Abstract
Background
The role of prophylactic gastrojejunostomy in patients with unresectable periampullary cancer is controversial.
Objectives
To determine whether prophylactic gastrojejunostomy should be performed routinely in patients with unresectable periampullary cancer.
Search methods
For the initial version of this review, we searched the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, issue 3), MEDLINE, EMBASE and Science Citation Index Expanded until April 2010. Literature searches were re‐run in August 2012.
Selection criteria
We included randomised controlled trials comparing prophylactic gastrojejunostomy versus no gastrojejunostomy in patients with unresectable periampullary cancer (irrespective of language or publication status).
Data collection and analysis
Two review authors independently assessed trials for inclusion and independently extracted data. We analysed data with both the fixed‐effect and the random‐effects models using Review Manager (RevMan). We calculated the hazard ratio (HR), risk ratio (RR), and mean difference (MD) with 95% confidence intervals (CI) based on an intention‐to‐treat or available case analysis.
Main results
We identified two trials (of high risk of bias) involving 152 patients randomised to gastrojejunostomy (80 patients) and no gastrojejunostomy (72 patients). In both trials, patients were found to be unresectable during exploratory laparotomy. Most of the patients also underwent biliary‐enteric drainage. There was no evidence of difference in the overall survival (HR 1.02; 95% CI 0.84 to 1.25), peri‐operative mortality or morbidity, quality of life, or hospital stay (MD 0.97 days; 95%CI ‐0.18 to 2.12) between the two groups. The proportion of patients who developed long‐term gastric outlet obstruction was significantly lower in the prophylactic gastrojejunostomy group (2/80; 2.5%) compared with no gastrojejunostomy group (20/72; 27.8%) (RR 0.10; 95%CI 0.03 to 0.37). The operating time was significantly longer in the gastrojejunostomy group compared with no gastrojejunostomy group (MD 45.00 minutes; 95%CI 21.39 to 68.61).
Authors' conclusions
Routine prophylactic gastrojejunostomy is indicated in patients with unresectable periampullary cancer undergoing exploratory laparotomy (with or without hepaticojejunostomy).
Keywords: Humans, Ampulla of Vater, Ampulla of Vater/surgery, Common Bile Duct Neoplasms, Common Bile Duct Neoplasms/surgery, Gastric Bypass, Gastric Bypass/methods, Gastric Bypass/mortality, Gastric Outlet Obstruction, Gastric Outlet Obstruction/prevention & control, Jaundice, Jaundice/prevention & control, Jaundice/surgery, Length of Stay, Quality of Life, Randomized Controlled Trials as Topic
Plain language summary
Routine diversion of food for patients with unresectable periampullary cancers without obstruction to the stomach outlet
Periampullary cancer is cancer that forms near the junction of the lower end of the common bile duct (the channel that transmits bile from the liver to the small bowel), pancreatic duct, and the upper part of the small bowel. Four‐fifths of these tumours are not amenable to surgical removal (unresectable periampullary cancer). Because of its close proximity to the stomach outlet, these periampullary cancers can cause obstruction to the stomach outlet and prevent the flow of food from the stomach to the small bowel. While diversion of food by way of joining the stomach to the upper small bowel (gastrojejunostomy) or inserting a duodenal stent across the obstructed part of the small bowel is necessary for patients who have established stomach outlet obstruction, the role of prophylactic gastrojejunostomy in patients without established stomach outlet obstruction is controversial. The aim of this review was to determine whether prophylactic gastrojejunostomy should be performed routinely in patients with unresectable periampullary cancer. We searched for randomised controlled trials comparing prophylactic gastrojejunostomy versus no gastrojejunostomy in patients with unresectable periampullary cancer. Two review authors independently assessed the studies for inclusion and extracted data.
We identified two trials (of high risk of bias or systematic error) involving 152 patients randomised to gastrojejunostomy (80) and no gastrojejunostomy (72). In both studies, patients were found to be unresectable during operations aimed at surgical removal i.e. the stomach was opened to remove the cancer but the cancer could not be removed. There was no evidence of any difference in the overall survival, surgical complications, quality of life, or hospital stay between the two groups. The proportion of patients who developed long‐term stomach outflow obstruction was significantly lower in the prophylactic gastrojejunostomy group (2.5%) compared with no gastrojejunostomy group (27.8%). The operating time was significantly longer in the gastrojejunostomy group compared with no gastrojejunostomy group by about 45 minutes. Routine prophylactic gastrojejunostomy is indicated in patients with unresectable periampullary cancer undergoing open operation of the stomach. There is no information available currently about the necessity for prophylactic gastrojejunostomy in patients with periampullary cancer diagnosed to be unresectable by investigations such as scans. Further trials of low risk of bias are necessary to assess the role of prophylactic gastrojejunostomy in patients with unresectable periampullary cancer.
Summary of findings
Summary of findings for the main comparison. Prophylactic gastrojejunostomy for unresectable periampullary carcinoma.
| Prophylactic gastrojejunostomy for unresectable periampullary carcinoma | ||||||
| Patient or population: patients with unresectable periampullary carcinoma Settings: inpatients Intervention: Prophylactic gastrojejunostomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prophylactic gastrojejunostomy | |||||
| Survival Follow‐up: 6 to 9 months | 40 per 1001 | 41 per 100 (35 to 48)1 | HR 1.02 (0.84 to 1.25) | 152 (2 studies) | ⊕⊝⊝⊝ very low2,3 | There was no difference in the long‐term survival between the patients undergoing prophylactic gastrojejunostomy and those who did not. |
| Gastric outlet obstruction Clinical symptoms of vomiting with confirmation of the gastric outlet obstruction by radiological or endoscopic investigations Follow‐up: 6 months | 28 per 100 | 3 per 100 (1 to 10) | RR 0.1 (0.03 to 0.37) | 152 (2 studies) | ⊕⊝⊝⊝ very low2,3,4 | Gastric outlet obstruction was significantly reduced in those undergoing gastrojejunostomy. |
| Operating time | The mean operating time in the control groups was 209 minutes | The mean operating time in the intervention groups was 45 minutes longer (21.39 to 68.61 longer) | 87 (1 study) | ⊕⊝⊝⊝ very low2,3,4 | The operating time was 45 minutes longer in the gastrojejunostomy group than the control group. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HR: Hazard ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 At six to nine months approximately 40% of patients in the control group were dead. This is equivalent to about 29 patients. Since all the included patients were followed until death, there was no censoring of patients and the figure is of actual survival of patients rather than actuarial survival. 2 Three patients in one trial were excluded from the trial report because of loss to follow‐up. 3 Small numbers of events 4 The existence or method of blinding was not reported in either trial.
Background
Description of the condition
Periampullary cancer is cancer that forms near the ampulla of Vater (National Cancer Institute 2009). This includes cancer of the head and neck of the pancreas, cancer of the distal end of the bile duct, cancer of the ampulla of Vater, and cancer of the second part of the duodenum. Pancreaticoduodenectomy is generally considered the only curative treatment for periampullary cancer. About 20% of periampullary cancers are resectable (Engelken 2003; Michelassi 1989; Smith 2008). In the remaining patients, the cancers are not resected because of infiltration of local structures or because of disseminated disease. This may be found during investigations performed for staging the cancer. In spite of all the pre‐operative staging investigations such as computerised tomogram (CT scan), endoscopic ultrasound (EUS), diagnostic laparoscopy (DL) or a combination of these investigations, between 8% and 33% of patients are found to have unresectable pancreatic cancer on laparotomy (Mayo 2009). Between 10% and 15% of those with unresected periampullary cancers who are considered to be at low risk of gastric outlet obstruction develop gastric outlet obstruction at a later stage (Watanapa 1992; Wong 2002) due to direct involvement of the second part of the duodenum by cancer.
Description of the intervention
Gastrojejunostomy is an anastomotic procedure involving anastomosis (surgical connection) of the stomach and the jejunum. Gastrojejunostomy can be performed either by open operation or by laparoscopic operation (Ly 2009).
How the intervention might work
By anastomosing the stomach and the jejunum prophylactically, the food can bypass the obstructed duodenum. This may allow the patients to eat food thereby providing adequate nutrition until eventual death due to disseminated cancer. As the patients are able to eat, the quality of life may be better.
Why it is important to do this review
About 70% of patients with unresectable ampullary cancer present with obstructive jaundice (Yokoyama 2005). Prior to the use of biliary stents for malignant obstructive jaundice, most of these patients used to undergo surgical bypass procedure for biliary obstruction. Currently, patients will undergo surgery when the staging investigations would suggest the possibility of curative resection. The British Society of Gastroenterology, however, recommends that prophylactic duodenal bypass surgery be performed during palliative biliary bypass surgery carried out on patients expected to survive longer than average (British Society of Gastroenterology 2005). This was based on evidence that little or no extra morbidity results from adding palliative gastrojejunostomy to palliative biliary surgery (Watanapa 1992). Gastrojejunostomy can, however, cause delayed gastric emptying and some authors suggest that there is no need to perform a palliative gastrojejunostomy routinely (Schantz 1984).
In spite of all the pre‐operative staging investigations such as computerised tomogram (CT scan), endoscopic ultrasound (EUS), diagnostic laparoscopy (DL) or a combination of these investigations, between 8% and 33% of patients are found to have unresectable pancreatic cancer on laparotomy (Mayo 2009). Whether prophylactic gastrojejunostomy is indicated at the time of laparotomy is not clear. With diagnostic laparoscopy being performed by some surgeons to decrease unnecessary laparotomy (Mayo 2009), it is also not clear whether prophylactic laparoscopic gastrojejunostomy is indicated if the cancer is considered unresectable during diagnostic laparoscopy.
In addition, with the recent success rates of about 80% for self‐expandable metallic stents in relieving malignant gastric outlet obstruction with low morbidity (Gaidos 2009), subjecting all the patients with unresectable periampullary cancers undergoing laparotomy to gastrojejunostomy (with associated morbidity) may be unnecessary.
There is no systematic review of randomised controlled trials investigating whether prophylactic gastrojejunostomy is routinely indicated at the time of laparotomy or laparoscopy.
Objectives
To determine whether prophylactic gastrojejunostomy should be performed routinely in people with unresectable periampullary cancer based on differences in survival, peri‐operative morbidity, quality of life, and the incidence of gastric outlet obstruction.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials of parallel design, irrespective of blinding, sample size, publication status, or language. We planned to exclude quasi‐randomised studies but did not identify any quasi‐randomised studies. We excluded studies of other study designs in the presence of randomised controlled trials. This is because of the potential for bias in quasi‐randomised studies and non‐randomised studies (Gurusamy 2009; Higgins 2008).
Types of participants
People with unresectable periampullary cancer undergoing laparotomy (for palliative biliary bypass or found to be unresectable at the time of laparotomy performed with an intention of resection) or laparoscopy (diagnostic laparoscopy to stage the cancer during which the cancers were found to be unresectable) or identified as unresectable by any other investigation such as CT scan or positron emission tomography (PET scan). We did not consider those with established gastric outlet obstruction in this review.
Types of interventions
Routine prophylactic gastrojejunostomy (open or laparoscopic) against a comparator of no prophylactic gastrojejunostomy.
We allowed co‐interventions including surgical biliary bypass or endoscopic biliary bypass if performed equally in both study arms.
Types of outcome measures
Primary outcomes
Survival.
Peri‐operative morbidity (as defined by study authors. As far as possible, we classified peri‐operative morbidity by Clavien‐Dindo classification (Clavien 2009; Dindo 2004)).
Secondary outcomes
Quality of life (as defined by authors).
Gastric outlet obstruction (as defined by authors).
Operating time.
Hospital stay.
Search methods for identification of studies
Electronic searches
We searched:
The Cochrane Upper Gastrointestinal and Pancreatic Diseases Group Controlled Trials Register (Forman 2009) (Appendix 1)
The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library 2012, issue 8) (Appendix 1)
MEDLINE via Pubmed (January 1951 to August 2012) (Appendix 2)
EMBASE via OVIDSP (January 1980 to August 2012) (Appendix 3)
Science Citation Index Expanded (Royle 2003) until August 2012 (Appendix 4)
Searching other resources
We searched the references of the identified trials to identify further relevant trials. We also searched the metaRegister of Controlled Trials (mRCT) for further trials (Appendix 5). This register includes the following trials registers: ISRCTN Register and NIH ClinicalTrials.gov Register, among others.
Data collection and analysis
We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008) and the Cochrane Upper GastrointestinaI and Pancreatic Diseases Group Module (Forman 2009).
Selection of studies
Review authors KG and SK independently identified the trials for inclusion. We have also listed the excluded trials with the reasons for the exclusion.
Data extraction and management
Review authors KG and SK independently extracted data for the review. In addition to the outcomes, we extracted the population characteristics (such as sex, age, disease aetiology), and the interventions used in each trial. We assessed the methodological quality of the trials independently, without masking the trial names. We sought any unclear or missing information by contacting the authors of the individual trials. If there was any doubt whether the trials shared participants ‐ completely or partially (by identifying common authors and centres) ‐ we planned to contact the authors of the trials to determine whether they had published multiple trial reports, but we found no such trials. For continuous outcomes, we imputed the standard deviation from P values according to the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008) and used the median for the meta‐analysis when the mean was not available. If it was not possible to calculate the standard deviation from the P value or confidence intervals, we planned to impute the standard deviation as the highest standard deviation noted for that group under that outcome.
For overall survival and disease‐free survival, we extracted the logarithm of hazard ratios (ln(HR)) and the standard error (SE) of ln(HR) according to the methods described by Parmar 1998 using the excel sheet provided by Tierney 2007.
We resolved any differences in opinion through discussion.
Assessment of risk of bias in included studies
According to empirical evidence (Kjaergard 2001; Moher 1998; Schulz 1995; Wood 2008), we assessed the methodological quality of the trials based on sequence generation, allocation concealment, blinding of participants, personnel, and outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias. We based quality components on the Cochrane Handbook for Systematic Reviews of Interventions (Gurusamy 2009; Higgins 2008).
Sequence generation
Low risk of bias (the method used was either adequate (e.g., computer‐generated random numbers, table of random numbers) or unlikely to introduce confounding).
Uncertain risk of bias (there was insufficient information to assess whether the method used was likely to introduce confounding).
High risk of bias (the method used (e.g., quasi‐randomised trials) was improper and likely to introduce confounding).
Allocation concealment
Low risk of bias (the method used (e.g., central allocation) was unlikely to induce bias on the final observed effect).
Uncertain risk of bias (there was insufficient information to assess whether the method used was likely to induce bias on the estimate of effect).
High risk of bias (the method used (e.g., open random allocation schedule) was likely to induce bias on the final observed effect).
Blinding of participants and outcome assessors
It is impossible to blind the surgeons as to whether prophylactic gastrojejunostomy was performed, but it is possible to blind the participants and the outcome assessors to the groups.
Low risk of bias (blinding was performed adequately, or the outcome measurement was not likely to be influenced by lack of blinding).
Uncertain risk of bias (there was insufficient information to assess whether the type of blinding used was likely to induce bias on the estimate of effect).
High risk of bias (no blinding or incomplete blinding, and the outcome or the outcome measurement was likely to be influenced by lack of blinding).
Incomplete outcome data
Low risk of bias (the underlying reasons for missingness were unlikely to make treatment effects depart from plausible values, or proper methods have been employed to handle missing data).
Uncertain risk of bias (there was insufficient information to assess whether the missing data mechanism in combination with the method used to handle missing data was likely to induce bias on the estimate of effect).
High risk of bias (the crude estimate of effects (e.g., complete case estimate) was clearly biased due to the underlying reasons for missingness, and the methods used to handle missing data were unsatisfactory).
Selective outcome reporting
Low risk of bias (the trial protocol was available and all of the trial's pre‐specified outcomes that are of interest in the review had been reported or similar).
Uncertain risk of bias (there was insufficient information to assess whether the magnitude and direction of the observed effect were related to selective outcome reporting).
High risk of bias (not all of the trial's pre‐specified primary outcomes had been reported or similar).
Other bias
Baseline imbalance
Low risk of bias (there was no baseline imbalance in important characteristics).
Uncertain risk of bias (the baseline characteristics were not reported).
High risk of bias (there was a baseline imbalance due to chance or due to imbalanced exclusion after randomisation).
Early stopping
Low risk of bias (sample size calculation was reported and the trial was not stopped or the trial was stopped early by a formal stopping rule at a point where the likelihood of observing an extreme intervention effect due to chance was low).
Uncertain risk of bias (sample size calculations were not reported and it was not clear whether the trial was stopped early or not).
High risk of bias (the trial was stopped early due to an informal stopping rule or the trial was stopped early by a formal stopping rule at a point where the likelihood of observing an extreme intervention effect due to chance was high).
Source of funding bias
Low risk of bias (the trial's source(s) of funding did not come from any parties that might have conflicting interest (e.g., instrument manufacturer)).
Uncertain risk of bias (the source of funding was not clear).
High risk of bias (the trial was funded by an instrument manufacturer).
We considered trials that were classified as low risk of bias in sequence generation, allocation concealment, blinding, incomplete data, and selective outcome reporting as being at low risk of bias.
Measures of treatment effect
We measured the hazard ratio with the 95% confidence intervals for survival. For dichotomous outcomes, we calculated the risk ratio (RR) with 95% confidence interval. For continuous outcomes, we calculated mean difference (MD) or standardised mean difference (SMD) with 95% confidence interval. We also calculated the risk difference with 95% confidence interval for dichotomous outcomes but we have not reported the results as they were not different from the RR.
Unit of analysis issues
The unit of analysis was each participant recruited into the trials.
Dealing with missing data
We performed the analysis on an 'intention‐to‐treat' basis (Newell 1992) whenever possible. Otherwise, we adopted the 'available case analysis'.
Assessment of heterogeneity
We used the Chi² test with significance set at P value 0.10 to explore heterogeneity, and we used I² to measure the quantity of heterogeneity (Higgins 2002). We considered an I² of > 30% to indicate statistically significant heterogeneity.
Assessment of reporting biases
We planned to use a funnel plot to explore bias (Egger 1997; Macaskill 2001), and use asymmetry in a funnel plot of trial size against treatment effect to assess bias. We planned to perform the linear regression approach described by Egger et al to determine the funnel plot asymmetry (Egger 1997), but we did not do this because we only included two trials.
Data synthesis
We performed the meta‐analyses according to the recommendations of The Cochrane Collaboration (Higgins 2008). We used the software package Review Manager (RevMan) 5 provided by The Cochrane Collaboration (RevMan 2008). We used a random‐effects model (DerSimonian 1986) and a fixed‐effect model (DeMets 1987). In case of discrepancy between the two models, we planned to report both results; otherwise we planned to report only the results from the fixed‐effect model. Since there was no difference in the models, we have reported the results of fixed‐effect model only. We combined the hazard ratios of overall survival and disease‐free survival obtained from the different trials using the generic inverse variance method. We did not plan to perform meta‐analysis of outcomes such as quality of life and gastric outlet obstruction if there were significant differences in the definitions or scales used. Only one trial reported the quality of life (Van Heek 2003). As there were no significant differences in the definitions used for gastric outlet obstruction, we performed the meta‐analysis. We were not able to classify peri‐operative morbidity by Clavien‐Dindo classification (Clavien 2009; Dindo 2004). Instead, we performed meta‐analysis of different peri‐operative morbidities (similar across trials) separately.
Subgroup analysis and investigation of heterogeneity
We planned the following sub‐group analysis.
Trials with low risk of bias versus those with high risk of bias.
Different types of gastrojejunostomy (open versus laparoscopic).
Absent biliary obstruction versus surgical biliary bypass versus endoscopic biliary bypass.
We planned to perform the Chi² test for subgroup differences set at a P value of 0.05 to identify any subgroup differences.
We did not perform a subgroup analysis, however, because only two trials were included in the review.
Sensitivity analysis
We planned to perform a sensitivity analysis by imputing the missing values using various scenarios such as good outcome analysis, poor outcome analysis, best case analysis, and worst case analysis (Gurusamy 2009). In cases where we found 'zero‐event' trials for statistically significant outcomes, we planned to perform a sensitivity analysis, with and without, empirical continuity correction factors as suggested by Sweeting 2004. In cases where we imputed the mean and standard deviation, we planned to perform another analysis excluding such imputed data to determine their influence on the results.
Results
Description of studies
Results of the search
We identified a total of 2352 references through the electronic searches of the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group Controlled Trials Register and the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE and Science Citation Index Expanded (literature searches were re‐run in August 2012). We also identified 439 references through searching clinical trials registers via the metaRegister of Controlled Trials (mRCT) for further trials. We have shown the flow of references in Figure 1. We excluded 1462 duplicates and 1314 clearly irrelevant references through reading abstracts. We retrieved five references for further assessment. No references were identified through scanning reference lists of the identified randomised trials. Of the five retrieved references, we excluded three for the reasons listed in the table 'Characteristics of excluded studies'. Two references of two randomised controlled trials fulfilled the inclusion criteria.
1.
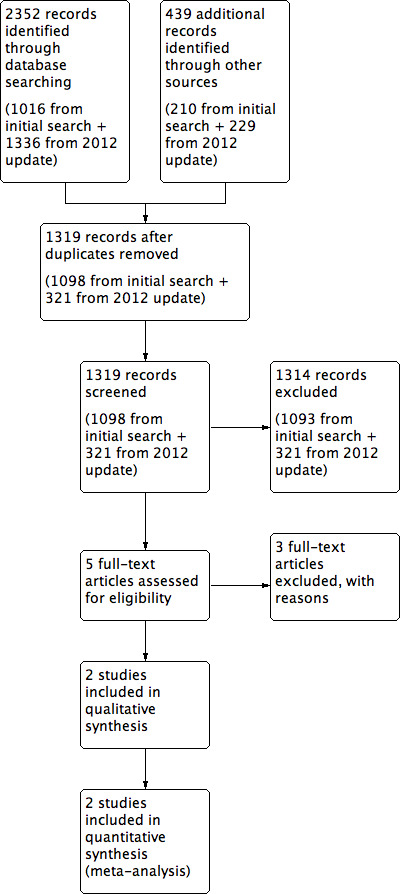
Study flow diagram.
Included studies
In the two trials included in this review, 152 patients were randomised to prophylactic gastrojejunostomy (n = 80) and no gastrojejunostomy (n = 72) (Lillemoe 1999; Van Heek 2003). Both trials included patients who were found to have unresectable periampullary cancers on exploratory laparotomy undertaken with an intention to perform a curative resection (Lillemoe 1999; Van Heek 2003). The patients did not have established gastric outlet obstruction prior to the exploratory laparotomy. In one trial, patients with biliary obstruction (approximately 75% of patients) underwent hepaticojejunostomy (Lillemoe 1999). In the other trial, all patients irrespective of whether they had biliary obstruction underwent hepaticojejunostomy (Van Heek 2003).
Excluded studies
The reasons for exclusion of studies are listed in the table 'Characteristics of excluded studies'. None of these studies were randomised controlled trials.
Risk of bias in included studies
Both trials were considered to be at high risk of bias (Lillemoe 1999; Van Heek 2003). The risk of bias is summarised in the 'Risk of bias' graph (Figure 2) and 'Risk of bias' summary (Figure 3). The risk of bias in the various domains are described below.
2.
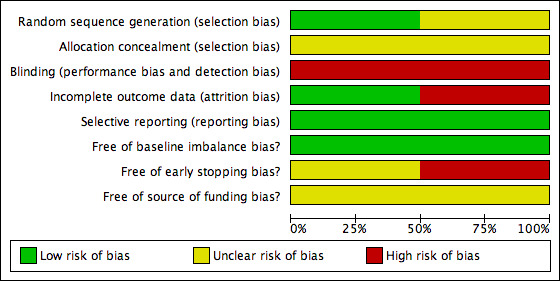
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
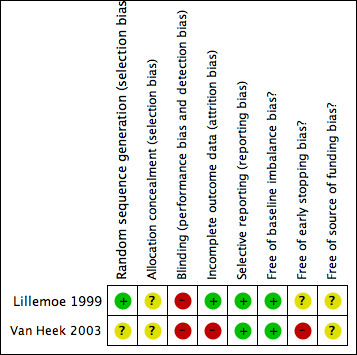
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The allocation sequence generation was adequate in one trial (Lillemoe 1999). The allocation concealment was unclear in both trials (Lillemoe 1999; Van Heek 2003).
Blinding
Blinding of participants and outcome assessors was not reported in either trial (Lillemoe 1999; Van Heek 2003).
Incomplete outcome data
There were no post‐randomisation drop‐outs in one trial (Lillemoe 1999). In the other trial, there were five post‐randomisation drop‐outs (Van Heek 2003). Of these, one patient was found to be resectable after the frozen section biopsy of the liver turned out to be benign. Another patient had benign disease on frozen section biopsy of the pancreas. Three patients were lost to follow‐up. These three patients lost to follow‐up could have affected the effect estimates obtained in this review.
Selective reporting
No protocol was available for the two trials (Lillemoe 1999; Van Heek 2003). Both trials reported the primary outcomes of this review and are free from bias due to selective reporting (Lillemoe 1999; Van Heek 2003).
Other potential sources of bias
There was no baseline imbalance in important prognostic factors between the groups in either trial (Lillemoe 1999; Van Heek 2003). One trial was stopped early because of evidence of benefit (Van Heek 2003). The authors state that they took into account the results of the first trial (Lillemoe 1999) and also in the decision to stop this trial (Van Heek 2003). There were no prespecified early stopping rules in the trial (Van Heek 2003). The other trial did not report sample size calculations and so it was not possible to judge whether the trial was stopped early. Neither trial reported the source of funding (Lillemoe 1999; Van Heek 2003).
Effects of interventions
See: Table 1
Primary outcomes
Survival
Analysis 1.1 Both trials reported this outcome in the form of Kaplan‐Meier curves (Lillemoe 1999; Van Heek 2003). There was no difference in the long‐term survival between the patients undergoing prophylactic gastrojejunostomy and those who did not undergo prophylactic gastrojejunostomy (hazard ratio (HR) 1.02; 95% confidence interval (CI) 0.84 to 1.25).
1.1. Analysis.
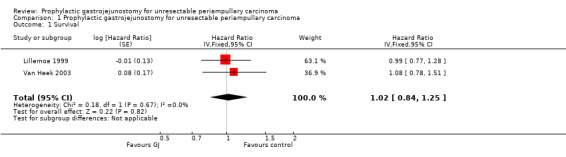
Comparison 1 Prophylactic gastrojejunostomy for unresectable periampullary carcinoma, Outcome 1 Survival.
Peri‐operative morbidity
Analysis 1.2 It was not possible to classify the peri‐operative morbidity according to the Clavien‐Dindo classification (Clavien 2009; Dindo 2004) in either trial (Lillemoe 1999; Van Heek 2003). Instead we analysed individual morbidities reported by the authors. There was no difference in peri‐operative mortality or morbidity between the two groups. Among the post‐operative morbidities, both trials reported delayed gastric emptying. By definition, delayed gastric emptying occurs in the immediate post‐operative period. It was unclear from either trial, however, whether the delayed gastric emptying was mechanical (i.e. due to gastric outlet obstruction) or functional (i.e. due to gastroparesis).
1.2. Analysis.
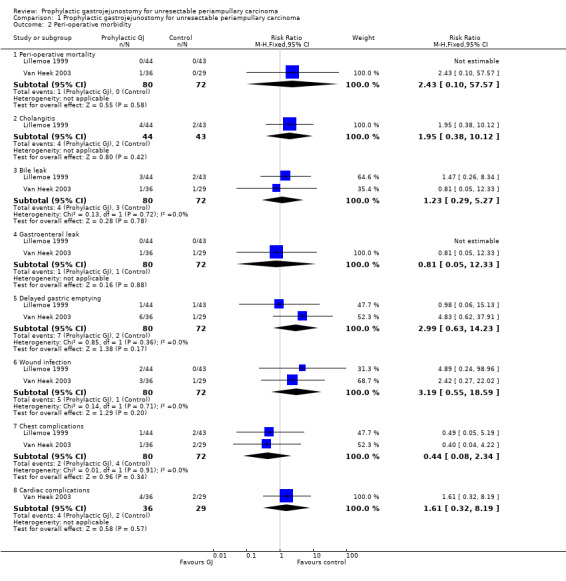
Comparison 1 Prophylactic gastrojejunostomy for unresectable periampullary carcinoma, Outcome 2 Peri‐operative morbidity.
Secondary outcomes
Quality of life
Analysis 1.3 One trial reported the quality of life of patients after surgery based on the European Organization for Research and Treatment of Cancer (EORTC) Quality‐of‐Life Questionnaire (Van Heek 2003). The authors of this trial report that there was no difference in the quality of life between the two groups at any time point. Although the mean and standard deviation could be imputed from the graphs containing upper and lower confidence intervals, the number of patients who reported their quality of life was not available at different time points. So, the quality of life information could not be represented as forest plots.
1.3. Analysis.
Comparison 1 Prophylactic gastrojejunostomy for unresectable periampullary carcinoma, Outcome 3 Quality of life.
| Quality of life | |
|---|---|
| Study | |
| Van Heek 2003 | There was no difference in the quality of life between the two groups at any time point. |
Gastric outlet obstruction
Analysis 1.4 In both trials, gastric outlet obstruction was defined as clinical symptoms of vomiting in the patient with confirmation of the gastric outlet obstruction by radiological or endoscopic investigations (Lillemoe 1999; Van Heek 2003). The proportion of patients who developed gastric outlet obstruction was 2/80 (2.5%) in the gastrojejunostomy group compared with 20/72 (27.8%) in the control group. This difference was statistically significant (risk ratio (RR) 0.10; 95% CI 0.03 to 0.37).
1.4. Analysis.
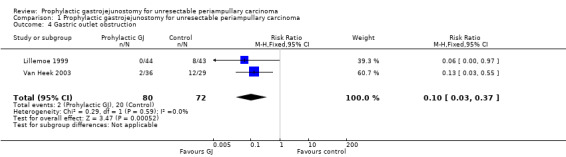
Comparison 1 Prophylactic gastrojejunostomy for unresectable periampullary carcinoma, Outcome 4 Gastric outlet obstruction.
Operating time
Analysis 1.5 The operating time was reported in one trial (Lillemoe 1999). The operating time was significantly longer in the gastrojejunostomy group than the control group (mean difference (MD) 45.00 minutes; 95% CI 21.39 to 68.61).
1.5. Analysis.
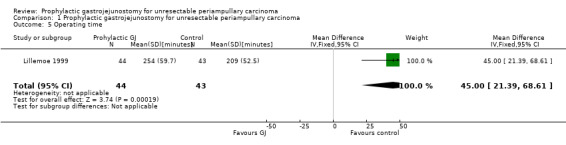
Comparison 1 Prophylactic gastrojejunostomy for unresectable periampullary carcinoma, Outcome 5 Operating time.
Hospital stay
Analysis 1.6 Both trials reported this outcome. There was no significant difference in the hospital stay between the two groups (MD 0.97 days; 95% CI ‐0.18 to 2.12).
1.6. Analysis.
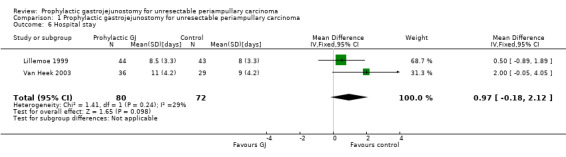
Comparison 1 Prophylactic gastrojejunostomy for unresectable periampullary carcinoma, Outcome 6 Hospital stay.
Subgroup analysis
We did not perform a subgroup analysis because only two trials were included in this review.
Variations in statistical analysis
We observed no change in results by adopting the random‐effects model or by calculating the risk difference. We did not perform the sensitivity analysis using empirical continuity correction factors as suggested by Sweeting et al (Sweeting 2004) as there were no statistically significant outcomes in the main comparison with zero event trials. We did not perform a sensitivity analysis excluding trials in which the standard deviation was imputed. This was because the standard deviation of hospital stay was not reported in both trials. Hospital stay was the only trial in which the standard deviation was imputed.
Reporting bias
We did not explore reporting bias using a funnel plot because only two trials were included in this review.
Discussion
Summary of main results
Prophylactic gastrojejunostomy decreased the incidence of late gastric outlet obstruction without any evidence of increase in the surgical morbidity in patients with unresectable periampullary cancer undergoing exploratory laparotomy performed for potential resection (of periampullary cancer). There was no evidence, however, of any survival benefit or an improvement in the quality of life as a result of the prophylactic gastrojejunostomy.
The long‐term incidence of gastric outlet obstruction in patients with unresectable periampullary cancers was 27.8% in the patients who did not undergo prophylactic gastrojejunostomy. The treatment options available for these patients with gastric outlet obstruction are surgical bypass (gastrojejunostomy) and endoscopic stent (Jeurnink 2010; Mehta 2006). Most of the patients who developed gastric outlet obstruction in the two trials included in this review underwent surgical bypass (Lillemoe 1999; Van Heek 2003). These trials were performed at a time when the duodenal stents were not readily used for the relief of malignant gastric outlet obstruction. Even if the duodenal stents were available, surgical bypass is the preferred option in the treatment of malignant gastric outlet obstruction because of the lower incidence of recurrent gastric outlet obstruction (Jeurnink 2010). If patients develop malignant gastric outlet obstruction, this will involve a second surgery. Palliative gastrojejunostomy for malignant gastric outlet obstruction cannot be undertaken lightly and can be associated with peri‐operative mortality (Lillemoe 1999; Mehta 2006). There is no evidence that the addition of gastrojejunostomy increases the operative risk for patients undergoing a surgical biliary‐enteric bypass. By adding gastrojejunostomy to exploratory laparotomy, the need for surgical bypass for gastric outlet obstruction that develops in more than a quarter of the patients with unresectable periampullary cancer can be avoided. Thus, it appears that prophylactic gastrojejunostomy can be beneficial in patients even though there was no evidence of any survival benefit or improvement in quality of life.
The hospital stay reported in the trials included only the post‐operative stay after the exploratory laparotomy (with or without hepaticojejunostomy and gastrojejunostomy) and did not include the hospital stay for the treatment of gastric outlet obstruction. Future trials should report the overall hospital stay, which would help in a cost‐effectiveness analysis of prophylactic gastrojejunostomy in patients with unresectable periampullary cancer. Future trials should also report the surgical morbidity in terms of the Clavien‐Dindo classification, which is a validated method of classifying post‐operative complications (Clavien 2009; Dindo 2004).
Overall completeness and applicability of evidence
Both trials included in this review included patients who underwent exploratory laparotomy. The results of this review are therefore applicable only in patients undergoing exploratory laparotomy. There are no randomised controlled trials assessing the role of prophylactic gastrojejunostomy in patients diagnosed to have unresectable periampullary cancers during staging procedures such as diagnostic laparoscopy or radiological imaging. Obstructive jaundice is the presenting symptom for most periampullary cancers (Lillemoe 1999; Van Heek 2003). Currently, endoscopic stenting is considered superior to open surgical bypass in patients with malignant obstructive jaundice (Moss 2006) because of the decreased risk of procedural complications. There is, however, a higher risk of recurrent obstructive jaundice in patients who received endoscopic stents versus those who underwent surgical bypass for malignant obstructive jaundice (Moss 2006). Patients with longer expected survival may therefore undergo surgical biliary‐enteric bypass, which can be performed either by conventional open method or laparoscopically (Tang 2007). A significant proportion of the patients found to have unresectable periampullary cancer on diagnostic laparoscopy or radiological imaging will, however, not be undergoing laparotomy or biliary bypass procedures. Based on the lack of evidence of survival benefit or improvement of quality of life in patients undergoing prophylactic gastrojejunostomy, there does not appear to be any reason for performing routine prophylactic gastrojejunostomy in patients with unresectable periampullary cancer who did not undergo exploratory laparotomy (with or without hepaticojejunostomy). Further randomised controlled trials are necessary to assess the role of prophylactic gastrojejunostomy (preferably laparoscopic) in patients with unresectable periampullary cancer not undergoing exploratory laparotomy.
Quality of the evidence
Both trials included in this review have a high risk of bias (Lillemoe 1999; Van Heek 2003). Lack of blinding can introduce bias in most of the outcomes included in the review. The only trial that assessed quality of life used the EORTC QOL, a validated quality of life scale (Aaronson 1993). Lack of observer and patient blinding can result in a biased estimate of the results. Patient blinding could have been easily achieved as there is no need for any major difference in the post‐operative management because of the addition of gastrojejunostomy to hepaticojejunostomy. The use of a second surgical team blinded to the groups would have enabled assessor blinding (Gurusamy 2009).
In one trial, there were five post‐randomisation drop‐outs (Van Heek 2003). While the exclusion of two patients (one who underwent pancreatic resection surgery after the frozen section biopsy of the liver turned out to be non‐cancerous and another patient who had benign tumour) would not have introduced any bias in the effect estimate, exclusion of three other patients due to lack of availability of follow‐up data could have introduced bias in the effect estimate.
Future trials should use patient blinding and assessor blinding and should randomise patients only after it is clear that patients have unresectable periampullary cancer.
Potential biases in the review process
Only two trials were included in this review. We were unable to assess reporting bias. The survival estimate was extracted from the Kaplan‐Meier curves. Since only two trials were included, the possibility of random errors (type I and type II errors) in the various outcomes exists.
Agreements and disagreements with other studies or reviews
This study agrees with the conclusions of the authors of the two trials (Lillemoe 1999; Van Heek 2003). It also agrees with a systematic review and meta‐analysis of prospective and retrospective studies on this topic (Huser 2009).
Authors' conclusions
Implications for practice.
Routine prophylactic gastrojejunostomy is indicated in patients with unresectable periampullary cancer undergoing exploratory laparotomy (with or without hepaticojejunostomy).
Implications for research.
Further trials of low risk of bias are necessary to assess the role of prophylactic gastrojejunostomy (including laparoscopic gastrojejunostomy) particularly in patients not undergoing laparotomy or biliary‐enteric bypass.
What's new
| Date | Event | Description |
|---|---|---|
| 4 September 2012 | New citation required but conclusions have not changed | Review updated. No new studies identified for inclusion. |
| 4 September 2012 | New search has been performed | Literature searches re‐run. No new studies identified for inclusion. |
History
Protocol first published: Issue 6, 2010 Review first published: Issue 10, 2010
| Date | Event | Description |
|---|---|---|
| 13 February 2012 | Amended | Contact details updated. |
Acknowledgements
The Cochrane Upper Gastrointestinal and Pancreatic Disease Group for their advice on this review.
This project was funded by the National Institute for Health Research.Disclaimer of the Department of Health: 'The views and opinions expressed in the review are those of the authors and do not necessarily reflect those of the National Institute for Health Research (NIHR), National Health Services (NHS), or the Department of Health.'
Appendices
Appendix 1. CENTRAL search strategy
#1 ((ampulla near/2 vater*) or ampullavateric or (papilla near/2 vater*) or choledoch* or alcholedoch* or bile duct* or biliary or cholangio* or gall duct or duoden* or small bowel or small intestin* or enter* or pancrea*) #2 (carcin* or cancer* or neoplasm* or tumour* or tumor* or cyst* or growth* or adenocarcin* or malign*) #3 Gastroenterostom* #4 (gastrojejun* or jejunogastr*) #5 (gastric near/2 bypass) #6 Billroth #7 (Roux Y or Roux en Y) #8 MeSH descriptor Anastomosis, Roux‐en‐Y explode all trees #9 (#3 OR #4 OR #5 OR #6 OR #7 OR #8) #10 (#1 AND #2 AND #9)
Appendix 2. MEDLINE search strategy
(Gastroenterost* OR Billroth OR gastrojejunost* OR gastric bypass OR ((gastrojejun* OR jejunogastric OR gastroenter* OR enterogastric OR Roux Y OR Roux en Y) AND (anastomo* OR fixation*)) OR "Gastroenterostomy"[Mesh] OR "Gastric Bypass"[Mesh]) AND (((ampulla vateri OR "Ampulla of Vater" [Mesh] OR ampullavateric OR papilla vateri OR vater papilla OR vater ampulla OR choledoch* OR alcholedoch* OR bile duct* OR biliary OR cholangiocarcinoma OR cholangiocarcinomas OR cholangiocellular OR cholangiolar OR gall duct OR duoden* OR small bowel OR small intestin* OR enter* OR pancrea*) AND (carcin* or cancer* or neoplasm* or tumour* or tumor* or cyst* or growth* or adenocarcin* or malign*)) OR "Duodenal Neoplasms"[Mesh] OR "Pancreatic Neoplasms"[Mesh] OR "Common Bile Duct Neoplasms"[Mesh]) AND ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT (humans[mh] AND animals[mh]))
Appendix 3. EMBASE search strategy
1 (Gastroenterost* or Billroth or gastrojejunost* or gastric bypass or ((gastrojejun* or jejunogastric or gastroenter* or enterogastric or Roux Y or Roux en Y) and (anastomo* or fixation*))).af. 2 exp gastrojejunostomy/ or exp gastroenterostomy/ 3 1 or 2 4 ((ampulla vateri or ampullavateric or papilla vateri or vater papilla or vater ampulla or choledoch* or alcholedoch* or bile duct* or biliary or cholangiocarcinoma or cholangiocarcinomas or cholangiocellular or cholangiolar or gall duct or duoden* or small bowel or small intestin* or enter* or pancrea*) and (carcin* or cancer* or neoplasm* or tumour* or tumor* or cyst* or growth* or adenocarcin* or malign*)).af. 5 exp duodenum cancer/ or exp duodenum carcinoma/ or exp Vater papilla tumor/ or exp Vater papilla carcinoma/ or pancreas carcinoma/ or exp pancreas cancer/ or exp bile duct carcinoma/ or exp bile duct cancer/ 6 4 or 5 7 Clinical trial/ 8 Randomized controlled trial/ 9 Randomization/ 10 Single‐Blind Method/ 11 Double‐Blind Method/ 12 Cross‐Over Studies/ 13 Random Allocation/ 14 Placebo/ 15 Randomi?ed controlled trial$.tw. 16 Rct.tw. 17 Random allocation.tw. 18 Randomly allocated.tw. 19 Allocated randomly.tw. 20 (allocated adj2 random).tw. 21 Single blind$.tw. 22 Double blind$.tw. 23 ((treble or triple) adj blind$).tw. 24 Placebo$.tw. 25 Prospective study/ 26 or/7‐25 27 Case study/ 28 Case report.tw. 29 Abstract report/ or letter/ 30 or/27‐29 31 26 not 30 32 3 and 6 and 31
Appendix 4. Science Citation Index Expanded search strategy
# 1 TS=(Gastroenterost* or Billroth or gastrojejunost* or gastric bypass or ((gastrojejun* or jejunogastric or gastroenter* or enterogastric or Roux Y or Roux en Y) and (anastomo* or fixation*))) # 2 TS=(((ampulla vateri or ampullavateric or papilla vateri or vater papilla or vater ampulla or choledoch* or alcholedoch* or bile duct* or biliary or cholangiocarcinoma or cholangiocarcinomas or cholangiocellular or cholangiolar or gall duct or duoden* or small bowel or small intestin* or enter* or pancrea*) and (carcin* or cancer* or neoplasm* or tumour* or tumor* or cyst* or growth* or adenocarcin* or malign*))) # 3 TS=(random* OR rct* OR crossover OR masked OR blind* OR placebo* OR meta‐analysis OR systematic review* OR meta‐analys*) # 4 #3 AND #2 AND #1
Appendix 5. MetaRegister of randomised controlled trials (mRCT)
(Gastroenterost* or Billroth or gastrojejunost* or gastric bypass or ((gastrojejun* or jejunogastric or gastroenter* or enterogastric or Roux Y or Roux en Y) and (anastomo* or fixation*)))
Data and analyses
Comparison 1. Prophylactic gastrojejunostomy for unresectable periampullary carcinoma.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Survival | 2 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.84, 1.25] | |
| 2 Peri‐operative morbidity | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Peri‐operative mortality | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [0.10, 57.57] |
| 2.2 Cholangitis | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.38, 10.12] |
| 2.3 Bile leak | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.29, 5.27] |
| 2.4 Gastroenteral leak | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.05, 12.33] |
| 2.5 Delayed gastric emptying | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.99 [0.63, 14.23] |
| 2.6 Wound infection | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.19 [0.55, 18.59] |
| 2.7 Chest complications | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.08, 2.34] |
| 2.8 Cardiac complications | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.32, 8.19] |
| 3 Quality of life | Other data | No numeric data | ||
| 4 Gastric outlet obstruction | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.37] |
| 5 Operating time | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 45.0 [21.39, 68.61] |
| 6 Hospital stay | 2 | 152 | Mean Difference (IV, Fixed, 95% CI) | 0.97 [‐0.18, 2.12] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Lillemoe 1999.
| Methods | Randomised controlled trial | |
| Participants | Country: USA. Sample size: 87. Post‐randomisation drop‐out: 0 (0%). Revised sample size: 87. Females: 37 (42.5%). Mean age: 67 years. Pancreatic cancer: 84 (96.6%). Ampullary cancer: 0 (0%). Duodenal cancer: 1 (1.1%). Bile duct cancer: 2 (2.3%). Biliary obstruction: 65 (74.7%). Open or laparoscopic gastrojejunostomy: Open. Histological confirmation: Yes. Minimum follow‐up: 6 months. Biliary obstruction management: Hepaticojejunostomy. Inclusion criteria: Patients undergoing surgery for peri‐ampullary cancer with intention to resect. Exclusion criteria: 1. Resectable disease on laparotomy. 2. Considered to be at high risk of gastric outlet obstruction based on radiological features or intraoperative findings | |
| Interventions | The participants were randomly assigned to two groups.
Group 1: Gastrojejunostomy (n = 44).
Further details: a retrocolic gastrojejunostomy performed to the most dependent portion of the gastric antrum.
Group 2: No gastrojejunostomy (n = 43). Patients with biliary tract obstruction underwent hepaticojejunostomy in both groups. |
|
| Outcomes | The outcomes reported were survival, peri‐operative morbidity, gastric outlet obstruction, operating time, hospital stay. | |
| Notes | Definition for gastric outlet obstruction: All patients had nausea and vomiting, with evidence of gastric outlet obstruction documented on endoscopy, upper gastrointestinal series, or computed tomography scan. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized (using a computer‐generated random number pattern)". |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | Low risk | Comment: All important outcomes were reported. |
| Free of baseline imbalance bias? | Low risk | Comment: Patients were well matched for important prognostic factors. |
Van Heek 2003.
| Methods | Randomised controlled trial | |
| Participants | Country: Netherlands. Sample size: 70. Post‐randomisation drop‐out: 5 (7.1%). Revised sample size: 65. Females: 30 (46.2%). Mean age: 64 years. Pancreatic cancer: 57 (87.7%). Ampullary cancer: 2 (3.1%). Duodenal cancer: 0 (0%). Bile duct cancer: 6 (9.2%). Biliary obstruction: 51 (78.5%). Open or laparoscopic gastrojejunostomy: Open. Histological confirmation: Yes. Minimum follow‐up: 9 months. Biliary obstruction management: Hepaticojejunostomy. Inclusion criteria: Patients undergoing surgery for peri‐ampullary cancer with intention to resect. Exclusion criteria: Resectable disease on laparotomy. | |
| Interventions | The participants were randomly assigned to two groups. Group 1: Gastrojejunostomy (n = 36). Further details: retrocolic. Group 2: No gastrojejunostomy (n = 29). Co‐interventions: All patients underwent hepaticojejunostomy irrespective of biliary tract obstruction | |
| Outcomes | The outcomes reported were survival, peri‐operative morbidity, gastric outlet obstruction, hospital stay, and quality of life. | |
| Notes | Reasons for post‐randomisation drop‐out: 1 patient benign; 1 patient became resectable after frozen section; 3 patients lost to follow‐up. Gastric outlet obstruction was defined as clinical symptoms of obstruction, such as nausea and vomiting, in combination with radiologic or endoscopic proof of gastric retention or stenosis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Post‐randomisation drop‐outs could influence the effect estimate. |
| Selective reporting (reporting bias) | Low risk | Comment: All important outcomes were reported. |
| Free of baseline imbalance bias? | Low risk | Comment: Patients were well matched for important prognostic factors. |
| Free of early stopping bias? | High risk | Comment: The trial was stopped early. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cho 2008 | Not a randomised controlled trial. |
| Lillemoe 2004 | Not a randomised controlled trial. |
| Tandon 1999 | Not a randomised controlled trial. |
Differences between protocol and review
The reasons for not carrying out any analyses planned in the protocol have been clearly stated.
Contributions of authors
KS Gurusamy selected the trials for inclusion, extracted the data from the trials, and wrote the review. S Kumar independently identified the trials for inclusion and extracted the data from the trials. BR Davidson critically commented on the review and provided advice for improving the review. All the authors agree with the conclusions of the review.
Sources of support
Internal sources
None, Not specified.
External sources
NIHR, UK.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Lillemoe 1999 {published data only}
- Lillemoe KD, Cameron JL, Hardacre JM, Sohn TA, Sauter PK, Coleman J, et al. Is prophylactic gastrojejunostomy indicated for unresectable periampullary cancer? A prospective randomized trial. Annals of Surgery 1999;230(3):322‐8. [10493479] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Heek 2003 {published data only}
- Heek NT, Castro SM, Eijck CH, Geenen RC, Hesselink EJ, Breslau PJ, et al. The need for a prophylactic gastrojejunostomy for unresectable periampullary cancer: A prospective randomized multicenter trial with special focus on assessment of quality of life. Annals of Surgery 2003;238(6):894‐902. [14631226] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Cho 2008 {published data only}
- Cho YK, Shin JH, Oh SY. Significance of palliative gastrojejunostomy for unresectable pancreatic head carcinoma. Hepato‐Gastroenterology 2008;55(81):254‐7. [18507119] [PubMed] [Google Scholar]
Lillemoe 2004 {published data only}
- Lillemoe KD, Grosfeld JL. Addition of prophylactic gastrojejunostomy to hepaticojejunostomy significantly reduces gastric outlet obstruction in people with unresectable periampullary cancer. Cancer Treatment Reviews 2004;30(4):389‐93. [ISI:000221959100007] [DOI] [PubMed] [Google Scholar]
Tandon 1999 {published data only}
- Tandon V. Prophylactic gastric bypass for unresectable periampullary cancer. Tropical Gastroenterology 1999;20(4):185‐6. [10769610] [PubMed] [Google Scholar]
Additional references
Aaronson 1993
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute 1993;85(5):365‐76. [PUBMED: 8433390] [DOI] [PubMed] [Google Scholar]
British Society of Gastroenterology 2005
- Pancreatic Section, British Society of Gastroenterology, Pancreatic Society of Great Britain and Ireland, Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland, Royal College of Pathologists, Special Interest Group for Gastro‐Intestinal Radiology. Guidelines for the management of patients with pancreatic cancer periampullary and ampullary carcinomas. Gut 2005;54(Suppl 5):v1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clavien 2009
- Clavien PA, Barkun J, Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien‐Dindo classification of surgical complications: five‐year experience. Annals of Surgery 2009;250(2):187‐96. [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dindo 2004
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery 2004;240(2):205‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Engelken 2003
- Engelken FJ, Bettschart V, Rahman MQ, Parks RW, Garden OJ. Prognostic factors in the palliation of pancreatic cancer. European Journal of Surgical Oncology 2003;29(4):368‐73. [DOI] [PubMed] [Google Scholar]
Forman 2009
- Forman D, Delaney B, Kuipers E, Malthaner R, Moayyedi P, Gardener E, et al. Cochrane Upper Gastrointestinal and Pancreatic Diseases Group. About The Cochrane Collaboration 2009, Issue 2. Art. No.: UPPERGI.
Gaidos 2009
- Gaidos JK, Draganov PV. Treatment of malignant gastric outlet obstruction with endoscopically placed self‐expandable metal stents. World Journal of Gastroenterology: WJG 2009;15(35):4365‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurusamy 2009
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. The British Journal of Surgery 2009;96(4):342‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Huser 2009
- Huser N, Michalski CW, Schuster T, Friess H, Kleeff J. Systematic review and meta‐analysis of prophylactic gastroenterostomy for unresectable advanced pancreatic cancer. The British Journal of Surgery 2009;96(7):711‐9. [DOI] [PubMed] [Google Scholar]
Jeurnink 2010
- Jeurnink SM, Steyerberg EW, Hooft JE, Eijck CH, Schwartz MP, Vleggaar FP, et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointestinal endoscopy 2010;71(3):490‐9. [PUBMED: 20003966] [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Ly 2009
- Ly J, O'Grady G, Mittal A, Plank L, Windsor JA. A systematic review of methods to palliate malignant gastric outlet obstruction. Surgical Endoscopy 2009;23:1‐8. [DOI: 10.1007/s00464-009-0577-1] [DOI] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Mayo 2009
- Mayo SC, Austin DF, Sheppard BC, Mori M, Shipley DK, Billingsley KG. Evolving preoperative evaluation of patients with pancreatic cancer: does laparoscopy have a role in the current era?. Journal of the American College of Surgeons 2009;208(1):87‐95. [DOI] [PubMed] [Google Scholar]
Mehta 2006
- Mehta S, Hindmarsh A, Cheong E, Cockburn J, Saada J, Tighe R, et al. Prospective randomized trial of laparoscopic gastrojejunostomy versus duodenal stenting for malignant gastric outflow obstruction. Surgical Endoscopy 2006;20(2):239‐42. [PUBMED: 16362479] [DOI] [PubMed] [Google Scholar]
Michelassi 1989
- Michelassi F, Erroi F, Dawson PJ, Pietrabissa A, Noda S, Handcock M, et al. Experience with 647 consecutive tumors of the duodenum, ampulla, head of the pancreas, and distal common bile duct. Annals of Surgery 1989;210(4):544‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Moss 2006
- Moss AC, Morris E, Mac Mathuna P. Palliative biliary stents for obstructing pancreatic carcinoma. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004200.pub4; PUBMED: 16625598] [DOI] [PMC free article] [PubMed] [Google Scholar]
National Cancer Institute 2009
- National Cancer Institute (U.S. National Institute of Health). Dictionary of Cancer terms. Periampullary cancer. http://www.cancer.gov/dictionary/?CdrID=543930 accessed on 20/08/10.
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [PUBMED: 9921604] [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Schantz 1984
- Schantz SP, Schickler W, Evans TK, Coffey RJ. Palliative gastroenterostomy for pancreatic cancer. American Journal of Surgery 1984;147(6):793‐6. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Smith 2008
- Smith RA, Bosonnet L, Ghaneh P, Sutton R, Evans J, Healey P, et al. The platelet‐lymphocyte ratio improves the predictive value of serum CA19‐9 levels in determining patient selection for staging laparoscopy in suspected periampullary cancer. Surgery 2008;143(5):658‐66. [DOI] [PubMed] [Google Scholar]
Sweeting 2004
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23(9):1351‐75. [DOI] [PubMed] [Google Scholar]
Tang 2007
- Tang CN, Siu WT, Ha JP, Tai CK, Tsui KK, Li MK. Laparoscopic biliary bypass ‐a single centre experience. Hepatogastroenterology 2007;54(74):503‐7. [PUBMED: 17523308] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [PUBMED: 17555582] [DOI] [PMC free article] [PubMed] [Google Scholar]
Watanapa 1992
- Watanapa P, Williamson RC. Surgical palliation for pancreatic cancer: developments during the past two decades. The British Journal of Surgery 1992;79(1):8‐20. [DOI] [PubMed] [Google Scholar]
Wong 2002
- Wong YT, Brams DM, Munson L, Sanders L, Heiss F, Chase M, et al. Gastric outlet obstruction secondary to pancreatic cancer: surgical vs endoscopic palliation. Surgical Endoscopy 2002;16(2):310‐2. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yokoyama 2005
- Yokoyama N, Shirai Y, Wakai T, Nagakura S, Akazawa K, Hatakeyama K. Jaundice at presentation heralds advanced disease and poor prognosis in patients with ampullary carcinoma. World Journal of Surgery 2005;29(4):519‐23. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gurusamy 2010
- Gurusamy KS, Kumar S, Davidson BR. Prophylactic gastrojejunostomy for unresectable periampullary carcinoma. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD008533.pub2] [DOI] [PubMed] [Google Scholar]


