Abstract
Context
Observational studies show discordant links between endogenous testosterone levels and cardiovascular diseases (CVD).
Objective
We assessed whether sex hormones and sex hormone–binding globulin (SHBG) are associated with CVD in community-dwelling elderly men.
Design, Setting and Participants
Prospective study of incident CVD among 552 men ≥ 65 years in the MrOS Sleep Study without prevalent CVD and no testosterone therapy at baseline.
Outcomes
Fasting serum levels of total testosterone and estradiol were measured using liquid chromatography-mass spectrometry, and SHBG by chemiluminescent substrate. The association of sex hormones and SHBG with incident coronary heart disease (CHD), cerebrovascular (stroke and transient ischemic attack) and peripheral arterial disease (PAD) events were assessed by quartile and per SD increase in proportional hazards models.
Results
After 7.4 years, 137 men (24.8%) had at least 1 CVD event: 90 CHD, 45 cerebrovascular and 26 PAD. The risk of incident CVD events was not associated with quartiles of baseline sex hormones or SHBG (all P ≥ 0.16). For +1 SD in total testosterone, the multivariate-adjusted hazard ratio was 1.04 (95% CI, 0.80-1.34) for CHD, 0.86 (0.60-1.25) for cerebrovascular, and 0.81 (0.52-1.26) for PAD events. When analyzed as continuous variables or comparing highest to low quartile, levels of bioavailable testosterone, total estradiol, testosterone/estradiol ratio and SHBG were not associated with CVD events.
Conclusions
In community-dwelling elderly men, endogenous levels of testosterone, estradiol, and SHBG were not associated with increased risk of CHD, cerebrovascular, or PAD events. These results are limited by the small number of events and should be explored in future studies.
Keywords: testosterone, cardiovascular events, aging, men
Cardiovascular diseases (CVD) are the most common cause of death and comorbidities. CVD predominate among men compared to premenopausal women, while postmenopausal women catch up in terms of CVD incidence and prevalence [1]. The exact mechanisms of this difference by sex are not known, but a potential effect of sex hormones on CVD risk has long been debated.
Multiple observational studies have explored the association of endogenous testosterone (T) and cardiovascular outcomes, albeit with inconsistent results and methodology. Even after focusing on community-dwelling populations, Araujo et al found multiple studies examining this association and more recent studies have been published since this review [2]. In the large EPIC-Norfolk study in Europe and the Rancho Bernardo Study in the United States, lower endogenous T levels were associated with increased risk of cardiovascular mortality [3, 4]. A large Swedish study showed a reduced 5-year cardiovascular risk among elderly men in the highest T quartile, overall and in separate analyses of coronary heart disease (CHD) and cerebrovascular diseases (hospitalization for stroke or transient ischemic attack, or death from stroke) [5]. In an Australian study of elderly men, higher T and dihydrotestosterone (DHT) levels were associated with a lower incidence of stroke, but not CHD [6]. At the other end of the spectrum, men with T below the 10th percentile had an increased risk of ischemic stroke [7]. In a smaller study of 171 Japanese men aged 30 to 69 years, those in the lowest T tertile had a higher overall CVD risk [8]. Taken together, these studies suggest an increased CVD risk associated with low T levels.
Other studies reported no association of T with CVD risk, but with other sex hormones. Among middle-aged men of the Framingham cohort, the highest quartile of estradiol (E2) was associated with a lower CVD risk, but there was no association across quartiles of T and dehydroepiandrosterone sulfate [9]. In the Cardiovascular Health Study, there was no association of T with CVD risk, but a curvilinear relationship of DHT with CVD risk [10]. In the Atherosclerosis Risk in Communities study, low T levels were associated with CVD classical risk factors only in the cross-sectional analysis, but not in the longitudinal analysis [11]. In contrast, postmenopausal women with high T/E2 ratios, or T absolute levels had increased CVD risk in the MESA study [12]. Other potential mechanisms would include the increased duration of the QTc interval in women, and linked with sex hormone levels and ratios [13], or mediated by differences in lipid levels.
In this context, we assessed whether endogenous T, in total or bioavailable forms, in addition to E2 and sex hormone–binding globulin (SHBG) were associated with incident CVD events in a prospective cohort of 552 community-dwelling elderly men.
1. Materials and Methods
A. Participants and design
From March 2000 to April 2002, the Osteoporotic Fractures in Men (MrOS) Study enrolled 5994 community-dwelling men aged ≥ 65 years at 6 study sites [14, 15]: Birmingham, AL; the Monongahela Valley near Pittsburgh, PA; Minneapolis, MN; Palo Alto, CA; San Diego, CA; and Portland, OR. Exclusion criteria were the inability to walk without assistance and bilateral hip replacement. Each site and coordinating center’s institutional review board approved the study protocol, and written informed consent was obtained from all participants.
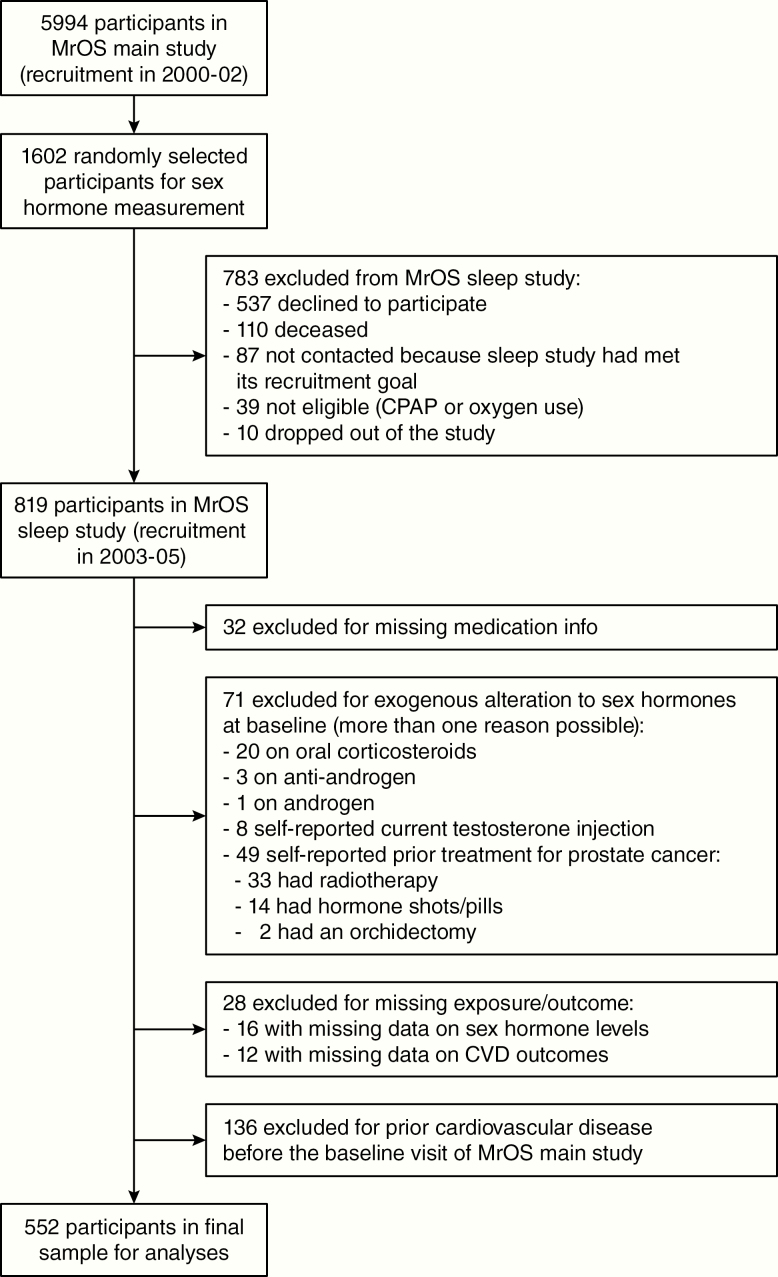
Subsequently a random sample of 1602 men was selected for baseline sex hormone assessment to determine the association with bone mineral density [16] (Fig. 1). Of this group, 819 (51.1%) participated in an ancillary study of sleep disorders (MrOS Sleep Study) from December 2003 to March 2005 [17]; the average time between the baseline and the sleep study visits was 3.4 years. Among these 819 participants, we excluded those with incomplete data on medications (n = 32); those with exogenous alterations to sex hormones at baseline (n = 71, reasons detailed in Fig. 1); those with incomplete data on sex hormone levels (n = 16) and cardiovascular outcomes (n = 12); and those who reported a history of CVD (n = 136). The final sample of the main analysis therefore included 552 men (Fig. 1). When compared to the 1602 men randomly selected for sex hormone measurement, the 552 men in our analysis were younger, more physically active, had higher low-density lipoprotein (LDL)-cholesterol and lower triglycerides, and were less likely to use anti-hypertensive, lipid-lowering, and diabetes medication. There was no major difference between the 2 groups in terms of smoking, body mass index (BMI), systolic blood pressure, high-density lipoprotein (HDL), or fasting glucose.
Figure 1.
Flow of participants of MrOS main study and MrOS sleep study. Participants of the MrOS main study were randomly selected for additional measurement of their sex hormone levels (see Methods), then a subset was randomly selected for an ancillary study on sleep, with exclusions depicted in the right boxes, thus leading to 552 participants in the final sample of this study. Abbreviations: CPAP, continuous positive airway pressure; CVD, cardiovascular diseases; MrOS, the Osteoporotic Fractures in Men Study.
B. Cardiovascular outcomes
After recruitment in the MrOS Sleep Study, participants were surveyed for incident CVD events every 4 months via postcard and/or phone contact with a 99% response rate [17]. When participants reported a potential event, the local study center gathered clinical documentation such as notes, laboratory results, diagnostic studies, and discharge summaries. Death certificates were obtained for all fatal events. All fatal and nonfatal cardiovascular events up until July 29, 2014, were adjudicated at the central coordinating center by a board-certified cardiologist blinded to laboratory results using standardized outcome definitions. An expert adjudicator independently reviewed a random subset of 5% of all reported events to ensure accuracy in diagnosis.
The primary outcomes of this analysis were (1) CHD events defined by ST or non-ST elevation acute myocardial infarction, CHD sudden death, coronary artery bypass surgery or revascularization, hospitalization for unstable angina, ischemic congestive heart failure, and other CHD events not listed above; 2) cerebrovascular events (stroke > 24 hours or transient ischemic attack < 24 hours); and 3) peripheral arterial disease (PAD) events defined as acute arterial occlusion, dissection or rupture, and vascular surgery. Multiple events in one hospitalization were categorized as separate events. In case of multiple CVD events, only the first (incident) event of the same category (CHD, cerebrovascular or PAD events) was retained for the analysis.
C. Clinical measurements
At the baseline visit, participants completed a questionnaire on demographic characteristics, medical history, smoking, and alcohol consumption. Physical activity was assessed using the Physical Activity Scale for the Elderly (PASE, score range 0-793 with higher scores representing greater physical activity) [18, 19]. To assess their functional status, men were asked how many of 5 instrumental activities of daily living (IADL, score range 0-5) they had any difficulty with. The IADLs consisted of walking 2 to 3 blocks outside on level ground, climbing 10 steps without resting, preparing meals, heavy housework, and shopping for groceries or clothes.
Participants were asked to bring all prescription medications used in the previous 30 days on a daily or almost daily basis. Each medication was matched to its active drug agent(s) based on the Iowa Drug Information Service Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA) [20].
Height was measured on Harpenden stadiometers and weight on standard balance beam or digital scales, with participants wearing light clothing without shoes. BMI was calculated as weight in kg/height in meters squared. Systolic blood pressure was measured once in each arm using a conventional mercury sphygmomanometer, and the average of the 2 measurements was obtained.
D. Lab assays
Serum samples were collected at baseline in the morning after an overnight fast and stored at −70 C degrees. Aliquots from each participant were assayed in duplicate and the values averaged. A combined gas chromatographic negative ionization tandem mass spectrometry and liquid chromatographic electrospray tandem mass spectrometry bioanalytical method were used to measure total testosterone, estradiol, and estrone in serum (Taylor Techonology, Princeton, NJ) [16]. Detection ranges were 2.5 to 320 ng/dL for testosterone, 0.625 to 80 pg/mL for estradiol and 1.56 to 200 pg/mL for estrone. Testosterone values above the detection range were reanalyzed after dilution. The intra-assay and inter-assay coefficients of variation were, respectively, 2.5% and 6% for testosterone, 6.4% and 10.1% for estradiol, and 5.2% and 12.9% for estrone. The total testosterone as measured in the MrOS Study was part of the cross-calibration process to develop harmonized testosterone measurement [21].
The testosterone-to-estradiol (T:E2) ratio was calculated by dividing the respective total serum levels (after converting both totals to ng/mL). SHBG concentration was determined on an Immulite Analyzer with chemiluminescent substrate (Diagnostic Products Corp., Los Angeles, CA). The standard curve range was 0.2 to 180 nmol/L, the intra-assay coefficient of variation 4.6% and the inter-assay coefficient of variation 5.8%. Albumin concentration was considered as a constant value of 4.3 g/dL. Bioavailable (non SHBG-bound) fractions of testosterone and estradiol were then calculated using mass action equations by Sodegard et al [22] and Vermeulen et al [23].
Total cholesterol, HDL-cholesterol and triglycerides were measured using a Roche COBAS Integra 800 automated analyzer which was calibrated daily (Roche Diagnostics Corp., Indianapolis, IN). LDL-cholesterol levels were calculated with the Friedewald equation [= Total cholesterol − HDL-cholesterol – (Triglycerides * 0.2) (all in mg/dL)] [24]. Fasting glucose was analyzed enzymically on a Hitachi 917 Autoanalyzer, and fasting insulin concentrations by a 2-site immune-enzymometric assay using a Tosoh 600 II auto-analyzer.
E. Statistical analysis
Participant baseline characteristics were compared across quartiles of total and bioavailable testosterone; total and bioavailable E2; the ratio of total T to total E2 (T:E2) and SHBG, using either analyses of variance or Kruskal-Wallis tests (continuous variables) or chi-squared tests of homogeneity (categorical variables) where appropriate. The percentage of participants with cardiovascular events were compared across total testosterone quartiles using chi-squared tests of homogeneity. The associations between baseline sex hormone levels and subsequent risk of incident cardiovascular events were determined for the composite cardiovascular outcome (incident CHD, cerebrovascular, and/or PAD event) and for each cardiovascular outcome individually: incident CHD event, incident cerebrovascular event, and incident PAD event. For participants who had more than one type of event (e.g., incident CHD and PAD), time to event was based on the first event to be experienced. Cox proportional hazards regression models were used to assess these associations. We estimated the hazard ratios (HR) with 95% confidence intervals (CI) of cardiovascular events by quartiles of sex hormone levels. The initial model was adjusted for age at inclusion and study enrollment site. To account for the potential effect of covariates, we assessed multivariate (MV) models adjusted for multiple variables (MV1 to MV5 models) and report results from the full multivariate (MV5) model. In addition to the analysis by quartiles detailed above, we assessed the HR per 1 standard deviation (SD) increase in the sex hormone levels. We also tested whether the relationship between the sex hormones and incident cardiovascular events was nonlinear by testing the significance of a quadratic term for the sex hormones in the models. Finally, we tested effect modification by age in analyses stratified by the median age for the study sample (< 72 vs ≥ 72 years). Statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC).
2. Results
The 552 male subjects without CVD at baseline had a mean age of 72.4 years (SD, 5.4) and a mean BMI of 27.6 kg/m2 (SD, 3.6) (Table 1). The majority were white (91.3%), with no difference of ethnic group distribution across T quartiles (P = 0.44). Participants within higher T quartiles had a lower weight and BMI (both P < 0.001), and a borderline lower prevalence of diabetes mellitus (P = 0.06), consistent with lower fasting glucose and insulin levels (both P < 0.001). Antihypertensive drugs were more often prescribed to those in lower quartiles of testosterone (P = 0.02), with no prominent drug class. Participants in lower quartiles of testosterone were also more frequently prescribed statins (P = 0.01), and had lower levels of total cholesterol, LDL, and HDL-cholesterol (all P ≤ 0.04) and higher levels of triglycerides (P < 0.001). After excluding those on lipid-lowering drugs (n = 126), only the associations with HDL-cholesterol and triglycerides remained significant (data not shown).
Table 1.
Baseline Characteristics by Quartile of Serum Total Testosterone Among Men With No Prior Cardiovascular Diseases
| Characteristic, mean ± SD or n (%) | Overall | T quartile 1 < 307 ng/dL | T quartile 2 307-391 ng/dL | T quartile 3 391-495 ng/dL | T quartile 4 ≥ 495 ng/dL | P- valuea |
|---|---|---|---|---|---|---|
| (N = 552) | (N = 139) | (N = 135) | (N = 136) | (N = 142) | ||
| Age at inclusion, years | 72.4 ± 5.4 | 72.8 ± 5.5 | 72.2 ± 5.5 | 72.3 ± 5.4 | 72.0 ± 5.1 | 0.63 |
| Self-reported race/ethnicity | 0.44 b | |||||
| White | 504 (91.3) | 132 (95.0) | 123 (91.1) | 125 (91.9) | 124 (87.3) | |
| African American | 16 (2.9) | 3 (2.2) | 3 (2.2) | 4 (2.9) | 6 (4.2) | |
| Asian/Hispanic/Other | 32 (5.8) | 4 (2.9) | 9 (6.7) | 7 (5.2) | 12 (8.5) | |
| Weight, kg | 84.6 ± 12.7 | 89.7 ± 13.2 | 85.2 ± 12.9 | 84.1 ± 12.1 | 79.6 ± 10.6 | <0.001 |
| Body mass index, kg/m2 | 27.6 ± 3.6 | 29.3 ± 4.0 | 27.6 ± 3.4 | 27.1 ± 3.2 | 26.3 ± 3.0 | <0.001 |
| Systolic blood pressure, mmHg | 137.9 ± 17.7 | 140.7 ± 19.1 | 136.3 ± 15.5 | 138.8 ± 17.6 | 136.1 ± 18.2 | 0.10 |
| Lifestyle | ||||||
| Current smoker | 18 (3.3) | 4 (2.9) | 1 (0.7) | 5 (3.7) | 8 (5.6) | 0.14 b |
| Alcohol consumption, standard drinks per week | 5.0 ± 8.1 | 5.4 ± 9.3 | 4.9 ± 6.8 | 5.1 ± 7.1 | 4.7 ± 8.9 | 0.63 |
| Physical activity, PASE score | 157.7 ± 66.9 | 155.2 ± 69.3 | 152.9 ± 64.1 | 162.4 ± 69.3 | 160.4 ± 65.0 | 0.61 |
| Number of IADL impairments (range 0-5) | 0.19 ± 0.60 | 0.22 ± 0.56 | 0.18 ± 0.56 | 0.15 ± 0.54 | 0.21 ± 0.72 | 0.38 |
| Medical history | ||||||
| Hypertension | 212 (38.4) | 65 (46.8) | 51 (37.8) | 48 (35.3) | 48 (33.8) | 0.11 |
| Diabetes mellitus | 63 (12.0) | 24 (18.3) | 15 (11.6) | 13 (9.9) | 11 (8.3) | 0.06 |
| Current medications c | ||||||
| Aspirin | 159 (28.8) | 42 (30.2) | 34 (25.2) | 44 (32.4) | 39 (27.5) | 0.58 |
| Lipid-lowering drugs | 126 (22.8) | 36 (25.9) | 40 (29.6) | 32 (23.5) | 18 (12.7) | 0.006 |
| Statin | 122 (22.1) | 34 (24.5) | 39 (28.9) | 31 (22.8) | 18 (12.7) | 0.01 |
| Gemfibrozil | 4 (0.7) | 2 (1.4) | 1 (0.7) | 1 (0.7) | 0 (0.0) | 0.57 b |
| Anti-hypertensive drugs | 211 (38.2) | 66 (47.5) | 53 (39.3) | 50 (36.8) | 42 (29.6) | 0.02 |
| Diabetes mellitus treatment | 32 (5.8) | 9 (6.5) | 11 (8.2) | 7 (5.2) | 5 (3.5) | 0.40 |
| Oral hypoglycemic agents | 32 (5.8) | 9 (6.5) | 11 (8.2) | 7 (5.2) | 5 (3.5) | 0.40 |
| Insulin | 6 (1.1) | 2 (1.4) | 1 (0.7) | 2 (1.5) | 1 (0.7) | 0.88 b |
| Lipid profile | ||||||
| Total cholesterol, mg/dL | 197.1 ± 32.5 | 194.6 ± 33.5 | 197.1 ± 34.0 | 192.9 ± 29.5 | 203.5 ± 32.1 | 0.04 |
| LDL-cholesterol, mg/dL | 118.7 ± 29.3 | 113.9 ± 30.1 | 117.0 ± 31.1 | 118.1 ± 27.8 | 125.7 ± 27.0 | 0.009 |
| HDL-cholesterol, mg/dL | 49.4 ± 13.5 | 46.7 ± 12.4 | 46.8 ± 11.6 | 50.5 ± 13.6 | 53.8 ± 14.9 | <0.001 |
| Triglycerides, mg/dL | 144.6 ± 84.9 | 170.3 ± 98.6 | 166.8 ± 95.9 | 121.6 ± 61.5 | 120.4 ± 64.1 | <0.001 |
| Glucose, mg/dL | 104.7 ± 24.8 | 110.5 ± 22.6 | 104.3 ± 25.1 | 103.6 ± 28.8 | 100.4 ± 21.2 | <0.001 |
| Insulin, µIU/mL | 9.16 ± 6.26 | 11.63 ± 6.35 | 10.03 ± 6.99 | 8.13 ± 6.31 | 6.92 ± 4.03 | <0.001 |
| Sex hormones | ||||||
| Total T, ng/dL | 415.0 ± 156.9 | 249.6 ± 52.0 | 346.6 ± 22.9 | 437.1 ± 29.8 | 620.7 ± 132.9 | <0.001 |
| Bioavailable T, ng/dL | 212.8 ± 62.5 | 148.8 ± 33.7 | 191.7 ± 24.0 | 224.5 ± 27.8 | 284.5 ± 55.4 | <0.001 |
| Total E2, pg/mL | 22.7 ± 7.3 | 19.1 ± 5.9 | 21.0 ± 5.4 | 22.8 ± 6.4 | 27.8 ± 8.0 | <0.001 |
| Bioavailable E2, pg/mL | 14.6 ± 4.4 | 13.7 ± 4.3 | 14.2 ± 3.9 | 14.5 ± 4.3 | 16.0 ± 4.8 | <0.001 |
| T:E2 ratio | 189.6 ± 65.8 | 138.2 ± 42.4 | 175.7 ± 45.6 | 207.4 ± 65.2 | 235.8 ± 62.0 | <0.001 |
| Estrone, pg/mL | 33.5 ± 11.9 | 32.0 ± 12.1 | 32.5 ± 11.1 | 33.9 ± 12.9 | 35.4 ± 11.4 | 0.07 |
| SHBG, nmol/L | 48.0 ± 17.8 | 34.6 ± 10.6 | 41.9 ± 10.5 | 50.2 ± 12.4 | 64.7 ± 19.4 | <0.001 |
Abbreviations: E2, estradiol; HDL, high-density lipoprotein; IADL, instrumental activities of daily living; LDL, low-density lipoprotein; PASE, physical activity scale for the elderly; SD, standard deviation; SHBG, sex hormone-binding globulin; T, total testosterone; T:E2 ratio, total testosterone / total estradiol ratio.
Footnotes: a P values are from ANOVA for normally distributed continuous variables and Kruskal-Wallis test for skewed continuous variables. P-values for categorical data are from a chi-squared test for homogeneity. bChi-squared may not be a valid test since too many cells have expected counts < 5 (unable to obtain Fisher’s exact P value). c Current medication use was based on review of prescription medications used in the previous 30 days.
During a mean follow-up of 8.4 years (SD 2.2) after inclusion in the MrOS Sleep Study, 23 (4.2%) of the 552 men terminated the study. Out of the 152 deaths, 49 (32.2%) were of cardiovascular origin (Table 2). Of the 552 participants, 137 (24.8%) participants had at least 1 CVD event of any type during a mean follow-up of 7.4 ± 3.0 years, 90 (16.3%) had at least 1 CHD event during a mean follow-up of 7.7 ± 2.8 years, 45 (8.2%) had at least 1 cerebrovascular event during a mean follow-up of 8.1 ± 2.5 years and 26 (4.7%) had at least 1 PAD event during a mean follow-up of 8.2 ± 2.4 years.
Table 2.
Testosterone and Cardiovascular Events in Older Men Cardiovascular Outcomes by Quartile of Serum Total Testosterone Among Men With No Prior Cardiovascular Disease Events
| Clinical outcomes during follow-up, n (%) | Overall | T quartile 1 < 307 ng/dL | T quartile 2 307-391 ng/dL | T quartile 3 391-495 ng/dL | T quartile 4 ≥ 495 ng/dL | P-valuea |
|---|---|---|---|---|---|---|
| (N = 552) | (N = 139) | (N = 135) | (N = 136) | (N = 142) | ||
| Cardiovascular outcomes | ||||||
| Incident CHD event | 90 (16.3) | 28 (20.1) | 17 (12.6) | 20 (14.7) | 25 (17.6) | 0.35 |
| Incident cerebrovascular event | 45 (8.2) | 9 (6.5) | 16 (11.9) | 8 (5.9) | 12 (8.5) | 0.27 |
| Incident PAD event | 26 (4.7) | 7 (5.0) | 8 (5.9) | 5 (3.7) | 6 (4.2) | 0.83 |
| Any of the incident cardiovascular events above | 137 (24.8) | 38 (27.3) | 34 (25.2) | 27 (19.9) | 38 (26.8) | 0.46 |
| Mortality outcomes | ||||||
| Cardiovascular death | 49 (9.0) | 13 (9.4) | 10 (7.5) | 17 (12.6) | 9 (6.5) | 0.31 |
| Death from any cause | 152 (27.9) | 37 (26.8) | 39 (29.3) | 39 (28.9) | 37 (26.6) | 0.94 |
Abbreviations: CHD, coronary heart disease; PAD, peripheral arterial disease; T, total testosterone.
Footnotes: aP values are from a chi-squared test for homogeneity.
The percentage of men experiencing cardiovascular events during follow-up did not differ across total testosterone quartiles (Table 2). Likewise, there was no difference in the percentage of men who died from cardiovascular causes or from any cause across the total testosterone quartiles. There was no difference in risk of incident CHD, incident cerebrovascular, or incident PAD events for men in quartiles 2, 3, and 4 of serum total testosterone compared to those in the lowest quartile of total testosterone in minimally adjusted or fully adjusted models (all P ≥ 0.18, Fig. 2). In analyses by quartiles of bioavailable testosterone, total estradiol and SHBG, there was again no difference in risk of any of the cardiovascular events for men in quartiles 2 to 4 compared with those in quartile 1 of the sex hormones in any of the models (data not shown).
Figure 2.
Hazard ratios of coronary heart disease, cerebrovascular and peripheral vascular events by quartiles of serum total testosterone. The hazard ratios (HR) of coronary heart disease events by quartiles of serum total testosterone (T) from fully-adjusted multivariate models are shown in panel A (P for trend 0.82), for cerebrovascular events in panel B (P for trend 0.72) and for peripheral vascular events in panel C (P for trend 0.37). Quartile 1 (Q1: T < 307 ng/dL) was the reference group (ref.) for comparisons with quartile 2 (Q2: T 307-391 ng/dL), quartile 3 (Q3: T 391-495 ng/dL) and quartile 4 (Q4: T ≥ 495 ng/dL). Vertical bars represent 95% confidence intervals.
The HR of incident cardiovascular events per 1 SD increase in levels of each sex hormone were not significant in age and study site-adjusted analyses, nor after adjustment for multiple cardiovascular risk factors (Table 3).
Table 3.
Risk of Cardiovascular Events Per Standard Deviation (SD) Increase in Serum Sex Steroids and Sex Hormone-Binding Globulin (SHBG) Among Men Without Cardiovascular Disease At Baseline
| Hazard ratio (95% CI) of coronary heart disease event | Hazard ratio (95% CI) of cerebrovascular event | Hazard ratio (95% CI) of peripheral arterial disease | |||||
|---|---|---|---|---|---|---|---|
| Hormone | 1 SD | Initial modela | Full multivariate modelb | Initial modela | Full multivariate modelb | Initial modela | Full multivariate modelb |
| Total T | 156.9 ng/dL | 1.01 (0.82, 1.26) | 1.04 (0.80, 1.34) | 0.92 (0.68, 1.24) | 0.86 (0.60, 1.25) | 0.92 (0.61, 1.39) | 0.81 (0.52, 1.26) |
| Bioavailable T | 62.5 ng/dL | 1.04 (0.84, 1.29) | 1.08 (0.84, 1.39) | 0.90 (0.66, 1.22) | 0.89 (0.61, 1.31) | 0.91 (0.60, 1.38) | 0.82 (0.51, 1.33) |
| Total E2 | 7.3 pg/mL | 1.03 (0.83, 1.28) | 1.05 (0.83, 1.32) | 0.84 (0.61, 1.14) | 0.81 (0.57, 1.16) | 1.07 (0.73, 1.56) | 0.97 (0.64, 1.48) |
| Bioavailable E2 | 4.4 pg/mL | 1.03 (0.83, 1.27) | 1.05 (0.84, 1.31) | 0.82 (0.60, 1.12) | 0.82 (0.57, 1.18) | 1.09 (0.75, 1.58) | 1.06 (0.68, 1.66) |
| T:E2 ratio | 65.8 | 0.92 (0.75, 1.14) | 0.90 (0.70, 1.17) | 1.02 (0.77, 1.34) | 1.00 (0.68, 1.46) | 0.77 (0.50, 1.19) | 0.69 (0.41, 1.16) |
| SHBG | 17.8 nmol/L | 0.95 (0.76, 1.19) | 0.92 (0.71, 1.20) | 1.04 (0.78, 1.38) | 0.95 (0.67, 1.34) | 0.96 (0.64, 1.44) | 0.84 (0.54, 1.31) |
Abbreviations: CI, confidence interval; E2, estradiol; PASE, physical activity scale for the elderly; SD, standard deviation; SHBG, sex hormone-binding globulin; T, testosterone; T:E2 ratio, total testosterone / total estradiol ratio.
Footnotes: aAdjusted for age at inclusion and study site. bAdjusted for age at inclusion, study site, smoking status, physical activity (PASE score), body mass index, systolic blood pressure, use of anti-hypertensive medication, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, use of lipid-lowering medication, fasting plasma glucose and use of diabetes mellitus treatment.
There was no evidence of interaction between sex hormone levels and age (<72 years vs ≥ 72 years) for prediction in risk of CVD outcomes (all P ≥ 0.08). There was no evidence of a non-linear relationship between sex hormone levels and the risk of any cardiovascular event, except for a possible non-linear relationship between the T:E2 ratio and risk of any cardiovascular event (Table 4, p for quadratic term = 0.06 in age and study site-adjusted model, and 0.09 in fully adjusted model).
Table 4.
Risk of Any Cardiovascular Event (Coronary Heart Disease Event, Cerebrovascular Event, or Peripheral Arterial Disease Event) by Quartile of Serum Sex Hormones and SHBG in Multivariate Models
| Hazard ratio (95% CI) by quartiles of sex hormone measures | P for linear trend | P for quadratic term | ||||
|---|---|---|---|---|---|---|
| Sex hormone and models | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Total Testosterone | < 307 ng/dL | 307-391 ng/dL | 391-495 ng/dL | ≥ 495 ng/dL | ||
| Initial model | 1.00 (ref.) | 0.94 (0.59, 1.50) | 0.75 (0.45, 1.22) | 1.10 (0.70, 1.73) | 0.94 | 0.94 |
| MV1 | 1.00 (ref.) | 0.98 (0.61, 1.57) | 0.71 (0.42, 1.18) | 1.03 (0.63, 1.67) | 0.80 | |
| MV2 | 1.00 (ref.) | 0.99 (0.61, 1.61) | 0.69 (0.41, 1.17) | 1.08 (0.66, 1.77) | 0.95 | |
| MV3 | 1.00 (ref.) | 0.93 (0.57, 1.51) | 0.74 (0.44, 1.27) | 1.06 (0.63, 1.78) | 0.97 | |
| MV4 | 1.00 (ref.) | 0.96 (0.58, 1.56) | 0.68 (0.40, 1.17) | 1.01 (0.60, 1.69) | 0.72 | |
| MV5 | 1.00 (ref.) | 0.93 (0.55, 1.55) | 0.64 (0.36, 1.14) | 1.04 (0.60, 1.79) | 0.82 | 0.84 |
| Total Estradiol | < 17.8 pg/mL | 17.8-21.9 pg/mL | 21.9-26.6 pg/mL | ≥ 26.6 pg/mL | ||
| Initial model | 1.00 (ref.) | 1.08 (0.68, 1.70) | 1.01 (0.63, 1.62) | 0.97 (0.59, 1.60) | 0.86 | 0.89 |
| MV1 | 1.00 (ref.) | 1.09 (0.69, 1.74) | 1.04 (0.65, 1.68) | 1.01 (0.61, 1.68) | 0.99 | |
| MV2 | 1.00 (ref.) | 1.09 (0.68, 1.73) | 0.97 (0.59, 1.59) | 0.98 (0.59, 1.62) | 0.82 | |
| MV3 | 1.00 (ref.) | 1.12 (0.70, 1.82) | 1.07 (0.65, 1.75) | 1.08 (0.64, 1.83) | 0.83 | |
| MV4 | 1.00 (ref.) | 1.24 (0.76, 2.02) | 1.12 (0.67, 1.87) | 1.04 (0.60, 1.79) | 0.98 | |
| MV5 | 1.00 (ref.) | 1.15 (0.69, 1.90) | 0.96 (0.56, 1.63) | 0.98 (0.56, 1.70) | 0.76 | 0.91 |
| T:E2 ratio | < 143 | 143-181 | 181-227 | ≥ 227 | ||
| Initial model | 1.00 (ref.) | 1.62 (1.02, 2.60) | 1.44 (0.89, 2.34) | 0.86 (0.51, 1.45) | 0.50 | 0.06 |
| MV1 | 1.00 (ref.) | 1.50 (0.93, 2.42) | 1.34 (0.81, 2.22) | 0.73 (0.42, 1.29) | 0.23 | |
| MV2 | 1.00 (ref.) | 1.53 (0.94, 2.51) | 1.39 (0.83, 2.33) | 0.81 (0.45, 1.44) | 0.38 | |
| MV3 | 1.00 (ref.) | 1.48 (0.90, 2.42) | 1.28 (0.76, 2.15) | 0.70 (0.39, 1.28) | 0.21 | |
| MV4 | 1.00 (ref.) | 1.50 (0.92, 2.47) | 1.23 (0.72, 2.08) | 0.68 (0.37, 1.25) | 0.16 | |
| MV5 | 1.00 (ref.) | 1.53 (0.92, 2.56) | 1.20 (0.69, 2.09) | 0.73 (0.39, 1.36) | 0.24 | 0.09 |
| SHBG | < 35.1 nmol/L | 35.1-45.1 nmol/L | 45.1-57.3 nmol/L | ≥ 57.3 nmol/L | ||
| Initial model | 1.00 (ref.) | 0.74 (0.45, 1.23) | 1.11 (0.70, 1.78) | 1.15 (0.72, 1.86) | 0.31 | 0.34 |
| MV1 | 1.00 (ref.) | 0.72 (0.43, 1.19) | 1.04 (0.64, 1.68) | 1.06 (0.64, 1.74) | 0.53 | |
| MV2 | 1.00 (ref.) | 0.76 (0.45, 1.27) | 1.07 (0.65, 1.76) | 1.10 (0.66, 1.84) | 0.49 | |
| MV3 | 1.00 (ref.) | 0.72 (0.43, 1.23) | 1.13 (0.68, 1.87) | 1.07 (0.63, 1.83) | 0.47 | |
| MV4 | 1.00 (ref.) | 0.71 (0.42, 1.19) | 0.99 (0.60, 1.64) | 0.91 (0.53, 1.55) | 0.98 | |
| MV5 | 1.00 (ref.) | 0.72 (0.42, 1.24) | 1.05 (0.62, 1.80) | 0.94 (0.53, 1.65) | 0.87 | 0.50 |
We estimated the hazard ratios (with 95% confidence interval) of cardiovascular events per quartile of sex hormones, compared to the lowest quartile used as the reference group. The initial model was adjusted for age at inclusion and study site, followed by multivariate (MV) models to account for the potential effect of covariates:
- Initial model: Adjusted for age at inclusion and study site.
- MV1 model: Initial model + further adjusted for smoking status, physical activity (PASE score) and body mass index.
- MV2 model: MV1 model + further adjusted for systolic blood pressure and the use of anti-hypertensive medication.
- MV3 model: MV1 model + further adjusted for LDL cholesterol, HDL cholesterol, triglycerides and the use of lipid-lowering medication.
- MV4 model: MV1 model + further adjusted for fasting plasma glucose and any current diabetes treatment.
- MV5 model: Initial model + further adjusted for all aforementioned covariates, i.e., age at inclusion, study site, smoking status, physical activity (PASE score), body mass index, systolic blood pressure, use of anti-hypertensive medication, LDL cholesterol, HDL cholesterol, triglycerides, use of lipid-lowering medication, fasting plasma glucose and any current diabetes treatment.
Abbreviations: CI, confidence interval; HDL, high density lipoprotein; LDL, low density lipoprotein; MV, multivariate model; PASE, physical activity scale for the elderly; SHBG, sex hormone-binding globulin; T:E2 ratio, total testosterone / total estradiol ratio.
3. Discussion
In our study of community-dwelling elderly men, the levels of endogenous testosterone or other sex hormones were not associated with incident cardiovascular events, including CHD, cerebrovascular, and PAD events, over 8.4 years of follow-up.
Our results contrast with those of other observational studies. The meta-analysis by Araujo et al [2] that concluded that lower T levels are associated with an increased risk of all cause and CVD mortality, but this review did highlight the large heterogeneity in findings across individual studies, and not specifically CVD events as done in the current study. These inconsistent results may be due to differences between the studied populations, the baseline cardiovascular risk, the analysis methods chosen, or the sex hormone assays [25]. The latter was the reason for a recent standardization effort of T assays, which relied on measurements by mass spectrometry, for which the MrOS Study was 1 of the 4 cohorts used to harmonize T levels [21]. Another potential explanation is the use of total vs free vs bioavailable T as the exposure parameter in different studies, which is still debated, although the latest recommendations suggest that it is best to rely on total T and to measure SHBG to evaluate hypogonadism in men [26]. In our study, there was no difference of HRs of CVD events between total and bioavailable T, contrary to other studies [27]. To avoid confusion, future studies or re-analysis of older studies would need to check for differences in the predictive power for CVD event risk with total T measured with mass spectrometry or a CDC-certified assay.
As the knowledge regarding CVD event risk and its link with sex hormones is still open to debate, it is important for clinicians caring for elderly men to screen for agreed-upon cardiovascular risk factors, i.e., hypertension, hyperlipidemia, smoking cessation, obesity, and/or diabetes mellitus. Interestingly, treating these other risk factors can lead to improvements of T levels. For example, weight loss led to increased T levels in the EMAS study [28]. In our study, adjustment for other conditions and their treatments did not change the null associations of sex hormones and SHBG with the risk of CVD events.
This study is observational and the small number of participants who were on hormone-altering treatments were excluded from the analysis (Fig. 1). Thus, this study does not address the effects of T prescription, which has surged over the past decade [29], with wide differences between countries [30]. As highlighted by the recently updated Endocrine Society guidelines on T replacement therapy [26], clinicians should be wary of potential cardiovascular complications of exogenous T in older men. The controversy about T replacement in older men has been ongoing for years and has faced increased scrutiny by the US Food and Drug Administration (FDA) [31, 32] and the European Medicines Agency [33]. Of note, other groups do not share these views and strongly advocate in favor of limiting T therapy for men with documented T deficiency [34].
Multiple meta-analyses have assessed the effect of T replacement on CVD with inconsistent results and criteria [35]. The Testosterone Trials grouped 7 randomized controlled trials (RCTs) assessing the effects of T replacement on multiple outcomes after 1 year: The Cardiovascular Trial showed an increased progression of coronary artery plaques after 1 year of T [36], but no difference in CVD events, although these studies were not designed to assess clinical cardiovascular endpoints and had low power [37]. Another RCT of T gel was terminated prematurely due to more cardiovascular adverse events compared to placebo, but the premature termination of the study, the low number of events and the inclusion of men ≥ 65 years of age with limited mobility and a high prevalence of comorbidities preclude any definite conclusion [38]. Another trial of T in older men by the same group showed no difference in carotid intima-media thickness or coronary artery calcium score after 3 years of T replacement, but was not powered to conclude on CVD events and cardiovascular safety [39]. On the other hand, an intention-to-treat observational cohort study reported benefits of T replacement on CVD outcomes [40]. Current clinical knowledge is therefore based on retrospective cohorts with their inherent limitations: Some observational studies reported increased CVD event risk with T replacement [41, 42], while other registries concluded that T replacement was not associated with higher rates of CVD events [43]. This open question on the potential benefits and harms of T replacement still needs more definite and longer RCTs [26]. The CVD outcomes will be assessed in an industry-sponsored RCT launched in 2018 to satisfy the safety concerns of the FDA (TRAVERSE trial, clinicaltrials.gov ID NCT03518034).
Our study has strengths and limitations. It was conducted at 6 different sites across the US, including more than 500 community-dwelling elderly white men followed up over 8.4 years with very low attrition rate. While a lot of other studies relied mostly on death certificates or registry [2], our study used death certificates as the primary source of data then all cardiovascular outcomes were adjudicated by a panel of cardiologists. The sample size of the study is limited to draw firm conclusions, especially in the context of a fairly healthy populations, with a low mean BMI, low smoking rates, and not many comorbidities. Because it is an observational study and participants on hormone-altering treatments were excluded, we cannot conclude on the causal pathway of endogenous T, nor can we assess the effects of T replacement. Moreover, the higher rate of statin users in participants whose T was below the median could be presumably due to a higher underlying CVD risk, but the lipid profile prior to statin use is not known in this study. Finally, as in most epidemiologic studies, sex hormones were measured at baseline only and measurement predated collection of CVD outcomes by an average of 3.5 years. We cannot assess the changes in sex hormone levels over time and their effects on cardiovascular outcomes. However, we used high-throughput liquid chromatography–tandem mass spectrometry method to measure sex hormones, which are less subject to bias and interference than other assays [21]. Statistical power was limited due to the relatively small number of outcomes events, especially stroke and PAD events, even prior to attempting to stratify the analysis according to age. Therefore the analyses stratified by age and adjusted with multiple variables (Table 4) should be seen as hypothesis-generating in the context of past and ongoing studies on the association of endogenous T levels and the risk of stroke and PAD events.
In conclusion, endogenous T levels were not associated with risk of CHD, cerebrovascular or PAD events in this observational study of elderly community-dwelling men. The effects of endogenous and exogenous T on the incidence of CVD events in older men require further study.
Acknowledgments
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), NIH Roadmap for Medical Research (grant numbers U01AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140), and the American Diabetes Association (1-04-JF-46, Strotmeyer ES).
Dr T-H Collet’s research is supported by grants from the Swiss National Science Foundation (P3SMP3-155318, PZ00P3-167826) and the Swiss Society of Endocrinology and Diabetes. The authors want to acknowledge the contributions of Prof. Elizabeth Barrett-Connor who recently passed away and reviewed a prior version of this manuscript. The authors wish to thank Dr. Christopher Dant for his assistance with a previous version of this manuscript.
Glossary
Abbreviations
- BMI
body mass index
- CHD
coronary heart disease
- CI
confidence interval
- CVD
cardiovascular disease
- DHT
dihydrotestosterone
- HDL
high-density lipoprotein
- HR
hazard ratio
- LDL
low-density lipoprotein
- MV
multivariate
- PAD
peripheral arterial disease
- SHBG
sex hormone–binding globulin
- T
testosterone
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: MrOS data are available at https://mrosdata.sfcc-cpmc.net/. The datasets analyzed during the current study are also available from the corresponding author upon request.
References
- 1. Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;111(2):383-390. [DOI] [PubMed] [Google Scholar]
- 2. Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(10):3007-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khaw KT, Dowsett M, Folkerd E, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116(23):2694-2701. [DOI] [PubMed] [Google Scholar]
- 4. Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93(1):68-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ohlsson C, Barrett-Connor E, Bhasin S, et al. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men. The MrOS (Osteoporotic Fractures in Men) study in Sweden. J Am Coll Cardiol. 2011;58(16):1674-1681. [DOI] [PubMed] [Google Scholar]
- 6. Yeap BB, Alfonso H, Chubb SA, et al. In older men, higher plasma testosterone or dihydrotestosterone is an independent predictor for reduced incidence of stroke but not myocardial infarction. J Clin Endocrinol Metab. 2014;99(12):4565-4573. [DOI] [PubMed] [Google Scholar]
- 7. Holmegard HN, Nordestgaard BG, Jensen GB, Tybjærg-Hansen A, Benn M. Sex hormones and ischemic stroke: a prospective cohort study and meta-analyses. J. Clin. Endocrinol. Metab. 2016;101(1):69-78. 10.1210/jc.2015-2687. [DOI] [PubMed] [Google Scholar]
- 8. Akishita M, Hashimoto M, Ohike Y, et al. Low testosterone level as a predictor of cardiovascular events in Japanese men with coronary risk factors. Atherosclerosis. 2010;210(1):232-236. [DOI] [PubMed] [Google Scholar]
- 9. Arnlöv J, Pencina MJ, Amin S, et al. Endogenous sex hormones and cardiovascular disease incidence in men. Ann Intern Med. 2006;145(3):176-184. [DOI] [PubMed] [Google Scholar]
- 10. Shores MM, Biggs ML, Arnold AM, et al. Testosterone, dihydrotestosterone, and incident cardiovascular disease and mortality in the cardiovascular health study. J Clin Endocrinol Metab. 2014;99(6):2061-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Srinath R, Hill Golden S, Carson KA, Dobs A. Endogenous testosterone and its relationship to preclinical and clinical measures of cardiovascular disease in the atherosclerosis risk in communities study. J Clin Endocrinol Metab. 2015;100(4):1602-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao D, Guallar E, Ouyang P, et al. Endogenous Sex Hormones and Incident Cardiovascular Disease in Post-Menopausal Women. J Am Coll Cardiol. 2018;71(22):2555-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abehsira G, Bachelot A, Badilini F, et al. Complex Influence of Gonadotropins and Sex Steroid Hormones on QT Interval Duration. J Clin Endocrinol Metab. 2016;101(7):2776-2784. [DOI] [PubMed] [Google Scholar]
- 14. Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials. 2005;26(5):557-568. [DOI] [PubMed] [Google Scholar]
- 15. Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569-585. [DOI] [PubMed] [Google Scholar]
- 16. Cauley JA, Ewing SK, Taylor BC, et al. ; Osteoporotic Fractures in Men Study (MrOS) Research Group Sex steroid hormones in older men: longitudinal associations with 4.5-year change in hip bone mineral density–the osteoporotic fractures in men study. J Clin Endocrinol Metab. 2010;95(9):4314-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bajaj A, Stone KL, Peters K, et al. Circulating vitamin D, supplement use, and cardiovascular disease risk: the MrOS Sleep Study. J Clin Endocrinol Metab. 2014;99(9):3256-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153-162. [DOI] [PubMed] [Google Scholar]
- 19. Washburn RA, Ficker JL. Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sports Med Phys Fitness. 1999;39(4):336-340. [PubMed] [Google Scholar]
- 20. Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405-411. [DOI] [PubMed] [Google Scholar]
- 21. Travison TG, Vesper HW, Orwoll E, et al. Harmonized Reference Ranges for Circulating Testosterone Levels in Men of Four Cohort Studies in the United States and Europe. J Clin Endocrinol Metab. 2017;102(4):1161-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Södergård R, Bäckström T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16(6):801-810. [DOI] [PubMed] [Google Scholar]
- 23. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666-3672. [DOI] [PubMed] [Google Scholar]
- 24. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499-502. [PubMed] [Google Scholar]
- 25. Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92(2):405-413. [DOI] [PubMed] [Google Scholar]
- 26. Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(5):1715-1744. [DOI] [PubMed] [Google Scholar]
- 27. Antonio L, Wu FCW, O’Neill TW, et al. the European Male Ageing Study Study Group Low free testosterone is associated with hypogonadal signs and symptoms in men with normal total testosterone. J. Clin. Endocrinol. Metab. 2016;101(7):2647-2657. 10.1210/jc.2015-4106. [DOI] [PubMed] [Google Scholar]
- 28. Camacho EM, Huhtaniemi IT, O’Neill TW, et al. ; EMAS Group Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013;168(3):445-455. [DOI] [PubMed] [Google Scholar]
- 29. Layton JB, Li D, Meier CR, Sharpless JL, Stürmer T, Jick SS, Brookhart MA. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J. Clin. Endocrinol. Metab. 2014;99(3):835-842. 10.1210/jc.2013-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Handelsman DJ. Global trends in testosterone prescribing, 2000–2011: expanding the spectrum of prescription drug misuse. Med J Aust.2013;199(8):548-551. 10.5694/mja13.10111. [DOI] [PubMed] [Google Scholar]
- 31. Garnick MB. Testosterone replacement therapy faces FDA scrutiny. JAMA. 2015;313(6):563-564. [DOI] [PubMed] [Google Scholar]
- 32. Nguyen CP, Hirsch MS, Moeny D, Kaul S, Mohamoud M, Joffe HV. Testosterone and “Age-Related Hypogonadism”–FDA Concerns. N Engl J Med. 2015;373(8):689-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wolfe SM. Increased heart attacks in men using testosterone: the UK importantly lags far behind the US in prescribing testosterone. BMJ. 2014;348:g1789. [DOI] [PubMed] [Google Scholar]
- 34. Morgentaler A. Testosterone deficiency and cardiovascular mortality. Asian J. Androl. 2015;17(1):26-31. 10.4103/1008-682X.143248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Onasanya O, Iyer G, Lucas E, Lin D, Singh S, Alexander GC. Association between exogenous testosterone and cardiovascular events: an overview of systematic reviews. Lancet Diabetes Endocrinol. 2016;4(11):943-956. [DOI] [PubMed] [Google Scholar]
- 36. Budoff MJ, Ellenberg SS, Lewis CE, et al. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA. 2017;317(7):708-70 9. 10.1001/jama.2016.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Snyder PJ, Bhasin S, Cunningham GR, et al. Lessons from the testosterone trials. Endocr Rev. 2018;39(3):369-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Basaria S, Harman SM, Travison TG, et al. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels: a randomized clinical trial. JAMA. 2015;314(6):570-581. [DOI] [PubMed] [Google Scholar]
- 40. Wallis CJ, Lo K, Lee Y, et al. Survival and cardiovascular events in men treated with testosterone replacement therapy: an intention-to-treat observational cohort study. Lancet Diabetes Endocrinol. 2016;4(6):498-506. [DOI] [PubMed] [Google Scholar]
- 41. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836. [DOI] [PubMed] [Google Scholar]
- 42. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. Plos One. 2014;9(1):e85805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saad F, Caliber M, Doros G, Haider KS, Haider A. Long-term treatment with testosterone undecanoate injections in men with hypogonadism alleviates erectile dysfunction and reduces risk of major adverse cardiovascular events, prostate cancer, and mortality. Aging Male 2019;13:1-12. 10.1080/13685538.2019.1575354. [DOI] [PubMed] [Google Scholar]