(CHEST 1995; 108:43S-52S)
The term “chronic bronchitis” appears to have been introduced in the United Kingdom in the early 19th century to describe chronic bronchial mucosal inflammation.1 The British population continues to suffer one of the highest mortality rates from chronic bronchitis in Europe, and in the 1960s, as many as 17% of adult men were thought to be affected.2 However, the problem is worldwide in distribution. In the mid-1980s, 6.4 million Americans per year suffered episodes of bronchitis,3 and in excess of 600,000 Canadians have symptoms of chronic obstructive pulmonary disease.4 It has been suggested that 25% of adults in their middle years in the United States are affected.5 The precise relationship of infective exacerbations to the progress of the disease in individuals remains uncertain, but huge sums are expended each year on the antibiotic treatment of exacerbations, indicating a widespread belief in the efficacy of such treatment among the medical profession. However, recent studies of lower respiratory tract infections and acute exacerbations of chronic bronchitis (AECB)6, 7 suggest that, while the majority of patients respond satisfactorily to traditional antibiotics such as the broad spectrum penicillins, others may not. Factors which distinguish this group of less responsive patients have only recently been investigated. This overview will examine the epidemiology of the disease, current chemotherapeutic modalities, and methods of assessing treatment response and will suggest avenues for more discriminatory evaluation of new agents.
EPIDEMIOLOGY
A classic study of 1,000 adult bronchitic patients from 1951 to 1953 in London indicated that, after onset mainly from the third decade onwards, the prevalence rose steadily in the fifth and sixth decades predominantly in smoking men from the lower social classes.8 This pattern of disease remained unchanged in a survey of 471 patients in the United Kingdom 40 years later,7 the medical history and clinical features of these patients confirming the classic definition of chronic bronchitis.9 United Kingdom government statistics indicate respiratory illnesses to account for 14% of cash sickness benefits and that 56% of this total was related to chronic obstructive airways disease, in comparison with 9% for asthma. Although the onset phase of this illness takes many years in most patients, once significant respiratory obstruction has developed, the prognosis is poor. The 10-year mortality of a cohort of 60-year-old nonatopic smokers rose to 60% compared with 15% in nonsmoking asthmatic subjects.10
Annual death rates from chronic bronchitis in various countries, taken from the World Health Organization Statistics Annual 1986 are shown in Figure 1 . In the United Kingdom in the 1980s, deaths from this disease and its exacerbations among adult men ranked third after myocardial infarction and lung cancer (WHO Statistics Annual, 1983), predominating in the lower social classes. In the 1970s, male death rates ranged from 33/100,000 in social class 1 to 97,115, and 191/100,000, respectively, in classes 3, 4, and 5.11 Epidemiologic surveys, for example in England and Wales,12 have suggested that recurrent respiratory tract infections in childhood, consequent upon the poor social conditions of the 1920s, may have predisposed to the development of chronic bronchitis in later life and that such factors may have had a greater influence on the geographic distribution of the disease than cigarette smoking. However, numerous studies have shown a positive correlation between mortality rates from chronic bronchitis and smoking, perhaps the most convincing describing this relationship among British doctors.13
Figure 1.
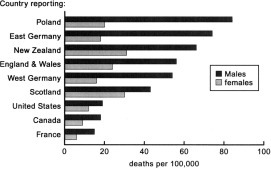
Death rates per 100,000 population: adults aged 55 to 65, 1983.
Industrial pollution is a further major precipitant, massive increases in mortality accompanying the London and Los Angeles “smogs” of the 1950s and 1960s. Reid and Fairbaim14 had previously defined the relationship between fog and work absenteeism due to bronchitis among London postmen during the 1940s. Since the Clean Air legislation in the United Kingdom, mortality from this disease has been dropping among younger people, death rates in the 1970s falling by 40 to 60% compared with those of the 1960s among patients aged 35 to 74 years.15 However, the cost to society of this illness remains high. In each of recent years, the total drug costs in the United Kingdom have exceeded £50M and the annual cost to Canadians is in excess of $33M.4
PATHOGENS ASSOCIATED WITH ACUTE BACTERIAL EXACERBATIONS OF CHRONIC BRONCHITIS
A variety of microorganisms have been shown to be associated with exacerbations of chronic bronchitis (Table 1 ). However, the preeminent pathogen is Haemophilus influenzae which has been recognized since the 1950s to cause more than 50% of all bacteriologically defined exacerbations.16, 17 Initial attempts to confirm the invasive nature of this pathogen by detection of serologic responses after exacerbations originally provided conflicting data but later evidence indicates a positive association and demonstrates the previously negative results to have been methodologic in origin.18 The frequency of isolation of H influenzae increases as respiratory obstruction worsens,19 and its role in the initiation and perpetuation of the “vicious circle” hypothesis of bronchial damage appears beyond doubt.20, 21 The role of Haemophilus parainfluenzae is less certain: Smith and colleagues19 identified the organism frequently but found no correlation with symptoms or decreasing pulmonary function. Another study of 214 patients found this organism in almost 30% of sputum cultures.22
Table 1.
Pathogens Associated With Exacerbations of Chronic Bronchitis
| Bacteria | |
|---|---|
H influenzae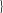
|
Accounting for 70% of all exacerbations and 85-95% of bacterial exacerbations |
| S pneumoniae V | |
| M catarrhalis | |
|
Staphylococcus aureus Pseudomonas aeruginosa Opportunist Gram-negatives Mycoplasma pneumoniae |
|
| Viruses | |
Influenza/parainfluenza viruses 
|
Accounting for 30% of all acute infective exacerbations |
| Respiratory syncytial virus | |
| Rhinoviruses | |
| Coronaviruses |
The proportions of major bacterial respiratory pathogens isolated from patients in recent clinical trials are indicated in Table 2 . Moraxella catarrhalis and Streptococcus pneumoniae account for approximately a further third of isolates from AECB. Although persistently present in respiratory secretions of established chronic bronchitic patients, H influenzae and pneumococci are isolated significantly more frequently during infective exacerbations.23 Further evidence suggests that they may persist in low numbers in sputum after apparently effective therapy of AECB and that the same phenotypic isolates are responsible for subsequent relapses.24, 25
Table 2.
Prevalence of Major Respiratory Pathogens in AECB
| Percentage of Total Isolates Accounted for by |
||||
|---|---|---|---|---|
| Study | No. of isolates | H influenzae | M catarrhalis | Pneumococci |
| Davies et al, 198678 | 127 | 58,5 | 15 | 16,5 |
| Basran et al, 199059 | 60 | 43.3 | 3.3 | 25 |
| Chodosh, 199222 | 214 | 37.9 | 22.4 | 22.4 |
| Aidons, 199155 | 53 | 70 | 13 | 15 |
| Bachand, 199176 | 84 | 30 | 10.7 | 21.4 |
| Lindsay et al, 199283 | 398 | 49.7 | 19 | 17 |
| Ball, 199462 | 85 | 52 | 13 | 16,5 |
Viruses and mycoplasmas are probably responsible for a third of all exacerbations.26 Chlamydiae, important precipitants of acute bronchitis, play a minor role, if any, in the causation of AECB.27
CHEMOTHERAPY FOR ACUTE EXACERBATIONS OF CHRONIC BRONCHITIS
The majority of patients with AECB presenting to primary care physicians receive antibiotic therapy. In the United Kingdom, this accounts for a substantial proportion of the 17.2 million antibiotic prescriptions issued per year (Intercontinental Medical Statistics, MDI Dec 1993, MAT). However, between 13 and 25% of such patients with lower respiratory tract infections treated on a domiciliary basis return within a matter of a few weeks because of persisting symptoms.6, 7, 28 It is thus necessary to question whether antibiotic therapy per se is effective in AECB, and if so, whether certain of the newer antibiotics, which have improved in vitro activity and pharmacokinetics, are likely to prove more effective than traditional agents such as the broad spectrum penicillins. It is perhaps even more important to assess the evidence for the efficacy of rapid, effective therapy of acute exacerbations in preventing the inexorable decline in respiratory function which characterises this disease. Anthonisen and colleagues29 found that the acute reduction in FEV1 associated with AECB improved more rapidly with antibiotic therapy than with placebo. However, a comprehensive literature review18 found only one study which indicated recurrent infective exacerbations to have any long-term detrimental effect on pulmonary function in chronic bronchitics.
EFFICACY OF ANTIBIOTIC THERAPY IN AECB
General Considerations
As depicted in Table 3 , the efficacy or otherwise of antimicrobial chemotherapy has remained for many years a controversial issue, with various placebo-controlled studies in relatively small numbers of patients having provided conflicting evidence for and against the benefit of antibiotics.18, 30, 31a In the late 1980s, Anthonisen and coworkers29 published a further, large scale, placebo-controlled study in over 350 patients which, in defined exacerbations, indicated significant benefit from antibiotic therapy. Patients allocated antibiotic therapy received either amoxycillin (40%), co-trimoxazole (40%), or doxycycline (20%). Although the results overall indicated a significant difference in favor of antibiotic treatment, optimal benefits were observed in patients who had exacerbations characterized by increases in dyspnea, sputum production, and sputum purulence (often termed the “Winnipeg criteria”). Subsequently, an Italian group31b studying a similarly sized population, showed a highly significant difference in favor of co-amoxyclav (Augmentin) compared with placebo in patients with standardized disease severity. This group based their assessment on an empiric scoring system which incorporated the Winnipeg criteria together with additional factors, including the presence and degree of pyrexia, severity of cough, and presence of coexistent cardiopulmonary disease. The results of these and earlier studies are shown in Table 3.
Table 3.
Results of Placebo-Controlled Trials of Efficacy of Antibiotic Therapy in AECB
| Comparators | No. of patients | Outcome of Therapy | Reference |
|---|---|---|---|
| Placebo vs | 179 | 50.3% vs | Allegra et al, 199131b |
| co-amoxyclav | 190 | 86.4% clinical success | |
| p<0.0l | |||
| Placebo vs | 180 | 55% vs | Anthonisen et al, 198729 |
| either TMP-SMX,* | 182 | 68% clinical success | |
| amoxycillin or doxycycline | p<0.0l | ||
| Placebo vs | 20 | 100% vs | Nicotra et al, 198284 |
| tetracycline | 20 | 100% clinical response† | |
| Placebo vs | 15 | 20% vs | Pines et al, 196885 |
| penicillin/ | 15 | 66% improvement‡ | |
| streptomycin | |||
| Placebo vs | 10 | No significant differences in either | Petersen et al, 196786 |
| physiotherapy, | 10 | group | |
| chloramphenicol | 9 | ||
| Placebo vs | 28 | No significant difference in clinical | Elmes et al, 196550 |
| ampicillin | 28 | response§ |
Trimethoprim-sulfamethoxazole.
Significant trends to more rapid response in oxygenation with tetracycline.
Nine patients deteriorated (three deaths) on placebo compared with two (one death) on active therapy.
The frequency of relapse of H influenzae infections was reduced by ampicillin.
The varying results of the historic and contemporary studies of chemotherapy in AECB may have a number of explanations, not least the nonhomogeneity of the patient populations studied and the improvements in chemotherapy over the 30 years in question. The increasing prevalence of bacterial resistance has also had a major influence on choices between traditional agents and novel antibiotics which are either insusceptible to or are capable of bypassing the common resistance mechanisms. However, insistence by many registration authorities that new drugs must be compared to traditional agents such as amoxycillin and that for standard evaluation, all isolates should be sensitive to both the investigational and comparator agents often results in failure to prove what should be clear advantages.
As frequently shown in pneumonia trials,32 intention to treat analyses may be far more indicative of the subsequent clinical role of the new agent. A further problem follows the use of such criteria as those proposed by the Winnipeg group,29 in that although these criteria define an exacerbation, they do not define its etiology. Thus noninfective or viral exacerbations may be being compared with bacterial exacerbations, with inevitably confusing results.
Influence of Bacterial Resistance
Sensitivity of respiratory tract pathogens to traditional antibiotics may no longer be assumed. Nationwide studies in the United States and Canada in the 1980s indicated beta-lactamase-mediated amoxycillin resistance among nonencapsulated strains of H influenzae to have risen to approximately 15 to 16%.33, 34 These figures were mirrored by rates of up to 20% in Poland in the 1990s35 and 8.3% in the United Kingdom36 where there was an associated increase in non-beta-lactamase-mediated beta-lactam resistance (5.8%), implying resistance to co-amoxyclav, and in resistance to sulphamethoxazole (16.9%), trimethoprim (8%), and cefaclor (5.2%).
Resistance rates in Europe vary widely, but in mainland Spain, 31% of isolates were resistant to ampicillin, 16.7% to chloramphenicol, 15% to erythromycin (27.9% in France), 17.2% to tetracycline, and 41.3% to co-trimoxazole.37 Methodologic differences may influence erythromycin sensitivity testing but in the United Kingdom, Powell et al38 found 86.6% to have minimum inhibitory concentrations (MICs) equal to or greater than 1 mg/L.
Most isolates of M catarrhalis produce beta-lactamase, 79% of UK isolates being considered resistant38 and penicillin resistance among S pneumoniae is burgeoning worldwide, reaching approximately one third of all isolates in Spain, 26% in some areas of France, and 15 to 20% in the United States.39, 40
Choice among appropriate agents for the management of AECB is now clearly constrained by local resistance rates, and broad recommendations for the use of standard beta-lactams, co-trimoxazole, tetracyclines, and erythromycin can no longer be justified.
Pharmacokinetic Considerations
There are profound differences in the penetration of different antibiotic classes into the tissues and secretions of the respiratory tract, and the implications of these factors for the treatment of acute exacerbations of chronic bronchitis merit initial consideration. Although precise relationships have not been clearly established, both sputum and bronchial mucosal concentrations of antibiotics are considered potentially predictive of the outcome of therapy in lower respiratory infections.41, 42 In general, beta-lactams attain only 5 to 25% of simultaneous serum concentrations in sputum and bronchial secretions, whereas erythromycin, chloramphenicol, and tetracyclines often achieve ratios of 50% or more, and quinolones produce concentrations in bronchial secretions which are 80 to 200% of those in serum.41, 42, 43 Azithromycin is highly concentrated in sputum and bronchial secretions.44 Levels of beta-lactams in bronchial mucosa are higher, perhaps 35 to 55% of those in serum, while concentrations of quinolones range up to 200% and those of azithromycin may be 50- to 100-fold greater.42 The ratios of the concentrations obtained in respiratory tissues and fluids to those in serum are shown in Table 4 .
Table 4.
Ratios of Sputum/Bronchial Secretion to Serum Concentrations for Selected Antibiotics at Dosages Shown
| Agent, dose, mg* | Concentration (mg/L) in Serum | Ratio of Sputum or B. Secretion/Serum | Ratio of Bronchial Mucosa/Serum |
|---|---|---|---|
| Amoxycillin, 1,000 | 6.9 | 0.06 | |
| Amoxycillin, 500 | 4.13 | 0.65 | |
| Amoxycillin, 500 | 5.1 | 1.41* | |
| Clavulanate, 125 | 2.3 | 1.04* | |
| Amoxycillin, 500 | 6.6 | 0.40† | |
| Clavulanate, 250 | 5.15 | 0.36† | |
| Cefaclor, 500 | 6.23 | 0.067 | |
| Cefaclor, 500 | 7.2 | 0.14 | |
| Cefaclor, 500 | 9.6 | 0.45 | |
| Cefuroxime, 1,000 | 12.8 | 0.14 | |
| Cefuroxime, 500 | 3.5 | 0.51 | |
| Cefixime, 400 | 6.6 | 0.36 | |
| Doxycycline, 200 | 3.8 | 0.18 | |
| Doxycycline, 100 | 2.0 | 0.17 | |
| Ciprofloxacin, 500 | 3.4 | 0.38 | |
| Ciprofloxacin, 500 | 3.1 | 1.29 | |
| Ofloxacin, 400 | 4.03 | 0.77 | |
| Temafloxacin, 400 | 6.9 | 1.77 | |
| Clindamycin, 300 | 2.6 | 0.61 | |
| Erythromycin, 500 | 4.3 | 0.05 | 1.67 |
| Clarithromycin, 500 | 2.3 | 4.43 | |
| Azithromycin, 500 | 0.4 | 9.75 | |
| (single dose) |
Data taken from references 43, 49, 87, 88, 89, 90, 91 (co-amoxiclav 67, 68) and various other references in the text.
See text.
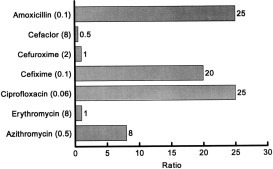
The ratios of the attainable sputum, bronchial secretion, and bronchial mucosal concentrations related to the MIC90 of H influenzae and the pneumococcus are depicted in Figures 2 and 3 . High ratios should predict greater clinical efficacy, and these relationships are referred to in the following paragraphs dealing with individual classes of antibiotic.
Figure 2.
Ratios of antibiotic concentrations in the bronchial mucosa to the MIC 90 (mg/L) of H influenzae (MICs are given in parentheses: kinetic data from Table 4).
Figure 3.
Ratios of antibiotic concentrations in the bronchial mucosa to the MIC 90 (mg/L) of S pneumoniae (MICs are given in parentheses: kinetic data from Table 4).
INDIVIDUAL CHEMOTHERAPEUTIC AGENTS
Tetracyclines
Many of the original trials of antibiotic therapy in AECB utilized tetracyclines. Two early studies of prophylaxis of exacerbations indicated a reduction in days lost from work in patients receiving Oxytetracycline.45, 46 However, the number of exacerbations in winter months was not reduced and such studies have not been pursued. Early treatment studies suggested that treated patients might recover sooner and deteriorate less often than control subjects.47
Pines48 performed many studies with tetracyclines in the 1960s and 1970s, concluding that, on the basis of his experience and that of others, tetracycline therapy was more effective than placebo in milder infections, derivatives were no more effective than tetracycline itself, severe infections required large doses and that prophylactic use was largely ineffective. In contrast, Nicotra et al31 found no differences between tetracycline and placebo at the end of treatment of moderately ill patients.
Maesen and colleagues49 repeated a 1960s study of doxycycline in the 1980s, attempting to reassess the place of this compound in contemporary therapy. Clinical results were excellent or good in 74% of patients but H influenzae, the major pathogen in AECB, presented difficulties in eradication. Nevertheless, the authors considered that doxycycline remained a useful oral antibiotic for management of AECB and was especially useful in infections caused by M catarrhalis. Tetracyclines are useful for minor AECB, but resistance dictates against their use in severely ill patients with minimal ventilatory reserve.
Oral Broad-Spectrum Penicillins and Cephalosporins
Although early placebo-controlled studies failed to show conclusive advantages for active therapy in AECB,50 ampicillin and its successor amoxycillin have become the most widely used agents for management of AECB.6, 7, 28 They are drugs of choice in patients with mild-to-moderate exacerbations in countries or counties where resistance among H influenzae and pneumococci remains at low levels.
May and Ingold51 considered amoxycillin superior to ampicillin although, on the basis of follow-up studies, Chodosh52 would undoubtedly disagree. Various regimens have been evaluated including a comparison of standard 7-day amoxycillin therapy with a 3-day course in which patients received 3 g twice daily.53 High dose amoxycillin was well tolerated, and there were no significant differences between the two groups. The mean number of exacerbations in the year following therapy was similar although the mean time to the first exacerbation was longer (21 weeks) after high dose therapy compared with 15 weeks for those receiving a standard treatment course.
An impressive array of data attesting the efficacy of these agents in mild to moderate AECB caused by sensitive organisms has accumulated from drug development studies which use them as comparators. In patients categorized as suffering from lower respiratory tract infections, the majority of which suffer from acute bronchitis or AECB, few differences have been demonstrated between ciprofloxacin, ofloxacin, azithromycin and clarithromycin, and oral broad-spectrum penicillins.54, 55, 56, 57 Studies in patients with more severe episodes of AECB, some fulfilling the Winnipeg criteria,29 have on occasion, shown advantages compared with ampicillin or amoxycillin for example, with azithromycin and ciprofloxacin.58, 59
Despite their relatively poor activity and suboptimal respiratory pharmacokinetics, cephalexin and cefaclor have been used extensively for the management of AECB, and therefore, until recently, have commonly been used as standard comparators in clinical trials of new agents. In severe infections, cefaclor gave poor results,60 and ciprofloxacin proved significantly superior to both.56 Newer cephalosporins, eg, cefixime61 and cefprozil,62 may have some advantages. Comparisons of newer oral cephalosporins with amoxycillin have rarely shown significant advantages, providing the organisms were fully sensitive to both agents.
Co-amoxiclav
Although most studies of patients with lower respiratory infection have shown co-amoxiclav to be the equivalent of standard comparators, a number have demonstrated superiority to amoxycillin63, 64 and to parenteral cefuroxime followed by oral cephalexin, cefaclor, and josamycin.65 Comparison with ciprofloxacin showed no significant differences.66 An overview of the data from clinical trials demonstrates co-amoxiclav to be a valuable agent for infections caused by beta-lactamase producing H influenzae and M catarrhalis.65 However, controversy still surrounds the degrees of penetration of amoxycillin and clavulanic acid into bronchial mucosa, which range from 40% of simultaneous serum levels when estimated by one group of investigators to over 100% with others.67, 68
Co-trimoxazole
In the early 1970s, few agents other than ampicillin/amoxycillin enjoyed the popularity of co-trimoxazole. The 12-hour administration schedule enhanced compliance, and original clinical trial results suggested that the combination might be more effective than amoxycillin and various tetracyclines.69 However, a follow-up study suggested that this benefit was not maintained into convalescence.70 Comparisons with the oral cephalosporins generally showed no significant differences.71, 72 Lacey and coworkers73 demonstrated equality between trimethoprim alone and co-trimoxazole among patients with a variety of lower respiratory tract infections. However, the recognition of the toxicity of co-trimoxazole, especially in elderly patients,74 and the increasing availability of safer agents with potentially enhanced activity, resulted in a dramatic decline in the use of the compound in Europe in the late 1980s.
Chloramphenicol
If not for its infrequent but well-recognized marrow toxicity, chloramphenicol would remain an extremely useful agent in chronic bronchitis, perhaps worthy of reappraisal in patients with severe exacerbations of this disease. It is highly active against H influenzae, the principal pathogen, among isolates of which resistance rates are low, ranging from 0.5 to 2.2% in most of Europe, although significantly higher, at 16.7%, in Spain.37 Concentrations in bronchial secretions exceed 50% of simultaneous serum levels.43 Anecdotal reports of benefit in severely ill patients with minimal respiratory reserve are commonplace, but definitive clinical studies are lacking. In the late 1990s, in the presence of widespread resistance to beta-lactams, it may be that a reassessment of the place of chloramphenicol for severe exacerbations is overdue.
New Macrolides and Azalides
Erythromycin has poor activity against H influenzae (MIC 4 to 8 mg/L) and cannot be considered one of the drugs of choice for AECB. Azithromycin and clarithromycin have improved pharmacokinetics (Table 4) and antibacterial activity. Azithromycin has much enhanced potency against H influenzae (MIC 0.5 mg/L) and compares favorably with amoxycillin and co-amoxiclav in patients with Winnipeg type 1 exacerbations.57 Two significant advantages for azithromycin are apparent: first, the once daily administration and abbreviated 3-day course, aiding compliance, and second, a reduced frequency of relapse during extended follow-up.58 Clarithromycin has only intermediate activity against H influenzae, but synergy with the human metabolite 14 hydroxyclarithromycin reduces the overall MIC to around 1 mg/L and thus into the therapeutic range.75 Clinical studies of clarithromycin involving 7 to 14-day regimens administered to patients with mild to moderate infections have shown equivalence with ampicillin.55, 76 A direct comparison of azithromycin (3-day course) and clarithromycin (10 days) showed no difference in response rates or adverse reactions.77
These agents may be considered for patients with moderate to severe exacerbations in whom standard agents have become compromised by multiple previous use and the emergence of resistance.
Fluoroquinolones
Despite gloomy prognostications which questioned the activity of fluoroquinolones against S pneumoniae and suggested that clinical response rates might not exceed 65%,78 agents such as ciprofloxacin and ofloxacin have proven remarkably effective in AECB. Their potency against H influenzae and M catarrhalis and superior penetration into sputum and bronchial mucosa are reflected by favorable comparisons with beta-lactams and other traditional agents. Reviews of the efficacy of fluoroquinolones in AECB indicate response rates of 80 to 95%, and trials of ciprofloxacin have frequently demonstrated superiority to standard agents such as ampicillin, cefaclor, cephalexin, and josamycin in terms of bacterial eradication, notably of H influenzae.56 Prior to its abrupt withdrawal, temafloxacin, which had enhanced activity against the pneumococcus, appeared to have a very promising future.79 Sparfloxacin also has similarly improved activity, but proved only equivalent to co-amoxiclav by both evaluables and intention to treat analyses in a double-blind, multicenter study of 734 adults with exacerbations conforming to Winnipeg type 1 criteria.80 The increasing prevalence of resistance to standard antibiotics among respiratory pathogens may soon elevate the fluoroquinolones to a position of primary choice in the management of moderate-to-severe exacerbations, an outcome supported by the development of new compounds, such as Bay 3118 and CP 99,219, which have even greater Gram-positive activity.
NEW APPROACHES TO OUTCOME ASSESSMENT
Since the landmark study by Anthonisen and colleagues29 suggested criteria which, on empiric grounds, were thought to be predictive of the severity of exacerbations, many studies have incorporated such assessments. Despite this, very few trials have succeeded in demonstrating the superiority of novel agents which offer significant improvements in antibacterial activity and respiratory tissue/fluid penetration, over standard antibiotics. Thus, ciprofloxacin, ofloxacin, clarithromycin, and azithromycin should prove superior to agents such as amoxycillin and cefaclor, but very few studies based on assessments at the end of therapy have confirmed more than equivalence.54, 56, 57, 75 In some cases, this may relate to the assessment criteria used. Thus if, as in the studies on fluoroquinolones by Davies and his group,78 clinical response and pathogen eradication are insisted upon, the reappearance of a pathogen in follow-up studies a week after therapy may be interpreted as overall failure. However, it is increasingly recognized that H influenzae and pneumococci may persist in bronchial secretions despite apparently satisfactory clinical response,24, 25 calling into question this method of assessment.
Clinical comparisons made 2 to 4 weeks after completion of treatment, may prove more discriminatory. For example, Pines and colleagues70 found no difference between amoxycillin and co-trimoxazole at the end of therapy but only a third receiving co-trimoxazole remained free of relapse at 2 to 4-week follow-up compared with 72% of those who had received amoxycillin. This difference was statistically significant.70 Chodosh,52 analyzing long-term follow-up data on a series of trials in AECB, has shown that the mean time from the end of therapy of one exacerbation to the onset of the next may allow more reliable assessment of comparative efficacy. Thus, if the time to relapse after ampicillin therapy (about 200 days) is taken as the index value, ratios for the same periods applicable to other agents can be calculated. Using this approach, the poor performance of oral cephalosporins is emphasized, the ratios for cephalexin and cefaclor being 0.31, indicating much earlier relapse after treatment with these compounds. In contrast, the efficacy of the quinolones is demonstrated by such methods. Ciprofloxacin (208 days to relapse) and temafloxacin (235 days) offer ratios equal to or in excess of unity when compared with ampicillin.79
A similar approach was taken by Bennet and colleagues53 who measured not only the time to the next exacerbation but also the number of exacerbations in the year following therapy. Alternatively, response can be assessed at a fixed interval. For example, Petrie and coworkers58 found the relapse rate 3 months after azithromycin therapy of hospitalized patients conforming to Winnipeg type 1 exacerbation criteria29 to be significantly lower (46 vs 62%) than that for patients treated with amoxycillin. Clearly, the advantages of quinolones, new macrolides, and azalides are augmented by the relative lack of resistance among respiratory pathogens: for example, a recent study noted that only 105 of 162 isolates from AECB were ampicillin-sensitive.76
However, although most studies comply with accepted definitions of chronic bronchitis and its exacerbations, interpretation of the results of clinical trials is complicated by a lack of standardization of the severity of illness in the populations studied. Empirically based systems such as the Winnipeg criteria29 and the Italian composite score31b may define acute exacerbations, but no studies were undertaken to correlate their observations with outcome and thus with severity. We have performed an observational study of 471 patients with acute exacerbations, recording historic and clinical parameters at presentation and follow-up, and relating these to outcome, utilizing stepwise logistic regression techniques.7 The results show that, while they may accurately define an exacerbation, none of the Winnipeg criteria (increase in dyspnea, sputum production, and sputum purulence) either singly or in combination predict outcome. The two factors found positively to predict return to the family practitioner with similar symptoms within 4 weeks of the initial presentation were (1) frequent exacerbations (>4) within the previous year, and (2) significant comorbidity (unrelated cardiopulmonary disease). The latter factor conforms to similar observations relating to outcome of bacterial pneumonia.81, 82 Trials comparing new quinolones, macrolides, azalides, and other antibiotic classes with traditional compounds should, in the future, be performed in patients who conform to these and the Winnipeg criteria and should involve assessments both at the end of therapy and through extended follow-up. Real differences might then appear in favor of the new agents which could be translated into improved patient care.
REFERENCES
- 1.Badham C. Observations on the inflammatory affections of the mucous membranes of the bronchiae. London, 1808
- 2.Report (College of General Practitioners) Chronic bronchitis in Great Britain. BMJ. 1961;2:973. [Google Scholar]
- 3.Garibaldi RA. Epidemiology of community acquired respiratory infections in adults. Am J Med. 1985;78(suppl 6B):32–37. doi: 10.1016/0002-9343(85)90361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balter MS, Hyland RH, Low DE. Recommendations on the management of chronic bronchitis: a practical guide for Canadian physicians. Can Med Assoc J. 1994;151:1–23. [Google Scholar]
- 5.Woolcock AJ. Epidemiology of chronic airways disease. Chest. 1989;96(suppl):302S–306S. doi: 10.1378/chest.96.3_supplement.302s. [DOI] [PubMed] [Google Scholar]
- 6.MacFarlane JT, Colville A, Guion A. Prospective study of aetiology and outcome of adult lower respiratory tract infections in the community. Lancet. 1993;341:511–514. doi: 10.1016/0140-6736(93)90275-l. [DOI] [PubMed] [Google Scholar]
- 7.Ball P, Harris JM, Lowson D. Acute infective exacerbations of chronic bronchitis: study of outcome by clinical parameters. Q J Med. 1995;88:61–68. [PubMed] [Google Scholar]
- 8.Oswald NC, Harold JT, Martin WJ. Clinical pattern of chronic bronchitis. Lancet. 1953;3:639–643. doi: 10.1016/s0140-6736(53)90369-9. [DOI] [PubMed] [Google Scholar]
- 9.Medical Research Council Definition and classification of chronic bronchitis for clinical and epidemiological purposes. Lancet. 1965;1:775–779. [PubMed] [Google Scholar]
- 10.Burrows B, Bloom JW, Traver GA. The course and prognosis of different forms of chronic airways obstruction in a sample from the general population. N Engl J Med. 1987;317:1309–1314. doi: 10.1056/NEJM198711193172103. [DOI] [PubMed] [Google Scholar]
- 11.Parliamentary Question Bronchitis and asthma deaths. BMJ. 1971;1:616. [Google Scholar]
- 12.Barker DJP, Osmond C. Childhood respiratory infection and adult chronic bronchitis in England and Wales. BMJ. 1986;293:1271–1275. doi: 10.1136/bmj.293.6557.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doll R, Peto R. Mortality in relation to smoking: 10 years observations on British doctors. BMJ. 1976;2:1525–1528. doi: 10.1136/bmj.2.6051.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reid DD, Fairbairn AS. The natural history of chronic bronchitis. Lancet. 1958;2:1147–1152. doi: 10.1016/s0140-6736(58)91950-0. [DOI] [PubMed] [Google Scholar]
- 15.Report (Royal College of Physicians of London) Health and smoking. Pitman; London: 1983. [Google Scholar]
- 16.Mulder J, Goslings WRO, Vaan der Plas MC. Studies on the treatment with antibacterial drugs of acute and chronic purulent bronchitis caused by Haemophilus influenzae. Acta Med Scand. 1952;143:32–49. doi: 10.1111/j.0954-6820.1952.tb14249.x. [DOI] [PubMed] [Google Scholar]
- 17.Lees AW, McNaught W. Bacteriology of lower respiratory tract secretions, sputum and upper respiratory tract secretions in <<normals>> and chronic bronchitics. Lancet. 1959;2:1112–1115. doi: 10.1016/s0140-6736(59)90099-6. [DOI] [PubMed] [Google Scholar]
- 18.Murphy TF, Sethi S. Bacterial infection in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992;146:1067–1083. doi: 10.1164/ajrccm/146.4.1067. [DOI] [PubMed] [Google Scholar]
- 19.Smith CB, Golden CA, Kanner RE. Haemophilus influenzae and Haemophilus parainfluenzae in chronic obstructive pulmonary disease. Lancet. 1976;1:1253–1255. doi: 10.1016/s0140-6736(76)91733-5. [DOI] [PubMed] [Google Scholar]
- 20.Cole P, Wilson R. Host-microbial interelationships in respiratory infection. Chest. 1989;95:217S–221S. [Google Scholar]
- 21.Wilson R. Infections of the airways. Curr Opin Infect Dis. 1991;4:166–167. [Google Scholar]
- 22.Chodosh S. Bronchitis and asthma. In: Gorbach SL, Bartlett JG, Blacklow NR, editors. Infectious diseases. WB Saunders; Philadelphia: 1992. pp. 476–485. [Google Scholar]
- 23.Fisher M, Akhtar AJ, Calder MA. Pilot study of factors associated with exacerbations in chronic bronchitis. BMJ. 1969;4:187–192. doi: 10.1136/bmj.4.5677.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calder MA, Schonell ME. Pneumococcal typing and the problem of endogenous or exogenous reinfection in chronic bronchitis. Lancet. 1971;1:1156–1159. doi: 10.1016/s0140-6736(71)91663-1. [DOI] [PubMed] [Google Scholar]
- 25.Groeneveld K, van Alphen L, Eijk PP. Endogenous and exogenous re-infections by Haemophilus influenzae in patients with chronic obstructive airways disease: the effect of antibiotic treatment on persistence. J Infect Dis. 1990;161:512–571. doi: 10.1093/infdis/161.3.512. [DOI] [PubMed] [Google Scholar]
- 26.Gump DW, Phillips CA, Forsyth BR. Role of infection in chronic bronchitis. Am Rev Respir Dis. 1976;113:465–473. doi: 10.1164/arrd.1976.113.4.465. [DOI] [PubMed] [Google Scholar]
- 27.Beaty CD, Grayston JT, Wang SP. Chlamydia pneumoniae, Strain TWAR, infection in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1991;144:1408–1410. doi: 10.1164/ajrccm/144.6.1408. [DOI] [PubMed] [Google Scholar]
- 28.Davey P, Rutherford D, Graham B. Repeat consultations after antibiotic prescribing for respiratory infection: a study in one general practice. Br J Gen Pract. 1994;44:509–513. [PMC free article] [PubMed] [Google Scholar]
- 29.Anthonisen NR, Manfreda J, Warren CPW. Antibiotic therapy in acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 30.Tager I, Speizer FE. Role of infection in chronic bronchitis. N Engl J Med. 1975;292:563–571. doi: 10.1056/NEJM197503132921105. [DOI] [PubMed] [Google Scholar]
- 31.Nicotra MB, Rivera M, Awe RJ. Antibiotic therapy of acute exacerbations of chronic bronchitis: a controlled study using tetracycline. Ann Intern Med. 1982;97:18–21. doi: 10.7326/0003-4819-97-1-18. [DOI] [PubMed] [Google Scholar]
- 31.Allegra L, Grassi C, Grossi E. Ruolo degli antibiotici nel trattamento delle riacutizza della bronchite cronica. Ital J Chest Dis. 1991;45:138–148. [Google Scholar]
- 32.Anderson G, Esmonde TS, Coles A. A comparative safety and efficacy study of clarithromycin and erythromycin stearate in community acquired pneumonia. J Antimicrob Chemother. 1991;27(suppl A):117–124. doi: 10.1093/jac/27.suppl_a.117. [DOI] [PubMed] [Google Scholar]
- 33.Jorgensen JH, Doern GV, Maher LA. Antimicrobial resistance among respiratory isolates of Haemophilus influenzae, Moraxella catarrhalis and Streptococcus pneumoniae in the United States. Antimicrob Agents Chemother. 1990;34:2075–2080. doi: 10.1128/aac.34.11.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tremblay LD, L’ecuyer J, Provencher P. Canadian study group: susceptibility of Haemophilus influenzae to antimicrobial agents used in Canada. Can Med Assoc J. 1990;143:895–901. [PMC free article] [PubMed] [Google Scholar]
- 35.Hryniewicz W. Bacterial resistance in Eastern Europe: selected problems. Scand J Infect Dis. 1993;93(suppl):33–39. [PubMed] [Google Scholar]
- 36.Powell M, Fah YS, Seymour A. Antimicrobial resistance in Haemophilus influenzae from England and Scotland in 1991. J Antimicrob Chemother. 1992;29:547–554. doi: 10.1093/jac/29.5.547. [DOI] [PubMed] [Google Scholar]
- 37.Kayser FH, Morenzoni G, Santanam P. The second European collaborative study on the frequency of antimicrobial resistance in Haemophilus influenzae. Eur J Clin Microbiol Infect Dis. 1990;9:810–817. doi: 10.1007/BF01967379. [DOI] [PubMed] [Google Scholar]
- 38.Powell M, McVey D, Kassim MH. Antimicrobial susceptibility of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella (Branhamella) catarrhalis isolated in the UK from sputa. J Antimicrob Chemother. 1991;28:249–259. doi: 10.1093/jac/28.2.249. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein FW, Garau J. Resistant pneumococci: a renewed threat in respiratory infections. Scand J Infect Dis. 1994;93(suppl):55–62. [PubMed] [Google Scholar]
- 40.Jacoby GA. Prevalence and resistance mechanisms of common bacterial respiratory pathogens. Clin Infect Dis. 1994;18:951–957. doi: 10.1093/clinids/18.6.951. [DOI] [PubMed] [Google Scholar]
- 41.Walstad RA. Concentrations of antibiotics in sputum. Res Clin Forums. 1990;12:87–100. [Google Scholar]
- 42.Baldwin DR, Andrews JM, Wise R. Bronchoalveolar distribution of cefuroxime axetil and in vitro efficacy of observed concentrations against respiratory pathogens. J Antimicrob Chemother. 1992;30:377–385. doi: 10.1093/jac/30.3.377. [DOI] [PubMed] [Google Scholar]
- 43.Bergogne-Berezin E. Penetration of antibiotics into the respiratory tree. J Antimicrob Chemother. 1981;8:171–174. doi: 10.1093/jac/8.3.171. [DOI] [PubMed] [Google Scholar]
- 44.Baldwin DR, Wise R, Andrews JM. Azithromycin concentrations at the sites of pulmonary infection. Eur Respir J. 1990;3:886–890. [PubMed] [Google Scholar]
- 45.Elmes PC, Fletcher CM, Dutton AAC. Prophylactic use of Oxytetracycline for exacerbations of chronic bronchitis. BMJ. 1957;4:1272–1275. doi: 10.1136/bmj.2.5056.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medical Research Council Value of chemoprophylaxis and chemotherapy in early chronic bronchitis. BMJ. 1966;1:1317–1322. doi: 10.1136/bmj.1.5499.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berry DG, Fry J, Hindley CP. Exacerbations of chronic bronchitis: treatment with Oxytetracycline. Lancet. 1960;1:137–139. doi: 10.1016/s0140-6736(60)90056-8. [DOI] [PubMed] [Google Scholar]
- 48.Pines A. The tetracyclines in purulent exacerbations of chronic bronchitis. J Antimicrob Chemother. 1982;9:333–335. doi: 10.1093/jac/9.5.333. [DOI] [PubMed] [Google Scholar]
- 49.Maesen FPV, Davies BI, van Noord JA. Doxycycline in respiratory infections: a re-assessment after 17 years. J Antimicrob Chemother. 1986;187:531–536. doi: 10.1093/jac/18.4.531. [DOI] [PubMed] [Google Scholar]
- 50.Elmes PC, King TC, Langlands JHM. Value of ampicillin in the hospital treatment of exacerbations of chronic bronchitis. BMJ. 1965;2:904–908. doi: 10.1136/bmj.2.5467.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.May JR, Ingold A. Amoxicillin in the treatment of chronic nontuberculous bronchial infections. Br J Dis Chest. 1972;66:185–191. doi: 10.1016/0007-0971(72)90029-0. [DOI] [PubMed] [Google Scholar]
- 52.Chodosh S. Treatment of acute exacerbations of chronic bronchitis: state of the art. Am J Med. 1991;91(suppl 6A):87S–92S. doi: 10.1016/0002-9343(91)90317-q. [DOI] [PubMed] [Google Scholar]
- 53.Bennet JB, Crook SJ, Shaw EJ. A randomised double blind controlled trial comparing two amoxycillin regimens in the treatment of acute exacerbations of chronic bronchitis. J Antimicrob Chemother. 1988;21:225–232. doi: 10.1093/jac/21.2.225. [DOI] [PubMed] [Google Scholar]
- 54.Ball P. Overview of experience with ofloxacin in respiratory tract infection. Scand J Infect Dis. 1990;68(suppl):56–63. [PubMed] [Google Scholar]
- 55.Aldons PM. A comparison of clarithromycin with ampieillin in the treatment of outpatients with acute bacterial exacerbation of chronic bronchitis. J Antimicrob Chemother. 1991;27(suppl A):101–108. doi: 10.1093/jac/27.suppl_a.101. [DOI] [PubMed] [Google Scholar]
- 56.Ball AP. Evidence for the efficacy of ciprofloxacin in lower respiratory tract infections. Rev Contemp Pharmacother. 1992;3:133–142. [Google Scholar]
- 57.Ball AP. Azithromycin in the treatment of lower respiratory tract infections. Rev Contemp Pharmacother. 1994;5:351–357. [Google Scholar]
- 58.Petrie GR, Choo-Kang J, Washton H. Proceedings of 18th International Congress of Chemotherapy, Stockholm. June 1993. Azithromycin: an open comparison with amoxycillin in severe exacerbations of chronic bronchitis (abstract 83). [Google Scholar]
- 59.Basran GS, Joseph J, Abbas AMA. Treatment of acute exacerbations of chronic obstructive airways disease: a comparison of amoxycillin and ciprofloxacin. J Antimicrob Chemother. 1990;26(suppl F):19–24. doi: 10.1093/jac/26.suppl_f.19. [DOI] [PubMed] [Google Scholar]
- 60.Maesen FPV, Geraedts WH, Davies BI. Cefaclor in the treatment of chronic bronchitis. J Antimicrob Chemother. 1990;26:456–458. doi: 10.1093/jac/26.3.456. [DOI] [PubMed] [Google Scholar]
- 61.Verghese A. Efficacy of cefixime in respiratory tract infections. Adv Ther. 1990;7:9–15. [Google Scholar]
- 62.Ball P. Efficacy and safety of cefprozil versus other beta-lactam antibiotics in the treatment of lower respiratory tract infections. Eur J Clin Microbiol Infect Dis. 1994;13:851–856. doi: 10.1007/BF02111352. [DOI] [PubMed] [Google Scholar]
- 63.Benard Y, Lemenager J, Morel C. A comparative study of amoxycillin and Augmentin in the treatment of bronchopulmonary infections. In: Croydon EAP, Michel ME, editors. Augmentin: clavulanate-potentiated amoxycillin. Excerpta Medica; Amsterdam: 1983. pp. 282–290. [Google Scholar]
- 64.Miki F, Takamatsu K, Kohno M. Comparison of BRL 25000 (clavulanic acid-amoxicillin) and amoxicillin in the treatment of respiratory tract infections. Chemother (Tokyo) 1983;31(suppl 2):1–43. [Google Scholar]
- 65.Todd PA, Benfield P. Amoxicillin/clavulanic acid: an update of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1990;39:264–307. doi: 10.2165/00003495-199039020-00008. [DOI] [PubMed] [Google Scholar]
- 66.Schmidt EW, Zimmerman I, Ritzerfeld W. Controlled prospective study of oral amoxycillin/clavulanate vs ciprofloxacin in acute exacerbations of chronic bronchitis. J Antimicrob Chemother. 1989;24(suppl B):185–193. doi: 10.1093/jac/24.suppl_b.185. [DOI] [PubMed] [Google Scholar]
- 67.Cook PJ, Andrews JM, Woodcock J. Concentrations of amoxicillin and clavulanate in lung compartments in adults without pulmonary infection. Thorax. 1994;49:1134–1138. doi: 10.1136/thx.49.11.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gould IM, Harvey G, Golder D. Penetration of amoxycillin/clavulanic acid into bronchial mucosa with differing dosing regimens. Thorax. 1994;49:999–1001. doi: 10.1136/thx.49.10.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hughes DTD. The use of combinations of trimethoprim and sulphonamides in the treatment of chest infections. J Antimicrob Chemother. 1983;12:423–434. doi: 10.1093/jac/12.5.423. [DOI] [PubMed] [Google Scholar]
- 70.Pines A, Nandi AR, Raafat H. Amoxycillin and co-trimoxazole in acute purulent exacerbations of chronic bronchitis. Chemotherapy (Basel) 1977;23:58–64. doi: 10.1159/000221972. [DOI] [PubMed] [Google Scholar]
- 71.Anderson G, Williams L, Pardoe T. Co-trimoxazole versus cefaclor in acute on chronic bronchitis. J Antimicrob Chemother. 1981;8:487–489. doi: 10.1093/jac/8.6.487. [DOI] [PubMed] [Google Scholar]
- 72.Mehtar S, Parr JH, Morgan DJR. A comparison of cefuroxime and co-trimoxazole in severe respiratory tract infection. J Antimicrob Chemother. 1982;9:479–484. doi: 10.1093/jac/9.6.479. [DOI] [PubMed] [Google Scholar]
- 73.Lacey RW, Lord VL, Gunasekera HKW. Comparison of trimethoprim alone with trimethoprim sulphamethoxazole in the treatment of respiratory and urinary tract infections with particular reference to selection of trimethoprim resistance. Lancet. 1980;1:1270–1273. doi: 10.1016/s0140-6736(80)91732-8. [DOI] [PubMed] [Google Scholar]
- 74.Ball P. Toxicity of sulphonamide-diaminopyrimidine combinations: implications for future use. J Antimicrob Chemother. 1986;17:694–696. doi: 10.1093/jac/17.6.694. [DOI] [PubMed] [Google Scholar]
- 75.Ball AP. Therapeutic considerations for the management of respiratory tract infections. Infect Med. 1993;8(suppl A):7–17. [Google Scholar]
- 76.Bachand RT. Comparative study of clarithromycin and ampicillin in the treatment of patients with acute bacterial exacerbations of chronic bronchitis. J Antimicrob Chemother. 1991;27(suppl A):91–100. doi: 10.1093/jac/27.suppl_a.91. [DOI] [PubMed] [Google Scholar]
- 77.Bradbury F. Comparison of azithromycin versus clarithromycin in treatment of patients with lower respiratory tract infection. J Antimicrob Chemother. 1993;31(suppl E):153–162. doi: 10.1093/jac/31.suppl_e.153. [DOI] [PubMed] [Google Scholar]
- 78.Davies BI, Maesen FPV, Teengs JP. The quinolones in chronic bronchitis. Pharm Weekbl Sci. 1986;8:53–59. doi: 10.1007/BF01975481. [DOI] [PubMed] [Google Scholar]
- 79.Chodosh S. Use of quinolones for the treatment of acute exacerbations of chronic bronchitis. Am J Med. 1991;91(suppl 6A):93S–100S. doi: 10.1016/0002-9343(91)90318-r. [DOI] [PubMed] [Google Scholar]
- 85.Pines A, Raafat H, Plucinski K. Antibiotic requirements in severe and acute purulent exacerbations of chronic bronchitis. BMJ. 1968;2:735–738. doi: 10.1136/bmj.2.5607.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Petersen ES, Esmann V, Honcke P. A continuing study of the effect of treatment on chronic bronchitis. Acta Med Scand. 1967;182:293–305. doi: 10.1111/j.0954-6820.1967.tb11527.x. [DOI] [PubMed] [Google Scholar]
Uncited references
- 80.Allegra L, Brumpt I. Programme and Abstracts, 5th International Symposium of New Quinolones, Singapore: August 1994. Sparfloxacin (S) in the management of acute exacerbations of chronic obstructive pulmonary disease (COPD): a comparison with Augmentin (abstract 139). [Google Scholar]
- 81.Farr BM, Sloman AJ, Fisch MJ. Predicting death in patients hospitalised for community acquired pneumonia. Ann Intern Med. 1991;115:429–443. doi: 10.7326/0003-4819-115-6-428. [DOI] [PubMed] [Google Scholar]
- 82.Niederman MS, Bass JB, Campbell GD. Guidelines for the initial management of adults with community-acquired pneumonia: diagnosis, assessment of severity and initial antimicrobial therapy. Am Rev Respir Dis. 1993;148:1418–1426. doi: 10.1164/ajrccm/148.5.1418. [DOI] [PubMed] [Google Scholar]
- 83.Lindsay G, Scorer HJN, Carnegie CMD. Safety and efficacy of temafloxacin versus ciprofloxacin in lower respiratory tract infections: a randomised, double-blind trial. J Antimicrob Chemother. 1992;30:89–100. doi: 10.1093/jac/30.1.89. [DOI] [PubMed] [Google Scholar]
- 84.Nicotra MB, Rivera M, Awe RJ. Antibiotic therapy of acute exacerbations of chronic bronchitis: a controlled study using tetracycline. Ann Intern Med. 1982;97:18–21. doi: 10.7326/0003-4819-97-1-18. [DOI] [PubMed] [Google Scholar]
- 87.Law MR, Holt HA, Reeves DS. Cefaclor and amoxycillin in the treatment of infective exacerbations of chronic bronchitis. J Antimicrob Chemother. 1983;11:83–88. doi: 10.1093/jac/11.1.83. [DOI] [PubMed] [Google Scholar]
- 88.Davies BI, Maesen FPV, Teengs JP. Cefuroxime axetil in acute purulent exacerbations of chronic bronchitis. Infection. 1987;15:253–256. doi: 10.1007/BF01644126. [DOI] [PubMed] [Google Scholar]
- 89.Wise R, Baldwin DR, Honeybourne D. Penetration of antibiotics into the bronchial mucosa: a review. Res Clin Forums. 1990;12:95–100. [Google Scholar]
- 90.Honeybourne D, Baldwin DR. The site concentrations of antimicrobial agents in the lung. J Antimicrob Chemother. 1992;30:249–260. doi: 10.1093/jac/30.3.249. [DOI] [PubMed] [Google Scholar]
- 91.Baldwin DR, Honeybourne D, Wise R. Pulmonary disposition of antimicrobial agents: in vivo observations and clinical relevance. Antimicrob Agent Chemother. 1992;36:1176–1180. doi: 10.1128/aac.36.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]


