Abstract
Introduction: Cannabidiol (CBD) as Epidiolex® (GW Pharmaceuticals) was recently approved by the U.S. Food and Drug Administration (FDA) to treat rare forms of epilepsy in patients 2 years of age and older. Together with the increased societal acceptance of recreational cannabis and CBD oil for putative medical use in many states, the exposure to CBD is increasing, even though all of its biological effects are not understood. Once such example is the ability of CBD to be anti-inflammatory and immune suppressive, so the purpose of this review is to summarize effects and mechanisms of CBD in the immune system. It includes a consideration of reports identifying receptors through which CBD acts, since the “CBD receptor,” if a single one exists, has not been definitively identified for the myriad immune system effects. The review then provides a summary of in vivo and in vitro effects in the immune system, in autoimmune models, with a focus on experimental autoimmune encephalomyelitis, and ends with identification of knowledge gaps.
Conclusion: Overall, the data overwhelmingly support the notion that CBD is immune suppressive and that the mechanisms involve direct suppression of activation of various immune cell types, induction of apoptosis, and promotion of regulatory cells, which, in turn, control other immune cell targets.
Keywords: cannabidiol, immune response, inflammation
Cannabidiol History and Therapeutic Uses
Cannabidiol (CBD) is a plant-derived cannabinoid that has structural similarity to the primary psychotropic congener in cannabis, Δ9-tetrahydrocannabinol (THC). While CBD was initially isolated in the 1940s, its structure was not elucidated until the 1960s.1,2 Unlike THC, CBD is bicyclic, comprised a terpene and an aromatic ring, and is a pentyl side chain.1 It exists as two enantiomers, and it is (−)CBD3 that is one of the major constituents found in Cannabis sp., and will be the focus of this review. For many years, THC and CBD were designated as psychoactive and nonpsychoactive, respectively, owing to the fact that THC produces the euphoric high associated with cannabis use, while CBD does not. However, since we know that CBD produces biological effects in the central nervous system (CNS), perhaps it is better defined as psychoactive, but not psychotropic, since it is active in the CNS without producing the euphoric high.
Perhaps it was the association of the euphoric high with THC that provided the initial focus on THC as opposed to CBD for potential medical use, since THC was originally identified as the active component of the plant.4 However, in recent years, researchers have begun to explore CBD more as a therapeutic addition or alternative to THC. In the United States, oral THC (dronabinol, Marinol®) was first approved in 1985 by the Food and Drug Administration (FDA) to treat nausea and vomiting associated with chemotherapy. In 1992, dronabinol was also approved to treat cachexia in AIDS patients.5 The next major advancement in cannabinoid pharmaceuticals was not until the mid-2000s when Sativex® (nabiximols), a combination of THC and CBD as an oromucosal spray, was approved in Canada and the EU for neuropathic pain in multiple sclerosis (MS) and intractable cancer pain.6 There are several reasons why combining THC and CBD in a single therapeutic could have value.6 First, additional therapeutic benefit might be gained from hitting multiple targets; for example, if THC alleviates pain and CBD alleviates anxiety,7–16 the combination therapy could be quite effective for chronic pain sufferers. Second, for disease states in which both THC and CBD are efficacious, a combination might allow for lower doses of THC, thereby potentially decreasing the psychotropic effects of THC. Third, there are some studies suggesting pharmacokinetic interactions between CBD and THC in which CBD treatment increases THC levels,17–20 thereby allowing longer duration of effects of THC. Sativex® has been evaluated in several clinical trials for spasticity associated with MS, neuropathic pain, and other conditions.21–37
The latest approved cannabinoid pharmaceutical in the United States is CBD as Epidiolex®. It was approved by the U.S. FDA in 2018 for epilepsy in children, in particular, for Dravet Syndrome and Lennox-Gastaut Syndrome.38–42 CBD is also being investigated for its effectiveness in other diseases, including Tuberous Sclerosis, a genetic condition that causes growth of benign tumors all over the body,43,44 schizophrenia,45 and refractory epileptic encephalopathy.46
In addition to the federally approved uses of CBD as Epidolex®, CBD, usually as CBD oil, is widely used for putative medical benefit in several states, and is certainly used in states in which cannabis has been decriminalized, or legalized, for recreational use.47 There are reports that CBD and other cannabinoids are beneficial for sleep, anxiety, pain, post-traumatic stress disorder, schizophrenia, neurodegenerative disorders, and immune-mediated diseases.48 Often these conditions are self-diagnosed and self-treated, so there can be issues with dosing, other drug interactions, and characterization of CBD safety and efficacy.
Overall, it is clear that exposures to CBD are increasing.47,49–51 It is also clear that CBD possesses therapeutic benefit, and in some cases, the beneficial effects of CBD are for diseases for which other available treatments have not been efficacious.52 Together, these observations demonstrate the critical need to continue research on CBD, and therefore the goal of this review is to provide a summary of the effects and mechanisms by which CBD alters immune function. The review will include an evaluation of the role for various receptors through which CBD acts in the immune system. There will also be a description of CBD effects in animal and human immune responses, a characterization of mechanisms by which CBD mediates immune effects, and identification of knowledge gaps regarding CBD's actions in the immune system.
Identification of CBD Receptors and Other Targets
Upon identification of the cannabinoid receptors, CBD was determined to exhibit low affinity for CB153 and CB2 receptors.54 Consistent with this, we showed CBD-induced suppression of cytokine production in mouse splenocytes in both wild-type and double cannabinoid receptor knockout mice (Cnr1−/−/Cnr2−/− mice).55 Another study demonstrated that ophthalmic administration of CBD following corneal inflammation reduced neutrophils in both wild-type and CB2 receptor knockout mice.56 CBD-mediated suppression of anti-CD3-mediated proliferation of T cells also occurred in both wild-type and CB2 receptor knockout splenocytes.57 However, there are a few reports using inflammatory stimuli in which CBD's actions have been attributed to either CB1 or CB2 receptors (Table 1). In a sepsis model induced with bacterial lipopolysaccharide (LPS), CBD-mediated inhibition of gastric emptying was reversed with the CB1 receptor antagonist, AM251.58 Similarly, CBD inhibited interleukin (IL)-1 in a hypoxia-ischemia brain insult model and this effect was reversed with the CB2 receptor antagonist, AM630.59 Use of ovalbumin to induce an asthma-like disease in mice demonstrated that some cytokines and chemokines induced in the lungs of mice that were suppressed by CBD (IL-4, IL-5, IL-13, and eotaxin) were differentially regulated by CB receptors.60 Specifically, CBD-induced suppression of IL-5 was reversed in the presence of the CB2 receptor antagonist in bronchoalveolar lavage fluid and lung tissue, but there was no clear receptor dependence identified for CBD's suppression of IL-4, IL-13, or eotaxin.60 Thus, several studies do suggest a possible role for cannabinoid receptors in CBD-mediated suppression of inflammatory effects. It should also be noted that there are several reports suggesting that CBD acts as an allosteric modulator of CB1 or CB2 receptors,61–64 although the role for CB1 or CB2 receptor allosteric modulation by CBD in immune function has not yet been determined.
Table 1.
Receptors Identified in Mediating Cannabidiol Immune Effects
| Receptor | Activity | References |
|---|---|---|
| CB1 | Agonist | 58 |
| CB2 | Agonist | 59,60 |
| FAAH | Inhibition | 58,65–67,84,157,160 |
| TRPV1 | Agonist | 65,66,74,82–88,105,148,194 |
| Adenosine A2A | Agonist | 89–91,125,164 |
| PPAR-γ | Activation | 92–94,96–98,136 |
| 5-HT1a | Agonist | 59 |
| GPR55 | Antagonist | 109,110 |
FAAH, fatty acid amide hydrolase; PPAR-γ, peroxisome proliferator-activated receptor gamma; TRPV1, transient receptor potential vanilloid 1.
Another mechanism by which CBD acts is through inhibition of fatty acid amide hydrolase (FAAH),65–67 suggesting that some of CBD's effects are mediated by anandamide elevation since FAAH is responsible for the breakdown of anandamide.65,66 Anandamide is an endogenous cannabinoid that exhibits affinity for CB1 and CB2 receptors.68,69 A recent study suggested that the mechanism by which CBD elevates anandamide involves CBD interaction with fatty acid binding proteins, which prevents anandamide binding to these proteins to block anandamide transport to FAAH.67 Since anandamide exhibits affinity for CB1 and CB2 receptors, and oxidation products of anandamide through cyclooxygenase or cytochrome P450 enzymes produce metabolites that also exhibit affinity for CB1 and CB2 receptors,70,71 anandamide or its metabolites could account for some of the reports that CBD acts through CB1 and/or CB2 receptors.58,61–64,72–84
Actions of CBD in immune function might also be mediated by the transient receptor potential V1, known as the vanilloid receptor (TRPV1), which was found to be activated by CBD.65 Specifically, CBD was found to increase intracellular calcium in HEK cells transfected with TRPV1, and the CBD-induced increase in calcium was blocked by the TRPV1 antagonist, capsazepine.65,66 Follow-up studies demonstrated that CBD desensitizes TRPV1 following activation.85 Other studies have suggested that CBD acts through TRPV1 in the immune system (Table 1). CBD can induce myeloid-derived suppressor cells (MDSCs), a type of regulatory cell, in the liver, and this effect is lost in TRPV1 knockout mice.86 Specifically, regarding inflammation, CBD attenuated thermal hyperalgesia in response to carrageenan injections or in a neuropathic pain model in a capsazepine-dependent manner.87,88 CBD suppression of cytokines in inflamed primary human colonic tissue was attenuated by the TRPV1 antagonist, SB366791.82 SB366791 was also effective in reversing CBD's suppression of rolling and adherent leukocytes in the sodium monoiodoacetate model of osteoarthritis in rats.83 Together, these data suggest that TRPV1 is a critical receptor through which CBD acts in the immune system.
There have been several critical articles in which adenosine A2A receptors have been shown to mediate CBD's effects in the immune system.89–91 CBD was shown to inhibit microglial cell proliferation, which was associated with inhibition of adenosine uptake into cells.89 The studies also demonstrated that CBD suppression of tumor necrosis factor-alpha (TNF-α) could be reversed using an adenosine A2A receptor antagonist, and CBD-induced suppression of LPS-stimulated TNF-α was not observed in adenosine A2A receptor knockout mice.89 The role for adenosine A2A receptor in CBD-mediated neuroprotection or suppression of neuroinflammation was demonstrated in a model of hypoxia-ischemia in newborn mouse brains.90 CBD inhibited adenosine uptake into rat microglial cells and CBD enhanced adenosine's ability to inhibit TNF-α, which was prevented by the adenosine A2A receptor antagonist, ZM241385.91 These studies show that CBD acts through the adenosine A2A receptor, especially in microglial cells.
CBD's effects have also been shown to be mediated by peroxisome proliferator-activated receptor gamma (PPAR-γ) using PPAR-γ antagonists in models of β amyloid neuroinflammation,92 apoptosis,93,94 dinitrobenzene sulfonic acid (DNBS)-induced colitis,95 human ulcerative colitis,96 LPS activation of microglial cells,97 and hypoxia-ischemia model of neuroinflammation.98
There are several reports that CBD acts through the serotonin 5-HT1a receptor (Table 1). Although most of the evidence for the involvement of this receptor comes from the attenuation of CBD's effects using the 5-HT1a antagonist, WAY100635, early studies demonstrated that CBD displaced binding of the 5-HT1a agonist, 8-OH-DPAT, in membranes from CHO cells expressing the human 5-HT1a receptor.99 Few of the CBD-mediated effects acting through the serotonin 5-HT1a receptor have been reported in immune cells, but immune cells do express 5-HT1a.100–103 One study showed that IL-1 produced in the brain in response to hypoxia-ischemia insult was inhibited by CBD, and reversed with the 5-HT1a receptor antagonist, WAY100635.59
Studies have suggested that CBD might act through other receptors, including other TRP receptors,66,85,104–107 or opioid receptors.108 There is also evidence that CBD acts through blockade of GPR55,109 and specifically that CBD modestly antagonized proinflammatory effects in human innate cells following GPR55 activation.110 Thus, together, the current data support that immune effects of CBD are mediated through activation of CB1, CB2, TRPV1, adenosine A2A, and PPAR-γ receptors, blockade of GPR55 receptors, and FAAH inhibition.
CBD Immune System Effects and Mechanisms
Immunity is maintained through various cell types acting together to provide protection against foreign invaders, and simultaneously avoid reactions against self-proteins. Thus, an appropriate immune response requires a regulated balance between robust reactions against non-self, but limited or no reactions against self. Cell types include neutrophils, macrophages, and other myeloid cells comprising the innate immune system, which reacts quickly to destroy pathogens. In the event that an innate response is insufficient, certain innate cells can activate the adaptive immune response, comprised predominantly of T and B cells. T cells can then provide signals that recruit and activate other immune cells, or directly lyse or induce apoptosis of infected cells. T cells can also help stimulate B cells, which produce antibodies to neutralize pathogens and/or enhance destruction of the pathogens. Communication between the various cell types, and therefore the innate and adaptive immune responses, is mediated by expressed or secreted proteins called cytokines or chemokines. Inflammation is the process commonly associated with the innate immune response since pathogen destruction can also cause tissue damage, although T cells certainly are proinflammatory as well. In fact, many cell types, regardless of whether they are immune cells, produce proinflammatory cytokines in response to inflammation.
The effects of CBD on immune responses can involve innate or adaptive responses. In assessing these responses, various cell types and their functions have been examined. For instance, a common end-point to examine regardless of cell type is cytokine or chemokine production. Typical proinflammatory cytokines include IL-1α, IL-1β, IL-6, TNF-α and IL-17A, while IL-10 is considered anti-inflammatory. Some cytokines are produced by specific T cell subsets; for instance, the Th1 subset produces interferon-gamma (IFN-γ) and promotes cell-mediated cytotoxicity, while the Th2 subset produces IL-4 and promotes B cell responses. Other end-points that might provide clues of disruption of immune competence are nitric oxide or myeloperoxidase (MPO) production from innate cells, as these are often released during pathogen destruction. Thus, the effects of CBD on immune function are presented by cell type, outlining known mechanisms by which CBD alters various end-points. Tables 2–4 include the studies described in the text (and others) and are organized by experimental approach. As indicated above, inflammation can induce proinflammatory cytokine production in nonimmune cells, so there are also a few of those examples included in the tables.
Table 2.
Cannabidiol-Induced Immune Suppression by Cell Type in Human Cells In Vitro
| Cell type | End-point(s) | References |
|---|---|---|
| PBMCs | ↓rosette formation | 138a |
| PBMCs | ↓cytokines | 111,112a |
| Human cell linesb | ↓cytokines | 186 |
| HL-60b | ↑apoptosis | 113 |
| Jurkat and MOLT-4 T cellsb | ↑apoptosis | 80a |
| Human coronary artery endothelial cells | ↓adhesion molecules, migration, transcription factors, nitrative stress | 119a |
| Jurkat T cellsb | ↓cytokines, transcription factors | 55a |
| Human neutrophils | ↓migration | 195 |
| PBMCs | ↓indoleamine-2,3-dioxygenase (IDO), ↓cytokines |
142 |
| THP-1 cellsb | ↓IDO | 142 |
| PBMCs | ↑apoptosis | 114a |
| Human intestine | ↓proteins and nitric oxide | 96 |
| Human liver sinusoidal endothelial cells | ↓adhesion molecules | 118 |
| Human gingival mesenchymal stem cells | ↓inflammatory genes | 79 |
| Caco-2 cellsb | ↓phosphoproteins | 82a |
| Primary colonic explants | ↓cytokines | 82a |
| Human neutrophils | ↓ROS | 185 |
| Human PBMCs | ↓proliferation and cytokines | 146a |
| HaCaT human keratinocytesb | ↓cytokines | 84 |
| Human monocytes | ↑apoptosis | 115a |
| Human plasmacytoid dendritic cells | ↓CD83 expression in HIV+ dendritic cells | 134a |
Discussed in review.
Cell line.
ROS, reactive oxygen species.
Table 3.
Cannabidiol-Induced Immune Suppression by Animal Cell Type In Vitro
| Cell type | End-point(s) | References |
|---|---|---|
| B6C3F1 female splenocytes | ↓IL-2 | 196 |
| EL-4 T cellsa | ↑apoptosis | 80b |
| Mouse EOC-20 microglial cellsa | ↓proliferation | 89b |
| BALB/c male splenocytes | ↓IL-4 and IFN-γ | 140b |
| B6C3F1 female splenocytes | ↓IL-2 and IFN-γ | 55b |
| BALB/c male thymocytes and EL-4 T cellsa | ↑apoptosis | 150b |
| BALB/c male splenocytes | ↑apoptosis | 151b |
| Sprague-Dawley rat microglial cellsc | ↓adenosine uptake, | 91b |
| ↓TNF-α | ||
| BV-2 cellsa | ↓cytokines, ↓NF-κB activation | 147b |
| Mouse brain slicesc | ↓cytokines | 90b |
| Rat male astroglial cells | ↓gliosis | 92b |
| C57BL/6 male Kupffer cells | ↓TNF-α | 118 |
| BALB/c microglial cellsc | ↑apoptosis | 156b |
| BV-2 cellsa | ↓oxidative stress, ↓Ccl2 | 159 |
| MOG-specific female T cells | ↓IL-17A and IL-6 | 144b |
| Mouse brain endothelial cellsa | ↓VCAM-1 and leukocyte adhesion | 164b |
| Rat astrocytesc | ↓Ccl2 | 164b |
| RAW cellsa | ↓TNF-α | 148 |
| MOG-specific female T cells | ↓cytokines | 143b |
| Rat male splenocytes and mesenteric lymph nodes | ↓proliferation and cytokines | 146b |
| Primary mouse male and female microglial cells | ↓activation | 97b |
| BV-2 cellsa | Alteration of circadian rhythm-associated genes | 197 |
| BV-2 cellsa | alteration of miRNAs | 161b |
| C57BL/6 or BALB/c female splenocytes | ↓proliferation and cytokines | 57 |
Cell line.
Discussed in review.
Sex not stated for cells derived from animals (or in the case of primary microglial cell isolates, not determined in newborn animals).
IFN-γ, interferon-gamma; IL, interleukin; miRNA, microRNA; MOG, myelin oligodendrocyte glycoprotein; NF-κB, nuclear factor-κB; TNF-α, tumor necrosis factor-alpha; VCAM-1, vascular cell adhesion molecule-1.
Table 4.
Cannabidiol-Induced Immune Suppression in Animals In Vivo
| Model | Disease model | Route, dose range, and duration/frequencya | Major effects | Reference |
|---|---|---|---|---|
| Male CD-1 mice | sRBC | i.p. | Modest ↓antibody production | 155 |
| 25 mg/kg | ||||
| 4 days | ||||
| Male DBA/2 mice | Collagen-induced arthritis | i.p. or oral | ↓disease, ↓TNF-α and IFN-γ | 139b |
| 2.5–20 mg/kg for i.p. | ||||
| 5–50 mg/kg for oral | ||||
| 10 days | ||||
| Male ICR mice | Carrageenan-induced inflammation | ethosome (CBD in ethosomal gel) | ↓inflammation | 198 |
| 100 mg of ethosomal CBD (3%) | ||||
| Male Wistar rats | Carrageenan-induced inflammation | Oral | ↓disease, ↓prostaglandin (PGE2) | 199 |
| 5–40 mg/kg | ||||
| 3 days | ||||
| Female NOD mice | Diabetes | i.p. | ↓disease incidence, ↓IL-12, TNF-α and IFN-γ, ↑IL-4 | 123 |
| 5 mg/kg/day | ||||
| 10–20 injections | ||||
| Female C57BL/6 mice | EL-4 leukemia growth | i.p. | ↑apoptosis of tumor cells | 80b |
| 12.5 or 25 mg/kg once | ||||
| Male Wistar rats | Sciatic nerve pain or CFA-induced inflammation | Oral | ↓pain, ↓TNF-α, ↓prostaglandin (PGE2) | 88 |
| 2.5–20 mg/kg | ||||
| 7 days | ||||
| Male Sprague-Dawley rats | Ischemia-reperfusion injury (myocardial) | i.p. | Modest ↓infarct size, ↓TNF-α | 200 |
| 5 mg/kg | ||||
| twice | ||||
| C57BL/6J micec | Aβ inflammation | i.p. | ↓IL-1β, ↓iNOS | 117 |
| 2.5 or 10 mg/kg | ||||
| 7 days | ||||
| Male BALB/c mice | Ovalbumin (asthma) | i.p. | ↓serum antibodies, ↓IL-2, IL-4, and IFN-γ | 140b |
| 5–20 mg/kg | ||||
| once | ||||
| Male ddY mice | Focal cerebral ischemia | i.p. | ↓infarct size, ↓neutrophil MPO activity | 129b |
| 3 mg/kg | ||||
| various times surrounding occlusion | ||||
| Female NOD mice | Diabetes | i.p. | ↓disease incidence, ↓IL-6 and IL-12, ↑IL-4 and IL-10 | 124b |
| 5 mg/kg/day | ||||
| 5 injections per week for 4 weeks | ||||
| Female B6C3F1 mice | sRBC | Oral | Modest ↓antibody production | 55b |
| 25–100 mg/kg/day | ||||
| 5 days | ||||
| Male ICR mice | DNBS colitis | i.p. | ↓inflammation, ↓colon weight:length ratio, ↓iNOS, IL-1β, ↑IL-10 | 95b |
| 1–10 mg/kg | ||||
| 6 days | ||||
| Male Wistar rats | None | i.p. | ↓blood leukocytes and lymphocytes, ↓B, T and CTL cells, ↑NK and NKT cells | 201 |
| 2.5 or 5 mg/kg | ||||
| 14 days | ||||
| Male CD-1 mice | Diabetes | i.p. or i.n. | ↓diabetic pain, ↓density of microglial cells | 81b |
| 0.1–2 mg/kg i.n. | ||||
| 1–20 mg/kg i.p. | ||||
| 3 months | ||||
| Male C57BL/6 mice | Streptozotocin-induced diabetes | i.p. | ↓disease, ↓TNF-α, NF-κB activity, ICAM-1, VCAM-1, iNOS, p-p38, p-JNK, ↑p-AKT | 120b |
| 1–20 mg/kg | ||||
| 11 weeks | ||||
| Male Wistar rats | TNBS colitis | i.p. | Modest ↓disease, ↓colonic contractions, ↓neutrophil MPO activity | 130 |
| 5–20 mg/kg | ||||
| once | ||||
| Male Wistar rats | Cecal ligation and puncture | i.p. | ↑disease survival | 184 |
| 2.5–10 mg/kg | ||||
| once or up to 9 days | ||||
| Female Sabra mice | Hepatic encephalopathy (bile duct ligation) | i.p. | Improved disease-associated cognitive impairments, ↓TNF-α | 202 |
| 5 mg/kg | ||||
| 4 weeks | ||||
| Male BALB/c mice | Ovalbumin (footpad) | i.p. | ↓footpad swelling, ↓TNF-α and IFN-γ, ↑IL-10 | 188 |
| 1–10 mg/kg | ||||
| 5 days | ||||
| Male Swiss OFI mice | LPS i.p. | i.p. | ↓mast cell infiltration, macrophage activation marker, ↓TNF-α | 96 |
| 10 mg/kg | ||||
| twice | ||||
| Female C57BL/6 mice | Experimental autoimmune hepatitis | i.p. | ↓hepatic inflammation, ↓IL-2, TNF-α, IFN-γ, IL-6, IL-17A, IL-12, MCP-1 (CCL-2), and eotaxin, ↑MDSCs | 86b |
| 10–50 mg/kg | ||||
| once | ||||
| Male C57BL/6 mice | Ischemia reperfusion injury (liver) | i.p. | ↓hepatic inflammation, ↓MIP-1α, ICAM, MIP-2, TNF-α, NF-κB activity, ICAM-1, iNOS, p-p38, p-JNK | 118 |
| 3 or 10 mg/kg | ||||
| once | ||||
| C57BL/6 micec | LPS i.v. | i.v. | ↓vasodilation, leukocyte margination, and extravasation, ↓COX-2, TNF-α, and iNOS | 121 |
| 1 or 3 mg/kg | ||||
| once | ||||
| Male C57BL/6 mice | LPS-induced pulmonary inflammation | i.p. | ↓BALF lymphocytes, macrophages, and neutrophils, ↓TNF-α, IL-6, MCP-1 (CCL-2), and MIP-2 | 125b |
| 0.3–80 mg/kg | ||||
| once | ||||
| Male Wistar rats | Meningitis (Streptococcus pneumoniae) | i.p. | Improved disease-associated cognitive impairments, ↓TNF-α | 203 |
| 2.5–10 mg/kg | ||||
| once or up to 9 days | ||||
| C57BL/6 micec | Cerulein (pancreatitis) | i.p. | ↓disease, ↓TNF-α and IL-6, ↓neutrophil MPO | 128b |
| 0.5 mg/kg | ||||
| twice | ||||
| Newborn pigsc | Hypoxia-ischemic brain injury | i.v. | neuroprotection, ↓IL-1 | 59b |
| 1 mg/kg | ||||
| once | ||||
| Male Wistar rats | Ovalbumin (asthma) | i.p. | ↓TNF-α, IL-6, IL-4, IL-5, and IL-13 | 127b |
| 5 mg/kg | ||||
| twice | ||||
| Male C57BL/6 mice | LPS-induced pulmonary inflammation | i.p. | ↓inflammation, ↓BALF lymphocytes, macrophages, and neutrophils, ↓TNF-α, IL-6, MCP-1 (CCL-2), and MIP-2 | 132 |
| 20–80 mg/kg | ||||
| once | ||||
| Female C57BL/6 mice | None | i.p. | ↑MDSCs | 136b |
| 20 mg/kg | ||||
| once | ||||
| Female C57BL/6 mice | Malaria (Plasmodium berghei) | i.p. | ↓IL-6 and TNF-α | 204 |
| 30 mg/kg | ||||
| 3–5 days | ||||
| Male Sprague Dawley rats | Freund's Adjuvant (osteoarthritis) | Transdermal | ↓inflammation, ↓TNF-α | 205 |
| 0.6–63.2 mg/day | ||||
| 4 days | ||||
| Male ICR mice | DNBS Colitis | i.p. or orald | ↓colon weight:length ratio, ↓neutrophil MPO | 131 |
| 5–30 mg/kg for i.p. 10–60 mg/kg oral | ||||
| 3 days | ||||
| Female NOD mice | Type 1 diabetes | i.p. | ↓disease | 206 |
| 5 mg/kg | ||||
| 5 injections/week for 10 weeks | ||||
| Male A/J mice | Experimental autoimmune myocarditis | i.p. | ↓disease, ↓lymphocyte populations in heart, ↓IL-6, IFN-γ, IL-1β, and MCP-1 (CCL-2) | 126b |
| 10 mg/kg | ||||
| 46 days | ||||
| Male Wistar rats | Middle cerebral artery occlusion | i.c.v. | ↓infarct size | 149 |
| 50–200 ng/rat | ||||
| 5 days | ||||
| Male Wistar rats | Middle cerebral artery occlusion | i.c.v. | ↓infarct size, ↓TNF-α | 207 |
| 50–200 ng/rat | ||||
| 5 days | ||||
| Male Wistar rats | Sodium monoiodoacetate (osteoarthritis) | Intra-arterial | ↓pain, ↓rolling and adherent leukocytes, ↓joint nerve demyelination | 83b |
| 100–300 μg/rat | ||||
| multiple doses | ||||
| Female C57BL/6 mice | Alcoholic liver disease | i.p. | ↓liver damage, ↓neutrophils, ↓TNF-α, MIP-1, IFN-γ, IL-1β, and MCP-1 (CCL-2) | 185 |
| 5 or 10 mg/kg | ||||
| 11 days | ||||
| Male and female dogs | Osteoarthritis | Orale | ↓pain | 208 |
| 2 and 8 mg/kg | ||||
| every 12 h for 4 weeks | ||||
| Male Wistar rats | Ulcerative tongue lesion | i.p. | ↓inflammation | 209 |
| 5 or 10 mg/kg | ||||
| 3 or 7 days | ||||
| Female C57BL/6 mice | Spinal cord contusion | i.p. | ↓spinal cord CD4 T cells, ↓IL-23A, IL-23R, IFN-γ, CXCL9, CLCL11, NOS2, and IL-10 | 189 |
| 1.5 mg/kg | ||||
| 1 and 24 h after injury, on day 3, then twice/week up to 10 weeks | ||||
| Male Sprague-Dawley rats | Carrageenan-induced inflammation | Oral | ↓hyperalgesia | 210 |
| 100 or 10,000 μg/kg | ||||
| once | ||||
| Male Swiss mice | Haloperidol-induced inflammation | i.p | ↓IL-1β and TNF-α, ↑IL-10 | 97b |
| 60 mg/kg | ||||
| twice/day up to 21 days | ||||
| Male BALB/c mice | Corneal inflammation | Topical (ophthalmic) | ↓pain, ↓neutrophils | 56b |
| 3% or 5% | ||||
| Male ICR mice | Ischemia-reperfusion injury (kidney) | i.p. | ↓kidney injury, ↓TH17 cells, ↑Tregs and Treg17 cells | 152b |
| 10 mg/kg | ||||
| once | ||||
| Female C57BL/6 and BALB/c mice | Syngeneic or allogeneic bone marrow transplant | i.p. | ↓lymphocyte recovery | 57 |
| 5 mg/kg | ||||
| every other day for 2 weeks | ||||
| BALB/c mice | Ovalbumin (asthma) | i.p. | ↓airway resistance; ↓IL-4, IL-5, IL-13, and eotaxin | 60b |
| 5 or 10 mg/kg | ||||
| three times at time of ovalbumin challenge |
Maximum duration or frequency noted; some studies in the article might have been shorter.
Discussed in review.
Sex not stated.
CBD or CBD BDS (botanical drug substance).
CBD oil.
CBD, Cannabidiol; DNBS, dinitrobenzene sulfonic acid; iNOS, inducible nitric oxide synthase; i.n. intranasal; i.p., intraperitoneal; JNK, c-jun N-terminal kinase; LPS, lipopolysaccharide; MDSCs, myeloid-derived suppressor cells; MPO, myeloperoxidase; sRBC, sheep red blood cell; TNBS, 2,4,6-trinitrobenzene sulfonic acid; Treg, regulatory T cell.
CBD effects and mechanisms of immune suppression in innate cells
One of earliest effects reported with CBD was in human mononuclear cells,111,112 in which TNF-α, IFN-γ, and IL-1α were all suppressed (0.01–20 μg/mL CBD or 0.03–64 μM CBD). Later studies focused on human monocytic cells revealed that CBD can induce apoptosis in either HL-60 (1–8 μg/mL CBD or 3.2–26 μM CBD)113 or primary human monocytic cells (1–16 μM CBD).114,115 Macrophages are also targets, although they have been studied more commonly in animal models. Peritoneal macrophages were used early on to demonstrate that CBD (3 μg/mL or 10 μM) targets nitric oxide,116 and this has also been a well-studied target of suppression by CBD in many tissues and cell types. The mechanism by which CBD suppressed nitric oxide involves suppression of endothelial87 or inducible nitric oxide synthase (iNOS)58,95,117–121 in response to various inflammatory stimuli. iNOS is known to be regulated by the transcription factor nuclear factor-κB (NF-κB),122 which is comprised of p65 and other proteins, and becomes active after degradation of the inhibitory protein, IκB. Decreased expression of iNOS by CBD correlated with stimulation of the inhibitory IκBα protein and inhibition of NF-κB p65 protein expression.119,120 Using peritoneal macrophages from diabetic mice stimulated ex vivo with LPS revealed that macrophages isolated from CBD-treated mice did not produce as much TNF-α or IL-6 as macrophages isolated from vehicle-treated mice.123,124 A direct effect of CBD decreasing macrophage numbers in the bronchoalveolar lavage fluid was shown following intranasal LPS administration to induce pulmonary inflammation.125 There was also decreased expression of F4/80 (a marker of macrophages) mRNA expression by CBD in heart tissue in experimental autoimmune myocarditis.126 Although this study identified CBD only affecting F4/80 mRNA expression as opposed to F4/80 cell surface staining, it does suggest a novel target (i.e., heart tissue) of CBD in a relatively understudied autoimmune model.
IL-6 is a proinflammatory cytokine produced by many cell types, predominantly innate cells. Many studies have shown that circulating IL-6 is readily inhibited by CBD in inflammatory models, including diabetes,124 asthma,127 pancreatitis,128 and hepatitis.86 CBD treatment in vivo resulted in lower IL-6 production in peritoneal macrophages stimulated ex vivo with LPS,124 in the pancreas in acute pancreatitis,128 and in bronchoalveolar lavage fluid in LPS-induced pulmonary inflammation.125
There have been some reports that CBD alters neutrophil function. Compromised MPO activity by CBD has been studied in several tissues, including brain,129 colon,130,131 lung,125,128,132 and pancreas.128 Interestingly, in the pulmonary inflammation studies with LPS, neutrophil cell counts in the bronchoalveolar lavage fluid were also decreased by CBD compared to LPS.125,132 Together, the results suggest that CBD's mechanism for neutrophil suppression involves both decreased numbers of neutrophils and compromised MPO activity.
There are two recent studies focused on CpG stimulation of IFN-α production from human plasmacytoid dendritic cells.133,134 While these studies are focused primarily on THC and other CB2 agonists, CBD was also used (1–10 μM) and did not affect IFN-α production.133,134 It was interesting, however, that CBD suppressed the CD83 dendritic cell activation marker on dendritic cells derived from HIV+, but not healthy, individuals.134 Reduction in dendritic cell CD83 signaling can compromise T cell function,135 although additional studies using CBD in human dendritic cells and T cells are needed to establish the consequences of CBD-induced reduction in CD83 on HIV+ dendritic cells.
Another mechanism by which CBD controls immune function is induction of regulatory cells. MDSC are innate, myeloid cells that possess the ability to control immune responses. Hegde et al. demonstrated that CBD induced CD11b+Gr-1+ MDSCs in the liver in a mouse hepatitis model.86 Importantly, the isolated MDSCs were functional, that is, they suppressed proliferation of responder T cells ex vivo and improved liver function when administered before hepatitis induction.86 CBD-induced MDSCs from the peritoneal cavity were able to attenuate inflammation in response to LPS.136 In the experimental autoimmune encephalomyelitis (EAE) model, CBD induced MDSCs in the peritoneal cavity, but decreased the infiltration of MDSCs in the spinal cord and brain.137 CBD-induced MDSCs from the peritoneal cavity were able to attenuate responder T cell proliferation ex vivo and attenuate EAE disease when administered in vivo.137
CBD effects and mechanisms of immune suppression in lymphocytes
The area in which most of the effects of CBD in the immune system have been studied is T cells. Early studies examining rosette formation in response to sheep red blood cells (sRBCs) (generally considered to be a T cell response) revealed that CBD (1 and 100 μM) reduced this response.138 Phytohemagglutinin (PHA)-stimulated IFN-γ production in T cells has also been shown to be inhibited by CBD (0.01–20 μg/mL or 0.03–64 μM).111,112 Other studies have provided further evidence that T cell-produced IFN-γ is a critical target of CBD suppression. CBD inhibited IFN-γ production from lymph node cells isolated from arthritic mice stimulated ex vivo with collagen,139 and from splenocytes isolated from NOD mice stimulated ex vivo with ConA.123,124 IFN-γ production from splenocytes isolated from untreated mice was suppressed by CBD following ex vivo stimulation with phorbol 12-myristate 13-acetate/ionomycin (PMA/Io).140 In the latter study, a 1-h exposure of CBD to the mice was meant to mimic the time for CBD distribution before receiving antigen sensitization with ovalbumin to induce asthma-like disease.140 Thus, CBD's ability to compromise various cytokines at the time of antigen sensitization might suggest that CBD affects primary activation of T cells, as has been suggested as part of the mechanism for other cannabinoids, such as THC.141 Indeed, we have shown that a 30-min pre-treatment with CBD (0.1–20 μM) suppressed IFN-γ production in mouse splenocytes in response to PMA/Io or anti-CD3/CD28.55 In these studies, it was shown that the mechanism by which CBD suppressed IFN-γ occurred at the level of transcription and that two important transcription factors for IFN-γ, activator protein-1 (AP-1) and nuclear factor of activated T cells (NFAT), were inhibited by CBD, suggesting a transcriptional mechanism for suppression.55 CBD-induced suppression (0.1–10 μg/mL or 0.3–32 μM) of Ifng mRNA expression was shown using PHA-stimulated human PBMCs.142 Given the many reports that IFN-γ seems to be a sensitive target of suppression by CBD, it was surprising that Ifng mRNA was not affected by CBD (5 μM) using encephalitogenic T cells stimulated by antigen-presenting cells (APCs) and myelin oligodendrocyte glycoprotein peptide (MOG35–55) in vitro.143 However, CBD did inhibit expression of IFN-γ receptor 1 and CBD increased several IFN-γ-responsive genes known to attenuate T cell proliferation.143 Overall, the data reveal that an important part of CBD's action in the immune system is its ability to affect IFN-γ in multiple ways. Not only did CBD directly suppress IFN-γ production through a transcriptional mechanism under several conditions55,142 but also suppressed IFN-γ receptor expression, and increased IFN-γ-induced genes that subsequently attenuate other immune targets.143
A few other T cell-derived cytokines have been shown to be targets of CBD. As noted above, IL-6 is a critical target of CBD in many cells and tissues,82,84,86,97,125–128,132 many of which are innate cells. However, IL-6 was also suppressed by CBD (5 μM) using encephalitogenic T cells stimulated by APCs and MOG35–55 in vitro,144 and “IL-6 signaling” as a critical pathway suppressed by CBD.143 Interestingly, “IL-17 signaling” was also identified as a critical pathway suppressed by CBD (5 μM) in T cells in vitro.143 It should be noted that IL-6 promotes the differentiation of TH17 cells,145 so the simultaneous suppression of IL-6 and IL-17A by CBD is consistent with CBD suppressing TH17 cell differentiation. Indeed, CBD (1–20 μg/mL or 3.2–64 μM) suppressed IL-17A production in human CD3+ T cells (derived from healthy patients or patients with MS or nonseminomatous germ cell tumors) stimulated ex vivo with PMA/Io.146 Taken together with the data described in innate cells above, it is clear that CBD's action in inflammation and immune function involves suppression of cytokine production from many different cell types.
The ability of CBD to suppress transcription factors such as NFAT, AP-1, and NF-κB likely accounts for its widespread suppression of many cytokines.74,82,118–120,147–149 Some of the studies suggest that CBD increased, or perhaps stabilized, expression of IκB as part of the mechanism by which it suppresses NF-κB.119,120,147 CBD (4 μM) stimulated IκB-α expression in high glucose-treated human coronary artery endothelial cells.119 CBD induced expression of IκB-α in heart tissue from diabetic mice in vivo120 and in LPS-stimulated microglial cells in vitro (CBD 1–10 μM).147 It is interesting that NF-κB activity has not yet been identified as a target in T cells, suggesting that CBD-mediated suppression of NF-κB plays a bigger role in mediating anti-inflammatory effects in non-T cells.
Certainly, some of the dysregulation of these transcription factors is the result of suppression of various kinases upstream of their activation. Extracellular signal-regulated kinase (ERK), c-jun N-terminal kinase (JNK), and p38 MAPKs have all been identified as targets of suppression by CBD in various cell types.74,80–82,118,120 Of these reports, one was conducted in human T cells.80 In these studies, CBD (5 μM) was shown to suppress expression of total and phosphorylated p38 at the 16-h timepoint following CBD treatment. The authors also showed that the CBD-mediated inhibition of phosphorylated p38 was reversed by SR1445328 or tocopherol, suggesting that CBD acts through CB2 and that the mechanism of suppression involves reactive oxygen species (ROS) production.80
Although well studied in cancer cell lines and primary tumor tissue, CBD-mediated apoptosis is also a contributor to the immune suppressive mechanism. Initially CBD-induced apoptosis in T cells was described in Jurkat and MOLT4 human T cells.80 In the same study, McKallip et al. observed increased apoptosis of mouse lymphoma cells injected into, and then recovered from, the peritoneal cavity of mice that were treated with CBD.80 Since then, there has been a series of studies characterizing the mechanisms by which CBD induced apoptosis in mouse immune cells. CBD (1–16 μM) was shown to induce apoptosis in mouse thymocytes and EL-4 T cells.150 The same group demonstrated that CBD (1–16 μM) induced apoptosis in mouse splenocytes, including assessment of CBD-induced apoptosis by cell type (B220+ B cells and CD4+ and CD8+ T cells).151 In both studies, CBD increased ROS, and CBD-mediated apoptosis was attenuated by N-acetylcysteine.150,151 Wu et al. further demonstrated that the CBD increased ROS-activated caspase-8 to mediate apoptosis.151 In follow-up studies in human monocytes, Wu et al. noted that CBD (1–16 μM) readily induced apoptosis, but that the effect of CBD on apoptosis was lost if the monocytes were pre-cultured for 72 h.114 The authors suggest that the differential responsiveness to CBD was due to an increase in antioxidant capacity in cultured cells, which is a thought consistent with the mechanism by which CBD induced apoptosis in mouse lymphocytes.150,151 CBD-induced apoptosis (1–16 μM CBD) in human monocytes was due to a cascade of intracellular events, including opening of the mitochondrial permeability transition pore, depolarization of the mitochondrial membrane potential, oxidation of a lipid in the mitochondrial inner membrane, and mitochondrial ROS generation, leading to cytochrome C release.115 Thus, this latest study demonstrates a critical role of the mitochondria in CBD-induced apoptosis.
Another important mechanism by which CBD acts to control immune responses is through regulatory T cell (Treg) induction. In the ConA model of hepatitis, CBD modestly enhanced Tregs in the liver as quantified by CD4+Foxp3+ cells.86 A confirmation of in vivo induced Tregs by CBD was noted in an ischemia-reperfusion injury model in the kidney, in which CBD returned the disease-induced reduction in CD3+Foxp3+ cells to baseline.152 Interestingly, in the ischemia-reperfusion kidney model, CBD also induced “TReg17 cells,” which were defined as CD3+Foxp3+CCR6+STAT3+.152 It has been suggested that Treg17 cells help control a TH17 response. In vitro, CBD (5 μM) induced a CD69+LAG+ population in CD4+CD25− cells, which were identified as one type of regulatory cell, and induced Il10 mRNA expression.153 We showed in vitro that CBD (1–15 μM) induced functional CD4+CD25+Foxp3+ T cells under conditions of suboptimal stimulation and that Il10 mRNA expression was induced.154
There are only a few studies in which B cells are identified as targets of CBD. CBD given at 25 mg/kg by intraperitoneal (i.p.) injection modestly reduced the sRBC-induced plaque-forming cells, which is a measure of antibody production.155 We conducted a similar study using oral administration of CBD and also found modest inhibition of antibody production.55 Other studies have shown that CBD robustly inhibited the sRBC-induced antibody production in vitro,55 suppressed ovalbumin-induced IgM, IgG1, and IgG2a in an in vivo asthma model,140 and reduced expression of activation markers such as major histocompatibility complex II, CD25, and CD69, on B cells.153 CBD has also been shown to induce apoptosis in B cells.151 Overall, the results suggest that B cells can be targets of suppression by CBD.
CBD-induced neuroprotection by suppression of microglial cell activation
There is no doubt that many of the mechanisms already identified for innate cells and lymphocytes also account for CBD's ability to decrease microglial cell activation. CBD (1–16 μM) induced apoptosis in microglial cells,156 which was dependent on activation of caspase 8 and 9, and was reversed in the presence of an agent that depletes cholesterol and disrupts lipid rafts.156 These results suggest that CBD-induced apoptosis is dependent on lipid raft formation,156 and indeed, this observation was confirmed by another group in BV-2 microglial cells.157
BV-2 microglial cells have been used as a model in several articles, in which detailed transcriptional effects of CBD have been evaluated.147,157–159 The mechanisms contributing to CBD (10 μM)-mediated suppression of LPS-stimulated cytokine production in microglial cells includes decreased activation of the Toll/IL-1 receptor domain-containing adapter-inducing IFN-β (TRIF)/IFN-β/signal transducer and activator of transcription (STAT) signaling pathway.147 CBD suppressed LPS-stimulated NF-κB activation, and induced LPS-stimulated STAT3 activation, which has been shown to suppress NF-κB activation.147 CBD (10 μM) was shown to affect several genes involved in lipid metabolism in unstimulated BV-2 cells,157 which might account for CBD's ability to increase anandamide58,65–67,84,157,160 or could account for CBD's dependence on lipid raft formation to induce apoptosis156,157 Follow-up studies examining CBD's effects in unstimulated BV-2 cells demonstrated that CBD (10 μM) alters zinc homeostasis, oxidative stress, and glutathione levels in microglial cells.158,159 A recent study demonstrated that CBD alters microRNA (miRNA) expression,161 and two of the CBD miRNA targets identified are discussed. First, CBD downregulated miR146-a, which acts as a negative regulator of inflammation, in both resting and LPS-stimulated cells, thereby contributing to CBD's ability to downregulate proinflammatory cytokines.161 Second, CBD upregulated miR-34a, which has several roles in cell survival, such as cell cycle, apoptosis, and differentiation.161 These results show that CBD-induced alterations in miRNA expression are involved in the mechanism by which CBD suppresses immune function.
In vivo, CBD has been shown to decrease microglial accumulation in the spinal cord in diabetic mice,81 which might contribute to attenuation of neuropathic pain, and CBD decreased haloperidol-induced activation of reactive microglial cells.97 CBD's suppression of TNF-α production from microglial cells in vitro was mediated by A2A adenosine receptors in EOC-20 mouse microglial cells (0.5–5 μm)89 or rat retinal microglial cells (1 μM).91
CBD Effects in Autoimmune Disease Models
EAE and MS
The immunosuppressive and neuroprotective mechanisms of CBD make it an ideal therapeutic candidate for MS, a neurodegenerative autoimmune disease of the CNS that affects ∼2.5 million people worldwide. The average age of onset is around 30 years, and symptoms can vary greatly for each patient based on the lesion locations within the CNS.162 Two models frequently used in the laboratory environment to study MS are the EAE and Theiler's murine encephalomyelitis virus (TMEV) models, and an increasing number of studies have shown promising results with CBD using these models (Table 5). In 2011, Kozela et al. successfully demonstrated that CBD (5 mg/kg i.p.) administered at the onset of disease attenuated clinical disease, microglial activation, and T cell infiltration into the CNS in EAE, and that CBD reduced T cell proliferation in vitro.163 CBD showed similar effects in the TMEV model, in which Mecha et al. demonstrated that CBD (5 mg/kg i.p.) administered for the first 10 days following disease onset reduced clinical disease and neuroinflammation by decreasing microglial activation and immune cell trafficking signals in the CNS.164 Use of MOG35–55-specific T cells isolated from EAE mice in vitro has also been extremely vital to determining how CBD might be affecting T cells in these and other disease models. As outlined above, in the T cell section, in vitro CBD treatment of MOG35–55-specific T cells co-cultured with APCs with CBD suppressed IL-17A and IL-6 production, suggesting CBD suppressed TH17 development; however, production of Il10 mRNA was potentiated with CBD treatment, suggesting that CBD may have multiple suppressive mechanisms.144 In vitro treatment of MOG35–55-specific T cells with CBD induced a Treg with a CD4+CD25−LAG3+CD69+ phenotype, promoted upregulation of anergy-associated genes, such as Lag3, Erg2, and Il10, and altered the balance between STAT3 and STAT5 activation.153 In another study, CBD administered during disease onset increased the number of functional MDSCs present within the peritoneal cavity, decreased neuroinflammation, and reduced IL-17A and IFN-γ in the serum.137 When splenocytes from these mice were restimulated ex vivo, the CBD-treated mice had significantly decreased levels of IL-17A and IFN-γ, and increased levels of IL-10 in the supernatants.137 Finally, a recent study using an adoptive transfer EAE model showed a reduction in neuroinflammation, demyelination, and axonal damage with CBD treatment during disease onset.165 Adoptive transfer EAE is a variation of the EAE model induced by transfer of encephalitogenic T cells into naive mice, which allows experiments performed with this model to focus more on the T cell-specific mechanisms of pathogenesis in the EAE model. From the accumulation of data, it is obvious that multiple immune cell types, proinflammatory and anti-inflammatory, within the EAE model are modulated by CBD, but overall, CBD appears to downregulate proinflammatory pathways and upregulate anti-inflammatory pathways in the EAE model.
Table 5.
Cannabidiol Effects in Experimental Autoimmune Encephalomyelitis
| Model | Approach | Dosage/concentration | Effects | Reference |
|---|---|---|---|---|
| EAE in ABH | In vivo | In vivo: 0.5–25 mg/kg i.p. | No effects | 211 |
| EAE in C57BL/6 | In vivo and in vitro | In vivo: 5 mg/kg i.p. in vitro: 1, 5, and 10 μM | in vivo: ↓disease severity, ↓T cell infiltration into the CNS, ↓microglial activation, ↓axonal damage in vitro: ↓T cell proliferation | 163a |
| TMEV in SJL/J | In vivo and in vitro | In vivo: 5 mg/kg i.p. in vitro: 1 and 5 μM | in vivo:↓disease severity, ↓leukocyte infiltration into the CNS, ↓microglial activation, ↓CCL2 (MCP-1), ↓CCL5, ↓IL-1β ↓TNF-α in vitro: ↓sVCAM-1 production from endothelial cells, ↓leukocyte adhesion, ↓CCL2 (MCP-1) | 164a |
| MOG35–55-specific T cells from EAE mice | In vitro | In vitro: 0.1, 1, and 5 μM | in vitro: ↓IL-17A, ↓IL-6, ↑IL-10 | 144a |
| MOG35–55-specific T cells from EAE mice | In vitro | In vitro: 5 μM | in vitro:↓IL-17A, ↓IL-6, ↑IL-10, ↑EGR2, ↑CD4+CD25−CD69+LAG3+ phenotype, ↑STAT5/↓STAT3, ↓B cell activity, ↑Nfatc1, ↑Casp4, ↑Cdkn1a, ↑Icos, ↑Fas | 153a |
| EAE in C57BL/6 | In vivo | In vivo: 5 mg/kg i.p. | in vivo: ↓disease severity, ↓leukocyte invasion, ↓demyelination, ↓TNF-α, ↓IFN-γ, ↓IL-17A | 212 |
| EAE in C57BL/6 | In vivo | In vivo: 10 mg/kg i.p. | in vivo: ↓disease severity, ↓FAS ligand, ↓ERK phosphorylation, ↓Caspase-3 activity, ↓Bax/↑Bcl-2, ↓p53-p21 activation, ↓apobody formation | 166a |
| MOG35–55-specific T cells from EAE mice | In vitro | In vitro: 5 μM | in vitro: ↓IL-1β, ↓IL-3, ↓Xcl1 mRNA, ↓IL-12a mRNA, ↑Dusp6 mRNA, ↑Btla mRNA, ↑Lag3 mRNA, ↑Irf4 mRNA, ↑IL-10 mRNA | 143a,b |
| EAE in C57BL/6 | In vivo | In vivo: 10 mg/kg i.p. | in vivo: ↓disease severity, ↓leukocyte infiltration, ↑PI3k/Akt/mTOR phosphorylation, ↑S6k phosphorylation, ↑BDNF expression, ↑PPAR-γ, ↓IFN-γ, ↓IL-17A, ↓JNK activity, ↓p38 MAP kinase activity | 167a |
| Adoptive Transfer EAE in C57BL/6 | In vivo and in vitro | In vivo: 5–50 mg/kg i.p in vitro: 1, 5 & 10 μM | in vivo: ↓disease severity, ↓leukocyte invasion, ↓demyelination, ↓axonal damage, ↓microglial activation, ↓CB2 receptor expression in CNS, ↓GPR55 receptor expression in CNS in vitro: ↓Cell viability, ↓IL-6, ↑apoptosis, ↑ROS | 165a |
| EAE in C57BL/6 | In vivo | In vivo: 20 mg/kg i.p | in vivo: ↓disease severity, ↓leukocyte invasion, ↓IL-17A, ↓IFN-γ, ↓RORγT, ↓T-bet, ↑IL-10, ↑MDSC ex vivo: ↓IL-17A, ↓IFN-γ, ↑IL-10 | 137a |
Discussed in review.
See140 for a full description of the microarray results.
CNS, central nervous system; EAE, experimental autoimmune encephalomyelitis; ERK, extracellular signal-regulated kinase; STAT, signal transducer and activator of transcription; TMEV, Theiler's murine encephalomyelitis virus.
In addition to its immunomodulatory effects, CBD's neuroprotective properties in the EAE model also indicate its therapeutic potential in MS. CBD has been shown to decrease the activation of proapoptotic proteins, such as caspase-3 and Bax,166 and to counteract the effects of EAE on the PI3K/Akt/mTOR pathway, JNK, and p38 MAP kinases in the CNS of EAE mice.167 Interestingly, the study from Giacoppo et al. found the PI3k/Akt/mTOR pathway was upregulated in neural tissues when EAE mice were treated with CBD.167 However, Kozela et al.153 observed a reduction in the activation of Akt in vitro in MOG35–55-reactive T cells, which might suggest a differential role for CBD's effects on the PI3K/Akt/mTOR pathway in various cell types.
Despite the growing number of studies involving the neuroprotective and immunosuppressive effects of CBD, the majority of human studies involving cannabinoids and MS have been focused on the use of THC:CBD mixtures, with a particular focus on Sativex. Clinical studies that have been performed have shown that Sativex has beneficial effects on spasticity, mobility, bladder function, and pain in MS patients, and is well tolerated22,25,28,31,168–175; however, there has been little focus on the neuroprotective and immunosuppressive effects of THC:CBD mixtures in MS, and so it is difficult to say at this point if the successful results observed with CBD in the animal models of MS will be observed in MS patients. For a more complete review on the effects of Sativex in MS, see Zettl et al.176
Other autoimmune disease states
CBD has been shown to attenuate experimental autoimmune hepatitis,86 experimental autoimmune myocarditis,126 and autoimmune diabetes123,124 in mice. There are few studies done with CBD only in human autoimmune diseases. In human patients, CBD at 20 mg/kg did not reduce clinical Crohn's disease.177 However, CBD is effective at attenuating intestinal inflammation in other models of human inflammatory bowel disease,82,96 so it is possible that CBD could be effective at higher doses. Indeed, CBD as Epidolex for epilepsy in children is being used as high as 20 mg/kg, but CBD doses as high as 300 mg/kg have been evaluated, and have not exhibited significant adverse effects.178
CBD Immune Enhancement Effects
Much of the data support the fact that CBD is immune suppressive and anti-inflammatory; however, there have been a few reports over the years that CBD has produced some immune enhancing effects (Table 6). The potential for CBD, and other cannabinoids, to produce immune enhancing effects has been attributed to differences in hormetic (i.e., biphasic) responses depending on CBD concentration/dose, cell culture conditions, including serum presence and/or percent, immune stimulant, and magnitude of cellular activation in response to the immune stimulant. Indeed, studies from our laboratory and others have shown that CBD either enhanced or suppressed cytokine production (IL-2 and IFN-γ) in response to relatively low or high degree of immune stimulation, respectively.154,179,180 The mechanism for the differential responsiveness likely involves alterations in intracellular calcium, as CBD increases intracellular calcium in mouse splenocytes regardless of the increase of intracellular calcium produced by the immune stimulant.179 In addition, the differential cytokine production was correlated with nuclear expression of the NFAT transcription factor,179 which is calcium responsive. Interestingly, CBD's ability to increase intracellular calcium also likely accounts for some of the other enhancing effects, including stimulation of neutrophil degranulation,181 chemotaxis,182 and mast cell/basophil activation.183
Table 6.
Immune Enhancement by Cannabidiol
| Cell type/model | In vivo | Effect | Reference |
|---|---|---|---|
| Male human subjects | X | ↑antibody response | 213 |
| Rabbit neutrophilsa | ↑neutrophil degranulation | 181b | |
| Female Hartley guinea pigs | X | ↑skin sensitization | 214 |
| Female B6C3F1 mouse splenocytes | ↑IL-2 production | 180 | |
| Mouse BV-2 microglial cellsc | ↑chemotaxis | 182b | |
| Male Swiss mouse peritoneal macrophages | ↑IL-12 production | 187 | |
| Male Swiss mouse peritoneal macrophages | X | ↑IL-12 production (stimulated ex vivo) | 187 |
| Rat RBL-2H3 mast cellsc | ↑mast cell/basophil activation | 183b | |
| Female B6C3F1 and C57BL/6 mouse splenocytes | ↑IL-2 and IFN-γ production | 179b | |
| Female C57BL/6 mice | X | ↑LPS-induced pulmonary inflammation | 191b |
| Mouse BV-2 microglial cellsc, mouse RAW264.7 macrophage cellsc, rat HAPI microglial cellsc, male C57BL/6 microglial cells | ↑phagocytosis | 105 | |
| Female C57BL/6 splenocytes | ↑IL-2 production | 154b |
Sex not stated.
Discussed in review.
Cell line.
In addition to the ones listed in Table 6, there are a few well-studied end-points for which CBD treatment has produced opposing effects, one of which is apoptosis. As described in detail above, part of the mechanism by which CBD produces immune suppression is induction of apoptosis; however, there are a few studies in which CBD has inhibited inflammation-induced apoptosis.118,120 Interestingly, the reports of CBD on oxidative stress are different across studies, with some articles identifying CBD as an antioxidant,59,118,184,185 and others reporting that CBD induces oxidative stress.114,115,150,151 CBD-mediated effects on IL-10 production also revealed opposing effects when compared across several studies.95,123,124,127,148,186–189 Effects of CBD on IL-10 could be tied to regulatory cell production (i.e., Tregs or MDSCs) or changes in T cell subpopulations.
Although it is not entirely clear why CBD produces opposing effects for many end-points, a critical part in understanding the mechanisms of CBD involves investigation of the consequences of all the changes. Let us consider two examples, the first of which was introduced in the section on cytokines (IFN-γ). We know that IFN-γ is a critical target of suppression by CBD,55,111,112,123,124,139,140,142,143 but there are some conditions under which CBD had no effect143 or enhanced it.179 Perhaps under some conditions, the CBD-induced enhancement of IFN-γ would increase the IFN-γ-responsive genes that attenuate T cell proliferation, as suggested by Kozela et al.143 Thus, although CBD increased a “proinflammatory” cytokine, its consequence could be immune suppression. The second example is IL-2, which was enhanced under conditions of low-level T cell stimulation.154,179,180 We recently showed that CBD, by producing IL-2 under some conditions, contributed to the appropriate milieu to drive Treg induction,154 again demonstrating that enhancement of seemingly proinflammatory cytokines by CBD still resulted in immune suppression.
Conclusions, Challenges, and Knowledge Gaps
Considering all the studies conducted on immune responses and inflammation, the data overwhelmingly demonstrate that CBD is immune suppressive and anti-inflammatory (Fig. 1). Critical targets of suppression include cytokines such as TNF-α, IFN-γ, IL-6, IL-1β, IL-2, IL-17A, and chemokines, such as CCL-2. The overall mechanism of CBD involves direct suppression of target cells, such as effector T cells and microglial cells, through suppression of kinase cascades and various transcription factors. An example of this is CBD-induced suppression of phosphorylated p38, leading to compromised AP-1 or NF-κB activity. Direct suppression of target cells also includes induction of IκB, which could contribute to decreased NF-κB activity. The involvement of regulatory cell induction by CBD is also a major part of the mechanism by which CBD controls immune responses, and CBD has been shown to induce Tregs and MDSCs. Finally, CBD-induced apoptosis is likely an important mechanism in many target cells.
FIG. 1.
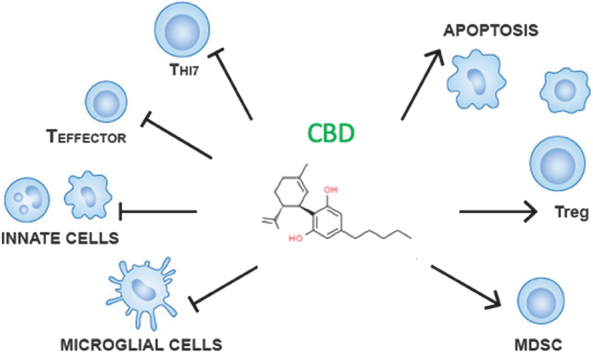
Summary of CBD's mechanisms of immune suppression. Overall, CBD's immune system suppression is mediated by direct inhibition of various cell types (microglial, innate, and T cells) and induction of apoptosis and regulatory cells (Tregs and MDSCs). CBD, Cannabidiol; MDSCs, myeloid-derived suppressor cells; Treg, regulatory T cell.
It is often argued that the concentrations/doses at which CBD acts in vitro/in vivo are high. However, it should be noted that CBD is highly lipophilic and subject to first-pass metabolism after oral dosing.190 In fact, we have shown that in mice at 6 h after oral CBD at 75 mg/kg/day for 3 days resulted in plasma CBD levels of ∼40 ng/mL and were not detectable by 24 h.191 This is ∼0.12 μM CBD, which is on the lower end of concentrations typically used in vitro to evaluate the effects of CBD as detailed in this review. On the other hand, recent data obtained in one human clinical trial that was used to support the indication of CBD as Epidiolex in epilepsy studies showed that plasma CBD levels were as high as 400 ng/mL following 20 mg/kg/day dosing for 22 days.192 This concentration is ∼1.2 μM CBD, which is a more common concentration used in vitro at which CBD effects are observed. These two studies in mice and humans191,192 suggest that the doses and concentrations of CBD used in many of the studies in this review are appropriate. There are still limitations on our knowledge about CBD dosing and plasma levels and how those relate to immune modulation. Some of these limitations might be clarified with many of the planned clinical trials with CBD in the coming years. Specifically related to immune effects of CBD, there is a planned randomized, open-label interventional study assessing CBD and THC on immune cell activation in HIV+ patients.193 Importantly, this trial will evaluate dose escalation of relatively high CBD doses compared to THC; the dose escalation for CBD will go from 45–225 mg/kg/day over a 5-week period and then maintain the highest dose for an additional 7 weeks.193
In addition to the need for more data on CBD dosing and pharmacokinetics, this broad summary of immune and inflammatory effects of CBD revealed a number of data gaps that should be addressed. First, identification of the receptor(s) through which CBD acts in the immune system remains a critical question. An important part of this question is whether CBD-induced FAAH inhibition generates anandamide metabolites that bind to various receptors to mediate some of the immune suppressive or anti-inflammatory effects of CBD. Coupled with the observation that some of the effects of CBD can be attenuated with PPAR-γ antagonists,92–98 the possibility exists that CBD-mediated anandamide production drives the subsequent production of (yet unidentified) metabolites that activate PPAR-γ. Another critical determination needed for many of the receptor studies is identification of the cell type(s) on which the receptors are expressed, which are mediating the CBD effects. Second, although combined cannabinoids were not a major focus of this review, it will be critical to determine the CBD contribution to immune function compromise in cannabis and/or combined pharmaceuticals such as Sativex. Third, there are still several cell types for which little data exist, notably B cells and dendritic cells. Even in the rich CBD-T cell literature, several well-established targets have not been extensively studied in T cells. In fact, there are limited data examining CBD's effects on various T cell subsets. Fourth, increasing our understanding of CBD's effects in response to a variety of immune stimuli and degrees of immune stimulation will help in interpreting effects of CBD in humans and other outbred species that are naturally exposed to a variety of pathogens. Thus, the last identified knowledge gap is the need for increased studies on the effects of CBD in human and veterinary immune responses. These include well-controlled studies considering differences with administration routes, dose, and pharmacokinetics.
Acknowledgments
The authors acknowledge ChemSpider for CBD structure. CSID:559095 (accessed 16:45, Oct 11, 2018). The authors also acknowledge Ms. Moyim Kim at Mississippi State University for help with illustrations. Research supported by Mississippi State University College of Veterinary Medicine.
Abbreviations Used
- AP-1
activator protein-1
- APC
antigen-presenting cell
- CBD
Cannabidiol
- CNS
central nervous system
- DNBS
dinitrobenzene sulfonic acid
- EAE
experimental autoimmune encephalomyelitis
- ERK
extracellular signal-regulated kinase
- FAAH
fatty acid amide hydrolase
- IFN-γ
interferon-gamma
- IL
interleukin
- i.n.
intranasal
- iNOS
inducible nitric oxide synthase
- i.p.
intraperitoneal
- JNK
c-jun N-terminal kinase
- LPS
lipopolysaccharide
- MDSCs
myeloid-derived suppressor cells
- miRNA
microRNA
- MOG
myelin oligodendrocyte glycoprotein
- MPO
myeloperoxidase
- MS
multiple sclerosis
- NFAT
nuclear factor of activated T cells
- NF-κB
nuclear factor-κB
- PHA
phytohemagglutinin
- PMA/Io
phorbol 12-myristate 13-acetate/ionomycin
- PPAR-γ
peroxisome proliferator-activated receptor gamma
- ROS
reactive oxygen species
- sRBC
sheep red blood cell
- STAT
signal transducer and activator of transcription
- THC
Δ9-tetrahydrocannabinol
- TMEV
Theiler's murine encephalomyelitis virus
- TNBS
2,4,6-trinitrobenzene sulfonic acid
- TNF-α
tumor necrosis factor-alpha
- Treg
regulatory T cell
- TRPV1
transient receptor potential vanilloid 1
- VCAM-1
vascular cell adhesion molecule-1
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Funding provided by Mississippi State University College of Veterinary Medicine.
Cite this article as: Nichols JM, and Kaplan BLF (2020) Immune responses regulated by cannabidiol, Cannabis and Cannabinoid Research 5:1, 12–31, DOI: 10.1089/can.2018.0073.
References
- 1. Mechoulam R, Hanus L. Cannabidiol: an overview of some chemical and pharmacological aspects. Part I: chemical aspects. Chem Phys Lipids. 2002;121:35–43 [DOI] [PubMed] [Google Scholar]
- 2. Mechoulam R, Shvo Y. Hashish. I. The structure of cannabidiol. Tetrahedron. 1963;19:2073–2078 [DOI] [PubMed] [Google Scholar]
- 3. Hanus LO, Tchilibon S, Ponde DE, et al. Enantiomeric cannabidiol derivatives: synthesis and binding to cannabinoid receptors. Org Biomol Chem. 2005;3:1116–1123 [DOI] [PubMed] [Google Scholar]
- 4. Gaoni Y, Mechoulam R. Isolation, structure, and partial suntheis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647 [Google Scholar]
- 5. Institute of Medicine; Joy JE, Watson SJ Jr., Benson JA. Marijuana and medicine: assessing the science base. National Academies Press: Washington, DC, 1999 [PubMed] [Google Scholar]
- 6. Tanasescu R, Rog D, Constantinescu CS. A drug discovery case history of “delta-9-tetrahydrocannabinol, cannabidiol.” Expert Opin Drug Discov. 2011;6:437–452 [DOI] [PubMed] [Google Scholar]
- 7. Guimaraes FS, Chiaretti TM, Graeff FG, et al. Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology (Berl). 1990;100:558–559 [DOI] [PubMed] [Google Scholar]
- 8. Zuardi AW, Cosme RA, Graeff FG, et al. Effects of ipsapirone and cannabidiol on human experimental anxiety. J Psychopharmacol. 1993;7:82–88 [DOI] [PubMed] [Google Scholar]
- 9. Moreira FA, Aguiar DC, Guimaraes FS. Anxiolytic-like effect of cannabidiol in the rat Vogel conflict test. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1466–1471 [DOI] [PubMed] [Google Scholar]
- 10. Campos AC, Guimaraes FS. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology (Berl). 2008;199:223–230 [DOI] [PubMed] [Google Scholar]
- 11. Crippa JA, Derenusson GN, Ferrari TB, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011;25:121–130 [DOI] [PubMed] [Google Scholar]
- 12. Bergamaschi MM, Queiroz RH, Chagas MH, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology. 2011;36:1219–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Almeida V, Levin R, Peres FF, et al. Cannabidiol exhibits anxiolytic but not antipsychotic property evaluated in the social interaction test. Prog Neuropsychopharmacol Biol Psychiatry. 2013;41:30–35 [DOI] [PubMed] [Google Scholar]
- 14. Fogaca MV, Campos AC, Coelho LD, et al. The anxiolytic effects of cannabidiol in chronically stressed mice are mediated by the endocannabinoid system: role of neurogenesis and dendritic remodeling. Neuropharmacology. 2018;135:22–33 [DOI] [PubMed] [Google Scholar]
- 15. De Gregorio D, McLaughlin RJ, Posa L, et al. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain. 2019;160:136–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Linares IM, Zuardi AW, Pereira LC, et al. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Braz J Psychiatry. 2019;41:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Britch SC, Wiley JL, Yu Z, et al. Cannabidiol-Delta(9)-tetrahydrocannabinol interactions on acute pain and locomotor activity. Drug Alcohol Depend. 2017;175:187–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bornheim LM, Kim KY, Li J, et al. Effect of cannabidiol pretreatment on the kinetics of tetrahydrocannabinol metabolites in mouse brain. Drug Metab Dispos. 1995;23:825–831 [PubMed] [Google Scholar]
- 19. Hlozek T, Uttl L, Kaderabek L, et al. Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur Neuropsychopharmacol. 2017;27:1223–1237 [DOI] [PubMed] [Google Scholar]
- 20. Nadulski T, Pragst F, Weinberg G, et al. Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Delta9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Ther Drug Monit. 2005;27:799–810 [DOI] [PubMed] [Google Scholar]
- 21. Blake DR, Robson P, Ho M, et al. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford). 2006;45:50–52 [DOI] [PubMed] [Google Scholar]
- 22. Collin C, Davies P, Mutiboko IK, et al. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol. 2007;14:290–296 [DOI] [PubMed] [Google Scholar]
- 23. Russo M, Naro A, Leo A, et al. Evaluating sativex(R) in neuropathic pain management: a clinical and neurophysiological assessment in multiple sclerosis. Pain Med. 2016;17:1145–1154 [DOI] [PubMed] [Google Scholar]
- 24. Nurmikko TJ, Serpell MG, Hoggart B, et al. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain. 2007;133:210–220 [DOI] [PubMed] [Google Scholar]
- 25. Rog DJ, Nurmikko TJ, Young CA. Oromucosal delta9-tetrahydrocannabinol/cannabidiol for neuropathic pain associated with multiple sclerosis: an uncontrolled, open-label, 2-year extension trial. Clin Ther. 2007;29:2068–2079 [DOI] [PubMed] [Google Scholar]
- 26. Selvarajah D, Gandhi R, Emery CJ, et al. Randomized placebo-controlled double-blind clinical trial of cannabis-based medicinal product (Sativex) in painful diabetic neuropathy: depression is a major confounding factor. Diabetes Care. 2010;33:128–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Collin C, Ehler E, Waberzinek G, et al. A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res. 2010;32:451–459 [DOI] [PubMed] [Google Scholar]
- 28. Kavia RB, De Ridder D, Constantinescu CS, et al. Randomized controlled trial of Sativex to treat detrusor overactivity in multiple sclerosis. Mult Scler. 2010;16:1349–1359 [DOI] [PubMed] [Google Scholar]
- 29. Novotna A, Mares J, Ratcliffe S, et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex((R))), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol. 2011;18:1122–1131 [DOI] [PubMed] [Google Scholar]
- 30. Schoedel KA, Chen N, Hilliard A, et al. A randomized, double-blind, placebo-controlled, crossover study to evaluate the subjective abuse potential and cognitive effects of nabiximols oromucosal spray in subjects with a history of recreational cannabis use. Hum Psychopharmacol. 2011;26:224–236 [DOI] [PubMed] [Google Scholar]
- 31. Notcutt W, Langford R, Davies P, et al. A placebo-controlled, parallel-group, randomized withdrawal study of subjects with symptoms of spasticity due to multiple sclerosis who are receiving long-term Sativex(R) (nabiximols). Mult Scler. 2012;18:219–228 [DOI] [PubMed] [Google Scholar]
- 32. Johnson JR, Lossignol D, Burnell-Nugent M, et al. An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J Pain Symptom Manage. 2013;46:207–218 [DOI] [PubMed] [Google Scholar]
- 33. Langford RM, Mares J, Novotna A, et al. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol. 2013;260:984–997 [DOI] [PubMed] [Google Scholar]
- 34. Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage. 2014;47:166–173 [DOI] [PubMed] [Google Scholar]
- 35. Flachenecker P, Henze T, Zettl UK. Nabiximols (THC/CBD oromucosal spray, Sativex(R)) in clinical practice—results of a multicenter, non-interventional study (MOVE 2) in patients with multiple sclerosis spasticity. Eur Neurol. 2014;71:271–279 [DOI] [PubMed] [Google Scholar]
- 36. Marinelli L, Balestrino M, Mori L, et al. A randomised controlled cross-over double-blind pilot study protocol on THC:CBD oromucosal spray efficacy as an add-on therapy for post-stroke spasticity. BMJ Open. 2017;7:e016843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Riva N, Mora G, Soraru G, et al. Safety and efficacy of nabiximols on spasticity symptoms in patients with motor neuron disease (CANALS): a multicentre, double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2019;18:155–164 [DOI] [PubMed] [Google Scholar]
- 38. Porter BE, Jacobson C. Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav. 2013;29:574–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hussain SA, Zhou R, Jacobson C, et al. Perceived efficacy of cannabidiol-enriched cannabis extracts for treatment of pediatric epilepsy: a potential role for infantile spasms and Lennox-Gastaut syndrome. Epilepsy Behav. 2015;47:138–141 [DOI] [PubMed] [Google Scholar]
- 40. Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15:270–278 [DOI] [PubMed] [Google Scholar]
- 41. Sands TT, Rahdari S, Oldham MS, et al. Long-term safety, tolerability, and efficacy of cannabidiol in children with refractory epilepsy: results from an expanded access program in the US. CNS Drugs. 2019;33:47–60 [DOI] [PubMed] [Google Scholar]
- 42. Thiele EA, Marsh ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391:1085–1096 [DOI] [PubMed] [Google Scholar]
- 43. Hess EJ, Moody KA, Geffrey AL, et al. Cannabidiol as a new treatment for drug-resistant epilepsy in tuberous sclerosis complex. Epilepsia. 2016;57:1617–1624 [DOI] [PubMed] [Google Scholar]
- 44. Serra I, Scheldeman C, Bazelot M, et al. Cannabidiol modulates phosphorylated rpS6 signalling in a zebrafish model of Tuberous Sclerosis Complex. Behav Brain Res. 2019;363:135–144 [DOI] [PubMed] [Google Scholar]
- 45. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175:225–231 [DOI] [PubMed] [Google Scholar]
- 46. Reithmeier D, Tang-Wai R, Seifert B, et al. The protocol for the Cannabidiol in children with refractory epileptic encephalopathy (CARE-E) study: a phase 1 dosage escalation study. BMC Pediatr. 2018;18:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Corroon J, Kight R. Regulatory status of cannabidiol in the United States: a perspective. Cannabis Cannabinoid Res. 2018;3:190–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The health effects of cannabis and cannabinoids: the current state of evidence and recommendataions for research. National Academies Press, Washington, DC, 2017 [Google Scholar]
- 49. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2016 National Survey on Drug Use and Health (HHS Publication No. SMA 17–5044, NSDUH Series H-52). 2017. Available at: https://www.samhsa.gov/data/data-we-collect/nsduh-national-survey-drug-use-and-health (accessed August25, 2019)
- 50. Hoffenberg EJ, McWilliams S, Mikulich-Gilbertson S, et al. Cannabis oil use by adolescents and young adults with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2019;68:348–352 [DOI] [PubMed] [Google Scholar]
- 51. Armour M, Sinclair J, Chalmers KJ, et al. Self-management strategies amongst Australian women with endometriosis: a national online survey. BMC Complement Altern Med. 2019;19:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sekar K, Pack A. Epidiolex as adjunct therapy for treatment of refractory epilepsy: a comprehensive review with a focus on adverse effects. F1000Res. 2019;8:F1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Matsuda LA, Lolait SJ, Brownstein MJ, et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564 [DOI] [PubMed] [Google Scholar]
- 54. Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65 [DOI] [PubMed] [Google Scholar]
- 55. Kaplan BL, Springs AE, Kaminski NE. The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT). Biochem Pharmacol. 2008;76:726–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thapa D, Cairns EA, Szczesniak AM, et al. The cannabinoids delta(8)THC, CBD, and HU-308 act via distinct receptors to reduce corneal pain and inflammation. Cannabis Cannabinoid Res. 2018;3:11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Khuja I, Yekhtin Z, Or R, et al. Cannabinoids reduce inflammation but inhibit lymphocyte recovery in murine models of bone marrow transplantation. Int J Mol Sci. 2019;20:E668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. de Filippis D, Iuvone T, d'amico A, et al. Effect of cannabidiol on sepsis-induced motility disturbances in mice: involvement of CB receptors and fatty acid amide hydrolase. Neurogastroenterol Motil. 2008;20:919–927 [DOI] [PubMed] [Google Scholar]
- 59. Pazos MR, Mohammed N, Lafuente H, et al. Mechanisms of cannabidiol neuroprotection in hypoxic-ischemic newborn pigs: role of 5HT(1A) and CB2 receptors. Neuropharmacology. 2013;71:282–291 [DOI] [PubMed] [Google Scholar]
- 60. Vuolo F, Abreu SC, Michels M, et al. Cannabidiol reduces airway inflammation and fibrosis in experimental allergic asthma. Eur J Pharmacol. 2019;843:251–259 [DOI] [PubMed] [Google Scholar]
- 61. Laprairie RB, Bagher AM, Kelly ME, et al. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172:4790–4805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tham M, Yilmaz O, Alaverdashvili M, et al. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br J Pharmacol. 2019;176:1455–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Navarro G, Reyes-Resina I, Rivas-Santisteban R, et al. Cannabidiol skews biased agonism at cannabinoid CB1 and CB2 receptors with smaller effect in CB1-CB2 heteroreceptor complexes. Biochem Pharmacol. 2018;157:148–158 [DOI] [PubMed] [Google Scholar]
- 64. Martinez-Pinilla E, Varani K, Reyes-Resina I, et al. Binding and signaling studies disclose a potential allosteric site for cannabidiol in cannabinoid CB2 receptors. Front Pharmacol. 2017;8:744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bisogno T, Hanus L, De Petrocellis L, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. De Petrocellis L, Ligresti A, Moriello AS, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163:1479–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Elmes MW, Kaczocha M, Berger WT, et al. Fatty acid-binding proteins (FABPs) are intracellular carriers for Delta9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J Biol Chem. 2015;290:8711–8721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949 [DOI] [PubMed] [Google Scholar]
- 69. Facci L, Dal Toso R, Romanello S, et al. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc Natl Acad Sci U S A. 1995;92:3376–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Alhouayek M, Muccioli GG. COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol Sci. 2014;35:284–292 [DOI] [PubMed] [Google Scholar]
- 71. Zelasko S, Arnold WR, Das A. Endocannabinoid metabolism by cytochrome P450 monooxygenases. Prostaglandins Other Lipid Mediat. 2015;116–117:112–123 [DOI] [PubMed] [Google Scholar]
- 72. Alhamoruni A, Lee AC, Wright KL, et al. Pharmacological effects of cannabinoids on the Caco-2 cell culture model of intestinal permeability. J Pharmacol Exp Ther. 2010;335:92–102 [DOI] [PubMed] [Google Scholar]
- 73. Alhamoruni A, Wright KL, Larvin M, et al. Cannabinoids mediate opposing effects on inflammation-induced intestinal permeability. Br J Pharmacol. 2012;165:2598–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stanley CP, Hind WH, Tufarelli C, et al. Cannabidiol causes endothelium-dependent vasorelaxation of human mesenteric arteries via CB1 activation. Cardiovasc Res. 2015;107:568–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Campos AC, Ortega Z, Palazuelos J, et al. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: involvement of the endocannabinoid system. Int J Neuropsychopharmacol. 2013;16:1407–1419 [DOI] [PubMed] [Google Scholar]
- 76. Sartim AG, Guimaraes FS, Joca SR. Antidepressant-like effect of cannabidiol injection into the ventral medial prefrontal cortex-Possible involvement of 5-HT1A and CB1 receptors. Behav Brain Res. 2016;303:218–227 [DOI] [PubMed] [Google Scholar]
- 77. Hwang YS, Kim YJ, Kim MO, et al. Cannabidiol upregulates melanogenesis through CB1 dependent pathway by activating p38 MAPK and p42/44 MAPK. Chem Biol Interact. 2017;273:107–114 [DOI] [PubMed] [Google Scholar]
- 78. Capasso R, Borrelli F, Aviello G, et al. Cannabidiol, extracted from Cannabis sativa, selectively inhibits inflammatory hypermotility in mice. Br J Pharmacol. 2008;154:1001–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Libro R, Scionti D, Diomede F, et al. Cannabidiol modulates the immunophenotype and inhibits the activation of the inflammasome in human gingival mesenchymal stem cells. Front Physiol. 2016;7:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. McKallip RJ, Jia W, Schlomer J, et al. Cannabidiol-induced apoptosis in human leukemia cells: a novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol Pharmacol. 2006;70:897–908 [DOI] [PubMed] [Google Scholar]
- 81. Toth CC, Jedrzejewski NM, Ellis CL, et al. Cannabinoid-mediated modulation of neuropathic pain and microglial accumulation in a model of murine type I diabetic peripheral neuropathic pain. Mol Pain. 2010;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Couch DG, Tasker C, Theophilidou E, et al. Cannabidiol and palmitoylethanolamide are anti-inflammatory in the acutely inflamed human colon. Clin Sci (Lond). 2017;131:2611–2626 [DOI] [PubMed] [Google Scholar]
- 83. Philpott HT, O'Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain. 2017;158:2442–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Petrosino S, Verde R, Vaia M, et al. Anti-inflammatory properties of cannabidiol, a nonpsychotropic cannabinoid, in experimental allergic contact dermatitis. J Pharmacol Exp Ther. 2018;365:652–663 [DOI] [PubMed] [Google Scholar]
- 85. Iannotti FA, Hill CL, Leo A, et al. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci. 2014;5:1131–1141 [DOI] [PubMed] [Google Scholar]
- 86. Hegde VL, Nagarkatti PS, Nagarkatti M. Role of myeloid-derived suppressor cells in amelioration of experimental autoimmune hepatitis following activation of TRPV1 receptors by cannabidiol. PLoS One. 2011;6:e18281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Costa B, Giagnoni G, Franke C, et al. Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br J Pharmacol. 2004;143:247–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Costa B, Trovato AE, Comelli F, et al. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol. 2007;556:75–83 [DOI] [PubMed] [Google Scholar]
- 89. Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci U S A. 2006;103:7895–7900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Castillo A, Tolon MR, Fernandez-Ruiz J, et al. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol Dis. 2010;37:434–440 [DOI] [PubMed] [Google Scholar]
- 91. Liou GI, Auchampach JA, Hillard CJ, et al. Mediation of cannabidiol anti-inflammation in the retina by equilibrative nucleoside transporter and A2A adenosine receptor. Invest Ophthalmol Vis Sci. 2008;49:5526–5531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Esposito G, Scuderi C, Valenza M, et al. Cannabidiol reduces Abeta-induced neuroinflammation and promotes hippocampal neurogenesis through PPARgamma involvement. PLoS One. 2011;6:e28668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ramer R, Heinemann K, Merkord J, et al. COX-2 and PPAR-gamma confer cannabidiol-induced apoptosis of human lung cancer cells. Mol Cancer Ther. 2013;12:69–82 [DOI] [PubMed] [Google Scholar]
- 94. Scuderi C, Steardo L, Esposito G. Cannabidiol promotes amyloid precursor protein ubiquitination and reduction of beta amyloid expression in SHSY5YAPP+ cells through PPARgamma involvement. Phytother Res. 2014;28:1007–1013 [DOI] [PubMed] [Google Scholar]
- 95. Borrelli F, Aviello G, Romano B, et al. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J Mol Med (Berl). 2009;87:1111–1121 [DOI] [PubMed] [Google Scholar]
- 96. De Filippis D, Esposito G, Cirillo C, et al. Cannabidiol reduces intestinal inflammation through the control of neuroimmune axis. PLoS One. 2011;6:e28159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sonego AB, Prado DS, Vale GT, et al. Cannabidiol prevents haloperidol-induced vacuos chewing movements and inflammatory changes in mice via PPARgamma receptors. Brain Behav Immun. 2018;74:241–251 [DOI] [PubMed] [Google Scholar]
- 98. Hind WH, England TJ, O'Sullivan SE. Cannabidiol protects an in vitro model of the blood-brain barrier from oxygen-glucose deprivation via PPARgamma and 5-HT1A receptors. Br J Pharmacol. 2016;173:815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Russo EB, Burnett A, Hall B, et al. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–1043 [DOI] [PubMed] [Google Scholar]
- 100. Abdouh M, Storring JM, Riad M, et al. Transcriptional mechanisms for induction of 5-HT1A receptor mRNA and protein in activated B and T lymphocytes. J Biol Chem. 2001;276:4382–4388 [DOI] [PubMed] [Google Scholar]
- 101. Fajardo O, Galeno J, Urbina M, et al. Serotonin, serotonin 5-HT(1A) receptors and dopamine in blood peripheral lymphocytes of major depression patients. Int Immunopharmacol. 2003;3:1345–1352 [DOI] [PubMed] [Google Scholar]
- 102. Xu J, Zhang G, Cheng Y, et al. Hypomethylation of the HTR1A promoter region and high expression of HTR1A in the peripheral blood lymphocytes of patients with systemic lupus erythematosus. Lupus. 2011;20:678–689 [DOI] [PubMed] [Google Scholar]
- 103. Hernandez-Torres G, Enriquez-Palacios E, Mecha M, et al. Development of a fluorescent bodipy probe for visualization of the serotonin 5-HT1A receptor in native cells of the immune system. Bioconjug Chem. 2018;29:2021–2027 [DOI] [PubMed] [Google Scholar]
- 104. Qin N, Neeper MP, Liu Y, et al. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci. 2008;28:6231–6238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hassan S, Eldeeb K, Millns PJ, et al. Cannabidiol enhances microglial phagocytosis via transient receptor potential (TRP) channel activation. Br J Pharmacol. 2014;171:2426–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. De Petrocellis L, Orlando P, Moriello AS, et al. Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol (Oxf). 2012;204:255–266 [DOI] [PubMed] [Google Scholar]
- 107. De Petrocellis L, Vellani V, Schiano-Moriello A, et al. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J Pharmacol Exp Ther. 2008;325:1007–1015 [DOI] [PubMed] [Google Scholar]
- 108. Kathmann M, Flau K, Redmer A, et al. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:354–361 [DOI] [PubMed] [Google Scholar]
- 109. Ryberg E, Larsson N, Sjogren S, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Chiurchiu V, Lanuti M, De Bardi M, et al. The differential characterization of GPR55 receptor in human peripheral blood reveals a distinctive expression in monocytes and NK cells and a proinflammatory role in these innate cells. Int Immunol. 2015;27:153–160 [DOI] [PubMed] [Google Scholar]
- 111. Watzl B, Scuderi P, Watson RR. Marijuana components stimulate human peripheral blood mononuclear cell secretion of interferon-gamma and suppress interleukin-1 alpha in vitro. Int J Immunopharmacol. 1991;13:1091–1097 [DOI] [PubMed] [Google Scholar]
- 112. Watzl B, Scuderi P, Watson RR. Influence of marijuana components (THC and CBD) on human mononuclear cell cytokine secretion in vitro. Adv Exp Med Biol. 1991;288:63–70 [DOI] [PubMed] [Google Scholar]
- 113. Gallily R, Even-Chena T, Katzavian G, et al. Gamma-irradiation enhances apoptosis induced by cannabidiol, a non-psychotropic cannabinoid, in cultured HL-60 myeloblastic leukemia cells. Leuk Lymphoma. 2003;44:1767–1773 [DOI] [PubMed] [Google Scholar]
- 114. Wu HY, Chang AC, Wang CC, et al. Cannabidiol induced a contrasting pro-apoptotic effect between freshly isolated and precultured human monocytes. Toxicol Appl Pharmacol. 2010;246:141–147 [DOI] [PubMed] [Google Scholar]
- 115. Wu HY, Huang CH, Lin YH, et al. Cannabidiol induced apoptosis in human monocytes through mitochondrial permeability transition pore-mediated ROS production. Free Radic Biol Med. 2018;124:311–318 [DOI] [PubMed] [Google Scholar]
- 116. Coffey RG, Yamamoto Y, Snella E, et al. Tetrahydrocannabinol inhibition of macrophage nitric oxide production. Biochem Pharmacol. 1996;52:743–751 [DOI] [PubMed] [Google Scholar]
- 117. Esposito G, Scuderi C, Savani C, et al. Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression. Br J Pharmacol. 2007;151:1272–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Mukhopadhyay P, Rajesh M, Horvath B, et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic Biol Med. 2011;50:1368–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Rajesh M, Mukhopadhyay P, Batkai S, et al. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am J Physiol Heart Circ Physiol. 2007;293:H610–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Rajesh M, Mukhopadhyay P, Batkai S, et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol. 2010;56:2115–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ruiz-Valdepenas L, Martinez-Orgado JA, Benito C, et al. Cannabidiol reduces lipopolysaccharide-induced vascular changes and inflammation in the mouse brain: an intravital microscopy study. J Neuroinflammation. 2011;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kleinert H, Pautz A, Linker K, et al. Regulation of the expression of inducible nitric oxide synthase. Eur J Pharmacol. 2004;500:255–266 [DOI] [PubMed] [Google Scholar]
- 123. Weiss L, Zeira M, Reich S, et al. Cannabidiol lowers incidence of diabetes in non-obese diabetic mice. Autoimmunity. 2006;39:143–151 [DOI] [PubMed] [Google Scholar]
- 124. Weiss L, Zeira M, Reich S, et al. Cannabidiol arrests onset of autoimmune diabetes in NOD mice. Neuropharmacology. 2008;54:244–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ribeiro A, Ferraz-de-Paula V, Pinheiro ML, et al. Cannabidiol, a non-psychotropic plant-derived cannabinoid, decreases inflammation in a murine model of acute lung injury: role for the adenosine A(2A) receptor. Eur J Pharmacol. 2012;678:78–85 [DOI] [PubMed] [Google Scholar]
- 126. Lee WS, Erdelyi K, Matyas C, et al. Cannabidiol limits T cell-mediated chronic autoimmune myocarditis: implications to autoimmune disorders and organ transplantation. Mol Med. 2016;22:136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Vuolo F, Petronilho F, Sonai B, et al. Evaluation of serum cytokines levels and the role of cannabidiol treatment in animal model of asthma. Mediators Inflamm. 2015;2015:538670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Li K, Feng JY, Li YY, et al. Anti-inflammatory role of cannabidiol and O-1602 in cerulein-induced acute pancreatitis in mice. Pancreas. 2013;42:123–129 [DOI] [PubMed] [Google Scholar]
- 129. Hayakawa K, Mishima K, Nozako M, et al. Delayed treatment with cannabidiol has a cerebroprotective action via a cannabinoid receptor-independent myeloperoxidase-inhibiting mechanism. J Neurochem. 2007;102:1488–1496 [DOI] [PubMed] [Google Scholar]
- 130. Jamontt JM, Molleman A, Pertwee RG, et al. The effects of Delta-tetrahydrocannabinol and cannabidiol alone and in combination on damage, inflammation and in vitro motility disturbances in rat colitis. Br J Pharmacol. 2010;160:712–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Pagano E, Capasso R, Piscitelli F, et al. An orally active cannabis extract with high content in cannabidiol attenuates chemically-induced intestinal inflammation and hypermotility in the mouse. Front Pharmacol. 2016;7:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ribeiro A, Almeida VI, Costola-de-Souza C, et al. Cannabidiol improves lung function and inflammation in mice submitted to LPS-induced acute lung injury. Immunopharmacol Immunotoxicol. 2015;37:35–41 [DOI] [PubMed] [Google Scholar]
- 133. Henriquez JE, Crawford RB, Kaminski NE. Suppression of CpG-ODN-mediated IFNalpha and TNFalpha response in human plasmacytoid dendritic cells (pDC) by cannabinoid receptor 2 (CB2)-specific agonists. Toxicol Appl Pharmacol. 2019;369:82–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Henriquez JE, Rizzo MD, Schulz MA, et al. Delta9-tetrahydrocannabinol suppresses secretion of IFNalpha by plasmacytoid dendritic cells from healthy and HIV-infected individuals. J Acquir Immune Defic Syndr. 2017;75:588–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Prechtel AT, Steinkasserer A. CD83: an update on functions and prospects of the maturation marker of dendritic cells. Arch Dermatol Res. 2007;299:59–69 [DOI] [PubMed] [Google Scholar]
- 136. Hegde VL, Singh UP, Nagarkatti PS, et al. Critical role of mast cells and peroxisome proliferator-activated receptor gamma in the induction of myeloid-derived suppressor cells by marijuana cannabidiol in vivo. J Immunol. 2015;194:5211–5222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Elliott DM, Singh N, Nagarkatti M, et al. Cannabidiol attenuates experimental autoimmune encephalomyelitis model of multiple sclerosis through induction of myeloid-derived suppressor cells. Front Immunol. 2018;9:1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Cushman P., Jr. Cannabinols and the rosette forming properties of lymphocytes in vitro. Life Sci. 1976;19:875–885 [DOI] [PubMed] [Google Scholar]
- 139. Malfait AM, Gallily R, Sumariwalla PF, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 2000;97:9561–9566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Jan TR, Su ST, Wu HY, et al. Suppressive effects of cannabidiol on antigen-specific antibody production and functional activity of splenocytes in ovalbumin-sensitized BALB/c mice. Int Immunopharmacol. 2007;7:773–780 [DOI] [PubMed] [Google Scholar]
- 141. Karmaus PW, Chen W, Kaplan BL, et al. Delta9-tetrahydrocannabinol suppresses cytotoxic T lymphocyte function independent of CB1 and CB2, disrupting early activation events. J Neuroimmune Pharmacol. 2012;7:843–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Jenny M, Santer E, Pirich E, et al. Delta9-tetrahydrocannabinol and cannabidiol modulate mitogen-induced tryptophan degradation and neopterin formation in peripheral blood mononuclear cells in vitro. J Neuroimmunol. 2009;207:75–82 [DOI] [PubMed] [Google Scholar]
- 143. Kozela E, Juknat A, Gao F, et al. Pathways and gene networks mediating the regulatory effects of cannabidiol, a nonpsychoactive cannabinoid, in autoimmune T cells. J Neuroinflammation. 2016;13:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kozela E, Juknat A, Kaushansky N, et al. Cannabinoids decrease the th17 inflammatory autoimmune phenotype. J Neuroimmune Pharmacol. 2013;8:1265–1276 [DOI] [PubMed] [Google Scholar]
- 145. Zhou L, Ivanov II, Spolski R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974 [DOI] [PubMed] [Google Scholar]
- 146. Zgair A, Lee JB, Wong JCM, et al. Oral administration of cannabis with lipids leads to high levels of cannabinoids in the intestinal lymphatic system and prominent immunomodulation. Sci Rep. 2017;7:14542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kozela E, Pietr M, Juknat A, et al. Cannabinoids Delta(9)-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-kappaB and interferon-beta/STAT proinflammatory pathways in BV-2 microglial cells. J Biol Chem. 2010;285:1616–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Rajan TS, Giacoppo S, Iori R, et al. Anti-inflammatory and antioxidant effects of a combination of cannabidiol and moringin in LPS-stimulated macrophages. Fitoterapia. 2016;112:104–115 [DOI] [PubMed] [Google Scholar]
- 149. Khaksar S, Bigdeli MR. Correlation between cannabidiol-induced reduction of infarct volume and inflammatory factors expression in ischemic stroke model. Basic Clin Neurosci. 2017;8:139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Lee CY, Wey SP, Liao MH, et al. A comparative study on cannabidiol-induced apoptosis in murine thymocytes and EL-4 thymoma cells. Int Immunopharmacol. 2008;8:732–740 [DOI] [PubMed] [Google Scholar]
- 151. Wu HY, Chu RM, Wang CC, et al. Cannabidiol-induced apoptosis in primary lymphocytes is associated with oxidative stress-dependent activation of caspase-8. Toxicol Appl Pharmacol. 2008;226:260–270 [DOI] [PubMed] [Google Scholar]
- 152. Baban B, Hoda N, Malik A, et al. Impact of cannabidiol treatment on regulatory T-17 cells and neutrophil polarization in acute kidney injury. Am J Physiol Renal Physiol. 2018;315:F1149–F1158 [DOI] [PubMed] [Google Scholar]
- 153. Kozela E, Juknat A, Kaushansky N, et al. Cannabidiol, a non-psychoactive cannabinoid, leads to EGR2-dependent anergy in activated encephalitogenic T cells. J Neuroinflammation. 2015;12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Dhital S, Stokes JV, Park N, et al. Cannabidiol (CBD) induces functional Tregs in response to low-level T cell activation. Cell Immunol. 2017;312:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zimmerman S, Zimmerman AM, Cameron IL, et al. delta1-tetrahydrocannabinol, cannabidiol and cannabinol effects on the immune response of mice. Pharmacology. 1977;15:10–23 [DOI] [PubMed] [Google Scholar]
- 156. Wu HY, Goble K, Mecha M, et al. Cannabidiol-induced apoptosis in murine microglial cells through lipid raft. Glia. 2012;60:1182–1190 [DOI] [PubMed] [Google Scholar]
- 157. Rimmerman N, Juknat A, Kozela E, et al. The non-psychoactive plant cannabinoid, cannabidiol affects cholesterol metabolism-related genes in microglial cells. Cell Mol Neurobiol. 2011;31:921–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Juknat A, Rimmerman N, Levy R, et al. Cannabidiol affects the expression of genes involved in zinc homeostasis in BV-2 microglial cells. Neurochem Int. 2012;61:923–930 [DOI] [PubMed] [Google Scholar]
- 159. Juknat A, Pietr M, Kozela E, et al. Differential transcriptional profiles mediated by exposure to the cannabinoids cannabidiol and Delta9-tetrahydrocannabinol in BV-2 microglial cells. Br J Pharmacol. 2012;165:2512–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Watanabe K, Kayano Y, Matsunaga T, et al. Inhibition of anandamide amidase activity in mouse brain microsomes by cannabinoids. Biol Pharm Bull. 1996;19:1109–1111 [DOI] [PubMed] [Google Scholar]
- 161. Juknat A, Gao F, Coppola G, et al. miRNA expression profiles and molecular networks in resting and LPS-activated BV-2 microglia-effect of cannabinoids. PLoS One. 2019;14:e0212039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15:545–558 [DOI] [PubMed] [Google Scholar]
- 163. Kozela E, Lev N, Kaushansky N, et al. Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in C57BL/6 mice. Br J Pharmacol. 2011;163:1507–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Mecha M, Feliu A, Inigo PM, et al. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: a role for A2A receptors. Neurobiol Dis. 2013;59:141–150 [DOI] [PubMed] [Google Scholar]
- 165. Gonzalez-Garcia C, Torres IM, Garcia-Hernandez R, et al. Mechanisms of action of cannabidiol in adoptively transferred experimental autoimmune encephalomyelitis. Exp Neurol. 2017;298:57–67 [DOI] [PubMed] [Google Scholar]
- 166. Giacoppo S, Soundara Rajan T, Galuppo M, et al. Purified Cannabidiol, the main non-psychotropic component of Cannabis sativa, alone, counteracts neuronal apoptosis in experimental multiple sclerosis. Eur Rev Med Pharmacol Sci. 2015;19:4906–4919 [PubMed] [Google Scholar]
- 167. Giacoppo S, Pollastro F, Grassi G, et al. Target regulation of PI3K/Akt/mTOR pathway by cannabidiol in treatment of experimental multiple sclerosis. Fitoterapia. 2017;116:77–84 [DOI] [PubMed] [Google Scholar]
- 168. Coghe G, Pau M, Corona F, et al. Walking improvements with nabiximols in patients with multiple sclerosis. J Neurol. 2015;262:2472–2477 [DOI] [PubMed] [Google Scholar]
- 169. Lorente Fernandez L, Monte Boquet E, Perez-Miralles F, et al. Clinical experiences with cannabinoids in spasticity management in multiple sclerosis. Neurologia. 2014;29:257–260 [DOI] [PubMed] [Google Scholar]
- 170. Messina S, Solaro C, Righini I, et al. Sativex in resistant multiple sclerosis spasticity: discontinuation study in a large population of Italian patients (SA.FE. study). PLoS One. 2017;12:e0180651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Patti F, Messina S, Solaro C, et al. Efficacy and safety of cannabinoid oromucosal spray for multiple sclerosis spasticity. J Neurol Neurosurg Psychiatry. 2016;87:944–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Rog DJ, Nurmikko TJ, Friede T, et al. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65:812–819 [DOI] [PubMed] [Google Scholar]
- 173. Vaney C, Heinzel-Gutenbrunner M, Jobin P, et al. Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled, crossover study. Mult Scler. 2004;10:417–424 [DOI] [PubMed] [Google Scholar]
- 174. Wade DT, Makela P, Robson P, et al. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004;10:434–441 [DOI] [PubMed] [Google Scholar]
- 175. Zajicek J, Fox P, Sanders H, et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomised placebo-controlled trial. Lancet. 2003;362:1517–1526 [DOI] [PubMed] [Google Scholar]
- 176. Zettl UK, Rommer P, Hipp P, et al. Evidence for the efficacy and effectiveness of THC-CBD oromucosal spray in symptom management of patients with spasticity due to multiple sclerosis. Ther Adv Neurol Disord. 2016;9:9–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Naftali T, Mechulam R, Marii A, et al. Low-dose cannabidiol is safe but not effective in the treatment for crohn's disease, a randomized controlled trial. Dig Dis Sci. 2017;62:1615–1620 [DOI] [PubMed] [Google Scholar]
- 178. Leo A, Russo E, Elia M. Cannabidiol and epilepsy: rationale and therapeutic potential. Pharmacol Res. 2016;107:85–92 [DOI] [PubMed] [Google Scholar]
- 179. Chen W, Kaplan BL, Pike ST, et al. Magnitude of stimulation dictates the cannabinoid-mediated differential T cell response to HIVgp120. J Leukoc Biol. 2012;92:1093–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Jan TR, Kaminski NE. Role of mitogen-activated protein kinases in the differential regulation of interleukin-2 by cannabinol. J Leukoc Biol. 2001;69:841–849 [PubMed] [Google Scholar]
- 181. Naccache PH, Volpi M, Becker EL, et al. Cannabinoid induced degranulation of rabbit neutrophils. Biochem Biophys Res Commun. 1982;106:1286–1290 [DOI] [PubMed] [Google Scholar]
- 182. Walter L, Franklin A, Witting A, et al. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Giudice ED, Rinaldi L, Passarotto M, et al. Cannabidiol, unlike synthetic cannabinoids, triggers activation of RBL-2H3 mast cells. J Leukoc Biol. 2007;81:1512–1522 [DOI] [PubMed] [Google Scholar]
- 184. Cassol OJ Jr., Comim CM, Silva BR, et al. Treatment with cannabidiol reverses oxidative stress parameters, cognitive impairment and mortality in rats submitted to sepsis by cecal ligation and puncture. Brain Res. 2010;1348:128–138 [DOI] [PubMed] [Google Scholar]
- 185. Wang Y, Mukhopadhyay P, Cao Z, et al. Cannabidiol attenuates alcohol-induced liver steatosis, metabolic dysregulation, inflammation and neutrophil-mediated injury. Sci Rep. 2017;7:12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Srivastava MD, Srivastava BI, Brouhard B. Delta9 tetrahydrocannabinol and cannabidiol alter cytokine production by human immune cells. Immunopharmacology. 1998;40:179–185 [DOI] [PubMed] [Google Scholar]
- 187. Sacerdote P, Martucci C, Vaccani A, et al. The nonpsychoactive component of marijuana cannabidiol modulates chemotaxis and IL-10 and IL-12 production of murine macrophages both in vivo and in vitro. J Neuroimmunol. 2005;159:97–105 [DOI] [PubMed] [Google Scholar]
- 188. Liu DZ, Hu CM, Huang CH, et al. Cannabidiol attenuates delayed-type hypersensitivity reactions via suppressing T-cell and macrophage reactivity. Acta Pharmacol Sin. 2010;31:1611–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Li H, Kong W, Chambers CR, et al. The non-psychoactive phytocannabinoid cannabidiol (CBD) attenuates pro-inflammatory mediators, T cell infiltration, and thermal sensitivity following spinal cord injury in mice. Cell Immunol. 2018;329:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Samara E, Bialer M, Mechoulam R. Pharmacokinetics of cannabidiol in dogs. Drug Metab Dispos. 1988;16:469–472 [PubMed] [Google Scholar]
- 191. Karmaus PW, Wagner JG, Harkema JR, et al. Cannabidiol (CBD) enhances lipopolysaccharide (LPS)-induced pulmonary inflammation in C57BL/6 mice. J Immunotoxicol. 2013;10:321–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Devinsky O, Patel AD, Thiele EA, et al. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90:e1204–e1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Costiniuk CT, Saneei Z, Routy JP, et al. Oral cannabinoids in people living with HIV on effective antiretroviral therapy: CTN PT028-study protocol for a pilot randomised trial to assess safety, tolerability and effect on immune activation. BMJ Open. 2019;9:e024793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Fonseca BM, Correia-da-Silva G, Teixeira NA. Cannabinoid-induced cell death in endometrial cancer cells: involvement of TRPV1 receptors in apoptosis. J Physiol Biochem. 2018;74:261–272 [DOI] [PubMed] [Google Scholar]
- 195. McHugh D, Tanner C, Mechoulam R, et al. Inhibition of human neutrophil chemotaxis by endogenous cannabinoids and phytocannabinoids: evidence for a site distinct from CB1 and CB2. Mol Pharmacol. 2008;73:441–450 [DOI] [PubMed] [Google Scholar]
- 196. Kaplan BL, Rockwell CE, Kaminski NE. Evidence for cannabinoid receptor-dependent and -independent mechanisms of action in leukocytes. J Pharmacol Exp Ther. 2003;306:1077–1085 [DOI] [PubMed] [Google Scholar]
- 197. Lafaye G, Desterke C, Marulaz L, et al. Cannabidiol affects circadian clock core complex and its regulation in microglia cells. Addict Biol. 2018. [Epub ahead of print]; DOI: 10.1111/adb.12660 [DOI] [PubMed] [Google Scholar]
- 198. Lodzki M, Godin B, Rakou L, et al. Cannabidiol-transdermal delivery and anti-inflammatory effect in a murine model. J Control Release. 2003;93:377–387 [DOI] [PubMed] [Google Scholar]
- 199. Costa B, Colleoni M, Conti S, et al. Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:294–299 [DOI] [PubMed] [Google Scholar]
- 200. Durst R, Danenberg H, Gallily R, et al. Cannabidiol, a nonpsychoactive Cannabis constituent, protects against myocardial ischemic reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;293:H3602–H3607 [DOI] [PubMed] [Google Scholar]
- 201. Ignatowska-Jankowska B, Jankowski M, Glac W, et al. Cannabidiol-induced lymphopenia does not involve NKT and NK cells. J Physiol Pharmacol. 2009;60 Suppl 3:99–103 [PubMed] [Google Scholar]
- 202. Magen I, Avraham Y, Ackerman Z, et al. Cannabidiol ameliorates cognitive and motor impairments in bile-duct ligated mice via 5-HT1A receptor activation. Br J Pharmacol. 2010;159:950–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Barichello T, Ceretta RA, Generoso JS, et al. Cannabidiol reduces host immune response and prevents cognitive impairments in Wistar rats submitted to pneumococcal meningitis. Eur J Pharmacol. 2012;697:158–164 [DOI] [PubMed] [Google Scholar]
- 204. Campos AC, Brant F, Miranda AS, et al. Cannabidiol increases survival and promotes rescue of cognitive function in a murine model of cerebral malaria. Neuroscience. 2015;289:166–180 [DOI] [PubMed] [Google Scholar]
- 205. Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Lehmann C, Fisher NB, Tugwell B, et al. Experimental cannabidiol treatment reduces early pancreatic inflammation in type 1 diabetes. Clin Hemorheol Microcirc. 2016;64:655–662 [DOI] [PubMed] [Google Scholar]
- 207. Khaksar S, Bigdeli MR. Intra-cerebral cannabidiol infusion-induced neuroprotection is partly associated with the TNF-alpha/TNFR1/NF-small ka, CyrillicB pathway in transient focal cerebral ischaemia. Brain Inj. 2017;31:1932–1943 [DOI] [PubMed] [Google Scholar]
- 208. Gamble LJ, Boesch JM, Frye CW, et al. Pharmacokinetics, safety, and clinical efficacy of cannabidiol treatment in osteoarthritic dogs. Front Vet Sci. 2018;5:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Klein M, de Quadros De Bortolli J, Guimaraes FS, et al. Effects of cannabidiol, a Cannabis sativa constituent, on oral wound healing process in rats: clinical and histological evaluation. Phytother Res. 2018;32:2275–2281 [DOI] [PubMed] [Google Scholar]
- 210. Rock EM, Limebeer CL, Parker LA. Effect of cannabidiolic acid and (9)-tetrahydrocannabinol on carrageenan-induced hyperalgesia and edema in a rodent model of inflammatory pain. Psychopharmacology (Berl). 2018;235:3259–3271 [DOI] [PubMed] [Google Scholar]
- 211. Maresz K, Pryce G, Ponomarev ED, et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat Med. 2007;13:492–497 [DOI] [PubMed] [Google Scholar]
- 212. Rahimi A, Faizi M, Talebi F, et al. Interaction between the protective effects of cannabidiol and palmitoylethanolamide in experimental model of multiple sclerosis in C57BL/6 mice. Neuroscience. 2015;290:279–287 [DOI] [PubMed] [Google Scholar]
- 213. Shapiro CM, Orlina AR, Unger PJ, et al. Marihuana-induced antibody response. J Lab Clin Med. 1976;88:194–201 [PubMed] [Google Scholar]
- 214. Watson ES, Murphy JC, Turner CE. Allergenic properties of naturally occurring cannabinoids. J Pharm Sci. 1983;72:954–955 [DOI] [PubMed] [Google Scholar]


