Abstract
Background
Rhinovirus-induced early wheezing has been suggested as a new important risk factor for recurrent wheezing.
Objective
We sought to investigate the risk factors for recurrent wheezing and to determine post hoc the efficacy of prednisolone in risk groups.
Methods
We followed for 1 year 118 children (median age, 1.1 years) who had had their first episode of wheezing and had participated in a trial comparing prednisolone with placebo in hospitalized children. Demographics and laboratory data were obtained at study entry. The follow-up outcome was recurrent wheezing (3 physician-confirmed episodes).
Results
Recurrent wheezing was diagnosed in 44 (37%) children. Independent risk factors were age < 1 year, atopy, and maternal asthma. The probability of recurrent wheezing was higher in rhinovirus than respiratory syncytial virus (RSV)–affected children among placebo recipients (hazard ratio, 5.05; 95% CI, 1.00-25.41). Prednisolone decreased the probability of recurrent wheezing in children with eczema (0.15; 95% CI, 0.04-0.63) but not in those without eczema (1.89; 95% CI, 0.83-4.29; P = .007 for interaction). Prednisolone was associated with less recurrent wheezing in the rhinovirus group (0.19; 95% CI, 0.05-0.71), but not in the RSV (2.12; 95% CI, 0.46-9.76) or in the RSV/rhinovirus-negative groups (2.03; 95% CI, 0.83-5.00; P = .017 for interaction).
Conclusion
Rhinovirus-induced early wheezing is a major viral risk factor for recurrent wheezing. Prednisolone may prevent recurrent wheezing in rhinovirus-affected first-time wheezers. The presence of eczema may also influence the response to prednisolone.
Clinical implications
A prospective trial is needed to test the hypothesis that prednisolone reduces recurrent wheezing in rhinovirus-affected wheezing children.
Key words: Recurrent wheezing, rhinovirus, eczema, atopy, prednisolone, child
Abbreviations used: HR, Hazard ratio; OR, Odds ratio; RSV, Respiratory syncytial virus
Acute wheezing affects one third of young children.1 Half of these children continue to wheeze, at least recurrently, until school age. The risk factors for recurrent wheezing include host, environment, and genetic factors such as male sex, eczema, atopy, rhinitis apart from colds, older siblings, maternal smoking, maternal asthma, and Hispanic ethnic background.1, 2
Acute wheezing is predominantly related to viral respiratory infection.2, 3, 4, 5 Respiratory syncytial virus (RSV) usually induces wheezing during infancy, and the episodes are followed by recurrent wheezing in 36% to 68% of cases and by school-age asthma in 18% to 37% of cases.6, 7, 8, 9, 10, 11, 12 The rates of recurrent wheezing associated with other respiratory viruses are not well known because viral diagnostic tools have not been widely available. Rhinovirus is particularly interesting, because it is the second most common virus, triggering early wheezing in as many as 45% of cases.3, 4, 5 Recently, rhinovirus-induced early wheezing has been suggested as a new major risk factor, because it has been followed by third-year wheezing in 65% of cases and school-age asthma in 60% of cases.2, 11
It would be of utmost importance to identify children at high risk for recurrent wheezing and provide them with effective intervention.13 Although systemic corticosteroids are effective in the management of childhood asthma exacerbations,14 many efficacy studies of early wheezing have been discouraging. First episodes of RSV-induced wheezing in infants do not respond to systemic corticosteroids at standard doses.15, 16, 17, 18 Furthermore, only 2 separate reports have also analyzed nonviral factors.15, 19 In these studies, atopy or systemic eosinophil priming has not been associated with efficacy of systemic corticosteroids in young children.
We recently showed that a short course of oral prednisolone reduces recurrences over a 2-month period after the first or second episode of wheezing associated with rhinovirus infection or with above-average blood eosinophils.20 We report here a 1-year follow-up of children who had their first episode of wheezing. Our aim was to determine risk factors for recurrent wheezing and to assess post hoc the long-term effects of prednisolone on the risk groups.
Methods
Study population
As part of an efficacy trial of oral prednisolone on wheezing requiring hospitalization, we conducted a prospective, 1-year follow-up of a cohort of children age 3 to 35 months who had experienced their first episode of wheezing in their lives. The exclusion criteria were inhaled or systemic corticosteroids within 4 weeks before the study, chronic disease, and need for intensive care. All participants of the efficacy trial were randomized double-blindly to receive either oral prednisolone (first dose 2 mg/kg, then 2 mg/kg/d in 3 divided doses) or placebo.20 No stratified randomization was done for participants eligible for the follow-up cohort because the follow-up protocol was made and implemented during the conduct of the efficacy trial. Written parental informed consent was obtained before commencing the study, and the study protocol was approved by the Ethics Committee of the Turku University Hospital.
Risk factor data
On initial admission, a nasopharyngeal mucus sample was aspirated and a venous blood sample was obtained during the acute (at study entry before randomization) and convalescent phases (2-3 weeks after discharge from the hospital). We used comprehensive viral diagnostics including culture, antigen detection, PCR, and/or serology to detect the following respiratory viruses: adenovirus, coronaviruses (strains OC43 and 229), enteroviruses, human metapneumovirus, influenza A and B viruses, parainfluenza viruses (types 1, 2, and 3), rhinovirus, and RSV.21 From the acute phase blood sample, blood eosinophils and IgE antibodies for common allergens (codfish, cow's milk, egg, peanut, soybean, wheat, cat, dog, horse, birch, mugwort, timothy, Cladosporium herbarum, and Dermatophagoides pteronyssinus; fluoroenzyme immunoassay, CAP FEIA, Phadiatop Combi, Phadia, Uppsala, Sweden) were measured by the Central Laboratory of Turku University Hospital. Age, sex, and prematurity were recorded. The parents filled out a questionnaire on other host and environment-related risk factors of recurrent wheezing: physician-diagnosed eczema, parental history of allergy and/or asthma, parental smoking, and day care.
Outcome
The primary outcome was time to recurrent wheezing—that is, time to the third physician-confirmed episode of wheezing within 12 months of the first episode at study entry. This was based on the Finnish practice to start continuous inhaled corticosteroids in all children at the time of the third episode of wheezing within 1 year. The outcome was measured by clinical examination and parental interview at a follow-up session 1 year after initial hospitalization. All episodes of wheezing were confirmed by reviewing each child's medical records.
Definitions
Prematurity meant a gestational age less than 37 weeks. The diagnosis of eczema had been made by the participants' personal physicians according to typical symptoms that included pruritus, typical morphology and distribution of eczematous lesions, and chronicity of disease.22 Atopy referred to positive IgE antibodies against at least 1 of the listed allergens (Phadiatop Combi; detection limit, 0.35 kU/L).22 Each study participant was divided into 1 of the 3 viral subgroups according to the viral etiology of the first episode of wheezing at study entry: rhinovirus group (rhinovirus diagnosed alone or together with any other viruses), RSV group (RSV diagnosed alone or together with any other viruses except rhinovirus), and RSV/rhinovirus negative group (any other viruses except RSV or rhinovirus diagnosed or no viruses found). This grouping was based on the a priori hypothesis that rhinovirus-associated wheezing is a stronger risk factor for recurrences than RSV-associated wheezing,2, 11 and it agrees with the viral grouping of Lemanske et al.2
Statistics
We analyzed the host and environment-related risk factors by Kruskal-Wallis ANOVA followed by the Mann-Whitney U test, and by the χ2 test, as appropriate. We did not calculate statistical significances for the proportions of outcomes in the risk factor groups because we aimed to avoid multiple testing and because the primary outcome included the time aspect. Instead, we used Cox proportional hazards regression to identify any risk factors of prognostic significance. In this study, the Cox hazard ratio (HR) indicated the probability of recurrent wheezing. First, we analyzed in separate models the interactions between treatment grouping and each risk factor. Then, we used stepwise multivariable model to identify independent risk factors for recurrent wheezing. We included all risk factors, and those interaction terms were included that showed a potential effect on outcome. We used a backward stepwise procedure in which we eliminated factors or interaction terms one by one according to the highest P value >.05. The analyses were made using SPSS/PC 13.0 software (SPSS Inc, Chicago, Ill).
Results
Of the 293 randomized patients, 131 children fulfilled the criteria for the long-term follow-up ( Fig 1). Lost to follow-up were 13 children. Thus, the final study cohort consisted of 118 children. Of them, 96 were clinically examined and their parents personally interviewed, and the parents of 22 children were interviewed by phone. Eleven children (9%) were treated for prolonged cough with a continuous inhaled corticosteroid. They were censored from analyses at the time of the initiation of continuous treatment. Of these 11 children, 4 had eczema, 5 were atopic, 7 were rhinovirus-positive, and 6 received prednisolone as the study drug. Their median age was 1.6 years, and median blood eosinophil count was 0.3 × 109/L. Cough resolved from all these children, but it is not known whether it reappeared after discontinuing the inhaled corticosteroid. The median age of the final study cohort was 1.1 years (range, 0.3-2.9). Table I shows the distributions of the host and environment-related risk factors. The risk factor characteristics of prednisolone and placebo groups were comparable (all P values > .28). However, the risk factors were interrelated. Children with eczema had atopic sensitization more often and RSV infection less often than those without eczema (P = .017 and P = .014, respectively). Atopic children were older (P < .001) and more often had blood eosinophilia (≥0.4 × 109/L; P = .006) and rhinovirus infection (P = .001) than nonatopic children. Within the 3 viral groups, the RSV group was younger (P = .002) and less often had eczema (P = .014) and eosinophilia (P < .001) than the 2 other viral groups combined, and the rhinovirus group had atopy more frequently than those without rhinovirus infection (P = .001).
Fig 1.
Study flow chart.
Table I.
Distributions of risk factors and children with recurrent wheeze in the entire cohort and in risk factor groups
| Children with recurrent wheeze |
||||
|---|---|---|---|---|
| Risk factor | Entire cohort (n = 118) | Entire cohort | Prednisolone | Placebo |
| 44 (37%) | 22 (37%) | 22 (38%) | ||
| Young age | ||||
| <1 y | 53 (45%) | 22 (42%) | 11 (42%) | 11 (41%) |
| ≥1 y | 65 (55%) | 22 (34%) | 11 (32%) | 11 (36%) |
| Sex | ||||
| Male | 78 (66%) | 31 (40%) | 15 (40%) | 16 (40%) |
| Female | 40 (34%) | 13 (33%) | 7 (32%) | 6 (33%) |
| Prematurity | ||||
| Yes | 20 (17%) | 10 (50%) | 5 (50%) | 5 (50%) |
| No | 98 (83%) | 34 (35%) | 17 (34%) | 17 (35%) |
| Eczema | ||||
| Yes | 35 (30%) | 15 (43%) | 4 (24%) | 11 (61%) |
| No | 82 (70%) | 29 (35%) | 18 (43%) | 11 (28%) |
| Atopy | ||||
| Yes | 24 (20%) | 11 (46%) | 6 (46%) | 5 (46%) |
| No | 93 (80%) | 32 (34%) | 15 (33%) | 17 (36%) |
| Blood eosinophils | ||||
| <0.4 × 109/L | 84 (72%) | 32 (38%) | 16 (37%) | 16 (39%) |
| ≥0.4 × 109/L | 32 (28%) | 12 (38%) | 6 (40%) | 6 (35%) |
| Maternal asthma | ||||
| Yes | 14 (12%) | 8 (57%) | 5 (71%) | 3 (43%) |
| No | 103 (88%) | 36 (35%) | 17 (33%) | 19 (37%) |
| Parental smoking | ||||
| Yes | 49 (41%) | 20 (41%) | 11 (50%) | 9 (33%) |
| No | 69 (59%) | 24 (35%) | 11 (29%) | 13 (42%) |
| Day care | ||||
| Yes | 70 (59%) | 28 (40%) | 12 (32%) | 16 (49%) |
| No | 48 (41%) | 16 (33%) | 10 (44%) | 6 (24%) |
| Viral etiology | ||||
| RSV | 43 (36%) | 10 (23%) | 7 (28%) | 3 (17%) |
| Rhinovirus | 37 (31%) | 14 (38%) | 5 (26%) | 9 (50%) |
| RSV/rhinovirus-negative | 38 (32%) | 20 (53%) | 10 (63%) | 10 (46%) |
Recurrent wheezing during the 1-year follow-up affected 44 (37%) of the 118 children equally often in the prednisolone group and the placebo group (Table I). Recurrent wheezing was most often related to prematurity (mean 33 gestational weeks) and atopy regardless of treatment. Among the placebo recipients, 61% of children with eczema and 50% of those with rhinovirus infection had recurrent wheezing. The proportions of children with recurrent wheezing seemed to be different among prednisolone recipients and placebo recipients in relation to eczema, maternal asthma, parental smoking, day care attendance, and viral etiology, which indicated a possible interaction between treatment and these risk factors. The separate Cox regression models further suggested an interaction between treatment grouping and eczema (P = .006), day care attendance (P = .023), and the viral etiology of the first episode of wheezing (P = .059). No interaction was identified between treatment grouping and any other risk factor (all P values > .3). The 3 suggestive interactions were included in the multivariable model together with all risk factors listed in Table I. After stepwise elimination, the final model showed that the independent risk factors for recurrent wheezing were young age (<1 year), atopy, and maternal asthma ( Table II). Furthermore, a significant interaction was detected between treatment grouping and eczema status (P = .007) as well as between treatment grouping and viral etiology (P = .017).
Table II.
Significant risk factors and interaction terms associated with recurrent wheeze according to the Cox multivariable regression model
| Risk factor or interaction term | HR (95% CI) | P value |
|---|---|---|
| Age <1 y | 3.04 (1.40-6.61) | .005 |
| Atopy | 4.71 (1.93-11.47) | .001 |
| Maternal asthma | 2.84 (1.19-6.79) | .019 |
| Treatment × eczema | .007∗ | |
| Placebo | ||
| No eczema | Reference | |
| Eczema | 3.29 (1.28-8.44) | |
| Prednisolone | ||
| No eczema | Reference | |
| Eczema | 0.46 (0.14-1.50) | |
| Treatment × viral etiology | .017† | |
| Placebo | ||
| RSV | Reference | |
| Rhinovirus | 5.05 (1.00-25.41) | |
| RSV/rhinovirus-negative | 3.64 (0.74-17.85) | |
| Prednisolone | ||
| RSV | Reference | |
| Rhinovirus | 0.93 (0.25-3.42) | |
| RSV/rhinovirus-negative | 13.67 (4.04-46.23) |
P value for interaction between treatment grouping and eczema status.
P value for interaction between treatment grouping and viral etiology.
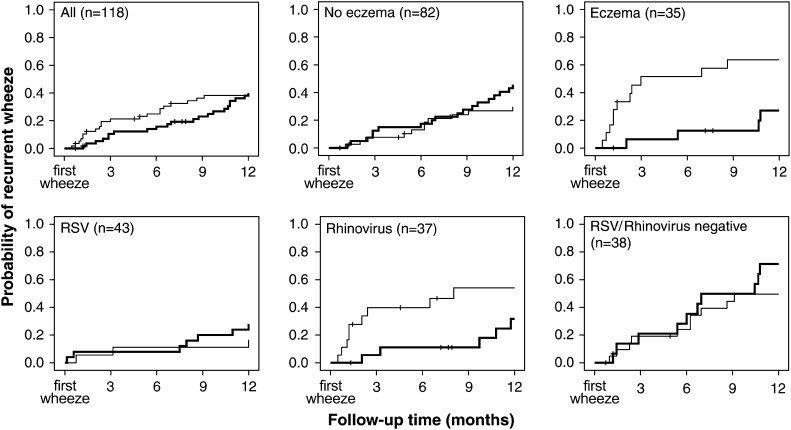
Subgroup analyses suggested that prednisolone, compared with placebo, decreased the probability of recurrent wheezing in children with eczema (HR adjusted for young age, atopy, maternal asthma, and viral etiology, 0.15; 95% CI, 0.04-0.63) but not in those without eczema (1.89; 95% CI, 0.83-4.29). Moreover, in children whose first episode of wheezing was induced by rhinovirus, prednisolone was associated with less recurrent wheezing (HR adjusted for young age, eczema, atopy, and maternal asthma, 0.19; 95% CI, 0.05-0.71). Treatment seemed to have no effect on recurrences in the RSV group (2.12; 95% CI, 0.46-9.76) or in the RSV/rhinovirus-negative group (2.03; 95% CI, 0.83-5.00). Fig 2 illustrates the interactions showing that prednisolone treatment provided no overall benefit, but the treatment effect showed differences related to the eczema and viral etiology of the first episode. Although in the eczema and rhinovirus groups, the difference between prednisolone and placebo recipients is most apparent during the first 3 months, the favorable effect of prednisolone on children with eczema or rhinovirus infection persisted for the entire 1-year follow-up.
Fig 2.
Probability of recurrent wheezing in prednisolone (bold line) and placebo recipients during a 1-year follow-up (censored cases marked with vertical lines).
Discussion
Our results indicate that rhinovirus-induced early wheezing is a major viral risk factor for recurrent wheezing. It seems to be a more important predictor than RSV-induced disease. In our study, 50% of placebo recipients infected by rhinovirus had recurrent wheezing within the first year after the initial episode, compared with 17% of RSV-positive and 46% of RSV/rhinovirus-negative children. This is in agreement with 2 other recent studies that have also used modern viral diagnostics.2, 11 In a previous Finnish study on early wheezing in hospitalized children (n = 66), 60% had asthma at school age if rhinovirus-positive, whereas only 18% of RSV-positive cases and 29% of rhinovirus-negative cases developed asthma (odds ratio [OR], 4.1 for rhinovirus-positive compared with rhinovirus-negative cases).11 A study from the United States followed a birth cohort with an increased risk of allergies or asthma.2 Of 275 infants, 65% with moderate-to-severe wheezing associated with rhinovirus infection continued to wheeze at the age of 3 years compared with 48% and 49% of infants infected by RSV or other viruses, respectively. The association of wheezing in infancy with persistent wheezing was significantly higher in the rhinovirus group (OR, 10) than in the RSV group (OR, 3.5) and in the RSV/rhinovirus-negative group (OR, 4.6). These 3 studies from different centers show that rhinovirus infection–induced wheezing in young children is a major viral risk factor for recurrent wheezing and/or development of asthma.
What is the plausible explanation for the increased tendency of recurrences after rhinovirus-induced wheezing? First, rhinovirus infections can invade the lower airways, increase their inflammatory responses, and enhance airway hyperresponsiveness.23, 24, 25, 26, 27 Second, as in the other Finnish wheeze study, rhinovirus etiology and risk factors for recurrences were interrelated in our population.11 This implies that these patient groups may be connected to other pre-existing immunologic or genetic factors that predispose to recurrent infections or wheezing.1, 2, 28, 29, 30, 31, 32, 33 This suggestion is supported by experimental studies in adults.34, 35 During rhinovirus infection, subjects with high IgE levels, blood eosinophilia, or increased expired nitric oxide had more severe lower respiratory tract symptoms. Third, age at the first infection may also be critical, because immature immune responses may be modified by environmental factors, such as viral infections. This suggestion derived from a neonatal mouse model agrees with our finding that young age at the first episode of wheezing was associated with an increased risk for recurrent wheezing.36
Our results on traditional risk factors for recurrent wheezing agree with previous studies.1, 37 Atopy and maternal asthma are strong predictors of persistent wheezing, whereas prematurity is more likely to be associated with transient wheezing in early life. Both these wheezing phenotypes were represented in our study because the participants were recruited from the general population, not from a specific risk population.
An unexpected and provocative finding was that a 3-day prednisolone treatment for the first episode of wheezing associated with rhinovirus infection showed benefit for as long as 12 months. In the rhinovirus group, 50% of placebo recipients had at least 2 recurrences, compared with 26% among prednisolone recipients. This finding is in agreement with our previous report of a 2-month follow-up.20 Contrary to our finding, no disease-modifying effect was seen in 2 recent studies using inhaled corticosteroid therapy either intermittently during wheezing in infants or continuously in children with a positive asthma-predictive index.38, 39 It is, however, important to note that the study by Guilbert et al39 included children who had experienced recurrent wheezing episodes, making their population very different from ours. The reasons for the discrepancy between our study and these earlier studies may lie in our systemic administration and higher dosage of corticosteroids. Thus, our finding suggests that early wheezing associated with rhinovirus is not only an important risk factor for recurrent wheezing but also might be a criterion for the selection of young children who are likely to respond to systemic corticosteroids.
We also found children with eczema to benefit from prednisolone, which is understandable, because atopic eczema is one of the main risk factors for asthma, and nonatopic eczema predicts sensitization in wheezing children.1, 40 Recently, Bisgaard et al38 found no interaction between response to intermittent inhaled corticosteroid therapy and eczema in infants. The treatment was started after a 3-day episode of wheezing in an outpatient setting. Their findings, however, do not necessarily override our results. As they suggested, higher doses and earlier timing of treatment may be necessary. The efficacy of systemic corticosteroids in children with eczema is important to be confirmed by others, because eczema diagnosis would be a useful clinical tool to identify wheezing children who respond to corticosteroids.
Our finding that heterogenous group of RSV/rhinovirus-negative children had increased tendency for recurrent wheezing compared with RSV-positive children is in agreement with the Tucson Children's Respiratory Study.9 They reported that at the age 13 years, there still was a significant link between bronchiolitis and asthma in the groups of children with virus other than RSV or with negative microbiology. However, it is possible that they missed many rhinovirus infections because rhinovirus PCR techniques were not used. Future studies should incorporate comprehensive microbiologic diagnostics to elucidate better the risk related to non-RSV/nonrhinovirus infections.
The limitations of this study must be taken into account. The study population was small. We studied only hospitalized children and had no nonwheezing children as a control group. The inclusion criteria for the long-term follow-up were first planned during the efficacy trial, and thus no stratified randomization was performed. Therefore, we regarded prednisolone versus placebo treatment as an equal covariate to risk factors such as maternal asthma. Our viral grouping can be criticized, although it is in line with that of the Childhood Origins of Asthma (COAST) study.2 There is no standard for etiologic grouping. In the regression model, we used the RSV group as a reference group because the probability of recurrent wheezing associated with RSV is well known. To avoid multiple testing and consequent false-positive results, we performed interaction tests instead of testing subgroups, for which we provided only estimates with CIs.41 Although our children had not had previous wheezing requiring outpatient visit or hospitalization, we cannot assure that previous viral infections had not induced lower respiratory symptoms insufficient to result in noticeable wheezing. Prolonged coughing alone can be the dominant symptom under such circumstances. The strengths of this study are careful recording of risk factors, detailed viral diagnostics, prospective follow-up, good adherence of study participants, and use of the general population.
In summary, our study raises rhinovirus as a major viral risk factor for recurrent wheezing. Furthermore, our data suggest that prednisolone may have long-term beneficial effects in young wheezing children affected by rhinovirus infection. The presence of eczema may also influence the response to prednisolone. A prospectively designed clinical trial appropriately powered is needed to test the hypothesis that prednisolone reduces recurrent wheezing associated with rhinovirus infection or eczema.
Turku, Finland
Footnotes
Supported by the Academy of Finland, the Foundation for Pediatric Research, the Finnish Cultural Foundation, the Paulo Foundation, and the Turku University Foundation. Prednisolone and placebo were provided by Leiras Pharmaceuticals Inc, Turku, Finland.
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
References
- 1.Martinez F.D., Wright A.L., Taussig L.M., Holberg C.J., Halonen M., Morgan W.J. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 2.Lemanske R.F., Jackson D.J., Gangnon R.E., Evans M.D., Li Z., Shult P.A. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 3.Papadopoulos N.G., Moustaki M., Tsolia M., Bossios A., Astra E., Prezerakou A. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am J Respir Crit Care Med. 2002;165:1285–1289. doi: 10.1164/rccm.200112-118BC. [DOI] [PubMed] [Google Scholar]
- 4.Jacques J., Bouscambert-Duchamp M., Moret H., Carquin J., Brodard V., Lina B. Association of respiratory picornaviruses with acute bronchiolitis in French infants. J Clin Virol. 2006;35:463–466. doi: 10.1016/j.jcv.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Malmström K., Pitkäranta A., Carpen O., Pelkonen A., Malmberg L.P., Turpeinen M. Human rhinovirus in bronchial epithelium of infants with recurrent respiratory symptoms. J Allergy Clin Immunol. 2006;118:591–596. doi: 10.1016/j.jaci.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 6.Sims D.G., Downham M.A., Gardner P.S., Webb J.K., Weightman D. Study of 8-year-old children with a history of respiratory syncytial virus bronchiolitis in infancy. BMJ. 1978;1:11–14. doi: 10.1136/bmj.1.6104.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pullan C.R., Hey E.N. Wheezing, asthma, and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. BMJ. 1982;284:1665–1669. doi: 10.1136/bmj.284.6330.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osundwa V.M., Dawod S.T., Ehlayel M. Recurrent wheezing in children with respiratory syncytial virus (RSV) bronchiolitis in Qatar. Eur J Pediatr. 1993;152:1001–1003. doi: 10.1007/BF01957225. [DOI] [PubMed] [Google Scholar]
- 9.Stein R.T., Sherrill D., Morgan W.J., Holberg C.J., Halonen M., Taussig L.M. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 10.Sigurs N., Bjarnason R., Sigurbergsson F., Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 11.Kotaniemi-Syrjänen A., Vainionpää R., Reijonen T.M., Waris M., Korhonen K., Korppi M. Rhinovirus-induced wheezing in infancy—the first sign of childhood asthma? J Allergy Clin Immunol. 2003;111:66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigurs N., Gustafsson P.M., Bjarnason R., Lundberg F., Schmidt S., Sigurbergsson F. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 13.Gold D.R., Fuhlbrigge A.L. Inhaled corticosteroids for young children with wheezing. N Engl J Med. 2006;354:2058–2060. doi: 10.1056/NEJMe068058. [DOI] [PubMed] [Google Scholar]
- 14.Storr J., Barrell E., Barry W., Lenney W., Hatcher G. Effect of a single oral dose of prednisolone in acute childhood asthma. Lancet. 1987;1:879–882. doi: 10.1016/s0140-6736(87)92857-1. [DOI] [PubMed] [Google Scholar]
- 15.Roosevelt G., Sheehan K., Grupp Phelan J., Tanz R.R., Listernick R. Dexamethasone in bronchiolitis: a randomised controlled trial. Lancet. 1996;348:292–295. doi: 10.1016/s0140-6736(96)02285-4. [DOI] [PubMed] [Google Scholar]
- 16.De Boeck K., Van der Aa N., Van Lierde S., Corbeel L., Eeckels R. Respiratory syncytial virus bronchiolitis: a double-blind dexamethasone efficacy study. J Pediatr. 1997;131:919–921. doi: 10.1016/s0022-3476(97)70044-1. [DOI] [PubMed] [Google Scholar]
- 17.Bülow S.M., Nir M., Levin E., Friis B., Thomsen L.L., Nielsen J.E. Prednisolone treatment of respiratory syncytial virus infection: a randomized controlled trial of 147 infants. Pediatrics. 1999;104:e77–e82. doi: 10.1542/peds.104.6.e77. [DOI] [PubMed] [Google Scholar]
- 18.Van Woensel J.B., Kimpen J.L., Sprikkelman A.B., Ouwehand A., van Aalderen W.M. Long-term effects of prednisolone in the acute phase of bronchiolitis caused by respiratory syncytial virus. Pediatr Pulmonol. 2000;30:92–96. doi: 10.1002/1099-0496(200008)30:2<92::aid-ppul3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 19.Oommen A., Lambert P.C., Grigg J. Efficacy of a short course of parent-initiated oral prednisolone for viral wheeze in children aged 1-5 years: randomized controlled trial. Lancet. 2003;362:1433–1438. doi: 10.1016/S0140-6736(03)14685-5. [DOI] [PubMed] [Google Scholar]
- 20.Jartti T., Lehtinen P., Vanto T., Hartiala J., Vuorinen T., Mäkelä M.J. Evaluation of the efficacy of prednisolone in early wheezing induced by rhinovirus or respiratory syncytial virus. Pediatr Infect Dis J. 2006;25:482–488. doi: 10.1097/01.inf.0000215226.69696.0c. [DOI] [PubMed] [Google Scholar]
- 21.Jartti T., Lehtinen P., Vuorinen T., Osterback R., van den Hoogen B., Osterhaus A.D. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis. 2004;10:1095–1101. doi: 10.3201/eid1006.030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson S.G., Bieber T., Dahl R., Friedmann P.S., Lanier B.Q., Lockey R.F. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 23.Lemanske R.F., Jr., Dick E.C., Swenson C.A., Vrtis R.F., Busse W.W. Rhinovirus upper respiratory infection increases airway hyperreactivity and late asthmatic reactions. J Clin Invest. 1989;83:1–10. doi: 10.1172/JCI113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calhoun W.J., Dick E.C., Schwartz L.B., Busse W.W. A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in allergic subjects. J Clin Invest. 1994;94:2200–2208. doi: 10.1172/JCI117581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gern J.E., Galagan D.M., Jarjour N.N., Dick E.C., Busse W.W. Detection of rhinovirus RNA in lower airway cells during experimentally induced infection. Am J Respir Crit Care Med. 1997;155:1159–1161. doi: 10.1164/ajrccm.155.3.9117003. [DOI] [PubMed] [Google Scholar]
- 26.Grunberg K., Sharon R.F., Sont J.K., In 't Veen J.C., Van Schadewijk W.A., De Klerk E.P. Rhinovirus-induced airway inflammation in asthma: effect of treatment with inhaled corticosteroids before and during experimental infection. Am J Respir Crit Care Med. 2001;164:1816–1822. doi: 10.1164/ajrccm.164.10.2102118. [DOI] [PubMed] [Google Scholar]
- 27.Mosser A.G., Vrtis R., Burchell L. Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am J Respir Crit Care Med. 2005;171:645–651. doi: 10.1164/rccm.200407-970OC. [DOI] [PubMed] [Google Scholar]
- 28.Ehlenfield D.R., Cameron K., Welliver R.C. Eosinophilia at the time of respiratory syncytial virus bronchiolitis predicts childhood reactive airway disease. Pediatrics. 2000;105:79–83. doi: 10.1542/peds.105.1.79. [DOI] [PubMed] [Google Scholar]
- 29.Copenhaver C.C., Gern J.E., Li Z., Shult P.A., Rosenthal L.A., Mikus L.D. Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am J Respir Crit Care Med. 2004;170:175–180. doi: 10.1164/rccm.200312-1647OC. [DOI] [PubMed] [Google Scholar]
- 30.Hoffjan S., Ostrovnaja I., Nicolae D., Newman D.L., Nicolae R., Gangnon R. Genetic variation in immunoregulatory pathways and atopic phenotypes in infancy. J Allergy Clin Immunol. 2004;113:511–518. doi: 10.1016/j.jaci.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 31.Hoebee B., Bont L., Rietveld E., van Oosten M., Hodemaekers H.M., Nagelkerke N.J. Influence of promoter variants of interleukin-10, interleukin-9, and tumor necrosis factor-alpha genes on respiratory syncytial virus bronchiolitis. J Infect Dis. 2004;189:239–247. doi: 10.1086/380908. [DOI] [PubMed] [Google Scholar]
- 32.Wark P.A., Johnston S.L., Bucchieri F., Powell R., Puddicombe S., Laza-Stanca V. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brooks G.D., Buchta K.A., Swenson C.A., Gern J.E., Busse W.W. Rhinovirus-induced interferon-gamma and airway responsiveness in asthma. Am J Respir Crit Care Med. 2003;168:1091–1094. doi: 10.1164/rccm.200306-737OC. [DOI] [PubMed] [Google Scholar]
- 34.Gern J.E., Calhoun W., Swenson C., Shen G., Busse W.W. Rhinovirus infection preferentially increases lower airway responsiveness in allergic subjects. Am J Respir Crit Care Med. 1997;155:1872–1876. doi: 10.1164/ajrccm.155.6.9196088. [DOI] [PubMed] [Google Scholar]
- 35.Zambrano J.C., Carper H.T., Rakes G.P., Patrie J., Murphy D.D., Platts-Mills T.A. Experimental rhinovirus challenges in adults with mild asthma: response to infection in relation to IgE. J Allergy Clin Immunol. 2003;111:1008–1016. doi: 10.1067/mai.2003.1396. [DOI] [PubMed] [Google Scholar]
- 36.Culley F.J., Pollott J., Openshaw P.J. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J Exp Med. 2002;196:1381–1386. doi: 10.1084/jem.20020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright A.L. Epidemiology of asthma and recurrent wheeze in childhood. Clin Rev Allergy Immunol. 2002;22:33–44. doi: 10.1007/s12016-002-0004-z. [DOI] [PubMed] [Google Scholar]
- 38.Bisgaard H., Northman Hermansen M., Loland L., Brydensholt Halkjaer L., Buchvald F. Intermittent inhaled corticosteroids in infants with episodic wheezing. N Engl J Med. 2006;354:1998–2005. doi: 10.1056/NEJMoa054692. [DOI] [PubMed] [Google Scholar]
- 39.Guilbert T.W., Morgan W.J., Zeiger R.S., Mauger D.T., Boehmer S.J., Szefler S.J. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 40.Guilbert T.W., Morgan W.J., Zeiger R.S., Bacharier L.B., Boehmer S.J., Krawiec M. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol. 2004;114:1282–1287. doi: 10.1016/j.jaci.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 41.Lagakos S.W. The challenge of subgroup analyses: reporting without distorting. N Engl J Med. 2006;354:1667–1669. doi: 10.1056/NEJMp068070. [DOI] [PubMed] [Google Scholar]