Abstract
Introduction: Synthetic cannabinoids (SCs) are commonly found in preparations used as recreational drugs. Although severe adverse health effects are not generally associated with cannabis use, a rising number of studies document seizures and even death after SC use. In this study, a mouse model is used to investigate the hypothesis that SCs are more toxic than Δ9-tetrahydrocannabinol (THC), the principal psychoactive constituent of cannabis.
Materials and Methods: Beginning with the SCs, JWH-073 and AM-2201, dose–response curves were generated to find the dose of each drug that was similarly efficacious to 50 mg/kg THC. Mice were given daily intraperitoneal (IP) injections of vehicle, 50 mg/kg THC, 30 mg/kg JWH-073, or 1 mg/kg AM-2201 until tolerance to the antinociceptive and hypothermic effects was complete, and then were assessed for spontaneous and antagonist-precipitated withdrawal and potential organ damage. No differences in tolerance were noted, but AM-2201 showed more rearing in the spontaneous and antagonist-precipitated withdrawal phases than either vehicle or the other two drug treatments. Histopathological examination of these mice revealed no drug-induced lesions. In a subsequent set of experiments, various doses of THC, methanandamide (mAEA), and of a variety of SCs (HU-210, CP55940, JWH-073, AM-2201, and PB-22) were given IP, and convulsions and change in body temperature were quantified.
Discussion: The treatments yielded varying numbers of convulsions and a range of changes in body temperature. JWH-073 and AM-2201 produced significantly more convulsions than THC, HU-210, mAEA, or cannabidiol (CBD) (the latter two producing none). HU-210, CP55940, JWH-073, and mAEA produced greater hypothermia than THC or CBD. Convulsions and hypothermia induced by several agonists were prevented by pretreatment with a CB1 antagonist, but not a CB2 antagonist.
Conclusions: In agreement with human studies and case reports, this study found that SCs generally produced more seizures than THC. Of particular significance was the finding that mAEA produced far greater hypothermia than THC (similar to most SCs), but unlike the SCs and THC, produced no seizures.
Keywords: AM-2201, HU-210, JWH-073, methanandamide, PB-22, tetrahydrocannabinol
Introduction
It has been estimated that synthetic cannabinoids (SCs) are 30 times more likely than cannabis to result in the need for emergency medical treatment.1 Increased use of SCs, sold under brand names such as Spice and K2, and their apparent toxicity2 has led to an urgent need for in vivo toxicological studies.
There are several chemical classes of cannabinoid receptor agonists. The phytocannabinoids from Cannabis sativa3 include Δ9-tetrahydrocannabinol (THC) and related compounds such as cannabidiol (CBD). Although THC is an agonist for the cannabinoid CB1 receptor, CBD appears to be a negative allosteric modulator of this receptor.4,5 Synthetic CB1 agonists belong to different chemical classes, and include close analogs of THC such as HU-210, and more distantly related classes such as alkyl phenols (e.g., CP55940) and aminoalkylindoles (e.g., JWH-073).
The endogenous cannabinoids termed “endocannabinoids” are the arachidonic acid derivatives, arachidonoyl ethanolamide (AEA or anandamide), and 2-arachidonoyl glycerol (2-AG).6 Methanandamide (mAEA), a semisynthetic metabolism-resistant methyl analog of anandamide, has similar pharmacology to anandamide.7
In animals, activation of CB1 produces hypothermia, antinociception, decreased spontaneous activity, and catalepsy.8 In humans, cannabinoid effects on the psyche and perception include fatigue, euphoria, anxiety or anxiolysis, increased appetite, decreased nausea, amnesia, depersonalization, and hallucinations.9 Cannabinoid somatic effects include tachycardia, analgesia, xerostomia, conjunctivitis, decreased intraocular pressure, and unsteady gait.9 Recreational use of SCs is reported to cause seizures,10 an acute toxic symptom seen with increasing prevalence in hospital emergency rooms.2,11–13
Two recent studies showed that THC and the aminoalkylindoles, JWH-018 and AM-2201, produced seizures in mice. The first showed that seizures, recorded visually and by electroencephalogram (EEG), were induced by THC or JWH-018 and prevented by the CB1-selective antagonist, AM-251.14 The second study found that AM-2201 produced seizures, which were prevented by AM-251 or a metabotropic glutamate receptor antagonist, but not CB2 or vanilloid receptor antagonists.15 These studies concluded that cannabinoid-induced seizures are mediated by CB1, and antagonists such as AM-251 are potential treatments for acute cannabinoid toxicity.
This study extends the findings of previous studies to include additional agonists. It began by comparing JWH-073 and AM-2201 with THC. JWH-073 and AM-2201 are among the SCs found in products sold to the public and in patients admitted to the emergency room.16 This study then examined the seizure-inducing activity of these and additional cannabinoids representing a range of chemical classes (Fig. 1), and exhibiting a range of affinities and efficacies for activating G-proteins through CB1.17,18 It also confirmed the roles of cannabinoid CB1 receptors in mediating seizure activity.
FIG. 1.
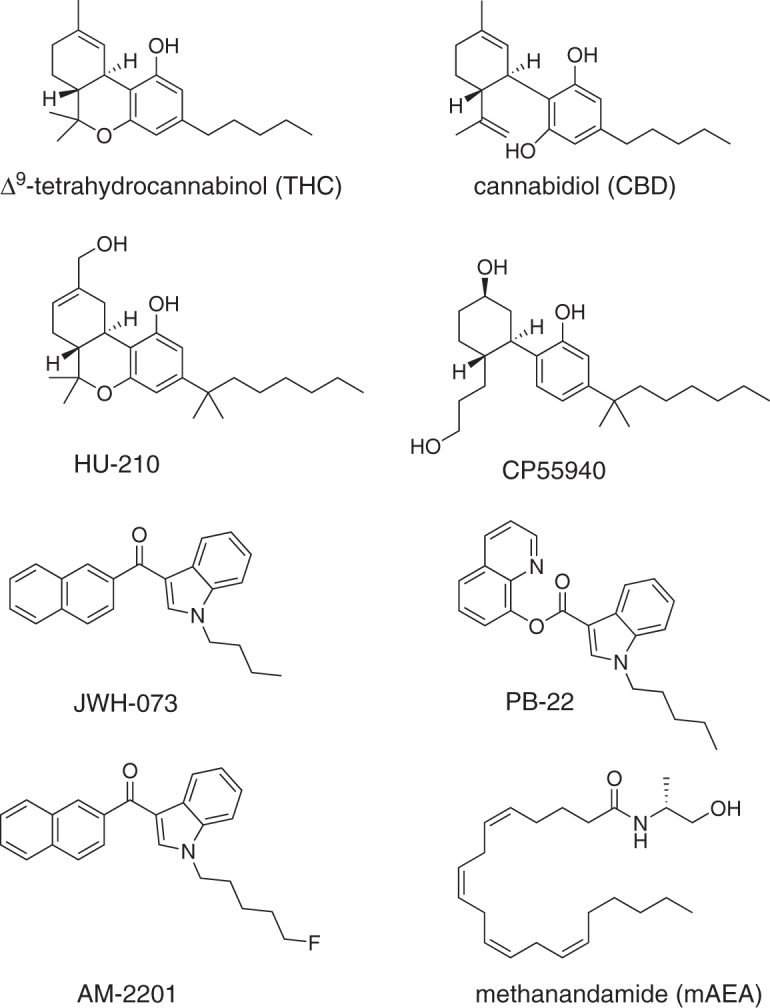
Structures of the cannabinoids used in this study. THC and CBD are phytocannabinoids. HU-210 is a synthetic dimethylheptyl and hydroxylated analog of THC. CP55940 belongs to the alkyl phenol class, and JWH-073, PB-22, and AM-2201 belong to the aminoalkylindole class of synthetic cannabinoids. Methanandamide is a semisynthetic metabolism-resistant analog of the endocannabinoid anandamide. CBD, cannabidiol; mAEA, methanandamide; THC, Δ9-tetrahydrocannabinol.
As the use of cannabis has not been associated with seizures (and may even decrease the risk of seizures)10 in humans or tissue damage in humans or animals, it was hypothesized that, compared with THC, SCs would cause greater or more rapid levels of tolerance, dependence, tissue damage, hypothermia, and seizures.
Materials and Methods
Materials
THC as dronabinol was obtained from Mutual Drug Company, and other cannabinoids were purchased from Cayman Chemical Company. Male C57BL/6 mice (Charles River Laboratories) were housed 2 per cage at 21°C and 50%±20% humidity on a 12 h light/dark cycle. Food and water were provided ad libitum. All experiments were conducted on mice between 5 and 13 weeks in age and in accordance with protocols approved by the Campbell University Institutional Animal Care and Use Committee. Drugs were prepared as emulsions in vehicle (5:1:44 of sesame oil: Tween-80: deionized water) and were administered through intraperitoneal (IP) injection at 0.005–0.01 mL/g.
Cumulative dose–response
Antinociception was assessed by latency to tail withdrawal from a water bath at 53°C using a 30 sec cutoff, and body temperature assessed from ∼2 cm from each animal's ventral thorax by a VeraTemp model 11–900 noncontact infrared thermometer (Brooklands Incorporated) as previously described.19 Previous studies have shown maximum effects in C57BL/6 mice in these assays of JWH-07317 and AM-2201 (unpublished observations) are at 30–60 min, and of THC17 are at 60–180 min, thus each measure was taken before the first injection and then at 30 min (JWH-073, AM-2201, or vehicle) or 60 min (THC) after each injection. Mice were given the next injection for cumulative doses of 3, 7.5, 15, 30, and 60 mg/kg JWH-073; 0.5, 1, 2, and 4 mg/kg AM-2201; or 12.5, 25, 50, and 100 mg/kg THC immediately after each measurement of the effect of the previous dose.
Assessment of tolerance, dependence, and pathology
Mice were administered vehicle, 50 mg/kg THC, 30 mg/kg JWH-073, or 1 mg/kg AM-2201 daily for 4 days at ∼9 am, and latency to tail withdrawal and body temperature were measured before the first and 60 min after each injection. On day 5, ∼24 h after the previous injection, each mouse was observed for 10 min before and 10–30 min after IP injection of 10 mg/kg SR141716A. The number of times each animal exhibited the following behaviors was counted: rear, scratch, groom, forepaw tremor, retropulsion, and head shake.
Within 1 h of the end of the aforementioned experiment, mice were euthanized and necropsied. Heart, lungs, thymus, liver, kidneys, spleen, brain, and skeletal muscle (right gastrocnemius) were collected and stored in 10% neutral buffered formalin. Postfixation, tissues were placed into cassettes (Simport). For the heart, a longitudinal section through both ventricles from base to apex created two halves and permitted visualization of ventricles and atria. For the brain, four coronal sections, anterior to posterior, were submitted. Routine histological processing was performed by the Histology Laboratory of the College of Veterinary Medicine at North Carolina State University. Slides with 5 μm-thick hematoxylin and eosin stained sections of the organs collected were evaluated by light microscopy (n=4–9) using a Nikon Eclipse Ci microscope.
Quantification of convulsions and hypothermia with antagonist blockade
Separate groups of mice were administered 25, 50, or 100 mg/kg THC; 65 mg/kg CBD; 1, 2, or 4 mg/kg HU-210; 2, 4, or 8 mg/kg CP55940; 15, 30, or 60 mg/kg JWH-073; 2, 4, or 8 mg/kg AM-2201; 1, 2, or 4 mg/kg PB-22; or 200 mg/kg mAEA (n=4–7). Dose ranges were based on previous rodent studies.17,18,20 Mice were weighed, and their temperature recorded as earlier before injection and placement into a spontaneous activity chamber. A video camera above the chamber recorded activity for 3 h. At 45 and 180 min after injection, body temperature was measured again.
The 3-h recordings were subsequently analyzed and the time and severity of each convulsion based on the Racine scale21 was noted by two separate observers on separate occasions. Only large clearly involuntary jerking (Racine scale 2) or stretching (Racine scale 3) movements were counted. Minor seizures (Racine scale 0–1) are not visually detectable, and all drugs that produced convulsions produced convulsions that were ranked 2 and 3; thus, the analysis was simplified to quantify total convulsions. The doses used in the subsequent experiment were the lowest that produced the maximum number of convulsions with each drug. The greatest change in temperature (at either 45 or 180 min after injection) is reported.
CB1 antagonist SR141716A or CB2 antagonist SR144528 at 10 mg/kg were administered IP at 0.005 mL/g 30 min before injection with CP55940 (4 mg/kg), THC (50 mg/kg), JWH-073 (30 mg/kg), or PB-22 (2 mg/kg) at 0.005 mL/g. Activity was recorded beginning at the time of antagonist injections and for 3 h after agonist injection. Body temperature was recorded before antagonist injection and 45 and 180 min after agonist injection.
Data analysis
Change in temperature was determined as postdrug temperature minus predrug temperature, and antinociception data were analyzed as % maximum possible effect (%MPE)=(postdrug latency minus predrug latency)/(30 sec minus predrug latency)×100%. Emax, logED50, and ED50 values were determined by nonlinear fitting.
Differences between %MPE and change in body temperature after daily treatments and differences between withdrawal signs were determined by two-way ANOVA (time versus treatment or sign versus treatment, respectively) followed by Tukey's multiple comparisons test. Differences between drugs in the number of convulsions and change in temperature were determined by one-way ANOVA followed by Tukey's multiple comparison test. Differences between each drug and drug+antagonist in the number of convulsions and change in temperature were determined by two-way ANOVA followed by Tukey's multiple comparison test. All analyses were performed using Prism (GraphPad Software) with statistical significance set at p<0.05.
Results
Cumulative dose–response
Figure 2 illustrates the average %MPE for latency to tail withdrawal and change in body temperature in mice after 12.5–100 mg/kg THC, 3–30 mg/kg JWH-073, and 0.5–4 mg/kg AM-2201. For %MPE with THC, JWH-073, and AM-2201, the ED50 values were 25.6, 9.40, and 0.366 mg/kg, respectively (Emax was set to 100%). For change in temperature by THC, JWH-073, and AM-2201 the ED50 values were 11.2, 16.5, and 0.745 mg/kg, and the Emax values were −4.0°C, −7.1°C, and −8.1°C, respectively. For subsequent experiments, 30 mg/kg THC, 50 mg/kg JWH-073, and 1 mg/kg AM-2201 were chosen because they produced roughly equivalent effects, although the SCs produced slightly greater effects than THC on antinociception and change in body temperature.
FIG. 2.
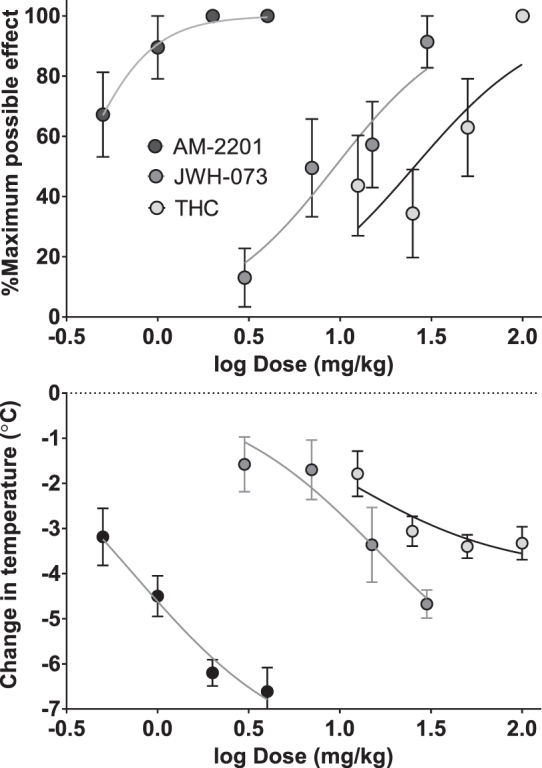
Dose–response curves showing antinociceptive (top) and hypothermic (bottom) effects produced by THC (n=8), JWH-073 (n=10), and AM-2201 (n=8) in male C57BL/6 mice. Data shown were calculated as described in methods and are the mean±SEM of each parameter for each treatment group. Data were fit by nonlinear regression analysis using Prism with %MPE and change in temperature bottom set to 0 and %MPE top (Emax) set to 100%. %MPE, % maximum possible effect.
Assessment of tolerance, dependence, and pathology
Daily treatments with vehicle, THC, JWH-073, or AM-2201 for 4 days resulted in rapid tolerance to the antinociceptive and hypothermic activities of all three agonists (Fig. 3). Each effect of all three agonists was significantly different from vehicle on day 1 (Fig. 3). On day 2, the antinociceptive effect of AM-2201 was decreased to about 60% of the effect on day 1, and was the only effect of any agonist significantly different from vehicle (Tukey's test, p<0.01).
FIG. 3.
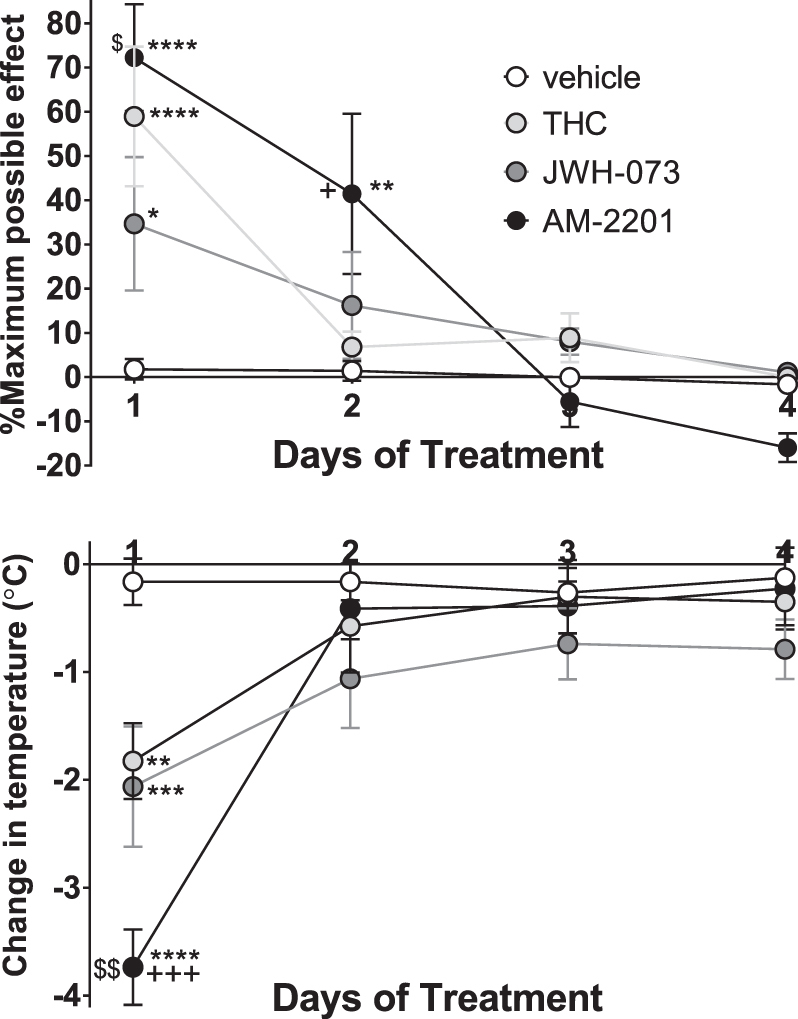
Effects of four daily injections of 50 mg/kg THC (n=8), 30 mg/kg JWH-073 (n=8), 1 mg/kg AM-2201 (n=9), or vehicle (n=6) on antinociceptive (top) and hypothermic (bottom) effects in male C57BL/6 mice. Data shown are the mean±SEM of each parameter for each treatment group. Data were analyzed by two-way ANOVA followed by Tukey's multiple comparisons test. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 versus vehicle; +p<0.05, +++p<0.001 versus THC; $p<0.05, $$p<0.01 versus JWH-073.
On day 5, mice were observed before and after injection of 10 mg/kg of SR141716A for signs of spontaneous and precipitated withdrawal, respectively (Fig. 4), using a modification of previously used methods.22,23 The only significant differences observed before treatment with SR141716A were in the number of times the mice reared (two-way ANOVA, treatment vs. sign, p<0.0001, Fig. 4, top panel). Mice treated with THC showed significantly less, and mice treated with AM-2201 showed significantly more rearing than mice treated with vehicle (Tukey's test, p<0.0001 each). Mice treated with JWH-073 reared more than mice treated with THC, and mice treated with AM-2201 reared significantly more than mice treated with either THC or JWH-073 (Tukey's test, p<0.0001, Fig. 4, top panel).
FIG. 4.
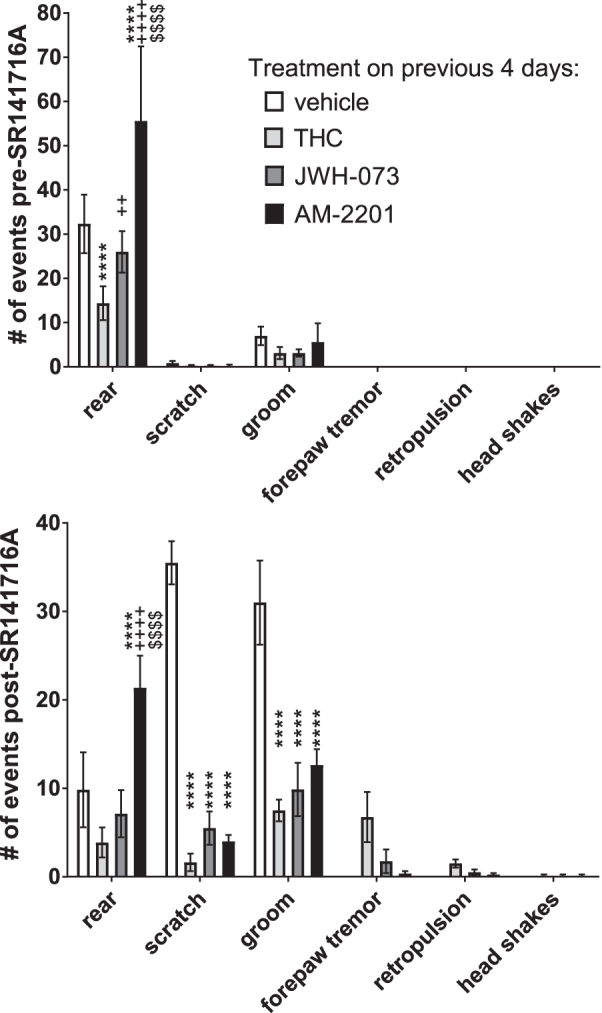
Spontaneous and antagonist-precipitated withdrawal behaviors of mice on day 5 after four daily injections of cannabinoid agonists. Male C57BL/6 mice were injected with 50 mg/kg THC (n=8), 30 mg/kg JWH-073 (n=8), 1 mg/kg AM-2201 (n=9), or vehicle (n=6) on days 1–4 (Fig. 3), then on day 5 were injected with 10 mg/kg of the CB1-selective antagonist, SR141716A. Behaviors were observed and each event noted. Data shown are the mean±SEM number of events observed for each treatment group during the 10 min before (spontaneous withdrawal) and 10–30 min after (antagonist-precipitated withdrawal) injection of 10 mg/kg SR141716A. Data were analyzed by two-way ANOVA followed by Tukey's multiple comparisons test. ****p<0.0001 versus vehicle; ++p<0.01, ++++p<0.0001 versus THC; $$$$p<0.0001 versus JWH-073.
Behaviors that appeared only after administration of antagonist and only in mice treated with agonists on days 1–4 included forepaw tremor, retropulsion, and headshakes (Fig. 4, bottom panel), and thus are the only behaviors considered signs of withdrawal from agonist. There were no statistically significant differences between any treatments in those antagonist-precipitated withdrawal signs. After treatment with SR141716A, mice treated with AM-2201 did significantly more rearing than vehicle-, THC-, or JWH-073-treated mice. There were no significant differences between agonist treatment groups in scratching or grooming, but mice treated with any of the three agonists performed fewer of these behaviors than mice treated with vehicle (Fig. 4, bottom panel).
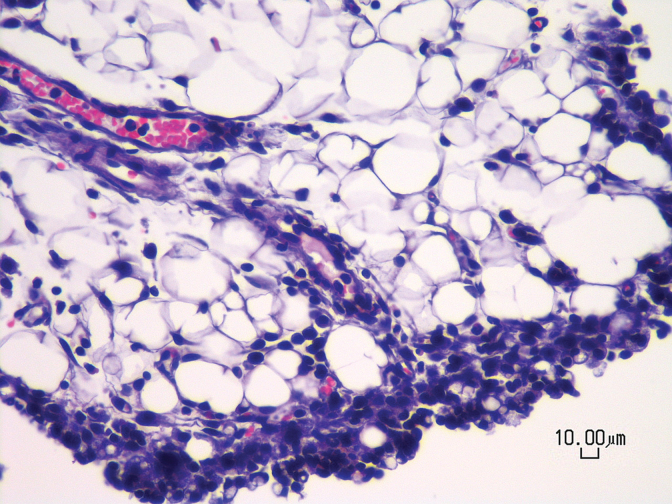
Necropsy and histopathology of these mice revealed no definitive drug-induced lesions. A consistent finding in all groups was mild to moderate inflammation in the peritoneal fat (Fig. 5) attached to the abdominal organs examined. The inflammatory infiltrate was composed of lymphocytes, plasma cells, and macrophages and often the mesothelial lining was plump (reactive mesothelial cells), consistent with chronic irritation and inflammation.
FIG. 5.
Photomicrograph of a vehicle-treated mouse showing mild to moderate chronic peritonitis characterized by the presence of lymphocytes, plasma cells, and macrophages within peritoneal fat. All animals receiving daily IP injections for 5 days (n=4–9/treatment) exhibited the same finding regardless of treatment with drug or vehicle. Hematoxylin and eosin stain, 400× magnification, scale bar=10 μm. IP, intraperitoneal.
Quantification of convulsions and hypothermia with antagonist blockade
To assess seizure activity, mice were observed after a single injection of one of a number of cannabinoids (Fig. 6, top panel). Change in temperature was measured as a control (Fig. 6, bottom panel). Every agonist, except mAEA and CBD, produced visually observable convulsions. Most occurred between 2 and 20 min after drug administration, with occasional events occurring up to ∼60 min. There were no differences in the number of convulsions or hypothermia between the highest two doses. The only significant difference in convulsions between any of the three doses was between 1 and 2 mg/kg PB-22. There were significant differences in hypothermia between the lowest and middle and/or highest doses for THC, CP55940, and PB-22. The highest doses did not cause more convulsions or hypothermia than the second highest, indicating the maximum effect of each agonist had been identified.
FIG. 6.
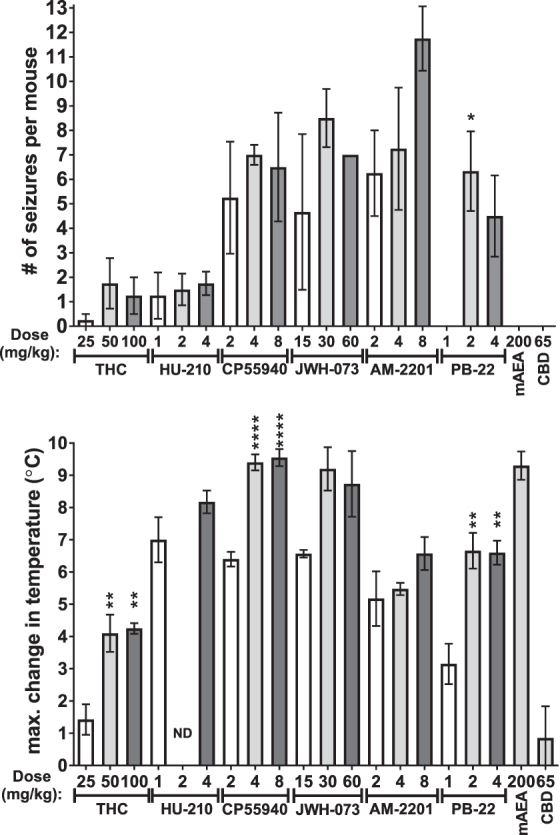
Convulsions and change in temperature caused by each cannabinoid at three doses of most. Mice were injected with 0.01 mL/g IP of each compound at the indicated dose. Activity was video-recorded and subsequently scored for the time of each convulsion as indicated by a jerk, series of jerks, and/or tonic extension of the fore and/or hindlimbs. Body temperature was measured before the injection and at 45 and 180 min after the injection and the greatest change in temperature is shown. Data shown are mean±SEM (n=4–7). Data were analyzed one drug at a time by one-way ANOVA (at p<0.05) followed by Tukey's post-test for differences between the effects of each dose. *p<0.05, **p<0.01, ****p<0.0001 versus lowest dose of same drug. ND, not determined.
Further statistical analysis comparing agonists was performed on the dose of each drug that produced the maximum number of convulsions (if any) and the temperature change observed with the same treatments (Fig. 7). JWH-073 and AM-2201 produced significantly more convulsions than THC, HU-210, mAEA, or CBD. The number of convulsions produced by CP55940 and PB-22 were not different than the other drugs.
FIG. 7.
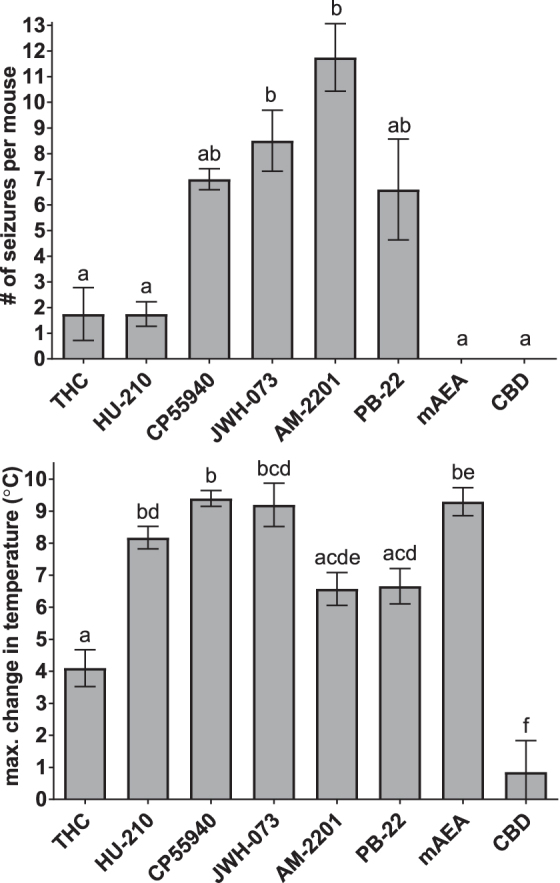
Convulsions at the dose of each drug that caused the greatest number of seizures and change in temperature at the same dose. Data (shown as mean±SEM) for a single dose of each drug shown in Figure 6 were subjected to statistical testing for differences between drugs. Columns marked with the same letter were not significantly different by one-way ANOVA followed by Tukey's post-test at p<0.05.
The changes in temperature (Fig. 7, bottom panel) with HU-210, CP55940, JWH-073, and mAEA were significantly greater than with THC or CBD. The effects of AM-2201 and PB-22 were not significantly different from THC (only a trend toward significance with p-values of 0.101 and 0.058), but were significantly greater than CBD. There was not a significant correlation between the number of convulsions and change in temperature across drugs (Pearson r=0.2504, p=0.5881).
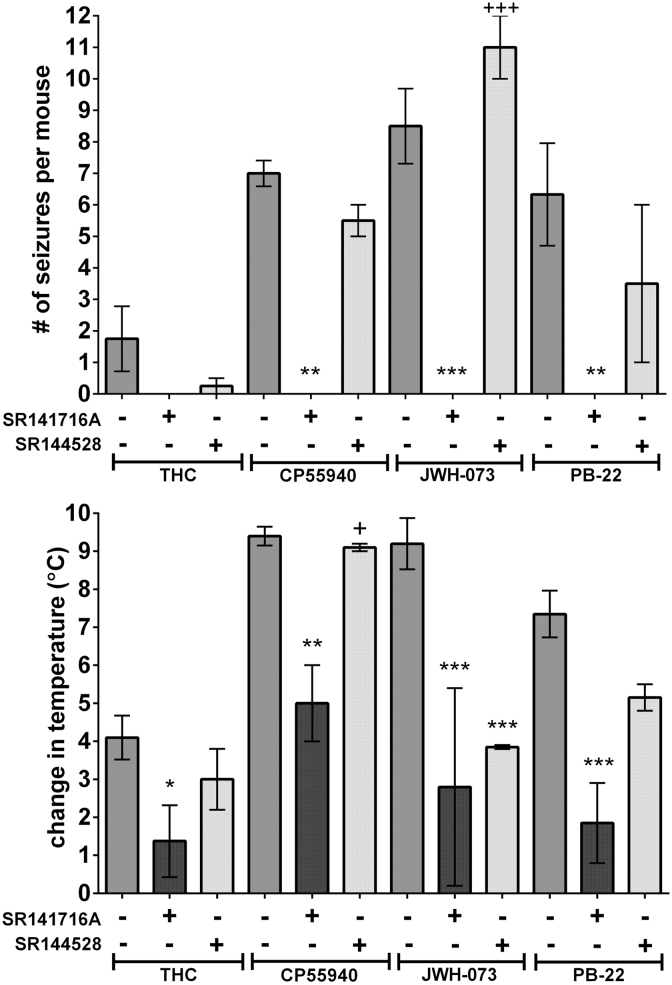
Representative agonists (THC, CP55940, JWH-073, and PB-22) were assessed for whether pretreatment with CB1 antagonist (SR141716A) or CB2 antagonist (SR144528) affected the number of convulsions or change in temperature (Fig. 8). No convulsions were seen with any agonist after pretreatment with SR141716A, and there were statistically significant differences in convulsions with CP55940, JWH-073, and PB-22, although not with THC. SR144528 did not significantly alter the number of convulsions. Change in temperature was significantly lower after SR141716A for all agonists. The only statistically significant difference in change in temperature after SR144528 was with JWH-073 (p<0.001). Although most convulsions induced by agonists occurred in the first 20 min, no convulsions were observed during the 30 min after SR141716A or SR144528 (n=10 for each antagonist) injection before agonist injection.
FIG. 8.
Effects of CB1-selective (SR141716A) and CB2-selective (SR144528) antagonist pretreatments on convulsions at the drug dose that caused the greatest number of seizures and change in temperature of four representative cannabinoids. Antagonists were administered at 10 mg/kg IP at 0.005 mL/g 30 min before the administration of each agonist at 0.005 mL/g IP. Seizure activity and body temperature were measured as earlier for Figure 6. Data (shown as mean±SEM) were analyzed by two-way ANOVA (agonist vs. antagonist treatment) followed by Tukey's post-test at p<0.05. *p<0.05, **p<0.01, ***p<0.001 versus agonist alone; +p<0.05, +++p<0.001 versus agonist+SR141617A.
Discussion
Several reports have shown the potential of SC use to induce seizures in humans,10–13 with some effects replicated in mice.14,15 We hypothesized that SCs produce more adverse effects than THC, the principal psychoactive constituent of cannabis, in a mouse model.
Among the significant findings of this study is that most of the SCs tested produced more seizures and hypothermia than THC. Notably, HU-210 and mAEA both produced hypothermia approximately twofold greater than THC, but the number of seizures induced was similar to THC (HU-210) or produced no seizures (mAEA). Since both produced significant hypothermia, it is unlikely that mAEA and HU-210 failed to produce many seizures due to lack of central nervous system penetration.
In agreement with previous studies,14,15 antagonist pretreatments indicated that seizure activity was mediated by CB1, and not CB2 receptors. Accordingly, all CB1 agonists tested, except mAEA, produced convulsions. JWH-073 and AM-2201 were more potent for antinociception and hypothermia than THC probably owing to their higher affinity for CB1 receptors.24 Most SCs were more efficacious than THC for hypothermia and seizures, likely because they are full agonists, while THC is a partial agonist, for CB1.25–27 However, using comparable doses of THC, JWH-073, and AM-2201, the development of tolerance was similar.
Lack of confirmatory EEG data was a significant limitation of this study. Recently, Malyshevskaya et al. reported that in addition to a significant decrease in locomotor and electromyographic activity, electrographic seizures (assessed as EEG seizure spikes) were evident in mice treated with THC or JWH-018.14 Based on those observations we conclude that the convulsions we observed represent seizures. Malyshevskaya et al. also found that JWH-018 produced greater numbers of and more severe seizures than THC.14 This is in agreement with our data that SCs produce more seizures than THC.
Similar to what has been reported previously for cannabinoid withdrawal,22,28 some behaviors were observed only in mice treated with agonists and then antagonist: forepaw tremor, retropulsion, and head shakes. These antagonist-precipitated withdrawal signs are attributed to dependence on the daily agonist treatments. In this study, none of these signs differed significantly between agonists. Also as reported previously,22,28 SR141716A treatment itself (in mice treated previously with vehicle) decreased rearing and increased scratching and grooming, and prior agonist treatments resulted in less scratching and grooming.
The only difference between mice treated with agonists was that mice treated with AM-2201 exhibited more rearing than THC or JWH-073 in both the preantagonist (spontaneous withdrawal) and the postantagonist (precipitated withdrawal) phases. Since rearing is a normal mouse behavior, it is unclear what this signifies, but may indicate a difference between the effect of AM-2201 versus THC or JWH-073. Alternatively, the difference might be that a greater effective dose of AM-2201 was used. Although care was taken to use equally effective doses, on day 1 of the 4-day treatment, the effects of AM-2201 were greater than the effects of THC and/or JWH-073.
The lack of pathological changes from cannabinoids is consistent with other studies in which animals had minimal to no pathology or the pathological changes were associated with factors such as route of administration.29–34 The chronic peritonitis observed in this study was attributed to the multiple IP injections.
One possible explanation for the discrepancy between inducing hypothermia and seizures is that different signal transduction proteins may mediate each effect. Biased agonism at G-protein coupled receptors is a well-established phenomenon where different agonists at the same receptor activate different signaling pathways by activating G-proteins rather than beta-arrestins or vice versa or even different subtypes of G-proteins or beta-arrestins. Since THC, HU-210, and mAEA are all known to activate G-proteins, perhaps they exhibit biased agonism toward G-proteins, whereas the other agonists are also efficacious at activating beta-arrestins.
Conclusions
Although most measured effects of SCs were not different from THC, most SCs exhibited greater capacity than THC to induce hypothermia and seizures. The trend appears to apply to SCs with chemical structures disparate from that of THC. This conclusion appears to be in agreement with the frequent clinical reports of seizures in humans after exposure to SC products.10 A finding worthy of further study was that mAEA produced more hypothermia than THC, but no seizures.
Acknowledgments
The authors thank Christina Stalder for technical assistance, Dr. Brianne Raccor for Figure 1 and medicinal chemistry expertise, and Dr. Victor Pulgar for assistance with the writing of the article.
Abbreviations Used
- %MPE
% maximum possible effect
- CBD
cannabidiol
- EEG
electroencephalograph
- IP
intraperitoneal
- mAEA
methanandamide
- SC
synthetic cannabinoid
- THC
Δ9-tetrahydrocannabinol
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Funding was provided by Campbell University.
Cite this article as: Breivogel CS, Wells JR, Jonas A, Mistry AH, Gravley ML, Patel RM, Whithorn BE, Brenseke BM (2020) Comparison of the neurotoxic and seizure-inducing effects of synthetic and endogenous cannabinoids with Δ9-tetrahydrocannabinol, Cannabis and Cannabinoid Research 5:1, 32–41, DOI: 10.1089/can.2019.0003.
References
- 1. Winstock A, Lynskey M, Borschmann R, et al. Risk of emergency medical treatment following consumption of cannabis or synthetic cannabinoids in a large global sample. J Psychopharmacol. 2015;29:698–703 [DOI] [PubMed] [Google Scholar]
- 2. Armenian P, Darracq M, Gevorkyan J, et al. Intoxication from the novel synthetic cannabinoids AB-PINACA and ADB-PINACA: a case series and review of the literature. Neuropharmacology. 2018;134(Pt A):82–91 [DOI] [PubMed] [Google Scholar]
- 3. Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647 [Google Scholar]
- 4. Morales P, Hurst DP, Reggio PH. Molecular targets of the phytocannabinoids: a complex picture. Prog Chem Org Nat Prod. 2017;103:103–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tham M, Yilmaz O, Alaverdashvili M, et al. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br J Pharmacol. 2019;176:1455–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mechoulam R, Ben Shabat S, Hanus L, et al. Endogenous cannabinoid ligands—chemical and biological studies. J Lipid Mediat Cell Signal. 1996;14:45–49 [DOI] [PubMed] [Google Scholar]
- 7. Abadji V, Lin S, Taha G, et al. (R)-methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. J Med Chem. 1994;37:1889–1893 [DOI] [PubMed] [Google Scholar]
- 8. Adams IB, Martin BR. Cannabis: pharmacology and toxicology in animals and humans. Addiction. 1996;91:1585–1614 [PubMed] [Google Scholar]
- 9. Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42:327–360 [DOI] [PubMed] [Google Scholar]
- 10. Wolfe CE, Wood DM, Dines A, et al. Seizures as a complication of recreational drug use: analysis of the Euro-DEN Plus data-set. Neurotoxicology. 2019;73:183–187 [DOI] [PubMed] [Google Scholar]
- 11. Tatusov M, Mazer-Amirshahi M, Abbasi A, et al. Clinical effects of reported synthetic cannabinoid exposure in patients admitted to the intensive care unit. Am J Emerg Med. 2019;37:1060–1064 [DOI] [PubMed] [Google Scholar]
- 12. Lapoint J, James LP, Moran CL, et al. Severe toxicity following synthetic cannabinoid ingestion. Clin Toxicol (Phila). 2011;49:760–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schneir AB, Baumbacher T. Convulsions associated with the use of a synthetic cannabinoid product. J Med Toxicol. 2012;8:62–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malyshevskaya O, Aritake K, Kaushik MK, et al. Natural (delta(9)-THC) and synthetic (JWH-018) cannabinoids induce seizures by acting through the cannabinoid CB1 receptor. Sci Rep. 2017;7:10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Funada M, Takebayashi-Ohsawa M. Synthetic cannabinoid AM2201 induces seizures: involvement of cannabinoid CB1 receptors and glutamatergic transmission. Toxicol Appl Pharmacol. 2018;338:1–8 [DOI] [PubMed] [Google Scholar]
- 16. Ford BM, Tai S, Fantegrossi WE, et al. Synthetic pot: not your grandfather's marijuana. Trends Pharmacol Sci. 2017;38:257–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Breivogel CS, Lambert JM, Gerfin S, et al. Sensitivity to delta9-tetrahydrocannabinol is selectively enhanced in beta-arrestin2 -/- mice. Behav Pharmacol. 2008;19:298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Banister SD, Stuart J, Kevin RC, et al. Effects of bioisosteric fluorine in synthetic cannabinoid designer drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135. ACS Chem Neurosci. 2015;6:1445–1458 [DOI] [PubMed] [Google Scholar]
- 19. Breivogel CS, Vaghela MS. The effects of beta-arrestin1 deletion on acute cannabinoid activity, brain cannabinoid receptors and tolerance to cannabinoids in mice. J Recept Signal Transduct Res. 2015;35:98–106 [DOI] [PubMed] [Google Scholar]
- 20. Breivogel CS, Griffin G, Di Marzo V, et al. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60:155–163 [PubMed] [Google Scholar]
- 21. Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294 [DOI] [PubMed] [Google Scholar]
- 22. Cook SA, Lowe JA, Martin BR. CB1 receptor antagonist precipitates withdrawal in mice exposed to Delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1998;285:1150–1156 [PubMed] [Google Scholar]
- 23. Lichtman AH, Fisher J, Martin BR. Precipitated cannabinoid withdrawal is reversed by Delta(9)-tetrahydrocannabinol or clonidine. Pharmacol Biochem Behav. 2001;69:181–188 [DOI] [PubMed] [Google Scholar]
- 24. Compton DR, Rice KC, De Costa BR, et al. Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther. 1993;265:218–226 [PubMed] [Google Scholar]
- 25. Brents LK, Gallus-Zawada A, Radominska-Pandya A, et al. Monohydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain intermediate to high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity. Biochem Pharmacol. 2012;83:952–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chimalakonda KC, Seely KA, Bratton SM, et al. Cytochrome P450-mediated oxidative metabolism of abused synthetic cannabinoids found in K2/Spice: identification of novel cannabinoid receptor ligands. Drug Metab Dispos. 2012;40:2174–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sim LJ, Hampson RE, Deadwyler SA, et al. Effects of chronic treatment with Δ9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPγS autoradiography in rat brain. J Neurosci. 1996;16:8057–8066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aceto MD, Scates SM, Lowe JA, et al. Cannabinoid precipitated withdrawal by the selective cannabinoid receptor antagonist, SR 141716A. Eur J Pharmacol. 1995;282:R1–R2 [DOI] [PubMed] [Google Scholar]
- 29. Fitzgerald KT, Bronstein AC, Newquist KL. Marijuana poisoning. Top Companion Anim Med. 2013;28:8–12 [DOI] [PubMed] [Google Scholar]
- 30. Thompson GR, Rosenkrantz H, Schaeppi UH, et al. Comparison of acute oral toxicity of cannabinoids in rats, dogs and monkeys. Toxicol Appl Pharmacol. 1973;25:363–372 [DOI] [PubMed] [Google Scholar]
- 31. Cha HJ, Seong YH, Song MJ, et al. Neurotoxicity of synthetic cannabinoids JWH-081 and JWH-210. Biomol Ther (Seoul). 2015;23:597–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thompson GR, Fleischman RW, Rosenkrantz H, et al. Oral and intravenous toxicity of delta9-tetrahydrocannabinol in rhesus monkeys. Toxicol Appl Pharmacol. 1974;27:648–665 [DOI] [PubMed] [Google Scholar]
- 33. Thompson GR, Mason MM, Rosenkrantz H, et al. Chronic oral toxicity of cannabinoids in rats. Toxicol Appl Pharmacol. 1973;25:373–390 [DOI] [PubMed] [Google Scholar]
- 34. Chan PC, Sills RC, Braun AG, et al. Toxicity and carcinogenicity of delta 9-tetrahydrocannabinol in Fischer rats and B6C3F1 mice. Fundam Appl Toxicol. 1996;30:109–117 [DOI] [PubMed] [Google Scholar]