Abstract
Background
Age‐related cataract is the opacification of the lens, which occurs as a result of denaturation of lens proteins. Age‐related cataract remains the leading cause of blindness globally, except in the most developed countries. A key question is what is the best way of removing the lens, especially in lower income settings.
Objectives
To compare two different techniques of lens removal in cataract surgery: manual small incision surgery (MSICS) and extracapsular cataract extraction (ECCE).
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2014, Issue 8), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to September 2014), EMBASE (January 1980 to September 2014), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to September 2014), Web of Science Conference Proceedings Citation Index‐ Science (CPCI‐S), (January 1990 to September 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 23 September 2014.
Selection criteria
We included randomised controlled trials (RCTs) only. Participants in the trials were people with age‐related cataract. We included trials where MSICS with a posterior chamber intraocular lens (IOL) implant was compared to ECCE with a posterior chamber IOL implant.
Data collection and analysis
Data were collected independently by two authors. We aimed to collect data on presenting visual acuity 6/12 or better and best‐corrected visual acuity of less than 6/60 at three months and one year after surgery. Other outcomes included intraoperative complications, long‐term complications (one year or more after surgery), quality of life, and cost‐effectiveness. There were not enough data available from the included trials to perform a meta‐analysis.
Main results
Three trials randomly allocating people with age‐related cataract to MSICS or ECCE were included in this review (n = 953 participants). Two trials were conducted in India and one in Nepal. Trial methods, such as random allocation and allocation concealment, were not clearly described; in only one trial was an effort made to mask outcome assessors. The three studies reported follow‐up six to eight weeks after surgery. In two studies, more participants in the MSICS groups achieved unaided visual acuity of 6/12 or 6/18 or better compared to the ECCE group, but overall not more than 50% of people achieved good functional vision in the two studies. 10/806 (1.2%) of people enrolled in two trials had a poor outcome after surgery (best‐corrected vision less than 6/60) with no evidence of difference in risk between the two techniques (risk ratio (RR) 1.58, 95% confidence interval (CI) 0.45 to 5.55). Surgically induced astigmatism was more common with the ECCE procedure than MSICS in the two trials that reported this outcome. In one study there were more intra‐ and postoperative complications in the MSICS group. One study reported that the costs of the two procedures were similar.
Authors' conclusions
There are no other studies from other countries other than India and Nepal and there are insufficient data on cost‐effectiveness of each procedure. Better evidence is needed before any change may be implemented. Future studies need to have longer‐term follow‐up and be conducted to minimize biases revealed in this review with a larger sample size to allow examination of adverse events.
Keywords: Adult; Aged; Aged, 80 and over; Humans; Middle Aged; Posterior Eye Segment; Age Factors; Cataract Extraction; Cataract Extraction/adverse effects; Cataract Extraction/methods; India; Lens Implantation, Intraocular; Lens Implantation, Intraocular/methods; Lenses, Intraocular; Nepal; Randomized Controlled Trials as Topic; Visual Acuity
Plain language summary
Comparison of two different methods of lens removal in cataract surgery, particularly relevant to lower income settings
Review question What is the best way of removing the lens in cataract surgery, especially in lower income settings?
This review considers two ways of removing the lens. In manual small incision surgery (MSICS) the lens is broken up and removed through a small incision. In extracapsular cataract extraction (ECCE) the lens is removed through a larger incision. ECCE is the standard way of doing cataract surgery in lower income countries.
Background As people get older, the lens in the eye can become cloudy ‐ this is known as a cataract. Cataract is the most common cause of blindness in the world. Vision can be restored by surgery to remove the cloudy lens. The lens is replaced with a plastic lens. This is known as an intraocular lens or IOL.
Study characteristics We found three randomised controlled trials. The searches are up to date to September 23rd 2014.
A total of 953 people with age‐related cataract in India and Nepal were randomly allocated to MSICS and ECCE in these trials.
Key results The data were limited. People whose lens was removed with MSICS were more likely to achieve good functional vision, however, overall not more than 50% of people achieved good functional vision in the two studies. 1.2% of people enrolled in two trials had a poor outcome after surgery with best‐corrected vision less than 6/60. There was no evidence of any difference between the two groups with respect to this outcome. Surgically induced astigmatism was more common with the ECCE procedure than MSICS in the two trials that reported this outcome. In one study there were more intra‐ and postoperative complications in the MSICS group. One study reported that the costs of the two procedures were similar.
Quality of the evidence We judged the quality of the evidence to be low or very low. There were only three studies and we could not combine the data because of differences in reporting and inconsistency between trials which meant that some of the results were imprecise.
Summary of findings
for the main comparison.
| MSICS compared with ECCE for age‐related cataract | |||
|
Patient or population: people with age‐related cataract Settings: hospital Intervention: MSICS Comparison: ECCE | |||
| Outcomes | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Good functional vision: presenting visual acuity of 6/12 or better at 6‐8 weeks | 806 (2) | ⊕⊕⊝⊝ low1,2 | More people in MSCIS group achieved good functional vision. Risk ratio (RR) (in favour of MSICS) of 1.29 (95% confidence intervals (CI) 1.08 to 1.53) (Gogate 2003) and 2.43 (1.10 to 5.34) (Gurung 2009). |
| Poor visual outcome after surgery: best‐corrected visual acuity of < 6/60 at 6‐8 weeks. | 806 (2) | ⊕⊕⊝⊝ low1,3 | Six people in MSICS group and four people in ECCE group had poor visual outcome (RR 1.58, 95% CI 0.45 to 5.55) (Gogate 2003). No participant had poor outcome in Gurung 2009 (100 participants) |
| Intraoperative and immediate post‐operative complications | 953 (3) |
⊕⊝⊝⊝ very low4 | There were no reported complications during surgery in the George 2005 and Gurung 2009 studies. In the PUNE study (Gogate 2003), 21 of the participants in the MSICS group were converted to ECCE either due to density of cataract or because of small pupil. 29/358 (8.1%) of the MSICS group and 17/383 (4.4%) of the ECCE group and had intraoperative complications (RR 1.83, 95% CI 1.02 to 3.26). 18/358 (6 with vitreous loss) in the MSICS group had posterior capsule tears compared to 10/383 (6 with vitreous loss) in the ECCE group (RR 1.93, 95% CI 0.90 to 4.12). Two participants in the MSICS group had iridodialysis. |
| Long‐term complications (one year or more after surgery) | No data: no trial reported long term follow‐up. | ||
| Quality of life | No data: no trial reported data on quality of life. | ||
| Cost‐effectiveness | 741 (1) |
⊕⊝⊝⊝ very low4 | In the PUNE study (Gogate 2003), there was no significant difference in surgical time or cost between the two procedures, even accounting for surgeon variation. The average cost of ECCE was USD 15.82, MSICS USD 15.68 of which USD 11.34 was a fixed facility cost common to both. |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
1. Risk of bias: generation of allocation schedule, allocation concealment and masking of participants, personnel and outcome assessors not clearly described. 2. Inconsistency in trial results. 3. Imprecision: wide confidence intervals. 4. Lack of data.
Background
Description of the condition
Age‐related cataract is the opacification of the lens, which occurs as a result of denaturation of lens proteins and this is not thought to be reversible. These changes are often bilateral although they can be asymmetric. Symptoms from cataracts include glare, blurred vision, progressive decrease in visual function and blindness.
Description of the intervention
Extracapsular cataract extraction (ECCE) was introduced with the development of microsurgical instrumentation in the early 1980s. The lens content is removed through a large 12 mm incision leaving the posterior lens capsule intact. A posterior chamber intraocular lens (IOL) can then be placed in the capsular bag (Apple 1989; Duane 1986). If no IOL is implanted, aphakic glasses or contact lenses must be used. Extracapsular surgery has become the preferred method of extraction in economically advantaged countries and most surgeons in developing countries have been trained in this technique.
Further technological development has led to a majority of surgeons in developed countries adopting sutureless ECCE surgery (Norregaard 1999). This surgery uses either ultrasonic fragmentation (phacoemulsification) of the lens nucleus (Mehta 1999), or a manual fragmentation technique (Blumenthal 1992; Hennig 1999). Both suture and sutureless ECCE leave in place the posterior capsule of the lens. This keeps the anatomical barrier between the posterior and anterior segments of the eye and may reduce the risk of posterior segment complications. The disadvantage of all the extracapsular techniques is that the posterior lens capsule can become cloudy (Apple 1992) with the need for a primary or secondary capsulotomy by surgery or using a YAG laser. This increases the costs of surgery and incurs the risk of secondary complications (Javitt 1992).
Manual small incision cataract surgery (MSICS) was first described by Blumenthal (Blumenthal 1992). In Asia and Africa there has been a renewal of interest in this technique (Ruit 2000) as an alternative to phacoemulsification because it is considerably less costly but has similar benefits of rapid visual recovery and reduced astigmatism (Yorston 2005). It involves a 6 mm to 6.5 mm scleral incision, just large enough to allow insertion of a 6 mm IOL. There are various different techniques described for performing the capsulotomy in MSICS, for example, the can‐opener (Gogate 2005), the continuous curvilinear capsulorhexis (Gogate 2003) and the endocapsular technique where the incision is from pupil margin to pupil margin. The cataract is delivered into the anterior chamber, hydroextracted and aspirated. The posterior capsule of the lens is left intact. This technique is technically more difficult than a standard manual ECCE.
Figure 1 is a flow diagram summarising the different types of cataract surgery.
1.
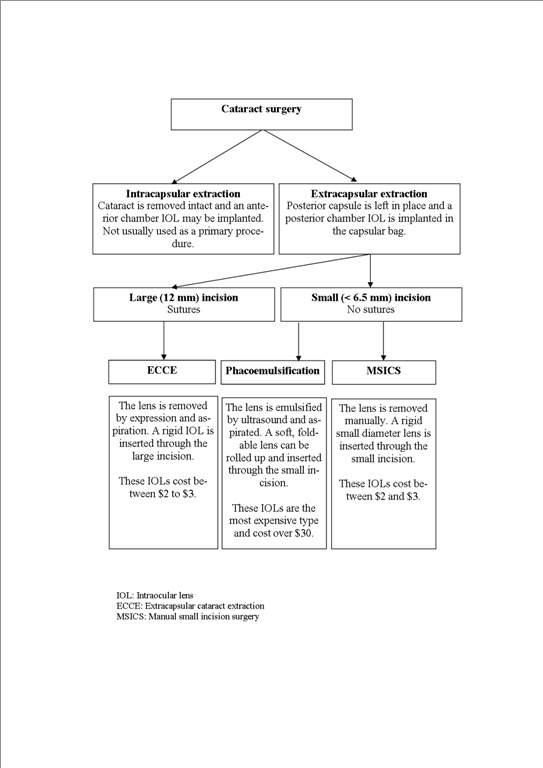
Types of cataract surgery
How the intervention might work
Cataract surgery works by removing the opacified lens and replacing it with a clear lens called an IOL. IOLs can be made from a range of materials, and they can be made of varying size, shape and refractive powers. Before cataract surgery the eye to be operated on is measured so that an IOL of the correct power (strength) can be inserted after the cataract has been removed. The IOL is usually placed inside the “bag” of the lens capsule inside the eye. Other options for lens replacement include contact lenses and glasses.
Surgery is currently the only treatment option once the lens has opacified and vision is decreasing. The indication for surgery is based on whether the patient's reduced visual function interferes with their quality of life.
Why it is important to do this review
The World Health Organization (WHO) recently reported that age‐related cataract is now responsible for 48% of world blindness, which represents about 18 million people currently. It was estimated that there were 37 million people worldwide who were blind in 2002 (Passolini 2004; Resnikoff 2004). Age‐related cataract remains the leading cause of blindness globally, except in the most developed countries. This is despite an increasing number of visually impaired and blind people gaining access to cataract surgical services due to the development of prevention of blindness programmes in many countries (Kupfer 1994). Despite these positive trends the number of people blind due to cataract is increasing because of the changing demographic structure of populations (Limburg 1996; Minassian 1990; Thylefors 1998). More than 82% of all blind people are 50 years of age or older.
It is estimated that the present number of 20 million cataract blind will double by the year 2020. The global initiative "Vision 2020: The Right to Sight" has suggested various strategies to reduce cataract blindness (Foster 2001). The WHO has called for a dramatic increase in surgical volumes worldwide, but the outcomes of cataract surgery are not always good and may depend on the surgical technique used (Venkatesh 2005).
The first published version of this review ‘Surgical interventions for age‐related cataract’ (Snellingen 2002) compared the outcomes of different cataract surgical techniques. The techniques included initially were intracapsular extraction (ICCE), ECCE and phacoemulsification. In 2006 the review was revised and a fourth surgical technique MSICS was added to the review (Riaz 2006).
Following consultation with the review authors and the Cochrane Eyes and Vision Group this update has been divided into three smaller reviews each using the same outcome measures but only comparing two surgical methods within each review. The ICCE technique is no longer included as this method is no longer used as a primary procedure.
The cataract surgical techniques compared in these three reviews are:
1. MSICS and ECCE (current review); 2. phacoemulsification and ECCE (de Silva 2014); 3. phacoemulsification and MSICS (Riaz 2013).
Although phacoemulsification is the most technologically advanced method providing small incision sutureless surgery, it requires considerable resources due to consumables, maintenance and training of surgeons. It is the procedure of choice for cataract surgery in developed countries.
From a global perspective phacoemulsification is too costly for many developing countries where there is the highest incidence of cataract blindness. Manual small incision surgery and ECCE are alternative techniques available at a lower cost. The aim of this review is to compare the relative effectiveness of ECCE and MSICS.
This review will help to establish which surgical method (MSICS or ECCE) should be performed for people with age‐related cataract, especially those living in low and middle‐income countries, where high volumes of cataract surgeries are performed.
Objectives
The aim of this review is to compare two different techniques of lens removal in cataract surgery: MSICS and ECCE.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) only.
Types of participants
Participants in the trials were people with age‐related cataract.
Types of interventions
We included trials where MSICS with a posterior chamber IOL implant was compared to ECCE with a posterior chamber IOL implant.
We also considered the different ways in which the lens was removed in MSICS or ECCE. We defined these as:
techniques requiring the placement of sutures;
techniques not requiring the placement of sutures with the lens removed after manual fragmentation.
We did not consider phacoemulsification in this review as this is the subject of the two separate Cochrane reviews (de Silva 2014; Riaz 2013) mentioned above.
Types of outcome measures
Primary outcomes
The primary outcome for this review was postoperative visual acuity. We considered both presenting* and best‐corrected visual acuity (BCVA) at the following cut‐points.
Proportion of people achieving good functional vision defined as presenting visual acuity better than or equal to 6/12 in the operated eye.
Proportion of people with a poor outcome after surgery defined as BCVA worse than 6/60 in the operated eye.
* Presenting visual acuity is vision that the person uses in normal life, i.e. with or without glasses, if worn.
Secondary outcomes
-
Intraoperative complications
capsular rupture with or without vitreous loss
iris prolapse
postoperative inflammation
other complications as reported
-
Long‐term complications (one year or more after surgery)
posterior capsule opacification
retinal detachment
glaucoma
cystoid macular oedema
corneal endothelial cell loss
corneal decompensation
other complications as reported
Quality of life (self‐care, mobility, social and mental function) as reported
Cost‐effectiveness
Follow up
We considered outcomes at three months and one year after surgery. As studies may not report outcomes exactly at these time points we defined the following time periods:
three months: from four weeks to less than six months
12 months: from six months to less than 18 months
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2014, Issue 8), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to September 2014), EMBASE (January 1980 to September 2014), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to September 2014), Web of Science Conference Proceedings Citation Index‐ Science (CPCI‐S), (January 1990 to September 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 23 September 2014.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), CPCI‐S (Appendix 5), mRCT (Appendix 6), ClinicalTrials.gov (Appendix 7) and the ICTRP (Appendix 8).
Searching other resources
We searched the reference lists of all included studies and pertinent reviews identified. We contacted the authors of the included studies to identify unpublished studies or studies sent for publication or in press.
Data collection and analysis
Selection of studies
Two authors assessed the search results for relevance and inclusion. We obtained full‐text copies of any report referring to definitely or possibly relevant trials. We assessed these full‐text copies according to the definitions in the 'Criteria for considering studies for this review' section. We only assessed trials meeting these criteria for methodological quality. Any trial that was excluded at this stage, was documented in the review and a reason for exclusion given.
Data extraction and management
We extracted data using a form developed by the Cochrane Eyes and Vision Group. Two authors extracted data and compared the results for differences. We resolved discrepancies by discussion. One author entered data in to Review Manager 5 (Review Manager 2011) and the second author checked for errors.
Assessment of risk of bias in included studies
We assessed the included studies using the Cochrane Collaboration's tool for risk of bias as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We considered the following parameters: sequence generation and allocation concealment, masking (blinding) of participants, personnel and outcome assessors, incomplete outcome data, selective outcome reporting and other sources of bias. We graded them as low risk of bias, high risk of bias, and unclear risk of bias indicating either lack of information or uncertainty over the potential for bias. Two review authors independently assessed the risk of bias for each of these parameters and disagreement was resolved by discussion. Authors were not masked to the report authors and trial results during the assessment.
Measures of treatment effect
The outcomes for this review are largely dichotomous (i.e. postoperative visual acuity and complications). Our measure of treatment effect is the risk ratio. For outcomes that occur rarely (in less than 10% of the cohort), we planned to use the odds ratio. We planned to analyse quality of lIfe, which may be reported as a continuous variable, using the weighted mean difference, but in the event no data were available on quality of life.
Unit of analysis issues
The main unit of analysis issue is how the trial investigators dealt with the fact that people have two eyes. There are several options here: a trial may randomise people to the intervention groups and then apply the intervention and/or measure the outcome in one eye (study eye) or both eyes. In the latter case it is incorrect to analyse eyes without taking into account the fact that the eyes for a person are not independent. Alternatively a trial may randomly allocate eyes to an intervention so each person has a different intervention in each eye. In this case, the pairing has to be taken into account in the analysis. In our protocol we planned the following:
At the review level, if the trial has been incorrectly analysed, we will contact the trial investigators for further information to enable calculation of a design effect (Perera 2007). If the trial does report estimates adjusted for within person correlation we will enter them in the review using the generic inverse variance method. Although cluster trials are a possibility we think they are unlikely because individual randomisation is relatively easy to do in this case.
However, we did not have enough data to include this in any formal meta‐analysis. Only Gurung 2009 mentioned that 100 eyes of 88 participants were randomised into two groups. For the other two trials it was unclear from the study report but contact with the investigators of Gogate 2003 confirmed only one eye per person was entered into the trial.
Dealing with missing data
We planned to collect data on the reason for missingness, with the caveat that this might not be reliably reported.
Our plan to deal with missing data was as follows but in the event we did not have enough data for any formal meta‐analysis:
Analyses based on available data assume that missing data are missing at random. We will investigate how reasonable this assumption is by doing a series of sensitivity analyses with different assumptions about the missing data using methods as set out by White et al (White 2008). The "informative missingness odds ratio" (IMOR) refers to the ratio of the odds of the outcome among participants for whom data are missing and the odds of the outcome among participants who are available. These IMORs can be assumed to be equal or different in the two trial arms. We plan to do four sensitivity analyses. Firstly we will assume the IMOR is 2 in treatment and control groups i.e. that people who were not seen were twice as likely to have the outcome. Secondly, we will assume that the IMOR was ½ in both treatment and control groups i.e. that people who were not seen were half as likely to have the outcome. For the third and fourth sensitivity analyses, we will assume that the IMOR was opposite in treatment and control groups ‐ i.e. 2 or ½.
All analyses will be done using the metamiss command in Stata (version 11.0, StataCorp LP, 4905 Lakeway Drive, College Station, TX 77845 USA).
If the pooled risk ratio in any of these sensitivity analyses differs substantially from the available case analysis (say by 10% or more) it is likely that the missing data in the included trials are a cause for concern. We will record this information in the risk of bias tables under "incomplete data".
Assessment of heterogeneity
Our plan for assessing heterogeneity was as follows but in the event we did not have enough data for any formal meta‐analysis:
We will assess heterogeneity in several ways. Firstly, by documenting clinical and methodological differences between the studies. Secondly by examining the forest plots to see whether the estimates of effect are consistent, and thirdly by considering the I2 value and χ2 test for heterogeneity (bearing in mind that the χ2 test has low power when the number of trials is small).
Assessment of reporting biases
Our plan for assessing reporting biases was as follows but in the event we did not have enough data to complete these:
The main reporting biases that we will consider are publication bias and outcome reporting bias. For publication bias, if there are enough trials we will do a funnel plot to assess whether small trials have different effects. To assess outcome reporting bias we will complete a review outcome matrix following the ORBIT classification (Kirkham 2010).
Data synthesis
Our plan for assessing data synthesis was as follows but in the event we did not have enough data for a formal meta‐analysis:
We will pool data from studies collecting comparable outcome measures with similar follow‐up times using a random‐effects model (unless there are three or fewer trials in which case we will use a fixed‐effect model). If there is evidence for substantial heterogeneity or inconsistency, for example an I2 value of 50% or more, we will not pool the results.
The outcomes for this review include a number of complications. Initially we will tabulate these data only. For outcomes that are commonly reported we will go on to do a meta‐analysis in order to provide a summary estimate of risk.
Subgroup analysis and investigation of heterogeneity
Our plan for subgroup analysis was as follows but in the event we did not have enough data for a formal subgroup analysis:
It is possible that the effect of the interventions will vary according to the setting (high/low volume) and whether or not suture/sutureless techniques are used. If there are enough data, we will explore heterogeneity focusing primarily on these subgroups.
Sensitivity analysis
Our plan for sensitivity analysis was as follows but in the event we did not have enough data for a formal sensitivity analysis:
If there are enough trials contributing to the meta‐analyses we will investigate the effect of excluding poorer quality trials. In particular, we will investigate the effect of excluding trials where allocation concealment was not properly reported and where there was no masking of outcome assessment.
Results
Description of studies
Results of the search
The electronic searches yielded a total of 148 titles and abstracts. After de‐duplication we screened the title and abstracts of 103 references. We rejected 100 abstracts as not eligible for inclusion in the review. We obtained and screened full‐text copies of three references and included them in the review.
An update search run in September 2014 identified a further 33 references (Figure 2). The Trials Search Co‐ordinator removed 14 duplicates and screened the remaining 19 references, of which eight were not relevant to the scope of the review. We reviewed the remaining 11 references and but none met the inclusion criteria for the review.
2.
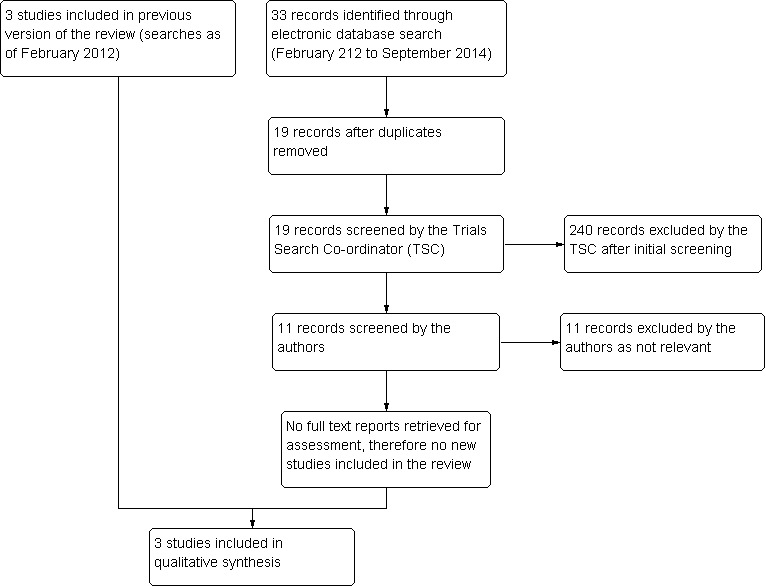
Results from searching for studies for inclusion in the review.
Included studies
We included three trials (George 2005; Gogate 2003; Gurung 2009) that met our inclusion criteria. We have provided a brief summary of the characteristics of the included studies and further details can be found in the 'Characteristics of included studies' table.
Size of study
Number of participants that underwent MSICS or ECCE were 124 (George 2005); 741 (Gogate 2003) and 88 (Gurung 2009) respectively.
Location of studies
Two studies were conducted in India (George 2005; Gogate 2003) and one in Nepal (Gurung 2009)
Age of participants
Participants were aged between 35 and 93 years of age. Specifically, the age of participants was a mean of 58±8.0 years (George 2005); 40 to 90 years (Gogate 2003) and 35 to 93 years (Gurung 2009).
Types of interventions
All three studies compared MSICS with ECCE; in one trial there was an additional phacoemulsification arm (George 2005).
Follow‐up
All three studies had a minimum follow‐up of six weeks. None of the trials reported data after eight weeks. For Gogate 2003 this was confirmed by contact with the investigator.
Outcomes
All three studies evaluated visual acuity and astigmatism as their main outcome; and complications as part of results of the study. Distance visual acuity was measured in all trials using either Snellen acuity or LogMAR scale with the EDTRS chart. One study specifically stated their primary and secondary outcomes, such as surgical time and vision related quality of life, patient satisfaction, and economic outcomes. Postoperative complications were graded according to the Oxford Cataract Treatment and Evaluation Team (OCTET) grading system (OCTET 1986)
Excluded studies
We did not exclude any studies after obtaining full‐text copies.
Risk of bias in included studies
3.
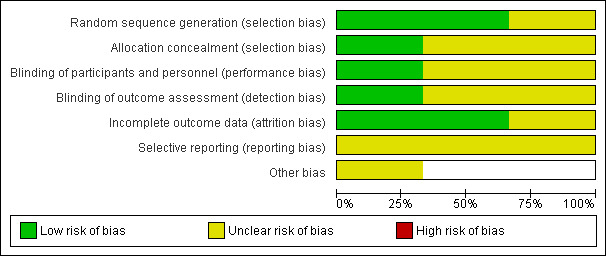
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
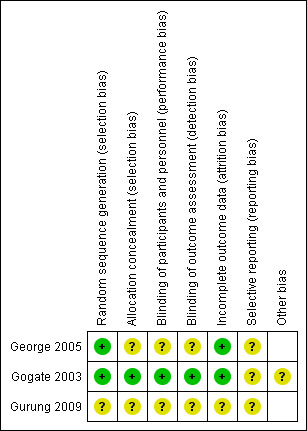
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In only one trial was it clearly stated how the allocation schedule was generated (George 2005). One trial described using drawing lots or ‘ballots’ to randomly assign the treatment and surgeon (Gogate 2003). Allocation concealment was not described in any trial.
Blinding
In assessing ECCE and MSICS, it may be difficult to mask the assessors due to the obvious presence of sutures in ECCE. Nonetheless, masking was stated in one study where internee doctors and optometrists did postoperative visual acuity testing and administering the questionnaires; participants were not told about the type of surgery done (Gogate 2003).
Incomplete outcome data
Follow‐up rates were good in all three trials: 85% (George 2005); 95% (Gogate 2003) and 100% (Gurung 2009) respectively. Exclusions were not clearly documented except in one trial (Gogate 2003).
Selective reporting
Postoperative complications were not described in the George 2005 study. Otherwise, all outcomes on visual acuity, astigmatism and complications were reported in all three studies.
Other potential sources of bias
In one trial, some surgeons performed more surgeries of one kind to increase the external validity of the study. Imbalance of surgeon assignment may have introduced bias, but this was dealt with by stratification by surgeon in the analysis (Gogate 2003).
Effects of interventions
See: Table 1
Visual outcomes
The data on visual outcomes is summarised in Table 2. The three studies only followed‐up to six weeks (George 2005; Gogate 2003) and six to eight weeks (Gurung 2009).
1. Visual acuity.
| Outcome | *3 months | 12 months | ||||
| MSICS n/N |
ECCE n/N |
***Risk ratio (95% CI) | MSICS n/N |
ECCE n/N |
Risk ratio (95% CI) | |
| **Presenting vision 6/12 or better | ||||||
| George 2005 | No data | No data | ||||
| Gogate 2003 | 165/344 | 135/362 | 1.29 (1.08 to 1.53) | No data | ||
| Gurung 2009 | 17/50 | 7/50 | 2.43 (1.10 to 5.34) | No data | ||
| BCVA < 6/60 | ||||||
| George 2005 | No data | No data | ||||
| Gogate 2003 | 6/344 | 4/362 | 1.58 (0.45 to 5.55) | No data | ||
| Gurung 2009 | 0/50 | 0/50 | No data | |||
BCVA: best‐corrected visual acuity
* In the protocol for the review we planned to measure outcomes at three months which we defined as any assessment between 4 weeks and 6 months. In fact both trials contributing data measured visual acuity a bit earlier than 3 months at six weeks (Gogate 2003) and six to eight weeks (Gurung 2009).
**In the protocol for the review, we planned to examine "presenting" vision but in fact both trials reported unaided or uncorrected vision here and Gogate 2003 only reported visual acuity of 6/18 or better.
*** Data from Gogate 2003; Gurung 2009 were inconsistent (I2 = 59%) therefore were not pooled.
In George 2005, 1/53 cases had BCVA < 6/18 in MSICS group compared to five in the ECCE group (three related to high astigmatism, one posterior capsule opacification and one anterior ischaemic optic neuropathy).
In the PUNE study (Gogate 2003),165/344 (48%) of the MSICS group and 135/362 (37%) of the ECCE group had a UCVA 6/18 or better (relative risk (RR) 1.29, 95% confidence intervals (CI) 1.08 to 1.53). Six (1.7%) people in the MSICS group and four participants (1.1%) in the ECCE group had poor visual outcome (BCVA < 6/60) in the operated eye (RR 1.58, 95% CI 0.45 to 5.55).
In Gurung 2009, UCVA of 6/12 and better was achieved in 17/50 (34%) of the MSICS group and 7/50 (14%) of people in the ECCE group (RR 2.43, 95% CI 1.10 to 5.34) at six to eight weeks postoperatively.
Surgically induced astigmatism (SIA)
In George 2005, SIA was greater in the ECCE group compared to MSICS (mean induced astigmatism in dioptres, 1.77±1.65 versus 1.1±0.95, P = 0.012). In Gurung 2009, astigmatism of ≥ 2D was found in 17/48 (35.4%) and 35/48 (72.9%) participants from MSICS and ECCE groups respectively (RR 0.49, 95% CI = 0.32 to 0.74) at eight weeks. Surgically induced astigmatism was not described in the George 2005 study.
Intraoperative surgical complications
There were no reported complications during surgery in George 2005 and Gurung 2009. In the PUNE study (Gogate 2003), 21 of the participants in the MSICS group were converted to ECCE either due to density of cataract or because of small pupil. 29/358 (8.1%) of the MSICS group and 17/383 (4.4%) of the ECCE group and had intraoperative complications (RR 1.83, 95% CI 1.02 to 3.26). 18/358 (six with vitreous loss) in the MSICS group had posterior capsule tears compared to 10/383 (six with vitreous loss) in the ECCE group (RR 1.93, 95% CI 0.90 to 4.12). Two participants in the MSICS group had iridodialysis.
Postoperative complications
Postoperative complications were not described in George 2005. In Gurung 2009, corneal oedema was present immediately postoperatively in 48% of MSICS and 62% in ECCE, which cleared by eight weeks postoperatively. One participant from MSICS group had Descemet membrane detachment that reattached by eight weeks with good vision. Posterior capsule opacification was present in 6% of MSICS and 4% of ECCE. In the PUNE study (Gogate 2003), 121/358 (33.8%) of the MSICS group and 94/383 (24.5%) of the ECCE group had postoperative complications in the first six weeks (RR 1.38, 95% CI 1.10 to 1.73); the majority were mild (27.1%) (OCTET grade 1). There were no severe complications (OCTET grade 3), moderate complications were seen in 5/358 in the MSICS group and 3/383 ECCE group (RR 1.78, 95% CI 0.43 to 7.41), there was no significant difference between the two groups. Mild complications e.g. Descemet’s folds, iritis and corneal oedema were more commonly seen in the MSICS group (32.4% versus 23.7% ECCE group). Posterior capsule opacification was seen equally in both groups (4/358 MSICS versus 3/383 ECCE).
Endothelial cell count
Gogate 2003 and Gurung 2009 did not study this outcome. In George 2005, there was no statistically significant difference in endothelial cell loss between the MSICS and ECCE groups. The sample size was adequate to detect a 7% difference in endothelial cell count between the groups, giving a power of 80%. There was a mean 4.72% (N = 52, SD 13.07) induced cell loss in ECCE at six weeks follow‐up compared with 4.21% (N = 53, SD 10.29) for MSICS.
Economic evaluation
In the PUNE study (Gogate 2003), there was no significant difference in surgical time or cost between the two procedures, even accounting for surgeon variation. The average cost of ECCE was USD 15.82, MSICS USD 15.68 of which USD 11.34 was a fixed facility cost common to both.
Discussion
Summary of main results
Overall, visual outcomes are comparable between MSICS and ECCE ('Table 1'). Although MSICS have better UCVA results, there is no difference in BCVA between the two methods. However, surgically induced astigmatism is significantly greater after ECCE compared to MSICS. There is suggestion that there are fewer intraoperative and postoperative complications after ECCE than MSICS but this requires further study based on the quality of evidence supporting this. Thus, in countries such as India where high surgical volumes are required, MSICS was suggested to be the surgical technique of choice due to better unaided visual outcomes but equal costs.
Overall completeness and applicability of evidence
As most study participants came from India or Nepal, the applicability to other populations or races may be limited. Moreover, within India there is a difference between the results from hospitals when compared to cataract camps (Singh 2000), which should be kept in mind when interpreting these results. Furthermore, evaluation of cataract surgery outcomes should not be based on postoperative visual acuity alone – and assessments of quality of life and quality of vision should be made. The studies in this review did not specifically measure these outcomes.
Quality of the evidence
We included three trials in this review, which compared two techniques for cataract surgery. Due to the small number of studies that actually examined our objectives, conclusions have to be interpreted with caution. The main outcome measure was visual acuity in the studies reviewed. However, it is not appropriate to compare MSICS and ECCE at six weeks, as suture techniques such as ECCE require a longer period for vision stabilisation due to suture induced astigmatism. Only one study had a follow‐up of up to one year (Gogate 2003) but did not report these data. Although long‐term follow‐up is always a challenge in developing countries, more studies with a longer‐follow‐up are required.
Potential biases in the review process
All studies included were from an extensive search with the above‐mentioned search and inclusion criteria. However, only three studies were included out of the many studies reviewed. Studies not published and indexed in the libraries included, or non‐English journals may have been omitted. While RCTs provide the highest level of evidence, cohort studies or observational studies could provide some information not included in this review. Finally, publication bias may exist if only studies with significant results are published, however, we did not have direct evidence of any publication bias in this case.
Agreements and disagreements with other studies or reviews
When evaluating cataract surgeries, cost‐effectiveness is an important outcome measure not studied frequently. In our review, included studies suggested that MSICS had better unaided visual acuity and equal cost. Another study not included in this review found that MSICS (USD 17.03) cost more than ECCE (USD 16.25) (Muralikrishnan 2004), but patients’ costs (direct and indirect) were highest for ECCE due to the increased number of days required for follow‐up, which incurs transportation and economic productivity loss. However, it is unclear if this study was adequately powered to study this and clearly, the need for a proper cost‐effectiveness study is required.
Authors' conclusions
Implications for practice.
This review, which only includes three RCTs, suggests that MSICS gives better uncorrected visual acuity and less surgically induced astigmatism compared to ECCE. Each surgical technique has its limitations, and should be chosen based on patients’ medical and ocular history. For example, relative contraindications to MSICS include zonular weakness, lack of corneal clarity with corneal decompensation and dense cataracts. There are no other studies from other countries other than India and Nepal and there are insufficient data on cost‐effectiveness of each procedure. Better evidence is needed before any change may be implemented.
Implications for research.
More studies are required to compare the visual outcomes between MSICS and ECCE. We suggest that visual outcomes at three and six months are the minimum follow‐up time for comparing ECCE and MSICS. Also, an adequately powered randomised controlled trial is required to assess cost‐effectiveness and the impact on quality of life. When executing these RCTs the study participant should be randomised to expert surgeons in each technique rather than having the same surgeon performing both procedures to reduce single surgeon bias. A single surgeon performing both procedures does not produce a surgeon effect. This is bias introduced by a surgeon having more expertise in one intervention as compared to the other.
What's new
| Date | Event | Description |
|---|---|---|
| 5 November 2014 | New citation required but conclusions have not changed | Plain language summary updated |
| 5 November 2014 | New search has been performed | Electronic searches updated but no new trials identified |
Notes
The updated version of the original published Cochrane review 'Riaz Y, Mehta JS, Wormald R, Evans JR, Foster A, Ravilla T, Snellingen T. Surgical interventions for age‐related cataract. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No.: CD001323. DOI: 10.1002/14651858.CD001323.pub2' has been divided into three smaller reviews each using the same outcome measures as the original review but only comparing two surgical methods within each review. The interventions being compared are ECCE, MSICS and phacoemulsification. Intracapsular extraction (ICCE) is no longer included in the reviews as this technique is no longer used as a primary procedure.
Acknowledgements
The Cochrane Eyes and Vision Group (CEVG) Trials Search Co‐ordinator created and ran the electronic search strategies. We thank Clare Gilbert, Catey Bunce and Richard Wormald for their comments on the review and Anupa Shah for editorial support.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Cataract #2 MeSH descriptor Cataract Extraction #3 MeSH descriptor Lens, Crystalline #4 MeSH descriptor Lenses, Intraocular #5 MeSH descriptor Lens Implantation, Intraocular #6 intraocular lens* or intra ocular lens* or IOL* #7 (#1 OR #2 OR #3 OR #4 OR #5 OR #6) #8 extracapsular near/2 cataract* #9 extra capsular near/2 cataract* #10 ECCE #11 (#8 OR #9 OR #10) #12 manual near/3 small near/3 incision near/3 cataract* #13 MISICS or SICS #14 MeSH descriptor Capsulorhexis #15 continuous near/3 curvilinear near/3 capsulor*hexis #16 continuous near/3 circular near/3 capsulor*hexis #17 CCC or CCS #18 can opener near/5 capsulotom* #19 endocapsular #20 (#12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19) #21 (#7 AND #11 AND #20)
Appendix 2. MEDLINE (OvidSP) search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp cataract/ 14. cataract extraction/ 15. exp lens crystalline/ 16. exp lenses intraocular/ 17. lens implantation intraocular/ 18. (intraocular lens$ or intra ocular lens$ or IOL$).tw. 19. or/13‐18 20. (extracapsular adj2 cataract$).tw. 21. (extra capsular adj2 cataract$).tw. 22. ECCE.tw. 23. or/20‐22 24. (manual adj3 small adj3 incision adj3 cataract$).tw. 25. (MISICS or SICS).tw. 26. capsulorhexis/ 27. (continuous adj3 curvilinear adj3 capsulor?hexis).tw. 28. (continuous adj3 circular adj3 capsulor?hexis).tw. 29. (CCC or CCS).tw. 30. (can opener adj5 capsulotom$).tw. 31. endocapsular.tw. 32. or/24‐31 33. 19 and 23 and 32 34. 12 and 33
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. EMBASE (OvidSP) search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp cataract/ 34. exp cataract extraction/ 35. exp lens/ 36. exp lens implant/ 37. exp lens implantation/ 38. (intraocular lens$ or intra ocular lens$ or IOLS).tw. 39. or/33‐38 40. exp extracapsular cataract extraction/ 41. (extracapsular adj2 cataract$).tw. 42. (extra capsular adj2 cataract$).tw. 43. ECCE.tw. 44. or/40‐43 45. (manual adj3 small adj3 incision adj3 cataract$).tw. 46. (MISICS or SICS).tw. 47. capsulorhexis/ 48. (continuous adj3 curvilinear adj3 capsulor?hexis).tw. 49. (continuous adj3 circular adj3 capsulor?hexis).tw. 50. (CCC or CCS).tw. 51. (can opener adj5 capsulotom$).tw. 52. endocapsular.tw. 53. or/45‐52 54. 39 and 44 and 53 55. 32 and 54
Appendix 4. LILACS search strategy
cataract$ and extracapsular or extra capsular or ECCE and manual small incis$ or MISICS or SICS or capsulorhexis or capsulorrhexis
Appendix 5. Web of Science CPCI‐S search strategy
#16 #3 and #4 and #15 #15 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 #14 TS=endocapsular #13 TS=can opener capsulotom* #12 TS=(CCC or CCS) #11 TS=(continuous circular capsulorrhexis) #10 TS=(continuous circular capsulorhexis) #9 TS=(continuous curvilinear capsulorrhexis) #8 TS=(continuous curvilinear capsulorhexis) #7 TS=capsulorhexis #6 TS=(MISICS or SICS) #5 TS=(manual small incision) #4 TS= (extracapsular or extra capsular or ECCE) #3 #1 OR #2 #2 TS=(intraocular lens* or intra ocular lens* or IOL*) #1 TS=cataract*
Appendix 6. metaRegister of Controlled Trials search strategy
cataract and extracapsular
Appendix 7. ClinicalTrials.gov search strategy
Cataract AND Extracapsular
Appendix 8. ICTRP search strategy
cataract and extracapsular
Characteristics of studies
Characteristics of included studies [ordered by study ID]
George 2005.
| Methods | Randomised controlled trial Masking of outcome assessment: not reported. ECCE: sutured; PHACO and MSICS: not routinely sutured unless wound leak | |
| Participants | Number randomised: 186 participants (total) Number of participants underwent ECCE: 62 Number of participants underwent MSICS: 62 Age: Mean age of ECCE group: 57.8±8.0 years Mean age of MSICS group: 58.8±8.7 years Inclusion criteria: participant undergoing planned cataract surgery; otherwise normal pre‐op examination; cataract < grade III Exclusion criteria: other potential causes of decreased vision; complicated cataracts; non age‐related cataracts; phacodenesis; glaucoma or retinal pathology Country: India |
|
| Interventions | PHACO versus ECCE versus MSICS Follow‐up: six weeks | |
| Outcomes | SIA; EC ‐ specular microscopy counts; visual acuity | |
| Notes | Two surgeons PHACO ‐ 5 mm incision rigid lens MSICS ‐ Blumenthal technique | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
"Cases were randomized into three groups based on computer‐generated random numbers. Randomization was carried out at the time of admission and used the hospital numbers (which were allotted at the time of the first hospital visit) for allocation into different groups." Page 294 "Cases were separately randomized for each surgeon so that equal numbers of each technique were performed by each surgeon". Page 294 |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The 6‐weeks follow‐up was completed by 52/62 cases of ECCE, 53/62 cases of SICS |
| Selective reporting (reporting bias) | Unclear risk | There were no intra‐operative complications and post‐operative complications were not described. Authors presented main outcomes, visual acuity, astigmatism and endothelial cell counts as described |
Gogate 2003.
| Methods | Randomised clinical trial: ECCE and MSICS | |
| Participants | 741 participants Age: 40 to 90 years Inclusion criteria: cataract participants within age 40 to 90 years old Exclusion criteria: any ocular co‐morbidity capable of compromising vision, if they needed combined surgical procedures, or if the axial length of the eye was more than 26 mm |
|
| Interventions | ECCE versus MSICS Follow‐up: one week, six weeks, and one year after surgery |
|
| Outcomes | Visual acuity Primary outcome was the proportion of participants having uncorrected and corrected visual acuity of 6/18 or better at 6 weeks by both techniques Secondary outcomes: 1. Complications, both intraoperative and postoperative, with either technique 2. The average surgical time for each technique 3. Vision related quality of life, patient satisfaction, and economic outcomes |
|
| Notes | Randomisation and blinding/masking of outcome assessment clearly described in Methods | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Each patient was randomly allocated to one of the two groups by drawing lots (ballots). There was always a 50% chance of the patient getting one particular kind of intervention." Page 669 |
| Allocation concealment (selection bias) | Low risk | "The operating surgeons also drew ballots for the type of surgery they were supposed to do that day, at the beginning of the theatre list immediately before scrubbing. This random assignment was done in the presence of the anaesthetist, operation theatre senior nurse, and another non‐operating ophthalmologist." Page 669 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The patients were not informed as to the type of intervention they would receive, in the OT and during follow up. The surgeons were unaware until scrubbing up which surgery they would perform that day. They were also unaware which patient would be brought to them for surgery and did not examine the patients the next day." Page 669 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Internee doctors and optometrists did postoperative visual acuity testing and administering the questionnaires. They were not told about the type of surgery done." Page 669 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 706/741 completed follow‐up. ECCE group 362/383 (94.5%) and MSICS group 344/358 (96.1%). Figure 1 page 668 |
| Selective reporting (reporting bias) | Unclear risk | None obvious |
| Other bias | Unclear risk | Some surgeons performed more surgeries of one kind if the operating list was more compared to the other technique when the list was shorter. This was done to increase the external validity of the study. Imbalance of surgeon assignment may have introduced bias, but this was dealt with by stratification by surgeon in the analysis |
Gurung 2009.
| Methods | Randomised clinical trial, 2 arms: ECCE and MSICS | |
| Participants | 100 eyes (88 participants) Age: 35 to 93 years Inclusion criteria: cataract participants with no local or systemic diseases Exclusion criteria: any ocular co‐morbidity capable of compromising vision, e.g., participants with central corneal opacity, glaucoma, diabetics with significant fundus changes, participants with inflammatory eye diseases, etc |
|
| Interventions | ECCE versus MSICS Follow‐up: six to eight weeks |
|
| Outcomes | Unaided and best‐corrected visual acuity and astigmatism | |
| Notes | Masking of outcome assessment: not reported. Analysed 100 eyes of 88 participants; did not adjust for within‐person correlation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Used "systematic randomization sampling technique" for allocation into two groups. Page 14 |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. At the end of six to eight weeks, the final unaided visual acuity was recorded. The best‐corrected visual acuity with the type of astigmatism was noted by objective and subjective refraction |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants completed follow‐up |
| Selective reporting (reporting bias) | Unclear risk | None obvious |
ECCE: extracapsular extraction MSICS: manual small incision cataract surgery PHACO: phacoemulsification SIA: surgically induced astigmatism
Contributions of authors
MA and JM were responsible for formulating the review question, writing the protocol for the review, undertaking manual searches, screening search results, screening retrieved papers against the inclusion criteria, writing to authors for additional information, obtaining and screening data on unpublished studies, providing a clinical and policy perspective MA, JM and JE were responsible for appraising the quality of the papers, extracting data from the trial reports, analysing the data, interpretation of data, providing a methodological perspective and writing the review. MA and JE were responsible for entering data in to RevMan. JM was responsible for checking the data that were entered in to RevMan.
For the update in November 2014 MA and JE screened search results and JE updated the Plain Language Summary to current standards.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Sightsavers, UK.
Provided funding to support JE to co‐author the first version of this review.
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision Group (CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEVG Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
George 2005 {published data only}
- George R, Rupauliha P, Sripriya AV, Rajesh PS, Vahan PV, Praveen S. Comparison of endothelial cell loss and surgically induced astigmatism following conventional extracapsular cataract surgery, manual small‐incision surgery and phacoemulsification. Ophthalmic Epidemiology 2005;12(5):293‐7. [DOI] [PubMed] [Google Scholar]
Gogate 2003 {published data only}
- Gogate PM, Deshpande M, Wormald RP, Deshpande R, Kulkarni SR. Extracapsular cataract surgery compared with manual small incision cataract surgery in community eye care setting in western India: a randomised controlled trial. British Journal of Ophthalmology 2003;87(6):667‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurung 2009 {published data only}
- Gurung A, Karki DB, Shrestha S, Rijal AP. Visual outcome of conventional extracapsular cataract extraction with posterior chamber intraocular lens implantation versus manual small‐incision cataract surgery. Nepalese Journal of Ophthalmology 2009;1(1):13‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Apple 1989
- Apple DH, Mamalis N, Olson RJ, Kincaid MC. Intraocular Lenses: Evolution, Designs, Complications and Pathology. Baltimore: Williams & Wilkins, 1989:225‐361. [Google Scholar]
Apple 1992
- Apple DJ, Solomon KD, Tetz MR, Assia EI, Holland EY, Legler UF, et al. Posterior capsule opacification. Survey of Ophthalmology 1992;37(2):73‐116. [DOI] [PubMed] [Google Scholar]
Blumenthal 1992
- Blumenthal M, Ashkenazi I, Assia E, Cahane M. Small‐incision manual extracapsular cataract extraction using selective hydrodissection. Ophthalmic Surgery 1992;23(10):699‐701. [PubMed] [Google Scholar]
de Silva 2014
- Silva SR, Riaz Y, Evans JR. Phacoemulsification with posterior chamber intraocular lens versus extracapsular cataract extraction (ECCE) with posterior chamber intraocular lens for age‐related cataract. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD008812.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duane 1986
- Duane T. Textbook of Ophthalmology. Lippincott‐Raven, 1986:25. [Google Scholar]
Foster 2001
- Foster A. Cataract and "Vision 2020‐the right to sight" initiative. British Journal of Ophthalmology 2001;85(6):635‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Gogate 2005
- Gogate PM, Kulkarni SR, Krishnaiah S, Deshpande RD, Joshi SA, Palimkar A, et al. Safety and efficacy of phacoemulsification compared with manual small‐incision cataract surgery by a randomized controlled clinical trial: six‐week results. Ophthalmology 2005;112(5):869‐74. [DOI] [PubMed] [Google Scholar]
Hennig 1999
- Hennig A. Tunnel sutureless high volume cataract surgery. IAPB 6th General Assembly. Beijing, September 6 1999.
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Javitt 1992
- Javitt JC, Tielch JM, Canner JK, Kolb MM, Sommer A, Steinberg EP. National outcomes of cataract extraction. Increased risk of retinal complications associated with Nd:YAG laser capsulotomy. The Cataract Patient Outcomes Research Team. Ophthalmology 1992;99(10):1487‐98. [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI] [PubMed] [Google Scholar]
Kupfer 1994
- Kupfer C. The International Agency for the Prevention of Blindness. American Journal of Ophthalmology 1994;117(2):253‐7. [DOI] [PubMed] [Google Scholar]
Limburg 1996
- Limburg H, Kumar R, Bachani D. Monitoring and evaluating cataract intervention in India. British Journal of Ophthalmology 1996;80(11):951‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehta 1999
- Mehta KR, Mehta CK. Teaching standards in phacoemulsification. How realistic are they?. Symposium on Phacoemulsification. VI Ophthalmological Congress of SAARC Countries. Kathmandu, November 20 1999.
Minassian 1990
- Minassian DC, Mehra V. 3.8 Million blinded by cataract each year: projections from the first epidemiological study of the incidence of cataract blindness in India. British Journal of Ophthalmology 1990;74(6):341‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muralikrishnan 2004
- Muralikrishnan R, Venkatesh R, Prajna NV, Frick KD. Economic cost of cataract surgery procedures in an established eye care centre in Southern India. Ophthalmic Epidemiology 2004;11(5):369‐80. [DOI] [PubMed] [Google Scholar]
Norregaard 1999
- Norregaard JC, Bernth‐Pettersen P, Bellan L, Alonso J, Black C, Dunn E, et al. Intraoperative clinical practice and risk of early complications after cataract extraction in the Unites States, Canada, Denmark and Spain. Ophthalmology 1999;106(1):42‐8. [DOI] [PubMed] [Google Scholar]
OCTET 1986
- Anonymous. Use of a grading system in the evaluation of complications in a randomised controlled trial on cataract surgery. Oxford Cataract Treatment and Evaluation Team (OCTET). British Journal of Ophthalmology 1986;70(6):411‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Passolini 2004
- Passolini D, Mariotti SP, Pokharel GP, Pararajasegarm R, Etyalale D, Negrel AD, et al. 2002 global update of available data on visual impairment:a compilation of population‐based prevalence studies. Ophthalmic Epidemiology 2004;11(2):67‐115. [DOI] [PubMed] [Google Scholar]
Perera 2007
- Perera R, Glasziou P. A simple method to correct for the design effect in systematic reviews of trials using paired dichotomous data. Journal of Clinical Epidemiology 2007;60(9):975‐8. [DOI] [PubMed] [Google Scholar]
Resnikoff 2004
- Resnikoff S, Passolini D, Etyalale D, Kocur I, Pararajasegarm R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bulletin of The World Health Organisation 2004;82(11):844‐51. [PMC free article] [PubMed] [Google Scholar]
Review Manager 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Riaz 2006
- Riaz Y, Mehta JS, Wormald R, Evans JR, Foster A, Ravilla T, et al. Surgical interventions for age‐related cataract. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD001323.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riaz 2013
- Riaz Y, Malik A, Evans JR. Manual small incision cataract surgery (MSICS) with posterior chamber intraocular lens versus phacoemulsification with posterior chamber intraocular lens for age‐related cataract. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD008813.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruit 2000
- Ruit S, Paudyal G, Gurung R, Tabin G, Moran D, Brian G. An innovation in developing world cataract surgery: sutureless extracapsular cataract extraction with intraocular lens implantation. Clinial and Experimental Ophthalmology 2000;28(4):274‐9. [DOI] [PubMed] [Google Scholar]
Singh 2000
- Singh AJ, Garner P, Floyd K. Cost‐effectiveness of public‐funded options for cataract surgery in Mysore, India. Lancet 2000;355(9199):180‐4. [DOI] [PubMed] [Google Scholar]
Snellingen 2002
- Snellingen T, Evans JR, Ravilla T, Foster A. Surgical interventions for age‐related cataract. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD001323] [DOI] [PubMed] [Google Scholar]
Thylefors 1998
- Thylefors B. A global initiative for the elimination of avoidable blindness. American Journal of Ophthalmology 1998;125(1):90‐3. [DOI] [PubMed] [Google Scholar]
Venkatesh 2005
- Venkatesh R, Muralikrishnan, Balent LC, Prakash SK, Prajna NV. Outcomes of high volume cataract surgeries in a developing country. British Journal of Ophthalmology 2005;89(9):1079‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2008
- White IR, Higgins JP, Wood AM. Allowing for uncertainty due to missing data in meta‐analysis‐Part 1: two‐stage methods. Statistics in Medicine 2008;27(5):711‐27. [DOI] [PubMed] [Google Scholar]
Yorston 2005
- Yorston D. High‐volume surgery in developing countries. Eye 2005;19(10):1083‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ang 2010
- Ang M, Mehta JS, Evans JR. Extracapsular cataract extraction (ECCE) with posterior chamber intraocular lens versus manual small incision cataract surgery (MSICS) with posterior chamber intraocular lens for age‐related cataract. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD008811] [DOI] [Google Scholar]
Ang 2012
- Ang M, Mehta JS, Evans JR. Manual small incision cataract surgery (MSICS) with posterior chamber intraocular lens versus extracapsular cataract extraction (ECCE) with posterior chamber intraocular lens for age‐related cataract. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD008811.pub2] [DOI] [PubMed] [Google Scholar]


