Abstract
Background
Caesarean section increases the risk of postpartum infection for women and prophylactic antibiotics have been shown to reduce the incidence; however, there are adverse effects. It is important to identify the most effective class of antibiotics to use and those with the least adverse effects.
Objectives
To determine, from the best available evidence, the balance of benefits and harms between different classes of antibiotic given prophylactically to women undergoing caesarean section.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 September 2014) and reference lists of retrieved papers.
Selection criteria
We included randomised controlled trials comparing different classes of prophylactic antibiotics given to women undergoing caesarean section. We excluded trials that compared drugs with placebo or drugs within a specific class; these are assessed in other Cochrane reviews.
Data collection and analysis
Two review authors independently assessed the studies for inclusion, assessed risk of bias and carried out data extraction.
Main results
We included 35 studies of which 31 provided data on 7697 women. For the main comparison between cephalosporins versus penicillins, there were 30 studies of which 27 provided data on 7299 women. There was a lack of good quality data and important outcomes often included only small numbers of women.
For the comparison of a single cephalosporin versus a single penicillin (Comparison 1 subgroup 1), we found no significant difference between these classes of antibiotics for our chosen most important seven outcomes namely: maternal sepsis ‐ there were no women with sepsis in the two studies involving 346 women; maternal endometritis (risk ratio (RR) 1.11, 95% confidence interval (CI) 0.81 to 1.52, nine studies, 3130 women, random effects, moderate quality of the evidence); maternal wound infection (RR 0.83, 95% CI 0.38 to 1.81, nine studies, 1497 women, random effects, low quality of the evidence), maternal urinary tract infection (RR 1.48, 95% CI 0.89 to 2.48, seven studies, 1120 women, low quality of the evidence) and maternal composite adverse effects (RR 2.02, 95% CI 0.18 to 21.96, three studies, 1902 women, very low quality of the evidence). None of the included studies looked for infant sepsis nor infant oral thrush.
This meant we could only conclude that the current evidence shows no overall difference between the different classes of antibiotics in terms of reducing maternal infections after caesarean sections. However, none of the studies reported on infections diagnosed after the initial postoperative hospital stay. We were unable to assess what impact, if any, the use of different classes of antibiotics might have on bacterial resistance.
Authors' conclusions
Based on the best currently available evidence, cephalosporins and penicillins have similar efficacy at caesarean section when considering immediate postoperative infections. We have no data for outcomes on the baby, nor on late infections (up to 30 days) in the mother. Clinicians need to consider bacterial resistance and women's individual circumstances.
Plain language summary
Comparing different types of antibiotics given routinely to women at caesarean section to prevent infections
Background
Women undergoing caesarean section have an increased likelihood of infection compared with women who give birth vaginally. These infections can be in the urine, surgical incision, or the lining of the womb (endometritis). The infections can become serious, causing, for example, an abscess in the pelvis or infection in the blood, and very occasionally can lead to the mother's death. Sound surgical techniques are important for reducing infections, along with skin antiseptics and antibiotics. However, antibiotics can cause adverse effects such as nausea, vomiting, skin rash and rarely allergic reactions in the mother, and the risk of thrush (candida) for the mother and the baby. Antibiotics, given to women around the time of giving birth, can also change the baby's gut flora and thus may interfere with the baby's developing immune system.
Our review question
We asked if cephalosporin antibiotics were better than penicillins for women having a caesarean section. We also looked to see how other groups of antibiotics compared.
What we found
When comparing cephalosporins against penicillins, we found 27 studies, involving 7299 women as of September 2014. The quality of the studies was generally unclear and three studies reported drug company funding. Cephalosporins and penicillins had similar effects in reducing infections after caesareans and similar adverse effects. However, none of the studies considered infections after the women left hospital. None of the studies looked at outcomes on the babies. Other evidence show tetracyclines can cause discolouration of teeth in children and are best avoided. Consideration also needs to be given to antibiotics compatible with breastfeeding. We were unable to assess bacterial resistance, and this is crucial when considering which antibiotic might be used.
What our results mean
At caesarean sections, cephalosporins and penicillins have similar benefits and side effects for mothers when considering infections immediately following the operation but there is no information on babies. Clinicians need to consider bacterial resistance and women's individual circumstances.
Summary of findings
Summary of findings for the main comparison. Cephalosporins versus penicillins ‐ all women for preventing infection at caesarean section.
| Cephalosporins versus penicillins ‐ all women for preventing infection at caesarean section | ||||||
| Population: Women undergoing caesarean section. Settings: Hospitals in Sudan, US, Thailand, Italy, Zimbabwe, Mozanbique, Switzerland, South Africa, Canada, Rwanda, Malaysia, Finland, United Arab Emirates, Netherlands, Argentina, UK, Greece. Intervention: Cephalosporins versus penicillins ‐ all women. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Cephalosporins versus penicillins ‐ all women | |||||
| Maternal sepsis ‐ Single cephalosporin vs single penicillin | See comment | See comment | Not estimable | 346 (2 studies) | See comment | The outcome was reported with no events. |
| Maternal endometritis ‐ Single cephalosporin vs single penicillin | Study population | RR 1.11 (0.81 to 1.52) | 3130 (9 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 109 per 1000 | 121 per 1000 (88 to 165) | |||||
| Moderate | ||||||
| 86 per 1000 | 95 per 1000 (70 to 131) | |||||
| Infant sepsis ‐ Single cephalosporin vs single penicillin | See comment | See comment | Not estimable | 0 (0) | See comment | This outcome was not reported in any of the included studies. |
| Infant oral thrush ‐ Single cephalosporin vs single penicillin | See comment | See comment | Not estimable | 0 (0) | See comment | This outcome was not reported in any of the included studies. |
| Maternal wound infection ‐ Single cephalosporin vs single penicillin | Study population | RR 0.83 (0.38 to 1.81) | 1497 (9 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 33 per 1000 | 27 per 1000 (12 to 59) | |||||
| Moderate | ||||||
| 34 per 1000 | 28 per 1000 (13 to 62) | |||||
| Maternal urinary tract infection ‐ Single cephalosporin vs single penicillin | Study population | RR 1.48 (0.89 to 2.48) | 1120 (7 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 49 per 1000 | 72 per 1000 (43 to 121) | |||||
| Moderate | ||||||
| 37 per 1000 | 55 per 1000 (33 to 92) | |||||
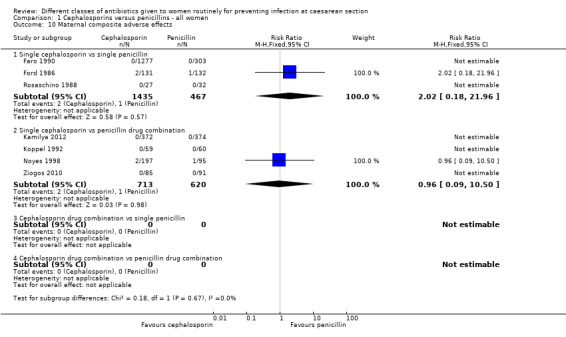
| Maternal composite adverse effects ‐ Single cephalosporin vs single penicillin | Study population | RR 2.02 (0.18 to 21.96) | 1902 (3 studies) | ⊕⊝⊝⊝ very low3,4 | ||
| 2 per 1000 | 4 per 1000 (0 to 47) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Most studies contributing data had design limitations. 2 Wide confidence interval crossing the line of no effect & small sample size. 3 One study with design limitations. 4 Wide confidence interval crossing the line of no effect & few events.
Background
Women undergoing caesarean section have an increased risk of postoperative infection and infectious morbidity compared with women giving birth vaginally (Declercq 2007). Since caesarean section rates are in excess of 20% in many high‐income countries, these infections are a major concern.
Description of the condition
Caesarean sections have been shown to have nearly five times the risk of postpartum infection as vaginal births (and this is with a policy of antibiotics at caesarean section) and just over 75% occur after hospital discharge (Leth 2009). The infectious complications that can occur after caesarean birth include infections of the wound/incision, endometritis (infection of the lining of the uterus) and urinary tract infection, although fever can occur after any operation and is not necessarily an indicator of infection (MacLean 1990). However, there can occasionally be more serious infectious complications such as pelvic abscess (collection of pus in the pelvis), bacteraemia (bacterial infection in the blood), septic shock (reduced blood volume due to infection), necrotising fasciitis (tissue destruction in the abdominal wall) and septic pelvic vein thrombophlebitis (inflammation and infection of the veins in the pelvis). These more serious infectious complications can lead to maternal mortality.
Description of the intervention
The potential for prophylactic antibiotics to reduce the incidence of maternal infectious morbidity following caesarean section has now been systematically investigated (Smaill 2002; Smaill 2008). Although evidence has existed for some time to support this practice (Pedersen 1996), it is not clear whether any one particular agent, dose or route of administration is superior. Many different drug regimens have been reported to be effective in decreasing immediate postoperative infectious morbidity. To date, various penicillins (ampicillin, ticarcillin, mezlocillin, piperacillin), cephalosporins (cefazolin, cephalothin, ceforanide, cefonicid, cefuroxime, ceftazidime, cefoxitin, cefamandole, cephradine, cefotetan, cefotaxime), fluoroquinolones etc. have been used for caesarean section prophylaxis and overall they have demonstrated some efficacy either alone or in combination with another drug (Smaill 2008). Some of these drugs have activity against a narrow range of potential pathogens (e.g. metronidazole, gentamicin), others have additional specific anaerobic activity (e.g. cefoxitin and cefotetan), and yet others have very broad‐spectrum coverage (imipenem). Their pharmacokinetic properties (e.g. serum half‐life) also differ. Some drugs used in the past are now associated with bacterial resistance.
In addition to the choice of drug there are differences in the route of administration and the timing of administration of prophylactic antibiotics. As well as systemic administration (intravenous and intramuscular), use of intra‐operative irrigation of the uterus and peritoneal cavity with an antibiotic solution has been reported. While some guidelines recommend multiple doses of antibiotics, a single dose at the time of the procedure may be adequate. These considerations will be covered in other Cochrane reviews ‐ see Differences between protocol and review for details.
How the intervention might work
Since penicillin was introduced during the 1940s, scientists have developed numerous other antibiotics. Today, over 100 different antibiotics are available. For the prevention of surgical infections it is generally considered that sound surgical technique is important along with skin antiseptics and the use of antibiotics (Owen 1994). Antibiotics act by either killing bacteria (bacteriocidal) or inhibiting bacterial replication (bacteriostatic) but the large variety of different types of bacteria mean a large variety of possible antibiotics may be used.
Classification of antibiotics
Antibiotics can be classified in a number of ways, but classifying by chemical structure is useful because antibiotics within a structural class will generally have similar patterns of effectiveness, toxicity and allergic potential (Bayarski 2006; eMedExpert 2009; Goodman 2008). The most commonly used types of antibiotics are penicillins, cephalosporins, fluoroquinolones, tetracyclines and macrolides, with each class including many drugs (Table 17). Penicillins have a common structure which they share with cephalosporins. Both classes of antibiotics are bactericidal, acting through inhibiting cell wall synthesis. Penicillins are grouped into four types and cephalosporins are grouped into four generations with each newer generation having a broader spectrum of activity (eMedExpert 2009). Fluoroquinolones are the newest class of antibiotics and are synthetic rather than derived from bacteria. These newer fluoroquinolones are broad‐spectrum bacteriocidal drugs chemically unrelated to penicillins or cephalosporins. They interfere with the ability of bacteria to make DNA. Tetracyclines are derived from streptomyces bacteria and are broad‐spectrum bacteriostatic antibiotics. Macrolides are also derived from streptomyces bacteria and are also bacteriostatic in action, binding to bacterial ribosomes. Aminoglycosides are used to treat gram‐negative bacteria and may be used alongside penicillins and cephalosporins (eMedExpert 2009).
1. Classification of antibiotics.
| A. Penicillins | Penicillins consist of a thiazolidine ring connected to a B‐lactam ring to which is attached a side chain. The penicillin nucleus itself is the chief structural requirement for biological activity. Penicillins are the oldest class of antibiotics and function by inhibiting cell wall synthesis (bactericidal). A1. Natural penicillins are based on the original penicillin‐G structure. Examples include: penicillin G; procaine, penicillin V; benzathine. A2. Penicillinase‐resistant penicillins are active even in the presence of the bacterial enzyme that inactivates most natural penicillins. Examples include: cloxacillin; dicloxacillin; methicillin; nafcillin; oxacillin. A3. Extended spectrum penicillins which are effective against a wider range of bacteria. Examples include: ticarcillin; piperacillin; carbenicillin; timentin. A4. Aminopenicillins also have an extended spectrum of action compared with the natural penicillins. Examples include: ampicillin; amoxicillin. A+. Penicillin combinations. Examples include: co‐amoxyclav = 'ampicillin+ clavulanic acid' (Trade names include: Augmentin; Clavamox; Tyclav); 'ampicillin + sulbactam' (Trade names include: Ampictam; Unasyn). |
| B. Cephalosporins | Cephalosporins have a similar basic structure to penicillins but with different side chains. They function by inhibiting cell wall synthesis. B1. First generation cephalosporins; examples include: cephalothin; cefazolin; cephapirin; cephradine; cephalexin; cefadroxil. B2. Second generation cephalosporins; examples include: cefoxitin; cefaclor; cefuroxime; cefotetan; cefprozil; cefamandole, cefonicid; ceforanide, cefotiam. B3. Third generation cephalosporins, examples include: cefotaxime; ceftizoxime; ceftriaxon; cefpodoxime; cefditoren; ceftibuten; ceftazidine; cefcapene; cefdaloxime; cefetamet; cefixime; cefmenoxime; cefodizime; cefoperazone; cefpimizole. B4. Fourth generation cephalosporins, examples include: cefepime; cefpirome; cefclidine; cefluprenam; cefozopran; cefquinome. B+: Cephalosporin combinations. Examples include: 'cephradine + metronidazole'; 'ceftriaxone + metronidazole'; 'cloxacillin + gentamicin'. |
| C. Fluoroquinolones | The fluoroquinolones target the bacterial DNA gyrase and topoisomerase. They are potent bacteriocidal agents against a broad variety of micro‐organisms. Examples include: ciprofloxacin; levofloxacin; lomefloxacin; norfloxacin; sparfloxacin; clinafloxacin; gatifloxacin; ofloxacin; trovafloxacin. |
| D. Tetracyclines | Tetracyclines are bacteriostatic antibiotics active against a wide range of aerobes and anaerobic gram‐positive and gram‐negative bacteria. They inhibit bacterial protein synthesis by binding to the 30S bacterial ribosome. Examples include: tetracycline; doxycycline; minocycline. Tetracyclines should not be used with children under 8 and specifically during teeth development as they can cause a permanent brown discolouration to the teeth. This antibiotic is, therefore, unlikely to be used at caesarean section. Chloramphenicol is considered to have similar action to tetracycline. |
| E. Macrolides | Macrolide antibiotics inhibit bacterial protein synthesis. Resistance can arise. Examples include: erythromycin; clarithromycin; azithromycin. |
| F. Other beta‐lactams (carbapenems) | Carbapenems are beta‐lactams that have a broader spectrum of activity than most other beta‐lactam antibiotics. Examples include: imipenem; meropenem; ertapenem; aztreonam, mezlocillin. |
| G. Aminoglycosides | Aminoglycosides are first‐line therapy for a limited number of very specific, often historically prominent infections, such as plague, turaremia and tuberculosis. Examples include: streptomycin; gentamicin, kanamycin. |
| H. Lincosamides | Lincosamides are protein synthesis inhibitors which bind to the 50s subunit of bacterial ribosomes and inhibit early elongation of peptide chain by inhibiting transpeptidase reaction. Examples include: lincomycin; clindamycin. |
| I. Nitroimidazoles | Nitroimidazole is an imidazole derivative that contains a nitro group. It is used for the treatment of infection with anaerobic organisms. Examples include: metronidazole, tinidazol. |
| J. Others |
SeeGoodman 2008 for more detailed information about the classification and BNF 2009. Also from Wikipedia (http://www.wikipedia.org/)
Potential adverse effects of antibiotics
On the mother
The benefits of antibiotics are well‐known, but there are potential adverse effects which also need to be considered. Antibiotic use is associated with some gastrointestinal symptoms (nausea, vomiting or diarrhoea), skin rashes, thrush/candidiasis (infection with candida which can affect both mother and baby), and joint pain (Dancer 2004). Occasionally there can also be blood problems, or kidney or liver damage (Dancer 2004; Westphal 1994), and very occasionally anaphylaxis (a hypersensitivity reaction leading to pallor, shock and collapse, which is sometimes fatal). Possible interactions with other drugs the mother may be taking also need to be considered.
On the infant
Some antibiotics can reach the baby during labour or through breastfeeding, and these may upset the pattern of friendly bacterial flora being established in the baby's gut as part of the baby's immune system (Bedford Russell 2006; Penders 2006). There is evidence that this impact can continue for up to six months after birth (Grolund 1999) and the consequences of this may occasionally be late‐onset serious bacterial infections (Glasgow 2005). It has been proposed that perinatal exposure to certain agents can cause irreversible changes to health conditions in adulthood through impact on hormonal imprinting (Csaba 2007; Tchernitchin 1999). It is also possible that babies born prematurely, with less mature immune systems, may be affected more.
Drug‐resistant strains of antibiotics
Resistance of bacteria to antibiotics is spreading and develops when a strain of bacteria evolves which is not destroyed by the antibiotic. The antibiotic kills off the non‐resistant bacteria allowing the resistant ones to colonise and spread. Widespread use of antibiotics can contribute to the development of drug‐resistant strains of bacteria, which means that these antibiotics become ineffective because of bacterial resistance (Dancer 2004). At a population level this is a critical problem which may cause increase in serious morbidity from hospital‐acquired drug‐resistant infections (Dancer 2004). The use of antibiotics in other areas of maternity care, e.g. anti‐Group B streptococcus prophylaxis, contribute further to this problem. This drug resistance is unlikely to be detected in randomised controlled trials and other types of research are needed to assess the potential problem of drug‐resistant strains (e.g. MRSA (Methicillin‐resistant Staphylococcus aureus), C difficile) in hospitals. The dose and number of antibiotic administrations given are a major consideration in relation to antibiotic resistance. These issues will be addressed in the other reviews ‐ seeDifferences between protocol and review for details.
Why it is important to do this review
Since there are an overwhelming number of effective drugs available, attempts to define an antibiotic regimen of choice have been problematic. Ideally, such a drug regimen should be: (1) proven to be effective in well‐designed prospective, randomised, double‐blind clinical trials, (2) active against the majority of pathogens likely to be involved, (3) able to attain adequate serum and tissue levels throughout the procedure, (4) not associated with the development of antimicrobial resistance, (5) inexpensive, and (6) well‐tolerated. In many respects penicillins and cephalosporins meet these criteria. Many investigators have used these drugs and have recommended that drugs from these classes represent the antibiotics of choice for caesarean section prophylaxis (Cartwright 1984). However, current knowledge of bacterial resistance may challenge these recommendations.
The past several decades have seen an increase in the incidence of caesarean section, associated with an increase in maternal postoperative infection. Studies indicate that wound infection can be as high as 30% and endometritis as high as 60% where prophylactic antibiotics have not been utilised (Smaill 2002). Therefore, infectious complications that occur following caesarean section are an important contributor to maternal morbidity and mortality (Henderson 1995). Such complications are also an important source of increased hospital stay and consumption of financial resources. Prophylactic antibiotics for caesarean section can be expected to result in a major reduction in postoperative infectious morbidity. The question that remains, therefore, is which regimen to use.
Objectives
To determine, from the best available evidence, the balance of benefits and harms between different class of antibiotic given prophylactically to women undergoing caesarean section, considering the effectiveness in reducing infectious complications for women and adverse effects on both mother and infant.
Other Cochrane reviews will address: effectiveness against placebo (Smaill 2008), dosage by the various classes of antibiotics, different routes of administration and various timings of administration.
We will consider factors that may affect antibiotic resistance in a future update of this review.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) where the intention was to allocate participants randomly to one of at least two alternative classes of regimens of antibiotic prophylaxis for caesarean section. We excluded quasi‐RCTs. Cluster‐RCTs were eligible for inclusion but none were identified. Cross‐over trials were not eligible for inclusion.
Types of participants
Women undergoing caesarean section, both elective and non‐elective.
Types of interventions
Prophylactic antibiotic regimens comparing different classes of antibiotics. We included studies where there was a comparison between two or more antibiotics from the different classes of antibiotics.
We excluded comparisons of different drugs within the same class of antibiotics as these will be assessed in other Cochrane reviews.
Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section
Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section
Assessment of the appropriate timing and route of administration of prophylactic antibiotics will also be considered in further reviews.
Timing of prophylactic antibiotics for preventing infectious morbidity in women undergoing caesarean section
Routes of administration for antibiotic given to women routinely for preventing infection after caesarean section
Types of outcome measures
Primary outcomes
Maternal
Maternal sepsis (suspected or proven)
Endometritis
Infant
Infant sepsis (suspected or proven)
Oral thrush
Secondary outcomes
Maternal
Fever (febrile morbidity)
Wound infection
Urinary tract infection
Thrush
Serious infectious complication (such as bacteraemia, septic shock, septic thrombophlebitis, necrotising fasciitis, or death attributed to infection)
Adverse effects of treatment on the woman (e.g. allergic reactions, nausea, vomiting, diarrhoea, skin rashes)
Maternal length of hospital stay
Infections ‐ post‐hospital discharge to 30 days postoperatively (not pre‐specified in the protocol)
Readmissions (not pre‐specified in the protocol)
Infant
Immediate adverse effects of antibiotics on the infant (unsettled, diarrhoea, rashes)
Infant length of hospital stay
Long‐term adverse effects (e.g. general health, frequency of visits to hospital)
Infant's immune system development (using a validated scoring assessment)
Additional outcomes
Development of bacterial resistance
Costs
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (30 September 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists at the end of papers for further studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, please see Alfirevic 2010.
For this update, the following methods were used. These methods are based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed the methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
For this update the quality of the evidence assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following key outcomes for the main comparison first subgroup, single cephalosporins versus single penicillins:
Maternal sepsis
Maternal endometritis
Infant sepsis
Infant oral thrush
Wound infection
Maternal urinary tract infection
Maternal composite adverse effects (e.g. allergic reactions; nausea, vomiting, diarrhoea, skin rashes)
GRADE profiler (GRADE 2008) was used to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
Had we identified any cluster‐RCTs we would have included them in the analyses along with individually‐randomised trials, following the methods described in Higgins 2009 and the Handbook [Section 16.3.4 or 16.3.6] using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. In future updates, if we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity subgroup analysis to investigate the effects of the randomisation unit.
Other unit of analysis issues
No special methods were used for trials with more than one treatment group.
Dealing with missing data
For included studies, levels of attrition were noted. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if the Tau² was greater than zero or the I² was greater than 30% and there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Had we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
Where there were 10 or more studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we explored possible reasons for this.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
Where there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. If we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We analysed separately, for all outcomes, penicillins and cephalosporins given alone as opposed to when they were given in combination with other drugs, for the main comparison of cephalosporins versus penicillins (Comparison 1).
We carried out the following subgroup analyses for the main comparison between penicillins and cephalosporins only and for primary outcomes only.
By type of surgery: elective caesarean section versus non‐elective caesarean section versus mixed or not defined. (Rupture of membranes for more than six hours or the presence of labour was used to differentiate a non‐elective caesarean section from an elective procedure.)
By time of administration: before cord clamping versus after cord clamping versus not defined.
By route of administration: systemic versus lavage.
We intended to undertake a subgroup analysis by the number of doses given but feel this is better assessed in other reviews (Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section and Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section). There will also be a review or reviews on the timing and routes of administration of the antibiotics where studies exist which compare directly, for example, before and after cord clamping (Timing of prophylactic antibiotics for preventing infectious morbidity in women undergoing caesarean section and Routes of administration for antibiotic given to women routinely for preventing infection after caesarean section).
We assessed subgroup differences by interaction tests (Deeks 2001) available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analysis to explore the effect of trial quality for important outcomes in the review. Where there was a high risk of bias associated with a particular aspect of study quality, for example, inadequate sequence generation and allocation concealment (Schultz 1995), we planned to explore this by sensitivity analysis (Higgins 2009) but we felt there were insufficient high‐quality trials (only three identified Figure 1) for a meaningful analysis.
1.
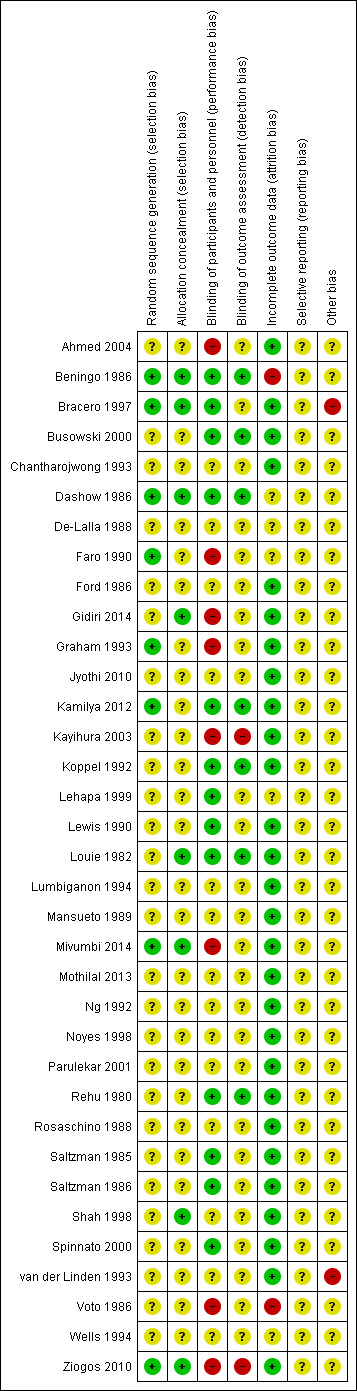
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Results
Description of studies
Results of the search
We identified 136 reports for 133 studies. For a detailed description of studies seeCharacteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification.
The 35 trials included in the review were conducted mostly in industrialised countries, for example, United States, Canada, Israel, Italy, Switzerland or The Netherlands (see Characteristics of included studies).. Criteria listed to define the presence of outcome variables of interest (e.g. endometritis) were remarkably consistent across trials.
Two studies await data extraction, either because they are being translated or we are awaiting information from the authors (see Studies awaiting classification).
Included studies
We included 35 studies, of which 31 provided data involving 7697 women and we undertook 37 meta‐analyses. Four studies were reported as conference abstracts only (De‐Lalla 1988; Lehapa 1999; Lumbiganon 1994; Wells 1994). Four studies did not provide data: two full papers (Graham 1993; Voto 1986) and two of the conference abstracts (De‐Lalla 1988; Wells 1994). Antibiotics for prophylaxis were administered after the cord was clamped in all but five of the studies, where four administered the prophylaxis before cord clamping (Ahmed 2004; Mivumbi 2014; Parulekar 2001; Rosaschino 1988) and one study did not provide the information (Gidiri 2014).
Cephalosporins versus penicillins
We included 30 studies, of which 27 provided data on 7299 women, where cephalosporins were compared with penicillins for prophylaxis at caesarean section (Ahmed 2004; Beningo 1986; Bracero 1997; Busowski 2000; Chantharojwong 1993; Dashow 1986; Faro 1990; Ford 1986; Gidiri 2014; Jyothi 2010; Kamilya 2012; Koppel 1992; Lehapa 1999; Lewis 1990; Louie 1982; Lumbiganon 1994; Mivumbi 2014; Ng 1992; Noyes 1998; Parulekar 2001; Rosaschino 1988; Saltzman 1985; Saltzman 1986; Shah 1998; Spinnato 2000; van der Linden 1993; Ziogos 2010). Four studies were included though they provide no usable data on the outcomes listed in the review (De‐Lalla 1988; Graham 1993; Voto 1986).
We looked at comparisons of the subgroups of :
single cephalosporins versus single penicillins
single cephalosporins versus penicillin combinations (e.g. co‐amoxyclav)
cephalosporin combinations versus single penicillins
cephalosporin combinations versus penicillin combinations
Other antibiotic classes
We found three studies comparing a cephalosporin or penicillin with another class of antibiotics (Busowski 2000; Mothilal 2013; Wells 1994).
Mixed antibiotics regimens that did not include cephalosporins versus penicillins
We included a further five studies that assessed other combined antibiotic regimens against penicillins or cephalosporins for prophylaxis at caesarean section (Kayihura 2003; Mansueto 1989; Mothilal 2013; Rehu 1980; Shah 1998 ) and one study already included which also compared a combination of other antibiotics with a cephalosporin (Parulekar 2001).
Excluded studies
We excluded 96 studies that compared different antibiotics within the same class, either singly or in combination (seeCharacteristics of excluded studies).
Risk of bias in included studies
See Figure 1 for a summary of 'Risk of bias' assessments.
Allocation
Five studies were considered to have adequate sequence generation and allocation concealment (Beningo 1986; Bracero 1997; Dashow 1986; Mivumbi 2014; Ziogos 2010). Three further studies were assessed as low risk of bias for sequence generation but were unclear on allocation concealment (Faro 1990; Graham 1993; Kamilya 2012). The remaining studies were unclear about how adequately they had addressed these aspects to minimise bias.
Blinding
Thirteen studies were assessed as low risk of bias for performance bias (Beningo 1986; Bracero 1997; Busowski 2000; Dashow 1986; Kamilya 2012; Koppel 1992; Lehapa 1999; Lewis 1990; Louie 1982; Rehu 1980; Saltzman 1985; Saltzman 1986; Spinnato 2000), eight were assessed as high risk (Ahmed 2004; Faro 1990; Gidiri 2014; Graham 1993; Kayihura 2003; Mivumbi 2014; Voto 1986; Ziogos 2010) and the remainder were unclear.
For detection bias, we assessed seven studies as low risk (Beningo 1986; Busowski 2000; Dashow 1986; Kamilya 2012; Koppel 1992; Louie 1982; Rehu 1980), two as high risk (Kayihura 2003; Ziogos 2010) and the remainder as unclear.
Incomplete outcome data
Twenty‐eight studies were assessed as low risk of attrition bias (Ahmed 2004; Bracero 1997; Busowski 2000; Chantharojwong 1993; Ford 1986; Gidiri 2014; Graham 1993; Jyothi 2010; Kamilya 2012; Kayihura 2003; Koppel 1992; Lewis 1990; Louie 1982; Lumbiganon 1994; Mansueto 1989; Mivumbi 2014; Mothilal 2013; Ng 1992; Noyes 1998; Parulekar 2001; Rehu 1980; Rosaschino 1988; Saltzman 1985; Saltzman 1986; Shah 1998; Spinnato 2000; van der Linden 1993; Ziogos 2010).
Selective reporting
This was unclear on all the included studies as we were not able to assess the trial protocols.
Other potential sources of bias
This was assessed as unclear for all but two of the included studies; many of the studies were quite old and it was difficult to assess if there were other biases. The two studies assessed as having high risk of other bias were studies funded by drug companies (Bracero 1997; van der Linden 1993). In one study the antibiotic drugs were donated by the drug company but this was considered not to necessarily increase the likelihood of bias (Ahmed 2004). The other included studies gave no mention of drug company involvement.
Effects of interventions
See: Table 1
The search identified 35 studies of which 31 provided data in a format that could be included in this review (Ahmed 2004; Beningo 1986; Bracero 1997; Busowski 2000; Chantharojwong 1993; Dashow 1986; Faro 1990; Ford 1986; Gidiri 2014; Jyothi 2010; Kamilya 2012; Kayihura 2003; Koppel 1992; Lehapa 1999; Lewis 1990; Louie 1982; Lumbiganon 1994; Mansueto 1989; Mivumbi 2014; Mothilal 2013; Ng 1992; Noyes 1998; Parulekar 2001; Rehu 1980; Rosaschino 1988; Saltzman 1985; Saltzman 1986; Shah 1998; Spinnato 2000; van der Linden 1993; Ziogos 2010). These studies included 7697 women. A further four studies also addressed the comparisons in this review but did not provide data that could be included in the meta‐analyses (De‐Lalla 1988; Graham 1993; Voto 1986; Wells 1994).
The classification of antibiotics is set out in Additional tables.
1. Cephalosporins (B) versus penicillins (A) ‐ all women, 27 studies, 7299 women
Twenty‐seven studies provided data for inclusion in this comparison (Ahmed 2004; Beningo 1986; Bracero 1997; Busowski 2000; Chantharojwong 1993; Dashow 1986; Faro 1990; Ford 1986; Gidiri 2014; Jyothi 2010; Kamilya 2012; Koppel 1992; Lehapa 1999; Lewis 1990; Louie 1982; Lumbiganon 1994; Mivumbi 2014; Ng 1992; Noyes 1998; Parulekar 2001; Rosaschino 1988; Saltzman 1985; Saltzman 1986; Shah 1998; Spinnato 2000; van der Linden 1993; Ziogos 2010 ). A further four studies addressed this comparison but did not provide data in a format suitable for inclusion (De‐Lalla 1988; Graham 1993; Voto 1986; Wells 1994).
Overall, the quality of studies was generally unclear for the critical aspects of selection bias, probably reflecting that they were mostly older studies undertaken in the 1980s and 1990s. Only five studies met the criteria for low risk of bias in terms of sequence generation and allocation concealment (Beningo 1986; Bracero 1997; Dashow 1986; Mivumbi 2014; Ziogos 2010). Three studies had adequate sequence generation but allocation concealment was unclear (Faro 1990; Graham 1993; Kamilya 2012; ). The remainder were unclear on both sequence generation and allocation concealment (Figure 1).
For this comparison we have pooled any cephalosporin or any penicillin, at any dose or doses and by any route of administration. Different cephalosporins, different penicillins and different doses will be assessed in other reviews on Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section and Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section.
Subgroup 1: A single cephalosporin (B) versus a single penicillin (A)
Of the included studies with data, 13 assessed a single cephalosporin versus a single penicillin and involved 4010 women (Beningo 1986; Chantharojwong 1993; Dashow 1986; Faro 1990; Ford 1986; Lehapa 1999; Lewis 1990; Louie 1982; Mivumbi 2014; Ng 1992; Rosaschino 1988; Saltzman 1986; Spinnato 2000).
The quality of these studies was generally unclear on selection bias. Three studies were assessed at low risk of bias for both sequence generation and allocation concealment (Beningo 1986; Dashow 1986; Mivumbi 2014). One study had adequate sequence generation but allocation concealment was unclear (Faro 1990).
Primary outcomes
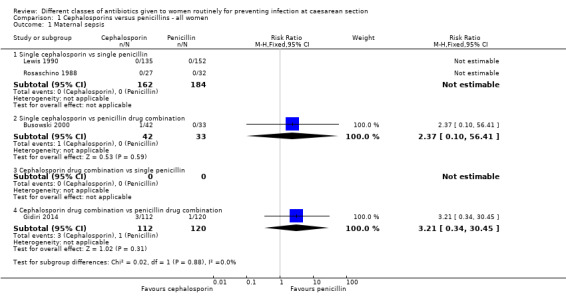
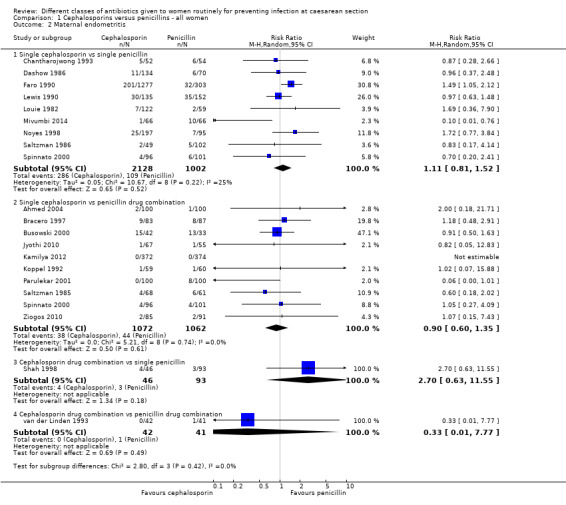
There was no maternal sepsis identified in the 346 women involved in two studies which looked at this outcome (Analysis 1.1). There was no significant difference identified in the incidence of endometritis between cephalosporins and penicillins, average risk ratio (RR) 1.11, and 95% confidence interval (CI) 0.81 to 1.52, nine studies, 3130 women, random effects (Tau² = 0.05; Chi² = 10.67, P = 0.22; I² = 25%, Analysis 1.2).
1.1. Analysis.
Comparison 1 Cephalosporins versus penicillins ‐ all women, Outcome 1 Maternal sepsis.
1.2. Analysis.
Comparison 1 Cephalosporins versus penicillins ‐ all women, Outcome 2 Maternal endometritis.
None of the included studies assessed either infant sepsis or infant oral thrush.
Secondary outcomes
There was no significant difference identified for the following:
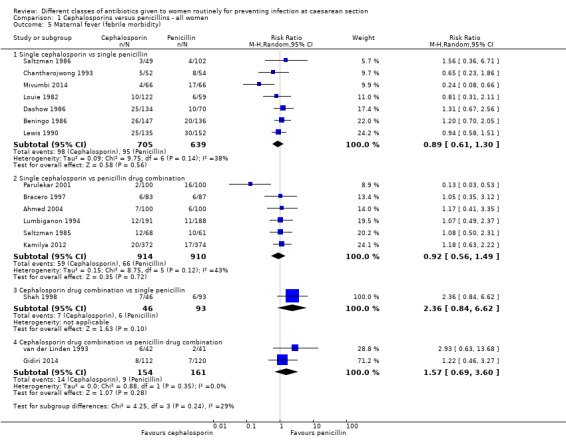
maternal fever (average RR 0.89, 95% CI 0.61 to 1.30, seven studies, 1344 women, random effects Tau² = 0.09; Chi² = 9.75, P = 0.14; I² = 38%, Analysis 1.5);
maternal wound infection (average RR 0.83 95% CI 0.38 to 1.81, nine studies, 1497 women, random effects (Tau² = 0.29; Chi² = 9.11, P = 0.24; I² = 23%, Analysis 1.6) ;
maternal urinary tract infection (average RR 1.48; 95% CI 0.89 to 2.48, seven studies, 1120 women, random effects (Tau² = 0.00; Chi² = 3.90, P = 0.56, I² = 0%, Analysis 1.7);
maternal composite adverse effects (RR 2.02, 95% CI 0.18 to 21.96, three studies, 1902 women, Analysis 1.10);
maternal skin rash (RR 1.45, 95% CI 0.06 to 35.38, two studies, 351 women, Analysis 1.15).
1.5. Analysis.
Comparison 1 Cephalosporins versus penicillins ‐ all women, Outcome 5 Maternal fever (febrile morbidity).
1.6. Analysis.
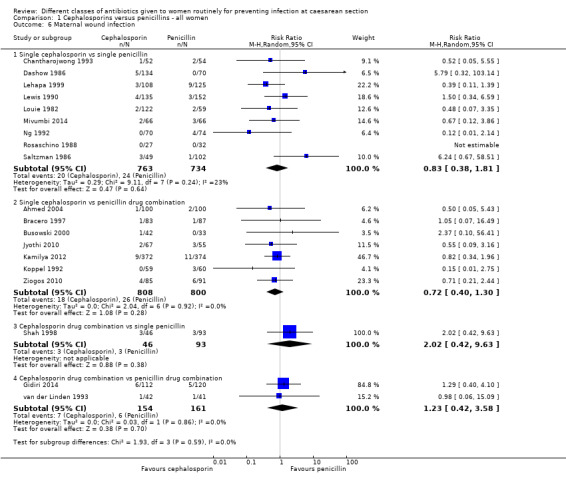
Comparison 1 Cephalosporins versus penicillins ‐ all women, Outcome 6 Maternal wound infection.
1.7. Analysis.
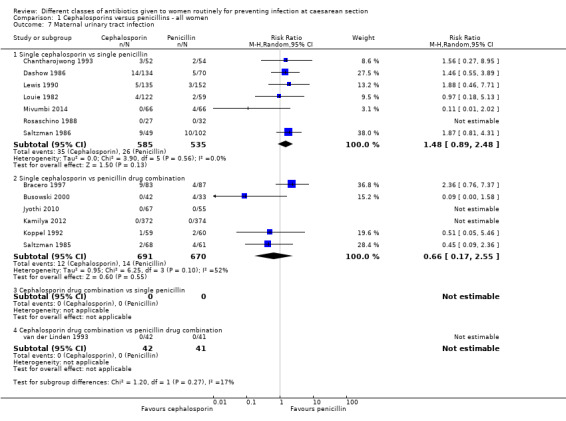
Comparison 1 Cephalosporins versus penicillins ‐ all women, Outcome 7 Maternal urinary tract infection.
1.10. Analysis.
Comparison 1 Cephalosporins versus penicillins ‐ all women, Outcome 10 Maternal composite adverse effects.
1.15. Analysis.
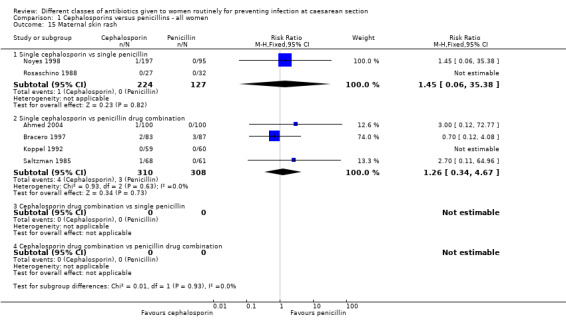
Comparison 1 Cephalosporins versus penicillins ‐ all women, Outcome 15 Maternal skin rash.
For the remaining outcomes, either there were no events or the studies did not asses the outcomes.
See Analysis 1.1 to Analysis 1.28.
Subgroup 2: A single cephalosporin (B) versus a penicillin combination (A+)
Twelve studies involving 2875 women compared a single cephalosporin with a penicillin combination. Six studies compared a cephalosporin alone with co‐amoxyclav (Ahmed 2004; Jyothi 2010; Kamilya 2012; Koppel 1992; Lumbiganon 1994; Saltzman 1985); five studies compared a cephalosporin alone with a penicillin plus sulbactam (Bracero 1997; Busowski 2000; Noyes 1998; Spinnato 2000; Ziogos 2010). One study compared a cephalosporin alone with a cocktail of drugs including a penicillin (Parulekar 2001).
The quality of these studies was generally unclear. The studies were all unclear for sequence generation and allocation concealment.
Primary outcomes
There was no significant difference identified between single cephalosporins and penicillin combinations in sepsis (RR 2.37, 95% CI 0.10 to 56.41, one study, 75 women, Analysis 1.1), nor in endometritis (average RR 0.90, 95% CI 0.60 to 1.35, ten studies, 2134 women, random effects Tau² = 0.00, Chi² = 5.21, P = 0.74, I² = 0%, Analysis 1.2).
None of the studies assessed infant sepsis or infant oral thrush.
Secondary outcomes
There was no significant difference identified between single cephalosporins and penicillin combinations for:
maternal fever (average RR 0.92, 95% CI 0.56 to 1.49, six studies, 1824 women, random effects, Tau² = 0.15; Chi² = 8.75, P = 0.12; I² = 43%, Analysis 1.5);
maternal wound infection (average RR 0.72, (95% CI 0.40 to 1,30, seven studies, 1608 women, random effects Tau² = 0.00; Chi² = 2.04, P = 0.92; I² = 0%, Analysis 1.6);
maternal urinary tract infection (average RR 0.66, 95% CI 0.17 to 2.55, six studies, 1361 women, random effects Tau² = 0.95, Chi² = 6.25, P= 0.10; I² = 52%, Analysis 1.7);
maternal composite adverse effects (RR 0.96, 95% CI 0.09 to 10.50, four studies, 1333 women, Analysis 1.10);
maternal vomiting (RR 7.00, 95% CI 0.37 to 133.78, two studies, 319 women, Analysis 1.13);
maternal skin rash (RR 1.26, 95% CI 0.34 to 4.67, four studies, 618 women, Analysis 1.15).
1.13. Analysis.
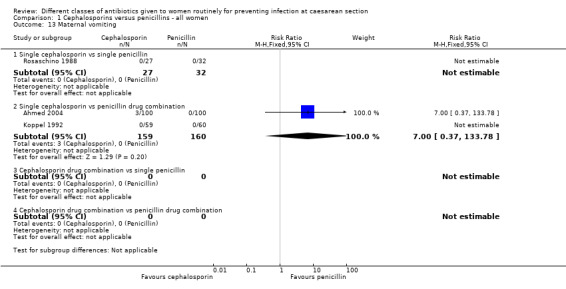
Comparison 1 Cephalosporins versus penicillins ‐ all women, Outcome 13 Maternal vomiting.
For the remaining outcomes, either there were no events or the studies did not asses the outcomes.
See Analysis 1.1 to Analysis 1.28.
Subgroup 3: A cephalosporin combination (B+) versus a single penicillin (A)
One study with 147 women compared a cephalosporin combination versus a single penicillin (Shah 1998).
The study was unclear about how the randomisation sequence was generated but was considered at low risk of bias for allocation concealment.
Primary outcomes
We found no significant difference between cephalosporin combination and single penicillins for maternal endometritis (RR 2.70, 95% CI 0.63 to 11.55, one study, 139 women, Analysis 1.2).
The study did not assess maternal sepsis, infant sepsis nor infant oral thrush.
Secondary outcomes
There was no significant difference identified between cephalosporin combination and single penicillins for:
maternal fever (RR 2.36, 95% CI 0.84 to 6.62, one study, 139 women, Analysis 1.5);
maternal wound infection (RR 2.02, 95% CI 0.42 to 9.63, one study, 139 women, Analysis 1.6).
For the remaining outcomes, either there were no events or the study did not assess the outcomes.
See Analysis 1.1 to Analysis 1.26.
Subgroup 4: A cephalosporin combination (B+) versus a penicillin combination (A+)
Two studies with 363 women compared a cephalosporin combination versus a penicillin combination (Gidiri 2014; van der Linden 1993).
In terms of quality, one study was generally unclear for most of the aspects of assessment of bias of these studies (van der Linden 1993) the other study was unclear on sequence generation and allocation concealment and was considered at high risk of bias for blinding (Gidiri 2014).
Primary outcomes
There was no significant difference identified between cephalosporins combinations and penicillins combinations for maternal sepsis (RR 3.21, 95% CI 0.34 to 30.45, one study, 232 women, Analysis 1.1) or endometritis (RR 0.33, 95% CI 0.01 to 7.77, one study, 83 women, Analysis 1.2).
The study did not assess infant sepsis nor infant oral thrush.
Secondary outcomes
There was no significant difference identified between cephalosporins combinations and penicillins combinations for:
maternal fever (RR 1.57, 95% CI 0.69 to 3.60, two studies, 315 women, Analysis 1.5);
maternal wound infection (RR 1.23, 95% CI 0.42 to 3.58, two studies, 315 women, Analysis 1.6).
For the remaining outcomes, either there were no events or the study did not assess the outcomes.
See Analysis 1.1 to Analysis 1.28.
Publication bias
We identified possible publication bias in the assessment of maternal endometritis (Figure 2) and urinary tract infection (Figure 3). However, there appeared to be no publication bias for maternal fever (Figure 4) nor wound infections (Figure 5). However, as we found no overall difference this is probably of little significance.
2.
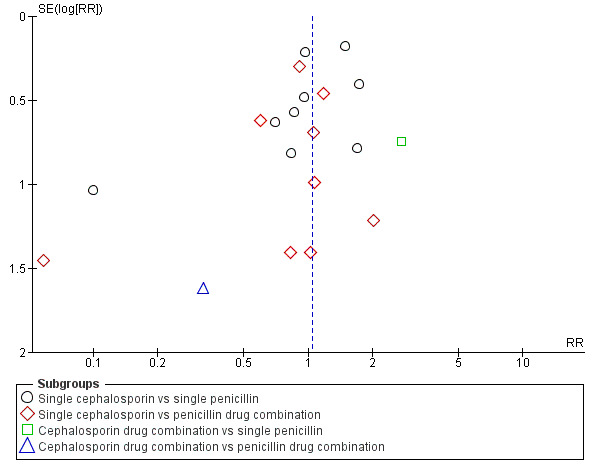
Funnel plot of comparison: 1 Cephalosporins versus penicillins ‐ all women, outcome: 1.2 Maternal endometritis.
3.
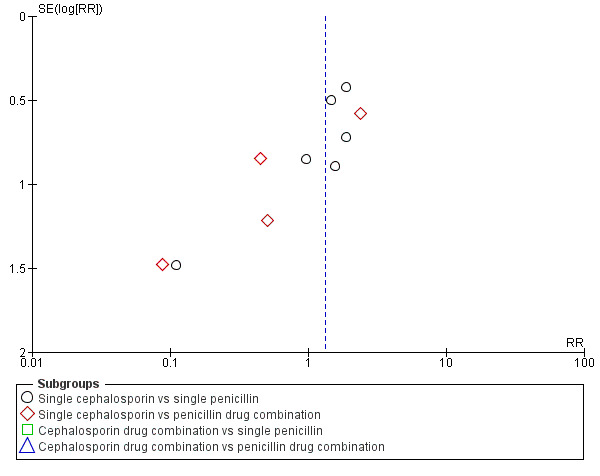
Funnel plot of comparison: 1 Cephalosporins versus penicillins ‐ all women, outcome: 1.7 Maternal urinary tract infection.
4.
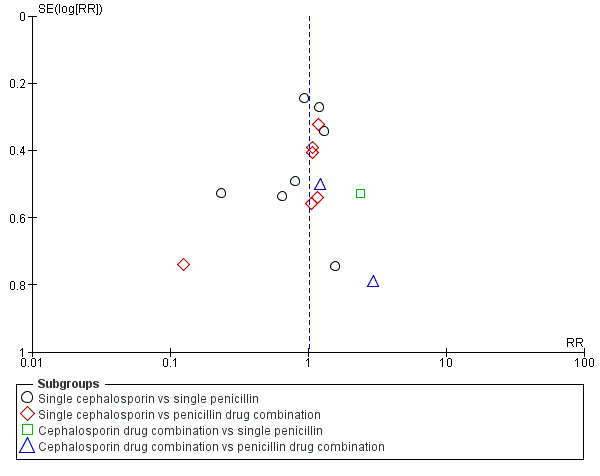
Funnel plot of comparison: 1 Cephalosporins versus penicillins ‐ all women, outcome: 1.5 Maternal fever (febrile morbidity).
5.
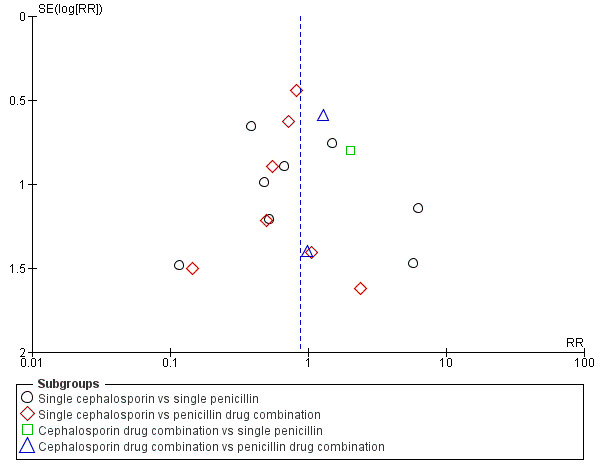
Funnel plot of comparison: 1 Cephalosporins versus penicillins ‐ all women, outcome: 1.6 Maternal wound infection.
2. Cephalosporins (B) versus penicillins (A) ‐ comparison by type of caesarean section, 22 studies, 5788 women
Twenty‐two studies provided data on at least one primary outcome for this subgroup comparison (seeCharacteristics of included studies. Three studies included women having an elective caesarean section (Ahmed 2004; Jyothi 2010; Shah 1998), five studies included women having a non‐elective caesarean section (Busowski 2000; Faro 1990; Louie 1982; Saltzman 1986; van der Linden 1993) and 14 studies included women having elective or non‐elective caesarean section or the studies did not specify the type of caesarean section (Bracero 1997; Chantharojwong 1993; Dashow 1986; Gidiri 2014; Kamilya 2012; Koppel 1992; Lewis 1990; Mivumbi 2014; Noyes 1998; Parulekar 2001; Rosaschino 1988; Saltzman 1985; Spinnato 2000; Ziogos 2010). A further five studies specified the type of caesarean section but did not provide data on at least one primary outcome (Beningo 1986; Ford 1986; Lehapa 1999; Lumbiganon 1994; Ng 1992).
Four studies were assessed as having low risk of bias in terms of adequate sequence generation and adequate allocation concealment (Bracero 1997; Dashow 1986; Mivumbi 2014; Ziogos 2010). Two studies had adequate sequence generation (Faro 1990; Kamilya 2012 ) but unclear allocation concealment;. The remainder of the studies were unclear for both sequence generation and allocation concealment (Figure 1).
We identified no real differences between the two groups of drugs in relation to the different types of caesarean section for maternal sepsis (RR 2.91, 95% CI 0.47 to 18.10, four studies, 653 women, Analysis 2.1) or endometritis (average RR 1.11, 95% CI 0.90 to 1.37, 20 studies, 5390 women, random effects Tau² = 0.01, Chi² = 18.60, P = 0.42, I² = 3%, Analysis 2.2). However, in the subgroup analysis for endometritis we found differences between groups of type of caesarean section (interaction test, Chi² = 5.18, P = 0.08, I² = 61.4%). Penicillins were more effective than cephalosporins for reducing endometritis among women undergoing non‐elective caesarean section (average RR 1.33, 95% CI 1.01 to 1.75, 6 studies, 2362 women, random effects Tau² = 0.00, Chi² = 3.68, P = 0.60, I² = 0%, Analysis 2.2).
2.1. Analysis.
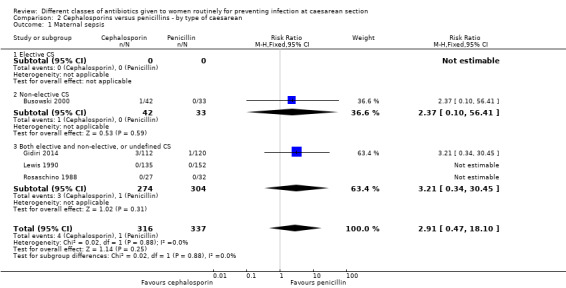
Comparison 2 Cephalosporins versus penicillins ‐ by type of caesarean, Outcome 1 Maternal sepsis.
2.2. Analysis.
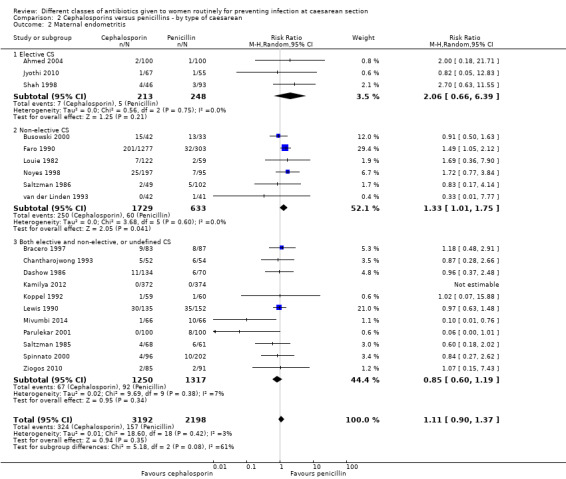
Comparison 2 Cephalosporins versus penicillins ‐ by type of caesarean, Outcome 2 Maternal endometritis.
None of the studies assessed infant sepsis nor infant oral thrush.
3. Cephalosporins (B) versus penicillins (A) ‐ comparison by timing of administration, 22 studies, 5788 women
Twenty‐two studies provided data on at least one primary outcome for inclusion in this subgroup comparison (seeCharacteristics of included studies). Two studies administered antibiotics before cord clamping (Ahmed 2004; Mivumbi 2014 ) and 19 studies administered the antibiotics after cord clamping (Bracero 1997; Busowski 2000; Chantharojwong 1993; Faro 1990; Jyothi 2010; Kamilya 2012; Koppel 1992; Lewis 1990; Louie 1982; Mivumbi 2014; Noyes 1998; Parulekar 2001; Rosaschino 1988; Saltzman 1985; Saltzman 1986; Shah 1998; Spinnato 2000; van der Linden 1993; Ziogos 2010) and two studies did not report the timing of administration with relation to cord clamping (Dashow 1986; Gidiri 2014). A further five studies addressed this comparison but did not provide data in a format suitable for inclusion (Beningo 1986; Ford 1986; Lehapa 1999; Lumbiganon 1994; Ng 1992).
Four studies were assessed as having low risk of bias in terms of adequate sequence generation and adequate allocation concealment (Bracero 1997; Dashow 1986; Mivumbi 2014; Ziogos 2010). Two studies had adequate sequence generation but unclear allocation concealment (Faro 1990; Kamilya 2012) The remainder of the studies were unclear for both sequence generation and allocation concealment (Figure 1).
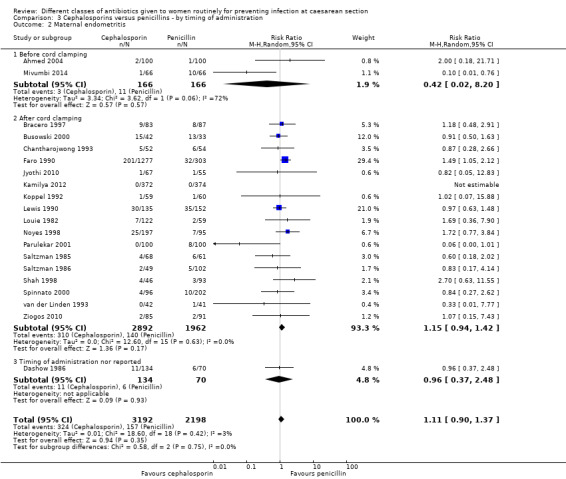
We identified no real differences between the two groups of drugs for maternal sepsis (RR 2.91, 95% CI 0.47 to 18.10, four studies, 653 women, Analysis 3.1) or endometritis (average RR 1.11, 95% CI 0.90 to 1.37, 20 studies, 5390 women, random effects Tau² = 0.01, Chi² = 18.6, P = 0.42, I² = 3%, Analysis 3.2) in relation to the timing of administration. A separate review will be undertaken where studies have compared directly the antibiotic being given before versus after cord clamping ('Timing of prophylactic antibiotics for preventing infectious morbidity in women undergoing caesarean section').
3.1. Analysis.
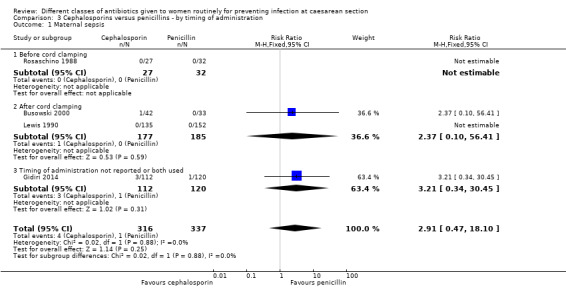
Comparison 3 Cephalosporins versus penicillins ‐ by timing of administration, Outcome 1 Maternal sepsis.
3.2. Analysis.
Comparison 3 Cephalosporins versus penicillins ‐ by timing of administration, Outcome 2 Maternal endometritis.
The interaction test for endometritis showed no significant difference between the groups (P = 0.75) and this was also the case visually.
None of the studies assessed infant sepsis nor infant oral thrush.
4. Cephalosporins (B) versus penicillins (A) ‐ comparison by route of administration, 22 studies, 5788 women
Twenty‐two studies provided data on at least one primary outcome for inclusion in this subgroup comparison. Twenty studies compared the antibiotics when given by intravenous administration (Ahmed 2004; Bracero 1997; Busowski 2000; Chantharojwong 1993; Faro 1990; Gidiri 2014; Jyothi 2010; Kamilya 2012; Koppel 1992; Louie 1982; Mivumbi 2014; Noyes 1998; Parulekar 2001; Rosaschino 1988; Saltzman 1985; Saltzman 1986; Shah 1998; Spinnato 2000; van der Linden 1993; Ziogos 2010). Two studies compared the antibiotics when administered as a lavage/irrigation (Dashow 1986; Lewis 1990). A further five studies addressed this comparison but did not provide data in a format suitable for inclusion (Beningo 1986; Ford 1986; Lehapa 1999; Lumbiganon 1994; Ng 1992).
Four studies were assessed as having low risk of bias in terms of adequate sequence generation and adequate allocation concealment (Bracero 1997; Dashow 1986; Mivumbi 2014; Ziogos 2010). Two studies had adequate sequence generation but unclear allocation concealment (Faro 1990; Kamilya 2012). The remainder of the studies were unclear for both sequence generation and allocation concealment (Figure 1).
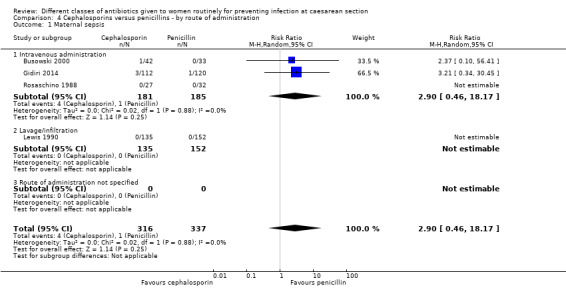
We identified no real differences between the two groups of drugs for maternal sepsis (average RR 2.90, 95% CI 0.46 to 18.17, four studies, 653 women, random effects Tau² = 0.00, Chi² = 0.02, P = 0.88, I² = 0%, Analysis 4.1) or endometritis (RR 1.12, 95% CI 0.92 to 1.37, 20 studies, 5390 women, Analysis 4.2) in relation to the route of administration. A separate review will be undertaken where studies have compared directly the different routes of antibiotic administration ('Routes of administration for antibiotic given to women routinely for preventing infection after caesarean section').
4.1. Analysis.
Comparison 4 Cephalosporins versus penicillins ‐ by route of administration, Outcome 1 Maternal sepsis.
4.2. Analysis.
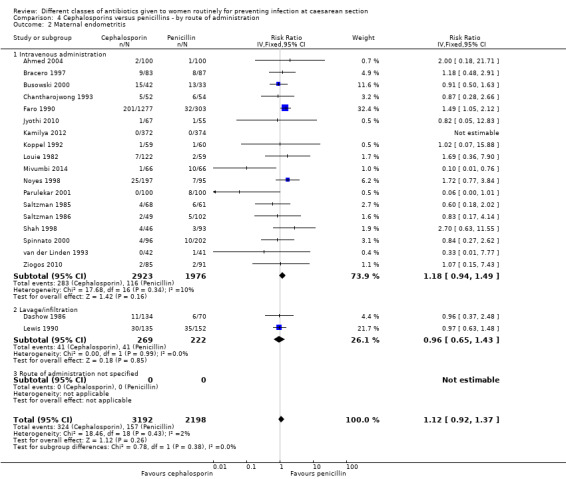
Comparison 4 Cephalosporins versus penicillins ‐ by route of administration, Outcome 2 Maternal endometritis.
The interaction test for endometritis showed no significant difference between the groups (P = 0.38) and this was also the case visually.
None of the studies assessed infant sepsis nor infant oral thrush.
5. First generation cephalosporins (B1) versus extended spectrum penicillins (A3) ‐ all women, two studies, 822 women
Two studies provided data for inclusion in this comparison (Faro 1990; Shah 1998). Cephalosporins included cefazolin (Faro 1990), cephazoline, (Faro 1990) and cephradine plus metronidazole (Shah 1998). Penicillins included only piperacillin (Faro 1990; Shah 1998). Both studies were of unclear quality, with one being unclear about the sequence generation (Shah 1998) and the other being unclear about concealment allocation (Faro 1990). (Figure 1).
Primary outcomes
There was a significantly higher incidence of maternal endometritis with first generation cephalosporins compared with extended spectrum penicillins (RR 2.18, 95% CI 1.30 to 3.66, two studies, 814 women, Analysis 5.2). None of the other primary outcomes (maternal sepsis, infant sepsis and infant thrush) were assessed in either of these studies.
5.2. Analysis.
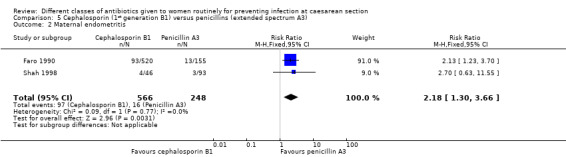
Comparison 5 Cephalosporin (1st generation B1) versus penicillins (extended spectrum A3), Outcome 2 Maternal endometritis.
Secondary outcomes
There was no statistically significant difference identified in maternal fever (RR 2.36, 95% CI 0.84 to 6.62, one study, 139 women, Analysis 5.5) nor maternal wound infection (RR 2.02, 95% CI 0.42 to 9.63, one study, 139 women, Analysis 5.6). None of the other secondary outcomes were assessed in this comparisons.
5.5. Analysis.
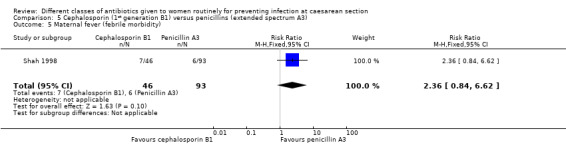
Comparison 5 Cephalosporin (1st generation B1) versus penicillins (extended spectrum A3), Outcome 5 Maternal fever (febrile morbidity).
5.6. Analysis.
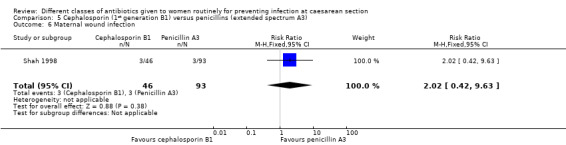
Comparison 5 Cephalosporin (1st generation B1) versus penicillins (extended spectrum A3), Outcome 6 Maternal wound infection.
Neither study assessed any outcomes on the infant, nor post‐discharge infections or readmissions for the mother.
6. First generation cephalosporins (B1) versus aminopenicillins (A4) ‐ all women, eight studies, 1882 women
Eight studies provided data for inclusion in this comparison (Chantharojwong 1993; Dashow 1986; Faro 1990; Jyothi 2010; Louie 1982; Lumbiganon 1994; Mivumbi 2014; Noyes 1998). Cephalosporins included cefazolin (Chantharojwong 1993; Faro 1990; Jyothi 2010; Louie 1982; Lumbiganon 1994; Noyes 1998; Mivumbi 2014) and cephapirin (Dashow 1986). Penicillins included ampicillin (Chantharojwong 1993; Dashow 1986; Faro 1990; Louie 1982; Mivumbi 2014), amoxicillin/clavulanic acid (Lumbiganon 1994) and ampicillin/sulbactam (Noyes 1998). A further study addressed this comparison but did not provide data in a format suitable for inclusion (Graham 1993). Two studies were assessed as adequate on sequence generation and allocation concealment (Dashow 1986; Mivumbi 2014) and one study was adequate on allocation concealment but because sequence generation was unclear overall uncertainty remains (Lumbiganon 1994). The remainder were assessed as unclear for allocation concealment (Figure 1).
Primary outcomes
There was no significant difference identified in maternal endometritis (average RR 1.09, 95% CI 0.69 to 1.71, seven studies, 1487 women, random effects, Tau² = 0.08, Chi² = 7.66, P = 0.26, I² = 22%, Analysis 6.2). None of the other primary outcomes (maternal sepsis, infant sepsis and infant thrush) were assessed in any of these studies.
6.2. Analysis.
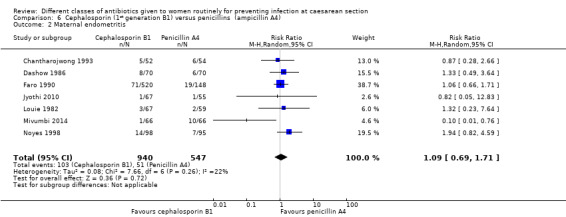
Comparison 6 Cephalosporin (1st generation B1) versus penicillins (ampicillin A4), Outcome 2 Maternal endometritis.
Secondary outcomes
There was no statistically significant difference identified in maternal fever (average RR 0.78, 95% CI 0.40 to 1.51, five studies, 883 women, random effects Tau² = 0.35, Chi² = 10.37, P = 0.03, I² = 61%, Analysis 6.5), wound infection (RR 0.85, 95% CI 0.36 to 2.01, five studies, 626 women, Analysis 6.6), urinary tract infections (average RR 1.41, 95% CI 0.54 to 3.70, five studies, 626 women, random effects Tau² = 0.28, Chi² = 4.17, P = 0.24, I² = 28%, Analysis 6.7), nor maternal composite adverse outcomes (RR 0.32, 95% CI 0.01 to 7.84, two studies, 861 women, Analysis 6.10). There was a significant reduction in the mothers' hospital stay with the cephalosporins (mean difference (MD) ‐1.50, 95% CI ‐2.46 to ‐0.54, one study, 132 women, Analysis 6.21). There were no events in the assessments of maternal allergic reactions not skin rashes. None of the other secondary outcomes were assessed in any of the studies.
6.5. Analysis.
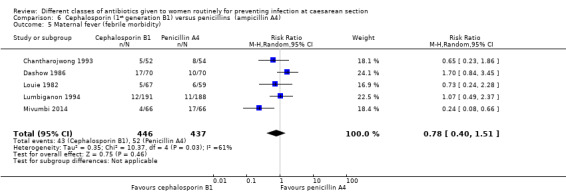
Comparison 6 Cephalosporin (1st generation B1) versus penicillins (ampicillin A4), Outcome 5 Maternal fever (febrile morbidity).
6.6. Analysis.
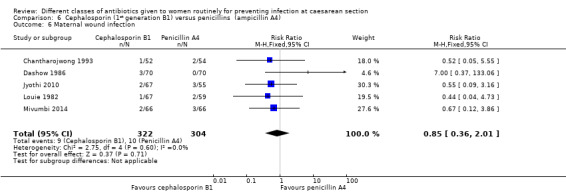
Comparison 6 Cephalosporin (1st generation B1) versus penicillins (ampicillin A4), Outcome 6 Maternal wound infection.
6.7. Analysis.
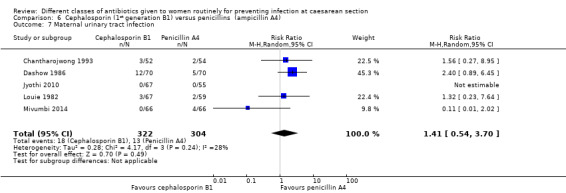
Comparison 6 Cephalosporin (1st generation B1) versus penicillins (ampicillin A4), Outcome 7 Maternal urinary tract infection.
6.10. Analysis.
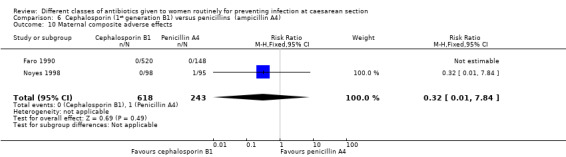
Comparison 6 Cephalosporin (1st generation B1) versus penicillins (ampicillin A4), Outcome 10 Maternal composite adverse effects.
6.21. Analysis.
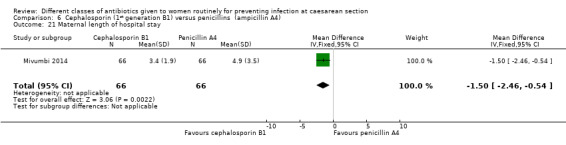
Comparison 6 Cephalosporin (1st generation B1) versus penicillins (ampicillin A4), Outcome 21 Maternal length of hospital stay.
None of the studies assessed any outcomes on the infant, nor post‐discharge infections or readmissions for the mother.
7. Second generation cephalosporins (B2) versus extended spectrum penicillins (A3) ‐ all women, six studies, 2077 women
Six studies provided data for inclusion in this comparison (Beningo 1986; Faro 1990; Ford 1986; Lewis 1990; Saltzman 1985; Saltzman 1986). Cephalosporins included cefoxitin (Beningo 1986; Faro 1990; Ford 1986; Lewis 1990; Saltzman 1985; Saltzman 1986), cefonicid (Faro 1990) and cefotetan (Faro 1990). Penicillins included piperacillin (Beningo 1986; Faro 1990; Ford 1986), ticarcillin (Lewis 1990; Saltzman 1985) and mezlocillin (Saltzman 1986). A further study addressed this comparison but did not provide data in a format suitable for inclusion (De‐Lalla 1988). Only one study was assessed as adequate on sequence generation and allocation concealment (Beningo 1986). One study was adequate for sequence generation but unclear on allocation concealment (Faro 1990) and the remainder were unclear on both criteria (Figure 1).
Primary outcomes
There was no sepsis in the 287 women included in the one study that reported it (Lewis 1990). We found no significant difference identified for maternal endometritis (average RR 1.10, 95% CI 0.78 to 1.54, four studies, 1334 women, random effects Tau² = 0.01, Chi² = 3.19, P = 0.36, I² = 6%, Analysis 7.2). None of the other primary outcomes (infant sepsis and infant thrush) were assessed in either of these studies.
7.2. Analysis.
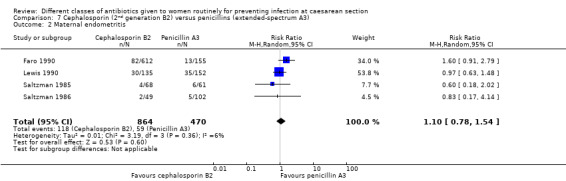
Comparison 7 Cephalosporin (2nd generation B2) versus penicillins (extended‐spectrum A3), Outcome 2 Maternal endometritis.
Secondary outcomes
There was no significant difference identified for maternal fever (RR 1.08, 95% CI 0.79 to 1.47, four studies, 850 women, Analysis 7.5), wound infection (average RR 2.37, 95% CI 0.64 to 8.73, two studies, 438 women, random effects Tau² = 0.08, Chi² = 2.37, P = 0.30, I² = 8%, Analysis 7.6), urinary tract infection (average RR 1.43, 95% CI 0.67 to 3.07, three studies, 567 women, random effects Tau² = 0.09, Chi² = 2.42, P = 0.30, I² = 17%, Analysis 7.7), maternal composite adverse effects (RR 2.02, 95% CI 0.18 to 21.96, two studies, 1030 women, Analysis 7.10) and skin rash (RR 2.70, 95% CI 0.11 to 64.96, one study, 129 women, Analysis 7.15).
7.5. Analysis.
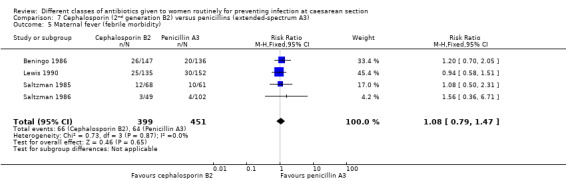
Comparison 7 Cephalosporin (2nd generation B2) versus penicillins (extended‐spectrum A3), Outcome 5 Maternal fever (febrile morbidity).
7.6. Analysis.
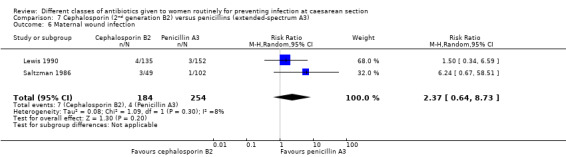
Comparison 7 Cephalosporin (2nd generation B2) versus penicillins (extended‐spectrum A3), Outcome 6 Maternal wound infection.
7.7. Analysis.
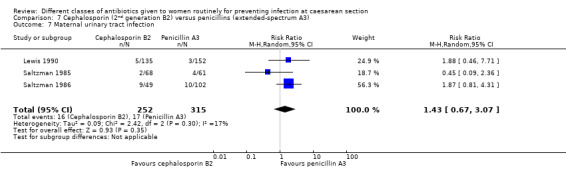
Comparison 7 Cephalosporin (2nd generation B2) versus penicillins (extended‐spectrum A3), Outcome 7 Maternal urinary tract infection.
7.10. Analysis.
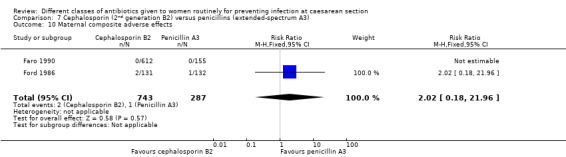
Comparison 7 Cephalosporin (2nd generation B2) versus penicillins (extended‐spectrum A3), Outcome 10 Maternal composite adverse effects.
7.15. Analysis.
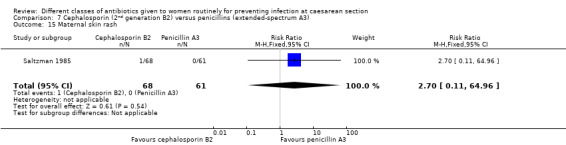
Comparison 7 Cephalosporin (2nd generation B2) versus penicillins (extended‐spectrum A3), Outcome 15 Maternal skin rash.
Three studies looked at infection rates after discharge (Beningo 1986; Saltzman 1985; Saltzman 1986). Two other studies reported no infections up to six weeks postoperatively based on 305 women participating in the studies (Saltzman 1985; Saltzman 1986).
None of the studies assessed any outcomes on the infant.
8. Second generation cephalosporins (B2) versus aminopenicillins (A4) ‐ all women, eight studies, 1921 women
Eight studies provided data for inclusion in this comparison (Bracero 1997; Busowski 2000; Dashow 1986; Faro 1990; Noyes 1998; Spinnato 2000; van der Linden 1993; Ziogos 2010). Cephalosporins included cefotetan (Bracero 1997; Busowski 2000; Faro 1990; Noyes 1998; Spinnato 2000; Ziogos 2010), cefamandole (Dashow 1986), cefonicid (Faro 1990), cefoxitin (Faro 1990) and cefuroxime (van der Linden 1993). Penicillins included ampicillin (Bracero 1997; Dashow 1986; Faro 1990; Spinnato 2000), ampicillin/sulbactam (Busowski 2000; Noyes 1998; Spinnato 2000) and amoxicillin/clavulanic acid (van der Linden 1993). A further study addressed this comparison but did not provide data in a format suitable for inclusion (Voto 1986). Two studies were assessed as adequate on sequence generation and allocation concealment (Bracero 1997; Dashow 1986). One study was assessed as adequate on sequence generation but unclear on allocation concealment (Faro 1990) and the remainder were unclear on both criteria (Figure 1).
Primary outcomes
There was no significant difference identified in maternal sepsis (RR 2.37, 95% CI 0.10 to 56.41, one study, 75 women, Analysis 8.1) nor endometritis (RR 1.01, 95% CI 0.75 to 1.35, eight studies, 1890 women, Analysis 8.2). None of the other primary outcomes (infant sepsis and infant thrush) were assessed in any of these studies.
8.1. Analysis.
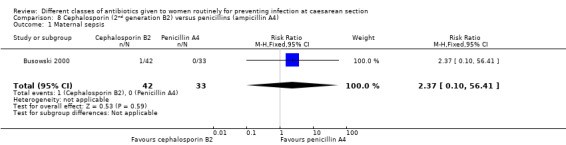
Comparison 8 Cephalosporin (2nd generation B2) versus penicillins (ampicillin A4), Outcome 1 Maternal sepsis.
8.2. Analysis.
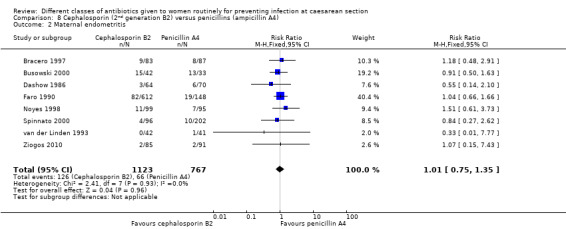
Comparison 8 Cephalosporin (2nd generation B2) versus penicillins (ampicillin A4), Outcome 2 Maternal endometritis.
Secondary outcomes
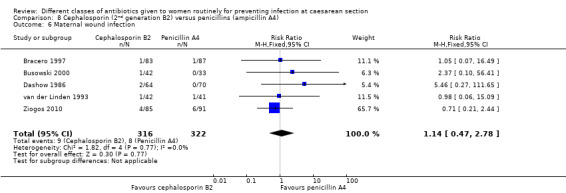
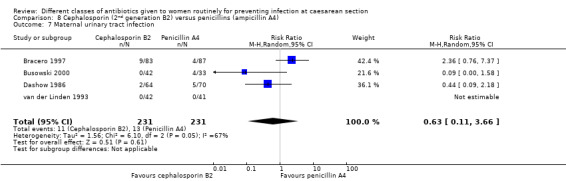
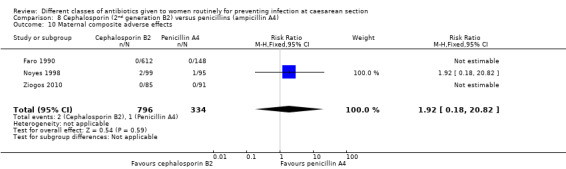
There was no significant difference identified for maternal fever (RR 1.17, 95% CI 0.64 to 2.15, three studies, 387 women, Analysis 8.5), wound infection (RR 1.14, 95% CI 0.47 to 2.78, five studies, 638 women, Analysis 8.6), urinary tract infection (average RR 0.63, 95% CI 0.11 to 3.66, four studies, 462 women, random effects Tau² = 1.56, Chi² = 6.10; P = 0.05, I² = 67%, Analysis 8.7), maternal composite adverse effects (RR 1.92, 95% CI 0.18 to 20.82, three studies, 1130 women, Analysis 8.10), nor skin rash (RR 1.02, 95% CI 0.23 to 4.46, two studies, 364 women, Analysis 8.15). For urinary tract infection there was high heterogeneity and studies showed effects in different directions but no overall difference was identified Analysis 8.7). None of the other secondary outcomes were assessed in any of the included studies.
8.5. Analysis.
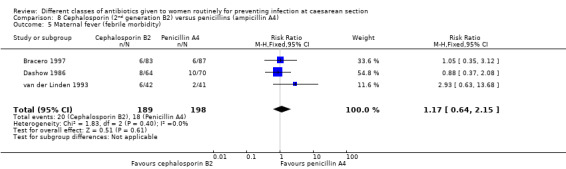
Comparison 8 Cephalosporin (2nd generation B2) versus penicillins (ampicillin A4), Outcome 5 Maternal fever (febrile morbidity).
8.6. Analysis.
Comparison 8 Cephalosporin (2nd generation B2) versus penicillins (ampicillin A4), Outcome 6 Maternal wound infection.
8.7. Analysis.
Comparison 8 Cephalosporin (2nd generation B2) versus penicillins (ampicillin A4), Outcome 7 Maternal urinary tract infection.
8.10. Analysis.
Comparison 8 Cephalosporin (2nd generation B2) versus penicillins (ampicillin A4), Outcome 10 Maternal composite adverse effects.
8.15. Analysis.
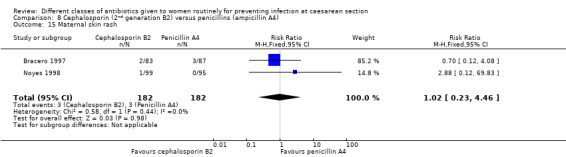
Comparison 8 Cephalosporin (2nd generation B2) versus penicillins (ampicillin A4), Outcome 15 Maternal skin rash.
None of the studies assessed any outcomes on the infants, nor post‐discharge infections or readmissions for the mother.
9. Third generation cephalosporins (B3) versus extended‐spectrum penicillins ( A3) ‐ all women, two studies, 359 women
Two studies provided data for inclusion in this comparison (Faro 1990; Rosaschino 1988). Cephalosporins included ceftizoxime (Faro 1990) and ceftriaxone (Rosaschino 1988). Penicillins included piperacillin (Faro 1990) and mezlocillin (Rosaschino 1988). Neither study was assessed as adequate on both sequence generation and allocation concealment. Only the Faro study (Faro 1990) had adequate sequence generation and all other criteria were assessed as unclear (Figure 1).
Primary outcomes
There was no sepsis in the 287 women included in the one study that reported it (Rosaschino 1988). We found significantly more women with endometritis when third generation cephalosporins were used compared with extended spectrum penicillin (RR 2.14, 95% CI 1.14 to 4.00, one study, 300 women, Analysis 9.2). None of the other primary outcomes were assessed.
9.2. Analysis.
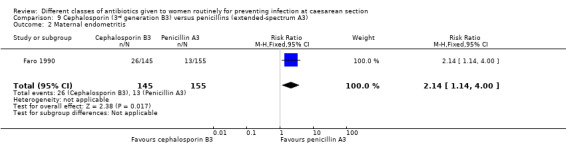
Comparison 9 Cephalosporin (3rd generation B3) versus penicillins (extended‐spectrum A3), Outcome 2 Maternal endometritis.
Secondary outcomes
Although some secondary outcomes were assessed, there were no events in the included studies.
Neither study assessed any outcomes on the infants, nor post‐discharge infections or readmissions for the mother.
10. Third generation cephalosporins (B3) versus aminopenicillins (A4) ‐ all women, seven studies, 1904 women
Seven studies provided data for inclusion in this comparison (Ahmed 2004; Faro 1990; Kamilya 2012; Koppel 1992; Lehapa 1999; Louie 1982; Ng 1992). Cephalosporins included ceftriaxone (Ahmed 2004; Lehapa 1999), ceftizoxime (Faro 1990), cefotamine (Kamilya 2012; Koppel 1992), cefotaxime (Louie 1982) and cefoperazone (Ng 1992). Penicillins ampicillin/cloxacillin (Ahmed 2004), amoxicillin/clavulanic acid (Koppel 1992) and ampicillin (Faro 1990; Lehapa 1999; Louie 1982; Ng 1992). None of the six studies were assessed as adequate on both sequence generation and allocation concealment. Only the Faro study (Faro 1990) had adequate sequence generation and all other criteria were assessed as unclear for the studies (Figure 1).
Primary outcomes
There was no significant difference identified for maternal endometritis (RR 1.47, 95% CI 0.89 to 2.42, five studies, 1472 women, Analysis 10.2). The other primary outcomes were not assessed in any of the studies.
10.2. Analysis.
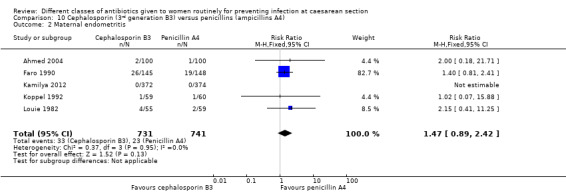
Comparison 10 Cephalosporin (3rd generation B3) versus penicillins (ampicillins A4), Outcome 2 Maternal endometritis.
Secondary outcomes
There was a significant reduction in maternal wound infection with third generation cephalosporins (B3) compared with aminopenicillins (A4) (RR 0.49, 95% CI 0.27 to 0.90, six studies, 1556 women, Analysis 10.6).
10.6. Analysis.
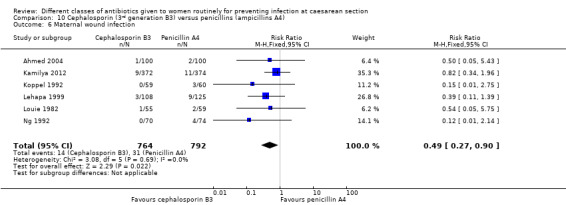
Comparison 10 Cephalosporin (3rd generation B3) versus penicillins (ampicillins A4), Outcome 6 Maternal wound infection.
We identified no significant difference in maternal fever (RR 1.12, 95% CI 0.69 to 1.83, three studies, 1060 women, Analysis 10.5), urinary tract infection (RR 0.52, 95% CI 0.10 to 2.80, two studies, 233 women, Analysis 10.7), maternal vomiting (RR 7.00, 95% CI 0.37 to 133.78, one study, 200 women, Analysis 10.13) or maternal skin rash (RR 3.00, 95% CI 0.12 to 72.77, one study, 200 women, Analysis 10.15). The other secondary outcomes were not assessed in any of the included studies. There was no significant difference identified in the length of hospital stay (MD ‐0.03, 95% CI ‐0.14 to 0.08, one study, 746 women, Analysis 10.21).
10.5. Analysis.
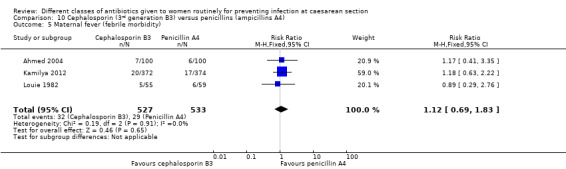
Comparison 10 Cephalosporin (3rd generation B3) versus penicillins (ampicillins A4), Outcome 5 Maternal fever (febrile morbidity).
10.7. Analysis.
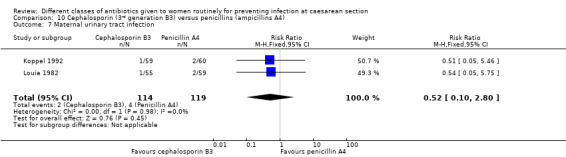
Comparison 10 Cephalosporin (3rd generation B3) versus penicillins (ampicillins A4), Outcome 7 Maternal urinary tract infection.
10.13. Analysis.
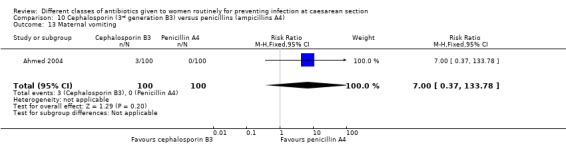
Comparison 10 Cephalosporin (3rd generation B3) versus penicillins (ampicillins A4), Outcome 13 Maternal vomiting.
10.15. Analysis.
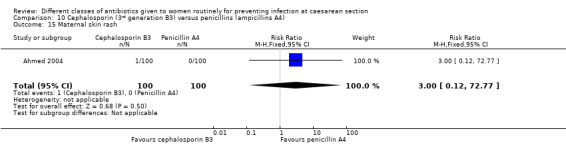
Comparison 10 Cephalosporin (3rd generation B3) versus penicillins (ampicillins A4), Outcome 15 Maternal skin rash.
10.21. Analysis.
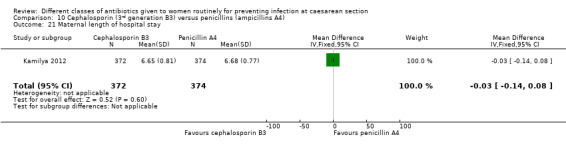
Comparison 10 Cephalosporin (3rd generation B3) versus penicillins (ampicillins A4), Outcome 21 Maternal length of hospital stay.
None of the studies assessed any outcomes on the infant, nor post‐discharge infections or readmissions for the mother.
11. Fluoroquinolones (C) versus penicillins (A) ‐ all women, one study, 72 women
One study provided data for inclusion in this comparison (Busowski 2000). This study compared the second generation fluoroquinolone (C2) ciprofloxacin with the ampicillin/sulbactam (A4). This study was of questionable quality as it provided no information on the sequence generation or allocation concealment.
There were insufficient data to provide good evidence on the only outcomes assessed (maternal sepsis, endometritis, wound infection and urinary tract infection) in this comparison (Analysis 11.1; Analysis 11.2; Analysis 11.6; Analysis 11.7).
11.1. Analysis.
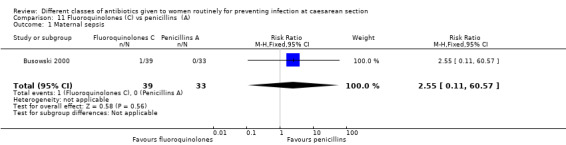
Comparison 11 Fluoroquinolones (C) vs penicillins (A), Outcome 1 Maternal sepsis.
11.2. Analysis.
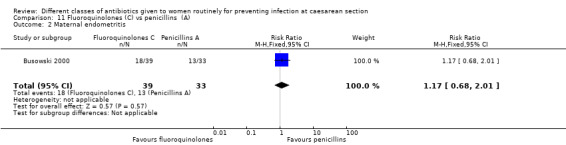
Comparison 11 Fluoroquinolones (C) vs penicillins (A), Outcome 2 Maternal endometritis.
11.6. Analysis.
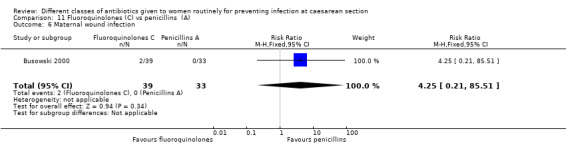
Comparison 11 Fluoroquinolones (C) vs penicillins (A), Outcome 6 Maternal wound infection.
11.7. Analysis.
Comparison 11 Fluoroquinolones (C) vs penicillins (A), Outcome 7 Maternal urinary tract infection.
This study did not address any outcomes on the infant, nor post‐discharge infections or readmissions for the mother.
12. Fluoroquinolones (C) versus cephalosporins (B) ‐ all women, one study, 81 women
One study provided data for inclusion in this comparison (Busowski 2000). This study compared the second generation fluoroquinolone (C2) ciprofloxacin with the second generation cephalosporin cefotetan (B2). This study was of questionable quality as it provided no information on the sequence generation or allocation concealment.
There were insufficient data to provide good evidence on the only outcomes assessed (maternal sepsis, endometritis, wound infection and urinary tract infection) in this comparison (Analysis 12.1; Analysis 12.2; Analysis 11.6; Analysis 12.7).
12.1. Analysis.
Comparison 12 Fluoroquinolones (C) vs cephalosporins (B), Outcome 1 Maternal sepsis.
12.2. Analysis.
Comparison 12 Fluoroquinolones (C) vs cephalosporins (B), Outcome 2 Maternal endometritis.
12.7. Analysis.
Comparison 12 Fluoroquinolones (C) vs cephalosporins (B), Outcome 7 Maternal urinary tract infection.
This study did not address any outcomes on the infant, nor post‐discharge infections or readmissions for the mother.
13. Other antibiotic regimens (D ‐ I) versus penicillins (A)
13.1 Lincosamide (H) plus aminoglycoside (G) versus penicillin (A) ‐ all women, one study, 88 women
One study involving 88 women provided data for this comparison (Rehu 1980). This study compared clindamicin (a lincosamide ‐ group H) plus gentamicin (an aminoglycoside ‐ group G) against benzylpenicillin penicillin (a penicillin ‐ group A). The sequence generation and allocation concealment was unclear.
There were insufficient data to provide good evidence on the only outcomes assessed (maternal endometritis and wound infection) in this comparison (Analysis 13.2; Analysis 13.6).
13.2. Analysis.
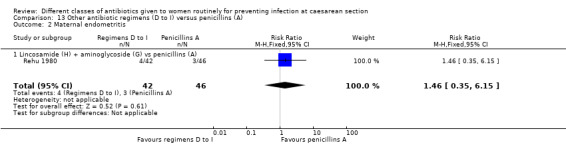
Comparison 13 Other antibiotic regimens (D to I) versus penicillins (A), Outcome 2 Maternal endometritis.
13.6. Analysis.
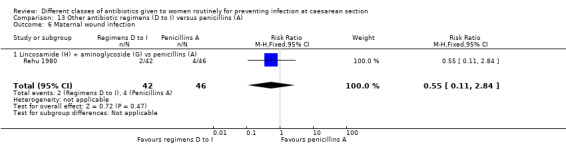
Comparison 13 Other antibiotic regimens (D to I) versus penicillins (A), Outcome 6 Maternal wound infection.
This study did not address any outcomes on the infant, nor post‐discharge infections or readmissions for the mother.
14. Other antibiotic regimens (D to I) versus cephalosporins (B)
14.1 Beta‐lactam (F) versus cephalosporin (B) ‐ all women, two studies, 118 women
Two studies involving 118 women provided data for this comparison (Mansueto 1989; Mothilal 2013). These studies compared azithromycin (a macrolide ‐ group E) against cefazolin (a cephalosporin ‐ group B1) (Mothilal 2013) and imipenem (a beta‐lactam ‐ group F) against cefotamine (a cephalosporin ‐ group B3) (Mansueto 1989). The sequence generation and allocation concealment were unclear in both studies.
There were insufficient data to provide good evidence on the only outcomes assessed (maternal endometritis, fever, wound infection and urinary tract infection) in this comparison (Analysis 14.2; Analysis 14.5; Analysis 14.6; Analysis 12.7).
14.2. Analysis.
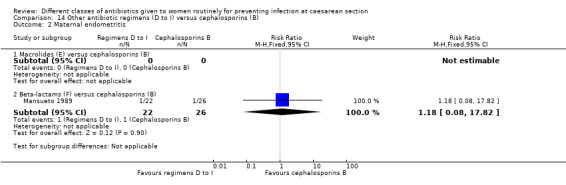
Comparison 14 Other antibiotic regimens (D to I) versus cephalosporins (B), Outcome 2 Maternal endometritis.
14.5. Analysis.
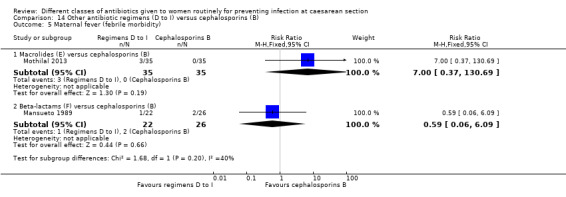
Comparison 14 Other antibiotic regimens (D to I) versus cephalosporins (B), Outcome 5 Maternal fever (febrile morbidity).
14.6. Analysis.
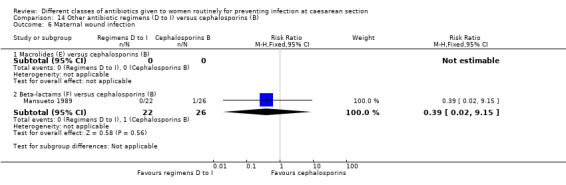
Comparison 14 Other antibiotic regimens (D to I) versus cephalosporins (B), Outcome 6 Maternal wound infection.
This study did not address any outcomes on the infant, nor post‐discharge infections or readmissions for the mother.
15. Other antibiotic regimens versus different antibiotic regimens
15.1 Aminoglycoside (G) plus nitroimidazole (I) versus standard antibiotic cocktail ‐ all women, one study, 241 women
One study involving 241 women provided data for this comparison (Kayihura 2003). This study compared gentamicin (an aminoglycoside ‐ group G) plus metronidazole (a nitroimidazole ‐ group I) versus a standard cocktail of antibiotics. The sequence generation and allocation concealment was unclear.
There were no differences identified in maternal endometritis (RR 0.81, 95% CI 0.29 to 2.26, one study, 241 women, Analysis 15.2), wound infection (RR 3.23, 95% CI 0.34 to 30.64, one study 241 women, Analysis 15.6) and urinary tract infection (RR 1.08, 95% CI 0.07 to 17.03, one study 241 women, Analysis 15.7).
15.2. Analysis.
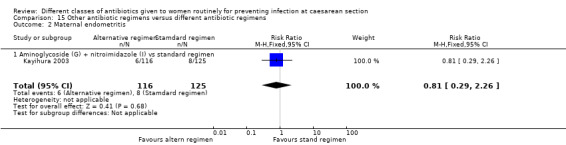
Comparison 15 Other antibiotic regimens versus different antibiotic regimens, Outcome 2 Maternal endometritis.
15.6. Analysis.
Comparison 15 Other antibiotic regimens versus different antibiotic regimens, Outcome 6 Maternal wound infection.
15.7. Analysis.
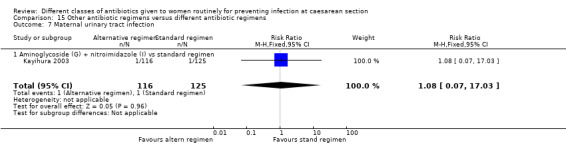
Comparison 15 Other antibiotic regimens versus different antibiotic regimens, Outcome 7 Maternal urinary tract infection.
This study did not address any outcomes on the infant, nor post‐discharge infections or readmissions for the mother.
Sensitivity analyses
There were insufficient data from high‐quality studies for an meaningful sensitivity analyses.
Discussion
Antibiotic prophylaxis can be expected to produce a significant reduction in the incidence of maternal infectious morbidity (Smaill 2008). The type of antibiotic used prophylactically, as well as the optimal timing of administration, have been widely studied and discussed in the literature. Here we have addressed the comparisons between the different classes of antibiotics.
Summary of main results
There was no conclusive evidence identified of any difference between cephalosporins and penicillins in the outcomes of maternal sepsis, endometritis, fever, wound infection, urinary tract infection and adverse effects. Endometritis seemed less common when extended‐spectrum penicillins were used rather than first or third generation cephalosporins, but more data are required to be sure of this.
Nor was there any difference identified between fluoroquinolones and penicillins or cephalosporins for maternal sepsis, endometritis, wound infection or urinary tract infection. However, there are clearly insufficient data on the comparisons with fluoroquinolones and further trials are needed.
None of the studies assessed any outcomes on the baby. This is a serious omission as women will want to know if this intervention has any adverse effect on their babies. There are also concerns about this lack of information even for studies where the antibiotic was given after the cord had been clamped and cut, as these drugs may pass to the baby through breastfeeding. In addition, none of the studies assessed readmissions and only three considered post‐discharge infections. This is a limitation of this analysis as late infections appear to constitute the majority of infections after caesarean section (Leth 2009). We have no information on whether prophylactic antibiotics impact on these infections and whether one class of antibiotic is better than another.
We also identified no overall differences between cephalosporins and penicillins by the subgroup analyses of type of caesarean, timing of administration (before or after cord clamping), nor route of administration. Other Cochrane reviews to be undertaken will address specifically the timing and routes of administration (Timing of prophylactic antibiotics for preventing infectious morbidity in women undergoing caesarean section and Routes of administration for antibiotic given to women routinely for preventing infection after caesarean section).
In terms of subsidiary drug classifications, there seemed to be no overall difference identified, except that there may be less wound infection with third generation cephalosporins compared with ampicillins and less endometritis with extended‐spectrum penicillins compared with first or third generation cephalosporins. However, since these are all likely to be underpowered, further studies are needed. The comparisons between the specific subclasses of penicillins and cephalosporins will also be addressed in further Cochrane reviews (Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section and Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section).
Overall completeness and applicability of evidence
There is uncertainty around the quality of the evidence and for the subclassification of the antibiotics there were insufficient data to draw any firm conclusions. Development of bacterial resistance is an important consideration which we plan to address as best we can in a subsequent update of this review.
Quality of the evidence
The quality of the evidence was generally unclear for most of the studies, possibly reflecting the lack of knowledge about the important criteria for minimising bias when many of the studies were undertaken back in the 1980s and 1990s. The quality of the evidence using GRADE was moderate for maternal endometritis, low for wound infection and maternal urinary tract infection, and very low for maternal composite adverse effects (Table 1). The outcomes were downgraded due to design limitations or wide confidence interval crossing the line of no effect, small sample sizes and few events.
Potential biases in the review process
We attempted to minimise bias in a number of ways: two review authors assessed eligibility for inclusion and carried out data extraction, and three authors assessed risk of bias. Each worked independently. Nevertheless, the process of assessing risk of bias, for example, is not an exact science and includes many personal judgements.
Agreements and disagreements with other studies or reviews
The previous version of this review concluded that there was no difference in efficacy between ampicillin and first generation cephalosporins and that the more costly extended‐spectrum penicillins and second and third generation cephalosporins had not been demonstrated to be any more effective (Hopkins 1999). This previous version of the review also found no benefit from multiple doses but this aspect will be addressed in the remaining two reviews to be undertaken (Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section and Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section). There is a published review on the timing of administration of the antibiotic that suggests that further studies are needed also assessing neonatal outcomes (Tita 2009). This question needs to be addressed in a Cochrane review so that it can be updated as new data come along (Timing of prophylactic antibiotics for preventing infectious morbidity in women undergoing caesarean section).
Authors' conclusions
Implications for practice.
Best current evidence suggests that both cephalosporins and penicillins represent good choices for prophylaxis in women undergoing caesarean section, although the impact on post‐discharge infections and on the infant are unknown, as is the impact on bacterial resistance. All are critical to decision‐making. The use of any antibiotic needs to be made on an individual basis, taking into account other medication the mother may be on. Impact on the baby, for which there is no formal evidence, also needs to be considered, as does bacterial resistance. More costly extended‐spectrum penicillins, second or third generation cephalosporins and combination regimens have not been demonstrated to be more effective but there are few data upon which to make a clear judgement.
Considering that all the antibiotic regimens have shown similar efficacy in terms of the measured outcomes, the decision of what antibiotic to use will depend on the woman's sensitivity to specific antibiotics, the physician's experience, the adverse events, the prevalence of pathogenic organisms according to previous epidemiological studies (if available), the availability and the costs in the different scenarios.
Implications for research.
There is a need for good‐quality trials to assess the most effective antibiotic to use at caesarean section and it is critical that outcomes on the babies are assessed. Trials should include the outcomes identified for this review, in particular outcomes on the baby and post‐discharge infections for the mother. There will continue to be debate both in the literature and in clinical practice regarding the optimal time for administration of prophylactic antibiotics and there is a need for a Cochrane review on this aspect of care. The impact of routine antibiotics at caesarean section on bacterial resistance needs to be investigated with some urgency.
What's new
| Date | Event | Description |
|---|---|---|
| 30 September 2014 | New citation required but conclusions have not changed | Six new trials included. Conclusions remain the same. |
| 30 September 2014 | New search has been performed | Methods updated to include GRADE. Search updated, 17 new reports identified. |
History
Protocol first published: Issue 3, 1998 Review first published: Issue 10, 2010
| Date | Event | Description |
|---|---|---|
| 13 July 2010 | New search has been performed | We have included further studies and re‐structured the review to address the comparisons between different classes of antibiotics. Further reviews will be undertaken to address comparisons within classes of antibiotics, including drug doses, and separate reviews will be undertaken on timings and routes of administration ‐ seeDifferences between protocol and review. |
Notes
The Hopkins 1999 published review on, Antibiotic prophylaxis regimens and drugs for caesarean section, has been subsequently ‘withdrawn’ from publication in The Cochrane Library because it has become out of date. The review has now been split into five separate reviews.
Different classes of antibiotics given to women routinely for preventing infection at caesarean section (this review)
Different regimens of penicillin antibiotics given to women routinely for preventing infection after caesarean section (Liu 2014)
Different regimens of cephalosporin antibiotic prophylaxis at caesarean section for reducing morbidity (protocol in preparation)
Timing of prophylactic antibiotics for preventing infectious morbidity in women undergoing caesarean section (Baxter 2011) (review in preparation)
Routes of administration of antibiotic prophylaxis for preventing infection after caesarean section (protocol in preparation)
Acknowledgements
We wish to thank Laura Hopkins and Fiona Smaill for all their work on the previous version of this review. Also, we would like to thank Tim Neal for providing guidance on the updating of this review, and Neil Hotham and Wendy Jones who provided guidance on the classification of the antibiotics. Also our thanks to Zarko Alfirevic for his contribution to previous versions of the review, particularly in helping to develop the structure and interpret the data.
Thanks to Austin Anderson Leirvik for translating Fugere 1983; Anne‐Marie Grant for translating Luttkus 1997; Danping He for translating Xu 1997; Alison Jenner for translating Warnecke 1982; Andrew MacDonald for translating Voto 1986; Cathryn Siegel‐Bergman for translating Koppel 1992; Tamara Stampalija for translating Mansani 1984 and Mansueto 1989; Caroline Summers for translating Wagner 2006; and Elizabeth Whiteley for translating Patacchiola 2000 and Rosaschino 1988.
As part of the pre‐publication editorial process for Alfirevic 2010, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
The review authors would like to thank Erika Ota for preparing the 'Summary of findings' table for this update. Erika Ota's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
We would also like to thank Sonja Henderson for her help.
Appendices
Appendix 1. Data collection for studies identified before October 1998
All potential trials were selected for eligibility according to the criteria specified in the protocol and data were extracted from each publication by two reviewers. Any discrepancies were resolved by discussion. In addition to the main outcome measures listed above, information on the setting of the study (country, type of population, socioeconomic status), a detailed description of the antibiotic regimen used (drug, dose, frequency and timing), and definitions of the outcomes (if provided) were collected. An intention‐to‐treat analysis was performed where possible.
Trials were assessed for methodological quality using the standard Cochrane criteria of adequacy of allocation concealment: adequate (A), unclear (B), inadequate (C), or that allocation concealment was not used (D). Information on blinding of outcome assessment and loss to follow‐up were collected.
The main comparison of any treatment versus another treatment was to be stratified according to the indication for caesarean section.
Separate comparisons of different classes of antibiotics and regimens, grouped where appropriate by spectrum of activity, were made. If there were sufficient trials, separate comparisons were made between the timing of antibiotic administration, the number of doses given and the route of administration (whether systemic or lavage).
Summary relative risks were calculated using a fixed‐effect model (if there was no significant heterogeneity between trials).
Data and analyses
Comparison 1. Cephalosporins versus penicillins ‐ all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal sepsis | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Single cephalosporin vs single penicillin | 2 | 346 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Single cephalosporin vs penicillin drug combination | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.10, 56.41] |
| 1.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Cephalosporin drug combination vs penicillin drug combination | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [0.34, 30.45] |
| 2 Maternal endometritis | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Single cephalosporin vs single penicillin | 9 | 3130 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.81, 1.52] |
| 2.2 Single cephalosporin vs penicillin drug combination | 10 | 2134 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.60, 1.35] |
| 2.3 Cephalosporin drug combination vs single penicillin | 1 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 2.70 [0.63, 11.55] |
| 2.4 Cephalosporin drug combination vs penicillin drug combination | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.77] |
| 3 Infant sepsis | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Single cephalosporin vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Single cephalosporin vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Infant oral thrush | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Single cephalosporin vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Single cephalosporin vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal fever (febrile morbidity) | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Single cephalosporin vs single penicillin | 7 | 1344 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.61, 1.30] |
| 5.2 Single cephalosporin vs penicillin drug combination | 6 | 1824 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.56, 1.49] |
| 5.3 Cephalosporin drug combination vs single penicillin | 1 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 2.36 [0.84, 6.62] |
| 5.4 Cephalosporin drug combination vs penicillin drug combination | 2 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.69, 3.60] |
| 6 Maternal wound infection | 19 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Single cephalosporin vs single penicillin | 9 | 1497 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.38, 1.81] |
| 6.2 Single cephalosporin vs penicillin drug combination | 7 | 1608 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.40, 1.30] |
| 6.3 Cephalosporin drug combination vs single penicillin | 1 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [0.42, 9.63] |
| 6.4 Cephalosporin drug combination vs penicillin drug combination | 2 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.42, 3.58] |
| 7 Maternal urinary tract infection | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Single cephalosporin vs single penicillin | 7 | 1120 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.89, 2.48] |
| 7.2 Single cephalosporin vs penicillin drug combination | 6 | 1361 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.17, 2.55] |
| 7.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Cephalosporin drug combination vs penicillin drug combination | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Maternal thrush | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Single cephalosporin vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Single cephalosporin vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal composite serious infectious complication | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Single cephalosporin vs single penicillin | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Single cephalosporin vs penicillin drug combination | 1 | 746 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal composite adverse effects | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Single cephalosporin vs single penicillin | 3 | 1902 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 21.96] |
| 10.2 Single cephalosporin vs penicillin drug combination | 4 | 1333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.09, 10.50] |
| 10.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal allergic reactions | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Single cephalosporin vs single penicillin | 2 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Single cephalosporin vs penicillin drug combination | 3 | 1041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal nausea | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Single cephalosporin vs single penicillin | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Single cephalosporin vs penicillin drug combination | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal vomiting | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Single cephalosporin vs single penicillin | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Single cephalosporin vs penicillin drug combination | 2 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.37, 133.78] |
| 13.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Maternal diarrhoea | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Single cephalosporin vs single penicillin | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Single cephalosporin vs penicillin drug combination | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Maternal skin rash | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Single cephalosporin vs single penicillin | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.06, 35.38] |
| 15.2 Single cephalosporin vs penicillin drug combination | 4 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.34, 4.67] |
| 15.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Immediate infant adverse effects | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Single cephalosporin vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Single cephalosporin vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Infant unsettled | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Single cephalosporin vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Single cephalosporin vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Infant diarrhoea | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 Single cephalosporin vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Single cephalosporin vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Infant skin rash | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Single cephalosporin vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 Single cephalosporin vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Development of bacterial resistance | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 Single cephalosporin vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Single cephalosporin vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Maternal length of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21.1 Single cephalosporin vs single penicillin | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Single cephalosporin vs penicillin drug combination | 1 | 746 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.14, 0.08] |
| 21.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Infant length of hospital stay | 0 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 22.1 Single cephalosporin vs single penicillin | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Single cephalosporin vs penicillin drug combination | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Infant's immune system development | 0 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 23.1 Single cephalosporin vs single penicillin | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Single cephalosporin vs penicillin drug combination | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Infant general health | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 24.1 Single cephalosporin vs single penicillin | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Single cephalosporin vs penicillin drug combination | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Infant frequency of hospital visits | 0 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 25.1 Single cephalosporin vs single penicillin | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Single cephalosporin vs penicillin drug combination | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Costs | 0 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 26.1 Single cephalosporin vs single penicillin | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Single cephalosporin vs penicillin drug combination | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 Cephalosporin drug combination vs single penicillin | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.4 Cephalosporin drug combination vs penicillin drug combination | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.9. Analysis.
Comparison 1 Cephalosporins versus penicillins ‐ all women, Outcome 9 Maternal composite serious infectious complication.
1.11. Analysis.
Comparison 1 Cephalosporins versus penicillins ‐ all women, Outcome 11 Maternal allergic reactions.
1.12. Analysis.
Comparison 1 Cephalosporins versus penicillins ‐ all women, Outcome 12 Maternal nausea.
1.14. Analysis.
Comparison 1 Cephalosporins versus penicillins ‐ all women, Outcome 14 Maternal diarrhoea.
1.21. Analysis.
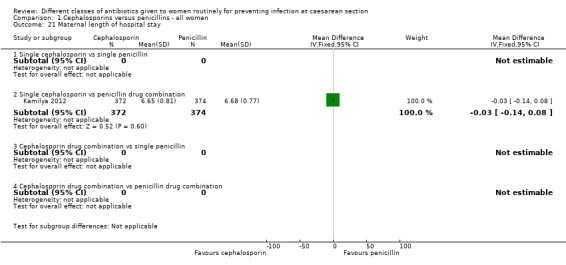
Comparison 1 Cephalosporins versus penicillins ‐ all women, Outcome 21 Maternal length of hospital stay.
Comparison 2. Cephalosporins versus penicillins ‐ by type of caesarean.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal sepsis | 4 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.47, 18.10] |
| 1.1 Elective CS | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Non‐elective CS | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.10, 56.41] |
| 1.3 Both elective and non‐elective, or undefined CS | 3 | 578 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [0.34, 30.45] |
| 2 Maternal endometritis | 20 | 5390 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.90, 1.37] |
| 2.1 Elective CS | 3 | 461 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.66, 6.39] |
| 2.2 Non‐elective CS | 6 | 2362 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.01, 1.75] |
| 2.3 Both elective and non‐elective, or undefined CS | 11 | 2567 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.60, 1.19] |
| 3 Infant sepsis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Elective CS | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Non‐elective CS | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Both elective and non‐elective, or undefined CS | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Infant oral thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Elective CS | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Non‐elective CS | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Both elective and non‐elective, or undefined CS | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Cephalosporins versus penicillins ‐ by timing of administration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal sepsis | 4 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.47, 18.10] |
| 1.1 Before cord clamping | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 After cord clamping | 2 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.10, 56.41] |
| 1.3 Timing of administration not reported or both used | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [0.34, 30.45] |
| 2 Maternal endometritis | 20 | 5390 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.90, 1.37] |
| 2.1 Before cord clamping | 2 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.02, 8.20] |
| 2.2 After cord clamping | 17 | 4854 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.94, 1.42] |
| 2.3 Timing of administration nor reported | 1 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.37, 2.48] |
| 3 Infant sepsis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Before cord clamping | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 After cord clamping | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Timing of administration nor reported | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Infant oral thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Before cord clamping | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 After cord clamping | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Timing of administration nor reported | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Cephalosporins versus penicillins ‐ by route of administration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal sepsis | 4 | 653 | Risk Ratio (M‐H, Random, 95% CI) | 2.90 [0.46, 18.17] |
| 1.1 Intravenous administration | 3 | 366 | Risk Ratio (M‐H, Random, 95% CI) | 2.90 [0.46, 18.17] |
| 1.2 Lavage/infiltration | 1 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Route of administration not specified | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Maternal endometritis | 20 | 5390 | Risk Ratio (IV, Fixed, 95% CI) | 1.12 [0.92, 1.37] |
| 2.1 Intravenous administration | 18 | 4899 | Risk Ratio (IV, Fixed, 95% CI) | 1.18 [0.94, 1.49] |
| 2.2 Lavage/infiltration | 2 | 491 | Risk Ratio (IV, Fixed, 95% CI) | 0.96 [0.65, 1.43] |
| 2.3 Route of administration not specified | 0 | 0 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Infant sepsis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Intravenous administration | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Lavage/infiltration | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Route of administration not specified | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Infant oral thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Intravenous administration | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Lavage/infiltration | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Route of administration not specified | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Cephalosporin (1st generation B1) versus penicillins (extended spectrum A3).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal sepsis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Maternal endometritis | 2 | 814 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [1.30, 3.66] |
| 3 Infant sepsis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Infant oral thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal fever (febrile morbidity) | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [0.84, 6.62] |
| 6 Maternal wound infection | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.42, 9.63] |
| 7 Maternal urinary tract infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Maternal thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal composite serious infectious complication | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal composite adverse effects | 1 | 675 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal allergic reactions | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal nausea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal vomiting | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Maternal diarrhoea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Maternal skin rash | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Immediate infant adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Infant unsettled | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Infant diarrhoea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Infant skin rash | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Development of bacterial resistance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Maternal length of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Infant length of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Infant's immune system development | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Infant general health | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Infant frequency of hospital visits | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Costs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.10. Analysis.
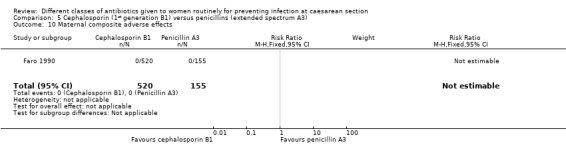
Comparison 5 Cephalosporin (1st generation B1) versus penicillins (extended spectrum A3), Outcome 10 Maternal composite adverse effects.
Comparison 6. Cephalosporin (1st generation B1) versus penicillins (ampicillin A4).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal sepsis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Maternal endometritis | 7 | 1487 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.69, 1.71] |
| 3 Infant sepsis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Infant oral thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal fever (febrile morbidity) | 5 | 883 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.40, 1.51] |
| 6 Maternal wound infection | 5 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.36, 2.01] |
| 7 Maternal urinary tract infection | 5 | 626 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.54, 3.70] |
| 8 Maternal thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal composite serious infectious complication | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal composite adverse effects | 2 | 861 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.84] |
| 11 Maternal allergic reactions | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal nausea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal vomiting | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Maternal diarrhoea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Maternal skin rash | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Immediate infant adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Infant unsettled | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Infant diarrhoea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Infant skin rash | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Development of bacterial resistance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Maternal length of hospital stay | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐2.46, ‐0.54] |
| 22 Infant length of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Infant's immune system development | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Infant general health | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Infant frequency of hospital visits | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Costs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.11. Analysis.
Comparison 6 Cephalosporin (1st generation B1) versus penicillins (ampicillin A4), Outcome 11 Maternal allergic reactions.
6.15. Analysis.
Comparison 6 Cephalosporin (1st generation B1) versus penicillins (ampicillin A4), Outcome 15 Maternal skin rash.
Comparison 7. Cephalosporin (2nd generation B2) versus penicillins (extended‐spectrum A3).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal sepsis | 1 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Maternal endometritis | 4 | 1334 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.78, 1.54] |
| 3 Infant sepsis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Infant oral thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal fever (febrile morbidity) | 4 | 850 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.79, 1.47] |
| 6 Maternal wound infection | 2 | 438 | Risk Ratio (M‐H, Random, 95% CI) | 2.37 [0.64, 8.73] |
| 7 Maternal urinary tract infection | 3 | 567 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.67, 3.07] |
| 8 Maternal thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal composite serious infectious complication | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal composite adverse effects | 2 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 21.96] |
| 11 Maternal allergic reactions | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal nausea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal vomiting | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Maternal diarrhoea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Maternal skin rash | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [0.11, 64.96] |
| 16 Immediate infant adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Infant unsettled | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Infant diarrhoea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Infant skin rash | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Development of bacterial resistance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Maternal length of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Infant length of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Infant's immune system development | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Infant general health | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Infant frequency of hospital visits | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Costs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
7.1. Analysis.
Comparison 7 Cephalosporin (2nd generation B2) versus penicillins (extended‐spectrum A3), Outcome 1 Maternal sepsis.
Comparison 8. Cephalosporin (2nd generation B2) versus penicillins (ampicillin A4).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal sepsis | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.10, 56.41] |
| 2 Maternal endometritis | 8 | 1890 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.75, 1.35] |
| 3 Infant sepsis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Infant oral thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal fever (febrile morbidity) | 3 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.64, 2.15] |
| 6 Maternal wound infection | 5 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.47, 2.78] |
| 7 Maternal urinary tract infection | 4 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.11, 3.66] |
| 8 Maternal thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal composite serious infectious complication | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal composite adverse effects | 3 | 1130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.18, 20.82] |
| 11 Maternal allergic reactions | 1 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal nausea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal vomiting | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Maternal diarrhoea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Maternal skin rash | 2 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.23, 4.46] |
| 16 Immediate infant adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Infant unsettled | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Infant diarrhoea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Infant skin rash | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Development of bacterial resistance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Maternal length of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Infant length of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Infant's immune system development | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Infant general health | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Infant frequency of hospital visits | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Costs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
8.11. Analysis.
Comparison 8 Cephalosporin (2nd generation B2) versus penicillins (ampicillin A4), Outcome 11 Maternal allergic reactions.
Comparison 9. Cephalosporin (3rd generation B3) versus penicillins (extended‐spectrum A3).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal sepsis | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Maternal endometritis | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.14, 4.00] |
| 3 Infant sepsis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Infant oral thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal fever (febrile morbidity) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Maternal wound infection | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal urinary tract infection | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Maternal thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal composite serious infectious complication | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal composite adverse effects | 2 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal allergic reactions | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal nausea | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal vomiting | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Maternal diarrhoea | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Maternal skin rash | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Immediate infant adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Infant unsettled | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Infant diarrhoea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Infant skin rash | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Development of bacterial resistance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Maternal length of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Infant length of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Infant's immune system development | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Infant general health | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Infant frequency of hospital visits | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Costs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
9.1. Analysis.
Comparison 9 Cephalosporin (3rd generation B3) versus penicillins (extended‐spectrum A3), Outcome 1 Maternal sepsis.
9.6. Analysis.
Comparison 9 Cephalosporin (3rd generation B3) versus penicillins (extended‐spectrum A3), Outcome 6 Maternal wound infection.
9.7. Analysis.
Comparison 9 Cephalosporin (3rd generation B3) versus penicillins (extended‐spectrum A3), Outcome 7 Maternal urinary tract infection.
9.9. Analysis.
Comparison 9 Cephalosporin (3rd generation B3) versus penicillins (extended‐spectrum A3), Outcome 9 Maternal composite serious infectious complication.
9.10. Analysis.
Comparison 9 Cephalosporin (3rd generation B3) versus penicillins (extended‐spectrum A3), Outcome 10 Maternal composite adverse effects.
9.11. Analysis.
Comparison 9 Cephalosporin (3rd generation B3) versus penicillins (extended‐spectrum A3), Outcome 11 Maternal allergic reactions.
9.12. Analysis.
Comparison 9 Cephalosporin (3rd generation B3) versus penicillins (extended‐spectrum A3), Outcome 12 Maternal nausea.
9.13. Analysis.
Comparison 9 Cephalosporin (3rd generation B3) versus penicillins (extended‐spectrum A3), Outcome 13 Maternal vomiting.
9.14. Analysis.
Comparison 9 Cephalosporin (3rd generation B3) versus penicillins (extended‐spectrum A3), Outcome 14 Maternal diarrhoea.
9.15. Analysis.
Comparison 9 Cephalosporin (3rd generation B3) versus penicillins (extended‐spectrum A3), Outcome 15 Maternal skin rash.
Comparison 10. Cephalosporin (3rd generation B3) versus penicillins (ampicillins A4).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal sepsis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Maternal endometritis | 5 | 1472 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.89, 2.42] |
| 3 Infant sepsis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Infant oral thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal fever (febrile morbidity) | 3 | 1060 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.69, 1.83] |
| 6 Maternal wound infection | 6 | 1556 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.27, 0.90] |
| 7 Maternal urinary tract infection | 2 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.10, 2.80] |
| 8 Maternal thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal composite serious infectious complication | 1 | 746 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal composite adverse effects | 2 | 1039 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal allergic reactions | 1 | 746 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal nausea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal vomiting | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.37, 133.78] |
| 14 Maternal diarrhoea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Maternal skin rash | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.77] |
| 16 Immediate infant adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Infant unsettled | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Infant diarrhoea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Infant skin rash | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Development of bacterial resistance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Maternal length of hospital stay | 1 | 746 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.14, 0.08] |
| 22 Infant length of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Infant's immune system development | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Infant general health | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Infant frequency of hospital visits | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Costs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
10.9. Analysis.
Comparison 10 Cephalosporin (3rd generation B3) versus penicillins (ampicillins A4), Outcome 9 Maternal composite serious infectious complication.
10.10. Analysis.
Comparison 10 Cephalosporin (3rd generation B3) versus penicillins (ampicillins A4), Outcome 10 Maternal composite adverse effects.
10.11. Analysis.
Comparison 10 Cephalosporin (3rd generation B3) versus penicillins (ampicillins A4), Outcome 11 Maternal allergic reactions.
Comparison 11. Fluoroquinolones (C) vs penicillins (A).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal sepsis | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [0.11, 60.57] |
| 2 Maternal endometritis | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.68, 2.01] |
| 3 Infant sepsis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Infant oral thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal fever (febrile morbidity) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Maternal wound infection | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.25 [0.21, 85.51] |
| 7 Maternal urinary tract infection | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.69] |
| 8 Maternal thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal composite serious infectious complication | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal composite adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal allergic reactions | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal nausea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal vomiting | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Maternal diarrhoea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Maternal skin rash | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Immediate infant adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Infant unsettled | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Infant diarrhoea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Infant skin rash | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Development of bacterial resistance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Maternal length of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Infant length of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Infant's immune system development | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Infant general health | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Infant frequency of hospital visits | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Costs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 12. Fluoroquinolones (C) vs cephalosporins (B).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal sepsis | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.07, 16.63] |
| 2 Maternal endometritis | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.76, 2.19] |
| 3 Infant sepsis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Infant oral thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal fever (febrile morbidity) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Maternal wound infection | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [0.20, 22.82] |
| 7 Maternal urinary tract infection | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Maternal thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal composite serious infectious complication | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal composite adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal allergic reactions | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal nausea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal vomiting | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Maternal diarrhoea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Maternal skin rash | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Immediate infant adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Infant unsettled | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Infant diarrhoea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Infant skin rash | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Development of bacterial resistance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Maternal length of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Infant length of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Infant's immune system development | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Infant general health | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Infant frequency of hospital visits | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Costs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
12.6. Analysis.
Comparison 12 Fluoroquinolones (C) vs cephalosporins (B), Outcome 6 Maternal wound infection.
Comparison 13. Other antibiotic regimens (D to I) versus penicillins (A).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal sepsis | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Maternal endometritis | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.35, 6.15] |
| 2.1 Lincosamide (H) + aminoglycoside (G) vs penicillins (A) | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.35, 6.15] |
| 3 Infant sepsis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Lincosamide (H) + aminoglycoside (G) vs penicillins (A) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Infant oral thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Lincosamide (H) + aminoglycoside (G) vs penicillins (A) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal fever (febrile morbidity) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Lincosamide (H) + aminoglycoside (G) vs penicillins (A) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Maternal wound infection | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.11, 2.84] |
| 6.1 Lincosamide (H) + aminoglycoside (G) vs penicillins (A) | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.11, 2.84] |
| 7 Maternal urinary tract infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Maternal thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal composite serious infectious complication | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal composite adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal allergic reactions | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal nausea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal vomiting | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Maternal diarrhoea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Maternal skin rash | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Immediate infant adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Infant unsettled | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Infant diarrhoea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Infant skin rash | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Development of bacterial resistance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Maternal length of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Infant length of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Infant's immune system development | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Infant general health | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Infant frequency of hospital visits | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Costs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 Lincosamide (H) + aminoglycoside (G) vs penicillin (A) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 14. Other antibiotic regimens (D to I) versus cephalosporins (B).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal sepsis | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Maternal endometritis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Beta‐lactams (F) versus cephalosporins (B) | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.08, 17.82] |
| 3 Infant sepsis | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Infant oral thrush | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal fever (febrile morbidity) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Macrolides (E) versus cephalosporins (B) | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.37, 130.69] |
| 5.2 Beta‐lactams (F) versus cephalosporins (B) | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.06, 6.09] |
| 6 Maternal wound infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Beta‐lactams (F) versus cephalosporins (B) | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.02, 9.15] |
| 7 Maternal urinary tract infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Beta‐lactams (F) versus cephalosporins (B) | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Maternal thrush | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal composite serious infectious complication | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal composite adverse effects | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal allergic reactions | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal nausea | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal vomiting | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Maternal diarrhoea | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Maternal skin rash | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Immediate infant adverse effects | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Infant unsettled | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Infant diarrhoea | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Infant skin rash | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Development of bacterial resistance | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Maternal length of hospital stay | 0 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Infant length of hospital stay | 0 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 22.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Infant's immune system development | 0 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 23.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Infant general health | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 24.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Infant frequency of hospital visits | 0 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 25.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Costs | 0 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 26.1 Macrolides (E) versus cephalosporins (B) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Beta‐lactams (F) versus cephalosporins (B) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
14.7. Analysis.
Comparison 14 Other antibiotic regimens (D to I) versus cephalosporins (B), Outcome 7 Maternal urinary tract infection.
Comparison 15. Other antibiotic regimens versus different antibiotic regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal sepsis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Maternal endometritis | 1 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.29, 2.26] |
| 2.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 1 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.29, 2.26] |
| 3 Infant sepsis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Infant oral thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal fever (febrile morbidity) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Maternal wound infection | 1 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.23 [0.34, 30.64] |
| 6.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 1 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.23 [0.34, 30.64] |
| 7 Maternal urinary tract infection | 1 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.07, 17.03] |
| 7.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 1 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.07, 17.03] |
| 8 Maternal thrush | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal composite serious infectious complication | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Maternal composite adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal allergic reactions | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal nausea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal vomiting | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Maternal diarrhoea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Maternal skin rash | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Immediate infant adverse effects | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Infant unsettled | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Infant diarrhoea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Infant skin rash | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Development of bacterial resistance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Maternal length of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Infant length of hospital stay | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Infant's immune system development | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Infant general health | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Infant frequency of hospital visits | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Costs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Stillbirth (not‐prespecified) | 1 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.38] |
| 27.1 Aminoglycoside (G) + nitroimidazole (I) vs standard regimen | 1 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.38] |
15.27. Analysis.
Comparison 15 Other antibiotic regimens versus different antibiotic regimens, Outcome 27 Stillbirth (not‐prespecified).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmed 2004.
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B3).
Comparison: penicillin (A4) combination.
|
|
| Outcomes | Post‐operative febrile morbidity; post‐operative infection; endometritis; wound infection; pelvic abscess; peritonitis; other febrile morbidity. | |
| Notes |
Dates: January to June 2001 Setting: Wad Medani Teaching Hospital, Central Sudan. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...were randomised...” |
| Allocation concealment (selection bias) | Unclear risk | “...were randomised...” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and the drug regimens were different, one a single dose, the other 3 doses. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol. |
| Other bias | Unclear risk | No statistical differences in admission variables between the two groups. Data and P values provided on temperature, weight, gestational age, pre‐operative Hb. However, other aspects of bias unclear. · "The drugs were donated by Alhikma Company, Wad Medani, Sudan." but it seems unclear whether this might give the company any influence or not. |
Beningo 1986.
| Methods | RCT. Individual women. Multi‐centre (6 centres). 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B2).
Comparison: penicillin (A3).
|
|
| Outcomes | Satisfactory prophylactic response; febrile morbidity (temperature > 38 ºC x 2 occasions, 6 hours apart, not included first 24 hours post‐operation; wound infection). | |
| Notes |
Setting: Women from hospitals and universities of San Francisco, Atlanta, Memphis, Los Angeles, Phoenix, New York. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “...a computer‐generated randomization schedule...” |
| Allocation concealment (selection bias) | Low risk | “...a computer‐generated randomization schedule maintained by each hospital pharmacy...” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The investigator and his (or her) staff were blinded as to antibiotic assignment. The code was not broken by the investigator until the last patient had been evaluated for prophylactic response.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | · “The investigator and his (or her) staff were blinded as to antibiotic assignment. The code was not broken by the investigator until the last patient had been evaluated for prophylactic response.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Excluded after randomisation: cephalosporin group 30/177 (16.9%) and penicillin group 33/119 (19.5%). Also differential loss from two groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol. |
| Other bias | Unclear risk | Study not stopped early; similar baseline characteristics for weight; height & race, but significant difference in mean age ‐ though not considered important. Other aspects of bias were unclear. No information on funding source of study. |
Bracero 1997.
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B2).
Comparison: penicillin (A4) combination.
|
|
| Outcomes | Treatment success; incision site infection; endometritis; UTI; febrile morbidity; peak recorded temperature; days in hospital. | |
| Notes |
Setting: Westchester County Medical Center, US. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A computer was used to generate a list of random numbers for two groups.” |
| Allocation concealment (selection bias) | Low risk | “Treatment assignments were placed in numbered, sealed and opaque envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Neither patient nor obstetrician was informed of the antibiotic assignment. The study drugs were administered by the anaesthesiologist in the operating room immediately after the umbilical cord was clamped” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | “Neither patient nor obstetrician was informed of the antibiotic assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 26/196 (13%) women were excluded because of protocol violations. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | High risk | "This work was supported by a grant (89‐S‐0591, R‐0102) from The Reorig Division of Pfizer Inc." Not stopped early; no imbalance in baseline characteristics assessed on: on age; race; weight; height; BP; temperature and pulse but other aspects of bias were unclear. |
Busowski 2000.
| Methods | RCT. Individual women. 3‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B2).
Comparison 1: penicillin (A4) combination
Comparison 2
|
|
| Outcomes | Endometritis; pneumonia; bacteraemia; UTI; would infection; postpartum stay > 6 days. | |
| Notes |
Setting: Tampa General Hospital, US. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “... prospectively randomised...” |
| Allocation concealment (selection bias) | Unclear risk | “... prospectively randomised...” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | · “Investigators were blinded to treatment.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | · “Investigators were blinded to treatment.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not asses trial protocol. |
| Other bias | Unclear risk | Baseline characteristics similar for: primaparous; weight; gestation; race; repeat CS; Hb but there was a statistically significant difference in age considered not to be clinically important. Other aspects of bias were unclear. No information about funding source of study. |
Chantharojwong 1993.
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B1).
Comparison: penicillin (A4).
|
|
| Outcomes | Febrile morbidity; endometritis; parametritis; UTI; wound infection. | |
| Notes |
Dates: 1st January 1990 to 31 December 1992. Setting: Inburi Hospital, Thailand. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “... assigned randomly...” |
| Allocation concealment (selection bias) | Unclear risk | “... assigned randomly...” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 3 women excluded. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Baseline characteristics similar between groups on: age; height; weight; gravidity; gestation; preoperative haematocrit. However, other aspects of bias unclear. No information about funding source of study. |
Dashow 1986.
| Methods | RCT. Individual women. 5‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention 1: cephalosporin (B1).
Intervention 2: cephalosporin (B2).
Comparison: penicillin (A4).
4th group ‐ moxalactam disodium (1‐oxa‐beta‐lactam antibiotic: N = 64 in Table 4 (but 79 in Table 2). 5th group ‐ placebo (N = 77). Vitamin added to each solution for disguise. |
|
| Outcomes | Endometritis (temperature > 37.8 ºC, uterine tenderness, pelvic irritation without other localizing signs); wound infection (breakdown, positive culture and/or cellulitis): febrile morbidity (temperature > 100.4 x 2. 6 hour apart, excluded first 24 hours); mean duration of hospital stay. | |
| Notes |
Dates: 1 December 1982 to 31 May 1984. Setting: Madigan Army Medical Center, US. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A computer‐generated table of pseudo‐random numbers using the mixed congruential method was used by the pharmacy to assign each patient to one of five groups.” |
| Allocation concealment (selection bias) | Low risk | “A computer‐generated table of pseudo‐random numbers using the mixed congruential method was used by the pharmacy to assign each patient to one of five groups.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Patients and physicians were unaware of the group assignment until after completion of the study...” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Patients and physicians were unaware of the group assignment until after completion of the study...” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No explanation for differences between the numbers of the initially randomised groups and the groups included in the morbidity analysis (cephapirin 79 vs 70, cefamandole 70 vs 64, moxalactam 64 vs 79. The total of women included is the same (360); therefore we can assume that women originally assigned to one group received other treatment and they were not analysed by intention to treat. The uneven numbers may be due to lack of block‐randomisation. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol. |
| Other bias | Unclear risk | Baseline characteristics similar on: age; parity; gestation. Small difference on gravidity but not considered clinically important. However, other aspects of bias unclear. No information about funding source of study. |
De‐Lalla 1988.
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B2).
Comparison: penicillin (A3).
|
|
| Outcomes | Fever > 38 ºC; endometritis; wound infection; UTIs; asymptomatic bacteriuria. | |
| Notes |
Setting: Obstetric and Gynecologic department of UCSC, Como, Italy. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | No information provided. Also no information about funding source of study. |
Faro 1990.
| Methods | RCT. Individual women. 10‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Interventions: cephalosporins (B1, B2, B3).
Total N = 1277. Comparisons: penicillins (A3, A4).
Total N = 303. |
|
| Outcomes | Endometritis (defined as temperature > 37.8 ºC x 2, 4 hours apart, excluding 24 hours after delivery plus tachycardia, white blood count > 14, uterine tenderness). | |
| Notes |
Dates: 7 December 1989 to 1 July 1989. Setting: Harris County Hospital, US. Mixed ethnic population. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “...randomised...according to a computer‐generated schedule.” |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Prospective, open, randomised study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Prospective, open, randomised study but assessors may have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No loss to follow‐up as yet but study still on‐going at time of publication. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol. |
| Other bias | Unclear risk | The difference in the numbers allocated to groups (ranging from 142 to 217) is reported as likely to be due to the study being on‐going and the randomisation schedule not complete or to some statistical issue, this si unclear. No information on funding source of study. |
Ford 1986.
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B2).
Comparison: penicillin (A3).
|
|
| Outcomes | Febrile morbidity; duration of hospitalisation; administration of systematic antibiotics post‐operatively; wound healing; infection at operation site. | |
| Notes |
Setting: UCLA Medical Center, US. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...randomly assigned...”. |
| Allocation concealment (selection bias) | Unclear risk | “...randomly assigned...”. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There appeared to be no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol. |
| Other bias | Unclear risk | No baseline imbalances on: age; weight; duration of surgery. However, other aspects of possible bias were unclear. No information on funding source of study. |
Gidiri 2014.
| Methods | RCT. 2‐arm parallel study. Women randomised individually. | |
| Participants |
Inclusion criteria
Exclusion criteria Women who declined to participate in the study; severe immunosuppression of any cause; stage 3 and 4 HIV infection; prolonged rupture of membranes more than 12 hours; surgery longer than 3 hours; chorioamnionitis diagnosed preoperatively and obvious concurrent infection that requires therapeutic antibiotics. |
|
| Interventions |
Intervention: Cephalosporin (B3) and metronidazole (I) combination.
Comparison: Penicillin (A1) + chloramphenicol + amoxicillin (A4) + metronidazole (I) combination.
|
|
| Outcomes | Pyrexia; admission with puerperal sepsis; wound sepsis; death; duration of hospital stay; laparotomy for pelvic abscess. | |
| Notes |
Dates: 2 February 2012 to 30 May 2012. Setting: Parirenyatwa and Harare (tertiary) hospitals, Zimbabwe. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Low risk | Randomisation was by taking a ticket from a box. There was an A4 envelope of tickets at each of the two maternity units. Each envelope contained 75 tickets marked Arm 1 and 75 marked Arm 2. The ticket for Arm 1 was identical to that of Arm 2 in size, shape and material. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not really possible to blind as one group had a single dose and the other had a weeks prescription. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information so most likely assessors were not blinded either. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Women lost to follow‐up were 24 in each group. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Baseline data showed no imbalance on: age; marital status; education; occupation; booking status; HIV. Otherwise unclear. Authors report 'No conflict of interest' and they alone are responsible for the content and writing of the paper. So appears to have no drug company involvement. |
Graham 1993.
| Methods | RCT. Individual women. Multi‐centre. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B1).
Comparison: penicillin (A4).
|
|
| Outcomes | Genital tract cultures. | |
| Notes |
Setting: University Medical Centre, Lubbock & LBJ General Hospital, Houston, Texas, US. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer‐generated number..." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Antibiotic administration was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Antibiotic administration was not blinded but outcome assessor could have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions of women were reported. |
| Selective reporting (reporting bias) | Unclear risk | We did not asses trial protocol. |
| Other bias | Unclear risk | Baseline characteristics across groups not reported and other aspects of possible bias unclear. No information about funding source of study. |
Jyothi 2010.
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B1).
Comparison: penicillin (A4) combination.
|
|
| Outcomes |
Outcomes: fever and infection, endometritis. Reported outcomes: postoperative hospital stay, wound infection, asymptomatic bacteriuria, total infection, postoperative urinary infection. |
|
| Notes |
Setting: Kasturba Hospital, Manipal, Karnataka, India, April 2004 to September 2005. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of follow‐up was reported. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is unavailable. |
| Other bias | Unclear risk | Baseline characteristics were similar for: age; BMI; associated disease; type of surgery (primary CS or not). Other possible biases were unclear. No information about funding source for study. |
Kamilya 2012.
| Methods | RCT two parallel treatment groups, women randomised individually. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B3).
Comparison: penicillin (A4) combination.
|
|
| Outcomes | Febrile morbidity, wound healing, endometritis, side effects of antibiotics. Reported outcomes: “fever, mild or moderate wound infection, endometritis, UTI or any serious infection, fever in the 5th post‐operative period, adverse reactions, duration of hospital stay. ” |
|
| Notes |
Setting Tertiary care teaching hospital in Kolkata, India. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was done a priori by computer in blocks of 40.” |
| Allocation concealment (selection bias) | Unclear risk | "The randomization list remained in the custody of the principal investigator." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Being a double blind study, the nature or medication being received by individual trial subjects was not known to the subject or the project clinician”. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Being a double blind study, the nature or medication being received by individual trial subjects was not known to the subject or the project clinician”. It seemed most likely considering the outcomes assessed that assessors were blinded as well. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “A total of 760 patients were recruited for the study. Eight patients in the cefotaxime group and six patients in the amoxicillin–clavulanic acid group had to be excluded from final analysis for various reasons”. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is unavailable. |
| Other bias | Unclear risk | Baseline characteristics were similar for: age; parity; gestation. Other possible biases were unclear. No information about funding source of trial. |
Kayihura 2003.
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: aminoglycoside (G) + nitroimidazole (I) combination.
Comparison: penicillins (A1) + nitroimidazole (I) + macrolide (E) combination.
|
|
| Outcomes | Maternal endometritis; would infection; UTI; peritonitis; evisceration; post‐operative infection; stillbirth. | |
| Notes |
Dates: January to June 2000. Setting: Maternity Unit, Hospital Central de Maputo, Mozambique. Quaternary level care. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...randomly constituted...”. |
| Allocation concealment (selection bias) | Unclear risk | “The anaesthetist administered the antibiotics according to the code written in the exercise book in the sequential order of admission to the theatre...the code was then written on the patient’s file so that the surgeon could prescribe...the doctor on duty was not aware of the group to which the patient was allocated. The principle investigator was not allowed either to select cases to be enrolled in the study or to follow the patients in the ward.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded comparative study. Although the doctor on duty was reported as not aware of the group to which the woman was allocated, the anaesthetist and surgeon were aware and overall the study was described as not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded comparative study. “Medical doctors allowing the patients to leave the maternity ward knew the regimen followed by the patients in order to not modify the antibiotics given…”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 288 women randomised and analysis on 241 so 47/288 (16.3%) loss. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol. |
| Other bias | Unclear risk | Baseline characteristics similar for:age; parity; gestation. However, other possible biases are unclear. No information about funding source of study. |
Koppel 1992.
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B3).
Comparison: penicillin (A4) combination.
|
|
| Outcomes | Endometritis (temperature > 37.5 ºC, uterine tenderness); UTI; wound infection. | |
| Notes |
Dates: 17 October 1987 to 24 February 1989. Setting: Kantonspital Hospital, Winterthur, Switzerland. Translation: from German. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The translation reports: "A midwife not involved in the study pulled the names from a randomised list and provided the medications in neutral syringes in the operating room." It is unclear if a 'randomised list', is the same as a 'random table' and until we are able to check this we are reporting this as unclear. |
| Allocation concealment (selection bias) | Unclear risk | A midwife who was not part of the study divided the women into 2 treatment groups, cefotaxim and amoxicillin/clavulanic acid, according to a randomised list. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol. |
| Other bias | Unclear risk | Baseline characteristics similar for age. However, other possible biases unclear. No information about funding source of study. |
Lehapa 1999.
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B3).
Comparison: penicillin (A4).
|
|
| Outcomes | Abdominal and/or wound sepsis; febrile morbidity; hospital stay; antibiotic and consumable costs. | |
| Notes |
Setting: Ga‐Rankuwa Hospital, South Africa. Subgroups
Conference abstract only. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind study although it is unclear whether assessors might have known allocation of not. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None reported, but there is no information on the denominators in either group for us to be sure there was no loss of participants. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol. |
| Other bias | Unclear risk | Two groups reported as similar in baseline characteristics on: age; gestation; type of incision; length of surgery, but no data provided. Other aspects of potential bias were unclear. No information about funding source of study. |
Lewis 1990.
| Methods | RCT. Individual women. Study in 2 parts. Study 1: compared a penicillin vs placebo (so not included in this review). Study 2: compared a cephalosporin vs a penicillin. |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B2).
Comparison: penicillin (A3).
|
|
| Outcomes | Endometritis; wound infection (criteria not specified): UTI (criteria not specified): sepsis. | |
| Notes |
Setting: Louisiana State University Hospital, US. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...random double‐blind fashion...”. |
| Allocation concealment (selection bias) | Unclear risk | “...random double‐blind fashion...”. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although a double‐blind study, it is unclear whether the outcome assessors might have not know allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 396 women were reported to be included with 383 providing data, 186 in cephalosporin group and 197 in penicillin group (loss of 13/396 = 3.3%). |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol. |
| Other bias | Unclear risk | Baseline characteristics were similar for age; gestation; gravidity; parity; length of labour. However, other potential biases were unclear. No information about funding source of study. |
Louie 1982.
| Methods | RCT. Individual women. 3‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention 1: cephalosporin (B1).
Intervention 2: cephalosporin (B3).
Comparison: penicillin (A4).
|
|
| Outcomes | Endometritis (temperature > 38 ºC, foul lochia, uterine tenderness); UTI; wound infection; febrile morbidity (temp > 38 ºC x 2, 6 hours apart, excluding first 24 hours). | |
| Notes |
Dates: December 1979 to December 1981. Setting: Women's Centre at the Health Sciences Winnipeg, Canada. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...randomised...”. |
| Allocation concealment (selection bias) | Low risk | “..unlabelled but number‐coded, previously randomised vials...”. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Only pharmacist was aware of the drug code.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/195 (3.6%) lost to follow‐up. Similar across groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol. |
| Other bias | Unclear risk | Baseline characteristics similar for age and race. However, other possible biases were unclear. No information about funding source of study. |
Lumbiganon 1994.
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B1).
Comparison: penicillin (A4) combination.
|
|
| Outcomes | Febrile and infectious morbidity. | |
| Notes |
Setting: Department of Obstetric and Gynecology, Khon Kaen University, Thailand. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | Although the published abstract provided no information, the registration of the study with the Oxford Database of Perinatal Trials reported allocation was "by sealed, numbered envelopes" but it is unclear if these had to be used in sequential order. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | ‘Partially blinded’. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | ‘Partially blinded’. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported. |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol not available and trial registration form only asks for principle outcomes (febrile morbidity & infectious morbidity) both of which are reported in the Conference abstract. |
| Other bias | Unclear risk | No baseline data provided and other possible biases unclear. No information about funding source of study. |
Mansueto 1989.
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: B‐lactam (F).
Comparison: cephalosporin (B3).
|
|
| Outcomes | Infective complications after the CS (endometritis, infection of the wound, peritonitis, urinary infections, other causes, fever morbidity). | |
| Notes |
Dates: 1 January 1988 to 30 September 1988. Setting: Umberto I Hospital, Frosinone, Italy. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women reported to be divided randomly into two groups. |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol. |
| Other bias | Unclear risk | No differences in baseline characteristics for: age, parity, length of PROM and number of vaginal examination between two groups. Other possible biases were unclear. No information about funding source of study. |
Mivumbi 2014.
| Methods | A prospective, randomised, open‐label, single‐site study,with two parallel arms. Women randomised individually. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B1).
Comparison (usual care): aminopenicillin (A4).
|
|
| Outcomes |
Outcomes: The primary outcome variable was postoperative febrile morbidity. Secondary outcomes were infection‐related complications defined as endometritis, wound infection, UTI, fever with unexplained source, need for therapeutic antibiotics, and length of postoperative days in hospital (starting the day after surgery and including the day of discharge). Reported outcomes: Febrile morbidity, endometritis, wound infection, UTI, unexplained fever(febrile morbidity), required therapeutic antibiotics, length of postoperative stay, allergic reactions. |
|
| Notes |
Dates: March 1 to May 31, 2012. Setting: The Centre Hospitalier Universitaire de Kigali/University Teaching Hospital of Kigali (CHUK), which is located in Kigali, the capital of Rwanda, is 1 of 3 tertiary care referral hospitals in the Rwandan healthcare system and, compared with district hospitals, receives a disproportionate number of women needing CS. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The cards inside the envelopes were randomized by the principle investigator using a random integer generator." |
| Allocation concealment (selection bias) | Low risk | “The women were preoperatively randomized to 1 of 2 study groups via numerically ordered cards in sealed envelopes...The allocated envelopes were opened by clinicians only after the decision for cesarean delivery was made." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “...open‐label...”. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | As open‐label RCT the investigators were not blinded but the assessors could have been blinded. . |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "No women were lost to follow‐up”. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Unclear risk | Not known. Reported that authors have no conflict of interest. Funding source of study not reported. |
Mothilal 2013.
| Methods | A randomised prospective study with two parallel arms. Women randomised individually. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: macrolide (E).
Comparison: cephalosporin (B1).
|
|
| Outcomes | Reported outcomes: post‐operative fever, wound healing duration (healing within 10 days, healing within 20 days), pain for 6 days, pain for 7‐9 days, infection, PV discharge. | |
| Notes |
Study dates: September 2011 to February 2012. Follow‐up of the cases were finished in March 2012. Setting: Department of Obstetrics & Gynaecology, SRM Medical Research Centre and Hospital in Kattankulathur, Kancheepuram District, India. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The pregnant women were randomly given either Azithromycin or cefazolinas prophylactic antibiotics” and "(Antibiotics) were given ... in a random order ...”. |
| Allocation concealment (selection bias) | Unclear risk | "The pregnant women were randomly given either Azithromycin or Cefazolinas prophylactic antibiotics". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on this. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on this. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses of follow‐up were reported. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Unclear risk | Not known. No information about funding source of study. |
Ng 1992.
| Methods | RCT. Individual women. 3‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention 1: cephalosporin (B3).
Intervention 2: penicillin (A4).
Comparison
|
|
| Outcomes | Febrile morbidity; wound infection. | |
| Notes |
Dates: March to August 1991. Setting: Ipoh General Hospital, Ipoh, Perak Darul Ridzuan. Subgroups
Inconsistency in data. There was inconsistency in the number of women reported in each group between the table and the text. We took the data from the tables and will write to the authors to seek clarification. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A randomised trial. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women excluded ‐ 1 from each group. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | Baseline characteristics similar for: age; race; parity; gestation. Other possible biases were unclear. No information about funding source of study. |
Noyes 1998.
| Methods | RCT. Individual women. 3‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention 1: cephalosporin (B1).
Intervention 2: cephalosporin (B2).
Comparison: penicillin (A4) combination.
|
|
| Outcomes | Endometritis. | |
| Notes |
Dates: July 1988 to November 1990. Setting: New York Hospital, university‐based, US. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Prospective randomized trial”. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 out of 300 women (2.7%) were excluded from the analysis. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | There was insufficient information to assess other possible biases. No information about funding source of study. |
Parulekar 2001.
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion
Exclusion
|
|
| Interventions |
Intervention: cephalosporin (B3).
Comparison: penicillin (A2) + aminoglycoside (G) combination.
|
|
| Outcomes | Postpartum infection; wound infection; fever; duration in hospital. | |
| Notes |
Setting: Naval Hospital INHS Asvini Colaba, Mumbai, India. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned...". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up was reported. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol unavailable. |
| Other bias | Unclear risk | Insufficient information to assess other possible biases. No information about funding source of study. |
Rehu 1980.
| Methods | RCT. Individual women. 4 groups. | |
| Participants |
Inclusion
Exclusion
|
|
| Interventions |
Intervention 1: penicillin (A1).
Intervention 2: lincosamide antibiotic (H) + aminoglycoside (G).
Comparison 1: placebo 1 (not included in this review).
Comparison 2: placebo 2 (not included in this review).
|
|
| Outcomes | Endometritis; wound infection; duration of hospital stay; number of women receiving post‐operative treatment. | |
| Notes |
Dates: September 1977 and January 1978. Setting: State/maternity hospital, Helsinki, Finland. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...assigned at random...". |
| Allocation concealment (selection bias) | Unclear risk | Antibiotic preparations for intravenous use were supplied in solution in bottles carrying code numbers. Still unclear if there was allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The code was kept secret for persons performing the operations and observing the patients in the postoperative period." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The code was kept secret for persons performing the operations and observing the patients in the postoperative period". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two out of the 147 women receiving antibiotics of other reasons during the preoperative period were later excluded from the series. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. |
| Other bias | Unclear risk | There was no information on baseline characteristics of the women in the groups, and other possible biases were unclear. No information about funding source of study. |
Rosaschino 1988.
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B3).
Comparison: penicillin (A3).
|
|
| Outcomes | Tolerability, wound infections, urinary or respiratory infections, complications, side effects. | |
| Notes |
Setting: Bolognini di Seriate (BG) hospital, obstetric and gynaecological clinic, Italy. Translation: paper in Italian with summary in English. We had information extracted for us in English. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised controlled trial. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol unavailable. |
| Other bias | Unclear risk | Unable to assess other possible biases. No information about funding source of study. |
Saltzman 1985.
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B2).
Comparison: penicillin (A3) combination.
|
|
| Outcomes | Febrile morbidity; endometritis; UTI. | |
| Notes |
Setting: Virginia, US. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...randomly assigned...”. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind study but no mention about whether assessors were blinded or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18 out of 147 women (12.2%) were excluded from the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol unavailable. |
| Other bias | Unclear risk | No information on baseline characteristics. Other possible biases were unclear. No information about funding source of study. |
Saltzman 1986.
| Methods | RCT. Individual women. 3‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B2).
Comparison 1: penicillin (A3).
Comparison 2: penicillin (A3).
|
|
| Outcomes | Febrile morbidity (temperature > 38 x 2, 8 hours apart, excluding first 24 hours post‐operatively; endometritis (temperature > 38 plus foul lochia or uterine tenderness); wound infection (wound surrounded by cellulitis and/or draining purulent material); UTI. | |
| Notes |
Dates: October 1982 to April 1983. Setting: Virginia, US. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...randomly assigned...”. |
| Allocation concealment (selection bias) | Unclear risk | “...randomly assigned...”. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “...double‐blind study...”. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind study but no mention of whether assessors were blinded or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 out of 158 women (4.4%) were removed for failure to fulfil the study criteria. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was unavailable. |
| Other bias | Unclear risk | Baseline characteristics similar on: age; parity; gestation. However other aspects of potential bias were unclear. No information about funding source of study. |
Shah 1998.
| Methods | RCT. Individual women. 4‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B1) combination.
Comparison 1: penicillin (A3).
Comparison 2: penicillin (A3).
Comparison 3: Data not included in this review.
|
|
| Outcomes | Post‐operative febrile morbidity; metritis with pelvic cellulitis; wound infection. | |
| Notes |
Dates: January 1995 to mid‐1996. Setting: Abu‐Dhabi, United Arab Emirates. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reports women were randomised and no further details. |
| Allocation concealment (selection bias) | Low risk | ‘...consecutively numbered sealed envelopes...'. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 out of 198 women (5.6%) were excluded during the course of the study. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was unavailable. |
| Other bias | Unclear risk | No information on the baseline characteristics of women in each group. Also other aspects of possible bias were unclear. No information about funding source of study. |
Spinnato 2000.
| Methods | RCT. Individual women. 3‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B2).
Comparison 1: penicillin (A4).
Comparison 2: penicillin (A4) combination.
|
|
| Outcomes | Endomyometritis; wound complications. | |
| Notes |
Dates: 24 January 1994 to 12 December 1996. Setting: University of Louisville Hospital, US. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...patients were randomized...”. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “...double‐blinded...”. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blinded but unclear if the outcome assessors were blinded or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three out of 301 women (1.0%) were not evaluated due to incomplete information data that restricted chart retrieval. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was unavailable. |
| Other bias | Unclear risk | Baseline characteristics were similar on: age; gestational age; height and weight. Other possible biases were unclear. No information about funding source of study. |
van der Linden 1993.
| Methods | RCT. Individual women. 2‐arm study, stratified by type of operation, CS or hysterectomy. We only report on women having CS here. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B2) combination.
Comparison: penicillin (A4) combination.
|
|
| Outcomes | UTI; febrile temperature; abdominal wound infection; endometritis and infiltrates at the top of the vaginal vault. | |
| Notes |
Dates: 1 August 1988 to 15 December 1989. Setting: Leyenburg Hospital, Netherlands. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were “randomly allocated to one of two treatment regimes”. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16 out of 215 (7.4%) women were excluded from the analysis. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was unavailable. |
| Other bias | High risk | Baseline characteristics were similar on: age and weight. Other possible biases were unclear. The study was sponsored by Smith Kline and Beecham Pharmaceuticals. |
Voto 1986.
| Methods | RCT. Individual women. 2‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B2).
Comparison: penicillin (A4).
|
|
| Outcomes | Analyses of cultures at endocervix, skin and tissue and urine. | |
| Notes |
Setting: maternity ward, the Hospital Juan A. Fernandez, Buenos Aires, Argentina. Translation: from Portuguese. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Women were divided, at random, into two groups of which one was administered cefoxitin and the other ampicillin.” |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 80 women included (GpA = 39 and GpB = 40) authors only report data on GpA = 37 and GpB = 17. Suggest loss too high and very uneven, so groups are not randomised groups. We have not used data in the meta‐analyses. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was unavailable. |
| Other bias | Unclear risk | No information on baseline characteristics and other possible biases unclear. No information about funding source of study. |
Wells 1994.
| Methods | RCT. Individual women. 3‐arm study. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention 1: cephalosporin (B) + nitroimidazole (I).
Intervention 2: nitroimidazole (I).
Comparison: placebo.
|
|
| Outcomes | Temperature; wound infection; offensive lochia; UTI. | |
| Notes |
Setting: authors from Grey's Hospital, London, UK. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...were randomised...". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess trial protocol. |
| Other bias | Unclear risk | No baseline data and other aspects of possible bias were unclear. No information about funding source of study. |
Ziogos 2010.
| Methods | Prospective RCT ‐ 2 parallel arms ‐ women randomised individually. | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: cephalosporin (B2).
Comparison: penicillin (A4) combination.
|
|
| Outcomes |
Outcomes: The primary outcome was development of an infection either at the surgical site or elsewhere e.g. UTI. Endometritis, Reported outcomes: postoperative infections, surgical site infection (SSI), endometritis, Duration of hospitalisation in days median (IQR), Duration of hospitalisation post‐operatively in days median (IQR), adverse drug reactions. |
|
| Notes |
Study dates July 2004 to December 2008 Setting Major tertiary care hospital, Nikaia’s Regional General Hospital “Agios Panteleimon”, Athens, Greece. Subgroups
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Using a random‐number generator”. |
| Allocation concealment (selection bias) | Low risk | “The sequence was obtained using a central telephone number and it was concealed until interventions were assigned.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “Participants ... were blinded to the intervention, however the physician administering the intervention and assessing the outcomes was not.” |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | “Participants ... were blinded to the intervention, however the physician administering the intervention and assessing the outcomes was not.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of follow‐up were reported |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is unavailable. |
| Other bias | Unclear risk | Not known. Authors reported that had no competing interests. |
BP: blood pressure CI: confidence interval CS: caesarean section Hb: haemoglobin IM: intramuscular IV: intravenous PROM: premature rupture of membranes RCT: randomised controlled trial RR: risk ratio t.d.s.: three times daily UTI: urinary tract infection vs: versus
A. Penicillins
A1. Natural penicillins A2. Penicillinase‐resistant penicillins A3. Extended‐spectrum penicillins A4. Aminopenicillins
B. Cephalosporins
B1. First generation cephalosporins B2. Second generation cephalosporins B3. Third generation cephalosporins B4. Fourth generation cephalosporins
C. Fluoroquinolones
D. Tetracyclines
E. Macrolides
F. Beta‐lactams/carbapenems
G. Aminoglycosides
H. Lincosamides
I. Nitroimidazoles
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andrews 2003 | Study compared 2 different 2nd generation cephalosporins. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Baheraie 1997 | Compares single vs multiple doses of same class of antibiotic (cephalosporin). Study will be considered in the review ‘Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section’. |
| Beksac 1989 | Quasi‐RCT ‐ "...randomly assigned according to the last number of her hospital notes..." |
| Berkeley 1990 | Study compared a cephalosporin (cefotaxime) by 2 different routes of administration, IV and lavage. |
| Bernstein 1994 | Study compared 2 different cephalosporins, cefotetan versus cefoxitin. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Bilgin 1998 | Quasi‐RCT, allocated women to groups according to last digit of hospital number. |
| Boothby 1984 | Study compared a cephalosporin (cefoxitin) by 2 different routes of administration, IV and lavage. |
| Carlson 1990 | Study compared 2 different cephalosporins, cefazolin versus cefotetan. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Chamberlain 1993 | Study compared ampicillins plus sulbactam versus ampicillin alone. Study will be considered in the review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Chittacharoen 1998 | Study compared 2 different ampicillins (augmentin vs ampicillin). Study will be considered in the review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Conover 1984 | Study compared a cephalosporin (cefoxitin) by 2 different routes of administration, IV and irrigation. |
| Crombleholme 1987 | Study compared 2 versus 3 doses of a penicillin (mezlocillin). Study will be considered in review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Crombleholme 1989 | Study compared 2 different 2nd generation cephalosporins. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Cunningham 1983 | Study compared 2 differing timings of giving the prophylactic antibiotics, before and after cord clamping. |
| D'Angelo 1980 | Comparison of short‐ versus long‐course prophylactic antibiotic treatment. Authors do not list dose of drug at time of first administration, nor do they indicate the time of administration (pre‐operative, cord clamp). The authors are not even clear about the identity of the drug which begins the prophylactic regimen. They state that it is a random study but provide no details of mechanism. |
| De Palma 1980 | At the start of the study 2 arms: 1 a no treatment arm, the other composed of women given either cefamandole or penicillin plus gentamicin. It would have been possible to try and dissect important information from the study except that they changed the antibiotic regimen after treating 57/105 women in the cefamandole subgroup. A co‐intervention (addition of chloramphenicol) was also applied to 3/105 women in the cefamandole subgroup and 4/104 women in the penicillin/gentamicin arm. |
| De Palma 1982 | Timing of delivery of antibiotics for prophylaxis not specified. Authors state antibiotics given within 90 minutes of delivery with no indication as to whether these might have been given pre‐, post‐ or intra‐operatively. Mechanism of randomisation clearly inadequate. |
| Digumarthi 2008 | Quasi‐RCT, allocated women to groups alternatively. |
| Ding 2000 | Study compared 2 different cephalosporins from 1st and 2nd generations. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Donnenfeld 1986 | Study compared a cephalosporin (cefazolin) by 2 different routes of administration, IV and lavage. |
| Duff 1987 | Study compared 2 different cephalosporins, cefazolin versus cefonicid; 1 g after cord clamped. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Elliot 1982 | Study compared a penicillin, ampicillin, given in differing multiple doses. Study will be considered in review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Elliot 1986 | Study compared a cephalosporin, cefoxitin, given in different ways, IV or lavage, or a combination of IV plus lavage. |
| Fejgin 1993 | This study compared 2 different cephalosporins from 2nd and 3rd generations. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Flaherty 1983 | Comparison of pharmacokinetics of cefoxitin when administered by intravenous versus intraperitoneal lavage. Outcome variable of interest: concentration of drug in decidua. No outcomes of interest in our review are listed or were collected (i.e. febrile morbidity, endometritis, etc). |
| Fugere 1983 | Study compared 2 different cephalosporins, cefoxitin (2 g) versus cefazolin (1g). Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Galask 1988 | Study compared 2 different cephalosporins, cefotetan (2 g, single dose, IV dose) versus cefoxitin (6 g, multiple doses (2 g each dose), IV); after cord clamped. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Galask 1989 | Study compared 2 different 2nd generation cephalosporins. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Gall 1987 | Study compared a penicillin, piperacillin (4 g, IV, after cord clamped) single dose versus multiple doses. Study will be considered in review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Gonen 1986 | Study compared a cephalosporin, cefamandole, by 2 different routes of administration, lavage and multiple doses IV. |
| Gonik 1985 | Study compared a cephalosporin, cefotaxime (IV, after cord clamped) single dose (1 g) versus multiple doses (3 x 1 g). Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Gonik 1994 | Study compared 2 different 2nd generation cephalosporins. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Gordon 1982 | Study compared 2 different cephalosporins from 2nd and 3rd generations. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Grujic 2009 | Study not randomised, authors report that women weer allocated to groups according to the type of antibiotics prophylaxis administered as a single dose. |
| Gul 1999 | Study compared different timings of the antibiotic prophylaxis (before versus after cord clamping). |
| Hager 1991 | Study compared 3 cephalosporins, cefazolin (1 g) versus cefoxitin (2 g) versus cefotaxime (1 g); IV; after cord clamped. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Hartert 1987 | Study compared 2 cephalosporins, single dose cefonicid (1 g) versus multiple doses cefoxitin (2 g each); IV; after cord clamped. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Hawrylyshyn 1983 | Study compared a cephalosporin, cefoxitin, single dose (2 g) versus multiple dosed (2 g each); IV; after cord clamped. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Ijarotimi 2012 | Comparing the same antibiotics but using different time scales (24 hours vs 48 hours + oral for 5 days). Study may be considered for possible inclusion in another review. |
| Itskovitz 1979 | Quasi‐RCT. Women were assigned to each of the 2 wings of the department according to the day of their admission. |
| Jakobi 1988 | Study compared a cephalosporin, cefazolin, single dose (1 g) versus multiple doses (2 g each); IV; after cord clamped. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Kreutner 1979 | Study compared 2 cephalosporins, cephalothin versus cefamandole; 1 g; IV at differing times of administration. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Lavery 1986 | Study compared penicillin (mezlocillin) by differing routes of administration and single versus multiple doses. Study will be considered in review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Leonetti 1989 | Study compared penicillin (piperacillin, IV after cord clamping) single (4 g) versus multiple doses (4 g each). Study will be considered in review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Leveno 1984 | Study compared a cephalosporin (cefamandole 2 g) by 2 routes of administration, lavage versus IV. |
| Levin 1983 | Study compared 2 different cephalosporins, cefoxitin versus cephapirin (2 g/L by irrigation). Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Luttkus 1997 | Compares different doses of the same cephalosporin. Study will be considered in the review on ‘Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section (0732)’. |
| Lyimo 2012 | Compares different doses of the same combination of antibiotics. Study may be considered for possible inclusion in another review. |
| Macones 2008 | Study compared different timings of giving the prophylactic antibiotic. |
| Maggioni 1998 | Study compared 2 different B‐lactams (F). |
| Major 1999 | Study compared 2 different cephalosporins from 1st and 2nd generations. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Mansani 1984 | Study on antibiotics for women undergoing hysterectomy. |
| Masse 1988 | Study compared a cephalosporin, cefoxitin (2 g IV after cord clamped) single dose versus multiple doses. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Mathelier 1992 | Study compared a cephalosporin, cefazolin, by different routes of administration, '2 g IV after cord clamped and saline irrigation of abdomen' versus '1 g IV after cord clamped and 1 g in 500 ml normal saline by irrigation'. |
| McGregor 1986 | Study compared 2 cephalosporins, cefotetan (2 g IV after cord clamped) versus cefoxitin (2 g IV and 2 further doses at 4 and 8 hours post‐operatively). |
| McGregor 1988 | Study compared 2 cephalosporins, cefotetan (2 g IV after cord clamped) versus cefoxitin (2 g IV and 2 further doses at 4 and 8 hours post‐operatively). |
| Meyer 2000 | This study compared a cephalosporin versus the same cephalosporin plus another antibiotic. More specifically, cefazolin versus cefalozin‐metronidazole. So this study compared B1 versus B1 + I and will be considered in the review 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Meyer 2003 | Study compared 2 different 1st generation cephalosporins 1 in combination with metronidazole versus the antibiotic alone. |
| Neuman 1990 | Study comparing penicillin G (10 million units IV after cord clamped) plus tetracycline (250 mg IM after cord clamped) versus ampicillin (2 g) plus tetracycline (1.5 g per day, to complete 3 days). Study will be considered in review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. |
| O'Leary 1986 | Study compared a penicillin (ampicillin 2 g IV intraoperatively and 7 further doses) versus the same penicillin regime plus another antibiotic (gentamicin 2 g IV after cord clamping and 6 further doses). So the study compared A4 versus A4 + G and will be considered in the review 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Ovalle 1996 | Quasi‐RCT as “Patients were distributed strictly by order of admission in five groups:”. |
| Parsons 1985 | Study compared 2 cephalosporins, cefonicid (1 g IV after cord clamped) versus cefoxitin (2 g IV after cord clamped and 4 additional doses). Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Patacchiola 2000 | Study compared 2 3rd generation penicillins at differing doses. Study will be considered in the review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Periti 1988 | Study compared 2 different cephalosporins from 1st and 2nd generations. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Peterson 1990 | Study compared 2 different cephalosporins by 2 different routes of administration. Cefazolin (2 g IV after cord clamped) versus cefamandole (2 g IV after cord clamped) versus cefazolin (2 g in 1 L in normal saline by lavage) versus cefamandole (2 g in 1 L normal saline by lavage). Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Pevzner 2009 | Compares giving prophylactic antibiotics before or after cord clamping. Study will be considered for review on 'Timing of prophylactic antibiotics for preventing infectious morbidity in women undergoing caesarean section'. |
| Prasuna 2011 | Quasi‐RCT, allocated women to groups alternately. |
| Puri 1991 | Quasi‐RCT, allocated women to groups alternately. |
| Rayburn 1985 | Study compared a 1st and 3rd generation cephalosporins. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Rijhsinghani 1995 | This study compares a single penicillin with a penicillin combination so should be considered for review ‘Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section’. |
| Rodriguez 1990 | Study compared different timings of giving prophylactic antibiotics. |
| Roex 1987 | Study compared a cephalosporin, cefoxitin (2 g IV after cord clamped) single dose versus multiple doses. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Roy 2003 | Study looked at women with acute pelvic infections. |
| Saravolatz 1985 | Study compared cephalosporin (ceforanide) by 2 routes of administration, 2 g IV after cord clamped versus 2 g in 1 L normal saline by irrigation. |
| Scarpignato 1982 | Study compared a cephalosporin (cefuroxime) by different length of administration, 750 mg IM 30 to 60 minutes pre‐operatively and again post‐operatively at 8 and 16 hours versus 750 mg IM to complete 5 days of therapy, first dose post‐operatively after return of woman to the ward. |
| Seton 1996 | Study looked at different timings of giving 3rd generation cephalosporins. |
| Shakya 2010 | Stuudy is unclear how women were allocated to groups and also compared single dose (Cefazolin + Metronidazole) vs multiple doses antibiotics |
| Stiver 1983 | Study compared a cephalosporin (cefoxitin) at different doses, 1 g IV after cord clamped versus 2 g IV after cord clamped. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Stiver 1984 | Study compared 2 different cephalosporins from 1st and 2nd generations. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Sullivan 2006 | Study looked at different timings of giving 1st generation cephalosporins for prophylaxis. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Sullivan 2007 | Study looked at different timings of giving 1st generation cephalosporins for prophylaxis. |
| Tassi 1987 | Study compared a cephalosporin (ceftazidime) by single (2 g IM 1 hour pre‐operative) versus multiple doses (2 g IM 1 hour pre‐operative plus 2 additional doses). Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Teansutikul 1993 | Study compared different doses of ampicillins. Study will be considered in the review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Thigpen 2005 | Study looked at different timings of giving 1st generation cephalosporins for prophylaxis. |
| van Beekhuizen 2008 | Study looked at single dose of ampicillin plus metronidazole versus multiple doses in low‐income setting. |
| van Velzen 2009 | Study compared a single dose of ampicillin + metronidazole versus multiple doses. |
| Varner 1986 | Study compared a cephalosporin (cefotetan) by single (2 g IV after cord clamping) versus multiple doses (2 g IV after cord clamping plus 2 additional doses). Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| von Mandach 1993 | Study compared 2 cephalosporins, ceftriaxone 1 g IV after cord clamped versus cefoxitin 1 g IV after cord clamped and 2 additional doses at 8 and 16 hours after first dose. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Wagner 2006 | Study compared 2 different 3rd generation cephalosporins. Study will be considered in the review on 'Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Warnecke 1982 | Study compares prophylactic antibiotics with what we presume is 'no treatment' as the English summary only refers to the ‘control group’. Study will be considered for review on 'Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section'. |
| Watts 1991 | Study looked at upper genital isolates at birth as predictors of infection. |
| Wax 1997 | Study looked at different timings of cephalosporin administration (before and after cord clamping). |
| Wu 1991 | Study compared a penicillin versus the same penicillin plus another antibiotic. More specifically, ampicillin versus ampicillin‐gentamicin. So the study compared A4 versus A4 + G and will be considered in the review on 'Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section'. |
| Xu 1997 | Study looked at cephalosporins versus penicillins but the penicillins were sometimes given with other classes of antibiotics and the data could not be separated. |
| Yildirim 2009 | Study compares administration of antibiotics before and after cord clamping. Study will be considered for review on ‘Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section. |
| Zutshi 2008 | Compared different dosage regimens of the same antibiotic, but the specific antibiotic is not given in this conference abstract. |
IM: intramuscular IV: intravenous RCT: randomised controlled trial vs: versus
Characteristics of studies awaiting assessment [ordered by study ID]
Deng 2007.
| Methods | RCT. 2 parallel‐arm study. Women randomised individually. |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: cephalosporin (B1) + metronidazole (I).
Comparison: penicillins (A1 + A4) + metronidazole (I).
|
| Outcomes | Infection, duration of medication and cost. |
| Notes |
Dates: No information. Setting: Central Hospital of Changnin District of Shanghai, China. Subgroups:
Paper in Chinese with abstract only in English. No data provided in abstract. Translation on main paper sought/we will write to authors for data. |
Ijarotimi 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Seeking full text. |
CS: caesarean section IV: intravenous RCT: randomised controlled trial
Differences between protocol and review
From the original protocol, this review has been separated into three reviews as described in the updated protocol sections of this review and a further two reviews will provide information on this topic.
Different classes of antibiotics given to women routinely for preventing infection after caesarean section (this review)
Different regimens of penicillin antibiotic given to women routinely for preventing infection after caesarean section (Liu 2014)
Different regimens of cephalosporin antibiotic given to women routinely for preventing infection after caesarean section (protocol in preparation)
Timing of prophylactic antibiotics for preventing infectious morbidity in women undergoing caesarean section (Baxter 2011) (review in preparation)
Routes of administration for antibiotic given to women routinely for preventing infection after caesarean section (protocol in preparation)
We have added subgroup analyses for all outcomes for Comparison 1, 'Cephalosporins versus penicillins', according to whether single or combination drugs were used.
We have added two further outcomes 'Post‐discharge infections ‐ to 30 days' and 'Maternal readmissions to hospital'.
Contributions of authors
Gill Gyte (GG) drafted the protocol with Zarko Alfirevic (ZA) and Lixia Dou (LD) providing comments. GG, LD and Juan C Vazquez (JCV) carried out the data extraction and GG entered the data with LD checking the data entry. GG drafted the results and conclusions and both LD and JCV checked and provided amendments.
For the 2014 update, GG, JVC and LD performed the data extraction, GG entered the data with LD and JVC checking the data entry. JCV provided the clinical input to the text of the update.
Sources of support
Internal sources
The University of Liverpool, UK.
External sources
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ahmed 2004 {published data only}
- Ahmed ET, Mirghani OA, Gerais AS, Adam I. Ceftriaxone versus ampicillin/cloxacillin as antibiotic prophylaxis in elective caesarean section. East Mediterranean Health Journal 2004;10(3):277‐88. [PubMed] [Google Scholar]
Beningo 1986 {published data only}
- Beningo B, Ford L, Lawrence W, Ledger W, Ling F, McNeeley S. A double‐blind, controlled comparison of piperacillin and cefoxitin in the prevention of postoperative infection in patients undergoing cesarean section. Surgery, Gynecology and Obstetrics 1986;162(1):1‐7. [PubMed] [Google Scholar]
Bracero 1997 {published data only}
- Bracero LA. Ampicillin/sulbactam versus cefotetan for the prevention of infection following cesarean delivery in high‐risk patients: a randomized double‐blind trial. Gynecologic and Obstetric Investigation 1997;44:21‐5. [DOI] [PubMed] [Google Scholar]
Busowski 2000 {published data only}
- Busowski JD, Porter KB, Pendergraft S, O'Brien F, Vodra J. Antibiotic prophylaxis for cesarean delivery: a randomized trial of cefotetan, ampicillin‐sulbactam and ciprofloxacin. Prenatal and Neonatal Medicine 2000;5:357‐62. [Google Scholar]
Chantharojwong 1993 {published data only}
- Chantharojwong P. An efficacy study of ampicillin vs cefazolin prophylaxis in patients undergoing cesarean section. Journal of the Medical Association of Thailand 1993;76:165‐70. [PubMed] [Google Scholar]
Dashow 1986 {published data only}
- Dashow E, Read J, Coleman F. Randomized comparison of five irrigation solutions at cesarean section. Obstetrics & Gynecology 1986;68(4):473‐8. [PubMed] [Google Scholar]
De‐Lalla 1988 {published data only}
- De‐Lalla F, Oliva GC, Fratoni A, Papadia LS, Mancuso S. Single‐dose mezlocillin versus single‐dose cefotetan for prophylaxis in cesarean section. 12th World Congress of Gynecology and Obstetrics; 1988 Oct 23‐28; Rio de Janeiro, Brazil. 1988.
Faro 1990 {published data only}
- Faro S, Martens M, Hammill H, Riddle G, Tortolero G. Antibiotic prophylaxis: is there a difference?. American Journal of Obstetrics and Gynecology 1990;162:900‐9. [DOI] [PubMed] [Google Scholar]
Ford 1986 {published data only}
- Ford L. Cost of antibiotic prophylaxis in cesarean section. Drug Intelligence and Clinical Pharmacy 1986;20:592‐3. [DOI] [PubMed] [Google Scholar]
Gidiri 2014 {published data only}
- Gidiri MF, Ziruma A. A randomised clinical trial evaluating prophylactic single‐dose versus a prolonged week‐long course of antibiotics for caesarean section at two teaching hospitals in Zimbabwe. BJOG: an international journal of obstetrics and gynaecology 2013;120(Suppl s1):12. [DOI] [PubMed] [Google Scholar]
- Gidiri MF, Ziruma A. A randomized clinical trial evaluating prophylactic single‐dose vs prolonged course of antibiotics for caesarean section in a high HIV‐prevalence setting. Journal of Obstetrics and Gynaecology 2014;34(2):160‐4. [DOI] [PubMed] [Google Scholar]
Graham 1993 {published data only}
- Graham JM, Blanco JD, Oshiro BT, Magee KP, Monga M, Eriksen N. Single‐dose ampicillin prophylaxis does not eradicate enterococcus from the lower genital tract. Obstetrics & Gynecology 1993;81:115‐7. [PubMed] [Google Scholar]
Jyothi 2010 {published data only}
- Jyothi S, Vyas M, Pratap K, Asha K. Antibiotic prophylaxis for hysterectomy and cesarean section: Amoxicillin‐clavulanic acid versus cefazolin. Journal of Obstetrics and Gynecology of India 2010;60(5):419‐23. [Google Scholar]
Kamilya 2012 {published data only}
- Kamilya G, Seal SL, Mukherji J, Roy H, Bhattacharyya SK, Hazra A. A randomized controlled trial comparing two different antibiotic regimens for prophylaxis at cesarean section. Journal of Obstetrics and Gynecology of India 2012;62(1):35‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kayihura 2003 {published data only}
- Kayihura V, Osman NB, Bugalho A, Bergstrom S. Choice of antibiotics for infection prophylaxis in emergency cesarean sections in low‐income countries: a cost‐benefit study in Mozambique. Acta Obstetricia et Gynecologica Scandinavica 2003;82(7):636‐41. [DOI] [PubMed] [Google Scholar]
Koppel 1992 {published data only}
- Koppel R, Kaehler D, Benz J. Effectiveness of single dose antibiotic prophylaxis in caesarean section: comparison between cefotaxim and amoxicillin plus clavulanic acid. Geburtshilfe und Frauenheilkunde 1992;52(2):113‐6. [DOI] [PubMed] [Google Scholar]
Lehapa 1999 {published data only}
- Lehapa AM, Towobola OA, Mahapa DH. The comparative efficacy and cost benefit of ceftriaxone versus ampicillin as prophylaxis in patients undergoing caesarean section. Women's Health ‐ into the new millennium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3‐6; Cape Town South Africa. RCOG, 1999:83.
Lewis 1990 {published data only}
- Lewis D, Otterson W, Dunnihoo D. Antibiotic prophylactic uterine lavage in cesarean section: a double‐blind comparison of saline, ticarcillin, and cefoxitin irrigation in indigent patients. Southern Medical Journal 1990;83(3):274‐6. [DOI] [PubMed] [Google Scholar]
Louie 1982 {published data only}
- Louie TJ, Binns B, Baskett T, Ross J, Koss J. Cefotaxime, cefazolin or ampicillin prophylaxis of febrile morbidity in emergency cesarean sections. Clinical Therapeutics 1982;5:83‐96. [PubMed] [Google Scholar]
Lumbiganon 1994 {published data only}
- Lumbiganon P, Sirivatanapa P, Prasertchareonsook W, Kumlertkit S, Anansuwanchai J. A randomized controlled trial of augmentin vs cefazolin in emergency cesarean section. International Journal of Gynecology & Obstetrics 1994;46:77. [Google Scholar]
Mansueto 1989 {published data only}
- Mansueto G, Tomaselli F. Antibiotic prophylaxis in non‐elective caesarean section with single dose imipenem versus multiple‐dose cefotaxime [Profilassi antibiotica in pazieti sottoposte a taglio cesareo non di elezione con Imipenem in "single dose" versus cefotaxime in "multiple doses"]. European Review for Medical and Pharmacological Sciences 1989;11:65‐8. [PubMed] [Google Scholar]
Mivumbi 2014 {published data only}
- Mivumbi V, Greenberg J, Rulisa S. Randomized trial of prophylactic ampicillin vs cefazolin for prevention of post cesarean delivery infectious morbidity in Rwanda. 1st FIGO African Regional Conference of Gynecology and Obstetrics; 2013 Oct 2‐5; Addis Ababa, Ethiopia. 2013.
- Mivumbi VN, Little SE, Rulisa S, Greenberg JA. Prophylactic ampicillin versus cefazolin for the prevention of post‐cesarean infectious morbidity in Rwanda. International Journal of Gynecology and Obstetrics 2014;124:244‐7. [DOI] [PubMed] [Google Scholar]
Mothilal 2013 {published data only}
- Mothilal M, Thivya R, Anjalakshi C, Ramesh A, Damodharan N. Comparison of effectiveness of azithromycin and cefazolin in post caesarean section infection. International Journal of Pharmacy and Pharmaceutical Sciences 2013;5(Suppl 3):92‐4. [Google Scholar]
Ng 1992 {published data only}
- Ng NK, Sivalingam N. The role of prophylactic antibiotics in caesarean section ‐ a randomized trial. Medical Journal of Malaysia 1992;47(4):273‐9. [PubMed] [Google Scholar]
Noyes 1998 {published data only}
- Noyes N, Berkeley AS, Freedman K, Ledger W. Incidence of postpartum endomyometritis following single‐dose antibiotic prophylaxis with either ampicillin/sulbactam, cefazolin, or cefotetan in high‐risk cesarean section patients. Infectious Diseases in Obstetrics and Gynecology 1998;6(5):220‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parulekar 2001 {published data only}
- Parulekar P, Kumar S, Awasthi RT, Tarneja P. A single dose of cefotoxime ‐ as a prophylaxis during caesarean section. Journal of Obstetrics and Gynecology of India 2001;51(5):118‐21. [Google Scholar]
Rehu 1980 {published data only}
- Rehu M, Jahkola M. Prophylactic antibiotics in caesarean section: effect of a short preoperative course of benzyl penicillin or clindamycin plus gentamicin on postoperative infectious morbidity. Annals of Clinical Research 1980;12:45‐8. [PubMed] [Google Scholar]
Rosaschino 1988 {published data only}
- Rosaschino P, Palmerio G, Ziliani A, Gambarini A, Zambetti E, Bagolan M. Antibiotic prophylaxis in cesarean section: a controlled randomized study of mezlocillin vs. ceftriaxone [La profilassi antibiotica nel taglio cesareo: mezlocillina vs. cetriaxone. Studio controllato randomizzato]. Giornale Italiano di Ostetricia e Ginecologia 1988;10(8):587‐8. [Google Scholar]
Saltzman 1985 {published data only}
- Saltzman DH, Eron LJ, Toy C, Protomastro LJ, Sites JG. Ticarcillin plus clavulanic acid versus cefoxitin in the prophylaxis of infection after cesarean section. American Journal of Medicine 1985;79(Suppl 5B):172‐3. [DOI] [PubMed] [Google Scholar]
Saltzman 1986 {published data only}
- Saltzman D, Eron L, Tuomala R, Protomastro L, Sites J. Single‐dose antibiotic prophylaxis in high‐risk patients undergoing cesarean section. A comparative trial. Journal of Reproductive Medicine 1986;31:709‐12. [PubMed] [Google Scholar]
Shah 1998 {published data only}
- Shah S, Mazher Y, John IS. Single or triple dose piperacillin prophylaxis in elective cesarean section. International Journal of Gynecology & Obstetrics 1998;62(1):23‐9. [DOI] [PubMed] [Google Scholar]
Spinnato 2000 {published data only}
- Spinnato JA, Youkilis B, Cook VD, Pietrantoni M, Clark AL, Gall SA. Antibiotic prophylaxis at cesarean delivery. Journal of Maternal‐Fetal Medicine 2000;9:348‐50. [DOI] [PubMed] [Google Scholar]
van der Linden 1993 {published data only}
- Linden MCGJ, Erp EJM, Ruijs GJHM, Holm JP. A prospective randomized study comparing amoxycillin/clavulanate with cefuroxime plus metronidazole for perioperative prophylaxis in gynaecological surgery. European Journal of Obstetrics & Gynecology and Reproductive Biology 1993;50:141‐5. [DOI] [PubMed] [Google Scholar]
Voto 1986 {published data only}
- Voto LS, Benoliel LA, Amadora Muñiz A, Trepat A, Balsechi EE, Margulies M. Prophylaxis of post cesarean section puerperal infection with the using of cefoxitin antibiotics [Profilaxis de la infección puerperal post‐cesarea mediante el uso del antibiótico cefoxitina: I ‐ Resultados de su empleo valorados a través del cultivo sistemático pre, intra y postquirúrgico]. Obstetricia y Ginecologia Latino‐Americanas 1986;44:419‐24. [Google Scholar]
Wells 1994 {published data only}
- Wells M, McCullough W, Rymer J. Antibiotic prophylaxis in emergency cesarean section. International Journal of Gynecology and Obstetrics 1994;46:77. [Google Scholar]
Ziogos 2010 {published data only}
- Ziogos E, Tsiodras S, Matalliotakis I, Giamarellou H, Kanellakopoulou K. Ampicillin/sulbactam versus cefuroxime as antimicrobial prophylaxis for cesarean delivery: a randomized study. BMC Infectious Diseases 2010;10:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Andrews 2003 {published data only}
- Andrews WW, Hauth JC, Cliver SP, Savage K, Goldenberg RL. Randomized clinical trial of extended spectrum antibiotic prophylaxis with coverage for ureaplasma urealyticum to reduce post‐cesarean delivery endometritis. Obstetrics & Gynecology 2003;101:1183‐9. [DOI] [PubMed] [Google Scholar]
Baheraie 1997 {published data only}
- Baheraie A, Modares M, Azimi Kh, Mahmodi M. Single‐dose cefazolin prophylaxis for cesarean section. Acta Obstetricia et Gynecologica Scandinavica 1997;76:28. [Google Scholar]
Beksac 1989 {published data only}
- Beksac MS, Urman CB, Akalin E, Baykal M. A randomized comparative study of a single‐dose 3rd generation cephalosporin (ceftriaxone) and a broad spectrum ureidopenicillin (mezlocillin) in a controlled group trial for cesarean section prophylaxis. International Journal of Experimental and Clinical Chemotherapy 1989;2(1):55‐7. [Google Scholar]
Berkeley 1990 {published data only}
- Berkeley A, Hirsch J, Freedman K, Ledger W. Cefotaxime for cesarean section prophylaxis in labor; intravenous administration vs lavage. Journal of Reproductive Medicine 1990;35(3):214‐8. [PubMed] [Google Scholar]
Bernstein 1994 {published data only}
- Bernstein P, Novosel S, Collins JA, Jarrell JF, Joly J, Moutquin JM. Cefotetan versus cefoxitin in cesarean section prophylaxis. Annals of the Royal College of Physicians and Surgeons of Canada 1994;27(7):401‐4. [Google Scholar]
Bilgin 1998 {published data only}
- Bilgin T, Ozan H, Dirgen A, Esmer A. Comparison of four different antibiotics as prophylaxis in caesarean section. Journal of Obstetrics and Gynaecology 1998;18(6):546‐7. [DOI] [PubMed] [Google Scholar]
Boothby 1984 {published data only}
- Boothby R, Benrubi G, Ferrell E. Comparison of intravenous cefoxitin prophylaxis with intraoperative cefoxitin irrigation for the prevention of post‐cesarean‐section endometritis. Journal of Reproductive Medicine 1984;29(11):830‐2. [PubMed] [Google Scholar]
Carlson 1990 {published data only}
- Carlson C, Duff P. Antibiotic prophylaxis for cesarean delivery: is an extended‐spectrum agent necessary?. Obstetrics & Gynecology 1990;76(3):343‐6. [PubMed] [Google Scholar]
Chamberlain 1993 {published data only}
- Chamberlain A, White S, Bawdon R, Thomas S, Larsen B. Pharmacokinetics of ampicillin and sulbactam in pregnancy. American Journal of Obstetrics and Gynecology 1993;168:667‐73. [DOI] [PubMed] [Google Scholar]
Chittacharoen 1998 {published data only}
- Chittacharoen A, Manonai J, Suthutvoravut S, Phaupradit W. Single‐dose amoxycillin‐clavulanic acid vs ampicillin prophylaxis in emergency cesarean section. International Journal of Gynecology & Obstetrics 1998;62:249‐54. [Google Scholar]
Conover 1984 {published data only}
- Conover W, Moore T. Comparison of irrigation and intravenous antibiotic prophylaxis at cesarean section. Obstetrics & Gynecology 1984;63(6):787‐91. [PubMed] [Google Scholar]
- Conover WB, Moore TR. Comparison of irrigation and intravenous antibiotic prophylaxis at cesarean section. Obstetrical and Gynecological Survey 1984;39:692‐3. [PubMed] [Google Scholar]
Crombleholme 1987 {published data only}
- Crombleholme W, Green J, Ohm‐Smith M, Dahrouge D, DeKay V, Rideout A, et al. Cesarean section prophylaxis: comparison of two doses with three doses of mezlocillin. American Journal of Reproductive Immunology 1987;13:71‐5. [DOI] [PubMed] [Google Scholar]
Crombleholme 1989 {published data only}
- Crombleholme WR, Green JR, Ohm‐Smith M, DeKay V, Klaisle C, Luft J, et al. Prophylaxis in caesarean section with cefmetazole and cefoxitin. Journal of Antimicrobial Chemotherapy 1989;23:97‐104. [DOI] [PubMed] [Google Scholar]
Cunningham 1983 {published data only}
- Cunningham FG, Leveno KJ, Palma RT, Roark ML, Rosenfeld CR. Perioperative antimicrobials for cesarean delivery: before or after cord clamping?. Obstetrics & Gynecology 1983;62:151‐4. [PubMed] [Google Scholar]
D'Angelo 1980 {published data only}
- D'Angelo L, Sokol R. Short‐ versus long‐course prophylactic antibiotic treatment in cesarean section patients. Obstetrics & Gynecology 1980;55(5):583‐6. [PubMed] [Google Scholar]
De Palma 1980 {published data only}
- Palma R, Leveno K, Cunningham FG, Pope T, Kappus S, Roark M, et al. Identification and management of women at high risk for pelvic infection following cesarean section. Obstetrics & Gynecology 1980;55(5):185S‐191S. [DOI] [PubMed] [Google Scholar]
De Palma 1982 {published data only}
- Palma R, Cunningham G, Leveno K, Roark M. Continuing investigation of women at high risk for infection following cesarean delivery. Obstetrics & Gynecology 1982;60(1):53‐9. [PubMed] [Google Scholar]
Digumarthi 2008 {published data only}
- Digumarthi L, Gupta S. A single dose of co‐amoxiclav vs. five‐day antibiotics in elective and emergency caesarean section. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):159. [Google Scholar]
Ding 2000 {published data only}
- Ding H, Wang Y, Liu X. Effects of elective cesarean section and antibiotics to the bacterial flora in female genital tract. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology] 2000;35(6):342‐4. [PubMed] [Google Scholar]
Donnenfeld 1986 {published data only}
- Donnenfeld A, Otis C, Weiner S. Antibiotic prophylaxis in cesarean section. Comparison of intrauterine lavage and intravenous administration. Journal of Reproductive Medicine 1986;31(1):15‐8. [PubMed] [Google Scholar]
Duff 1987 {published data only}
- Duff P, Robertson A, Read J. Single‐dose cefazolin versus cefonicid for antibiotic prophylaxis in cesarean delivery. Obstetrics & Gynecology 1987;70(5):718‐21. [PubMed] [Google Scholar]
Elliot 1982 {published data only}
- Elliott J, Freeman R, Dorchester W. Short versus long course of prophylactic antibiotics in cesarean section. American Journal of Obstetrics and Gynecology 1982;143:740‐4. [DOI] [PubMed] [Google Scholar]
Elliot 1986 {published data only}
- Elliott J, Flaherty J. Comparison of lavage or intravenous antibiotics at cesarean section. Obstetrics & Gynecology 1986;67(1):29‐32. [PubMed] [Google Scholar]
Fejgin 1993 {published data only}
- Fejgin MD, Markov S, Goshen S, Segal J, Arbel Y, Lang R. Antibiotic for cesarean section: the case for 'true' prophylaxis. International Journal of Gynecology & Obstetrics 1993;43:257‐61. [DOI] [PubMed] [Google Scholar]
Flaherty 1983 {published data only}
- Flaherty J, Boswell G, Winkel C, Elliott J. Pharmacokinetics of cefoxitin in patients at term gestation; lavage versus intravenous administration. American Journal of Obstetrics and Gynecology 1983;146:760‐6. [DOI] [PubMed] [Google Scholar]
Fugere 1983 {published data only}
- Fugere P, Turgeon P, Boucher M, Verscheiden G, Lemay M. Utilisation des cephalosporines comme antibioprophylaxie lors de cesariennes. Canadian Medical Association Journal 1983;129:132‐5. [PMC free article] [PubMed] [Google Scholar]
Galask 1988 {published data only}
- Galask R, Benigno B, Cunningham G, Elliott J, Makowski E, McGregor J, et al. Results of a multicenter comparative study of single‐dose cefotetan and multiple‐dose cefoxitin as prophylaxis in patients undergoing cesarean section. American Journal of Surgery 1988;155:86‐90. [DOI] [PubMed] [Google Scholar]
Galask 1989 {published data only}
- Galask RP, Weiner C, Petzold CR. Comparison of single‐dose cefmetazole and cefotetan prophylaxis in women undergoing primary caesarean section. Journal of Antimicrobial Chemotherapy 1989;23:105‐8. [DOI] [PubMed] [Google Scholar]
Gall 1987 {published data only}
- Gall S, Hill G. Single‐dose versus multiple‐dose piperacillin prophylaxis in primary cesarean operation. American Journal of Obstetrics and Gynecology 1987;157:502‐6. [DOI] [PubMed] [Google Scholar]
Gonen 1986 {published data only}
- Gonen R, Samberg I, Levinski R, Levitan Z, Sharf M. Effect of irrigation or intravenous antibiotic prophylaxis on infectious morbidity at cesarean section. Obstetrics & Gynecology 1986;67:545‐8. [PubMed] [Google Scholar]
Gonik 1985 {published data only}
- Gonik B. Single‐ versus three‐dose cefotaxime prophylaxis for cesarean section. Obstetrics & Gynecology 1985;65:189‐93. [PubMed] [Google Scholar]
Gonik 1994 {published data only}
- Gonik B, Mcgregor J. Comparison of short vs. long half‐life single‐dose prophylactic antibiotics for cesarean section. Infectious Diseases in Obstetrics & Gynecology 1994;2:120‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gordon 1982 {published data only}
- Gordon SF, Russell J. A randomized controlled study comparing ceftizoxime, cefamandole, and cefoxitin in obstetric and gynaecological surgery: a preliminary report. Journal of Antimicrobial Chemotherapy 1982;10 Suppl C:289‐92. [DOI] [PubMed] [Google Scholar]
Grujic 2009 {published data only}
- Grujic Z, Bogavac M, Ilija G, Nikolic A. Is this necessary to give antibiotic prophylaxis in elective caesarean sections?. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(S1):230. [Google Scholar]
- Grujic Z, Sabo A, Grujic I, Kopitovic V, Papovic M. Single dose of antibiotic prophylaxis in elective cesarean sections. Medicinski Pregled 2009;62(3‐4):101‐6. [DOI] [PubMed] [Google Scholar]
Gul 1999 {published data only}
- Gul A, Zeteroilu I, Surucu R. The comparison of piperacillin use for prophylaxis of post cesarean section infection before and after clamping umbilical cord [abstract]. Infectious Diseases in Obstetrics & Gynecology 1999;7(6):306. [Google Scholar]
Hager 1991 {published data only}
- Hager W, Rapp R, Billeter M, Bradley B. Choice of antibiotic in nonelective cesarean section. Antimicrobial Agents and Chemotherapy 1991;35(9):1782‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartert 1987 {published data only}
- Hartert R, Benrubi G, Thompson R, Nuss R. Cefonicid vs. cefoxitin for cesarean section prophylaxis. Journal of Reproductive Medicine 1987;32(12):907‐10. [PubMed] [Google Scholar]
Hawrylyshyn 1983 {published data only}
- Hawrylyshyn P, Bernstein P, Papsin F. Short‐term antibiotic prophylaxis in high‐risk patients following cesarean section. American Journal of Obstetrics and Gynecology 1983;145:285‐9. [DOI] [PubMed] [Google Scholar]
Ijarotimi 2012 {published data only}
- Ijarotimi OA, Badejoko OO, Ijarotimi OO, Orji EO, Loto OM, Fasubaa OB. Comparison of short versus long term antibiotic prophylaxis in elective caesarean section‐the ile‐ife experience. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S375. [Google Scholar]
Itskovitz 1979 {published data only}
- Itskovitz J, Paldi E, Katz M. The effect of prophylactic antibiotics on febrile morbidity following cesarean section. Obstetrics & Gynecology 1979;53(2):162‐5. [PubMed] [Google Scholar]
Jakobi 1988 {published data only}
- Jakobi P, Weissman A, Zimmer E, Paldi E. Single‐dose cefazolin prophylaxis for cesarean section. American Journal of Obstetrics and Gynecology 1988;158:1049‐52. [DOI] [PubMed] [Google Scholar]
Kreutner 1979 {published data only}
- Kreutner K, Bene V, DeLamar D, Bodden J, Loadholt C. Perioperative cephalosporin prophylaxis in cesarean section: effect on endometritis in the high‐risk patient. American Journal of Obstetrics and Gynecology 1979;134:925‐33. [DOI] [PubMed] [Google Scholar]
Lavery 1986 {published data only}
- Lavery J, Huang K, Koontz W, Reinstine J, Marcell C, Rosenberg N. Mezlocillin prophylaxis against infection after cesarean section: a comparison of techniques. Southern Medical Journal 1986;79(10):1248‐351. [DOI] [PubMed] [Google Scholar]
Leonetti 1989 {published data only}
- Leonetti H, Yun H, O'Leary J, Greenberg A. Single versus multiple dose piperacillin in high risk primary cesarean section. American Journal of Gynecologic Health 1989;3(6):29‐32. [Google Scholar]
Leveno 1984 {published data only}
- Leveno K, Quirk J, Cunningham F, Nelson S, Bawdon R. Perioperative antimicrobials at cesarean section: lavage versus three intravenous doses. American Journal of Obstetrics and Gynecology 1984;149:463‐4. [DOI] [PubMed] [Google Scholar]
Levin 1983 {published data only}
- Levin DK, Gorchels C, Andersen R. Reduction of post‐cesarean section infectious morbidity by means of antibiotic irrigation. American Journal of Obstetrics and Gynecology 1983;147(3):273‐6. [DOI] [PubMed] [Google Scholar]
Luttkus 1997 {published data only}
- Luttkus A, Fiebelkorn J, Dudenhausen W. Antibiotic prophylaxis in cases of emergency caesarean section. Geburtshilfe und Frauenheilkunde 1997;57(9):510‐4. [Google Scholar]
Lyimo 2012 {published data only}
- Lyimo FM, Massinde AN, Kidenya BR, Konje E, Mshana SE. Efficacy of single dose of gentamicin in combination with metronidazole versus multiple doses for prevention of post‐caesarean infection: study protocol for a randomized controlled trial. Trials 2012;13:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macones 2008 {published data only}
- Macones G, Cleary K, Odibo A, Gross G, Parry S, Rampersad R, et al. Timing of antibiotic prophylaxis at cesarean ‐ results of an RCT. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S33. [Google Scholar]
Maggioni 1998 {published data only}
- Maggioni P, Stefano F, Facchini V, Irato S, Mancuso S, Colombo M, et al. Treatment of obstetric and gynecologic infections with meropenem: comparison with imipenem/cilastatin. Journal of Chemotherapy 1998;10:114‐21. [DOI] [PubMed] [Google Scholar]
Major 1999 {published data only}
- Major CA, Reimbold T, Morgan MA. Cephararin versus cefoxitin prophylaxis for cesarean sections. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S75. [Google Scholar]
Mansani 1984 {published data only}
- Mansani FE, Caltabiano M, Condemi V, Scarpignato C. Short‐ or long‐term antibiotic prophylaxis in obstetrical and gynecological surgery?. Acta Bio‐Medica de l Ateneo Parmense 1984;55:147‐51. [PubMed] [Google Scholar]
Masse 1988 {published data only}
- Masse A, Turgeon P, Gay N, Verschelden G. To compare the efficacy of antibiotic prophylaxis with cefoxitine using one or three doses at caesarean [Efficacite comparee de l'antibioprophylaxie par la cefoxitine en une ou trois doses dans la cesarienne]. Canadian Medical Association Journal 1988;138:921‐4. [PMC free article] [PubMed] [Google Scholar]
Mathelier 1992 {published data only}
- Mathelier A. A comparison of postoperative morbidity following prophylactic antibiotic administration by combined irrigation and intravenous route or by intravenous route alone during cesarean section. Journal of Perinatal Medicine 1992;20:177‐82. [DOI] [PubMed] [Google Scholar]
McGregor 1986 {published data only}
- McGregor J, French J, Makowski E. Single‐dose cefotetan versus multidose cefoxitin for prophylaxis in cesarean section in high‐risk patients. American Journal of Obstetrics and Gynecology 1986;154:955‐60. [DOI] [PubMed] [Google Scholar]
McGregor 1988 {published data only}
- McGregor J, Gordon S, Krotec J, Poindexter A. Results of a randomized, multicenter, comparative trial of a single dose of cefotetan versus multiple doses of cefoxitin as prophylaxis in cesarean section. American Journal of Obstetrics and Gynecology 1988;158:701‐6. [DOI] [PubMed] [Google Scholar]
Meyer 2000 {published data only}
- Meyer NL, Scott K, Hosier K, Sibai B. Cefazolin versus cefazolin/metronidazole for antibiotic prophylaxis at cesarean section [abstract]. Obstetrics & Gynecology 2000;95(4 Suppl):74S. [Google Scholar]
Meyer 2003 {published data only}
- Meyer NL, Hosier KV, Scott K, Lipscomb GH. Cefazolin versus cefazolin plus metronidazole for antibiotic prophylaxis at cesarean section. Southern Medical Journal 2003;96(10):992‐5. [DOI] [PubMed] [Google Scholar]
Neuman 1990 {published data only}
- Neuman M, Langer R, Bachar R, Golan A, Bukovsky I, Caspi E. Penicillin‐tetracycline prophylaxis in cesarean delivery: prospective and randomized comparison of short and long term therapy. Journal of Perinatal Medicine 1990;18:145‐8. [DOI] [PubMed] [Google Scholar]
O'Leary 1986 {published data only}
- O'Leary J, Mullins J, Andrinopoulos G. Ampicillin vs. ampicillin‐gentamicin prophylaxis in high‐risk primary cesarean section. Journal of Reproductive Medicine 1986;31(1):27‐30. [PubMed] [Google Scholar]
Ovalle 1996 {published data only}
- Ovalle SA, Robinovich Tannenbaum J, Leyton H, Nilo C, Nasra J, Aliaga J, et al. Antibiotic prophylaxis in the cesarean surgery [Profilaxis antibiótica en la operación cesárea]. Revista Chilena De Obstetricia y Ginecología 1996;61(4):243‐9. [Google Scholar]
Parsons 1985 {published data only}
- Parsons M, Gibson R, Rimando‐Ramos J, Spellacy W. Comparison of single‐dose cefonicid and multiple‐dose cefoxitin for surgical prophylaxis in women undergoing cesarean section. Advances in Therapy 1985;2(5):233‐9. [Google Scholar]
Patacchiola 2000 {published data only}
- Patacchiola F, Paolantonio L, Palermo P, Stefano L, Mascaretti G, Moscarini M. Antibiotic prophylaxis of infective complications after cesarean section. Our experience [Profilassi antibiotica delle complicanze infettive dopo taglio cesareo. Nostra esperienza]. Minerva Ginecologica 2000;52(10):385‐9. [PubMed] [Google Scholar]
Periti 1988 {published data only}
- Periti P, Mazzei T, Periti E. Prophylaxis in gynaecological and obstetric surgery: a comparative randomised multicentre study of single‐dose cefotetan versus two doses of cefazolin. Chemioterapia 1988;7(4):245‐52. [PubMed] [Google Scholar]
Peterson 1990 {published data only}
- Peterson CM, Medchill M, Gordon D, Chard H. Cesarean prophylaxis: a comparison of cefamandole and cefazolin by both intravenous and lavage routes, and risk factors associated with endometritis. Obstetrics & Gynecology 1990;75:179‐82. [PubMed] [Google Scholar]
Pevzner 2009 {published data only}
- Pevzner L, Chan K. Optimal timing for antibiotic prophylaxis during elective cesarean: before or after cord clamping. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S225‐S226. [Google Scholar]
Prasuna 2011 {published data only}
- Prasuna NJL. Comparative study between prophylactic and routine antibiotic in elective and emergency caesarean section, using coamoxyclav vs ampicillin and gentamicin respectively. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:124.
Puri 1991 {published data only}
- Puri M, Chadda P, Nagrani R, Tandon G. Single dose cephexin prophylaxis for caesarean section. Journal of Obstetrics and Gynaecology of India 1991;41(5):643‐6. [Google Scholar]
Rayburn 1985 {published data only}
- Rayburn W, Varner M, Galask R, Petzold CR, Piehl E. Comparison of moxalactam and cefazolin as prophylactic antibiotics during cesarean section. Antimicrobial Agents and Chemotherapy 1985;27:337‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rijhsinghani 1995 {published data only}
- Rijhsinghani A, Savapoulos SE, Walters JK, Huggins G, Hibbs JR. Ampicillin/sulbactam vs ampicillin alone for Cesarean section prophylaxis: a randomized double‐blind trial. American Journal of Perinatology 1995;12:322‐4. [DOI] [PubMed] [Google Scholar]
Rodriguez 1990 {published data only}
- Rodriguez GC, Gall SA, Parsons MT. Timing of prophylactic antibiotic administration at cesarean section. Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 Jan 23‐27; Houston, Texas, USA. 1990:311.
Roex 1987 {published data only}
- Roex AJM, Puyenbroek JI, Loenen AC, Arts NFT. Single‐ versus three‐dose cefoxitin prophylaxis in caesarean section: a randomized clinical trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 1987;25:293‐8. [DOI] [PubMed] [Google Scholar]
Roy 2003 {published data only}
- Roy S, Higareda I, Angel‐Muller E, Ismail M, Hague C, Adeyi B, et al. Ertapenem once a day versus piperacillin‐tazobactam every 6 hours for treatment of acute pelvic infections: a prospective, multicenter, randomized, double‐blind study. Infectious Diseases in Obstetrics and Gynecology 2003;11(1):27‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saravolatz 1985 {published data only}
- Saravolatz L, Lee C, Drukker B. Comparison of intravenous administration with intrauterine irrigation with ceforanide for nonelective cesarean section. Obstetrics & Gynecology 1985;66(4):513‐6. [PubMed] [Google Scholar]
Scarpignato 1982 {published data only}
- Scarpignato C, Caltabiano M, Condemi V, Mansani F. Short‐term versus long‐term cefuroxime prophylaxis in patients undergoing emergency cesarean section. Clinical Therapeutics 1982;5(2):186‐2. [PubMed] [Google Scholar]
Seton 1996 {published data only}
- Seton DN, Vellema DMM, Schoon MG, Grobler JM. Prophylactic antibiotics and caesarean section: comparing ceftriaxone administration pre‐ and post‐ umbilical cord clamping in terms of maternal and neonatal morbidity. 15th Conference on Priorities in Perinatal Care in Southern Africa; 1996 March 5‐8; Goudini Spa, South Africa. 1996.
Shakya 2010 {published data only}
- Shakya A, Sharma J. Comparison of single versus multiple doses of antibiotic prophylaxis in reducing post‐elective caesarean section infectious morbidity. Kathmandu University Medical Journal 2010;8(30):179‐84. [DOI] [PubMed] [Google Scholar]
Stiver 1983 {published data only}
- Stiver HG, Forward K, Livingstone R, Fugere P, LeMay M, Verschelden G, et al. Muliticenter comparison of cefoxitin versus cefazolin for prevention of infectious morbidity after nonelective cesarean section. Obstetrics & Gynecology 1983;143:158‐63. [DOI] [PubMed] [Google Scholar]
Stiver 1984 {published data only}
- Stiver HG, Forward KR, Tyrrell DL, Livingstone RA, Fugere P, Lemay M, et al. Comparative cervical microflora shifts after cefoxitin or cefazolin prophylaxis against infection following cesarean section. American Journal of Obstetrics and Gynecology 1984;149:718‐21. [DOI] [PubMed] [Google Scholar]
Sullivan 2006 {published data only}
- Sullivan S, Smith T, Chang E, Hulsey T, Dorsten J, Soper D. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S17. [DOI] [PubMed] [Google Scholar]
Sullivan 2007 {published data only}
- Sullivan SA, Smith T, Chang E, Hulsey T, Vandorsten JP, Soper D. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity: a randomized, controlled trial. American Journal of Obstetrics and Gynecology 2007;196(5):455.e1‐455.e5. [DOI] [PubMed] [Google Scholar]
Tassi 1987 {published data only}
- Tassi P, Tarantini M, Cadenelli G, Gastaldi A, Benedetti M. Coftazidime in antibiotic prophylaxis for emergency cesarean section: a randomized prospective study. International Journal of Clinical Pharmacology, Therapy and Toxicology 1987;25(10):582‐8. [PubMed] [Google Scholar]
Teansutikul 1993 {published data only}
- Teansutikul C. Comparative study of 2 gram single dose versus 1 gram three doses of ampicillin prophylaxis in high risk emergency cesarean section patients. Chon Buri Hospital Journal 1993;18(1):1‐18. [Google Scholar]
Thigpen 2005 {published data only}
- Thigpen BD, Hood WA, Chauhan S, Bufkin L, Bofill J, Magann E, et al. Timing of prophylactic antibiotic administration in the uninfected laboring gravida: a randomized clinical trial. American Journal of Obstetrics and Gynecology 2005;192:1864‐71. [DOI] [PubMed] [Google Scholar]
van Beekhuizen 2008 {published data only}
- Beekhuizen H. Prevention of post‐caesarean infections in low resource countries: is a single dose as adequate as a multiple dose antibiotic regiment? A randomised controlled trial. Current Controlled Trials (http://controlled‐trials.com/) (accessed 20 February 2008).
van Velzen 2009 {published data only}
- Velzen C, Kolk P, Westen E, Mmuni N, Hamisi A, Nakua R, et al. Comparison of a single prophylactic dose of ampicillin and metronidazole before caesarean section with a multiple day regimen of these antibiotics in prevention of postpartum maternal infection in a low resource setting: a randomized controlled trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S369. [Google Scholar]
Varner 1986 {published data only}
- Varner M, Weiner C, Petzold R, Galask R. Comparison of cefotetan and cefoxitin as prophylaxis in cesarean section. American Journal of Obstetrics and Gynecology 1986;154:951‐4. [DOI] [PubMed] [Google Scholar]
von Mandach 1993 {published data only}
- Mandach U, Huch R, Malinverni R, Huch A. Ceftriaxone (single dose) versus cefoxitin (multiple doses): success and failure of antibiotic prophylaxis in 1052 cesarean sections. Journal of Perinatal Medicine 1993;21:385‐97. [DOI] [PubMed] [Google Scholar]
Wagner 2006 {published data only}
- Wagner KJ, Bier U, Callies R, Regidor PA, Schindler AE. Antibiotic prophylaxis in cesarean section ‐ piperacillin 4 g versus piperacillin/tazobactam 4.5 g in 300 cesarean sections [Antibiotikaprophylaxe bei Sectio Caesarea ‐ Piperacillin 4 g versus Piperacillin/Tazobactam 4.5 g]. Zentralblatt fur Gynakologie 2006;128(3):149‐52. [DOI] [PubMed] [Google Scholar]
Warnecke 1982 {published data only}
- Warnecke HH, Graeff H, Selbmann HK, Preac‐Mursic V, Adam D, Gloning K, et al. Perioperative short‐term prophylaxis with antibiotics in caesarean section. Geburtshilfe und Frauenheilkunde 1982;42(9):654‐61. [DOI] [PubMed] [Google Scholar]
Watts 1991 {published data only}
- Watts DH, Hillier SL, Eschenbach DA. Upper genital tract isolates at delivery as predictors of post‐Cesarean infections among women receiving antibiotic prophylaxis. Obstetrics & Gynecology 1991;77:287‐92. [DOI] [PubMed] [Google Scholar]
Wax 1997 {published data only}
- Wax JR, Hersey K, Philput C, Wright MS, Nichols KV, Eggleston MK, et al. Single dose cefazolin prophylaxis for postcesarean infections: before vs after cord clamping. Journal of Maternal Fetal Medicine 1997;6(1):61‐5. [DOI] [PubMed] [Google Scholar]
Wu 1991 {published data only}
- Wu Y, Zhan L, Jing Y. Prevention of post‐operative infection by uterine and intraperitoneal irrigation with ampicillin during cesarean section. International Journal of Experimental and Clinical Chemotherapy 1991;4(3):132‐6. [Google Scholar]
Xu 1997 {published data only}
- Xu X, Zhang W, He X. Use of cefotaxime to control post‐operative infection in patients who underwent C‐section. Acta Academiae Medicinae Hubei 1997;18:74‐6. [Google Scholar]
Yildirim 2009 {published data only}
- Yildirim G, Gungorduk K, Guven HZ, Aslan H, Celikkol O, Sudolmus S, et al. When should we perform prophylactic antibiotics in elective cesarean cases?. Archives of Gynecology and Obstetrics 2009;280(1):13‐8. [DOI] [PubMed] [Google Scholar]
Zutshi 2008 {published data only}
- Zutshi V. Prophylactic antibiotics ‐ a concept forgotten by gynaecologists?. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):104. [Google Scholar]
References to studies awaiting assessment
Deng 2007 {published data only}
- Deng DM, Huang Z, Yuan WJ, Ding LZ, Chen XY. Clinical study on coadministration of cefazolin sodium and metronidazole for infection prophylaxis in perioperative period of cesarean section. Pharmaceutical Care and Research 2007;7(4):278‐80. [Google Scholar]
Ijarotimi 2013 {published data only}
- Ijarotimi AO, Badejoko OO, Ijarotimi O, Loto OM, Orji EO, Fasubaa OB. Comparison of short versus long term antibiotic prophylaxis in elective caesarean section at the Obafemi Awolowo University Teaching Hospitals Complex, Ile‐Ife, Nigeria. Nigerian Postgraduate Medical Journal 2013;20(4):325‐30. [PubMed] [Google Scholar]
Additional references
Baxter 2011
- Baxter JK, Berghella V, Mackeen AD, Ohly NT, Weed S. Timing of prophylactic antibiotics for preventing postpartum infectious morbidity in women undergoing cesarean delivery. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009516] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bayarski 2006
- Bayarski Y. Antibiotics classification and side effects. http://ezinearticles.com/?Antibiotics‐Classification‐And‐Side‐Effects&id=288137 (accessed 14 April 2010).
Bedford Russell 2006
- Bedford Russell A, Murch S. Could peripartum antibiotics have delayed health consequences for the infant?. BJOG: an international journal of obstetrics and gynaecology 2006;113:758‐65. [DOI] [PubMed] [Google Scholar]
BNF 2009
- British National Formulary. British Medical Association and Royal Pharmaceutical Society of Great Britain. London: BMJ Group, September 2009. [Google Scholar]
Cartwright 1984
- Cartwright PS, Pittaway DE, Jones HW, Entman SS. The use of prophylactic antibiotics in obstetrics and gynecology. A review. Obstetrical and Gynecological Survey 1984;39(9):537‐54. [DOI] [PubMed] [Google Scholar]
Csaba 2007
- Csaba G. Hormonal imprinting: phylogeny, ontogeny, diseases and possible role in present‐day human evolution. Cell Biochemistry and Function 2007;26(1):1‐10. [DOI] [PubMed] [Google Scholar]
Dancer 2004
- Dancer SJ. How antibiotics can make us sick: the less obvious adverse effects of antimicrobial chemotherapy. Lancet Infectious Diseases 2004;4:611‐9. [DOI] [PubMed] [Google Scholar]
Declercq 2007
- Declercq E, Barger M, Cabral HJ, Evans SR, Kotelchuck M, Simon C, et al. Maternal outcomes associated with planned primary cesarean births compared with planned vaginal births. Obstetrics and Gynecology 2007;109(3):669‐77. [DOI: 10.1097/01.AOG.0000255668.20639.40] [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd Edition. London (UK): BMJ Publications Group, 2001. [Google Scholar]
eMedExpert 2009
- eMedExpert. Antibiotics: types and side effects. http://www.emedexpert.com/classes/antibiotics.shtml (accessed 14 April 2010).
Glasgow 2005
- Glasgow TS, Young PC, Wallin J, Kwok C, Stoddard G, Firth S, et al. Association of intrapartum antibiotic exposure and late‐onset serious bacterial infections in infants. Pediatrics 2005;116(3):696‐702. [DOI] [PubMed] [Google Scholar]
Goodman 2008
- Goodman L, Gilman A. Section V111: Chemotherapy of microbial diseases. In: Brunton L, Parker K, Blumenthal D, Buxton I editor(s). Goodman & Gillman's Manual of Pharmacology and Therapeutics. 11th Edition. New York: McGraw‐Hill, 2008:707‐852. [Google Scholar]
GRADE 2008 [Computer program]
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro. Version 3.6. The Cochrane Collaboration, 2008.
Grolund 1999
- Grolund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. Journal of Pediatric Gastroenterology and Nutrition 1999;28(1):19‐25. [DOI] [PubMed] [Google Scholar]
Henderson 1995
- Henderson E, Love EJ. Incidence of hospital‐acquired infections associated with caesarean section. Journal of Hospital Infection 1995;29:245‐55. [DOI] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Leth 2009
- Leth RA, Møller JK, Thomsen RW, Uldbjerg N, Nørgaard M. Risk of selected postpartum infections after cesarean section compared with vaginal birth: a five‐year cohort study of 32,468 women. Acta Obstetricia et Gynecologica Scandinavica 2009;88(9):976‐83. [DOI] [PubMed] [Google Scholar]
Liu 2014
- Liu D, Zhang L, Zhang C, Chen M, Zhang L, Li J, et al. Different regimens of penicillin antibiotics given to women routinely for preventing infection after caesarean section. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD011362] [DOI] [Google Scholar]
MacLean 1990
- MacLean AB. Clinical Infection in Obstetrics and Gynecology. Oxford: Blackwell Scientific Publications, 1990. [Google Scholar]
Owen 1994
- Owen J, Andrews WW. Wound complications after cesarean sections. Clinical Obstetrics and Gynecology 1994;37(4):842‐55. [DOI] [PubMed] [Google Scholar]
Pedersen 1996
- Pedersen TK, Blaakaer J. Antibiotic prophylaxis in cesarean section. Acta Obstetricia et Gynecologica Scandinavica 1996;75:537‐9. [DOI] [PubMed] [Google Scholar]
Penders 2006
- Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006;118(2):511‐21. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schultz 1995
- Schultz KF. Subverting randomization in controlled trials. JAMA 1995;274:1456‐8. [PubMed] [Google Scholar]
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [DOI] [PubMed] [Google Scholar]
Smaill 2002
- Smaill F, Hofmeyr GJ. Antibiotic prophylaxis for cesarean section. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD000933] [DOI] [PubMed] [Google Scholar]
Smaill 2008
- Smaill FM, Gyte GML. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007482] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tchernitchin 1999
- Tchernitchin AN, Tchernitchin NN, Mena MA, Unda C, Soto J. Imprinting: perinatal exposures cause the development of diseases during the adult age. Acta Biologica Hungarica 1999;50(4):425‐40. [PubMed] [Google Scholar]
Tita 2009
- Tita ATN, Rouse DJ, Blackwell S, Saade GR, Spong CY, Andrews WW. Emerging concepts in antibiotic prophylaxis for cesarean delivery: a systematic review. Obstetrics and Gynecology 2009;113(3):675‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Westphal 1994
- Westphal JF, Vetter D, Brogard JM. Hepatic side‐effects of antibiotics. Journal of Antimicrobial Chemotherapy 1994;33:387‐401. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Alfirevic 2010
- Alfirevic Z, Gyte GML, Dou L. Different classes of antibiotics given to women routinely for preventing infection at caesarean section. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD008726] [DOI] [PubMed] [Google Scholar]
Hopkins 1999
- Hopkins L, Smaill F. Antibiotic prophylaxis regimens and drugs for cesarean section. Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI: 10.1002/14651858.CD001136] [DOI] [PubMed] [Google Scholar]


