Abstract
Background
Idiopathic intracranial hypertension (IIH) has an estimated incidence of one to three people per 100,000 people per year, and occurs most commonly in obese, young women. IIH is associated with severe morbidity, notably due to a significant threat to sight and severe headache. Several different management options have been proposed. Conservative measures centre on weight loss. Pharmacological therapy includes use of diuretics. Refractory and sight‐threatening cases demand surgical intervention, most often in the form of cerebrospinal fluid (CSF) diversion or optic nerve sheath fenestration. Other treatments include venous sinus stenting and bariatric surgery.
Objectives
To assess the effects of any intervention for IIH in any patient group.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2015 Issue 6), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to July 2015), EMBASE (January 1980 to July 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 22 July 2015.
Selection criteria
We included only randomised controlled trials (RCTs) in which any intervention was compared to placebo, or to another form of treatment, for people with a clinical diagnosis of IIH.
Data collection and analysis
Two review authors independently assessed the search results for trials to be included in the review. We resolved any discrepancies by third party decision.
Main results
We identified two completed RCTs (enrolling a total of 211 participants and conducted in the UK and US) and two ongoing trials that met the inclusion criteria. Both completed trials compared acetazolamide to placebo, in conjunction with a weight loss intervention in both groups. Attrition bias was a problem in both trials with high loss to follow‐up, in one study this loss to follow‐up occurred particularly in the acetazolamide arm. One trial was unmasked and we judged it to be at risk of performance and detection bias.
In these studies, change in visual acuity was similar in the treatment and control groups as measured by logMAR acuity. In one study people in the acetalomazide group had a similar change in logMAR acuity compared to the placebo group between baseline and 12 months in the right eye (MD 0.04 logMAR, 95% CI ‐0.08 to 0.16) and left eye (MD 0.03 logMAR, 95% CI ‐0.09 to 0.15). In the other study people in the acetalomazide group had a similar change in vision over six months compared with people in the placebo group (mean difference in change in letters read was 0.01 (95% CI ‐1.45 to 1.46). One study reported no cases of visual loss in 21 people treated with acetalomazide compared to 2/20 cases in the placebo group (odds ratio 0.17, 95% CI 0.01, 3.82).
The prespecified outcome for this review was reduction in CSF pressure to normal levels which was not reported by the two trials. One trial reported that, in a subsample of 85 participants who agreed to lumbar puncture at 6 months, people in the acetalomazide group on average had a greater reduction in CSF pressure (MD ‐59.9 mmH2O, 95% CI ‐96.4, ‐23.4).
In one study, people in the acetalozamide group on average experienced a greater reduction in papilloedema as assessed by fundus photographs MD ‐0.70 (95% CI ‐1.00 to ‐0.40) and by clinical grading MD ‐0.91 (95% CI ‐1.27 to ‐0.54) between baseline and six months in the study eye.
Headache was recorded as present/absent in one study at 12 months (OR 0.42, 95% CI 0.12,1.41, 41 participants). Both studies reported headache on visual analogue scales (different ones) but results were inconclusive (MD for change in headache score measured on 10‐point visual analogue scale at 12 months was 1.0 (‐1.80, 3.70, 41 participants) and MD for change in headache score on a 6 point scale measured at 6 months was ‐0.45 (‐3.5,2.6, number of participants unclear).
In one study, a similar proportion of people in the acetalomazide group were in remission (however, the trial authors did not state their definition of this term) at 12 months compared to the placebo group. However, the 95% CIs were wide and there is considerable uncertainty as to the effect (OR 1.13 (95% CI 0.32 to 3.90, 41 participants).
In one study of 185 participants, people in the acetalomazide group had an increased risk of decreased CO2, diarrhoea, dysgeusia, fatigue, nausea, paresthesia, tinnitus and vomiting compared to people in the placebo group. In general, the estimates of effect were uncertain with wide 95% CIs. Adverse effects were not reported in the other study.
One study reported that quality of life was better in acetazolamide‐treated patients based on the visual quality of life (VFQ‐25) (MD 6.35, 95% CI 2.22 to 10.47) and the physical (MD 3.02, 95% CI 0.34 to 5.70) and mental (MD 3.45, 95% CI 0.35 to 6.55) components of the 36‐Item Short Form Health Survey tool at six months. Costs were not reported in either study.
We judged the evidence to be low certainty (GRADE) downgrading for imprecision and risk of bias.
Authors' conclusions
Although the two included RCTs showed modest benefits for acetazolamide for some outcomes, there is insufficient evidence to recommend or reject the efficacy of this intervention, or any other treatments currently available, for treating people with IIH. Further high‐quality RCTs are required in order to adequately assess the effect of acetazolamide therapy in people with IIH.
Plain language summary
Interventions for idiopathic intracranial hypertension
Review question We attempted to find all of the published randomised controlled trials (RCT, a type of rigorous study that compares one treatment option against another) that investigated any treatment for idiopathic intracranial hypertension (IIH) in any patient group. We looked at a number of outcomes including reduction in vision, improvement of headache and quality of life.
Background IIH is a condition in which there is increased pressure inside the head without any detectable cause. IIH occurs most commonly in young women who are obese. Increased pressure inside the head often results in swelling of the optic disc (the point where the optic nerve meets the eye), which is called papilloedema. This swelling in turn causes a potential threat to sight. Different management options or treatments have been suggested for treating people with IIH, such as weight loss, drugs (e.g. diuretics) and surgery (e.g. surgery to the optic nerve, brain surgery to reduce the pressure or weight loss surgery). However, there is no consensus for how IIH should best be treated.
Search date We searched for all available trials up to 22 July 2015.
Key results We included two completed RCTs from the UK and US with a total of 211 participants and two ongoing studies. Both completed trials compared acetazolamide to placebo, together with a weight loss intervention in both groups.
In these studies, change in the participant’s central vision was similar in the treatment and control groups as measured by a logMAR chart (a chart with rows of letters). The outcome for this review was reduction in CSF pressure to normal levels which was not reported by the two trials. In one study, people in the acetalozamide group on average experienced a greater reduction in papilloedema as assessed by fundus photographs between baseline and six months in the study eye. Both studies reported headache on visual analogue scales but results were inconclusive. In the study that reported adverse effects, the acetazolamide group was found to have a greater number of adverse effects compared to the placebo controls including diarrhoea, nausea, tinnitus and fatigue. One study reported that quality of life was better for the acetazolamide‐treated group. Costs were not reported in either study.
Quality of evidence Both completed trials had issues such as high loss to follow up and in one trial the participants and trial investigators knew what treatment was being received. The evidence was therefore judged to be of low quality.
More higher‐quality RCTs are required in order to adequately assess the effect of acetazolamide therapy in IIH.
Background
Description of the condition
Idiopathic intracranial hypertension (IIH), also known as benign intracranial hypertension or pseudotumour cerebri, is a syndrome that exhibits the symptoms and signs of increased intracranial pressure (ICP) but in which there is no evidence of an intracranial mass lesion on neuroimaging (Galgano 2013).
The incidence of IIH in the developed world is one to three people per 100,000 people per year (Durcan 1988; Radhakrishnan 1993a; Radhakrishnan 1993b; Radhakrishnan 1994). IIH is most common in obese women (Corbett 1982; Radhakrishnan 1993a). Disease onset occurs at any age but most commonly between 20 and 40 years. IIH also occurs in children where incidence is equal between males and females (Phillips 2012).
The aetiology of IIH is unknown. Individual case reports of IIH have been associated with many different medical conditions. For example, IIH has been associated with endocrine disturbances (Klein 2013), raised vitamin A and its derivatives (Donahue 2000; Tabassi 2005; Warner 2002; Warner 2007), and drugs (most notably tetracyclines) (Lochhead 2003). In case control studies, factors associated with IIH are female, being of reproductive age, menstrual irregularity, obesity and recent weight gain (Giuseffi 1991; Ireland 1990). The most popular theory of causation is that obstruction to cerebrospinal fluid (CSF) outflow through the arachnoid villi or venous sinuses causes a rise in ICP. It has been suggested that elevated intracranial venous pressure may be a universal mechanism in cases of IIH from multiple aetiologies (Karahalios 1996). Sugerman 1997 suggests that raised intra‐abdominal pressure due to obesity leads to increased pleural pressure and cardiac filling pressure, impeding venous return from the brain, causing elevated intracranial venous pressure and ICP. It is possible that several different mechanisms may be involved.
IIH is an important condition to recognise and treat because it can cause visual loss. The degree of visual loss ranges from an enlarged blind spot to permanent total blindness. Visual obscurations (episodes of transient loss of vision) are common. Corbett 1982 suggested that blindness occurred in 10% of patients, but 75% to 87% of all affected eyes exhibit some degree of visual loss if carefully monitored (Rowe 1998; Wall 1991).
IIH was first described in 1897 (Quincke 1896), but the precise definition has varied. Currently, the following criteria are generally accepted as necessary for IIH diagnosis (Friedman 2002):
Symptoms and signs only reflect those of generalised intracranial hypertension or papilloedema.
Elevated ICP must be documented, measured in the lateral decubitus position.
Normal CSF composition.
No evidence of hydrocephalus, mass, structural or vascular lesion on structural neuroimaging for typical patients, and magnetic resonance imaging (MRI) and magnetic resonance venography for all others.
No other cause of intracranial hypertension identified.
Most patients have papilloedema (swelling of the optic disc), but the papilloedema may be unilateral (Maxner 1987; Sher 1983). Cases have been described in which there was no evidence of papilloedema (Lipton 1972; Mathew 1996). Headache is a major cause of morbidity (Johnston 1974a; Johnston 1974b; Weisberg 1975). IIH may be spontaneously self‐limiting or it may continue for many years. Features identical to IIH can occur as a result of cerebral sinus thrombosis. By definition, this cerebral sinus thrombosis is not idiopathic and we will not consider it further in this Cochrane review.
Description of the intervention
Consequent to a poorly defined disease entity and an unknown aetiology, a number of varied treatments for IIH have been suggested. Reducing CSF pressure with a lumbar puncture transiently helps symptoms (particularly headache and visual obscurations). As such, repeated lumbar punctures have been advocated as a form of IIH management. Conservative strategies include weight loss (in some cases by means of weight‐loss surgery) and, in children, a low‐salt diet. Alongside, simple analgesics for headaches and a range of other drugs, including acetazolamide, topiramate, other diuretics, octreotide, oral glycerol, cardiac glycosides and corticosteroids, have been used. Surgical management options include CSF diversion and optic nerve sheath fenestration. Lumbo‐peritoneal shunting is used most often. However, ventriculo‐peritoneal, cisterno‐peritoneal and cisterno‐pleural shunts have also been employed.
How the intervention might work
Treatments attempt to reduce ICP and have two main aims: treatment of headache and prevention of visual loss.
Why it is important to do this review
There is no consensus on how IIH should be optimally managed. Some treatment options have significant complications, are very expensive, or both. Due to the heterogeneity of presenting symptoms and signs, patients may present to neurologists, ophthalmologists, neurosurgeons, otolaryngological surgeons or general physicians. Each specialty has its own preferred approach to management. Very few of the currently used therapies have been subjected to critical assessment in the form of a randomised controlled trial (RCT).
Objectives
To assess the effects of any intervention for IIH in any patient group.
Methods
Criteria for considering studies for this review
Types of studies
RCTs.
Types of participants
We included RCTs of participants with a clinical diagnosis of IIH. A particular problem arises because the syndrome is often referred to as idiopathic even when it occurs in the context of a proposed aetiological factor (e.g. the administration of tetracyclines). This leads to potential confusion in the literature. Further confusion may arise because of the presence or absence of papilloedema. For the purposes of this Cochrane review, we defined three categories:
True IIH: participants with papilloedema (unilateral or bilateral) and raised CSF pressure (> 250 mmH2O; Friedman 2002). There had to be no other abnormal neurological sign other than the presence of a sixth nerve palsy. Cerebral imaging by either computed tomography (CT) or MRI and examination of CSF constituents had to be completely normal. There had to be no determinable underlying cause (infection, medication, etc.) and no abnormality of cerebral sinuses.
IIH with underlying cause: participants as defined in (a) above but with an associated recognised contributory factor. This factor could be determined on the basis of history (e.g. consumption of tetracyclines, oral contraceptive medication or vitamin A) or on investigation (e.g. thrombophilic disorder or anti‐cardiolipin syndrome). As above, studies of individuals with the syndrome of IIH occurring in the context of a cortical sinus thrombosis were not included. If studies included this group plus those with true IIH we planned to separate them for the purposes of analysis.
IIH without papilloedema: participants as defined in 1. or 2. above but without papilloedema. Investigations may or may not have determined an underlying cause.
Types of interventions
We included RCTs in which any intervention used to treat IIH had been compared to placebo or another form of treatment. These included:
Dietary (weight loss, dietary advice, low‐salt diet).
Bariatric surgery.
Medication (diuretics, octreotide, corticosteroids, other).
Repeated lumbar puncture.
Optic nerve sheath fenestration (optic nerve decompression).
Stenting of transverse intracerebral venous sinus.
CSF diversion procedures.
Any other interventions (e.g. hyperbaric oxygen, complementary medicine).
Types of outcome measures
Primary outcomes
Reduction in vision (visual field, visual acuity, blind registration). Three cut‐offs of visual acuity were used (6/12 (20/40) or worse; 6/60 (20/200) or worse; and loss of three lines (or more) on a Snellen chart). It is possible to measure reduction in visual field in various ways. We standardised this by using an endpoint that would prevent a person from driving legally in the UK. This involved encroachment on the binocular visual field such that a person no longer had a full binocular field of at least ± 20º above and below fixation, extending a total of 120º in the horizontal direction.
Reduction of CSF pressure into the normal range (< 250 mmH2O; Friedman 2002).
Resolution of papilloedema or oculomotor disorder or both (assessed clinically).
Improvement of headache, defined as a reduction in patient‐reported frequency/severity of headaches by 50%. The method of measurement varied depending on the study concerned.
Remission rate, where participants must have been declared cured of the condition in terms of headache and papilloedema. CSF pressure reduction was not required as part of the definition as in many cases this is not routinely measured.
In each primary outcome we were particularly interested in outcome at six months and 12 months.
Secondary outcomes
Side effects of interventions.
Cost of intervention, as calculated based on information derived from the Western General Hospital (Edinburgh, UK) (the review authors' base hospital). While costs will vary from one centre to another, the relative information can reasonably be used to generate figures for resource use and the relative cost‐effectiveness of the various treatments.
Quality of life, as measured by the scale used in the included study.
Again, we were particularly interested in each outcome at six and 12 months.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2015 Issue 6), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to July 2015), EMBASE (January 1980 to July 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 22 July 2015.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), ISRCTN (Appendix 4), ClinicalTrials.gov (Appendix 5) and the ICTRP (Appendix 6).
Searching other resources
We did not handsearch any journals, reference lists or conference proceedings for this review.
Data collection and analysis
Selection of studies
Two review authors (RJP, AMHY) assessed the titles and abstracts of all references identified by the electronic searches. We excluded studies that did not report RCTs. When a disagreement arose, we resolved the issue by consensus or by consulting a third review author (AABJ). We obtained the full‐text copies of possible or completed RCTs of treatment of IIH. Two review authors (RJP, AABJ) independently assessed suitability for inclusion according to the definitions in the Criteria for considering studies for this review section. We contacted trial authors for further information if necessary.
Data extraction and management
Two review authors (RJP, AABJ) independently extracted data from studies and resolved discrepancies by discussion. One review author entered the agreed data into RevMan 2014 but all review authors confirmed that the data included in the review were correct.
Assessment of risk of bias in included studies
Two review authors independently assessed the quality of the trial according to methods set out in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The review authors were unmasked to the trial authors and results during the assessment. We considered the following parameters:
Selection bias.
Performance bias.
Detection bias.
Attrition bias.
Reporting bias.
We graded each parameter of RCT quality as at either 'low', 'unclear' or 'high' risk of bias. Although unnecessary in this review update, in future updates we will contact the trial authors for clarification of any parameter graded at 'unclear' risk of bias.
Measures of treatment effect
We did not detect a sufficient number of RCTs in order to allow meta‐analyses. However, if we identify sufficient studies in future review updates, we will summarise data from studies collecting the same outcome measure. We will present dichotomous data as odds ratios (ORs) or risk ratios. We will present continuous variables as mean differences (MDs). We will calculate the number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) if appropriate. We will use a random‐effects model unless there are fewer than three RCTs included in the analysis, in which case we will use the fixed‐effect model.
Unit of analysis issues
Trials may randomise one or both eyes of a participant to the intervention or comparator, which may pose a unit of analysis problem. Both trials included in this review update randomised people to treatment. Ball 2011 reported data on left and right eyes separately and Wall 2014 reported data for the eye with the worst visual acuity at baseline (study eye) and fellow eye separately. In future review updates, when people are randomly allocated to treatment but only one eye per person is reported in the trial, we will document how the eye was selected. If people are randomly allocated to treatment but data on both eyes are reported together, we will analyse as 'clustered data' (i.e. adjust for within‐person correlation). We may contact the trial authors for further information to do this. If the study is a within‐person study (i.e. one eye is randomly allocated to intervention and the other eye receives the comparator), then we will analyse as paired data. Again, we may contact the trial authors for further information.
Dealing with missing data
Where possible, we conducted an intention‐to‐treat (ITT) analysis but only using imputed data if computed by the trial authors using an appropriate method. Where ITT data were not available, we performed an available case analysis. This assumes that data were missing at random. We assessed whether this assumption was reasonable by collecting data from each included trial on the number of participants excluded or lost to follow‐up, and reasons for loss to follow‐up by treatment group, if reported.
Assessment of heterogeneity
We did not assess heterogeneity as only two trials met the inclusion criteria. In future review updates we will examine the overall characteristics of the included studies, in particular the type of participants and types of interventions, to assess the extent to which the studies are similar enough to make pooling study results sensible. We will look at the forest plots of study results to see how consistent the trial results are, in particular looking at the size and direction of effects. We will calculate the I² statistic which estimates the percentage of variation across studies that is due to heterogeneity rather than chance (Higgins 2011). We will consider I² statistic values over 50% to indicate substantial inconsistency, and we will also consider the Chi² test P value. As this may have low power when the number of included studies are few, we will consider P < 0.1 to indicate statistical significance.
Assessment of reporting biases
In future review updates, if we include 10 or more trials in a meta‐analysis, we will construct funnel plots and consider tests for asymmetry for assessment publication bias according to Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data synthesis
We did not perform any data synthesis due to the limited number of included trials. In future review updates, we will pool data using a random‐effects model in RevMan 2014. If there are fewer than three trials in a comparison, we will use a fixed‐effect model. If there is inconsistency between individual study results such that a pooled result may not be a good summary of the individual trial results (e.g. the effects are in different directions or the I² statistic > 50% and P < 0.1), we will not pool the data but will describe the pattern of the individual study results. If the I² statistic is > 50% but all the effect estimates are in the same direction such that a pooled estimate would seem to provide a good summary of the individual trial results, we may pool the data.
'Summary of findings' tables
In future review updates we will prepare a 'Summary of findings' table presenting relative and absolute risks. We will include the following outcomes in the 'Summary of findings' table: visual fields, visual acuity, blind registration, CSF pressure, papilloedema, headache and remission rate.
GRADE assessment
We assessed the overall quality of the evidence for each outcome using the GRADE classification (GRADEpro 2014).
Subgroup analysis and investigation of heterogeneity
In future review updates we will perform subgroup analyses according to type of treatment and participant group (true IIH, IIH with underlying cause, and IIH without papilloedema) where sufficient RCTs are included. We will assess heterogeneity between RCTs using the Chi² test.
Sensitivity analysis
We will perform a sensitivity analysis to assess the effect of excluding RCTs at high risk of bias on any aspect of RCT quality.
Results
Description of studies
Results of the search
The original electronic searches performed in 2002 identified 550 articles (Lueck 2002). Most articles were not directly relevant as they concerned raised ICP due to head injury, stroke or other neurosurgical cause, such as tumour. Of the 550 articles, 49 referred to dural sinus thrombosis and were excluded. A total of 51 studies were identified which related to IIH. Of these, only seven were concerned with treatment; two were retrospective reviews, and none of the others had a control group (see Characteristics of excluded studies). Consequently, the searches failed to identify any RCT suitable for inclusion in the review.
Updated searches
The searches were updated in January 2005, and 352 further articles were identified, of which 50 related to IIH (Lueck 2005). Of these, two referred to treatment but neither had a control group (Bynke 2004; Owler 2003) (see Characteristics of excluded studies).
The searches were updated again in January 2007. A further 438 articles were found but none were relevant to this Cochrane review.
The searches were further updated in December 2008, finding 53 further references of which 32 were related to IIH. Only one was a RCT (Çelebisoy 2007). Çelebisoy 2007 compared treatment with acetazolamide to treatment with topiramate, but participants were alternately assigned to the treatments, not randomly, and there was no placebo control group.
We performed an update search in July 2015 which yielded a total of 2886 references (Figure 1). The Trials Search Co‐ordinator scanned the search results, removed 219 duplicates and then removed 2418 references which were not relevant to the scope of this Cochrane review. We screened the remaining 249 reports and discarded 218 reports as irrelevant. We obtained 31 full‐text reports for potential inclusion. After assessment, we excluded 24 references (see Characteristics of excluded studies for reasons). We identified four reports of two new trials which met the inclusion criteria (Ball 2011; Wall 2014). Also, we identified three reports of two ongoing studies and will assess these data when they become available (NCT02017444; NCT02124486).
1.
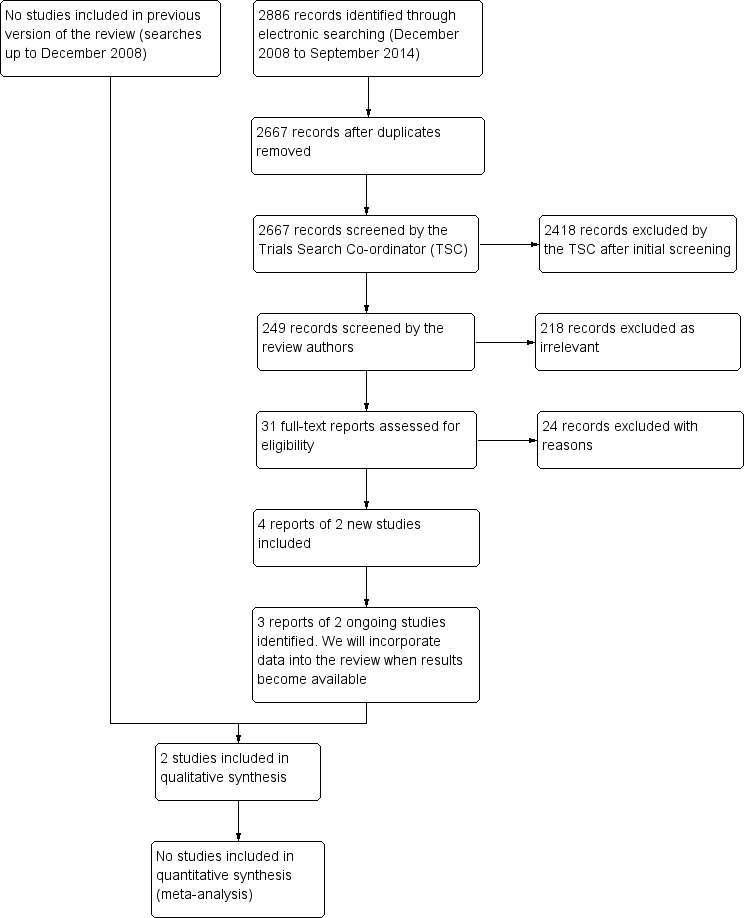
Study flow diagram.
Included studies
We identified two RCTs that met the inclusion criteria (Ball 2011; Wall 2014). Below is a summary of the included studies.
Design
In Ball 2011, the open‐label, parallel‐group RCT investigated the effectiveness of treating IIH participants with acetazolamide in comparison to placebo. Wall 2014 was a multi‐centre double‐masked RCT comparing patients treated with acetazolamide (plus low sodium and weight reduction diet) against patients managed with low sodium and weight reduction diet alone.
Sample size
Ball 2011 initially recruited 25 participants to the acetazolamide group and 25 participants to the placebo control. Wall 2014 recruited a total of 165 participants (86 patients in the acetazolamide group and 79 placebo control). From those recruited, 69 (80%) and 57 (72%) participants completed follow‐up, respectively.
Setting
Ball 2011 was conducted in six UK ophthalmology centres and Wall 2014 in 38 centres throughout North America.
Participants
In Ball 2011, all participants had a clinical diagnosis of IIH in accordance with the criteria reported in Friedman 2002 and consistent with the type 1 participants as described in the Types of participants section. In the acetazolamide group, participants were 29 years old on average (range: 18 to 66), and included a female:male ratio of 22:3. In the placebo group, participants were 33 years old on average (range: 18 to 63) and included a female:male ratio of 24:1.
For Wall 2014, all IIH patients met the modified Dandy criteria for IIH (consistent with the criteria reported in Friedman 2002 and consistent with the type 1 participants as described in our Types of participants section) and were required to have mild visual loss with a perimetric mean deviation (PMD) between ‐2 dB and ‐7 dB. Participants were also required to be between the ages of 18 and 60, have bilateral papilloedema and elevated CSF opening pressure.
Interventions
Participants were treated with either acetazolamide (clinician decided dosing schedule) or placebo, and all Ball 2011 participants were encouraged to lose weight. For Wall 2014, the intervention was low‐sodium weight‐reduction diet plus the maximally tolerated dosage of acetazolamide (up to 4 g/d) or matching placebo for six months.
Outcomes
For Ball 2011, outcome measures included: headache (subjective patient‐reported 10‐point scale); tinnitus (subjective patient‐reported presence versus absence); visual acuity (measured on LogMAR chart); visual obscurations (patient reported: absent, present, or deteriorating); visual fields (measured on automated Humphrey perimetry); contrast sensitivity (measured on Pelli‐Robson chart); papilloedema (absent or present); anxiety/depression (measured on Hospital Anxiety and Depression Scale); patient rated health status (measured on EuroQoL and Short Form 36). All outcomes were measured at baseline, 3, 6, 9 and 12 months. At 12 months, each participant was ranked according to performance on an aggregate score of outcome measures (headache, tinnitus, visual obscurations, visual acuity, optic disc appearance, visual field). The aggregate score ranged from 0 to 15 (15 being the worst possible outcome measured). In addition, clinicians were asked to describe the condition of the participant (IIH in remission, active IIH improving, active IIH but stable, or active IIH deteriorating).
The primary outcome for Wall 2014 was a change in PMD from baseline to six months in eye with most severe visual loss. Secondary outcomes included PMD changes in least affected eye, papilloedema grade (Frisén scale), visual acuity, visual quality of life (VFQ‐25), 6‐item headache impact test (HIT‐6), quality of life (36‐Item Short Form Health Survey), CSF pressure, weight, vital signs and laboratory results. Outcome measurements were reported at baseline, 1, 2, 3, 4, 5 and 6 months (apart from HIT‐6 and quality of life, which were only at six months).
Ongoing studies
We also identified two ongoing RCTs that met the inclusion criteria. These data will be included once available. The first ongoing trial is a placebo controlled trial assessing the safety and effectiveness of a 11‐βhydroxysteroid dehydrogenase type 1 inhibitor (NCT02017444). The second RCT is a study comparing bariatric surgery versus a community weight loss programme in people with IIH (NCT02124486).
Excluded studies
See Characteristics of excluded studies for further details.
Risk of bias in included studies
The included trials exhibited risk of bias on a number of fronts. Ball 2011 was underpowered and unmasked. Participants were allocated to the trial only if the managing clinician felt that they should or should not receive another form of therapy. Wall 2014 suffered from a high withdrawal rate (19%) which could act as a source of bias in the results.
Allocation
In Ball 2011, participants were excluded if the treating clinician felt there was or was not a particular therapy that the patient should get. This selection bias is likely to have meant participants on the milder spectrum of IIH were included in this study.
Blinding
In Ball 2011, neither participants nor treating clinicians were masked to allocation. This lack of masking increased the risk of performance and detection bias. This was exacerbated by clinicians being free to dictate the dosing schedules for those treated with acetazolamide. Wall 2014 had a more robust masking policy with the managing staff masked to treatment status except for a programmer who generated the randomisation plan, a statistician and a member of staff involved with packaging the drugs.
Incomplete outcome data
Both studies suffered from significant attrition bias. In Ball 2011, a fifth of participants did not complete the 12‐month assessment. Similarly, in Wall 2014 there was a 19% withdrawal rate.
Selective reporting
In both studies, the primary outcome data were provided in full. However, Ball 2011 did not provide data for anxiety, depression and patient‐reported health status assessments. Wall 2014 provided data on all the secondary outcomes mentioned.
Other potential sources of bias
In Ball 2011, five participants from the placebo arm of the RCT crossed over and were started on acetazolamide therapy and two participants (one from each of the two arms) underwent surgical intervention during the study period. Furthermore, 12 participants from the acetazolamide arm discontinued due to: patient preference (four participants), non‐compliance from the outset (three participants), adverse events (two participants), planned pregnancy (two participants) and insertion of VP shunt (one participant). Notably, the final aggregate outcome score used to assess 12‐month efficacy of the intervention is an unvalidated endpoint in the context of IIH.
Risk of bias is summarised in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Primary outcomes
Reduction in vision
Wall 2014 reported a change in PMD over six months and showed that PMD in the acetalozamide group on average increased by more decibels than the placebo group. However, the relevance of this difference in clinical practice is unknown (Table 1).
1. Reduction in vision according to change in perimetric mean deviation.
| Outcome | Acetalomazide | Placebo | MD (95% CI) adjusted for centre, baseline value of outcome and baseline papilloedema grade, with multiple imputation for missing data | ||||
| Mean | SD | N | Mean | SD | N | ||
| Wall 2014: Change in perimetric mean deviation (decibels) between baseline and 6 months | 1.43 | 2.23 | 86 | 0.71 | 2.49 | 69 | 0.71 (0 to 1.43) |
Abbreviations: MD = mean difference; SD = standard deviation; CI = confidence interval.
People in the acetalomazide group of Ball 2011 had a similar change in logMAR acuity compared to the placebo group between baseline and 12 months in the right eye (MD 0.04 logMAR, 95% CI ‐0.08 to 0.16) and left eye (MD 0.03 logMAR, 95% CI ‐0.09 to 0.15; Table 2). In Wall 2014 people in the acetalomazide group had a similar change in vision over six months compared with people in the placebo group. The MD value in change in letters read was 0.01 (95% CI ‐1.45 to 1.46; Table 2).
2. Reduction in vision according to change in logMAR acuity.
| Outcome | Acetalomazide | Placebo | MD (95% CI) | ||||
| Mean | SD | N | Mean | SD | N | ||
| Ball 2011: Change in logMAR acuity in the right eye between baseline and 12 months | Not reported | Not reported | 21 | Not reported | Not reported | 20 | 0.04 (‐0.08, 0.16) |
| Ball 2011: Change in logMAR acuity in the left eye between baseline and 12 months | Not reported | Not reported | 21 | Not reported | Not reported | 20 | 0.03 (‐0.09, 0.15) |
| Wall 2014a: Change in visual acuity (number of correct letters) between baseline and 6 months | 2.65 | Not reported (SE 0.49) | b | 2.64 | Not reported (SE 0.51) | b | 0.01 (‐1.45, 1.46) |
aIn Wall 2014 MD adjusted for centre, baseline value of outcome and baseline papilloedema grade. bIt is unclear how many patients were included in these analyses. Abbreviations: MD = mean difference; SD = standard deviation; CI = confidence interval; SE = standard error.
No cases of visual loss were observed in the acetalomazide group of Ball 2011 at 12 months compared to two cases in the placebo group. However, the effect was uncertain with wide 95% CIs (OR 0.17, 95% CI 0.01 to 3.82; Table 3).
3. Reduction in patients with visual loss according to change in logMAR acuity.
| Outcome | Acetalomazide | Placebo | OR (95% CI) | ||
| n | N | n | N | ||
| Ball 2011: Visual loss (logMAR 0.2 or more) at 12 months | 0 | 21 | 2 | 20 | 0.17 (0.01, 3.82) |
Abbreviations: MD = mean difference; SD = standard deviation; CI = confidence interval; n = number of events; N = number of participants; OR = odds ratio.
Reduction of CSF pressure
In Wall 2014, only 85 participants (55% in the acetazolamide group and 48% in the placebo group) agreed to a lumbar puncture at six months. People in the acetalomazide group on average had a greater reduction in CSF pressure. However, the clinical significance of this difference is unclear (Table 4). Notably, the pre‐specified outcome in this review is reduction of CSF to normal levels, a dichotomous outcome, which was not reported by the two included trials.
4. Reduction in cerebrospinal fluid pressure.
| Outcome | Acetalomazide | Placebo | MD (95% CI) adjusted for centre, baseline value of outcome and baseline papilloedema grade | ||||
| Mean | SD | N | Mean | SD | N | ||
| Wall 2014: Change in CSF pressure (mmH20) between baseline and 6 months | ‐112.3 | Not reported | 47 | ‐52.4 | Not reported | 38 | ‐59.9 (‐96.4 to ‐23.4) |
Resolution of papilloedema or oculomotor disorder or both
In Wall 2014 people in the acetalozamide group on average experienced a greater reduction in papilloedema as assessed by fundus photographs MD ‐0.70 (95% CI ‐1.00 to ‐0.40) and by clinical grading MD ‐0.91 (95% CI ‐1.27 to ‐0.54) between baseline and six months in the study eye (Table 5).
5. Resolution of papilloedema or oculomotor disorder or both.
| Outcome | Acetalomazide | Placebo | MD (95% CI) adjusted for centre and baseline papilloedema grade | ||||
| Mean | SD | N | Mean | SD | N | ||
| Wall 2014: Change in papilloedema grade between baseline and 6 months, graded by fundus photographs | ‐1.31 | SE 0.11a | a | ‐0.61 | SE 0.11a | a | ‐0.70 (‐1.00 to ‐0.40) |
| Wall 2014: Change in papilloedema grade between baseline and 6 months, clinical grading | ‐1.75 | SE 0.13a | a | ‐0.85 | SE 0.14a | a | ‐0.91 (‐1.27 to ‐0.54) |
aIt is unclear how many patients were included in these analyses. Abbreviations: MD = mean difference; SD = standard deviation; SE = standard error; CI = confidence interval; N = number of participants.
Improvement of headache
Headache was recorded as present/absent in Ball 2011 and also rated by the patient on a 10‐point visual analogue score. In Wall 2014 the 6‐item Headache Impact Test (HIT‐6) Inventory was used to assess headache effect; scores range from 36 to 78 with higher scores indicating worse headache severity. Based on both included trials, the effect of treatment on headaches was uncertain (Table 6; Table 7).
6. Improvement in headache.
| Outcome | Acetalomazide | Placebo | MD (95% CI) adjusted for centre, baseline value of outcome and baseline papilloedema grade | ||||
| Mean | SD | N | Mean | SD | N | ||
| Ball 2011: Change in headache score as measured on a 10‐point visual analogue score at 12 months | Not reported | Not reported | 21 | Not reported | Not reported | 20 | 1.0 (‐1.80 to 3.70) |
| Wall 2014: Change in HIT‐6 total score between baseline and 6 months | ‐9.56 | Not reporteda | a | ‐9.11 | Not reporteda | a | ‐0.45 (‐3.50 to 2.60) |
aIt is unclear how many patients were included in these analyses. Abbreviations: MD = mean difference; SD = standard deviation; CI = confidence interval; N = number of participants.
7. Resolution of headache.
| Outcome | Acetalomazide | Placebo | OR (95% CI) | ||
| n | N | n | N | ||
| Ball 2011: Number of people with headache at 12 months | 9 | 21 | 13 | 20 | 0.42 (0.12 to 1.41) |
Abbreviations: CI = confidence interval; n = number of events; N = number of participants; OR = odds ratio.
Remission rate
In Ball 2011, a similar proportion of people in the acetalomazide group were in remission (however, the trial authors did not state their definition of this term) at 12 months compared to the placebo group. However, the 95% CIs were wide and there is considerable uncertainty as to the effect (OR 1.13 (95% CI 0.32 to 3.90; Table 8).
8. Remission rate.
| Outcome | Acetalomazide | Placebo | OR (95% CI) | ||
| n | N | n | N | ||
| Ball 2011: Number of people in remission at 12 months | 9 | 21 | 8 | 20 | 1.13 (0.32 to 3.90) |
Abbreviations: CI = confidence interval; n = number of events; N = number of participants; OR = odds ratio.
Secondary outcomes
Side effects
We have listed the adverse effects reported in Wall 2014 in Table 9. People in the acetalomazide group had an increased risk of decreased CO2, diarrhoea, dysgeusia, fatigue, nausea, paresthesia, tinnitus and vomiting compared to people in the placebo group. In general, the estimates of effect were uncertain with wide 95% CIs. Adverse effects were not reported in Ball 2011.
9. Side effects.
| Outcome | Acetalomazide | Placebo | OR (95% CI) | ||
| n | N | n | N | ||
| Elevated ALT | 6 | 69 | 3 | 79 | 1.90 (0.46 to 7.87) |
| Decreased CO2 | 9 | 69 | 0 | 79 | 19.49 (1.12 to 340.66) |
| Diarrhea | 12 | 69 | 3 | 79 | 4.11 (1.11 to 15.15) |
| Dizziness | 8 | 69 | 3 | 79 | 2.60 (0.66 to 10.16) |
| Dysgeusia | 13 | 69 | 0 | 79 | 29.20 (1.71 to 500.07) |
| Dyspepsia | 7 | 69 | 1 | 79 | 6.91 (0.83 to 57.49) |
| Dyspnea | 7 | 69 | 2 | 79 | 3.41 (0.69 to 16.94) |
| Fatigue | 14 | 69 | 1 | 79 | 15.17 (1.94 to 118.27) |
| Headache | 13 | 69 | 11 | 79 | 1.10 (0.46 to 2.62) |
| Nasopharyngitis | 5 | 69 | 8 | 79 | 0.55 (0.17 to 1.75) |
| Nausea | 26 | 69 | 10 | 79 | 2.99 (1.33 to 6.70) |
| Paresthesia | 41 | 69 | 5 | 79 | 13.48 (4.96 to 36.64) |
| Post‐LP syndrome | 5 | 69 | 6 | 79 | 0.75 (0.22 to 2.56) |
| Rash | 7 | 69 | 2 | 79 | 3.41 (0.69 to 16.94) |
| Sinusitis | 3 | 69 | 6 | 79 | 0.44 (0.11 to 1.82) |
| Tinnitus | 11 | 69 | 3 | 79 | 3.72 (1.00 to 13.85) |
| Vomiting | 12 | 69 | 3 | 79 | 4.11 (1.11 to 15.15) |
This data is from one study (Wall 2014).
Abbreviations: CI = confidence interval; n = number of events; N = number of participants; OR = odds ratio.
Costs
Quality of life
Data from the Wall 2014 RCT indicated that quality of life was better in acetazolamide‐treated patients based on the visual quality of life (VFQ‐25) (MD 6.35, 95% CI 2.22 to 10.47) and the physical (MD 3.02, 95% CI 0.34 to 5.70) and mental (MD 3.45, 95% CI 0.35 to 6.55) components of the 36‐Item Short Form Health Survey tool at six months (Table 10).
10. Quality of life.
| Outcome | Acetalomazide | Placebo | MD (95% CI) adjusted for centre, baseline value of outcome and baseline papilloedema grade | ||||
| Mean | SD | N | Mean | SD | N | ||
| Wall 2014: Change in VFQ‐25 total score between baseline and 6 months | 8.33 | SE 1.47a | a | 1.98 | SE 1.53a | a | 6.35 (2.22 to 10.47) |
| Wall 2014: Change in VFQ‐25 10‐item neuro‐ophthalmic supplement between baseline and 6 months | 9.82 | SE 1.55a | a | 1.59 | SE 1.62a | a | 8.23 (3.89 to 12.56) |
| Wall 2014: Change in SF‐36 Physical Component Summary between baseline and 6 months | 5.84 | SE 1.01a | a | 2.82 | SE 1.03a | a | 3.02 (0.34 to 5.70) |
| Wall 2014: Change in SF‐36 Mental Component Summary between baseline and 6 months | 5.62 | SE 1.16a | a | 2.17 | SE 1.17a | a | 3.45 (0.35 to 6.55) |
aIt is unclear how many patients were included in these analyses. Abbreviations: CI = confidence interval; N = number of participants; SE = standard error; SD = standard deviation.
GRADE assessment
We downgraded for risk of bias and imprecision for all outcomes and judged all data included in this review to be low quality (GRADEpro 2014).
Discussion
Summary of main results
Two RCTs met the inclusion criteria of our review (Ball 2011; Wall 2014). Ball 2011 detected a small benefit for the use of acetazolamide based upon an aggregate outcome score at 12 months. Alongside this, the RCT demonstrated an improvement in clinician‐decided outcome at 12 months (remission/improving versus stable/deteriorating) in the treatment group compared with the control group. The data showed that there was a reduction in headache (presence versus absence), visual loss, transient visual obscuration and improvements on binocular contrast sensitivity. However, Ball 2011 did not find evidence of reduction in papilloedema, headache severity, improvement in visual acuity and visual field outcomes. Wall 2014, on the other hand, demonstrated an improvement in PMD at six months in the acetazolamide group compared to placebo. Alongside this, the trial demonstrated an improvement in the treatment group's papilloedema, quality of life, weight loss and CSF opening pressure compared to the placebo group. However, it did not find a benefit in visual acuity or symptomatic headache relief.
Overall completeness and applicability of evidence
In Ball 2011, participants were excluded if the treating clinician felt there was a more appropriate therapy than acetazolamide. There is considerable risk that the group of participants included in this RCT were not representative of the IIH population but instead represent IIH patients in which the management decision seems controversial. As such, the trial authors suggest that the participants selected may have had a relatively benign disease course. The trial authors stated that the design and size of the trial was not to detect treatment effect, but rather to assist the design in future RCT. Comparatively, Wall 2014 has a significantly more robust design. However, the trial suffered from a high (19%) withdrawal rate bringing into question the applicability of the results. It is also important to highlight that the trial only included participants with mild (‐2 to ‐7 dB) visual loss, meaning that evidence for the use of acetazolamide in participants with moderate to severe visual loss is lacking.
Quality of the evidence
There is now evidence for the use of acetazolamide in IIH patients with mild visual loss (Wall 2014). The benefit demonstrated in the RCT is modest and the trial suffered from a high attrition rate. However, as indicated in the results of our GRADE assessment, further research is required for us to be confident in the estimate of effect for several outcomes.
Potential biases in the review process
As indicated in the Methods for this review, we did not handsearch references or consider references from conference proceedings. We may have failed to detect all relevant, and perhaps negative, studies.
Agreements and disagreements with other studies or reviews
To our knowledge, there has been no other systematic review on interventions specifically for people with IIH.
Authors' conclusions
Implications for practice.
There remains insufficient evidence on the efficacy of acetazolamide or any other treatments currently available for treating people with IIH.
Implications for research.
This Cochrane review continues to demonstrate the lack of well‐designed RCTs of the various treatments used for treating people with IIH. Many of the treatments have significant potential complications, as well as major resource implications. Existing studies do not allow quantification of either relative or absolute benefit of any of the treatments and there is a compelling need for large RCTs that can provide this information.
What's new
| Date | Event | Description |
|---|---|---|
| 3 August 2015 | New search has been performed | Issue 8, 2015: A new review team has taken over the update of this review. |
| 3 August 2015 | New citation required but conclusions have not changed | Issue 8, 2015: We performed updated searches in July 2015 and two trials met the inclusion criteria (Ball 2011; Wall 2014). |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 3, 2002
| Date | Event | Description |
|---|---|---|
| 10 August 2009 | New search has been performed | Issue 4, 2009: Updated searches yielded no new trials. |
| 15 October 2008 | Amended | Converted to new review format. |
| 19 May 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We have used text adapted from a standard template used by the Cochrane Eyes and Vision Review Group (CEVG) in the Methods section. We thank James Acheson for peer review comments on the original version of this review. The CEVG developed and performed the electronic searches. We thank Jennifer Evans and Anupa Shah from the CEVG editorial team for their assistance with the review process. We thank Christian Lueck (CL) and Gawn McIllwaine (GM) for their work on the original published versions of this review (Lueck 2002; Lueck 2005).
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Pseudotumor Cerebri #2 MeSH descriptor Sinus Thrombosis, Intracranial #3 MeSH descriptor Intracranial Hypertension #4 MeSH descriptor Papilledema #5 (#1 OR #2 OR #3 OR #4) #6 intracranial #7 intra cranial #8 intercranial #9 inter cranial #10 (#6 OR #7 OR #8 OR #9) #11 hypertens* or pressur* #12 increas* or elevat* or high* #13 benign* or idiopathic* or secondary #14 (#10 AND #11 AND #12 AND #13) #15 pseudotumor or pseudo tumor* near cerebr* #16 pseudoabscess* or pseudo abscess* #17 otitic or toxic* near hydroceph* #18 sinus near thrombosis #19 meningeal near hydrop* #20 (#15 OR #16 OR #17 OR #18 OR #19) #21 (#5 OR #14 OR #20)
Appendix 2. MEDLINE (Ovid) search strategy
1 randomized controlled trial.pt. 2 (randomized or randomised).ab,ti. 3 placebo.ab,ti. 4 dt.fs. 5 randomly.ab,ti. 6 trial.ab,ti. 7 groups.ab,ti. 8 or/1‐7 9 exp animals/ 10 exp humans/ 11 9 not (9 and 10) 12 8 not 11 13 exp pseudotumor cerebri/ 14 exp sinus thrombosis intracranial/ 15 exp intracranial hypertension/ 16 exp papilledema/ 17 or/13‐16 18 intra?cranial.tw. 19 intra cranial.tw. 20 inter?cranial.tw. 21 inter cranial.tw. 22 or/18‐21 23 (hypertens$ or pressur$).tw. 24 (increas$ or elevat$ or high$).tw. 25 (benign$ or idiopathic$ or secondary).tw. 26 22 and 23 and 24 and 25 27 ((pseudotumor or pseudo tumor$) adj3 cerebr$).tw. 28 (pseudoabscess$ or pseudo abscess$).tw. 29 ((otitic or toxic$) adj5 hydroceph$).tw. 30 (sinus adj3 thrombosis).tw. 31 (meningeal adj3 hydrop$).tw. 32 or/13‐15 33 or/26‐32 34 12 and 33
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. EMBASE (Ovid) search strategy
1 exp randomized controlled trial/ 2 exp randomization/ 3 exp double blind procedure/ 4 exp single blind procedure/ 5 random$.tw. 6 or/1‐5 7 (animal or animal experiment).sh. 8 human.sh. 9 7 and 8 10 7 not 9 11 6 not 10 12 exp clinical trial/ 13 (clin$ adj3 trial$).tw. 14 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15 exp placebo/ 16 placebo$.tw. 17 random$.tw. 18 exp experimental design/ 19 exp crossover procedure/ 20 exp control group/ 21 exp latin square design/ 22 or/12‐21 23 22 not 10 24 23 not 11 25 exp comparative study/ 26 exp evaluation/ 27 exp prospective study/ 28 (control$ or prospectiv$ or volunteer$).tw. 29 or/25‐28 30 29 not 10 31 30 not (11 or 23) 32 11 or 24 or 31 33 exp brain pseudotumor/ 34 exp cerebral sinus thrombosis/ 35 exp intracranial hypertension/ 36 exp papilledema/ 37 or/33‐36 38 intra?cranial.tw. 39 intra cranial.tw. 40 inter?cranial.tw. 41 inter cranial.tw. 42 or/38‐41 43 (hypertens$ or pressur$).tw. 44 (increas$ or elevat$ or high$).tw. 45 (benign$ or idiopathic$ or secondary).tw. 46 42 and 43 and 44 and 45 47 ((pseudotumor or pseudo tumor$) adj3 cerebr$).tw. 48 (pseudoabscess$ or pseudo abscess$).tw. 49 ((otitic or toxic$) adj5 hydroceph$).tw. 50 (sinus adj3 thrombosis).tw. 51 (meningeal adj3 hydrop$).tw. 52 or/47‐51 53 37 or 46 or 52 54 32 and 53
Appendix 4. ISRCTN search strategy
(benign or idiopathic) and intracranial hypertension
Appendix 5. Clinicaltrials.gov search strategy
(Benign OR Idiopathic) AND Intracranial Hypertension
Appendix 6. ICTRP search strategy
Idiopathic Intracranial Hypertension
Benign Intracranial Hypertension
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ball 2011.
| Methods | Multicentre, open‐label, parallel‐group RCT. | |
| Participants | Participants had IIH that met Friedman's criteria (Friedman 2002). 50 participants from six UK centres. Two arms: 25 randomised to treatment with acetazolamide and 25 randomised to placebo. |
|
| Interventions | Participants were randomised to either the acetazolamide group or the placebo group. All participants were encouraged to lose weight. |
|
| Outcomes | Measured at baseline, 3, 6, 9 and 12 months. Primary outcomes were measured as an aggregate score (out of 15) on final visit (each outcome measure was ranked as being: absent (0); present, stable (1); deteriorating (2)):
Secondary outcomes (and methods):
Right and left eye data were reported separately. |
|
| Notes | In addition, at 12 months, clinicians were asked to select the term that best described the participant from the following options:
Date study conducted:
Funding:
Conflict of interest:
Trial registration ID:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised by computer‐generated random list. No difference in baseline characteristics detected between arms. |
| Allocation concealment (selection bias) | Low risk | Neither participant or treating clinician was masked to allocation. Allocation was communicated to treating clinicians via telephone. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither participant or treating clinician was masked to allocation. Dosing schedules for acetazolamide decided by prescribing clinician. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No masking of assessor reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Five participants from the placebo arm started on acetazolamide therapy. Two participants (one from each of the two arms) underwent surgical intervention. 12 participants from the acetazolamide arm discontinued treatment during the study period. |
| Selective reporting (reporting bias) | Low risk | Primary outcome data provided in full. Data not provided for anxiety, depression, patient reported health status assessment. |
Wall 2014.
| Methods | Multicentre, double‐masked, parallel‐group RCT | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes | A study eye was decided: this was the eye with poorer visual acuity. Measurement of outcomes was performed at baseline, 1, 2, 3, 4, 5 and 6 months (apart from HIT‐6 and quality of life, which were only at six months). Primary outcomes (and methods):
Secondary outcomes (and methods):
|
|
| Notes | Date study conducted:
Funding:
Conflict of interest:
Trial registration ID:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed per site, but the method has not been described. |
| Allocation concealment (selection bias) | Low risk | Both participants and clinicians were masked to allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Robust masking policy with the managing staff blinded to treatment status except for programmer who generated the randomization plan, a statistician and a member of staff involved with packaging the drugs. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 19% withdrawal rate. In the treatment group 16 patients withdrew for the following reasons: lost to follow‐up (6), withdrew consent (4), time commitment (3), desired active treatment (1), moved (1), and treatment failure later adjudicated to be performance failure (1). Sixteen patients in the placebo group were withdrawn for the following reasons: lost to follow‐up (9), time commitment (5), adverse event (1), needed disallowed medication (1). |
| Selective reporting (reporting bias) | Low risk | Data provided on all the secondary outcomes. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abubaker 2011 | Retrospective review of 25 patients treated with either lumboperitoneal shunt or ventriculoperitoneal shunt. |
| Aguilar Perez 2013 | Prospective study of 29 patients undergoing endovascular treatment of IIH. No control group. |
| Ahmed 2011 | No control group. Retrospective study of 52 patients with IIH and venous sinus stenosis treated with transverse sinus stenting. |
| Ahmed 2014 | Retrospective analysis of 86 adults with IIH who underwent transverse sinus stenting compared with 110 children treated with CSF shunting for hydrocephalus. |
| Albuquerque 2011 | No control group. Retrospective review of 19 patients who underwent venous sinus stenting. |
| Alsuhaibani 2011 | Retrospective review of 78 patients who underwent optic nerve fenestration and 20 control patients who did not. |
| Bussière 2010 | No control group. Retrospective review of 13 female patients who underwent venous sinus stenting. |
| Bynke 2004 | No control group. Retrospective study. 17 patients treated with ventriculo‐peritoneal shunting. |
| Egan 2011 | No control group. Prospective study of four patients who underwent laparoscopic adjustable gastric banding. |
| Fonseca 2014 | Case series of 33 patients undergoing either optic nerve sheath fenestration or cerebrospinal fluid diversion. No control group. |
| Gates 2013 | No control group. Prospective following of seven patients and retrospective review of a different six patients. Total of 13 patients treated with CSF drainage at low pressure. |
| Halmagyi 2010 | No control group. Prospective study of 38 patients treated with venous sinus stenting. |
| Herzau 1998 | No control group. Late review of 14 patients (23 eyes) treated by optic nerve sheath fenestration. |
| Heyman 2013 | No control group. Retrospective review of 10 children treated with ventriculo‐peritoneal shunt (VPS) insertion using frameless stereotaxy. |
| Johnson 1998 | No control group. Retrospective study. 15 patients treated with acetazolamide and weight loss. |
| Kandasamy 2011 | No control group. Retrospective review and three‐year prospective follow‐up of 17 patients treated with custom‐designed electromagnetic image‐guided ventriculoperitoneal shunt placement. |
| Lin 2012 | Technical validation of a novel 'bi‐corporal' pump to provide intermittent CSF drainage. |
| NCT01407809 | No control group. Prospective trial of venous sinus stenting in IIH patients refractory to medical therapy. |
| NCT02143258 | No control group. Single assignment trial of venous sinus stenting for refractory IIH. |
| Nemeth 1995 | Retrospective review of the outcomes after three years in 39 cases of refenestration, 15 cases of acetazolamide and 14 cases of neurosurgical shunt insertion. |
| Nithyanandam 2008 | No controls. Retrospective review of 21 patients treated with optic nerve sheath decompression. |
| Owler 2003 | No control group. 4 patients treated with endoluminal stent insertion into transverse venous sinus. |
| Raoof 2010 | Retrospective review of 31 patients treated with CSF diversion surgery. |
| Salman 2001 | Retrospective review of 32 patients. |
| Sesenna 1996 | No control group. 8 patients (10 eyes) treated with optic nerve sheath fenestration. |
| Sinclair 2010 | Prospective cohort study following 25 women adhering to a low energy diet. |
| Sinclair 2014 | Prospective cohort study investigating outcomes before and after weight loss. No control group. |
| Sugerman 1999 | No control group. 24 obese patients underwent surgery for weight loss. |
| Sugerman 2001 | No control group, treatment not standardised, numbers small. 7 patients treated with external device to generate negative intra‐abdominal pressure. |
| Tacke 2012 | Single case report. |
| Tarnaris 2011 | Retrospective review of 34 patients who underwent CSF fluid diversion. |
| Teleb 2015 | Case series of 18 patients. No control group. |
| Warman 2000 | Retrospective review of 22 patients. |
| Çelebisoy 2007 | No placebo control group. No randomisation: 41 patients alternately allocated to treatment with acetazolamide or topiramate. |
Characteristics of ongoing studies [ordered by study ID]
NCT02017444.
| Trial name or title | Lowering Intracranial Pressure in Idiopathic Intracranial Hypertension: Assessing the Therapeutic Efficacy and Safety of an 11β‐hydroxysteroid Dehydrogenase Type 1 Inhibitor (AZD4017). Phase II Study. |
| Methods | Double‐blind, parallel assignment RCT |
| Participants |
|
| Interventions | AZD4017 (11b‐HSD1 inhibitor) 400 mg tablet twice daily for 12 weeks versus matched placebo tablet twice‐daily for 12 weeks |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
Other outcome measures:
|
| Starting date | January 2014 |
| Contact information | Alexandra Sinclair; a.b.sinclair@bham.ac.uk |
| Notes | NCT02017444 |
NCT02124486.
| Trial name or title | A Randomised Controlled Trial of Bariatric Surgery Versus a Community Weight Loss Programme for the Sustained Treatment of Idiopathic Intracranial Hypertension: the IIH:WT Trial |
| Methods | Open‐label, parallel assignment RCT |
| Participants | Inclusion criteria:
|
| Interventions | Patients will be assigned to one of four arms:
|
| Outcomes | Primary outcomes:
Secondary outcomes:
Other outcome measures:
|
| Starting date | March 2014 |
| Contact information | Alexandra Sinclair; a.b.sinclair@bham.ac.uk |
| Notes |
Differences between protocol and review
We have updated the protocol of this review in line with current methodological expectations for Cochrane reviews. Therefore there are some differences compared with the original published protocol. However, we did not use these updated methods in this review version because only two trials met the inclusion criteria and we were unable to perform meta‐analysis. We may use the updated methods in future review updates.
Contributions of authors
CL and GM conceived and designed the review (Lueck 2002; Lueck 2005).
Original review
CL wrote the protocol and discussed the search strategy with the Cochrane Eyes and Vision Group editorial team. He was responsible for reading and assessing studies identified by the search (with regard to both inclusion criteria and quality), and for writing the definitive review. GM also read and assessed studies identified by the search (with regard to both inclusion criteria and quality), and assisted in the writing of the definitive review. CL coordinated the review and screened the search results. CL and GM screened the retrieved papers against inclusion criteria, and appraised the quality of the papers. CL wrote the review.
2015 update
RJP coordinated the review update. RJP and AMHY screened the search results. AABJ acted as a third party decision on screening disagreements. RJP and AABJ screened retrieved papers against inclusion criteria and appraised the quality of the papers. RJP, AVK, AMHY, MAH, AABJ and IPF wrote the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision Group (CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEVG Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
RJP, AMHY, AABJ, AVK, MAH, IPF: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ball 2011 {published data only}
- Ball AK, Howman A, Wheatley K, Burdon MA, Matthews T, Jacks AS, et al. A randomised controlled trial of treatment for idiopathic intracranial hypertension. Journal of Neurology 2011;258(5):874‐81. [DOI] [PubMed] [Google Scholar]
Wall 2014 {published data only}
- Digre KB, Bruce BB, McDermott MP, Galetta KM, Balcer LJ, Wall M. Quality of life in idiopathic intracranial hypertension at diagnosis IIH Treatment Trial results. Neurology 2015;84(24):2449‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam B, Falardeau J, Fletcher W, Granadier R, Longmuir R, Patel A, et al. Risk factors for poor visual outcome in idiopathic intracranial hypertension patients with mild visual loss. 67th American Academy of Neurology Annual Meeting; 2015 April 18‐25, Washington DC.
- NORDIC Idiopathic Intracranial Hypertension Study Group Writing Committee, Wall M, McDermott MP, Kieburtz KD, Corbett JJ, Feldon SE, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA 2014;311(16):1641‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abubaker 2011 {published data only}
- Abubaker K, Ali Z, Raza K, Bolger C, Rawluk D, O'Brien D. Idiopathic intracranial hypertension: lumboperitoneal shunts versus ventriculoperitoneal shunts ‐ case series and literature review. British Journal of Neurosurgery 2011;25(1):94‐9. [DOI] [PubMed] [Google Scholar]
Aguilar Perez 2013 {published data only}
- Aguilar Perez M, Kurre K, Fischer S, Horward‐Rizea D, Unsold R, Bazner H, et al. [Endovascular treatment in "Bening" intracranial hypertension. Clinical result and long‐term follow‐up]. Neuroradiology. 37th European Society of Neuroradiology Annual Meeting; 2013 28 Sept‐1 Oct; Frankfurt. 2013.
Ahmed 2011 {published data only}
- Ahmed RM, Wilkinson M, Parker GD, Thurtell MJ, Macdonald J, McCluskey PJ, et al. Transverse sinus stenting for idiopathic intracranial hypertension: a review of 52 patients and of model predictions. AJNR. American Journal of Neuroradiology 2011;32(8):1408‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahmed 2014 {published data only}
- Ahmed RM, Zmudzki F, Parker GD, Owler BK, Halmagyi GM. Transverse sinus stenting for pseudotumor cerebri: a cost comparison with CSF shunting. American Journal of Neuroradiology 2014;35(5):952‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Albuquerque 2011 {published data only}
- Albuquerque FC, Dashti SR, Hu YC, Newman CB, Teleb M, McDougall CG, et al. Intracranial venous sinus stenting for benign intracranial hypertension: clinical indications, technique, and preliminary results. World Neurosurgery 2011;75(5‐6):648‐52. [DOI] [PubMed] [Google Scholar]
Alsuhaibani 2011 {published data only}
- Alsuhaibani AH, Carter KD, Nerad JA, Lee AG. Effect of optic nerve sheath fenestration on papilledema of the operated and the contralateral nonoperated eyes in idiopathic intracranial hypertension. Ophthalmology 2011;118(2):412‐4. [DOI] [PubMed] [Google Scholar]
Bussière 2010 {published data only}
- Bussière M, Falero R, Nicolle D, Proulx A, Patel V, Pelz D. Unilateral transverse sinus stenting of patients with idiopathic intracranial hypertension. AJNR. American Journal of Neuroradiology 2010;31(4):645‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bynke 2004 {published data only}
- Bynke G, Zemack G, Bynke H, Romner B. Ventriculoperitoneal shunting for idiopathic intracranial hypertension. Neurology 2004;63(7):1314‐6. [DOI] [PubMed] [Google Scholar]
Çelebisoy 2007 {published data only}
- Çelebisoy N, Gökçay F, Sirin H, Akyürekli O. Treatment of idiopathic intracranial hypertension: topiramate vs acetazolamide, an open‐label study. Acta Neurologica Scandinavica 2007;116(5):322‐7. [DOI] [PubMed] [Google Scholar]
Egan 2011 {published data only}
- Egan RJ, Meredith HE, Coulston JE, Bennetto L, Morgan JD, Norton SA. The effects of laparoscopic adjustable gastric banding on idiopathic intracranial hypertension. Obesity Surgery 2011;21(2):161‐6. [DOI] [PubMed] [Google Scholar]
Fonseca 2014 {published data only}
- Fonseca PL, Rigamonti D, Miller NR, Subramanian PS. Visual outcomes of surgical intervention for pseudotumour cerebri: optic nerve sheath fenestration versus cerebrospinal fluid diversion. British Journal of Ophthalmology 2014;90(10):1360‐3. [DOI] [PubMed] [Google Scholar]
Gates 2013 {published data only}
- Gates P, Christensen J. Immediate resolution of idiopathic intracranial hypertension with drainage of CSF at low pressure. www.neurology.org/cgi/content/meeting_abstract/80/1_MeetingAbstracts/P02.246 (accessed 5 April 2014).
Halmagyi 2010 {published data only}
- Halmagyi M, Parker G, Thurtell M, Macdonald J, Ahmed R, Wilkinson M, et al. Management of idiopathic intracranial hypertension with transverse sinus stenting. Journal of Neurology; Conference: 20th Meeting of the European Neurological Society; 2010 June 19‐23; Berlin. 2010.
Herzau 1998 {published data only}
- Herzau V, Baykal HE. Long‐term results of optic nerve sheath fenestration in pseudotumor cerebri [Langzeitergebnisse nach optikusscheidenfensterung bei pseudotumor cerebri]. Klinische Monatsblätter für Augenheilkunde 1998;213(3):154‐60. [DOI] [PubMed] [Google Scholar]
Heyman 2013 {published data only}
- Heyman J, Amato‐Watkins A, Water Naude J, Gibbon F, Leach P. Frameless stereotactic navigation for ventriculoperitoneal shunt insertion for the treatment of idiopathic intracranial hypertension in children. 2013 Spring Meeting of the Society of British Neurological Surgeons; 2013 May 22‐24; Sheffield. 2013.
Johnson 1998 {published data only}
- Johnson LN, Krohel GB, Madsen RW, March GA Jr. The role of weight loss and acetazolamide in the treatment of idiopathic intracranial hypertension (pseudotumor cerebri). Ophthalmology 1998;105(12):2313‐7. [DOI] [PubMed] [Google Scholar]
Kandasamy 2011 {published data only}
- Kandasamy J, Hayhurst C, Clark S, Jenkinson MD, Byrne P, Karabatsou K, et al. Electromagnetic stereotactic ventriculoperitoneal CSF shunting for idiopathic intracranial hypertension: a successful step forward?. World Neurosurgery 2011;75(1):155‐60. [DOI] [PubMed] [Google Scholar]
Lin 2012 {published data only}
- Lin J, Cole D, Flatt M, Kroeter B, Warren S, Morris M. Design and testing of a 'Bi‐corporal' pump for the treatment of hydrocephalus and idiopathic intracranial hypertension. Journal of Neurosurgery: Pediatrics; Conference: 35th Annual Meeting of the American Society of Pediatric Neurosurgeons; 2012 Jan 31‐Feb 3; Rio Grande. 2012.
NCT01407809 {published data only}
- NCT01407809. Venous sinus stenting for idiopathic intracranial hypertension refractory to medical therapy (VSSIIH). clinicaltrials.gov/ct2/show/NCT01407809 (accessed 14 January 2015).
NCT02143258 {published data only}
- NCT02143258. Stenting of venous sinus stenosis for medically refractory idiopathic intracranial hypertension. clinicaltrials.gov/ct2/show/NCT02143258 (accessed 15 January 2015).
Nemeth 1995 {published data only}
- Nemeth GG, McHenry JG, Tinoosh F, Spoor TC, Blum LR. Comparison of acetazolamide, shunts, and refenestration for failed decompressions for pseudotumor cerebri. American Academy of Ophthalmology. 1995:140.
Nithyanandam 2008 {published data only}
- Nithyanandam S, Manayath GJ, Battu RR. Optic nerve sheath decompression for visual loss in intracranial hypertension: report from a tertiary care center in South India. Indian Journal of Ophthalmology 2008;56(2):115‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Owler 2003 {published data only}
- Owler BK, Parker G, Halmagyi GM, Dunne VG, Grinnell V, McDowell D, et al. Pseudotumor cerebri syndrome: Venous sinus obstruction and its treatment with stent placement. Journal of Neurosurgery 2003;98(5):1045‐5. [DOI] [PubMed] [Google Scholar]
Raoof 2010 {published data only}
- Raoof N, Panesar H, McMullan J, Sharrack B, Pepper IM, Hickman SJ. Outcome from CSF diversion surgery for idiopathic intracranial hypertension. Neuro‐Ophthalmology. Conference: 18th International Neuro‐Ophthalmology Society; 2010 June 19‐22; Lyon. 2010.
Salman 2001 {published data only}
- Salman MS, Kirkham FJ, MacGregor DL. Idiopathic "benign" intracranial hypertension: case series and review. Journal of Child Neurology 2001;16(7):465‐70. [DOI] [PubMed] [Google Scholar]
Sesenna 1996 {published data only}
- Sesenna E, Monteverdi R, Gandolfi S, Nizzoli V. Optic nerve sheath decompression (perioptic meningectomy) through a lateral orbitotomy approach: Indications and technique. Rivista di Neurobiologia 1996;42(4):313‐21. [Google Scholar]
Sinclair 2010 {published data only}
- Sinclair AJ, Burdon MA, Nightingale PG, Ball AK, Good P, Matthews TD, et al. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ 2010;340:c2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinclair 2014 {published data only}
- Sinclair A, Mulla Y, Markey K, Mitchell J, Patel S. Headache determines quality of life in idiopathic intracranial hypertension.. Journal of Headache and Pain Conference: 4th European Headache and Migraine Trust International Congress. Copenhagen, 2014. [DOI] [PMC free article] [PubMed]
Sugerman 1999 {published data only}
- Sugerman HJ, Felton WL 3rd, Sismanis A, Kellum JM, DeMaria EJ, Sugerman EL. Gastric surgery for pseudotumor cerebri associated with severe obesity. Annals of Surgery 1999;229(5):634‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sugerman 2001 {published data only}
- Sugerman HJ, Felton III WL 3rd, Sismanis A, Saggi BH, Doty JM, Blocher C, et al. Continuous negative abdominal pressure device to treat pseudotumor cerebri. International Journal of Obesity and Related Metabolic Disorders 2001;25(4):486‐90. [DOI] [PubMed] [Google Scholar]
Tacke 2012 {published data only}
- Tacke U, Budde J, Korinthenberg R. Pseudotumor cerebri under prednisolone treatment for infantile spasms. Neuropediatrics. Conference: 38th Annual Meeting of the Society of Neuropediatrics; 2012 Apr 19‐22; Munster. 2012.
Tarnaris 2011 {published data only}
- Tarnaris A, Toma AK, Watkins LD, Kitchen ND. Is there a difference in outcomes of patients with idiopathic intracranial hypertension with the choice of cerebrospinal fluid diversion site: a single centre experience. Clinical Neurology and Neurosurgery 2011;113(6):477‐9. [DOI] [PubMed] [Google Scholar]
Teleb 2015 {published data only}
- Teleb MS, Cziep ME, Issa M, Lazzaro M, Asif K, Hun Hong S, et al. Stenting and angioplasty for idiopathic intracranial hypertension: a case series with clinical, angiographic, ophthalmological, complication, and pressure reporting. Journal of Neuroimaging 2015;25(1):72‐80. [DOI] [PubMed] [Google Scholar]
Warman 2000 {published data only}
- Warman R. Management of pseudotumor cerebri in children. International Pediatrics 2000;15(3):147‐50. [Google Scholar]
References to ongoing studies
NCT02017444 {published data only}
- NCT02017444. Lowering intracranial pressure in idiopathic intracranial hypertension: assessing the therapeutic efficacy and safety of an 11β‐hydroxysteroid dehydrogenase type 1 inhibitor (AZD4017). Phase II study. clinicaltrials.gov/ct2/show/NCT02017444 (accessed 16 January 2015).
NCT02124486 {published data only}
- ISRCTN40152829. Bariatric surgery versus a community weight loss programme for the sustained treatment of Idiopathic Intracranial Hypertension. www.isrctn.com/ISRCTN40152829 (accessed 23 July 2015).
- NCT02124486. A randomised controlled trial of bariatric surgery versus a community weight loss programme for the sustained treatment of idiopathic intracranial hypertension: the IIH:WT trial. clinicaltrials.gov/ct2/show/NCT02124486 (accessed 16 January 2015).
Additional references
Corbett 1982
- Corbett JJ, Savino PJ, Thompson HS, Kansu T, Schatz NJ, Orr LS, et al. Visual loss in pseudotumor cerebri. Follow‐up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Archives of Neurology 1982;39(8):461‐74. [DOI] [PubMed] [Google Scholar]
Donahue 2000
- Donahue SP. Recurrence of idiopathic intracranial hypertension after weight loss: the carrot craver. American Journal of Ophthalmology 2000;130(6):850‐1. [DOI] [PubMed] [Google Scholar]
Durcan 1988
- Durcan FJ, Corbett JJ, Wall M. The incidence of pseudotumor cerebri. Population studies in Iowa and Louisiana. Archives of Neurology 1988;45(8):875‐7. [DOI] [PubMed] [Google Scholar]
Friedman 2002
- Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology 2002;59(10):1492‐5. [DOI] [PubMed] [Google Scholar]
Galgano 2013
- Galgano MA, Deshaies EM. An update on the management of pseudotumor cerebri. Clinical Neurology and Neurosurgery 2013;115(3):252‐9. [DOI] [PubMed] [Google Scholar]
Giuseffi 1991
- Giuseffi V, Wall M, Siegel PZ, Rojas PB. Symptoms and disease associations in idiopathic intracranial hypertension (pseudotumor cerebri): a case‐control study. Neurology 1991;41(2 Pt 1):239‐44. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- Macmasters University. Computer program on www.gradepro.org. 2014: Macmasters University, (accessed 20 March 2015).
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ireland 1990
- Ireland B, Corbett JJ, Wallace RB. The search for causes of idiopathic intracranial hypertension. A preliminary case‐control study. Archives of Neurology 1990;47(3):315‐20. [DOI] [PubMed] [Google Scholar]
Johnston 1974a
- Johnston I, Paterson A. Benign intracranial hypertension. I. Diagnosis and prognosis. Brain 1974;97(2):289‐300. [DOI] [PubMed] [Google Scholar]
Johnston 1974b
- Johnston I, Paterson A. Benign intracranial hypertension. II. CSF pressure and circulation. Brain 1974;97(2):301‐12. [DOI] [PubMed] [Google Scholar]
Karahalios 1996
- Karahalios DG, Rekate HL, Khayata MH, Apostolides PJ. Elevated intracranial venous pressure as a universal mechanism in pseudotumor cerebri of varying etiologies. Neurology 1996;46(1):198‐202. [DOI] [PubMed] [Google Scholar]
Klein 2013
- Klein A, Stern N, Osher E, Kliper E, Kesler A. Hyperandrogenism is associated with earlier age of onset of idiopathic intracranial hypertension in women. Current Eye Research 2013;38(9):972‐6. [DOI] [PubMed] [Google Scholar]
Lipton 1972
- Lipton HL, Michelson PE. Pseudotumor cerebri syndrome without papilledema. JAMA 1972;220(12):1591‐2. [PubMed] [Google Scholar]
Lochhead 2003
- Lochhead J, Elston JS. Doxycycline induced intracranial hypertension. BMJ 2003;326(7390):641‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathew 1996
- Mathew NT, Ravishankar K, Sanin LC. Coexistence of migraine and idiopathic intracranial hypertension without papilloedema. Neurology 1996;46(5):1226–30. [DOI] [PubMed] [Google Scholar]
Maxner 1987
- Maxner CE, Freedman MI, Corbett JJ. Asymmetric papilledema and visual loss in pseudotumour cerebri. Canadian Journal of Neurological Sciences 1987;14(4):593‐6. [PubMed] [Google Scholar]
Phillips 2012
- Phillips PH. Pediatric pseudotumor cerebri. International Ophthalmology Clinics 2012;52(3):51‐9, xii. [DOI] [PubMed] [Google Scholar]
Quincke 1896
- Quincke H. Über Meningitis serosa und verwandte Zustände [German]. Deutsche Zeitschrift für Nervenheilkunde 1896;9(3‐4):149‐68. [Google Scholar]
Radhakrishnan 1993a
- Radhakrishnan K, Ahlskog JE, Cross SA, Kurland LT, O'Fallon WM. Idiopathic intracranial hypertension (pseudotumor cerebri). Descriptive epidemiology in Rochester, Minnesota, 1976 to 1990. Archives of Neurology 1993;50(1):78‐80. [DOI] [PubMed] [Google Scholar]
Radhakrishnan 1993b
- Radhakrishnan K, Thacker AK, Bohlaga NH, Maloo JC, Gerryo SE. Epidemiology of idiopathic intracranial hypertension: a prospective and case‐control study. Journal of the Neurological Sciences 1993;116(1):18‐28. [DOI] [PubMed] [Google Scholar]
Radhakrishnan 1994
- Radhakrishnan K, Ahlskog JE, Garrity JA, Kurland LT. Idiopathic intracranial hypertension. Mayo Clinic Proceedings 1994;69(2):169‐80. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rowe 1998
- Rowe FJ, Sarkies NJ. Assessment of visual function in idiopathic intracranial hypertension: a prospective study. Eye 1998;12(Pt 1):111‐8. [DOI] [PubMed] [Google Scholar]
Sher 1983
- Sher NA, Wirtschafter J, Shapiro SK, See C, Shapiro I. Unilateral papilledema in 'benign' intracranial hypertension (pseudotumor cerebri). JAMA 1983;250(17):2346‐7. [PubMed] [Google Scholar]
Sugerman 1997
- Sugerman HJ, DeMaria EJ, Felton WL 3rd, Nakatsuka M, Sismanis A. Increased intra‐abdominal pressure and cardiac filling pressures in obesity‐associated pseudotumor cerebri. Neurology 1997;49(2):507‐11. [DOI] [PubMed] [Google Scholar]
Tabassi 2005
- Tabassi A, Salmasi AH, Jalali M. Serum and CSF vitamin A concentrations in idiopathic intracranial hypertension. Neurology 2005;64(11):1893‐6. [DOI] [PubMed] [Google Scholar]
Wall 1991
- Wall M, George D. Idiopathic intracranial hypertension. A prospective study of 50 patients. Brain 1991;114(Pt 1A):155‐80. [PubMed] [Google Scholar]
Warner 2002
- Warner JE, Bernstein PS, Yemelyanov A, Alder SC, Farnsworth ST, Digre KB. Vitamin A in the cerebrospinal fluid of patients with and without idiopathic intracranial hypertension. Annals of Neurology 2002;52(5):647‐50. [DOI] [PubMed] [Google Scholar]
Warner 2007
- Warner JE, Larson AJ, Bhosale P, Digre KB, Henley C, Alder SC, et al. Retinol‐binding protein and retinol analysis in cerebrospinal fluid and serum of patients with and without idiopathic intracranial hypertension. Journal of Neuro‐Ophthalmology 2007;27(4):258‐62. [DOI] [PubMed] [Google Scholar]
Weisberg 1975
- Weisberg LA. Benign intracranial hypertension. Medicine 1975;54(3):197‐207. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lueck 2002
- Lueck C, McIlwaine G. Interventions for idiopathic intracranial hypertension. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003434] [DOI] [PubMed] [Google Scholar]
Lueck 2005
- Lueck C, McIlwaine G. Interventions for idiopathic intracranial hypertension. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003434.pub2] [DOI] [PubMed] [Google Scholar]


