Were we but able to explain
The fiefdom of the microbe—
Why one man is his serf,
Another is his lord
When all are his domain.
C.B.H.
Respiratory syncytial virus (RSV) is the major cause of lower respiratory tract illness in young children.1, 2, 3, 4 So effectively does RSV spread that essentially all persons have experienced RSV infection within the first few years of life. Immunity, however, is not complete, and reinfection is common. Although life-threatening infections most commonly occur during the first couple of years of life, RSV infections contribute an appreciable share of the morbidity caused by acute upper and lower respiratory tract infections among older children and adults. The health care costs associated with outpatient infections add appreciably to the estimated cost of $300 to $600 million for hospitalized infants with RSV infection.2, 5, 6 Of concern and increasing recognition are the growing morbidity and costs associated with RSV infections in older adults.7, 8, 9
History
RSV was discovered in 1956 but was not initially associated with respiratory illness among infants. Indeed, when a group of 14 chimpanzees were noted to be suffering from colds and coryza, Morris and co-workers10 isolated a new virus originally named chimpanzee coryza agent (CCA).1 Subsequently, Chanock and co-workers11confirmed that the agent caused respiratory illness in humans when they obtained isolates from two children, one with laryngotracheobronchitis and the other with bronchopneumonia, that were indistinguishable from CCA. When specific neutralizing antibody to CCA was found to be present in most school-aged children, “chimpanzee coryza agent” was more appropriately renamed respiratory syncytial virus to denote its clinical and laboratory manifestations.
Description
Classification
RSV belongs to the order Mononegavirales, family Paramyxoviridae, and the subfamily Pneumovirinae.12 The two genera within the Pneumovirinae subfamily are Metapneumovirus, containing human metapneumovirus (hMPV), and Pneumovirus, which contains human RSV and the morphologically and biologically similar murine pneumovirus and canine pneumovirus, bovine RSV, ovine RSV, and caprine RSV.13 Distinctive features of RSV include the number and order of genes and the lack of hemagglutinin and neuraminidase activity.
Viral Structure and Characteristics
RSV is an enveloped, medium-sized (120 to 300 nm) RNA virus with a nonsegmented, single-stranded, negative-sense genome that is associated with viral proteins throughout its length, which form the nucleocapsid (Figs. 160-1 and 160-2 ).6, 12 The viral envelope has a bilipid layer derived from the plasma membrane of the host cells. Its surface has transmembrane surface glycoprotein spikes 11 to 12 nm in length and 6 to 10 nm apart, which on electron microscopy give it a thistle-like appearance (Fig. 160-3 ).12
FIGURE 160-1.
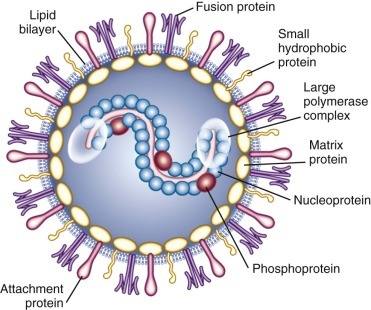
Structure of respiratory syncytial virus.
(Modified from Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917-1928.)
FIGURE 160-2.
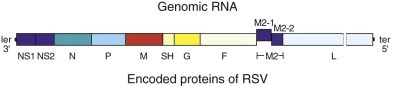
Simplified representation of the negative-sense RNA genome of respiratory syncytial virus (RSV) and the encoded proteins.
The genome is shown 3′ with the leader extragenic region (ler) and with the 5′ trailer extragenic region (ter). The viral genes are depicted by the divided bars. Each viral gene transcribes a single messenger RNA (mRNA) encoding a single protein, with the exception of M2 mRNA, which transcribes two proteins, M2-1 and M2-2.
FIGURE 160-3.
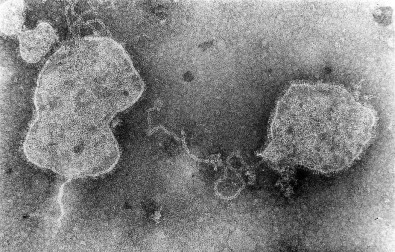
Negative contrast electron micrograph of respiratory syncytial virus.
The complete genome of RSV (A2 strain) has been sequenced (see Fig. 160-2).12, 14 The viral RNA consists of 15,222 nucleotides containing 10 genes. Each gene encodes for a single protein, except for the M2 gene, which possesses two overlapping open reading frames that encode for two separate proteins (M2-1 and M2-2, the transcription processivity factor and a transcriptional regulatory protein, respectively). Four proteins—N (nucleoprotein), P (phosphoprotein), L (polymerase), and M2-1—are associated with the RNA-containing nucleocapsid complex. Associated with the envelope are three transmembrane surface proteins, F (fusion), G (attachment), and SH (proposed viroporin protein), that are important for viral infectivity.15 In addition, a truncated secreted form of G (Gs) is transcribed from a second start codon. The M (matrix) protein accumulates at the inner surface of the envelope and is important in viral morphogenesis. Two proteins, NS1 and NS2, are nonstructural proteins that inhibit cellular type I interferon activity and subsequently effect the adaptive immune response to RSV.12, 16
The two major glycosylated surface proteins, F and G, are the major immunoprotective antigens and are targets for antibody-mediated neutralization. The G protein is the primary mediator of attachment of the virus to host cells, although the F protein also can facilitate viral attachment.17 After attachment in the pretriggered form, F undergoes structural changes into a post-triggered form that initiates viral penetration by fusing viral and host cellular membranes and promotes fusion of infected cells to adjacent uninfected cells, thereby resulting in the characteristic RSV syncytia.18 Maximally efficient fusion requires participation of all three of the surface glycoproteins, F, G, and SH.
Laboratory Properties
RSV poorly withstands slow freezing and thawing and changes in pH. At 55° C, infectivity is rapidly diminished, and at room temperature (25° C), only 10% infectivity remains at 48 hours, and even at 4° C only 1% remains after 7 days.19 The optimal pH for RSV is 7.5. Inactivation occurs quickly with acidic media (pH < 5), ether, and chloroform and with detergents such as sodium dodecyl sulfate and Triton X-100.
The survival of RSV in the environment appears to depend in part on the drying time and dew point. At room temperature, RSV in the secretions of patients may survive on nonporous surfaces, such as countertops, for 3 to 30 hours.20 On porous surfaces, such as cloth and paper tissue, survival is generally shorter, usually less than 1 hour. The infectivity of RSV on the hands is variable from person to person but is usually less than 1 hour.
Human heteroploid cell lines are usually preferred for primary isolation. Most commonly used are HEp-2, HeLa, and A549 cell lines. RSV may also be recovered in human kidney, amnion, diploid fibroblastic cells, and monkey kidney cells. With primary isolation in sensitive cell cultures, characteristic cytopathic effect may be first detected after an average of 3 to 5 days. The typical syncytia develop about 10 to 24 hours later and progress until the cell sheet is completely destroyed, which is usually within 4 days. Cell-free virus may be demonstrated in the culture medium, but up to 90% of the virus remains cell-associated.12
Infection in Animals
Humans and chimpanzees are the only known natural hosts for human RSV, although a variety of small animal species may be experimentally infected with RSV.21 Cows are the natural hosts of bovine RSV (BRSV), which is antigenically and genetically closely related to human RSV, and some antibodies directed against the F, N, P, and M proteins of either virus recognize the heterologous virus. Ovine and caprine strains of RSV also have been recovered, and genetic analysis suggests that caprine RSV is more closely related than ovine RSV to BRSV and human RSV.12
Although many animal models develop upper respiratory tract infection, their lack of symptomatic lower respiratory tract disease comparable to that observed with infants limits their utility. Closest is disease in chimpanzees because they readily acquire infection from infected contacts and shed moderate levels of RSV in respiratory secretions.12 Nevertheless, their disease is generally mild and without the degree of lower respiratory tract involvement observed in infants. Rodents are the most commonly used models, particularly cotton rats and mice, but replication of RSV is only semipermissive and highly variable.22
Epidemiology
Distribution
In every geographic area studied, RSV infections are ubiquitous and clinically similar in causing the most severe disease during infancy and repetitive infections throughout life.6, 23, 24 The seasonal occurrence, however, varies according to geography and climate.25
Seasonal Occurrence
RSV is singular in its ability to produce reliably a major burden of infections every year.3, 26 In temperate climates, the outbreaks occur primarily in the late fall through early spring and spread across the United States over a period of 20 or more weeks, generally from October to May. In warmer climates, RSV activity may be more prolonged or even present throughout the year.24, 25, 27, 28, 29 What factors initiate and terminate the recurring patterns of RSV activity has been a subject of ongoing curiosity and investigation but remains a conundrum.29, 30, 31 A complex, and currently incompletely understood, interaction of local meteorologic conditions may explain part of the geographically variable epidemiologic patterns.32 Among these are the ambient temperature, humidity, and sunlight, and their effects on RSV's stability and infectivity. However, because the only source of RSV infection is an infected individual, human behavior is an indefinable factor integral to its transmission.29, 33
Antigenic Variation
Strain differences among RSV isolates may also affect the intensity, severity, and diversity of RSV outbreaks.34, 35, 36 RSV isolates are divided into two major antigenic groups, A and B, each of which can be further divided into multiple subgroups containing separate genotypes.12, 14, 37 The two major groups have 81% nucleotide identity, but some of the proteins between the A and B strains vary appreciably. The major genetic diversity between groups A and B resides with the G protein, followed by M2-2 and SH proteins. This is reflected in the relative low antigenic relatedness between the two groups of only 1% to 7% for the G proteins, compared with 50% for the F proteins. Nucleotide and amino acid sequence analysis within both A and B strain groups has further revealed subtypes of distinctive lineage, or clades.12, 14
Strains of both groups circulate simultaneously during outbreaks, but the proportions that are A and B vary, as do the subtypes.34, 35, 36, 37, 38 Even in widely separated geographic areas, the cocirculating strains may have similar genotypes and parallel evolutionary lineages. Analyses of the proteins of strains collected over decades and from diverse areas suggest that the pressure of the population's immunity may result in a selective advantage for the dominance of strains that are most divergent from those that have recently circulated in the area. Evidence indicates that a recently emerged RSV B strain containing a unique 20-amino-acid duplication in the G protein has spread throughout the world and currently is the dominant variant among group B viruses, suggesting an evolutionary advantage over prior strains.39 However, the relationship of the circulating strain groups and subtypes to the clinical severity and manifestations of the RSV infections among young children has been inconsistent and thus inconclusive.35, 36, 38, 40, 41 The relationship between the substantial antigenic diversity of the G protein to reinfection with RSV has not been established.
Epidemiologic Manifestations
The characteristic epidemiologic ramifications of RSV's presence within a community are notable rises in the number of cases of bronchiolitis, pneumonia, and hospital admissions of young children, especially infants with acute lower respiratory tract disease.2, 42, 43 RSV outbreaks may vary year to year in size and intensity. Severe lower respiratory tract illness from RSV in previously healthy children occurs most frequently in the first year of life and is almost always associated with primary infection. Essentially all children have experienced RSV infection within the first several years of life one or more times. Repeated infections are common not only among young children but throughout life.7, 44, 45, 46
Prevalence and Incidence
RSV is the most frequent cause of bronchiolitis and is estimated to cause 40% to 90% of bronchiolitis hospitalizations and up to 50% of pneumonia admissions among infants.2, 3, 5, 43 Ten to 30% of tracheobronchitis cases have been associated with RSV infection, but only 2% to 10% of croup cases. Bronchiolitis is the leading cause of all hospitalizations among infants in the United States. Overall, as many as 172,000 children younger than 5 years are reported to be hospitalized annually with RSV infection in the United States.3, 4, 47 The yearly rates of RSV hospitalizations estimated from national databases have been variable, ranging from about 2 to 44 per 1000 children within the first 2 to 5 years of life.42, 48, 49 Children within the first year of life consistently had the highest rates of hospitalization for bronchiolitis and other RSV-associated illnesses, and the preponderance of admissions were among infants younger than 6 months.4 Recent population-based studies in the United States have indicated the current hospitalization rates are 17 per 1000 children younger than 6 months and 3 per 1000 children younger than 5 years.2, 43 These rates, which are similar to those derived from RSV-coded hospitalization data, were more than three times the rates from parainfluenza or influenza viral infections over 4 years of surveillance among the same population of children.50, 51 The reported RSV hospitalization rates from European countries have been generally similar, ranging from 2.5 to 11 per 1000 children within the first 4 years of life and highest among those younger than 12 months of age—19 to 22 per 1000 children.5, 52, 53, 54 Estimates of lower respiratory tract infection from RSV in developing countries are estimated to be twice that of developed countries.27
The rates of hospitalization among children, however, vary according to the presence of risk factors. Many host, socioeconomic, and environmental factors have been associated with a greater likelihood of young children developing more severe RSV infection and requiring hospitalization (see “Patients at High Risk for Severe Infection”).
Population-based surveillance during 2000 to 2004, examining laboratory-proven RSV hospitalizations and emergency department and outpatient visits in the counties surrounding Rochester, New York; Nashville, Tennessee; and Cincinnati, Ohio, indicated that outpatient visits constituted a major proportion of the health care burden attributable to RSV infection.2 Of all visits for acute respiratory illnesses (ARI) among children within the first 5 years of life, RSV infection was documented among 20% of ARI hospitalizations, 18% of emergency department ARI visits, and 15% of office ARI visits. The estimated rates of ARI visits from RSV among children younger than 5 years in pediatric practices (80 per 1000 children) was about 3 times higher than that observed among emergency department patients and 26 times the rates among hospitalized children.
Less information exists on the health care burden imposed by RSV infection among older children and adults. Recurrent RSV infections are common among school-aged children and adults who are exposed to RSV at school, home, work, or during military training.49, 55 In urban Rochester, 44% of the families with young children who were followed became infected with RSV during the winter months, when RSV was prevalent in the community.46 Of the exposed family members, 46% acquired RSV infection. Although the attack rate was highest among infants, 38% to 47% of older children and adults developed RSV infection. In the Houston family study, in which children were followed from birth, the infection rate was 68.8 per 100 children in the first year of life, and during the second year, at least half were reinfected.44
More recently recognized is the frequency of RSV infections among older adults and the resulting appreciable clinical and economic impact.7, 9, 56 In one study spanning 4 years, rates of RSV infection ranged from 2% to 10% per year in persons 65 years of age or older and in high-risk persons with underlying cardiopulmonary disease.7 RSV infection among older individuals is remarkably similar to influenza with respect to clinical manifestations and as a cause of hospitalization. In London, the rate of hospitalization attributable to RSV infection among the elderly has been estimated at 0.7 per 1000 adults 65 years of age or older, compared with 1.1 per 1000 estimated for influenza.57
Pathogenesis
Infection with RSV is primarily acquired through close contact with an infected individual or direct inoculation into the eyes and nose of infectious secretions.33, 58 Large-particle aerosols engendered by coughs and sneezes of an ill person may transmit RSV to others within a radius of about 3 feet. Longer-distance spread by small-particle (droplet nuclei) aerosols appears to be much less likely.33 However, infection occurring from touching objects that have been contaminated with infectious secretions, followed by self-inoculation into the eyes or nose, is an important mode of transmission.
Experimental infection occurs in adult volunteers after an average incubation period of 3 to 5 days.59, 60, 61, 62, 63 In naturally acquired infection, the average incubation period appears similar, with a range of 2 to 8 days. The mucosa of the nose and eye appears to be equally sensitive portals of entry, in contrast to the mouth.61 In hospitalized infants with primary RSV infection, peak viral titers range from 101 to 107 plaque-forming units (pfu)/mL of nasal secretions (mean of 105) and fall slowly.64, 65 Shedding duration is typically 7 to 10 days, but virus can occasionally be detected as long as 30 days later. Increased viral load has variably been associated with greater disease severity.64, 65, 66
RSV replicates in respiratory epithelium, primarily involving the ciliated columnar cells, but additional cells, such as type I and II pneumocytes, may be involved.67 During primary infection, lower respiratory tract infection usually is manifest by bronchiolitis, and the initial pathologic findings are a lymphocytic peribronchiolar infiltration, predominantly CD69+ monocytes, with edema of the walls and surrounding tissue.67, 68, 69 Subsequently, the characteristic proliferation and necrosis of the epithelium of the bronchioles develop. The lumina of these small airways become obstructed from the sloughed epithelium and from the increased mucus secretion. The airway of the young infant is particularly vulnerable to any degree of inflammation and obstruction because resistance to the flow of air is related inversely to the cube of the radius. Impedance to the flow of air occurs during both inspiration and expiration but is greater in the latter when the lumen is narrowed further by the positive expiratory pressure. Hyperinflation, therefore, results from air trapping peripheral to the sites of partial occlusion. With complete obstruction, the trapped air eventually becomes absorbed, producing the characteristic multiple areas of atelectasis. Young infants are at increased risk for developing such areas of atelectasis because the collateral channels that maintain alveolar expansion in the presence of airway obstruction are not yet well developed. These changes result in an increase in lung volume and expiratory resistance.
Infants with lower respiratory tract disease from RSV often have pathologic evidence of both pneumonia and bronchiolitis. Patients with pneumonia demonstrate an interstitial infiltration of mononuclear cells that may be accompanied by edema and necrotic areas that lead to alveolar filling.67, 68 Some histologic evidence of recovery is present in most children with bronchiolitis within the first week of illness and is marked by the beginning regeneration of the bronchiolar epithelium. However, ciliated cells may not be present for weeks.
Immunity and Pathogenesis of Disease
Diverse theories and supporting data have been offered to explain how RSV engenders these pathogenic findings. Much of our current knowledge regarding the immunologic responses to RSV is from studies in vitro and in animal models. In humans, the immune response cannot be separated from the confounding and poorly understood influence of genetics, environment, age, and antigenic experience.70, 71, 72, 73 Consensus exists that during primary infection, disease is both virally and immunologically mediated. RSV produces its most devastating illness when specific, maternally derived antibody is present at variable amounts. The severity of RSV infection in the young infant with high levels of specific antibody and the augmented disease induced by the inactivated RSV vaccine developed in the 1960s first suggested the putative singular role of the immune response in RSV's pathogenesis in infants.73, 74, 75, 76, 77
The potential importance of the host's immune response to the development of disease and the subsequent rowen of long-term complications has been further supported by the observation that RSV is not very invasive or cytopathogenic.73 Although viral loads correlate with disease severity in humans, reducing viral replication with the administration of neutralizing antibody to the F protein has not ameliorated clinical disease in infants.78, 79 However, recent data in murine models of RSV indicate that administration of non-neutralizing antibodies to the centrally conserved CX3C chemokine motif of the G protein can reduce inflammatory responses and illness even after infection is established.80, 81
The relative contribution of the immune response to disease in infants, however, has been questioned by the observation that among children with bronchiolitis, those with RSV bronchiolitis produced a more robust response of proinflammatory cytokines in their nasal washes than did children with non-RSV bronchiolitis, but it was not associated with more severe disease and, indeed, appeared protective against hypoxia.82 Furthermore, examination of fatal RSV cases has shown that RSV antigen was extensively present in the pulmonary tissue, indicating abundant viral replication.83 Cytokine production, on the other hand, was nearly absent, and the expression of apoptosis was increased, with the conclusion that the patients had an inadequate immune response and unchecked viral replication. Whether the innate or adaptive immune responses, or both, are enhanced or suppressed in association with more severe disease remains controversial, but most likely the pathogenicity of RSV infection results from varying and currently ill-defined combinations of the contributions of the virus and host.
Innate Immunity
The first barrier of defense against RSV infection in infants is the innate immune response, which is initiated by infection of the respiratory epithelium.73, 77, 84, 85 RSV is a potent inhibitor of cellular antiviral type I interferons (α, β, and λ) via the production of the NS1 and NS2 proteins.16 In addition, RSV interacts with the Toll-like receptor (TLR)2, 3, 4, and 7, which triggers secretion of inflammatory cytokines.77 Infection of the respiratory epithelium, antigen-processing cells (dendrites), and macrophages produces multiple alterations in gene expression, which result in the production of cell surface markers and the release of cytokines and chemokines (Fig. 160-4 ). Inflammatory cells are recruited to the respiratory tract and consist of neutrophils as well as macrophages, mononuclear cells, T cells, natural killer (NK) cells, and eosinophils. Intubated infants with severe primary RSV infection have an early and robust neutrophilic infiltration into the airway, as determined by bronchoalveoloar lavage, and this correlated with a decline in viral load before the development of T-cell responses.86 The variability in the endowed innate defense and susceptibility of the host are also being increasingly correlated with polymorphisms in genes that are integral to various components of innate immunity.72, 87
FIGURE 160-4.
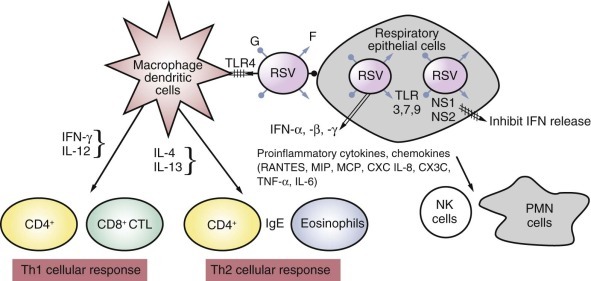
Early innate and adaptive immunity response to respiratory syncytial virus (RSV).
RSV's envelope glycoprotein G attaches to the respiratory tract epithelial cells by glucosamine glycans expressed on the cell surface, and F interacts with antigen-presenting cells (macrophages, dendritic cells) through Toll-like receptor 4 (TLR4) protein. This triggers the production and release of antiviral interferons (IFN-α, IFN-β, IFN-γ) and a cascade of proinflammatory cytokines and chemokines. Two early nonstructural RSV gene products (NS1, NS2) antagonize interferon production. The chemokines recruit polymorphonuclear neutrophils (PMN cells), natural killer (NK) cells, and CD4+ and CD8+ T cells. A Th1-type cellular response becomes dominant under the influence of IFN-γ and interleukin-12 (IL-12), whereas under the influence of IL-4 and IL-13, the cellular response is skewed toward Th2, with the production of immunoglobulin E (IgE) and eosinophils. CTL, cytotoxic lymphocyte; CXC, cysteine X cysteine; MCP, monocyte chemotactic protein; MIP, macrophage inhibitory protein; RANTES, regulated on activation, normal T-cell expressed and secreted.
(Modified from Hall CB, Walsh EE. Respiratory syncytial virus. In: Feigin R, Cherry J, Demmler G, Kaplan S, eds. Textbook of Pediatric Infectious Diseases. 6th ed. Philadelphia: Saunders; 2009:2462-2487.)
Adaptive Immunity
The relative contributions and interactions of different arms of the immune response to either a primary or recurrent exposure to RSV or after immunization with experimental vaccines are complex and unclear. Considerable evidence suggests that an effective, nondetrimental immune response to RSV infection requires a fine balance of the multiple components of immunity, and that balance is determined by both host and viral factors.70, 73
Serum neutralizing antibody provides some, but not complete, protection against RSV infection. Higher titers of antibody generally correlate with better resistance to infection, but no defined level of neutralizing antibody is predictive of the risk for infection, the severity of illness, or recovery in children or adults.8, 88, 89, 90 Higher levels of maternal antibody have been correlated with lower infection rates and with less severe illness in some studies, but not in others.91, 92 More recently, the trials of administration of RSV monoclonal antibody to high-risk infants have demonstrated protection against more severe RSV disease.93
Passively derived maternal antibody usually declines to undetectable levels by 6 months of age, but occasionally, it may remain up to 9 to 12 months of age. During primary infection, serum immunoglobulin M (IgM) antibody appears within several days, but it is transient and detectable usually only for a few weeks.73 During the second week, IgG antibody appears, usually peaks in the fourth week, and begins to decline after 1 to 2 months. The IgA serum antibody response is more variable in infants. An anamnestic response involving all three immunoglobulin classes occurs after reinfection, and after about three infections, the titers reach levels similar to those in adults.
Primary and subsequent infection produce antibody to many of the RSV proteins. However, the major immunoprotective antigens are two large surface glycoproteins, F and G.12 Both contain neutralizing epitopes, but those on the F protein are conserved between the two strain groups, resulting in antibody to the F protein being cross-reactive between group A and B strains. In contrast, the response to the variable G protein is group and genotype specific.94 Most adults with RSV infection develop IgG responses to the centrally conserved chemokine motif of G, although their role in recovery or protection from infection or illness is not clear.95
Although young children are able to produce neutralizing antibodies directed against both the F and G proteins, the neutralizing antibody responses are blunted in infants younger than 6 months because of a dampening effect of maternal antibody.96 In infants, the antibody response to the F and G proteins mainly involves the subclasses IgG1 and IgG3. However, adults respond to the G protein with antibodies in both the IgG1 and the IgG2 subclasses, and the adult response to the F protein is predominantly IgG1. Antibodies after primary and recurrent infection usually decline substantially within months. Subsequent to natural infection, 75% of adults demonstrate a fourfold or greater drop in titer, returning to preinfection titers within 2 years in most.97 A protective role for local antibody in RSV infection has been suggested because animal studies indicate that circulating IgG does not prevent viral replication in the nasal passages.90, 98 RSV-specific IgA antibody, which is produced in nasal secretions during primary and subsequent infection, has been associated with protecting the upper respiratory tract from infection in adults and has been correlated with clearance of viral shedding in infants.88, 90, 99 Children with RSV infection may also produce transient specific IgE antibody responses in the respiratory tract. Levels in the nasal secretions of both RSV-specific IgE antibodies and of cysteinyl leukotrienes have been correlated with increased risk for more severe acute infection, with wheezing and with later episodes of recurrent wheezing.100, 101
Cell-mediated immunity is likely to be pivotal in the clearance of virus and in recovery, but has not been definitively shown to protect against reinfection and illness in humans. Adults and children with deficiencies of cellular immunity, as well as experimentally immunosuppressed animals, have more severe disease and prolonged virus shedding.102, 103 Most information delineating the specific components of the cellular response induced by RSV are derived from rodent models and, to a much lesser extent, from humans.70, 73
RSV infection has multiple suppressive effects on the cellular immune response. Diminished in vitro lymphoproliferative responses during initial and repeated infections suggest impaired RSV-specific helper T-cell responses. Furthermore, RSV-infected dendritic cells have diminished ability to activate CD4+ T cells, and enhanced apoptosis of CD4+ and CD8+ lymphocytes is observed among infants with RSV bronchiolitis.104, 105
RSV infection in both animals and humans engenders a spectrum of both Th1- and Th2-dominant responses (see Fig. 160-4). Th1-dominant responses, characterized by the production of CD8+ cytotoxic lymphocyte and Th1 CD4+ cells secreting interleukin-2 (IL-2), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) are associated with viral clearance and minimal pulmonary cytopathology. In contrast, Th2-dominant responses induce Th2 CD4+ cells, which stimulate IL-4, IL-5, IL-10, and IL-13 secretion. These Th2 cytokines affect CD8+ T-cell function and impair viral clearance.22 IL-4 and IL-13 also augment isotype switching to IgE, and a Th2-biased response has been correlated with wheezing, more severe disease, and greater cellular inflammation and eosinophilia in the lung.100
Whether RSV evokes predominantly a Th1- or Th2-biased response has been shown to be influenced by the individual's genetic background, age, and previous RSV experience and the specific antigen. Infants, within the first several months of life, have been observed to have higher levels of Th2-type cytokines in nasal secretions than older infants.106 Inactivated virus, as used in the initial formalin-inactivated vaccine and even in subunit vaccines, is more likely to induce a Th2-like response in experiments with unprimed animals than is live virus.22 Antigenic stimulation with the F protein is associated with a predominantly Th1 response, whereas the G protein produces a Th2-biased response, possibly because its CX3C motif adversely affects the CD8+ T-cell response.107
Clinical Manifestations
Clinical observations over the half century of RSV's recognition have confirmed the important immunologic correlates that naturally acquired immunity to RSV infection is incomplete, variable, and not durable. Repeated infections are common, but severe disease rarely occurs after the primary encounter in normal healthy children. Lower respiratory tract involvement and severe disease may occur during repeat infections, but it is generally confined to those at either end of the age spectrum.7, 44, 45
Infection among Young Children
Primary infections with RSV frequently involve the lower respiratory tract, particularly among infants within the first several months of life. Most commonly, these present as bronchiolitis, followed by pneumonia and tracheobronchitis.2, 44, 45 Croup is the least common form of clinical illness and usually accounts for less than 2% to 10% of cases. Upper respiratory tract signs almost always accompany lower respiratory tract disease, or the infection may be confined to the upper respiratory tract, which in young children is commonly associated with fever and otitis media (Fig. 160-5 ). Rarely is the first infection asymptomatic.44, 45, 46 The risk for lower respiratory tract involvement occurring with the first infection is high. Pneumonia or bronchiolitis has been estimated to occur in 30% to 71%, depending on the age and population.2, 44, 45, 108 Among infants younger than 6 months, those with underlying cardiopulmonary disease, and those in close contact with other young children, such as those attending daycare, the proportion developing lower respiratory tract disease may be even higher.45 Furthermore, the severity of illness among outpatient preschool-aged children is considerable, with two thirds manifesting wheezing and three fourths developing labored breathing (see Fig. 160-5).
FIGURE 160-5.
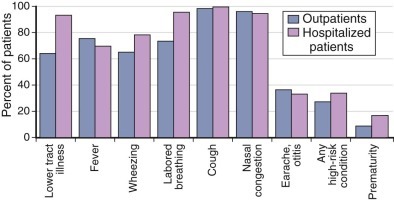
Clinical characteristics of children younger than 5 years with respiratory syncytial virus (RSV) who were outpatients, compared with those who were hospitalized.
RSV infection was laboratory confirmed during population-based surveillance of acute respiratory illnesses conducted in counties surrounding Nashville, Tennessee; Rochester, New York; and Cincinnati, Ohio, during 2000 to 2004.
(Data from Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588-598.)
RSV infection among young children and older individuals usually starts with an upper respiratory tract illness with nasal congestion and a cough. Hoarseness and laryngitis are not prominent features. A low-grade fever, lasting for 2 to 4 days, occurs in most young children early in the course of the illness. The height or duration of the fever does not correlate with the severity of the disease and is frequently absent in the presence of lower respiratory tract involvement and at the time of hospitalization. With progression of disease to the lower respiratory tract, the cough may become more prominent and productive, followed by an increased respiratory rate, dyspnea, and retractions of the intercostal muscles. With bronchiolitis, both expiratory and inspiratory obstruction may be evident. On auscultation, the infant may have crackles and wheezing. The rapid variability in the presence and intensity of these physical findings is characteristic of bronchiolitis, with marked transient swings in oxygen saturation often noted. Repeated observations of the infant, therefore, are required for adequate assessment of clinical severity.109
Infants hospitalized with RSV lower respiratory tract infection commonly have some impairment of oxygenation.109 This reflects diffuse viral involvement of the lung parenchyma, which causes an abnormally low ratio of ventilation to perfusion. Usually, this is not clinically apparent, and mild degrees of desaturation may persist despite clinical improvement. Approximately 10% of hospitalized infants will develop alveolar hypoventilation and progressive hypercarbia and may require assisted ventilation. Hospitalization, if required, averages 3 days, but prolonged hospitalization is not uncommon.2 For most infants, however, the duration of the acute illness is 3 to 10 days, but respiratory signs, especially cough, may be present for 4 or more weeks.
The abnormalities observed on chest radiograph may be minimal, irrespective of the severity of the child's illness. Hyperaeration has been shown to be especially indicative of RSV infection and may be associated with peribronchial thickening.110, 111 Most children exhibit only airway disease. Less than 10% of bronchiolitis cases have evidence of both airway and airspace disease, and only about 1% have parenchymal consolidation.110 However, about 20% to 35% of children with RSV lower respiratory tract disease have opacities, which are commonly misdiagnosed as bacterial pneumonia. These most commonly are subsegmental in the right upper or middle lobe and result from atelectasis. Pleural fluid is rarely demonstrated.
Otitis Media
Acute otitis media is a common complication of RSV infection among young children.112, 113, 114 Among children within the first 3 years of life who developed acute otitis media, as many as 74% have had RSV detected in their middle ear fluid. Acute otitis media usually develops about 5 days after the onset of the respiratory illness and is more common among children older than 1 year than in infants. Even among older children and previously healthy adults with RSV infection, otitis and earache are common complications, noted in approximately half of hospitalized infants in one study.46, 55, 114 RSV has been recovered from the middle ear fluid as the sole pathogen, with bacteria, or with another virus in up 2% to 22% of cases.112, 114 RSV increases adherence of bacteria to respiratory epithelial cells, and the most frequent concurrent bacterial pathogens are those commonly found in the respiratory tract, especially Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. 114 Clinical and experimental evidence suggests that coinfection of RSV with a bacterial pathogen may prolong the duration and worsen the outcome of otitis media, resulting in a greater chance of treatment failure with antibiotics and persistent effusion.
Infections among Older Children
The frequency of RSV infections at all ages is well illustrated among families with young children and among those in contact with young children, as in schools and daycare facilities.44, 45, 46 Among longitudinally followed children attending daycare facilities who have had primary RSV infection in their first winter, 75% and 65% develop infection during their second and third years, respectively.45 Recurrent infections commonly are upper respiratory tract illnesses, but 20% to 50% of recurrent infections among preschool-aged children involve the lower respiratory tract.44 Although second episodes of lower respiratory tract illness among children are rarely as severe as the initial episode, many are associated with wheezing. The overall burden of repeated infection in children younger than 5 years is substantial.2
Infections among Adults
Adults also may have repetitive RSV infection occurring within sequential years, especially those living or working with children or in confined military and university settings.46, 55, 58, 115, 116 The lack of durable immunity evoked by natural infection is illustrated by a study of 15 healthy adults who became ill with a community-acquired RSV A strain and were subsequently challenged with an RSV A strain at repetitive intervals over 2 to 26 months.88 Within 2 months of their natural infection, 47% became reinfected, and by 8 months, two thirds had been reinfected. However, successive infections, especially those occurring within close intervals, were associated with decreasing symptoms and shedding. Nevertheless, observational studies have noted that both infection and severity of RSV infection in adults are reduced by higher titers of serum and mucosal antibody.8, 90
The extent of the burden RSV places on the health care of this growing population has only recently been appreciated fully.3, 7, 9, 56, 117, 118, 119 Those at greatest risk of severe illness include the frail elderly; those with underlying cardiopulmonary disease, especially chronic obstructive pulmonary disease (COPD); and the severely immunocompromised. Adults, even those with severe disease, shed RSV at titers considerably lower than infants, and thus estimates of the incidence of RSV infection in this population have been hampered until recently by the lack of sensitive diagnostic tests. The incidence of RSV hospitalization, found by using a regression model incorporating viral culture data from infants and hospital coding data from adults, has been estimated at 86 per 100,000, or 28% of the incidence of influenza hospitalizations.3 Using similar methods from the Netherlands to model data, RSV mortality was 64% of that attributable to influenza.9 Others have noted similar hospitalization and mortality rates in comparison to influenza.120, 121, 122
In long-term care facilities, 5% to 27% of respiratory infections have been reported to be caused by RSV. The attack rate generally has been estimated as 1% to 15%, the rate of pneumonia as 10% to 20%, and the mortality as 2% to 5%.117 A retrospective cohort analysis estimated that among Tennessee nursing home patients, RSV was associated with 15 hospitalizations, 17 deaths, and 76 antibiotic courses per 1000 people, and 7% of hospitalizations and 9% of deaths from cardiopulmonary disease.122 In a study of older adults admitted with cardiopulmonary conditions, 10% had RSV infection, compared with 13% with influenza.119 Illness was equally severe among the two groups: 18% required intensive care, and 10% of the RSV patients died, compared with 6% of the influenza patients. Similarly, among a prospectively studied population of older adults living in the community, RSV was identified as the cause of 7% and influenza as the cause of 9% of respiratory illnesses with identifiable pathogens.123 The morbidity associated with the two viruses was also comparable, with 82% of the RSV patients and 79% of the influenza patients developing lower respiratory tract disease. Among adults attending daycare facilities, 10% of acute respiratory infections were found to be caused by RSV, a rate similar to the rates for influenza and coronaviruses.124
The highest risk for severe RSV infection is in persons with underlying cardiopulmonary disease, especially COPD and congestive heart failure.7, 56, 119, 125, 126, 127 A 4-year study of respiratory illnesses among prospectively followed elderly and high-risk adults living in the community emphasizes the epidemiologic and clinical impact of RSV infection among these populations (Table 160-1 ).7 In this study, 608 healthy persons age 65 and older and 504 high-risk people with cardiopulmonary conditions were evaluated for RSV infection by culture, reverse-transcriptase polymerase chain reaction (RT-PCR), and serology. A third group of 1388 adults hospitalized with acute cardiopulmonary conditions was also evaluated. During 4 years, RSV infection was identified in 3% to 7% of the elderly cohort, 4% to 10% of the high-risk cohort, and 10% of the hospitalized group. In comparison, influenza infection was identified among 4% of the individuals in the prospectively followed cohorts and among 11% of the hospitalized patients. Clinically, the illnesses from RSV and influenza were remarkably similar. Among the healthy elderly outpatients, those with RSV infection and those with influenza were ill for an average of 16 days. Of those with RSV infection, 39% were unable to perform their activities of daily living, only slightly less than with influenza. Among the hospitalized RSV patients, intensive care was required for 15%, and the mortality rate was 8%, compared with 12% and 7%, respectively, among influenza patients. RSV was identified in 5.4% of admissions for congestive heart failure, 11.4% of COPD exacerbations, and 10.6% of pneumonia diagnoses. Among the prospective cohorts, almost all RSV infections were symptomatic and frequently associated with appreciable functional impairment and use of health care services. The clinical illness caused by RSV in elderly persons is nonspecific and not readily distinguishable from other common respiratory viruses.118 Typical upper respiratory symptoms are more frequent than with influenza and precede lower respiratory symptoms by several days. Wheezing is more common with RSV infection, especially in patients with COPD, and chest radiographic findings are often minimal.
TABLE 160-1.
Respiratory Syncytial Virus (RSV) Compared with Influenza among Hospitalized Adults*
| CHARACTERISTICS | RSV (N = 132) | Influenza A (N = 144) |
|---|---|---|
| Age (yr) | 76 ± 13 | 76 ± 12 |
| Female sex—N (%) | 84 (64) | 81 (56) |
| Chronic illness—N (%) | ||
| Any cardiac disease | 71 (54) | 71 (49) |
| Congestive heart failure | 39 (30) | 33 (23) |
| Any lung disease | 77 (58) | 79 (55) |
| Any heart or lung disease | 106 (80) | 113 (78) |
| Diabetes mellitus | 35 (27) | 28 (19) |
| Residence in a long-term care facility—N (%) | 16 (12) | 15 (10) |
| Smoking (current or past)—N (%) | 88 (67) | 98 (68) |
| Influenza vaccination—N (%) | 99 (75) | 98 (68) |
| Katz ADL score—mean ± SD | 1.2 ± 2.4 | 1.3 ± 3.0 |
| IADL score—mean ± SD | 4.1 ± 4.1 | 3.3 ± 4.0 |
| Length of hospital stay—days | 14 ± 41 | 8 ± 5 |
| Findings on chest radiography—N (%) | ||
| Infiltrate found | 41 (31) | 43 (30) |
| Congestive heart failure | 17 (13) | 15 (10) |
| Other | 24 (18) | 27 (19) |
| Admission to intensive care unit—N (%) | 20 (15) | 17 (12) |
| Use of mechanical ventilation—N (%) | 17 (13) | 15 (10) |
| Higher level of care at discharge than at admission—N (%) | 7 (5) | 8 (6) |
| Death—N (%) | 10 (8) | 10 (7) |
SD, standard deviation.
Plus-minus values are means plus or minus standard deviation. The Katz Activities of Daily Living (ADL) score and the Instrumental Activities of Daily Living (IADL) score are functional assessments based on a 12-point scale, with 0 representing total independence and 12 representing total dependence. Percentage may not sum to 100 because of rounding.
Modified from Falsey A, Hennessey PA, Formica MA, et al. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749-1759.
Peak viral titers in nasal secretions in nonhospitalized adults with RSV infection are substantially lower than in infants, ranging from 1 to 105 pfu/mL of nasal secretions (mean, 102.3), and slightly higher among hospitalized persons and those older than 65 years.128 When simultaneously measured, virus titers in sputum are generally higher than those found in nasal secretions. The duration of virus shedding in adults averages about 10 days but can be detected for as long as 20 to 30 days in some patients. In individual patients, RSV cannot be distinguished from other viral infections in this population. However, as in infants, wheezing is more common with RSV infection than influenza but similar to that caused by human metapneumovirus. Bacterial coinfection can occur during RSV infection, although the precise incidence is unknown. There are no controlled data on antiviral treatment for RSV in adults, and thus treatment is rarely considered except in immunocompromised patients (see later).
The increased morbidity associated with RSV infection observed among older adults is only partly explained by the presence of comorbid conditions and age-associated decline in pulmonary function.129 Evidence is accumulating that among healthy older adults, age adversely affects the immune response to RSV infection. Humoral immunity to RSV among elderly people, however, is equal to or greater than that produced by young adults.56, 89 Age may have its greatest effect on the cellular adaptive response, which demonstrates diminished RSV-specific CD8+ T-cell function in elderly persons.130, 131, 132 Experiments with rodents have shown specific defects in the cellular response to RSV infection related to senescence, including the timing and type of the pulmonary cytokine response.133
The burden of RSV among young healthy adults may be considerable and comparable to that from influenza.46, 55, 115 Among working, healthy adults, RSV infection was symptomatic in 84%, and 22% had lower respiratory tract manifestations (Table 160-2 ). Compared with influenza infection occurring in these same individuals, fever was less frequent, but earache and sinus pain and a persistent productive cough were significantly more common with RSV infection (Table 160-3 ). Thirty-eight percent of those with RSV infection missed work, and the average duration of illness was 9.5 days, which was significantly longer than that produced by influenza.115, 116 Wheezing was more frequently associated with RSV infection, and the proportion of military recruits with RSV infection requiring ward confinement was the same as for influenza. Among university students with cough persisting for 6 or more days, RSV was identified more often than the parainfluenza viruses, adenoviruses, pertussis, Mycoplasma pneumoniae, or Chlamydia pneumoniae. In more than half of those with RSV infection, the clinical manifestations were indistinguishable from those of pertussis, and most were treated with a macrolide.116
TABLE 160-2.
Types of Acute Respiratory Infection in Adults Who Are Infected with Respiratory Syncytial Virus (RSV)
| TYPE OF ACUTE RESPIRATORY ILLNESS | NO. OF PATIENTS | PERCENTAGE OF PATIENTS WITH SYMPTOMATIC RSV INFECTION (n = 177) | PERCENTAGE OF ALL PATIENTS WITH RSV INFECTION (n = 211) |
|---|---|---|---|
| Asymptomatic | 34 | — | 16 |
| Symptomatic | 177 | — | 84 |
| Upper respiratory tract | 131 | 74 | 62 |
| With fever | 52 | 29 | 25 |
| Without fever | 79 | 45 | 37 |
| Lower respiratory tract | 46 | 26 | 22 |
| Tracheobronchitis | 36 | 20 | 17 |
| Wheezing | 10 | 6 | 5 |
From Hall CB, Long CE, Schnabel KC. Respiratory syncytial virus infections in previously healthy working adults. Clin Infect Dis. 2001;33:792-796. Copyright 2001 with permission from the Infectious Diseases Society of America.
© 2015
Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. The COVID-19 resource centre is hosted on Elsevier Connect, the company's public news and information website. Elsevier hereby grants permission to make all its COVID-19-related research that is available on the COVID-19 resource centre - including this research content - immediately available in PubMed Central and other publicly funded repositories, such as the WHO COVID database with rights for unrestricted research re-use and analyses in any form or by any means with acknowledgement of the original source. These permissions are granted for free by Elsevier for as long as the COVID-19 resource centre remains active.
TABLE 160-3.
Clinical Characteristics of Illness Caused by Influenza or Respiratory Syncytial Virus (RSV) among 211 Previously Healthy Adults
| NO. (%) OF ADULTS WITH ILLNESS CAUSED BY: |
|||
|---|---|---|---|
| CHARACTERISTIC | RSV (n = 177) | Influenza (n = 59) | P |
| Sign or symptom | |||
| Fever (temperature, >37.8° C) | 50 (58) | 43 (73) | <.001 |
| Nasal congestion, rhinorrhea | 157 (89) | 46 (78) | <.04 |
| Sore throat | 102 (58) | 32 (54) | .65 |
| Ear pain | 35 (20) | 3 (5) | <.01 |
| Headache | 70 (40) | 48 (81) | <.001 |
| Sinus pain | 55 (31) | 8 (14) | <.01 |
| Cough | |||
| Nonproductive | 150 (85) | 47 (80) | .36 |
| Productive | 92 (52) | 14 (24) | <.001 |
| Lower respiratory tract signs, wheezing | 28 (16) | 5 (9) | .16 |
| Work absence | 67 (38) | 39 (66) | <.001 |
| Duration of illness, mean days (range) | 9.5 (1-20) | 6.8 (39-66) | <.001 |
From Hall CB, Long CE, Schnabel KC. Respiratory syncytial virus infections in previously healthy working adults. Clin Infect Dis. 2001;33:792-796. Copyright© Infectious Diseases Society of America.
© 2015
Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. The COVID-19 resource centre is hosted on Elsevier Connect, the company's public news and information website. Elsevier hereby grants permission to make all its COVID-19-related research that is available on the COVID-19 resource centre - including this research content - immediately available in PubMed Central and other publicly funded repositories, such as the WHO COVID database with rights for unrestricted research re-use and analyses in any form or by any means with acknowledgement of the original source. These permissions are granted for free by Elsevier for as long as the COVID-19 resource centre remains active.
Complications
Patients at High Risk for Severe Infection
Children with underlying conditions affecting cardiopulmonary function or immunity are most likely to increase the risk for developing complicated RSV infection among individuals of any age. Among young children, those most likely to require hospitalization are those who are premature and those with underlying chronic lung disease, cyanotic or complicated congenital heart disease, immunosuppressive conditions, or other chronic diseases that affect the handling of respiratory secretions, such as neuromuscular disease.2, 93, 134, 135 About one third of children who are hospitalized with RSV infection within the first 5 years of life have one or more of these underlying conditions, and the proportion is greater among those older than 2 years.2 Among children with RSV infection evaluated in pediatric practices and emergency departments, about one fourth have a preexisting chronic condition.
Preterm gestation, with or without associated chronic lung disease, is clearly a major risk factor for more severe RSV disease.2, 93, 135 Hospitalization rates among infants with gestational ages of less than 36 weeks are three or more times higher than the rate for full-term infants. As the gestational age decreases below 32 weeks, the chance of admission with RSV infections rises, and the need for intensive care significantly increases. Recently recognized, however, is the disproportionate economic and clinical burden contributed by late preterm infants—those with gestational ages of 33 to 35 weeks—with RSV infections who represent about three fourths of all preterm infants.93, 136 These late preterm infants, compared with full-term infants, not only have significantly increased costs and risk for hospitalization but also have a significantly greater need for medical resources for at least the subsequent year.
Congenital heart conditions, especially cyanotic heart conditions and those accompanied by pulmonary hypertension, have ranked among the top three major conditions among infants hospitalized with RSV infection, and about one fourth to one third require intensive care, and one fifth require mechanical ventilation.137, 138, 139 Infants hospitalized in the first few months of life with uncorrected cyanotic congenital heart disease are at particular peril. However, the increased rate of hospitalization for RSV among children with congenital heart disease remains beyond infancy and appears greatest during the second year of life.137, 139 Early surgical correction of cardiac defects have appreciably reduced the mortality from RSV infection among infants with congenital heart disease from 30% in the 1970s to less than 2% currently.
Multiple demographic and environmental factors also have been evaluated for augmenting the risk for more severe RSV infection.2, 140, 141 Those most frequently associated with RSV disease requiring hospitalization among young children are male gender, crowded living conditions, lower socioeconomic status, exposure to other young children in the home or daycare, exposure to tobacco smoke, and lack of breast-feeding. However, the degree of risk these factors play in the expression of RSV disease in children has been difficult to quantify, and the reported data have been inconsistent and conflicting. The two independent risk factors that appear to be most important for hospitalization with RSV infection are prematurity and young age, especially within the first 3 months of life.2, 93
The genetic background of an individual has important, but mostly undefined, effects on the susceptibility to RSV infection and more severe disease. Groups of children with certain racial and ethnic backgrounds have notably increased rates of hospitalization and severe RSV infection, including Native American Indian and Alaskan Native infants, especially those living in the Yukon-Kuskokwim Delta region who have RSV hospitalization rates three to four times that observed for other U.S. infants.93, 142 Severe disease with primary RSV infection has been correlated with specific polymorphisms in certain genetic loci affecting the immune response, including expression of cytokines and inflammatory chemokines.70, 72, 87
Immunocompromised Patients
Greater awareness of RSV as a cause of morbidity and mortality among immunocompromised patients has resulted from the increasing numbers of patients receiving solid-organ and hematopoietic cell transplantation (HCT) with more intensive chemotherapy regimens and also from the greater availability of sensitive techniques for identification of RSV infection, such as PCR assays.143, 144 The reported frequency of RSV infection among immunocompromised patients varies widely from about 2% to 50%, depending on the population and type of immunosuppression.103, 143, 144, 145, 146, 147, 148, 149, 150 Retrospective case series suggest a mortality rate of about 9% to 18%.
RSV is often introduced onto wards that house immunocompromised patients by medical staff or visitors, and spread may be rapid and difficult to control and accompanied by appreciable morbidity and mortality.103, 145, 147, 148, 149 The severity of the RSV infection among these patients is primarily related to the degree of immunosuppression103, 148, 149, 151; severe combined immunodeficiency states and HCT recipients are particularly at risk for a poor outcome. Among transplant recipients, factors associated with poor outcomes include use of bone marrow cells (in contrast to peripheral stem cells), occurrence of RSV infection before engraftment, the presence of acute or chronic graft-versus-host disease, and lymphopenia. Among children, young age also is associated with a poorer prognosis.
RSV infection in these patients may clinically mimic other opportunistic agents, and the correct etiology may not be suspected. Sinusitis and wheezing may be more indicative of RSV infection than other pathogens. Concurrent infections by other infectious agents, including community-acquired infections, may further confound or delay the diagnosis of RSV infection.149 In adult HCT recipients with RSV, upper respiratory tract signs precede pneumonia in 80% to 90% of patients, with progression to pneumonia in 30% to 40% after a median of 7 days.143, 144 The need for oxygen supplementation at the time of presentation is associated with significantly higher rates of respiratory failure and mortality.152 With lower respiratory tract involvement, radiographic findings range from focal interstitial infiltrates, sometimes with hyperinflation or with lobar consolidation, to generalized alveolar and interstitial infiltrates, or even to a picture of acute respiratory distress syndrome.147 With high-resolution computed tomography, the characteristic findings are airspace consolidation, small centrilobular nodules, ground-glass opacities, and thickening of the bronchial walls. These manifestations tend to have an asymmetrical but bilateral distribution.153 Long-term complications include bronchiolitis obliterans syndrome (BOS) in nearly 10%, although rates as high as 50% can be seen in lung transplant recipients with RSV lower respiratory infection.154 Overall mortality with RSV pneumonia is as high as 45%, although treatment with ribavirin, with or without immunoglobulin (intravenous immune globulin [IVIG] or monoclonal antibody), has been thought by some observers to reduce morbidity and mortality (see “Therapy”).
Among children with human immunodeficiency virus (HIV) infection, RSV has been the most frequently identified cause of viral respiratory disease, either acquired in the community or nosocomially.145 The manifestations of RSV infection vary according to the stage and severity of the HIV infection, and although most develop lower respiratory tract disease, it generally is not as severe as that occurring among the more highly immunosuppressed transplant recipients.155 HIV patients may shed RSV for prolonged periods. Confounding this, however, is the observation that children with HIV infection and concurrent viral respiratory infections also have a higher rate of bacterial coinfections.155 Of importance, the clinical outcome of respiratory viral infections has not been consistently different between children with and those without HIV infection.
Although many authorities generally recommend treatment of severely immunocompromised adults with ribavirin and antibody therapy, definitive guidelines are not available because of the lack of prospective controlled trials (see “Therapy”). Most important in the management of immunocompromised patients is prevention of RSV infection by strict adherence to infection control policies. The Centers for Disease Control and Prevention guidelines for preventing opportunistic infections in hematopoietic stem cell transplant recipients provides evidence-based recommendations for control of RSV infection and transmission. These guidelines emphasize preventing the introduction of community respiratory viruses, including RSV, onto units with compromised patients and stress the importance of early diagnosis.143, 156, 157
Acute Complications in Infants
Apnea is one of the most frightening and striking of acute complications among young infants with RSV infection. Up to 20% of infants hospitalized with RSV infection have been admitted with apnea.157, 158 Among infants evaluated in the emergency department with unspecified bronchiolitis, 3% had a diagnosis of apnea. Most at risk for developing apnea are preterm infants with a gestational age of 32 weeks or less, those with a history of apnea of prematurity, and infants of young postnatal age of less than 44 weeks after conception. Characteristically, the apnea occurs at the onset of the RSV infection and may be the initial sign before respiratory symptoms are noted. The pathophysiology of apnea associated with RSV is not clear, although it is nonobstructive. The prognosis is generally good after the acute RSV infection with no subsequent episodes, even with later-acquired respiratory infections.
Infants admitted with RSV lower respiratory tract disease may be at increased risk for aspiration, which can appear clinically similar to bronchiolitis with airway hyperreactivity.159 In one study of infants hospitalized with the diagnosis of RSV bronchiolitis and followed over a 12-month period, 83% developed reactive airway disease if they received neither ribavirin nor therapy for aspiration. However, the development of hyperreactive airways was reduced to 45% in infants who were given thickened feedings along with early ribavirin therapy. Furthermore, the decrease in episodes of reactive airway disease was greater in infants who received both ribavirin and thickened feedings than in infants who received either therapy alone.
Coexistent bacterial infection is a frequent concern in infants hospitalized with RSV lower respiratory tract disease, and many children receive unnecessary antibiotic treatment. In part, this is because of their young age, the presence of fever, and, particularly, the relatively common appearance on the chest radiograph of opacities from viral infiltrates and atelectasis, which are commonly mistaken for consolidation of bacterial pneumonia. However, multiple studies in the United States have shown that a secondary bacterial infection is an unusual complication of RSV infection.160, 161, 162 A 9-year prospective study of infants hospitalized with RSV lower respiratory tract disease identified secondary bacterial pneumonia in less than 1%.160 Furthermore, antibiotic therapy has not been shown to improve the rate of recovery among infants with RSV lower respiratory tract disease.163 More frequent is coinfection with another viral or bacterial agent. Most common of these coinfections are other respiratory viruses, such as adenoviruses, influenza, parainfluenza viruses, human metapneumovirus, and bocavirus.164 However, there is no definitive evidence that viral coinfection carries a worse outcome. Urinary tract infections are the most frequently identified concurrent bacterial infections.165 In developing countries, however, complicating bacterial infections are more common and may contribute appreciably to the high mortality rate from RSV.
Long-term Complications
Recurrent wheezing after RSV lower respiratory tract disease and bronchiolitis in infancy has long been recognized as a frequent sequela, but a causal link between the two remains unclear. About 30% to 50% of children hospitalized with RSV infection later develop repeated occurrences of wheezing.164, 166, 167 For many children, the severity of the recurrent wheezing episodes decreases with age, although in some, pulmonary function abnormalities may persist without clinical manifestations.168, 169 Others may have persistent wheezing into adolescence, or have wheezing cease during childhood, but recur as adults. The frequency of this long-term sequela in the general population is confounded by most studies having focused on children with more severe illness.170 Similarities between the immune responses after RSV infection and the responses observed with reactive airway disease have been offered as a possible explanation for the immunopathogenesis of the putative link between RSV bronchiolitis reactive airways in adolescence and adulthood.
Epidemiologic evidence indicates that atopy in the child or family is not a major cause of this link. However, in a murine model of allergen-induced airway inflammation and remodeling, previous RSV infection could induce airway abnormalities in mice exposed to allergen through the airway, even though these mice had not been previously sensitized to the allergens.171 This suggests that even without an atopic family background, RSV infection may provoke an allergic phenotype. In addition, RSV infection has been shown both in vitro and in children infected with RSV to produce an immunologic response similar to that observed with allergic sensitization, one with a predominantly Th2 T-cell profile and release of proinflammatory mediators, IgE, and neuropeptides70, 170, 172 (see “Immunity and Pathogenesis of Disease”). A similar response, however, may be produced by viruses other than RSV.170 The direct correlation between RSV lower respiratory tract disease and pulmonary sequelae likely remains elusive because the clinical syndrome of hyperreactive airways results from many different genetic, developmental, and environmental disorders.173
Diagnosis
RSV infection among young children is most often diagnosed clinically in the setting of the community's RSV season. Among adults, however, the findings are less specific, and RSV is commonly not suspected. Laboratory diagnosis may be made by viral isolation, by one of the rapid diagnostic tests, by RT-PCR, or by serology.
Viral isolation, once the standard technique, has been supplanted by one of the rapid antigen assays or by nucleic acid amplification, RT-PCR. The sensitivity of each of these tests is dependent on the viral load, and thus the choice of test must take into consideration the population under study. The sensitivity of viral isolation is highly dependent on the sensitivity of the cell lines, the specific laboratory's expertise, and the quality and handling of the specimen. RSV is a relatively labile virus and requires prompt inoculation without subjecting the specimen to major temperature changes during transportation. Nasopharyngeal washes or tracheal secretions are generally better than nasal swabs, although combined throat swab and swabs of both nares improve the rate of recovery over a single nasal swab.174, 175 Specific cytopathic changes usually appear within 2 to 5 days, but the range is 2 to 10 days. Shell vial culture assays allow diagnosis within 48 hours.
Multiple rapid direct antigen detection tests are widely available and are used by most laboratories.174 In hospitalized infants, direct and indirect immunofluorescent assays are both sensitive (93% to 98%) and specific (92% to 97%) but require several hours and skilled laboratory personnel. Most frequently used for infants are the commercially available rapid detection tests using an enzyme immunoassay (EIA) method and, to a lesser extent, the optical immunoassay (OIA), which has a sensitivity of 88% to 95% and specificity of 97% to 100% when compared with culture.174 The accuracy of these tests may vary according to the manufacturer and the viral strain but are highly dependent on the adequacy of the specimen and whether it was obtained during the peak of RSV activity in the community. The positive predictive value of these assays falls precipitously when the prevalence of RSV is low in the community. These tests perform poorly in adults, including the immunocompromised and elderly populations, because of the low viral titers in secretions.117, 174, 176
RT-PCR assay for the diagnosis of RSV infections has consistently demonstrated much higher rates of specificity and sensitivity than the rapid antigen diagnostic assays.174 Among 496 specimens obtained from children with RSV infection determined by viral isolation or duplicate positive RT-PCR assays, about 50% were positive by both RT-PCR and culture, and 50% by RT-PCR alone. Less than 1% were positive only by viral isolation. The RT-PCR assays also allow the strain group of RSV to be concurrently determined. The rapidly expanding microassay technology allows the simultaneous highly sensitive detection of potentially innumerable agents.175, 177
Serologic diagnosis of RSV infection is primarily useful for epidemiologic studies rather than for patient management because of the delay required to obtain convalescent sera and because young infants and immunocompromised patients may not produce a significant rise in antibody titer. Serologic diagnosis is most useful in elderly adults, who curiously have the most vigorous antibody responses to RSV.178
Therapy
Most children and adults with RSV infection require no more than the usual care given to ensure comfort, fever control, and adequate fluid intake. For bronchiolitis, the most commonly administered medications are those used for exacerbations of hyperreactive airway disease, primarily bronchodilators and corticosteroids, and also antibiotics.109, 179, 180 Multiple studies and meta-analyses have shown these agents are not consistently effective for RSV disease or bronchiolitis of unspecified cause among previously healthy young children and thus are not routinely recommended (see Chapter 68).
Antibiotic therapy for children with RSV lower respiratory tract disease should be reserved for those with specific evidence of a coexisting bacterial infection.109 Preemptive administration of antibiotics to children with RSV infection or bronchiolitis has not been associated with an improved outcome. Furthermore, complicating or secondary bacterial infections, other than otitis media among children with RSV infection in developed countries, is unusual.160, 161
Ribavirin (1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide), a synthetic nucleoside, is the only currently approved specific treatment for RSV lower respiratory tract disease in hospitalized infants (see Chapter 44). The drug, administered as a small-particle aerosol, has shown modest clinical benefit and improved levels of oxygenation in some studies, but an improvement in the duration of hospitalization or short-term outcome has not been consistently demonstrated. An analysis of the studies examining the effect of ribavirin therapy among children with RSV lower respiratory tract disease included 11 relatively small, randomized controlled trials. Of these, 7 showed clinical benefit, and 4 did not.109 A Cochrane Review analysis of randomized placebo-controlled trials evaluating ribavirin for the therapy of children hospitalized with RSV lower respiratory tract disease found that ribavirin reduced the duration of mechanical ventilation and length of hospitalization.181 Significant reduction in respiratory failure or deaths was not shown, but few children progressed to either. Ribavirin has teratogenic properties, and precautions must be taken to guard against exposing women of potential childbearing capacity.
In view of the unclear degree of benefit relative to the considerable cost of aerosolized ribavirin, the American Academy of Pediatrics recommends that ribavirin should not be used routinely in the management of RSV lower respiratory tract infections.93, 109 Ribavirin therapy should be considered on an individual basis for children who have severe disease or who are at high risk for developing complicated or severe disease.
The data supporting ribavirin treatment of RSV infection in severely immunocompromised persons with RSV infection is primarily drawn from retrospective case series.103, 143, 182, 183 In a large retrospective single-center analysis of 280 HCT patients with RSV infection, progression to lower respiratory disease and mortality was reduced significantly in patients in whom administration of inhaled ribavirin was started when symptoms were limited to the upper respiratory tract.182 The only prospective placebo-controlled study was performed in 14 HCT patients with RSV upper respiratory tract infection. Although ribavirin was safe and showed a trend of decreasing viral load during therapy, the number of subjects was too small to make firm conclusions.184 Inhaled ribavirin has been given either continuously (6 g over 18 hours) or intermittently (2 g over 2 to 3 hours three times per day). In a prospective randomized trial comparing these two dosing schedules in immunocompromised patients with RSV infection, the intermittent schedule appeared more effective in preventing development of lower respiratory disease.185
The efficacy of systemic ribavirin, administered either by the oral or the intravenous route, is unknown in the immunocompromised patient with RSV infection, although retrospective case studies suggest they may provide some benefit.186, 187, 188 Experimental therapies, such as one described in a recent report of inhaled RNA interference therapy in lung transplant patients, or newer inhibitors of the RSV fusion protein, may offer possible future therapy.154
The benefit of immunoglobulin therapy for RSV infection among highly immunocompromised patients is based primarily on retrospective observational studies. The results with either polyclonal IVIG or the anti-F monoclonal antibody (palivizumab) alone or in combination with ribavirin have been mixed, with some studies suggesting a trend toward diminished morbidity and progression to lower respiratory tract disease when used therapeutically.* However, consensus exists that these monoclonals appear safe and well tolerated by highly immunosuppressed patients. Prophylactic administration of palivizumab to immunocompromised patients has not been adequately evaluated and is not recommended.93 The American Academy of Pediatrics, nevertheless, states that children with severe immunodeficiencies may benefit from prophylaxis. Using a decision analysis model to evaluate the effect of palivizumab prophylaxis to prevent RSV mortality after bone marrow transplantation in children, the survival rate was estimated to increase by 10%, and it was calculated that 12 children would need to be treated to prevent one fatal RSV infection.191 Palivizumab is extraordinarily expensive in dosages administered to adults and is approved only for intramuscular use.
New approaches are being investigated to develop therapies specific for RSV disease.12, 192 Among these include the development of inhibitors of attachment and fusion, including small peptide fusion inhibitors, N protein inhibitors, and RNA-dependent RNA polymerase inhibitors.193 Much interest and effort recently has been focused on developing oligonucleotides that interfere with viral RNA, antisense/small interfering RNA (siRNA) inhibitors.154 Some of these have shown promise in clinical trials.
Prevention
The viper's venom,
the serpent's spell
daunts not the tortoise
beneath his shell.
C.B.H.
Infection Control
Prevention rather than treatment is the preferable, but yet unattained, goal for the control of RSV infection. Avoiding infection at home through interruption of the transmission of the virus is difficult and unlikely to be effective. However, general precautions may be useful against the spread of infectious secretions on hands and fomites. These include good hand hygiene, use of hand-rub antiseptic products, and care of contaminated tissues, toys, and other objects likely to be contaminated with secretions.194
On hospital wards, however, RSV poses a particular hazard for nosocomial spread.58, 147, 195, 196, 197 Yearly outbreaks occur with widespread infection among both children and adults, including medical personnel who may continue to work despite upper respiratory tract signs. Considerable morbidity and mortality have been associated with nosocomial RSV infection among those with underlying conditions, especially prematurity, and cardiopulmonary and immunocompromised conditions. Strict adherence to recommended guidelines, therefore, is essential and cost-effective.
RSV may be spread by close contact and by direct inoculation of large droplets from the secretions of an infected person, as well as by indirect spread from hands that touch infectious secretions in the environment.58, 196 Careful hand hygiene by all personnel, therefore, is integral to preventing nosocomial transmission (Table 160-4 ). Additional procedures aimed at preventing self-inoculation include the wearing of eye-nose goggles and gloves. Procedures aimed at reducing the risk for the introduction and spread of RSV to other personnel and patients include the wearing gowns for close contact with infected patients, isolation or cohorting of infected patients, and use of rapid diagnostic techniques. In addition, during the RSV season, staff with signs of respiratory illness should not care for high-risk patients, and visitors should be screened for respiratory illness.58
TABLE 160-4.
Infection Control Procedures, Both Standard Precautions and Contact Precautions, for Prevention of Respiratory Syncytial Virus (RSV) Infection Recommended by Centers for Disease Control and Prevention
| RECOMMENDATION CATEGORY, PROCEDURE | COMMENTS |
|---|---|
| Category 1-B Recommendations* | |
| Hand washing | Water with soap or antibacterial agent or waterless antiseptic hand rub |
| Wearing gloves | Combined with hand washing before and after each glove change; may diminish self-inoculation |
| Wearing gowns | When direct contact with patient or patient secretions is likely |
| Wearing masks plus eye protection | Eyes and nose are major sites for inoculation |
| Housing patient in private room or in a cohort isolated from other patients | Patients with documented infection can be grouped and isolated from other patients; beds should be separated by >0.9 m |
| Use of dedicated patient care equipment | Equipment, including toys, assigned to specific patients |
| Sometimes Recommended with Less or No Supporting Evidence | |
| Staff assigned according to patient's RSV status | Specific staff care only for patients with RSV infection |
| Visitor restrictions during RSV season | Some qualify by restricting young children only |
| Screening visitors for illness during RSV season | Visitor assessed by trained personnel or advised by use of an educational patient information list |
From Garner JS. Guidelines for isolation precautions in hospitals. Infect Control Hosp Epidemiol. 1996;17:53-80.
From Hall CB. Nosocomial respiratory syncytial virus infections: the “Cold War” has not ended. Clin Infect Dis. 2000;360:588-598.
Prophylaxis
Prophylaxis using the passive administration of RSV-specific antibody currently is available primarily for those groups most at risk for developing severe or complicated RSV disease. High-risk children administered monthly doses of intravenous immune globulin containing high levels of RSV neutralizing antibody (RSV-IVIG) or intramuscular monoclonal antibody have shown reduced rates of hospitalization from RSV infection.93 RSV-IVIG is no longer available and has been replaced by palivizumab, a humanized monoclonal antibody. Palivizumab was developed from a mouse monoclonal antibody directed against a protective epitope of the RSV fusion (F) protein. Immunoprophylaxis of five monthly intramuscular doses of 15 mg/kg initiated a month before the RSV season has been shown to reduce hospitalization rates by 34% to 82% among selected groups of high-risk infants.93 Palivizumab administration does not prevent infection with RSV but is associated with diminished clinical severity, the risk for developing lower respiratory tract disease, and need for hospitalization.
Currently, palivizumab is recommended for selected children who are younger than 2 years and are considered to have a high risk for developing severe RSV disease. Included are those with prematurity (<32 weeks' gestation), chronic lung disease, functionally important cardiac disease, and chronic conditions that interfere with the handling of respiratory secretions.93 Infants born between 32 and 35 weeks' gestation also have significantly greater risk for hospitalization and use of medical services for RSV disease than full-term infants.93 However, considering that most premature infants are between 32 and 35 weeks' gestation and that palivizumab prophylaxis is very expensive, the American Academy of Pediatrics recommends that infants born from 32 to 35 weeks' gestation be considered for prophylaxis if they are younger than 3 months at the start of the RSV season and have additional risk factors.93
Controversy exists concerning the extent of the use of palivizumab prophylaxis based primarily on the concern of its considerable cost relative to its benefit. Economic analyses in general have not shown an overall savings in health care costs for prophylaxis of all infants less than 32 weeks' gestation and with underlying high-risk conditions, but the benefit relative to the cost among those at highest risk increases.198, 199 Palivizumab prophylaxis has not been shown to reduce the mortality from RSV, but currently in the United States, the mortality from RSV infection, even among high-risk infants, is very low. The effect of palivizumab on the long-term sequelae of RSV infection has not been adequately evaluated.200
Additional products are being evaluated for the prevention of RSV disease in high-risk individuals, including a newer humanized monoclonal antibody, motavizumab.201 Motavizumab is derived from palivizumab and is also directed against the F protein. In vitro, it exhibits a 20-fold improvement in RSV neutralization. A recent multicenter study of more than 6000 high-risk infants showed that infants receiving motavizumab, compared with those receiving palivizumab, had a 26% relative reduction in RSV hospitalization, and motavizumab was superior to palivizumab in reducing RSV-related outpatient visits for lower respiratory tract disease.202 However, the U.S. Food and Drug Administration panel raised questions regarding motavizumab's safety (primarily allergic reactions), and it has not been approved for prophylaxis or treatment of RSV infection in high-risk infants currently.
Immunization
Currently, an effective vaccine for prevention of RSV for use in infants or in adults is not available. Ideally, a vaccine should provide better protection than natural disease, which does not confer durable immunity. This is compounded by the lack of identifiable correlates of solid immunity to infection. Also, concern still exists that an unpredictable abnormal immune-mediated response could occur in some vaccinees during their first RSV infection, such as was observed during the trials with the initial formalin-inactivated RSV vaccine.74 Furthermore, immunization would have to be initiated within the first weeks of life because most hospitalization for RSV occurs in the first several months of life. Even the response to a vaccine administered to adults and healthy older children may not predict the vaccine's safety or protection among very young infants.
Experimental live-attenuated vaccines, produced by chemical mutagenesis and reverse-genetic techniques, have thus far proven to be poorly immunogenic or underattenuated, resulting in upper airway obstruction from increased production of mucus and secretions. Among infants administered a candidate live-attenuated RSV vaccine, all seronegative children older than 6 months developed at least a fourfold antibody response after a dose of vaccine, whereas only 44% of infants 2 to 4 months of age were able to do so after two doses.203 In addition, the induction of a cytotoxic cellular response from RSV immunization appears variable.203 Of the children given a live candidate RSV vaccine, less than 40% of those younger than 5 months produced a detectable, but transient, cytotoxic T-cell response to RSV, compared with 65% of those 6 to 24 months of age. RSV immunization in early life is further complicated by the apparent bias toward a Th2-type immunologic response to RSV that exists during the first 3 months of life.106
The efficacy of a candidate RSV vaccine may also relate to its ability to stimulate an adequate local antibody response in the respiratory tract, which may be the first line of defense.12 Despite high levels of RSV antibodies in the serum, little reaches the respiratory secretions. Transudation of serum antibodies is poor, resulting in RSV antibody levels being 80 to 160 times less in the respiratory tract secretions or even less in the nasal secretions, compared with the antibody levels in the serum. Nevertheless, a major potential advantage of a live-attenuated intranasal vaccine in comparison to a subunit vaccine is that it is more apt to mimic naturally evoked immunity and induce local respiratory tract antibodies.
Subunit candidate vaccines have focused on F and G proteins, primarily using the F protein because of its considerable cross-reactivity between group A and B RSV strains. These purified F protein vaccines have been safe and immunogenic among individuals who are seropositive from previous natural RSV infection and should theoretically boost neutralizing serum antibodies, especially in adults.204, 205, 206 They may offer the possibility of boosting the immunity among some groups of older children and adults who have an increased risk for developing more severe RSV infection and among pregnant women as a means of augmenting maternal antibody levels in the infant.207 However, because of the aforementioned concerns about the initial experience with RSV as elicited by nonreplicating virus, live-attenuated vaccines have been judged a more appropriate approach for RSV-naïve infants.
The use of reverse genetics has resulted in generations of newly designed candidate strains that contain mutations specifically chosen for their attenuating, immunogenic, and other advantageous characteristics.208 These candidate “designer gene” vaccines are potentially safer, have increased breadth of antigenic expression, and allow combination vaccines with parainfluenza and other respiratory pathogens.209
Footnotes
Key References
The complete reference list is available online at Expert Consult.
- 2.Hall C, Weinberg G, Iwane M. The burden of respiratory syncytial virus infection among healthy children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou H, Thompson WW, Viboud CG. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993-2008. Clin Infect Dis. 2012;54:1427–1436. doi: 10.1093/cid/cis211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falsey A, Hennessey P, Formica M. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 9.van Asten L, van den Wijngaard C, van Pelt W. Mortality attributable to 9 common infections: significant effect of influenza A, respiratory syncytial virus, influenza B, norovirus, and parainfluenza in elderly persons. J Infect Dis. 2012;206:628–639. doi: 10.1093/infdis/jis415. [DOI] [PubMed] [Google Scholar]
- 11.Chanock R, Roizman B, Myers R. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA), I. Isolation, properties and characterization. Am J Hyg. 1957;66:281–290. doi: 10.1093/oxfordjournals.aje.a119901. [DOI] [PubMed] [Google Scholar]
- 12.Collins P, Crowe J., Jr . Respiratory syncytial virus and metapneumovirus. In: Knipe DM, Howley PM, Griffin DE, editors. Fields Virology. 5th ed. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 73–82. [Google Scholar]
- 20.Hall C, Douglas RJ, Geiman J. Possible transmission by fomites of respiratory syncytial virus. J Infect Dis. 1980;141:98–102. doi: 10.1093/infdis/141.1.98. [DOI] [PubMed] [Google Scholar]
- 22.Graham B, Rutigliano J, Johnson T. Respiratory syncytial virus immunobiology and pathogenesis. Virology. 2002;297:1–7. doi: 10.1006/viro.2002.1431. [DOI] [PubMed] [Google Scholar]
- 33.Hall C, Douglas RJ. Modes of transmission of respiratory syncytial virus. J Pediatr. 1981;99:100–103. doi: 10.1016/s0022-3476(81)80969-9. [DOI] [PubMed] [Google Scholar]
- 34.Peret T, Hall C, Hammond G. Circulation patterns of group A and B human respiratory syncytial virus genotypes in 5 communities in North America. J Infect Dis. 2000;181:1891–1896. doi: 10.1086/315508. [DOI] [PubMed] [Google Scholar]
- 36.Jafri HS, Wu X, Makari D, Henrickson KJ. Distribution of respiratory syncytial virus subtypes A and B among infants presenting to the emergency department with lower respiratory tract infection or apnea. Pediatr Infect Dis J. 2013;32:335–340. doi: 10.1097/INF.0b013e318282603a. [DOI] [PubMed] [Google Scholar]
- 39.Trento A, Casas I, Calderon A. Ten years of global evolution of the human respiratory syncytial virus BA genotype with a 60-nucleotide duplication in the G protein gene. J Virol. 2010;84:7500–7512. doi: 10.1128/JVI.00345-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glezen W, Taber L, Frank A. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 46.Hall C, Geiman J, Biggar R. Respiratory syncytial virus infections within families. N Engl J Med. 1976;294:414–419. doi: 10.1056/NEJM197602192940803. [DOI] [PubMed] [Google Scholar]
- 55.Hall C. Respiratory syncytial virus infections in previously healthy working adults. Clin Infect Dis. 2001;33:792–796. doi: 10.1086/322657. [DOI] [PubMed] [Google Scholar]
- 58.Hall C. Nosocomial respiratory syncytial virus infections: the “cold war” has not ended. Clin Infect Dis. 2000;31:590–596. doi: 10.1086/313960. [DOI] [PubMed] [Google Scholar]
- 61.Hall C, Douglas RJ, Schnabel K. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect Immun. 1981;33:779–783. doi: 10.1128/iai.33.3.779-783.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Houben ML, Coenjaerts FE, Rossen JW. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J Med Virol. 2010;82:1266–1271. doi: 10.1002/jmv.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DeVincenzo JP, El Saleeby CM, Bush AJ. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J Infect Dis. 2005;191:1861–1868. doi: 10.1093/infdis/jir494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson J, Gonzales R, Olson S. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol. 2007;20:108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- 72.Miyairi I, DeVincenzo JP. Human genetic factors and respiratory syncytial virus disease severity. Clin Microbiol Rev. 2008;21:686–703. doi: 10.1128/CMR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Collins P, Graham B. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82:2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim H, Canchola J, Brandt C. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 76.Kapikian A, Mitchell R, Chanock R. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 83.Welliver T, Reed J, Welliver R., Sr Respiratory syncytial virus and influenza virus infections: observations from tissues of fatal infant cases. Pediatr Infect Dis J. 2008;27:S92–S96. doi: 10.1097/INF.0b013e318168b706. [DOI] [PubMed] [Google Scholar]
- 86.Lukens MV, van de Pol AC, Coenjaerts FE. A systemic neutrophil response precedes robust CD8(+) T-cell activation during natural respiratory syncytial virus infection in infants. J Virol. 2010;84:2374–2383. doi: 10.1128/JVI.01807-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Glezen W, Paredes A, Allison J. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981;98:708–715. doi: 10.1016/s0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- 93.Committee on Infectious Diseases From the American Academy of Pediatrics: policy statements—modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics. 2009;124:1694–1701. doi: 10.1542/peds.2009-2345. [DOI] [PubMed] [Google Scholar]
- 100.Welliver R, Wong D, Sun M. The development of respiratory syncytial virus-specific IgE and the release of histamine in naso-pharyngeal secretions after infection. N Engl J Med. 1981;305:841–846. doi: 10.1056/NEJM198110083051501. [DOI] [PubMed] [Google Scholar]
- 103.Kim Y, Boeckh M, Englund J. Community respiratory virus infections in immunocompromised patients: hematopoietic stem cell and solid organ transplant recipients, and individuals with human immunodeficiency virus infection. Semin Respir Crit Care Med. 2007;28:222–242. doi: 10.1055/s-2007-976494. [DOI] [PubMed] [Google Scholar]
- 109.American Academy of Pediatrics Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–1793. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- 111.Friis B, Eiken M, Hornsleth A. Chest x-ray appearances in pneumonia and bronchiolitis. Acta Paediatr Scand. 1990;79:219–225. doi: 10.1111/j.1651-2227.1990.tb11442.x. [DOI] [PubMed] [Google Scholar]
- 114.Marom T, Nokso-Koivisto J, Chonmaitree T. Viral-bacterial interactions in acute otitis media. Curr Allergy Asthma Rep. 2012;12:551–558. doi: 10.1007/s11882-012-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Camargo CA, Ginde AA, Clark S. Viral pathogens in acute exacerbations of chronic obstructive pulmonary disease. Intern Emerg Med. 2008;3:355–359. doi: 10.1007/s11739-008-0197-0. [DOI] [PubMed] [Google Scholar]
- 128.Walsh EE, Peterson DR, Kalkanoglu AE. Viral shedding and immune responses to respiratory syncytial virus infection in older adults. J Infect Dis. 2013;207:1424–1432. doi: 10.1093/infdis/jit038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Romero J. Palivizumab prophylaxis of respiratory syncytial virus disease from 1998 to 2002: results from four years of palivizumab usage. Pediatr Infect Dis J. 2003;22:S46–S54. doi: 10.1097/01.inf.0000053885.34703.84. [DOI] [PubMed] [Google Scholar]
- 139.Altman C, Englund J, Demmler G. Respiratory syncytial virus in patients with congenital heart disease: a contemporary look at epidemiology and success of preoperative screening. Pediatr Cardiol. 2000;21:433–438. doi: 10.1007/s002460010103. [DOI] [PubMed] [Google Scholar]
- 143.Hirsch HH, Martino R, Ward KN. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis. 2013;56:258–266. doi: 10.1093/cid/cis844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Boeckh M. The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br J Haematol. 2008;143:455–467. doi: 10.1111/j.1365-2141.2008.07295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.El Saleeby C, Somes G, DeVincenzo J. Risk factors for severe respiratory syncytial virus disease in children with cancer: the importance of lymphopenia and young age. Pediatrics. 2008;121:235–243. doi: 10.1542/peds.2007-1102. [DOI] [PubMed] [Google Scholar]
- 161.Purcell K, Fergie J. Concurrent serious bacterial infections in 2396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156:322–324. doi: 10.1001/archpedi.156.4.322. [DOI] [PubMed] [Google Scholar]
- 166.Szabo SM, Levy AR, Gooch KL. Elevated risk of asthma after hospitalization for respiratory syncytial virus infection in infancy. Paediatr Respir Rev. 2013;13(suppl 2):S9–S15. doi: 10.1016/S1526-0542(12)70161-6. [DOI] [PubMed] [Google Scholar]
- 170.Gern J. Viral respiratory infection and the link to asthma. Pediatr Infect Dis J. 2008;27:S97–S103. doi: 10.1097/INF.0b013e318168b718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.American Academy of Pediatrics . Respiratory syncytial virus. In: Pickering LK, Bucher CJ, Kimberlin DW, Long SS, editors. Red Book: 2012 Report of the Committee on Infectious Diseases. American Academy of Pediatrics; Elk Grove Village, IL: 2012. pp. 609–618. [Google Scholar]
- 181.Ventre K, Randolph A. Ribavirin for respiratory syncytial virus infection of the lower respiratory tract in infants and young children. Cochrane Database Syst Rev. 2007;(1) doi: 10.1002/14651858.CD000181.pub3. [DOI] [PubMed] [Google Scholar]
- 182.Shah DP, Ghantoji SS, Shah JN. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother. 2013;68:1872–1880. doi: 10.1093/jac/dkt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chemaly RF, Torres HA, Munsell MF. An adaptive randomized trial of an intermittent dosing schedule of aerosolized ribavirin in patients with cancer and respiratory syncytial virus infection. J Infect Dis. 2012;206:1367–1371. doi: 10.1093/infdis/jis516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Goldmann D. Epidemiology and prevention of pediatric viral respiratory infections in health-care institutions. Emerg Infect Dis. 2001;7:249–253. doi: 10.3201/eid0702.010220. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Shay D, Holman R, Newman R. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 2.Hall C, Weinberg G, Iwane M. The burden of respiratory syncytial virus infection among healthy children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou H, Thompson WW, Viboud CG. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993-2008. Clin Infect Dis. 2012;54:1427–1436. doi: 10.1093/cid/cis211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stockman LJ, Curns AT, Anderson LJ, Fischer-Langley G. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997-2006. Pediatr Infect Dis J. 2012;31:5–9. doi: 10.1097/INF.0b013e31822e68e6. [DOI] [PubMed] [Google Scholar]
- 5.Forster J, Ihorst G, Rieger C. Prospective population-based study of viral lower respiratory tract infections in children under 3 years of age (the PRI.DE study) Eur J Pediatr. 2004;163:709–716. doi: 10.1007/s00431-004-1523-9. [DOI] [PubMed] [Google Scholar]
- 6.Hall C. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 7.Falsey A, Hennessey P, Formica M. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 8.Walsh E, Peterson D, Falsey A. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis. 2004;189:233–238. doi: 10.1086/380907. [DOI] [PubMed] [Google Scholar]
- 9.van Asten L, van den Wijngaard C, van Pelt W. Mortality attributable to 9 common infections: significant effect of influenza A, respiratory syncytial virus, influenza B, norovirus, and parainfluenza in elderly persons. J Infect Dis. 2012;206:628–639. doi: 10.1093/infdis/jis415. [DOI] [PubMed] [Google Scholar]
- 10.Morris J, Blount R, Savage R. Recovery of cytopathogenic agent from chimpanzees with coryza. Proc Soc Exp Biol Med. 1956;92:544–549. doi: 10.3181/00379727-92-22538. [DOI] [PubMed] [Google Scholar]
- 11.Chanock R, Roizman B, Myers R. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA), I. Isolation, properties and characterization. Am J Hyg. 1957;66:281–290. doi: 10.1093/oxfordjournals.aje.a119901. [DOI] [PubMed] [Google Scholar]
- 12.Collins P, Crowe J., Jr . Respiratory syncytial virus and metapneumovirus. In: Knipe DM, Howley PM, Griffin DE, editors. Fields Virology. 5th ed. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 73–82. [Google Scholar]
- 13.Renshaw RW, Zylich NC, Laverack MA. Pneumovirus in dogs with acute respiratory disease. Emerg Infect Dis. 2010;16:993–995. doi: 10.3201/eid1606.091778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rebuffo-Scheer C, Bose M, He J. Whole genome sequencing and evolutionary analysis of human respiratory syncytial virus A and B from Milwaukee, WI, 1998-2010. PLoS One. 2011;6:e25468. doi: 10.1371/journal.pone.0025468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gan SW, Ng L, Lin X. Structure and ion channel activity of the human respiratory syncytial virus (hRSV) small hydrophobic protein transmembrane domain. Protein Sci. 2008;17:813–820. doi: 10.1110/ps.073366208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spann KM, Tran KC, Chi B. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages [corrected] J Virol. 2004;78:4363–4369. doi: 10.1128/JVI.78.8.4363-4369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tayyari F, Marchant D, Moraes TJ. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat Med. 2011;17:1132–1135. doi: 10.1038/nm.2444. [DOI] [PubMed] [Google Scholar]
- 18.Chaiwatpongsakorn S, Epand RF, Collins PL. Soluble respiratory syncytial virus fusion protein in the fully cleaved, pretriggered state is triggered by exposure to low-molarity buffer. J Virol. 2011;85:3968–3977. doi: 10.1128/JVI.01813-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hambling M. Survival of the respiratory syncytial virus during storage under various conditions. Br J Exp Pathol. 1964;45:647–655. [PMC free article] [PubMed] [Google Scholar]
- 20.Hall C, Douglas RJ, Geiman J. Possible transmission by fomites of respiratory syncytial virus. J Infect Dis. 1980;141:98–102. doi: 10.1093/infdis/141.1.98. [DOI] [PubMed] [Google Scholar]
- 21.Domachowske J, Bonville C, Rosenberg H. Animal models for studying respiratory syncytial virus infection and its long term effects on lung function. Pediatr Infect Dis J. 2004;23:S228–S234. doi: 10.1097/01.inf.0000144672.81955.a4. [DOI] [PubMed] [Google Scholar]
- 22.Graham B, Rutigliano J, Johnson T. Respiratory syncytial virus immunobiology and pathogenesis. Virology. 2002;297:1–7. doi: 10.1006/viro.2002.1431. [DOI] [PubMed] [Google Scholar]
- 23.Berman S. Epidemiology of acute respiratory infections in children of developing countries. Rev Infect Dis. 1991;13:S454–S462. doi: 10.1093/clinids/13.supplement_6.s454. [DOI] [PubMed] [Google Scholar]
- 24.Shek L, Lee B. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatr Respir Rev. 2003;4:105–111. doi: 10.1016/s1526-0542(03)00024-1. [DOI] [PubMed] [Google Scholar]
- 25.Bloom-Feshbach K, Alonso WJ, Charu V. Latitudinal variations in seasonal activity of influenza and respiratory syncytial virus (RSV): a global comparative review. PLoS One. 2013;8:e54445. doi: 10.1371/journal.pone.0054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meissner C. Summary: seasonal and geographic variation in respiratory syncytial virus outbreaks across the United States. Pediatr Infect Dis J. 2007;26:S60. [Google Scholar]
- 27.Okiro EA, Ngama M, Bett A, Nokes DJ. The incidence and clinical burden of respiratory syncytial virus disease identified through hospital outpatient presentations in Kenyan children. PLoS One. 2012;7:e52520. doi: 10.1371/journal.pone.0052520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Light M. Respiratory syncytial virus seasonality in southeast Florida: results from three area hospitals caring for children. Pediatr Infect Dis J. 2007;26:S55–S59. doi: 10.1097/INF.0b013e318157dac1. [DOI] [PubMed] [Google Scholar]
- 29.Welliver R. Temperature, humidity, and ultraviolet b radiation predict community respiratory syncytial virus activity. Pediatr Infect Dis J. 2007;26:S29–S35. doi: 10.1097/INF.0b013e318157da59. [DOI] [PubMed] [Google Scholar]
- 30.Donaldson G. Climate change and the end of the respiratory syncytial virus season. Clin Infect Dis. 2006;42:677–679. doi: 10.1086/500208. [DOI] [PubMed] [Google Scholar]
- 31.Noyola D, Mandeville P. Effect of climatological factors on respiratory syncytial virus epidemics. Epidemiol Infect. 2008;136:1328–1332. doi: 10.1017/S0950268807000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sloan C, Moore ML, Hartert T. Impact of pollution, climate, and sociodemographic factors on spatiotemporal dynamics of seasonal respiratory viruses. Clin Transl Sci. 2011;4:48–54. doi: 10.1111/j.1752-8062.2010.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall C, Douglas RJ. Modes of transmission of respiratory syncytial virus. J Pediatr. 1981;99:100–103. doi: 10.1016/s0022-3476(81)80969-9. [DOI] [PubMed] [Google Scholar]
- 34.Peret T, Hall C, Hammond G. Circulation patterns of group A and B human respiratory syncytial virus genotypes in 5 communities in North America. J Infect Dis. 2000;181:1891–1896. doi: 10.1086/315508. [DOI] [PubMed] [Google Scholar]
- 35.Walsh EE, McConnochie KM, Long CE, Hall CB. Severity of respiratory syncytial virus infection is related to virus strain. J Infect Dis. 1997;175:814–820. doi: 10.1086/513976. [DOI] [PubMed] [Google Scholar]
- 36.Jafri HS, Wu X, Makari D, Henrickson KJ. Distribution of respiratory syncytial virus subtypes A and B among infants presenting to the emergency department with lower respiratory tract infection or apnea. Pediatr Infect Dis J. 2013;32:335–340. doi: 10.1097/INF.0b013e318282603a. [DOI] [PubMed] [Google Scholar]
- 37.Matheson J, Rich F, Cohet C. Distinct patterns of evolution between respiratory syncytial virus subgroups A and B from New Zealand isolates collected over thirty-seven years. J Med Virol. 2006;78:1354–1364. doi: 10.1002/jmv.20702. [DOI] [PubMed] [Google Scholar]
- 38.Hall C, Walsh E, Schnabel K. Occurrence of groups A and B of respiratory syncytial virus over 15 years: associated epidemiologic and clinical characteristics in hospitalized and ambulatory children. J Infect Dis. 1990;162:1283–1290. doi: 10.1093/infdis/162.6.1283. [DOI] [PubMed] [Google Scholar]
- 39.Trento A, Casas I, Calderon A. Ten years of global evolution of the human respiratory syncytial virus BA genotype with a 60-nucleotide duplication in the G protein gene. J Virol. 2010;84:7500–7512. doi: 10.1128/JVI.00345-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papadopoulos N, Gourgiotis D, Javadyan A. Does respiratory syncytial virus subtype influence the severity of acute bronchiolitis in hospitalized infants? Respir Med. 2004;98:879–882. doi: 10.1016/j.rmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Brandenburg A, van Beek R, Moll H. G protein variation in respiratory syncytial virus group A does not correlate with clinical severity. J Clin Microbiol. 2000;38:2849–2852. doi: 10.1128/jcm.38.10.3849-3852.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyce T, Mellen B, Mitchel EJ. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr. 2000;137:865–870. doi: 10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- 43.Iwane M, Edwards K, Szilagyi P. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics. 2004;113:1758–1764. doi: 10.1542/peds.113.6.1758. [DOI] [PubMed] [Google Scholar]
- 44.Glezen W, Taber L, Frank A. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 45.Henderson F, Collier A, Clyde W. Respiratory syncytial virus infections, reinfections and immunity: A prospective, longitudinal study in young children. N Engl J Med. 1979;300:530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- 46.Hall C, Geiman J, Biggar R. Respiratory syncytial virus infections within families. N Engl J Med. 1976;294:414–419. doi: 10.1056/NEJM197602192940803. [DOI] [PubMed] [Google Scholar]
- 47.Pelletier A, Mansbach J, Camargo CJ. Direct medical costs of bronchiolitis hospitalizations in the United States. Pediatrics. 2006;118:2418–2423. doi: 10.1542/peds.2006-1193. [DOI] [PubMed] [Google Scholar]
- 48.Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997-1999. Pediatr Infect Dis J. 2002;21:629–632. doi: 10.1097/00006454-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Schanzer D, Langley J, Tam T. Hospitalization attributable to influenza and other viral respiratory illnesses in Canadian children. Pediatr Infect Dis J. 2006;25:795–800. doi: 10.1097/01.inf.0000232632.86800.8c. [DOI] [PubMed] [Google Scholar]
- 50.Poehling K, Edwards K, Weinberg G. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355:31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 51.Weinberg G, Hall C, Iwane M. Parainfluenza virus infections in children. J Pediatr. 2009;154:694–699. doi: 10.1016/j.jpeds.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 52.Weigl J, Puppe W, Schmitt H. Incidence of respiratory syncytial virus-positive hospitalizations in Germany. Eur J Clin Microbiol Infect Dis. 2001;20:452–459. doi: 10.1007/s100960100527. [DOI] [PubMed] [Google Scholar]
- 53.Nicholson K, McNally T, Silverman M. Rates of hospitalisation for influenza, respiratory syncytial virus and human metapneumovirus among infants and young children. Vaccine. 2006;24:102–108. doi: 10.1016/j.vaccine.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 54.Fjaerli H, Farstad T, Bratlid D. Hospitalisations for respiratory syncytial virus bronchiolitis in Akershus, Norway, 1993-2000: a population-based retrospective study. BMC Pediatr. 2004;25:1–7. doi: 10.1186/1471-2431-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hall C. Respiratory syncytial virus infections in previously healthy working adults. Clin Infect Dis. 2001;33:792–796. doi: 10.1086/322657. [DOI] [PubMed] [Google Scholar]
- 56.Widmer K, Zhu Y, Williams JV. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis. 2012;206:56–62. doi: 10.1093/infdis/jis309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mangtani P, Hajat S, Kovats S. The association of respiratory syncytial virus infection and influenza with emergency admissions for respiratory disease in London: an analysis of routine surveillance data. Clin Infect Dis. 2006;42:640–646. doi: 10.1086/499810. [DOI] [PubMed] [Google Scholar]
- 58.Hall C. Nosocomial respiratory syncytial virus infections: the “cold war” has not ended. Clin Infect Dis. 2000;31:590–596. doi: 10.1086/313960. [DOI] [PubMed] [Google Scholar]
- 59.Johnson K, Chanock R, Rifkind D. Respiratory syncytial virus. IV. Correlation of virus shedding, serologic response, and illness in adult volunteers. JAMA. 1961;176:663–667. [PubMed] [Google Scholar]
- 60.Kravtez H, Knight V, Chanock R. Respiratory syncytial virus, III. Production of illness and clinical observations in adult volunteers. JAMA. 1961;176:657–663. [PubMed] [Google Scholar]
- 61.Hall C, Douglas RJ, Schnabel K. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect Immun. 1981;33:779–783. doi: 10.1128/iai.33.3.779-783.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee FE, Walsh EE, Falsey AR. Experimental infection of humans with A2 respiratory syncytial virus. Antiviral Res. 2004;63:191–196. doi: 10.1016/j.antiviral.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 63.DeVincenzo J, Wilkinson T, Vaishnaw A. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med. 2010;182:1305–1314. doi: 10.1164/rccm.201002-0221OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hall CB, Douglas RG, Jr, Geiman JM. Quantitative shedding patterns of respiratory syncytial virus in infants. J Infect Dis. 1975;132:151–156. doi: 10.1093/infdis/132.2.151. [DOI] [PubMed] [Google Scholar]
- 65.Houben ML, Coenjaerts FE, Rossen JW. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J Med Virol. 2010;82:1266–1271. doi: 10.1002/jmv.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DeVincenzo JP, El Saleeby CM, Bush AJ. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J Infect Dis. 2005;191:1861–1868. doi: 10.1093/infdis/jir494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson J, Gonzales R, Olson S. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol. 2007;20:108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- 68.Visscher D, Myers J. Bronchiolitis: the pathologist's perspective. Proc Am Thorac Soc. 2006;3:41–47. doi: 10.1513/pats.200512-124JH. [DOI] [PubMed] [Google Scholar]
- 69.Adams J, Imagawa D, Zike K. Epidemic bronchiolitis and pneumonia related to respiratory syncytial virus. JAMA. 1961;176:1037–1039. doi: 10.1001/jama.1961.63040250020020b. [DOI] [PubMed] [Google Scholar]
- 70.Openshaw P, Tregoning J. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin Microbiol Rev. 2005;18:541–555. doi: 10.1128/CMR.18.3.541-555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peebles RJ, Graham B. Pathogenesis of respiratory syncytial virus infection in the murine model. Proc Am Thorac Soc. 2005;2:110–115. doi: 10.1513/pats.200501-002AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miyairi I, DeVincenzo JP. Human genetic factors and respiratory syncytial virus disease severity. Clin Microbiol Rev. 2008;21:686–703. doi: 10.1128/CMR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Collins P, Graham B. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82:2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim H, Canchola J, Brandt C. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 75.Fulginiti V, Eller J, Sieber O. Respiratory virus immunization, I. A field trial of two inactivated respiratory virus vaccines: an aqueous trivalent parainfluenza virus vaccine, and an alumprecipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969;89:435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- 76.Kapikian A, Mitchell R, Chanock R. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 77.Lotz MT, Peebles RS., Jr Mechanisms of respiratory syncytial virus modulation of airway immune responses. Curr Allergy Asthma Rep. 2012;12:380–387. doi: 10.1007/s11882-012-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Prince G, Mathews A, Curtis S. Treatment of respiratory syncytial virus bronchiolitis and pneumonia in a cotton rat model with systemically administered monoclonal antibody (palivizumab) and glucocorticosteroid. J Infect Dis. 2000;182:1326–1330. [Google Scholar]
- 79.Malley R, DeVincenzo J, Ramilo O. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. J Infect Dis. 1998;178:1555–1561. doi: 10.1086/314523. [DOI] [PubMed] [Google Scholar]
- 80.Johnson CH, Miao C, Blanchard EG. Effect of chemokine receptor CX3CR1 deficiency in a murine model of respiratory syncytial virus infection. Comp Med. 2012;62:14–20. [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang W, Choi Y, Haynes LM. Vaccination to induce antibodies blocking the CX3C-CX3CR1 interaction of respiratory syncytial virus G protein reduces pulmonary inflammation and virus replication in mice. J Virol. 2010;84:1148–1157. doi: 10.1128/JVI.01755-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bennett B, Garofalo R, Cron S. Immunopathogenesis of respiratory syncytial virus bronchiolitis. J Infect Dis. 2007;195:1532–1540. doi: 10.1086/515575. [DOI] [PubMed] [Google Scholar]
- 83.Welliver T, Reed J, Welliver R., Sr Respiratory syncytial virus and influenza virus infections: observations from tissues of fatal infant cases. Pediatr Infect Dis J. 2008;27:S92–S96. doi: 10.1097/INF.0b013e318168b706. [DOI] [PubMed] [Google Scholar]
- 84.Finberg R, Wang J, Kurt-Jones E. Toll like receptors and viruses. Rev Med Virol. 2007;17:35–43. doi: 10.1002/rmv.525. [DOI] [PubMed] [Google Scholar]
- 85.Crowe J, Williams J. Immunology of viral respiratory tract infection in infancy. Paediatr Respir Rev. 2003;4:112–119. doi: 10.1016/s1526-0542(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 86.Lukens MV, van de Pol AC, Coenjaerts FE. A systemic neutrophil response precedes robust CD8(+) T-cell activation during natural respiratory syncytial virus infection in infants. J Virol. 2010;84:2374–2383. doi: 10.1128/JVI.01807-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hull J. Genetic susceptibility to RSV disease. In: Cane PA, editor. Respiratory Syncytial Virus. Elsevier; Amsterdam: 2007. pp. 115–140. [Google Scholar]
- 88.Hall C, Walsh E, Long C. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 89.Walsh E, Falsey A. Age-related differences in humoral immune response to respiratory syncytial virus infection in adults. J Med Virol. 2004;73:295–299. doi: 10.1002/jmv.20090. [DOI] [PubMed] [Google Scholar]
- 90.Walsh EE, Falsey AR. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis. 2004;190:373–378. doi: 10.1086/421524. [DOI] [PubMed] [Google Scholar]
- 91.Glezen W, Paredes A, Allison J. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981;98:708–715. doi: 10.1016/s0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- 92.Parrott R, Kim H, Arrobio J. Epidemiology of respiratory syncytial virus infection in Washington, DC, II. Infection and disease with respect to age, immunologic status, race, and sex. Am J Epidemiol. 1973;98:289–300. doi: 10.1093/oxfordjournals.aje.a121558. [DOI] [PubMed] [Google Scholar]
- 93.Committee on Infectious Diseases From the American Academy of Pediatrics: policy statements—modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics. 2009;124:1694–1701. doi: 10.1542/peds.2009-2345. [DOI] [PubMed] [Google Scholar]
- 94.Scott P, Ochola R, Sande C. Comparison of strain-specific antibody responses during primary and secondary infections with respiratory syncytial virus. J Med Virol. 2007;79:1943–1950. doi: 10.1002/jmv.20999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Murata Y, Lightfoote PM, Biear JN. Humoral response to the central unglycosylated region of the respiratory syncytial virus attachment protein. Vaccine. 2010;28:6242–6246. doi: 10.1016/j.vaccine.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wright P, Gruber W, Peters M. Illness severity, viral shedding, and antibody responses in infants hospitalized with bronchiolitis caused by respiratory syncytial virus. J Infect Dis. 2002;185:1011–1018. doi: 10.1086/339822. [DOI] [PubMed] [Google Scholar]
- 97.Falsey A, Singh H, Walsh E. Serum antibody decay in adults following natural respiratory syncytial virus infection. J Med Virol. 2006;78:1493–1497. doi: 10.1002/jmv.20724. [DOI] [PubMed] [Google Scholar]
- 98.Prince G, Horswood R, Chanock R. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J Virol. 1985;55:517–520. doi: 10.1128/jvi.55.3.517-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McIntosh K, McQuillin J, Gardner PS. Cell-free and cell-bound antibody in nasal secretions from infants with respiratory syncytial virus infection. Infect Immun. 1979;23:276–281. doi: 10.1128/iai.23.2.276-281.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Welliver R, Wong D, Sun M. The development of respiratory syncytial virus-specific IgE and the release of histamine in naso-pharyngeal secretions after infection. N Engl J Med. 1981;305:841–846. doi: 10.1056/NEJM198110083051501. [DOI] [PubMed] [Google Scholar]
- 101.Welliver R, Garofalo R, Ogra P. Beta-chemokines, but neither T helper type 1 nor T helper type 2 cytokines, correlate with severity of illness during respiratory syncytial virus infection. Pediatr Infect Dis J. 2002;21:457–561. doi: 10.1097/00006454-200205000-00033. [DOI] [PubMed] [Google Scholar]
- 102.Hall C, Powell K, MacDonald N. Respiratory syncytial virus infection in children with compromised immune function. N Engl J Med. 1986;315:77–81. doi: 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- 103.Kim Y, Boeckh M, Englund J. Community respiratory virus infections in immunocompromised patients: hematopoietic stem cell and solid organ transplant recipients, and individuals with human immunodeficiency virus infection. Semin Respir Crit Care Med. 2007;28:222–242. doi: 10.1055/s-2007-976494. [DOI] [PubMed] [Google Scholar]
- 104.Chi B, Dickensheets H, Spann K. Alpha and lambda interferon together mediate suppression of CD4 T cells induced by respiratory syncytial virus. J Virol. 2006;80:5032–5040. doi: 10.1128/JVI.80.10.5032-5040.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guerrero-Plata A, Casola A, Suarez G. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am J Respir Cell Mol Biol. 2006;34:320–329. doi: 10.1165/rcmb.2005-0287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kristjansson S, Bjarnarson S, Wennergren G. Respiratory syncytial virus and other respiratory viruses during the first 3 months of life promote a local TH2-like response. J Allergy Clin Immunol. 2005;116:805–811. doi: 10.1016/j.jaci.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 107.Harcourt J, Alvarez R, Jones L. Respiratory syncytial virus G protein and G protein CX3C motif adversely affect CX3CR1+ T cell responses. J Immunol. 2006;176:1600–1608. doi: 10.4049/jimmunol.176.3.1600. [DOI] [PubMed] [Google Scholar]
- 108.Glezen W, Denny F. Epidemiology of acute lower respiratory disease in children. N Engl J Med. 1973;288:498–505. doi: 10.1056/NEJM197303082881005. [DOI] [PubMed] [Google Scholar]
- 109.American Academy of Pediatrics Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–1793. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- 110.Schuh S, Lalani A, Allen U. Evaluation of the utility of radiography in acute bronchiolitis. J Pediatr. 2007;150:429–433. doi: 10.1016/j.jpeds.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Friis B, Eiken M, Hornsleth A. Chest x-ray appearances in pneumonia and bronchiolitis. Acta Paediatr Scand. 1990;79:219–225. doi: 10.1111/j.1651-2227.1990.tb11442.x. [DOI] [PubMed] [Google Scholar]
- 112.Chonmaitree T, Revai K, Grady J. Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis. 2008;46:815–823. doi: 10.1086/528685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Heikkinen T, Thint M, Chonmaitree T. Prevalence of various respiratory viruses in the middle ear during acute otitis media. N Engl J Med. 1999;340:260–264. doi: 10.1056/NEJM199901283400402. [DOI] [PubMed] [Google Scholar]
- 114.Marom T, Nokso-Koivisto J, Chonmaitree T. Viral-bacterial interactions in acute otitis media. Curr Allergy Asthma Rep. 2012;12:551–558. doi: 10.1007/s11882-012-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Louie J, Hacker J, Gonzales R. Characterization of viral agents causing acute respiratory infection in a San Francisco University Medical Center Clinic during the influenza season. Clin Infect Dis. 2005;41:822–828. doi: 10.1086/432800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Harris J, Erdman D, Dhashemi S, et al. Respiratory syncytial virus (RSV) and persistent cough at a University Health Service. Presented at the 39th Annual Meeting of the Infectious Diseases Society of America. San Francisco, Oct 25-28, 2001.
- 117.Falsey A, Walsh E. Respiratory syncytial virus infection in elderly adults. Drugs Aging. 2005;22:577–587. doi: 10.2165/00002512-200522070-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Walsh E, Peterson D, Falsey A. Is clinical recognition of respiratory syncytial virus infection in hospitalized elderly and high-risk adults possible? J Infect Dis. 2007;195:1046–1051. doi: 10.1086/511986. [DOI] [PubMed] [Google Scholar]
- 119.Falsey A, Cunningham C, Barker W. Respiratory syncytial virus and influenza A infections in the hospitalized elderly. J Infect Dis. 1995;172:389–394. doi: 10.1093/infdis/172.2.389. [DOI] [PubMed] [Google Scholar]
- 120.Thompson WW, Shay DK, Weintraub E. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 121.Mullooly JP, Bridges CB, Thompson WW. Influenza- and RSV-associated hospitalizations among adults. Vaccine. 2007;25:846–855. doi: 10.1016/j.vaccine.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 122.Ellis SE, Coffey CS, Mitchel EF., Jr Influenza- and respiratory syncytial virus-associated morbidity and mortality in the nursing home population. J Am Geriatr Soc. 2003;51:761–767. doi: 10.1046/j.1365-2389.2003.51254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nicholson K, Kent J, Hammersley V. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. BMJ. 1997;315:1060–1064. doi: 10.1136/bmj.315.7115.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Falsey A, McCann R, Hall W. Acute respiratory tract infection in daycare centers for older persons. J Am Geriatr Soc. 1995;43:30–36. doi: 10.1111/j.1532-5415.1995.tb06238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mehta J, Walsh EE, Mahadevia PJ, Falsey AR. Risk factors for respiratory syncytial virus illness among patients with chronic obstructive pulmonary disease. COPD. 2013;10:293–299. doi: 10.3109/15412555.2012.744741. [DOI] [PubMed] [Google Scholar]
- 126.De Serres G, Lampron N, La Forge J. Importance of viral and bacterial infections in chronic obstructive pulmonary disease exacerbations. J Clin Virol. 2009;46:129–133. doi: 10.1016/j.jcv.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Camargo CA, Ginde AA, Clark S. Viral pathogens in acute exacerbations of chronic obstructive pulmonary disease. Intern Emerg Med. 2008;3:355–359. doi: 10.1007/s11739-008-0197-0. [DOI] [PubMed] [Google Scholar]
- 128.Walsh EE, Peterson DR, Kalkanoglu AE. Viral shedding and immune responses to respiratory syncytial virus infection in older adults. J Infect Dis. 2013;207:1424–1432. doi: 10.1093/infdis/jit038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Falsey A. Respiratory syncytial virus infection in adults. Semin Respir Crit Care Med. 2007;28:171–181. doi: 10.1055/s-2007-976489. [DOI] [PubMed] [Google Scholar]
- 130.Cherukuri A, Patton K, Gasser RA., Jr Adults 65 years old and older have reduced numbers of functional memory T cells to respiratory syncytial virus fusion protein. Clin Vaccine Immunol. 2013;20:239–247. doi: 10.1128/CVI.00580-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cusi MG, Martorelli B, Di Genova G. Age-related changes in T cell mediated immune response and effector memory to respiratory syncytial virus (RSV) in healthy subjects. Immun Ageing. 2010;7:14. doi: 10.1186/1742-4933-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.de Bree GJ, Heidema J, van Leeuwen EM. Respiratory syncytial virus-specific CD8+ memory T cell responses in elderly persons. J Infect Dis. 2005;191:1710–1718. doi: 10.1086/429695. [DOI] [PubMed] [Google Scholar]
- 133.Boukhvalova M, Yim K, Kuhn K. Age-related differences in pulmonary cytokine response to respiratory syncytial virus infection: modulation by anti-inflammatory and antiviral treatment. J Infect Dis. 2007;195:511–518. doi: 10.1086/510628. [DOI] [PubMed] [Google Scholar]
- 134.Panitch H. Viral respiratory infections in children with technology dependence and neuromuscular disorders. Pediatr Infect Dis J. 2004;23:S222–S227. doi: 10.1097/01.inf.0000144670.78558.c7. [DOI] [PubMed] [Google Scholar]
- 135.Simon A, Ammann R, Wilkesmann A. Respiratory syncytial virus infection in 406 hospitalized premature infants: results from a prospective German multicentre database. Eur J Pediatr. 2007;166:1273–1283. doi: 10.1007/s00431-007-0426-y. [DOI] [PubMed] [Google Scholar]
- 136.McLaurin K, Hall C, Jackson E. Persistence of morbidity and cost differences between late-preterm and term infants during the first year of life. Pediatrics. 2009;123:653–659. doi: 10.1542/peds.2008-1439. [DOI] [PubMed] [Google Scholar]
- 137.MacDonald N, Hall C, Suffin S. Respiratory syncytial viral infection in infants with congenital heart disease. N Engl J Med. 1982;307:397–400. doi: 10.1056/NEJM198208123070702. [DOI] [PubMed] [Google Scholar]
- 138.Romero J. Palivizumab prophylaxis of respiratory syncytial virus disease from 1998 to 2002: results from four years of palivizumab usage. Pediatr Infect Dis J. 2003;22:S46–S54. doi: 10.1097/01.inf.0000053885.34703.84. [DOI] [PubMed] [Google Scholar]
- 139.Altman C, Englund J, Demmler G. Respiratory syncytial virus in patients with congenital heart disease: a contemporary look at epidemiology and success of preoperative screening. Pediatr Cardiol. 2000;21:433–438. doi: 10.1007/s002460010103. [DOI] [PubMed] [Google Scholar]
- 140.Simoes E. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr. 2003;143:S118–S126. doi: 10.1067/s0022-3476(03)00511-0. [DOI] [PubMed] [Google Scholar]
- 141.Iwane MK, Chaves SS, Szilagyi PG. Disparities between black and white children in hospitalizations associated with acute respiratory illness and laboratory-confirmed influenza and respiratory syncytial virus in 3 US counties—2002-2009. Am J Epidemiol. 2013;177:656–665. doi: 10.1093/aje/kws299. [DOI] [PubMed] [Google Scholar]
- 142.Singleton R, Bruden D, Bulkow L. Respiratory syncytial virus season and hospitalizations in the Alaskan Yukon-Kuskokwim Delta. Pediatr Infect Dis J. 2007;26:S46–S50. doi: 10.1097/INF.0b013e318157da9b. [DOI] [PubMed] [Google Scholar]
- 143.Hirsch HH, Martino R, Ward KN. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis. 2013;56:258–266. doi: 10.1093/cid/cis844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Boeckh M. The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br J Haematol. 2008;143:455–467. doi: 10.1111/j.1365-2141.2008.07295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mendoza Sanchez M, Ruiz-Contreras J, Vivanco J. Respiratory virus infections in children with cancer or HIV infection. J Pediatr Hematol Oncol. 2006;28:154–159. doi: 10.1097/01.mph.0000210061.96075.8e. [DOI] [PubMed] [Google Scholar]
- 146.Raboni S, Nogueira M, Tsuchiya L. Respiratory tract viral infections in bone marrow transplant patients. Transplantation. 2003;76:142–146. doi: 10.1097/01.TP.0000072012.26176.58. [DOI] [PubMed] [Google Scholar]
- 147.Champlin R, Whimbey E. Community respiratory virus infections in bone marrow transplant recipients: the M.D. Anderson Cancer Center experience. Biol Blood Marrow Transplant. 2001;7:8S–10S. doi: 10.1053/bbmt.2001.v7.pm11777103. [DOI] [PubMed] [Google Scholar]
- 148.Khanna N, Widmer A, Decker M. Respiratory syncytial virus infection in patients with hematological diseases: single-center study and review of the literature. Clin Infect Dis. 2008;46:402–412. doi: 10.1086/525263. [DOI] [PubMed] [Google Scholar]
- 149.El Saleeby C, Somes G, DeVincenzo J. Risk factors for severe respiratory syncytial virus disease in children with cancer: the importance of lymphopenia and young age. Pediatrics. 2008;121:235–243. doi: 10.1542/peds.2007-1102. [DOI] [PubMed] [Google Scholar]
- 150.Whimbey E, Champlin RE, Couch RB. Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin Infect Dis. 1996;22:778–782. doi: 10.1093/clinids/22.5.778. [DOI] [PubMed] [Google Scholar]
- 151.Chemaly R, Ghosh S, Bodey G. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore) 2006;85:278–287. doi: 10.1097/01.md.0000232560.22098.4e. [DOI] [PubMed] [Google Scholar]
- 152.Seo S, Campbell AP, Xie H. Outcome of respiratory syncytial virus lower respiratory tract disease in hematopoietic cell transplant recipients receiving aerosolized ribavirin: significance of stem cell source and oxygen requirement. Biol Blood Marrow Transplant. 2013;19:589–596. doi: 10.1016/j.bbmt.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gasparetto E, Escuissato D, Marchiori E. High-resolution CT findings of respiratory syncytial virus pneumonia after bone marrow transplantation. AJR Am J Roentgenol. 2004;182:1133–1137. doi: 10.2214/ajr.182.5.1821133. [DOI] [PubMed] [Google Scholar]
- 154.Zamora MR, Budev M, Rolfe M. RNA interference therapy in lung transplant patients infected with respiratory syncytial virus. Am J Respir Crit Care Med. 2011;183:531–538. doi: 10.1164/rccm.201003-0422OC. [DOI] [PubMed] [Google Scholar]
- 155.Madhi S, Venter M, Madhi A. Differing manifestations of respiratory syncytial virus-associated severe lower respiratory tract infections in human immunodeficiency virus type 1-infected and uninfected children. Pediatr Infect Dis J. 2001;20:164–170. doi: 10.1097/00006454-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 156.Dykewicz C. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients: focus on community respiratory virus infections. Biol Blood Marrow Transplant. 2001;7:19S–22S. doi: 10.1053/bbmt.2001.v7.pm11777100. [DOI] [PubMed] [Google Scholar]
- 157.Church N, Anas N, Hall C. Respiratory syncytial virus-related apnea in infants: demographics and outcome. Am J Dis Child. 1984;138:247–250. doi: 10.1001/archpedi.1984.02140410027010. [DOI] [PubMed] [Google Scholar]
- 158.Willwerth B, Harper M, Greenes D. Identifying hospitalized infants who have bronchiolitis and are at high risk for apnea. Ann Emerg Med. 2006;48:441–447. doi: 10.1016/j.annemergmed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 159.Hernandez E, Khoshoo V, Thoppil D. Aspiration: a factor in rapidly deteriorating bronchiolitis in previously healthy infants? Pediatr Pulmonol. 2002;33:30–31. doi: 10.1002/ppul.10022. [DOI] [PubMed] [Google Scholar]
- 160.Hall C, Powell K, Schnabel K. Risk of secondary bacterial infection in infants hospitalized with respiratory syncytial viral infection. J Pediatr. 1988;113:266–271. [PubMed] [Google Scholar]
- 161.Purcell K, Fergie J. Concurrent serious bacterial infections in 2396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156:322–324. doi: 10.1001/archpedi.156.4.322. [DOI] [PubMed] [Google Scholar]
- 162.Oray-Schrom P, Phoenix C, St Martin D. Sepsis workup in febrile infants 0-90 days of age with respiratory syncytial virus infection. Pediatr Emerg Care. 2003;19:314–319. doi: 10.1097/01.pec.0000092576.40174.28. [DOI] [PubMed] [Google Scholar]
- 163.Kneyber MC, van Woensel J, Uijtendaal E. Azithromycin does not improve disease course in hospitalized infants with respiratory syncytial virus (RSV) lower respiratory tract disease: a randomized equivalence trial. Pediatr Pulmonol. 2008;43:142–149. doi: 10.1002/ppul.20748. [DOI] [PubMed] [Google Scholar]
- 164.Choi E, Lee H, Kim S. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000-2005. Clin Infect Dis. 2006;43:585–592. doi: 10.1086/506350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Purcell K, Fergie J. Lack of usefulness of an abnormal white blood cell count for predicting a concurrent serious bacterial infection in infants and young children hospitalized with respiratory syncytial virus lower respiratory tract infection. Pediatr Infect Dis J. 2007;26:311–315. doi: 10.1097/01.inf.0000258627.23337.00. [DOI] [PubMed] [Google Scholar]
- 166.Szabo SM, Levy AR, Gooch KL. Elevated risk of asthma after hospitalization for respiratory syncytial virus infection in infancy. Paediatr Respir Rev. 2013;13(suppl 2):S9–S15. doi: 10.1016/S1526-0542(12)70161-6. [DOI] [PubMed] [Google Scholar]
- 167.Regnier S, Huels J. Association between respiratory syncytial virus hospitalizations in infants and respiratory sequelae: systematic review and meta-analysis. Pediatr Infect Dis J. 2013;32:820–826. doi: 10.1097/INF.0b013e31829061e8. [DOI] [PubMed] [Google Scholar]
- 168.Taussig L, Wright A, Holberg C. Tucson Children's Respiratory Study: 1980 to present. J Allergy Clin Immunol. 2003;11:661–675. doi: 10.1067/mai.2003.162. [DOI] [PubMed] [Google Scholar]
- 169.Sigurs N. Respiratory syncytial virus lower respiratory tract illness in infancy and subsequent morbidity. Acta Paediatr. 2007;96:156–157. doi: 10.1111/j.1651-2227.2007.00105.x. [DOI] [PubMed] [Google Scholar]
- 170.Gern J. Viral Respiratory infection and the link to asthma. Pediatr Infect Dis J. 2008;27:S97–S103. doi: 10.1097/INF.0b013e318168b718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tourdot S, Mathie S, Hussell T. Respiratory syncytial virus infection provokes airway remodelling in allergen-exposed mice in absence of prior allergen sensitization. Clin Exp Allergy. 2008;38:1016–1024. doi: 10.1111/j.1365-2222.2008.02974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Welliver R. Respiratory syncytial virus and other respiratory viruses. Pediatr Infect Dis J. 2003;22:S6–S12. doi: 10.1097/01.inf.0000053880.92496.db. [DOI] [PubMed] [Google Scholar]
- 173.Patino C, Martinez F. Interactions between genes and environment in the development of asthma. Allergy. 2001;56:279–286. doi: 10.1034/j.1398-9995.2001.00135.x. [DOI] [PubMed] [Google Scholar]
- 174.Henrikson K, Hall C. Diagnostic assays for respiratory syncytial virus disease. Pediatr Infect Dis J. 2007;26:S36–S40. doi: 10.1097/INF.0b013e318157da6f. [DOI] [PubMed] [Google Scholar]
- 175.Lambert S, Whiley D, O’Neill N. Comparing nose-throat swabs and nasopharyngeal aspirates collected from children with symptoms for respiratory virus identification using real-time polymerase chain reaction. Pediatrics. 2008;122:e615–e620. doi: 10.1542/peds.2008-0691. [DOI] [PubMed] [Google Scholar]
- 176.Casiano-Colon AE, Hulbert BB, Mayer TK. Lack of sensitivity of rapid antigen tests for the diagnosis of respiratory syncytial virus infection in adults. J Clin Virol. 2003;28:169–174. doi: 10.1016/s1386-6532(03)00002-7. [DOI] [PubMed] [Google Scholar]
- 177.Kodani M, Yang G, Conklin LM. Application of TaqMan low-density arrays for simultaneous detection of multiple respiratory pathogens. J Clin Microbiol. 2011;49:2175–2182. doi: 10.1128/JCM.02270-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Walsh EE, Falsey AR. Age related differences in humoral immune response to respiratory syncytial virus infection in adults. J Med Virol. 2004;73:295–299. doi: 10.1002/jmv.20090. [DOI] [PubMed] [Google Scholar]
- 179.Behrendt C, Decker M, Burch D. International variation in the management of infants hospitalized with respiratory syncytial virus. Eur J Pediatr. 1998;157:215–220. doi: 10.1007/s004310050798. [DOI] [PubMed] [Google Scholar]
- 180.American Academy of Pediatrics . Respiratory syncytial virus. In: Pickering LK, Bucher CJ, Kimberlin DW, Long SS, editors. Red Book: 2012 Report of the Committee on Infectious Diseases. American Academy of Pediatrics; Elk Grove Village, IL: 2012. pp. 609–618. [Google Scholar]
- 181.Ventre K, Randolph A. Ribavirin for respiratory syncytial virus infection of the lower respiratory tract in infants and young children. Cochrane Database Syst Rev. 2007;(1) doi: 10.1002/14651858.CD000181.pub3. [DOI] [PubMed] [Google Scholar]
- 182.Shah DP, Ghantoji SS, Shah JN. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother. 2013;68:1872–1880. doi: 10.1093/jac/dkt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood. 2011;117:2755–2763. doi: 10.1182/blood-2010-08-263400. [DOI] [PubMed] [Google Scholar]
- 184.Boeckh M, Englund J, Li Y. Randomized controlled multicenter trial of aerosolized ribavirin for respiratory syncytial virus upper respiratory tract infection in hematopoietic cell transplant recipients. Clin Infect Dis. 2007;44:245–249. doi: 10.1086/509930. [DOI] [PubMed] [Google Scholar]
- 185.Chemaly RF, Torres HA, Munsell MF. An adaptive randomized trial of an intermittent dosing schedule of aerosolized ribavirin in patients with cancer and respiratory syncytial virus infection. J Infect Dis. 2012;206:1367–1371. doi: 10.1093/infdis/jis516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Park SY, Baek S, Lee SO. Efficacy of oral ribavirin in hematologic disease patients with paramyxovirus infection: analytic strategy using propensity scores. Antimicrob Agents Chemother. 2013;57:983–989. doi: 10.1128/AAC.01961-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pelaez A, Lyon GM, Force SD. Efficacy of oral ribavirin in lung transplant patients with respiratory syncytial virus lower respiratory tract infection. J Heart Lung Transplant. 2009;28:67–71. doi: 10.1016/j.healun.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li L, Avery R, Budev M. Oral versus inhaled ribavirin therapy for respiratory syncytial virus infection after lung transplantation. J Heart Lung Transplant. 2012;31:839–844. doi: 10.1016/j.healun.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 189.de Fontbrune F, Robin M, Porcher R. Palivizumab treatment of respiratory syncytial virus infection after allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2007;45:1019–1024. doi: 10.1086/521912. [DOI] [PubMed] [Google Scholar]
- 190.Chavez-Bueno S, Mejias A, Merryman R. Intravenous palivizumab and ribavirin combination for respiratory syncytial virus disease in high-risk pediatric patients. Pediatr Infect Dis J. 2007;26:1089–1093. doi: 10.1097/INF.0b013e3181343b7e. [DOI] [PubMed] [Google Scholar]
- 191.Thomas N, Hollenbeak C, Ceneviva G. Palivizumab prophylaxis to prevent respiratory syncytial virus mortality after pediatric bone marrow transplantation: a decision analysis model. J Pediatr Hematol Oncol. 2007;29:227–232. doi: 10.1097/MPH.0b013e3180437ded. [DOI] [PubMed] [Google Scholar]
- 192.Sidwell R, Barnard D. Respiratory syncytial virus infections: recent prospects for control. Antiviral Res. 2006;71:379–390. doi: 10.1016/j.antiviral.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 193.Sun Z, Pan Y, Jiang S, Lu L. Respiratory syncytial virus entry inhibitors targeting the F protein. Viruses. 2013;5:211–225. doi: 10.3390/v5010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Centers for Disease Control and Prevention Guideline for hand hygiene in health-care settings. Recommendations of the Healthcare Infections Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. MMWR Morb Mortal Wkly Rep. 2002;51(RR-16):1–45. [PubMed] [Google Scholar]
- 195.Hall C, Douglas RJ, Geiman J. Nosocomial respiratory syncytial virus infections. N Engl J Med. 1975;293:1343–1346. doi: 10.1056/NEJM197512252932604. [DOI] [PubMed] [Google Scholar]
- 196.Goldmann D. Epidemiology and prevention of pediatric viral respiratory infections in health-care institutions. Emerg Infect Dis. 2001;7:249–253. doi: 10.3201/eid0702.010220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Halasa N, Williams J, Wilson G. Medical and economic impact of a respiratory syncytial virus outbreak in a neonatal intensive care unit. Pediatr Infect Dis J. 2005;24:1040–1044. doi: 10.1097/01.inf.0000190027.59795.ac. [DOI] [PubMed] [Google Scholar]
- 198.Elhassan N, Sorbero M, Hall C. Cost-effectiveness analysis of palivizumab in premature infants without chronic lung disease. Arch Pediatr Adolesc Med. 2006;160:1070–1076. doi: 10.1001/archpedi.160.10.1070. [DOI] [PubMed] [Google Scholar]
- 199.Reeve C, Whitehall J, Buettner P. Cost-effectiveness of respiratory syncytial virus prophylaxis with palivizumab. J Paediatr Child Health. 2006;42:248–252. doi: 10.1111/j.1440-1754.2006.00850.x. [DOI] [PubMed] [Google Scholar]
- 200.Simoes E, Groothuis J, Carbonell-Estrany X. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151:34–42. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 201.Wu H, Pfarr D, Johnson S. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J Mol Biol. 2007;368:652–665. doi: 10.1016/j.jmb.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 202.Carbonell-Estrany X, Genevieve L, Micki H, Edward C. Phase 3 trial of motavizumab (MEDI-524), an enhanced potency respiratory syncytial virus (RSV) specific monoclonal antibody (Mab) for the prevention of serious RSV disease in high-risk infants. Presented at Pediatric Academic Societies Annual Meeting. Toronto, May 5, 2007.
- 203.Karron R, Wright P, Belshe R. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis. 2005;191:1093–1104. doi: 10.1086/427813. [DOI] [PubMed] [Google Scholar]
- 204.Falsey AR, Walsh EE, Capellan J. Comparison of the safety and immunogenicity of 2 respiratory syncytial virus (RSV) vaccines—nonadjuvanted vaccine or vaccine adjuvanted with alum–given concomitantly with influenza vaccine to high-risk elderly individuals. J Infect Dis. 2008;198:1317–1326. doi: 10.1086/592168. [DOI] [PubMed] [Google Scholar]
- 205.Magro M, Mas V, Chappell K. Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc Natl Acad Sci U S A. 2012;109:3089–3094. doi: 10.1073/pnas.1115941109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Swanson KA, Settembre EC, Shaw CA. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci U S A. 2011;108:9619–9624. doi: 10.1073/pnas.1106536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Greenberg H, Piedra P. Immunization against viral respiratory disease: a review. Pediatr Infect Dis J. 2004;23:S254–S261. doi: 10.1097/01.inf.0000144756.69887.f8. [DOI] [PubMed] [Google Scholar]
- 208.Collins P, Murphy B. New generation live vaccines against human respiratory syncytial virus designed by reverse genetics. Proc Am Thorac Soc. 2005;2:166–173. doi: 10.1513/pats.200501-011AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rudraraju R, Jones BG, Sealy R. Respiratory syncytial virus: current progress in vaccine development. Viruses. 2013;5:577–594. doi: 10.3390/v5020577. [DOI] [PMC free article] [PubMed] [Google Scholar]


