Highlights
-
•
Three Pseudomonas-derived lectins: PFL, PML, and PTL, have been examined for anti-influenza virus activity against several strains of influenza virus.
-
•
These lectins would bind high-mannose glycan and blocked the entry of influenza virus into the host cells.
-
•
It is expected that these lectins could have an antiviral activity against not only influenza virus but also other enveloped viruses including HIV as described by many other studies.
-
•
These three lectins will be applicable to a novel microbicide.
Abbreviations: m.o.i, multiplicity of infection; hpi, hour post-infection; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; IPTG, isopropyl β-d-thiogalactoside
Keywords: Pseudomonas-derived lectin, Mannose binding, Antiviral activity, Influenza virus
Abstract
Lectin PFL binding high-mannose glycan derived from Pseudomonas fluorescens and other homologous lectins: PML derived from Pseudomonas mandelii and PTL derived from Pseudomonas taiwanensis were examined for antiviral activity. The cDNA of these lectin genes were synthesized, cloned, expressed in Escherichia coli. The expressed lectins were purified by gel filtrations, and supplied to cultures infected with several strains of influenza virus. These three lectins have inhibited propagation of influenza viruses with a similar extent, 50% of inhibition-dose was around ten nanomolar concentration. An immunofluorescent microscopy, a microarray analysis, and several infection experiments with different time periods of lectin addition or using the competitor substrates indicated that binding of these lectins with high-mannose glycan on HA protein of influenza virus could block the virus entry into the host cells, thereby resulting in inhibition of the virus propagation. These Pseudomonas-derived lectins would be protential and attractive antiviral agents targeting glycoproteins of enveloped viruses including influenza virus.
1. Introduction
Lectins are glycoprotein- or glycolipid-associated carbohydrate-binding proteins. These proteins can be found in many kinds of organisms, including prokaryotes, sea corals, algae, fungi, plants, invertebrates, and vertebrates (François and Balzarini, 2012). Lectins are involved in many biological processes, host-pathogen interactions, cell–cell communication, induction of apoptosis, cancer metastasis and differentiation, targeting of cells, as well as recognizing and binding carbohydrates (Huskens and Schols, 2012). These lectins are characterized by different carbohydrate-binding specificities, including mannose, glucose, galactose, N-acetylglucosamine, N-acetylgalactosamine, sialic acid and fucose (Balzarini, 2007). As for mannose-specificity in mammals, mannose-binding lectin (MBL) in the serum, characterized as C-type lectin, is well known to function for pathogen recognition in the innate immune system. Binding of this lectin with foreign surface molecule or pathogen can activate the complement cascade as lectin pathway and/or lead directly to phagocytosis of microorganisms via macrophage mannose receptor (Weis et al., 1998, Mason and Tarr, 2015).
N-linked glycans are added co-translationally to newly synthesized polypeptides in the endoplasmic reticulum (ER). The N-linked glycan is assembled as a high-mannose type glycan. In the ER, the addition of these glycans on the native peptide plays a pivotal role in protein folding. The correctly folded protein then migrates to the Golgi apparatus, where glycosidases and glycosyltransferases process the glycans by trimming and adding new sugars, creating hybrid and complex type glycans (Helenius and Aebi, 2001, Huskens and Schols, 2012). Therefore, mannose is not common in terminal positions of N-linked glycan on surface proteins of mammalian cells but is frequently found on the surfaces of microorganisms (Weis et al., 1998). The mannose can be one of target molecules, which are recognized and excluded as an alien in the mammalian cells.
In tumor cells, it is known that an N-linked glycan on a specific tumor antigen (like epidermal growth factor receptor) is transported to the surface with high-mannose form once its overexpression causes a specific glycosylation pathway to become saturated (Johns et al., 2005). N-linked glycan on structural surface protein of a matured enveloped virus contains high-mannose form, its limited oligosaccharide processing is due to the inaccessibility of the relevant enzymes to the site (Mir-Shekari et al., 1997). It was reported that high-mannose form of N-linked glycan was present on glycoproteins of other enveloped viruses, including human immunodeficiency virus (HIV), and hepatitis C virus, (Leonard et al., 1990, Iacob et al., 2008). Glycoproteins in the envelope of many different viruses play an instrumental role in virus entry. Lectins binding to glycan of the viral glycoprotein, therefore, should inhibit viral entry into its host cells, thereby, being considered as attractive targets for antiviral drug research (Balzarini, 2007).
Recently, antiviral activities of those lectins have been widely investigated specially in algal lectins: cyanovirin-N (CV-N) derived from cynobacterium Nostoc ellipsosporum, cynobacterium Oscillatoria agardhii agglutinin (OAA), Burkholderia oklahomensis agglutinin (BOA) and griffithsin (GRFT) from a red alga Griffithsia sp. (Balzarini, 2007, François and Balzarini, 2012, Huskens and Schols, 2012, Whitley et al., 2013). These lectins that specifically bind to high-mannose glycan on viral envelope have been shown to inhibit infection of HIV (Boyd et al., 1997, Mori et al., 2005, Férir et al., 2014) as well as other enveloped viruses, including influenza virus (O’Keefe et al., 2003), hepatitis C virus (Helle et al., 2006, Kachko et al., 2013), Ebola virus (Barrientos et al., 2003, Barrientos et al., 2004), herpesvirus 6 (Dey et al., 2000), measles virus (Dey et al., 2000), coronaviruses (van der Meer et al., 2007, O’Keefe et al., 2010), and Japanese encephalitis virus (Ishag et al., 2013). Also in our studies, KAA-2 from a red alga Kappaphycus alvarezii, ESA-2 from red algae Eucheuma serra, BCA from a green alga Boodlea coacta, OAA from a cyanobacterium, and PFL from bacterium Peudomonas fluorescence have been shown to inhibit infection of HIV and/or influenza viruses (Sato et al., 2007, Sato et al., 2011a, Sato et al., 2011b, Sato et al., 2012, Sato et al., 2015).
In this study, we have found homologous genes from other Pseudomonas species, PML from Pseudomonas mandelii and PTL from Pseudomonas taiwanensis, and cloned both genes. All three lectins including PFL from Pseudomonas fluorescence have been produced and purified as recombinant proteins in Escherichia coli (E. coli), and examined for antiviral activity against several strains of Influenza virus.
2. Materials and methods
2.1. Viruses and cells
Virus stocks of influenza A/H1N1 subtype: FM/1/47, Bangkok/10/83, Beijing/262/95, Oita/OU1P3-3/09, and A/H3N2 subtype: Udorn/72, Aichi/2/68, and B/Ibaragi/2/85 were used for virus infection. All strains were grown in the chorioallantonic fluid of 10-day-old chicken eggs. The aliquots of each virus preparation were stored at −80 °C until use. Human lung carcinoma, NCI-H292 cells (ATCC CRL1848) or A549 cells (ATCC CCL185) were used for the host cells. These cells were grown in RPMI-1640 supplemented with 10% fetal bovine serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin (GIBCO, NY, USA). Influenza HA vaccine “SEIKEN” containing a mixture of A/California/7/2009 (H1N1), A/Victoria/210/2009 (H3N2), and B/Brisbane/60/2008 was used as HA protein preparation (Denka-Seiken, Tokyo, Japan).
2.2. cDNA cloning of PML and PTL genes and purification of PML and PTL proteins
Homologous proteins of PFL (lectin OAA [Pseudomonas fluorescens]: accession no. WP_011332171.1; the complete genome: accession no. NC_007492.2) were searched by BLAST analysis. Two homologous proteins were picked with Pseudomonas mandelii hypothetical protein: accession no. AHZ70030.1 (the complete genome: accession no. CP005960.1) (termed as PML) and Pseudomonas taiwanensis hypothetical protein: accession no. WP_027907962 (the complete genome: accession no. NZ_KE384450.1) (termed as PTL). The cDNAs (PML, PTL) of the corresponding lectin genes (402 bases length) were chemically made by the GeneArt (Life technologies Inc., Tokyo, Japan). The cDNAs were amplified by Taq polymerase using primers with PML-F: 5-CACCATGTCTAGATACGTAGTG-3 (underlined ATG is start codon of the PML gene) and PML-R: 5-TTACTCTATCTGCCCACGAAAG-3 (underlined TTA is complementary codon of TAA stop codon of the PML gene) for PML gene, or primers with PTL-F: 5-CACCATGTTCAAGTACGCAGTGGA-3 (underlined ATG is start codon of the PTL gene) and PTL-R: 5-CTAACCGAGCAGGCTGCGGAAG-3 (underlined CTA is complementary codon of TAG stop codon of the PTL gene) for PTL gene. The amplified cDNAs were cloned into pET101/D-TOPO vector and transformed in E.coli K12 TOP10 strain by Champion pET Directional TOPO Expression kit (Invitrogen, CA, USA). The colonies harboring the cloned vector were grown, and the vectors were examined for the existence of the desired correct cDNA. The desired clones were named as pET-PML from Pseudomonas mandelii and pET-PTL from Pseudomonas taiwanensis, respectively. The obtained lectin genes were confirmed for the absence of any mutations by DNA sequencing. Cloning of pET-PFL from Pseudomonas fluorescens was previously described (Sato et al., 2012).
To expression the lectin proteins, the plasmids were transformed into E. coli K12 BL21 Star (DE3) strain (Invitrogen, CA, USA). The transformed E. coli cells were grown and inducibly expressed under the lac operon with addition of 0.8 mM IPTG. After 6 h incubation at 37 °C, the E. coli cells were collected by centrifugation at 5800 g for 20 min, were lysed with SDS-PAGE lysis buffer, and were subjected with 10–15% gel electrophoresis for examining the existence of the desired lectin proteins. For purification of the lectins: PML and PTL, the E. coli cells were suspended in phosphate buffered saline (PBS: 20 mM phosphate buffer containing 0.15 M NaCl, pH 7.0), then were disrupted by sonication, and were subjected to Superose 12 column (GE Healthcare, Tokyo, Japan) as described by our previous PFL purification (Sato et al., 2012).
2.3. Virus infection and detection of the inhibition by lectins
NCI-H292 cells grown in 48-wells plate were infected with several strains of influenza virus including A/H1N1/Oita/OU1P3-3/09 and A/H3N2/Udorn/72 at an m.o.i. of 2.5. The lectins were added prior to, simultaneously with, or with indicated times of virus exposure into the cell cultures. At 24 hpi, the infected cells were fixed with 80% acetone, and stained with 0.5% amide black in 45% ethanol and 10% acetic acid. The stained plates were pictured with a gray scale. The color densities of the pictures were quantitated by densitometry with the NHI-ImageJ 1.48 v software. The infected cell cultures in the absence of lectin exhibited severe cytopathic effect, almost cells on the wells were gone, in which percent of the cell viability was shown as 0%. On the other hand, cells in the mock-infected cell cultures were intact, in which percent of the cell viability was shown as 100%.
2.4. Direct immunofluorescence of virus-infected cells
The virus-infected cells were fixed with 80% acetone at 8 hpi, and stained with a diluted fluorescein isothiocyanate (FITC)-conjugated influenza A NP monoclonal antibody (D67J) (Pierce-Antibodies, MA, USA). The pictures were taken under fluorescence microscope (OLYMPUS CKX41, Tokyo, Japan) with ×400 magnification.
2.5. Microarray analysis of virus-infected cells in the presence and absence of PFL lectin
A549 cells were infected with A/H3N2/Udorn/72 virus at an m.o.i. of 2.5 in the presence or absence of 200 nM PFL. At 4 hpi, total RNAs of the virus-infected cells were extracted by the RNeasy Kit (Qiagen Inc., CA, USA). A part of the RNAs were subjected with Agilent Expression Array SurePrint G3 Human Gene Expression 8 × 60k ver. 2 (Agilent Technologies, CA, USA). The expression levels of mRNAs were analyzed by using approximately 50,000 probes. Furthermore, Keyword Gene Analysis in “data mining light” software was performed (Takara Bio Inc.; Shiga; Japan). Among them; human 410 genes were picked up by a keyword searching “virus infection” in Entrez Gene Database (NCBI). Among these 410 genes; the expression levels of mRNAs were compared and discussed for the interaction of the lectin with virus infection.
3. Results and discussion
3.1. Cloning of lectins derived from genus Pseudomonas and their expression in E. coli
In our previous study, a lectin PFL derived from Pseudomonas fluorescens Pf0-1 has been demonstrated to block infection by influenza viruses (Sato et al., 2012). Carbohydrate-binding specificity of the PFL was determined by a glycan array analysis. It exhibited exclusive specificity for high-mannose glycan with α1-3 Man that was highly exposed at the D2 position. Our prior data suggested that binding of the PFL with high-mannose glycan on HA glycoprotein of influenza virus would interrupt the virus entry into the host cells. Homologous search of the PFL using BLAST (basic local alignment search tool) has found a hypothetical protein OU5_2951 from Pseudomonas mandelii JR-1 (termed PML) and a lectin from Pseudomonas taiwanensis (termed PTL) with 96% and 72% homology, respectively, compared with the PFL. The amino acid sequences (133 amino acids in length) compared with that of the PFL were shown in Fig. 1 A. Molecular weight of PFL, PML, and PTL was estimated as 14,012, 14,070, and 14,102, respectively. The corresponding gene-regions were detected in their complete genomes deposited in GenBank, they comprised 402 bases (Fig. 1B). The nucleotide homology of PML and PTL genes with PFL gene was 88% and 73%, respectively. The actual expression levels for these lectin genes and the original intrinsic functions of these lectins still remain unclear.
Fig. 1.
Comparisons of amino acid sequence (A) and nucleotide sequence (B) of genus Pseudomonas-derived lectins: PFL, PML, and PTL.
PFL shows Pseudomonas fluorescens Pf0-1 derived lectin (gene accession no. WP_011332171.1, protein accession no. NC_007492.2). PML shows Pseudomonas mandelii derived lectin (gene accession no. AHZ70030.1, protein accession no. CP005960.1). PTL shows Pseudomonas taiwanensis derived lectin (gene accession no. WP_027907962, protein accession no. NZ_KE384450.1). The identical base and amino acid were shown as asterisks.
In this study, we have synthesized the cDNAs of the corresponding genes of PML and PTL, cloned the genes, expressed the proteins in E. coli, and finally purified PML and PTL as lectin preparations for examining their anti-influenza activity. Each lectin preparation has been shown as a single band with an apparent mobility of 12 kDa on SDS-PAGE (Fig. 2 ).
Fig. 2.
SDS-PAGE of purified lectins: PFL, PML, and PTL.
PFL, PML, and PTL, purified by gel filtration on Superose 12 column, were subjected with 10–15% linear gradient gel electrophoresis. The gel was strained with coomassie brilliant blue.
3.2. Inhibition of influenza virus infection by lectins: PFL, PML, and PTL
To examine anti-influenza activity of the lectins: PFL, PML, and PTL, infections with several strains of influenza virus were performed using human lung carcinoma cells, NCI-H292, in the presence or absence of 100 nM lectin. The NCI-H292 cells exert a profound cytopathic effect by influenza virus, therefore, a high m.o.i. infection results in death of almost all cells in the culture until 24 hpi. Under these infections with A/H1N1 subtypes: FM/1/47, Bangkok/10/83, Beijing/262/95, and Oita/OU1P3-3/09, or A/H3N2 subtypes: Udorn/72 and Aichi/2/68, or B/Ibaragi/2/85, almost all cells were dead at 24 hpi (except for B/Ibaragi/2/85 strain, it required 48 h). An addition of each 100 nM lectin simultaneously with virus exposure in to the culture promoted survival of the cells (Fig. 3 ). Here we demonstrated that these lectins: PFL, PML, and PTL, were potently active against all tested strains of influenza virus.
Fig. 3.
Anti-influenza virus activity by lectins: PFL, PML, and PTL.
NCI-H292 cells were infected with several influenza virus strains: A/H1N1 subtype: FM/1/47, Bangkok/10/83, Beijing/262/95, Oita/OU1P3-3/09, or A/H3N2 subtype: Udorn/72, Aichi/2/68, or B/Ibaragi/2/85 in the presence or absence of 100 nM lectins. At 24 hpi, the infected cell cultures were fixed with 80% acetone and stained with amide black. For the B/Ibaragi/2/85 infection, the infected cultures were fixed at 48 hpi.
Furthermore, dose-effects of these lectins on the inhibition of infection were investigated with two influenza virus strains, A/H1N1/Oita/OU1P3-3/09 and A/H3N2/Udorn/72 (Fig. 4 ). The extent of the infection-inhibition correlated with the increased concentrations of these lectins in the cultures. Almost similar inhibition modes were detected in both strains. The lectin concentration of 50% inhibition of infection was obtained by reading the point cross the 50% line. Those curves in the H1N1 strain for PFL, PML, and PTL crossed the 50% line at 12.4, 8.9, and 6.6 nM, respectively. Those curves in the H3N2 strain crossed the 50% line at 10.5, 10.5, 9.5 nM (Fig. 4).
Fig. 4.
Dose-dependent anti-influenza virus activity by lectins: PFL, PML, PTL.
NCI-H292 cells were infected with A/H1N1/Oita/OU1P3-3/09 (A, B) or A/H3N2/Udorn/72 (C) at an m.o.i. of 2.5 in the presence of serial diluted lectins (200, 100, 50, 25, 12.5, 6.25 nM) or absence of the lectins. At 24 hpi, the infected cell cultures were fixed with 80% acetone and stained with amide black. A representative picture of the H1N1 strain infection was shown (A). Color densities of the stained culture-wells were quantitated, and the extent of infection-inhibition was shown as a percentage of the cell viability (B, C). Mock-infected culture-wells were shown as 100% of cell viability, the infected cell culture-wells without lectin (almost all the cells were gone) were shown as 0% of cell viability. Closed circles: PFL; open circles: PML; open triangles: PTL.
3.3. Inhibition properties of virus infection caused by binding of lectins: PFL, PML, and PTL
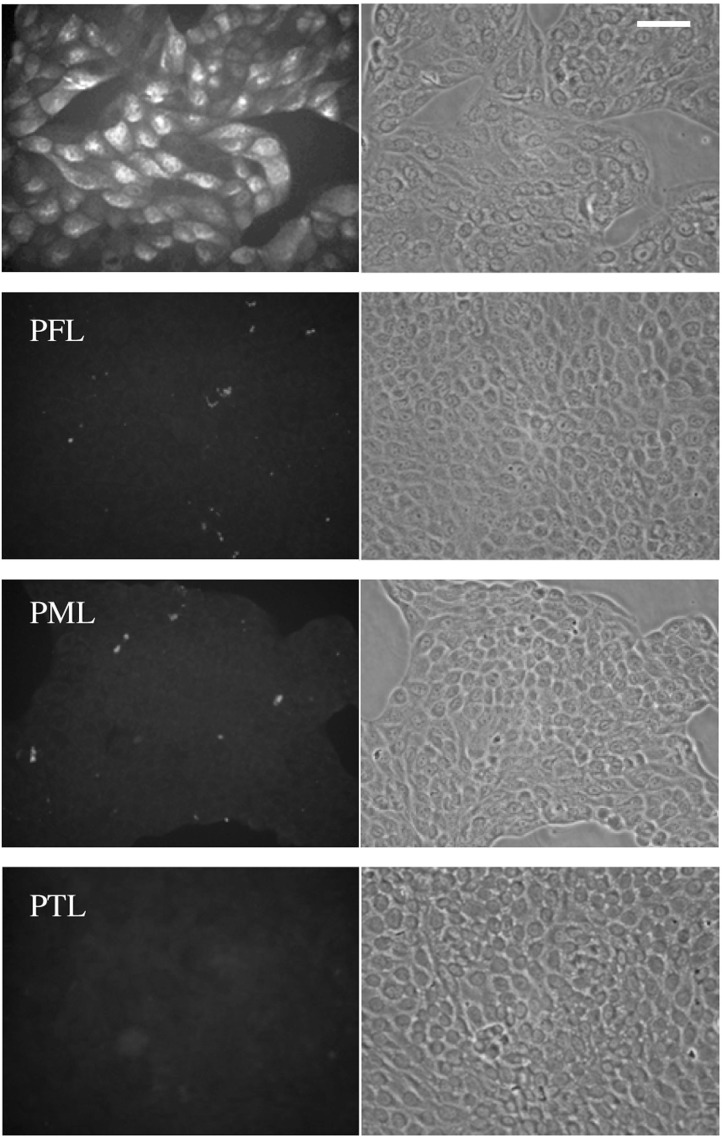
To explore the antiviral properties, viral antigens in the virus-infected cells were observed by using an immunofluorescence microscopy with anti-NP antibody (Fig. 5 ). At 8 hpi, viral antigens produced in the infected cells were apparently detected in the absence of lectin. At an addition of 100 nM PFL, PML, or PTL into the infected culture at 0 hpi, no viral antigens were detected in the host cells. These data indicated that the lectins efficiently inhibited propagation of the viruses in the cells at the early stage of infection.
Fig. 5.
Immunofluorescent microscopy of NCI-H292 cells infected with A/H1N1/Oita/OU1P3-3/09 strain.
NCI-H292 cells were infected with A/H1N1/Oita/OU1P3-3/09 at an m.o.i. of 2.5 in the presence or absence of 100 nM lectins. At 8 hpi, the infected cultures were fixed with 80% acetone, and subjected with a direct immunofluorescence. FITC-conjugated anti-NP protein antibody was used to detect viral antigens in the cells. Both fluorescence (left panels) and bright-field (right panels) images were shown. Scale bar, 100 μm.
To investigate whether the inhibition of infection would be caused by binding of the lectins with virus particles or cellular factors, experiments with pre-incubation of the lectins with virus solution or cell culture were performed, then, the extent of infection was monitored (Fig. 6 ). Pre-incubations of all these 100 nM lectins with virus solution for 30 min completely inhibited the infection by influenza virus. On the other hand, in pre-incubation of them with cell cultures, the virus propagations proceeded to kill the cells as same as in the absence of the lectins. Binding of the lectins with any cellular factors had little, if any, effect on the influenza infection. The lectins would bind influenza virus particles and blocked the virus entry into the host cells, resulting in inhibition of virus propagations.
Fig. 6.
Effect of pre-incubation of lectin with virus or cell on inhibition of the virus infection.
V: virus solutions of A/H3N2/Udorn/72 were pre-incubated with 100 nM lectins for 30 min at RT, the mixed solutions were exposed into the NCI-H292 cells for infection. C: the cells were pre-incubated with 100 nM lectins for 30 min at RT, the cell cultures were washed with PBS twice. The pre-treated cells were infected with A/H3N2/Udorn/72. At 24 hpi, both infected cultures were fixed with 80% acetone and stained with amide black. The extent of infection-inhibition was shown as a percentage of cell viability. M: mock-infected cells, I: virus-infected cells without lectin. (A): pre-incubated with PFL, (B): pre-incubated with PML, (C): pre-incubated with PTL.
It was examined whether additions of lectin simultaneously with or at 3 h after the virus input into the cultures could influence the extent of infection (Fig. 7 ). Simultaneously additions of the lectin potently exhibited the inhibition of infection. However, additions of the lectin at 3 hpi, when almost the influenza viruses had bound and penetrated into the host cells, reduced the inhibition of infection. Taken together with the above results, we have concluded that these lectins: PFL, PML, and PTL, could directly bind to the virus particles, prohibited entry of the virus particles in the host cells, and consequently inhibited the subsequent virus propagations. These data indicate that the likely target for these lectins was present on the influenza virus particle.
Fig. 7.
Effect of addition of lectin at 0 hpi or 3 hpi on inhibition of the virus infection.
PFL at 0 hpi: virus solution of A/H3N2/Udorn/72 was mixed with 80 nM of PFL, immediately the mixtures were added into NCI-H292 cell cultures. PFL at 3 hpi: the cells were infected with the virus in the absence of PFL. A final concentration of 80 nM PFL was added into the infected cultures at 3 hpi. At 24 hpi, the infected cell cultures were fixed with 80% acetone and stained with amide black. The extent of infection-inhibition was shown as a percentage of cell viability. Mock-infection: mock-infected cultures were shown as 100% of cell viability, Infection-PFL: virus- infected cultures without lectin were shown as 0% of cell viability.
3.4. Involvement of mannose glycan and HA protein on the infection-inhibition by the lectins: PFL, PML, and PTL
A glycan analysis showed that the PFL exhibited exclusive specificity for high-mannose glycan with α1-3 Man (Sato et al., 2012). However, the carbohydrate-binding specificities of PML and PTL remain undefined. It is expected that the specificities were same or quite similar to that of PFL from similar mode of the inhibition by these lectins. Whether binding of the lectins might indeed be mediated by protein-carbohydrate interactions on envelope of viruses was explored. To examine involvement of mannose in the inhibition of infection by these lectins, each 100 nM lectin was pre-incubated for 15 min with 100 μg/ml yeast mannan, a glycoprotein bearing abundant high-mannose glycans, then the mixture and a virus solution were simultaneously added into the cell culture (Fig. 8 ). Pre-incubation of yeast mannan apparently reduced the inhibitory effect by the lectins to a similar extent, which indicated that these lectins: PFL, PML and PTL, could bind to high-mannose glycans on the yeast mannan.
Fig. 8.
Effect of addition of yeast mannan on the infection-inhibition by lectins.
One hundred nanomolar lectins were pre-incubated with 100 μg/ml of yeast mannan for 15 min at RT (shown as +YM), the solutions were mixed with virus solution of A/H3N2/Udorn/72. The mixtures were added into NCI-H292 cell cultures for infection. At 24 hpi, the infected cell cultures were fixed with acetone and stained with amide black. The extent of infection-inhibition was shown as a percentage of cell viability. Mock: mock-infected cultures were shown as 100% of cell viability, Inf: virus-infected cultures without lectin were shown as 0% of cell viability.
Furthermore, to confirm that the infection-inhibition was mediated by binding of the lectins with HA protein on influenza virus, we performed a similar experiment using influenza HA vaccine preparation. The vaccine preparation exclusively contained HA proteins as their HA1 and HA2 cleaved forms (Fig. 9 A). Pre-incubation of these lectins with the vaccine preparation for 15 min also reduced the extent of inhibition as similar as the pre-incubation with yeast mannan (Fig. 9B). Taken together, these data indicated that these lectins: PFL, PML, and PTL, exert their antiviral effect on the influenza virus particle, they blocked the viral entry into the host cells by direct interaction with high-mannose glycan on HA protein of influenza virus. These data indicate that the carbohydrate-binding specificities of PML and PTL would be same or closely similar to that of PFL that is high-mannose glycan.
Fig. 9.
Effect of addition of influenza HA protein on the infection-inhibition by lectins.
(A) SDS-PAGE of influenza HA vaccine preparation. (B) One hundred nanomolar lectins were pre-incubated with 400 μg/ml of influenza HA vaccine preparation for 15 min at RT (shown as +Vac), then the solutions were mixed with virus solution of A/H3N2/Udorn/72. The mixtures were added into NCI-H292 cell cultures for infection. At 24 hpi, the infected cell cultures were fixed with 80% acetone and stained with amide black. The extent of infection-inhibition was shown as a percentage of cell viability. Mock: mock-infected cultures were shown as 100% of cell viability, INF: virus- infected cultures without lectin were shown as 0% of cell viability.
3.5. Inhibition of the virus entry into the host cells by binding of PFL with influenza virus
We concluded that the lectins: PFL, PML, and PTL, could bind high-mannose glycan on HA protein of influenza virus, resulting in inhibition of virus entry the into the host cells. The validity of this conclusion has been demonstrated by another study with a microarray analysis. Expression of mRNAs in cells infected with influenza virus in the presence of lectin PFL, where the virus invasion was prohibited, was compared with that in cells infected with influenza virus in the absence of the lectin, where the virus can invade and propagate in the host cells. Expression levels of 410 genes, which were chosen with keyword “virus infection” in Entrez data bank (provided by Keyword Gene Analysis in data mining light (Aligent Technology, CA, USA)), were analyzed in the microarray analysis. Among these 410 genes, 19 genes were found to be expressed differently by 4 times between the infected cells and the infection-inhibited cells by the lectin (Table 1 ). It was indicated that the expression levels of 13 genes were decreased and those of 6 genes were increased in the presence of PFL compared with that in the absence of PFL. These decreased 13 genes were mostly responsible for cytokines, chemokines or antiviral-related functions, including IL-2, IL-28B (IFN-λ3), IFN-β, TNF super family, IFN-induced proteins IFIT1, IFIT2, OAS2, Granzyme A, and MX1 (Iwasaki and Pillai, 2014). The result of gene expression patterns indicated that the host cells did not evoke responses against the virus invasion because the lectin completely blocked the virus entry.
Table 1.
Comparative expression analysis of mRNAs in the virus-infected cells in the presence or absence of PFL.
| Description [GenBank accession no.] | INF ± PFL | ±INF | ±PFL | Gene Symbol |
Remarks* |
|---|---|---|---|---|---|
| Fas ligand (TNF superfamily, member 6) [NM_000639] | – | + | FASLG | CK | |
| Interferon, beta 1, fibroblast [NM_002176] | – | + | IFNB1 | CK | |
| Interleukin 2 [NM_000586] | – | + | IL2 | CK | |
| Interleukin 28 B (interferon, lambda 3) [NM_172139] | – | + | IL28B | CK | |
| Granzyme A (granzyme 1, cytotoxic T-lymphocyte-associated serine esterase 3) [NM_006144] | – | + | GZMA | AV | |
| Interferon-induced protein with tetratricopeptide repeats 1, transcript variant 2 [NM_001548] | – | + | IFIT1 | AV | |
| Interferon-induced protein with tetratricopeptide repeats 2 [NM_001547] | – | + | IFIT2 | AV | |
| MX dynamin-like GTPase 1, transcript variant 2 [NM_002462] | – | + | MX1 | AV | |
| 2′-5′-oligoadenylate synthetase 2, 69/71 kDa, transcript variant 3 [NM_001032731] | – | + | OAS2 | AV | |
| MDM2 proto-oncogene, E3 ubiquitin ligase, transcript variant 1 [NM_002392] | – | + | MDM2 | ||
| Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta, transcript variant 2 [NM_145690] | – | – | YWHAZ | ||
| Lymphotoxin alpha (TNF superfamily, member 1), transcript variant 2 [NM_000595] | – | LTA | CK | ||
| Indoleamine 2,3-dioxygenase 1 [NM_002164] | – | IDO1 | AV | ||
| Chemokine (C-C motif) ligand 2 [NM_002982] | + | CCL2 | CK | ||
| Nitric oxide synthase 2, inducible [NM_000625] | + | NOS2 | AV | ||
| Poliovirus receptor-related 4 [NM_030916] | + | + | PVRL4 | ||
| Interleukin 12 B (natural killer cell stimulatory factor 2, cytotoxic lymphocyte maturation factor 2, p40) [NM_002187] | + | + | IL12B | CK | |
| Interferon, gamma [NM_000619] | + | + | IFNG | CK | |
| Tumor protein p53, transcript variant 1 [NM_000546] | + | + | TP53 |
A549 cells were infected with A/H3N2/Udorn/72 virus in the presence or absence of 200 nM PFL. At 4 hpi, RNAs of the virus-infected cells or mock-infected cells were extracted and subjected with a microarray analysis. INF ± PFL: the levels of mRNA expression were compared between those of virus-infected cells with and without 200 nM PFL. ±INF: the levels of mRNA expression were compared between those of virus-infected cells and mock-infected cells. ±PFL: the levels of mRNA expression were compared between those of mock-infected cells with and without 200 nM PFL. +: more than 4 times increased, −: less than one fourth decreased. *CK: cytokines or chemokines related genes, AV: interferon-induced or other antiviral related genes.
Antiviral drugs for viruses against which vaccines have not yet been developed (HIV, hepatitis C virus) or for new emerging viruses (Ebola virus, SARS/MERS coronaviruses) play an important role in therapeutic and prophylactic applications. As an antiviral drug with a novel mode of action, carbohydrate-binding agents (CBAs) that show specificity for high-mannose glycan, especially, cyanobacterium- and alga-derived lectins: CV-N and GRFT have been actively investigated for drugs targeting the initial entry stage of HIV infection. The CV-N and GRFT have been shown to inhibit in vivo transmission of HIV when used as a topical microbicide that can be applied vaginally or rectally, and they have generated interest as a promising new generation of microbicides characterized by specific and potent activity (Tsai et al., 2004, O’Keefe et al., 2009, Kouokam et al., 2011). It is possible that treatment with these bacteria-derived lectins may trigger undesirable side effects or immunogenicity including secretion of inflammatory cytokines and activation of host T-cells. In fact, the treatment with PFL disturbed various cellular responses such as proliferation or autophagy. The change of expression in many genes was detected to a varying degree (Sato et al., 2016). However, the GRFT treatment induces only minimal changes in secretion of inflammatory cytokines and chemokines. The GRFT exhibits superior safety profile for use as a tropical microbicide (O’Keefe et al., 2009, Kouokam et al., 2011).
For antiviral drug of influenza virus, zanamivir and oseltamivir are useful worldwide and have proven clinically effective. But the resistant strains of influenza virus can arise rapidly in the presence of the agents (Gubareva et al., 2000, McKimm-Breschkin, 2000). They target on the NA protein of influenza virus. The CBAs binding high-mannose glycan, including PFL, PML, and PTL, as well as CV-N and griffithsin, target on the HA protein. These lectins are apparently promising anti-influenza drugs with different modes of action. The virus itself does not control the structure of the specific oligosaccharides added to those sites of virus proteins (O’Keefe et al., 2003). It was reported that a relatively long time-period of drug exposure was required to afford marked phenotypic resistance against the CBA (Bolmstedt et al., 2001, Balzarini, 2007, François and Balzarini, 2012). Thus, the agents targeted to high-mannose glycan on glycoprotein of enveloped viruses including Pseudomonas-derived lectins: PFL, PML, and PTL, might be active and attractive agents against not only a wide variety of influenza virus strains but also other enveloped viruses.
4. Conclusion
We have indicated that three Pseudomonas-derived lectins: PFL, PML, and PTL, have potent anti-influenza virus activity against several strains of influenza virus with a similar extent. These lectins would bind high-mannose glycan as previously indicated with the PFL. These three lectins blocked entry of influenza virus into the host cells, by binding with high-mannose glycan on HA protein of influenza virus. Therefore, these lectins could not exhibit any inhibition after virus had invaded into the infected cells. It is expected that these lectins could have potent antiviral activity against not only influenza virus but also HIV and/or other enveloped viruses as described by many other studies. These three lectins will be applicable to a novel microbicide.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgments
This work was partly supported by Grant-in-Aid for Scientific Research (C), JSPS KAKENHI Grant Number 24590167. We thank to Rei Isagi, Sayako Kodama, Arisa Kubo, and Marie Wada for their technical assistance.
References
- Balzarini J. Carbohydrate-binding agents: a potential future cornerstone for the chemotherapy of enveloped viruses. Antivir. Chem. Chemother. 2007;18:1–11. doi: 10.1177/095632020701800101. [DOI] [PubMed] [Google Scholar]
- Barrientos L.G., O’Keefe B.R., Bray M., Sanchez A., Gronenborn A.M., Boyd M.R. Cyanovirin-N binds to the viral surface glycoprotein, GP1, 2 and inhibits infectivity of Ebola virus. Antivir. Res. 2003;58(1):47–56. doi: 10.1016/S0166-3542(02)00183-3. [DOI] [PubMed] [Google Scholar]
- Barrientos L.G., Lasala F., Otero J.R., Sanchez A., Delgado R. In vitro evaluation of cyanovirin-N antiviral activity, by use of lentiviral vectors pseudotyped with filovirus envelope glycoproteins. J. Infect. Dis. 2004;189:1440–1443. doi: 10.1086/382658. [DOI] [PubMed] [Google Scholar]
- Bolmstedt A.J., O’Keefe B.R., Shenoy S.R., McMahon J.B., Boyd M.R. Cyanovirin-N defines a new class of antiviral agent targeting N-linked, high-mannose glycans in an oligosaccharide-specific manner. Mol. Pharmacol. 2001;59(5):949–954. doi: 10.1124/mol.59.5.949. [DOI] [PubMed] [Google Scholar]
- Boyd M.R., Gustafson K.R., McMahon J.B., Shoemaker R.H., O’Keefe B.R., Mori T., Gulakowski R.J., Wu L., Rivera M.I., Laurencot C.M., Currens M.J., Cardellina J.H.I.I., Buckheit R.W., Jr., Nara P.L., Pannell L.K., Sowder R.C., II, Henderson L.E. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob. Agents Chemother. 1997;41(7):1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey B., Lerner D.L., Lusso P., Boyd M.R., Elder J.H., Berger E.A. Multiple antiviral activities of cyanovirin-N: blocking of human immunodeficiency virus type 1 gp120 interaction with CD4 and coreceptor and inhibition of diverse enveloped viruses. J. Virol. 2000;74(10):4562–4569. doi: 10.1128/JVI.74.10.4562-4569.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Férir G., Huskens D., Noppen S., Koharudin L.M., Gronenborn A.M., Schols D. Broad anti-HIV activity of the Oscillatoria agardhii agglutinin homologue lectin family. J. Antimicrob. Chemother. 2014;69:2746–2758. doi: 10.1093/jac/dku220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François K.O., Balzarini J. Potential of carbohydrate-binding agents as therapeutics against enveloped viruses. Med. Res. Rev. 2012;32(2):349–387. doi: 10.1002/med.20216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubareva L.V., Kaiser L., Hayden F.G. Influenza virus neuraminidase inhibitors. Lancet. 2000;355(9206):827–835. doi: 10.1016/S0140-6736(99)11433-8. [DOI] [PubMed] [Google Scholar]
- Helenius A., Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291(5512):2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- Helle F., Wychowski C., Vu-Dac N., Gustafson K.R., Voisset C., Dubuisson J. Cyanovirin-N inhibits hepatitis C virus entry by binding to envelope protein glycans. J. Biol. Chem. 2006;281(35):25177–25183. doi: 10.1074/jbc.M602431200. [DOI] [PubMed] [Google Scholar]
- Huskens D., Schols D. Algal lectins as potential HIV microbicide candidates. Mar. Drugs. 2012;10:1476–1497. doi: 10.3390/md10071476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacob R.E., Perdivara I., Przybyiski M., Tomer K.B. Mass spectrometric characterization of glycosyaltion of hepatitis C virus E2 envelope glycoprotein reveals extended microheterogenicity of N-glycans. J. Am. Soc. Mass Spectrom. 2008;19(3):428–444. doi: 10.1016/j.jasms.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishag H.Z., Li C., Huang L., Sun M.X., Wang F., Ni B., Malik T., Chen P.Y., Mao X. Griffithsin inhibits Japanese encephalitis virus infection in vitro and in vivo. Arch. Virol. 2013;158:349–358. doi: 10.1007/s00705-012-1489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A., Pillai P.S. Innate immunity to influenza virus infection. Nat. Rev. Immunol. 2014;14:315–328. doi: 10.1038/nri3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns T.G., Mellman I., Cartwright G.A., Ritter G., Old L.J., Burgess A.W., Scott A.M. The antitumor monoclonal antibody 806 recognizes a high-mannose form of the EGF receptor that reaches the cell surface when cells over-express the receptor. FASEB J. 2005;19:780–782. doi: 10.1096/fj.04-1766fje. (express article) [DOI] [PubMed] [Google Scholar]
- Kachko A., Loesgen S., Shahzad-Ul-Hussan S., Tan W., Zubkova I., Takeda K., Wells F., Rubin S., Bewley C.A., Major M.E. Inhibition of hepatitis C virus by the cyanobacterial protein Microcystis viridis lectin, mechanistic differences between the high-mannose specific lectins MVL, CV-N, and GNA. Mol. Pharm. 2013;10(2):4590–4602. doi: 10.1021/mp400399b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouokam J.C., Huskens D., Schols D., Johannemann A., Riedell S.K., Walter W., Walker J.M., Matoba N., O'Keefe B.R., Palmer K.E. Investigation of griffithsin’s interactions with human cells confirms its outstanding safety and efficacy profile as a microbicide candidate. PLoS One. 2011;6:e22635. doi: 10.1371/journal.pone.0022635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard C.K., Spellman M.W., Riddle L., Harris R.J., Thomas J.N., Gregory T.J. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J. Biol. Chem. 1990;265:10373–10382. [PubMed] [Google Scholar]
- Mason C.P., Tarr A.W. Human lectins and their roles in virus infections. Molecules. 2015;20:2229–2271. doi: 10.3390/molecules2002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKimm-Breschkin J.L. Resistance of influenza viruses to neuraminidase inhibitors–a review. Antivir. Res. 2000;47:1–17. doi: 10.1016/S0166-3542(00)00103-0. [DOI] [PubMed] [Google Scholar]
- Mir-Shekari S.Y., Ashford D.A., Harvey D.J., Dwek R.A., Schulze I.T. The glycosylation of the influenza A virus hemagglutinin by mammalian cells. A site-specific study. J. Biol. Chem. 1997;272(7):4027–4036. doi: 10.1074/jbc.272.7.4027. [DOI] [PubMed] [Google Scholar]
- Mori T., O’Keefe B.R., Sowder R.C., Bringans I.I., Gardella S., Berg R., Cochran S., Turpin P., Buckheit J.A., Jr R.W., McMahon J.B., Boyd M.R. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 2005;280(10):9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- O’Keefe B.R., Smee D.F., Turpin J.A., Saucedo C.J., Gustafson K.R., Mori T., Blakeslee D., Buckheit R., Boyd M.R. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob. Agents Chemother. 2003;47(8):2518–2525. doi: 10.1128/AAC.47.8.2518-2525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe B.R., Vojdani F., Buffa V., Shattock R.J., Montefiori D.C., Bakke J., Mirsalis J., d'Andrea A.L., Hume S.D., Bratcher B., Saucedo C.J., McMahon J.B., Pogue G.P., Palmer K.E. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc. Natl. Acad. Sci. U. S. A. 2009;106:6099–6104. doi: 10.1073/pnas.0901506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe B.R., Giomarelli B., Barnard D.L., Shenoy S.R., Chan P.K., McMahon J.B., Palmer K.E., Barnett B.W., Meyerholz D.K., Wohlford-Lenane C.L., McCray P.B., Jr. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J. Virol. 2010;84:2511–2521. doi: 10.1128/JVI.02322-09. (Erratum in: J Virol. 84 5456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Okuyama S., Hori K. Primary structure and carbohydrate binding specificity of a potent anti-HIV lectin isolated from the filamentous cyanobacterium Oscillatoria agardhii. J. Biol. Chem. 2007;282(15):11021–11029. doi: 10.1074/jbc.M701252200. [DOI] [PubMed] [Google Scholar]
- Sato Y., Morimoto K., Hirayama M., Hori K. High mannose-specific lectin (KAA-2) from the red alga Kappaphycus alvarezii potently inhibits influenza virus infection in a strain-independent manner. Biochem. Biophys. Res. Commun. 2011;405:291–296. doi: 10.1016/j.bbrc.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Hirayama M., Morimoto K., Yamamoto N., Okuyama S., Hori K. High mannose-binding lectin with preference for the cluster of alpha1-2-mannose from the green alga Boodlea coacta is a potent entry inhibitor of HIV-1 and influenza viruses. J. Biol. Chem. 2011;286:19446–19458. doi: 10.1074/jbc.M110.216655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Morimoto K., Kubo T., Yanagihara K., Seyama T. High mannose-binding antiviral lectin PFL from Pseudomonas fluorescens Pf0-1 promotes cell death of gastric cancer cell MKN28 via interaction with alpha2-integrin. PLoS One. 2012;7(9):e45922. doi: 10.1371/journal.pone.0045922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Morimoto K., Kubo T., Sakaguchi T., Nishizono A., Hirayama M., Hori K. Entry inhibition of influenza viruses with high mannose binding lectin ESA-2 from the red alga Eucheuma serra through the recognition of viral hemagglutinin. Mar. Drugs. 2015;13:3454–3465. doi: 10.3390/md13063454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Kubo T., Morimoto K., Yanagihara K., Seyama T. High mannose-binding Pseudomonas fluorescens lectin (PFL) downregulates cell surface integrin/EGFR and induces autophagy in gastric cancer cells. BMC Cancer. 2016;16:63. doi: 10.1186/s12885-016-2099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C.C., Emau P., Jiang Y., Agy M.B., Shattock R.J., Schmidt A., Morton W.R., Gustafson K.R., Boyd M.R. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res. Hum. Retrovir. 2004;20:11–18. doi: 10.1089/088922204322749459. [DOI] [PubMed] [Google Scholar]
- van der Meer F.J., de Haan C.A., Schuurman N.M., Haijema B.J., Peumans W.J., Van Damme E.J., Delputte P.L., Balzarini J., Egberink H.F. Antiviral activity of carbohydrate-binding agents against Nidovirales in cell culture. Antivir. Res. 2007;76:21–29. doi: 10.1016/j.antiviral.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis W.I., Taylor M.E., Drickamer K. The C-type lectin superfamily in the immune system. Immunol. Rev. 1998;163:19–34. doi: 10.1111/j.1600-065X.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- Whitley M.J., Furey W., Kollipara S., Gronenborn A.M. Burkholderia oklahomensis agglutinin is a canonical two-domain OAA-family lectin: structures, carbohydrate binding and anti-HIV activity. FEBS J. 2013;280:2056–2067. doi: 10.1111/febs.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]