Abstract
Objectives:
Exportin 1(XPO1), a nuclear exporter protein, has been gaining recognition in cancer progression and treatment. This study aimed to evaluate the association between the overexpression of XPO1 with NF-κB, Ki67 and clinicopathological characteristics in colorectal cancer (CRC) tissue samples and to explore the anti-proliferative effect of KPT-330, as XPO1 inhibitor, in colorectal cancer cell line.
Methods:
Forty CRC tissue samples were analyzed by immunostaining for the expressions of XPO1, NF-κB and Ki67 and then the anti-proliferative effect of the KPT-330 was also evaluated in HT29 colorectal cancer cell line.
Results:
XPO1 overexpression was observed in 52.5% of CRC and significantly apparent with strong intensity in tumor cells compared to the normal adjacent epithelium (P<0.001). Regarding to the histopathological characteristics, the XPO1 overexpression significantly associated with advanced tumor stages (P=0.049) and has great tendency towards moderate/poorly differentiated tumors. Although the XPO1 overexpression was strongly associated with high Ki67 expression (P=0.001), only Ki67 expression showed significant association with tumor size (P=0.012). No significant association was detected between the XPO1 overexpression and NF-κB, while the NF-κB positive expression was significantly associated with lymph node metastasis and Ki67 expression at P=0.027 and P= 0.007, respectively. The in vitro experiments showed a great impact of KPT-330, as XPO1 inhibitor, to inhibit cancer growth in dose and time dependent manner and significantly diminished the colony formation (P<0.001) of HT29 cells- associated with the expression of Ki67 (P<0.001).
Conclusion:
XPO1 overexpression and NF-κB expression may serve as potential biomarker associated with CRC pathogenesis and proliferation, while the KPT-330 is effectively inhibited-colon cancer growth in vitro. Further studies considering the prognostication role of XPO1 overexpression in CRC are required.
Key Words: Colorectal cancer (CRC), Exportin 1 (XPO1), NF-κB, Ki67, KPT-330
Introduction
Colorectal cancer (CRC) is one of the most common cancers, implicated as the third causative cancer morbidity and the fourth of cancer mortality worldwide (Torre et al., 2015). Despite recent advances in therapeutic approaches that have considerable practical value, the prognosis of more than 50% of CRC patients still remains poor (Bai et al., 2015). The uncontrolled expansion of proliferative activity propelled by the disruption of cellular homeostasis may play a major role to increase the growth and survival rates of CRC tumor cells (Sancho et al., 2004). Exportin 1 (XPO1), also known as chromosome region maintenance 1 (CRM1) protein, is involved in the homeostatic of nucleocytoplasmic transport of over 200 known cargos, most of them are tumor suppressor and cell cycle regulatory proteins such as p53, pRb, FOXOs, BRCA1/2 and inhibitor of NF-κB (Kau et al., 2004; Kashyap et al., 2016). In cancer, the disruption of XPO1 activity may be involved in the increasing of survival and proliferative rate activity of tumor cells (Turner and Sullivan, 2008). Preclinical data and many clinical trials used the developed new generation of selective inhibitor of nuclear export (SINE) compounds as XPO1 inhibitor, such as KPT-330, has a property of low toxicity and good patients’ tolerability as it was seen in both hematological malignancies and solid tumors. This provided an excellent model in translational medicine for targeting of XPO1 activity in cancer therapy with promising efficacy in recent years (Abdul Razak et al., 2016).
Association of XPO1 overexpression with high tumor grade and advanced tumor stage (Noske et al., 2008; Shen et al., 2009) as well as with poor prognosis was noted in some tumor cancers such as gastric (Zhou et al., 2013), ovarian (Noske et al., 2008) and acute leukemia (Kojima et al., 2013), but little is known about such association between the XPO1 overexpression and clinicopathological characteristics in CRC. In colon cancer cell lines, the XPO1 increases the oncogenic activity of tumor cells through mislocalization of some proteins such as p27 and survivin (Ferreiro-Neira et al., 2016; Heong et al., 2016). On the other hand, In CRC, NF-κB over-expression was considered as biomarker associated with worse 3 and 5 years overall survival (Wu et al., 2015). Therefore, exploration, in to what extent, the involvement of XPO1 overexpression in CRC characteristics in tissue samples, considering the histopathological characteristics and proliferation activity, in association with the NF-κB may be helpful in line of individualizing of patients treatment using XPO1 inhibitor. Accordingly, in the present study, we tried to address the differences between the overexpression of XPO1 in CRC tumor cells and adjacent normal epithelium. Then we explored the association between the XPO1 overexpression and clinico-histopathological features as well as with NF-κB and proliferative marker, Ki67, in CRC tissue samples. Furthermore, we demonstrated the anti-proliferative effect of XPO1 inhibitor, KPT-330, on HT29 colrectal cancer cell line.
Materials and Methods
Patients and clinicopathological data
Samples in the form of formalin fixed paraffin embedded (FFPE) tissue blocks were collected randomly from 40 patients who underwent surgical resection and diagnosed as colorectal carcinoma between December 2016 and October 2017 at National Oncology Center (NOC), Sana’a, Republic of Yemen. Age and sex of the patients as well as tumor size were retrieved from the histopathological reports. The degree of histological differentiation of tumors were categorized according to percentage of the gland-like structures formation and grouped in to well and moderately/poorly differentiation (Xiao et al., 2013). The lymph node metastasis was also categorized in to negative (no regional lymph node metastasis) and positive (metastasis in to regional lymph nodes). The tumor stages were classified according to the TNM staging system, the American Joint Committee of Cancer (AJCC), and grouped in to stage I-II and stage III-IV. Hematoxylin and eosin (H&E) stained sections were reviewed to confirm the presence of >50% of tumor cells with adjacent normal epithelial for further study with immunohistochemistry (IHC) staining. Ethical approval for this study was obtained from National Health and Medical Research Committee (NHMRC), Republic of Yemen (B2/10-2017) and the informed consent was taken from all the CRC patients.
Antibodies and Reagents
The anti-XPO1 antibody was obtained from Santa Cruz Biotechnology, XPO1/CRM1 (sc-74454), and the monoclonal anti-NF-κB p65 antibody (phosphor S536) purchased from abcam, USA, while the anti-Ki67 antibody (MIB-1) was obtained from Cell Marque, USA. For in vitro experiments, colorectal cancer cell line HT29 was obtained from the American Type Culture Collection (ATCC). The HT29 cell was maintained in DMEM/F12 supplemented with 10% fetal bovine serum (FBS) (Sigma) and was cultured at 37°C in a humidified incubator of 5% CO2. XPO1 inhibitor (KPT-330) was obtained from Karyopharm Therapeutics. DMSO was used as diluent control for all in vitro studies.
Immunohistochemical analysis
Tissue sections from FFPE tissue blocks with 4μm thickness were cut and mounted on positive charge glass slides and processed by standard procedures for IHC in parallel with positive and negative controls for each antibody as described previously (Noske et al., 2008). Endogenous peroxidase activity was blocked using 3% hydrogen peroxide (H2O2) and antigen retrieval was done in citrate buffer (pH 6.0) using microwave oven. Then, the sections were incubated with monoclonal primary antibodies against XPO1 (1:400), NF-κB (1:200) and Ki-67 (1:500) overnight at 4°C, followed by a detection system HiDef Detection™ Amplifier and then a HRP Polymer Detector (anti-mouse/rabbit, Cell Marque, USA) for 10 minutes in each step. The DAB chromogen (3,3-diaminobenzidine tetrahydrochloride) then added with substrate for 5 minutes and the sections were washed in distilled water and then counterstained with hematoxylin for 2 minutes. The tissue sections were rinsed with PBS (pH 7.3) between each step during IHC processing.
IHC evaluation of XPO1, NF-κB and Ki67 expression
Two independent investigators evaluated each section. The intensity scoring and positive staining cells were used to define the XPO1 expression in CRC tumor cells and adjacent normal epithelium. The XPO1 expression was calculated by using the intensity score multiplied with positive cells score. The intensity of XPO1 immunostaining scored as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong) for either the nuclear, nuclear membranous and/or cytoplasmic area. The localization staining that diagnosed with positive cells scored according to the percentage of immunoreactive cells as 0 (none), 1 (<10%), 2 (10–50%) and 3 (>50%) and multiplied by the intensity score to give 0 score (negative expression), 1-3 (weak expression), 4-6 (moderate expression) and 7-9 (strong expression). The XPO1 expression levels were categorized in to non-overexpression (negative, weak or moderate expressions) and over-expression (strong expression) (Gravina et al., 2015).
The evaluation of NF-κB (p65) was done according the percentage of immunoreactive cells (quantity score) with the staining intensity in 10 high power visual fields of tumor cells. The percentage of immunoreactive tumor cells was evaluated as follow: no staining as 0, 1%-10% of cells stained as 1, 11%-50% cells stained as 2, 51%-80% cells stained as 3, and 81%-100% cells stained as 4. Staining intensity was rated on a scale of 0-3, with 0 = negative, 1 = weak, 2 = moderate, and 3= strong. The IHC staining of NF-κB was considered positive if the multiplied scored >3/12 (Long et al., 2008). The Ki67 expression was assessed in 10 representative high power visual fields of tumor cells with cutoff value of <40% considered as low and ≥40% as high nuclear Ki67 expression (Salminen et al., 2005).
Cell growth inhibition assay
Cells were seeded at density 104 cells/well in 96-well plates, then the cells were treated next day with series doses below and above their IC50 concentrations of KPT-330, 0.25, 0.5, 1, and 2μm/L and then further incubated for 24, 48 and 72 hours respectively. Thereafter, the cells were subjected to cell proliferation analysis in consecutive days using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay and incubated for 4 hours. After that, the cells were lysed by 200 µL of DMSO. Then the spectrophotometric absorbance of the samples was determined using a microplate reader (Bio-Rad) (Ferreiro-Neira et al., 2016).
Colony formation assay
The ability of KPT-330 to inhibit the HT29 cell lines colonies formation was assessed by the colony formation assay as described previously (Niu et al., 2015). Briefly, 102 HT29 cells were seeded into 24-well plates in triplicates and then treated with vehicle control (DMSO) and series doses of KPT-330, 0.5, 1, 2µmol/L) for 24 hours. The culture medium was changed and the cells were incubated to allow colonies formation. After two weeks, cells were fixed with 5% glutaraldehyde in PBS and stained with 0.1% crystal violet. The numbers of colonies formed were confirmed by manual counting for each dose.
Immunocytochemical (ICC) expressions of Ki67 in HT29-KPT-330-treated cells
The HT29 cells seeded on cover slips in 6-well at density of 105 cells, incubated overnight and then subjected to increasing doses of KPT-330 and incubated for further 48 hours. The cells then washed with PBS, fixed in cold acetone immersed in hydrogen peroxide for 10 minutes. After that, the cells incubated with anti-Ki67 (1:1,000) antibody for one hour followed by incubation in the HiDef Detection™ Amplifier and HiDef Detection™ HRP Polymer Detector for 10 minutes in each. The cells then incubated with DAB-chromogen system for 3-5 minutes followed by counterstain in hematoxylin. Scoring of Ki67 was done depending on the percentage of positive cells (0% - 100%) of the total cells numbers in high-power fields, as in previous study (Zu et al., 2012).
Statistical analysis
In this study, all analysis was performed using SPSS version 18 statistical software program (SPSS Inc., Chicago, IL, USA). Chi-square and Fisher’s exact tests were used to find the association between the overexpression of XPO1, NF-κB and Ki67 with clinico-histopathological factures. Mann-Whitney U test was used with continuous variables. All cell culture experiments were performed in triplicate and repeated at least three times while the one-way ANOVA was used to compare the mean between groups. The P values <0.05 was considered statistically significant difference.
Results
Expressions of XPO1, NF-κB and Ki67 in CRC
The average age of the patients was 49.9 years (range 24-80), with 55% (22/40) were females. After we used the IHC analysis, the majority of XPO1 expressions were nuclear and/or nuclear membrane with weak to moderate diffuse cytoplasmic staining. The XPO1 expression was identified and scored as negative (15%), weak (12.5%), moderate (20%) and strong (52.5%). The only strong score was considered as XPO1 overexpression (52.5%) while the 47.5% were considered as XPO1 non-overexpression. Although there was no big differences in the frequency of the XPO1 immunostaining between the tumor cells and normal adjacent epithelium, the intensity of XPO1 expression was abundant, intense and more apparent in tumor cells compared to the adjacent normal epithelial with significant difference (P<0.001) (Table 1 and Figure 1). The positive NF-κB expression was noted in 32.5% (13/40) while the Ki67 was observed as high expression in 72.5% (29/40) of CRC tumors (Table 2).
Table 1.
Expression of XPO1 in CRC Tumors Cells and Adjacent Normal Epithelium
| XPO1 | Number of patients (%) |
P value | |
|---|---|---|---|
| expression | Normal (%) | Cancer (%) | |
| Negative | 3 (7.5) | 6 (15.0) | |
| Weak | 18 (45.0) | 5 (12.5) | |
| Moderate | 15 (37.5) | 8 (20.0) | |
| Strong | 4 (10.0) | 21 (52.5) | <0.001* |
Figure 1.
XPO1 Expression in Adjacent Normal Epithelium (A1 and B) and Tumor Cells (A2 and C). (Original Magnification- 200x and 400x, respectively).
Table 2.
Association of XPO1 Overexpression and NF-κB with Clinicopathological Features and Ki67 Expression
| Clinicopathological | Patients | XPO1 expression |
P-value | NF-κB expression |
P-value | ||
|---|---|---|---|---|---|---|---|
| features and Ki67 expression | No (%) | Nonoverex-pressio | Overexpr-ession | -ve | +ve | ||
| Tumor differentiation | 0.059 | 0.906 | |||||
| Well | 19 (47.5) | 12 | 7 | 13 | 6 | ||
| Moderate/Poor | 21 (52.5) | 7 | 14 | 14 | 7 | ||
| Lymph node metastasis | 0.366 | 0.027* | |||||
| Negative | 16 (40) | 9 | 7 | 14 | 2 | ||
| Positive | 24 (60) | 10 | 14 | 13 | 11 | ||
| Tumor stage | 0.049* | 0.286 | |||||
| I–II | 11 (27.5) | 8 | 3 | 4 | 7 | ||
| III-IV | 29 (72.5) | 11 | 18 | 7 | 22 | ||
| Ki67 | 0.001** | 0.007** | |||||
| <40% | 11 (27.5) | 10 | 1 | 11 | 0 | ||
| ≥40% | 29 (72.5) | 9 | 20 | 16 | 13 | ||
*,** represent the statistical P<0.001 and P<0.0001, respectively.
Association between the XPO1 overexpression and NF-κB, Ki67 with clinicopathological features of CRC tissue samples
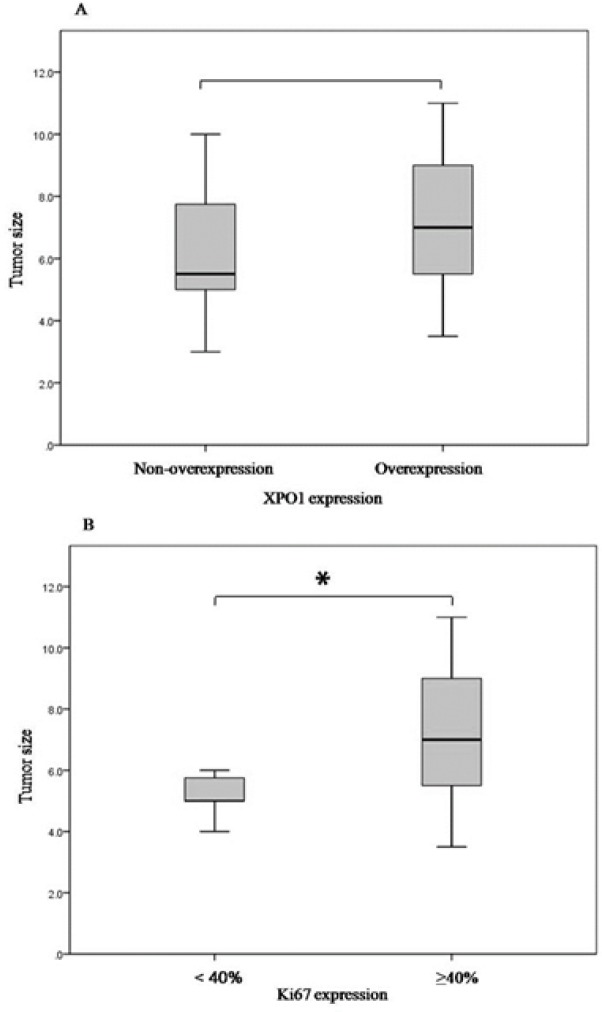
In this study we identified a high tendency of XPO1 overexpression in moderately/poorly differentiated tumors and was significantly associated with advanced tumor stage (III/IV) (P=0.059 and P=0.049, respectively) (Table 2). Although the XPO1 overexpression was frequently noted in tumors with increasing numbers of positive lymph nodes metastasis, this tendency did not reach to the statistical significance difference (P=0.308). Significantly, we found a strong concordance between the XPO1 overexpression and high Ki67 expression in the tumor cells within the most tissue sections that showed immunoreactivity for both XPO1 and Ki67, and the XPO1 overexpression was strongly associated with the high Ki67 expression (P=0.001) (Table 2 and Figure 2). On the same manner, the CRC tumors sizes in this study ranges from 3 to 11 cm with mean 6.6 cm and we noted that the high nuclear Ki67 expression was associated with an increase tumor size (P=0.012) but not XPO1 (P=0.168), with Mann-Whitney test, as it is illustrated in Figure 3. In this study, although the XPO1 overexpression was noticed in 8/13 of positive NF-κB expression tumors, the statistical significant association between the XPO1 overexpression and NF-κB was not found (P=0.427) (Table 3). However, most of the NF-κB positive tumors (11/13) were involved with positive lymph nodes metastasis with significant difference (P=0.027) and showed strong association with the high Ki67 expression (P=0.007) (Table 2 and Figure 2)
Figure 2.
Association of XPO1 Overexpression and NF-κB with Ki67. The IHC expressions of both XPO1 (A) and NF-κB (B) show highly concordance of immunoreactivity within the tissue sections expressed Ki67 (C and D respectively) (original magnification- 400x).
Figure 3.
Correlation between the XPO1 overexpression and Ki67 with tumor size. Mann-Whitney test demonstrate that the tumors with larger size exhibit significant high Ki67 expression (A) (P=0.012) and to some extent the XPO1 overexpression (B) (P=0.168). * represents the significant statistical differences between groups at P<0.05
Table 3.
Association between the XPO1 Overexpression and NF-κB in CRC
| NF-κB expression |
Patients No (%) |
XPO1 expression |
P value | |
|---|---|---|---|---|
| Nonoverex-pressio | Overexpr-ession | |||
| Negative | 27 (67.5) | 14 | 13 | |
| Positive | 13 (32.5) | 5 | 8 | 0.427 |
KPT-330 inhibits cell proliferation, colony formation and Ki67 expression of HT29 cells
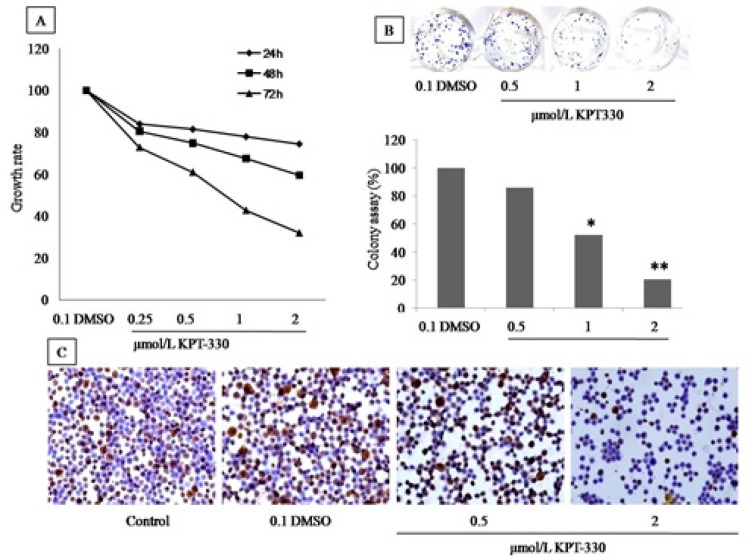
As shown at Figure 4A, the increased of KPT-330 serial concentrations from 0.25, 0.5 and 1 to 2 µmol/L induced the cells growth inhibition after 72 hours of incubation. The IC50 value was 0.9 µmol/L in HT29 cells. These results revealed that, when the KPT-330 dose exceeds 1 µmol/L and the action of time was >24 hours, the growth of cells was inhibited in a time and dose-dependent manner. Further evaluation of the long-term effect of KPT-330 on HT29 growth, the clonogenic assay was performed for 2 weeks. The results revealed that the colonies formation decreased significantly by 86%, 52% and 21% in response to KPT-330 doses of 0.5, 1 and 2 μmol/L, respectively (Figure 4B). Moreover, the Ki67 ICC staining of KPT-330-treated-HT29 cells showed that the Ki67 positive cells in the KPT-330 treated groups was significantly reduce compared to the untreated (Figure 4C). These results were consistent with the MTT and colony formation assays results, confirming that the KPT-330 inhibits the proliferation of HT29 colon cancer cell line in dose and time dependent manner.
Figure 4.
KPT-330 Inhibits the Growth and Proliferation of HT29 Cell Line. MTT assay (A), note the decreases of rate cell growth after 24, 48 and 72 hours of incubation while the colony formation assay (B) evince the ability of KPT-300 to inhibit the colonies formation. The ICC (C) staining shows reversible results of cell-expressed-ki67 and KPT-330 at doses 0.5 to 2µmol/L. *,** represent the statistical differences between the treated and untreated cells (P<0.001 and P<0.0001, respectively)
Discussion
XPO1 is the major mammalian exporter protein that facilitates the nucleocytoplasmic transport of over 200 tumor suppressors and cell cycle regulatory proteins. Its overexpression reported in several types of tumors and was correlated with aggressive behavior and poor survival (Sun et al., 2016). Furthermore, in recent years the XPO1 gains a great attention after launching a next-generation selinexor (KPT-330) as selective inhibition of nuclear export of XPO1 with good properties regarding low toxicity in vivo and good tolerability by the patients (Lapalombella et al., 2012; Hing et al., 2016). In CRC, little is known about the overexpression of XPO1 in tissue samples and its association with histopathological features, NF-κB and Ki67. Our previous study concerned of p53, reported that the XPO1 positivity was associated with loss of p53 expression in CRC tumors with lymph node metastasis (Aladhraei et al., 2019). In the current study, we noticed a significant apparent of XPO1 overexpression in CRC tumor cells compared to the adjacent normal epithelium as it was reported in many other types of cancers such as esophageal (van der Watt et al., 2014), gastric (Subhash et al., 2018), lung (Gao et al., 2015), and ovarian (Noske et al., 2008) cancers, as well as leukemic cells (Kojima et al., 2013). The overexpression of XPO1 within the tumor cells may reflect its abundance and suggests a gain-of-function or oncogenic activity in line of further CRC pathogenesis (Conforti et al., 2015). Crochiere et al., (2016) suggested that the abundance of XPO1 within the tumor cells might be able to predict the drug resistance.
As it is known, the tumor grade is considered as stage-independent prognostic factor in CRC, and the poorly differentiated tumors are associated with poor patient survival (Fleming, et al., 2012). In this study we identified a high tendency of XPO1 overexpression towards the moderate/poorly differentiated tumors as well as increase of XPO1 overexpression frequency in advance tumor stages III-IV with significant difference. Although Shintani et al., (2016) studied the XPO1 expression in CRC tissue samples, they did not find such association in CRC tissues which may attribute to the large differences in the sample sizes in each of well and poorly differentiated tumor groups; others were found such association in other types of cancers such as ovarian cancer (Noske et al., 2008), glioma (Shen et al., 2009) and the XPO1 expression was increased from well to poorly differentiated breast tumors (Yue et al., 2018). Additionally, we noted that the poorly differentiated tumors were significantly increased numbers of metastatic lymph nodes, which goes in line with a cohort study results of 124,180 CRC patients by Ricciardi et al., (2006) who concluded that the poorly differentiated tumors were much more likely to be with lymph node positive than well-differentiated tumors. In this study, most of moderately/ poorly differentiated tumors with XPO1 overexpression were involved by lymph node metastasis. Moreover, the clinical trial carried by Mau-Soerensen et al., (2014) among advanced metastatic CRC patients and used oral KPT-330 for 28 days confirmed the valuable of XPO1 inhibitors in line of disease stability for advanced staged CRC patients. Collectively, these results may add insights of XPO1 involvement in the CRC pathogenesis and progression leading to poorly differentiated tumors and advance tumor stages. In some tumors such as lung cancer and mantle cell lymphoma cells, the XPO1 inhibitors modulate the NF-κB activity through trapping of IκB in the nucleus, which is target of XPO1 for nucleocytoplasmic transportation, leading to repression of NF-κB activity over time which contributes in the growth suppression and apoptosis induction (Zhang et al., 2013). In this study we found that the XPO1 overexpression was associated, to some extent, with the NF-κB positive expression without significant difference. On the other hand, it has been proved that the activated NF-κB contributes in the progression of CRC through upregulation expression of diverse target genes that are involved in inflammation (cytokines), cell proliferation (Cyclin D1), angiogenesis (VEGF, IL-8, COX2), and metastasis (MMP9) (Wang et al., 2009; Xie et al., 2019) make it an interesting tumor marker in CRC pathogenesis. In this regard, the results of our study showed that the NF-κB expression was significantly associated with positive lymph node metastasis. Furthermore, strong significant association between the expressions of NF-κB and high Ki67 expression was identified. These findings were consistent with in vitro studies (Lu et al., 2016) as well as with NF-κB activity role in CRC tumors (Meteoglu et al., 2015). These results add an insight in line of NF-κB involvement in CRC metastasis and proliferation makes a rational for targeting of NF-κB in CRC as it was reported (Sakamoto and Maeda, 2010). It is well known that the sustained proliferation is one of the cancer hallmarks acquired during cancer development and progression (Hanahan and Weinberg, 2011). In this regard, we identified that the overexpression of XPO1 was strongly associated with Ki67 expression, which in turn reflected the implication of XPO1 overexpression in mislocalization of essential cell cycle inhibitory proteins such as p27, p53, cyclins and some apoptotic proteins (Nguyen et al., 2012; Niu et al., 2015) lead to unregulated cell division and increased tumor size. Despite ascending tendency of XPO1 expression was noticed with increased tumor size, our study did not find a direct association between the XPO1 overexpression and tumor size; while the high Ki67 expression was significantly noticed in larger tumor sizes. The strong association between the overexpression of XPO1 and high Ki67 expression in CRC patients’ tumors was in concordance with our in vitro results that confirmed the anti-proliferative effect of KPT-330. The KPT-330 induced the growth inhibition and suppressed the HT29 colorectal cancer cell line proliferation in dose and time dependent manner as shown by the MTT assay. Furthermore, the experiments showed a great stability of KPT-330 in vitro, which means a continuous inhibition of growth over the time during incubation and persisted for up to 72 hours. This explains the powerful binding of KPT-330 in slowly reversible action with XPO1 leading to inhibition the binding between the cargoes proteins and XPO1 in HT29 cancer cell line which in turn induce nuclear retention of cell cycle regulatory proteins, inhibits the proliferation and may initiate the apoptosis (Draetta et al., 2011; Senapedis et al., 2014). The long-term effect of KPT-330 on the HT29 colony formation was significantly seen as a decrease of colonies formation with increase of KPT-330 concentration. These findings were further supported by the evaluation of Ki67 expression by ICC in HT29-KPT-330-treated cells. The number of HT29 cells with Ki67 expression was dramatically and significantly decreased in HT29 KPT-330-treated groups compared to the control, and this confirmed the anti-proliferative effect of KPT-330. In fact, the inhibition of XPO1 activity induced G1 cell cycle arrest with the loss of S, G2, and M phases within hours of application followed by increase of nuclear cell cycle inhibitory proteins such as p27 and p53 (Niu et al., 2015). Normally, the Ki67 is degraded and decreased continuously in G0 and G1, which indicate the decrease of protein synthesis and then accumulate from S to M phases, which indicate an increase of cells proliferative activity. The increase of the sub-G1 fraction within the cells may an indicative of apoptosis that explains the KPT-330 action in time dependent manner (Sobecki et al., 2017; Miller et al., 2018). This result explains the reduction of HT29-cells-expressed-Ki67 with increase doses from 0.5 µmol/L to 2 µmol/L of KPT-330 after 48 hours of incubation. The above results fall in-line with the published data about the KPT-330 ability to sequester the cargoes such as tumor suppressor proteins within the nucleus and leads to cell cycle arrest and proliferation inhibition. The anti-proliferative effect of XPO1 inhibitors were reported in different types of cancer cell lines such as pancreatic cells (Azmi et al., 2017), liver cells (Zheng et al., 2014), prostate cells (Gravina et al., 2015), and gastric cells (Subhash et al., 2018) as well as in colon cancer cell lines (Draetta et al., 2011; Niu et al., 2015). In conclusion, an apparent of XPO1 overexpression in CRC tumor cells compared to the adjacent normal epithelium as well as the association of XPO1 overexpression with advance tumor stages, tumor differentiation and high Ki67 expression may reflect its potential involvement in CRC pathogenesis which can be inhibited by KTP-330. The XPO1 overexpression and NF-κB expression may serve as potential biomarker associated with CRC proliferation and pathogenesis, therefore, further studies regarding the XPO1 overexpression prognostication in CRC patients may be recommended.
Acknowledgements
The authors are grateful to the Department of Pathobiology, Faculty of Science, Mahidol University for the providing the laboratory space and facilities as well as the excellent technical assistance assisted by Mrs. Pranom Puchadapirom and Mrs. Maliwan Emyeam. Ethically, the study protocol followed the guidelines approved by the Ethical Committee of the Medical Research of the National Health and Medical Research Committee (NHMRC), Republic of Yemen (Approval No. B2/10-2017). No potential conflict of interest relevant to this article was reported.
References
- Abdul Razak AR, Mau-Soerensen M, Gabrail NY, et al. First-in-class, first-in-human phase i study of selinexor, a selective inhibitor of nuclear export, in patients with advanced solid tumors. J Clin Oncol. 2016;34:4142–50. doi: 10.1200/JCO.2015.65.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aladhraei M, Al-Salami E, Poungvarin N, Suwannalert P. The roles of p53 and XPO1 on colorectal cancer progression in Yemeni patients. J Gastrointest Oncol. 2019;10:437–44. doi: 10.21037/jgo.2019.01.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmi AS, Li Y, Muqbil I, et al. Exportin 1 (XPO1) inhibition leads to restoration of tumor suppressor miR-145 and consequent suppression of pancreatic cancer cell proliferation and migration. Oncotarget. 2017;8:82144–55. doi: 10.18632/oncotarget.19285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai W, Wu Y, Zhang P, Xi Y. Correlations between expression levels of thymidylate synthase, thymidine phosphorylase and dihydropyrimidine dehydrogenase, and efficacy of 5-fluorouracil-based chemotherapy for advanced colorectal cancer. Int J Clin Exp Pathol. 2015;8:12333–45. [PMC free article] [PubMed] [Google Scholar]
- Conforti F, Wang Y, Rodriguez JA, et al. Molecular pathways: Anticancer activity by inhibition of nucleocytoplasmic shuttling. Clin Cancer Res. 2015;21:4508–13. doi: 10.1158/1078-0432.CCR-15-0408. [DOI] [PubMed] [Google Scholar]
- Crochiere ML, Baloglu E, Klebanov B, et al. A method for quantification of exportin-1 (XPO1) occupancy by Selective Inhibitor of Nuclear Export (SINE) compounds. Oncotarget. 2016;7:1863–77. doi: 10.18632/oncotarget.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draetta G, Shacham S, Kauffman M, et al. Cytotoxicity of novel, small molecule, CRM1-selective inhibitors of nuclear export (SINE) in colorectal cancer (CRC) cells. J Clin Oncol. 2011;29:e14091. [Google Scholar]
- Ferreiro-Neira I, Torres NE, Liesenfeld LF, et al. XPO1 inhibition enhances radiation response in preclinical models of rectal cancer. Clin Cancer Res. 2016;22:1663–73. doi: 10.1158/1078-0432.CCR-15-0978. [DOI] [PubMed] [Google Scholar]
- Fleming M, Ravula S, Tatishchev SF, Wang HL. Colorectal carcinoma: Pathologic aspects. J Gastrointest Oncol. 2012;3:153–73. doi: 10.3978/j.issn.2078-6891.2012.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Lu C, Chen L, Keohavong P. Overexpression of CRM1: a characteristic feature in a transformed phenotype of lung carcinogenesis and a molecular target for lung cancer adjuvant therapy. J Thor Oncol. 2015;10:815–25. doi: 10.1097/JTO.0000000000000485. [DOI] [PubMed] [Google Scholar]
- Gravina GL, Mancini A, Sanita P, et al. KPT-330, a potent and selective exportin-1 (XPO-1) inhibitor, shows antitumor effects modulating the expression of cyclin D1 and survivin in prostate cancer models. BMC Cancer. 2015;15:941. doi: 10.1186/s12885-015-1936-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Heong V, Koe P, Yong W, et al. RAS/AKT pathway mutations as predictive biomarkers in patients with colorectal cancer treated with the exportin 1 (XPO1) inhibitor selinexor (SEL)–inhibition of nuclear-cytoplasmic translocation of p27 as a mechanism of anti-tumour activity. Ann Oncol. 2016;27:383. [Google Scholar]
- Hing ZA, Fung HY, Ranganathan P, et al. Next-generation XPO1 inhibitor shows improved efficacy and in vivo tolerability in hematological malignancies. Leukemia. 2016;30:2364–72. doi: 10.1038/leu.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap T, Argueta C, Aboukameel A, et al. Selinexor, a selective inhibitor of nuclear Export (SINE) compound, acts through NF-kappaB deactivation and combines with proteasome inhibitors to synergistically induce tumor cell death. Oncotarget. 2016;7:78883–95. doi: 10.18632/oncotarget.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau TR, Way JC, Silver PA. Nuclear transport and cancer: from mechanism to intervention. Nat Rev Cancer. 2004;4:106–17. doi: 10.1038/nrc1274. [DOI] [PubMed] [Google Scholar]
- Kojima K, Kornblau SM, Ruvolo V, et al. Prognostic impact and targeting of CRM1 in acute myeloid leukemia. Blood. 2013;121:4166–74. doi: 10.1182/blood-2012-08-447581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapalombella R, Sun Q, Williams K, et al. Selective inhibitors of nuclear export show that CRM1/XPO1 is a target in chronic lymphocytic leukemia. Blood. 2012;120:4621–34. doi: 10.1182/blood-2012-05-429506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Y M, Ye S, Rong J, Xie WR. Nuclear factor kappa B: a marker of chemotherapy for human stage IV gastric carcinoma. World J Gastroenterol. 2008;14:4739–44. doi: 10.3748/wjg.14.4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YX, Ju HQ, Wang F, et al. Inhibition of the NF-kappaB pathway by nafamostat mesilate suppresses colorectal cancer growth and metastasis. Cancer Lett. 2016;380:87–97. doi: 10.1016/j.canlet.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Mau-Soerensen M, Razak AR, Mahipal A, et al. Safety and antitumor activity of selinexor (KPT-330), a first-in-class, oral XPO1 selective inhibitor of nuclear export: A phase I study expanded with colon cancer cohort. J Clin Oncol. 2014;32:482. [Google Scholar]
- Meteoglu I, Erdogdu IH, Tuncyurek P, et al. Nuclear factor kappa B, matrix metalloproteinase-1, p53, and Ki-67 expressions in the primary tumors and the lymph node metastases of colorectal cancer cases. Gastroenterol Res Pract. 2015;2015:945392. doi: 10.1155/2015/945392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller I, Min M, Yang C, et al. Ki67 is a graded rather than a binary marker of proliferation versus quiescence. Cell Rep. 2018;24:1105–12. doi: 10.1016/j.celrep.2018.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Holloway MP, Altura RA. The CRM1 nuclear export protein in normal development and disease. Int J Biochem Mol Biol. 2012;3:137–51. [PMC free article] [PubMed] [Google Scholar]
- Niu M, Chong Y, Han Y, Liu X. Novel reversible selective inhibitor of nuclear export shows that CRM1 is a target in colorectal cancer cells. Cancer Biol Ther. 2015;16:1110–8. doi: 10.1080/15384047.2015.1047569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noske A, Weichert W, Niesporek S, et al. Expression of the nuclear export protein chromosomal region maintenance/exportin 1/Xpo1 is a prognostic factor in human ovarian cancer. Cancer. 2008;112:1733–43. doi: 10.1002/cncr.23354. [DOI] [PubMed] [Google Scholar]
- Ricciardi R, Madoff RD, Rothenberger DA, Baxter NN. Population-based analyses of lymph node metastases in colorectal cancer. Clin Gastroenterol Hepatol. 2006;4:1522–7. doi: 10.1016/j.cgh.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Maeda S. Targeting NF-kappaB for colorectal cancer. Expert Opin Ther Targets. 2010;14:593–601. doi: 10.1517/14728221003769903. [DOI] [PubMed] [Google Scholar]
- Salminen E, Palmu S, Vahlberg T, Roberts PJ, Soderstrom KO. Increased proliferation activity measured by immunoreactive Ki67 is associated with survival improvement in rectal/recto sigmoid cancer. World J Gastroenterol. 2005;11:3245–9. doi: 10.3748/wjg.v11.i21.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723. doi: 10.1146/annurev.cellbio.20.010403.092805. [DOI] [PubMed] [Google Scholar]
- Senapedis WT, Baloglu E, Landesman Y. Clinical translation of nuclear export inhibitors in cancer. Semin Cancer Biol. 2014;27:74–86. doi: 10.1016/j.semcancer.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Shen A, Wang Y, Zhao Y, et al. Expression of CRM1 in human gliomas and its significance in p27 expression and clinical prognosis. Neurosurgery. 2009;65:153–9. doi: 10.1227/01.NEU.0000348550.47441.4B. [DOI] [PubMed] [Google Scholar]
- Shintani M, Tashiro A, Sangawa A, Yamao N, Kamoshida S. Expression of chromosomal regional maintenance protein-1 may be associated with subcellular survivin expression in human gastric and colorectal carcinoma. Oncol Lett. 2016;12:4630–4. doi: 10.3892/ol.2016.5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobecki M, Mrouj K, Colinge J, et al. Cell-Cycle Regulation Accounts for Variability in Ki-67 Expression Levels. Cancer Res. 2017;77:2722–34. doi: 10.1158/0008-5472.CAN-16-0707. [DOI] [PubMed] [Google Scholar]
- Subhash VV, Yeo MS, Wang L, et al. Anti-tumor efficacy of Selinexor (KPT-330) in gastric cancer is dependent on nuclear accumulation of p53 tumor suppressor. Sci Rep. 2018;8:12248. doi: 10.1038/s41598-018-30686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Chen X, Zhou Q, et al. Inhibiting cancer cell hallmark features through nuclear export inhibition. Signal Transduct Target Ther. 2016;1:16010. doi: 10.1038/sigtrans.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Turner JG, Sullivan DM. CRM1-mediated nuclear export of proteins and drug resistance in cancer. Curr Med Chem. 2008;15:2648–55. doi: 10.2174/092986708786242859. [DOI] [PubMed] [Google Scholar]
- van der Watt PJ, Zemanay W, Govender D, et al. Elevated expression of the nuclear export protein, Crm1 (exportin 1), associates with human oesophageal squamous cell carcinoma. Oncol Rep. 2014;32:730–8. doi: 10.3892/or.2014.3231. [DOI] [PubMed] [Google Scholar]
- Wang S, Liu Z, Wang L, ZhangX NF-κB signaling pathway, inflammation and colorectal cancer. Cell Mol Immunol. 2009;6:327–34. doi: 10.1038/cmi.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Wu P, Zhao L, et al. NF-kappaB expression and outcomes in solid tumors: A asystematic review and metaanalysis. Medicine (Baltimore) 2015;94:e1687. doi: 10.1097/MD.0000000000001687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Yoon YS, Hong SM, et al. Poorly differentiated colorectal cancers: correlation of microsatellite instability with clinicopathologic features and survival. Am J Clin Pathol. 2013;140:341–7. doi: 10.1309/AJCP8P2DYNKGRBVI. [DOI] [PubMed] [Google Scholar]
- Xie B, Nie S, Hu G, et al. The involvement of NF-kappaB/Klotho signaling in colorectal cancer cell survival and invasion. Pathol Oncol Res. 2019;25:1553–65. doi: 10.1007/s12253-018-0493-6. [DOI] [PubMed] [Google Scholar]
- Yue L, Sun ZN, Yao YS, et al. CRM1, a novel independent prognostic factor overexpressed in invasive breast carcinoma of poor prognosis. Oncol Lett. 2018;15:7515–22. doi: 10.3892/ol.2018.8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Wang M, Tamayo AT, et al. Novel selective inhibitors of nuclear export CRM1 antagonists for therapy in mantle cell lymphoma. Exp Hematol. 2013;41:67–78. doi: 10.1016/j.exphem.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Gery S, Sun H, et al. KPT-330 inhibitor of XPO1-mediated nuclear export has anti-proliferative activity in hepatocellular carcinoma. Cancer Chemother Pharmacol. 2014;74:487–95. doi: 10.1007/s00280-014-2495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Qiu W, Yao R, et al. CRM1 is a novel independent prognostic factor for the poor prognosis of gastric carcinomas. Med Oncol. 2013;30:726. doi: 10.1007/s12032-013-0726-1. [DOI] [PubMed] [Google Scholar]
- Zu YF, Wang XC, Chen Y, et al. Thyroid transcription factor 1 represses the expression of Ki-67 and induces apoptosis in non-small cell lung cancer. Oncol Rep. 2012;28:1544–50. doi: 10.3892/or.2012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]