Highlights
-
•
HIV-infected and uninfected children with LRTIs were tested for WUPyV and KIPyV.
-
•
At least one polyomavirus was identified in 16% of specimens.
-
•
Polyomaviruses were frequently identified in association with other viruses.
-
•
Polyomaviruses may contribute to the pathogenesis of pneumococcal pneumonia.
Abbreviations: CFR, case fatality rate; hBoV, human-bocavirus; CoV, human-coronavirus; hMPV, human metapneumovirus; hRV, human-rhinovirus; LRTIs, lower respiratory tract infections; NPAs, nasopharyngeal aspirates; PIV, parainfluenza viruses; PCP, Pneumocystis jiroveci; PyV, polyomaviruses; RCT, randomized placebo-controlled trial; rRT-PCR, real-time reverse transcriptase–polymerase chain reaction; RSV, respiratory syncytial virus; VE, vaccine efficacy; PCV9, 9-valent pneumococcal conjugate vaccine
Keywords: Polyomaviruses, HIV, Pneumonia, Respiratory infections, PCV9, Pneumococcal vaccine
Abstract
Background
Two recently discovered polyomaviruses (PyV), WU and KI, have been identified in respiratory-tract specimens from children with acute respiratory infections, although there are limited data in HIV-infected children.
Objectives
To determine the prevalence and clinical manifestations of WUPyV and KIPyV-associated lower respiratory tract infections (LRTIs) hospitalization in HIV-infected and -uninfected children; and probe the role of pneumococcal co-infection.
Study design
Nasopharyngeal aspirates were collected from a cohort of 39,836 children randomized to receive 9-valent pneumococcal conjugate vaccine (PCV9) or placebo when hospitalized for LRTIs, and were screened by PCR for WUPyV, KIPyV and other respiratory viruses.
Results
In placebo-recipients the prevalence of WUPyV was 6.3% (18/285) in HIV-infected and 13.9% (66/476) in HIV-uninfected children (p = 0.002). In WUPyV-positive LRTIs HIV-infected children had lower oxygen saturation at admission and a higher case fatality rate (11.1% vs. 0%; p = 0.04). KIPyV was identified in 10.2% (29/285) of HIV-infected and in 7.4% (35/476) of HIV-uninfected placebo-recipients with LRTIs (p = 0.13). HIV-infected compared to HIV-uninfected children with KIPyV-positive LRTIs had lower oxygen saturation, higher respiratory rate and longer duration of hospitalization. Co-infections with other respiratory-viruses were detected in 65.5% of WUPyV-positive LRTIs and in 75.0% of KIPyV-positive LRTIs. Among HIV-uninfected children, there was a lower incidence of hospitalization for clinical pneumonia episodes in which KIPyV (80%; 95% CI: 41, 93) and WUPyV (49%; 95% CI: 9, 71) were identified among PCV9-recipients compared to placebo-recipients.
Conclusions
Polyomaviruses were commonly identified in HIV-infected and -uninfected children hospitalized for LRTIs, frequently in association with other viruses and may contribute to the pathogenesis of pneumococcal pneumonia.
1. Background
Lower respiratory tract-infections (LRTIs) are a major cause of hospitalizations during childhood [1]. Determining pathogen specific causality of LRTIs is hampered by lack of sensitive methods for diagnosing bacterial pneumonia, as well as the concurrent identification of multiple respiratory-viral pathogens, particularly when using molecular assays [2]. Nevertheless, worldwide studies attribute a large proportion of LRTIs to viral infections [3], [4]. Large-scale molecular screening based technologies have contributed to the discovery of new infectious pathogens, including in 2007 two polyomaviruses (PyV), WU- and KI-polyomavirus [5], [6]. These PyV belong to the Polyomaviridae family and have been associated with respiratory disease in humans, although direct evidence of causality is lacking [7]. Two other polyomaviruses (BKPyV and JCPyV) have been implicated in disease in immunocompromised patients [8], [9], [10]. There are, however, conflicting data with regard to the role of WUPyV and KIPyV in immunocompromised individuals [11], [12], [13].
Previous studies have used pneumococcal-conjugate-vaccine (PCV) randomized placebo-controlled trials (RCT) as a probe to establish the probability of co-infections by Streptococcus pneumoniae (pneumococcus) vaccine-serotypes and respiratory-virus in children hospitalized for pneumonia [14], [15]. The rational of this approach is that any biological-plausible difference between PCV- and placebo-recipients in the incidence of any disease which could be associated with vaccine-serotype pneumococcal infection would indicate a role of vaccine-serotypes in the outcome of interest (for review [16]). For example, we have previously reported that PCV-recipients, had a 45% lower incidence of pneumonia hospitalizations in which influenza-virus was identified, and as such concluded that at least a similar proportion of the influenza-associated pneumonias among placebo-recipients was precipitated by co-infection with PCV-serotypes [14].
2. Objectives
The aim of this study was to determine the burden and clinical features of WUPyV and KIPyV infections in HIV-infected and HIV-uninfected children hospitalized for LRTIs. Furthermore, as an exploratory analysis we used the design of a RCT of a 9-valent PCV (PCV9) to probe whether pneumococcal co-infection may contribute to hospitalization for PyV-associated pneumonia.
3. Study design
We analyzed respiratory specimens collected from children who participated in RCT in South Africa as previously described [14], [17]. Briefly, 39,836 children were randomized (1:1) from 1998 to 2000 to receive 3 doses of PCV9 or placebo [17]. Hospital-based surveillance for all-cause hospitalization was undertaken, all hospitalized children underwent HIV testing [17]. Nasopharyngeal aspirates (NPAs) were obtained from children hospitalized with LRTIs for identification of selected respiratory-viruses [14] and archived from February 2000 onward. In this study only NPAs collected from February 2000 to January 2002 from children <2 years old were analyzed. If a child had recurrent LRTI hospitalizations, only NPAs collected >28 days apart were included in the analysis. These samples had been previously investigated for respiratory syncytial virus (RSV), influenza A/B, parainfluenza viruses (PIV) and adenovirus using immunofluorescence and for human-metapneumovirus (hMPV) by nested-PCR, as described [14], [15].
3.1. Viral testing
Archived NPAs were tested by real-time reverse transcriptase–PCR (rRT-PCR) using the primers and probes as described [18]. We tested for WU- and KI-polyomavirus, as well as for human-bocavirus (hBoV), human-rhinovirus (hRV), and four human-coronaviruses (CoV). A comprehensive overview of the identified individual viruses has been reported separately [18]. The current analysis details the epidemiology of WUPyV and KIPyV-positive LRTIs in the study cohort. The clinical definitions used in this study have been described [14].
3.2. Statistical analysis
The analyses on the epidemiology of WUPyV and KIPyV in children hospitalized for LRTIs were restricted to placebo-recipients in the context of the initial trial [17].
Proportions were compared by Chi-square or Fisher's exact tests and continuous variables by Student's t-test or Mann–Whitney test. Regression analyses were performed to compare clinical features between HIV-infected and -uninfected children. Multiple regressions were controlled for age at hospitalization, detection of a virus previously-tested and year of collection. In an exploratory analysis using the concept of vaccine-probe studies [14], [19], we explored whether there was any association between PCV9 and the risk of hospitalization for LRTIs in which WUPyV or KIPyV were detected by estimating vaccine efficacy (VE) based on the formula:
Children were included in the per-protocol analysis if they received all the study-vaccines as per planned schedule and the LRTI event occurred >14 days after the third dose of study-vaccine. Only the first episode of viral detection was included in the VE calculation for an individual participant. p-values <0.05 were considered significant. Analyses were performed using STATA version 12.1 (College Station, TX, USA).
4. Results
A total of 1460 NPAs were analyzed by rRT-PCR, including 699 from PCV9-recipients (48.8%) and 761 (52.1%) from placebo-recipients [18].
4.1. WUPyV in PCV-unvaccinated children hospitalized with LRTIs
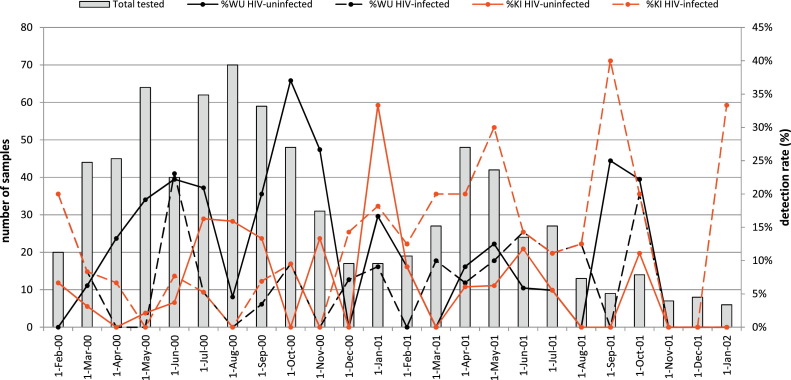
WUPyV was detected less frequently in HIV-infected (6.3%) compared to HIV-uninfected children (13.9%, p = 0.002) (Table 1 ). One HIV-infected and three HIV-uninfected children had at least two episodes of WUPyV-positive LRTIs more than 28 days apart. WUPyV was detected every month except from November 2001 to January 2002, with a higher detection rate among HIV-uninfected children in 2000 (16.6%, 53/319) compared to 2001 (8.3%, [13/157]; p = 0.01) (Fig. 1 ). In HIV-infected children there was a similar detection rate during 2000 (5.6%, 198/187) and 2001 (8.0%, [7/80]; p = 0.43).
Table 1.
Demographic, clinical and laboratory characteristics of HIV-infected and HIV-uninfected pneumococcal conjugate-unvaccinated children hospitalized for lower respiratory tract infections where polyomaviruses WU or KI were detected.
| Overall | HIV-infected | HIV-uninfected | OR 95% CI p-value |
aORa 95% CI p-value |
|
|---|---|---|---|---|---|
| Total number of specimens studied | 761 | 285 | 476 | – | – |
| Positive samples, n (%) | |||||
| for WUPyV | 84 (11.0) |
18 (6.3) |
66 (13.9) |
0.42 (0.24–0.72) 0.002 |
0.40b (0.23–0.72) 0.002 |
| for KIPyV | 64 (8.4) |
29 (10.2) |
35 (7.4) |
1.43 (0.85–2.39) 0.176 |
1.52b (0.88–2.63) 0.131 |
| Demographic characteristics | |||||
| Mean age in months, (SD) | |||||
| WUPyV-positive cases | 13.2 (5.9) |
15.0 (5.3) |
12.7 (6.0) |
1.07 (0.98–1.18) 0.150 |
1.06b (0.96–1.18) 0.220 |
| KIPyV-positive cases | 11.7 (5.5) |
11.7 (5.8) |
11.7 (5.3) |
1.00 (0.91–1.09) 0.982 |
0.95 (0.85–1.06) 0.397 |
| Male, n (%) | |||||
| WUPyV-positive cases | 53 (63.1) |
10 (55.6) |
43 (65.2) |
0.67 (0.23–1.93) 0.456 |
0.66 (0.21–2.05) 0.469 |
| KIPyV-positive cases | 28 (43.8) |
10 (34.5) |
18 (51.4) |
0.50 (0.18–1.37) 0.176 |
0.56 (0.19–1.65) 0.290 |
| Clinical characteristicse | |||||
| Mean oxygen saturation on room air <90%, n (%) | |||||
| WUPyV-positive cases | 17 (20.2) |
7 (38.9) |
10 (15.2) |
3.56 (1.11–11.39) 0.032 |
3.88 (1.06–14.21) 0.041 |
| KIPyV-positive cases | 16/61 (26.2) |
11/27 (40.7) |
5/34 (14.7) |
3.99 (1.18–13.52) 0.026 |
5.00 (1.27–19.65) 0.021 |
| Mean respiratory rate; breaths per minute, (SD) | |||||
| WUPyV-positive cases | 50.0 (12.1) |
53.3 (112.2) |
49.1 (12.0) |
1.03 (0.99–1.07) 0.193 |
1.03 (0.99–1.08) 0.184 |
| KIPyV-positive cases | 52.5 (14.5) |
58.1 (16.8) |
47.9 (10.6) |
1.06 (1.01–1.10) 0.009 |
1.07 (1.02–1.12) 0.004 |
| Clinical pneumonia, n (%) | |||||
| WUPyV-positive cases | 55 (65.5) |
16 (88.9) |
39 (59.1) |
5.54 (1.18–26.09) 0.030 |
5.67 (1.14–28.16) 0.034 |
| KIPyV-positive cases | 51 (79.7) |
26 (89.7) |
25 (71.4) |
3.47 (0.85–14.09) 0.082 |
3.04 (0.68–13.56) 0.145 |
| Wheezing, n (%) | |||||
| WUPyV-positive cases | 37 (44.1) |
3 (16.7) |
34 (51.5) |
0.19 (0.05–0.71) 0.014 |
0.21 (0.05–0.84) 0.028 |
| KIPyV-positive cases | 19 (29.7) |
6 (20.7) |
13 (37.1) |
0.44 (0.14–1.37) 0.156 |
0.57 (0.17–1.92) 0.360 |
| Bronchial breathing, n (%) | |||||
| WUPyV-positive cases | 7 (8.3) |
4 (22.2) |
3 (4.6) |
6.00 (1.21–29.87) 0.029 |
3.79 (0.61–23.50) 0.152 |
| KIPyV-positive cases | 11 (17.2) |
8 (27.6) |
3 (8.6) |
4.06 (0.97–17.09) 0.056 |
3.04 (0.64–14.39) 0.161 |
| Hospital stay ≥5 days, n (%) | |||||
| WUPyV-positive cases | 14 (16.7) |
6 (33.3) |
8 (12.1) |
3.63 (1.06–12.37) 0.040 |
3.17 (0.83–12.02) 0.090 |
| KIPyV-positive cases | 21 (32.8) |
15 (51.7) |
6 (17.1) |
5.18 (1.65–16.22) 0.005 |
10.41 (2.48–43.71) 0.001 |
| Deaths during LRTIs hospitalization, n (%) | |||||
| WUPyV-positive cases | 2 (2.4) |
2 (11.1) |
0 | 0.044c | |
| KIPyV-positive cases | 6 (9.4) |
4 (13.8) |
2 (5.7) |
2.64 (0.45–15.58) 0.284 |
2.77 (0.41–18.63) 0.296 |
| Laboratory and other investigationsf | |||||
| Alveolar consolidation on chest X-ray, n/N (%) | |||||
| WUPyV-positive cases | 22/77 (28.6) |
8/16 (50.0) |
14/61 (23.0) |
3.36 (1.07–10.57) 0.039 |
4.43 (1.15–17.10) 0.031 |
| KIPyV-positive cases | 25/59 (42.4) |
15/24 (62.5) |
10/35 (28.6) |
4.17 (1.38–12.58) 0.011 |
4.15 (1.24–13924) 0.021 |
| Bacterial infection, n/N (%) | |||||
| WUPyV-positive casesg | 3/79 (3.8) |
1/17 (5.9) |
2/62 (3.2) |
1.88 (0.16–22.01) 0.617 |
1.76 (0.10–30.24) 0.696 |
| KIPyV-positive casesh | 6/63 (9.5) |
6/29 (20.7) |
0/34 | 0.005c | |
| Co-detection with other respiratory viruses, N (%) | |||||
| WUPyV-positive cases | 55 (65.5) |
7 (38.9) |
48 (72.7) |
0.24 (0.08–0.71) 0.010 |
0.18d (0.06–0.60) 0.005 |
| KIPyV-positive cases | 48 (75.0) |
22 (75.9) |
26 (74.3) |
1.09 (0.35–3.40) 0.885 |
1.07d (0.32–3.52) 0.916 |
In parenthesis percent of number studied, unless otherwise indicated. n: number of children with the analyzed characteristic; N: number of children with information available if different from overall tested; SD: standard deviation; OR: odds ratio; aOR: adjusted odds ratio; LRTIs: lower respiratory tract infections.
aOR adjusted for year of sampling and detection of viruses previously-tested unless otherwise indicated.
aOR adjusted for age, year of sampling and detection of viruses previously-tested.
p-value not adjusted.
p-value not adjusted for detection of viruses previously-tested.
Other clinical characteristics assessed that were not significantly different between HIV-infected and HIV-uninfected children included: percentage of children presenting with axillary temperature ≥38 °C, percentage of children with a history of fever, vomiting, seizures or cyanosis, percentage of children needing mechanical ventilation and percentage of children presenting with World Health Organization severe pneumonia definition.
Other laboratory investigations assessed that were not significantly different between HIV-infected and HIV-uninfected children included: percentage of children presenting with C-reactive protein levels ≥40 mg/l or procalcitonin levels ≥2 ng/ml and mean white cell count.
Streptococcus pneumoniae was isolated from one HIV-infected child and in two HIV-uninfected children in whom WUPyV was detected.
Bacteria isolated from HIV-infected children in whom KIPyV was detected included: Streptococcus pneumoniae (n = 3), Escherichia coli (n = 2) and Haemophilus parainfluenzae (n = 1).
Fig. 1.
Seasonal variation in identifying WU- and KI-polyomavirus in children hospitalized for lower respiratory tract infection between February 2000 until January 2002.
Among HIV-uninfected children, WUPyV was generally detected concurrently with other respiratory-viruses (72.7%), which was at a higher frequency compared to HIV-infected children (38.9%, p = 0.005) (Table 1). The most common co-detected viruses were hRV (31.8%, n = 21), hBoV (16.7%, n = 11), RSV (12.1%, n = 8) and hMPV (10.6%, n = 7) in HIV-uninfected children; and hRV (27.8%, n = 5) and KIPyV (16.7%, n = 3) in HIV-infected children.
By multivariate analysis of WUPyV-associated LRTIs, HIV-infected compared to HIV-uninfected children were more likely to have oxygen saturation <90%, present clinically as pneumonia rather than bronchiolitis. Pneumococci were isolated by blood culture in one HIV-infected child and two HIV-uninfected children with WUPyV-associated LRTI (Table 1). HIV-infected (11.1%) compared to HIV-uninfected children (0%, p = 0.04) had a higher case fatality rate (CFR). The two HIV-infected children who died were 13 and 15 months old, both presented with pneumonia and WUPyV was the only respiratory-virus identified, neither had evidence of bacteraemia nor pulmonary tuberculosis and the one child tested for Pneumocystis jiroveci (PCP) infection was negative (Table 2 ).
Table 2.
Characteristics of the eight pneumococcal conjugate-unvaccinated children who died during hospitalization for lower respiratory tract infections where polyomaviruses WU or KI were detected.
| HIV status | Virus detecteda | Age at hospitalization (months) | Gender | Diagnosis | Chest X-ray result | CRP (mg/l) | PCT (ng/ml) | Bacteria isolated from blood | P. jiroveci infection |
|---|---|---|---|---|---|---|---|---|---|
| Infected | WUPyV | 13 | Female | Pneumonia | Normal | 2 | Unknown | No | No |
| Infected | WUPyV | 15 | Male | Pneumonia | Alveolar consolidation | Unknown | Unknown | No | Not test |
| Uninfected | KIPyV+CoV-229E | 3 | Female | Pneumonia | Uninterpretable | 1 | Unknown | No | Yes |
| Uninfected | KIPyV+PIV | 3 | Male | Pneumonia | Alveolar consolidation | 39 | 0.4 | No | Not test |
| Infected | KIPyV | 2 | Female | Bronchiolitis | Normal | 142 | 62.2 | No | Not test |
| Infected | KIPyV | 11 | Female | Pneumonia | Unknown | 258 | 26.8 | Escherichia coli | Yes |
| Infected | KIPyV+CoV-OC43 | 2 | Male | Pneumonia | Alveolar consolidation | 21 | 0.6 | No | Yes |
| Infected | KIPyV+hRV+hBoV | 12 | Female | Bronchiolitis | Unknown | 371 | 84.3 | Escherichia coli | No |
CRP: C-reactive protein; PCT: procalcitonin. None of the children who died had pulmonary tuberculosis.
Including viruses previously-tested by immunofluorescence assay [Influenza A, respiratory syncytial virus (RSV), parainfluenza viruses (PIV) and adenovirus] and by nested-PCR (human metapneumovirus) and newly-tested viruses [human bocavirus (hBoV), human rhinovirus (hRV), human coronaviruses (CoV)-OC43, -NL63, -HKU1 and -229E and polyomavirus-WU (WUPyV) and -KI (KIPyV)].
4.2. KIPyV-associated LRTI hospitalization in PCV-unvaccinated children
KIPyV was detected in 10.2% of the specimens available from HIV-infected and in 7.4% from HIV-uninfected children (p = 0.13) (Table 1). Recurrent KIPyV-associated LRTI episodes occurred in five HIV-infected children, including three children with two episodes each, one child with 3 episodes and one child with 5 episodes. There was a single HIV-uninfected child with two KIPyV-positive LRTIs. Identification of KIPyV was perennial, albeit uncommon during November and December 2001 (Fig. 1). There was a lower prevalence of KIPyV detection in 2000 compared to 2001 among HIV-infected (6.6% [13/198] vs. 18.4% [16/87]; p = 0.002); no difference was evident in HIV-uninfected children between the two calendar years (8.2% in 2000 vs. 5.7% in 2001; p = 0.34).
KIPyV was frequently detected in combination with other respiratory-viruses in both HIV-infected (75.9%) and HIV-uninfected children (74.3%) (Table 1). In HIV-infected the most common co-detected viruses included hRV (51.7%, n = 15), WUPyV and hMPV (10.3%, n = 3 each). In HIV-uninfected the most common viruses associated with KIPyV were hRV (31.4%, n = 11), hMPV (14.3%, n = 5) and WUPyV (11.4%, n = 4).
HIV-infected compared to HIV-uninfected children with KIPyV-associated LRTIs were more likely to have oxygen saturation <90%, higher respiratory rate, longer duration of hospitalization (median [range]: 5.0 days [1–29] vs. 1.0 day [1–15]; p = 0.008) and were more likely to have alveolar consolidation on chest X-ray (CXR-AC) and concurrent bacteraemia (Table 1). Pneumococci were isolated from three HIV-infected children.
Four HIV-infected (13.8%) and two HIV-uninfected (5.7%) children in whom KIPyV was detected died during hospitalization. HIV-uninfected children with KIPyV-associated LRTI who died compared with those who survived were younger (3 vs. 12 months; p = 0.03). Both HIV-uninfected children, aged 3 months, presented with pneumonia, had co-infections with other viruses and one child (who was malnourished) also had PCP infection (Table 2). Of the four HIV-infected children who died, KIPyV was the only respiratory-virus identified in two of them although at least one was co-infected with PCP. Overall, five of the six children who died had indirect markers of bacterial infection, including three HIV-infected with raised C-reactive protein and procalcitonin levels (two of whom also had Escherichia coli bacteraemia) and the other two (one HIV-infected and one HIV-uninfected) with CXR-AC (Table 2).
4.3. Effect of PCV9 on the incidence of polyomavirus-positive LRTIs
As an exploratory analysis the effect of PCV9-vaccination on the incidence of polyomavirus-associated pneumonia hospitalizations was evaluated. In fully vaccinated HIV-uninfected children, the incidence of WUPyV-positive clinical pneumonia hospitalizations was 48.5% (95% CI: 9.1, 70.8) lower in children who received PCV9 compared to placebo-recipients (Table 3 ). A similar VE point-estimate was observed in cases restricted to episodes in which WUPyV was the sole detected virus (45.4%, 95% CI: −47.6, 79.8).
Table 3.
Differences in incidence of WUPyV and KIPyV-associated lower respiratory tract infections between fully-immunized children who received 9-valent pneumococcal conjugated vaccine and placebo recipients; per-protocol analysis.
| HIV-infected |
HIV-uninfected |
Overall |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCV9 (n = 1180) | Placebo (n = 1182) | Vaccine efficacy (95% CI) | p-value | PCV9 (n = 17,065) | Placebo (n = 17,086) | Vaccine efficacy (95% CI) | p-value | PCV9 (n = 18,245) | Placebo (n = 18,268) | Vaccine efficacy (95% CI) | p-value | |
| WUPyV-positive clinical pneumonia | 18 | 15 | −20.2 | 0. 60 | 18 | 35 | 48.5 | 0.02 | 36 | 50 | 27.9 | 0.13 |
| (−101.7, 61.2) | (9.1, 70.8) | (−10.6, 53.0) | ||||||||||
| WUPyV single-detection clinical pneumoniaa | 5 | 9 | 44.4 | 0.42 | 6 | 11 | 45.4 | 0.23 | 11 | 20 | 44.9 | 0.11 |
| (−65.6, 81.3) | (−47.6, 79.8) | (−14.9, 73.6) | ||||||||||
| WUPyV-positive bronchiolitis | 2 | 1 | −100.3 | 0.63 | 22 | 23 | 4.2 | 0.89 | 24 | 24 | −0.13 | 0.10 |
| (−2106.4, 81.8) | (−71.8, 46.6) | (−76.2, 43.1) | ||||||||||
| WUPyV-positive LRTI | 20 | 16 | −25.2 | 0.50 | 40 | 56 | 28.5 | 0.10 | 60 | 72 | 16.6 | 0.30 |
| (−140.4, 34.8) | (−7.3, 52.3) | (−17.5, 40.7) | ||||||||||
| WUPyV single-detection LRTIa | 5 | 9 | 44.4 | 0.42 | 11 | 15 | 26.6 | 0.43 | 16 | 24 | 33.2 | 0.21 |
| (65.6, 81.3) | (−59.8, 66.3) | (−25.6, 64.5) | ||||||||||
| KIPyV-positive clinical pneumonia | 14 | 17 | 17.5 | 0.59 | 4 | 20 | 80.0 | 0.001 | 18 | 37 | 51.3 | 0.01 |
| (−66.7, 59.2) | (41.4, 93.2) | (14.5, 72.3) | ||||||||||
| KIPyV single-detection clinical pneumoniaa | 3 | 4 | 24.9 | 0.50 | 1 | 6 | 83.3 | 0.13 | 4 | 10 | 59.9 | 0.18 |
| (−234.9, 83.1) | (−38.6, 98.0) | (−27.7, 87.4) | ||||||||||
| KIPyV-positive bronchiolitis | 0 | 2 | 100 | 0.50 | 5 | 8 | 37.4 | 0.58 | 5 | 10 | 49.9 | 0.30 |
| (−91.2, 79.5) | (−46.4, 82.9) | |||||||||||
| KIPyV-positive LRTI | 14 | 17 | 17.5 | 0.59 | 9 | 27 | 66.6 | 0.003 | 23 | 44 | 47.7 | 0.01 |
| (−66.6, 59.2) | (29.1, 84.3) | (13.4, 68.4) | ||||||||||
| KIPyV single-detection LRTIa | 3 | 4 | 24.9 | 0.50 | 1 | 8 | 87.5 | 0.04 | 4 | 12 | 66.6 | 0.08 |
| (−234.9, 83.1) | (0.0, 98.4) | (−3.5, 89.2) | ||||||||||
LRTI: lower respiratory tract infections; PCV9: 9-valent pneumococcal conjugate vaccine.
Single-detection: if only one single virus was detected in nasopharyngeal aspirates.
There were also reductions in the incidence of hospitalization for KIPyV-associated clinical pneumonia in PCV9-recipients compared to placebo-recipients overall (51.3%, 95% CI: 14.5, 72.3) and specific in HIV-uninfected children (80.0%, 95% CI: 41.4, 93.2). Similar reductions were observed when KIPyV was the only identified virus (Table 3).
No differences in incidence were observed between PCV9- and placebo-recipients in hospitalizations for WUPyV- or KI-associated clinical pneumonia in HIV-infected children and in hospitalizations for WUPyV- or KIPyV-associated bronchiolitis.
5. Discussion
To our knowledge, this is the most detailed report on the prevalence and clinical features of LRTIs where polyomaviruses were detected in HIV-infected and -uninfected children. Among hospitalized HIV-infected children, WUPyV was detected in 6% of the cases and KIPyV in 10%. In HIV-uninfected children the prevalence of WUPyV was 14% and of KIPyV was 7%. In HIV-infected children 11–14% of the cases positive for at least one polyomavirus were fatal and in 67% (4 cases in total) of these WUPyV or KIPyV were identified as single-detections. Our study also established a possible interaction between polyomaviruses and pneumococcus in HIV-uninfected children, although this may have been masked in HIV-infected children in whom PCV9-vaccination was demonstrated to be less efficacious against pneumonia [17].
WUPyV was detected in 9% of respiratory samples from children presenting with upper or LRTI in Germany, 7% in South Korea and South Africa and 6% in Thailand; KIPyV has been identified in 1–6% of children presenting with respiratory-tract infections [12], [13], [20], [21], [22]. Another study from South Africa reported that 57% of 21 children with LRTIs with WUPyV and 33% of 3 with KIPyV were HIV-infected, however, the HIV-status was unknown in 50% of the cases [12]. In that same study, both polyomaviruses were absent among 50 healthy immunocompetent controls [12]. The role of WU/KIPyV as more serious pathogens in immunocompromised individuals is uncertain. Higher viral loads of PyV were documented in lymphoid tissues and in the brains of HIV-infected adults compared to those from immunocompetent individuals [23], [24]. Similarly higher KIPyV viral loads were found in respiratory-tract specimens from haematology/oncology paediatric patients with respiratory infections, however, whether the viral loads correlated with disease severity was not established [13]. These observations suggest that there may be more viral replication and/or that polyomaviruses might reactivate in immunosuppressed individuals, implying that T-cell impairment might be a factor in facilitating polyomavirus replication. Norja et al. found that WU/KIPyV detection on respiratory samples occurred predominantly in two groups of subjects: immunocompetent <2 years of age with LRTIs (7%) in whom there was a high frequency of co-infection (75%), and among older, generally immunocompromised individuals without respiratory illness (11%) or with mild upper respiratory-tract infections (7%) [7].
Although our study was not designed to establish whether polyomavirus infections caused more severe disease in HIV-infected children, among hospitalized children in whom polyomaviruses were detected HIV-infected were more likely to present with pneumonia rather than bronchiolitis, had a longer duration of hospitalization and higher CFR compared to HIV-uninfected children. Similar observations have been detected between HIV-infected and HIV-uninfected children for other viruses [18], and consequently may not necessarily infer causality for polyomaviruses precipitate more severe illness in HIV-infected children. The increased morbidity and mortality in HIV-infected children could have been related to other co-morbidities such as bacterial or other non-viral co-infections. In the absence of sensitive tools for diagnosing bacterial pneumonia, as well as lack of investigating for other non-viral causes of pneumonia, our study is unable to conclude the actual role of polyomaviruses to more severe disease in HIV-infected children. Nonetheless, the higher CFR, as well as that four fatal cases with polyomavirus as the sole respiratory-virus detected were all HIV-infected children suggest a possible association of polyomavirus causing severe disease in HIV-infected. Furthermore, five HIV-infected children had recurrent KIPyV-positive LRTIs compared to only one HIV-uninfected child. This could have been due to extended viral shedding, viral reactivation or reinfection. A study from Germany detected KIPyV DNA in the respiratory-tract of an immunocompromised child for 7 months and hypothesized that a KIPyV infection during childhood could result in latency of the virus in normal individuals, however, an immune impairment could result in viral reactivation [25]. In 2001 the detection rate of KIPyV in HIV-infected children was higher than in 2000 this may be because of the older cohort in 2001, hence possibly different risk of infection, or differences in year-to-year epidemics.
The concept of a vaccine-probe analysis was initially used in attributing the contribution of Haemophilus influenza-type b in the aetiology of radiological-confirmed pneumonia, as well as subsequently in PCV trials [14], [16], [19]. We have used the same approach in probing the likelihood of co-infection by PCV9-serotypes in children hospitalized for pneumonia associated with influenza virus, PIV and hMPV [14], [15]; and we demonstrated that 44–58% of children hospitalized for pneumonia-associated with these viruses were likely to have pneumococcus co-infection [14], [15]. Using this type of analysis, our study suggests that co-infections with PCV9-serotypes contribute to hospitalizations for clinical pneumonia in which WU/KIPyV were identified. The imputed rate of co-infection of pneumococci in children with polyomaviruses-positive pneumonia from our study provides a conservative estimate of this possible interaction as only 9 serotypes were included in the vaccine and VE even against vaccine-serotypes pneumococcal pneumonia was not 100%. The high VE estimate for KIPyV-positive disease needs to be contextualized within the wide uncertainty bounds of this estimate. The suggested interaction between polyomaviruses and pneumococcus warrants further study.
High co-infection rates with other respiratory-viruses were found for WUPyV and KIPyV which are similar to previous reports [6], [13], [20]. In the absence of a control group of children without LRTIs a definitive causal relationship between polyomavirus detection and disease could not be inferred and this constitutes a limitation of our study. Another limitation of our study is that we relied on NPAs, where identification of an organism does not necessarily imply infection. Also in our study the HIV-infected children were not treated with anti-retroviral treatment (ART), which may have contributed to their clinical course and may differ to HIV-infected children treated with ART.
Determining the aetiology of pneumonia remains a challenge, especially in children and the pathogenic potential of some of the newly-described respiratory-viruses is difficult to address in the context of multiple infections and without specific symptoms. Although polyomaviruses were frequently detected in children hospitalized for LRTIs in our study, further studies which include autopsy samples from fatal LRTI cases, lung aspirate ant-mortem and also enrolment of healthy controls [26], are required to clarify the role of polyomaviruses in the pathogenesis of pneumonia.
Funding
This work is based upon research supported in-part by the South African Research Chairs Initiative of the Department of Science and Technology (DST) and National Research Foundation (NRF) in Vaccine Preventable Diseases. Additional funding support was received from the National Health Laboratory Service Research Fund and Medical Research Council (Respiratory and Meningeal Pathogens Research Unit). Any opinion, findings and conclusions or recommendations expressed in this material are those of the author(s) and therefore the NRF and DST do not accept any liability with regard thereto. MCN had financial support from the University of the Witwatersrand.
Competing interests
The authors have no conflicts of interest to disclose.
Ethical approval
The main efficacy trial and subsequent retrospective analyses were approved by the Human Research Ethics Committee (HREC) of the University of the Witwatersrand. Signed written informed consent was obtained from the parent/legal guardians as part of the trial. HREC did not require additional consent for this analysis. The main study was not registered under any clinical trial registry as it was undertaken prior to registration being mandatory.
Acknowledgments
The authors thank the essential contribution of the members of the Vaccine Trialist Group [17] for their involvement in the original study, all the trial participants and all RMPRU staff involved in the study. The authors also thank John W. Rossen for technical assistance and BioMérieux South Africa for providing reagents.
References
- 1.Nair H., Simoes E.A., Rudan I., Gessner B.D., Azziz-Baumgartner E., Zhang J.S. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;381(9875):1380–1390. doi: 10.1016/S0140-6736(12)61901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juven T., Mertsola J., Waris M., Leinonen M., Meurman O., Roivainen M. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;19(4):293–298. doi: 10.1097/00006454-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Pavia A.T. Viral infections of the lower respiratory tract: old viruses, new viruses, and the role of diagnosis. Clin Infect Dis. 2011;52(Suppl. 4):S284–S289. doi: 10.1093/cid/cir043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruuskanen O., Lahti E., Jennings L.C., Murdoch D.R. Viral pneumonia. Lancet. 2011;377(9773):1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allander T., Andreasson K., Gupta S., Bjerkner A., Bogdanovic G., Persson M.A. Identification of a third human polyomavirus. J Virol. 2007;81(8):4130–4136. doi: 10.1128/JVI.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaynor A.M., Nissen M.D., Whiley D.M., Mackay I.M., Lambert S.B., Wu G. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007;3(5):e64. doi: 10.1371/journal.ppat.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norja P., Ubillos I., Templeton K., Simmonds P. No evidence for an association between infections with WU and KI polyomaviruses and respiratory disease. J Clin Virol. 2007;40(4):307–311. doi: 10.1016/j.jcv.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman D.V., Mackenzie E.F., Gardner S.D., Poulding J.M., Amer B., Russell W.J. Human polyomavirus (BK) infection and ureteric stenosis in renal allograft recipients. J Clin Pathol. 1978;31(4):338–347. doi: 10.1136/jcp.31.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Silva L.M., Bale P., de Courcy J., Brown D., Knowles W. Renal failure due to BK virus infection in an immunodeficient child. J Med Virol. 1995;45(2):192–196. doi: 10.1002/jmv.1890450214. [DOI] [PubMed] [Google Scholar]
- 10.Padgett B.L., Walker D.L., ZuRhein G.M., Eckroade R.J., Dessel B.H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971;1(7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- 11.Mourez T., Bergeron A., Ribaud P., Scieux C., de Latour R.P., Tazi A. Polyomaviruses KI and WU in immunocompromised patients with respiratory disease. Emerg Infect Dis. 2009;15(1):107–109. doi: 10.3201/1501.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venter M., Visser A., Lassauniere R. Human polyomaviruses: WU and KI in HIV exposed children with acute lower respiratory tract infections in hospitals in South Africa. J Clin Virol. 2009;44(3):230–234. doi: 10.1016/j.jcv.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao S., Garcea R.L., Robinson C.C., Simoes E.A. WU and KI polyomavirus infections in pediatric hematology/oncology patients with acute respiratory tract illness. J Clin Virol. 2011;52(1):28–32. doi: 10.1016/j.jcv.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madhi S.A., Klugman K.P. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10(8):811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madhi S.A., Ludewick H., Kuwanda L., Niekerk N., Cutland C., Little T. Pneumococcal coinfection with human metapneumovirus. J Infect Dis. 2006;193(9):1236–1243. doi: 10.1086/503053. [DOI] [PubMed] [Google Scholar]
- 16.Feikin D.R., Scott J.A., Gessner B.D. Use of vaccines as probes to define disease burden. Lancet. 2014;383(9930):1762–1770. doi: 10.1016/S0140-6736(13)61682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klugman K.P., Madhi S.A., Huebner R.E., Kohberger R., Mbelle N., Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349(14):1341–1348. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 18.Nunes M.C., Kuschner Z., Rabede Z., Madimabe R., Van Niekerk N., Moloi J. Clinical epidemiology of bocavirus, rhinovirus, two polyomaviruses and four coronaviruses in HIV-infected and HIV-uninfected South African children. PLoS One. 2014;9(2):e86448. doi: 10.1371/journal.pone.0086448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulholland K., Hilton S., Adegbola R., Usen S., Oparaugo A., Omosigho C. Randomised trial of Haemophilus influenzae type-b tetanus protein conjugate vaccine [corrected] for prevention of pneumonia and meningitis in Gambian infants. Lancet. 1997;349(9060):1191–1197. doi: 10.1016/s0140-6736(96)09267-7. [DOI] [PubMed] [Google Scholar]
- 20.Bialasiewicz S., Whiley D.M., Lambert S.B., Jacob K., Bletchly C., Wang D. Presence of the newly discovered human polyomaviruses KI and WU in Australian patients with acute respiratory tract infection. J Clin Virol. 2008;41(2):63–68. doi: 10.1016/j.jcv.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han T.H., Chung J.Y., Koo J.W., Kim S.W., Hwang E.S. WU polyomavirus in children with acute lower respiratory tract infections, South Korea. Emerg Infect Dis. 2007;13(11):1766–1768. doi: 10.3201/eid1311.070872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Payungporn S., Chieochansin T., Thongmee C., Samransamruajkit R., Theamboolers A., Poovorawan Y. Prevalence and molecular characterization of WU/KI polyomaviruses isolated from pediatric patients with respiratory disease in Thailand. Virus Res. 2008;135(2):230–236. doi: 10.1016/j.virusres.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharp C.P., Norja P., Anthony I., Bell J.E., Simmonds P. Reactivation and mutation of newly discovered WU: KI, and Merkel cell carcinoma polyomaviruses in immunosuppressed individuals. J Infect Dis. 2009;199(3):398–404. doi: 10.1086/596062. [DOI] [PubMed] [Google Scholar]
- 24.Barzon L., Squarzon L., Militello V., Trevisan M., Porzionato A., Macchi V. WU and KI polyomaviruses in the brains of HIV-positive patients with and without progressive multifocal leukoencephalopathy. J Infect Dis. 2009;200(11):1755–1758. doi: 10.1086/648095. [DOI] [PubMed] [Google Scholar]
- 25.Falcone V., Panning M., Strahm B., Vraetz T., Bierbaum S., Neumann-Haefelin D. Prolonged KI polyomavirus infection in immunodeficient child. Emerg Infect Dis. 2012;18(4):706–708. doi: 10.3201/eid1804.111588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine O.S., Bhat N., Crawley J., Deloria-Knoll M., DeLuca A.N., Driscoll A.J. Pneumonia etiology research for child health. Introduction. Clin Infect Dis. 2012;54(Suppl. 2):S87–S88. doi: 10.1093/cid/cir1050. [DOI] [PMC free article] [PubMed] [Google Scholar]