Abstract
Background
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND), is a progressive neurodegenerative disease without effective therapies. Several studies have suggested that repetitive transcranial magnetic stimulation (rTMS) may have positive benefit in ALS. However, the efficacy and safety of this therapy remain uncertain. This is the first update of a review published in 2011.
Objectives
To determine the clinical efficacy and safety of rTMS for treating ALS.
Search methods
On 30 July 2012, we searched the Cochrane Neuromuscular Disease Group Specialized Register, CENTRAL (2012, issue 7 in The Cochrane Library), MEDLINE (1966 to July 2012), EMBASE (1980 to July 2012), CINAHL (1937 to July 2012), Science Citation Index Expanded (January 1945 to July 2012), AMED (January 1985 to July 2012). We searched the Chinese Biomedical Database (1979 to August 2012). We also searched for ongoing studies on clinicaltrials.gov (August 2012).
Selection criteria
Randomised and quasi‐randomised controlled trials assessing the therapeutic efficacy and safety of rTMS for patients with a clinical diagnosis of ALS.
Comparisons eligible for inclusion were:
1. rTMS versus no intervention;
2. rTMS versus sham rTMS;
3. rTMS versus physiotherapy;
4. rTMS versus medications;
5. rTMS + other therapies or drugs versus sham rTMS + the same therapies or drugs;
6. different methods of application of rTMS such as high‐frequency (> 1Hz) compared to low‐frequency (≤ 1Hz) rTMS.
Data collection and analysis
Two authors independently selected papers, assessed risk of bias and extracted data. We resolved disagreements through discussion. We contacted study authors for additional information.
Main results
Three randomised, placebo‐controlled trials with a total of 50 participants were included in the review. All three trials compared rTMS with sham TMS. All the trials were of poor methodological quality and were insufficiently homogeneous to allow the pooling of results. Moreover, the high rate of attrition further increased the risk of bias. None of the trials provided detailed data on the ALS Functional Rating Scale‐Revised (ALSFRS‐R) scores at six months follow‐up which was pre‐assigned as our primary outcome. One trial contained data in a suitable form for quantitative analysis of our secondary outcomes. No difference was seen between rTMS and sham rTMS using the ALSFRS‐R scores and manual muscle testing (MMT) scores at 12 months follow‐up in this trial. Additionally, none of the trials reported any adverse events associated with the use of rTMS. However, in view of the small sample size, the methodological limitations and incomplete outcome data, treatment with rTMS cannot be judged as completely safe.
Authors' conclusions
There is currently insufficient evidence to draw conclusions about the efficacy and safety of rTMS in the treatment of ALS. Further studies may be helpful if their potential benefit is weighed against the impact of participation in a randomised controlled trial on people with ALS.
Keywords: Female, Humans, Male, Middle Aged, Amyotrophic Lateral Sclerosis, Amyotrophic Lateral Sclerosis/therapy, Motor Neuron Disease, Motor Neuron Disease/therapy, Randomized Controlled Trials as Topic, Transcranial Magnetic Stimulation, Transcranial Magnetic Stimulation/methods
Plain language summary
Repetitive transcranial magnetic stimulation (rTMS) for treating amyotrophic lateral sclerosis (ALS)
Amyotrophic lateral sclerosis (ALS), which is also known as motor neuron disease (MND), is a fatal disease in which the nerves in the brain and spinal cord that control movement degenerate. Treatments have little effect on how the disease progresses. People with ALS develop muscle weakness and paralysis of limb muscles and muscles involved in swallowing and breathing. Repetitive transcranial magnetic stimulation (rTMS) is a method for exciting nerve cells in superficial areas of the brain. It applies pulsed magnetic fields to the surface of the brain via an electrode on the scalp. There have been trials to see if rTMS is effective in people with ALS.
For this review we searched widely for clinical trials of rTMS in people with ALS and found three studies, which involved 50 participants in total. All three compared rTMS with sham (inactive) rTMS. None of the three studies reported on disability or limitation in activity as assessed by a specific ALS scale (ALSFRS‐R) at six months follow‐up, which was what we chose as our primary measure of the effectiveness of rTMS. Other outcome measures were only available from 12 participants in one poor quality trial, in which there was no difference between rTMS and sham rTMS in ALSFRS‐R or a test of muscle strength at 12 months’ follow‐up. None of the studies reported any adverse effects with rTMS. The trials in this review had small numbers of participants and some problems of design, so larger, well‐designed trials should be considered, to determine the efficacy and safety of rTMS in ALS. However, the potential benefit should be balanced against the impact of taking part in trials for people with ALS.
Summary of findings
Summary of findings for the main comparison. rTMS compared to sham rTMS for the treatment of amyotrophic lateral sclerosis or motor neuron disease.
| rTMS compared to sham rTMS for the treatment of amyotrophic lateral sclerosis or motor neuron disease | ||||||
| Patient or population: patients with the treatment of amyotrophic lateral sclerosis or motor neuron disease Settings: hospital setting in Italy Intervention: rTMS Comparison: sham rTMS | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| sham rTMS | rTMS | |||||
| Changes to the ALSFRS‐R scores at 12 months follow‐up Changes to the ALSFRS‐R scores at 12 months | The mean changes to the ALSFRS‐R scores at 12 months follow‐up in the control groups was 10.1 | The mean changes to the ALSFRS‐R scores at 12 months follow‐up in the intervention groups was 1.2 lower3 (8.78 lower to 6.38 higher) | 12 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| Changes to the MMT scores at 12 months follow‐up Changes to the MMT scores at 12 months4 | The mean changes to the MMT scores at 12 months follow‐up in the control groups was 1.1 | The mean changes to the MMT scores at 12 months follow‐up in the intervention groups was 0.2 higher4 (0.77 lower to 1.17 higher) | 12 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| Changes to FSS at 12 months follow‐up | See comment | See comment | Not estimable | 0 (0 study) |
See comment | No detailed data on the changes to FSS were available in the included studies at this time point. |
| Adverse events leading to cessation of treatment | See comment | See comment | Not estimable | 50 (3 studies) | See comment | The rTMS was reported to be well tolerated and produced no adverse events in 3 studies. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; ALSFRS‐R: Amyotrophic Lateral Sclerosis Functional Rating Scale‐Revised; MMT: manual muscle testing; FSS: Fatigue Severity Scale; rTMS: repetitive transcranial magnetic stimulation | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Random sequence generation and allocation concealment were unclear. Numbers lost to follow‐up and reasons for this were given, but intention‐to‐treat analysis was not performed. 2 Very few patients were included. 3 Better is indicated by lower. 4 MMT score was measured using the UK Medical Research Council (MRC) scale. Better is indicated by higher.
Background
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND), is one of the major neurodegenerative diseases alongside Alzheimer's disease and Parkinson's disease. It is an adult‐onset neurodegenerative disorder that causes premature loss of motor neurons at all levels, including the cerebral cortex, brainstem and spinal cord, and gives rise to both upper and lower motor neuron signs.
Description of the condition
ALS is considered a rare disease worldwide, with a reported incidence between 1.5 and 2.0 per 100,000 population per year and a prevalence of 1 per 20,000 (Mandrioli 2003; Sorenson 2002). The average age of ALS onset is 55 years and males are usually affected more than females (ratio about 1.6:1). Five to ten per cent of patients have a positive family history for ALS. These families usually show an autosomal‐dominant pattern of inheritance. The other 90% of patients without a family history are said to have sporadic disease, the cause of which is unknown.
The clinical features of ALS can be considered in relation to neurological regions. Bulbar‐onset patients often present with dysarthria, dysphagia, or both. Cervical‐onset patients often present with muscle atrophy and upper‐limb weakness, associated with brisk reflexes. Lumbar‐onset patients always have lower motor neuron symptoms and signs in the legs, such as a tendency to trip (distal weakness) or difficulty on stairs (proximal weakness). Death occurs in most patients within two to five years of diagnosis, usually from respiratory failure caused by ventilatory muscle weakness (Forsgren 1983; Rowland 2001).
While the aetiology of ALS is unknown, current evidence suggests that multiple interacting factors contribute to motor neuron injury in ALS. One of the hypothesised pathogenetic mechanisms is glutamate‐driven excitotoxicity in the motor cortex (Zanette 2002). This mechanism provides a rationale for clinical trials which have demonstrated that riluzole, as a glutamate antagonist, improves clinical outcomes in patients with ALS (Miller 2007). However, some other treatments may also have a treatment effect in this condition (Andersen 2005).
Description of the intervention
Transcranial magnetic stimulation (TMS) is a non‐invasive means of stimulating nerve cells in superficial areas of the brain by applying a high‐energy magnetic field at the skull surface which induces a perpendicular electrical field in the vertical plane through the cortex. It was first introduced by Barker et al. in 1985 (Barker 1985). Since then, numerous studies have found that TMS provides a non‐invasive approach to condition the excitability and activity of neurons (Kobayashi 2003; Siebner 2003) and can be used as a tool to evaluate the motor system and study the function of brain regions. Moreover, some clinical trials have shown that TMS might be effective in treating various diseases, including Parkinson's disease, epilepsy, dystonia depression and schizophrenia (Borich 2009; Elahi 2009; Fitzgerald 2003; Klein 1999; Theodore 2002).
Repetitive transcranial magnetic stimulation (rTMS) is a type of TMS that occurs in a rhythmic and repetitive form. If the stimulation occurs at a frequency equal to or less than once per second (1 Hz) it is called low‐frequency rTMS and if the speed of stimulation is faster than 1 Hz it is called high‐frequency rTMS (Wassermann 1998). Some previous studies have demonstrated that low‐frequency rTMS produces a decrease of motor cortex excitability lasting up to 30 minutes post stimulation (Chen 1997; Plewnia 2003; Romero 2002) and brings a reduction in glutamate‐induced excitotoxicity, which may benefit people with ALS (Ziemann 2004). Conversely, high‐frequency rTMS provokes a short‐term increase in cortical excitability (Di Lazarro 2002), which may be detrimental in ALS. However, high‐frequency rTMS may have neuroprotective effects (Fujiki 2003) and increase the expression of neurotrophic factors such as brain‐derived neurotrophic factor (BDNF) (Angelucci 2004), which could counteract the neuronal damage in ALS. These experimental findings suggest that both low‐ and high‐frequency rTMS might be effective therapies for ALS.
Different rTMS protocols have been developed. The theta burst magnetic stimulation (TBS) of the human motor cortex, a kind of novel rTMS technique, which can swiftly produce powerful and controllable long‐term changes in the excitability of cortical circuits, is widely used nowadays (Huang 2004). The basic TBS pattern is of a train containing three pulses at 50 Hz delivered every 200 ms. On the basis of this, two different paradigms of TBS with different effects on corticospinal excitability are employed: continuous TBS (cTBS, a 40 second train of three pulses of 50 Hz stimulation repeated every 200 ms for a total of 600 stimuli) and intermittent TBS (iTBS, 10 bursts of high frequency stimulation, three pulses at 50 Hz, are applied at 5 Hz every 10 s for a total of 600 pulses) (Huang 2004). As cTBS can lead to a marked depression of cortical excitability and a significant reduction of glutamatergic‐related intracortical facilitation (Huang 2005), some studies have adopted cTBS as a therapeutic tool to aid the treatment of ALS (Di Lazarro 2006; Di Lazarro 2009; Di Lazarro 2010). This limited evidence has suggested that this kind of rTMS may have a positive benefit in ALS.
Therefore, this review will consider rTMS as a therapeutic intervention for ALS.
Why it is important to do this review
In recent years, several clinical trials have been conducted to investigate the efficacy and safety of rTMS in treating ALS. The initial results of these studies have suggested that rTMS could improve motor function in ALS (Di Lazarro 2006; Zanette 2008), while other studies have demonstrated that rTMS may worsen the clinical course of the disease (Di Lazarro 2004). However, we know of no systematic review of rTMS for the treatment of ALS. For this reason, a systematic review to assess the benefits and harms of rTMS for ALS is needed.
We therefore planned to perform a systematic review which would be based on evidence from randomised controlled trials (RCTs) and quasi‐randomised controlled trials (quasi‐RCTs) that examine the efficacy and safety of rTMS for ALS. We updated the search in 2012.
This review has a published protocol (He 2010).
Objectives
The primary objective of the review was to examine the efficacy and safety of rTMS in the treatment of patients with ALS.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs and quasi‐RCTs assessing the therapeutic efficacy and safety of rTMS for patients with ALS.
Types of participants
We included participants of any age or sex with a clinical diagnosis of definite, probable, probable with laboratory support or possible ALS according to the El Escorial criteria.(Brooks 1994; Brooks 2000).
Types of interventions
(a) We carried out comparisons of:
1. rTMS versus no intervention;
2. rTMS versus sham rTMS;
3. rTMS versus physiotherapy;
4. rTMS versus medications;
5. rTMS + other therapies or drugs versus sham rTMS + the same therapies or drugs.
(b) We also compared different methods of application of rTMS such as high‐frequency (> 1Hz) compared to low‐frequency (≤ 1Hz) rTMS.
Types of outcome measures
Primary outcomes
The primary outcome focused on disability or limitation in activity as assessed by the ALS Functional Rating Scale‐Revised (ALSFRS‐R) (Cedarbaum 1999) at six months.
Secondary outcomes
Secondary outcome measures included:
1. Changes to the ALSFRS‐R scores at 12 months or longer follow‐up.
2. Changes to muscle strength as measured by manual muscle testing (MMT) at one month and six months or longer follow‐up.
3. Changes to fatigue as measured by the Fatigue Severity Scale (FSS) (Krupp 1989) at one month and six months or longer follow‐up.
4. Adverse events that may have resulted from the intervention. We defined serious adverse events as life‐threatening conditions, prolonged hospitalisation or death. Nonserious adverse events included: tinnitus; dizziness; and progression of ALS‐related weakness beyond that expected as a result of typical disease progression, with events leading to cessation of treatment.
Search methods for identification of studies
Electronic searches
On 30 July 2012, we searched the Cochrane Neuromuscular Disease Group Specialized Register (30 July 2012), CENTRAL (2012, issue 7 in The Cochrane Library), MEDLINE (1966 to July 2012), EMBASE (1980 to July 2012), CINAHL (1937 to July 2012), ISI (Science Citation Index Expanded, January 1945 to July 2012), AMED (the Allied and Complementary Medicine Database, January 1985 to July 2012), We also searched the Chinese Biomedical Database (1979 to August 2012) and www.ClinicalTrials.gov (http://clinicaltrials.gov/) (August 2012).
The detailed search strategies are in the appendices: MEDLINE (Appendix 1), EMBASE (Appendix 2), AMED (Appendix 3), CINAHL Plus (Appendix 4), CENTRAL (Appendix 5), ISI (Appendix 6), Chinese Biomedical Database (Appendix 7) and www.ClinicalTrials.gov (Appendix 8). We screened search results for RCTs as well as well‐designed published observational studies.
Searching other resources
We checked the reference lists of published studies to identify more trials. We also searched for unpublished data and grey literature through personal communication with researchers and others with an interest in the field, and contacted the corresponding authors of identified RCTs for additional information about other relevant studies. We did not include any restrictions with respect to language.
Data collection and analysis
Selection of studies
Two authors (Jian Guo (JG) and MY for the original review, JF and MY at this update) independently scrutinised the titles, abstract sections, and keywords of every record retrieved to determine if the studies had to be assessed further. Two authors (JG or JF, MY) obtained and assessed full copies of potentially relevant trials as per the 'Criteria for considering studies for this review'. The authors decided which trials fitted the inclusion criteria and graded their risk of bias. There were no disagreements about inclusion.
Data extraction and management
Two review authors (JG or JF, CZ) independently extracted data on participants, methods, interventions, outcomes and results using a data extraction form. Where data were missing, we contacted the study authors via e‐mail and requested this information. One review author (JG or JF) entered data into the Cochrane Review Manager (RevMan) 5 software (RevMan 2008, updated to RevMan 2012), and two other review authors (MY, MZ) checked the data entered. We resolved disagreements on data extraction by discussion.
Assessment of risk of bias in included studies
Two review authors (JG, MZ for the original review) independently assessed the risk of bias in each included study and completed the 'Risk of bias' table according to the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). JF and MZ reassessed blinding for this update. The following six methodological domains were considered for each trial.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessors.
Incomplete outcome data.
Selective outcome reporting.
Other sources of bias.
We made a judgement on each of these criteria relating to the risk of bias of 'Low risk', 'High risk' or 'Unclear risk'. We resolved disagreements by discussion with a third author (CZ).
Measures of treatment effect
We used the Cochrane statistical software RevMan 5 to measure the treatment effect according to the recommendations of the Cochrane Collaboration. We calculated the weighted treatment effect using a fixed‐effect model across trials. Results were expressed as risk ratios (RRs) with 95% confidence intervals (CIs), risk differences (RDs) with 95% CIs for dichotomous outcomes and as mean differences (MDs) with 95% CIs for continuous outcomes.
We constructed a 'Summary of findings' table using GRADEpro software and included the following outcomes.
1. Changes to the ALSFRS‐R scores at 12 months follow‐up.
2. Changes to the MMT scores at 12 months follow‐up.
3. Changes to FSS at 12 months follow‐up.
4. Adverse events leading to cessation of treatment.
Dealing with missing data
We planned to perform analyses on an intention‐to‐treat basis whenever possible. We included all participants with available data in the analysis in the group to which they were allocated, regardless of whether or not they received the allocated intervention. If in the original reports participants were not analysed in the group to which they were randomised, and there was sufficient information in the trial report, we attempted to restore them to the correct group. We dealt with missing data through an attempt to contact the study authors via email.
Assessment of heterogeneity
We used the Chi2 test for heterogeneity to provide an indication of between‐study heterogeneity. In addition, we quantified the degree of heterogeneity observed in the results using the I2 statistic with significance being set at P < 0.1 (Higgins 2002; Higgins 2003). We planned to assess possible sources of heterogeneity by sensitivity and subgroup analyses as described below. For trials that were clinically heterogeneous or presented insufficient information for pooling, we planned to perform a descriptive analysis (Higgins 2011).
Assessment of reporting biases
As there were insufficient studies included in the review, we used a descriptive analysis to evaluate the potential reporting biases.
Data synthesis
We planned to perform meta‐analysis using RevMan 5 software if trials were clinically similar enough; otherwise we would analyse the trials separately.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses in order to explore effect size difference according to the following methodological sources of heterogeneity if sufficient trials specifying these parameters were available.
(1) State of ALS at baseline: mild, moderate, severe or terminal (Riviere 1998).
(2) Clinical pattern of the disease: bulbar onset or limb onset.
(3) Type of TMS: high‐frequency (> 1Hz) or low‐frequency (≤ 1Hz) stimulation.
Sensitivity analysis
If heterogeneity was found across the trials, we intended to undertake sensitivity analyses in order to determine the effect of omitting trials with a high risk of bias.
Results
Description of studies
Results of the search
The number of possibly relevant studies found on each database were as follows: Cochrane Neuromuscular Disease Group Register: 5 records; the Cochrane Central Register of Controlled Trials (CENTRAL): 24 records; MEDLINE: 76 records; EMBASE: 239 records; CINAHL plus: 16 records; AMED: 1 record; Science Citation Index Expanded: 40 records; the Chinese Biomedical Database: 0 records; Clinicaltrials.gov: 1 record.
For the 2013 update we excluded 124 studies after we screened the title or the abstract of the article, and found 14 duplicated studies.
We initially identified nine potentially eligible trials. Six trials were excluded after we screened the full text of all the possible relevant references and three met the inclusion criteria. See Characteristics of included studies and Characteristics of excluded studies.
For the 2013 update we did not find any new trials for inclusion. We moved an abstract of Di Lazarro 2006 from excluded studies.
Included studies
Trial location and participants
All three included trials were conducted in a single centre in Italy (Di Lazarro 2006;Di Lazarro 2009; Zanette 2008). Of the three included trials, two were carried out by the same group of researchers investigating the effects of rTMS on the progression of ALS (Di Lazarro 2006; Di Lazarro 2009). Both trials had similar design, intervention, efficacy and safety assessments.
Baseline demographic and clinical characteristics of the participants in the included trials are shown in Table 2. The included trials had 50 participants with ALS ranging from 5 to 70 months duration. The mean age of the intervention group ranged from 59.4 to 60.6 years and control group ranged from 55.1 to 65.7 years. The ratio of males to females in intervention group ranged from 2.5 to 4 and control group ranged from 0.6 to 2.3. Two studies included in the review recruited patients who fulfilled the EI Escorial criteria for definite or probable ALS (Di Lazarro 2009; Zanette 2008) and one study only recruited patients with criteria for definite ALS (Di Lazarro 2006). One trial (Di Lazarro 2009) excluded participants that had (1) seizure history; (2) concomitant severe medical problems; (3) history of tracheostomy; and (4) contraindications for rTMS (for example, pacemakers or metallic objects in the body). The other two trials did not mention the exclusion criteria (Di Lazarro 2006; Zanette 2008).
1. Baseline demographic and clinical characteristics of the participants in included trials.
| Study | Participants | Female/male | Age (years) Mean (SD) |
Disease duration (months) Mean (SD) |
Site of onset (spinal/bulbar) |
El Escorial category (definite/probable) |
ALSFRS‐R score Mean (SD) |
|||||||
| real | sham | real | sham | real | sham | real | sham | real | sham | real | sham | real | sham | |
| Di Lazarro 2006 | N = 10 | N = 10 | unclear | unclear | unclear | unclear | 15.3 (8.2) | 14.8 (8.9) | unclear | unclear | N = 10/0 | N =10/0 | 38.3 (7.5) | 37.93 (7.9) |
| Di Lazarro 2009 | N =10 | N = 10 | N = 2/8 | N = 3/7 | 60.2 (6.7) | 55.1 (14.0) | 32.2 (18.3) | 31.4 (18.6) | N = 8/2 | N = 7/3 | N = 4/6 | N = 4/6 | 32.0 (7.1) | 31.3 (6.9) |
| Zanette 2008 | N = 5 | N = 5 | N = 1/4 | N = 2/3 | 59.4 (9.2) | 60.2 (8.7) | 11.4 (3.0) | 12.2 (4.0) | N = 3/2 | N = 3/2 | unclear | unclear | 36.0 (3.4) | 34.4 (2.3) |
ALSFRS: Amyotrophic Lateral Sclerosis Functional Rating Scale; SD: standard deviation.
Interventions
All the three trials were randomised, parallel‐group trials designed to test the effectiveness of active rTMS versus sham rTMS in reducing the progression of ALS. In two studies, the participants and assessors were blinded to the treatment assignment (Di Lazarro 2006; Di Lazarro 2009 ). In one study, it was not stated whether the assessors were blinded (Zanette 2008).
Table 3 lists the rTMS parameters used in the three included studies. Among the three randomised controlled studies there was heterogeneity in the rTMS technique with respect to three variables: duration of treatment, frequency of rTMS and intensity of rTMS. The study by Di Lazarro 2006 delivered rTMS for five consecutive days per month for six months to the motor cortex of each hemisphere. The stimulation protocol was cTBS, in which three pulses of stimulation are given at 50 Hz, repeated every 200 ms for a total of 600 pulses. The stimulus intensity was 80% of action motor threshold. Another of the included trials carried out subsequently by the same group delivered rTMS for five consecutive days per month for 12 months, using the same stimulation protocol (Di Lazarro 2009). The study by Zanette 2008 delivered the rTMS for five consecutive days per week for two weeks. Stimulation protocol was 20 trains of 15 stimuli at 5 Hz, 60 s interval between trains. The stimulus intensity was 110% resting motor threshold.
2. rTMS parameters of included trials.
| Study | Duration | Stimulator and Coil | rTMS placement | rTMS frequency | Intensity % motor threshold | Medications |
| Di Lazarro 2006 | Five consecutive days per month for six months | MagPro stimulator and fig 8 coil | Motor cortex of both hemispheres | 50 Hz | 80% of action motor threshold | All participants were taking riluzole |
| Di Lazarro 2009 | Five consecutive days per month for 12 months | MagPro stimulator and fig 8 coil | Motor cortex of both hemispheres | 50 Hz | 80% of action motor threshold | All participants were taking riluzole |
| Zanette 2008 | Five consecutive days per week for two weeks | Super Rapid magnetic stimulator and fig 8 coil | Motor cortex of thenar muscles and tibialis anterior muscles were stimulated separately on both hemispheres | 5 Hz | 110% of resting motor threshold | All participants were taking riluzole |
rTMS: repetitive transcranial magnetic stimulation
Outcomes
The primary outcome of all the three trials was rate of decline of ALSFRS‐R scores. But time points linked to the primary outcome measures were heterogeneous: four weeks in Zanette 2008, six months in Di Lazarro 2006 and six months and 12 months in Di Lazarro 2009. Two studies used the rate of decline of MMT as their secondary outcome (performed by means of the Medical Research Council (MRC) Scale) after the end of rTMS treatment (Di Lazarro 2006; Di Lazarro 2009 ), while another study used the changes in muscle strength (assessed by the MRC scale) and fatigue (assessed by Fatigue Severity Scale (FSS)) after the treatment as the secondary outcome (Zanette 2008). All studies clearly reviewed and recorded serious adverse events and events leading to discontinuations.
Excluded studies
Six studies were excluded from the analysis of this review. For full details see Characteristics of excluded studies. One study was not a randomised trial and measured different outcomes from our protocol (Di Lazarro 2004). One study was a research report to investigate the effects of rTMS on BDNF plasma levels in ALS patients (Angelucci 2004). One study enrolled only two participants (Di Lazarro 2010). Two studies investigated rTMS measures as clinical correlates and longitudinal markers of ALS (Floyd 2009; Khedr 2011).The last report was a review of rTMS as a treatment for ALS (Dileone 2010).
Risk of bias in included studies
Overall, there was little information on methods provided by study authors. For full details, see Characteristics of included studies and 'Risk of bias' summary (Figure 1).
1.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Key: green = low risk of bias; yellow = unclear risk of bias; red = high risk of bias.
Allocation
In Di Lazarro 2006, randomisation was performed using a stratified block allocation scheme (block size: unclear, allocation ratio: 1:1) and in Di Lazarro 2009, randomisation was performed using a dynamic allocation strategy. However, the method the investigators used to carry out the stratified block allocation and the dynamic allocation strategy was not stated. In Zanette 2008, randomisation was stated to be performed but the method of random sequence generation was unclear.
None of the included studies reported explicitly on concealment of allocation. Di Lazarro 2006 and Di Lazarro 2009 stated that randomisation was performed by independent physicians. However, in neither study was there mention of any attempted method of allocation concealment.
Blinding
Two of the studies were double‐blinded, with participants and the neurologists who carried out the Interventions unaware of the study group. Both of the studies used the same stimulator connected to the placebo, with the same site of stimulation. The number of placebo stimuli was identical to the number used for the real rTMS, to ensure the interventions were the same in both groups and in order to avoid bias in the outcome assessors (Di Lazarro 2006;Di Lazarro 2009). The remaining study was stated to be a randomised trial, and used the same measurement method in both groups. However, the report did not provide details on the method of blinding or adequate information about whether outcome assessors were blinded or not (Zanette 2008). To sum up, blinding of participants and personnel was clearly described in all three studies. All three studies used the same stimulator in both active and sham groups to blind the neurologists administering the interventions and the participants (Di Lazarro 2006; Di Lazarro 2009; Zanette 2008). The reports of two studies clearly described blinding of outcome assessment: these stated that the neurologists assessing the outcomes were blinded to group assignment (Di Lazarro 2006; Di Lazarro 2009). The remaining study provided insufficient information on blinding of outcome assessors (Zanette 2008).
Incomplete outcome data
All studies adequately disclosed withdrawals and drop‐outs. Drop‐out rates were high in both intervention and control groups and varied between the three studies. In Di Lazarro 2006, three participants (30%) discontinued treatment in the real rTMS group (one for an unrelated medical condition (breast cancer), one because of difficulty attending hospital and one for protocol violation) and two participants (20%) discontinued treatment in the sham rTMS group (one for an unrelated medical condition (ileus), and one died from ALS‐related respiratory failure). In Di Lazarro 2009, three participants (30%) discontinued treatment in the real rTMS group (one for an unrelated medical condition (myocardial infarction), one for ALS‐related respiratory failure and one because of difficulty attending the hospital) and five participants (50%) discontinued treatment in the sham rTMS group (three for ALS‐related respiratory failure and two because of difficulty attending the hospital). No intention‐to‐treat analysis with regard to efficacy was performed in these two studies. The remaining study reported no loss to follow‐up (Zanette 2008).
Selective reporting
No evidence of this.
Other potential sources of bias
In all studies baseline data were similar for all study groups. No other potential sources of bias could be identified.
Effects of interventions
See: Table 1
Comparison: rTMS versus sham rTMS for the treatment of ALS
We planned to carry out comparisons of: 1. rTMS versus no intervention; 2. rTMS versus sham rTMS; 3. rTMS versus physiotherapy; 4. rTMS versus medications; 5. rTMS + other therapies or drugs versus sham rTMS + the same therapies or drugs. But all three included studies were designed to compare rTMS versus sham rTMS treatment. All studies demonstrated that there were no significant differences in the baseline values of the two therapy groups. Therefore, they statistically compared the outcome values between the two groups at a given point in time. In Di Lazarro 2009, mean scores of ALSFRS‐R and MMT with standard deviations at 12 months follow‐up were presented. But in Di Lazarro 2006 and Zanette 2008, no raw numerical data were available. We tried to contact the authors to query the data, but received no response. Given the significant clinical and methodological diversity in the studies and the different lengths of follow‐up, we did not attempt a meta‐analysis for the primary and secondary outcomes. Thus, sensitivity analyses, subgroup analyses or assessment of heterogeneity could not be undertaken for this version of the review.
Primary outcome
Our primary outcome was disability or limitation in activity as assessed by the ALSFRS‐R at six months. The ALSFRS‐R was published as a primary outcome measure in all studies and two trials (Di Lazarro 2006; Di Lazarro 2009), with a sample size of 40 participants (20 in the treatment group and 20 in the placebo group), evaluated it at six months follow‐up. However, no raw numerical data were reported in either trial at this time point. In Di Lazarro 2006, the study reported only statistical summary data indicating a significant benefit from real rTMS for ALSFRS‐R at six months (see Table 4; repeated measures analysis of variance: F(1, 5) = 5.16; P = 0.0005). In Di Lazarro 2009, the statistical summary data of ALSFRS‐R scores in the two treatment groups at six months follow‐up were also stated, and the difference was nonsignificant between rTMS‐treated and placebo‐treated participants (Mann–Whitney test, P = 0.92).
3. rTMS versus sham rTMS: self‐reported statistical summary data.
| Study | Outcome | Statistic (TIME x TREATMENT interaction) |
| Di Lazarro 2006 | ALSFRS‐R score, at six months | F (1, 5) = 5.16; P = 0.0005 |
| Di Lazarro 2006 | MMT score, at six months | F (1, 5) = 2.43; P = 0.044 |
| Zanette 2008 | ALSFRS‐R score, at four weeks | F (2, 16) = 2.7; P > 0.05 |
| Zanette 2008 | MMT score, at four weeks | F(2, 16) = 2.8; P > 0.05 |
| Zanette 2008 | FSS score, at four weeks | F (2, 16) = 4.0; P = 0.04 |
| Zanette 2008 | SF‐36 score, at four weeks | F (2, 16) = 18.1; P = 0.001 |
| Zanette 2008 | Maximum voluntary isometric contraction, at four weeks | F (2, 16) = 20.0; P = 0.0001 |
ALSFRS‐R: Amyotrophic Lateral Sclerosis Functional Rating Scale Revised; MMT: manual muscle testing; FSS: Fatigue Severity Scale; SF‐36: Short Form‐36.
Secondary outcomes
1. Changes to the ALSFRS‐R scores at 12 months or longer follow‐up
Di Lazarro 2009 provided data on ALSFRS‐R scores at 12 months follow‐up. There was no significant difference between real rTMS and sham rTMS group (MD ‐1.20; 95% CI ‐8.78 to 6.38, see Analysis 1.1, Figure 2). None of the included studies in the review reported longer follow‐up times.
1.1. Analysis.

Comparison 1 rTMS versus sham rTMS, Outcome 1 Changes to the ALSFRS‐R scores at 12 months follow‐up.
2.
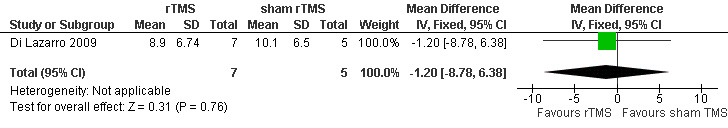
Forest plot of comparison: 1 rTMS versus sham rTMS, outcome: 1.1 Changes to the ALSFRS‐R scores at 12 months follow‐up.
2. Changes to muscle strength as measured by MMT at one month and six months or longer follow‐up
All the three included studies evaluated participants using the MMT, which was performed by means of the MRC scale. However, the muscles they tested were different. In Di Lazarro 2006 and Di Lazarro 2009, the authors assessed MRC scale scores in thirteen muscles on each side (biceps brachii, deltoid, triceps brachii, extensor carpi radialis, extensor digitorum communis, abductor digiti minimi, abductor pollicis brevis, opponens pollicis, iliopsoas, rectus femoris, tibialis anterior, extensor hallucis longus, and gastrocnemius); but in Zanette 2008, the authors assessed eight muscles on each side (biceps brachii, triceps brachii, extensor carpi radialis, first dorsal interosseus, quadriceps, tibialis anterior, gastrocnemius, and extensor hallucis longus). Only one study provided detailed data of MMT scores at the follow‐up at 12 months (Di Lazarro 2009). There was no significant difference between real rTMS and sham rTMS groups (MD 0.20; 95% CI ‐0.77 to 1.17, see Analysis 1.2, Figure 3). Zanette 2008 and Di Lazarro 2006 reported the statistical summary data (see Table 4) for the MMT scores at four weeks (repeated measures analysis of variance: F(2, 16) = 2.8; P > 0.05) and six months (repeated measures analysis of variance: F(1, 5) = 2.43; P = 0.044).
1.2. Analysis.

Comparison 1 rTMS versus sham rTMS, Outcome 2 Changes to the MMT scores at 12 months follow‐up.
3.
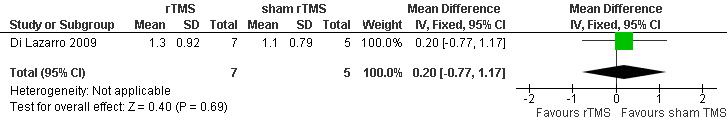
Forest plot of comparison: 1 rTMS versus sham rTMS, outcome: 1.2 Changes to the MMT scores at 12 months follow‐up.
3. Changes to fatigue as measured by FSS at one month and six months or longer follow‐up
Only Zanette 2008 used the FSS as an outcome measure. This study reported skewed summary data (see Table 4) for FSS scores at four weeks indicating a statistically significant slower deterioration in FSS score overall for the real rTMS group compared with the placebo group (repeated measures analysis of variance: F(2, 16) = 4.0; P = 0.04).
4. Adverse events that may have resulted from the intervention
Treatment appeared safe in all included studies. There was no report of any adverse events related to rTMS. Side effects that are considered to be associated with rTMS, such as headache, tinnitus, dizziness and convulsions, were not observed in any of the studies.
Discussion
The aim of this review was to examine the effectiveness and safety of rTMS for the treatment of ALS. The evidence from the randomised controlled trials (RCTs) included in our review does not support rTMS as a treatment for patients with ALS. All the trials we identified were designed to compare real rTMS versus sham rTMS treatment. We did not find any trials reporting comparisons with other therapies or drugs, nor any trials comparing high‐frequency rTMS to low‐frequency rTMS. All the three included studies had relatively small sample sizes, 20 in the Di Lazarro 2006, 20 in the Di Lazarro 2009 and 10 in the Zanette 2008 trial. They were also at potential risk of bias from various aspects related to protocol design. Furthermore, among the three studies, only Di Lazarro 2009 provided mean baseline and follow‐up scores with standard deviations. The other two studies merely presented summaries of statistical data which could not be pooled to perform a valid meta‐analysis.
Summary of main results
See: Table 1.
We found three randomised trials with 50 participants recruited; 20 with definite ALS and 30 with definite or probable ALS. Given the heterogeneity of the studies reviewed, combining our pre‐specified outcomes among the studies was not possible. Moreover, these studies generally had small sample sizes and high drop‐out rates, and as a result may provide unreliable evidence about the comparative effects of rTMS for people with ALS. In Di Lazarro 2009, scores of ALSFRS‐R and MMT were presented, with no significant differences between real and sham rTMS groups (MD ‐1.2; 95% CI ‐8.78 to 6.38 and MD 0.20; 95% CI ‐0.77 to 1.17). But in Di Lazarro 2006 and Zanette 2008, no raw numerical data were available.
Two studies reported statistically significant improvement in the real rTMS group compared with the sham rTMS group. The first study (Di Lazarro 2006) showed slower deterioration in score of ALSFRS‐R and MMT in the real‐treatment group at six months (Table 4). The second study (Zanette 2008) claimed that rTMS may improve motor function (measured by maximum voluntary isometric contraction) and quality of life (QoL) (measured by SF‐36) in people with ALS at four weeks (Table 4). However, there is still insufficient evidence to reach conclusions on the effectiveness of rTMS for the treatment of ALS.
The treatment was reported to be well tolerated and seemed not to produce significant adverse events in any of the included studies, even in the study with long‐term follow‐up (12 months). The possible side effects of rTMS observed in other reports, including headache, neck pain, tinnitus, dizziness, convulsions and memory loss, were not reported in the three studies included in this review. However, this finding should be interpreted with caution. In view of the small sample size and the methodological limitations, treatment with rTMS cannot be judged as completely safe.
Overall completeness and applicability of evidence
The data and the applicability of rTMS to treating ALS are questionable for several reasons. Firstly, small numbers of studies with relatively small sample sizes were included in our review. The search strategy of the review identified 296 citations, of which only three trials fulfilled all the selection criteria. The included studies used sample sizes of between 10 and 20 participants (median 16). Therefore the process of randomisation may not have been able to guarantee the equality of groups in all their variables. Secondly, the rTMS protocols and clinical pictures of the participants in the studies reviewed were heterogeneous. As a new technique for the treatment of ALS, there are no standard rTMS protocols that can be used for this disease. More exploratory investigations are warranted to examine different combinations of rTMS parameters in the treatment of ALS, and how these could be integrated with conventional therapies. Thirdly, the data for several important outcomes were lacking in some studies. Among the three included studies, only Di Lazarro 2009 provided detailed data of outcome measures before and after the rTMS treatment. This meant that we could not do further analyses on the other studies.
Quality of the evidence
There is only limited evidence on the effects of rTMS in people with ALS. Only three trials with 37 participants of the initially 50 eligible patients randomised were included in the review. Overall methodological quality of the included trials was low and is described in detail in the 'Risk of bias' table (Characteristics of included studies). For one study, sequence generation and allocation concealment were adequate (Di Lazarro 2009). For the other two studies, it was unclear whether these were adequate. Two studies included in this review were described as double‐blind (Di Lazarro 2006;Di Lazarro 2009). However, when administering rTMS, it is impossible to blind the person who administers the technique, because they must have known whether each participant belonged to the treatment or the control group and used the right magnetic stimulator. Double‐blind therefore refers to the participants, outcome assessors and investigators involved in other processes of the study and may increase the probability of biases related to the performance of the studies.
Two studies in the review reported drop‐outs adequately (Di Lazarro 2006;Di Lazarro 2009), with high rates in both intervention and control groups. The reasons for drop‐outs in the trials included ALS‐related problems, developing unrelated medical conditions, unwillingness to travel to the clinic for follow‐up and protocol violation. Moreover, as the outcome measures were continuous variables, the trial investigators did not perform any intention‐to‐treat analyses at the end time‐point of the studies, which may have affected the final results.
Potential biases in the review process
We applied a very comprehensive search strategy to identify all potential studies and found three trials for inclusion, all of which were performed in Italy. However, research in this field has been performed outside Italy, as we noticed that experiments with rTMS for ALS have been widely conducted in other countries such as Japan and France. It is possible that some studies may not have been identified, if they were not published or if they were published in journals not indexed in widely accessible databases. We will include such studies in updates of this review if they are identified in the future.
Agreements and disagreements with other studies or reviews
Dileone and co‐authors conducted a narrative review on rTMS for ALS (Dileone 2010), which summarised the results of four published studies. All studies were adequately described but no pooled analysis was performed due to the lack of sufficient data from these research studies. The authors concluded that rTMS is a safe and tolerable procedure for ALS patients and further studies should be pursued to determine if rTMS has applicable effects on ALS, which is similar to the conclusions of our systematic review. However, we note that any potential benefits of rTMS need to be balanced with the demands of trial participation for ALS patients (Di Lazarro 2009).
Authors' conclusions
Implications for practice.
The included trials failed to use adequate methodology, examined only small number of participants, had incomplete outcome data and presented little raw numerical data. Low quality evidence from one small trial showed no significant differences between rTMS and sham rTMS for the ALSFRS‐R scores and MMT scores at 12 months follow‐up. There is currently insufficient evidence of the efficacy and safety of rTMS in the treatment of ALS from the included trials.
Implications for research.
Further studies may be helpful if their potential benefit is weighed against the impact of participation in a randomised controlled trial on people with ALS. A rigorous method of randomisation should be used and the allocation adequately concealed. Missing outcome data should be avoided wherever possible and data should be analysed according to intention‐to‐treat principles.
Some other important points relating to rTMS therapy should be taken into account before considering a new trial. With variation in frequency, intensity and duration of the magnetic field, further research is warranted to evaluate the potential efficacy of different protocols of motor cortex stimulation, particularly during the early stages of the disease when the progression rate is more pronounced (Dileone 2010). Some techniques and indicators could be used to explore neurochemical effects of the stimulation to develop a more effective protocol, such as magnetic resonance spectroscopy (MRS) (Stagg 2009), diffusion tensor MRI (Sach 2004), the triple‐stimulation technique (Magistris 1999) and BDNF production (Angelucci 2004). This type of human trial would likely require that intermittent rTMS be sustained over a long period of time until a dose‐response curve was established, before proceeding to a larger scale clinical trial.
What's new
| Date | Event | Description |
|---|---|---|
| 1 March 2013 | New citation required but conclusions have not changed | Results of new search incorporated. Published notes added. |
| 3 February 2013 | New search has been performed | For the 2013 update we updated the searches but found no new trials. We also updated the methods of assessment of the risk of bias. There are minor revisions and the plain language summary has been rewritten. |
Notes
New research on this intervention in ALS/MND is slow to emerge. The next update of this review is scheduled four years from the date of search instead of the usual two years. If there are new trials in the interim, an earlier update will be planned.
Acknowledgements
The authors would like to acknowledge editors and staff of the Cochrane Neuromuscular Disease Group for advice and helpful comments on this review.
The Cochrane Neuromuscular Disease Group editorial base is supported by the MRC Centre for Neuromuscular Diseases and the Motor Neurone Disease Association.
Appendices
Appendix 1. MEDLINE (OvidSP) search strategy
Database: Ovid MEDLINE(R) <1946 to July Week 3 2012> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp Motor Neuron Disease/ (17583) 2 (moto$1 neuron$1 disease$1 or moto?neuron$1 disease$1).mp. (5865) 3 (Lou Gehrig$1 adj5 (disease or syndrome$1)).mp. (65) 4 charcot disease.tw. (11) 5 Amyotrophic Lateral Sclerosis.mp. (14247) 6 or/1‐5 (21212) 7 Transcranial Magnetic Stimulation/ (5071) 8 rtms.tw. (1736) 9 ((transcranial magnetic stimulation or tms) adj5 repetitive).tw. (1897) 10 ((transcranial magnetic stimulation or tms) adj5 rhythmic).tw. (12) 11 or/7‐10 (5539) 12 6 and 11 (77) 13 remove duplicates from 12 (76)
Appendix 2. EMBASE (OvidSP) search strategy
Database: Embase <1980 to 2012 Week 30> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 Motor Neuron Disease/ or amyotrophic lateral sclerosis/ (23039) 2 (moto$1 neuron$1 disease$1 or moto?neuron$1 disease$1).mp. (8530) 3 (Lou Gehrig$1 adj5 (syndrome$ or disease$)).mp. (109) 4 charcot disease.tw. (19) 5 Amyotrophic Lateral Sclerosis.tw. (14187) 6 or/1‐5 (25642) 7 transcranial magnetic stimulation/ (10026) 8 rtms.tw. (2577) 9 ((transcranial magnetic stimulation or tms) adj5 repetitive).tw. (2665) 10 ((transcranial magnetic stimulation or tms) adj5 rhythmic).tw. (16) 11 or/7‐10 (10360) 12 6 and 11 (256) 13 limit 12 to embase (239) 14 remove duplicates from 13 (239)
Appendix 3. AMED (OvidSP) search strategy
Database: AMED (Allied and Complementary Medicine) <1985 to July 2012> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 Motor Neuron Disease/ (90) 2 (moto$1 neuron$1 disease$1 or moto?neuron$1 disease$1).mp. (164) 3 (Lou Gehrig$1 adj5 (syndrome$ or disease)).mp. (2) 4 charcot disease.tw. (1) 5 Amyotrophic lateral sclerosis/ or Amyotrophic Lateral Sclerosis.tw. (246) 6 or/1‐5 (386) 7 (Transcranial Magnetic Stimulation or tms).tw. (202) 8 rtms.tw. (35) 9 7 or 8 (205) 10 6 and 9 (1)
Appendix 4. CINAHL Plus (EBSCOhost) search strategy
Monday, July 30, 2012 9:53:33 AM S11 S5 and S10 16 S10 S6 or S7 or S8 or S9 1429 S9 ( transcranial magnetic stimulation or tms ) and rhythmic 6 S8 ( transcranial magnetic stimulation or tms ) and repetitive 340 S7 rtms 258 S6 ("Transcranial Magnetic Stimulation") or (MH "Magnet Therapy") 1393 S5 S1 or S2 or S3 or S4 4795 S4 ("Amyotrophic Lateral Sclerosis") 1958 S3 Lou Gehrig* and ( disease* or syndrome* ) 31 S2 (moto* neuron* disease* or moto?neuron* disease) 856 S1 MH Motor Neuron Diseases+ 4535
Appendix 5. CENTRAL search strategy
#1 MeSH descriptor Motor Neuron Disease explode all trees #2 (moto* neuron* disease* or moto?neuron* disease) #3 "Amyotrophic Lateral Sclerosis" #4 ("Lou Gehrig*" and (disease* or syndrome*)) #5 (#1 OR #2 OR #3 OR #4) #6 "transcranial magnetic stimulation" #7 rtms #8 tms NEAR repetitive #9 tms NEAR rhythmic #10 (#6 OR #7 OR #8 OR #9) #11 (#5 AND #10)
Appendix 6. Science Citation Index Expanded (ISI) search strategy
# 7 #3 and #6# 6 #4 OR #5 # 5 TS=Lou Gehrig$ OR TS=Charcot disease # 4 TS=motor neuron$ disease$ OR TS=moto$neuron$ disease$ OR TS= amyotrophic lateral sclerosis # 3 #1 OR #2 # 2 TS=rTMS # 1 TS=(transcranial magnetic stimulation) SAME TS=(repetitive) Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S Timespan=All Years
Appendix 7. Chinese Biomedical Database search strategy
(amyotrophic lateral sclerosis or motor neuron disease) and transcranial magnetic stimulation
Appendix 8. ClinicalTrials.gov
(amyotrophic lateral sclerosis or motor neuron disease or motor neurone disease or motorneurone disease or motorneuron disease or motoneuron disease or motoneurone disease) and (transcranial magnetic stimulation or rTMS)
Data and analyses
Comparison 1. rTMS versus sham rTMS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Changes to the ALSFRS‐R scores at 12 months follow‐up | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐8.78, 6.38] |
| 2 Changes to the MMT scores at 12 months follow‐up | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.77, 1.17] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Di Lazarro 2006.
| Methods | Prospective, double‐blind, randomised, placebo‐controlled trial Six months treatment |
|
| Participants | Twenty participants (10 men and 10 women; mean age 61.2 ± 10.7 years) with definite ALS were enrolled and allocated to real (n = 10) and sham (n = 10) stimulation group (no more than 24 months of disease duration) | |
| Interventions | Experimental group: rTMS was performed using a butterfly coil held over motor cortex on each hemisphere. Stimulation protocol was the cTBS in which three pulses of stimulation are given at 50 Hz, repeated every 200 ms for a total of 600 pulses. The stimulus intensity was 80% of action motor threshold. rTMS was delivered for 5 consecutive days per month for 6 months Control group: Sham rTMS was performed using the same stimulator connected to the placebo butterfly coil which has no stimulating effect on the cortex but produces similar auditory and tactile sensations as the active coil. The site of stimulation and the number of stimuli was identical to those used for the active magnetic rTMS rTMS was delivered for 5 consecutive days per month for 6 months All participants were taking riluzole |
|
| Outcomes | Primary outcomes: rate of decline of ALSFRS‐R score after the end of rTMS treatment (6 months) Secondary outcomes: rate of decline of MMT score after the end of rTMS treatment (6 months) |
|
| Notes | It was a single centre trial carried out in Italy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Patients were randomly allocated by one of the authors (VD) not involved in follow‐up evaluations and data analysis. Stratified block randomisation was performed' ‐ the way the stratified block randomisation was carried out was not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Allocation 'by one of the authors (VD) not involved in follow‐up evaluations and data analysis' ‐ no further details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical stimulator used for the real rTMS and placebo rTMS, to ensure participants and the neurologists administering the intervention were unaware of the study group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The neurologists assessing the outcomes were stated to be blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Numbers lost to follow‐up and reasons for this were given, but intention‐to‐treat analysis was not performed |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in the results section of the manuscript |
| Other bias | Low risk | There were no other concerns regarding the risk of bias in this study |
Di Lazarro 2009.
| Methods | Prospective, double‐blind, randomised, placebo‐controlled trial 12 months treatment |
|
| Participants | Twenty patients, 5 women and 15 men aged 25 to 69 years, were enrolled and allocated to real (n = 10) and sham (n = 10) stimulation group (disease duration ranged from 8 to 70 months) Inclusion criteria: a diagnosis of definite or probable ALS according to the revised El Escorial criteria and age 18 years or older Exclusion criteria: seizure history; concomitant severe medical problems; history of tracheostomy; and contraindications for TMS |
|
| Interventions | Experimental group: rTMS was performed using a butterfly coil held over motor cortex on each hemisphere. Stimulation protocol was the cTBS in which three pulses of stimulation are given at 50 Hz, repeated every 200 ms for a total of 600 pulses. The stimulus intensity was 80% of action motor threshold. rTMS was delivered for 5 consecutive days per month for 12 months Control group: Sham rTMS was performed using the same stimulator connected to the placebo butterfly coil which has no stimulating effect on the cortex but produces similar auditory and tactile sensations as the active coil. The site of stimulation and the number of stimuli will be identical to those used for the active magnetic rTMS rTMS was delivered for 5 consecutive days per month for 12 months All participants were taking riluzole |
|
| Outcomes | Primary outcomes: rate of decline of ALSFRS‐R score after the end of rTMS treatment (12 months) Secondary outcomes: rate of decline of MMT score after the end of rTMS treatment (12 months) |
|
| Notes | It was a single centre trial carried out in Italy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Patients were randomly allocated by one of the authors (VD) not involved in follow‐up evaluations and data analysis. Randomisation was performed using a dynamic allocation strategy' ‐ the way dynamic allocation strategy was carried out was not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Allocation 'by one of the authors (VD) not involved in follow‐up evaluations and data analysis' ‐ no further details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical stimulator used for the real rTMS and placebo rTMS, to ensure participants and the neurologists administering the intervention were unaware of the study group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The neurologists assessing the outcomes were state to be blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Numbers lost to follow‐up and reasons for this were given, but intention‐to‐treat analysis was not performed |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in the results section of the manuscript |
| Other bias | Low risk | There were no other concerns regarding the risk of bias in this study |
Zanette 2008.
| Methods | Prospective RCT 2 weeks treatment and 2 weeks follow‐up |
|
| Participants | 10 patients with probable or definite ALS were enrolled. 5 participants in active stimulation group and 5 participants in sham stimulation group Age: 43 to 72 years Men: 7 Women: 3 Disease duration: 7 to 18 months |
|
| Interventions | Experimental group: for upper‐limb cortical areas on both sides, a Super Rapid magnetic stimulator (The Magstim Co., UK) connected to a figure‐of‐eight focal coil was performed (5Hz, 20 trains of 15 stimuli, 60 s interval between trains; 110% resting motor threshold). rTMS was delivered for 5 consecutive days per week for 2 weeks Control group: performed using the same stimulator connected to a specific sham coil that have no stimulating effect on the cortex but produces similar auditory and scalp sensations as the active coil treatment with manipulation, which delivered 5 consecutive days per week for 2 weeks All participants were taking riluzole |
|
| Outcomes | Changes in ALSFRS‐R scores, muscle strength and fatigue 2 weeks after the end of rTMS | |
| Notes | It was a single centre trial carried out in Italy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "a randomised trial on a group of ALS patients", the detailed method was not stated |
| Allocation concealment (selection bias) | Unclear risk | "a randomised trial on a group of ALS patients", the detailed method was not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical stimulator used for the real rTMS and placebo rTMS, to ensure participants and the neurologists administering the intervention were unaware of the study group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study report does not state whether investigators were blinded to treatment assignment until completion of the final analyses. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four weeks assessments were available for all randomised participants |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in the results section of the manuscript |
| Other bias | Low risk | There were no other concerns regarding the risk of bias in this study |
ALS: amyotrophic lateral sclerosis; ALSFRS‐R: Amyotrophic Lateral Sclerosis Functional Rating Scale‐Revised; BDNF: brain derived neurotrophic factor; RCT: randomised controlled trial; rTMS: repetitive transcranial magnetic stimulation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Angelucci 2004 | Not a RCT. The aim was to investigate the effects of suprathreshold 1 Hz rTMS on BDNF plasma levels in 10 healthy subjects and the effects of either 1 Hz or 20 Hz rTMS in 4 ALS patients |
| Di Lazarro 2004 | Not a RCT. The purpose of this study was to investigate the efficacy of different protocols of rTMS in the treatment of ALS. Therefore, 4 patients with ALS were included and each patient received a different kind of rTMS |
| Di Lazarro 2010 | Only 2 patients were included in the study |
| Dileone 2010 | Not a RCT, a review of rTMS as a treatment for ALS |
| Floyd 2009 | Investigates rTMS measures as clinical correlates and longitudinal markers of ALS |
| Khedr 2011 | Investigates rTMS measures as clinical correlates and longitudinal markers of ALS |
RCT: randomised controlled trial; rTMS: repetitive transcranial magnetic stimulation; BDNF: brain derived neurotrophic factor.
Differences between protocol and review
In the protocol we specified that six month rather than 12 month changes in ALSFRS‐R scores and muscle strength would be included in the 'Summary of findings' table.
Jinghuan Fang joined the review team for the first update of the review in 2012 and Jian Guo withdrew from authorship.
Contributions of authors
For the original review, Jian Guo selected studies, assessed risk of bias, performed data extraction, compiled the draft version of the review and modified the review in accordance with feedback provided by the other authors.
JF ‐ updated the review, selected studies, assessed risk of bias, performed data extraction, compiled the draft version of the review, modified the review in accordance with feedback provided by the other authors.
LH ‐ conceived and designed the review.
MZ ‐ assessed risk of bias.
MY ‐ selected studies.
CZ ‐ performed data extraction and data analysis.
Sources of support
Internal sources
West China Hospital of Sichuan University, Sichuan, China.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Di Lazarro 2006 {published data only}
- Lazzaro V, Dileone M, Pilato F, Profice P, Ranieri F, Musumeci G, et al. Repetitive transcranial magnetic stimulation for ALS. A preliminary controlled study. Neuroscience Letters 2006;408(2):135‐40. [PUBMED: 16979292] [DOI] [PubMed] [Google Scholar]
- Tagliente D, Dileone M, Pilato F, Profice P, Ranieri F, Capone F. Repetitive transcranial magnetic stimulation for ALS. A preliminary controlled study;:.. European Journal of Neurology 2008;15 (Suppl 3):351. [Google Scholar]
Di Lazarro 2009 {published data only}
- Lazzaro V, Pilato F, Profice P, Ranieri F, Musumeci G, Florio L, et al. Motor cortex stimulation for ALS: a double blind placebo‐controlled study. Neuroscience Letters 2009;464(1):18‐21. [PUBMED: 19682544] [DOI] [PubMed] [Google Scholar]
Zanette 2008 {published data only}
- Zanette G, Forgione A, Manganotti P, Fiaschi A, Tamburin S. The effect of repetitive transcranial magnetic stimulation on motor performance, fatigue and quality of life in amyotrophic lateral sclerosis. Journal of Neurological Sciences 2008;270(1‐2):18‐22. [PUBMED: 18304580] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Angelucci 2004 {published data only}
- Angelucci F, Oliviero A, Pilato F, Saturno E, Dileone M, Versace V, et al. Transcranial magnetic stimulation and BDNF plasma levels in amyotrophic lateral sclerosis. Neuroreport 2004;15(4):717‐20. [PUBMED: 15094483] [DOI] [PubMed] [Google Scholar]
Di Lazarro 2004 {published data only}
- Lazzaro V, Oliviero A, Saturno E, Pilato F, Dileone M, Sabatelli M, et al. Motor cortex stimulation for amyotrophic lateral sclerosis. Time for a therapeutic trial?. Clinical Neurophysiology 2004;115(6):1479‐85. [DOI] [PubMed] [Google Scholar]
Di Lazarro 2010 {published data only}
- Lazzaro V, Dileone M, Pilato F, Profice P, Cioni B, Meglio M, et al. Long‐term motor cortex stimulation for amyotrophic lateral sclerosis. Brain Stimulation 2010;3(1):22‐7. [PUBMED: 20633427] [DOI] [PubMed] [Google Scholar]
Dileone 2010 {published data only}
- Dileone M, Profice P, Pilato F, Ranieri F, Capone F, Musumeci G, et al. Repetitive transcranial magnetic stimulation for ALS. CNS and Neurological Disorders Drug Targets 2010;9(3):331‐4. [PUBMED: 20406177] [DOI] [PubMed] [Google Scholar]
Floyd 2009 {published data only}
- Floyd AG, Yu QP, Piboolnurak P, Tang MX, Fang Y, Smith WA, et al. Transcranial magnetic stimulation in ALS: utility of central motor conduction tests. Neurology 2009;72(6):498‐504. [PUBMED: 19204259] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khedr 2011 {published data only}
- Khedr EM, Ahmed MA, Hamdy A, Shawky OA. Cortical excitability of amyotrophic lateral sclerosis:transcranial magnetic stimulation study. Neurophysiologie Clinique 2011;41(2):73‐9. [PUBMED: 21624709] [DOI] [PubMed] [Google Scholar]
Additional references
Andersen 2005
- Andersen PM, Borasio GD, Dengler R, Hardiman O, Kollewe K, Leigh PN, et al. EFNS task force on management of amyotrophic lateral sclerosis: guidelines for diagnosing and clinical care of patients and relatives. European Journal of Neurology 2005;12(12):921‐38. [PUBMED: 16324086] [DOI] [PubMed] [Google Scholar]
Barker 1985
- Barker AT, Jalinous R, Freeston IL. Non‐invasive magnetic stimulation of human motor cortex. Lancet 1985;1(8437):1106‐7. [PUBMED: 2860322] [DOI] [PubMed] [Google Scholar]
Borich 2009
- Borich M, Arora S, Kimberley TJ. Lasting effects of repeated rTMS application in focal hand dystonia. Restorative Neurology and Neuroscience 2009;27(1):55‐65. [PUBMED: 19164855] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brooks 1994
- Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial "Clinical limits of amyotrophic lateral sclerosis" workshop contributors. Journal of Neurological Sciences 1994;124 Suppl:96‐107. [PUBMED: 7807156] [DOI] [PubMed] [Google Scholar]
Brooks 2000
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders 2000;1(5):293‐9. [PUBMED: 11464847] [DOI] [PubMed] [Google Scholar]
Cedarbaum 1999
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS‐R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). Journal of Neurological Sciences 1999;169(1‐2):13‐21. [PUBMED: 10540002] [DOI] [PubMed] [Google Scholar]
Chen 1997
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, et al. Depression of motor cortex excitability by low‐frequency transcranial magnetic stimulation. Neurology 1997;48(5):1398‐403. [PUBMED: 9153480] [DOI] [PubMed] [Google Scholar]
Di Lazarro 2002
- Lazzaro V, Oliviero A, Berardelli A, Mazzone P, Insola A, Pilato F, et al. Direct demonstration of the effects of repetitive transcranial magnetic stimulation on the excitability of the human motor cortex. Experimental Brain Research 2002;144(4):549‐53. [PUBMED: 12037639] [DOI] [PubMed] [Google Scholar]
Elahi 2009
- Elahi B, Chen R. Effect of transcranial magnetic stimulation on Parkinson motor function‐systematic review of controlled clinical trials. Movement Disorders 2009;24(3):357‐63. [PUBMED: 18972549] [DOI] [PubMed] [Google Scholar]
Fitzgerald 2003
- Fitzgerald PB, Brown TL, Marston NA, Daskalakis ZJ, Castella A, Kulkarni J. Transcranial magnetic stimulation in the treatment of depression: a double‐blind, placebo‐controlled trial. Archives of General Psychiatry 2003;60(10):1002‐8. [PUBMED: 14557143] [DOI] [PubMed] [Google Scholar]
Forsgren 1983
- Forsgren L, Almay B G, Holmgren G, Wall S. Epidemiology of motor neuron disease in northern Sweden. Acta Neurologica Scandinavica 1983;68(1):20‐9. [PUBMED: 6604389] [DOI] [PubMed] [Google Scholar]
Fujiki 2003
- Fujiki M, Kobayashi H, Abe T, Kamida T. Repetitive transcranial magnetic stimulation for protection against delayed neuronal death induced by transient ischemia. Journal of Neurosurgery 2003;99(6):1063‐9. [PUBMED: 14705735] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 10578460] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated March 2011]. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011.
Huang 2004
- Huang YZ, Rothwell JC. The effect of short‐duration bursts of high‐frequency, low‐intensity transcranial magnetic stimulation on the human motor cortex. Clinical neurophysiology 2004;115(5):1069‐75. [PUBMED: 15066532] [DOI] [PubMed] [Google Scholar]
Huang 2005
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron 2005;45(2):201‐6. [PUBMED: 15664172] [DOI] [PubMed] [Google Scholar]
Klein 1999
- Klein E, Kolsky Y, Puyerovsky M, Koren D, Chistyakov A, Feinsod M. Right prefrontal slow repetitive transcranial magnetic stimulation in schizophrenia: a double‐blind sham‐controlled pilot study. Biological Psychiatry 1999;46(10):1451‐4. [PUBMED: 10578460] [DOI] [PubMed] [Google Scholar]
Kobayashi 2003
- Kobayashi M, Pascual‐Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurology 2003;2(3):145‐56. [PUBMED: 12849236] [DOI] [PubMed] [Google Scholar]
Krupp 1989
- Krupp LB, LaRocca NG, Muir‐Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology 1989;46(10):1121‐3. [PUBMED: 2803071] [DOI] [PubMed] [Google Scholar]
Magistris 1999
- Magistris MR, Rosler KM, Truffert A, Landis T, Hess CW. A clinical study of motor evoked potentials using a triple stimulation technique. Brain 1999;122 ( Pt 2):265‐79. [PUBMED: 10071055] [DOI] [PubMed] [Google Scholar]
Mandrioli 2003
- Mandrioli J, Faglioni P, Merelli E, Sola P. The epidemiology of ALS in Modena, Italy. Neurology 2003;60(4):683‐9. [PUBMED: 12601112] [DOI] [PubMed] [Google Scholar]
Miller 2007
- Miller RG, Mitchell JD, Lyon M, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD001447.pub2; PUBMED: 17253460] [DOI] [PubMed] [Google Scholar]
Plewnia 2003
- Plewnia C, Lotze M, Gerloff C. Disinhibition of the contralateral motor cortex by low‐frequency rTMS. Neuroreport 2003;14(4):609‐12. [PUBMED: 12657896] [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Riviere 1998
- Riviere M, Meininger V, Zeisser P, Munsat T. An analysis of extended survival in patients with amyotrophic lateral sclerosis treated with riluzole. Archives of Neurology 1998;55(4):526‐8. [PUBMED: 9561981] [DOI] [PubMed] [Google Scholar]
Romero 2002
- Romero JR, Anschel D, Sparing R, Gangitano M, Pascual‐Leone A. Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clinical Neurophysiology 2002;113(1):101‐7. [PUBMED: 11801430] [DOI] [PubMed] [Google Scholar]
Rowland 2001
- Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. The New England Journal of Medicine 2001;344(22):1688‐700. [PUBMED: 11386269] [DOI] [PubMed] [Google Scholar]
Sach 2004
- Sach M, Winkler G, Glauche V, Liepert J, Heimbach B, Koch MA, et al. Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain 2004;127(Pt 2):340‐50. [PUBMED: 14607785] [DOI] [PubMed] [Google Scholar]
Siebner 2003
- Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Experimental Brain Research 2003;148(1):1‐16. [PUBMED: 12478392] [DOI] [PubMed] [Google Scholar]
Sorenson 2002
- Sorenson EJ, Stalker AP, Kurland LT, Windebank AJ. Amyotrophic lateral sclerosis in Olmsted County, Minnesota, 1925 to 1998. Neurology 2002;59(2):280‐2. [PUBMED: 12136072] [DOI] [PubMed] [Google Scholar]
Stagg 2009
- Stagg CJ, Wylezinska M, Matthews PM, Johansen‐Berg H, Jezzard P, Rothwell JC, et al. Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. Journal of Neurophysiology 2009;101(6):2872‐7. [PUBMED: 19386916] [DOI] [PMC free article] [PubMed] [Google Scholar]
Theodore 2002
- Theodore WH, Hunter K, Chen R, Vega‐Bermudez F, Boroojerdi B, Reeves‐Tyer P, et al. Transcranial magnetic stimulation for the treatment of seizures: a controlled study. Neurology 2002;59(4):560‐2. [PUBMED: 12196649] [DOI] [PubMed] [Google Scholar]
Wassermann 1998
- Wassermann EM, Wedegaertner FR, Ziemann U, George MS, Chen R. Crossed reduction of human motor cortex excitability by 1‐Hz transcranial magnetic stimulation. Neuroscience Letters 1998;250(3):141‐4. [PUBMED: 9708852] [DOI] [PubMed] [Google Scholar]
Zanette 2002
- Zanette G, Tamburin S, Manganotti P, Refatti N, Forgione A, Rizzuto N. Different mechanisms contribute to motor cortex hyperexcitability in amyotrophic lateral sclerosis. Clinical Neurophysiology 2002;113(11):1688‐97. [PUBMED: 12417221] [DOI] [PubMed] [Google Scholar]
Ziemann 2004
- Ziemann U, Eisen A. TMS for ALS: why and why not. Clinical Neurophysiology 2004;115(6):1237‐8. [PUBMED: 15134689] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Guo 2010
- Guo J, Zhou M, Yang M, Zhu C, He L. Repetitive transcranial magnetic stimulation for the treatment of amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD008554.pub2] [DOI] [PubMed] [Google Scholar]
He 2010
- He L, Guo J, Zhou M, Yang M, Zhu C. Repetitive transcranial magnetic stimulation for the treatment of amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD008554] [DOI] [PubMed] [Google Scholar]


