Abstract
Clinical research in neurodevelopmental disorders remains reliant upon clinician and caregiver measures. Limitations of these approaches indicate a need for objective, quantitative, and reliable biomarkers to advance clinical research. Extant research suggests the potential utility of multiple candidate biomarkers; however, effective application of these markers in trials requires additional understanding of replicability, individual differences, and intra-individual stability over time. The Autism Biomarkers Consortium for Clinical Trials (ABC-CT) is a multi-site study designed to investigate a battery of electrophysiological (EEG) and eye-tracking (ET) indices as candidate biomarkers for autism spectrum disorder (ASD). The study complements published biomarker research through: inclusion of large, deeply phenotyped cohorts of children with ASD and typical development; a longitudinal design; a focus on well-evidenced candidate biomarkers harmonized with an independent sample; high levels of clinical, regulatory, technical, and statistical rigor; adoption of a governance structure incorporating diverse expertise in the ASD biomarker discovery and qualification process; prioritization of open science, including creation of a repository containing biomarker, clinical, and genetic data; and use of economical and scalable technologies that are applicable in developmental populations and those with special needs. The ABC-CT approach has yielded encouraging results, with one measure accepted into the FDA’s Biomarker Qualification Program to date. Through these advances, the ABC-CT and other biomarker studies in progress hold promise to deliver novel tools to improve clinical trials research in ASD.
Keywords: autism spectrum disorder, biomarker, neuroscience, clinical trial methodology/study design, EEG, ERP, eye-tracking
Introduction
There are currently no validated biomarkers for use in clinical trials in autism spectrum disorder (ASD). Clinical research remains reliant upon standardized but intrinsically subjective clinician and caregiver/self-report measures. These tools have supported significant but incomplete progress in diagnosis, selection of intervention, and measurement of treatment response; however, advancement on other key objectives, such as designation of subgroups of individuals (i.e., stratification) within this heterogeneous neurodevelopmental condition, have stagnated. Notably, the most recent diagnostic taxonomy for ASD (American Psychiatric Association, 2013) discarded behaviorally defined subtypes because they were not reliable and had limited utility for treatment selection or determination of prognosis (Lord et al., 2012a). As highlighted by other articles in this collection (Ewen et al., 2019), there is a widely recognized and urgent need for biomarkers to support clinical research in ASD (McPartland, 2017). Improved understanding of biomarkers may also provide a framework to bridge understanding of mechanisms across human and animal models, in areas in which behavior may be insufficiently informative (Modi and Sahin, 2017).
This Frontiers in Neuroscience Perspective highlights the specific challenges that have impeded progress in biomarker research in ASD and presents the rationale, design, and progress of the Autism Biomarkers Consortium for Clinical Trials (ABC-CT). The ABC-CT is a multisite study specifically designed to evaluate a set of promising electrophysiological (EEG) and eye-tracking (ET) markers while addressing shortcomings of prior research and establishing a comprehensive approach to biomarker validation in ASD. Within this context, we describe the study design of the ABC-CT in terms of specific strategies implemented to address limitations of published research and to provide opportunities for enhancing understanding of ASD biomarkers. We highlight recent advances that have been made in the context of this project and describe recommended directions for future investigation.
Scientific Context: Challenges to Biomarker Development in ASD
A primary factor slowing progress in biomarker development for ASD is the heterogeneity associated with the disorder. The diagnosis of ASD is based on a constellation of widely variable behaviors (American Psychiatric Association, 2013). Additional phenotypic variability is introduced by associated non-diagnostic features, such as intellectual disability, and comorbidities, such as epilepsy and attention-deficit/hyperactivity disorder. Myriad genetic, epigenetic, and environmental factors contribute to the etiology of ASD. While there is some neurobiological convergence in common neural circuits, many upstream molecular pathways lead to this disruption of network function (Jeste and Geschwind, 2014). Given that biomarker development strategies frequently focus on measurement of an identified mechanism, the challenge in ASD is significant, as candidate biological factors are selected, in large part, by purported connection to behavior rather than a clearly defined biological pathway. For example, impaired social-communication is a hallmark and universal feature of ASD, but there is neither a single neural pathway for nor standard means of quantifying social-communication. The lack of clear target mechanisms is further complicated by the dynamic and variable nature of human development. In a neurodevelopmental condition in which symptoms evolve and change throughout the lifespan, applicability of biomarkers across ages is uncertain.
Other impediments to biomarker development in ASD reflect elements of the research enterprise itself. Multiple factors, such as high costs of human subjects research and limitations on recruitment in single site studies, encourage dissemination with the minimal viable sample size, often permitting assessment of group discrimination or simple associations but not analysis of complex interactions or stratification. Such small studies may also be prone to generation of spurious or idiosyncratic results that are unlikely to replicate. Even in biomarker studies utilizing large samples, the task of understanding individual differences and relationships to the clinical phenotype is only possible with deep phenotyping of these behavioral and clinical correlates, which is resource intensive. Publication and procurement of research funding explicitly value innovation, creating a pressure to explore novel biomarkers that is, to some degree, at odds with the goal of examining the replicability and reproducibility of well-studied biomarkers to provide more conclusive evidence of viability. Even fewer studies include a designated replication sample to verify findings in an independent group.
For even the most well-studied biomarkers in ASD, there are several near universal gaps in understanding. Methodological rigor, such as variation among studies, is a significant and poorly understood concern. Factors such as stimulus presentation, experimental design, and variation in hardware and software could all influence biomarker measurement in unpredictable ways. For most biomarkers, it is not understood whether or how such factors contribute to observed variability in results. Few biomarker studies have included multiple sampling points in a longitudinal design, preventing inference regarding the stability of measurement in a person over time (i.e., test-retest reliability, developmental stability). This is critical information for the potential use of biomarkers in clinical trials.
Responding to Challenges in ASD Biomarker Development: ABC-CT Study Design
The scientific objectives of the ABC-CT were to evaluate a set of candidate EEG and ET biomarkers, alongside lab-based tasks, in terms of: (1) feasibility of administration in children with ASD; (2) reliability of data collection across sites; (3) construct validity of the assays (i.e., whether they manipulated neural processes as predicted in typically developing (TD) children); (4) test-retest reliability; (5) ability to discriminate children with ASD from those with TD; (6) utility for stratification into meaningful subgroups of children with ASD; (7) association with clinical phenotype; and (8) developmental stability/sensitivity to change in symptom severity. Below we describe specific elements of ABC-CT study design intended to address the aforementioned challenges for biomarker development in ASD (see sections “Study Population”, “Deep Phenotyping”, “Well-studied Biomarkers”, “Replication Sample”, “Methodological Rigor”, and “Longitudinal Design”), as well as additional features of the study innovated for this purpose (see sections “Study Governance”, “Formation of a Repository”, and “Scalability of Biomarkers”).
Study Population
A considerable strength of the ABC-CT was the administration of the selected paradigms in a large sample of children with ASD and TD. The study enrolled 280 children with ASD and 119 children with TD. Heterogeneity in the sample was considered carefully. Age range was constrained from 6 to 11 years to limit age-related confounds and to focus on an age-group in which biomarker data could be acquired reliably and validly. Presence of a known genetic syndrome or neurological condition putatively causally related to ASD or known metabolic disorder and/or mitochondrial dysfunction were exclusionary criteria. Because medication use may influence biomarker measurement, a stable regimen was required for 8 weeks prior to enrollment; all medications were allowed in order to enroll a representative sample. Cognitive ability spanned full scale IQ from 60 to 150, as assessed by the Differential Ability Scales (DAS) – 2nd Edition (Elliott, 2007), to permit evaluation of the feasibility of biomarker ascertainment procedures across a range of intellectual abilities. In this way, the sample provided strong statistical power for analyses, while constraining developmental and cognitive heterogeneity. Given the likelihood of significant developmental changes between 6 and 11 years, both chronological age and developmental level are considered in all statistical analyses.
Deep Phenotyping
An extensive phenotyping battery provided rigorous characterization, including observation, interview, and multiple perspectives (i.e., clinician and caregiver). Diagnostic characterization relied upon research gold standard instruments: DSM-5 diagnosis of ASD based on the Autism Diagnostic Observation Schedule (Lord et al., 2012b) and the Autism Diagnostic Interview-Revised (Rutter et al., 2003). Clinician administered assessments also included the DAS, 2nd Edition (Elliott, 2007), and the Vineland Adaptive Behavior Scales, 3rd Edition (Sparrow et al., 2016). Caregiver questionnaires included the Aberrant Behavior Checklist (Aman et al., 1985), the Autism Impact Measure (Kanne et al., 2014), the Pervasive Developmental Disorder Behavior Inventory (Cohen and Sudhalter, 2005), and the Social Responsiveness Scale, 2nd Edition (Constantino and Gruber, 2012). To assess clinical status, the Clinical Global Impression Scale (Guy, 1976) was employed, as this scale is widely used as an outcome measure in pharmacologic treatment studies. Finally, interventions and medications utilized both prior to and during the course of study participation were carefully recorded. The study was thus positioned to evaluate biomarkers with respect to current best practices in terms of clinical assessment.
Well-Studied Biomarkers
Candidate biomarkers were selected to measure social-communicative function or related processes, to be feasible in children with ASD across a wide range of functioning, and to be scalable for clinical trials (section “Scalability of Biomarkers”). Importantly, all biomarkers had been studied in prior research and had shown strong potential to distinguish between children with ASD and TD children or to correlate with clinical characteristics. Four EEG paradigms and five ET paradigms were included in the ABC-CT main study biomarker battery. EEG tasks included: resting state, with eyes open, acquired during viewing of abstract videos (Wang et al., 2013); N170 event-related potential (ERP) to upright human faces, compared to inverted faces and non-social stimuli (McPartland et al., 2004); ERPs to biological motion, contrasting signal between coherent and scrambled point-light animations of walking adults (Kroger et al., 2014); and visual evoked potentials, in response to presentation of phase-reversing black and white checkerboards (Siper et al., 2016). ET tasks included: activity monitoring, comparing percentage of ocular focus (POF) to human faces and heads during videos of highly structured shared activities (Shic et al., 2011); visual attention to biological motion, quantified as POF to biological motion versus scrambled and rotating point-light animations (Klin and Jones, 2008; Annaz et al., 2012); pupillary light reflex (PLR), measuring relative pupil constriction amplitude and latency in response to a flash of light (Nystrom et al., 2015); an interactive social task, measuring POF to human heads and faces during videos of two children at play (Chevallier et al., 2015); and static scenes, measuring POF to human heads and faces during images showing naturalistic scenes of children and adults (Loth et al., 2017; Ness et al., 2017).
Replication Sample
The ABC-CT coordinated study design and analyses with other networks engaged in ASD biomarker studies. For several biomarker assays (N170 ERP, ET static scenes, ET biological motion, ET PLR), acquisition paradigms were harmonized with the European Autism Interventions Multicenter Study for Developing New Medications project (EU-AIMS) (Loth et al., 2014, 2017) to permit replication in a separate sample. Likewise, data analytic teams from both groups coordinated processing pipelines and analytic strategies to ensure comparability of study results. The Janssen Autism Knowledge Engine (JAKE) (Ness et al., 2017) study applied several conceptually analogous assays (e.g., a face ERP biomarker with a different acquisition paradigm), enabling evaluation of robustness of results across different assays.
Methodological Rigor
The study design incorporated a high level of methodological rigor in terms of both clinical and biomarker data acquisition. Identical equipment was used for EEG (EGI 128 channel system) and ET (SR Research EyeLink System) data acquisition and processing at all five data collection sites; equipment was installed and tested by a central data acquisition team to ensure identical setup parameters. Detailed manuals of procedures (MOPs) were established for all biomarker paradigms and standardized protocols were adopted for data collection, processing, and analysis (Webb et al., 2020). Likewise, MOPs guided clinical data collection, and all staff underwent comprehensive training, addressing participant screening, clinical measurement, biomarker data collection procedures, data entry, and study management processes. Fidelity in procedures was maintained for clinical measurement through regular conference calls and monitoring of clinical interview reliability within and across sites. Rigor was enhanced via conduct of the study according to Good Clinical Practice standards, optimizing ABC-CT infrastructure for the conduct of clinical trials.
Longitudinal Design
The naturalistic, longitudinal design of the ABC-CT allowed for the examination of test-retest reliability and stability over time, paralleling the structure and timeline of a clinical trial. Children were assessed across three time points (Time 1: Baseline, Time 2: 6 weeks after baseline, and Time 3: 24 weeks after baseline). At each time point, clinical assessments, parent-rated measures of social impairment, independent ratings of clinical status, and the biomarker battery were completed. These time points were selected to provide information about short-term test-retest reliability (6 weeks) and developmental stability/change over a time period consistent with a potential clinical trial (24 weeks).
Study Governance
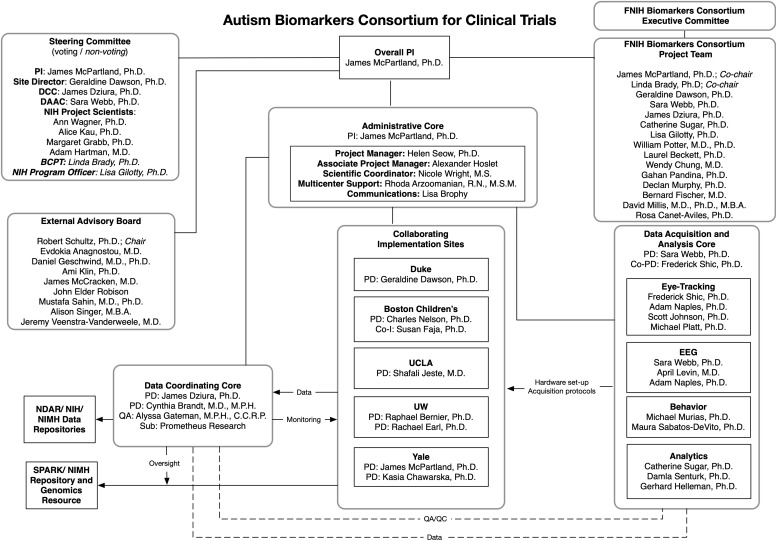
The ABC-CT adopted a complex governance structure to incorporate expertise relevant to biomarker development (see Figure 1). Funded through a NIH U19 collaborative agreement, the project was a public/private partnership that brought together specialists spanning academia, government agencies, and industry. Administration of the project was overseen by a Steering Committee including ABC-CT members, as well as the Program Officer and project scientists associated with the National Institute of Child Health and Human Development (NICHD), the National Institute of Neurological Disorders and Stroke (NINDS), and the National Institute of Mental Health (NIMH). The ABC-CT was designated a project of the FNIH Biomarkers Consortium, and a Biomarkers Consortium Project Team was assembled to provide additional guidance from experts from the Alzheimer’s Disease Neuroimaging Initiative, drug development and neuroscience, autism biomarker projects in industry (JAKE), EU-AIMS, the Simons Foundation, and FDA scientists from the Division of Psychiatry Products. An External Advisory Board included specialists in ASD clinical trial design, an individual with ASD, a family member of individuals with ASD, neurogeneticists, and experts in the conduct of large scale ASD biomarker studies. These three groups informed study design, study conduct, interpretation of results, and preparation of biomarker qualification documentation.
FIGURE 1.
ABC-CT organizational chart.
Formation of a Repository
Efficient sharing of all study data was a priority for the ABC-CT. All data were uploaded to the National Database for Autism Research (NDAR, a database within the NIMH Data Archive) on a quarterly basis and made publicly available within four months of uploading (permitting time for quality assurance and control). Blood samples collected from participants and available biological parents have been shared via the NIMH Repository and Genomics Resource1. Through a collaboration facilitated by the FNIH Biomarkers Consortium, samples are being genotyped, creating a publicly available repository with complete clinical, biomarker, and genotypic information across the large, longitudinal sample.
Scalability of Biomarkers
Biomarker acquisition modalities utilized in the ABC-CT were selected based on their potential to yield high public health impact. Both EEG and ET are relatively economical biomarker assays, particularly within the class of neurophysiological or neurobehavioral measurements. These methods are also highly scalable and accessible, with EEG recording facilities widely available in existing health care systems, supporting efficient large-scale implementation with extant infrastructure. Though ET is not readily available in most health care settings, commercially available products can be obtained at low cost. These technologies are applicable across a developmental range (e.g., infancy though adulthood) and to individuals with neurodevelopmental conditions and intellectual disabilities.
ABC-CT Progress and Future Directions
The ABC-CT was initiated in July 2015. After a series of in-person meetings and teleconferences involving project governance, a feasibility study of 25 children with ASD and 26 TD children was conducted between December 2015 and March 2016. Based on results of the Feasibility Study, the Main Study design (described in this manuscript) was finalized (for details of review of feasibility and transition to main study, see Webb et al., 2020, sections 2.6 and 2.7). The first subject in the Main study was enrolled in October 2016, data collection was completed in May 2019, and final analyses of the complete data set are in progress, with planned dissemination in Spring 2020.
The N170 biomarker showed strong performance in terms of reliable and valid data acquisition and demonstration of predicted between-group differences at interim analyses conducted in April 2018. Based on these results, a Letter of Intent (LOI) for the N170 latency to upright human faces was submitted to the FDA’s Center for Drug Evaluation and Research Biomarker Qualification Program (BQP) in November, 2018; the proposed context of use was identifying a biologically homogeneous subgroup within ASD to enrich clinical trials by reduction of ASD-associated heterogeneity. In May, 2019, this index was accepted into the Biomarker Qualification Program2, marking a milestone for the field as the first biomarker for a neurodevelopmental disorder or psychiatric condition accepted into the BQP. A Biomarker Qualification Plan, the second step in the program, for the N170 is in development. In October 2019, a second LOI was submitted for the ET biomarker, Oculomotor index of orienting to human faces. Ongoing analyses will determine the appropriateness of other candidate biomarkers for potential submission to the BQP.
As outlined above, the ABC-CT was designed to evaluate promising biomarkers in several areas. The large sample and thorough characterization enable inference regarding group discrimination and relationships among the biomarkers, as well as evaluation of individual differences in clinical characteristics and demographic factors. The longitudinal design provides information about test-retest reliability and developmental stability over a length of time intended to align with a clinical trial; future research is needed to investigate the reliability of these biomarkers over longer periods of time. However, there are several biomarker properties that the ABC-CT was not designed to address. Because it was a naturalistic longitudinal study, without an active treatment, there is limited clinical change observed in participants during the 6 month course of the study, limiting the ability to evaluate biomarker sensitivity to change. This key objective may be addressed in future research by studies that evaluate biomarkers in the context of intervention or through naturalistic studies in younger cohorts, receiving initial diagnoses and being channeled into their first interventions, when significant progress in a six month span may be more likely. It is important to recognize that generalizability of the ABC-CT results to other populations has not yet been established; although extant research provides strong evidence of the potential utility of these biomarkers in other cohorts (e.g., younger/older children and adults, individuals with IQ below 60), studies of the scope and rigor of the ABC-CT have yet to be conducted and may be required before biomarker qualification in these groups can be pursued. The ABC-CT biomarker battery focused primarily on the visual domain because these measures were the most well-researched at the time of study design. Given the centrality of other sensory modalities (e.g., audition) to social-communication, investigation of these modalities is warranted.
Conclusion
The ABC-CT represents a comprehensive, collaborative approach to biomarker development in ASD. Building upon a strong foundation of prior research that has put forward candidate markers, the ABC-CT has advanced understanding by innovating in terms of study design and scope. The field of neurodevelopmental disorders has emerged as a leader within psychiatry, with the first biomarker of this nature accepted into the FDA’s BQP. We move closer to a scientific reality in which clinical research may rely upon objective and sensitive biological measurements to bolster the clinical instruments on which we currently rely. The ABC-CT seeks to provide a foundation upon which novel treatments for ASD can be rigorously evaluated and that, ultimately, may lead to more effective methods for diagnosing and treating ASD.
Data Availability Statement
ABC-CT data are publicly available via the National Database for Autism Research https://ndar.nih.gov/, #2288.
Ethics Statement
The studies involving human participants were reviewed and approved by the Yale Institutional Review Board which served as Central Institutional Review Board for the study. Written informed consent to participate in this study was provided by the participants’ legal guardian.
Author Contributions
All authors made substantial contributions to the conception or design of the work or to acquisition, to analysis or interpretation of the data, to drafting or revision of the work, and provided approval for publication of the content, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. JM, SW, SJ, and RB drafted the work.
Conflict of Interest
JM has received funding from Janssen Research and Development, receives book Royalties from Guilford, Springer, and Lambert Press, and is a consultant with Blackthorn Therapeutics. LR was employed by Prometheus Research during the conduct of this research. RB was employed at the University of Washington during the conduct of this study and authoring of this manuscript; he is currently employed by Apple. GD is on the Scientific Advisory Boards of Janssen Research and Development, Akili, Inc., LabCorp, Inc., and Roche Pharmaceutical Company, a consultant for Apple, Inc., Gerson Lehrman Group, and Axial Ventures, has received grant funding from Janssen Research and Development, is CEO of DASIO, LLC, which focuses on digital phenotyping tools, and receives book royalties from Guilford Press, Springer, and Oxford University Press. FS consults for Roche Pharmaceutical Company and Janssen Research and Development. LS consults for Roche and Neuren Pharmaceuticals.
No company contributed to funding of this study. A representative from Janssen served on the FNIH Biomarkers Consortium Project Team and provided in kind support in terms of sharing experiences and preliminary results of the JAKE study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We extend gratitude to all of the families and participants who participated in this research. In addition, we thank the ABC-CT Project Management Team, the ABC-CT External Advisory Board, NIH project scientists, and colleagues from the FNIH Biomarkers Consortium.
Funding. Support was provided by NIMH U19 MH108206 (JM), the Autism Biomarkers Consortium for Clinical Trials.
References
- Aman M. G., Singh N. N., Stewart A. W., Field C. J. (1985). The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. Am. J. Ment. Deficiency 89 485–491. [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th Edn Washington, D.C: American Psychiatric Association. [Google Scholar]
- Annaz D., Campbell R., Coleman M., Milne E., Swettenham J. (2012). Young children with autism spectrum disorder do not preferentially attend to biological motion. J. Autism Dev.Disord. 42 401–408. 10.1007/s10803-011-1256-3 [DOI] [PubMed] [Google Scholar]
- Chevallier C., Parish-Morris J., McVey A., Rump K. M., Sasson N. J., Herrington J. D., et al. (2015). Measuring social attention and motivation in autism spectrum disorder using eye-tracking. Stimulus type matters. Autism Res. 8 620–628. 10.1002/aur.1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I. L., Sudhalter V. (2005). PDD Behavior Inventory (PDDBI). Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Constantino J. N., Gruber C. (2012). The Social Responsiveness Scale Manual, (SRS-2), 2nd Edn Los Angeles: CA: Western Psychological Services. [Google Scholar]
- Elliott (2007). Differential Ability Scales:(DAS-II), 2nd Edn San Antonio, TX: Psychological Corporation. [Google Scholar]
- Ewen J. B., Sweeney J. A., Potter W. Z. (2019). Conceptual, regulatory and strategic imperatives in the early days of EEG-Based biomarker validation for neurodevelopmental disabilities. Front. Integr. Neurosci. 13:45. 10.3389/fnint.2019.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. (1976). ECDEU Assessment Manual for Psychopharmacology. US Department of Health, and Welfare. Rockville, MD: National Institute of Mental Health, 76–338. [Google Scholar]
- Jeste S. S., Geschwind D. H. (2014). Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nat. Rev. Neurol. 10 74–81. 10.1038/nrneurol.2013.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanne S. M., Mazurek M. O., Sikora D., Bellando J., Branum-Martin L., Handen B., et al. (2014). The autism impact measure (AIM): initial development of a new tool for treatment outcome measurement. J. Autism Dev. Disord. 44 168–179. 10.1007/s10803-013-1862-3 [DOI] [PubMed] [Google Scholar]
- Klin A., Jones W. (2008). Altered face scanning and impaired recognition of biological motion in a 15-month-old infant with autism. Dev. Sci. 11 40–46. 10.1111/j.1467-7687.2007.00608.x [DOI] [PubMed] [Google Scholar]
- Kroger A., Bletsch A., Krick C., Siniatchkin M., Jarczok T. A., Freitag C. M., et al. (2014). Visual event-related potentials to biological motion stimuli in autism spectrum disorders. Soc. Cogn. Affect. Neurosci. 9 1214–1222. 10.1093/scan/nst103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Petkova E., Hus V., Gan W., Lu F., Martin D. M., et al. (2012a). A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch. Gen. Psychiatry 69 306–313. 10.1001/archgenpsychiatry.2011.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Rutter M., DiLavore P. C., Risi S., Gotham K., Bishop S. (2012b). Autism Diagnostic Observation Schedule, 2ed Edn Torrance, CA: Western Psychological Services. [Google Scholar]
- Loth E., Charman T., Mason L., Tillmann J., Jones E. J. H. J. H., Wooldridge C., et al. (2017). The EU-AIMS longitudinal european autism project (LEAP): design and methodologies to identify and validate stratification biomarkers for autism spectrum disorders. Mol. Autism. 8 24. 10.1186/s13229-017-0146-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loth E., Spooren W., Murphy D. G. Eu-Aims consortium, (2014). New treatment targets for autism spectrum disorders: EU-AIMS. Lancet Psychiatry 1 413–415. 10.1016/S2215-0366(14)00004-2 [DOI] [PubMed] [Google Scholar]
- McPartland J. (2017). Developing clinically practicable biomarkers for autism spectrum disorder. J. Autism. Dev. Disord. 47 2935–2937. 10.1007/s10803-017-3237-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland J., Dawson G., Webb S. J., Panagiotides H., Carver L. J. (2004). Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. J. Child Psychol. Psychiatry 45 1235–1245. 10.1111/j.1469-7610.2004.00318.xJCPP318 [DOI] [PubMed] [Google Scholar]
- Modi M. E., Sahin M. (2017). Translational use of event-related potentials to assess circuit integrity in ASD. Nat. Rev. Neurol. 13 160. 10.1038/nrneurol.2017.15 [DOI] [PubMed] [Google Scholar]
- Ness S. L., Manyakov N. V., Bangerter A., Lewin D., Jagannatha S., Boice M., et al. (2017). JAKE(R) multimodal data capture system: insights from an observational study of autism spectrum disorder. Front. Neurosci. 11:517. 10.3389/fnins.2017.00517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystrom P., Gredeback G., Bolte S., Falck-Ytter T. Ease team, (2015). Hypersensitive pupillary light reflex in infants at risk for autism. Mol. Autism. 6 10. 10.1186/s13229-015-0011-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M., LeCouteur A., Lord C. (2003). Autism Diagnostic Interview - Revised. Los Angeles: Western Psychological Services. [Google Scholar]
- Shic F., Bradshaw J., Klin A., Scassellati B., Chawarska K. (2011). Limited activity monitoring in toddlers with autism spectrum disorder. Brain Res. 1380 246–254. 10.1016/j.brainres.2010.11.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siper P. M., Zemon V., Gordon J., George-Jones J., Lurie S., Zweifach J., et al. (2016). Rapid and objective assessment of neural function in autism spectrum disorder using transient visual evoked potentials. PLoS One 11:e0164422. 10.1371/journal.pone.0164422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow S., Cicchetti D., Saulnier C. A. (2016). Vineland Adaptive Behavior Scales: Third Edition. Bloomington: Pearson. [Google Scholar]
- Wang J., Barstein J., Ethridge L. E., Mosconi M. W., Takarae Y., Sweeney J. A. (2013). Resting state EEG abnormalities in autism spectrum disorders. J. Neurodev. Disord. 5 24. 10.1186/1866-1955-5-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S. J., Shic F., Murias M., Sugar C. A., Naples A. J., Barney E., et al. (2020). Biomarker acquisition and quality control for multisite studies: the autism biomarkers consortium for clinical trials. Front. Integr. Neurosci. 13:71 10.3389/fnint.2019.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
ABC-CT data are publicly available via the National Database for Autism Research https://ndar.nih.gov/, #2288.