Abstract
Background
Pelvic organ prolapse is common, with some degree of prolapse seen in up to 50% of parous women in a clinic setting, although many are asymptomatic. The use of pessaries (a passive mechanical device designed to support the vagina) to treat prolapse is very common, and up to 77% of clinicians use pessaries for the first line management of prolapse. A number of symptoms may be associated with prolapse and treatments include surgery, pessaries and conservative therapies. A variety of pessaries are described which aim to alleviate the symptoms of prolapse and avert or delay the need for surgery.
Objectives
To determine the effectiveness of pessaries (mechanical devices) for pelvic organ prolapse.
Search methods
We searched the Cochrane Incontinence Group Specialised Register of trials (searched 13 March 2012), which includes searches of CENTRAL, MEDLINE, PREMEDLINE and handsearching of conference proceedings, and handsearched the abstracts of two relevant conferences held in 2011. We also searched the reference lists of relevant articles.
Selection criteria
Randomised and quasi‐randomised controlled trials which included a pessary for pelvic organ prolapse in one arm of the study.
Data collection and analysis
Abstracts were assessed independently by two authors with arbitration from a third if necessary. Data extraction was completed independently for included studies by two review authors.
Main results
To date there is only one published randomised controlled trial assessing the use of pessaries in the treatment of pelvic organ prolapse.
Authors' conclusions
The review authors identified one randomised controlled trial comparing ring and Gellhorn pessaries. The results of the trial showed that both pessaries were effective for the approximately 60% of women who completed the study with no significant differences identified between the two types of pessary. However, methodological flaws were noted in the trial, as elaborated under risk of bias assessment. There is no consensus on the use of different types of device, the indications nor the pattern of replacement and follow‐up care. There is an urgent need for randomised studies to address the use of pessaries in comparison with no treatment, surgery and conservative measures.
Plain language summary
Pessaries (mechanical devices) for managing pelvic organ prolapse in women
Pelvic organs, such as the uterus, bladder or bowel, may protrude into the vagina because of weakness in the tissues that normally support them. The symptoms that they cause vary depending on the type of prolapse. Pessaries (mechanical devices such as latex or silicone pessaries) can be used to try to restore the prolapsed organs to their normal position and hence to relieve symptoms. They are commonly used when conservative treatment, like physiotherapy, and surgery have either failed or are not suitable. The review found one randomised trial which compared two types of pessary, the ring pessary and the Gellhorn pessary. Both pessaries worked for the 60% of women who completed the study and there were no differences between the two types of pessary.
Summary of findings
Summary of findings for the main comparison. Pessaries (mechanical devices) for pelvic organ prolapse in women.
| Mechanical devices for pelvic organ prolapse in women | ||||||
| Patient or population: patients with pelvic organ prolapse in women Settings: Intervention: mechanical devices | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Mechanical devices | |||||
| Patients perceived improvement in symptoms of prolapse assessed using validated symptom questionnaire at 1 year ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Acceptability/satisfaction with treatment at 1 year ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Grade of prolapse with device in situ at 1 year ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Pelvic organ prolapse is common and is seen in up to 50% of parous women in a clinic setting (Swift 2000). In the general population, an estimated 30% of women will have signs of prolapse although the majority are asymptomatic (Samuelsson 1999). MacLennan found in telephone interviews that only 8.8% of women in the general population are symptomatic (MacLennan 2000). Pelvic organ prolapse includes anterior vaginal wall prolapse (cystocoele, urethrocoele), posterior vaginal wall prolapse (enterocoele, rectocoele, perineal deficiency) and uterine or vaginal vault prolapse. A woman can present with prolapse of one or more of these sites. The International Continence Society has standardised the nomenclature using the POP‐Q evaluation (Bump 1996), but in this document we have also used the descriptive terms above as these are compatible with searches of the literature.
The aetiology of pelvic organ prolapse is complex and multi‐factorial. Risk factors include pregnancy, childbirth, congenital or acquired connective tissue abnormalities, denervation or weakness of the pelvic floor, ageing, menopause and factors associated with chronically raised intra‐abdominal pressure such as obesity, cough and heavy lifting (Bump 1998; MacLennan 2000; Dietz 2008).
Women with prolapse may have a variety of pelvic floor symptoms (Hagen 2009; Lone 2011). Only some of the symptoms are directly related to the prolapse, including pelvic heaviness, a dragging sensation in the vagina, a bulge, lump or protrusion coming down from the vagina and backache. Symptoms of bladder, bowel or sexual dysfunction are frequently present. These symptoms may be directly related to the prolapsed organ, for example a poor urinary stream when a cystocoele is present or obstructed defecation when a rectocoele is present. They may also be independent of the prolapse, for example symptoms of detrusor overactivity when a cystocoele is present. Symptoms may negatively affect body image, quality of life and a woman's ability to perform day to day activities (Lowder 2011).
Description of the intervention
Prolapse treatment may be dependent on a number of factors including the severity of prolapse, the bothersomeness of the associated symptoms, the woman's general health and the woman's treatment preference (Kapoor 2009; Basu 2011). Options available for treatment are conservative (for example pelvic floor muscle training), mechanical support (such as vaginal pessaries), oestrogens and surgery. Previously, conservative or pessary treatment was only considered for women with a mild degree of prolapse, for those who wished to have more children, and the frail or those unwilling to undergo surgery. However, 87% to 98% of clinicians report using pessaries in their clinical practice (Cundiff 2000; Pott‐Grinstein 2001; Gorti 2009) and 77% of gynaecologists report using pessaries as first line treatment for prolapse (Cundiff 2000).
This is a review of treatment with pessaries (mechanical devices) for prolapse. We will use the term 'pessary' throughout the review, with a pessary been defined as a passive mechanical device designed to support the vagina. Other Cochrane reviews assess the effectiveness of surgical, conservative and oestrogen based treatments for prolapse (Ismail 2010; Maher 2010; Hagen 2011b) and mechanical devices for urinary incontinence (Lipp 2011).
An extensive range of pessaries have been described for the treatment of prolapse (Poma 2000; Oliver 2011). These consist mainly of latex or silicone pessaries which are shaped devices that are inserted into and left in the vagina to support the prolapsed pelvic organs. Two main groups of pessaries are used, support pessaries and space filling pessaries (Oliver 2011). Study findings suggest that ring pessaries are the type most commonly used in practice (Cundiff 2000) but these may not be effective for all types of prolapse.
How the intervention might work
Pessaries are used in pelvic organ prolapse in order to physically support the vaginal walls and the pelvic organs behind them. The pessary is inserted into the vagina with a view to holding the prolapsed organs inside the vagina, supporting the pelvic structures, and relieving pressure on the bladder and bowel (Hay‐Smith 2009). The aims of using a pessary in the management of pelvic organ prolapse include to:
prevent the prolapse becoming worse;
help decrease the frequency or severity of symptoms of prolapse;
avert or delay the need for surgery (Oliver 2011).
Variable patterns of follow‐up care are reported (Gorti 2009), however pessaries do need to be removed regularly and the vaginal mucosa checked for erosions. Some patients will be able to remove and replace the pessary themselves, which may lengthen the intervals between gynaecological examinations, while others will return to the clinic for removal and replacement. Descriptive data suggest that local oestrogens may be beneficial in successful pessary fitting or in maintenance of treatment with pessaries (Hanson 2006) but more evidence is needed about ongoing pessary management. Pessaries are cheap and complications are reported to be rare (Hanson 2006). The majority of evidence for the use of pessaries comes from level II and III studies (Clemons 2004; Clemons 2 2004; Clemons 3 2004; Hanson 2006; Kapoor 2009; Lone 2011; Manchana 2012) thus the efficacy of pessary use in the management of prolapse still requires to be clearly established.
Why it is important to do this review
The wide variety of treatments available for prolapse indicates the lack of consensus as to the optimal treatment. Provided that sufficient numbers of trials of adequate quality have been conducted, the most reliable evidence is likely to come from the consideration of randomised controlled trials, and this is the basis for the present review. The aim is to help identify optimal practice and highlight where there is a need for further research.
Objectives
To determine the effectiveness of pessaries (mechanical devices) for pelvic organ prolapse in women. The following comparisons were considered:
1. any mechanical device versus control, waiting list or no active treatment;
2. any mechanical device versus another treatment (lifestyle interventions, oestrogen treatment, physical interventions such as pelvic floor muscle training, surgery); 3. any mechanical device plus another treatment versus the other treatment alone;
4. any mechanical device plus another treatment versus the mechanical device alone;
5. one mechanical device versus another mechanical device; 6. differing frequencies of device review or device change.
Other treatments could include: lifestyle interventions; oestrogen treatment; physical interventions such as pelvic floor muscle training; surgery.
This review was designed to assess the effects of mechanical devices which support prolapse. It did not assess devices designed to improve pelvic floor muscle tone.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials in which at least one arm was a pessary (mechanical device intervention) for pelvic organ prolapse.
Types of participants
Adult women seeking treatment for symptomatic pelvic organ prolapse.
Pelvic organ prolapse included:
anterior vaginal wall prolapse (cystocoele, urethrocoele);
posterior vaginal wall prolapse (enterocoele, rectocoele, perineal deficiency);
uterine or vaginal vault prolapse.
Types of interventions
One arm of a trial involved allocation to a pessary (mechanical device) for prolapse. Comparison or concomitant interventions included no treatment, lifestyle interventions, oestrogen treatment, physical interventions such as pelvic floor muscle training, or surgery.
The types of devices included:
1. support pessaries for unspecified prolapse including ring, ring with support, Regula, Shaatz, Gellhorn, Gehrung, Hodge, Shelf, Falk, and others; 2. space filling devices including donut, cube, inflatable ball, and others.
Types of outcome measures
Primary outcomes
Women's perceived improvement in symptoms of prolapse (e.g. assessed using validated symptom questionnaires)
Secondary outcomes
Subjective
Acceptability or satisfaction with treatment
Objective
Grade of prolapse with device in situ
Site‐specific grading of prolapse using Baden Walker or Pelvic Organ Prolapse‐Quantification (POP‐Q) classification (Bump 1996)
Quality of life
Prolapse‐specific quality of life questionnaire (e.g. P‐QOL) (Bump 1996)
Generic quality of life or health status measures (e.g. SF 36) (Ware 1993)
Psychological outcome measures (e.g. HADS) (Zigmond 1983)
Measures (objective or subjective) of associated symptoms
Bladder problems, including urinary incontinence, occult urinary incontinence, and relief of voiding difficulty
Bowel problems, including relief of obstructed defecation
Sexual problems, including acceptability of device to both partners
Complications
Ulceration, bleeding, discharge, need for removal, fistula
Dislodgement, discomfort
Urinary tract or bowel obstruction
Incontinence, occult incontinence
Incarceration
Carcinoma
Need for and reasons for device removal
Economic outcomes
Costs of interventions
Resource implications of the effects of treatment
Measures of formal economic evaluations
Primary and secondary outcomes, as defined above, were classified by the review authors as 'critical', 'important' or 'not important' for decision making from the woman’s perspective. The GRADE working group strongly recommends including up to seven critical outcomes in a systematic review (Guyatt 2011a; Guyatt 2011b; Guyatt 2013a; Guyatt 2013b).
In this systematic review, GRADE methodology was adopted for assessing the quality of evidence for the following outcomes:
• woman's perceived improvement in prolapse symptoms assessed using validated symptom questionnaire at one year;
• acceptability of or satisfaction with treatment at one year;
• grade of prolapse with device in situ at one year.
Search methods for identification of studies
We did not impose any language or other restrictions on any of the searches.
Electronic searches
This review has drawn on the search strategy developed for the Cochrane Incontinence Review Group. Relevant trials were identified from the Incontinence Group Specialised Register of controlled trials, which is described under the Incontinence Group module in The Cochrane Library. The Register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, CINAHL, and handsearching of journals and conference proceedings. The date of the most recent search of the Specialised Register for this review was 13 March 2012. The trials in the Incontinence Group Specialised Register are also contained in CENTRAL. The terms used to search the Incontinence Group trials register are given below:
({design.cct*} OR (design.rct*}) AND {topic.prolapse*}) (All searches were of the keywords field of Reference Manager 12, Thomson Reuters).
The search methods and strategies used for the previous version of this review are given in Appendix 1.
Searching other resources
We handsearched the proceedings of two relevant conferences:
Proceedings of the 41st Annual Meeting of the International Continence Society (ICS), 2011, Aug 29 to Sept 2, Glasgow, Scotland;
Proceedings of the 36th Annual Meeting of the International Urogynecological Association (IUGA), 2011, Jun 28 to Jul 2, Lisbon, Portugal.
The reference lists of relevant articles were also searched for other possibly relevant trials.
Data collection and analysis
Data collection and analysis were conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Our search generated a list of abstracts.Two review authors (DG and CB) independently screened these abstracts. A third review author (FMR) was nominated to arbitrate in the event of disagreement. Studies which were not relevant were excluded at this stage. The full text articles of relevant studies identified were obtained. If there was any uncertainty on the eligibility of the studies based on title and abstract, the full paper was obtained and reviewed by the same two review authors. Studies formally considered for the review but excluded were listed with reasons given for their exclusion (see Characteristics of excluded studies). Our search is summarised in Figure 1.
1.
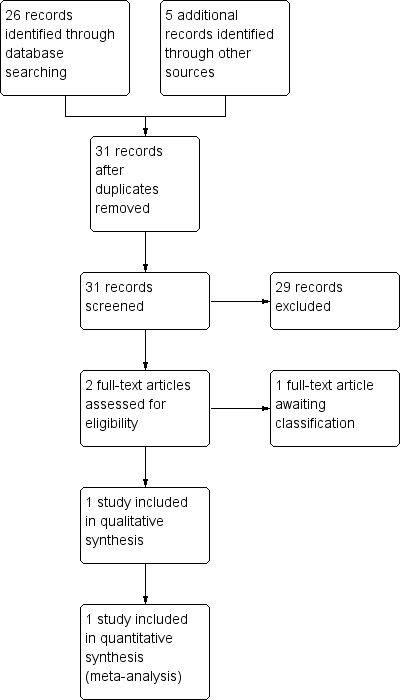
PRISMA study flow diagram (for the current version of the review).
Data extraction and management
Data extraction was undertaken independently by two review authors (FMR and DG) using a predefined data extraction form (Appendix 2) and comparisons made to ensure accuracy. Discrepancies were resolved by discussion with, or referral to, a third party (EA).
Assessment of risk of bias in included studies
The risk of bias within the study was assessed using the Cochrane Incontinence Group risk of bias form (Appendix 3).
Measures of treatment effect
The primary outcome was a woman‐reported outcome, the resolution of or improvement in the symptoms of prolapse (lump or bulge). This can be assessed by standardised, validated patient symptom questionnaires. Given there was only one trial included, the treatment effect from that trial is described below and no meta‐analysis of treatment effect was possible.
Unit of analysis issues
The primary analysis was per woman randomised.
Dealing with missing data
Missing data is a common problem within trials, which can bias the results. We have described missing outcomes (with reasons). We addressed the potential impact of the missing outcomes and we describe in the discussion section their impact on the findings of the review.
Assessment of heterogeneity
Heterogeneity was not assessed as there was only one trial.
Assessment of reporting biases
Using the risk of bias form, we assessed for data that should have been collected but were not reported.
Data synthesis
Our aim had been to try to combine the outcome measures from the individual trials in a meta‐analysis to provide a pooled effect estimate for each outcome, if the studies were clinically and methodologically comparable. However, as only one trial was included this was not possible.
Subgroup analysis and investigation of heterogeneity
We would have carried out subgroup analysis according to different prolapse compartments (for example upper versus lower) if data had been available. An investigation of heterogeneity could not be undertaken as there was only one trial.
Sensitivity analysis
Sensitivity analysis was not undertaken as there was only one trial.
Results
Description of studies
See 'Characteristics of included studies'; 'Characteristics of studies awaiting classification'.
Results of the search
In the previous review no randomised controlled trials (RCTs) of pessaries for women with pelvic organ prolapse were identified. In the current review we assessed a further 31 records and found one eligible RCT (Cundiff 2007) and one (Hagen 2011) that is awaiting classification. The flow of literature through the assessment process for the update of this review is shown in Figure 1.
Included studies
Cundiff 2007 reported a randomised crossover trial comparing symptom relief and the change in life impact for women using ring with support versus Gellhorn pessaries. The study was multicentre and performed in the USA. The trial included 134 women presenting with symptomatic pelvic organ prolapse, stage II or greater on the POP‐Q classification (Bump 1996). Women were randomised to each pessary using computer generated random numbers allocated in sealed opaque envelopes. Neither women nor researchers were blinded to the allocated pessary, but data were coded to permit blinding during analysis. Those women who were successfully fitted were asked to wear the pessary for three months, but if they discontinued prior to three months the data collection was accelerated. Outcome measures included changes in the Pelvic Floor Distress Inventory (PFDI) and the Pelvic Floor Impact Questionnaire (PFIQ) (Barber 2001). The study reported both statistically significant and clinically significant changes in the PFDI and PFIQ. Clinically significant changes were defined as a change greater than half the standard deviation of the pre‐intervention score (Sloan 2005).
Excluded studies
Studies awaiting classification
One study (Hagen 2011) is being considered for inclusion. Both intervention and control groups had a pessary fitted, with the intervention group also having pelvic floor muscle training (PFMT). This trial is awaiting classification for possible inclusion in the next update of the review.
Risk of bias in included studies
Please see Figure 2 for a visual representation of the risk of bias factors in the included trial.
2.
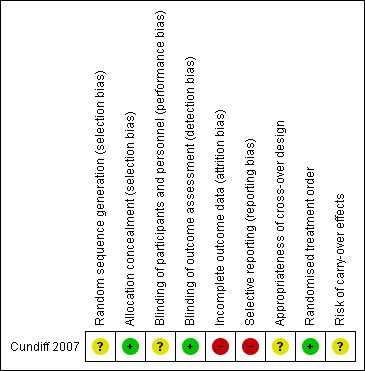
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In the trial, randomisation assigned participants to one of two groups that differed in the sequence of pessary use, and participants were assigned to the two groups with equal probability. Randomisation used computer generated random numbers in permuted blocks of variable size (six to 10) and allocated by sealed opaque envelopes.
Blinding
Blinding of participants and clinicians was not feasible however the trial did code data collection to permit blinding during analysis.
Incomplete outcome data
Attrition rates in the study were very high with only 85 of the 134 women completing the study and some of these had data collection accelerated that is collected before the primary end point of three months.
Selective reporting
The authors described many outcome measures in the methods of the study. These included POP‐Q, pelvic muscle grading, assessment of perineal descent, atrophy, erosions, wet prep, PFDI and PFIQ, a validated sexual function questionnaire, and a patient satisfaction visual analogue scale. However only the PFDI and PFIQ and associated subscales were reported.
Bias related to crossover design
One small study published in the literature (Handa 2002) suggested that pessaries may improve the stage of prolapse. Although Handa measured outcomes at one year, the findings did raise the possibility of a carryover effect in the crossover design.
Other potential sources of bias
No other potential sources of bias were identified.
Effects of interventions
See: Table 1
Trial results were reported under the appropriate comparison heading.
1. Any mechanical device versus control, waiting list or no active treatment
No trials identified.
2. Any mechanical device versus another treatment (lifestyle interventions, oestrogen treatment, physical interventions such as pelvic floor muscle training, surgery).
No trials identified.
3. Any mechanical device plus another treatment versus the other treatment alone.
No trials identified.
4. Any mechanical device plus another treatment versus a mechanical device alone.
One trial awaiting classification (Hagen 2011).
5. One mechanical device versus another mechanical device.
One trial was identified (Cundiff 2007) comparing a ring with support versus a Gellhorn pessary. The outcome measures reported were the PFDI (3 subscales: POPDI, CRADI, UDI) and PFIQ (3 scales: CRAIQ, POPIQ, IIQ each with 4 subscales: travel, social, emotional, physical).
Both the POPDI and POPIQ scales and subscales measured statistically significant changes from baseline for both pessaries. Clinically significant changes from baseline were also found in the POPDI for both the ring and the Gellhorn and a clinically significant change from baseline in POPIQ was found for the Gellhorn. However, there were no significant differences in terms of improvement in POPDI or POPIQ, statistical or clinical, in direct comparisons between the ring with support and the Gellhorn pessaries.
The Urinary Distress Inventory (UDI) and Urinary Impact Questionnaire (UIQ) scales and subscales showed statistical and clinical improvement from baseline for both pessaries but no difference between the two pessaries. The Colorectal‐Anal Distress Inventory (CRADI) showed a statistically and clinically significant improvement from baseline but no difference between the two pessaries. However, the Colorectal‐Anal Impact Questionnaire (CRAIQ) only showed a statistically significant difference but not a clinically significant improvement for both pessaries.
Cundiff and colleagues did not report the outcomes selected for GRADE, as illustrated in the Table 1.
6. Differing frequencies of device review or device change.
No trials identified.
Discussion
This updated review considers whether pessaries (mechanical devices) are effective in the management of pelvic organ prolapse. The scope of the review has not been changed. Reviews relating to alternative forms of treatment for pelvic organ prolapse are covered in other Cochrane reviews, surgery (Maher 2010), conservative methods such as pelvic floor muscle training and lifestyle changes (Hagen 2011b) and oestrogen (Ismail 2010).
Since the last review only one randomised controlled trial has been identified (Cundiff 2007). This trial had a high attrition rate leading to high risk of bias. However, the trial was not underpowered due to the crossover design. In this study it was reported that approximately 60% of women offered a pessary would continue with the treatment in the long term regardless of which device they used. This low continuation and high attrition factor should be considered in future study designs.
The use of pessaries has become commonplace over many years without full evaluation of their efficacy in comparison to other modes of treatment such as surgery, oestrogens or pelvic floor muscle training (PFMT). There is a need for trials of the effectiveness of pessaries in comparison to other treatments. There is a need for trials to assess whether early use of pessaries prevents progression of prolapse. There is also a need for trials which address the indication for pessary use and the care of pessaries; at present there is no consensus on the intervals between pessary changes, the treatment of complications, the role of local oestrogens or other concomitant treatments such as PFMT, nor which pessaries are indicated in specific types of pelvic organ prolapse. Despite their common usage, there are wide gaps in our knowledge of the outcomes of treatment using pessaries, which should be remedied with well‐designed RCTs.
Summary of main results
The only RCT reported to date (Cundiff 2007) comparing ring pessaries with support to Gellhorn pessaries found no statistically significant difference in symptom scores (PFDI and PFQI) between the two pessaries.
Overall completeness and applicability of evidence
With only one relatively small, USA based trial, the data lack the completeness to be widely applicable to international practice.
Quality of the evidence
Cundiff and colleagues did not report the outcomes selected for GRADE, as illustrated in the Table 1.
Potential biases in the review process
None noted.
Agreements and disagreements with other studies or reviews
The previous Cochrane review did not identify any trials for inclusion. This review provides evidence from one relevant trial.
Authors' conclusions
Implications for practice.
No good quality evidence from randomised controlled trials was identified on which to base the management by pessaries of women with pelvic organ prolapse. The only randomised controlled trial that was reported highlighted a high attrition rate of up to 40%, which needs to be taken into account in the design of randomised controlled trials of pessaries for pelvic organ prolapse in the future.
Implications for research.
There is a need for well‐designed randomised controlled trials in this area. Specifically the following comparisons should be made: 1. a pessary versus control, waiting list or no active treatment; 2. a pessary versus surgery; 3. a pessary versus physical interventions such as pelvic floor muscle training (PFMT) or lifestyle changes.
These trials should also evaluate whether there is any additional risk or benefit from the use of local oestrogen therapy or PFMT in conjunction with a pessary. In addition, trials are needed to inform the best ways to manage long term pessary use.
What's new
| Date | Event | Description |
|---|---|---|
| 22 January 2013 | New search has been performed | Identified one new study Cundiff 2007 |
| 22 January 2013 | New citation required but conclusions have not changed | Review updated, one new study identified Cundiff 2007 |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 7 August 2008 | Amended | Converted to new review format. |
| 26 January 2006 | New search has been performed | Minor update: 26/01/06 New studies sought but none found: 26/10/05 |
| 25 February 2004 | New citation required and conclusions have changed | Review first published |
Acknowledgements
We thank Sheila Wallace (Cochrane Incontinence Group) for assistance with searches for this review and Muhammad Imran Omar (Cochrane Incontinence Group) for advice on undertaking and reporting the review.
Appendices
Appendix 1. Search methods and strategies for the extra specific searches conducted for the previous version of this review (October 2005)
Electronic Databases MEDLINE (January 1966 to Week 5 August 2005) was searched on 14 September 2005 and PREMEDLINE (15 September 2005) was searched on 19 September 2005, both on OVID, using the following search terms: 1.prolapse/ 2.uterine prolapse/ 3.Rectocele/ 4.(prolaps$ adj5 (pelvi$ or vagin$ or genit$ or uter$ or vault$ or apical or urethr$ or segment$ or wall$)).tw. 5.cystoc?ele$.tw. 6.rectoc?ele$.tw. 7.urethroc?ele$.tw. 8.enteroc?ele$.tw. 9.proctoc?ele$.tw. 10.sigmoidoc?ele$.tw. 11.(pelvi$ adj3 dysfunct$).tw. 12.(pelvi$ adj3 (disorder$ or relax$)).tw. 13.(vagin$ adj3 defect$).tw. 14.(urogenital adj5 prolaps$).tw. 15.(cervi$ adj5 prolaps$).tw. 16.or/1‐15 This set of terms was combined with the first two parts of the Cochrane Highly Sensitive Search Strategy for randomised controlled trials (Appendix 5b.2, Cochrane Handbook, version 4.2, March 2003) using the Boolean operator 'AND'.
EMBASE (January 1996 to Week 43 2005) was searched on 25 October 2005, on OVID, using the following search terms:
1.pelvic adj5 prolaps$.tw. 2.uterus prolapse/ 3.rectocele/ 4.vagina prolapse/ 5.cystocele/ 6.or/1‐5 7.randomised controlled trial/ 8.controlled study/ 9.clinical study/ 10.major clinical study/ 11.prospective study/ 12.meta analysis/ 13.exp clinical trial/ 14.randomisation/ 15.crossover procedure/ or double blind procedure/ or parallel design/ or single blind procedure/ 16.placebo/ 17.latin square design/ 18.exp comparative study/ 19.follow up/ 20.pilot study/ 21.family study/ or feasibility study/ or study/ 22.placebo$.tw. 23.random$.tw. 24.(clin$ adj25 trial$).tw. 25.((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw. 26.factorial.tw. 27.crossover.tw. 28.latin square.tw. 29.(balance$ adj2 block$).tw. 30.or/7‐29 31.(nonhuman not human).sh. 32.30 not 31 33.6 and 32
CINAHL (January 1982 to February Week 4 2003) was searched on 13 March 2003, on OVID, using the following search terms:
1.exp pelvic organ prolapse/ 2.genital diseases, female/ 3.prolapse/ 4.uterine prolapse/ 5.Rectocele/ 6.(prolaps$ adj5 (pelvi$ or vagin$ or genit$ or uter$ or vault$ or apical or urethr$ or segment$ or wall$)).tw. 7.cystoc?ele$.tw. 8.rectoc?ele$.tw. 9.urethroc?ele$.tw. 10.enteroc?ele$.tw. 11.proctoc?ele$.tw. 12.sigmoidoc?ele$.tw. 13.(pelvi$ adj3 dysfunct$).tw. 14.(pelvi$ adj3 (disorder$ or relax$)).tw. 15.(vagin$ adj3 defect$).tw. 16.(urogenital adj5 prolaps$).tw. 17.(cervi$ adj5 prolaps$).tw. 18.((descen$ adj2 (uter$ or genit$ or pelv$)).tw. 19.procident$.tw. 20.(vagin$ adj2 (eversion$ or evert$)).tw. 21.(hernia$ adj2 (bladder$ or cystic or vesico$)).tw. 22.(bladder$ adj2 protru$).tw. 23.(viscer$ adj2 prolap$).tw. 24.hysteropex$.tw. 25.or/1‐2 26.placebo$.tw. 27.random$.tw. 28.(clin$ adj25 trial$).tw. 29.((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw. 30.factorial.tw. 31.crossover.tw. 32.latin square.tw. 33.(balance$ adj2 block$).tw. 34.or/26‐33 35.25 and 34
PEDro (the Physiotherapy Evidence Database) (url: www.pedro.fhs.usyd.edu.au) produced by the Centre for Evidence‐Based Physiotherapy (CEBP), University of Sydney, Australia was searched on 13 October 2003 using the search term "prolapse".
The UK National Research Register (Issue 3, 2003), Controlled Clinical Trials (April 2003) and ZETOC database of conference abstracts (April 2003) were searched using the search terms cystocele, urethrocele, rectocele, vault prolapse, uterine prolapse, vaginal prolapse, pelvic organ prolapse, pelvic floor.
The reference lists of relevant articles were searched for other possibly relevant trials, including the Cochrane review (Hay‐Smith 2001) of pelvic floor muscle training for urinary incontinence.
We did not impose any language or other restrictions on any of the searches.
Appendix 2. Data extraction form
MECHANICAL DEVICES FOR PROLAPSE: TRIAL CHARACTERISTIC FORM
REF ID ______________
REF NO_____
| TYPE OF PUBLICATION (abstract, proceeding, full text, translated, etc) |
|
|
METHODS | |
| Description of randomization |
|
| Stratification? |
|
| No. of treatment arms? |
|
| Allocation concealment? |
|
| Blinding? Patient Care giver Assessor |
|
| Power calculation? |
|
| Intention to treat analysis? |
|
| Follow up |
|
|
PARTICIPANTS | |
| Total study population |
|
| Withdrawals |
|
| Diagnosis by : Symptom /VE/POP‐Q/ultrasound |
|
| Type of prolapse | |
| Severity of prolapse | |
| Urinary incontinence present | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Baseline comparison of treatment groups? | |
| Characteristic of population (age, parity, etc) |
|
| No. of centres |
|
| Type of centre Location |
|
|
INTERVENTION | |
| Comparisons |
|
| Description / Variation |
|
| Types of pessary fitted? |
|
| Who fitted pessary? (surgeon/physiotherapist/ nurse)? |
|
| Follow up care? Who? Frequency? |
|
| Is pessary refitted? Frequency of refitting? |
|
|
OUTCOME | |
| List all |
|
| Definition of symptom alleviation |
|
| Other definitions |
|
| Proportion of women who progress to surgery [timescale and surgery type] |
|
| OTHER NOTES / COMMENTS |
|
MECHANICAL DEVICES FOR PROLAPSE: DATA ABSTRACTION FORM
REF ID _______________________
REF NO _______
COMPARISONS I : _________________ II : ____________________ III : ____________________
| Outcome | Units | I | II | III |
| A. Patients' observations | ||||
| perceived alleviation of prolapse symptoms | ||||
| acceptability/ satisfaction with outcome of treatment | ||||
| B. Objective measures | ||||
| grade of prolapse with device in situ judged on clinical examination e.g. (which system?? ‐ ICS POP‐Q system (Bump 1996) | ||||
| Site‐specific grading of prolapse judged on clinical examination e.g. ICS POP‐Q system (Bump 1996) | ||||
| C. Quality of Life | ||||
| prolapse‐specific quality of life questionnaire e.g. P‐QoL (Bump 1996) | ||||
| generic quality of life or health status measures e.g. SF‐36 (Ware 1992) | ||||
| psychological outcome measures e.g. Hospital Anxiety and Depression Score (Zigmond 1983) | ||||
|
D. Measures of Associated Symptoms |
||||
| bladder problems (including UI, occult UI, relief of voiding difficulty) | ||||
| bowel problems (including relief of obstructed defaecation) | ||||
| sexual function (including acceptability to both partners) | ||||
| E. Complications | ||||
| associated with pessary use: fistula formation, ulceration, bleeding, discharge etc. [record all complications] | ||||
| Reasons for device removal | ||||
| F. Socio‐economic evaluations | ||||
| cost comparisons | ||||
NOTES
Appendix 3. Risk of Bias assessment form
| The Cochrane Incontinence Group |
Risk of Bias Form
| TITLE OF POTENTIAL INCLUDED STUDY: _____________________ FIRST AUTHOR: ____________________________________ JOURNAL: ____________________________________ YEAR: ____________________________________ VOLUME/NUMBER: ____________________________________ |
PAGES: ____________________________________
Name of review: ____________________________________
Name of review author: ____________________________________
To be completed by the review author
Is the study relevant to the above review?
Yes o No o
Is the study a randomised or quasi‐randomised trial?
(quasi‐randomised = alternation, day of week etc)
Yes o Unclear o No o
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Cundiff 2007.
| Methods | Multicentre randomised crossover trial | |
| Participants | 134 women with symptomatic pelvic organ prolapse of stage II or greater on POP‐Q (Bump 1996). 54% of women had a predominantly anterior compartment prolapse; 35% a predominantly apical compartment and 10% a predominately posterior compartment prolapse. | |
| Interventions | Ring pessary with support or Gellhorn pessary | |
| Outcomes | Outcomes were assessed at 1 week, 6 weeks and 12 weeks and at time of drop out. Multiple outcome measures were reported in the methods (PFDI, PfIQ, VAS, POP‐Q, perineal descent, perineal reflexes, atrophy, erosions and wet prep). The study power calculation was based on the outcome "symptom relief", which probably equates to the POP‐DI outcome. However no primary outcome was formally stated. | |
| Notes | This study had a very high drop‐out rate: only 85 of the original 134 completed the study. However some results appear to have been reported on the total number 134. Most results do not specify the number of subjects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization used computer‐generated random numbers in permuted blocks of variable size (6‐10)." Initial randomisation appropriate however then patient preference took priority over randomisation. Although these patients were then excluded from the analysis it may affect the results. |
| Allocation concealment (selection bias) | Low risk | "allocated by sealed opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was not possible to blind patients to the type of pessary used because they were taught to remove them. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The data collected were coded to permit blinding during the analysis of results. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The study had a high drop‐out rate: only 85 of the 134 completed the study. |
| Selective reporting (reporting bias) | High risk | There was no formally stated primary outcome. Not all of the outcomes described in the methods have been reported in the results. There is no report on vaginal discharge or bleeding. |
| Appropriateness of cross‐over design | Unclear risk | There is some suggestion in the literature (Handa 2002) that pessaries may improve the stage of prolapse therefore there is the potential for a carryover effect. |
| Randomised treatment order | Low risk | Random allocation was by computer generated random numbers using permuted blocks of variable size. Allocation was stored in opaque sealed envelopes. |
| Risk of carry‐over effects | Unclear risk | Given the possibility of pessaries improving stage of prolapse (Handa 2002) there may be some risk of carryover effect. |
Characteristics of studies awaiting assessment [ordered by study ID]
Hagen 2011.
| Methods | RCT |
| Participants | 16 women with symptomatic pelvic organ prolapse of POP‐Q stage I‐IV (8 intervention and 8 control) |
| Interventions | Pessary plus PFMT versus pessary alone |
| Outcomes | |
| Notes |
Differences between protocol and review
None
Contributions of authors
FMR reviewed documents, extracted data and co‐produced the final review.
CB co‐produced the final review.
DG extracted data and along with CB reviewed abstracts and included studies.
DG and EJA contributed to writing the final review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR, UK.
The Cochrane Incontinence Review Group is supported by NIHR UK.
Declarations of interest
None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Cundiff 2007 {published data only}
- Cundiff GN, Amundsen CL, Bent AE, Coates KW, Schaffer JI, Strohbehn K, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. American Journal of Obstetrics and Gynecology 2007;196(4):405.e1‐8. [SR‐INCONT23081; PUBMED: 17403437] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Hagen 2011 {published data only}
Additional references
Barber 2001
- Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition‐specific quality of life instruments for women with pelvic floor disorders. American Journal of Obstetrics and Gynecology 2001;185(6):1388‐95. [DOI] [PubMed] [Google Scholar]
Basu 2011
- Basu M, Wise B, Duckett J. A qualitative study of women’s preferences for treatment of pelvic floor disorders. BJOG 2011;118:338‐44. [DOI] [PubMed] [Google Scholar]
Bump 1996
- Bump RC, Mattiasson A, Bo K, Brubaker LP, Delancey JOL, Klarskov P, et al. The standardisation of terminology of female pelvic organ prolapse and pelvic floor dysfunction. American Journal of Obstetrics and Gynecology 1996;175(1):10‐7. [DOI] [PubMed] [Google Scholar]
Bump 1998
- Bump R, Norton P. Epidemiology and natural history of pelvic floor dysfunction. Obstetrics and Gynecology Clinics of North America 1998;25(4):723‐46. [DOI] [PubMed] [Google Scholar]
Clemons 2 2004
- Clemons JL, Aguilar VC, Tillinghast TA, Jackson ND, Myers DL. Risk factors associated with an unsuccessful pessary fitting trial in women with pelvic organ prolapse. American Journal of Obstetrics and Gynecology 2004;190(2):345‐50. [DOI] [PubMed] [Google Scholar]
Clemons 2004
- Clemons JL, Aguilar VC, Tillinghast TA, Jackson ND, Myers DL. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. American Journal of Obstetrics and Gynecology 2004;190(4):1025‐9. [DOI] [PubMed] [Google Scholar]
Clemons 3 2004
- Clemons JL, Aguilar VC, Sokol ER, Jackson ND, Myers DL. Patient characteristics that are associated with continued pessary use versus surgery after 1 year. American Journal of Obstetrics and Gynecology 2004;191(1):159‐64. [DOI] [PubMed] [Google Scholar]
Cundiff 2000
- Cundiff GW, Weidner AC, Visco AG, Bump RC, Addison WA. A survey of pessary use by members of the American Urogynecologic Society. American Journal of Obstetrics and Gynecology 2000;95(6):931‐5. [DOI] [PubMed] [Google Scholar]
Dietz 2008
- Dietz HP. The aetiology of prolapse. International Urogynecology Journal 2008;19:1323‐9. [DOI] [PubMed] [Google Scholar]
Gorti 2009
- Gorti M, Hundelist G, Simons A. Evaluation of vaginal pessary management: A UK‐based survey. Journal of Obstetrics and Gynaecology 2009;29(2):129‐31. [DOI] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380‐2. [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing Summary of Findings tables‐binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing Summary of Findings tables and evidence profiles‐continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173‐83. [DOI] [PubMed] [Google Scholar]
Hagen 2009
- Hagen S, Glazener C, Sinclair L, Stark D, Bugge C. Psychometric properties of the Pelvic Organ Prolapse Symptom Score (POP‐SS). British Journal of Obstetrics and Gynaecology 2009;116(1):25‐31. [DOI] [PubMed] [Google Scholar]
Hagen 2011b
- Hagen S, Stark D. Conservative prevention and management of pelvic organ prolapse in women. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD003882.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Handa 2002
- Handa VL, Jones M. Do pessaries prevent the progression of pelvic organ prolapse?. International Urogynecological Journal 2002;13(6):349‐51. [DOI] [PubMed] [Google Scholar]
Hanson 2006
- Hanson LM, Schultz J, Flood CG, Cooley B, Tam F. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence:patient characteristics and factors contributing to success. International Urogynecological Journal 2006;17:155‐9. [DOI] [PubMed] [Google Scholar]
Hay‐Smith 2009
- Hay‐Smith J, Berghmans B, Burgio K, Dumoulin C, Hagen S, Moore K, Nygaard I. Adult Conservative Management. Incontinence, Fourth International Consultation on Incontinence. Plymouth, UK: Health Publication Ltd, 2009. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ismail 2010
- Ismail SI, Bain C, Hagen S. Oestrogens for treatment or prevention of pelvic organ prolapse in postmenopausal women. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD007063.pub2] [DOI] [PubMed] [Google Scholar]
Kapoor 2009
- Kapoor DS, Thakar R, Sultan AH, Oliver R. Conservative versus surgical management of prolapse: what dictates patient choice?. International Urogynecology Journal and Pelvic Floor Dysfunction 2009;20(10):1157‐61. [DOI] [PubMed] [Google Scholar]
Lipp 2011
- Lipp A, Shaw C, Glavind K. Mechanical devices for urinary incontinence in women. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD001756.pub5] [DOI] [PubMed] [Google Scholar]
Lone 2011
- Lone F, Thakar R, Sultan AH, Karamalis G. A 5‐year prospective study of vaginal pessary use for pelvic organ prolapse. International Journal of Gynaecology and Obstetrics 2011;114(1):56‐9. [DOI] [PubMed] [Google Scholar]
Lowder 2011
- Lowder JL, Ghetti C, Nikolajski C, Oliphant SS, Zyczynski HM. Body image perceptions in women with pelvic organ prolapse: a qualitative study. American Journal of Obstetrics and Gynecology 2011;204(441):e1‐5. [DOI] [PubMed] [Google Scholar]
MacLennan 2000
- MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. British Journal of Obstetrics and Gynaecology 2000;107(12):1460‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Maher 2010
- Maher C, Feiner B, Baessler K, Glazener CMA. Surgical management of pelvic organ prolapse in women. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD004014.pub4] [DOI] [PubMed] [Google Scholar]
Manchana 2012
- Manchana T, Bunyavejchevin S. Impact on quality of life after ring pessary use for pelvic organ prolapse. International Urogynecological Journal 2012;23(7):873‐7. [DOI] [PubMed] [Google Scholar]
Oliver 2011
- Oliver R, Thakar R, Sultan AH. The history and usage of the vaginal pessary: a review. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2011;156:125‐30. [DOI] [PubMed] [Google Scholar]
Poma 2000
- Poma PA. Nonsurgical management of genital prolapse. A review and recommendations for clinical practice. Journal of Reproductive Medicine 2000;45(10):789‐97. [MEDLINE: ] [PubMed] [Google Scholar]
Pott‐Grinstein 2001
- Pott‐Grinstein E, Newcomer JR. Gynecologists' patterns of prescribing pessaries. Journal of Reproductive Medicine 2001;46(3):205‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Samuelsson 1999
- Samuelsson EC, Victor FTA, Tibblin G, Svardsudd KF. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. American Journal of Obstetrics and Gynecology 1999;180(2):299‐305. [DOI] [PubMed] [Google Scholar]
Sloan 2005
- Sloan JA. Cella D, Hays RD. Clinical significance of patient‐reported questionnaire data: another step towards consensus. Journal of Clinical Epidemiology 2005;58:1217‐9. [DOI] [PubMed] [Google Scholar]
Swift 2000
- Swift SE. The distribution of pelvic organ support in a population of female subjects seen for routine gynecologic health care. American Journal of Obstetrics and Gynecology 2000;183(2):277‐85. [DOI] [PubMed] [Google Scholar]
Ware 1993
- Ware JE. Measuring patients' views: the optimum outcome measure. SF36: a valid, reliable assessment of health from the patient's point of view. British Medical Journal 1993;306(6890):1429‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. [DOI] [PubMed] [Google Scholar]


