Abstract
BACKGROUND:
Disruptive behaviors are prevalent in children with autism spectrum disorder (ASD) and often cause substantial impairments. However, the underlying neural mechanisms of disruptive behaviors remain poorly understood in ASD. In children without ASD, disruptive behavior is associated with amygdala hyperactivity and reduced connectivity with the ventrolateral prefrontal cortex (vlPFC). This study examined amygdala reactivity and connectivity in children with ASD with and without co-occurring disruptive behavior disorders. We also investigated differential contributions of externalizing behaviors and callous-unemotional traits to variance in amygdala connectivity and reactivity.
METHODS:
This cross-sectional study involved behavioral assessments and neuroimaging in three groups of children 8 to 16 years of age: 18 children had ASD and disruptive behavior, 20 children had ASD without disruptive behavior, and 19 children were typically developing control participants matched for age, gender, and IQ. During functional magnetic resonance imaging, participants completed an emotion perception task of fearful versus calm faces. Task-specific changes in amygdala reactivity and connectivity were examined using whole-brain, psychophysiological interaction, and multiple regression analyses.
RESULTS:
Children with ASD and disruptive behavior showed reduced amygdala–vlPFC connectivity compared with children with ASD without disruptive behavior. Externalizing behaviors and callous-unemotional traits were associated with amygdala reactivity to fearful faces in children with ASD after controlling for suppressor effects.
CONCLUSIONS:
Reduced amygdala–vlPFC connectivity during fear processing may differentiate children with ASD and disruptive behavior from children with ASD without disruptive behavior. The presence of callous-unemotional traits may have implications for identifying differential patterns of amygdala activity associated with increased risk of aggression in ASD. These findings suggest a neural mechanism of emotion dysregulation associated with disruptive behavior in children with ASD.
Keywords: Aggression, Amygdala, Autism, Emotion regulation, Functional connectivity, Ventrolateral prefrontal cortex
Disruptive behaviors such as anger and/or irritability, aggression, and noncompliance are common and impairing among children with autism spectrum disorder (ASD) (1,2). Co-occurrence of ASD with disruptive behavior disorders (DBDs), including oppositional defiant disorder and conduct disorder, is estimated at 28% (3), and more than 50% of children with ASD exhibit clinically significant levels of disruptive behaviors (4) that are a primary reason for referral to psychiatric services (5). While neuroimaging studies have investigated the neural underpinnings of disruptive behaviors in youths without ASD (6), similar types of studies are lagging, and the neural mechanisms of disruptive behaviors remain poorly understood in ASD.
Studies of children with DBDs indicate hyperactive circuitry of emotional reactivity paired with reduced activity in prefrontal regulatory circuits (7), particularly increased amygdala reactivity (8–10) and reduced prefrontal response to emotional stimuli (11–14). Of relevance to this study, reduced connectivity between the amygdala and prefrontal regions implicated in the cognitive control of emotion (15,16), such as the ventromedial and ventrolateral prefrontal cortices (vmPFC and vlPFC, respectively), was reported during emotional perception tasks in children with DBDs (11,14). The emotion dysregulation model of disruptive behavior, therefore, suggests abnormal reactivity in the circuitry of experience and regulation of emotions (17); specifically, amygdala hyperactivity and reduced connectivity with the ventral prefrontal cortex (18). Similarly, emotion dysregulation in ASD (19,20) can be associated with reduced amygdala connectivity with the vlPFC (21) and vmPFC (22) during emotional face perception tasks. However, no study to date has investigated patterns of amygdala connectivity and reactivity that differentiate children with ASD with and without disruptive behavior. A better understanding of the underlying neural mechanisms of disruptive behaviors in ASD may also contribute to the development of neuromarkers that can inform clinical interventions. Therefore, this study sought to examine shared and distinct neural signatures of emotional face perception in samples of children with ASD with and without co-occurring DBDs to probe amygdala reactivity and connectivity with prefrontal regions.
In children with DBDs, the presence of callous-unemotional (CU) traits, defined by a lack of guilt, empathy, or remorse, can be associated with higher levels of aggressive and antisocial behaviors (23). Children with ASD can also exhibit elevated levels of CU traits relative to typically developing children (24–27) and impaired recognition of distress cues common to children with DBDs plus CU traits (28,29). The association of disruptive behavior with amygdala reactivity to emotional faces can also be moderated by the presence of CU traits; specifically, aggressive behavior in children with DBDs with CU traits is linked with amygdala hypoactivity in response to fearful facial expressions (11,30–32). Amygdala hypoactivity to socioemotional cues, including faces, is also well documented in ASD (33–37) and was shown to be a shared neural profile in both children with ASD and children with DBDs with CU traits (25). However, amygdala hyperactivity during emotional face perception is also reported in ASD (37–41), which may indicate heightened sensitivity to threat. Given that CU traits and disruptive behaviors such as emotionally driven aggression can have differential associations with amygdala reactivity to fearful faces (i.e., amygdala reactivity to fear is negatively associated with CU traits and positively associated with disruptive behaviors) (42,43), it is important to account for suppressor effects, which can mask the unique contribution of each predictor variable to the dependent variable. Therefore, including predictor variables that are correlated with each other in the same regression model enhances the association of one or both of the predictors with the dependent variable (44,45). However, an understanding of differential patterns of amygdala reactivity based on the presence of CU traits and disruptive behaviors has not been tested in children with ASD.
To our knowledge, this study is the first to use functional magnetic resonance imaging (fMRI) with categorical and dimensional approaches to investigate shared and distinct neural profiles of amygdala connectivity and reactivity as a possible mechanism of disruptive behaviors in well-characterized samples of children with ASD with and without co-occurring DBDs. We used an emotional face perception task of fearful expressions to allow comparison with prior fMRI work in non-ASD youths with DBDs (10,30,43) and because fearful expressions signal potential threat in the environment that preferentially engages the amygdala relative to other regions (46). Our first aim was to examine differential patterns of amygdala–prefrontal connectivity comparing three groups: subjects with ASD and disruptive behavior (ASD+DB ), subjects with ASD without disruptive behavior (ASD-only), and typically developing (TD) control participants. Based on prior work (11,13,14), we hypothesized that the ASD+DB group would show reduced connectivity between the amygdala and ventral prefrontal regions when viewing fearful versus calm faces compared with that of the ASD-only and TD groups. We then examined the association between amygdala–prefrontal connectivity and parent ratings of disruptive behaviors using the Child Behavior Checklist (CBCL) (47) Externalizing Behavior Problems scale and CU traits using the Inventory of Callous-Unemotional Traits (ICU) (48) modeled as dimensional variables in children with ASD. Based on previous fMRI studies in children with DBDs (42,43), we expected that after controlling for suppressor effects between externalizing behavior problems and CU traits, these variables would emerge as significant predictors of amygdala–prefrontal connectivity in ASD. Our second aim was to investigate amygdala reactivity and its association with CBCL externalizing behavior problems and CU traits. We predicted that the ASD+DB group would show greater amygdala response relative to the ASDwoBD and TD groups. We then examined the association between amygdala reactivity and CBCL externalizing behavior problems and CU traits in ASD. Prior studies of non-ASD youths with conduct problems (42,43) informed our hypothesis that after controlling for suppressor effects between externalizing behavior and CU traits, the variance associated with these two variables would differentially predict amygdala reactivity to fearful faces.
METHODS AND MATERIALS
Participants
Three groups of children 8 to 16 years of age were included: 18 children with ASD+DB; 20 children with ASD-only; and 19 TD healthy control participants matched for age, gender, and IQ. Participants with ASD had a DSM-5–defined ASD diagnosis confirmed with the Autism Diagnostic Interview–Revised (49) and Autism Diagnostic Observation Schedule, second edition (ADOS-2) (50). Table 1 shows demographic and clinical characterization data. Details regarding participant inclusion and exclusion are provided in the Supplement. To recruit a representative group of children with ASD and aggression, common co-occurring psychiatric diagnoses (e.g., attention-deficit/hyperactivity disorder, anxiety disorders) and treatment with psychotropic medications were not used as exclusion criteria. However, given that psychotropic medication may influence functional brain connectivity (51), we examined the effects of medication status in a separate post hoc analysis (see Supplement).
Table 1.
Participant Demographics and Clinical Characteristics
| Variable | ASD+DB (n = 18) | ASD-only (n = 20) | TD (n = 19) | p Value |
|---|---|---|---|---|
| Age, Years, Mean (SD) | 12.7 (2.0) | 12.6 (2.1) | 12.9 (2.2) | .895 |
| Male, % | 88.9 | 80.0 | 73.7 | .501 |
| IQa, Mean (SD) | 104.4 (15.4) | 104.3 (19.1) | 112.8 (10.8) | .168 |
| Race, % | .264 | |||
| White | 77.8 | 95.0 | 68.4 | |
| Black | 11.1 | 0 | 21.1 | |
| Asian/Pacific Islander | 5.6 | 0 | 0 | |
| Other> race | 5.6 | 5 | 10.5 | |
| Ethnicity, % | .593 | |||
| Hispanic | 5.6 | 5 | 0 | |
| Non-Hispanic | 94.4 | 95 | 100 | |
| CBCL Aggression T Score, Mean (SD) | 73.6 (9.1) | 50.7 (1.3) | 51.2 (3.0) | <.001b,c |
| CBCL Externalizing T Score, Mean (SD) | 69.3 (5.8) | 44.9 (7.1) | 41.1 (7.8) | <.001b,c |
| CBCL Internalizing T Score, Mean (SD) | 67.1 (8.6) | 54.3 (6.9) | 43.1 (6.5) | <.001b,d |
| ICU Total Score, Mean (SD) | 31.2 (10.5) | 22.5 (10.7) | 14.3 (6.7) | <.001b,c |
| ADOS-2 Module 3 Total Score, Mean (SD) | 15.7 (3.8) | 13.8 (6.1) | .263 | |
| Social Affect subscale score, mean (SD) | 11.9 (3.2) | 10.0 (3.5) | .086 | |
| Restricted and Repetitive Behaviors subscale score, mean (SD) | 3.78 (1.3) | 3.80 (4.2) | .983 | |
| ADI-R Subscale Scores, Mean (SD) | ||||
| Social interaction | 19.3 (5.2) | 18.6 (4.3) | .670 | |
| Communication | 15.2 (3.2) | 14.8 (4.1) | .727 | |
| Primary Comorbid Diagnosis (K-SADS), % | ||||
| Oppositional defiant disorder | 100 | 0 | <.001b | |
| Attention-deficit/hyperactivity disorder | 83.3 | 35 | .003b | |
| Anxiety disorder | 27.8 | 0 | .011b | |
| Currently Taking Medication, % | 66.7 | 20 | .004b | |
| Type of Medication, % | ||||
| Stimulant | 33.3 | 10 | ||
| Antidepressant | 27.8 | 10 | ||
| Neuroleptic | 22.2 | 0 | ||
| Nonstimulant | 22.2 | 10 | ||
| Mood stabilizer | 16.7 | 0 | ||
| Benzodiazepine | 11.1 | 0 |
ASD+DB, autism spectrum disorder with co-occurring disruptive behavior disorder; ASD-only, autism spectrum disorder (without disruptive behavior); ADI-R, Autism Diagnostic Interview–Revised; ADOS-2, Autism Diagnostic Observation Schedule, second edition; CBCL, Child Behavior Checklist; ICU, Inventory of Callous-Unemotional Traits; K-SADS, Schedule for Affective Disorders and Schizophrenia for School-Age Children; TD, typically developing.
Full-scale IQ measured by the Wechsler Abbreviated Scale of Intelligence.
Significant group differences at p < .05, Bonferroni corrected, except for χ2 test for categorical variables.
ASD+DB > ASD-only; ASD+DB > TD.
ASD+DB > ASD-only; ASD+DB > TD; ASD-only > TD.
Participants with ASD were recruited from the Yale Child Study Center Autism Program. Healthy control participants were recruited from the community via advertisements. Each participant’s parent provided informed consent according to specifications by the institutional review board at the Yale University School of Medicine. Each child provided assent.
Clinical Assessment
Children received a comprehensive diagnostic evaluation that included the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (52). Parents also completed the ICU (48) and the CBCL (47). The CBCL externalizing behavior scores were used to assess levels of disruptive behaviors, such as aggression, in analyses. Additional clinical assessments and measures are described in the Supplement.
Experimental Paradigm
Children underwent a block design fMRI task where they viewed emotionally expressive faces from the NimStim face stimulus set (53) that depicted fearful or calm expressions with an equal number of male and female faces (see Supplement for details). Participants were instructed to perform a gender identification task. To examine amygdala reactivity, we used fearful versus calm faces as the contrast of interest. Additional task details are also reported elsewhere (54). Prior to the fMRI session, a mock scanner was used to acclimate participants to the scanning environment (see Supplement).
Imaging Acquisition/Preprocessing
fMRI data were collected using a Siemens MAGNETOM Tim Trio 3T scanner (Siemens, Erlangen, Germany). More detail regarding data acquisition and preprocessing is provided in the Supplement. No between-group differences were observed in mean head motion detected during the functional scan (Supplemental Table S1).
Psychophysiological Interaction Analysis
Our primary aim was to test differential patterns of amygdala–prefrontal coupling comparing ASD+DB, ASD-only, and TD groups. Thus, we conducted a psychophysiological interaction (PPI) analysis (55) to measure changes in connectivity modulated by fear processing. Whole-brain PPI tests were conducted for the fearful versus calm contrast of interest to examine connectivity between the structurally defined left and right amygdala region of interest (ROI) and the rest of the brain. We did not have a priori hypotheses regarding a specific hemisphere, and therefore a single analysis was conducted for both the right and left amygdala. A detailed description of the PPI analysis is provided in the Supplement. We created a general linear model that included four regressors: psychological (task), physiological (amygdala ROI time series), PPI, and nuisance (six motion parameters).
To assess the differential contributions of disruptive behavior problems (CBCL externalizing behavior scores) and CU traits (ICU scores) to the strength of amygdala connectivity from regions identified in the PPI analysis, we conducted a multiple regression analysis in SPSS version 24 (IBM Corp., Armonk, NY) restricted to the ASD groups (n = 38). FSL Featquery was used to extract β coefficients for each subject of the mean parameter estimate values for the contrast of interest (fearful vs. calm) from ventral prefrontal regions that were identified in the PPI analysis. Externalizing behaviors and CU traits were modeled as continuous variables to identify the contributions of each variable (42,43,56). To test whether social deficits conferred by ASD contribute to the variance in amygdala–prefrontal connectivity, we conducted a separate exploratory analysis including the social affect scores from the ADOS-2 in the last step of the regression model. We repeated the same exploratory analysis to test whether internalizing symptoms, which commonly co-occur with disruptive behavior in children with ASD, are associated with amygdala–prefrontal connectivity by entering the CBCL internalizing score in the last step of the regression model.
General Linear Model fMRI Data Analysis
Our second aim was to interrogate the role of amygdala reactivity to fearful versus calm faces between the three groups and its association with CBCL externalizing behaviors and CU traits in children with ASD. We conducted a whole-brain analysis to develop β coefficients to conduct an ROI analysis to test between-group differences in amygdala reactivity and explore patterns of activity in other areas with no a priori hypotheses. We extracted β coefficients for the right and left amygdala to focus our analyses on this region, because it might be advantageous to understand any lateralization effects and a recent meta-analysis showed reduced activity of the bilateral amygdala in conduct disorders (6). A general linear model analysis that included the three groups was conducted for the fearful versus calm comparison on regional activation plus six motion parameters as covariates of no interest. Details regarding fMRI data analysis are described in the Supplement.
To assess the differential contributions of externalizing behavior scores and CU traits to amygdala reactivity to fearful versus calm faces, we conducted a multiple regression analysis in SPSS version 24 restricted to ASD groups (n = 38). Featquery was used to extract β coefficients of the mean parameter estimate values for the contrast fearful > calm using a bilateral amygdala anatomical ROI mask from the Harvard–Oxford Structural Atlas in FSL, which were then entered into a multiple regression analysis. To test whether social deficits in ASD contribute to the variance in amygdala reactivity, we conducted a separate exploratory analysis including the social affect scores from the ADOS-2 in the last step of the regression model. We repeated the same exploratory analysis to test whether internalizing symptoms are associated with amygdala reactivity by entering the CBCL internalizing score in the last step of the regression model. We report data from the amygdala ROI in the main text. For completeness, we report results from the exploratory whole-brain analyses in the Supplement. Behavioral results are reported in the Supplement.
RESULTS
Amygdala Functional Connectivity
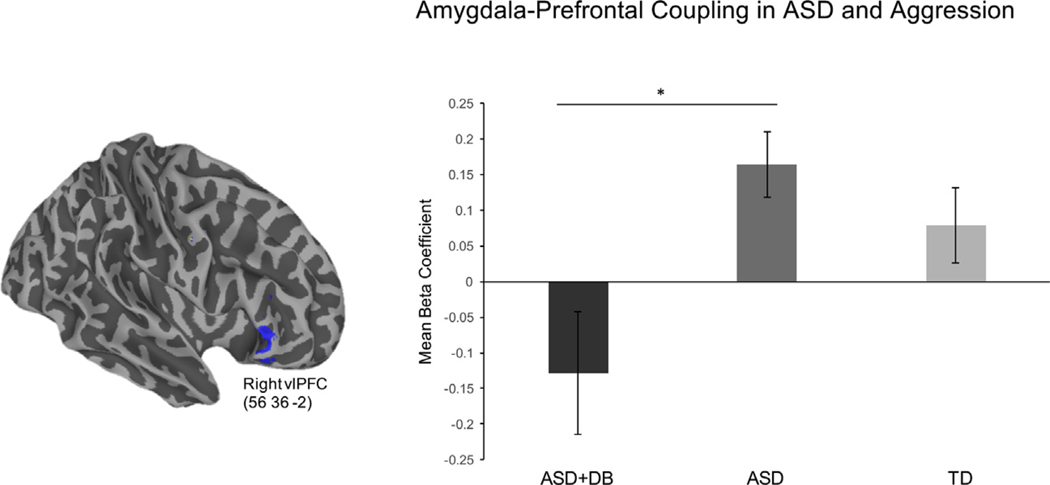
Our hypotheses centered on connectivity between the amygdala and ventral prefrontal regions implicated in emotion regulation circuitry in children with ASD. Therefore, we first examined between-group differences in amygdala–prefrontal connectivity. The PPI contrast revealed that children with ASD+DB showed reduced connectivity between the amygdala and right vlPFC relative to children with ASD when viewing faces with fearful versus calm expressions (Figures 1 and 2). Reduced amygdala connectivity was also observed in the left middle frontal gyrus, right inferior frontal gyrus, parietal lobules, and subcortical regions including the right globus pallidus, which extended to the thalamus, caudate, and insula. Peak coordinates are reported in the Supplement. There were no areas that showed greater connectivity with the amygdala for the ASD+DB versus ASD-only contrast. Relative to the TD group, children with ASD+DB showed reduced connectivity between the amygdala and middle temporal gyrus and between the amygdala and a cluster in the precuneus that extended to the superior parietal cortex (Figure 3). No significant differences in connectivity were found for the reverse contrast (ASD+DB vs. TD).
Figure 1.
Reduced levels of connectivity with the amygdala (inset) were observed for children with autism spectrum disorder (ASD) with co-occurring disruptive behavior disorders (ASD+DB group) in a region of the right ventrolateral prefrontal cortex (vlPFC) compared with children with ASD without co-occurring disruptive behaviors (ASD group) when viewing fearful vs. calm faces (z > 2.3, p < .05). The figure shows psychophysiological interaction results for the contrast ASD > ASD+DB. L, left; R, right.
Figure 2.
Mean β coefficients for the right ventrolateral prefrontal cortex (vlPFC) cluster observed in the psychophysiological interaction analysis (left panel) are shown for descriptive purposes (right panel). Children with autism spectrum disorder (ASD) and co-occurring disruptive behaviors (ASD+DB group) showed reduced amygdala–vlPFC connectivity relative to children with ASD without disruptive behaviors (ASD group) for the fearful vs. calm contrast. Standard error is represented in error bars. *p < .05. TD, typically developing group.
Figure 3.
Children with autism spectrum disorder with co-occurring disruptive behavior disorders showed reduced amygdala connectivity (z > 2.3, p < .05) with the parietal cortex relative to that of typically developing children for the fearful vs. calm contrast. Rendered brains show psychophysiological interaction results for the contrast typically developing > autism spectrum disorder with co-occurring disruptive behavior disorders. L, left; MTG, middle temporal gyrus; R, right.
Next, we assessed the relationship between amygdala connectivity and parent-rated measures of externalizing behaviors and CU traits across ASD groups (n = 38) while controlling for possible suppressor effects between these variables. Consistent with the definition of suppressor effects (44,45), CBCL externalizing problem scores and ICU total scores were correlated with each other (r = .53, p < .01). Bivariate correlations between each of these variables and amygdala–vlPFC connectivity were not significant (for CBCL externalizing behavior score: r = −.317, p = .053; for ICU total score: r = .018, p = .912). However, results of a regression analysis revealed that externalizing problems, after we controlled for CU traits, negatively predicted amygdala–vlPFC connectivity (p = .015) (Figure 4 and Table 2). Additionally, CU traits did not significantly predict amygdala–vlPFC connectivity after controlling for externalizing problems (p = .132). As an exploratory analysis, including the ADOS-2 social affect score could not explain these effects (R2 change = .003), and this variable did not make a significant independent contribution to the variance in amygdala–vlPFC connectivity (p = .74). A separate exploratory analysis including CBCL internalizing problems scores also could not explain these effects (R2 change = .09) and did not make a significant independent contribution to the variance in amygdala–vlPFC connectivity (p = .054).
Figure 4.
Results of a regression analysis for amygdala–ventrolateral prefrontal cortex (vlPFC) connectivity in the combined sample of children with autism spectrum disorder. The leverage plot shows the relationship between the Child Behavior Checklist externalizing behavior score (x-axis) with functional connectivity, quantified as the average bilateral amygdala psychophysiological interaction β coefficient (y-axis) for the right vlPFC (peak coordinates = 56, 36, −2) for the fearful vs. calm contrast after accounting for the variance of callous-unemotional traits.
Table 2.
Multiple Regression Analysis for Amygdala– Ventrolateral Prefrontal Cortex Functional Connectivity in the Combined Sample of Children With Autism Spectrum Disorder When Viewing Fearful Versus Calm Faces
| Variable | B (SE) | β | p Value | Semipartial r |
|---|---|---|---|---|
| Constant | −20.029 (0.128) | |||
| CBCL Externalizing Behaviors Score | −20.012 (0.005) | −.481 | .015 | −.397 |
| ICU Total Score | 0.008 (0.005) | .290 | .132 | .240 |
Model predicting amygdala-vlPFC connectivity: F2,35 = 3.278, R2 = .158, p = .05.
CBCL, Child Behavior Checklist; ICU, Inventory of Callous-Unemotional Traits.
Amygdala Activation to Fearful Versus Calm Faces
We tested whether amygdala reactivity to fearful versus calm faces was associated with CBCL externalizing behavior problems and CU traits. First, β coefficients of amygdala activation to fearful versus calm faces were extracted using an anatomical ROI mask in Featquery. Results of a one-way analysis of variance conducted in SPSS revealed no significant differences in amygdala response to fearful versus calm faces between groups (ASD+DB vs. ASD-only vs. TD) in the right hemisphere (F2,54 = 0.205, p = .815) or left hemisphere (F2,54 = 0.579, p = .564) (see Supplement).
Next, amygdala β coefficients were used to conduct a regression analysis in SPSS restricted to children with ASD (n = 38) to examine the relationship between externalizing behaviors and CU traits in predicting amygdala reactivity to fearful faces. Bivariate correlations between CBCL externalizing problems, CU traits, and amygdala response were not significant for the right hemisphere (for CBCL externalizing problems score: r = .113, p = .501; for ICU total score: r = −.265, p = .108) or the left hemisphere (for CBCL externalizing problems: r = .096, p = .567; for ICU total score: r = −.277, p = .092). However, results from a multiple regression analysis within the right amygdala showed suppressor effects between externalizing problems and CU traits (Figure 5): that is, externalizing problems positively predicted right amygdala response after controlling for CU traits (p = .046), while CU traits negatively predicted right amygdala response after controlling for externalizing problems (p = .014) (Table 3). For the left amygdala, externalizing problems did not significantly predict amygdala response after controlling for CU traits (p = .055), while CU traits negatively predicted amygdala response after controlling for externalizing problems (p = .013). As an exploratory analysis, including the ADOS-2 social affect score could not explain these effects for the right (R2 change = .017) or left (R2 change = .039) amygdala, and the score did not make significant independent contributions to the variance in response for the right (p = .404) or left (p = .207) amygdala. A separate exploratory regression analysis including CBCL internalizing problems scores could not explain these effects for the right amygdala (R2 change = .001) or the left amygdala (R2 change = .001), and did not make significant independent contributions to the variance in response for the right amygdala (p = .847) or left amygdala (p = .814).
Figure 5.
Results of a regression analysis in the right amygdala across the combined autism spectrum disorder group. The partial regression plots show the associations between mean β coefficients extracted from the right amygdala anatomical mask (y-axis) and (A) callous-unemotional traits and (B) Child Behavior Checklist externalizing problems score after accounting for the variance of the other variable.
Table 3.
Multiple Regression Results Showing Externalizing Behavior Problems and Callous-Unemotional Traits as Predictors of Amygdala Response to Fearful Versus Calm Faces in the Combined Autism Spectrum Disorder Group
| Dependent Variable | B (SE) | β | p Value | Semipartial r |
|---|---|---|---|---|
| Right Amygdalaa | ||||
| Constant | 25.013 (13.134) | |||
| CBCL externalizing behaviors score | 0.999 (0.483) | .386 | .046 | .318 |
| ICU total score | −1.399 (0.540) | 2.483 | .014 | −.398 |
| Left Amygdalab | ||||
| Constant | 25.0429 (12.011) | |||
| CBCL externalizing behaviors score | 0.878 (0.442) | .371 | .055 | .306 |
| ICU total score | −1.288 (0.494) | −.487 | .013 | −.401 |
CBCL, Child Behavior Checklist; ICU, Inventory of Callous-Unemotional Traits.
Model predicting right amygdala reactivity: F2,35 = 3.62, R2 = .171, p = .037.
Model predicting left amygdala reactivity: F2,35 = 3.59, R2 = .170, p = .038.
DISCUSSION
This study investigated distinct and shared patterns of amygdala connectivity and reactivity in children with ASD with and without co-occurring DBDs. To our knowledge, this is the first study to examine amygdala–prefrontal connectivity and amygdala reactivity in well-characterized samples of children with ASD+DB and ASD-only. The primary aim of this study was to test amygdala–prefrontal connectivity in children with ASD+DB during a task of emotional face processing. Consistent with our hypotheses, children with ASD+DB showed reduced connectivity between the amygdala and the right vlPFC compared with children with ASD-only. These findings are in line with studies of non-ASD youths with DBDs reporting reduced amygdala coupling with the ventral prefrontal cortex (11–14,32). Our findings of reduced amygdala–vlPFC connectivity are also consistent with earlier studies of children with ASD showing weaker amygdala connectivity with the vmPFC (22,39,57) and vlPFC (21,58) during emotional face perception tasks. Given that the top-down control of amygdala reactivity to aversive or emotional stimuli is modulated by coupling with the vmPFC (16,59–61) and vlPFC (15,62), our finding of reduced amygdala–vlPFC connectivity in children with ASD+DB suggests reduced cognitive control of reactions to emotional stimuli that is consistent with the emotion dysregulation view of ASD (63) and pathophysiology of aggression (17,18).
Children with ASD+DB also showed diminished connectivity in regions of the posterior parietal cortex, which is implicated in disruptive behavior, compared with that found in the ASD-only and TD groups. However, we did not have a priori hypotheses relevant to these regions. Children with ASD+DB also showed reduced connectivity with frontotemporal regions relative to that of the ASD-only group (see Supplement). Parietal regions implicated in memory and attention are recruited during cognitive control processes along with ventral prefrontal regions to modulate amygdala reactivity (15,60,62). Reduced activation of parietal regions during executive control tasks was associated with conduct problems and aggressive behavior (7,25,64). Similarly, reduced parietal cortex activity (65) and connectivity with the vlPFC (66) was reported in individuals with ASD during both social perception and executive functioning tasks. As such, the recruitment of parietal regions may support adaptive responding to threat and emotionally salient stimuli. Taken together, it is possible that our findings of reduced connectivity with the parietal cortex could reflect weaker modulation of amygdala reactivity to negative emotional stimuli in children with ASD+DB compared with that in ASD-only and TD groups.
Contrary to our predictions, however, there were no significant differences in amygdala–vlPFC connectivity between the ASD+DB and TD groups. One possibility is that the emotional face perception task did not elicit negative affect in TD children and thereby required fewer neural resources of emotion regulation circuitry. Another interpretation is that the task was indeed successful in eliciting amygdala activation, and the differences in amygdala–vlPFC connectivity could be specific to externalizing behaviors in the context of ASD, which could be valuable in identifying subgroups of children with ASD. In support of this, our regression results showed that externalizing behavior was negatively associated with amygdala–vlPFC connectivity across ASD groups controlling for CU traits. Recent studies have also reported no significant differences in brain activation and/or connectivity in children with disruptive behaviors versus those in TD groups using a similar paradigm of fear processing (32,43), which could lend support to the contribution of a dimensional framework to understand disruptive behaviors in non-ASD and ASD youths (67). Alternatively, null findings could indicate increased amygdala–vlPFC coupling to fearful versus calm faces for ASD+DB and TD groups.
We also explored the relationship between CBCL externalizing problems and CU traits with amygdala–vlPFC coupling. Greater levels of externalizing behavior problems were associated with weaker amygdala–vlPFC coupling across ASD groups after controlling for CU traits, which could suggest that the presence of CU traits in children with ASD+DB can mask the neural mechanisms of emotion dysregulation. This finding is also consistent with prior work in children with DBDs indicating that reduced amygdala–prefrontal coupling is associated with greater levels of conduct problems (11,14,68). However, CU traits did not significantly predict amygdala–vlPFC connectivity in children with ASD after controlling for externalizing behavior problems. Including ASD social deficits did not make a significant contribution to the model predicting amygdala–vlPFC connectivity, which suggests that neural mechanisms of disruptive behaviors in ASD could be distinct from core symptoms in ASD. However, future studies with larger samples should examine social perception processes to disentangle the overlap of ASD symptoms with disruptive behaviors as related to amygdala–prefrontal cortex connectivity.
It is also noteworthy that in our sample of children with ASD+DB , there was a high correlation between externalizing and internalizing problem behavior scales of the CBCL and 28% of children in this group also met criteria for anxiety disorders. Further, high rates of co-occurrence of disruptive behavior and anxiety are common in children with ASD. For example, in a recent study of co-occurring psychopathology in children with ASD seeking treatment for disruptive behavior, 44% of children met criteria for one or more internalizing disorders (69). In the current study, children with ASD+DB were recruited based on clinically significant levels of disruptive behavior, and all met criteria for oppositional defiant disorder as a primary diagnosis. When entered in the regression model as a last step after CU traits, CBCL externalizing problems, and ADOS-2 social affect scores, CBCL internalizing scores did not contribute significantly to amygdala–vlPFC connectivity. However, because anxiety can also be associated with reduced amygdala–ventral prefrontal cortex connectivity (70–72), larger studies of children with ASD with and without disruptive behavior and anxiety are needed to dissociate the role of externalizing and internalizing psychopathology.
Our second aim was to test amygdala reactivity to fearful versus calm faces and its association with externalizing behaviors and CU traits. We did not observe significant differences in amygdala activation between the three groups when modeled categorically. However, we found that amygdala reactivity to fearful versus calm faces was differentially associated with externalizing behaviors and CU traits when modeled dimensionally across ASD groups. We modeled these regression-based analyses on a recent study of children with disruptive behaviors (43). Moreover, to allow comparison with prior fMRI studies of children with disruptive behaviors (10,42,43) and those comparing children with ASD (24,25,73), we relied on the same measures of externalizing problems and CU traits. We also used the fearful versus calm faces contrast of the emotional perception task, because the expression of these affective states is most sensitive in detecting effects of CU traits. While there were no significant differences in amygdala activation for fearful > calm faces in the current study, other studies have reported similar amygdala responses to fearful faces in children (33) and adults (74) with ASD compared with TD control participants as well as in response to facial expressions of different emotions (75). A recent study using a similar paradigm of fearful faces also found no significant group differences in amygdala responses between children with conduct problems and TD control participants (43). Larger studies are necessary to understand the role of externalizing behaviors and CU traits in modulating amygdala reactivity to fear in children with ASD. Nonetheless, this study is a first step in investigating amygdala connectivity and reactivity in a subgroup of children with ASD+DB .
Consistent with prior studies of children with conduct problems (42,43), we found that right amygdala reactivity was negatively associated with CU traits and positively associated with externalizing behaviors after controlling for suppressor effects across ASD groups. Further, neither CBCL externalizing behaviors nor CU traits were significantly associated with right or left amygdala reactivity in zero-order correlations. Therefore, our findings support the use of a dimensional approach to modeling externalizing behaviors and CU traits in ASD that is consistent with prior studies in children with DBDs (42,43). Additionally, core ASD symptoms did not contribute significantly or change the results of the regression model. However, future studies of aggression in ASD should include different tasks of social information processing to disambiguate the overlap of core ASD symptoms (e.g., deficits in theory of mind) with disruptive behaviors. Further, including CBCL internalizing problem behaviors as a last step in the model did not make a significant independent contribution to the variance in amygdala reactivity. In sum, our finding of a differential association between amygdala reactivity and externalizing problem behaviors and CU traits is consistent with previous evidence in children with DBDs (10,11,30,32,43). Further, results of this study could suggest that when externalizing behaviors are held constant, patterns of amygdala responses to negative affect may reflect a neuroendophenotype that links CU traits with increased risk of disruptive behaviors in ASD.
Study Limitations
First, the sample size was relatively small. However, the samples of children with ASD with and without DBDs were well matched on age, IQ, gender, and ASD symptom severity. Nonetheless, the replication of results with larger samples is needed. Second, eye-tracking data were not collected and we cannot therefore assess whether there were group differences in fixation that may have influenced amygdala responses in participants with ASD. However, the authors took steps to ensure that participants were attending to faces, such as prescan training and gender identification. Future studies should include eye tracking to avoid this limitation. Third, the PPI method does not indicate directional information and whether modulatory interactions reflect direct or indirect pathways. Fourth, it will be important to control for long-term use of psychotropic medication to understand its effects on patterns of brain connectivity in children with ASD and co-occurring DBDs. Fifth, this study was not powered for rigorous testing of the effects of co-occurring internalizing disorders that can also be associated with aberrant amygdala–vlPFC connectivity. Studies with larger samples are needed to disambiguate the overlap between internalizing and externalizing psychopathology with amygdala–prefrontal cortex connectivity in children with ASD. Finally, this study consisted of predominately boys, and it will be important to understand gender differences and neural signatures of aggression in girls with ASD.
Conclusions
In summary, children with ASD+DB showed reduced amygdala–vlPFC connectivity that was associated with levels of externalizing behaviors. These findings provide unique insights into a putative mechanism of emotion dysregulation in ASD that could pose increased risk of disruptive behavior in children with ASD. This study also provides the first evidence of a possible distinct neural mechanism of correlates of emotion processing in children with ASD and co-occurring disruptive behaviors, which is consistent with studies of children with DBDs without ASD. Further, the presence of CU traits may have implications for identifying neural mechanisms of aggression in ASD. These findings also support the development of biomarkers that could serve as neural targets for next-generation personalized treatments in ASD.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by the National Institute of Mental Health (Grant No. R01MH101514 [to DGS and KAP]) and Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant No. R01HD083881 [to DGS and KAP]). KI is a Fellow of the Translational Developmental Neuroscience Training Program (T32 MH18268) directed by Dr. Michael Crowley.
We thank Mr. Jesse Reynolds and Mr. Fangyong Li for their assistance in data analysis, Dr. Wuyi Wang and Dr. Rachael Grazioplene for assistance in data visualization, Dr. Megan Tudor for subject characterization assessments, and Ms. Emilie Bertschinger, Ms. Tess Gladstone, and Ms. Carolyn Marsh for study coordination.
Footnotes
DGS receives royalties from Guilford Press for a treatment manual on cognitive behavioral therapy for anger and aggression in children. KI, GH, PV, GM, KAP, and JAE report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at http://doi.org/10.1016/j.bpsc.2019.01.009.
Contributor Information
Karim Ibrahim, Child Study Center, Yale University School of Medicine, New Haven, Connecticut.
Jeffrey A. Eilbott, Child Study Center, Yale University School of Medicine, New Haven, Connecticut
Pamela Ventola, Department of Psychology, Yale University, New Haven, Connecticut.
George He, Department of Neurology, University of Virginia School of Medicine, Charlottesville, Virginia.
Kevin A. Pelphrey, Department of Neurology, University of Virginia School of Medicine, Charlottesville, Virginia
Gregory McCarthy, Department of Psychology, Yale University, New Haven, Connecticut.
Denis G. Sukhodolsky, Child Study Center, Yale University School of Medicine, New Haven, Connecticut
REFERENCES
- 1.American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders, 5th ed Washington, DC: American Psychiatric Association. [Google Scholar]
- 2.Kanne SM, Mazurek MO (2011): Aggression in children and adolescents with ASD: Prevalence and risk factors. J Autism Dev Disord 41:926–937. [DOI] [PubMed] [Google Scholar]
- 3.Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G (2008): Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 47:921–929. [DOI] [PubMed] [Google Scholar]
- 4.Mazurek MO, Kanne SM, Wodka EL (2013): Physical aggression in children and adolescents with autism spectrum disorders. Res Autism Spectr Disord 7:455–465. [Google Scholar]
- 5.Arnold LE, Vitiello B, McDougle C, Scahill L, Shah B, Gonzalez NM, et al. (2003): Parent-defined target symptoms respond to risperidone in RUPP autism study: Customer approach to clinical trials. J Am Acad Child Adolesc Psychiatry 42:1443–1450. [DOI] [PubMed] [Google Scholar]
- 6.Noordermeer SD, Luman M, Oosterlaan J (2016): A systematic review and meta-analysis of neuroimaging in oppositional defiant disorder (ODD) and conduct disorder (CD) taking attention-deficit hyperactivity disorder (ADHD) into account. Neuropsychol Rev 26:44–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alegria AA, Radua J, Rubia K (2016): Meta-analysis of fMRI studies of disruptive behavior disorders. Am J Psychiatry 173:1119–1130. [DOI] [PubMed] [Google Scholar]
- 8.Herpertz SC, Huebner T, Marx I, Vloet TD, Fink GR, Stoecker T, et al. (2008): Emotional processing in male adolescents with childhood-onset conduct disorder. J Child Psychol Psychiatry 49:781–791. [DOI] [PubMed] [Google Scholar]
- 9.Passamonti L, Fairchild G, Goodyer IM, Hurford G, Hagan CC, Rowe JB, et al. (2010): Neural abnormalities in early-onset and adolescence-onset conduct disorder. Arch Gen Psychiatry 67: 729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CA, De Brito SA, et al. (2012): Amygdala response to preattentive masked fear in children with conduct problems: The role of callous-unemotional traits. Am J Psychiatry 169:1109–1116. [DOI] [PubMed] [Google Scholar]
- 11.Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, et al. (2008): Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry 165:712–720. [DOI] [PubMed] [Google Scholar]
- 12.Decety J, Michalska KJ, Akitsuki Y, Lahey BB (2009): Atypical empathic responses in adolescents with aggressive conduct disorder: A functional MRI investigation. Biol Psychol 80:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL (2007): Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol Psychiatry 62:168–178. [DOI] [PubMed] [Google Scholar]
- 14.Aghajani M, Klapwijk ET, van der Wee NJ, Veer IM, Rombouts SA, Boon AE, et al. (2017): Disorganized amygdala networks in conduct-disordered juvenile offenders with callous-unemotional traits. Biol Psychiatry 82:283–293. [DOI] [PubMed] [Google Scholar]
- 15.Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin RE, et al. (2016): VlPFC–vmPFC–amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cereb Cortex 27: 3502–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD (2002): Rethinking feelings: An FMRI study of the cognitive regulation of emotion. J Cogn Neurosci 14:1215–1229. [DOI] [PubMed] [Google Scholar]
- 17.Davidson RJ, Putnam KM, Larson CL (2000): Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science 289:591–594. [DOI] [PubMed] [Google Scholar]
- 18.Coccaro EF, Sripada CS, Yanowitch RN, Phan KL (2011): Corticolimbic function in impulsive aggressive behavior. Biol Psychiatry 69:1153–1159. [DOI] [PubMed] [Google Scholar]
- 19.Samson AC, Hardan AY, Lee IA, Phillips JM, Gross JJ (2015): Maladaptive behavior in autism spectrum disorder: The role of emotion experience and emotion regulation. J Autism Dev Disord 45:3424–3432. [DOI] [PubMed] [Google Scholar]
- 20.Samson AC, Hardan AY, Podell RW, Phillips JM, Gross JJ (2015): Emotion regulation in children and adolescents with autism spectrum disorder. Autism Res 8:9–18. [DOI] [PubMed] [Google Scholar]
- 21.Pitskel NB, Bolling DZ, Kaiser MD, Pelphrey KA, Crowley MJ (2014): Neural systems for cognitive reappraisal in children and adolescents with autism spectrum disorder. Dev Cogn Neurosci 10: 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swartz JR, Wiggins JL, Carrasco M, Lord C, Monk CS (2013): Amygdala habituation and prefrontal functional connectivity in youth with autism spectrum disorders. J Am Acad Child Adolesc Psychiatry 52:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowe R, Maughan B, Moran P, Ford T, Briskman J, Goodman R (2010): The role of callous and unemotional traits in the diagnosis of conduct disorder. J Child Psychol Psychiatry 51:688–695. [DOI] [PubMed] [Google Scholar]
- 24.O’Nions E, Sebastian CL, McCrory E, Chantiluke K, Happe F, Viding E (2014): Neural bases of Theory of Mind in children with autism spectrum disorders and children with conduct problems and callous-unemotional traits. Dev Sci 17:786–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klapwijk ET, Aghajani M, Colins OF, Marijnissen GM, Popma A, van Lang ND, et al. (2016): Different brain responses during empathy in autism spectrum disorders versus conduct disorder and callous-unemotional traits. J Child Psychol Psychiatry 57:737–747. [DOI] [PubMed] [Google Scholar]
- 26.Bours C, Bakker-Huvenaars M, Tramper J, Bielczyk N, Scheepers F, Nijhof K, et al. (2018): Emotional face recognition in male adolescents with autism spectrum disorder or disruptive behavior disorder: An eye-tracking study. Eur Child Adolesc Psychiatry 27:1143–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tye C, Bedford R, Asherson P, Ashwood K, Azadi B, Bolton P, et al. (2017): Callous-unemotional traits moderate executive function in children with ASD and ADHD: A pilot event-related potential study. Dev Cogn Neurosci 26:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter Leno V, Charman T, Pickles A, Jones CR, Baird G, Happe F, et al. (2015): Callous-unemotional traits in adolescents with autism spectrum disorder. Br J Psychiatry 207:392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers J, Viding E, Blair RJ, Frith U, Happe F (2006): Autism spectrum disorder and psychopathy: Shared cognitive underpinnings or double hit? Psychol Med 36:1789–1798. [DOI] [PubMed] [Google Scholar]
- 30.White SF, Marsh AA, Fowler KA, Schechter JC, Adalio C, Pope K, et al. (2012): Reduced amygdala response in youths with disruptive behavior disorders and psychopathic traits: Decreased emotional response versus increased top-down attention to nonemotional features. Am J Psychiatry 169:750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E (2009): Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. Am J Psychiatry 166:95–102. [DOI] [PubMed] [Google Scholar]
- 32.Cardinale EM, Breeden AL, Robertson EL, Lozier LM, Vanmeter JW, Marsh AA (2018): Externalizing behavior severity in youths with callous–unemotional traits corresponds to patterns of amygdala activity and connectivity during judgments of causing fear. Dev Psychopathol 30:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Dapretto M, Hariri AR, Sigman M, Bookheimer SY (2004): Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 43:481–490. [DOI] [PubMed] [Google Scholar]
- 34.Pelphrey KA, Morris JP, McCarthy G, Labar KS (2007): Perception of dynamic changes in facial affect and identity in autism. Soc Cogn Affect Neurosci 2:140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nomi JS, Uddin LQ (2015): Face processing in autism spectrum disorders: From brain regions to brain networks. Neuropsychologia 71:201–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perlman SB, Hudac CM, Pegors T, Minshew NJ, Pelphrey KA (2011): Experimental manipulation of face-evoked activity in the fusiform gyrus of individuals with autism. Soc Neurosci 6:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kleinhans NM, Richards T, Johnson LC, Weaver KE, Greenson J, Dawson G, et al. (2011): FMRI evidence of neural abnormalities in the subcortical face processing system in ASD. Neuroimage 54:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kleinhans NM, Richards T, Weaver K, Johnson LC, Greenson J, Dawson G, et al. (2010): Association between amygdala response to emotional faces and social anxiety in autism spectrum disorders. Neuropsychologia 48:3665–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monk CS, Weng S-J, Wiggins JL, Kurapati N, Louro HM, Carrasco M, et al. (2010): Neural circuitry of emotional face processing in autism spectrum disorders. J Psychiatry Neurosci 35:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tottenham N, Hertzig ME, Gillespie-Lynch K, Gilhooly T, Millner AJ, Casey B (2013): Elevated amygdala response to faces and gaze aversion in autism spectrum disorder. Soc Cogn Affect Neurosci 9:106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kliemann D, Dziobek I, Hatri A, Baudewig J, Heekeren HR (2012): The role of the amygdala in atypical gaze on emotional faces in autism spectrum disorders. J Neurosci 32:9469–9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sebastian CL, McCrory EJ, Cecil CA, Lockwood PL, De Brito SA, Fontaine NM, et al. (2012): Neural responses to affective and cognitive theory of mind in children with conduct problems and varying levels of callous-unemotional traits. Arch Gen Psychiatry 69:814–822. [DOI] [PubMed] [Google Scholar]
- 43.Lozier LM, Cardinale EM, VanMeter JW, Marsh AA (2014): Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry 71:627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen J, Cohen P, West SG, Aiken LS (2003): Applied Multiple Correlation/Regression Analysis for the Behavioral Sciences. New York, NY: Routledge. [Google Scholar]
- 45.Hicks BM, Patrick CJ (2006): Psychopathy and negative emotionality: Analyses of suppressor effects reveal distinct relations with emotional distress, fearfulness, and anger-hostility. J Abnorm Psychol 115:276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. (2009): Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci 34:418–432. [PMC free article] [PubMed] [Google Scholar]
- 47.Achenbach TM, Rescorla LA (2001): Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families. [Google Scholar]
- 48.Frick PJ (2003): The Inventory of Callous-Unemotional Traits. New Orleans, LA: University of New Orleans. [Google Scholar]
- 49.Le Couteur A, Lord C, Rutter M (2003): The Autism Diagnostic Interview-Revised (ADI-R). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- 50.Lord C, Rutter M, DiLavore PC, Risi S (2012): Autism Diagnostic Observation Schedule, 2nd ed Los Angeles, CA: Western Psychological Services. [Google Scholar]
- 51.Linke AC, Olson L, Gao Y, Fishman I, Müller R-A (2017): Psychotropic medication use in autism spectrum disorders may affect functional brain connectivity. Biol Psychiatry Cogn Neurosci Neuroimaging 2:518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaufman J, Birmaher B, Axelson D, Perepletchikova F, Brent D, Ryan N (2016): Schedule for Affective Disorders and Schizophrenia for School Aged Children: Present and Lifetime Version for DSM-5 (K-SADS-PL DSM-5). Available at: https://www.pediatricbipolar.pitt.edu/resources/instruments Accessed July 8, 2018.
- 53.Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. (2009): The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res 168:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sukhodolsky DG, Vander Wyk BC, Eilbott JA, McCauley SA, Ibrahim K, Crowley MJ, et al. (2016): Neural mechanisms of cognitive-behavioral therapy for aggression in children and adolescents: Design of a randomized controlled trial within the National Institute for Mental Health Research domain criteria construct of frustrative non-reward. J Child Adolesc Psychopharmacol 26:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friston K, Buechel C, Fink G, Morris J, Rolls E, Dolan R (1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6:218–229. [DOI] [PubMed] [Google Scholar]
- 56.Markon KE, Chmielewski M, Miller CJ (2011): The reliability and validity of discrete and continuous measures of psychopathology: A quantitative review. Psychol Bull 137:856–879. [DOI] [PubMed] [Google Scholar]
- 57.von dem Hagen EA, Stoyanova RS, Rowe JB, Baron-Cohen S, Calder AJ (2014): Direct gaze elicits atypical activation of the theory-of-mind network in autism spectrum conditions. Cereb Cortex 24:1485–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Green SA, Hernandez L, Tottenham N, Krasileva K, Bookheimer SY, Dapretto M (2015): Neurobiology of sensory overresponsivity in youth with autism spectrum disorders. JAMA Psychiatry 72:778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL (2007): Amygdala–frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci 2:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. (2014): Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cereb Cortex 24:2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diekhof EK, Geier K, Falkai P, Gruber O (2011): Fear is only as deep as the mind allows: A coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage 58: 275–285. [DOI] [PubMed] [Google Scholar]
- 62.McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, et al. (2012): The development of emotion regulation: An fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc Cogn Affect Neurosci 7:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mazefsky CA, Herrington J, Siegel M, Scarpa A, Maddox BB, Scahill L, et al. (2013): The role of emotion regulation in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 52:679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.White SF, Williams WC, Brislin SJ, Sinclair S, Blair KS, Fowler KA, et al. (2012): Reduced activity within the dorsal endogenous orienting of attention network to fearful expressions in youth with disruptive behavior disorders and psychopathic traits. Dev Psychopathol 24:1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP (2009): Functional brain correlates of social and nonsocial processes in autism spectrum disorders: An activation likelihood estimation meta-analysis. Biol Psychiatry 65:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rudie JD, Shehzad Z, Hernandez LM, Colich NL, Bookheimer SY, Iacoboni M, et al. (2011): Reduced functional integration and segregation of distributed neural systems underlying social and emotional information processing in autism spectrum disorders. Cereb Cortex 22:1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ibrahim K, Sukhodolsky DG (2018): RDoC and autism In: Volkmar FR, editor. Encyclopedia of Autism Spectrum Disorders. New York, NY: Springer. [Google Scholar]
- 68.Ewbank MP, Passamonti L, Hagan CC, Goodyer IM, Calder AJ, Fairchild G (2018): Psychopathic traits influence amygdala–anterior cingulate cortex connectivity during facial emotion processing. Soc Cogn Affect Neurosci 13:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lecavalier L, McCracken CE, Aman MG, McDougle CJ, McCracken JT, Tierney E, et al. (2019): An exploration of concomitant psychiatric disorders in children with autism spectrum disorder. Compr Psychiatry 88:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ball TM, Ramsawh HJ, Campbell-Sills L, Paulus MP, Stein MB (2012): Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorders. Psychol Med 43:1475–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ (2010): Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex 21:1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Makovac E, Meeten F, Watson DR, Herman A, Garfinkel SN, Critchley HD, et al. (2016): Alterations in amygdala-prefrontal functional connectivity account for excessive worry and autonomic dysregulation in generalized anxiety disorder. Biol Psychiatry 80:786–795. [DOI] [PubMed] [Google Scholar]
- 73.Yang YD, Sukhodolsky DG, Lei J, Dayan E, Pelphrey KA, Ventola P (2017): Distinct neural bases of disruptive behavior and autism symptom severity in boys with autism spectrum disorder. J Neurodev Disord 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zurcher NR, Rogier O, Boshyan J, Hippolyte L, Russo B, Gillberg N, et al. (2013): Perception of social cues of danger in autism spectrum disorders. PLoS One 8:e81206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aoki Y, Cortese S, Tansella M (2015): Neural bases of atypical emotional face processing in autism: A meta-analysis of fMRI studies. World J Biol Psychiatry 16:291–300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.