Publisher Summary
The arboviruses constitute a set defined by the epidemiologic fact that they are transmitted between vertebrate hosts through the agency of biting, blood-sucking arthropods. The word arbovirus is an abbreviation for arthropod-borne virus of vertebrates; it defines a concept not related to the chemical, physical, or morphological properties of the virion. Arboviruses are viruses that are maintained in nature principally, or to an important extent, through biological transmission between susceptible vertebrate hosts by hematophagous arthropods. In the strict sense, the definition of arbovirus requires that the cycle of biological propagation from arthropod to vertebrate and back to arthropod be observed under controlled conditions as it occurs in nature. Properties of a virus that are not directly related to the transmission cycle, though helpful at times for orientation, should not be entertained as defining criteria. Easy isolation and propagation by intracerebral inoculation into newborn or adult mice or the inactivation of the virus by lipid solvents are properties shared with other viruses not remotely suspected of being arthropod-borne such as herpes and rabies.
I. Introduction
The arboviruses constitute a set defined by the epidemiologic fact, that they are transmitted between vertebrate hosts through the agency of biting, blood-sucking arthropods (Casals, 1957; WHO Study Group, 1967). Attempts have been made to incorporate them into universal systems of classification of viruses (Lwoff et al., 1962); the attempts have resulted at times in confusion arising, in part, from the use of the term, arbovirus, to designate both an epidemiologic set and a set in a system based on properties of the virion.
Efforts toward clearing this confusion were initiated at least as far back as 1966 (Casals, 1966). It has been all along evident that the arboviruses constitute a heterogeneous set when the properties of the virion and the activity of the virion at the cellular level are considered. Since in the various systems of viral classification advanced (Lwoff et al., 1962; Committee, 1965; Melnick and McCombs, 1966; Gibbs et al., 1966; Bellet, 1967) the properties of the virion are the only, or main, basis for classifying, it follows that the arboviruses cannot as an undivided body be incorporated in those systems. The solution was to dismantle the arbovirus set and redistribute its parts into the pertinent groups of the universal system.
The International Committee on Nomenclature of Viruses (Wildy et al., 1967) designated a Subcommittee on Viruses of Vertebrates which has made certain proposals (Andrewes, 1970) to end the confusion that had heretofore prevailed. These proposals are: to use the term arbovirus only as an epidemiologic designation; to distribute the arboviruses into the classes of the universal system to which they belong; and to create a name to designate the set of viruses that have RNA, cubic symmetry, and an envelope, regardless of whether they are or not arboviruses.
II. Arboviruses
A. DEFINITIONS
The word arbovirus is an abbreviation for “arthropod-borne virus of vertebrates”; it defines a concept not related to the chemical, physical, or morphological properties of the virion. That these properties may in the last resort determine all activities of a virus goes without saying; this, however, is irrelevant to the present definition.
The definition to which we suscribe (WHO Study Group, 1967) is: “arboviruses are viruses which are maintained in nature principally, or to an important extent, through biological transmission between susceptible vertebrate hosts by hematophagous arthropods.” In the natural cycle are involved, in addition to virus, vertebrate host, and vector, vertebrate reservoir and amplifier; possibly the vector acts in some instances also as reservoir.
Essential to the definition is the expression “biological transmission,” by which it is meant that a period of time, from 5 or 6 to 10 or 12 days, elapses between the moments when the vector becomes infected by biting a viremic host and when it can transmit the virus to a new vertebrate host. During this period, designated extrinsic incubation, the arthropod though infected cannot transmit the virus by bite; the virus multiplies in the tissues of the arthropod causing—with hardly an exception—no damage, ill effects or recognizable lesions; and finds its way to the salivary glands of the arthropod which can then transmit it by biting a new vertebrate host. Viremia ensues in the vertebrate setting up conditions for infection of a new arthropod and continuation of the cycle.
The definition excludes mechanical transmission—usually immediate—by an arthropod whose mouth parts have become contaminated on biting an infected host. It must not be concluded, however, that an arbovirus can be transmitted in nature only through biological transmission; infection of man by direct contact with patients, probably through droplet infection, or by ingestion of contaminated food are known to occur (Downs, 1970).
The recognized range of natural vectors has been so far confined to mosquitoes, ticks, Phlebotomus and Culicoides; mites, though on occasion suspected, have not been conclusively proved.
B. ANTIGENIC GROUPS
There are at present between 250 and 300 virus serotypes which with various degrees of legitimacy are assembled in the arbovirus set. The currently published, most comprehensive listing and description of these agents (Taylor, 1967) has 204 entries to which an additional 44 are being added (Berge, 1970, personal communication); another publication (WHO Study Group, 1967) lists, either by name or strain designation, 252 viruses some of which are not included in the former.
About 4/5 of the arboviruses are assembled in antigenic groups (Table I ), on the basis of serological overlaps (Casals, 1957; WHO Study Group, 1967); according to the established concept, all cross-reacting viruses constitute a group. Several groups have been bound in a supergroup owing to the fact that occasional viruses from a group reproducibly show low titered antigenic overlaps with viruses from another group (Whitman and Shope, 1962; Casals, 1963). Under a strict application of the definition of antigenic group all such groups and viruses thus related should be assembled in one single group. Practical considerations have, however, made it advisable thus far to maintain the separate groups as given in Table I.
TABLE I.
AN ANTIGENIC CLASSIFICATION OF ARBOVIRUSES
| Group | Number of viruses | Group | Number of viruses |
|---|---|---|---|
| A | 18 | Changuinola | 2 |
| African Horsesickness | 9 | Congo | 2 |
| Anopheles A | 3 | EHDD | 2 |
| Anopheles B | 2 | Ganjam | 2 |
| B | 39 | Hughes | 3 |
| Bakau | 2 | Kaisodi | 3 |
| Bluetongue of sheep | 12 | Kemerovo | 5 |
| Bunyamwera Supergroup | Mapputta | 2 | |
| Bunyamwera | 13 | Mossuril | 2 |
| Bwamba | 2 | Nyando | 2 |
| C | 11 | Phlebotomus Fever | 11 |
| California | 9 | Qalyub | 2 |
| Capim | 7 | Quaranfil | 2 |
| Guama | 6 | Timbo | 2 |
| Koongol | 2 | Turlock | 3 |
| Patois | 4 | Uukuniemi | 4 |
| Simbu | 15 | Vesicular stomatitis | 5 |
| Tete | 2 | Ungrouped | 43 |
| Unassigned | 1 | Tacaribea | 6 |
Evidence for an arthropod cycle in nature is scant for all the viruses in the group.
Number of viruses in the groups is in some instances, approximate. There are at this time well over 40 additional serotypes as yet incompletely identified and unreported, not included in the table.
Some groups include viruses for which there is little or no evidence that they are arboviruses. One of the groups, Tacaribe, is listed separately; while it has been assumed in the past that the viruses in the group may be arthropod-borne in nature or that they had a cycle, perhaps not the main one, involving an arthropod, current evidence seems not to support this assumption (see Section II,C,4).
C. CRITERIA USED IN PRACTICE FOR DEFINING AN ARBOVIRUS
In the strict sense the definition of arbovirus requires that the cycle of biological propagation from arthropod to vertebrate and back to arthropod be observed under controlled conditions as it occurs in nature.
Properties of a virus which are not directly related to the transmission cycle, though helpful at times for orientation, should not be entertained as defining criteria. Easy isolation and propagation by intracerebral inoculation into newborn or adult mice, or inactivation of the virus by lipid solvents are properties shared with other viruses not remotely suspected of being arthropod-borne, such as herpes and rabies. It is perhaps time even to ask critically whether antigenic relationship with well-established arboviruses is an acceptable norm.
Only properties relating to the transmission cycle should therefore be considered in attempting to determine whether a virus is an arbovirus. The amount of information available concerning the natural transmission of the arboviruses varies greatly from one to the other with the result that viruses are considered to be arthropod-borne with varying degrees of conformity with, or fulfillment of, the defining criteria (WHO Study Group, 1967; Downs, 1970). Even disregarding that the mere fact of investigating transmission as it occurs in nature may disrupt events, consequently transmission is no longer natural: it is a matter of record that complete observation and reproduction of the cycle, relatively undisturbed, in nature has been secured only in few instances, considering the large number of arboviruses accepted.
The degrees of information required to fulfill the criteria for definition of an arbovirus fall in three categories:
-
1.Criteria fulfilled:
-
a.Observation of the complete natural cycle achieved.
-
b.Observation of the natural cycle achieved in a less complete or uninterrupted manner; overwhelming epidemiological and laboratory evidence is available.
-
a.
-
2.Criteria less adequately fulfilled:
-
a.Artificial reproduction of the complete cycle in laboratory animals.
-
b.Artificial reproduction of parts of the cycle, usually coupled with detection of virus in animal tissues by inoculation of experimental hosts.
-
c.Serial propagation by experimental inoculation of blood-sucking arthropods.
-
a.
-
3.
Circumstances of isolation of the virus.
-
4.
Other criteria independent from association with arthropods.
Whatever experimental procedures or ecological considerations are employed, it is generally agreed that multiplication of the virus in an arthropod and its transmission by bite to a vertebrate are the minimum criteria necessary before it can be accepted as biologically arthropod-borne.
1.a. Three viruses or virus diseases fall in this class; urban yellow fever, sandfly fever, and dengue. While in the early studies the actual viral serotype is unknown, the observations with these three diseases remain as classical epidemiological examples that established a complete natural cycle. Undoubtedly the fact that they are diseases of man, one of them very serious, may account for early efforts made to solve the cycle for, by interrupting it, epidemics might be stopped. It must not be assumed from these examples, however, that human involvement is an essential feature of the arboviruses' ecology. Rather the opposite; infection of man is generally accidental, not essential to the perpetuation of the cycle and, moreover, usually leads to a deadend (Downs, 1970).
The natural cycle for urban yellow fever was definitely established by the work of the U.S. Yellow Fever Commission, in 1900-1901. Well-controlled observation showed that the disease was transmitted to healthy persons by mosquitoes, Aedes aegypti, that had fed on patients in the acute stage of their illness; these patients had contracted the disease through natural exposure. An interval of some 10–12 days—somewhat variable depending on circumstances—must elapse from the time a mosquito takes an infected blood meal to the moment when it can transmit it by bite to a well person, extrinsic incubation. Inoculation of blood taken from a patient during the first days of illness to a healthy person resulted in disease. Finally, it was later shown that control of Aedes aegypti prevented urban epidemics; this observation completed the case for the arthropod-vertebrate cycle with this illness.
A similar clear-cut proof of the natural cycle was observed (Doerr et al., 1909) with sandfly fever. Transmission by the bite of a midge, Phlebotomus papatasi, was authenticated through the use of human volunteers. Midges were fed during the first days of illness on patients who had acquired the disease through natural exposure in an endemic area; 7–10 days later the arthropod transmitted the disease, by bite, to human volunteers in an area far removed from the endemic zone.
The association between dengue fever and a vector, Aedes aegypti, was established (Siler et al., 1926) indicating the capacity of the mosquito to become infected by feeding on a patient in the acute stage of illness and its ability to transmit it from 10 to 11 days later, but not before, to a healthy volunteer.
1.b. There are a number of viruses for which the evidence that they are arthropod-borne in the natural infections that they cause is nearly as complete as that shown above. With these agents, the complete cycle in nature has not been observed in one continuous operation, mainly due to the fact that no such attempts (which are always difficult to carry out) were made, or found necessary, in view of other, overwhelming evidence that established them as arthropod-borne. With the agents in question the association in nature between a vector, the virus, and the disease in man or lower animals has been so well and repeatedly established; and the experimental transmission in the laboratory between vertebrate hosts by the vector, with a required extrinsic incubation, so well documented, that one must consider the natural cycle as adequately proved.
Within this category are included: Western equine encephalitis (WEE) and St. Louis encephalitis (SLE) and their vector, Culex tarsalis, in the western United States; Japanese encephalitis (JE) and Culex tritaeniorhynchus, in Japan; Russian tick-borne encephalitis of group B(RSSE) and the tick, Ixodes persulcatus, in the far eastern regions of the Soviet Union; Colorado tick fever (CTF) and Dermacentor andersoni in the Mountain States.
In this class may also be included, with in some instances less complete evidence, the following viruses and some of their associated vectors: Kyasanur Forest disease (KFD) and Haemaphysalis spinigera in Mysore, India; Nairobi sheep disease and Ripicephalus appendiculatus, in Kenya, Uganda; Murray Valley encephalitis (MVE) and Culex annulirostris in Australia; Rift Valley fever (RVF) and Eretmapodites chrysogaster, also Aedes caballus, in East Africa; West Nile (WN) and Culex univittatus in Egypt; Eastern equine encephalitis (EEE) and Culiseta melanura (in birds), in eastern United States; o'nyong-nyong (ONN) and Anopheles funestus, in eastern Africa; chikungunya and Aedes mosquitoes in Africa and Thailand.
A few additional viruses may also be included in the present category on the basis of good epidemiological association between virus, disease, and particular arthropods, even though studies on laboratory transmission may be lacking or unsatisfactory; the reason for the latter being the difficulty in colonizing the arthropod. The diseases are: African horsesickness and bluetongue of sheep which have as vectors Culicoides species; bovine ephemeral fever is also considered to have Culicoides as vectors. Recently (Plowright et al., 1969) evidence has been adduced to suggest that African swine fever may be a vector-transmitted disease, Ornithodoros moubata, in Tanzania.
2. A large proportion of the arboviruses are so considered with evidence less complete than that shown with the viruses in Section 1.; the criteria for definition have been less adequately fulfilled. Within this partial fulfillment there are various degrees.
2.a.b. With some viruses the evidence consists in the reproduction in the laboratory of an artificial transmission cycle between vertebrate hosts by means of an arthropod. Mosquitoes are infected by inoculation of a viral suspension, or by feeding on a viremic animal experimentally inoculated, or are wild-caught infected; after a sufficient number of days to allow for an extrinsic incubation, the arthropod is made to feed on a new animal which is then observed for signs of illness, viremia, or antibody development. The cycle is completed, at least once, by allowing new mosquitoes to feed on the vertebrate at the time it circulates virus and determining whether the mosquito is infected, either by further transmission by bite or by inoculation of its tissues into susceptible animals.
With some viruses the experimental evidence does not reproduce the complete cycle; thus, after feeding on an infected vertebrate host and allowing for time to elapse, the arthropod is tested for evidence of viral multiplication by inoculation into a host system. Or, conversely, an artificially infected arthropod is set upon a susceptible vertebrate host and the tissues of the latter tested for presence of virus.
The artificiality of the system consists in that the host employed is seldom the natural one, but often newborn mice; the mosquito may have no or little epidemiological significance in nature; and in interrupting the cycle and testing for virus by inoculation of tissue suspensions.
There are in this class at least 29 viruses and among them, Bunyamwera, California encephalitis, Ilheus, Mayaro, Middelburg, Nodamura, Oriboca, Semliki, Sindbis, vesicular stomatitis Indiana (VSV-I) and New Jersey (VSV-NJ), Wesselsbron, and Zika. All have also been isolated from wild-caught arthropods and eight or ten have, in addition, been propagated serially by mosquito inoculation.
2.c. With other viruses the evidence for their arboviral nature, while still pertaining to the transmission cycle, is more artificial than thus far considered; it consists on the observation that a virus can be maintained serially by parenteral inoculation of arthropods, usually mosquitoes. Inoculation is done intrathoracically and passage to the next arthropod is done a few days later either by grinding the entire mosquito or, better yet, its salivary glands only. Maintenance of a virus in this fashion for several consecutive passages is considered good evidence that it is an arbovirus. Strictly considered, this evidence is valid only if positive; a true arbovirus may fail to multiply in the wrong kind of arthropod.
Also included in this group are a few viruses which on inoculation to mosquitoes or fed to mosquitoes have been found to multiply and persist for some time in the body of the arthropod, determined by mouse inoculation.
At least 50 viruses have been proved to propagate by serial passage in mosquitoes and one in ticks; or have shown to multiply and persist. With few exceptions—8 or 10—all have also been isolated from wild-caught arthropods. Representative viruses are: Anopheles A and B, Apeu, Bussuquara, Bwamba, Cocal, Corriparta, epizootic hemorrhagic disease of deer (EHD-NJ), Eubenangee, Guaroa, Hart Park, Marituba, Melao, Oropouche, Quaranfil, Tacaiuma, Uganda S, Venkatapuran, Witwatersrand, and Wyeomyia.
3. With a large number of viruses, over 100, the only criterion at the moment available for consideration as arboviruses is circumstances of isolation. Viruses are classed as arboviruses that have been isolated, some once, others repeatedly, from wild-caught mosquitoes, ticks, midges, or other blood-sucking arthropods which when ground up and inoculated into susceptible hosts had digested their last blood meal. Viruses isolated from sentinel animals exposed in such a manner that their only likely source of infection are hematophagous arthropods are provisionally considered or suspected of being arboviruses.
4. In another category, viruses are included as arboviruses solely on the grounds of an antigenic relationship with established arboviruses. From the total number, 240, considered in this analysis, 26 distinct serotypes belong in this category; in addition, there are 6 to 8 viruses of the antigenic group Tacaribe that deserve special consideration.
Attempts to passage or detect multiplication of the virus in arthropods have been reported as negative with some: bat salivary gland and Modoc viruses of group B and Candiru and Icoaraci of phlebotomus fever group. No reported attempts to test for arthropod susceptibility are available with 22 among which are included seven group B agents, four from Simbu group, and three from phlebotomus fever group.
An antigenic group, Tacaribe, presents special problems. It includes the etiological agents of several important diseases of man: Junin (Argentinian hemorrhagic fever), Machupo (Bolivian hemorrhagic fever), and Lassa (Lassa fever) viruses. In addition (Rowe et al., 1970b), a virus not known to have an arthropod cycle in nature, lymphocytic choriomeningitis, is antigenically part of the group; as are other agents not known to be associated with human disease, Amapari, Pichinde, Tacaribe, Tamiami, and two other viruses isolated in South America, as yet incompletely characterized (Johnson, personal communication, 1970). Junin virus has been isolated from mites on a few occasions (Mettler, 1969); Tacaribe from mosquitoes once (Downs et al., 1963); and Amapari on a few occasions from mites taken off rodents that were aviremic at the time (Woodall, personal communication, 1968). With these exceptions, there is nothing in the epidemiology of the diseases that indicates arthropod transmission to man or between lower animals; determined efforts to isolate Machupo virus from arthropods in the course of a large epidemic failed (Kuns, 1965), while its presence in the urine of chronically infected rodents points to a different mode of transmission than by the bite of an arthropod (Johnson, 1965). In view of the importance of the human diseases involved, inclusion of this antigenic group with the arboviruses is of more than passing importance and should no longer be done unless there is definite evidence in its favor. As it now stands the evidence fails to support an arthropod cycle in the maintenance of the viruses, nor do they appear to fit the definition of an arbovirus by any of the currently accepted criteria.
Two viruses, Kern Canyon and Lagos bat are, so far, antigenically unrelated to any established arboviruses; attempts to passage them in mosquitoes have failed. These agents fulfill none of the requirements for consideration as arboviruses and should, therefore, be excluded from the set, until otherwise established.
The existence of viruses antigenically related to proved arboviruses but which are themselves not able to propagate in arthropods, raises a doubt with respect to the adequacy of antigenic relatedness as a criterion for inclusion; the solution is to accept the fact that nonarboviruses can be related to arboviruses. In this connection Baker's comments (1943) on the evolution of the arthropod-borne viruses may be relevant; successive steps in the evolution may lead from an originally latent virus of an arthropod to a virus that has a primary arthropod-vertebrate cycle, then to another with a side, secondary arthropod-vertebrate cycle and, finally, to a virus with a vertebrate-vertebrate propagation. In the latter case the virus would eventually lose its potential to multiply in arthropod vectors. There are instances in which arthropod-borne viruses can maintain themselves, in limited conditions, in nature in the absence of an arthropod vector, for example, EEE in pheasants (Holden, 1955); VEE in man (Briceño-Rossi, 1964), and group B tick-borne encephalitis (CETB) virus between cow and man by the ingestion of contaminated milk (Blaskovic, 1967).
III. Properties of the Virions
In systems of universal viral classification currently under consideration the properties of the virion are the criteria used for establishing different taxons; those of the arboviruses are analyzed in this section.
Since arboviruses are defined on epidemiological-ecological grounds, there is no a priori reason why the agents included in the set should all be alike in other respects, for example, in the properties of their virions; nor why nonarboviruses could not share basic properties with arboviruses.
In the following analysis, African horsesickness and bluetongue viruses are each considered as one virus, disregarding their numerous antigenic types; tick-borne virus of group B (Russian spring-summer encephalitis and central European tick-borne encephalitis) is also considered as one agent. Viruses of the antigenic group Tacaribe are included even though there are reasons (see Section II,C,4) for excluding them from the arbovirus set. The total number of viruses considered is 240.
A. NUCLEIC ACID TYPE
Nucleic acid examination has been reported in at least 40 arboviruses; in a number, infectious nucleic acid has been extracted and the type determined by the effect of the corresponding nucleases; in others the type is inferred from the effect of bromodeoxyuridine (BUDR) on viral multiplication in cell cultures.
All these viruses contain RNA with the exception of African swine fever, from which infectious DNA has been extracted (Plowright et al., 1966). Infectious RNA has been extracted from at least the following 16 viruses: bluetongue, CETB, chikungunya, dengue 1, dengue 2, EEE, JBE, MVE, Semliki, Sindbis, SLE, VEE, VSV-I, WEE, West Nile, and yellow fever.
In another 23 viruses the presence of RNA has been deduced from the lack of inhibition by BUDR of virus multiplication in cell cultures. The viruses are: African horsesickness, Cocal, CTF, Congo (Crimean hemorrhagic fever), Corriparta, bovine ephemeral fever, Gamboa, Guaroa, Juan Diaz, Junin, Kemerovo, Lipovnik, Lone Star, Louping ill, Machupo, Matucare, Mayaro, Mermet, Omsk hemorrhagic fever, Tamiami, Tribec, Uukuniemi, and VSV-NJ.
B. MORPHOLOGY
Considerable advances have been accomplished in this sector during the last 3 or 4 years (see Casals, 1966; WHO Study Group, 1967). Electron microscopy of infected cells in thin sections and negative contrast staining of sedimented viral suspensions has been extended to arboviruses of several antigenic groups as well as to ungrouped ones. As a consequence several patterns are now discerned in these viruses with respect to symmetry of capsid, shape, size, envelope, and morphogenesis. The emergence of these patterns amply documents the claim (Casals, 1966; WHO Study Group, 1967) that the arboviruses are a mixed set that does not fit as a whole in any of the taxons of the proposed systems of classification based on properties of the virion.
Even with these recent contributions, data are not available on the large majority of arboviruses. Results of electron microscopy have been reported in 63 of the 240 viruses considered in this survey; in 20, a statement is made on the type of symmetry as well as giving information on size, shape, envelope, and site of development and maturation of the viron. The reports on the remaining 43 agents fail to state type of symmetry or the statement is to the effect that none was discernible; the description of these viruses is limited to the other morphological characteristics.
1. Symmetry
The capsid's symmetry has been clearly stated in 15 viruses; stated less definitely in 4; and hinted at in one instance.
Clearly, definitely stated symmetries are: cubic, in African horsesickness (Breese et al., 1969), African swine fever, bluetongue, Eubenangee, Kemerovo, and Sindbis viruses; helical, in Batai, Inkoo, and Uukuniemi; helical-complex, in Cocal, bovine ephemeral fever, Hart Park-Flanders, Kern Canyon, VSV-I, and VSV-NJ viruses.
The type of symmetry has been stated less categorically but, it appears, still convincingly in four viruses: Chenuda, CTF (Murphy et al., 1968a), and Middelburg and Semliki (Simpson and Hauser, 1968); these four viruses show cubic symmetry.
Observations with Powassan virus (Abdelwahar et al., 1964) showed under the envelope a surface geometric arrangement compatible with cubic symmetry.
With an additional virus, VEE, it was reported (Klimenko et al., 1965) that helical structures presumably representing tightly packed nucleoprotein components of the virus were seen; no claim was made on symmetry.
2. Other Morphological Characteristics
Morphological details other than symmetry have been detected in 43 additional arboviruses: shape, size, envelope, and density or lightness of different areas—core or halo. These details were also noted with the viruses in the preceeding subsection (Section III,B,1).
a. Shape. The virion's shape is given for nearly all the viruses examined by electron microscopy.
The six viruses with helical-complex symmetry are bullet-shaped, stubby rods with a round and a blunt end. The 11 viruses with cubic symmetry are reported as being round, polygonal or, more precisely, icosahedral in shape.
The shape of 41 viruses, including those with helical symmetry, is described by one, or several, of the following words: round, oval, spherical, or polygonal. Two viruses, Machupo and Tacaribe (Murphy et al., 1969), are reported to be round, oval, and pleomorphic.
b. Size. Reported size determinations by electron microscopy show that the arboviruses are heterogeneous (Table II ). The viruses with small diameter, between 30 and 40 nm, all belong thus far in group B. The distribution of other grouped viruses in size categories does not correspond precisely with their antigenic groups. Inconsistencies in reported sizes may, in part, be due to differences in technique; also, some reports give by preference the internal diameter of enveloped viruses (Bastardo et al., 1968; Bergold et al., 1969).
TABLE II.
SIZE OF ARBOVIRUSES DETERMINED BY ELECTRON MICROSCOPY
| Virus | Diameter, or width × length (nm) |
|---|---|
| African swine fever | 200 |
| Machupo, Tacaribea | 60–260; mean 110 |
| Bunyamwera, California, Guaroa, Gumbo Limbo, Keystone, La Crosse, Lone Star, Marituba, San Angelo, Shark River, Uukuniemi | 90–100 (Shark River, 104) |
| Chenuda, Colorado tick fever | 80 |
| African horsesickness, Eubenangee, Kemerovo, Middelburg, Rift Valley fever, Semliki, Sindbis, Tribec, VEE | 60–80 (AHS, also 94; Eubenangee, enveloped, 101) |
| Nodamura | 55–75 |
| Aura, Batai, bluetongue, Cache Valley, chikungunya, EEE, Getah, Inkoo (Tahyna-like), Kairi, Manzanilla, Melao, Oriboca, Pacui, Restan, WEE, Wesselsbron, Whataroa, Wyeomyia | 45–58 |
| Mayaro | 40 |
| Dengue 1, dengue 2, CETB-RSSE, Cowbone ridge, JBE, Langat, MVE, Omsk HF, Powassan, SLE, West Nile, yellow fever | 30–40 (a few extremes 17–50) |
| Bullet-shaped: bovine ephemeral fever, Cocal, Hart Park, Kern Canyon, VSV-I, VSV-NJ | 60–73 × 120–220 |
See footnote Table I.
In addition to the more precise determinations by electron microscopy, estimates have been reported on the size of other arboviruses based on their filterability through commercially available graded membranes. The diameter of these viruses, on the assumption that they are spherical, is considered to be between the average pore diameter (APD) of a membrane that allows passage with no loss of infectivity and that of the next, tighter membrane that substantially reduces infectivity; a correction factor gives the estimated size range (Casals, 1968). The estimates between relatively wide margins for a number of viruses are: Mt. Elgon bat, between membranes of APD 450 and 220 nm; Bahig, Bhanja, Bwamba, Caraparu, Congo, Gamboa, Ganjam, Grand Arbaud, Junin, Kaisodi, Mapputta, Murutucu, Quaranfil, Silverwater, Tensaw, Thogoto, and Wad Medani, between 220 and 100 nm; Corriparta, Koongol, Matucare, and Ntaya, between 100 and 50 nm.
Other size estimates, also by filtration, have been given for additional viruses: Zika, between 18 and 25 nm; Chagres, between 55 and 110; and Turlock, between 120 and 128 nm.
c. Envelope. The presence or absence of an envelope surrounding the capsid has been settled with those arboviruses whose symmetry has been determined (see Section III,B,1) and with nearly all in which electron microscopy showed the shape, if not the structure, of the viral particle. As with respect to other properties of the virion, the arboviruses fall in distinct categories.
Statements that an envelope was present have been made for 46 viruses of the 59 examined, including viruses from several antigenic groups and ungrouped ones. That no envelope was visible has been reported for: African horsesickness, bluetongue, and Rift Valley fever viruses. It has also been stated that, on the whole, Chenuda, CTF, Eubenangee, and Kemerovo viruses had no envelopes, although an occasional extracellular enveloped virion could be detected. Finally, no statements are made for 6 more viruses, dengue 1, dengue 2, Lone Star, MVE, Tribec, and Wesselsbron; presumably, the ones belonging in group B are enveloped.
d. Morphogenesis; Maturation. Electron microscopy of thin sections of infected cell cultures or mouse brain tissue reveals a diversity in the details of development and maturation of arboviruses.
Based on available descriptions on about 35 viruses there appears to be a common property: all replicate in the cytoplasm; beyond this several distinct patterns have been observed.
Group A nucleoids appear to form at cytoplasmic membranes, sometimes are scattered throughout the cytoplasm; crystalline arrays have been observed. The nucleoids migrate to the plasma membrane where complete virions are formed by budding and extrusion into intercellular spaces (Morgan et al., 1961; Acheson and Tamm, 1967; Lascano et al., 1969).
Viruses of group B appear to replicate and bud almost exclusively on thickened membranes of cytoplasmic organelles which envelope the viral particle; these accumulate in the lumina of the distended endoplasmic reticulum membranes and are released into intercellular spaces either by migration or cell disruption (Ota, 1965; Murphy et al., 1968b; Filshie and Rehacek, 1968).
Several members of the Bunyamwera and California groups have been examined (Southam et al., 1964; Murphy et al., 1968c,d) and observed to have similar morphology and mode of maturation. The virion matures by budding from cytoplasmic membranes into cisternae or vacuoles, predominantly in the golgi area; no budding is prominent at the cell surface membrane. Virus release is by cell disruption or through transport by migration of the vacuole to the cell margin, fusion, and expulsion.
Certain viruses, CTF and Chenuda, share some developmental characteristics which are similar to those of reoviruses (Murphy et al., 1968a). The virus particles are associated with intracytoplasmic granular matrices, and with arrays of intracytoplasmic filaments and kinky threads; these formations are distributed throughout the cytoplasm. The virus is, for quite a while, almost exclusively intracellular; later, it is released by cell disruption. No budding was observed, although a few enveloped particles could be seen in vacuoles; there were no crystalline arrays.
The arboviruses with bullet shapes characteristically mature at marginal cytoplasmic membranes and bud through the cell membrane to accumulate extracellularly (Howatson and Whitmore, 1962).
C. LIPID SOLVENT SUSCEPTIBILITY
Andrewes and Horstmann (1949) proposed that the susceptibility of viruses to the action of lipid solvents be used as a criterion for classification. Ethyl ether and sodium deoxycholate (SDC) and, to a lesser extent, chloroform have been used (Theiler, 1957; Sunaga et al., 1960; Feldman and Wang, 1961). Inactivation of viral infectivity by these chemicals indicates the presence of essential lipids in the virion and is generally accepted as equivalent to possession of an envelope by the virus particle.
Determination of the action of these chemicals on the virus is technically simple; a large proportion of the arboviruses have been studied. Of the 240 viruses surveyed, 191 have been tested; 44 of the remaining belong in antigenic groups most of whose members have been tried and it is assumed that they would behave like their group mates. Information is lacking in 5 ungrouped viruses.
The majority of the viruses have been tested with SDC at 0.1% final dilution, others at a dilution of 0.5%. It has been shown (Sunaga et al., 1960) that the effect of SDC is dependent on concentration; also that chloroform has a stronger inactivating effect than either ether of SDC. The effects of the latter are considered similar.
The dosage-dependent effect of SDC may lead to contradictory conclusions concerning the resistance of a virus; resistance ought to be stated in connection with the concentration of chemical used. A clear demonstration of differing degrees of susceptibility is given in Table III , based on tests carried out in our laboratory. The procedure used in all cases was to incubate at 37°C mixtures of equal volumes of a virus suspension, 10−2, and of an adequate dilution of SDC; the residual virus was titrated by the intracerebral route of inoculation in newborn mice. The amount of inactivated virus by comparison to that present in the control suspension is expressed in dex (Haldane, 1960).
TABLE III.
INACTIVATION OF SELECTED VIRUSES BY SODIUM DEOXYCHOLATE
| Sodium deoxycholate (%)a |
||
|---|---|---|
| Virus, strain | 0.5 | 0.1 |
| Polioencephalitis, mouse (GD7) | –0.4 | |
| Encephalomyocarditis, mouse (CDC) | –0.3 | |
| Reovirus 3 (hepatoencephalitis, mouse) | 0.8 | |
| Chenuda (Eg Ar 1152) | +4.6 | –0.4 |
| Colorado tick fever | +4.5 | 0.9 |
| EHD of deer, New Jersey | 4.1 | 0.1 |
| Kemerovo (R10) | +4.5 | –0.5 |
| Sindbis (Eg Ar 339) | + 5.2 | 2.2 |
| Bunyamwera (Smithburn) | +4.7 | 4.7 |
| Junin (XJ) | +4.0 | +4.0 |
| Nyamanini (Eg Ar 1304) | +4.4 | +4.3 |
| Oriboca (Be An 17) | 5.1 | 5.4 |
| Uukuniemi (S-21) | +2.6 | +3.6 |
| West Nile (Eg 101) | +7.0 | |
Dex of virus inactivated; –, indicates that treatment by SDC increased the virus titer; +, indicates that no end-point of inactivation was reached.
As shown in Table III, mouse polioencephalitis and encephalomyocarditis viruses, picornaviruses, and mouse hepatoencephalitis virus, reovirus type 3, are resistant to the action of 0.5% SDC. Some arboviruses, Chenuda, CTF, EHD-NJ, and Kemerovo are inactivated by 0.5% SDC but resist a 0.1% solution; the remaining listed viruses are inactivated by SDC diluted 0.1%. It appears, therefore, that on the basis of the effect of SDC viruses should, properly, be classified as resistant, partially resistant, and susceptible.
All the 191 arboviruses tested are reported to be susceptible to the action of one or more of the chemicals, usually SDC, with the following exceptions: Nodamura virus, tested in the form of a suspension of infected mouse brain tissue, is resistant (Scherer, 1968); African horsesickness and bluetongue of sheep are resistant or partially resistant (Howell, 1962; Studdert, 1965); Chenuda, CTF, EHD-NJ, and Kemerovo (Table III; also Borden et al., 1971), Corriparta (Carley and Standfast, 1969), Eubenangee (Schnagl et al., 1969), and Tribec are partially resistant.
IV. Arboviruses in a General System of Classification
A. CRITERIA FOR SYSTEMS OF CLASSIFICATION
Systems for differentiation of major groups of viruses and classification are, essentially, particle oriented. An International Committee on Nomenclature of Viruses (1963) recommended that a system be established with a hierarchy of criteria in which were considered: nucleic acid type, symmetry of capsid, presence of envelope, and size. Subdivisions within the major groups were to be made on cytopathological, immunological, biochemical, and other criteria. In general, most of the systems proposed (see Chapter 1, by Dr. Lwoff; also Section I), have accepted these criteria at least in part.
The principle on which these systems are based has been adversely criticized on the grounds that since the visible characteristics of the virion are a phenotypic expression they are less important than knowledge of the genotype. A system based on properties of the virion may not represent phylogeny, it assumes an unproved sequence of values or hierarchy, creates artificial groups with no valid relationships discernible among them (Cooper, 1967; Gibbs et al., 1966).
While recognizing the possible flaws of currently proposed systems they are, nevertheless, the only ones available; an attempt will be made in the next section (see Section IV,B) to fit the arboviruses in a virion-based general system.
B. INCORPORATION OF THE ARBOVIRUSES IN A UNIVERSAL SYSTEM
Analysis of the virion's properties (Section III) makes it abundantly clear that the arboviruses are heterogeneous. Any attempt to fit them into generally accepted taxonomic groups of a universal system requires that the arbovirus set be disassembled and the resulting subsets or individual viruses be then placed in the corresponding taxonomic groups. One such attempt is represented schematically in Fig. 1 and given in some detail in Table IV .*
FIG. 1.
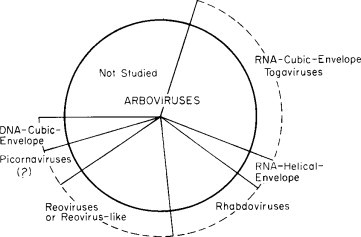
Distribution of the arboviruses among groups of a universal system of viral classification.
TABLE IV.
INCORPORATION OF THE ARBOVIRUSES IN A UNIVERSAL SYSTEM OF CLASSIFICATION
| Arboviruses |
Togaviruses (RNA-cubic-enveloped) |
||
|---|---|---|---|
| Not togaviruses | Togaviruses | Arboviruses | Not arboviruses |
| African horsesickness | Group A | Antigenically, group A | Antigenically, group Ba |
| Bluetongue | Sindbis | Sindbis | Modocc |
| Kemerovo | Middelburgb | Middelburg | Bat salivary glandc |
| Tribec | Semlikib | Semliki | |
| Colorado tick fever | Other (β) | Other | Antigenically, Tacaribe group: |
| Chenuda (? NA) | Arenoviruses | ||
| Corriparta (? NA) | LCMc | ||
| Other | Group B | Antigenically, group B | Machupoc |
| RNA-cubic-naked | Tick-borne enc.b | Tick-borne enc. | Tacaribec |
| Reovirus, reoviruslike; diplornavirus | Omsk HFb | Omsk HF | Lassac |
| St. Louisb | St. Louis | Otherc | |
| Nodamura (cubic ?) | Powassanc | Powassan | |
| RNA-cubic-naked | Other (β) | Other (β) | Antigenically, other arbovirus groups |
| Picornavirus | ? | ||
| Other groups | Other antigenic groups | ||
| Batai (?NA) | ? | Some arboviruses | |
| Inkoo (?NA) | Some not arboviruses | Rubella | |
| Uukuniemi (RNA) RNA-helical-enveloped Myxoviruslike | Ungrouped ? | Ungrouped Some arboviruses Some not arboviruses | Other RNA-cubic-enveloped (?) or some of the RNA-undetermined symmetry-enveloped (?) |
| Cocal | |||
| VSV-NJ | |||
| VSV-I | |||
| Bovine ephemeral fever | |||
| Hart Park (? NA) | |||
| Other | |||
| RNA-helical: complex-enveloped | |||
| Rhabdovirus | |||
| African swine fever | |||
| DNA-cubic-enveloped | |||
| Iridovirus | |||
| Cotia | |||
| Poxviruslike | |||
Serial propagation by inoculation in mosquitoes unsuccessfully attempted with Modoc and bat salivary gland viruses. There are at least 6 additional viruses in antigenic group B which may be togaviruses for which there is no proof of transmission by arthropods in nature nor recorded attempts to serial passage in the laboratory.
Likely to be a togavirus; however, no categorical statement on its symmetry available.
No evidence that it is arthropod-borne; possibly a togavirus but there is no knowledge on its symmetry.
Figure 1, not drawn to scale, is given in order to show that arboviruses belong in different taxons in a classification based on properties of the virion; these taxons in turn comprise viruses that are not arboviruses. The designation “togavirus” was suggested by the Study Group as a temporary expedient to prevent the confusion arising from the use of a word, arbovirus, to designate both an epidemiological set (arthropod-borne viruses of vertebrates) and a taxon in the general system (viruses with RNA-cubic or presumed cubic symmetry-envelope). As the figure also shows, there are arboviruses that cannot be classified for lack of available information.
In Table IV an effort is made to present a synthesis even though much information is still not at hand. Considering first the known or accepted arboviruses (left half of the table) they fall into the following divisions.
1. Arboviruses that are not togaviruses (RNA-cubic-enveloped) but fit in other established or proposed taxons:
a. Reovirus, reoviruslike (Borden et al., 1971), diplornavirus (Verwoerd, 1969). Absence of an envelope, resistance or partial resistance to SDC and characteristic virion structure place the listed viruses, probably additional ones not listed for lack of information, in this group. Chenuda and CTF viruses have, in addition, some features in their development and maturation that are similar to those of reoviruses (Murphy et al., 1968a).
b. Picornavirus. Nodamura virus has been maintained serially in mosquitoes by parenteral inoculation and transmitted by bite; is resistant to ether and SDC, contains RNA, and is relatively small (Scherer, 1968). Since, however, there are no published reports on the type of its symmetry, inclusion in the picornaviruses is tentative.
c. Myxoviruslike. The viruses in this set, Batai, Inkoo, and Uukuniemi have helical symmetry (von Bonsdorff et al., 1969); the aspect of the helix differs from that of the myxoviruses. They have, presumably, RNA although no report on the type of nucleic acid is available for all.
d. Rhabdovirus. Vesicular stomatitis viruses (New Jersey and Indiana serotypes), Cocal, Hart Park, and, probably, a few others are bullet-shaped, enveloped viruses containing RNA and showing a complex structure with an internal helix characteristic of the rhabdoviruses. The criteria for considering bovine ephemeral fever an arbovirus are not totally fulfilled; however, the epidemiological circumstances justify inclusion in the set.
e. Iridovirus. This name had been suggested (Committee, 1965) to designate large DNA viruses, with an envelope and cubic symmetry of the compound type; to this description answer tipula iridescent and African swine fever viruses (Almeida et al., 1967). It may be premature to consider African swine fever virus an arbovirus; there is, however, good epidemiological evidence to this effect, including mention of transmission by the bite of a tick, Ornithodoros erraticus (Plowright et al., 1969).
f. Poxviruslike. A virus, Cotia, isolated repeatedly from wild-caught mosquitoes has been observed to have a virion morphology similar to the poxviruses (Borden, Shope, and Murphy, personal communication, 1970).
2. Arboviruses that are togaviruses. This is a set that has been usually referred to as the “typical or true” arboviruses. As indicated in Table IV, lack of definite information on capsid's symmetry prevents at this time inclusion in this set of many arboviruses that, presumably, will in time be included. Sindbis virus is definitely a member of this taxon (Horzinek and Mussgay, 1969); so are, most likely, Middelburg and Semliki viruses (Simpson and Houser, 1968) and, probably, the listed viruses of antigenic group B. At the moment it is not justified to assign other arboviruses, grouped or ungrouped, to this taxon for lack of knowledge of capsid symmetry.
Having distributed a number of arboviruses in accepted divisions of a general system and having placed others in a taxon, togavirus, heretofore recognized but unnamed, it remains to see whether viruses exist which answer the general description of togaviruses but are not arboviruses. (This analysis appears on the right half of Table IV.)
3. Togaviruses that are arboviruses. This set should be, of course, an exact duplicate of the converse, i.e., arboviruses that are togaviruses. If electron microscopy reveals in the future that the symmetry of the listed viruses of antigenic groups A and B, as well as of other unlisted grouped or ungrouped viruses is of cubic type, the viruses so characterized being arboviruses will also be included in this set.
4. Togaviruses that are not arboviruses. This is a particularly difficult set to consider due to lack of information on virion properties, particularly capsid symmetry, and on the natural epidemiological cycle of the agents involved.
The two viruses of antigenic group B listed, Modoc and bat salivary gland, have not been isolated from wild-caught arthropods; attempts to passage them by parenteral inoculation of mosquitoes have failed (Whitman, personal communication, 1969); nor are there any epidemiological associations that favor the view that they have an arthropod cycle in nature. There is no evidence by any of the criteria used that these two viruses are arboviruses. There are 6 additional viruses, antigenically belonging in group B, for which there is a similar lack of evidence that they are arthropod-borne; no reported information is available as to whether attempts have been made to passage them by parenteral inoculation in arthropods. The viruses are: Cowbone ridge, Dakar bat, Entebbe bat, Israel turkey meningoencephalitis, MML, and Negishi.
It cannot be stated whether Modoc, bat salivary gland, and the other viruses are togaviruses. Their nucleic acid type has not been reported, nor is there knowledge about their morphology; should these viruses by analogy to other group B agents be considered or proved to be togaviruses, they might well be examples of togaviruses that are not arboviruses.
The viruses of antigenic group Tacaribe are included in a newly defined taxon for which the name arenoviruses has been proposed (Rowe et al., 1970a). The arenoviruses have antigens in common, contain RNA, are susceptible to lipid solvents, and have an envelope; no capsid symmetry has been discerned. If future investigations were to reveal cubic symmetry a decision would be needed concerning the position of the arenoviruses with respect to the larger, inclusive taxon of the togaviruses. The lack of convincing evidence that the viruses of the Tacaribe group are arthropod-borne has been reviewed (see Section II,C,4).
Rubella virus appears to be a togavirus that is not arthropod-borne. It contains RNA; by electron microscopy it shows an electron-dense core surrounded by a clear halo, interpreted as an envelope; it develops by budding through the cytoplasmic membrane or sometimes into cytoplasmic vacuoles; its diameter is 60 nm and it is susceptible to ether and SDC (Holmes and Warburton, 1967; Holmes et al., 1969). Recent studies (Mussgay, personal communication, 1970) indicate that rubella virus has an isometrical core strongly suggesting icosahedral symmetry. There is no evidence that rubella virus is arthropod-borne; nor has it been found antigenically related to any of the arboviruses (Mettler et al., 1968).
The position in the present scheme of classification of nonarthropod-borne viruses that have RNA, an envelope, and undetermined type of symmetry, perhaps even no symmetry, is much too vague in all respects to warrant a discussion.
V. Conclusions
There are various reasons why it may be premature to try to incorporate such a large and heterogeneous group, the arboviruses, into a general system of viral classification. As the preceeding sections have shown there is on the whole scant information on the properties of their virion; in hardly 10% of the arboviruses is there complete knowledge of the type of nucleic acid, capsid symmetry, and envelope, properties whose knowledge is indispensable for current viral taxonomy.
Furthermore, a system of classification has not yet been fully agreed upon; while, in general, a system based only on properties of the virion is accepted, there is no complete agreement concerning the divisions and subdivisions in the scheme, nor on the proper value to assign to each of the virion's properties in the hierarchy.
In the group consisting of viruses with RNA, cubic symmetry, and no envelope there are two main divisions, picornaviruses and reoviruses. On the basis of recent work with African horsesickness and bluetongue viruses (Verwoerd, 1969; Breese et al., 1969) and with Chenuda, Colorado tick fever, Kemerovo, and other viruses (Borden et al., 1971) it has been proposed that new taxons be recognized, diplornavirus and reoviruslike. If these proposals are found acceptable, should there be or not an all encompassing label for all the viruses that have these three basic properties, RNA, cubic symmetry, and no envelope: picornaviruses (including enteroviruses and rhinoviruses), reoviruses, reoviruslike viruses, and diplorna-viruses?
Still more complex and, seemingly, more unresolved is the situation with RNA, helical (or helical component) symmetry, enveloped viruses (Waterson and Almeida, 1966). Agents so defined are a mixed collection for which there is no overall designation; the term myxovirus, obviously, does not apply to the collection, at least as the term was first used (Andrewes et al., 1955). Possibly an overall designation is meaningless except as a shortcut for “RNA-helical-enveloped.”
The morphology and other properties of certain arboviruses, Batai, Inkoo, and Uukuniemi seem to place them in the RNA-helical-enveloped collection; these viruses are distinct from myxoviruses (von Bonsdorff et al., 1969) and probably from other similar agents. The taxonomic problem arising here is whether these arboviruses should be assigned to a new taxon in the RNA-helical-enveloped collection, on a par with myxoviruses, paramyxoviruses, coronaviruses, and rhabdoviruses.
Similar complications may appear in the set of viruses defined by RNA, cubic symmetry, and envelope, to which the designation togavirus has been provisionally given. In this taxon are now included, or proposed, arboviruses from groups A and B and a nonarbovirus, rubella virus. Arboviruses of the Bunyamwera supergroup differ sufficiently in morphology from those in groups A and B so that even if some were shown to possess cubic symmetry, they might not be placed in the togavirus group, unless the latter is interpreted as equivalent to RNA-cubic-enveloped with no regard to other characteristics. A similar comment could be made with respect to the arenoviruses (Rowe et al., 1970a); if they were resolved into cubic symmetry a decision would be needed as to whether they are a subdivision of the togaviruses or a taxon of equal rank with them.
The problem to solve is whether an all-inclusive group, RNA-cubic-enveloped, serves any meaningful purpose in a general system or should taxons be defined by the above plus other properties—size, number of capsomeres, presence of inclusionlike bodies in the core; togavirus would then be used in the more restricted sense.
Difficulties of detail inherent in an attempt to incorporate the arboviruses in a general system of classification, therefore, exist and derive mainly from lack of knowledge of the virion's properties and lack of a well-systematized, generally accepted scheme of taxonomy. There is little doubt, however, that the following applies to the classification of the arboviruses:
-
1.
The term arbovirus designates an epidemiological concept which is irrelevant to the criteria on which current general systems of viral classification are based; the term arbovirus should not appear in these systems of classification
-
2.
The set of the arboviruses cannot be incorporated as a whole into a division of the universal system; the set must be disassembled and individual viruses, or antigenically related ones, should be distributed among the pertinent taxons of the system.
-
3.
A taxon defined by virions with RNA, cubic symmetry, and envelope is recognized in the general systems. Some arboviruses, at the moment mainly from antigenic groups A and B, belong in the taxon; it appears that viruses that are not arthropod-borne also belong in it.
ACKNOWLEDGMENT
The author gratefully acknowledges the helpful advice and constructive criticism given by Dr. Wilbur G. Downs and Dr. Robert E. Shope; the latter, in addition, supplied me with much information as yet unpublished.
Footnotes
Figure 1 and Table IV, as well as the discussion that follows, represent in a general manner, if not in detail, the substance of a report by an ad hoc Study Group for the Arboviruses to Dr. C. H. Andrewes, Chairman, Vertebrate Virus Subcommittee, International Committee on Nomenclature of Viruses (Andrewes, personal communication, 1969). The Study Group consisted of: Dr. J. S. Porterfield, Chairman, Dr. J. Casals, Prof. M. P. Chumakov, Dr. Claude Hannoun, and Prof. M. Mussgay.
REFERENCES
- Abdelwahar K.S.E., Almeida J.D., Doane F.W., McLean D.M. Can. Med. Ass. J. 1964;90:1068. [PMC free article] [PubMed] [Google Scholar]
- Acheson N.H., Tamm I. Virology. 1967;32:128. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- Almeida J.D., Waterson A.P., Plowright W. Arch. Gesamte Virusforsch. 1967;20:392. doi: 10.1007/BF01241958. [DOI] [PubMed] [Google Scholar]
- Andrewes C.H. Virology. 1970;40:1070. [Google Scholar]
- Andrewes C.H., Horstmann D.M. J. Gen. Microbiol. 1949;3:290. doi: 10.1099/00221287-3-2-290. [DOI] [PubMed] [Google Scholar]
- Andrewes C.H., Bang F.B., Burnet F.M. Virology. 1955;1:176. doi: 10.1016/0042-6822(55)90014-3. [DOI] [PubMed] [Google Scholar]
- Baker A.C. Amer. J. Trop. Med. 1943;23:559. [Google Scholar]
- Bastardo J.W., Bergold G.H., Munz K. Amer. J. Trop. Med. Hyg. 1968;17:115. doi: 10.4269/ajtmh.1968.17.115. [DOI] [PubMed] [Google Scholar]
- Bellett A.J.D. J. Virol. 1967;1:245–259. doi: 10.1128/jvi.1.2.245-259.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergold G.H., Graf T., Munz K. In: Arboviruses of the California Complex and the Bunyamwera Group, Proceedings of a Symposium. Bardos V., editor. Publishing House of the Slovak Academy of Sciences, page 41; Bratislava: 1969. [Google Scholar]
- Blaskovic D. Bull. W.H.O. [Suppl. 1] 1967;36:5. [PMC free article] [PubMed] [Google Scholar]
- Borden E.C., Murphy F., Shope R., Harrison A. To be published. 1971 [Google Scholar]
- Breese S.S., Jr., Ozawa Y., Dardiri A.H. J. Amer. Vet. Med. Ass. 1969;155 [2]:391. [PubMed] [Google Scholar]
- Briceño-Rossi A.L. Rev. Venezolana Sanidad Asistencia Social. 1964;29:351. [PubMed] [Google Scholar]
- Carley J.G., Standfast H.A. Amer. J. Epidemiol. 1969;89:583. doi: 10.1093/oxfordjournals.aje.a120971. [DOI] [PubMed] [Google Scholar]
- Casals J. Trans. N. Y. Acad. Sci. 1957;19 [Ser. 2]:219. doi: 10.1111/j.2164-0947.1957.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Casals J. Anais Microbiol. 1963;11A:13. [Google Scholar]
- Casals J. 9th Int. Congr. Microbiol. Moscow, p. 441. Ivanovsky Institute of Virology; Moscow: 1966. [Google Scholar]
- Casals J. Nature (London) 1968;217:648. doi: 10.1038/217648a0. [DOI] [PubMed] [Google Scholar]
- Committee Ann. Inst. Pasteur, Paris. 1965;109:625. [PubMed] [Google Scholar]
- Cooper P.D. Brit. Med. Bull. 1967;23:155. doi: 10.1093/oxfordjournals.bmb.a070537. [DOI] [PubMed] [Google Scholar]
- Doerr R., Franz K., Taussig S. Das Pappatacifieber. Deuticke; Leipzig: 1909. [Google Scholar]
- Downs W.G. In: Arboviruses: Epidemiological Considerations. Mudd S., editor. Saunders, Philadelphia; Pennsylvania: 1970. page 538. [Google Scholar]
- Downs W.G., Anderson C.R., Spence L., Aitken T.H.G., Greenhall A.H. Amer. J. Trop. Med. Hyg. 1963;12:640. doi: 10.4269/ajtmh.1963.12.640. [DOI] [PubMed] [Google Scholar]
- Feldman H.A., Wang S.S. Proc. Soc. Exp. Biol. Med. 1961;106:736. doi: 10.3181/00379727-106-26459. [DOI] [PubMed] [Google Scholar]
- Filshie B.K., Rehacek J. Virology. 1968;34:435. doi: 10.1016/0042-6822(68)90063-9. [DOI] [PubMed] [Google Scholar]
- Gibbs A.J., Harrison B.D., Watson D.H., Wildy P. Nature (London) 1966;209:450. doi: 10.1038/209450a0. [DOI] [PubMed] [Google Scholar]
- Haldane J.B.S. Nature (London) 1960;187:879. [Google Scholar]
- Holden P. Proc. Soc. Exp. Biol. Med. 1955;88:607. doi: 10.3181/00379727-88-21668. [DOI] [PubMed] [Google Scholar]
- Holmes I.H., Warburton M.F. Lancet. 1967;ii:1233. doi: 10.1016/s0140-6736(67)90568-5. [DOI] [PubMed] [Google Scholar]
- Holmes I.H., Wark M.C., Warburton M.F. Virology. 1969;37:15. doi: 10.1016/0042-6822(69)90301-8. [DOI] [PubMed] [Google Scholar]
- Horzinek M., Mussgay M. J. Virol. 1969;4:514. doi: 10.1128/jvi.4.4.514-520.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howatson A.F., Whitmore G.F. Virology. 1962;16:466. doi: 10.1016/0042-6822(62)90228-3. [DOI] [PubMed] [Google Scholar]
- Howell P.G. Onderstepoort J. Vet. Res. 1962;29:139. [PubMed] [Google Scholar]
- Johnson K.M. Amer. J. Trop. Med. Hyg. 1965;14:816. doi: 10.4269/ajtmh.1965.14.816. [DOI] [PubMed] [Google Scholar]
- Klimenko S.M., Yershov F.I., Gofman Y.P., Nabatnikov A.P., Zhdanov V.M. Virology. 1965;27:125. doi: 10.1016/0042-6822(65)90152-2. [DOI] [PubMed] [Google Scholar]
- Kuns M.L. Amer. J. Trop. Med. Hyg. 1965;14:813. doi: 10.4269/ajtmh.1965.14.813. [DOI] [PubMed] [Google Scholar]
- Lascano E.F., Berria M.I., Oro J.G.B. J. Virol. 1969;4:271. doi: 10.1128/jvi.4.3.271-282.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwoff A., Horne R.W., Tournier P. Cold Spring Harbor Symp. Quant Biol. 1962;27:51. doi: 10.1101/sqb.1962.027.001.008. [DOI] [PubMed] [Google Scholar]
- Melnick J.L., McCombs R.M. Progr. Med. Virol. 1966;8:400. [PubMed] [Google Scholar]
- Mettler N.E. Pan Amer. Health Organ., Sci. Publ. No. 1969;183 [Google Scholar]
- Mettler N.E., Petrelli R.L., Casals J. Virology. 1968;36:503. doi: 10.1016/0042-6822(68)90175-x. [DOI] [PubMed] [Google Scholar]
- Morgan C., Howe C., Rose H.M. J. Exp. Med. 1961;113:219–234. doi: 10.1084/jem.113.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F.A., Coleman P.H., Harrison A.K., Gary G.W., Jr. Virology. 1968;35:28. doi: 10.1016/0042-6822(68)90302-4. [DOI] [PubMed] [Google Scholar]
- Murphy F.A., Harrison A.K., Gary W.G., Jr., Whitfield S.G., Forrester F.T. Lab. Invest. 1968;19:652–662. [PubMed] [Google Scholar]
- Murphy F.A., Harrison A.K., Tzianabos T. J. Virol. 1968;2:1315. doi: 10.1128/jvi.2.11.1315-1325.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F.A., Whitfield S.G., Coleman P.H., Calisher C.H., Rabin E.R., Jenson A.B., Melnick J.L., Edwards M.R., Whitney E. Exp. Mol. Pathol. 1968;9:44. doi: 10.1016/0014-4800(68)90049-x. [DOI] [PubMed] [Google Scholar]
- Murphy F.A., Webb P.A., Johnson K.M., Whitfield S.G. J. Virol. 1969;4:535. doi: 10.1128/jvi.4.4.535-541.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota Z. Virology. 1965;25:372. doi: 10.1016/0042-6822(65)90057-7. [DOI] [PubMed] [Google Scholar]
- Plowright W., Brown F., Parker J. Arch. Gesamte Virusforsch. 1966;19:289. [Google Scholar]
- Plowright W., Parker J., Peirce M.A. Nature (London) 1969;221:1071. doi: 10.1038/2211071a0. [DOI] [PubMed] [Google Scholar]
- Rowe W.P., Murphy F.A., Bergold G.H., Casals J., Hotchin J., Johnson K.M., Lehmann-Grube F., Mims C.A., Traub E., Webb P.A. J. Virol. 1970;5:651. doi: 10.1128/jvi.5.5.651-652.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W.P., Pugh W.E., Webb P.A., Peters C.J. J. Virol. 1970;5:289. doi: 10.1128/jvi.5.3.289-292.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer W.F. Proc. Soc. Exp. Biol. Med. 1968;129:194. doi: 10.3181/00379727-129-33282. [DOI] [PubMed] [Google Scholar]
- Schnagl R.D., Holmes I.H., Doherty R.L. Virology. 1969;38:347. doi: 10.1016/0042-6822(69)90377-8. [DOI] [PubMed] [Google Scholar]
- Siler J.F., Hall M.W., Hitchens A.P. Manila Bur. Sci. Monogr. No. 1926;20:62–170. [Google Scholar]
- Simpson R.W., Hauser R.E. Virology. 1968;34:358. doi: 10.1016/0042-6822(68)90248-1. [DOI] [PubMed] [Google Scholar]
- Southam C.M., Shipkey F.H., Babcock V.I., Bailey R., Erlandson R.A. J. Bacteriol. 1964;88:187. doi: 10.1128/jb.88.1.187-199.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studdert M.J. Proc. Soc. Exp. Biol. Med. 1965;118:1006. doi: 10.3181/00379727-118-30030. [DOI] [PubMed] [Google Scholar]
- Subcommittee Virology. 1963;21:516. [Google Scholar]
- Sunaga H., Taylor R.M., Henderson J.R. Amer. J. Trop. Med. Hyg. 1960;9:419. doi: 10.4269/ajtmh.1960.9.419. [DOI] [PubMed] [Google Scholar]
- Taylor R.M. U.S. Dept. Health, Education and Welfare Public Health Service Publ. No. Washington, D.C. 1967;1760 [Google Scholar]
- Theiler M. Proc. Soc. Exp. Biol. Med. 1957;96:380. doi: 10.3181/00379727-96-23483. [DOI] [PubMed] [Google Scholar]
- Verwoerd D.W. Virology. 1969;38:203. doi: 10.1016/0042-6822(69)90361-4. [DOI] [PubMed] [Google Scholar]
- von Bonsdorff C.-H., Saikku P., Oker-Blom N. Virology. 1969;39:342. doi: 10.1016/0042-6822(69)90057-9. [DOI] [PubMed] [Google Scholar]
- Waterson A.P., Almeida J.D. Nature (London) 1966;210:1138. doi: 10.1038/2101138a0. [DOI] [PubMed] [Google Scholar]
- Whitman L., Shope R.E. Amer. J. Trop. Med. Hyg. 1962;11:691. doi: 10.4269/ajtmh.1962.11.691. [DOI] [PubMed] [Google Scholar]
- WHO Scientific Group. W.H.O. Tech. Rept. Ser. No. 1967;369 [Google Scholar]
- Wildy P., Ginsberg H.S., Brandes J., Maurin J. Progr. Med. Virol. 1967;9:476. [PubMed] [Google Scholar]


