Abstract
Background
There is insufficient evidence to guide stent usage following angioplasty in subclavian artery stenosis. This is an update of a review first published in 2011.
Objectives
The aim of this review was to determine whether stenting is more effective than angioplasty alone for stenosis of the subclavian artery.
Search methods
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator searched the Specialised Register (last searched February 2014) and CENTRAL (2014, Issue 1). There was no restriction on language.
Selection criteria
Randomised controlled trials of endovascular treatment of subclavian artery lesions comparing angioplasty alone and stent implantation.
Data collection and analysis
Two authors independently evaluated studies to assess eligibility. Discrepancies were resolved by discussion. If there was no agreement, the third author was asked to assess the study for inclusion.
Main results
To date we have not identified any completed or ongoing randomised controlled trials comparing percutaneous transluminal angioplasty and stenting for subclavian artery stenosis.
Authors' conclusions
There is currently insufficient evidence to determine whether stenting is more effective than angioplasty alone for stenosis of the subclavian artery.
Plain language summary
Angioplasty versus stenting for subclavian artery stenosis
The subclavian arteries are two major arteries of the upper chest, below the collar bone, that come from the arch of the aorta. The left subclavian artery supplies blood to the left arm and the right subclavian artery supplies blood to the right arm, with some branches supplying blood to the head and chest. A history of smoking, high blood pressure, lower levels of 'good' (high density lipoprotein) cholesterol and peripheral arterial disease are associated with an increased risk of subclavian artery narrowing or stenosis. Subclavian artery stenosis is often without symptoms. Symptoms when they occur include short‐lasting vertigo, commonly described as the environment spinning, due to decreased blood flow in the back part of the brain and blood circulation problems in the hands and arms. Endovascular treatment for stenosis of the subclavian arteries includes angioplasty alone and with stenting. We could not find any randomised controlled trials in the medical literature that compared the effectiveness and safety of stent implantation with angioplasty alone for treatment of subclavian artery lesions. We conclude that there is currently insufficient evidence to determine whether stenting is more effective than angioplasty alone.
Background
Description of the condition
Arterial reconstruction is less frequently performed in the upper limbs than in the lower limbs, probably because of the lower muscular mass, rich collateral circulation and the lesser burden of the weight carried by the upper limbs compared with the lower limbs. The prevalence of subclavian artery stenosis in the general population is estimated at approximately 2%. In a clinical population (that is patients recruited from vascular laboratories or medical institutions) the prevalence is about 7% (Shadman 2004).
Subclavian artery stenosis may be asymptomatic. When symptoms occur they are mainly due to vertebrobasilar insufficiency (that is decreased blood flow in the posterior circulation of the brain), upper limb ischaemia, or both.
A history of smoking, higher levels of systolic blood pressure, lower levels of high density lipoprotein cholesterol and the presence of peripheral arterial diseases are associated with an increased risk of subclavian artery stenosis (Shadman 2004).
Description of the intervention
Bachman et al (Bachman 1980) reported the first case of successful subclavian angioplasty. Lyon et al suggested that the placement of metallic stents in supra‐aortic arteries represented an effective adjunct to percutaneous balloon angioplasty of atherosclerotic stenosis in these vessels, and that primary stent placement may be an effective treatment for selected lesions (Lyon 1996).
How the intervention might work
Elastic recoil of the vessel wall is a common cause of failure of percutaneous transluminal angioplasty. Expandable metal stents are used to oppose such recoil (Palmaz 1987).
Why it is important to do this review
There is insufficient evidence to guide stent usage following angioplasty in subclavian artery stenosis. This review will address effectiveness and safety issues that could help with establishing practical guidelines.
Objectives
The aim of this review was to determine whether stenting is more effective than angioplasty alone for stenosis of the subclavian artery.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing stenting and angioplasty for stenosis of the subclavian artery. We considered trials that had been published in full or had results presented in abstract form. We included abstracts only if there were sufficient data for analysis or there were plans for full publication, or the authors had available unpublished data on file for review and analysis. In addition, we considered randomised trials conducted by the stent device manufacturers (on file but not published). There was no restriction on language.
Types of participants
We planned for the studies selected for review to include:
men and women with an indication for revascularization of the subclavian artery (stenosis > 70%);
patients with symptomatic subclavian artery or brachiocephalic trunk disease; claudication of the upper limb or vertebrobasilar insufficiency, or both;
patients with symptomatic coronary‐subclavian steal syndrome;
patients with documented occlusions or stenoses of the subclavian artery or brachiocephalic trunk (diagnosed by duplex ultrasound scan or angiogram);
patients with lesions who were allocated to treatment with balloon angioplasty or stenting;
patients receiving medical therapy for associated pre‐morbid conditions e.g. diabetes mellitus, hyperlipidaemia or hypertension, pre‐ or post‐intervention (e.g. antiplatelets, antihypertensive drugs, lipid‐lowering drugs);
patients receiving anticoagulation pre‐ or post‐intervention (e.g. heparin or warfarin);
patients not receiving any medical or anticoagulation therapy.
Types of interventions
The primary interventions of interest were angioplasty without a stent and the use of stents after restoration of patency in occlusions or stenoses of the subclavian artery or brachiocephalic trunk that is primary stenting.
We planned to include interventional studies fulfilling the following criteria:
studies in which stenting was done for secondary purposes i.e. to treat post‐angioplasty vessel wall dissections;
studies using balloon angioplasty techniques to restore patency (transluminal or subintimal);
studies including various types of stent and configuration e.g. uncovered or covered, steel or reinforced nitinol, drug eluting or simple stents.
Types of outcome measures
Primary outcomes
We planned to analyse the following primary outcome.
Vessel patency rate i.e. restenosis or re‐occlusion rates, both primary and secondary, including duration to restenosis or re‐occlusion as defined by an imaging modality such as duplex ultrasound, magnetic resonance angiography or computed tomography angiography.
Secondary outcomes
We planned to analyse the following secondary outcomes.
Improvement of symptoms i.e. disappearance of the symptoms of upper limb claudication or vertebrobasilar insufficiency, or both.
Follow up of restenosis (1, 6,12 months or longer).
We planned for the secondary outcomes to include the following.
Decrease in the difference of blood pressure between the upper limbs post‐treatment.
Morbidity rates: (a) stent related ‐ stent failure or fracture, stent migration, stent infection, stent occlusion; (b) procedural related ‐ groin or brachial hematoma, wound infection, wound bleeding, vessel rupture or perforation, vessel wall dissection, distal emboli; (c) general morbidity ‐ development of acute myocardial infarction, acute renal failure, chronic renal failure, or cerebrovascular events.
Mortality at 30 days, one year, and two years.
Measures of efficacy such as quality of life scores at 30 days, one year, and two years.
We planned to perform subgroup analyses of patients stratified by the following factors.
Stent types: drug eluting versus non‐drug eluting, steel versus reinforced (nitinol), covered (PTFE) versus uncovered.
Stent location: brachiocephalic trunk versus subclavian artery stenting.
Search methods for identification of studies
Electronic searches
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched February 2014) and the Cochrane Central Register of Controlled Trials (CENTRAL) 2014, Issue 1, part of The Cochrane Library, (www.thecochranelibrary.com). See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Peripheral Vascular Diseases Group module in The Cochrane Library (www.thecochranelibrary.com).
In addition, the authors searched the following electronic databases.
Ovid MEDLINE® (1946 to February Week 2 2014), see Appendix 2 for the search strategy.
Ovid EMBASE (1980 to Week 08, 2014), see Appendix 3 for the search strategy.
LILACS (1985 to 10 April 2014), see Appendix 4 for the search strategy.
Data collection and analysis
Selection of studies
Two review authors (AP and JEM) independently evaluated studies to assess eligibility. Discrepancies were resolved by discussion. If there was no agreement, a third review author (FS) was asked to assess the study for inclusion.
Data extraction and management
We intended that three review authors would extract data from published reports using a data collection form. We intended to resolve disagreements by consensus.
We intended to extract the following data.
The method of randomisation and whether the person undertaking the randomisation was blinded to the allocated treatment.
The number of patients originally allocated to each treatment group, to allow an intention‐to‐treat analysis.
The method of measuring outcomes and whether outcome assessment was independent or blinded, or both.
The number of exclusions and losses to follow‐up.
Intervention characteristics.
Outcome measures, as defined above.
We also planned to extract the following data to allow a number of subgroup analyses.
The proportion of symptomatic versus asymptomatic patients in each treatment group.
The degree of baseline stenosis in each treatment group.
Assessment of risk of bias in included studies
We planned to assess risk of bias of the included studies using the Cochrane risk of bias tool (Higgins 2011) assessing sequence generation; allocation concealment; blinding of participants, providers and outcome assessors; completeness of outcome data; selective outcome reporting; and other potential sources of bias. Three review authors planned to assess these domains by assigning a judgement of either low, high or unclear risk of bias according to Higgins 2011. We planned to resolve disagreements by consensus.
Measures of treatment effect
We intended to use the following measures for the effect of treatment.
For time‐to‐event data, we would use the hazard ratio, if possible.
For dichotomous outcomes, we would use the odds ratio (OR).
For continuous outcomes, we would use the mean difference between treatment arms.
We intended to report the results as OR with 95% confidence intervals (CI), which we planned to calculate using the Peto fixed‐effect model method. In view of expected heterogeneity between trials, we also planned to calculate the OR using the Mantel‐Haenszel random‐effects model. We intended to use P = 0.05 as the level of significance.
Unit of analysis issues
We planned to individually analyse each cluster‐randomised trial before deciding whether or not to include it in the review. Because of the nature of the procedures involved, it was not expected that there would be any cross‐over trials. In studies with multiple treatment groups, we planned to assess the possibility of including the subgroups with the interventions of interest.
Dealing with missing data
We did not plan to impute missing outcome data for the primary outcome. If, in future updates of this review, data are missing or only imputed data are reported, we plan to contact the trial authors to request data on the outcomes only for those participants who were assessed.
Assessment of heterogeneity
We planned to assess heterogeneity between studies by visual inspection of forest plots; by estimation of the percentage heterogeneity between trials which could not be ascribed to sampling variation (Higgins 2003); by a formal statistical test of the significance of the heterogeneity (Deeks 2001) using a standard Chi2 test with P = 0.1 as the level of significance; and, if possible, by subgroup analyses (see below). We planned to investigate and report the possible reasons where there was evidence of substantial heterogeneity.
Assessment of reporting biases
We planned to examine funnel plots corresponding to the meta‐analysis of the primary outcome to assess the potential for small study effects such as publication bias.
Data synthesis
If sufficient clinically similar studies were available, we planned to pool their results in a meta‐analysis.
For time‐to‐event data, hazard ratios would be pooled using the generic inverse variance facility of RevMan 5.
For any dichotomous outcomes, the OR would be calculated for each study and these would then be pooled.
For continuous outcomes, the mean difference between the treatment arms at the end of follow up would be pooled if all trials measured the outcome on the same scale, otherwise standardised mean differences would be pooled.
If any trials had multiple treatment groups, we planned to divide the 'shared' comparison group into the number of treatment groups and comparisons between each treatment group and we planned to treat the split comparison group as an independent comparison.
We planned to use random‐effects models with inverse variance weighting for all meta‐analyses (DerSimonian 1986).
If possible, we would synthesize the studies making different comparisons using the methods of Bucher (Bucher 1997).
Subgroup analysis and investigation of heterogeneity
We intended to perform subgroup analyses, grouping the trials by:
symptomatic and asymptomatic patients in each treatment group;
degree of baseline stenosis in each treatment group.
We planned to consider factors such as age, clinical stage, type of intervention, length of follow up, adjusted or unadjusted analysis in the interpretation of any heterogeneity.
Sensitivity analysis
We planned to perform sensitivity analyses by excluding studies at high risk of bias.
If sufficient trials were available, we also planned to performed sensitivity analyses by excluding studies which did not report adequate concealment of allocation or blinding of the outcome assessor.
Results
Description of studies
Results of the search
See Figure 1.
1.
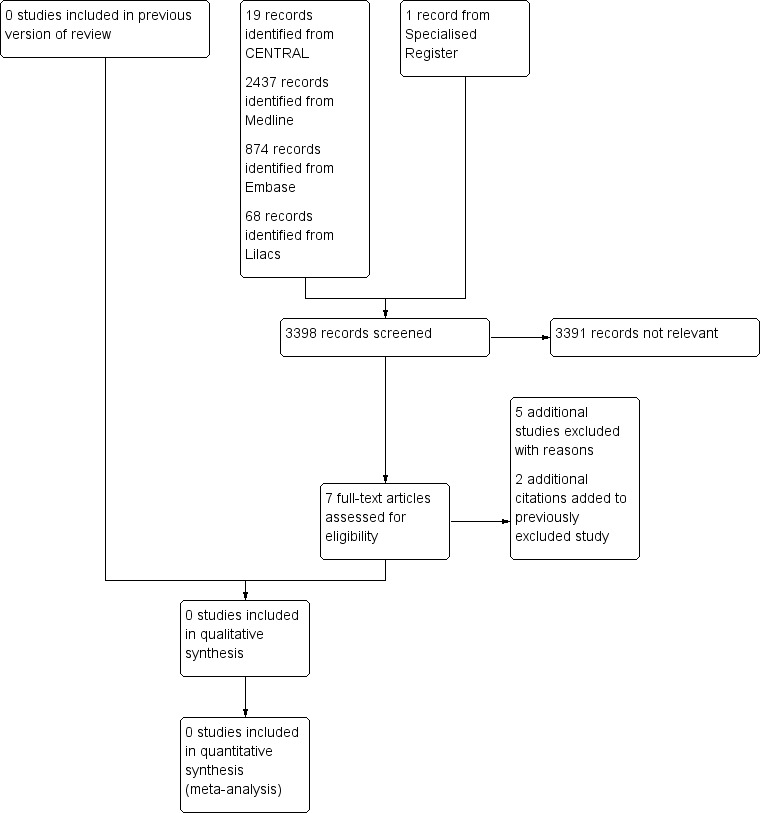
Study flow diagram.
Included studies
We did not find any randomised controlled trials, therefore no studies were included in the review.
Excluded studies
For this update an additional five studies were excluded (Angle 2003; De Vries 2005; Mathias 1993; Motarjeme 1996; Westerband 2003) and two additional citations were added to a study which had previously been excluded (Henry 1999).
In total eight studies were excluded (Angle 2003; De Vries 2005; Henry 1999; Mathias 1993; Motarjeme 1996; Schillinger 2001; Sixt 2009; Westerband 2003). All were comparative observational studies comparing results of PTA and stenting for subclavian artery stenosis.
Risk of bias in included studies
There are no included studies.
Effects of interventions
Results based on RCTs were not available as no RCTs were identified. Information regarding whether stenting is more effective than angioplasty alone for stenosis of the subclavian artery is currently based on observational studies only.
Discussion
Summary of main results
There are no randomised controlled trials (RCTs) to support that stenting is better than angioplasty alone for subclavian artery stenosis.
Overall completeness and applicability of evidence
The studies that assessed the interventions, outcomes and types of participants of interest to this review were not randomised controlled trials. Therefore there is a lack of evidence to support changes in current practice.
Quality of the evidence
The best evidence regarding whether stenting is more effective than angioplasty alone for stenosis of the subclavian artery stems from retrospective observational studies. This type of study presents biases inherent in non‐randomised studies.
Potential biases in the review process
Although we have conducted extensive literature searches only observational studies were identified.
Agreements and disagreements with other studies or reviews
Despite the absence of randomised controlled trials, there have been publications of observational studies comparing angioplasty and stenting for subclavian artery lesions (Angle 2003; De Vries 2005; Henry 1999; Mathias 1993; Motarjeme 1996; Schillinger 2001; Sixt 2009; Westerband 2003. Most of these have been summarised in a systematic review with meta‐analysis by Chatterjee 2013.
Chatterjee 2013 also did not find RCTs. Their review included 544 participants of whom 307 underwent angioplasty alone and 237 had stents implanted. The results of the observational studies suggests that stenting after PTA is superior to angioplasty alone for treatment of subclavian artery stenosis and maintenance of patency at one year without significant complication rates for either procedure.
Still, the best evidence regarding whether stenting is more effective than angioplasty alone for stenosis of the subclavian artery stems from retrospective observational studies.
Authors' conclusions
Implications for practice.
There is currently insufficient evidence to determine whether stenting is more effective than angioplasty alone for stenosis of the subclavian artery.
Implications for research.
A randomised controlled trial comparing the two intervention modalities would be desirable. Future research should focus on clinical endpoints, technical endpoints and complications. We envisage that the main clinical endpoints should be improvement of the symptoms of upper limb ischaemia, vertebrobasilar insufficiency and the subclavian steal phenomenon. Complications should include distal embolisation, stroke and transient ischaemic attack and technical endpoints should be assessed by primary and secondary patency, and restenosis or re‐occlusion rates in short, mid and long‐term (Rodriguez‐Lopez 1999; White 2007) follow‐up.
What's new
| Date | Event | Description |
|---|---|---|
| 25 February 2014 | New search has been performed | Searches rerun. No new included studies were identified, five additional studies were excluded. |
| 25 February 2014 | New citation required but conclusions have not changed | New authors have joined the review team. Searches rerun. No new included studies were identified, five additional studies were excluded. Minor changes to the text of the review. Conclusions not changed. |
Acknowledgements
We would like to thank Prof Dr Álvaro Nagib Atallah, Dr Edina Mariko Koga da Silva and Dr Marlene Stewart for their guidance, and Dr Karen Welch for her essential assistance in the preparation of strategies for searching the electronic databases.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MeSH descriptor: [Angioplasty] explode all trees | 4361 |
| #2 | (angioplas* or percutan* or PTA):ti,ab,kw (Word variations have been searched) | 10285 |
| #3 | recanali* or revascular*:ti,ab,kw (Word variations have been searched) | 5040 |
| #4 | dilat*:ti,ab,kw (Word variations have been searched) | 5792 |
| #5 | balloon or baloon:ti,ab,kw (Word variations have been searched) | 6059 |
| #6 | MeSH descriptor: [Endovascular Procedures] explode all trees | 6017 |
| #7 | endovascular:ti,ab,kw (Word variations have been searched) | 935 |
| #8 | #1 or #2 or #3 or #4 or #5 or #6 or #7 | 21464 |
| #9 | MeSH descriptor: [Blood Vessel Prosthesis] explode all trees | 452 |
| #10 | MeSH descriptor: [Blood Vessel Prosthesis Implantation] explode all trees | 508 |
| #11 | MeSH descriptor: [Stents] explode all trees | 3314 |
| #12 | *stent* or graft* or endograft* or endoprosthe*:ti,ab,kw (Word variations have been searched) | 45533 |
| #13 | powerlink or talent or excluder or aorfix or zenith or endologix or anaconda or Triascular or Cordis or Endurant or Quantum or Aneurx or Ancure or Advanta or Intracoil or Zilver or Luminex:ti,ab,kw (Word variations have been searched) | 292 |
| #14 | #9 or #10 or #11 or #12 or #13 | 45928 |
| #15 | #8 and #14 | 5618 |
| #16 | MeSH descriptor: [Subclavian Artery] explode all trees | 19 |
| #17 | MeSH descriptor: [Brachiocephalic Trunk] explode all trees | 3 |
| #18 | MeSH descriptor: [Brachiocephalic Veins] explode all trees | 14 |
| #19 | subclav* or sub‐clav*:ti,ab,kw (Word variations have been searched) | 305 |
| #20 | brachioceph*:ti,ab,kw (Word variations have been searched) | 32 |
| #21 | MeSH descriptor: [Vertebrobasilar Insufficiency] explode all trees | 46 |
| #22 | vertebro* near/3 insuff*:ti,ab,kw (Word variations have been searched) | 79 |
| #23 | steal:ti,ab,kw (Word variations have been searched) | 85 |
| #24 | #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 | 483 |
| #25 | #15 and #24 in Trials | 19 |
Appendix 2. Authors' MEDLINE search strategy
Database: Ovid MEDLINE(R) <1946 to February Week 2 2014>
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp Angioplasty/ (53773)
2 Vascular Surgical Procedures/ (23182)
3 Blood Vessel Prosthesis Implantation/ (15107)
4 exp Balloon Dilatation/ (0)
5 exp Stents/ (51225)
6 (angioplasty or stent$ or PTA or revasculari?ation or dilatation or endovascular).ti,ab. (159919)
7 1 or 2 or 3 or 4 or 5 or 6 (203672)
8 arterial occlusive diseases/ or arteriosclerosis/ or arteriolosclerosis/ or arteriosclerosis obliterans/ or atherosclerosis/ (100175)
9 (isch?emia or insufficiency or arteriosclerosis or atherosclerosis or occlus$ or claudic$ or steno$).ti,ab. (504210)
10 8 or 9 (545465)
11 Subclavian Artery/ (6372)
12 Brachiocephalic Trunk/ (2240)
13 Brachiocephalic Veins/ (1252)
14 (subclav$ or sub‐clav$).ti,ab. (12695)
15 brachioceph$.ti,ab. (2378)
16 exp Vertebrobasilar Insufficiency/ (4160)
17 (vertebro$ adj3 insuff$).ti,ab. (765)
18 steal.ti,ab. (3131)
19 or/11‐18 (23347)
20 7 and 10 and 19 (2437)
Appendix 3. Authors' EMBASE search strategy
Database: Ovid EMBASE <1980 to 2014 Week 08>
Database: Embase <1980 to 2014 Week 08>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp angioplasty/ (68370)
2 vascular surgery/ (27727)
3 exp blood vessel prosthesis/ (14224)
4 exp balloon dilatation/ (12940)
5 exp stent/ (99626)
6 (angioplasty or stent$ or PTA or revasculari?ation or dilatation or endovascular).ti,ab. (239308)
7 1 or 2 or 3 or 4 or 5 or 6 (310964)
8 exp arteriosclerosis/ (188137)
9 exp peripheral occlusive artery disease/ (116627)
10 (isch?emia or insufficiency or arteriosclerosis or atherosclerosis or occlus$ or claudic$ or steno$*).ti,ab. (672294)
11 8 or 9 or 10 (793215)
12 subclavian artery/ (8725)
13 brachiocephalic trunk/ (3232)
14 brachiocephalic vein/ (1750)
15 (subclav$ or sub‐clav$).ti,ab. (16835)
16 brachioceph$.ti,ab. (3255)
17 vertebrobasilar insufficiency/ (2293)
18 (vertebro$ adj3 insuff$).ti,ab. (1025)
19 steal.ti,ab. (3931)
20 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 (29140)
21 7 and 13 and 20 (874)
Appendix 4. Authors' Lilacs search strategy
("vascular surgical procedures") or "blood vessel prosthesis implantation" [Subject descriptor] or ("Balloon Dilatation") or (Stents) or (Stent) or (angioplasty) or (PTA) or (revascularization) or (dilatation) or (endovascular) [Words] and (Subclavian) or ("Subclavian Artery") or ("Brachiocephalic Trunk") or ("Brachiocephalic Veins") or ("Vertebrobasilar Insufficiency") [Words] (68)
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Angle 2003 | Not a randomised controlled trial. |
| De Vries 2005 | Not a randomised controlled trial. |
| Henry 1999 | Not a randomised controlled trial. |
| Mathias 1993 | Not a randomised controlled trial. |
| Motarjeme 1996 | Not a randomised controlled trial. |
| Schillinger 2001 | Not a randomised controlled trial. |
| Sixt 2009 | Not a randomised controlled trial. |
| Westerband 2003 | Not a randomised controlled trial. |
Contributions of authors
AP and JEM selected trials for inclusion. DSC and WI assessed methodological quality and extracted data. FS resolved any disagreements in trial selection, assessed methodological quality and extracted data. WI and DSC wrote the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The PVD Group editorial base is supported by the Chief Scientist Office.
Declarations of interest
None known
New search for studies and content updated (no change to conclusions)
References
References to studies excluded from this review
Angle 2003 {published data only}
- Angle FJ, Matsumoto AH, McGraw JK, Spinosa DJ, Hagspiel KD, Leung DA, et al. Percutaneous angioplasty and stenting of left subclavian artery stenosis in patients with left internal mammary‐coronary bypass grafts: clinical experience and long‐term follow‐up. Vascular and Endovascular Surgery 2003;37:89‐97. [DOI] [PubMed] [Google Scholar]
De Vries 2005 {published data only}
- Vries JP, Jager LC, Berg JC, Overtoom TT, Ackerstaff RG, Pavoordt ED, et al. Durability of percutaneous transluminal angioplasty for obstructive lesions of proximal subclavian artery: long‐term results. Journal of Vascular Surgery 2005;41(1):19‐23. [DOI] [PubMed] [Google Scholar]
Henry 1999 {published data only}
- Henry IP, Benjelloun A, Henry MC. Percutaneous transluminal angioplasty of the subclavian arteries. Long‐term follow up. Journal of the American College of Cardiology 2013;62(18 Suppl 1):B156. [Google Scholar]
- Henry M, Armor M, Henry I, Ethevenot G, Tzvetanov K, Chati Z. Percutaneous transluminal angioplasty of the subclavian arteries. Journal of Endovascular Surgery 1999;6:33‐41. [DOI] [PubMed] [Google Scholar]
- Henry M, Hugel M, Henry I, Polydorou A. Percutaneous transluminal angioplasty of the subclavian arteries. Long‐term followup. Journal of the American College of Cardiology 2010;56(13):B95. [PubMed] [Google Scholar]
Mathias 1993 {published data only}
- Mathias KD, Lüth I, Haarmann P. Percutaneous transluminal angioplasty of proximal subclavian artery occlusions. Cardiovascular and Interventional Radiology 1993;16:214‐8. [DOI] [PubMed] [Google Scholar]
Motarjeme 1996 {published data only}
- Motarjeme A. Percutaneous transluminal angioplasty of supra‐aortic vessels. Journal of Endovascular Surgery 1996;3(2):171‐81. [DOI] [PubMed] [Google Scholar]
Schillinger 2001 {published data only}
- Schillinger M, Haumer M, Schillinger S, Ahmadi R, Minar E. Risk stratification for subclavian artery angioplasty: is there an increased rate of restenosis after stent implantation?. Journal of Endovascular Therapy 2001;8(6):550–7. [DOI] [PubMed] [Google Scholar]
Sixt 2009 {published data only}
- Sixt S, Rastan A, Schwarzwalder U, Burgelin K, Noory E, Schwarz T, et al. Results after balloon angioplasty or stenting of atherosclerotic subclavian artery obstruction. Catheterization and Cardiovascular Interventions 2009;73(3):395–403. [DOI] [PubMed] [Google Scholar]
Westerband 2003 {published data only}
- Westerband A, Rodriguez JA, Ramaiah VG, Diethrich EB. Endovascular therapy in prevention and management of coronary‐subclavian steal. Journal of Vascular Surgery 2003;38(4):699‐703. [DOI] [PubMed] [Google Scholar]
Additional references
Bachman 1980
- Bachman DM, Kim RM. Transluminal dilatation for subclavian steal syndrome. American Journal of Roentgenology 1980;135(5):995‐6. [DOI] [PubMed] [Google Scholar]
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. [DOI] [PubMed] [Google Scholar]
Chatterjee 2013
- Chatterjee S, Nerella N, Chakravarty S, Shani J. Angioplasty alone versus angioplasty and stenting for subclavian artery stenosis ‐ a systematic review and meta‐analysis. American Journal of Therapeutics 2013;20(5):520‐3. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in seta‐Analysis. Systematic reviews in health care: Meta‐analysis in context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lyon 1996
- Lyon RD, Shonnard KM, McCarter DL, Hammond SL, Ferguson D, Rholl KS. Supra‐aortic arterial stenoses: management with Palmaz balloon‐expandable intraluminal stents. Journal of Vascular and Interventional Radiology 1996;7(6):825‐35. [DOI] [PubMed] [Google Scholar]
Palmaz 1987
- Palmaz JC, Kopp DT, Hayashi H, Schatz RA, Hunter G, Tio FO, et al. Normal and stenotic renal arteries: experimental balloon‐expandable intraluminal stenting. Radiology 1987;164(3):705‐8. [DOI] [PubMed] [Google Scholar]
Rodriguez‐Lopez 1999
- Rodriguez‐Lopez JA, Werner A, Martinez R, Torruella LJ, Ray LI, Diethrich EB. Stenting for atherosclerotic occlusive disease of the subclavian artery. Annals of Vascular Surgery 1999;13(3):254‐60. [DOI] [PubMed] [Google Scholar]
Shadman 2004
- Shadman R, Criqui MH, Bundens WP, Fronek A, Denenberg JO, Gamst AC, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. Journal of the American College of Cardiology 2004;44(3):618‐23. [DOI] [PubMed] [Google Scholar]
White 2007
- White CJ. Non‐surgical treatment of patients with peripheral vascular disease. British Medical Bulletin 2001;59:173‐92. [DOI] [PubMed] [Google Scholar]


