Abstract
Background
The morbidity and socioeconomic costs of fractures are considerable. The length of time to healing is an important factor in determining a person's recovery after a fracture. Ultrasound may have a therapeutic role in reducing the time to union after fracture. This is an update of a review previously published in February 2012.
Objectives
To assess the effects of low‐intensity ultrasound (LIPUS), high‐intensity focused ultrasound (HIFUS) and extracorporeal shockwave therapies (ECSW) as part of the treatment of acute fractures in adults.
Search methods
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (2 June 2014), the Cochrane Central Register of Controlled Trials (The Cochrane Library 2014, Issue 5), MEDLINE (1946 to May Week 3 2014), EMBASE (1980 to 2014 Week 22), trial registers and reference lists of articles.
Selection criteria
Randomised and quasi‐randomised controlled trials evaluating ultrasound treatment in the management of acute fractures in adults. Studies had to include participants over 18 years of age with acute fractures, reporting outcomes such as function; time to union; non‐union; secondary procedures such as for fixation or delayed union or non‐union; adverse effects; pain; costs; and patient adherence.
Data collection and analysis
Two authors independently extracted data from the included studies. Treatment effects were assessed using mean differences, standardised mean differences or risk ratios using a fixed‐effect model, except where there was substantial heterogeneity, when data were pooled using a random‐effects model. Results from 'worst case' analyses, which gave more conservative estimates of treatment effects for time to fracture union, are reported in preference to those from 'as reported' analyses.
Main results
We included 12 studies, involving 622 participants with 648 fractures. Eight studies were randomised placebo‐controlled trials, two were randomised controlled trials without placebo controls, one was a quasi‐randomised placebo‐controlled trial and one was a quasi‐randomised controlled trial without placebo control. Eleven trials tested LIPUS and one trial tested ECSW. Four trials included participants with conservatively treated upper limb complete fractures and six trials included participants with lower limb complete fractures; these were surgically fixed in four trials. The remaining two trials reported results for conservatively treated tibial stress fractures.
'Risk of bias' assessment of the included studies was hampered by the poor reporting of methods, frequently resulting in the risk of bias of individual domains being judged as ‘unclear’. Both quasi‐randomised studies were at high risk of bias, including selection and attrition bias. Three studies were at low risk of selection bias relating to allocation concealment the majority of studies were at low risk of performance bias as they employed a form of intervention blinding.
Only limited data were available from three of only four studies reporting on functional outcome. One study of complete fractures found little evidence of a difference between the two groups in the time to return to work (mean difference (MD) 1.95 days favouring control, 95% confidence interval (CI) ‐2.18 to 6.08; 101 participants). Pooled data from two studies found LIPUS did not significantly affect the time to return to training or duty in soldiers or midshipmen with stress fractures (MD ‐8.55 days, 95% CI ‐22.71 to 5.61; 93 participants).
We adopted a conservative strategy for data analysis that was more likely to underestimate than to overestimate a benefit of the intervention. After pooling results from eight studies (446 fractures), the data showed no statistically significant reduction in time to union of complete fractures treated with LIPUS (standardised mean difference (SMD) ‐0.47, 95% CI ‐1.14 to 0.20). This result could include a clinically important benefit or harm, and should be seen in the context of the highly significant statistical heterogeneity (I² = 90%). This heterogeneity was not explained by the a priori subgroup analyses (upper limb versus lower limb fracture, smoking status). An additional subgroup analysis comparing conservatively and operatively treated fractures raised the possibility that LIPUS may be effective in reducing healing time in conservatively managed fractures, but the test for subgroup differences did not confirm a significant difference between the subgroups.
Pooled results from five of the eight trials (333 fractures) reporting proportion of delayed union or non‐union showed no significant difference between LIPUS and control (10/168 versus 13/165; RR 0.75; 95% CI 0.24 to 2.28). Adverse effects directly associated with LIPUS and associated devices were found to be few and minor, and compliance with treatment was generally good. One study reporting on pain scores found no difference between groups at eight weeks (101 participants).
One quasi‐randomised study found no significant difference in non‐union at 12 months between internal fixation supplemented with ECSW and internal fixation alone (3/27 versus 6/30; RR 0.56, 95% CI 0.15 to 2.01). There was a clinically small but statistically significant difference in the visual analogue scores for pain in favour of ECSW at three month follow‐up (MD ‐0.80, 95% CI ‐1.23 to ‐0.37). The only reported complication was infection, with no significant difference between the two groups.
Authors' conclusions
While a potential benefit of ultrasound for the treatment of acute fractures in adults cannot be ruled out, the currently available evidence from a set of clinically heterogeneous trials is insufficient to support the routine use of this intervention in clinical practice. Future trials should record functional outcomes and follow‐up all trial participants.
Plain language summary
Ultrasound and shockwave treatment for recently broken bones in adults
Broken bones (fractures) are a major cause of disability in adults. The time taken for a bone to heal (achieve "union") is an important factor in determining recovery after an injury. A minority of fractures fail to heal at all or their healing takes considerably longer than expected. This review set out to find out whether treatment with ultrasound, in a variety of forms, accelerates fracture healing and reduces complications associated with new (acute) fractures. A related intervention, shockwave therapy, was also examined. Typically, ultrasound treatment involves placing a special device in contact with the skin overlying the fracture site for around 20 minutes on a daily basis.
This is an update of a review previously published in February 2012. We did a new literature search up till 2 June 2014 but did not find any new studies. There are 12 studies, involving 622 participants with 648 fractures, included in this review. In all the studies we included, participants were assigned randomly to one of two groups, one group receiving treatment by ultrasound and the other group receiving no treatment or sham treatment. Most participants had a recent complete fracture of a single bone. The participants of two trials had 110 incomplete or stress fractures that resulted from heavy exercise. Four trials tested the effects of ultrasound on healing of 203 upper limb fractures and the other trials, on 130 lower limb fractures. The most commonly investigated bone was the tibia (shin bone). Eleven trials tested low‐intensity pulsed ultrasound and one trial with 59 fractures tested shockwave therapy.
Most trials compared a working ultrasound device with a sham device and thus protected against placebo effects. The placebo effect is a phenomenon whereby patients experience a treatment effect that is not objectively attributable to the treatment itself. However, studies varied substantially in terms of quality and risk of having biased results. In many cases the quality of reporting was poor, which made it difficult to determine which biases might have affected each study. The risk of bias across many domains therefore had to be judged as 'unclear'. The results of many trials were probably biased because of missing data from several trial participants. Additionally, the trials were very different from each other; for example, they varied in the bone that was broken and whether or not the fractures were also treated surgically. Based on analyses that adjusted for these missing data, the available evidence did not confirm that ultrasound improved the time taken for bone healing or prevented the problem of the bone failing to heal at all (eight trials with 333 fractures). The results from one low quality trial (with 59 fractures) testing shockwave therapy were inconclusive.
Few complications were reported in any of the studies and these were not related to the ultrasound or shockwave therapy.
While a potential benefit of ultrasound for the treatment of acute fractures in adults cannot be ruled out, the currently available evidence from 12 quite different trials is insufficient to support the routine use of ultrasound in clinical practice. Future studies should measure return to full function and normal activity and should try to ensure all participants are followed up.
Background
Description of the condition
The morbidity and socioeconomic cost of fractures (broken bones) is considerable. Whilst most fractures unite, between 5% and 10% of long bone fractures are associated with delayed or non‐union, resulting in significant morbidity, loss of independence and loss of productivity (Aaron 2004). The length of time to healing is also an important factor in determining recovery after a fracture (Heckman 1997). Several interventions, including ultrasound, have been proposed to enhance and accelerate bone healing, and potentially reduce the incidence of the complications associated with fractures and their treatment (Einhorn 1995; Hadjiargyrou 1998).
Description of the intervention
Ultrasound, comprising high frequency sound waves, is a form of mechanical stimulation that is delivered via a special device to the fracture site. For closed fractures (where the overlying soft tissue envelope remains intact), the device is typically placed in contact with the skin overlying the fracture site and left in position for around 20 minutes on a daily basis.
There are three modalities of ultrasound used in clinical practice.
Low‐intensity pulsed ultrasound (LIPUS)
High‐intensity focused ultrasound (HIFUS)
Extracorporeal shock wave therapy (ECSW)
How the intervention might work
It is known that bone formation and fracture healing are influenced by mechanical factors. It is possible that ultrasound might work by reproducing the effect of functional loading by inducing low level mechanical forces at the fracture site. The mechanisms have not been fully elucidated (Hadjiargyrou 1998), but it is likely that ultrasound influences healing at multiple points during the fracture healing process.
Although it is thought that all three ultrasound modalities work in a similar way in the body, the effectiveness of each modality does appear to be different (Reher 1997; Wang 1994). Thus, these three modalities are considered separately in this review.
Why it is important to do this review
The ability to improve fracture healing would have a large clinical and socioeconomic impact. Whilst there is currently no consensus on the role of ultrasound, its use is becoming increasingly widespread (Victoria 2009). A recent systematic review identified a broad evidence base concerning the use of ultrasound in the management of acute fractures (Griffin 2008). This review updates the summary of the available best evidence on the use of ultrasound for acute fractures in order to inform practice and highlight areas in need of further research.
Objectives
To assess the effects of any ultrasound therapy used as part of the treatment of acute fractures in adults.
We planned to make the following comparisons.
Low‐intensity pulsed ultrasound (LIPUS) versus control (sham ultrasound or none)
High‐intensity focused ultrasound (HIFUS) versus control (sham ultrasound or none)
Extracorporeal shockwave therapy (ECSW) versus control (sham ultrasound or none)
Participants might additionally receive a standard‐of‐care treatment, which would be the treatment routinely used in clinical practice for the treatment of the fracture. This might include, but not be limited to, non‐surgical treatment such as plaster cast immobilisation, or surgical treatment such as external or internal fixation, using various devices, for example, intramedullary nailing.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised (a method of allocating participants to a treatment which was not strictly random, e.g. by date of birth, hospital record number, alternation) controlled clinical studies evaluating any type of ultrasound treatment in the management of acute fractures in adults.
Types of participants
Any skeletally mature adults, over the age of 18 years, with acute traumatic fractures. We excluded trials evaluating treatment for delayed union, non‐union or post‐corticotomy (e.g. distraction osteogenesis).
Types of interventions
Trials of all three types of ultrasound (low‐intensity pulsed ultrasound (LIPUS), high‐intensity focused ultrasound (HIFUS) and extracorporeal shock wave therapy (ECSW)) were eligible provided the treatment was compared with either no additional treatment or a placebo (sham ultrasound). Ultrasound could be the only treatment, but would more usually be an adjunct to a standard‐of‐care treatment applied to all trial participants. The standard‐of‐care treatment could be non‐surgical or surgical. Trials comparing ultrasound with other interventions were excluded. Each modality of ultrasound treatment was considered in a separate comparison as described in the Objectives.
Types of outcome measures
Functional recovery, including return to former activities, was the prime focus of the review. However, we anticipated that most trials would not report patient‐reported outcome measures but would focus instead on fracture healing outcomes.
The definition of a healed fracture is contentious. For the purpose of this review we adopted the widely accepted definitions in the literature. A fracture is healed when callus is present bridging three of four cortices on orthogonal radiographs, or there is an absence of pain and movement at the fracture site, or both. It was expected that most studies would report the time to union for each participant. These are the most frequently reported statistics when studies are published in this field. However, it was possible that some studies might have presented a proportional analysis of healed fractures at a number of fixed time points after treatment.
Primary outcomes
Overall quantitative functional improvement of the participant using recognised patient‐reported outcome measures and the return to normal activities, including work
Time to fracture union
Secondary outcomes
Confirmed non‐union or secondary procedure, such as for failure of fixation or for delayed or non‐union
Adverse effects
Pain using validated pain scores
Costs
Patient adherence
Timing of outcome assessment
We anticipated that some studies might have reported proportional incidence of union at several time points rather than a time‐to‐event analysis. We planned to try to group these assessments into three categories: short‐ (up to three months), medium‐ (between three and 12 months) and long‐term follow‐up (greater than one year) (see Unit of analysis issues). These time points were a necessary compromise to encompass data from studies that included different bones with different typical healing times.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (2 June 2014), the Cochrane Central Register of Controlled Trials (The Cochrane Library 2014, Issue 5), MEDLINE (1950 to May Week 3 2014) and EMBASE (1980 to 2014 Week 22). There were no constraints based on language or publication status. For this update, the search results were limited to 2011 onwards. Details of the search strategies used for the previous review are given in Griffin 2012. No language or publication restrictions were applied.
In MEDLINE, the subject‐specific search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials: sensitivity‐maximising version (Lefebvre 2011). In EMBASE, the subject‐specific search was combined with the SIGN strategy for randomised controlled trials (seeAppendix 1 for all strategies).
Current Controlled Trials and the WHO International Clinical Trials Registry Platform were also searched (15 October 2013) using the term "fracture" and items manually screened to identify ongoing and recently completed trials.
Searching other resources
We searched reference lists of articles retrieved from the electronic search. We contacted experts in the field for any additional or unpublished articles.
Data collection and analysis
Selection of studies
In the original review, two authors independently selected the studies for inclusion based upon the criteria defined above. Initially the titles and abstracts of all the retrieved studies were screened to determine potential eligibility. The full text of each study in this shortlist was then read to determine which studies were eligible for inclusion in the review. Any disagreement was settled by consensus between all authors of the review. For the current update, items retrieved from database searches were screened by a single author (DM).
Data extraction and management
Data were extracted from the included studies using a pre‐piloted version of the Cochrane Bone, Joint and Muscle Trauma Group's data extraction form. The review statistician (NP), who was independent from study selection, collated the extracted outcome data and entered it into Review Manager software.
Assessment of risk of bias in included studies
Risk of bias was assessed by two authors in the original review and two (XG and DM) in this updated review. We assessed each of the included studies for the risk of bias using The Cochrane Collaboration's 'Risk of bias' tool (Higgins 2008). This tool incorporates assessment of randomisation (sequence generation and allocation concealment), blinding (trial participants and personnel, and outcome assessors), completeness of outcome data, selection of outcomes reported and other sources of bias. We assessed the risk of bias associated with a) blinding and b) proportion of follow‐up separately for patient‐reported outcomes and objective outcomes. Other sources of bias included the risk of bias from major imbalances in key baseline characteristics (age, sex and smoking behaviour).
In our protocol, we suggested that different considerations apply to the primary outcome of fracture healing (i.e. 'time to union'), which is variably defined in the literature. We anticipated that studies may define healing clinically and radiographically. We anticipated that other bias might be introduced by inter‐ and intra‐observer error in the reading of radiographs. We planned to ascribe a low risk of bias to studies in which a blinded panel of specialist radiologists or orthopaedic surgeons read the radiographs; and a high risk of bias to studies employing other methodologies, such as multiple independent observers. As noted in Risk of bias in included studies, we decided subsequently that this was not a source of other bias.
Measures of treatment effect
We had intended to assess time to fracture union after treatment using a (log) hazard ratio and 95% confidence intervals. However, as we had anticipated, fracture union was either reported as a proportion of fractures healed at each follow‐up time point or the mean time to union. Where studies reported a proportion of fractures healed, we calculated the mean time to union (and standard deviation) assuming that each fracture had healed at the end of the interval between follow‐up time points. From the reported and calculated mean times to union, we calculated standardised mean differences and 95% confidence intervals. This reflected the widely differing mean times to union in different studies including different bones. Risk ratios with 95% confidence intervals were used to express the intervention effect for dichotomous outcomes. For continuous data, such as pain scores, we calculated mean differences with 95% confidence intervals.
Unit of analysis issues
It was expected that most studies would report functional improvement scores at a number of follow‐up times; for example, at six and 12 weeks. Dependent on the nature of reporting, we planned to make separate analyses at each of the commonly reported occasions, representing perhaps, short‐, medium‐ and long‐term follow‐up. It was expected that all studies would report simple parallel group designs. However, if other designs had been reported (e.g. cluster‐randomised designs), we would have used generic inverse variance methods to combine data where appropriate.
Dealing with missing data
We sought additional information from the authors of the included studies where the published information or data were incomplete. Where standard deviations were not specifically reported, we attempted to determine these, if available, from standard errors, confidence intervals or exact P values. We did not expect there to be substantial missing data for studies in this research area. Where small amounts of data were missing for proportional outcomes, which could not be reliably determined from the authors, then these outcomes were initially classed as treatment failures and a sensitivity analysis conducted to test the effect of this assumption. For continuous measures, in order to determine a conservative estimate of any treatment effect, we assumed that healing times of participants in the treatment group for whom data were missing lay at the extreme of the distribution (two standard deviations from the reported mean). Conversely, for participants in the control group, we assumed the distribution was unaffected by the missing data. Pooled effect sizes were presented with and without these adjustments to check the effect of these assumptions. We refer to the adjusted analyses as 'worst case' analyses and the unadjusted as 'as reported' analyses.
Assessment of heterogeneity
The degree of statistical heterogeneity between studies was assessed graphically using the Chi² test and I² statistic (Higgins 2003). A conservative P value for Chi² of < 0.1 was set to indicate significant heterogeneity between studies. Where the heterogeneity statistic indicated significant heterogeneity and one or more studies appeared to be clear outliers, then data for these studies were checked carefully for errors or other methodological reasons why they might differ from the other studies. Where we found good reasons why outlier studies differed from the majority, we removed the study from the pooled analysis; however, we performed all analyses with and without outlier studies where any were excluded (sensitivity analysis).
Assessment of reporting biases
Our search strategy attempted to reduce the risk of missing relevant studies. We had planned to complete a funnel plot but an insufficient number of studies were available.
Data synthesis
Treatment effects from studies reporting proportional outcomes were summarised using risk ratios and combined using the Mantel‐Haenszel method. Continuous outcome measures were converted to standardised mean differences to assess the treatment effect and generic inverse variance methods were used to combine data. Confidence intervals were reported at the 95% level and initially the fixed‐effect model was used for meta‐analyses. Where there was significant heterogeneity, we used the random‐effects model.
Subgroup analysis and investigation of heterogeneity
When significant heterogeneity was present, we planned to conduct subgroup analysis to explore possible sources of the heterogeneity. Two possible subgroup analyses were identified a priori.
Upper versus lower limb fractures. This was a pragmatic proxy for weight bearing bones versus bones that are not weight bearing.
Smokers versus non‐smokers.
Sensitivity analysis
We conducted post hoc sensitivity analyses to explore the causes of statistical heterogeneity. We explored the effect of excluding a study which seemed to differ both clinically and statistically from the majority of studies.
Results
Description of studies
Results of the search
The search was updated from November 2011 to June 2014. We screened a total of 3582 records from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (one record); Cochrane Central Register of Controlled Trials (239), MEDLINE (76), EMBASE (153), the WHO International Clinical Trials Registry Platform (1181) and Current Controlled Trials (1933). We did not identify any additional studies from reference lists or other sources.
The search update did not result in the identification of any new studies available for inclusion. We identified three papers associated with already included studies, one for Handolin 2005a, one for Kristiansen 1997 and one for Strauss 1999. We also found one new excluded study (Urita 2013). We obtained further information on the status of ISRCTN90844675 that continues to await assessment. This trial, which appears to have been presented in an oral presentation in 2013, was reported to be completed and is actively under consideration for publication (Seifert 2013).
Two days after date of last search (2 June 2014), we were notified by the Trial Search Co‐ordinator of a prepublication report of the 'pilot' study for the TRUST trial (Busse 2014). This clarified that one study previously awaiting assessment (ISRCTN98682811, formerly TRUST (Pilot)) that aimed to recruit conservatively managed fractures of the tibia was abandoned through lack of recruitment. We have now excluded ISRCTN98682811. In correspondence in 2013 (Busse 2013), Busse had indicated that the pilot trial was being considered for publication and that the full trial (NCT00667849, formerly TRUST (Full)) had been completed but that the data were not yet available. Given the very recent publication of the pilot study (Busse 2014) and the completion of the full study, we transferred NCT00667849 from ongoing studies to studies awaiting classification. Of note is that both Busse 2014 and a related publication (Dijkman 2011) report recruitment dates prior to that listed in the trial registration document for this trial.
Overall, there are now 12 included studies, five excluded studies, two studies awaiting assessment and no ongoing trials. A summary of the search process is given in Figure 1.
1.
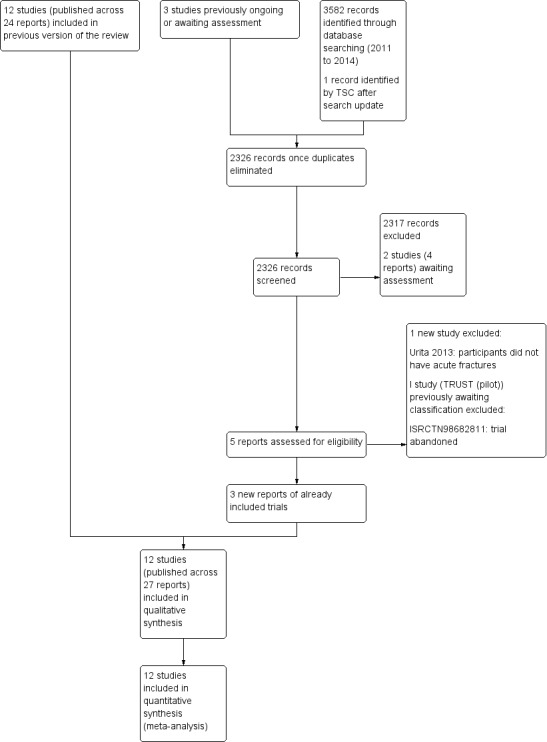
Study flow diagram
Included studies
We included 12 studies, involving 622 participants with 648 fractures. Most studies recruited only adults although one (Wang 2007) included adolescent participants as well; the age range of participants across all the studies was 15 to 81 years. Details of the individual studies are shown in the Characteristics of included studies.
Design
Eight studies were randomised placebo‐controlled trials and two studies (Mayr 2000; Strauss 1999) were randomised controlled trials without placebo controls. Of the two quasi‐randomised studies, one was placebo‐controlled (Leung 2004) and the other (Wang 2007) was not.
Cook 1997 reported a subgroup analysis by smoking status of participants recruited in the trials reported by Heckman 1994 and Kristiansen 1997. These data were not doubly entered into the analyses but have been used to inform an a priori subgroup analysis in this review.
Sample sizes
Each of the included studies included relatively few participants.
Emami 1999: 30 participants (15:15, ultrasound:control)
Handolin 2005: 30 participants (15:15, ultrasound:control)
Handolin 2005a: 22 participants (11:11, ultrasound:control)
Heckman 1994: 97 participants (48:49, ultrasound:control)
Kristiansen 1997: 85 fractures in 83 participants (40:45, ultrasound:control)
Leung 2004: 30 fractures in 28 participants (16:14, ultrasound:control)
Lubbert 2008: 120 participants (61:59 ultrasound:control)
Mayr 2000: 30 fractures in 29 participants (15:15, ultrasound:control)
Rue 2004: 58 fractures in 40 participants (21:19, ultrasound:control)
Strauss 1999: 20 participants (10:10, ultrasound:control)
Wang 2007: 59 fractures in 56 participants (28:31, ECSW:control)
Yadav 2008: 67 participants (39:28, ultrasound:control)
Settings
The studies that reported outcomes in participants with complete fractures were set in hospital trauma and orthopaedic departments. These studies were based in a wide variety of countries: Sweden (Emami 1999), Finland (Handolin 2005; Handolin 2005a), USA (Heckman 1994; Kristiansen 1997; Strauss 1999), China (Leung 2004), Netherlands (Lubbert 2008), Germany (Mayr 2000) and Taiwan (Wang 2007). The study reported by Kristiansen 1997 was a multicentre study. Rue 2004 reported outcomes in American midshipmen with stress fractures presenting to a military clinic. Yadav 2008 reported outcomes in Indian soldiers with stress fractures presenting to a military clinic.
Participants
The majority of studies reported outcomes from participants with conservatively managed fresh fractures; of these, Heckman 1994 reported data from fractures of the tibia, Strauss 1999 fractures of the fifth metatarsal, and the remainder from upper limb fractures (Kristiansen 1997: distal radius; Lubbert 2008: clavicle; Mayr 2000: scaphoid). Three studies reported outcomes from participants with operatively managed fractures of the tibia (Emami 1999; Leung 2004) or tibia and femur (Wang 2007), and two reported results from participants following internal fixation of lateral malleolus (ankle) fractures (Handolin 2005; Handolin 2005a).
Two studies reported outcomes from participants with acute stress fractures of the tibia (Rue 2004; Yadav 2008).
Interventions
All the included studies reported the use of LIPUS except Wang 2007, which tested ECSW therapy. The nine placebo‐controlled LIPUS trials used a deactivated (sham) ultrasound machine.
The LIPUS treatments were very similar across the included studies. Participants given the test treatment received ultrasound treatment for 20 minutes each day for a total cumulative time of approximately 24 hours. The ultrasound signal was composed of a 200 µs burst of 1.5 MHz sine waves, with a repetition rate of 1 kHz and a spatial average intensity of 30 mW/cm².
All 10 studies of participants with complete fractures reported the effectiveness of the test treatment in addition to a method of bony stabilisation. In five studies, stabilisation was achieved with either a plaster or a brace (Heckman 1994; Kristiansen 1997; Lubbert 2008; Mayr 2000; Strauss 1999). Internal fixation was used in the remaining studies (Emami 1999; Handolin 2005; Handolin 2005a; Leung 2004; Wang 2007).
Outcomes
A mixture of outcomes were reported. In terms of our primary outcomes, the majority of studies reported time to radiographic union using plain radiographs as the primary measure of efficacy. Exceptionally, Mayr 2000 used computed tomography to determine fracture union. Each of these studies measured union at multiple time points at various intervals (related to fracture site) from which mean time to union was derived. Wang 2007 and Strauss 1999 reported the proportion of fractures achieving radiographic union only. Four studies (Handolin 2005; Lubbert 2008; Rue 2004; Yadav 2008) presented patient‐reported functional outcomes: Handolin 2005 reported a region‐specific functional score, and the other three trials on return to work or training. Details about other outcomes measured in each study can be found in the Characteristics of included studies tables.
Excluded studies
The reasons for exclusion of five studies are detailed in the Characteristics of excluded studies. Two studies reporting on costs were excluded because the data for the economic analysis were not obtained from a randomised trial (Busse 2005; Heckman 1997). Basso 1998, a quasi‐randomised trial, did not focus on fracture healing nor report relevant outcomes to this review; similarly Urita 2013 did not include any participants with acute fractures. ISRCTN98682811 was a pilot randomised trial that was stopped after failure to recruit any patients with conservatively managed tibial fractures.
Risk of bias in included studies
The quality of reporting of the studies was varied. A summary of the assessment of the risk of bias in each study can be found in Figure 2.
2.
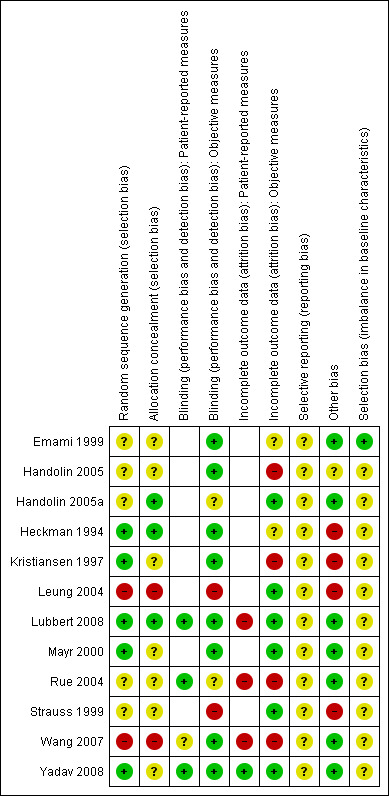
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
(Empty cells = not applicable as no patient‐reported outcomes in study)
Allocation
Both quasi‐randomised trials (Leung 2004; Wang 2007) were considered to be at high risk of selection for both sequence generation and allocation concealment.
Sequence generation and methods of allocation were often poorly reported in the other 10 studies; an absence of details of methods resulted in a judgement of 'unclear risk' for one or both domains. Five studies (Emami 1999; Handolin 2005; Handolin 2005a; Rue 2004; Strauss 1999) were judged to be at 'unclear' risk of selection bias relating to sequence generation and five at 'low risk' (Heckman 1994; Kristiansen 1997; Lubbert 2008; Mayr 2000; Yadav 2008). Seven studies were at 'unclear' risk of selection bias due to insufficient allocation concealment (Emami 1999; Handolin 2005; Kristiansen 1997; Mayr 2000; Rue 2004; Strauss 1999; Yadav 2008) and three at 'low risk' (Handolin 2005a; Heckman 1994; Lubbert 2008).
Blinding
For blinding in relation to patient‐reported outcomes, three studies had a rating of 'low risk' (Lubbert 2008; Rue 2004; Yadav 2008) and one was at 'unclear' risk (Wang 2007). Eight studies did not include any patient‐reported measures (Emami 1999; Handolin 2005; Handolin 2005a; Heckman 1994; Kristiansen 1997; Leung 2004; Mayr 2000; Strauss 1999).
For blinding in relation to objective outcome measures, two studies were rated as 'unclear' (Handolin 2005a; Rue 2004) and eight as 'low risk' (Emami 1999; Handolin 2005; Heckman 1994; Kristiansen 1997; Lubbert 2008; Mayr 2000; Wang 2007; Yadav 2008). Two studies were rated as 'high risk' (Leung 2004; Strauss 1999). The majority of studies used a deactivated ultrasound unit to blind the allocation, but, the unit used by Leung 2004 was externally dissimilar to the intervention unit and therefore the blinding in this study may have been compromised. While three studies (Mayr 2000; Strauss 1999; Wang 2007) were not placebo‐controlled, only Strauss 1999 did not blind outcome assessment of objective outcomes.
Incomplete outcome data
None of the included studies explicitly reported, or justified where absent, all of the outcome data. We were successful in contacting authors of three trials (Heckman 1994; Kristiansen 1997; Lubbert 2008) for missing data.
Eight studies did not include any patient‐reported measures (Emami 1999; Handolin 2005; Handolin 2005a; Heckman 1994; Kristiansen 1997; Leung 2004; Mayr 2000; Strauss 1999). One study was assessed to be at 'low risk' (Yadav 2008) and three 'high risk' (Lubbert 2008; Rue 2004; Wang 2007) of attrition bias for patient‐reported outcomes.
In terms of incomplete data for objective outcomes, two studies were at 'unclear' risk of attrition bias (Emami 1999; Heckman 1994) and six at 'low risk' (Handolin 2005a; Leung 2004; Lubbert 2008; Mayr 2000; Strauss 1999; Yadav 2008). Overall, we judged four studies to be at high risk of attrition bias for objective outcomes: Handolin 2005 because of the high (47%) and unaccounted loss to follow‐up at 18 months follow‐up; Kristiansen 1997 because of a high attrition (30%) and difficulty assessing the primary measure with plaster casts in‐situ; Rue 2004 because of a high attrition rate (35%), in part resulting from post‐hoc selection decision to limit their analysis to tibial stress fractures; and Wang 2007 because of inappropriate handling of participants with adverse events.
Selective reporting
The overall quality of the reporting of the included studies was poor. No protocols were available for comparing with the trial reports. The reporting of the methods and results was frequently mixed so that determining the risk of bias from selective reporting of outcomes was very difficult and we had to assess all as 'unclear'. However, there was no clear evidence of selective outcome reporting.
Other potential sources of bias
It was clear from all of the studies that, for obvious practical reasons, it was impossible to assess healing in each participant every day. Typically, participants were assessed at fixed follow‐up intervals that varied between studies. This inevitably led to a lack of precision in estimates of healing times. However, we see no reason why this process should have differed between treatment groups in any study, so would not expect there to be any bias in estimates of the treatment effects. However, this may, at least in part, explain the significant heterogeneity in observed healing times between studies.
Four studies (Heckman 1994; Kristiansen 1997; Leung 2004; Strauss 1999) were judged at 'high risk' of other bias. This related to the reporting of a per protocol analysis only in Heckman 1994; unit of analysis issues relating to bilateral fractures in Kristiansen 1997 and Leung 2004; and the very incomplete nature on the trial methods and results in Strauss 1999.
There were often insufficient data, in particular relating to smoking status, to judge whether there were major imbalances between the treatment and control groups in baseline characteristics. Only Emami 1999 was considered at low risk for this item.
Effects of interventions
Low‐intensity pulsed ultrasound versus control
Primary outcomes
Functional outcomes
Complete fractures
Only Lubbert 2008 provided data on 120 participants for return to work. There was no significant difference between the treatment and control groups using either 'as reported' data (mean difference (MD) 1.95 days, 95% confidence interval (CI) ‐2.18 to 6.08 days) or when based upon a 'worst case' analysis (MD 1.42 days, 95% CI ‐2.40 to 5.24) (see Analysis 1.1).
1.1. Analysis.
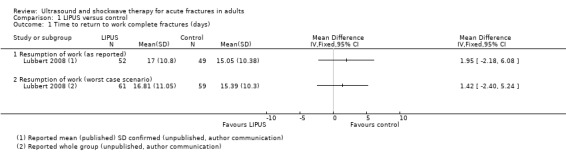
Comparison 1 LIPUS versus control, Outcome 1 Time to return to work complete fractures (days).
Handolin 2005 reported no significant difference in the Olerud‐Molander score between treatment and control groups in 16 participants (53% of the 30 randomised participants) at 18 months follow‐up. However, insufficient data were reported to be able to confirm this report and efforts to contact the authors were unsuccessful.
Stress fractures
Rue 2004 and Yadav 2008 both reported time to return to training or duty in 40 midshipmen and 67 military recruits, respectively. There was no significant benefit of LIPUS in the treatment of stress fractures of the tibia using 'as reported' data (MD ‐8.55 days, 95% CI ‐22.71 to 5.61) (see Analysis 1.2). There were insufficient baseline data from Rue 2004 to conduct a 'worst‐case scenario' analysis. There was considerable heterogeneity in the pooled estimate (I² = 78%); the difference in the findings of the two trials is also clearly visible in the analysis.
1.2. Analysis.
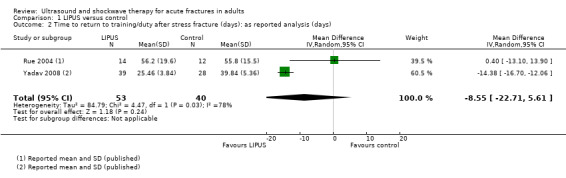
Comparison 1 LIPUS versus control, Outcome 2 Time to return to training/duty after stress fracture (days): as reported analysis (days).
Time to union
Although time to union data were available in most studies, the definition of union, timing of assessment and statistical analysis were variable. Efforts made to contact authors from each study in order to carry out appropriate intention‐to‐treat analyses were partly successful.
Four studies of 164 participants defined union radiographically (Emami 1999; Handolin 2005a; Kristiansen 1997; Mayr 2000). Where data were presented from surgeons and radiologists, we report only those based upon radiologists' opinions. Three studies, which included 208 participants, defined union as a combined clinical and radiographic finding with similar definitions of radiographic union (Heckman 1994; Kristiansen 1997; Leung 2004). Lubbert 2008 defined union based upon participants' self‐reports.
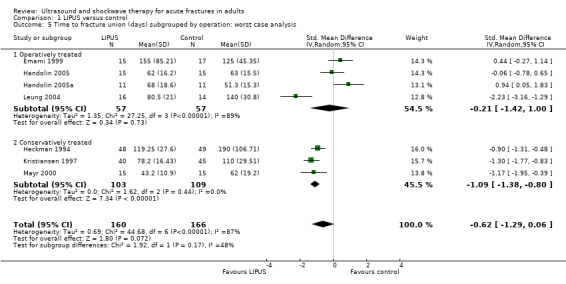
Each of the studies reporting this outcome only reported a per‐protocol analysis, where the reported data are for those participants who complied with the protocol, including follow‐up. These 'as reported' data are presented in Figure 3 (Analysis 1.3). We contacted the trial authors who explained that such an analysis was necessary because the data were missing due to the haphazard follow‐up of some participants. This 'as reported' analysis of 355 fractures demonstrated a significant benefit from LIPUS therapy (standardised mean difference (SMD) ‐0.69, 95% CI ‐1.31 to ‐0.07). However, the highly significant and substantial heterogeneity overall and for the upper and lower limb subgroups is also evident (P < 0.00001; I² = 86%). A conservative or 'worst case' analysis of 446 fractures, which attempted to include the missing data is presented in Figure 4 (Analysis 1.4; details of the imputation method described in Dealing with missing data), shows no significant difference between the treatment and control groups (SMD ‐0.47; 95% CI ‐1.14 to 0.20). The subgroup analysis by upper (235 fractures) and lower limb (211 fractures) did not significantly alter this finding.
3.
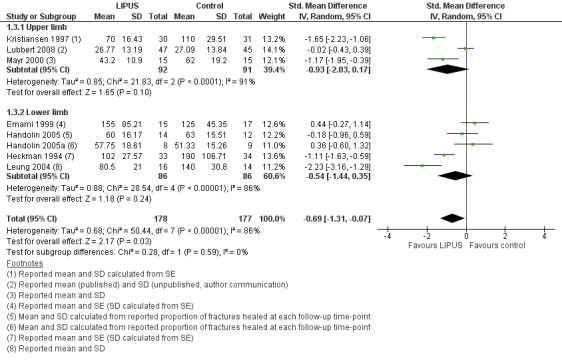
Forest plot of comparison: 1 LIPUS versus control, outcome: 1.3 Time to fracture union (days): 'as reported' analysis
1.3. Analysis.
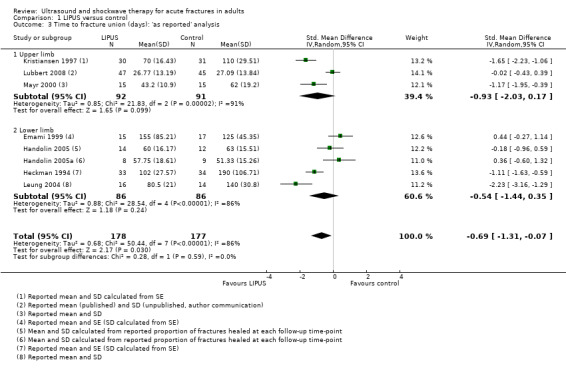
Comparison 1 LIPUS versus control, Outcome 3 Time to fracture union (days): 'as reported' analysis.
4.
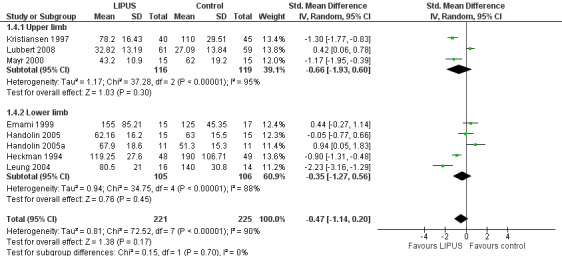
Forest plot of comparison: 1 LIPUS versus control, outcome: 1.4 Time to fracture union (days): worst case analysis
1.4. Analysis.
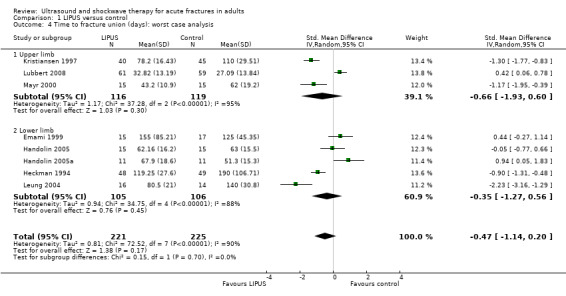
Comparison 1 LIPUS versus control, Outcome 4 Time to fracture union (days): worst case analysis.
As reported above, there was very substantial statistical heterogeneity both in the pooled estimate of effect from all the studies and in the subgroup analyses (I² = 90% for worst case analysis). We considered that this may be explained by the clinical variation in the treatment (operative versus conservative) of the participants between studies. We thus subgrouped the worst case analysis data by operative and conservative management (Figure 5; Analysis 1.5). The effect estimates from studies of participants with operatively treated fractures were substantially heterogenous and the precision of the estimate poor. The majority of the data from participants whose fractures were managed conservatively was consistent, excepting those from one study (Lubbert 2008). Importantly, Lubbert 2008 defined union quite differently from the other studies based upon participants' self‐reports and this may be a reason for the observed heterogeneity in the estimate of effect. Excluding these data from Lubbert 2008 suggested a significant treatment effect due to ultrasound in this subgroup of 202 conservatively managed fractures (SMD ‐1.09, 95% CI ‐1.38 to ‐0.80). However, the test for subgroup differences did not indicate that the findings of the operatively‐ and conservatively‐managed subgroups were statistically significantly different from each other (Chi² = 1.92, df = 1 (P = 0.17), I² = 47.8%).
5.
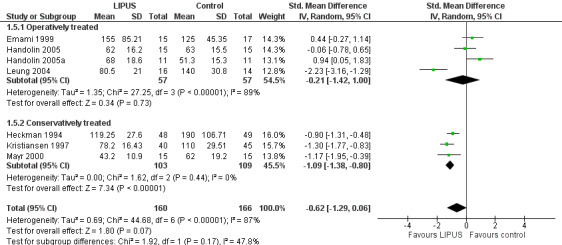
Forest plot of comparison: 1 LIPUS versus control, outcome: 1.5 Time to fracture union (days) subgrouped by operation: worst case analysis
1.5. Analysis.
Comparison 1 LIPUS versus control, Outcome 5 Time to fracture union (days) subgrouped by operation: worst case analysis.
The trial reports of the included studies failed to present adequate data to conduct the two described a priori subgroup analyses in this review. However, Cook 1997 reported a retrospective subgroup analysis split by smoking status of the data from Heckman 1994 and Kristiansen 1997 (see Analysis 1.6). This analysis of 111 fractures show that the effects of LIPUS were similar in both smokers and non‐smokers (test for subgroup differences: Chi² = 0.05, df = 1 (P = 0.83), I² = 0%).
1.6. Analysis.
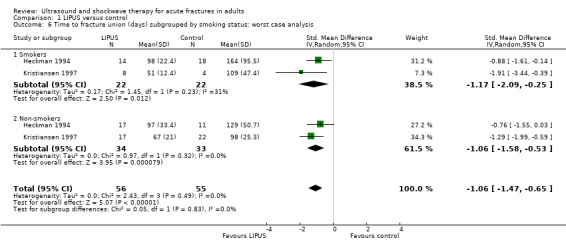
Comparison 1 LIPUS versus control, Outcome 6 Time to fracture union (days) subgrouped by smoking status: worst case analysis.
Secondary outcomes
Delayed union and non‐union
Figure 6 (Analysis 1.7) shows the available data for delayed or non‐union in 333 fractures. The different follow‐up times and definition of this outcome are shown in the footnotes. The available data for all three upper limb fracture trials (203 fractures) indicated that all fractures had eventually healed. Overall, the pooled data from five lower limb studies (130 fractures) showed no significant difference between the treatment and control groups in this outcome (10/168 versus 13/165; RR 0.75; 95% CI 0.24 to 2.28).
6.
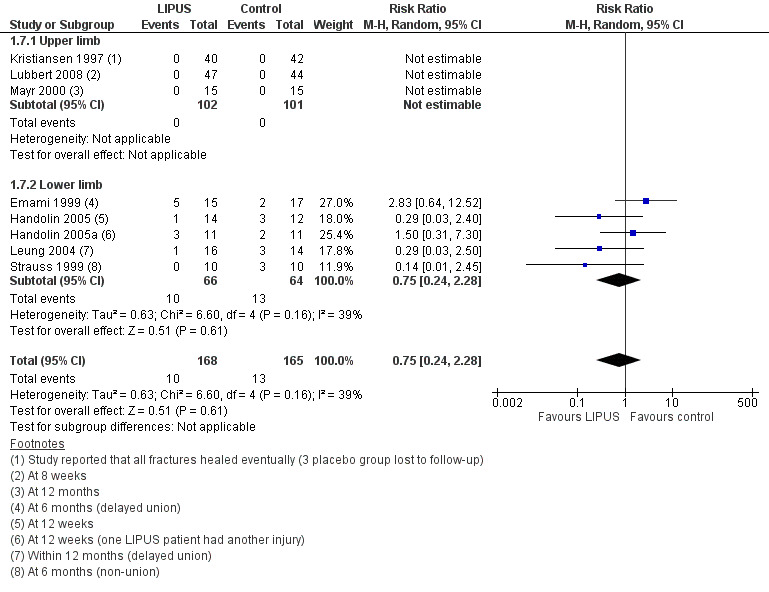
Forest plot of comparison: 1 LIPUS versus control, outcome: 1.7 Delayed or non‐union (as reported analysis)
1.7. Analysis.
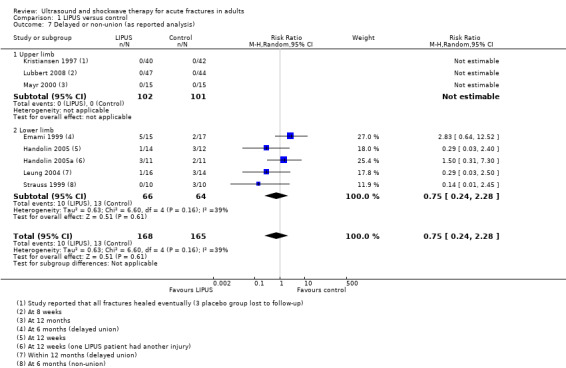
Comparison 1 LIPUS versus control, Outcome 7 Delayed or non‐union (as reported analysis).
Adverse events
Seven studies reported on adverse events (Emami 1999; Handolin 2005; Handolin 2005a; Heckman 1994; Kristiansen 1997; Leung 2004; Lubbert 2008). Emami 1999 reported no difference in the numbers of participants developing compartment syndrome (one in the treatment group versus two in the placebo group) or deep infection (none versus two) or requiring the removal of locking screws (revision dynamisation: two versus one). Several venous thromboembolic events were reported. Handolin 2005 found four deep vein thromboses, three of which were in the placebo group; Handolin 2005a reported one deep vein thrombosis in the placebo group, and one participant suffered a pulmonary embolus in Heckman 1994. Three studies (Heckman 1994; Leung 2004; Lubbert 2008) reported a low incidence of self‐resolving conditions (skin irritation, erythema and swelling), which did not lead to any trial protocol violations, and occurred in both treatment and placebo groups. Kristiansen 1997 reported that there had been no adverse reactions or complications attributable to the device reported during their study.
Pain
Lubbert 2008 reported visual analogue scores for pain from 101 participants (Analysis 1.8). An estimate from both the 'as reported' analysis and 'worst case' analysis showed no significant treatment effect (worst case analysis: MD ‐0.02, 95% CI ‐0.54 to 0.50) (Analysis 1.8).
1.8. Analysis.
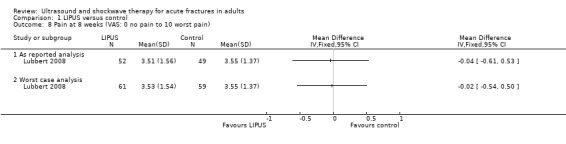
Comparison 1 LIPUS versus control, Outcome 8 Pain at 8 weeks (VAS: 0 no pain to 10 worst pain).
Cost
None of the studies reported a health economics analysis.
Adherence
Several studies reported the recordings from both internal timers, contained within the devices, and participant treatment diaries. Emami 1999 reported good adherence to the trial protocol, with no significant difference between the treatment and placebo groups' usage or diary records, both of which closely matched the protocol requirements (ultrasound: mean 23.4 hours, SD 0.8; placebo: 22.3 hrs SD 1.0; participant diary: mean 24.6 hours). Kristiansen 1997 reported similar findings (ultrasound: mean 62 hours; placebo 64 hours), which compared favourably with the trial protocol requirement. Other studies (Handolin 2005; Heckman 1994) reported adherence less formally but did highlight good participant compliance. For instance, Handolin 2005 reported comparable duration of use of the ultrasound device (mean: 40.7 days versus 39.9 days). Participants of Rue 2004 were administered treatments by trial personnel so that adherence was easily determined. Both LIPUS and control groups missed a similar proportion of treatments, which was less than approximately 20% of all treatments in each group.
Extracorporeal shock wave therapy (ECSW) versus control
ECSW was tested only in Wang 2007, which compared ECSW with no ECSW in 56 participants with 59 fractures of the tibia or femur. Results in this trial were reported for fractures instead of participants; however, it was not possible to correct for the unit of analysis discrepancy.
Primary outcomes
Wang 2007 did not report on functional outcome or time to union.
Secondary outcomes
Delayed union and non‐union
Wang 2007 found there was no significant effect of ECSW on non‐union (all cases involved fractures of the femur) at 12 months (seeAnalysis 2.1: risk ratio (RR) 0.56; 95% CI 0.15 to 2.01). A sensitivity analysis where the fractures of the two excluded participants were assumed not to have united at 12 months gave similar results (RR 0.63, 95% CI 0.21 to 1.93).
2.1. Analysis.
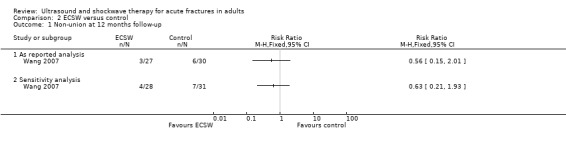
Comparison 2 ECSW versus control, Outcome 1 Non‐union at 12 months follow‐up.
Adverse events
Wang 2007 reported one case of deep infection and osteomyelitis in each group (both patients were excluded from the final analyses) and five cases of superficial infection (2/27 versus 3/30), all of which resolved with antibiotics and wound care. There were no other complications, including those directly related to shockwave treatment.
Pain
Wang 2007 found a clinically small but statistically significant difference in visual analogue scores for pain in favour of ECSW, from both the 'as reported' analysis (MD ‐0.87, 95% CI ‐1.31 to ‐0.43) and the 'worst case' analysis (MD ‐0.80, 95% CI ‐1.23 to ‐0.37) (Analysis 2.2). Similarly, pain scores were significantly lower in the ECSW group at six (1.19 versus 2.47) and 12 months (0.15 versus 0.77).
2.2. Analysis.
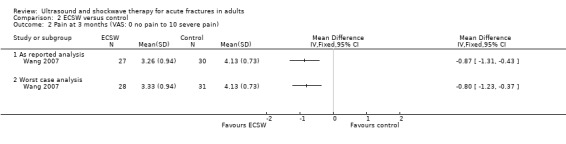
Comparison 2 ECSW versus control, Outcome 2 Pain at 3 months (VAS: 0 no pain to 10 severe pain).
Others
Wang 2007 reported neither measures of cost nor adherence.
Discussion
Summary of main results
The review presented evidence from 11 trials comparing low‐intensity pulsed ultrasound (LIPUS) versus control, and one trial comparing extracorporeal shock wave therapy (ECSW) versus control. We found no trials evaluating high‐intensity focused ultrasound. The included trials form a clinically heterogeneous group of studies, which included participants with a range of acute fractures, treated in a variety of ways. The fractures were complete fractures in 10 trials and stress fractures in two trials.
Low‐intensity pulsed ultrasound versus control
Primary outcomes
Neither of the two studies of complete fractures reporting functional outcomes found a difference between LIPUS compared with placebo control. Pooled results from two studies found that LIPUS had no significant effect on the time to return to training for soldiers or midshipmen with tibial stress fractures.
Data were pooled from eight small studies that reported the time to union of a complete fracture as a primary outcome following LIPUS. While the 'as reported' analysis indicated a significant benefit of LIPUS on time to union, a purposefully conservative 'worst case' analysis showed there was no statistically significant reduction in healing time of fresh fractures treated with LIPUS. However, a potential for greater benefit than harm from LIPUS should not be ruled out and the highly significant heterogeneity in the results indicates this potential may apply for some categories of patients. The two prespecified subgroup analyses by upper and lower limb fractures and smoking status did not show any differences between the subgroups. An additional subgroup analysis, comparing conservatively with surgically treated fractures, raised the possibility that LIPUS may be more effective in reducing healing time in conservatively managed fractures. However, while the results from the subset of the three trials of conservatively treated fractures (that measured time to union radiographically) were homogeneous, the test for differences between the conservative and surgical treatment subgroups was not statistically significantly different.
Secondary outcomes
Several studies reported the proportion of participants experiencing delayed union or non‐union. However, the reporting, measurement and definition of these outcomes varied. We found no significant difference between the treatment and control groups in the pooled result.
Importantly, the compliance with LIPUS treatment was found to be good and the adverse effects directly associated with its use (or associated devices) were found to be few and minor. Thus this review provides reasonably good evidence that such a treatment would be acceptable to patients in general clinical practice.
Only one study reported pain scores using visual analogue scales, finding no difference between groups at eight weeks. There were no data on costs.
Extracorporeal shock wave therapy (ECSW) versus control
The small quasi‐randomised trial evaluating ECSW for tibia and femur fractures did not report on functional outcomes nor time to union. It found no significant improvement in the proportion of people achieving union following ECSW at 12 months. There were, however, clinically small but statistically significant differences in the visual analogue scores for pain in favour of ECSW at three‐, six‐ and 12‐month follow‐ups. The only reported complication was infection, with no significant differences between the two groups.
Overall completeness and applicability of evidence
Completeness of the evidence
This review includes data from 12 studies, conducted in seven countries. Eleven of the studies tested the use of ultrasound for acute fractures in a total of 566 adults: nine studies concerned the treatment of complete fractures and two (Rue 2004 and Yadav 2008) reported the outcomes in participants with stress fractures. The evidence for ECSW therapy was restricted to that from one small study involving 56 adults (Wang 2007). We found no trials evaluating high‐intensity focused ultrasound. While the total population represents a small proportion of the acute fractures occurring annually, the fractures included in the studies are some of those characterised by higher incidences of delayed and non‐union.
The general quality of trial reporting was poor. Only four of the studies reported one of our primary outcomes: patient‐reported functional outcome measures or return to limb function or work. There was a considerable proportion of data missing for time to union, the other primary outcome of this review. This may, in part, reflect the difficulties in measuring this outcome. There was little evidence of adverse effects, although it is unclear whether this is due to under‐reporting or a positive safety profile for the intervention. Participant adherence to the treatment protocols was frequently commented upon but inconsistently reported.
Application of the evidence to current practice
The included studies reported the use of ultrasound in a wide variety of settings and participants. Most settings were typical hospital settings, such as in Europe and the United States. The participants included those with fractures of the upper or lower limbs, which were treated either surgically or conservatively. Although these populations were highly heterogeneous, they are still representative of the type of fracture populations, generally at higher risk of delayed healing and non‐union, for which treatment adjuncts might be considered.
Clinical practice varies worldwide but LIPUS remains a specialist treatment usually only considered for, or administered to, patients with fractures at risk of delayed union or non‐union. The evidence from this review would not seem to encourage the wider clinical application of LIPUS at this time.
Quality of the evidence
Sources of systematic error
The quality of reporting of the included studies was generally poor, with insufficient details to judge risk of bias. All bar two studies were randomised, but in only three could we assign a low risk of selection bias relating to allocation concealment. Most trials were blinded through the use of sham devices but, even so, the lack of identical devices in Leung 2004 put this quasi‐randomised trial at high risk of performance and detection bias. Overall, both quasi‐randomised studies were at high risk of bias, reflecting also high risks of selection and attrition bias.
All of the studies were small and therefore the likelihood of an imbalance in the baseline characteristics is increased. However, there were often insufficient data, in particular relating to smoking status, to judge whether there were major imbalances between the treatment and control groups in baseline characteristics. Only one trial was considered at low risk for this item.
There was also a considerable proportion of data missing. Several of the lead authors of the studies were contacted during this review and each reported that they had struggled to maintain participant compliance with the demanding follow‐up schedule required to determine time to union. We have chosen to handle the missing data in such a way to make a conservative estimate of any treatment effect.
Other sources of error
The individual studies reported here are small and underpowered. This is reflected in the imprecision and heterogeneity of the study estimates of treatment effects. The largest pool of data concerned the time to fracture union. Reported data from 355 participants were available to determine the effect of LIPUS on the time to fracture union. This might be sufficient to detect a large, clinically relevant effect, although the problem of missing data cannot be ignored. The number of participants included in the other reported pooled analyses is lower, and therefore the conclusions about these important outcomes are necessarily even more tentative.
The primary outcome of fracture healing is variably defined in the literature. As anticipated, we found that studies defined healing clinically and radiographically. This reflects the difficulty that is inherent in the assessment of union. The choice of measurement tool and the timing of assessments of union varied between studies. Radiographic evidence of union often takes longer to appear than assessment of fracture union by clinical means, e.g. physical examination. Fracture union can also be difficult to determine from plain radiographs. None of the included studies used a panel of independent and blinded radiologists or orthopaedic surgeons to assess radiographic union for plain radiographs. We note, however, that a blinded assessment by two independent radiologists and one hand surgeon of CT scans was performed in Mayr 2000.
Potential biases in the review process
None of the authors of this review have been involved in any of the included trials or have any commercial or other conflict of interest.
We predominantly searched the published literature. Despite efforts to contact experts, we have not been able to access any unpublished data. Given that trial registration was limited during the period over which most of these studies were conducted, it is possible that commercially sponsored negative trials were not published. We have also not searched conference abstracts. It is therefore possible that there are other trials and trial data that have not been included in this review. It is not possible to estimate the potential effects of these on the review findings. However, some reassurance can be gained from the finding that a recent systematic review by Busse 2009 found no additional studies that fulfilled our inclusion criteria.
There was significant heterogeneity in the meta‐analyses. We conducted a post hoc sensitivity analysis to try to explore the sources of heterogeneity between the studies. We made these decisions through consensus with a view to dealing with the available data in a pragmatic manner. However, the decisions regarding data pooling were necessarily subjective and may be a cause of bias. The rational for our conjecture of a difference between conservatively and operatively (where rigid fixation methods are used) treated fractures is that in the former, ultrasound might cause micromotion at the fracture site leading to accelerated union whereas, in the latter, such micromotion might be impossible and any benefit of ultrasound lost. This hypothesis was not confirmed by the data available for this review.
We have attempted to contact the authors of included studies to retrieve missing data with mixed success. There may be a systematic difference between those authors who we have been able to contact and those that we have not.
Agreements and disagreements with other studies or reviews
The findings of this review are in keeping with a systematic review on the effects of LIPUS for fractures by Busse 2009. Busse 2009 also included trials testing the effects of LIPUS on non‐union (one trial) and 'distraction osteogenesis' (three trials). In keeping with our review, Busse 2009 observed the conflicting results from the included trials and concluded that the evidence, while promising, was not enough to establish the role of LIPUS in the management of fractures.
Authors' conclusions
Implications for practice.
This review highlights the limitations of the available evidence on therapeutic ultrasound for acute fractures in adults. Currently, the best assessment of the clinical effectiveness of LIPUS for complete or stress fractures in adults does not support the routine use of this intervention in clinical practice.
Implications for research.
The identification of two currently unpublished trials emphasises the importance of both the timely publication of the results of these trials and the regular updating of this review. Both trials involve surgically treated tibial fractures. While conclusive evidence on time to union may result from the largest trial (NCT00667849) should it have recruited 500 participants, it seems that the opportunity to collect patient‐reported outcome measures was overlooked. Any future research, which should involve secure randomisation and placebo controls, on the use of ultrasound for acute fractures should focus on patient‐reported outcome measures to determine if the possible benefit of ultrasound in terms of fracture healing translates into a tangible benefit to patients. Trials should conform to reporting standards as set out in the CONSORT statement, including reporting the results of all trial participants (Boutron 2008).
Feedback
Issues concerning choice of analysis, 12 December 2014
Summary
Comment: This review contains several errors, some of them serious. As a result, the treatment effect calculated for low‐intensity pulsed ultrasound (LIPUS) is inconsistent with the real effect shown in the reported data. 1. In an effort to eliminate bias, the authors rejected the results of published fresh‐fracture studies on LIPUS. Instead, they re‐analyzed data from each paper based on their own criteria. As part of this re‐analysis, they inserted outcomes data for patients lost to follow‐up (‘Data collection and analysis’; ‘Dealing with missing data’). This might have been acceptable if the authors had treated patients equally in the active and placebo groups, but they did not. In the LIPUS group, patients lost to follow‐up were assigned a time‐to‐heal equal to two standard deviations greater than the mean for that group. In the placebo group, however, missing patients were assumed to have healed normally and were assigned a time‐to‐heal equal to the group mean. The authors called this a “worst case” analysis, and it effectively increased heal times in the LIPUS group by an average of 9%. Based on the skewed data, it was concluded that LIPUS and control groups were not significantly different (Abstract). This conclusion is not supported by the actual data. When Griffin et al. analyzed the literature without unequal imputation of heal rates, LIPUS was shown to significantly accelerate fracture healing (text and Figure 3; p=0.03). However, this “as reported” analysis was neither included in the abstract nor discussed in detail in the text. The authors also asserted, incorrectly, that the results of the “worst case” and “as reported” analyses were similar (‘Risk of bias in included studies’; ‘Incomplete outcome data’). Both the biased analysis and its burial deep within the text are concerning. Unless readers purchase and closely review the full text, they will remain unaware that the authors’ “worst case” analysis changed the data and, indeed, the main finding of the study. 2. Other errors in the manuscript include: a) Incomprehension of normal fracture‐healing heterogeneity. It is universally recognized that different bones heal at different rates. Thus, variation is unavoidable when comparing healing times at different fracture locations. The broad generalizations applied by the authors to probe this variation, such as upper limb vs. lower, or smoker vs. nonsmoker, are inadequate. b) Mischaracterization of the normal heterogeneity in healing by bone. The authors misinterpreted the inherent heterogeneity of fracture healing as evidence of bias, which was then used to justify the rejection of “as reported” heal‐rate data from the literature (‘Effects of interventions’). c) Unacknowledged, unsupported, a priori assumptions that all forms of ultrasound are comparable, and all low‐intensity ultrasound is equivalent. d) Unmerited inclusion of extracorporeal shockwave treatment (1 study) and high‐intensity focused ultrasound (0 studies) in the review. e) Inappropriate analysis of the evidence for delayed union and nonunion (Figure 6). By design, the authors’ search criteria only identified acute‐fracture studies (‘Methods’: ‘Types of participants’). Having excluded delayed‐union and nonunion studies at the start, no valid analysis was possible for this clinical population. f) Inappropriate inclusion of the study by Lubbert et al., which lacked radiographic outcome data, in analyses of time to radiographic union (Figures 3 and 4). g) Unspecified criteria for the weighting of results from different studies (Figures 3‐6). This practice was not discussed in the methods or body of the paper, and no explanation or algorithm was presented. The given weights were not based on the number of patients per study or other obvious criteria. h) Unrealistic criteria for radiological review. In 6 of 7 LIPUS studies (Heckman 1994, Kristiansen 1997, Emami 1999, Mayr 2000, Leung 2004, Handolin 2005a), radiographs were assessed by multiple, blinded reviewers. In 5 of 7 studies, the review team included both surgeons and radiologists. The authors’ criticism that “none of the included studies used a panel of independent radiologists to assess radiographic union,” (‘Discussion’; ‘Quality of the evidence’) represents an unrealistic standard that is not demonstrably superior to the joint efforts of surgeons and radiologists. The fact that radiographs in Mayr et al. 2000 actually were reviewed by a panel of independent, blinded radiologists, suggests an inadequate review of the literature. In light of these serious issues, we recommend withdrawal of the current review and publication of a revised version in which these errors are corrected. Conflict of interest statement: Both authors are affiliated with Bioventus LLC*, Durham, NC.
* Bioventus is the manufacturer of Exogen® device, which was tested in several trials in this review.
Reply
We thank Drs Heeckt and Brodie for their interest and careful consideration of our Cochrane Review.
1. We agree that there are multiple ways to report the pooled data and that our ‘worst case’ analysis sets a higher standard than an ‘as reported’ analysis. The ‘worst case’ analysis is however important so that readers can discern one possible and conservative interpretation that is consistent with the data. In this case, it shows that the data could be consistent with no treatment effect if the missing data in each study were not missing at random. In essence, the variation in estimates between the ‘worst case’ and ‘as reported’ analyses simply highlights the critical importance of good follow‐up in clinical studies. Our approach to handling these types of data issues, and their effect on study outcomes, are not novel.(1) A fuller discussion of the effects and means of handling missing data can be found in the Cochrane Handbook.(2)
We agree that it is also important to describe the ‘as reported’ analysis and we already do this in the ‘Effects of interventions’ section as well as, as you point out, presenting the data in Figure 3 and Analysis 1.3.
The statement that reads ‘the proportion of missing data was sufficiently low, that “as reported” and “worst case” analyses were similar’ is incomplete. We recognise that whilst the effect is significant in the ‘as reported’ analysis, the effect estimate and confidence intervals are approximately comparable. We have removed this statement to avoid any confusion.
Both the methods and the abstract were clear that our review was designed to report, and draw conclusions from, the ‘worst case’ analysis. Importantly, the abstract also stated that the likely impact of this design was to give “more conservative estimates of treatment effects for time to fracture union”. We believe that this should be sufficient to alert readers to look deeper within the article for a more in depth analysis.
2. With regards to the other observations:
a) We certainly agree that the rate of fracture healing varies between anatomical sites. However, this should not have biased the within‐study estimates of treatment effect because participants within each trial were drawn from the same fracture populations. The intervention and control groups should therefore include a similar number of tibial, fifth metatarsal, scaphoid fractures etc. We recognise that the effect may not necessarily be linearly related to control healing times, and we sought to explore the observed heterogeneity between studies with some pre‐specified subgroup analyses. Whilst the categories for these subgroups were coarsely defined the source studies did not report baseline demographics in sufficient detail to facilitate a more in‐depth analysis.
b) As discussed earlier our preference for a ‘worst case’ analysis is due to the important possibility of bias from attrition. Heterogeneity is not relevant here. Our division of the fractures into upper and lower limb subgroups was a means to try to explore possible causes for the observed heterogeneity.
c) It is certainly possible that different forms of low‐intensity ultrasound vary in effectiveness. However, this raises a more general criticism of pooling data from multiple studies. This is why, for example, we determined a priori to analyse LIPUS, HIFUS, and ECSW separately.
d) It is not clear why the inclusion of HIFUS and ECSW should be ‘unmerited’ as the review was designed to consider all ultrasound technologies, not simply LIPUS. In any event, as you point out, there was only one ECSW trial and none investigating HIFUS. The ECSW trial data was analysed, reported, and discussed separately from the data concerning LIPUS.
e) We agree that it would have been improper for us to draw conclusions about the role of ultrasound for treating delayed and/or non‐unions. This is because our review only included studies of patients with acute fractures. However, non‐union is an important outcome of acute fracture and it was appropriate for us to comment on whether ultrasound reduced the risk of delayed and/or non‐union in this population.
f) We recognise the difficulty of including data from Lubbert 2008 in pooled analyses from other studies. We defined union a priori as radiographic or clinical or both (see types of outcome measures). We have modified the figure legends (Figures 3 and 4) to more accurately reflect our pre‐specified methodology.
g) The weighting of studies is a feature of the Mantel‐Haenszel method of producing a pooled estimate of the effect. This is the default method for pooling data within Cochrane Reviews where data are sparse due to small study size or low event rates or both. A fuller discussion can be found in the Cochrane Handbook.(3)
h) We agree that a ‘panel of independent radiologists’ is not in itself demonstrably superior to surgeons and radiologists. However, radiologists are more likely to be more removed from the study in other ways, e.g. not also performing the clinical assessment of fracture union in the same patient.
The important details are really whether appropriate assessors (surgeons or radiologists) were multiple, independent, and blinded. We do not think that this would be an unfairly high standard against which to hold a modern randomised controlled trial. These three standards were not met in a number of cases, e.g. Leung 2004 did not blind assessors and Enami 1999 did not make pooled results available for analysis.
We also recognise that Mayr 2000 made efforts to assess outcome more formally: blinded, independent radiologists and a surgeon assessed CTs for fracture union. We have added further detail to the Characteristics of Included Studies table for Mayr 2000 and expanded our discussion to highlight this aspect of the study.
References
1. Akl EA, Briel M, You JJ, Sun X, Johnston BC, Busse JW et al. Potential impact on estimated treatment effects of information lost to follow‐up in randomised controlled trials (LOST‐IT): systematic review. BMJ 2012;344:e2809
2. Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
3. Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Contributors
Comment from Peter Heeckt and Christopher Brodie, Bioventus LLC Reply from Xavier Griffin, Contact Author, on behalf of the review team, and Helen Handoll, acting Feedback Editor, Cochrane Bone, Joint and Muscle Trauma Group
What's new
| Date | Event | Description |
|---|---|---|
| 22 January 2015 | Feedback has been incorporated | Prompted by feedback, received 12 December 2014, minor amendments made as detailed in the reply (Feedback 1). |
History
Protocol first published: Issue 7, 2010 Review first published: Issue 2, 2012
| Date | Event | Description |
|---|---|---|
| 2 June 2014 | New search has been performed | New search. No additional studies included. Since the original review, one potentially eligible study has been completed and is awaiting publication. Another is completed but the data are not yet available for analysis. Review edited to provide more information about included trials. |
| 2 June 2014 | New citation required but conclusions have not changed | No additional studies included and no changes made to the conclusions. |
Acknowledgements
We would like to thank the trial authors Jason Busse, Joan McCabe, James Heckman, Pieter Lubbert and Julia Seifert, each of whom responded positively to our requests for further information about their studies. We would also like to thank Anette Bluemle and Juliane Ried from the German Cochrane Centre for their help in translation of one of the studies.
The authors would like to thank Prof William Gillespie, Dr Helen Handoll, Dr James D Heckman, Prof Peter Herbison and Dr Vicki Livingstone for valuable comments on the protocol and review. We also acknowledge the help of Mrs Lesley Gillespie and Dr Joanne Elliott in developing the search strategies, and the editorial base staff Lindsey Elstub and Laura MacDonald for their help in the processes of writing the protocol and review. We would also thank Dr Nick Smith for previous contributions to earlier versions of the review.
Appendices
Appendix 1. Search strategies (2011 to June 2014)
The Cochrane Central Register of Controlled Trials (Wiley Online Library)
#1 MeSH descriptor: [Ultrasonics] this term only (256) #2 MeSH descriptor: [Ultrasonic Therapy] this term only (695) #3 MeSH descriptor: [High‐Energy Shock Waves] this term only (122) #4 (ultraso* or LIPUS or HIPUS or HIFU* or shock wave* or shockwave* or ESWT):ti,ab 13436 #5 #1 or #2 or #3 or #4 (13598) #6 MeSH descriptor: [Fractures, Bone] explode all trees (4104) #7 MeSH descriptor: [Fracture Healing] this term only (401) #8 MeSH descriptor: [Bone Remodeling] explode all trees (2005) #9 MeSH descriptor: [Bony Callus] this term only (19) #10 fractur*:ti,ab (8747) #11 #6 or #7 or #8 or #9 or #10 (10986) #12 #5 and #11 in Trials (239)
MEDLINE (OvidSP interface)
1 Ultrasonics/ or Ultrasonic Therapy/ or High‐Energy Shock Waves/ (28543) 2 (ultraso$ or LIPUS or HIPUS or HIFU$ or shock wave$ or shockwave$ or ESWT).tw. (234899) 3 or/1‐2 (242363) 4 exp Fractures, Bone/ or Fracture Healing/ or exp Bone Remodeling/ or Bony Callus/ (183256) 5 fractur$.tw. (158314) 6 or/4‐5 (238609) 7 and/3,6 (3761) 8 (dental or tooth or oral).mp. (833436) 9 7 not 8 (3494) 10 Randomized controlled trial.pt. (373718) 11 Controlled clinical trial.pt. (88349) 12 randomized.ab. (272196) 13 placebo.ab. (146010) 14 Drug therapy.fs. (1699810) 15 randomly.ab. (193278) 16 trial.ab. (282269) 17 groups.ab. (1242462) 18 or/10‐17 (3190172) 19 exp Animals/ not Humans/ (3940677) 20 18 not 19 (2714563) 21 and/9,20 (567) 22 (201111* or 201112* or 2012* or 2013* or 2014*).ed. (2036541) 23 21 and 22 (76)
EMBASE (OvidSP interface)
1 Ultrasound/ or Ultrasound Therapy/ or Low Intensity Pulsed Ultrasound/ or Extracorporeal Lithotripsy/ (115795) 2 (ultraso$ or LIPUS or HIPUS or HIFU* or shock wave$ or shockwave$ or ESWT).tw. (331812) 3 or/1‐2 (358801) 4 exp Fracture/ or Fracture Treatment/ or Bone Remodeling/ (207418) 5 fractur$.tw. (194826) 6 or/4‐5 (265145) 7 and/3,6 (5567) 8 (dental or tooth or oral).mp. (1166475) 9 7 not 8 (5245) 10 Clinical trial/ (831266) 11 Randomized controlled trial/ (342545) 12 Randomization/ (62132) 13 Single blind procedure/ (18308) 14 Double blind procedure/ (113378) 15 Crossover procedure/ (39030) 16 Placebo/ (239992) 17 Randomi?ed controlled trial$.tw. (98361) 18 Rct.tw. (13831) 19 Random allocation.tw. (1303) 20 Randomly allocated.tw. (20116) 21 Allocated randomly.tw. (1918) 22 (allocated adj2 random).tw. (712) 23 Single blind$.tw. (14205) 24 Double blind$.tw. (140073) 25 ((treble or triple) adj blind$).tw. (365) 26 Placebo$.tw. (196721) 27 Prospective study/ (251129) 28 or/10‐27 (1355532) 29 Case study/ (26024) 30 Case report.tw. (257343) 31 Abstract report/ or Letter/ (890113) 32 or/29‐31 (1167900) 33 28 not 32 (1318017) 34 limit 33 to human (1208158) 35 and/9,34 (548) 36 (2011* or 2012* or 2013* or 2014*).em. (4525121) 37 35 and 36 (153)
Data and analyses
Comparison 1. LIPUS versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to return to work complete fractures (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Resumption of work (as reported) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Resumption of work (worst case scenario) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Time to return to training/duty after stress fracture (days): as reported analysis (days) | 2 | 93 | Mean Difference (IV, Random, 95% CI) | ‐8.55 [‐22.71, 5.61] |
| 3 Time to fracture union (days): 'as reported' analysis | 8 | 355 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.31, ‐0.07] |
| 3.1 Upper limb | 3 | 183 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐2.03, 0.17] |
| 3.2 Lower limb | 5 | 172 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.44, 0.35] |
| 4 Time to fracture union (days): worst case analysis | 8 | 446 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.14, 0.20] |
| 4.1 Upper limb | 3 | 235 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.93, 0.60] |
| 4.2 Lower limb | 5 | 211 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐1.27, 0.56] |
| 5 Time to fracture union (days) subgrouped by operation: worst case analysis | 7 | 326 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.29, 0.06] |
| 5.1 Operatively treated | 4 | 114 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐1.42, 1.00] |
| 5.2 Conservatively treated | 3 | 212 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.38, ‐0.80] |
| 6 Time to fracture union (days) subgrouped by smoking status: worst case analysis | 2 | 111 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐1.47, ‐0.65] |
| 6.1 Smokers | 2 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐2.09, ‐0.25] |
| 6.2 Non‐smokers | 2 | 67 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐1.58, ‐0.53] |
| 7 Delayed or non‐union (as reported analysis) | 8 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.24, 2.28] |
| 7.1 Upper limb | 3 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Lower limb | 5 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.24, 2.28] |
| 8 Pain at 8 weeks (VAS: 0 no pain to 10 worst pain) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 As reported analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Worst case analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. ECSW versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐union at 12 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 As reported analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sensitivity analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pain at 3 months (VAS: 0 no pain to 10 severe pain) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 As reported analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Worst case analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Emami 1999.
| Methods | Randomised, placebo‐controlled study. | |
| Participants | Setting: Uppsala University Hospital, Sweden. Size: 30 participants in total, with 15 in each arm. Baseline characteristics: mean (range) age 39 years (19 to 73), 21 males and 9 females. Inclusion criteria: patients aged over 16 years with a closed or Gustillo and Anderson grade I open fracture of the tibial diaphysis treated with closed reduction and fixation with a reamed, intra‐medullary, locked nail. Exclusion criteria: history of alcohol or drug dependency; current steroid, anticoagulant, NSAID or bisphosphonate use; past medical history of neuropathy, arthritis, malignant disease; radiographs that showed severe comminution or open physes. |
|
| Interventions | Participants underwent closed reduction and reamed, intramedullary nailing of the fracture. Surgery was performed by one of six experienced trauma surgeons. The fracture site was marked with a permanent skin marker.
Test: ultrasound treatment was started within three days of fixation and was continued for 75 days. The treatment consisted of one 20‐minute period daily with a maximum exposure of 25 hours. The transducer head was coupled to the skin with a standard gel. The ultrasound signal was composed of a 200 µs burst of 1.5 MHz sine waves, with a repetition rate of 1 kHz and a spatial average intensity of 30 mW/cm². Control: sham ultrasound treatment was started within three days of fixation and was continued for 75 days. The treatment consisted of one 20‐minute period daily with a maximum exposure of 25 hours. The sham device was a deactivated, identical model to that provided to the test group. |
|
| Outcomes | Follow‐up schedule: every third week until union. Additional follow‐up at 26 and 52 weeks irrespective of union status. Primary: time to radiographic union. Secondary: time to first radiographic evidence of callus, proportion of fractures united at six months, adverse events. |
|
| Notes | Outcomes were assessed by a single‐blinded radiologist and an orthopaedic surgeon independently, but were not pooled. The data used in this review are derived from the single independent radiologist. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was ... randomized" Comment: No specific report of how the sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | The allocation method was not reported. |
| Blinding (performance bias and detection bias) Objective measures | Low risk | Quotes: "The codes were not broken for any device until the radiographic reviews for all patients had been completed." "...devices were identical in every way..." Comment: All measures were adequately blinded. |
| Incomplete outcome data (attrition bias) Objective measures | Unclear risk | Quote: "In one patient, it became obvious during the course of the study that he did not fulfil the inclusion/exclusion criteria." Comment: No data were reported for this single participant and he was excluded from the analysis. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | No other bias detected. |
| Selection bias (imbalance in baseline characteristics) | Low risk | Baseline data for age, sex and smoking status are reported and show a balanced distribution of these confounders between groups. |
Handolin 2005.
| Methods | Randomised, placebo‐controlled study. | |
| Participants | Setting: Helsinki University Central Hospital, Finland. Size: 30 patients in total, 15 in each arm. Baseline characteristics: mean age 41.4 years (5 male/10 female) in intervention group and 39.4 years (8 male/7 female) in the control group. Inclusion criteria: patients aged between 18 and 65 years with displaced Weber B fractures of the lateral malleolus. Exclusion criteria: widening of the distal tibiofibular joint; open fracture; inability to co‐operate with the requirements of the trial. |
|
| Interventions | Participants underwent open reduction and internal fixation with a 4.5 mm self‐reinforced poly‐L‐lactic acid screw. Surgery was carried out by one of two surgeons. The fracture was approached through a lateral incision. Post‐operatively the ankle was immobilised for six weeks with a removable Soft Cast brace. Partial weight bearing was allowed at two weeks and full weight bearing at four weeks. Test: participants self‐administered daily ultrasound treatment for 20 minutes from the 3rd to 9th post‐operative weeks directly over the fracture marked with an intraoperatively placed marker. Appropriate contact between the probe and the skin was maintained with standard ultrasound coupling gel. The ultrasound signal was composed of a 200 µs burst of 1.5 MHz sine waves, with a repetition rate of 1 kHz and a spatial average intensity of 30 mW/cm². Control: participants in the control group were given a similar treatment regimen but had an externally similar sham machine instead. |
|
| Outcomes | Follow‐up schedule was at 2, 6, 9 and 12 weeks and, in a separate publication, 18 months. At 18 months, the clinical outcome was assessed using the Olerud‐Molander scoring as well as clinical examination; this was reported in a separate article for 16 (8 versus 8) participants. Plain radiographic assessment at 2, 6, 9 and 12 weeks and at 18 months. Multi detector computed tomography (MDCT) at 18 months and dual‐energy X‐ray absorptiometry (DEXA) scan post operatively and at 18 months. |
|
| Notes | Based on overlapping, but not matching, dates of recruitment we have assumed that a publication (Handolin 2005b) reporting 18 month results for 16 participants is a long‐term follow‐up of this trial. These reports share a common methodology and reporting framework. Efforts to contact the authors were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...prospective, randomised ... study." Comment: The method of sequence generation is not reported. |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment is not reported |
| Blinding (performance bias and detection bias) Objective measures | Low risk | Quote: " double blind; half of the devices were active ... half were sham." Comment: Likely to be the same device but placebo devices were deactivated. |
| Incomplete outcome data (attrition bias) Objective measures | High risk | All outcome data reported up to 12 weeks, but data from only 16 participants reported at 18 months |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | It is not reported how the radiographic outcomes were assessed |
| Selection bias (imbalance in baseline characteristics) | Unclear risk | Age and sex similarly distributed, but smoking status not reported |
Handolin 2005a.
| Methods | Randomised, placebo‐controlled study | |
| Participants | Setting: Helsinki Unversity Central Hospital, Finland. Size: 22 patients, 11 in each arm. Baseline characteristics: mean (range) age 37.5 years (18 to 54), 9 males and 2 females in intervention group. Mean (range) age 45.5 years (26 to 59), 6 males and 5 females in the control group. Inclusion criteria: patients aged between 18 and 65 years with displaced Weber B fractures of the lateral malleolus. Exclusion criteria: widening of the distal tibiofibular joint; open fracture; inability to co‐operate with the requirements of the trial. |
|
| Interventions | Participants underwent open reduction and internal fixation with a 4.5 mm self‐reinforced poly‐L‐lactic acid screw. Surgery was carried out by one of two surgeons. The fracture was approached through a lateral incision. Post‐operatively the ankle was immobilised for six weeks with a removable Soft Cast brace. Partial weight bearing was allowed at two weeks and full weight bearing at four weeks. Test: participants self‐administered daily ultrasound treatment for 20 minutes from the third to ninth post‐operative weeks directly over the fracture marked with an intra‐operatively placed marker. Appropriate contact between the probe and the skin was maintained with standard ultrasound coupling gel. The ultrasound signal was composed of a 200 µs burst of 1.5 MHz sine waves, with a repetition rate of 1 kHz and a spatial average intensity of 30 mW/cm². Control: participants in the control group were given a similar treatment regimen but had an externally similar sham machine instead. |
|
| Outcomes | Fracture healing was assessed by anterior and lateral radiographs taken immediately and at 2, 6, 9 and 12 weeks postoperatively In addition, fracture healing was assessed by multiplanar computed tomography and 2 and 9 weeks postoperatively |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "..prospective, randomized, double‐blind and placebo controlled study". No comment on sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients were randomly provided with either an active or sham ultrasound device in a double‐blind manner". |
| Blinding (performance bias and detection bias) Objective measures | Unclear risk | Quote: "The patients were randomly provided with either an active or sham ultrasound device in a double‐blind manner". Comment: Likely to be the same device but placebo devices were deactivated. |
| Incomplete outcome data (attrition bias) Objective measures | Low risk | "One patient was excluded... because of a new injury" but the group to which he was randomised is unreported |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No additional biases identified |
| Selection bias (imbalance in baseline characteristics) | Unclear risk | Smoking status not reported |
Heckman 1994.
| Methods | Randomised, placebo‐controlled study | |
| Participants | Setting: University of Texas Health Science Centre, USA. Size: 97 patients were enrolled. Of the 48 patients in the test group, 11 violated the protocol and 4 were lost to follow‐up, leaving 33 patients completing the study. Of the 49 patients in the control group, 6 violated the protocol and 9 were lost to follow‐up, leaving 34 patients completing the study. Baseline characteristics: mean age was 36 years, with 25 males and 8 females in the intervention group, and mean age 31 years with 29 males to 5 females in the control group. Inclusion criteria: skeletally mature men and non‐pregnant women aged less than 76 years with closed or grade I open, transverse or short oblique/spiral, fractures of the tibial diaphysis that could be treated with closed reduction and cast immobilisation. Exclusion criteria: post‐reduction findings of long oblique/spiral fracture, length of fracture line greater than twice the diameter of the diaphysis; fracture displacement greater than 50%; fracture gap greater than 0.5 cm or persistent shortening; persistent angulation greater than 10 degrees; metaphyseal fracture; large butterfly fragment; pathological fracture; comminution; participant inability to comply with trial procedures; current prescription of NSAID, calcium channel blockers, bisphosphonates; history of thrombophlebitis, vascular insufficiency, alcoholism or nutritional deficiency. |
|
| Interventions | Participants were treated with closed reduction and above‐knee casting. An alignment window was placed in the cast at the level of the fracture over the antero‐medial aspect of the leg. Reduction of the casting to a below‐knee cast, any subsequent splintage and weight bearing status was at the discretion of the clinician. Test: participants underwent ultrasound treatment for 20 minutes each day from the second to twentieth week, or earlier if the clinician believed there was adequate evidence of union. The ultrasound signal was composed of a 200 µs burst of 1.5 MHz sine waves, with a repetition rate of 1 kHz and a spatial average intensity of 30 mW/cm². Control: participants in the control group were given a similar treatment regimen but had an externally similar sham machine instead. |
|
| Outcomes | Follow‐up schedule: plain radiographs at 4, 6, 8, 10, 12, 14, 20, 33 and 52 weeks. Clinical examination at times of cast change and at the time of union. Outcomes: time to combined radiographic and clinical union. |
|
| Notes | The weight bearing status of the patients was strictly described initially but subsequently handed over to the discretion of the treating clinician part way through the trial. It was confirmed in personal communication with James Heckman that there was no time to union data on participants who violated protocol. Cook 1997 describes a subgroup analysis of the study by Heckman 1994. Smoking status was collected prospectively during the study for half the participants and retrospectively for the other half. There were 33 participants in the active group and 34 in the control group. These numbers correspond with the numbers of participants that successfully completed the study by Heckman 1994. Of these smoking status was not determined in 7 participants due to loss to follow‐up. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...predetermined computer generated code." Comment: Likely to have been a robust method. |
| Allocation concealment (selection bias) | Low risk | Quote: "...the patients were randomized, in groups of four, at each study site..." Comment: It is likely that the sequence was held centrally and allocations were given to the distant study centres. |
| Blinding (performance bias and detection bias) Objective measures | Low risk | Quote: "The active and placebo devices were identical in every way..." Comment: Likely to have been a robust method. |
| Incomplete outcome data (attrition bias) Objective measures | Unclear risk | Quote: "...patients who adhered to the study protocol ... inferences were drawn" Only data from 67 fractures were presented, which represents a loss to follow‐up of 31%. (From JDH: 13 lost to follow‐up, 17 did not present in a timely manner so only certainty is ultimate successful union, no time to event data available.) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | High risk | Quote: "Ninety‐six patients, who had ... ninety‐seven fractures..." Comment: Per protocol analysis only. Also, there was no adjustment for recruiting related fractures |
| Selection bias (imbalance in baseline characteristics) | Unclear risk | Smoking status is not reported as part of the baseline characteristics of the participants. |
Kristiansen 1997.
| Methods | Randomised, placebo‐controlled study | |
| Participants | Setting: multicentre trial, USA. Size: a total of 85 fractures in 83 patients. Of the 40 fractures in the test group, there were 10 withdrawn, leaving 30. Of the 45 fractures in the control group, 3 were lost to follow‐up and 11 were withdrawn, leaving 31. Baseline characteristics: there were 6 males and 24 females in the intervention group and 4 males and 27 females in the control group. Inclusion: men and non‐pregnant women who were at least 20 years old, who had closed dorsally angulated metaphyseal fractures of the distal radius. Exclusion: fracture extending beyond 4 cm proximally from the tip of the radial styloid, failure to satisfactorily reduce closed and immobilise in a below elbow cast, requirement for additional reduction after ultrasound treatment had begun, associated fracture of the ulnar shaft, current prescription of steroids or anticoagulant, any medical history of thrombophlebitis or vascular insuffiencey of the upper limb, current nutritional deficiency or alcohol dependency. |
|
| Interventions | Patients underwent closed reduction and immobilisation of the limb in a cast with volar flexion and ulnar deviation. A window was created on the dorsal aspect of the cast overlying the fracture and a retaining alignment fixture was placed in the window. The patients were given a device within 7 days of the fracture, were told to use it for 20 minutes a day, until their 10‐week appointment. Test: ultrasound probe that fitted into the retaining fixture was given to each participant. The ultrasound signal was composed of a 200 µs burst of 1.5 MHz sine waves, with a repetition rate of 1 kHz and a spatial average intensity of 30 mW/cm². Control: a visually and audibly similar device was given to each participant. |
|
| Outcomes | Follow‐up schedule was weekly until week 6 and then 8, 10, 12 and 16 weeks. End point was defined as combined clinical and radiographic healing. Primary: time to radiographic union. Secondary: time to early trabecular healing, time to cortical bridging, percentage of organised trabecular healing, loss of reduction. |
|
| Notes | The protocol specified combined clinical and radiographic healing, but investigators were reluctant to remove casts, therefore no clinical data are reported and radiographic union was used as the primary outcome measure. It was confirmed in personal communication with Joan McCabe that multiple reports with similar titles were all from the same study. Cook 1997 describes a subgroup analysis of the study by Kristiansen 1997. Smoking status before and during the study was retrospectively collected. There were 30 participants in the active group and 31 in the control group. These numbers correspond with the numbers of participants that successfully completed the study by Kristiansen 1997. There were 10 participants who could not be located for a retrospective analysis of smoking status. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomly assigned...according to a computer generated code, developed by an independent consultant". |
| Allocation concealment (selection bias) | Unclear risk | Comments: Concealment of the codes is not reported. |
| Blinding (performance bias and detection bias) Objective measures | Low risk | Quote: "The placebo device...was identical to the active unit". "The principle investigator and the independent radiologist...were blinded...performed independent central assessments...of the radiographic parameters of union. |
| Incomplete outcome data (attrition bias) Objective measures | High risk | Comments: The protocol specified combined clinical and radiographic healing, but investigators were reluctant to remove casts, therefore no clinical data is reported. All patients lost to follow‐up accounted for but approximately 30% loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | High risk | Two patients had bilateral fractures and they were treated with alternate devices. These fractures were analysed as independent events |
| Selection bias (imbalance in baseline characteristics) | Unclear risk | Gender, age and fracture characteristics were similar. Smoking status is not reported. |
Leung 2004.
| Methods | Quasi‐randomised, placebo‐controlled study | |
| Participants | Setting: Chinese University of Hong Kong, China. Size: a total of 30 fractures in 28 patients. The test group had 16 fractures in 15 patients and the control group had 14 fractures in 13 patients. Baseline characteristics: mean (range) age 35.3 years (22 to 61), 25 males and 3 females. Inclusion: patients with open or comminuted tibial fractures. Exclusion: simple fractures, fractures of sites other than the tibia. |
|
| Interventions | Patients with closed fractures or Gustillo grade 1 or 2 open fractures in the diaphysis underwent fixation with reamed, locked intramedullary nail. Participants with fractures in the metaphysis or Gustillo grade 3 open fractures were treated with an external fixator. All open fractures were treated with emergency debridement and delayed closure. Test: LIPUS machine was given to the patients as soon as the soft tissues were closed. The ultrasound signal was composed of a 200 µs burst of 1.5 MHz sine waves, with a repetition rate of 1 kHz and a spatial average intensity of 30 mW/cm² and was given for 20 minutes a day, for 90 days using coupling gel applied directly over the fracture site. Control: a sham device that was externally identical to the LIPUS machine was given to the participants as soon as the soft tissues were closed. |
|
| Outcomes | End point was combined clinical and radiographic union. Clinical union was defined as full painless weight bearing. Radiographic union was defined as 3 out of 4 cortices were bridged with bone on plain orthogonal radiograph. Follow‐up times were every 3 weeks for the first 3 months, every 6 weeks for the following 3 months and every 8 weeks for the last 6 months. The radiographs were assessed by 3 independent surgeons and a mean time of union was used. Primary: time to union. Secondary: bone mineral density and plasma bone specific alkaline phosphatase, adverse events. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "...assigned...according to the sequence of admission". Comments: Quasi‐randomised. |
| Allocation concealment (selection bias) | High risk | Quote: "...assigned...according to the sequence of admission". Comments: No list provided. Quasi‐randomised. |
| Blinding (performance bias and detection bias) Objective measures | High risk | Quote: "Control group were given a dummy machine". Comments: Efforts were made to blind the patients, but the assessors were not blind as the machines were not identical and the patients were quasi‐randomly allocated. |
| Incomplete outcome data (attrition bias) Objective measures | Low risk | The complete dataset was presented. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | High risk | Quote: "Four patients had segmental fractures..." Some participants had two fractures, which may have been randomised independently. No statistical adjustments were reported to allow for this |
| Selection bias (imbalance in baseline characteristics) | Unclear risk | Age, gender and smoking status not separately reported. |
Lubbert 2008.
| Methods | Randomised, placebo‐controlled study | |
| Participants | Setting: multicentre trial, Netherlands. Size: there were 120 patients. Of the 61 in the test group, 9 were lost to follow‐up, leaving 52 patients. Of the 59 in the control group, 7 were lost to follow‐up and 3 did not complete the intervention, leaving 49 patients. Baseline characteristics: 46 males and 6 females in the intervention group and 39 males and 10 females in the control group. Inclusion: over 18 years of age, diaphyseal fracture of the clavicle (Allman group 1), treatment begun within 5 days of trauma. Exclusion: multiple trama, re‐fracture, pathological fracture, open fracture or threatened soft tissue envelope, metaphyseal fracture. |
|
| Interventions | All participants were treated non‐operatively with a collar and cuff sling for symptom control. Free arm movements within a range allowed by pain were allowed from day 1. Participants maintained a treatment diary. Test: a LIPUS machine was given to the patients at the first visit. The ultrasound signal was given for 20 minutes a day, for 28 days using coupling gel applied directly over the fracture site. The unit was an Exogen 2000 battery powered Main Operating Unit and a Smith and Nephew Treatment Head Module transducer that delivered an ultrasound signal composed of a 200 µs burst of 1.5 MHz sine waves, with a repetition rate of 1 kHz and a spatial average intensity of 30 mW/cm². Control: a sham device that was externally identical to the LIPUS machine was given to the participants with similar instructions for use. |
|
| Outcomes | Follow‐up schedule: 1, 2, 4, 6, 8 weeks.
Primary: patient‐reported subjective clinical fracture healing. Secondary: pain (VAS and painkiller use), operation, adverse events, resumption of sport/professional activities/sport. |
|
| Notes | Data from the patients excluded from the study was provided by Pieter Lubbert in personal communication; these allowed an intention‐to‐treat analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For each participating hospital consecutive numbered transducers were delivered in packs of four." "Randomisation took place at the site of the manufacturer." Comment: Distant block randomisation. |
| Allocation concealment (selection bias) | Low risk | Quotes: "Each hospital supply contained two randomly assigned active transducers and two placebo transducers." "The placebo transducers looked identical..." Comment: Allocation was concealed at a distant site. |
| Blinding (performance bias and detection bias) Patient‐reported measures | Low risk | Quote: "The placebo transducers looked identical..." |
| Blinding (performance bias and detection bias) Objective measures | Low risk | Quote: "The placebo transducers looked identical..." |
| Incomplete outcome data (attrition bias) Patient‐reported measures | High risk | Trial flow diagram presented clearly. Only a per‐protocol analysis was presented |
| Incomplete outcome data (attrition bias) Objective measures | Low risk | Need for operation following delayed or non‐union thoroughly described |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No additional biases identified |
| Selection bias (imbalance in baseline characteristics) | Unclear risk | Age and smoking status not separately reported. |
Mayr 2000.
| Methods | Randomised controlled trial | |
| Participants | Setting: German emergency outpatient department. Single‐centre study. Size: 29 patients, 30 fractures; 15 fractures in each group. Baseline characteristics: mean age (SD) age 37 (14) years; 5 to 1 male / female ratio. Inclusion: skeletally mature adults with a fresh stable scaphoid fracture (AO B1 and B2). Exclusion: unstable fractures, generalised skeletal disease, pathological fracture, fracture more than 10 days old at diagnosis. |
|
| Interventions | A forearm plaster splint was applied to include the thumb to the interphalangeal joint. After detumescence, the splint was replaced with a circular restraining forearm bandage to include the thumb to the interphalangeal joint. Test: after appliance of the circular immobilising forearm bandage, daily 20‐minute pulsed low‐intensity ultrasound treatment (SAFHS, Exogen, Piscataway, NJ, USA; frequency: 1.5 MHz, pulsed with 1 kHz, signal length: 200 µsec, intensity: 30 mW/cm²) was conducted. Control: no additional placebo treatment. |
|
| Outcomes | Follow‐up schedule: CT at 6 weeks and then every 2 weeks until union.
Primary outcome: time to union by CT assessment of fracture union. Secondary outcome: percentage of ossification of the fracture gap. |
|
| Notes | The follow‐up schedule was changed after 6 patients had been scanned at 6 weeks, when 3 of them had already achieved union. From that point onwards in the trial, first follow‐up was at 4 weeks. Translated from German. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated by a random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Objective measures | Low risk | CT scans were blinded before reporting by a panel of independent radiologists and surgeons. |
| Incomplete outcome data (attrition bias) Objective measures | Low risk | There was no loss of outcome data. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | No additional biases identified |
| Selection bias (imbalance in baseline characteristics) | Unclear risk | Smoking status is not reported. |
Rue 2004.
| Methods | Randomised, placebo‐controlled trial | |
| Participants | Setting: US Naval Academy Size: 40 midshipmen with 58 stress fractures; data reported for 26 (14 in the treatment group and 12 in the control group) midshipmen with tibial stress fractures. Baseline characteristics: 23 men and 17 women; mean age 19 years; fractures sites were tibia, metatarsal, femur and fibula (74%, 9%, 5% and 5% respectively) Inclusion: new midshipmen sustaining stress fractures diagnosed on radiographic and scintigraphic examinations during initial training. Informed consent. Exclusion: none |
|
| Interventions | While not stated explicitly it is likely that all participants received the standard‐of‐care treatment that included protected weight bearing if normal walking reproduced symptoms, alternative aerobic exercise, a daily multivitamin and calcium supplementation (twice daily 500 mg). Test: daily 20‐minute LIPUS treatment (Exogen Inc, Piscataway, NJ) administered by sports medicine personnel until stress fracture had healed. Control: similar protocol with a sham unit. |
|
| Outcomes | Follow‐up schedule: daily treatments until fit to return to duty (work) defined as no pain on palpation, the ability to do a single leg hop on the affected side without pain and radiographic evidence of healing. Primary outcome: time to return to duty (work). Secondary outcome: adherence. |
|
| Notes | Although 40 participants were enrolled with a variety of injured bones, only 33 were able to comply with the protocol for a variety of reasons. Of these 33, 7 further participants were excluded from the analysis as only those with fractures of the tibia were analysed (total attrition: 14 of 40). The 26 participants had 43 tibial stress fractures ‐ time to return to duty was based on stress fracture site with the longest duration of symptoms. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...and were randomized into one of two treatment protocols..." Comment: No description of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...and were randomized into one of two treatment protocols..." Comment: No description of allocation concealment. |
| Blinding (performance bias and detection bias) Patient‐reported measures | Low risk | Quote: "The placebo group underwent the identical protocol, except that the stimulator unit was non‐functional. This study was a double‐blind, placebo‐controlled investigation." Comment: Participants were blinded to intervention. |
| Blinding (performance bias and detection bias) Objective measures | Unclear risk | Quote: "This study was a double‐blind ... investigation." Comment: Trial personnel administered the treatments and documented adherence. It is not explicit that they were also blind to the allocation although the study was 'double‐blind'. |
| Incomplete outcome data (attrition bias) Patient‐reported measures | High risk | Overall attrition proportion was 14 of 40 and the loss was explicitly systematic. |
| Incomplete outcome data (attrition bias) Objective measures | High risk | Overall attrition proportion was 14 of 40 and the loss was explicitly systematic. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | No additional biases identified |
| Selection bias (imbalance in baseline characteristics) | Unclear risk | Smoking status is not reported. |
Strauss 1999.
| Methods | Randomised controlled trial | |
| Participants | Setting: USA hospital. Size: 20 participants, 20 fractures; 10 fractures in each group. Baseline characteristics: not reported. Inclusion: patients with a fracture of the fifth metatarsal (zone II). Exclusion: not stated. |
|
| Interventions | All fractures were initially treated with short leg cast and weightbearing as tolerated for a mean of 10 days. All casts were converted to a hinged ankle foot orthosis and patients continued with weightbearing until fracture union. Test: participants were given LIPUS therapy for 20 minutes twice each day. Control: participants were given no additional placebo treatment. |
|
| Outcomes | Follow‐up schedule: not reported Primary: time to clinical and radiographic union Secondary: proportion of union within 20 weeks |
|
| Notes | Inadequate data were presented to include the primary outcome in the analysis in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...were studied in a prospective randomized setting. The twenty fractures were randomly divided..." Comment: Method of randomisation is unclear. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...were studied in a prospective randomized setting. The twenty fractures were randomly divided..." Comment: Method of randomisation is unclear. |
| Blinding (performance bias and detection bias) Objective measures | High risk | Quote: "...Group B (control or no ultrasound treatment)." Comment: Control group received no sham LIPUS machine. |
| Incomplete outcome data (attrition bias) Objective measures | Low risk | All participants were followed up to the final time point of the study. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | High risk | This study was only reported as a poster abstract. The detail contained within this report is minimal and evaluation of the risk of bias is extremely limited |
| Selection bias (imbalance in baseline characteristics) | Unclear risk | Baseline characteristics were not reported. |
Wang 2007.
| Methods | Quasi‐randomised controlled trial | |
| Participants | Setting: Taiwan Size: a total of 59 fractures in 56 patients. There was one exclusion in each group, leading to 27 fractures in 27 patients in the test and 30 fractures in 27 patients in the control. Baseline characteristics: mean (range) age was 34.2 years (15 to 81), 40 males and 16 females. Inclusion: patients with acute, displaced, high energy trauma diaphyseal fractures of the femur and tibia that required reduction and internal or external fixation. Exclusion: pathological fracture, active infection, coagulopathy, immunosuppression, pregnancy, cardiac pacemaker, skeletal immaturity, poor compliance. |
|
| Interventions | All closed fractures were treated with open or closed reduction and internal fixation with intra‐medullary nailing or plate fixation. Patients with type III‐C open fractures were initially treated with surgical debridement of the wounds and external fixator for fracture stabilization. Delayed open or closed reduction and internal fixation was performed when the soft tissues were optimised. All other open fractures were treated with primary open reduction and internal fixation. Postoperative management included early ambulation with no weight bearing allowed through the affected limb; quadriceps and hamstring and lower limb joint range of motion exercises. Test: participants in the study group received shockwave treatment immediately after surgery under the same anaesthesia. For patients with type III‐C open fractures, shockwave treatment was performed after delayed open reduction and internal fixation for the fractures. The source of shockwaves was from an OssaTron (High Medical Technology, Kreulingen, Switzerland). Shockwaves were performed with patients on the fracture table. The fracture site was verified with C‐arm X‐rays, and the depth of treatment was confirmed with the control guide of the device under C‐arm imaging. Surgical lubrication gel was applied to the area of skin in direct contact with the shockwave tube. Each fracture site was treated with 6,000 impulses of shockwave at 28 kV (equivalent to 0.62 mJ/mm² energy flux density). Shockwaves were applied in two planes with equal dosage in each plane as a single session. Control: participants in the control group received open reduction and internal fixation without shockwave treatment after surgery. |
|
| Outcomes | Follow‐up schedule: 1, 3, 6 and 12 months. Primary: proportion of union at 12 months. Secondary: proportion of union at earlier time points, fracture alignment, pain (VAS), weight bearing status, adverse events. |
|
| Notes | Authors have assumed independence between observations from multiple fractures in a single participant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "[The study group] who had surgery on odd days of the week, and the control group ... who had surgery performed on even days of the week" Comment: Quasi‐randomised study. |
| Allocation concealment (selection bias) | High risk | Quote: "[The study group] who had surgery on odd days of the week, and the control group..who had surgery performed on even days of the week" Comment: Unclear whether method of randomisation known, but would be easy to identify pattern. |
| Blinding (performance bias and detection bias) Patient‐reported measures | Unclear risk | Quote: "Patients in the control group ... without shockwave treatment after surgery." Comment: It is not reported whether the participants were blind to their allocation. |
| Blinding (performance bias and detection bias) Objective measures | Low risk | Quote: "An independent examiner blinded to the nature of the study protocol performed the examination." |
| Incomplete outcome data (attrition bias) Patient‐reported measures | High risk | Quote: "Two patients were excluded from the final analysis because of postoperative deep infection and osteomyelitis." Comment: This was consistent with the eligibility criteria but is an unusual means to handle data from participants developing adverse events. |
| Incomplete outcome data (attrition bias) Objective measures | High risk | Quote: "Two patients were excluded from the final analysis because of postoperative deep infection and osteomyelitis." Comment: This was consistent with the eligibility criteria but is an unusual means to handle data from participants developing adverse events. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | Quote: "56 patients with 59 .... fractures" Some participants had two fractures which may have been randomised independently. No statistical adjustments were reported to allow for this |
| Selection bias (imbalance in baseline characteristics) | Unclear risk | The distribution of smoking status between the groups is not reported. |
Yadav 2008.
| Methods | Randomised, placebo‐controlled trial | |
| Participants | Setting: Indian military recruits in training. Size: 67 cases with stress fracture; with 39 in the treatment group and 28 in the control group. Baseline characteristics: age not reported, gender data not reported. Inclusion: history and examination consistent with a diagnosis of stress fracture. Exclusion: none. |
|
| Interventions | All participants were managed non‐operatively and prescribed paracetamol and ice‐packs. Test: treated with 10 min/day using a ultrasound probe emitting a 3 MHz, 1 W/cm² ultrasound signal pulsed with a duty cycle of 50%. Control: similar treatment with a sham unit which was identical to the test unit. |
|
| Outcomes | Time to return to training. No radiological outcome measures assessed. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...were randomly assigned ... by chit method." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...were randomly assigned ... by chit method." Comment: It is not clear whether this was done on or off site and who had access to the results. |
| Blinding (performance bias and detection bias) Patient‐reported measures | Low risk | Quotes: "... nonfunctioning unit identical in appearance." "... patients ... study's researchers were blinded..." |
| Blinding (performance bias and detection bias) Objective measures | Low risk | Quotes: "... nonfunctioning unit identical in appearance." "... patients ... study's researchers were blinded..." |
| Incomplete outcome data (attrition bias) Patient‐reported measures | Low risk | There were no missing data. |
| Incomplete outcome data (attrition bias) Objective measures | Low risk | There were no missing data. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | No additional biases identified |
| Selection bias (imbalance in baseline characteristics) | Unclear risk | Quote: "... matched in terms of age, height, demographics, and delay from symptom onset to diagnosis." Comment: Sex and smoking status not reported. |
CT = computed tomography LIPUS = low‐intensity pulsed ultrasound NSAID = non‐steroidal anti‐inflammatory drug VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Basso 1998 | This RCT involving a single application of ultrasound to conservatively treated distal radius fractures reported on range of motion and referral for physiotherapy at 8 weeks. It is excluded because its focus was not on fracture healing; it also not did not report any outcome measures pertinent to this review. |
| Busse 2005 | This study is a health economic analysis, which is informed using data from a systematic review. |
| Heckman 1997 | This study is a cost analysis based upon models developed from clinical data and specified assumptions. It is not a formal health economics analysis within a RCT. |
| ISRCTN98682811 | This trial ('TRUST (pilot)' in the first version of our review (Giffin 2011) was intended to be a pilot study comparing LIPUS versus sham in 50 patients with conservatively managed fractures of the tibia. It was abandoned after four months (Busse 2014) as a survey of practice showed a shift to surgical management of tibial fractures. A new pilot of surgically managed tibial fractures was set up under a new trial registration number (NCT00667849). |
| Urita 2013 | This study only included patients undergoing shortening osteotomies of the upper limb. |
RCT = randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
ISRCTN90844675.
| Methods | Randomised controlled multicentre trial |
| Participants | Adults with closed or type I open fractures of the tibia that had been treated by reamed or unreamed locking intramedullary nails less than 10 days prior to randomisation. Patients with fractures of the lateral malleolus, fixed by plates, as well as patients with minor concomitant injuries (bruises, sprains) were offered trial participation. Intended target population: 250 |
| Interventions | Test: pulsed, low‐energetic ultrasound (Exogen, Smith & Nephew), applied daily for three months Control: standard of care |
| Outcomes | Follow‐up: 1 year Primary: bony union three months (+/‐ 1 week) after randomisation, as assessed on plain radiographs by independent, blinded raters Secondary (assessed after 6 weeks, 3, 6, and 12 months): 1. Delayed union and non‐union rates 2. Health‐related quality of life (36‐item Short Form Health Survey [SF‐36], EuroQoL instrument [EQ‐5D]) 3. Functional outcomes (Western Ontario and McMaster Universities Osteoarthritis Index [WOMAC]) 4. Duration of sick leave 5. Cost‐utility 6. Serious adverse events (SAE) |
| Notes | Trial registration identified after preparation of the review. Indicated as a completed trial (01/10/2008 to 01/10/2010). Efforts to learn its current status were unsuccessful for the first version of this review but Dr Seifert indicated that data were under analysis during the update of the review. (Seifert 2013)
Contact: Dr Julia Seifert, Berlin (julia.seifert@ukb.de) We also found that the results may have been presented in an oral presentation but have not obtained a copy of this: Froese E, Gümbel D, Stengel D. Pulsed ultrasound to speed healing after internal fixation of tibia fractures‐ Results from the randomized PUSH‐IT trial (ISRCTN90844675). 12th Congress European Forum For Research In Rehabilitation; 11‐14 September 2013, Istanbul Turkey. |
NCT00667849.
| Methods | Randomised controlled trial |
| Participants | Patients with tibial fractures treated with intramedullary nailing. Target population = 500 |
| Interventions |
Test: LIPUS (low‐intensity pulsed ultrasound) ‐ Exogen (Piscataway, New Jersey) Bone Healing System Control: Sham ultrasound unit |
| Outcomes | Primary: radiographs at 6, 12, 18, 26, 38 and 52 weeks Secondary: rates of nonunion of tibial fractures (6, 12, 18, 26, 38 and 52 weeks) |
| Notes | This multicentre study, involving centres in USA and Canada, is sponsored by Smith & Nephew (changed to BioventusLLC). It has the same name as the other trial on conservatively treated tibia fractures: TRUST (Pilot). TRUST (Full) has been completed but the data are not yet available (Busse 2013). On 28/06/2013, the trial registration record update on status reported: "The Study was terminated due to futility". The actual population was reported as "501" but a linked report reported: "From July 5th, 2005, to June 22nd, 2007, 51 patients with 14 open and 37 closed tibial fractures were treated with reamed intramedullary nailing". However, the study start date was listed as September 2008 in the trial registration document. The pilot study for this trial (Busse 2014), which recruited 51 patients between March 2006 to June 2007, was published after our date of last search. |
Differences between protocol and review
Data analysis
We anticipated that the primary analysis in the included studies was likely to be a survival analysis using the time to fracture union as the outcome measure. Therefore, it seemed likely that the majority of studies would report either log‐rank statistics or estimates of hazard ratios, after fitting Cox's proportional hazards regression model, as an estimate of the intervention effect. However, the majority of studies reported mean healing times. We specified that we would deal with such continuous data by estimating the mean differences and 95% confidence intervals. Since the included studies report outcomes from participants with a variety of long bone injuries, which are well understood to have widely varying healing times normally, we chose to combine these data using standardised mean differences.
Dealing with missing data
We altered our method of dealing with missing continuous data where these remained unavailable after attempting to contact trial authors. In order to determine a conservative estimate of any treatment effect, we assumed that the missing data from participants in the treatment group lay at the extreme of the distribution (two standard deviations from the reported mean). Conversely, for participants in the control group we assumed the distribution was unaffected by the missing data.
Sensitivity analyses
We anticipated that outcomes may have been reported at a number of time points (e.g. six months and 12 months). We planned to include these outcomes in order to provide some sensitivity to the selection of an appropriate follow‐up time for assessment of the treatment effect. Given these data were not available, such an analysis was not necessary.
Contributions of authors
XL Griffin is responsible for the conception, design and writing of the review. He is the co‐guarantor of the review. ML Costa is involved in conception and design of the review. He is the co‐guarantor of the review. N Parsons is the review statistician. He is responsible for the data management and analysis plan. D Metcalfe is responsible for database searching and updating the review.
Sources of support
Internal sources
-
National Institute for Health Research (NIHR), UK.
Provision of salaries to XL and DM
-
University of Warwick, UK.
Provision of salaries to NP and MC
External sources
No sources of support supplied
Declarations of interest
XL Griffin: XG, nor his institution, have received any grants pertaining to this published work. XG has been in receipt of several institutional grants to support other academic interests. ML Costa: MC, nor his institution, have received any grants pertaining to this published work. MC has been in receipt of several institutional grants to support other academic interests. N Parsons: none known D Metcalfe: none known
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Emami 1999 {published data only}
- Emami A, Petren‐Mallmin M, Larsson S. No effect of low‐intensity ultrasound on healing time of intramedullary fixed tibial fractures. Journal of Orthopaedic Trauma 1999;13(4):252‐7. [DOI] [PubMed] [Google Scholar]
Handolin 2005 {published data only (unpublished sought but not used)}
- Handolin L, Kiljunen V, Arnala I, Kiuru MJ, Pajarinen J, Partio EK, et al. No long‐term effects of ultrasound therapy on bioabsorbable screw‐fixed lateral malleolar fracture. Scandinavian Journal of Surgery 2005;94(3):239‐42. [DOI] [PubMed] [Google Scholar]
- Handolin L, Kiljunen V, Arnala I, Pajarinen J, Partio EK, Rokkanen P. The effect of low intensity ultrasound and bioabsorbable self‐reinforced poly‐L‐lactide screw fixation on bone in lateral malleolar fractures. Archives of Orthopaedic and Trauma Surgery 2005;125(5):317‐21. [DOI] [PubMed] [Google Scholar]
Handolin 2005a {published data only (unpublished sought but not used)}
- Handolin L, Kiljunen V, Arnala I, Kiuru M, Pajarinen J, Partio E, et al. The effects of low‐intensity ultrasound on 22 bioabrorbable self‐reinforced poly‐L‐lactic acid (SR‐PLLA) screw fixed lateral malleolar fracture. Suomen Ortopedia ja Traumatologia 2004; Vol. 27, issue 4:236‐8.
- Handolin L, Kiljunen V, Arnala I, Kiuru MJ, Pajarinen J, Partio EK, et al. Effect of ultrasound therapy on bone healing of lateral malleolar fractures of the ankle joint fixed with bioabsorbable screws. Journal of Orthopaedic Science 2005;10(4):391‐5. [DOI] [PubMed] [Google Scholar]
Heckman 1994 {published and unpublished data}
- Cook S, Ryaby JP, Heckmann JD, Kristiansen TK. Low‐intensity pulsed ultrasound accelerates tibia and distal radius fracture healing in smokers. Hefte zur der Unfallchirurg 1996;262:336. [Google Scholar]
- Cook SD, Ryaby JP, McCabe J, Frey JJ, Heckman JD, Kristiansen TK. Acceleration of tibia and distal radius fracture healing in patients who smoke. Clinical Orthopaedics & Related Research 1997;(337):198‐207. [DOI] [PubMed] [Google Scholar]
- Cook SD, Ryaby JP, McCabe J, Frey JJ, Heckman JD, Kristiansen TK. Low intensity pulsed ultrasound accelerates tibia and distal radius fracture healing in smokers [abstract]. Orthopaedic Transactions 1996;20(1):56. [Google Scholar]
- Heckman JD. Personal communication September 2 2010.
- Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF. Acceleration of tibial fracture‐healing by non‐invasive, low‐intensity pulsed ultrasound. Journal of Bone and Joint Surgery ‐ American Volume 1994;76(1):26‐34. [DOI] [PubMed] [Google Scholar]
Kristiansen 1997 {published and unpublished data}
- Cook S, Ryaby JP, Heckmann HD, Kristiansen TK. Low‐intensity pulsed ultrasound accelerates tibia and distal radius fracture healing in smokers. Hefte zur der Unfallchirurg 1996;262:336. [Google Scholar]
- Cook SD, Ryaby JP, McCabe J, Frey JJ, Heckman JD, Kristiansen TK. Acceleration of tibia and distal radius fracture healing in patients who smoke. Clinical Orthopaedics and Related Research 1997;337:198‐207. [DOI] [PubMed] [Google Scholar]
- Cook SD, Ryaby JP, McCabe J, Frey JJ, Heckman JD, Kristiansen TK. Low intensity pulsed ultrasound accelerates tibia and distal radius fracture healing in smokers [abstract]. Orthopaedic Transactions 1996;20(1):56. [Google Scholar]
- Kristiansen TK. The effect of low power specifically programmed ultrasound on the healing time of fresh fractures using a Colles model [abstract]. Journal of Orthopaedic Trauma 1990;4(2):227. [Google Scholar]
- Kristiansen TK, Ryaby JP, McCabe J, Frey J. Controlling loss of reduction in distal radius fractures in an randomized, double‐blind study using low‐intensity ultrasound. Hefte zur der Unfallchirurg. 1996; Vol. 262:369.
- Kristiansen TK, Ryaby JP, McCabe J, Frey J. Controlling loss of reduction in distal radius fractures with low intensity pulsed ultrasound [abstract]. Orthopaedic Transactions 1997;21(1):141. [Google Scholar]
- Kristiansen TK, Ryaby JP, McCabe J, Frey JJ, Roe LR. Accelerated healing of distal radial fractures with the use of specific, low‐intensity ultrasound: A multicenter, prospective, randomized, double‐blind, placebo‐controlled study. Journal of Bone and Joint Surgery ‐ American Volume 1997;79(7):961‐73. [DOI] [PubMed] [Google Scholar]
- McCabe J. Personal communication 22 September 2010.
Leung 2004 {published data only}
- Leung KS, Lee WS, Tsui HF, Liu PPL, Cheung WH. Complex tibial fracture outcomes following treatment with low‐intensity pulsed ultrasound. Ultrasound in Medicine and Biology 2004;30(3):389‐95. [DOI] [PubMed] [Google Scholar]
Lubbert 2008 {published and unpublished data}
- Lubbert PH. Personal communication November 7 2010.
- Lubbert PHW, Rijt RHH, Hoorntje LE, Werken C. Low‐intensity pulsed ultrasound (LIPUS) in fresh clavicle fractures: A multi‐centre double blind randomised controlled trial. Injury 2008;39(12):1444‐52. [DOI] [PubMed] [Google Scholar]
Mayr 2000 {published data only}
- Mayr E. Accelerated healing of scaphoid fracture ‐ a randomized study [abstract]. Journal of Bone and Joint Surgery ‐ British Volume 1999;81 Suppl 2:206. [Google Scholar]
- Mayr E, Rudzki M, Borchardt B, Ruter A. Accelerated healing of scaphoid fractures ‐ A randomized study [abstract]. Journal of Orthopaedic Trauma 1999;13(4):310. [Google Scholar]
- Mayr E, Rudzki M‐M, Rudzki M, Borchardt B, Hausser H, Ruter A. Does pulsed low‐Intensity ultrasound accelerate healing of scaphoid fractures? [Beschleunigt niedrig intensive, gepulster Ultraschall die Heilung von Skaphoidfrakturen?]. Handchirurgie Mikrochirurgie Plastische Chirurgie 2000;32(2):115‐22. [DOI] [PubMed] [Google Scholar]
Rue 2004 {published data only}
- Rue JP, Armstrong DW 3rd, Frassica FJ, Deafenbaugh M, Wilckens JH. The effect of pulsed ultrasound in the treatment of tibial stress fractures. Orthopedics 2004;27(11):1192‐5. [DOI] [PubMed] [Google Scholar]
Strauss 1999 {published data only (unpublished sought but not used)}
- Strauss E, McCabe J. Treatment of Jones fractures of the foot with adjunctive use of pulsed low‐intensity ultrasound stimulation [abstract]. American Academy of Orthopaedic Surgeons Annual Meeting; 1998 Mar 13‐14; New Orleans. 1998.
- Strauss E, Ryaby JP, McCabe J. Treatment of Jones' fractures of the foot with adjunctive use of low‐pulsed ultrasound stimulation. Journal of Orthopaedic Trauma 1999;13(4):310. [Google Scholar]
Wang 2007 {published data only}
- Liu HC, Fu TH. Effects of shockwave on acute high‐energy fractures of the femur and tibia [abstract]. American Academy of Orthopaedic Surgeons Annual Meeting; 2007 Feb 14–18; San Diego. 2007.
- Wang CJ, Liu HC, Fu TH. The effects of extracorporeal shockwave on acute high‐energy long bone fractures of the lower extremity. Archives of Orthopaedic and Trauma Surgery 2007;127(2):137‐42. [DOI] [PubMed] [Google Scholar]
Yadav 2008 {published data only}
- Yadav YK, Salgotra KR, Banerjee A. Role of ultrasound therapy in the healing of tibial stress fractures. Medical Journal Armed Forces India 2008;64(3):234‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Basso 1998 {published data only}
- Basso O, Pike JM. The effect of low frequency, long‐wave ultrasound therapy on joint mobility and rehabilitation after wrist fracture. Journal of Hand Surgery ‐ British Volume 1998;23(1):136‐9. [DOI] [PubMed] [Google Scholar]
Busse 2005 {published data only}
- Busse JW, Bhandari M, Sprague S, Johnson‐Masotti AP, Gafni A. An economic analysis of management strategies for closed and open grade I tibial shaft fractures. Acta Orthopaedica 2005;76(5):705‐12. [DOI] [PubMed] [Google Scholar]
Heckman 1997 {published data only}
- Heckman JD, Sarasohn‐Kahn J. The economics of treating tibia fractures: The cost of delayed unions. Bulletin of The Hospital for Joint Dieases 1997;56(1):63‐72. [PubMed] [Google Scholar]
ISRCTN98682811 {unpublished data only}
- Bhandari M, Busse J, Guyatt G. Trial to re‐evaluate ultrasound in the treatment of tibial fractures (TRUST). http://www.controlled‐trials.com/ISRCTN98682811 (accessed 14 June 2011). [DOI] [PMC free article] [PubMed]
- Busse J. Personal communication 15 October 2013.
Urita 2013 {published data only}
- Urita A, Iwasaki N, Kondo M, Nishio Y, Kamishima T, Minami A. Effect of low‐intensity pulsed ultrasound on bone healing at osteotomy sites after forearm bone shortening. Journal of Hand Surgery (American) 2013;38(3):498‐503. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ISRCTN90844675 {unpublished data only}
- Seifert J. Personal communication. 30 October 2013.
- Seifert J. Pulsed ultrasound to speed‐up healing after intramedullary nailing of tibia fractures (PUSH‐IT). http://controlled‐trials.com/ISRCTN90844675/ISRCTN90844675 (accessed 9 December 2011).
NCT00667849 {published and unpublished data}
- Bhandari M. Trial to re‐evaluate ultrasound in the treatment of tibial fractures (TRUST). http://www.clinicaltrials.gov/ct2/show/NCT00667849 (accessed 7 December 2011). [DOI] [PMC free article] [PubMed]
- Busse J. Personal communication 15 October 2013.
- Busse J, Bhandari M, Einhorn TA, Heckman JD, Leung K‐S, Schemitsch E, et al. Trial to re‐evaluate ultrasound in the treatment of tibial fractures (TRUST): a multicenter randomized pilot study. Trials 2014; Vol. 15, issue 206. [DOI] [PMC free article] [PubMed]
- Dijkman BG, Busse JW, Walter SD, Bhandari M, for TRUST Investigators. The impact of clinical data on the evaluation of tibial fracture healing. Trials 2011;12:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aaron 2004
- Aaron RK, Ciombor DM, Simon BJ. Treatment of nonunions with electric and electromagnetic fields. Clinical Orthopaedics & Related Research 2004;(419):21‐9. [PUBMED: 15021127] [DOI] [PubMed] [Google Scholar]
Boutron 2008
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P, CONSORT Group. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Annals of Internal Medicine 2008;148(4):295‐309. [DOI] [PubMed] [Google Scholar]
Busse 2009
- Busse JW, Kaur J, Mollon B, Bhandari M, Tornetta P 3rd, Schunemann HJ, et al. Low intensity pulsed ultrasonography for fractures: systematic review of randomised controlled trials. BMJ 2009;338:b351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Busse 2013
- Busse J. Personal communication 15 October 2013.
Busse 2014
- Busse J, Bhandari M, Einhorn TA, Heckman JD, Leung K‐S, Schemitsch E, et al. Trial to re‐evaluate ultrasound in the treatment of tibial fractures (TRUST): a multicenter randomized pilot study. Trials 2014; Vol. 15, issue 206. [DOI] [PMC free article] [PubMed]
Cook 1997
- Cook SD, Ryaby JP, McCabe J, Frey JJ, Heckman JD, Kristiansen TK. Acceleration of tibia and distal radius fracture healing in patients who smoke. Clinical Orthopaedics & Related Research 1997;(337):198‐207. [DOI] [PubMed] [Google Scholar]
Dijkman 2011
- Dijkman BG, Busse JW, Walter SD, Bhandari M, for TRUST Investigators. The impact of clinical data on the evaluation of tibial fracture healing. Trials 2011;12:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Einhorn 1995
- Einhorn TA. Enhancement of fracture healing. Journal of Bone & Joint Surgery ‐ American Volume 1995;77(6):940‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Griffin 2008
- Griffin XL, Costello I, Costa ML. The role of low intensity pulsed ultrasound therapy in the management of acute fractures: a systematic review. Journal of Trauma‐Injury Infection & Critical Care 2008;65(6):1446‐52. [PUBMED: 19077640] [DOI] [PubMed] [Google Scholar]
Hadjiargyrou 1998
- Hadjiargyrou M, McLeod K, Ryaby JP, Rubin C. Enhancement of fracture healing by low intensity ultrasound. Clinical Orthopaedics & Related Research 1998;(355 Suppl):S216‐29. [PUBMED: 9917641] [DOI] [PubMed] [Google Scholar]
Handolin 2005b
- Handolin L, Kiljunen V, Arnala I, Kiuru MJ, Pajarinen J, Partio EK, et al. No long‐term effects of ultrasound therapy on bioabsorbable screw‐fixed lateral malleolar fracture. Scandinavian Journal of Surgery 2005;94(3):239‐42. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008. [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Reher 1997
- Reher P, Elbeshir el‐NI, Harvey W, Meghji S, Harris M. The stimulation of bone formation in vitro by therapeutic ultrasound. Ultrasound in Medicine & Biology 1997;23(8):1251‐8. [PUBMED: 9372573] [DOI] [PubMed] [Google Scholar]
Seifert 2013
- Seifert J. Personal communication. 30 October 2013.
Victoria 2009
- Victoria G, Petrisor B, Drew B, Dick D. Bone stimulation for fracture healing: What's all the fuss?. Indian Journal of Orthopaedics 2009;43(2):117‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 1994
- Wang SJ, Lewallen DG, Bolander ME, Chao EY, Ilstrup DM, Greenleaf JF. Low intensity ultrasound treatment increases strength in a rat femoral fracture model. Journal of Orthopaedic Research 1994;12(1):40‐7. [PUBMED: 8113941] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Griffin 2012
- Griffin XL, Smith N, Parsons N, Costa ML. Ultrasound and shockwave therapy for acute fractures in adults. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD008579.pub2] [DOI] [PubMed] [Google Scholar]


