Supplemental Digital Content is available in the text.
Abstract
The severe acute respiratory syndrome Coronavirus 2 (Coronavirus Disease 2019 [COVID-19]) pandemic has challenged medical systems and clinicians globally to unforeseen levels. Rapid spread of COVID-19 has forced clinicians to care for patients with a highly contagious disease without evidence-based guidelines. Using a virtual modified nominal group technique, the Pediatric Difficult Intubation Collaborative (PeDI-C), which currently includes 35 hospitals from 6 countries, generated consensus guidelines on airway management in pediatric anesthesia based on expert opinion and early data about the disease. PeDI-C identified overarching goals during care, including minimizing aerosolized respiratory secretions, minimizing the number of clinicians in contact with a patient, and recognizing that undiagnosed asymptomatic patients may shed the virus and infect health care workers. Recommendations include administering anxiolytic medications, intravenous anesthetic inductions, tracheal intubation using video laryngoscopes and cuffed tracheal tubes, use of in-line suction catheters, and modifying workflow to recover patients from anesthesia in the operating room. Importantly, PeDI-C recommends that anesthesiologists consider using appropriate personal protective equipment when performing aerosol-generating medical procedures in asymptomatic children, in addition to known or suspected children with COVID-19. Airway procedures should be done in negative pressure rooms when available. Adequate time should be allowed for operating room cleaning and air filtration between surgical cases. Research using rigorous study designs is urgently needed to inform safe practices during the COVID-19 pandemic. Until further information is available, PeDI-C advises that clinicians consider these guidelines to enhance the safety of health care workers during airway management when performing aerosol-generating medical procedures. These guidelines have been endorsed by the Society for Pediatric Anesthesia and the Canadian Pediatric Anesthesia Society.
Coronavirus Disease 2019 (COVID-19), a pandemic infection caused by a positive-sense ribonucleic acid (RNA) virus named the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has strained health care systems, ignited fear, and dramatically changed the daily lives of people around the world. Clinicians must care for patients with a highly communicable disease while protecting themselves from a potentially lethal disease. Anesthesiologists are at particularly high risk of being exposed to SARS-CoV-2 because airway management, particularly tracheal intubation, positive pressure ventilation through a mask, and management of tracheostomy tubes, causes widespread aerosolization of thevirus (Table 1).1,2 Though the virus appears to have its most damaging clinical effects in adult patients, infection does occur in children.3–6 Indeed, in the Chinese experience, asymptomatic transmission of the virus from children to health care workers (HCWs) emerged as a significant risk.4,5 How to manage the pediatric airway in patients who may or may not be symptomatic is the focus of this report. Further many routine pre–COVID-19 practices, such as mask induction of general anesthesia in anxious, crying, and agitated children or carrying them into the operating room (OR), may be less desirable because of the risk of viral exposure to the clinical staff.
Table 1.
Risk of Transmission of SARS-CoV-1 to Health Care Workers Exposed and Not Exposed to Aerosol-Generating Procedures During the 2003 SARS Outbreak1
| Aerosol-Generating Medical Procedure | Odds Ratio |
|---|---|
| Tracheal intubation | 6.6 |
| Tracheostomy | 4.2 |
| Suction before intubation | 3.5 |
| Noninvasive ventilation | 3.1 |
| Manual ventilation before intubation | 2.8 |
| Chest compression/defibrillation | 2.5 |
| Bronchoscopy | 1.9 |
Abbreviations: SARS, severe acute respiratory syndrome; SARS-CoV-1, severe acute respiratory syndrome Coronavirus 1.
Robust, evidence-based research to advise safe airway practices during the COVID-19 pandemic isnot yet available. Therefore, we sought to develop consensus guidelines from airway experts in pediatric anesthesiology. The Pediatric Difficult Intubation Collaborative (PeDI-C; pediregistry.org) is a special interest group of the Society for Pediatric Anesthesia (pedsanesthesia.org). The mission of PeDI-C is to advance the safety of pediatric airway management by facilitating multicenter research, quality improvement, and education. PeDI-C has led several multicenter studies on pediatric airway management.7–10 PeDI-Ccurrently includes 35 hospitals from 6 countries and 10 additional hospitals that are being onboarded. The more than 100 PeDI-C members include many internationally recognized experts in pediatric airway management. PeDI-C hosts biannual in-person meetings and communicates year-round using an online active chat group on WhatsApp (https://www.whatsapp.com). Members discuss real-time management of difficult intubation cases and disseminate clinical best practices through the forum. Average messages increased from 4 per day in March 2019 to 77 per day during March 2020. The surge in messaging highlighted the global need for guidance about airway management during this COVID-19 pandemic. To help address this need, PeDI-C leadership organized a webinar to generate consensus guidelines about airway management during aerosol-generating medical procedures (AGMPs) in pediatric anesthesia.
METHODOLOGY
On March 23, 2020, PeDI-C members on the WhatsApp forum hosted a webinar to discuss and identify themes and formulate best practice guidelines for AGMPs during the COVID-19 pandemic based on expert opinion. We used a modified nominal group technique (NGT). The classic NGT technique yields prompt results but requires a face-to-face meeting.11 Due to the travel restrictions and requirement for social distancing during the unfolding COVID-19 pandemic, PeDI-Cheld a virtual NGT. PeDI-C leadership advertised the virtual meeting on its WhatsApp channel. Participation was voluntary and open to all members. Zoom (https://www.zoom.us), an online video conferencing service for the virtual NGT, was used,allowing members to interact using audio and video. J.E.F. moderated the session. Preplanned topics for discussion included appropriate use of personal protective equipment (PPE), conduct of anesthesia, and management of at-risk care teams. Forty-four pediatric anesthesiologists and one otolaryngologist from 33 institutions attended the virtual NGT (Supplemental Digital Content 1, Material 1, http://links.lww.com/AA/D84). We analyzed audio, chat messages, and video recordings to identify themes and compared these themes to notes taken in realtime during the meeting by 3 PeDI-C members (J.E.F., P.C., andC.T.M.). We summarized the identified themes and shared them with PeDI-C membership for review, further input, and refinement (Table 2). Additionally, an investigator (C.T.M.) conducted a literature search using Ovid Medline, PubMed, and Google on March 24 and 25, 2020, using the search terms “COVID-19,” “SARS-CoV-2,” “children,” AND “pediatrics” to identify publications relevant to airway management in children with COVID-19.
Table 2.
PeDI-C Recommendations for Airway Management in Pediatric Patients During the COVID-19 Pandemic
| Theme | Recommendations | Example Comments |
|---|---|---|
| Training | Context-sensitive simulation. | Pediatric patients; needs to be relevant to the perioperative and out of operating room procedures. |
| Cognitive aids | Develop, test, and share. | Need to address challenges related to processes, workflows, and clinical management. Development and testing should include nurses and other stakeholders. |
| Patient safety and clinical management | Use of sedation. Parental presence at induction of anesthesia. |
Coughing and crying can increase aerosolization. Should be avoided or minimized. |
| IV induction. Use of neuromuscular blockers for intubations. Extubation. Avoid nasal prongs. |
Should minimize coughing and crying. Should be smooth and under clear plastic is needed. They can cause aerosolization, but a simple oxygen mask covering may prevent or reduce dispersion. |
|
| Staff safety | Personal protection equipment. | Needs to protect health care workers who are a scarce resource. |
| Minimizing staff in the room. Continued use of personal protection equipment during high-risk procedures or patients. |
Should work for the context of the operating room. | |
| High-risk staff (age, immunodeficiency, andpregnancy). | ||
| Anesthesia trainees. |
Abbreviations: COVID-19, Coronavirus Disease 2019; IV, intravenous; PeDI-C, Pediatric Difficult Intubation Collaborative.
RESULTS
Literature Search
We identified 30articles from the literature search (Supplemental Digital Content 1, Material 2, http://links.lww.com/AA/D84), none of which provided details on airway management during AGMPs.
Training and Context-Sensitive Simulation
As COVID-19 continues to spread all over the world, many organizations have implemented simulation sessions to train clinicians on basic donning and doffing of PPE. This training is commendable; however, PeDI-C identified the need for context-specific simulation (ie, simulation that reflects their specific role in the health care team).12,13 For example, anesthesia clinicians should design simulation sessions focused on intubating and extubating COVID-19 patients in full appropriate PPE while minimizing exposure to and spread of the virus in the perioperative environment. Similarly, otolaryngologists would simulate performing an aerosolizing procedure with a clinical team while also minimizing OR exposure using various barrier techniques and PPE.
Protecting Clinicians
PeDI-Cagreed that clinicians who are at higher risk of morbidity and mortality from COVID-19 should be protected from clinical exposure.14 Some suggestions included delegating these at-risk clinicians to staff telemedicine clinics or contribute to scholarly and administrative tasks while maintaining adequate physical distancing. PeDI-C discussed the importance of PPE for anesthesia clinicians; specifically, there was consensus that airway manipulation, such as endotracheal intubation or extubation, isAGMPs and therefore requires maximum protection.1,15–19 The group also acknowledged that during times of crisis, such as the current pandemic, institutions might have PPE shortages. Several members emphasized that although equipment shortages are essential to consider, the highest priority should be the safety of care teams. Prioritizing the safety of HCWs can maximize the delivery of care for patients during a pandemic. PPE supplies are becoming available from manufacturers, donations, and release from national strategic stockpiles; however, clinicians are impossible to replace if quarantined, severely ill, or, worse yet, dead. PeDI-Cfelt that centers should err on the side of overprotection rather than underprotection. Nearly10% of HCWs in Italy and 14% in Spain have contracted COVID-19 with associated morbidity and mortality.20 Inadequate PPE, deeper lung penetration of aerosolized viral particles, and a high burden of exposure may contribute to these infections. In centers with limited PPE supplies, PeDI-C felt that teams should be pared down to the minimum necessary, and cases should be consolidated into the fewest possible rooms to conserve PPE.
PeDI-C recognized that children infected with SARS-CoV-2 could shed the virus asymptomatically, even in stool, and infect others.21–24 A case report of an asymptomatic well infant reported high viral loads for 16 days.5 Anywhere from 18% to 31% of COVID-19-positive passengers (mostly adults) isolated on the Diamond Cruise ship never developed symptoms. Early periods of SARS-CoV-2 can lead to lower levels of sensitivity on screening tests.25 Therefore, PeDI-C recommends appropriate PPE (N95 and face shield and powered, air-purifying respirator [PAPR]) for AGMPs in all children in areas with high community spread. PeDI-Calso recognized the importance of balancing the need for ideal PPE for AGMPs against the current global shortage of PPE. A PPE coach should be available to ensure correct donning and doffing of PPE. The US Centers for Disease Control and Prevention (CDC) offers educational videos of proper donning and doffing technique at https://www.cdc.gov/vhf/ebola/hcp/ppe-training/index.html.
Cognitive Aids
PeDI-Cidentified the need for cognitive aids to support clinical care and workflow during the pandemic.26 PeDI-C encourages the creation and sharing of such cognitive aids for current and future pandemics. Careful attention must be paid to the design and composition of these aids so that they are easily readable and comprehensible. Figure 1 demonstrates an example of a cognitive aid for managing COVID-19 pediatric patients. Cognitive aids (eg, checklists) should be printed, laminated, and mounted in care locations.
Figure 1.

A cognitive aid summarizing the recommendations of PeDI-C for airway management of pediatric patients during the COVID-19 pandemic. AGMP indicates aerosol-generating medical procedure; COVID-19, Coronavirus Disease 2019; FONA, XXX; HEPA, high-efficiency particulate air; LMA, laryngeal mask airway; PeDI-C, Pediatric Difficult Intubation Collaborative; TIVA, total intravenous anesthesia.
Case Preparation
All drugs and equipment should be prepared and readily available before starting an anesthetic. This preparation reduces the need for clinicians to reach into the anesthesia workstation drawers and bins once the patient has entered the procedure room. Trash cans and sharps containers should be readily available and open to avoid dropping equipment on the floor, which increases viral dispersion. For anesthesia drug dispensing workstations that require touching the screen, a plastic shield should be placed over the screen to minimize contamination. Clinicians should leave badges, keys, cell phones, pagers, and pens outside the OR. Emergency phones may be kept in sealed bags to facilitate communication with other clinicians.
Premedication
Clinicians should consider the routine use of preprocedural sedatives to reduce anxiety and increase compliance when an intravenous (IV) is placed awake. Additionally, premedication may reduce the risk of vigorous crying and the need for physical restraints during inhalational inductions. Nasal administration of premedication is undesirable because of the potential for high viral loads and the risk of coughing and sneezing. PeDI-C did not recommend parental presence for the induction of anesthesia to conserve PPE and reduce clinician exposure to SARS-CoV-2. However, this will depend on the local infrastructure and practice especially in areas where PPE shortages are not of concern.
Intravenous Placement and Induction of Anesthesia
Because inhalational induction may increase exposure to respiratory droplets and aerosols, PeDI-Cmembers agreed that IV induction is preferred. However, clinicians should assess the child’s disposition to IV catheter placement as struggling to place a catheter may result in higher exposure to respiratory droplets if the child cries. PeDI-C recommended rapid sequence induction or modified rapid sequence to reduce the risk of reflex airway activation during intubation with associated aerosolization. Rapid Sequence Induction may not be feasible without severe hypoxemia in small children and patients with severe lung pathology. These patients should receive gentle positive pressure ventilation with the goal of using just enough tidal volume to achieve chest rise while maintaining a tight mask seal.
Mask Induction if Required
Clinicians should induce anesthesia with the lowest possible flow rates and maintain a tight mask seal.PeDI-C recommended avoiding bag-mask ventilation if feasible to reduce aerosolization. Several PeDI-C members recommended mask induction and direct laryngoscopy–assisted and video laryngoscopy–assisted intubation (Figure 2), using a transparent plastic barrier around the anesthesia elbow to minimize extensive contamination of the OR. A simulation using a 3-layer transparent plastic technique with a simulated cough with particles of similar size to the SARS-COV-2 virus indicates that this barrier may trap virus under the plastic drape creating a hot zone around the patient but reducing the exposure of clinicians.27 The World Health Organization (WHO) guide for rational use of PPE encourages the use of “physical barriers to reduce exposure to COVID-19 virus.”28
Figure 2.
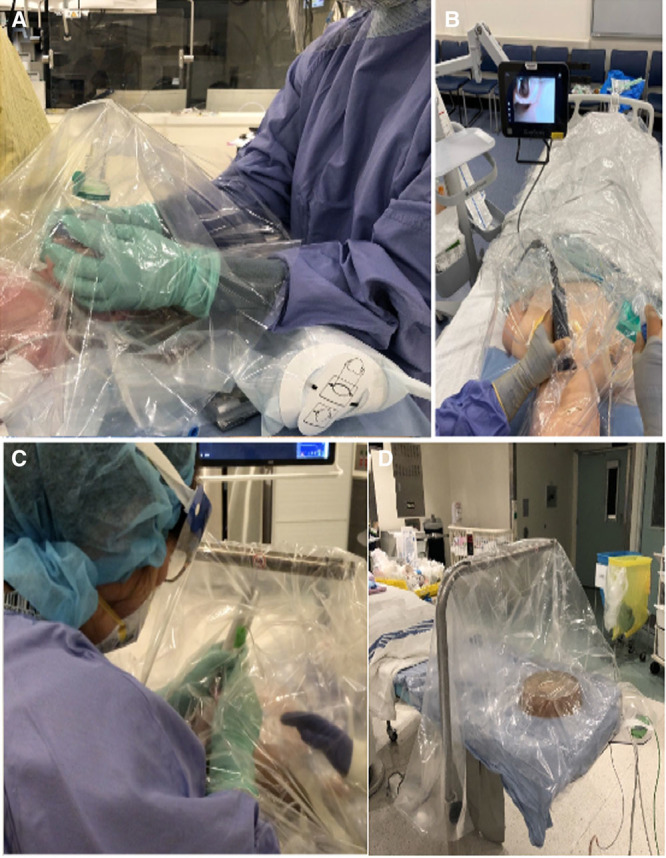
A depiction of transparent drapes being used as an aerosolization barrier during mask induction in a patient (A); video laryngoscopy intubation in a manikin (B); direct laryngoscopy in a real patient (C); and 3-drape technique using an anesthesia elbow and suction under the transparent drapes (D).
Airway Device Placement
The PeDI-C agreed that a cuffed tracheal tube was the ideal device to secure the airway in children with COVID-19. PeDI-C recommends using video laryngoscopy for all intubations, if available, to reduce the laryngoscopist’s proximity to the patient’s airway.29 The most experienced laryngoscopist should attempt tracheal intubation to minimize laryngoscopy time and the number of attempts. Open suctioning may create aerosols, and an in-line closed suction system is preferred.1,17,30 If clinically appropriate, patients in the intensive care unit (ICU) should be intubated in the ICU (preferably in a negative pressure room) before transfer to the OR. PeDI-C felt that a supraglottic airway device with a good seal was acceptable in some cases. A simulated cough in a manikin model with a supraglottic airway device in place showed minimal aerosol dispersion (Supplemental Digital Content 2, Video 1, http://links.lww.com/AA/D85). Second-generation supraglottic airway devices have higher leak pressures than first-generation masks and should be considered.31 PeDI-C agreed that the least desirable approaches were high- or low-flow nasal cannula or bag-mask ventilation, though these techniques may be unavoidable at times. A simple oxygen mask placed on top of a nasal cannula may reduce the risk of aerosol dispersion (Supplemental Digital Content 3, Video 2, http://links.lww.com/AA/D86). PeDI-C recommended avoiding techniques that bring the clinician’s face or stethoscope near the patient to verify leak pressures for endotracheal tube (ETT) and supraglottic airway (SGA). Clinicians can use the ventilator’s measurements of expired and inspired tidal volume and handheld manometers to titrate cuff inflation. Wireless stethoscopes and point-of-care ultrasound can be used to confirm bilateral ventilation of the lungs. A “high-quality” viral filter should be placed between the breathing circuit and the patient’s airway and another one at the end of the expiratory limb at the connection to the anesthesia machine as illustrated at
Maintenance of Anesthesia
PeDI-C recommended that clinicians use full PPE during the entire operative case given the risk of accidental ventilator circuit disconnection, accidental extubation, and unquantified aerosolization from the procedure, especially airway, laparoscopic, and endoscopic procedures. PeDI-C recommended a transparent barrier over the airway device and patient’s head to trap any aerosolized virus. Others have used wet towels and gauze for the same purpose.32
Emergence and Extubation
If available, the clinician should use closed in-line suction to minimize aerosolization during tracheal tube suctioning (Figure 3A). Clinicians should consider deep extubation using techniques that minimize coughing and bucking during emergence, such as total IV anesthesia or dexmedetomidine.33,34 Still, patients may cough during the subsequent emergence and recovery. Protective barriers can be helpful in all phases of care, and the WHO has recommended using them to reduce viral dispersion.35 Supplemental Digital Content 4, Video 3, http://links.lww.com/AA/D87, demonstrates an example of transparent barrier techniques used by PeDI-C members during extubation.27 In addition, PeDI-C agreed thatclinicians should consider placing a suction device under the barrier to create a negative pressure microenvironment which may scavenge droplets and aerosolized materials.
Figure 3.
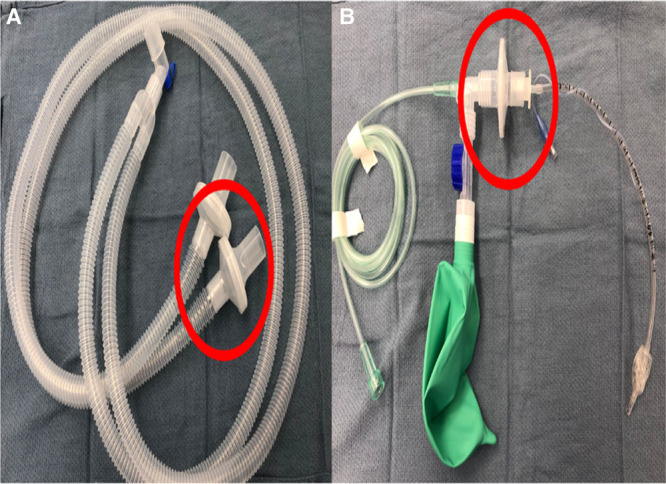
A, Standard viral filters (red circle) present on inspiratory and expiratory limbs of an anesthesia circuit depending on manufacturer can be removed and used as a viral filter for a transport circuit for patient transport. B, Viral filter (red circle) removed from anesthesia circuit and inserted between the endotracheal tube adapter and transport circuit.
PeDI-C recommends emerging and recovering COVID-19 patients and patients under investigation for COVID-19 in the OR, followed by direct transfer of the patient to the inpatient ward bypassing the postanesthesia care unit if possible. This change in workflow minimizes the number of exposed HCWs and nearby patients. For patients being admitted to the ICU postoperatively, extubating in the ICU is considered.
Pediatric anesthesiologists may be called upon to assist with the extubation of critically ill COVID-19 patients in the ICU. Advanced planning of workflows and procedures for emergent intubations outside the OR is critical and should include representatives from anesthesiology, critical care, respiratory therapists, hospitalists and nursing, and respiratory therapists.
Prepare for Adult Care
Pediatric anesthesiologists may be involved in providing care to adults using anesthesia ventilators. The Anesthesia Patient Safety Foundation and the American Society of Anesthesiologists have provided detailed guidance on how to repurpose anesthesia ventilators for ICU care.36 In preparation for this possibility, we encourage anesthesiologists to familiarize themselves with these processes in collaboration with other key stakeholders.
Transporting Intubated Patients
Mechanically ventilated children with COVID-19 should be transported with a ventilator with viral filters on the patient side of the y-piece and the expiratory limb of the breathing circuit (Figure 3).36 If a transport ventilator is not available, a viral filter should be placed on the tracheal tube during transport with the caveat that they increase the dead space in the circuit which may be significant in small children. Figure 4 demonstrates 2 positions of the viral filter with this consideration in mind. Tracheal intubation should be confirmed with capnography and visual observation of bilateral chest rise. Samples lines for capnography should be placed after viral filters. In some cases, viral filters can be removed from the anesthesia circuit and repurposed as a viral filter for a manual transport circuit (Figure 3A). Before transport, clinicians should weigh the risks and benefits of administering additional sedation or neuromuscular blockade to prevent coughing and bucking.
Figure 4.
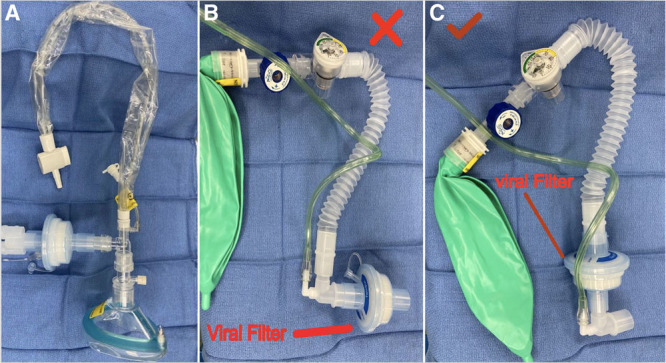
A, A Mapleson D breathing circuit with an in-line suction catheter. B, A Mapleson D breathing circuit with a viral filter at the distal end. Not suitable for infants, neonates, and small children because of the dead space of the filter and potential rebreathing. C, A Mapleson D breathing circuit with a viral filter proximal to the fresh gas flow. Preferred in infants, neonates, and small children.
Infrastructure
The use of a negative pressure OR for AGMPs is recommended for all proven or suspected COVID-19 patients if feasible. Ensure adequate air exchange and filtration time of the ORs used for patients with COVID-19 and suspected cases before cleaning and preparing for the next case.37 A chart of time needed for adequate air exchange is available at https://www.cdc.gov/infectioncontrol/guidelines/environmental/appendix/air.html#tableb1 (scroll down to Table B1). If negative pressure ORs are not available, high-efficiency particulate air (HEPA) filters that sufficiently filter the OR’s square footage were used. Also, try to avoid rooms with connected ventilation systems.
Difficult Airways
PeDI-C members identified the unique challenges involved in managing difficult airways in patients with known or suspected COVID-19. Many of the recommendations described above for tracheal intubation of normal airways apply to difficult airways as well. An airway team should be assembled, and all equipment should be setup in the OR and checked. The team should consider a just-in-time review before beginning airway management. The clinician with the most experience with the selected airway device should perform the tracheal intubation. PeDI-C ranked difficult airway management approaches as follows: video laryngoscopy as the primary technique followed by fiberoptic intubation through a supraglottic airway device, combined video laryngoscopy and fiberoptic bronchoscopy and finally freehand fiberoptic. Oral fiberoptic intubation is preferred over nasal fiberoptic intubation and minimizes passive oxygenation as tolerated. Hypoxia can be addressed with intermittent 2-hand mask ventilation to maintain a good seal and low tidal volumes. If safe to do so, consider administering a neuromuscular blocking agent after IV induction. Sugammadex should be immediately available to antagonize the neuromuscular blocking agent if needed. If warranted, perform mask ventilation with low tidal volumes using a 2-person technique to maintain a good seal. If nasal fiberoptic intubation is required, using an endoscopy mask (Supplemental Digital Content 1, Material 3, http://links.lww.com/AA/D84) is considered. Endoscopy masks have a diaphragm that seals around the fiberoptic scope but allows the tracheal tube to be advanced into the airway. It may be prudent to call early for personnel and equipment for a surgical airway. Prolonged attempts at intubation may be associated with increased aerosolization of the virus.
DISCUSSION
PeDI-C generated consensus guidelines for multiple aspects of pediatric anesthesia care during the COVID-19 pandemic. Our literature search yielded no articles investigating pediatric airway management in patients known or suspected of having COVID-19, further supporting the need for such guidelines. Additionally, rigorous studies of COVID-19 patients may be challenging, given the high infectivity of the disease.
PeDI-C identified the critical importance of protecting HCWs. SARS-CoV-2 infected 3300 HCWs in China and 20% of Italian HCWs with associated morbidity and mortality.19,20,38 The PeDI-C recommendations regarding the use of PPE for all AGMPs are in line with consensus guidelines from the American Society of Anesthesiologists, Anesthesia Patient Safety Foundation, American Academy of Anesthesiologist Assistants, and the Association of Nurse Anesthetists.39 PeDI-C recognized that many institutions are already facing inadequate PPE supplies and need to balance PPE use for routine care in asymptomatic patients with future demand for COVID-19 patients.40 Some have argued that a surgical facemask and standard universal precautions are sufficient in asymptomatic untested patients for AGMPs and cite published literature to support this practice. One study compared the efficacy of N95 respirators to medical masks in preventing HCWs from acquiring influenza and other viral respiratory infections. They randomly assigned 2862 HCWs to N95 or a medical mask and found no difference in laboratory-confirmed influenza infections between the 2 groups: 207 (8.2%) in the N95 group, and 193 (7.2%) in the medical mask group (difference, 1%; 95% confidence interval [CI], −0.5% to 2.5%; P= .18).41 Clinicians should not extrapolate these data to COVID-19 for 2 reasons. First, the SARS-CoV-2 virus is more contagious than influenza. The R0, also known as the basic reproductive number, is a measure of how contagious an infectious disease is, and indicates the expected number of people who will get the disease from an infected individual. The R0 of influenza is 1.2, while that of COVID-19 is estimated to be between 2 and 3.42 Second, SARS-CoV-2 may aerosolize more readily than influenza and remain airborne longer.43 Clinicians should err on the side of overprotection in asymptomatic patients until more rigorous data areavailable in children. Fortunately, new data from Italy and China suggest that the pediatric burden of the disease might be low (2,4). Data from the Virtual pediatric intensive care unit systems (www.myvps.org), a collaboration of 135 North American Pediatric Intensive Care Units, indicate that of 609 patients tested, 30 were COVID-19 positive as of March 29, 2020. These data should be considered in the context of limited testing and exponential spread, which means a low disease prevalence can change rapidly. We caution clinicians not to lower their guard because of a perceived low burden of disease in children.
There remain areas of controversy and need for future exploration, including the value of preprocedural testing for COVID-19, and ethical and legal considerations for the use of innovative but unapproved PPE. Some centers use COVID-19 screening tests to determine the type of PPE for AGMPs, reserving N95 masks for COVID-19 patients and standard surgical masks for negative patients. Using reverse transcription polymerase chain reaction (RT-PCR) testing for COVID-19 to determine PPE use in a limited resource setting makes sense. However, sensitivity for detection of COVID-19 can vary based on how the sample was obtained, the duration from infection to testing, and how the laboratory performed the test. Further, the potential for false negatives should be recognized and clinicians should not be falsely reassured by a negative test. If high-sensitivity testing for COVID-19 is negative in the child, PPE may not be required. Another area of concern is the potential for asymptomatic spread of SARS-CoV-2 from HCWs to patients. It may become necessary for all HCWs to undergo COVID-19 testing to prevent nosocomial infection.
We acknowledge several limitations. The guidelines outlined in this document are based on expert opinions from a diverse group of clinicians. Supportive evidence is referenced when available; however, there are few rigorous studies given the novelty of this disease. Although we searched multiple online repositories and search engines for articles, publication delays could have made some reports unavailable to us at the time of this writing, and not all articlesfrom the global medical community are available in the repositories we queried. Information is evolving quickly during this pandemic, and PeDI-C recognizes that an update to this document may be warranted as we learn more. Most importantly, each hospital will need to adapt these guidelines based on local regulations, availability of equipment, and the prevalence of the disease. These guidelines are meant to help clinicians deliver care that is safe for children as well as staff.
CONCLUSIONS
The Society for Pediatric Anesthesia’s PeDI-C developed consensus guidelines based on expert opinion and the limited available data to guide pediatric airway management during the COVID-19 pandemic. Pandemics of this magnitude are rare. We hope that these guidelines will support clinical care, workflow, and decision-making that maintain patient-centered care while protecting HCWs, our most valuable resource to fight the pandemic. Finally, PeDI-C hopes that these guidelines help prepare clinicians to safely and effectively fight this pandemic.
ACKNOWLEDGMENTS
The authors thank the Department of Anesthesia and Pain Medicine, Hospital for Sick Children, Toronto, Ontario, Canada (R.J. Williams).
DISCLOSURES
Name: Clyde T. Matava, MBCHB, MMed, MHSC.
Contribution: This author helped design the project, implement the study, and write the manuscript.
Conflicts of Interest: None.
Name: Pete G. Kovatsis, MD.
Contribution: This author helped write and edit the manuscript.
Conflicts of Interest: Dr Pete G. Kovatsis is a medical advisor to Verathon Medical, Inc, outside the submitted work.
Name: Jennifer Lee Summers, MD, FRCPC.
Contribution: This author helped write and edit the manuscript.
Conflicts of Interest: None.
Name: Pilar Castro, MD.
Contribution: This author helped implement the study and edit the manuscript.
Conflicts of Interest: None.
Name: Simon Denning, BMBS, FRCA.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Julie Yu, MD, FRCPC.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Justin L. Lockman, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Britta Von Ungern-Sternberg, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Stefano Sabato, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Lisa K. Lee, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Ihab Ayad, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Sam Mireles, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: David Lardner, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Simon Whyte, MBBS.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Judit Szolnoki, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Narasimhan Jagannathan, MD, MBA.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Nicole Thompson, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Mary Lyn Stein, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Nicholas Dalesio, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Robert Greenberg, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: John McCloskey, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: James Peyton, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Faye Evans, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Bishr Haydar, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Paul Reynolds, MD.
Contribution: XXX.
Conflicts of Interest: None.
Name: Franklin Chiao, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Brad Taicher, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Thomas Templeton, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Tarun Bhalla, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Vidya T. Raman, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Annery Garcia-Marcinkiewicz, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Jorge Gálvez, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Jonathan Tan, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Mohamed Rehman, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Christy Crockett, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Patrick Olomu, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Peter Szmuk, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Chris Glover, MD, MBA.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Maria Matuszczak, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Ignacio Galvez.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Agnes Hunyady, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: David Polaner, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: Dr David Polaner reports personal fees from John Wiley and Sons, Inc, and personal fees from Wolters Kluwer, outside the submitted work.
Name: Cheryl Gooden, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Grace Hsu, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Harshad Gumaney, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Caroline Pérez-Pradilla, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Edgar E. Kiss, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Mary C. Theroux, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Jennifer Lau, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Saeedah Asaf, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Pablo Ingelmo, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Thomas Engelhardt, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Mónica Hervías.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Eric Greenwood, MD.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Luv Javia.
Contribution: This author helped edit the manuscript.
Conflicts of Interest: None.
Name: Nicola Disma.
Conflicts of Interest: None.
Name: Myron Yaster, MD.
Contribution: This author helped write and edit the manuscript.
Conflicts of Interest: None.
Name: John E. Fiadjoe, MD.
Contribution: This author helped design the project, implement the study, and write the manuscript.
Conflicts of Interest: Dr John E. Fiadjoe reports receiving a grant from the Anesthesia Patient Safety Foundation, outside the submitted work.
This manuscript was handled by: James A. DiNardo, MD, FAAP.
Supplementary Material
FOOTNOTES
GLOSSARY
- AGMP
- aerosol-generating medical procedure
- CDC
- US Centers for Disease Control and Prevention
- CI
- confidence interval
- COVID-19
- Coronavirus Disease 2019
- ETT
- endotracheal tube
- FONA
- XXX
- HCW
- health care worker
- HEPA
- high-efficiency particulate air
- ICU
- intensive care unit
- IV
- intravenous
- LMA
- laryngeal mask airway
- NGT
- nominal group technique
- OR
- operating room
- PAPR
- powered, air-purifying respirator
- PeDI
- Pediatric Difficult Intubation
- PeDI-C
- Pediatric Difficult Intubation Collaborative
- PICU
- pediatric intensive care unit
- PPE
- personal protective equipment
- RT-PCR
- reverse transcription polymerase chain reaction;
- RNA
- = ribonucleic acid;
- SARS
- severe acute respiratory syndrome
- SARS-CoV-1
- severe acute respiratory syndrome Coronavirus 1
- SARS-CoV-2
- severe acute respiratory syndrome Coronavirus 2
- SGA
- supraglottic airway
- TIVA
- total intravenous anesthesia
- WHO
- World Health Organization
Funding: None.
Conflicts of Interest: See Disclosures at the end of the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.anesthesia-analgesia.org).
Contribution: This author helped edit the manuscript.
Reprints will not be available from the authors.
REFERENCES
- 1.Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7:e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JY, Han MS, Park KU, Kim JY, Choi EH. First pediatric case of coronavirus disease 2019 in Korea. J Korean Med Sci. 2020;35:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020[Epub ahead of print]. [Google Scholar]
- 5.Kam KQ, Yung CF, Cui L, et al. A well infant with coronavirus disease 2019 (COVID-19) with high viral load. Clin Infect Dis. 2020;ciaa201 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiadjoe JE, Nishisaki A, Jagannathan N, et al. Airway management complications in children with difficult tracheal intubation from the Pediatric Difficult Intubation (PeDI) registry: a prospective cohort analysis. Lancet Respir Med. 2016;4:37–48. [DOI] [PubMed] [Google Scholar]
- 8.Burjek NE, Nishisaki A, Fiadjoe JE, et al. PeDI Collaborative Investigators. Videolaryngoscopy versus fiber-optic intubation through a supraglottic airway in children with a difficult airway: an analysis from the Multicenter Pediatric Difficult Intubation Registry. Anesthesiology. 2017;127:432–440. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Marcinkiewicz AG, Adams HD, Gurnaney H, et al. A retrospective analysis of neuromuscular blocking drug use and ventilation technique on complications in the pediatric difficult intubation registry using propensity score matching. Anesth Analg. 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10.Park R, Peyton JM, Fiadjoe JE, et al. PeDI Collaborative Investigators; PeDI Collaborative Investigators. The efficacy of GlideScope® videolaryngoscopy compared with direct laryngoscopy in children who are difficult to intubate: an analysis from the paediatric difficult intubation registry. Br J Anaesth. 2017;119:984–992. [DOI] [PubMed] [Google Scholar]
- 11.Delbecq AL, Van de Ven AH. A group process model for problem identification and program planning. J Appl Behav Sci. 1971;7:466–492. [Google Scholar]
- 12.Lind MM, Corridore M, Sheehan C, Moore-Clingenpeel M, Maa T. A multidisciplinary approach to a pediatric difficult airway simulation course. Otolaryngol Head Neck Surg. 2018;159:127–135. [DOI] [PubMed] [Google Scholar]
- 13.Fehr JJ, Boulet JR, Waldrop WB, Snider R, Brockel M, Murray DJ. Simulation-based assessment of pediatric anesthesia skills. Anesthesiology. 2011;115:1308–1315. [DOI] [PubMed] [Google Scholar]
- 14.COVID-19 (coronavirus). Lymphat Res Biol. 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Ranney ML, Griffeth V, Jha AK. Critical supply shortages: the need for ventilators and personal protective equipment during the COVID-19 pandemic. N Engl J Med. 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Ng K, Poon BH, Kiat Puar TH, et al. COVID-19 and the risk to health care workers: a case report. Ann Intern Med. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan MTV, Chow BK, Lo T, et al. Exhaled air dispersion during bag-mask ventilation and sputum suctioning - implications for infection control. Sci Rep. 2018;8:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung FF, Lin HL, Liu HE, et al. Aerosol distribution during open suctioning and long-term surveillance of air quality in a respiratory care center within a medical center. Respir Care. 2015;60:30–37. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Huang Y. To protect healthcare workers better, to save more lives. Anesth Analg. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nadeau BL. Coronavirus Is Killing Italy’s Doctors. The U.S. Could Be Next. Daily Beast. Published 2020. Available at: https://www.thedailybeast.com. https://www.thedailybeast.com/COVID-19-is-killing-italys-doctors-the-us-could-be-next. Accessed March 27, 2020.
- 21.Zhang T, Cui X, Zhao X, et al. Detectable SARS-CoV-2 viral RNA in feces of three children during recovery period of COVID-19 pneumonia. J Med Virol. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong SH, Lui RN, Sung JJ. COVID-19 and the digestive system. J Gastroenterol Hepatol. 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 23.Su L, Ma X, Yu H, et al. The different clinical characteristics of corona virus disease cases between children and their families in China - the character of children with COVID-19. Emerg Microbes Infect. 2020;9:707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L, Lou J, Bai Y, Wang M. COVID-19 disease with positive fecal and negative pharyngeal and sputum viral tests. Am J Gastroenterol. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR Testing. Radiology. 2020;200343 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renna TD, Crooks S, Pigford AA, et al. Cognitive Aids for Role Definition (CARD) to improve interprofessional team crisis resource management: an exploratory study. J Interprof Care. 2016;30:582–590. [DOI] [PubMed] [Google Scholar]
- 27.Matava CT, Yu J, Denning S. Clear plastic drapes may be effective at limiting aerosolization and droplet spray during extubation: implications for COVID-19. Can J Anesth. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organisation. Rational Use of Personal Protective Equipment (PPE) for Coronavirus Disease (COVID-19): Interim Guidance, 19 March 2020. 2020Geneva, Switzerland: World Health Organization; [Google Scholar]
- 29.Hall D, Steel A, Heij R, Eley A, Young P. Videolaryngoscopy increases ‘mouth-to-mouth’ distance compared with direct laryngoscopy. Anaesthesia. 2020[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30.Loh N-HW, Tan Y, Taculod J, et al. The impact of high-flow nasal cannula (HFNC) on coughing distance: implications on its use during the novel coronavirus disease outbreak. Can J Anesth. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin HW, Yoo HN, Bae GE, et al. Comparison of oropharyngeal leak pressure and clinical performance of LMA ProSeal™ and i-gel® in adults: meta-analysis and systematic review. J Int Med Res. 2016;44:405–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng L, Qiu H, Wan L, et al. Intubation and ventilation amid the COVID-19 outbreak: Wuhan’s experience. Anesthesiology. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hohlrieder M, Tiefenthaler W, Klaus H, et al. Effect of total intravenous anaesthesia and balanced anaesthesia on the frequency of coughing during emergence from the anaesthesia. Br J Anaesth. 2007;99:587–591. [DOI] [PubMed] [Google Scholar]
- 34.Lee JS, Choi SH, Kang YR, Kim Y, Shim YH. Efficacy of a single dose of dexmedetomidine for cough suppression during anesthetic emergence: a randomized controlled trial. Can J Anaesth. 2015;62:392–398. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organisation. Rational use of personal protective equipment (PPE) for coronavirus disease (COVID-19). World Health Organization; Available at: https://apps.who.int/iris/bitstream/handle/10665/331498/WHO-2019-nCoV-IPCPPE_use-2020.2-eng.pdf. Published 2020. Accessed March 31, 2020. [Google Scholar]
- 36.APSF/ASA. APSF/ASA guidance on purposing anesthesia machines as ICU ventilators. APSF/ASA. Available at: https://www.asahq.org/in-the-spotlight/coronavirus-covid-19-information/purposing-anesthesia-machines-for-ventilators. Published 2020. Accessed March 31, 2020.
- 37.Dexter F, Parra MC, Brown JR, Loftus RW. Perioperative COVID-19 defense: an evidence-based approach for optimization of infection control and operating room management. Anesth Analg. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang, Xu H, Rebaza A, Sharma L, Dela Cruz CS. Protecting health-care workers from subclinical coronavirus infection. Lancet Respir Med. 2020;8:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anesthesiologist ASf. UPDATE: The use of personal protective equipment by anesthesia professionals during the COVID-19 pandemic | American Society of Anesthesiologists (ASA). American Society for Anesthesiologists; Available at: https://www.asahq.org/about-asa/newsroom/news-releases/2020/03/update-the-use-of-personal-protective-equipment-by-anesthesia-professionals-during-the-COVID-19-pandemic. Published 2020. Accessed March 31, 2020. [Google Scholar]
- 40.Feng S, Shen C, Xia N, Song W, Fan M, Cowling BJ. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radonovich LJ, Jr, Simberkoff MS, Bessesen MT, et al. ResPECT Investigators. N95 respirators vs medical masks for preventing influenza among health care personnel: a randomized clinical trial. JAMA. 2019;322:824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang S, Diao M, Yu W, Pei L, Lin Z, Chen D. Estimation of the reproductive number of novel coronavirus (COVID-19) and the probable outbreak size on the Diamond Princess cruise ship: a data-driven analysis. Int J Infect Dis. 2020;93:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kormuth KA, Lin K, Prussin AJ, II, et al. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity. J Infect Dis. 2018;218:739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


