Abstract
Viruses survive in nature only if they are able to pass from one host to another, whether of the same or another species. Viral epidemiology is the study of the factors that determine the frequency and distribution of viral diseases in an animal population. In the broadest sense, epidemiology may be viewed as a part of population biology, involving not only environmental factors but also genetic factors in both the virus and the host. The terms incidence and prevalence are used to describe quantitative aspects of the occurrence of infections in populations. The incidence of infection is defined as the proportion of a population contracting that infection during a specified period, whereas prevalence refers to the proportion infected at a particular point in time. The comparisons of incidence and prevalence at different times and places are made by relating the appropriate numerator to a denominator that may be as general as the total population of the animal species concerned or may be specified as the susceptible population at risk. A disease is said to be enzootic when there are continuous chains of transmission in the region involved; epizootics are peaks in disease incidence. The size of the peak required to constitute an epizootic is arbitrary and is related to the background enzootic rate, the morbidity, and the anxiety that the disease arouses.
Viruses survive in nature only if they are able to pass from one host to another, whether of the same or another species. Viral epidemiology is the study of the factors that determine the frequency and distribution of viral diseases in an animal population. In the broadest sense, epidemiology may be viewed as a part of population biology, involving mainly environmental (ecological) factors, but also genetic factors, in both the virus (e.g., new strains of influenza virus) and the host (differences in genetic resistance of different receptive hosts).
The terms incidence and prevalence are used to describe quantitative aspects of the occurrence of infections in populations. The incidence of infection (or of disease) is defined as the proportion of a population contracting that infection (or disease) during a specified period (usually a year), whereas prevalence refers to the proportion infected (or sick, or immune) at a particular point in time. Comparisons of incidence and prevalence at different times and places (incidence and prevalence rates) are made by relating the appropriate numerator to a denominator which may be as general as the total population of the animal species concerned (in a region or country), or may be specified as the susceptible population at risk (exposed and susceptible, the latter usually being defined as those lacking antibodies).
Deaths from a disease can be categorized in two ways: as a cause-specific mortality rate (the number of deaths from the disease in a given year, divided by the total population at midyear), usually expressed as per 100,000; or the case fatality rate (the percentage of those animals with a particular disease that die from it). These rates vary with age, sex, genetic constitution, immune status, and nutrition.
A disease is said to be enzootic (endemic in humans) when there are continuous chains of transmission in the region involved; epizootics (epidemics) are peaks in disease incidence. The size of the peak required to constitute an epizootic is arbitrary and is related to the background enzootic rate, the morbidity (frequency of illness), and the anxiety that the disease arouses; e.g., a few cases of velogenic Newcastle disease in poultry might be regarded as an epizootic, whereas a few cases of infectious bronchitis would not be. A panzootic (pandemic) is a worldwide epizootic.
TRANSMISSION OF VIRUSES
Transmission involves both the entry of viruses into the body and, after replication, their shedding from the body surfaces (see Chapter 7).
Direct transmission involves actual contact between an infected and a susceptible animal, such as by licking or rubbing. Two special cases of direct transmission are contacts occurring during coitus (sexually transmitted diseases) and those occurring between mother and the fetus via the placenta (transplacental). There are several modes of indirect transmission. Respiratory infections are transmitted both by contact and by droplets (aerosols) emitted during coughing or sneezing. Such aerosols contain large droplets which settle before evaporating and microdroplets which evaporate before settling, and thus produce dry droplet nuclei less than 5 μm in diameter. Droplets may travel only a meter or so, while droplet nuclei may remain airborne for long periods and hence travel long distances—many kilometers if wind and other weather conditions are favorable.
Enteric infections are often transmitted via a fecal–oral cycle, that may include fecal contamination of food and water supplies; diarrheic feces may also splash to give rise to aerosols or dry to give rise to infected airborne dust. Indirect transmission may also occur via fomites, i.e., any object that may be contaminated by a virus, such as bedding, harness, grooming or surgical equipment, multiple-use syringes, vehicles, books, and clothing. Some diseases are transmitted via milk (e.g., caprine arthritis encephalitis virus), meat-contaminated garbage (e.g., vesicular exanthema virus, hog cholera virus), or dander (Marek' disease virus). Some are spread by bite (e.g., rabies virus), while a number of viruses are transmitted by arthropod vectors (see p. 300).
Veterinarians (or other persons) may themselves be responsible for the transmission of viruses. Such iatrogenic infections include transmission of equine infectious anemia virus via multiple-use syringes and of reticuloendotheliosis virus via contaminated Marek's disease vaccine. Nosocomial infections are those acquired while in a hospital or clinic. During the peak incidence of canine parvovirus in the late 1970s many pups brought to veterinary hospitals acquired canine parvovirus there. Kennel cough and feline respiratory virus infections are also often nosocornial. Certain viruses are vertically transmitted, either as virions via the placenta or the egg, or as provirus integrated in the DNA of the gamete (germ-line transmission; see Fig. 31-2). Some viruses are transmitted by several routes; others are transmitted in nature exclusively by one route (Table 15-1 ).
TABLE 15-1.
Common Routes of Transmission of Viruses of Animals
| Virus family | Routes |
|---|---|
| Papovaviridae | Contact, skin abrasion |
| Adenoviridae | Respiratory, fecal–oral |
| Herpesviridae | Sexual (e.g., equine coital exanthema) |
| Respiratory (e.g., infectious bovine rhinotracheitis) | |
| Transplacental (e.g., canine herpesvirus) | |
| African swine | Fecal–oral, respiratory, arthropod |
| fever virus | |
| Poxviridae | Contact (e.g., orf, cowpox) |
| Arthropod (mechanical, e.g., myxoma virus) | |
| Contact, respiratory (e.g., sheeppox) | |
| Parvoviridae | Fecal–oral, respiratory, contact, transplacental |
| Picornaviridae | Fecal–oral, respiratory (Enterovirus) |
| Respiratory (Aphthovirus, Rhinovirus) | |
| Caliciviridae | Respiratory, fecal–oral, contact |
| Togaviridae | Arthropod (Alphavirus) |
| Respiratory, fecal–oral, transplacental (e.g., hog cholera) | |
| Flaviviridae | Arthropod |
| Orthomyxoviridae | Respiratory |
| Paramyxoviridae | Respiratory, contact |
| Coronaviridae | Fecal–oral, respiratory, contact |
| Arenaviridae | Contact with contaminated urine, respiratory |
| Bunyaviridae | Arthropod (Rift Valley fever) |
| Rhabdoviridae | Arthropod and contact (vesicular stomatitis), animal bite (rabies) |
| Retroviridae | Contact, in ovo (germ line) |
| Birnaviridae | Fecal–oral, water (fish) |
| Reoviridae | Fecal–oral (Rotavirus and Reovirus) |
| Arthropod (Orbivirus) |
SURVIVAL OF VIRUSES IN NATURE
Some of the factors that affect the survival of viruses in nature are set out in Table 15-2 and are discussed below.
TABLE 15-2.
Factors Affecting Survival of Viruses in Nature
| Factor | Example |
|---|---|
| Population size | Canine distemper virus |
| Respiratory viruses | |
| Virulence and transmissiblity | Myxoma virus |
| Antigenic variation | Influenza viruses |
| Foot-and-mouth disease viruses | |
| Subclinical infections | Enteroviruses, many others |
| Persistent infections | Herpesviruses |
| Vertical transmission | Retroviruses |
| Reservoir hosts | Alphaviruses |
| Resistant virus | Parvoviruses |
Transmissibility and Virulence
The best-documented example of how the virulence of a virus may directly affect the probability of transmission is myxomatosis of rabbits (see Chapter 5). Here mechanical transmission by biting arthropods is most effective when the diseased rabbit maintains highly infectious skin lesions for a long period. Very virulent strains of myxoma virus kill rabbits too quickly and very attenuated strains cause lesions that heal too rapidly, so that viruses at either extreme of the virulence range do not survive in nature.
Subclinical Infections
Survival of a virus in nature depends on the maintenance of serial infections, i.e., a chain of transmission; the occurrence of disease is neither required nor necessarily advantageous. Infection without recognizable disease is called subclinical or clinically inapparent. Overall, subclinical infections are much more common than those that result in disease. Their relative frequency accounts for the difficulty of tracing chains of transmission, even with the help of laboratory aids. Although clinical cases may be somewhat more productive sources of virus than subclinical infections, the latter are more numerous and, because they do not restrict the movement of infected animals, can be an important source of viral dissemination. In most acute infections, whether clinically apparent or not, virus is shed at highest titers during the late stages of the incubation period, before the influence of the host immune response takes effect.
The infrequency of subclinical infections in some diseases, such as rinderpest or velogenic Newcastle disease, is an important factor in the implementation of control programs, because it makes surveillance and quarantine an efficient strategy for interrupting chains of transmission.
Persistent Infections
Persistent viral infections, whether or not they are associated with episodes of clinical disease, also play an important role in the perpetuation of many viruses in nature. For example, persistent virus shedding by an animal can introduce virus into a population of susceptible animals all of which have been born since the last episode of acute infections. This transmission pattern is important for the survival of bovine virus diarrhea virus, hog cholera virus, equine arteritis virus, and the herpesviruses.
Sometimes the effects of persistent infections in the production of disease and the transmission of infection are dissociated. Thus togaviruses and arenavirus infections seem to have little adverse effect on their reservoir hosts (arthropods, birds, and rodents), but virus shedding and transmission are very efficient. On the other hand, persistent infection of the central nervous system with canine distemper virus is of no epidemiological significance, since no infectious virus is shed; infection of the central nervous system may have a severe effect on the dog, but is of no consequence for the virus.
Infection with viruses of some viral families is characteristically associated with continuous or intermittent shedding; certain other viruses cause acute infections which are associated with transient intense shedding. Table 15-3 lists some of the more important groups of viruses that are associated with persistent or recurrent virus shedding.
TABLE 15-3.
Some Viral Infections Associated with Persistence
| Family | Virus | Comments |
|---|---|---|
| Herpesviridae | Infectious bovine rhinotracheitis virus | Persistence in nerve ganglia |
| Pseudorabies virus | Recurrent shedding in oral and/or genital secretions | |
| Unclassified | African swine fever virus | Persistence in hematopoietic system Persistence in infected ticks |
| Picornaviridae | Foot-in-mouth disease virus | Sometimes, persistence in pharynx of ruminants, but transmission from persistently infected cattle is rare and therefore mainly important as possible means of introduction of virus into a disease-free area |
| Togaviridae | Hog cholera virus | Persistence in hematopoietic system |
| Bovine virus diarrhea virus | Congenital persistent infection with chronic shedding after birth | |
| Arenaviridae | All species | Long-term excretion in urine of reservoir rodent hosts |
| Retroviridae | Bovine leukemia virus | Persistence in hematopoietic system |
| Visna/maedi virus | Persistence in central nervous and respiratory systems | |
| Reoviridae | Bluetongue viruses | Persistence in infected Culicoides |
Population Size
It is self-evident that the long-term survival of a virus requires that it should continue to be transmitted from one host to another. In general, for rapidly and efficiently transmitted viruses such as the respiratory viruses, survival of the virus requires that the susceptible host population is very large. A virus may disappear from a population because it exhausts its potential supply of susceptible hosts. Depending on the breeding characteristics (population turnover rate), duration of immunity, and the pattern of virus shedding, the critical population size varies considerably with different viruses and with different host species.
Acute Infections
The most precise data on the importance of population size in acute, nonpersistent infections come from studies of measles, which is a cosmopolitan human disease caused by a virus which is related to rinderpest and canine distemper viruses. Measles has long been a favorite disease for modeling epidemics, because it is one of the few common human diseases in which subclinical infections are rare and clinical diagnosis is easy. Persistence of measles virus in a population depends on a continuous supply of susceptible hosts. Analyses of the incidence of measles in large cities and in island communities have shown that a population of about half a million persons is needed to ensure a large enough annual input of new susceptible hosts, by birth or immigration, to maintain measles virus in the population.
Because infection depends on respiratory transmission, the duration of epidemics of measles is correlated inversely with population density. If a population is dispersed over a large area, the rate of spread is reduced and the epidemic will last longer, so that the number of susceptible persons needed to maintain transmission chains is reduced. On the other hand, in such a situation a break in the transmission chain is much more likely. When a large proportion of the population is initially susceptible, the intensity of the epidemic builds up very quickly and attack rates are almost 100% (a virgin-soil epidemic). There are many examples of similar transmission patterns among animal viruses, but the quantitative data are not as complete as those for measles. Exotic viruses, i.e., those which are not present in a particular country or region, represent the most important group of viruses with a potential for causing virgin-soil epizootics.
The history of rinderpest in cattle in Africa in the early twentieth century shows many parallels with measles in isolated human populations. When it was first introduced into virgin populations in various parts of Africa in the late nineteenth and early twentieth centuries, the initial impact was devastating. Cattle and wild ruminants of all ages were susceptible, and the mortality was so high that in northern Tanzania the ground was so littered with the carcasses of cattle that a Masai tribesman commented that “the vultures had forgotten how to fly.” The development of effective vaccines in the 1920s changed the epidemiology of rinderpest, leading to a period in the 1960s when its global eradication was anticipated. Unfortunately, in the 1970s vaccination programs in West Africa were poorly maintained and by the 1980s the disease had once more become rampant and the cause of major losses in many parts of Africa. The cyclical nature of the occurrence of such diseases is determined by several variables, including the rate of buildup of susceptible animals, introduction of the virus, and environmental conditions which promote viral spread.
Persistent Infections
Although acute herpesvirus infections are not unlike acute measles or rinderpest, each has a much smaller critical population size, as small as a single farm, kennel, or cattery. This is because herpesviruses establish lifelong latent infections, from which virus may be reactivated and initiate infection in susceptible animals born or introduced into the population since the last acute cases (see Chapters 11 and 19).
Vertical Transmission
Vertical transmission refers to the transfer of virus from parent to offspring, usually before birth, but occasionally in the immediate perinatal period. Other modes of transmission are designated horizontal. Vertical transmission of a virus may occur via the germ line, via the egg (especially in birds), across the placenta, or during the perinatal period via milk. Vertical transmission may be associated with congenital disease, and occurs in all domestic animals and with viruses belonging to several families (see Table 7-4): all arenaviruses, several herpesviruses, parvoviruses, and retroviruses, some orbiviruses and togaviruses, and a few bunyaviruses and coronaviruses may be transmitted in this way. Germ-line transmission (i.e., via the egg or sperm) occurs only with certain retroviruses, in which the viral genome is integrated as provirus in the DNA of the gametes (see Chapter 31).
Zoonoses
Because most viruses are host-restricted, most viral infections are maintained in nature within populations of the same or related species. However, there are a number of viruses that may have multiple hosts and spread naturally between several different species of vertebrate hosts, e.g., rabies and eastern equine encephalitis viruses. The term zoonosis is used to describe multiple-host infections that are transmissible from animals to humans (Tables 15-4 and 15-6 ). Most viral zoonoses are caused by arboviruses (see Table 15-6). The non-arthropod-borne zoonoses listed in Table 15-4 are primarily infections of domestic or wild animals transmissible only under special conditions to humans engaged in activities involving close contact with animals.
TABLE 15-4.
Non-Arthropod-Borne Viral Zoonoses
| Family | Virus | “Reservoir” host | Mode of transmission to humans |
|---|---|---|---|
| Poxviridae |
|
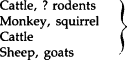 |
Contact, through skin abrasions |
| Herpesviridae | B virus | Monkey | Animal bite |
| Bunyaviridae | Hantaan virus | Rodents | Contact with rodent urine |
| Rhabdoviridae | Rabies virus | Terrestrial mammals and bats | Animal bite, scratch |
| Vesicular stomatitis virus | Cattle | Contact with oral secretions, vesicular fluid | |
| Filoviridae | Ebola, Marburg viruses | Unknown | Contact; iatrogenic (injection) human-to-human spread |
|
|
Swine, horses, birds | Respiratory |
 |
 |
Contact with rodent urine |
TABLE 15-6.
Arthropod-Borne Viral Zoonoses
| Family | Genus | Virus | Principal vertebrate hosts | Arthropod vector |
|---|---|---|---|---|
| Togaviridae | Alphavirus |
|
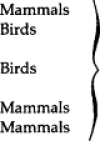 |
|
| Western equine | Mosquitoes | |||
| encephalitis | ||||
| Ross River | ||||
| Venzuelan equine | ||||
| encephalitis | ||||
| Flaviviridae | Flavivirus | Japanese encephalitis |  |
|
| Murray Valley | Mosquitoes | |||
| encephalitis | ||||
| Yellow fever | ||||
| Kyasanur Forest disease |  |
|||
| Louping ill | ||||
| Omsk hemorrhagic fever | Ticks | |||
| Powassan | ||||
| Tick-borne encephalitis | ||||
| Bunyaviridae | Bunyavirus | California encephalitis | ||
| La Crosse | Mammals | Mosquitoes | ||
| Tahyna | ||||
| Phlebovirus | Sandfly fever | Gerbils | Sandflies | |
| Rift Valley fever | Mammals | Mosquitoes | ||
| Nairovirus | Crimean-Congo hemorrhagic fever | Mammals | Ticks | |
| Reoviridae | Unclassified | Colorado tick fever | Mammals | Ticks |
SEASONAL VARIATIONS IN DISEASE INCIDENCE
Many viral infections show pronounced seasonal variations in incidence. In temperate climates, arbovirus infections transmitted by mosquitoes or sandflies occur mainly during the summer months, when vectors are most numerous and active. Infections transmitted by ticks occur most commonly during the spring and early summer months. Other biological reasons for seasonal disease include both virus and host factors. Influenza viruses and poxviruses survive better in air at low rather than at high humidity, and in aerosols all viruses survive better at lower temperatures. It has also been suggested, without much supporting evidence, that there may be seasonal changes in the susceptibility of the host, perhaps associated with changes in the physiological status of nasal and oropharyngeal mucous membranes.
More important in veterinary medicine than any natural seasonal effects are the changes in housing and management practices that occur in different seasons. Housing animals such as cattle and sheep for the winter often increases the incidence of respiratory and enteric diseases. These diseases often have obscure primary etiologies, usually viral, followed by secondary infections caused by opportunistic pathogens (see Chapter 10). In such cases, infectious disease diagnosis, prevention, and treatment must be integrated into an overall system for the management of facilities as well as husbandry practices. In areas where animals are moved—e.g., to feedlots or seasonally to distant pasturage—there are two major problems: animals are subjected to the stress of transportation and the diseases associated with stress, and they are brought into contact with new populations carrying and shedding different infectious agents. Often summer pasturage is at high altitude, adding the stress of pulmonary vascular dysfunction and pulmonary edema to the insult of respiratory virus infections. Secondary Pasteurella pneumonia, shipping fever, is not limited to animals subjected to the stress of transportation to feedlots.
In areas of the world where cattle are moved hundreds of miles each year, such as in the Sahelian zone of western Africa, viral diseases such as rinderpest are associated with the contact between previously separate populations brought about by this traditional husbandry practice. In southern Africa, the communal use of waterholes during the dry season promotes the exchange of viruses such as foot-and-mouth disease virus between different species of wildlife, and in certain circumstances between wildlife and domestic animals.
EPIDEMIOLOGICAL ASPECTS OF IMMUNITY
Immunity from prior infection or from vaccination plays a vital role in the epidemiology of viral diseases. Two examples are given here: (1) canine distemper, caused by a monotypic virus, for which immunity is very effective, and (2) feline respiratory disease due to feline calicivirus and feline rhinotracheitis virus (a herpesvirus), in which lasting immunity does not follow either natural infection or vaccination. In industrialized countries, widespread vaccination of puppies with attenuated live canine distemper virus vaccine has sharply diminished the incidence of canine distemper and of its complications, old dog encephalitis and hard-pad disease.
Feline respiratory infection in a cattery is a good example of the failure of immunity, for disease occurs not only in previously unexposed cats, but also in persistently infected cats. Both feline calicivirus and feline viral rhinotracheitis virus produce persistent infections. Only partial immunity follows primary infection with either of these viruses. The succession of respiratory problems experienced in many catteries reflects a series of minor epizootics. Reactivation and recurrent infection leading to transmission to young kittens accounts for the maintenance of feline rhinotracheitis virus, whereas long-term carrier status and continuous or intermittent shedding account for the transmission of feline calicivirus (possibly assisted by the existence of antigenic variants of the virus).
Seroepidemiology
Seroepidemiology is useful in veterinary public health operations and in research. Because of the expense of collecting and properly storing sera, advantage is taken of a wide range of sources, such as abattoirs, culling from overstocked wildlife populations, and vaccination programs. Such sera can be used to determine the prevalence or incidence of particular infections, to evaluate eradication and immunization programs, and to assess past history when a “new” virus is discovered.
Estimates of Prevalence
Traditional surveillance is based on the reporting of clinical disease. However, examination of appropriate numbers of sera by appropriate serological methods gives a more accurate index of the prevalence of a particular virus. By detecting antibodies to selected viruses in various age groups of the population, it is possible to determine how effectively enzootic viruses have spread, or how long it has been since the last appearance of epizootic viruses.
Estimates of Incidence
When pairs of serum specimens are obtained from individual animals several weeks apart, the initial appearance of antibody in the second specimen or a rise in antibody titer (usually a fourfold or greater rise is considered significant) indicates recent infection. Likewise, the presence of specific IgM antibody (or, with some infections, significant titers of complement-fixing antibody) in a single serum sample often indicates recent infection. Correlation of serological tests with clinical observations makes it possible to determine the ratio of clinical to subclinical infections.
Evaluation of Eradication or Immunization Programs
Serological surveys to detect infected breeding herds of swine have been of major importance in hog cholera eradication campaigns. Vaccination programs were restricted to fattening herds, so that serological surveys could be used to monitor breeding herds and destroy these if they were infected. Serological surveys are also valuable in determining how well immunization programs have succeeded in various populations. For example, the gradual decrease in the percentage of animals with antibodies to rinderpest virus heralded the reemergence of epidemic rinderpest in Africa in the late 1970s.
Emergence of “New” Diseases or Viruses
Sometimes “new” viruses are discovered for which no clinical disease is recognized, e.g., bluetongue virus 20 in Australia in 1977 and bluetongue virus 2 in Florida in 1983. Serological surveys can be used to determine their distribution. Serology is also valuable in determining retrospectively the geographical and secular distribution (i.e., the distribution over time) of newly discovered viruses.
ANALYTICAL EPIDEMIOLOGY
Analytical epidemological techniques are used to investigate the relationships between cause and effect and to evaluate risk factors of disease. There are two basic methods, the case–control study and the cohort study. In the case-control study, investigation starts after the disease has occurred and it attempts to identify the cause; thus it is a retrospective study, going back in time to determine cause. This is the most common type of study. Although it does not require the creation of new data or records, it does require careful selection of the control group, matched to the test group so as to avoid bias. The advantages of the retrospective study are that it lends itself to quick analysis and it is relatively inexpensive to carry out.
In cohort studies, investigation starts with a presumed cause and a population exposed to the causative virus is followed into the future to identify correlated resulting effects—a prospective study. This type of study requires the creation of new data and records, and the careful selection of the control group to be as similar as possible to the exposed group, except for the absence of contact with the presumed causative virus. It usually does not lend itself to quick analysis as groups must be followed until disease is observed, which makes such studies expensive. However, when cohort studies are successful, proof of cause-effect relationship is often incontrovertible.
The production of congenital defects by Akabane virus in cattle provides examples of both the case-control and prospective (cohort) types of epidemiological studies. Epizootics of congenital defects in calves, characterized by deformed limbs and abnormal brain development referred to as congenital arthrogryposis–hydranencephaly (see Chapter 29), occurred in Australia in the 1950s and 1960s, but the cause was not identified. During the summer and early winter months from 1972 to 1975, approximately 42,000 calves were born with these congenital defects in central and western Japan, causing significant economic loss. Japanese scientists postulated that the disease was infectious but were unable to isolate a virus from affected calves. However, when pre-colostral sera from such calves were tested for antibody to a number of viruses, antibody to Akabane virus, an arbovirus (Simbu serogroup, family Bunyaviridae), which was first isolated from mosquitoes in Akabane Prefecture in 1959, was found in almost all sera tested. A retrospective serological survey indicated a very high correlation between the geographical distribution of the disease and the presence of antibody to the virus, suggesting that Akabane virus was the etiological agent of congenital arthrogryposis–hydranencephaly in cattle.
Prospective studies were then organized. Sentinel herds were established (see long-term herd studies, below) in Japan and Australia, and it was soon found that the virus could be isolated from fetuses obtained by slaughter or caesarian section for only a short period after infection, thus explaining the earlier failures in attempts to isolate virus after calves were born. Experimental inoculation of pregnant cows with Akabane virus during the first two trimesters of pregnancy induced congenital abnormalities in calves similar to those seen in natural cases of the disease; clinical disease was not seen in the cows.
Vaccine Trials
The immunogenicity, potency, safety, and efficacy of vaccines are first studied in laboratory animals, followed by small-scale closed trials in the definitive animal species, and finally in large-scale field trials. Such studies employ epidemiological methods rather like those of the cohort (prospective) study described above. There is no alternative way to evaluate new vaccines, and the design of field trials has now been developed so that they yield maximum information with minimum risk and cost. Even with this system, however, a serious problem may be recognized only after a vaccine has passed into commercial use. This occurred after the introduction of attenuated live-virus vaccines for infectious bovine rhinotracheitis in the United States in the 1950s. Surprisingly, the vaccines had been in use for 5 years before it was recognized that abortion was a common sequel to vaccination (see Chapter 19).
Long-Term Herd Studies
Another kind of epidemiological investigation that can provide etiological information and data on the value of vaccines or therapeutic agents is the long-term herd study. Because of the present advanced state of diagnostic and serological virology, such studies now yield a much greater array of valuable data than was possible a few years ago, but they are very expensive and require long-term dedication of both personnel and money. When used principally for the detection of virus in an area, such investigations are referred to as sentinel studies, and are widely used for studying the prevalence of arbovirus infections. For example, sentinel chickens are used for the early detection of eastern equine and St. Louis encephalitis viruses in the southern United States. When used for the estimation of the value of vaccines or therapeutic agents, long-term herd studies have the exceptional advantage that they include all of the variables attributable to the entire husbandry system.
MOLECULAR EPIDEMIOLOGY
The term molecular epidemiology has been applied to the use of molecular biological methods for epidemiological investigation of viral diseases. All the techniques described in Chapter 13 can be used. With DNA viruses and retroviruses, restriction endonuclease fingerprinting or mapping provides a means of identification of viral strains with a specificity that often surpasses serological methods, e.g., with pseudorabies virus. With viruses that have segmented genomes, like orbiviruses and rotaviruses, polyacrylamide gel electrophoresis provides a method of genome analysis that is a valuable supplement to serological typing of these viruses. The genome of a poliovirus isolate recovered from a paralyzed (or normal) person can be sequenced to determine whether it is a wild-type strain, an attenuated vaccine strain, or a vaccine strain that has reacquired neurovirulence as a result of subsequent mutations. Other techniques such as nucleic acid hybridization and oligonucleotide fingerprinting can also distinguish strains of virus within the same serotype. For example, the 1981 outbreak of foot-and-mouth disease in the United Kingdom was traced by oligonucleotide fingerprinting to the presence of live virus in an inactivated vaccine (see Chapter 23). Monoclonal antibodies provide a powerful method of distinguishing viruses that cannot be readily differentiated by serology employing polyclonal antibodies. This method has been particularly useful in elucidating the relationships between rabies virus and rabieslike rhabdoviruses and in distinguishing geographical variants of rabies virus. We can expect to see all these techniques become incorporated into standard epidemiological investigations in the future; properly used, they become tools for much more precise analyses of the role of specific viruses in particular disease outbreaks.
MATHEMATICAL MODELING
From the time of William Farr, who studied both medical and veterinary problems in the 1840s, mathematicians have been interested in “epidemic curves” and secular trends in the incidence of infectious diseases. With the development of mathematical modeling techniques there has been a resurgence of interest in the population dynamics of infectious diseases. Models are now being developed that allow estimates of (1) the patterns of disease transmission, and (2) the critical population size for animal viruses with short or long incubation periods, persistent or recurrent infectivity, and/or age-dependent pathogenicity.
Computer modeling also provides useful insights into the effectiveness of disease control programs. In this regard, most attention has been given to the potential national and international spread of exotic diseases in susceptible populations. Figure 15-1 illustrates results obtained in modeling the spread of foot-and-mouth disease across the United States, commencing at the stage where the disease becomes well established and traditional control measures (quarantine, slaughter, disinfection) are no longer effective. The model suggests that under these conditions—the “worst scenario” model—60% of the cattle herds in the United States could become infected within a period as short as 30 weeks. In the absence of vaccination, the disease would increase again in incidence after 60 weeks and begin a series of enzootic cycles.
FIG. 15-1.

(A) A state transition model of epizootic foot-and-mouth disease. In constructing this model, the aim was to simulate the spread of foot-and-mouth disease across the United States commencing at the stage where the disease became established and traditional control measures (quarantine, slaughter, and disinfection) were no longer effective. The model was based on data collected from the 1967–1968 epizootic of foot-and-mouth disease in the United Kingdom during which outbreaks of disease were recorded on 2364 farms. The objective was to simulate a major epizootic. The state transition model is a general model that permits a variety of disease control strategies to be examined. It illustrates several key characteristics of a useful model: (1) its pathways are intuitively acceptable, (2) it behaves in a biologically and mathematically logical way (i.e., it is sensitive to appropriate variables), (3) it mimics real-life situations, and (4) it is simple enough to be rigorously tested, but complex enough to represent the system being studied.
(B) Pathways of transitions in the model. The basic unit is the herd (or farm), which is in one of four mutually exclusive categories: “susceptible,” “infectious,” “immune,” or “removed/dead.” Each week the probability of each transition is calculated and the number of herds in each category during the next week are derived. A key factor in determining the probability of a susceptible herd becoming infected in a particular week is the dissemination rate. This depends on a number of factors such as topography, weather, husbandry, animal movement, and quarantine. The dissemination rate used for the “scenario” shown in the figure is based on that calculated for the 1967–1968 epidemic in the United Kingdom. With a dissemination rate diminishing at a rate only slightly more slowly than in the United Kingdom, a situation whereby the traditional controls would be abandoned could be reached 4–5 weeks after introduction of the virus to the United States.
(From W. M. Miller, 1979.)
© 1987
Models such as this make no special claim for reliability, but their construction and use brings a number of issues into focus. The results are often unexpected, pointing to the need for better data and different strategies for disease control.
EPIDEMIOLOGY OF ARTHROPOD-BORNE VIRAL DISEASES
Arboviruses have two classes of hosts: vertebrate and invertebrate. Over 400 arboviruses are known, of which at least 66 cause disease in domestic animals or humans (Tables 15-5 and 15-6). Fourteen of these pathogenic arboviruses are tick-borne, and 52 are transmitted by mosquitoes, phlebotomine flies (sandflies), or Culicoides spp. (midges).
TABLE 15-5.
Arthropod-Transmitted Viral Diseases of Domestic Animals
| Vector |
|||||
|---|---|---|---|---|---|
| Family | Subfamily, genus, or virus | Disease | Domestic animals | Vector Species | Replicationa |
| Unclassified | African swine fever virus | African swine fever | Swine | Tick | + |
| Poxiviridae | Avipoxvirus | Fowlpox, pigeonpox, etc. | Poultry, pigeons | Mosquito, other | – |
| Leporipoxivirus | Myxomatosis | Rabbit | Mosquito, flea, | – | |
| other | |||||
| Togaviridae | Alphavirus | Eastern equine encephalitis | Horse | Mosquito | + |
| Western equine encephalitis | Horse | Mosquito | + | ||
| Venezuelan equine encephalitis | Horse | Mosquito | + | ||
| Getah virus disease | Horse | Mosquito | + | ||
| Flaviviridae | Flavivirus | Louping ill | Sheep | Tick | + |
| Wesselsbron disease | Sheep | Mosquito | + | ||
| Japanese encephalitis | Swine | Mosquito | + | ||
| Bunyaviridae | Phlebovirus | Rift Valley fever | Sheep, cattle | Mosquito | + |
| Bunyavirus (Akabane) | Arthrogryposis–hydraencephaly | Sheep, cattle | Mosquito | + | |
| Nairovirus | Nairobi sheep disease | Sheep | Mosquito | + | |
| Reoviridae | Orbivirus | Bluetongue | Sheep, cattle | Culicoides | + |
| Ibaraki infection | Cattle | ? | + | ||
| Epizootic hemorrhagic disease of deer | Deer | Culicoides | + | ||
| African horse sickness | Horse | Culicoides, mosquito | + | ||
| Equine encephalosis | Horse | ? | + | ||
| Retroviridae | Lentivirinae | Equine infectious anemia | Horse | Biting flies | – |
Only viruses that replicate in the vector are classed as arboviruses.
Arthropod transmission may be mechanical, as in myxomatosis and fowlpox, in which mosquitoes act as “flying pins,” or, more commonly, biological, involving replication of the virus in the arthropod vector. The arthropod vector acquires virus by feeding on the blood of a viremic animal. Replication of the ingested virus, initially in the insect gut, and its spread to the salivary gland takes several days (the extrinsic incubation period); the interval varies with different viruses and is influenced by ambient temperature. Virions in the salivary secretions of the vector are injected into animal hosts during all blood meals. Arthropod transmission provides a way for a virus to cross species barriers, since the same arthropod may bite birds, reptiles, and mammals that rarely or never come into close contact in nature.
Ecology of Arboviruses
Most arboviruses have localized natural habitats in which specific receptive arthropod and vertebrate hosts are involved in the viral life cycle. Vertebrate reservoir hosts are usually wild mammals or birds; domestic animals and humans are rarely involved in primary transmission cycles, although the exceptions to this generalization are important (e.g., Venezuelan equine encephalitis virus in horses, yellow fever and dengue viruses in humans). Domestic animal species and humans are in most cases infected incidentally, for example by the geographical extension of a reservoir vertebrate host and/or a vector arthropod.
Most arboviruses that cause periodic epizootics have ecologically complex enzootic maintenance cycles; often these enzootic cycles involve different arthropod as well as different vertebrate hosts from those involved in epizootic cycles. Enzootic cycles provide for the amplification of virus and therefore are critical in dictating the magnitude of epizootics. Enzootic cycles are generally poorly understood and inaccessible to effective control measures.
When arthropods are active, arboviruses replicate alternately in vertebrate and invertebrate hosts. A problem that has concerned many investigators has been to understand what happens to these viruses during the winter months in temperate climates, when the arthropod vectors are inactive. An important mechanism for “overwintering” is transovarial transmission from one generation of arthropods to the next. This is necessarily associated with transstadial transmission (from one larval stage to the next). Transovarial transmission has long been known to occur with the tick-borne flaviviruses and has more recently been discovered to occur with some mosquito-borne bunyaviruses and flaviviruses. For example, some bunyaviruses are found in high northern latitudes where the mosquito breeding season is too short to allow virus survival by horizontal transmission cycles alone; many of the first mosquitoes to emerge each summer carry virus as a result of transovarial transmission, and the pool of virus is rapidly amplified by horizontal transmission in mosquito–mammal–mosquito cycles.
Vertical transmission in arthropods does not explain all arbovirus overwintering, but other possibilities are still unproven or speculative. For example, hibernating vertebrates have been thought to play a role in overwintering. In cold climates, bats and some small rodents, as well as snakes and frogs, hibernate during the winter months; their low body temperature has been thought to favor persistent infection, with recrudescent viremia occurring when normal body temperature returns in the spring. Though demonstrated in the laboratory, this mechanism has never been proven to occur in nature.
Effects of Human Activities on Arbovirus Cycles
Many ecological changes produced by human activities disturb natural arbovirus life cycles and have been incriminated in the geographical spread or increased prevalence of the diseases they cause:
-
1.
Population movements and the intrusion of humans and domestic animals into new arthropod habitats, notably tropical forests.
-
2.
Deforestation, with development of new forest-farmland margins and exposure of domestic animals to new arthropods.
-
3.
Irrigation, especially primitive irrigation systems, which pay no attention to arthropod control.
-
4.
Uncontrolled urbanization, with vector populations breeding in accumulations of water and sewage.
-
5.
Increased rapid and long-distance air travel, with potential for carriage of arthropod vectors.
-
6.
Increased long-distance livestock transportation, with potential for carriage of viruses and arthropods (especially ticks).
-
7.
New routing of long-distance bird migrations brought about by new man-made water impoundments.
As an example, horses may become infected in the eastern part of North America with eastern equine encephalitis virus when their pasturage is made to overlap the natural swamp-based mosquito-bird-mosquito cycle of this virus. Similarly, in Japan and southeastern Asian countries, swine may become infected with Japanese encephalitis virus and become important amplifying hosts when they are bitten by mosquitoes that breed in improperly irrigated rice fields.
Life Cycles of Arboviruses
Examples of the complexity of the life cycles of arboviruses are given in Chapters 20, 25, 29, 30, and 32. Mosquito-borne and tick-borne arboviruses have some features worth comment here.
Mosquito-Borne Encephalitis
Several togaviruses and bunyaviruses cause encephalitis in domestic animals (see Table 15-6). Most of these viruses cycle through wild birds or small mammals, with domestic animals being only incidental or “dead-end” hosts. However, in its epizootic cycle Venezuelan equine encephalitis virus can be maintained in a horse-mosquito-horse cycle, from which humans are easily infected by the same species of mosquitoes.
Tick-Borne Encephalitis
Central European tick-borne flavivirus encephalitis illustrates two features not found in mosquito-transmitted viral infections. First, transovarial infection in ticks is sufficient to ensure survival of the virus, independently of a cycle in vertebrates; vertebrate infection serves to amplify the population of infected ticks. Second, transmission of this arbovirus from one vertebrate host to another can also occur by a mechanism not involving an arthropod at all, namely, via milk. Tick-borne encephalitis is widespread in central Europe and the eastern part of the Soviet Union. A variety of mammals may be infected in nature; small rodents are the most important. Goats, cows, and sheep are incidental hosts and sustain inapparent infections, but they excrete virus in their milk. Adult and juvenile animals may acquire the virus during grazing on tick-infested pastures, and suckling animals may be infected by drinking infected milk. Humans may be infected in two ways; they may enter natural foci of infection in pursuit of work or recreation and become infected by tick bite, or they may acqure infection from milk. All milkborne outbreaks have been associated with goat's milk.
FURTHER READING
- Anderson R.M., May R.M. Life Sciences Research Report 25 of Dahlem Workshop. Springer-Verlag; Berlin and New York: 1982. “Population Biology of Infectious Diseases.”. [Google Scholar]
- Bailey N.T.J. “The Mathematical Theory of Infectious Diseases and its Applications,”. 2nd edn. Griffin; London: 1975. [Google Scholar]
- Beran G.W. “CRC Handbook Series in Zoonoses,”. CRC Press; Boca Raton, Florida: 1981. “Viral Zoonoses,” (2 volumes) Section B. [Google Scholar]
- Burridge M.J. Epidemiological approaches to disease control. In “Diseases of Cattle in the Tropics.” Curr. Top. Vet. Med. Anim. Sci. 1990;6(53) [Google Scholar]
- Evans A.S. Plenum Press; New York: 1982. “Viral Infections of Humans. Epidemiology and Control,” 2nd edition. [Google Scholar]
- Gibbs E.P.J. Vol. 2, “Disease Monographs.”. Academic Press; London: 1981. “Virus Diseases of Food Animals. A World Geography of Epidemiology and Control.” Vol. 1, “International Perspectives”. [Google Scholar]
- Gloster J. Forecasting the airborne spread of foot-and-mouth disease and Newcastle disease. Phil. Trans. R. Soc. Lond. 1983;B302(535) [Google Scholar]
- Miller W.M. A state-transition model of epidemic foot-and-mouth disease. In: McCauley E.H., Aulaqi N.A., New J.C., Sundquist W.B., Miller W.B., editors. In “A Study of the Potential Economic Impact of Foot-and-Mouth Disease in the United States”. U.S. Government Printing Office; Washington, D.C.: 1979. [Google Scholar]
- Mims C.A. Vertical transmission of viruses. Microbiol. Rev. 1981;41(267) doi: 10.1128/mr.45.2.267-286.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J.R., White C. “Serological Epidemiology.”. Academic Press; New York: 1973. [Google Scholar]
- Riemann H.P., Burridge M.J. Dev. Anim. Vet. Sci., Academic Press; New York: 1984. “Impact of Diseases on Livestock Production in the Tropics.”. 15. [Google Scholar]
- Schwabe C., Riemann H.P., Franti C.E. “Epidemiology in Veterinary Practice.”. 2nd edn. Lea and Febiger; Philadelphia: 1977. [Google Scholar]
- Smith C.E.G. Major factors in the spread of infections. In: Edwards M.A., McDonnell U., editors. In “Animal Disease in Relation to Animal Conservation” Symp. Zool. Soc. Lona. Academic Press; London: 1982. 50,. 207 . [Google Scholar]
- WHO (1982). Report of a WHO Expert Committee with the participation of FAO. “Bacterial and Viral Zoonoses.” WHO Tech. Rep. Ser.No. 682, World Health Organization, Geneva. [PubMed]


