Abstract
Background
Gastrectomy remains the primary therapeutic method for resectable gastric cancer. Thought of as an important measure to reduce post‐operative complications and mortality, abdominal drainage has been used widely after gastrectomy for gastric cancer in previous decades. The benefits of abdominal drainage have been questioned by researchers in recent years.
Objectives
The objectives of this review were to assess the benefits and harms of routine abdominal drainage post‐gastrectomy for gastric cancer.
Search methods
We searched the Cochrane Upper Gastrointestinal and Pancreatic Diseases (UGPD) Group Specialised Register and the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2014, Issue 11); MEDLINE (via PubMed) (1950 to November 2014); EMBASE (1980 to November 2014); and the Chinese National Knowledge Infrastructure (CNKI) Database (1979 to November 2014).
Selection criteria
We included randomised controlled trials (RCTs) comparing an abdominal drain versus no drain in patients who had undergone gastrectomy (not considering the scale of gastrectomy and the extent of lymphadenectomy); irrespective of language, publication status, and the type of drain. We excluded RCTs comparing one drain with another.
Data collection and analysis
We adhered to the standard methodological procedures of The Cochrane Collaboration. From each included trial, we extracted the data on the methodological quality and characteristics of the participants, mortality (30‐day mortality), re‐operations, post‐operative complications (pneumonia, wound infection, intra‐abdominal abscess, anastomotic leak, drain‐related complications), operation time, length of post‐operative hospital stay, and initiation of a soft diet. For dichotomous data, we calculated the risk ratio (RR) and 95% confidence interval (CI). For continuous data, we calculated mean difference (MD) and 95% CI. We tested heterogeneity using the Chi2 test. We used a fixed‐effect model for data analysis with RevMan software, but we used a random‐effects model if the P value of the Chi2 test was less than 0.1.
Main results
We included four RCTs involving 438 patients (220 patients in the drain group and 218 in the no‐drain group). There was no evidence of a difference between the two groups in mortality (RR 1.73, 95% CI 0.38 to 7.84); re‐operations (RR 2.49, 95% CI 0.71 to 8.74); post‐operative complications (pneumonia: RR 1.18, 95% CI 0.55 to 2.54; wound infection: RR 1.23, 95% CI 0.47 to 3.23; intra‐abdominal abscess: RR 1.27, 95% CI 0.29 to 5.51; anastomotic leak: RR 0.93, 95% CI 0.06 to 14.47); or initiation of soft diet (MD 0.15 days, 95% CI ‐0.07 to 0.37). However, the addition of a drain prolonged the operation time (MD 9.07 min, 95% CI 2.56 to 15.57) and post‐operative hospital stay (MD 0.69 day, 95% CI 0.18 to 1.21) and led to drain‐related complications. Additionally, we should note that 30‐day mortality and re‐operations are very rare events and, as a result, very large numbers of patients would be required to make any sensible conclusions about whether the two groups were similar. The overall quality of the evidence according to the GRADE approach was 'very low' for mortality and re‐operations, and 'low' for post‐operative complications, operation time, and post‐operative length of stay.
Authors' conclusions
We found no convincing evidence to support routine drain use after gastrectomy for gastric cancer.
Plain language summary
Inserting a drain after gastrectomy for gastric cancer
Background
Gastrectomy remains the primary therapeutic method for resectable gastric cancer. It is believed that abdominal drains can help in the earlier detection and drainage of anastomotic fistulas and the prevention of intra‐abdominal abscesses. There is no consensus on the routine placement of abdominal drainage after gastrectomy for gastric cancer.
Review question
To assess the benefits and harms of routine abdominal drainage post‐gastrectomy for gastric cancer, we included randomised controlled trials (RCTs) that compared inserting an abdominal drain versus no drain in patients with gastric cancer who had undergone gastrectomy. The main outcomes included deaths (30‐day mortality), re‐operations, post‐operative complications, operation time, length of post‐operative hospital stay, and time of initiation of a soft diet.
Study characteristics
This review included four RCTs involving 438 patients that investigated the benefits and harms of routine abdominal drainage post‐gastrectomy for gastric cancer.
Key results
There was no evidence of a difference between the two groups in deaths, post‐operative complications, and initiation of a soft diet. The results showed that drains increased harms by prolonging operation time and post‐operative hospital stay, and led to drain‐related complications without providing any additional benefit for patients with gastric cancer undergoing gastrectomy. There was no convincing evidence to support the routine use of drains after gastrectomy for gastric cancer.
Quality of the evidence
The overall quality of the evidence according to the GRADE approach was 'very low' for deaths and re‐operations, and 'low' for post‐operative complications, operation time, and post‐operative length of stay. This review included only four RCTs, and not all of the included studies reported all outcomes that we were assessing. Therefore, the quality was mainly limited by insufficient data.
Summary of findings
Summary of findings for the main comparison. Drain versus no drain for gastric cancer.
| Drain versus no drain for gastric cancer | ||||||
| Patient or population: gastric cancer Settings: hospital Intervention: drain versus no drain | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Drain versus no drain | |||||
| 30‐day mortality Death during the follow‐up Follow‐up: mean 30 days | 9 per 1000 | 16 per 1000 (3 to 71) | RR 1.73 (0.38 to 7.84) | 438 (4 studies) | ⊕⊝⊝⊝ very low1,2,3 | |
| Post‐operative complications | See comment | See comment | Not estimable | 0 (4 studies) | ⊕⊕⊝⊝ low3,4 | |
| Re‐operations Re‐operations during the follow‐up Follow‐up: mean 30 days | 26 per 1000 | 65 per 1000 (18 to 227) | RR 2.49 (0.71 to 8.74) | 230 (2 studies) | ⊕⊝⊝⊝ very low3,5,6 | |
| Operation time The time from the start to the end of operation. Scale from: 0 to 100. Follow‐up: mean 30 days7 | The mean operation time ranged across control groups from 132 to 159 minutes8 | The mean operation time in the intervention groups was 9.07 minutes higher (2.56 to 15.57 higher) | 378 (3 studies) | ⊕⊕⊝⊝ low3,9,10,11,12,13 | ||
| Length of post‐operative hospital stay The time from the day of operation to the day of discharge. Scale from: 0 to 100. Follow‐up: mean 30 days | The mean length of post‐operative hospital stay ranged across control groups from 8.39 to 12.9 days | The mean length of post‐operative hospital stay in the intervention groups was 0.69 days higher (0.18 to 1.21 higher) | 438 (4 studies) | ⊕⊕⊝⊝ low1,3,9 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The four included trials did not report the study design and implementation clearly, so most of the items of methodological quality were unclear. 2 30‐day mortality is a zero event for the Kim 2004 and Jiang 2008 trials and as a result less than half the total patients contribute to the meta‐analysis. 3 We only included four trials in this review, and all the four studies concluded that use of a drain had no additional benefits. Funnel plot was not drawn because of the limited numbers of studies. 4 Not all the included studies reported the complication data. And blinding was not clearly stated. 5 The two included trials did not report the study design and implementation clearly, so most of the items of methodological quality were unclear. 6 Only two included studies reported this result. 7 The follow‐up time of the three included trials was 30 days. 8 They were the lowest and the highest estimates of the scores in the no‐drain groups 9 The three included trials did not report the study design and implementation clearly, so most of the items of methodological quality were unclear. 10 Although the operation time was closely associated with the surgeon, the three trials reported that all the surgical procedures were by the same surgeon or the same group of surgeons. 11 All three trials compared drain with no drain directly. 12 The sample size of the three included studies was not very small and the operation was measured based on every included patient. 13 All the three studies were randomised controlled trials.
Background
Description of the condition
Although the incidence of, and mortality rates for, gastric cancer have decreased markedly in most areas of the world in the past few decades (Jemal 2002), the prognosis of gastric cancer remains poor and mortality remains high. After lung cancer, gastric cancer is at present considered the main cause of cancer‐related death (Crew 2006). As a result of trends in global aging and population growth, the potential incidence of gastric cancer for 2010 was estimated to increase to 1.1 million. Developing countries are expected to carry most of the disease burden (Parkin 2005), amounting to about two‐thirds of all cases of gastric cancer (Lochhead 2008). Treatments for gastric cancer include surgery, adjuvant chemotherapy, chemoradiotherapy, palliative chemotherapy, and complete surgical resection of the tumour, which offers the best opportunity for a full recovery (Phan 2004). In recent decades progress has been made in the treatment of gastric cancer, however the survival remains poor outside of Japan (Lochhead 2008) where the five‐year survival rate is greater than 50%, largely owing to the introduction of population‐based endoscopic screening in the 1960s (Parkin 2005).
Description of the intervention
Thought to be an important measure to reduce post‐operative complications and mortality, for a long time prophylactic drainage of the peritoneal cavity was considered efficient and was used widely after various abdominal surgeries, although not much data existed to scientifically support the practice (Robinson 1986; Dougherty 1992). Drainage of the abdominal cavity was first described by Ambroise Pare but as the practice had been in use earlier it has a long historical tradition (Karliczek 2006). Surgical drains can be classified into two categories, open drains and closed drains. An open drain is where surgeons place an artificial conduit in the wound after an operation to drain fluids from the patient’s body (for example a Penrose drain). There are two types of closed drains; passive drains, which are gravity assisted (for example a Robinson drain), and suction drains which work on negative pressure (for example a Redon drain) (Gurusamy 2007).
How the intervention might work
Although surgical techniques have improved, abdominal drainage is still used routinely in most centres (Bona 1994). The aims of prophylactic drainage are to prevent repeated infection (for example by discharging remnant blood and preventing abscess formation), control possible leakage from the surgical seam (by drainage of the digestive closure, for example a colonic anastomosis), and to provide a warning of potential complications (for example by providing evidence of pancreatic leakage or post‐operative bleeding, just like an alarm bell) (Schein 2008). The benefits of routine drainage have been questioned by some researchers (Sager 1993) who argue that drains might be associated with increased rates of intra‐abdominal and wound infection, increased abdominal pain, decreased pulmonary function, and prolonged hospital stay (Liu 2004; Yeh 2005). Furthermore, recent studies have shown that for a variety of routine intra‐abdominal procedures where drains were once routinely placed, such as pancreatic resection, partial hepatectomy, cholecystectomy, splenectomy, colorectal or other gastrointestinal surgery, the surgery can be completed securely without prophylactic drainage (Cerise 1970; Monson 1991; Merad 1999; Conlon 2001). Therefore, routine drainage after abdominal surgeries is an issue of considerable debate.
Why it is important to do this review
Currently, there is no consensus on prophylactic drainage post‐gastrectomy. A 2004 systematic review, which concluded that prophylactic drainage remains indicated after total gastrectomy, was not based on any prospective studies of prophylactic drainage versus no drainage (Petrowsky 2004). Nevertheless, regarding abdominal drainage post‐gastrectomy for gastric cancer there has been some recent progress (Wronski 2007). Advances in the surgical technique, anaesthesia, and peri‐operative patient care have consistently decreased the number of post‐operative complications after gastric cancer surgery, especially in countries such as Korea and Japan where gastrectomy with D2 lymphadenectomy is the standard surgery (Maruyama 1998). From the perspective of evidence‐based medicine, it has become necessary for us to update our old conceptions with respect to abdominal drainage post‐gastrectomy for gastric cancer.
Objectives
The objectives of this review were to assess the benefits and harms of routine abdominal drainage post‐gastrectomy for gastric cancer.
Methods
Criteria for considering studies for this review
Types of studies
We included only parallel group design, randomised controlled trials (RCTs) comparing drain use and no‐drain use post‐gastrectomy for gastric cancer (irrespective of language or publication status).
Types of participants
We included only patients who had undergone gastrectomy for gastric cancer (irrespective of whether it was a total, subtotal, radical, or palliative gastrectomy), not considering the extent of lymph node dissection. We excluded patients who had undergone gastrectomy for any other gastric diseases (other benign or malignant diseases).
Types of interventions
We included only trials comparing abdominal drainage versus no drainage post‐gastrectomy for gastric cancer (irrespective of the type of the drain). We excluded trials comparing the two types of drains (open and closed) and studies without a control group.
Types of outcome measures
We assessed the benefits and harms of routine abdominal drainage post‐gastrectomy for gastric cancer.
Primary outcomes
Mortality (30‐day mortality).
Re‐operations.
-
Post‐operative complications:
pneumonia,
wound infection,
intra‐abdominal abscess,
anastomotic leak,
drain‐related complications.
Secondary outcomes
Operation time
Length of post‐operative hospital stay
Initiation of soft diet
Search methods for identification of studies
We considered all eligible published and unpublished studies, irrespective of language.
Electronic searches
We conducted electronic searches of:
the Cochrane Upper Gastrointestinal and Pancreatic Diseases (UGPD) Group Specialised Registe;
Cochrane Central Register of Controlled Trials (CENTRAL) (Appendix 1) in the The Cochrane Library (2014, Issue 11);
MEDLINE via PubMed (1946 to 26 November 2014) (Appendix 2);
EMBASE (1980 to 26 November 2014) (Appendix 3); and
Chinese National Knowledge Infrastructure (CNKI) Database (1979 to 26 November 2014).
We established the search strategy for the review using a combination of subject headings and text words relating to the use of drains in patients undergoing gastrectomy for gastric cancer. In addition, we modified the search strategies for CENTRAL, MEDLINE and EMBASE to suit the CNKI Database.
Searching other resources
We handsearched the reference lists of eligible trials that were retrieved by electronic searching to identify further relevant trials. In addition, we contacted authors of relevant studies, members of the Cochrane UGPD Group, and experts in the field for additional published or unpublished data. We also handsearched published abstracts from the conference proceedings of the United European Gastroenterology Week (published in Gut) and Digestive Disease Week (published in Gastroenterology).
Data collection and analysis
Selection of studies
Three review authors (Junqiang Chen (J‐Q C), Zhen Wang (ZW), and Ka Su (KS)) selected relevant articles and independently assessed their eligibility based on the inclusion and exclusion criteria. For further assessment, we obtained the full texts for those trials identified from their titles and abstracts as relevant or possibly relevant. We resolved disagreements by discussion.
Data extraction and management
Two authors (J‐Q C and ZW) independently extracted the following information from eligible studies, using a predefined data extraction form, and entered the data into the Review Manager 5 software (RevMan 2012).
Year of publication and conduct of the study.
Country and publication language of the study.
Sample size.
Inclusion and exclusion criteria.
Baseline characteristics of the participants (age, gender, comparability between two groups, etc).
Type of operations and drains used.
Pre‐operative and post‐operative antibiotics.
Primary and secondary outcomes.
Quality of methodology (method of randomisation, allocation concealment, details of blinding, completeness of outcome data, selective reporting, and others).
We resolved any differences in opinion through discussion, and contacted the authors responsible for studies if data were unpublished or where confirmation of results was required.
Assessment of risk of bias in included studies
Three authors (J‐Q C, ZW, and KS) independently assessed the quality of the methodology of the included studies, following the quality checklist supplied in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This contains six items: method of randomisation, adequacy of concealment allocation, blinding, completeness of outcome data, selective reporting of outcomes, and other bias. Taking into account the risk of overestimating intervention effects in randomised trials with inadequate methodological quality (Kjaergard 2001), we assessed the methodological quality of the trials according to the information available in the published articles. If information was not available in the published article, we tried to contact the authors for more information. We reached consensus by discussion.
For all items listed in the sections below, a result of 'Yes' indicated a low risk of bias, 'No' indicated a high risk of bias, and 'Probably yes' indicated an uncertain risk of bias.
Sequence generation
In each trial, we assessed risk of bias with respect to method of randomisation as follows.
For a result of ‘Yes’, the method for allocating interventions to participants must be specified and based on some chance (random) process, such as computer‐generated or random numbers, throwing dice, or coin tossing.
For a result of ‘Probably yes’, the study must be described as randomised but with the method used for randomisation not clearly described.
For a result of ‘No’, the method of sequence generation should be non‐randomised, for example, by using participants’ birth dates, dates of admission, or their inpatient or outpatient numbers.
Adequacy of concealment allocation
In each trial, we assessed risk of bias with respect to how adequately allocation was concealed as follows.
For a result of ‘Yes’, an effective approach should be used that will ensure that participants and investigators cannot predict the assignment, such as using sealed or opaque envelopes, a central independent unit, or an on‐site locked computer.
For a result of ‘Probably yes’, the trial must be described as randomised but with the method used to conceal the allocation not reported.
For a result of ‘No’, this means that the allocation sequence was known to the investigators who recruited the participants.
Blinding
We did not assess double blinding as we considered that it was impractical in our review. However, we recorded whether or not the drain was placed by a second surgeon who was not otherwise involved in the operation.
Completeness of outcome data
In each trial, we assessed risk of bias with respect to completeness of outcome data as follows.
For a result of ‘Yes’, there should be no missing data, unless missing data were balanced in numbers across intervention groups or had been imputed using appropriate methods, or if the reasons for missing outcome data did not affect the true outcome of the trial, etc.
For a result of ‘Probably yes’, there should be insufficient reporting of missing data to permit a judgement of ‘Yes’ or ‘No’.
For a result of ‘No’, it should be clear that the true outcome was affected by the missing data, with either an imbalance in numbers or reasons for missing data across the intervention groups, or with inappropriate application of imputation, etc.
Selective reporting of outcomes
In each trial, we assessed risk of bias with respect to selective reporting of outcomes as follows.
For a result of ‘Yes’, all of the study's pre‐specified outcomes that were of interest to the review should be reported.
For a result of ‘Probably yes’, there should be insufficient information to permit a judgement of ‘Yes’ or ‘No’.
For a result of ‘No’, some of the study's pre‐specified outcomes were missing, some primary outcomes might be reported using methods or subsets of the data that were not pre‐specified, some primary outcomes that were not pre‐specified might be reported, outcomes of interest in the review might be reported incompletely, or the study might fail to report a key outcome.
Other bias
In each trial, we assessed risk of other bias as follows.
For a result of ‘Yes’, the study should appear to be free of other sources of bias such as conflict of interests.
For a result of ‘Probably yes’, there should be insufficient information to assess whether an important risk of bias existed, or insufficient rationale or evidence that an identified issue caused bias.
For a result of ‘No’, there should be at least one important source of risk of bias (potential source of bias related to specific study design used, extreme baseline imbalance, or some other problem etc.).
Measures of treatment effect
To secure accuracy of data, one review author (ZW) entered all data into Review Manager (RevMan 2012) and another review author (J‐Q C) checked them. For continuous data, we presented the results as mean differences (MD) and 95% confidence intervals (CI); for dichotomous data, we calculated risk ratio (RR) and 95% CI.
Unit of analysis issues
Cluster‐randomised and cross‐over trials did not appear in our review. And there was no study with multiple treatment arms.
Dealing with missing data
If data were missing or incomplete, we contacted the study authors to help find relevant information for the review. We used the data available from the articles if there was no response from the authors.
Assessment of heterogeneity
We explored the clinical and methodological heterogeneity of included studies from the aspects of baseline characteristics of participants, interventions, and study designs, and we found clinical and methodological heterogeneity was not significant in our review. We explored statistical heterogeneity using the Chi2 test (Higgins 2011).
Assessment of reporting biases
We did not explore bias by using a funnel plot because of the small number of included studies, according to the instructions of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data synthesis
We calculated pooled statistics using a fixed‐effect model. We did not use a random‐effects model because the P values of the heterogeneity tests (Chi2 test) were all greater than 0.1.
Subgroup analysis and investigation of heterogeneity
We investigated clinical heterogeneity according to the scale of gastrectomy. Methodological heterogeneity (study design) and statistical heterogeneity were not significant in this review. We used subgroup analysis according to the scale of gastrectomy.
Sensitivity analysis
We performed sensitivity analyses to investigate the effect on outcome, by:
comparing fixed‐effect model results to random‐effects model results;
excluding studies published in different languages; and
using different statistical parameters.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
We identified a total of 1196 references through electronic searches of CENTRAL in The Cochrane Library (n = 57), PubMed (n = 489), EMBASE (n = 474), and CNKI (n = 176). After reading the titles and abstracts, we found 503 duplicates and 687 clearly irrelevant references. From the remaining six articles we excluded two trials (Lerut 2001; Petrowsky 2004) and the reasons are listed in the Characteristics of excluded studies table. Finally, we included four RCTs in this review (Kim 2004; Álvarez 2005; Kumar 2007; Jiang 2008). See Figure 1.
1.
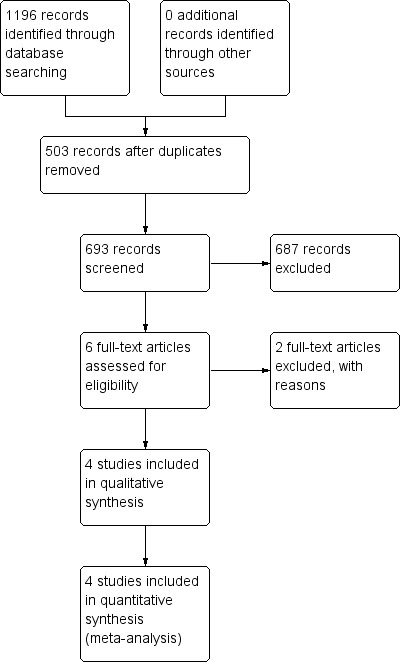
Study flow diagram.
Included studies
We included four RCTS with a total of 438 patients (220 patients in the drain group and 218 in the no‐drain group) in this review (please see Characteristics of included studies). Three studies were from Asia (Kim 2004; Kumar 2007; Jiang 2008) and one study was from South America (Álvarez 2005). The publication dates were from 2004 to 2008, and the sample sizes were from 60 to 170. Three studies were published in English (Kim 2004; Álvarez 2005; Kumar 2007) and one in Chinese (Jiang 2008). None of the included studies carried out a sample size estimation. Two trials clearly described the inclusion criteria in the reports (Kim 2004; Jiang 2008) but the other two did not (Álvarez 2005; Kumar 2007). Only one study included patients who received a palliative operation (Kumar 2007).
Design
All four included studies were parallel group RCTs.
Sample sizes
The sample sizes of included studies were from 60 to 170.
Participants
All the included participants were patients who have undergone gastrectomy for gastric cancer.
Interventions
All included studies compared drain with no drain, but the scale of gastrectomy and lymphadenectomy, combined organ resection (splenectomy or pancreatectomy), as well as drain type in the included studies were different. Kim 2004 performed subtotal or total gastrectomy with D2 or more lymph node dissection depending on the extent and location of the primary tumour, and calculated the results according to the scale of gastrectomy. In this review we labelled the study as Kim 2004 T (total gastrectomy) and Kim 2004 S (subtotal gastrectomy) respectively. Splenectomy was performed if there was suspicion of cancer involvement, and distal pancreatectomy was not performed for any of the patients. A two‐armed closed suction drain was used in the subtotal gastrectomy subgroup, and two two‐armed closed suction drains were used in the total gastrectomy subgroup. Drains were removed when the total output was < 100 ml/24 hours. All procedures was performed by a single surgeon (SHN). Álvarez 2005 performed total gastrectomy with D2 lymphadenectomy. Three patients (5.0%) underwent splenectomy and one (1.7%) underwent spleno‐pancreatectomy. Two gross tubular drains were used in the drain group. Drains were removed after the radiological study on the eighth post‐operative day. Kumar 2007 performed subtotal gastrectomy with D1 or D2 lymph node dissection. Splenectomy and pancreatectomy were not clearly recorded in the article. A single tube drain (28‐F) was used in the drain group. Drains were removed when the total output was < 50 ml/24 hours. All surgical procedures were performed by consultant surgeons. Jiang 2008 performed total or subtotal gastrectomy with D2 lymphadenectomy according to the location of the primary tumour, but did not calculate the results per group. None of the included patients underwent splenectomy or pancreatectomy. Drain type was not clearly recorded. Drains were removed when the total output was < 100 ml/24 hours. Operations were performed by the same group of surgeons. None of the included studies clearly recorded the antibiotics used.
Comparisons
All the included studies compared drain with no drain post‐gastrectomy for gastric cancer.
Outcomes
The primary outcomes (30‐day mortality, re‐operations, and post‐operative complications) were reported in the included studies except for Kumar 2007, which did not report on re‐operations. Our secondary outcomes (operation time, length of post‐operative hospital stay, initiation of soft diet) were reported in the included RCTs with the exception of Álvarez 2005, which did not report on operation time. The overall quality of the evidence according to the GRADE approach was 'very low' for mortality and re‐operations, and 'low' for post‐operative complications, operation time, and post‐operative length of stay (Summary of findings table 1).
Excluded studies
We excluded two articles for the following reasons: Lerut 2001 involved gastric drainage and not abdominal drainage; Petrowsky 2004 was a systematic review article. Please see Characteristics of excluded studies.
Risk of bias in included studies
Three review authors (J‐Q C, ZW, and KS) independently performed methodological quality assessments according to the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not rate the studies; we accepted or rejected each one based on the six items noted above. We resolved disagreements by consensus. A summary of the 'risk of bias' assessments can be found in Figure 2 and Figure 3.
2.
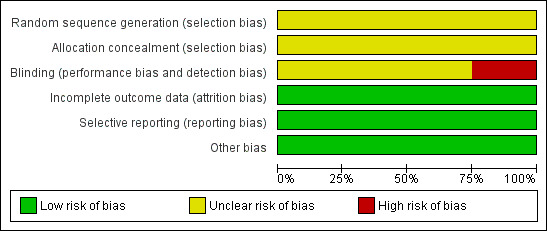
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
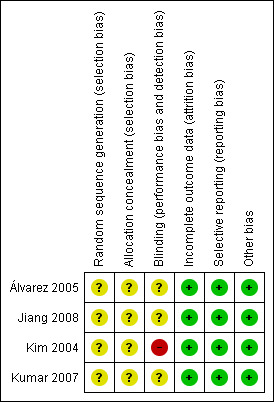
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
None of the included trials described the method used to generate allocation sequence. Only one trial (Kim 2004) mentioned that randomisation was performed after determining that the primary tumour was resectable and the surgery was curative. We considered that the method of allocation concealment was inadequate in the included studies.
Blinding
Double‐blinding was impractical in our review but we recorded whether or not the drain was placed by a second surgeon who was not otherwise involved in the operation. Operations were completed by a single surgeon (SHN) in one trial (Kim 2004). The other three studies (Álvarez 2005; Kumar 2007; Jiang 2008) did not record whether or not the drain was placed by a second surgeon.
Incomplete outcome data
There were no dropouts or cross‐overs in the included studies. We considered the included studies to have a low risk of incomplete outcome data bias.
Selective reporting
Because we could not obtain the protocols of the included trials, it was difficult to confirm whether all pre‐specified outcomes were reported. Considering that clinical results, whether favourable or unfavourable, were reported in the included trials, we classified them as having a low risk of selective reporting bias.
Other potential sources of bias
None of the included studies were supported by any company, therefore we considered that there was no conflict of interest and assessed them as low risk of other potential sources of bias.
We have presented the findings of our review in 'summary of findings' tables. We used the GRADEpro software to construct the tables (GRADEpro 2014). The tables present information on the body of evidence (for example number of studies), the judgements about the underlying quality of evidence, key statistical results, and a grade for the quality of evidence for each outcome. We used the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) system to describe the quality of the evidence and the strength of the recommendation (Guyatt 2011; GRADE 2013). We expressed the quality of evidence on a four‐point adjectival scale from 'high' to 'very low'.
Effects of interventions
See: Table 1
Comparison 1: drain versus no drain
Primary outcomes
The 30‐day mortality
All included studies (438 patients) reported post‐operative mortality, without statistical significance. The overall 30‐day mortality was 1.4% and there was no clear evidence of a difference between the two groups (1.8% in the drain group and 0.9% in the no‐drain group). No heterogeneity existed using the Chi2 test (I2 statistic = 0%) when we used the fixed‐effect model. There was no clear evidence of a difference in mortality between the two groups (RR 1.73, 95% CI 0.38 to 7.84; Analysis 1.1). The result did not change when we calculated the risk difference (RD), adopted the random‐effects model, or excluded the study published in a different language. We performed a subgroup analysis to investigate the effect of a different scale of gastrectomy. The result of the subgroup analysis suggested there was no clear evidence of a difference between the two groups (RR 3.20, 95% CI 0.14 to 75.55 in the total gastrectomy subgroup; and RR 1.39, 95% CI 0.24 to 8.01 in the subtotal gastrectomy subgroup). Details of mortality are listed in Table 2. According to Table 2, we can see that 30‐day mortality was a zero event outcome for Kim 2004 and Jiang 2008 and, as a result, less than half of the total number of patients contributed to the meta‐analysis. A very large numbers of patients would be required to make any sensible conclusions about whether the two groups were similar.
1.1. Analysis.
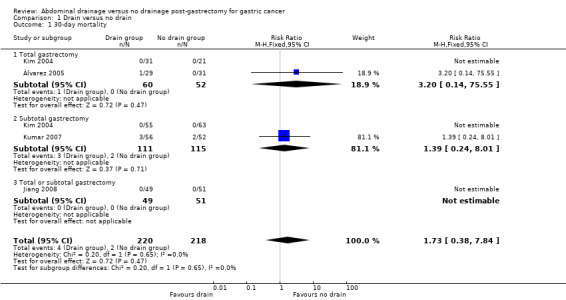
Comparison 1 Drain versus no drain, Outcome 1 30‐day mortality.
1. Mortality.
| Study name | Drain group | No‐drain group | Note |
| Álvarez 2005 | 1/29 | 0/31 | The cause of death was jejunal necrosis due to microembolisation of the mesenteric vessels |
| Jiang 2008 | 0/49 | 0/51 | ‐ |
| Kim 2004 S | 0/55 | 0/63 | ‐ |
| Kim 2004 T | 0/31 | 0/21 | ‐ |
| Kumar 2007 | 3/56 | 2/52 | P = 0.284, the cause of death was unclear |
Re‐operations
Only two studies (230 patients) reported re‐operations (Kim 2004; Álvarez 2005). None of the patients in Kim 2004 underwent re‐operation. Álvarez 2005 reported that the number of re‐operations was higher in the drain group but with no clear evidence of a difference between the two groups (24.1% in the drain group and 9.7% in the no‐drain group, P = 0.12). A test for heterogeneity was not applicable and we used the fixed‐effect model. There was no clear evidence of a difference between the two groups for this outcome (RR 2.49, 95% CI 0.71 to 8.74; Analysis 1.2). The result did not change when we calculated the RD or adopted the random‐effects model. Subgroup analysis was not applicable to the reported data.
1.2. Analysis.
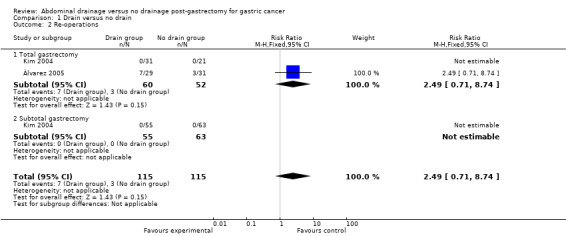
Comparison 1 Drain versus no drain, Outcome 2 Re‐operations.
Post‐operative complications
All included studies (438 patients) reported data on post‐operative complications, but the results of the four studies were not consistent. Álvarez 2005 reported that complications were less frequent in the no‐drain group (37.9% in the drain group and 9.7% in the no‐drain group, P = 0.02). The other three trials reported no significant difference between the two groups (Kim 2004; Kumar 2007; Jiang 2008).
Pneumonia
All trials (438 patients) reported this outcome. The Chi2 test indicated no significant heterogeneity (I2 statistic = 0%) using the fixed‐effect model. The result of meta‐analysis suggested that there was no clear evidence of a difference between the two groups for this outcome (RR 1.18, 95% CI 0.55 to 2.54; Analysis 1.3). There was no change in the result when we calculated the RD, adopted the random‐effects model, or excluded the study published in a different language. Subgroup analysis did not reveal any statistically significant difference when considering the scale of gastrectomy (RR 2.37, 95% CI 0.39 to 14.23 for the total gastrectomy subgroup; RR 0.95, 95% CI 0.36 to 2.50 for the subtotal gastrectomy subgroup; Analysis 1.3).
1.3. Analysis.
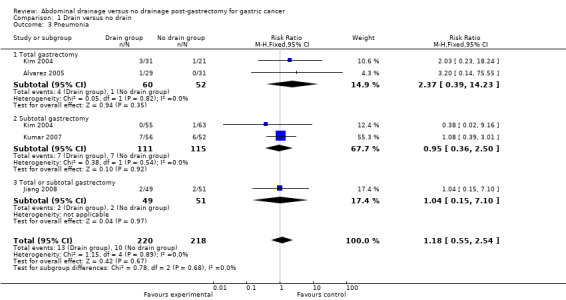
Comparison 1 Drain versus no drain, Outcome 3 Pneumonia.
Wound infection
Three studies (378 patients) reported data for this outcome (Kim 2004; Kumar 2007; Jiang 2008). There was no significant heterogeneity (I2 statistic = 0%) so we applied the fixed‐effect model. Meta‐analysis did not reveal any statistically significant difference between the two groups (RR 1.23, 95% CI 0.47 to 3.23; Analysis 1.4). The result did not change when we calculated the RD, adopted the random‐effects model, or excluded the study published in a different language. Subgroup analysis according to the scale of gastrectomy did not reveal any statistically significant difference in this outcome (RR 0.23, 95% CI 0.01 to 5.37 for the total gastrectomy subgroup; and RR 1.41. 95% CI 0.45 to 4.46 for the subtotal gastrectomy subgroup; Analysis 1.4).
1.4. Analysis.
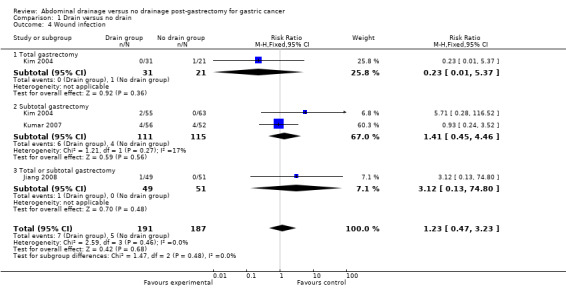
Comparison 1 Drain versus no drain, Outcome 4 Wound infection.
Intra‐abdominal abscess
Only two studies (230 patients) reported intra‐abdominal abscesses (Kim 2004; Álvarez 2005). The Chi2 test indicated no significant heterogeneity (I2 statistic = 0%) and we used the fixed‐effect model. The result of the meta‐analysis suggested there was no clear difference between the two groups for this outcome (RR 1.27, 95% CI 0.29 to 5.51). There was no change in the result by calculating the RD or adopting the random‐effects model. Subgroup analysis considering the scale of gastrectomy did not reveal any statistically significant difference (RR 0.68, 95% CI 0.04 to 10.24 for the total gastrectomy subgroup; and RR 1.65, 95% CI: 0.28 to 9.88 for the subtotal gastrectomy subgroup; Analysis 1.5).
1.5. Analysis.
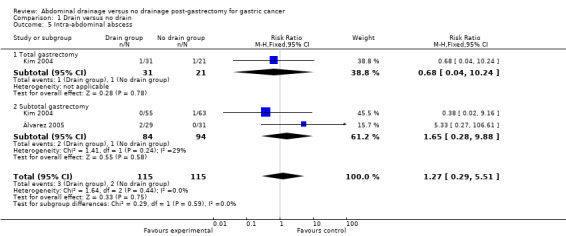
Comparison 1 Drain versus no drain, Outcome 5 Intra‐abdominal abscess.
Anastomotic leak
Only one trial (108 patients) reported this outcome (Kumar 2007). There was no statistically significant difference between the two groups (RR 0.93, 95% CI 0.06 to 14.47; Analysis 1.6).
1.6. Analysis.
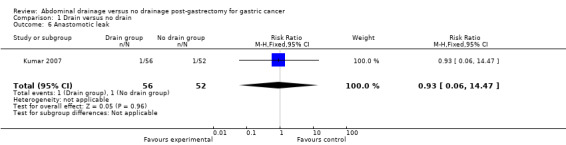
Comparison 1 Drain versus no drain, Outcome 6 Anastomotic leak.
Drain‐related complications
Drain‐related complications were mainly complications caused by the placement of an abdominal drain (for example drain‐site infection, omentum coming out through the drain site after removal of the drain, etc.). Only two studies (208 patients) reported this outcome (Kumar 2007; Jiang 2008). There were four drain‐related complications in one trial (Kumar 2007) and one drain‐related complication in the other (Jiang 2008).
Secondary outcomes
Operation time
Three studies (378 patients) reported this outcome (Kim 2004; Kumar 2007; Jiang 2008). There was no significant heterogeneity (I2 statistic = 0%) so we applied the fixed‐effect model. The result suggested that operation time was significantly longer in the drain group (MD 9.07 min, 95% CI 2.56 to 15.57). There was no change in the result by adopting the random‐effects model or excluding the study published in a different language. Subgroup analysis indicated that there was no statistically significant difference in the total gastrectomy subgroup (MD 2.00 min, 95% CI ‐12.16 to 16.16) but the operation time was significantly longer in the subtotal gastrectomy subgroup (MD 12.43 min, 95% CI 4.09 to 20.78; Analysis 1.7).
1.7. Analysis.
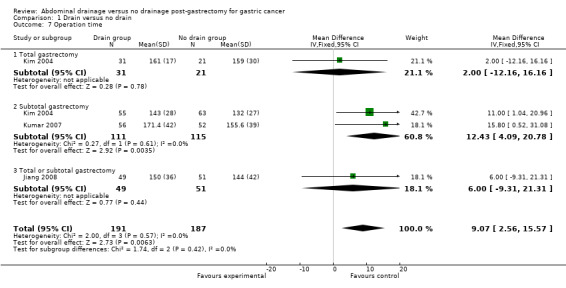
Comparison 1 Drain versus no drain, Outcome 7 Operation time.
Length of post‐operative hospital stay
All included studies (438 patients) reported this outcome. The Chi2 test indicated no significant heterogeneity (I2 statistic = 19%) and we applied the fixed‐effect model. The result suggested that the length of post‐operative hospital stay was significantly longer in the drain group (MD 0.69 day, 95% CI 0.18 to 1.21). There was no change in the result by adopting the random‐effects model or excluding the study published in a different language. Subgroup analysis indicated that there was no clear difference between the two groups in the total gastrectomy subgroup (MD 0.77 day, 95% CI ‐2.13 to 3.68) but the length of hospital stay was significantly longer in the subtotal gastrectomy subgroup (MD 0.64 day, 95% CI 0.01 to 1.27; Analysis 1.8).
1.8. Analysis.
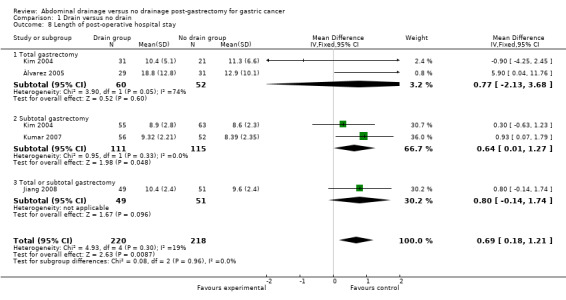
Comparison 1 Drain versus no drain, Outcome 8 Length of post‐operative hospital stay.
Initiation of soft diet
All included studies (438 patients) reported this outcome. No heterogeneity existed using the Chi2 test (I2 statistic = 0%) and we used the fixed‐effect model. There was no statistical significance in the time for initiation of a soft diet between the two groups (MD 0.15 days, 95% CI ‐0.07 to 0.37). The result did not change when adopting the random‐effects model or excluding the study published in a different language. Subgroup analysis did not reveal any statistically significant difference between the two groups (MD 0.44 day, 95% CI ‐0.87 to 1.76 for the total gastrectomy subgroup; and MD 0.11 day, 95% CI ‐0.12 to 0.34 for the subtotal gastrectomy subgroup; Analysis 1.9).
1.9. Analysis.
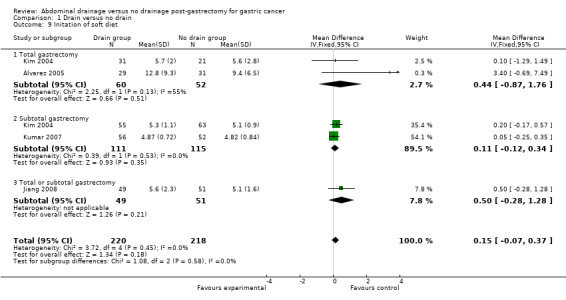
Comparison 1 Drain versus no drain, Outcome 9 Initation of soft diet.
Discussion
Prophylactic placement of drains after abdominal surgery has been used routinely for many years. To our knowledge there is little information assessing the scientific evidence for prophylactic drainage placement after gastrectomy for gastric cancer. In this review we evaluated the value of prophylactic drainage placement in gastric cancer surgery.
Summary of main results
We included four RCTs involving 438 patients and found that routine drain use after gastrectomy for gastric cancer had no significant advantage. However, drains prolonged operation time and post‐operative hospital stay and led to drain‐related complications without providing any additional benefits.
The main purpose of abdominal drains after gastrectomy is earlier detection and drainage of anastomotic fistulas, reducing the number of re‐operations. If it is not detected in time, fatal complications such as peritonitis can result. However, in this review we found no clear evidence of a difference in anastomotic leak between the drain group and the no‐drain group. Additionally, no death caused by anastomotic leak was reported. On the contrary, one study reported higher numbers of re‐operations in the drain group (Álvarez 2005). Anastomotic leak was reported as one for each group in one trial (Kumar 2007), suspected clinically and confirmed after re‐exploration. This suggests that the drain did not help in the identification and management of anastomotic leak.
Another purpose of abdominal drains in gastric cancer surgery is to remove blood and other exudates and so prevent intra‐abdominal abscesses. Two of the four included studies reported on intra‐abdominal abscesses, without any significant difference between the two groups (Kim 2004; Álvarez 2005). Additionally, patients who developed intra‐abdominal abscesses had no evidence of anastomotic leak and were managed with ultrasound‐guided percutaneous drainage or surgical drainage, but not abdominal drainage.
Compared with the no‐drain group, operation time and post‐operative hospital stay were significantly longer in the drain group, and initiation of soft diet was later although without statistical significance. This will lead to waste of medical resources and an increased economic burden on patients. Additionally, as reported in the literature (Fong 1996; Nakajima 2002), drain‐related complications such as drain‐site infection, omentum coming out through the drain site after removal of the drain will cause more pain for patients. However, long term complications such as tumour recurrence in the drain site were not studied in the included trials.
Critics of prophylactic drain placement have claimed that the drain caused more post‐operative infections, such as pneumonia and wound infection (Budd 1982; Monson 1991). We found that the rate of pneumonia and wound infections were higher in the drain group although without statistical significance. This result was stable in sensitivity analyses changing the statistical model and using different statistical parameters. This suggested that our result is reliable. The possible reason for the higher rate of pneumonia is associated with the pain induced by the drain. One included study showed increased analgesic use after surgery in the drain group compared with the no‐drain group (Kim 2004). Indirectly, this confirmed our speculation.
In order to evaluate the value of drains after gastrectomy for gastric cancer without potential confounding by differences in the extent of surgery, and to increase the reliability of our results, we performed a subgroup analysis according to the scale of gastrectomy. It is reported that post‐operative complications after gastric cancer surgery are related to pancreatico‐splenectomy and the extent of lymphadenectomy (Sano 1996; Wang 2010). In the four included studies, pancreatico‐splenectomy and the extent of lymphadenectomy were confounding and were not clearly reported, so we could not perform a subgroup analysis. The value of drain placement after gastrectomy combined with pancreatico‐splenectomy and extended lymphadenectomy is worthy of further study. A Cochrane review comparing the value of different drains in other surgical procedures failed to show any significant difference in any of the outcomes measured, except pain at the drain site (Cochrane 2009). So far, there are no RCTs comparing the value of different drains (such as a suction drain versus a passive closed drain) after gastrectomy for gastric cancer, therefore we did not compare different types of drains.
With advances in the technology of surgery (such as the increasingly common use of stapled anastomosis), anaesthesia, and peri‐operative care, the incidence of anastomotic leak and intra‐abdominal abscesses have been decreasing internationally (Csendes 1990; Csendes 2002). This makes routine drain use after gastrectomy in order to identify and manage anastomotic leak and intra‐abdominal abscesses less urgent. In our review we found that prophylactic drains do not help in the detection or treatment of these complications. Thus we do not recommend routine drain use after gastrectomy for gastric cancer.
Overall completeness and applicability of evidence
We are unable to give any recommendation for the clinical practice of routine drain placement after gastrectomy for gastric cancer, whether subtotal or total gastrectomy. Because of inadequate and confounding data, we could not perform a subgroup analysis for additional pancreatico‐splenectomy and the extent of lymphadenectomy, use of routine antibiotic prophylaxis (whether they are used or not), and types of drains used, as planned in the protocol. Therefore, we can not ascertain the necessity of routine drain placement after pancreatico‐splenectomy and extended lymphadenectomy, as well as gastrectomy for other stomach diseases.
Quality of the evidence
We included only four RCTs involving 438 patients in this review, and not all included studies reported all the outcomes selected for this review. The methodological quality of the included studies was moderate, even low.
All included studies did not clearly report the method and time of randomisation and of allocation concealment. One trial (Kim 2004) reported that all surgical procedures were performed by one surgeon, which increased the risk of both performance and measurement bias to some extent. Three other studies (Álvarez 2005; Kumar 2007; Jiang 2008) did not record whether or not the drain was placed by a second surgeon. We assessed selective reporting and other potential sources of bias based on the reporting of results and funding, and judged them as low risk of bias. This is subjective and may lead to bias. Additonally, we must note that 30‐day mortality and re‐operations are very rare events (for example 30‐day mortality was a zero event for Kim 2004 and Jiang 2008 and, as a result, less than half of the total number of patients contributed to the meta‐analysis). Very large numbers of patients would be required to make any sensible conclusions about whether the two groups were similar. Moreover, operation time and hospital stay are very unlikely to follow a normal distribution, so the analyses of mean differences are not necessarily accurate. Therefore, these results should be interpreted cautiously as the assumption of normality may not be met for these outcomes.
The overall quality of the evidence according to the GRADE approach was 'very low' for mortality and re‐operations, and 'low' for post‐operative complications, operation time, and post‐operative length of stay.
Potential biases in the review process
Potential biases from the included studies
Even though we made great efforts to find suitable trials, only four RCTs were eligible for inclusion. Three studies were from Asia (Kim 2004; Kumar 2007; Jiang 2008) and one from South America (Álvarez 2005); this may suggest possible publication bias. Additionally, we did not use a funnel plot to test for publication bias due to the small number of included studies. As is known, the use of antibiotics can effectively prevent post‐operative infections. However, not all included studies recorded the details of antibiotics usage. Similarly, post‐operative complications are closely associated with the nutritional condition of patients (especially the albumin levels). The pre‐operative nutritional condition of patients was unclear and we did not know whether it was the same in the two groups of each study. These factors could potentially lead to both selection and performance bias.
Potential biases from the review authors
Three review authors independently selected studies, extracted data, and assessed study quality. Where necessary, we reached consensus by discussion. We consider that these methods minimised potential biases in the development of this review. Additionally, we declare no conflicts of interest. Based on the above, we consider potential biases from the review authors to be negligible.
Agreements and disagreements with other studies or reviews
As far as we know, there is only one systematic review that compared drain with no drain in gastrointestinal surgery, which concluded that prophylactic drainage remains indicated after total gastrectomy (Petrowsky 2004). This conclusion was not based on any prospective studies so we can not readily accept this conclusion.
Authors' conclusions
Implications for practice.
Drains increase harm by prolonging both operation time and post‐operative hospital stay, and lead to drain‐related complications without providing any additional benefit for patients with gastric cancer undergoing gastrectomy. We found no convincing evidence to support routine drain use after gastrectomy for gastric cancer.
Although there are many surgical interventions where double blinding is not possible, the intervention of abdominal drainage or no abdominal drainage can be blinded if adequate measures are taken. For example, in the group without drainage a drain can be used with one end connected to a drain bag and the other end outside the skin with the drain side covered by opaque dressing. Thus, we can know if any potential increased risk of chest complications is due to the perceived fear of the patients or because patients experience real pain that inhibits breathing.
Quality of life after surgery is an important index to evaluate treatment effectiveness for patients with malignant tumours. Unfortunately, none of the included studies reported this clinical outcome; so quality of life should be taken into consideration in future clinical practice.
Implications for research.
The necessity of drain placement in gastrectomy for other reasons (such as laparoscopic gastrectomy, gastrectomy combined with pancreatico‐splenectomy and extended lymphadenectomy) and in surgery for other stomach diseases (such as perforated gastric ulcer) is worthy of study in the future.
We consider that the accumulation of exudates in the abdominal cavity without a drain could induce chronic intestinal obstruction and the tumour to seed in the drain site. So the follow‐up time should be longer in future research to observe long term complications in the two groups.
Quality of life after surgery should be included as an outcome in future studies.
In order to improve the quality of RCTs, studies should be conducted and reported according to the CONSORT Statement (www.consort‐statement.org).
We can not interpret non‐significant results as 'equivalent' in this review. Consequently, more trials are necessary to investigate this comparison.
What's new
| Date | Event | Description |
|---|---|---|
| 23 February 2015 | New citation required but conclusions have not changed | Literature searches rerun. No new studies identified for inclusion. Conclusions unchanged. |
| 23 February 2015 | New search has been performed | Updated according to the current standards for the conduct and reporting of Cochrane systematic reviews and the latest search results. |
Acknowledgements
We thank Karin Dearness (Managing Editor of the Cochrane Upper Gastrointestinal and Pancreatic Diseases (UGPD) Group) and Racquel Simpson (former Trials Search Co‐ordinator of the UGPD Group) for their guidance in developing our review. We thank the UGPD Group editorial board for their direction in preparing our review. We thank Liu Guanjian and Li Youping (The Chinese Cochrane Center) for their advice in amending our review. We also thank Cao Yunfei and colleagues from The First Affiliated Hospital of Guangxi Medical University for their warmhearted assistance in developing this review.
Appendices
Appendix 1. CENTRAL search strategy
1. (carcin$ or cancer$ or neoplas$ or tumour$ or tumor$ or cyst$ or growth$ or adenocarcin$ or malig$).mp.
2. (Intestin$ or Digest$ or Gastr$ or gut or epigastr$ or stomach$ or abdomin$).mp.
3. 1 or 2
4. exp abdominal neoplasms/ or exp intestinal neoplasms/ or exp stomach neoplasms/
5. 3 or 4
6. drain$.mp. [mp=title, original title, abstract, mesh headings, heading words, keyword]
7. exp Drainage/
8. or/6‐7
9. gastrectomy.mp. [mp=title, original title, abstract, mesh headings, heading words, keyword]
10. 5 and 8 and 9
Appendix 2. MEDLINE (PubMed) search strategy
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. drug therapy.fs.
6. randomly.ab.
7. trial.ab.
8. groups.ab.
9. or/1‐8
10. (animals not (humans and animals)).sh.
11. 9 not 10
12. (carcin$ or cancer$ or neoplas$ or tumour$ or tumor$ or cyst$ or growth$ or adenocarcin$ or malig$).mp.
13. (Intestin$ or Digest$ or Gastr$ or gut or epigastr$ or stomach$).mp.
14. 12 and 13
15. exp abdominal neoplasms/ or exp intestinal neoplasms/ or exp stomach neoplasms/
16. 14 or 15
17. exp drainage/ or exp negative‐pressure wound therapy/
18. ((abdomin$ or gastric) adj2 drain$).mp.
19. Abdominal Abscess/pc, su, th [Prevention & Control, Surgery, Therapy]
20. or/17‐19
21. exp Gastrectomy/
22. gastrectomy.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
23. or/21‐22
24. 16 and 20 and 23
25. 11 and 24
Appendix 3. EMBASE search strategy
1. Clinical trial/
2. Randomized controlled trial/
3. Randomization/
4. Single‐Blind Method/
5. Double‐Blind Method/
6. Cross‐Over Studies/
7. Random Allocation/
8. Placebo/
9. Randomi?ed controlled trial$.tw.
10. Rct.tw.
11. Random allocation.tw.
12. Randomly allocated.tw.
13. Allocated randomly.tw.
14. (allocated adj2 random).tw.
15. Single blind$.tw.
16. Double blind$.tw.
17. ((treble or triple) adj blind$).tw.
18. Placebo$.tw.
19. Prospective study/
20. or/1‐19
21. Case study/
22. Case report.tw.
23. Abstract report/ or letter/
24. or/21‐23
25. 20 not 24
26. (carcin$ or cancer$ or neoplas$ or tumour$ or tumor$ or cyst$ or growth$ or adenocarcin$ or malig$).mp.
27. exp neoplasm/ or exp tumor/
28. 26 or 27
29. (Intestin$ or Digest$ or Gastr$ or gut or epigastr$ or stomach$).mp.
30. 28 and 29
31. exp stomach tumor/ or exp abdominal tumor/ or exp stomach cancer/ or exp abdominal cancer/ or exp intestine cancer/ or exp stomach carcinoma/ or exp stomach carcinogenesis/ or exp stomach carcinoid/
32. 30 or 31
33. exp drain/ or exp abscess drainage/ or exp abdominal drainage/ or exp wound drainage/ or exp surgical drainage/ or exp vacuum assisted closure/
34. ((abdomin$ or gastric) adj2 drain$).mp.
35. exp abdominal abscess/pc, su, th [Prevention, Surgery, Therapy]
36. or/33‐35
37. exp gastrectomy Billroth I/ or exp gastrectomy/ or exp partial gastrectomy/ or exp gastrectomy Billroth II/
38. gastrectomy.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
39. or/37‐38
40. 32 and 36 and 39
41. 25 and 40
Data and analyses
Comparison 1. Drain versus no drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 30‐day mortality | 4 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.38, 7.84] |
| 1.1 Total gastrectomy | 2 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.2 [0.14, 75.55] |
| 1.2 Subtotal gastrectomy | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.24, 8.01] |
| 1.3 Total or subtotal gastrectomy | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Re‐operations | 2 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.49 [0.71, 8.74] |
| 2.1 Total gastrectomy | 2 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.49 [0.71, 8.74] |
| 2.2 Subtotal gastrectomy | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Pneumonia | 4 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.55, 2.54] |
| 3.1 Total gastrectomy | 2 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.39, 14.23] |
| 3.2 Subtotal gastrectomy | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.36, 2.50] |
| 3.3 Total or subtotal gastrectomy | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.15, 7.10] |
| 4 Wound infection | 3 | 378 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.47, 3.23] |
| 4.1 Total gastrectomy | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 5.37] |
| 4.2 Subtotal gastrectomy | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.45, 4.46] |
| 4.3 Total or subtotal gastrectomy | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.13, 74.80] |
| 5 Intra‐abdominal abscess | 2 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.29, 5.51] |
| 5.1 Total gastrectomy | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.04, 10.24] |
| 5.2 Subtotal gastrectomy | 2 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.28, 9.88] |
| 6 Anastomotic leak | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.06, 14.47] |
| 7 Operation time | 3 | 378 | Mean Difference (IV, Fixed, 95% CI) | 9.07 [2.56, 15.57] |
| 7.1 Total gastrectomy | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐12.16, 16.16] |
| 7.2 Subtotal gastrectomy | 2 | 226 | Mean Difference (IV, Fixed, 95% CI) | 12.43 [4.09, 20.78] |
| 7.3 Total or subtotal gastrectomy | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐9.31, 21.31] |
| 8 Length of post‐operative hospital stay | 4 | 438 | Mean Difference (IV, Fixed, 95% CI) | 0.69 [0.18, 1.21] |
| 8.1 Total gastrectomy | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | 0.77 [‐2.13, 3.68] |
| 8.2 Subtotal gastrectomy | 2 | 226 | Mean Difference (IV, Fixed, 95% CI) | 0.64 [0.01, 1.27] |
| 8.3 Total or subtotal gastrectomy | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.14, 1.74] |
| 9 Initation of soft diet | 4 | 438 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.07, 0.37] |
| 9.1 Total gastrectomy | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | 0.44 [‐0.87, 1.76] |
| 9.2 Subtotal gastrectomy | 2 | 226 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.12, 0.34] |
| 9.3 Total or subtotal gastrectomy | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.28, 1.28] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jiang 2008.
| Methods | Randomised clinical trial with parallel design. Generation of the allocation sequence and allocation concealment: were not clearly described in the original article Follow‐up: adequate Duration of follow‐up: 30 days | |
| Participants | Patients who were diagnosed with gastric cancer Country: China Year of study: July 2005 to June 2006 Number randomised: 100 Mean age (years): Group 1 (57.3 ± 15.4), Group 2 (56.7 ± 12.4) Sex (M/F): Group 1 (29/20), Group 2 (30/21) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Group 1: drain (n = 49), type of drains was unclear Group 2: no drain (n = 51) Antibiotic use: not stated All patients in the two groups received total or subtotal gastrectomy with D2 lymphadenectomy according to the location of primary tumour. The same group of surgeons performed the operation | |
| Outcomes | Mortality (30‐day mortality) Re‐operations Post‐operative complications Operation time Length of post‐operative hospital stay Initiation of soft diet |
|
| Notes | 10 ml fibrin glue was used in abdominal cavity after the operation Patients with subtotal or total gastrectomy were not analysed separately Drains were removed when the total output < 100 ml/24 hours |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The authors did not record whether or not the drain was placed by a second surgeon |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Yes |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Low risk | Yes |
Kim 2004.
| Methods | Randomised clinical trial with parallel design Generation of the allocation sequence and allocation concealment: were not clearly described in the original article Follow‐up: adequate Duration of follow‐up: 30 days | |
| Participants | Patients who were diagnosed with gastric cancer Country: Korea Year of study: between 1 February 2001 and 31 July 2001 Number randomised: 170 Mean age (years): Group 1S (58.5 ± 7.5), Group 2S (54.9 ± 11.4); Group 1T (56.1.3 ± 10.1), Group 2T (55.9 ± 12.5). Sex (M/F): Group 1S (36/18), Group 2S (41/23); Group 1T (24/5), Group 2T (15/6). Inclusion criteria
|
|
| Interventions | Group 1S: subtotal gastrectomy with drain (n = 55), a two‐armed closed suction drain was used
Group 2S: subtotal gastrectomy without drain (n = 63) Group 1T: total gastrectomy with drain (n = 31), two two‐armed closed suction drains were placed Group 2T: total gastrectomy without drain (n = 21) Antibiotic use: not stated All patients underwent either total or subtotal gastrectomy with D2 or more lymph node dissection depending on the extent and location of the primary tumour. A single surgeon (SHN) performed all procedures. Splenectomy was not performed unless there was suspicion of cancer involvement. Distal pancreatectomy was not performed for any of the patients in the study |
|
| Outcomes | Mortality (30‐day mortality) Re‐operations Post‐operative complications Operation time Length of post‐operative hospital stay Initiation of soft diet |
|
| Notes | Drains were removed when the total output < 100 ml/24 hours Patients with subtotal or total gastrectomy were analysed separately Dose of analgesic use after surgery was compared between the drain group and the no‐drain group Liquid diet was started after confirmation of return of bowl function with passage of flatus and advanced to soft diet as tolerated Patients were discharged from the hospital after tolerating soft diet for 3 to 4 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | All procedures were performed by a single surgeon (SHN) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Yes |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Low risk | Yes |
Kumar 2007.
| Methods | Randomised clinical trial with parallel design Generation of the allocation sequence and allocation concealment: were not clearly described in the original article Follow‐up: adequate Duration of follow‐up: 4 weeks | |
| Participants | Patients who were diagnosed with gastric cancer Country: Nepal Year of study: between January 2001 and December 2005 Number randomised: 108 Mean age (years): Group 1 (54.3 ± 11.2), Group 2 (57.5 ± 13.4) Sex (M/F): Group 1 (36/20), Group 2 (33/19) Inclusion criteria: not clearly record in the article | |
| Interventions | Group 1: drain (n = 56), a single tube drain (28‐F) was used
Group 2: no drain (n = 52)
Antibiotic use: not stated All patients underwent subtotal gastrectomy, regardless whether it was radical or palliative, or D1 or D2 lymph node dissection. All surgical procedures were performed by consultant surgeons in the Surgical Department, Patan Hospital. Splenectomy or pancreatectomy was not clearly described |
|
| Outcomes | Mortality (30‐day mortality) Post‐operative complications Operation time Length of post‐operative hospital stay Initiation of soft diet |
|
| Notes | Drains were removed when the total output < 50 ml/24 hours Liquid diet was started after confirmation of bowel sound with passage of flatus and advanced to soft diet when the patients tolerated the liquid diet for at least 12 hours Patients were discharged from the hospital after tolerating a soft diet for at least 2 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The authors did not record whether or not the drain was placed by a second surgeon |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Yes |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Low risk | Yes |
Álvarez 2005.
| Methods | Randomised clinical trial with parallel design Generation of the allocation sequence and allocation concealment: were not clearly described in the original article Follow‐up: adequate | |
| Participants | Patients who were diagnosed with gastric cancer Country: Chile Year of study: between 2000 and 2003 Number randomised: 60 Mean age (years): Group 1 (mean: 60.6, range: 36 to 78), Group 2 (mean: 61.2, range: 42 to 79) Sex (M/F): Group 1 (22/7), Group 2 (22/9) Inclusion criteria: not clearly recorded in the article | |
| Interventions | Group 1: drain (n = 29), two gross tubular drains were used Group 2: no drain (n = 31) Antibiotic use: not stated All patients in the two groups received total gastrectomy with D2 lymphadenectomy except for palliative gastrectomies | |
| Outcomes | Mortality (30‐day mortality) Re‐operations Post‐operative complications Length of post‐operative hospital stay Initiation of soft diet |
|
| Notes | In 19% of patients an additional surgery was performed, with cholecystectomy being most frequent (8.3%) Drains were removed until the radiological study on the eighth post‐operative day |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Yes |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Low risk | Yes |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Lerut 2001 | The author studied gastric drainage, not abdominal drainage |
| Petrowsky 2004 | Systematic review article |
Differences between protocol and review
We did not identify any quasi‐RCTS or unpublished studies. In addition, details of randomisation, drain and antibiotics use were unclear in included studies, therefore we did not perform subgroup and sensitivity analyses as planned in the protocol.
Contributions of authors
Wang Zhen, Chen Junqiang, Su Ka and Dong Zhiyong drafted this review. Wang Zhen and Su Ka developed the search strategy. Chen Junqiang, Wang Zhen and Dong Zhiyong selected studies in accord with our predefined inclusion criteria. Chen Junqiang, Su Ka and Dong Zhiyong extracted relevant data of included studies. Su Ka and Dong Zhiyong entered data into RevMan. Chen Junqiang, Wang Zhen and Dong Zhiyong carried out the analysis. Chen Junqiang, Wang Zhen and Su Ka interpreted the results. Wang Zhen, Chen Junqiang, Su Ka and Dong Zhiyong drafted the review. Chen Junqiang and Wang Zhen will update the review.
Sources of support
Internal sources
The first affiliated hospital of Guangxi Medical University, China.
External sources
No sources of support supplied
Declarations of interest
ZW: none known
JC: none known
KS: none known
ZD: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Álvarez 2005 {published data only}
- Álvarez Uslar R, Molina H, Torres O, Cancino A. Total gastrectomy with or without abdominal drains. A prospective randomized trial. Revista Espanola de Enfermedades Digestivas 2005;97(8):562‐9. [PUBMED: 16266223] [DOI] [PubMed] [Google Scholar]
Jiang 2008 {published data only}
- Jiang ZW, Li JS, Wang ZM, Li N, Diao YQ, Huang XJ. Prospective randomized study of abdominal drains in gastric cancer surgery. Chinese Journal of Practical Surgery 2008;28(9):761‐2. [Google Scholar]
Kim 2004 {published data only}
- Kim J, Lee J, Hyung WJ, Cheong JH, Chen J, Choi SH, et al. Gastric cancer surgery without drains: A prospective randomized trial. Journal of Gastrointestinal Surgery 2004;8(6):727‐32. [PUBMED: 15358335] [DOI] [PubMed] [Google Scholar]
Kumar 2007 {published data only}
- Kumar M, Yang SB, Jaiswal VK, Shah JN, Shreshtha M, Gongal R. Is prophylactic placement of drains necessary after subtotal gastrectomy?. World Journal of Gastroenterology 2007;13(27):3738‐41. [PUBMED: 17659736] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Lerut 2001 {published data only}
- Lerut T, Coosemans W, De Leyn P, Van Raemdonck D. Gastroplasty: Yes or no to gastric drainage procedure. Diseases of the Esophagus 2001;14(3‐4):173‐7. [PUBMED: 11869315] [DOI] [PubMed] [Google Scholar]
Petrowsky 2004 {published data only}
- Petrowsky H, Demartines N, Rousson V, Clavien PA. Evidence‐based value of prophylactic drainage in gastrointestinal surgery: A systematic review and meta‐analyses. Annals of Surgery 2004;240(6):1074‐85. [PUBMED: 15570212] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Bona 1994
- Bona S, Gavelli A, Huguet C. The role of abdominal drainage after major hepatic resection. American Journal of Surgery 1994;167(6):593‐5. [DOI] [PubMed] [Google Scholar]
Budd 1982
- Budd DC, Cochran RC, Fouty WJ Jr. Cholecystectomy with and without drainage. A randomized, prospective study of 300 patients. American Journal of Surgery 1982;143(3):307–9. [DOI] [PubMed] [Google Scholar]
Cerise 1970
- Cerise EJ, Pierce WA, Diamond DL. Abdominal drains: their role as a source of infection following splenectomy. Annals of Surgery 1970;171(5):764‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane 2009
- Gurusamy KS, Samraj K. Routine abdominal drainage for uncomplicated open cholecystectomy. Cochrane Database of Systematic Reviews 2009, Issue Issue 1. [DOI: 10.1002/14651858.CD006003.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Conlon 2001
- Conlon KC, Labow D, Leung D, Smith A, Jarnagin W, Coit DG, et al. Prospective randomized clinical trial of the value of intraperitoneal drainage after pancreatic resection. Annals of Surgery 2001;234(4):487‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crew 2006
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World Journal of Gastroenterology 2006;12(3):354‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Csendes 1990
- Csendes A, Diaz JC, Burdiles P, Braghetto I, Maluenda F, Nava O, et al. Classification and treatment of anastomotic leakage after extended total gastrectomy in gastric carcinoma. Hepato‐Gastroenterology 1990;37 Suppl 2:174‐7. [PubMed] [Google Scholar]
Csendes 2002
- Csendes A, Burdiles P, Rojas J, Braghetto I, Diaz JC, Maluenda F. A prospective randomized study comparing D2 total gastrectomy versus D2 total gastrectomy plus splenectomy in 187 patients with gastric carcinoma. Surgery 2002;131(4):401‐7. [DOI] [PubMed] [Google Scholar]
Dougherty 1992
- Dougherty SH, Simmons RL. The biology and practice of surgical drains. Part 1. Current Problems in Surgery 1992;29(8):559‐623. [DOI] [PubMed] [Google Scholar]
Fong 1996
- Fong Y, Brennan MF, Brown K, Heffernan N, Blumgart LH. Drainage is unnecessary after elective liver resection. American Journal of Surgery 1996;171(1):158‐62. [DOI] [PubMed] [Google Scholar]
GRADE 2013
- Schünemann H, Brozek J, Guyatt G, Oxman A (editors). GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from www.guidelinedevelopment.org/handbook.
GRADEpro 2014 [Computer program]
- McMaster University. GRADEpro.. Version 3.6. McMaster University, 2014.
Gurusamy 2007
- Gurusamy KS, Samraj K, Mullerat P, Davidson BR. Routine abdominal drainage for uncomplicated laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2007, Issue Issue 4. [DOI: 10.1002/14651858.CD006004.pub3] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org.
Jemal 2002
- Jemal A, Thomas A, Murray T. Cancer Statistics, 2002. CA: a Cancer Journal for Clinicians 2002;52(1):23‐47. [DOI] [PubMed] [Google Scholar]
Karliczek 2006
- Karliczek A, Jesus EC, Matos D, Castro AA, Atallah AN, Wiggers T. Drainage or non‐drainage in elective colorectal anastomosis: A systematic review and meta‐analysis. Colorectal Disease 2006;8(4):259‐65. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Liu 2004
- Liu CL, Fan ST, Lo CM, Wong Y, Ng IOL, Lam CM, et al. Abdominal drainage after hepatic resection is contraindicated in patients with chronic liver diseases. Annals of Surgery 2004;239(2):194‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lochhead 2008
- Lochhead P, El‐Omar E. Gastric cancer. British Medical Bulletin 2008;85(1):87‐100. [DOI] [PubMed] [Google Scholar]
Maruyama 1998
- Maruyama K, Sasako M, Kinoshita T, Sano T, Katai H, Hada M, et al. Should systematic lymph node dissection be recommended for gastric cancer?. European Journal of Cancer 1998;34(10):1480‐9. [DOI] [PubMed] [Google Scholar]
Merad 1999
- Merad F, Hay JM, Fingerhut A, Yahchouchi E, Laborde Y, Pelissier E, et al. Is prophylactic pelvic drainage useful after elective rectal or anal anastomosis? A multicenter controlled randomized trial. Surgery 1999;125(5):529‐35. [PubMed] [Google Scholar]
Monson 1991
- Monson JRT, Guillou PJ, Keane FBV, Tanner WA, Brennan TG. Cholecystectomy is safer without drainage: the results of a prospective, randomized clinical trial. Surgery 1991;109(6):740‐6. [PubMed] [Google Scholar]
Nakajima 2002
- Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer 2002;5(1):1‐5. [DOI] [PubMed] [Google Scholar]
Parkin 2005
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: a Cancer Journal for Clinicians 2005;55(2):74‐108. [DOI] [PubMed] [Google Scholar]
Phan 2004
- Phan AT, Ajani JA. Gastric Carcinoma. Current Oncology Reports 2004;6(3):192‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Robinson 1986
- Robinson JO. Surgical drainage: An historical perspective. British Journal of Surgery 1986;73(6):422‐6. [DOI] [PubMed] [Google Scholar]
Sager 1993
- Sager PM, Couse N, Kerin M, May J, MacFie J. Randomized trial of drainage of colorectal anastomosis. British Journal of Surgery 1993;80(6):769‐71. [DOI] [PubMed] [Google Scholar]
Sano 1996
- Sano T, Martin IG. Lymphadenectomy and pancreatico‐splenectomy in gastric cancer surgery. Lancet 1996;348(9021):195‐6. [DOI] [PubMed] [Google Scholar]
Schein 2008
- Schein M. To drain or not to drain? The role of drainage in the contaminated and infected abdomen: an international and personal perspective. World Journal of Surgery 2008;32(2):312‐21. [DOI] [PubMed] [Google Scholar]
Wang 2010
- Wang Z, Chen J‐Q, Cao Y‐F. Systematic review of D2 lymphadenectomy versus D2 with para‐aortic nodal dissection for advanced gastric cancer. World Journal of Gastroenterology 2010;16(9):1138‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wronski 2007
- Wronski K, Okraszewski J, Gorski A, Kunecki M, Bocian R. Is it necessary to perform routine drainage of the peritoneal cavity after a gastrectomy caused by a neoplasm?. Wspolczesna Onkologia 2007;11(10):487‐91. [Google Scholar]
Yeh 2005
- Yeh CY, Changchien CR, Wang JY, Chen JS, Chen HH, Chiang JM, et al. Pelvic drainage and other risk factors for leakage after elective anterior resection in rectal cancer patients: a prospective study of 978 patients. Annals of Surgery 2005;241(1):9‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]


