Abstract
Background: Growing evidence suggests that cannabis and alcohol (and especially binge alcohol drinking) use independently alters neural structure and functioning, particularly during sensitive developmental time periods (e.g., emerging adulthood). However, few studies have investigated the effects of same-day use of these two substances. Here, white matter (WM) integrity was investigated in relation to binge alcohol drinking, cannabis, and same-day binge and cannabis co-use in adolescents and emerging adults.
Methods: FreeSurfer's TRACULA was used to assess WM in emerging adults (n=75; 16–26 years old). Timeline Followback calculated past month cannabis use, binge episodes, and same-day cannabis and binge drinking co-use. Multiple regressions investigated WM by past month cannabis, binge, and co-use.
Results: Results revealed co-use episodes were related to lower fractional anisotropy (FA), an overall measure of neuronal integrity, in three tracts (left inferior longitudinal fasciculus [ILF], p=0.02; right anterior thalamic radiation [ATR], p=0.01; and left cingulum cingulate gyrus [CCG], p=0.01); and lower axial diffusivity in left ILF (p=0.03). Cannabis use was significantly related to greater FA in left ILF (p=0.005), left ATR (p=0.02), right ATR (p=0.05), left CCG (p=0.006), right CCG (p=0.03), and right superior longitudinal fasciculus parietal (p=0.03). Binge episodes related to greater FA in right ATR (p=0.03).
Conclusions: These findings suggest that co-use was associated with lower WM integrity across frontolimbic tracts. In addition, greater FA was related to greater cannabis use across several tracts and binge alcohol use in one tract. Co-users also appeared to be more severe substance users. Future research should investigate the potential independent and interactive effects of these substances on pre-clinical and clinical levels.
Keywords: alcohol, cannabis, co-occurring substance use, emerging adults, white matter
Introduction
Adolescents and emerging adults undergo ongoing neurodevelopment, including structural (e.g., white matter [WM]) and functional changes,1–3 placing them at increased vulnerability to neurotoxins during this period (see ref.4). Around 6% of 12th graders smoke cannabis daily and 25% have engaged in heavy episodic drinking (≥5 standard drinks on one occasion) in the past 2 weeks.5 Furthermore, cannabis use is positively correlated with alcohol use6 and 23% of high school seniors and 15% of young adults (age 18–29) report simultaneously using cannabis and alcohol in the past year.7,8
The main psychoactive component of cannabis, delta-9-tetrahydrocannabinol (THC), binds to the cannabinoid receptor 1 (CB1) in the cortical, limbic, and striatal regions.9 The endocannabinoid (eCB) system also undergoes neuromaturation during adolescence and emerging adulthood, making it more vulnerable to exogenous cannabinoids and their deleterious effects on the eCB system, morphological changes, and overall functioning (see ref.10).
Much remains to be discovered regarding co-occurring alcohol and cannabis use. Preliminary studies suggest a potential negative additive effect of combined cannabis and alcohol use. CB1 activity is downregulated by chronic cannabis11 and alcohol use.12 Disrupted CB1 activity may, in turn, affect WM development, as healthy oligodendrocyte development requires that CB1 receptors protect progenitors from apoptosis.13 Combined, alcohol and cannabis may result in cross-tolerance (see ref.14), THC levels may increase while conflicting studies have shown reduced blood-alcohol content (BAC)15 and increased BAC,16 and studies of co-users (but not co-use patterns) reveal conflicting WM results.17–20 Given the potential for underlying mechanistic changes that may relate to anatomical and functional changes, additional research into the effects of co-occurring cannabis and alcohol use is needed.
The present study examined structural connectivity in cannabis and alcohol using male and female adolescents and emerging adults utilizing diffusion tensor imaging (DTI) tractography to assess WM integrity in conjunction with a wide range of substance use patterns. The potential independent dose-dependent effects of binge drinking, cannabis, and cannabis + binge co-use on WM integrity were assessed.
It was hypothesized that greater past month cannabis use and greater past month binge episodes would independently predict decreased fractional anisotropy (FA), a putative marker of neuronal integrity, and increased mean diffusivity (MD), a measure of rate and free diffusion, in frontoparietal tracts and frontolimbic tracts.17–19,21–24 In addition, we conducted exploratory analyses of axial diffusivity (AD), a measure of axon abnormalities, and radial diffusivity (RD), a sign of demyelination, as these have been limitedly studied in cannabis users. Further, given the attenuation of THC metabolism25–27 and the proposed potentiation of alcohol on THC neurotoxicity,28 it was predicted that co-occurring cannabis and binge use would have an additive effect, resulting in greater decrements in WM integrity in frontoparietal and frontolimbic tracts than due to binge drinking or cannabis use alone.
Materials and Methods
Overview and participants
Data from 75 individuals with varying recent cannabis and alcohol use, ages 16–26, were used for the present study. The Institutional Review Boards at the University of Wisconsin-Milwaukee (UWM) and the Medical College of Wisconsin (MCW) approved all aspects of this study, research was conducted in accordance with the Declaration of Helsinki, and all participants provided written informed consent. Participants were recruited through flyers in the community and newspaper ads. Full participant eligibility assessment and study description have been published elsewhere29; the present analyses included all participants who had completed a substance use interview (including those who had not used any substances) and DTI data.
Inclusion/exclusion criteria
Exclusion criteria for all participants
Current use of psychotropic medication, lifetime history of serious neurologic injuries or disorders (including loss of consciousness >2 min), major medical illness, diagnosis of an independent Axis I psychiatric disorder in the past year (except for substance abuse or dependence) as measured by the Mini International Psychiatric Interview,30 pregnancy, left-handedness, or magnetic resonance imaging (MRI) contraindications (e.g., metal anywhere in or on the body).
Selection of covariates
Although the study focused on utilizing dose-dependent binge alcohol and cannabis effects, participants were classified for selection of covariates (those who had a co-use binge + cannabis episode in the past month, also known as co-users or “CO”; and those who did not have a co-use episode, or “NO”). CO and NO were assessed for sociodemographic and other factors that may differentiate individual substance use patterns; no significant differences were revealed. All regressions were then run with continuous substance use variables.
Procedure
Eligible participants completed a parent study protocol (PI: K.M.L., R01 DA03035429); 75 participants who had valid MRI scan and data were included in the present analyses. Participants were asked to remain abstinent for 3 weeks leading up to the MRI session; this was confirmed through weekly urine toxicology screens and sweat patch analysis to monitor reducing levels of drug metabolites. Female participants were also given a pregnancy test. Positive results on any test deemed the participant ineligible. Participants were given questionnaires to assess mood and psychological variables, and then completed the neuroimaging and neurocognitive testing protocols. All participants were compensated for their time. Neuroimaging and substance use data are presented here.
Recent drug use
Drug use history was collected using the Timeline Followback.31 Using a calendar to cue special dates and holidays, participants were asked to recount when they used cannabis (grams), alcohol (standard drinks), or engaged in binge drinking (≥4 standard drinks for females, ≥5 standard drinks for males, in one time period), or co-occurring binge and cannabis use episodes (days of co-use).
MRI and DTI data acquisition
Participants were scanned on a 3 T GE scanner at MCW.
Structural image acquisition
A T1-weighted, three-dimensional (3D) anatomical brain scan was obtained with a modified driven equilibrium Fourier transform sequence (TMD=1.1 sec, repetition time [TR]=13 msec, field of view [FOV]=25.6×19.2 ×19.2 cm, matrix 256×192×96 pixels, flip angle=20°).
DTI acquisition
DTI was obtained using 48 diffusion directions with b≈700 sec/mm2 (FOV=25.6 cm, 128×128 matrix, resolution=4×4×4 mm, TR=9300 msec, echo time [TE]=81.4 msec (minimum), flip angle 90°).
MRI processing
Structural images were initially preprocessed using the Analysis of Functional Neuroimages software package. This sequence included converting 2D slice data into a 3D data set (BRIK and HEAD files), and then converting into a file readable by FreeSurfer (.mgz). Using FreeSurfer software (Version 5.3), all T1-weighted 3D anatomical data sets underwent motion correction, nonparametric nonuniform intensity normalization, Montreal Neurological Institute (MNI) transformation, removal of nonbrain materials, and skull-stripping. This was followed by whole-brain segmentation of WM and gray matter and registration of anatomical brain regions. Automatic subcortical segmentation included registration to a template, canonical normalization, canonical registration, neck removal, registration w/skull, and subcortical labeling. All automated FreeSurfer steps were inspected for processing errors, and manual edits were made as needed. For each case, automatic segmentation and parcellation masks were manually edited for accurate segmentation, using multiple views for visual inspection.
DTI processing
TRACULA was used to reconstruct WM pathways from DTI images using a global probabilistic tractography program, which has been found to be valid and reliable.32 This yields measures of WM integrity, including FA, MD, RD, and AD.32 Each image underwent the following preprocessing steps. (1) Image corrections (e.g., for B0 inhomogeneities, eddy currents, and simple head motion through correcting volume to volume translation and rotation), (2) Further head motion correction through detection of outliers based on signal-drop out due to excessive subject head motion, with slices with excessive motion being replaced by nonparametric prediction by Gaussian process33,34). (3) Intrasubject registration between T1 and DTI images and intersubject registration with the MNI template. (4) Mask creation (WM is extracted from the prior intra- and inter-registration step). (5) Tensor fit. (6) Estimation of pathways by combining the individual's data with an atlas. Following preprocessing, a ball-and-stick model of diffusion was fitted to the images. Markov Chain Monte Carlo sampling was used to measure diffusion in each voxel, and then establishing the likelihood of locations of tract for each subject. From these estimated pathways, statistics on diffusion measures (average weighted FA, MD, RD, and AD) within each individual was extracted and exported into SPSS for regression analysis.
Sixteen tracts were located for analysis using TRACULA: forceps major, forceps minor, left inferior longitudinal fasciculus (ILF), right ILF, left uncinate, right uncinate, left anterior thalamic radiation (ATR), right ATR, left cingulum angular bundle (CAB), right CAB, left cingulum cingulate gyrus (CCG), right CCG, left superior longitudinal fasciculus (SLF) parietal, right SLF parietal, left SLF temporal, and right SLF temporal.
Data analysis
Preliminary analyses
Although the primary analyses used dose-dependent independent variables, differences in demographic data were examined with analysis of variance and chi-square analyses between those who co-use and those who do not. As no variables differed by substance use pattern, no demographic covariates were included in subsequent analyses.
Primary analyses
A series of multiple regressions examined the study aims of WM integrity (measured by FA, MD, RD, and AD), using the whole sample so as to examine dose-dependent relationships; no groups were used in analyses. For the primary analysis, the independent variables included past month binge episodes (≥4 drinks for females, ≥5 drinks for males), past month cannabis use (total grams), and co-occurring binge/cannabis days. Raw values were used in the analyses. Each of the 16 tracts was first assessed for significance by FA value; tracts that were significantly related to any of the three predictors (cannabis, binge, or co-use) were then assessed by MD, AD, and RD, for a total of 34 regression analyses. Variance of inflation factor for each regression model was monitored, with no variables presenting with concern for multicollinearity. Data were also assessed for outliers using DFBETAS, with no outliers found.
Results
Demographics
To determine the need to covary for important demographic variables, differences between substance use patterns were assessed; CO and NO participants did not differ by length of abstinence [F(1, 64)=2.12, p=0.15], age [F(1, 73)=0.64, p=0.43], education [F(1, 73)=0.06, p=0.81], ethnicity [χ2=2.54, p=0.77], gender [χ2=0.1, p=0.75], and race [χ2=0.45, p=0.80], precluding the need to include any demographic variables as covariates (Table 1).
Table 1.
Demographic and Substance Use Patterns Between Those Who Co-Use and Do Not Co-Use Participants
| |
NO |
CO |
|---|---|---|
| n=61 |
n=14 |
|
| % or M (SD) |
% or M (SD) |
|
| Range | Range | |
| Age | 21.11 (2.64) | 21.71 (1.98) |
| 16–25 | 18–26 | |
| Education | 14.34 (2.28) | 14.50 (1.65) |
| 9–21 | 11–18 | |
| Gender (% female) | 47 | 43 |
| Race (% Caucasian) | 69 | 64 |
| Ethnicity (% Hispanic) | 10 | 14 |
| Alcohol*** | 10.75 (14.52) | 56.50 (36.11) |
| 0–64.84 | 15.48–132.58 | |
| Binge*** | .76 (1.48) | 5.81 (4.03) |
| 0–6.77 | .97–11.61 | |
| Cannabis*** | 1.24 (3.73) | 12.59 (14.26) |
| 0–20.56 | .08–47.42 | |
| Co-use*** | 0 (0) | 2.35 (1.81) |
| 0–0 | .97–5.81 | |
| # Drinking days in past month** | 3.88 (4.68) | 8.64 (3.95) |
| 0–20 | 2–14 | |
| # Cannabis use days in past month*** | 2.73 (7.60) | 14.36 (12.46) |
| 0–31 | 1–30 | |
| Age of regular alcohol use onset*,a | 19.69 (1.76) | 18.31 (1.44) |
| 16–25 | 16–21 | |
| Age of regular CAN use onseta | 17.43 (1.76) | 17.77 (1.64) |
| 13–21 | 15–20 |
p<0.05, **p<0.01,***p<0.001.
As not all participants have regularly (weekly) used either cannabis or alcohol, age of onset for regular cannabis NO has n=20, age of regular alcohol in NO has n=35. For CO group, n=13 for either variable; data were missing for one participant.
Demographic and substance use information by those who do (CO) and do not (NO) co-use cannabis and binge drinking. These groups were not use in any analyses, but to better approximate patterns of use and to establish appropriate covariates. Substance use information is from the past month and is presented in standardized units. Alcohol: standard drinks (1 ounce of liquor, 4 ounces of wine, 12 ounces of beer). Binge: occasions when individuals drank >4 drinks for females and >5 drinks for males. Cannabis: grams. Co-use: co-occurring binge and cannabis, days of co-use.
M, mean; SD, standard deviation; CO, co-users; NO, non-co-users; CAN, cannabis.
Substance use patterns
Participants exhibited a range of substance use in the past month (overall cannabis use in grams: mean=3.36, standard deviation (SD)=8.17, range=0–47.42; overall alcohol use in standard drinks: mean=19.29, SD=26.87, range=0–132.58; binge episodes: mean=1.73, SD=2.96, range=0–11.61; co-use episodes: mean=0.44, SD=1.19, range=0–5.81; Table 2). Substance use differed significantly by those who co-used relative to those who did not in past month cannabis use [F(1, 73)=30.85, p<0.001], alcohol use [F(1, 73)=58.74, p<0.001], binge episodes [F(1, 73)=65.11, p<0.001], and co-use binge episodes [F(1, 73=107.92, p<0.001]. Groups also differed by number of drinking days [F(1, 72)=12.38, p=0.001] and cannabis use days [F(1, 72)=20.35, p<0.001] in the past month. Individuals who co-use had significantly more substance use by each category. Of those who initiated regular (weekly) substance use, CO individuals initiated regular alcohol use at an earlier age than NO [F(1, 46)=5.42, p=0.02], but did not differ by regular age of cannabis use onset [F(1, 31)=0.32, p=0.58].
Table 2.
Multiple Regression Fractional Anisotropy Results for Cannabis, Binge Drinking, and Cannabis and Binge Co-Use Days As Primary Variables
| |
BG |
CAN |
CO |
|||
|---|---|---|---|---|---|---|
| Tract | Beta | p | Beta | p | Beta | P |
| Forceps major | −0.08 | 0.60 | −0.14 | 0.41 | −0.08 | 0.68 |
| Forceps minor | −0.01 | 0.93 | −0.11 | 0.50 | 0.22 | 0.28 |
| Left ILF | 0.03 | 0.84 | 0.48 | 0.003* | −0.46 | 0.02* |
| Right ILF | −0.13 | 0.37 | 0.12 | 0.47 | −0.10 | 0.61 |
| Left uncinate | −0.06 | 0.68 | 0.14 | 0.41 | −0.18 | 0.36 |
| Right uncinate | 0.02 | 0.87 | 0.13 | 0.44 | −0.17 | 0.38 |
| Left ATR | 0.17 | 0.23 | 0.39 | 0.02* | −0.37 | 0.054 |
| Right ATR | 0.32 | 0.03* | 0.32 | 0.048* | −0.50 | 0.01* |
| Left CAB | 0.09 | 0.55 | 0.20 | 0.24 | −0.30 | 0.13 |
| Right CAB | 0.08 | 0.60 | −0.13 | 0.45 | 0.001 | 0.99 |
| Left CCG | 0.19 | 0.16 | 0.53 | 0.001* | −0.48 | 0.01* |
| Right CCG | 0.06 | 0.66 | 0.36 | 0.03* | −0.26 | 0.19 |
| Left SLF parietal | 0.20 | 0.17 | 0.28 | 0.10 | −0.30 | 0.13 |
| Right SLF parietal | 0.18 | 0.21 | 0.36 | 0.03* | −0.32 | 0.10 |
| Left SLF temporal | 0.24 | 0.10 | 0.18 | 0.27 | −0.20 | 0.31 |
| Right SLF temporal | 0.21 | 0.16 | 0.17 | 0.31 | −0.26 | 0.18 |
p<0.05.
ILF, inferior longitudinal fasciculus; ATR, anterior thalamic radiation; CCG, cingulum cingulate gyrus; CAB, cingulum angular bundle; SLF, superior longitudinal fasciculus.
Supplementary substance use information
To aid in interpretation of results, participants were also categorized as controls, alcohol only, cannabis only, and alcohol + cannabis CO, with demographic, substance use, and DTI information, included in the Supplementary Data (Supplementary Tables S1, S2, S3). Groups were still not used in any analyses.
Primary analysis: TRACULA
All hypothesized tracts were analyzed first by FA. If FA tracts significantly related to any substance use pattern, MD, RD, and AD were then assessed in those significant tracts to determine more specific types of tract abnormalities. Full DTI metrics are available in the Supplementary Data.
Fractional anisotropy
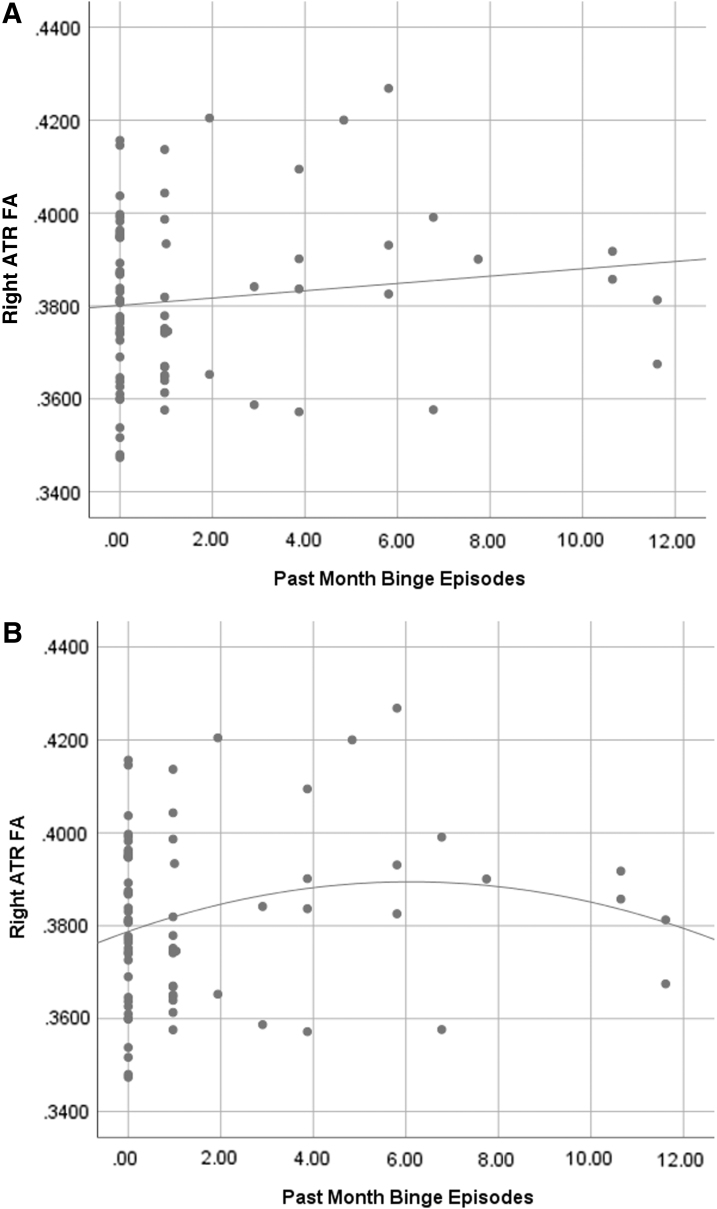
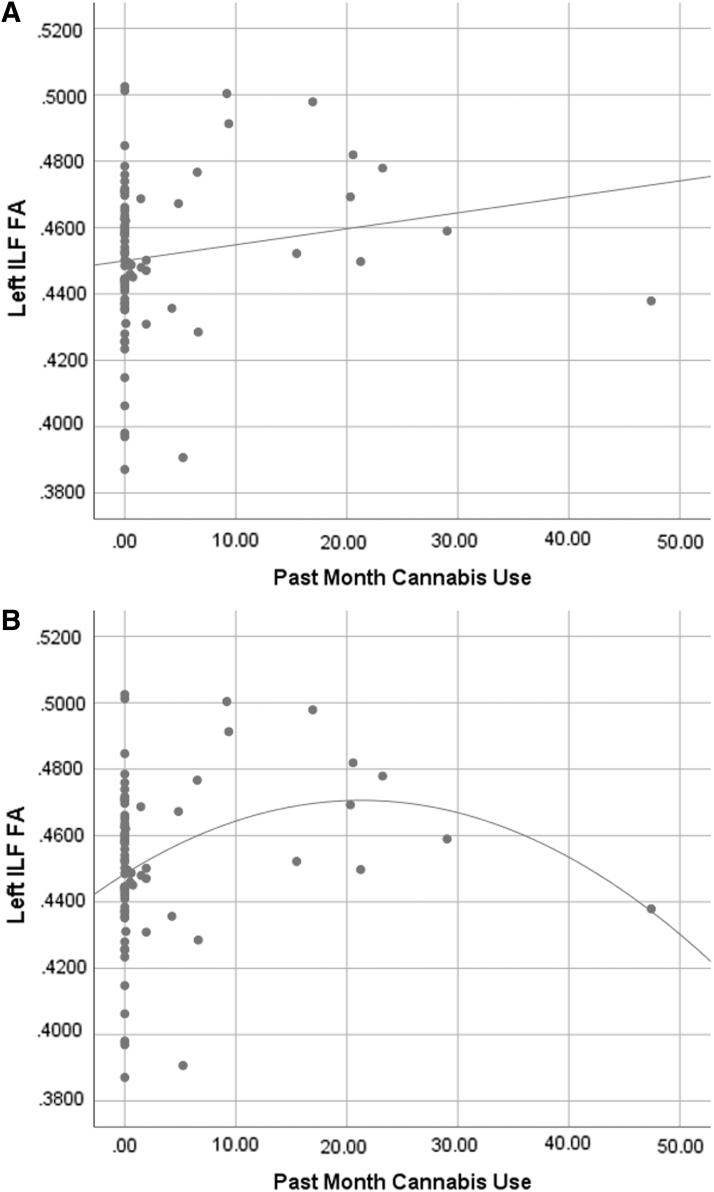
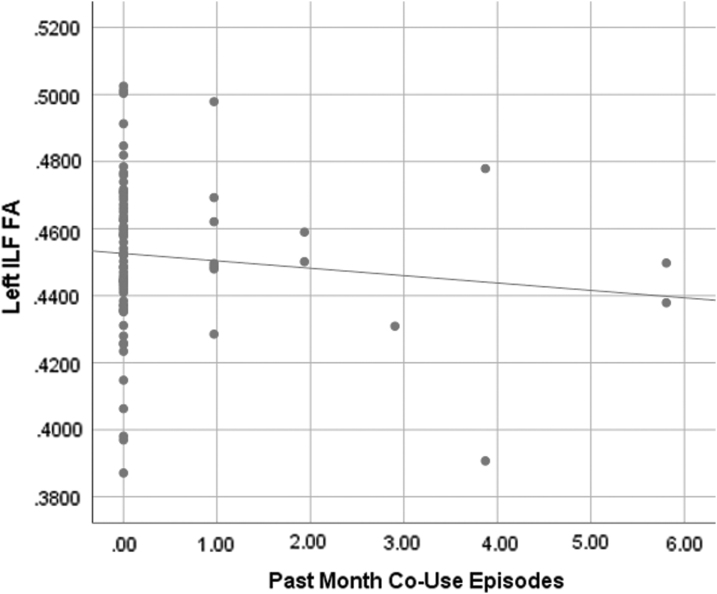
Past month binge drinking was associated with higher FA in the right ATR (f2=0.07; Fig. 1). Past month cannabis use was significantly associated with higher FA in the left ILF (f2=0.13; Fig. 2), left CCG (f2=0.16), right CCG (f2=0.07), left ATR (f2=0.08), right ATR (f2=0.06), and right SLF parietal (f2=0.07). Past month co-use was significantly associated with lower FA in the left ILF (f2=0.09; Fig. 3), right ATR (f2=0.10), and left CCG (f2=0.09) (Table 2).
FIG. 1.
(A) Scatterplot of binge drinking episodes by right ATR FA values (R2=0.016) with linear fit line. (B) Scatterplot of binge drinking episodes by right ATR FA values (R2=0.039) with quadratic fit line. Both fit lines are presented to aid in interpretation, as the higher R2 value in the quadratic fit suggests there may be a nonlinear relationship between binge drinking and right ATR FA value. As the primary analyses used linear multiple regressions, it would be beneficial to assess nonlinear relationships in a future study. Note: Data were assessed for outliers, with no outliers revealed. ATR, anterior thalamic radiation; FA, fractional anisotropy.
FIG. 2.
(A) Scatterplot of cannabis use (grams) by left ILF FA values (R2=0.026) with linear fit line. (B) Scatterplot of cannabis use (grams) by left ILF FA values (R2=0.083) with quadratic fit line. Both fit lines are presented to aid in interpretation, as the higher R2 value in the quadratic fit suggests there may be a nonlinear relationship between cannabis use and left ILF FA value. As the primary analyses used linear multiple regressions, it would be beneficial to assess nonlinear relationships in a future study. Note: Data were assessed for outliers, with no outliers revealed. ILF, inferior longitudinal fasciculus.
FIG. 3.
Scatterplot of co-use episodes by left ILF values (R2=0.012) with linear fit line. Note: Data were assessed for outliers, with no outliers revealed.
Scatterplots suggest relationships between binge drinking and right ATR, and cannabis use and left ILF, left CCG, and right CCG may be nonlinear. Examples of these relationships are shown in Figures 1 and 2, as the R2 values are higher for nonlinear fit lines. However, we note that the statistical analyses conducted were linear, thus restricting these figures to be used only for aiding in interpretation.
Mean diffusivity
Neither past month cannabis use, binge episodes, nor co-use episodes were significantly associated with MD in any tract (Table 3).
Table 3.
Multiple Regression Mean Diffusivity Results for Cannabis, Binge Drinking, and Cannabis and Binge Co-Use Days As Primary Variables
| |
BG |
CAN |
CO |
|||
|---|---|---|---|---|---|---|
| Tract | Beta | p | Beta | p | Beta | P |
| Left ILF | 0.19 | 0.19 | −0.07 | 0.66 | −0.12 | 0.56 |
| Left ATR | 0.04 | 0.79 | −0.03 | 0.85 | −0.24 | 0.23 |
| Right ATR | 0.05 | 0.72 | 0.03 | 0.88 | −0.22 | 0.27 |
| Left CCG | −0.19 | 0.20 | −0.02 | 0.93 | −0.07 | 0.74 |
| Right CCG | 0.02 | 0.92 | 0.001 | 0.99 | −0.19 | 0.35 |
| Right SLF Parietal | 0.002 | 0.99 | −0.12 | 0.48 | 0.11 | 0.59 |
Radial diffusivity
Neither past month cannabis use, binge episodes, nor co-use episodes were significantly associated with RD in any tract (Table 4).
Table 4.
Multiple Regression Radial Diffusivity Results for Cannabis, Binge Drinking, and Cannabis and Binge Co-Use Days As Primary Variables
| |
BG |
CAN |
CO |
|||
|---|---|---|---|---|---|---|
| Tract | Beta | p | Beta | p | Beta | P |
| Left ILF | 0.06 | 0.70 | −0.09 | 0.61 | −0.09 | 0.67 |
| Left ATR | −0.09 | 0.50 | −0.11 | 0.44 | 0.08 | 0.58 |
| Right ATR | −0.07 | 0.60 | −0.12 | 0.42 | −0.04 | 0.76 |
| Left CCG | −0.20 | 0.13 | −0.09 | 0.54 | 0.05 | 0.71 |
| Right CCG | −0.03 | 0.81 | −0.07 | 0.64 | 0.03 | 0.83 |
| Right SLF Parietal | −0.04 | 0.76 | −0.03 | 0.86 | −0.09 | 0.52 |
Axial diffusivity
In assessing AD in tracts that were previously indicated by FA, past month co-use episodes were associated with lower AD in left ILF (f2=0.07) (Table 5).
Table 5.
Multiple Regression Axial Diffusivity Results for Cannabis, Binge Drinking, and Cannabis and Binge Co-Use Days As Primary Variables
| |
BG |
CAN |
CO |
|||
|---|---|---|---|---|---|---|
| Tract | Beta | p | Beta | p | Beta | P |
| Left ILF | 0.18 | 0.21 | 0.16 | 0.33 | −0.43 | 0.03* |
| Left ATR | −0.02 | 0.88 | −0.09 | 0.60 | −0.04 | 0.83 |
| Right ATR | 0.03 | 0.83 | 0.009 | 0.96 | −0.13 | 0.51 |
| Left CCG | −0.004 | 0.98 | 0.05 | 0.75 | −0.08 | 0.70 |
| Right CCG | 0.20 | 0.18 | −0.06 | 0.72 | −0.05 | 0.79 |
| Right SLF Parietal | 0.17 | 0.24 | −0.19 | 0.26 | 0.007 | 0.97 |
p<0.05.
Discussion
Given high rates of substance use among adolescents and emerging adults and the unknown impact of combined substance use on neuroanatomical structure, the present study sought to assess the relationship between substance use patterns and WM integrity. Results suggest significant associations between greater past month cannabis and binge drinking co-use episodes and lower WM integrity in three tracts (left ILF, right ATR, and left CCG). In addition, greater past month cannabis use was related to higher FA in left ILF, left and right CCG, left and right ATR, and right SLF parietal, and past month binge episodes independently related to higher FA in right ATR. Notably, some of these findings may represent nonlinear relationships. Also, those who engaged in co-use reported significantly more substance use over the past month. As such, these findings highlight the need to carefully look at patterns of substance use to delineate unique effects on neuroanatomy.
Our primary aim and finding regarding co-use episodes suggest a negative relationship between increased co-use episodes and lower WM integrity in frontolimbic tracts, while controlling for patterns of each individual substance used. Consistent with this, prior studies investigating co-use on a group level have found poorer WM integrity across frontal, limbic, and parietal areas17,18 and morphological changes in frontal regions,20 including when specifically considering cannabis and binge group status.19 While others have previously shown acute additive cognitive deficits35–38 and chronic cognitive deficits due to co-use,39 none of these studies investigated WM differences. Although there remain somewhat equivocal results of human pharmacological studies of co-use,15,16,25,26 the scant literature that does exist appears to suggest a more negative impact of combined cannabis and alcohol (here, binge drinking) use. Together, these findings highlight the great need for more research in this area. Studies should consist of pre-clinical work, as well as carefully categorizing and accounting for patterns of substance use and co-use in human subjects.
The disrupted WM revealed in relation to co-use may be indicative of damage to myelin due to the combination of cannabis use and binge drinking. Indeed, both cannabis and alcohol use independently downregulate CB1 activity,11 cannabinoid receptors are important for WM development,13 and earlier age of onset of cannabis use is related to poorer WM integrity.23 Changes in CB1 signaling thus suggest a potential underlying mechanism of these findings during the neurodevelopmentally sensitive time period of emerging adulthood. Perhaps underlying microstructural integrity is altered by substance use patterns, including co-use episodes, and such microstructural changes lead to functional impairment.
Importantly, conflicting and null results in this study do not mean that either cannabis or alcohol independently is not having an impact, nor is either substance inherently beneficial when consumed alone. Indeed, as suggested in Figure 1, findings may be nonlinear, such that moderate substance use may be driving more beneficial findings. For example, the socially facilitative nature of initial alcohol experimentation40 may lead to better social and, by extension, WM outcomes.41 Thus, these findings do not negate the importance of investigating potential effects of cannabis or alcohol independently, as others have previously found poorer WM associated with either substance (e.g., refs.17,42) and quadratic effects may mask real differences. Rather, careful assessment of patterns of use is needed, and pharmacological interactions may alter the effects of each substance. Findings such as these suggest complex relationships that need more careful study of patterns, dosages, and other factors that may be influencing outcomes, as well as investigations into the mechanisms that shape neuroanatomical structure.
Individuals who co-use substances may also be qualitatively different in how they approach substance consumption. Our CO here had an earlier onset of regular alcohol use and reported significantly more alcohol and cannabis consumption over the past month. Subjectively, when cannabis and alcohol are consumed together at low doses, the alcohol may be prolonging the effects of THC43; however, this relationship has not been assessed at a higher dose. Perhaps the elevated level of use by CO in this study, in addition to combining substances and earlier onset of regular alcohol use, led to decrements in WM related to co-use. Lower levels of use likely have less of a detrimental effect on neuroanatomy.
Although this study has strengths in its novelty and measurement of patterns of use, a number of limitations should be noted. As a first attempt at investigating patterns of co-use episodes, this study covers an important and understudied topic; however, the sample size was relatively small, limiting the power to detect differences or control for multiple comparisons, making replication crucial. In addition, the sample size and reported substance use patterns did not allow for investigating individuals who used primarily only one substance. Regression analyses were used to assess dose-dependent relationships while simultaneously controlling for other patterns of substance use; thus, the results cannot be used to create a predictive algorithm, as is sometimes done using full regression equations. In addition, linear regressions were conducted, although the relationship between substance use and WM may be nonlinear, and this should be investigated in the future. Although nonsubstance using controls were included in the study and are represented in each variable, as groups were not used, there is no pure “control” in the data analyses. Participants had extended periods of time of abstinence (at least 2 weeks of monitored abstinence), which is important for showing more chronic effects of substance use. However, with only one time point of data, it limits the ability to ascertain how abstinence may impact structure, as abstinence from substances may lead to WM recovery.44,45 While every effort was made to carefully measure past month of substance use, we were not able to account for whether the substances were used simultaneously or even within hours of one another. Future research should determine simultaneous and long-term use patterns. Finally, as a cross-sectional study, causal relationships cannot be established; longitudinal studies are needed to assess causality. One such study, the Adolescent Brain Cognitive Development study (abcdstudy.org), is well designed for future analyses assessing the structural and functional impact of patterns of substance use over time.
In conclusion, the present study found that greater past month binge drinking and cannabis co-use episodes were associated with poorer WM integrity, as measured by FA and AD across three frontolimbic tracts, while cannabis and binge drinking alone were related to better WM in some tracts. Given ongoing movements for greater access to and legalization of cannabis, methods and patterns of use should be considered in all policies and legislation. The present results underscore the need to measure cannabis and alcohol use with careful, sensitive, quantitative techniques that facilitate assessment of the unique influence of each substance. Furthermore, more careful research into the combined effects of cannabis and alcohol and their potential psychopharmacological interactions is also needed on both pre-clinical and clinical levels.
Supplementary Material
Acknowledgments
The IDEAA consortium was supported by NIDA (R01 DA032646, PI: S.A.G.; R01 DA030354, PI: K.M.L.). Dr. S.A.G.'s work was supported by DA032646, DA016695, and DA021241. Dr. S.F.T.'s work was supported by R01 AA03419, P20 DA024194, and R01 DA021182. Dr. F.M.F.'s work was supported by R01 DA042490, R01 DA030344, R01 AA023658, and the Bert Moore Chair in BrainHealth. Dr. K.M.L.'s work was supported by R01 DA030354 and article preparation was supported by funding from the National Institutes of Health (U01DA041025; P.I.: K.M.L.). Dr. N.E.W.'s article preparation was supported by the National Institute On Alcohol Abuse And Alcoholism of the National Institutes of Health under award number T32AA013525. The funding sources had no further role in the study design, data collection, analysis, interpretation, the writing of the report, or in the decision to submit the article for publication. All authors have contributed significantly to the design of this study, and have read and approved the final article.
Abbreviations Used
- 3D
three-dimensional
- AD
axial diffusivity
- ATR
anterior thalamic radiation
- CAB
cingulum angular bundle
- CAN
cannabis
- CB1
cannabinoid receptor 1
- CCG
cingulum cingulate gyrus
- CO
co-users
- DTI
diffusion tensor imaging
- eCB
endocannabinoid
- FA
fractional anisotropy
- ILF
left inferior longitudinal fasciculus
- MCW
Medical College of Wisconsin
- MD
mean diffusivity
- MRI
magnetic resonance imaging
- NO
non-co-users
- RD
radial diffusivity
- SD
standard deviation
- SLF
superior longitudinal fasciculus
- THC
tetrahydrocannabinol
- WM
white matter
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
Cite this article as: Wade NE, Thomas AM, Gruber SA, Tapert SF, Filbey FM, Lisdahl KM (2020) Binge and cannabis co-use episodes in relation to white matter integrity in emerging adults, Cannabis and Cannabinoid Research 5:1, 62–72, DOI: 10.1089/can.2018.0062.
References
- 1. Giedd JN, Snell JW, Lange N, et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6:551–560 [DOI] [PubMed] [Google Scholar]
- 2. Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shaw P, Kabani NJ, Lerch JP, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bava S, Tapert SF. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychol Rev. 2010;20:398–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miech RA, Johnston LD, O'Malley PM, et al. Monitoring the future national survey results on drug use, 1975–2014: Volume I secondary school students. Ann Arbor: Institute for Social Research, The University of Michigan, 2015 [Google Scholar]
- 6. Johnston LD, O'Malley PM, Bachman JG, et al. Monitoring the future national survey results on drug use,1975–2012: Volume 1, secondary school students. Ann Arbor: Institute for Social Research, The University of Michigan, 2013 [Google Scholar]
- 7. Subbaraman MS, Kerr WC. Simultaneous versus concurrent use of alcohol and cannabis in the National Alcohol Survey. Alcohol Clin Exp Res. 2015;39:872–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Terry-McElrath YM, O'Malley PM, Johnston LD. Simultaneous alcohol and marijuana use among U.S. high school seniors from 1976 to 2011:trends, reasons, and situations. Drug Alcohol Depend. 2013;133:71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sim-Selley LJ. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol. 2003;15:91–119 [DOI] [PubMed] [Google Scholar]
- 10. Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol. 2008;13:253–263 [DOI] [PubMed] [Google Scholar]
- 11. Hirvonen J, Goodwin RS, Li CT, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17:642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirvonen J, Zanotti-Fregonara P, Umhau JC, et al. Reduced cannabinoid CB1 receptor binding in alcohol dependence measured with positron emission tomography. Mol Psychiatry. 2013;18:916–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Molina-Holgado E, Vela JM, Arevalo-Martin A, et al. Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/akt signaling. J Neurosci. 2002;22:9742–9753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pava MJ, Woodward JJ. A review of the interactions between alcohol and the endocannabinoid system: implications for alcohol dependence and future directions for research. Alcohol. 2012;46:185–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lukas SE, Benedikt R, Mendelson JH, et al. Marihuana attenuates the rise in plasma ethanol levels in human subjects. Neuropsychopharmacology. 1992;7:77–81 [PubMed] [Google Scholar]
- 16. Chesher GB, Franks HM, Hensley VR, et al. The interaction of ethanol and delta9-tetrahydrocannabinol in man effects of perceptual, cognitive, and motor functions. Med J Aust. 1976;2:159–163 [PubMed] [Google Scholar]
- 17. Jacobus J, Squeglia LM, Bava S, et al. White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: a 3-year investigation. Psychiatry Res. 2013;214:374–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bava S, Jacobus J, Thayer RE, et al. Longitudinal changes in white matter integrity among adolescent substance users. Alcohol Clin Exp Res. 2013;37 Suppl 1:E181–E189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jacobus J, McQueeny T, Bava S, et al. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol Teratol. 2009;31:349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Squeglia LM, Tapert SF, Sullivan EV, et al. Brain development in heavy-drinking adolescents. Am J Psychiatry. 2015;172:531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ashtari M, Cervellione K, Cottone J, et al. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. J Psychiatr Res. 2009;43:189–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clark DB, Chung T, Thatcher DL, et al. Psychological dysregulation, white matter disorganization and substance use disorders in adolescence. Addiction. 2012;107:206–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gruber SA, Dahlgren MK, Sagar KA, et al. Worth the wait: effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacology (Berl). 2014;231:1455–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shollenbarger SG, Price J, Wieser J, et al. Poorer frontolimbic white matter integrity is associated with chronic cannabis use, FAAH genotype, and increased depressive and apathy symptoms in adolescents and young adults. Neuroimage Clin. 2015;8:117–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lukas SE, Orozco S. Ethanol increasess plasma delta9-tetrahydrocannbinol (THC) levels and subjective effects after marihuana smoking in human volunteers. Drug Alcohol Depend. 2001;64:143–149 [DOI] [PubMed] [Google Scholar]
- 26. Hartman RL, Brown TL, Milavetz G, et al. Controlled cannabis vaporizer administration: blood and plasma cannabinoids with and without alcohol. Clin Chem. 2015;61:850–869 [DOI] [PubMed] [Google Scholar]
- 27. Ronen A, Chassidim HS, Gershon P, et al. The effect of alcohol, THC and their combination on perceived effects, willingness to drive and performance of driving and non-driving tasks. Accid Anal Prev. 2010;42:1855–1865 [DOI] [PubMed] [Google Scholar]
- 28. Hansen HH, Krutz B, Sifringer M, et al. Cannabinoids enhance susceptibility of immature brain to ethanol neurotoxicity. Ann Neurol. 2008;64:42–52 [DOI] [PubMed] [Google Scholar]
- 29. Wade NE, Wallace AL, Swartz AS, et al. Aerobic fitness level moderates the association between cannabis use and executive functioning and psychomotor speed following abstinence in adolescents and young adults. J Int Neuropsychol Soc. 2018;25:134–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sheehan DV, Lecrubier Y, Sheehan H, et al. The mini-international neuropsychiatric interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33 [PubMed] [Google Scholar]
- 31. Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ (eds) Measuring alcohol consumption: psychosocial and biological methods. Humana Press, Totowa, NJ, 1992:41–72 [Google Scholar]
- 32. Yendiki A, Panneck P, Srinivasan P, et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinform. 2011;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andersson JLR, Graham MS, Drobnjak I, et al. Towards a comprehensive framework for movement and distortion correction of diffusion MR images: within volume movement. Neuroimage. 2017;152:450–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Andersson JLR, Soritopoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Belgrave BE, Bird KD, Chesher GB, et al. The effect of (−) trans-delta9-tetrahydrocannnabinol, alone and in combination with ethanol, on human performance. Psychopharmacology. 1979;62:53–60 [DOI] [PubMed] [Google Scholar]
- 36. Chait LD, Perry JL. Acute and residual effects of alcohol and marijuana, alone and in combination, on mood and performance. Psychopharmacology. 1994;115:340–349 [DOI] [PubMed] [Google Scholar]
- 37. Chesher GB, Franks HM, Jackson DM, et al. Ethanol and delta9-tetrahydrocannabinol: interactive effects on human perceptual, cognitive, and motor functions. Med J Aust. 1977;1:478–481 [PubMed] [Google Scholar]
- 38. Marks DF, MacAvoy MG. Divided attention performance in cannabis users and non-users following alcohol and cannabis separately and in combination. Psychopharmacology. 1989;99:397–401 [DOI] [PubMed] [Google Scholar]
- 39. Winward JL, Hanson KL, Tapert SF, et al. Heavy alcohol use, marijuana use, and concomitant use by adolescents are associated with unique and shared cognitive decrements. J Int Neuropsychol Soc. 2014;20:784–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Varlinskaya EI, Spear LP. Social consequences of ethanol: impact of age, stress, and prior history of ethanol exposure. Physiol Behav. 2015;148:145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Y, Metoki A, Alm KH, et al. White matter pathways and social cognition. Neurosci Biobehav Rev. 2018;90:350–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lisdahl KM, Thayer R, Squeglia LM, et al. Recent binge drinking predicts smaller cerebellar volumes in adolescents. Psychiatry Res. 2013;211:17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hartman RL, Brown TL, Milavetz G, et al. Controlled vaporized cannabis, with and without alcohol: subjective effects and oral fluid-blood cannabinoid relationships. Drug Test Anal. 2016;8:690–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brown GG, Jacobus J, McKenna B. Structural imaging for addiction medicine: from neurostructure to neuroplasticity. Prog Brain Res. 2016;224:105–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zahr NM, Pfefferbaum A. Alcohol's effects on the brain: neuroimaging results in human and animal models. Alcohol Res. 2017;38:183–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.