Highlights
-
•
25% of hematological patients with a positive HMPV test have pneumonia.
-
•
HMPV pneumonia can occur in the course of several hematological conditions.
-
•
HMPV can cause pneumonia as a single pathogen.
-
•
Lung HRCT scan may be suggestive of HMPV pneumonia.
-
•
The outcome of HMPV pneumonia is good despite no antiviral treatment.
Abbreviations: HMPV, human metapneumovirus; HSCT, hematopoietic stem cell transplant; URTID, upper respiratory tract infection disease; BAL, bronchoalveolar lavage; RV, respiratory virus; HRCT, high resolution computed tomography
Keywords: Metapneumovirus, Emerging virus, Respiratory infection, Immunocompromised, Hematological patients
Abstract
Background
Human metapneumovirus (HMPV) has recently emerged as a cause of respiratory infections in hematological patients. Clinical data are lacking to guide the management of HMPV pneumonias.
Objectives
To characterize the clinical and radiographic presentation and outcome of HMPV pneumonias diagnosed in hematological patients.
Study design
We screened the patients with a positive HMPV respiratory test in two French teaching hospitals between 2007 and 2011. Among them, the medical charts from the hematological patients who presented with HMPV pneumonia were reviewed.
Results
Among the 54 patients with several underlying hematological conditions who were positive for HMPV, we found 13 cases of HMPV pneumonias. HMPV could be the cause of pneumonia as a single pathogen without associated upper respiratory infection. Centrilobular nodules were constant on lung computed tomography scans. No patients died despite the absence of administration of antiviral treatments.
Conclusions
Our data provide further insights in the diagnosis and management of HMPV pneumonias in this setting.
1. Background
The development of molecular techniques has highlighted the role of emerging respiratory viruses (RV), particularly in immunocompromised patients. Human metapneumovirus (HMPV) is a paramyxovirus identified in the early 2000s that is closely related to the respiratory syncytial virus (RSV). The clinical spectrum of HMPV infections ranges from asymptomatic carriage in the airways to upper respiratory tract infection disease (URTID) and severe pneumonia [1], [2], [3], [4], [5], [6]. To date, the few published epidemiological or clinical data on HMPV pneumonias in hematological patients have focused on hematopoietic stem cell transplant (HSCT) recipients [2], [3], [4], [5], [6], and this virus is still poorly recognized. Due to the lack of clinical data on HMPV infections, the fourth European conference on infections in leukemia could not make any recommendations for the management of HMPV infections in hematological patients in recent guidelines [7].
2. Objective
We sought to characterize the clinical and radiographic presentation and outcome of HMPV pneumonias diagnosed in hematological patients over a four-year period in two French teaching hospitals.
3. Study design
These cases were diagnosed in two French centers between January 1, 2007, and October 31, 2011. Hospital Saint Louis, Paris is a 650 bed teaching hospital that includes 350 beds dedicated to onco-hematology adult patients. The Poitiers teaching hospital has 1413 beds for both adults and children including 64 beds for onco-hematology. In both centers, RVs are screened in the nasopharyngeal aspirate and/or bronchoalveolar lavage (BAL) in all hematological patients who present with acute respiratory tract symptoms. In Hospital Saint Louis, Paris, from October 2007 to October 2009 HMPV was detected using immunofluorescence testing (Argene, Verniolle, France). Since November 2009, the detection of HMPV is performed using 200 μL of respiratory specimen using a molecular multiplex assay, RespiFinder®, (Pathofinder, Maastricht, The Netherlands), which enables the detection and differentiation of 14 RVs including the following: influenza viruses A and B; parainfluenza viruses, RSV; rhinovirus; human coronaviruses, adenovirus and HMPV [8]. In the Poitiers teaching hospital, during the study period, HMPV and other respiratory pathogens (Influenza viruses A and B, parainfluenza viruses, RSV, rhinovirus, human coronaviruses 229E and OC43, adenovirus, Chlamydophila pneumoniae and Mycoplasma pneumoniae) were detected using multiplex or monoplex nucleic acid amplification [9], [10], [11].
Pneumonia was defined as the presence of dyspnea and/or hypoxia with a new infiltrate on imaging. URTID was defined as the presence of coryza, pharyngitis, sinusitis and/or cough with or without fever, no hypoxemia and clear chest imaging. BAL samples were tested for bacteria (direct examination and culture) and fungi (immunofluorescence and molecular biology for Pneumocystis jiroveci; direct examination and culture for other species). When bronchoscopy could not be performed, the presence of bacteria and fungi was evaluated in induced sputum samples. To describe the initial high resolution computed tomography (HRCT) findings in patients with HMPV pneumonia, we reviewed the HRCT scans performed shortly (<5 days) after the onset of symptoms.
4. Results
During the study period, a total of 11267 HMPV tests were performed in both centers (1677 immunofluorescence HMPV tests and 9590 molecular HMPV tests). HMPV was detected in a total of 171 respiratory samples (1.5%) in 131 patients including 77 patients of the general hospital population and 54 hematological patients.
Thirteen of these hematological patients (24%) had a diagnosis of HMPV pneumonia whereas the diagnosis of URTID was retained for the others. All the nonhematological patients positive for HMPV were diagnosed with URTID. The clinical features of the patients with pneumonia are described in Table 1 . Six patients were allogeneic HSCT recipients, with a median time of 4.3 months [1.8–5.8] after HSCT and two had previously experienced an autologous HSCT. Nine of the HMPV infections occurred during the winter (69%), one in spring, one in autumn and two in summer. The infection was considered to be community acquired in 10 patients and nosocomial in three. Eleven patients had HMPV as a single pathogen, and 2 patients had rhinovirus co-infection of the lung. No bacteria or fungus was found in the respiratory samples at the time of HMPV pneumonia diagnosis. Seven patients with HMPV pneumonia presented no URTID (54%). However, among the seven patients who did not present with URTI symptoms, a nasopharyngeal aspirate was available for five patients that were all positive for HMPV. Six patients presented with both URTID and pneumonia. All of the six patients who initially presented with URTID progressed to pneumonia with a mean interval of 4 days (range, 3–5 days). The most frequent symptoms were cough and dyspnea in all patients, fever in 11 (85%), and wheezing in 11 (85%). In 12 patients, a lung HRCT scan was performed at a mean time of 2.5 days after the onset of symptoms (range, 1–5 days). HRCT findings were mainly small blurred ill-defined centrilobular nodules, branching centrilobular nodules and ground glass opacities (Table 1, Fig. 1 ). Bilateral involvement was observed in all patients, predominantly in the lower lobes. Nine patients (69%) had lymphopenia (≤0.6 cells/μL), whereas 5 (38%) had neutropenia (<500 cells/μL). Two patients were admitted to an intensive care unit, but none required ventilation.
Table 1.
Clinical characteristics of patients at diagnosis of HMPV pneumonia.
| Age (years)/sex | Underlying disease/stage of disease | Significant medications in the past 3 months | Type of conditioning allogeneic HSCT | GVHD | Time from HSCT to HMPV (months) | Immunosuppressive treatment | Lymphocytes count G/L | CT-scan findings | Co-pathogens | Treatment O2/ICU | Outcome at 3 months |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 66/F | CLL/progression | 0 | 60.4 | Ill-defined nodules, ground-glass opacities, | Rhinovirus | Supportive +/− |
Alive | ||||
| 74/F | MDS | 0 | 0.6 | Ill-defined nodules, ground-glass opacities, | 0 | Supportive −/− |
Alive | ||||
| 63/M | AML/induction | Aracytin, daunorubicin | <0.1 | Ill-defined nodules, ground-glass opacities | 0 | Supportive +/− |
Alive | ||||
| 62/M | CLL/progression | 0 | 33.3 | Confluent ill-defined nodules, ground-glass opacities pleural effusion |
0 | Supportive +/+ |
Alive | ||||
| 74/M | NHL/relapse | Rituximab Bendamustine |
0.1 | Reticulations, ground grass opacities | 0 | Supportive +/+ |
Alive | ||||
| 43/M | CLL/autologous | 14 | 0 | 3.1 | Ill-defined nodules, ground-glass opacities | 0 | Supportive −/− |
Alive | |||
| 62/M | MM/autologous | 24 | Dexamethasone, thalidomide | 0.5 | Ill-defined nodules, ground-glass opacities | 0 | Supportive −/− |
Alive | |||
| 55/F | AML/allogeneic related PBSC | NMA | + | 5 | Prednisone 5 mg/d, Cy | 0.4 | NA/diffuse alveolar opacities on chest X-ray | 0 | Ig −/− |
Alive | |
| 59/M | NHL/allogeneic related PBSC | NMA | + | 6 | Prednisone 110 mg/d, Cy | 0.4 | Ill-defined nodules, ground-glass opacities | 0 | Supportive −/− |
Alive | |
| 41/M | AML/allogeneic related PBSC | MA | − | 1 | Cy | 0.6 | Ill-defined nodules, ground-glass opacities | 0 | Supportive +/− |
Alive | |
| 27/M | AML/allogeneic related PBSC | MA | + | 4 | Prednisone 2.5 mg/d, Cy | 0.3 | Ill-defined nodules, ground-glass opacities, coalescence of nodules | 0 | Supportive +/− |
Alive | |
| 58/M | NHL/allogeneic related PBSC | NMA | + | 36 | Prednisone 5 mg/d, Cy | 1.9 | Ill-defined nodules, ground-glass opacities | 0 | Supportive +/− |
Alive | |
| 14/M | ALL/allogeneic related BM | MA | − | <1 | Cy | <0.1 | Ill-defined nodules, ground-glass opacities | Rhinovirus | Supportive +/− |
Alive |
HSCT, hematopoietic stem cell transplant; HMPV, human metapneumovirus; M, male; F, female; GVHD, graft-versus-host disease; CLL, chronic lymphocytic leukemia; MDS: myelodysplastic syndrome; AML, acute myeloid leukemia; NHL, non Hodgkin lymphoma; ALL, acute lymphocytic leukemia; CLL, chronic lymphocytic leukemia; MM, multiple myeloma; PBSC, peripheral blood stem cell; BM, bone marrow; MA, myeloablative; NMA, non myeloablative; Cy, cyclosporine; CT, computed tomography; O2, need for oxygen; ICU, intensive care unit; Ig, immunoglobulins.
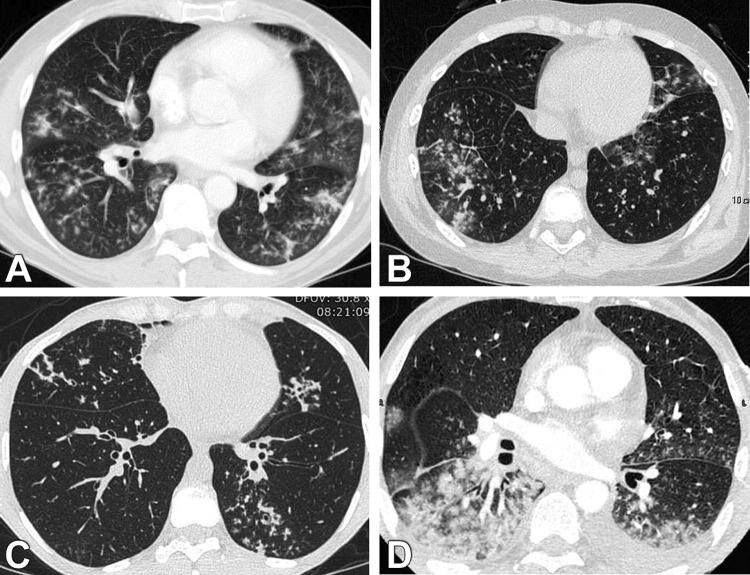
Fig. 1.
Lung CT scans from four hematological patients with HMPV pneumonia. Ill-defined centrilobular nodules and ground glass opacities were most frequently noted and were predominantly located in the lower lobes (A–D). Centrilobular nodules could be extensive and confluent (D). Bilateral pleural effusion could be present (D).
None of the patients were treated with any antiviral therapy, such as inhaled or oral ribavirin. All patients received broad-spectrum antibiotics. HMPV pneumonia resolved in all patients, and they were all alive three months after HMPV pneumonia diagnosis.
5. Discussion
We found that HMPV pneumonia may occur in several hematological conditions although previous studies have focused on HSCT. Whatever the underlying condition, the clinical and radiological presentation was similar. Contrary to previously described findings, half of our patients had no signs of URTID in the days that preceded the diagnosis of HMPV pneumonia.
Lung CT scan is a cornerstone in the management of respiratory infections in hematological patients [12]. The presence of centrilobular nodules was strikingly noted in almost all of our cases of HMPV pneumonia and should suggest the diagnosis, especially in the winter. The fact that only rhinovirus was detected as copathogens in only two patients suggests that the HPMV could by itself be the cause of respiratory infection and confirms previous data [2].
The striking feature of our study is the good prognosis of our patients. In previous series that focused on HSCT recipients, mortality has been reported to be between 10 and 40% [4], [6]. In our series over a period of 4 years, including all consecutive hematological patients with a diagnosis of HMPV pneumonia, no patients died, even though 8/13 patients required oxygen and among them two needed intensive care. Our data are even more striking in that none of our patients had received any anti-viral treatment. There is little data to determine risk factors for mortality of HMPV pneumonia in hematological patients. When available individual data are analyzed, a number of deaths are not directly related to the virus: relapse of hematological disease, lung cancer, other severe infection, or, in a few cases, the patient's desire to limit care [4], [6]. Other factors are likely to be involved such as an underlying lung disease, especially bronchiolitis obliterans in the case of allogeneic HSCT recipients. The very early onset of HMPV pneumonia after transplantation is also a factor likely to impact mortality. Renaud et al. identified several risk factors for HMPV-related mortality in a population of HSCT recipients including the severity of the pneumonia, peripheral blood stem cell or cord blood as stem cell sources and the use of corticosteroids [6].
Although some authors have suggested that ribavirin could be an effective option for treating HMPV infection, preliminary data regarding the effect of ribavirin on HMPV pneumonia do not support a significant effect [6], [13]. In summary, we showed that HMPV pneumonia can occur in hematological patients other than HSCT recipients with a suggestive radiological pattern. Although the majority of our patients had a severe respiratory infection requiring oxygen, the clinical outcome was good despite the absence of a specific antiviral treatment. Randomized studies are needed to determine the effect of any antiviral drug on HMPV pneumonia.
Funding
None.
Competing interests
None declared.
Ethical approval
This study was approved by the institutional review board of the French Learned Society for Respiratory Medicine CEPRO 2013-20.
Authors’ contribution
CG collected the data, analyzed the data and wrote the manuscript. JL and ABD performed and analyzed the virological testings, and wrote the manuscript. MR, ER, BA, FR, JPF, NM and AB participated in patients’ recruitment and data collection. AT analyzed the data and critically revised the manuscript. AB supervised the study, analyzed the data and wrote the manuscript. All authors approved the final manuscript.
Acknowledgment
CG, AT and AB are members of the GREPI (French Group for Research and Education in Respiratory Infectious Diseases).
References
- 1.Martino R., Porras R.P., Rabella N., Williams J.V., Rámila E., Margall N. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant. 2005;11:781–796. doi: 10.1016/j.bbmt.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Debur M.C., Vidal L.R., Stroparo E., Nogueira M.B., Almeida S.M., Takahashi G.A. Human metapneumovirus infection in hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2010;12:173–179. doi: 10.1111/j.1399-3062.2009.00465.x. [DOI] [PubMed] [Google Scholar]
- 3.Oliveira R., Machado A., Tateno A., Boas L.V., Pannuti C., Machado C. Frequency of human metapneumovirus infection in hematopoietic SCT recipients during 3 consecutive years. Bone Marrow Transplant. 2008;42:265–269. doi: 10.1038/bmt.2008.153. [DOI] [PubMed] [Google Scholar]
- 4.Egli A., Bucher C., Dumoulin A., Stern M., Buser A., Bubendorf L. Human metapneumovirus infection after allogeneic hematopoietic stem cell transplantation. Infection. 2012;40:677–684. doi: 10.1007/s15010-012-0279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cane P.A., van den Hoogen B.G., Chakrabarti S., Fegan C.D., Osterhaus A.D. Human metapneumovirus in a haematopoietic stem cell transplant recipient with fatal lower respiratory tract disease. Bone Marrow Transplant. 2003;31:309–310. doi: 10.1038/sj.bmt.1703849. [DOI] [PubMed] [Google Scholar]
- 6.Renaud C., Xie H., Seo S., Kuypers J., Cent A., Corey L. Mortality rates of human metapneumovirus and respiratory syncytial virus lower respiratory tract infections in hematopoietic cell transplantation recipients. Biol Blood Marrow Transplant. 2013;19:1220–1226. doi: 10.1016/j.bbmt.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsch H.H., Martino R., Ward K.N., Boeckh M., Einsele H., Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis. 2013;56:258–266. doi: 10.1093/cid/cis844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnell D., Legoff J., Mariotte E., Seguin A., Canet E., Lemiale V. Molecular detection of respiratory viruses in immunocopromised ICU patients: incidence and meaning. Respir Med. 2012;106:1184–1191. doi: 10.1016/j.rmed.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellau-Pujol S., Vabret A., Legrand L., Dina J., Gouarin S., Petitjean-Lecherbonnier J. Development of three multiplex RT-PCR assays for the detection of 12 respiratory RNA viruses. J Virol Methods. 2005;126:53–63. doi: 10.1016/j.jviromet.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stockton J., Ellis J.S., Saville M., Clewley J.P., Zambon M.C. Multiplex PCR for typing and subtyping influenza and respiratory syncytial viruses. J Clin Microbiol. 1998;36:2990–2995. doi: 10.1128/jcm.36.10.2990-2995.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hierholzer J.C., Halonen P.E., Dahlen P.O., Bingham P.G., McDonough M.M. Detection of adenovirus in clinical specimens by polymerase chain reaction and liquid-phase hybridization quantitated by time-resolved fluorometry. J Clin Microbiol. 1993;31:1886–1891. doi: 10.1128/jcm.31.7.1886-1891.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergeron A. The pulmonologist's point of view on lung infiltrates in haematological malignancies. Diagn Interv Imaging. 2013 doi: 10.1016/j.diii.2012.12.004. pii:S2211-5684(12)00399-3. [DOI] [PubMed] [Google Scholar]
- 13.Park S.Y., Baek S., Lee S.O., Choi S.H., Kim Y.S., Woo J.H. Efficacy of oral ribavirin in hematologic disease patients with paramyxovirus infection: analytic strategy using propensity scores. Antimicrob Agents Chemother. 2013;57:983–989. doi: 10.1128/AAC.01961-12. [DOI] [PMC free article] [PubMed] [Google Scholar]