Abstract
Background
Concern about estrogen‐related adverse effects has led to progressive reductions in the estrogen dose in combination oral contraceptives (COCs). However, reducing the amount of estrogen to improve safety could result in decreased contraceptive effectiveness and unacceptable changes in bleeding patterns.
Objectives
To test the hypothesis that COCs containing ≤ 20 μg ethinyl estradiol (EE) perform similarly as those containing > 20 μg in terms of contraceptive effectiveness, bleeding patterns, discontinuation, and side effects.
Search methods
In July 2013, we searched CENTRAL, MEDLINE, and POPLINE, and examined references of potentially eligible trials. We also searched for recent clinical trials using ClinicalTrials.gov and ICTRP. No new trials met the inclusion criteria. Previous searches included EMBASE. For the initial review, we wrote to oral contraceptive manufacturers to identify trials.
Selection criteria
English‐language reports of randomized controlled trials were eligible that compare a COC containing ≤ 20 μg EE with a COC containing > 20 μg EE. We excluded studies where the interventions were designed to be administered for less than three consecutive cycles or to be used primarily as treatment for non‐contraceptive conditions. Trials had to report on contraceptive effectiveness, bleeding patterns, trial discontinuation due to bleeding‐related reasons or other side effects, or side effects to be included in the review.
Data collection and analysis
One author evaluated all titles and abstracts from literature searches to determine whether they met the inclusion criteria. Two authors independently extracted data from studies identified for inclusion. We wrote to the researchers when additional information was needed. Data were entered and analyzed with RevMan.
Main results
No differences were found in contraceptive effectiveness for the 13 COC pairs for which this outcome was reported. Compared to the higher‐estrogen pills, several COCs containing 20 μg EE resulted in higher rates of early trial discontinuation (overall and due to adverse events such as irregular bleeding) as well as increased risk of bleeding disturbances (both amenorrhea or infrequent bleeding and irregular, prolonged, frequent bleeding, or breakthrough bleeding or spotting).
Authors' conclusions
While COCs containing 20 μg EE may be theoretically safer, this review did not focus on the rare events required to assess this hypothesis. Data from existing randomized controlled trials are inadequate to detect possible differences in contraceptive effectiveness. Low‐dose estrogen COCs resulted in higher rates of bleeding pattern disruptions. However, most trials compared COCs containing different progestin types, and changes in bleeding patterns could be related to progestin type as well as estrogen dose. Higher follow‐up rates are essential for meaningful interpretation of results.
Keywords: Female; Humans; Contraceptives, Oral, Combined; Contraceptives, Oral, Combined/administration & dosage; Contraceptives, Oral, Combined/adverse effects; Contraceptives, Oral, Hormonal; Contraceptives, Oral, Hormonal/administration & dosage; Contraceptives, Oral, Hormonal/adverse effects; Desogestrel; Desogestrel/administration & dosage; Desogestrel/adverse effects; Estrogens; Estrogens/administration & dosage; Estrogens/adverse effects; Ethinyl Estradiol; Ethinyl Estradiol/administration & dosage; Ethinyl Estradiol/adverse effects; Menstruation Disturbances; Menstruation Disturbances/chemically induced; Randomized Controlled Trials as Topic
Plain language summary
Birth control pills with 20 µg estrogen versus more than 20 µg estrogen
Concerns about safety have led to making birth control pills with less of the hormone estrogen. Pills with less estrogen might not work as well to prevent pregnancy and could cause bleeding problems. This review looked at studies that compared pills with 20 µg ethinyl estradiol versus pills that have more estrogen.
In July 2013, we did computer searches for randomized trials of pills with 20 µg estrogen versus more estrogen. We did not find any new trials. For the initial review, we also wrote to researchers and makers of birth control pills to find other trials.
Studies had to be written in the English language, include at least three cycles, and focus on birth control. The trials had to report on pregnancy, bleeding problems, or stopping the pills early. We also looked at side effects.
More women taking the pills with less estrogen quit the studies early. The women on less estrogen also had more bleeding problems than those taking pills with more estrogen. Pregnancy rates seemed to be the same between groups, but the studies may not have been large enough to know for sure. This review did not focus on the rare events needed to test whether birth control pills with 20 µg estrogen were safer. Also, most trials compared pills with different types of the hormone progestin, which could also affect bleeding patterns. The high losses in many trials make the results hard to interpret.
Background
Since the introduction of combined estrogen and progestin oral contraceptives (COCs) in the early 1960s, the dose of estrogen has been reduced progressively. COCs containing 50 μg of estrogen or more comprised over 99% of OC retail prescriptions in the United States (USA) in 1968 (Gerstman 1991). Twenty years later, less than 2% of these prescriptions were for 50‐μg estrogen COCs. The reduction in the estrogen dose has been in response to two main discoveries. First, concern about estrogen‐related adverse effects has driven the search for lower‐dose estrogen COCs. COC use has been linked in epidemiological studies to breast cancer (CGHFBC 1996), and estrogen has been associated with a number of adverse events, including cerebrovascular complications, thromboembolic incidents, and myocardial infarction (Anonymous 2000). Low‐estrogen COCs have been formulated in an attempt to reduce the risk of these rare events. Second, the discovery that estrogen and progestin act synergistically to inhibit ovulation revealed that contraceptive efficacy could be maintained with lower doses of each component. COCs with less than 50 μg estrogen contain ethinyl estradiol (EE) as the estrogenic component in combination with a progestin (Nelson 2007). Twenty‐μg EE COCs (Table 1) first became available in the 1970s and, by 1998, accounted for about 8% of COC prescriptions in the USA (Wallach 2000). COC pills with 15 μg of estrogen contain the lowest estrogen dose available (Table 2) and have been approved for use in some countries in Europe, South America and elsewhere (IPPF 2013).
1. Twenty µg estrogen combination oral contraceptive.
| Formulation | Brand name | Manufacturer |
| 150 µg desogestrel and 20 µg EE | Ciclidon 20 | Lafi S.A. |
| Cycleane‐20 | G D Searle & Co | |
| Dal | Osteolab | |
| Desmin 20 | Grunenthal GmbH | |
| Desoren 20 | Beta‐Grunenthal | |
| Femilon | Organon International NV | |
| Lovelle | ||
| Marvelon 20 | ||
| Mercilon | ||
| Mircette | ||
| Myralon | ||
| Securgin | ||
| Segurin | ||
| Suavuret | ||
| Femina | Ache Laboratorios Farmaceuticos S/A | |
| Gedarel 20/150 | Consilient Health Ltd | |
| Gynostat‐20 | Pharmatrade S.A. | |
| Lovina 20 | Hexal | |
| Midalet 20 | Silesia | |
| Neolette | ||
| Novynette | Gedeon Richter | |
| 75 µg gestodene and 20 µg EE | Ciclomex 20 | Pharmafina S.A. |
| Diminut | Libbs | |
| Estinette 20 | Effik Group | |
| Fedra | Schering AG | |
| Femiane | ||
| Femodette | ||
| Gynera 75/20 | ||
| Gynovin 20 | ||
| Logest | ||
| Meliane | ||
| Meloden | ||
| Melodene | ||
| Minigeste | ||
| Femadiol‐Mepha 20 | Mepha Pharma AG | |
| Feminol‐20 | Pharmatrade S.A. | |
| Gyselle 20 | Spirig Pharma Ltd | |
| Harmonet | Wyeth‐Ayerst International Inc | |
| Lerogin 20 | Recalcine | |
| Lindynette 20 | Gedeon Richter, Ltd | |
| Microgen | Silesia | |
| Millinette | Consilient Health Ltd | |
| Minifem | Urufarma | |
| Sunya | Stratgen Pharma SA | |
| 100 µg levonorgestrel and 20 µg EE | Alesse | Wyeth‐Ayerst International Inc |
| Leois | ||
| Loette | ||
| Loette 21 | ||
| Lovette | ||
| Anulette 20 | Silesia | |
| Aprll | Gador | |
| Elyfem 20 | Berlis | |
| Femexin | Urufarma | |
| Levlite | Berlex Inc ‐ subsidiary of Schering AG | |
| Microgynon 20 | Schering AG | |
| Microgynon 20 ED | ||
| Microgynon Suave | ||
| Microlite | ||
| Miranova | ||
| Microlevlen | Bayer HealthCare | |
| Minisiston | ||
| Norvetal | Recalcine | |
| 500 µg norethisterone and 20 µg EE | Eve | Grunenthal |
| 1000 µg norethisterone acetate and 20 µg EE | Loestrin | Pfizer Inc |
| Loestrin 1/20 | ||
| Loestrin 20 | ||
| Loestrin 21 1/20 | ||
| Loestrin Fe 1/20+ | ||
| Minestril‐20 | ||
| Minestrin 1/20 | ||
| 3 mg drospirenone and 20 µg EE | Aliane | Bayer HealthCare |
| Beyaz | ||
| Dschess | ||
| Dzhess | ||
| Eloine | ||
| Jasminelle | ||
| Jasminellecontinu | ||
| Liofora | ||
| Yasmin 24/4 | ||
| Yasminelle | ||
| Yasminique | ||
| YAZ | ||
| Diva | Urufarma |
2. Fifteen µg estrogen combination oral contraceptive.
| Formulation | Brand name | Manufacturer |
| 60 µg gestodene and 15 µg EE | Arianna | Bayer HealthCare |
| Careza | Silesia | |
| Meliane Light | Schering AG | |
| Melodene 15 | ||
| Melodia | ||
| Mirelle | ||
| Minesse | Wyeth‐Ayerst International Inc | |
| Secret 28 | Urufarma |
Reducing the estrogen dose to improve safety also could decrease contraceptive effectiveness and cycle control. Contraceptive effectiveness depends both on individual susceptibility and compliance. Determining the optimal estrogen dose required is complicated by high biological variation. The inter‐individual variation in the blood levels of exogenous hormones has been estimated to vary tenfold (Guillebaud 1989) and intra‐individual differences also occur. Consequently, the lowest estrogen dose needed to prevent pregnancy while also maintaining acceptable cycle control and minimal adverse side effects could vary substantially both among women and within an individual woman at different times. Also, the contraceptive effectiveness of low‐estrogen COCs could be influenced more by missed pills or drug interactions (Elstein 1994).
Contraceptives with lower doses of estrogen also may compromise cycle control. Evaluating these concerns is difficult due to the lack of uniformity in the analyses of bleeding patterns. Recognizing the ambiguities in the interpretation of trial results and the comparison of contraceptive products, the World Health Organization (WHO) issued recommendations to standardize the collection and analysis of bleeding pattern data (Belsey 1986). Recently, Mishell 2007a reviewed methods used for collecting and analyzing bleeding data for trial reports of combined hormonal contraceptives. Mishell 2007b then developed recommendations for designing trials and for data collection and analysis of bleeding data. However, when this review was initially conducted, Belsey 1986 was considered the standard. 'Bleeding' was defined as a bloody vaginal discharge that requires sanitary protection whereas 'spotting' does not require protection. A 'bleeding or spotting episode' consists of one or more days with bleeding or spotting that is bounded by days without bleeding or spotting. The WHO advised that the woman, rather than the cycle, be used as the unit of analysis; this avoids disproportionate weight in the analysis from women who contribute more cycles and also prevents artificially precise confidence intervals (Belsey 1986). Outcomes should be measured using reference periods of at least 90 days, an amount of time that was sufficiently short to allow the identification of changes over time, while also long enough for bleeding patterns to be characterized properly. The reference period was modified to 84 days in a recent trial of COCs to correspond to the typical length of three pill cycles (Miller 2001). The WHO identified five bleeding outcomes to be included: the proportion of women with prolonged, frequent, infrequent, or irregular bleeding or spotting episodes and the proportion with amenorrhea during the reference period (Belsey 1986). They noted that the timing of bleeding is an additional issue particular to COC research and that terms for assessing bleeding associated with the pill‐free interval (e.g., intermenstrual or breakthrough bleeding) should be defined and evaluated. However, the reporting of trial results often does not conform to the WHO recommendations. Instead many trials report only cyclical data for outcomes related to the control of the menstrual cycle, often without specifying precise definitions for the terms used.
Low‐estrogen COCs have been attributed with more breakthrough bleeding and spotting (Nelson 2007). Although bleeding irregularities do not threaten health, sub‐optimal cycle control impairs the acceptance of and adherence to the contraceptive. A large, six‐month USA study found that 46% of pill users discontinued the method due to side effects or physician recommendations, and about 12% of these women identified bleeding irregularities as the primary reason for discontinuation (Rosenberg 1998). The progestin type and dosing regimen are also thought to affect cycle control. Reviews of COCs suggest that levonorgestrel results in better cycle control than norethindrone and that gestodene performs better than desogestrel and levonorgestrel (Rosenberg 1992; Maitra 2004). Given the possible relationship between progestin type and bleeding patterns, a meta‐analysis of low‐estrogen contraceptives should combine only trials that compare identical drugs, dosages, and regimens.
A secondary concern related to reducing the estrogenic component involves possible decreases in the non‐contraceptive benefits of COCs. Combined oral contraception confers health benefits, such as the prevention of ovarian cancer and endometrial cancer and the reduction of acne (Nelson 2007), and the effect of reducing the estrogen level on these preventive benefits is unclear. Ness 2000 found that the reduction in the risk of ovarian cancer was similar for COCs containing less than 50 μg estrogen compared to those with 50 μg or more. Because 20‐μg estrogen COCs were not analyzed separately from the other low‐estrogen pills, the question remains as to whether this low dose is sufficient to maintain the protective effect. Contemporary low‐estrogen COC pills may grant no protection against functional ovarian cysts, as was observed with COCs containing higher doses of estrogen (Holt 1992). As to acne reduction, a COC containing 20 μg estrogen and 100 μg levonorgestrel had better results than a placebo in Thiboutot 2001. However, whether the low‐dose estrogen contraceptive is as effective in reducing acne as a higher‐dose estrogen COC is unknown. Furthermore, low‐estrogen contraceptives might vary in their ability to improve acne.
Combined contraceptives should have high contraceptive effectiveness, while maintaining cycle control and causing minimal adverse effects. The present review evaluates COCs containing 20 μg EE or less with those containing a higher dose of EE regarding these key outcomes. Rare adverse events, though, were not a focus because randomized controlled trials generally do not have sufficient power to study infrequent events, even when combined.
Objectives
To test the hypothesis that COCs containing ≤ 20 μg of EE perform similarly as those containing > 20 μg in terms of contraceptive effectiveness, bleeding patterns, discontinuation, and side effects.
Methods
Criteria for considering studies for this review
Types of studies
Published or unpublished randomized controlled trials that compare a COC containing 20 μg of EE or less with a COC containing more than 20 μg EE were eligible. Trials were restricted to those reported in English (Higgins 2011).
Types of participants
Women of reproductive age without medical contraindications to COCs, irrespective of previous COC history were eligible.
Types of interventions
Eligible interventions included any COC containing ≤ 20 μg of EE that was compared with a COC containing > 20 μg EE. Trial interventions had to be designed to be administered for a minimum of three consecutive cycles to be eligible for inclusion. Studies were excluded if the interventions were used primarily as treatment for non‐contraceptive conditions (e.g., acne, anovulation, dysmenorrhea, menorrhagia, pelvic pain, or endometriosis).
Types of outcome measures
To be included, trials had to report on contraceptive effectiveness, bleeding patterns, side effects, or trial discontinuation due to bleeding‐related reasons or other side effects. Those that measured only biochemical changes were not eligible. The outcomes were measured as follows:
1) Contraceptive effectiveness
Cumulative life‐table or Kaplan‐Meier pregnancy rate
Pregnancy Pearl index
Proportion of women pregnant
2) Discontinuation (overall, due to bleeding‐related reasons, and due to other side effects)
Cumulative life‐table or Kaplan‐Meier discontinuation rate
Proportion of women who did not complete the trial
Discontinuation Pearl index
3) Bleeding patterns
Cycle control during reference periods of 90 days (Belsey 1986) or 84 days (Miller 2001)
Proportion of women with amenorrhea
Proportion of women with prolonged (i.e., longer than 14 days) bleeding or spotting episodes
Proportion of women with frequent (i.e., more than 5) bleeding or spotting episodes
Proportion of women with infrequent (i.e., less than 3) bleeding or spotting episodes
Proportion of women with irregular bleeding (i.e., 3 to 5 bleeding or spotting episodes and less than 3 bleeding or spotting‐free intervals of at least 14 days)
Proportion of women with amenorrhea or breakthrough (also known as intermenstrual) bleeding or spotting for cycle three or cycle six, or if data for these cycles were not reported, for the last cycle of follow up
4) Side effects
Proportion of women experiencing any side effect reported
Search methods for identification of studies
Electronic searches
We searched the computerized databases the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and POPLINE for eligible trials using a list of brand names of COCs containing 20 μg of EE or less (IPPF 2013). We also searched for trials via ClinicalTrials.gov and ICTRP. The strategies are shown in Appendix 1. Previous strategies, which also included EMBASE, can be found in Appendix 2.
Searching other resources
We assessed the references of eligible trials. For the initial review, we wrote to COC manufacturers to request information about any other published or unpublished trials not discovered in our search.
Data collection and analysis
One author evaluated the titles and abstracts located in the literature searches to determine whether they met the inclusion criteria. Two authors independently extracted data from the studies identified for inclusion. We wrote to the researchers when clarifications or additional data were needed. Data were entered and analyzed with RevMan. For trials that included more than two intervention groups, regardless of whether the authors identified a control group, we treated the ≤ 20 μg EE contraceptive as the referent and compared it to the other study groups.
We calculated Peto odds ratios (ORs) with 95% confidence intervals (CIs) for all dichotomous outcomes and mean differences using fixed‐effect models with 95% CIs for all continuous outcomes. Contraceptive effectiveness and early discontinuation also were presented as Pearl indices or survival analysis estimates when these measures were available. Pearl indices are calculated by the number of events divided by the total person‐time at risk of the event (Trussell 1998). Because contraceptive failure rates typically decrease with duration of use, the Pearl index rate tends to decline as the amount of person‐time contributed by each woman increases. Thus, rates from two studies will not be comparable if the studies differed in duration. Although the Pearl method is a sub‐optimal measurement, it was included in the review because its use remains prevalent. Survival analyses, which include life‐table and Kaplan‐Meier methods, are preferred measures. Because RevMan 4.2, used for the initial review, was not designed to include Pearl indices or measures of survival analysis, these estimates were presented in Table 3 and Table 4. Most of the studies that included contraceptive effectiveness data simply reported the number of women who became pregnant, and we used these proportions of women to calculate ORs in RevMan. However, these measures potentially are biased in favor of OCs that result in higher discontinuation rates since women who discontinue the study treatment or study participation or who are lost to follow up were unlikely to have been followed to determine whether they became pregnant.
3. Pregnancy.
| Study ID | Effect measure | OC | Rate | 95% CI | P value log rank test |
| Akerlund 1993 | Pearl index (12 cycle) | EE 20 µg and desogestrel 150 µg | 0.4 | (0.0 to 1.5) | |
| EE 30 µg and desogestrel 150 µg | 0.6 | (0.1 to 1.6) | |||
| Hampton 2001 | Kaplan‐Meier (13 cycle) | EE 20 µg and norethindrone acetate 1 mg | 2.6 | (1.1 to 4.2) | |
| EE 25 µg and norgestimate 180‐215‐250 µg | 1.9 | (0.8 to 2.9) | |||
| WHO 1982 | Life‐table (676 days) | EE 20 µg and norethindrone acetate 1 mg | 5.0 | 0.07 | |
| EE 30 µg and levonorgestrel 150 µg | 5.1 | ||||
| EE 50 µg and levonorgestrel 150 µg | 4.2 | ||||
| Mestranol 50 µg and norethistrone 1 mg | 1.0 | ||||
| EE 35 µg and norethindrone acetate 400 µg | 6.0 | ||||
| EE 50 µg and norethindrone acetate 1 mg | 4.2 |
4. Lifetable discontinuation at 676 days per 100 women (WHO 1982).
| Effect estimate | OC | Rate | P value log rank test |
| Overall | EE 20 µg and norethisterone acetate 1 mg | 68.8 | 0.04 |
| EE 30 µg and levonorgestrel 150 µg | 62.0 | ||
| EE 50 µg and levonorgestrel 150 µg | 60.6 | ||
| Mestranol 50 µg and norethistrone 1 mg | 60.4 | ||
| EE 35 µg and norethisterone acetate 400 µg | 63.5 | ||
| EE 50 µg and norethindrone acetate 1 mg | 64.9 | ||
| Due to medical reasons | EE 20 µg and norethisterone acetate 1 mg | 46.4 | 0.00 |
| EE 30 µg and levonorgestrel 150 µg | 35.0 | ||
| EE 50 µg and levonorgestrel 150 µg | 33.8 | ||
| Mestranol 50 µg and norethistrone 1 mg | 30.9 | ||
| EE 35 µg and norethisterone acetate 400 µg | 39.4 | ||
| EE 50 µg and norethindrone acetate 1 mg | 40.3 | ||
| Due to amenorrhea | EE 20 µg and norethisterone acetate 1 mg | 13.7 | 0.00 |
| EE 30 µg and levonorgestrel 150 µg | 1.5 | ||
| EE 50 µg and levonorgestrel 150 µg | 0.5 | ||
| Mestranol 50 µg and norethistrone 1 mg | 3.3 | ||
| EE 35 µg and norethisterone acetate 400 µg | 2.6 | ||
| EE 50 µg and norethindrone acetate 1 mg | 3.3 | ||
| Due to irregular bleeding | EE 20 µg and norethisterone acetate 1 mg | 4.2 | 0.00 |
| EE 30 µg and levonorgestrel 150 µg | 2.8 | ||
| EE 50 µg and levonorgestrel 150 µg | 2.6 | ||
| Mestranol 50 µg and norethistrone 1 mg | 2.8 | ||
| EE 35 µg and norethisterone acetate 400 µg | 8.5 | ||
| EE 50 µg and norethindrone acetate 1 mg | 6.2 | ||
| Due to prolonged bleeding | EE 20 µg and norethisterone acetate 1 mg | 1.8 | 0.26 |
| EE 30 µg and levonorgestrel 150 µg | 0.3 | ||
| EE 50 µg and levonorgestrel 150 µg | 0.7 | ||
| Mestranol 50 µg and norethistrone 1 mg | 0.3 | ||
| EE 35 µg and norethisterone acetate 400 µg | 1.5 | ||
| EE 50 µg and norethindrone acetate 1 mg | 1.2 | ||
| Due to light bleeding | EE 20 µg and norethisterone acetate 1 mg | 2.4 | 0.13 |
| EE 30 µg and levonorgestrel 150 µg | 0.0 | ||
| EE 50 µg and levonorgestrel 150 µg | 1.2 | ||
| Mestranol 50 µg and norethistrone 1 mg | 2.2 | ||
| EE 35 µg and norethisterone acetate 400 µg | 1.6 | ||
| EE 50 µg and norethindrone acetate 1 mg | 2.0 | ||
| Due to spotting | EE 20 µg and norethisterone acetate 1 mg | 5.3 | 0.00 |
| EE 30 µg and levonorgestrel 150 µg | 2.8 | ||
| EE 50 µg and levonorgestrel 150 µg | 2.9 | ||
| Mestranol 50 µg and norethistrone 1 mg | 2.8 | ||
| EE 35 µg and norethisterone acetate 400 µg | 9.0 | ||
| EE 50 mcg and norethindrone acetate 1 mg | 6.8 |
We included all bleeding‐related outcomes reported in the eligible trials (e.g., WHO bleeding terms, breakthrough bleeding, spotting, and amenorrhea). Following WHO recommendations (Belsey 1986), we used women, rather than cycles, as the unit of analysis when possible. For studies that did not use the recommended 90‐ or 84‐day reference period, we extracted bleeding‐related data from the third and the sixth cycle, or when neither of these was available, from the last cycle of follow up. Although the choice of these cycles was arbitrary, the standardization in the data extraction facilitated comparisons across trials. Definitions of bleeding‐related outcomes were described in Characteristics of included studies.
Interval estimation considers both the magnitude of the estimate and the width of the confidence intervals when assessing potential relationships (Rothman 1998). While all available data are presented in the tables, we highlighted the findings that suggest possible differences in COCs based on interval estimation as well as P values.
The review was limited to the analytic method (e.g., intent‐to‐treat, per‐protocol, or a modification of either type) used in the trial report. We combined study results for meta‐analysis only when identical drugs, dosages, and regimens were compared. Homogeneity was assessed by examining the results of both a fixed‐effect model and a random‐effects model. Because the chi‐squared test for heterogeneity is a low‐power test, the alpha‐level was set at 0.10. We conducted sensitivity analyses by examining the effect of deleting each study in turn to test the robustness of any result that appeared to be based on heterogeneous combinations. We critically appraised validity of trials by assessing the potential for bias resulting from the study design, blinding, randomization method, group allocation concealment, and loss to follow up.
Results
Description of studies
Results of the search
The 2013 search resulted in 201 unduplicated references: 162 from the main databases, 10 from other sources such as reference lists, and 29 ongoing or recent clinical trials. We did not find any RCTs that met our eligibility criteria for this review. One new study was excluded because it reported on pooled analysis from two uncontrolled trials (Marr 2012).
Included studies
The search strategy yielded 21 primary articles that were eligible for the review. Most trials recruited healthy, reproductive‐age women without contraindications to COC use. Trials ranged in duration from 3 to 24 treatment cycles with most designed for 6 or 12 treatment cycles. The location was not described for 7 trials; the remaining 14 trials were conducted in North America, South America, Europe or Asia. The trials ranged from a single site (four trials) to 110 sites except for three trials with unspecified number of sites. Five trials included more than two eligible intervention arms. The trials compared 20 different COC pairs.
Risk of bias in included studies
The reporting of randomized clinical trials should include details on the participants, blinding, randomization method, group allocation concealment method, participant flow, and statistical methods (CONSORT 2009). One trial blinded only the study observer (Appel 1987). Three trials blinded the participants and either the clinic staff (Bounds 1979; WHO 1982) or the investigator (Endrikat 2001). Two articles described double‐blinding but did not specify who was blinded (Akerlund 1993; Endrikat 1997). The remaining trials either were open (12 trials) or did not mention blinding (3 trials). Fourteen reports did not provide any details of the method of randomization. Reisman 1999 reported the use of sealed envelopes for allocation concealment; the remaining trials did not provide any details regarding attempts to conceal the allocation process. None of the articles described the person responsible for implementing the randomization process or the use of a centralized location for randomization.
The number of participants who were recruited, randomized, received treatment, lost to follow up, discontinued early, and excluded from the analysis should be reported in the details of the participant flow (CONSORT 2009). Only WHO 1982 reported the number of recruited women who were screened for trial participation. All trials reported the total number of women randomized, although these figures may have been the total numbers analyzed rather than randomized. The number of women reportedly randomized in the trials ranged from 20 to 2894. Four trials did not specify the number of women randomized to each study group (Bounds 1979; Appel 1987; Teichmann 1995; Endrikat 2001). Eleven trials reported excluding randomized women from the analysis, usually for protocol violations. Kluft 2006 used intent‐to‐treat analysis. Hampton 2001 reported using an intent‐to‐treat analysis for contraceptive effectiveness but did not specify the analytic method used for the remaining outcomes. Endrikat 2001 reported both modified intent‐to‐treat and per‐protocol analyses without specifying which method was used for the results presented. Seven trials described using a per‐protocol or modified per‐protocol analysis based on the exclusion of women or treatment cycles from the analysis for failure to comply with the protocol (Akerlund 1993; Brill 1996; Winkler 1996; Chavez 1999; Kaunitz 2009; Reisman 1999; Skouby 2005). However, the decision to exclude the woman or the cycle was unclear in Chavez 1999 because different sections of the article reported conflicting procedures. Inauen 1991 had complete follow up and no early discontinuation. The remaining trials did not specify the analytic method used. Three trials did not report the proportion of women completing the study (Bounds 1979; Brill 1996; Winkler 1996), and the proportion ranged from 46% to 94% in the remaining trials.
Effects of interventions
Contraceptive effectiveness
No significant differences were found in contraceptive effectiveness for the 13 COC pairs for which this outcome was reported (Table 3).
Early Discontinuation
The COC containing EE 20 μg and norethindrone acetate 1 mg had higher life‐table rates of early discontinuation (overall, due to medical reasons, and due to amenorrhea) than its five comparison COCs (Table 4), but P values were the only measure of variability provided for the estimates (WHO 1982). The remaining six comparisons with data for overall discontinuation found no significant differences between groups. Differences in bleeding‐related discontinuations were apparent for three comparisons. Women in the EE 20 μg and desogestrel 150 μg group had an OR of discontinuation due to irregular bleeding 2.59 (95% CI 1.35 to 5.00) times that of the EE 30 μg and desogestrel 150 μg group (Akerlund 1993). Women assigned to use the COC with EE 20 μg and desogestrel 150 μg were 2.4 times as likely to discontinue due to metrorrhagia 2.35 (95% CI 1.16 to 4.77) than those in the EE 30 μg and gestodene 75 μg group (Kirkman 1994; Bruni 2000). In addition, women on EE 20 μg and norethindrone acetate 1 mg were more likely to discontinue due to bleeding than women on EE 30 μg and levonorgestrel 150 μg (Bounds 1979).
Bleeding Patterns
EE 20 mg and desogestrel COCs versus higher‐estrogen COCs
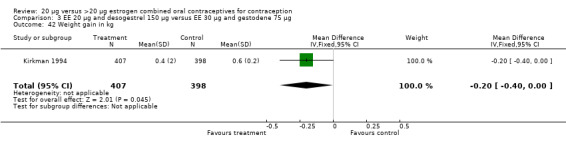
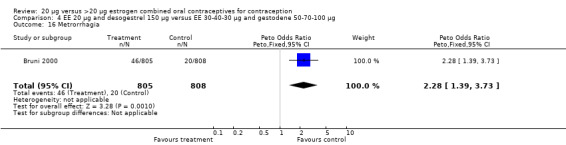
Women in the EE 20 μg and desogestrel 150 μg group were more likely than those in the EE 30 μg and desogestrel 150 μg group to report irregular bleeding (OR 1.56; 95% CI 1.10 to 2.20) and a longer duration of irregular bleeding during the third cycle (mean difference 0.70 days; 95% CI 0.30 to 1.10) (Akerlund 1993). No significant differences in amenorrhea or the duration of irregular bleeding remained by the sixth cycle. The lower‐dose estrogen group was also more likely to report the occurrence of irregular bleeding (OR 1.69; 95% CI 1.07 to 2.69) or prolonged withdrawal bleeding (OR 1.98; 95% CI 1.03 to 3.78) at least once throughout the trial than the higher‐dose estrogen group. The EE 20 μg and desogestrel 150 μg group versus the EE 30 μg and gestodene 75 μg had an OR of irregular bleeding of 2.51 (95% CI 1.77 to 3.56) during the third cycle and 1.72 (95% CI 1.15 to 2.55) during the sixth cycle (Kirkman 1994). Women assigned to use EE 20 μg and desogestrel 150 μg were also more likely to report metrorrhagia (i.e., bleeding from the uterus that is not associated with menstruation) at least once during the trial than women using EE 30 μg and gestodene 75 μg (OR 1.67; 95% CI 1.05 to 2.66) (Brill 1996) or women using EE 30‐40‐30 μg and gestodene 50‐70‐100 μg (OR 2.28; 95% CI 1.39 to 3.73) (Bruni 2000).
Inauen 1991 compared a COC containing EE 20 μg and desogestrel to a second COC and also reported bleeding outcomes. However, the trial had insufficient power to detect major differences between the groups.
EE 20μg and gestodene COCs versus higher‐estrogen COCs
Five trials compared the same gestodene dose (75 μg) but differing EE doses (20 μg versus 30 μg) with a standard (Brill 1996; Winkler 1996; Endrikat 1997; Taneepanichskul 2002) or an extended cycle length (Endrikat 2001). Limitations to assessing bleeding pattern changes included small sample sizes, insufficient data reported, and varying definitions.
EE 20 μg and levonorgestrel COCs versus higher‐estrogen COCs
The trials comparing a low‐estrogen and levonorgestrel COC to a triphasic norethindrone COC found no difference in amenorrhea between the groups at the third cycle (Chavez 1999; Reisman 1999).
EE 20 μg and drospirenone COC versus higher‐estrogen COC
In Kaunitz 2009, the low dose group (EE 20 µg plus drospirenone) had more unscheduled bleeding days than the group with EE 25 μg and norgestimate 180‐215‐250 µg (mean difference 1.00; 95% CI 0.44 to 1.56).
EE 20 μg and norethindrone acetate COCs versus higher‐estrogen COCs
The low‐dose COC containing EE 20 μg and norethindrone acetate 1 mg fared worse than the COC EE 30 μg and levonorgestrel 150 μg for three bleeding outcomes during the first to third cycles: 1) frequent bleeding (OR 2.92; 95% CI 2.08 to 4.09); 2) infrequent bleeding (OR 2.84; 95% CI 1.80 to 4.46); and 3) irregular bleeding (OR 4.01; 95% CI 2.12 to 7.61) (WHO 1982). The low‐dose COC also had worse bleeding outcomes during the first to third cycles when compared to a COC with the same norethindrone acetate dose (1 mg) but more estrogen (EE 50 μg): 1) frequent bleeding (OR 4.59; 95% CI 3.24 to 6.51); 2) infrequent bleeding (OR 3.08; 95% CI 1.95 to 4.86); 3) irregular bleeding (OR 5.33; 95% CI 2.74 to 10.34); and 4) prolonged bleeding (OR 3.11; 95% CI 1.83 to 5.27).
Similarly, EE 20 μg and norethindrone acetate 1 mg resulted in a longer duration of bleeding or spotting days during the third cycle when compared to three higher‐dose COCs containing the same progestin type: 1) EE 30 μg and norethindrone acetate 1.5 μg (mean difference 1.10 days; 95% CI 0.37 to 1.83); 2) EE 50 μg and norethindrone acetate 1 mg (mean difference 1.20 days; 95% CI 0.43 to 1.97); and 3) EE 20‐30‐50 μg and norethindrone acetate 1‐1.5‐1 mg (mean difference 1.60 days; 95% CI 0.94 to 2.26) (Appel 1987). Women in the low‐dose COC group (EE 20 μg and norethindrone acetate 1 mg) also were more likely to report frequent bleeding (OR 1.97; 95% CI 1.42 to 2.73), infrequent bleeding (OR 1.95; 95% CI 1.27 to 3.00), and irregular bleeding (OR 2.38; 95% CI 1.31 to 4.31) during the first to third cycles than those in the EE 50 μg and norethindrone acetate 1 mg group (WHO 1982). (The groups did not differ significantly in the frequency of prolonged bleeding during the first three cycles.) Likewise, women assigned to this low‐dose COC reported more infrequent bleeding (OR 1.88; 95% CI 1.22 to 2.90) and irregular bleeding (OR 1.92; 95% CI 1.08 to 3.43) than those using EE 35 μg and norethindrone acetate 400 μg, but no difference in frequent or prolonged bleeding (WHO 1982).
The sole trial to compare EE 20 μg and norethindrone acetate 1 mg to mestranol 50 μg and norethindrone 1 mg found the low‐dose COC group had a higher risk of frequent bleeding (OR 2.82; 95% CI 2.00 to 3.97), infrequent bleeding (OR 2.49; 95% CI 1.58 to 3.91), irregular bleeding (OR 4.85; 95% CI 2.49 to 9.43), and prolonged bleeding (OR 2.67; 95% CI 1.58 to 4.52) during the first to third cycles (WHO 1982). Finally, the COC with EE 20 μg and norethindrone acetate 1 mg resulted in more breakthrough bleeding during the third cycle (OR 2.79; 95% CI 2.09 to 3.74) and during the sixth cycle (OR 2.40; 95% CI 1.78 to 3.24) compared to the COC containing EE 25 μg and norgestimate 180‐215‐250 μg (Hampton 2001). When breakthrough spotting was included with the outcome breakthrough bleeding, the ORs were similar to those for breakthrough bleeding alone. The bleeding data were re‐analyzed with new criteria for a secondary article; the pattern was the same. However, the percentages with unscheduled bleeding at cycle six were 33.5% for EE 20 μg and norethindrone acetate 1 mg versus 21% for EE 25 μg and norgestimate 180‐215‐250 μg, while the earlier estimates of breakthrough bleeding or spotting were 22.2% and 10.3%, respectively.
Side effects
Side effects were measured as the proportion of women experiencing the event during the study. Three of the six COC pairs with side effects reported found differences. The ORs of headache (1.71; 95% CI 0.94 to 3.11), dizziness (7.65; 95% CI 1.54 to 38.08), mood changes (1.93; 95% CI 1.05 to 3.56), and weight gain (2.46; 95% CI 1.04 to 5.84) were higher for the COC EE 20 μg and desogestrel 150 μg group than the COC EE 30 μg and desogestrel 150 μg group (Akerlund 1993). However, women in the COC EE 20 μg and desogestrel 150 μg group were less likely to report breast pain (OR 0.70; 95% CI 0.47 to 1.05) and dizziness (OR 0.40; 95% CI 0.17 to 0.93), than those in the EE 30‐40‐30 μg and gestodene 50‐70‐100 μg group (Bruni 2000). Also, compared to women in the 35 μg and norethindrone 500‐750‐1000 μg, group women in the 20 μg and levonorgestrel 100 μg were less likely to report breast pain (OR 0.45; 95% CI 0.22 to 0.93) or vomiting (OR 0.33; 95% CI 0.11 to 0.96) (Chavez 1999).
Heterogeneity
Few comparisons could be combined in meta‐analysis because most studies differed in the COC pairs that were compared. Only two of the comparisons that were eligible for meta‐analyses appeared to combine heterogeneous estimates with either fixed‐effect or random‐effects models. The risk of headache differed in the two studies that compared COCs with gestodene 75 μg but different EE doses (Brill 1996; Endrikat 1997), but neither trial found significant ORs for this outcome. The increased rate of discontinuation due to adverse events in the EE 20 μg and levonorgestrel 100 μg group versus the EE 35 μg and norethindrone 500‐750‐1000 μg group was the result of Reisman 1999; Chavez 1999 did not show a difference in discontinuation for this reason between the two study groups.
Discussion
Summary of main results
The included trials provide insufficient evidence to determine whether the contraceptive effectiveness of COCs containing 20 μg of EE differs from those with higher estrogen doses. Most studies were underpowered to study pregnancy as a primary outcome; larger sample sizes are required because COCs are highly effective and few pregnancies occurred. Furthermore, most pregnancy estimates could not be combined in meta‐analysis because the study COCs contained different progestins. Research using ultrasonography to measure the growth of follicle‐like structures suggests that low‐estrogen pills could have a reduced margin of safety from missed pills or drug interactions (Teichmann 1995; Spona 1996). Increased risk of pregnancy from this possible lack of 'forgiveness' for missed pills among the low‐dose estrogen users might not have been detected in the present review. Also, many studies excluded women from the analysis for protocol violations, including failure to adhere to the daily pill intake, which could have biased the results.
Early discontinuation from the trial can be considered a proxy measure of method acceptability. Overall discontinuation rates did not vary substantially for most COCs compared. Four comparisons showed higher rates of discontinuation due to adverse events for EE 20 μg pills than their higher‐estrogen comparison COCs, and one trial found more medical‐related discontinuations for the low‐dose estrogen COC than its five high‐dose estrogen counterparts. However, discontinuation due to adverse events or medical reasons provides limited information because this outcome can combine disparate reasons for quitting the trial early. For example, amenorrhea is different than excessive or irregular bleeding, and studies should separate these. On the other hand, the included trials might have been underpowered to detect differences between specific reasons for discontinuation. Few trials reported data for discontinuation due to specific adverse events.
Several COCs containing 20 μg EE resulted in higher rates of outcomes related to lack of bleeding (amenorrhea and infrequent bleeding) as well as changes in bleeding (irregular, prolonged, frequent, and breakthrough bleeding) than their higher‐estrogen comparison pills. Comparing bleeding data from studies is complicated by lack of uniformity in the outcomes used. Also, determining the clinical importance of changes in bleeding patterns is difficult since women in different cultures may view menstrual patterns and assess the acceptability of any changes differently. For example, amenorrhea or infrequent bleeding may be more acceptable in certain cultural settings than others. Finally, the progestin type and dose as well as the ratio of the progestin and estrogen doses could also affect bleeding patterns. Research suggests that certain progestin types could result in better cycle control than others (Rosenberg 1992). While bleeding irregularities do not pose a health risk, they reduce method acceptability and adherence by users.
Overall completeness and applicability of evidence
The randomized controlled trial design, generally, is not suited for evaluating the risk of rare adverse events (e.g., thromboembolic events or myocardial infarction). Studies assessing various hemostatic outcomes as markers for these rare events indicate that the effects on these intermediate variables might be less with 20 μg pills (Basdevant 1993; Norris 1996; Winkler 1996). However, usual tests of hemostasis do not predict clinical events, so these intermediate outcomes have no clinical utility.
Quality of the evidence
Trials often failed to report a measure of variability for outcome data (e.g., reporting dichotomous outcomes in percentages, rather than absolute numbers, not including standard deviation or confidence interval for continuous outcomes). The lack of variability estimates severely constrains the interpretation of a point estimate and, consequently, prevented their inclusion in this review. A second limitation was that none of the trials described using adequate steps to conceal the allocation process during randomization. Failure to conceal the allocation sequence can lead to biased results (Schulz 2002). Furthermore, most trials were unblinded or did not report blinding of group assignment, and the knowledge of the group assignment could have introduced bias. Losses to follow up were high in many trials, which can bias the results (Strauss 2005). Finally, all of the trials appear to have been funded by pharmaceutical companies except for WHO 1982 and two that did not specify an industry relationship (Basdevant 1993; Teichmann 1995). Pharmaceutical sponsorship represents a potential conflict of interest and can introduce bias in terms of the study design, analysis or reporting of unfavorable results (Lexchin 2003).
Authors' conclusions
Implications for practice.
While COCs containing 20 μg EE may be theoretically safer, this review did not focus on the rare events required to assess this hypothesis. Data from existing randomized controlled trials are inadequate to detect possible differences in contraceptive effectiveness. Twenty μg EE COCs resulted in higher rates of bleeding pattern disruptions. However, most trials compared oral contraceptives containing different progestin types, and changes in bleeding patterns could be related to progestin type as well as estrogen dose.
Implications for research.
Large randomized controlled trials comparing regimens with the same progestin type are needed to determine whether the contraceptive effectiveness of 20‐μg EE COCs is similar to that of their higher‐estrogen counterparts. Likewise, studies of bleeding patterns should compare pills containing the same progestin type but different estrogen doses. Trials should use standardized methods for collecting and analyzing bleeding data (Mishell 2007b). Trials should also employ better research methods, e.g., adequate allocation concealment (Schulz 2002), and follow CONSORT guidelines for reporting the results (CONSORT 2009). Higher follow‐up rates are essential for meaningful interpretation of the results.
What's new
| Date | Event | Description |
|---|---|---|
| 15 July 2013 | New citation required but conclusions have not changed | Searches updated. No new trials were eligible for inclusion. |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 3 November 2010 | New citation required but conclusions have not changed | One new trial was included (Kaunitz 2009). A secondary report (Hampton 2009) from Hampton 2001 was also added. |
| 3 November 2010 | New search has been performed | Searches were updated; searches were added for ClinicalTrials.gov and ICTRP. In the original review, 37 studies did not have eligible outcomes and were listed as 'excluded.' Under our current procedures, we would consider them 'discarded' and not list them. Hence, we removed them for brevity. |
| 14 April 2008 | Amended | Converted to new review format. |
| 6 February 2008 | New citation required and conclusions have changed | Substantive amendment |
| 6 February 2008 | New citation required but conclusions have not changed | Two new trials were found (Skouby 2005; Kluft 2006. A secondary report was identified (Burkman 2007) from an earlier trial (Hampton 2001) and the relevant data were added. |
| 31 January 2008 | New search has been performed | Searches were updated in Dec 2007 and Jan 2008. |
Acknowledgements
Carol Manion of FHI 360 helped develop and execute the literature searches.
Appendices
Appendix 1. Search strategies 2013
OC BRAND LIST (used within MEDLINE and CENTRAL searches) Adoless OR Alesse OR Aliane OR Allestra OR Anulette 20 OR April OR Arianna OR Beyaz OR Careza OR Ciclidon OR Ciclomex OR Ciclotab OR Cycleane‐20 OR Dal OR Desmin OR Desorelle OR Desoren OR Diminut OR Diva OR Dschess OR Dzhess OR Eloine OR Elyfem OR Estinette OR Eve OR Fedra OR Femadiol‐Mepha OR Femexin OR Femiane OR Femilon OR Femina OR Feminet OR Feminol‐20 OR Femodette OR Gedarel OR Ginelea OR Ginesse OR Gynera OR Gynostat‐20 OR Gynovin OR Gyselle OR Harmonet OR Jasminelle OR Jasminellecontinu OR Leois OR Lerogin OR Levlite OR Lindynette OR Liofora OR Loestrin OR Loette OR Logest OR Lovelle OR Lovette OR Lowette OR Marvelon OR Meliane OR Meloden OR Melodene OR Melodia OR Mercilon OR Microgen OR Microgynon OR Microlevlen OR Microlite OR Midalet OR Millinette OR Minesse OR Minestril‐20 OR Minestrin OR Minian OR Minima OR Minifem OR Minigeste OR Minisiston OR Miranova OR Mirelle OR Myralon OR Neolette OR Norvetal OR Novinet OR Novynette OR Ortho Tri‐cyclen Lo OR Primera OR Secret OR Securgin OR Siblima OR Suavuret OR Sunya OR Tamisa OR Vivelle OR Yasmin OR Yasminelle OR Yaz
MEDLINE via PubMed (01 Mar 2010 to 10 Jul 2013)
contraceptives, oral[MeSH] AND ("low dose" OR "low‐dose"[title/abstract word] OR "ultra low dose" OR "ultra‐low‐dose"[title/abstract word] OR [OC BRAND LIST]) AND (Clinical Trial[ptyp])
CENTRAL (2010 to 10 Jul 2013)
Title, abstract, keywords: oral AND contracept* AND Title, abstract, keywords: low dose OR low‐dose OR ultra low dose OR ultra‐low‐dose OR [OC BRAND LIST]
POPLINE (2010 to 11 Jul 2013)
oral contraceptives, low‐dose OR (contraceptive agents, female) Filter by keywords: clinical trials, oral contraceptives combined
ClinicalTrials.gov (01 Sep 2010 to 08 Jul 2013)
Search terms: 20 μg OR 15 μg Study type: Interventional studies Condition: NOT (HIV OR acne OR PMDD OR post‐menopausal OR postmenopausal OR polycystic OR PCOS OR dysmenorrhea OR cancer OR anorexia) Intervention: oral AND (contraceptive OR contraception) Gender: Studies with female participants
ICTRP (01 Sep 2010 to 08 Jul 2013)
Condition: contraceptive OR contraception Intervention: 20 μg OR 15 μg
Appendix 2. Previous search strategies
OC BRAND LIST (used within database searches below) Alesse OR Allestra OR Anulette OR April OR Arianna OR Careza OR Ciclidon OR Ciclomex OR Ciclotab OR Cycleane‐20 OR Dal OR Desmin OR Desorelle OR Desoren OR Diminut OR Estrostep Eve OR Fedra OR Femexin OR Femiane OR Femilon OR Femina OR Feminet OR Feminol‐20 OR Femodette OR Ginelea OR Ginesse OR Gynera OR Gynostat‐20 OR Gynovin OR Harmonet OR Lamuna OR Leois OR Lerogin OR Levlite OR Lindynette OR Loestrin OR Leotte OR Logest OR Lovelle OR Lovette OR Lowette OR Lovina OR Marvelon OR Meliane OR Meloden OR Melodene OR Melodia OR Mercilon OR Microdosis OR Microgen OR Microgynon OR Microlevlen OR Microlite OR Midalet OR Minesse OR Minestril‐20 OR Minestrin OR Minian OR Minima OR Minifem OR Minigeste OR Miranova OR Mircette OR Mirelle OR Myralon OR Neolette OR Norvetal OR Novinet OR Novynette OR Primera OR Secret OR Securgin OR Segurin OR Siblima OR Suavuret OR Tamisa OR YAZ
MEDLINE via PubMed (to 21 Sep 2010)
((randomized controlled trials [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ("latin square" [tw]) OR placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR cross‐over studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animal [mh] NOT human [mh])) AND (eng [la] AND contraceptives, oral[MeSH] AND ("low dose" OR "low‐dose"[title/abstract word] OR "ultra low dose" OR "ultra‐low‐dose"[title/abstract word] OR [OC BRAND LIST])
CENTRAL (to 21 Sep 2010)
(oral AND (contracept*) in Title, Abstract or Keywords AND (low dose OR low‐dose OR ultra low dose OR ultra‐low‐dose OR [OC BRAND LIST]) in Title, Abstract or Keywords
EMBASE (to 03 Nov 2010)
((low dose oral contraceptive OR (oral contraceptive agent AND low(W)dose)) OR (oral contraceptive agent AND [OC BRAND LIST])) AND (clinical trial OR controlled study OR randomized controlled trial OR (controlled(W)clinical(W)trial) OR (random(W)allocation) OR multicenter study OR (comparative(W)study) OR (evidence(W)based(W)medicine) OR (research(W)design) OR (double(W)blind(W)procedure) OR (single(W)blind(W)procedure) OR random) AND human
POPLINE (to 01 Nov 2010)
(oral contraceptives, low‐dose) / (contraceptive agents, female & [OC BRAND LIST])
ClinicalTrials.gov (to 28 Sep 2010)
Search terms: 20 μg OR 15 μg Condition: NOT (HIV OR acne OR PMDD OR post‐menopausal OR postmenopausal OR polycystic OR PCOS OR dysmenorrhea OR cancer OR anorexia) Intervention: oral AND (contraceptive OR contraception) Study type: Interventional studies Gender: Studies with female participants
ICTRP (to 28 Sep 2010)
Condition: contraceptive OR contraception Intervention: 20 μg OR 15 μg
Data and analyses
Comparison 1. EE 20 µg and desogestrel 150 µg versus EE 30 µg and desogestrel 150 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman | 1 | 982 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.12, 3.97] |
| 2 Discontinuation ‐ overall | 1 | 1000 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [0.92, 1.56] |
| 3 Discontinuation ‐ mood changes | 1 | 1000 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.51 [0.68, 3.33] |
| 4 Discontinuation ‐ irregular bleeding | 1 | 1000 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.59 [1.35, 5.00] |
| 5 Discontinuation ‐ nausea | 1 | 58 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.04, 12.64] |
| 6 Amenorrhea ‐ cycle 3 | 1 | 778 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.49 [0.75, 2.97] |
| 7 Amenorrhea ‐ cycle 6 | 1 | 721 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.43 [0.65, 3.12] |
| 8 Irregular bleeding ‐ cycle 3 | 1 | 778 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.56 [1.10, 2.20] |
| 9 Duration of irregular bleeding in days ‐ cycle 3 | 1 | 778 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.30, 1.10] |
| 10 Duration of irregular bleeding in days ‐ cycle 6 | 1 | 721 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.46, 0.26] |
| 11 Dizziness | 1 | 982 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.65 [1.54, 38.08] |
| 12 Dysmenorrhea | 1 | 982 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.46 [0.70, 3.06] |
| 13 Headache | 1 | 982 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.71 [0.94, 3.11] |
| 14 Increased weight | 1 | 982 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.46 [1.04, 5.84] |
| 15 Irregular bleeding | 1 | 982 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.69 [1.07, 2.69] |
| 16 Mood change | 1 | 982 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.93 [1.05, 3.56] |
| 17 Nausea, diarrhea, vomiting | 1 | 982 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.42 [0.74, 2.72] |
| 18 Prolonged withdrawal bleeding | 1 | 982 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.98 [1.03, 3.78] |
1.1. Analysis.
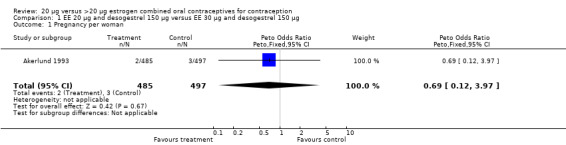
Comparison 1 EE 20 µg and desogestrel 150 µg versus EE 30 µg and desogestrel 150 µg, Outcome 1 Pregnancy per woman.
1.2. Analysis.
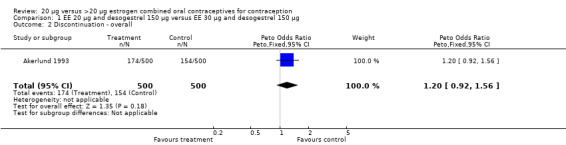
Comparison 1 EE 20 µg and desogestrel 150 µg versus EE 30 µg and desogestrel 150 µg, Outcome 2 Discontinuation ‐ overall.
1.3. Analysis.
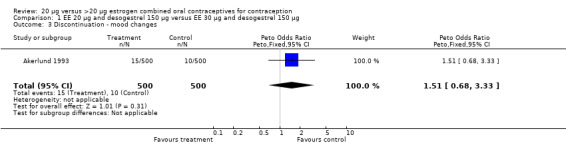
Comparison 1 EE 20 µg and desogestrel 150 µg versus EE 30 µg and desogestrel 150 µg, Outcome 3 Discontinuation ‐ mood changes.
1.4. Analysis.
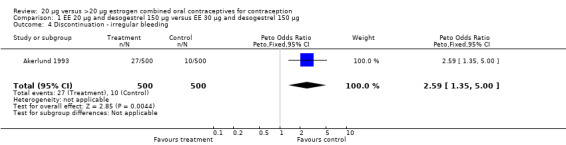
Comparison 1 EE 20 µg and desogestrel 150 µg versus EE 30 µg and desogestrel 150 µg, Outcome 4 Discontinuation ‐ irregular bleeding.
1.5. Analysis.
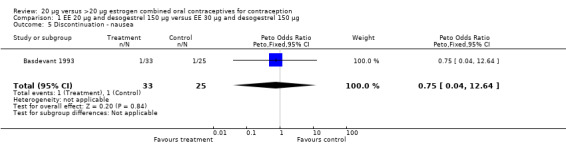
Comparison 1 EE 20 µg and desogestrel 150 µg versus EE 30 µg and desogestrel 150 µg, Outcome 5 Discontinuation ‐ nausea.
1.6. Analysis.
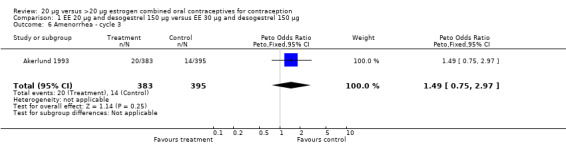
Comparison 1 EE 20 µg and desogestrel 150 µg versus EE 30 µg and desogestrel 150 µg, Outcome 6 Amenorrhea ‐ cycle 3.
1.7. Analysis.
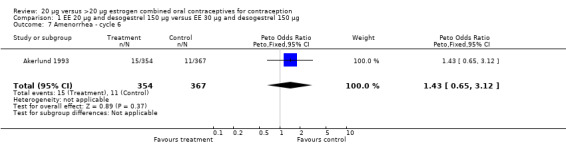
Comparison 1 EE 20 µg and desogestrel 150 µg versus EE 30 µg and desogestrel 150 µg, Outcome 7 Amenorrhea ‐ cycle 6.
1.8. Analysis.
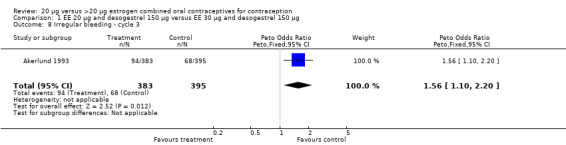
Comparison 1 EE 20 µg and desogestrel 150 µg versus EE 30 µg and desogestrel 150 µg, Outcome 8 Irregular bleeding ‐ cycle 3.
1.9. Analysis.
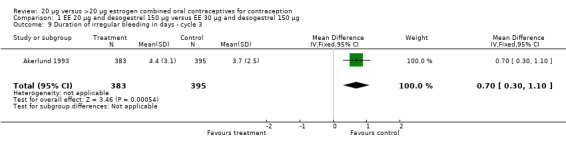
Comparison 1 EE 20 µg and desogestrel 150 µg versus EE 30 µg and desogestrel 150 µg, Outcome 9 Duration of irregular bleeding in days ‐ cycle 3.
1.10. Analysis.
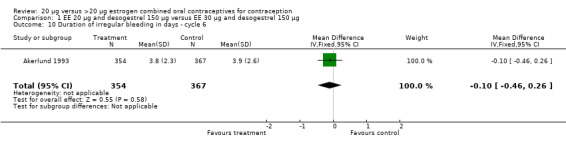
Comparison 1 EE 20 µg and desogestrel 150 µg versus EE 30 µg and desogestrel 150 µg, Outcome 10 Duration of irregular bleeding in days ‐ cycle 6.
1.11. Analysis.
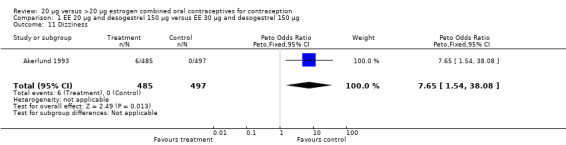
Comparison 1 EE 20 µg and desogestrel 150 µg versus EE 30 µg and desogestrel 150 µg, Outcome 11 Dizziness.
1.12. Analysis.
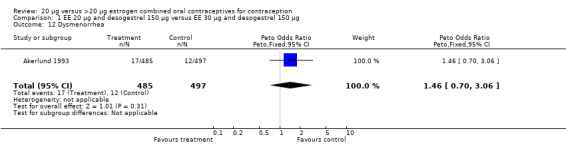
Comparison 1 EE 20 µg and desogestrel 150 µg versus EE 30 µg and desogestrel 150 µg, Outcome 12 Dysmenorrhea.
1.13. Analysis.
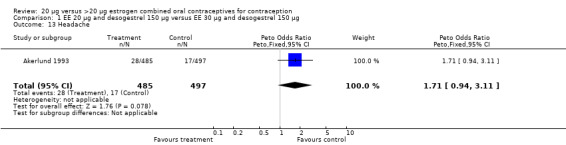
Comparison 1 EE 20 µg and desogestrel 150 µg versus EE 30 µg and desogestrel 150 µg, Outcome 13 Headache.
1.14. Analysis.
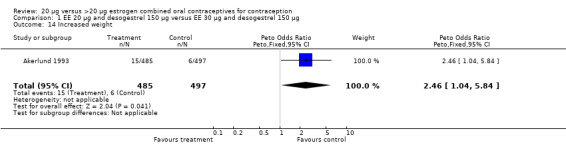
Comparison 1 EE 20 µg and desogestrel 150 µg versus EE 30 µg and desogestrel 150 µg, Outcome 14 Increased weight.
1.15. Analysis.
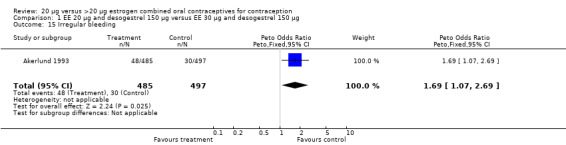
Comparison 1 EE 20 µg and desogestrel 150 µg versus EE 30 µg and desogestrel 150 µg, Outcome 15 Irregular bleeding.
1.16. Analysis.
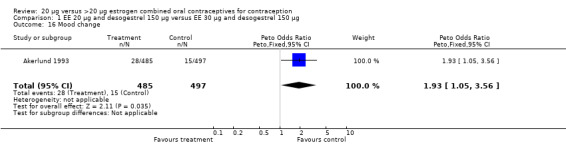
Comparison 1 EE 20 µg and desogestrel 150 µg versus EE 30 µg and desogestrel 150 µg, Outcome 16 Mood change.
1.17. Analysis.
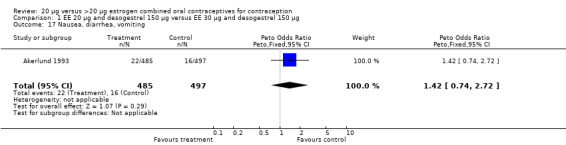
Comparison 1 EE 20 µg and desogestrel 150 µg versus EE 30 µg and desogestrel 150 µg, Outcome 17 Nausea, diarrhea, vomiting.
1.18. Analysis.
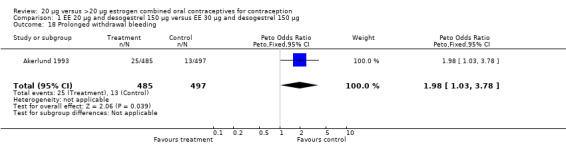
Comparison 1 EE 20 µg and desogestrel 150 µg versus EE 30 µg and desogestrel 150 µg, Outcome 18 Prolonged withdrawal bleeding.
Comparison 2. EE 20 µg and desogestrel 150 µg versus EE 50 µg and desogestrel 125 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Breakthough bleeding ‐ cycles 1 to 3 | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.23 [0.81, 84.07] |
| 2 Breakthrough spotting ‐ cycles 1 to 3 | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.79 [0.47, 129.11] |
| 3 Acne | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.13] |
| 4 Breast tenderness | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.13, 7.69] |
| 5 Headache | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.82] |
| 6 Weight gain | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.13] |
2.1. Analysis.
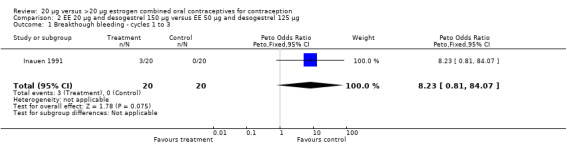
Comparison 2 EE 20 µg and desogestrel 150 µg versus EE 50 µg and desogestrel 125 µg, Outcome 1 Breakthough bleeding ‐ cycles 1 to 3.
2.2. Analysis.
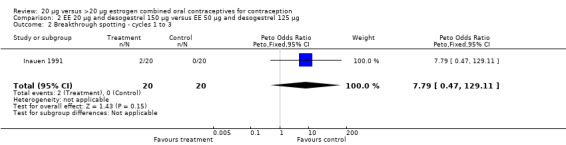
Comparison 2 EE 20 µg and desogestrel 150 µg versus EE 50 µg and desogestrel 125 µg, Outcome 2 Breakthrough spotting ‐ cycles 1 to 3.
2.3. Analysis.
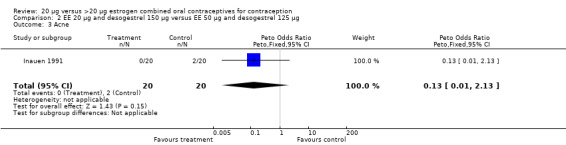
Comparison 2 EE 20 µg and desogestrel 150 µg versus EE 50 µg and desogestrel 125 µg, Outcome 3 Acne.
2.4. Analysis.
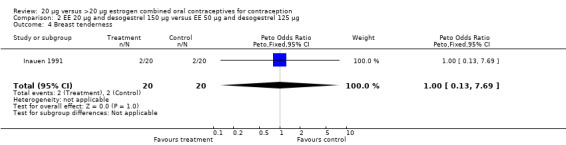
Comparison 2 EE 20 µg and desogestrel 150 µg versus EE 50 µg and desogestrel 125 µg, Outcome 4 Breast tenderness.
2.5. Analysis.
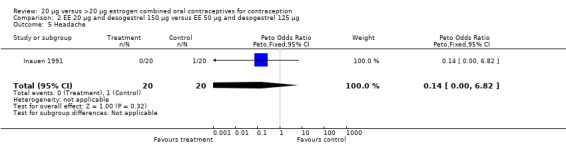
Comparison 2 EE 20 µg and desogestrel 150 µg versus EE 50 µg and desogestrel 125 µg, Outcome 5 Headache.
2.6. Analysis.
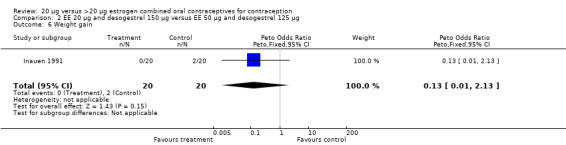
Comparison 2 EE 20 µg and desogestrel 150 µg versus EE 50 µg and desogestrel 125 µg, Outcome 6 Weight gain.
Comparison 3. EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman | 2 | 2027 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.20, 4.96] |
| 2 Discontinuation ‐ overall | 3 | 3033 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.03 [0.85, 1.26] |
| 3 Discontinuation ‐ abdominal pain | 1 | 416 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.49 [0.43, 5.22] |
| 4 Discontinuation ‐ adverse event | 3 | 3033 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.28 [0.98, 1.68] |
| 5 Discontinuation ‐ breast tension | 1 | 416 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.05, 4.90] |
| 6 Discontinuation ‐ colpitis | 1 | 416 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.06, 15.89] |
| 7 Discontinuation ‐ depressive mood | 1 | 416 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.05, 4.90] |
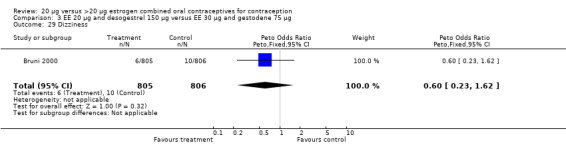
| 8 Discontinuation ‐ dizziness | 1 | 416 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.43 [1.04, 53.09] |
| 9 Discontinuation ‐ headache | 1 | 416 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [0.33, 4.65] |
| 10 Discontinuation ‐ hypertension | 1 | 416 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.32 [0.15, 368.86] |
| 11 Discontinuation ‐ hypomenorrhea | 1 | 1006 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.46 [0.47, 119.49] |
| 12 Discontinuation ‐ intermenstrual bleeding | 1 | 416 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.17, 3.30] |
| 13 Discontinuation ‐ menorrhagia | 1 | 1006 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.14, 7.18] |
| 14 Discontinuation ‐ menstrual disorder | 1 | 1006 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.05, 4.98] |
| 15 Discontinuation ‐ metrorrhagia | 2 | 2617 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.35 [1.16, 4.77] |
| 16 Discontinuation ‐ nausea | 1 | 416 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.24, 4.01] |
| 17 Discontinuation ‐ nervousness | 1 | 416 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.76, 71.43] |
| 18 Discontinuation ‐ pruritus | 1 | 416 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.32 [0.15, 368.86] |
| 19 Discontinuation ‐ vomiting | 1 | 416 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.82 [0.76, 19.10] |
| 20 Irregular bleeding ‐ cycle 3 | 1 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.51 [1.77, 3.56] |
| 21 Irregular bleeding ‐ cycle 6 | 1 | 823 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.72 [1.15, 2.55] |
| 22 Amenorrhea ‐ cycle 3 | 1 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.38 [0.83, 6.82] |
| 23 Amenorrhea ‐ cycle 6 | 1 | 823 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.37 [0.09, 1.47] |
| 24 Abdominal pain | 1 | 1611 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.19 [0.71, 2.01] |
| 25 Acne | 1 | 1611 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.46, 1.91] |
| 26 Breast pain | 1 | 1611 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.56, 1.30] |
| 27 Decreased libido | 1 | 1611 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.25, 1.62] |
| 28 Depression | 1 | 1611 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.40, 1.46] |
| 29 Dizziness | 1 | 1611 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.60 [0.23, 1.62] |
| 30 Dysmenorrhea | 1 | 1611 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.48, 1.85] |
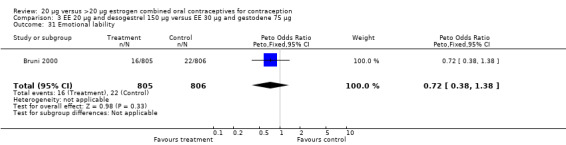
| 31 Emotional lability | 1 | 1611 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.38, 1.38] |
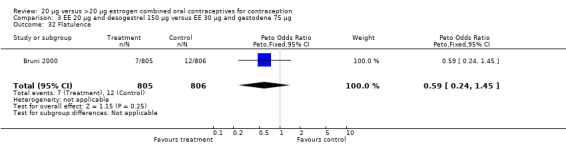
| 32 Flatulence | 1 | 1611 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.24, 1.45] |
| 33 Headache | 1 | 1611 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.81, 1.42] |
| 34 Menstrual disorder | 1 | 1611 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.41, 2.42] |
| 35 Metrorrhagia | 1 | 1611 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.67 [1.05, 2.66] |
| 36 Migraine | 1 | 1611 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.38 [0.83, 6.80] |
| 37 Nausea | 1 | 1611 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.68, 1.95] |
| 38 Pain | 1 | 1611 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.37 [0.63, 2.97] |
| 39 Vaginal moniliasis | 1 | 1611 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.45 [0.62, 3.36] |
| 40 Vomiting | 1 | 1611 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.19, 1.17] |
| 41 Weight gain | 1 | 1611 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.34, 1.38] |
| 42 Weight gain in kg | 1 | 805 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.40, ‐0.00] |
3.1. Analysis.
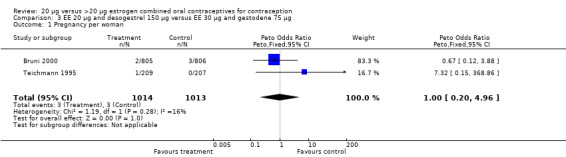
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 1 Pregnancy per woman.
3.2. Analysis.
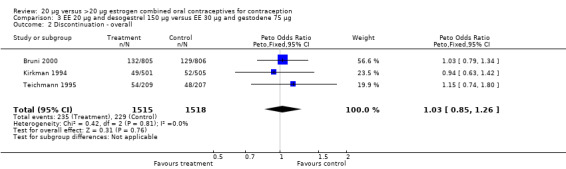
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 2 Discontinuation ‐ overall.
3.3. Analysis.
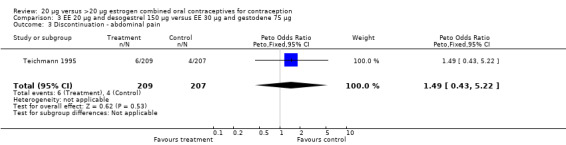
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 3 Discontinuation ‐ abdominal pain.
3.4. Analysis.
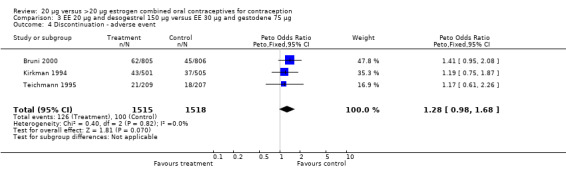
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 4 Discontinuation ‐ adverse event.
3.5. Analysis.
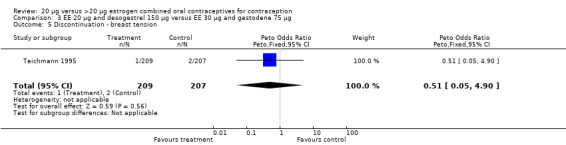
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 5 Discontinuation ‐ breast tension.
3.6. Analysis.
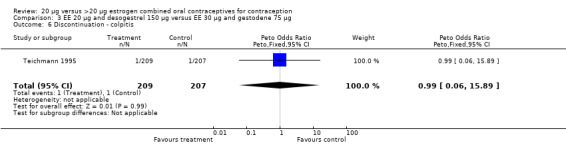
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 6 Discontinuation ‐ colpitis.
3.7. Analysis.
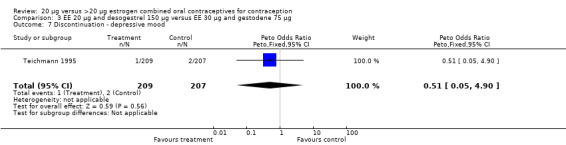
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 7 Discontinuation ‐ depressive mood.
3.8. Analysis.
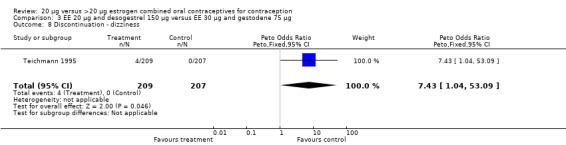
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 8 Discontinuation ‐ dizziness.
3.9. Analysis.
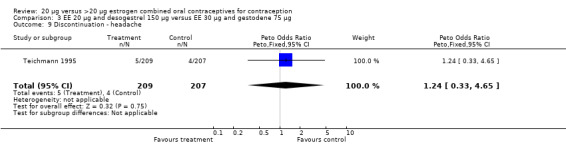
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 9 Discontinuation ‐ headache.
3.10. Analysis.
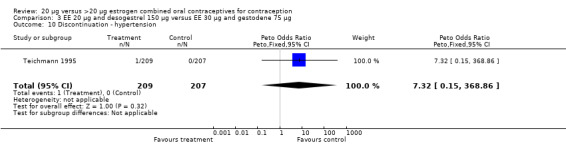
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 10 Discontinuation ‐ hypertension.
3.11. Analysis.
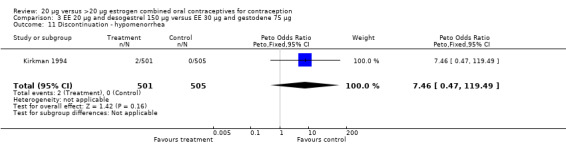
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 11 Discontinuation ‐ hypomenorrhea.
3.12. Analysis.
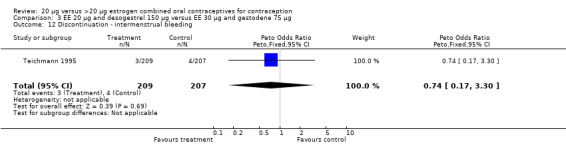
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 12 Discontinuation ‐ intermenstrual bleeding.
3.13. Analysis.
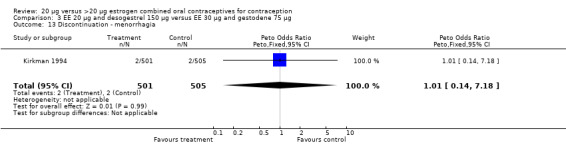
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 13 Discontinuation ‐ menorrhagia.
3.14. Analysis.
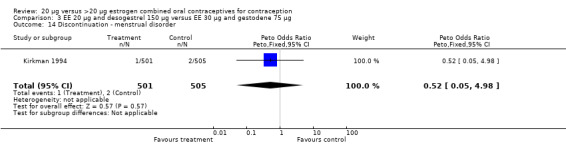
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 14 Discontinuation ‐ menstrual disorder.
3.15. Analysis.
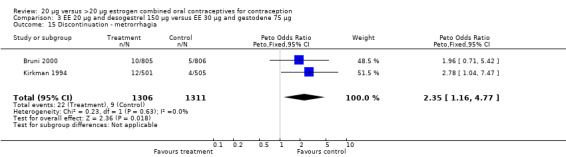
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 15 Discontinuation ‐ metrorrhagia.
3.16. Analysis.
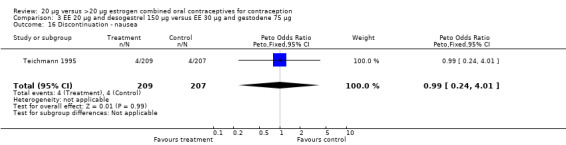
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 16 Discontinuation ‐ nausea.
3.17. Analysis.
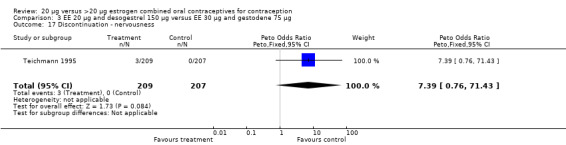
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 17 Discontinuation ‐ nervousness.
3.18. Analysis.
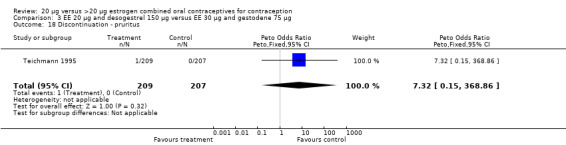
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 18 Discontinuation ‐ pruritus.
3.19. Analysis.
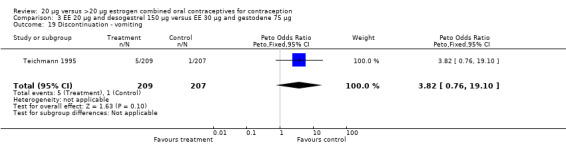
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 19 Discontinuation ‐ vomiting.
3.20. Analysis.
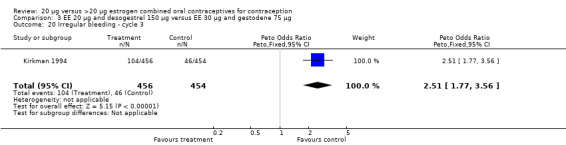
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 20 Irregular bleeding ‐ cycle 3.
3.21. Analysis.
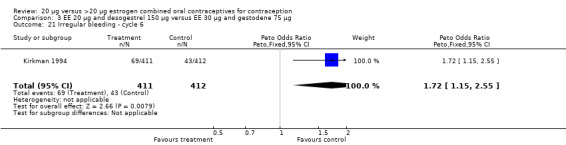
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 21 Irregular bleeding ‐ cycle 6.
3.22. Analysis.
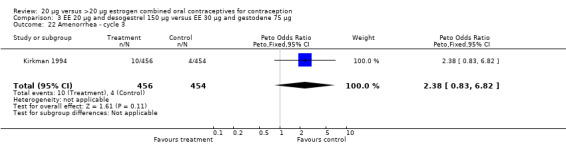
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 22 Amenorrhea ‐ cycle 3.
3.23. Analysis.
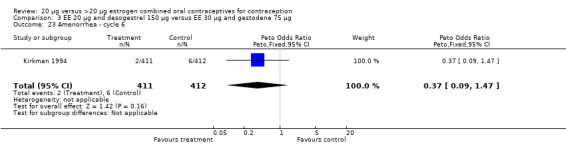
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 23 Amenorrhea ‐ cycle 6.
3.24. Analysis.
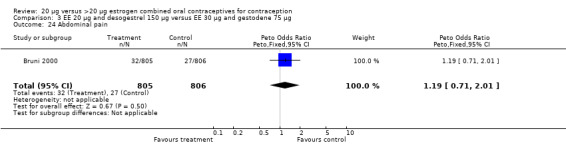
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 24 Abdominal pain.
3.25. Analysis.
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 25 Acne.
3.26. Analysis.
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 26 Breast pain.
3.27. Analysis.
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 27 Decreased libido.
3.28. Analysis.
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 28 Depression.
3.29. Analysis.
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 29 Dizziness.
3.30. Analysis.
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 30 Dysmenorrhea.
3.31. Analysis.
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 31 Emotional lability.
3.32. Analysis.
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 32 Flatulence.
3.33. Analysis.
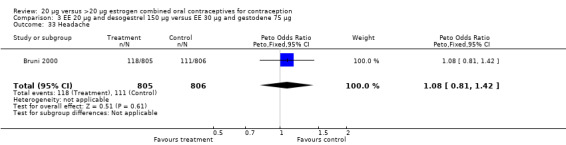
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 33 Headache.
3.34. Analysis.
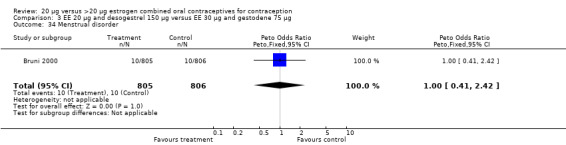
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 34 Menstrual disorder.
3.35. Analysis.
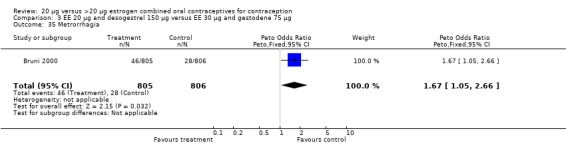
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 35 Metrorrhagia.
3.36. Analysis.
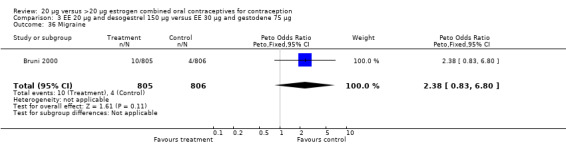
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 36 Migraine.
3.37. Analysis.
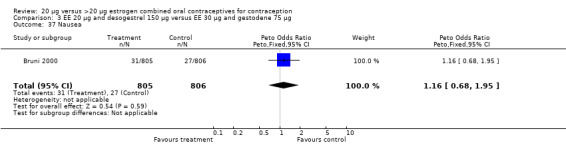
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 37 Nausea.
3.38. Analysis.
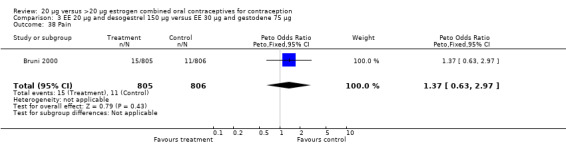
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 38 Pain.
3.39. Analysis.
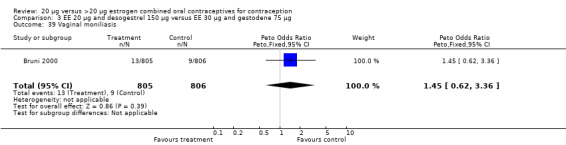
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 39 Vaginal moniliasis.
3.40. Analysis.
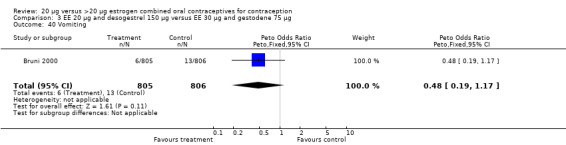
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 40 Vomiting.
3.41. Analysis.
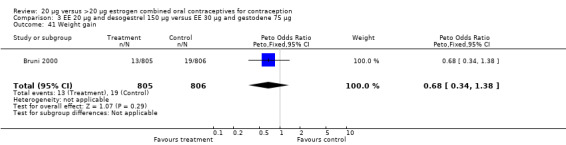
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 41 Weight gain.
3.42. Analysis.
Comparison 3 EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg, Outcome 42 Weight gain in kg.
Comparison 4. EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.14, 7.14] |
| 2 Discontinuation ‐ overall | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.82, 1.40] |
| 3 Discontinuation ‐ adverse reaction | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [0.91, 1.99] |
| 4 Discontinuation ‐ metrorrhagia | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.97 [1.00, 8.85] |
| 5 Abdominal pain | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [0.71, 2.01] |
| 6 Acne | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.38, 1.46] |
| 7 Breast pain | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.47, 1.05] |
| 8 Decreased libido | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.35, 2.87] |
| 9 Depression | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.53, 2.18] |
| 10 Dizziness | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.40 [0.17, 0.93] |
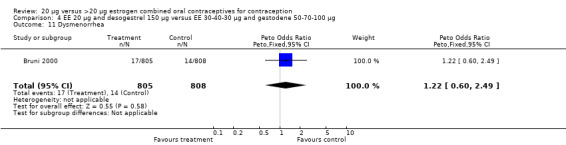
| 11 Dysmenorrhea | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.22 [0.60, 2.49] |
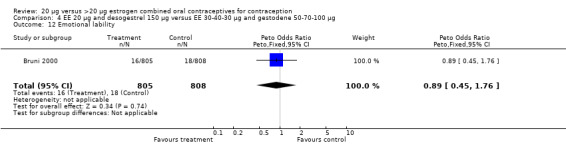
| 12 Emotional lability | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.45, 1.76] |
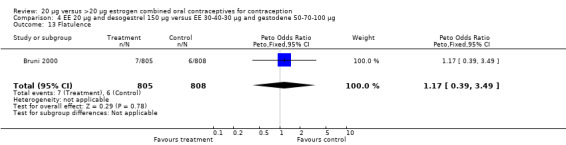
| 13 Flatulence | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.39, 3.49] |
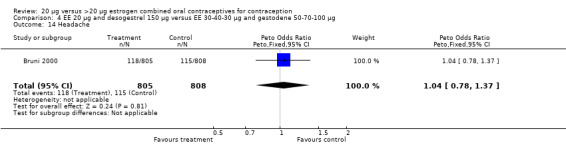
| 14 Headache | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.78, 1.37] |
| 15 Menstrual disorder | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.43 [0.55, 3.73] |
| 16 Metrorrhagia | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.28 [1.39, 3.73] |
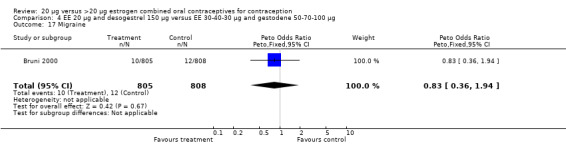
| 17 Migraine | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.36, 1.94] |
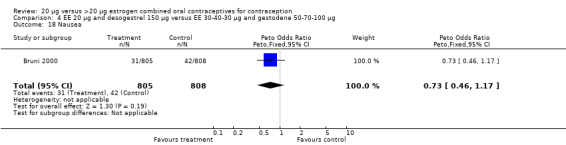
| 18 Nausea | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.46, 1.17] |
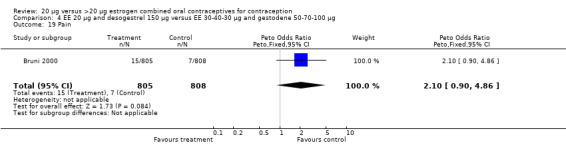
| 19 Pain | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.10 [0.90, 4.86] |
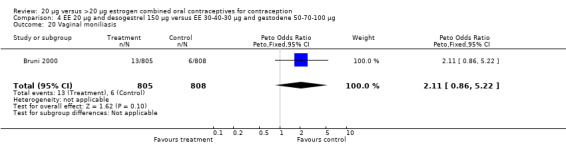
| 20 Vaginal moniliasis | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.11 [0.86, 5.22] |
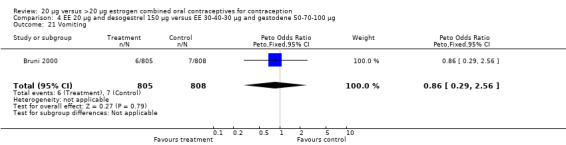
| 21 Vomiting | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.29, 2.56] |
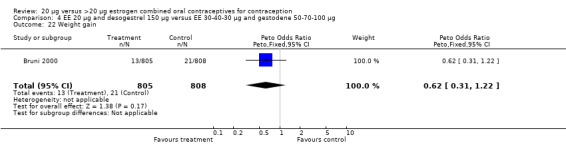
| 22 Weight gain | 1 | 1613 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.31, 1.22] |
4.1. Analysis.
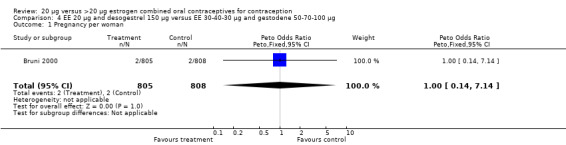
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 1 Pregnancy per woman.
4.2. Analysis.
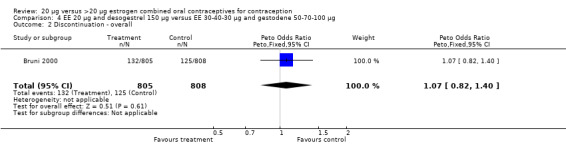
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 2 Discontinuation ‐ overall.
4.3. Analysis.
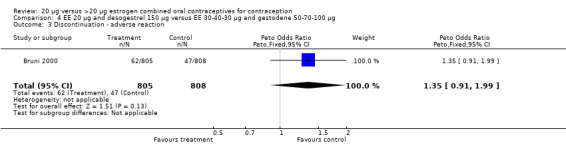
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 3 Discontinuation ‐ adverse reaction.
4.4. Analysis.
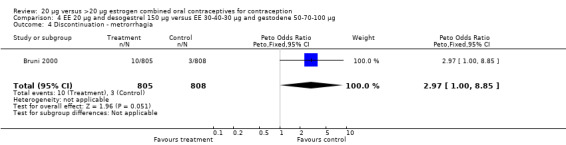
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 4 Discontinuation ‐ metrorrhagia.
4.5. Analysis.
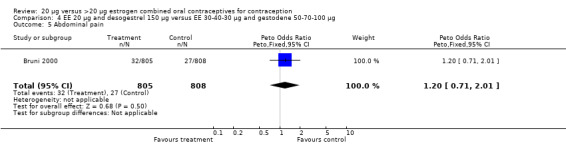
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 5 Abdominal pain.
4.6. Analysis.
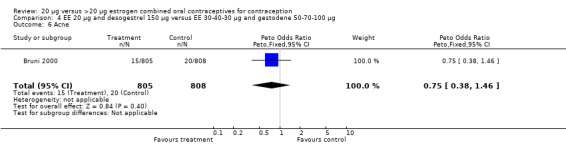
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 6 Acne.
4.7. Analysis.
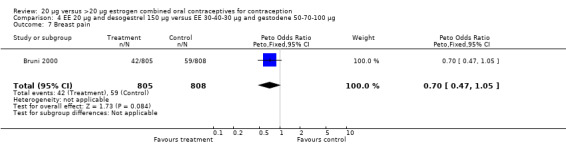
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 7 Breast pain.
4.8. Analysis.
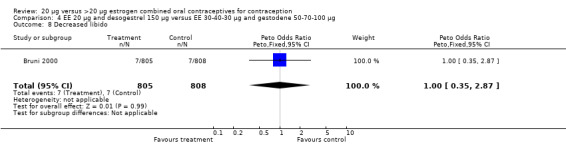
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 8 Decreased libido.
4.9. Analysis.
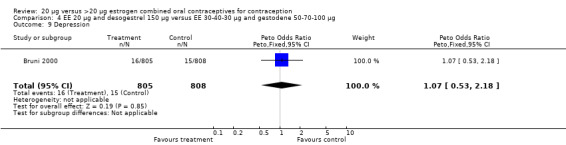
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 9 Depression.
4.10. Analysis.
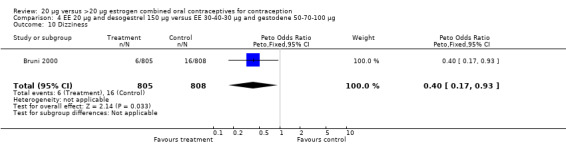
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 10 Dizziness.
4.11. Analysis.
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 11 Dysmenorrhea.
4.12. Analysis.
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 12 Emotional lability.
4.13. Analysis.
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 13 Flatulence.
4.14. Analysis.
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 14 Headache.
4.15. Analysis.
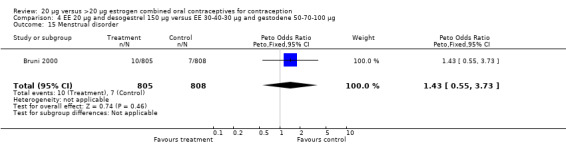
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 15 Menstrual disorder.
4.16. Analysis.
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 16 Metrorrhagia.
4.17. Analysis.
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 17 Migraine.
4.18. Analysis.
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 18 Nausea.
4.19. Analysis.
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 19 Pain.
4.20. Analysis.
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 20 Vaginal moniliasis.
4.21. Analysis.
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 21 Vomiting.
4.22. Analysis.
Comparison 4 EE 20 µg and desogestrel 150 µg versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg, Outcome 22 Weight gain.
Comparison 5. EE 20 µg and desogestrel 150 µg versus EE 35 µg and norgestimate 180‐215‐250 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman | 1 | 309 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 1.30] |
5.1. Analysis.
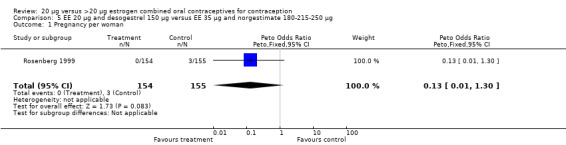
Comparison 5 EE 20 µg and desogestrel 150 µg versus EE 35 µg and norgestimate 180‐215‐250 µg, Outcome 1 Pregnancy per woman.
Comparison 6. EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman | 2 | 799 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.02, 2.55] |
| 2 Discontinuation ‐ overall | 2 | 799 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.14 [0.80, 1.63] |
| 3 Discontinuation ‐ adverse event | 3 | 753 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.46 [0.86, 2.46] |
| 4 Discontinuation ‐ intermenstrual bleeding | 1 | 64 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Discontinuation ‐ metrorrhagia | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.82] |
| 6 Breakthrough bleeding ‐ cycle 3 | 1 | 114 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.90 [0.14, 348.82] |
| 7 Breakthrough bleeding ‐ cycle 6 | 1 | 114 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.36] |
| 8 Spotting ‐ cycle 3 | 1 | 114 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.61 [0.10, 3.66] |
| 9 Spotting ‐ cycle 6 | 1 | 114 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.06, 15.10] |
| 10 Acne | 2 | 707 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [0.60, 3.08] |
| 11 Breast tension or tenderness | 3 | 821 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.68, 2.05] |
| 12 Change in libido | 1 | 649 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.72 [0.64, 4.61] |
| 13 Chloasma | 1 | 114 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.13, 6.79] |
| 14 Depressive moods | 2 | 707 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.12 [0.80, 5.66] |
| 15 Diarrhea | 1 | 114 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.05, 2.43] |
| 16 Dizziness | 2 | 763 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.52 [0.57, 4.02] |
| 17 Edema | 1 | 649 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.49 [0.09, 2.66] |
| 18 Headache | 2 | 707 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.60, 1.59] |
| 19 Nausea | 2 | 707 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.66, 2.45] |
| 20 Nausea and vomiting | 1 | 114 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.84 [0.19, 18.04] |
| 21 Nervousness | 1 | 649 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.51 [0.59, 3.87] |
| 22 Varicose conditions | 1 | 649 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.20, 3.72] |
| 23 Vomiting | 2 | 707 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.20, 2.25] |
| 24 Weight gain >2 kg | 1 | 452 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.63, 1.81] |
| 25 Weight gain in kg | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐4.23, 1.23] |
6.1. Analysis.
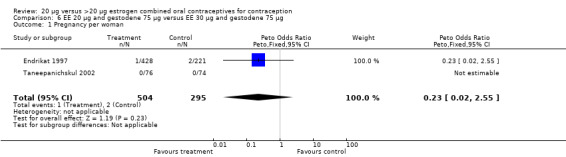
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 1 Pregnancy per woman.
6.2. Analysis.
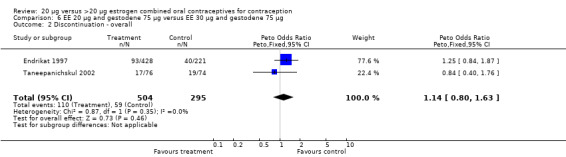
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 2 Discontinuation ‐ overall.
6.3. Analysis.
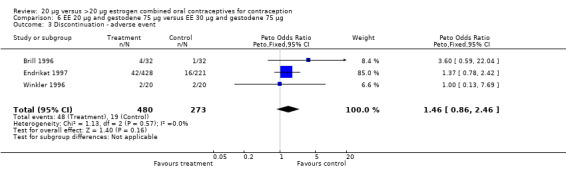
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 3 Discontinuation ‐ adverse event.
6.4. Analysis.
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 4 Discontinuation ‐ intermenstrual bleeding.
6.5. Analysis.
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 5 Discontinuation ‐ metrorrhagia.
6.6. Analysis.
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 6 Breakthrough bleeding ‐ cycle 3.
6.7. Analysis.
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 7 Breakthrough bleeding ‐ cycle 6.
6.8. Analysis.
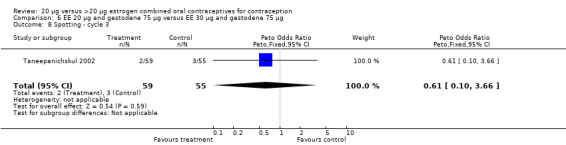
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 8 Spotting ‐ cycle 3.
6.9. Analysis.
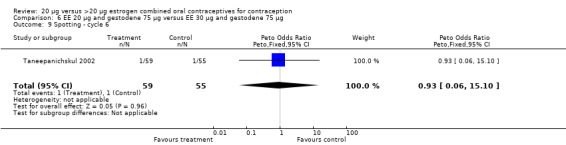
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 9 Spotting ‐ cycle 6.
6.10. Analysis.
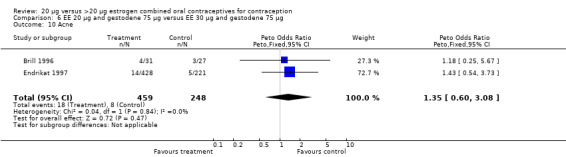
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 10 Acne.
6.11. Analysis.
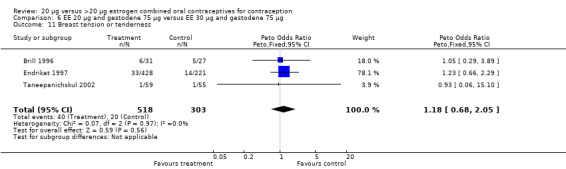
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 11 Breast tension or tenderness.
6.12. Analysis.
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 12 Change in libido.
6.13. Analysis.
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 13 Chloasma.
6.14. Analysis.
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 14 Depressive moods.
6.15. Analysis.
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 15 Diarrhea.
6.16. Analysis.
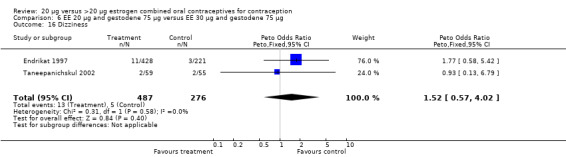
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 16 Dizziness.
6.17. Analysis.
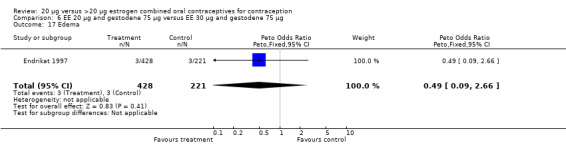
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 17 Edema.
6.18. Analysis.
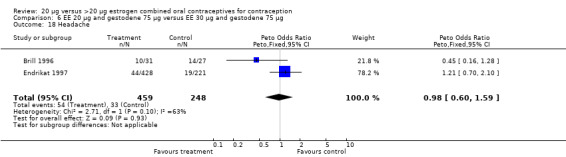
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 18 Headache.
6.19. Analysis.
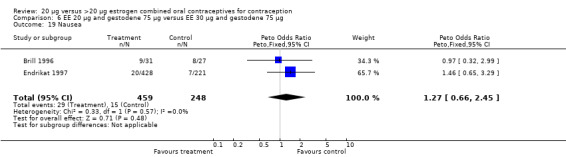
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 19 Nausea.
6.20. Analysis.
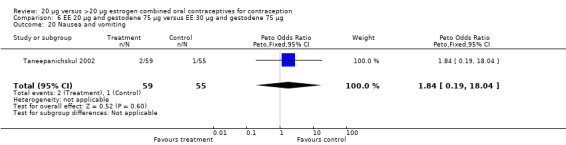
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 20 Nausea and vomiting.
6.21. Analysis.
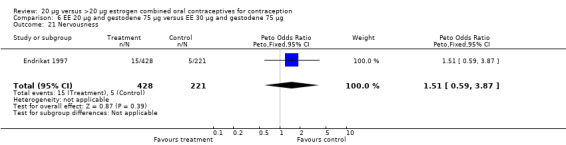
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 21 Nervousness.
6.22. Analysis.
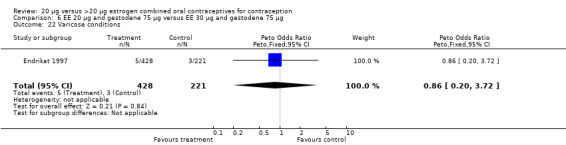
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 22 Varicose conditions.
6.23. Analysis.
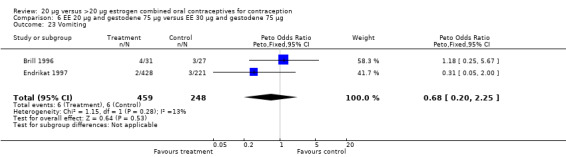
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 23 Vomiting.
6.24. Analysis.
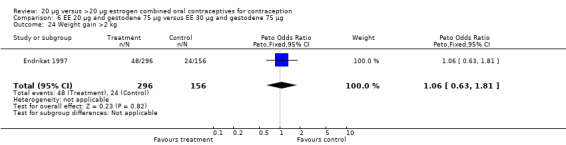
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 24 Weight gain >2 kg.
6.25. Analysis.
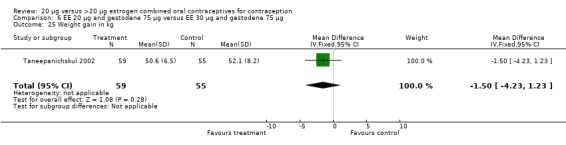
Comparison 6 EE 20 µg and gestodene 75 µg versus EE 30 µg and gestodene 75 µg, Outcome 25 Weight gain in kg.
Comparison 7. EE 20 µg and gestodene 75 µg (23‐day) versus EE 30 µg and gestodene 75 µg (21‐day).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman | 1 | 69 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.63] |
| 2 Intracyclic bleeding ‐ cycle 1 and at least once during cycles 2 to 6 | 1 | 66 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.11 [0.02, 0.46] |
7.1. Analysis.
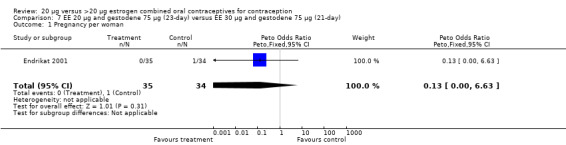
Comparison 7 EE 20 µg and gestodene 75 µg (23‐day) versus EE 30 µg and gestodene 75 µg (21‐day), Outcome 1 Pregnancy per woman.
7.2. Analysis.
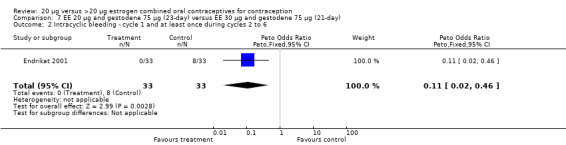
Comparison 7 EE 20 µg and gestodene 75 µg (23‐day) versus EE 30 µg and gestodene 75 µg (21‐day), Outcome 2 Intracyclic bleeding ‐ cycle 1 and at least once during cycles 2 to 6.
Comparison 8. EE 20 µg and levonorgestrel 100 µg versus EE 35 µg and norethindrone 500‐750‐1000 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman | 2 | 729 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.10, 2.60] |
| 2 Discontinuation ‐ overall | 2 | 729 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.78, 1.73] |
| 3 Discontinuation ‐ adverse events | 2 | 729 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.44 [0.86, 2.41] |
| 4 Discontinuation ‐ breakthrough bleeding | 1 | 387 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.78 [0.39, 19.91] |
| 5 Discontinuation ‐ headache | 1 | 387 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.42 [0.59, 19.90] |
| 6 Discontinuation ‐ nausea or vomiting | 1 | 387 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.34, 4.77] |
| 7 Intermenstrual bleeding ‐ cycle 3 | 2 | 420 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.57, 1.26] |
| 8 Amenorrhea ‐ cycle 3 | 2 | 420 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.43 [0.32, 6.34] |
| 9 Breast pain | 1 | 289 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.45 [0.22, 0.93] |
| 10 Dysmenorrhea | 1 | 289 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.82 [0.49, 1.36] |
| 11 Headache | 1 | 289 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.57, 1.56] |
| 12 Nausea | 1 | 289 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.40, 1.36] |
| 13 Vomiting | 1 | 289 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.11, 0.96] |
8.1. Analysis.
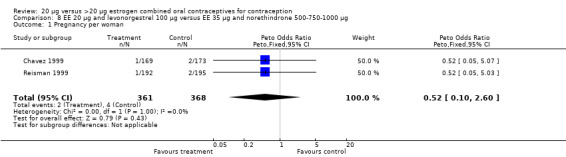
Comparison 8 EE 20 µg and levonorgestrel 100 µg versus EE 35 µg and norethindrone 500‐750‐1000 µg, Outcome 1 Pregnancy per woman.
8.2. Analysis.
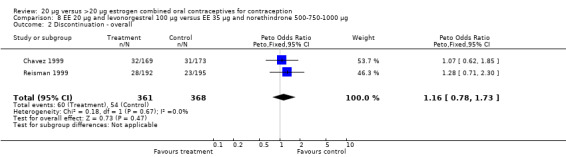
Comparison 8 EE 20 µg and levonorgestrel 100 µg versus EE 35 µg and norethindrone 500‐750‐1000 µg, Outcome 2 Discontinuation ‐ overall.
8.3. Analysis.
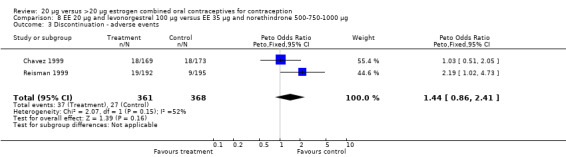
Comparison 8 EE 20 µg and levonorgestrel 100 µg versus EE 35 µg and norethindrone 500‐750‐1000 µg, Outcome 3 Discontinuation ‐ adverse events.
8.4. Analysis.
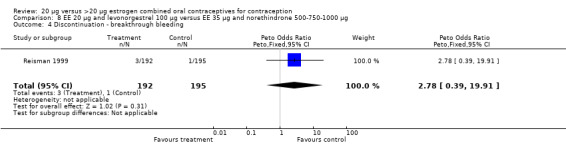
Comparison 8 EE 20 µg and levonorgestrel 100 µg versus EE 35 µg and norethindrone 500‐750‐1000 µg, Outcome 4 Discontinuation ‐ breakthrough bleeding.
8.5. Analysis.
Comparison 8 EE 20 µg and levonorgestrel 100 µg versus EE 35 µg and norethindrone 500‐750‐1000 µg, Outcome 5 Discontinuation ‐ headache.
8.6. Analysis.
Comparison 8 EE 20 µg and levonorgestrel 100 µg versus EE 35 µg and norethindrone 500‐750‐1000 µg, Outcome 6 Discontinuation ‐ nausea or vomiting.
8.7. Analysis.
Comparison 8 EE 20 µg and levonorgestrel 100 µg versus EE 35 µg and norethindrone 500‐750‐1000 µg, Outcome 7 Intermenstrual bleeding ‐ cycle 3.
8.8. Analysis.
Comparison 8 EE 20 µg and levonorgestrel 100 µg versus EE 35 µg and norethindrone 500‐750‐1000 µg, Outcome 8 Amenorrhea ‐ cycle 3.
8.9. Analysis.
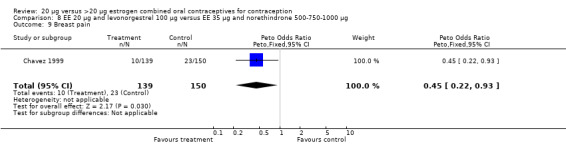
Comparison 8 EE 20 µg and levonorgestrel 100 µg versus EE 35 µg and norethindrone 500‐750‐1000 µg, Outcome 9 Breast pain.
8.10. Analysis.
Comparison 8 EE 20 µg and levonorgestrel 100 µg versus EE 35 µg and norethindrone 500‐750‐1000 µg, Outcome 10 Dysmenorrhea.
8.11. Analysis.
Comparison 8 EE 20 µg and levonorgestrel 100 µg versus EE 35 µg and norethindrone 500‐750‐1000 µg, Outcome 11 Headache.
8.12. Analysis.
Comparison 8 EE 20 µg and levonorgestrel 100 µg versus EE 35 µg and norethindrone 500‐750‐1000 µg, Outcome 12 Nausea.
8.13. Analysis.
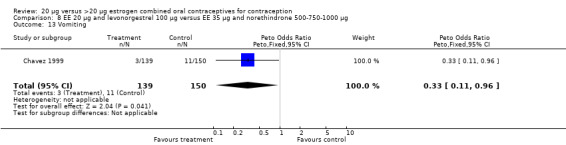
Comparison 8 EE 20 µg and levonorgestrel 100 µg versus EE 35 µg and norethindrone 500‐750‐1000 µg, Outcome 13 Vomiting.
Comparison 9. EE 20 µg and levonorgestrel 100 µg versus EE 35 µg and norgestimate 180‐215‐250 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman | 1 | 309 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.37 [0.05, 2.63] |
9.1. Analysis.
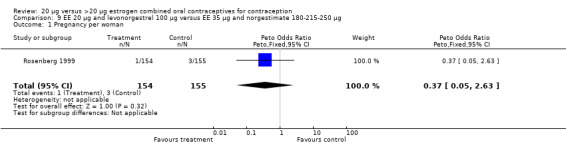
Comparison 9 EE 20 µg and levonorgestrel 100 µg versus EE 35 µg and norgestimate 180‐215‐250 µg, Outcome 1 Pregnancy per woman.
Comparison 10. EE 20 µg and norethindrone acetate 1 mg versus EE 30 µg and levonorgestrel 150 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman | 1 | 110 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.80 [0.38, 20.41] |
| 2 Discontinuation ‐ bleeding | 1 | 110 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.38 [1.82, 15.91] |
| 3 Frequent bleeding ‐ cycles 1 to 3 | 1 | 629 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.92 [2.08, 4.09] |
| 4 Infrequent bleeding ‐ cycles 1 to 3 | 1 | 629 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.84 [1.80, 4.46] |
| 5 Irregular bleeding ‐ cycles 1 to 3 | 1 | 629 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.01 [2.12, 7.61] |
| 6 Prolonged bleeding ‐ cycles 1 to 3 | 1 | 629 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.51 [0.94, 2.43] |
10.1. Analysis.
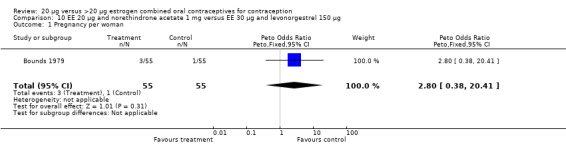
Comparison 10 EE 20 µg and norethindrone acetate 1 mg versus EE 30 µg and levonorgestrel 150 µg, Outcome 1 Pregnancy per woman.
10.2. Analysis.
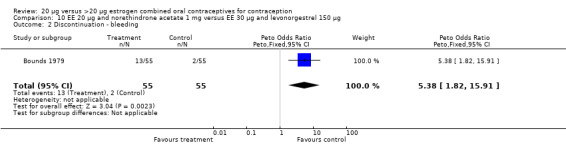
Comparison 10 EE 20 µg and norethindrone acetate 1 mg versus EE 30 µg and levonorgestrel 150 µg, Outcome 2 Discontinuation ‐ bleeding.
10.3. Analysis.
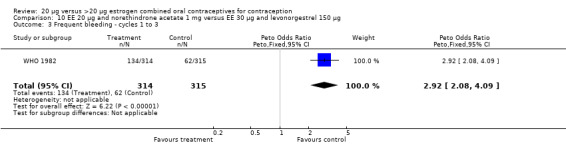
Comparison 10 EE 20 µg and norethindrone acetate 1 mg versus EE 30 µg and levonorgestrel 150 µg, Outcome 3 Frequent bleeding ‐ cycles 1 to 3.
10.4. Analysis.
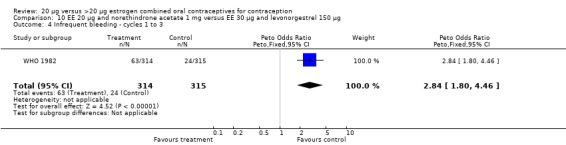
Comparison 10 EE 20 µg and norethindrone acetate 1 mg versus EE 30 µg and levonorgestrel 150 µg, Outcome 4 Infrequent bleeding ‐ cycles 1 to 3.
10.5. Analysis.
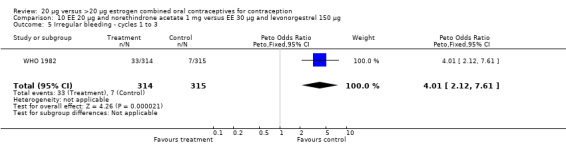
Comparison 10 EE 20 µg and norethindrone acetate 1 mg versus EE 30 µg and levonorgestrel 150 µg, Outcome 5 Irregular bleeding ‐ cycles 1 to 3.
10.6. Analysis.
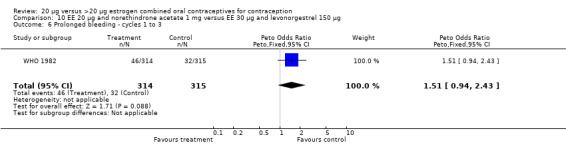
Comparison 10 EE 20 µg and norethindrone acetate 1 mg versus EE 30 µg and levonorgestrel 150 µg, Outcome 6 Prolonged bleeding ‐ cycles 1 to 3.
Comparison 11. EE 20 µg and norethindrone acetate 1 mg versus EE 50 µg and levonorgestrel 150 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Frequent bleeding ‐ cycles 1 to 3 | 1 | 631 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.59 [3.24, 6.51] |
| 2 Infrequent bleeding ‐ cycles 1 to 3 | 1 | 631 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.08 [1.95, 4.86] |
| 3 Irregular bleeding ‐ cycles 1 to 3 | 1 | 631 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.33 [2.74, 10.34] |
| 4 Prolonged bleeding ‐ cycles 1 to 3 | 1 | 631 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.11 [1.83, 5.27] |
11.1. Analysis.
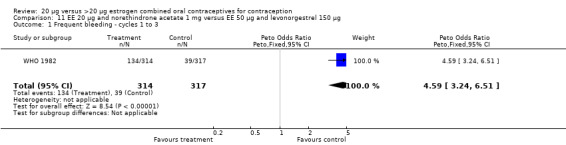
Comparison 11 EE 20 µg and norethindrone acetate 1 mg versus EE 50 µg and levonorgestrel 150 µg, Outcome 1 Frequent bleeding ‐ cycles 1 to 3.
11.2. Analysis.
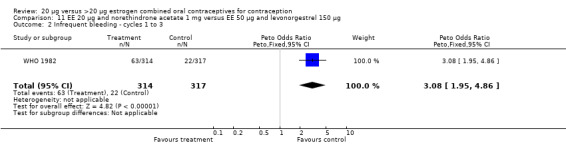
Comparison 11 EE 20 µg and norethindrone acetate 1 mg versus EE 50 µg and levonorgestrel 150 µg, Outcome 2 Infrequent bleeding ‐ cycles 1 to 3.
11.3. Analysis.
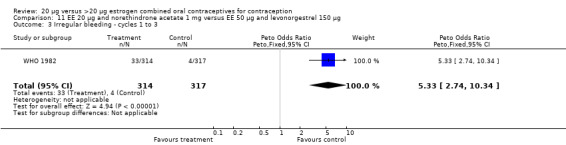
Comparison 11 EE 20 µg and norethindrone acetate 1 mg versus EE 50 µg and levonorgestrel 150 µg, Outcome 3 Irregular bleeding ‐ cycles 1 to 3.
11.4. Analysis.
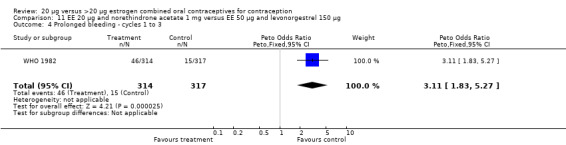
Comparison 11 EE 20 µg and norethindrone acetate 1 mg versus EE 50 µg and levonorgestrel 150 µg, Outcome 4 Prolonged bleeding ‐ cycles 1 to 3.
Comparison 12. EE 20 µg and norethindrone acetate 1 mg versus EE 30 µg and norethindrone acetate 1.5 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of breakthrough bleeding or spotting in days ‐ cycle 3 | 1 | 228 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [0.37, 1.83] |
| 2 Amenorrhea ‐ cycle 3 | 1 | 228 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.17 [0.68, 6.93] |
12.1. Analysis.
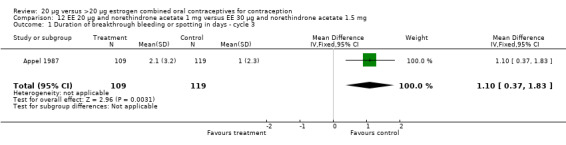
Comparison 12 EE 20 µg and norethindrone acetate 1 mg versus EE 30 µg and norethindrone acetate 1.5 mg, Outcome 1 Duration of breakthrough bleeding or spotting in days ‐ cycle 3.
12.2. Analysis.
Comparison 12 EE 20 µg and norethindrone acetate 1 mg versus EE 30 µg and norethindrone acetate 1.5 mg, Outcome 2 Amenorrhea ‐ cycle 3.
Comparison 13. EE 20 µg and norethindrone acetate 1 mg versus EE 50 µg and norethindrone acetate 1 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of breakthrough bleeding or spotting in days ‐ cycle 3 | 1 | 214 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [0.43, 1.97] |
| 2 Amenorrhea ‐ cycle 3 | 1 | 215 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.34 [0.94, 11.84] |
| 3 Frequent bleeding ‐ cycles 1 to 3 | 1 | 626 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.97 [1.42, 2.73] |
| 4 Infrequent bleeding ‐ cycles 1 to 3 | 1 | 626 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.95 [1.27, 3.00] |
| 5 Irregular bleeding ‐ cycles 1 to 3 | 1 | 626 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.38 [1.31, 4.31] |
| 6 Prolonged bleeding ‐ cycles 1 to 3 | 1 | 626 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.80, 2.02] |
13.1. Analysis.
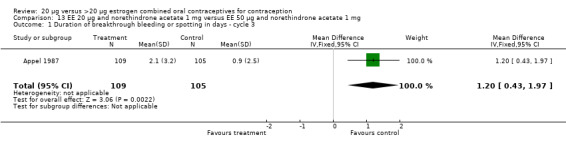
Comparison 13 EE 20 µg and norethindrone acetate 1 mg versus EE 50 µg and norethindrone acetate 1 mg, Outcome 1 Duration of breakthrough bleeding or spotting in days ‐ cycle 3.
13.2. Analysis.
Comparison 13 EE 20 µg and norethindrone acetate 1 mg versus EE 50 µg and norethindrone acetate 1 mg, Outcome 2 Amenorrhea ‐ cycle 3.
13.3. Analysis.
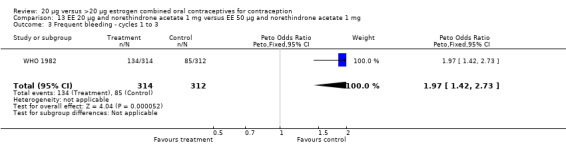
Comparison 13 EE 20 µg and norethindrone acetate 1 mg versus EE 50 µg and norethindrone acetate 1 mg, Outcome 3 Frequent bleeding ‐ cycles 1 to 3.
13.4. Analysis.
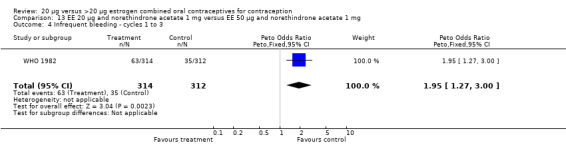
Comparison 13 EE 20 µg and norethindrone acetate 1 mg versus EE 50 µg and norethindrone acetate 1 mg, Outcome 4 Infrequent bleeding ‐ cycles 1 to 3.
13.5. Analysis.
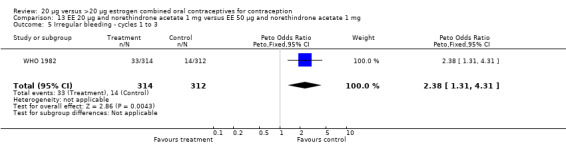
Comparison 13 EE 20 µg and norethindrone acetate 1 mg versus EE 50 µg and norethindrone acetate 1 mg, Outcome 5 Irregular bleeding ‐ cycles 1 to 3.
13.6. Analysis.
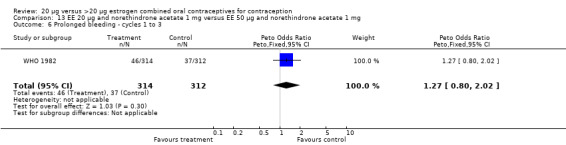
Comparison 13 EE 20 µg and norethindrone acetate 1 mg versus EE 50 µg and norethindrone acetate 1 mg, Outcome 6 Prolonged bleeding ‐ cycles 1 to 3.
Comparison 14. EE 20 µg and norethindrone acetate 1 mg versus EE 20‐30‐50 µg and norethindrone acetate 1‐1.5‐1 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of bleeding or spotting in days ‐ cycle 3 | 1 | 219 | Mean Difference (IV, Fixed, 95% CI) | 1.6 [0.94, 2.26] |
| 2 Amenorrhea ‐ cycle 3 | 1 | 221 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.04 [0.64, 6.50] |
14.1. Analysis.
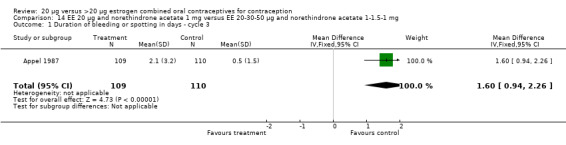
Comparison 14 EE 20 µg and norethindrone acetate 1 mg versus EE 20‐30‐50 µg and norethindrone acetate 1‐1.5‐1 mg, Outcome 1 Duration of bleeding or spotting in days ‐ cycle 3.
14.2. Analysis.
Comparison 14 EE 20 µg and norethindrone acetate 1 mg versus EE 20‐30‐50 µg and norethindrone acetate 1‐1.5‐1 mg, Outcome 2 Amenorrhea ‐ cycle 3.
Comparison 15. EE 20 µg and norethindrone acetate 1 mg versus EE 35 µg and norethindrone acetate 400 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Frequent bleeding ‐ cycles 1 to 3 | 1 | 616 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.64, 1.20] |
| 2 Infrequent bleeding ‐ cycles 1 to 3 | 1 | 616 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.88 [1.22, 2.90] |
| 3 Irregular bleeding ‐ cycles 1 to 3 | 1 | 616 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.92 [1.08, 3.43] |
| 4 Prolonged bleeding ‐ cycles 1 to 3 | 1 | 616 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.59, 1.41] |
15.1. Analysis.
Comparison 15 EE 20 µg and norethindrone acetate 1 mg versus EE 35 µg and norethindrone acetate 400 µg, Outcome 1 Frequent bleeding ‐ cycles 1 to 3.
15.2. Analysis.
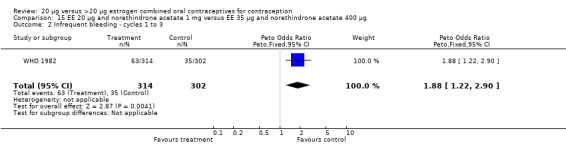
Comparison 15 EE 20 µg and norethindrone acetate 1 mg versus EE 35 µg and norethindrone acetate 400 µg, Outcome 2 Infrequent bleeding ‐ cycles 1 to 3.
15.3. Analysis.
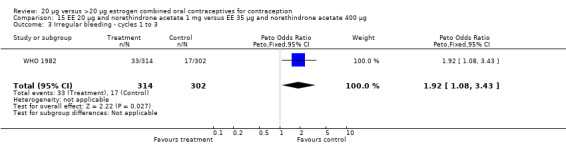
Comparison 15 EE 20 µg and norethindrone acetate 1 mg versus EE 35 µg and norethindrone acetate 400 µg, Outcome 3 Irregular bleeding ‐ cycles 1 to 3.
15.4. Analysis.
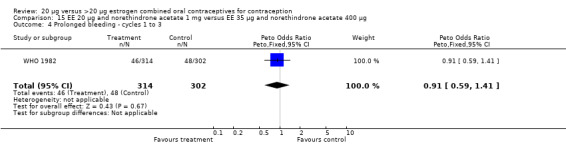
Comparison 15 EE 20 µg and norethindrone acetate 1 mg versus EE 35 µg and norethindrone acetate 400 µg, Outcome 4 Prolonged bleeding ‐ cycles 1 to 3.
Comparison 16. EE 20 µg and norethindrone acetate 1 mg versus mestranol 50 µg and norethindrone 1 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Frequent bleeding ‐ cycles 1 to 3 | 1 | 602 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.82 [2.00, 3.97] |
| 2 Infrequent bleeding ‐ cycles 1 to 3 | 1 | 602 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.49 [1.58, 3.91] |
| 3 Irregular bleeding ‐ cycles 1 to 3 | 1 | 602 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.85 [2.49, 9.43] |
| 4 Prolonged bleeding ‐ cycles 1 to 3 | 1 | 602 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.67 [1.58, 4.52] |
16.1. Analysis.
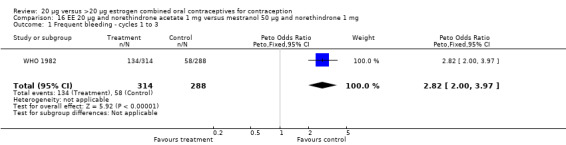
Comparison 16 EE 20 µg and norethindrone acetate 1 mg versus mestranol 50 µg and norethindrone 1 mg, Outcome 1 Frequent bleeding ‐ cycles 1 to 3.
16.2. Analysis.
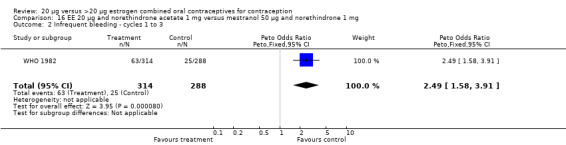
Comparison 16 EE 20 µg and norethindrone acetate 1 mg versus mestranol 50 µg and norethindrone 1 mg, Outcome 2 Infrequent bleeding ‐ cycles 1 to 3.
16.3. Analysis.
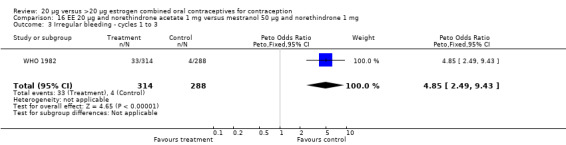
Comparison 16 EE 20 µg and norethindrone acetate 1 mg versus mestranol 50 µg and norethindrone 1 mg, Outcome 3 Irregular bleeding ‐ cycles 1 to 3.
16.4. Analysis.
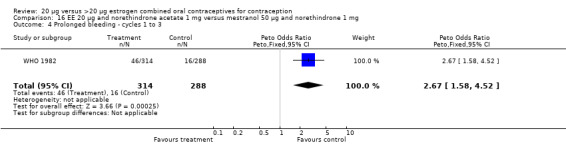
Comparison 16 EE 20 µg and norethindrone acetate 1 mg versus mestranol 50 µg and norethindrone 1 mg, Outcome 4 Prolonged bleeding ‐ cycles 1 to 3.
Comparison 17. EE 20 µg and norethindrone acetate 1 mg versus EE 25 µg and norgestimate 180‐215‐250 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman | 1 | 2814 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.41 [0.74, 2.68] |
| 2 Discontinuation ‐ overall | 1 | 2894 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.81, 1.18] |
| 3 Discontinuation ‐ adverse events | 1 | 2894 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.54, 1.16] |
| 4 Breakthrough bleeding ‐ cycle 3 | 1 | 2330 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.79 [2.09, 3.74] |
| 5 Breakthrough bleeding ‐ cycle 6 | 1 | 2118 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.40 [1.78, 3.24] |
| 6 Breakthrough bleeding or spotting ‐ cycle 3 | 1 | 2330 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.32 [1.85, 2.90] |
| 7 Breakthrough bleeding or spotting ‐ cycle 6 | 1 | 2118 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.51 [1.97, 3.20] |
| 8 Unscheduled bleeding ‐ cycle 3 | 1 | 2478 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.92 [1.61, 2.29] |
| 9 Unscheduled bleeding ‐ cycle 6 | 1 | 2222 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.91 [1.58, 2.32] |
| 10 Weight loss, maintenance, or gain <5% ‐ cycle 6 | 1 | 2157 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.92, 1.46] |
17.1. Analysis.
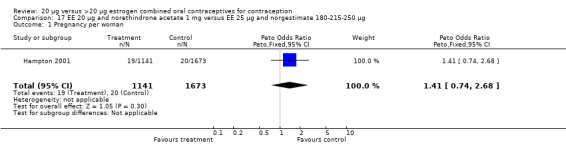
Comparison 17 EE 20 µg and norethindrone acetate 1 mg versus EE 25 µg and norgestimate 180‐215‐250 µg, Outcome 1 Pregnancy per woman.
17.2. Analysis.
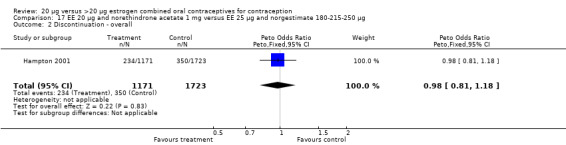
Comparison 17 EE 20 µg and norethindrone acetate 1 mg versus EE 25 µg and norgestimate 180‐215‐250 µg, Outcome 2 Discontinuation ‐ overall.
17.3. Analysis.
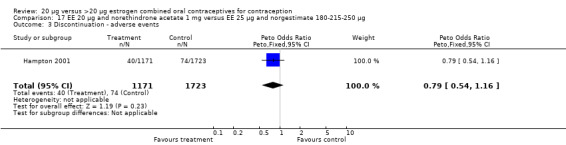
Comparison 17 EE 20 µg and norethindrone acetate 1 mg versus EE 25 µg and norgestimate 180‐215‐250 µg, Outcome 3 Discontinuation ‐ adverse events.
17.4. Analysis.
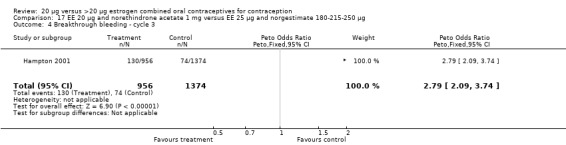
Comparison 17 EE 20 µg and norethindrone acetate 1 mg versus EE 25 µg and norgestimate 180‐215‐250 µg, Outcome 4 Breakthrough bleeding ‐ cycle 3.
17.5. Analysis.
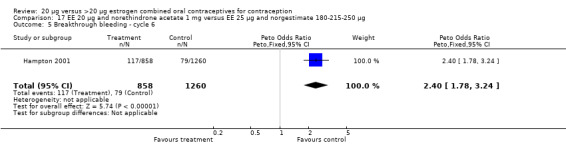
Comparison 17 EE 20 µg and norethindrone acetate 1 mg versus EE 25 µg and norgestimate 180‐215‐250 µg, Outcome 5 Breakthrough bleeding ‐ cycle 6.
17.6. Analysis.
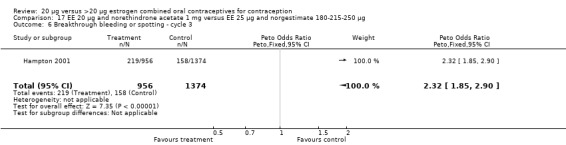
Comparison 17 EE 20 µg and norethindrone acetate 1 mg versus EE 25 µg and norgestimate 180‐215‐250 µg, Outcome 6 Breakthrough bleeding or spotting ‐ cycle 3.
17.7. Analysis.
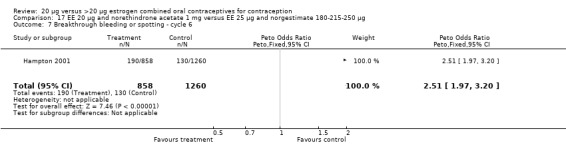
Comparison 17 EE 20 µg and norethindrone acetate 1 mg versus EE 25 µg and norgestimate 180‐215‐250 µg, Outcome 7 Breakthrough bleeding or spotting ‐ cycle 6.
17.8. Analysis.
Comparison 17 EE 20 µg and norethindrone acetate 1 mg versus EE 25 µg and norgestimate 180‐215‐250 µg, Outcome 8 Unscheduled bleeding ‐ cycle 3.
17.9. Analysis.
Comparison 17 EE 20 µg and norethindrone acetate 1 mg versus EE 25 µg and norgestimate 180‐215‐250 µg, Outcome 9 Unscheduled bleeding ‐ cycle 6.
17.10. Analysis.
Comparison 17 EE 20 µg and norethindrone acetate 1 mg versus EE 25 µg and norgestimate 180‐215‐250 µg, Outcome 10 Weight loss, maintenance, or gain <5% ‐ cycle 6.
Comparison 18. EE 20 µg and drospirenone 3 mg versus EE 30 µg and drospirenone 3 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman | 1 | 50 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
18.1. Analysis.
Comparison 18 EE 20 µg and drospirenone 3 mg versus EE 30 µg and drospirenone 3 mg, Outcome 1 Pregnancy per woman.
Comparison 19. EE 20 µg and drospirenone 3 mg versus EE 25 µg and norgestimate 180‐215‐250 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Unscheduled bleeding days ‐ cycle 3 | 1 | 332 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [0.44, 1.56] |
19.1. Analysis.
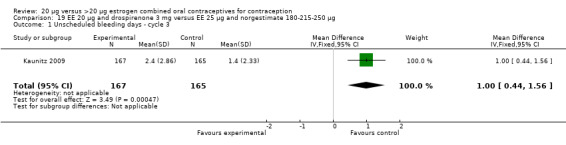
Comparison 19 EE 20 µg and drospirenone 3 mg versus EE 25 µg and norgestimate 180‐215‐250 µg, Outcome 1 Unscheduled bleeding days ‐ cycle 3.
Comparison 20. EE 20 µg and levonorgestrel 100 µg versus EE 30 µg and levonorgestrel 150 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy per woman | 1 | 70 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Adverse events | 1 | 70 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.57 [0.62, 3.98] |
20.1. Analysis.
Comparison 20 EE 20 µg and levonorgestrel 100 µg versus EE 30 µg and levonorgestrel 150 µg, Outcome 1 Pregnancy per woman.
20.2. Analysis.
Comparison 20 EE 20 µg and levonorgestrel 100 µg versus EE 30 µg and levonorgestrel 150 µg, Outcome 2 Adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akerlund 1993.
| Methods | Six sites in Norway, two in Sweden and one in Denmark. 12 treatment cycles. Double‐blinded but did not specify who was blinded. | |
| Participants | Women aged 18 to 35 (Norway sites) or 18 to 40 (Sweden and Denmark sites) years. Excluded heavy smoking among women 35 years of age; risk factors for or history of certain diseases; lactation; and certain antibiotics. | |
| Interventions | EE 20 µg and desogestrel 150 µg (N=500) versus EE 30 µg and desogestrel 150 µg (N=500). | |
| Outcomes | Contraceptive efficacy, cycle control, and side effects. 'Withdrawal' bleeding defined as bleeding that began within the pill‐free period and did not exceed eight days. 'Irregular' bleeding defined as any other bleeding. | |
| Notes | Allocated with simple random table. Excluded randomized women from the analysis. 67% (672/1000) completed study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
Appel 1987.
| Methods | 30 sites in unreported location(s). Four treatment cycles. Observer blinded. Tablets were supplied from manufacturer in standard, unmarked packs. | |
| Participants | Healthy women aged 18 to 36 years with regular menses. Excluded pregnancy; history of certain diseases; and certain drugs. | |
| Interventions | EE 20 µg and norethindrone acetate 1.0 mg versus EE 30 µg and norethindrone acetate 1.5 µg versus EE 50 µg and norethindrone acetate 1.0 µg versus EE 20‐30‐50 µg and norethindrone acetate 1.0‐1.5‐1.0 mg. 564 women randomized; initial number assigned to each study group not reported. | |
| Outcomes | Contraceptive efficacy, cycle control, side effects, blood pressure, body weight and hemoglobin. 'Breakthrough' bleeding or spotting defined as bleeding or spotting occurring during the pill‐taking period. | |
| Notes | Randomization method not reported. 76% (426/564) completed study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
Basdevant 1993.
| Methods | Number and location of sites not reported. Six treatment cycles. Blinding not described. | |
| Participants | Healthy, non‐obese women with regular menses. Excluded lactation; recent birth or abortion; recent steroid treatment; venous or arterial disease; diabetes; hyperlipidemia; eating disorders; smokers; hypertension; gynecological tumors; cancer; and certain drugs. | |
| Interventions | EE 20 µg and desogestrel 150 µg (N=33) versus EE 30 µg and desogestrel 150 µg (N=25). | |
| Outcomes | Plasma lipid levels, glucose tolerance, blood pressure, hemostatic values, and discontinuation. Did not report bleeding outcomes. | |
| Notes | Randomization method not reported. 76% (426/564) completed study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
Bounds 1979.
| Methods | Seven initial sites in the U.K. planned but increased to 12 due to slow enrollment. 12 treatment cycles. Participants and clinic doctors blinded. Pre‐coded, sealed envelopes with unmarked blister packs used for pills. | |
| Participants | Sexually active women aged 16 to 39 years at risk for pregnancy with regular menses. Excluded contraindications to oral contraceptive use; lactation; and irregular bleeding and spotting. | |
| Interventions | EE 20 µg and norethisterone acetate 1.0 mg versus EE 30 µg and levonorgestrel 150 µg. 143 women randomized; initial number assigned to each study group not reported. | |
| Outcomes | Contraceptive efficacy, discontinuation, cycle control, and side effects. 'Menstrual' bleeding defined as any bleeding requiring sanitary protection regardless of timing in cycle. | |
| Notes | Randomization method not reported. Excluded randomized women from the analysis. Number completing study was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
Brill 1996.
| Methods | One site in unreported location. Three pill‐free pretreatment and 13 treatment cycles. Unblinded. | |
| Participants | Women aged 18 to 35 years with regular menses. Excluded smokers over 30 years of age; pregnancy; certain diseases; certain drugs; intrauterine device use; overweight or dieting; and heavy alcohol use. | |
| Interventions | EE 20 µg and gestodene 75 µg (N=32) versus EE 30 µg and gestodene 75 µg (N=32). | |
| Outcomes | Lipid metabolism, cycle control, and adverse events. Did not report bleeding outcomes. | |
| Notes | Randomization method not reported. Excluded randomized women from the analysis. Number completing study was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
Bruni 2000.
| Methods | Unreported number of sites in 18 nations. 13 treatment cycles. Unblinded. | |
| Participants | Women 'over the legal age of consent' and less than 42 years of age with regular menses. Excluded estrogen or progestogen hypersensitivity; pregnancy; lactation; and certain disorders. | |
| Interventions | EE 20 µg and desogestrel 150 µg (N=805) versus EE 30 µg and gestodene 75 µg (N=806) versus EE 30‐40‐30 µg and gestodene 50‐70‐100 µg (N=808). | |
| Outcomes | Cycle control and effect on well‐being. Bleeding terms not defined. | |
| Notes | Randomization method not reported. Excluded randomized women from the analysis. 71% (1721/2419) completed study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
Chavez 1999.
| Methods | 11 sites in the USA Four treatment cycles. Unblinded. | |
| Participants | Healthy women at risk for pregnancy with regular menses. Excluded contraindications for oral contraceptive use; smokers aged 35 years or older; heavy smoking; recent oral injectable, implantable, or intrauterine contraceptive use; and drug or alcohol abuse. | |
| Interventions | EE 20 µg and levonorgestrel 100 µg (N=169) versus EE 35 µg and norethindrone 500‐750‐1000 µg (N=173). | |
| Outcomes | Cycle control. 'Withdrawal' bleeding or spotting defined as bleeding or spotting beginning in pill‐free interval and stopping by fourth day of the next cycle. 'Intermenstrual' bleeding defined as all other bleeding. | |
| Notes | Randomization method not reported. Excluded randomized women from the analysis. 56% (191/342) completed study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
Endrikat 1997.
| Methods | 10 sites in Germany. 12 treatment cycles. Double‐blinded but did not specify who was blinded. | |
| Participants | Healthy, sexually active women aged 18 to 39 years who wanted contraception for at least 12 months. Excluded recent depot‐contraceptives; certain diseases; and contraindications for oral contraceptive use. | |
| Interventions | EE 20 µg and gestodene 75 µg (N=428) versus EE 30 µg and gestodene 75 µg (N=221). | |
| Outcomes | Contraceptive efficacy, cycle control, and tolerance. 'Intermenstrual' bleeding was defined as either spotting or breakthrough bleeding. The definition for 'intermenstrual' bleeding did not specify cycle days. | |
| Notes | Randomization method not reported. Excluded randomized women from the analysis. 75% (488/649) completed study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
Endrikat 2001.
| Methods | Two sites in The Netherlands. Six treatment cycles. Participants and investigators blinded. | |
| Participants | Healthy women aged 18 to 35 years who wanted contraception for at least six months. Excluded contraindications to oral contraceptive use; recent depot contraceptive use; genital bleeding; and menses‐related migraines. | |
| Interventions | EE 20 µg and gestodene 75 µg (23 pill days; N=35) versus EE 30 µg and gestodene 75 µg (21 pill days; N=34). 70 women randomized; the group assignment for one woman was not specified. | |
| Outcomes | Hemostatic values, lipids, carbohydrate metabolism, and tolerability. 'Intracyclic' bleeding defined as any bleeding during cycle days 4 to 21 for the 21‐day regimen and cycle days 6 to 23 for the 23‐day regimen. | |
| Notes | Randomization method not reported. 94% (66/70) completed study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
Hampton 2001.
| Methods | 100 sites in USA and 10 in Canada. First 1/3 of participants were to have 13 treatment cycles and the remaining 2/3 were to have 6 treatment cycles. Allocated in block sizes of 11; blinded. | |
| Participants | Sexually active, healthy women aged 18 to 45 years at risk for pregnancy with regular menses. Excluded recent pregnancy; recent lactation; contraindications to oral contraceptives; certain diseases; smokers aged 35 or more years; certain drugs or devices; recent DMPA use; and recent alcohol or substance abuse. | |
| Interventions | EE 20 µg and norethindrone acetate 1.0 mg with 75 mg ferrous fumarate on days 22‐28 (N=853 for 6 cycles / 318 for 13 cycles) versus EE 25 µg and norgestimate 180‐215‐250 µg (N=1236 for 6 cycles / 487 for 13 cycles). | |
| Outcomes | Contraceptive efficacy, cycle control, and safety. 'Breakthrough' bleeding or spotting defined as bleeding or spotting that occurred during active pill days unless contiguous with menses. 'Amenorrhea' defined as two consecutive cycles without any bleeding or spotting. Secondary report (Burkman 2007) included weight change. Secondary report (Hampton 2009) re‐analyzed bleeding data with new criteria (Mishell 2007b); unscheduled bleeding data presented here. | |
| Notes | Cyclophasic regimens discontinued early and not reported. 74% (2130/2894) completed study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods not reported. |
Inauen 1991.
| Methods | Number and location of sites not reported. Cross‐over trial with 3 treatment cycles on each oral contraceptive. Blinding not described. | |
| Participants | Healthy women aged 18 to 30 years without oral contraceptive use during prior four months. Excluded blood coagulation disorders. | |
| Interventions | EE 20 µg and desogestrel 150 µg (N=20) versus EE 50 µg and desogestrel 125 µg (N=20). | |
| Outcomes | Blood coagulation, thrombogenesis, and side effects. Bleeding terms were not defined. | |
| Notes | Randomization method not reported. 100% completed study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
Kaunitz 2009.
| Methods | RCT at 20 centers in the USA. Computer‐generated randomization schedule; stratified by center and recent hormonal contraceptive use (new user or switcher). 3 treatment cycles. Open label. Sample size based on prior studies for total bleeding days for cycles 1 to 3; 90% power to detect difference between groups. | |
| Participants | 334 healthy women, nonpregnant, non‐lactating, sexually active, aged 18 to 45 years. Inclusion criteria: nonsmokers, regular menstrual cycles, negative Chlamydia test, normal Pap test in past 12 months. Exclusion criteria: contraindication to hormonal therapy, untreated thyroid disorder, body mass index (BMI) > 40 kg/m2, previously discontinued one of the treatments due to breakthrough bleeding; received injectable contraceptive in past 6 months, implant in past 60 days, or hormonal IUD in past 3 months. | |
| Interventions | EE 20 µg and drospirenone 3 mg (24/4‐day regimen) (N=167) versus EE 25 µg and norgestimate 180‐215‐250 µg (21/7‐day regimen) (N=167) | |
| Outcomes | Bleeding data were recorded daily via a voice‐response system. Bleeding and unscheduled bleeding (days) defined as per Mishell 2007b. Unscheduled bleeding episode was defined as per Belsey 1986. | |
| Notes | Of 355 randomized, 21 were screen failures (10 EE 20 µg and 11 EE 25 µg). The remaining 334 women who received study drug were included in safety analysis. Completed study: EE 20 µg group, (156/167) 93%; EE 25 µg group, 154/167 (92%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Kirkman 1994.
| Methods | 66 sites in Denmark, Italy, New Zealand and the UK. Six treatment cycles. Unblinded. | |
| Participants | Healthy women over 30 years of age with regular menses. Excluded smokers over 34 years of age, select drug use, and lactation. | |
| Interventions | EE 20 µg and desogestrel 150 µg (N=501) versus EE 30 µg and gestodene 75 µg (N=505). | |
| Outcomes | Cycle control, discontinuation, body weight, blood pressure, and adverse events. 'Withdrawal' bleeding episode was defined as a sequence of one or more days of bleeding or spotting that began during the pill‐free period and was bounded by two consecutive days without bleeding. Results, though, were reported for 'irregular' bleeding (with and without withdrawal bleeding), which was never defined. | |
| Notes | Allocated with pre‐distributed schedules. Excluded randomized women from the analysis. 87% (874/1006) completed study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
Kluft 2006.
| Methods | Open‐label, randomized trial at one center in The Netherlands from 1992 to 1993. | |
| Participants | 75 healthy women, 18 to 35 years. Inclusion criteria: desiring contraception for at least 6 cycles, new OC users or switchers with at least 2 OC‐free cycles before study. Exclusion criteria: contraindications to OC use, family history of coagulation disorders, use of parenteral depot contraceptive in past 6 months, concomitant diseases (not specified in report), diagnostically unclassified genital bleeding, history of migraine with menstruation. | |
| Interventions | Drospirenone 3 mg + EE 30 µg or drospirenone 3 mg + EE 20 µg versus desogestrel + EE 30 µg (N=25 in each group); 6 treatment cycles. | |
| Outcomes | For pregnancy and serious adverse events, the researchers reported none occurred. | |
| Notes | No information on method of randomization. No losses reported; N=75 in per protocol analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
Reisman 1999.
| Methods | 11 sites in the USA Four treatment cycles. Unblinded. | |
| Participants | Healthy women over aged 18 years at risk for pregnancy with regular menses. Excluded smokers over age 35 years; contraindications for oral contraceptive use; and recent oral contraceptive, intrauterine device, injectable, or implantable estrogens, progestins, or androgens. | |
| Interventions | EE 20 µg and levonorgestrel 100 µg (N=192) versus EE 35 µg and norethindrone 500‐750‐1000 µg (N=195). | |
| Outcomes | Cycle control and safety. 'Withdrawal' bleeding or spotting was defined as bleeding or spotting beginning in pill‐free interval and stopping by fourth day of the next cycle. 'Intermenstrual' bleeding was defined as all other bleeding. | |
| Notes | Excluded randomized women from the analysis. 57% (220/387) completed study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocated with sequentially numbered, sealed envelopes. |
Rosenberg 1999.
| Methods | 15 sites in US. Six‐month wash‐out period for injectable or implant contraceptive users. Six treatment cycles. Unblinded. | |
| Participants | Sexually active, normal weight women aged 18 to 50 years with regular menses. Excluded contraindications to oral contraceptive use; smokers over 35 years of age; heavy alcohol use; and lactation. | |
| Interventions | EE 20 µg and levonorgestrel 100 µg (N=154) versus EE 20 µg and desogestrel 150 µg (N=154) versus EE 35 µg and norgestimate 180‐215‐250 µg (N=155). | |
| Outcomes | Cycle control. 'Unscheduled' bleeding was defined as bleeding not continuous with withdrawal bleeding. 'Withdrawal bleeding' was not defined. | |
| Notes | Allocated with masked randomization lists at each site generated with block size of six. 86% (398/463) completed study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
Skouby 2005.
| Methods | Open‐label, randomized trial at one center in Denmark | |
| Participants | 70 healthy women, 18 to 35 years. Inclusion criteria: desiring contraception for at least 13 cycles, new OC users or switchers with at least 2 OC‐free cycles before study. Exclusion criteria: contraindications to OC use, parenteral depot contraceptive in past 6 months, co‐existing diseases (not specified in report), diagnostically unclassified genital bleeding, history of migraine with menstruation. | |
| Interventions | Levonorgestrel 100 µg + EE 20 µg (N=35) versus levonorgestrel 150 µg + EE 30 µg (N=35); 13 treatment cycles. | |
| Outcomes | Pregnancy and adverse events. | |
| Notes | No information on method of randomization. Losses: 1 took no study medication (excluded) and 7 discontinued early (8/70 = 11%); no information about which groups these women were assigned to. Full analysis reportedly had N=69; 49 in per protocol analysis (22 in EE 20 µg group and 27 in 30 µg). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
Taneepanichskul 2002.
| Methods | One site in Thailand. Three‐month wash‐out period for OC users. 12 treatment cycles. Unblinded. | |
| Participants | Women aged 18 to 35 years, willing to use contraception for over 12 complete cycles with at least a three‐month washout period. Excluded contraindications to OC use; liver, vascular or metabolic diseases; tumor; pregnancy; unclassified and genital bleeding. | |
| Interventions | EE 20 µg and gestodene 75 µg (N=76) versus EE 30 µg and gestodene 75 µg (N=74). | |
| Outcomes | Contraceptive efficacy, cycle control, and side effects. 'Regular' cycle was defined as periodic withdrawal bleeding every 28±7days. 'Breakthrough bleeding' was defined as intermenstrual bleeding that did not require sanitary protection. | |
| Notes | Randomization method not reported. 76% (114/150) completed study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
Teichmann 1995.
| Methods | One site in Poland. Two pretreatment and 12 treatment cycles. Blinding not described. | |
| Participants | Healthy, normal‐weight, sexually active women aged 19 to 40 years seeking oral contraception with regular menses. Excluded recent hormonal medication and certain other drugs; smokers; and contraindications to oral contraception. | |
| Interventions | EE 20 µg and desogestrel 150 µg versus EE 30 µg and gestodene 75 µg. 500 women randomized; initial number assigned to each study group not reported. | |
| Outcomes | Contraceptive efficacy, discontinuation, adverse events, and follicle growth. Bleeding terms not defined. | |
| Notes | Allocated according to a randomization list in chronological order. Excluded randomized women from the analysis. 63% (314/500) completed study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
WHO 1982.
| Methods | 10 sites in unreported location(s). 24 treatment cycles. Clinic staff and participants blinded. Pills were repackaged in packets and identified only by random number. | |
| Participants | Healthy women aged 18 to 38 with regular menses. Excluded contraindications for oral contraceptive use; postpartum; lactation; and recent oral or injectable contraception. | |
| Interventions | EE 20 µg and norethisterone acetate 1.0 mg (N=448) versus EE 35 µg and norethisterone acetate 400 µg (N=434) versus EE 50 µg and levonorgestrel 150 µg (N=435) versus EE 30 µg and levonorgestrel 150 µg (N=430) versus mestranol 50 µg and norethisterone 1.0 mg (N=436) versus EE 50 µg and norethisterone acetate 1.0 mg (N=431). | |
| Outcomes | Contraceptive efficacy, discontinuation, and cycle control. 'Segments' were defined as the start of one menstrual‐like bleeding episode to the start of the next. 'Infrequent' bleeding defined as when the longest menstrual segment was greater than 35 days and not over 60 days. 'Frequent' bleeding defined as when the shortest complete menstrual segment was less than 24 days. 'Irregular' bleeding defined as when the shortest complete segment was less than 24 days and the longest segment was greater than 35 days. 'Prolonged' bleeding defined as when bleeding or spotting episode was longer than 7 days. | |
| Notes | Randomization method not reported. Excluded randomized women from the analysis. 46% (1196/2614) completed study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
Winkler 1996.
| Methods | One site in unreported location. Duration: 2 pretreatment cycles, 6 treatment cycles, and 1 post‐treatment cycle. Unblinded. | |
| Participants | Healthy women aged 18 to 30 with regular menses. Excluded contraindications to oral contraceptive use; smoking; and certain drugs. | |
| Interventions | EE 20 µg and gestodene 75 µg (N=20) versus EE 30 µg and gestodene 75 µg (N=20). | |
| Outcomes | Hemostatic values. Did not report bleeding outcomes. | |
| Notes | Randomization method not reported. Number completing study not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
EE = ethinyl estradiol OC = oral contraceptive
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bassol 2000 | The sum of the percentages of women completing the study and withdrawing appears to be greater than 100%. |
| Lawson 1979 | Initiated as randomized controlled trial but some participants were re‐assigned treatments in a nonrandom manner during the trial. |
| Marr 2012 | Not an RCT; analysis involved pooling data from two uncontrolled trials. |
| Rosenberg 1996 | Reported bleeding and spotting outcomes by percentages without providing absolute numbers. |
| Westhoff 2005 | Ring released EE < 20 mcg; OC contained EE 25 mcg |
| Wiegratz 2003 | Reported relevant outcomes by percentages without providing absolute numbers. |
Contributions of authors
M Gallo extracted data for the initial review and drafted the original review. K Nanda developed the idea and extracted data for the initial review. K Nanda, D Grimes and K Schulz revised and approved the initial review. L Lopez conducted the updates from 2008 to 2013, extracted new data, and revised the review accordingly. D Grimes did the second data extraction for the 2008 and 2010 updates.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute of Child Health and Human Development, USA.
Support for conducting the review at FHI 360
-
US Agency for International Development, USA.
Support for conducting the review at FHI 360
Declarations of interest
DA Grimes has consulted with the pharmaceutical companies Bayer Healthcare Pharmaceuticals and Merck & Co, Inc.
Edited (no change to conclusions)
References
References to studies included in this review
Akerlund 1993 {published data only}
- Akerlund M. Clinical experience of a combined oral contraceptive with very low dose ethinyl estradiol. Acta Obstetricia et Gynecologica Scandinavica 1997;164(Suppl):63‐5. [PubMed] [Google Scholar]
- Akerlund M, Rode A, Westergaard J. Comparative profiles of reliability, cycle control and side effects of two oral contraceptive formulations containing 150 µg desogestrel and either 30 µg or 20 µg ethinyl oestradiol. British Journal of Obstetrics and Gynaecology 1993;100(9):832‐8. [DOI] [PubMed] [Google Scholar]
Appel 1987 {published data only}
- Appel TB, Kambi AA, Birdsall C, Christenson DA, Clark DO, DeKoning JR, et al. A comparison of a new graduated estrogen formulation with three constant‐dosed oral contraceptives. Contraception 1987;35(6):523‐32. [DOI] [PubMed] [Google Scholar]
Basdevant 1993 {published data only}
- Basdevant A, Conard J, Pelissier C, Guyene TT, Lapousterle C, Mayer M, et al. Hemostatic and metabolic effects of lowering the ethinyl‐estradiol dose from 30 mcg to 20 mcg in oral contraceptives containing desogestrel. Contraception 1993;48(3):193‐204. [DOI] [PubMed] [Google Scholar]
Bounds 1979 {published data only}
- Bounds W, Vessey M, Wiggins P. A randomized double‐blind trial of two low dose combined oral contraceptives. British Journal of Obstetrics and Gynaecology 1979;86(4):325‐9. [DOI] [PubMed] [Google Scholar]
Brill 1996 {published data only}
- Brill K, Then A, Beisiegel U, Jene A, Wunsch C, Leidenberger F. Investigation of the influence of two low‐dose monophasic oral contraceptives containing 20 µg ethinylestradiol/75 µg gestodene and 30 µg ethinylestradiol/75 µg gestodene, on lipid metabolism in an open randomized trial. Contraception 1996;54(5):291‐7. [DOI] [PubMed] [Google Scholar]
Bruni 2000 {published data only}
- Bruni V, Croxatto H, Cruz J, Dhont M, Durlot F, Fernandes MT, et al. A comparison of cycle control and effect on well‐being of monophasic gestodene‐, triphasic gestodene‐ and monophasic desogestrel‐containing oral contraceptives. Gestodene Study Group. Gynecological Endocrinology 2000;14(2):90‐8. [DOI] [PubMed] [Google Scholar]
Chavez 1999 {published data only}
- Chavez A, DelConte A. A comparison of cycle control with monophasic levonorgestrel/ethinylestradiol 100 µg/20 µg versus triphasic norethindrone/ethinylestradiol 500‐750‐1000 µg/35 µg: a multicenter, randomized, open‐label study. The European Journal of Contraception and Reproductive Health Care 1999;4(2):75‐83. [DOI] [PubMed] [Google Scholar]
Endrikat 1997 {published data only}
- Endrikat J, Müller U, Düsterberg B. A twelve‐month comparative clinical investigation of two low‐dose oral contraceptives containing 20 µg ethinylestradiol/75 µg gestodene and 30 µg ethinylestradiol/75 µg gestodene, with respect to efficacy, cycle control, and tolerance. Contraception 1997;55(3):131‐7. [DOI] [PubMed] [Google Scholar]
Endrikat 2001 {published data only}
- Endrikat J, Klipping C, Gerlinger C, Ruebig A, Schmidt W, Holler T, et al. A double‐blind comparative study of the effects of a 23‐day oral contraceptive regimen with 20 µg ethinyl estradiol and 75 µg gestodene and a 21‐day regimen with 30 µg ethinyl estradiol and 75 µg gestodene on hemostatic variables, lipids, and carbohydrate metabolism. Contraception 2001;64(4):235‐41. [DOI] [PubMed] [Google Scholar]
Hampton 2001 {published data only}
- Burkman RT, Fisher AC, LaGuardia KD. Effects of low‐dose oral contraceptives on body weight. Journal of Reproductive Medicine 2007;52(11):1030‐4. [PubMed] [Google Scholar]
- Hampton RM, Fisher AC, Pagano S, LaGuardia KD. Scheduled and unscheduled bleeding patterns with two combined hormonal contraceptives: application of new recommendations for standardization. Fertility and Sterility 2009;92(2):434‐40. [DOI] [PubMed] [Google Scholar]
- Hampton RM, Short M, Bieber E, Bouchard C, Ayotte N, Shangold G, et al. Comparison of a novel norgestimate/ethinyl estradiol oral contraceptive (Ortho Tri‐Cyclen Lo) with the oral contraceptive Loestrin Fe 1/20. Contraception 2001;63(6):289‐95. [DOI] [PubMed] [Google Scholar]
Inauen 1991 {published data only}
- Inauen W, Stocker G, Haeberli A, Straub PW. Effects of low and high dose oral contraceptives on blood coagulation and thrombogenesis induced by vascular subendothelium exposed to flowing human blood. Contraception 1991;43(5):435‐46. [DOI] [PubMed] [Google Scholar]
Kaunitz 2009 {published data only}
Kirkman 1994 {published data only}
- Kirkman RJ. Clinical comparison of two low dose oral contraceptives in women over 30 years of age [abstract]. Advances in Contraception 1992; Vol. 8, issue 3:170‐1. [DOI] [PubMed]
- Kirkman RJ. Clinical comparison of two low‐dose oral contraceptives in women older than 30 years. Advances in Contraception 1991;7(Suppl 2):63‐76. [Google Scholar]
- Kirkman RJ, Pedersen JH, Fioretti P, Roberts HE. Clinical comparison of two low‐dose oral contraceptives, Minulet® and Mercilon®, in women over 30 years of age. Contraception 1994;49(1):33‐46. [DOI] [PubMed] [Google Scholar]
- Kirkman RJ, Pedersen JH, Fioretti P, Roberts HG. Comparison of acceptability and efficacy of low dose oral contraceptives containing gestodene or desogestrel [abstract]. Advances in Contraception 1995;11(1):36‐7. [Google Scholar]
Kluft 2006 {published data only}
- Kluft C, Endrikat J, Mulder SM, Gerlinger C, Heithecker R. A prospective study on the effects on hemostasis of two oral contraceptives containing drospirenone in combination with either 30 or 20 µg ethinyl estradiol and a reference containing desogestrel and 30 µg ethinyl estradiol. Contraception 2006;73(4):336‐43. [DOI] [PubMed] [Google Scholar]
Reisman 1999 {published data only}
- Reisman H, Martin D, Gast MJ. A multicenter randomized comparison of cycle control and laboratory findings with oral contraceptive agents containing 100 µg levonorgestrel with 20 µg ethinyl estradiol or triphasic norethindrone with ethinyl estradiol. American Journal of Obstetrics and Gynecology 1999;181(5 Pt 1):45‐52. [DOI] [PubMed] [Google Scholar]
Rosenberg 1999 {published data only}
- Rosenberg MJ, Meyers A, Roy V. Efficacy, cycle control, and side effects of low‐ and lower‐dose oral contraceptives: a randomized trial of 20 µg and 35 µg estrogen preparations. Contraception 1999;60(6):321‐9. [DOI] [PubMed] [Google Scholar]
Skouby 2005 {published data only}
- Jespersen J, Endrikat J, Dusterberg B, Schmidt W, Gerlinger C, Wessel J, et al. A 1‐year study to compare the hemostatic effects of oral contraceptive containing 20 µg of ethinylestradiol and 100 µg of levonorgestrel with 30 µg of ethinylestradiol and 100 µg of levonorgestrel. Contraception 2005;72(2):98‐104. [DOI] [PubMed] [Google Scholar]
- Skouby SO, Endrikat J, Dusterberg B, Schmidt W, Gerlinger C, Wessel J, et al. A 1‐yr randomized study to evaluate the effects of a dose reduction in oral contraceptives on lipids and carbohydrate metabolism: 20 µg ethinyl estradiol combined with 100 µg levonorgesterel. Contraception 2005;71(2):111‐7. [DOI] [PubMed] [Google Scholar]
Taneepanichskul 2002 {published data only}
- Taneepanichskul S, Kriengsinyot R, Jaisamrarn U. A comparison of cycle control, efficacy, and side effects among healthy Thai women between two low‐dose oral contraceptives containing 20 µg ethinylestradiol/75 µg gestodene (Meliane) and 30 µg ethinylestradiol/75 µg gestodene (Gynera). Contraception 2002;66(6):407‐9. [DOI] [PubMed] [Google Scholar]
Teichmann 1995 {published data only}
- Teichmann AT, Brill K, Albring M, Schnitker J, Wojtynek P, Kustra E. The influence of the dose of ethinylestradiol in oral contraceptives on follicle growth. Gynecological Endocrinology 1995;9(4):299‐305. [DOI] [PubMed] [Google Scholar]
WHO 1982 {published data only}
- Task Force on Oral Contraceptives, WHO Special Programme of Research, Development and Research Training in Human Reproduction. A randomized, double‐blind study of six combined oral contraceptives. Contraception 1982;25(3):231‐41. [DOI] [PubMed] [Google Scholar]
Winkler 1996 {published data only}
- Winkler UH, Schindler AE, Endrikat J, Dusterberg B. A comparative study of the effects of the hemostatic system of two monophasic gestodene oral contraceptives containing 20 µg and 30 µg ethinylestradiol. Contraception 1996;53(2):75‐84. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bassol 2000 {published data only}
- Bassol S, Alvarado A, Celis C, Cravioto MC, Peralta O, Montano R, et al. Latin American experience with two low‐dose oral contraceptives containing 30 µg ethinylestradiol/75 µg gestodene and 20 µg ethinylestradiol/150 µg desogestrel. Contraception 2000;62(3):131‐5. [DOI] [PubMed] [Google Scholar]
Lawson 1979 {published data only}
- Lawson JS, Yuliano SE, Pasquale SA, Osterman JJ. Optimum dosage of an oral contraceptive. American Journal of Obstetrics and Gynecology 1979;134(3):315‐20. [DOI] [PubMed] [Google Scholar]
Marr 2012 {published data only}
- Bachmann G, Sulak PJ, Sampson‐Landers C, Benda N, Marr J. Efficacy and safety of a low‐dose 24‐day combined oral contraceptive containing 20 micrograms ethinylestradiol and 3 mg drospirenone. Contraception. 2004/08/25 2004; Vol. 70, issue 3:191‐8. [DOI] [PubMed]
- Marr J, Gerlinger C, Kunz M. A historical cycle control comparison of two drospirenone‐containing combined oral contraceptives: ethinylestradiol 30 µg/drospirenone 3 mg administered in a 21/7 regimen versus ethinylestradiol 20 µg/drospirenone 3 mg administered in a 24/4 regimen. European Journal of Obstetrics & Gynecology and Reproductive Biology 2012;162(1):91‐5. [DOI] [PubMed] [Google Scholar]
- Parsey KS, Pong A. An open‐label, multicenter study to evaluate Yasmin, a low‐dose combination oral contraceptive containing drospirenone, a new progestogen. Contraception. 2000/05/10 2000; Vol. 61, issue 2:105‐11. [DOI] [PubMed]
Rosenberg 1996 {published data only}
- Benagiano G. Comparison of two monophasic oral contraceptives: gestodene/ethinyl estradiol versus desogestrel/ethinyl estradiol. International Journal of Fertility 1989;34(Suppl):31‐9. [PubMed] [Google Scholar]
- Rosenberg MJ, Waugh MS, Higgins JE. The effect of desogestrel, gestodene, and other factors on spotting and bleeding. Contraception 1996;53(2):85‐90. [DOI] [PubMed] [Google Scholar]
Westhoff 2005 {published data only}
- Westhoff C, Osborne LM, Schafer JE, Morroni C. Bleeding patterns after immediate initiation of an oral compared with a vaginal hormonal contraceptive. Obstetrics and Gynecology 2005;106(1):89‐96. [DOI] [PubMed] [Google Scholar]
Wiegratz 2003 {published data only}
- Wiegratz I, Kutschera E, Lee JH, Moore C, Mellinger U, Winkler UH, et al. Effect of four different oral contraceptives on various sex hormones and serum‐binding globulins. Contraception 2003;67(1):25‐32. [DOI] [PubMed] [Google Scholar]
Additional references
Anonymous 2000
- Anonymous 2000. Oral contraceptives and cardiovascular risk. Drug and Therapeutics Bulletin 2000;38(1):1‐5. [DOI] [PubMed] [Google Scholar]
Belsey 1986
- Belsey EM, Machin D, d'Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. Contraception 1986;34(3):253‐60. [DOI] [PubMed] [Google Scholar]
CGHFBC 1996
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet 1996;347(9017):1713‐27. [DOI] [PubMed] [Google Scholar]
CONSORT 2009
- CONSORT group. CONSORT: Transparent reporting of trials. http://www.consort‐statement.org/ (accessed 15 Jul 2009).
Elstein 1994
- Elstein M. Low dose contraceptive formulations: is further reduction in steroid dosage justified?. Advances in Contraception 1994;10(1):1‐4. [DOI] [PubMed] [Google Scholar]
Gerstman 1991
- Gerstman BB, Gross TP, Kennedy DL, Bennett RC, Tomita DK, Stadel BV. Trends in the content and use of oral contraceptives in the United States, 1964‐88. American Journal of Public Health 1991;81(1):90‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guillebaud 1989
- Guillebaud J. Practical prescribing of the combined oral contraceptive pill. In: Filshie M, Guillebaud J Filshie, John Guillebaud editor(s). Contraception: science and practice. London: Butterworth & Co., 1989:69‐93. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. John Wiley & Sons, Ltd, (accessed 08 Jul 2013).
Holt 1992
- Holt VL, Daling JR, McKnight B, Moore D, Stergachis A, Weiss NS. Functional ovarian cysts in relation to the use of monophasic and triphasic oral contraceptives. Obstetrics and Gynecology 1992;79(4):529‐33. [PubMed] [Google Scholar]
IPPF 2013
- International Planned Parenthood Federation. Directory of Hormonal Contraceptives. http://contraceptive.ippf.org/search (accessed 10 Jul 2013).
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. British Medical Journal 2003;326(7400):1167‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maitra 2004
- Maitra N, Kulier R, Bloemenkamp KWM, Helmerhorst FM, Gülmezoglu AM. Progestogens in combined oral contraceptives for contraception. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004861] [DOI] [PubMed] [Google Scholar]
Miller 2001
- Miller L, Notter KM. Menstrual reduction with extended use of combination oral contraceptive pills: randomized controlled trial. Obstetrics and Gynecology 2001;98(5 Pt 1):771‐8. [DOI] [PubMed] [Google Scholar]
Mishell 2007a
- Mishell DR, Guillebaud J, Westhoff C, Nelson AL, Kaunitz AM, Trussell J, et al. Combined hormonal contraceptive trials: variable data collection and bleeding assessment methodologies influence study outcomes and physician perception. Contraception 2007;75(1):4‐10. [DOI] [PubMed] [Google Scholar]
Mishell 2007b
- Mishell DR, Guillebaud J, Westhoff C, Nelson AL, Kaunitz AM, Trussell J, et al. Recommendations for standardization of data collection and analysis of bleeding in combined hormone contraceptive trials. Contraception 2007;75(1):11‐5. [DOI] [PubMed] [Google Scholar]
Nelson 2007
- Nelson AL. Combined oral contraceptives. In: Hatcher RA, Trussell J, Nelson AL, Cates W Jr, Stewart F, Kowal D editor(s). Contraceptive Technology. 19th Edition. New York: Ardent Media, Inc., 2007:193‐270. [Google Scholar]
Ness 2000
- Ness RB, Grisso JA, Klapper J, Schlesselman JJ, Silberzweig S, Vergona R, et al. Risk of ovarian cancer in relation to estrogen and progestin dose and use characteristics of oral contraceptives. American Journal of Epidemiology 2000;152(3):233‐41. [DOI] [PubMed] [Google Scholar]
Norris 1996
- Norris LA, Bonnar J. The effect of oestrogen dose and progestogen type on haemostatic changes in women taking low dose oral contraceptives. British Journal of Obstetrics and Gynaecology 1996;103(3):261‐7. [DOI] [PubMed] [Google Scholar]
Rosenberg 1992
- Rosenberg MJ, Long SC. Oral contraceptives and cycle control: a critical review of the literature. Advances in Contraception 1992;8(Suppl 1):35‐45. [DOI] [PubMed] [Google Scholar]
Rosenberg 1998
- Rosenberg MJ, Waugh MS. Oral contraceptive discontinuation: a prospective evaluation of frequency and reasons. American Journal of Obstetrics and Gynecology 1998;179(3 Pt 1):577‐82. [DOI] [PubMed] [Google Scholar]
Rothman 1998
- Rothman KJ, Greenland S. Approaches to statistical analysis. In: Rothman KJ, Greenland S editor(s). Modern epidemiology. 2nd Edition. New York: Lippincott Williams and Wilkins, 1998:183‐99. [Google Scholar]
Schulz 2002
- Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. Lancet 2002;359(9306):614‐700. [DOI] [PubMed] [Google Scholar]
Spona 1996
- Spona J, Elstein M, Feichtinger W, Sullivan H, Ludicke F, Muller U, et al. Shorter pill‐free interval in combined oral contraceptives decreases follicular development. Contraception 1996;54(2):71‐7. [DOI] [PubMed] [Google Scholar]
Strauss 2005
- Strauss SE, Richardson WS, Glasziou P, Haynes RB. Evidence‐based Medicine: How to Practice and Teach EBM. Third Edition. New York: Churchill Livingstone, 2005. [Google Scholar]
Thiboutot 2001
- Thiboutot D, Archer DF, Lemay A, Washenik K, Roberts J, Harrison DD. A randomized, controlled trial of a low‐dose contraceptive containing 20 µg of ethinyl estradiol and 100 µg of levonorgestrel for acne treatment. Fertility and Sterility 2001;76(3):461‐8. [DOI] [PubMed] [Google Scholar]
Trussell 1998
- Trussell J. Dynamics of reproductive behavior and population change. In: Hatcher RA, Trussell J, Stewart F, Cates W Jr, Stewart GK, Guest F, et al. editor(s). Contraceptive technology. 17th Edition. New York: Ardent Media Inc., 1998:745‐77. [Google Scholar]
Wallach 2000
- Wallach M, Grimes DA. Modern oral contraception. 1st Edition. Totowa, NJ: Emron, 2000. [Google Scholar]
References to other published versions of this review
Gallo 2005
- Gallo MF, Nanda K, Grimes DA, Schulz KF. 20 mcg versus > 20 mcg Estrogen combined oral contraceptives for contraception. Contraception 2005;71(3):162‐9. [DOI] [PubMed] [Google Scholar]


