Abstract
Background
Health information systems such as electronic health records (EHR), computerized decision support systems, and electronic prescribing are potentially valuable components to improve the quality and efficiency of clinical interventions for tobacco use.
Objectives
To assess the effectiveness of electronic health record‐facilitated interventions on smoking cessation support actions by clinicians, clinics, and healthcare delivery systems and on patient smoking cessation outcomes.
Search methods
We searched the Cochrane Tobacco Addiction Group Specialised Register, CENTRAL, MEDLINE, EMBASE, PsycINFO, CINAHL, and reference lists and bibliographies of included studies. We searched for studies published between January 1990 and July 2014.
Selection criteria
We included both randomized studies and non‐randomized studies that reported interventions targeting tobacco use through an EHR in healthcare settings. The intervention could include any use of an EHR to improve smoking status documentation or cessation assistance for patients who use tobacco, either by direct action or by feedback of clinical performance measures.
Data collection and analysis
Characteristics and content of the interventions, participants, outcomes and methods of the included studies were extracted by one author and checked by a second. Because of wide variation in measurement of outcomes, we were not able to conduct a meta‐analysis.
Main results
We included six group randomized trials, one patient randomized study, and nine non‐randomized observational studies of fair to good quality that tested the use of an existing EHR to improve documentation and/or treatment of tobacco use. None of the studies included a direct assessment of patient quit rates. Overall, these studies found only modest improvements in some of the recommended clinician actions on tobacco use.
Authors' conclusions
Documentation of tobacco status and referral to cessation counselling appears to increase following EHR modifications designed to prompt the recording and treating of tobacco use at healthcare visits. There is a need for additional research to enhance the potential of EHRs to prompt additional tobacco use treatment and cessation outcomes in healthcare settings.
Keywords: Humans, Electronic Health Records, Observational Studies as Topic, Randomized Controlled Trials as Topic, Smoking, Smoking/therapy, Smoking Cessation, Smoking Cessation/methods, Tobacco Use Cessation
Plain language summary
Does use of an electronic health record improve the delivery of stop smoking treatment to patients?
In many countries a large investment is being made in technology to computerize patient medical records. One potential of electronic health records (EHR) is that they could be used to remind doctors and other clinic staff to record tobacco use, to give brief advice to quit, to prescribe medications and to refer to stop smoking counselling. They could also help refer people to these services and be used to measure how well a clinic was doing. EHRs could also help make the delivery of tobacco use treatments standard practice by providing electronic referrals for additional treatment services (e.g., referral to a telephone tobacco quit line). We included 16 studies in this review, nine of which were observational studies so were lower quality than randomized controlled trials. Of the recommended actions for doctors with tobacco using patients we found only modest improvements associated with the EHR changes. Specifically, documentation of tobacco use and referral to cessation counselling appear to increase following EHR changes. However, these studies did not test for and/or demonstrate an increase in the number of people who quit smoking.
Summary of findings
for the main comparison.
| Use of electronic health records to support smoking cessation | ||||
|
Patient or population: People who smoke Settings: Healthcare clinics Intervention: Any use of an Electronic Health Record (EHR) to improve smoking status documentation or cessation assistance for patients who use tobacco, either by direct action or by feedback of clinical performance measures. Comparison: No EHR, or EHR without support for smoking cessation intervention | ||||
| Outcomes | Effect | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Smoking cessation | More intervention clinic than control clinic smokers quit (5.3% vs 1.9%, p < 0.001) | 1 cluster RCT, 26 clinics | ⊕⊝⊝⊝ very low1 |
Indirect measurement based on EHR documentation of smoking status |
| Guideline recommended actions | Studies typically showed positive effects on outcomes including documenting smoking status, giving advice to quit, assessing interest in quitting, and providing assistance including referral. | 6 cluster RCTs, 98 clinics | ⊕⊕⊕⊝ Moderate2 |
Studies did not all assess the same outcomes. Non randomized and uncontrolled studies also showed positive effects |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
1 Only one study reported the outcome, and did not use direct patient report of cessation
2 Heterogeneity in the interventions and targeted behaviours
Background
Description of the condition
In 2012, an estimated 31.1% of men and 6.2% of women worldwide were daily smokers (Ng 2014). Although daily smoking has reduced among men and women, population growth has led to a significant increase in the number of smokers around the world (Ng 2014). Tobacco use currently kills more than five million people each year and this number is expected to increase substantially (WHO 2009). Even if prevalence rates remain unchanged, an estimated 500 million people will die as a direct result of tobacco usage over the next fifty years (WHO 2002).
The healthcare setting remains an underused venue to provide cessation assistance to tobacco users, particularly in developing countries. Recognizing this, Article 14 of the World Health Organization (WHO) Framework Convention on Tobacco Control emphasizes the necessity of promoting evidence‐based tobacco cessation and disseminating comprehensive guidelines and best practices. To achieve the goals of Article 14, such evidence‐based clinical practice guidelines exist, outlining strategies that healthcare settings can use to help smokers quit (Fiore 2008; NHS 2011).
Evidence‐based clinical practice guidelines for tobacco cessation support recommend systematic identification and intervention for tobacco use. Changes in health systems operations that institutionalise the identification and clinical treatment of patients using tobacco are a particularly promising way to take advantage of the primary care visit to help patients quit tobacco use (Fiore 2008).
A system level change that might increase the frequency of effective cessation delivery is to take advantage of the electronic medical record for clinician reminders, linking patients to cessation services, monitoring performance, and providing feedback.
Description of the intervention
We included both direct and indirect types of electronic health record (EHR)‐based interventions. EHRs could be used directly to remind clinicians to document tobacco use, to deliver brief advice, and to prescribe cessation medications, as well as to facilitate other cessation support such as referral to counselling.They also could be used indirectly to provide performance measures of cessation support by clinics or individual clinicians that are then publicly reported or fed back to those studied or to leaders for quality improvement.
How the intervention might work
Treatment for tobacco use in a healthcare setting first requires an assessment of tobacco use and patient willingness to stop using tobacco (Fiore 1991). Healthcare clinician advice has a small effect on cessation ‐ leading to approximately three to six per cent of patients stopping using tobacco (Stead 2013). However, higher rates of cessation are achieved when a coordinated system within the healthcare setting facilitates evidence‐based actions such as cessation counselling and use of cessation medications (Fiore 2008). In the absence of electronic records, a stamp or similar visual aid in a paper chart can serve as a clinician reminder to discuss tobacco use, to provide treatment, and to facilitate referrals. Chart audits by hand can also provide performance measure information needed for quality improvement. However, these paper‐based methods are time and resource expensive and unlikely to be performed consistently. EHRs provide a systematic mechanism to improve the fidelity of following clinical practice guidelines consistently (Hesse 2010).
Why it is important to do this review
Health information systems such as EHRs, computerized decision support systems, and electronic prescribing are increasingly identified as potentially valuable components to improve the quality and efficiency of patient care. EHRs are also very likely to disseminate rapidly, at least in developed countries, as healthcare systems modernize away from paper records.
Two occurrences ‐ inadequate tobacco cessation support during clinical encounters (Agaku 2014) and the rapid dissemination of EHRs ‐ create a need to evaluate the evidence for any beneficial connections between the two, and to identify any gaps in this evidence requiring additional research.
Objectives
To assess the effectiveness of electronic health record‐facilitated interventions on smoking cessation support actions by clinicians, clinics and healthcare delivery systems, and on patient smoking cessation outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials and observational studies (cohort, case‐control, cross sectional) were included. We included observational studies that included sufficient transparency in design, analysis, and reporting results.
Rationale for including non‐randomized studies: Our primary aim in this review is to determine the extent of evidence supporting EHRs as a means of enhancing the delivery of effective tobacco use cessation treatments in healthcare settings. Most clinical research in healthcare settings, including preventive measures such as smoking treatment, have involved observational rather than randomized studies. In part this reflects the challenges of conducting research in the healthcare setting. Therefore it is especially important to learn what we can from observational studies. Well‐done observational designs have the potential to fill the need for evidence when it is unavailable from randomized trials as well as to supplement those trials. Journal editors are now providing guidance for the publishing of observational studies, for example, the STROBE checklist (strobe‐statement.org) is now required for publication in some journals (The PLOS Medicine Editors 2014)
Types of participants
Participation can be considered at the individual patient level and at the clinic level where a group of clinics is the unit of randomization.
Types of interventions
We included any interventions that involved electronic health record systems in healthcare settings that were intended to improve documentation or assistance for patients who use tobacco, either by directly prompting clinician, clinic, or health system action or by measuring and reporting on clinical performance.
Types of outcome measures
Primary outcomes
Included studies measured abstinence from smoking at a minimum of six months from the date of the intervention. Smoking status might be measured directly from patient self‐reports or indirectly from patient medical records. We did not require biochemical validation of quit rates.
In addition to quit rates we included changes in smoking cessation support actions by clinicians, clinics, and health systems. These steps include: Ask ‐ systematically identify all tobacco users; Advise ‐ advise all users to quit; Assess ‐ determine willingness to make a quit attempt; Assist ‐ provide tobacco cessation counselling and medications; and Arrange ‐ ensure follow‐up contact. Changes in the rates of these action steps are important outcomes, since there is good evidence that they are associated with increased quit rates (Fiore 2008).
Search methods for identification of studies
Electronic searches
We searched the Specialised Register of the Cochrane Tobacco Addiction Group: this register includes controlled studies identified by systematic electronic searches of various databases including CENTRAL, MEDLINE, EMBASE, PsycINFO, hand searching of relevant specialty journals, conference proceedings and ’grey literature’ (e.g. unpublished reports, literature which is not covered by most electronic databases). We searched for the following keywords; 'Medical Records Systems*' OR 'Electronic Health Records*', or the following combinations of terms in title or abstract: '(electronic or automated or medical) AND record*'. See Appendix 1 for full strategy. The Register search was updated in July 2014. At the time of the search the Register included the results of searches of the Cochrane Central Register of Controlled trials (CENTRAL), issue 6, 2014; MEDLINE (via OVID) to update 20140627; EMBASE (via OVID) to week 201427; PsycINFO (via OVID) to update 20140630. See the Tobacco Addiction Group Module in the Cochrane Library for full search strategies and list of other resources searched.
In addition, we searched the following electronic databases without study design term limits in order to identify observational studies; The Cochrane Central Register of Controlled Trials (CENTRAL) via Cochrane Library, PUBMED (MEDLINE), OVID CINAHL, ISI Web of Science, Engineering Village, EMBASE, and Academic Search Premier. In each database we searched for the combination of the following key terms: (1) 'medical records' or 'health records'; (2) 'electronic' or 'automated'; (3) 'smoking or tobacco'; (4) 'cessation or quitting'; (5) 'feedback or reminders'. We limited these searches to records where at least the abstract was published in English from January 1990 through March 2014.
Searching other resources
In addition, we scanned the reference lists of retrieved studies for additional papers.
Data collection and analysis
Patient randomized or group randomized trials were analysed separately from non‐randomized studies.
Selection of studies
The title and abstract of records identified using the keyword searches were read independently by two of the authors. We looked for studies of interventions involving adult smokers and an electronic medical or health record that was used to directly or indirectly facilitate cessation support (e.g. by providing audit and feedback, by increasing rates of tobacco user identification and documentation).
Data extraction and management
The full text of each relevant article was read and study quality was assessed using a data abstraction form. Two authors independently extracted data about the research design, outcomes, and analysis, and all three authors adjudicated any significant differences between the two extracts.
Assessment of risk of bias in included studies
We estimated the risk of bias (ROB), including both the direction and magnitude. We independently assessed the ROB in randomized trials using the following ROB items:
(1) The presence of any sequence generation during randomizations
(2) Allocation sequence concealment
(3) Blinding of providers, participants and outcome assessors
(4) The completeness of outcome data
(5) Selective outcome reporting
We categorized each trial as being at low, unclear, or high risk of bias according to the standards described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We recognise that the potential biases are likely to be greater in observational studies. We used the ROB items as a starting point to assess included observational studies.
Measures of treatment effect
For the randomized trials we examined the treatment methods to determine if there was an acceptable level of inter‐study homogeneity to enable us to draw any inference.
Unit of analysis issues
For group randomized trials we determined if appropriate multilevel or other statistical methods were used to correct for non‐independence within groups.
Dealing with missing data
For a clinical trial that did not specify, we assumed an intention to treat analysis was followed ‐ this assumes missing participants have not quit smoking but are still included in the denominator.
Data synthesis
Rather than pool the included studies we reported descriptively the relationships between and within studies because there was no commonality among the outcomes reported.
Subgroup analysis and investigation of heterogeneity
We did not test for statistical heterogeneity or perform any subgroup analyses. The majority of studies involved patients seen in general medicine or primary care clinics. One study involved patients seen in primary dental care clinics.
Sensitivity analysis
We did not conduct a sensitivity analysis of included studies.
Results
Description of studies
We screened 69 records in the most recent update. A total of 16 studies met the eligibility criteria (Figure 1). Details of the design, intervention, and measures are presented in the Characteristics of included studies table. All of the studies except one (Frank 2004, Australia) were conducted in the United States. Fourteen of the studies were conducted in general practice/primary care medical clinics. One study (Rindal 2013) was conducted in dental clinics and another (Koplan 2008) was conducted in a single, large hospital. Overall six studies were group randomized trials (Bentz 2007; Linder 2009; Rindal 2013; Sherman 2008; Vidrine 2013; Vidrine 2013a), and one was a patient randomized study conducted in a single clinic (Frank 2004). A further two studies included a control or comparison group, and seven measured outcomes using a before and after design.
1.
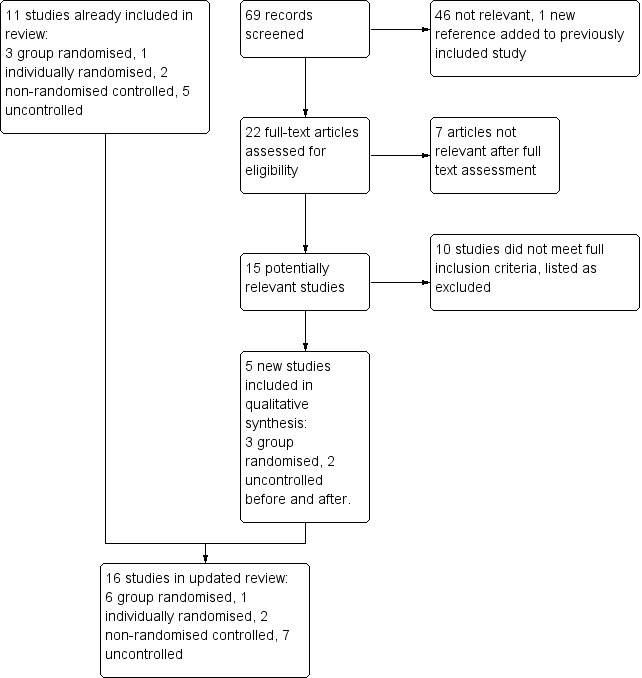
Study flow diagram for update 2014
Group Randomized Studies
We found six group randomized clinical trials (Bentz 2007; Linder 2009; Rindal 2013; Sherman 2008; Vidrine 2013; Vidrine 2013a) that assigned medical or dental clinics to either intervention or control conditions. One of the benefits of randomizing clinics rather than individual patients is the added protection against contamination of the control conditions when patients are seen in the same clinic (Campbell 2000). In each of these studies treatment conditions tested an electronic health record (EHR) with enhancements intended to facilitate the provider interaction with a smoker patient. Linder 2009 provided intervention clinics with additional tools within the EHR and clinical staff were reminded to use them. In Bentz 2007, the enhancement was based on information in an existing EHR. Clinical staff (physicians and medical assistants) in the intervention clinics received feedback reports on their use of the existing tools with smoking patients. Sherman 2008 also provided additional tools for clinical staff in the EHR system with some restrictions on use of the tools by the control clinics. The dental study (Rindal 2013) created text boxes or scripts within the intervention clinic dental record. The scripts served as language the dental providers could use based on patient‐specific information obtained during the dental encounter. In the other studies (Vidrine 2013; Vidrine 2013a) the intervention clinics were able to link patients through the EHR to a telephone quitline, and the quitline proactively called the patient.
Other studies
Of the other ten studies, three used a control condition or comparison clinic (Bentz 2002; Frank 2004; Szpunar 2006). In Bentz 2002, the comparison clinic was a paper records‐based clinic without an electronic health record. Szpunar 2006 used four control clinics, two were based on usual care and two had access to a new electronic health record vital sign screen but were provided no training or support on the use of the screen. Frank 2004 randomly assigned patients in one clinic to either intervention or usual care based on their family medical record number.
The seven additional studies (Adsit 2014; Koplan 2008; Lindholm 2010; Mathias 2012; McCullough 2009; Ragucci 2009; Spencer 1999) measured outcomes before and after the introduction of an enhancement to an existing electronic health record, without any comparison group. Adsit 2014 was conducted in a family practice clinic and a pulmonary specialty clinic within the same health system. Spencer 1999 was conducted in a single primary care clinic. Koplan 2008 studied the intervention in a single hospital. The McCullough 2009 and Ragucci 2009 studies each involved 3 clinics, and Lindholm 2010 studied one large health system with 18 primary care clinics. Ragucci 2009 and Mathias 2012 involved retrospective cohorts.
Length of follow‐up
There was wide variation in the type and length of follow‐up across the studies. For example, Rindal 2013 made telephone contact with dental patients a few days after a dental visit to measure intervention effects but no other follow‐up was conducted. Szpunar 2006 collected follow‐up data through a patient survey about two weeks after a medical care visit during an eight month study period. Bentz 2002 collected data during a three month period, and Frank 2004 collected 12 month outcome data. Spencer 1999 followed patients to 19 months. Lindholm 2010 provided data one year before and one year after the intervention. Koplan 2008 examined outcomes four months before and after implementation. McCullough 2009 followed a cohort for eight months. Mathias 2012 included patients with two or more visits during a six month post intervention period. Adsit 2014 collected outcome data from electronic records 6 months post intervention.
Excluded studies
We list seven excluded studies. See the Characteristics of excluded studies table for more detail.
Risk of bias in included studies
Allocation
All six group randomized studies described a method for matching or stratifying clinics prior to randomization, and patient populations were defined by clinic with no risk of differential patient recruitment, so all six were rated low risk for selection bias. Two studies (Bentz 2007; Linder 2009) were conducted in large health systems and, prior to randomizations, groups of clinics were created based on predetermined criteria such as the proportion of payment from government versus private insurance payers (Bentz 2007) or practice type (hospital based, community based or community health centre) (Linder 2009). In both of these studies all patients in a medical practice were included in the study. The Sherman 2008 study was conducted in a government funded health system, and clinics were randomly assigned, stratified by region (Northern vs Southern California) and size (large vs small). Vidrine 2013 and Vidrine 2013a pair matched community‐based primary care clinics based on patient demographics and clinic characteristics such as patient volume and smoking prevalence. Rindal 2013 stratified all dental clinics in one system by size and smoking volume then randomized clinics into intervention or control conditions.
In the other randomized trial, Frank 2004 randomly assigned patients within a single medical clinic. This was rated low risk for selection bias.
Among the controlled observational studies, there was no consistent method for choosing the control group. Bentz 2002 selected two clinics that were willing to participate, one used a paper chart and the other had recently switched to an electronic health record. Szpunar 2006 selected clinics based on a variety of criteria, including number of patients (population size), willingness to participate, and technical ability to complete the study. Control clinics were selected to match the intervention clinics based on a combination of number of patients and number of clinical providers. These studies were rated as high risk for selection bias.
Blinding
Since the intention of the intervention was to change provider performance, intervention providers could not be blind in any study design. Providers in control clinics may or may not have been aware of the study hypothesis but if they were and it acted as a prompt to change their behaviour the impact would be to reduce the intervention effect. Clinic patients (participants) would have been unlikely to be aware of any change in the procedure. For these reasons we rated most studies as low risk of bias. Frank 2004 was judged at high risk for performance bias since doctors saw patients allocated to both conditions. Sherman 2008 was judged at high risk because the change to the electronic record could not be restricted to intervention clinics so might have acted as a prompt in control clinics.
Outcomes were largely objective and obtained from medical records using similar procedures for intervention and control clinics so we judged most studies at low risk of detection bias. Ragucci 2009 was rated as high risk because patients self‐reported reported smoking cessation. Sherman 2008 was rated at high risk because providers self‐reported their referral patterns via surveys.
Incomplete outcome data
We considered both exclusions and attrition and found no concerns in most studies, Sherman 2008 surveyed clinicians about their activities and did not get 100% response.
Selective reporting
We examined studies for the completeness of their results. Sherman 2008 reported an increase within intervention clinics but failed to describe the comparable referral rate within control clinics.
Other potential sources of bias
We examined the group randomized trials for the potential of recruitment bias. Linder 2009 included all the medical clinics that belonged to a practice‐based research network. Rindal 2013 included all the clinics within a large system of dental clinics. Bentz 2007 reported the inclusion of 19 medical clinics that were part of a large health system. How clinics were selected to participate was not described but all patients in the selected clinics were included. The Sherman 2008 study involved 18 clinics but the criteria for study inclusion were not reported. Vidrine 2013 and Vidrine 2013a included 10 primary care clinics that were part of larger health systems but the selection criteria for the included clinics were not described.
Effects of interventions
See: Table 1
Outcomes are tabulated in Analysis 1.1
1.1. Analysis.
Comparison 1 Study results, Outcome 1 All outcomes.
| All outcomes | ||
|---|---|---|
| Study | Smoking cessation | Guideline recommended actions |
| Randomized controlled trials | ||
| Bentz 2007 | Guideline actions increased within the intervention clinics for smoking status (94.5% vs 88.1% p<0.05), advised to quit (71.6% vs 52.7%, p<0.001), assessed interest in quitting 65.5% vs 40.1% p<0.001), and provided assistance (20.1% vs 10.5%, p < 0.001). Quitline referral increased in the intervention clinics (adjusted OR 1.53) |
|
| Linder 2009 | Significantly more smokers in the intervention clinics were subsequently documented as nonsmokers compared to smokers in the control clinics (5.3% vs 1.9%, p < 0.001) | Significantly more smokers were referred to cessation counselling in the intervention clinics (4.5% vs 0.4% in control clinics, p<0.001), and significantly more smokers from intervention clinics made contact with a cessation counsellor (3.9% vs 0.3% in control clinics, p<0.001). No difference in the proportion of documented smokers from control or intervention clinics prescribed any cessation medication (2.0% vs 2.0%). |
| Rindal 2013 | Significantly more smoking patients from intervention clinics versus control clinic patients reported dental provider actions: discussed interest in quitting (87% vs 70%); discussed quitting (47% vs 26%); and referral to quitline (37% vs 17%). | |
| Sherman 2008 | The average number of smokers per month referred to telephone counselling increased from 1.0 to 15.6 (p< 0.001) among intervention clinic providers, and from 0.2 to 0.7 (p<0.04) among control clinic providers. | |
| Vidrine 2013 | Patients from intervention clinics were more likely to enroll in quitline treatment compared to control clinics (15% vs 0.5%). | |
| Vidrine 2013a | Patients from intervention clinics were more likely to enroll in quitline treatment compared to control clinics (8% vs 0.6%). | |
| Controlled trials | ||
| Bentz 2002 | Documentation of tobacco use was unchanged in the paper chart clinic, but increased from 79% to 88% in the enhanced EHR clinic. | |
| Frank 2004 | Assessment of smoking status was unchanged between intervention and control patient visits (2.0% vs 1.8%, RR 1.12 , 95% CI 0.90 to 1.39). | |
| Szpunar 2006 | Asking about tobacco use increased in the intervention clinics from 88.4% to 92.8%. | |
| Uncontrolled trials | ||
| Adsit 2014 | The proportion of patients referred to the quitline increased from <1% to 14%. 5% enrolled in quitline treatment. | |
| Koplan 2008 | The proportion of smoking patients referred to cessation counselling increased from 0.8% to 2.1% (p < 0.001); medication ordered increased from 1.6% to 2.5% (p < 0.001). | |
| Lindholm 2010 | Tobacco use status in the EHR increased from 71.6% to 78.4% (p < 0.001). | |
| Mathias 2012 | The percentage of documented smokers with a change in smoking status from active to quit during the pre‐ or postintervention period increased from 17.1% in the preintervention cohort to 20.5% in the postintervention cohort (p = .06) | In the post enhancement period, cessation medication prescribing did not change (14.4% vs. 13.4%, p = .5), but quitline referral increased from 2% to 7% (p < 0.001). |
| McCullough 2009 | Tobacco use status increased from 71% to 84% (p < 0.001). Assessement of plan to quit increased from 25.% to 51% (p < 0.005), and smokers assessed for a plan to quit were more likely to receive cessation counselling (46% vs 14% among smokers not assessed, p < 0.001). | |
| Ragucci 2009 | Of 90 smokers in the study, 29 were quit at 6 months (32%) | |
| Spencer 1999 | Recording of tobacco use status increased from 18.4% to 80.3%. | |
Evidence from group randomized studies
Smoking cessation
Only Linder 2009 reported a comparison of quit rates between control and intervention measured indirectly based on changes in the EHR documentation of smoking status. Significantly more smokers in the intervention clinics were subsequently documented as nonsmokers as compared to smokers in the control clinics (5.3% vs 1.9%, p < 0.001).
Clinical guideline recommended actions
Smoking status
Bentz 2007 and Linder 2009 measured documentation of smoking and found significantly higher rates after the intervention. Bentz 2007 reported no difference at baseline, but documentation increased among providers in the intervention clinics (94.5%) compared to control (88.1%) (p < 0.05). In Linder 2009 the comparable rates were 46% vs 54% (p < 0.001). However Rindal 2013 found no increase in already very high levels of dental care provider documentation of smoking status (97.5%).
Advise and assess interest in quitting
Bentz 2007 found higher rates of advice (71.6% vs 52.7%), and assessment (65.5% vs 40.1%), when comparing intervention and control clinics. Rindal 2013 reported only post intervention data and found an increase in dental provider actions. Compared to control clinics, providers in the intervention clinics were more likely to ask about interest in quitting (70% vs 87%) and to discuss strategies for quitting smoking (26% vs 47%).
Cessation assistance
A logical approach to increase the number of smokers who make attempts to quit smoking is to connect patients during a medical visit to the necessary resources to assist quitting. These resources might include physician assistance with a quitting plan or medications, or a referral to cessation counselling.
Linder 2009 found more smokers in the intervention clinics were referred to cessation counselling compared to the control clinics (4.5% vs 0.4% p<0.001) and making a contact with a cessation counsellor was more likely among intervention clinic smokers compared to control (3.9% vs 0.3%, p<0.001). However, they found smokers in the intervention clinics were no more likely to be prescribed a cessation medication. Based on similar clinic rates at baseline, Bentz 2007 reported an increase in documented assistance in the intervention clinics compared to the control clinics (20.1% vs 10.5%, p<0.001). However, referrals to the telephone‐based quitline did not increase. The researchers found variation across clinics and therefore adjusted their analysis for two factors: the presence of a "clinic champion" advocating for cessation support and the proportion of patients with more documented illnesses. This adjustment revealed an increase in referrals from intervention clinics (adjusted OR 1.5). Sherman 2008 found an increase in the last month estimated number of patients referred to telephone counselling from clinician self‐reports (15.6 vs 0.7), but no difference in the likelihood of patients from intervention clinics to receive a prescription for cessation medications. Using only post intervention data, Rindal 2013 reported significantly more dental patient smokers from intervention clinics reporting a quitline referral compared to control clinics (37% vs 17%). A similar intervention effect was found in Vidrine 2013 and Vidrine 2013a, although no baseline rates were reported. A significantly higher proportion of smokers enrolled in treatment with a quitline compared to control (Vidrine 2013; 15% vs 0.5%; Vidrine 2013a; 8% vs 0.6%).
Evidence from observational and patient randomized studies
Of the other studies, documentation of smoking status was the most commonly measured outcome. In the single patient randomized study (Frank 2004) that assessed smoking status, a preventive care reminder did not increase documentation of smoking status. However, many of the observational studies found that using an EHR system change to prompt the identification and documentation of smoking status boosted such interventions. These studies provided additional evidence of clinician assistance to smokers following an amended EHR. Four measured assistance with quitting at baseline and follow‐up (Koplan 2008; McCullough 2009; Spencer 1999; Szpunar 2006). All four found the intervention increased the rate of assistance provided to smokers. McCullough 2009 found an increase in documented assistance among smokers who were also asked about plans to quit smoking. After the EHR change, Koplan 2008 found an increase in both the proportion of admitted smokers referred to cessation counselling and an increase in physician orders for cessation medication. Adsit 2014 found more electronic referral to a quitline compared to a fax referral comparison (14% vs 0.3%). Mathias 2012 observed no change in prescriptions for cessation medications, but referrals for cessation counselling increased from 2% to 7%. They also reported a post intervention change in EHR documented 'not smoking' status from 17% to 20% (p < 0.06).
Discussion
Summary of main results
We included randomized and non‐randomized studies that tested the use of an electronic health record (EHR) to improve documentation and treatment of tobacco use among medical and dental patients. None of the studies included a direct assessment of patient quit rates. At least in the short term, documentation of tobacco status and quit assistance to smokers does appear to increase following the introduction of an electronic reminder to provide clinical support for patients who smoke (Table 1).
Overall completeness and applicability of evidence
The goal of this review was to evaluate available evidence supporting computerized medical record systems as a method to enhance the delivery of effective tobacco use treatments.
The most common study design measured changes in clinician, clinic, or health system actions before and after the introduction of an enhancement to an electronic health record, but often without a control or comparison condition. Randomized controlled trials in real‐world settings such as medical or dental clinics are often designed as group randomized designs. Indeed this review included six studies with randomizations at the clinic level. Such designs provide an advantage for clinic settings but at the expense of complexity in design and sample size.
The most common enhancement of the EHR was the linking of smoking patients with a telephone based quitline (9/16 studies). The most recent studies had as their primary outcome the measurement of the rate of smokers receiving quitline treatment.
Quality of the evidence
Overall the studies included in this review were heterogeneous in design and intervention. For example, patient surveys, provider surveys, medical record reviews, and reports from quitline vendors were used to measure outcomes across the studies. Each of these methods introduces a different view of outcomes and each introduces a potential bias. Moreover, there was great variation in type of outcome. Therefore we were not able to perform statistical analysis or a meta‐analysis of the included studies.
Although 16 studies were included, we determined that five were high quality group randomized controlled trials. The sixth group randomized trial (Sherman 2008) demonstrated the difficulty of conducting such research within an existing healthcare system. In this study the researchers were unable to restrict the enhancement of the medical record system only to the intervention clinics. Instead, they relied on a visual request to maintain the delivery of the intervention.
There were various limitations to the non‐randomized studies. Most (7/9) lacked a control group and adopted a before and after design. Other limitations included small sample sizes and convenience sampling of included clinics which increases the potential risk of selection bias.
Authors' conclusions
Implications for practice.
Adding tobacco use as an electronic vital sign collected during a medical visit appears to increase some of the recommended clinician actions for treating patients who use tobacco.
Implications for research.
The findings of this review highlight the need for well designed randomized controlled studies that also include direct measurement of cessation to better assess the promise of EHRs to enhance the clinical treatment of tobacco dependence. While the findings to date support the potential of EHRs to facilitate some evidence‐based tobacco use clinical interventions (e.g., some of the 5 As), we were unable to conclude that EHR support has a definite impact on cessation rates.
What's new
| Date | Event | Description |
|---|---|---|
| 25 September 2014 | New search has been performed | Updated with the inclusion of five new studies. |
| 25 September 2014 | New citation required but conclusions have not changed | Conclusions remain unchanged |
Acknowledgements
The authors wish to acknowledge the helpful search assistance from Wendy Theobald, University of Wisconsin, and Lindsay Stead.
Appendices
Appendix 1. Register Search Strategy
#1 MeSH DESCRIPTOR Medical Records #2 MeSH DESCRIPTOR Medical Records Systems, Computerized #3 MeSH DESCRIPTOR Electronic Health Records #4 Medical record*:MH,EMT,KY,KW,XKY #5 (electronic health*):MH,EMT,KY,KW,XKY #6 (health* records):MH,EMT,KY,KW,XKY #7 ((electronic or automated or medical) ADJ3 record*):MH,EMT,KY,KW,XKY #8 ((electronic or automated or medical) ADJ3 record*):TI #9 ((electronic or automated or medical) ADJ3 record*):AB #10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9
(MH,EMT,KY,KW,XKY are keyword fields)
Data and analyses
Comparison 1. Study results.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All outcomes | Other data | No numeric data | ||
| 1.1 Randomized controlled trials | Other data | No numeric data | ||
| 1.2 Controlled trials | Other data | No numeric data | ||
| 1.3 Uncontrolled trials | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adsit 2014.
| Methods | Country: USA Setting: Madison, Wisconsin Design: 18 month before and after study measuring change in referral to a telephone quitline. |
|
| Participants | Two clinics within a healthcare system: Primary care clinic with 7 physicians; Pulmonary care clinic with 6 physicians. | |
| Interventions | EHR was modified to prompt clinic staff to offer a quitline referral. Secure link was established between patient record and quitline. | |
| Outcomes | Proportion of patients who smoke referred to quitline; acceptance rate by the patient. Data obtained from medical and administrative records. | |
| Notes | Designed as a case study without empirical testing. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No control group |
| Allocation concealment (selection bias) | High risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel not blind to change in EHR by design, risk of bias judged low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective data obtained from medical and administrative records using an automated reporting system. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were based on electronic records for all patients with a visit. |
Bentz 2002.
| Methods | Country: USA Setting: Portland, Oregon Design: Tracking codes to measure and report tobacco cessation guideline provider activities were introduced in two primary care clinics. One clinic was using an electronic health record and the comparison clinic a paper chart. |
|
| Participants | 2 Primary care clinics, one using an internally developed, web‐based electronic health record and another using a paper medical record. | |
| Interventions | The EHR clinic was prompted to ask patients about smoking, give advice to quit, and to document these actions in the EHR. A tracking form was attached to the paper chart in the non‐EHR clinic. | |
| Outcomes | Documentation of tobacco use was collected from a sample of 50 patient charts. Billing and claims databases were used to measure code utilization. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Clinics not randomly allocated |
| Allocation concealment (selection bias) | High risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel not blind to change in EHR by design, risk of bias judged low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective data extracted by research staff based on a random sample of clinic patients |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | EMR data included all charts; the comparison clinic was based on a random sample. |
Bentz 2007.
| Methods | Country: USA Setting: Primary care clinics, Portland, Oregon Design: Cluster randomized controlled trial. Clinics were grouped by business affiliation, payer mix, and baseline rate of recorded smoking status (ask rate); then randomized into intervention and control. A case‐mix score was calculated to control for age and illness diagnosis. Regression analysis was performed using generalized estimating equations. Intra‐cluster correlation coefficients (ICC) were calculated for the analysis. |
|
| Participants | 19 Primary care clinics (n=10 intervention) using a common electronic health record within one health system. | |
| Interventions | Intervention group clinics received written reports showing individual provider, and clinic performance on tobacco clinical guideline actions: ask, advise, assess, assist, arrange. Written reports were provided monthly to the clinic manager. Control clinics used same EHR without feedback |
|
| Outcomes | Outcomes were obtained from electronic files and included estimates of asking about tobacco use, advising to quit, assessing interest in quitting, and assistance with referral to the telephone quitline. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Group randomized, clinics were grouped by pre‐determined criteria prior to randomization |
| Allocation concealment (selection bias) | Low risk | Clinics assigned at baseline, defined patient populations, no risk of differential patient recruitment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intervention personnel not blind to change in EHR by design, risk of bias judged low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data were extracted from the EMR on a monthly basis based on all patient visits |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected at the patient level and included all patients |
Frank 2004.
| Methods | Country: Australia Setting: Urban general practice clinic of 10 physicians; Adelaide Design: Patient randomized study. Data were analysed with regression using generalized estimating equations. |
|
| Participants | Intervention sample n=5118; Control sample n=5389; 56% female | |
| Interventions | Reminders for preventive activities including recording smoking status appeared as a field in the electronic health record. | |
| Outcomes | Documentation of smoking status in the electronic health record. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomized by family medical record number. 'The baseline characteristics of the patients allocated to the two experimental groups were similar'. Judged at low risk of selection bias. |
| Allocation concealment (selection bias) | Low risk | No opportunity to change allocation, all clinic patients included, no potential for selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | All physicians in a single clinic were included. 'The GPs were not blinded to the allocation of patients to the intervention or control groups.' The risk is that all patients could be treated the same regardless of study assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'Automatic recording of preventive care opportunities and their uptake' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data collected electronically for all patients. |
Koplan 2008.
| Methods | Country: USA Setting: Boston, Massachusetts Design: Uncontrolled before‐and‐after study. Pre‐intervention period (4 months) was compared to post‐intervention (4 months). |
|
| Participants | Admitted hospital patients in a large multi‐specialty hospital affiliated with a University. Records for 17,530 admissions were examined. | |
| Interventions | A series of check boxes (tobacco order set) was added to admission screens of the hospital computerized order‐entry system. The assessment included smoking/nonsmoking, cessation materials, cessation consultation, and orders for nicotine replacement medications or bupropion. | |
| Outcomes | Referral to smoking cessation counselling and ordering cessation medications. | |
| Notes | Effects on smoking status identification could not be assessed as available only in the postintervention period; previously these data were not electronically collected. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No control group |
| Allocation concealment (selection bias) | High risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel not blind to change in EHR by design, risk of bias judged low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'NRT orders were obtained from hospital pharmacy records. Smoking counselor consults were obtained from the electronic database kept by hospital smoking counselors.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Same methods of data collection pre and postintervention |
Linder 2009.
| Methods | Country: USA Setting: Boston, Massachusetts Design: Cluster randomized controlled trial. Clinics were matched based on size (number of annual visits) and practice type (hospital based, community based, or community health centre) then randomly assigned to intervention or usual care. Intra‐cluster correlation coefficients (ICC) were calculated for the analysis. A generalized linear model controlled for the clusters and possible interactions. |
|
| Participants | Documented smokers (n=9589) in 26 Primary care clinics (n=12 intervention) using an internally developed, web‐based electronic health record. | |
| Interventions | Intervention group clinicians experienced three changes to the electronic health record: a cigarette icon on the top of the health record was either black when smoking status was missing or scarlet for current smokers; tobacco treatment reminders were listed in the patient record; and treatment order forms for cessation medication and telephone Quitline referral were added. | |
| Outcomes | Primary outcome was documented smoking cessation counselling (smoking counsellor reached a patient by telephone, or a patient attended a program, or the Quitline reached a patient by telephone). Secondary outcomes included documentation of smoking status, prescribing cessation medication, and referral to cessation treatment, and smokers subsequently documented as non smokers. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Group randomized; clinics were matched on pre‐determined criteria then randomized |
| Allocation concealment (selection bias) | Low risk | Clinics assigned at baseline, defined patient populations, no risk of differential patient recruitment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intervention personnel not blind to change in EHR by design, risk of bias judged low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Same methods of data collection from electronic records for intervention and control clinics |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Methods of data collection makes differential attrition unlikely |
Lindholm 2010.
| Methods | Country: USA Setting: Madison, Wisconsin Design: Uncontrolled before‐and‐after study. Pre‐intervention period (12 months) was compared to post‐intervention (12 months). Chi² tests were performed. |
|
| Participants | Primary care patients attending 18 general internal medicine and family medicine clinics. About 250,000 patient visits were examined pre and post intervention. | |
| Interventions | A tobacco use box was added to the vital signs patient window; if a tobacco user was identified, the patient was asked if they were willing to talk to the doctor about quitting: if yes, a three question paper survey asked about past cessation medication use, cigarettes used per day, and a possible quit date. This survey was left for the physician to review during the visit. | |
| Outcomes | Assessment of smoking status pre‐post from the electronic health record; proportion provided medication (post intervention only), clinician documentation of smoking cessation counselling (post intervention only). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No control group |
| Allocation concealment (selection bias) | High risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel not blind to change in EHR by design, risk of bias judged low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data automatically generated from EHR |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data collected electronically. |
Mathias 2012.
| Methods | Country: USA Setting: Chicago, Illinois Design: before and after study involving cohort and cross sectional smokers. |
|
| Participants | Single urban primary care practice; 37 attending and 78 resident physicians. 1,349 documented smokers in preintervention cohort, 1,346 in postintervention cohort. 764 included in both cohorts | |
| Interventions | A smoking cessation alert was added to the EHR. The alert prompted physician actions including a medication order set. | |
| Outcomes | Change in orders of prescription for cessation medications; change in referral to cessation counselling. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No control group |
| Allocation concealment (selection bias) | High risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel not blind to change in EHR, risk of bias judged low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective data extracted from EHR, although assessors not blind to whether pre‐ or post‐intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Same methods of data extraction pre and postintervention |
McCullough 2009.
| Methods | Country: USA Setting: Chapel Hill, North Carolina Design: Uncontrolled before‐and‐after study. Pre‐intervention period (4 months) was compared to post‐intervention (8 months). Chi² tests were performed. |
|
| Participants | Primary care patients attending 3 family medicine clinics (n=899) | |
| Interventions | Two questions were added to the patient vital signs in the electronic health record – “Current smoker?” and “Plan to quit?” | |
| Outcomes | Documented smoking status, assessment of quit plan, and smoking cessation counselling recorded in the electronic health record. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No control group |
| Allocation concealment (selection bias) | High risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel not blind to change in EHR, risk of bias judged low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers extracted objective data from randomly selected medical records |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Same methods of data collection pre and postintervention |
Ragucci 2009.
| Methods | Country: USA Setting: Columbia, South Carolina Design: Uncontrolled before‐and‐after study. Pre‐intervention period (4 months) was compared to post‐intervention (8 months). Pharmacist delivered intervention during drug therapy management. |
|
| Participants | Anticoagulation patients or diabetes patients who were current smokers (n=90) attending 3 University‐based primary care clinics. | |
| Interventions | A smoking template was added to the pharmacy‐related progress notes within the electronic health record. The template queried on smoking status, type of tobacco, amount of tobacco, years of tobacco use, past quit attempts, desire to quit, and assessment of nicotine addiction. Based on smoking status, pharmacist provided a message on the benefits of smoking cessation and education on cessation medications. | |
| Outcomes | Smoking cessation and readiness to quit smoking if not quit. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled study |
| Allocation concealment (selection bias) | High risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel not blind to change in EHR by design, risk of bias judged low |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Patients reported their smoking status without validation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether any smokers identified at baseline were not followed up |
Rindal 2013.
| Methods | Country: USA Setting: Minneapolis, Minnesota Design: A two‐arm group randomized trial with dental clinics assigned to an enhanced dental record intervention or usual care control. |
|
| Participants | 15 dental clinics. Prior to randomizations, clinics were stratified by size, proportion smoking, and public vs private insurance. | |
| Interventions | In the enhanced condition, the EDR was modified to prompt providers to ask and discuss smoking and interest in quitting. | |
| Outcomes | Change in provider actions ‐‐ ask, discuss quitting, refer to quitline. | |
| Notes | No follow‐up as patients were called a few days after their visit. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified and group randomization of all clinics in a dental system |
| Allocation concealment (selection bias) | Low risk | Clinics assigned at baseline, defined patient populations, no risk of differential patient recruitment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Personnel not blind to change in EHR by design, dentists only worked at a single clinic, risk of bias judged low |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Patient reported outcomes. Unclear whether patients or assessors blind to EDR condition |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar proportion of patients of intervention and control clinics reached |
Sherman 2008.
| Methods | Country: USA Setting: Los Angeles, California and Palo Alto, California Design: group randomized clinical trial. Regression analysis performed; no assessment of group correlation. |
|
| Participants | 18 Primary care clinics (n=10 intervention) affiliated with the Veterans Health Administration (VA). | |
| Interventions | A simplified method was added to an existing electronic health record for referral to telephone‐based cessation counselling. Electronic mail reminders were sent to providers. Project staff promoted the referral tool during visits to the intervention clinics. | |
| Outcomes | Primary outcome was provider self reported referrals to telephone‐based cessation counselling. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned, stratified by region (Northern vs Southern California) and size (large vs small) |
| Allocation concealment (selection bias) | Low risk | Clinics assigned at baseline, defined patient populations, no risk of differential patient recruitment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The change to the electronic record could not be restricted in control clinics so access to the intervention was incompletely controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self reported outcome from providers surveyed about their referral patterns |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not all providers responded to surveys. |
| Selective reporting (reporting bias) | High risk | Referral rate was reported for intervention but not control clinics |
Spencer 1999.
| Methods | Country: USA Setting: Eau Claire, Wisconsin Design: Uncontrolled before‐and‐after study. Pre‐intervention period (9 months) was compared to post‐intervention (19 months). |
|
| Participants | Primary care patients attending a single family medicine clinic affiliated with a university. | |
| Interventions | Smoking status was documented in a single location – the major problem list in the electronic health record. Medical Assistants were assigned the role of documenting smoking status and providing cessation education. | |
| Outcomes | Documentation of smoking status and cessation counselling by clinicians. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No control group |
| Allocation concealment (selection bias) | High risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel not blind to change in EHR by design, risk of bias judged low |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Different source of data before and after the EHR change |
Szpunar 2006.
| Methods | Country: USA Setting: Detroit, Michigan Design: Controlled before‐and‐after study. Pre‐intervention data collection (9 weeks) and post intervention data collection (14 weeks). Two intervention clinics were compared to 4 control clinics. Regression analysis controlled for baseline demographics and co‐morbidities. Patient surveys were completed at baseline and 2 weeks following a visit in the post‐intervention period. |
|
| Participants | Primary care patients attending 6 primary care clinics (2 intervention, 2 vital sign check in screen only, 2 control). These clinics form part of a large healthcare system. Clinics were selected based on convenience and size. | |
| Interventions | Screens were added to the electronic health record. A vital sign entry recorded smoking status and willingness to quit. Further screens were automated to provide information to the provider, suggested dialogue to use, and encouraged referral to a smoking cessation program. | |
| Outcomes | Documentation of clinician actions – ask, advise, assess, assist, arrange based on patient surveys. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non randomized. Clinics were selected on ability to participate; there were baseline differences in patient characteristics |
| Allocation concealment (selection bias) | Low risk | Clinics assigned at baseline, defined patient populations, no risk of differential patient recruitment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Patients interviewed by telephone after clinic visits, unclear whether they or interviewers were blind to clinic condition |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details about proportion of clinic attenders who were successfully contacted |
Vidrine 2013.
| Methods | Country: USA Setting: Houston, Texas Design: pair‐matched two‐arm group randomized trial |
|
| Participants | 10 community health clinics (described as safety net clinics) matched on patient volume, age, gender, race/ethnicity, and SES. | |
| Interventions | Intervention clinics trained nursing staff to assess quitting interest and connect interested smokers to the quitline. The quitline then called smokers. Control clinics gave smokers a quitline referral card. | |
| Outcomes | Proportion of smokers enrolled in treatment with the quitline. | |
| Notes | Clinic selection not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized after pairing on patient volume, smoking prevalence, mean age and sex distribution |
| Allocation concealment (selection bias) | Low risk | Clinics assigned at baseline, defined patient populations, no risk of differential patient recruitment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel not blind to change in EHR by design, risk of bias judged low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective outcomes using data supplied by quitline |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Control clinics patient outcomes relied on names of quitline referrals being correctly matched to quitline callers. |
Vidrine 2013a.
| Methods | Country: USA Setting: Houston, Texas Design: Pair‐matched two arm group randomized trial |
|
| Participants | 10 family practice clinics from a large network of clinics. | |
| Interventions | Intervention clinics linked interested smokers through the EHR to the quitline. The quitline proactively called the smokers. | |
| Outcomes | Proportion of smokers enrolled in treatment with the quitline. | |
| Notes | Clinic selection not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized after pairing on patient volume, smoking prevalence, mean age and sex distribution |
| Allocation concealment (selection bias) | Low risk | Clinics assigned at baseline, defined patient populations, no risk of differential patient recruitment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel not blind to change in EHR by design, risk of bias judged low |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective outcomes using data supplied by quitline |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Control clinics patient outcomes relied on names of quitline referrals being correctly matched to quitline callers. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bunik 2013 | Single clinic with non random surveys of patients |
| Greenwood 2012 | The EHR effect was confounded by staff training effect |
| Herrin 2012 | Outcome measure not well defined; limited to documentation of smoking |
| Herrin 2014 | Observational study with significant selection bias and without a focus on smoking |
| Kruse 2013 | EHR changes could not be separated from pay for performance changes |
| Levine 2013 | Single site pilot study with selection bias |
| Mundra 2012 | Single site, observational study with no useful data on smoking intervention |
| Onders 2014 | EHR improvements coincided with staffing changes and patient volume changes |
| Wang 2013 | Study was a test of performance feedback with the EHR as the source of the feedback |
| Warren 2013 | This was a feasability study that did not test EHR changes |
Contributions of authors
RB completed the search and screening.
RB, LS, and MF completed study selection.
RB extracted the data; checked by LS and MF.
RB, LS, and MF wrote the text of the review.
Declarations of interest
Dr Boyle has no competing financial interest. He is an investigator on an included study.
Dr Solberg has no competing interest.
Dr Fiore has no competing financial interest. He is an investigator on two included studies.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Adsit 2014 {published data only}
- Adsit RT, Fox BM, Tsiolis T, Ogland C, Simerson M, Vind LM, et al. Using the electronic health record to connect primary care patients to evidence‐based telephonic tobacco quitline services: a closed‐loop demonstration project. Translational Behavioral Medicine 2014 Sep;4(3):324‐32. [DOI: 10.1007/s13142-014-0259-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bentz 2002 {published data only (unpublished sought but not used)}
- Bentz CJ, Davis N, Bayley B. The feasibility of paper‐based Tracking Codes and electronic medical record systems to monitor tobacco‐use assessment and intervention in an Individual Practice Association (IPA) Model health maintenance organization (HMO). Nicotine & Tobacco Research 2002;4(Suppl 1):S9‐17. [DOI] [PubMed] [Google Scholar]
Bentz 2007 {published data only}
- Bentz CJ, Bayley KB, Bonin KE, Fleming L, Hollis JF, Hunt JS, et al. Provider feedback to improve 5A's tobacco cessation in primary care: a cluster randomized clinical trial. Nicotine & Tobacco Research 2007;9(3):341‐9. [DOI] [PubMed] [Google Scholar]
Frank 2004 {published data only}
- Frank O, Litt J, Beilby J. Opportunistic electronic reminders. Improving performance of preventive care in general practice. Australian Family Physician 2004;33:87‐90. [PubMed] [Google Scholar]
Koplan 2008 {published data only}
- Koplan KE, Regan S, Goldszer RC, Schneider LI, Rigotti NA. A computerized aid to support smoking cessation treatment for hospital patients. Journal of General Internal Medicine 2008;23(8):1214‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linder 2009 {published data only}
- Linder JA, Rigotti NA, Schneider LI, Kelley JH, Brawarsky P, Haas JS. An electronic health record‐based intervention to improve tobacco treatment in primary care: a cluster‐randomized controlled trial. Archives of Internal Medicine 2009;169(8):781‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder JA, Rigotti NA, Schneider LI, Kelley JH, Brawarsky P, Schnipper JL, et al. Clinician characteristics and use of novel electronic health record functionality in primary care. Journal of the American Medical Informatics Association : JAMIA 2011;18 Suppl 1:i87‐90. [CENTRAL: 841364; CRS: 9400123000015269; PUBMED: 21900702] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindholm 2010 {published data only}
- Lindholm C, Adsit R, Bain P, Reber PM, Brein T, Redmond L, et al. A demonstration project for using the electronic health record to identify and treat tobacco users. WMJ 2010;109(6):335‐40. [PMC free article] [PubMed] [Google Scholar]
Mathias 2012 {published data only}
- Mathias JS, Didwania AK, Baker DW. Impact of an electronic alert and order set on smoking cessation medication prescription. Nicotine & Tobacco Research 2012;14(6):674‐81. [CRS: 9400123000016464; PUBMED: 22180576] [DOI] [PubMed] [Google Scholar]
McCullough 2009 {published data only}
- McCullough A, Fisher M, Goldstein AO, Kramer KD, Ripley‐Moffitt C. Smoking as a vital sign: prompts to ask and assess increase cessation counseling. Journal of the American Board of Family Medicine: JABFM 2009;22(6):625‐32. [DOI] [PubMed] [Google Scholar]
Ragucci 2009 {published data only}
- Ragucci KR, Shrader SP. A method for educating patients and documenting smoking status in an electronic medical record. Annals of Pharmacotherapy 2009;43(10):1616‐20. [DOI] [PubMed] [Google Scholar]
Rindal 2013 {published data only}
- Rindal DB, Rush WA, Schleyer TK, Kirshner M, Boyle RG, Thoele MJ, et al. Computer‐assisted guidance for dental office tobacco‐cessation counseling: a randomized controlled trial. American Journal of Preventive Medicine 2013;44(3):260‐4. [CENTRAL: 853436; CRS: 9400123000018009; EMBASE: 2013118212; PUBMED: 23415123] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sherman 2008 {published data only (unpublished sought but not used)}
- Sherman SE, Takahashi N, Kalra P, Gifford E, Finney JW, Canfield J, et al. Care coordination to increase referrals to smoking cessation telephone counseling: a demonstration project. American Journal of Managed Care 2008;14(3):141‐8. [PubMed] [Google Scholar]
Spencer 1999 {published data only}
- Spencer E, Swanson T, Hueston WJ, Edberg DL. Tools to improve documentation of smoking status. Continuous quality improvement and electronic medical records. Archives of Family Medicine 1999;8(1):18‐22. [DOI] [PubMed] [Google Scholar]
Szpunar 2006 {published data only}
- Szpunar SM, Williams PD, Dagroso D, Enberg RN, Chesney JD. Effects of the tobacco use cessation automated clinical practice guideline. American Journal of Managed Care 2006;12(11):665‐73. [PubMed] [Google Scholar]
Vidrine 2013 {published data only}
- Vidrine JI, Shete S, Cao Y, Greisinger A, Harmonson P, Sharp B, et al. Ask‐Advise‐Connect: A new approach to smoking treatment delivery in health care settings. JAMA Internal Medicine 2013;173(6):458‐64. [CENTRAL: 870587; CRS: 9400107000000013; EMBASE: 2013215566; PUBMED: 23440173] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vidrine 2013a {published data only}
- Vidrine JI, Shete S, Li Y, Cao Y, Alford MH, Michelle Galindo‐Talton R, et al. The ask‐advise‐connect approach for smokers in a safety net healthcare system: A group‐randomized trial. American Journal of Preventive Medicine 2013;45(6):737‐41. [CENTRAL: 915204; CRS: 9400129000000250; EMBASE: 2013729163] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bunik 2013 {published data only}
- Bunik M, Cavanaugh KL, Herrick D, Mehner L, Venugopalakrishnan J, Crane LA, et al. The ONE step initiative: quality improvement in a pediatric clinic for secondhand smoke reduction. Pediatrics 2013;132(2):e502‐11. [CRS: 9400126000000028; PUBMED: 23858424] [DOI] [PubMed] [Google Scholar]
Greenwood 2012 {published data only}
- Greenwood DA, Parise CA, MacAller TA, Hankins AI, Harms KR, Pratt LS, et al. Utilizing clinical support staff and electronic health records to increase tobacco use documentation and referrals to a state quitline. Journal of Vascular Nursing 2012;30(4):107‐11. [CRS: 9400126000000506; PUBMED: 23127426] [DOI] [PubMed] [Google Scholar]
Herrin 2012 {published data only}
- Herrin J, Graca B, Nicewander D, Fullerton C, Aponte P, Stanek G, et al. The effectiveness of implementing an electronic health record on diabetes care and outcomes. Health Services Research 2012;47(4):1522‐40. [CRS: 9400123000016383; PUBMED: 22250953] [DOI] [PMC free article] [PubMed] [Google Scholar]
Herrin 2014 {published data only}
- Herrin J, Graca B, Aponte P, Stanek HG, Cowling T, Fullerton C, et al. Impact of an EHR‐Based Diabetes Management Form on Quality and Outcomes of Diabetes Care in Primary Care Practices. American Journal of Medical Quality 2014;January; epub ahead of print. [DOI] [PubMed] [Google Scholar]
Kruse 2013 {published data only}
- Kruse GR, Chang Y, Kelley JH, Linder JA, Einbinder JS, Rigotti NA. Healthcare system effects of pay‐for‐performance for smoking status documentation. American Journal of Managed Care 2013;19(7):554‐61. [CRS: 9400129000002774; PUBMED: 239194195] [PMC free article] [PubMed] [Google Scholar]
Levine 2013 {published data only}
- Levine L, Chang J, Merkatz IR, Bernstein PS. enhamced physician prompts in prenatal electronic medical records impact documentation on smoking cessation. Open J Obstet Gynecol 2013;3(10):717‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mundra 2012 {published data only}
- Mundra V, Shadi Yaghoubian S, Nguyen V, Sider D, Jose Muniz J, Villabona CV. Impact of the Use of an Electronic Template on CliniciansAdherence to Follow Guidelines for Diabetes Care. Eur J Biomed Informatics 2012;8(1):20‐33. [Google Scholar]
Onders 2014 {published data only}
- Onders R, Spillane J, Reilley B, Leston J. Use of electronic clinical reminders to increase preventive screenings in a primary care setting: blueprint from a successful process in Kodiak, Alaska. Journal of Primary Care & Community Health 2014;5(1):50‐4. [CRS: 9400129000002776; PUBMED: 24327588] [DOI] [PubMed] [Google Scholar]
Wang 2013 {published data only}
- Wang JJ, Sebek KM, McCullough CM, Amirfar SJ, Parsons AS, Singer J, et al. Sustained improvement in clinical preventive service delivery among independent primary care practices after implementing electronic health record systems. Preventing Chronic Disease 2013;10:E130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Warren 2013 {published data only}
- Warren GW, Marshall JR, Cummings KM, Zevon MA, Reed R, Hysert P, Mahoney MC, Hyland AJ, Nwogu C, Demmy T, Dexter E, Kelly M, O'Connor RJ, Houstin T, Jenkins D, Germain P, Singh AK, Epstein J, Dobson Amato KA, Reid ME. Automated tobacco assessment and cessation support for cancer patients. Cancer 2014;120(4):562‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Agaku 2014
- Agaku IT, Ayo‐Yusuf OA, Vardavas CI. A comparison of cessation counseling received by current smokers at US dental and physician offices during 2010‐2011. American Journal of Public Health 2014;104(8):e67‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2000
- Campbell MJ. Cluster randomized trials in general (family) practice research. Statistical Methods for Medical Research 2000;9(2):81‐94. [DOI] [PubMed] [Google Scholar]
Fiore 1991
- Fiore MC. The New Vital Sign. JAMA 1991;266:3183‐84. [PubMed] [Google Scholar]
Fiore 2008
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating Tobacco Use and Dependence: 2008 Update U.S. Public Health Service Clinical Practice Guideline. Treating Tobacco Use and Dependence: 2008 Update Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services, 2008. [Google Scholar]
Hesse 2010
- Hesse BW. Time to reboot: resetting healthcare to support tobacco dependency treatment services. American Journal of Preventive Medicine 2010;39(6S1):S85‐S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S [eds]. Cochrane Handbook for Systematic Reviews of Interventions, 5.1.0. [updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]
Ng 2014
- Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer‐lindgren L, Thomson B, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980‐2112. JAMA 2014;311:183‐192. [DOI] [PubMed] [Google Scholar]
NHS 2011
- National Institute for Health and Clinical Excellence (NICE). Brief interventions and referral for smoking cessation in primary care and other settings. http://www.nice.org.uk/guidance/PH1 March 2006. [website accessed May 2011]
Stead 2013
- Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann‐Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD000165.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
The PLOS Medicine Editors 2014
- The PLOS Medicine Editors. Observational Studies: Getting Clear about Transparency. PloS Med 2014;11(8):e1001711. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2002
- WHO. The Tobacco Atlas. 1st Edition. Geneva, Switzerland: WHO, 2002. [Google Scholar]
WHO 2009
- WHO. WHO Report on the Global Tobacco Epidemic. Geneva, Switzerland: WHO, 2009. [Google Scholar]
References to other published versions of this review
Boyle 2010
- Boyle R, Solberg L, Fiore M. Use of electronic health records to support smoking cessation. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD008743.pub2] [DOI] [PubMed] [Google Scholar]
Boyle 2011
- Boyle R, Solberg L, Fiore M. Use of electronic health records to support smoking cessation. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD008743.pub2] [DOI] [PubMed] [Google Scholar]


