Abstract
Background
Total knee replacement (TKR) is a common and often painful operation. Femoral nerve block (FNB) is frequently used for postoperative analgesia.
Objectives
To evaluate the benefits and risks of FNB used as a postoperative analgesic technique relative to other analgesic techniques among adults undergoing TKR.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 1, MEDLINE, EMBASE, CINAHL, Web of Science, dissertation abstracts and reference lists of included studies. The date of the last search was 31 January 2013.
Selection criteria
We included randomized controlled trials (RCTs) comparing FNB with no FNB (intravenous patient‐controlled analgesia (PCA) opioid, epidural analgesia, local infiltration analgesia, and oral analgesia) in adults after TKR. We also included RCTs that compared continuous versus single‐shot FNB.
Data collection and analysis
Two review authors independently performed study selection and data extraction. We undertook meta‐analysis (random‐effects model) and used relative risk ratios (RRs) for dichotomous outcomes and mean differences (MDs) or standardized mean differences (SMDs) for continuous outcomes. We interpreted SMDs according to rule of thumb where 0.2 or smaller represents a small effect, 0.5 a moderate effect and 0.8 or larger, a large effect.
Main results
We included 45 eligible RCTs (2710 participants) from 47 publications; 20 RCTs had more than two allocation groups. A total of 29 RCTs compared FNB (with or without concurrent treatments including PCA opioid) versus PCA opioid, 10 RCTs compared FNB versus epidural, five RCTs compared FNB versus local infiltration analgesia, one RCT compared FNB versus oral analgesia and four RCTs compared continuous versus single‐shot FNB. Most included RCTs were rated as low or unclear risk of bias for the aspects rated in the risk of bias assessment tool, except for the aspect of blinding. We rated 14 (31%) RCTs at high risk for both participant and assessor blinding and rated eight (18%) RCTs at high risk for one blinding aspect.
Pain at rest and pain on movement were less for FNB (of any type) with or without a concurrent PCA opioid compared with PCA opioid alone during the first 72 hours post operation. Pooled results demonstrated a moderate effect of FNB for pain at rest at 24 hours (19 RCTs, 1066 participants, SMD ‐0.72, 95% CI ‐0.93 to ‐0.51, moderate‐quality evidence) and a moderate to large effect for pain on movement at 24 hours (17 RCTs, 1017 participants, SMD ‐0.94, 95% CI ‐1.32 to ‐0.55, moderate‐quality evidence). Pain was also less in each FNB subgroup: single‐shot FNB, continuous FNB and continuous FNB + sciatic block, compared with PCA. FNB also was associated with lower opioid consumption (IV morphine equivalent) at 24 hours (20 RCTs, 1156 participants, MD ‐14.74 mg, 95% CI ‐18.68 to ‐10.81 mg, high‐quality evidence) and at 48 hours (MD ‐14.53 mg, 95% CI ‐20.03 to ‐9.02 mg), lower risk of nausea and/or vomiting (RR 0.47, 95% CI 0.33 to 0.68, number needed to treat for an additional harmful outcome (NNTH) four, high‐quality evidence), greater knee flexion (11 RCTs, 596 participants, MD 6.48 degrees, 95% CI 4.27 to 8.69 degrees, moderate‐quality evidence) and greater patient satisfaction (four RCTs, 180 participants, SMD 1.06, 95% CI 0.74 to 1.38, low‐quality evidence) compared with PCA.
We could not demonstrate a difference in pain between FNB (any type) and epidural analgesia in the first 72 hours post operation, including pain at 24 hours at rest (six RCTs, 328 participants, SMD ‐0.05, 95% CI ‐0.43 to 0.32, moderate‐quality evidence) and on movement (six RCTs, 317 participants, SMD 0.01, 95% CI ‐0.21 to 0.24, high‐quality evidence). No difference was noted at 24 hours for opioid consumption (five RCTs, 341 participants, MD ‐4.35 mg, 95% CI ‐9.95 to 1.26 mg, high‐quality evidence) or knee flexion (six RCTs, 328 participants, MD ‐1.65, 95% CI ‐5.14 to 1.84, high‐quality evidence). However, FNB demonstrated lower risk of nausea/vomiting (four RCTs, 183 participants, RR 0.63, 95% CI 0.41 to 0.97, NNTH 8, moderate‐quality evidence) and higher patient satisfaction (two RCTs, 120 participants, SMD 0.60, 95% CI 0.23 to 0.97, low‐quality evidence), compared with epidural analgesia.
Pooled results of four studies (216 participants) comparing FNB with local infiltration analgesia detected no difference in analgesic effects between the groups at 24 hours for pain at rest (SMD 0.06, 95% CI ‐0.61 to 0.72, moderate‐quality evidence) or pain on movement (SMD 0.38, 95% CI ‐0.10 to 0.86, low‐quality evidence). Only one included RCT compared FNB with oral analgesia. We considered this evidence insufficient to allow judgement of the effects of FNB compared with oral analgesia.
Continuous FNB provided less pain compared with single‐shot FNB (four RCTs, 272 participants) at 24 hours at rest (SMD ‐0.62, 95% CI ‐1.17 to ‐0.07, moderate‐quality evidence) and on movement (SMD ‐0.42, 95% CI ‐0.67 to ‐0.17, high‐quality evidence). Continuous FNB also demonstrated lower opioid consumption compared with single‐shot FNB at 24 hours (three RCTs, 236 participants, MD ‐13.81 mg, 95% CI ‐23.27 to ‐4.35 mg, moderate‐quality evidence).
Generally, the meta‐analyses demonstrated considerable statistical heterogeneity, with type of FNB, allocation concealment and blinding of participants, personnel and outcome assessors reducing heterogeneity in the analyses. Available evidence was insufficient to allow determination of the comparative safety of the various analgesic techniques. Few RCTs reported on serious adverse effects such as neurological injury, postoperative falls or thrombotic events.
Authors' conclusions
Following TKR, FNB (with or without concurrent treatments including PCA opioid) provided more effective analgesia than PCA opioid alone, similar analgesia to epidural analgesia and less nausea/vomiting compared with PCA alone or epidural analgesia. The review also found that continuous FNB provided better analgesia compared with single‐shot FNB. RCTs were insufficient to allow definitive conclusions on the comparison between FNB and local infiltration analgesia or oral analgesia.
Keywords: Humans; Arthroplasty, Replacement, Knee; Femoral Nerve; Analgesia; Analgesia/methods; Analgesia, Epidural; Analgesia, Patient‐Controlled; Analgesics, Opioid; Analgesics, Opioid/therapeutic use; Nerve Block; Nerve Block/methods; Pain, Postoperative; Pain, Postoperative/drug therapy; Randomized Controlled Trials as Topic
Plain language summary
Femoral nerve blocks for acute postoperative pain after knee replacement surgery
Total knee replacement is a common and often painful orthopaedic operation. Femoral nerve block (FNB) is an analgesic technique that blocks sensation to the knee to reduce pain following surgery. FNB is given as a single injection or as continuous infusion of numbing medication in the groin area.
This review compared FNB with other common analgesic techniques (patient‐controlled analgesia (PCA) with opioids, epidural analgesia, local infiltration analgesia and oral analgesia). A total of 45 randomized trials with 2710 participants were included. Among the included studies, 30 treatment groups compared FNB with PCA opioids, 10 compared FNB with epidural analgesia, five compared FNB with local infiltration analgesia, one compared FNB with oral analgesia and four compared continuous FNB with single‐injection FNB. The average methodological quality of the included studies was moderate.
FNB (any type) resulted in less pain at rest and on movement during the first 72 hours after surgery, compared with PCA opioid alone. FNB also resulted in lower opioid consumption, fewer patients with nausea and vomiting, greater knee flexion and higher patient satisfaction, compared with PCA opioid. Additionally, continuous FNB provided better pain relief than was attained with single‐injection FNB.
No differences in postoperative pain and opioid consumption at 24 hours were noted between FNB and epidural analgesia, although the former resulted in fewer patients with nausea and vomiting, and higher patient satisfaction was reported with analgesia. Similarly, we found no difference in postoperative pain between participants given FNB as opposed to local infiltration analgesia. Information was insufficient for review authors to conclude on the comparison of FNB with local infiltration analgesia or oral analgesia, or on the safety of the various analgesic techniques.
Summary of findings
Summary of findings for the main comparison. FNB (any type) compared with PCA opioid for knee replacement surgery.
| FNB (any type) compared with PCA opioid for knee replacement surgery | ||||||
| Patient or population: patients with knee replacement surgery Settings: hospital Intervention: FNB (any type) Comparison: PCA opioid | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| PCA opioid | FNB (any type) | |||||
| Pain at rest at 24 hours Visual analogue scale Scale from zero to 10 Follow‐up: median two days | Mean pain at rest at 24 hours ranged across control groups from 0.7 to 5.5 points | Mean pain at rest at 24 hours in the intervention groups was 1.20 lower (1.60 to 0.87 lower) | 1066 (19 studies) | ⊕⊕⊕⊝ moderate1 | SMD ‐0.72 (‐0.93 to ‐0.51), representing moderate effects between groups. Lower score indicates less pain2 | |
| Pain on movement at 24 hours Visual analogue scale Scale from zero to 10 Follow‐up: median two days | Mean pain on movement at 24 hours ranged across control groups from 2.8 to 8 points | Mean pain on movement at 24 hours in the intervention groups was 1.66 lower (2.32 to 0.97 lower) | 1017 (17 studies) | ⊕⊕⊕⊝ moderate1 | SMD ‐0.94 (‐1.32 to ‐0.55), representing moderate to large effect between groups. Lower score indicates less pain2 | |
| Neurological injury Follow‐up: during hospitalization | See comment | See comment | Not estimable | 390 (4 studies) | See comment | Effect is uncertain as neurological injuries are extremely rare. No events were reported in four studies |
| Opioid consumption at 24 hours using morphine equivalents Scale from zero to 150 mg Follow‐up: 24 hours | Mean opioid consumption 24 hours ranged across control groups from 19.3 to 99.8 mg | Mean opioid consumption at 24 hours in the intervention groups was 14.17 lower (18.10 to 10.23 lower) | 1156 (20 studies) | ⊕⊕⊕⊕ high3 | MD ‐14.74 mg (‐18.68 to ‐10.81 mg) | |
| Nausea and/or vomiting Follow‐up: median two days | Study population4 | RR 0.47 (0.33 to 0.68) | 1100 (16 studies) | ⊕⊕⊕⊕ high3 | ||
| 47 per 100 | 22 per 100 (15 to 32) | |||||
| Low4 | ||||||
| 10 per 100 |
5 per 100 (3 to 7) |
|||||
| High4 | ||||||
| 93 per 100 | 44 per 100 (31 to 63) | |||||
| Knee flexion range of motion (degree)5 Follow‐up: median three days | Mean knee flexion range of motion ranged across control groups from 55 to 85 degrees | Mean knee flexion in the intervention group was 6.48 higher (4.27 to 8.69 higher) | 596 (11 studies) | ⊕⊕⊕⊝ moderate1 | MD 6.48 degrees (4.27 to 8.69 degrees) | |
|
Participant satisfaction with analgesia
(point) Scale from zero to 10 Follow‐up: during hospitalization |
Mean participant satisfaction ranged across control groups from 6.3 to 8.7 points | Mean participant satisfaction was 1.79 higher (1.25 to 2.32 higher) | 180 (4 studies) | ⊕⊕⊝⊝ low6 | SMD 1.06 (0.74 to 1.38), representing large effects. Higher score indicates greater satisfaction2 | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded for significant heterogeneity. 2Rule of thumb for interpreting SMD: 0.2 small effect, 0.5 moderate effect, 0.8 large effect (Cohen 1988). 3Downgrading for significant heterogeneity balanced by upgrading for large effect based on consistent evidence from more than two studies.
4Assumed risk was based on control group risk in the included studies.
5Knee flexion of 90 degrees is usually required for patients to be able to climb stairs. 6Downgraded for relatively few participants and for lack of blinding of participants, personnel and/or outcome assessors in some trials.
Summary of findings 2. FNB (any type) compared with epidural analgesia for knee replacement surgery.
| FNB (any type) compared with epidural analgesia for knee replacement surgery | ||||||
| Patient or population: patients with knee replacement surgery Settings: hospital Intervention: FNB (any type) Comparison: epidural | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Epidural | FNB (any type) | |||||
| Pain at rest at 24 hours Visual analogue scale Scale from zero to 10 Follow‐up: median two days | Mean pain at rest at 24 hours ranged across control groups from 0.2 to 3.9 points | Mean pain at rest at 24 hours in the intervention groups was0.10 lower (0.70 lower to 0.67 higher) | 328 (6 studies) | ⊕⊕⊕⊝ moderate1 | SMD ‐0.05 (‐0.43 to 0.32), representing small and non‐significant effects. Lower score indicates less pain2 | |
| Pain on movement at 24 hours Visual analogue scale Scale from zero to 10 Follow‐up: median two days | Mean pain on movement at 24 hours ranged across control groups from 1.8 to 5.5 points | Mean pain on movement at 24 hours in the intervention groups was 0.02 lower (0.48 lower to 0.54 higher) | 317 (6 studies) | ⊕⊕⊕⊕ high | SMD 0.01 (‐0.21 to 0.24), representing small and non‐significant effects. Lower score indicates less pain2 | |
| Neurological injury Follow‐up: during hospitalization | See comment | See comment | Not estimable | 306 (4 studies) | See comment | Effect is uncertain as neurological injuries are extremely rare. Only one event was reported in the epidural group and none in the FNB group |
| Opioid consumption at 24 hours using morphine equivalents Scale from zero to 150 mg Follow‐up: 24 hours | Mean opioid consumption 24 hours ranged across control groups from 8 to 50.7 mg | Mean opioid consumption at 24 hours in the intervention groups was 4.35 lower (9.95 lower to 1.26 higher) |
341 (5 studies) | ⊕⊕⊕⊕ high | MD ‐4.35 (‐9.95 to 1.26) | |
| Nausea and/or vomiting Follow‐up: median two days | Study population3 | RR 0.63 (0.41 to 0.97) | 183 (4 studies) | ⊕⊕⊕⊝ moderate4 | ||
| 38 per 100 | 24 per 100 (15 to 37) | |||||
| Low3 | ||||||
| 13 per 100 |
8 per 100 (5 to 13) |
|||||
| High3 | ||||||
| 67 per 100 | 42 per 100 (27 to 65) | |||||
| Knee flexion range of motion (degrees)5 Follow‐up: median three days | Mean knee flexion range of motion ranged across control groups from 48 to 84 degrees | Mean knee flexion in the intervention groups was 1.65 lower (5.14 lower to 1.84 higher) | 328 (6 studies) | ⊕⊕⊕⊕ high | MD ‐1.65 (‐5.14 to 1.84). | |
|
Participant satisfaction with analgesia
(point) Scale from zero to 10 Follow‐up: during hospitalization |
Mean participant satisfaction ranged across control groups from 8.2 to 8.7 points | Mean participant satisfaction in the intervention groups was 1.03 higher (0.39 to 1.66 higher) | 120 (2 studies) | ⊕⊕⊝⊝ low6 | SMD 0.60 (0.23 to 0.97), representing small to moderate effects. Higher score indicates greater satisfaction2 | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded for significant heterogeneity attributable to inadequate allocation concealment. 2Rule of thumb for interpreting SMD: 0.2 small effect, 0.5 moderate effect, 0.8 large effect (Cohen 1988). 3Assumed risk was based on control group risks in the included studies.
4Downgraded for relatively few participants.
5Knee flexion of 90 degrees is usually required for mobility independence.
6Downgraded for relatively few participants and for lack of blinding of participants, personnel and/or outcome assessors.
Summary of findings 3. FNB (any type) compared with local infiltration analgesia for knee replacement surgery.
| FNB (any type) compared with local infiltration analgesia for knee replacement surgery | ||||||
| Patient or population: patients with knee replacement surgery Settings: hospital Intervention: FNB (any type) Comparison: local infiltration analgesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Local infiltration analgesia | FNB (any type) | |||||
| Pain at rest at 24 hours Visual analogue scale Scale from zero to 10 Follow‐up: median two days | Mean pain at rest at 24 hours ranged across control groups from 1.6 to 5 points | Mean pain at rest at 24 hours in the intervention groups was 0.10 higher (1.04 lower to 1.22 higher) |
216 (4 studies) | ⊕⊕⊕⊝ moderate1 | SMD 0.06 (‐0.61 to 0.72), representing small and non‐significant effects. Lower score indicate less pain3 | |
| Pain on movement at 24 hours Visual analogue scale Scale from zero to 10 Follow‐up: median two days | Mean pain on movement at 24 hours ranged across control groups from 2.4 to 7 points | Mean pain on movement at 24 hours in the intervention groups was 0.63 higher (0.17 lower to 1.43 higher) | 153 (3 studies) | ⊕⊕⊝⊝ low1,2 | SMD 0.38 (‐0.10 to 0.86), representing small and non‐significant effects. Lower score indicates less pain3 | |
| Neurological injury: not reported | See comment | See comment | Not estimable | ‐ | See comment | Effect is uncertain as neurological injuries are extremely rare. No study reported on neurological injury |
| Opioid consumption at 24 hours using morphine equivalents Scale from zero to 150 mg Follow‐up: 24 hours | Mean opioid consumption at 24 hours in the control groups was 26 mg | Mean opioid consumption at 24 hours in the intervention group was 11.50 lower (24.08 lower to 1.08 higher) |
40 (1 study) | ⊕⊕⊕⊝ moderate2 | MD ‐11.50 (‐24.08 to 1.08) | |
| Nausea and/or vomiting Follow‐up: median two days | Study population4 | RR 1.71 (0.64 to 4.62) | 177 (3 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 27 per 100 | 47 per 100 (18 to 100) | |||||
| Low4 | ||||||
| 6 per 100 |
10 per 100 (4 to 28) |
|||||
| High4 | ||||||
| 55 per 100 | 94 per 100 (35 to 100) | |||||
|
Participant satisfaction with analgesia Scale from zero to 10 Follow‐up not reported |
See comment | See comment | Not estimable | ‐ | See comment | Mean (SD) = 7.8 (0) in FNB and 9 (0) in local infiltration reported in 1 RCT. Unable to generate effect estimate, as SD = 0 for both interventions. Higher scores indicate greater satisfaction |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded for significant heterogeneity. 2Downgraded for relatively few participants. 3Rule of thumb for interpreting SMD: 0.2 small effect, 0.5 moderate effect, 0.8 large effect (Cohen 1988).
4Assumed risk was based on control group risks in the included studies.
Summary of findings 4. Continuous FNB compared with single‐shot FNB for knee replacement surgery.
| Continuous FNB compared with single‐shot FNB for knee replacement surgery | ||||||
| Patient or population: patients with knee replacement surgery Settings: hospital Intervention: continuous FNB Comparison: single‐shot FNB | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Single‐shotFNB | Continuous FNB | |||||
|
Pain at rest at 24 hours
Visual analogue scale Scale from zero to 10 Follow‐up: median two days |
Mean pain at rest at 24 hours ranged across control groups from 0.8 to 3.9 points | Mean pain at rest at 24 hours in the intervention group was 0.95 lower (1.78 to 0.11 lower) | 272 (4 studies) | ⊕⊕⊕⊝ moderate1 | SMD ‐0.62 (‐1.17 to ‐0.07), representing small to moderate effects. Lower score indicates less pain2 | |
|
Pain on movement at 24 hours
Visual analogue scale Scale from zero to 10 Follow‐up: median two days |
Mean pain on movement at 24 hours ranged across control groups from 4.5 to 7.2 points | Mean pain on movement at 24 hours was 0.74 lower (1.18 to 0.30 lower) |
272 (4 studies) | ⊕⊕⊕⊕ high | SMD ‐0.42 (‐0.67 to ‐0.17), representing small effects. Lower score indicates less pain2 | |
| Neurological injury: not reported | See comment | See comment | Not estimable | ‐ | See comment | Effect is uncertain as neurological injuries are extremely rare. No events were reported in two trials |
| Opioid consumption at 24 hours using morphine equivalents Scale from 0 to 150 mg Follow‐up: 24 hours | Mean opioid consumption at 24 hours ranged across control groups from 14 to 42 mg | Mean opioid consumption at 24 hours in the intervention group was 13.81 lower (23.27 to 4.35 lower) |
236 (3 studies) | ⊕⊕⊕⊝ moderate1 | MD ‐13.81 (‐23.27 to ‐4.35) | |
| Nausea and/or vomiting Follow‐up: median two days | Study population3 | RR 0.55 (0.2 to 1.56) | 214 (2 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 36 per 100 | 20 per 100 (7 to 56) | |||||
| Low3 | ||||||
| 33 per 100 |
18 per100 (7 to 51) |
|||||
| High3 | ||||||
| 45 per 100 | 25 per 100 (9 to 70) | |||||
| Participant satisfaction with analgesia: not reported | See comment | See comment | Not estimable | ‐ | See comment | Effect is uncertain, as no study reported on this outcome |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded for significant heterogeneity. 2Rule of thumb for interpreting SMD: 0.2 small effect, 0.5 moderate effect, 0.8 large effect (Cohen 1988).
3Assumed risk was based on control group risks in the included studies.
Background
Description of the condition
Total knee replacement (TKR) is a common orthopaedic operation, consisting of replacing diseased or damaged knee joint surfaces to relieve the pain and disability of osteoarthritis. The significant public health burden of osteoarthritis is reflected in the findings that nearly half of all adults in the United States are at risk of developing symptomatic knee osteoarthritis by 85 years of age (Murphy 2008). The number of knee replacements undertaken annually around the world will increase exponentially as the population ages and becomes more obese. Indeed, the number of primary TKR procedures in the United States was projected to increase almost eight‐fold, from 450,000 in 2005 to 3.48 million in 2030 (Kurtz 2007).
TKR is one of the most painful surgical procedures. Effective analgesia in the immediate postoperative phase is important to allow the patient to exercise and regain mobility, thereby facilitating recovery and decreasing the length of hospital stay (Capdevila 1999; Chelly 2001). Unrelieved severe postoperative pain can result in pathophysiological responses causing adverse postsurgical outcomes. These adverse outcomes have medical and economic implications, such as impaired early rehabilitation, delayed discharge, unscheduled rehospitalization, impaired health‐related quality of life and increased risk of chronic pain (American Society of Anesthesiologists 2004; Carr 1999; Sinatra 2009; Twersky 1997).
Various analgesic techniques are used to relieve postoperative pain following TKR. Analgesic options include patient‐controlled analgesia (PCA) using opioids, epidural analgesia, local infiltration analgesia and femoral nerve block (FNB) with local anaesthetic agents. PCA opioids have been associated with significant adverse effects such as respiratory depression, nausea, urinary retention and constipation (Chelly 2001; Sinatra 2009). Epidural analgesia, when given simultaneously with an anticoagulant, has been associated with spinal epidural haematoma as well as hypotension, urinary retention and pruritus (Capdevila 1999; Choi 2003; Sinatra 2009). Epidural analgesia also causes bilateral motor blockade to the same extent, which may interfere with early mobilization (Barrington 2005). Some randomized controlled trials (RCTs) have suggested that FNB provides better pain control and fewer opioid‐related adverse effects compared with PCA opioid or epidural analgesia (Chelly 2001; Sundarathiti 2009; Szczukowski 2004; Wang 2002). Local infiltration analgesia is an emerging technique. The advantage of local infiltration analgesia is that pain conduction is blocked at its origin. A study evaluating the efficacy of a perioperative local infiltration analgesia consisting of local anaesthetic and morphine found a significant reduction in pain and opioid consumption during the first postoperative 48 hours compared with PCA morphine (Vendittoli 2006). To date, no systematic review has compared the effects of FNB with those of local infiltration analgesia.
Description of the intervention
Femoral, psoas (lumbar plexus) and fascia iliaca blocks have been used as postoperative analgesic techniques for TKR surgery. Among these nerve blocks, the FNB is used most commonly. An FNB may be given as a single shot or as a continuous block via a catheter and an infusion.
One method that is commonly used to deliver an FNB is the Winnie paravascular technique (Winnie 1973), in which a peripheral nerve stimulator or ultrasound guidance is often used to locate the nerve. When a peripheral nerve stimulator needle is used, the common femoral artery is palpated first with the patient in the supine position, and the stimulating needle is then inserted at the inguinal crease, approximately 1 cm lateral to the femoral artery pulse. Stimulation of the femoral nerve generates contraction of the quadriceps muscle. The needle position is optimized when contractions persist at an output of 0.3 mA to 0.5 mA. Approximately 20 ml to 30 ml of local anaesthetic is then injected. When using ultrasound, the operator places the transducer in the inguinal crease to locate the hyperechoic femoral nerve, which can be visualized lateral to the hypoechoic pulsative common femoral artery. Successful FNB is attained when the local anaesthetic is seen circumferentially around the nerve (Ballantyne 2010).
An FNB can also be performed as part of a more extensive nerve block, termed the three‐in‐one block (femoral, lateral femoral cutaneous and obturator nerves). In the three‐in‐one block, a greater volume of local anaesthetic is used, and pressure is applied just distal to the needle during administration of the local anaesthetic to help spread the local anaesthetic to the lateral femoral cutaneous and obturator nerves. However, the femoral nerve is the only nerve that is consistently blocked (Lang 1993). Consequently, the three‐in‐one block frequently is referred to simply as the FNB (Enneking 2009).
How the intervention might work
An FNB blocks sensation to the anteromedial aspect of the knee, thus reducing pain and muscle spasms. Compared with a single shot, continuous FNB provides a longer duration of postoperative analgesia (Sinatra 2009) with a lower concentration of local anaesthetic. These features may allow earlier rehabilitation with a continuous FNB, compared with a single‐shot FNB, in that the degree of motor block is reduced. An FNB does not block sensation to the posterior aspect of the knee, as it is innervated by the sciatic nerve. To improve postoperative analgesia, a sciatic and/or obturator nerve blockade is sometimes added to the FNB (McNamee 2002; Morin 2005).
Why it is important to do this review
Traditionally, PCA opioid and epidural analgesia were the postoperative analgesic methods of choice following TKR. Recent years have seen a growing interest in the use of FNB to minimize the adverse effects associated with PCA opioid or epidural analgesic techniques. However, FNB after TKR surgery is not without risk; concerns about prolonged quadriceps weakness (Kandasami 2009) and complications such as femoral neuropathy or neuritis have been reported; an increased risk of falls has also been associated with continuous FNB, compared with single‐shot block or no block (Feibel 2009; Johnson 2013; Sharma 2010). Additionally, continuous FNB catheters require specialized skills and additional time for insertion and management.
A sciatic nerve block is sometimes combined with an FNB to improve analgesia (Morin 2005). However, this combination could lead to complications such as increased risk of falls and heel ulceration. The combination could also mask peroneal nerve injury or an evolving sciatic nerve injury from compartment syndrome (Ben‐David 2004; Kadic 2009; Todkar 2005). A selective obturator nerve block is added at times to an FNB, but the additional analgesic benefit is conflicting (Macalou 2004; McNamee 2002).
To date, no strong evidence has been obtained from large RCTs evaluating the comparative efficacy and safety of FNB versus other forms of postoperative analgesia. A recent systematic review limited to English language publications up to 2009 concluded that single‐shot or continuous FNB (with PCA opioid) was superior to PCA opioid alone for acute pain control in the first 72 hours after knee replacement (Paul 2010). Another systematic review compared nerve blockade with epidural analgesia and found no significant difference in pain in the first 24 hours post operation (Fowler 2008). Our work will extend that of these reviews by looking at all comparator analgesic regimens, including the emerging analgesic technique of local infiltration analgesia; by examining outcomes beyond the initial postoperative period and by not limiting our search to English language publications. The rapidly rising number of TKRs performed annually worldwide will considerably increase the burden on healthcare resources (March 2004). A systematic review is needed to evaluate current evidence for the short‐ and long‐term safety and efficacy of FNB after TKR.
Objectives
To evaluate the benefits and risks of FNB used as a postoperative analgesic technique relative to other analgesic techniques among adults undergoing TKR.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs comparing FNB (inserted preoperatively, operatively or postoperatively) versus postoperative analgesic techniques not requiring an FNB (intravenous PCA opioids, epidural analgesia, local infiltration analgesia and oral analgesia).
We excluded quasi‐randomized trials (e.g. using alternation) and observational studies.
Types of participants
We included adults who have undergone TKR surgery.
Types of interventions
FNB involving groin injections of any type (performed in isolation or as part of a three‐in‐one block) used to provide postoperative analgesia after TKR surgery versus no FNB. We also included RCTs that compared continuous versus single‐shot FNB.
Types of outcome measures
In this review, we intend to examine the following outcomes across a range of potential alternatives to FNB. We will present sequentially all outcomes for each comparison with FNB as follows: (1) PCA opioid; (2) epidural analgesia; (3) local Infiltration analgesia and (4) oral analgesia; we will also compare two types of FNB: (5) continuous FNB versus (6) single‐shot FNB.
Primary outcomes
1. Pain at rest and on movement
We considered pain at rest and on movement within the following postoperative time frames: first two hours, three to 12 hours, 24 hours, 48 hours, 72 hours and more than 72 hours. Pain outcomes were based on measures with reliable and validated psychometric properties such as the visual analogue scale (VAS). We converted data to a zero to 10 scale. Unless otherwise reported, pain was assumed to be experienced at rest.
2. Serious adverse events
We defined a serious adverse event as any untoward occurrence that results in death, is life threatening, requires rehospitalization or prolongation of existing hospitalisation, results in persistent or significant disability or is considered a medically important event or reaction. Examples include neurological injury, postoperative falls and thrombotic events. We used the time frame set by the study authors.
Secondary outcomes
3. Proportion of participants in significant pain postoperatively, as defined by the study authors
4. Time from end of surgery to first rescue analgesic request
5. Opioid consumption
We converted intravenous fentanyl and oral oxycodone to intravenous morphine dosing equivalents using the following computations: 1 mg oral oxycodone = 0.6 mg intravenous morphine; and 1 µg intravenous fentanyl = 0.067 mg intravenous morphine (Allman 2006; Berdine 2006). Data on opioid consumption were expected to be skewed in distribution, and it is recommended that opioid consumption be dichotomzed (Moore 2011; PaPaS document 2011). However, opioid consumption was not reported as above or below a certain threshold. Instead, the included studies reported opioid consumption as mean (standard deviation (SD)) milligrams, and we had to follow suit. Some studies reported opioid consumption as cumulative consumption since its commencement, whereas others used consumption over 24 hours. When available, we analysed total cumulative consumption since commencement.
6. Adverse effects
a. Nausea or vomiting, or both, in the first 72 hours. If more than one time point was reported, we chose the data closest to the first 24 hours, when the adverse effects were most pronounced. When both nausea and vomiting were reported, we used the data for vomiting, with the assumption that all participants who vomited would have also experienced nausea. When nausea and vomiting were reported as mild, moderate or severe, the numbers experiencing severe nausea or vomiting were used.
b. Sedation in the first 72 hours using sedation score of at least one on a four‐point scale where zero = alert; one = drowsy; two = sleeping, easy to arouse and three = sleeping, difficult to arouse; or using the author's definition of event/no event.
c. Urinary retention requiring catheterization or using the author's definition of event/no event in the first 72 hours.
d. Technical failure of the blocks.
7. Physical function
a. Knee flexion range of motion during the first four postoperative days.
b. Knee extension range of motion during the first four postoperative days.
c. Time to first ambulation during the first four postoperative days.
When more than one time point was reported for range of motion, we chose the time point closest to the fourth day, when ambulation ability is more critical with impending hospital discharge.
8. Participant satisfaction with analgesia during the hospital stay
Participant satisfaction was reported on a continuous scale or as dichotomous data (i.e. number of participants in a study satisfied with treatment). We normalized continuous data to a zero to 10 scale.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 1 (see Appendix 1); MEDLINE (Ovid SP) (1948 to January 2013) (see Appendix 2); EMBASE (Ovid SP) (1980 to January 2013) (see Appendix 3); CINAHL (EBSCO host) (1982 to January 2013) (see Appendix 4); ISI Web of Science (1973 to January 2013) (see Appendix 5) and dissertation abstracts.
We combined the sensitive strategies suggested in Section 6.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to search for trials in MEDLINE. We adopted the search strategy for MEDLINE to search all other databases. The search strategies used are reported in Appendices 1 to 5. We applied no language restriction.
Searching other resources
One review author (EC) reviewed the references of relevant articles and textbooks for additional citations. We also searched trial registers (www.clinicaltrials.gov, www.controlled‐trials.com and http://www.anzctr.org.au), Google Scholar and the Procedure Specific Postoperative Pain Management (PROSPECT) Website (www.postoppain.org) to ensure that significant papers were not missed. The date of the last search was 31 January 2013.
Data collection and analysis
Selection of studies
Two review authors (EC and MF) independently screened for eligibility the titles and abstracts of publications identified in the literature search.. We obtained and assessed the full published manuscripts of clinical trials that appeared to be eligible to assess their relevance on the basis of prespecified inclusion criteria. Each of these review authors documented the reasons for study exclusion.The fifth review author (NC) resolved disagreements regarding study exclusion. All review authors have participated in trials that could potentially be eligible for this review (see Declarations of interest). To prevent conflict of interest, two independent reviewers (MH and FP; see Acknowledgements) determined the eligibility of these trials.
A copy of the 'Study selection form' is provided in Appendix 6.
Data extraction and management
Two review authors (EC and MF) independently performed data extraction using a data extraction form (see Appendix 7). The fifth review author (NC) resolved disagreements. Two independent reviewers extracted the data of an included trial when the review authors were among the study investigators. We contacted trial authors to ask for additional details of their studies.
Assessment of risk of bias in included studies
Two review authors (EC and MF) independently assessed the methodological quality of eligible trials using the tool stated in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (see Appendix 8). We resolved disagreements by discussion with the fifth review author (NC). Two independent reviewers assessed the risk of bias of included trials when the review authors were among the study investigators.
In cases of insufficient reporting to enable judgement of 'low risk' or 'high risk,' we contacted the respective study authors to ask for more information. We rated the method as 'unclear' if we could not reach the study authors.
We conducted sensitivity analyses to determine the effect of excluding studies considered to be at high or unclear risk of bias. The results of studies with high and unclear risk of bias were not considered when the results of our sensitivity analyses were interpreted, although we presented them in the meta‐analyses for completeness.
1. Random sequence generation
We considered random sequence generation as low risk if it was generated by using a computer, by using a random number table algorithm or by drawing lots or shuffling cards.
2. Concealment of allocation
We considered allocation concealment as low risk if participant recruiters, investigators and participants were unable to anticipate treatment assignment. Adequate methods included a central allocation system (telephone, Web‐based or pharmacy controlled randomisation) or sequentially numbered sealed opaque envelopes.
3. Blinding of participants and personnel
We considered blinding as low risk of bias if:
a. participants and personnel (healthcare providers) were blinded to the allocated intervention; and
b. it was unlikely that the blinding could have been broken.
We considered blinding as high risk if:
a. blinding of participants and personnel was attempted, but it was likely that the blinding could have been broken; and
b. no blinding or incomplete blinding was applied.
4. Blinding of outcome assessment
We considered blinding as low risk if:
a. outcome assessors were blinded to the allocated intervention, and it was unlikely that the blinding could have been broken.
We considered blinding as high risk if:
a. outcome assessors were blinded, but it was likely that the blinding could have been broken; and
b. outcome assessors were not blinded.
5. Incomplete outcome data
We considered a study as having low risk of incomplete outcome data if:
a. no outcome data were missing;
b. the proportion of missing outcomes compared with observed event risks was not enough to have a clinically relevant impact on the intervention effect estimate (i.e. the percentage of missing data was small relative to the incidence proportion of the outcome);
c. reasons for missing outcome data were unlikely to be related to true outcomes and the percentage of missing data was small; and
d. missing outcome data were balanced in quantities across intervention groups with similar reasons for missing data across groups, and the percentage of missing data was small.
A study was considered as having high risk of incomplete outcome data if:
a. reasons for missing outcome data were likely to be related to true outcomes with imbalance in numbers or reasons for missing data across intervention groups;
b. the proportion of missing outcomes compared with the proportion of observed events was enough to induce clinically relevant bias in intervention effect estimates; and
c. 'as treated' analysis was performed with substantial differences between the numbers of participants contributing to the analysis and the numbers of participants randomly assigned.
6. Selective reporting
We considered low risk of selective reporting if all of the a priori outcomes of a study that are of interest in the review were reported in the prespecified way.
We considered high risk of selective reporting for any of the following.
a. Not all of the a priori outcomes of a study were reported.
b. One or more primary outcomes were reported using only measurements, analysis methods or subsets of data that were not prespecified.
c. One or more reported primary outcomes were not prespecified, unless clear justification of their reporting was provided.
d. One or more outcomes of interest in the review were reported incompletely and therefore could not be included in a meta‐analysis.
7. Other sources of bias
We also assessed whether the study used intention‐to‐treat (ITT) analysis methods (Hollis 1999) for the primary outcomes.
Measures of treatment effect
We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes and mean differences (MDs) or standardized mean differences (SMDs) with 95% CIs for continuous outcomes. For comparisons based on continuous outcomes consisting of trials that reported only differences between groups, we converted the data for all relevant trials to estimate the intervention effect and its corresponding standard error (Borenstein 2009) and used generic inverse variance outcome type. A similar approach was used for comparisons including RCTs that reported a zero SD in one of the intervention arms.
For continuous outcomes, we interpreted intervention effects based on SMDs according to the rule of thumb where 0.2 or smaller represents a small effect, 0.5 a moderate effect and 0.8 or larger a large effect (Higgins 2011; see Section 12.6.2). To further assist in interpreting SMDs, we reexpressed them in their original units by multiplying the pooled SMD with the pooled SD of the control group differences (Higgins 2011; see Section 12.6.4).
SMD is a more sensible measure for synthesizing pain outcomes because one cannot verify whether 2‐cm changes in VAS are equivalent across the scale. Thus the combination of VAS results that have sampled different parts of the scale might be misrepresented by combining MDs and might be better represented by using SMDs. We have used SMDs to report the outcome of participant satisfaction (measured using scale zero to 10) for the same reason..
We calculated number needed to treat for an additional beneficial outcome (NNTB) or an additional harmful outcome (NNTH) as and where appropriate. We obtained confidence intervals for NNTB/NNTH by inverting and exchanging the confidence limits for the absolute risk reduction (ARR). We used the Wilson score method to calculate the confidence limits of ARR and NNTB/NNTH (Bender 2001; Wilson 1927).
Unit of analysis issues
Some RCTs had three or four allocation groups. When the study contributed several independent comparisons (e.g. FNB vs PCA and FNB vs epidural), the interventions were analysed separately in the appropriate meta‐analyses as if they were from different studies.
When the RCTs contributed several correlated comparisons, with similar type of FNB (e.g. continuous FNB with ropivacaine vs PCA and continuous FNB with bupivacaine vs PCA), to the same meta‐analysis, we combined the groups to create a single pair‐wise comparison, as recommended in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
If the RCTs contributed several correlated comparisons, with different types of FNB (e.g. single‐shot FNB vs PCA and continuous FNB vs PCA) to a meta‐analysis, we split the control group into two groups (shared) and included two 'reasonably independent' comparisons. This was done to address the issues of double‐counting and unit of analysis errors in the control group (Higgins 2011; see Chapter 16).
Dealing with missing data
We contacted the study authors if any required data were missing or unclear.
We calculated the missing estimates of SDs from other relevant statistics reported in the study (e.g. standard error, 95% CI, P value). If these statistics were reported, the missing SDs were imputed according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011; see Chapter 16) using the RevMan calculator. Some studies presented results using a combination of median, interquartile range (25th and 75th quartiles) and/or range (minimum and maximum values). We calculated corresponding means and SDs from the presented results based on the recommendation of Hozo 2005. The required data were estimated as follows.
Mean = (Minimum + 2 * Median + Maximum)/4, if median and range were reported for each group; otherwise, Mean = Median.
SD = SQRT (1/12(((Min ‐ 2 * Median + Max)2)/4 + (Min ‐ Max)2), if group size was less than 15 and median and range were reported for each group.
SD = SQRT((Min – Max)/4), if the range was reported and the group size was greater than 15 but equal to or less than 70.
SD = SQRT((Min – Max)/6), if the range was reported and the group size was greater than 70.
SD = (75th Quartile – 25th Quartile)/1.35, if the interquartile range was reported.
Assessment of heterogeneity
We assessed heterogeneity of included RCTs on the basis of their clinical and methodological diversity (risk of bias assessment). Our a priori hypothesis for sources of clinical heterogeneity were as follows.
Different analgesic regimens (e.g. FNB as single‐shot or continuous infusion, using different types of local anaesthetics, concentrations, infusion rates and timings of injections; and with or without sciatic or obturator nerve blocks).
Different standard co‐analgesic regimens.
We presented primary analyses using the random‐effects model to account for the impact of anticipated clinical and methodological heterogeneity. We assessed statistical heterogeneity using the Chi2 test for heterogeneity and quantified heterogeneity using the I2 statistic (Higgins 2011). We considered values of I2 greater than 50% to represent significant between‐study heterogeneity (Higgins 2003). If significant heterogeneity was demonstrated, we explored the data to test whether our planned subgroup analyses explained this heterogeneity.
Assessment of reporting biases
We used Orwin's fail‐safe N test to evaluate the impact of potential publication bias on the robustness of the overall observed analgesic effect (Orwin 1983). We determined how many missing studies without an intervention effect (magnitude of SMD ≤ 0.01) would render a significant overall or pooled effect non‐significant or trivial. The trivial effect was defined by an SMD value of 0.2 (Orwin 1983).
Data synthesis
We conducted a meta‐analysis using Review Manager 5.2 (RevMan 5.2) when data from two or more RCTs were sufficient. We used the random‐effects model of DerSimonian and Laird, as we anticipated that heterogeneity would be present in the interventions and in the outcomes (DerSimonian 1986).
VAS results that sampled different parts of the scale were combined using SMDs. As the dichotomous data for opioid consumption were not available, the means of opioid consumption were synthesized using SMDs. When it was not possible to conduct a meta‐analysis, we discussed the results narratively.
Primary analyses include the following.
Single‐shot or continuous FNB (± sciatic/obturator block ± PCA opioid) versus PCA opioid.
Single‐shot or continuous FNB (± sciatic/obturator block) versus epidural analgesia.
Single‐shot or continuous FNB versus local infiltration analgesia.
Single‐shot or continuous FNB versus oral analgesia.
Continuous versus single‐shot FNB.
We stratified the meta‐analyses of included RCTs according to type of FNB (single‐shot FNB, single‐shot FNB + sciatic block, single‐shot FNB + obturator block, continuous FNB, continuous FNB + sciatic block). For subgroup and sensitivity analyses, we combined the various types of FNB.
We did not adjust for multiplicity of the multiple primary analyses as, in general, this is not recommended (see Section 16.7.2 of the Cochrane Handbook for Systematic Reviews of Interventions) (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
We limited subgroup and sensitivity analyses to the outcomes of pain at rest and on movement at 24 hours.
We performed the following subgroup analyses.
Type of FNB (i.e. single‐shot FNB and continuous FNB, with or without an additional sciatic/obturator nerve block).
FNB (any type) with or without a concurrent parenteral opioid.
Type of local anaesthetic (i.e. FNB (any type) using ropivacaine and FNB (any type) using bupivacaine).
Sensitivity analysis
We performed sensitivity analyses to evaluate the effect on the overall primary result of removing trials with low and unclear methodological quality (i.e. allocation concealment, as well as blinding of participants, personnel and outcome assessors). We did not conduct sensitivity analyses to determine the effect of missing data imputation, as no substantial missing data issues were identified in the included RCTs. Also, sensitivity analysis based on multiple interventions within studies was not performed because we followed the recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (see Chapter 16; Higgins 2011), as discussed in the Unit of analysis issues section.
Summary of findings tables
We used the principles of the GRADE system (Guyatt 2008) to assess the quality of the body of evidence associated with specific outcomes (pain at rest at 24 hours, pain on movement at 24 hours, neurological injury, opioid consumption at 24 hours, nausea and/or vomiting, knee flexion range of motion, participant satisfaction with analgesia) in our review, and we constructed a 'Summary of findings' (SoF) table using the GRADE software (see Appendix 9). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias (see Chapter 12) (Higgins 2011).
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
Figure 1 shows the results of the literature search. We identified 87 publications for potential inclusion, of which we excluded 36 after reviewing the full‐text reports and assigned as awaiting assessment to four conference abstracts. We identified 45 eligible RCTs from 47 publications (two sets of publications reported the same randomized trials (Bergeron 2009 and Kardash 2007; Seet 2006 and Shum 2009). We managed to contact 21 authors of the included studies to clarify the study methodology or to obtain additional data (three of the authors no longer have the original data).
1.
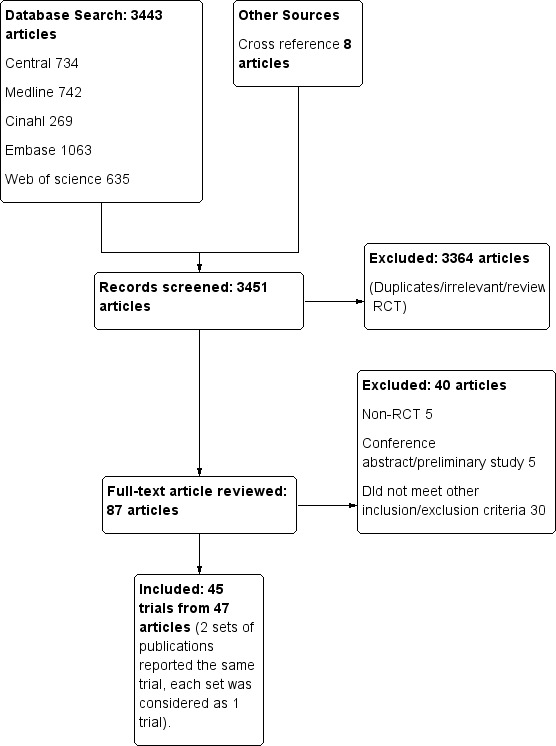
Study flow diagram.
Included studies
We included 45 RCTs with a total of 2710 participants. Details of the individual RCTs are provided in the Characteristics of included studies table. Three RCTs were reported in Chinese (Tang 2010; Wang 2010; Yu 2010), and one RCT was published in German (Fritze 2009). Two RCTs (Chan 2013; Widmer 2012), for which some of the review authors were named, were judged eligible for inclusion by two independent reviewers (MH and FP). In two RCTs (Martin 2008; Mistraletti 2006), the effects of FNB on inflammation and postoperative suppression of gluconeogenesis, respectively, were the main focus of the studies.
The sample size of the individual RCTs ranged from 18 (Ng 2012) to 200 (Chan 2013) participants, the age of study participants ranged from 29 to 86 years (Affas 2011) and the proportion of female participants ranged from 38% (Good 2007) to 100% (Park 2010).
Of the included RCTs, 20 (Adams 2002; Allen 1998; Chan 2012; Chan 2013; de Lima e Souza 2008; Fritze 2009; Ganapathy 1999; Hirst 1996; Hunt 2009; Kaloul 2004; Kardash 2007; Macalou 2004; McNamee 2001; Mistraletti 2006; Ng 2001; Park 2010; Seet 2006; Singelyn 1998; Tugay 2006; Xie 2012) evaluated more than two treatment groups (see Unit of analysis issues section).
In three of these RCTs, one of the treatment groups was excluded from the review because it was a non‐randomized group (Hunt 2009) or because the treatment did not meet the review's inclusion criteria (psoas block group in Kaloul 2004; obturator block group in Kardash 2007).
The included RCTs made the following comparisons according to the aims of this review.
1. FNB (with or without PCA opioid) versus PCA opioid (29 RCTs with two RCTs having three allocation groups)
Of the included RCTs, 14 (Adams 2002; Allen 1998; Chan 2012; Chan 2013; Good 2007; Hirst 1996; Hunt 2009; Kardash 2007; Macalou 2004; Ng 2001; Ozen 2006; Szczukowski 2004; Tugay 2006; Wang 2002) compared single‐shot FNB versus PCA.
Two RCTs (Allen 1998; McNamee 2001) compared single‐shot FNB + sciatic block versus PCA, and one RCT (Macalou 2004) compared single‐shot FNB + obturator versus PCA.
Of the included RCTs, 12 (Baranovic 2011; Chan 2013; Ganapathy 1999; Hirst 1996; Kadic 2009; Kaloul 2004; Seet 2006; Serpell 2001; Singelyn 1998; Tang 2010; Wang 2010; Yu 2010) compared continuous FNB versus PCA.
Two RCTs (Martin 2008; Mistraletti 2006) compared continuous FNB + sciatic versus PCA.
All except six RCTs (Adams 2002; Baranovic 2011; Chan 2013; Mistraletti 2006; Singelyn 1998; Yu 2010) had a concurrent PCA opioid in the FNB intervention.
2. FNB versus epidural analgesia (10 RCTs with one RCT contributing two comparisons)
One RCT (Adams 2002) compared single‐shot FNB versus epidural, and another RCT (Davies 2004) compared single‐shot FNB + sciatic versus epidural. One RCT (Lee 2011) compared single‐shot FNB + epidural versus epidural; the results of this study were not pooled, as a concurrent epidural was included in the FNB intervention.
Five RCTs (Barrington 2005; Fritze 2009; Long 2006; Singelyn 1998; Sundarathiti 2009) compared continuous FNB versus epidural.
Three RCTs (Fritze 2009; Mistraletti 2006; Zaric 2006) compared continuous FNB + sciatic versus epidural.
3. FNB versus local infiltration analgesia (six RCTs)
One RCT (Parvataneni 2007) compared single‐shot FNB versus local infiltration analgesia.
Three RCTs (Affas 2011; Carli 2010; Toftdahl 2007) compared continuous FNB versus local infiltration analgesia.
One RCT (Widmer 2012) compared single‐shot FNB + local infiltration analgesia versus local infiltration analgesia alone; the results of this study were not pooled, as a concurrent local infiltration analgesia was included in the FNB intervention.
One cross‐over RCT (Ng 2012) with knees as the unit of comparison compared continuous FNB versus local infiltration analgesia. The data from this RCT were not included in the meta‐analysis, as we were uncertain about the cross‐over effects and issues with analysis.
4. FNB versus oral analgesia (one RCT)
One RCT (Nader 2012) compared continuous FNB + oral analgesia versus oral analgesia.
5. Continuous FNB versus single‐shot FNB (four RCTs)
Four RCTs (Chan 2013; Hirst 1996; Park 2010; Salinas 2006) made the comparison between continuous and single‐shot FNB. Three of these studies (Hirst 1996; Park 2010; Salinas 2006) had a concurrent PCA opioid intervention in both the continuous and single‐shot FNB groups, and one study (Chan 2013) had a concurrent PCA opioid intervention only in the single‐shot FNB group.
Excluded studies
We excluded 36 trials for the reasons given in the Characteristics of excluded studies. We assigned four trials reported as conference abstracts as awaiting assessment as they do not contain enough information to allow determination of eligibility, or they do not provide quantifiable outcome data (Characteristics of studies awaiting classification).
Risk of bias in included studies
The risk of bias assessment for the individual RCTs is shown in the 'Risk of bias' graph and the 'Risk of bias' summary in Figure 2 and Figure 3, respectively. Details are provided in the risk of bias tables in the Characteristics of included studies.
2.
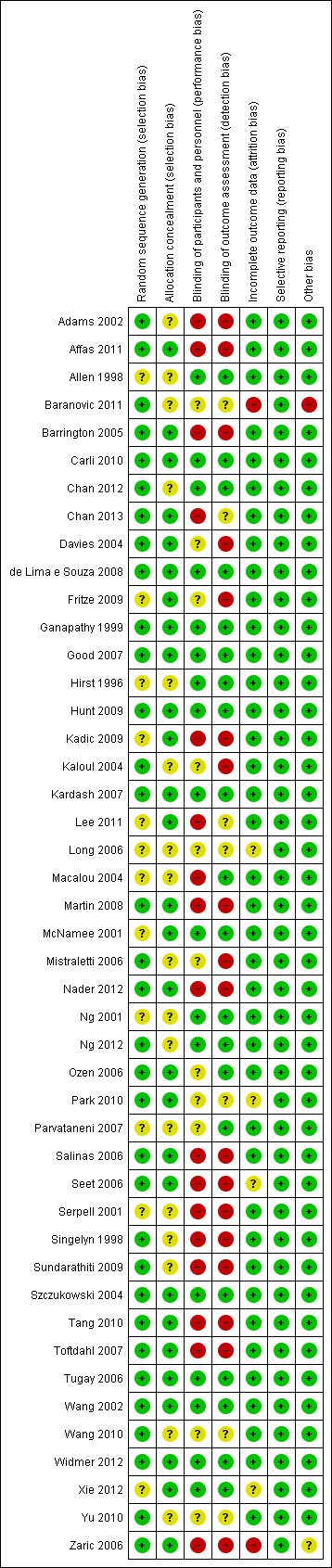
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
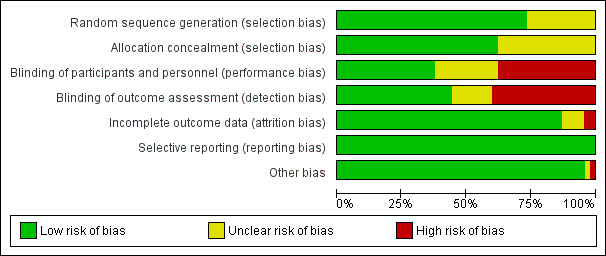
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All studies stated that the participants were randomly allocated to treatment groups. The method of randomization was judged to have a low risk of bias for 33 (73%) RCTs and unclear risk for 12 (27%) RCTs, as the methods were not stated and we were unable to reach the study authors for details.
Concealment of allocation was adequate in 28 (62%) and unclear in 17 (38%) included RCTs. Concealment using sealed envelopes without mention of whether they were sequentially numbered and opaque was rated unclear.
Blinding
Blinding of participants and personnel was judged to be at low risk of bias for 17 (38%) RCTs, high risk of bias for 17 (38%) RCTs and unclear risk of bias for 11 (24%) RCTs. Twenty RCTs (44%) reported using blinded outcome assessors, 18 (40%) RCTs did not blind outcome assessors and seven (16%) RCTs were judged as having unclear risk, as they provided no information on assessor blinding.
Incomplete outcome data
The short follow‐up period (first 72 hours post operation) in most of the RCTs reduced the risk of loss to follow‐up. Hence, attrition bias was low in all except four (9%) RCTs (Long 2006; Park 2010; Seet 2006; Xie 2012), which were rated as unclear, as no information on post randomization exclusions was provided. Two (4%) RCTs (Baranovic 2011; Zaric 2006) were rated as high risk, as 18% to 20% post randomization exclusions were determined. See Characteristics of included studies.
Selective reporting
We judged that selective reporting bias was avoided by the reporting of results for all outcomes listed in the methods section and by the provision of additional data on request. The risk of reporting bias was low to moderate for the included RCTs.
Other potential sources of bias
All RCTs except two (4%) (Baranovic 2011; Zaric 2006) had a low risk of bias for intention‐to‐treat analysis, as the number of participants lost to follow‐up for the outcomes of interest in this review was very small.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
See Data and analyses; Table 1 (FNB with or without PCA opioid vs PCA opioid); Table 2 (FNB vs epidural); Table 3 (FNB vs local infiltration analgesia) and Table 4 (continuous vs single‐shot FNB).
For pain and participant satisfaction outcomes, we interpreted intervention effects based on SMDs according to the rule of thumb where 0.2 or smaller represents a small effect, 0.5 a moderate effect and 0.8 or larger a large effect (Higgins 2011; see Section 12.6.2).
I. FNB (with or without PCA opioid) versus PCA opioid
Primary outcomes
1. Pain at rest and on movement
(See Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Figure 4; Analysis 1.9; Analysis 1.10.)
1.1. Analysis.

Comparison 1 FNB versus PCA opioid, Outcome 1 Pain at rest first 2 hours.
1.2. Analysis.
Comparison 1 FNB versus PCA opioid, Outcome 2 Pain at rest 3 to 12 hours.
1.3. Analysis.
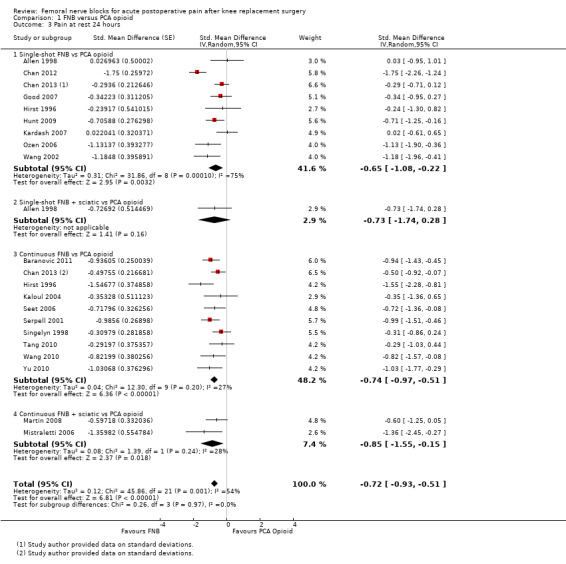
Comparison 1 FNB versus PCA opioid, Outcome 3 Pain at rest 24 hours.
1.4. Analysis.
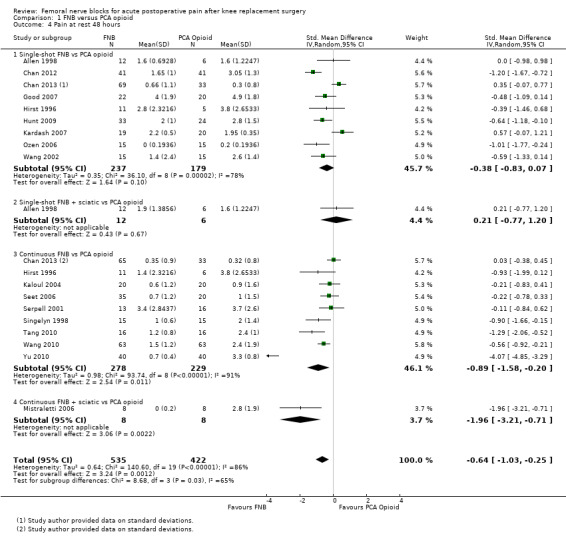
Comparison 1 FNB versus PCA opioid, Outcome 4 Pain at rest 48 hours.
1.5. Analysis.
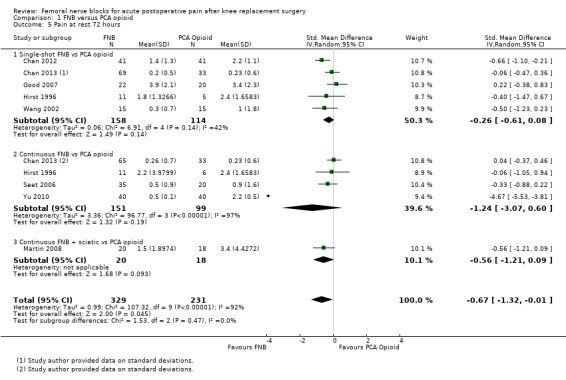
Comparison 1 FNB versus PCA opioid, Outcome 5 Pain at rest 72 hours.
1.6. Analysis.
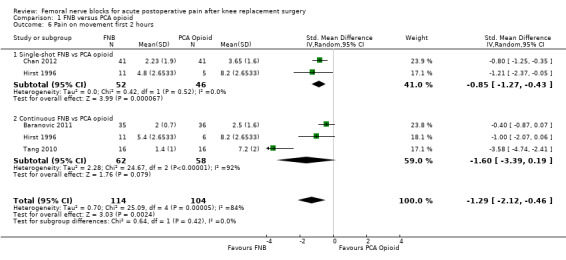
Comparison 1 FNB versus PCA opioid, Outcome 6 Pain on movement first 2 hours.
1.7. Analysis.

Comparison 1 FNB versus PCA opioid, Outcome 7 Pain on movement 3 to 12 hours.
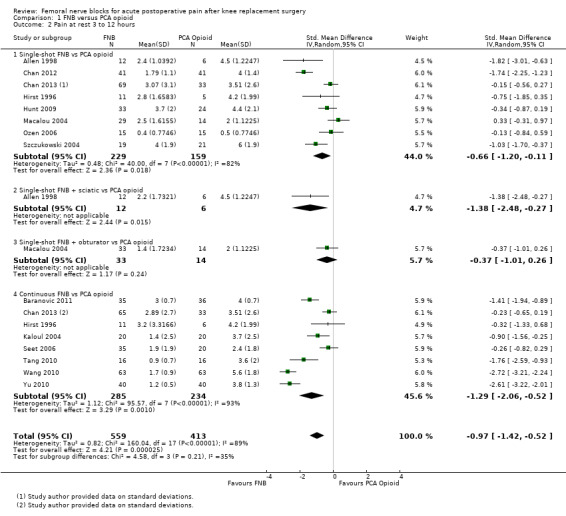
4.
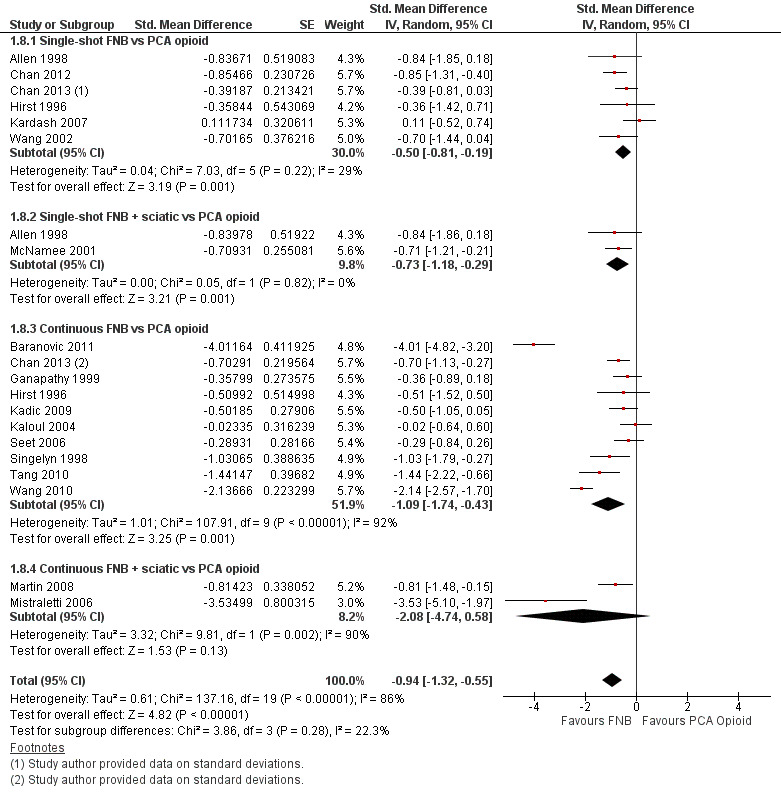
Forest plot of comparison: 1 FNB versus PCA opioid. Outcome: 1.8 Pain on movement at 24 hours.
1.9. Analysis.
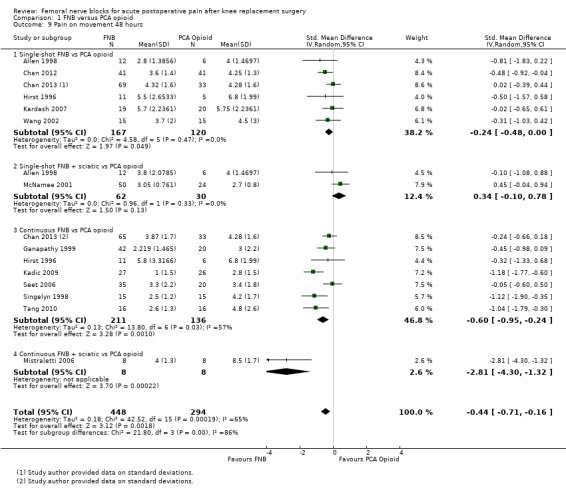
Comparison 1 FNB versus PCA opioid, Outcome 9 Pain on movement 48 hours.
1.10. Analysis.
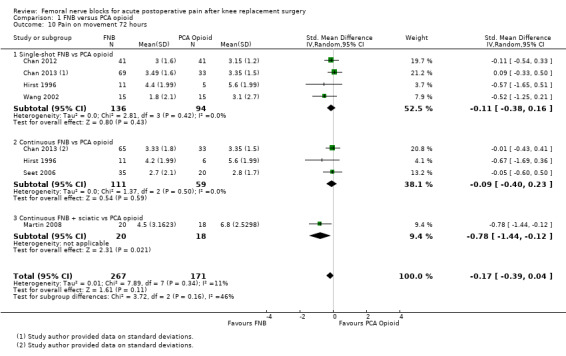
Comparison 1 FNB versus PCA opioid, Outcome 10 Pain on movement 72 hours.
Pooled results for FNB (any type, with or without a concurrent PCA opioid) vs PCA opioid demonstrated significantly lower pain at rest for FNB from zero to 72 hours: first two hours (11 RCTs, 706 participants, SMD ‐0.58, 95% CI ‐1.00 to ‐0.16, I2 = 84%), three to 12 hours (14 RCTs, 972 participants, SMD ‐0.97, 95% CI ‐1.42 to ‐0.52, I2 = 89%), 24 hours (19 RCTs, 1066 participants, SMD ‐0.72, 95% CI ‐0.93 to ‐0.51, I2 = 54%), 48 hours (17 RCTs, 957 participants, SMD ‐0.64, 95% CI ‐1.03 to ‐0.25, I2 = 86%), and 72 hours (eight RCTs, 560 participants, SMD ‐0.67, 95% CI ‐1.32 to ‐0.01, I2 = 92%).
Similarly, the pooled results for pain on movement also demonstrated significantly less pain for FNB (any type, with or without a concurrent PCA opioid) from zero to 48 hours: first two hours (four RCTs, 218 participants, SMD ‐1.29, 95% CI ‐2.12 to ‐0.46, I2 = 84%), three to 12 hours (eight RCTs, 462 participants, SMD ‐1.06, 95% ‐1.68 to ‐0.43, I2 = 88%), 24 hours (17 RCTs, 1017 participants, SMD ‐0.94, 95% CI ‐1.32 to ‐0.55, I2 = 86%) and 48 hours (13 RCTs, 742 participants, SMD ‐0.44, 95% CI ‐0.71 to ‐0.16, I2 = 65%). The results for 72 hours also favoured FNB, although not significantly (six RCTs, 438 participants, SMD ‐0.17, 95% CI ‐0.39 to 0.04, I2 = 11%).
Three RCTs (264 participants) (Chan 2013; Martin 2008; Mistraletti 2006) assessed pain intensity after 72 hours. Of these, one RCT (Chan 2013) compared continuous FNB (catheter duration less than 72 hours) and single‐shot FNB with PCA, and two RCTs (Martin 2008; Mistraletti 2006) compared continuous FNB + sciatic versus PCA (catheter duration 48 hours). Chan 2013 found no significant difference in pain scores at rest and on movement at day five, week two and month three; Mistraletti 2006 reported no difference in pain at rest on discharge between the allocation groups. Martin 2008 reported significantly less pain at rest for FNB at day seven, but not at month one and month three, compared with PCA opioid.
For pain at rest and on movement at 48 hours, the result of test for differences between subgroups is statistically significant (P < 0.05), suggesting that analgesic effects at 48 hours are not consistent between the different types of FNB subgroups.
Pain at rest and on movement at 24 hours: subgroup analysis by type of FNB
(See Analysis 1.3; Analysis 1.8.)
1.8. Analysis.
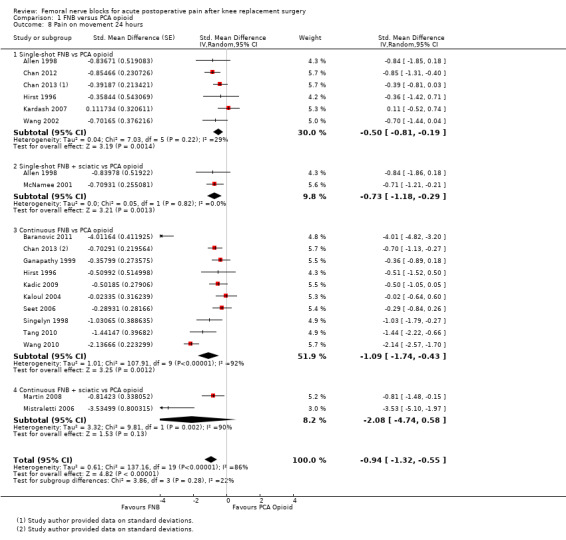
Comparison 1 FNB versus PCA opioid, Outcome 8 Pain on movement 24 hours.
We categorized RCTs according to type of FNB (i.e. single‐shot FNB, single‐shot FNB + sciatic/obturator block, continuous FNB, continuous FNB + sciatic block). For the single‐shot FNB subgroup, pain at rest at 24 hours (nine RCTs, 416 participants, SMD ‐0.65, 95% CI ‐1.08 to ‐0.22, I2 = 75%) and pain on movement at 24 hours (six RCTs, 287 participants, SMD ‐0.50, 95% CI ‐0.81 to ‐0.19, I2 = 29%) were significantly less in the FNB subgroup compared with PCA. Pain scores were also significantly lower in the continuous FNB subgroup at rest at 24 hours (10 RCTs, 578 participants, SMD ‐0.74, 95% CI ‐0.97 to ‐0.51, I2 = 27%) and on movement at 24 hours (10 RCTs, 584 participants, SMD ‐1.09, 95% CI ‐1.74 to ‐0.43, I2 = 92%).
Only a few small RCTs evaluated the addition of sciatic or obturator block to the single‐shot or continuous FNB. The single‐shot FNB + sciatic block subgroup had significantly lower pain scores compared with the PCA opioid subgroup on movement at 24 hours (two RCTs, 92 participants, SMD ‐0.73, 95% CI ‐1.18 to ‐0.29, I2 = 0%) but not at rest at 24 hours (one RCT, 24 participants, SMD ‐0.73, 95% CI ‐1.74 to 0.28). The continuous FNB + sciatic block subgroup favoured FNB for pain at rest at 24 hours (two RCTs, 54 participants, SMD ‐0.85, 95% CI ‐1.55 to ‐0.15, I2 = 28%) but not for pain on movement at 24 hours (two RCTs, 54 participants, SMD ‐2.08, 95% CI ‐4.74 to 0.58, I2 = 90%).
Generally, results of the subgroup analyses demonstrated that the type of FNB explained some of the heterogeneity of the overall results (I2 < 30% for continuous FNB and continuous FNB + sciatic block for pain at rest at 24 hours; and I2 < 30% for single‐shot FNB and single‐shot FNB + sciatic block for pain on movement at 24 hours).
Results of the various types of FNB at other timings are shown in these analyses (see Analysis 1.4; Analysis 1.5; Analysis 1.9; Analysis 1.10).
Pain at rest and on movement at 24 hours: subgroup analysis by FNB with and without concurrent PCA opioid
(See Analysis 1.11; Analysis 1.12.)
1.11. Analysis.
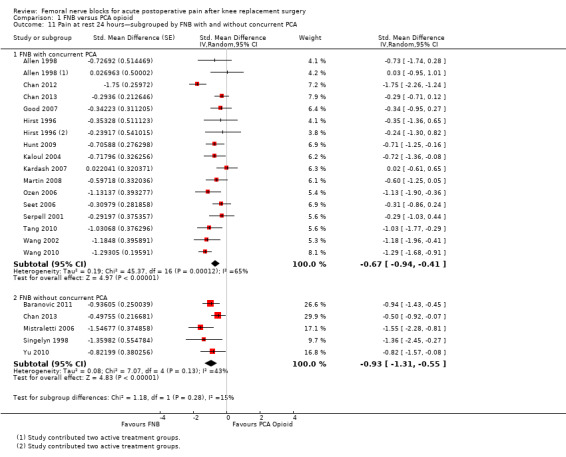
Comparison 1 FNB versus PCA opioid, Outcome 11 Pain at rest 24 hours—subgrouped by FNB with and without concurrent PCA.
1.12. Analysis.
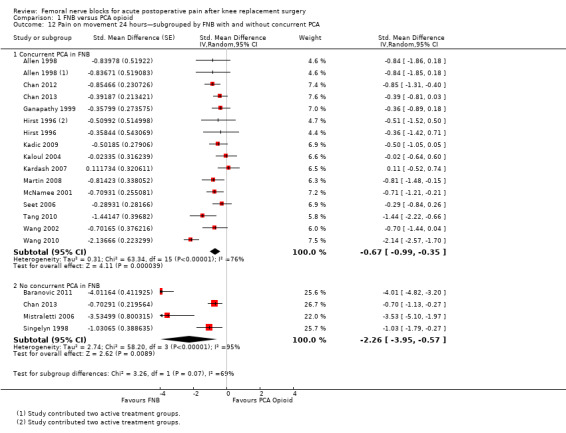
Comparison 1 FNB versus PCA opioid, Outcome 12 Pain on movement 24 hours—subgrouped by FNB with and without concurrent PCA.
For pain at rest at 24 hours, FNB (any type) both with a concurrent PCA opioid (15 RCTs, 771 participants, SMD ‐0.67, 95% CI ‐0.94 to ‐0.41, I2 = 65%) and without a concurrent PCA opioid (five RCTs, 295 participants, SMD ‐0.93, 95% CI ‐1.31 to ‐0.55, I2 = 43%) demonstrated significantly less pain compared with PCA opioid alone. Similarly, for pain on movement at 24 hours, the groups given FNB with a concurrent PCA opioid (14 RCTs, 802 participants, SMD ‐0.67, 95% CI ‐0.99 to ‐0.35, I2 = 76%) and without a concurrent PCA opioid (four RCTs, 215 participants, SMD ‐2.26, 95% CI ‐3.95 to ‐0.57, I2 = 95%) had significantly less pain when compared with the group given PCA opioid alone.
Pain at rest and on movement at 24 hours: subgroup analysis by FNB with ropivacaine versus bupivacaine
(See Analysis 1.13; Analysis 1.14.)
1.13. Analysis.
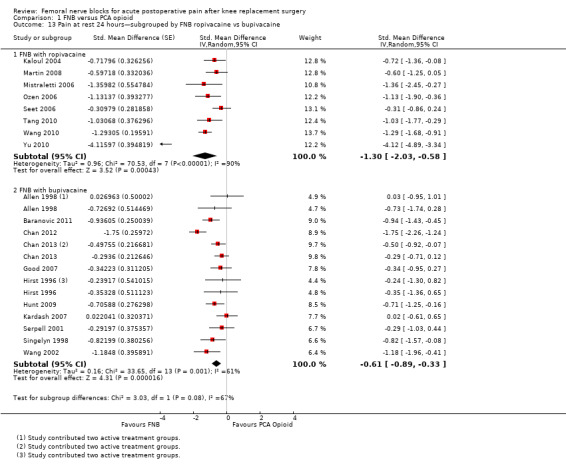
Comparison 1 FNB versus PCA opioid, Outcome 13 Pain at rest 24 hours—subgrouped by FNB ropivacaine vs bupivacaine.
1.14. Analysis.
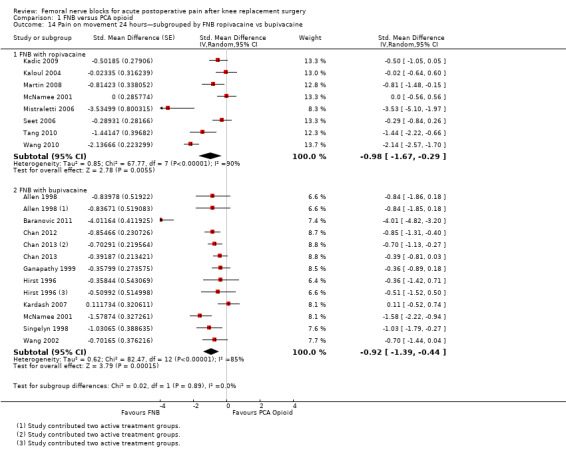
Comparison 1 FNB versus PCA opioid, Outcome 14 Pain on movement 24 hours—subgrouped by FNB ropivacaine vs bupivacaine.
For pain at rest at 24 hours, both FNB (any type) with ropivacaine (eight RCTs, 379 participants, SMD ‐1.30, 95% CI ‐2.03 to ‐0.58, I2 = 90%) and FNB (any type) with bupivacaine (11 RCTs, 649 participants, SMD ‐0.61, 95% CI ‐0.89 to ‐0.33, I2 = 61%) demonstrated significantly less pain compared with PCA opioid. For pain on movement at 24 hours, the group given FNB with ropivacaine (eight RCTs, 409 participants, SMD ‐0.98, 95% CI ‐1.67 to ‐0.29, I2 = 90%) and FNB with bupivacaine (10 RCTs, 632 participants, SMD ‐0.92, 95% CI ‐1.39 to ‐0.44, I2 = 85%) also had significantly less pain compared with the group given PCA opioid.
This subgroup analysis did not explain the heterogeneity in the main results.
Pain at rest and on movement at 24 hours: sensitivity analysis by adequacy of allocation concealment and blinding
(See Analysis 1.15; Analysis 1.16; Analysis 1.17; Analysis 1.18.)
1.15. Analysis.

Comparison 1 FNB versus PCA opioid, Outcome 15 Pain at rest 24 hours—sensitivity analysis by low bias for allocation concealment.
1.16. Analysis.
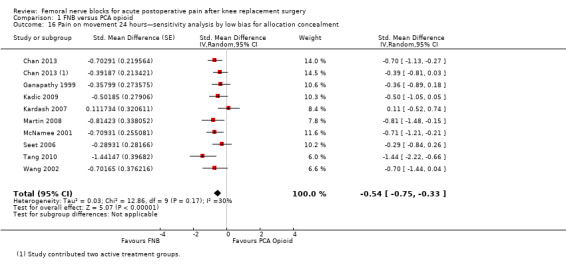
Comparison 1 FNB versus PCA opioid, Outcome 16 Pain on movement 24 hours—sensitivity analysis by low bias for allocation concealment.
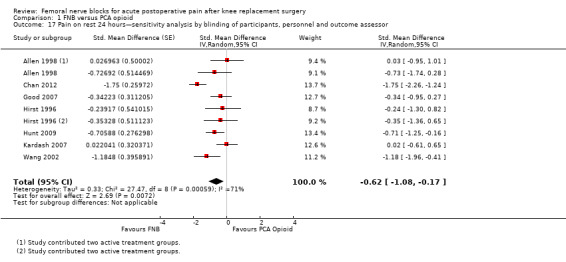
1.17. Analysis.
Comparison 1 FNB versus PCA opioid, Outcome 17 Pain on rest 24 hours—sensitivity analysis by blinding of participants, personnel and outcome assessor.
1.18. Analysis.
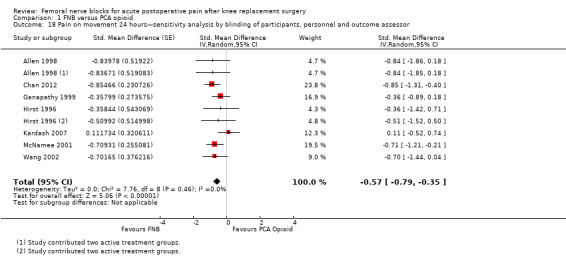
Comparison 1 FNB versus PCA opioid, Outcome 18 Pain on movement 24 hours—sensitivity analysis by blinding of participants, personnel and outcome assessor.
Pooling only the RCTs with low risk of bias regarding allocation concealment showed a significant reduction in the FNB (any type) for pain at rest (nine RCTs, 523 participants, SMD ‐0.54, 95% CI ‐0.76 to ‐0.32, I2 = 28%) and on movement (nine RCTs, 583 participants, SMD ‐0.54, 95% CI ‐0.75 to ‐0.33, I2 = 30%) at 24 hours.
Pooling only the results of trials with low risk of bias for adequate blinding of participants, personnel and outcome assessors also demonstrated significantly less pain at rest at 24 hours (seven RCTs, 319 participants, SMD ‐0.62, 95% CI ‐1.08 to ‐0.17, I2 = 71%) and on movement at 24 hours (seven RCTs, 356 participants, SMD ‐0.57, 95% CI ‐0.79 to ‐0.35, I2 = 0). Heterogeneity was explained by adequate blinding for pain on movement but not at rest at 24 hours.
2. Serious adverse events
Few RCTs reported on serious adverse events.
Five RCTs (474 participants) reported no neurological injury in the FNB group (Chan 2012; Chan 2013; Kadic 2009; McNamee 2001; Seet 2006). Persistent numbness of the anterior thigh at 48 hours was reported in one participant in the single‐shot FNB group compared with none in the PCA opioid group (one RCT, 40 participants) (Kardash 2007). One RCT (42 participants) reported that in the single‐shot FNB group, a participant had knee numbness, and in the PCA group, two participants had infection and one participant had dyspnoea (Good 2007). The only RCT (200 participants) that reported on thrombotic events had one event in the continuous FNB group (Chan 2013). Two RCTs (282 participants) reported no falls in the FNB or PCA group (during the hospital stay (Chan 2013) or during the first three postoperative days (Chan 2012)). Three RCTs (192 participants) reported that no local anaesthetic toxicity was noted in the FNB or PCA group (Kadic 2009; McNamee 2001, Seet 2006). Five RCTs reported on technical failure: 27% technical failure with continuous FNB 0.2% bupivacaine and 55% with continuous FNB 0.1% bupivacaine in one RCT (62 participants) (Ganapathy 1999), no technical failure with the continuous FNB in one RCT (58 participants) (Kadic 2009) and no technical failure with the single‐shot FNB in three RCTs (171 participants) (Allen 1998; de Lima e Souza 2008; McNamee 2001).
Secondary outcomes
1. Proportion of participants with significant pain postoperatively
Six comparisons from five RCTs (511 participants) reported the proportion of participants with moderate/severe pain. FNB demonstrated significantly fewer participants with moderate/severe pain compared with PCA opioid (RR 0.73, 95% CI 0.65 to 0.82, I2 = 0). The NNTB was five (95% CI four to 11).
2. Time from end of surgery to first rescue opioid request
Two RCTs (Chan 2012; McNamee 2001) reported that participants receiving single‐shot FNB took significantly longer time to first opioid request compared with those receiving PCA. One RCT (McNamee 2001) (74 participants, MD 7.21 hour, 95% CI 6.88 to 7.54 hour) demonstrated a much larger FNB effect compared with the other (Chan 2012) (82 participants, MD 3.23 hour, 95% CI 1.88 to 4.58 hour). The former RCT (McNamee 2001) had the addition of a sciatic block. The findings of these two RCTS were not pooled because of the high heterogeneity (I2 = 99%).
3. Opioid consumption
(See Analysis 1.19; Analysis 1.20.)
1.19. Analysis.
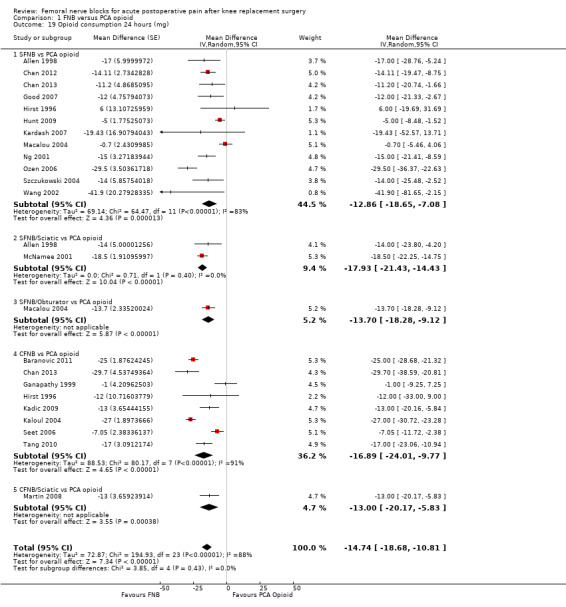
Comparison 1 FNB versus PCA opioid, Outcome 19 Opioid consumption 24 hours (mg).
1.20. Analysis.
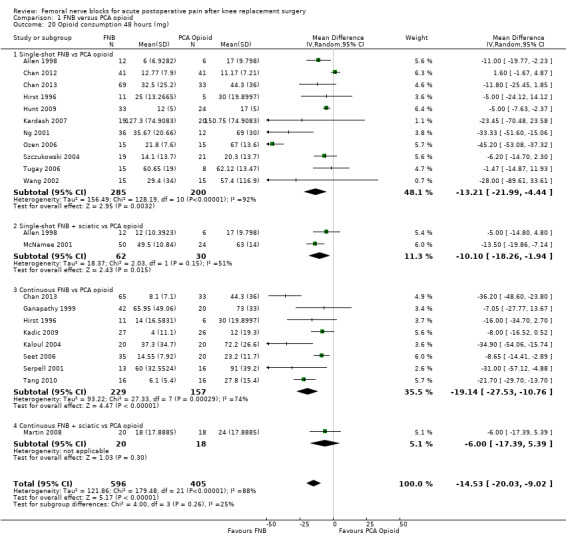
Comparison 1 FNB versus PCA opioid, Outcome 20 Opioid consumption 48 hours (mg).
Pooled results for opioid consumption (IV morphine equivalent) were significantly reduced for FNB at 24 hours (20 RCTs, 1152 participants, MD ‐14.74 mg, 95% ‐18.68 to ‐10.81 mg, I2 = 88%) and at 48 hours (19 RCTs, 1001 participants, MD ‐14.53 mg, 95% ‐20.03 to ‐9.02 mg, I2 = 88%).
4. Adverse effects
4.1 Nausea and/or vomiting
(See Analysis 1.21.)
1.21. Analysis.
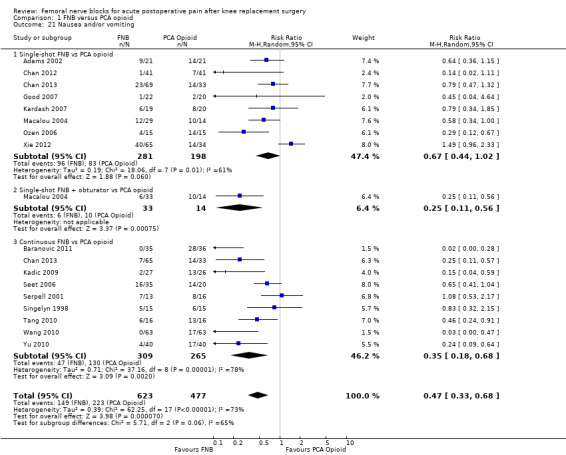
Comparison 1 FNB versus PCA opioid, Outcome 21 Nausea and/or vomiting.
Pooled results showed that the risk of nausea and/or vomiting was significantly lower for FNB compared with PCA opioid (16 RCTs, 1100 participants, RR 0.47, 95% CI 0.33 to 0.68, I2 = 73%). The NNTH was four (95% CI three to six).
4.2 Sedation
(See Analysis 1.22.)
1.22. Analysis.
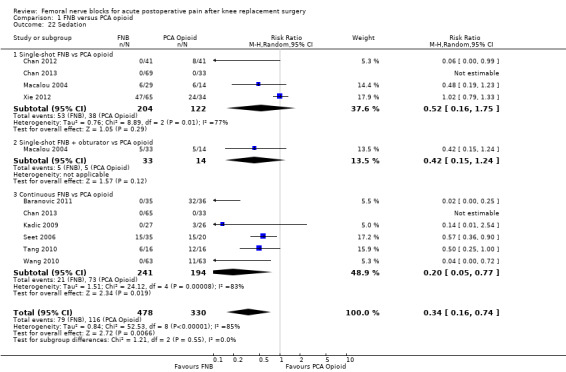
Comparison 1 FNB versus PCA opioid, Outcome 22 Sedation.
Pooled results showed that the risk of sedation was significantly lower in the FNB group as compared with the PCA opioid group (nine RCTs, 808 participants, RR 0.34, 95% CI 0.16 to 0.74, I2 = 85%; NNTH five, 95% CI five to eight).
4.3 Urinary retention
(See Analysis 1.23.)
1.23. Analysis.
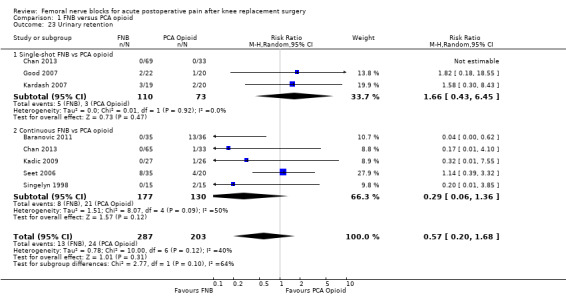
Comparison 1 FNB versus PCA opioid, Outcome 23 Urinary retention.
Seven RCTs with 37 events from 490 participants evaluated urinary retention. These results showed no difference in risk of urinary retention between FNB and PCA opioid (RR 0.57, 95% CI 0.20 to 1.68, I2 = 40%).
5. Physical function
(See Analysis 1.24.)
1.24. Analysis.
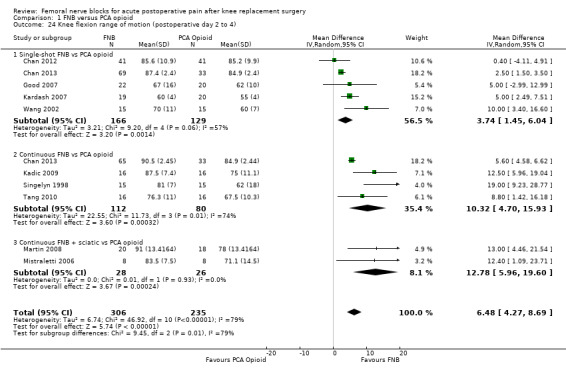
Comparison 1 FNB versus PCA opioid, Outcome 24 Knee flexion range of motion (postoperative day 2 to 4).
During postoperative day two to four, the FNB group achieved greater knee flexion (10 RCTs, 541 participants, MD 6.48 degrees, 95% CI 4.27 to 8.69 degrees, I2 = 79%) and knee extension (three RCTs, 297 participants, MD ‐0.16 degrees, 95% CI ‐0.30 to ‐0.01 degrees, I2 = 0%) compared with the PCA opioid group. Two RCTs evaluated time to first ambulation and found no differences between groups (71 participants, MD 1.86 degrees, 95% CI ‐7.40 to 11.12 degrees, I2 = 0%).
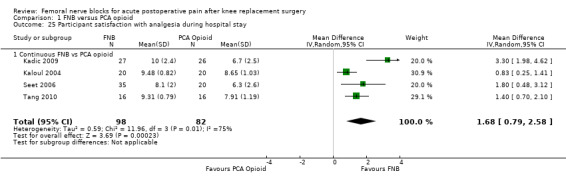
6. Participant satisfaction with analgesia during hospital stay
(See Analysis 1.25.)
1.25. Analysis.
Comparison 1 FNB versus PCA opioid, Outcome 25 Participant satisfaction with analgesia during hospital stay.
Four studies assessed participant satisfaction on a continuous scale, and their pooled results indicated greater participant satisfaction with FNB versus PCA opioid (180 participants, SMD 1.06, 95% CI 0.74 to 1.38, I2 = 0%). Two small RCTs reported participant satisfaction as a dichotomous variable, and their results were not pooled because of high heterogeneity (I2 = 96%). The findings of both studies were not statistically significant (42 participants, RR 1.00, 95% CI 0.91 to 1.09) (Adams 2002); (62 participants, RR 1.64, 95% CI 1.02 to 2.64) (de Lima e Souza 2008).
II. FNB versus epidural analgesia
Primary outcomes
1. Pain at rest and on movement
(See Analysis 2.3; Analysis 2.5.)
2.3. Analysis.
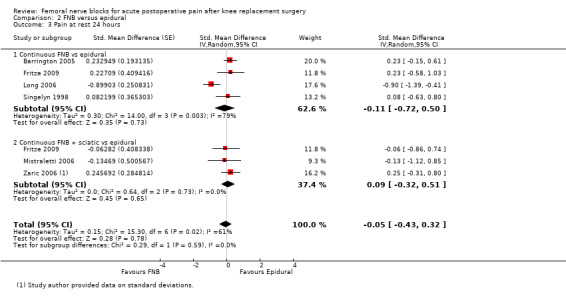
Comparison 2 FNB versus epidural, Outcome 3 Pain at rest 24 hours.
2.5. Analysis.
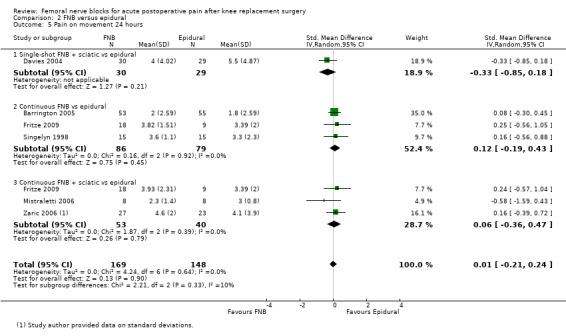
Comparison 2 FNB versus epidural, Outcome 5 Pain on movement 24 hours.
We found no differences in pooled pain scores at rest and on movement for all measured time points in the first 72 hours post operation between FNB and epidural analgesia. Pooled results for FNB (any type) versus epidural for pain at rest at 24 hours included the following: six RCTs, 328 participants, SMD ‐0.05, 95% CI ‐0.43 to 0.32, I2 = 61%; and for pain on movement at 24 hours: six RCTs, 317 participants, SMD 0.01, 95% CI ‐0.21 to 0.24, I2 = 0%. The data from one study with a concurrent epidural in the FNB intervention showed significantly lower pain scores for the FNB + epidural intervention for pain on movement at 24 hours compared with epidural alone (78 participants, SMD ‐2.94, 95% CI ‐3.59 to ‐2.29) but not for pain at rest at 24 hours (SMD ‐0.34, 95% CI ‐0.79 to 0.10) (Lee 2011). Details of the pooled results at rest at two hours, three to 12 hours and 48 hours are shown in Analysis 2.1; Analysis 2.2; Analysis 2.4 and Analysis 2.6. The other non‐significant findings were for pain at rest at 72 hours (two RCTs, 124 participants, SMD 0.18, 95% CI ‐0.35 to 0.71, I2 = 45%); pain on movement at three to 12 hours (one RCT, 59 participants, SMD 0.20, 95% CI ‐0.31 to 0.71) and pain on movement at 72 hours (one RCT, 54 participants, SMD 0.61, 95% CI ‐0.06 to 1.09).
2.1. Analysis.
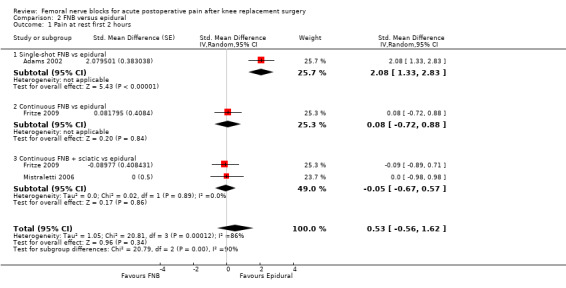
Comparison 2 FNB versus epidural, Outcome 1 Pain at rest first 2 hours.
2.2. Analysis.
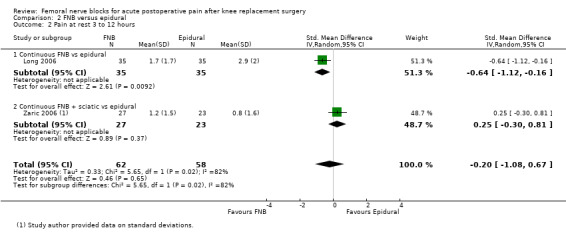
Comparison 2 FNB versus epidural, Outcome 2 Pain at rest 3 to 12 hours.
2.4. Analysis.
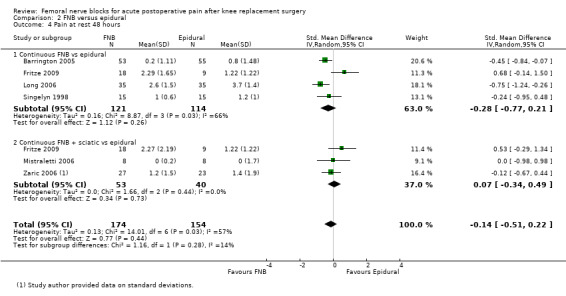
Comparison 2 FNB versus epidural, Outcome 4 Pain at rest 48 hours.
2.6. Analysis.
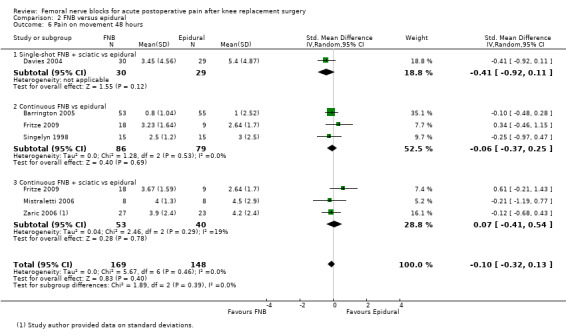
Comparison 2 FNB versus epidural, Outcome 6 Pain on movement 48 hours.
The test results for subgroup differences were significant (P < 0.05) for pain at rest at two hours and at three to 12 hours,
Pain at rest and on movement at 24 hours: subgroup analysis by type of FNB
(See Analysis 2.3; Figure 5.)
5.
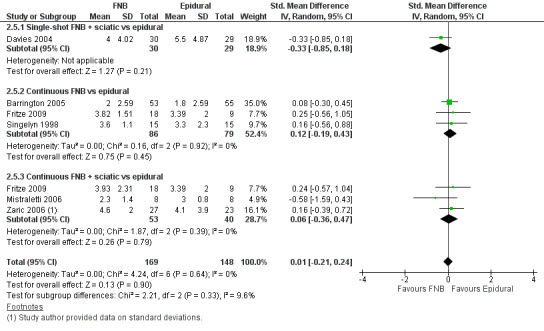
Forest plot of comparison: 2 FNB versus epidural. Outcome: 2.5 Pain on movement at 24 hours.
No significant differences were detected between pain scores at rest and on movement at 24 hours for the subgroups of single‐shot FNB + sciatic block, continuous FNB and continuous FNB + sciatic block. For pain at rest at 24 hours, statistical heterogeneity was reduced in the analyses by type of FNB for the continuous FNB + sciatic block subgroup (three RCTs, 93 participants, SMD 0.09, 95% CI ‐0.32 to 0.51, I2 = 0%). For pain on movement at 24 hours, no heterogeneity was found (I2 = 0%) in the subgroups of continuous FNB (three RCTs, 165 participants, SMD 0.12, 95% CI ‐0.19 to 0.43) and continuous FNB + sciatic block (three RCTs, 93 participants, SMD 0.06, 95% CI ‐0.36 to 0.47). Only one RCT was identified in the subgroup of. single‐shot FNB + sciatic block (59 participants, SMD ‐0.33, 95% CI ‐0.85 to 0.18) for this outcome.
Pain at rest and on movement at 24 hours: subgroup analysis by FNB with ropivacaine versus bupivacaine
(See Analysis 2.7; Analysis 2.8.)
2.7. Analysis.
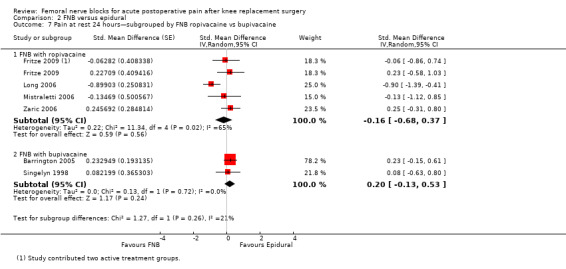
Comparison 2 FNB versus epidural, Outcome 7 Pain at rest 24 hours—subgrouped by FNB ropivacaine vs bupivacaine.
2.8. Analysis.

Comparison 2 FNB versus epidural, Outcome 8 Pain on movement 24 hours—subgrouped by FNB ropivacaine vs bupivacaine.
For pain at rest at 24 hours, no significant differences were detected for FNB with ropivacaine (four RCTs, 190 participants, SMD ‐0.16, 95% CI ‐0.68 to 0.37, I2 = 65%) or FNB with bupivacaine (two RCTs, 216 participants, SMD 0.20, 95% CI ‐0.13 to 0.53, I2 = 0%) compared with epidural, respectively. Similarly, for pain on movement at 24 hours, no significant differences were found for FNB with ropivacaine (four RCTs, 120 participants, SMD 0.10, 95% CI ‐0.27 to 0.47, I2 = 0%) or FNB with bupivacaine (three RCTs, 197 participants, SMD ‐0.03, 95% CI ‐0.31 to 0.25, I2 = 0%) compared with epidural, respectively.
Pain at rest and on movement at 24 hours: sensitivity analysis by adequacy of allocation concealment and blinding
(See Analysis 2.9; Analysis 2.10.)
2.9. Analysis.
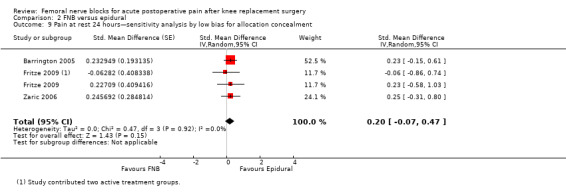
Comparison 2 FNB versus epidural, Outcome 9 Pain at rest 24 hours—sensitivity analysis by low bias for allocation concealment.
2.10. Analysis.
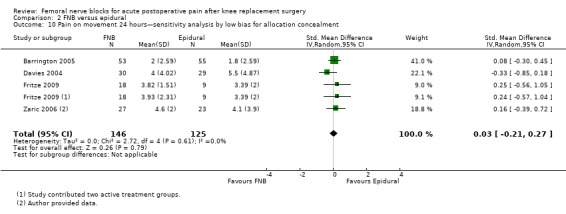
Comparison 2 FNB versus epidural, Outcome 10 Pain on movement 24 hours—sensitivity analysis by low bias for allocation concealment.
No significant difference between the groups was detected at 24 hours on the basis of pooled results from RCTs with low risk of bias for allocation concealment for pain at rest (three RCTs, 194 participants, SMD 0.20, 95% CI ‐0.07 to 0.47, I2 = 0%) and for pain on movement (four RCTs, 271 participants, SMD 0.03, 95% CI ‐0.21 to 0.27, I2 = 0%). Statistical heterogeneity was reduced in analyses by adequate allocation concealment for pain at rest and on movement at 24 hours. Sensitivity analysis on the adequacy of blinding was not conducted, as no RCTs with low risk of bias for blinding were identified.
2. Serious adverse events
Four RCTs reported on neurological injury: One RCT (59 participants) reported that a participant with epidural developed unilateral foot drop and sphincteric disturbance after operation, but the aetiology was not stated (Davies 2004), and three RCTs (247 participants) reported no incidence of neurological injury in either allocation group (Barrington 2005; Lee 2011; Sundarathiti 2009). One RCT (70 participant) with a follow‐up of six months post operation reported a participant with a fall at home with wound dehiscence requiring reoperation in the continuous FNB group and none in the epidural group (Long 2006). This RCT also reported two participants in the continuous FNB group requiring close manipulation, one participant in the epidural group with bladder inflammation from reaction to latex of the Foley catheter and no incidence of haematoma formation of the knee or drainage in either group (Long 2006). Another RCT (50 participants) reported a participant with atrial fibrillation in the epidural group (Zaric 2006). There was no incidence of local anaesthetic toxicity from two RCTs (111 patients) (Sundarathiti 2009; Zaric 2006), and no incidence of catheter‐related infection from one RCT (61 patients) (Sundarathiti 2009). One RCT (108 patients) reported three cardiac events (one non‐ST elevation myocardiac infarction and one cardiac conduction defect requiring permanent pacemaker) in the continuous FNB group and one hypotension (60/40 mmHg and bradycardic,spinal‐epidural block reached dermatome T3 upon 2 hours of the epidural infusion) in the epidural group (Barrington 2005). Another RCT (59 patients) reported frequent hypotension in both allocation groups (37% in the single‐shot FNB+sciatic group and 27% in the epidural group) (Davies 2004). Conversely, another RCT (78 patients) reported no incidence of severe hypotension or cardiac complications (Lee 2011).
Secondary outcomes
1. Proportion of participants with significant pain postoperatively
The result from one small RCT (61 participants) favoured epidural between groups for the proportion of participants with moderate/severe pain (RR 2.07, 95% CI 1.28 to 3.33) (Sundarathiti 2009).
2. Time from end of surgery to first rescue analgesic request
We found no data on this outcome.
3. Opioid consumption
(See Analysis 2.11; Analysis 2.12.)
2.11. Analysis.
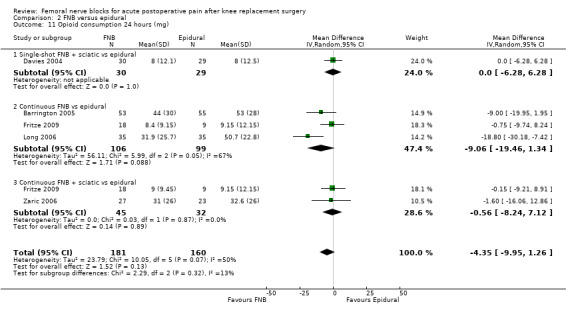
Comparison 2 FNB versus epidural, Outcome 11 Opioid consumption 24 hours (mg).
2.12. Analysis.

Comparison 2 FNB versus epidural, Outcome 12 Opioid consumption 48 hours (mg).
Results from five RCTs indicate that the FNB group consumed less opioid (IV morphine equivalent) compared with the epidural group at 24 hours (341 participants, MD ‐4.35 mg, 95% CI ‐9.95 to 1.26 mg, I2 = 50%). Similarly, pooled results at 48 hours also favoured FNB (four RCTs, 233 participants, MD ‐1.28 mg, 95% CI ‐5.30 to 2.74 mg, I2 = 0%), although this finding was not statistically significant.
We did not pool the data from three RCTs because the data at 24 and 48 hours were not available (Barrington 2005; Sundarathiti 2009) or because the analgesics were too varied (Singelyn 1998). In the first RCT (108 participants) (Barrington 2005), over 72 hours, the continuous FNB group required less morphine (mean (SD) 44 (30) vs 53 (28) mg, P value 0.45), but more oxycodone (mean (SD) 21 (15) vs 13 (12) mg, P value 0.005) compared with the epidural group. However the epidural group also received a mean dosage of fentanyl of 1.74 mg. In the second RCT (61 participants) (Sundarathiti 2009), the cumulative IV tramadol over 72 hours was higher in the continuous FNB group compared with the epidural group (median (range) = 150 (0 to 350) vs 50 (0 to 150) mg, P value 0.001). In the third RCT (30 participants) (Singelyn 1998), both the FNB and epidural groups received infusion bupivacaine with sufentanil and clonidine, and the supplemental analgesics required in the first 48 hours were comparable (propacetamol 1.7 (1.1) vs 1.1 (1.5) g, P value 0.37; IM piritramide 1.9 (4.1) vs 2.3 (6.2) mg, P value 0.29).
4. Adverse effects
4.1 Nausea and/or vomiting
(See Analysis 2.13.)
2.13. Analysis.
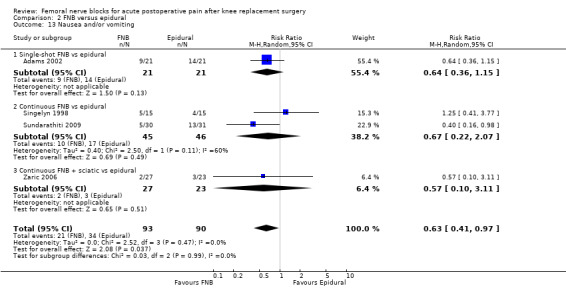
Comparison 2 FNB versus epidural, Outcome 13 Nausea and/or vomiting.
Pooled results from four RCTs demonstrated that the risk of nausea and/or vomiting in the FNB group was less than in the epidural group (183 participants, RR 0.63, 95% CI 0.41 to 0.97, I2 = 0%). The NNTH was eight, 95% CI four to 53.
4.2 Sedation
The only RCT that evaluated sedation found no significant difference in risk of sedation between the allocation groups (50 participants, RR 0.43, 95% CI 0.04 to 4.40) (Zaric 2006).
4.3 Urinary retention
Pooled results from four RCTs revealed no significant difference between groups in risk of urinary retention (200 participants, RR 0.36, 95% CI 0.07 to 1.88, I2 = 49%).
4.4 Technical failure of the blocks
The meta‐analysis of four RCTs (287 participants) found no difference in technical failure of the block (RR 0.87, 95% CI 0.49 to 1.55, I2 = 0%) or catheter disconnection/problem (RR 0.73, 95% CI 0.24 to 2.22, I2 = 15%) between the FNB and epidural groups.
5. Physical function
Pooled data of six RCTs revealed no significant difference in knee flexion during postoperative day two to four (328 participants, MD ‐1.65 degrees, 95% CI ‐5.14 to 1.84 degrees, I2 = 33%), and the results from one RCT (Long 2006) showed no significant difference in knee extension on postoperative day three between groups (115 participants, MD 5.00 degrees, 95% CI 1.62 to 8.38 degrees).
One small RCT evaluated the time to first ambulation and found no differences between groups (16 participants, MD 8.50 hours, 95% CI ‐5.50 to 22.50 hours) (Mistraletti 2006).
6. Participant satisfaction with analgesia
Two RCTs reported on participant satisfaction as a continuous variable (scale zero to 10), and their pooled results indicate that participants with FNB were more satisfied (120 participants, SMD 0.60, 95% CI 0.23 to 0.97, I2 = 0%). The only RCT that evaluated participant satisfaction as a dichotomous outcome reported that all participants in both FNB and epidural groups were satisfied with their treatment (30 participants, RR 1, 95% CI 0.91 to 1.09) (Singelyn 1998).
III. FNB versus local infiltration analgesia
Primary outcomes
1. Pain at rest and on movement
(See Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4.)
3.1. Analysis.

Comparison 3 FNB versus local infiltration analgesia, Outcome 1 Pain at rest 24 hours.
3.2. Analysis.
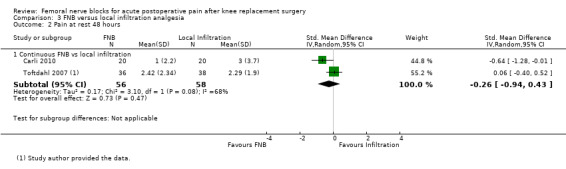
Comparison 3 FNB versus local infiltration analgesia, Outcome 2 Pain at rest 48 hours.
3.3. Analysis.
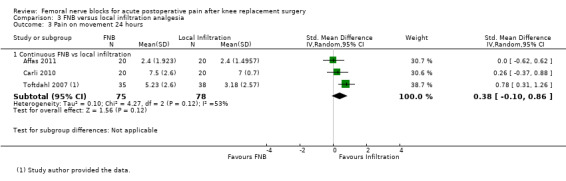
Comparison 3 FNB versus local infiltration analgesia, Outcome 3 Pain on movement 24 hours.
3.4. Analysis.
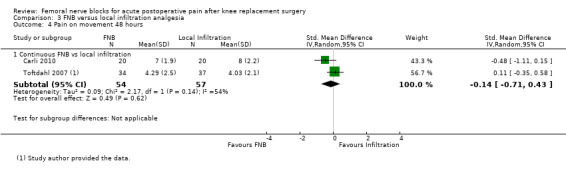
Comparison 3 FNB versus local infiltration analgesia, Outcome 4 Pain on movement 48 hours.
Pooled results revealed no difference between FNB and local infiltration analgesia for pain at rest at 24 hours (four RCTs, 216 participants, SMD 0.06, 95% CI ‐0.61 to 0.72, I2 = 82%) and at 48 hours (two RCTs, 114 participants, SMD ‐0.26, 95% CI ‐0.94 to 0.43, I2 = 68%) or for pain on movement at 24 hours (three RCTs, 153 participants, SMD 0.38, 95% CI ‐0.10 to 0.86, I2 = 53%) and at 48 hours (two RCTs, 111 participants, SMD ‐0.14, 95% CI ‐0.71 to 0.43, I2 = 54%).
One RCT with FNB + local infiltration analgesia versus local infiltration analgesia also found no difference in pain at rest at 24 hours between allocation groups (55 participants, SMD ‐0.11, 95% CI ‐0.64 to 0.42) (Widmer 2012). Similarly, one cross‐over RCT (16 participants) comparing collateral knees reported no significant differences between FNB and local infiltration analgesia for pain at rest and on movement at 24 hours, 48 hours and 72 hours (Ng 2012). One RCT assessed pain at rest at 12 hours and found that local infiltration analgesia yielded lower pain scores compared with FNB (76 participants, SMD 0.56, 95% CI 0.10 to 1.02) (Toftdahl 2007).
Pain at rest and on movement at 24 hours: sensitivity analysis by adequacy of blinding
Only one small RCT (40 participants) described adequate blinding of participants, personnel and outcome assessors for pain at rest and on movement at 24 hours (Carli 2010). The RCT favoured FNB for pain at rest at 24 hours (SMD ‐1.12, 95% CI ‐1.79 to ‐0.45) but not for pain on movement at 24 hours (SMD 0.26, 95% CI ‐0.37 to 0.88).
2. Serious adverse event
One RCT (76 participants) reported the following adverse events in the local infiltration group (40 participants) and none in the FNB group: two participants requiring open surgical intervention (one for deep surgical site infection and another for tissue necrosis), two participants with a cardiac event (one syncopal attack of cardiac origin and one episode of chest pain justifying investigation), two participants with gastric ulcer and two participants with urinary tract infection (Toftdahl 2007). Another RCT (40 participants) reported that one participant in the local infiltration group had a cardiac event (atrial fibrillation requiring treatment) (Carli 2010). Four RCTs (171 participants) reported no incidence of neurological injury, wound infection, surgical intervention, cardiac events or falls in both FNB and infiltration groups (Affas 2011; Ng 2012; Parvataneni 2007; Widmer 2012).
Secondary outcomes
1. Proportion of participants with significant pain postoperatively
No statistical significance was detected in the proportion of participants with moderate/severe pain from the pooling of two RCTs (114 participants, RR 1.86, 95% CI 0.50 to 6.92, I2 = 45%).
2. Time from end of surgery to first rescue analgesic request
We found no data on this outcome.
3. Opioid consumption
At 24 hours, a small RCT found that FNB had lower opioid consumption compared with local infiltration analgesia, but the results were not significant (40 participants, MD ‐11.50 mg, 95% CI ‐24.08 mg to 1.08) (Carli 2010). Another RCT found that FNB + local infiltration analgesia resulted in lower opioid consumption at 24 hours compared with local infiltration analgesia alone (55 participants, MD ‐35.44 mg, 95% CI ‐56.02 to ‐14.86 mg) (Widmer 2012). These two RCTs were not pooled, as one of them (Widmer 2012) had a concurrent local infiltration analgesia in the FNB group. At 48 hours, pooled results of two RCTs on opioid consumption revealed no significant differences (117 participants, MD 3.51 mg, 95% CI ‐23.43 to 30.46 mg). Similarly, one cross‐over RCT (16 participants) reported no significant differences in opioid consumption on postoperative day one or day two between the allocation groups (Ng 2012).
4. Adverse effects
4.1 Nausea and/or vomiting
(See Analysis 3.5.)
3.5. Analysis.
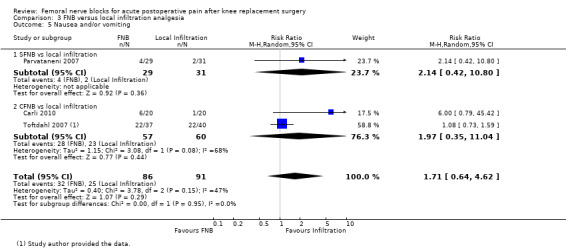
Comparison 3 FNB versus local infiltration analgesia, Outcome 5 Nausea and/or vomiting.
Pooled results of three RCTs showed no statistical differences between groups in risk of nausea and vomiting (177 participants, RR 1.71, 95% CI 0.64 to 4.62, I2 = 47%).
5. Physical function
One RCT found no significant differences in knee flexion on postoperative day two between groups (40 participants, MD ‐0.50 degrees, ‐5.28 to 4.28 degrees) (Carli 2010). Another RCT failed to find any significant difference between groups in knee extension on postoperative day two (76 participants, MD 1.15 degrees, 95% CI ‐1.73 to 4.03 degrees) (Toftdahl 2007).
6. Participant satisfaction with analgesia
One RCT found that participants with FNB had lower satisfaction scores compared with participants with local infiltration analgesia (60 participants, mean 7.8 vs 9.0, SD not reported) (Parvataneni 2007).
IV. FNB versus oral analgesia
Primary outcomes
1. Pain at rest and on movement
Only one RCT (62 participants) compared FNB versus oral analgesia. In this RCT (Nader 2012), continuous FNB + oral analgesia was compared with oral analgesia alone on postoperative day one at discontinuation of the epidural analgesia. Investigators found that continuous FNB + oral analgesia led to significantly lower pain scores at rest at 24 hours (MD ‐3.03, 95% CI ‐4.00 to ‐2.06) and 48 hours (MD ‐2.00, 95% CI ‐3.20 to ‐0.80), as well as on movement at 24 hours (MD ‐2.00, 95% CI ‐3.10 to ‐0,90), when compared with oral analgesia alone (Nader 2012). At 72 hours, no significant difference was detected for pain at rest (MD ‐1.00, 95% CI ‐2.18 to 0.18).
2. Serious adverse event
Following epidural, four of 31 participants in the oral opioid analgesia group had deep venous thrombosis or pulmonary embolus versus none in the continuous FNB + oral analgesia group (Nader 2012).
Secondary outcomes
Results from one RCT (62 participants) showed that following epidural, the FNB + oral analgesia group consumed less opioid at 24 hours (MD ‐15.00 mg, 95% CI ‐24.50 to ‐5.50 mg) and at 48 hours (MD ‐7.00 mg, 95% CI ‐21.25 to 7.25 mg), although the results were not statistically significant. FNB + oral analgesia also led to greater knee flexion compared with oral analgesia alone (MD 15.00 mg, 95% CI 5.96 to 24.04 mg). However, no significant difference was detected in participant satisfaction between FNB + oral analgesia and oral analgesia alone (MD 2.00 mg, 95% CI 1.06 to 2.94 mg) (Nader 2012). We found no data on the other secondary outcomes.
V. Continuous versus single‐shot FNB
Primary outcomes
1. Pain at rest and on movement
(See Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4; Figure 6; Analysis 4.6.)
4.1. Analysis.
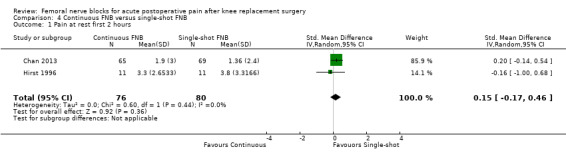
Comparison 4 Continuous FNB versus single‐shot FNB, Outcome 1 Pain at rest first 2 hours.
4.2. Analysis.
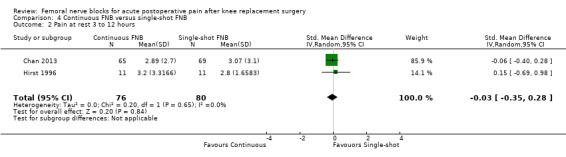
Comparison 4 Continuous FNB versus single‐shot FNB, Outcome 2 Pain at rest 3 to 12 hours.
4.3. Analysis.
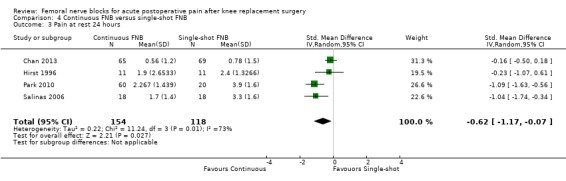
Comparison 4 Continuous FNB versus single‐shot FNB, Outcome 3 Pain at rest 24 hours.
4.4. Analysis.
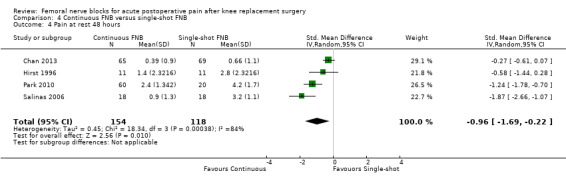
Comparison 4 Continuous FNB versus single‐shot FNB, Outcome 4 Pain at rest 48 hours.
6.
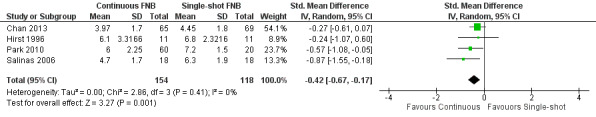
Forest plot of comparison: 4 Continuous FNB versus single‐shot FNB. Outcome: 4.5 Pain on movement at 24 hours.
4.6. Analysis.
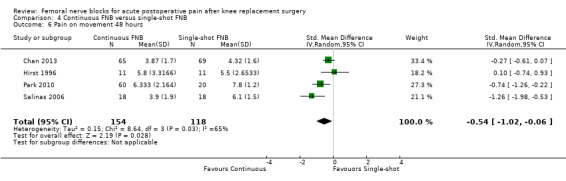
Comparison 4 Continuous FNB versus single‐shot FNB, Outcome 6 Pain on movement 48 hours.
Continuous FNB showed significantly lower pain intensity when compared with single‐shot FNB at rest at 24 hours (four RCTs, 272 participants, SMD ‐0.62, 95% CI ‐1.17 to ‐0.07, I2 = 73%) and at 48 hours (four RCTs, 272 participants, SMD ‐0.96, 95% CI ‐1.69 to ‐0.22, I2 = 84%), as well as on movement at 24 hours (four RCTs, 272 participants, SMD ‐0.42, 95% CI ‐0.67 to ‐0.17, I2 = 0%) and at 48 hours (four RCTs, 272 participants, SMD ‐0.54, 95% CI ‐1.02 to ‐0.06, I2 = 65%).
Pooled results of two RCTs (156 participants) at the following time points found no significant differences between allocation groups: pain at rest at two hours (SMD 0.15, 95% CI ‐0.17 to 0.46, I2 = 0), at three to 12 hours (SMD ‐0.03, 95% CI ‐0.35 to 0.28, I2 = 0) and at 72 hours (SMD 0.10, 95% CI ‐0.21 to 0.42, I2 = 0); and pain on movement at 72 hours (SMD ‐0.09, 95% CI ‐0.41 to 0.22, I2 = 0).
Pain at rest and on movement at 24 hours: subgroup analysis by continuous FNB with and without a PCA opioid intervention
(See Analysis 4.7; Analysis 4.8.)
4.7. Analysis.
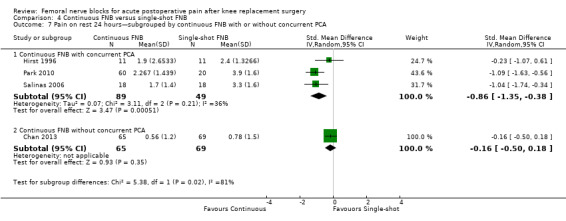
Comparison 4 Continuous FNB versus single‐shot FNB, Outcome 7 Pain on rest 24 hours—subgrouped by continuous FNB with or without concurrent PCA.
4.8. Analysis.
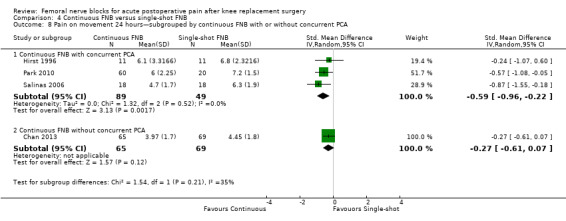
Comparison 4 Continuous FNB versus single‐shot FNB, Outcome 8 Pain on movement 24 hours—subgrouped by continuous FNB with or without concurrent PCA.
For pain at rest at 24 hours, pooled RCTs with a concurrent PCA opioid intervention in both continuous and single‐shot FNB groups (three RCTs, 138 participants, SMD ‐0.86, 95% CI ‐1.35 to ‐0.38, I2 = 36%) and the one RCT with a concurrent PCA opioid intervention only in the single‐shot FNB group (134 participants, SMD ‐0.16, 95% CI ‐0.50 to 0.18), respectively, showed that continuous FNB resulted in less pain compared with single‐shot FNB. For pain on movement at 24 hours, results for continuous FNB with a concurrent PCA opioid (three RCTs, 138 participants, SMD ‐0.59, 95% CI ‐0.96 to ‐0.22, I2 = 0) and without a concurrent PCA opioid (one RCT, 134 participants, SMD ‐0.27, 95% CI ‐0.61 to 0.07), respectively, also showed continuous FNB to lead to less pain compared with single‐shot FNB.
Pain at rest and on movement at 24 hours: sensitivity analysis by adequacy of allocation concealment and blinding
(See Analysis 4.9; Analysis 4.10.)
4.9. Analysis.
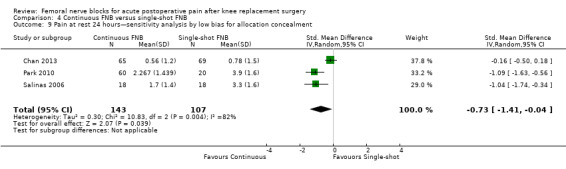
Comparison 4 Continuous FNB versus single‐shot FNB, Outcome 9 Pain at rest 24 hours—sensitivity analysis by low bias for allocation concealment.
4.10. Analysis.
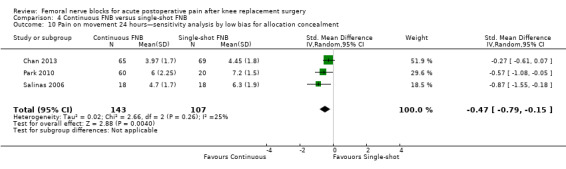
Comparison 4 Continuous FNB versus single‐shot FNB, Outcome 10 Pain on movement 24 hours—sensitivity analysis by low bias for allocation concealment.
Pooling of only RCTs with low risk of bias for allocation concealment did not change the magnitude or the direction of the effect estimate for pain at 24 hours, at rest (three RCTs, 250 participants, SMD ‐0.73, 95% CI ‐1.41 to ‐0.04, I2 = 82%) or on movement (three RCTs, 250 participants, SMD ‐0.47, 95% CI ‐0.79 to ‐0.15, I2 = 25%).
Only one small RCT (Hirst 1996) (22 participants) had low risk of bias pertaining to blinding. Results indicated that differences between groups for pain at 24 hours were not statistically significant at rest (MD ‐0.50, 95% CI ‐2.25 to 1.25) and on movement (MD ‐0.70, 95% CI ‐3.09 to 1.69).
2. Serious adverse events
Two RCTs (170 participants) that compared continuous versus single‐shot FNB reported no incidence of neurological injury (Chan 2013; Salinas 2006). One RCT (38 participants) reported no catheter‐related infection (Salinas 2006).
Secondary outcomes
1. Proportion of participants in significant pain postoperatively
We found no data on this outcome.
2. Time from end of surgery to first rescue analgesic request
We found no data on this outcome.
3. Opioid consumption
(See Analysis 4.11; Analysis 4.12.)
4.11. Analysis.
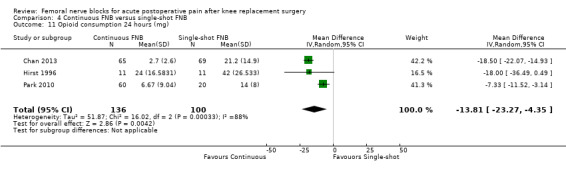
Comparison 4 Continuous FNB versus single‐shot FNB, Outcome 11 Opioid consumption 24 hours (mg).
4.12. Analysis.
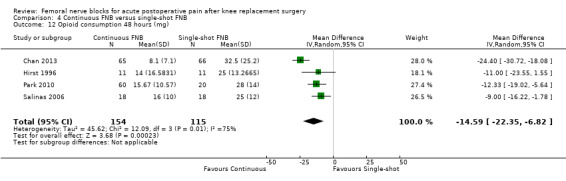
Comparison 4 Continuous FNB versus single‐shot FNB, Outcome 12 Opioid consumption 48 hours (mg).
Opioid consumption was significantly less in the continuous FNB group compared with the single‐shot FNB group at 24 hours (three RCTs, 236 participants, MD ‐13.81 mg, 95% CI ‐23.27 to ‐4.35 mg, I2 = 88%) and at 48 hours (four RCTs, 269 participants, MD ‐14.59 mg, 95% CI ‐22.35 to ‐6.82 mg, I2 = 75%).
4. Adverse effects
4.1 Nausea and/or vomiting
Pooled results from two RCTs showed no differences in risk of nausea/vomiting between continuous and single‐shot FNB groups (214 participants, RR 0.55, 95% CI 0.20 to 1.56, I2 = 78%).
4.2 Sedation
Two RCTs (214 participants) presented results on sedation (Chan 2013; Park 2010). One of these RCTs (134 participants) reported zero events in both groups and did not contribute to the analysis (Chan 2013). Thus, only the results of one RCT were considered; these revealed no differences in risk of sedation between groups (80 participants, RR 4.33, 95% CI 0.60 to 31.07) (Park 2010).
4.3 Technical failure of the blocks
One RCT (36 participants) reported that no technical failure occurred (Salinas 2006).
5. Physical function
We found no data on this outcome.
6. Participant satisfaction with analgesia
We found no data on this outcome.
Discussion
Summary of main results
This review has examined the published evidence comparing the use of FNB (any type) with or without concurrent treatments including PCA opioid versus a variety of alternative methods for reducing postoperative pain following knee replacement surgery. We found that pain at rest and pain on movement were less for FNB at all time intervals considered up to 72 hours post operation. At 24 hours, FNB (any type) had a moderate to large analgesic effect at rest and on movement compared with PCA. The moderate to large effects (SMD), when re‐expressed as mean differences (zero to 10 scale), represented at 24 hours a 1.20‐point decrease in mean pain at rest and a 1.66‐point decrease in mean pain on movement for FNB compared with PCA (Table 1). FNB also resulted in less opioid consumption at 24 hours and 48 hours, and correspondingly lower risk of nausea/vomiting or sedation, compared with PCA opioid.
When compared with an epidural block, no differences in pain at rest or on movement were noted when FNB was used, although FNB demonstrated lower opioid consumption at 24 hours, lower risk of nausea and/or vomiting and greater participant satisfaction compared with epidural. Similarly, no differences were detected between FNB and local infiltration analgesia for pain at rest and on movement at any evaluated time point.
The single study comparing FNB versus oral analgesia after TKR suggested that the former offered superior analgesia at rest from 24 to 48 hours, and on movement at 24 hours.
Using a continuous FNB was superior to using a single‐shot technique, with moderate effects on pain intensity noted at 24 hours and 48 hours both at rest and on movement. Reexpressing SMD as MD on a zero to 10 scale suggests, at 24 hours, a 0.95‐point reduction in mean pain at rest and a 0.74‐point reduction in mean pain on movement (Table 4). Continuous FNB also led to lower opioid consumption at 24 hours and at 48 hours compared with single‐shot FNB.
Serious adverse events are rare with FNB, and we have been unable to report definitive results concerning the risk of serious adverse events for any of the analgesic techniques examined. Nevertheless, the adverse events in the local infiltration analgesia group reported by Toftdahl 2007 are a cause of concern. Larger trials examining two main issues are needed before this technique can be recommended. First, two reinterventions out of 40 participants for infection or tissue necrosis at the surgical site seems unusually high. Second, although blood concentrations have been reported to be low, the total amount of local anaesthetic administered with this technique is high. Cardiac toxicity in patients with underlying cardiac disease may occur with blood concentrations that would be well tolerated in normal patients. In the RCT, two participants given local infiltration analgesia had cardiac events (Toftdahl 2007). Therefore, before widespread use of this technique is considered, especially in centres with very short hospital stays (less than 24 hours), studies evaluating the effects of this technique on cardiac conduction may be warranted.
Overall completeness and applicability of evidence
Generally, application of the review's finding to clinical practice is possible, as adequate descriptions of inclusion and exclusion criteria were provided. Nevertheless, the included RCTs have variable reporting or inconsistent definitions for some of the secondary outcomes. Subgroups involving single‐shot or continuous FNB with the addition of sciatic or obturator block should be interpreted cautiously because of the small numbers of trials and participants. For relatively uncommon events such as urinary retention, differences between groups would have been difficult to detect. Additionally, serious adverse events were rare, were reported in very few RCTs and most often were reported anecdotally.
In this review, the overall summary effects of FNB compared with PCA opioid, epidural or local infiltration analgesia, respectively, were derived from the combination of data from any type of FNB including single‐shot FNB, if the data were derived from similar postoperative periods. Since the effects of the single‐shot FNB possibly last less than 24 hours, the magnitude of the overall summary effect of the FNB (any type) after 24 hours likely underestimates the intervention effect of the continuous FNB.
Quality of the evidence
Generally, most RCTs were at low to moderate risk of bias for the aspects rated in our risk of assessment tool, except for the aspect of blinding (see Figure 3). We rated 14 (31%) RCTs at high risk for both participant and assessor blinding, and eight (18%) RCTs were rated at high risk for one blinding aspect. The high risk of bias for blinding was expected, as the nature of the analgesic regimens in this review is such that it is challenging to implement blinding effectively. To do so would have required the insertion of sham catheters. Even with sham catheters, clinical signs of numbness of the affected areas would have broken the blinding to participants. The open‐label nature of some of the RCTs may potentially bias the measurement of subjective outcomes such as pain and participant satisfaction.
For allocation concealment, sensitivity analyses pooling only RCTs at low risk of bias demonstrated that the primary results for comparisons of FNB (with or without PCA opioid) versus PCA opioid, FNB versus epidural and continuous FNB versus single‐shot FNB were robust. Pooling only RCTs at low risk of bias for blinding of participants/personnel and outcome assessors also showed the findings to be robust for the comparison of FNB versus PCA opioid. As expected, pooling results of trials with low risk of bias resulted in a slight reduction of the magnitude of the mean intervention effect but no change in statistical significance. We were unable to assess the effect of blinding for the comparison of FNB versus epidural, as all included RCTs were at high risk of blinding bias. We also could not conduct sensitivity analyses for the other comparisons because of the limited number of available RCTs.
Potential biases in the review process
Publication bias
We may have missed trials that were not indexed in CENTRAL, MEDLINE, EMBASE, CINAHL or Web of Science or that remain unpublished in journals. The fail‐safe N test indicated that our results were robust to publication bias (see Appendix 10). Nonetheless, we did approach trialists for information on existing RCTs. Although the possibility of publication bias cannot be excluded, a well‐conducted non‐significant trial would have had a reasonable chance of being published. The procedure we used to retrieve all RCTs was comprehensive and systematic, and two review authors conducted independent selection from results obtained by the searches. Thus the risk of publication bias is low.
Pooling and heterogeneity
We pooled data from RCTs testing the same comparisons (i.e. FNB vs PCA opioid, FNB vs epidural and FNB vs local infiltration analgesia) and presented the meta‐analyses stratified by different types of FNB (single‐shot or continuous FNB, with or without sciatic/obturator block). Notable differences were reported in the analgesic regimen across pooled FNB subgroups such as FNB with different local anaesthetics, drug concentrations and infusion rates; control groups with variations in drugs and dosages and differences in the supplemental analgesics used in these trials. These differences likely contributed to high heterogeneity in many of the meta‐analyses in this review. The type of FNB and the methodological quality of the trials have explained some of the heterogeneity of pooled data.
We used the random‐effects model to account for the anticipated heterogeneity of included RCTs. We also used risk ratios rather than odds ratios to report dichotomous outcomes. These decisions resulted in more conservative estimates of treatment effects with wider confidence intervals, compared with outcomes when fixed‐effect models and odds ratios were used. We did not reanalyse the data using a fixed‐effect model because of the high heterogeneity of the included trials. Sensitivity analyses demonstrated that the primary findings were robust to potentially influential allocation concealment and blinding bias.
Small‐study effects
We did not assess the influence of small trials on estimated treatment effects. Nevertheless, given that all the RCTs included in this review had fewer than 100 participants per arm, small‐study effects could potentially have biased the results of meta‐analyses towards an inflated treatment effect (Nuesch 2010).
Agreements and disagreements with other studies or reviews
The conclusions of the current review are similar to those provided by a previously published meta‐analysis comparing FNB and opioids (Paul 2010). Both reviews found single‐shot FNB to lead to lower pain scores during movement at 24 hours and at 48 hours; lower opioid requirement at 24 hours and at 48 hours and lower risk of nausea and vomiting. Unlike Paul's review, the current review also found that single‐shot FNB and continuous FNB have lower pain intensity at rest at 24 hours and 48 hours, lower risk of sedation, greater knee range of motion and higher participant satisfaction. Both Fischer 2008 and the authors of the current review found single‐shot or continuous FNB to have superior effects on pain up to 48 hours. The current review also demonstrated that FNB reduced the risk of nausea and/or vomiting, although this outcome was not significant in the Fischer 2008 review. Additionally, we demonstrated that continuous FNB was superior to single‐shot FNB for postoperative analgesia and opioid consumption, unlike in Paul 2010..
Any discordance with the two past reviews (Fischer 2008; Paul 2010) may be due to the fact that the current review included a larger number of RCTs for meta‐analysis and therefore had a greater possibility of detecting statistical significance. Moreover, for the continuous versus single‐shot FNB comparisons, we conducted direct (head‐to‐head) comparisons of parallel trials, while Paul 2010 used an indirect comparison.
Another review compared continuous peripheral nerve block and opioids (Richman 2006). That review concluded that continuous peripheral nerve block provided superior postoperative analgesia compared with opioids at 24 hours, 48 hours and 72 hours. It also reported that nerve block led to fewer minor complications, including nausea or vomiting, pruritus and sedation, and improved participant satisfaction. These conclusions were similar to ours, despite the broader inclusion criteria provided in the review by Richman 2006, which extended inclusion to both upper and lower extremity operations, and catheter locations in places other than the femoral nerve.
Our results for FNB versus epidural were somewhat in agreement with those of Fowler 2008, who compared FNB and epidural analgesia for major knee surgery. Both reviews found no differences in pain scores in the first 48 hours, or in physical activities, between FNB and epidural. Both reviews also found that participant satisfaction was greater with FNB compared with epidural analgesia. In contrast to Fowler 2008, our review additionally demonstrated lower opioid consumption at 24 hours and lower risk of nausea and/or vomiting in the FNB group. These differing results could have resulted from differences in the participant populations (TKR compared with major knee surgery).
Authors' conclusions
Implications for practice.
This review found evidence that following TKR, FNB (with or without concurrent treatments including PCA opioid) provided superior analgesia compared with PCA opioid alone, similar analgesia compared with epidural and less nausea/vomiting compared with both PCA opioid alone and epidural analgesia. The review also found that continuous FNB provided a better analgesia and adverse effects profile compared with single‐shot FNB. The analgesic benefits of FNB over PCA are moderate to large, and those of continuous FNB over single‐shot FNB are small to moderate (Cohen 1988). These findings favour the use of FNB, in particular continuous FNB, over PCA opioid or epidural. The analgesic benefit offered by FNB, however, did not appear to extend beyond the first 72 hours.
We do not have enough evidence in this review to draw conclusions about the additional analgesic benefit of adding sciatic or obturator nerve blocks to the FNB, although results suggest that little analgesic benefit is gained by the addition of the sciatic block. Nevertheless, it was not the intent of this review to compare FNB + sciatic or FNB + obturator versus FNB. Any effects of FNB on serious adverse events were unclear because of the limited available evidence. Information from the identified RCTs was insufficient for definitive conclusions to be drawn on the comparisons between FNB and local infiltration analgesia or oral analgesia.
Implications for research.
The findings of this review on local infiltration analgesia and oral analgesia were based on the results of a few small RCTs. More RCTs comparing FNB and local infiltration analgesia are warranted, given the increasing popularity of the latter and the increasing number of total knee replacement operations conducted annually worldwide. One problem of the existing evidence involves the varied components of the local infiltration analgesia used in the different studies, making it unclear which particular components of the technique are the determinants of any observed effects. The consistent components have been the local anaesthetic (with ropivacaine or bupivacaine) and epinephrine, although in varying dosages. Conversely, the roles of the other components such as ketorolac, morphine and corticosteroid are less clear, and great variance in usage has been noted between studies. Moreover, safety concerns regarding surgical site infection or tissue necrosis have arisen, along with occurrences of cardiac toxicity in cardiac patients with the use of local infiltration analgesia. Hence, an important area for research is to determine the ideal components and safety of local infiltration analgesia. Indeed, large prospective observational studies are needed to quantify the risk of serious adverse events associated with the local infiltration analgesia.
What's new
| Date | Event | Description |
|---|---|---|
| 6 January 2016 | Amended | Typo corrected in main objectives section (an additional full stop was removed). |
Acknowledgements
We would like to thank Associate Prof Mike Bennett (content editor), Prof Nathan Pace (statistical editor), Prof Joanne Guay, Prof Andrew Moore (peer reviewers) and Dr Janet Wale (consumer editor) for help and editorial advice provided during the preparation of this systematic review.
We would like to thank Dr John Carlisle (content editor), Dr Marialena Trivella (statistical editor), Prof Nathan Pace (co‐ordinating editor); Prof Joanne Guay, Prof Andrew Moore, Dr Gustavo Zanoli and Associate Prof Fiona Blyth (peer reviewers) and Dr Janet Wale and Suzanne Cunliffe (consumers) for help and editorial advice provided during the preparation of this systematic review protocol. We also thank the CARG Trials Search Co‐ordinator, Dr Karen Hovhannisyan, for help with the search strategy, and the Managing Editor, Mrs Jane Cracknell, for efficient administrative support.
We would like to thank the 21 study authors who responded to our request for additional information, including three authors who regretted that the data were no longer available. We are grateful to Dr Markus Huebscher (MH) and Fereshteh Pourkazemi (FP) for determining the study inclusion and extracting data from studies for which the review authors are the named authors, Isabel Ng HL and Dr Zhang Jinbin for their help in extracting data from the Chinese studies (Tang 2010; Wang 2010; Yu 2010) and Dr Markus Huebscher for extracting data from the German study (Fritze 2009).
Appendices
Appendix 1. Search strategy for CENTRAL, part of The Cochrane Library
#1 MeSH descriptor Arthroplasty, Replacement, Knee explode all trees #2 MeSH descriptor Arthroplasty, Replacement explode all trees #3 MeSH descriptor Arthroplasty explode all trees #4 arthroplast* or (knee near (replacement or surg*)) #5 (#1 OR #2 OR #3 OR #4) #6 MeSH descriptor Nerve Block explode all trees #7 nerve block* or FNB or ((femoral or psoas or (lumbar plexus) or (fascia iliaca)) near block*) #8 (#6 OR #7) #9 (#5 AND #8)
Appendix 2. Search strategy for MEDLINE (Ovid SP)
1. exp Arthroplasty, Replacement, Knee/ or exp Arthroplasty, Replacement/ or exp Arthroplasty/ or arthroplast*.af. or (knee adj3 (replacement or surg*)).af. 2. nerve block*.af. or ((femoral or psoas or lumbar plexus or fascia iliaca) adj3 block*).mp. or FNB.mp. or exp Nerve block/ 3. 1 and 2 4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 3. Search strategy for EMBASE (Ovid SP)
1. exp knee arthroplasty/ or exp arthroplasty/ or arthroplast*.af. or (knee adj3 (replacement or surg*)).mp. 2. nerve block*.af. or ((femoral or psoas or lumbar plexus or fascia iliaca) adj3 block).mp. or FNB.mp. or exp nerve block/ 3. 1 and 2 4. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 4. Search strategy for CINAHL (EBSCO host)
(((MH "Arthroplasty, Replacement, Knee+") OR (MH "Arthroplasty, Replacement") OR (MH "Arthroplasty") ) or arthroplast* or (knee and (replacement or surg*))) AND ((MM "Nerve Block") or (nerve block* or FNB) or ((femoral or psoas or (lumbar plexus) or (fascia iliaca)) and block*))
Appendix 5. Search strategy for Web of Science
#1 TS= arthroplast* or TS=(knee SAME (replacement or surg*)) #2 TS= (nerve block* or FNB) or TS=((femoral or psoas or (lumbar plexus) or (fascia iliaca)) SAMEblock*)) #3 (#1 and #2) #4 TS=random* or TS=((clinical or controlled) SAME trial*) #5 (#4 and #3)
Appendix 6. Study selection form
| Title of article | ||||
| Study ID (surname of first author and year published): | ||||
| Report ID (if different from Study ID) (e.g. duplicate publications, follow‐up studies) | ||||
| Publication type (e.g. full report, abstract) | ||||
| Study author contact details | ||||
| Date form completed (dd/mm/yy) | ||||
| Name of person extracting data | ||||
| Eligibility criteria | ||||
| Type of study | Participants | Interventions | Comparisons | Outcomes |
| RCT | Total knee replacement surgery |
FNB (single‐shot or continuous) +/‐ sciatic/obturator block |
|
Circle: Pain intensity, neurological injury, proportion of participants in pain postoperatively, time to first rescue analgesia, opioid consumption, supplemental postoperative analgesic requirements, knee range of motion, early mobilization, adverse effects, adverse events (postoperative falls, technical failures of block, reintervention), time to achieve discharge criteria and participant satisfaction with analgesia |
| Criterai met? Yes/No/Unclear |
Criteria met? Yes/No/Unclear |
Criteria met? Yes/No/Unclear |
Criteria met? Yes/No/Unclear |
Other outcome/s? |
| Include: Yes/No/Unclear | ||||
| If unclear, comment: | ||||
| Reason for exclusion: | ||||
| Notes: | ||||
Appendix 7. Data extraction form
| Study ID (surname of first author and year published) | |||
| Study characteristics | Descriptions (include comparative information for each intervention or comparison group if available) | Further details | Source (pg and/ fig/table/other) |
| Single centre/Multi‐centre | |||
| Start and end dates of data collection | |||
| Duration of participation (from recruitment to last follow‐up) | |||
| Power (e.g. power and sample size calculation, level of power achieved) | |||
| Notes: | |||
| Participant characteristics (record all reported measures) | |||
| Population description (from which study participants were drawn) | |||
| Setting (include location) | |||
| Inclusion criteria | |||
| Exclusion criteria | |||
| Method of recruitment (e.g. phone, mail, clinic patients) | |||
| Total no. randomly assigned | |||
| Baseline imbalances | |||
| Withdrawals and exclusions | |||
| Age | |||
| Sex | |||
| BMI | |||
| No. of participants in each group | Intervention 1:_____ ; Intervention 2:_____ Control 1: _____; Control 2: _____ |
||
| No. of participants who received intended treatment | Intervention 1: _____ ; Intervention 2:_____ Control 1:_____; Control 2:______ |
||
| No. of participants who were analysed | Intervention 1: _____; Intervention 2:_____ Control 1:______; Control 2:______ |
||
| Notes: | |||
| Interventions | |||
| Intervention group 1 | |||
| FNB technique and type (i.e. continuous or single‐shot FNB) | |||
| Additional blocks (i.e. sciatic or obturator) | |||
| Description of FNB (include type of drug, dosage and regimen used) | |||
| Duration of treatment period | |||
| PCA opioid in FNB group? | Yes/No. If yes, describe | ||
| Type of co‐analgesia/Supplemental analgesia | |||
| Loss to follow‐up | |||
| Others | |||
| Intervention group 2 | |||
| FNB technique and type (i.e. continuous or single‐shot FNB) | |||
| Additional blocks (i.e. sciatic or obturator) | |||
| Description of FNB (include type of drug, dosage and regimen used) | |||
| Duration of treatment period | |||
| PCA opioid in FNB group? | Yes/No. If yes, describe | ||
| Type of co‐analgesia/Supplemental analgesia | |||
| Loss to follow‐up | |||
| Others | |||
| Control group 1 | |||
| Technique and type (i.e. PCA, epidural, infiltration +/‐ intra‐articular injection, oral or placebo) | |||
| Drugs, dosage and regimen used | |||
| Placebo block in PCA group? | Yes/No. If yes, describe | ||
| Type of co‐analgesia/Supplemental analgesia | |||
| Loss to follow‐up | |||
| Others | |||
| Control group 2 | |||
| Technique and type (i.e. PCA, epidural, infiltration +/‐ intra‐articular injection, oral or placebo) | |||
| Drugs, dosage and regimen used | |||
| Placebo block in PCA group? | Yes/No. If yes, describe | ||
| Type of co‐analgesia/Supplemental analgesia | |||
| Loss to follow‐up | |||
| Others | |||
*NA: not appropriate; NR: not required.
| Outcomes | Description (include outcome definition) | Time points reported | Person measuring/reporting | Imputation of missing data | Further details |
|
Pain intensity (describe scale used, scale upper and lower limits and if high or low score is good, and whether scale is validated) |
|||||
| Proportion of participants in pain | |||||
| Time to first rescue analgesia | |||||
| Total opioid consumption | |||||
| Adjunct oral analgesia | |||||
| Knee range of motion | |||||
| Early mobilization | |||||
| Time to achieve discharge criteria | |||||
| Adverse effects (nausea, vomiting, urinary retention, etc) | |||||
| Technical failures of the block | |||||
| Neurological injury | |||||
| Mortality | |||||
| Postoperative falls | |||||
| Reintervention | |||||
|
Participant satisfaction with analgesia (include scale used, scale upper and lower limits and if high or low score is good, and whether scale is validated) |
|||||
| Others: | |||||
| Others: | |||||
|
Any outcomes collected but not reported? Yes/No If yes, what outcome/s: | |||||
| For continuous data (with a separate copy for each relevant intervention/control) | |||||||||
| Outcome | Unit of measurement/Time point | Intervention group | Control group | No. of missing participants in each group. Reasons missing? | No. of participants moved from other group. Reasons moved? | Details if outcome only described in text, or other summary statistics (SE, 95% CI)/If required statistics are not reported | Source (pg and/ fig/table/other) | ||
| n | Mean (SD) (specify if other variance) |
n | Mean (SD) (specify if other variance) | ||||||
| Pain intensity on movement (first 2 hours) | |||||||||
| Pain intensity on movement (> 2 to 12 hours) | |||||||||
| Pain intensity on movement (> 12 to 24 hours) | |||||||||
| Pain intensity on movement (> 24 to 72 hours) | |||||||||
| Pain intensity on movement (> 72 hours) | |||||||||
| Pain intensity at rest (first 2 hours) | |||||||||
| Pain intensity at rest (> 2 to 12 hours) | |||||||||
| Pain intensity at rest (> 12 to 24 hours) | |||||||||
| Pain intensity at rest (> 24 to 72 hours) | |||||||||
| Pain intensity at rest (> 72 hours) | |||||||||
| Time to first rescue analgesia | |||||||||
| Total opioid consumption | |||||||||
| Supplemental postoperative analgesic requirements | |||||||||
| Knee range of motion | |||||||||
| Early mobilization | |||||||||
| Time to achieve discharge criteria | |||||||||
| Participant satisfaction with analgesia | |||||||||
| Others | |||||||||
| For dichotomous data (with a separate copy for each relevant intervention/control) | |||||
|
Outcomes |
Intervention group (n/N) where n = no. of participants with outcome, N = no. of participants randomly assigned |
Control group (n/N) where n = no. of participants with outcome, N = no. of participants randomly assigned |
No. of missing participants in each group. Reasons missing? | No. of participants moved from other group. Reasons moved? | Location in text or source (pg and/ fig/table/other) |
| Proportion of participants in pain | |||||
| Nausea and/or vomiting | |||||
| Sedation | |||||
| Urinary retention | |||||
| Technical failure of blocks | |||||
| Neurological injury | |||||
| Mortality | |||||
| Postoperative falls | |||||
| Reintervention | |||||
| Others: | |||||
|
Other information relevant to the results Indicate whether any data were obtained from the primary author and whether results were estimated from graphs etc or were calculated by you using a formula (the formula should be given). Provide information here if results not reported in the article are obtained. |
| |
| Key conclusions of study authors: |
| Study funding sources: |
| Correspondence required for further study information (from whom, what and when) |
| |
References to other RCTs:
| References to published reports of potentially eligible RCTs not already identified for this review? If yes, give details. | ||
| First author | Journal/Conference | Year of publication |
| References to unpublished data from potentially eligible RCTs not already identified for this review? If yes, give details. | ||
Appendix 8. Quality assessment of eligible trials form
| Domain |
Risk of bias (Low/High/Unclear) |
Support for judgement (Quotes/Comments) |
Location in text or source (pg and/fig/table/ other) |
| Random sequence generation | |||
| Allocation concealment | |||
| Blinding of participants and personnel | |||
| Blinding of outcome assessment | |||
| Incomplete outcome data addressed (short‐term outcomes—during hospital admission) | |||
| Incomplete outcome data addressed (long‐term outcomes—after discharge) | |||
| Selective outcome reporting | |||
| Intention‐to‐treat | |||
| Other bias | |||
| Notes: |
Were withdrawals described? Yes/No/Unclear
Comment if any:
Appendix 9. Summary of findings
| Outcomes | Illustration comparative risks (95% CI) |
Relative effect (95% CI) |
No. of participants (studies) |
Quality of the evidence (GRADE) |
Comments |
|
| Assumed risk | Corresponding risk | |||||
| Pain at rest at 24 hours | ||||||
| Pain on movement at 24 hours | ||||||
| Neurological injury | ||||||
| Total opioid consumption | ||||||
| Nausea and/or vomiting | ||||||
| Knee range of motion | ||||||
| Participant satisfaction with analgesia | ||||||
Appendix 10. Fail‐safe‐N analysis
| Comparison | Outcome | Subgroup | Effect size (SMD) | No. of studies (N) | Fail‐safe‐n |
| 1. FNB vs PCA opioid | Analysis 1.1—Pain at rest at 24 hours | Overall | ‐0.72 | 19 | 60 |
| 1. FNB vs PCA opioid | Analysis 1.2—Pain at rest at 24 hours by allocation concealment | Low risk of bias RCTs | ‐0.54 | 9 | 18 |
| 1. FNB vs PCA Opioid | Analysis 1.5—Pain on movement at 24 hours | Overall | ‐0.94 | 17 | 78 |
| 1. FNB vs PCA opioid | Analysis 1.6—Pain on movement at 24 hours by allocation concealment | Low risk of bias RCTs | ‐0.54 | 9 | 18 |
| 2. FNB vs epidural | Analysis 2.1—Pain at Rrest at 24 hours | < not significant, not applicable > | |||
| 2. FNB vs epidural | Analysis 2.5—Pain on movement at 24 hours | < not significant, not applicable > | |||
| 3. FNB vs local infiltration | Analysis 2.1—Pain at rest at 24 hours | < not significant, not applicable > | |||
| 3. FNB vs local infiltration | Analysis 2.5—Pain on movement at 24 hours | < not significant, not applicable > | |||
| 4. Continuous vs single‐shot FNB | Analysis 4.1—Pain at rest at 24 hours | Overall | ‐0.62 | 4 | 9 |
| 4. Continuous vs single‐shot FNB | Analysis 4.3—Pain on movement at 24 hours | Overall | ‐0.42 | 4 | 5 |
Data and analyses
Comparison 1. FNB versus PCA opioid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain at rest first 2 hours | 11 | Std. Mean Difference (Random, 95% CI) | ‐0.58 [1.00, ‐0.16] | |
| 1.1 Single‐shot FNB vs PCA opioid | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.44 [‐1.16, 0.28] | |
| 1.2 Single‐shot FNB + obturator vs PCA opioid | 1 | Std. Mean Difference (Random, 95% CI) | ‐1.25 [‐1.92, ‐0.57] | |
| 1.3 Continuous FNB vs PCA opioid | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.62 [‐1.25, 0.00] | |
| 1.4 Continuous FNB + sciatic vs PCA opioid | 1 | Std. Mean Difference (Random, 95% CI) | ‐0.63 [‐1.63, 0.38] | |
| 2 Pain at rest 3 to 12 hours | 14 | 972 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.42, ‐0.52] |
| 2.1 Single‐shot FNB vs PCA opioid | 8 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.20, ‐0.11] |
| 2.2 Single‐shot FNB + sciatic vs PCA opioid | 1 | 18 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐2.48, ‐0.27] |
| 2.3 Single‐shot FNB + obturator vs PCA opioid | 1 | 47 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐1.01, 0.26] |
| 2.4 Continuous FNB vs PCA opioid | 8 | 519 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐2.06, ‐0.52] |
| 3 Pain at rest 24 hours | 19 | Std. Mean Difference (Random, 95% CI) | ‐0.72 [‐0.93, ‐0.51] | |
| 3.1 Single‐shot FNB vs PCA opioid | 9 | Std. Mean Difference (Random, 95% CI) | ‐0.65 [‐1.08, ‐0.22] | |
| 3.2 Single‐shot FNB + sciatic vs PCA opioid | 1 | Std. Mean Difference (Random, 95% CI) | ‐0.73 [‐1.74, 0.28] | |
| 3.3 Continuous FNB vs PCA opioid | 10 | Std. Mean Difference (Random, 95% CI) | ‐0.74 [‐0.97, ‐0.51] | |
| 3.4 Continuous FNB + sciatic vs PCA opioid | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.85 [‐1.55, ‐0.15] | |
| 4 Pain at rest 48 hours | 17 | 957 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.03, ‐0.25] |
| 4.1 Single‐shot FNB vs PCA opioid | 9 | 416 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.83, 0.07] |
| 4.2 Single‐shot FNB + sciatic vs PCA opioid | 1 | 18 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.77, 1.20] |
| 4.3 Continuous FNB vs PCA opioid | 9 | 507 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.58, ‐0.20] |
| 4.4 Continuous FNB + sciatic vs PCA opioid | 1 | 16 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.96 [‐3.21, ‐0.71] |
| 5 Pain at rest 72 hours | 8 | 560 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.32, ‐0.01] |
| 5.1 Single‐shot FNB vs PCA opioid | 5 | 272 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.61, 0.08] |
| 5.2 Continuous FNB vs PCA opioid | 4 | 250 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐3.07, 0.60] |
| 5.3 Continuous FNB + sciatic vs PCA opioid | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.21, 0.09] |
| 6 Pain on movement first 2 hours | 4 | 218 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐2.12, ‐0.46] |
| 6.1 Single‐shot FNB vs PCA opioid | 2 | 98 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.27, ‐0.43] |
| 6.2 Continuous FNB vs PCA opioid | 3 | 120 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐3.39, 0.19] |
| 7 Pain on movement 3 to 12 hours | 8 | Std. Mean Difference (Random, 95% CI) | ‐1.06 [‐1.68, ‐0.43] | |
| 7.1 Single‐shot FNB vs PCA opioid | 2 | Std. Mean Difference (Random, 95% CI) | ‐1.22 [‐1.66, ‐0.79] | |
| 7.2 Single‐shot FNB + sciatic vs PCA opioid | 1 | Std. Mean Difference (Random, 95% CI) | ‐0.34 [‐0.83, 0.15] | |
| 7.3 Continuous FNB vs PCA opioid | 6 | Std. Mean Difference (Random, 95% CI) | ‐1.18 [‐2.13, ‐0.23] | |
| 8 Pain on movement 24 hours | 17 | Std. Mean Difference (Random, 95% CI) | ‐0.94 [‐1.32, ‐0.55] | |
| 8.1 Single‐shot FNB vs PCA opioid | 6 | Std. Mean Difference (Random, 95% CI) | ‐0.50 [‐0.81, ‐0.19] | |
| 8.2 Single‐shot FNB + sciatic vs PCA opioid | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.73 [‐1.18, ‐0.29] | |
| 8.3 Continuous FNB vs PCA opioid | 10 | Std. Mean Difference (Random, 95% CI) | ‐1.09 [‐1.74, ‐0.43] | |
| 8.4 Continuous FNB + sciatic vs PCA opioid | 2 | Std. Mean Difference (Random, 95% CI) | ‐2.08 [‐4.74, 0.58] | |
| 9 Pain on movement 48 hours | 13 | 742 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.71, ‐0.16] |
| 9.1 Single‐shot FNB vs PCA opioid | 6 | 287 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.48, ‐0.00] |
| 9.2 Single‐shot FNB + sciatic vs PCA opioid | 2 | 92 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.10, 0.78] |
| 9.3 Continuous FNB vs PCA opioid | 7 | 347 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.95, ‐0.24] |
| 9.4 Continuous FNB + sciatic vs PCA opioid | 1 | 16 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.81 [‐4.30, ‐1.32] |
| 10 Pain on movement 72 hours | 6 | 438 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.39, 0.04] |
| 10.1 Single‐shot FNB vs PCA opioid | 4 | 230 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.38, 0.16] |
| 10.2 Continuous FNB vs PCA opioid | 3 | 170 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.40, 0.23] |
| 10.3 Continuous FNB + sciatic vs PCA opioid | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.44, ‐0.12] |
| 11 Pain at rest 24 hours—subgrouped by FNB with and without concurrent PCA | 19 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 11.1 FNB with concurrent PCA | 15 | Std. Mean Difference (Random, 95% CI) | ‐0.67 [‐0.94, ‐0.41] | |
| 11.2 FNB without concurrent PCA | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.93 [‐1.31, ‐0.55] | |
| 12 Pain on movement 24 hours—subgrouped by FNB with and without concurrent PCA | 17 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 12.1 Concurrent PCA in FNB | 14 | Std. Mean Difference (Random, 95% CI) | ‐0.67 [‐0.99, ‐0.35] | |
| 12.2 No concurrent PCA in FNB | 4 | Std. Mean Difference (Random, 95% CI) | ‐2.26 [‐3.95, ‐0.57] | |
| 13 Pain at rest 24 hours—subgrouped by FNB ropivacaine vs bupivacaine | 19 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 13.1 FNB with ropivacaine | 8 | Std. Mean Difference (Random, 95% CI) | ‐1.30 [‐2.03, ‐0.58] | |
| 13.2 FNB with bupivacaine | 11 | Std. Mean Difference (Random, 95% CI) | ‐0.61 [‐0.89, ‐0.33] | |
| 14 Pain on movement 24 hours—subgrouped by FNB ropivacaine vs bupivacaine | 17 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 14.1 FNB with ropivacaine | 8 | Std. Mean Difference (Random, 95% CI) | ‐0.98 [‐1.67, ‐0.29] | |
| 14.2 FNB with bupivacaine | 10 | Std. Mean Difference (Random, 95% CI) | ‐0.92 [‐1.39, ‐0.44] | |
| 15 Pain at rest 24 hours—sensitivity analysis by low bias for allocation concealment | 9 | Std. Mean Difference (Random, 95% CI) | ‐0.54 [‐0.76, ‐0.32] | |
| 16 Pain on movement 24 hours—sensitivity analysis by low bias for allocation concealment | 9 | Std. Mean Difference (Random, 95% CI) | ‐0.54 [‐0.75, ‐0.33] | |
| 17 Pain on rest 24 hours—sensitivity analysis by blinding of participants, personnel and outcome assessor | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.62 [‐1.08, ‐0.17] | |
| 18 Pain on movement 24 hours—sensitivity analysis by blinding of participants, personnel and outcome assessor | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.57 [‐0.79, ‐0.35] | |
| 19 Opioid consumption 24 hours (mg) | 20 | Mean Difference (Random, 95% CI) | ‐14.74 [‐18.68, ‐10.81] | |
| 19.1 SFNB vs PCA opioid | 12 | Mean Difference (Random, 95% CI) | ‐12.86 [‐18.65, ‐7.08] | |
| 19.2 SFNB/Sciatic vs PCA opioid | 2 | Mean Difference (Random, 95% CI) | ‐17.93 [‐21.43, ‐14.43] | |
| 19.3 SFNB/Obturator vs PCA opioid | 1 | Mean Difference (Random, 95% CI) | ‐13.7 [‐18.28, ‐9.12] | |
| 19.4 CFNB vs PCA opioid | 8 | Mean Difference (Random, 95% CI) | ‐16.89 [‐24.01, ‐9.77] | |
| 19.5 CFNB/Sciatic vs PCA opioid | 1 | Mean Difference (Random, 95% CI) | ‐13.0 [‐20.17, ‐5.83] | |
| 20 Opioid consumption 48 hours (mg) | 19 | 1001 | Mean Difference (IV, Random, 95% CI) | ‐14.53 [‐20.03, ‐9.02] |
| 20.1 Single‐shot FNB vs PCA opioid | 11 | 485 | Mean Difference (IV, Random, 95% CI) | ‐13.21 [‐21.99, ‐4.44] |
| 20.2 Single‐shot FNB + sciatic vs PCA opioid | 2 | 92 | Mean Difference (IV, Random, 95% CI) | ‐10.10 [‐18.26, ‐1.94] |
| 20.3 Continuous FNB vs PCA opioid | 8 | 386 | Mean Difference (IV, Random, 95% CI) | ‐19.14 [‐27.53, ‐10.76] |
| 20.4 Continuous FNB + sciatic vs PCA opioid | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐6.0 [‐17.39, 5.39] |
| 21 Nausea and/or vomiting | 16 | 1100 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.33, 0.68] |
| 21.1 Single‐shot FNB vs PCA opioid | 8 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.44, 1.02] |
| 21.2 Single‐shot FNB + obturator vs PCA opioid | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.11, 0.56] |
| 21.3 Continuous FNB vs PCA opioid | 9 | 574 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.18, 0.68] |
| 22 Sedation | 9 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.16, 0.74] |
| 22.1 Single‐shot FNB vs PCA opioid | 4 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.16, 1.75] |
| 22.2 Single‐shot FNB + obturator vs PCA opioid | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.15, 1.24] |
| 22.3 Continuous FNB vs PCA opioid | 6 | 435 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.05, 0.77] |
| 23 Urinary retention | 7 | 490 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.20, 1.68] |
| 23.1 Single‐shot FNB vs PCA opioid | 3 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [0.43, 6.45] |
| 23.2 Continuous FNB vs PCA opioid | 5 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.06, 1.36] |
| 24 Knee flexion range of motion (postoperative day 2 to 4) | 10 | 541 | Mean Difference (IV, Random, 95% CI) | 6.48 [4.27, 8.69] |
| 24.1 Single‐shot FNB vs PCA opioid | 5 | 295 | Mean Difference (IV, Random, 95% CI) | 3.74 [1.45, 6.04] |
| 24.2 Continuous FNB vs PCA opioid | 4 | 192 | Mean Difference (IV, Random, 95% CI) | 10.32 [4.70, 15.93] |
| 24.3 Continuous FNB + sciatic vs PCA opioid | 2 | 54 | Mean Difference (IV, Random, 95% CI) | 12.78 [5.96, 19.60] |
| 25 Participant satisfaction with analgesia during hospital stay | 4 | 180 | Mean Difference (IV, Random, 95% CI) | 1.68 [0.79, 2.58] |
| 25.1 Continuous FNB vs PCA opioid | 4 | 180 | Mean Difference (IV, Random, 95% CI) | 1.68 [0.79, 2.58] |
Comparison 2. FNB versus epidural.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain at rest first 2 hours | 3 | Std. Mean Difference (Random, 95% CI) | 0.53 [‐0.56, 1.62] | |
| 1.1 Single‐shot FNB vs epidural | 1 | Std. Mean Difference (Random, 95% CI) | 2.08 [1.33, 2.83] | |
| 1.2 Continuous FNB vs epidural | 1 | Std. Mean Difference (Random, 95% CI) | 0.08 [‐0.72, 0.88] | |
| 1.3 Continuous FNB + sciatic vs epidural | 2 | Std. Mean Difference (Random, 95% CI) | ‐0.05 [‐0.67, 0.57] | |
| 2 Pain at rest 3 to 12 hours | 2 | 120 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.08, 0.67] |
| 2.1 Continuous FNB vs epidural | 1 | 70 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.12, ‐0.16] |
| 2.2 Continuous FNB + sciatic vs epidural | 1 | 50 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.30, 0.81] |
| 3 Pain at rest 24 hours | 6 | Std. Mean Difference (Random, 95% CI) | ‐0.05 [‐0.43, 0.32] | |
| 3.1 Continuous FNB vs epidural | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.11 [‐0.72, 0.50] | |
| 3.2 Continuous FNB + sciatic vs epidural | 3 | Std. Mean Difference (Random, 95% CI) | 0.09 [‐0.32, 0.51] | |
| 4 Pain at rest 48 hours | 6 | 328 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.51, 0.22] |
| 4.1 Continuous FNB vs epidural | 4 | 235 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.77, 0.21] |
| 4.2 Continuous FNB + sciatic vs epidural | 3 | 93 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.34, 0.49] |
| 5 Pain on movement 24 hours | 6 | 317 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.21, 0.24] |
| 5.1 Single‐shot FNB + sciatic vs epidural | 1 | 59 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.85, 0.18] |
| 5.2 Continuous FNB vs epidural | 3 | 165 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.19, 0.43] |
| 5.3 Continuous FNB + sciatic vs epidural | 3 | 93 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.36, 0.47] |
| 6 Pain on movement 48 hours | 6 | 317 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.32, 0.13] |
| 6.1 Single‐shot FNB + sciatic vs epidural | 1 | 59 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.92, 0.11] |
| 6.2 Continuous FNB vs epidural | 3 | 165 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.37, 0.25] |
| 6.3 Continuous FNB + sciatic vs epidural | 3 | 93 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.41, 0.54] |
| 7 Pain at rest 24 hours—subgrouped by FNB ropivacaine vs bupivacaine | 6 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 7.1 FNB with ropivacaine | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.16 [‐0.68, 0.37] | |
| 7.2 FNB with bupivacaine | 2 | Std. Mean Difference (Random, 95% CI) | 0.20 [‐0.13, 0.53] | |
| 8 Pain on movement 24 hours—subgrouped by FNB ropivacaine vs bupivacaine | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 FNB with ropivacaine | 3 | 120 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.27, 0.47] |
| 8.2 FNB with bupivacaine | 3 | 197 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.31, 0.25] |
| 9 Pain at rest 24 hours—sensitivity analysis by low bias for allocation concealment | 3 | Std. Mean Difference (Random, 95% CI) | 0.20 [‐0.07, 0.47] | |
| 10 Pain on movement 24 hours—sensitivity analysis by low bias for allocation concealment | 4 | 271 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.21, 0.27] |
| 11 Opioid consumption 24 hours (mg) | 5 | 341 | Mean Difference (IV, Random, 95% CI) | ‐4.35 [‐9.95, 1.26] |
| 11.1 Single‐shot FNB + sciatic vs epidural | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐6.28, 6.28] |
| 11.2 Continuous FNB vs epidural | 3 | 205 | Mean Difference (IV, Random, 95% CI) | ‐9.06 [‐19.46, 1.34] |
| 11.3 Continuous FNB + sciatic vs epidural | 2 | 77 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐8.24, 7.12] |
| 12 Opioid consumption 48 hours (mg) | 4 | 233 | Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐5.30, 2.74] |
| 12.1 Single‐shot FNB + sciatic vs epidural | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐18.88, 10.88] |
| 12.2 Continuous FNB vs epidural | 2 | 97 | Mean Difference (IV, Random, 95% CI) | ‐3.54 [‐12.42, 5.34] |
| 12.3 Continuous FNB + sciatic vs epidural | 2 | 77 | Mean Difference (IV, Random, 95% CI) | 0.62 [‐5.51, 6.75] |
| 13 Nausea and/or vomiting | 4 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.41, 0.97] |
| 13.1 Single‐shot FNB vs epidural | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.36, 1.15] |
| 13.2 Continuous FNB vs epidural | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.22, 2.07] |
| 13.3 Continuous FNB + sciatic vs epidural | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.10, 3.11] |
Comparison 3. FNB versus local infiltration analgesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain at rest 24 hours | 4 | 216 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.61, 0.72] |
| 1.1 Single‐shot FNB vs local infiltration | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.01, 1.02] |
| 1.2 Continuous FNB vs local infiltration | 3 | 156 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐1.01, 0.80] |
| 2 Pain at rest 48 hours | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Continuous FNB vs local infiltration | 2 | 114 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.94, 0.43] |
| 3 Pain on movement 24 hours | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Continuous FNB vs local infiltration | 3 | 153 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.10, 0.86] |
| 4 Pain on movement 48 hours | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Continuous FNB vs local infiltration | 2 | 111 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.71, 0.43] |
| 5 Nausea and/or vomiting | 3 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.64, 4.62] |
| 5.1 SFNB vs local infiltration | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [0.42, 10.80] |
| 5.2 CFNB vs local infiltration | 2 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 1.97 [0.35, 11.04] |
Comparison 4. Continuous FNB versus single‐shot FNB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain at rest first 2 hours | 2 | 156 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.17, 0.46] |
| 2 Pain at rest 3 to 12 hours | 2 | 156 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.35, 0.28] |
| 3 Pain at rest 24 hours | 4 | 272 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.17, ‐0.07] |
| 4 Pain at rest 48 hours | 4 | 272 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.69, ‐0.22] |
| 5 Pain on movement 24 hours | 4 | 272 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.67, ‐0.17] |
| 6 Pain on movement 48 hours | 4 | 272 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.02, ‐0.06] |
| 7 Pain on rest 24 hours—subgrouped by continuous FNB with or without concurrent PCA | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Continuous FNB with concurrent PCA | 3 | 138 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.35, ‐0.38] |
| 7.2 Continuous FNB without concurrent PCA | 1 | 134 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.50, 0.18] |
| 8 Pain on movement 24 hours—subgrouped by continuous FNB with or without concurrent PCA | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Continuous FNB with concurrent PCA | 3 | 138 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.96, ‐0.22] |
| 8.2 Continuous FNB without concurrent PCA | 1 | 134 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.61, 0.07] |
| 9 Pain at rest 24 hours—sensitivity analysis by low bias for allocation concealment | 3 | 250 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.41, ‐0.04] |
| 10 Pain on movement 24 hours—sensitivity analysis by low bias for allocation concealment | 3 | 250 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.79, ‐0.15] |
| 11 Opioid consumption 24 hours (mg) | 3 | 236 | Mean Difference (IV, Random, 95% CI) | ‐13.81 [‐23.27, ‐4.35] |
| 12 Opioid consumption 48 hours (mg) | 4 | 269 | Mean Difference (IV, Random, 95% CI) | ‐14.59 [‐22.35, ‐6.82] |
4.5. Analysis.
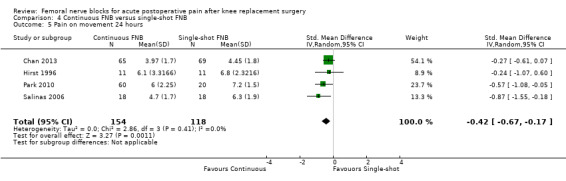
Comparison 4 Continuous FNB versus single‐shot FNB, Outcome 5 Pain on movement 24 hours.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adams 2002.
| Methods | Randomized trial: drawing lots | |
| Participants | Single centre in Hannover, Germany 63 participants undergoing unilateral TKR Inclusions: ASA I to III Exclusion: age younger than 18 years, endocrine disorders except diabetes mellitus, glucocorticoid therapy, use of etomidate up to 48 hours before the beginning of study and allergy towards one of the test substances. Patients requesting another method than the randomly intended postoperative pain management Mean age (years): 69 (range 56 to 86) Female: 70% Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. Three‐in‐one single‐shot FNB with bupivacaine 40 ml 0.375% solution after detection of the nerve using a nerve stimulator 2. Continuous epidural with perioperative bupivacaine test dose 3 ml 0.25% solution and postoperative bupivacaine 0.375% (1 ml per 10 cm body height, maximum 15 ml). Started at first expression of pain at PACU 3. IV pirinitramide with initial small bolus injections of 0.1 to 0.2 mg/kg and diclofenac 100 mg rectally, and subsequently single doses of pirinitramide—about 0.025 mg/kg (2 mg at 80 kg) from a PCA pump with ‘lockout’ time of 10 minutes. Started at first expression of pain at PACU. Diclofenac 100 mg was administered rectally |
|
| Outcomes | Pain intensity at rest: visual analogue scale at one hour, two hours, three hours Nausea/vomiting: first postoperative day Participant satisfaction with postoperative management: first postoperative day |
|
| Notes | Study author provided additional information on methods of randomization and blinding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random." Drawing lots (study author provided information) |
| Allocation concealment (selection bias) | Unclear risk | No mention (study author unable to provide additional information) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | "None were blinded" (study author provided information) |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | "None were blinded" (study author provided information) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Affas 2011.
| Methods | Randomized trial: mixing 40 tickets and drawing one envelope at a time | |
| Participants | Orthopaedics clinic at Karolinska University Hospital in Solna, Sweden 40 participants with osteoarthritis or rheumatoid arthritis scheduled for primary unilateral elective TKR under spinal anaesthesia Inclusion criteria: ASA I to III and older than 18 years of age Exclusion criteria: allergy or intolerance to one of the study drugs, renal insufficiency, epilepsy, language difficulty, mental illness, dementia, QT interval on electrocardiogram > 450 ms before start Mean age (years): 68 (range 29 to 88) Female: 53% Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. Continuous FNB with 30 ml ropivacaine (2 mg/ml) was injected, followed by 15 ml of the same concentration every four‐hourly for 24 hours (total dose 240 mg/24 h). Nerve identified via nerve stimulation 2. Periarticular and intra‐articular infiltration of a solution containing 150 ml ropivacaine (2 mg/ml), 1 ml ketorolac (30 mg/ml) and 5 ml epinephrine (0.1 mg/ml). Total dose of ropivacaine was 300 mg Adjunct analgesics: IV ketorolac 30 mg given during the first 24 hours post operation, IV PCA morphine (2 mg/dose) given on demand with a lock‐out time of six minutes and maximum dose of 35 mg over four hours and paracetamol 1 g × 4 over 24 hours |
|
| Outcomes | Pain intensity at rest and on movement: visual analogue scale at 24 hours Total opioid consumption (mg) at 24 hours Proportion of participants in pain at 24 hours Adverse events |
|
| Notes | One participant from the FNB group had a history of insensitivity to pain. He did not demand any PCA morphine, and pain severity was assessed as no pain at all time points | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization sequence was determined by mixing 40 tickets, 20 labelled 'F' and 20 labelled 'LIA' in sealed opaque envelopes, and drawing one envelope at a time. The anaesthesiologist performing the spinal anaesthesia and femoral block or supervising the local infiltration analgesia technique did not participate in the randomization procedure" |
| Allocation concealment (selection bias) | Low risk | Quote: "...sealed opaque envelopes, and drawing one envelope at a time" |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote: "open labelled" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: "open labelled" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Allen 1998.
| Methods | Randomized trial: method not stated | |
| Participants | Single centre from Washington, USA 36 participants undergoing unilateral TKR Exclusion criteria: age < 40 or > 80 years; ASA physical status greater than III; allergy to local anaesthetics; history of opioid dependence; contraindications to spinal anaesthesia, femoral nerve block or sciatic nerve block (coagulation defects, infection at puncture site, preexisting neurological deficits in the lower extremities); inability to use patient‐controlled analgesia (IV PCA), contraindications for NSAID use (NSAID or aspirin allergy, severe liver disease or serum creatinine 21.7 mg/dl), weight > 140 kg and recent steroid use Mean age (years): 68 Female: 61% Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. Single‐shot FNB (with sham sciatic) with 30 ml of 0.25% bupivacaine with 1:400,000 epinephrine was injected. Nerve identified via nerve stimulation. IV PCA morphine initial bolus of 1 mg and lockout interval of 10 minutes with no limit or background infusion; further adjustments at the discretion of the orthopaedic doctor 2. Single‐shot FNB + sciatic with 30 ml of 0.25% bupivacaine with 1:400,000 epinephrine was injected. Nerve identified via nerve stimulation. IV PCA morphine initial bolus of 1 mg and lockout interval of 10 minutes with no limit or background infusion; further adjustments at the discretion of the orthopaedic doctor 3. IV PCA morphine initial bolus of 1 mg and lockout interval of 10 minutes with no limit or background infusion; further adjustments at the discretion of the orthopaedic doctor Adjunct analgesics: IV ketorolac either 15 (< 65 years or < 50 kg) or 30 mg every six hours for at least 24 hours. If oral medications were tolerated after 24 hours, then oral ibuprofen 600 mg every eight hours was substituted for ketorolac |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity at rest and on movement: visual analogue scale at one hour upon arrival on ward and every four hours until 10 pm, and morning and evening of postoperative days one to five Total opioid consumption: postoperative days one and two Participant satisfaction: two weeks after discharge Nausea/vomiting Sedation Pruritus Other outcomes: Technical block to pinprick of saphenous nerve Technical block to pinprick of sural nerve |
|
| Notes | Additional information regarding the study method sought, but study authors could not be reached. For pain at rest, the data presented in the figures were not used for the meta‐analysis, as quoted from the report: "Data were incomplete after the 8h measurement and are not presented" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "random." Method not stated |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Control group had sham injections with dressings. All participants told to expect numbness and motor weakness |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Blinded investigators collected data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For pain severity at rest: report quoted: ''Data were incomplete after the 8 h measurement and are not presented." Apart from this, no dropouts/withdrawals reported |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Baranovic 2011.
| Methods | Randomized trial: computer‐generated list | |
| Participants | Single centre from Zagreb, Croatia 80 participants undergoing TKR under spinal anaesthesia Inclusion criteria: ASA II to III Exclusion criteria: previous vascular surgery in the region of femoral veins or arteries; confirmed coagulopathy; local infection; hepatic and renal insufficiency; dementia; BMI > 30 kg/m2; allergy to local anaesthetic, morphine and NSAID drugs; a previously diagnosed neurological deficit Mean age (years): 70 (range 66 to 73) Female: 48% Number excluded post randomization: nine in continuous FNB because femoral catheter fell out Number lost to follow‐up: none |
|
| Interventions | 1. Continuous FNB using nerve stimulation. Bolus dose 8 ml 0.35% levobupivacaine, then 5 to 6ml per hour of 0.25% levobupivacaine via catheter in addition to IV morphine 5 mg (wt < 60 kg) or 10 mg (wt > 60 kg), if posterior knee pain > three. Femoral catheter in situ for 24 hours 2. PCA morphine concentration 1 mg/ml, basal rate 3 mg/h, bolus upon request 2 mg, lockout interval eight minutes Adjunct analgesics: IV diclofenac 75 mg 12‐hourly |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity at rest and on movement: visual analogue scale at two‐hourly × 24 hours Opioid consumption: first 24 hours Nausea/vomiting: first 24 hours Sedation: first 24 hours Urinary retention Knee flexion: postoperative day two Participant satisfaction Other outcomes: Haemodynamic instability Length of stay |
|
| Notes | Additional information regarding study method sought, but study authors could not be reached | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomized using statistical software Medcalc for Windows" |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | No mention |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Quote: "The VAS scores were assessed by persons who were not involved in setting the femoral nerve catheter." It may be obvious that the FNB group did not receive an IV PCA |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Nine participants (20%) in the continuous FNB group excluded post randomization because their catheter fell out |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | High risk | ITT an issue with the high risk of attrition bias |
Barrington 2005.
| Methods | Randomized trial: computer‐generated in permuted blocks of four | |
| Participants | Single centre from St Vincent’s Hospital, Melbourne, Australia 112 participants undergoing primary TKR Exclusion criteria: inability to give informed consent for language or cognitive reasons; contraindications to neuraxial blockade (including participant refusal, platelet count 100 × 109/L or coagulopathy); contraindications to continuous FNB (e.g. infection overlying injection site or previous femoro‐popliteal bypass surgery); and contraindication to any study drugs Mean age (years): 70 ± 10 Female: 53% Number excluded post randomization: none Number lost to follow‐up: four (three protocol violations from continuous FNB group and one data lost from epidural group) |
|
| Interventions | 1. Continuous FNB with femoral infusion of bupivacaine 0.2% commenced at 0.1 ml/kg/h, with PCA bolus of 0.05 ml/kg and 60‐minute lockout period. Nerve identified via nerve stimulation 2. Continuous epidural with infusion of ropivacaine 0.2% plus fentanyl 4 g/ml commenced at 6 to 10 ml/h; subsequently the acute pain service varied the infusion rate to maintain sensory blockade of the surgical site. Both interventions continued until postoperative day three morning Adjunct analgesics: rofecoxib 50 mg daily and oxycodone 10 to 15 mg every four hours, as required. If analgesia was inadequate despite oral adjuncts, an IV morphine infusion was commenced |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity at rest and on movement: visual analogue scale at postoperative days one and two at rest, and during CPM and active physiotherapy Nausea and vomiting (0 = no nausea, 1 = nausea only, 2 = nausea and vomiting) Knee flexion: postoperative days one and two Hypotension Neurological injury Technical failure of blocks Catheter dislodgement Sit out of bed on postoperative day one Other outcomes: Drainage blood Number of participants who required rescue analgesics Length of stay |
|
| Notes | Different local anaesthetics used in the two allocation groups (bupivacaine in continuous FNB group and ropivacaine in epidural group). Also epidural group had fentanyl in the infusion group but not in the continuous FNB group. Different modalities included continuous infusion in the epidural group and PCA in the continuous FNB group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated in permuted blocks of four |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants and treated clinicians not blinded to study group randomization |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quotes: "The motor block was assessed...by unblinded physiotherapists..."; "The limitations of this study include its non‐blinded nature..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four participants not included in the analysis (three protocol violations from continuous FNB group and one data lost from epidural group). Apart from this, no other dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes prespecified |
| Other bias | Low risk | Appears to be free of other sources of bias |
Carli 2010.
| Methods | Randomized trial: computer‐generated tables | |
| Participants | Single centre from McGill University Health Centre (MUHC), Canada 40 participants undergoing primary, unilateral, tricompartmental cemented TKR Inclusion criteria: older than 18 years of age and referred for TKR secondary to osteoarthritis Exclusion criteria: ASA IV and V, history of abnormal liver enzymes, hepatic failure, renal insufficiency, cardiac failure, organ transplant, morbid obesity (BMI 40 kg/m2), neuropathic pain, history of stroke or major neurological deficit, sensory and motor disorders in the operated limb, previous drug dependency, long‐term use of opioids, allergy to local anaesthetics, inability to walk independently and inability to comprehend pain assessment Mean age (years): 71 (range 55 to 85) Female: 73% Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. Continuous FNB with loading dose of 8 ml of ropivacaine 0.2%, continuous infusion of ropivacaine 0.2% at a rate of 8 ml/h for 48 hours. Infiltration of the posterior capsule of the knee with a solution containing 100 mg of ropivacaine (50 ml of ropivacaine 0.2%), 0.5 ml of ketorolac (30 mg/ml) and 0.25 ml of epinephrine (1 mg/ml) (after all bony cuts and before cementing of implants). Sham periarticular and intra‐articular infiltration with saline 0.9% 2. Sham continuous FNB with saline 0.9%. Infiltration of posterior capsule of the knee with a solution containing 100 mg of ropivacaine (50 ml of ropivacaine 0.2%), 0.5 ml of ketorolac (30 mg/ml) and 0.25 ml of epinephrine (1 mg/ml) (after all bony cuts and before cementing of implants) Intra‐articular injection with 100 ml of ropivacaine 0.2%, 1 ml of ketorolac (30 mg/ml) and 0.5 ml of epinephrine (1 mg/ml) infiltrated into remainder of capsule, cut quadriceps tendon, patellar ligament and soft tissues surrounding joint and subcutaneous tissues after closure of medial parapatellar arthrotomy; 50 ml of ropivacaine 0.5% with ketorolac (30 mg/ml) and 0.25 ml of epinephrine (1 mg/ml) infused over a two‐hour period at 24 hours after the end of the operation via intra‐articular catheter Both groups: solution containing 100 mg of ropivacaine (50 ml of ropivacaine 0.2%), 0.5 ml of ketorolac (30 mg/ml) and 0.25 ml of epinephrine (1 mg/ml) was injected into the posterior capsule of the knee PCA pump to deliver incremental doses of 1 mg of morphine, with lockout of seven minutes and no background infusion, discontinued 48 hours after surgery Adjunct analgesics: slow‐release oral oxycodone 10 mg every 12 hours. For breakthrough pain with NRS > 3, oral oxycodone 5 to 10 mg every three hours was prescribed for three days. Celecoxib 100 mg every 12 hours and acetaminophen 1 g every six hours, respectively, for five days |
|
| Outcomes |
Outcomes of interest for the review: Pain severity at rest and on movement: numerical rating scale zero to 10 at first three days, upon discharge, six weeks after discharge Total opioid consumption: first 48 hours Nausea/vomiting: first 48 hours Knee flexion: postoperative days one and two Other outcomes: Length of stay Knee flexion and extension: week six SF‐12 WOMAC Knee Society Score Two‐minute walking text Six‐minute walking text Ambulated > 30 m |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated tables |
| Allocation concealment (selection bias) | Low risk | Sealed brown envelopes |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Clinical staff and participants not aware of treatment allocations |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Research assistant unaware of the study hypothesis. Different treatment groups may not be obvious, as both groups received IV PCA opioid |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Chan 2012.
| Methods | Randomized trial: computer random number generator | |
| Participants | Single centre from Taipei, Taiwan 88 participants undergoing unilateral primary TKR Exclusion criteria: < 40 and > 80 years old, ASA > III, known hypersensitivities to test substances used in study, history of substance abuse, contraindications to spinal anaesthesia, having femoral neuropathy or communication difficulties Mean age (years): single‐shot FNB 67.7 ± 8.9 versus PCA 71.4 ± 7.9 Female: single‐shot FNB 75.6% versus PCA 70.7% Number excluded post randomization: six excluded because of severe allergy to morphine, communication difficulties, multiple operations and loss to follow‐up Number lost to follow‐up: unclear (from the number of withdrawals, could range from one to three participants) |
|
| Interventions | 1. Single‐shot FNB 0.4 ml/kg 0.375% bupivacaine and 1:200,000 epinephrine after spinal anaesthesia but before surgery. Used ultrasound imaging and nerve stimulators. IV PCA morphine bolus 1 mg with five‐minute lockout (four‐hour maximum dose of 30 mg) started at PACU. Dosage adjusted by 0.4 mg every one to two hours, if pain control was unsatisfactory 2. Single‐shot FNB 0.4 ml/kg 0.375% bupivacaine and 1:200,000 epinephrine immediately after surgery. Used ultrasound imaging and nerve stimulators. IV PCA morphine bolus 1 mg with five‐minute lockout (four‐hour maximum dose of 30 mg) started at PACU. Dosage adjusted by 0.4 mg every one to two hours, if pain control was unsatisfactory 3. Sham FNB 0.4 ml/kg saline and 1:200,000 epinephrine after spinal anaesthesia but before surgery. IV PCA morphine bolus 1 mg with five‐minute lockout (four‐hour maximum dose of 30 mg) started at PACU. Dosage adjusted by 0.4 mg every one to two hours, if pain control was unsatisfactory. Sham FNB with saline given 4. Sham FNB 0.4 ml/kg saline and 1:200,000 epinephrine immediately after surgery. IV PCA morphine bolus 1 mg with five‐minute lockout (four‐hour maximum dose of 30 mg) started at PACU. Dosage adjusted by 0.4 mg every one to two hours, if pain control was unsatisfactory. Sham FNB with saline given Adjunct analgesics: not mentioned |
|
| Outcomes | Pain intensity at rest and on movement: visual analogue scale at two hours, four hours, six hours, 24 hours, 48 hours, 72 hours Opioid consumption: 24 hours, 48 hours Time to first morphine request Nausea/vomiting Pruritus Dizziness Knee flexion Neuropathy of FNB Postoperative falls |
|
| Notes | For the meta‐analysis, we combined single‐shot FNB groups (before and after surgery); similarly we combined sham FNB groups (before and after surgery) (i.e. interventions one and two vs interventions three and four) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. Not mentioned whether sequentially labelled and opaque |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quote: "Double blind"; "The patients, the pain management team, and the chart analysts were not informed of the patients’ group assignment" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: "Double blind"; "The patients, the pain management team, and the chart analysts were not informed of the patients’ group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Six participants excluded. Missing outcome data due to not meeting inclusion criteria |
| Selective reporting (reporting bias) | Low risk | Analysis predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Chan 2013.
| Methods | Randomized trial: computer random number generator | |
| Participants | Single centre from Singapore 200 participants undergoing unilateral primary TKR Inclusion criteria: 40 to 85 years of age; ASA I to III; BMI < 35 kg/m2; competent in using VAS pain score Exclusion criteria: contraindication to femoral block; abnormal coagulation studies; thrombocytopenia < 100,000/cc; renal insufficiency; lower extremities thromboembolic diseases; allergy to study medications; taking daily opioid past two weeks before surgery Mean age (years): continuous FNB: 66.4 ± 8.3 versus single‐shot FNB: 66.1 ± 7.6 versus PCA: 64.7 ± 8.4 Female: continuous FNB 81.5% versus single‐shot FNB 82.6% versus PCA 80.3% Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. Continuous FNB 20 ml of 0.25% bupivacaine with 1:400,000 adrenaline (2.5 mcg/ml) followed by continuous infusion bupivacaine 0.125% 4 ml/h started at PACU for 72 hours. Oral oxycodone 5 mg four‐hourly when necessary 2. Single‐shot FNB 20 ml of 0.25% bupivacaine with 1:400,000 adrenaline (2.5 mcg/ml); IV PCA morphine bolus 1 mg with five‐minute lockout (maximum 10 mg/h) until day three; for participants at risk of developing renal impairment, fentanyl 20 mcg dose with five‐minute lockout (maximum dose of 240 mcg/h) 3. IV PCA morphine bolus 1 mg with five‐minute lockout (maximum 10 mg/h) until day three; for participants at risk of developing renal impairment, fentanyl 20 mcg dose with five‐minute lockout (maximum dose of 240 mcg/h) Adjunct analgesics: days one to six: oral paracetamol 1 g six‐hourly; and equianalgesic dose of ibuprofen or COX‐2 inhibitor. From 72 hours: oral oxycodone 5 mg six‐hourly when necessary |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity at rest and on movement: visual analogue scale at one hour, six hours, 12 hours, 24 hours, 48 hours, 72 hours, 120 hours, week two, month three Opioid consumption: postoperative days one and two Proportion of participants in significant pain: 24 hours, 48 hours Nausea/vomiting: 24 hours, 48 hours Pruritus Urinary retention Adverse events Participant satisfaction Knee flexion: daily until discharge Knee extension: daily until discharge Other outcomes: Day achieved 90 degrees knee flexion Day achieved rehabilitation indices Straight leg raised Length of stay |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Data collector unaware of study objectives. May be obvious that continuous FNB group did not receive an IV PCA |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Davies 2004.
| Methods | Randomized trial: computer random number generator | |
| Participants | Single centre from Exeter, UK 60 participants undergoing unilateral primary TKR Exclusion criteria: ASA > III or had a contraindication to use of non‐steroidal anti‐inflammatory drugs, local anaesthetic agent, neuraxial blockade or tourniquet usage; painful polyarthralgia Mean age (years): 72 ± 9 Female: 70% Number excluded post randomization: one from epidural group because of insertion failure Number lost to follow‐up: none |
|
| Interventions | 1. Single‐shot FNB + sciatic with 0.375% bupivacaine 30 ml for the femoral component and 25 ml for the sciatic component 2. Continuous lumbar epidural analgesia 0.5% bupivacaine, test dose 3 ml, a further 7 ml and an infusion of bupivacaine 0.25% until postoperative day two Adjunct analgesics: 1‐mg bolus of morphine sulphate with lockout of five minutes; diclofenac 50 mg every eight hours |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity on movement: visual analogue scale at six hours, 24 hours. 48 hours Opioid consumption: 24 hours, 48 hours Urinary retention Hypotension Participant satisfaction with analgesics: 24 hours, 48 hours Straight leg raised Knee flexion Other outcomes: Motor block Complete analgesia at recovery unit Total perioperative blood loss Total drain loss Length of stay |
|
| Notes | Additional information regarding the study method sought, but study authors could not be reached | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator |
| Allocation concealment (selection bias) | Low risk | Codes stored by third party in opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Surgeon blinded. Not stated for participants |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Clinical staff uninvolved with the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant excluded. No other dropouts/exclusions |
| Selective reporting (reporting bias) | Low risk | Analysis predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
de Lima e Souza 2008.
| Methods | Randomized trial: computer‐assisted randomization | |
| Participants | Single centre from Minas Gerais, Brazil 61 participants undergoing unilateral TKR Inclusion criteria: ASA II to III Exclusion criteria: patient refusal of spinal anaesthesia or FNB; age < 18 or > 90 years; weight less than 40 kg; BMI > 45 kg/m2; allergy to local anaesthetics; preexisting neurological deficit; or inability to understand VAS scoring Mean age (years): 65 ± 9,2 (author provided information) Female: 72% (author provided information) Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. Single‐shot FNB with 40 ml of 0.25% ropivacaine 100 mg, nerve stimulation; IV PCA morphine 2 mg on participant demand 2. Single‐shot FNB with 40 ml of 0.25% bupivacaine with epinephrine 1:200,000 (100 mg); nerve stimulation; IV PCA morphine 2 mg on participant demand 3. IV PCA morphine 2 mg on participant demand; no sham FNB Adjunct analgesics: 30 mg oral codeine plus acetaminophen 0.5 g every six hours starting at the end of surgery, then 20 mg of tenoxicam 12/12 hours |
|
| Outcomes |
Outcomes of interest for the review: Proportion of pain at rest and on movement: visual analogue scale, zero to six hours, six to 10 hours, 10 to 24 hours Adverse effects: urinary retention requiring catheterization, nausea, vomiting and sedation Other outcomes: Pruritus Use of morphine (yes/no) Participant satisfaction (yes/no) |
|
| Notes | Study author provided additional information on proportion of participants in pain, use of morphine and pain satisfaction | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐assisted, randomized treatment assignments |
| Allocation concealment (selection bias) | Low risk | Sequentially ordered sealed envelopes |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quote (from report): "double blind." Participants and anaesthesiologist blinded (data from study author) |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Blinded research assistant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Fritze 2009.
| Methods | Randomized trial: method not stated | |
| Participants | Single hospital (Orthopaedic Department) in Vienna, Austria 54 participants undergoing TKR Inclusion criteria: ASA I to III, no contraindications against the procedure (allergies, skin alterations, blood‐clotting disorder, previous hip or spine surgery, no contraindications against used medications), over 50 years Exclusion criteria: insufficient cooperation, major surgery (e.g. osteotomy, replacement TKR implant), contraindications against used medications, scheduled spinal anaesthesia Mean age (years): 70 (range 50 to 85) Female: 78% Number excluded post randomization: two (catheter dislocation) Number lost to follow‐up: none |
|
| Interventions | 1. Continuous FNB with initial 10 ml 1% prilocaine and 20 ml 0.75% ropivacaine; postoperative infusion 0.2% ropivacaine 5 ml/h until postoperative day three 2. Continuous FNB + sciatic with initial 10 ml 1% prilocaine and 20 ml 0.75% ropivacaine; postoperative infusion 0.2% ropivacaine 5 ml/h until postoperative day three 3. Continuous epidural catheter with test dose 4 ml 1% xylocaine, bolus 10 ml 0.75% ropivacaine; postoperative infusion 0.2% ropivacaine 7 ml/h Adjunct analgesics: oral lornoxicam twice 8 mg, tramadol thrice 100 mg, in cases of severe pain post operation: IV piritramid 3 mg every 10 minutes (if in intensive care unit during first post operation night) until pain relief or SC 15 mg every four hours until pain relief in general ward |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity at rest and on movement: visual analogue scale at one hour, 24 hours, 48 hours, 72 hours Knee flexion: 48 hours Pulmonary embolism Other outcomes: Time in bed (hours) Haematoma at catheter site |
|
| Notes | Additional information regarding the study method sought, but study authors could not be reached | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Performed by physician on duty |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Ganapathy 1999.
| Methods | Randomized trial: closed envelope system by the pharmacy | |
| Participants | Single centre from London, Ontario, Canada 62 participants undergoing TKR Inclusion criteria: > 18 and < 80 years, ASA I to III Exclusion criteria: significant medical or psychiatric problems, unable to co‐operate with study protocol, contraindications to spinal anaesthesia or had allergy to bupivacaine, indomethacin, morphine or codeine Mean age (years): 70 ± 9 Female: 50% Number excluded post randomization:none Number lost to follow‐up: none |
|
| Interventions | 1. Continuous FNB with 0.2% bupivacaine via the femoral catheter 10 ml/h and continued for 48 hours. Nerve identified via nerve stimulation. IV PCA morphine 1.5 mg with lockout time of six minutes, increased to 2 mg if pain control poor and VAS pain score > 50/100‐mm scale 2. Continuous FNB with 0.1% bupivacaine via the femoral catheter 10 ml/h and continued for 48 hours. Nerve identified via nerve stimulation. IV PCA morphine 1.5 mg with lockout time of six minutes, increased to 2 mg if pain control poor and VAS pain score > 50/100‐mm scale; sham block 3. IV PCA 1.5 mg with lockout time of six minutes, increased to 2 mg if pain control poor and VAS pain score > 50/100‐mm scale; sham block Adjunct analgesics: 100 mg of indomethacin rectally every 12 hours for 48 hours |
|
| Outcomes | Pain intensity: visual analogue scale at day of surgery, at postoperative days one and two, on discharge Total opioid consumption: day of surgery to postoperative day one Range of flexion Nausea and vomiting |
|
| Notes | Study author provided additional information on methods of randomization, allocation concealment and blinding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Closed envelope system by the pharmacy" (study author provided information) |
| Allocation concealment (selection bias) | Low risk | Drug labelled as study drug by the pharmacy. Randomization not broken unless medical emergencies (study author provided information) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | "Patients and personnel were blinded" (study author provided information) |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | A blinded independent observer evaluated VAS scores, a blinded physiotherapist recorded range of flexion and a blinded surgeon assessed range of flexion at six weeks |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Good 2007.
| Methods | Randomized trial: coded and supplied by hospital pharmacy | |
| Participants | Single centre from Bryn Mawr Hospital, Pennsylvania, USA 42 participants undergoing TKR Inclusion criteria: not stated Exclusion criteria: not stated Mean age (years): 65 Female: 38% Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. Single‐shot FNB with 40 ml 0.5% bupivacaine with epinephrine 1:200,000 before surgery; nerve identified via nerve stimulator. IV morphine 1 mg/h, max 10 mg/h, continued until postoperative day one 2. IV morphine 1 mg/h, max 10 mg/h, continued until postoperative day one with 40 ml 0.5% saline given as placebo block Adjunct analgesics: not stated |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity on movement: visual analogue scale at every eight hours on day of surgery, daily from postoperative day one to three Total opioid consumption Nausea/vomiting Urinary retention Infection Dyspnoea Knee flexion and extension: postoperative days two and three Other outcomes: Difficulty in ambulation Fever Ambulation distance based on graded scale |
|
| Notes | Study author provided additional information on method of randomization | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coded and supplied by hospital pharmacy (study author provided information) |
| Allocation concealment (selection bias) | Low risk | Coded and supplied by hospital pharmacy. Unblinded at end of study |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Blinded ortho surgeon, anaesthesiologist, physical therapist, clinical nursing staff, participants |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Blinded outcome assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Hirst 1996.
| Methods | Randomized trial: method not stated | |
| Participants | Single centre from Saskatchewan, Canada. 33 participants undergoing TKR Inclusion criteria: ASA I to III Exclusion criteria: not stated Mean age (years): 69 Female: 79% Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. Single‐shot FNB with 20 ml 0.05% bupivacaine with 1:200,000 adrenaline. Nerve identified via nerve stimulation. IV PCA morphine 1 mg/ml, dose 1.5 mg, lockout seven minutes, four‐hour dose limit 30 mg. If inadequate, increase to 2 mg/ml, lockout five minutes 2. Continuous FNB with 20 ml 0.05% bupivacaine with 1:200,000 adrenaline and Infusion 0.125% bupivacaine 6 ml/h for 48 hours. IV PCA morphine 1 mg/ml, dose 1.5 mg, lockout seven minutes, four‐hour dose limit 30 mg. If inadequate, increase to 2 mg/ml, lockout five minutes 3. Sham FNB. IV PCA morphine 1 mg/ml, dose 1.5 mg, lockout seven minutes, four‐hour dose limit 30 mg. If inadequate, increase to 2 mg/ml, lockout five minutes Adjunct analgesics: not stated |
|
| Outcomes | Pain intensity at rest and on movement: visual analogue scale two hours, six hours, 12 hours, 18 hours, 24 hours, 48 hours, 72 hours Total opioid consumption: 24 hours, 48 hours Nausea: reported as incidence |
|
| Notes | Study author contacted for additional information but no longer has original data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated. Study author unable to provide additional information |
| Allocation concealment (selection bias) | Unclear risk | Method not stated. Study author unable to provide additional information |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quote: "Double blind." Study author unable to provide additional information |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Not stated but presumably low because sham FNB in control group and IV PCA in all groups. Study author unable to provide additional information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Hunt 2009.
| Methods | Randomized trial: Web‐based random number generator | |
| Participants | Single centre from Salt Lake City, Utah, USA 57 participants TKR for degenerative joint disease Exclusion criteria: allergy to local anaesthetics, inability to use a PCA pump, history of opioid dependence, severe liver disease, renal insufficiency, bilateral procedure and contraindication to non‐steroidal anti‐inflammatory drugs Mean age (years): 69 ± 7 Female: 67% Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. Single‐shot FNB with 10 to 15 ml 0.5% bupivacaine. Nerve identified via nerve stimulation. IV PCA morphine 1 mg to 2 mg every 10 minutes (lockout 10 minutes) with maximum of 30 mg in four hours 2. IV PCA morphine 1 mg to 2 mg every 10 minutes (lockout 10 minutes) with maximum of 30 mg in four hours until postoperative day two; sham FNB with 10 to 15 ml saline Adjunct analgesics: standard dose of vicodin. In cases of allergy, an alternate analgesic was used |
|
| Outcomes | Pain severity at rest: visual analogue scale at day of surgery, postoperative days one and two Total opioid consumption: postoperative days one and two |
|
| Notes | Single‐shot FNB + sciatic group excluded from data extraction, as it was a non‐random arm. Study author provided additional information on method of randomization, allocation concealment, persons blinded and co‐analgesics used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based random number generator (study author provided information) |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes (study author provided information) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Participants and surgeon blinded (study author provided information) |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Ward nurses blinded to treatment groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Kadic 2009.
| Methods | Randomized trial: method not stated | |
| Participants | Single centre from Radboud University Nijmegen Medical Centre, The Netherlands 58 participants undergoing primary unilateral TKR Exclusion criteria: preexisting neurological deficit, anticoagulant therapy, long‐term use of opioids, bilateral TKR, revision knee surgery, infection at the puncture site or known intolerance to any of the drugs to be used according to the study protocol Mean age (years): 67 Female: 67% Number excluded post randomization: five (one from continuous FNB and two from PCA due to protocol violations, one from PCA due to severe adverse events and one from continuous FNB due to not meeting inclusion criteria) Number lost to follow‐up: for month three: 41% in continuous FNB and 38% in PCA |
|
| Interventions | 1. Continuous FNB with 20 to 25 ml ropivacaine 0.75%, postoperatively, a continuous infusion of 0.2% ropivacaine at 5 ml/h to a max of 10 ml/h for the next 48 hours. Nerve identified via nerve stimulation. IV PCA morphine 1 mg on demand, lockout period of six minutes 2. IV PCA morphine 1 mg on demand, lockout period of six minutes Adjunct analgesics: paracetamol 1000 mg four times daily and diclofenac 50 mg three times daily |
|
| Outcomes |
Outcomes of interest for the review: Pain severity on movement: numerical rating scale of zero to 10. Day of surgery, days one and two Total opioid consumption: first 48 hours Nausea/vomiting Sedation Constipation Urinary retention Neurological injury (not applicable for PCA group) Local anaesthetic toxicity Infection Participant satisfaction with analgesia: on discharge Other outcomes: Knee flexion Knee Society Score: knee; function WOMAC: pain; stiffness 90 degrees knee flexion Blood loss during surgery Technical failure of block (not applicable to PCA group) |
|
| Notes | Additional information regarding the study method sought, but study authors could not be reached | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "random." Method not stated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Unblinded trial |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Unblinded independent pain team: Physician and physical therapist conducted assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up for month three; knee flexion and functional outcome measures were 41% in continuous FNB and 38% in PCA. However, the review authors judged that this will not bias the review because these outcomes are not of interest for the review |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Kaloul 2004.
| Methods | Randomized trial: blocks of six with allocation drawn from envelopes | |
| Participants | Single centre in Montreal, Canada 40 participants with osteoarthritis for primary TKR Inclusion: 20 to 80 years of age, ASA I to III Exclusion: allergic to amide local anaesthetics, fentanyl or midazolam; had a history of hepatic or renal failure (serum creatinine > 150 μmol/L); had a contraindication to regional anaesthesia (acquired or congenital coagulopathy, systemic or local infection, neurological disease affecting lower limbs) or use of PCA (drug dependence, inability to understand the use of the PCA device), or weight greater than 110 kg Mean age (years): 68 Female: 63% Number excluded post randomization: nine in continuous FNB as catheter fell out Number lost to follow‐up: none |
|
| Interventions | 1. Continuous FNB with 30 ml of ropivacaine 0.5% with epinephrine 1:200,000 injected, followed by ropivacaine 0.2% infusion at 12 ml/h for 48 hours. IV morphine 1 mg infused over two minutes with five‐minute lockout period 2. IV morphine 1 mg infused over two minutes with five‐minute lockout period Adjunct analgesics: rectal indomethacin 100 mg twice daily for 48 hours after surgery. Subsequently, IV PCA or oral analgesia (oxycodone or a combination of acetaminophen and codeine) |
|
| Outcomes | Pain intensity at rest and on movement: visual analogue scale at six hours, 24 hours, 48 hours and daily during physiotherapy for postoperative days one and two Total opioid consumption: 24 hours, 48 hours Participant satisfaction |
|
| Notes | Allocation group with psoas block was excluded from data extraction. Data on morphine consumption at 48 hours in abstract differed from graph: data from abstract used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned in blocks of six with allocation drawn from an envelope to one of three allocation groups |
| Allocation concealment (selection bias) | Unclear risk | Allocation drawn from an envelope. Not stated whether envelopes were opaque and sealed |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Unblinded research assistant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Kardash 2007.
| Methods | Randomized trial: computer‐generated in blocks of four | |
| Participants | Single centre from Montreal, Canada 40 participants undergoing elective unilateral TKR Exclusion criteria: < 18 years, inability to communicate lucidly in English or French, revision knee replacement, morbid obesity (body mass index > 35), contraindication to spinal anaesthesia (back fusion, coagulopathy, local infection), renal failure or hypersensitivity to bupivacaine, fentanyl or NSAIDs Mean age (years): 65 ± 2 (single‐shot FNB); 67 ± 1 (IV PCA) Female: 75% Number excluded post randomization: none Number lost to follow‐up: 29 for outcomes measured at week six and year one |
|
| Interventions | 1. Single‐shot FNB with 20 ml bupivacaine 0.5% with 1/200,000 epinephrine, nerve stimulator; IV PCA fentanyl 50 g/ml set to deliver 25 g every five minutes as needed 2. IV PCA fentanyl 50 g/ml set to deliver 25 g every five minutes as needed; sham FNB (area prepared and dressing applied, no injection) 3. Obturator nerve block (not considered for this review) Adjunct analgesics: celecoxib 100 mg and acetaminophen 650 mg PO were given in the recovery room, then every 12 and six hours. If pain at rest was rated > six/10, breakthrough medication with IM ketorolac 10 mg given every four hours prn |
|
| Outcomes |
Outcomes of interest for the review: Pain severity at rest and on movement: visual analogue scale at 24 hours, four hours, discharge Total opioid consumption (fentanyl): every four hours for 48 hours Time to first dose of PCA Time to first breakthrough analgesics Nausea/vomiting at 48 hours Pruritus at 48 hours Urinary retention at 48 hours Persistent numbness at 48 hours Knee flexion: postoperative day two and at discharge Other outcomes: Knee scores at six weeks and one year Length of stay |
|
| Notes | Data for the obturator nerve block group were not extracted, as they are not of interest for the review. Study author provided additional information on methods of randomization and allocation concealment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization computer‐generated in blocks of four according to a list prepared by the study epidemiologist (study author provided information) |
| Allocation concealment (selection bias) | Low risk | "Allocation was concealed in sealed envelopes" (study author provided information) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quotes (from report): "double‐blind"; "Both patients and nurse observers collecting data were blinded to the intervention group"; "For all groups, a bandage was placed over the inguinal area, to maintain double‐blinding in the recovery period" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Outcome assessors blinded to the intervention groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 29 participants lost to follow‐up for the outcome of knee scores at six weeks and one year. However the review authors judged that this will not bias the review because these outcomes are not of interest for the review |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Lee 2011.
| Methods | Randomized trial: method not stated | |
| Participants | Single centre from Seoul, Korea 80 participants undergoing unilateral TKR Exclusion criteria: study drug allergy, opioid dependence, history of previous postoperative nausea, vomiting (PONV) and motion sickness, contraindication for epidural block (previous back surgery, bleeding diathesis and neurological dysfunction), contraindication to FNB (infection at injection site), inability to use the patient‐controlled epidural analgesia device and to comprehend the VAS for pain assessment Mean age (years): 68 (single‐shot FNB group) versus 70 (epidural group) Female: 91% Number excluded post randomization: two participants from FNB group excluded from analysis because of catheter dislodgement Number lost to follow‐up: none |
|
| Interventions | 1. Single‐shot FNB with 0.25% levobupivacaine (30 ml) with 5 μg/ml epinephrine; patient‐controlled epidural analgesia (PCEA) via epidural catheter for 48 hours 2. PCEA via epidural catheter; infusion comprised 0.2% ropivacaine plus fentanyl 3 μg/ml, and programmed to deliver at basal rate of 4 ml/h, 3‐ml bolus dose, with lockout duration of 10 minutes, for 48 hours Adjunct analgesics: meperidine (50 mg) IV if participants reported pain ≥ 50 mm on VAS at rest |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity at rest and on movement: visual analogue scale at zero to six hours, six to 24 hours, 24 to 48 hours Proportion of participants in pain: from zero to 48 hours Nausea/vomiting Participant satisfaction: 48 hours Neurological injury Other outcomes: Total patient‐controlled epidural analgesia consumption at 24 hours and 48 hours Supplementary meperidine |
|
| Notes | Additional information regarding study methods sought, but study authors could not be reached | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quotes: "Patients and treating clinicians were not blinded as to study group randomization"; "Anesthesiologist who visited patients during the postoperative period was not aware of patients’ assignments" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Long 2006.
| Methods | Randomized trial: method not stated | |
| Participants | Single centre from California, USA 80 participants undergoing unilateral TKR Exclusion criteria: systemic arthritis, history of substance abuse, chronic pain syndromes, diabetes mellitus peripheral neuropathy, allergy to study medication Mean age (years): 69 (range 41 to 85) Female: 50% Number excluded post randomization: 10 because of catheter failure Number lost to follow‐up: none |
|
| Interventions | 1. Continuous FNB with bolus 0.2% ropivacaine 100 mg, infusion 5 ml/h × 36 hours 2. Epidural catheter inserted with 20.2% ropivacaine infused at 5 ml/h × 36 hours. FNB and epidural catheters discontinued at 6 am postoperative day two Adjunct analgesics: hydrocodone or hydrocodone bitartrate and acetaminophen given four hourly for postoperative day, then on request for the rest of the hospitalization. Oral oxycodone 6 pm day of surgery unless comfortable. IV ketorolac 30 mg six‐hourly × first 24 hours, as needed, for second 24 hours for breakthrough pain. COX‐2 morning of hospitalization |
|
| Outcomes |
Outcomes of interest for the review: Pain severity at rest: visual analogue scale, four‐hourly Total opioid consumption: daily until postoperative day three Knee flexion at days two and three, on discharge Knee extension at days two and three, on discharge Postoperative falls Deep vein thrombosis Other outcomes: Bladder infection Straight leg raise Walking distance |
|
| Notes | Additional information regarding the study method sought, but study authors could not be reached | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "10 were eliminated from the study because of catheter failures"; unknown if numbers were equal across groups |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Macalou 2004.
| Methods | Randomized trial: "randomly assigned by envelope by clinician in charge" | |
| Participants | Single centre from Nancy, France 90 participants undergoing unilateral TKR Exclusion criteria: age < 18 or >85 years, ASA I to III, morbid obesity, allergy to local anaesthetics or other medications used in this study, contraindications to regional anaesthesia, preexisting neurological deficits in lower extremities, pregnancy, breast‐feeding and inability to use a PCA or to comprehend pain scales Mean age (years): 68 ± 9 (single‐shot FNB) versus 71 ± 9 (single‐shot FNB + obturator) versus 70 ± 7 (IV PCA) Female: 79% Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. Single‐shot FNB with 0.5% bupivacaine and 2% lidocaine with 1:200 000 epinephrine. Nerve identified via nerve stimulator. IV PCA morphine delivering 1‐mg doses with seven‐minute lockout period and maximum dose of 25 mg in four hours 2. Single‐shot FNB with selective obturator nerve block. Nerve identified via nerve stimulator. IV PCA morphine delivering 1‐mg doses with seven‐minute lockout period and maximum dose of 25 mg in four hours 3. IV PCA morphine delivering 1‐mg doses with seven‐minute lockout period and maximum dose of 25 mg in four hours; sham FNB Adjunct analgesics: During the first 24 hours after surgery, all participants received 2 g propacetamol and 50 mg ketoprofen infused IV over 15 minutes at six‐hour intervals |
|
| Outcomes | Pain intensity at rest: visual analogue scale, hourly × six Total morphine consumption: first six hours Nausea/vomiting Sedation Hypotension Bradycardia Respiratory depression |
|
| Notes | Additional information regarding the study method sought, but study authors could not be reached | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned by envelope (unable to reach study authors for additional information) |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...assigned by envelope .." (unable to reach study authors for additional information). Unclear whether envelopes were sequentially numbered, sealed and opaque |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quotes: "single‐blind"; "Pain was evaluated at rest by another blinded investigator" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quotes: "single‐blind"; "Pain was evaluated at rest by another blinded investigator" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Martin 2008.
| Methods | Randomized trial: randomization list | |
| Participants | Two institutions in Paris, France 40 participants undergoing TKR, regardless of age Inclusion criteria: indication for TKR due to knee arthritis and surgery performed during general anaesthesia Exclusion criteria: previous surgery or trauma of the knee or preoperative use of corticosteroids Mean age (years): 67 ± 2 (continuous FNB); 70 ± 2 (IV PCA) Female: 70% Number excluded post randomization: two participants in PCA group excluded for absence of preoperative blood measurement Number lost to follow‐up: none |
|
| Interventions | 1. Continuous FNB + sciatic (single‐shot) with bolus 20 ml of 0.75% ropivacaine each for femoral and sciatic blocks, followed by continuous infusion of 0.2% ropivacaine, 0.15 ml/kg/h via femoral catheter for 48 hours, performed preoperatively; IV PCA morphine 1‐mg bolus with five‐minute lockout time; if insufficient, increased to 2 mg. Discontinued 72 hours after surgery 2. IV PCA morphine 1‐mg bolus with five‐minute lockout time; if insufficient, increased to 2 mg. Discontinued 72 hours after surgery Adjunct analgesics: IV acetaminophen (1 g every six hours) |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity at rest and on movement: visual analogue scale, days one, four, seven, months one, three Total opioid consumption: 24 hours. 48 hours, 72 hours Knee flexion: days one, four, seven, months one and three Other outcomes: Stand with and without assistance Uses toilet with and without assistance |
|
| Notes | Main aim of the study was to evaluate the effects of FNB on inflammation (clinical, cytokine levels). Study author provided additional information on method of allocation concealment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization list |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes (study author provided information) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote: "We could not perform a blinded study because of the sensory effects of the block and the visibility of the catheter during postoperative evaluation"; review authors judge that these outcomes are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: "We could not perform a blinded study because of the sensory effects of the block and the visibility of the catheter during postoperative evaluation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
McNamee 2001.
| Methods | Randomized trial: method not stated | |
| Participants | Single centre from Belfast, UK 75 participants undergoing TKR, regardless of age Inclusion criteria: ASA I to III, 40 to 85 years of age, weighed 40 to 95 kg and had no contraindication to regional anaesthesia Mean age (years): 69 (range 54 to 84) Female: 64% Number excluded post randomization: one participant excluded because of vasovagal collapse Number lost to follow‐up: none |
|
| Interventions | 1. Single‐shot FNB + sciatic with 2 mg/kg of bupivacaine 7.5 mg/ml divided equally between femoral and sciatic nerves. Nerve identified via nerve stimulator. IV PCA morphine 1‐mg bolus with five‐minute lockout time 2. Single‐shot FNB + sciatic with 2 mg/kg ropivacaine 7.5 mg/ml divided equally between femoral and sciatic nerves. Nerve identified via nerve stimulator. IV PCA morphine 1‐mg bolus with five‐minute lockout time 3. IV PCA morphine 1‐mg bolus with five‐minute lockout time. No femoral nerve blockade, but the area was prepared and a dressing applied Co‐analgesics: not stated |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity on movement: visual analogue scale, four‐hourly × 48 hours Total opioid consumption at 24 hours and 48 hours TIme to first morphine request Motor blockade Proportion of participants in pain > 30/100 mm Other outcomes: Emesis scores |
|
| Notes | Additional information regarding study methods sought, but study authors could not be reached | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quotes: "…blinding of the patient"; "All blocks performed by two authors blinded to which local anaesthetic was being administered" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: "…observations performed by nursing staff, unaware as to which group the patient had been allocated" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Mistraletti 2006.
| Methods | Randomized trial: computer‐generated randomization | |
| Participants | Single centre from McGill, Montreal, Quebec, Canada 27 participants undergoing primary unilateral TKR Exclusion criteria: age < 18 or > 85 years; ASA > III; morbid obesity (BMI > 40); diabetes or any metabolic disease; loss of body weight > 10% over the preceding three months; serum albumin < 35 g/l; cardiac, renal or hepatic failure; anaemia (haematocrit < 30%); organ transplant; rheumatoid arthritis; allergy to local anaesthetics or other medications used in the study; contraindication to use of PCA, epidural and CPNB; preexisting neurological deficits in lower extremities; and inability to use PCA or to comprehend pain scales Mean age (years): continuous FNB + sciatic: 67 ± 8; epidural: 64 ± 11; IV PCA: 71 ± 11 Female: 59% Number excluded post randomization/lost to follow‐up: three participants excluded from analysis (one participant in epidural group for removal of epidural catheter, one participant in continuous FNB group for cutting of femoral catheter and one participant in PCA group for refusal to continue the study) |
|
| Interventions | 1. Continuous FNB + sciatic with bolus, lignocaine 2% with epinephrine 2.5 μg/ml, 0.25 ml/kg. Infusion ropivacaine 0.2% initially at 8 ml/h in femoral catheter and 4 ml/h in sciatic. Subsequent adjustments performed to obtain VAS score < four and least possible motor block. Administered for 48 hours. Nerve identified via nerve stimulation 2. Epidural with bupivacaine 0.1% with fentanyl 3 μg/ml starting at 10 ml/h and adjusted if VAS at rest > three or VAS at knee flexion > four 3. IV PCA morphine with bolus doses from 2.5 to 5 mg (max 30 mg) until VAS score at rest < four, infusion at incremental doses of 1 mg, with lockout of seven minutes Adjunct analgesics: oral naproxen 500 mg every eight hours and oral acetaminophen 650 mg every six hours |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity at rest and on movement: visual analogue scale, 24 hours, 48 hours, at discharge Knee flexion: 48 hours TIme to walking |
|
| Notes | Main aim of study was to assess whether postoperative suppression of glyconeogenesis by dextrose infusion would be influenced by continuous nerve blocks compared with epidural or PCA. Additional information regarding the study method sought, but study authors could not be reached | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Investigators not involved in perioperative management collected VAS and other data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three participants excluded from analysis (one participant in epidural group for removal of epidural catheter, one participant in continuous FNB group for cutting of femoral catheter and one participant in PCA group for refusal to continue the study) |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Nader 2012.
| Methods | Randomized trial: computer‐generated randomization | |
| Participants | Single centre from Chicago, USA 62 participants undergoing elective unilateral TKR by a single surgeon Exclusion criteria: contraindication to the use of neuraxial analgesia, pregnancy, infection, prior surgery in inguinal/lumbar region, haemostatic abnormality, preexisting neuropathy, currently taking antidepressant or antiepileptic drugs, opioid‐tolerant patients or neuromuscular disease that could interfere with data collection Median (IQR) age (years): continuous FNB: 65 (60 to 76) versus IV PCA: 64 (60‐71) Female: 67.7% Number excluded post randomization/loss to follow‐up: two participants excluded from analysis for data > one month (one participant in each group) |
|
| Interventions | 1. Continuous FNB 10‐ml bolus of ropivacaine 0.25%, 5 ml/h infusion of ropivacaine 0.1%, commenced on postoperative day one morning at discontinuation of epidural analgesia (infusion bupivacaine 1 mg/ml and hydromorphone 10 mg/ml, bolus 3 ml of the same solution, lockout of 15 minutes, maximum 15 ml/h). Oral opioid hydrocodone 10 mg plus acetaminophen 325 mg every four to six hours 2. Oral opioid hydrocodone 10 mg plus acetaminophen 325 mg every four to six hours, commenced on postoperative day one morning after discontinuation of epidural analgesia Adjunct analgesics: If unable to achieve verbal rating score for pain < four, analgesic regimen was switched to sustained‐release oxycodone 10 mg every 12 hours with oral hydromorphone 2 mg every four hours for breakthrough pain |
|
| Outcomes | Pain intensity at rest and on movement: visual analogue scale, hospitalisation, months one, six and 12 Opioid consumption: 24 hours, 48 hours, 72 hours Knee flexion: postoperative days one and two, months one, six and 12 Participant satisfaction: at discharge Postoperative falls Venous thrombotic events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelope, not opened until after informed consent was obtained |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals for short‐term data. One dropout from each group after one month |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Ng 2001.
| Methods | Randomized trial: method not stated | |
| Participants | National University Hospital, Singapore 48 participants undergoing primary unilateral TKR Inclusion criteria: ASA I to II Exclusion criteria: age < 40 or > 80 years, weight < 50 or > 100 kg, allergy to local anaesthetics or acetaminophen, preexisting neurological deficit or inability to understand pain scale or PCA device usage Mean age (years): 64 Female: 85% Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. Single‐shot FNB with 30 ml of ropivacaine 0.25% (75 mg), nerve identified via stimulation; IV PCA morphine 1 mg with lockout time of five minutes, continuous background infusion at 0.01 mg/kg/h set for first 24 hours 2. Single‐shot FNB with 30 ml of ropivacaine 0.5% (150 mg), nerve identified via nerve stimulation; IV PCA morphine 1 mg with lockout time of five minutes, continuous background infusion at 0.01 mg/kg/h set for first 24 hours 3. Single‐shot FNB with 30 ml of bupivacaine 0.25% (75 mg), nerve identified via nerve stimulation. IV PCA morphine 1 mg with lockout time of five minutes, continuous background infusion at 0.01 mg/kg/h set for first 24 hours 4. Sham block. IV PCA morphine 1 mg with lockout time of five minutes, continuous background infusion at 0.01 mg/kg/h set for first 24 hours Adjunct analgesics: oral acetaminophen 1 g six‐hourly started 24 hours after surgery |
|
| Outcomes |
Outcomes of interest for the review: Proportion of pain at rest and on movement: four‐point verbal pain score, one hour, four hours, eight hours, 24 hours, 48 hours Total opioid consumption: 24 hours Respiratory depression Sedation Other outcomes: Length of stay |
|
| Notes | Study author contacted for additional information but could not provide requested information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope. Unclear whether envelopes were sequentially numbered and opaque |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Presumably low risk as quote: "double blind." Also, sterile syringes containing test solutions were prepared by one of the authors not involved in the nerve block, and FNBs were performed by anaesthesiologists unaware of the test solution used |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Assessment done by an investigator blinded to participant grouping |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Ng 2012.
| Methods | Randomized trial: randomization table | |
| Participants | Hong Kong 16 participants undergoing bilateral osteoarthritis of the knee scheduled for staged total knee (staged operations performed at a minimum interval of three months) Exclusion criteria: known allergies to any of the test drugs, those with major systemic illnesses (heart failure, renal impairment and coagulopathy), long‐term users of opioids and participants who had failed femoral catheter insertions Mean age (years): 70 ± 7.6 Female: 83% Number excluded post randomization: two Number lost to follow‐up: none |
|
| Interventions | 1. Continuous FNB 20‐ml bolus 0.2% ropivacaine, followed by continuous infusion at 10 ml/h, starting in the recovery room and continuing for 72 hours after the operation. Sham multimodal periarticular injection 2. Multimodal periarticular soft tissue injection with ropivacaine (300 mg in 30 ml), adrenaline (1 mg in 0.5 ml), saline (70 ml), triamcinolone acetonide (40 mg in 1 ml), injected into posterior joint capsules, periarticular soft tissue. Triamcinolone infiltrated into the skin to avoid fat atrophy. Sham continuous FNB Adjunct analgesics: patient‐controlled intravenous morphine (1‐mg bolus, lockout time of five minutes, maximum 6 mg/h) |
|
| Outcomes | Pain intensity: visual analogue scale, postoperative days one, two and three Morphine consumption: postoperative days one, two and three Knee flexion: daily × three Participant satisfaction with pain management at 72 hours |
|
| Notes | Cross‐over trial comparing collateral knees. Pair analysis not reported. Means (SDs) are available only for the two groups separately. Unit of analysis error may exist if we consider the data as from a parallel‐group trial, and carry‐over effect may exist for outcomes such as functionality and participant satisfaction. The population in this trial may be quite different (i.e. fitter) from that of parallel‐arm RCTs. Hence, the review authors decided not to extract the data for meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization table to allow 50% of participants treated to receive true FNB first and 50% of participants to receive true MPI first, using SPSS software (version 14.0; SPSS Inc, Chicago, Illinois) |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Participants and nurses responsible for recording pain scores blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Nurses recording pain scores blinded. Sham FNB/local infiltration analgesia used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants withdrew but unlikely to affect study conclusions as the study recruited two extra participants compared with required sample size |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Ozen 2006.
| Methods | Randomized trial: computer assisted | |
| Participants | Single centre from Ankara, Turkey 34 participants undergoing unilateral TKR Inclusion criteria: 50 to 75 years of age, ASA I to III Exclusion criteria: weight < 50 or > 100 kg, undergoing tumour surgery, immunosuppressive therapy, preexisting neuropathy or neurological deficits, allergy to local anaesthetics, inability to understand pain scale or PCA device usage Mean age (years): 64 Female: 62% Number excluded post randomization: four Number lost to follow‐up: none |
|
| Interventions | 1. Three‐in‐one single‐shot FNB with 40 ml 0.375% ropivacaine. Nerve identified via nerve stimulation. IV PCA morphine 0.3 mg/ml, 1‐mg bolus, 15‐minute lockout 2. IV PCA morphine 0.3 mg/ml, 1‐mg bolus, 15‐minute lockout Co‐analgesics: not stated |
|
| Outcomes | Pain intensity at rest. Visual analogue scale, hourly × four, then two‐hourly × three, 18 hours, 24 hours, 36 hours, 48 hours Total opioid consumption: 24 hours, 48 hours Nausea/vomiting Hypotension |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer assisted |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Team monitoring postop pain blind to treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Park 2010.
| Methods | Randomized trial: drawing of lots | |
| Participants | Single centre from Daejeon, Korea 80 participants undergoing elective unilateral TKR Inclusion criteria: female, ASA I to II under spinal anaesthesia Exclusion criteria: contraindications to regional anaesthetic technique (e.g. local infection, sepsis, coagulation abnormality), allergy to local anaesthetic or morphine, contraindications for NSAID use, preexisting neurological deficit in lower extremities and inability to comprehend pain scales or to use IV PCA device Mean age (years): 67 ± 6 Female: 100% Number excluded post randomization: eight Number lost to follow‐up: none |
|
| Interventions | Three arms of different concentrations of continuous FNB versus single‐shot FNB 1. Continuous FNB with bolus 20 ml of 0.25% bupivacaine with epinephrine 1:200,000, followed by continuous infusion 0.125% bupivacaine 2 ml/h until postoperative day three or four 2. Continuous FNB with bolus 20 ml of 0.25% bupivacaine with epinephrine 1:200,000, followed by continuous infusion 0.125% bupivacaine 4 ml/h until postoperative day three or four 3. Continuous FNB with bolus 20 ml of 0.25% bupivacaine with epinephrine 1:200,000, followed by continuous infusion 0.125% bupivacaine 6 ml/h until postoperative day three or four 4. Single‐shot FNB with bolus 20 ml of 0.25% bupivacaine with epinephrine 1:200,000, followed by continuous infusion 0.125% bupivacaine 0 ml/h until postoperative day three or four Adjunct analgesics: IV PCA morphine‐ketorolac at 1‐ml bolus of 0.5 mg/ml morphine, 1.5 mg/ml ketorolac solution, eight‐minute lockout interval and no continuous background infusion, bolus dose on demand when VAS > three out of 10 Rescue doses of 90 mg IM diclofenac |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity at rest and on movement: visual analogue scale, PACU, 6 pm on day of surgery, postoperative days one and two Total opioid consumption: postoperative days one and two Nausea/vomiting Sedation Pruritus Rescue analgesics required |
|
| Notes | Study author provided additional information on methods of randomization and allocation concealment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing of lots (study author provided information) |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes (study author provided information) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | No mention |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Eight participants excluded post randomization; not mentioned whether they were equally distributed across groups |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Parvataneni 2007.
| Methods | Randomized trial: method not stated | |
| Participants | Centre in USA 60 participants undergoing TKR Inclusion criteria: diagnosis of non‐inflammatory osteoarthritis. Participant factors such as BMI and co‐morbidities were not considered during the enrolment process Mean age (years): 69 Female: 48% Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. Single‐shot FNB with IV PCA morphine for 24 hours 2. Intra‐articular injection with 0.5% bupivacaine 200 to 400 mg, morphine sulfate (0.4 to 1.0 ml) 4 to 10 mg, epinephrine 1:1000 (0.3 ml) 300 μg, methylprednisolone acetate 40 mg, cefuroxime (10 ml) 750 mg, normal saline 22 ml. Clonidine transdermal patch applied in operating room: 100 μg/24 h Adjunct analgesics: IM ketorolac every six hours as needed (15 mg if > 65 years, 30 mg if < 65 years, hold if renal Impairment), if ketorolac is ineffective, IM morphine 2 to 4 mg every two to four hours, celecoxib 200 mg orally daily for 10 days, oxycodone SR 10/20 mg orally every 12 hours for 48 hours, oxycodone 5 mg orally every six hours as needed, acetaminophen 1000 mg orally every six hours |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity at rest: visual analogue scale, postoperative day one Knee ROM: postoperative day two Nausea/vomiting Participant satisfaction: postoperative day one Other outcomes: Straight leg raised Pain less than expected Confusion Discharged home |
|
| Notes | Additional information regarding the study method sought, but study authors could not be reached | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Research personnel blinded to randomization |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Salinas 2006.
| Methods | Randomized trial: computer random number generator | |
| Participants | Single centre in Seattle, USA 36 participants undergoing unilateral TKR under spinal anaesthesia Inclusion criteria: ASA I to III participants Exclusion criteria: age < 18 or > 85 years; BMI > 45; renal insufficiency; contraindications (localized infection, sepsis, preexisting lower extremity neurological abnormality) or refused spinal anaesthesia or femoral nerve block; allergy to local anaesthetics, morphine or oxycodone; long‐term opioid use; and difficulties in comprehending VAS pain scores or in using IV PCA device Mean age (years): 67 (9) versus 68 (6) Female: 50% Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. Contnuous FNB with 30 ml of ropivacaine 0.375% with epinephrine 2.5 g/ml, followed by continuous infusion of ropivacaine 0.2% at 10 ml/h via catheter six hours later until 8 am on the morning of postoperative day two; nerve stimulation 2. Single‐shot FNB with 30 ml of ropivacaine 0.375% with epinephrine 2.5 g/ml; nerve stimulation Adjunct analgesics: IV PCA morphine 1‐mg bolus with five‐minute lockout, to titrate VAS scores to ≤ three until morning postoperative day one. Oral ibuprofen 600 mg three times a day (starting date of surgery). Breakthrough pain (> four/10) treated on demand with oral oxycodone 5 mg |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity at rest and on movement: visual analogue scale, twice daily Total opioid consumption: 48 hours Nerve injury Catheter‐related infection |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Presumably low, as no mention of withdrawals/dropouts |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Seet 2006.
| Methods | Randomized trial: on‐line randomization software | |
| Participants | Single centre at Singapore General Hospital, Singapore 60 participants undergoing elective unilateral TKR under subarachnoid block Inclusion criteria: ASA I to III Exclusion criteria: contraindication to a lumbar subarachnoid block (e.g. coagulopathy, local infection, sepsis, previous lumbar spine operation), patients who preferred general anaesthesia, body weight < 40 kg or > 120 kg, allergies to study drugs, rheumatoid arthritis, preexisting lower limb neurological deficits Mean age (years): 66 ± 7 Female: 73% Number excluded post randomization: three from continuous FNB 0.15% ropivacaine and two from continuous FNB 0.2% ropivacaine (one unable to locate the subarachnoid space, one inadequate subarachnoid, three slipped femoral catheters postoperatively) Number lost to follow‐up: none |
|
| Interventions | 1. Three‐in‐one continuous FNB with 0.15% ropivacaine at 10 ml/h first 24 hours and 5 ml/h next 24 hours. Nerve identified via nerve stimulation. IV PCA morphine 1‐mg/ml bolus doses with lockout period of five minutes and maximum dose of 8 mg/h 2. 3‐in‐1 continuous FNB with 0.2% ropivacaine at 10 ml/h first 24 hours and 5 ml/h next 24 hours. Nerve identified via nerve stimulation. IV PCA morphine 1‐mg/ml bolus doses with lockout period of five minutes and maximum dose of 8 mg/h 3. IV PCA morphine 1‐mg/ml bolus doses with lockout period of five minutes and maximum dose of 8 mg/h Adjunct analgesics: oral paracetamol 1 g six‐hourly. Oral rofecoxib 50 mg each morning |
|
| Outcomes |
Outcomes of interest for the review: Pain severity at rest and on movement: visual analogue scale, six hours, 12 hours, 24 hours, 48 hours, 72 hours Total opioid consumption: 24 hours, 48 hours, 72 hours Nausea/vomiting Urinary retention Pruritus Sedation Hypotension Giddiness Participant satisfaction: 72 hours Knee flexion Time to ambulation Time to discharge Length of stay Other outcomes: Knee Society Clinical Rating System: knee scores, function scores |
|
| Notes | Study author provided additional information on methods of randomization and allocation concealment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "On‐line randomization software" (study author provided information) |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes (study author provided information) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Five participants excluded post randomization |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Serpell 2001.
| Methods | Randomized trial: method not stated | |
| Participants | Single centre in Ninewells Hospital, Dundee, UK 30 participants undergoing elective TKR under spinal anaesthesia Inclusion criteria: ASA I to II, 18 to 85 years Exclusion criteria: history of neurological disease, hypersensitivity to bupivacaine or unsuitable for spinal anaesthesia Mean age (years): 69 ± 3 Female: 77% Number excluded post randomization: one participant from continuous FNB excluded because of postoperative urinary retention Number lost to follow‐up: none |
|
| Interventions | 1. Continuous FNB with 0.3 ml/kg 0.5% bupivacaine and adrenaline 1:200,000, administered in 5‐ml increments. Nerve identified via nerve stimulation, block inserted post surgery, cannula left in situ to enable top‐ups of same dose of bupivacaine at six‐ to eight‐hour intervals during the next 48 hours; IV PCA morphine 2‐mg boluses to a maximum of 12 mg/h with lockout interval of nine minutes 2. IV PCA morphine 2‐mg boluses to a maximum of 12 mg/h with lockout interval of nine minutes Adjunct analgesics: IM morphine 10 mg or paracetamol 1 g orally |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity at rest: visual analogue scale, 24 hours, 48 hours Total morphine consumption: 48 hours Nausea/vomiting Other outcomes: Total number of IM supplements 10 mg Total number of paracetamol supplements 1 g Total number of doses of prochlorperazine |
|
| Notes | Additional information regarding study method sought, but study authors could not be reached | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant from continuous FNB excluded because of postoperative urinary retention. No other dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Singelyn 1998.
| Methods | Randomized trial: computer‐generated list of random permutations | |
| Participants | Single centre in Brussels, Belgium 45 participants undergoing elective TKR under general anaesthesia Inclusion criteria: ASA I to III Exclusion criteria: contraindications to regional anaesthetic technique (e.g. local infection, sepsis, coagulation abnormality), age < 18 or > 80 years, weight < 50 or > l00 kg, allergy to local anaesthetic and/or opioid, preexisting neurological deficit, diabetes or inability to comprehend pain scales or to use a PCA device Mean age: not stated Female: percentage not stated Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. Continuous FNB with bolus 37 ml of 0.25% bupivacaine with epinephrine 1:200,000, followed by continuous infusion 0.125% bupivacaine with sufentanil 0.1 μg/ml and clonidine 1 μg/ml, 10 ml/h, for 48 hours. Nerve identified via nerve stimulator 2. IV PCA with morphine 2 mg/ml, dose 1.5 mg, lockout time of eight minutes, for 48 hours 3. Epidural with test dose 3 ml 0.25% bupivacaine with epinephrine 1:200,000, 10 ml of same solution and 10 μg/ml sufentanil, followed by infusion 0.125% bupivacaine with sufentanil 0.1 μg/ml and clonidine 1 μg/ml 10 ml/h, for 48 hours Adjunct analgesics: If postoperative pain score ≥ one (ranged from zero to three) in FNB and epidural groups: Ig IV propacetamol (Prodafalgan), followed by 10 to 20 mg of IM piritramide, a synthetic p‐agonist opioid (Dipidolor) (15 mg piritramide = 10 mg morphine—information provided by study author) |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity at rest and on movement: visual analogue scale, four hours, 24 hours, 48 hours Knee flexion: daily until discharge Nausea/vomiting Urinary retention Hypotension Technical problem: kinked cath Time to achieve 90 degrees knee flexion Day able to walk Other outcomes: Hospital stay (including rehabilitation) Supplemental analgesia (propacetamol) IM piritramide |
|
| Notes | Study author contacted for additional information but no longer has original data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random permutations |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Not stated but likely unblinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Not stated but likely unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Sundarathiti 2009.
| Methods | Randomized trial: random number of tables | |
| Participants | Single centre in Bangkok, Thailand 61 participants undergoing elective unilateral TKR under spinal anaesthesia Inclusion criteria: ASA physical status I to III Exclusion criteria: < 35 or > 80 years of age, BMI > 45, renal insufficiency (creatine > 1.5 mg/dl), preexisting neurological deficit, inability to comprehend pain scales, long‐term opioid use and contraindications to neuraxial block or FNB Mean age (years): 68 ± 9 Female: 85% Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. Continuous FNB with bolus 20 ml of 0.25% levobupivacaine, continuous infusion of 0.125% levobupivacaine at 8 ml/h for 24 hours postoperatively, then reduced to 6 ml/h if VAS > three, nerve identified via nerve stimulator 2. Continuous epidural with bolus 0.125% levobupivacaine 10 ml plus 2 mg morphine, continuous infusion of 0.125% levobupivacaine with morphine 0.0125 mg/ml at 4 ml/h for 24 hours postop, then reduced to 3 ml/h if VAS > three Adjunct analgesics: oral ultracet one tab three times a day, oral acetaminophen 500 mg four times a day. Breakthrough pain (defined as VAS > four): IV tramadol 50 mg every four hours until discharge |
|
| Outcomes |
Outcomes of interest for the review: Proportion of participants in pain. At PACU, six hours, 12 hours, 24 hours, 36 hours, 48 hours and 72 hours Nausea/vomiting Pruritus Urinary retention Dizziness Numbness Catheter‐related infection Nerve injury Local anaesthesia toxicity Complete motor blockade Participant satisfaction (one = poor, two = fair, three = good, four = excellent) Other outcomes: Stand with help Walker mobilization and transfer to chair with and without help Hospital length of stay |
|
| Notes | Study author contacted but could not provide additional information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number of tables |
| Allocation concealment (selection bias) | Unclear risk | Method not mentioned |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Szczukowski 2004.
| Methods | Randomized trial: research randomizer application | |
| Participants | Single centre in Maryland, USA 40 participants undergoing unilateral TKR Inclusion criteria: age 40 to 80 years, ASA I to III Exclusion criteria: weighed > 300 lb or < 110 lb, or had an allergy to medications such as bupivacaine, epinephrine, morphine or meperidine. Other exclusions included history of narcotic abuse, preexisting neurological deficit in the lower extremity, inability to use the PCA, a bleeding disorder, a local or systemic infection, a bilateral procedure, uncontrolled hypertension or history of severe arrhythmia Mean age (years): 67 Female: 63% Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. Single‐shot FNB with 30 ml of 0.5% bupivacaine with epinephrine 1:200,000. Nerve identified via stimulation. IV PCA morphine loading dose of 3 mg and initial bolus dose of 1.5 mg, with lockout interval of 15 minutes and four‐hour maximum of 30 mg; morphine infusion discontinued after 24 hours 2. Sham block with 30 ml of preservative‐free normal saline. IV PCA morphine loading dose of 3 mg and initial bolus dose of 1.5 mg, with lockout interval of 15 minutes and four‐hour maximum of 30 mg; morphine infusion discontinued after 24 hours Adjunct analgesics: Oxycodone (5 mg)/acetaminophen (325 mg) oral tablets (1 tablet) every four hours as needed for breakthrough pain while on the PCA. When PCA morphine was discontinued, started on oxycodone (5 mg)/acetaminophen (325 mg) (one to two tablets) every four hours as needed |
|
| Outcomes | Pain severity at rest: visual analogue scale, day of surgery, daily post operation until discharge Total opioid consumption: 24 hours after surgery Sedation Nausea/vomiting Urinary retention Disorientation Pruritus and any other complications Sensory block Participant satisfaction |
|
| Notes | Study author contacted for additional information but no longer has original data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Research randomizer application (www.randomizer.org/form.htm) |
| Allocation concealment (selection bias) | Low risk | Quote: "Each vial was labelled in a similar manner, and the surgeons, anaesthesiologists, nurses, and all operating room staff were blinded to the contents of the vials. Each vial was labelled with a patient protocol number. The records were maintained and locked in the pharmacy‐services research closet" |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quote: "...surgeons, anaesthesiologists, nurses, and all operating room staff were blinded to the contents of the vials" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: "double‐blind." Presumably low risk as it will not be obvious which was the intervention group because both groups were given IV PCA |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Tang 2010.
| Methods | Randomized trial: computer‐generated randomization | |
| Participants | Single centre in Beijing, China 34 participants undergoing elective TKR under general anaesthesia Inclusion criteria: ASA I to II, age 18 to 80 years, weight 40 to 70 kg Exclusion criteria: impaired blood clotting, generalized sensitive skin, hypersensitivity to study drugs, uncooperative patients, impaired immune system Mean age (years): 64 ± 7 Female: 79% Number excluded post randomization: one from continuous FNB group because catheter fell out Number lost to follow‐up: one dropout from PCA morphine group |
|
| Interventions | 1. Continuous FNB with bolus 25 ml 0.4% ropivacaine, post operation infusion 0.2% ropivacaine loading dose 10 ml, background infusion 10 ml/h; IV PCA morphine bolus 1 mg/ml; lockout period of five minutes, no background infusion 2. IV PCA morphine bolus 1 mg/ml; lockout period of five minutes, no background infusion Adjunct analgesics: not stated |
|
| Outcomes |
Outcomes of interest to the study: Pain intensity at rest and on movement: visual analogue scale, PACU, four hours, eight hours, 12 hours, 24 hours, 48 hours Total opioid consumption: PACU, four hours, eight hours, 12 hours, 24 hours, 48 hours Nausea/vomiting Sedation Prutitus Knee flexion: 24 hours, 48 hours Participant satisfaction Other outcomes: Haemodynamic status Blood results |
|
| Notes | Study author provided additional information on methods of allocation concealment and blinding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Excel to generate the sequence |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque, sealed envelopes (study author provided information) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Unblinded (study author provided information) |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Unblinded (study author provided information) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant from continuous FNB group withdrew from the study because the catheter slipped out, and one participant from the PCA group dropped out after randomization. Apart from these, no other dropouts/withdrawals reported |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Toftdahl 2007.
| Methods | Randomized trial: method: thorough shuffling of sealed envelopes | |
| Participants | Department of Orthopaedic Surgery, Aarhus Hospital, Denmark 80 participants undergoing primary TKR under spinal anaesthesia Exclusion criteria: lack of mental ability to provide informed consent, neuropathic pain or sensory disorders in the leg to be operated, previous major bone surgery in the knee joint, intolerance to study drugs, failure of spinal anaesthesia and reoperation or trauma to the knee within the study period Mean age (years): continuous FNB: 72 ± 9; periarticular injections: 70 ± 9 Female: 61% Number excluded post randomization: three from continuous FNB group because of conversion of failed spinal anaesthesia to general anaesthesia. Number lost to follow‐up: none |
|
| Interventions | 1. Continuous FNB with bolus 20 ml ropivacaine 10 mg/ml, infusion 10 ml/h ropivacaine (2 mg/ml) for 48 hours. If needed, a bolus of 20 ml could be given every eight hours. After skin closure, 4 mg morphine (0.4 mg/ml) and 50 mg bupivacaine (5 mg/ml) given intra‐articularly through the drain 2. Periarticular injections: 150 ml ropivacaine (2 mg/ml), 1 ml ketorolac (30 mg/ml) and 1 ml epinephrine (0.5 mg/ml) by infiltration of the knee at the end of surgery and two postoperative injections of these substances through an intra‐articular catheter Adjunct analgesics: Paracetamol (1 g × 4), ibuprofen (400 mg × 3) and controlled‐release oxycodone (20 mg × 2) daily. Immediate‐release oxycodone (5 to 10 mg) used for pain treatment if pain at rest exceeded three on the zero to 10 numeric rating scale. IV morphine (0.05 to 0.1 mg/kg) was used for the treatment of severe pain (> seven) |
|
| Outcomes |
Outcomes of interest for the review: Pain severity at rest and on movement: visual analogue scale, one hour, two hours, thrice daily until postoperative day three and discharge Number of participants in pain (scores ≥ three from surgery to the evening of postoperative day one) Total opioid consumption: postoperative day one Nausea/vomiting Dizziness Pruritus Urinary track infection Constipation (on the evening of day three) Wound infection Flexion > 90 degrees Other outcomes: Bullae around wounds Knee ROM: median extension defect postoperative days one and two Walking distance Quadriceps function Ability to hold quadriceps tension > five seconds postoperative day one Ability to lift leg for > five seconds postoperative day two Length of stay |
|
| Notes | Study author provided additional information/data on method of randomization and individual participant data on pain intensity scores, nausea and vomiting, opioid consumption and knee range of motion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Thorough shuffling of sealed envelopes in batches of 20 envelopes per shuffle (study author provided information) |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote: "Blinding of patients and caregivers was not attempted. It was considered impossible to do properly, as the partial motor block present in patients in group F would be obvious to patients and staff" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: "Data collection and analysis was carried out by assessor who was not blinded since catheter placement was obvious" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four participants who discontinued their allocated interventions were included in the data analysis |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Tugay 2006.
| Methods | Randomized trial: drawing envelopes | |
| Participants | Single centre: Department of Orthopaedics and Traumatology, Hacettepe Hospital, Turkey 23 participants undergoing TKR for osteoarthritis Inclusion criteria: ASA I to III Exclusion criteria: significant medical/ psychiatric problems, unable to co‐operate with study protocol, contraindication to RA Mean age (years): 66 (range 51 to 78) Female: 87% Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. Single‐shot FNB (preemptive) with 40 ml 0.25% bupivacaine administered before muscle relaxant. Nerve identified via nerve stimulator. IV PCA morphine infusion 1 mg, lockout time of five minutes 2. Single‐shot FNB (post operation) with 40 ml 0.25% bupivacaine administered after reversal of muscle relaxant. Nerve identified via nerve stimulator. IV PCA morphine infusion 1 mg, lockout time of five minutes 3. IV PCA morphine infusion 1 mg, lockout time of five minutes Adjunct analgesics: none (study author provided information) |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity: changes in group means. Visual analogue scale, one hour, four hours, six hours, 12 hours, 24 hours, 48 hours Total opioid consumption: one hour, four hours, six hours, 12 hours, 24 hours, 48 hours Other outcomes: Ambulation of velocity Iowa University Level of Assistance scale Length of stay |
|
| Notes | No difference in pain reduction between preemptive and postop FNB. Study author provided additional information on co‐analgesics and methods of randomization, allocation concealment and blinding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The femoral nerve block protocols and control were written and enveloped. Prior to each operation an envelope was taken by the anaesthesiologist ..." (study author provided information) |
| Allocation concealment (selection bias) | Low risk | "Only the anaesthesiologists knew the femoral nerve block method used on each patient" (study author provided information) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | "Both patients and the rehabilitation staff were all blinded to the femoral nerve block protocol" (study author provided information) |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | "The physiotherapists were not informed about which protocol was applied to which patients until the end of the study. The functional evaluations and pain evaluations were done by these physiotherapist authors" (study author provided information) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Wang 2002.
| Methods | Randomized trial: drawing marked cards from a deck | |
| Participants | Single centre in Henry Ford Hospital, Detroit, USA 30 participants undergoing TKR Inclusion criteria: age 40 to 70 years Exclusion criteria: hypersensitivity to the test substance or co‐morbid condition that might compromise safety or ability to comply with study procedures Mean age (years): single‐shot FNB: 67 ± 8; IV PCA: 66 ± 10 Female: 63% Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. Single‐shot FNB with 0.25% bupivacaine with epinephrine 1:100,000. Nerve identified via nerve stimulator. IV PCA morphine 1‐mg doses with five‐minute lockout 2. IV PCA morphine 1‐mg doses with five‐minute lockout; placebo FNB with 40 ml N/saline Co‐analgesics: not stated |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity at rest and on movement: visual analogue scale, PACU, postoperative days one to three Total opioid consumption: postoperative days one and two Morphine related adverse effects Knee flexion postoperative day one, discharge Other outcomes: Distance of ambulation Distance goal met Length of stay |
|
| Notes | Study author contacted for additional data on morphine consumption but could not provide the information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing marked cards from mixed pack with equal numbers of each designation |
| Allocation concealment (selection bias) | Low risk | Blindly drawn marked cards from mixed pack with equal numbers of each designation |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quote: "placebo‐controlled, double‐blind study" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | All study personnel including outcome assessors blinded to treatment group assignment . |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Wang 2010.
| Methods | Randomized trial: computer‐generated list | |
| Participants | Single centre in Beijing, China 126 participants undergoing unilateral TKR Inclusion criteria: ASA I to II, 40 to 84 years, 45 to 95 kg Exclusion criteria: hypersensitivity to anaesthetics used for the study, congenital myopathy and neuropathies, severe obesity (BMI > 35), electrolyte derangement, severe diabetes mellitus, redo surgery, liver/renal failure, bleeding diathesis Mean age (years): 67 ± 13 Female: 64% Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. Continuous FNB with bolus 20 ml 0.5% ropivacaine, infusion: 0.2% ropivacaine 5 ml/h, PCA bolus 5 ml, lockout of 30 minutes for 48 hours 2. IV PCA with fentanyl 30 mcg/kg + tropisetron 5 mg in normal saline 100 ml, infusing 2 ml/h, bolus 2 ml, lockout time of 10 minutes Adjunct analgesics: IM pethidine 50 mg or oral Celebrex 200 mg |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity at rest and on movement: visual analogue scale, four hours, eight hours, 12 hours, 16 hours for pain at rest, 24 hours, 36 hours, 48 hours for pain at rest and on movement Proportion of participants in pain Nausea/vomiting Participant satisfaction Other outcomes: Supplemental analgesia (pethidine) Supplemental analgesia (Celebrex) |
|
| Notes | Study author provided additional information on method of randomization | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list (study author provided information) |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Not stated. It would have been difficult to blind participants without a placebo block |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Outcome assessor unaware of participant allocation. However it may be obvious that the FNB group has a catheter and the other group does not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Widmer 2012.
| Methods | Randomized trial: method not stated | |
| Participants | Single centre in Sydney, Australia 55 participants undergoing unilateral TKR Inclusion criteria: 18 to 85 years Exclusion criteria: known allergies to study medication, anatomical aberrations in inguinal area preventing FNB, history of drug or alcohol abuse, significant cognitive impairment or psychiatric disorder, postoperative endotracheal intubation, preoperative use > 40 mg oral morphine or severe cardiac, hepatic or renal disease Median (IQR) age (years): 72.1 (64.4 to 76.5) versus 69.4 (63.4 to 75.5) Female: 44% Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. FNB with bolus 30 ml 0.375% ropivacaine and periarticular anaesthetic infiltration 100 ml 0.2% ropivacaine with 0.5 mg adrenaline, to posterior, medial, lateral and anterior capsule, as well as arthrotomy margins, subcutaneous tissue and skin. IV PCA fentanyl 20 μg at five‐minute intervals on demand until postoperative day two. Additional boluses allowed 2. Sham setup for FNB with skin preparation and dressing, and periarticular anaesthetic infiltration 100 ml 0.2% ropivacaine with 0.5 mg adrenaline, to posterior, medial, lateral and anterior capsule, as well as arthrotomy margins, subcutaneous tissue and skin. IV PCA fentanyl 20 μg at five‐minute intervals on demand until postoperative day two. Additional boluses allowed Adjunct analgesics: COX‐2 inhibitor and paracetamol 1 g six‐hourly. Postoperative day two: oral oxycodone SR 10 mg 12‐hourly. Breakthrough pain with oxycodone immediate release 5 to 10 mg three‐hourly as needed |
|
| Outcomes |
Outcomes of interest for the review: Pain at rest (most severe pain during first 24 hours on scale of one to four). Visual analogue scale, first 24 hours Total opioid consumption (fentanyl) 24 hours Adverse events Other outcomes: Knee function scores at one year SF‐36 |
|
| Notes | Study author provided additional information on methods of randomization and allocation concealment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization (study author provided information) |
| Allocation concealment (selection bias) | Low risk | Coded and opaque envelopes (study author provided information) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quote: "Both the investigators and patients were blinded to treatment modality, and the procedural anaesthetists did not reveal group allocation to any party associated with the study" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: "blinded study personnel" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Xie 2012.
| Methods | Randomized trial: method not stated | |
| Participants | Single centre in Chicago, USA 105 participants undergoing unilateral TKR Exclusion criteria: age < 18 or > 90 years, ASA > III, coagulopathy, cellulitis at the injection site, severe morbid obesity defined as BMI > 40, seizure disorder, severe liver or renal disease, opioid dependency, mental or psychiatric disease affecting ability to comprehend pain scale, preexisting neurological deficits or neuropathy in the lower extremities Mean age (years): 69 Female: 72% Number excluded post randomization: six for surgery cancelled, PCA not ordered or missing PCA data sheet Number lost to follow‐up: none |
|
| Interventions | 1. Single‐shot FNB with 30 ml 0.25% bupivacaine with 1:200,000 epinephrine. Nerve identified via nerve stimulation. IV PCA morphine converted to oral pain medication on postoperative day one or two 2. Single‐shot FNB with 30 ml 0.50% bupivacaine with 1:200,000 epinephrine. Nerve identified via nerve stimulation. IV PCA morphine converted to oral pain medication on postoperative day one or two 3. Sham FNB with 30 ml normal saline. IV PCA morphine converted to oral pain medication on postoperative day one or two Co‐analgesics: not stated |
|
| Outcomes | Pain severity: six‐point Likert scale from zero to five “none or minimal” (scores zero and one), “moderate” (scores two and three) and “considerable” (scores four and five) Total opioid consumption: 24 hours Nausea Sedation Participant satisfaction |
|
| Notes | Additional information regarding study method sought, but study authors could not be reached | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Low risk | Formulated by pharmacists and labelled with numbers only |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Anaesthesiologists, surgeons and participants blinded to treatment assignment |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Six participants withdrawn for surgery cancelled, PCA not ordered or missing PCA data sheet |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Yu 2010.
| Methods | Randomized trial: random number table | |
| Participants | Single centre in Beijing, China 80 participants undergoing TKR Exclusion criteria: lumbar spondylosis, diabetes mellitus, mentally subnormal, insomnia, unable to understand VAS Mean age (years): 65.0 ± 4 Female: 88% Number excluded post randomization: none Number lost to follow‐up: none |
|
| Interventions | 1. Continuous FNB with initial dose of 5 ml 2% lignocaine, infusion 0.2% ropivacaine 200 ml, 4 ml/h for 72 hours 2. PCA with sufentanil 200 μg, ondansetron 12 mg in saline 200 ml, infusing 2 ml/h Adjunct analgesics: COX‐2 100 mg BD, NSAIDs or opioids |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity at rest: visual analogue scale, two hours, six hours, 24 hours, 36 hours, 48 hours, 56 hours, 72 hours Supplemental analgesics: number of times opioid used Supplemental analgesics: number of times NSAIDs used Nausea/vomiting Giddiness Postoperative active motion of knee joint Other outcomes: Abdominal discomfort Hospital for Special Surgery Knee Score: motor function Restful sleep at 72 hours |
|
| Notes | Additional information regarding study method sought, but study authors could not be reached | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Outcome assessor not involved in surgery. Group with catheter would have been obvious |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Low risk | Appears to be free of other sources of bias |
Zaric 2006.
| Methods | Randomized trial: computerized random number tables | |
| Participants | Single centre in Copenhagen, Denmark 60 participants undergoing TKR with general anaesthesia Inclusion criteria: ASA I to III Exclusion criteria: morphine intolerance, neurological disease, coagulation disturbance and chronic pain and rheumatoid arthritis Mean age (years): 66 ± 7 Female: 53% Number excluded post randomization: 11 participants (four in continuous FNB and seven in epidural group) Number lost to follow‐up: none |
|
| Interventions | 1. Continuous FNB + sciatic with bolus 30 ml of ropivacaine 7.5 mg/ml and Infusion ropivacaine 2 mg/ml and sufentanil 1 μg/ml at 5 ml/h through femoral nerve catheter; and bolus 30 ml of ropivacaine 7.5 mg/ml and Infusion ropivacaine 0.5 mg/ml at 5 ml/h through sciatic nerve catheter 2. Epidural with ropivacaine 7.5 mg/ml given in 5‐ml aliquots to attain a level of analgesia at Th 10, and infusion ropivacaine 2 mg/ml and sufentanil 1 μg/ml at 5 ml/h All infusions were continued for 55 hours Adjunct analgesics: IV PCA morphine bolus 2 mg with lockout period of six minutes and maximum dose of 20 mg/h, paracetamol 1 g four times daily. If VAS > three at rest, 5 ml ropivacaine 7.5‐mg/ml bolus given through catheters. If pain score > five, 5 ml lidocaine 20‐mg/ml bolus given. After cessation of infusions, rofecoxib 25 mg, sustained‐release morphine (Contalgin) 20 mg (10 mg for participants > 70 years) twice daily and morphine 10 mg |
|
| Outcomes |
Outcomes of interest for the review: Pain intensity at rest and on movement: visual analogue scale, four hours post operation, 11 am and 14.30 pm on first, second and morning of third postoperative days Total opioid consumption: postoperative days one and two Nausea/vomiting Dizziness (moderate to severe) Sedation Pruritus Urinary retention Adverse events Knee flexion: postoperative day one, discharge Other outcomes: Rehab indices Length of stay |
|
| Notes | Study author provided additional data on mean (SD) for pain intensity on movement and at rest with knee ROM flexion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized random number tables |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Blinding of groups not attempted |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Blinding of groups not attempted |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11 participants (18%) excluded post randomization (four in continuous FNB and six in epidural group because of insufficient analgesia;.one in epidural group had an acute myocardial infarction followed by coronary artery bypass graft) |
| Selective reporting (reporting bias) | Low risk | Outcomes predefined |
| Other bias | Unclear risk | Potential impact of high attrition on ITT bias may be an issue, as the study did not use ITT analysis |
Abbreviations:
ASA: American Society of Anaesthesiologists; BD: twice a day; BMI: body mass index; COX: cyclo‐oxygenase; CPM: continuous passive motion; CPNB: continuous peripheral nerve block; FNB: femoral nerve block; IQR: interquartile range; ITT: intention‐to‐treat; NSAID: non‐steroidal anti‐inflammatory drug; PACU: postanaesthesia care unit; PCA: patient‐controlled analgesia; PCEA: patient‐controlled epidural analgesia; ROM: range of motion; SD: standard deviation; SF: Short Form; TKR: total knee replacement; VAS: visual analogue scale; WOMAC: Western Ontario and McMaster Osteoarthritis Index.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Affas 2012 | Secondary analysis of Affas 2011 (an included study). Wrong outcomes. |
| Bagry 2008 | Wrong intervention: lumbar plexus versus PCA opioid |
| Blisard 2012 | Not an RCT |
| Cappelleri 2011 | Population included arthrolysis participants with no separate data for TKR participants |
| Carvalho 2012 | No non‐FNB group (continuous FNB vs continuous FNB + sciatic) |
| Chelly 2001 | Not an RCT |
| Combes 2000 | Population included knee surgery participants with no separate data for TKR participants |
| Dang 2005 | No non‐FNB group (continuous FNB vs continuous FNB + sciatic) |
| Edwards 1992 | Wrong comparator (intramuscular papaveretum) |
| Frassanito 2009 | Wrong intervention (single‐shot lumbar plexus block vs continuous lumbar plexus/sciatic blocks) |
| Frassanito 2010 | Wrong comparator (intrathecal morphine) |
| Hakkalamani 2008 | Not an RCT. No non‐FNB comparator (FNB vs FNB + sciatic) |
| Ilfeld 2008 | Wrong intervention |
| Ilfeld 2009 | Wrong intervention |
| Ilfeld 2010 | Wrong intervention |
| Johnson 2011 | Wrong comparator (DepoDur extended‐release epidural morphine) |
| Koh 2011 | Wrong intervention (periarticular multimodal drug injection + multimodal pain control vs multimodal pain control) |
| McMeniman 2010 | No non‐FNB group (FNB vs fascia iliaca block) |
| Meftah 2012 | Wrong intervention (FNB/epidural) |
| Morin 2005 | No non‐FNB group (continuous posterior lumbar plexus block vs continuous FNB vs continuous FNB + sciatic nerve block) |
| Ng 2012b | Not an RCT |
| Niskanen 2005 | Wrong intervention (bedside femoral blocks on first postoperative day vs IM oxycodone/tramadol/epidural and additional femoral blocks) |
| Rais 2009 | No non‐FNB comparator |
| Rajeev 2007 | No non‐FNB comparator |
| Rasiah 2012 | Compared collateral knees in participants undergoing simultaneous bilateral total knee replacement |
| Safa 2011 | Interim analysis |
| Serrano 2011 | No non‐FNB comparator |
| Sites 2004 | Wrong control group (intrathecal morphine) |
| Taninishi 2011 | No non‐FNB comparator |
| Tarkkila 1998 | Population included major knee surgery participants with no separate data for TKR participants. Wrong comparator (intrathecal morphine) |
| Tricarico 2009 | Population included major knee surgery participants with no separate data for TKR participants |
| Watson 2005 | Wrong intervention (continuous vs single‐shot lumbar plexus blocks) |
| Wegener 2011 | No non‐FNB comparator (FNB vs FNB + single‐shot sciatic nerve block vs FNB/continuous sciatic nerve block) |
| Weston‐Simons 2012 | No non‐FNB comparator |
| Zhang 2010 | Not an RCT. Population included artificial joint replacement participants with no separate data for TKR participants |
| Ziwenga 2010 | No non‐FNB comparator (continuous infusion vs patient‐controlled FNB) |
Abbreviations:
FNB: femoral nerve block; PCA: patient‐controlled analgesia; RCT: randomized controlled trial; TKR: total knee replacement.
Characteristics of studies awaiting assessment [ordered by study ID]
Grider 2011.
| Methods | Randomized trial: method not stated |
| Participants | Number of centre: not stated. Study done in USA 80 participants undergoing knee replacements |
| Interventions | 1. Continuous FNB with bolus 30 ml 0.5% ropivacaine followed by postoperative infusion 8 ml/h 0.2% ropivacaine 2. Single‐shot FNB with bolus 30 ml 0.5% ropivacaine Postoperation: Both groups received rescue IV PCA morphine first 48 hours followed by oral oxycodone |
| Outcomes | VAS pain scores at rest Morphine consumption Knee range of motion Local anaesthetic or opioid analgesic complications Hospital length of stay |
| Notes | Conference abstract: possibly eligible but not in format that is easy to appraise |
Mullen 2008.
| Methods | Unable to obtain abstract or to contact study author for details |
| Participants | Ditto |
| Interventions | Ditto |
| Outcomes | Ditto |
| Notes | Conference abstract: awaiting information to determine eligibility |
Tobin 2011.
| Methods | Randomized trial: method not stated |
| Participants | Number of centre: not stated. Study done in India 63 participants undergoing unilateral TKR Inclusions: ASA I to II |
| Interventions | 1. Continuous FNB with bolus 30 ml 0.25% bupivacaine, followed by postoperative infusion 8 ml/h 0.125% bupivacaine 2. Epidural with bolus 8 ml 0.25% bupivacaine, infusion 8 ml/h 0.125% bupivacaine |
| Outcomes | VAS pain scores Adverse effects Motor blockade Fentanyl and bupivacaine consumption Rehabilitation indices Oxford knee scores |
| Notes | Conference abstract with no quantifiable outcome data provided |
Yuksel 2011.
| Methods | Randomized trial: method not stated. |
| Participants | Number of centre: not stated. Study done in Turkey 100 participants undergoing unilateral TKR Inclusions: ASA I to III |
| Interventions | 1. Continuous FNB with single‐shot sciatic, and PCA 0.125% bupivacaine, bolus 0.05 ml/kg, lockout time of 30 minutes, infusion 0.1 ml/kg/h for 48 hours 2. Continuous epidural with PCA 0.125% bupivacaine, bolus 0.05 ml/kg, lockout time of 30 minutes, infusion 0.1 ml/kg/h Both groups: if VAS > four: subcutaneous morphine 0.1 mg/kg |
| Outcomes | VAS pain scores Total bupivacaine consumption Degree of knee flexion Participant satisfaction |
| Notes | Conference abstract with no quantifiable outcome data provided |
Abbreviations:
ASA: American Society of Anaesthesiologists; FNB: femoral nerve block; PCA: patient‐controlled analgesia; TKR: total knee replacement; VAS: visual analogue scale.
Differences between protocol and review
We made the following changes to the published protocol (Chan 2012).
1. Types of interventions.
We added a new comparison called continuous versus single‐shot FNB for a more comprehensive review on FNB.
2. Primary outcomes: pain at rest and on movement.
We have reclassified pain at rest and on movement for the postoperative time frame ‘> 24 to 72’ to two categories: ’48 hours’ and ’72 hours.’
3. Data synthesis.
For better clarity, our text in the ‘Data synthesis’ section was changed as follows.
The primary analyses include:
single‐shot or continuous FNB ± sciatic/obturator block versus PCA opioid;
single‐shot or continuous FNB ± sciatic/obturator block versus epidural analgesia;
single‐shot or continuous FNB versus local infiltration analgesia;
single‐shot or continuous FNB versus oral analgesia; and
continuous versus single‐shot FNB.
4. Unit of analysis issues.
A total of 20 RCTs were included with three intervention arms: multiple independent comparisons (e.g. FNB vs PCA and FNB vs epidural); multiple correlated comparisons with similar types of FNB (e.g. continuous FNB with ropivacaine vs PCA and continuous FNB with bupivacaine vs PCA) or multiple correlated comparisons with different types of FNB (e.g. single‐shot FNB vs PCA and continuous FNB vs PCA). We have reworded our text in Unit of analysis issues to clarify.
5. Dealing with missing data.
Some of the included RCTs presented results using summary statistics other than mean (SD). In the review, we added details on how we handled these missing data.
6. Subgroup analysis.
Because of limited data, subgroup analysis was restricted to:
type of FNB (single‐shot or continuous FNB, with or without an additional sciatic/ obturator nerve block);
FNB with and without a concurrent parenteral opioid; and
type of FNB local anaesthetics (i.e. ropivacaine or bupivacaine).
7. Sensitivity analysis.
Sensitivity analysis based on multiple interventions within studies was not performed because we followed the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (see Chapter 16; Higgins 2011), as discussed in the Unit of analysis issues section.
8. SoF tables.
For the SoF tables, we have reduced the number of outcomes to seven: pain at rest at 24 hours, pain on movement at 24 hours, neurological injury, opioid consumption, nausea and vomiting, knee range of motion and participant satisfaction with analgesia, by excluding ‘time to first rescue analgesia’ and ‘early mobilisation’.
9. We changed the term ‘surgical infiltration with/without intra‐articular injection’ to ‘local infiltration analgesia,’ as the latter appears to be commonly used in recent literature.
10. We defined ‘early ambulation’ as ‘time to first ambulation.’
Contributions of authors
Ee‐Yuee Chan (EC), Marlene Fransen (MF), David A Parker (DA), Pryseley N Assam (PA), Nelson Chua (NC)
Conceiving of the review: EC
Co‐ordinating the review: EC
Undertaking manual searches: EC
Screening search results: EC, MF
Organizing retrieval of papers: EC
Screening retrieved papers against inclusion criteria: EC, MF, NC
Appraising quality of papers: EC, MF
Abstracting data from papers: EC, MF, NC
Writing to authors of papers to ask for additional information: EC
Providing additional data about papers: EC
Obtaining and screening data on unpublished studies: EC, MF
Managing data for the review: EC
Entering data into Review Manager (RevMan 5.2): EC
Handling RevMan statistical data: EC, PA
Performing other statistical analysis not using RevMan: EC, PA
Interpreting data: EC, MF, PA, NC, DP
Making statistical inferences: EC, MF, PA, NC, DP
Writing the review: EC with support from the other review authors
Performing previous work that was the foundation of the present study: EC
Serving as guarantor for the review (one review author): EC
Taking responsibility for reading and checking the review before submission: EC
Sources of support
Internal sources
Ee‐Yuee Chan was supported by an International Postgraduate Research Scholarship, University of Sydney, Australia.
External sources
No sources of support supplied
Declarations of interest
Ee‐Yuee Chan, Marlene Fransen and Nelson Chua were the study authors of an RCT (Chan 2013) that was eligible for inclusion in this Cochrane review.
Pryseley N Assam is a co‐author of a possible publication arising from the RCT authored by Chan et al (Chan 2013).
David A Parker co‐authored an RCT (Widmer 2012) that was also included in this review.
Edited (no change to conclusions)
References
References to studies included in this review
Adams 2002 {published data only}
- Adams HA, Saatweber P, Schmitz CS, Hecker H. Postoperative pain management in orthopaedic patients: no differences in pain score, but improved stress control by epidural anaesthesia. European Journal of Anaesthesiology 2002;19(9):658‐65. [PUBMED: 12243289] [DOI] [PubMed] [Google Scholar]
Affas 2011 {published data only}
- Affas F, Nygrds EB, Stiller CO, Wretenberg P, Olofsson C. Pain control after total knee arthroplasty: a randomized trial comparing local infiltration anesthesia and continuous femoral block. Acta Orthopaedica 2011;82(4):441‐7. [PUBMED: 21561303] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 1998 {published data only}
- Allen HW, Liu SS, Ware PD, Nairn CS, Owens BD. Peripheral nerve blocks improve analgesia after total knee replacement surgery. Anesthesia and Analgesia 1998;87(1):93‐7. [PUBMED: 9661553 ] [DOI] [PubMed] [Google Scholar]
Baranovic 2011 {published data only}
- Baranovic S, Maldini B, Milosevic M, Golubic R, Nikolic T. Peripheral regional analgesia with femoral catheter versus intravenous patient controlled analgesia after total knee arthroplasty: a prospective randomized study. Collegium Antropologicum 2011;35(4):1209‐14. [PUBMED: 22397261] [PubMed] [Google Scholar]
Barrington 2005 {published data only}
- Barrington MJ, Olive D, Low K, Scott DA, Brittain J, Choong P. Continuous femoral nerve blockade or epidural analgesia after total knee replacement: a prospective randomized controlled trial. Anesthesia and Analgesia 2005;101(6):1824‐9. [PUBMED: 16301267] [DOI] [PubMed] [Google Scholar]
Carli 2010 {published data only}
- Carli F, Clemente A, Asenjo JF, Kim DJ, Mistraletti G, Gomarasca M, et al. Analgesia and functional outcome after total knee arthroplasty: periarticular infiltration vs continuous femoral nerve block. British Journal of Anaesthesia 2010;105(2):185‐95. [PUBMED: 20551021] [DOI] [PubMed] [Google Scholar]
Chan 2012 {published data only}
- Chan M‐H, Chen W‐H, Tung Y‐W, Liu K, Tan P‐H, Chia Y‐Y. Single‐injection femoral nerve block lacks preemptive effect on postoperative pain and morphine consumption in total knee arthroplasty. Acta Anaesthesiologica Taiwanica 2012;50:54‐8. [PUBMED: 22769858] [DOI] [PubMed] [Google Scholar]
Chan 2013 {published and unpublished data}
- Chan EY, Fransen M, Sathappan S, Chua NH, Chan YH, Chua N. Comparing the analgesia effects of single‐injection and continuous femoral nerve blocks with patient controlled analgesia after total knee arthroplasty. Journal of Arthroplasty 2013;28(4):608‐13. [PUBMED: 23142441 ] [DOI] [PubMed] [Google Scholar]
Davies 2004 {published data only}
- Davies AF, Segar EP, Murdoch J, Wright DE, Wilson IH. Epidural infusion or combined femoral and sciatic nerve blocks as perioperative analgesia for knee arthroplasty. British Journal of Anaesthesia 2004;93(3):368‐74. [PUBMED: 15247111] [DOI] [PubMed] [Google Scholar]
de Lima e Souza 2008 {published and unpublished data}
- Lima e Souza R, Correa CH, Henriques MD, Oliveira CB, Nunes TA, Gomez RS. Single‐injection femoral nerve block with 0.25% ropivacaine or 0.25% bupivacaine for postoperative analgesia after total knee replacement or anterior cruciate ligament reconstruction. Journal of Clinical Anesthesia 2008;20(7):521‐7. [PUBMED: 19019663] [DOI] [PubMed] [Google Scholar]
Fritze 2009 {published data only}
- Fritze P, Anderl S, Marouf A, Cumlivski R, Muller C, Pernicka E, et al. Pain therapy using stimulating catheters after total knee arthroplasty. Schmerz 2009;23(3):292‐8. [PUBMED: 19308464] [DOI] [PubMed] [Google Scholar]
Ganapathy 1999 {published data only}
- Ganapathy S, Wasserman RA, Watson JT, Bennett J, Armstrong KP, Stockall CA, et al. Modified continuous femoral three‐in‐one block for postoperative pain after total knee arthroplasty. Anesthesia and Analgesia 1999;89(5):1197‐202. [PUBMED: 10553834] [PubMed] [Google Scholar]
Good 2007 {published data only}
- Good RP, Snedden MH, Schieber FC, Polachek A. Effects of a preoperative femoral nerve block on pain management and rehabilitation after total knee arthroplasty. American Journal of Orthopedics 2007;36(10):554‐7. [PUBMED: 18033568] [PubMed] [Google Scholar]
Hirst 1996 {published data only}
- Hirst GC, Lang SA, Dust WN, Cassidy JD, Yip RW. Femoral nerve block. Single injection versus continuous infusion for total knee arthroplasty. Regional Anesthesia 1996;21(4):292‐7. [PUBMED: 8837185] [PubMed] [Google Scholar]
Hunt 2009 {published and unpublished data}
- Hunt KJ, Bourne MH, Mariani EM. Single‐injection femoral and sciatic nerve blocks for pain control after total knee arthroplasty. Journal of Arthroplasty 2009;24(4):533‐8. [PUBMED: 19026519 ] [DOI] [PubMed] [Google Scholar]
Kadic 2009 {published data only}
- Kadic L, Boonstra MC, Waal Malefijt MC, Lako SJ, Egmond J, Driessen JJ. Continuous femoral nerve block after total knee arthroplasty?. Acta Anaesthesiologica Scandinavica 2009;53(7):914‐20. [PUBMED: 19388886] [DOI] [PubMed] [Google Scholar]
Kaloul 2004 {published data only}
- Kaloul I, Guay J, Cote C, Fallaha M. The posterior lumbar plexus (psoas compartment) block and the three‐in‐one femoral nerve block provide similar postoperative analgesia after total knee replacement. Canadian Journal of Anaesthesia 2004;51(1):45‐51. [PUBMED: 14709460] [DOI] [PubMed] [Google Scholar]
Kardash 2007 {published and unpublished data}
- Bergeron SG, Kardash KJ, Huk OL, Zukor DJ, Antoniou J. Functional outcome of femoral versus obturator nerve block after total knee arthroplasty. Clinical Orthopaedics & Related Research 2009;467(6):1458‐62. [PUBMED: 19224305] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardash K, Hickey D, Tessler MJ, Payne S, Zukor D, Velly AM. Obturator versus femoral nerve block for analgesia after total knee arthroplasty. Anesthesia and Analgesia 2007;105(3):853‐8. [PUBMED: 17717250] [DOI] [PubMed] [Google Scholar]
Lee 2011 {published data only}
- Lee AR, Choi DH, Ko JS, Choi SJ, Hahm TS, Kim GH, et al. Effect of combined single‐injection femoral nerve block and patient‐controlled epidural analgesia in patients undergoing total knee replacement. Yonsei Medical Journal 2011;52(1):145‐50. [PUBMED: 21155047 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Long 2006 {published data only}
- Long WT, Ward SR, Dorr LD, Raya J, Boutary M, Sirianni LE. Postoperative pain management following total knee arthroplasty: a randomized comparison of continuous epidural versus femoral nerve infusion. The Journal of Knee Surgery 2006;19(2):137‐43. [PUBMED: 16642893] [DOI] [PubMed] [Google Scholar]
Macalou 2004 {published data only}
- Macalou D, Trueck S, Meuret P, Heck M, Vial F, Ouologuem S, et al. Postoperative analgesia after total knee replacement: the effect of an obturator nerve block added to the femoral 3‐in‐1 nerve block. Anesthesia and Analgesia 2004;99(1):251‐4. [PUBMED: 15281539] [DOI] [PubMed] [Google Scholar]
Martin 2008 {published data only}
- Martin F, Martinez V, Mazoit JX, Bouhassira D, Cherif K, Gentili ME, et al. Antiinflammatory effect of peripheral nerve blocks after knee surgery: clinical and biologic evaluation. Anesthesiology 2008;109(3):484‐90. [PUBMED: 18719447] [DOI] [PMC free article] [PubMed] [Google Scholar]
McNamee 2001 {published data only}
- McNamee DA, Convery PN, Milligan KR. Total knee replacement: a comparison of ropivacaine and bupivacaine in combined femoral and sciatic block. Acta Anaesthesiologica Scandinavica 2001;45(4):477‐81. [PUBMED: 11300387] [DOI] [PubMed] [Google Scholar]
Mistraletti 2006 {published data only}
- Mistraletti G, Cuadra‐Fontaine JC, Asenjo FJ, Donatelli F, Wykes L, Schricker T, et al. Comparison of analgesic methods for total knee arthroplasty: metabolic effect of exogenous glucose. Regional Anesthesia & Pain Medicine 2006;31(3):260‐9. [PUBMED: 16701193] [DOI] [PubMed] [Google Scholar]
Nader 2012 {published data only}
- Nader A, Kendall MC, Wixson RL, Chung B, Polakow LM, Mccarthy RJ. A randomized trial of epidural analgesia followed by continuous femoral analgesia compared with oral opioid analgesia on short‐ and long‐term functional recovery after total knee replacement. Pain Medicine 2012;13(7):937‐47. [PUBMED: 22680916] [DOI] [PubMed] [Google Scholar]
Ng 2001 {published data only}
- Ng HP, Cheong KF, Lim A, Lim J, Puhaindran ME. Intraoperative single‐shot "3‐in‐1" femoral nerve block with ropivacaine 0.25%, ropivacaine 0.5% or bupivacaine 0.25% provides comparable 48‐hr analgesia after unilateral total knee replacement. Canadian Journal of Anaesthesia 2001;48(11):1102‐8. [PUBMED: 11744586] [DOI] [PubMed] [Google Scholar]
Ng 2012 {published data only}
- Ng FY, Ng JKF, Chiu KY, Yan CH, Chan CW. Multimodal periarticular injection vs continuous femoral nerve block after total knee arthroplasty. A prospective, crossover, randomized clinical trial. Journal of Arthroplasty 2012;27(6):1234‐8. [PUBMED: 22325963 ] [DOI] [PubMed] [Google Scholar]
Ozen 2006 {published data only}
- Ozen M, Inan N, Tumer F, Uyar A, Baltaci B. The effect of 3‐in‐1 femoral nerve block with ropivacaine 0.375% on postoperative morphine consumption in elderly patients after total knee replacement surgery. Agri Dergisi 2006;18(4):44‐50. [PUBMED: 17457713] [PubMed] [Google Scholar]
Park 2010 {published data only}
- Park CK, Cho CK, Lee GG, Lee JH. Optimizing dose infusion of 0.125% bupivacaine for continuous femoral nerve block after total knee replacement. Korean Journal of Anesthesiology 2010;58(5):468‐76. [PUBMED: 20532056 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parvataneni 2007 {published data only}
- Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. Journal of Arthroplasty 2007;22(6):33‐8. [PUBMED: 17823012] [DOI] [PubMed] [Google Scholar]
Salinas 2006 {published data only}
- Salinas FV, Liu SS, Mulroy MF. The effect of single‐injection femoral nerve block versus continuous femoral nerve block after total knee arthroplasty on hospital length of stay and long‐term functional recovery within an established clinical pathway. Anesthesia and Analgesia 2006;102(4):1234‐9. [PUBMED: 16551930] [DOI] [PubMed] [Google Scholar]
Seet 2006 {published and unpublished data}
- Seet E, Leong WL, Yeo ASN, Fook‐Chong S. Effectiveness of 3‐in‐1 continuous femoral block of differing concentrations compared to patient controlled intravenous morphine for post total knee arthroplasty analgesia and knee rehabilitation. Anaesthesia and Intensive Care 2006;34(1):25‐30. [PUBMED: 16494145 ] [DOI] [PubMed] [Google Scholar]
- Shum CF, Lo NN, Yeo SJ, Yang KY, Chong HC, Yeo SN. Continuous femoral nerve block in total knee arthroplasty: immediate and two‐year outcomes. Journal of Arthroplasty 2009;24(2):204‐9. [PUBMED: 18534496] [DOI] [PubMed] [Google Scholar]
Serpell 2001 {published data only}
- Serpell MG, Millar FA, Thomson MF. Comparison of lumbar plexus block versus conventional opioid analgesia after total knee replacement. Anaesthesia 1991;46(4):275‐7. [PUBMED: 2024744] [DOI] [PubMed] [Google Scholar]
Singelyn 1998 {published data only}
- Singelyn FJ, Deyaert M, Joris D, Pendeville E, Gouverneur JM. Effects of intravenous patient‐controlled analgesia with morphine, continuous epidural analgesia, and continuous three‐in‐one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesthesia and Analgesia 1998;87(1):88‐92. [PUBMED: 9661552] [DOI] [PubMed] [Google Scholar]
Sundarathiti 2009 {published data only}
- Sundarathiti P, Ruananukul N, Channum T, Kitkunasathean C, Mantay A, Thammasakulsiri J, et al. A comparison of continuous femoral nerve block (CFNB) and continuous epidural infusion (CEI) in postoperative analgesia and knee rehabilitation after total knee arthroplasty (TKA). Journal of the Medical Association of Thailand 2009;92(3):328‐34. [PUBMED: 19301724 ] [PubMed] [Google Scholar]
Szczukowski 2004 {published data only}
- Szczukowski MJ Jr, Hines JA, Snell JA, Sisca TS. Femoral nerve block for total knee arthroplasty patients: a method to control postoperative pain. Journal of Arthroplasty 2004;19(6):720‐5. [PUBMED: 15343531] [DOI] [PubMed] [Google Scholar]
Tang 2010 {published and unpublished data}
- Tang S, Xu ZH, Huang YG, He K, Ren LY, Qian WW, et al. Comparison of the influences of continuous femoral nerve block and patient controlled intravenous analgesia on total knee arthroplasty. [Chinese]. Acta Academiae Medicinae Sinicae 2010;32(5):574‐8. [PUBMED: 21050565] [DOI] [PubMed] [Google Scholar]
Toftdahl 2007 {published and unpublished data}
- Toftdahl K, Nikolajsen L, Haraldsted V, Madsen F, Tonnesen EK, Soballe K. Comparison of peri‐ and intraarticular analgesia with femoral nerve block after total knee arthroplasty: a randomized clinical trial. Acta Orthopaedica 2007;78(2):172‐9. [PUBMED: 17464603 ] [DOI] [PubMed] [Google Scholar]
Tugay 2006 {published and unpublished data}
- Tugay N, Saricaoglu F, Satilmis T, Alpar U, Akarcali I, Citaker S, et al. Single‐injection femoral nerve block. Effects on the independence level in functional activities in the early postoperative period in patients with total knee arthroplasty. Neurosciences 2006;11(3):175‐9. [PUBMED: 22266616 ] [PubMed] [Google Scholar]
Wang 2002 {published data only}
- Wang H, Boctor B, Verner J. The effect of single‐injection femoral nerve block on rehabilitation and length of hospital stay after total knee replacement. Regional Anesthesia and Pain Medicine 2002;27(2):139‐44. [PUBMED: 11915059 ] [DOI] [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang H‐J, Zhang D‐Z, Li S‐Z. Comparing the analgesic efficacy of continuous femoral nerve blockade and continuous intravenous analgesia after total knee arthroplasty. [Chinese]. Chung Hua I Hsueh Tsa Chih [Chinese Medical Journal] 2010;90(33):2360‐2. [PUBMED: 21092500] [PubMed] [Google Scholar]
Widmer 2012 {published data only}
- Widmer BJ, Scholes CJ, Pattullo GG, Oussedik SI, Parker DA, Coolican MRJ. Is femoral nerve block necessary during total knee arthroplasty? A randomized controlled trial. Journal of Arthroplasty 2012;27:1800‐5. [PUBMED: 22658231] [DOI] [PubMed] [Google Scholar]
Xie 2012 {published data only}
- Xie Z, Hussain W, Cutter TW, Apfelbaum JL, Drum ML, Manning DW. Three‐in‐one nerve block with different concentrations of bupivacaine in total knee arthroplasty. Randomized, placebo‐controlled, double‐blind trial. Journal of Arthroplasty 2012;27(5):673‐8.e1. [PUBMED: 21945081] [DOI] [PubMed] [Google Scholar]
Yu 2010 {published data only}
- Yu H‐P, Liu Z‐H, Guo W‐S, Jiang H‐Y, Zhao J. Effect of continuous femoral nerve block in analgesia and the early rehabilitation after total knee replacement. [Chinese]. Zhongguo Gushang [China Journal of Orthopaedics and Traumatology] 2010;23(11):825‐7. [PUBMED: 21254673] [PubMed] [Google Scholar]
Zaric 2006 {published and unpublished data}
- Zaric D, Boysen K, Christiansen C, Christiansen J, Stephensen S, Christensen B. A comparison of epidural analgesia with combined continuous femoral‐sciatic nerve blocks after total knee replacement. Anesthesia and Analgesia 2006;102(4):1240‐6. [PUBMED: 16551931] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Affas 2012 {published data only}
- Affas F, Stiller CO, Nygards EB, Stephanson N, Wretenberg P, Olofsson C. A randomized study comparing plasma concentration of ropivacaine after local infiltration analgesia and femoral block in primary total knee arthroplasty. Scandinavian Journal of Pain 2012;3(1):46‐51. [DOI: 10.1016/j.sjpain.2011.09.001] [DOI] [PubMed] [Google Scholar]
Bagry 2008 {published data only}
- Bagry H, Cuadra Fontaine JC, Asenjo JF, Bracco D, Carli F. Effect of a continuous peripheral nerve block on the inflammatory response in knee arthroplasty. Regional Anesthesia and Pain Medicine 2008;33(1):17‐23. [PUBMED: 18155052] [DOI] [PubMed] [Google Scholar]
Blisard 2012 {published data only}
- Blisard R, Haddad FS. Pre‐emptive peripheral nerve block delays recovery after total knee arthroplasty. Orthopedics Today 2012; Vol. 32, issue 2:31‐2.
Cappelleri 2011 {published data only}
- Cappelleri G, Ghisi D, Fanelli A, Albertin A, Somalvico F, Aldegheri G. Does continuous sciatic nerve block improve postoperative analgesia and early rehabilitation after total knee arthroplasty? A prospective, randomized, double‐blinded study. Regional Anesthesia and Pain Medicine 2011;36(5):489‐92. [PUBMED: 21857276] [DOI] [PubMed] [Google Scholar]
Carvalho 2012 {published data only}
- Carvalho R, Braganca JP, Calixto L. Effect of a single shot sciatic nerve block combined with a continuous femoral block on pain scores after knee arthroplasty. A randomized controlled trial. British Journal of Anaesthesia 2012;108:23‐4. [DOI: 10.4236/ojanes.2012.24025] [DOI] [Google Scholar]
Chelly 2001 {published data only}
- Chelly JE, Greger J, Gebhard R, Coupe K, Clyburn TA, Buckle R, et al. Continuous femoral blocks improve recovery and outcome of patients undergoing total knee arthroplasty. Journal of Arthroplasty 2001;16(4):436‐45. [PUBMED: 11402405]] [DOI] [PubMed] [Google Scholar]
Combes 2000 {published data only}
- Combes X, Cerf C, Bouleau D, Duvaldestin P, Dhonneur G. The effects of residual pain on oxygenation and breathing pattern during morphine analgesia. Anesthesia and Analgesia 2000;90(1):156‐60. [PUBMED: 10624997] [DOI] [PubMed] [Google Scholar]
Dang 2005 {published data only}
- Pham Dang C, Gautheron E, Guilley J, Fernandez M, Waast D, Volteau C, et al. The value of adding sciatic block to continuous femoral block for analgesia after total knee replacement. Regional Anesthesia and Pain Medicine 2005;30(2):128‐33. [PUBMED: 15765454] [DOI] [PubMed] [Google Scholar]
Edwards 1992 {published data only}
- Edwards ND, Wright EM. Continuous low‐dose 3‐in‐1 nerve blockade for postoperative pain relief after total knee replacement. Anesthesia and Analgesia 1992;75(2):265‐7. [PUBMED: 1632541] [DOI] [PubMed] [Google Scholar]
Frassanito 2009 {published data only}
- Frassanito L, Vergari A, Messina A, Pitoni S, Puglisi C, Chierichini A. Anaesthesia for total knee arthroplasty: efficacy of single‐injection or continuous lumbar plexus associated with sciatic nerve blocks—a randomized controlled study. European Review for Medical and Pharmacological Sciences 2009;13(5):375‐82. [MEDLINE: ] [PubMed] [Google Scholar]
Frassanito 2010 {published data only}
- Frassanito L, Vergari A, Zanghi F, Messina A, Bitondo M, Antonelli M. Post‐operative analgesia following total knee arthroplasty: comparison of low‐dose intrathecal morphine and single‐shot ultrasound‐guided femoral nerve block: a randomized, single blinded, controlled study. European Review for Medical & Pharmacological Sciences 2010;14(7):589‐96. [PUBMED: 20707248] [PubMed] [Google Scholar]
Hakkalamani 2008 {published data only}
- Hakkalamani S, Carroll FA, Ford C, Mereddy P, Jefferies G, Parkinson RW. Analgesia in total knee replacement: a comparison between femoral versus combined femoral and sciatic nerve block. Journal of Bone and Joint Surgery (Proceedings) 2008;90b (supp):328‐E0A. [Google Scholar]
Ilfeld 2008 {published data only}
- Ilfeld BM, Le LT, Meyer RS, Mariano ER, Vandenborne K, Duncan PW, et al. Ambulatory continuous femoral nerve blocks decrease time to discharge readiness after tricompartment total knee arthroplasty: a randomized, triple‐masked, placebo‐controlled study. Anesthesiology 2008;108(4):703‐13. [PUBMED: 18362603] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ilfeld 2009 {published data only}
- Ilfeld BM, Meyer RS, Le LT, Mariano ER, Williams BA, Vandenborne K, et al. Health‐related quality of life after tricompartment knee arthroplasty with and without an extended‐duration continuous femoral nerve block: a prospective, 1‐year follow‐up of a randomized, triple‐masked, placebo‐controlled study. Anesthesia and Analgesia 2009;108(4):1320‐5. [PUBMED: 19299806] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ilfeld 2010 {published data only}
- Ilfeld BM, Mariano ER, Girard PJ, Loland VJ, Meyer RS, Donovan JF, et al. A multicenter, randomized, triple‐masked, placebo‐controlled trial of the effect of ambulatory continuous femoral nerve blocks on discharge‐readiness following total knee arthroplasty in patients on general orthopaedic wards. Pain 2010;150(3):477‐84. [PUBMED: 20573448] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2011 {published data only}
- Johnson CB, Steele‐Moses SK. The use of continuous femoral nerve blocks versus extended release epidural morphine: a study comparing outcomes in total knee arthroplasty procedures. Orthopedic Nursing 2011;30(1):44‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Koh 2011 {published data only}
- Koh IJ, Kang YG, Chang CB, Do SH, Seong SC, Kim TK. Does periarticular injection have additional pain relieving effects during contemporary multimodal pain control protocols for TKA? A randomised, controlled study. Knee 2012;19(4):253‐9. [PUBMED: 21507661] [DOI] [PubMed] [Google Scholar]
McMeniman 2010 {published data only}
- McMeniman TJ, McMeniman PJ, Myers PT, Hayes DA, Cavdarski A, Wong M, et al. Femoral nerve block vs fascia iliaca block for total knee arthroplasty postoperative pain control: a prospective, randomized controlled trial. Journal of Arthroplasty 2010;25(8):1246‐9. [PUBMED: 20178889] [DOI] [PubMed] [Google Scholar]
Meftah 2012 {published data only}
- Meftah M, Wong AC, Nawabi DH, Yun RJ, Ranawat AS, Ranawat CS. Pain management after total knee arthroplasty using a multimodal approach. Orthopedics 2012;35(5):e660‐4. [PUBMED: 22588407] [DOI] [PubMed] [Google Scholar]
Morin 2005 {published data only}
- Morin AM, Kratz CD, Eberhart LHJ, Dinges G, Heider E, Schwarz N, et al. Postoperative analgesia and functional recovery after total‐knee replacement: comparison of a continuous posterior lumbar plexus (psoas compartment) block, a continuous femoral nerve block, and the combination of a continuous femoral and sciatic nerve block. Regional Anesthesia and Pain Medicine 2005;30(5):434‐45. [PUBMED: 16135347] [DOI] [PubMed] [Google Scholar]
Ng 2012b {published data only}
- Ng F‐Y, Chiu K‐Y, Yan CH, Ng K‐FJ. Continuous femoral nerve block versus patient‐controlled analgesia following total knee arthroplasty. Journal of Orthopaedic Surgery 2012;20:23‐6. [PUBMED: 22535806] [DOI] [PubMed] [Google Scholar]
Niskanen 2005 {published data only}
- Niskanen RO, Strandberg N. Bedside femoral block performed on the first postoperative day after unilateral total knee arthroplasty: a randomized study of 49 patients. The Journal of Knee Surgery 2005;18(3):192‐6. [PUBMED: 16152867] [DOI] [PubMed] [Google Scholar]
Rais 2009 {published data only}
- Rais K, Ben Said A, Soussi M, Chahed S, Kaabachi O. Multimodal regional and systemic approach for postoperative analgesia in total knee arthroplasty. European Journal of Anaesthesiology 2009;26:123. [Google Scholar]
Rajeev 2007 {published data only}
- Rajeev S, Batra YK, Panda NB, Kumar M, Nagi ON. Combined continuous "3‐in‐1" and sciatic nerve blocks provide improved postoperative analgesia with no correlation to catheter tip location after unilateral total knee arthroplasty. Journal of Arthroplasty 2007;22(8):1181‐6. [PUBMED: 18078888] [DOI] [PubMed] [Google Scholar]
Rasiah 2012 {published data only}
- Rasiah R, Azhar AA, Thevanthiran MN. Single injection of femoral nerve block for bilateral total knee arthroplasty for post‐operative pain management. British Journal of Anaesthesia 2012;108:ii411. [Google Scholar]
Safa 2011 {published data only}
- Safa B, Haslam L, Gollish J, McCartney C. A prospective, randomized trial, comparing analgesic efficacy and postoperative functional recovery of either single shot sciatic nerve block or posterior capsule infiltration combined with femoral block for total knee arthroplasty. Regional Anesthesia and Pain Medicine 2011;36:508. [Google Scholar]
Serrano 2011 {published data only}
- Serrano A, Santiveri X, Bisbe E, Ortiz P, Puig L, Castillo J. Analgesic efficacy of associating a sciatic block to a femoral block in the postoperative period of total knee arthroplasty. European Journal of Anaesthesiology 2011;28:119‐20. [Google Scholar]
Sites 2004 {published data only}
- Sites BD, Beach M, Gallagher JD, Jarrett RA, Sparks MB, Lundberg CJF. A single injection ultrasound‐assisted femoral nerve block provides side effect‐sparing analgesia when compared with intrathecal morphine in patients undergoing total knee arthroplasty. Anesthesia and Analgesia 2004;99(5):1539‐43; table of contents. [PUBMED: 15502061] [DOI] [PubMed] [Google Scholar]
Taninishi 2011 {published data only}
- Taninishi H, Sato K, Morita K. Effects of anterior sciatic nerve block on intraoperative hemodynamics and pain relief in the postanesthesia care unit for patients undergoing total knee arthroplasty. Regional Anesthesia and Pain Medicine 2011;36(5):E263. [Google Scholar]
Tarkkila 1998 {published data only}
- Tarkkila P, Tuominen M, Huhtala J, Lindgren L. Comparison of intrathecal morphine and continuous femoral 3‐in‐1 block for pain after major knee surgery under spinal anaesthesia. European Journal of Anaesthesiology 1998;15(1):6‐9. [PUBMED: 9522133] [DOI] [PubMed] [Google Scholar]
Tricarico 2009 {published data only}
- Tricarico E, Tomasino S, D'Orlando L. Epidural analgesia compared with peripheral nerve blockade after major knee surgery. Critical Care 2009;13:S160. [Google Scholar]
Watson 2005 {published data only}
- Watson MW, Mitra D, McLintock TC, Grant SA. Continuous versus single‐injection lumbar plexus blocks: comparison of the effects on morphine use and early recovery after total knee arthroplasty. Regional Anesthesia & Pain Medicine 2005;30(6):541‐7. [PUBMED: 16326339] [DOI] [PubMed] [Google Scholar]
Wegener 2011 {published data only}
- Wegener JT, Ooij B, Dijk CN, Hollmann MW, Preckel B, Stevens MF. Value of single‐injection or continuous sciatic nerve block in addition to a continuous femoral nerve block in patients undergoing total knee arthroplasty: a prospective, randomized, controlled trial. Regional Anesthesia & Pain Medicine 2011;36(5):481‐8. [PUBMED: 21857273] [DOI] [PubMed] [Google Scholar]
Weston‐Simons 2012 {published data only}
- Weston‐Simons JS, Pandit H, Haliker V, Dodd CA, Popat MT, Murray DW. Intra‐articular local anaesthetic on the day after surgery improves pain and patient satisfaction after unicompartmental knee replacement: a randomised controlled trial. Knee 2012;19:352‐5. [PUBMED: 21669534] [DOI] [PubMed] [Google Scholar]
Zhang 2010 {published data only}
- Zhang HH, Yan SH, Li X, Jin Y, Liu ZC. Analgesia following artificial joint replacement joint replacement: nerve block based on gait analysis. Journal of Clinical Rehabilitative Tissue Engineering Research 2010;14(22):4018‐22. [Google Scholar]
Ziwenga 2010 {published data only}
- Ziwenga O, Tsui B, Sriramatr D. Comparison of quadriceps weakness following total knee arthroplasty using analgesia by femoral nerve block: continuous vs patient controlled techniques. Regional Anesthesia and Pain Medicine 2010;35(5):E194. [Google Scholar]
References to studies awaiting assessment
Grider 2011 {published data only}
- Grider JS, Harned ME, Paul S, Mauro G. Comparison of single shot versus continuous femoral local anesthetic nerve block in patients undergoing knee arthroplasty. Regional Anesthesia and Pain Medicine 2011;36(5):508‐20. [Google Scholar]
Mullen 2008 {published data only}
- Mullen M, Rooney BP, Kelly MP, Storey N. 24 hour infusion femoral and sciatic nerve block following total knee arthroplasty. Scottish Medical Journal 2008;2:59. [Google Scholar]
Tobin 2011 {published data only}
- Tobin R, Singh MK, Sharma P, Girotra G, Arora D, Panigrahi B. Ultrasound guided in‐plane continuous femoral nerve block for postoperative analgesia & early mobilisation in patients undergoing unilateral total knee arthroplasty—an Indian experience. Regional Anesthesia and Pain Medicine 2011;36(5):E263. [Google Scholar]
Yuksel 2011 {published data only}
- Yuksel BE, Doger C, Erdogan N, Ornek D, Kadiogullari N. Continuous epidural analgesia versus combination of single dose sciatic nerve block and continuous femoral analgesia in total knee arthroplasty. Regional Anesthesia and Pain Medicine 2011;36(5):E183‐E4. [Google Scholar]
Additional references
Allman 2006
- Allman KG, Wilson IH (editors). Oxford Handbook of Anesthesia. 2nd edition, Chapter 39. New York: Oxford University Press, 2006. [Google Scholar]
American Society of Anesthesiologists 2004
- No authors listed. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on acute pain management. Anesthesiology 2004;100(6):1573‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ballantyne 2010
- Ballantyne JC, Rathmell JP, Fishman SM (editors). Bonica's Management of Pain. 4th Edition. Philadelphia: Lippincott, Williams & Wilkins, 2010. [Google Scholar]
Ben‐David 2004
- Ben‐David B, Schmalenberger K, Chelly JE. Analgesia after total knee arthroplasty: is continuous sciatic blockade needed in addition to continuous femoral blockade?. Anesthesia and Analgesia 2004;98(3):747‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bender 2001
- Bender R. Calculating confidence intervals for the number need to treat. Controlled Clinical Trials 2001;22:102‐10. [DOI] [PubMed] [Google Scholar]
Berdine 2006
- Berdine HJ, Nesbit SA. Equianalgesic dosing of opioids. Journal of Pain and Palliative Care Pharmacotherapy 2006;20(4):79‐84. [PUBMED: 17182514] [PubMed] [Google Scholar]
Bergeron 2009
- Bergeron SG, Kardash KJ, Huk OL, Zukor DJ, Antoniou J. Functional outcome of femoral versus obturator nerve block after total knee arthroplasty. Clinical Orthopaedics and Related Research 2009;467(6):1458‐62. [PUBMED: 19224305] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borenstein 2009
- Borenstein M. Effect sizes for continuous data. In: Cooper HM, Hedges LV, Valentine JC editor(s). The Handbook of Research Synthesis and Meta‐analysis. Second Edition. New York:: Russell Sage Foundation, 2009:225‐7. [Google Scholar]
Capdevila 1999
- Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d'Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology 1999;91(1):8‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carr 1999
- Carr DB, Goudas LC. Acute pain. Lancet 1999;353(9169):2051‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Choi 2003
- Choi PT, Bhandari M, Scott J, Douketis J. Epidural analgesia for pain relief following hip or knee replacement. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003071] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Second Edition. Hillsdale, NJ: Lawrence Erlbaum Associates, 1988. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Enneking 2009
- Enneking FK, Wedel DJ, Horlocker TT. Chapter 14. The lower extremity: somatic blockade. In: Cousins MJ, Carr DB, Horlocker TT, Bridenbaugh PO editor(s). Cousins and Bridenbaugh's Neural Blockade in Clinical Anesthesia and Pain Medicine. Fourth. Philadelphia: Lippincott Williams & Wilkins, 2009:349‐50. [Google Scholar]
Feibel 2009
- Feibel RJ, Dervin GF, Kim PR, Beaule PE. Major complications associated with femoral nerve catheters for knee arthroplasty: a word of caution. The Journal of Arthroplasty 2009;24:132‐7. [PUBMED: 19553071] [DOI] [PubMed] [Google Scholar]
Fischer 2008
- Fischer HBJ, Simanski CJP, Sharp C, Bonnet F, Camu F, Neugebauer EAM, et al. A procedure‐specific systematic review and consensus recommendations for postoperative analgesia following total knee arthroplasty. Anaesthesia 2008;63(10):1105‐23. [PUBMED: 18627367] [DOI] [PubMed] [Google Scholar]
Fowler 2008
- Fowler SJ, Symons J, Sabato S, Myles PS. Epidural analgesia compared with peripheral nerve blockade after major knee surgery: a systematic review and meta‐analysis of randomized trials. British Journal of Anaesthesia 2008;100(2):154‐64. [PUBMED: 18211990] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336:995‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319:670–4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005; Vol. 5, issue 13. [PUBMED: 15840177 ] [DOI] [PMC free article] [PubMed]
Johnson 2013
- Johnson RL, Kopp SL, Hebl JR, Erwin PJ, Mantilla CB. Falls and major orthopaedic surgery with peripheral nerve blockade: a systematic review and meta‐analysis. British Journal of Anaesthesia 2013;110(4):518‐28. [23440367 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kandasami 2009
- Kandasami M, Kinninmonth AW, Sarungi M, Baines J, Scott NB. Femoral nerve block for total knee replacement: a word of caution. Knee 2009;16:98‐100. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kurtz 2007
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. Journal of Bone and Joint Surgery 2007;89:780‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lang 1993
- Lang SA, Yip RW, Chang PC, Gerard MA. The femoral 3‐in‐1 block revisited. Journal of Clinical Anesthesia 1993;5(4):292‐6. [PUBMED: 8373606] [DOI] [PubMed] [Google Scholar]
March 2004
- March LM, Bagga H. Epidemiology of osteoarthritis in Australia. Medical Journal of Australia 2004;180:S6‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McNamee 2002
- McNamee DA, Parks L, Milligan KR. Post‐operative analgesia following total knee replacement: an evaluation of the addition of an obturator nerve block to combined femoral and sciatic nerve block. Acta Anaesthesiologica Scandinavica 2002;46(1):95‐9. [PUBMED: 11903080] [DOI] [PubMed] [Google Scholar]
Moore 2011
- Moore RA, Mhuircheartaigh RJ, Derry S, McQuay HJ. Mean analgesic consumption is inappropriate for testing analgesic efficacy in post‐operative pain: analysis and alternative suggestion. European Journal of Anaesthesiology 2011;28(6):427‐32. [PUBMED: 21445017] [DOI] [PubMed] [Google Scholar]
Murphy 2008
- Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis and Rheumatism 2008;59(9):1207‐13. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nuesch 2010
- Nuesch E, Trelle S, Reichenbach S, Rutjes A, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. British Medical Journal 2010;341:c3515. [PUBMED: 20639294] [DOI] [PMC free article] [PubMed] [Google Scholar]
Orwin 1983
- Orwin RG. A fail‐safe N for effect size in meta‐analysis. Journal of Educational Statistics 1983;8(2):157‐9. [Google Scholar]
PaPaS document 2011
- Cochrane Pain, Palliative and Supportive Care Review Group. Authoring or Assessing a Cochrane Protocol, Review, or Review Update for the PaPaS Review Group. Version 1.0 [2011]. The Cochrane Collaboration, 2011. http://papas.cochrane.org/sites/papas.cochrane.org/files/uploads/L%20‐%20PaPaSAuthor%26RefereeGuidance.pdf. Accessed 29 Mar 2013.
Paul 2010
- Paul JE, Arya A, Hurlburt L, Cheng J, Thabane L, Tidy A, et al. Femoral nerve block improves analgesia outcomes after total knee arthroplasty: a meta‐analysis of randomized controlled trials. Anesthesiology 2010;113(5):1144‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 5.2 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Richman 2006
- Richman JM, Liu SS, Courpas G, Wong R, Rowlingson AJ, McGready J, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta‐analysis. Anesthesia and Analgesia 2006;102:248‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sharma 2010
- Sharma S, Iorio R, Specht LM, Davies‐Lepie S, Healy WL. Complications of femoral nerve block for total knee arthroplasty. Clinical Orthopaedics Related Research 2010;468:135‐40. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shum 2009
- Shum CF, Lo NN, Yeo SJ, Yang KY, Chong HC, Yeo SN. Continuous femoral nerve block in total knee arthroplasty: immediate and two‐year outcomes. Journal of Arthroplasty 2009;24(2):204‐9. [PUBMED: 18534496] [DOI] [PubMed] [Google Scholar]
Sinatra 2009
- Sinatra RS, Leon‐Casasola OA, Ginsberg B, Viscuri ER (editors). Acute Pain Management. Cambridge, MA: Cambridge University Press, 2009. [Google Scholar]
Todkar 2005
- Todkar M. Sciatic nerve block causing heel ulcer after total knee replacement in 36 patients. Acta Orthopaedica Belgica 2005;71(6):724‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Twersky 1997
- Twersky R, Fishman D, Homel P. What happens after discharge? Return hospital visits after ambulatory surgery. Anesthesia and Analgesia 1997;84(2):319‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vendittoli 2006
- Vendittoli P‐A, Makinen P, Drolet P, Lavigne M, Fallaha M, Guertin M‐C, et al. A multimodal analgesia protocol for total knee arthroplasty. A randomized, controlled study. Journal of Bone and Joint Surgery 2006;88(2):282‐9. [PUBMED: 16452738] [DOI] [PubMed] [Google Scholar]
Wilson 1927
- Wilson EB. Probably inference, the law of succession, and statistical inference. Journal of American Statistical Association 1927;22:209‐12. [Google Scholar]
Winnie 1973
- Winnie AP, Ramamurthy S, Durrani Z. The inguinal paravascular technic of lumbar plexus anesthesia: the "3‐in‐1 block". Anesthesia and Analgesia 1973;52:989‐96. [MEDLINE: ] [PubMed] [Google Scholar]
References to other published versions of this review
Chan 2012
- Chan E‐Y, Fransen M, Parker DA, Assam PN, Chua N. Femoral nerve blocks for acute postoperative pain after knee replacement surgery. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD009941] [DOI] [PMC free article] [PubMed] [Google Scholar]


