Abstract
Objectives
The primary objective was to evaluate whether a molecular point-of-care test (POCT) for viral and atypical pathogens added to routine real-time PCR could reduce duration of intravenous antibiotics in hospitalized patients with lower respiratory tract infection (LRTI) compared with routine real-time PCR.
Methods
In this single-centre, open-label, randomized controlled study, we enrolled hospitalized adults diagnosed with LRTI. Patients were randomized to an intervention group (POCT FilmArray Panel for 20 viruses, atypical pathogens and bacteria plus routine real-time PCR) or a control group (routine real-time PCR for ten pathogens). The primary outcome was duration of intravenous antibiotics during hospitalization. The secondary outcomes included length of stay, cost of hospitalization and de-escalation within 72 hours and between 72 hours and 7 days. Intention-to-treat analysis was used.
Results
Between October 2017 and July 2018, we enrolled 800 eligible patients (398 in the intervention group and 402 in the control group). Duration of intravenous antibiotics in the intervention group was shorter than in the control (7.0 days (interquartile range (IQR) 5.0–9.0) versus 8.0 days (IQR 6.0–11.0); p <0.001). Length of hospital stay in the intervention group was significantly shorter (8.0 days (IQR 7.0–11.0) versus 9.0 days (IQR 7.0–12.0; p <0.001) and the cost of hospitalization in the intervention group was significantly lower ($1804.7 (IQR 1298.4–2633.8) versus $2042.5 (IQR 1427.4–2926.2); p 0.002) than control group. More patients in the intervention group achieved de-escalation within 72 hours (7.9%, 29/367 versus 3.2%, 12/377; p 0.005) and between 72 hours and 7 days (29.7%, 109/367 versus 22.0%, 83/377; p 0.024).
Conclusions
Use of molecular POCT testing for respiratory viruses and atypical pathogens might help to reduce intravenous antibiotic use in hospitalized LRTI patients.
Clinical Trial Registration
clinicaltrials.gov Identifier: NCT03391076.
Keywords: Acute exacerbation of bronchiectasis, Acute exacerbation of chronic obstructive pulmonary disease, Community-acquired pneumonia, FilmArray Respiratory Panel, Lower respiratory tract infection, Molecular point-of-care testing
Introduction
Lower respiratory tract infection (LRTI) is the leading infectious disease in the world [1]. It is also the fourth commonest cause of death globally, accounting for about 3.0 million deaths worldwide in 2016 [2]. Viral infection is one of the most important causes of LRTI [3]. Because of large overlap in symptoms and clinical presentation between bacterial and viral LRTI, antibiotics are inappropriately prescribed to patients with viral infection. This may result in potential risks of antimicrobial resistance with a corresponding financial burden and environmental pollution [4]. Furthermore, inappropriate prescription of antibiotics is even more critical in China, which ranks as the world's most frequent user of antibiotics [5], [6]. Overuse of intravenous antibiotics in patients hospitalized with LRTI constitutes an important part of the inappropriate prescription of antibiotics [7]. In one retrospective study in a teaching hospital in Beijing [8], the median duration of intravenous antibiotics was 10 days (interquartile range 8–14 days) among hospitalized individuals with mild to moderate community-acquired pneumonia (CAP). Diagnostic uncertainty regarding the lack of microbiological evidence may be one of the most important reasons.
Laboratory-developed PCR testing is highly accurate for the diagnosis of microbial aetiology, with the turnaround time generally being 1–2 days [9], [10], [11]. However, experienced specialists are required for this test and the instruments have to be installed in a central laboratory. FilmArray Respiratory Panel (BioFire; Salt Lake City, UT, USA) is a new molecular point-of-care test (POCT) platform, which can simultaneously detect 20 viruses and atypical pathogens and provide results in about 1 hour [12], [13]. The sensitivity and specificity of this new molecular POCT for detecting pathogens were high, with the sensitivity and specificity ranging from 92.3% to 97.9% and 96.1% to 99.1%, respectively [12], [13].
A recently published randomized controlled trial showed that use of the FilmArray Respiratory Panel was associated with reduction in proportion of antibiotic use among patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD) and asthma [9]. We speculated that molecular POCT for the detection of viruses might reduce the duration of antibiotic use in adult LRTI patients [14], [15]. Considering predominant overuse of intravenous antibiotics in China, the aim of this study was to evaluate whether combination of POCT and routine real-time PCR for pathogen detection could reduce duration and improve de-escalation of intravenous antibiotics in individuals with LRTI compared with routine real-time PCR only.
Methods
Study design and participants
This was a single-centre, open-label, parallel randomized controlled study that took place between October 2017 and July 2018 in the China–Japan Friendship Hospital (CJFH), Beijing, China (clinicaltrials.gov identifier: NCT03391076). CJFH is a large teaching hospital with 1600 beds. Patients were recruited from the general ward of the Department of Pulmonary and Critical Care Medicine, Department of Traditional Chinese Medicine Lung Disease and Department of Infectious Disease in CJFH. Hospitalized patients aged ≥18 years who were preliminarily diagnosed as radiographically confirmed CAP, AECOPD or acute exacerbation of bronchiectasis were recruited on the day of hospitalization. Patients were excluded if they were <18 years old, pregnant, had hospital-acquired pneumonia, or lung tuberculosis. We also excluded patients with human immunodeficiency virus infection, haematological cancer or solid tumour treated with chemotherapy or radiotherapy in the previous 3 months, organ or bone marrow transplantation, splenectomy, or autoimmune diseases including systemic lupus erythematosus, rheumatoid arthritis, rheumatic polymyalgia and interstitial lung disease treated with immunosuppressive therapy for >3 weeks. In addition, patients with any other condition that may have increased serum procalcitonin levels, including severe burns, major surgical procedures, major trauma, long-term or severe cardiogenic shock, invasive fungal infection, or an acute attack of Plasmodium falciparum, were also excluded.
This study was conducted in accordance with the Declaration of Helsinki. The study was approved by the ethics committee of CJFH (2017-29). Written informed consent was obtained from each participant after meeting inclusion criteria and before randomization.
Randomization and masking
Random allocation sequence was generated using SPSS 22.0 software (Statistical Product and Service Solutions, IBM Co. Ltd, Armonk, NY, USA) with a fixed random seed. Simple randomization was conducted subsequently by sealing the group allocation cards into envelopes according to the sequence number. Each envelope was opened only when patients met inclusion criteria and signed informed consent, with allocation of patients to intervention or control group accordingly. Study participants, research staff and clinical care providers were not blinded to the group allocations. Allocation of patients was blinded to data analysts.
Procedures
Demographics and clinical characteristics were collected on enrolment. Before the study commenced, research personnel were trained on how to take nasopharyngeal swabs and how to operate the FilmArray Respiratory Panel instrument. Routine diagnosis, treatment and microorganism detection of CAP, AECOPD and acute exacerbation of bronchiectasis followed Chinese guidelines and consensus for these diseases [16], [17], [18]. In the intervention group, research staff took nasopharyngeal swabs from patients according to standard protocols within 4 hours of admission. The samples were analysed immediately using the FilmArray Respiratory Panel. The panel can detect 17 viruses (influenza A (H1 and H3) virus, influenza B virus, respiratory syncytial virus, rhinovirus or enterovirus, human metapneumovirus, parainfluenza virus types 1 4, coronaviruses (OC43, 229E, HKU1 and NL63) and adenovirus), two atypical pathogens (Chlamydia pneumoniae and Mycoplasma pneumoniae) and one bacterium (Bordetella pertussis). The results were reported and explained to physicians via telephone, sending text messages (with mandatory feedback) or face-to-face communication on the day of admission. In both the intervention and control groups, routine real-time PCR assays for the detection of viral pathogens (including influenza A (H1N1, H7N9) virus, influenza B virus, respiratory syncytial virus, parainfluenza virus, adenovirus, Epstein–Barr virus, herpes simplex virus and human cytomegalovirus) were performed in the CJFH microbiology laboratory with sputum or nasopharyngeal swab samples (see Supplementary material, Table S1). The results were reported and explained to physicians once obtained. Other diagnostic tests such as blood gas analysis, C reactive protein, erythrocyte sedimentation rate, procalcitonin and routine microbiological testing were prescribed by physicians in both groups.
The responsible attending physicians decided on antibiotic administration (including moxifloxacin, levofloxacin, Types I/II/III/IV generation cephalosporin, carbapenem, β-lactamase/β-lactamase-inhibitors, macrolide, penicillins, tetracycline), de-escalation or cessation of use in both groups without intervention of the research staff. Management system and technical support framework for antimicrobial stewardship have been established in China. The Medical Department and Pharmacy Department regularly assess of the use of antibiotics in CJFH. All data were collected on a standard case report form and then input into an electronic medical database by an authorized assistant.
Outcomes
The primary outcome was the duration of intravenous antibiotics during hospitalization. Duration of intravenous antibiotics was defined as the total number of calendar days when one or more than one dose of intravenous antibiotics was used. The secondary outcomes included the proportion of patients who received intravenous antibiotics, the proportion of patients with antibiotics de-escalation within the first 72 hours and between 72 hours and 7 days, length of hospital stay, cost of intravenous antibiotics and cost of hospitalization. De-escalation of antibiotics was defined as reduction of antibiotic types, change from intravenous antibiotics to oral antibiotics, or from broad-spectrum antibiotics to narrower-spectrum antibiotics (see Supplementary material, Table S2). Cost of hospitalization from the perspective of the hospital before deduction of benefits consist of six parts, including laboratory test (radiation, pathology and blood biochemistry test), medical care (oxygen therapy and doctor visit), surgery, blood storage or processing, drug and other (such as medical material) costs (see Supplementary material, Table S3). All outcomes were measured until discharge from hospital. Participants were followed up in person at day 30 by trained research staff if the length of hospitalization was <30 days, with six participants in the intervention group and eight participants in the control group lost to follow up. Adverse outcomes included admission to intensive care unit (ICU), death during hospitalization, readmission within 30 days, and death within 30 days.
Statistical analysis
According to findings of Branche et al. [14], we assumed that a 1-day reduction of antibiotics use in the intervention group would be clinically significant. We estimated that 340 patients would be required in each group to yield a statistical power of 80% to detect a 1-day reduction in antibiotic use in the intervention group at a significance level of p 0.05. We further assumed that 10%–15% of the study participants would be non-adherent or lost to follow up and set a total target recruitment number of 400 patients in each group.
Data analyses were performed according to intention-to-treat analysis. Per-protocol analysis by excluding participants whose diagnosis was ascertained not to be LRTI after randomization or who were withdrawn for refusing nasopharyngeal swab was also performed.
Baseline characteristics were expressed as numbers (proportion), median (interquartile range) or mean ± standard deviation and comparisons were made using the χ2 test, Wilcoxon rank-sum test or Student's t-test where appropriate. The median and interquartile range of the primary outcome (duration of intravenous antibiotics) and secondary outcomes, including length of hospital stay, cost of intravenous antibiotics and cost of hospitalization, were calculated and the difference between intervention and control group was compared using the Wilcoxon rank-sum test. For other secondary outcomes (proportion of intravenous antibiotic use, de-escalation within the first 72 hours and de-escalation between 72 hours and 7 days), any significant differences in the proportions calculated with the χ2 test and unadjusted odds ratios calculated with a logistic regression model were used to look for differences between the intervention and control groups. Differences and 95% CIs involved in this study were absolute differences expressed as means or proportions. Data analyses were performed using SAS version 9.4 (SAS Institute Inc.,).
Results
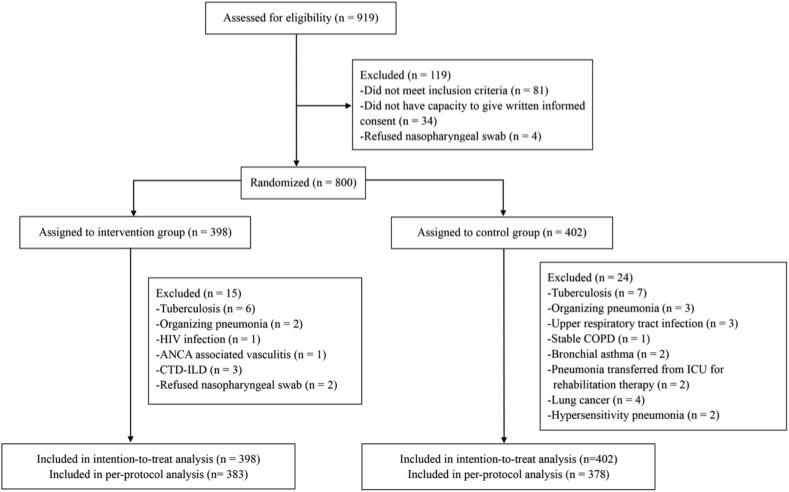
Between 16 October 2017 and 13 July 2018, we assessed 919 patients for eligibility, and 800 of them were eligible to participate in the study (Fig. 1 ). A total of 398 patients were randomly assigned to the intervention group and 402 to the control group and included in the intention-to-treat analysis, with 15 patients in the intervention group and 24 patients in the control group who were ascertained not to have LRTI after randomization or who were withdrawn for refusing nasopharyngeal swab.
Fig. 1.
Trial profile. Abbreviations: ANCA, anti-neutrophil cytoplasmic antibody; COPD, chronic obstructive pulmonary disease; CTD-ILD, connective tissue diseases–interstitial lung disease; HIV, human immunodeficiency virus; POCT, point-of-care testing.
Baseline characteristics of study participants in intervention and the control group included in intention-to-treat analysis and per-protocol are shown in Table 1 and the Supplementary material (Table S4), respectively. All patients in the intervention group were tested using the FilmArray Respiratory Panel and 46.0% (183/398) of patients were also tested using routine real-time PCR, whereas 47.8% (192/402) of patients in the control group were tested using routine real-time PCR for viral pathogens (see Supplementary material, Tables S1 and S5).
Table 1.
Demographic and clinical characteristics of patients with lower respiratory tract infection
| Variable | Intervention group (n = 398) | Control group (n = 402) |
|---|---|---|
| Age, years | 60.8 ± 18.2 | 60.9 ± 17.7 |
| Male (%) | 232 (58.3) | 224 (55.7) |
| Observation | ||
| Body mass index (kg/cm2) | 23.3 ± 3.8 | 23.5 ± 5.2 |
| Temperature (°C) | 37.0 (36.5–37.7) | 36.8 (36.5–37.5) |
| Respiratory frequency (breaths/min) | 20.0 (20.0–22.0) | 20.0 (20.0–22.0) |
| Heart rate (beats/min) | 90.0 (80.0–100.0) | 90.0 (80.0–100.0) |
| Systolic blood pressure (mmHg) | 125.5 (116.0–140.0) | 128.0 (117.0–142.0) |
| Final diagnosis (%) | ||
| CAP | 242 (60.8) | 214 (53.2) |
| CURB-65 score a | ||
| 0–1 | 213/240 (88.8) | 180/214 (84.5) |
| 2 | 22/240 (9.2) | 29/214 (13.6) |
| 3–5 | 5/240 (2.1) | 4/214 (1.9) |
| AECOPD | 98 (24.6) | 108 (26.9) |
| AE of bronchiectasis | 45 (11.3) | 56 (13.9) |
| Onset of illness to admission (days) | 7.0 (5.0–14.0) | 7.0 (5.0–14.0) |
| Symptoms (%) | ||
| Fever | 266 (66.8) | 254 (63.2) |
| Cough | 383 (96.2) | 393 (97.8) |
| Chest pain | 103 (25.9) | 122 (30.3) |
| Dyspnoea | 306 (76.9) | 309 (76.9) |
| Catarrhal symptoms | 89 (22.4) | 97 (24.1) |
| Headache | 79 (19.8) | 66 (16.4) |
| Diarrhoea | 53 (13.3) | 47 (11.7) |
| Co-morbidity (%) | ||
| Chronic respiratory disease | 168 (42.2) | 180 (44.8) |
| Cardiovascular disease | 176 (44.2) | 182 (45.3) |
| Diabetes | 80 (20.1) | 101 (25.1) |
| Renal disease | 14 (3.5) | 18 (4.5) |
| Liver disease | 8 (2.0) | 4 (1.0) |
| Cancer | 14 (3.5) | 17 (4.2) |
| Current smoker | 73 (18.3) | 83 (20.6) |
| Influenza vaccine (<1 year) | 53 (13.3) | 51 (12.7) |
| Antibiotics use before admission | 6 (1.5) | 7 (1.7) |
| Laboratory test | ||
| Procalcitonin (ng/mL) b | ||
| <0.1 | 19/280 (6.8) | 20/262 (7.6) |
| 0.1–0.24 | 124/280 (44.3) | 120/262 (45.8) |
| ≥0.25 | 137/280 (48.9) | 122/262 (46.6) |
| White blood cell count (×109/L) | 6.6 (5.3–9.4) | 7.0 (5.3–9.3) |
| Neutrophil count (×109/L) | 4.6 (3.1–7.0) | 4.5 (3.1, 6.9) |
| Lymphocyte count (×109/L) | 1.3 (1.0–1.9) | 1.5 (1.0–1.9) |
| Haemoglobin (g/L) | 130.0 (120.0–141.0) | 128.0 (118.0–140.0) |
| Platelet count (G/L) | 222.5 (177.0–277.0) | 237.0 (185.0–292.0) |
| Albumin (g/L) | 38.7 ± 4.4 | 38.7 ± 4.5 |
| Aspartate aminotransferase (U/L) | 20.0 (16.0–30.0) | 19.0 (15.0–24.0) |
| Lactate dehydrogenase (U/L) | 186.0 (162.0–230.0) | 187.0 (157.0–221.0) |
| Alkaline phosphatase (U/L) | 66.0 (56.0–86.0) | 68.0 (58.0–86.0) |
| Total bilirubin (μmol/L) | 9.7 (6.9–13.3) | 9.6 (6.6–13.1) |
| Direct bilirubin (μmol/L) | 3.3 (2.4–4.2) | 3.1 (2.5–4.2) |
| Blood glucose (mmol/L) | 5.5 (4.9–6.5) | 5.5 (5.0–6.7) |
| Creatinine (μmol/L) | 64.5 (55.4–78.2) | 64.1 (53.4–76.6) |
| Blood urea nitrogen (mmol/L) | 4.4 (3.4–5.7) | 4.5 (3.6–5.9) |
| Oxygenation index (mmHg) | 338.0 (290.0–381.0) | 343.0 (300.0–388.0) |
AE, acute exacerbation; AECOPD, acute exacerbation of chronic obstructive pulmonary disease; CAP, community-acquired pneumonia; CURB-65, a pneumonia severity score calculator (measured by 5 risk factors in total, with 1 point for each criterion satisfied: confusion defined as an abbreviated mental test score ≤8; blood urea nitrogen ≥ 7mmol/l; respiratory rate ≥30 bpm; systolic blood pressure < 90 mmHg or diastolic blood pressure ≤60 mmHg; age ≥65 years).
Data are presented as mean ± SD (standard deviation) or as median (interquartile range) for continuous variables and as percent for categorical variables. Categorical variables were compared using χ2 tests, and continuous variables were compared using Wilcoxon rank-sum test or Student's t-test.
The denominator is the number of community-acquired pneumonia.
The denominator is the number of study participants that received the procalcitonin test.
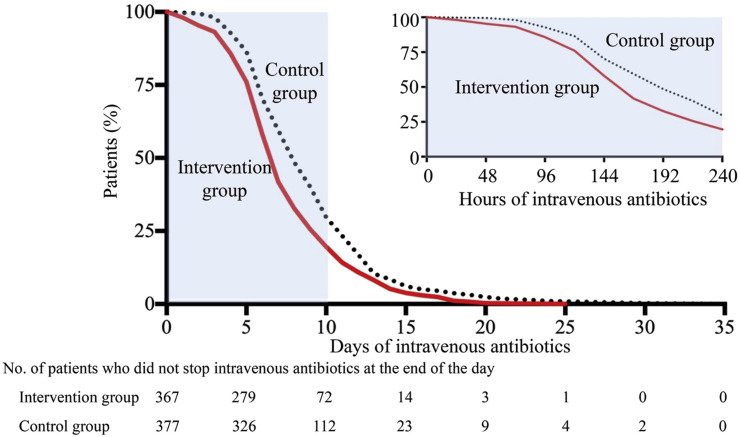
The median duration of intravenous antibiotic treatment in the intervention group was significantly shorter than in the control group (7.0 days (5.0–9.0 days) versus 8.0 days (6.0–11.0 days); difference –1.5 days, 95% CI –2.1 to –0.8 days; p <0.001) (Table 2 ). The proportion of participants who were given intravenous antibiotics was high in both the intervention and control groups, but with no significant differences observed between the two groups (92.1%, 367/398 versus 93.8%, 377/402; difference –1.6%, 95% CI –5.1% to 2.0%; p 0.38). Patients in the intervention group stopped having intravenous antibiotics earlier compared with the control group (p < 0.001 for log-rank test) (Fig. 2 ). Eight patients in the intervention group stopped intravenous antibiotic use on the same day as receiving the POCT test result (data not shown). More patients in the intervention group achieved de-escalation within 72 hours (7.9%, 29/367 versus 3.2%, 12/377; difference 4.7%, 95% CI 1.4%–8.0%; p 0.005) and between 72 hours and 7 days (29.7%, 109/367 versus 22.0%, 83/377; difference 7.7%, 95% CI 1.4% to 14.0%; p 0.024) than in the control group. The per-protocol analysis also showed shorter intravenous antibiotic treatment and earlier de-escalation of intravenous antibiotics in the intervention group compared with the control group.
Table 2.
Comparison of intravenous antibiotic duration and cost of hospitalization
| Variable | Intervention group | Control group | Difference (95% CI) | Odds ratio (95% CI) | p value |
|---|---|---|---|---|---|
| Intention-to-treat analysis | |||||
| No. of study participants | 398 | 402 | |||
| Intravenous antibiotics | |||||
| Duration of intravenous antibiotics (days) | 7.0 (5.0–9.0) | 8.0 (6.0–11.0) | –1.5 (–2.1 to –0.8)a | NA | <0.001 |
| Given (%) | 367/398 (92.1) | 377/402 (93.8) | –1.6 (–5.1 to 2.0) | 0.79 (0.46–1.36) | 0.38 |
| De-escalation within the first 72 h (%)b | 29/367 (7.9) | 12/377 (3.2) | 4.7 (1.4–8.0) | 2.61 (1.31–5.20) | 0.005 |
| De-escalation between 72 h and 7 days (%)b | 109/367 (29.7) | 83/377 (22.0) | 7.7 (1.4–14.0) | 1.50 (1.08–2.08) | 0.024 |
| Length of hospital stay (days) | 8.0 (7.0–11.0) | 9.0 (7.0–12.0) | –1.0 (–1.6 to –0.4)a | NA | <0.001 |
| Cost of intravenous antibiotics ($) | 189.9 (103.5–316.5) | 245.8 (138.1–397.8) | NA | NA | <0.001 |
| Cost of hospitalization ($) | 1804.7 (1298.4–2633.8) | 2042.5 (1427.4–2926.2) | NA | NA | 0.002 |
| Per-protocol analysis | |||||
| No. of study participants | 383 | 378 | |||
| Intravenous antibiotics | |||||
| Duration of intravenous antibiotics (days) | 7.0 (5.0–9.0) | 8.0 (6.0–11.0) | –1.6 (–2.2 to –1.0)a | NA | <0.001 |
| Given (%) | 353 (92.2) | 356 (94.2) | –2.0 (–5.6 to 1.6) | 0.73 (0.41–1.29) | 0.27 |
| De-escalation within the first 72 h (%)b | 27/353 (7.7) | 12/356 (3.4) | 4.3 (0.9–7.6) | 2.37 (1.18–4.77) | 0.013 |
| De-escalation between 72 h and 7 days (%)b | 105/353 (29.8) | 81/356 (22.8) | 7.0 (0.5–13.5) | 1.44 (1.03–2.01) | 0.034 |
| Length of hospital stay (days) | 8.0 (6.0–10.0) | 9.0 (7.0–12.0) | –1.1 (–1.7 to –0.5)a | NA | <0.001 |
| Cost of intravenous antibiotics ($) | 188.3 (103.5–312.7) | 251.7 (147.5–411.4) | NA | NA | <0.001 |
| Cost of hospitalization ($) | 1792.6 (1261.1–2581.3) | 2027.2 (1436.5–2903.3) | NA | NA | <0.001 |
NA, not applicable.
Data are presented as median (interquartile range) for continuous variables and as per cent for categorical variables. Difference of continuous variable between intervention and control group was compared using the Wilcoxon rank-sum test. Difference of categorical variable between intervention and control group was compared using χ2 tests and logistic regression model was used to calculate unadjusted odds ratios.
Mean difference.
The denominator is the number of study participants that received intravenous antibiotics.
Fig. 2.
Time to withdrawal of intravenous antibiotics (intention-to-treat analysis).
The median length of hospital stay in the intervention group was significantly shorter than in the control group (8.0 days (7.0–11.0 days) versus 9.0 days (7.0–12.0 days)]; difference –1.0 days, 95% CI –1.6 to –0.4 days; p <0.001). The median cost of intravenous antibiotics and hospitalization were also significantly less in the intervention group ($189.9 (103.5–316.5) versus $245.8 (138.1–397.8); p <0.001 and $1804.7 (1298.4–2633.8) versus $2042.5 (1427.4–2926.2); p 0.002). The per-protocol analysis also showed shorter length of hospital stay and lower cost of intravenous antibiotics and hospitalization in the intervention group compared with the control group.
The proportions of adverse outcomes, including ICU admission, death during hospitalization, readmission within 30 days, and death within 30 days were not found to be significantly different between intervention and control groups (all p ≥0.05) (Table 3 ).
Table 3.
Adverse outcomes
| Variable | Intervention group | Control group | Difference (95% CI) | Odds ratio (95% CI) |
|---|---|---|---|---|
| Intention-to-treat analysis | ||||
| No. of study participants | 398 | 402 | ||
| ICU admission | 4 (1.0) | 6 (1.5) | –0.5 (–2.0 to 1.1) | 0.67 (0.19–2.39) |
| Death during hospitalization | 2 (0.5) | 3 (0.8) | –0.2 (–1.3 to 0.9) | 0.67 (0.11–4.04) |
| Readmission within 30 daysa | 23/392 (5.9) | 27/394 (6.9) | –1.0 (–4.4 to 2.4) | 0.85 (0.48–1.51) |
| Death within 30 daysa | 3/392 (0.8) | 4/394 (1.0) | –0.3 (–1.6 to 1.1) | 0.75 (0.17–3.38) |
| Per-protocol analysis | ||||
| No. of study participants | 383 | 378 | ||
| ICU admission | 4 (1.0) | 5 (1.3) | –0.3 (–1.8 to 1.3) | 0.79 (0.21–2.96) |
| Death during hospitalization | 2 (0.5) | 3 (0.8) | –0.3 (– 1.4 to 0.9) | 0.66 (0.11–3.95) |
| Readmission within 30 daysa | 22/377 (5.8) | 26/370 (7.0) | –1.2 (–4.7 to 2.3) | 0.82 (0.46–1.47) |
| Death within 30 daysa | 3/377 (0.8) | 4/370 (1.1) | –0.3 (–1.7 to 1,1) | 0.73 (0.16–3.30) |
ICU, intensive care unit.
Data are presented as per cent for categorical variables. Difference between intervention and control group was compared using χ2 tests and logistic regression model was used to calculate unadjusted odds ratios.
The denominator is the number of study participants that were followed up at day 30.
Discussion
In our study adding POCT to routine real-time PCR testing shortened the duration of intravenous antibiotic treatment, reduced the length of stay and cost of hospitalization, and improved early de-escalation of intravenous antibiotics compared with routine real-time PCR assays in hospitalized LRTI patients. Admission to the ICU and fatality rates were similar between groups.
The efficacy of this new POCT has been evaluated in retrospective studies [15], [19], [20], [21], but most of them were conducted among paediatric patients [19], [20], [21]. A few randomized controlled trials have explored the impact of POCT among adults, but with small samples [10], [22]. One recent randomized controlled trial with a relatively large sample size evaluated the effect of the FilmArray Respiratory Panel among adult patients with acute respiratory illness [9]. However, only half of them were diagnosed with LRTI, including pneumonia and AECOPD. The heterogeneity of the study population, including asthma and upper respiratory infections, limited extrapolation to LRTI. The advantage of our study is that only individuals with LRTI were enrolled, excluding acute upper respiratory infection and non-infectious respiratory illness. Another advantage of our study is that we enrolled patients throughout four consecutive seasons.
In this study, we focused on duration of antibiotics, but not on withdrawal of antibiotics once the POCT result was available. It is easy to understand that physicians can safely stop antibiotics as soon as they know the positive viral results for patients with upper respiratory infections or asthma, as demonstrated in the study by Brendish et al. [9]. However, for patients with pneumonia and other kinds of LRTI, most Chinese physicians refer to local clinical guidelines for duration of antibiotics, such as 5–7 days for CAP [16], 10–14 days for CAP with atypical pathogen [16], 5–10 days for AECOPD [17] and 14 days for acute bronchiectasis [18]. Although it is difficult to exclude bacterial co-infection, the knowledge of viral aetiology will help physicians to decide whether to stop intravenous antibiotics earlier [10]. Considering that length of hospital stay for LRTI patients was directly related to duration of intravenous antibiotics, we finally chose duration of intravenous antibiotics as the primary outcome. With the median cost around $225 per hospital-day for patients with LRTI, 1 day less of intravenous antibiotics and 1 day less of hospitalization could save billions of dollars in China.
There are a number of limitations in our study. First, it was a single-centre study, the results of which need to be verified by future multicentre studies. Second, the cost of the FilmArray Respiratory Panel was not considered in the total costs of hospitalization since the FilmArray Respiratory Panel is not commercially available in China. Our post-hoc analysis indicated that the cost during hospitalization in the intervention group would be lower than, or at least equal to, that in the control group if the FilmArray Respiratory Panel test cost less than $360. Third, because the proportion of patients who received intravenous antibiotic therapy was high and duration of intravenous antibiotics was relatively long in both groups, extrapolation of our results should be carefully interpreted. Fourth, the study was conducted in general wards, without including patients from ICUs. The effect of POCT needs further rigorous evaluation in patients who are more severely ill, including ICU patients.
In conclusion, this study found the addition of molecular POCT testing to routine real-time PCR testing for respiratory viruses and atypical pathogens might help to reduce intravenous antibiotic use in LRTI patients without resulting in adverse outcomes. More multicentre studies will be required to verify these findings.
Transparency declaration
We declare that we have no competing interests.
Notation of prior abstract publication/presentation
The content of the manuscript has not been published, or submitted for publication elsewhere. The abstract has been accepted for presentation at the 2019 ATS International Conference.
Funding
This work was supported by the National Science Grant for Distinguished Young Scholars (grant number 81425001/H0104) and Chinese Academy of Medical Science Innovation Fund for Medical Sciences (2018-I2M-1-003). BioMérieux provided, free-of-charge, FilmArray panel punches and FilmArray instruments. They did not participate in the design, conduction and analysis of the study.
Author contributions
BC conceived and designed the trial, supervised the trial and allocated staff, had full access to all of the data in the study and takes responsibility for the content of the manuscript. SD and DY participated in the recruitment of patients and data acquisition. The randomization of participants into intervention or control group according to random sequence number was done by RS, and allocation of patients was finished by HL and YW. XG and GF performed the data analysis. SD, BC and XG drafted and revised the manuscript, and ZX and BL ensured the quality control and running environment of the FilmArray instrument. All authors reviewed the manuscript and contributed to the study report during the whole progress. All authors approved the final version of the manuscript.
Acknowledgements
We thank all the patients and clinical staff in the general wards of the Respiratory Critical Medical Department, Traditional Chinese Medicine lung disease department, Infectious Disease Department and Laboratory of Clinical Microbiology and Infectious Diseases in the China–Japan Friendship Hospital, including physicians, nurses and laboratory technicians.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2019.06.012.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization. World health report: the ten most common infections. Available at: http://www.who.int/whr/1996/media_centre/press_release/en, accessed 11 September 2017.
- 2.World Health Organization. Fact sheet 310: the top 10 causes of death. Available at: http://www.who.int/mediacentre/factsheets/fs310/en, accessed 11 September 2017.
- 3.World Health Organization. Battle against respiratory viruses initiative. Available at: www.who.int/influenza/patient_care/clinical/brave/en, accessed 11 September 2017.
- 4.Crotty M.P., Meyers S., Hampton N., Bledsoe S., Ritchie D.J., Buller R.S. Impact of antibacterials on subsequent resistance and clinical outcomes in adult patients with viral pneumonia: an opportunity for stewardship. Crit Care. 2015;19:404. doi: 10.1186/s13054-015-1120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao Y.H. Bacterial resistance: challenge and strategies. China Lic Pharm. 2011;8:3–8. [Google Scholar]
- 6.Wang Z., Zhang H., Han J., Xing H., Wu M.C., Yang T. Deadly sins of antibiotic abuse in China. Infect Contr Hosp Epidemiol. 2017;38:758–759. doi: 10.1017/ice.2017.60. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q.Q., Ying G.G., Pan C.G., Liu Y.S., Zhao J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ Sci Technol. 2015;49:6772–6782. doi: 10.1021/acs.est.5b00729. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Q., He B., Zhu H. Potential for cost-savings in the care of hospitalized low-risk community-acquired pneumonia patients in China. Value in Health. 2009;12:40–46. doi: 10.1111/j.1524-4733.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 9.Brendish N.J., Malachira A.K., Armstrong L., Houghton R., Aitken S., Nyimbili E. Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open-label, randomised controlled trial. Lancet Respir Med. 2017;5:401–411. doi: 10.1016/S2213-2600(17)30120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelfer G., Leggett J., Myers J., Wang L., Gilbert D.N. The clinical impact of the detection of potential etiologic pathogens of community-acquired pneumonia. Diagn Microbiol Infect Dis. 2015;83:400–406. doi: 10.1016/j.diagmicrobio.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews D., Chetty Y., Cooper B.S., Virk M., Glass S.K., Letters A. Multiplex PCR point of care testing versus routine, laboratory-based testing in the treatment of adults with respiratory tract infections: a quasi-randomised study assessing impact on length of stay and antimicrobial use. BMC Infect Dis. 2017;17:671. doi: 10.1186/s12879-017-2784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poritz M.A., Blaschke A.J., Byington C.L., Meyers L., Nilsson K., Jones D.E. FilmArray, an automated nested multiplex PCR system for multi-pathogen detection: development and application to respiratory tract infection. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan M., Koo S.H., Jiang B., Lim P.Q., Tan T.Y. Comparison of the biofire FilmArray respiratory panel, Seegene AnyplexII RV16, and Argene for the detection of respiratory viruses. J Clin Virol. 2018;106:13–17. doi: 10.1016/j.jcv.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Branche A.R., Walsh E.E., Vargas R., Hulbert B., Formica M.A., Baran A. Serum procalcitonin measurement and viral testing to guide antibiotic use for respiratory infections in hospitalized adults: a randomized controlled trial. J Infect Dis. 2015;212:1692–1700. doi: 10.1093/infdis/jiv252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rappo U., Schuetz A.N., Jenkins S.G., Calfee D.P., Walsh T.J., Wells M.T. Impact of early detection of respiratory viruses by multiplex PCR assay on clinical outcomes in adult patients. J Clin Microbiol. 2016;54:2096–2103. doi: 10.1128/JCM.00549-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao B., Huang Y., She D.Y., Cheng Q.J., Fan H., Tian X.L. Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese Thoracic Society, Chinese Medical Association. Clin Respir J. 2018;12:1320–1360. doi: 10.1111/crj.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai B.Q., Cai S.X., Chen R.C., Cui L.Y., Feng Y.L., Gu Y.T. Expert consensus on acute exacerbation of chronic obstructive pulmonary disease in the People's Republic of China. Int J Chron Obstruct Pulmon Dis. 2014;9:381–395. doi: 10.2147/COPD.S58454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai B.Q., He Q.Y., Gao Z.C., Cao Z.L., Ma Y.L., Yang R.H. [Expert consensus on bronchiectasis for adults in China] Zhonghua Jie He He Hu Xi Za Zhi. 2012;35:485–492. [Google Scholar]
- 19.Subramony A., Zachariah P., Krones A., Whittier S., Saiman L. Impact of multiplex polymerase chain reaction testing for respiratory pathogens on healthcare resource utilization for pediatric inpatients. J Pediatr. 2016;173:196–201.e2. doi: 10.1016/j.jpeds.2016.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers B.B., Shankar P., Jerris R.C., Kotzbauer D., Anderson E.J., Watson J.R. Impact of a rapid respiratory panel test on patient outcomes. Arch Pathol Lab Med. 2015;139:636–641. doi: 10.5858/arpa.2014-0257-OA. [DOI] [PubMed] [Google Scholar]
- 21.Keske S., Ergonul O., Tutucu F., Karaaslan D., Palaoglu E., Can F. The rapid diagnosis of viral respiratory tract infections and its impact on antimicrobial stewardship programs. Eur J Clin Microbiol Infect Dis. 2018;37:779–783. doi: 10.1007/s10096-017-3174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilbert D., Gelfer G., Wang L., Myers J., Bajema K., Johnston M. The potential of molecular diagnostics and serum procalcitonin levels to change the antibiotic management of community-acquired pneumonia. Diagn Microbiol Infect Dis. 2016;86:102–107. doi: 10.1016/j.diagmicrobio.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.