Abstract
Introduction: Literature on the association between cannabis use and body mass index (BMI) among adults suggests that greater cannabis use is associated with a lower BMI. However, results are mixed among adolescents, with both cross-sectional and longitudinal studies finding positive, negative, and nonsignificant associations between cannabis use and BMI. This longitudinal study aims to shed light on these associations by prospectively examining the associations between cannabis use and BMI across a 2-year window in a large sample of adolescent cannabis users.
Methods: Participants were 401 adolescents ages 14–17 at baseline who were at risk for escalation in their use of cannabis. We conducted a parallel process latent growth curve model to examine associations between the cannabis use intercept, BMI intercept, cannabis use slope, and BMI slope.
Results: Results showed that baseline BMI predicted a positive and significant association with cannabis use slope. In addition, there was a significant and negative correlation between the cannabis use slope and the BMI slope. These significant associations remained after controlling for relevant covariates.
Conclusions: Results are consistent with the adult literature that reports a negative association between cannabis use and BMI. Future research should focus on uncovering the mechanisms that may drive the association between cannabis use and BMI.
Keywords: adolescence, body mass index, cannabis
Introduction
The high prevalence of cannabis use among adolescents, particularly among older adolescents, highlights the importance of understanding outcomes during this sensitive developmental period. In 2018, ∼14%, 33%, and 44% of 8th, 10th, and 12th grade students, respectively, reported using cannabis at least once in their lifetime. Furthermore, ∼6–22% reported using cannabis at least once in the past month.1 Although a little <1/4 of high school seniors report using cannabis in the past month, not much is known about the impact of cannabis use on adolescents' physical health, including body mass index (BMI). This is particularly important considering appetite stimulation and increased caloric intake are reported as acute effects of cannabis.2 Thus, it is possible that cannabis use may impact BMI among adolescents.
Perhaps counterintuitively, there is overwhelming evidence of an association between cannabis use and lower BMI across both cross-sectional and longitudinal studies and various samples of adults. Numerous studies have found significant differences between current cannabis users compared to nonusers on BMI, with results suggesting that cannabis users have a lower BMI.3–5 Cannabis users also report a higher caloric intake but still have a lower BMI compared to those who do not use cannabis.6 Longitudinal studies also indicate that greater cannabis use over time is associated with a lower BMI.7,8 In addition to BMI, there are studies that suggest cannabis users have smaller waist circumference,9 lower prevalence of diabetes,10 more unhealthy weight control behaviors,11 a greater likelihood of reaching recommended levels of exercise,12 and greater reported enjoyment from exercise.13
The mechanism through which cannabis use impacts BMI is unclear and may be the result of several different influential factors. There is increasing evidence that the endocannabinoid system, a signaling system consisting of CB1 receptors and endogenous ligands, is involved with food intake. The primary psychoactive compound in cannabis is Δ9-tetrahydrocannabinol, a partial agonist of the CB1 receptor, has long been known for its effects on appetite stimulation known as the “munchies” during acute intoxication. As a result of this known effect, Rimonabant, a selective antagonist/inverse agonist of the CB1 receptor, was developed to decrease appetite among obese individuals. Although patients taking Rimonabant for weight loss had, on average, a 10% reduction in weight, Rimonabant was denied approval by the Food and Drug Administration because of psychiatric side effects.14
Although numerous studies have been conducted to examine whether cannabis use impacts BMI among adults, few studies have attempted to replicate this finding among adolescent cannabis users. Moreover, results from the limited body of available adolescent studies are equivocal, with some reporting that cannabis use is associated with higher BMI,7,15,16 others reporting normal BMI among cannabis users,17 and others finding no significant association.18 Furthermore, all the adolescent studies have been cross-sectional, with the exception of Huang et al.7 Huang et al.7 examined adolescent substance use and young adult clinical weight status, therefore the concurrent longitudinal association between cannabis use and BMI was not examined. Research that investigates the association between cannabis use and BMI among adolescents is critically needed, considering differences between adolescents and adults basal metabolism and rates of obesity. Basal metabolism decreases with age resulting in increased weight,19 and obesity rates among adolescents are lower than adults with 37% of adults categorized as obese compared to 17% of youths.20 Because adults have slower basal metabolism and greater rates of obesity, cannabis may differentially impact adolescent's BMI.
This study aims to shed light on the longitudinal associations between cannabis use and BMI in a relatively large sample of adolescent cannabis users. This is the first study, that we are aware of, that examines the longitudinal and concurrent associations between cannabis and BMI among a sample of adolescents. Adolescents in this study were a part of another study examining cognitive functioning, mental health, and substance use longitudinally. Adolescents, 14–17 years of age at baseline, were assessed five times over 2 years at 6-month intervals. More specifically, this study examines (1) the associations between cannabis use and BMI at baseline, (2) whether baseline cannabis use predicts changes in BMI over time, (3) whether baseline BMI predicts changes in cannabis use over time, and (4) whether changes in cannabis use are associated with changes in BMI, while controlling for relevant demographic, mental health, and substance use confounds. We hypothesize that, based on evidence from our previous study,16 cannabis use will be positively associated with BMI at baseline, and that although greater baseline cannabis use will predict increases in BMI, baseline BMI will not predict changes in cannabis use. We also predict that increases in cannabis use will be associated with increases in BMI over time.
Methods
Participants
Participants were 401 adolescents recruited from Miami-Dade County middle and high schools, and through flyers posted throughout the community and word-of-mouth referrals. The sample consisted of participants from a longitudinal study examining associations between decision-making, episodic memory, and cannabis use trajectories (R01 DA031176, PI: Gonzalez). Eligibility for the parent study was ascertained via a phone screen. Participants were between the ages of 14–17 years at baseline, able to read and write English, and reported some use, even if minimal, of alcohol, cigarettes, or other drugs (although ∼10% of the sample was allowed to have no history of substance use). Exclusion criteria included self-reported developmental disorders, birth complications, neurological disorders, or a history of diagnosed significant mood or thought disorders (excluding attention deficit/hyperactivity disorder). Participants who reported frequent or recent use of drugs other than alcohol, nicotine, or cannabis, or whose answers at the time of screening suggested the presence of an alcohol or cannabis use disorder were also excluded. Participant characteristics are given in Table 1.
Table 1.
Participant Characteristics
| Visit | T1 |
T2 |
T3 |
T4 |
T5 |
|---|---|---|---|---|---|
| n=401 | n=391 | n=383 | n=381 | n=387 | |
| Age | 15.4 (0.7) | 16.0 (0.8) | 16.4 (0.7) | 16.9 (.8) | 17.4 (0.8) |
| Years of education | 9.1 (0.8) | 9.7 (0.9) | 10.1 (0.9) | 10.7 (0.9) | 11.1 (0.8) |
| Years of education (mother) | 14.2 (2.5) | — | — | — | — |
| WRAT-4 reading standard score | 108.3 (14.7) | — | — | — | — |
| Race/ethnicity (%) | |||||
| Hispanic/Latino | 89.8 | — | — | — | — |
| Caucasian | 76.8 | — | — | — | — |
| African-American/Black | 7.7 | — | — | — | — |
| More than one race | 12.0 | — | — | — | — |
| Other/unknown | 3.4 | — | — | — | — |
| Male (%) | 54.1 | — | — | — | — |
| BMI raw score | 23.6 (4.7) | — | 24.5 (4.9) | — | 24.8 (5.5) |
| Ever used cannabis (%) | 78.6 | 79.5 | 80.4 | 80.7 | 81.9 |
| Ever used alcohol (%) | 82.0 | 86.7 | 90.2 | 92.4 | 94.2 |
| Ever used nicotine (%) | 40.6 | 44.8 | 52.1 | 55.4 | 63.8 |
| Ever used other drugs (%) | 36.2 | 40.4 | 47.6 | 49.2 | 52.6 |
| Frequency of substance use (days in past 6 months) | |||||
| Cannabis use, median (IQR) | 6.0 [0.0, 30.0] | 8.0 [8.0, 49.0] | 10.0 [0.0, 73.0] | 7.0 [0.0, 84.5] | 14.0 [0.0, 105.0] |
| Alcohol use, median (IQR) | 1.0 [0.0, 5.0] | 2.0 [0.0, 8.0] | 3.0 [1.0, 9.0] | 3.0 [0.0, 11.0] | 4 [1.0, 15.0] |
| Nicotine use, median (IQR) | 0.0 [0.0, 1.0] | 0 [0.0, 0.0] | 0.0 [0.0, 1.0] | 0.0 [0.0, 0.0] | 0.0 [0.0, 2.0] |
| Current cannabis abuse diagnosis (%) | 11.0 | 8.7 | 15.4 | 14.2 | 21.0 |
| Current cannabis dependence diagnosis (%) | 2.2 | 2.8 | 4.1 | 3.4 | 2.3 |
| Current alcohol abuse diagnosis (%) | 1.2 | 2.6 | 1.8 | 1.0 | 2.8 |
| Current alcohol dependence diagnosis (%) | 0.0 | 0.5 | 0.8 | 0.3 | 0.3 |
| Current other drug abuse diagnosis (%) | 0.2 | 0.3 | 0.8 | 0.0 | 1.5 |
| Current other drug dependence diagnosis (%) | 0.0 | 0.0 | 0.8 | 0.5 | 0.3 |
| DASS—depression subscale z-score | −0.3 (0.9) | −0.4 (0.8) | −0.3 (1.0) | −0.4 (0.8) | −0.3 (0.9) |
Note: All values are given as mean and standard deviation unless noted.
BMI, body mass index; IQR, interquartile range; T1, baseline visit; T2, 6-month follow-up visit; T3, 1-year follow-up visit; T4, 18-month follow-up visit; T5, 2-year follow-up visit; WRAT, Wide Range Achievement Test.
Procedures
Participant assent and parental consent were obtained for all participants before the baseline assessment. If participants turned 18 years old during the course of the study, a new participant consent was obtained. Study procedures and protocols were approved by the Institutional Review Board at Florida International University. The parent study involved five assessment waves conducted at 6-month intervals over a 2-year period, which involved in-person assessments at baseline (T1), 1-year follow-up (T3), and 2-year follow-up (T5), and telephonic assessments at the 6-month follow-up (T2) and 18-month follow-up (T4). Substance use and mental health data were collected at all measurement waves (T1–T5), whereas BMI data were only collected during in-person assessments (T1, T3, and T5).
Measures
Demographic information
Gender, ethnicity/race, age, and parental education were collected at the baseline assessment through a questionnaire designed to obtain demographic information.
Substance use
The Drug Use History Questionnaire is a detailed semistructured interview used to assess frequency and amount of use of 16 different drug classes (alcohol, nicotine, cannabis, synthetic cannabinoids, cocaine, methamphetamine, other stimulants, heroin, other opiates, barbiturates, benzodiazepines, ecstasy, hallucinogens, other club drugs, phencyclidine, and inhalants) during a participant's lifetime and the past 6 months.21,22 Consistent with previous studies,23 past 6-month frequency of cannabis use (in days) was our primary measure of cannabis, alcohol, and nicotine use.
Body mass index
Adolescents' height and weight were measured at three separate times using a wall-mounted stadiometer and a scale, respectively. The median height and weight were used to calculate raw BMI during each in-person assessment (T1, T3, and T5). Raw BMI scores were then converted into sex- and age-adjusted BMI z-scores, which were used as our measure of BMI.24
Mental health
The Depression, Anxiety, and Stress Scale was used to assess symptoms of depression, anxiety, and stress during the past week. Items were rated in a 4-point scale, ranging from 0 (Did not apply to me at all) to 3 (Applied to me very much, or most of the time).25,26 The 7-item depression subscale z-score was used as our measure of depressive symptoms.
Statistical analyses
All statistical analyses were conducted using Mplus 8.27 We first identified theoretically relevant covariates that may influence associations between cannabis use and BMI. Approximately 90% of the participants identified as Hispanic/Latino, a population that tends to have a higher BMI compared to other ethnicities.28 Because BMI z-scores are already adjusted for age and sex, we controlled for the effects of ethnicity on BMI in our adjusted model. Furthermore, because of known associations between depression and BMI, we covaried for the effects of depressive symptoms on BMI at each annual assessment in our adjusted model.29 Finally, we covaried for past 6-month alcohol and nicotine use frequency, as use of these substances has been shown to impact BMI,30,31 and we were specifically interested in the effects of cannabis in this study. In addition, given known sex differences in the effects of cannabis use32 we also controlled for the effects of sex and ethnicity on cannabis use.
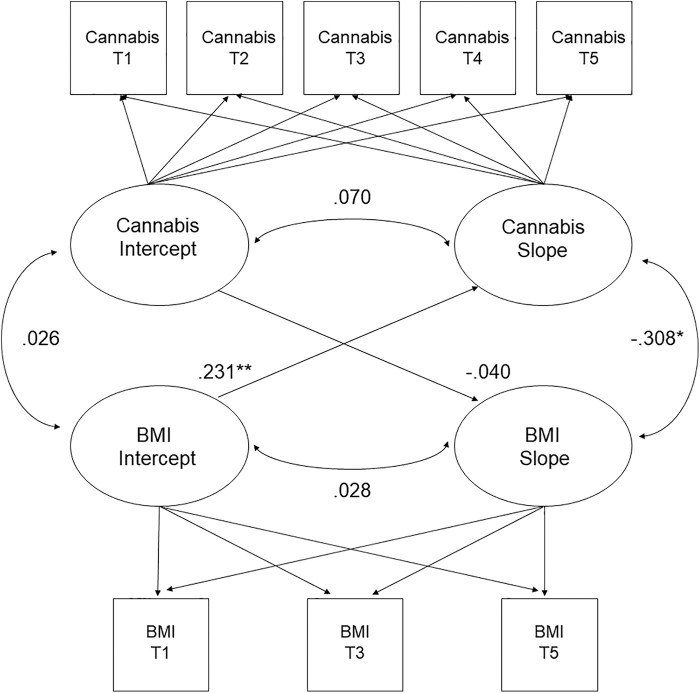
A latent growth curve modeling (LGCM) approach was used to characterize trajectories of cannabis use and BMI over time. Specifically, we generated separate unconditional linear growth curve models to delineate change in cannabis use across the five assessment waves and BMI across three assessment waves. We then ran a combined (i.e., parallel process) LGCM, which simultaneously estimated the growth curves of cannabis use and BMI within a single model. As illustrated in Figure 1, the following parameters were specified to address the study hypotheses: (1) the cannabis use intercept was correlated with the BMI intercept to examine associations at baseline; (2) the BMI slope was regressed on the cannabis use intercept to examine whether baseline cannabis use predicted changes in BMI; (3) the cannabis use slope was regressed on the BMI intercept to examine whether baseline BMI predicted changes in cannabis use; and (4) the cannabis use slope was correlated with the BMI slope to determine whether changes in cannabis use were associated with changes in BMI over time. Finally, we reran this model controlling for theoretically relevant confounds (sex, ethnicity, depressive symptoms, and concurrent use of alcohol and nicotine).
FIG. 1.
Estimates of the latent growth curve model. Note: BMI, body mass index; Cannabis, frequency of cannabis use; T1, baseline visit; T2, 6-month follow-up visit; T3, 1-year follow-up visit; T4, 18-month follow-up visit; T5, 2-year follow-up visit. *p<0.05, **p<0.01.
Models were estimated using maximum likelihood estimation with a chi-squared statistic and standard errors that are robust to non-normality. Model fit was evaluated using common indices of absolute model fit, including the comparative fit index (CFI) and root mean square error of approximation (RMSEA), as well as indices of relative fit, such as the sample-size-adjusted Bayesian information criterion (SABIC) and the Akaike information criterion (AIC). For the CFI, cutoff values of 0.90 or greater were used to indicate acceptable fit, and 0.95 or greater to indicate excellent fit.33,34 For the RMSEA, values between 0.05 and 0.10 were considered to represent acceptable fit, whereas values <0.05 were considered to indicate excellent fit.34 When comparing models with and without covariates, smaller values indicate better fit for the SABIC and AIC fit indices.
Cannabis use data were available for 100% of participants at T1 (N=401), 97% of participants at T2 (n=391), 96% of participants at T3 (n=383), 95% of participants at T4 (n=380), and 97% of participants at T5 (n=387). Because BMI data collection began after parent study onset, 106 participants did not have BMI data at T1. These 106 participants with missing BMI data at T1 did not differ from participants with complete data on age, gender, ethnicity, race, and BMI at T3 and T5, or alcohol, nicotine, and cannabis frequency at T1. BMI data were available for 74% of participants at T1 (n=295), 95% of participants at T3 (n=382), and 95% of participants at T5 (n=382). One participant was missing BMI data at T1, T3, and T5 (0.3%) and 3% of participants were missing BMI data for two time points. To handle missing data, we used full-information maximum likelihood estimates, as this procedure uses all available data points to construct parameter estimates without imputing individual values.35 All included participants had at least one data point and were thus used to inform the maximum likelihood estimates.
Results
Patterns of change in cannabis use and BMI over time
The unconditional linear growth models of cannabis use and BMI demonstrated excellent to acceptable fit (Table 2). On average, there was a significant moderately sized increase in cannabis use frequency over time (β=0.56, p<0.001). Of importance, however, there was significant individual variability in participants' rates of change in their use of cannabis over time. On average, BMI z-scores showed a small decrease (β=−0.22, p=0.041) over time, suggesting that participants' BMI became lower relative to the population mean over time. The variability of the slope was not significant, suggesting that participants showed similar decreases in BMI over time.
Table 2.
Fit Indices and Estimates for Unconditional Linear Growth Models of Cannabis Use and Motivation Indices
| χ2 | df | CFI | RMSEA | AIC | SABIC | Intercept ݲ | Slope ݲ | Intercept σ2 | Slope σ2 | Cov (I/S) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabis use | 21.87 | 10 | 0.98 | 0.05 | 19,685.88 | 19,694.09 | 0.70** | 0.56** | 1502.36** | 122.91** | 0.08 |
| BMI | 0.02 | 1 | 1.00 | 0.00 | 1508.25 | 1514.49 | 0.90** | −0.22* | 0.69** | 0.07 | 0.01 |
Variance (σ2) estimates are unstandardized. All other estimates are presented in standardized metric.
p<0.001, *p<0.05.
AIC, Akaike information criterion; CFI, comparative fit index; Cov (I/S), covariance between intercept and slope; CU, cannabis use; df, degrees of freedom; RMSEA, root mean square error of approximation; SABIC, sample-size-adjusted Bayesian information criterion.
Associations between cannabis use and BMI over time
Results from the unadjusted model are given in Figure 1. The correlation between the intercepts was not significant, indicating that cannabis use and BMI were not significantly associated at baseline (r=0.029, p=0.622). The cannabis use intercept did not predict the BMI slope (r=0.076, p=0.425), suggesting that, contrary to our hypotheses, baseline cannabis use failed to predict changes in BMI over time. Furthermore, there was a small, significant effect of the BMI intercept on the cannabis use slope (r=0.225, p=0.001), which indicated that a higher BMI at baseline predicted greater increases in cannabis use over time. The association between the cannabis and BMI slopes was negative and significant (β=−0.257, p=0.004), suggesting that increases in cannabis use were accompanied by greater decreases in BMI over time. These findings remained unchanged after adjusting for sex, ethnicity, depression, and use of alcohol and nicotine. Results are displayed in Table 3.
Table 3.
Estimates for Cannabis Use and Body Mass Index Growth Curve Models
| Path | Standardized estimate (standard error) | Unstandardized estimate (standard error) | p | Model fit |
|||
|---|---|---|---|---|---|---|---|
| CFI | RMSEA | AIC | SABIC | ||||
| Unadjusted model | |||||||
Cannabis use slope  BMI slope BMI slope |
−0.256 (0.090) | −0.004 (0.001) | 0.004 | 0.98 | 0.047 | 21,181.350 | 21,197.768 |
Cannabis use slope 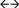 cannabis use intercept cannabis use intercept |
0.076 (0.095) | 32.498 (38.375) | 0.425 | ||||
BMI intercept 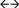 cannabis use intercept cannabis use intercept |
0.029 (0.059) | 0.936 (1.907) | 0.622 | ||||
BMI intercept 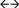 cannabis use slope cannabis use slope |
0.224 (0.065) | 2.073 (0.638) | 0.001 | ||||
| Demographic-adjusted model | |||||||
Cannabis use slope  BMI slope BMI slope |
−0.257 (0.090) | −0.004 (0.001) | 0.004 | 0.98 | 0.052 | 20,350.026 | 20,368.19 |
Cannabis use slope 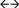 cannabis use intercept cannabis use intercept |
0.076 (0.095) | 32.714 (38.412) | 0.394 | ||||
BMI intercept 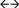 cannabis use intercept cannabis use intercept |
0.028 (0.059) | 0.911 (1.908) | 0.632 | ||||
BMI intercept 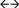 cannabis use slope cannabis use slope |
0.225 (0.065) | 2.077 (0.638) | 0.001 | ||||
Ethnicity  BMI slope BMI slope |
0.048 (0.090) | 0.027 (0.050) | 0.596 | ||||
| Depression-adjusted model | |||||||
Cannabis use slope  BMI slope BMI slope |
−0.251 (0.094) | −0.004 (0.002) | 0.007 | 0.97 | 0.049 | 20,350.026 | 20,368.19 |
Cannabis use slope 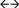 cannabis use intercept cannabis use intercept |
0.108 (0.101) | 45.739 (38.990) | 0.281 | ||||
BMI intercept 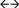 cannabis use intercept cannabis use intercept |
0.027 (0.062) | 0.862 (1.961) | 0.653 | ||||
BMI intercept 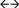 cannabis use slope cannabis use slope |
0.209 (0.066) | 1.874 (0.625) | 0.002 | ||||
Ethnicity  BMI slope BMI slope |
0.047 (0.092) | 0.027 (0.051) | 0.608 | ||||
Depression T1  BMI T1 BMI T1 |
−0.005 (0.023) | −0.005 (0.023) | 0.840 | ||||
Depression T3  BMI T3 BMI T3 |
0.002 (0.024) | 0.002 (0.022) | 0.946 | ||||
Depression T5  BMI T5 BMI T5 |
−0.006 (0.028) | −0.006 (0.029) | 0.839 | ||||
| Alcohol and tobacco-adjusted model | |||||||
Cannabis use slope  BMI slope BMI slope |
−0.212 (0.093) | −0.003 (0.001) | 0.023 | 0.95 | 0.048 | 20,358.170 | 20,380.876 |
Cannabis use slope 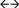 cannabis use intercept cannabis use intercept |
0.106 (0.101) | 44.807 (39.274) | 0.294 | ||||
BMI intercept 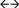 cannabis use intercept cannabis use intercept |
0.026 (0.062) | 0.817 (1.974) | 0.678 | ||||
BMI intercept  cannabis use slope cannabis use slope |
0.206 (0.066) | 1.847 (0.622) | 0.002 | ||||
Ethnicity  BMI slope BMI slope |
0.057 (0.095) | 0.032 (0.51) | 0.545 | ||||
Depression T1  BMI T1 BMI T1 |
−0.004 (0.023) | −0.004 (0.022) | 0.848 | ||||
Depression T3  BMI T3 BMI T3 |
0.002 (0.025) | 0.002 (0.023) | 0.921 | ||||
Depression T5  BMI T5 BMI T5 |
0.001 (0.026) | 0.001 (0.027) | 0.964 | ||||
Alcohol use T1  BMI T1 BMI T1 |
−0.008 (0.023) | −0.001 (0.002) | 0.727 | ||||
Alcohol use T3  BMI T3 BMI T3 |
−0.018 (0.023) | −0.001 (0.002) | 0.433 | ||||
Alcohol use T5  BMI T5 BMI T5 |
−0.012 (0.021) | −0.001 (0.001) | 0.567 | ||||
Tobacco use T1  BMI T1 BMI T1 |
0.020 (0.024) | 0.001 (0.001) | 0.409 | ||||
Tobacco use T3  BMI T3 BMI T3 |
0.006 (0.020) | 0.000 (0.001) | 0.784 | ||||
Tobacco use T5  BMI T5 BMI T5 |
−0.039 (0.025) | −0.001 (0.001) | 0.112 | ||||
Bolded paths indicate significance at p<0.05. Unidirectional arrows represent regression paths and bidirectional arrows represent correlations.
Discussion
This study examined bidirectional influences between cannabis frequency and BMI z-score over 2 years while controlling for sex, ethnicity, depressive symptoms, and alcohol and nicotine frequency among adolescents. We found that higher baseline BMI z-score (i.e., relative to the general adolescent population) is associated with escalation in cannabis frequency, whereas baseline cannabis frequency did not predict changes in BMI z-score. Next, we found that increases in cannabis frequency were associated with a small, significant decrease in BMI z-scores over 2 years. Together, our results present the provocative conclusion that higher BMI z-scores may be associated with increases in cannabis use (the mechanisms on which we speculate below). Yet, consistent with much of the literature, cannabis use seems to be associated with decreasing BMI z-score. These complex associations may help to shed light on existing disparate findings.
First, we found that BMI z-score at the baseline visit was associated with increases in cannabis use. This association may be accounted for by other variables. For instance, obese/overweight adolescents have poorer impulse control compared to normal weight adolescents.36 In addition, poor impulse control is related to greater use of substances like cannabis.37 Thus, this association may be accounted for by other variables not assessed in this study.
Our results suggest that increases in cannabis frequency are associated with decreases in BMI z-score. Throughout development, a normal or healthy BMI raw score for an adolescent increases with age. For example, the BMI raw score that corresponds to a BMI z-score of 0 for adolescent males and females increase by 0.68 and 0.45, respectively, from ages 16 to 17 years. The mean BMI raw score in our sample increased each year, 23.6 at baseline to 24.8 at the 2-year follow-up. Although our sample had an increasing BMI raw score, the BMI z-score suggests that our sample had decreases in BMI relative to the general adolescent population. The correlation between increases in cannabis frequency and decreases in BMI may be the result of several potential mechanisms. A possible mechanism in teens may be the result of repeated cannabis abstinence in between cannabis use sessions. Cannabis withdrawal syndrome (CWS) symptoms include decreased appetite, stomach pains, and nausea. On average, abstinent cannabis users report six symptoms of CWS after 1 day of abstinence.38 It is possible that after sleeping for 6–8 h, chronic cannabis users may experience prodromal symptoms or subthreshold symptoms of CWS. That said, the actual mechanism through which chronic cannabis exposure may result in weight loss remains unknown. It is also important to note that we cannot ascertain if decreasing BMI among teens in this study, and the attributable underlying mechanisms, is beneficial or harmful to participants' health.
This study has several notable strengths, including a relatively large sample of diverse adolescents who primarily use cannabis, and a prospective longitudinal design. This study also has several limitations to consider. First, cannabis use and BMI may be related to another variable not captured in this study, which contributes to the association. For instance, levels of physical activity, diet, and caloric intake are all associated with BMI. Thus, future studies should examine potential mediators of the association between cannabis use and BMI. In addition, cannabis use and BMI may share genetic influences, which may be driving these significant associations. A twin study would be able to determine the variance attributed to genetic and environmental influences and to examine causality more directly. Further research is also warranted to understand the mechanism behind the association between cannabis and BMI. For example, decreases in BMI after cannabis exposure could be the result of nausea from chronic cannabis administration, greater physical activity, effects of cannabis on the endocannabinoid system, or a combination of these reasons.
The results of this study suggest that cannabis use and BMI are associated over time and may have bidirectional influences on one another. These results may help clinicians identify adolescents who are at a greater risk for escalation in cannabis use; adolescents with a higher BMI may benefit from greater prevention efforts to decrease or delay cannabis use. Furthermore, weight could be targeted concurrently with treatment for substance use disorders among adolescents. Research has also demonstrated that adolescents in inpatient substance use treatment centers tend to gain weight during prolonged periods of abstinence.39 Of interest, focus groups, consisting of adult women who are currently in treatment for substance use disorders, found that most patients were interested in learning more about healthy eating and exercise, while being treated for a substance use disorder and remaining abstinent. During the same focus groups, staff at these treatment facilities reported that there are currently no programs that focus on educating residents about healthy diets and exercise.40 Perhaps substance use treatments would benefit from including components on healthy eating and exercise.
Abbreviations Used
- AIC
Akaike information criterion
- BMI
body mass index
- CFI
comparative fit index
- Cov (I/S)
covariance between intercept and slope
- CU
cannabis use
- CWS
cannabis withdrawal syndrome
- df
degrees of freedom
- IQR
interquartile range
- LGCM
latent growth curve modeling
- RMSEA
root mean square error of approximation
- SABIC
sample-size-adjusted Bayesian information criterion
- T1
baseline visit
- T2
6-month follow-up visit
- T3
1-year follow-up visit
- T4
18-month follow-up visit
- T5
2-year follow-up visit
- WRAT
Wide Range Achievement Test
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
The authors have no other conflicts of interest to declare.
Funding Information
This study was supported by the National Institute on Drug Abuse [R01 DA031176 (PI: R.G.), R01 DA033156 (PI: R.G.), F31 DA047750 (PI: I.P.-C.), and T32 DA017637 (PI: Hewitt)]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Cite this article as: Ross JM, Pacheco-Colón I, Hawes SW, Gonzalez R (2020) Bidirectional longitudinal associations between cannabis use and body mass index among adolescents, Cannabis and Cannabinoid Research 5:1, 81–88, DOI: 10.1089/can.2019.0091.
References
- 1. Johnston LD, Miech RA, O'Malley PM, et al. Monitoring the future national survey results on drug use, 1975–2017: overview, key findings on adolescent drug use. Institute for Social Research, University of Michigan: Ann Arbor, MI, 2018 [Google Scholar]
- 2. Foltin RW, Fischman MW, Byrne MF. Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite. 1988;11:1–14 [DOI] [PubMed] [Google Scholar]
- 3. Gerberich SG, Sidney S, Braun BL, et al. Marijuana use and injury events resulting in hospitalization. Ann Epidemiol. 2003;13:230–237 [DOI] [PubMed] [Google Scholar]
- 4. Le Strat Y, Le Foll B. Obesity and cannabis use: results from 2 representative national surveys. Am J Epidemiol. 2011;174:929–933 [DOI] [PubMed] [Google Scholar]
- 5. Ngueta G, Bélanger RE, Laouan-Sidi EA, et al. Cannabis use in relation to obesity and insulin resistance in the inuit population. Obesity (Silver Spring). 2015;23:290–295 [DOI] [PubMed] [Google Scholar]
- 6. Rodondi N, Pletcher MJ, Liu K, et al. Marijuana use, diet, body mass index, and cardiovascular risk factors (from the CARDIA study). Am J Cardiol. 2006;98:478–484 [DOI] [PubMed] [Google Scholar]
- 7. Huang DY, Lanza HI, Anglin MD. Association between adolescent substance use and obesity in young adulthood: a group-based dual trajectory analysis. Addict Behav. 2013;38:2653–2660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jin LZ, Rangan A, Mehlsen J, et al. Association between use of cannabis in adolescence and weight change into midlife. PLoS One. 2017;12:e0168897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Penner EA, Buettner H, Mittleman MA. The impact of marijuana use on glucose, insulin, and insulin resistance among US adults. Am J Med. 2013;126:583–589 [DOI] [PubMed] [Google Scholar]
- 10. Alshaarawy O, Anthony JC. Cannabis smoking and diabetes mellitus: results from meta-analysis with eight independent replication samples. Epidemiology. 2015;26:597–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Korn L, Haynie DL, Luk JW, et al. Prospective associations between cannabis use and negative and positive health and social measures among emerging adults. In J Drug Policy. 2018;58:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rajavashisth TB, Shaheen M, Norris KC, et al. Decreased prevalence of diabetes in marijuana users: cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open. 2012;2:e000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. YorkWilliams SL, Gust CJ, Mueller R, et al. The new runner's high? Examining relationships between cannabis use and exercise behavior in states with legalized cannabis. Front Public Health. 2019;7:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Christensen R, Kristensen PK, Bartels EM, et al. Efficacy and safety of the weight-loss drug Rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713 [DOI] [PubMed] [Google Scholar]
- 15. Farhat T, Iannotti RJ, Simons-Morton BG. Overweight, obesity, youth, and health-risk behaviors. Am J Pre Med. 2010;38:258–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ross JM, Graziano P, Pacheco-Colón I, et al. Decision-making does not moderate the association between cannabis use and body mass index among adolescent cannabis users. J Int Neuropsychol Soc. 2016;20:944–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Denoth F, Sicilliano V, Iozzo P, et al. The association between overweight and illegal drug consumption in adolescents: is there an underlying influence of the sociocultural environment? PLoS One. 2011;6:e27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blackstone SR, Herrmann LK. Relationships between illicit drug use and body mass index among adolescents. Health Educ Behav. 2016;43:21–24 [DOI] [PubMed] [Google Scholar]
- 19. Keys A, Taylor HL, Grande R. Basal metabolism and age of adult man. Metabolism. 1973;22:579–587 [DOI] [PubMed] [Google Scholar]
- 20. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015–2016. National Center for Health Statistics: Hyattsville, MD, 2017 [Google Scholar]
- 21. Gonzalez R, Schuster RM, Mermelstein RJ, et al. Performance of young adult cannabis users on neurocognitive measures of impulsive behavior and their relationship to symptoms of cannabis use disorders. J Clin Exp Neuropsychol. 2012;34:962–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rippeth JD, Heaton RK, Carey CL, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10:1–14 [DOI] [PubMed] [Google Scholar]
- 23. Hawes SW, Trucco EM, Duperrouzel JC, et al. Developmental pathways of adolescent cannabis use: risk factors, outcomes and sex-specific differences. Subst Use Misuse. 2019;54:271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. National Center for Health Statistics. Vital Health Stat Ser. 11 2002;246:1–190 [PubMed] [Google Scholar]
- 25. Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br J Clin Psychol. 2005;44:227–239 [DOI] [PubMed] [Google Scholar]
- 26. Lovibond SH, Lovibond PF. Manual for the depression anxiety stress scales. Psychology Foundation of Australia: Sydney, Australia, 1996 [Google Scholar]
- 27. Muthén LK, Muthén B. Mplus user's guide: statistical analysis with latent variables. Muthén & Muthén: Los Angeles, California, 2017 [Google Scholar]
- 28. Clarke P, O'Malley PM, Johnston LD, et al. Social disparities in BMI trajectories across adulthood by gender, race/ethnicity and lifetime socio-economic position: 1986–2004. Int J Epidemiol. 2008;38:499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lev-Ran S, Roerecke M, Le Foll B, et al. The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol Med. 2014;44:797–810 [DOI] [PubMed] [Google Scholar]
- 30. Akbartabartoori M, Lean M, Hankey C. Relationships between cigarette smoking, body size and body shape. Int J Obes. 2005;29:236–243 [DOI] [PubMed] [Google Scholar]
- 31. French MT, Norton EC, Fang H, et al. Alcohol consumption and body weight. Health Econ. 2010;19:814–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crane NA, Schuster RM, Fusar-Poli P, et al. Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychol Rev. 2013;23:117–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model. 1999;6:1–55 [Google Scholar]
- 34. McDonald RP, Ho MHR. Principles and practice in reporting structural equation analyses. Psychol Methods. 2002;7:64–82 [DOI] [PubMed] [Google Scholar]
- 35. Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct Equ Model. 2001;8:430–457 [Google Scholar]
- 36. Nederkoorn C, Braet C, Van Eijs Y, et al. Why obese children cannot resist food: the role of impulsivity. Eat Behav. 2006;7:315–322 [DOI] [PubMed] [Google Scholar]
- 37. Nigg JT, Wong MM, Martel MM, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45:468–475 [DOI] [PubMed] [Google Scholar]
- 38. Bonnet U, Specka M, Stratmann U, et al. Abstinence phenomena of chronic cannabis-addicts prospectively monitored during controlled inpatient detoxification: cannabis withdrawal syndrome and its correlation with delta-9-tetrahydrocannabinol and-metabolites in serum. Drug Alcohol Depend. 2014;143:189–197 [DOI] [PubMed] [Google Scholar]
- 39. Hodgkins CC, Cahill KS, Seraphine AE, et al. Adolescent drug addiction treatment and weight gain. J Addict Dis. 2004;23:55–65 [DOI] [PubMed] [Google Scholar]
- 40. Emerson MH, Glovsky E, Amaro H, et al. Unhealthy weight gain during treatment for alcohol and drug use in four residential programs for Latina and African American women. Subst Use Misuse. 2009;44:1553–1565 [DOI] [PubMed] [Google Scholar]