Abstract
Most RNA viruses have evolved strategies to regulate cellular translation in order to promote preferential expression of the viral genome. Positive strand RNA viruses express large portions, or all of their proteome via translation of large polyproteins that are processed by embedded viral proteinases or host proteinases. Several of these viral proteinases are known to interact with host proteins, particularly with the host translation machinery, and thus, encompass the dual functions of processing of viral polyproteins and exerting translation control. Picornaviruses are perhaps the best characterized in regards to interaction of their proteinases with the host translation machinery and will be emphasized here. However, new findings have shown that similar paradigms exist in other viral systems which will be discussed.
Keywords: Proteinase, Translation inhibition, eIF4G, PABP
1. Brief overview cap-dependent translation initiation
Translation can be divided into the three phases of initiation, elongation and termination. Most translation regulation mechanisms regulate the initiation phase, including viral regulation schemes, thus, initiation will be emphasized here. Most cellular mRNAs are translated by mechanisms that are dependent on the 5′ cap structure. De novo initiation of typical mRNA requires recognition of the 5′ m7GpppN cap structure by the trimeric translation factor complex eIF4F, and subsequent recruitment of a 43S ribosomal subunit (containing a 40S ribosomal subunit, eukaryotic initiation factors (eIFs) eIF1, eIF1a, eIF2, eIF5, eIF3 and Met-tRNAiMet) and other initiation factors to form a 48S ribosomal preinitiation complex. The 48S complex is functional for scanning the mRNA sequence in a 5′–3′ direction for initiation codons in a favorable consensus sequence. There is no clear evidence that the cap structure is released by eIF4E during the scanning process, although it is often depicted this way (see Fig. 3). The initiation phase of translation is completed when the 60S ribosomal subunit has joined, and then the 80S ribosome completes the translation of the mRNA. For recent reviews on this complex topic, see (Gebauer and Hentze, 2004, Gingras et al., 1999, Merrick, 2004, Preiss and Hentze, 2003, Rogers et al., 2002, Schneider and Mohr, 2003). Note that for clarity, only initiation factors that play a role in viral proteinase-mediated translation regulation mechanisms are discussed further below.
Fig. 3.
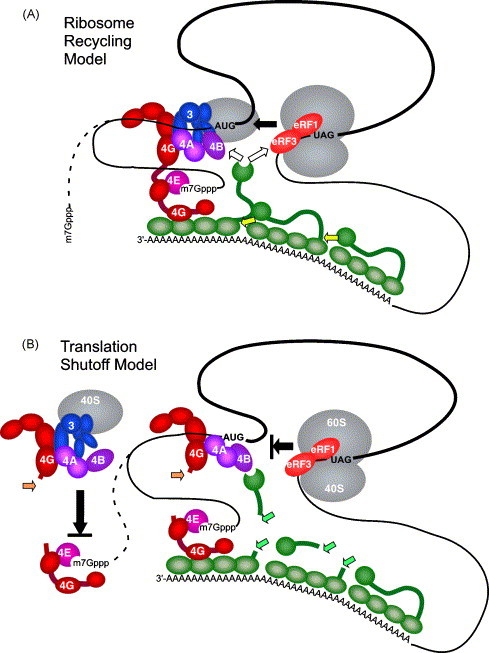
A working model for dual roles of 2Apro and 3Cpro in inhibition of de novo translation and ribosome recycling on capped mRNAs. (A) Model for ribosome recycling. 40S subunit is depicted on the AUG codon after completing scanning. It is unclear if the cap structure is released by eIF4F during scanning (dashed line) or the 5′ UTR is looped out (solid line). Ribosomes that have reached the stop codon bind eRF3 which may interact with PABP-CTD to facilitate recycling of 60S subunits to waiting 40S subunits on initiation codons, or alternatively, both subunits may recycle. The PABP-CTD may dynamically switch back and forth between binding eRF3 and eIF4B (white arrows). This recycling step can function after eIF4G is cleaved. PABP oligomerization involving the CTD (yellow arrows) may prevent other PABP-CTD from interactions with eIF4B and eRF3. (B) Model for translation shutoff in PV-infection. After cleavage of eIF4G by 2Apro (orange arrows), de novo binding of 40S subunits to mRNA via the cap structure is blocked and recycling of ribosomes is blocked by cleavage of PABP by 3Cpro (green arrows). Cleaved eIF4G may still retain mRNA in closed loop configuration.
eIF4F is a heterotrimeric complex consisting of eIF4G, eIF4E and eIF4A and can be isolated as a salt-stable complex from mammalian cells. eIF4G is a multivalent scaffolding protein that contains binding domains for cap-binding protein eIF4E and the prototype DEAD-box helicase eIF4A. eIF4G also contains binding sites for poly(A)-binding protein (PABP) and MNK-1 kinase (Fig. 1 ). eIF4F is also associated with eIF4B, which interacts with eIF4A in RNA unwinding assays but may not be required for cap-binding functions (Grifo et al., 1984, Ray et al., 1985, Rozen et al., 1990). There are two major forms of eIF4G, termed eIF4GI and eIF4GII that share only 46% homology but are highly conserved in key regions that bind other translation factors (Gradi et al., 1998a). eIF4GI is the dominant form in HeLa cells, comprising approximately 90% of total eIF4GI (Marissen and Lloyd, 1998). Further, a complex translation initiation scheme involving alternate initiation codon selection at five AUGs and alternate splicing produces a set of five isoforms of eIF4GI that vary at the N-terminus. The smallest isoform lacks the PABP-binding site (Byrd et al., 2002, Byrd et al., 2005).
Fig. 1.
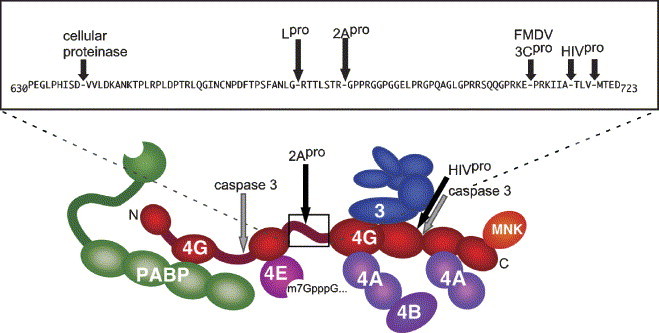
Schematic illustrating scaffolding protein eIF4GI (and eIF4GII) (shown in red) as an extended structure bearing a series of binding domains for other translation factors PABP, eIF4E, eIF4A (two sites), eIF3 and mnk-1. The NH2 and COOH-termini of eIF4G are shown. The locations of known protease cleavage sites are depicted with arrows. A central region of eIF4G linking eIF4E- and eIF3-binding domains has been expanded in the box to illustrate locations of individual proteinase cleavage sites.
The primary function of eIF4F is to facilitate binding of 40S ribosomal subunits to the 5′ cap structure of mRNA and then aid ribosomal scanning. Because eIF4G can simultaneously bind all these initiation factors, it performs two critical linking or bridging functions. First, eIF4G-mediated linkage of eIF3 (which is bound to 40S ribosomal subunits), and eIF4E, completes a molecular bridge which binds the mRNA to the ribosome (Fig. 3A). Second, eIF4G-mediated linkage of PABP and eIF4E simultaneously provides a second molecular bridge linking 5′ and 3′ ends of the mRNA in a pseudo-circularized structure (Wells et al., 1998). Thus, eIF4G is in many ways the centerpiece of the translation initiation complex. Therefore, it is not surprising that many viruses have evolved mechanisms to modify eIF4G functions in their bid to control cellular translation. Several non-proteolytic translation regulation mechanisms involving eIF4GI are detailed in other chapters herein. This chapter reviews only mechanisms involving cleavage of eIF4G and other factors.
2. IRES-mediated cap-independent translation
Picornavirus RNA does not contain a cap structure to aid ribosome binding. To compensate for this, the 5′ untranslated region (5′ UTR) contains a large RNA structure called an internal ribosome entry sequence (IRES) that recruits ribosomes to bind to internal sites in the RNA. IRES structures have been found in a wide range of virus and cellular mRNAs and are quite variable in sequence and structure. The mechanism of ribosome binding by HCV and picornavirus IRESs are best understood and they involve variable subsets of the canonical initiation factors, depending on the IRES. In addition, certain RNA-binding proteins such as La, PTB, UNR and PCBP2 have been described that stimulate the functional activity of certain IRES elements in biochemical assays (Bedard et al., 2004, Blyn et al., 1996, Blyn et al., 1997, Boussadia et al., 2003, Costa-Mattioli et al., 2004, Hellen et al., 1993, Hunt et al., 1999, Meerovitch et al., 1989, Meerovitch et al., 1993). Such IRES-transactivating factors (ITAFs) are thought to play a role in the selective pathogenesis and variable replication of several picornaviruses in different cell types and tissues (Pilipenko et al., 2000, Pilipenko et al., 2001).
3. 5′–3′ interactions in translation and ribosome recycling
Poly(A)-binding protein (PABP) binds the poly(A) tail on mRNA via four conserved RNA-recognition motifs (RRMs) and contains a highly conserved C-terminal domain (CTD) linked by a proline-rich domain. Like eIF4GI, PABP also binds a large number of proteins, including eIF4G, eIF4B, translation termination factor eRF3, and three regulatory proteins, UNR, and PABP-interacting proteins 1 and 2 (Paip1 and Paip2) (Fig. 2 ) (Bushell et al., 2001, Chang et al., 2004, Imataka et al., 1997, Imataka et al., 1998, Khaleghpour et al., 2001, Kozlov et al., 2004, Roy et al., 2002). The interaction between eIF4E/eIF4G and PABP is sufficient to circularize the mRNA, and provides the primary basis for 5′–3′ interactions in mRNAs (Wells et al., 1998). Many, if not most translating mRNAs in the cell are thought to be arranged in such a quasi-circular configuration. This “closed loop model” was suggested over 30 years ago as the most efficient means to recycle ribosomes during translation (Baglioni et al., 1969, Philipps, 1965). It has been proposed that circularization of mRNA stimulates translation by increasing the binding affinity of eIF4E for the cap structure or by providing a mechanism to recycle terminating ribosomes to the 5′ end of the mRNA (Kahvejian et al., 2001, Sachs and Varani, 2000). Further, enhanced translation on circularized mRNAs promotes translation of only intact mRNAs, and PABP–eIF4G interaction may stabilize mRNA by inhibiting cap- and poly(A)-oriented mRNA decay mechanisms (Coller and Parker, 2004, Jacobson, 2004, Meyer et al., 2004).
Fig. 2.
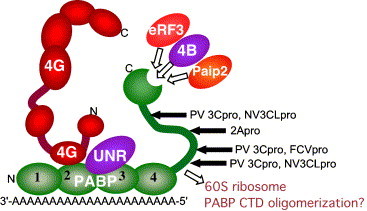
Schematic illustrating PABP (shown in green) with its multiple binding partners. The structure of PABP is illustrated with four numbered RRM domains that interact with RNA, a flexible proline-rich linking domain and a structured COOH-terminal domain that contains a binding cleft for eRF3, eIF4B and PAIP2. RRMs 2 and 3 interact with eIF4G and UNR. A region near RRM 4 interacts with 60S ribosomal subunits in yeast and may be involved in PABP oligomerization. The location of viral proteinase cleavage sites is indicated. Other binding sites for PAIP1 and PAIP2 are not shown for clarity.
The presence of poly(A) tails (with PABP bound), stimulate joining of both the 60S ribosomal subunit and 40S ribosomal subunit to mRNA during initiation in yeast and reticulocyte lysates (Munroe and Jacobson, 1990, Sachs and Davis, 1989). Also, PABP stimulates translation of mRNA that is missing a cap structure, during so-called poly(A)-dependent initiation, even if the 5′ end of mRNA is blocked. This 3′-mediated translation still requires eIF4G (Preiss and Hentze, 1998, Preiss et al., 1998). Thus, functional interaction between 5′ and 3′ ends of the mRNA is considered to be crucial, particularly in conditions in vivo where mRNAs must compete for ribosomes. The confirmed interaction between PABP and eRF3 is very interesting as it may place PABP in space and time in the same location as a terminating ribosome and the stop codon (Hoshino et al., 2000, Kozlov et al., 2001, Uchida et al., 2002). This suggests that often lengthy 3′ UTR may be looped out to allow for this interaction (Fig. 3A). This configuration may also enhance translation by facilitating recycling of ribosomes. It may be possible for ribosome subunits to hop or shunt from stop codons, or 3′ UTR regions to the 5′ UTR, thus providing a way for ribosomes to continually translate the same mRNA without undergoing more rounds of de novo initiation. Direct evidence for ribosome recycling has not been demonstrated, however, has been inferred from some experiments that will be discussed below. It is not known if the biochemical requirements for ribosome recycling would differ from the requirements for de novo ribosome binding and scanning. Therefore, the concept and mechanism of re-initiation of ribosomes is under investigation. These new models of translation control impact our understanding of viral regulation of translation.
4. Viral 2A proteinases cleave eIF4GI and eIF4GII
Virus infection of most cell types by human enteroviruses such as poliovirus (PV), rhinovirus (HRV) and Coxsackie B viruses (CVB3) induces a rapid and nearly complete inhibition of host cell protein synthesis. The mechanism that blocks host translation does not affect translation of virus RNA during the first 4–5 h of infection, however, virus translation does suffer a rapid decline about 2 h after host translation is inhibited. The early translational switch is temporally accompanied by disaggregation of polysomes containing cellular mRNA followed by reformation of larger polysomes containing exclusively viral mRNA. The mechanism of the translational switch is largely based on lack of a cap structure on viral RNA and the presence of an IRES that mediates cap-independent translation initiation.
The actual site for host translation inhibition was long ago localized to the initiation step of protein synthesis, prompting a search for inactivated initiation factors in infected cells. In 1982 Etchison and Hershey made the seminal discovery that eIF4G (then called p220) was cleaved during PV infection, thus inactivating eIF4F complexes in cells (Etchison et al., 1982). Other reports demonstrated that eIF4E, eIF4A, eIF4B, eIF3 were not cleaved in PV-infected cells (Duncan et al., 1983, Etchison et al., 1984, Lee et al., 1985), however, PABP was independently reported by two groups to be cleaved by PV and CVB3 in 1999 (Joachims et al., 1999, Kerekatte et al., 1999). No other canonical translation factors are known to be cleaved during PV infection.
eIF4GI is cleaved by 2A proteases of PV, HRV and CVB3 at position (amino acid 681/682) (Lamphear et al., 1993, Sommergruber et al., 1994). Cleavage of eIF4G effectively splits the eIF4F complex in half, separating the eIF4E-binding domain of eIF4G from the eIF3-binding domain (Lamphear et al., 1995) (Fig. 1). Thus, the bridging function of eIF4G that brings capped mRNAs to the 40S ribosome is neatly abrogated by the virus. However, the second bridging function of eIF4G that connects 5′–3′ ends of mRNA is retained. PV infection also activates at least two cellular proteinase activities that cleave eIF4GI very near the 2Apro cleavage site and at another site 43 amino acids upstream (Zamora et al., 2002). Combined, these multiple proteinase activities rapidly cleave eIF4GI during the first 2–3 h of infection. Efforts to purify eIF4G cleavage activities from PV-infected cells revealed that fractions enriched with 2Apro had weak eIF4G cleavage activity and most cleavage activity associated with cellular proteins (Bovee et al., 1998a, Bovee et al., 1998b). The preferred molecular target of HRV 2Apro was recognized to be eIF4F (eIF4E bound to eIF4G) instead of purified eIF4GI, likely due to formation of a protease-sensitive conformation (Haghighat et al., 1996). This model is supported by structural analysis of the region of eIF4G that binds eIF4E. This region is unstructured before binding but becomes folded into an alpha helix with two turns after binding eIF4E (Gross et al., 2003, Marcotrigiano et al., 1999). The issue of why PV 2Apro isolated from infected cells contains so little eIF4GI cleavage activity has not been resolved, nor have the cellular eIF4G-proteases been identified. HRV 2Apro cleaves eIF4GI relatively rapidly in translation reactions in rabbit reticulocytes, requiring only 15 min and 1:12 molar ratios of enzyme to substrate (Glaser and Skern, 2000). But recombinant PV and CVB 2Apro are less efficient in in vitro cleavage assays (Bovee et al., 1998a). eIF4G is also cleaved by caspase 3 at two other sites during apoptosis (Fig. 1) (Marissen and Lloyd, 1998). Viral infection activates some apoptosis pathways, however, the caspase inhibitor zVAD-fmk did not diminish eIF4G cleavage during PV infection (Roberts et al., 2000). In contrast, another investigation found that zVAD-fmk did inhibit eIF4G cleavage if viral RNA replication was also blocked with 2 mM guanidine in order to limit 2Apro expression (Zamora et al., 2002). The eIF4G-protease is clearly not caspase 3, which cleaves at different sites and is not activated early in infection (Agol et al., 2000, Belov et al., 2003, Carthy et al., 1998, Romanova et al., 2005), however, this protease may be part of an early apoptotic response to viral infection.
The eIF4GI homolog, eIF4GII, is also cleaved by 2Apro, however, cleavage kinetics are slower during HRV or PV infection than cleavage of eIF4GI (Gradi et al., 1998b). The cleavage site on eIF4GII has been mapped to amino acid 699, which is in the same region as the 2Apro cleavage site in eIF4GI (Gradi et al., 2003). Based on kinetics studies in infected cells, cleavage of both eIF4GI and eIF4GII was proposed to be required for complete translation shutoff during PV or HRV infection (Gradi et al., 1998b, Svitkin et al., 1999). However, no mechanistic studies of eIF4GII's contribution to cellular translation in comparison to eIF4GI or PABP have been performed.
5. FMDV L-proteinase cleaves eIF4GI and eIF4GII
The animal picornavirus foot-and-mouth disease virus (FMDV) also causes rapid cleavage of eIF4G during virus infections. The 2A gene of FMDV is not a functional proteinase, rather cleavage of eIF4G is accomplished by the leader proteinase (L-pro), an alternate papain-like protease encoded at the N-terminus of the viral polyprotein. This proteinase cleaves eIF4GI at a distinct site, seven amino acids upstream of the 2Apro cleavage site, thereby accomplishing the same functional scission of eIF4E- and eIF3-binding domains (Kirchweger et al., 1994). This cleavage reaction has been very well characterized and occurs extremely rapidly, presumably while ribosomes are translating the FMDV polyprotein (Glaser and Skern, 2000). L-proteinase also cleaves eIF4GII, however, this scission is very rapid and efficient, unlike PV or HRV 2Apro, which cleave more slowly. The Lpro cleavage site is located one amino acid downstream of the 2Apro cleavage site on eIF4GII (Gradi et al., 2004).
Interesting work has shown that both FMDV Lpro and HRV 2Apro initially recognize and bind to eIF4GI in regions away from the scissile bond, between amino acids 600–674 (Fig. 1), which are located immediately to the N-terminal side, but do not include the amino acids where cleavage occurs. Similarly, mutagenesis and binding studies have located eIF4G-binding domains on each protease that are located away from the substrate binding cleft, thus representing exosites (Foeger et al., 2002, Foeger et al., 2003). Comparison of the 2Apro and Lpro exosites on each protease reveal no obvious similarity, despite the fact they interact with the same general region of eIF4GI. Phylogenetic analysis of key residues in the HRV2 2Apro exosite shows a lack of conservation among PV and other enteroviruses in this region, suggesting that other viral 2Apro molecules may not share this feature (Foeger et al., 2003).
Thus, FMDV has evolved a separate L-proteinase in place of 2Apro to cleave eIF4G, affirming the importance of this translation control strategy for the virus. However, it is important to recognize that not all picornaviruses encode proteinases that cleave eIF4G, such as the cardioviruses encephalomycarditis virus (EMCV) and mengovirus. The EMCV 2A gene, which is not a proteinase, is localized to nucleoli during infection and may instead interfere with or alter ribosome assembly and transport to indirectly regulate translation. (Aminev et al., 2003a, Aminev et al., 2003b).
6. Other viral proteases that cleave eIF4GI
Recently several reports have described cleavage of eIF4G by proteases of other viruses. Human immunodeficiency virus-1 (HIV-1) infection of cells resulted in partial cleavages of eIF4GI that was mapped to three sites in two regions on either side of the eIF3-binding domain (Fig. 1) (Ohlmann et al., 2002, Ventoso et al., 2001). Similarly, proteases of HIV-2, human T-cell leukemia virus (HTLV-1), simian immunodeficiency virus (SIV), Moloney murine leukemia virus and mouse mammary tumor virus, also caused partial cleavage of eIF4GI (Alvarez et al., 2003). Cleavage of eIF4GI at the downstream site inhibits de novo initiation of both capped and IRES-driven mRNAs in reticulocyte lysate assays (Ohlmann et al., 2002). Translation assays based on luciferase reporter constructs in cells indicated that expression of HIV protease (HIV PR) primarily inhibited translation of capped mRNAs. Interestingly, comparison of translation function of eIF4GI C-terminal cleavage products produced by Lpro and HIV PR revealed that the slightly shorter HIV PR-derived fragment was defective in supporting translation of the PV-IRES but not the EMCV IRES. This 40-amino acid segment of eIF4G binds RNA and was suggested to be critical for scanning (Prevot et al., 2003).
HIV mRNAs are capped, so why would a virus that expresses capped mRNAs encode a function that cleaves eIF4G and represses cap-dependent translation and scanning? Similar to picornaviruses, several retroviruses, including HIV and SIV, contain IRES elements in the leader or gag gene that may help them escape this translation restriction or maintain expression in quiescent cells or during mitosis (Brasey et al., 2003, Buck et al., 2001, Ohlmann et al., 2000). HIV proteinase stimulated translation of HIV mRNA in vitro (Ventoso et al., 2001) and cleavage of eIF4G by Lpro activated the HIV leader IRES (Ohlmann et al., 2000). Interestingly, eIF4GII is not cleaved by HIV PR (Alvarez et al., 2003, Ohlmann et al., 2002). Thus, the combined weak and late cleavage of eIF4GI and lack of eIF4GII cleavage insures that significant levels of intact eIF4F will always be present to support cap-dependent translation during HIV infection. Accordingly, HIV may be seen to modulate cap-dependent translation, though probably not aggressively shutoff translation like picornaviruses.
Although FMDV Lpro is the most active eIF4G-protease described, an alternate secondary cleavage of eIF4GI, along with partial cleavage of eIF4A occurs in infected cells late in infection in BHK cells (Belsham et al., 2000). These cleavage events were shown to correlate with the shutdown of viral translation more than host translation and were induced by 3Cpro instead of Lpro. Similar secondary cleavage does not occur with human eIF4GI due to an amino acid change at the active site. The FMDV 3Cpro cleavage site on eIF4G was mapped 39 amino acids downstream of the FMDV Lpro cleavage site (Fig. 1). The functional consequences of further cleavage of eIF4GI are unclear, however, cleavage of eIF4A, though partial, may produce a dominant negative mutant that blocks functions required for FMDV IRES activity (Belsham et al., 2000, Pause et al., 1994).
7. Effects of eIF4G cleavage on translation
2Apro-mediated cleavage of eIF4G separates the NH2-terminal eIF4E-binding domain (mRNA binding) and COOH-terminal eIF3-binding domain (ribosome binding) of eIF4G (Lamphear et al., 1995). This finding formed the basis for the attractive “eIF4G cleavage model” for the mechanism of PV-induced shutoff of cap-dependent translation. eIF4F complexes that are cleaved by 2Apro are not able to recruit ribosomes to capped mRNAs (Borman et al., 1997, Liebig et al., 1993). This hypothesis was influenced by early reports that transient expression of 2Apro alone in cells was sufficient to cause eIF4G cleavage and translation inhibition in cells (Aldabe et al., 1995, Davies et al., 1991) and a drastic 50-fold decrease in translation rate of a reporter gene (Sun and Baltimore, 1989), a finding that has not been repeated by others. More typically, expression of 2Apro in cells has been associated with less drastic effects on translation (e.g. 2–3 fold) and proposed to lead to apoptosis which also leads to translation inhibition via a variety of alternate mechanisms (Aldabe et al., 1995, Barco et al., 2000, Tee and Proud, 2002, Zhao et al., 2003). Recently, the 2Apro cleavage site was mutated on eIF4GI and cells ectopically expressing cleavage-resistant eIF4G were able to partly restore translation inhibition from expressed 2Apro (Zhao et al., 2003). These data provide strong evidence that eIF4GI cleavage is an important aspect of the shutoff mechanism, but do not indicate that other factors and events are not also required.
Many findings suggest that a revision of this eIF4GI cleavage model for shutoff is in order. Aaron Shatkin was first to point out that the kinetics of eIF4G cleavage in infected HeLa cells did not correlate closely with the onset of host cell shutoff during infection (Shatkin, 1985). Instead, cleavage of eIF4GI precedes translation shutoff by 30 or more minutes. Several groups have shown that poliovirus infections carried out in the presence of inhibitors of viral RNA replication (e.g. 2 mM guanidine–HCl, quercitin, monensin) result in complete cleavage of eIF4GI, yet cap-dependent translation is only partly inhibited, usually by about 50% (Bonneau and Sonenberg, 1987, Bovee et al., 1998b, Irurzun et al., 1995, Pérez and Carrasco, 1992). Thus, some fundamental event was missing from the shutoff models that did not occur in the presence of guanidine. Further, the cleaved C-terminal fragment of eIF4GI can still support cap-dependent translation initiation, although with a limited efficiency (Ali et al., 2001). These results demonstrate that cleavage of eIF4GI alone is not sufficient to completely block host cap-dependent translation in vivo.
What could be the missing part(s) of the translation shutoff model? It is important to note that many biochemical experiments that tested the function of cleaved eIF4G used conditions where only de novo translation was measured and there was less opportunity to measure the fate of translating polysomes, e.g. ribosomes that may be able to recycle. Initially, Gradi et al. demonstrated that eIF4GII is more resistant to 2Apro cleavage during poliovirus infection than eIF4GI, particularly in infections containing guanidine. Similar correlations were observed in rhinovirus-infected cells (Svitkin et al., 1999). Thus, earlier results in which eIF4GI was found to be cleaved but translation continued at 40–50% levels was proposed to result from incomplete cleavage of eIF4GII, retaining enough functional eIF4F in the cell to sustain cap-dependent translation. This seems reasonable at first, however, eIF4GII comprises only a small portion, approximately 10%, of the total cellular eIF4G. It is unclear if a portion of uncleaved eIF4GII, can support such high translation rates (50% of normal) in guanidine–PV-infected cells. However, the cleavage of both eIF4GI and eIF4GII would likely be required for complete translation shutoff to occur.
8. Enterovirus proteinases cleave PABP
What else could have been missing from the shutoff model? Alternatively, it has been shown that PABP is also cleaved in PV- and CVB3-infected cells (Joachims et al., 1999, Kerekatte et al., 1999). Importantly, since cleavage of PABP is blocked in PV infections carried out with 2 mM guanidine–HCl, PABP cleavage also may be an important “missing event” in the model of translation shutoff. In infected cells the cleavage of PABP is substantial, but is not complete at times when the host translation shutoff is maximal. For instance, host translation is effectively shutoff by 3 h post-infection in PV-infected cells yet only 35% of PABP is cleaved at this timepoint. However, PABP cleavage typically progresses to 60–70% by 6 h post-infection (Joachims et al., 1999, Kuyumcu-Martinez et al., 2002).
Purified PABP can be directly cleaved in vitro with purified 2Apro and a single cleavage site for both PV and CVB3 2Apro was mapped in the C-terminal proline-rich region of PABP, splitting the M487–G488 peptide bond (Joachims et al., 1999, Kerekatte et al., 1999). This cleavage separates the four N-terminal RNA-recognition motifs (RRMs) from the CTD and homodimerization domains (Fig. 2). PABP cleavage correlated with translation inhibition in infected HeLa cells and in some instances PABP cleavage correlated better than eIF4G cleavage (Kerekatte et al., 1999) since it is less abrupt. Both initial reports focused on 2Apro as the mediator of PABP cleavage partly because expression of 2Apro alone was thought to be sufficient for translation inhibition (Aldabe et al., 1995, Davies et al., 1991, Sun and Baltimore, 1989). However, all picornaviruses contain another proteinase, 3Cpro, that performs most of the processing of viral polyproteins. New experimental results have established a significant role for 3C proteinase in translation regulation.
In fact, most PABP in PV-infected cells is processed by 3Cpro, not 2Apro. Further mapping studies and examination of infected HeLa cell extracts with better PABP antibodies revealed that PV 3Cpro can cleave PABP at three sites that flank the 2Apro cleavage site. These sites were mapped to Q/T413, Q/G438 and Q/G538 (Fig. 2) (Kuyumcu-Martinez et al., 2002). Thus, cleavage of PABP by 3Cpro at any site and 2Apro at its single site, all result in separation of the RRMs from the C-terminal peptide interaction domain (Fig. 2). The fact that PABP cleavage correlated with significant inhibition of translation, yet only a subset of cellular PABP was processed in cells, suggested that compartmentalization or alternate conformation may regulate PABP cleavage. Indeed, PABP fractionates into several subcellular compartments. About a third of HeLa cell PABP was found in a soluble compartment that was not associated with other initiation factors or polysomes. This PABP fraction was very refractory to cleavage with either 2Apro or 3Cpro (Kuyumcu-Martinez et al., 2002). In contrast, PABP in ribosome-enriched fractions was preferentially cleaved in vitro and in vivo compared to PABP in other fractions. An interesting proteinase-selectivity toward certain PABP pools was noted. 3Cpro preferentially processes most of the PABP in polysome fractions, not 2Apro. In addition, binding of PABP to poly(A) RNA stimulated 3Cpro-mediated cleavage and inhibited 2Apro-mediated cleavage (Kuyumcu-Martinez et al., 2002). These findings provide evidence that PABP conformational changes or association with other factors in translation complexes activates PABP cleavage. The molecular details of the complexes and specific factors that modulate proteinase cleavage have not been identified.
9. Calicivirus 3C-like proteinases cleaves PABP
Caliciviruses are single-stranded RNA viruses that cause a wide range of diseases in both humans and animals, but little is known about the regulation of cellular translation during infection. Like picornaviruses, calicivirus RNA contains an 5′-linked VPg protein and a 3′ poly(A) tail, however, there is only a very short 4–5 nucleotide 5′ leader region and currently no evidence for an IRES. Calicivirus VPg is much larger (∼15 kDa) than picornavirus VPg (∼2–5 kDa) and has been shown to bind directly to eIF3 (Daughenbaugh et al., 2003). This novel interaction may play a positive selective role in translation initiation by recruiting the 40S ribosomal subunit preferentially to calicivirus RNA. Further, calicivirus VPg itself contains sequence homology to translation factor eIF1a, indicating that it potentially may complete with cellular host factors for ribosome binding (Sosnovtseva et al., 1999). eIF1a is a small, highly conserved factor that functions in binding Met-tRNAi to 40S ribosomes and in mRNA binding and scanning (Hershey and Merrick, 2000).
The overall genomic arrangement of structural and non-structural proteins of caliciviruses is reversed from picornaviruses, yet small regions of homology exist in non-structural proteins and caliciviruses encode a 3C-like proteinase that processes its ORF1 polyprotein. There is no homologous 2Apro in caliciviruses. Experiments with human norovirus (NV) or feline calicivirus (FCV) 3C-like proteinases show they do not cleave human or mouse eIF4G in in vitro assays (Kuyumcu-Martinez et al., 2004a). However, some eIF4G cleavage does occur in FCV-infected feline kidney cells. The eIF4GI processing occurred late in infection and only partly cleaved eIF4GI at the timepoints when host translation shutoff was evident (Willcocks et al., 2004). The eIF4GI processing profile was quite different than that generated by PV 2Apro. The identity of the eIF4G-proteinase is unknown, though it could be a cellular activity activated by the infection.
In contrast to the lack of 2Apro and drastic eIF4GI cleavage, PABP is readily targeted by FCV and NV 3C-like proteinases (Kuyumcu-Martinez et al., 2004a). Interestingly, the NV and FCV proteinases cleave PABP at different sites, however, these sites are identical to the three cleavage sites used by PV 3Cpro. Thus, caliciviruses also remove the C-terminal domain of PABP that binds eIF4B and eRF3, establishing a common translation regulation theme between two distinct classes of RNA viruses. Cleavage of PABP correlated well with shutoff of host translation in FCV-infected cells (Kuyumcu-Martinez et al., 2004a).
10. Effects of PABP cleavage on translation
The functional consequences to translation resulting from PABP cleavage during virus infection are only beginning to emerge. The primary effect of cleavage of PABP would be to remove the CTD, and thus separate the binding domains for eRF3, eIF4B and PAIP from the mRNA/RNP. How would this be expected to affect translation? It is known that PABP-binding to eIF4G transduces conformation changes through eIF4G that enhance the binding of eIF4E to the cap structure (Gross et al., 2003). Similarly, in plants PABP–eIF4G binding stimulates translation and can transduce changes that increase the binding affinity of eIF4E to the cap structure (Borman et al., 2000, Luo and Goss, 2001, Wei et al., 1998). However, recombinant fragments of PABP and eIF4G bind tightly and 3Cpro cleavage of PABP has little effect on the PABP N-terminal fragment binding to eIF4GI in pull-down assays, which occurs via RRM2 (Imataka et al., 1998, Kuyumcu-Martinez et al., 2004a). Further, cleavage of both eIF4G and PABP are not expected to interrupt 5′–3′ circularization of the mRNA (Fig. 3B).
Since PV 3Cpro does not cleave eIF4G, the relative impact of PABP cleavage alone on cap-dependent translation can be measured by expression of 3Cpro in translation assays. The use of HeLa cell lysate translation system that is both cap-dependent and poly(A)-tail-dependent has allowed evaluation of the importance of these effects. Interestingly, when translation of endogenous HeLa mRNA was measured in this system, cleavage of eIF4GI and eIF4GII by addition of excess 2Apro resulted in only about a 60% decline in total translation (Kuyumcu-Martinez et al., 2004b). A portion of PABP was also cleaved by 2Apro. When 3Cpro was added to the same system, over 60% decline in translation was also observed. This suggested that partial cleavage of the 3Cpro-sensitive pool of PABP or complete cleavage of both eIF4GI and eIF4GII were equally effective in blocking translation.
However, cleavage of either eIF4G or PABP alone was insufficient to shutdown capped translation more than two–three-fold (Kuyumcu-Martinez et al., 2004b), significantly less than the drastic inhibition observed in infected cells. Further experiments with capped reporter RNAs showed that 3Cpro specifically inhibited translation of RNA containing poly(A) tails and that addition of PABP to HeLa extracts treated with 3Cpro restored translation (Kuyumcu-Martinez et al., 2004b). Addition of PABP to reticulocyte lysates treated with 2Apro also partly restored translation (Kerekatte et al., 1999). In related work, NV 3C-like proteinases added to in vitro translation systems caused inhibition of capped mRNAs in a poly(A)-dependent manner, similar to PV 3Cpro (Kuyumcu-Martinez et al., 2004a). Further, transient expression of 3Cpro in HeLa cells resulted in partial PABP cleavage and similar inhibition of translation (Kuyumcu-Martinez et al., 2004b). These findings illustrate the importance of the CTD of PABP in poly(A)-dependent translation in mammalian cells and suggest that blockage of CTD function can impact translation on polysomes to a similar extent as cleavage of eIF4G.
So how does proteinase cleavage of PABP affect translation? The results of kinetics experiments suggest that inhibition of CTD function by 3Cpro might inhibit late steps in translation (Kuyumcu-Martinez et al., 2004b, Uchida et al., 2002), and it is tantalizing to speculate that the removal of the CTD of PABP may block ribosome recycling. Very little is known about ribosome recycling via 5′–3′ interactions, however, evidence is accumulating that recycling may account for a substantial proportion of total translation initiation or may compensate for loss of de novo initiation in certain circumstances. It was actually proposed years ago that de novo initiation and recycling (called re-initiation then) were distinct processes and that recycling was cap-independent. This was demonstrated by showing that m7GDP cap analog could effectively block initiation of translation in nucleased lysates in which globin mRNA was added back, however, cap analog did not effectively block endogenous globin translation in non-nucleased lysates (Asselbergs et al., 1978). PABP is now recognized as an initiation factor since it participates in several steps in the translation initiation pathway and stimulates formation of 80S ribosomes, possibly by stimulating 60S subunit joining (Kahvejian et al., 2005). The recognition that PABP can influence these steps in de novo translation initiation experiments suggests that PABP could have similar effect in stages of ribosome recycling. It is difficult to distinguish de novo initiation and ribosome recycling experimentally.
The C-terminal domain of PABP cleaved away by 3Cpro interacts with initiation factor eIF4B, PAIP1, PAIP2, and interestingly, translation termination factor eRF3. Since the same binding cleft of PABP interacts with peptides from eIF4B and eRF3, it suggests that PABP can only bind one factor at a time (Kozlov et al., 2001, Kozlov et al., 2004). One can speculate that PABP toggles back and forth between eIF4B and eRF3 in transient reactions that are associated with ribosome recycling. If this is true in HeLa cells, it predicts that cleavage of eIF4G by 2Apro may not block recycling ribosomes, thus 50–60% continued translation in guanidine–PV-infected cells after eIF4G cleavage may actually reflect the percentage of total cellular translation that is due to ribosome recycling. Some support for this model was suggested by kinetics experiments in vitro that showed cleavage of eIF4G after polysomes were fully formed had only a slight inhibitory effect on translation whereas cleavage of eIF4GI before polysomes formed strongly blocked initiation (Fig. 4 ). In contrast, 3Cpro was more effective in inhibiting translation after polysomes formed. Drastic translation inhibition required both 2Apro and 3Cpro. This result provides some evidence for the tantalizing idea that translating ribosomes can recycle on formed polysomes and that this recycling does not require intact eIF4G. Thus, the biochemical requirements for de novo initiation may be distinct from the requirements for ribosome recycling. Finally, it is likely that enteroviruses use a dual strategy for host translation shutoff, requiring cleavage of eIF4G by 2Apro to block de novo ribosome initiation and cleavage of PABP by 3Cpro to interrupt recycling ribosomes.
Fig. 4.
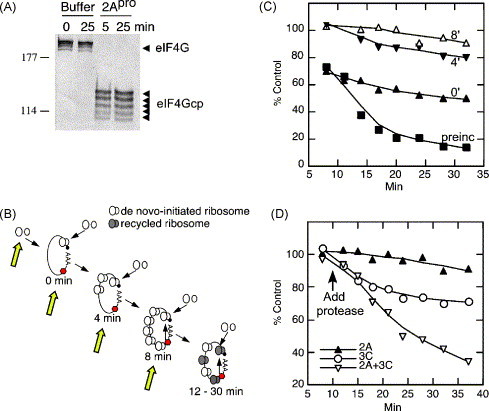
Kinetics experiments reveal severely diminished ability of 2Apro to inhibit translation after polysomes form. (A) Immunoblot shows rapid cleavage of eIF4GI in HeLa translation lysates incubated with excess CVB3 2Apro. (B) Schematic depicting ribosome loading of capped/polyadenylated luciferase RNA after addition of RNA to translation lysates at timepoint zero. Luciferase enzymatic activity takes 8 min to appear, marking the time when fully loaded polysomes first appear on Luc RNA. Ribosome recycling cannot occur until after 8 min. (C) Effect of adding 2Apro to lysates at various times before or after Luc RNA. 2Apro was preincubated with lysate 5 min, or added at 0, 4 or 8 min after Luc RNA (depicted in panel B by yellow arrows). The graph shows the accumulation of LUC relative light units plotted as percentage of the translation in mock-treated control lysate. Continued efficient polysome translation after eIF4G cleavage is likely due to ribosome recycling since de novo initiation is blocked. (D) Effect of addition of 2Apro or 3Cpro to translation lysate at 11 min after RNA was added (arrow). 3Cpro inhibited translation more effectively than 2Apro when added late. Drastic translation inhibition requires both 2Apro and 3Cpro.
11. Proteinases cleave ITAFs and regulate viral translation
What effects do viral proteinases have on translation of viral mRNA? During infection the virus must first insure that its mRNA is efficiently or preferentially translated in cells, thus many schemes to regulate host translation have been described. For picornaviruses it has been well documented that cleavage of eIF4G can stimulate IRES-dependent translation (Hambidge and Sarnow, 1992). Only the C-terminal region of eIF4G (spanning amino acids 676–1600), is required for IRES activity and may bind with other factors to the IRES (Borman et al., 1997, Ohlmann et al., 1996, Pestova et al., 1996). 5′–3′ interactions have been reported to stimulate PV-IRES-mediated translation (Bergamini et al., 2000). However, cleavage of eIF4G may interrupt this type of translation stimulation (Svitkin et al., 2001), even though overall IRES translation is upregulated by eIF4G cleavage.
In contrast to mechanisms to stimulate virus translation, plus-strand RNA viruses such as PV must also interrupt translation of the viral RNA genome at some point in the infection to allow the RNA replicase to utilize the genomic mRNA as a template. Viral polysomes must be cleared of ribosomes before RNA replication can occur. For PV it is likely that 3Cpro achieves this function by cleaving PABP and some of the ITAFs that are important for supporting IRES-dependent translation. For instance, PTB, La autoantigen and PCBP2 have been shown to play important roles in stimulating PV-IRES-mediated translation and may function as RNA chaperones, stabilizing the IRES in translationally active tertiary configurations (Costa-Mattioli et al., 2004, Hellen et al., 1993, Pilipenko et al., 2001). Both La and PTB are cleaved by 3Cpro in infected cells but result in opposing effects on PV-IRES function (Back et al., 2002, Shiroki et al., 1999). Both ITAFs are only partly cleaved during infection, similar to PABP. PTB is expressed as three isoforms (isoforms 1, 2, 4) and predominately isoforms 2 and 4 are cleaved. PTB cleavage was associated with a two-fold loss of ITAF function in translation assays with luciferase reporter RNAs in vivo (Back et al., 2002). In contrast, La autoantigen was found to be processed near its C-terminus by 3Cpro, removing a domain containing a nuclear localization signal. This truncated form of La correlated with stimulated PV-IRES translation, and 3Cpro cleavage was proposed to result from an increased concentration of La in the cytoplasm to support viral translation (Shiroki et al., 1999). In contrast, PCBP2 is cleaved in PV infection into a form that no longer supports PV-IRES-mediated translation (personal communication, Semler). It will be interesting to determine if there is similar truncation of UNR. Because ITAF cleavage generally inhibits viral translation, it is not surprising these cleavages are observed late during infection cycles. The relative importance of these ITAFs in supporting virus translation, and hence the relative effect of their cleavage has yet to be determined.
12. Indirect effects of proteinases on translation
Viruses utilize some other mechanisms to regulate host translation that are indirect. Theoretically, any attack on transcription, mRNA processing and export or increase in mRNA turnover may indirectly inhibit translation. Human enteroviruses downregulate transcription within 2 h of infection, thus reducing the flow of mRNA to the cytoplasm and upsetting homeostasis. Dasgupta and colleagues showed that poliovirus 3Cpro was able to inactivate Pol 1, Pol II and Pol III transcription in HeLa cells (Clark and Dasgupta, 1990, Clark et al., 1991, Das and Dasgupta, 1993, Kliewer et al., 1990, Rubinstein et al., 1992, Yalamanchili et al., 1997a, Yalamanchili et al., 1997b, Yalamanchili et al., 1996). In the case of rhinoviruses and PV, 3CD proteases are localized to the nucleus and concentrated in the nucleolus via a localization signal in the N-terminal domain of the 3Dpolymerase domain of 3CD (Aminev et al., 2003a, Aminev et al., 2003b, Amineva et al., 2004, Sharma et al., 2004). 3CD is the precursor for 3Cpro and 3Dpolymerase but its protease domain is very active in this precursor form and has a slightly altered substrate specificity from fully processed 3Cpro.
13. Other viruses?
While proteases of many picornaviruses and caliciviruses are known to cleave several translation factors, what of other RNA viruses? Essentially all plus-strand RNA viruses encode proteinases for polyprotein processing and potentially any of these could interact with translation factors and regulate translation, yet cleavage of translation factors has not been reported for other virus families such as coronaviruses, flaviviruses and pestiviruses. Interestingly, not all picornavirus infections result in eIF4GI cleavage. Hepatitis A virus does not encode a 2A protease and its IRES requires intact eIF4GI for translation function (Borman and Kean, 1997). Similarly, echovirus 22 does not cleave eIF4GI during infection (Coller et al., 1991). Infection of HeLa cells with Sindbis virus, an alphavirus, also do not result in eIF4GI cleavage (Lloyd, unpublished data).
14. Conclusions
Although much is known about how picornaviruses control translation via their proteinases, much remains to be elucidated. By discovering many of the cellular substrates of viral proteinases we have learned a great deal about the mechanism and regulation of the translation process. Manipulation of viral proteinases in kinetics experiments has provided new clues that ribosomes may indeed recycle. In turn, the old paradigm that eIF4GI cleavage is the essential event for translation shutoff in PV-infected cells must be modified to account for new data. A model is emerging that many RNA viruses that encode IRES elements may also manipulate cap-dependent translation via eIF4G cleavage, though cleavage may occur to various extents. Also, a new paradigm has emerged that PABP cleavage may be used by many viruses to manipulate poly(A)-dependent translation. Since most plus-strand RNA viruses have poly(A) tails on their genomes and mRNAs, PABP cleavage would have the dual effect of inhibiting both cellular translation and viral translation. These viruses must eventually block translation on their genomes to allow RNA replication. One important aspect of this model yet to be discovered is how the virus regulates cleavage of PABP or other factors so that viral translation is promoted just long enough to produce ample replicase proteins and then shutoff translation. The relatively weak cleavage of PABP and ITAFs by 3Cpro (compared to eIF4G cleavage) combined with local concentration effects from the accumulation of protease and replicase proteins at the microenvironment of viral polysomes may accomplish this regulation. Further, viruses that cleave PABP would also be expected to have other mechanisms to promote viral translation over cellular translation. This is represented by eIF4G cleavage (PV) or specific binding of VPg-RNA to eIF3 (calicivirus).
Finally, translation is a highly dynamic process that has evolved to regulate what, where and when proteins are synthesized. Work with 2A proteinase did much to uncover mechanistic steps in de novo initiation process on the 5′ end of mRNA. Now, the 3′ end of mRNA has also turned out to be surprisingly important in regulating translation. Important future work with viral proteinases that inactivate specific translation functions will hopefully elucidate more secrets about how the recycling of ribosomes for multiple rounds of translation on the same mRNA may occur and be regulated.
Acknowledgements
This work was supported by NIH grant AI50237. The author would like to thank Dr. Kyle Sherrill for critical review.
References
- Agol V.I., Belov G.A., Bienz K., Egger D., Kolesnikova M.S., Romanova L.I., Sladkova L.V., Tolskaya E.A. Competing death programs in poliovirus-infected cells: commitment switch in the middle of the infectious cycle. J. Virol. 2000;74(12):5534–5541. doi: 10.1128/jvi.74.12.5534-5541.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldabe R., Feduchi E., Novoa I., Carrasco L. Expression of poliovirus 2Apro in mammalian cells: effects on translation. FEBS Lett. 1995;377(1):1–5. doi: 10.1016/0014-5793(95)01269-9. [DOI] [PubMed] [Google Scholar]
- Ali I.K., McKendrick L., Morley S.J., Jackson R.J. Truncated initiation factor eIF4G lacking an eIF4E binding site can support capped mRNA translation. Embo J. 2001;20(15):4233–4242. doi: 10.1093/emboj/20.15.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez E., Menendez-Arias L., Carrasco L. The eukaryotic translation initiation factor 4GI is cleaved by different retroviral proteases. J. Virol. 2003;77(23):12392–12400. doi: 10.1128/JVI.77.23.12392-12400.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminev A.G., Amineva S.P., Palmenberg A.C. Encephalomyocarditis viral protein 2A localizes to nucleoli and inhibits cap-dependent mRNA translation. Virus Res. 2003;95(1–2):45–57. doi: 10.1016/s0168-1702(03)00162-x. [DOI] [PubMed] [Google Scholar]
- Aminev A.G., Amineva S.P., Palmenberg A.C. Encephalomyocarditis virus (EMCV) proteins 2A and 3BCD localize to nuclei and inhibit cellular mRNA transcription but not rRNA transcription. Virus Res. 2003;95(1–2):59–73. doi: 10.1016/s0168-1702(03)00163-1. [DOI] [PubMed] [Google Scholar]
- Amineva S.P., Aminev A.G., Palmenberg A.C., Gern J.E. Rhinovirus 3C protease precursors 3CD and 3CD’ localize to the nuclei of infected cells. J. Gen. Virol. 2004;85(Pt 10):2969–2979. doi: 10.1099/vir.0.80164-0. [DOI] [PubMed] [Google Scholar]
- Asselbergs F., Peters W., van Venrooij W., Bloemendal H. Diminished sensitivity of re-initiation of translation to inhibition by cap analogues in reticulocyte lysates. Eur. J. Biochem. 1978;88:483–488. doi: 10.1111/j.1432-1033.1978.tb12473.x. [DOI] [PubMed] [Google Scholar]
- Back S.H., Kim Y.K., Kim W.J., Cho S., Oh H.R., Kim J.E., Jang S.K. Translation of polioviral mRNA is inhibited by cleavage of polypyrimidine tract-binding proteins executed by polioviral 3C(pro) J. Virol. 2002;76(5):2529–2542. doi: 10.1128/jvi.76.5.2529-2542.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglioni C., Vesco C., Jacobs-Lorena M. The role of ribosomal subunits in mammalian cells. Cold Spring Harb. Symp. Quant. Biol. 1969;34:55–566. doi: 10.1101/sqb.1969.034.01.063. [DOI] [PubMed] [Google Scholar]
- Barco A., Feduchi E., Carrasco L. A stable HeLa cell line that inducibly expresses poliovirus 2Apro: effects on cellular and viral gene expression. J. Virol. 2000;74:2383–2392. doi: 10.1128/jvi.74.5.2383-2392.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K.M., Walter B.L., Semler B.L. Multimerization of poly(rC) binding protein 2 is required for translation initiation mediated by a viral IRES. RNA. 2004;10(8):1266–1276. doi: 10.1261/rna.7070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belov G.A., Romanova L.I., Tolskaya E.A., Kolesnikova M.S., Lazebnik Y.A., Agol V.I. The major apoptotic pathway activated and suppressed by poliovirus. J. Virol. 2003;77(1):45–56. doi: 10.1128/JVI.77.1.45-56.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsham G.J., McInerney G.M., Ross-Smith N. Foot-and-mouth disease virus 3C protease induces cleavage of translation initiation factors eIF4A and eIF4G within infected cells. J. Virol. 2000;74(1):272–280. doi: 10.1128/jvi.74.1.272-280.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamini G., Preiss T., Hentze M.W. Picornavirus IRESes and the poly(A) tail jointly promote cap-independent translation in a mammalian cell-free system. RNA. 2000;6:1781–1790. doi: 10.1017/s1355838200001679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyn L.B., Swiderek K.M., Richards O., Stahl D.C., Semler B.L., Ehrenfeld E. Poly(rC) binding protein 2 binds to stem-loop IV of the poliovirus RNA 5′ noncoding region: identification by automated liquid chromatography–tandem mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 1996;93(20):11115–11120. doi: 10.1073/pnas.93.20.11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyn L.B., Towner J.S., Semler B.L., Ehrenfeld E. Requirement of poly(rC) binding protein 2 for translation of poliovirus RNA. J. Virol. 1997;71(8):6243–6246. doi: 10.1128/jvi.71.8.6243-6246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau A.-M., Sonenberg N. Proteolysis of the p220 component of the cap-binding protein complex is not sufficient for complete inhibition of host cell protein synthesis after poliovirus infection. J. Virol. 1987;61:986–991. doi: 10.1128/jvi.61.4.986-991.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman A.M., Kean K.M. Intact eukaryotic initiation factor 4G is required for hepatitis a virus internal initiation of translation. Virology. 1997;237(1):129–136. doi: 10.1006/viro.1997.8761. [DOI] [PubMed] [Google Scholar]
- Borman A.M., Kirchweger R., Ziegler E., Rhoads R.E., Skern T., Kean K.M. elF4G and its proteolytic cleavage products: effect on initiation of protein synthesis from capped, uncapped, and IRES-containing mRNAs. RNA. 1997;3(2):186–196. [PMC free article] [PubMed] [Google Scholar]
- Borman A.M., Michel Y.M., Kean K.M. Biochemical characterisation of cap-poly(A) synergy in rabbit reticulocyte lysates: the eIF4G–PABP interaction increases the functional affinity of eIF4E for the capped mRNA 5′-end. Nucleic Acids Res. 2000;28(21):4068–4075. doi: 10.1093/nar/28.21.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussadia O., Niepmann M., Creancier L., Prats A.C., Dautry F., Jacquemin-Sablon H. Unr is required in vivo for efficient initiation of translation from the internal ribosome entry sites of both rhinovirus and poliovirus. J. Virol. 2003;77(6):3353–3359. doi: 10.1128/JVI.77.6.3353-3359.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovee M.L., Lamphear B., Rhoads R.E., Lloyd R.E. Direct cleavage of eIF4G by poliovirus 2A protease is inefficient in vitro. Virology. 1998;245:241–249. doi: 10.1006/viro.1998.9172. [DOI] [PubMed] [Google Scholar]
- Bovee M.L., Marissen W.E., Zamora M., Lloyd R.E. The predominant eIF4G-specific cleavage activity in poliovirus-infected HeLa cells is distinct from 2A protease. Virology. 1998;245:229–240. doi: 10.1006/viro.1998.9171. [DOI] [PubMed] [Google Scholar]
- Brasey A., Lopez-Lastra M., Ohlmann T., Beerens N., Berkhout B., Darlix J.L., Sonenberg N. The leader of human immunodeficiency virus type 1 genomic RNA harbors an internal ribosome entry segment that is active during the G2/M phase of the cell cycle. J. Virol. 2003;77(7):3939–3949. doi: 10.1128/JVI.77.7.3939-3949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck C., Shen X., Egan M., Pierson T., Walker C., Siliciano R. The human immunodeficiency virus type 1 gag gene encodes an internal ribosome entry site. J. Virol. 2001;75:181–191. doi: 10.1128/JVI.75.1.181-191.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushell M., Wood W., Carpenter G., Pain V.M., Morley S.J., Clemens M.J. Disruption of the interaction of mammalian protein synthesis initiation factor 4B with the poly(A) binding protein by caspase- and viral protease-mediated cleavages. J. Biol. Chem. 2001;276:23922–23928. doi: 10.1074/jbc.M100384200. [DOI] [PubMed] [Google Scholar]
- Byrd M.P., Zamora M., Lloyd R.E. Generation of multiple isoforms of eukaryotic translation initiation factor 4GI by use of alternate translation initiation codons. Mol. Cell. Biol. 2002;22(13):4499–4511. doi: 10.1128/MCB.22.13.4499-4511.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd M.P., Zamora M., Lloyd R.E. Translation of eIF4GI proceeds from multiple mRNAs containing a novel cap-dependent IRES that is active during poliovirus infection. J. Biol. Chem. 2005 doi: 10.1074/jbc.M414014200. [DOI] [PubMed] [Google Scholar]
- Carthy C.M., Granville D.J., Watson K.A., Anderson D.R., Wilson J.E., Yang D.C., Hunt D.W.C., McManus B.M. Caspase activation and specific cleavage of substrates after Coxsackievirus B3-induced cytopathic effect in HeLa cells. J. Virol. 1998;72(9):7669–7675. doi: 10.1128/jvi.72.9.7669-7675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T.C., Yamashita A., Chen C.Y., Yamashita Y., Zhu W., Durdan S., Kahvejian A., Sonenberg N., Shyu A.B. UNR, a new partner of poly(A)-binding protein, plays a key role in translationally coupled mRNA turnover mediated by the c-fos major coding-region determinant. Gene Dev. 2004;18(16):2010–2023. doi: 10.1101/gad.1219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M.E., Dasgupta A. A transcriptionally active form of TFIIIC is modified in poliovirus-infected HeLa cells. Mol. Cell. Biol. 1990;10(10):5106–5113. doi: 10.1128/mcb.10.10.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M.E., Hammerle T., Wimmer E., Dasgupta A. Poliovirus proteinase 3C converts an active form of transcription factor IIIC to an inactive form: a mechanism for inhibition of host cell polymerase III transcription by poliovirus. EMBO J. 1991;10(10):2941–2947. doi: 10.1002/j.1460-2075.1991.tb07844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B.A.G., Tracy S.M., Etchison D. Cap-binding complex protein-p220 is not cleaved during echovirus-22 replication in HeLa cells. J. Virol. 1991;65(7):3903–3905. doi: 10.1128/jvi.65.7.3903-3905.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller J., Parker R. Eukaryotic mRNA decapping. Annu. Rev. Biochem. 2004;73:861–890. doi: 10.1146/annurev.biochem.73.011303.074032. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M., Svitkin Y., Sonenberg N. La autoantigen is necessary for optimal function of the poliovirus and hepatitis C virus internal ribosome entry site in vivo and in vitro. Mol. Cell. Biol. 2004;24(15):6861–6870. doi: 10.1128/MCB.24.15.6861-6870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Dasgupta A. Identification of the cleavage site and determinants required for poliovirus 3CPro-catalyzed cleavage of human TATA-binding transcription factor TBP. J. Virol. 1993;67(6):3326–3331. doi: 10.1128/jvi.67.6.3326-3331.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughenbaugh K.F., Fraser C.S., Hershey J.W., Hardy M.E. The genome-linked protein VPg of the Norwalk virus binds eIF3, suggesting its role in translation initiation complex recruitment. EMBO J. 2003;22:2852–2859. doi: 10.1093/emboj/cdg251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M.V., Pelletier J., Meerovitch K., Sonenberg N., Kaufman R.J. The effect of poliovirus proteinase 2Apro expression on cellular metabolism. J. Biol. Chem. 1991;266:14714–14720. [PubMed] [Google Scholar]
- Duncan R., Etchison D., Hershey J.W.B. Protein synthesis eukaryotic initiation factors 4A and 4B are not altered by poliovirus infection of HeLa cells. J. Biol. Chem. 1983;258(11):7236–7239. [PubMed] [Google Scholar]
- Etchison D., Hansen J., Ehrenfeld E., Edery I., Sonenberg N., Milburn S., Hershey J.W.B. Demonstration in vitro that eucaryotic initiation factor 3 is active but a cap-binding protein complex is inactive in poliovirus-infected HeLa cells. J. Virol. 1984;51(3):832–837. doi: 10.1128/jvi.51.3.832-837.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison D., Milburn S.C., Edery I., Sonenberg N., Hershey J.W.B. Inhibition of HeLa cell protein synthesis following poliovirus infection correlates with the proteolysis of a 220,000-dalton polypeptide associated with eukaryotic initiation factor 3 and a cap binding protein complex. J. Biol. Chem. 1982;257:14806–14810. [PubMed] [Google Scholar]
- Foeger N., Glaser W., Skern T. Recognition of eukaryotic initiation factor 4G isoforms by picornaviral proteinases. J. Biol. Chem. 2002;277(46):44300–44309. doi: 10.1074/jbc.M208006200. [DOI] [PubMed] [Google Scholar]
- Foeger N., Schmid E.M., Skern T. Human rhinovirus 2 2Apro recognition of eukaryotic initiation factor 4GI involvement of an exosite. J. Biol. Chem. 2003;278(35):33200–33207. doi: 10.1074/jbc.M304007200. [DOI] [PubMed] [Google Scholar]
- Gebauer F., Hentze M.W. Molecular mechanisms of translational control. Nat. Rev. Mol. Cell. Biol. 2004;5(10):827–835. doi: 10.1038/nrm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras A.C., Raught B., Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- Glaser W., Skern T. Extremely efficient cleavage of eIF4G by picornaviral proteinases L and 2A in vitro. FEBS Lett. 2000;480(2–3):151–155. doi: 10.1016/s0014-5793(00)01928-1. [DOI] [PubMed] [Google Scholar]
- Gradi A., Foeger N., Strong R., Svitkin Y.V., Sonenberg N., Skern T., Belsham G.J. Cleavage of eukaryotic translation initiation factor 4GII within foot-and-mouth disease virus-infected cells: identification of the L-protease cleavage site in vitro. J. Virol. 2004;78(7):3271–3278. doi: 10.1128/JVI.78.7.3271-3278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradi A., Imataka H., Svitkin Y.V., Rom E., Raught B., Morino S., Sonenberg N. A novel functional human eukaryotic translation initiation factor 4G. Mol. Cell. Biol. 1998;18(1):334–342. doi: 10.1128/mcb.18.1.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradi A., Svitkin Y.V., Imataka H., Sonenberg N. Proteolysis of human eukaryotic translation initiation factor eIF4GII, but not eIF4GI, coincides with the shutoff of host protein synthesis after poliovirus infection. Proc. Natl. Acad. Sci. U.S.A. 1998;95(19):11089–11094. doi: 10.1073/pnas.95.19.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradi A., Svitkin Y.V., Sommergruber W., Imataka H., Morino S., Skern T., Sonenberg N. Human rhinovirus 2A proteinase cleavage sites in eukaryotic initiation factors (eIF) 4GI and eIF4GII are different. J. Virol. 2003;77(8):5026–5029. doi: 10.1128/JVI.77.8.5026-5029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifo J.A., Abramson R.D., Satler C.A., Merrick W.C. RNA-stimulated ATPase activity of eukaryotic initiation factors. J. Biol. Chem. 1984;259(13):8648–8654. [PubMed] [Google Scholar]
- Gross J.D., Moerke N.J., von der Haar T., Lugovskoy A.A., Sachs A.B., McCarthy J.E., Wagner G. Ribosome loading onto the mRNA cap is driven by conformational coupling between eIF4G and eIF4E. Cell. 2003;115(6):739–750. doi: 10.1016/s0092-8674(03)00975-9. [DOI] [PubMed] [Google Scholar]
- Haghighat A., Svitkin Y., Novoa I., Kuechler E., Skern T., Sonenberg N. The eIF4G-eIF4E complex is the target for direct cleavage by the rhinovirus 2A proteinase. J. Virol. 1996;70(12):8444–8450. doi: 10.1128/jvi.70.12.8444-8450.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambidge S.J., Sarnow P. Translational enhancement of the poliovirus 5′ noncoding region mediated by virus-encoded polypeptide-2A. Proc. Natl. Acad. Sci. U.S.A. 1992;89(21):10272–10276. doi: 10.1073/pnas.89.21.10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellen C.U.T., Witherell G.W., Schmid M., Shin S.H., Pestova T.V., Gil A., Wimmer E. A cytoplasmic 57-kDa protein that is required for translation of picornavirus RNA by internal ribosomal entry is identical to the nuclear pyrimidine tract-binding protein. Proc. Natl. Acad. Sci. U.S.A. 1993;90(16):7642–7646. doi: 10.1073/pnas.90.16.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey J.W.B., Merrick W.C. Pathway and mechanisms of initiation of protein synthesis. In: Sonenberg J.W.B.H.N., Matthews M.B., editors. Translational Control of Gene Expression. Cold Spring Harbor Press; Cold Spring Harbor, NY: 2000. pp. 33–88. [Google Scholar]
- Hoshino S., Hosoda N., Araki Y., Kobayashi T., Uchida N., Funakoshi Y., Katada T. Novel function of the eukaryotic polypeptide-chain releasing factor 3 (eRF3/GSPT) in the mRNA degradation pathway. Biochemistry (Moscow) 2000;64(12):1367–1372. [PubMed] [Google Scholar]
- Hunt S.L., Hsuan J.J., Totty N., Jackson R.J. unr, a cellular cytoplasmic RNA-binding protein with five cold-shock domains, is required for internal initiation of translation of human rhinovirus RNA. Gene Dev. 1999;13(4):437–448. doi: 10.1101/gad.13.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imataka H., Gradi A., Sonenberg N. A newly identified N-terminal amino acid sequence of human eIF4G binds poly(A)-binding protein and functions in poly(A)-dependent translation. EMBO J. 1998;17(24):7480–7489. doi: 10.1093/emboj/17.24.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imataka H., Olsen H.S., Sonenberg N. A new translational regulator with homology to eukaryotic translation initiation factor 4G. EMBO J. 1997;16:817–825. doi: 10.1093/emboj/16.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irurzun A., Sanchez-Palomino S., Novoa I., Carrasco L. Monensin and nigericin prevent the inhibition of host translation by poliovirus, without affecting p220 cleavage. J. Virol. 1995;69(12):7453–7460. doi: 10.1128/jvi.69.12.7453-7460.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A. Regulation of mRNA decay: decapping goes solo. Mol. Cell. 2004;15(1):1–2. doi: 10.1016/j.molcel.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Joachims M., van Breugel P.C., Lloyd R.E. Cleavage of poly(A)-binding protein by enterovirus proteases concurrent with inhibition of translation in vitro. J. Virol. 1999;73:718–727. doi: 10.1128/jvi.73.1.718-727.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahvejian A., Roy G., Sonenberg N. The mRNA closed-loop model: the function of PABP and PABP-interacting proteins in mRNA translation. Cold Spring Harb. Symp. Quant. Biol. 2001;66:293–300. doi: 10.1101/sqb.2001.66.293. [DOI] [PubMed] [Google Scholar]
- Kahvejian A., Svitkin Y.V., Sukarieh R., M’Boutchou M.N., Sonenberg N. Mammalian poly(A)-binding protein is a eukaryotic translation initiation factor, which acts via multiple mechanisms. Gene Dev. 2005;19(1):104–113. doi: 10.1101/gad.1262905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerekatte V., Keiper B.D., Bradorff C., Cai A., Knowlton K.U., Rhoads R.E. Cleavage of poly(A)-binding protein by Coxsackievirus 2A protease in vitro and in vivo: another mechanism for host protein synthesis shutoff? J. Virol. 1999;73:709–717. doi: 10.1128/jvi.73.1.709-717.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaleghpour K., Svitkin Y.V., Craig A.W., DeMaria C.T., Deo R.C., Burley S.K., Sonenberg N. Translational repression by a novel partner of human poly(A) binding protein, Paip2. Mol. Cell. 2001;7:205–216. doi: 10.1016/s1097-2765(01)00168-x. [DOI] [PubMed] [Google Scholar]
- Kirchweger R., Ziegler E., Lamphear B.J., Waters D., Liebig H.D., Sommergruber W., Sobrino F., Hohenadl C., Blaas D., Rhoads R.E., Skern T. Foot-and-mouth disease virus leader proteinase: purification of the lb form and determination of its cleavage site on eIF-4 gamma. J. Virol. 1994;68(9):5677–5684. doi: 10.1128/jvi.68.9.5677-5684.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S., Muchardt C., Gaynor R., Dasgupta A. Loss of a phosphorylated form of transcription factor CREB/ATF in poliovirus-infected cells. J. Virol. 1990;64(9):4507–4515. doi: 10.1128/jvi.64.9.4507-4515.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov G., De Crescenzo G., Lim N.S., Siddiqui N., Fantus D., Kahvejian A., Trempe J.F., Elias D., Ekiel I., Sonenberg N., O’Connor-McCourt M., Gehring K. Structural basis of ligand recognition by PABC, a highly specific peptide-binding domain found in poly(A)-binding protein and a HECT ubiquitin ligase. EMBO J. 2004;23(2):272–281. doi: 10.1038/sj.emboj.7600048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov G., Trempe J.-F., Khaleghpour K., Kahvejian A., Ekiel I., Gehring K. Structure and function of the C-terminal PABC domain of human poly(A)-binding protein. Proc. Natl. Acad. Sci. U.S.A. 2001;98:4409–4413. doi: 10.1073/pnas.071024998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyumcu-Martinez M., Belliot G., Sosnovtsev S.V., Chang K.O., Green K.Y., Lloyd R.E. Calicivirus 3C-like proteinase inhibits cellular translation by cleavage of poly(A)-binding protein. J. Virol. 2004;78(15):8172–8182. doi: 10.1128/JVI.78.15.8172-8182.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyumcu-Martinez N.M., Joachims M., Lloyd R.E. Efficient cleavage of ribosome-associated poly(A)-binding protein by enterovirus 3C protease. J. Virol. 2002;76:2062–2074. doi: 10.1128/jvi.76.5.2062-2074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyumcu-Martinez N.M., Van Eden M.E., Younan P., Lloyd R.E. Cleavage of poly(A)-binding protein by poliovirus 3C protease inhibits host cell translation: a novel mechanism for host translation shutoff. Mol. Cell. Biol. 2004;24:1779–1790. doi: 10.1128/MCB.24.4.1779-1790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamphear B.J., Kirchweger R., Skern T., Rhoads R.E. Mapping of functional domains in eukaryotic protein synthesis initiation factor 4G (eIF4G) with picornaviral proteases — implications for cap-dependent and cap-independent translational initiation. J. Biol. Chem. 1995;270(37):21975–21983. doi: 10.1074/jbc.270.37.21975. [DOI] [PubMed] [Google Scholar]
- Lamphear B.J., Yan R.Q., Yang F., Waters D., Liebig H.D., Klump H., Kuechler E., Skern T., Rhoads R.E. Mapping the cleavage site in protein synthesis initiation factor-eIF-4G of the 2A proteases from human Coxsackievirus and rhinovirus. J. Biol. Chem. 1993;268(26):19200–19203. [PubMed] [Google Scholar]
- Lee K.A.W., Edery I., Sonenberg N. Isolation and structural characterization of cap-binding proteins from poliovirus-infected HeLa cells. J. Virol. 1985;54(2):515–524. doi: 10.1128/jvi.54.2.515-524.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebig H.D., Ziegler E., Yan R., Hartmuth K., Klump H., Kowalski H., Blaas D., Sommergruber W., Frasel L., Lamphear B., Rhoads R., Kuechler E., Skern T. Purification of two picornaviral 2A proteinases — interaction with eIF-4G and influence on in vitro translation. Biochemistry. 1993;32(29):7581–7588. doi: 10.1021/bi00080a033. [DOI] [PubMed] [Google Scholar]
- Luo Y., Goss D.J. Homeostasis in mRNA initiation: wheat germ poly(A)-binding protein lowers the activation energy barrier to initiation complex formation. J. Biol. Chem. 2001;276(46):43083–43086. doi: 10.1074/jbc.M104970200. [DOI] [PubMed] [Google Scholar]
- Marcotrigiano J., Gingras A.C., Sonenberg N., Burley S.K. Cap-dependent translation initiation in eukaryotes is regulated by a molecular mimic of eIF4G. Mol. Cell. 1999;3(6):707–716. doi: 10.1016/s1097-2765(01)80003-4. [DOI] [PubMed] [Google Scholar]
- Marissen W.E., Lloyd R.E. Eukaryotic translation initiation factor 4G is targeted for proteolytic cleavage by caspase 3 during inhibition of translation in apoptotic cells. Mol. Cell. Biol. 1998;18(12):7565–7574. doi: 10.1128/mcb.18.12.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerovitch K., Pelletier J., Sonenberg N. A cellular protein that binds to the 5′- noncoding region of poliovirus RNA: implications for internal translation initiation. Gene Dev. 1989;3:1026–1034. doi: 10.1101/gad.3.7.1026. [DOI] [PubMed] [Google Scholar]
- Meerovitch K., Svitkin Y.V., Lee H.S., Lejbkowicz F., Kenan D.J., Chan E.K.L., Agol V.I., Keene J.D., Sonenberg N. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J. Virol. 1993;67:3798–3807. doi: 10.1128/jvi.67.7.3798-3807.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick W.C. Cap-dependent and cap-independent translation in eukaryotic systems. Gene. 2004;332:1–11. doi: 10.1016/j.gene.2004.02.051. [DOI] [PubMed] [Google Scholar]
- Meyer S., Temme C., Wahle E. Messenger RNA turnover in eukaryotes: pathways and enzymes. Crit. Rev. Biochem. Mol. Biol. 2004;39(4):197–216. doi: 10.1080/10409230490513991. [DOI] [PubMed] [Google Scholar]
- Munroe D., Jacobson A. Messenger RNA poly(A) tail, a 3′ enhancer of translational initiation. Mol. Cell. Biol. 1990;10(7):3441–3455. doi: 10.1128/mcb.10.7.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlmann T., Lopez-Lastra M., Darlix J.L. An internal ribosome entry segment promotes translation of the simian immunodeficiency virus genomic RNA. J. Biol. Chem. 2000;275(16):11899–11906. doi: 10.1074/jbc.275.16.11899. [DOI] [PubMed] [Google Scholar]
- Ohlmann T., Prevot D., Decimo D., Roux F., Garin J., Morley S.J., Darlix J.L. In vitro cleavage of eIF4GI but not eIF4GII by HIV-1 protease and its effects on translation in the rabbit reticulocyte lysate system. J. Mol. Biol. 2002;318(1):9–20. doi: 10.1016/S0022-2836(02)00070-0. [DOI] [PubMed] [Google Scholar]
- Ohlmann T., Rau M., Pain V.M., Morley S.J. The C-terminal domain of eukaryotic protein synthesis initiation factor (eIF) 4G is sufficient to support cap-independent translation in the absence of eIF4E. EMBO J. 1996;15(6):1371–1382. [PMC free article] [PubMed] [Google Scholar]
- Pause A., Methot N., Svitkin Y., Merrick W.C., Sonenberg N. Dominant negative mutants of mammalian translation initiation factor eIF-4A define a critical role for eIF-4F in cap-dependent and cap-independent initiation of translation. EMBO J. 1994;13(5):1205–1215. doi: 10.1002/j.1460-2075.1994.tb06370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova T.V., Shatsky I.N., Hellen C.U.T. Functional dissection of eukaryotic initiation factor 4F: the 4A subunit and the central domain of the 4G subunit are sufficient to mediate internal entry of 43S preinitiation complexes. Mol. Cell. Biol. 1996;16(12):6870–6878. doi: 10.1128/mcb.16.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipps G. Haemoglobin synthesis and polysomes in intact reticulocytes. Nature. 1965;205:567–570. doi: 10.1038/205567a0. [DOI] [PubMed] [Google Scholar]
- Pilipenko E.V., Pestova T.V., Kolupaeva V.G., Khitrina E.V., Poperechnaya A.N., Agol V.I., Hellen C.U. A cell cycle-dependent protein serves as a template-specific translation initiation factor. Gene Dev. 2000;14(16):2028–2045. [PMC free article] [PubMed] [Google Scholar]
- Pilipenko E.V., Viktorova E.G., Guest S.T., Agol V.I., Roos R.P. Cell-specific proteins regulate viral RNA translation and virus-induced disease. EMBO J. 2001;20(23):6899–6908. doi: 10.1093/emboj/20.23.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss T., Hentze M.W. Dual function of the messenger RNA cap structure in poly(A)-tail-promoted translation in yeast. Nature. 1998;392(6675):516–520. doi: 10.1038/33192. [DOI] [PubMed] [Google Scholar]
- Preiss T., Hentze M.W. Starting the protein synthesis machine: eukaryotic translation initiation. Bioessays. 2003;25:1201–1211. doi: 10.1002/bies.10362. [DOI] [PubMed] [Google Scholar]
- Preiss T., Muckenthaler M., Hentze M.W. Poly(A)-tail-promoted translation in yeast: implications for translational control. RNA. 1998;4(11):1321–1331. doi: 10.1017/s1355838298980669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevot D., Decimo D., Herbreteau C.H., Roux F., Garin J., Darlix J.L., Ohlmann T. Characterization of a novel RNA-binding region of eIF4GI critical for ribosomal scanning. EMBO J. 2003;22(8):1909–1921. doi: 10.1093/emboj/cdg175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez L., Carrasco L. Lack of direct correlation between p220 cleavage and the shut-off of host translation after poliovirus infection. Virology. 1992;189:178–186. doi: 10.1016/0042-6822(92)90693-j. [DOI] [PubMed] [Google Scholar]
- Ray B.K., Lawson T.G., Kramer J.C., Cladaras M.H., Grifo J.A., Abramson R.D., Merrick W.C., Thach R.E. ATP-dependent unwinding of messenger RNA structure by eukaryotic initiation factors. J. Biol. Chem. 1985;260(12):7651–7658. [PubMed] [Google Scholar]
- Roberts L.O., Boxall A.J., Lewis L.J., Belsham G.J., Kass G.E. Caspases are not involved in the cleavage of translation initiation factor eIF4GI during picornavirus infection. J. Gen. Virol. 2000;81(Pt 7):1703–1707. doi: 10.1099/0022-1317-81-7-1703. [DOI] [PubMed] [Google Scholar]
- Rogers G.W., Jr., Komar A.A., Merrick W.C. eIF4A: the godfather of the DEAD box helicases. Prog. Nucleic Acid Res. Mol. Biol. 2002;72:307–331. doi: 10.1016/s0079-6603(02)72073-4. [DOI] [PubMed] [Google Scholar]
- Romanova L.I., Belov G.A., Lidsky P.V., Tolskaya E.A., Kolesnikova M.S., Evstafieva A.G., Vartapetian A.B., Egger D., Bienz K., Agol V.I. Variability in apoptotic response to poliovirus infection. Virology. 2005;331(2):292–306. doi: 10.1016/j.virol.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Roy G., De Crescenzo G., Khaleghpour K., Kahvejian A., O’Conner-McCourt M., Sonenberg N. Paip1 interacts with poly(A) binding protein through two independent binding motifs. Mol. Cell. Biol. 2002;22:3769–3782. doi: 10.1128/MCB.22.11.3769-3782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen F., Edery I., Meerovitch K., Dever T.E., Merrick W.C., Sonenberg N. Bidirectional RNA helicase activity of eucaryotic translation initiation factors 4A and 4F. Mol. Cell. Biol. 1990;10(3):1134–1144. doi: 10.1128/mcb.10.3.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein S.J., Hammerle T., Wimmer E., Dasgupta A. Infection of HeLa cells with poliovirus results in modification of a complex that binds to the rRNA promoter. J. Virol. 1992;66(5):3062–3068. doi: 10.1128/jvi.66.5.3062-3068.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs A.B., Davis R.W. The poly(A)-binding protein is required for poly(A) shortening and 60S ribosomal subunit dependent translation initiation. Cell. 1989;58:857–867. doi: 10.1016/0092-8674(89)90938-0. [DOI] [PubMed] [Google Scholar]
- Sachs A.B., Varani G. Eukaryotic translation initiation: there are (at least) two sides to every story. Nat. Struct. Biol. 2000;7(5):356–361. doi: 10.1038/75120. [DOI] [PubMed] [Google Scholar]
- Schneider R.J., Mohr I. Translation initiation and viral tricks. TIBS. 2003;28:130–136. doi: 10.1016/S0968-0004(03)00029-X. [DOI] [PubMed] [Google Scholar]
- Sharma R., Raychaudhuri S., Dasgupta A. Nuclear entry of poliovirus protease-polymerase precursor 3CD: implications for host cell transcription shut-off. Virology. 2004;320(2):195–205. doi: 10.1016/j.virol.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Shatkin A.J. mRNA cap binding proteins: essential factors for initiating translation. Cell. 1985;40:223–224. doi: 10.1016/0092-8674(85)90132-1. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Isoyama T., Kuge S., Ishii T., Ohmi S., Hata S., Suzuki K., Takasaki Y., Nomoto A. Intracellular redistribution of truncated La protein produced by poliovirus 3Cpro-mediated cleavage. J. Virol. 1999;73(3):2193–2200. doi: 10.1128/jvi.73.3.2193-2200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommergruber W., Ahorn H., Klump H., Seipelt J., Zoephel A., Fessl F., Krystek E., Blaas D., Kuechler E., Liebig H.D., Skern T. 2A proteinases of Coxsackie and rhinovirus cleave peptides derived from eIF-4G via a common recognition motif. Virology. 1994;198(2):741–745. doi: 10.1006/viro.1994.1089. [DOI] [PubMed] [Google Scholar]
- Sosnovtseva S.A., Sosnovtsev S.V., Green K.Y. Mapping of the feline calicivirus proteinase responsible for autocatalytic processing of the nonstructural polyprotein and identification of a stable proteinase-polymerase precursor protein. J. Virol. 1999;73(8):6626–6633. doi: 10.1128/jvi.73.8.6626-6633.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X.-H., Baltimore D. Human immunodeficiency virus tat-activated expression of poliovirus protein 2A inhibits mRNA translation. Proc. Natl. Acad. Sci. U.S.A. 1989;86:2143–2146. doi: 10.1073/pnas.86.7.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkin Y.V., Gradi A., Imataka H., Morino S., Sonenberg N. Eukaryotic initiation factor 4GII (eIF4GII), but not eIF4GI, cleavage correlates with inhibition of host cell protein synthesis after human rhinovirus infection. J. Virol. 1999;73(4):3467–3472. doi: 10.1128/jvi.73.4.3467-3472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkin Y.V., Imataka H., Khaleghpour K., Kahvejian A., Liebig H.-D., Sonenberg N. Poly(A)-binding protein interaction with eIF4G stimulates picornavirus IRES-dependent translation. RNA. 2001;7:1743–1752. [PMC free article] [PubMed] [Google Scholar]
- Tee A.R., Proud C.G. Caspase cleavage of initiation factor 4E-binding protein 1 yields a dominant inhibitor of cap-dependent translation and reveals a novel regulatory motif. Mol. Cell. Biol. 2002;22(6):1674–1683. doi: 10.1128/MCB.22.6.1674-1683.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N., Hoshino S., Imataka H., Sonenberg N., Katada T. A novel role of the mammalian GSPT/eRF3 associating with poly(A)-binding protein in Cap/Poly(A)-dependent translation. J. Biol. Chem. 2002;277(52):50286–50292. doi: 10.1074/jbc.M203029200. [DOI] [PubMed] [Google Scholar]
- Ventoso I., Blanco R., Perales C., Carrasco L. HIV-1 protease cleaves eukaryotic initiation factor 4G and inhibits cap-dependent translation. Proc. Natl. Acad. Sci. U.S.A. 2001;98(23):12966–12971. doi: 10.1073/pnas.231343498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C.C., Balasta M.L., Ren J., Goss D.J. Wheat germ poly(A) binding protein enhances the binding affinity of eukaryotic initiation factor 4F and (iso)4F for cap analogues. Biochemistry. 1998;37(7):1910–1916. doi: 10.1021/bi9724570. [DOI] [PubMed] [Google Scholar]
- Wells S.E., Hillner P.E., Vale R.D., Sachs A.B. Circularization of mRNA by eukaryotic translation initiation factors. Mol. Cell. 1998;2:135–140. doi: 10.1016/s1097-2765(00)80122-7. [DOI] [PubMed] [Google Scholar]
- Willcocks M.M., Carter M.J., Roberts L.O. Cleavage of eukaryotic initiation factor eIF4G and inhibition of host-cell protein synthesis during feline calicivirus infection. J. Gen. Virol. 2004;85(Pt 5):1125–1130. doi: 10.1099/vir.0.19564-0. [DOI] [PubMed] [Google Scholar]
- Yalamanchili P., Datta U., Dasgupta A. Inhibition of host cell transcription by poliovirus: cleavage of transcription factor CREB by poliovirus-encoded protease 3Cpro. J. Virol. 1997;71(2):1220–1226. doi: 10.1128/jvi.71.2.1220-1226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalamanchili P., Harris K., Wimmer E., Dasgupta A. Inhibition of basal transcription by poliovirus: a virus-encoded protease (3Cpro) inhibits formation of TBP-TATA box complex in vitro. J. Virol. 1996;70(5):2922–2929. doi: 10.1128/jvi.70.5.2922-2929.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalamanchili P., Weidman K., Dasgupta A. Cleavage of transcriptional activator Oct-1 by poliovirus encoded protease 3Cpro. Virology. 1997;239(1):176–185. doi: 10.1006/viro.1997.8862. [DOI] [PubMed] [Google Scholar]
- Zamora M., Marissen W.E., Lloyd R.E. Multiple eIF4GI-specific protease activities present in uninfected and poliovirus-infected cells. J. Virol. 2002;76:165–177. doi: 10.1128/JVI.76.1.165-177.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Lamphear B., Xiong D., Knowlton K., Rhoads R. Protection of cap-dependent protein synthesis in vivo and in vitro with an eIF4G-1 variant highly resistant to cleavage by Coxsackievirus 2A protease. J. Biol. Chem. 2003;278(7):4449–4457. doi: 10.1074/jbc.M212393200. [DOI] [PubMed] [Google Scholar]


