Abstract
Background
This is an update of a Cochrane review first published in 2002, and previously updated in 2007. Late radiation rectal problems (proctopathy) include bleeding, pain, faecal urgency, and incontinence and may develop after pelvic radiotherapy treatment for cancer.
Objectives
To assess the effectiveness and safety of non‐surgical interventions for managing late radiation proctopathy.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 11, 2015); MEDLINE (Ovid); EMBASE (Ovid); CANCERCD; Science Citation Index; and CINAHL from inception to November 2015.
Selection criteria
We included randomised controlled trials (RCTs) comparing non‐surgical interventions for the management of late radiation proctopathy in people with cancer who have undergone pelvic radiotherapy for cancer. Primary outcomes considered were: episodes of bowel activity, bleeding, pain, tenesmus, urgency, and sphincter dysfunction.
Data collection and analysis
Study selection, 'Risk of bias' assessment, and data extraction were performed in duplicate, and any disagreements were resolved by involving a third review author.
Main results
We identified 1221 unique references and 16 studies including 993 participants that met our inclusion criteria. One study found through the last update was moved to the 'Studies awaiting classification' section. We did not pool outcomes for a meta‐analysis due to variation in study characteristics and endpoints across included studies.
Since radiation proctopathy is a condition with various symptoms or combinations of symptoms, the studies were heterogeneous in their intended effect. Some studies investigated treatments targeted at bleeding only (group 1), some investigated treatments targeted at a combination of anorectal symptoms, but not a single treatment (group 2). The third group focused on the treatment of the collection of symptoms referred to as pelvic radiation disease. In order to enable some comparison of this heterogeneous collection of studies, we describe the effects in these three groups separately.
Nine studies assessed treatments for rectal bleeding and were unclear or at high risk of bias. The only treatments that made a significant difference on primary outcomes were argon plasma coagulation (APC) followed by oral sucralfate versus APC with placebo (endoscopic score 6 to 9 in favour of APC with placebo, risk ratio (RR) 2.26, 95% confidence interval (CI) 1.12 to 4.55; 1 study, 122 participants, low‐ to moderate‐quality evidence); formalin dab treatment (4%) versus sucralfate steroid retention enema (symptom score after treatment graded by the Radiation Proctopathy System Assessments Scale (RPSAS) and sigmoidoscopic score in favour of formalin (P = 0.001, effect not quantified, 1 study, 102 participants, very low‐ to low‐quality evidence), and colonic irrigation plus ciprofloxacin and metronidazole versus formalin application (4%) (bleeding (P = 0.007, effect not quantified), urgency (P = 0.0004, effect not quantified), and diarrhoea (P = 0.007, effect not quantified) in favour of colonic irrigation (1 study, 50 participants, low‐quality evidence).
Three studies, of unclear and high risk of bias, assessed treatments targeted at something very localised but not a single pathology. We identified no significant differences on our primary outcomes. We graded all studies as very low‐quality evidence due to unclear risk of bias and very serious imprecision.
Four studies, of unclear and high risk of bias, assessed treatments targeted at more than one symptom yet confined to the anorectal region. Studies that demonstrated an effect on symptoms included: gastroenterologist‐led algorithm‐based treatment versus usual care (detailed self help booklet) (significant difference in favour of gastroenterologist‐led algorithm‐based treatment on change in Inflammatory Bowel Disease Questionnaire–Bowel (IBDQ‐B) score at six months, mean difference (MD) 5.47, 95% CI 1.14 to 9.81) and nurse‐led algorithm‐based treatment versus usual care (significant difference in favour of the nurse‐led algorithm‐based treatment on change in IBDQ‐B score at six months, MD 4.12, 95% CI 0.04 to 8.19) (1 study, 218 participants, low‐quality evidence); hyperbaric oxygen therapy (at 2.0 atmospheres absolute) versus placebo (improvement of Subjective, Objective, Management, Analytic ‐ Late Effects of Normal Tissue (SOMA‐LENT) score in favour of hyperbaric oxygen therapy (HBOT), P = 0.0019) (1 study, 150 participants, moderate‐quality evidence, retinol palmitate versus placebo (improvement in RPSAS in favour of retinol palmitate, P = 0.01) (1 study, 19 participants, low‐quality evidence) and integrated Chinese traditional plus Western medicine versus Western medicine (grade 0 to 1 radio‐proctopathy after treatment in favour of integrated Chinese traditional medicine, RR 2.55, 95% CI 1.30 to 5.02) (1 study, 58 participants, low‐quality evidence).
The level of evidence for the majority of outcomes was downgraded using GRADE to low or very low, mainly due to imprecision and study limitations.
Authors' conclusions
Although some interventions for late radiation proctopathy look promising (including rectal sucralfate, metronidazole added to an anti‐inflammatory regimen, and hyperbaric oxygen therapy), single small studies provide limited evidence. Furthermore, outcomes important to people with cancer, including quality of life (QoL) and long‐term effects, were not well recorded. The episodic and variable nature of late radiation proctopathy requires large multi‐centre placebo‐controlled trials (RCTs) to establish whether treatments are effective. Future studies should address the possibility of associated injury to other gastro‐intestinal, urinary, or sexual organs, known as pelvic radiation disease. The interventions, as well as the outcome parameters, should be broader and include those important to people with cancer, such as QoL evaluations.
Keywords: Humans, Anti‐Inflammatory Agents, Anti‐Inflammatory Agents/therapeutic use, Electrocoagulation, Electrocoagulation/methods, Fatty Acids, Fatty Acids/therapeutic use, Formaldehyde, Formaldehyde/therapeutic use, Hyperbaric Oxygenation, Pelvic Neoplasms, Pelvic Neoplasms/radiotherapy, Proctitis, Proctitis/therapy, Radiation Injuries, Radiation Injuries/therapy, Randomized Controlled Trials as Topic, Rectum, Rectum/radiation effects, Sucralfate, Sucralfate/therapeutic use
Plain language summary
Non‐surgical interventions for late rectal consequences of radiotherapy in people who have received radical radiotherapy to the pelvis
Background
Radiotherapy is often used to treat cancer in the pelvic area. Several organs in the pelvis, such as the anus, rectum, bladder, prostate, gynaecological organs (womb, ovaries, cervix, and vagina), small bowel, and pelvic bones may be exposed to the effects of radiotherapy, which can lead to pelvic radiation disease. Symptoms from pelvic radiation disease may occur around the time of treatment (early effects) or over a period of time, often many years after treatment (late effects) due to long‐term changes secondary to scarring (fibrosis), narrowing (stenosis), and bleeding due to new blood vessel formation (telangiectasia). Damage to the rectum (radiation proctopathy) is the most often investigated late radiation effect to the pelvis, which affects a small but but still important group of people who undergo pelvic radiotherapy. The common symptoms are rectal urgency, rectal incontinence, pain, mucus discharge, and rectal bleeding.
The aim of the review
The aim of this review was to assess the effect of non‐surgical treatments on late rectal damage.
Main Findings
We found 16 (quasi) randomised controlled trials (RCTs) including 993 participants that assessed non‐surgical treatments for radiation proctopathy. Although some treatments look promising (including rectal sucralfate, adding metronidazole to an anti‐inflammatory regimen, and hyperbaric oxygen therapy), the quality of evidence was low to very low. Furthermore, outcomes important to people with cancer, including quality of life (QoL), and long‐term effects were often not addressed in these studies.
Conclusions
Although some interventions for late radiation rectal damage are promising, the evidence was of low quality and we can draw no firm conclusions. We could not combine data from the studies to compare different treatments, since the trial designs and outcome measures differed. The episodic and variable nature of late radiation rectal damage requires larger RCTs to establish whether treatments are effective. Future studies should address the possibility of associated injury to other pelvic structures, collectively known as pelvic radiation disease. Ideally outcome measures should be standardised across studies and include QoL evaluations and other outcomes important to people with cancer .
Quality of the evidence
The quality of the evidence for the majority of outcomes was low or very low, mainly due to the small size of most studies and study limitations.
Background
This review is an update of the previously published Cochrane review 'Non‐surgical interventions for late radiation proctopathy in people who have received radical radiotherapy to the pelvis' (Denton 2002), which was last updated in 2007.
Description of the condition
Radiotherapy is often used to treat cancer in the pelvic area. It can be used as single therapy or in combination with chemotherapy, as primary treatment or before or after surgery. Examples of cancer in the pelvis that can be treated with radiotherapy include prostate, bladder, cervix, endometrial, vaginal, rectal, and anal cancer. One drawback of radiotherapy is the development of late radiation toxicity. Several organs in the pelvis, including the anus, rectum, bladder, prostate, gynaecological organs, pelvic bones, and sometimes small bowel may be exposed to the effects of radiotherapy, which can lead to a variety of symptoms. The term 'pelvic radiation disease' has been proposed, defined as “transient or long term problems, ranging from mild to severe, arising in non‐cancerous tissues resulting from radiotherapy treatment to a tumour of pelvic origin” (Andreyev 2010; Denham 2002). If symptoms are confined to the rectum or the anorectal complex, we tend to speak of radiation‐associated proctopathy, or radiation‐induced proctopathy, formerly referred to as late radiation proctopathy. The current review, which is an update of a Cochrane review first published in 2002 and subsequently assessed as up to date in 2008, about the treatment of late radiation proctopathy is mainly focused on late radiation‐associated proctopathy, further referred to as radiation proctopathy, although more recent studies may examine treatment for pelvic radiation disease (Andreyev 2013). Radiation proctopathy is the most often investigated late‐radiation effect to the pelvis. The common symptoms are rectal urgency, rectal incontinence, pain, strictures, mucus discharge, and rectal bleeding.
There have been extensive investigations into the association between rectal dose and the occurrence of late radiation proctopathy when treating the prostate gland (Boersma 1998; Peeters 2006; Pollack 2002). Since several dose‐escalation studies found an improved biochemical control for prostate cancer at higher radiation doses, the standard care for treatment is a prescribed dose of at least 75 Gy (Peeters 2006b; Pollack 2002; Zietman 2005). However, in these dose‐escalation studies, an increase of radiation proctopathy was found with higher doses to the prostate (and therefore also to the rectum). It is reasonable to assume that the risk of radiation proctopathy also applies to other cancer sites in the same region that are being radiated. Toxic effects of radiotherapy are often graded according to the Radiotherapy Therapy Oncology Group/European Organisation for Research and Treatment of Cancer (RTOG/EORTC) Late Radiation Morbidity Scoring Schema on a scale of 0 to 5 (www.rtog.org, Appendix 1). The Common Terminology Criteria for Adverse Events (CTCAE) and other adjusted local scorings schemas are also often used. Radiation proctopathy greater than grade 2 has been reported in 20% to 35% of men treated for prostate cancer (Al‐Mamgani 2008; Pollack 2002; Zietman 2005). However, these are physician‐reported outcomes. Patient‐reported outcomes (outcomes derived directly from people about how they function or feel in relation to a health condition and its therapy, without interpretation of the patient’s responses by a clinician) could be very different, and thus these toxicity outcomes could be underestimated (Higgins 2011).
The pathophysiology and symptomatology of radiation proctopathy are rather complex because different anorectal subregions can be involved (Heemsbergen 2005; Smeenk 2012b). For example, rectal incontinence and urgency are caused by reduced rectal capacity and tissue compliance resulting in impaired anal sphincter function (Kushwaha 2003; Smeenk 2012b; Yeoh 2009). Incontinence and urgency symptomatology originates from different muscle groups of the anorectal sphincter complex (Smeenk 2012). However, in this patient group incontinence can also be induced by loose stool consistency and speed of transit through the small bowel (Putta 2005). Rectal bleeding is usually the result of vulnerable mucosa combined with loss of submucosal capillaries, leading to new, but abnormal, blood vessels formation (teleangiectasia) in the rectal wall.
With modern radiotherapy techniques such as intensity modulated radiotherapy (IMRT) and volumetric modulated arc therapy (VMAT), higher conformal dose distribution to the prostate gland is reached with consequently lower doses to the anorectal complex. Yet, even with IMRT radiation, proctopathy incidence is 5% to 65% (Bekelman 2011; De Meerleer 2007; Zelefsky 2008).
Description of the intervention
The medical treatment of radiation proctopathy is not clearly defined and in the absence of recommendations, management is often unsatisfactory. This is due in part to difficulties in recognising and establishing the diagnosis and also because a proportion of the biological changes are not reversible. At present there is no 'best' treatment for this clinical scenario, and the outcomes of both medical and surgical management can be disappointing. The literature suggests a number of treatment options, including low‐residue or elemental diets, pain control, and replacement transfusion. Other therapies are reported to be of variable benefit in controlling symptoms, with the option of surgery if medical management fails or is inappropriate (Babb 1996).
Current non‐surgical treatment options include:
-
Aminosalicylic acid derivatives: Anti‐inflammatory agents in this group, such as sulfasalazine and mesalazine, have been reported to have a role in the management of this condition (Baum 1989). Another agent with anti‐inflammatory properties is WF10 (Veerasarn 2006).
Sulfasalazine is a prodrug that is composed of 5‐aminosalicylic acid (5‐ASA) and sulfapyridine. In the colon, sulfasalazine is divided by the bacterial enzyme azoreductase into the two components. 5‐ASA (mesalazine), the active component, is poorly absorbed from the colon and is largely secreted in the stool. Sulfapyridine is largely absorbed in the colon and is associated with many side effects. Sulfasalazine is available as an oral and as a topical (rectal) compound. Intake is distributed in two to three times over day. At the beginning of therapy, a dose of 2 g to 4 g a day is given, which can be lowered to 1 g to 2 g a day for maintainance. Sulfazalasine, 5‐ASA (mesalazine), is the active component against inflammatory bowel disease. The precise mechanism of action is not known, but is mainly attributed to anti‐inflammatory and immunosuppressive properties. The considered mechanisms of action are: inhibition of cytokine synthesis (Bantel 2000; Rousseaux 2005), inhibition of prostaglandin and leukotriene synthesis (Hawkey 1985), free radical scavenging (Ahnfelt‐Ronne 1990), immunosuppressive activity by inhibition of T‐cell and B‐cell activation (MacDermott 1989a; Stevens 1995), and impairement of white cell adhesion and function (Neal 1987).
Mesalazine is the active component of sulfasalazine. It is rapidly absorbed in the jejunum. Several compounds are on the market with delayed release of mesalazine to increase the availability for the colon.
Short chain fatty acid (SCFA) preparations: SCFA enema is a solution of sodium acetate, sodium propionate, sodium n‐butyrate with additional sodium chloride. As butyrate is the most effective SCFA for colonic regeneration, an only‐butyrate enema as a 80 mmol solution is also effective (Hille 2008; Vernia 2000). This solution is placed endorectally once or twice a day. Vernia et al. found that an application course of three weeks or more is needed for optimal result (Vernia 2000). An improvement can be observed after four to six weeks (Cook 1998). SCFAs are organic fatty acids that are produced by colonic bacterial metabolism. SFCAs are produced predominantly in the colon by anaerobic bacterial fermentation of non‐absorbed carbohydrates. The majority is absorbed in the colon. When SCFA is absorbed by colonocytes, sodium and water absorption is stimulated. Besides this, SCFA dilates resistance arteries, increase mucosal blood flow and oxygen uptake, reduces mucosal permeability, and enhances production and release of mucus (Kvietys 1981; Mortensen 1990). SCFAs are necessary for optimal growth of the colonic mucosa and stimulate cell proliferation.
Sucralfate preparations: Sucralfate is an aluminium salt of sucrose octasulfate. It can be used twice a day in a 1 g to 2 g enema as a 10% suspension in water (Kochhar 1991; McElvanna 2014). Relief of symptoms can be expected after one to two weeks. Other possible suspensions are in propylcellulose or glycerine. The dose varies from 1 g to 10 g (Carling 1986; Kochhar 1988). Sucralfate works by a cytoprotective effect because of an adherent complex binding to tissue proteins of the mucosa and protecting the colonic mucosa. There is evidence for promoting angiogenesis and reduction of microvascular injury (O'Brien 1997). Another possible mechanism is because of lowering of prostaglandin‐E2 levels (Zahavi 1989).
-
Coagulation therapy:
Bipolar electrocoagulation: Coagulation is achieved with a probe by heating the contact tissue. Direct contact of the probe with the treated tissue is necessary. At the tip of the probe positive and negative electrodes are located that pass electricity through the tissue. Coagulation depth is controlled by probe size, duration of heating, and choice of energy. The applied current is locally between the electrodes of the probe. The coagulation is aimed at bleeding telangiectatic areas. At 70ºC tissue coagulation will occur and bleeding is stopped. Once the tissue is desiccated, tissue resistance increases and prevents deep coagulation. Bipolar electrocoagulation is effective for superficial lesions. Penetration depth can be regulated by the probe size, applied energy level, and pressure of the probe on tissue.
Thermal coagulation therapy: The heater probe is a thermocouple to heat up the exposed tissue by cauterisation. At the tip of the probe there is a heat‐generating device that converts electric energy to heat energy (Protell 1978).
Argon plasma coagulation uses ionised argon gas for a thermal reaction. A probe is introduced to the treatment area, and argon gas, which itself is non‐flammable, is sprayed on the surface to be treated and ionised by 6000 volts via electric wires in the probe. A high‐frequency current develops between the electrode and the underlying tissue, resulting in coagulation and desiccation. Desiccated tissue loses electric conductivity, prohibiting deep devitalization of the treated tissue. Coagulation is achieved by heating up the treated tissue. Heating is not influenced by tissue resistance, making deep coagulation possible. The mechanism of coagulation and desiccation of the treated tissue is by direct heat transfer.
Corticosteroids: Several steroid enemas are available for radiation proctopathy treatment. Hydrocortisone, prednisolone, and betamethasone were used in this review. The enema is introduced into the rectum for several minutes once a day. The treatment can be continued for 2 to 4 weeks. Hydrocortisone is available as, for example, 100 mg in a 60 ml aqueous solution. Betamethasone, which has a more powerful action than hydrocortisone, is available as, for example, 5 mg in a 100 ml aqueous solution. The working mechanism of topical corticosteroid application (steroid enema) is an anti‐inflammatory effect with the inhibition of prostaglandin synthesis.
Formalin applications: Formalin is applied topically in a 4% to 10% solution on the affected rectal mucosa. The application can be either by irrigation of the rectum or direct application with a soaked gauze. Formalin acts by hydrolysing proteins leading to chemical cauterisation. Another mechanism is by coagulation of the tissue (Haas 2007; Leiper 2007; Parikh 2003).
Pentoxyfilline: Pentoxyfilline is a xanthine derivative. It is almost completely resorbed by oral admission. It is also used for intermittent claudication and administered orally three times a day 400 mg. Pentoxifylline increases the deformability of erythrocytes, inhibits the aggregation of thrombocytes, and reduces fibrinogen level in plasma. In this way microvascular blood flow is enhanced.
Antibiotic treatment: Metronidazole is an antibiotic that is especially useful in the treatment of anaerobic infections. Metronidazole is effective against obligate anaerobes and against facultative anaerobes such as Helicobacter pylori and Gardnerella vaginalis. It is orally well absorbed and distributed evenly into body tissues. The dosage is dependent on the indication for treatment. Usual oral administration is 1 g to 2 g distributed in one to four intakes. The working mechanism of metronidazole is by its anti‐inflammatory action to improve mucosal healing in combination with other therapeutic measures.
Hyperbaric oxygen: Hyperbaric oxygen therapy is performed in specially designed chambers. One or more people are treated in the chamber with 100% oxygen. Pressure is increased to 25 x 104 to 30 x 104 Pa. Treatment duration is approximately 60 to 90 minutes, 5 to 7 days a week. Number of treatments varies with the degree of severity and response effect, usually 30 to 40 treatments. Plasma oxygen level increases by inhalation of 100% high‐pressure oxygen. As a result, tissue oxygenation will improve. Hyperbaric oxygen facilitates fibroblast proliferation, angiogenesis, and wound healing (Marx 1990; Roth 1994; Wattel 1998).
Retinol palmitate: Retinol palmitate, or vitamin A, is completely absorbed via the bowel. The recommended daily allowance for an adult male is 3000 IU (900 micrograms) and for an adult female 2300 IU (700 micrograms). Therapeutic dosage for retinol in case of deficiency is 25,000 to 50,000 IU per day for a limited period. The prophylactic dosage is 2500 to 5000 IU per day. Retinol palmitate increases fibroblast secretion of mucopolysaccharide and collagen and increases fibronectin synthesis. These mechanisms assist in wound healing (Hein 1984).
Chinese traditional medicine in combination with Western medicine: consists of Shen Ling Bai Zhu powders (herbal ingredients) and Western medicine (smectite powder 6 g, dexamethasone 5 mg, levofloxacin hydrochloride 0.2 g, anisodamine 10 mg, and physiological saline 100 ml).
How the intervention might work
Why it is important to do this review
As described above, late radiation proctopathy is common, with incidence rates of 5% to 65% (Bekelman 2011; De Meerleer 2007; Zelefsky 2008), and potentially a major burden to people with cancer. The medical treatment of radiation proctopathy is not clearly defined, and in the absence of recommendations, management of the condition is often unsatisfactory. While the literature suggests a number of treatment options, there is no 'best' treatment choice. We are therefore assessing the effectiveness of various non‐surgical treatment options in managing late radiation proctopathy in this review.
Objectives
To assess the effectiveness and safety of non‐surgical interventions for managing late radiation proctopathy.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) or quasi‐RCTs, irrespective of language and publication status, that compared any non‐surgical intervention for late radiation proctopathy to no intervention, placebo, or any other intervention were eligible for inclusion. In previous versions of this review, non‐randomised studies were also eligible. For this update, as RCT data was available, we decided to focus on RCTs and quasi‐RCTs only, as non‐randomised studies suffer from high risk of bias.
Types of participants
People diagnosed with a pelvic malignancy, who had undergone pelvic radiotherapy as part of their treatment schedule (primary radiotherapy, pre‐ or postoperative radiotherapy, with or without chemotherapy, or as a palliative treatment) and subsequently developed late radiation proctopathy, defined as radiation proctopathy of any grade, continuing from completion of radiotherapy for more than three months, or occurring more than three months after completion of radiotherapy.
Types of interventions
Experimental: any non‐surgical intervention for late radiation proctopathy, such as:
Aminosalicylic acid derivatives
Short chain fatty acid (SCFA) preparations
Sucralfate preparations
Coagulation therapy
Corticosteroids
Formalin applications
Pentoxyfilline
Hyperbaric oxygen
Antibiotic treatment
Control: no intervention, placebo, or any other non‐surgical intervention.
Types of outcome measures
Primary outcomes
We determined primary outcome measures by the presenting symptoms as recorded retrospectively or prospectively with diaries and scoring systems, for example:
Episodes of bowel activity
Bleeding
Pain
Tenesmus
Urgency
Sphincter dysfunction
Secondary outcomes
Mortality
Morbidity
Quality of life (QoL): 12‐Item Short Form Health Survey (Ware 1996), Subjective, Objective, Management, Analytic ‐ Late Effects of Normal Tissue (SOMA‐LENT) score (Pavy 1995), Visual Analogue Scale, (Scott 1976), mean Inflammatory Bowel Disease Questionnaire–Bowel subset score (IBDQ‐B) (Cheung 2000)
Search methods for identification of studies
Electronic searches
The literature searches from inception to 2007 have been updated and were re‐run in November 2015.
The Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 11, 2015) (Appendix 2)
MEDLINE (Ovid) (April 2007 to Nov week 1 2015) (Appendix 3)
EMBASE (Ovid) (April 2007 to 2015 week 45) (Appendix 4)
We did not apply a search filter due to the range of interventions searched for. This basic strategy was expanded for text and MeSH terms before being applied to the described databases.
Searching other resources
In addition, we searched the prospective trial register ClinicalTrials.gov with the key words 'proctitis' AND 'radiation' and 'proctopathy' AND 'radiation', and checked reference lists.
Data collection and analysis
We downloaded all titles and abstracts retrieved by the electronic searches to a reference management database (Reference Manager) and removed duplicate references. Two pairs of review authors (FW and RS; LV and JV) independently examined the remaining references. We excluded those studies that clearly did not meet the inclusion criteria, and we obtained copies of the full text of potentially relevant references. Two pairs of review authors (FW and RS; LV and JV) independently assessed the eligibility of the retrieved reports/publications. We resolved any disagreements through discussion or, if required, by consulting a third review author (RS). We identified and excluded duplicates. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of excluded studies table.
Data extraction and management
We (FW, LV, JV) independently abstracted the data using a pre‐designed data abstraction form (Appendix 5). A second review author (LV, JV, or RS) checked data abstraction for accuracy. One review author (FW) transferred data into the RevMan 5 file (Review Manager 2014). We double‐checked that data was entered correctly by comparing the data presented in the systematic review with the study reports.
Assessment of risk of bias in included studies
Two review authors (FW, RS) independently assessed the risk of bias of all included studies according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We grouped outcomes as ‘subjective’ and ‘objective’ for the purposes of assessing blinding and incomplete outcome data. We resolved disagreements through consensus. For each relevant comparison we summarised the evidence per outcome in an additional table. We allocated the level of evidence using the the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system (GRADEpro 2014).
Measures of treatment effect
We described dichotomous data using the risk ratio (RR) with 95% confidence interval (CI). We expressed continuous data, that is symptom scores, as mean differences (MDs).
Dealing with missing data
If outcome data were missing, we planned to contact trial authors.
Assessment of heterogeneity
We planned to formally test statistical heterogeneity using the natural approximate Chi2 test, which provides evidence of variation in effect estimates beyond that of chance. Since the Chi2 test has low power to assess heterogeneity where a small number of participants or trials are included, we planned to set the P value conservatively at 0.1. We planned to test heterogeneity using the I2 statistic, which calculates the percentage of variability due to heterogeneity rather than chance.
We planned to interpret the I2 statistics in relation to the size of the included studies. We used the following interpretation as a rough guide:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
We planned to examine potential sources of clinical heterogeneity through the use of analyses as specified above. We planned to examine potential sources of methodological heterogeneity through the use of sensitivity analyses (Sensitivity analysis).
Assessment of reporting biases
If more than 10 included studies were available, we planned to use funnel plots to assess the potential for small‐study effects such as selective publication.
Data synthesis
We had planned to pool outcome data from studies that were sufficiently similar in participant characteristics and methodology followed (length of follow‐up, diagnostic criteria) and to use the random‐effects model for meta‐analysis, as we expected diversity in cancer types, interventions, and definitions of radiation proctopathy across included studies. However, due to the heterogeneous nature of the included studies, this was not feasible for this update but may be implemented in the future.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses to investigate possible differences between groups, considering factors such as age, stage, type of intervention, and length of follow‐up in interpreting any heterogeneity.
Sensitivity analysis
We planned sensitivity analyses by excluding studies with high risk of bias. However as no meta‐analyses were performed, no sensitivity analyses were undertaken.
Results
Description of studies
Results of the search
In the previous version of this review (Denton 2002), which was assessed as up to date in 2008, seven trials were included (Cavcic 2000; Ehrenpreis 2005; Jensen 1997; Kochhar 1991; Pinto 1999; Rougier 1992; Talley 1997). Through the update, we found a total of 1443 trials as a result of the literature search. After we removed duplicates, 1221 were left for evaluation. We (FW, LV, JV) identified a total of 48 potential titles and abstracts for full‐text evaluation. After review of full text and discussion (FW, LV, JV, RS), we excluded a further 31 trials (Characteristics of excluded studies). We (FW, LV, JV, RS) eventually decided to include an additional nine trials (Figure 1), increasing the total number of included trials to 16 (including 993 participants). One eligible study found through the last update was moved to the Studies awaiting classification section (Guo 2015). Details of the participants, interventions, and outcomes in these trials are in the Characteristics of included studies table.
1.
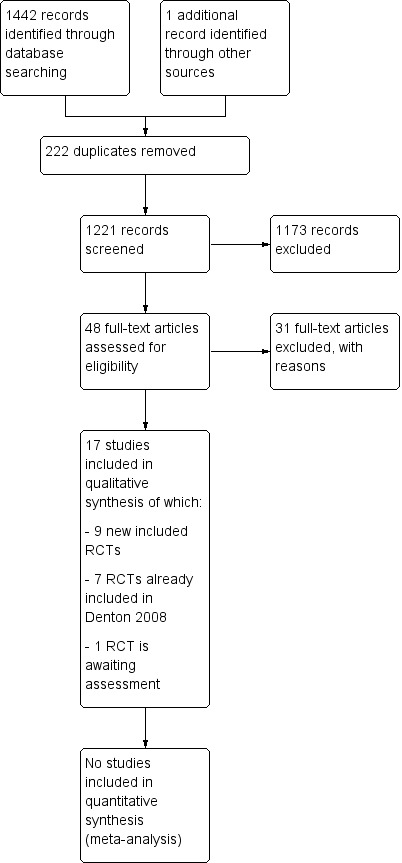
Study flow diagram.
Included studies
Study design
Five trials were placebo controlled (Chruscielewska 2012; Clarke 2008; Ehrenpreis 2005; Pinto 1999; Talley 1997), three trials assessed the effect of the addition of one intervention to another intervention, (Cavcic 2000; Kochhar 1991; Venkitaraman 2008) and eight trials compared two or more active interventions (Andreyev 2013; Jensen 1997; Lenz 2010; Nelamangala 2012; Rougier 1992; Sahakitrungruang 2012; Tian 2008; Yeoh 2013). All but two trials addressed single comparisons (Pinto 1999; Talley 1997).
Types of interventions
Four trials compared different types of anti‐inflammatory agents as a treatment for late radiation proctopathy (Cavcic 2000; Kochhar 1991; Rougier 1992; Venkitaraman 2008). In two trials, short‐chain fatty acids (SCFA) enemas were compared to placebo (Pinto 1999; Talley 1997). Three trials assessed the effects of sucralfate compared to either anti‐inflammatory agents (Kochhar 1991), formalin therapy (Nelamangala 2012), or placebo (Chruscielewska 2012). Another three trials assessed the effects of formalin therapy compared to colonic irrigation (Sahakitrungruang 2012), sucralfate steriod (Nelamangala 2012), or argon plasma coagulation (Yeoh 2013). Two trials assessed the effect of thermal coagulation therapy to either heater probe, in Jensen 1997, or bipolar coagulation, in Lenz 2010. One trial assessed the effect of hyperbaric oxygen therapy versus placebo (Clarke 2008), and three trials assessed other interventions: integrated Chinese traditional medicine (Tian 2008), retinol palmitate (Ehrenpreis 2005), and a gastroenterologist‐led algorithm‐based treatment with a nurse‐led algorithm‐based treatment or with usual care (Andreyev 2013).
Participant characteristics
Three trials included participants with prostate cancer (Cavcic 2000; Venkitaraman 2008; Yeoh 2013), one trial included participants with cervical cancer (Nelamangala 2012), seven trials included a mixed population of participants with prostate, cervical, endometrial, uterine, vaginal, or rectal cancer (Andreyev 2013; Chruscielewska 2012; Jensen 1997; Kochhar 1991; Lenz 2010; Sahakitrungruang 2012; Talley 1997), and five trials did not specify cancer types of their participants (Clarke 2008; Ehrenpreis 2005; Pinto 1999; Rougier 1992; Tian 2008).
Fourteen trials were published in English, one was published in French, and one in Chinese.
In addition, we searched the prospective trial register ClinicalTrials.gov with the key words 'proctitis' AND 'radiation' and 'proctopathy' AND 'radiation' and found four potentially relevant registered trials that are ongoing (Characteristics of ongoing studies).
Excluded studies
A total of 47 titles and abstracts seemed to fulfil our inclusion criteria and required further discussion. Through discussion we excluded 31 of these studies (Characteristics of excluded studies). We excluded 11 studies because of their design (no randomised studies), 10 studies because the population did not fulfil our inclusion criteria (no late rectal consequences of radiotherapy), 9 studies as they were prevention studies, and 1 study because the intervention did not fulfil our inclusion criteria.
Risk of bias in included studies
The results of the 'Risk of bias' assessment of the included studies are presented in the Characteristics of included studies tables and summarised in Figure 2 and Figure 3.
2.
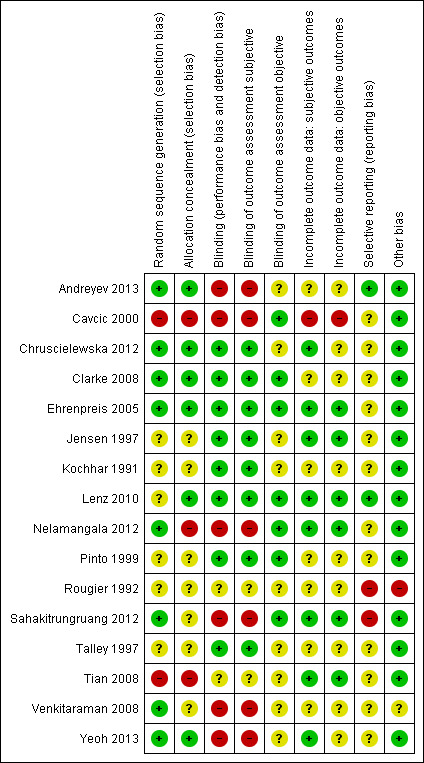
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
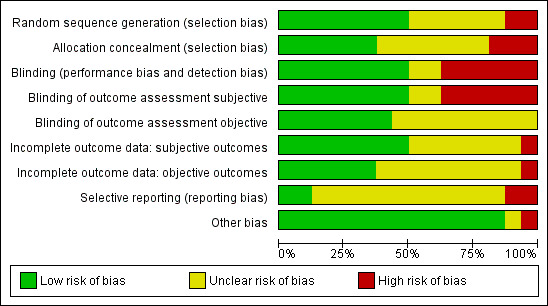
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Appropriate sequence generation to ensure randomisation seemed likely in eight studies (Andreyev 2013; Chruscielewska 2012; Clarke 2008; Ehrenpreis 2005; Nelamangala 2012; Sahakitrungruang 2012; Venkitaraman 2008; Yeoh 2013). Cavcic 2000 and Tian 2008 had a high‐risk score as they were quasi‐randomised trials (treatment was allocated according to the date on which their treatment began, in Cavcic 2000, and according to treatment order, in Tian 2008). The remaining six studies did not adequately describe the method of randomisation, and thus were judged to be of unclear risk of selection bias (Jensen 1997; Kochhar 1991; Lenz 2010; Pinto 1999; Rougier 1992; Talley 1997).
Allocation concealment was adequate in six studies (Andreyev 2013; Chruscielewska 2012; Clarke 2008; Ehrenpreis 2005; Lenz 2010; Yeoh 2013; ). We judged the method of allocation concealment to be inappropriate, resulting in a high risk of bias, in three studies, as two used quasi‐randomisation (Cavcic 2000; Tian 2008), and one used an open list of random numbers (Nelamangala 2012). The method of allocation concealment was not clearly described in the remaining seven studies.
Blinding
The participants and personnel were not blinded in six studies (Andreyev 2013; Cavcic 2000; Nelamangala 2012; Sahakitrungruang 2012; Venkitaraman 2008; Yeoh 2013), which we therefore judged as having a high risk of performance bias. Participants and personnel were adequately blinded in eight studies (Chruscielewska 2012; Clarke 2008; Ehrenpreis 2005; Jensen 1997; Kochhar 1991; Lenz 2010; Pinto 1999; Talley 1997). For the remaining two studies, insufficient information was provided to judge the potential risk of performance bias and we therefore considered them to be at an unclear risk of bias.
Outcome assessors for subjective outcomes were not blinded in six studies (Andreyev 2013; Cavcic 2000; Nelamangala 2012; Sahakitrungruang 2012; Venkitaraman 2008; Yeoh 2013), and blinded in eight studies (Chruscielewska 2012; Clarke 2008; Ehrenpreis 2005; Jensen 1997; Kochhar 1991; Lenz 2010; Pinto 1999; Talley 1997). In the remaining two studies, the risk of bias on this item was unclear due to insufficient information.
As for the objective outcomes, the outcome assessors were blinded in seven studies (Cavcic 2000; Clarke 2008; Ehrenpreis 2005; Lenz 2010; Nelamangala 2012; Pinto 1999; Sahakitrungruang 2012;). In six studies the risk of bias on this item was again unclear due to insufficient information, and three studies did not assess objective outcomes (Andreyev 2013; Chruscielewska 2012; Yeoh 2013).
Incomplete outcome data
For subjective outcomes, we judged eight studies as at low risk of attrition bias (Chruscielewska 2012; Ehrenpreis 2005; Jensen 1997; Lenz 2010; Nelamangala 2012; Pinto 1999; Sahakitrungruang 2012; Tian 2008). We scored one study as at high risk of attrition bias, as a substantial number of participants (25% after three months, 40% after one year, 52% after two years) dropped out of the study for unknown reasons (Cavcic 2000). For the remaining seven studies, insufficient information was provided to draw a safe conclusion.
As for the objective outcomes, we judged six studies as at low risk of attrition bias (Ehrenpreis 2005; Jensen 1997; Lenz 2010; Nelamangala 2012; Sahakitrungruang 2012; Tian 2008). We scored one study as at high risk for attrition bias because the number of dropouts was not evenly distributed between the two groups (at three months: 10% versus 40%; after one year: 20% versus 60%; after two years: 37% versus 67%), and reasons for lost to follow‐up were not reported (Cavcic 2000). In three studies (Andreyev 2013; Chruscielewska 2012; Yeoh 2013), objective outcomes were not assessed. The remaining six studies provided insufficient information to draw a safe conclusion and we therefore judged them as at unclear risk of bias.
Selective reporting
We judged only two studies to be at low risk of selective reporting bias (Andreyev 2013; Lenz 2010). We judged two studies to be at high risk of reporting bias (Rougier 1992; Sahakitrungruang 2012). In the remaining studies no protocol was available, and we therefore judged the risk of bias on this item as unclear.
Other potential sources of bias
We judged all but two of the studies to be at low risk of other bias. In one study there were substantial differences in baseline characteristics (more aggressive grade of disease in the betamethasone group), resulting in a high risk of bias (Rougier 1992). We judged a second study to be at unclear risk of bias, as they failed to achieve the expected rate of enrolment, which may have influenced the results (Venkitaraman 2008).
Effects of interventions
Since radiation proctopathy is a condition with various symptoms or combinations of symptoms, the studies are heterogeneous in their intended effect. Some studies investigated treatments targeted at bleeding only (group 1), some investigated treatments targeted at a combination of anorectal symptoms, but not a single treatment (group 2). The third group focused on the treatment of the collection of symptoms referred to as pelvic radiation disease. In order to enable some comparison of this heterogeneous collection of studies, we described the effects in these three groups separately.
1. Treatments for rectal bleeding
(see Table 1)
1. Treatment for bleeding only.
| Study ID | Participant characteristics | Intervention(s) | Results | Level of evidence (GRADE) |
| Chruscielewska 2012 | Adults with chronic radiation proctopathy or proctosigmoiditis were included if all of the following criteria were met: radiotherapy for a pelvic tumour completed at least 3 months before enrolment, the presence of rectal bleeding, radiation‐induced telangiectasia in the rectum and/or sigmoid colon on endoscopy, informed written consent by the person to participate in the study |
Endoscopic APC followed by oral sucralfate (6 g twice daily) for 4 weeks (n = 60) versus APC with placebo administration for 4 weeks (n = 62) |
Changes in chronic radiation proctopathy severity score (based on scales previously proposed by Chutkan et al. and Kochhar et al., median (interquartile range)) Overall severity score Week 8: 2 (1.3) versus 2 (1.3) Week 16: 2 (1.3) versus 2 (1.3) Week 52: 1 (1.2) versus (1.2) P > 0.05 for all between‐group comparisons |
Moderate due to imprecision (precision not quantified) |
|
Diarrhoea score Week 8: 1 (1.1) versus 1 (1.1) Week 16: 1 (1.1) versus 1 (1.1) Week 52: 1 (1.1) versus 1 (1.1) P > 0.05 for all between‐group comparisons |
Moderate due to imprecision (precision not quantified) | |||
|
Bleeding score Week 8: 1 (0.1) versus 0 (0.1) Week 16: 0 (0.1) versus 0 (0.1) Week 52: 0 (0.1) versus 0 (0.0) P > 0.05 for all between‐group comparisons |
Moderate due to imprecision (precision not quantified) | |||
|
Tenesmus ⁄ rectal pain score Week 8: 0 (0.1) versus 0 (0.1) Week 16: 0 (0.0) versus 0 (0.1) Week 52: 0 (0.0) versus 0 (0.0) P > 0.05 for all between‐group comparisons |
Moderate due to imprecision (precision not quantified) | |||
|
Endoscopy scores (graded according to the Gilinsky scale as endoscopic severity score) Score 6 to 9: Week 8: 20 to 59 versus 9 to 60 (RR 2.26, 95% CI 1.12 to 4.55) Week 16: 10 to 57 versus 8 to 60 (RR 1.32, 95% CI 0.56 to 3.10) |
‐ | |||
|
Complications Number (%) of people with complications: 38/60 (63%) versus 36/62 (58%) (RR 1.09, 95% CI 0.82 to 1.45) |
Moderate due to imprecision (CI includes both benefits and harms) | |||
|
APC‐related complications Asymptomatic rectal ulcer: 30 versus 30 Symptomatic rectal ulcers: 7 versus 5 Rectovaginal fistula: 2 versus 0 Adynamic ileus: 0 versus 1 |
Low due to very serious limitations for imprecision (CI includes both benefits and harms) | |||
|
Other complications Severe constipation: 4 versus 0 Urticaria: 1 versus 0 |
‐ | |||
|
Complication severity (APC‐related) Severe + fatal: 2/60 versus 0/62 (RR 5.17, 95% CI 0.25 to 105.4) P > 0.05 for all between‐group comparisons regarding complications |
Low due to very serious imprecision (CI includes both benefits and harms) | |||
| Jensen 1997 | People being considered for surgery, having failed 1 year of medical therapy, pelvic radiotherapy for a cancer at least 2 years earlier, rectal bleeds at least 3 times per week, anaemia, and a life expectancy of at least 2 years |
Bipolar electrocoagulation (n = 12) versus Heater probe (n = 9) |
Severe bleeding episodes after 1 year (measured by participant interview) 3/12 (33%) versus 1/9 (11%) (RR 2.25, 95% CI 0.28 to 18.22) |
Low due to very serious imprecision (CI includes both benefits and harms) |
|
Mean number of severe bleeds after 1 year, mean (SD) (measured by participant interview) 0.3 (0.3) versus 0.4 (0.9) (MD ‐0.10, 95% CI ‐0.71 to 0.51) |
Low due to very serious imprecision (CI includes both benefits and harms) | |||
|
Mean haematocrits after 1 year, mean (SD) 38.2 (4.8) versus 37.6 (3.0) (MD 0.60, 95% ‐2.75 to 3.95) |
Low due to very serious imprecision (CI includes both benefits and harms) | |||
|
Mean units of red blood cells transfused after 1 year, mean (SD) 0.0 (0.0) versus 0.2 (0.6) (MD not estimable) |
Low due to very serious imprecision (OIS not reached) | |||
|
Complications No major complications occurred |
||||
|
QoL QoL informally assessed with participant responses, which improved with treatment |
||||
|
Kochhar 1991 |
Symptomatic radiation‐induced proctosigmoiditis | Oral sulfasalazine (500 mg tds) + rectal prednisolone (20 mg bd) for 4 weeks (n = 8) versus Oral placebo + rectal sucralfate suspension (2 g bd) for 4 weeks (n = 19) |
Clinical improvement at 4 weeks (an in‐house scoring system was used measuring diarrhoea, bleeding per rectum, bleeding requiring blood transfusion, and tenesmus) 8 to 15 versus 16 to 17 (RR 0.57, 95% CI 0.35 to 0.92) |
Low due to unclear RoB and imprecision (OIS not reached) |
|
Endoscopic improvement at 4 weeks (injury was graded according to the criteria of Gilinsky et al.) 7 to 15 versus 12 to 17 (RR 0.66, 95% CI 0.35 to 1.23) |
Very low due to unclear RoB and very serious imprecision (CI includes both benefits and harms) | |||
|
Side effects at 4 weeks (participants were questioned and examined for any side effects of the medication) 2 participants in the sulfasalazine group did not tolerate the drugs due to myalgia, nausea, and headaches |
Very low due to unclear RoB and very serious imprecision (very small sample size) | |||
| Lenz 2010 | Recurrent rectal bleeding, started 6 months after radiotherapy with at least 1 bleeding episode in the week before and endoscopically confirmed radiation telangiectasias | APC (n = 15) versus Bipolar electrocoagulation (n = 15) |
Eradication of all telangiectasias at 1 year 12/15 versus 14/15 (RR 0.86, 95% CI 0.64 to 1.14) |
Low due to indirectness and imprecision (CI includes both benefits and harms) |
|
Complications Minor: 5/15 versus 10/15 (RR 0.50, 95% CI 0.22 to 1.11) Major: 1/15 versus 5/15 (RR 0.20, 95% CI 0.03 to 1.51) |
Low due to very serious imprecision (CI includes both benefits) | |||
|
Relapse 1/12 versus 2/14 (RR 0.58, 95% CI 0.06 to 5.66) |
Low due to very serious imprecision (CI includes both benefits and harms) | |||
| Nelamangala 2012 | Rectal bleeding as a result of chronic haemorrhagic radiation proctopathy, following radiotherapy for carcinoma of the cervix |
Formalin dab treatment (4%) as an outpatient procedure (formalin applied directly to each lesion via a rigid sigmoidoscope or a proctoscope under local anaesthesia. Under direct vision, a small piece of gauze soaked in 4% formalin was applied to the haemorrhagic areas for 2 min until the mucosa turned pale) (n = 51) versus Sucralfate steroid retention enema (100 mg of prednisolone and 1 g of sucralfate in 100 ml of normal saline), twice daily for 7 to 10 days (n = 51) |
Symptom score after treatment (graded by the RPSAS), median (range) 9 (6 to 24) versus 13 (8 to 27) (P < 0.001) | Low due to high risk of bias and imprecision (OIS not reached) |
| Sigmoidoscopic score (median (range) after treatment) 1 (0 to 3) versus 2 (0 to 3) (P < 0.001) | Very low due to high risk of bias, imprecision and indirectness (OIS not reached) | |||
|
Adverse events “Mild pain occurred in 33.3% patients in Group 1 during the application of formalin but this subsided within 1 day. There were no complications in Group 2.” |
Low due to high risk of bias and imprecision (OIS not reached) | |||
| Pinto 1999 | Clinical and histological diagnosis of chronic radioproctitis: Grade III Pourquier classification with rectal bleeding at least once a week, persistent symptoms for a minimum of 12 months (except 1 participant) |
SCFA enema (as per Harig preparation 60 ml bd 5 weeks) (n = 10) versus Placebo: identically appearing enemas containing saline isotonic solution, bd 5 weeks (n = 9) |
Mean endoscopic score after 5 weeks, mean (SD) 2.6 (1.8) versus 1.6 (4.2) (MD 1.00, 95% CI ‐2.33 to 4.33) |
Low due to indirectness and imprecision (CI includes both benefits and harms) |
|
Number of days of rectal bleeding at the end of treatment (after 5 weeks), mean (SD) (participant diary) 1.4 (2.2) versus 3.4 (2.6) (MD ‐2.00, 95% CI ‐4.40 to 0.40) |
Low due to very serious imprecision (CI includes both benefits and harms) | |||
|
Haemoglobin levels after 5 weeks, mean (SD) 13.1 ± 0.9 g/dl versus 10.7 ± 2.1 g/dl (MD 2.40, 95% CI 0.74 to 4.06) |
Low due to imprecision (very small sample size) | |||
| Rougier 1992 | Chronic radiation proctopathy confirmed and graded on sigmoidoscopy |
Hydrocortisone acetate mousse 90 mg bd for 4 weeks (n = 16) versus Betamethasone lavage 5 mg od for 4 weeks (n = 16) |
Improvement of endoscopic appearance 12/16 versus 5/14 (RR 2.10, 95% CI 0.98 to 4.48) |
Very low due to high RoB, indirectness, and imprecision (CI includes both benefits and harms) |
|
Reduction of degree of bleeding (an in‐house scoring system was used) 6/16 versus 3/14 (RR 1.75, 95% CI 0.53 to 5.73) |
Very low due to high RoB and very serious imprecision (CI includes both benefits and harms) | |||
|
Poor tolerance of enema 2/16 versus 10/14 (RR 0.17, 95% CI 0.05 to 0.67) |
Low due to high RoB and imprecision (OIS not reached) | |||
| Sahakitrungruang 2012 | Symptomatic haemorrhagic radiation proctopathy for more than 6 months without complications of rectal stricture, deep ulceration, fistula formation, or sepsis | Colonic irrigation (with 1000 ml of tap water via a 20F Foley catheter) plus ciprofloxacin (500 mg twice daily) and metronidazole (500 mg 3 times daily) by mouth for 1 week (n = 25) versus Formalin application in an office setting (4% formalin‐soaked gauze applied for 3 minutes under direct vision by using proctoscopy followed by immediate cleansing with water irrigation) (n = 25) |
Comparison between the 2 treatment groups after 8 wks of treatment (measured by participant survey) Bleeding (days/week) median (min,max) ‐5 (‐7,0) versus ‐2 (‐7,4) (P = 0.007) |
Low due to high RoB and imprecision (OIS not reached) |
|
Frequency (times/day) ‐2 (‐8,2) versus ‐2 (‐4,2) (P = 0.09) |
Low due to high RoB and imprecision (OIS not reached) | |||
|
Urgency (days/week) ‐2 (‐7,3) versus 0 (‐2,7) (P = 0.0004) |
Low due to high RoB and imprecision (OIS not reached) | |||
|
Diarrhoea (days/week) ‐2 (‐6,0) versus 0 (‐7,2) (P = 0.007) |
Low due to high RoB and imprecision (OIS not reached) | |||
|
Tenesmus (days/week) ‐2 (‐7,0) versus 0 (‐4,4) (P = 0.07) |
Low due to high RoB and imprecision (OIS not reached) | |||
|
Haematocrit (mg/dL) 0 (‐9,10) versus 0 (‐7,18) (P = 0.86) |
Low due to high RoB and imprecision (OIS not reached) | |||
|
Vienna rectoscopy score after 8 wks of treatment No significant differences between groups (P = 0.78) |
Low due to high RoB and imprecision (OIS not reached) | |||
|
Adverse events after 8 wks of treatment "There were no serious adverse drug reactions." "Four patients in the irrigation group and 8 patients in the formalin group required blood transfusion during the study, but this finding did not reach the statistic difference." |
Low due to high RoB and imprecision (OIS not reached) | |||
|
Patient satisfaction 20/24 versus 10/23 (RR 1.92, 95% CI 1.16 to 3.16) |
Low due to high RoB and imprecision (OIS not reached) | |||
| Venkitaraman 2008 | Symptomatic late morbidity with at least 1 episode of rectal bleeding more than 6 months since pelvic radiotherapy |
Standard therapies for late radiation‐induced bleeding plus oral pentoxifylline (400 mg) 3 times daily for 6 months (n = 20) versus Standard treatment for late radiation‐induced bleeding (n = 20) |
Cessation of rectal bleeding (measured using a daily participant symptom diary) 16/20 versus 12/20 (RR 1.33, 95% CI 0.88 to 2.03) |
Very low due to high RoB and very serious imprecision (CI includes both benefits and harms) |
|
Median time to cessation of bleeding (measured using a daily participant symptom diary) 22 days (range 1 to 119 days) versus 95 days (range 13 to 172) (P = 0.12) “However, at least one episode of recurrent bleeding occurred in 14 of the 16 patients in the study group and in all the 12 patients who had cessation of bleeding in the control group.” |
Low due to high RoB and imprecision (OIS not reached) | |||
|
The median duration of freedom from bleeding (measured using a daily participant symptom diary) 12 days (range 8 to 290) versus 11 days (range 7 to 133) |
Very low due to high RoB and very serious imprecision (precision not quantified; very small sample size) | |||
|
Adverse events “Pentoxifylline was well tolerated with minor side‐effects and the compliance rate was satisfactory. Seven patients required a dose reduction or temporary discontinuation of pentoxifylline, due to dyspepsia in five patients and rashes and chest pain in one patient each, whereas one patient required permanent discontinuation of pentoxifylline due to dyspepsia. Contrary to expectations, one patient had a transient, but significant, increase in serum fibrinogen levels.” |
Very low due to high RoB and very serious imprecision (OIS not reached) |
APC: argon plasma coagulation bd: 2 times a day CI: confidence interval MD: mean difference od: once a day QoL: quality of life RoB: risk of bias RPSAS: Radiation Proctopathy System Assessments Scale RR: risk ratio SCFA: short chain fatty acid SD: standard deviation tds: 3 times a day OIS: Optimal Information Size
Short‐chain fatty acid (SCFA) versus placebo
One study randomised 19 participants with late radiation proctopathy (grade III of the Pourquier classification and with persistent symptoms for a minimum of 12 months) to SCFA enema (60 ml twice a day for 5 weeks) or a placebo (Pinto 1999). We considered the risk of bias of this study to be unclear. At the end of the treatment period, the difference in reduction in endoscopic score was 1 (95% confidence interval (CI) ‐2.33 to 4.33) (Analysis 1.1), and the difference between the mean number of days of rectal bleeding per week was ‐2 (95% CI ‐4.4 to 0.4) (Analysis 1.2), but this was not statistically significant. In long‐term follow‐up, two people were dropped from the placebo group because of severe bleeding, presumably representing treatment failures. At the end of the six months, the mean number of days of rectal bleeding per week and the endoscopic score was similar in the two groups.
1.1. Analysis.
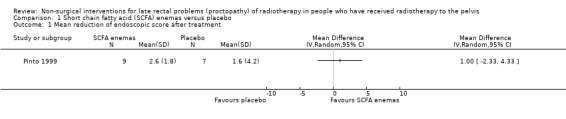
Comparison 1 Short chain fatty acid (SCFA) enemas versus placebo, Outcome 1 Mean reduction of endoscopic score after treatment.
1.2. Analysis.
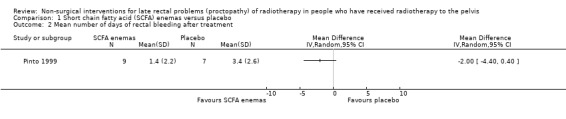
Comparison 1 Short chain fatty acid (SCFA) enemas versus placebo, Outcome 2 Mean number of days of rectal bleeding after treatment.
Sucralfate versus placebo following argon plasma coagulation
One study compared the efficacy of sucralfate with placebo following argon plasma coagulation (APC) for late haemorrhagic radiation proctopathy (Chruscielewska 2012). This study included 122 participants with haemorrhagic late radiation proctopathy after irradiation for prostate, uterine, cervix, rectal, or vaginal cancer. All participants received APC, and were then randomised to oral sucralfate (6 g twice a day) or placebo treatment for four weeks. APC was repeated every eight weeks, if necessary, after the first session. We considered the risk of bias of this study to be unclear. At all time points (week 8, 16, and 52), no differences between the two groups were found with regard to changes in late radiation proctopathy severity score. Significant differences in changes in endoscopy scores in favour of the placebo group were found at week 8 (score 6 to 9, week 8: risk ratio (RR) 2.26, 95% CI 1.12 to 4.55), but not at week 16 (score 6 to 9, week 16: RR 1.32, 95% CI 0.56 to 3.10) (Analysis 2.1; Analysis 2.2). The number of participants with complications did not significantly differ between groups (RR 1.09, 95% CI 0.82 to 1.45) (Analysis 2.3).
2.1. Analysis.
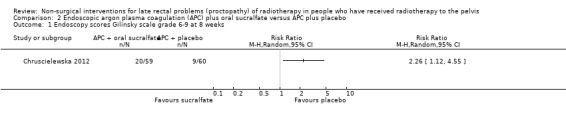
Comparison 2 Endoscopic argon plasma coagulation (APC) plus oral sucralfate versus APC plus placebo, Outcome 1 Endoscopy scores Gilinsky scale grade 6‐9 at 8 weeks.
2.2. Analysis.
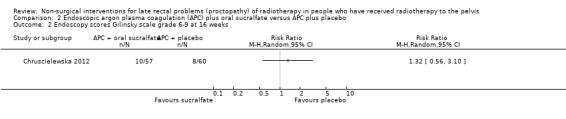
Comparison 2 Endoscopic argon plasma coagulation (APC) plus oral sucralfate versus APC plus placebo, Outcome 2 Endoscopy scores Gilinsky scale grade 6‐9 at 16 weeks.
2.3. Analysis.
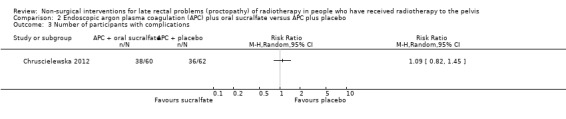
Comparison 2 Endoscopic argon plasma coagulation (APC) plus oral sucralfate versus APC plus placebo, Outcome 3 Number of participants with complications.
Endoscopic bipolar electrocoagulation versus heater probe
One study randomised 21 participants with late radiation proctopathy that after one year was resistant medical treatment, to either a heater probe or a bipolar electrocoagulation probe (Jensen 1997). We considered the risk of bias of this study to be unclear. Severe bleeding episodes, defined as a bleeding that provoked an unscheduled hospital assessment, occurred in 33% (3/12) of the bipolar probe group and in 11% (1/9) of the heater probe group (RR 2.25, 95% CI 0.28 to 18.22) (Analysis 3.1). There was no difference between the two treatment groups with regard to mean number of severe bleeds (mean difference (MD) ‐0.10, 95% CI ‐0.71 to 0.51) (Analysis 3.2). Mean units of blood transfused after one year was greater for the heater probe group, however the MD was not estimable (Analysis 3.4). No major complications occurred. Participants all expressed an improvement in their general health as a result of their controlled bleeding, which the study authors considered to be an informal quality of life assessment. During follow‐up endoscopy there was resolution of the telangiectasia, scarring, or epithelial replacement in all cases in both groups. Participant interviews, pretreatment and six months after treatment, revealed that rectal bleeds and tenesmus had improved in all participants so that they were encouraged to resume going out with less worry.
3.1. Analysis.
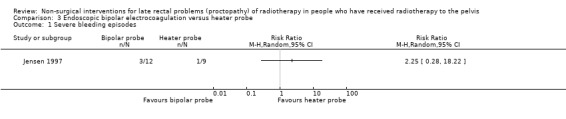
Comparison 3 Endoscopic bipolar electrocoagulation versus heater probe, Outcome 1 Severe bleeding episodes.
3.2. Analysis.
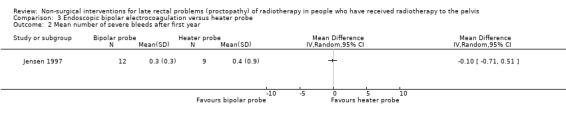
Comparison 3 Endoscopic bipolar electrocoagulation versus heater probe, Outcome 2 Mean number of severe bleeds after first year.
3.4. Analysis.
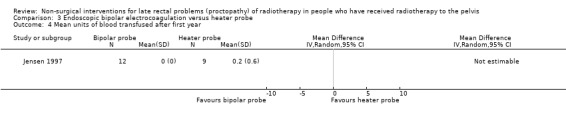
Comparison 3 Endoscopic bipolar electrocoagulation versus heater probe, Outcome 4 Mean units of blood transfused after first year.
Bipolar eletrocoagulation versus argon plasma coagulation
One study randomised 30 participants with recurrent rectal bleeding that had started six months or more after radiotherapy, to either argon plasma coagulation (APC) or bipolar electrocoagulation (BEC) (Lenz 2010). We considered the risk of bias of this study to be unclear. There were no significant differences between the groups with respect to rectal bleeding. Based on an intention‐to‐treat analysis the success rates (defined as eradication of all telangiectasias) were 12/15 (80.0%) for APC and 14/15 (93.3%) for BEC (RR 0.86, 95% CI 0.64 to 1.14) (Analysis 4.1). In a per‐protocol analysis, these results were 92.3% and 93.3% respectively (P = 1.000). There was no difference between the groups with regard to mean number of sessions needed for eradication (APC 3.7 (standard deviation (SD) 1.7), BEC 2.9 (1.9); P = 0.313). Minor complications were recorded in 5 to 15 in the APC group and 10 to 15 in the BEC group (RR 0.50, 95 % CI 0.22 to 1.11) (Analysis 4.2), and major haemorrhagic complications in 1 and 5, respectively (RR 0.20, 95% CI 0.03 to 1.51) (Analysis 4.3). No other major adverse effects, such as fistula, extensive necrosis, perforation, or bowel explosion were observed. Relapse of rectal bleeding occurred in 1 to 12 after APC and in 2 to 14 after BEC (RR 0.58, 95% CI 0.06 to 5.66) (Analysis 4.4).
4.1. Analysis.
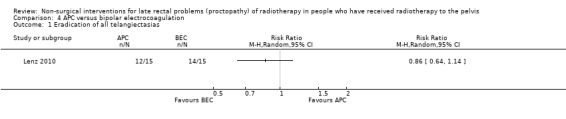
Comparison 4 APC versus bipolar electrocoagulation, Outcome 1 Eradication of all telangiectasias.
4.2. Analysis.
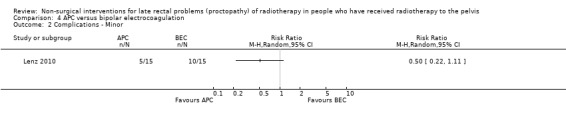
Comparison 4 APC versus bipolar electrocoagulation, Outcome 2 Complications ‐ Minor.
4.3. Analysis.
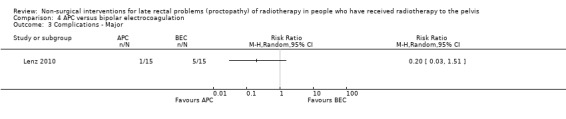
Comparison 4 APC versus bipolar electrocoagulation, Outcome 3 Complications ‐ Major.
4.4. Analysis.
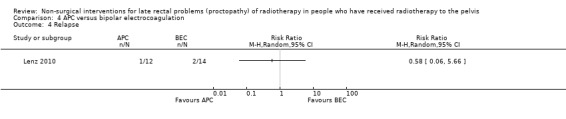
Comparison 4 APC versus bipolar electrocoagulation, Outcome 4 Relapse.
Hydrocortisone versus betamethasone
One study involved 32 participants with radiation proctopathy who received either a rectally administered hydrocortisone acetate mousse or betamethasone enema (Rougier 1992). We considered the risk of bias of this study to be high. Over the four weeks of treatment, the endoscopic appearance improved more in the hydrocortisone group (12/16) than in the betamethasone group (5/14) (RR 2.10, 95% CI 0.98 to 4.48) (Analysis 5.1). The degree of bleeding was reduced in 6 out of 16 in the hydrocortisone group and in 3 out of 14 of the betamethasone group, but this did not show a significant difference (RR 1.75, 95% CI 0.53 to 5.73) (Analysis 5.2). The duration of this response was only reported for the four‐week follow‐up period and not thereafter. Potential reasons for the difference in effect may be the more aggressive grade of disease in the betamethasone group at baseline, which would have been less likely to respond to any treatment, and also the fact that the betamethasone enema was poorly tolerated in 10/14 participants compared with 2/16 in the hydrocortisone group (RR 0.17, 95% CI 0.05 to 0.67 in favour of the hydrocortisone group) (Analysis 5.3).
5.1. Analysis.
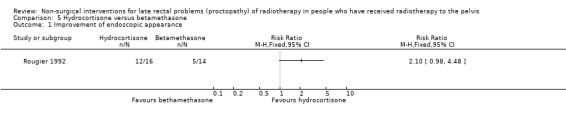
Comparison 5 Hydrocortisone versus betamethasone, Outcome 1 Improvement of endoscopic appearance.
5.2. Analysis.
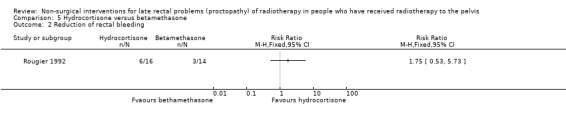
Comparison 5 Hydrocortisone versus betamethasone, Outcome 2 Reduction of rectal bleeding.
5.3. Analysis.
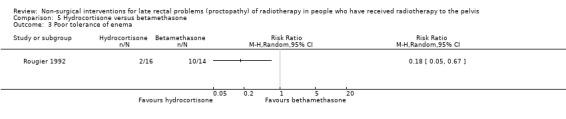
Comparison 5 Hydrocortisone versus betamethasone, Outcome 3 Poor tolerance of enema.
Formalin dab versus sucralfate steroid retention enema
One study compared the efficacy of formalin dab versus sucralfate steroid retention enema (Nelamangala 2012). This study randomly allocated 102 participants with late radiation proctopathy, presenting as rectal bleeding after radiotherapy for carcinoma of the cervix, to either formalin dab or sucralfate steroid retention enema. We considered the risk of bias of this study to be high. Ninety per cent of participants treated with formalin dab and 74.5% of participants treated with sucralfate retention enema responded to treatment (P = 0.038). In spite of having a higher median symptom score (graded by the Radiation Proctopathy System Assessments Scale) before treatment, participants treated with formalin dab demonstrated a marked decrease in symptom score after treatment compared with participants treated with sucralfate retention enema, and the difference once again was statistically significant (P = 0.001). Similarly, the median sigmoidoscopic grade was significantly lower for participants in group 1 compared with participants in group 2 after treatment (P = 0.000). There were no specific treatment‐related complications in either group.
Formalin application versus colonic irrigation and oral antibiotics
Another study randomised 50 participants with haemorrhagic radiation proctopathy to either 4% formalin application or colonic irrigation and oral antibiotics (Sahakitrungruang 2012). We considered the risk of bias of this study to be high. The study revealed greater improvement in rectal bleeding, urgency, and diarrhoea in the irrigation group. Twenty out of 24 participants in the irrigation group were satisfied with the treatment compared to ten out of 23 participants in the formalin group (RR 1.92, 95% CI 1.16 to 3.16).
Pentoxifylline in addition to standard therapy
In one study, 40 participants were randomised to either local standard therapies (including blood transfusion, analgesics, anti‐inflammatory agents, dietary modification, local steroids applications, or sucralfate enemas) or identical therapies plus oral pentoxifylline 400 mg three times daily for six months (study group) (Venkitaraman 2008). We considered the risk of bias of this study to be high. Sixteen participants in the pentoxifylline group and 12 in the control group had cessation of rectal bleeding for a week or more (RR 1.33, 95% CI 0.88 to 2.03) (Analysis 6.1). The median time to cessation of bleeding was 22 days (range 1 to 119 days) in the study group and 95 days (range 13 to 172) in the control group (P = 0.12). At least one episode of recurrent bleeding occurred in 14 of the 16 participants in the study group and in all the 12 participants who had cessation of bleeding in the control group. The median duration of freedom from bleeding was 12 days (range 8 to 290) in the study group and 11 days (range 7 to 133) in the control group. There was an overall trend of a reduction of rectal bleeding episodes with time in both groups, as judged by the proportion of days in which one or more rectal bleeding episode was reported. This study could not show a statistically significant advantage with six months of pentoxifylline compared with the used standard measures for late radiation‐induced rectal bleeding.
6.1. Analysis.
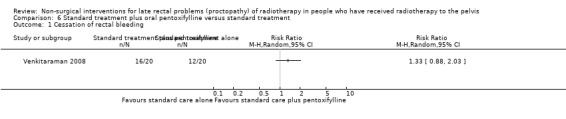
Comparison 6 Standard treatment plus oral pentoxifylline versus standard treatment, Outcome 1 Cessation of rectal bleeding.
Oral sulfasalazine plus rectal steroids versus oral placebo plus rectal sucralfate
One study included 32 participants with radiation‐induced proctosigmoidopathy (Kochhar 1991). Participants were randomised to either oral sulfasalazine 500 mg and rectal prednisolone 20 mg or oral placebo and rectal sucralfate suspension. We considered the risk of bias of this study to be unclear. Eight out of 15 participants in the sulfasalazine/steroid group showed a clinical improvement compared to 16/17 in the sucralfate group (RR 0.57, 95% CI 0.35 to 0.92) (Analysis 7.1). Seven out of 15 participants in the sulfasalazine/steroid group showed endoscopic improvement compared to 12/17 in the sucralfate group (RR 0.66, 95% CI 0.35 to 1.23) (Analysis 7.2). Two participants in the sulfasalazine/steroid group did not tolerate the drugs and were excluded due to myalgia, nausea, and headaches.
7.1. Analysis.
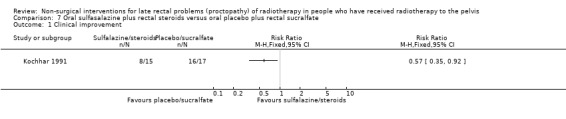
Comparison 7 Oral sulfasalazine plus rectal steroids versus oral placebo plus rectal sucralfate, Outcome 1 Clinical improvement.
7.2. Analysis.
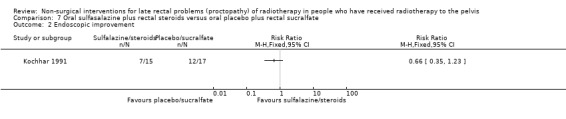
Comparison 7 Oral sulfasalazine plus rectal steroids versus oral placebo plus rectal sucralfate, Outcome 2 Endoscopic improvement.
2. Treatments targeted at a combination of anorectal symptoms
(see Table 2)
2. Treatment targeted at something very localised but not a single pathology.
| Study ID | Participant characteristics | Intervention(s) | Results | Level of evidence (GRADE) |
| Cavcic 2000 | Chronic radiation proctopathy producing diarrhoea and rectal bleeding | Metronidazole (3 x 400 mg orally per day), mesalazine (3 x 1 g orally per day), and betamethasone enema (once a day) (n = 30) versus Same doses of mesalazine and betamethasone enema, but without metronidazole (n = 30) |
Rectal bleeding score < 2, 1 year after treatment (an in‐house scoring system was used) 22/24 versus 5/12 (RR 1.57, 95% CI 0.96 to 2.57) |
Very low due to high RoB and very serious imprecision (single study/OIS not reached) |
|
Diarrhoea score < 2, 1 year after treatment (an in‐house scoring system was used) 23/24 versus 8/12 (RR 1.44, 95% CI 0.96 to 2.16) |
Very low due to high RoB and very serious imprecision (single study/CI includes both benefits and harms) | |||
|
No rectal ulceration, 1 year after treatment (an in‐house scoring system was used) 22/24 versus 7/12 (RR 1.57, 95% CI 0.96 to 2.57) |
Very low due to high RoB and very serious imprecision (single study/CI includes both benefits and harms) | |||
| Talley 1997 | Symptoms consistent with chronic radiation proctopathy (≥ 2 months) |
Butyric acid (SCFA) enema (60 ml containing 40 mmol butyric acid), twice a day, used for 2 weeks versus Normal saline placebo enema NB: this was a cross‐over study with a 1‐week wash‐out period before giving the alternate enema (n = 15 participants were randomised and 12 completed both arms of the study, number of participants per group not specified) |
Symptom scores (in‐house scoring system: total symptom score was calculated
combining 6 items: rectal pain, rectal bleeding episodes
per week, quantity of blood passed, number of
days of diarrhoea per week, number of stools per
week, and urgency) Mean score 3.5 (range 3 to 5) versus 4.5 (range 3 to 6) (P = 0.3) |
Very low due to unclear RoB and very serious imprecision (precision not quantified/small sample size) |
| Bleeding 38% versus 38% | Very low due to unclear RoB and very serious imprecision (precision not quantified and OIS not reached) | |||
| Urgency (time in min able to defer defecation) 5 (1 to 42.5) in the placebo group versus 10 (5 to 20) in the treatment group | Very low due to unclear RoB and very serious imprecision (precision not quantified and OIS not reached) | |||
| Days with diarrhoea 1 (1 to 5.5) in the placebo group versus 1 (1 to 2) in the treatment group | Very low due to unclear RoB and very serious imprecision (precision not quantified and OIS not reached) | |||
| Number of stools 1.5 (1 to 3) in the placebo group versus 1.5 (1 to 2.5) in the treatment group | Very low due to unclear RoB and very serious imprecision (precision not quantified and OIS not reached) | |||
| Pain (rectal) 33% in the placebo group versus 8% in the treatment group | Very low due to unclear RoB and very serious imprecision (precision not quantified and OIS not reached) | |||
| “Neither changes in the symptom score nor changes in the individual symptoms were statistically significant” | ‐ | |||
| “No side effects were reported and there was no QOL assessment” (2 participants experienced problems inserting the enema) | ‐ |
|||
| Yeoh 2013 | People who had (1) completed external beam radiation therapy for prostate carcinoma ≥ 6 months previously; (2) intractable rectal bleeding, defined as a frequency of ≥ 1x per week and/or requiring blood tranfusions, attributed to chronic radiation proctopathy at colonoscopy; (3) no constant requirement for medications likely to influence anorectal motility, such as opioid analgesic and antidiarrhoeal agents; and (4) provided written informed consent |
APC (outpatient procedure) (n = 17) versus Topical formalin (n = 13) NB: cross‐over to the other therapy was allowed if the treatment endpoint was not reached after 4 treatment sessions. 2 microlax enemas were administered before each session of either APC or topical formalin therapy |
Anorectal symptom parameters (after treatment), median (range) (measured by SOMA‐LENT and VAS) No. of bowel actions per week: 16 (7 to 46) versus 14 (4 to 42) Faecal incontinence scores: 0 (0 to 4) versus 0 (0 to 2) Urgency of defaecation scores: 4 (0 to 6) versus 4 (0 to 6) Rectal bleeding scores: 1 (0 to 2) versus 1 (0 to 2) VAS for rectal bleeding (mm): 14 (0 to 34) versus 13 (0 to 25) No between‐group comparisons were made |
Low due to high risk of bias and imprecision (precision not quantified) |
|
Comparisons of participant outcomes after APC and topical formalin treatment, median (range) (measured by SOMA‐LENT and VAS) Rectal bleeding scores: 1 (0 to 2) versus 1 (0 to 2), NS VAS for rectal bleeding (mm): 14 (0 to 34) versus 13 (0 to 25), NS |
Low due to high risk of bias and imprecision (precision not quantified) |
APC: argon plasma coagulation CI: confidence interval RR: risk ratio SCFA: short chain fatty acid VAS: visual analogue scale NB: nota bene NS: not significant OIS: Optimal Information Size
Short‐chain fatty acid (SCFA) versus placebo
The first study was a prospective randomised, double‐blind, cross‐over pilot study that randomised 15 participants with late radiation proctopathy, to either a normal saline placebo enema or a SCFA enema, which was 60 ml in volume and administered twice a day (Talley 1997). It contained 40 mM of butyrate in the Harig preparation, used for two weeks with a one‐week wash‐out period before giving the alternate enema. We considered the risk of bias of this study to be unclear. The total symptom score at baseline ranged from 2 to 11 (median 5.5). Symptom scores improved slightly on the active treatment (median score 3.5 (range 3 to 5) compared with 4.5 median score (range 3 to 6) for those receiving placebo. Neither changes in the symptom score nor changes in the individual symptoms were statistically significant.
Topical formalin versus argon plasma coagulation
The second study compared the effect of topical formalin and argon plasma coagulation (APC) for intractable rectal bleeding and anorectal dysfunction associated with late radiation proctopathy (Yeoh 2013). Thirty men with intractable rectal bleeding (defined as one per week or more or requiring blood transfusions, or both) after radiotherapy for prostate carcinoma were randomised to treatment with APC or topical formalin. We considered this study to be at high risk of bias. Control of rectal bleeding was achieved in 100% of the topical formalin group and 94% of the APC group after a median of two sessions of the respective treatment. No significant differences in efficacy and durability of rectal bleeding between the two groups (rectal bleeding scores, median (range): 1 (0 to 2) versus 1 (0 to 2); visual analogue scale for rectal bleeding (mm), median (range): 13 (0 to 25) versus 14 (0 to 34)) were found. There were no differences between topical formalin and APC for anorectal symptoms and function, nor for anal sphincteric morphology. The treatments were well tolerated. No skin toxicity was noted in participants who needed more than one formalin treatment session.
Metronidazole in addition to mesalazine and betamethasone
In the third study participants with rectal bleeding and diarrhoea were randomly allocated to either metronidazole (3 x 400 mg orally per day), mesalazine (3 x 1 g orally per day), and betamethasone enema (once a day) or to the same doses of mesalazine and betamethasone enema, but without metronidazole (Cavcic 2000). We considered the risk of bias of this study to be high. The incidence of rectal bleeding and mucosal ulcers was significantly lower in the metronidazole group at 4 weeks (P = 0.009), 3 months (P = 0.031), and 12 months (P = 0.029). There was also a significant decrease in diarrhoea and oedema in the metronidazole group at 4 weeks (P = 0.044), 3 months (P = 0.045), and 12 months (P = 0.034) after treatment. One year after treatment, 22/24 participants in the metronidazole group had demonstrated a reduction in the grade of their rectal bleeding compared to 5/12 in the group treated with mesalazine and betamethasone (RR 1.57, 95% CI 0.96 to 2.57) (Analysis 8.1). Similarly, 23 out of 24 participants in the metronidazole group compared to 8/12 in the control group had experienced reduction in their diarrhoea and rectal erythema (RR 1.44, 95% CI 0.96 to 2.16) (Analysis 8.2; Analysis 8.3). The degree of rectal ulceration at 1 year had decreased in 22/24 participants in the metronidazole group compared with 7/12 of the group treated with anti‐inflammatories alone (RR 1.57, 95% CI 0.96 to 2.57) (Analysis 8.4).
8.1. Analysis.
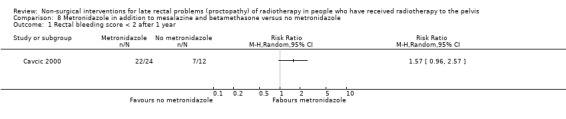
Comparison 8 Metronidazole in addition to mesalazine and betamethasone versus no metronidazole, Outcome 1 Rectal bleeding score < 2 after 1 year.
8.2. Analysis.
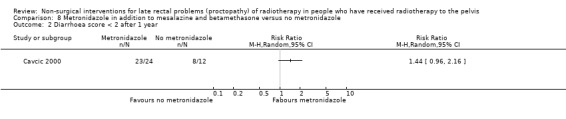
Comparison 8 Metronidazole in addition to mesalazine and betamethasone versus no metronidazole, Outcome 2 Diarrhoea score < 2 after 1 year.
8.3. Analysis.
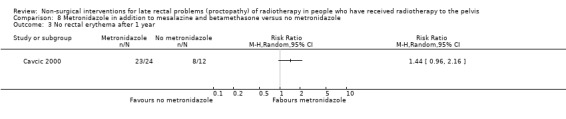
Comparison 8 Metronidazole in addition to mesalazine and betamethasone versus no metronidazole, Outcome 3 No rectal erythema after 1 year.
8.4. Analysis.
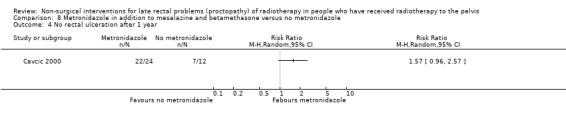
Comparison 8 Metronidazole in addition to mesalazine and betamethasone versus no metronidazole, Outcome 4 No rectal ulceration after 1 year.
3. Treatments targeted at pelvic radiation disease
(see Table 3)
3. Treatments targeted at the more global collection of symptoms.
| Study ID | Participant characteristics | Intervention(s) | Results | Level of evidence (GRADE) |
| Andreyev 2013 (ORBIT) | Adults (aged ≥ 18 years) who had troublesome, persisting gastrointestinal symptoms, started during or after radiotherapy given with curative intent for histologically proven prostatic, bladder, vulval, vaginal, cervical, endometrial, anal, or rectal malignant neoplasia or para‐aortic irradiation for metastatic disease from any of those primary sites or the testis. Radiotherapy should have been completed at least 6 months before enrolment | Gastroenterologist group (management according to an algorithm by a consultant gastroenterologist) (n = 70) versus Nurse group (management according to an algorithm by a specially trained research nurse) (n = 80) versus Booklet group (detailed advice booklet on self management of bowel symptoms) (n = 68) N.B.: the algorithm provides a step‐by‐step approach along a care pathway from initial identification of symptoms to long‐term management. Participants in the booklet group whose symptoms continued 6 months after recruitment were offered consultation with the gastroenterologist and, if appropriate, investigation and treatment. Participants in the nurse‐led care group were crossed over to the gastroenterologist‐led care group if they had gastrointestinal issues that were beyond the scope of the algorithm |
Pair‐wise mean difference in change in IBDQ‐B score between groups: Gastroenterologist versus booklet: 5.47 (95% CI 1.14 to 9.81; P = 0.01) Nurse versus booklet: 4.12 (95% CI 0.04 to 8.19; P = 0.04) “Outcomes in the nurse group were not inferior to outcomes in the gastroenterologist group (mean difference 1.36; one sided 95% CI –1.48).” “No statistical analysis between the three groups was planned for the second time point at 12 months as a result of the crossover from booklet to gastroenterologist group.” |
Low due to high risk of bias and imprecision (lower boundary of CI seems to be lower than the minimal important difference) |
|
Mean IBDQ‐B (SD) (10 = worst possible outcome, 70 = best possible outcome) At 6 months: Gastroenterologist group versus booklet group: 62.3 (8.4) versus 57.5 (12.9) (MD 4.80, 95% CI ‐0.52 to 10.12) |
Low due to high risk of bias and imprecision (CI includes both benefits and harms) | |||
|
Mean IBDQ‐B (SD) (10 = worst possible outcome, 70 = best possible outcome) At 6 months: Nurse group versus booklet group: 62.0 (10.2) versus 57.5 (12.9) (MD 4.50, 95% CI ‐0.99 to 9.99) Gastroenterologist group versus nurse group: 62.3 (8.4) versus 62.0 (10.2) (MD 0.30, 95% CI ‐2.99 to 3.59) |
Moderate due to high risk of bias | |||
|
St Mark’s incontinence score, median (range) (0 = perfect continence, 24 = total incontinence) At 6 months: Booklet group: 6.5 (0 to 22) Gastroenterologist group: 5 (0 to 20) Nurse group: 4.5 (0 to 18) |
Moderate due to high risk of bias | |||
|
Change in rectal SOMA‐LENT, mean (SD) (best possible score = 0, worst = 56) At 6 months: Gastroenterologist group versus booklet group: ‐0.10 (0.17) versus ‐0.04 (0.2) (MD ‐0.06, 95% CI ‐0.15 to 0.03) Nurse group versus booklet group: ‐0.12 (0.17) versus ‐0.04 (0.2) (MD ‐0.08, 95% CI ‐0.17 to 0.01) Gastroenterologist group versus nurse group: ‐0.10 (0.17) versus ‐0.12 (0.17) (MD 0.02, 95% CI ‐0.04 to 0.08) |
Low due to high risk of bias and imprecision (CI includes both benefits and harms) | |||
|
Change in small intestine SOMA‐LENT, mean (SD) (best possible score = 0, worst = 52) At 6 months: Gastroenterologist group versus booklet group: ‐0.10 (0.12) versus –0.04 (0.15) (MD ‐0.06, 95% CI ‐0.12 to 0.00) Nurse group versus booklet group: ‐0.09 (0.12) versus –0.04 (0.15) (MD ‐0.05, 95% CI ‐0.11 to 0.01) Gastroenterologist group versus nurse group: ‐0.10 (0.12) versus ‐0.09 (0.12) (MD ‐0.01, 95% CI ‐0.05 to 0.03) |
Low due to high risk of bias and imprecision (CI includes both benefits and harms) | |||
| 12‐Item Short Form Health Survey quality of life Physical component summary scales, mean change in score (SD) At 6 months: Gastroenterologist group versus booklet group: 3.30 (10.18) versus ‐0.26 (6.79) (MD 3.56, 95% CI ‐0.09 to 7.21) Nurse group versus booklet group: ‐0.57 (6.63) versus ‐0.26 (6.79) (MD ‐0.31, 95% CI ‐3.36 to 2.74) Gastroenterologist group versus nurse group: 3.30 (10.18) versus ‐0.57 (6.63) (MD 3.87, 95% CI 0.79 to 9.95) |
Low due to high risk of bias and imprecision (lower boundary of CI seems to be lower than the minimal important difference) | |||
|
Mental component summary scales, mean change in score (SD) At 6 months: Gastroenterologist group versus booklet group: ‐1.42 (9.44) versus 0.32 (8.01) (MD ‐1.74, 95% CI ‐5.60 to 2.12) Nurse group versus booklet group: 0.53 (8.06) versus 0.32 (8.01) (MD 0.21, 95% CI ‐3.42 to 3.84) Gastroenterologist group versus nurse group: ‐1.42 (9.44) versus 0.53 (8.06) (MD ‐1.95, 95% CI ‐5.08 to 1.18) “HAD anxiety seemed to improve at 6 months, but HAD depression seemed to worsen, which is possibly related to very large changes in scores from baseline in a small number of individuals at 6 months” |
Low due to high risk of bias and imprecision (CI includes both benefits and harms) | |||
| Clarke 2008 | Late rectal radiation tissue injury present for >= 3 months that has not responded sufficiently to other therapies | 100% oxygen at 2.0 ATA for 90 min, once daily, 5 times weekly (n = 75) (30 sessions) versus 21% oxygen (normal air) at 1.1 ATA for 90 min (sham treatment), once daily, 5 times weekly (n = 75) (30 sessions) |
SOMA‐LENT score (improvement) 5.00 versus 2.61 (MD (based on repeated measurements model) 1.93, 95% CI 0.38 to 3.48) The decrease was greater in the hyperbaric oxygen group (P = 0.0019) |
Moderate due to imprecision (OIS not reached) |
|
Clinical evaluation Proportion healed or improved: 56/63 (88.9%) versus 35/65 (62.5%) (RR 1.42, 95% CI 1.14 to 1.77) (OR for improvement (based on repeated measurements model) 5.93, 95% CI 2.04 to 17.24) |
Moderate due to imprecision (OIS not reached) | |||
| QoL Improvement on Expanded Prostate Cancer Index Composite QoL: Bowel bother: 14% versus 5% Bowel function: 9% versus 6% |
Low due to imprecision (results not quantified and OIS not reached) | |||
|
Harms 19 (15.8%) participants complained of ear pain or discomfort, of which 7 had tympanic membrane changes consistent with with barotrauma, and 1 had both tympanic membrane injury and middle ear effusion |
Low due to imprecision (results not presented per group and OIS not reached) | |||
| Ehrenpreis 2005 | Eligible people were more than 6 months post‐pelvic radiotherapy and had significant symptoms as measured with the RPSAS |
Retinol palmitate 10,000 IU by mouth for 90 days (n = 10) versus Identical placebo capsules (n = 9) |
Response to treatment 7/9 versus 2/8 (RR 3.11, 95% CI 0.89 to 10.86) |
Low due to very serious imprecision (CI includes both benefits and harms; very small sample size) |
|
Mean pre‐post‐treatment change in RPSAS 11 +/‐ 5 versus 2.5 +/‐ 3.6 (P = 0.013, Mann‐Whitney U test) "One patient from each group enrolled in the study but did not take a single dose of medication and was therefore excluded from analysis" |
Low due to very serious imprecision (OIS not reached and very small sample size) | |||
| Tian 2008 | People diagnosed with chronic rectal radiation damage | Intergrated Chinese traditional plus Western medicine (smectite powder 6 g, dexamethasone 5 mg, levofloxacin hydrochloride 0.2 g, anisodamine 10 mg, and physiological saline 100 ml) (n = 32) versus Western medicine (smectite powder 6 g, dexamethasone 5 mg, levofloxacin hydrochloride 0.2 g, anisodamine 10 mg, and physiological saline 100 ml) (n = 26) |
Grade of radioproctitis after treatment (defined according to colonoscopy test results) Grade 0 to 1: 22/32 versus 7/26 (RR 2.55, 95% CI 1.30 to 5.02) |
Low due to high RoB and imprecision (OIS not reached) |
|
Treatment effect after 37 days Cured‐better: 30 to 32 versus 14 to 26 (RR 1.74, 95% CI 1.21 to 2.51) |
Low due to high RoB and imprecision (OIS not reached) |
ATA: atmosphere absolute CI: confidence interval IBDQ‐B: Inflammatory Bowel Disease Questionnaire–Bowel MD: mean difference OR: odds ratio QoL: quality of life RPSAS: Radiation Proctopathy System Assessments Scale RR: risk ratio SD: standard deviation SOMA‐LENT: Subjective, Objective, Management, Analytic ‐ Late Effects of Normal Tissue HAD: Hospital Anxiety Depression OIS: Optimal Information Size
Hyperbaric oxygen therapy versus placebo
One study included 150 participants with refractory radiation proctopathy who were randomised to hyperbaric oxygen at 2.0 atmospheres absolute or air at 1.1 atmospheres absolute (sham treatment) (Clarke 2008). The sham participants were subsequently crossed to group 1 (however, only the results before the cross‐over are reported here). We considered this study to be at unclear risk of bias. A decrease (improvement) of the Subjective, Objective, Management, Analytic ‐ Late Effects of Normal Tissue (SOMA‐LENT) score of 5.00 points occurred in the intervention group, and a decrease of 2.61 points in the sham group. The decrease was significantly larger in the intervention group than in the sham group (P = 0.0019). The proportion of responders (healed, significant improvement, or modest improvement versus no improvement) in the intervention group was higher than in the sham group (88.9% versus 62.5%, respectively; RR 1.42, 95% CI 1.14 to 1.77) (Analysis 9.1). Based on a repeated measures logistic model, the odds ratio for improvement was 5.93 (95% CI 2.04 to 17.24), of which a risk difference was derived of 0.32 (32%) resulting in a number needed to treat for an additional beneficial outcome of 3. With respect to bowel‐specific QoL marked improvement was noted for the intervention group after treatment but not for the sham group (14% for bowel bother and 9% for bowel function versus 5% and 6%, respectively).
9.1. Analysis.
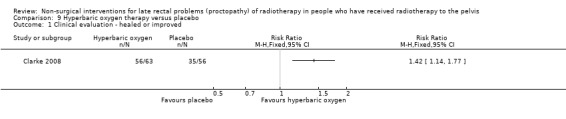
Comparison 9 Hyperbaric oxygen therapy versus placebo, Outcome 1 Clinical evaluation ‐ healed or improved.
Retinol palmitate (Vitamin A) versus placebo
One study randomised 19 participants with radiation proctopathy to either retinol palmitate (10,000 IU by mouth for 90 days) or placebo (Ehrenpreis 2005). We considered this study to be at unclear risk of bias. Response was defined as a reduction in two or more symptoms by at least two Radiation Proctopathy System Assessments Scale (RPSAS) points. Seven out of nine retinol palmitate participants responded, whereas 2/8 placebo participants responded (RR 3.11, 95% CI 0.89 to 10.86) (Analysis 10.1). Mean pre‐post‐treatment change in RPSAS score in the retinol palmitate group was 6 to 16 and ‐1.1 to 6.1 in the placebo group (P = 0.013).
10.1. Analysis.
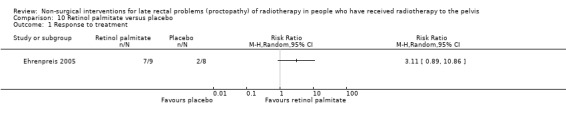
Comparison 10 Retinol palmitate versus placebo, Outcome 1 Response to treatment.
Gastroenterologist‐led algorithm‐based treatment versus nurse‐led algorithm‐based treatment versus usual care
One study compared the efficacy of a gastroenterologist‐led algorithm‐based treatment with a nurse‐led algorithm‐based treatment or with usual care (a detailed self help booklet) (Andreyev 2013). The algorithm provided a step‐by‐step approach along a care pathway from initial identification of symptoms to long‐term management. The study randomised 218 participants (18 years of age and older) with new‐onset gastrointestinal symptoms persisting 6 months after pelvic radiotherapy were randomised to one of the three arms. We considered the risk of bias of this study to be high. The primary endpoint was change in Inflammatory Bowel Disease Questionnaire–Bowel subset score (IBDQ‐B) at six months, on which the following pair‐wise mean differences in change in IBDQ‐B score between groups were recorded: nurse versus booklet 4.12 (95% CI 0.04 to 8.19), gastroenterologist versus booklet 5.47 (1.14 to 9.81). Outcomes in the nurse group were not inferior to outcomes in the gastroenterologist group (MD 1.36, one‐sided 95% CI –1.48). When considering IBDQ‐B at six months, a MD of 4.80 (95% CI ‐0.52 to 10.12) was found for the gastroenterologist versus booklet group; a MD of 4.50 (95% CI ‐0.99 to 9.99) for the nurse versus booklet group; and a MD of 0.30 (95% CI ‐2.99 to 3.59) for the gastroenterologist versus nurse group (Analysis 11.1). No significant differences were found on change in rectal and small intestine SOMA‐LENT at six months (Analysis 11.2; Analysis 11.3). A significant difference on the SF12 QoL physical component summary scales was found for the gastroenterologist group versus nurse group (MD 3.87, 95% CI 0.79 to 9.95) (Analysis 11.4). No other significant differences were found for QoL outcomes (Analysis 11.4; Analysis 11.5).
11.1. Analysis.
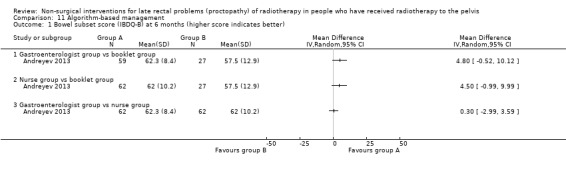
Comparison 11 Algorithm‐based management, Outcome 1 Bowel subset score (IBDQ‐B) at 6 months (higher score indicates better).
11.2. Analysis.
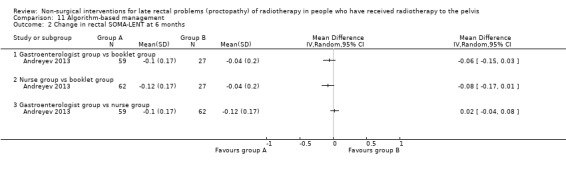
Comparison 11 Algorithm‐based management, Outcome 2 Change in rectal SOMA‐LENT at 6 months.
11.3. Analysis.
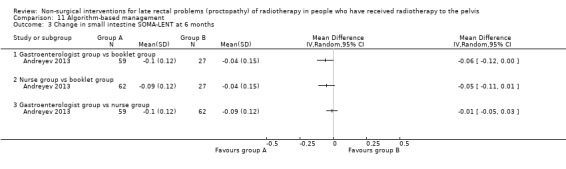
Comparison 11 Algorithm‐based management, Outcome 3 Change in small intestine SOMA‐LENT at 6 months.
11.4. Analysis.
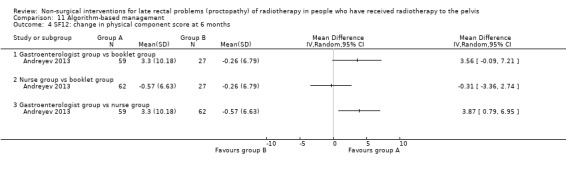
Comparison 11 Algorithm‐based management, Outcome 4 SF12: change in physical component score at 6 months.
11.5. Analysis.
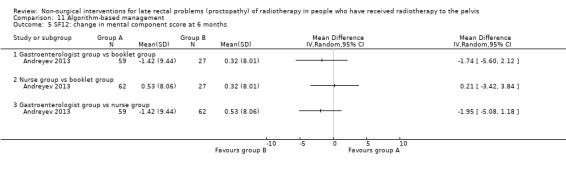
Comparison 11 Algorithm‐based management, Outcome 5 SF12: change in mental component score at 6 months.
Chinese traditional medicine plus Western medicine versus Western medicine alone
One study published in Chinese randomised 58 participants with rectal radiation proctopathy to either integrated Chinese traditional medicine plus Western medicine or Western medicine alone (Tian 2008). We considered this study to be at high risk of bias. Significant differences in grade of proctopathy after treatment (defined according to colonoscopy test results) in favour of the treatment group (Chinese traditional medicine) were found (Grade 0 to 1: RR 2.55, 95% CI 1.30 to 5.02) (Analysis 12.1). Also, the treatment effect after 37 days showed a significant difference in favour of the treatment group (Cured‐better: RR 1.74, 95% CI 1.21 to 2.51) (Analysis 12.2).
12.1. Analysis.
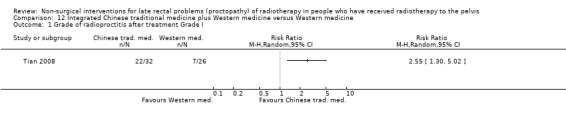
Comparison 12 Integrated Chinese traditional medicine plus Western medicine versus Western medicine, Outcome 1 Grade of radioproctitis after treatment Grade I.
12.2. Analysis.
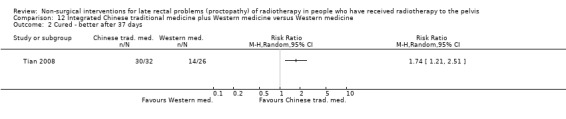
Comparison 12 Integrated Chinese traditional medicine plus Western medicine versus Western medicine, Outcome 2 Cured ‐ better after 37 days.
Discussion
This review included 13 studies that assessed non‐surgical interventions for the management of late radiation proctopathy, 2 that assessed non‐surgical interventions for a broader spectrum of symptoms, and 1 that evaluated a gastroenterologist‐led algorithm for the broader pelvic radiation disease.
Summary of main results
Nine studies assessed treatments for bleeding only, of which five were at unclear risk of bias (Chruscielewska 2012; Jensen 1997; Kochhar 1991; Lenz 2010; Pinto 1999), and four were at high risk of bias (Nelamangala 2012; Rougier 1992; Sahakitrungruang 2012; Venkitaraman 2008). Of these, five studies compared different active interventions with each other, three studies assessed the effect of the addition of one intervention to another intervention, and one study was placebo controlled. We only found significant differences in our primary outcomes for the studies that assessed argon plasma coagulation (APC) followed by oral sucralfate versus APC with placebo (significant difference in endoscopic score 6 to 9 at week 8 in favour of APC with placebo) (Chruscielewska 2012); formalin dab treatment (4%) versus sucralfate steroid retention enema (significant difference in favour of formalin on symptom score after treatment (graded by the Radiation Proctopathy System Assessments Scale and sigmoidoscopic score)) (Nelamangala 2012); colonic irrigation plus ciprofloxacin and metronidazole versus formalin application (4%) (significant difference in favour of colonic irrigation on the outcomes bleeding, urgency, and diarrhoea) (Sahakitrungruang 2012).
Three studies assessed treatments targeted at more than one symptom, yet confined to the anorectal region, of which one was at unclear risk of bias (Talley 1997), and two were at high risk of bias (Cavcic 2000; Yeoh 2013). Of these, one study assessed the effect of interventions in addition to other interventions (Cavcic 2000), one study compared two active interventions with each other (Yeoh 2013), and another was placebo controlled (Talley 1997). We identified no significant differences in our primary outcomes.
Four studies assessed treatments targeted at the collection of symptoms referred to as pelvic radiation disease, of which two studies were at unclear risk of bias (Clarke 2008; Ehrenpreis 2005), and two were at high risk of bias (Andreyev 2013; Tian 2008). Two studies compared various interventions with each other (Andreyev 2013; Tian 2008), and two studies were placebo controlled (Clarke 2008; Ehrenpreis 2005). Significant differences were found for the studies that assessed gastroenterologist‐led algorithm‐based treatment versus usual care (a detailed self help booklet) (significant difference in favour of gastroenterologist‐led algorithm‐based treatment on the outcome change in Inflammatory Bowel Disease Questionnaire–Bowel subset score (IBDQ‐B) score at six months) and nurse‐led algorithm‐based treatment versus usual care (significant difference in favour of the nurse‐led algorithm‐based treatment on the outcome change in IBDQ‐B score at six months), hyperbaric oxygen therapy (at 2.0 atmospheres absolute) versus sham treatment (SOMA‐LENT score (improvement) in favour of hyperbaric oxygen therapy), retinol palmitate versus placebo (significant difference in favour of retinol palmitate on the outcome improvement in RPSAS) and integrated Chinese traditional plus Western medicine versus Western medicine (significant difference in favour of integrated Chinese traditional medicine with respect to grade 0 to 1 radioproctitis after treatment (defined according to colonoscopy test results)).
Pooling outcomes for a meta‐analysis was impossible due to the lack of a common standard therapy and variation in study characteristics and endpoints across the included studies.
Overall completeness and applicability of evidence
Well‐conducted randomised trials investigating non‐surgical interventions for late radiation proctopathy are scarce. All included RCTs reported our primary outcome 'response of the presenting symptoms as recorded with diaries and scoring systems'. Interpretation and applicability of the evidence was limited, since many of the reported trials tended to focus mainly on rectal bleeding, whereas radiation‐associated proctopathy is more complex, and in itself only one aspect of what may be labelled pelvic radiation disease (Andreyev 2010). A number of studies have explicitly examined rectal bleeding, which may be a very dominant and well‐measurable symptom of radiation proctopathy. Though the results from these studies only relate to the reduction of rectal bleeding, they are still relevant. One of the treatments often used in clinical practice to reduce rectal bleeding is argon plasma coagulation. We found no placebo‐controlled trials investigating the effect of this treatment. However, it is remarkable that the studies comparing this treatment to other forms of coagulation or formalin showed no significant differences, but very high effect rates on rectal bleeding in both treatment arms (Jensen 1997; Lenz 2010; Yeoh 2013). Other studies also investigated the improvement of other clinical symptoms, but used different scales to score this, so comparison is difficult.
Some interventions showed a significant improvement of symptoms, such as hyperbaric oxygen therapy, retinol palmitate, and adding metronidazole to the anti‐inflammatory regimen. Chinese traditional medicine improved colonoscopic results. Some treatments were significantly better than others, suggesting at least some efficacy (such as rectal sucralfate suspension, colonic irrigation, and formalin dab). Since there is no common standard for the treatment of late radiation proctopathy, these results are even more difficult to interpret or compare. We could not compare the largest study in this review, Andreyev 2013, with 218 participants, to any of the other studies because the study investigates the logistic application of an algorithm (by medical specialist, nurse, or a self help booklet), in which a number of the aforementioned treatments are represented as 'standard', dependent of the symptoms of the participants.
Quality of the evidence
Using GRADE we downgraded the level of evidence for the majority of outcomes to low or very low, mainly due to imprecision and study limitations. Most included studies had small sample sizes. Only 4 out of 16 studies included more than 100 participants, and 9 of 16 fewer than 40 participants. Eight studies compared two active interventions against each other without a placebo‐controlled group, making it difficult to draw conclusions about the results of these studies. The best evidence for the effectiveness were the placebo‐controlled studies. Of the two placebo‐controlled studies with a positive result, the study investigating hyperbaric oxygen therapy has the highest level of evidence given the sample size (150 participants), but had an uncertain risk of bias (Clarke 2008). The other positive placebo‐controlled study (retinol palmitate) had a low risk of bias, but included only 19 participants.
Potential biases in the review process
We performed a comprehensive search in several electronic databases, however we did not search for conference abstracts. To overcome this, we did search a prospective trial register. In addition, the previous version of this review included both randomised and non‐randomised studies. In this update, we included only RCTs, as we felt that the benefit of including non‐randomised studies did not outweigh the risk of including them, as non‐randomised studies suffer from very high risk of bias.
Agreements and disagreements with other studies or reviews
Although we did not systematically search for reviews assessing non‐surgical interventions for late radiation proctopathy, we did identify the reviews of Do 2011, Hanson 2012, and Wilson 2006, which concluded that studies assessing treatments for late radiation proctopathy are few and suffer from small sample sizes and short follow‐up times, and that more and larger randomised placebo‐controlled studies are needed to prospectively look at both the prevention and treatment of late radiation proctopathy. The study of Wilson 2006 also concluded that a standardised system of classification of late radiation proctopathy would assist with interpretation of study results. These findings are in line with the results and conclusions of the present review.
The previous version of this review also included a phase II study, which we moved to the ongoing studies section. The conclusions of the previous versions of this review are in line with the conclusions of the present review.
Authors' conclusions
Implications for practice.
One of the most commonly used treatments against rectal bleeding is argon plasma coagulation (APC). Though APC is very effective against rectal blood loss and considered standard, to our knowledge it had never been tested in a randomised fashion. APC works to reduce rectal blood loss but not other symptoms. The treatments tested in addition to APC showed no benefit. The significant results of formalin dab treatment (4%) versus sucralfate steroid retention enema and colonic irrigation plus ciprofloxacin and metronidazole versus formalin application (4%) are hampered by the fact that they were not compared to APC and may only be indicated in case of contraindications to APC. The same applies to the three studies investigating a broader spectrum of anorectal symptoms, one of which showed an advantage of adding metronidazole to mesalazine and betamethasone enema (only for the symptom rectal blood loss). The remaining four studies focused on the entire symptom complex that may be described as pelvic radiation disease. One of these (the largest) showed that specialistic care in the form of a gastroenterologist‐ or nurse‐led algorithm is better then a self help booklet. The remaining three studies showed a benefit from the investigated treatment, but two of these were very small. Only hyperbaric oxygen showed an advantage over placebo in a reasonably sized randomised study, hence this was the most convincing evidence.
Overall, we conclude for general practice that radiation proctopathy is more complex than rectal blood loss, which many studies focussed upon. The studies focusing only or mainly on rectal blood loss are hampered by the fact that the treatment considered standard for this condition was never investigated in a randomised manner. The broader radiation proctopathy and pelvic radiation disease likely require specialistic care, for instance in the form of a gastroenterologist‐led algorithm. The most convincing evidence for improvement of the symptom complex of radiation proctopathy was shown for hyperbaric oxygen therapy.
Implications for research.
The evidence for the effectiveness of non‐surgical interventions for late radiation proctopathy is limited and hampered by a lack of common standards. Although certain interventions look promising, single small studies (even if well conducted) provide limited evidence. Some commonly used treatments have not been investigated in RCTs. This review is furthermore hampered by the inability to compare the different studies.
Pelvic radiation disease is often not confined to one organ, and symptomatology is based on physiological disorders. Radiation proctopathy, which was the focus of this review, is but one aspect of pelvic radiation disease. The true incidence of the disease is not clear. Before setting up future trials, a widely used uniform definition of this disorder is warranted to serve as a basis. Secondly, there is an urgent need to clearly define the endpoints to investigate and to use a unified grading system by which these endpoints can be categorised, such as the CTCAE or the LENT‐SOMA for late radiation effects, to our knowledge the most widely used and well‐validated classification system of (late) toxicity symptoms, which however is not as sensitive as some other scales (Khalid 2006; Olopade 2005). Without such a system, it is unlikely that meaningful randomised studies can be designed. Future RCTs should also include major important patient‐reported outcome measures’, such as long‐term effects and quality of life evaluations.
What's new
| Date | Event | Description |
|---|---|---|
| 12 November 2015 | New citation required but conclusions have not changed | Review updated and new studies added, but overall conclusions unchanged. |
| 12 November 2015 | New search has been performed | Nine new studies (RCTs) included. We have assessed the risk of bias of all included studies with the Cochrane 'Risk of bias' tool and divided the review into treatments targeted at bleeding, treatments targeted at very localised signs or symptoms, and treatments targeted at the more global collection of symptoms. In addition, we have changed the title from 'Non‐surgical interventions for late radiation proctitis in patients who have received radical radiotherapy to the pelvis' to 'Non‐surgical interventions for late rectal problems (proctopathy) of radiotherapy in people who have received radiotherapy to the pelvis'. |
History
Review first published: Issue 1, 2002
| Date | Event | Description |
|---|---|---|
| 12 November 2007 | New search has been performed | New studies found and included or excluded: 25 April 2007. The literature search as described in the search strategy sections was updated on 25 April 2007. The time frame used was from the April 2007 back to 1999. Fifteen publications were identified that had not been previously included, apparently dealing with the various therapeutic options for management of late radiation proctitis. Initial examination confirmed that 10 were case reports or case series, two were reviews, one was a phase II study, and one was a systematic review containing an as yet unpublished RCT of hyberbaric oxygen in the management of late radiotherapy proctitis. The other reports were excluded, leaving the phase II prospective series and one possible randomised comparative trial. Hence seven controlled studies are currently reported: three compared different rectal steroid preparations and anti‐inflammatory agents versus rectal sucralfate, and were not placebo controlled; two evaluated short chain fatty acid enemas and were placebo controlled but of different designs; one compared the effect of bipolar electrocoagulation versus the heater probe; one unpublished study examined the effect of hyberbaric oxygen versus placebo; another reported a double‐blind placebo RCT using vitamin A; and lastly a phase II series was included on people who had received WF10 therapy. As in the original review, all the sections describe different interventions and outcome parameters so that they cannot be compared and a summary statistic cannot be derived. |
| 29 October 2003 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
We wish to thank Arshi Denton and the original author team who produced the first two versions of this review. Furthermore, we would like to thank Jane Hayes for designing the search strategy, Clare Jess for the contribution to the editorial process, and Ms S Yu Han and Mr J Wang, who were kind enough to assist in the translations of studies.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. RTOG/EORTC Late Radiation Morbidity Scoring Schema
RTOG/EORTC Late Radiation Morbidity Scoring Schema
| Organ tissue | 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | 5 |
| Skin | None | Slight atrophy Pigmentation change Some hair loss |
Patch atrophy Moderate telangiectasia Total hair loss |
Marked atrophy Gross telangiectasia |
Ulceration | D E A T H D I R E C T L Y R E L A T E D T O R A D I A T I O N L A T E E F F E C T S |
| Subcutaneous tissue | None | Slight induration (fibrosia) and loss of subcutaneous fat | Moderate fibrosis but asymptomatic Slight field contracture < 10% linear reduction |
Severe induration and loss of subcutaneous tissue Field contracture > 10% linear measurement |
Necrosis | |
| Mucous membrane | None | Slight atrophy and dryness | Moderate atrophy and telangiectasia Little mucous |
Marked atrophy with complete dryness Severe telangiectasia |
Ulceration | |
| Salivary glands | None | Slight dryness of mouth Good response on stimulation |
Moderate dryness of mouth Poor response on stimulation |
Complete dryness of mouth No response on stimulation |
Fibrosis | |
| Spinal cord | None | Mild Lhermitte's syndrome | Severe Lhermitte's syndrome | Objective neurological findings at or below cord level treated | Mono, para, quadraplegia | |
| Brain | None | Mild headache Slight lethargy |
Moderate headache Great lethargy |
Severe headaches Severe central nervous system dysfunction (partial loss of power or dyskinesia) |
Seizures or paralysis Coma |
|
| Eye | None | Asymptomatic cataract Minor corneal ulceration or keratitis |
Symptomatic cataract Moderate corneal ulceration Minor retinopathy or glaucoma |
Severe keratitis Severe retinopathy or detachment Severe glaucoma |
Panopthalmitis/Blindness | |
| Larynx | None | Hoarseness Slight arytenoid oedema |
Moderate arytenoid oedema Chondritis |
Severe oedema Severe chondritis |
Necrosis | |
| Lung | None | Asymptomatic or mild symptoms (dry cough) Slight radiographic appearances |
Moderate symptomatic fibrosis or pneumonitis (severe cough) Low‐grade fever Patchy radiographic appearances |
Severe symptomatic fibrosis or pneumonitis Dense radiographic changes |
Severe respiratory insufficiency/Continuous O2/Assisted ventilation | |
| Heart | None | Asymptomatic or mild symptoms Transient T wave inversion and ST changes Sinus tachycardia > 110 (at rest) |
Moderate angina on effort Mild pericarditis Normal heart size Persistent abnormal T wave and ST changes Low ORS |
Severe angina Pericardial effusion Constrictive pericarditis Moderate heart failure Cardiac enlargement electrocardiogram abnormalities |
Tamponade/Severe heart failure/Severe constrictive pericarditis | |
| Esophagus | None | Mild fibrosis Slight difficulty in swallowing solids No pain on swallowing |
Unable to take solid food normally Swallowing semi‐solid food Dilatation may be indicated |
Severe fibrosis Able to swallow only liquids May have pain on swallowing Dilatation required |
Necrosis/Perforation Fistula |
|
| Small/large intestine | None | Mild diarrhoea Mild cramping Bowel movement 5 times daily Slight rectal discharge or bleeding |
Moderate diarrhoea and colic Bowel movement > 5 times daily Excessive rectal mucus or intermittent bleeding |
Obstruction or bleeding requiring surgery |
Necrosis/Perforation Fistula |
|
| Liver | None | Mild lassitude Nausea, dyspepsia Slightly abnormal liver function |
Moderate symptoms Some abnormal liver function tests Serum albumin normal |
Disabling hepatitic insufficiency Liver function tests grossly abnormal Low albumin Oedema or ascites |
Necrosis/Hepatic coma or encephalopathy | |
| Kidney | None | Transient albuminuria No hypertension Mild impairment of renal function Urea 25 to 35 mg% Creatinine 1.5 to 2.0 mg% Creatinine clearance > 75% |
Persistent moderate albuminuria (2+) Mild hypertension No related anaemia Moderate impairment of renal function Urea > 36 to 60 mg% Creatinine clearance (50% to 74%) |
Severe albuminuria Severe hypertension Persistent anaemia (< 10 g%) Severe renal failure Urea > 60 mg% Creatinine > 4.0 mg% Creatinine clearance < 50% |
Malignant hypertension Uraemic coma/Urea > 100% |
|
| Bladder | None | Slight epithelial atrophy Minor telangiectasia (microscopic haematuria) |
Moderate frequency Generalised telangiectasia Intermittent macroscopic haematuria |
Severe frequency and dysuria Severe generalised telangiectasia (often with petechiae) Frequent haematuria Reduction in bladder capacity (< 150 cc) |
Necrosis/Contracted bladder (capacity < 100 cc) Severe haemorrhagic cystitis |
|
| Bone | None | Asymptomatic No growth retardation Reduced bone density |
Moderate pain or tenderness Growth retardation Irregular bone sclerosis |
Severe pain or tenderness Complete arrest of bone growth Dense bone sclerosis |
Necrosis/Spontaneous fracture | |
| Joint | None | Mild joint stiffness Slight limitation of movement |
Moderate stiffness Intermittent or moderate joint pain Moderate limitation of movement |
Severe joint stiffness Pain with severe limitation of movement |
Necrosis/Complete fixation |
Appendix 2. The Cochrane Library (CENTRAL)
#1 MeSH descriptor Proctitis explode all trees #2 (proctitis or proctitides or proctopathy or proctocolitis or proctosigmoiditis or rectitis or rectocolitis or rectocolitides or rectosigmoiditis) #3 ((rect* or anus or anal or anorectal) near/5 (injur* or inflam* or diseas* or bleed* or rupture* or discharge* or pain* or discomfort* or irritat*)) #4 (#1 OR #2 OR #3) #5 MeSH descriptor Radiotherapy explode all trees #6 Any MeSH descriptor with qualifier: RT #7 Any MeSH descriptor with qualifier: RE #8 MeSH descriptor Radiation Injuries explode all trees #9 (radiotherap* or radiat* or irradiat* or radiochemo* or chemoradio*) #10 (#5 OR #6 OR #7 OR #8 OR #9) #11 (#4 AND #10)
Appendix 3. MEDLINE (OvidSP) search strategy
1 exp Proctitis/ 2 (proctitis or proctitides or proctopathy or proctocolitis or proctosigmoiditis or rectitis or rectocolitis or rectocolitides or rectosigmoiditis).mp. 3 ((rect* or anus or anal or anorectal) adj5 (injur* or inflam* or diseas* or bleed* or rupture* or discharge* or pain* or discomfort* or irritat*)).mp. 4 1 or 2 or 3 5 exp Radiotherapy/ 6 radiotherapy.fs. 7 radiation effects.fs. 8 exp Radiation Injuries/ 9 (radiotherap* or radiat* or irradiat* or radiochemo* or chemoradio*).mp. 10 5 or 6 or 7 or 8 or 9 11 4 and 10 12 randomized controlled trial.pt. 13 controlled clinical trial.pt. 14 randomized.ab. 15 placebo.ab. 16 drug therapy.fs. 17 randomly.ab. 18 trial.ab. 19 groups.ab. 20 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 21 11 and 20 22 exp animals/ not humans.sh. 23 21 not 22
key:
mp = title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier fs = floating subheading
Appendix 4. EMBASE (OvidSP) search strategy
1 proctitis/ 2 (proctitis or proctitides or proctopathy or proctocolitis or proctosigmoiditis or rectitis or rectocolitis or rectocolitides or rectosigmoiditis).mp. 3 ((rect* or anus or anal or anorectal) adj5 (injur* or inflam* or diseas* or bleed* or rupture* or discharge* or pain* or discomfort* or irritat*)).mp. 4 1 or 2 or 3 5 exp radiotherapy/ 6 rt.fs. 7 exp radiation injury/ 8 radiation response/ 9 (radiotherap* or radiat* or irradiat* or radiochemo* or chemoradio*).mp. 10 5 or 6 or 7 or 8 or 9 11 4 and 10 12 crossover procedure/ 13 double‐blind procedure/ 14 randomized controlled trial/ 15 single‐blind procedure/ 16 random*.mp. 17 factorial*.mp. 18 (crossover* or cross over* or cross‐over*).mp. 19 placebo*.mp. 20 (double* adj blind*).mp.| 21 (singl* adj blind*).mp. 22 assign*.mp. 23 allocat*.mp. 24 volunteer*.mp. 25 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 26 11 and 25 27 (exp Animal/ or Nonhuman/ or exp Animal Experiment/) not Human/ 28 26 not 27
key:
mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword
Appendix 5. Data extraction form
Data collection form
Intervention review – RCTs only
| Study author, year and study ID(surname of first author and year first full report of study was published e.g. Smith 2001) |
|
Notes: Interventieduur: Meetmomenten: |
1. General Information
| Date form completed(dd/mm/yyyy) | |
| Name/ID of person extracting data |
2. Population and setting
|
Description Include comparative information for each group (i.e. intervention and controls) if available |
|
|
Population description (from which study participants are drawn) |
|
|
Setting (including location and social context) |
|
| Inclusion criteria | |
| Exclusion criteria | |
| Notes |
3. Methods
| Descriptions as stated in report/paper | |
| Aim of study | |
| Design(e.g. parallel, crossover, cluster) | |
|
Period of study (year of study) |
|
| Notes |
4. Risk of Bias assessment à In Review Manager
See Chapter 8 of the Cochrane Handbook
| Domain | Risk of bias | Support for judgement | ||
| Low risk | High risk | Unclear | ||
|
Random sequence generation (selection bias) |
||||
|
Allocation concealment (selection bias) |
||||
|
Blinding of participants and personnel Subjective outcomes (performance bias) |
||||
|
Blinding of participants and personnel Objective outcomes (if required) |
||||
|
Blinding of outcome assessment Subjective outcomes (detection bias) |
Outcome group: All/ | |||
|
Blinding of outcome assessment Objective |
Outcome group: | |||
|
Incomplete outcome data Subjective outcomes – short term (attrition bias) |
||||
|
Incomplete outcome data Subjective outcomes –long term (attrition bias) |
||||
|
Incomplete outcome data Objective outcomes – short term |
||||
|
Incomplete outcome data Objective outcomes – long term |
||||
|
Selective outcome reporting? (reporting bias) |
||||
|
Other bias (baseline imbalances, early study ending …) |
||||
5. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
| Description as stated in report/paper | |
|
Total no. randomised (or total pop. at start of study for NRCTs, or total per cluster) |
|
|
Total no. analysed Loss to FU beschrijven |
|
| Age | |
| Sex | |
| Race/Ethnicity | |
| Smoking/non smoking | |
|
Severity of illness (type of malignancy and grade of radiation proctitis) |
|
| Weight | |
| Co‐morbidities | |
| Other relevant sociodemographics |
6. Intervention groups
Copy and paste table for each intervention and comparison group
Intervention Group 1
|
Description as stated in report/paper Intervention |
Control | |
| Group name | ||
|
No. randomised to group (specify whether no. people or clusters) |
||
|
Description (which category of agent used, method of administration and dose) |
||
| Duration of treatment period | ||
|
Providers (e.g. no., by physiotherapist , unclear, profession, training, ethnicity etc. if relevant) |
||
| Co‐interventions | ||
| Notes: | ||
7. Outcomes
Copy and paste table for each outcome.
Outcome 1
| Description as stated in report/paper | |
| Outcomes | |
| Time points measured |
8. Results
Copy and paste the appropriate table for each outcome, including additional tables for each time point and subgroup as required.
A vs placebo Short term (< X weken)
| Description as stated in report/paper | ||||
| Results | Intervention | Comparison | ||
| No. events | No. participants | No. events | No. participants | |
Continuous outcome A vs placebo Short term (< X weken)
| Description as stated in report/paper | |||||||
| Results | Intervention | Comparison | |||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | ||
Other outcome A vs placebo Short term (< X weken)
| Description as stated in report/paper | ||||
| Results | Intervention result | SD (or other variance) | Control result | SD (or other variance) |
| Overall results | SE (or other variance) | |||
| No. participants | Intervention | Control | ||
Data and analyses
Comparison 1. Short chain fatty acid (SCFA) enemas versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean reduction of endoscopic score after treatment | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Mean number of days of rectal bleeding after treatment | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Mean haemoglobin levels at the end of treatment period (5 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
1.3. Analysis.
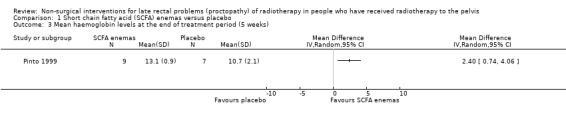
Comparison 1 Short chain fatty acid (SCFA) enemas versus placebo, Outcome 3 Mean haemoglobin levels at the end of treatment period (5 weeks).
Comparison 2. Endoscopic argon plasma coagulation (APC) plus oral sucralfate versus APC plus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Endoscopy scores Gilinsky scale grade 6‐9 at 8 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Endoscopy scores Gilinsky scale grade 6‐9 at 16 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Number of participants with complications | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Endoscopic bipolar electrocoagulation versus heater probe.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe bleeding episodes | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Mean number of severe bleeds after first year | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Mean haematocrit after first year | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Mean units of blood transfused after first year | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
3.3. Analysis.
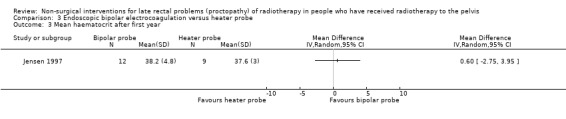
Comparison 3 Endoscopic bipolar electrocoagulation versus heater probe, Outcome 3 Mean haematocrit after first year.
Comparison 4. APC versus bipolar electrocoagulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eradication of all telangiectasias | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Complications ‐ Minor | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Complications ‐ Major | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 5. Hydrocortisone versus betamethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement of endoscopic appearance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Reduction of rectal bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Poor tolerance of enema | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 6. Standard treatment plus oral pentoxifylline versus standard treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cessation of rectal bleeding | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 7. Oral sulfasalazine plus rectal steroids versus oral placebo plus rectal sucralfate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Endoscopic improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 8. Metronidazole in addition to mesalazine and betamethasone versus no metronidazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rectal bleeding score < 2 after 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Diarrhoea score < 2 after 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 No rectal erythema after 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 No rectal ulceration after 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 9. Hyperbaric oxygen therapy versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical evaluation ‐ healed or improved | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 10. Retinol palmitate versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Response to treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 11. Algorithm‐based management.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bowel subset score (IBDQ‐B) at 6 months (higher score indicates better) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Gastroenterologist group vs booklet group | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Nurse group vs booklet group | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Gastroenterologist group vs nurse group | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in rectal SOMA‐LENT at 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Gastroenterologist group vs booklet group | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Nurse group vs booklet group | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Gastroenterologist group vs nurse group | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in small intestine SOMA‐LENT at 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Gastroenterologist group vs booklet group | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Nurse group vs booklet group | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Gastroenterologist group vs nurse group | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 SF12: change in physical component score at 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Gastroenterologist group vs booklet group | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Nurse group vs booklet group | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Gastroenterologist group vs nurse group | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 SF12: change in mental component score at 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Gastroenterologist group vs booklet group | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Nurse group vs booklet group | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Gastroenterologist group vs nurse group | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 12. Integrated Chinese traditional medicine plus Western medicine versus Western medicine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Grade of radioproctitis after treatment Grade I | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Cured ‐ better after 37 days | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andreyev 2013.
| Methods | Single‐centre, prospective, 3‐arm, non‐blinded, randomised controlled trial Duration of study: 2007 to 2012 Follow‐up: 12 months |
|
| Participants | 218 people (aged over 18 years) who had troublesome, persisting gastrointestinal symptoms that started during or after radiotherapy given with curative intent for histologically proven prostatic, bladder, vulval, vaginal, cervical, endometrial, anal, or rectal malignant neoplasia or para‐aortic irradiation for metastatic disease from any of those primary sites or the testis. Radiotherapy should have been completed at least 6 months before enrolment. Sex (M/F): 168/50 Similar groups with respect to demographic and clinical characteristics. Dropouts: 25 (11%) participants were withdrawn from the study before completion. |
|
| Interventions | Group I: gastroenterologist group (management according to the algorithm by a consultant gastroenterologist) (n = 70) Group II: nurse group (management according to the algorithm by a specially trained research nurse) (n = 80) Group III: booklet group (detailed advice booklet on self management of bowel symptoms) (n = 68) N.B.: participants in the booklet group whose symptoms continued 6 months after recruitment were offered consultation with the gastroenterologist and, if appropriate, investigation and treatment. Participants in the nurse‐led care group were crossed over to the gastroenterologist‐led care group if they had gastrointestinal issues that were beyond the scope of the algorithm. |
|
| Outcomes | Change in IBDQ‐B subset score, QoL, anxiety and depression scores, and pelvic symptom scores | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a computer‐generated randomization sequence and random permuted blocks, we allocated patients to one of the three groups." |
| Allocation concealment (selection bias) | Low risk | Central randomisation was performed (the randomisation office of the Institute of Cancer Research, which had no further involvement in the trial, generated the randomisation sequence) |
| Blinding (performance bias and detection bias) | High risk | Non‐blinded trial (interventions are impossible to blind), high risk of performance and detection bias |
| Blinding of outcome assessment subjective | High risk | Non‐blinded trial (interventions are impossible to blind), high risk of subjective outcome assessment (participant‐reported outcomes) |
| Blinding of outcome assessment objective | Unclear risk | Study item not assessed in the trial |
| Incomplete outcome data: subjective outcomes | Unclear risk | The study was analysed on an ITT basis, but 25 (11%) participants were withdrawn from the study before completion (if a participant had a recurrence of cancer requiring treatment or was admitted to hospital for gastrointestinal symptoms, they were withdrawn from the study). However, it is unclear what happened with the data of these withdrawn participants (such as LOCF, etc.). The number of withdrawn participants is not similar between the groups, so this could have led to bias |
| Incomplete outcome data: objective outcomes | Unclear risk | Study item not assessed in the trial |
| Selective reporting (reporting bias) | Low risk | This trial is registered with ClinicalTrials.gov, number NCT00737230. All assumed outcomes except cost‐effectiveness are reported in the paper. The authors state that a full cost‐effectiveness analysis was embedded within the trial, but that the results will be reported separately |
| Other bias | Low risk | No indications of other bias |
Cavcic 2000.
| Methods | Quasi‐randomised study. Duration of study: 1990 to 2000 Follow‐up: 12 months |
|
| Participants | 60 people with chronic radiation proctopathy and cytologically proven prostatic carcinoma staged to the TNM classification as T2N0M0 stage Sex (M/F): not reported Comparable groups with respect to age and previous treatment time Dropouts (after 3 months) IG: 3 CG: 12. Reasons for dropouts not explained |
|
| Interventions | Intervention: metronidazole (3 x 400 mg orally per day), mesalazine (3 x 1 g orally per day), and betamethasone enema (once a day) Comparator: same doses of mesalazine and betamethasone enema, but without metronidazole |
|
| Outcomes | The efficacy of metronidazole was assessed using rectal bleeding, diarrhoea, and endoscopy | |
| Notes | The incidence of diarrhoea, rectal bleeding, ulcers and oedema was significantly reduced in the metronidazole group up to 12 months after treatment. No QoL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were allocated to 2 groups according to the date on which their treatment began (quasi random) |
| Allocation concealment (selection bias) | High risk | Allocation concealment could be foreseen, as participants were allocated according to the date on which their treatment began |
| Blinding (performance bias and detection bias) | High risk | Participants in intervention group received their medication orally, it seemed the intervention had not been blinded |
| Blinding of outcome assessment subjective | High risk | Participants in intervention group received their medication orally, it seemed the intervention had not been blinded |
| Blinding of outcome assessment objective | Low risk | Objective participant response was documented by rectal bleeding score and diarrhoea score. The participants were scored the same way before and after treatment. The same physician interviewed participants once a week during the treatment period, however unclear whether outcome assessment was blinded |
| Incomplete outcome data: subjective outcomes | High risk | Assessments before and after 4 weeks treatment: I: N = 30; C: N = 30. After 3 months dropouts: I: N = 3; C: = 12 (25%). After 1 year dropouts: I: N = 6; C: N = 18 (40%). After 2 years dropouts: I: N = 11; C: N = 20 (52%) Reasons for lost to follow‐up not reported. Not all participants were followed up for the same period of time: "The longest follow‐up period after 4 weeks treatment was 3 years, whereas the shortest one was 2 years." |
| Incomplete outcome data: objective outcomes | High risk | Assessments before and after 4 weeks treatment: I: N = 30; C: N = 30. After 3 months dropouts: I: N = 3; C: = 12 (25%). After 1 year dropouts: I: N = 6; C: N = 18 (40%). After 2 years dropouts: I: N = 11; C: N = 20 (52%) Reasons for lost to follow‐up not reported. Not all participants were followed up for the same period of time: "The longest follow‐up period after 4 weeks treatment was 3 years, whereas the shortest one was 2 years." |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available, however all outcomes mentioned in the methods sections have been addressed in the results section |
| Other bias | Low risk | No indications of other bias |
Chruscielewska 2012.
| Methods | RCT. Duration of study: participants were recruited between June 2003 and March 2006. Follow‐up: 52 weeks |
|
| Participants | 122 adults with chronic radiation proctopathy or proctosigmoiditis were included if all of the following criteria were met: radiotherapy for a pelvic tumour completed at least 3 months before enrolment, the presence of rectal bleeding, radiation‐induced telangiectasia in the rectum or sigmoid, or both. The baseline characteristics of the 2 groups were comparable | |
| Interventions | Intervention: endoscopic APC followed by oral sucralfate (6 g twice daily) for 4 weeks (n = 60) Comparator: APC with placebo |
|
| Outcomes | Changes in chronic radiation proctopathy severity score, changes in endoscopy scores, and complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator was used |
| Allocation concealment (selection bias) | Low risk | The study investigators were not involved in the preparation of the computer‐generated allocation code or in the generation of consecutively numbered containers with the study medication (drug or identical‐appearing placebo tablets) |
| Blinding (performance bias and detection bias) | Low risk | Study participants and investigators remained blinded to group assignment until the conclusion of the study, which was the visit at week 52 |
| Blinding of outcome assessment subjective | Low risk | Study participants and investigators remained blinded to group assignment until the conclusion of the study, which was the visit at week 52 |
| Blinding of outcome assessment objective | Unclear risk | Study item not assessed in the trial |
| Incomplete outcome data: subjective outcomes | Low risk | ITT analysis was used throughout the study. Only 3 dropouts in intervention group |
| Incomplete outcome data: objective outcomes | Unclear risk | Study item not assessed in the trial |
| Selective reporting (reporting bias) | Unclear risk | No registered study protocol available, however, all outcomes mentioned in the methods sections have been addressed in the results section |
| Other bias | Low risk | No indications of other bias |
Clarke 2008.
| Methods | Multi‐centre RCT. Duration of study: unclear Follow‐up: 5 years |
|
| Participants | 120 people with rectal late radiation tissue injury. The diagnosis must have been present for more than 3 months and responded insufficiently to other therapies Sex (F/M): 106/14 Comparable groups with respect to demographic and clinical characteristics Dropouts: at 1 year, 5 participants (4%) had died and 9 (8%) had been lost to follow‐up |
|
| Interventions | Intervention: 100% oxygen at 2.0 ATA for 90 min, once daily, 5 times weekly Comparator: 21% oxygen (normal air) at 1.1 ATA for 90 min, once daily, 5 times weekly |
|
| Outcomes | The primary outcome measures were the SOMA‐LENT score and standardised clinical assessment. The secondary outcome was the change in QoL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Biostatisticians at the University of South Carolina generated the randomisation sequence, which was uploaded into, and concealed within, the study database software. Participants were randomly assigned (1:1) to receive HBO or normobaric air, using a "blocking" process. The block size was 4 and was equally stratified with 2 of each treatment options (A or B). |
| Allocation concealment (selection bias) | Low risk | Not reported on how the allocation concealment was done, but referring physicians (who also were the outcome assessors) as well as the participants are described as being unaware of the allocation |
| Blinding (performance bias and detection bias) | Low risk | Both assessors and participants had been blinded |
| Blinding of outcome assessment subjective | Low risk | Participants had been blinded (sham treatment for control group) |
| Blinding of outcome assessment objective | Low risk | Referring physicians (who were also the outcome assessors) are described as being unaware of the allocation |
| Incomplete outcome data: subjective outcomes | Unclear risk | At 1 year (minimum follow‐up time), 5 participants (4%) had died and 9 (8%) had been lost to follow‐up. No clear reasons for these dropouts provided (reasons only well described for dropouts before randomisation) |
| Incomplete outcome data: objective outcomes | Unclear risk | At 1 year (minimum follow‐up time), 5 participants (4%) had died and 9 (8%) had been lost to follow‐up. No clear reasons for these dropouts provided (reasons only well described for dropouts before randomisation) |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available, however all outcomes reported in the methods section have been addressed in the results section |
| Other bias | Low risk | No indications of other bias |
Ehrenpreis 2005.
| Methods | Double‐blind RCT. Duration of study: not reported Follow‐up: at 90 days |
|
| Participants | 19 people with radiation proctopathy (at least 2 symptoms with a severity score of 3 on at least a weekly basis), however, one participant from each group did not take a single dose of study medication and were therefore excluded from analysis (n = 17). Sex (M/F): 15/2 Comparable groups with respect to baseline RPSAS Dropouts: 1 (reason not explained), but included in final analysis |
|
| Interventions | Intervention: Retinol palmitate 10,000 IU by mouth for 90 days Comparator: Identical placebo capsules |
|
| Outcomes | Reduction in the RPSAS scores after 30 days for 90 days | |
| Notes | 5 placebo non‐responders who were crossed over to Tx responded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number system |
| Allocation concealment (selection bias) | Low risk | Neither the investigators nor participants were aware of who was receiving intervention treatment or placebo |
| Blinding (performance bias and detection bias) | Low risk | Double‐blinded study (participants and investigators were blinded) |
| Blinding of outcome assessment subjective | Low risk | Double‐blinded study (participants and investigators were blinded). |
| Blinding of outcome assessment objective | Low risk | Double‐blinded study |
| Incomplete outcome data: subjective outcomes | Low risk | ITT analyses was performed, number of drop‐outs reported, however reason for drop‐out not reported |
| Incomplete outcome data: objective outcomes | Low risk | ITT analyses was performed, number of drop‐outs reported, however reason for drop‐out not reported |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available, however all outcomes mentioned in the methods section had been addressed in the results section |
| Other bias | Low risk | No indications of other bias |
Jensen 1997.
| Methods | RCT. Duration of study: not reported Follow‐up, days (mean): 770 versus 704. However, major outcomes were compared at 12 months after randomisation |
|
| Participants | 21 people with CRP, RT 2 years ago, failed medical treatment, and per rectum bleeds at least 3 times a week Sex (M/F): 18/3 Similar groups with respect to demographic and clinical characteristics Dropouts: none |
|
| Interventions | Intervention: Heater probe (9) Comparator: Bipolar electrocoagulation probe (12) Treatment with the same probe till the bleeding stopped, mean 4 treatments |
|
| Outcomes | Sigmoidoscopies 4 to 6 weekly till bleeds stopped and follow‐up requirements Fall in severe bleeds and the need for follow‐up in both groups. Significant decrease in both groups | |
| Notes | No side effects reported. QoL informally assessed with participant responses, which improved with treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) | Low risk | Unclear whether participants had been blinded, but as both arms received a similar active intervention it seems unlikely that this would have introduced performance bias |
| Blinding of outcome assessment subjective | Low risk | Unclear whether participants had been blinded, but as both arms received a similar active intervention it seems unlikely that this would have introduced bias for subjective outcomes |
| Blinding of outcome assessment objective | Unclear risk | Managing physician was blinded to treatment, however, it was not clearly described whether the research nurse who evaluated and followed all participants was blinded. |
| Incomplete outcome data: subjective outcomes | Low risk | No loss to follow‐up |
| Incomplete outcome data: objective outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Study was published in 1997, so no study protocol available. However, all outcomes prespecified in the methods section were reported in the results section |
| Other bias | Low risk | No indications of other bias |
Kochhar 1991.
| Methods | Double‐blind RCT. Duration of study: 4 weeks Follow‐up: at 4 weeks |
|
| Participants | 37 people with CRP, 36 cervix and 1 prostate Dx confirmed on symptoms (graded) and mucosal appearance (graded). Cumulative score | |
| Interventions | Intervention (group 1): oral sulfasalzine 1 g tds + prednisolone enemas 20 mg bd for 4 weeks 18 people entered, 1 dropped out (unclear why), and 2 did not tolerate the drug Comparator (group 2): oral placebo + rectal sucralfate 2 g bd for 4 weeks 19 entered, with 2 dropouts (unclear why) | |
| Outcomes | Groups' responses: 1. Clin improvement 8/15, P < 0.01; endoscopic changes 7/15, P < 0.01 2. Clin improvement 16/17, P < 0.001 (clin response better P < 0.05); endoscopic changes 12/17, P < 0.001 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but methods of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) | Low risk | Both the participants and the physician were blinded to the type of therapy |
| Blinding of outcome assessment subjective | Low risk | Participants were blinded, and filled out their own diary |
| Blinding of outcome assessment objective | Unclear risk | Not described |
| Incomplete outcome data: subjective outcomes | Unclear risk | Group 1: 1 drop out, reasons not discussed. 2 others dropped out because they did not tolerate the drug. Group 2: 2 drop outs, reasons not discussed |
| Incomplete outcome data: objective outcomes | Unclear risk | Group 1: 1 drop out, reasons not discussed. 2 others dropped out because they did not tolerate the drug. Group 2: 2 drop outs, reasons not discussed |
| Selective reporting (reporting bias) | Unclear risk | Study was published in 1991, so no study protocol available. However, all outcomes prespecified in the methods section were reported in the results section |
| Other bias | Low risk | No indications of other bias |
Lenz 2010.
| Methods | RCT. Duration: 2005 to 2008 Follow‐up: 1 year |
|
| Participants | 30 people with recurrent rectal bleeding, started 6 months after radiotherapy Mean age 67.4 (SD 11.8); 10% grade 1; 43.3% grade 2; 26.7% grade 3; 20% grade 4. Comparable groups Dropouts: APC group: 2 (1 died, 1 refused further therapy after successful reduction of her rectal bleeding (score from 4 to 2 points)) |
|
| Interventions | Intervention: BEC Comparator: APC |
|
| Outcomes | Efficacy, complications, relapse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was done by sequential opening of numbered, opaque envelopes |
| Allocation concealment (selection bias) | Low risk | Randomisation was done by sequential opening of numbered, opaque envelopes |
| Blinding (performance bias and detection bias) | Low risk | Unclear whether participants had been blinded, but as both arms received a similar active intervention, it seems unlikely that this would have introduced performance bias |
| Blinding of outcome assessment subjective | Low risk | Unclear whether participants had been blinded, but as both arms received a similar active intervention, it seems unlikely that this would have introduced performance bias |
| Blinding of outcome assessment objective | Low risk | No information on blinding. However, it is unlikely that objective outcomes were influenced |
| Incomplete outcome data: subjective outcomes | Low risk | 2 participants (APC group) did not complete the treatment, despite clinical improvement. The clinical score of 1 of these participants improved from 4 to 3 points, but he died due to pneumonia. The other refused further therapy after successful reduction of her rectal bleeding (score from 4 to 2 points) |
| Incomplete outcome data: objective outcomes | Low risk | 2 participants (APC group) did not complete the treatment, despite clinical improvement. The clinical score of 1 of these participants improved from 4 to 3 points, but he died due to pneumonia. The other refused further therapy after successful reduction of her rectal bleeding (score from 4 to 2 points) |
| Selective reporting (reporting bias) | Low risk | Protocol was registered on ClinicalTrials.gov (NCT00725244), and all prespecified outcomes have been reported in the article |
| Other bias | Low risk | There was no significant difference between the groups regarding pelvic cancer, age, gender, aspirin use, previous medical therapy, or disease severity |
Nelamangala 2012.
| Methods | RCT. Duration of study: August 2005 to May 2007 Follow‐up: 6.3 (range 2 to 18) months |
|
| Participants | 102 participants with rectal bleeding as a result of chronic haemorrhagic radiation proctopathy, following radiotherapy for carcinoma of the cervix Mean age: 50.8 ± 5.0 years in Group 1 (formaline dab); 51.8 ± 5.1 years in Group 2 (sacralfate steroid enema) Symptoms:
Comparable groups Dropouts: none |
|
| Interventions | Intervention: formalin dab Comparator: sucralfate steroid retention enema |
|
| Outcomes | Symptom score and sigmoidoscopic grade | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block randomisation, fixed |
| Allocation concealment (selection bias) | High risk | An open list of random numbers was used, participants or investigators could possibly foresee assignments and thus introduce selection bias (information retrieved from registered protocol) |
| Blinding (performance bias and detection bias) | High risk | No information on blinding described, but blinding most likely impossible considering the characteristics of the interventions |
| Blinding of outcome assessment subjective | High risk | No information on blinding described, but blinding most likely impossible considering the characteristics of the interventions |
| Blinding of outcome assessment objective | Low risk | Sigmoidoscopy was repeated by a consultant who was blinded to the treatment received, and the sigmoidoscopic grading was recorded |
| Incomplete outcome data: subjective outcomes | Low risk | None were lost to follow‐up |
| Incomplete outcome data: objective outcomes | Low risk | None were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Trial was registered in the Clinical Trials Registry of India (CTRI/2011/12/002184). Adverse events were not pre‐specified in the protocol. In the protocol it was also stated that all participants were female, which was not reported in the article (gender was not mentioned at all) |
| Other bias | Low risk | No indications of other bias |
Pinto 1999.
| Methods | Double‐blind RCT. Duration of study: 1992 to 1994 Follow‐up: at 5 weeks |
|
| Participants | 19 people with Grade III CRP Pourquier classification, persistent symptoms for a minimum of 12 months Mean age 59 years (range 36 to 75), Sex (F/M): 18:1 Both groups had equivalent baseline characteristics 1 (SCFA arm) and 2 (placebo) arm dropped out due to heavy per rectum bleeds |
|
| Interventions | Intervention: SCFA enema (as per Harig preparation 60 ml bd, 5 wks) Comparator: placebo |
|
| Outcomes | No. of days of per rectum bleeding, colonoscopic scores, and DNA and protein content Reduction of days/week of bleeding in SCFA P = 0.001. Increased Hb in SCFA vs placebo P = 0.02. Colonoscopic scores reduced in both arms, but more in SCFA P = 0.02 | |
| Notes | Side effects were recorded and there were none. No QoL assessment either | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated, but randomisation method not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) | Low risk | Study was double blinded "allocated to receive either SCFA enemas or an indentically appearing saline isotonic solution..." |
| Blinding of outcome assessment subjective | Low risk | Participants were blinded to the treatment |
| Blinding of outcome assessment objective | Low risk | Outcome assessors were blinded to the treatment "...by two well‐trained physicians who were not aware of the study phase or the nature of the treatment administered." "All biopsy specimens were interpreted in a random and blinded manner by two pathologists." |
| Incomplete outcome data: subjective outcomes | Unclear risk | 3 drop outs: 1 in the intervention group and 2 in the placebo group. Reasons for dropouts not discussed |
| Incomplete outcome data: objective outcomes | Unclear risk | 3 drop outs; 1 in the intervention group and 2 in the placebo group. Reasons for dropouts not discussed |
| Selective reporting (reporting bias) | Unclear risk | Study was published in 1999, so no study protocol available. However, all outcomes prespecified in the methods section were reported in the results section |
| Other bias | Low risk | No indications of other bias |
Rougier 1992.
| Methods | Double‐blind RCT. Duration of study: not reported Follow‐up: unclear, possibly at 4 weeks |
|
| Participants | 32 people (3 male and 29 female) all with CRP, baseline characteristics comparable using t test Dx made on symptoms and grade of proctopathy | |
| Interventions | Intervention (group 1): 16 people entered and 2 were lost (unclear why); betamethasone lavage 5 mg od for 4 weeks. (Higher numbers of Grade 3 CRP) Comparator (group 2): 16 people entered and none were lost; hydrocortisone acetate mousse 90 mg od for 4 weeks |
|
| Outcomes | Clinically the response was better in the hydrocortisone group, P < 0.01 and also endoscopically, with 5 to 14 responding in group 1 compared to 12 to 16 in group 2, P < 0.05. The betamethasone lavage was poorly tolerated in 10 to 14. | |
| Notes | No standard error recorded. Lavage in group 1 poorly tolerated; group 1 also had a slightly higher proportion of Grade 3 CRP, which may be harder to treat | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) | Unclear risk | Not described, however both arms received different active interventions. It is therefore unclear whether this would have introduced performance bias |
| Blinding of outcome assessment subjective | Unclear risk | Not described, however both arms received different active interventions. It is therefore unlikely that this would have introduced bias for subjective outcomes |
| Blinding of outcome assessment objective | Unclear risk | Not described |
| Incomplete outcome data: subjective outcomes | Unclear risk | 2 participants were lost to follow‐up from group 1 (the betamethasone group), reasons not explained |
| Incomplete outcome data: objective outcomes | Unclear risk | 2 participants were lost to follow‐up from group 1 (the betamethasone group), reasons not explained |
| Selective reporting (reporting bias) | High risk | Study was published in 1992, so no study protocol available. However, not all outcomes described in the methods section appear in the results section |
| Other bias | High risk | More aggressive grade of disease in group 1 (betamethasone group) |
Sahakitrungruang 2012.
| Methods | RCT. Duration of study: October 2010 to January 2012 Follow‐up: at 2 months |
|
| Participants | 50 participants with symptomatic haemorrhagic radiation proctopathy for more than 6 months without complications of rectal stricture, deep ulceration, fistula formation, and sepsis. People who were allergic to ciprofloxacin and metronidazole were excluded. Mean age (range): 64 (31 to 85) versus 64 (27 to 80) Comparable groups Dropouts: 3 participants were lost to follow‐up: 1 in the irrigation group and 2 in the formalin group |
|
| Interventions | Intervention: Colonic irrigation Comparator: Formalin application |
|
| Outcomes | Episodes of rectal bleeding (days/week), frequency (times/week), urgency (days/week), diarrhoea (days/week), and tenesmus (days/ week) before and after 8 weeks of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were consecutively assigned to each treatment group according to a computer‐generated randomization list.” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) | High risk | No information on blinding, no placebo |
| Blinding of outcome assessment subjective | High risk | No information on blinding, no placebo, and different active interventions. As most outcomes were participant reported, high risk of bias. |
| Blinding of outcome assessment objective | Low risk | Repeated sigmoidoscopy (VRS) and haematocrit values and number of people requiring packed red blood cell transfusion are ‘objective’, however these outcomes are not of interest to this review |
| Incomplete outcome data: subjective outcomes | Low risk | 3 participants were lost to follow‐up: 1 in the irrigation group and 2 in the formalin group |
| Incomplete outcome data: objective outcomes | Low risk | 3 participants were lost to follow‐up: 1 in the irrigation group and 2 in the formalin group |
| Selective reporting (reporting bias) | High risk | No study protocol available. Outcomes every 2 months after treatment not reported |
| Other bias | Low risk | There are no indications of other bias |
Talley 1997.
| Methods | Double‐blind, randomised, placebo‐controlled, cross‐over pilot. Duration of study: not reported Follow‐up: at 5 weeks |
|
| Participants | 15 people: 12 prostate, 1 cervix, and 2 rectal carcinomas diagnosed as chronic radiation proctopathy (over 2 months). Mean age 67.7 years, Sex (F or M) 2 to 13 Group comparability: unclear (no separated group characteristics presented) Dropouts: 3 participants were lost without explanation, so 12 completed |
|
| Interventions | Intervention: Butyric acid enema (60 ml containing 40 mmol butyric acid) twice a day Comparator: Normal saline placebo enema Interventions for 2 weeks with a 1‐week wash‐out period and then alternate enema |
|
| Outcomes | Scores of symptoms, endoscopy, and biopsy. No significant change in the SCFA or placebo arm for any of the 3 scores | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated, but randomisation method not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) | Low risk | Study was double blind study |
| Blinding of outcome assessment subjective | Low risk | Both participants and outcome assessors were unaware of the treatment given |
| Blinding of outcome assessment objective | Unclear risk | Both participants and outcome assessors were unaware of the treatment given |
| Incomplete outcome data: subjective outcomes | Unclear risk | 3 dropouts (not specified why and in which group) |
| Incomplete outcome data: objective outcomes | Unclear risk | 3 dropouts (not specified why and in which group) |
| Selective reporting (reporting bias) | Unclear risk | Study was published in 1997, so no study protocol available. However, all outcomes prespecified in the methods section were reported in the results section |
| Other bias | Low risk | No indications of other bias |
Tian 2008.
| Methods | Quasi‐randomised study. Duration of study: not reported Follow‐up: at 37 days |
|
| Participants | 58 women diagnosed as having rectal radiation damage Mean age in years (range): Group 1: 46 (36 to 68) Group 2: 45 (35 to 66) Comparable groups with respect to demographics and clinical characteristics Dropouts: none |
|
| Interventions | Intervention: Shen Ling Bai Zhu powders Comparator: Shen Ling Bai Zhu powders combined with rectal administration of Western drugs |
|
| Outcomes | Grade of radioproctitis after treatment, treatment effect after 37 days | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Women were divided into 2 groups according to treatment order (quasi random) |
| Allocation concealment (selection bias) | High risk | Investigators may have foreseen the assignments, as women were divided into 2 groups according to treatment order (quasi random) |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Blinding of outcome assessment subjective | Unclear risk | Not described |
| Blinding of outcome assessment objective | Unclear risk | Not described |
| Incomplete outcome data: subjective outcomes | Low risk | No dropouts |
| Incomplete outcome data: objective outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. However, all outcomes prespecified in the methods section were reported in the results section |
| Other bias | Low risk | No indications of other bias |
Venkitaraman 2008.
| Methods | RCT. Duration of study: 1994 to 2002 Follow‐up: at 6 months |
|
| Participants | 40 people with symptomatic late morbidity with at least 1 episode of rectal bleeding more than 6 months since pelvic radiotherapy, no evidence of disease progression at the primary tumour site or rectum, had normal serum fibrinogen levels, were not on anticoagulants or antiplatelet medications, and had a life expectancy of more than 6 months No demographic or baseline disease characteristics reported, but groups were described as "well balanced" with regard to participant and disease characteristics |
|
| Interventions | Intervention: standard therapies for late radiation‐induced bleeding plus oral pentoxifylline (400 mg) 3 times daily for 6 months Comparator: standard treatment for late radiation‐induced bleeding |
|
| Outcomes | Frequency and severity of rectal bleeding. No statistically significant advantage with 6 months of pentoxifylline compared with standard measures for late radiation‐induced rectal bleeding were found | |
| Notes | Investigators had planned to accrue 80 participants over a period of 4 years for the study to have an 80% power of detecting a reduction in the incidence of people with recurrent symptoms from 60% with standard conservative management to 30% with the addition of pentoxifylline at a 5% statistical significance. However, they failed to achieve the expected rate of enrolment (N = 40), which may have influenced the results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out using randomised computed blocks software |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) | High risk | The study was non‐blinded, and the participants in the control group did not receive a placebo treatment |
| Blinding of outcome assessment subjective | High risk | The study was non‐blinded, and the participants in the control group did not receive a placebo treatment |
| Blinding of outcome assessment objective | Unclear risk | The study was non‐blinded |
| Incomplete outcome data: subjective outcomes | Unclear risk | Of the 50 randomised participants, 10 participants were excluded due to inadequate baseline morbidity and follow‐up information, and data from 40 participants were analysed. Thereafter, 20 people were randomised to each group |
| Incomplete outcome data: objective outcomes | Unclear risk | Of the 50 randomised participants, 10 were excluded due to inadequate baseline morbidity and follow‐up information, and data from 40 were analysed. Thereafter, 20 people were randomised to each group |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available, however all outcomes mentioned in the methods sections were addressed in the results section |
| Other bias | Unclear risk | “We had planned to accrue 80 patients over a period of 4 years, for the study to have an 80% power of detecting a reduction in the incidence of patients with recurrent symptoms from 60% with standard conservative management to 30% with the addition of pentoxifylline at a 5% statistical significance.” However, they failed to achieve the expected rate of enrolment (N = 40), which may have influenced the results |
Yeoh 2013.
| Methods | RCT. Duration of study: 10 years Follow‐up: 111 (29 to 170) months |
|
| Participants | 30 men (median age 72 years; range 49 to 87 years) with intractable rectal bleeding (defined as 1 per week or requiring blood transfusions, or both) after radiotherapy for prostate carcinoma. No significant differences in pretreatment characteristics reported |
|
| Interventions | Intervention: APC (n = 17) Comparator: topical formalin (n = 13) |
|
| Outcomes | Evaluations of:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by a clerk opening 1 envelope from 3 batches of 20 previously sealed envelopes kept in a central office between January 2000 and April 2010. Each batch of 20 sealed envelopes had 10 + 10 participants assigned in random order to APC and topical formalin, respectively |
| Allocation concealment (selection bias) | Low risk | Central allocation with sealed envelopes |
| Blinding (performance bias and detection bias) | High risk | No information on blinding, no placebo, and different active interventions |
| Blinding of outcome assessment subjective | High risk | No information on blinding, no placebo, and different active interventions. As all relevant outcomes were participant reported, high risk of bias |
| Blinding of outcome assessment objective | Unclear risk | Study item not assessed in the trial |
| Incomplete outcome data: subjective outcomes | Low risk | The data were analysed on an ITT basis. Apparently no dropouts |
| Incomplete outcome data: objective outcomes | Unclear risk | Study item not assessed in the trial |
| Selective reporting (reporting bias) | Unclear risk | No registered study protocol available, however all outcomes mentioned in the methods section have been addressed in the results section |
| Other bias | Low risk | No indications of other bias |
APC: argon plasma coagulation ATA: atmosphere absolute bd: 2 times a day BEC: bipolar eletrocoagulation CG: comparator group CRP: chronic radiation proctopathy DNA: deoxyribonucleic acid Dx: diagnosis Hb: haemoglobin HBO: hyperbaric oxygen IBDQ‐B: Inflammatory Bowel Disease Questionnaire–Bowel IG: intervention group ITT: intention‐to‐treat LOCF: last observation carried forward od: once a day QoL: quality of life RCT: randomised controlled trial RPSAS: Radiation Proctopathy System Assessments Scale SCFA: short chain fatty acid SD: standard deviation SOMA‐LENT: Subjective, Objective, Management, Analytic ‐ Late Effects of Normal Tissue tds: 3 times a day Tx: treatment VRS: Vienna Rectoscopy Score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Sabbagh 1996 | Prospective open pilot study of 7 cases treated with SCFA enemas daily for 1 month, for CRP. Per rectum bleeding showed significant reduction. Not randomised |
| Alvaro‐Villegas 2010 | Participants were not strictly randomised but were allocated into each group according to random referrals/conference abstract |
| Alvaro‐Villegas 2011 | No RCT |
| Barnett 2011 | Prognostic model |
| Baughan 1993 | RCT for the use of oral 5‐ASA during pelvic RT to prevent acute radiation proctopathy |
| Edsmyr 1976 | Does not concern late radiation proctopathy |
| Freund 1987 | Prevention study |
| Freund 1987a | Prevention study |
| Fuccio 2010 | Prevention study |
| Fuccio 2011 | Prevention study |
| Fuccio 2011a | Prevention study |
| Gheorghe 2003 | Does not concern chronic radiation proctopathy |
| Gonzalez 2009 | Intervention does not fulfil our inclusion criteria |
| Henriksson 1992 | Prevention study |
| Khan 2000 | Prevention study |
| Kneebone 2004 | Prevention study |
| Kneebone 2005 | The result was considered inconclusive, because the study was unable to exclude clinically important differences in the late toxicity rates |
| Lodge 1995 | Randomised cross‐over study for the treatment of acute radiation proctitis with either ispaghula husk or codeine phosphate |
| Menander‐Huber 1978 | Does not concern late radiation proctopathy |
| O'Brien 1997 | Rectal sucralfate is given in a RCT prophylactically to prevent the development of acute radiation proctopathy |
| Pilepich 2006 | Mixed population of acute and late radiation proctopathy, results for people with late radiation proctopathy are not presented seperately |
| Pironi 2013 | No RCT |
| Samalavicius 2013 | No RCT |
| Sherman 1971 | Controlled comparative study, and unclear whether it applies to late radiation proctopathy |
| Sidik 2007 | Participants do not fulfil our inclusion criteria |
| Stojcev 2013 | Letter to editor |
| Triantifillidis 1990 | High doses of 5‐ASA enemas in CRP: cf betamethasone enemas. Unrandomised cross‐over study of 6 people with CRP. 5‐ASA 4 g enemas bd for 2 weeks. Wash‐out period of 3 months, and betamethasone enemas 5 g bd 2 weeks. No significant change with either treatment |
| Tsibouris 1999 | Commentary |
| Vernia 2000 | RCT cross‐over study for the treatment of acute radiaiton proctitis with SCFA enema |
| Wen 2012 | Not all participants had subsequently developed late radiation complications |
| Yeoh 2013a | Comparative study |
ASA: aminosalicylic acid bd: 2 times a day CRP: chronic radiation proctopathy RCT: randomised controlled trial RT: radiotherapy SCFA: short chain fatty acid
Characteristics of studies awaiting assessment [ordered by study ID]
Chen 2002.
| Methods | No PDF available |
| Participants | No PDF available |
| Interventions | No PDF available |
| Outcomes | No PDF available |
| Notes |
Guo 2015.
| Methods | RCT |
| Participants | 120 women over 18 years of age with chronic haemorrhagic radiation proctopathy following radiotherapy for cervical carcinoma |
| Interventions | Randomised to receive 4% or 10% formalin |
| Outcomes | Symptom and rectoscopy scores were evaluated before and at 12 weeks after treatment |
| Notes |
Sharma 2013.
| Methods | Purposes were to (1) evaluate efficacy and safety of bipolar heater probe endoscopic coagulation compared to prior medical therapy for bleeding radiation telangiectasia, and (2) consider the impact of treatments on peoples' impression of their overall health and activity. 6 months of medical management had failed in 2 men and 9 women with chronic, recurrent haematochezia and anaemia after radiation treatment of pelvic malignancies. Participants were followed for 6 months |
| Participants | Participants had multiple rectal telangiectasias coagulated with bipolar heater probes CD 120 U with Olympus HPU 20 unit in a randomised, prospective study |
| Interventions | Bipolar heater probe endoscopic coagulation compared to prior medical therapy |
| Outcomes | Rectal bleeding, mean haematocrits, overall health, complications |
| Notes |
Veerasarn 2006.
| Methods | Phase II study |
| Participants | 30 cases with grade 2 to 3 late radiation proctopathy |
| Interventions | All received 0.5 ml/kg of WF10 diluted in 250 ml 5% dextrose iv over 2 hours every 3 weeks for 2 to 4 cycles and combined with standard therapy |
| Outcomes | Healing or significant improvement in SOMA‐LENT scores |
| Notes | Long‐term follow‐up data obtained from participants who had received WF10 |
Xie 1995.
| Methods | No PDF available |
| Participants | No PDF available |
| Interventions | No PDF available |
| Outcomes | No PDF available |
| Notes |
ATA: atmosphere absolute CTCAE: Common Terminology Criteria for Adverse Events HBO: hyperbaric oxygen IBDQ: Inflammatory Bowel Disease Questionnaire iv: intravenously RT: radiotherapy RCT: randomised controlled trial SOMA‐LENT: Subjective, Objective, Management, Analytic ‐ Late Effects of Normal Tissue
Characteristics of ongoing studies [ordered by study ID]
Botten 2011a.
| Trial name or title | Randomized trial of argon plasma coagulation therapy versus topical formalin for persistent rectal bleeding and anorectal dysfunction after radiotherapy for carcinoma of the prostate |
| Methods | The aims of the study were to evaluate and compare the effects of APC and topical formalin on anorectal bleeding and other anorectal symptoms, including urgency of defaecation and faecal incontinence, as well as anorectal function and anal sphincteric morphology, in people with persistent rectal bleeding associated with CRP. Methods: People with persistent rectal bleeding due to CRP were randomised to receive either APC or topical formalin. Anorectal symptoms (including rectal bleeding) were assessed by questionnaire (modified SOMA‐LENT questionnaire); rectal bleeding before and after treatment was also assessed by a VAS (cross‐over to the other treatment was allowed if the study endpoint of a reduction in rectal bleeding to 1x/month or better or to a VAS of 25 mm or less, or both, was not reached after 4 treatment sessions). Anorectal motor and sensory function (anorectal manometry with sleeve sensor and graded balloon distension) and anal sphincteric morphology (endoanal ultrasound) were also evaluated before and after APC and topical formalin treatment. Data were compared before and after treatment and between the treatment groups (paired and unpaired t tests, respectively). |
| Participants | 29 participants (median age 74 (58 to 87) years) with persistent rectal bleeding (defined as more than 1x/week with or without need for blood transfusion) due to CRP |
| Interventions | APC (n = 16) or topical formalin (n = 13) |
| Outcomes | Anorectal symptoms (including rectal bleeding) were assessed by questionnaire (modified SOMA‐LENT questionnaire); rectal bleeding before and after treatment was also assessed by a VAS (cross‐over to the other treatment was allowed if the study endpoint of a reduction in rectal bleeding to 1x/month or better or to a VAS of 25 mm or less, or both, was not reached after 4 treatment sessions). Anorectal motor and sensory function (anorectal manometry with sleeve sensor and graded balloon distension) and anal sphincteric morphology (endoanal ultrasound) were also evaluated before and after APC and topical formalin treatment. Data were compared before and after treatment and between the treatment groups (paired and unpaired t tests, respectively) |
| Starting date | |
| Contact information | |
| Notes |
Engen 2009.
| Trial name or title | Vitamin E and C decrease tissue oxidation and nitration in people with chronic radiation proctopathy |
| Methods | During a randomised double‐blind controlled trial of antioxidants in the treatment of RP, we obtained rectal and/or sigmoid biopsies with limited sigmoidoscopy, before and after 8 weeks of vitamin E (400 IU tid) and vitamin C (500 mg tid) from some of the participants treated for symptomatic RP. The biopsies were snap frozen in liquid nitrogen in the endoscopy suite as soon as procurement. They were analysed for tissue nitration and oxidation by measurement of protein nitrotyrosine and carbonyl levels, respectively, using a quantitative slot immunoblot. Monoclonal rabbit anti‐nitrotyrosine (1:10000) and rabbit anti‐carbonyl antibodies (1:2000) were used and relative levels of oxidized proteins were quantified by measuring the optical density of the bands corresponding to anti‐nitrotyrosine and anti‐carbonyl immunoreactivity with a laser densitometer |
| Participants | Participants treated for symptomatic RP |
| Interventions | Vitamins E and C |
| Outcomes | Levels of oxidation and nitration |
| Starting date | |
| Contact information | |
| Notes |
Oliner 2012.
| Trial name or title | Efficacy of radiofrequency coagulation in managing chronic radiation proctopathy |
| Methods | A prospective randomised study comparing standard APC and RFE in people with symptomatic CRP and their effect on common outcomes was performed. Participants enrolled in the study had pelvic malignancy treated with radiotherapy and subsequent history of chronic radiation proctopathy (defined as development of proctopathy at least 90 days from end of radiation treatment). Proctopathy was scored based on RTOG and Vienna endoscopic scoring system. Primary study endpoint was reduction or absence of bleeding episodes requiring no further endoscopic treatment after 6 months. Secondary endpoints included time to resolution of symptoms and need for further blood transfusions. Independent Student's t‐test was utilised to compare mean primary and secondary endpoints. P value of 0.05 was considered statistically significant |
| Participants | Participants enrolled in the study had pelvic malignancy treated with radiotherapy and subsequent history of chronic radiation proctopathy (defined as development of proctopathy at least 90 days from end of radiation treatment) |
| Interventions | RFE in treating CRP compared to that of the traditional coagulation modality of APC |
| Outcomes | Primary study endpoint was reduction or absence of bleeding episodes requiring no further endoscopic treatment after 6 months. Secondary endpoints included time to resolution of symptoms and need for further blood transfusions. Independent Student's t‐test was utilised to compare mean primary and secondary endpoints |
| Starting date | |
| Contact information | |
| Notes |
Tam 2001.
| Trial name or title | Prospective randomized treatment trial of argon plasma coagulation and topical formalin for radiation proctopathy [abstract] |
| Methods | 15 treatment‐naive people (13M, median age 74 (38 to 83) years) with proven radiation proctopathy and significant daily bleeding were randomised to receive day‐case APC or 4% topical formalin. A VAS was used to assess rectal bleeding (0 to 10; 0 = no bleeding, 10 = severe bleeding) and well‐being (0 to 10; 0 = very unwell, 10 = very well). Anorectal function (urgency, incontinence) was assessed with the modified Wexner/Cleveland Clinic Continence Score (0 to 24; 0 = normal anorectal function, 24 = complete incontinence with severe urgency). Haemoglobin and transfusion requirements were recorded. Treatment was given at 6‐weekly intervals until rectal bleeding had improved to VAS ≤ 2.5 |
| Participants | 15 treatment‐naive people (13M, median age 74 (38 to 83) years) with proven radiation proctopathy and significant daily bleeding |
| Interventions | Day‐case APC or 4% topical formalin |
| Outcomes | Median follow‐up was 51 weeks (19 to 114). The effect of APC and topical formalin was similar, with significant improvement in rectal bleeding after a median of 2 (1 to 3) treatment sessions. Haemoglobin increased in participants treated with APC. 4 participants were transfusion‐dependent before treatment, all of whom did not require transfusions after treatment (3 APC, 1 formalin). Participant well‐being and continence score did not change significantly after treatment. 2 participants treated with APC developed minor rectal strictures, which were readily treated with dilatation |
| Starting date | |
| Contact information | |
| Notes |
APC: argon plasma coagulation CRP: chronic radiation proctopathy RFE: radiofrequency energy RT: radiotherapy RTOG: Radiation Therapy Oncology Group SOMA‐LENT: Subjective, Objective, Management, Analytic ‐ Late Effects of Normal Tissue tid: 3 times a day VAS: visual analogue scale
Differences between protocol and review
We changed the title to make it more explicit. We discussed interventions according to symptoms or combinations of symptoms (treatments targeted at bleeding only (group 1), treatments targeted at a combination of anorectal symptoms, but not a single treatment (group 2), or treatment of the collection of symptoms referred to as pelvic radiation disease (group 3)) rather than according to intervention. We added safety considerations to Objectives. Unlike in the previous version, we included only (quasi) randomised trials. Due to developments in Cochrane methodology since the review was completed and last updated, the risk of bias was assessed using the new Cochrane 'Risk of bias' tool, and the GRADE approach was used to assess of the overall quality of evidence for each outcome (GRADEpro 2014).
Contributions of authors
FW, RS, LV, and JV assessed possible studies for inclusion. FW requested full papers, and FW, RS, LV, and JV extracted data and assessed risk of bias. FW and RS prepared the manuscript, BP wrote the Background section, and LV, JA, JM, JV, BP, and GV commented on drafts and edited the final manuscript.
Sources of support
Internal sources
None, Other.
External sources
No sources of support supplied
Declarations of interest
Fleur T van de Wetering: nothing to declare Leen Verleye: nothing to declare H. Jervoise N Andreyev: first author of one of the included studies Jane Maher: nothing to declare Joan Vlayen: nothing to declare Bradley R Pieters: nothing to declare Geertjan van Tienhoven: nothing to declare Rob JPM Scholten: nothing to declare
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Andreyev 2013 {published data only}
- Andreyev HJN, Benton BE, Lalji A, Norton C, Mohammed K, Gage H, et al. Algorithm‐based management of patients with gastrointestinal symptoms in patients after pelvic radiation treatment (ORBIT): a randomised controlled trial. The Lancet 2013;382:2084–92. [DOI] [PubMed] [Google Scholar]
Cavcic 2000 {published data only}
- Cavcic J, Turcic J, Martinac P, Jelincic Z, Zupancic B, Panijan‐Pezerovic R, et al. Metronidazole in the treatment of chronic radiation proctitis: clinical trial. Croatian Medical Journal 2000;41(3):314‐8. [PubMed] [Google Scholar]
Chruscielewska 2012 {published data only}
- Chruscielewska‐Kiliszek MR, Regula J, Polkowski M, Rupinski M, Kraszewska E, Pachlewski J, et al. Sucralfate or placebo following argon plasma coagulation for chronic radiation proctitis: a randomized double blind trial. Colorectal Disease. The Association of Coloproctology of Great Britain and Ireland 2012;15:e48–e55. [DOI] [PubMed] [Google Scholar]
Clarke 2008 {published data only}
- Clarke RE, Catalina Tenorio LM, Hussey JR, Toklu AS, Cone DL, Hinojosa JG, et al. Hyperbaric oxygen treatment of chronic refractory radiation proctitis: a randomised and controlled double‐blind crossover trial with long‐term follow‐up. International Journal of Radiation Oncology, Biology, Physics 2008;72:134–43. [DOI] [PubMed] [Google Scholar]
Ehrenpreis 2005 {published data only}
- Ehrenpreis ED, Jani A, Levitsky J, Ahn J, Hong J. A prospective randomised double‐blind, placebo‐controlled trial of vitamin A for symptomatic chronic radiation proctopathy. Diseases of the Colon & Rectum 2005;48(1):1‐8. [DOI] [PubMed] [Google Scholar]
Jensen 1997 {published data only}
- Jensen DM, Machicado GA, Cheng S, Jensen ME, Jutahaba R. A randomised prospective study of endoscopic bipolar electrocoagulation and heater probe treatment of chronic rectal bleeding from radiation telangiectasia. Gastrointestinal Endoscopy 1997;45(1):20‐5. [DOI] [PubMed] [Google Scholar]
Kochhar 1991 {published data only}
- Kochhar R, Patel F, Dhar A, Sharma SC, Ayyagari S, Aggarwal R, et al. Radiation‐induced proctosigmoiditis. Prospective, randomized, double‐blind controlled trial of oral sulfasalazine plus rectal steroids versus rectal sucralfate. Digestive Diseases & Sciences 1991;36(1):103‐7. [DOI] [PubMed] [Google Scholar]
Lenz 2010 {published data only}
- Lenz L, Tafarel J, Correia L, Boniha D, Santos M, Rodrigues R, et al. Comparative study of bipolar eletrocoagulation versus argon plasma coagulation for rectal bleeding due to chronic radiation coloproctopathy. Endoscopy 2011;43(8):697‐701. [DOI] [PubMed] [Google Scholar]
Nelamangala 2012 {published data only}
- Nelamangala Ramakrishnaiah VP, Javali TD, Dharanipragada K, Reddy KS, Krishnamachari S. Formalin dab, the effective way of treating haemorrhagic radiation proctitis: A randomized trial from a tertiary care hospital in South India. Colorectal Disease 2012;14:876‐82. [DOI] [PubMed] [Google Scholar]
Pinto 1999 {published data only}
- Pinto A, Fidalgo P, Cravo M, Midões J, Chaves P, Rosa J, et al. Short chain fatty acids are effective in short term treatment of chronic radiation proctitis. Diseases of the Colon & Rectum 1999;42:788‐96. [DOI] [PubMed] [Google Scholar]
Rougier 1992 {published data only}
- Rougier P, Zimmerman P, Pignon JP, Kac J, Crespon B, Zrihen E, et al. Radiation proctitis: comparing the effect of two rectal steroid preparations [Rectites radiques: efficate comparee de deux types de corticoides adminstre localement]. Medecine & Chirurgie Digestives 1992;21:91‐3. [Google Scholar]
Sahakitrungruang 2012 {published data only}
- Sahakitrungruang C, Patiwongpaisarn A, Kanjanasilp P, Malakorn S, Atittharnsakul P. A randomized controlled trial comparing colonic irrigation and oral antibiotics administration versus 4% formalin application for treatment of hemorrhagic radiation proctitis. Diseases of the Colon & Rectum 2012;55:1053‐8. [DOI] [PubMed] [Google Scholar]
Talley 1997 {published data only}
- Talley NA, Chen F, King D, Jones M, Talley NJ. Short‐chain fatty acids in the treatment of radiation proctitis: a randomized, double‐blind, placebo‐controlled, cross‐over pilot trial. Diseases of the Colon & Rectum 1997;40(9):1046‐50. [DOI] [PubMed] [Google Scholar]
Tian 2008 {published data only}
- Tian YP, Wang QC. Rectal radiation injuries treated by Shen Ling Bai Zhu powders combined with rectal administration of Western drugs [Chinese]. Chinese Journal of Integrated Traditional & Western Medicine 2008;28:159‐60. [PubMed] [Google Scholar]
Venkitaraman 2008 {published data only}
- Venkitaraman R, Coffey J, Norman AR, James FV, Huddart RA, Horwich, et al. Pentoxifylline to treat radiation proctitis: a small and inconclusive randomised trial. Clinical Oncology 2008;20:288‐92. [DOI] [PubMed] [Google Scholar]
Yeoh 2013 {published data only}
- Yeoh E, Tam W, Schoeman M, Moore J, Thomas M, Botten R, et al. Argon plasma coagulation therapy versus topical formalin for intractable rectal bleeding and anorectal dysfunction after radiation therapy for prostate carcinoma. International Journal of Radiation Oncology * Biology * Physics 2013;87:954‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐Sabbagh 1996 {published data only}
- Al‐Sabbagh R, Sinicrope FA, Sellin JH, Shen Y, Roubein L. Evaluation of short‐chain fatty acid enemas: treatment of radiation proctitis. American Journal of Gastroenterology 1996;91(9):1814‐6. [PubMed] [Google Scholar]
Alvaro‐Villegas 2010 {published data only}
- Alvaro‐Villegas J, Sobrino‐Cossio S, Tenorio‐TeLlez L, Hernandez‐Guerrero A, Alonso‐Larraga JO, Mora Levy JG. Effectiveness, safety and quality of life of argon plasma coagulation vs. hyperbaric oxygen therapy in chronic radiation proctopathy. Gastrointestinal Endoscopy 2010;Conference(var.pagings):AB326. [Google Scholar]
Alvaro‐Villegas 2011 {published data only}
- Alvaro‐Villegas JC, Sobrino‐Cossio S, Tenorio‐Tellez LC, Mora‐Levy JG, Hernandez‐Guerrero A, Alonso‐Larraga JO, et al. Argon plasma coagulation and hyperbaric oxygen therapy in chronic radiation proctopathy, effectiveness and impact on tissue toxicity. Revista Espanola de Enfermedades Digestivas 2011;103:(11):576‐81. [DOI] [PubMed] [Google Scholar]
Barnett 2011 {published data only}
- Barnett GCD. The impact of clinical factors on the development of late radiation toxicity: results from the Medical Research Council RT01 Trial (ISRCTN47772397). Clinical Oncology 2011;23(9):613‐24. [DOI] [PubMed] [Google Scholar]
Baughan 1993 {published data only}
- Baughan CA, Canney PA, Buchanan RB, Pickering RM. A randomised trial to assess the efficacy of 5‐Aminosalicylic acid for the prevention of radiation enteritis. Clinical Oncology 1993;5:19‐24. [DOI] [PubMed] [Google Scholar]
Edsmyr 1976 {published data only}
- Edsmyr F, Huber W, Menander KB. Orgotein efficacy in ameliorating side effects due to radiation therapy. Double‐blind, placebo‐controlled trial in patients with bladder tumors. Current Therapeutic Research, Clinical and Experimental 1976;19:198‐211. [PubMed] [Google Scholar]
Freund 1987 {published data only}
- Freund U, Scholmerich J, Siems H, Kluge F, Schafer HE, Wannenmacher M. Severe side effects with the application of Mesalazine (5‐ aminosalicylic acid) during radiotherapy. Strahlentherapie und Onkologie 1987;163:678‐80. [PubMed] [Google Scholar]
Freund 1987a {published data only}
- Freund U, Scholmerich J, Siems H, Kluge F, Schafer HE, Wannenmacher M. Unwanted side‐effects in using mesalazine (5‐aminosalicylic acid) during radiotherapy. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft, et al 1987;163:678‐80. [PubMed] [Google Scholar]
Fuccio 2010 {published data only}
- Fuccio A. Topical rectal beclometasone dypropionate for the prevention of radiation‐induced proctopathy: A prospective, randomized, double‐blind, placebo controlled, endoscopy‐based study. Radiotherapy and Oncology 2010;Conference(var.pagings):September. [Google Scholar]
Fuccio 2011 {published data only}
- Fuccio L, Guido A, Laterza L, Eusebi LH, Busutti L, Bunkheila F, et al. Preventive treatment with topical rectal beclo‐methasone dipropionate reduces post‐radiation risk of bleeding in patients irradiated for prostate cancer: A randomized controlled trial. Digestive and Liver Disease 2011;Conference(var.pagings):March. [DOI] [PubMed] [Google Scholar]
Fuccio 2011a {published data only}
- Fuccio L, Guido A, Laterza L, Eusebi LH, Busutti L, Bunkheila F, et al. Randomised clinical trial: preventive treatment with topical rectal beclomethasone dipropionate reduces post‐radiation risk of bleeding in patients irradiated for prostate cancer. Alimentary Pharmacology & Therapeutics 2011;34(6):628‐37. [DOI] [PubMed] [Google Scholar]
Gheorghe 2003 {published data only}
- Gheorghe C, Gheorghe L, Iacob R, Iacob S, Simionov I, Bancilla I. Argon plasma coagulation for radiation proctitis. Romanian Journal of Gastroenterology 2003;12:107‐12. [PubMed] [Google Scholar]
Gonzalez 2009 {published data only}
- Gonzalez N, Hernandez‐Bernal F. Epidermal growth factor in the treatment of radiogenic proctitis. Biotecnologia Aplicada 2009;26(1):49‐52. [Google Scholar]
Henriksson 1992 {published data only}
- Henriksson R, Franzen L, Littbrand B. Prevention and therapy of radiation‐induced bowel discomfort. Scandinavian Journal of Gastroenterology. Supplement 1992;191:7‐11. [DOI] [PubMed] [Google Scholar]
Khan 2000 {published data only}
- Khan AM, Birk JW, Anderson JC, Georgsson M, Park TL, Smith CJ, et al. A prospective randomized placebo‐controlled double‐blinded pilot study of misoprostol rectal suppositories in the prevention of acute and chronic radiation proctitis symptoms in prostate cancer patients. The American Journal of Gastroenterology 2000;95:1961‐6. [DOI] [PubMed] [Google Scholar]
Kneebone 2004 {published data only}
- Kneebone A, Mameghan H, Bolin T, Berry M, Turner S, Kearsley J, et al. Effect of oral sucralfate on late rectal injury associated with radiotherapy for prostate cancer: A double‐blind, randomized trial. International Journal of Radiation Oncology * Biology * Physics 2004;60:1088‐97. [DOI] [PubMed] [Google Scholar]
Kneebone 2005 {published data only}
- Kneebone A, Mameghan H, Bolin T, Berry M, Turner S, Kearsley J, et al. Effect of oral sucralfate on late rectal injury associated with radiotherapy for prostate cancer: A double‐blind, randomized trial. International Journal of Radiation Oncology, Biology, Physics 2004;60(4):1088‐97. [DOI] [PubMed] [Google Scholar]
Lodge 1995 {published data only}
- Lodge N, Evans MK, Wilkins M, Blake PR, Fryatt I. A randomised cross‐over study of the efficacy of codeine phosphate versus Isphagula husk in patients with gynaecological cancer experiencing diarrhoea during radiotherapy. European Journal of Cancer Care 1995;4:8‐10. [DOI] [PubMed] [Google Scholar]
Menander‐Huber 1978 {published data only}
- Menander‐Huber KB, Edsmyr F, Huber W. Orgotein (superoxide dismutase): a drug for the amelioration of radiation‐induced side effects. A double‐blind, placebo‐controlled study in patients with bladder tumours. Urological Research 1978;6:255‐7. [DOI] [PubMed] [Google Scholar]
O'Brien 1997 {published data only}
- O'Brien PC, Franklin CI, Dear KBG, Hamilton CC, Poulsen M, Joseph DJ, et al. A phase III double‐blind randomised study of rectal sucralfate suspension in the prevention of acute radiation procititis. Radiotherapy & Oncology 1997;45:117‐23. [DOI] [PubMed] [Google Scholar]
Pilepich 2006 {published data only}
- Pilepich MV, Paulus R, Clair W, Brasacchio RA, Rostock R, Miller RC. Phase III study of pentosanpolysulfate (PPS) in treatment of gastrointestinal tract sequelae of radiotherapy. American Journal of Clinical Oncology 2006;29:132‐7. [DOI] [PubMed] [Google Scholar]
Pironi 2013 {published data only}
- Pironi D, Panarese A, Vendettuoli M, Pontone S, Candioli S, Manigrasso A, et al. Chronic radiation‐induced proctitis: the 4 % formalin application as non‐surgical treatment. International Journal of Colorectal Disease 2013;28(2):261‐6. [DOI] [PubMed] [Google Scholar]
Samalavicius 2013 {published data only}
- Samalavicius NE, Dulskas A, Kilius A, Petrulis K, Norkus D, Burneckis A, et al. Treatment of hemorrhagic radiation‐induced proctopathy with a 4% formalin application under perianal anesthetic infiltration. World Journal of Gastroenterology 2013;19(30):4944‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sherman 1971 {published data only}
- Sherman LF, Prem KA, Mensheha NM. Factitial proctitis: a restudy at the University of Minnesota. Diseases of the Colon & Rectum 1971;14:281‐5. [DOI] [PubMed] [Google Scholar]
Sidik 2007 {published data only}
- Sidik S, Hardjodisastro D, Setiabudy R, Gondowiardjo S. Does hyperbaric oxygen administration decrease side effect and improve quality of life after pelvic radiation?. Acta Medica Indonesiana 2007;39:169‐73. [PubMed] [Google Scholar]
Stojcev 2013 {published data only}
- Stojcev Z, Krokowicz L, Krokowicz P, Szczepkowski M, Borycka‐Kiciak K, Kiciak A, et al. Early treatment and prevention of the radiation proctitis ‐ Composite enemas containing sodium butyrate. International Journal of Colorectal Disease 2013;28:1731‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Triantifillidis 1990 {published data only}
- Triantafillidis JK, Dadioti P, Nicolakis D, Mericas E. High doses of 5‐aminosalicylic acid enemas in chronic radiation proctitis: comparison with betamethasone enemas [letter]. American Journal of Gastroenterology 1990;85(11):1537‐8. [PubMed] [Google Scholar]
Tsibouris 1999 {published data only}
- Tsibouris P. A randomized prospective study of endoscopic bipolar electrocoagulation and heater probe treatment of chronic rectal bleeding from radiation telangiectasia. Hellenic Journal of Gastroenterology 1999;12:69‐71. [DOI] [PubMed] [Google Scholar]
Vernia 2000 {published data only}
- Vernia P, Fracasso PL, Casale V, Villotti G, Marcheggiano A, Stigliano V, et al. Topical butyrate for acute radiation proctitis: randomised cross‐over trial. The Lancet 2000;356(9237):1232‐5. [DOI] [PubMed] [Google Scholar]
Wen 2012 {published data only}
- Wen Q, Jiang X‐M. Protective effect of compound sophorae injection on radiation proctitis. Chinese Journal of New Drugs 2012;21:2063‐4. [Google Scholar]
Yeoh 2013a {published data only}
- Yeoh E, Tam W, Schoeman M, Moore J, Thomas M, Botten R, et al. Argon plasma coagulation therapy versus topical formalin for intractable rectal bleeding and anorectal dysfunction after radiation therapy for prostate carcinoma. International Journal of Radiation Oncology, Biology, Physics 2013;87(5):954‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Chen 2002 {published data only}
- Chen J, Zhang H, Yan ZP, Wang XQ, Jin ZX. Treatment of radiation proctitis hemorrhage. China Journal of Cancer Prevention and Treatment. 2002;9:463‐4. [Google Scholar]
Guo 2015 {published data only}
- Guo GH, Yu FY, Wanf XJ, Lu F. A randomized controlled clinical trial of formalin for treatment of chronic hemorrhagic radiation proctopathy in cervical carcinoma patients. Support Care Cancer 2015;23:441–6. [DOI] [PubMed] [Google Scholar]
Sharma 2013 {published data only}
- Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double‐blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy.. American Journal of Gastroenterology 2013;108(9):Epub 2013 Jul 23. [DOI] [PubMed] [Google Scholar]
Veerasarn 2006 {published data only}
- Veerasarn V, Boonnuch W, Kakanaporn C. A phase II study to evaluate WF10 in patients with late radiation haemorrhagic radiation cystitis and proctitis. Gynecologic Oncology 2006;100:179‐84. [DOI] [PubMed] [Google Scholar]
Xie 1995 {published data only}
- Xie XF, Wu YA, Liu Y, Che H. Effect analysis of Huoxue Huayu Shengji Jianji and keeping enema therapy for radiation proctitis. Chinese Journal of Integrated Traditional and Western Medicine 1995;15:565‐6. [Google Scholar]
References to ongoing studies
Botten 2011a {published data only}
- Botten RJD. Randomised trial of argon plasma coagulation (APC) therapy verses topical formalin for persistent rectal bleeding and anorectal dysfunction after radiation therapy (RT) for carcinoma of the prostate (CAP). Gastroenterology. 2011; Vol. 140 (5):S796.
Engen 2009 {published data only}
- Engen P, Kwasny M, Millner T, Mutlu E. Vitamin E and C decrease tissue oxidation and nitration in patients with chronic radiation proctitis. AGA Abstracts. Gastroenterology. May 2009:A370.
Oliner 2012 {published data only}
- Oliner C, Pinkhasov M, Lawlor G. Efficacy of radiofrequency coagulation in managing chronic radiation proctitis. American Journal of Gastroenterology. Proceedings of the 77th Annual Scientific Meeting of the American College of Gastroenterology; Oct 19‐24; Las Vegas. Las Vegas, 2012:S735‐6.
Tam 2001 {published data only}
- Tam W, Moore J, Schoeman M, Conroy Hiller T. Prospective randomised treatment trial of argon plasma coagulation and topical formalin for radiation proctitis [abstract]. ANZ Journal of Surgery 2001;71:A24. [Google Scholar]
Additional references
Ahnfelt‐Ronne 1990
- Ahnfelt‐Ronne I, Nielsen OH, Christensen A, Langholz E, Binder V, Riis P. Clinical evidence supporting the radical scavenger mechanism of 5‐aminosalicylic acid. Gastroenterology 1990;98(5 Pt 1):1162‐9. [PUBMED: 1969825] [DOI] [PubMed] [Google Scholar]
Al‐Mamgani 2008
- Al‐Mamgani A, Putten WLJ, Heemsbergen WD, Leenders GJLH, Slot A, Dielwart MFH, et al. Update of Dutch multicenter dose‐escalation trial of radiotherapy for localized prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2008;72:980‐8. [DOI] [PubMed] [Google Scholar]
Andreyev 2010
- Andreyev HJN, Wotherspoon A, Denham JW, Hauer‐Jensen M. Defining pelvic‐radiation disease for the survivorship era. The Lancet Oncology 2010;11:310‐2. [DOI] [PubMed] [Google Scholar]
Bantel 2000
Bekelman 2011
- Bekelman JE, Mitra N, Efstathiou J, Liao K, Sunderland R, Yeboa DN, et al. Outcomes after intensity‐modulated versus conformal radiotherapy in older men with nonmestastatic prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2011;81:325‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boersma 1998
- Boersma LJ, Brink M, Bruce AM, Shouman T, Gras L, Velde A, et al. Estimation of the incidence of late bladder and rectum complications after high‐dose (70‐78 Gy) conformal radiotherapy for prostate cancer, using dose‐volume histograms. International Journal of Radiation Oncology, Biology, Physics 1998;41:83‐92. [DOI] [PubMed] [Google Scholar]
Carling 1986
Cheung 2000
- Cheung W, Garratt A, Russell I, Williams J. The UK IBDQ ‐ a British version of the Inflammatory Bowel Disease questionnaire: development and validation. Journal of Clinical Epidemiology 2000;53(3):297‐306. [DOI] [PubMed] [Google Scholar]
Cook 1998
- Cook SI, Sellin JH. Review article: short chain fatty acids in health and disease. Alimentary Pharmacology & Therapeutics 1998;12(6):499‐507. [PUBMED: 9678808] [DOI] [PubMed] [Google Scholar]
De Meerleer 2007
- Meerleer GO, Fonteyne VH, Villeirs GM, Denoyette L, Verbaeys A, Lummen N, et al. Intensity‐modulated radiation therapy for prostate cancer: late morbidity and results on biochemical control. Radiotherapy and Oncology 2007;82:160‐6. [DOI] [PubMed] [Google Scholar]
Denham 2002
- Denham JW, Hauer‐Jensen M. The radiotherapeutic injury ‐ a complex 'wound'. Radiotherapy and Oncology 2002;63:129‐45. [DOI] [PubMed] [Google Scholar]
Do 2011
- Do NL, Nagle D, Poylin VY. Radiation proctitis: current strategies in management. Gastroenterology Research and Practice 2011;2011:9. [917941] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- McMaster University. GRADEpro. [www.gradepro.org]. McMaster University, 2014.
Haas 2007
- Haas EM, Bailey HR, Farragher I. Application of 10 percent formalin for the treatment of radiation‐induced hemorrhagic proctitis. Diseases of the Colon & Rectum 2007;50(2):213‐7. [PUBMED: 17080283] [DOI] [PubMed] [Google Scholar]
Hanson 2012
- Hanson B, MacDonald R, Shaukat A. Endoscopic and medical therapy for chronic radiation proctopathy: a systematic review. Diseases of the Colon & Rectum 2012;55:1081‐95. [DOI] [PubMed] [Google Scholar]
Hawkey 1985
- Hawkey CJ, Boughton‐Smith NK, Whittle BJ. Modulation of human colonic arachidonic acid metabolism by sulfasalazine. Digestive Diseases and Sciences 1985;30(12):1161‐5. [PUBMED: 2866075] [DOI] [PubMed] [Google Scholar]
Heemsbergen 2005
- Heemsbergen WD, Hoogeman MS, Hart GA, Lebesque JV, Koper PC. Gastrointestinal toxicity and its relation to dose distributions in the anorectal region of the prostate cancer patients treated with radiotherapy. International Journal of Radiation Oncology, Biology, Physics 2005;61:1011‐8. [DOI] [PubMed] [Google Scholar]
Hein 1984
- Hein R, Mensing H, Muller PK, Braun‐Falco O, Krieg T. Effect of vitamin A and its derivatives on collagen production and chemotactic response of fibroblasts. The British Journal of Dermatology 1984;111(1):37‐44. [PUBMED: 6234916] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Hille 2008
- Hille A, Herrmann MK, Kertesz T, Christiansen H, Hermann RM, Pradier O, et al. Sodium butyrate enemas in the treatment of acute radiation‐induced proctitis in patients with prostate cancer and the impact on late proctitis. A prospective evaluation. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft ... [et al] 2008;184(12):686‐92. [PUBMED: 19107351] [DOI] [PubMed] [Google Scholar]
Khalid 2006
- Khalid U, McGough C, Hackett C, Blake P, Harrington KJ, Khoo VS, et al. A modified inflammatory bowel disease questionnaire and the Vaizey Incontinence questionnaire are more sensitive measures of acute gastrointestinal toxicity during pelvic radiotherapy than RTOG grading. International Journal of Radiation Oncology, Biology, Physics 2006;64(5):1432‐41. [DOI] [PubMed] [Google Scholar]
Kochhar 1988
Kushwaha 2003
- Kushwaha RS, Hayne D, Valzey CJ, Wrightham E, Payne H, Boulos PB. Physiologic changes of the anorectum after pelvic radiotherapy for the treatment of prostate and bladder cancer. Diseases of the Colon & Rectum 2003;46:1182‐8. [DOI] [PubMed] [Google Scholar]
Kvietys 1981
- Kvietys PR, Granger DN. Effect of volatile fatty acids on blood flow and oxygen uptake by the dog colon. Gastroenterology 1981;80(5 pt 1):962‐9. [PUBMED: 7202979] [PubMed] [Google Scholar]
Leiper 2007
- Leiper K, Morris AI. Treatment of radiation proctitis. Clinical Oncology (Royal College of Radiologists (Great Britain)) 2007;19(9):724‐9. [PUBMED: 17728120] [DOI] [PubMed] [Google Scholar]
MacDermott 1989a
- MacDermott RP, Schloemann SR, Bertovich MJ, Nash GS, Peters M, Stenson WF. Inhibition of antibody secretion by 5‐aminosalicylic acid. Gastroenterology 1989;96(2 Pt 1):442‐8. [PUBMED: 2562949] [DOI] [PubMed] [Google Scholar]
Marx 1990
- Marx RE, Ehler WJ, Tayapongsak P, Pierce LW. Relationship of oxygen dose to angiogenesis induction in irradiated tissue. American Journal of Surgery 1990;160(5):519‐24. [PUBMED: 2240387] [DOI] [PubMed] [Google Scholar]
McElvanna 2014
- McElvanna K, Wilson A, Irwin T. Sucralfate paste enema: a new method of topical treatment for haemorrhagic radiation proctitis. Colorectal Disease: the official journal of the Association of Coloproctology of Great Britain and Ireland 2014;16(4):281‐4. [PUBMED: 24299100] [DOI] [PubMed] [Google Scholar]
Mortensen 1990
- Mortensen FV, Nielsen H, Mulvany MJ, Hessov I. Short chain fatty acids dilate isolated human colonic resistance arteries. Gut 1990;31(12):1391‐4. [PUBMED: 2265780] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neal 1987
- Neal TM, Winterbourn CC, Vissers MC. Inhibition of neutrophil degranulation and superoxide production by sulfasalazine. Comparison with 5‐aminosalicylic acid, sulfapyridine and olsalazine. Biochemical Pharmacology 1987;36(17):2765‐8. [PUBMED: 2888463] [DOI] [PubMed] [Google Scholar]
Olopade 2005
- Olopade FA, Norman A, Blake P, Dearnaley DP, Harrington KJ, Khoo V, et al. A modified Inflammatory Bowel Disease questionnaire and the Vaizey Incontinence questionnaire are simple ways to identify patients with significant gastrointestinal symptoms after pelvic radiotherapy. British Journal of Cancer 2005;92:1663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parikh 2003
- Parikh S, Hughes C, Salvati EP, Eisenstat T, Oliver G, Chinn B, et al. Treatment of hemorrhagic radiation proctitis with 4 percent formalin. Diseases of the Colon & Rectum 2003;46(5):596‐600. [PUBMED: 12792434] [DOI] [PubMed] [Google Scholar]
Pavy 1995
- Pavy JJ, Denekamp J, Letschert J, Littbrand B, Mornex F, Bernier J, et al. EORTC Late Effects Working Group. Late Effects toxicity scoring: the SOMA scale. International Journal of Radiation Oncology, Biology, Physics 1995;31(5):1043‐7. [DOI] [PubMed] [Google Scholar]
Peeters 2006
- Peeters ST, Lebesque JV, Heemsbergen WD, Putten WL, Slot A, Dielwart MF, et al. Localized volume effects for late rectal and anal toxicity after radiotherapy for prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2006;64:1151‐61. [DOI] [PubMed] [Google Scholar]
Peeters 2006b
- Peeters STH, Heemsbergen WD, Koper PCM, Putten WLJ, Slot A, Dielwart MFH, et al. Dose‐response in radiotherapy for localized prostate cancer: Results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. Journal of Clinical Oncology 2006;24:1990‐6. [DOI] [PubMed] [Google Scholar]
Pollack 2002
- Pollack A, Zagras GK, Starkschall G, Antolak JA, Lee JJ, Huang E, et al. Prostate cancer radiation dose response: Results of the M.D. Anderson phase III randomized trial. International Journal of Radiation Oncology, Biology, Physics 2002;53:1097‐105. [DOI] [PubMed] [Google Scholar]
Protell 1978
- Protell RL, Rubin CE, Auth DC, Silverstein FE, Terou F, Dennis M, et al. The heater probe: a new endoscopic method for stopping massive gastrointestinal bleeding. Gastroenterology 1978;74(2 Pt 1):257‐62. [PUBMED: 620899] [PubMed] [Google Scholar]
Putta 2005
- Putta S, Andreyev HJ. Faecal incontinence: A late side‐effect of pelvic radiotherapy. Clinical Oncology (Royal College of Radiologists (Great Britain)) 2005;6:469‐77. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roth 1994
- Roth RN, Weiss LD. Hyperbaric oxygen and wound healing. Clinics in Dermatology 1994;12(1):141‐56. [PUBMED: 8180937] [DOI] [PubMed] [Google Scholar]
Rousseaux 2005
- Rousseaux C, Lefebvre B, Dubuquoy L, Lefebvre P, Romano O, Auwerx J, et al. Intestinal antiinflammatory effect of 5‐aminosalicylic acid is dependent on peroxisome proliferator‐activated receptor‐gamma. The Journal of Experimental Medicine 2005;201(8):1205‐15. [PUBMED: 15824083] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 1976
- Scott J, Huskisson EC. Graphic representation of pain. Pain 1976;2:175–84. [PubMed] [Google Scholar]
Smeenk 2012
- Smeenk RJ, Hoffmann AL, Hopman WP, Lin EN Kaanders JH. Dose‐effect relationships for individual pelvic floor muscles and anorectal complaints after prostate radiotherapy. International Journal of Radiation Oncology, Biology, Physics 2012;83:636‐44. [DOI] [PubMed] [Google Scholar]
Smeenk 2012b
- Smeenk RJ, Hopman WP, Hoffmann AL, Lin EN, Kaanders JH. Differences in radiation dosimetry and anorectal function testing imply that anorectal symptoms may arise from different anatomic substrates. International Journal of Radiation Oncology, Biology, Physics 2012;82:145‐52. [DOI] [PubMed] [Google Scholar]
Stevens 1995
- Stevens C, Lipman M, Fabry S, Moscovitch‐Lopatin M, Almawi W, Keresztes S, et al. 5‐Aminosalicylic acid abrogates T‐cell proliferation by blocking interleukin‐2 production in peripheral blood mononuclear cells. The Journal of Pharmacology and Experimental Therapeutics 1995;272(1):399‐406. [PUBMED: 7815356] [PubMed] [Google Scholar]
Ware 1996
- Ware J Jr, Kosinski M, Keller S D. A 12‐Item short‐form health survey: Construction of scales and preliminary tests of reliability and validity. Medical Care 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
Wattel 1998
- Wattel F, Mathieu D, Neviere R, Bocquillon N. Acute peripheral ischaemia and compartment syndromes: a role for hyperbaric oxygenation. Anaesthesia 1998;53 Suppl 2:63‐5. [PUBMED: 9659073] [DOI] [PubMed] [Google Scholar]
Wilson 2006
- Wilson SA, Rex DK. Endoscopic treatment of chronic radiation proctopathy. Current Opinion in Gastroenterology 2006;22:536‐40. [DOI] [PubMed] [Google Scholar]
Yeoh 2009
- Yeoh EK, Holloway RH, Fraser RJ, Botten R, Matteo A, Moore JW, et al. Anorectal function after three‐ versus two‐dimensional radiation therapy for carcinoma of the prostate. International Journal of Radiation Oncology, Biology, Physics 2009;73:46‐52. [DOI] [PubMed] [Google Scholar]
Zahavi 1989
- Zahavi I, Avidor I, Marcus H, Rosenbach Y, Waisman Y, Ligumsky M, et al. Effect of sucralfate on experimental colitis in the rat. Diseases of the Colon & Rectum 1989;32(2):95‐8. [PUBMED: 2914534] [DOI] [PubMed] [Google Scholar]
Zelefsky 2008
- Zelefsky MJ, Levin EJ, Hunt M, Yamada Y, Shippy AM, Jackson A, et al. Incidence of late rectal and urinary toxicities after three‐dimensional conformal radiotherapy and intensity‐modulated radiotherapy for localized prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2008;70:1124‐9. [DOI] [PubMed] [Google Scholar]
Zietman 2005
- Zietman AL, DeSilvio ML, Slater JD, Rossi CJ, Miller DW, Adams JA, et al. Comparison of conventional‐dose vs high‐dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate. JAMA 2005;294:1233‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Denton 2002
- Denton AS, Andreyev JJ, Forbes A, Maher J. Non surgical interventions for late radiation proctitis in patients who have received radical radiotherapy to the pelvis. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD003455] [DOI] [PubMed] [Google Scholar]


