Abstract
Introduction: Recent studies have suggested that cannabidiol (CBD) could interconvert into Delta-8- and Delta-9- tetrahydrocannabinol.
Materials and Methods: Thus, we tested the plasma samples of 120 healthy human subjects (60 male and 60 female), 60 in fasting and the other 60 under normal feeding conditions after acute administration of an oral solution containing CBD 300 mg. To do this, we developed a bioanalytical method to determine CBD and the presence of THC in plasma samples by Ultra-High Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry.
Results: The results showed that THC was not detected in plasma after the administration of CBD, and those study participants did not present psychotomimetic effects.
Conclusions: The findings presented here are consistent with previous evidence suggesting that the oral administration of CBD in a corn oil formulation is a safe route for the administration of the active substance without bioconversion to THC in humans.
Keywords: cannabidiol, delta-8-tetrahydrocannabinol, delta-9-tetrahydrocannabinol, interconversion, pharmacokinetics, protein precipitation, UHPLC-ESI-MS/MS
Introduction
Cannabis sativa (cannabis) contains more than 100 compounds with similar chemical structures, known as phytocannabinoids. The main psychoactive compound in cannabis is Delta-9-tetrahydrocannabinol (Δ9-THC), responsible for most of the usual psychotomimetic effects linked to the use of the plant. Another major phytocannabinoid present in cannabis is cannabidiol (CBD), a compound that does not produce the typical subjective effects of cannabis induced by Δ9-THC. CBD was shown to have a wide range of pharmacological properties of great therapeutic interest such as anxiolytic, antiepileptic, and neuroprotective actions, among others, without causing significant adverse effects.1–3 Interestingly, although CBD and Δ9-THC show entirely different clinical and central actions, they share very similar chemical structures.
A recent study demonstrated that CBD could convert to Δ9-THC in an acidic medium in vitro that simulates gastric fluid. This led the authors to conclude that oral treatment with CBD could expose patients to the risk of ingesting significant levels of THC.4 A comment to this article contested the clinical relevance in its conclusion based on available clinical data that showed no effects characteristic of THC in subjects after the ingestion of CBD, even at high doses.5 These articles initiated a scientific controversy that generated more publications since then, with reports presenting animal data arguing for6,7 and against8–10 the risk of CBD conversion into Δ9-THC after oral ingestion. In humans, the only two studies that provided data regarding the conversion of CBD into Δ9-THC11,12 did not detect Δ9-THC in the plasma samples of any of the patients included.
While definitive conclusions remain to be reached, this scientific debate may have significant clinical and practical implications. Preliminary pre-clinical and more extensive clinical trials have shown that orally-administered CBD has antiseizure properties, particularly in children with treatment-resistant epilepsies such as Lennox-Gastaut and Dravet syndrome.13,14 Since it is well known that chronic exposure to Δ9-THC has long-lasting harmful effects, such as the onset of severe psychiatric disorders and cognitive impairment, the possible conversion of CBD into Δ9-THC raises important safety concerns, particularly in the developing brain of children and adolescents.15 Therefore, to contribute to the elucidation of the controversy about the conversion of oral CBD to THC, we investigated the pharmacokinetics of a single dose of CBD administered to a large sample of healthy human volunteers (male and female).
Materials and Methods
Subjects
To evaluate the possibility of interconversion of CBD to Δ8-THC or Δ9-THC, we carried out two studies with the participation of healthy volunteers after acute administration of an oral solution of CBD. The first study (fed condition) had the initial participation of 60 healthy adult volunteers of both sexes (30 male and 30 female) between the ages of 18 and 50 years (mean age 30.6±7.88). The administration of the formulation occurred after a standardized breakfast for all volunteers, and blood samples were collected at predetermined times (00:30, 01:00, 01:30, 02:00, 02:30, 03:00, 03:30, 04:00, 04:30, 05:00, 05:30, 06:00, 07:00, 08:00, 10:00, 12:00, 24:00, 48:00, 72:00, 120:00, 168:00, and 216:00). Fifty-three volunteers (26 males, 27 female) completed the first trial, with 7 dropouts.
The second trial had the initial participation of 60 healthy adult volunteers of both sexes (30 male and 30 female) between the ages of 18 and 50 years (mean age 32.88±8.36). In this trial, the CBD formulation was administered during fasting, and blood samples were collected at the same predetermined times as in the first trial (00:20, 00:40, 01:00, 01:30, 02:00, 02:30, 03:00, 03:30, 04:00, 04:30, 05:00, 06:00, 07:00, 08:00, 10:00, 12:00, 24:00, 48:00, 72:00, 120:00, 168:00, and 216:00). Forty-seven volunteers completed the second trial.
The two trials included only subjects with no history or presence of drug or alcohol dependence or positive urine drug screening results, both during the qualification phase and in the period of CBD administration. Subjects who tested positive for any drug at screening were not eligible for enrollment in the study. In addition, all volunteers were nonsmokers and had not taken any medications for at least 3 months before the study. Moreover, none of them had used cannabis in their lives, and none had ever used any other illegal drug. Pregnant women or those planning to become pregnant were also excluded. In addition, subjects were excluded if they had a history or presence of clinically significant general medical conditions such as cardiovascular, pulmonary, hepatic, renal, hematologic, gastrointestinal, endocrine, immunologic, dermatologic, neurologic, oncologic, or psychiatric disorder or any other condition that could, in the opinion of the investigators, affect the safety or validity of the study results. All subjects gave their written consent to participate after being fully informed of the research procedures, which were approved by the local ethics committee (process numbers CAAE 69028517.5.0000.0104 and 69024917.9.0000.0104).
Drugs
CBD (300 mg; 99.5% purity; Prati-Donaduzzi Pharm, Toledo, Brazil) was dissolved in corn oil. The 300 mg dose was chosen based on previous studies that detected the acute anxiolytic effect of this dose16,17 and reports18,19 in which this dose caused a reduction in the frequency of rapic eye movement sleep behavioral events and improved the quality of life in patients with Parkinson's disease.
Chemicals and reagents
(a) Acetonitrile—high-performance liquid chromatography (HPLC) grade (Merck, Darmstadt, Germany).
(b) Ammonia solution 30%—for analysis-ACS (Carlo Erba, Milan, Italy).
(c) Anticoagulant—Vacutainer® tubes with sodium heparin (Becton & Dickson).
(d) Methanol—for HPLC, ≥99.9% (Sigma-Aldrich, Oakville, Canada).
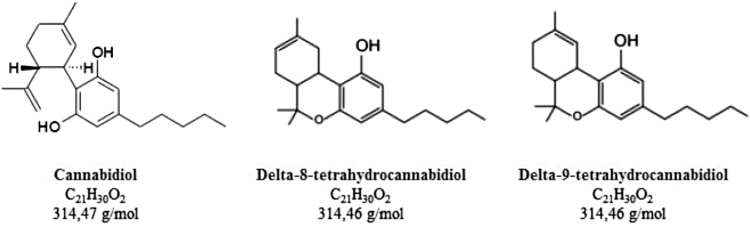
(e) CBD—Reference standard 99.78% (Specialità Fine Chemicals, Toledo, Brazil) (please see Fig. 1).
(f) Δ8-THC—Reference standard 99.4% (THC Pharm, Frankfurt, Germany) (please see Fig. 1).
(g) Δ9-THC—Reference standard 98.9% (THC Pharm) (please see Fig. 1).
(h) Internal Standard (IS), a molecule derived from CBD, with very similar structural and physicochemical properties to the analyte.
-
(i)
Water—Obtained from Milli-Q/Elix system (Millipore, Bedford, MA).
FIG. 1.
Nomenclature, molecular formulas, and molar masses of the compounds analyzed.
Procedure
In the fed trial, subjects were instructed to abstain from alcohol and caffeine for at least 48 h before each visit to the laboratory. After at least 8 h of fasting, subjects were instructed to have a light standardized meal (Table 1) 30 min before an oral dose of CBD (300 mg) was administered. Blood samples were collected at 00:30, 01:00, 01:30, 02:00, 02:30, 03:00, 03:30, 04:00, 04:30, 05:00, 05:30, 06:00, 07:00, 08:00, 10:00, 12:00, 24:00, 48:00, 72:00, 120:00, 168:00, and 216:00.
Table 1.
Composition of the Standard Breakfast
| Composition | Cho (g) | Ptn (g) | Lpds (g) | Kcal (g) |
|---|---|---|---|---|
| Industrial milk bread (two slices) | 23 | 3.7 | 1.2 | 136.1 |
| Turkey breast (two slices) | 1.52 | 8.0 | 0.72 | 44 |
| One cereal bar of oatmeal and honey | 17 | 1.0 | 0.5 | 79 |
| A small strawberry muffin | 18 | 2.2 | 5.4 | 129 |
| 200 mL of pineapple juice | 19 | — | — | 76 |
| Total | 78.52 | 14.9 | 7.82 | 466.1 |
Cho, carbohydrates; Kcal, kilocalorie; Lpds, lipids; Ptn, proteins.
Before the hospitalization of the volunteers, a clinical evaluation was performed, in which all the inclusion and exclusion criteria of the study protocol were evaluated. Laboratory tests and an electrocardiogram were also evaluated. Vital signs (pulse, blood pressure, and temperature) were checked between 30 and 60 min before the administration of the drug and 1:20, 4:00, and 11 h later. During all blood collections, volunteers were questioned about their health status to identify the occurrence of adverse events. The same procedure was performed with the fasting trial volunteers, with the difference that volunteers did not receive the standardized breakfast 30 min before the drug was given. Blood samples were collected at: 00:20, 00:40, 01:00, 01:30, 02:00, 02:30, 03:00, 03:30, 04:00, 04:30, 05:00, 06:00, 07:00, 08:00, 10:00, 12:00, 24:00, 48:00, 72:00, 120:00, 168:00, and 216:00.
Sample preparation
All frozen human plasma samples were thawed at room temperature. To perform sample extraction, 100 μL of plasma was added into microtubes, and 100 μL of 120 ng/mL IS solution and 200 μL of methanol were added to all tubes. The extraction was performed by vortex mixing for 3 min, followed by centrifugation of the mixture at 14,000 rpm, for 7 min at 4°C. The organic phase was transferred completely to another microtube and evaporated under a compressed air stream. The residue was reconstituted in 300 μL of solution composed of acetonitrile:water (6:4 v/v) followed by vortexing for 2 min. The supernatant was transferred into the sample insert vials, and an aliquot of 6 μL was injected into the HPLC-MS/MS system.
Apparatus and analytical conditions
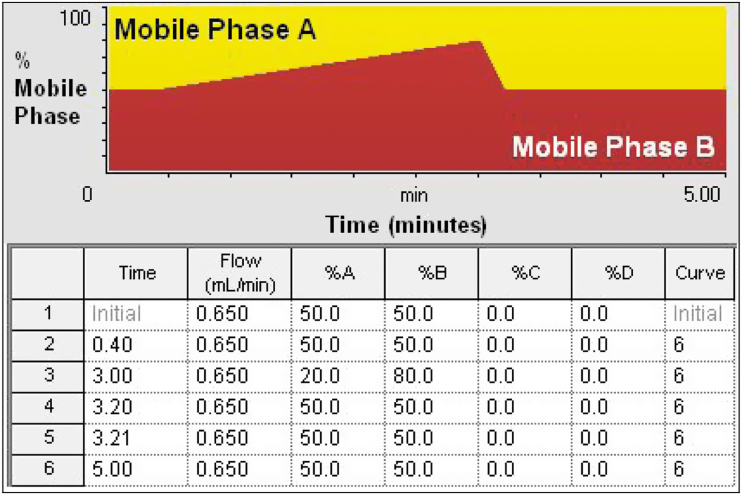
The ultra-performance liquid chromatography (UPLC) system utilized was a Waters Acquity H-Class FTN system (Milford, MA). The experiments were carried out on a reversed-phase BEH Waters C8 column (30×2.1 mm i.d.; 1.7 μm). The UPLC system was operated at 50°C. The flow rate of the mobile phase under gradient condition was kept at 0.65 mL/min (Fig. 2). The autosampler was set at 12°C, and the injection volume was 3 μL. The mobile phase consisted of two solutions: acetonitrile and 0.1% ammonium hydroxide. The mobile phases were sonicated for 5 min under vacuum.
FIG. 2.
Gradient conditions applied to the chromatographic method.
The analyte and IS were detected on a mass spectrometer (Xevo TQ-S Atmospheric Pressure Ionization; Waters) equipped with an electrospray ion source, operating in the negative ion mode. MassLynx software version 4.1 was used to control all parameters of LC and MS. Quantitation was performed using selective reaction monitoring (SRM) mode to study precursor → product ion transitions for CBD (m/z 313→245) and for IS (m/z 371→339). Delta-8 and delta-9-THC are isomers of CBD that present physical and chemical properties like CBD, including the MS/MS ionization fragmentation. Thus, Δ8-THC and Δ9-THC compounds were analyzed in the same SRM transitions (Broecker and Pragst).20 Consequently, the retention times of them were used to identify each analyte, as illustrated in the chromatograms of Figure 3.
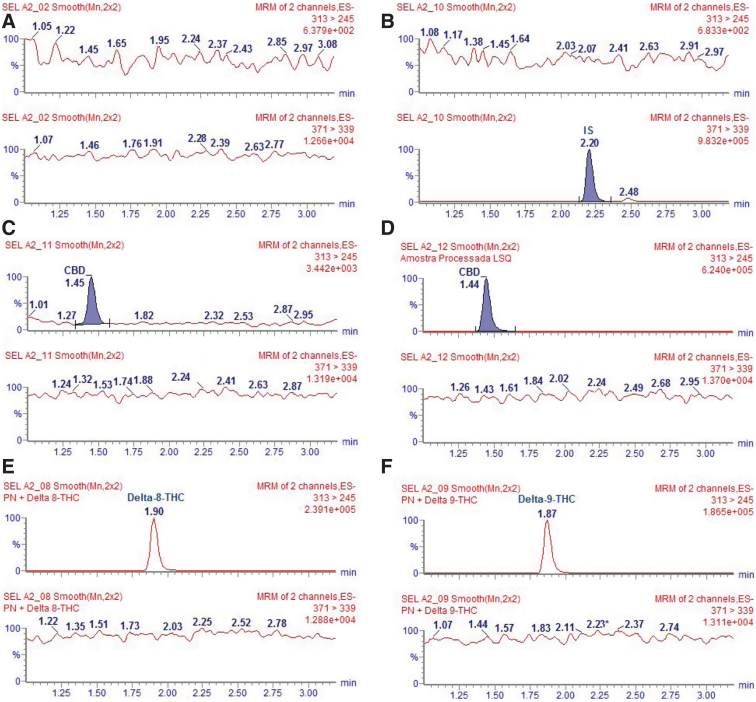
FIG. 3.
Selectivity of the LC-MS/MS method. (A) Blank plasma sample, (B) blank plasma sample spiked with IS, (C) blank plasma spiked with LLOQ: 1.5 ng/mL, (D) blank plasma spiked with ULQ: 500 ng/mL, (E) blank plasma spiked with Δ8-THC, and (F) blank plasma spiked with Δ9-THC. IS, Internal Standard; LC-MS/MS, chromatography triple quadrupole mass spectrometry; LLOQ, lower limit of quantification; THC, tetrahydrocannabinol.
Source-dependent parameters optimized were gas 1 (nebulizer gas): 150 L/h; gas 2 (heater gas): 650 L/h flow; ion spray voltage: 2.4 kV; and temperature: 450°C. Compound-dependent parameters were decluttering potential, 30 eV (CBD and IS; collision energy, 24 eV [CBD] and 16 eV [IS]). Argon was used as a collision-induced dissociation gas at 4.0 mbar, and the electron multiplier was set at 527 eV, with gain at 1.0. The MS was maintained at unit resolution, and dwell time was set at 0.2 sec.
The peak areas were measured, and the peak area of the analyte to IS ratios and the concentration were calculated using the MassLynx software (version 4.1; Waters). Each analysis required <5 min.
Bioanalytical validation
The method was validated according to international guidelines (FDA, ICH, and EMA16). The working solutions in the concentration range of 75–20,000 ng/mL were prepared by serial dilution of the stock solution (100,000 ng/mL of CBD in methanol) in methanol. The quality control (QC) standards were prepared by spiking blank human plasma samples (100 μL) with working solution that result in plasma concentration range from 1.5 to 400 ng/mL (calibration curve). These control standards (CQLLOQ: 1.5 ng/mL; low QC: 4.5 ng/mL; middle QC: 150 ng/mL; high QC: 300 ng/mL; upper limit of quantification [ULQ]: 400 ng/mL dilution QC: 480 ng/mL) of CBD were used for the analytical validation.
Under the conditions described, the lower limit of quantitation was 1.5 ng/mL for 0.1 mL of plasma. The relationship between the concentration and peak area ratio was found to be linear within the range of 1.5–400 ng/mL. The intraday accuracy of the method for CBD ranged from 89.3% to 107.4%, while the intraday precision ranged from 0.6% to 10.4%. The interday accuracy ranged from 96.3% to 101.9%, while the interday precision ranged from 2.2% to 7.1%. The absolute recovery was 96.4%, while the relative recovery ranged from 90.1% to 104.0%. A stability study showed that CBD was stable in plasma for 87 days when stored at −20°C.
The calibration curve fulfilled the following quality criteria: coefficient of variation (CV) ≤20% in relation to the nominal concentration for the lower limit of quantification (LLOQ). The calibration QC should be within ±15% (CV) of the nominal concentration. Furthermore, at least 75% of the calibration QC (at least six calibration QC with different concentrations) were approved according to these criteria, including LLOQ (1.5 ng/mL) and ULQ (400 ng/mL).
Precision was determined in a same run (intra-run precision) and in at least three different runs (inter-run precision). These parameters were approved with CV below 15%, except for LLOQ, for which values below or equal to 20% are accepted.
Accuracy was determined in a same run (intra-run accuracy) and in at least three different runs (inter-run accuracy). These parameters were approved by obtaining Relative Standard Errors below ±15% of the nominal value, except for LLOQ, for which no values out of the ±20% range are accepted.
Analysis of interconversion of CBD into THC
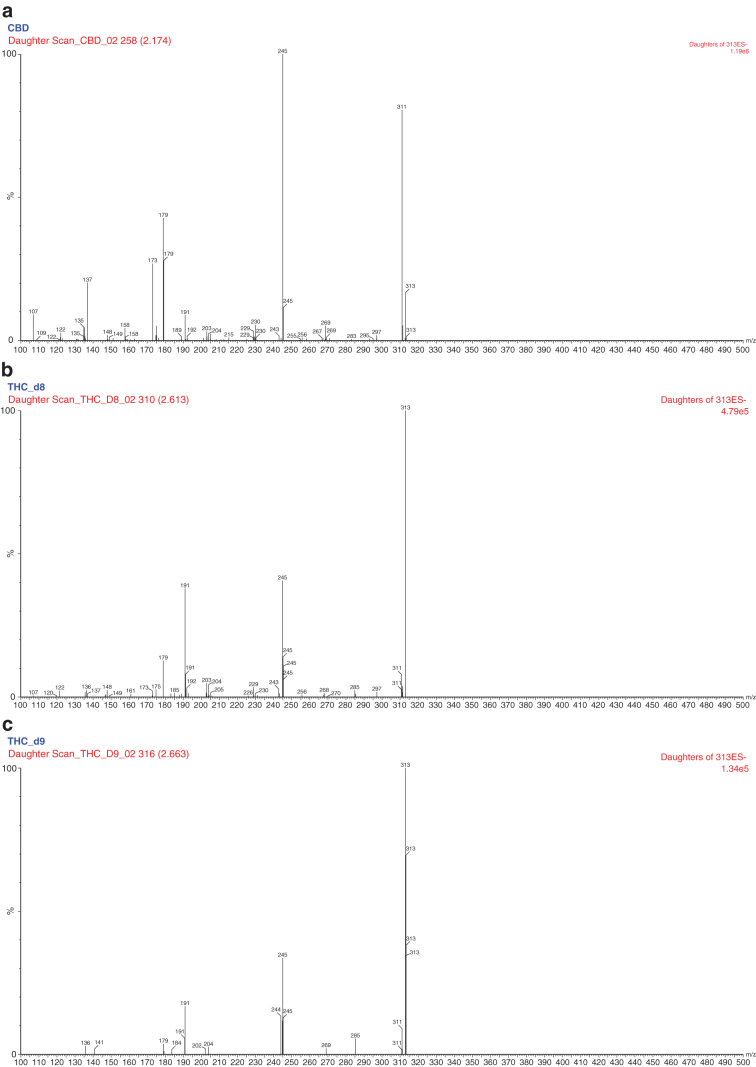
Due to the possibility of interconversion of CBD into THC during the study with humans, THC was assessed as a possible agent of interference on the selectivity and matrix effect of the bioanalytical method. Since they are isomers and have quite similar molecular structures, THC and CBD present the same fragmentation pattern when analyzed in mass spectrometry (MS), that is, they share the same main ions and product. Figure 4 illustrates the MS/MS spectrums (precursor → product) of the CBD and Δ8-THC and Δ9-THC compounds. Thus, CBD and THC have the same transition. The chromatographic part, therefore, must be developed with the aim of differentiating between these two molecules as a possible interference and in vivo metabolite for the assessment of interconversion. To achieve this, we performed the following steps.
FIG. 4.
MS/MS spectrums (precursor → product) of the (a) CBD, (b) Δ8-THC, and (c) Δ9-THC compounds, respectively. CBD, cannabidiol.
Selectivity
- Samples of blank plasma with Δ8-THC and Δ9-THC were contaminated individually to a final concentration of 200 ng/mL.
- The main objective was to verify the co-elution of CBD to THC because they have very similar molecular structures (isomers).
- If there is no co-elution of CBD in THC, there is no impact on the quantification of plasma concentration levels of CBD chromatographically, even in the presence of THC.
- If there is no co-elution of CBD in THC, it is possible to monitor the presence of THC in the samples of volunteers considering a semiquantitative method; that is, by knowing the relative retention time (RRT) and area/concentration ratio of Δ8-THC or Δ9-THC.
- Any peak eluting in the RRT of Δ8-THC or Δ9-THC characterizes that there is interconversion.
Matrix effect
Matuszewski et al.21 reported that matrix components that co-elute with analytes might adversely affect the reproducibility of analyte ionization in a mass spectrometer's electrospray ionization source. The response of the mean peak area from the six different sample sources was compared with those from standard solutions with concentrations of low quality control (4.5 ng/nL) and high quality control (300 ng/mL). In addition, a new set of samples with Δ8-THC or Δ9-THC at a concentration of 200 ng/mL was contaminated to verify if the presence of THC in the plasma sample interfered with the quantification of CBD. For both tests, we assumed a CV of 15% between samples for a controlled matrix effect.
Results
The reasons for dropping out of the fed condition trial were: the abandonment of study without subjects' justification (n=3); positive drug test (exclusion criterion; n=2); no total intake of the medication (n=1); and pharmacodermia (n=1). The reasons for dropping out of the fasting condition trial were: abandonment of study without subjects' justification (n=6); positive drug test (exclusion criterion; n=2); use of medication not allowed (n=2); no complete intake of the medication (n=1); pharmacodermia (n=1); and foot injury unrelated to the medicine (n=1). No dropouts were related to psychotropic effects.
The selectivity of this method (Fig. 4) was proved by the different retention times of them during the chromatographic separation, CBD (retention time [RT]=1.44 min), delta-8-THC (RT=1.90 min), and delta-9-THC (RT=1.87). Considering the retention time of the CBD, the Δ8-THC RRT is 1.31, and the Δ9-THC RRT is 1.29. Figure 3 illustrates the selectivity of the chromatography triple quadrupole mass spectrometry (LC-MS/MS) method. To improve the chromatograms visualization, the mass transition m/z 313>245 for CBD was filtered.
It is important to note that CBD elutes in a RT different from those of Δ8-THC and Δ9-THC, without affecting the chromatographic quality of CBD. Δ8-THC and Δ9-THC elute at RT 1.90 and 1.97, with partial co-elution that does not affect the evaluation of CBD interconversion into THC.
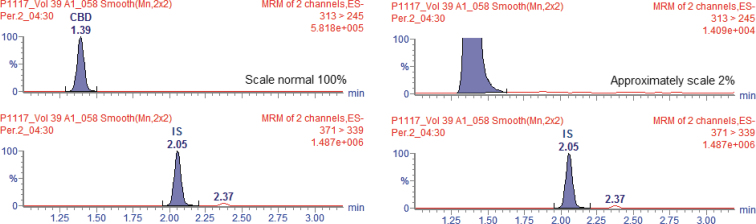
Figure 5 shows the chromatogram of a biological sample from the volunteer with the maximum concentration reached after the administration of the CBD formulation in the trial with fed volunteers. If interconversion of CBD into THC occurred, it would be possible to identify in this chromatogram. The Figure 5, even in the approximate scale in 2%, shows the absence of secondary peaks in RT 1.87 and 1.90 corresponding to Δ8-THC and Δ9-THC, except for the peak in RT 1.39 referring to CBD. The same behavior was observed for the other volunteers, demonstrating the absence of Δ8-THC or Δ9-THC in the analyzed samples.
FIG. 5.
Chromatograms of Cmax of a volunteer at collection time 04:30 h in normal and approximate scale for the fed-pharmacokinetic study.
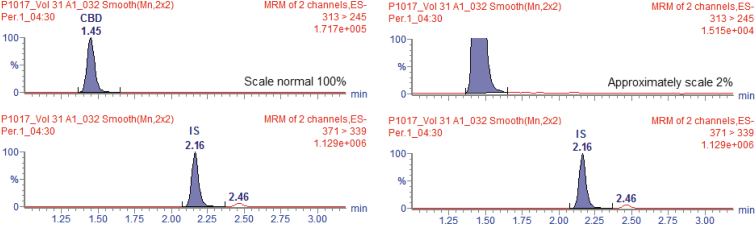
Figure 6 shows the chromatogram of a biological sample collected from the volunteer with the maximum concentration following administration of the formulation in the fasting condition. Similar behavior to the fed state was observed, with the absence of CBD interconversion in THC.
FIG. 6.
Chromatograms of Cmax of a volunteer at collection time 04:30 h on the average and approximate scale for the fasting pharmacokinetic study.
The matrix effect was evaluated with and without THC. In both cases, there was no significant matrix effect on the method, with CV <15%. With possible interferences and without potential interferences, CV values were 4.0% and 2.5%.
Discussion
The main results of our study show that a standard clinical dose of CBD in corn oil formulation did not convert into THC in humans under fasting and normal feeding conditions. We found no signs of Δ8-THC or Δ9-THC in whole blood at 3 and 6 h after oral administration of CBD.
Our findings are supported by the results of previous, methodologically adequate animal studies using oral CBD. In a study with male rats (Sprague Dawley), oral CBD (50 mg/kg) dissolved in olive oil and ethyl alcohol was administered, and the animals were sacrificed after 3 or 6 h.9 Despite the use of a highly sensitive and selective LC-MS/MS method, the authors found no signs of Δ9-THC or its metabolites (11-hydroxy-D9-tetrahydrocannabinol [11-OH-THC], THCCOOH, and THCCOOH-gluc) in whole blood at 3 or 6 h after oral CBD. The possibility of the conversion of CBD to Δ9-THC has also been investigated in minipigs, which is considered a suitable animal model for the human gastrointestinal tract. Synthetic CBD (15 mg/kg) dissolved in sesame oil was administered by gavage to male minipigs twice a day for 4 days, and blood samples were collected from the jugular vein on days 1 and 5, just before, and 1, 2, 4, and 6 h after CBD administration.10 No detectable levels of both Δ9-THC and 11-OH-THC were found in any of the plasma samples collected.
In a recent experiment, CBD dissolved in sunflower oil was administered by oral gavage to male Wistar rats deprived of food for 12 h at a dose of 10 mg/kg, and the animals were euthanized 0.5, 1, 2, 4, 8, and 24 h later.8 Although the authors described the presence of Δ9-THC in the animals' serum following the oral administration of CBD, no detectable levels of the substance were found in brain samples. In vitro studies tested the interconversion of CBD into Δ8-THC or Δ9-THC using simulated gastric fluid (SGF) and high doses of CBD; however, these methodologies were not relevant enough to confirm the interconversion.4,22 Although animal and in vitro experiments are essential in drug development, only the appropriate human studies can confirm whether CBD interconversion into THC occurs. It is due to the complexity of the human organism and its metabolic routes.
To evaluate the possibility of CBD interconversion into Δ8-THC or Δ9-THC in humans, we developed a bioanalytical method to evaluate the monitored transition of individual molecules in the same chromatograms. The bioanalytical method was developed and validated and showed that these compounds do not co-elute, that is, they have adequate chromatographic separation to enable the identification of the interconversion of these compounds in plasma samples of the volunteers. It is important to note that both the participants in the fasting branch and in the fed branch tolerated well the administration of the formulation and did not present any adverse events related to psychotropic effects.
The findings of the present study are also consonant with the few results available from human studies that investigated the possibility of the conversion of CBD into Δ9-THC. Consroe et al.11 conducted a double-blind study comparing the effects of CBD (10 mg/kg/day) and placebo for 6 weeks in 14 patients (both sexes) with Huntington disease and did not detect Δ9-THC in the plasma samples of any of the patients included. More recently, a double-blind crossover study administered single doses of Δ9-THC (10 mg), CBD (600 mg), and placebo in separated sessions to 16 healthy male volunteers.12 As expected, there was a clear increase of Δ9-THC, 11-OH-THC, and carboxy-THC (THCCOOH) levels following the intake of Δ9-THC, but the same did not occur after the administration of CBD or placebo. In addition, recent and more extensive clinical trials13,14 have shown that orally-administered CBD did not induce symptoms traditionally associated with THC in children with treatment-resistant epilepsies, such as changes in food intake, psychomotricity, physiological parameters (heart rate, blood pressure, and body temperature), and/or psychological functions. The safety findings of these trials contrast with reports of intoxication by Δ9-THC in epileptic children who used uncontrolled artisanal cannabis-based medicines not produced according to good manufacturing and laboratory practices.23
Conclusion
The findings presented here are consonant with previous evidence suggesting that the oral administration of CBD in a corn oil formulation is a safe route for the administration of the active substance without bioconversion to THC in humans under different conditions (fasting and normal feeding). The results also add to the knowledge built over 40 years of research that CBD-based therapies are safe and well tolerated in humans. Finally, these results may have clinical and forensic implications considering the potentially toxic effects of Δ9-THC and other cannabis constituents, including cognitive impairment and chronic psychiatric disturbances, especially in younger patients who might be more prone to potential long-term harmful effects of the substance on their developing brains.
Acknowledgment
The authors thank the pharmaceutical company Prati Donaduzzi for their support.
Abbreviations Used
- 11-OH-THC
11-hydroxy-D9-tetrahydrocannabinol
- CBD
cannabidiol
- CV
coefficient of variation
- HPLC
high-performance liquid chromatography
- IS
Internal Standard
- LC-MS/MS
chromatography triple quadrupole mass spectrometry
- LLOQ
lower limit of quantification
- QC
quality control
- RRT
relative retention time
- SRM
selective reaction monitoring
- THC
tetrahydrocannabinol
- THCCOOH
carboxy-THC
- ULQ
upper limit of quantification
- UPLC
ultra-performance liquid chromatography
Author Disclosure Statement
J.A.S.C., A.W.Z., and J.E.C.H. are coinventors of the patent “Fluorinated CBD compounds, compositions and uses thereof. Pub. No.: WO/2014/108899. International Application No.: PCT/IL2014/050023,” Def. US number Reg. 62193296; July 29, 2015; INPI on August 19, 2015 (BR1120150164927; Mechoulam R, Zuardi A.W., Kapczinski F, Hallak J.E.C, Guimarâes F.S., Crippa J.A.S., Breuer A). The University of São Paulo has licensed the patent to Phytecs Pharm (USP Resolution No. 15.1.130002.1.1). The University of São Paulo has an agreement with PratiDonaduzzi Pharm to “develop a pharmaceutical product containing synthetic CBD and prove its safety and therapeutic efficacy in the treatment of epilepsy, schizophrenia, Parkinson's disease, and anxiety disorders.” J.A.S.C. is a member of the International Advisory Board of the Australian Centre for Cannabinoid Clinical and Research Excellence (ACRE – National Health and Medical Research Council, NHMRC).
Funding Information
J.A.S.C. and J.E.C.H. have received travel support from BSPG-Pharm. J.A.S.C., A.W.Z., and J.E.C.H. are recipients of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) productivity fellowships (1 A). J.A.S.C. received a grant from the University Global Partnership Network (UGPN)—Global priorities in cannabinoid research excellence program.
Cite this article as: Crippa JAS, Zuardi AW, Hallak JEC, Miyazawa B, Bernardo SA, Donaduzzi CM, Guzzi S, Favreto WAJ, Campos A, Queiroz MEC, Guimarães FS, Zimmermann PMR, Rechia LM, Filho VJT, Brum Junior L (2020) Oral cannabidiol does not convert to Δ8-THC or Δ9-THC in humans: a pharmacokinetic study in healthy subjects, Cannabis and Cannabinoid Research 5:1, 89–98, DOI: 10.1089/can.2019.0024.
References
- 1. Zuardi AW, Crippa JA, Hallak JE, et al. A critical review of the antipsychotic effects of cannabidiol: 30 years of a translational investigation. Curr Pharm Des. 2012;18:5131–5140 [DOI] [PubMed] [Google Scholar]
- 2. de Mello Schier AR, de Oliveira Ribeiro NP, Coutinho DS, et al. Antidepressant-like and anxiolytic-like effects of cannabidiol: a chemical compound of Cannabis sativa. CNS Neurol Disord Drug Targets. 2014;13:953–960 [DOI] [PubMed] [Google Scholar]
- 3. dos Santos RG, Hallak JE, Leite JP, et al. Phytocannabinoids and epilepsy. J Clin Pharm Ther. 2015;40:135–143 [DOI] [PubMed] [Google Scholar]
- 4. Merrick J, Lane B, Sebree T, et al. Identification of psychoactive degradants of cannabidiol in simulated gastric and physiological fluid. Cannabis Cannabinoid Res. 2016;1:102–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grotenhermen F, Russo E, Zuardi AW. Even high doses of oral cannabidol do not cause THC-like effects in humans: comment on Merrick et al. 2016. Cannabis Cannabinoid Res. 2017;2:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonn-Miller MO, Banks SL, Sebree T. Conversion of cannabidiol following oral administration: authors' response to Grotenhermen et al. Cannabis Cannabinoid Res. 2017;2:5–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hložek T, Uttl L, Kadeřábek L, et al. Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur Neuropsychopharmacol. 2017;27:1223–1237 [DOI] [PubMed] [Google Scholar]
- 8. Nahler G, Grotenhermen F, Zuardi AW, et al. A Conversion of oral cannabidiol to delta9-tetrahydrocannabinol seems not to occur in humans. Cannabis Cannabinoid Res. 2017;2:81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palazzoli F, Citti C, Licata M, et al. Development of a simple and sensitive liquid chromatography triple quadrupole mass spectrometry (LC-MS/MS) method for the determination of cannabidiol (CBD), Δ9-tetrahydrocannabinol (THC) and its metabolites in rat whole blood after oral administration of a single high dose of CBD. J Pharm Biomed Anal. 2017;28;150:25–32 [DOI] [PubMed] [Google Scholar]
- 10. Wray L, Stott C, Jones N, et al. Cannabidiol does not convert to D9-tetrahydrocannabinol in an in vivo animal model. Cannabis Cannabinoid Res. 2017;2:282–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Consroe P, Kennedy K, Schram K. Assay of plasma cannabidiol by capillary gas chromatography/ion trap mass spectroscopy following high-dose repeated daily oral administration in humans. Pharmacol Biochem Behav. 1991;40:517–522 [DOI] [PubMed] [Google Scholar]
- 12. Martin-Santos R, Crippa JA, Batalla A, et al. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr Pharm Des. 2012;18:4966–4979 [DOI] [PubMed] [Google Scholar]
- 13. Devinsky O, Cross JH, Laux L, et al. ; Cannabidiol in Dravet Syndrome Study Group. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011–2020 [DOI] [PubMed] [Google Scholar]
- 14. Devinsky O, Patel AD, Cross JH, et al. ; GWPCARE3 Study Group. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378:1888–1897 [DOI] [PubMed] [Google Scholar]
- 15. Volkow ND, Swanson JM, Evins AE, et al. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA Psychiatry. 2016;73:292–297 [DOI] [PubMed] [Google Scholar]
- 16. Linares IM, Zuardi AW, Pereira LC, et al. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Braz J Psychiatry. 2019;41:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zuardi AW, Rodrigues NP, Silva AL, et al. Inverted U-shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Front Pharmacol. 2017;8:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chagas MH, Zuardi AW, Tumas V, et al. Effects of cannabidiol in the treatment of patients with Parkinson's disease: an exploratory double-blind trial. J Psychopharmacol. 2014;28:1088–1098 [DOI] [PubMed] [Google Scholar]
- 19. Chagas MH, Eckeli AL, Zuardi AW, et al. Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson's disease patients: a case series. J Clin Pharm Ther. 2014;39:564–566 [DOI] [PubMed] [Google Scholar]
- 20. Broecker S, Pragst F. Isomerization of cannabidiol and Δ9-tetrahydrocannabinol during positive electrospray ionization. In-source hydrogen/deuterium exchange experiments by flow injection hybrid quadrupole-time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2012;26:1407–1414 [DOI] [PubMed] [Google Scholar]
- 21. Matuszewski BK, Constanzer ML, Chavez-Eng CM. Matrix effect in quantitative LC/MS/MS analyses of biological fluids: a method for determination of finasteride in human plasma at picogram per milliliter concentrations. Anal Chem. 1998;70:882–889 [DOI] [PubMed] [Google Scholar]
- 22. Lalit VS, Bhagwat NP, Sharad VU, et al. Bioanalytical method validation and its pharmaceutical application—a review. Pharm Anal Acta. 2014;5:1–7 [Google Scholar]
- 23. Crippa JA, Crippa AC, Hallak JE, et al. Δ9-THC intoxication by cannabidiol-enriched cannabis extract in two children with refractory epilepsy: full remission after switching to purified cannabidiol. Front Pharmacol. 2016;7:359. [DOI] [PMC free article] [PubMed] [Google Scholar]