Abstract
Background
Because of the disability associated with surgery for anal fissure and the risk of incontinence, medical alternatives for surgery have been sought. Most recently, pharmacologic methods that relax the anal smooth muscle, to accomplish reversibly what occurs in surgery, have been used to obtain fissure healing.
Objectives
To assess the efficacy and morbidity of various medical therapies for anal fissure.
Search methods
Search terms include "anal fissure randomized". Timing from 1966 to August 2010. Further details of the search below.
Selection criteria
Studies in which participants were randomized to a non‐surgical therapy for anal fissure. Comparison groups may include an operative procedure, an alternate medical therapy or placebo. Chronic fissure, acute fissure and fissure in children are included in the review. Atypical fissures associated with inflammatory bowel disease or cancer or anal infection are excluded.
Data collection and analysis
Data were abstracted from published reports and meeting abstracts, assessing method of randomization, blinding, "intention to treat" and drop‐outs, therapies, supportive measures (applied to both groups), dosing and frequency and cross‐overs. Dichotomous outcome measures included Non‐healing of the fissure (a combination of persistence and recurrence), and Adverse events (including incontinence, headache, infection, anaphylaxis). Continuous outcome measures included measures of pain relief and anorectal manometry.
Main results
In this update 23 studies including 1236 participants is added to the 54 studies and 3904 participants in the 2008 publication, however 2 studies were from the last version reclassified as un included, so the final number of participants is 5031.
49 different comparisons of the ability of medical therapies to heal anal fissure have been reported in 75 RCTs. Seventeen agents were used (nitroglycerin ointment (GTN), isosorbide mono & dinitrate, Botulinum toxin (Botox), diltiazem, nifedipine (Calcium channel blockers or CCBs), hydrocortisone, lignocaine, bran, minoxidil, indoramin, clove oil, L‐arginine, sitz baths, sildenafil, "healer cream" and placebo) as well as Sitz baths, anal dilators and surgical sphincterotomy. GTN was found to be marginally but significantly better than placebo in healing anal fissure (48.9% vs. 35.5%, p < 0.0009), but late recurrence of fissure was common, in the range of 50% of those initially cured. Botox and CCBs were equivalent to GTN in efficacy with fewer adverse events. No medical therapy came close to the efficacy of surgical sphincterotomy, though none of the medical therapies in these RCTs were associated with the risk of incontinence.
Authors' conclusions
Medical therapy for chronic anal fissure, currently consisting of topical glyceryl trinitrate, botulinum toxin injection or the topical calcium channel blockers nifedipine or diltiazem in acute and chronic fissure and fissure in children may be applied with a chance of cure that is marginally better than placebo. For chronic fissure in adults all medical therapies are far less effective than surgery. A few of the newer agents investigated show promise based only upon single studies (clove oil, sildenifil and a "healer cream") but lack comparison to more established medications.
Plain language summary
Non surgical therapy for anal fissure.
Anal fissure is a painful ulcer usually occurring in the posterior midline of the skin just outside the entry to the rectum. Its persistence is due to spasm of the internal sphincter muscle. The typical pain of this condition is pain on moving one's bowels that persists for some time afterward. Relief with healing of chronic fissures until very recently has been achieved by surgical procedures aimed at ablation of the sphincter spasm. Because of the risk of incontinence resulting from surgery, medical alternatives for surgery have been sought. Among the older medications, bran is effective in preventing recurrence of acute fissure. Local application of muscle relaxing therapy is effective in healing chronic anal fissure, though not as well as surgery, and with considerable risk of adverse events during therapy. There is a Cochrane review related to this review dealing only with surgical procedures.
Summary of findings
Background
Anal fissure is an ulcer in the squamous epithelium of the anus located just distal to the muco‐cutaneous junction and usually in the posterior midline. It typically causes pain during defecation and for one to two hours afterwards (Goligher 1975). Atypical fissures may be multiple or off the midline, or be large and or irregular. These may be caused by inflammatory bowel disease, local or systemic malignancy, venereal infection, trauma, tuberculosis, or chemotherapy. The etiology of the typical or benign fissure is not so clear, nor are there accepted methods for fissure prevention. The most consistent finding in typical fissures is hypertonia of the internal anal sphincter, which is so severe that the pain caused by fissure is thought to be due to ischemia (Schouten 1994). Relief of the spasm has been associated with relief of pain and healing of the fissure without recurrence. Historically the most common approach for relieving the spasm is surgical. Operative techniques commonly used for fissure in ano include: anal stretch, open lateral sphincterotomy, closed lateral sphincterotomy, posterior midline sphincterotomy and to a lesser extent dermal flap coverage of the fissure. Morbidity from these procedures, being principally incontinence, was once thought to be extremely rare (Abcarian 1980), but has been substantial in some recent reports (Garcia‐Aguilar 1996), generating enthusiasm for therapies that do not involve sphincter division. A recent Cochrane review has assessed the efficacy and morbidity of operative therapy for anal fissure Nelson 2006. In this review non‐operative approaches will be addressed and assessed.
Objectives
To assess the efficacy and morbidity of various medical therapies for anal fissure.
Methods
Criteria for considering studies for this review
Types of studies
Studies in which participants were randomized to a non‐surgical therapy for anal fissure are the focus of this review. Comparison groups in each of these studies may include an operative procedure, an alternate medical therapy or placebo.
Types of participants
Participants in this review are principally adult patients with chronic anal fissure. Patients with acute fissures and fissure in children are also included in some reports, and are the exclusive focus of others, but atypical fissures (multiple, irregular, off the midline or not associated with hypertonia of the anal sphincter, often associated with inflammatory bowel disease and cancer), were not included in any RCT and will not be included in this analysis. Chronic fissure has both anatomic and temporal definitions. Chronicity is inferred with a history of pain lasting more than 4 weeks or with pain of less duration but similar episodes in the past. Physical findings of chronicity include a sentinel pile at the distal margin of the fissure, heaped up edges of the fissure, visible sphincter fibers at the base of the fissure, or an inflammatory polyp at the inner margin of the fissure. Any single sign or symptom of chronicity is sufficient to define chronicity. It is not certain whether fissure in children is exactly comparable to chronic fissure in adults, or that chronic hypertonia of the anal sphincter, hypertrophy and ischemia play a role in its persistence. For that reason surgery has rarely been applied to children with anal fissure and, until recently, laxatives and lubricants have formed the basis of therapy (Goligher 1975). The failure of these medications has led to the investigation of newer therapies in children (Kenny 2001; Oglesby 2001; Sonmez 2002; Tander 1999). Acute anal fissure in adults is thought to precede chronic fissure, to be more analogous to pediatric anal fissure in its pathologic anatomy and, if treated aggressively medically, can be healed preventing the development of chronic fissure. The differentiation between acute and chronic anal fissure is in fact a bit problematic, without much data to support those methods of telling acute from chronic fissure. It may depend largely on how carefully a patient is asked about past episodes of anal pain. Six reports focused exclusively on acute fissure (Antropoli 1999; Jensen 1986; Jensen 1987; McDonald 1983, Gaj 2006, Gupta 2006) and four more report included both patients with acute and chronic fissure (Bacher 1997, Ahmad 2007, Eshghi 2007, Yakoot 2009).
Types of interventions
The specific non‐surgical therapies tested in the identified studies, reviewed in this study, include nitroglycerin ointment or dermal patch ‐ also known as NTG, GTN or glyceryl trinitrate (or analogues such as isosorbide dinitrate), botulinum toxin injection (Botox), anal dilators, calcium channel inhibitors (CCBs) delivered as ointment or tablets (diltiazem or nifedipine), bulk aperients (bran or other forms of fiber), hydrocortisone or topical anaesthetic ointments, principally lignocaine and clove oil, an amino acid (L‐arginine), sitz baths and three additional smooth muscle relaxants, indoramin, sildenafil and minoxidil. In some reports the medical therapy was compared to the outcome of the gold standard therapy for anal fissure, partial lateral internal sphincterotomy. In some cases the comparisons were to placebo, others to standard palliative medical therapy and in others two new therapies were directly compared. Placebo therapy in most reports meant "best supportive care", which might include fiber supplements, Sitz baths or lubricants, applied sometimes equally to both groups (e.g. Chaudhuri 2001), and sometimes only to the control group (Perrotti 2002; Antropoli 1999).
Types of outcome measures
The two most broadly used outcomes of therapy were persistence of the fissure (which is used synonymously with persistence of anal pain, the measure of efficacy) and post treatment minor incontinence (the most commonly reported morbidity of operations for anal fissure; used synonymously with incontinence to flatus or anal seepage). Several authors have treated persistence and recurrence as separate outcomes. The natural history of anal fissure makes this a difficult distinction. Anal fissures typically wax and wane, even with morphologic healing occurring between "attacks". So a recurrence of pain and the anatomic finding of a fissure after a period of healing and amelioration of symptoms following treatment may be a "recurrence" or "persistence". The differentiation seems trivial and in either case amounts to treatment failure. In addition, more major defecation dysfunction was assessed, including incontinence to liquid and solid stool. Other adverse events analysed specific to the medical therapies included headache with nitroglycerin, allergy or anaphylaxis in patients having repeated botulinum toxin injection or pain or infection at the injection site. Though mortality or haemorrhage have not been reported in this condition, these were sought. Additional endpoints frequently reported are relief of pain and anorectal manometric measurement of sphincter resting and squeeze pressure. Both these endpoints are difficult to compare between studies since different scales, equipment and standards of observation were used in each of the studies in which they were employed. Since anal fissure has such a distinctive appearance, its healing is the most objective and standardizable measure of efficacy available and will be the principal measure of effect in the meta‐analysis. The timing of the observation is problematic because of the cyclical nature of fissure described above. The best studies had follow‐up periods that lasted over a year, though it was unusual for 100% of study participants to be followed that long in any study.
Search methods for identification of studies
The National Library of Medicine online PubMed search engine (www.nlm.nih.gov) was used to locate all published reports using the key words: "anal fissure, randomized". English language was not a restriction in the search. In this review PubMed was searched from 1966 to January, 2010. The list of cited references in all included reports also were used to find additional comparative studies. The Cochrane Library was searched in May 2010 (issue 2), and the CCCG specialised trials register was searched in May 2010. In addition proceedings of relevant meetings were screened for presentations not yet in print, focusing on the last three years and prospectively. Such meetings included the annual meetings of the American Society of Colon & Rectal Surgeons, The Int. Soc. of Univ. Colon & Rectal Surgeons, Digestive Disease Week and other regional colorectal surgical societies. Authors of some published reports were contacted, querying their awareness of ongoing studies.
The following search strategy was used to locate studies in the NLM, EMBASE and CLIB
MEDLINE 01/2010:
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. clinical trial.sh.
6. randomly.ab.
7. trial.ti.
8. 1 or 2 or 3 or 4 or 5 or 6 or 7
9. humans.sh.
10. 8 and 9
11. exp Fissure in Ano/
12. anal fissure*.mp. [mp=title, original title, abstract, name of substance word, subject heading word]
13. 11 or 12
14. 10 and 13
EMBASE 01/2010:
1. randomized controlled trial/
2. randomisation/
3. controlled study/
4. multicenter study/
5. phase 3 clinical trial/
6. phase 4 clinical trial/
7. double blind procedure/
8. single blind procedure/
9. ((single* or double* or treble* or triple*) adj (blind* or mask*)).ti,ab.
10. (random* or cross* over* or factorial* or placebo* or volunteer*).ti,ab.
11. 6 or 3 or 7 or 9 or 2 or 8 or 4 or 1 or 10 or 5
12. "human*".ti,ab.
13. (animal* or nonhuman*).ti,ab.
14. 13 and 12
15. 13 not 14
16. 11 not 15
17. exp anus fissure/
18. anal fissure*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
19. 18 or 17
20. 16 and 19
Search strategy CLib 08/2010
| ID | Search | Hits | Edit | Delete |
| #1 | MeSH descriptor Fissure in Ano, this term only | 140 | edit | delete |
| #2 | (ulcer) and (anus or anal) | 29 | edit | delete |
| #3 | (anal fissure*) | 223 | edit | delete |
| #4 | (#1 OR #2 OR #3) | 248 | edit | delete |
| #5 | (non surg* or non operat*) | 30820 | edit | delete |
| #6 | (medical therap*) | 243011 | edit | delete |
| #7 | (#5 OR #6) | 256574 | edit | delete |
| #8 | (#4 AND #7) | 168 | edit | delete |
Data collection and analysis
All reports in which there was a direct comparison between at least two treatments for anal fissure, at least one of which was non‐surgical, were reviewed and when more than one report exists for any given pair, that report was included in the meta‐analysis. If crude data were not presented in the report, the authors were contacted and crude data obtained. Revman is used to evaluate randomized studies only. To assess homogeneity, Revman was used as well. Sensitivity analyses were done using the following screens:
22‐1 as 1‐1, for GTN, exclusion of studies with placebo response rates more than 2 standard deviations below the mean‐placebo response rate (Lund 1997). 12‐1 as 1‐1, but only children 13‐1 as 1‐1, but only adults 22‐2 as 22‐1 for children, excluding studies with very low placebo response rates or high drop out rates 22‐3 as 22‐1 for adults, excluding studies with very low placebo response rates or high drop out rates, 22‐4 as 17‐1 exclusions (Sonmez 2002) for the same criteria as 22‐1, but for lignocaine instead of GTN 22‐5 as 3‐1, but excluding a study that had > 90% healing rate for Botox, to investigate heterogeneity (Brisinda 1999). 22‐6 as 16‐1, excluding (Mishra 2005), a clinical outlier, to investigate heterogeneity. 22‐7 as 1‐1, GTn vs. Placebo, looking only at the 3 largest studies (Altomare 2000, Bailey 2002, Scholefield 2003), to investigate heterogeneity. 29‐1 as 16‐1, but only studies with > 1 year follow up for most of their patients (Arroyo 2005, Libertiny 2002, Parellada 2004).
Results
Description of studies
75 randomized controlled trials were included in this review. The data available from some of these studies were sparse since they exist so far only in abstract from medical meeting booklets (Gecim 2001; Oglesby 2001). True cross‐over designs were rare and were usually limited to treatment failures (Bassotti 2000; Brisinda 1999). More frequently, treatment failures received partial lateral internal sphincterotomy, the gold standard therapy for anal fissure whether (Evans 2001; Libertiny 2002; Oettle 1997) , or not (Altomare 2000; Gough 1983; Jonas 2001; McDonald 1983; Zuberi 2000), if sphincterotomy was an arm of the protocol. The total number of patients encompassed by these 75 RCTs was 5031. This is the second update of this review,which is rapidly growing. Of the 23 new studies, 15 are GTN based. Insofar as GTN may be considered the gold standard medical therapy, this is appropriate, though its superiority to placebo is marginal enough that placebo controlled trials of new medications are justifiable. Six of the trials repeat previously published comparisons (Brisinda 2007, Jawaid 2009, Suknaic 2008, Shrivastava 2007, Nasr 2010, Siddique 2008,). Four investigate new medications (Elwkeel 2007, Eshghi 2007,Moghimi 2006, Yakoot 2009). The remainder investigated new procedures such as methods of dilation and combinations of previously published therapies.
Risk of bias in included studies
By far the most prevalent quality problem encountered in this review was failure to analyze results of the investigations on an "intention to treat" basis. Authors' conclusions were based far more often on broken randomizations. Fortunately crude data were presented in almost all of the reports so that, in this meta‐analysis, "intention to treat" will be used. It is noted when adherence to this technique is not possible in both situations when this occurs (Tander 1999; Kenny 2001), both studies focused upon children. The technique of randomization was specified in 46.7 % of the RCTs. Allocation concealment was specified in 74.7% of the studies. Blinding of the person rating outcome was used in 46.7 % of investigations, though it was clearly not possible when, for instance, surgery was compared to ointment (Figure 1; Figure 2). Drop‐outs were less frequent in this review than in some reports of the Surgery for Anal Fissure Nelson 2011 review, and when they occurred, they were counted as treatment failures in the meta‐analysis. Two reports had them at a high frequency (Ho 2005; Weinstein 2004). One major problem arose in two studies comparing surgery to GTN (Evans 2001; Richard 2000) regarding estimates of the efficacy of surgical sphincterotomy in curing anal fissure. Using "intention to treat" and categorizing all unevaluated patients as treatment failures, a number of individuals were categorized as treatment failures because they did not get a sphincterotomy after randomization, due either to refusal or the fissures were found to be healed in these individuals. The surgical procedures therefore were terminated and they were excluded from follow‐up. This was an error on both authors' parts. The individuals, if sphincterotomy could not be rationalized at the first setting, should have had continued follow‐up and sphincterotomy applied if needed at a later date. Even if the operation were never done, their outcomes should have been recorded. To find a healed fissure at surgery is not an unexpected course of events, because of the waxing/waning nature of anal fissures, and indeed the rate at which this occurred in these reports (Evans 2001; Richard 2000) approximates the expected placebo response rate (35%). The result of this error is an underestimate of the efficacy of surgery in curing fissure (71% and 74% respectively, compared to > 95% in most reports) , though in the meta‐analysis, surgery still fared much better than medical alternatives (Comparison & Data Tables (CDT) 2‐1, 16‐1, 22‐6, 29‐1). When, in some reports, placebo response rates for fissure healing were far below the expected level, quality concern also arose. This was especially true in four reports (Lund 1997; Sonmez 2002; Perrotti 2002, Moghimi 2006), in which the placebo response rate was far less than 10%, more than two standard deviations below the mean response rate for the entire group in which they resided: placebo, or the overall placebo response rate for all studies ‐ 34%. Exclusion of these reports did not have a significant effect on the outcome of GTN vs. Placebo in adults (13‐1, 22‐3) but it did in GTN vs. Placebo in children (12‐1, 22‐2). Both ends of the Perrotti comparison (Perrotti 2002) (CDT 10‐1) are outliers, nifedipine appearing far more efficacious than in other studies, and hydrocortisone falling well below the placebo response rate, and so this a result is not to be given any weight.
1.
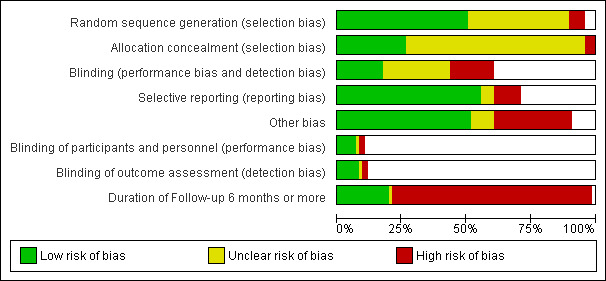
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
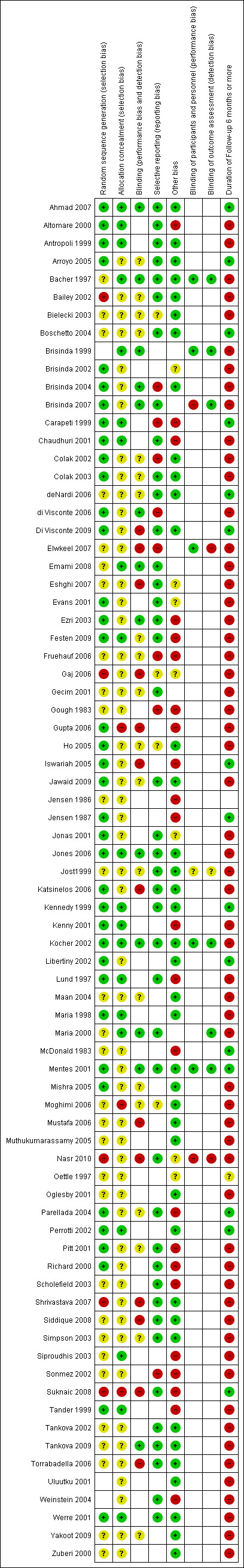
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
There are a number of interventions that have only been investigated in single trials, with few patients and short follow up. They include indoramine (Pitt 2001), arginine (Eshghi 2007), sildenifil (Moghimi 2006), Sitrz baths (Gupta 2006), "healer cream", the precise nature of which is not specified by the authors (Yakoot 2009), oral diltiazem (Jonas 2001), botox injection site (Maria 2000), anal injectors for GTN (Torrabadella 2006), minoxidil (Muthukumarassamy 2005), home dilation (Gaj 2006), clove oil (Elwkeel 2007) The Moghimi trial was the only one to analyse the efficacy of sildenafil vs placebo. Significant statistical heterogeneity was encountered in 4 primary analyses (CDTs 1‐1 GTN vs. Placebo; 3‐1 GTN vs. Botox; 7‐1 Botox vs. Placebo; 16‐1 Any Operation vs. Any Medical Therapy ). The sensitivity analyses described above were done to investigate the source of the heterogeneity.
Statistical heterogeneity was found in only four comparisons; GTN versus placebo (Analysis 1.1), All medical therapies versus surgery (Analysis 16.1), GTN versus botox (Analysis 3.1) and botox versus placebo (Analysis 7.1). The first of these, being the most important comparison in this review, was investigated in a number of sensitivity analyses to locate possible clinical differences such as age, duration of follow up and study size (Analysis 12.1, Analysis 13.1, Analysis 22.1, Analysis 22.3,Analysis 22.4, Analysis 22.7 ), with no success. A sensitivity analysis of (Analysis 16.1), eliminating (Mishra 2005) resolved the heterogeneity without altering the summary statistics. In a sensitivity analysis of (Analysis 3.1), eliminating studies with abnormally high non‐healing rates (Analysis 22.5) did not completely resolve the heterogeneity. The source of the heterogeneity in (Analysis 7.1) is clearly the study by (Siproudhis 2003), though clinical justification for elimination of this study were not found.
1.1. Analysis.
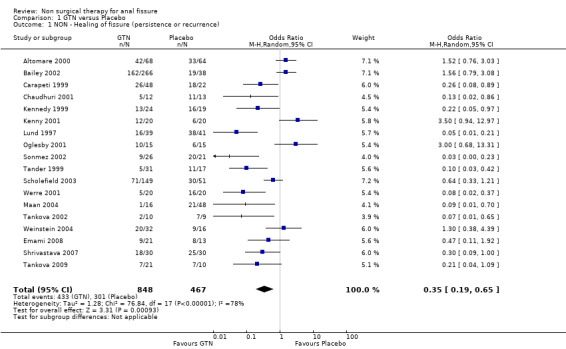
Comparison 1 GTN versus Placebo, Outcome 1 NON ‐ Healing of fissure (persistence or recurrence).
16.1. Analysis.
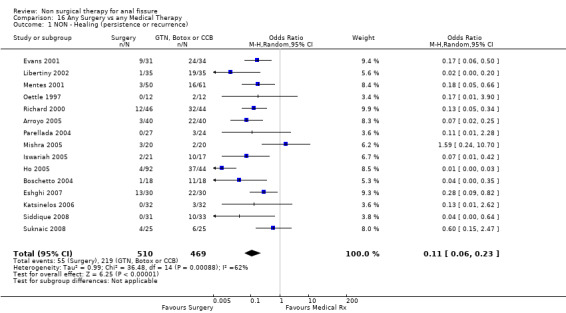
Comparison 16 Any Surgery vs any Medical Therapy, Outcome 1 NON ‐ Healing (persistence or recurrence).
3.1. Analysis.
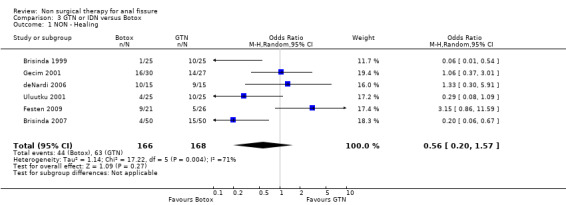
Comparison 3 GTN or IDN versus Botox, Outcome 1 NON ‐ Healing.
7.1. Analysis.
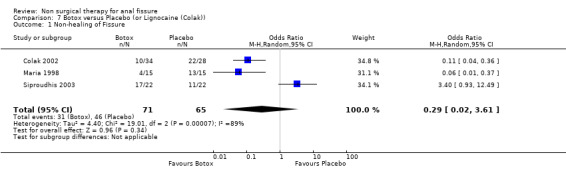
Comparison 7 Botox versus Placebo (or Lignocaine (Colak)), Outcome 1 Non‐healing of Fissure.
12.1. Analysis.
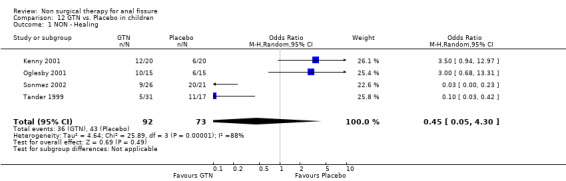
Comparison 12 GTN vs. Placebo in children, Outcome 1 NON ‐ Healing.
13.1. Analysis.
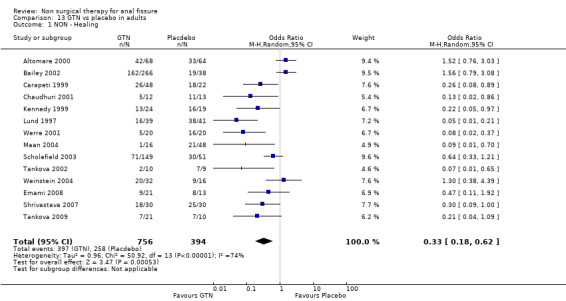
Comparison 13 GTN vs placebo in adults, Outcome 1 NON ‐ Healing.
22.1. Analysis.

Comparison 22 Sensitivity analyses, Outcome 1 Sensitivity analysis: Excluding GTN/Placebo RCTs with very low placebo response rates (<10%): NON‐healing.
22.3. Analysis.
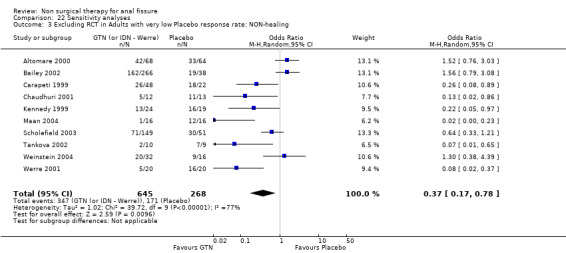
Comparison 22 Sensitivity analyses, Outcome 3 Excluding RCT in Adults with very low Placebo response rate: NON‐healing.
22.4. Analysis.
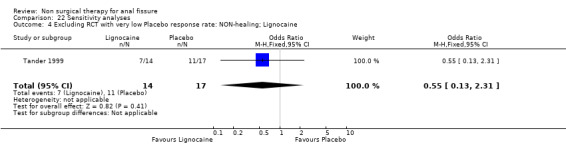
Comparison 22 Sensitivity analyses, Outcome 4 Excluding RCT with very low Placebo response rate: NON‐healing; Lignocaine.
22.7. Analysis.
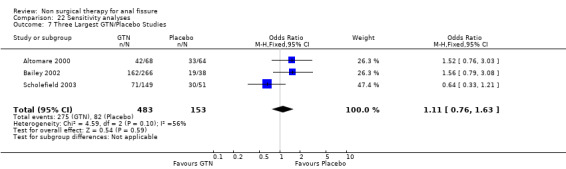
Comparison 22 Sensitivity analyses, Outcome 7 Three Largest GTN/Placebo Studies.
22.5. Analysis.
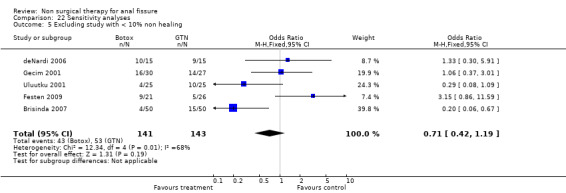
Comparison 22 Sensitivity analyses, Outcome 5 Excluding study with < 10% non healing.
Combining all CDTs in which a placebo is used as the comparison group, the healing rate in the placebo group is 33%, a level of response that is fairly uniform across studies (standard deviation < 10%). The medications being tested in this meta‐analysis must have their efficacy viewed in this context of placebo effect and also in the context of a cure rate of surgery that exceeds 95% (Nelson 2001). When a reported placebo cure rate (or inversely non‐healing rates) is less than 10% (or exceeds 90%), the quality of that study must be questioned (Lund 1997; Sonmez 2002, Perrotti 2002, Moghimi 2006) and analyses conducted both with and without (Analysis 22.1, Analysis 22.2; Analysis 22.3; Analysis 22.4) inclusion of these studies. The high reported placebo response rate is most likely due to the waxing/waning nature of anal fissure, so a reported fissure healing only 6 weeks after an intervention may have had little to do with the intervention. This effect is best demonstrated in the (Arroyo 2005) trial in which a botox group had in 40 patients, six recurrences at two months, six more at six months and 10 more at one year. Thus a cure rate of 85% at two months became 45% at one year. Duration of follow up therefore is a major quality issue in fissure trials and only 20% of the included trials reported follow‐up data of 6 months or more (Figure 1; Figure 2).
22.2. Analysis.
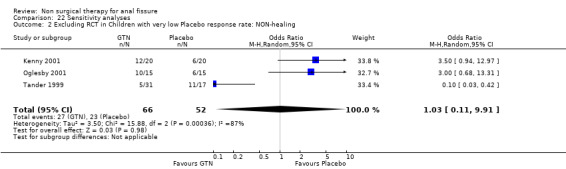
Comparison 22 Sensitivity analyses, Outcome 2 Excluding RCT in Children with very low Placebo response rate: NON‐healing.
Effects of interventions
Summary of findings for the main comparison. GTN versus Placebo for anal fissure.
| GTN versus Placebo for anal fissure | ||||||
| Patient or population: patients with anal fissure Settings: Intervention: GTN versus Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | GTN versus Placebo | |||||
| NON ‐ Healing of fissure (persistence or recurrence) Follow‐up: median 2 months | Study population | OR 0.35 (0.19 to 0.65) | 1315 (18 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 645 per 1000 | 388 per 1000 (256 to 541) | |||||
| Moderate | ||||||
| 674 per 1000 | 420 per 1000 (282 to 573) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 RANDOMIZATION SELDOM SPECIFIED AND FOLLOW UP WAY TOO SHORT 2 VARIABLE RESULTS
Summary of findings 2. Any Surgery compared to any Medical Therapy for anal fissure.
| Any Surgery compared to any Medical Therapy for anal fissure | ||||||
| Patient or population: patients with anal fissure Settings: Intervention: Any Surgery Comparison: any Medical Therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Any Medical Therapy | Any Surgery | |||||
| NON ‐ Healing (persistence or recurrence) Follow‐up: median 2 months | Study population | OR 0.11 (0.06 to 0.23) | 979 (15 studies) | ⊕⊕⊕⊕ high1,2 | ||
| 467 per 1000 | 88 per 1000 (50 to 168) | |||||
| Moderate | ||||||
| 543 per 1000 | 116 per 1000 (67 to 215) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Randomization method seldom specified and follow up too short 2 Consistant large effect of surgery across all but one study.
A total of 75 different Forrest plots are contained in this meta‐analysis to describe the ability of medical therapies to heal anal fissure that have been reported in 75 RCTs. The total number of pharmacologic agents employed includes 15 (glyceryl trinitrate (GTN), isosorbide mono & dinitrate, botulinum toxin (botox), the calcium channel blockers (CCB) diltiazem and nifedipine, hydrocortisone, lignocaine, bran, indoramin, minoxidil, clove oil, L‐arginine, sildenafil, "healer cream" and placebo) as well as dilators, sitz baths and surgical sphincterotomy. One RCT compared different GTN preparations on a manometric assessment of the anal canal in patients with fissure without assessing healing (Bassotti 2000). Many of these RCTs can be divided temporally into two groups. The first are those published 1997 and before. In these the test medication principally lubricates, numbs or decreases inflammation in the anal canal. In those published 1997 and after medications that are thought to decrease the hypertonia of the anal canal muscles ‐ specifically the internal anal sphincter, though comparisons in children lagged a bit beyond this date (Analysis 17.1). Lignocaine, bran and hydrocortisone are generally regarded as no more curative than placebo today. Though they were investigated with some success, especially for acute fissure in the 1980s (Jensen 1986; Jensen 1987), they have fared no better than placebo in more recent trials (Analysis 14.1, Analysis 15.1, Analysis 31.1, Analysis 39.1 )
17.1. Analysis.
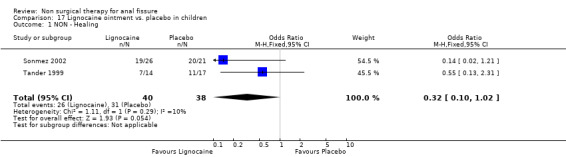
Comparison 17 Lignocaine ointment vs. placebo in children, Outcome 1 NON ‐ Healing.
14.1. Analysis.
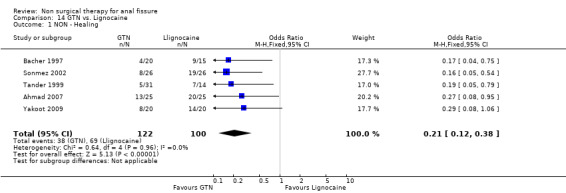
Comparison 14 GTN vs. Lignocaine, Outcome 1 NON ‐ Healing.
15.1. Analysis.
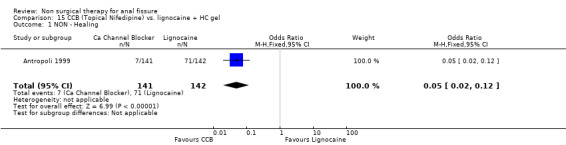
Comparison 15 CCB (Topical Nifedipine) vs. lignocaine + HC gel, Outcome 1 NON ‐ Healing.
31.1. Analysis.
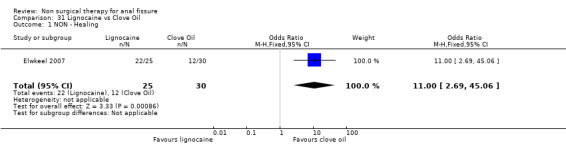
Comparison 31 Lignocaine vs Clove Oil, Outcome 1 NON ‐ Healing.
39.1. Analysis.
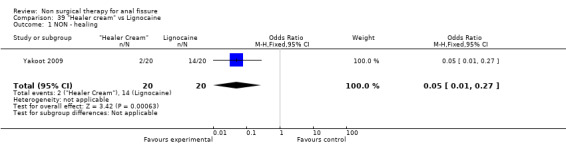
Comparison 39 "Healer cream" vs Lignocaine, Outcome 1 NON ‐ healing.
GTN vs. Placebo
The largest study group is GTN compared with placebo (Analysis 1.1; Analysis 1.2). There are 18 RCTs (1315 patients) of which 4 include only children (165 children). GTN is a vasodilator smooth muscle relaxant used traditional to dilate coronary arteries. In a diluted form, 0.2% ‐ 0.4%, it is applied directly to the anus to dilate the internal sphincter two to three times daily for six to eight weeks. GTN is found to be significantly better than placebo in healing anal fissure in the combined analysis and in all sensitivity analyses related to adults (13‐1, 22‐3), except when only the 3 largest studies are considered (Analysis 22.7), the only comparison in adults that does not have statistical heterogeneity. In children the significant benefit of GTN therapy is lost when a study with an abnormally low placebo response rate is excluded (Sonmez 2002; Analysis 22.2). The overall healing rate for GTN in these 18 studies is 48.9 % and the placebo healing rate reported is 35.5%, so the advantage of GTN, though significant, is not great. All studies looked only at chronic anal fissure and all studies were plagued by short follow up. Two case series with long follow up have reported recurrence rates of patients apparently cured of fissure by GTN of 51% (Jonas 2002) and 67% (Graziano 2001)
1.2. Analysis.
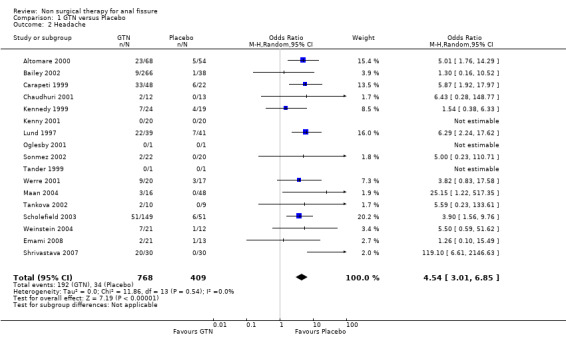
Comparison 1 GTN versus Placebo, Outcome 2 Headache.
GTN vs. Other Comparisons: Botox, CCBs, Lignocaine, home dilators, Surgery
Six other comparisons are made with GTN in this review: vs. Botox, CCBs, Lignocaine, "healer cream", home dilators and partial lateral internal sphincterotomy. There was no statistical advantage to either Botox or CCBs (or disadvantage) when compared to GTN (Analysis 3.1, 334 patients; Analysis 4.1, 365 patients) and the statistical heterogeneity seen in the Botox comparison (Analysis 3.1) diminishes a biy when a single outlier study is excluded, due to a response rate in excess of 90% (Brisinda 1999; Analysis 22.5). The heterogeneity does not disappear unless all studies favoring botox are excluded (Brisinda 2007, Uluutku 2001) Comparing GTN to Lignocaine (Analysis 14.1) there is a statistical advantage to GTN therapy, confirming the placebo status of lignocaine, i.e., pain relief alone is insufficient to heal a fissure (see below). Patients having an operation for anal fissure have a far greater likelihood of cure than after GTN therapy (6 studies, 343 patients: Analysis 2.1, Analysis 16.1, Analysis 22.6 ) a cure that will securely be maintained over time (Analysis 29.1, 204 patients). Similarly all studies looked at chronic anal fissure and with short follow up, except for di Visconte 2006, Libertiny 2002, and Parellada 2004 (Analysis 29.1), and (deNardi 2006) (Analysis 3.1 ). In (Analysis 30.1, 72 patients) GTN is compared to self anal dilation at home, not classified as anal dilator therapy in other studies (where it was done in surgery: Boschetto 2004, Gough 1983, McDonald 1983). In this analysis there was statistical advantage to dilator therapy over GTN. Receiving both together also demonstrated a benefit (Analysis 43.1). In addition, there was significantly more headaches in the GTN group and no reported minor incontinence in either group.
4.1. Analysis.
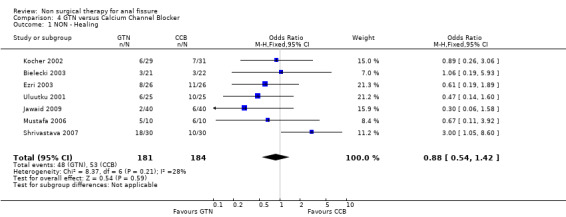
Comparison 4 GTN versus Calcium Channel Blocker, Outcome 1 NON ‐ Healing.
2.1. Analysis.
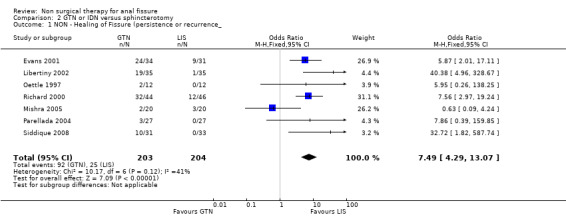
Comparison 2 GTN or IDN versus sphincterotomy, Outcome 1 NON ‐ Healing of Fissure (persistence or recurrence_.
22.6. Analysis.
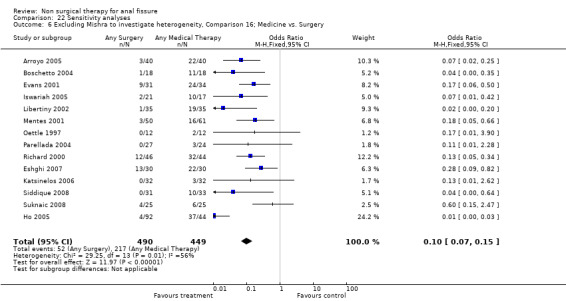
Comparison 22 Sensitivity analyses, Outcome 6 Excluding Mishra to investigate heterogeneity, Comparison 16; Medicine vs. Surgery.
29.1. Analysis.
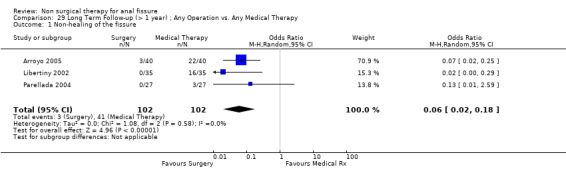
Comparison 29 Long Term Follow‐up (> 1 year) ; Any Operation vs. Any Medical Therapy, Outcome 1 Non‐healing of the fissure.
30.1. Analysis.
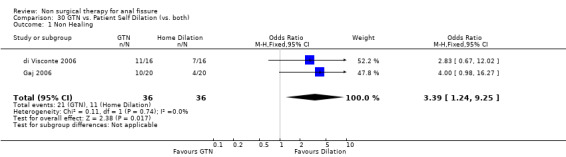
Comparison 30 GTN vs. Patient Self Dilation (vs. both), Outcome 1 Non Healing.
43.1. Analysis.

Comparison 43 GTN vs GTN + cryothermal dilators, Outcome 1 NON ‐ Healing.
GTN Dose and location of application
Three studies looked at the ability of various doses of topical GTN ointment to cure anal fissure ( 278 patients; Analysis 27.1) and found that dose made no difference in cure, doses varying between 0.05% and 0.4% GTN. One study compared topical vs intra‐anal injection of GTN (Analysis 35.1, 22patients) with no difference in results. Two studies compared GTN applied topical around the anus or by dermal patch at a distant location (Analysis 5.1, 131 patients). No difference was seen in efficacy or risk of adverse events (Analysis 5.2), being headache with GTN. This is a very significant analysis.
27.1. Analysis.
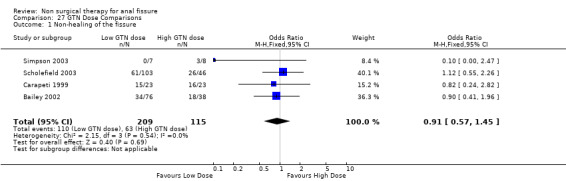
Comparison 27 GTN Dose Comparisons, Outcome 1 Non‐healing of the fissure.
35.1. Analysis.
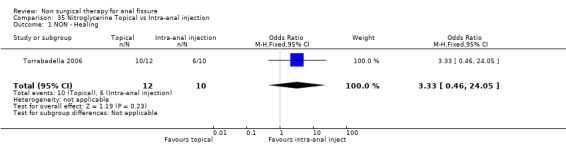
Comparison 35 Nitroglycerine Topical vs Intra‐anal injection, Outcome 1 NON ‐ Healing.
5.1. Analysis.
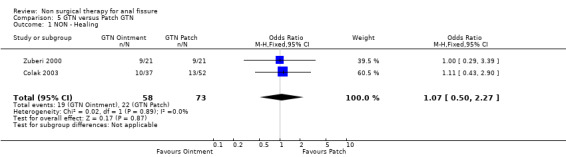
Comparison 5 GTN versus Patch GTN, Outcome 1 NON ‐ Healing.
5.2. Analysis.

Comparison 5 GTN versus Patch GTN, Outcome 2 Headache.
GTN Headache
The principal adverse event related to GTN use, besides lack of efficacy and recurrence of fissure, is headache; a headache so severe the it causes many patients to abandon therapy. In all comparison of GTN with other therapies, GTN was associated with a statistical increased risk of headache (Table 47). The risk of headache in the studies combined is 30%. Headache was also reported as a problem effecting compliance with Indoramin (Pitt 2001), oral Nifedipine (Ho 2005) and oral Diltiazem (Jonas 2001).
1. Adverse Events of interventions.
| HEADACHE RATE | INCONTINENCE RATE | |
| GTN all studies | 434/1425; 30.05% | |
| SURG all studies | 3/253; 1.2% | 37/378; 9.8% |
| ARG | 0/30 | |
| ISMN | 2/41; 4.9% | |
| HEALER CREAM | 1/20; 5% | |
| BTX | 7/138; 5.1% | |
| ORAL DILTIAZEM | 9/24; 37.5% | |
| TOPICAL CCB | 27/169; 16% | |
| INDORAMINE | 7/14; 50% | |
| GTN patch | 25/73; 34.2% | |
| LIGNOCAINE | 4/45; 8.9% | |
| DILATOR | 0/20 | |
| PLACEBO | 36/428; 8.4% |
Botox
Botulinum toxin is thought of principally as a striated muscle relaxant, used to treat muscle hypertonia and cosmetic disorders. For fissure there are many published techniques involving injection of anywhere from 10 to 100 units at various locations around the anal canal, though it is usually applied on either side of the fissure directly into the internal sphincter, a smooth muscle. Botulinum toxin (botox) injection into the internal sphincter curiously was found in combined analyses to be no better or worse than GTN (Analysis 3.1, 334 patients), and surprisingly also no better than placebo (Analysis 7.1, 136 patients), though a sensitivity analysis of this comparison did favour Botox over placebo by excluding (Siproudhis 2003). There is however no clinical reason to exclude (Siproudhis 2003). Botox did not fare as well as surgery in curing fissure (Analysis 8.1, 365 patients, 5 studies). In addition it has been found that recurrence of healed fissure exceeds 50% after one year (Arroyo 2005) in one RCT and 40% in a case series (Minguez 2002). Neither the dose (Analysis 9.1) or the type of Botox (Analysis 23.1) injected has been found to alter healing rates. Both these latter analyses had healing rates far greater than 90%, a level not seen by most investigators, and greatly affected the overall healing rate related to Botox in all studies in which Botox formed one arm (76.8%). Without the studies in these two analyses the overall healing rate was 67.5%. Anaphylaxis has not been reported with repeated Botox use (Brisinda 2002).
8.1. Analysis.
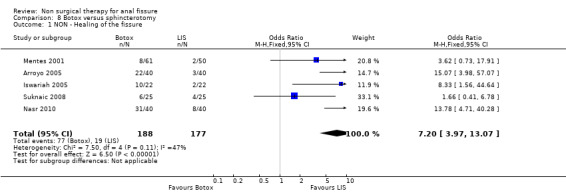
Comparison 8 Botox versus sphincterotomy, Outcome 1 NON ‐ Healing of the fissure.
9.1. Analysis.
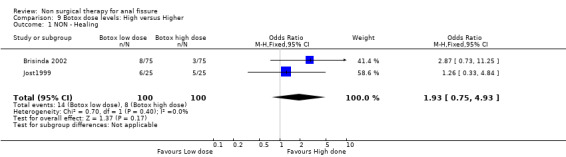
Comparison 9 Botox dose levels: High versus Higher, Outcome 1 NON ‐ Healing.
23.1. Analysis.
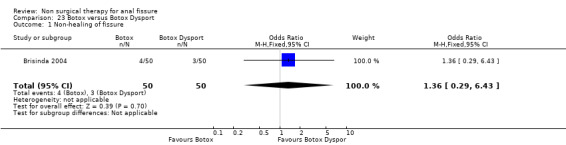
Comparison 23 Botox versus Botox Dysport, Outcome 1 Non‐healing of fissure.
Botox is clearly problematic, working far better in some investigators' hands than others. This may in part be explained by what is perhaps the most interesting study in this update: (Maria 2000). This looks at the effect of injecting botox either posteriorly, where most fissures are located, or anteriorly in the anal canal, with the anterior site being more effective (Analysis 44.1). This result is not widely known.
44.1. Analysis.
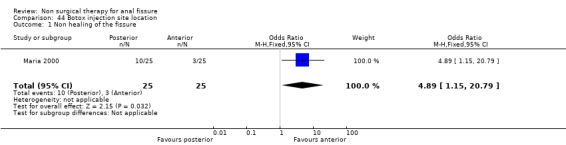
Comparison 44 Botox injection site location, Outcome 1 Non healing of the fissure.
Calcium Channel Blockers (CCBs)
The two drugs used in this classification are diltiazem and nifedipine, both antihypertensive vasodilators, each given for fissure either orally or topically in different studies. In comparison to GTN there was no significant difference in efficacy, though this was a clinically heterogeneous group of studies (Analysis 4.1; 365 patients), with 4 using diltiazem, 1 using nifedipine topically and 2 using nifedipine orally. In a single report there was not a significant benefit to either Botox or CCB (oral nifedipine) (Analysis 28.1; 50 patients). There were insignificant trends that favoured both Botox and GTN over CCBs in the above comparisons. CCBs fared far better in comparison to lignocaine (Analysis 15.1; 283 patients) and hydrocortisone (Analysis 10.1; 110 patients ‐ a study with significant quality issues ‐ see (Perrotti 2002)above in Risk of Bias) ointments. Both these latter studies had cure rates well above all other studies. Cure rates were on the other hand consistently far higher with surgical sphincterotomy than with CCBs (Analysis 24.1, 196 patients), however quality issues are raised with the Katsinelos 2006 study, with its 100% cure rate for surgery and >90% cure rate for topical nifedipine. If excluding this study for sensitivity analysis, we are left with only Ho 2005, with a very high drop out rate (17/41) in the oral nifedipine group due to side effects and continued anal pain. There are no studies with follow‐up over 1 year of CCBs to assess their recurrence rates accurately.
28.1. Analysis.
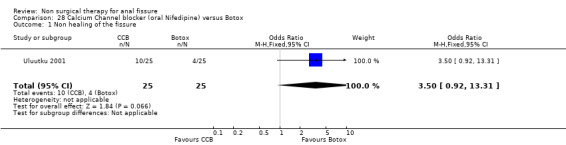
Comparison 28 Calcium Channel blocker (oral Nifedipine) versus Botox, Outcome 1 Non healing of the fissure.
10.1. Analysis.
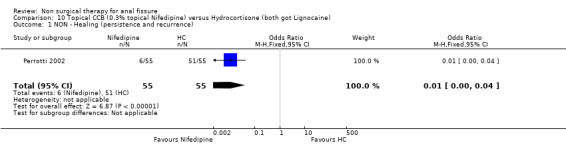
Comparison 10 Topical CCB (0.3% topical Nifedipine) versus Hydrocortisone (both got Lignocaine), Outcome 1 NON ‐ Healing (persistence and recurrence).
24.1. Analysis.

Comparison 24 CCB versus LIS, Outcome 1 Non‐Healing of the Fissure.
Surgery
Special aspects of surgery are the subject of a separate review. As noted above patients having an operation had a much higher cure rate than with any form of medical therapy, this being true in spite of the cure rate being understated in the combined analysis due to drop outs for the surgical group (see above). The combined healing rate is 89% in these analyses and one would expect it to be in excess of 95%. The risk of anal incontinence was 9% in the surgical group and not significantly different from the GTN group (Analysis 2.2; 384 patients). In the Botox comparison, incontinence occurred in 10% (Analysis 8.2), though in one report (Iswariah 2005) the incontinence rates were reported as equal between the Botox and surgery groups, though no numbers are given. In the comparison of Surgery to CCBs (Ho 2005) incontinence scores were lower (better) in the surgery group. Recurrence developed in 3/102 patients with follow‐up more than 1 year (Analysis 29.1) and in no patients in a case series (Rotholtz 2005) after 2 years. Statistical heterogeneity was not a great problem in the surgical comparisons. Where it did occur (Analysis 16.1), exclusion of one small study resolved the heterogeneity (Mishra 2005; Analysis 22.6).
2.2. Analysis.

Comparison 2 GTN or IDN versus sphincterotomy, Outcome 2 Minor Incontinence.
8.2. Analysis.
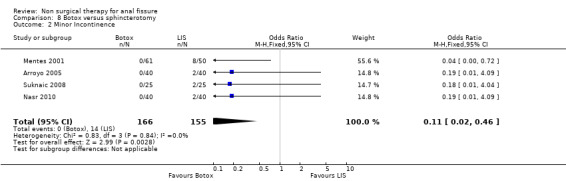
Comparison 8 Botox versus sphincterotomy, Outcome 2 Minor Incontinence.
Indoramin & Minoxidil
These two smooth muscle relaxers were tested in small RCTs and neither found to be effective in healing fissure (Pitt 2001, Muthukumarassamy 2005). The same is true of arginine (Eshghi 2007), whereas results are strong enough for clove oil, sildenifil and healer cream to suggest that further studies of these agents may be worth doing (Elwkeel 2007, Moghimi 2006, Yakoot 2009, Analysis 31.1, Analysis 34.1, Analysis 39.1)
34.1. Analysis.
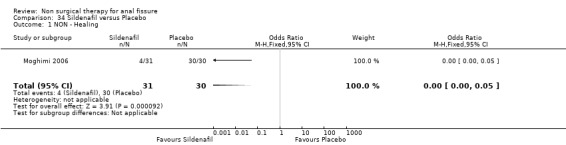
Comparison 34 Sildenafil versus Placebo, Outcome 1 NON ‐ Healing.
Acute Anal Fissure
Acute Fissure was the focus of five reports (Jensen 1986; Jensen 1987; McDonald 1983, Gaj 2006, Gupta 2006) and comprised 2/3rds of the patients in another (Bacher 1997). These are for the most part older studies and/or involving pretty much obsolete therapies: lignocaine, bran, hydrocortisone, sitz baths and dilators (Analysis 6.1, Analysis 19.1, Analysis 20.1, Analysis 21.1, Analysis 30.1, Analysis 33.1) . There is also the only prophylaxis study in this group (Analysis 18.1, 60 patients), in which bran was found to be more effective than placebo in preventing acute fissure recurrence.
6.1. Analysis.
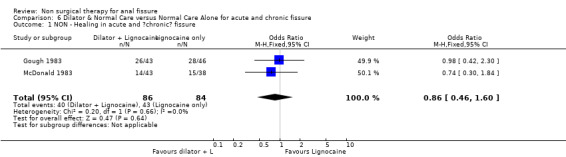
Comparison 6 Dilator & Normal Care versus Normal Care Alone for acute and chronic fissure, Outcome 1 NON ‐ Healing in acute and ?chronic? fissure.
19.1. Analysis.
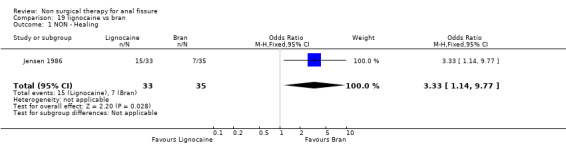
Comparison 19 lignocaine vs bran, Outcome 1 NON ‐ Healing.
20.1. Analysis.
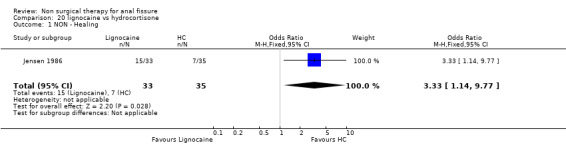
Comparison 20 lignocaine vs hydrocortisone, Outcome 1 NON ‐ Healing.
21.1. Analysis.
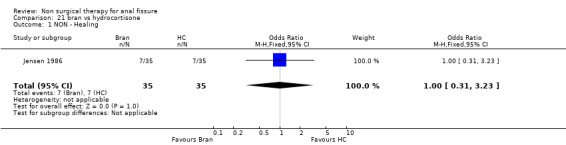
Comparison 21 bran vs hydrocortisone, Outcome 1 NON ‐ Healing.
33.1. Analysis.
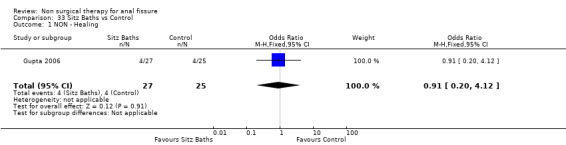
Comparison 33 Sitz Baths vs Control, Outcome 1 NON ‐ Healing.
18.1. Analysis.
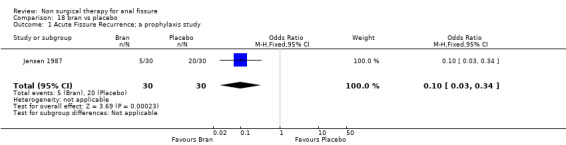
Comparison 18 bran vs placebo, Outcome 1 Acute Fissure Recurrence; a prophylaxis study.
Additional outcomes were often presented, but in scales that differed between reports, making quantitative amalgamation and analyses of these endpoints inadvisable. The outcomes included amount of or time to pain relief, which generally correlated well with fissure healing, and anorectal manometry. The reason for inclusion of manometric measurement was to demonstrate that the test medication could lower sphincter pressure as well as heal the fissure. This test was employed at different times in the course of therapy in almost every report in which it was presented, usually without specifying equipment used or normal ranges or blinding of the investigators as to subject status. Further emphasizing the importance of the placebo effect in these trials, in the original report of medical therapy, 7 of 11 RCTs in which manometry was done (Altomare 2000; Antropoli 1999; Brisinda 1999; Brisinda 2002; Kennedy 1999; Maria 1998; Werre 2001), the resting pressure fell more than 10 millimetres of mercury in the control group during the trial. Significant pain relief was also noted in the control groups during most trials. A golden opportunity was missed in not presenting individual patient data of fissure outcome and manometric data. This could have served to validate a physiologic assessment that is broadly employed, but not previously well validated in this setting. That is, is there an absolute sphincter pressure that is associated with fissure presence and a specific pressure drop associated with fissure healing? Are similar individual pressures and responses encountered in acute fissure or in children?
The Summary of Findings Tables of the two key outcomes of this review are below (Table 1; Table 2). Publication bias is assessed with funnel plots for these two outcomes (Figure 3; Figure 4) showing symmetry but not a clear funnel, especially in Figure 3.
3.
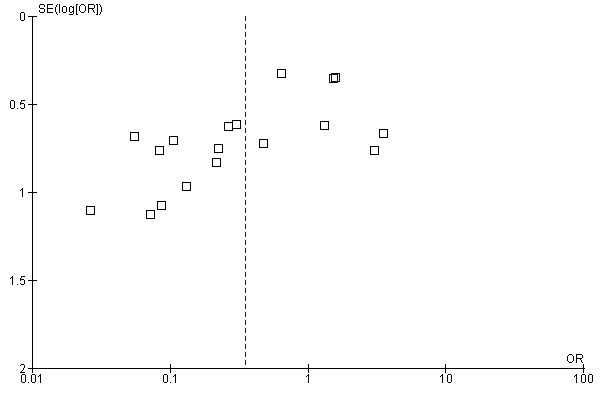
Funnel plot of comparison: 1 GTN versus Placebo, outcome: 1.1 NON ‐ Healing of fissure (persistence or recurrence).
4.
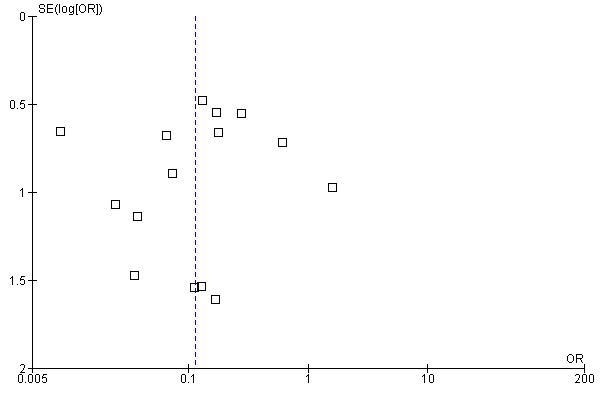
Funnel plot of comparison: 16 Any Surgery vs any Medical Therapy, outcome: 16.1 NON ‐ Healing (persistence or recurrence).
Discussion
Medical therapies applied to anal fissure prior to the use of GTN, Botox and CCBs were generally thought of as short term palliation for fissure symptoms, inefficient in obtaining a long term cure, and were replaced by surgery for the long term management of anal fissure (Goligher 1975; Nelson 2001), except for acute fissure and fissure in children. By the late 1990s, when alternatives to surgery were sought because of cost, time for recovery and risk of incontinence, rather than turn back to these older therapies, newer medications were therefore sought, in each case a medication that is known to relieve hypertonia of the anal sphincter muscle.
This has become a large and very complex review. Because of the large number of comparisons, with 75 Forest plots, an attempt has been made to summarize the results in (Table 48, Table 47). In some cases apparent outstanding results were found due to abberent results in the comparison group, as with sildenafil (Moghimi 2006, Table 48, Analysis 34.1 ) Because of the large number and diffuse nature of the comparisons in this review, the temptation exists to infer significant relationships by analogy, i.e., if "a" is better than "b" and "b" is better than or equivalent to "c", then "a" must be better than "c". So one might infer that GTN, CCBs, Botox, hydrocortisone and bran are all effective therapies for anal fissure because they have been found to be superior to lignocaine in a series of comparisons or equivalent to each other and lignocaine is reported to be nearly equivalent to a placebo. Yet placebo controlled examinations of GTN and Botox would suggest otherwise, and bran and hydrocortisone have not been examined in this regard in many years. In fact the mathematical basis for that assumption has been discussed and found to be lacking (Baker 2003) Cost comparisons were not done in any study, though Botox is known to be quite expensive. In light of the efficacy demonstrated in these analyses, cost needs to be assessed, including the costs related to late recurrence.
2. Nutshell of Effects of Interventions.
| Placebo | GTN only |
GTN injector |
GTN + dilator |
BTX | CCB | LIGN | HC | Surgery | ||
| GTN | 0.35; 0.19 ‐ 0.66 |
3.33; 0.46 ‐ 24 |
3.7; 104 ‐ 13.65 |
0.66; 0.2 ‐ 1.57 |
0.88; 0.54 ‐ 1.42 |
0.21; 0.12 ‐ 0.38 |
7.49; 4.29 ‐ 13.07 |
|||
| BTX | 0.29; 0.02 ‐ 3.61 |
1.91; 0.62 ‐ 5.88 |
7.20; 3.97 ‐ 13.07 |
|||||||
| CCB | 0.10; 0.03 ‐ 0.34 |
3.52; 0.92 ‐ 13.31 |
0.05; 0.02 ‐ 0.12 |
59.8; 15.5 ‐ 231 |
||||||
| Surgery | ||||||||||
| LIGN | 0.32; 0.03 ‐ 1.02 |
|||||||||
| HC | 3.33; 1.14 ‐ 9.77 |
|||||||||
| BRAN | 0.1; 0.03 ‐ 0.34 |
3.33; 1.14 ‐ 9.77 |
1.00; 0.31 ‐ 3.23 |
|||||||
| dilator | 3.39; 1.24 ‐ 9.25 |
|||||||||
| GTN patch |
1.07; 0.5 ‐ 2.27 |
|||||||||
| GTN dose * |
0.91; 0.57 ‐ 1.45 |
|||||||||
| BTX dose * |
1.93: 0.75 ‐ 4.93 |
|||||||||
| BTX site ** |
4.89; 1.1 ‐ 20.8 |
|||||||||
| SIL *** | 0.00; 0 ‐ 0.05 |
|||||||||
| IND | 2.40; 0.16 ‐ 34.9 |
|||||||||
| MIN | 1.04; 0.35 ‐ 3.12 |
|||||||||
| Clove Oil |
0.09; 0.02 ‐ 0.37 |
|||||||||
| ARG | 3.60; 1.22 ‐ 10.64 |
|||||||||
| GTN + BTX |
2.41; 0.52 ‐ 11.1 |
|||||||||
| ALL MEDs |
10; 6.67 ‐ 14.3 |
|||||||||
| healer cream |
0.17; 0.34 ‐ 0.92 |
0.05; 0.01 ‐ 0.27 |
||||||||
| ISMN | 0.17; 0.03 ‐ 0.89 |
1.25; 0.34 ‐ 4.64 |
||||||||
| CCB pill |
3.15; 0.99 ‐ 3.12 |
GTN: glyceryl trinitrate. CCB: calcium channel blocker, either nifedipine or diltiazem. LIGN: lignocaine. IND: indoramine. ARG: arginine. SIL: sildenafil. ISMN: isosorbide mononitrate. MIN: minoxidil. HC: hydrocortisone.
All of the above as well as clove oil and "healer cream" applied topically around or in the anus.
BTX: botulinum toxin, applied by injection between anal sphincters or in the internal sphincter.
Surgery: Any surgical procedure except simple non‐manual dilation. In all cases this is some form of internal anal sphincterotomy
ALL MEDs: any medication compared in an RCT to any surgical procedure ‐ which is some form of sphincterotomy.
*; Dose comparisons are simply high versus low. In an additional non‐comparable BTX study, the OR was 1.36; 0.3‐6.43
**; BTX site compared injection into the internal sphincter either antriorly, which was preferred, or posteriorly.
***; The outstanding results of this intervention are largely due to a 0% placebo response rate (see text).
Authors' conclusions
Implications for practice.
Medical therapy for chronic anal fissure, acute fissure and fissure in children may be applied with a chance of cure that is marginally but significantly better than placebo. The risk of using such therapies is not great, being mainly headache during GTN, or oral CCB use, and without apparent long term adverse effect. But these adverse events can be debilitating during therapy. GTN, Botox or CCBs might therefore be used in individuals wanting to avoid surgical therapy, with surgery being reserved for treatment failures. Late recurrence after medical therapy is common. There is no evidence that surgery should be used as definitive therapy for fissure in children or acute anal fissure. It is worth noting that GTN applied as a dermal patch remote from the anus was as effective as GTN applied to the area of the fissure. Why do people have to apply GTN to their anus? Might it not be just as effective applied to the thigh or abdomen, and cleaner?
Despite an almost 50% increase in the number of included studies in this update, there is very little to suggest in clinical practice that differs from the previous review. The only possible change might be the location of Botox injection into the anterior anal canal, though this is based upon only one small study.
Implications for research.
Botox and topical application of CCBs have been shown to be as effective as GTN in the treatment of anal fissure, usually without the risk of headache, which many patients find unacceptably painful. This is now a well studied field and it is unlikely that further placebo controlled trials of GTN will change its record of rather mediocre ability to cure fissure, nor is it likely that Botox or CCBs will be found in the future to be much more effective. Newer agents are being tested, but two of them have been found ineffective; minoxidil and indoramin. Does that mean the smooth muscle relaxation using pharmacologic agents will never be more than 50% effective in curing fissure? It would seem with the number and breadth of studies performed that this is the case and future research should be directed towards a different mechanism of fissure healing. This is especially true since the risk of incontinence related to surgery is declining in a recently updated Cochrane review (Nelson 2011). Also, though more appropriately placed in the surgery review, the nature and optimal therapy of incontinence after partial lateral internal sphincterotomy for anal fissure needs to be investigated. There is too much disparity between reported incontinence rates cited above and quality of life assessments after sphincterotomy (Hyman 2004; Mentes 2006) which demonstrates the high satisfaction patients have with surgery. .
Feedback
Herxheimer comments
Summary
1. This large and complex review usefully assesses the efficacy of the various medical treatments for chronic anal fissure, but it omits some important details. 2. The conclusion is too general to help clinicians or patients in choosing a medical treatment. The first sentence of the conclusion lumps together all the treatments for acute and chronic anal fissure in adults and children. I suggest that it would be better to discuss the trials in adults and children separately, because the natural history of CAF (and AAF) differs in these groups; they also concern different (though overlapping) groups of clinicians. The word 'marginally' seems grudging, and a personal value judgment. It would be desirable to use the best and most reliable trials of each treatment to calculate the Number Needed to Treat. This is especially important for the treatment tested in the largest number of trials, GTN ointment. The finding that GTN offers the possibility of avoiding surgery distinguishes it from other applications, which have only symptomatic lubricant, local anaesthetic, or anti‐inflammatory effects. This deserves explicit discussion in the conclusion. 3. Background This seems too compressed and could be written in a more logical sequence. The important factors in the causation of anal fissures and in their chronicity do not come out clearly: they are spasm, ischaemia, ulceration and inflammation. 4. Methodological Quality The various important and interesting methodological shortcomings discussed pointed to heterogeneity among several groups of trials, but the review does not explore possible explanations for apparently discordant results. Some are obvious: CDT 1‐1 GTN vs placebo includes two trials (Tankova and Werre) of isosorbide, which is not GTN and should be considered separately. There were other specific reasons for excluding a particular trial from a meta‐analysis. For example the trial by Altomare (2000) found no difference between 4 weeks treatment with GTN 0.2% ointment 12‐hourly and placebo. Two features which could explain this finding are that (1) the total quantity of ointment applied was much less than in other trials: 200mg twice a day; (2) the results were evaluated after 4 weeks of treatment, sooner than in the trials that found a difference. The identification of heterogeneity is only one step towards clarifying why trial results differ. A further step is needed to find reasons for the differences that make some trials less reliable than others. If such reasons are found, then it seems justified to exclude the less reliable ones in estimating treatment effectiveness. Eg, in CDT 1 exclusion of the Altomare trial would raise the estimate of GTN effectiveness from the better quality trials. 5. Headache is a well known effect of GTN and is dose‐related. It is not adequately reported and discussed, partly because the included trials give little or no detail of when it occurred in the course of treatment, how it was managed, and with what results. Were any patients told, for example, that if headache was troublesome, they should reduce the dose for a day or two? Severe headache with GTN is largely avoidable if patients are helped to titrate the dose; the remark in the conclusions that "these adverse events can be debilitating" wrongly implies that nothing can be done to minimise them. 6. The Perrotti study compared nifedipine ointment with 1% hydrocortisone ointment. The difference between them was extreme, but the review suggests no explanation, merely noting "this a result is not to be given any weight" [sic]. However it was unwise to use topical hydrocortisone for comparison because it is well known to inhibit healing: it is therefore not a suitable control treatment. The study would be better excluded from the review. [7a. The review contains no acknowledgements: did the author have no help from anyone? 7b. The text and tables of the review contain many typographical errors ‐ I will send details to the CRG office.]
Reply
Some of the comments relate to writing style, and will be dealt with shortly. Regarding the worries about heterogeneity, this is a large and unwieldy review with a tremendously heterogenious group of studies. A total of 48 Forest plots are presented, the vast majority of them being sub‐group analyses and sensitivity analyses exploring many of the aspects of heterogeneity encountered in the review, both relating to clinical variations between groups and quality issues such as length of follow‐up. None of these analyses change the basic findings: that GTN works some of the time, but not often achieves permanent cure. Botox and calcium channel blockers are no better nor have any studies carefully examined an ideal sequence of therapies in those people not wanting surgery. Headache? As stated, it is briefly mentioned and never studied. There is much common practice, especially in the US directed at diminishing headache incidence or severity, none of it subjected to clinical trials. Perotti? There have in the past been excellent rationales for using hydrocortisone for those that regarded fissure as having an inflammatory etiology. Getting back to quality, there is not among these studies a nice dichotomous division between high and low quality studies. Most have significant flaws. In any case, though quality assessment is central to the Cochrane process, its implementation remains controversial. Again this review is replete (though many more subgroup analyses could have been depicted ‐ and in fact are alluded to in the text) with subgroup analyses that explore how results might vary. And the results don't vary much. I think the clinical guidelines offered at the end are crystal clear. The data certainly support the term "marginal" benefit of GTN, with less than a 50% early healing rate, compared to 37% healing with placebo and only a 25% long term healing rate.
Richard L Nelson
Contributors
Andrew Herxheimer: a.herxheimer@ntlworld.com
Rick Nelson: altohorn@btinternet.com
What's new
| Date | Event | Description |
|---|---|---|
| 4 January 2012 | New search has been performed | updated October 2011, 23 new trials included |
| 1 October 2011 | New citation required but conclusions have not changed | updated October 2011, 23 new trials included |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 14 May 2008 | Feedback has been incorporated | Comments inserted |
| 14 May 2008 | Amended | Converted to new review format. |
| 30 July 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
With thanks to Miss Zorica Vujovic for assisting in the translation process of the Croatian paper Suknaic 2008.
Data and analyses
Comparison 1. GTN versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing of fissure (persistence or recurrence) | 18 | 1315 | Odds Ratio (M‐H, Random, 95% CI) | 0.35 [0.19, 0.65] |
| 2 Headache | 17 | 1177 | Odds Ratio (M‐H, Random, 95% CI) | 4.54 [3.01, 6.85] |
Comparison 2. GTN or IDN versus sphincterotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing of Fissure (persistence or recurrence_ | 7 | 407 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.49 [4.29, 13.07] |
| 2 Minor Incontinence | 7 | 384 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.22, 1.16] |
| 3 Headache | 7 | 381 | Odds Ratio (M‐H, Fixed, 95% CI) | 29.06 [10.30, 82.04] |
2.3. Analysis.

Comparison 2 GTN or IDN versus sphincterotomy, Outcome 3 Headache.
Comparison 3. GTN or IDN versus Botox.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 6 | 334 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.20, 1.57] |
| 2 Headache | 5 | 284 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.10, 0.49] |
| 3 Minor Incontinence | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.44 [0.37, 147.92] |
3.2. Analysis.
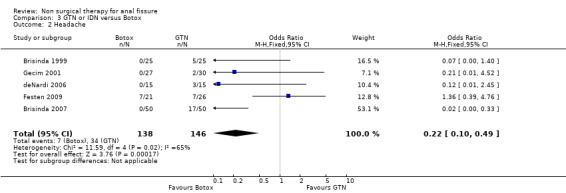
Comparison 3 GTN or IDN versus Botox, Outcome 2 Headache.
3.3. Analysis.
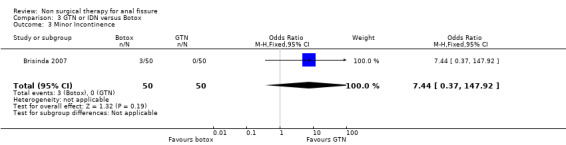
Comparison 3 GTN or IDN versus Botox, Outcome 3 Minor Incontinence.
Comparison 4. GTN versus Calcium Channel Blocker.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 7 | 365 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.54, 1.42] |
| 2 Adverse Events | 2 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.57 [1.28, 9.97] |
| 3 Headache | 5 | 272 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.90 [3.89, 12.25] |
4.2. Analysis.
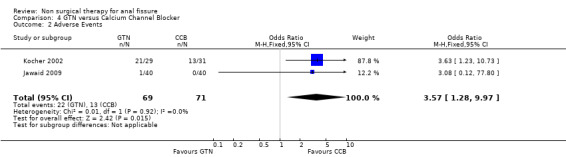
Comparison 4 GTN versus Calcium Channel Blocker, Outcome 2 Adverse Events.
4.3. Analysis.
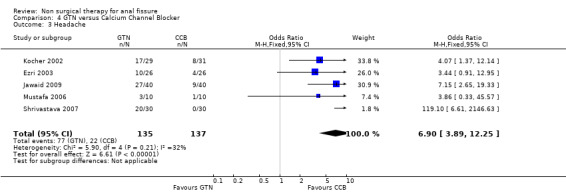
Comparison 4 GTN versus Calcium Channel Blocker, Outcome 3 Headache.
Comparison 5. GTN versus Patch GTN.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 2 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.50, 2.27] |
| 2 Headache | 2 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.46, 2.45] |
Comparison 6. Dilator & Normal Care versus Normal Care Alone for acute and chronic fissure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing in acute and ?chronic? fissure | 2 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.46, 1.60] |
Comparison 7. Botox versus Placebo (or Lignocaine (Colak)).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐healing of Fissure | 3 | 136 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.02, 3.61] |
| 2 Adverse Events | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.24, 4.10] |
7.2. Analysis.
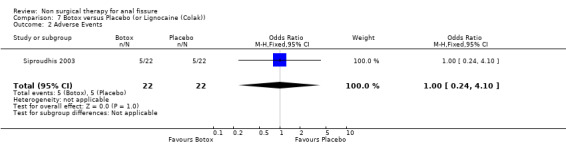
Comparison 7 Botox versus Placebo (or Lignocaine (Colak)), Outcome 2 Adverse Events.
Comparison 8. Botox versus sphincterotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing of the fissure | 5 | 365 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.20 [3.97, 13.07] |
| 2 Minor Incontinence | 4 | 321 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.02, 0.46] |
Comparison 9. Botox dose levels: High versus Higher.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 2 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.75, 4.93] |
Comparison 10. Topical CCB (0.3% topical Nifedipine) versus Hydrocortisone (both got Lignocaine).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing (persistence and recurrence) | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.01 [0.00, 0.04] |
Comparison 11. Diltiazem Oral versus Topical.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing (persistence & recurrence) | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.15 [0.99, 10.00] |
| 3 Adverse Events | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 32.48 [1.77, 597.53] |
11.1. Analysis.
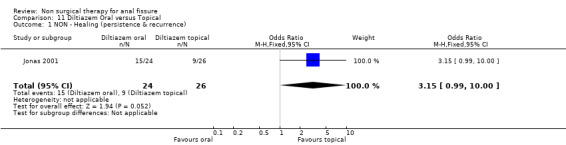
Comparison 11 Diltiazem Oral versus Topical, Outcome 1 NON ‐ Healing (persistence & recurrence).
11.3. Analysis.

Comparison 11 Diltiazem Oral versus Topical, Outcome 3 Adverse Events.
Comparison 12. GTN vs. Placebo in children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 4 | 165 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.05, 4.30] |
Comparison 13. GTN vs placebo in adults.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 14 | 1150 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.18, 0.62] |
Comparison 14. GTN vs. Lignocaine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 5 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.12, 0.38] |
| 2 Adverse Events | 5 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.88 [1.35, 6.13] |
14.2. Analysis.
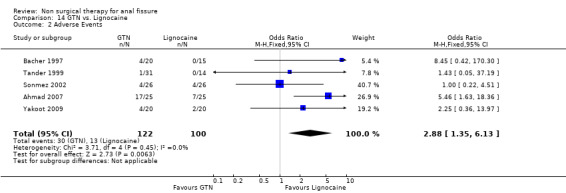
Comparison 14 GTN vs. Lignocaine, Outcome 2 Adverse Events.
Comparison 15. CCB (Topical Nifedipine) vs. lignocaine + HC gel.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 1 | 283 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.02, 0.12] |
Comparison 16. Any Surgery vs any Medical Therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing (persistence or recurrence) | 15 | 979 | Odds Ratio (M‐H, Random, 95% CI) | 0.11 [0.06, 0.23] |
Comparison 17. Lignocaine ointment vs. placebo in children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 2 | 78 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.10, 1.02] |
Comparison 18. bran vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute Fissure Recurrence; a prophylaxis study | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.1 [0.03, 0.34] |
Comparison 19. lignocaine vs bran.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.33 [1.14, 9.77] |
Comparison 20. lignocaine vs hydrocortisone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.33 [1.14, 9.77] |
Comparison 21. bran vs hydrocortisone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.31, 3.23] |
Comparison 22. Sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sensitivity analysis: Excluding GTN/Placebo RCTs with very low placebo response rates (<10%): NON‐healing | 13 | 1063 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.25, 0.96] |
| 2 Excluding RCT in Children with very low Placebo response rate: NON‐healing | 3 | 118 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.11, 9.91] |
| 3 Excluding RCT in Adults with very low Placebo response rate: NON‐healing | 10 | 913 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.17, 0.78] |
| 4 Excluding RCT with very low Placebo response rate: NON‐healing; Lignocaine | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.13, 2.31] |
| 5 Excluding study with < 10% non healing | 5 | 284 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.42, 1.19] |
| 6 Excluding Mishra to investigate heterogeneity, Comparison 16; Medicine vs. Surgery | 14 | 939 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.07, 0.15] |
| 7 Three Largest GTN/Placebo Studies | 3 | 636 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.76, 1.63] |
Comparison 23. Botox versus Botox Dysport.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐healing of fissure | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.29, 6.43] |
Comparison 24. CCB versus LIS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐Healing of the Fissure | 2 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 59.77 [15.47, 230.96] |
| 2 Incontinence | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.89] |
| 3 Headache | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 13.0 [0.69, 245.72] |
24.2. Analysis.
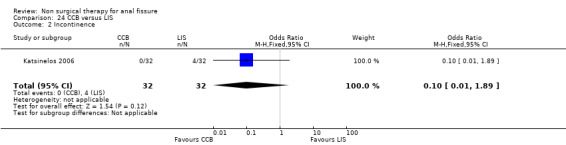
Comparison 24 CCB versus LIS, Outcome 2 Incontinence.
24.3. Analysis.
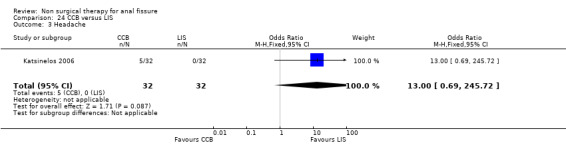
Comparison 24 CCB versus LIS, Outcome 3 Headache.
Comparison 25. Minoxidil versus Lidocaine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐healing of the fissure | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.35, 3.12] |
25.1. Analysis.
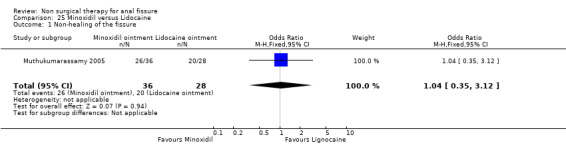
Comparison 25 Minoxidil versus Lidocaine, Outcome 1 Non‐healing of the fissure.
Comparison 26. Indoramine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐healing of the fissure | 1 | 14 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.4 [0.16, 34.93] |
| 2 Headache | 1 | 23 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.5 [0.53, 23.14] |
26.1. Analysis.
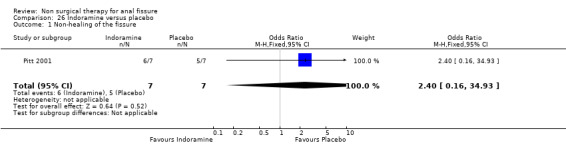
Comparison 26 Indoramine versus placebo, Outcome 1 Non‐healing of the fissure.
26.2. Analysis.
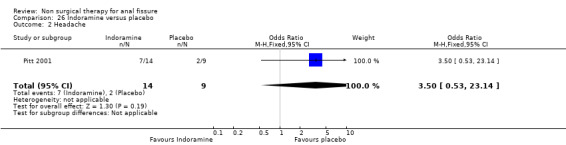
Comparison 26 Indoramine versus placebo, Outcome 2 Headache.
Comparison 27. GTN Dose Comparisons.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐healing of the fissure | 4 | 324 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.57, 1.45] |
Comparison 28. Calcium Channel blocker (oral Nifedipine) versus Botox.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non healing of the fissure | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.50 [0.92, 13.31] |
Comparison 29. Long Term Follow‐up (> 1 year) ; Any Operation vs. Any Medical Therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐healing of the fissure | 3 | 204 | Odds Ratio (M‐H, Random, 95% CI) | 0.06 [0.02, 0.18] |
Comparison 30. GTN vs. Patient Self Dilation (vs. both).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non Healing | 2 | 72 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.39 [1.24, 9.25] |
| 2 Headache | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 27.88 [1.48, 526.12] |
30.2. Analysis.
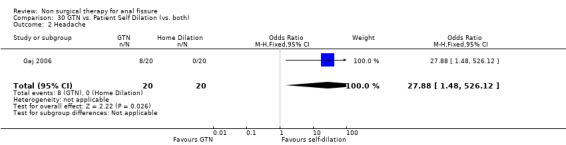
Comparison 30 GTN vs. Patient Self Dilation (vs. both), Outcome 2 Headache.
Comparison 31. Lignocaine vs Clove Oil.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.0 [2.69, 45.06] |
Comparison 32. L‐Arginine vs Surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.60 [1.22, 10.64] |
| 2 Headache | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.24] |
32.1. Analysis.
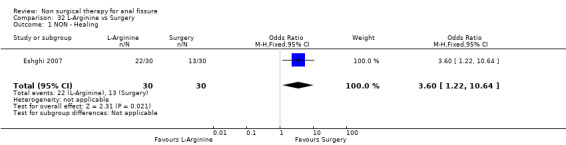
Comparison 32 L‐Arginine vs Surgery, Outcome 1 NON ‐ Healing.
32.2. Analysis.
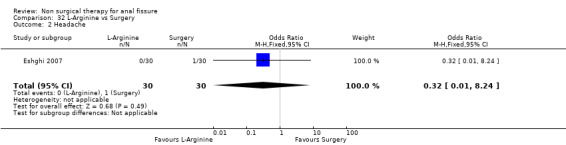
Comparison 32 L‐Arginine vs Surgery, Outcome 2 Headache.
Comparison 33. Sitz Baths vs Control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.20, 4.12] |
Comparison 34. Sildenafil versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 1 | 61 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.00 [0.00, 0.05] |
Comparison 35. Nitroglycerine Topical vs Intra‐anal injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.46, 24.05] |
| 2 Headache | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 45.0 [3.47, 584.34] |
35.2. Analysis.
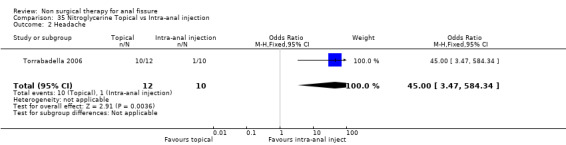
Comparison 35 Nitroglycerine Topical vs Intra‐anal injection, Outcome 2 Headache.
Comparison 36. diltiazem vs. no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.1 [0.03, 0.34] |
36.1. Analysis.
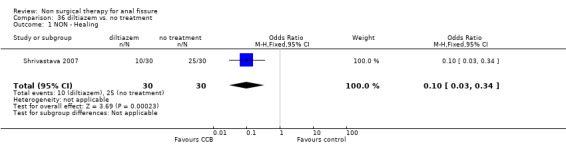
Comparison 36 diltiazem vs. no treatment, Outcome 1 NON ‐ Healing.
Comparison 37. GTN vs ISMN.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.34, 4.64] |
| 2 Headache | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.26] |
37.1. Analysis.
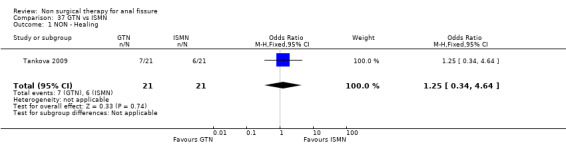
Comparison 37 GTN vs ISMN, Outcome 1 NON ‐ Healing.
37.2. Analysis.
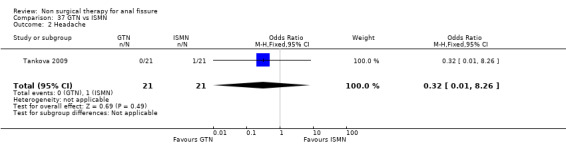
Comparison 37 GTN vs ISMN, Outcome 2 Headache.
Comparison 38. ISMN vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.03, 0.89] |
| 2 Headache | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.06, 41.08] |
38.1. Analysis.
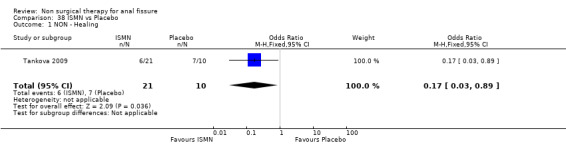
Comparison 38 ISMN vs Placebo, Outcome 1 NON ‐ Healing.
38.2. Analysis.
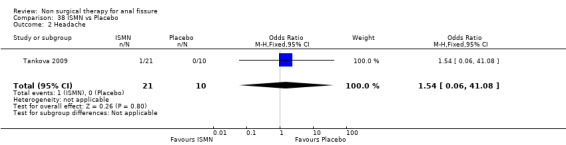
Comparison 38 ISMN vs Placebo, Outcome 2 Headache.
Comparison 39. "Healer cream" vs Lignocaine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ healing | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.27] |
| 2 Headache | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.04, 5.69] |
39.2. Analysis.
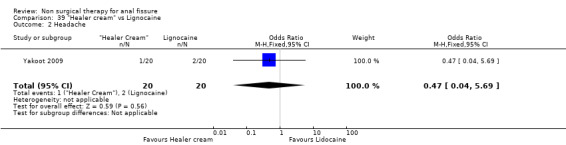
Comparison 39 "Healer cream" vs Lignocaine, Outcome 2 Headache.
Comparison 40. "Healer Cream" vs GTN.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.03, 0.92] |
| 2 Headache | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.03, 3.15] |
40.1. Analysis.
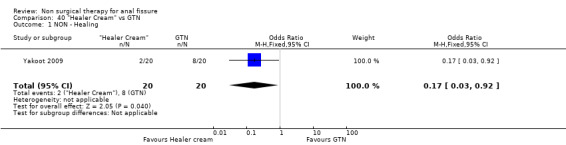
Comparison 40 "Healer Cream" vs GTN, Outcome 1 NON ‐ Healing.
40.2. Analysis.
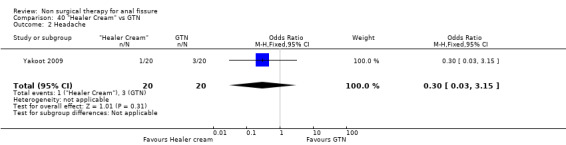
Comparison 40 "Healer Cream" vs GTN, Outcome 2 Headache.
Comparison 41. Lignocaine + Botox vs Lignocaine + GTN.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.62, 5.88] |
| 2 Headache | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.54] |
41.1. Analysis.

Comparison 41 Lignocaine + Botox vs Lignocaine + GTN, Outcome 1 NON ‐ Healing.
41.2. Analysis.

Comparison 41 Lignocaine + Botox vs Lignocaine + GTN, Outcome 2 Headache.
Comparison 42. Botox vs Botox + GTN.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.41 [0.52, 11.10] |
| 2 Minor incontinence | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.05, 1.93] |
42.1. Analysis.
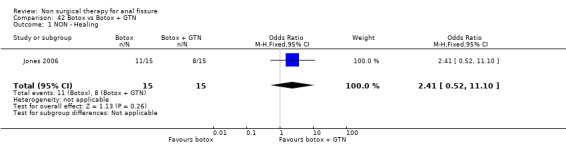
Comparison 42 Botox vs Botox + GTN, Outcome 1 NON ‐ Healing.
42.2. Analysis.

Comparison 42 Botox vs Botox + GTN, Outcome 2 Minor incontinence.
Comparison 43. GTN vs GTN + cryothermal dilators.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NON ‐ Healing | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.76 [1.04, 13.65] |
| 2 Headache | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 23.04 [1.26, 420.37] |
43.2. Analysis.
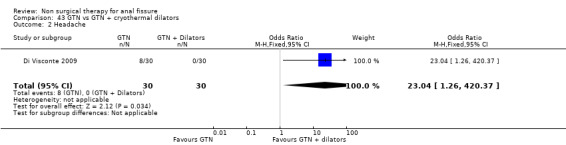
Comparison 43 GTN vs GTN + cryothermal dilators, Outcome 2 Headache.
Comparison 44. Botox injection site location.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non healing of the fissure | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.89 [1.15, 20.79] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmad 2007.
| Methods | RCT (Randomized Controlled Trial). n=50 | |
| Participants | Adults & Children. CAF (Chronic Anal Fissure) & AAF (Acute Anal Fissure) | |
| Interventions | GTN (Glyceryl TriNitrate) 0.2% bid 8 weeks vs Lidnocaine 5% bid 8 weeks | |
| Outcomes | Healing Headache Postural Hypotension Pruritis Ani |
|
| Notes | ROK (Randomization specified and acceptable) 5 Drop outs ITT + (analysis on Intention to Treat basis) 6 months F/U |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | colour coded card in a thick white envelope |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | neither patient nor examining consultant aware of treatment offered ‐ double blind |
| Selective reporting (reporting bias) | Low risk | appropriate side effects/outcomes reported |
| Other bias | Low risk | less than 10% dropout rate, ITT |
| Duration of Follow‐up 6 months or more | Low risk | 6 months follow up, |
Altomare 2000.
| Methods | RCT (Randomized Controlled Trial) n= 132 | |
| Participants | CAF (Chronic Anal Fissure) | |
| Interventions | GTN (Glyceryl TriNitrate) 0.2% bid 4 weeks vs placebo | |
| Outcomes | Healing Pain relief ARM (Anorectal Manometry) Headache | |
| Notes | Cross to LIS (lateral internal sphincterotomy) ITT ‐ (Intention to Treat not done) ROK (Randomization specified and acceptable) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | High risk | ITT not done |
| Duration of Follow‐up 6 months or more | High risk | 4 weeks |
Antropoli 1999.
| Methods | RCT n= 283 | |
| Participants | AAF (Acute Anal Fissure) | |
| Interventions | Nifedipine 0.2% bid 3 weeks vs. lignocaine and HC | |
| Outcomes | Healing Pain relief ARM | |
| Notes | ROK ITT + | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | Low risk | ITT+ |
| Duration of Follow‐up 6 months or more | High risk | 21 days to 3 months |
Arroyo 2005.
| Methods | RCT n=80 | |
| Participants | CAF | |
| Interventions | lateral internal sphincterotomy (LIS) vs. Botox 25 u. n=80 | |
| Outcomes | Healing anal incontinence (AI) | |
| Notes | 3 year f/u AS ok, AC ns, B ns, SS no 0 drop outs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not possible |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not stated |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | Low risk | 0 drop outs |
| Duration of Follow‐up 6 months or more | Low risk | 3 year follow up, |
Bacher 1997.
| Methods | RCT n=35 | |
| Participants | CAF & AAF | |
| Interventions | GTN 0.2% tid vs Xylocaine | |
| Outcomes | Healing Pain Relief ARM Headache | |
| Notes | R?OK ITT ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | R?OK |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | Low risk | ITT |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Duration of Follow‐up 6 months or more | High risk | 4 weeks |
Bailey 2002.
| Methods | RCT n=304 | |
| Participants | CAF | |
| Interventions | GTN in multiple doses and schedules | |
| Outcomes | Healing Pain relief Headache | |
| Notes | RNS ITT + | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | not specified |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | unclear |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | Low risk | ITT+ |
| Duration of Follow‐up 6 months or more | High risk | 8 weeks |
Bielecki 2003.
| Methods | RCT n=43 | |
| Participants | CAF | |
| Interventions | GTN 0.5% vs. diltiazem 2% | |
| Outcomes | healing | |
| Notes | 8 wk f/u AS, AC,B. SS ns. Exc. n.s. 0 drop outs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not specified |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not specified |
| Selective reporting (reporting bias) | Unclear risk | SEs not reported |
| Other bias | Low risk | 0 drop outs |
| Duration of Follow‐up 6 months or more | High risk | 8 weeks |
Boschetto 2004.
| Methods | RCT n=36 | |
| Participants | CAF | |
| Interventions | GTN 0.2% vs. hydrodynamic dilator x 1 n=36 | |
| Outcomes | healing, pain relief, headache, AI | |
| Notes | 2 y f/u, AS,AC, B, SS n.s. Exc. n.s. 0 drop outs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not specified |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not specified |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | Low risk | 0 drop outs |
| Duration of Follow‐up 6 months or more | Low risk | 24 months |
Brisinda 1999.
| Methods | RCT n=50 | |
| Participants | CAF | |
| Interventions | Botox 20u vs GTN 0.2% bid 6 weeks | |
| Outcomes | Healing ARM | |
| Notes | ROK ITT ? Cross over failures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Duration of Follow‐up 6 months or more | High risk | 2 months |
Brisinda 2002.
| Methods | RCT n=150 | |
| Participants | CAF | |
| Interventions | Botox (Botulinum Toxin Injection) dose variation | |
| Outcomes | Healing ARM | |
| Notes | ROK ITT ? | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Other bias | Unclear risk | unclear ITT |
| Duration of Follow‐up 6 months or more | High risk | 2 months |
Brisinda 2004.
| Methods | RCT n=100 | |
| Participants | CAF | |
| Interventions | Botox 50 u vs Botox dysport 150 u n=100 | |
| Outcomes | healing, pain relief | |
| Notes | f/u AS,AC, B OK. SS no 0 dropouts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | OK |
| Selective reporting (reporting bias) | High risk | only reports healing and pain relief |
| Other bias | Low risk | 0 drop outs |
| Duration of Follow‐up 6 months or more | High risk | 2 months |
Brisinda 2007.
| Methods | RCT n=100 | |
| Participants | CAF (adults) | |
| Interventions | Botox (either Botox 30u or Botox dysport 90u) vs Nitroglycerine 0.2% tid 8 weeks | |
| Outcomes | healing incontinence headache anal burning |
|
| Notes | ROK AS ok, AC (single blinded), SS not done, 0 dropouts reported F/U 2 months ITT+ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer generated list in sealed envelopes |
| Allocation concealment (selection bias) | Unclear risk | single blinding |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients were aware of the treatment but the two clinical assessors were blinded |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Duration of Follow‐up 6 months or more | High risk | follow up only 2 months |
Carapeti 1999.
| Methods | RCT n=70 | |
| Participants | CAF | |
| Interventions | GTN 0.2% tid vs higher doses | |
| Outcomes | Healing Pain Relief ARM Recurrence | |
| Notes | ROK ITT ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Selective reporting (reporting bias) | High risk | side effects not reported |
| Other bias | High risk | ITT‐ |
| Duration of Follow‐up 6 months or more | Low risk | 12 months |
Chaudhuri 2001.
| Methods | RCT n=25 | |
| Participants | CAF | |
| Interventions | GTN 0.2% bid 6 weeks vs placebo | |
| Outcomes | Healing Pain relief Headache Recurrence | |
| Notes | ROK ITT ‐ 6/26 ltf | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | High risk | ITT‐ |
| Duration of Follow‐up 6 months or more | High risk | 6 weeks |
Colak 2002.
| Methods | RCT n=62 | |
| Participants | CAF | |
| Interventions | Botox 25 u x 2 vs. lidocaine pomade | |
| Outcomes | healing, pain relief | |
| Notes | 2 mo. f/u AS ok, ACns, B ns, SS no 0 drop outs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | OK |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not specified |
| Selective reporting (reporting bias) | High risk | side effects not reported |
| Other bias | Low risk | 0 drop outs |
| Duration of Follow‐up 6 months or more | High risk | 2months follow up, |
Colak 2003.
| Methods | RCT n=89 | |
| Participants | CAF | |
| Interventions | GTN 0.2% ointment versus GTN dermal patch n=89 | |
| Outcomes | Healing Headache recurrence | |
| Notes | 12 w. f/u AS ok, AC ns, B ns, SS no 7 drop outs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | OK |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not specified |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | Low risk | 7 drop outs, |
| Duration of Follow‐up 6 months or more | High risk | 12 weeks follow up |
deNardi 2006.
| Methods | RCT n=30 | |
| Participants | CAF | |
| Interventions | GTN 0.2% o. vs. Botox 20 u n=30 | |
| Outcomes | healing recurrence headache | |
| Notes | 36 mo. follow‐up (f/u). Allocation sequence (AS), allocation concealment (AC), Blinding (B) and sample size (SS) not stated. 0 drop outs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not stated |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | Low risk | 0 drop outs, |
| Duration of Follow‐up 6 months or more | Low risk | 36 months follow up |
di Visconte 2006.
| Methods | RCT n=32 | |
| Participants | CAF | |
| Interventions | GTN 0.25%, cryothermal dilator to 27mm and both n=48 | |
| Outcomes | healing, recurrence | |
| Notes | 2 year f/u ASok,ACns, B ok, SS no. 1 DO @ for GTN alone and CTD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | OK |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | OK |
| Selective reporting (reporting bias) | High risk | SEs not reported |
| Duration of Follow‐up 6 months or more | High risk | 6 weeks |
Di Visconte 2009.
| Methods | RCT n=60 | |
| Participants | CAF Adults | |
| Interventions | GTN 0.4% bid 6/52 vs GTN 0.4% bid + anal dilators bid for 6 weeks | |
| Outcomes | Healing Headache Pruritis ani Orthostatic hypotension |
|
| Notes | AS adequate, AC no, B no, SS not stated ITT+ ROK 2 drop outs F/U 1 year |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Unclear risk | D ‐ not used |
| Blinding (performance bias and detection bias) All outcomes | High risk | not used |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | Low risk | ITT+, 2 drop outs, |
| Duration of Follow‐up 6 months or more | Low risk | follow up 1 year |
Elwkeel 2007.
| Methods | RCT n=65 | |
| Participants | CAF (adults) | |
| Interventions | lignocaine 5% tid 6 weeks vs clove oil 1% tid 6 weeks | |
| Outcomes | healing itching |
|
| Notes | AS unclear, AC unclear, B unclear, SS not stated ITT+ RNS 8 drop outs F/U 3 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | single blinded |
| Selective reporting (reporting bias) | High risk | side effects reported only itching |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Duration of Follow‐up 6 months or more | High risk | 6 weeks |
Emami 2008.
| Methods | RCT n=34 | |
| Participants | CAF Adults | |
| Interventions | GTN 0.2% bid 6 weeks vs placebo | |
| Outcomes | healing headache anal irritation |
|
| Notes | Crossover at 6 weeksAS unclear, AC adequate, B double blind, SS adequateITT+RNS2 drop outsF/U 6 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | crossover study |
| Allocation concealment (selection bias) | Low risk | double blind |
| Blinding (performance bias and detection bias) All outcomes | Low risk | double blind |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Duration of Follow‐up 6 months or more | High risk | 6 weeks follow up |
Eshghi 2007.
| Methods | RCT n=60 | |
| Participants | CAF & AAF adults | |
| Interventions | L‐Arginine 5% bid 3 months vs LIS | |
| Outcomes | healing headache pain relief bleeding |
|
| Notes | AS not stated, AC unclear, B not blinded, SS no ITT+ RNS drop outs not stated F/U 12 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | not blinded |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | Unclear risk | drop outs not stated |
| Duration of Follow‐up 6 months or more | High risk | 12 weeks follow up, |
Evans 2001.
| Methods | RCT n=65 | |
| Participants | CAF | |
| Interventions | GTN 0.2% tid 8 weeks vs LIS | |
| Outcomes | Healing Headache | |
| Notes | ROK GTN failure got LIS ITT ? | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | Unclear risk | ITT? |
| Duration of Follow‐up 6 months or more | High risk | 8 weeks |
Ezri 2003.
| Methods | RCT n=52 | |
| Participants | CAF | |
| Interventions | GTN 0.2% vs. nifedipine 0.2% n=52 = variable duration up to 24 w (m = 11.7 w | |
| Outcomes | healing, recurrence, headache, pain relief | |
| Notes | variable f/u m 8 and 7 mo AS,AC, B SS ok 8 drop outs, 7 from Nif. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | OK |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | OK |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | High risk | >10% drop outs, variable follow ups |
| Duration of Follow‐up 6 months or more | High risk | 12‐18 weeks |
Festen 2009.
| Methods | RCT n=73 | |
| Participants | CAF Adults | |
| Interventions | Botox 20u with placebo ointment vs Placebo injection with ISDN 1% ointment 6 time daily for 2 months | |
| Outcomes | Healing incontinence headache |
|
| Notes | AS adequate, AC adequate, B not stating, SS calculated but not achieved ITT+ ROK 26 drop outs F/U 4 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | appropriate |
| Allocation concealment (selection bias) | Low risk | intricate study design, complex mathematical reporting of results |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | participants blinded, not stated whether outcome assessor blinded |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | High risk | ITT+, SS calculated but not achieved, >30% drop outs |
| Duration of Follow‐up 6 months or more | High risk | follow up 4 months |
Fruehauf 2006.
| Methods | RCT n=50 | |
| Participants | CAF Adults | |
| Interventions | Botox 30u + lignocaine 4% vs nitroglycerine 0.2% bid 2 weeks + lignocaine 4% | |
| Outcomes | Healing | |
| Notes | AS not stated, AC not stated, B not stated, SS ok ITT+ RNS 4 drop outs F/U 2 weeks Crossover at 2 weeks if not healed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | RNS |
| Allocation concealment (selection bias) | Unclear risk | Indadequate information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not stated whether participants or outcome assessor blinded |
| Selective reporting (reporting bias) | High risk | side effects not reported |
| Other bias | High risk | ITT? as cross over at 2 weeks if not healed |
| Duration of Follow‐up 6 months or more | High risk | follow up 2 weeks |
Gaj 2006.
| Methods | RCT n=40 | |
| Participants | AAF Adults | |
| Interventions | GTN 0.2% bid 4 weeks vs anal dilators bid 30 days | |
| Outcomes | healing headache |
|
| Notes | AS inadequate, AC unclear, B no, SS unclear ITT+ R NO Drop outs unclear F/U 12 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | inadequate |
| Allocation concealment (selection bias) | Unclear risk | randomisation sequence not stated, drop outs not stated, ?groups comparable |
| Blinding (performance bias and detection bias) All outcomes | High risk | not blinded |
| Selective reporting (reporting bias) | Unclear risk | appropriate outcomes/side effects reported |
| Other bias | Unclear risk | drop outs unclear |
| Duration of Follow‐up 6 months or more | High risk | follow up 12 weeks, |
Gecim 2001.
| Methods | RCT n=57 | |
| Participants | CAF | |
| Interventions | GTN 0.3% tid 6 weeks Botox 5 units LL only (low dose) | |
| Outcomes | Healing, Recurrence, Headache, infection | |
| Notes | Abstract only Randomization NS 25% healed recurred in each group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not specified |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not stated |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Duration of Follow‐up 6 months or more | High risk | 3‐18 months |
Gough 1983.
| Methods | RCT n=89 | |
| Participants | CAF | |
| Interventions | Lignocaine +/‐ Dilator | |
| Outcomes | Healing Pain relief | |
| Notes | RNS ITT ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not specified |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Selective reporting (reporting bias) | High risk | side effects not reported |
| Other bias | High risk | ITT‐ |
| Duration of Follow‐up 6 months or more | High risk | 1 month |
Gupta 2006.
| Methods | RCT n=52 | |
| Participants | AAF Adults | |
| Interventions | Sitz baths bid 4 weeks vs none | |
| Outcomes | Healing rash |
|
| Notes | AS adequate, AC inadequate, B no, SS ok ITT+ ROK 6 drop outs F/U 4 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | High risk | inadequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | not blinded |
| Other bias | High risk | >10% drop outs, ITT+ |
| Duration of Follow‐up 6 months or more | High risk | 4 weeks |
Ho 2005.
| Methods | RCT n=132 | |
| Participants | ||
| Interventions | Nifedipine 20 mg p.o. 6 weeks vs.LIS, vs. taylored sphincterotomy n = 132 (136?) | |
| Outcomes | healing, recurrence, pain relief, AI satisfaction | |
| Notes | 4 mo f/u AS ok, AC ns, B ok, SS yes. 4 drop outs, 3 from nif. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | OK |
| Selective reporting (reporting bias) | Unclear risk | side effects unclear |
| Other bias | Low risk | 4 drop outs |
| Duration of Follow‐up 6 months or more | High risk | 4 months |
Iswariah 2005.
| Methods | RCT n=44 | |
| Participants | CAF | |
| Interventions | LIS vs. Botox 20 u x 2 n = 44 = | |
| Outcomes | healing, pain relief, AI, | |
| Notes | 26 w f/u AS ok, AC no, B, SS no 6 drop outs, 5 botox | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Blinding (performance bias and detection bias) All outcomes | High risk | not blinded |
| Other bias | High risk | 6 drop outs |
| Duration of Follow‐up 6 months or more | Low risk | 26 weeks follow up, |
Jawaid 2009.
| Methods | RCT n=80 | |
| Participants | CAF Adults | |
| Interventions | Diltiazem 2% bid 8 weeks vs GTN 0.2% bid 8 weeks | |
| Outcomes | Healing Headache Itch GI side effects |
|
| Notes | AS adequate, AC unclear, B unclear, SS not stated ITT+ ROK 7 drop outs F/U 8 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not stated whether participants or outcome assessor blinded |
| Selective reporting (reporting bias) | Low risk | Appropriate outcomes/SEs reported |
| Other bias | Low risk | 9% drop out, |
| Duration of Follow‐up 6 months or more | High risk | 8 weeks follow up |
Jensen 1986.
| Methods | RCT n=68 | |
| Participants | CAF | |
| Interventions | Lignocaine ointment vs. Topical Hydrocortisone (HC) vs Bran & Sitz Baths | |
| Outcomes | Pain Relief Healing | |
| Notes | ITT ‐ RNS (Randomization technique not specified) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | RNS |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Other bias | High risk | ITT‐ |
Jensen 1987.
| Methods | RCT n=60 | |
| Participants | AAF | |
| Interventions | Bran 5g tid vs. bran 2.5g tid vs placebo | |
| Outcomes | Fissure recurrence at 1 year | |
| Notes | ROK ITT ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Other bias | High risk | ITT‐ |
| Duration of Follow‐up 6 months or more | Low risk | 1 year |
Jonas 2001.
| Methods | RCT n=50 | |
| Participants | CAF | |
| Interventions | Diltiazem applied as 2% gel or oral tablet 60 mg 8 weeks | |
| Outcomes | Healing Headache other AE | |
| Notes | ROK Failures to GTN and then LIS ITT ? | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | Unclear risk | ITT? |
| Duration of Follow‐up 6 months or more | High risk | 8 weeks |
Jones 2006.
| Methods | RCT n=30 | |
| Participants | CAF Adults | |
| Interventions | Botox 25u + GTN 0.2% bid 8 weeks vs Botox 25u | |
| Outcomes | Healing Incontinence |
|
| Notes | AS adequate, AC adequate, B yes, SS stated but not achieved ITT+ ROK 0 drop outs F/U 8 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Low risk | Adequate randomisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | outcome assessor blinded |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | Low risk | ITT+, 0 drop outs, |
| Duration of Follow‐up 6 months or more | High risk | 8 weeks |
Jost1999.
| Methods | RCT | |
| Participants | CAF, n=50 | |
| Interventions | 20 vs 40 u Botox | |
| Outcomes | Healing/Pain relief/Incontinence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no method described |
| Allocation concealment (selection bias) | Unclear risk | " |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | " |
| Selective reporting (reporting bias) | Low risk | no |
| Other bias | Low risk | no drop outs |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | " |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | " |
| Duration of Follow‐up 6 months or more | High risk | 3 months |
Katsinelos 2006.
| Methods | RCT n=64 | |
| Participants | CAF Adults | |
| Interventions | Nifedipine 0.5% tid 8 week vs LIS | |
| Outcomes | Healing Headache Flushing Anal irritation |
|
| Notes | AS adequate, AC not blinded, B no, SS not stated ITT+ ROK 1 drop outs F/U 8 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Blinding (performance bias and detection bias) All outcomes | High risk | not blinded |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | Low risk | 1 drop out, ITT+, |
| Duration of Follow‐up 6 months or more | High risk | follow up 8 weeks |
Kennedy 1999.
| Methods | RCT n=43 | |
| Participants | CAF | |
| Interventions | GTN 0.2% 4 weeks vs placebo | |
| Outcomes | Healing Pain relief ARM Headache | |
| Notes | ROK ITT + Crossovers | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | Low risk | ITT+ |
| Duration of Follow‐up 6 months or more | Low risk | 25 months |
Kenny 2001.
| Methods | RCT n=40 | |
| Participants | CAF & AAF Kids only | |
| Interventions | GTN 0.2% bid vs placebo | |
| Outcomes | Time to painless defecation Bleeding | |
| Notes | ROK ITT ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Other bias | High risk | ITT‐ |
| Duration of Follow‐up 6 months or more | High risk | 16 weeks |
Kocher 2002.
| Methods | RCT n=60 | |
| Participants | CAF | |
| Interventions | GTN 0.2% bid vs. diltiazem 2% bid 6 weeks | |
| Outcomes | Healing Pain relief recurrence Headache | |
| Notes | ROK ITT + | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | Low risk | ITT+ |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Duration of Follow‐up 6 months or more | High risk | 12 weeks |
Libertiny 2002.
| Methods | RCT n=70 | |
| Participants | CAF | |
| Interventions | GTN 0.2% vs LIS | |
| Outcomes | Healing Fissure recurrence | |
| Notes | ROK ITT + | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Other bias | Low risk | ITT+ |
| Duration of Follow‐up 6 months or more | Low risk | 24 months |
Lund 1997.
| Methods | RCT n=80 | |
| Participants | CAF | |
| Interventions | GTN 0.2% bid 8 weeks vs. Placebo | |
| Outcomes | Healing Headache ARM, recurrence Pain relief | |
| Notes | ROK ITT ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | High risk | ITT‐ |
| Duration of Follow‐up 6 months or more | High risk | 8 weeks |
Maan 2004.
| Methods | RCT n=64 | |
| Participants | CAF | |
| Interventions | GTN 0.2% 6 wks vs. Vaseline, lidocaine, Proctsedyl n = 64 = | |
| Outcomes | healing, pain relief | |
| Notes | 6 wk f/u AS, AC, B, SS no 0 drop outs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not stated |
| Other bias | Low risk | 0 drop outs, |
| Duration of Follow‐up 6 months or more | High risk | 6 weeks |
Maria 1998.
| Methods | RCT n=30 | |
| Participants | CAF | |
| Interventions | Botox 20 u vs NaCl | |
| Outcomes | Healing ARM | |
| Notes | ROK ITT+ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Other bias | Low risk | ITT+ |
| Duration of Follow‐up 6 months or more | High risk | 1‐2 months |
Maria 2000.
| Methods | RCT n=50, 25@ | |
| Participants | CAF | |
| Interventions | Botox injection either anterior or posteriorly into the internal sphincter | |
| Outcomes | Fissure healing | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization Code |
| Allocation concealment (selection bias) | Low risk | yes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | double blinded |
| Selective reporting (reporting bias) | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | yes |
| Duration of Follow‐up 6 months or more | High risk | 2 months, then a rescue therapy for non‐healers |
McDonald 1983.
| Methods | RCT n=81 | |
| Participants | AAF | |
| Interventions | Dilator | |
| Outcomes | Referral to LIS | |
| Notes | RNS ITT ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Other bias | High risk | ITT‐ |
| Duration of Follow‐up 6 months or more | Low risk | 6 months |
Mentes 2001.
| Methods | RTC n=111 | |
| Participants | CAF | |
| Interventions | Botox 0.3u/kg vs. LIS n = 111 | |
| Outcomes | Healing Incontinence | |
| Notes | 6 m0 f/u0 drop outs, but 6 cross over to LIS at 2 mo. RNS ITT + | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RNS |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | Low risk | ITT+, 0 drop outs, |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Duration of Follow‐up 6 months or more | Low risk | 6 months |
Mishra 2005.
| Methods | RCT n=40 | |
| Participants | CAF | |
| Interventions | GTN 0.2 % vs LIS n = 40 = | |
| Outcomes | healing, pain relief, AI | |
| Notes | 6 wk f/u AS ok, AC, B, SS no 0 drop outs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not stated |
| Other bias | Low risk | 0 drop outs, |
| Duration of Follow‐up 6 months or more | High risk | 6 weeks |
Moghimi 2006.
| Methods | RCT n=61 | |
| Participants | CAF Adults | |
| Interventions | Sildenafil 10% tid 7 days vs placebo | |
| Outcomes | Healing Itching |
|
| Notes | AS unclear, AC unclear, B unclear, SS not stated ITT+ RNS 6 drop outs F/U 3 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | unclear |
| Allocation concealment (selection bias) | High risk | Methods not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not described |
| Selective reporting (reporting bias) | Unclear risk | not described |
| Other bias | Low risk | ITT+, 6 drop outs, |
| Duration of Follow‐up 6 months or more | High risk | 3 months |
Mustafa 2006.
| Methods | RCT n=20 | |
| Participants | CAF Adults | |
| Interventions | Nifedipine 20mg orally bid vs GTN 0.2% bid 8 weeks | |
| Outcomes | Healing Headache |
|
| Notes | AS unclear, AC no, B no, SS not stated ITT+ RNS ? drop outs F/U 8 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | not blinded |
| Other bias | Low risk | , ITT+ |
| Duration of Follow‐up 6 months or more | High risk | 2 months |
Muthukumarassamy 2005.
| Methods | RCT n=64 | |
| Participants | CAF | |
| Interventions | lidocaine 5% vs minoxidil 0.5% vs both n = 90 | |
| Outcomes | healing, pain relief | |
| Notes | 6 wk f/u AS, AC no, B yes SS no 7 drop outs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Other bias | Low risk | 7 drop outs, |
| Duration of Follow‐up 6 months or more | High risk | 6 weeks |
Nasr 2010.
| Methods | RCT n=80 | |
| Participants | Adults with CAF | |
| Interventions | LIS vs 20 U botulinum toxin n=80 | |
| Outcomes | non healing, incontinence | |
| Notes | 18 weeks follow up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | randomised according to whether registration number was odd or even |
| Allocation concealment (selection bias) | Unclear risk | no |
| Blinding (performance bias and detection bias) All outcomes | High risk | not blinded |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes reported |
| Other bias | Unclear risk | drop outs not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | not blinded ‐ surgical intervention |
| Duration of Follow‐up 6 months or more | High risk | 18 weeks |
Oettle 1997.
| Methods | RCT n=24 | |
| Participants | CAF | |
| Interventions | GTN ? dose vs. LIS | |
| Outcomes | Healing | |
| Notes | R?? ITT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Other bias | Unclear risk | ITT |
| Duration of Follow‐up 6 months or more | Unclear risk | confusing ‐ 4 weeks or 22 months, but |
Oglesby 2001.
| Methods | RCT n=30 | |
| Participants | CAF | |
| Interventions | GTN 0.2% vs placebo | |
| Outcomes | Healing | |
| Notes | RNS ITT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Other bias | Low risk | ITT |
| Duration of Follow‐up 6 months or more | High risk | 8 weeks |
Parellada 2004.
| Methods | RCT n=54 | |
| Participants | CAF | |
| Interventions | isosorbide dinitrate 0.2% tid for 6 wks vs.LIS n = 63 | |
| Outcomes | healing, recurrence, headache, AI | |
| Notes | 2 y f/u AS ok, AC, B, SS no 9 drop outs? leaving 27 @ group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not blinded |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | High risk | >10% drop outs |
| Duration of Follow‐up 6 months or more | Low risk | 2 years |
Perrotti 2002.
| Methods | RCT n=110 | |
| Participants | CAF | |
| Interventions | Nifedipine 0.3% topical vs. 1% HC | |
| Outcomes | Healing ARM Pain relief recurrence | |
| Notes | ROK ITT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Other bias | Low risk | ITT |
| Duration of Follow‐up 6 months or more | Low risk | 13 months |
Pitt 2001.
| Methods | RCT n=23 | |
| Participants | CAF | |
| Interventions | Indoramine 20 mg 6 wks vs. Placebo n = 23 | |
| Outcomes | healing, headache | |
| Notes | 14 wks f/u AS,AC ok, B, SS no 9 drop out, 7 from Ind. nasal congestion, dry mouth | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not stated |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | High risk | 9 drop outs |
| Duration of Follow‐up 6 months or more | High risk | 14 weeks |
Richard 2000.
| Methods | RCT n=90 | |
| Participants | CAF | |
| Interventions | GTN 0.5% to 0.25% tid vs LIS | |
| Outcomes | Headache Healing Incontinence QOL (Quality of Life) Anal irritation | |
| Notes | ROK ITT ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | High risk | ITT‐ |
| Duration of Follow‐up 6 months or more | High risk | 6 weeks |
Scholefield 2003.
| Methods | RCT n=200 | |
| Participants | CAF | |
| Interventions | GTN dosing: 0, 0.1%, 0.2%, 0.4% 8 wks n = 200 | |
| Outcomes | healing, headache, bad headache | |
| Notes | f/u only to end of Rx AS,AC no, B,SS ok 18 drop outs erroneous definition of ITT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | High risk | erroneous ITT, |
| Duration of Follow‐up 6 months or more | High risk | 8 weeks |
Shrivastava 2007.
| Methods | RCT n=60 | |
| Participants | CAF adults | |
| Interventions | Diltiazem 2% bid 6 weeks vs GTN 0.2% bid 6 weeks vs no treatment | |
| Outcomes | Healed Minor incontinence Headache Other adverse events |
|
| Notes | AS inadequate, AC unclear, B no, SS not stated ROK ITT+ Drop‐outs 0 Follow‐up 3 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | inadequate |
| Allocation concealment (selection bias) | Unclear risk | unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | not blinded |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | Low risk | ITT+, 3 drop outs, |
| Duration of Follow‐up 6 months or more | High risk | 3 months |
Siddique 2008.
| Methods | RCT n=64 | |
| Participants | CAF Adults | |
| Interventions | GTN 0.2% bid 8 weeks vs LIS | |
| Outcomes | Healing Headache Minor incontinence |
|
| Notes | AS unclear, AC no, B no, SS unclear ITT+ RNS 0 drop outs F/U 10 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | unclear |
| Allocation concealment (selection bias) | Unclear risk | not concealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | not blinded |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | Low risk | 0 drop outs, ITT+, |
| Duration of Follow‐up 6 months or more | High risk | 10 weeks |
Simpson 2003.
| Methods | RCT n=15 | |
| Participants | CAF in children age 3‐14 | |
| Interventions | GTN dosing: 0.1% & 0.05% bid 8 wk.s n = 15 | |
| Outcomes | healing, recurrence, headache | |
| Notes | ? f/u 0, 4 mo, 1 y. AS,AC, B, SS no 0 drop outs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | unclear |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | unclear |
| Selective reporting (reporting bias) | Low risk | appropriate side effects/outcomes reported |
| Other bias | Low risk | 0 drop outs, |
| Duration of Follow‐up 6 months or more | High risk | 8 weeks |
Siproudhis 2003.
| Methods | RCT n=44 | |
| Participants | CAF | |
| Interventions | Botox 20 u vs placebo | |
| Outcomes | Healing Pain relief local AE | |
| Notes | RNS ITT ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | RNS |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Other bias | High risk | ITT‐ |
| Duration of Follow‐up 6 months or more | High risk | 12 weeks |
Sonmez 2002.
| Methods | RCT n=47 | |
| Participants | AAF Kids only | |
| Interventions | GTN 0.2% bid vs lidocaine or mixed 'caines, or placebo | |
| Outcomes | Healing Anal seepage Anal irritation Pain relief | |
| Notes | RNS ITT ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | RNS |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Selective reporting (reporting bias) | High risk | appropriate outcomes/side effects not reported |
| Other bias | High risk | ITT‐ |
| Duration of Follow‐up 6 months or more | High risk | 8 weeks |
Suknaic 2008.
| Methods | RCT n=50 | |
| Participants | CAF adults and children | |
| Interventions | Botox 10u vs LIS | |
| Outcomes | Healing Minor incontinence |
|
| Notes | AS inadequate, AC no, B no, SS not stated R ‐ no (first 30 patients in one arm, second 30 in second arm) ITT+ Drop‐outs 10 Follow‐up 6 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Inadequate randomisation process. |
| Allocation concealment (selection bias) | High risk | not concealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded due to injection vs surgery |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | High risk | >10% drop outs, |
| Duration of Follow‐up 6 months or more | Low risk | 6 months |
Tander 1999.
| Methods | RCT n=48 | |
| Participants | AF (Anal Fissure unspecified) | |
| Interventions | GTN 0.2% 8 weeks vs. lidocaine or placebo | |
| Outcomes | Persistence Pain relief | |
| Notes | ROK ITT ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Other bias | High risk | ITT‐ |
| Duration of Follow‐up 6 months or more | High risk | 8 weeks |
Tankova 2002.
| Methods | RCT n=19 | |
| Participants | CAF | |
| Interventions | Isosorbide mononitrate bid 3 wk.s n = 19 | |
| Outcomes | healing, headache | |
| Notes | 3 mo f/u AS,AC,B,SS no 0 drop outs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | Low risk | 0 drop outs, |
| Duration of Follow‐up 6 months or more | High risk | 3 months |
Tankova 2009.
| Methods | RCT n=31 | |
| Participants | CAF Adults | |
| Interventions | ISMN 0.1% bid 6 weeks vs GTN 0.1% bid 6 weeks vs Placebo bid 6 weeks | |
| Outcomes | Healing Headache Anal burning |
|
| Notes | AS unclear, AC unclear, B yes, SS not stated RNS ITT+ Drop‐outs 0 Follow‐up 3 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | unclear |
| Allocation concealment (selection bias) | Unclear risk | unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | patient blinded but outcome assessor blinding not described |
| Selective reporting (reporting bias) | Low risk | appropriate side effects/ocutome reported |
| Other bias | Low risk | ITT+, 0 drop outs, |
| Duration of Follow‐up 6 months or more | High risk | 3 months |
Torrabadella 2006.
| Methods | RCT n=22 | |
| Participants | CAF adults | |
| Interventions | Nitroglycerine 0.3% topical tid vs nitroglycerine 0.3% intra‐anal injection tid | |
| Outcomes | Healing Headache |
|
| Notes | AS unclear, AC unclear, B no, SS not stated RNS ITT+ Drop‐outs 4 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not specified |
| Allocation concealment (selection bias) | Unclear risk | unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | not blinded |
| Selective reporting (reporting bias) | Low risk | appropriate side effects/outcomes reported |
| Other bias | Low risk | ITT+, 4 drop outs |
| Duration of Follow‐up 6 months or more | High risk | 2 months |
Uluutku 2001.
| Methods | RCT n=50 | |
| Participants | CAF | |
| Interventions | Nifedipine 20 mg po 5 d. vs. GTN 0.2% bid 30 days vs.. Botox 25 u n = 75 = | |
| Outcomes | healing, pain relief | |
| Notes | f/u 30 d 5 drop outs |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Other bias | Low risk | 5 drop outs, |
| Duration of Follow‐up 6 months or more | High risk | 30 day |
Weinstein 2004.
| Methods | RCT n=48 | |
| Participants | CAF | |
| Interventions | GTN dose: 0, 0.2% , 0.4% n = 48 = | |
| Outcomes | healing, pain relief, headache | |
| Notes | 15 drop outs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
| Selective reporting (reporting bias) | Low risk | appropriate outcomes/side effects reported |
| Other bias | High risk | 15 drop outs |
| Duration of Follow‐up 6 months or more | High risk | confusing again ‐ 8 weeks or 12 months |
Werre 2001.
| Methods | RCT n=40 | |
| Participants | AF | |
| Interventions | Isosorbide dinitrate 1% 5x/d 10 weeks vs. placebo | |
| Outcomes | Healing ARM Headache recurrence | |
| Notes | ROK ITT + | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ROK |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Selective reporting (reporting bias) | Low risk | Appropriate outcomes/side effects reported |
| Other bias | Low risk | ITT+ |
| Duration of Follow‐up 6 months or more | High risk | 10 weeks |
Yakoot 2009.
| Methods | RCT n=40 | |
| Participants | Adults CAF & AAF | |
| Interventions | ISDN 1% + lidocaine 2% + rupioides 5% tid 30 days vs Nitroglycerine 0.25% tid 30 days vs lidocaine 2% tid 30 days |
|
| Outcomes | ||
| Notes | AS not stated, AC unclear, B yes, SS no RNS ITT+ Drop‐outs 0 Follow‐up 30 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | double blinded |
| Other bias | Low risk | 0 drop outs, ITT+ |
| Duration of Follow‐up 6 months or more | High risk | 30 days |
Zuberi 2000.
| Methods | RCT n=42 | |
| Participants | CAF | |
| Interventions | GTN 0.2% ointment vs. GTN patch | |
| Outcomes | Healing Headache | |
| Notes | RNS ITT ‐ Pain not specified at entry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not specified |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not possible |
| Other bias | Low risk | ITT‐ |
| Duration of Follow‐up 6 months or more | High risk | 8 weeks |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Algaithy 2008 | Non‐randomized trial |
| Bassotti 2000 | Manometry only was the measured outcome |
| Coskun 2000 | Non‐randomized study |
| Filingeri 2005 | Only surgical procedures involved |
| Jonas 1999 | Non randomised study |
| Kocher 2001 | Abstract only, published in full in Kocher 2002 |
| Massoud 2005 | Non‐randomized study |
| Thornton 2005 | Fissure outcome was not a measured outcome |
Contributions of authors
This update has been carried out by KT, JM, RLN in addition to the work originally carried out by RLN. AJ on data collection.
Sources of support
Internal sources
NONE, Not specified.
External sources
NONE, Not specified.
Declarations of interest
none
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ahmad 2007 {published data only}
- Ahmad J, Andrabi S.I.H, Rathore M.A. Comparison of topical glyceryl trinitrate with lignocaine ointment for treatment of anal fissure: A randomised controlled trial.. International Journal of Surgery 2007;5(6):429‐432. [DOI] [PubMed] [Google Scholar]
Altomare 2000 {published data only}
- Altomare DF, Rinaldi M, Milito G, Arcana F, et al. Glyceryl trinitrate for chronic anal fissure ‐ ‐ healing or headache? results of a multicenter, randomized, placebo‐controlled double blind trial.. Dis Colon & Rectum 2000;43(2):174‐179. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Antropoli 1999 {published data only}
- Antripoli C, Perrotti P, Rubino M, Martino A, et al. Nifedipine for local use in conservative treatment of anal fissures: preliminary results of a multicenter study. Dis Colon & Rectum 1999;42(8):1011‐1015. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arroyo 2005 {published data only}
- Arroyo A, Perez F, Serrano P, Candela F, Lacueva J, Calpena R. Surgical versus chemical (botulinum toxin) sphincterotomy for chronic anal fissure: long‐term results of a prospective randomized clinical and manometric study. Am J Surg 2005;189:429‐34. [DOI] [PubMed] [Google Scholar]
Bacher 1997 {published data only}
- Bacher H, Mischinger HJ, Werkgartner G, Cerwenka H, et al. Local nitroglycerin for treatment of anal fissures: an alternative to lateral sphincterotomy?. Dis Colon & Rectum 1997;40(7):840‐845. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bailey 2002 {published data only}
- Bailey HR, Beck DE, Billingham RP, Binderow SR, et al. A study to determine the nitroglycerin ointment dose and dosing interval that best promote the healing of chronic anal fssures. Dis. Colon & Rectum 2002;45(9):1192‐1199. [DOI] [PubMed] [Google Scholar]
Bielecki 2003 {published data only}
- Bielecki K, Kolodziejczak M. A prospective randomized trial of diltiazem and glyceryltrinitrate ointment in the treatment of chronic anal fissure. Colorectal Disease 2003;5:256‐7. [DOI] [PubMed] [Google Scholar]
Boschetto 2004 {published data only}
- Boschetto S, Giovannone M, Tosoni M, Barberani F. Hydropneumatic anal dilation in conservative treatment of Chronic anal fissure: Clinical outcomes and randomized comparison with topical nitroglycerin. Tech Coloproctology 2004;8(2):89‐92. [DOI] [PubMed] [Google Scholar]
Brisinda 1999 {published data only}
- Brisinda G, Maria G, Bentivoglio AR, Cassetta E. Gui D, Albanese A. A comparison of injections of botulinum toxin and topical nitroglycerin ointment for the treatment of chronic anal fissure. New Eng J Med 1999;341(2):65‐69. [DOI] [PubMed] [Google Scholar]
Brisinda 2002 {published data only}
- Brisinda G, Maria G, Sganga G, Bentivoglio AR, et al. Effectiveness of higher doses of botulinum toxin to induce healing in patient with chronic anal fissures. Surgery 2002;131(2):179‐184. [DOI] [PubMed] [Google Scholar]
Brisinda 2004 {published data only}
- Brisinda G, Albanese A, Cadeddu F, Bentivoglio AR, Mabisonbi A, Maringa G, Maria G. Botulinum neurotoxin to treat anal fissure: Results of a randomized Botox vs Disport controlled trial. Alimentary Pharm and Therapeutics 2004;19(6):695‐701. [DOI] [PubMed] [Google Scholar]
Brisinda 2007 {published data only}
- Brisinda, G, Cadeddu, F, Brandara, F, Marniga, G, Maria. G. Randomized clinical trial comparing botulinum toxin injections with 0.2 per cent nitroglycerin ointment for chronic anal fissure. British Journal of Surgery 2007;94(2):162‐7. [DOI] [PubMed] [Google Scholar]
Carapeti 1999 {published data only}
- Carapeti EA, Kamm MA, McDonald PJ, Chadwick, et al. Randomised controlled trial shows that glyceral trinitrate heals anal fissures, higher doses are not more effective and there is a high recurrence rate. Gut 1999;44:727‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaudhuri 2001 {published data only}
- Chaudhuri S, Pal AK, Acharya A, Dey A, et al. Treatment of chronic anal fissure with topical glyceral trinitrate: a double blind, placebo controlled trial. Indian J Gastroent 2001;20(3):101‐102. [PubMed] [Google Scholar]
Colak 2002 {published data only}
- Colak T, Ipek T, Kanik A, Aydin S. A randomized trial of botulinum toxin vs lidocaine pomade for chronic anal fissure. Acta Gastro. Enterologica Belgica 2002;65(4):187‐90. [PubMed] [Google Scholar]
Colak 2003 {published data only}
- Colak T, Ipek T, Urkaya N, Kanik A, Dirlik M. A randomized study comparing systemic transdermal treatment and local application of glyceryl trinitrate in the management of chronic anal fissure. European J of Surgery, Suppl. 2003;168(588):18‐22. [PubMed] [Google Scholar]
deNardi 2006 {published data only}
- deNardi P, Ortolano E, Radaelli G, Staudacher C. Comparison of glycerine trinitrate and botulinum toxin‐A for the treatment of chronic anal fissure: Long term results. Dis. Colon & Rectum 2006;49(4):427‐32. [DOI] [PubMed] [Google Scholar]
di Visconte 2006 {published data only}
- Visconte MS, Bella R, Munegato G. Randomised prospective trial comparing 0.25% glycerine trinitrate ointment and anal cryothermal dilators with 0.25% glycerine trinitrate ointment alone and with anal cryothermal dilators alone in the treatment of chronic anal fissure: a two year follow up.. Dis Colon & Rectum 2006;49(12):1822. [DOI] [PubMed] [Google Scholar]
Di Visconte 2009 {published data only}
- Schiano di Visconte M, Munegato G. Glyceryl trinitrate ointment (0.25%) and anal cryothermal dilators in the treatment of chronic anal fissures.. Journal of Gastrointestinal Surgery. 2009;13(7):1283‐1291. [DOI] [PubMed] [Google Scholar]
Elwkeel 2007 {published data only}
- Elwakeel, H. A, Moneim, H. A, Farid, M, Gohar. A. A. Clove oil cream: a new effective treatment for chronic anal fissure.. Colorectal Disease 2007;9(6):549‐52. [DOI] [PubMed] [Google Scholar]
Emami 2008 {published data only}
- Emami, M. H, Sayedyahossein, S, Aslani. A. Safety and efficacy of new glyceryl trinitrate suppository formula: first double blind placebo‐controlled clinical trial.. Diseases of the Colon and Rectum. 2008;51(7):1079‐83. [DOI] [PubMed] [Google Scholar]
Eshghi 2007 {published data only}
- Eshghi F. The efficacy of L‐arginine gel for treatment of chronic anal fissure compared to surgical sphincterotomy.. Journal of Medical Sciences 2007;7(3):481‐484. [Google Scholar]
Evans 2001 {published data only}
- Evans J, Luck A, Hewett P. Glyceral trinitrate vs. lateral sphincterotomy for chronic anal fissure. Dis. Colon & Rectum 2001;44(1):93‐97. [DOI] [PubMed] [Google Scholar]
Ezri 2003 {published data only}
- Exri T, Susmallian S. Topical nifedipine vs. topical glyceryl trinitrate for treatment of chronic anal fissure. Dis. Colon & Rectum 2003;46(6):805‐8. [DOI] [PubMed] [Google Scholar]
Festen 2009 {published data only}
- Festen S, Gisbertz S.S, Schaagen F, Gerhards M.F. Blinded randomized clinical trial of botulinum toxin versus isosorbide dinitrate ointment for treatment of anal fissure.. BJS 2009;96(12):1393‐1399. [DOI] [PubMed] [Google Scholar]
Fruehauf 2006 {published data only}
- Fruehauf, H, Fried, M, Wegmueller, B, Bauerfeind, P, Thumshirn. M. Efficacy and safety of botulinum toxin a injection compared with topical nitroglycerin ointment for the treatment of chronic anal fissure: a prospective randomized study.. The American Journal of Gastroenterology. 2006;101(9):2107‐12. [DOI] [PubMed] [Google Scholar]
Gaj 2006 {published data only}
- Gaj, F, Trecca, A, Crispino. P. Efficacy of anal dilators in the treatment of acute anal fissure. A controlled clinical trial. Chirurgia Italiana 2006;58(6):761‐5. [PubMed] [Google Scholar]
Gecim 2001 {unpublished data only}
- Gecim I. Comparison of glyceryl trinitrate and botulinum toxin A in treatment of chronic anal fissure: a prospective randomized study. Program Abstracts ASCRS San Diego, 2001. 2001.
Gough 1983 {published data only}
- Gough MJ, Lewis A. The conservative treatment of fissure in ano. Br J Surg 1983;70(3):175‐176. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gupta 2006 {published data only}
- Gupta P. Randomized, controlled study comparing sitz‐bath and no‐sitz‐bath treatments in patients with acute anal fissures.. ANZ Journal of Surgery. 2006;76(8):718‐721. [DOI] [PubMed] [Google Scholar]
Ho 2005 {published data only}
- Ho KS, Ho YH. Randomized clinical trial comparing oral nifedipine with lateral anal sphincterotomy and tailored sphincterotomy in the treatment of chronic anal fissure. Brit J Surg. 2005;92(4):403‐8. [DOI] [PubMed] [Google Scholar]
Iswariah 2005 {published data only}
- Iswariah H, Stephens J, Reiger N, Rodda D, Hewett P. Randomized prospective controlled trial of lateral internal sphincterotomy versus injections of botulinum toxin for the treatment of idiopathic fissure in ano. ANZ J Surg 2005;75(7):553‐5. [DOI] [PubMed] [Google Scholar]
Jawaid 2009 {published data only}
- Jawaid M, Masood Z, Salim M. Topical diltiazem hydrochloride and glyceryl trinitrate in the treatment of chronic anal fissure.. Journal of the College of Physicians & Surgeons ‐ Pakistan. 2009 Oct;19(10):614‐7. [DOI] [PubMed] [Google Scholar]
Jensen 1986 {published data only}
- Jensen, SL. Treatment of first episodes of acute anal fissure: a prospective randomised study of lignocaine ointment versus hydrocortisone ointment or warm sitz baths plus bran. Br Med J 1986;292(3 May):1167‐1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 1987 {published data only}
- Jensen SL. Maintenance therapy with unprocessed bran in the prevention of acute anal fissure recurrence. J Roy. Soc. Med 1987;80(5):296‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jonas 2001 {published data only}
- Jonas M, Neal KR, Abercrombie JF, Scholefield JH. A randomized trial of oral vs. topical diltiazem for chronic anal fissures. Dis. Colon & Rectum 2001;44:1074‐1078. [DOI] [PubMed] [Google Scholar]
Jones 2006 {published data only}
- Jones O.M, Ramalingam T, Merrie A, Cunningham C, George B.D, Mortensen N.J.McC, Lindsey I. Randomized clinical trial of botulinum toxin plus glyceryl trinitrate vs. botulinum toxin alone for medically resistant chronic anal fissure: Overall poor healing rates.. Diseases of the Colon and Rectum 2006;49(10):1574‐1580. [DOI] [PubMed] [Google Scholar]
Jost1999 {published data only}
- Jost WH, Schrank B. Chronic anal fissures treated with botulinum toxin injections: a dose‐finding study with Dysport. Colorectal Dis. 1999;1:26‐8. [DOI] [PubMed] [Google Scholar]
Katsinelos 2006 {published data only}
- Katsinelos P, Papaziogas B, Koutelidakis I, Paroutoglou G, Dimiropoulos S, Souparis A, Atmatzidis K. Topical 0.5% Nifedipine versus Lateral Internal Sphincterotomy for the Treatment of Chronic Anal Fissure. International Journal of Colorectal Disease 2006;21(2):179‐83. [DOI] [PubMed] [Google Scholar]
Kennedy 1999 {published data only}
- Kennedy ML, Sowter S, Nguyen H, Lubowski DZ. Glyceryl trinitrate ointment for the treatment of chronic anal fissure: results of a placebo‐controlled trial and long term follow‐up. Dis. Colon & Rectum 1999;42(8):1000‐1006. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kenny 2001 {published data only}
- Kenny SE, Irvine T, Driver CP, Nunn AT, et al. Double blind randomised controlled trial of topical glyceral trinitrate in anal fissure. Arch Dis Childhood 2001;85:404‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kocher 2002 {published data only}
- Kocher HM, Steward M, Leather AJM, Cullen PT. Randomized clinical trial assessing the side effects of glyceral trinitrate and diltiazem hydrochloride in the treatment of chronic anal fissure. Brit. J Surgery 2002;89:413‐417. [DOI] [PubMed] [Google Scholar]
Libertiny 2002 {published data only}
- Libertiny G, Knight JS, Farouk R. Randomised trial of topical 0.2% glyceryl trinitrate and lateral internal sphincterotomy for the treatment of patients with chronic anal fissures: long term follow‐up. Eur J Surg 2002;168(7):418‐421. [DOI] [PubMed] [Google Scholar]
Lund 1997 {published data only}
- Lund JN, Scholefield JH. A randomized, prospective, double‐blind, placebo‐controlled trial of glyceryl trinitrate in treatment of anal fissure. Lancet 1997;349(9044):11‐14. [DOI] [PubMed] [Google Scholar]
Maan 2004 {published data only}
- Maan MS, Mishra R, Thomas S, Hadke N. Randomized double blind trial comparing topical nitroglycerin with xylocaine and Proctosedyl in idiopathic chronic anal fissure. Indian J. Gastroenterology 2004;23(91‐3):91‐3. [PubMed] [Google Scholar]
Maria 1998 {published data only}
- Maria G, Cassetta E, Gui D, Brisinda G, et al. A comparison of botulinum toxin and saline for the treatment of chronic anal fissure. New Engl J Med 1998;338:217‐220. [DOI] [PubMed] [Google Scholar]
Maria 2000 {published data only}
- Maria G, Brisinda G, Bentivoglio AR, Cassetta E, Gui D, Albanese A. Influewnce of botulinum toxin site of injections on healing rate in patients with chronic anal fissure. Am J Surgery 2000;179:46‐50. [DOI] [PubMed] [Google Scholar]
McDonald 1983 {published data only}
- MacDonald P, Driscoll AM, Nichollds RJ. The anal dilator in the conservative management of acute anal fissure. Br J Surg 1983;70(1):25‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mentes 2001 {unpublished data only}
- Mentes BB, Irkorucu O, Akin M, Leventoglu S, et al. Botulinum toxin injection compares favorably with lateral internal sphincterotomy in the treatment of chronic anal fissure. Dis. Colon & Rectum 2003;46:232‐237. [PMID 12576897] [DOI] [PubMed] [Google Scholar]
Mishra 2005 {published data only}
- Mishra R, Thomas S, Maan MS, Hadke NS. Topical nitroglycerin versus lateral internal sphincterotomy for chronic anal fissure: prospective randomized trial. ANZ J Surg 2005;75:1032‐5. [DOI] [PubMed] [Google Scholar]
Moghimi 2006 {published data only}
- Moghimi M, Ghodosi I. Topical sildenafil (Viagra) in the treatment of chronic anal fissure: A randomized double blind controlled trial.. International Journal of Pharmacology. 2006;2(6):608‐612. [Google Scholar]
Mustafa 2006 {published data only}
- Mustafa, N. A, Cengiz, S, Türkyilmaz, S, Yücel. Y. Comparison of topical glyceryl trinitrate ointment and oral nifedipine in the treatment of chronic anal fissure.. Acta Chirurgica Belgica. 2006;106(1):55‐8. [DOI] [PubMed] [Google Scholar]
Muthukumarassamy 2005 {published data only}
- Muthukumarassamy R, Robinson SS, Sarath SC, Raveendran R. Treatment of anal fissures using a combination of minoxidil and lignocaine: a randomized, double blind trial. Indian J Gastroenterology 2005;24(4):158‐60. [PubMed] [Google Scholar]
Nasr 2010 {published data only}
- Nasr M, Ezzat H, Elsebae M. Botulinum toxin injection versus lateral internal sphincterotomy in the treatment of chronic anal fissure: a randomised controlled trial. World J Surg 2010;34:2730‐2734. [DOI] [PubMed] [Google Scholar]
Oettle 1997 {published data only}
- Oettle GJ. Glyceryl trinitrate vs. sphincterotomy for treatment of chronic fissure in ano: a randomized controlled trial. Dis Colon & Rectum 1997;40(11):1318‐1320. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oglesby 2001 {unpublished data only}
- Oglesby S, Wilson‐Storey D, Munro F. A placebo controlled randomized trial of 0.2% GTN in the treatment of chronic anal fissures in children. Digestive Disease Week‐ USA. 2001.
Parellada 2004 {published data only}
- Parellada C. Randomized prospective trial comparing 0.2 percent isosorbide dinitrate ointment with sphincterotomy in treatment of chronic anal fissure: two year follow up. Dis. Colon & Rectum 2004;47(4):437‐43. [DOI] [PubMed] [Google Scholar]
Perrotti 2002 {published data only}
- Perrotti P, Bove A, Antropoli C, Molino D, et al. Topical nifedipine with lidocaine vs. active control for the treatment of anal fissure. Dis. Colon & Rectum 2002;45(11):1468‐1475. [DOI] [PubMed] [Google Scholar]
Pitt 2001 {published data only}
- Pitt J, Dawson PM, Hallan RI, Boulos PB. A double‐blind randomized placebo controlled trial of oral indoramine to treat chronic anal fissure. Colorectal Dis. 2001;3(3):165‐8. [DOI] [PubMed] [Google Scholar]
Richard 2000 {published data only}
- Richard CS, Gregroire R, Plewes EA, Silverman R, et al. Internal sphincterotomy is superior to topical nitroglycerin in the treatment of chronic anal fissure: results of a randomized controlled trial. Dis Colon & Rectum 2000;43(8):1048‐1057. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Scholefield 2003 {published data only}
- Scholefield JH, Bock JU, Marla B, Richter HJ, Athanasiadis S, Prois M, Herold A. A dose finding study with 0.1%, 0.2%, and 0.4% glyceryl trinitrate ointment inpatients with chronic anal fissure. Gut 2003;52:264‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shrivastava 2007 {published data only}
- Shrivastava UK, Jain BK, Kumar P, Saifee Y. A comparison of the effects of diltiazem and glyceryl trinitrate ointment in the treatment of chronic anal fissure: a randomized clinical trial.. Surgery Today 2007;37(6):482‐5. [DOI] [PubMed] [Google Scholar]
Siddique 2008 {published data only}
- Siddique, M. I, Murshed, K. M, Majid. M. A. Comparative study of lateral internal sphincterotomy versus local 0.2% glyceryl trinitrate ointment for the treatment of chronic anal fissure.. Bangladesh Medical Research Council Bulletin. 2008;34(1):12‐15. [DOI] [PubMed] [Google Scholar]
Simpson 2003 {published data only}
- Simpson J, Lund JN, Thompson RJ, Kapila L, Scholefield JH. The use of glyceryl trinitrate (GTN) in the treatment of chronic anal fissure in children. Medical Science Monitor 2003;9(10):PI123‐6. [PubMed] [Google Scholar]
Siproudhis 2003 {unpublished data only}
- Siproudhis L, Sebille V, Pigot F, Hemerey P, Juguet F, Bellissant E. Lack of efficacy of botulinum toxin in chronic anal fissure. Alimentary Pharmacology & Therapeutics 2003;18(5):515‐24. [DOI] [PubMed] [Google Scholar]
Sonmez 2002 {published data only}
- Sonmez K, Demmirogullan B, Ekingen G, Turkyilmaz Z, et al. Randomized Placebo controlled treatment of anal fissure by lidocaine, EMLA and GTN in children. J Ped Surg 2002;37(9):1313‐1316. [DOI] [PubMed] [Google Scholar]
Suknaic 2008 {published data only}
- Suknaic S, Patrlj L, Steresinic M, Skopljanac Macina A, Erdelez L. Surgical or biologic sphincterotomy in the treatment of chronic anal fissure. Acta Medica Croatica. 2008;62(1):73‐80. [PubMed] [Google Scholar]
Tander 1999 {published data only}
- Tander B, Guven A, Demirbag S, Ozkan Y, et al. A prospective, randomized, double‐blind, placebo‐controlled trial of glceryl‐trinitrate ointment in the treatment of children with anal fissure. J Pediatr Surg 1999;34(12):1810‐1812. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tankova 2002 {published data only}
- Tankova L, Yoncheva K, Muhtarov M, Kadyan H, Draganov V. Topical mononitrate treatment in patients with anal fissure. Aliment. Pharm & Therapeutics 2002;16(1):101‐3. [DOI] [PubMed] [Google Scholar]
Tankova 2009 {published data only}
- Tankova, L, Yoncheva, K, Kovatchki, D, Doytchinova. I. Topical anal fissure treatment: placebo‐controlled study of mononitrate and trinitrate therapies. International Journal of Colorectal Disease 2009;24(4):461‐4. [DOI] [PubMed] [Google Scholar]
Torrabadella 2006 {published data only}
- Torrabadella, L, Salgado. G. Controlled dose delivery in topical treatment of anal fissure: pilot study of a new paradigm.. Diseases of the Colon and Rectum. 2006;49(6):865‐8. [DOI] [PubMed] [Google Scholar]
Uluutku 2001 {published data only}
- Uluutku H, Akin ML, Erenglu C, Yildiz M, Ukraya N, Celen K. Efficacy of nifedipine, glyceryl trinitrate and botulinum toxin in treatment of chronic anal fissure. Turkish J Surg 2001;17(6):343‐50. [Google Scholar]
Weinstein 2004 {published data only}
- Weinstein D, Halevy A, Negri M, Levy N, Gelertner I, Ziv Y. A prospective randomized double‐blind study on the treatment of anal fissures with nitroglycderin ointment.. Harefuah 2004;143(10):713‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Werre 2001 {published data only}
- Werre AJ, Palamba HW, Bilgen EJS, Eggink WF. Isosorbide dinitrate in the treatment of anal fissure: a randomized, prospective double blind placebo controlled trial.. Eur J Surg 2001;167:382‐85. [DOI] [PubMed] [Google Scholar]
Yakoot 2009 {published data only}
- Yakoot M, Salaam M.A. Study of efficacy and safety of a new local cream ("healer") in the treatment of chronic anal fissure. A prospective, randomized, single‐blind, comparative study.. Arquivos De Gastroenterologia. 2009;46(3):179‐182. [DOI] [PubMed] [Google Scholar]
Zuberi 2000 {published data only}
- Zuberi BF, Rajput MR, Abro H, Shaikh SA. A randomized trial of glyceryl trinitrate ointment and nitroglycerin patch in healing of anal fissures. Int J Colorectal Dis 2000;15(4):243‐245. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Algaithy 2008 {published data only}
- Algaithy Z.K. Botulinum toxin versus surgical sphincterotomy in females with chronic anal fissure.. Saudi Medical Journal 2008;29(9):1260‐63. [PubMed] [Google Scholar]
Bassotti 2000 {published data only}
- Bassotti G, Clementi M, Ceccarelli F, Pelli MA. Double blind manometric assessment of two topical glyceral trinitrate formulations in patients with chronic anal fissures. Digest Liver Dis 2000;32:699‐702. [DOI] [PubMed] [Google Scholar]
Coskun 2000 {published data only}
- Coskun A, Ukunkoy A, Akinci OF. The treatment efficacy of medical sphincterotomy with glycerol trinitrate on acute and chronic anal fissures. Ulusal C errhi Dergisi 2000;16(3):176‐181. [Google Scholar]
Filingeri 2005 {published data only}
- Filingeri V, Gravante G. A prospective randomized trial between subcutaneous lateral internal sphincterotomy with radiofrequency bistoury and conventional Parks' operation in the treatment of anal fissure. Eur. Review for Medical and Pharmacolog. Sciences 2005;9(3):175‐8. [PubMed] [Google Scholar]
Jonas 1999 {published data only}
- Jonas M, Lobo DN, Gudgeon AM. Lateral internal sphincterotomy is not redundant in the era of glyceryl trinitrate therapy for chronic anal fissure. J R Soc Med 1999;92(4):186‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kocher 2001 {published data only}
- Kocher HM, Steward M, Leather AJ, Cullen PT. Topical glyceryl trinitrate (GTN ‐ 0.2%) vs topical diltiazem (2%) in the treatment of chronic fissure in ano: a prospective double blind randomized trial.. DDW Abstract CD‐ROM. Alanta: DDW, 2001.
Massoud 2005 {published data only}
- Massoud BW, Mehrdad V, Baharak T, Alireza Z. Botulinum toxin injection versus internal anal sphincterotomy for the chronic anal fissure. Ann Saudi Med 2005;25(2):140‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thornton 2005 {published data only}
- Thronton MJ, Kennedy ML, King DW. Manometric effect of topical glyceryl trinitrate and its impact on chronic anal fissure healing. Dis Colon & Rectum 2005;48(6):1207‐12. [DOI] [PubMed] [Google Scholar]
Additional references
Abcarian 1980
- Abcarian H. Surgical correction of chronic anal fissure: results of pateral internal sphincterotomy vs. fissurectomy ‐ midline sphincterotomy.. Dis Colon & Rectum 1980;23:31‐36. [DOI] [PubMed] [Google Scholar]
Baker 2003
- Baker SG, Kramer BS. A perfect correlate does not a surrogate make.. BMC Med Res. Methodol. 2003;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia‐Aguilar 1996
- Garcia‐Aguilar J, Belmonte C, Wong D, Lowry AC, Madoff RD. Open vs Closed sphincterotomy for chronic anal fissure: long term results. Dis Colon & Rectum 1996;39:440‐443. [DOI] [PubMed] [Google Scholar]
Goligher 1975
- Goligher JC. Surgery of the Anus, Rectum & Colon. 3rd Edition. London: Balliere & Tindall, 1975. [Google Scholar]
Graziano 2001
- Graziano A, Svidler Lopez L, Lecinas S, Masciangioloi G, Gualdrini U, Bisisio O. Long term results of topical nitroglycerine in the treatment of shronic anal fissures are disappointing. Techniques in Coloproctology 2001;5(3):143‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hyman 2004
- Hyman N. Incontinence after lateral internal sphincterotomy: a prospective study and quality of life assessment. Dis Colon & Rectum 2004;47:35‐8. [DOI] [PubMed] [Google Scholar]
Jonas 2002
- Jonas M, Lund JN, Scholefield JH. Topical 0.2% glyceryl trinitrate ointment for anal fissure: Long‐term efficacy in routine clinical practice. Colorectal Disease 2002;4(5):317‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mentes 2006
- Mentes BB, Tezcaner T, Yilmaz U, Leventoglu S, Oguz M. Results of lateral internal sphincterotomy for chronic anal fissure with particular reference to quality of life.. Dis Colon & Rectum 2006;49(7):1045‐51. [DOI] [PubMed] [Google Scholar]
Minguez 2002
- Minguez M, Herreros B, Espi A, Garcia‐Granero E, Sanchiz V, Mora F, Lledo S, Benages A. Long‐term follow‐up (42 months) of chronic anal fissure after healing with botulinum toxin. Gastroenterology 2002;123:112‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nelson 2001
- Nelson RL. Outcome of operative procedures for fissure in ano. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD002199.pub2] [DOI] [PubMed] [Google Scholar]
Nelson 2011
- Nelson RL, Chattopadhyay A, Brooks W, Platt I, Paavana T, Earl S. Operative Procedures for fissure in ano. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD002199.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rotholtz 2005
- Rotholtz NA, Bun M, Mauri MV, Bosio R, Peczan CE, Mezzadri NA. Long‐term assessment of fecal incontinence after lateral internal sphincterotomy. Tech. Coloproctology 2005;9:115‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schouten 1994
- Schouten WR, Briel HW, Auwerda JJA. Relationship between anal pressure and anodermal blood flow. Dis Colon & Rectum 1994;37:664‐669. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Nelson 2006
- Nelson RL. Non‐surgical therapy for anal fissure. Cochrane Database of Systematic Reviews July 30 2006, Issue 4. [DOI: 10.1002/14651858.CD003431.pub2] [DOI] [Google Scholar]


