Highlights
-
•
We used limiting-dilution passage to rapidly attenuated a QX-like IBV strain.
-
•
The attenuated strain presented a decreased pathogenicity in 1-day-old chickens.
-
•
The attenuated strain could serve as a vaccine candidate through spray route.
Keywords: Infectious bronchitisvirus, Limiting-dilution passage attenuation, Attenuated vaccine, 1-day-old chickens, Spray
Abstract
Infectious bronchitis (IB) is an acute, highly contagious disease, which causes economic losses to the poultry industry worldwide. To control the disease, biosecurity and vaccination are required. In the current research, we rapidly attenuated a QX-like IBV field strain ZYY-2014 using passage in embryos at limiting dilution and tested the safety and efficacy of the attenuated Chinese QX-like IBV strain ZYYR-2014 in 1-day-old specific-pathogen-free (SPF) chickens through spray route. Our result revealed that the attenuated strain presented a decreased pathogenicity in 1-day-old chickens. The strain ZYY-2014 inoculated birds presented typical IBV clinical signs with a mortality of 43%, while the attenuated strain ZYYR-2014 inoculated birds remained healthy. The strain ZYYR-2014 also presented stronger antibody responses and lower viral loads in tracheas, lungs and kidneys. When vaccinated through spray route into 1-day-old SPF chickens, our data suggest a potential of the attenuated ZYYR-2014 strain as a vaccine candidate applied in hatchery, which can contribute in preventing the QX-like IBV infections. Furthermore, attenuation by passage at limiting dilution could be applied for rapid vaccine development against emerging strains.
1. Introduction
Infectious bronchitis (IB) is an acute, highly contagious disease affecting respiratory, renal, and reproductive systems of chickens, thus resulting in egg production drop, a decline in carcass weight and bird death. Since its first identification in 1931, the disease has been circulating in different regions of the world, causing severe economic losses [1], [2].
The characterized pathogen of IB is infectious bronchitis virus (IBV), which belongs to the genus Gammacoronavirus in the family Coronaviridae, order Nidovirales. The virus genome is a linear, single-stranded, positive-sense RNA with a length of approximately 27 kilobases (kb) [3]. Genes encoding four structural proteins are identified in the genome and designated as spike (S), envelope (E), matrix (M), and nucleocapsid (N). Among the four structural proteins, the S protein, consisting of S1 and S2 subunits, plays an important role in receptor binding, virus fusion and antigenic neutralization [4], [5], [6]. Furthermore, small changes (<5%) in amino acid sequence of the S1 protein may affect cross-protection [7], thus contributing to complexity of disease epidemiology and control.
Vaccination is considered to be the most effective way to control the disease. Various live attenuated vaccines and inactivated vaccines derived from classical serotypes are often used in the field, showing little or no cross protection against heterologous IBV challenges when used alone [8], [9]. Effective strategies including applications of vaccines containing different antigenic types and multiple immunizations could provide broad protection [9]. However, due to high evolutionary rates, virus replication errors and recombination [10], [11], emerging IBV strains belonging to different genotypes and serotypes have been reported worldwide [12]. Facing the complex situation of IBV infections, effective vaccines to control the epidemic disease are highly required [13].
Since the first detection of QX-like or GI-19 [14] IBV strain in China in 1996, the QX-like IBV has become the predominant genotype in China. An epidemiological investigation of IBV isolates collected from 2013 to 2015 showed that 46.1% of the IBV strains isolated were characterized as QX-like genotype [15]. Not restricted to China, the QX-like genotype has spread widely to other countries in Asia, Africa and Europe [12]. Current vaccine strains used in China are derived from classical strains of Mass genotype. However, phylogenetic analysis indicates that the QX-like genotype is genetically distant from the Mass-type, which may explain the poor performance of these classical vaccines [16]. In Europe, QX-based vaccines were developed [17] and commercial vaccines such as POULVAC IB QX (Pfizer, France) are produced against endemic IBV strains. In China, there were also several attempts to develop an efficient QX-like IBV vaccine through serial passages of field isolates in embryonated specific pathogen free (SPF) eggs, take YX10-D90, aYN and P110 for instance. These vaccine strains were proved to be efficient in SPF chickens through intranasal vaccination [18], [19], [20]. However, the process of attenuation requires a long passage time in embryonated eggs, which usually takes up to 1-year, thus is time consuming.
To rapidly develop an effective vaccine, which could be applied in hatchery through spray, we developed a QX-like attenuated strain through rapid attenuation by embryo passage at limiting dilution in the current study. This method was shown to be able to attenuate the virulence of field strain within 5 passages. The attenuated strain presented a reduction of virulence. Furthermore, safety and efficacy tests in 1-day-old SPF chickens through spray route showed that, with a desired level of immunogenicity, the attenuated strain could be used as a promising vaccine candidate against IBV infection.
2. Materials and methods
2.1. Virus isolation
The IBV QX-like strain ZYY-2014 (GenBank accession no.: MG544178) was isolated from an H120 vaccinated flock presenting IB symptoms in Guangdong province, China, 2014, as described before [21]. Briefly, collected kidneys were homogenized and centrifuged at 11.5g at 4 °C for 10 min. The supernatant was filtered through 0.22 μm cellulose esters membranes (Merck Millipore Ltd., Ireland) and inoculated into 10-day-old SPF chicken embryonated eggs via allantoic cavity route with a quantity of 0.2 ml and the eggs were incubated at 37 °C for 48 h. Embryos that died within 24 h of inoculation were discarded, and lesions of embryos in the rest of the eggs were monitored. After 48 h, allantoic fluids were harvested under sterile condition for subsequent passages. After five passages, the allantoic fluid was examined using reverse transcription polymerase (RT-PCR) to detect the virus. The embryo 50% infectious doses (EID50) were calculated by the Reed and Muench method.
The IBV QX-like strain HSJ-2016 (GenBank accession no.: MG544176) was isolated from H120 vaccinated broiler flock as well in Guangdong province, 2016. It was used as a homologous IBV stain to determine the efficacy of the attenuated ZYY-2014. The M41 strain was used as a heterologous challenge strain.
2.2. Limiting dilution passage attenuation
The EID50 for the strain ZYY-2014 was 10−6.39 EID50/0.2 ml. According to the EID50, the allantoic fluid of embryonated eggs inoculated with ZYY-2014 was diluted 105, 106, 107 times. The EID50 of the virus of the first passage was 10−6.65/0.2 ml. For the 2nd passage, the allantoic fluid of embryonated eggs inoculated with the virus of the first passage was diluted 105, 106 and 107 times. The diluted allantoic fluid was inoculated for the next passage. For the 3rd passage, the allantoic fluid was diluted 106, 107 and 108 times, since the EID50 was 10−7.00/0.2 ml. The diluted allantoic fluid of each dilution ratio was inoculated into embryonated eggs (5 eggs for each dilution) via allantoic cavity route at a quantity of 0.2 ml/egg and the eggs were incubated at 37 °C for 48 h and the embryos that died within 24 h of inoculation were discarded. After 48 h, the allantoic fluid of each inoculated embryonated egg was collected and examined for the presence of IBV by RT-PCR. The allantoic fluid from the egg inoculated with the highest dilution ratio that was positive for IBV was diluted and used for the next dilution passage. The EID50 of the passaged virus was calculated by the Reed and Muench method. After five passages, the allantoic fluid positive for IBV was collected and the virus strain was cultured and designated as ZYYR-2014. The EID50 of the strain ZYYR-2014 was calculated by the Reed and Muench method.
2.3. RNA extraction and molecular characterization
Viral RNA was extracted from allantoic fluids applying Trizol (Invitrogen, Carlsbad, CA, USA) according to manufacturer's instruction. RT-PCR was performed using a PrimerScriptTM one step RT-PCR kit Ver.2 (TaKaRa, Otsu, Shiga, Japan) according to the manufacturer’s instruction. PCR primers for IBV detection were designed specifically according to the conserved sequence of the S1 gene (forward-primer: TTGAAAACTGAACAAAAGACCG, reverse-primer: TACAAAACCTGCCATAACTAACAT), resulting in a product of about 1740 bp. The amplified S1 genes of strain ZYY-2014 and ZYYR-2014 were sequenced by BGI (BGI Shenzhen, Shenzhen, China) and analyzed together with S1 gene sequences of 25 other IBV strains of different genotypes from GenBank (Supple. Table 1) using Clustalx alignment (DNASTAR Inc., Madison, Wisconsin, USA). Phylogenetic tree was constructed by the neighbor-joining method utilizing MEGA6.0 (http://www.megasoftware.net). Bootstrap values were determined from 1000 replicates of the original data.
2.4. Safety test
A total of 120 1-day-old SPF chickens were assigned to 4 groups (30 birds/ group). The experimental groups were intranasally inoculated with 200 μL of 104.5EID50 of the ZYY-2014 or ZYYR-2014 strains. Vaccine strain YX10-D90 at a dose of 104.5EID50 was used as a positive control. The control group was inoculated with 200 μL of phosphate buffered saline (PBS). All birds were reared in isolators with positive pressure in air-conditioned rooms. After inoculation, all birds were monitored daily for clinical signs such as hunched posture, depression, diarrhea, soiled vent etc. up to 21 days. At day 21, sera of 15 of the birds in each group were collected and antibodies were tested using commercial enzyme-linked immunosorbent assay (ELISA) according to manufacturer’s instruction (IDEXX, Westbrook, Maine, USA). Necropsies were performed in all birds. Lesions in trachea like punctate hemorrhages and catarrhal exudates were monitored and noted. Nephritis was characterized by pale and marbled kidneys with urate deposits in the ureters and cloaca. Tracheas and kidneys from birds that died and 3 other randomly selected birds in each group were collected and further processed for histopathology and electron microscopy.
2.5. Virulence reversion test
Ten 1-day-old SPF chickens were divided into 2 groups with 5 chickens per group. At day 1, the inoculated group was intranasally inoculated with 200 μL of 104.5EID50 containing the ZYYR-2014 strain and the control group was inoculated with 200 μL of phosphate buffered saline (PBS). At 5 day-post-infection (dpi), the tracheas and kidneys were collected and tissue homogenates were centrifuged at 11.5g at 4 °C for 10 min. The supernatant was intranasally inoculated into the next group of 5 chickens at a dose of 0.2 ml/bird via eye-drop. IBV was detected in tissue homogenates using RT-PCR with the primers described before. After 5 passages, chickens inoculated with the supernatant of the tissue homogenates were monitored daily up to 21 days. Tracheas and kidneys of 3 randomly selected birds were collected and processed further for pathological examination. At 5 dpi of each passage, tracheas and kidneys from all birds were collected. Viral RNA was extracted from the collected organs and the S1 genes was sequenced as described in 2.3. The sequences of the S1 gene were compared using Clustalx alignment (DNASTAR Inc., Madison, Wisconsin, USA). This experiment was repeated 3 times.
2.6. Efficacy test
A total of 165 one-day-old SPF chickens were divided into 11 groups (15 birds/group). Each bird in the vaccinated groups was inoculated by spray with 103.5, 104.5 and 105.5 EID50 of ZYYR-2014 and 103.5 EID50 of YX10-D90 as the manufacturer suggested. The spray was performed by a poultry vaccine sprayer (Vland Biotech Inc., Qingdao, China). The flow rate of the sprayer was approximately 3 ml/sec. Before vaccination, the sprayer was disinfected with 70% ethanol and rinsed 3 times with distilled water. During vaccination, 15 chickens were placed in the container of the sprayer and sprayed twice with a dose of 0.4 ml/bird. After spraying, the chickens were kept in the container for 20 min and then released to the housing facilities.
All vaccinated birds were vaccinated at day one through spray route and kept in isolators with positive pressure in air-conditioned rooms. Three groups vaccinated with ZYYR-2014 at different dosages, one group vaccinated with YX10-D90 and one unvaccinated group were intranasally challenged later with homologous field strain HSJ-2016 (105.0 EID50/bird) at 21 days of age. Simultaneously, the other vaccinated groups and one unvaccinated group were challenged with heterologous strain M41 (105.0 EID50/bird). The NC group was kept as negative control. The details of groups are shown in Table 2. At day 21, sera of all birds were collected and antibodies were tested using commercial enzyme-linked immunosorbent assay (ELISA) (IDEXX, Westbrook, Maine, USA) according to manufacturer’s instruction. At day 5 post challenge, all birds were sacrificed for post-mortem examination.
Table 2.
Efficacy test of ZYYR-2014.
| Group | Vaccination | Vaccine dosage | Challenge strain | Renal protection | Airway protection | Morbidity | Mortality |
|---|---|---|---|---|---|---|---|
| 1 | ZYYR-2014 | 103.5EID50 | M41 | 13/15 | 12/15 | 0 | 0 |
| 2 | ZYYR-2014 | 104.5EID50 | M41 | 14/15 | 13/15 | 0 | 0 |
| 3 | ZYYR-2014 | 105.5EID50 | M41 | 14/15 | 14/15 | 0 | 0 |
| 4 | ZYYR-2014 | 103.5EID50 | HSJ-2016 | 15/15 | 15/15 | 0 | 0 |
| 5 | ZYYR-2014 | 104.5EID50 | HSJ-2016 | 15/15 | 15/15 | 0 | 0 |
| 6 | ZYYR-2014 | 105.5EID50 | HSJ-2016 | 15/15 | 15/15 | 0 | 0 |
| 7 | YX10-D90 | 103.5EID50 | M41 | 11/15 | 12/15 | 2/15 | 0 |
| 8 | YX10-D90 | 103.5EID50 | HSJ-2016 | 10/15 | 11/15 | 1/15 | 0 |
| 9 | None | 0 | M41 | 2/15 | 0/15 | 12/15 | 0 |
| 10 | None | 0 | HSJ-2016 | 0/15 | 3/15 | 14/15 | 3/15 |
| 11 | None | 0 | none | 15/15 | 15/15 | 0 | 0 |
2.7. Histopathology
The trachea and kidney samples collected in the safety test and the virulence reversion test were fixed in 10% formalin, routinely processed and embedded in paraffin wax. 5 μm thin sections were cut and stained with hematoxylin and eosin. The slides were examined with light microscopy for lesions.
2.8. Real time RT-PCR
To examine viral replication ability in chicken embryos, 70 embryonated eggs (35 eggs/group) inoculated with ZYY-2014 or ZYYR-2014 (200 μL/egg) were used for replication curve experiment. Allantoic fluids were collected separately from at least 3 inoculated embryonated eggs per group at 6-hour intervals.
A total of 30 SPF chickens were divided into 3 groups (10 birds/group). 0.2 ml 104.5 EID50 of ZYY-2014 or ZYYR-2014 was inoculated intranasally into each bird in group 1 and 2, respectively. Group 3 was inoculated with PBS and remained as negative control. Tracheas, lungs and kidneys from the inoculated birds at 5 and 7 dpi were collected to detect viral loads.
cDNA was obtained by reverse transcription using a PrimerScript® RT Master Mix Perfect Real Time kit (TaKaRa, Otsu, Shiga, Japan) according to the manufacturer’s instruction and used later. Primers were designated using Primer Express 3.0 (Thermo Fisher Scientific, Waltham, MA, USA) based on the conserved region of 1a gene to detect a 127-bp fragment (IBV-F: CAAGAGCTTGCTGCATATCGTAAA; IBV-R: GCGCTTCCTTATACATAGTTGTCATAGC). The 20 μL PCR mixture was composed of 10 μL SYBR® Premix EX TaqTM II (Tli RNaseH Plus) Kit (TaKaRa Bio, Mountain View, CA, USA), 0.5 μmol of each primer, 0.4 μL ROX II, 100 ng cDNA template and 8 μL double-distilled water. Real-time PCR was performed on an Applied Biosystems 7500 Fast Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). Statistical data was converted to a linear form by the 2−CT calculation and the relative RNA copy numbers was analyzed by GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA). The C T values were obtained from each reaction containing the standard RNA with copies from 101 to 107. The standard curve was plotted against the log of the template copy number. A linear equation (y = -0.258x + 11.235) with a R2 value of 0.997 was generated.
All experiments were performed in triplicate and repeated at least twice.
2.9. Scanning electron microscopy
The trachea rings were cut into quarter-circles under aseptic conditions. The samples were immersed in 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4 and fixed at 4 °C overnight. After fixation, the samples were dehydrated in an increasing series of ethanol with an interval of 10 min. The dehydrated samples were point-dried with carbon dioxide Samdri-PVT-3D (Tousimis, Rockville, MD, USA) immediately and then gold–palladium sputtered by sputter coater E1010 (Hitachi Ltd., Tokyo, Japan). Subsequently, the samples were imaged by SEM Hitachi S-3400N (Hitachi Ltd., Tokyo, Japan).
2.10. Transmission electron microscopy
Three trachea and kidney sections of each bird were fixed in 2.5% glutaraldehyde in PBS overnight. After washing three times with PBS(0.1 M, pH7.4), the fixed tissue was dehydrated with an ascending sequence of ethanol (30%, 50%, 70%, 90%, 100%), dried by critical point drying method, and prepared to analyze by TEM Hitachi S-3400N (Hitachi Ltd., Tokyo, Japan) after gold–palladium sputtering.
2.11. Ciliostasis
A total of 120 one-day-old SPF chickens were divided into 6 groups (20 birds/group). In addition, 10 SPF chickens were kept as negative control. Each bird in the vaccinated groups was inoculated by spray with 103.5 EID50 of ZYYR-2014 or 103.5 EID50 of YX10-D90 as described in 2.6. The details of groups are shown in Table 3. At 21 days of age, two groups vaccinated with ZYYR-2014 or YX10-D90 and one unvaccinated group were intranasally challenged with strain HSJ-2016 (105.0 EID50/bird). Simultaneously, the other vaccinated groups and one unvaccinated group were challenged with strain M41 (105.0 EID50/bird). All birds from each group were sacrificed at 5 day post challenge for evaluation of tracheal ciliostasis as described before [19]. Briefly, three sections of the upper, middle and lower part of the trachea per bird (nine rings/bird) were analyzed. The ciliary activity was scored using the flowing criteria: 0 if the cilia in whole tracheal section showed ciliary activity, 1 if the cilia of 67–100% of the tracheal section showed ciliary activity, 2 if the cilia of 33–67% of the tracheal section showed ciliary activity, 3 if the cilia of 0–33% of the tracheal section showed ciliary activity and 4 if the cilia in the complete tracheal section showed no ciliary movement [17]. For each group, the average ciliostasis score was calculated. A chicken was considered protected if more than eight out of nine rings/bird showed > 50% ciliary activity.
Table 3.
Tracheal ciliostasis.
| Group | Vaccination | Vaccine dosage | Challenge strain | Protectiona |
|---|---|---|---|---|
| 1 | ZYYR-2014 | 103.5EID50 | HSJ-2016 | 18/20 |
| 2 | ZYYR-2014 | 103.5EID50 | M41 | 17/20 |
| 3 | YX10-D90 | 103.5EID50 | HSJ-2016 | 16/20 |
| 4 | YX10-D90 | 103.5EID50 | M41 | 17/20 |
| 5 | None | 0 | HSJ-2016 | 0/20 |
| 6 | None | 0 | M41 | 0/20 |
| 7 | None | 0 | none | 10/10 |
A chicken was considered protected if more than eight out of nine rings/bird showed >50% ciliary activity.
2.12. Statistics
All data were analyzed utilizing two-way ANOVA and unpaired t-test in GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA) to obtain a statistical analysis of the differences. The significance was considered as significant at P < 0.05 (*) and highly significant at P < 0.01 (**) and P < 0.001 (***).
2.13. Ethics statement
All experiments were approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University, concerning the handling of chicken embryos as well as animal experiments. And all experiments were performed in accordance with the relevant guidelines and regulations.
3. Results
3.1. Virus isolation and attenuation
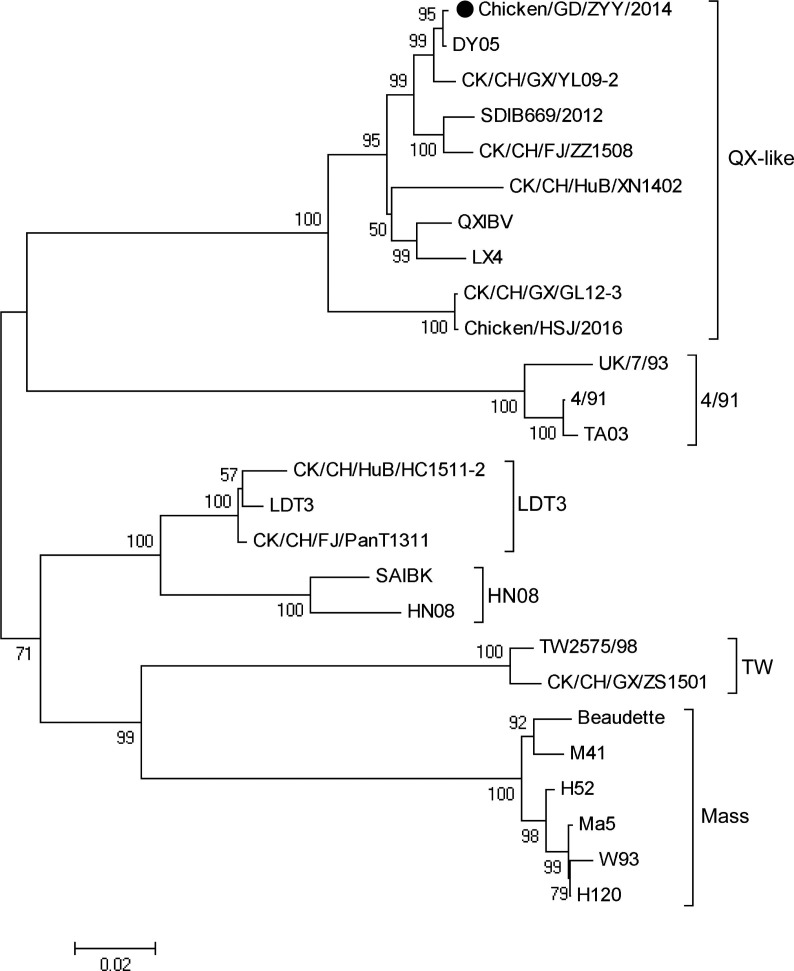
The ZYY-2014 IBV strain (GenBank accession no.: MG544178) was first isolated from field samples in Guangdong, China in 2014. Chicken embryonated eggs inoculated with the isolated strain presented typical IB lesions such as curling, dwarfing and growth stunting. The titer of the virus was determined as 105.69EID50/ml. The S1 gene of the virus was amplified and sequenced. The virus designated ZYY-2014 was identified as IBV and phylogenetic analysis reveals that it belongs to the QX-like genotype (Fig. 1 ).
Fig. 1.
Phylogenetic analysis based on S1 gene sequences of IBV strains. Bootstrap values were determined from 1000 replicates of the original data. Black circle indicates the isolated ZYY-2014 strain. Scale bar indicates amino acid substitution rate.
To obtain a higher viral titer in embryonated eggs, we passaged the strain ZYY-2014 at limiting dilution in embryonated eggs. After passage at limiting dilution, the titer of the virus, designated as ZYYR-2014, increased to 107.19EID50/ml. When the S1 gene sequences of the two viruses were compared, 2 nucleotide substitutions result in substitutions of 2 amino acids (Table 1 ). The ZYY-2014 and ZYYR-2014 strains tested negative for bacteria, fungi, mycoplasma and other chicken exogenous viruses (data not shown) were used in subsequent safety and efficacy experiments.
Table 1.
Nucleotide and amino acid substitutions in S1 gene of ZYY-2014 and ZYYR-2014 strains.
| Gene | Position (nt) | Nucleotide substitution | Position (aa) | Amino acid substitution |
|---|---|---|---|---|
| S1 | 578 | A → T | 193 | Y → F |
| 755 | C → T | 252 | T → I |
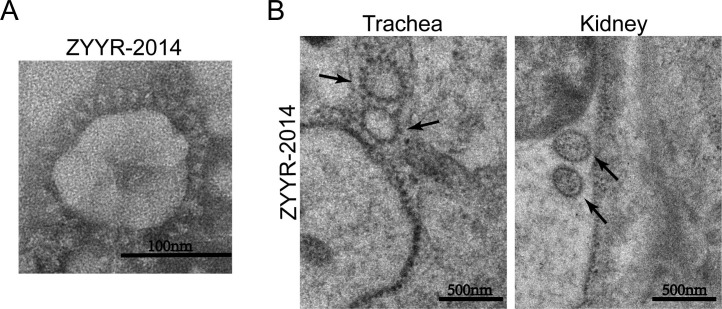
To further explore the characteristics of the strain ZYYR-2014, generated by passage at limiting dilution, we performed electron microscopy to compare morphology of the viruses. The ZYYR-2014 strain presented a spheroidal shape with a diameter of about 100 nm. Typical spikes could be observed on the surface of the viral particles (Fig. 2 A). In infected animals, virus particles of both ZYY-2014 and ZYYR-2014 could be observed in both tracheas and kidneys as well (Fig. 2B).
Fig. 2.
Morphological characteristics of the attenuated ZYYR-2014 strain. (A) Transmission electron micrographs of the virus particle. Scale bar = 100 nm. (B) Scanning electron micrographs of the virus in tracheal lumen and kidney. Black arrows indicate viral particles. Scale bar = 500 nm.
3.2. Virus propagation in embryos
When amplified in chicken embryos, the replication of both viruses reached their maximum at 24 h-post-infection (hpi) and declined afterwards. At 30 hpi, the viral RNA level of the ZYY-2014 was lower than the ZYYR-2014 and increased again at 54 h. However, the viral RNA level of ZYYR-2014 dropped continuously until 42 hpi, and remained at a lower level at 54 hpi, compared to the original virus ZYY-2014 (Fig. 3 ).
Fig. 3.
Viral multiplication in SPF chicken embryonated eggs. All data are presented as mean ± standard deviation (SD). Some of the error bars are too small to be seen. ** indicates significant at P ≤ 0.01.
3.3. Safety
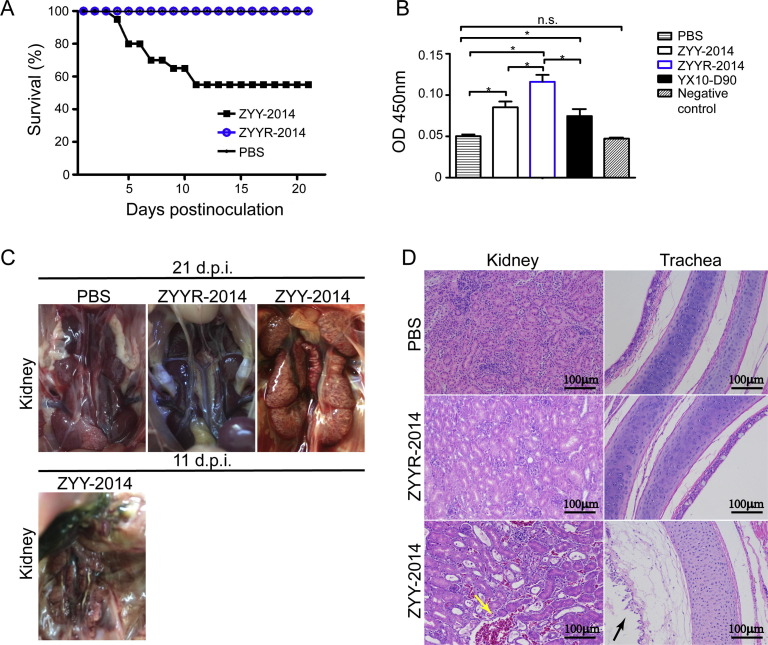
To test the safety of the two viruses in young birds, 1-day old SPF chickens were inoculated with a dosage of 104.5EID50 of ZYY-2014 and ZYYR-2014. The commercial vaccine strain YX10-D90 was used as a positive control. More than half the chickens inoculated with ZYY-2014 presented symptoms like tracheal and bronchiolar rales 24 h after infection. 40% (12/30) of the chickens showed symptoms like slight watery diarrhea, depression and reluctance to move at an early date of 2 dpi. The mortality rate was 6.67% (2/30) at 4 dpi and continuously increased to 43.33% (13/30) until 11 dpi (Fig. 4 A). However, all chickens inoculated with attenuated ZYYR-2014 remained healthy and the survival rate was 100% until 21 dpi (Fig. 4A). 2 chickens inoculated with the same dosage of YX10-D90 died at 6 dpi and the rest were healthy and survived for 21 days (Supple. Fig. 1A). To better understand the effect of inoculations of the viruses, we analyzed antibody responses by ELISA. Antibody against IBV was not detected in the mock-infected group. Chickens in all inoculated groups showed antibody responses against IBV and the ZYYR-2014-inoculated group presented higher antibody levels than the ZYY-2014-inoculated group (p < 0.05), while antibody levels of the YX10-D90-inoculated group were comparable to those of the ZYY-2014-inoculated group (Fig. 4B).
Fig. 4.
Pathogenicity of the IBV ZYY-2014 and ZYYR-2014 strains. (A) Survival percentage of the inoculated 1-day-old SPF chickens. (B) Mean antibody OD values at 21 dpi. All data are presented as mean ± standard deviation (SD) (n = 15); * indicates significant at P ≤ 0.05. (C) Gross lesions on kidneys of the inoculated chickens at 11 dpi and 21 dpi. (D) Histopathologic changes of kidneys and tracheas of the inoculated chickens at 21 dpi. Yellow arrow indicates abnormal bleeding in the kidney. Black arrow indicates lesions on trachea cilia. Scale bar = 100 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
At necropsy, all dead animals in the ZYY-2014 and YX10-D90 infected groups presented lesions in the urinary system (Fig. 4C). The kidneys of some of the surviving chickens in the ZYY-2014 group were also pale, mottled, and swollen. The renal tubules and ureters were distended with excess urates (Fig. 4C). Lesions in the respiratory system such as thickening of the wall and mucosal congestion were also observed by necropsy (data not shown). In the YX10-D90-inoculated group, 2/30 of the birds died at day 6. In anatomy, gross lesions like mottled kidneys were observed in the dead birds and 39.3% (11/28) of the surviving birds, suggesting this vaccine may not be suitable for 1-day-old chicken vaccination (Supple. Fig. 1B). To further confirm this data, we performed tissue pathological analysis from tissues collected from surviving animals in all groups (3 birds/group) at 21 dpi. The kidney pathological sections of ZYY-2014 group showed interstitial congestions with tubular dilations and renal interstitium hemorrhage, while trachea sections presented damage to the pseudostratified ciliated columnar epithelium, compared to the control group (Fig. 4D). Foci of interstitial congestion with tubular dilation and hemorrhages in kidneys were also observed in the YX10-D90 inoculated birds (one sample) (Supple. Fig. 1C). The pathological sections of the ZYYR-2014 inoculated group showed no lesions, which is consistent with the necropsy data, further suggesting a low pathogenicity of the ZYYR-2014 strain in the inoculated 1-day-old chickens (Fig. 4D).
3.4. Virus replication
Low pathogenicity indicates a change of the virulence, which may due to an alteration in virus replication kinetics or interaction with host immune responses. To analyze virus replications in different infected tissues, we examined viral loads in different tissues using real time RT-PCR. Consistent with our assumption, 5 days after inoculation, the virus loads in trachea, kidney and lung tissues of the ZYY-2014 inoculated group were significantly higher than those of the ZYYR-2014 inoculated group (Fig. 5 A). 7 days after inoculation, the viral loads in tracheas and lungs of each inoculated group decreased, while the viral loads in kidneys increased. In all three organs examined, the viral loads of the ZYY-2014 inoculated group were significantly higher than those of the ZYYR-2014 inoculated group (Fig. 5B). This data suggests that the low virus productivity of the ZYYR-2014 strain may contribute to its low pathogenicity.
Fig. 5.
Viral loads in different tissues after infection and scanning electron micrographs of respiratory epithelium from infected chickens. (A) Viral load in tracheas, lungs and kidneys at 5 dpi. (B) Viral load in tracheas, lungs and kidneys at 7 dpi. All data are presented as mean ± standard deviation (SD); *** indicates significant at P ≤ 0.001. (C) Scanning electron micrographs of respiratory epithelium at 7 dpi. Scale bar = 50 μm, 20 μm and 10 μm, respectively.
Furthermore, we performed SEM to examine lesions in the respiratory mucosa. Gross erosion was observed in any samples of the ZYY-2014 inoculated group with severe cilia desquamation, while no lesions were observed in all tracheas of the control and ZYYR-2014 infected groups (Fig. 5B).
Taken together, with a lower virus replication in different organs, ZYYR-2014 presented lower pathogenicity, which makes it a possible vaccine candidate against IBV. Furthermore, the alteration of the virus replication kinetics may affect the pathogenicity.
3.5. Virulence reversion
To further explore the possibility of ZYYR-2014 as a vaccine candidate, we inoculated the ZYYR-2014 strain into SPF chickens for five passages. IBV could be detected in tissue homogenates of inoculated groups for all five passages by RT-PCR. During the experimental period, all the inoculated birds remained healthy and did not show any IBV clinical signs. We also performed histological examination and did not observe any lesions (Supple. Fig. 2). Furthermore, the S1 gene sequences in each passage were as same as the ZYYR-2014 S1 gene sequence. These data suggest that no reversion to virulence of the ZYYR-2014 occurred in the host animals within 5 passages.
3.6. Protective efficacy
Since the ZYYR-2014 could elicit higher IBV-specific antibody levels, presented attenuated pathogenicity and no sign for virulence reversion, we further tested the efficacy of the ZYYR-2014 as a vaccine candidate in 1-day-old chickens through spray. In addition, host immune responses were analyzed though ELISA to check the antibody levels of birds with different vaccine dosages (103.5EID50, 104.5EID50, and 105.5EID50). The antibody level was increased when higher vaccine dosage was given (Fig. 6 A). Later, birds vaccinated with different vaccine dosages were challenged with hetero- and homologous IBV viruses (Fig. 6B). After challenge, the ZYYR-2014 vaccination groups showed full clinical protection against IBV viruses of QX-like (HSJ-2016) and Mass (M41) types. The YX10-D90 group presented a morbidity of 6.67% (1/15) and 13.33% (2/15), with symptoms of depression, reluctance to move and hunched posture, against Mass and QX-like IBV challenges (Table 2 ). Unvaccinated birds challenged with M41 and HSJ-2016 showed clinical signs at 2 day-post-challenge (dpc) such as reluctance to move, hunched posture, huddling, ruffled feathers and slight watery diarrhea. The mortality in the control-HSJ group was 20% (3/15) and the morbidity was 80% (12/15), while all birds in the other control-M41 group survived till the end of the experiment with a morbidity of 60% (9/15).
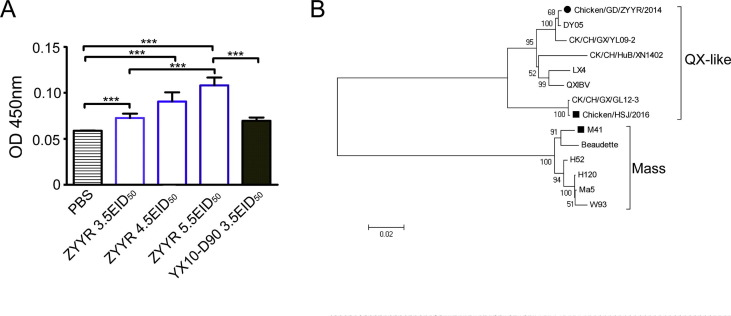
Fig. 6.
Efficacy test of ZYYR-2014 strain of different vaccine dosages. (A) Mean antibody OD values at 21 dpi. All data are presented as mean ± standard deviation (SD) (n = 20); *** indicates significant at P ≤ 0.001. (B) Phylogenetic tree of different IBV strains used in efficacy test. Black circle indicates the vaccine ZYYR-2014 strain. Black squares indicate challenge strains.
At necropsy, in birds inoculated with different dosages of the ZYYR-2014 strain, minor lesions in kidneys and tracheas were observed in M41 challenged birds. No gross lesions were found in HSJ-2016 challenged groups of the ZYYR-2014 vaccinated groups (Table 2). These data suggest the ZYYR-2014 could provide better protection against homologous IBV challenge. Swollen kidneys with urate deposits and catarrhal exudates in the tracheas were observed in the YX10-D90 group, when challenged with the HSJ-2016 strain (Table 2).
For tracheal ciliostasis, after challenge, the birds in the unvaccinated groups showed highest possible ciliostasis score, while the birds in the vaccinated groups as well as the non-challenged group presented normal tracheas. The vaccinated chickens showed hardly any damage in the tracheas, when challenged with either homologous or heterologous IBV strains (Table 3 ).
Taken together, our data indicate that the attenuated ZYYR-2014 strain could provide efficient protection against hetero- and homologous IBV infections in one-day-old chickens by spray, thus could serve as IBV vaccine candidate in young chickens.
4. Discussion
Avian infectious bronchitis virus is a single stranded RNA virus. Limited proofreading capacity of viral RNA-dependent RNA polymerase results in a high mutation rate in the viral genome. The average mutation rate of coronaviruses is approximately 1.2 × 10−3 substitutions/site/year [22]. Moreover, high recombination rate as well as multiple insertions and deletions in the viral genome also contribute to broad genetic diversity of the virus [2]. In IBV, researchers found that the evolutionary rate of the S1 gene is 2.93 × 10−5 substitutions/site/year [10]. Therefore, the genes of IBV undergoing selective pressures contribute to the rapid evolution of IBV in the past decades, producing a mass of genotypes, serotypes and pathogenic types.
Among various genotypes of IBV, previous research showed that QX-like is still the dominant genotype of IBV in China, while other genotypes such as Mass, TW etc. are also circulating in the region [15]. Live attenuated vaccine is used widely in IBV control. However, because of the lack of cross-protection between different serotypes, the widespread use of attenuated vaccine strains such as H120, LDT3, etc. has failed to achieve a complete protection against the challenge of the field IBV stains. Furthermore, to develop an optimal live attenuated vaccine, continuous passages of an IBV strain through SPF chicken embryonated eggs is applied [3], [18], [19], [20], [23]. The passage times could be as much as to hundred times [18], [19], thus is time consuming. Therefore, to rapidly develop an efficient vaccine against the endemic IBV strains is in urgent need.
In this research, we isolated an IBV strain designated ZYY-2014. The strain was isolated in the field, where outbreaks of IBV emerged frequently. In addition, we rapidly attenuated the isolated ZYYR-2014 strain by passage at limiting dilution. After 5 passages, the attenuated strain, designated as ZYYR-2014, presented a decrease of virulence in chickens. Whether the method could be applied for rapid vaccine development against emerging strains requires future work.
The S1 gene sequencing data provide some clues of virulence reduction in the ZYYR-2014 strain that several polar amino acids are substituted with nonpolar amino acids. However, based on reverse genetics data, some researchers suggest that the S1 gene is not responsible for IBV virulence determination [6], [24]. To determine whether these sequence changes in the ZYYR-2104 strain are critical for viral attenuation, future work in reverse genetics is required. Furthermore, since the genetic characteristics of the strain require further elaboration, full genome sequencing could be applied to elucidate changes in other viral genes and whether these changes might be responsible for attenuation.
To further explore the difference in virulence between ZYY-2014 and ZYYR-2014, viral replication kinetics were examined in embryos as well as different tissues of the birds. In embryos, the ZYYR-2014 presented different replication kinetics. The higher copies of the ZYYR-2014 RNA at 30 hpi and the decreased copies of the viral RNA at 54 hpi suggest a different replication pattern than the parental virus. Future work is required to illustrate the differences. Further, in samples of tracheas, lungs and kidneys, ZYY-2014 presented significantly greater viral loads at 5 dpi and 7 dpi compared to ZYYR-2014, which might be the reason for the higher virulence of the parental strain. Consistent with the high viral loads in trachea, a strong cilium desquamation in trachea of the ZYY-2014 group was observed by EM, which further confirms a higher virulence of ZYY-2014, compared to ZYYR-2014.
Young birds are more sensitive to IBV infections and spray vaccination in hatcheries would be practical in the field. Since ZYYR-2014 did not cause pathological damage to the host animals, to evaluate whether it could be applied as a suitable vaccine strain, we performed safety tests and virulence reversion tests in 1-day-old SPF chickens through spray. None of the ZYYR-2014 vaccinated animals showed clinical signs of IBV or death. When a vaccine YX10-D90 [18] was applied in 1-day-old SPF chickens, lesions in kidneys and tracheas could be observed. This vaccine was proved to be safe in 7-day-old SPF chickens [18]. Therefore, our data suggest that ZYYR-2014 is more suitable for 1-day-old chicken vaccination. Furthermore, virulence reversion test showed that the ZYYR-2014 strain did not revert to a virulent IBV strain after five passages in chickens. Therefore, the ZYYR-2014 strain could be considered to be a safe vaccine candidate for young chickens.
In order to evaluate the efficiency of the ZYYR-2014 strain, different dosages of the vaccine candidate were used in vaccination. The antibody levels increased with the dosage of the vaccine and our data indicate that ZYYR-2014 could provide full protection against hetero- and homologous IBV challenge and 103.5EID50 would be the minimum immune dosage. ZYYR-2014 and M41 might belong to the same protectotype, which may explain the high heterologous protection against M41. However, further experiments are required.
In summary, we rapidly attenuated an IBV field strain ZYY-2014 by passage at limiting dilution. Our data of safety and efficiency tests on 1-day-old chickens through spray route meet the standard of the safety and efficacy of IBV vaccines defined by the Chinese Veterinary Pharmacopoeia (CVP) 2010 [25]. Thus, ZYYR-2014 may be considered as a vaccine candidate for protection against hetero- and homologous IBV infection in young chickens of 1-day-old.
5. Author’s contributions
Yongchang Cao designed the experiments. Yun Zhang, Songjian Huang, Yuyao Zeng carried out the experiments. Yun Zhang wrote the manuscript and prepared the figures. Chunyi Xue and Yongchang Cao critically revised the manuscript. All authors read and approved the final version of the manuscript.
Acknowledgments
Acknowledgement
This study was supported by the “Zhujiang talent program” overseas youth talent introduction program (post-doctoral program).
Conflict of interest
The authors have no conflict of interest to declare.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.vaccine.2018.05.123.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Supplementary Fig. 2.
References
- 1.Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet Res. 2007;38(2):281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- 2.Jackwood M.W. Review of infectious bronchitis virus around the world. Avian Dis. 2012;56(4):634–641. doi: 10.1637/10227-043012-Review.1. [DOI] [PubMed] [Google Scholar]
- 3.Jordan B. Vaccination against infectious bronchitis virus: a continuous challenge. Vet Microbiol. 2017;206:137–143. doi: 10.1016/j.vetmic.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Cavanagh D., Davis P.J. Coronavirus IBV: removal of spike glycopolypeptide S1 by urea abolishes infectivity and haemagglutination but not attachment to cells. J Gen Virol. 1986;67:1443–1448. doi: 10.1099/0022-1317-67-7-1443. [DOI] [PubMed] [Google Scholar]
- 5.Promkuntod N., van Eijndhoven R.E., de Vrieze G. Mapping of the receptor-binding domain and amino acids critical for attachment in the spike protein of avian coronavirus infectious bronchitis virus. Virology. 2014;448:26–32. doi: 10.1016/j.virol.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casais R., Dove B., Cavanagh D. Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism. J Virol. 2003;77(16):9084–9089. doi: 10.1128/JVI.77.16.9084-9089.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bande F., Arshad S.S., Bejo M.H. Progress and challenges toward the development of vaccines against avian infectious bronchitis. J Immunol Res. 2015;2015:424860. doi: 10.1155/2015/424860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Wit J.J. Detection of infectious bronchitis virus. Avian Pathol. 2000;29(2):71–93. doi: 10.1080/03079450094108. [DOI] [PubMed] [Google Scholar]
- 9.Jackwood M.W., Hall D., Handel A. Molecular evolution and emergence of avian gammacoronaviruses. Infect Genet Evol. 2012;12(6):1305–1311. doi: 10.1016/j.meegid.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y., Zhang H., Zhao J. Evolution of infectious bronchitis virus in China over the past two decades. J Gen Virol. 2016;97(7):1566–1574. doi: 10.1099/jgv.0.000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffy S., Shackelton L.A., Holmes E.C. Rates of evolutionary change in viruses: patterns and determinants. Nat Rev Genet. 2008;9(4):267–276. doi: 10.1038/nrg2323. [DOI] [PubMed] [Google Scholar]
- 12.Lin S.Y., Chen H.W. Infectious bronchitis virus variants: molecular analysis and pathogenicity investigation. Int J Mol Sci. 2017;18(10) doi: 10.3390/ijms18102030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meeusen E.N., Walker J., Peters A. Current status of veterinary vaccines. Clin Microbiol Rev. 2007;20(3):489–510. doi: 10.1128/CMR.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valastro V., Holmes E.C., Britton P. S1 gene-based phylogeny of infectious bronchitis virus: an attempt to harmonize virus classification. Infect Genet Evol. 2016;39:349–364. doi: 10.1016/j.meegid.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng K., Wang F., Xue Y. Epidemiology and characterization of avian infectious bronchitis virus strains circulating in southern China during the period from 2013–2015. Sci Rep. 2017;7(1):6576. doi: 10.1038/s41598-017-06987-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S., Zhang X., Wang Y. Molecular characterization and pathogenicity of infectious bronchitis coronaviruses: complicated evolution and epidemiology in china caused by cocirculation of multiple types of infectious bronchitis coronaviruses. Intervirology. 2009;52(4):223–234. doi: 10.1159/000227134. [DOI] [PubMed] [Google Scholar]
- 17.Geerligs H.J., Boelm G.J., Meinders C.A. Efficacy and safety of an attenuated live QX-like infectious bronchitis virus strain as a vaccine for chickens. Avian Pathol. 2011;40(1):93–102. doi: 10.1080/03079457.2010.542742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng K., Xue Y., Wang J. Development and efficacy of a novel live-attenuated QX-like nephropathogenic infectious bronchitis virus vaccine in China. Vaccine. 2015;33(9):1113–1120. doi: 10.1016/j.vaccine.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y., Cheng J.L., Liu X.Y. Safety and efficacy of an attenuated Chinese QX-like infectious bronchitis virus strain as a candidate vaccine. Vet Microbiol. 2015;180(1–2):49–58. doi: 10.1016/j.vetmic.2015.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huo Y.F., Huang Q.H., Lu M. Attenuation mechanism of virulent infectious bronchitis virus strain with QX genotype by continuous passage in chicken embryos. Vaccine. 2016;34(1):83–89. doi: 10.1016/j.vaccine.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Picault J.P., Drouin P., Guittet M. Isolation, characterisation and preliminary cross-protection studies with a new pathogenic avian infectious bronchitis virus (strain PL-84084) Avian Pathol. 1986;15(3):367–383. doi: 10.1080/03079458608436300. [DOI] [PubMed] [Google Scholar]
- 22.Hanada K., Suzuki Y., Gojobori T. A large variation in the rates of synonymous substitution for RNA viruses and its relationship to a diversity of viral infection and transmission modes. Mol Biol Evol. 2004;21(6):1074–1080. doi: 10.1093/molbev/msh109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y.P., Wang C.H. Sequence changes of infectious bronchitis virus isolates in the 3' 7.3 kb of the genome after attenuating passage in embryonated eggs. Avian Pathol. 2007;36(1):59–67. doi: 10.1080/03079450601110015. [DOI] [PubMed] [Google Scholar]
- 24.Toro H., Espinoza C., Ponce V. Infectious bronchitis: effect of viral doses and routes on specific lacrimal and serum antibody responses in chickens. Avian Dis. 1997;41(2):379–387. [PubMed] [Google Scholar]
- 25.Yan J.L., Xiao B., editors. Chinese Veterinary Pharmacopoeia 2010. China Agriculture Press; Beijing: 2011. pp. 47–49. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.