is associated with alkaline phosphatase expression. Tissue Cell. 2003;35:47–58. doi: 10.1016/s0040-8166(02)00103-9. [DOI] [PubMed] [Google Scholar]
- Yu Q.H., Yang Q. Diversity of tight junctions (TJs) between gastrointestinal epithelial cells and their function in maintaining the mucosal barrier. Cell Biol. Int. 2009;33:78–82. doi: 10.1016/j.cellbi.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Zoumpopoulou G., Tsakalidou E., Dewulf J., Pot B., Grangette C. Differential crosstalk between epithelial cells, dendritic cells and bacteria in a co-culture model. Int. J. Food Microbiol. 2009;131:40–51. doi: 10.1016/j.ijfoodmicro.2008.12.037. [DOI] [PubMed] [Google Scholar]


