Abstract
Objective:
There is inconsistent evidence and limited data in the Hispanic population concerning fruit and vegetable intake and cancer risk. This study explored the effect of fruit and vegetable intake on cancer risk in Mexican-Americans.
Methods:
Participants in this cross-sectional study were drawn from the Cameron County Hispanic Cohort. Consumption of fruits and vegetables were assessed using a validated questionnaire. Cancer was self-reported by the participants based on being told by a health care provider that they had cancer.
Results:
Among 2,381 participants with available dietary data, 82 reported a diagnosis of cancer. Participants who met recommendations of 5 or more servings of fruit and vegetable per day had a significantly 86% lower risk for reported cancer compared with those who did not meet recommendations, after adjusting for other covariates. Every portion increment of total fruit and vegetable intake was significantly associated with the reduced cancer risk by 11% with the adjustment of other covariates.
Conclusions:
Fruit and vegetable intake was inversely associated with cancer risk in Mexican-Americans. Improving the consumption of fruit and vegetable might be an effective area for further research as part of a strategy for cancer prevention and control among Mexican-Americans independent of other factors.
Keywords: Fruit, vegetable, cancer, Mexican-Americans
INTRODUCTION
Two key components of lifestyle that most profoundly affect susceptibility to chronic disease are diet and physical activity. We previously reported that physical activity has an inverse effect on both metabolic conditions as well as risk for cancer (1). We now turn to a study of diet characterized by a high intake of fruits and vegetables that are associated with a lower risk of developing cardiovascular disease (2), hypertension (3) and diabetes (4). However, the evidence for the association between fruit and vegetable intake and cancer risk is controversial. A critical review suggested an inconsistent inverse relation between the consumption of fruit and vegetable and the risk of cancer based on the results from case-control and cohort studies (5). A meta-analysis of 13 cohort studies reported that the summary relative risk was 0.96 (95% confidence interval [CI]: 0.95–0.97) for any cancer incidence for each increase of 200 g/day fruit and vegetable intake (6). Only a few studies reported the associations in the Hispanic population and the evidence also showed inconsistent results (7–11). A case-control study also observed that low fruit and vegetable consumption did not contribute (independent of cigarette smoking) to the excess lung cancer risk in Mexican-Americans (8), while the multiethnic cohort study reported increased risks of prostate cancer were observed in relation to higher intakes of light green lettuce and dark leafy green vegetables among different ethnic groups including Hispanic people (11). In addition, some studies (12–15) included a small portion of Hispanic population and reported the associations of fruit and vegetable intake and bladder cancer risk. Castelao et al found inverse associations of vegetables and citrus fruits/juices with bladder cancer risk (15). Wu et al did not find significant associations between intake of fruits or vegetables and bladder cancer risk (14), and Park et al reported a significant finding in Hispanics (13). The two studies (13, 14) were limited by not including repeated diet measurements during long time follow-up as participants may have changed their diets substantially over time. The study by Lin et al did not find a significant association for intakes of total fruits or citrus fruits, but a significant one for cruciferous vegetable intake with bladder cancer risk (12). Given the inconsistent evidence and limited data in the Hispanic population, this study aimed to explore the association of fruit and vegetable intake on the occurrence of self-reported cancer in a randomly selected cohort of Mexican-American participants.
METHOD
Study Participants
This study was approved by the Committee for the Protection of Human Subjects of the University of Texas, Health Science Center at Houston and the Institutional Review Board of the University of Texas Health Science Center, San Antonio. All study participants gave written informed consent. This cross-sectional analysis used data from the Cameron County Hispanic Cohort (CCHC), an ongoing homogenous community-dwelling Mexican-American cohort study (16, 17). Study participants were recruited from randomly selected tract/blocks according to the 2000 Census as described previously (16, 17). At the baseline survey conducted between 2003 and 2016, 4,071 participants aged 18 years or older were recruited from their households in three predominantly Mexican-American cities along the Rio Grande Border with Mexico.
All participants responded to a detailed baseline survey of demographic characteristics, lifestyle including diet, physical activity, family and medical history, and other exposures. Participants were asked to fast for at least 10 hours overnight before a study visit at the Clinical Research Unit. Anthropometric measurements, including current weight and height, were taken (16, 17). Body mass index (BMI) was calculated as weight in kilograms divided by height squared in meters (kg/m2) (16). Waist circumference (WC) was measured at the level of the umbilicus and hip circumference (HC) at the level of the maximum width of the buttocks with participants in a standing position and breathing normally, to the nearest 0.2 cm. Waist-to-Hip (WHR) ratio was calculated as waist circumference divided by hip circumference. The average of three blood pressures (BP) measurements taken 5 minutes apart was used. Physical activity in a typical week according to intensity, frequency (times / week) and duration (minutes / time) was assessed using the International Physical Activity Questionnaire short-form (IPAQ)(18) before April 2010 or the Godin Leisure-Time Exercise Questionnaire instruments (19) after April 2010 as reported previously (20). The metabolic equivalent adjusted minutes (MET adjusted minutes) of moderate and vigorous physical activity in the past week was calculated based on responses (21). The MET intensity of physical activity was classified as light intensity (< 3 METs), moderate intensity (3–6 METs), and vigorous intensity (> 6 METs) (21). Physical activity ≥ 600 MET adjusted minutes per week was considered meeting United States physical activity guidelines (USDHHS, 2008)(21).
Fruit and vegetable intake
Using a validated Two-item Dietary Questionnaire (22, 23), fruit and vegetable consumption was assessed by asking participants how many portions of fruits and vegetables they consumed daily since 2008. A portion size was generally considered a 1/2 cup of fresh, frozen, or canned produce or a medium sized piece of produce (20). Consumption of five or more fruit and vegetable portions a day was considered meeting US dietary guidelines (24).
Identification of Cancer
Cancer diagnosis was identified in participants at their enrollment and initial interview as being told by a health care provider that they had cancer.
Laboratory Measurements
All participants provided a blood sample at their initial baseline visit. After collection, fasting samples were placed on ice and centrifuged within 30 minutes of collection. All plasma samples were stored at −80°C until laboratory analyses were conducted. Fasting lipid panel and plasma glucose analyses were performed by a local CLIA certified laboratory.
Definition of metabolic risk
Given the close relationship between metabolic risk and cancer (25), metabolic risk will be considered as a covariate in the analysis. Metabolic risk was defined as having ≥ 3 of the following metabolic abnormalities: WC ≥ 102 cm in men or ≥ 88 cm in women; systolic BP (SBP) ≥ 130 mmHg and/or diastolic BP (DBP) ≥ 85 mmHg or on antihypertensive medication; triglyceride ≥ 150 mg/dL; high-density lipoprotein cholesterol < 40 mg/dL in men or < 50 mg/dL in women; fasting glucose ≥ 100 mg/dL or on diabetes medication (26, 27).
Statistical analysis
Descriptive results and the models were adjusted for the probability sampling weights also taking into consideration clustering effects arising from the census block and household (16). Survey-weighted linear regression was used to obtain the t-test statistics to make comparisons for continuous data. Survey-weighted chi-square test was used to obtain the Rao-Scott F adjusted chi-square statistic to make comparisons for categorical data. Survey-weighted logistic regression modeling was performed to estimate the odds ratios (OR)s for cancer risk and their 95% CIs meeting recommendations of ≥ 5 servings of fruit and vegetable per day (yes vs no) after adjusting for other covariates, respectively. Similar logistic regression modeling was also used to estimate the effect (ORs and their 95% CIs) of total portions of fruit and vegetable intake per day on cancer risk. In order to compare if the effect of fruit and vegetable intake is more important than physical activity on cancer risk (1) as they are two important lifestyle factors, logistic regression modeling was used to estimate the combined effect of meeting recommendations of both fruit and vegetable intake ≥ 5 servings per day and physical activity ≥ 600 MET adjusted minutes on the risk of cancer, the effect of meeting recommendations of fruit and vegetable intake alone, and the effect of meeting recommendations of physical activity alone. Potential confounders adjusted for in multivariable-adjusted survey-weighted logistic regression models included age, gender, BMI, cigarette smoking status, meeting physical activity guideline, metabolic risk, and per capita income. The possible interaction terms such as income with fruit and vegetable intake, and other possible terms were also included in the model.
To illustrate the dose-response relationship between fruit and vegetable intake and cancer risk, we also used a restricted cubic spline logistic regression analysis (28) to evaluate the risk of cancer with daily total portions of fruit and vegetable intake. Knots were placed at the 5th, 50th, and 95th percentiles of the distribution of fruit and vegetable intake.
Statistical analyses were carried out by using SAS version 9.4 (SAS Institute, Cary, NC). All statistical tests were based on 2-sided probability.
RESULTS
At the time of this study, a total of 4,071 individuals were enrolled in the CCHC. Based on the availability of data, 1,608 participants without data on cancer/fruit and vegetable intake were excluded from the analyses. The characteristics of participants included in the analysis were not different from those excluded from the analysis. Of the remaining 2,463 participants, the mean age of this subset was 45.4 (standard deviation: 16.2) years; geometric mean per capita annual income was 5,480 (standard deviation: 2.6) US dollars, and 35% were males (Table 1). A total of 19.8% (n=153) of the participants met minimum recommendations for ≥ 5 servings of fruit and vegetable per day.
Table 1.
Cohort Demographics and Metabolic Characteristics Stratified by Cancer Risk: Cameron County Hispanic Cohort Study (2003–2016)a,b
| Cancer | |||
|---|---|---|---|
| Variable | Yes (n=82, 3.33%) | No (n=2,381, 96.67%) | P-value |
| Categorical variables, n (%) | |||
| Men | 28 (34.15) | 837 (35.15) | 0.58 |
| Employed | 27 (32.93) | 1144 (48.05) | 0.21 |
| Education, below high school | 43 (52.44) | 1126 (47.29) | 0.26 |
| Met minimum recommendations for physical activity of ≥ 600 MET-minutes/week | 12 (14.63) | 712 (29.90) | <.0001 |
| Met recommendations of ≥ 5 servings of fruit & vegetables per day | 10 (12.20) | 475 (19.95) | <.0001 |
| Ever smokers | 26 (31.71) | 713 (29.95) | 0.99 |
| Ever alcohol drinkers | 19 (23.17) | 872 (36.62) | 0.06 |
| Family history of cancer | 33 (40.24) | 665 (27.93) | 0.23 |
| Metabolically healthy | 45 (54.88) | 1126 (47.29) | 0.11 |
| Language used in the interview | |||
| English | 31 (43.06) | 736 (34.52) | 0.72 |
| Spanish | 41 (56.94) | 1396 (66.48) | |
| Continuous variables, mean (standard error) | |||
| Age at enrollment (years) | 63.42 (2.8) | 43.65 (1.3) | <0.0001 |
| Per capita annual income (US dollars)c | 8639 (1.15) | 7499 (1.08) | 0.37 |
| Body mass index (kg/m2) | 30.25 (1.16) | 31.54 (0.30) | 0.27 |
| Waist circumference (cm) | 105.12 (2.82) | 102.78 (1.05) | 0.43 |
| Waist-to-hip ratio | 0.96 (0.02) | 0.93 (0.01) | 0.03 |
| Total portions of fruit and vegetable intake per day | 2.38 (0.19) | 2.75 (0.12) | 0.09 |
Abbreviation: MET: metabolic equivalent
All descriptive results and the models were adjusted for the probability of sampling using weights taking into consideration clustering effects arising from the same census block and household. Linear regression models were used for continuous variables and Rao-Scott F adjusted chi-square statistic for categorical variables.
Geometric means. Annual income was calculated from the subject and spouse’s income.
Eighty-two participants of the cohort (3.33%) reported the history of cancers and 2,381 reported no cancers (Table 1). Participants with history of cancers were more likely to be older and to have higher WHR, and less likely to meet the recommended guidelines for consuming fruit and vegetable more than 5 servings per day, or to meet the recommended guidelines for physical activity of more than 600 MET adjusted minutes per week (all Ps<0.0001). There was no statistically significant difference in gender, employment status, ever cigarette smoking status, ever alcohol drinking, per capita annual household income, education, language used in the interview, family history of cancer, BMI, WC, and metabolically healthy status between participants with and without cancers. Table 2 shows the frequency of specific cancer sites. Seven had breast cancer among 38 subjects who provided 16 cancer sites and 44 subjects did not give cancer sites. Among 82 participants with cancers, 1 had both brain and uterus cancer.
Table 2.
Cancer types by specific sites: Cameron County Hispanic Cohort Study (2003–2016)
| Cancer Type | Frequency | Percentage |
|---|---|---|
| Breast cancer | 7 | 8.43% |
| Skin cancer | 5 | 6.02% |
| Cervical cancer | 4 | 4.82% |
| Uterine cancer | 4 | 4.82% |
| Colon cancer | 3 | 3.61% |
| Prostate cancer | 3 | 3.61% |
| Brain cancer | 2 | 2.41% |
| Esophagus cancer | 2 | 2.41% |
| Testicular cancer | 2 | 2.41% |
| Endometrial cancer | 1 | 1.20% |
| Kidney cancer | 1 | 1.20% |
| Leukemia | 1 | 1.20% |
| Lung cancer | 1 | 1.20% |
| Non-Hodgkin’s lymphoma | 1 | 1.20% |
| Cancer of the spine | 1 | 1.20% |
| Thyroid cancer | 1 | 1.20% |
| Unknown cancer | 44 | 53.01% |
| Total | 83 | 100.00% |
Compared with those who did not meet recommendations, participants who met recommendations of 5 or more servings of fruit and vegetable per day had 87% lower odds of reporting for cancers (OR=0.13; 95% CI: 0.05–0.31) after adjusting for age and gender (Table 3). The multivariable-adjusted model showed that the inverse association between meeting fruit and vegetable intake recommendation and cancer remained to be statistically significant (adjusted OR=0.14; 95% CI: 0.06–0.36) after further adjusting for smoking, BMI, meeting physical activity guideline, metabolic risk, per capita income and census tracts and blocks. Total portions of fruit and vegetable intake a day were significantly associated with the reduced risk of cancer after adjusting for age and gender; when daily total intake of fruit and vegetable increased 1 portion, the risk for cancer decreased 13% (OR=0.87; 95% CI: 0.78–0.97). The association did not materially change in the multivariable-adjusted model (OR=0.89; 95% CI: 0.80–0.99); when daily total intake of fruit and vegetable increased one portion, the risk for cancer decreased 11%. The multivariable-adjusted results did not materially change replacing BMI with WHR, or further adjusted for the language used in the interview. All interaction terms were not statistically significant and did not affect the results.
Table 3.
Association between fruit and vegetable intake and cancer risk: Cameron County Hispanic Cohort Study (2003–2016)
| Model | Met recommendations of ≥ 5 servings of fruit & vegetable per day | Total portions of fruit and vegetable intake per day | |||
|---|---|---|---|---|---|
| No | Yes | ||||
| OR | OR (95% CI) | P | OR (95% CI) | P | |
| Age, gender-adjusted model | 1.00 [Ref] | 0.13 (0.05–0.31) | < 0.0001 | 0.87 (0.78–0.97) | 0.01 |
| Multivariable-adjusted modela | 1.00 [Ref] | 0.14 (0.06–0.36) | <0.0001 | 0.89 (0.80–0.99) | 0.03 |
| Multivariable-adjusted modelb | 1.00 [Ref] | 0.15 (0.06–0.36) | <0.0001 | 0.89 (0.80–0.99) | 0.04 |
| Multivariable-adjusted modelc | 1.00 [Ref] | 0.10 (0.03–0.28) | <0.0001 | 0.88 (0.79–0.99) | 0.04 |
OR: odds ratio; CI: confidence interval; Ref: reference
Multivariable-adjusted model: adjusted for age, gender, smoking, body mass index, meeting physical activity guideline, metabolic risk, per capita income, and census tracts and blocks.
Multivariable-adjusted model: adjusted for age, gender, smoking, waist-to-hip ratio, meeting physical activity guideline, metabolic risk, per capita income, and census tracts and blocks.
Multivariable-adjusted model: adjusted for age, gender, smoking, body mass index, meeting physical activity guideline, metabolic risk, per capita income, language used in the interview, and census tracts and blocks.
Table 4 showed the combined effect of fruit & vegetable and physical activity on cancer frequency. Compared with those who met neither fruit & vegetable nor physical activity recommendations, participants who met fruit & vegetable recommendations alone had a 92% reduced frequency of cancer, participants who met physical activity recommendations alone had an 84% reduced frequency of cancer, and participants met both recommendations had an 80% reduced the risk of cancer. These results might reflect a small increase in the association of fruit & vegetable intake on cancer occurrence than physical activity alone.
Table 4.
Association between combined effect of fruit & vegetable intake and physical activity on cancer risk: Cameron County Hispanic Cohort Study (2003–2016)
| Groups | Age, gender-adjusted model | Multivariable-adjusted modela | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Reference group: met neither fruit & vegetable nor physical activity guidelines | 1.00 | 1.00 | ||
| Met recommendations of ≥ 5 servings of fruit & vegetable per day | 0.08 (0.03–0.23) | <0.0001 | 0.08 (0.03–0.23) | <0.0001 |
| Met minimum recommendations for physical activity of ≥ 600 MET-minutes/week | 0.15 (0.06–0.40) | 0.0001 | 0.16 (0.06–0.43) | 0.0003 |
| Met both guidelines of fruit & vegetable intake and physical activity | 0.19 (0.06–0.60) | 0.005 | 0.20 (0.06–0.61) | 0.005 |
OR: odds ratio; CI: confidence interval
Multivariable-adjusted model: adjusted for age, gender, smoking, body mass index, meeting physical activity guideline, metabolic risk, per capita income, and census tracts and blocks.
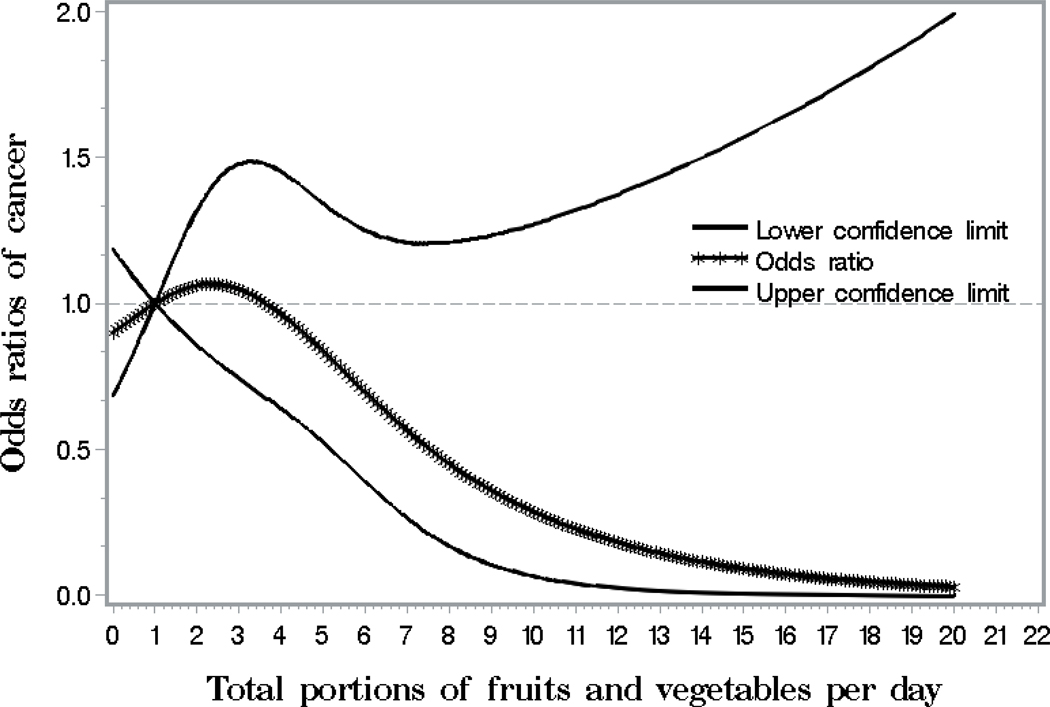
Figure 1 visually depicts the shape of the dose-response relationship between total portions of fruit and vegetable intake per day and cancer risk after adjusting for potential confounding variables in a restricted cubic spline model. Total portions of fruit and vegetable intake per day were inversely but not significantly associated with the risk of cancer (P for overall association = 0.26). If the total portions of fruit and vegetable intake were more than 4 per day, the ORs for cancer decreased with the increasing daily intake of fruit and vegetable.
Figure 1.
Smoothed plot for odds ratios (ORs) of the cancer risk according to total portions of fruit and vegetable intake per day. The ORs were estimated by using the restricted cubic-spline logistic regression models with knots placed at the 5th, 50th, and 95th percentiles of total portions of fruit and vegetable intake per day. The overall association between total portions of fruit and vegetable intake and the risk of cancer was not significant (P = 0.26). The model was adjusted for age, gender, smoking, body mass index, meeting physical activity guideline, metabolic risk, per capita income, and census tracts and blocks.
DISCUSSION
In a Mexican-American cohort, participants who reported 5 or more servings of fruit and vegetable per day had a significantly 87% lower odds of reporting history of cancer compared with those who did not meet recommendations, after adjusting for covariates. Total portions of fruit and vegetable intake per day were inversely associated with cancer risk; when total intake of fruit and vegetable per day increased by one portion, the risk of cancer decreased by 11% after adjusting for covariates.
The current evidence of the association of fruit and vegetable intake with cancer risk is inconsistent. A meta-analysis including 13 cohort studies reported an inverse association between fruit and vegetable intake and total cancer with a relative risk of 0.97 (95% CI: 0.95–0.99) (6), while another meta-analysis of 16 prospective cohort studies reported a null association (2). Only a few studies reported the associations in the Hispanic population (7–10). Some intervention studies reported that increasing fruit/vegetable intake increased biomarkers associated with the decreased breast cancer recurrence risk (9, 10), the other observational studies reported null associations between fruit and vegetable consumption and cancer risk (7, 8). According to the American Heart Association dietary guidelines, the recommendations for healthful food consumption include 5 or more portions of fruit and vegetable daily (24). Our study provides further evidence by showing the statistically significant inverse association between the consumption of fruit and vegetable and the risk of cancer in Mexican-Americans. Although further longitudinal data are needed, our findings still provide important support for further research on diet in the prevention and control of cancer among Mexican-Americans.
Obesity and type 2 diabetes are becoming increasingly prevalent worldwide, and both are associated with an increased incidence and mortality from many cancers (29). In our study, metabolic risk incorporating obesity, fasting glucose or on diabetes medication, blood pressure or on antihypertensive medication, triglyceride, and high-density lipoprotein cholesterol, was considered as a covariate in the analysis. As our previous study reported that meeting or exceeding recommended levels of moderate and vigorous physical activity was associated with a significantly reduced risk of cancer by Mexican-Americans (OR: 0.13; 95% CI: 0.03–0.54) in the CCHC (1), as well as our study showing the impact of physical activity on metabolic syndrome (30), meeting physical activity guideline is also considered as a covariate. Thus, our current study findings showed the independent association of fruit and vegetable intake and the risk of cancer after excluding the effect of obesity, type 2 diabetes, physical activity and other covariates.
This Two-Item Dietary Questionnaire (22) was developed as a short questionnaire to estimate fruit and vegetable intake. It categorizes whether respondents achieve the recommended daily intake of five portions of fruit and vegetables. The Two-item Dietary Questionnaire was confirmed by biomarkers (plasma ascorbic acid, β-carotene and α-tocopherol 24-hour urinary potassium excretion) for those eating less than five portions or fruit and vegetables a day (22). It is short which should allow it to be completed swiftly and useful for monitoring dietary preventive approaches in primary care without the use of invasive and costly biochemical measurements. It has also been used in a number of studies evaluating changes in dietary behavior following nutritional counselling. Therefore, it was recommended in 2010 by the National Obesity Observatory as a useful tool in measuring dietary intake (31).
The following underlying mechanisms might support the relationship between consumption of fruit and vegetable and cancer risk. As vegetable and fruit and the phytochemicals therein particularly influence not only inflammatory processes, but also cellular redox processes as well as endothelial and metabolic processes (32–36), which are involved in the pathogenesis of various diseases, it is assumed that these mechanisms are primarily responsible for the risk-reducing effect of vegetable and fruit consumption regarding the single diseases such as cancer. Fruit and vegetables contain a myriad of nutrients and phytochemicals, including fiber, vitamin C, carotenoids, antioxidants, potassium, flavonoids and other unidentified compounds which are likely to act synergistically through several biological mechanisms to reduce the risk of chronic diseases such as cancer (37). Dietary fiber and fruit and vegetable intakes have been shown to reduce inflammation and platelet aggregation, and to improve vascular and immune function (38–40). Antioxidants in fruit and vegetables may neutralize reactive oxygen species and reduce DNA damage (40), glucosinolates in cruciferous vegetables induce detoxifying enzymes (41) and intake of fruits, and vegetables and fiber may modulate steroid hormone concentrations and hormone metabolism (40). In addition, fruits and vegetables also contain a high water content and thus a low energy density. Consuming foods of low energy density assist in reducing energy intake and may help in weight control (24).
The study had several limitations. The study was cross-sectional in design; thus, only associations but not causal relationship may be inferred due to lack of a temporal relationship between exposure and disease such as fruit and vegetable changes after diagnosis or the influence of survival on cancer prevalence. One of the advantages of cross-sectional studies is that since data is collected all at once, it’s less likely that participants will quit the study before data is fully collected. Prospective studies are needed to further investigate the effect of fruit and vegetable intake on cancer risk. Our longitudinal data currently being collected will provide that opportunity once the sample size is sufficient for analysis. Fruit and vegetable intake was self-reported, which may affect its precision as a predictor. We only examined the association of fruit and vegetable consumption but other foods consumed by the participant were not accounted for and thus we are unable to make broad conclusions about eating patterns generally. Further, our measure of fruit and vegetable did not adjust for overall diet quality or energy consumption as we do not have full dietary data. Multi-pass and multiple day 24-hour recall data is certainly the gold standard in dietary assessment. As administering the 24-hour recalls would bring the participant, financial, analysis and staff burden for large population studies such as the Cameron County Hispanic Cohort, we did not use the 24-hour recalls but the more suitable Two-Item Dietary Questionnaire. Therefore, more brief options have been established, of which the Two-item Dietary Questionnaire is a recommended tool. Our results clearly indicate that fruit and vegetable consumption is clearly implicated in the health outcomes for our population that we can’t dismiss it (23, 42). Also, our results are consistent with those from other studies using the 24-hour recalls and Food Frequency Questionnaires (6). Cancer diagnosis was self-reported, which may not include those who had cancers but did not know their status due to no health insurance, not visiting health providers, death from cancers, or any other reasons. However, the self-reported approach we used has been validated in one study by correlating up to 84% of self-reported cancers with pathology from medical records (43) and cancer prevalence from our results were consistent with those from our previous reports in the same cohort (44). The recall bias could not be ruled out but the information we have collected in the study is the best available data in a large sample size study of Mexican-Americans. The fruit and vegetable intake is collected for a typical week, thus, the recall bias is also minimized. Per capita income was obtained based on the total income of participants and their spouses, but not everyone living in the same household, thus the current calculation of per capita income might cause inaccurate estimation. We could not completely rule out the possibility of residual confounding due to unmeasured or inadequately measured covariates.
There were some strengths in our research. First, this is a general population-based randomly selected Mexican-American cohort with relatively large sample size, thus avoiding bias inherent in studies drawing from clinic populations or other non-randomly selected populations with established disease or mixed ethnicity. Second, we first found an inverse relationship between fruit and vegetable intake and cancer risk in observational studies in Mexican-Americans, and more importance of fruit and vegetable intake on cancer risk than physical activity. Finally, detailed information on a wide range of factors related to cancer was available, allowing us to get a relatively comprehensive analysis of the relevant factors. If having cancer might have increased fruit and vegetable consumption, as a result, we did not observe this in our robust baseline cohort population.
CONCLUSION
Increased fruit and vegetable intake was associated with a significant reduction in cancer risk after excluding the effect of other confounding factors. We have an aging population with more chronic diseases including cancers. Any research that can lead to potential mitigation of these trends is not only warranted but should be strongly supported. Fruit and vegetable intake might be a modifiable protective factor for which Mexican-Americans can make changes to reduce their risk of cancers. Efforts need to be focused on improving fruit and vegetable intake intervention and to devising high-quality studies to measure the effect on cancer risk.
ACKNOWLEDGEMENT
We thank our cohort team, particularly, Rocio Uribe (Brownsville), Claudia Tamez (Laredo), Ariana Garza (Harlingen) and their teams, who recruited and documented the participants. We also thank Marcela Morris and other laboratory staff for their contributions, and Christina Villarreal for administrative support. We thank Valley Baptist Medical Center, Brownsville, Texas for providing us space for our Center for Clinical and Translational Science Clinical Research Unit. We also thank the community of Brownsville, Laredo, and Harlingen and the participants who so willingly participated in this study in their city.
Sources of support: This work was supported by MD000170 P20 funded from the National Center on Minority Health and Health Disparities, the Centers for Translational Science Award 1U54RR023417–01 from the National Center and the Centers for Disease Control Award RO1 DP000210–01 for Research Resources.
Footnotes
Disclosure statement: No potential conflict of interest was reported by the authors.
References
- 1.Wu S, Fisher-Hoch SP, Reninger B,McCormick JB. Meeting or Exceeding Physical Activity Guidelines is Associated with Reduced Risk for Cancer in Mexican-Americans. Am.J.Cancer Prev. 4:1–7,2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 349:g4490,2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu L, Sun D,He Y. Fruit and vegetables consumption and incident hypertension: dose-response meta-analysis of prospective cohort studies. J.Hum.Hypertens. 30:573–580,2016. [DOI] [PubMed] [Google Scholar]
- 4.Wang PY, Fang JC, Gao ZH, Zhang C,Xie SY. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta-analysis. J.Diabetes Investig. 7:56–69,2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boeing H, Bechthold A, Bub A, Ellinger S, Haller D, et al. Critical review: vegetables and fruit in the prevention of chronic diseases. Eur.J.Nutr. 51:637–663,2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int.J.Epidemiol. 46:1029–1056,2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma S, Vik S, Pakseresht M, Shen L,Kolonel LN. Diet impacts mortality from cancer: results from the multiethnic cohort study. Cancer Causes Control 24:685–693,2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pillow PC, Hursting SD, Duphorne CM, Jiang H, Honn SE, et al. Case-control assessment of diet and lung cancer risk in African Americans and Mexican Americans. Nutr.Cancer 29:169–173,1997. [DOI] [PubMed] [Google Scholar]
- 9.Delgado-Cruzata L, Zhang W, McDonald JA, Tsai WY, Valdovinos C, et al. Dietary modifications, weight loss, and changes in metabolic markers affect global DNA methylation in Hispanic, African American, and Afro-Caribbean breast cancer survivors. J.Nutr. 145:783–790,2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenlee H, Ogden GA, Aycinena AC, Koch P, Contento I, et al. Long-term Diet and Biomarker Changes after a Short-term Intervention among Hispanic Breast Cancer Survivors: The inverted exclamation markCocinar Para Su Salud! Randomized Controlled Trial. Cancer Epidemiol.Biomarkers Prev. 25:1491–1502,2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stram DO, Hankin JH, Wilkens LR, Park S, Henderson BE, et al. Prostate cancer incidence and intake of fruits, vegetables and related micronutrients: the multiethnic cohort study* (United States). Cancer Causes Control 17:1193–1207,2006. [DOI] [PubMed] [Google Scholar]
- 12.Lin J, Kamat A, Gu J, Chen M, Dinney CP, et al. Dietary intake of vegetables and fruits and the modification effects of GSTM1 and NAT2 genotypes on bladder cancer risk. Cancer Epidemiol.Biomarkers Prev. 18:2090–2097,2009. [DOI] [PubMed] [Google Scholar]
- 13.Park SY, Ollberding NJ, Woolcott CG, Wilkens LR, Henderson BE, et al. Fruit and vegetable intakes are associated with lower risk of bladder cancer among women in the Multiethnic Cohort Study. J Nutr. 143:1283–1292,2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu JW, Cross AJ, Baris D, Ward MH, Karagas MR, et al. Dietary intake of meat, fruits, vegetables, and selective micronutrients and risk of bladder cancer in the New England region of the United States. Br.J Cancer 106:1891–1898,2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castelao JE, Yuan JM, Gago-Dominguez M, Skipper PL, Tannenbaum SR, et al. Carotenoids/vitamin C and smoking-related bladder cancer. Int.J Cancer 110:417–423,2004. [DOI] [PubMed] [Google Scholar]
- 16.Fisher-Hoch SP, Rentfro AR, Salinas JJ, Perez A, Brown HS, et al. Socioeconomic status and prevalence of obesity and diabetes in a Mexican American community, Cameron County, Texas, 2004–2007. Prev.Chronic.Dis. 7:A53,2010. [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher-Hoch SP, Vatcheva KP, Laing ST, Hossain MM, Rahbar MH, et al. Missed opportunities for diagnosis and treatment of diabetes, hypertension, and hypercholesterolemia in a Mexican American population, Cameron County Hispanic Cohort, 2003–2008. Prev.Chronic.Dis. 9:110298,2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, et al. International physical activity questionnaire: 12-country reliability and validity. Med.Sci.Sports Exerc. 35:1381–1395,2003. [DOI] [PubMed] [Google Scholar]
- 19.Gofin GShephard RJ. Godin leisure-time exercise questionnaire. Med.Sci.Sports Exerc 29:S36–S38,2015. [Google Scholar]
- 20.Reininger BM, Mitchell-Bennett L, Lee M, Gowen RZ, Barroso CS, et al. Tu Salud, inverted exclamation markSi Cuenta!: Exposure to a community-wide campaign and its associations with physical activity and fruit and vegetable consumption among individuals of Mexican descent. Soc.Sci.Med. 143:98–106,2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.USDHHS. 2008 Physical activity guidelines for Americans. U.S Department of Health and Human Services; 2008. [Google Scholar]
- 22.Cappuccio FP, Rink E, Perkins-Porras L, McKay C, Hilton S, et al. Estimation of fruit and vegetable intake using a two-item dietary questionnaire: a potential tool for primary health care workers. Nutr.Metab Cardiovasc.Dis. 13:12–19,2003. [DOI] [PubMed] [Google Scholar]
- 23.Reininger BM, Wang J, Fisher-Hoch SP, Boutte A, Vatcheva K, et al. Non-communicable diseases and preventive health behaviors: a comparison of Hispanics nationally and those living along the US-Mexico border. BMC.Public Health 15:564,2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, et al. AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 102:2284–2299,2000. [DOI] [PubMed] [Google Scholar]
- 25.Esposito K, Chiodini P, Colao A, Lenzi A,Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care 35:2402–2411,2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112:2735–2752,2005. [DOI] [PubMed] [Google Scholar]
- 27.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch.Intern.Med. 168:1617–1624,2008. [DOI] [PubMed] [Google Scholar]
- 28.Harrell FJ Jr. Regression modeling strategies: with applications to linear models, logistic regression, survival analysis. New York, NY: Springer-Verlag; 2001. [Google Scholar]
- 29.Gallagher EJLeRoith D Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol Rev. 95:727–748,2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu S, Fisher-Hoch SP, Reininger B,McCormick JB. Recommended Levels of Physical Activity Are Associated with Reduced Risk of the Metabolic Syndrome in Mexican-Americans. PLoS.One. 11:e0152896,2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kath R, Flaherty SJ Review of dietary assessment methods in public health. Oxford: National Obesity Observatory; 2010. [Google Scholar]
- 32.Hubbard GP, Wolffram S, de VR, Bovy A, Gibbins JM, et al. Ingestion of onion soup high in quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in man: a pilot study. Br.J.Nutr. 96:482–488,2006. [PubMed] [Google Scholar]
- 33.O’Kennedy N, Crosbie L, Whelan S, Luther V, Horgan G, et al. Effects of tomato extract on platelet function: a double-blinded crossover study in healthy humans. Am.J.Clin.Nutr. 84:561–569,2006. [DOI] [PubMed] [Google Scholar]
- 34.Erlund I, Koli R, Alfthan G, Marniemi J, Puukka P, et al. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am.J.Clin.Nutr. 87:323–331,2008. [DOI] [PubMed] [Google Scholar]
- 35.Watzl B, Kulling SE, Moseneder J, Barth SW,Bub A. A 4-wk intervention with high intake of carotenoid-rich vegetables and fruit reduces plasma C-reactive protein in healthy, nonsmoking men. Am.J.Clin.Nutr. 82:1052–1058,2005. [DOI] [PubMed] [Google Scholar]
- 36.Kelley DS, Rasooly R, Jacob RA, Kader AA,Mackey BE. Consumption of Bing sweet cherries lowers circulating concentrations of inflammation markers in healthy men and women. J.Nutr. 136:981–986,2006. [DOI] [PubMed] [Google Scholar]
- 37.Bohn SK, Myhrstad MC, Thoresen M, Holden M, Karlsen A, et al. Blood cell gene expression associated with cellular stress defense is modulated by antioxidant-rich food in a randomised controlled clinical trial of male smokers. BMC.Med. 8:54,2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson JW, Baird P, Davis RH Jr., Ferreri S, Knudtson M, et al. Health benefits of dietary fiber. Nutr.Rev. 67:188–205,2009. [DOI] [PubMed] [Google Scholar]
- 39.Broekmans WM, Klopping-Ketelaars IA, Schuurman CR, Verhagen H, van den Berg H, et al. Fruits and vegetables increase plasma carotenoids and vitamins and decrease homocysteine in humans. J.Nutr. 130:1578–1583,2000. [DOI] [PubMed] [Google Scholar]
- 40.Lampe JW. Health effects of vegetables and fruit: assessing mechanisms of action in human experimental studies. Am.J.Clin.Nutr. 70:475S–490S,1999. [DOI] [PubMed] [Google Scholar]
- 41.Steinmetz KAPotter JD. Vegetables, fruit, and cancer. II. Mechanisms. Cancer Causes Control 2:427–442,1991. [DOI] [PubMed] [Google Scholar]
- 42.Wu S, Fisher-Hoch SP, Reininger BM,McCormick JB. Association between fruit and vegetable intake and symptoms of mental health conditions in Mexican Americans. Health Psychol. 37:1059–1066,2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Airewele G, Adatto P, Cunningham J, Mastromarino C, Spencer C, et al. Family history of cancer in patients with glioma: a validation study of accuracy. J.Natl.Cancer Inst. 90:543–544,1998. [DOI] [PubMed] [Google Scholar]
- 44.Garza A, Vatcheva P, Pan JJ, Rahbar MH, Fallon MB, et al. Liver and Other Gastrointestinal Cancers Are Frequent in Mexican Americans. J.Racial and Ethnic Health Disparities :1–10,2014. [DOI] [PMC free article] [PubMed] [Google Scholar]