Abstract
Background
PRO 140 (a humanized form of the PA14 antibody, a monoclonal CCR5 antibody) inhibits CCR5‐tropic (R5) type 1 human immunodeficiency virus (HIV). This may be an effective new treatment with the potential to address the limitations of currently available therapies for HIV‐infected patients.
Objectives
We aimed to assess the efficacy, safety, clinical disease progression and immunologic (CD4 count/percentage) and virologic (plasma HIV RNA viral load) markers of PRO 140 for HIV‐infected patients in randomized controlled trials (RCTs) and quasi‐randomized controlled trials (quasi‐RCTs).
Search methods
We searched databases including The Cochrane Central Register of Controlled Trials (The Cochrane Library 2014, Issue 4), MEDLINE (PubMed, January 1966 to April 2014), EMBASE (January 1978 to April 2014) and ISI Web of Knowledge (January 1966 to April 2014), online trials registries and other sources. We also screened the reference lists of related literature and eligible studies, and presentations from major HIV/AIDS (human immunodeficiency virus/acquired immunodeficiency syndrome) conferences.
Selection criteria
We included RCTs and quasi‐RCTs comparing PRO 140 with placebo or other antiretroviral drugs, or different doses of PRO 140 for individuals infected with HIV.
Data collection and analysis
Two reviewers (L Li and JH Tian) independently screened all retrieved citations and selected eligible studies. Two authors (P Zhang and WQ Jia) independently extracted data. Any disagreements when selecting studies and extracting data were adjudicated by the review mentor (KH Yang). We used Review Manager (RevMan) software for statistical analysis based on an intention‐to‐treat analysis. We examined heterogeneity using the Chi2 statistic. We regarded I2 estimates greater than 50% as moderate or high levels of heterogeneity. According to the level of heterogeneity, we used either a fixed or random‐effects model.If significant heterogeneity existed and the reasons could not be found, we reported the results qualitatively.
Main results
We included three trials comparing PRO 140 with placebo in adult patients with HIV infection. Our review indicates that PRO 140 may offer significant dose‐dependent HIV‐1 RNA suppression with tolerable side effects. PRO 140 2 mg/kg, 5 mg/kg, 10 mg/kg, 162 mg weekly, 324 mg biweekly, and 324 mg weekly showed statistically significant differences in the changes of HIV‐1 RNA levels. HIV‐1 RNA levels were reduced by intravenous (IV) infusion of PRO 140 2 mg/kg or 5 mg/kg on day 10, 5 mg/kg or 10 mg/kg on day 12, 162 mg weekly, 324 mg biweekly, or 324 mg weekly on day 22. PRO 140 2 mg/kg, 5 mg/kg, 10 mg/kg, 162 mg weekly, 324 mg biweekly, and 324 mg weekly demonstrated greater antiviral response. PRO 140 324 mg weekly, 5 mg/kg, and 10 mg/kg showed more patients with ≦ 400 copies/mL HIV‐1 RNA. Only PRO 140 5 mg/kg showed greater change in CD4+ cell count on day eight. Headache, lymphadenopathy, diarrhoea, fatigue, hypertension, nasal congestion and pruritus were reported to be the most frequent adverse events.
Authors' conclusions
Limited evidence from three small trials suggests that PRO 140 might demonstrate potent, short‐term, dose‐dependent, highly significant antiviral activity. However, as the evidence is insufficient, recommendations cannot yet be made. Larger, longer‐term, double‐blind RCTs are required to provide conclusive evidence.
Plain language summary
PRO 140 for treatment of people with HIV infection
PRO 140 (a humanized form of the PA14 antibody, a monoclonal CCR5 antibody) is a laboratory made antibody that blocks the CCR5 receptor on CD4 cells. By blocking CCR5, PRO 140 prevents the HIV virus from infecting healthy cells. PRO 140 may be an effective new treatment drug because it has the potential to address the limitations of currently available therapies for HIV‐infected patients. PRO 140 has emerged as an important new therapy and has entered testing.
We reviewed the efficacy, safety, clinical disease progression and immunologic (CD4 count/percentage) and virologic (plasma HIV RNA viral load) markers of PRO 140 for HIV‐infected patients. We included three randomized controlled trials (RCTs). The evidence is current to April, 2014. These three RCTs were of unclear risk of bias, as the details of methodological items were not adequately reported. All patients in these three studies were adult HIV‐infected patients, PRO 140 was adminstrated subcutaneous or intravenous infusion with different doses. These three studies adressed the immunologic (CD4 count/percentage) and virologic (plasma HIV RNA viral load) markers. There may be potential conflicts of interest in all studies, as some of the authors are current or past employees of Progenics Pharmaceuticals, the producer of PRO 140.
Our systematic review showed that PRO 140 may offer significant short‐term dose‐dependent HIV‐1 RNA suppression with tolerable side effects. PRO 140 2 mg/kg, 5 mg/kg, 10 mg/kg, 162 mg weekly, 324 mg biweekly, and 324 mg weekly could reduce HIV‐1 RNA levels and demonstrate antiviral response. And PRO 140 5 mg/kg showed greater change in CD4+ cell count on day eight. Headache, lymphadenopathy, diarrhoea, fatigue, hypertension, nasal congestion and pruritus were reported to be the most frequent adverse events. Even though available evidence from the three trials suggests that PRO 140 may be effective, the number of patients in these three studies was very small, and the results of these three studies may be influenced by potential biases. So the quality of the evidence from available RCTs was low.
PRO 140 has been granted fast‐track approval status by the United States Food and Drug Administration (FDA), but the efficacy of PRO 140 still needs to be proven in large, long‐term high quality RCTs. The three studies reviewed here only evaluated the short‐term (58 or 59 days) efficacy. The long‐term efficacy was not evaluated, and adverse events data were not reported adequately for each group. So any recommendations cannot yet be made for applying this evidence. Whether PRO 140 could be used in clinical practice as first‐line treatment for HIV‐infected patients or not depends on the results of high quality future RCTs.
Summary of findings
Summary of findings for the main comparison. PRO 140 compared to placebo for treatment of people with HIV infection.
| PRO 140 compared to placebo for treatment of people with HIV infection | ||||||
| Patient or population: patients being treated for HIV infection Intervention: PRO 140 Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | PRO 140 | |||||
| Antiviral response: 0.5 mg/kg | Study population | OR 3.00 (0.11 to 83.36) | 19 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Antiviral response: 2 mg/kg | Study population | OR 27.44 (1.25 to 601.57) | 19 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Antiviral response: 5 mg/kg | Study population | OR 439.09 (25.73 to 7493.14) | 40 (2 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Antiviral response: 10 mg/kg | Study population | OR 145.67 (5.3 to 4004.91) | 21 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Antiviral response: 162 mg weekly | Study population | OR 24.82 (1.17 to 527.12) | 21 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Antiviral response: 324 mg weekly | Study population | OR 147.00 (5.35 to 4037.48) | 21 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Antiviral response: 324 mg biweekly | Study population | OR 57.00 (2.59 to 1253.22) | 22 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Randomization: method not specified; Allocation concealment: unclear; Blinding: method not specified. 2 Total number of events is less than 300.
Summary of findings 2. PRO 140 compared to placebo for treatment of people with HIV infection.
| PRO 140 compared to placebo for treatment of people with HIV infection | ||||||
| Patient or population: patients being treated for HIV infection Intervention: PRO 140 Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | PRO 140 | |||||
| Individual nadir changes in HIV‐1 RNA level: 0.5 mg/kg | The mean individual nadir changes in HIV‐1 RNA level: 0.5 mg/kg in the control groups was ‐0.39 copies/mL | The mean individual nadir changes in HIV‐1 RNA level: 0.5 mg/kg in the intervention groups was 0.19 lower (0.42 lower to 0.04 higher) | 19 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| Individual nadir changes in HIV‐1 RNA level: 2 mg/kg | The mean individual nadir changes in HIV‐1 RNA level: 2 mg/kg in the control groups was ‐0.39 copies/mL | The mean individual nadir changes in HIV‐1 RNA level: 2 mg/kg in the intervention groups was 0.81 lower (1.22 to 0.4 lower) | 19 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| Individual nadir changes in HIV‐1 RNA level: 5 mg/kg | The mean individual nadir changes in HIV‐1 RNA level: 5mg/kg in the control groups was ‐0.305 copies/mL | The mean individual nadir changes in HIV‐1 RNA level: 5 mg/kg in the intervention groups was 1.49 lower (1.65 to 1.32 lower) | 40 (2 studies) | ⊕⊝⊝⊝ very low1,2 | ||
| Individual nadir changes in HIV‐1 RNA level: 10 mg/kg | The mean individual nadir changes in HIV‐1 RNA level: 10 mg/kg in the control groups was ‐0.32 copies/mL | The mean individual nadir changes in HIV‐1 RNA level: 10 mg/kg in the intervention groups was 1.51 lower (1.8 to 1.22 lower) | 21 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| Individual nadir changes in HIV‐1 RNA level: 162 mg weekly | The mean individual nadir changes in HIV‐1 RNA level: 162 mg weekly in the control groups was ‐0.23 copies/mL | The mean individual nadir changes in HIV‐1 RNA level: 162 mg weekly in the intervention groups was 0.76 lower (1.14 to 0.38 lower) | 21 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| Individual nadir changes in HIV‐1 RNA level: 324 mg weekly | The mean individual nadir changes in HIV‐1 RNA level: 324 mg weekly in the control groups was ‐0.23 copies/mL | The mean individual nadir changes in HIV‐1 RNA level: 324 mg weekly in the intervention groups was 1.42 lower (1.8 to 1.04 lower) | 21 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| Individual nadir changes in HIV‐1 RNA level: 324 mg biweekly | The mean individual nadir changes in HIV‐1 RNA level: 324 mg biweekly in the control groups was ‐0.23 copies/mL | The mean individual nadir changes in HIV‐1 RNA level: 324 mg biweekly in the intervention groups was 1.14 lower (1.67 to 0.61 lower) | 22 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Randomization: method not specified; Allocation concealment: unclear; Blinding: method not specified. 2 Total population size is less than 400.
Background
Description of the condition
Although there have been important advances in preventing new human immunodeficiency virus (HIV) infections and lowering the number of acquired immunodeficiency syndrome (AIDS)‐related deaths, the number of people living with HIV continues to increase. In 2008, the number reached an estimated 33.4 million, which was 20% higher than in 2000; the prevalence was roughly three‐fold higher than it was in 1990 (UNAIDS 2009). Also in 2008, approximately 2.7 million people became newly infected HIV patients, and about 2 million died from AIDS‐related illnesses worldwide (UNAIDS 2009;Appendix 1). In 2012, there was an estimated 35.3 million people living with HIV, 2.3 million new HIV infections, 1.6 million AIDS deaths in 2012 globally (UNAIDS 2013; Appendix 1).The continuing rise in the number of people living with HIV is due to factors such as the beneficial effects of antiretroviral therapy (ART) and population growth. Recent epidemiological data indicated that the spread of HIV appeared to have peaked globally in 1996, when 3.5 million new HIV infections occurred (UNAIDS 2009).
Description of the intervention
Highly active antiretroviral therapy (HAART) is the main treatment for HIV infection and has significantly demonstrated efficacy in suppressing HIV replication and improving survival of people living with HIV infection and AIDS (Mocroft 1998; Palella 1998; Pujari 2004; Sivadasan 2009). Antiretroviral agents are drawn from four treatment classes: nucleoside reverse transcriptase inhibitors (NRTI), nonnucleoside reverse transcriptase inhibitors (NNRTI), protease inhibitors, and fusion inhibitors (Murga 2006). Standard ART consists of at least three antiretroviral drugs to maximally suppress the HIV virus and stop the progression of the disease. Although very large reductions in death rates have been seen with use of a potent antiretroviral regimen, there is no cure for HIV infection. Drug toxicity of HAART may be life‐threatening, disfiguring or distressing, thus adversely affecting quality of life and the potential for optimum adherence to antiretroviral therapy. Lack of adherence ultimately leads to the emergence of resistance to antiretroviral drugs, and treatment failure (Sivadasan 2009). Therefore, new agents and treatment strategies that can be deployed in novel combination regimens need to be developed (Murga 2006).
PRO 140 (a humanized form of the PA14 antibody, a monoclonal CCR5 antibody) is a chemokine receptor CCR5 monoclonal antibody that broadly and potently inhibits CCR5‐tropic (R5) HIV‐1 at concentrations that do not antagonize the natural activity of CCR5 in vitro (Rusert 2005; Shearer 2006). PRO 140 may be an effective new treatment drug because it has the potential to address the limitations of currently available therapies for HIV‐infected patients, including the emergence of multidrug resistant viruses, significant side effects, drug‐drug or drug‐food interactions, and often complex daily treatment regimens (Richard 2006). PRO 140 has emerged as an important target for novel HIV‐1 therapies and has entered testing. In a 39‐person safety and efficacy study (Jacobson 2008), the drug reduced HIV viral loads by an average maximal decrease of 1.83 log10, which means that 98.5% of the virus was cleared from patients' blood. As a comparison, Gilead's HIV drug Viread, which received marketing approval in 2001, reduced HIV viral loads by an average of 1.5 log10 in one early stage trial among previously untreated HIV patients (Louie 2002). PRO 140 generally was well tolerated at all dose levels in the 39‐person study (Jacobson 2008). The largest individual HIV RNA reductions ranged up to ‐2.5 log10 among patients receiving both 2 mg/kg and 5 mg/kg doses (Biswas 2007).
How the intervention might work
The basic mechanism of PRO 140 is to stop HIV from entering cells and stop viral replication. Entry of HIV‐1 proceeds through a cascade of events mediated by the HIV‐1 envelope glycoproteins gp120 and gp41. The glycoprotein gp120 sequentially binds CD4 and then CCR5 or another coreceptor molecule, thereby triggering gp41‐mediated fusion of the viral and cellular membranes (Murga 2006). CCR5 is a requisite fusion coreceptor used by the virus as a portal by which to enter and infect healthy cells. PRO 140 is an anti‐CCR5 monoclonal antibody that potently inhibits HIV‐1 entry and replication at concentrations that do not affect CCR5's chemokine receptor activity in vitro (Trkola 2001). PRO 140 inhibits entry of HIV into cells by preventing virus‐cell binding at a distinct site on the CCR5 co‐receptor without interfering with the natural activity of CCR5. It binds an extracellular (not a transmembrane) site, inhibiting HIV via a competitive, rather allosteric, mechanism (AIDS info).
Why it is important to do this review
Although HAART showed improved efficacy in HIV‐infected patients, multidrug resistance or treatment related toxicities are the main problems in the treatment. Therefore, new antiretroviral drugs are needed, such as PRO 140, that have no multidrug resistance or toxicities for HIV‐infected patients. This review is necessary because we must make clear the exact efficacy and safety of PRO 140 for HIV‐infected patients based on available RCTs.
Objectives
The primary objective of this review was to assess the efficacy and safety of PRO 140 in HIV‐infected patients (including adults and children). The second objective was to assess clinical disease progression and immunologic (CD4 count/percentage) and virologic (plasma HIV RNA viral load) markers of PRO 140.
Methods
Criteria for considering studies for this review
Types of studies
We only included RCTs and quasi‐RCTs. It is impossible for PRO 140 to be administered to HIV‐infected patients in cluster‐randomized trials or cross‐over trials; therefore, we only included individual RCTs with parallel design that compared PRO 140 with placebo or other HIV medications (including other CCR5 antagonists), or different doses for HIV‐infected patients.
Types of participants
Individuals infected with HIV regardless of age, race/ethnicity, sexual orientation (gay/homosexual, bisexual, heterosexual), gender identity (including transsexuals), nationality, etc.
Types of interventions
PRO 140 administrated intravenously or subcutaneously, as compared with placebo or other antiretroviral drugs, including other CCR5 antagonists such as vicriviroc and the entry inhibitors enfuvirtide and maraviroc. Comparisons between different doses or administrations of PRO 140 were considered. We excluded interventions that combined PRO 140 with other therapies for HIV patients.
PRO 140 versus placebo.
Different doses or administrations of PRO 140.
PRO 140 versus other HIV medications (including other CCR5 antagonists).
Types of outcome measures
Primary outcomes
Clinical laboratory results (including change of log10 HIV RNA viral load from baseline, maximum change in viral load following initiation of treatment, antiviral response which was defined as a ≧ 1.0 log10 copies/mL reduction in HIV RNA level at any time after treatment, change of CD4+ cell count from baseline, and other parameters following PRO 140 administration, etc.).
Number of subjects with ≦ 400 copies/mL HIV RNA.
Survival rates.
Secondary outcomes
Safety and tolerability parameters (including adverse events).
Progression to AIDS.
Any death.
Quality of life: ability to perform daily activities, cognitive function, etc.
Search methods for identification of studies
Electronic searches
We searched databases including The Cochrane Central Register of Controlled Trials (The Cochrane Library 2014, Issue 4), MEDLINE (PubMed, January 1966 to April 2014), EMBASE (January 1978 to April 2014) and ISI Web of Knowledge (January 1966 to April 2014) for randomized trials in HIV‐infected patients who were treated with PRO 140 to the date of the search. We conducted all searches on 17 May 2010 and updated them on 30 April 2014. We did not impose any language restrictions. We combined the MEDLINE search string with the Cochrane Highly Sensitive Search Strategy for identifying RCTs in all the databases (Higgins 2011). The detailed search strategies for each database searched were presented in Appendix 2.
Searching other resources
Online trial searches
We searched the following databases for ongoing RCTs.
ClinicalTrials.gov (http://clinicaltrials.gov/).
Current Controlled Trials (http://www.controlled‐trials.com/isrctn/).
WHO International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/ictrp/en/).
Chinese Clinical Trial Registry (www.chictr.org).
Australian New Zealand Clinical Trials Registry (http://www.anzctr.org.au/default.aspx).
Clinical Trials Registry ‐ India (CTRI) (http://ctri.nic.in/Clinicaltrials/login.php).
Association of the British Pharmaceutical Industry (ABPI) Pharmaceutical Industry Clinical Trials database (http://www.abpi.org.uk/our‐work/library/Pages/default.aspx).
Manual searches
In addition, we searched the reference lists of related literature reviews and eligible articles. We performed a handsearch for abstracts published from 1995 to 2008 for presentations at the International Conference on HIV/AIDS in Africa (ICASA). We also searched abstracts from other important HIV meetings conducted by the Conference on Retroviral and Opportunistic Infections (CROI), European Aids Clinical Society (EACS), and International AIDS Society (IAS).
Data collection and analysis
Selection of studies
Two reviewers (L Li and JH Tian) independently screened all titles and abstracts of the citations identified through the searches. If both reviewers believed that the abstracts were potentially relevant, they screened the full‐text articles independently to determine whether the study was eligible for inclusion or not. We applied inclusion and exclusion criteria using a standard form to determine eligibility based on the types of participants, interventions, outcome measures and study designs to select studies. We rejected studies on initial screening if it could be determined that they were not RCTs or relevant to PRO 140 for HIV infections. We excluded other papers that did not meet the inclusion criteria after applying prespecified eligibility criteria (see Figure 1). A third review author (KH Yang) was available to resolve any disagreements.
1.
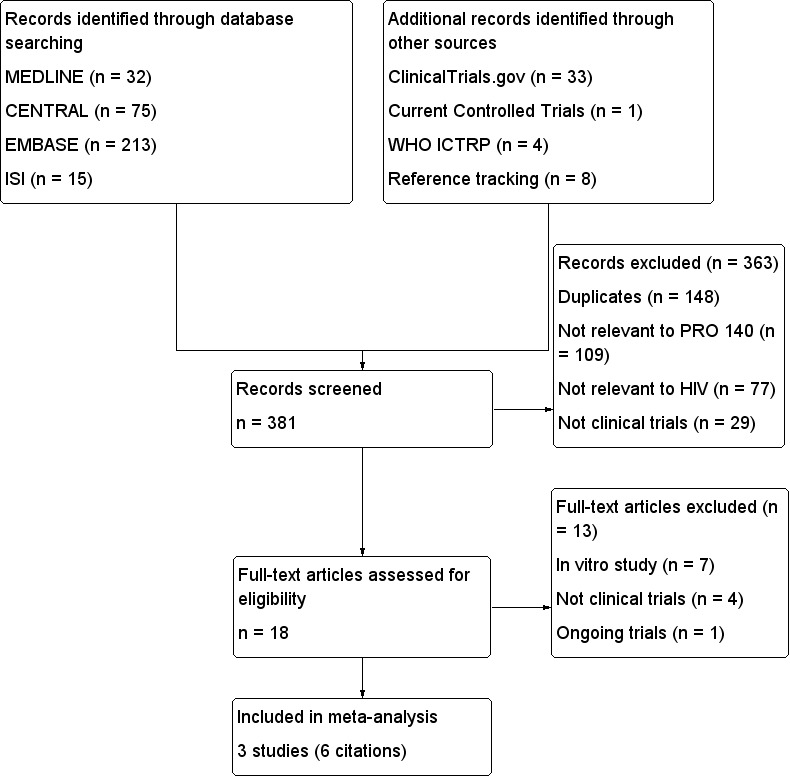
Study flow diagram.
Data extraction and management
In keeping with the guidance of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we used a standardized study record form in data extraction. Two non‐blinded authors (P Zhang and WQ Jia) independently extracted the data using a standardized data extraction form. We gathered the following information from each included study.
Administrative details ‐ titles, authors, publication, year of publication, volume number, issue number, and page numbers (if published); or titles, conductors, year in which the study was conducted (if not published); and details of other relevant papers.
Details of study ‐ study design, inclusion and exclusion criteria, number of participants, characteristics of participants (including age, sex, CD4‐cell count; prior use of antiretroviral drugs); number excluded, number enrolled, number analyzed; dropouts and losses; type, duration, frequency and completeness of follow‐up; country and location of the study.
Details of intervention ‐ doses, and routes of administration.
Details of outcomes ‐ primary and secondary outcomes.
Any disagreements about data extraction were resolved by the adjudication of a third reviewer (KH Yang).
Assessment of risk of bias in included studies
Two review authors (L Li and P Zhang) independently assessed the quality of each included trial according to the Cochrane Collaboration's tool for assessing risk of bias (Chapter 8 of Higgins 2011). We resolved discrepancies through discussion. If there was insufficient information about the study methods, we contacted the first author or the corresponding author for further information. If the trial authors did not respond within four or more weeks, we assessed risk of biases from the available information. We assessed these items as 'low risk' of bias, 'unclear risk' of bias, or 'high risk' of bias (see Appendix 3).
Measures of treatment effect
In keeping with the guidance of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we defined measures of treatment effects as follows.
For dichotomous outcomes, results were expressed as odds ratios (ORs) with 95% confidence intervals (CIs). For continuous variables, we used recommended methods to collect and combine the data. We used the mean difference (MD), or a standardized mean difference (SMD) if different scales. For quality of life, we measured it as ordinal data, which was reported qualitatively.
Unit of analysis issues
PRO 140 cannot be administered to HIV‐infected patients in cluster‐randomized trials or cross‐over trials; therefore, we only included individual RCTs with parallel design. As a result, individual participants were the unit of analysis.
Dealing with missing data
We tried our best to contact the authors (by email, telephone or fax when available) of the original studies for missing data. If all the authors of the study did not respond within four or more weeks, we extracted all the available data from the published report. We used sensitivity analyses to explore the impact of missing data in the assessment of treatment effect. If data were missing because of drop‐out of participants or loss to follow‐up, we conducted a primary analysis based on intention‐to‐treat analysis.
Assessment of heterogeneity
We examined heterogeneity among trials using the Chi2 statistic on N‐1 degrees of freedom with a significance level of 0.05 and the I2 statistic. I2 values of 0% to 40% correspond to heterogeneity which might be important; I2 values of 30% to 60% correspond to heterogeneity which may represent moderate heterogeneity; I2 values of 50% to 90% correspond to heterogeneity which may represent substantial heterogeneity; I2 values of 75% to 100% correspond to heterogeneity which may represent considerable heterogeneity (Higgins 2011). For I2 estimates greater than 50% we regarded them as moderate or high levels of heterogeneity and investigated its causes. If heterogeneity persisted, we presented results separately and reported reasons for persistence as possible.
Assessment of reporting biases
If it was possible, we had planned to assess reporting biases by using funnel plots in our review.
Data synthesis
We used the Review Manager software (RevMan 2012) provided by the Cochrane Collaboration for statistical analysis on an intention‐to‐treat basis. Meta‐analysis was considered to measure the appropriate measure of effect if the search yielded a group of trials sufficiently homogeneous in terms of measured outcomes. According to the level of heterogeneity between trials, we used either fixed‐ or random‐effects models where appropriate, and after careful consideration we pooled the outcomes and examined the differences between the two models. If significant heterogeneity existed and the reasons for heterogeneity could not be found, we reported the results qualitatively. For dichotomous outcomes, we expressed results as ORs with 95% CI. We pooled data using the fixed‐effect model but also considered the random‐effects model to ensure robustness of the model. If there were continuous scales of measurement, we used the MD to assess the effects of treatment, or the SMD if different scales had been used.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned to explore possible sources of heterogeneity, including routes of administration, dose, duration of therapy, and the different kinds of patients (adults or children). If significant heterogeneity existed, the causes of heterogeneity would be investigated. If the trends of all subgroups were the same, we would combine the results of subgroup analyses to find the overall trend of the interventions. If not, we would combine the overall results.
Sensitivity analysis
Sensitivity analyses would have been made to explore the influence of possible sources and studies of low methodological quality. If significant heterogeneity had existed after subgroup and sensitivity analysis, at the same time the reasons for heterogeneity could not be found, we would have had to report the results qualitatively.
Results
Description of studies
(see Characteristics of included studies)
Results of the search
After comprehensive searches (conducted on 17 May 2010 and updated on 30 April 2014), we found 381 citations identified through electronic databases (Cochrane Central Register of Controlled Trials (CENTRAL): 75 citations; MEDLINE (PubMed): 31 citations; EMBASE: 213 citations; and ISI Web of Science: 15 citations. We identified additional records (46 citations) through other sources (WHO ICTRP: four citations; ClinicalTrials.gov: 33 citations; Current Controlled Trials: one citation, and reference tracking eight citations).
After screening based on titles and abstracts, we identified 17 potential citations for further review, as we excluded 148 citations which were duplicates, 109 citations which were not relevant to PRO 140, 77 citations which were not relevant to HIV/AIDS, and 29 citations which were not clinical trials. We excluded seven citations that were in vitro studies (Ketas 2003; Ketas 2007; Morrow 2003; Murga 2006; Pugach 2008; Rusert 2005; Shearer 2006), four citations which were not clinical trials (Boesecke 2012; Khatib 2010; Tenorio 2011; Trkola 2001), and one citation that were not published ( NCT01272258). We have tried to contacted the investigators of the three trials, but they did not respond. We ultimately included three trials (six citations, Jacobson 2008; Jacobson 2010a; Jacobson 2010b). (See Figure 1 for flowchart of screening process).
Included studies
We included three RCTs (Jacobson 2008; Jacobson 2010a; Jacobson 2010b), which fulfilled our inclusion criteria. The characteristics of the included trials are presented in Characteristics of included studies. The baseline characteristics of treated subjects for each study are in Appendix 4,Appendix 5 and Appendix 6.
Excluded studies
Based on titles and abstracts, 148 citations which were duplicates, 109 citations which were not relevant to PRO 140, 77 citations which were not relevant to HIV/AIDS, and 29 citations which were not clinical trials were excluded. Based on full‐texts, we excluded seven citations that were in vitro studies (Ketas 2003; Ketas 2007; Morrow 2003; Murga 2006; Pugach 2008; Rusert 2005; Shearer 2006), four citations which were not clinical trials (Boesecke 2012; Khatib 2010; Tenorio 2011; Trkola 2001), and one citation that were not published (NCT01272258). See Characteristics of excluded studies.
Risk of bias in included studies
(see Characteristics of included studies, Figure 2)
2.
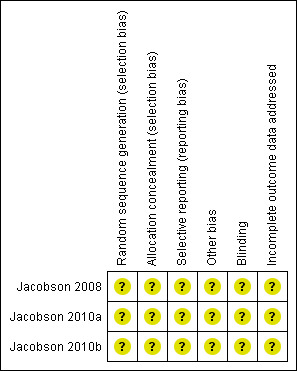
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We tried to contact all the authors of the original studies for information about missing risk of bias data, but they did not respond. We analyzed the studies based on available information. All studies (Jacobson 2008; Jacobson 2010a; Jacobson 2010b) mentioned randomization and double‐blinded, but any details about the methods were not mentioned. Allocation concealment, incomplete outcome data, and selective reporting were also not specified. There may be potential conflicts of interest in all studies (Jacobson 2008; Jacobson 2010a; Jacobson 2010b), as some of the authors are current or past employees of Progenics Pharmaceuticals, the producer of PRO 140. As such, they may hold stock in the company.
Allocation
All studies (Jacobson 2008; Jacobson 2010a; Jacobson 2010b) mentioned randomization, but they did not describe the method in detail. These three studies did not mention allocation concealment. The study authors did not respond to our requests for clarification.
Blinding
All studies (Jacobson 2008; Jacobson 2010a; Jacobson 2010b) mentioned blinding, but they did not describe the method in detail. The study authors did not respond to our requests for clarification.
Incomplete outcome data
Unclear. The study authors did not respond to our requests for clarification.
Selective reporting
Unclear. The study authors did not respond to our requests for clarification.
Other potential sources of bias
There may be potential conflicts of interest in all studies (Jacobson 2008; Jacobson 2010a; Jacobson 2010b), as some of the authors are current or past employees of Progenics Pharmaceuticals, the producer of PRO 140. The three included studies were supported by the US National Institutes of Health (Public Health Service grant AI066329).
Effects of interventions
Antiviral effects
Changes in HIV‐1 RNA level
Single IV infusion of PRO 140 0.5 mg/kg did not show any beneficial effects (MD ‐0.19, 95% CI ‐0.42 to 0.04) in the maximum changes in HIV‐1 RNA level after the 58‐day follow‐up period. The other doses (single IV infusion of PRO 140 2 mg/kg, 5 mg/kg, 10 mg/kg, 162 mg weekly, 324 mg weekly, 324 mg biweekly) showed significantly statistical differences between the subjects who were treated with PRO 140 and patients treated with placebo (Analysis 1.1;Figure 3).
1.1. Analysis.
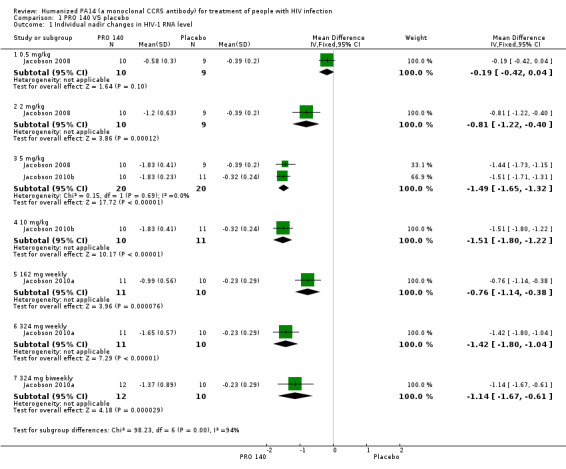
Comparison 1 PRO 140 VS placebo, Outcome 1 Individual nadir changes in HIV‐1 RNA level.
3.
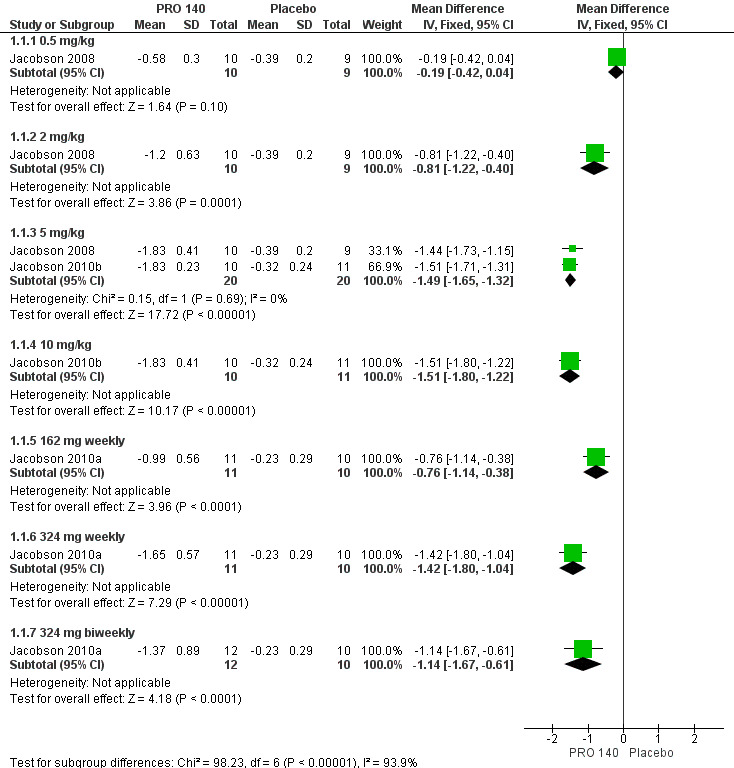
Forest plot of comparison: 1 PRO 140 versus placebo, outcome: 1.1 Individual nadir changes in HIV‐1 RNA level.
One trial (Jacobson 2008) reported the changes in HIV‐1 RNA levels on day 10. There was not a significantly statistical difference in the reductions of HIV‐1 RNA level on day 10 between patients with single IV infusion of PRO 140 0.5 mg/kg and placebo (MD ‐0.24, 95% CI ‐0.61 to 0.13). The other two doses (single IV infusion of PRO 140 2 mg/kg, 5 mg/kg) showed that PRO 140 significantly reduced HIV‐1 RNA level on day 10 (Analysis 1.2;Figure 4).
1.2. Analysis.
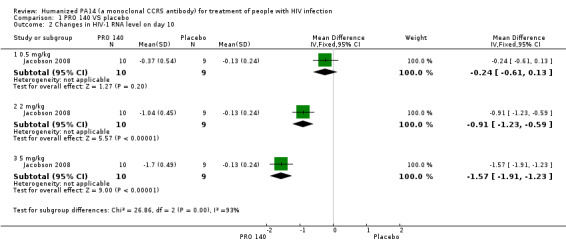
Comparison 1 PRO 140 VS placebo, Outcome 2 Changes in HIV‐1 RNA level on day 10.
4.
Forest plot of comparison: 1 PRO 140 versus placebo, outcome: 1.2 Changes in HIV‐1 RNA level on day 10.
One trial (Jacobson 2010b) reported the changes in HIV‐1 RNA levels on day 12. The result revealed that single IV infusion of PRO 140 5 mg/kg (MD ‐1.71, 95% CI ‐1.96 to ‐1.46) and 10 mg/kg (MD ‐1.75, 95% CI ‐2.00 to ‐1.50) was associated with reduced HIV‐1 RNA levels on day 12. And the combined results showed that PRO 140 could reduce HIV‐1 RNA levels on day 12 (MD ‐1.73, 95% CI ‐1.91 to ‐1.55) without any statistical heterogeneity among the studies (I2 = 0%, P = 0.82) (Analysis 1.3; Figure 5).
1.3. Analysis.
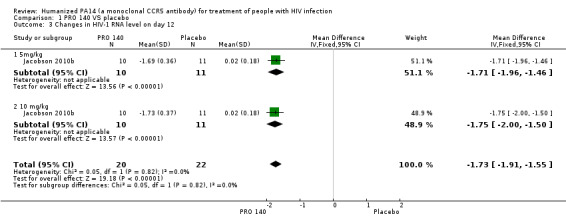
Comparison 1 PRO 140 VS placebo, Outcome 3 Changes in HIV‐1 RNA level on day 12.
5.
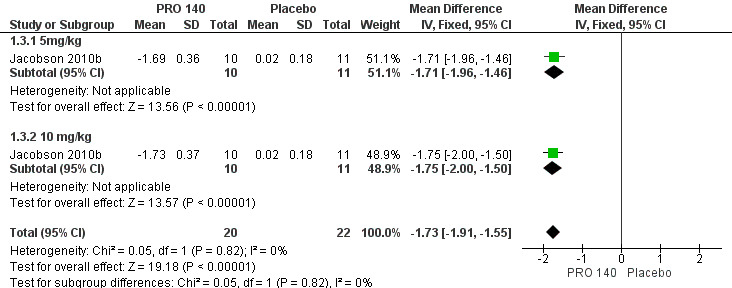
Forest plot of comparison: 1 PRO 140 versus placebo, outcome: 1.3 Changes in HIV‐1 RNA level on day 12.
One trial (Jacobson 2010a) reported the changes in HIV‐1 RNA levels on day 22. All the doses (162 mg weekly, 324 mg biweekly, 324 mg weekly) showed that PRO 140 significantly reduced HIV‐1 RNA levels on day 22. The pooled estimate of PRO 140 in the reductions of HIV‐1 RNA level on day 22 was ‐0.87 (95% CI ‐1.08 to ‐0.66) with a high amount of statistical heterogeneity among the studies (I2 = 80%, P = 0.007) (Analysis 1.4; Figure 6).
1.4. Analysis.
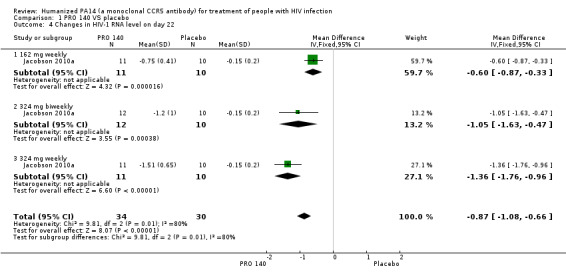
Comparison 1 PRO 140 VS placebo, Outcome 4 Changes in HIV‐1 RNA level on day 22.
6.
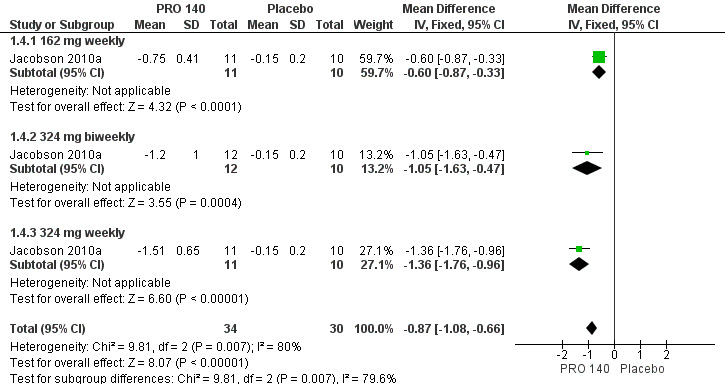
Forest plot of comparison: 1 PRO 140 versus placebo, outcome: 1.4 Changes in HIV‐1 RNA level on day 22.
Antiviral response
Antiviral response was defined as a >1.0 log10 copies/mL reduction in HIV‐1 RNA level at any time after treatment. Single IV infusion of PRO 140 0.5 mg/kg did not show any beneficial antiviral response effects (OR 3.00, 95% CI 0.11 to 83.36). The other doses of PRO 140 (single IV infusion of PRO 140 2 mg/kg, 5 mg/kg, 10 mg/kg, 162 mg weekly; 324 mg weekly; 324 mg biweekly) demonstrated greater antiviral response in PRO 140‐treated patients than those treated with placebo (Analysis 1.5).
1.5. Analysis.
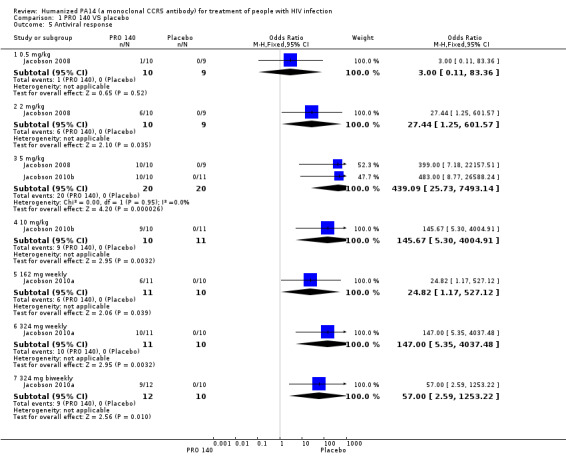
Comparison 1 PRO 140 VS placebo, Outcome 5 Antiviral response.
Number of subjects with ≦ 400 copies/mL HIV‐1 RNA
There were 0 subjects with ≦ 400 copies/mL HIV‐1 RNA for single IV infusion of PRO 140 0.5 mg/kg in both groups. There were significantly statistical differences in the number of subjects with ≦ 400 copies/mL HIV‐1 RNA between the PRO 140 324 mg weekly (OR 51.00, 95% CI 2.30 to 1129.95), 5 mg/kg (OR 43.99, 95% CI 4.22 to 459.01; I2 = 49%, P = 0.16), 10 mg/kg (OR 145.67, 95% CI 5.30 to 4004.91) and placebo group. The other three doses (single IV infusion of PRO 140 2 mg/kg, 162 mg weekly, 324 mg biweekly) did not show any beneficial effects in the number of subjects with ≦ 400 copies/mL HIV‐1 RNA (Analysis 1.6).
1.6. Analysis.
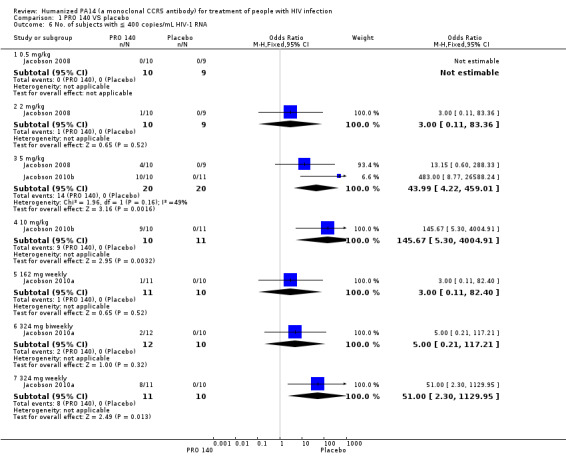
Comparison 1 PRO 140 VS placebo, Outcome 6 No. of subjects with ≦ 400 copies/mL HIV‐1 RNA.
Change in CD4+ cell count
As two studies (Jacobson 2010a; Jacobson 2010b) did not supply sufficient data of the doses of 162 mg weekly, 324 mg biweekly, 324 mg weekly, 5 mg/kg, and 10 mg/kg, we just analyzed the data of PRO 140 in the doses of 0.5 mg/kg, 2 mg/kg, and 5 mg/kg in the changes of CD4+ cell count on day eight from the study conducted in 2008 (Jacobson 2008). Only the 5 mg/kg dose of PRO 140 showed greater changes in CD4+ cell count on day eight than placebo‐treated patients (MD 110.00, 95% CI 3.14 to 216.86) (Analysis 1.7).
1.7. Analysis.
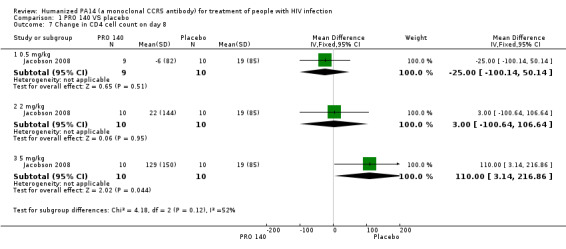
Comparison 1 PRO 140 VS placebo, Outcome 7 Change in CD4 cell count on day 8.
Safety
The authors of the three studies did not report enough information about adverse events for each group. In Jacobson 2008, the most frequently reported adverse events were headache in 12 subjects; lymphadenopathy in 11 subjects; and diarrhoea and fatigue in eight subjects. In Jacobson 2010a, the most frequently reported systemic adverse events were diarrhoea (6 of 44), headache (6 of 44), lymphadenopathy (5 of 44), and hypertension (4 of 44). Jacobson 2010b reported that the most frequently reported systemic adverse events were headache in one subject in the 5 mg/kg group and two subjects in the 10 mg/kg group, nasal congestion in two subjects in the placebo group and one subject in the 10 mg/kg group, and pruritus in three subjects in the placebo group. As sufficient data of adverse events to allow for statistical pooling were lacking, we did not pool the data of adverse events.
Other outcomes
Other outcomes (including progression to AIDS, any death, quality of life issues, survival rates) were not reported in these three trials.
Discussion
Summary of main results
This review indicates that PRO 140 might offer significant dose‐dependent HIV‐1 RNA suppression and tolerable side effects. All the doses except single IV infusion of PRO 140 0.5mg/kg (single IV infusion of PRO 140 2 mg/kg, 5 mg/kg, 10 mg/kg, 162 mg weekly, 324 mg biweekly and 324 mg weekly) showed significantly statistical differences in the nadir changes in HIV‐1 RNA level between the two groups. Two doses (single IV infusion of PRO 140 2 mg/kg and 5 mg/kg) showed that PRO 140 significantly reduced HIV‐1 RNA levels on day 10. Two doses (single IV infusion of PRO 140 10 mg/kg and 5 mg/kg) showed that PRO 140 significantly reduced HIV‐1 RNA levels on day 12. The three doses (162 mg weekly, 324 mg biweekly and 324 mg weekly) showed that PRO 140 significantly reduced HIV‐1 RNA levels on day 22. All the doses except single IV infusion of PRO 140 0.5 mg/kg (single IV infusion of PRO 140 2 mg/kg, 5 mg/kg, 10 mg/kg, 162 mg weekly, 324 mg biweekly and 324 mg weekly) demonstrated greater antiviral responses than placebo. Three doses of the PRO 140 group (324 mg weekly, 5 mg/kg, 10 mg/kg) showed more patients with ≦ 400 copies/mL HIV‐1 RNA than placebo‐treated patients. Only the single IV infusion of PRO 140 showed greater changes in CD4+ cell count on day eight than placebo.
The authors of the three studies did not report sufficient information about adverse events for each group. As a result, we did not pool the results. Headache, lymphadenopathy, diarrhoea, fatigue, hypertension, nasal congestion and pruritus were reported to be the most frequent adverse events.
Overall completeness and applicability of evidence
Although PRO 140 has been granted fast‐track approval status by the United States FDA, the efficacy of PRO 140 has not yet been proven in large double‐blind RCTs. The numbers of patients in these three studies (Jacobson 2008; Jacobson 2010a; Jacobson 2010b) were very small, and the results of these three studies may be influenced by potential biases. Their durations of follow‐up were very short, only 58 or 59 days. The long‐time efficacy was not evaluated, and the data of adverse events were not reported adequately in each group. Although available evidnece showed that PRO 140 might offer significant short‐term dose‐dependent HIV‐1 RNA suppression with tolerable side effects, but any recommendations could not be made based on these three studies. Whether PRO 140 could be used in clinical practice as front‐line treatment for HIV‐infected patients or not depends on the effects in the future of large double‐blind RCTs.
Quality of the evidence
All studies (Jacobson 2008; Jacobson 2010a; Jacobson 2010b) mentioned randomization and blinding, but any details about the methods were not found. And allocation concealment, incomplete outcome data, and selective reporting were unclear at the same time. The methodological quality of these three studies was low, as there were so many methodological problems, which may reduce the quality of evidence. The big problem was that there may be potential conflicts of interest in these three studies, which make the research results unreliable and unbelievable. The number of patients in these three studies (Jacobson 2008; Jacobson 2010a; Jacobson 2010b) was very small, and the results of these three studies may be influenced by potential biases. Thus, the quality of the evidence from available RCTs was low (Table 1, Table 2).
Potential biases in the review process
We conducted electronic, online trial and manual searches to search for relevant articles, but there may still have been papers we did not find. This review only includes published data; unpublished data of PRO 140 were not included (although we requested the data from the study authors). As a result, selective biases may exist in our review.
Agreements and disagreements with other studies or reviews
Available published data about PRO 140 in these three studies (Jacobson 2008; Jacobson 2010a; Jacobson 2010b) showed positive results and were consistent with each other. All (Jacobson 2008; Jacobson 2010a; Jacobson 2010b) showed that PRO 140 offered the potential for significant dose‐dependent HIV‐1 RNA suppression and infrequent patient self‐administration. Any studies or reviews disagreeing with such results for PRO 140 have not been published.
Authors' conclusions
Implications for practice.
These three small trials suggested PRO 140 may demonstrate potent, dose‐dependent, highly significant antiviral activity. But the frequency of adverse effects could not be determined from the evidence available. Meanwhile, the methodological quality of these three studies was low, and the number of patients in these three studies was very small, which made the quality of the evidence from available RCTs low. That is why available evidence is not sufficient and conclusive to determine whether PRO 140 was effective in HIV patients or not. Thus, at this stage, we cannot recommend PRO 140 as a therapy for the treatment of HIV patients based on available insufficient evidence.
Implications for research.
This review analyses three small, preliminary phase I/IIa clinical trials. Larger, longer‐term, double‐blind, randomized phase IIb and III trials need to be performed in order to determine the potential role of PRO 140 as a therapy for HIV infection and to provide conclusive evidence of the efficacy and safety of PRO 140 for HIV patients. Not only should the short‐term anti‐HIV activity and safety of PRO 140 be evaluated in future studies, but also its long‐term efficacy and safety. The methodological quality of these three trials (Jacobson 2008; Jacobson 2010a; Jacobson 2010b) were too low, and therefore the evidence they produced was also of low quality. As a result, in the future the RCTs related to PRO 140 for HIV‐infected patients should be well conducted (adapting strict randomization, blinding and allocation concealment methods) and transparently reported with a long follow‐up.
What's new
| Date | Event | Description |
|---|---|---|
| 25 July 2014 | New citation required but conclusions have not changed | Updated searches and systematic review process. Conclusions not changed. |
History
Protocol first published: Issue 3, 2010 Review first published: Issue 12, 2010
| Date | Event | Description |
|---|---|---|
| 18 January 2011 | Amended | To re‐publish with correct author affiliation |
Notes
No
Acknowledgements
The review authors would like to thank the following people (in addition to the peer referees) for commenting on the draft protocol and full‐text: Angela Broad, Tara Horvath, JinHui Tian, and ShaoLiang Sun.
Appendices
Appendix 1. Appendix 1
Part 1: Global summary of the AIDS epidemic December 2008 (UNAIDS 2009 )
| Number of people living with HIV in 2008 | Total | 33.4 million | 31.1 million to 35.8 million |
| Adults | 31.3 million | 29.2 million to 33.7 million | |
| Women | 15.7 million | 14.2 million to 17.2 million | |
| Children under 15 years | 2.1 million | 1.2 million to 2.9 million | |
| People newly infected with HIV in 2008 | Total | 2.7 million | 2.4 million to 3.0 million |
| Adults | 2.3 million | 2.0 million to 2.5 million | |
| Children under 15 years | 430 000 | 240 000 to 610 000 | |
| AIDS‐related deaths in 2008 | Total | 2.0 million | 1.7 million to 2.4 million |
| Adults | 1.7 million | 1.4 million to 2.1 million | |
| Children under 15 years | 280,000 | 150,000 to 410,000 |
Part 2: Global summary of the AIDS epidemic in 2012 (UNAIDS 2013)
| Number of people living with HIV in 2008 | Total | 35.3 million | 32.2 million to 38.8 million |
| Adults | 32.1 million | 29.1 million to 35.3 million | |
| Women | 17.7 million | 16.4 million to 19.3 million | |
| Children under 15 years | 3.3 million | 3.0 million to 3.7 million | |
| People newly infected with HIV in 2008 | Total | 2.3 million | 1.9 million to 2.7 million |
| Adults | 2.0 million | 1.7 million to 2.4 million | |
| Children under 15 years | 260 000 | 230 000 to 320 000 | |
| AIDS‐related deaths in 2008 | Total | 1.6 million | 1.4 million to 1.9 million |
| Adults | 1.4 million | 1.2 million to 1.7 million | |
| Children under 15 years | 210 000 | 190 000 to 250 000 |
Appendix 2. Search strategy
CENTRAL
#1 PRO 140
#2 PA14
#3 #1 or #2
#4 HIV
#5 MeSH descriptor HIV explode all trees
#6 HIV infections
#7 MeSH descriptor HIV infections explode all trees
#8 Human immunodeficiency virus
#9 AIDS
# 10 MeSH descriptor Acquired Immunodeficiency Syndrome explode all trees
#11 Acquired Immunodeficiency Syndrome
#12 #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11
#13 #3 and #12
MEDLINE search strategy (PubMed)
#1 randomized controlled trial [pt]
#2 controlled clinical trial [pt]
#3 randomized [ti/ab]
#4 placebo [ti/ab]
#5 drug therapy [sh]
#6 randomLy [ti/ab]
#7 trial [ti/ab]
#8 groups [ti/ab]
#9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8
#10 animals [mh] not (humans [mh] and animals [mh])
#11 #9 not #10
#12 PRO 140 [mh]
#13 PA14 [ti/ab]
#14 PRO 140 [ti/ab]
#15 #12 or #13 or #14
#16 HIV [mh]
#17 HIV [ti/ab]
#18 Human immunodeficiency virus [ti/ab]
#19 HIV infections [mh]
#20 HIV infections [ti/ab]
#21 AIDS [mh]
#22 Acquired Immunodeficiency Syndrome [ti/ab]
#23 AIDS [ti/ab]
#24 #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23
#25 #11 and #14 and #24
EMBASE search strategy (via EMBASE.COM)
#1 'randomized controlled trial': it
#2 'controlled clinical trial': it
#3 randomized: ab
#4 placebo: ab
#5 randomLy: ab
#6 trial: ab
#7 groups: ab
#8 #1 or #2 or #3 or #4 or #5 or #6 or #7
#9 'PRO 140'/exp
#10 'PRO 140'
#11 'PA 14'
#12 #9 or #10 or #11
#13 'HIV'
#14 'human immunodeficiency virus'
#15 'human immunodeficiency virus'/exp
#16 'HIV infections'
#17 'human immunodeficiency virus infection'/exp
#18 'AIDS'
#19 'Acquired Immunodeficiency Syndrome'
#20 'Acquired Immunodeficiency Syndrome'/exp
#21 #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20
#22 #8 and #12 and #21
ISI Web of Knowledge
#1 Topic= (PRO 140) OR Topic = (PA 14)
#2 Topic= (HIV) OR Topic = (HIV infections) OR Topic = (human immunodeficiency virus) OR Topic = (AIDS) OR Topic = (Acquired Immunodeficiency Syndrome)
#3 Topic= (random*) OR Topic= (groups) OR Topic= (controlled clinical trial) AND Topic= (randomised controlled trial)
# 4 #1 AND #2 AND #3
Appendix 3. Bias assessment
|
Selection Bias: Randomization method |
Low risk of bias | The method allows each study participant to have the same chance of receiving each intervention and provides some assurance that allocations are not known until the point of allocation, for example the randomization procedure are preformed by computer random number generator; random number table; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; etc |
|
High risk of bias |
Randomization by odd or even date of birth, or date of admission or hospital or clinic record number; allocation by judgement of the clinician or preference of the participant or the results of a laboratory test or a series of tests or availability of the intervention. If an open random allocation schedule (e.g. a list of random numbers) is used or assignment envelopes are used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered) or alternation or rotation or date of birth or case record number or any other explicitly unconcealed procedure, we define it as 'high risk' of bias | |
| Unclear | Insufficient information about the randomization procedure such as allocation concealment stated but no information on method used is available | |
|
Selection Bias: Allocation Concealment |
Low risk of bias | If randomization method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered the study (e.g. central allocation, including telephone, web‐based and pharmacy controlled randomization; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes) will be considered at 'low risk' of bias |
| High risk of bias | If an open random allocation schedule (e.g. a list of random numbers) is used or assignment envelopes are used without appropriate safeguards (e.g. if envelopes are unsealed or non‐opaque or not sequentially numbered) or alternation or rotation or date of birth or case record number or any other explicitly unconcealed procedure, we define it as 'high risk' of bias | |
| Unclear | Insufficient information about allocation concealment such as allocation concealment stated but no information on method used is available or the authors either did not report an allocation | |
| Performance bias | Low risk of bias | The study described methods of blinding patients and people administering the treatment that were appropriate so that participants and people administering the treatment do not know the exact treatment for each group until the blinding is broken; either participants or some key study personnel are not blinded, but outcome assessment is blinded and the non‐blinding of others is unlikely to introduce bias |
| High risk of bias | No blinding is used for participants and people administering the treatment | |
| Unclear | Insufficient information to permit judgment of bias or no useful information received after we contacted the authors | |
| Attrition bias | Low risk of bias | < 20% of participants withdrawn or lost to follow‐up because of the side effects of treatment or other reasons and also why some people were missed and withdrawn are stated. Intention‐to‐treat analysis was specifically reported |
| High risk of bias | > 20% of participants withdrawn or lost to follow‐up because of the side effects of treatment or other reasons and also the reasons why some people were missed and withdrawn are stated. Intention‐to‐treat analysis was not used if there were participants withdrawn or lost to follow‐up | |
| Unclear | The loss to follow‐up was not reported and cannot be judged from the article | |
| Detection bias | Low risk of bias | The same method of ascertainment for both groups and blinding of the outcome assessor for assessing the outcomes |
| High risk of bias | The different methods of ascertainment for both groups, or non‐blinding of outcome assessor | |
| Unclear | The method of ascertainment for both groups and blinding of outcome assessor for assessing the outcomes were not reported | |
| Incomplete outcome data | Low risk of bias | Missing outcome data balanced between two groups with similar reasons; missing outcomes not enough to have a clinically relevant impact on the final results; missing data have been imputed using appropriate methods |
| High risk of bias | The reason for missing outcome data related to true outcome, with either imbalance in numbers or reasons for each groups; missing outcomes enough to induce clinically relevant bias in the results; inappropriate methods were used to deal with the missing data | |
| Unclear | Cannot judge from the information taken from the article | |
| Selective outcome reporting | Low risk of bias | All the study's prespecified (primary and secondary) outcomes were not reported in the article (if the study protocol is available) or all expected outcomes were not mentioned in published reports (the study protocol is not available) |
| High risk of bias | One or more of the study's prespecified primary or expected outcomes fail to be included or were not reported | |
| Unclear | There is insufficient information to judge the level of bias |
Appendix 4. Baseline characteristics of Jacobson JM 2008 study
| Characteristic | Placebo (N = 9) | PRO 140 | All subjects (N = 39) | ||
| 0.5 mg/kg (N = 10) | 2 mg/kg (N = 10) | 5 mg/kg (N = 10) | |||
| Age (yrs) | 40.3 (23.8 to 50.2) | 37.1 (24.1 to 53.2) | 37.6 (23.2 to 51.5) | 42.8 (22.9 to 61.1) | 40.3 (22.9 to 61.1) |
| Sex (no. male/no. female) | 8/1 | 10/0 | 8/2 | 5/5 | 31/8 |
| Race (no. black/no. white/ no. other) | 4/5/0 | 4/4/2 | 4/6/0 | 5/4/1 | 17/19/3 |
| Prior antiretroviral therapy (no.) | 3 | 6 | 3 | 3 | 15 |
| HCV seropositive (no.) | 1 | 1 | 3 | 2 | 7 |
| Weight (kg) | 81.4 (57.3 to 101.7) | 81.0 (54.2 to 111.4) | 81.7 (55.9 to 142.9) | 73.4 (52.7 to 86.8) | 80.9 (52.7 to 142.9) |
| CD4+ cell count, cells/µL | 439 (281 to 555) | 493 (443 to 762) | 438 (269 to 613) | 535 (303 to 853) | 484 (269 to 853) |
| HIV‐1 RNA level, log10 copies/mL | 4.44 (3.98 to 5.61) | 4.45 (3.79 to 5.54) | 4.44 (3.89 to 4.94) | 4.37 (3.81 to 5.36) | 4.43 (3.79 to 5.61) |
Appendix 5. Baseline characteristics of Jacobson JM 2010a study
| Characteristic | Treatment with placebo (N = 10) | Treatment with PRO 140 | All subjects (N = 44) | ||
| 162 mg weekly (N = 11) | 324 mg biweekly (N = 12) | 324 mg weekly (N = 11) | |||
| Age (yrs) | 44.9 (32.3 to 51.6) | 40.0 (29.1 to 44.6) | 45.9 (31.0 to 59.6) | 41.1 (34.8 to 53.6) | 42.3 (29.1 to 59.6) |
| Sex (no. male/no. female) | 9/1 | 10/1 | 11/1 | 10/1 | 40/4 |
| Race (no. black/no. white/ no. other) | 3/7 | 5/6 | 5/7 | 4/7 | 17/27 |
| Weight (kg) | 82.3 (59.4 to 107) | 77.0 (59.3 to 94.4) | 88.3 (58.9 to 102) | 69.0 (60.8 to 83.6) | 79.1 (58.9 to 107) |
| CD4+ cell count, cells/µL | 410 (312 to 878) | 352 (307 to 611) | 493 (357 to 911) | 389 (341 to 638) | 410 (307 to 911) |
| HIV‐1 RNA level, log10 copies/mL | 4.09 (3.94 to 5.13) | 4.43 (3.92 to 4.97) | 4.60 (4.03 to 6.68) | 4.19 (3.61 to 4.77) | 4.40 (3.61 to 6.68) |
Appendix 6. Baseline characteristics of Jacobson JM 2010b study
| Characteristic | Placebo (N = 11) | 5 mg/kg PRO 140 (N = 10) | 10 mg/kg PRO 140 (N = 10) | All subjects (N = 31) |
| Age (yrs) | 40.2 (22.3 to 56.6) | 44.7 (28.0 to 55.9) | 45.3 (25.9 to 57.2) | 42.7 (22.3 to 57.2) |
| Sex (no. male/no. female) | 9/2 | 10/0 | 10/0 | 29/2 |
| Race (no. black/no. white/ no. other) | 4/7/0 | 2/8/0 | 3/6/1 | 9/21/1 |
| Weight (kg) | 82.4 (65.7 to 101.5) | 79.1 (62.2 to 126.0) | 82.3 (67.5 to 95.0) | 81.4 (62.2 to 126.0) |
| CD4+ cell count, cells/µL | 414.5 (316 to 738) | 389 (321 to 519) | 368 (264 to 595) | 382 (264 to 738) |
| HIV‐1 RNA level, log10 copies/mL | 4.52 (3.76 to 5.12) | 4.58 (3.88 to 4.75) | 4.63 (3.79 to 5.53) | 4.52 (3.76 to 5.53) |
Data and analyses
Comparison 1. PRO 140 VS placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Individual nadir changes in HIV‐1 RNA level | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 0.5 mg/kg | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.42, 0.04] |
| 1.2 2 mg/kg | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.81 [‐1.22, ‐0.40] |
| 1.3 5 mg/kg | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐1.49 [‐1.65, ‐1.32] |
| 1.4 10 mg/kg | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐1.51 [‐1.80, ‐1.22] |
| 1.5 162 mg weekly | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.76 [‐1.14, ‐0.38] |
| 1.6 324 mg weekly | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐1.42 [‐1.80, ‐1.04] |
| 1.7 324 mg biweekly | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐1.67, ‐0.61] |
| 2 Changes in HIV‐1 RNA level on day 10 | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 0.5 mg/kg | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.61, 0.13] |
| 2.2 2 mg/kg | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.91 [‐1.23, ‐0.59] |
| 2.3 5 mg/kg | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐1.57 [‐1.91, ‐1.23] |
| 3 Changes in HIV‐1 RNA level on day 12 | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐1.73 [‐1.91, ‐1.55] |
| 3.1 5mg/kg | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐1.71 [‐1.96, ‐1.46] |
| 3.2 10 mg/kg | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐1.75 [‐2.00, ‐1.50] |
| 4 Changes in HIV‐1 RNA level on day 22 | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.87 [‐1.08, ‐0.66] |
| 4.1 162 mg weekly | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.6 [‐0.87, ‐0.33] |
| 4.2 324 mg biweekly | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐1.05 [‐1.63, ‐0.47] |
| 4.3 324 mg weekly | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐1.36 [‐1.76, ‐0.96] |
| 5 Antiviral response | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 0.5 mg/kg | 1 | 19 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.11, 83.36] |
| 5.2 2 mg/kg | 1 | 19 | Odds Ratio (M‐H, Fixed, 95% CI) | 27.44 [1.25, 601.57] |
| 5.3 5 mg/kg | 2 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 439.09 [25.73, 7493.14] |
| 5.4 10 mg/kg | 1 | 21 | Odds Ratio (M‐H, Fixed, 95% CI) | 145.67 [5.30, 4004.91] |
| 5.5 162 mg weekly | 1 | 21 | Odds Ratio (M‐H, Fixed, 95% CI) | 24.82 [1.17, 527.12] |
| 5.6 324 mg weekly | 1 | 21 | Odds Ratio (M‐H, Fixed, 95% CI) | 147.0 [5.35, 4037.48] |
| 5.7 324 mg biweekly | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 57.00 [2.59, 1253.22] |
| 6 No. of subjects with ≦ 400 copies/mL HIV‐1 RNA | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 0.5 mg/kg | 1 | 19 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 2 mg/kg | 1 | 19 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.11, 83.36] |
| 6.3 5 mg/kg | 2 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 43.99 [4.22, 459.01] |
| 6.4 10 mg/kg | 1 | 21 | Odds Ratio (M‐H, Fixed, 95% CI) | 145.67 [5.30, 4004.91] |
| 6.5 162 mg weekly | 1 | 21 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.11, 82.40] |
| 6.6 324 mg biweekly | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.21, 117.21] |
| 6.7 324 mg weekly | 1 | 21 | Odds Ratio (M‐H, Fixed, 95% CI) | 51.0 [2.30, 1129.95] |
| 7 Change in CD4 cell count on day 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 0.5 mg/kg | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐25.0 [‐100.14, 50.14] |
| 7.2 2 mg/kg | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐100.64, 106.64] |
| 7.3 5 mg/kg | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 110.0 [3.14, 216.86] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jacobson 2008.
| Methods | Randomized, double‐blind, placebo‐controlled, dose‐escalating, phase 1b study Randomization: method not specified Allocation concealment: unclear Blinding: the objects that were blinded to were not mentioned Maximum follow‐up: 58 days |
|
| Participants | HIV‐infected adults Subjects were ≧ 18 years of age with HIV‐1 RNA levels 5000 ≧ copies/mL, only CCR5‐tropic (R5) HIV‐1 detectable, CD4+ cell counts ≧ 250 cells/μL with no documented nadir ≧200/μL, and no antiretroviral therapy for ≧ 3 months |
|
| Interventions | Single IV doses of 0.5 mg/kg (N = 10) Single IV doses of 2 mg/kg (N = 10) Single IV doses of 5 mg/kg (N = 10) Placebo (N = 9) |
|
| Outcomes | Antiviral effects (Change in HIV‐1 RNA level from baseline, log10 copies/mL; the proportion of subjects with ≧1 log10 copies/mL decrease in HIV RNA level; the proportion of subjects with < 400 HIV‐1 RNA copies/mL; change in CD4 cell count on day 8, cells/μL) Safety (adverse events): headache, lymphadenopathy, diarrhoea and fatigue |
|
| Notes | Potential conflicts of interest: some authors are current or past employees of Progenics Pharmaceuticals and may hold stock or stock options in the company Financial support: National Institutes of Health (Public Health Service grant AI066329) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | This article did not mention |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Blinding | Unclear risk | Blinding: the objects that were blinded to were not mentioned |
| Incomplete outcome data addressed | Unclear risk | Unclear |
Jacobson 2010a.
| Methods | Randomized, double‐blind, placebo‐controlled, phase 2a study Randomization: method not specified Allocation concealment: unclear Blinding: the objects that were blinded to were not mentioned Maximum follow‐up: 59 days |
|
| Participants | Adults with asymptomatic HIV‐1 infection Entry criteria included age of ≧ 18 years, plasma HIV‐1 RNA level of ≧ 5000 copies/mL, CD4+ lymphocyte count of ≧ 300 cells/μL with no documented count of ≦ 250 cells/μL, no antiretroviral therapy for ≧ 12 weeks, no history of AIDS‐defining illness, and only R5 HIV‐1 detectable |
|
| Interventions | 162 mg of PRO 140 on days 1, 8, and 15 (162 mg weekly) (N = 11) 324 mg of PRO 140 on days 1 and 15 and placebo on day 8 (324 mg biweekly) (N = 12) 324 mg of PRO 140 on days 1, 8, and 15 (324 mg weekly) (N = 11) Placebo on days 1, 8, and 15 (Placebo) (N = 10) |
|
| Outcomes | Antiviral effects (mean log10 reductions in HIV‐1 RNA level at virologic nadir, Day 22 log10 change in HIV‐1 RNA level, number of subjects with HIV‐1 RNA decrease, number of subjects with < 400 copies/mL HIV‐1 RNA, change in CD4+ cell count, cells/μL, at day 8, day 15, day 22) Safety (adverse events): diarrhoea, headache, lymphadenopathy, hypertension |
|
| Notes | Potential conflicts of interest: some authors are current or past employees of Progenics Pharmaceuticals and may hold stock or stock options in the company Financial support: National Institutes of Health (Public Health Service grant AI066329) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | This article did not mention |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Blinding | Unclear risk | The objects that were blinded to were not mentioned |
| Incomplete outcome data addressed | Unclear risk | Unclear |
Jacobson 2010b.
| Methods | Randomized, double‐blind, placebo‐controlled, parallel‐group study Randomization: method not specified Allocation concealment: unclear Blinding: the objects that were blinded to were not mentioned Maximum follow‐up: 58 days |
|
| Participants | Age of ≧ 18 years, plasma HIV‐1 RNA level of ≧ 5000 copies/mL, CD4+ lymphocyte counts of ≧ 300/μl and no documented count being 250/μL, no antiretroviral therapy for ≧ 12 weeks, no history of an AIDS‐defining illness, and only R5 HIV‐1 detectable in the original Trofile assay (Monogram Biosciences, Inc.) | |
| Interventions | Single IV doses of 10 mg/kg (N = 10) Single IV doses of 5 mg/kg (N = 10) Placebo (N = 11) |
|
| Outcomes | Antiviral effects (Change in HIV‐1 RNA level from baseline, log10 copies/mL; the proportion of subjects with ≧ 1 log10 copies/mL decrease in HIV RNA level; the proportion of subjects with ≧ 2 log10 copies/mL decrease in HIV RNA level; change in CD4 cell count on at days 8, 12,
15, and 22, cells/μL) Safety (adverse events): headache, nasal congestion and pruritus |
|
| Notes | Potential conflicts of interest: some authors are current or past employees of Progenics Pharmaceuticals and may hold stock or stock options in the company Financial support: National Institutes of Health (Public Health Service grant AI066329) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | This article did not mention |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Blinding | Unclear risk | The objects that were blinded to were not mentioned |
| Incomplete outcome data addressed | Unclear risk | Unclear |
AIDS: acquired immune deficiency syndrome; HIV: human immunodeficiency virus; IV: intravenous; N: number of patients
Characteristics of excluded studies [ordered by year of study]
| Study | Reason for exclusion |
|---|---|
| Trkola 2001 | It is not a clinical trial |
| Ketas 2003 | It is an in vitro study |
| Morrow 2003 | It is an in vitro study |
| Rusert 2005 | It is an in vitro study |
| Murga 2006 | It is an in vitro study |
| Shearer 2006 | It is an in vitro study |
| Ketas 2007 | It is an in vitro study |
| Khatib 2010 | It is not a clinical trial |
| Tenorio 2011 | It is a comment |
| Boesecke 2012 | It is a review |
| Pugach 2008 | It is an in vitro study |
Characteristics of ongoing studies [ordered by study ID]
NCT01272258.
| Trial name or title | A Phase 2b, Randomized, Double‐Blind, Placebo‐Controlled Clinical Trial of Observed Systemic, Long‐Acting, Anti‐HIV Treatment With a Monoclonal Anti CCR5 Antibody (PRO 140) as an Adjunct to a New, Optimized, Oral Antiretroviral Regimen in HIV‐Infected Injection Drug Users With Viral Rebound and Documented Poor Adherence to the Previous Antiretroviral Regimen |
| Methods | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Double‐Blind (Subject, Caregiver, Investigator, Outcomes Assessor) Primary Purpose: Treatment |
| Participants |
Key Inclusion Criteria:
Key Exclusion Criteria:
|
| Interventions |
|
| Outcomes |
|
| Starting date | December 2010 |
| Contact information | United States, Pennsylvania Drexel University College of Medicine Recruiting Philadelphia, Pennsylvania, United States, 19102 Contact: Sharon Lewis 215‐762‐3251 sharon.lewis@drexelmed.edu Principal Investigator: Jeff Jacobson, MD |
| Notes | Sponsors: Progenics Pharmaceuticals, Inc. |
Differences between protocol and review
Due to few studies we included, we did not assess reporting biases, conduct subgroup analyses of duration of therapy, the different kinds of patients (adults or children), and conduct sensitivity analysis.
Contributions of authors
| Draft the protocol | Lun Li |
| Develop and run the search strategy | KeHu Yang |
| Obtain copies of studies | Lun Li |
| Select which studies to include (2 people) | Lun Li; Peng Zhang |
| Extract data from studies (2 people) | Peng Zhang; WenQin Jia |
| Enter data into RevMan | Lun Li; WenQin Jia |
| Carry out the analysis | KeHu Yang; Lun Li |
| Interpret the analysis | KeHu Yang; Peng Zhang |
| Draft the final review | Lun Li; KeHu Yang |
| Update the review | Lun Li; KeHu Yang |
Declarations of interest
All authors have nothing to declare, none of them was funded.
Edited (no change to conclusions)
References
References to studies included in this review
Jacobson 2008 {published data only (unpublished sought but not used)}
- ISRCTN45537485. Study of intravenous PRO 140 or placebo in adult patients with HIV [A phase Ib, double‐blind, randomized, dose‐cohort escalation study of intravenous PRO 140 or placebo in adult patients with HIV‐1 infection]. Current Controlled Trials (http://controlled‐trials.com/ISRCTN45537485/ISRCTN45537485) accessed 23 July 2014.
- Jacobson JM, Saag MS, Thompson MA, Fischl MA, Liporace R, Reichman RC, et al. Antiviral activity of single‐dose PRO 140, a CCR5 monoclonal antibody, in HIV‐infected adults. Journal of Infectious Diseases 2008;198(9):1345‐52. [PUBMED: 18771406] [DOI] [PubMed] [Google Scholar]
Jacobson 2010a {published data only (unpublished sought but not used)}
- Jacobson JM, Thompson MA, Lalezari JP, Saag MS, Zingman BS, D'Ambrosio P, et al. Anti‐HIV‐1 activity of weekly or biweekly treatment with subcutaneous PRO 140, a CCR5 monoclonal antibody. Journal of Infectious Diseases 2010;201(10):1481‐7. [PUBMED: 20377413] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00642707. Study of PRO 140 by Subcutaneous Administration in Adult Subjects With HIV ‐1 Infection. ClinicalTrials.gov (http://www.clinicaltrials.gov/ct2/show/NCT00642707?term=NCT00642707&rank=1) accessed 23 July 2014.
Jacobson 2010b {published data only}
- Jacobson JM, Lalezari JP, Thompson MA, Fichtenbaum CJ, Saag MS, Zingman BS, et al. Phase 2a study of the CCR5 monoclonal antibody PRO 140 administered intravenously to HIV‐infected adults. Antimicrobrial Agents and Chemotherapy 2010;54(10):4137‐42. [PUBMED: 20660677] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00613379. A phase 2a, randomized, double‐blind, placebo‐controlled study of PRO 140 by intravenous administration in adult subjects with human immunodeficiency virus type 1 infection. clinicaltrials.gov (http://www.clinicaltrials.gov/ct2/show/NCT00613379?term=NCT00613379&rank=1) accessed 23 July 2014.
References to studies excluded from this review
Boesecke 2012 {published data only}
- Boesecke C, Pett SL. Clinical studies with chemokine receptor‐5 (CCR5)‐inhibitors. Current Opinion in HIV and AIDS 2012;7(5):456‐62. [DOI] [PubMed] [Google Scholar]
Ketas 2003 {published data only}
- Ketas TJ, Frank I, Klasse PJ, Sullivan BM, Gardner JP, Spenlehauer C, et al. Human immunodeficiency virus type 1 attachment, coreceptor, and fusion inhibitors are active against both direct and trans infection of primary cells. Journal of Virology 2003;77(4):2762‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ketas 2007 {published data only}
- Ketas TJ, Kuhmann SE, Palmer A, Zurita J, He W, Ahuja SK, et al. Cell surface expression of CCR5 and other host factors influence the inhibition of HIV‐1 infection of human lymphocytes by CCR5 ligands. Virology 2007;364(2):281‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khatib 2010 {published data only}
- Khatib N, Das S. PRO 140‐a novel CCR5 co‐receptor inhibitor. Recent Patents on Anti‐infective Drug Discovery 2010;5(1):18‐22. [DOI] [PubMed] [Google Scholar]
Morrow 2003 {published data only}
- Morrow K, Rosen R, Richter L, Emans A, Forbes A, Day J, et al. The acceptability of an investigational vaginal microbicide, PRO 2000 Gel, among women in a phase I clinical trial. Journal of Womens Health 2003;12(7):655‐66. [DOI] [PubMed] [Google Scholar]
Murga 2006 {published data only}
- Murga JD, Franti M, Pevear DC, Maddon PJ, Olson WC. Potent antiviral synergy between monoclonal antibody and small‐molecule CCR5 inhibitors of human immunodeficiency virus type 1. Antimicrobial Agents and Chemotherapy 2006;50(10):3289‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pugach 2008 {published data only}
- Pugach P, Ketas TJ, Michael E, Moore JP. Neutralizing antibody and anti‐retroviral drug sensitivities of HIV‐1 isolates resistant to small molecule CCR5 inhibitors. Virology 2008;377(2):401‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rusert 2005 {published data only}
- Rusert P, Kuster H, Joos B, Misselwitz B, Gujer C, Leemann C, et al. Virus isolates during acute and chronic human immunodeficiency virus type 1 infection show distinct patterns of sensitivity to entry inhibitors. Journal of Virology 2005;79(13):8454‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shearer 2006 {published data only}
- Shearer WT, DeVille JG, Samson PM, Moye JH Jr, Fletcher CV, Church JA, et al. Susceptibility of pediatric HIV‐1 isolates to recombinant CD4‐IgG2 (PRO 542) and humanized mAb to the chemokine receptor CCR5 (PRO 140). Journal of Allergy and Clinical Immunology 2006;118(2):518‐21. [DOI] [PubMed] [Google Scholar]
Tenorio 2011 {published data only}
- Tenorio AR. The monoclonal CCR5 antibody PRO‐140: the promise of once‐weekly HIV therapy. Current HIV/AIDS Reports 2011;8(1):1‐3. [DOI] [PubMed] [Google Scholar]
Trkola 2001 {published data only}
- Trkola A, Ketas TJ, Nagashima KA, Zhao L, Cilliers T, Morris L, et al. Potent, broad‐spectrum inhibition of human immunodeficiency virus type 1 by the CCR5 monoclonal antibody PRO 140. Journal of Virology 2001;75(2):579‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT01272258 {unpublished data only}
- NCT01272258. A trial of observed long‐acting, anti‐HIV treatment with a monoclonal CCR5 antibody (PRO 140) as an adjunct to a new, optimized, oral antiretroviral regimen in HIV‐infected injection drug users with viral rebound and documented poor adherence. clinicaltrials.gov/show (http://www.clinicaltrials.gov/ct2/show/NCT01272258?term=NCT01272258&rank=1) accessed 23 July 2014.
Additional references
AIDS info
- AIDS info. PRO 140. http://www.aidsinfo.nih.gov/ accessed 23 July 2014.
Biswas 2007
- Biswas P, Tambussi G, Lazzarin A. Access denied? The status of co‐receptor inhibition to counter HIV entry. Expert Opinion on Pharmacotherapy 2007;8(7):923‐33. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Louie 2002
- Louie M, Hogan C, Hurley A, Captan B, Flaherty J, Lamy P, et al. Determining the relative efficacy of Tenofovir DF using frequent measurements of HIV‐1 RNA during a short course of monotherapy in antiretroviral drug naive individuals. 9th Conference on Retroviruses and Opportunistic Infections. Seattle, 2002.
Mocroft 1998
- Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, et al. EuroSIDA Study Group. Changing patterns of mortality across Europe in patients infected with HIV‐1. Lancet 1998;352(9142):1725‐30. [DOI] [PubMed] [Google Scholar]
Palella 1998
- Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. New England Journal of Medicine 1998;338(13):853‐60. [DOI] [PubMed] [Google Scholar]
Pujari 2004
- Pujari SN, Patel AK, Naik E, Patel KK, Dravid A, Patel JK, et al. Effectiveness of generic fixed‐dose combinations of highly active antiretroviral therapy for treatment of HIV infection in India. Journal of Acquired Immune Deficiency Syndromes 2004;37(5):1566‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Richard 2006
- Richard WK. Progenics Pharmaceuticals' HIV drug, PRO 140, receives FDA fast‐track designation. www.natap.org/2006/HIV/022406_02.htm (accessed 18 June 2009).
Sivadasan 2009
- Sivadasan A, Abraham OC, Rupali P, Pulimood SA, Rajan J, Rajkumar S, et al. High rates of regimen change due to drug toxicity among a cohort of South Indian adults with HIV infection initiated on generic, first‐line antiretroviral treatment. Journal of the Association of Physicians of India 2009;57:384‐8. [PubMed] [Google Scholar]
UNAIDS 2009
- UNAIDS 2009. 09 AIDS epidemic update. www.search.unaids.org/search.asp?lg=en&search=09%20AIDS%20epidemic%20update (accessed 18 November 2009).
UNAIDS 2013
- UNAIDS 2013. UNAIDS report on the global AIDS epidemic 2013. http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf accessed 23 July 2014. [Google Scholar]


