Abstract
Background
The UK prevalence of abdominal aortic aneurysm (AAA) is estimated at 4.9% in over 65‐year olds. Progressive and unpredictable enlargement can lead to rupture. Endovascular repair of AAAs involves a stent graft system being introduced via the femoral artery and manipulated within the aorta under radiological guidance. Following endograft deployment, a seal is formed at the proximal and distal landing zones to exclude the aneurysm sac from the circulation. With the increasing popularity of endovascular repair there has been an increase in the number of commercially available stent graft designs on the market. This is an update of the review first published in 2013.
Objectives
This review aimed to assess the different stent graft types for endovascular repair of AAA.
Search methods
The Cochrane Vascular Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched February 2015) and the Cochrane Register of Studies (2015, Issue 1). Trial databases were searched by the TSC for details of ongoing and unpublished studies.
Selection criteria
All published and unpublished randomised controlled trials (RCTs) of stent graft types in the repair of AAAs were sought without language restriction and in consultation with the Cochrane Vascular Group TSC.
Data collection and analysis
We planned to conduct data collection and analysis in accordance with the Cochrane Handbook for Systematic Reviews of Interventions.
Main results
No studies were identified that met the inclusion criteria. It was not possible to review the quality of the evidence in the absence of studies eligible for inclusion in the review.
Authors' conclusions
Unfortunately, no data exist regarding direct comparisons of the performance of different stent graft types. High quality randomised controlled trials evaluating stent graft types in abdominal endovascular aneurysm repair are required.
Plain language summary
Different stent grafts for repair of abdominal aortic aneurysms
Background
An aneurysm is a localised widening of an artery. The abdominal aorta is the largest artery in the body, delivering blood from the heart to the organs in the abdomen and the legs. If an aneurysm occurs in the abdominal aorta it can expand and may rupture, resulting in death. Open surgery can treat these aneurysms; this involves opening the abdomen and placing an artificial graft over the widening. A new alternative treatment involves an artifical stent graft, delivered through an arterial blood vessel in the groin, fixed over the widening. This technique is called endovascular repair. There are many different types of stent graft available. They differ in how they are inserted in/access the blood vessel, how they attach to the walls of the artery and the design and materials they are made from.
Study characteristics and key results
We searched for evidence directly comparing the different types of stent grafts in aneurysm repair (current until February 2015). This review found no randomised controlled trial evidence investigating if any specific stent graft performs better than another type of stent graft. More research is required to help surgeons decide which specific type of stent graft to use.
Quality of the evidence It was not possible to review the quality of the evidence in the absence of studies eligible for inclusion in the review.
Background
Description of the condition
An aneurysm is a localised pathological dilatation of an arterial wall. Aneurysms most commonly affect the abdominal aorta.
The UK prevalence of abdominal aortic aneurysm (AAA) is estimated at 4.9% in over 65‐year olds (Ashton 2002), which increases with advanced age. With a burgeoning elderly population in developed counties, AAAs represent an increasing public health burden to society.
Abdominal aortic aneurysm has a multifactorial pathology involving environmental and genetic risk factors. The disease process shares a number of common risk factors with occlusive atherosclerotic disease, namely smoking, male gender and elevated diastolic blood pressure, but there is an inverse association with diabetes mellitus and no risk attributed to hypercholesterolaemia (Blanchard 2000). A genetic predisposition is supported by a relative risk of 1.9 in people with a family history (Larsson 2009).
Abdominal aortic aneurysms develop without any symptoms and gradually expand over time. They are often diagnosed incidentally when patients undergo ultrasonic or computed tomography (CT) imaging for unrelated symptoms. The natural history of AAA is expansion followed by eventual rupture with subsequent haemodynamic compromise, organ ischaemia and death. Mortality from aortic rupture is high (80% to 90%), with an in‐hospital mortality rate after surgical repair of approximately 50% (Campbell 1991; Visser 2005). Current practice advocates surgical intervention for aortic aneurysms exceeding 5.5 cm, or at any size if the patient becomes symptomatic or if the AAA ruptures.
Conventional open surgical repair involves a large abdominal incision to gain access to the aorta followed by the insertion of a prosthetic graft under general anaesthesia with the aim of excluding the aneurysmal section from the systemic circulation to prevent aortic rupture. This operation is associated with significant mortality and morbidity. Thirty‐day mortality associated with an elective open procedure has been reported in the range of 2% to 12% in the UK (Brown 2012).
Description of the intervention
Endovascular repair of AAAs was initially described by Parodi and colleagues in animal models and has become established as an alternative to open surgical repair (Parodi 1991). Access to the aorta is achieved via two incisions in the groin, with guidewires, catheters and then a stent graft system being introduced via the femoral artery and manipulated within the aorta under radiological guidance. Following endograft deployment, a seal is formed at the proximal and distal landing zones to exclude the aneurysm sac from the circulation. A number of anatomical criteria are used to assess the patient’s suitability for EVAR including the morphology of the proximal and distal landing zones and the dimensions of the access vessels.
Stent graft design has progressed quickly since the first prototype devices. With the increasing popularity of endovascular repair there has been an increase in the number of commercially available stent graft designs on the market. Many factors influence the way surgeons select stent grafts and there is now a need for a robust comparative evaluation of their performance.
How the intervention might work
A number of studies have compared elective endovascular aortic aneurysm repair (EVAR) with open surgical repair for AAAs including the EVAR‐1 randomised controlled trial (RCT), which showed that EVAR has a significantly lower 30‐day and in‐hospital mortality than open repair (Greenhalgh 2004; Visser 2005). Similar early outcomes were reported in the smaller Dutch RCT (Prinssen 2004), although there was no difference in survival between the two groups at two years (66%) (Prinssen 2004).
Following EVAR, patients are at risk of a number of graft‐related complications (Visser 2005). Device‐related complications include endoleak, graft kink or fracture, migration and visceral ischaemia secondary to partial coverage of aortic branches. Ongoing aneurysm sac enlargement is of primary concern as the sac may rupture over time. Serial computed tomography (CT) imaging post‐procedure is recommended to survey for device‐related complications and sac expansion which may warrant re‐intervention. This exposes EVAR patients to radiation levels higher than their open surgical counterpoints, although recent studies have shown that less frequent CT imaging post‐EVAR is appropriate in the majority of patients (Dias 2009).
Stent graft design has evolved significantly since the first devices were handmade in theatre. Several different grafts are currently available on the market from various manufacturers. There are many differences between stents, including their design, component materials and deployment techniques. Different device designs are likely to have different complication rates (Kelso 2009). For example, fabric porosity led to the withdrawal of the original Gore device in 2004 (Tanski 2007). Secondary intervention rates may differ depending on the graft type, including late conversion to open repair (Harris 2000; Sampram 2003).
The evolution of stent graft design is driven towards both short‐ and long‐term aims. In the short term, designs aim to allow introduction of the device, particularly through challenging iliac anatomy, accurate graft positioning and deployment and successful immediate sealing at the landing zones. In the long term, the aim is to reduce late migration and disconnection or other device‐related failure that would lead to secondary interventions.
Device performance has been carefully regulated both in the US and Europe, and this has led to detection of device failure with subsequent withdrawal or modification of the device. For example, the early designs for the Ancure device had fixational hook fractures reported (Najibi 2001) and the AneuRx (Medtronic) stent graft, a modular heavily stented and sutured device, suffered from reports of type IV endoleak in the follow‐up period and were withdrawn from the market (Katzen 2005).
Devices rely on a combination of radial force provided by metal stents (usually self expanding) and hooks or barbs to engage the vessel wall. The graft is also oversized relative to the vessel diameter in order to enhance frictional attachment.
Manufacturers produce different stent grafts for different market demands. For example, the Excluder (Gore) has no sutures and has the smallest delivery system for limited iliac access (12F sheath). The Zenith (Cook) stent has supra‐renal barb fixation for reduced proximal graft migration and increased main body diameters to enable more patients to be considered for endovascular repair who would otherwise be unsuitable.
A number of anatomical constraints for patient suitability for endovascular repair are detailed in each device instruction manual. These include increased sac diameter and angulation, reduced neck length (< 15 mm) and increased angulation (> 60 °). Newer stent graft devices are being manufactured in response to these anatomical challenges. For example, the Endurant stent graft system (Medtronic) has anchoring pins for proximal fixation and wire‐formed M‐shaped body stents for increased flexibility and conformability for severe angulation of aneurysm necks (Verhagen 2009).
Why it is important to do this review
Several established stent graft types are available for use in abdominal aortic aneurysm repair and new devices are emerging. Many patients have an anatomy suitable for repair with a number of the available grafts. Comparison of early and late complications is needed to allow clinicians an informed choice. In addition, patient factors that affect the outcome with certain graft types may be identified.
Objectives
This review aimed to assess the different stent graft types for endovascular repair of abdominal aortic aneurysms.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials (RCTs) were eligible for inclusion. The review authors planned to include cross‐over trials in the review for completeness but data from the first phase only would have been included in meta‐analyses as the cross‐over is not a valid design in this context.
Types of participants
Individuals with abdominal aortic aneurysms (AAA) visualised by CT or ultrasonographic techniques, or both.
Types of interventions
Any stent graft type versus another stent graft type.
Types of outcome measures
We intended to consider the following outcome measures.
Primary outcomes
Short‐term mortality (30‐day or in‐hospital mortality, i.e. procedure‐related).
Aneurysm exclusion (no flow in the aneurysm sac, or further extravasation (escape of blood from the vessel into the tissues) beyond the sac on follow‐up imaging 30 days after the procedure).
Secondary outcomes
Major complications, e.g. open conversion, haemorrhage, myocardial infarction, stroke, paraplegia, renal failure (20% rise in creatinine levels above normal reference limits), respiratory failure (need for post‐operative mechanical ventilation), pneumonia, bowel ischaemia, lower limb ischaemia, etc.
Minor complications, e.g. access site haematoma, wound infection, etc.
Long‐term complications and mortality; device‐related, re‐intervention rates, cause of death.
Search methods for identification of studies
All published and unpublished RCTs of stent graft types in the repair of abdominal aortic aneurysms were sought without language restriction.
Electronic searches
The Cochrane Vascular Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched February 2015) and the Cochrane Register of Studies (CRS) (http://www.metaxis.com/CRSWeb/Index.asp) (CENTRAL) (2015, Issue 1). See Appendix 1 for details of the search strategy used to search the CRS. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED; and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Vascular Group module in the Cochrane Library (www.cochranelibrary.com).
The following trials databases were searched by the TSC for details of ongoing and unpublished studies using the terms abdominal and aneurysm and stent*:
World Health Organization International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/);
ClinicalTrials.gov (http://clinicaltrials.gov/);
Current Controlled Trials (http://www.controlled‐trials.com/).
Searching other resources
The reference lists of articles retrieved by the searches were checked.
Data collection and analysis
We planned to conduct data collection and analysis in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Selection of studies
Two of three review authors (RR, JMD, RC) scanned the titles and abstracts of articles retrieved by the search and removed those that were irrelevant. The full text of all potentially eligible studies was retrieved. Two of three review authors (RR, JMD, RC) independently examined the full text articles for compliance with the inclusion criteria and selected studies eligible for inclusion in the review. Review authors corresponded with study investigators, when required, to clarify study eligibility (for example with respect to participant eligibility criteria and allocation method). Disagreements as to study eligibility were resolved by consensus.
Data extraction and management
The aim was to extract data from eligible studies using a data extraction form designed and pilot‐tested by the review authors. Where studies had multiple publications, the main trial report would have been used as the reference and additional details supplemented from secondary papers. The review authors would have corresponded with study investigators in order to resolve any data queries, as required. Two review authors (one a topic area specialist) would have independently extracted the data and any disagreement between these review authors would have been resolved by a third review author.
Assessment of risk of bias in included studies
The aim was to assess the included studies for risk of bias using the Cochrane risk of bias tool assessing sequence generation; allocation concealment; blinding of participants, providers and outcome assessors; completeness of outcome data; selective outcome reporting; and other potential sources of bias. Two review authors planned to assess these domains with any disagreements resolved by consensus or by discussion with a third author.
All judgments would have been fully described.
Measures of treatment effect
For dichotomous data the number of events in the control and intervention groups of each study would have been used to calculate Peto odds ratios. For continuous data, mean differences between treatment groups would have been calculated if all studies reported exactly the same outcomes. If similar outcomes were reported on different scales, the standardised mean difference would have been calculated. Ordinal data would be treated as continuous data and such data would be analysed appropriately depending on the number of categories. We aimed to present 95% confidence intervals for all outcomes.
Unit of analysis issues
The primary analysis was intended to be per individual randomised. Only first‐phase data from cross‐over trials would have been included.
Dealing with missing data
The aim was to analyse the data on an intention‐to‐treat basis as far as possible and, where necessary, attempts would have been made to obtain missing data from the original investigators. Where these were unobtainable, imputation of individual values would have been undertaken for the primary outcomes only. If studies reported sufficient detail to calculate mean differences but gave no information on associated standard deviations (SD), the outcome would have been assumed to have an SD equal to the highest SD from other studies within the same analysis. For other outcomes, only the available data would have been analysed. Any imputation undertaken would have been subjected to sensitivity analysis (see below).
Assessment of heterogeneity
The review authors planned to consider whether the clinical and methodological characteristics of the studies were sufficiently similar for a meta‐analysis to provide a meaningful summary. Statistical heterogeneity would have been assessed by the measure of the I2 statistic. An I2 measurement greater than 50% would have been taken to indicate substantial heterogeneity (Higgins 2008). If substantial heterogeneity had been detected, possible explanations would have been explored in sensitivity analyses (see below).
Assessment of reporting biases
In view of the difficulty in detecting and correcting for publication bias and other reporting biases, the authors aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. If we had included 10 or more studies in an analysis, a funnel plot would have been used to explore the possibility of small study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
The data from primary studies would have been combined using random‐effects models in the following comparison:
any stent graft type versus another stent graft type.
An increase in the odds of a particular outcome, which may be beneficial or detrimental, would have been displayed graphically in the meta‐analyses to the right of the centre‐line and a decrease in the odds of an outcome to the left of the centre‐line.
Subgroup analysis and investigation of heterogeneity
No subgroup analysis was expected. If substantial heterogeneity had been detected, possible explanations would have been explored in sensitivity analyses (see below).
Sensitivity analysis
Sensitivity analyses would have been conducted for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made regarding the eligibility and analysis. These analyses would have included consideration of whether conclusions would have differed if:
eligibility were restricted to studies without high risk of bias;
studies with outlying results had been excluded;
alternative imputation strategies had been adopted.
Results
Description of studies
No studies were identified that met the inclusion criteria.
Results of the search
See Figure 1.
1.
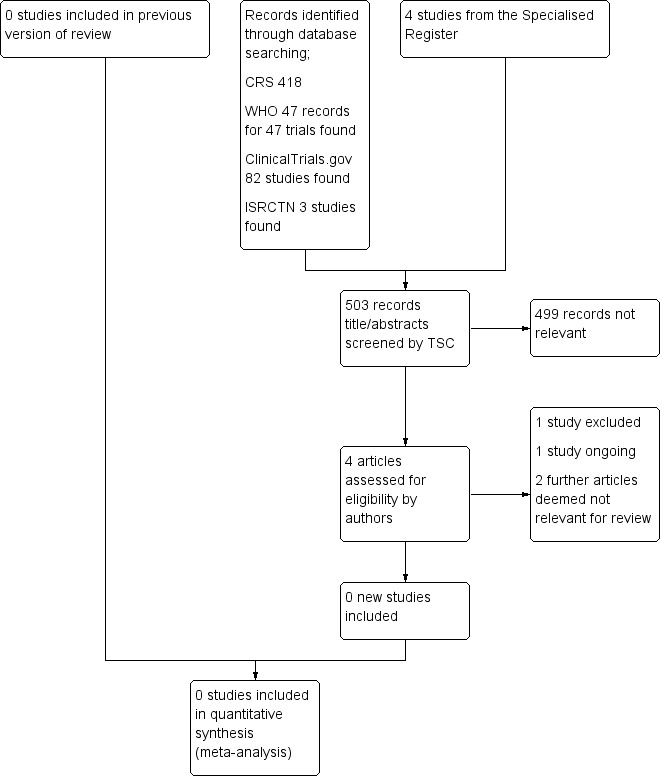
Study flow diagram.
The search results were independently reviewed by the review authors (JMD, RR, RC). Unfortunately, no RCTs were identified for inclusion in this review.
A single ongoing study, NCT00922454, is described within the prospective registration as both a case control study and "cross over study". The study investigators have been contacted for clarification. No response has been received. This study will be assessed further during subsequent review updates.
Excluded studies
ChiCTR‐TRC‐12002844 was excluded as the study is recruiting participants diagnosed with thoracic aortic aneurysm or aortic dissection.
Risk of bias in included studies
It was not possible to review methodological quality in the absence of studies eligible for inclusion in the review.
Effects of interventions
Unfortunately, no published or unpublished RCTs were found comparing stent graft types in the treatment of abdominal aortic aneurysms.
Discussion
Summary of main results
This review documents that there are no published or pending RCTs that compare different stent graft types for abdominal aortic aneurysm repair, assessing early and late mortality and major complications. This paucity of evidence is also reflected in other topics considering endovascular repair for abdominal aortic aneurysms. The most recent Cochrane review comparing open surgery versus endovascular repair for ruptured abdominal aortic aneurysms included three studies, with a total of 761 participants (Badger 2014). The risk of bias was generally low and from these data currently available there appears to be no difference in 30 day mortality. These data on complications are not robust enough at this point to make any conclusions on superiority of either repair technique.
Endovascular repair is associated with late complications that are not encountered with open surgery, including endoleaks, graft migration and stent fractures. The specific contribution of the stent graft type to these outcome measures is poorly characterised, with no direct comparisons between stent graft types. It is imperative that high quality data are produced to characterise the influence of stent graft type selection with regards to these outcome measures and therefore improve patient care.
Overall completeness and applicability of evidence
There are no high quality RCTs comparing one stent graft type with another for the repair of abdominal aortic aneurysms. When designing these trials, careful consideration of outcome measures is required. Trials would need to assess all relevant outcomes and should be divided into two categories:
(i) durability of stent graft type e.g. endoleak rate, re‐intervention rate, open‐conversion rate, and rupture‐free survival;
(ii) clinical outcome measures e.g. early and late mortality, major complications, hospital stay.
Given that elective endovascular repair of abdominal aortic aneurysms is often the treatment of choice in specialist vascular centres, there is scope for a multicentre RCTs to be performed comparing stent graft types. The patients would need to be standardised across groups and have detailed aortic imaging to confirm that their aortic anatomy was 'straightforward' and comparable, assessing the following criteria for inclusion into the trial: (i) adequate proximal and distal landing zones for the stent; (ii) length and diameter of device; (iii) angulation of the aorta; (iv) access vessel diameters.
Further trials would be beneficial in assessing the performance of more complex stent graft procedures for abdominal aortic aneurysms where the anatomy is not favourable for the majority of commercial stents and where custom‐made fenestrated stent grafts are required on an individual basis.
Quality of the evidence
It was not possible to review the quality of the evidence in the absence of studies eligible for inclusion in the review.
Potential biases in the review process
None.
Agreements and disagreements with other studies or reviews
A study was identified which compares the performance of Zenith and Talent stent grafts in elective open repair of abdominal aortic aneurysms within the context of the UK EVAR Trials (UK EVAR Trial 2007). This was an analysis of stent graft performance within the EVAR 1 and 2 multicentre trials; however, the use of stent graft type was not randomised and often was determined based upon centre preference to either device. The study reports that there was no evidence to support a significant difference in performance, as measured by secondary intervention rates (aneurysm‐related mortality and all‐cause mortality), between the Zenith and Talent devices. The study length to follow‐up was limited to an average of 3.8 years, which is relatively short, and thus cannot predict if longer‐term differences between the stent graft types would emerge.
This review agrees with the Cochrane review on stent graft types for endovascular repair of thoracic aortic aneurysms (Rolph 2015), which also found high quality evidence to be lacking for the use of one stent graft versus another.
Authors' conclusions
Implications for practice.
Endovascular repair is associated with endoleaks, graft migration and stent fractures requiring secondary intervention. Unfortunately, no data exist regarding comparisons of the performance of different stent graft types in reducing these complications. Therefore, this review cannot recommend guidance to clinicians in their selection of stent graft type.
Implications for research.
High quality randomised controlled trials evaluating stent graft types in abdominal endovascular aneurysm repair for abdominal aortic aneurysms are needed.
Feedback
1 Fixed‐effect or random‐effects model, 4 June 2010
Summary
In the section on 'Data synthesis', the authors state that they will use a fixed‐effect model in their comparison of their desired outcomes. I feel that this will not account for the risk of inherent heterogeneity caused by the fact that the trial results are influenced by the centre at which they are done, case volume and interventionalist's experience. I feel they should use the random‐effects model as it will give allowance for this heterogeneity and then calculate risk of chance heterogeneity. Or they should do it both ways and then discuss the difference in results for example as in the protocol for 'Stent placement versus surgery for coarctation of the aorta'.
Reply
Thank you Dr Bit for your constructive feedback. Reflecting on your feedback we think it would be appropriate to use the random‐effects model to take into account heterogeneity. Many thanks for your input.
Contributors
Feedback: Dr Nupur Bit, general surgeon interested in a career in vascular surgery.
Reply: James MN Duffy, Rachel Rolph, Rachel Clough, Bijan Modarai, Peter Taylor, Matthew Waltham
What's new
| Date | Event | Description |
|---|---|---|
| 4 June 2015 | New search has been performed | New search run. No new studies included. One new study excluded and one ongoing study identified. |
| 4 June 2015 | New citation required but conclusions have not changed | New search run. No new studies included. One new study excluded and one ongoing study identified. Minor text changes made. No change to conclusions. |
History
Protocol first published: Issue 4, 2010 Review first published: Issue 3, 2013
| Date | Event | Description |
|---|---|---|
| 4 June 2010 | Feedback has been incorporated | Feedback added to protocol |
Acknowledgements
We would like to thank members of Cochrane Vascular for their assistance. We would also like to thank Rachel Clough (RC), who reviewed the search results. We would like to thank Rachel Clough, Bijan Modarai, and Peter Taylor for contributing to the original review.
Appendices
Appendix 1. Search strategy for CRS
| #1 | MESH DESCRIPTOR Aortic Aneurysm | 98 |
| #2 | MESH DESCRIPTOR Aortic Aneurysm, Abdominal | 382 |
| #3 | MESH DESCRIPTOR Aortic Aneurysm, Thoracic | 44 |
| #4 | aort*:TI,AB,KY | 5838 |
| #5 | (juxta renal):TI,AB,KY | 0 |
| #6 | juxtarenal:TI,AB,KY | 2 |
| #7 | (juxta renal or juxtarenal ):TI,AB,KY | 2 |
| #8 | (pararenal or para renal ):TI,AB,KY | 2 |
| #9 | (suprarenal or supra renal):TI,AB,KY | 23 |
| #10 | (short neck* or shortneck*):TI,AB,KY | 9 |
| #11 | (visceral aortic segment):TI,AB,KY | 0 |
| #12 | thorac*:TI,AB,KY | 7993 |
| #13 | abdominal:TI,AB,KY | 16097 |
| #14 | #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 | 27859 |
| #15 | aneur?sm*:TI,AB,KY | 2243 |
| #16 | #14 AND #15 | 924 |
| #17 | (JRAAA or JRAAAs or PAAA or PAAAs or TAAA or TAAAs or JPAA or JPAAs or SRA or SRAs or SRAA or SRAAs):TI,AB,KY | 59 |
| #18 | ((aort* near3 (ballon* or dilat* or bulg* or expan*))):TI,AB,KY | 64 |
| #19 | #1 OR #2 OR #3 | 516 |
| #20 | #16 OR #17 OR #18 OR #19 | 1009 |
| #21 | MESH DESCRIPTOR Stents EXPLODE ALL TREES | 2697 |
| #22 | (stent* or graft* or tevar or endograft* or evar or fevar or f‐evar):TI,AB,KY | 19771 |
| #23 | (powerlink or talent or excluder or aorfix or zenith or endologix or anaconda or Triascular or Cordis or Endurant or Quantum or Aneurx or Ancure):TI,AB,KY | 253 |
| #24 | MESH DESCRIPTOR Blood Vessel Prosthesis EXPLODE ALL TREES | 381 |
| #25 | MESH DESCRIPTOR Blood Vessel Prosthesis Implantation EXPLODE ALL TREES | 364 |
| #26 | MESH DESCRIPTOR Vascular Surgical Procedures | 479 |
| #27 | endovascular:TI,AB,KY | 967 |
| #28 | fenestrat*:TI,AB,KY | 84 |
| #29 | chimney:TI,AB,KY | 12 |
| #30 | #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 | 21023 |
| #31 | #20 AND #30 | 418 |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ChiCTR‐TRC‐12002844 | Recruiting participants diagnosed with thoracic aortic aneurysm, thoracoabdominal aortic aneurysm or aortic dissection |
Characteristics of ongoing studies [ordered by study ID]
NCT00922454.
| Trial name or title | Acute Technical Outcomes of the Talent Abdominal Aortic Aneurysm (AAA) Stent‐Graft Versus Cook Zenith Stent‐Graft |
| Methods | Acute Technical Outcomes of the Talent AAA Stent ‐Graft vs. Cook Zenith Stent‐ Graft: A Case‐Control Study. Later described as a "cross over study" |
| Participants | Subject with abdominal aortic aneurysm, with or without iliac involvement |
| Interventions | Talent AAA Stent ‐Graft vs. Cook Zenith Stent‐ Graft |
| Outcomes | Acute technical success, successful exclusion of the aneurysm |
| Starting date | 15/04/2003. Prospective registration states trial is still recruiting |
| Contact information | Deborah Hill hilld@albanyvascular.com |
| Notes | Confusing study design. Sponsor contacted for trial update. No response received |
Contributions of authors
JD: main reviewer, involved in all aspects of the review. RR: main reviewer, involved in all aspects of the review. MW: involved in revising the manuscript for important intellectual content and final approval of the version for publication.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the National Institute for Health Research, via Cochrane Programme Grant funding to the Cochrane Vascular Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
JD: none known RR: none known MW: none known
New search for studies and content updated (no change to conclusions)
References
References to studies excluded from this review
ChiCTR‐TRC‐12002844 {unpublished data only}
- ChiCTR‐TRC‐12002844. Evaluate the safety and efficacy of Ankura Ⅱstent graft for the treatment of aortic endovascular: a prospective, multi‐center, randomized clinical trial. http://www.chictr.org/en/proj/show.aspx?proj=3768 (accessed June 2015).
References to ongoing studies
NCT00922454 {unpublished data only}
- NCT00922454. Acute technical outcomes of the Talent abdominal aortic aneurysm (AAA) stent‐graft versus Cook Zenith stent‐graft. https://clinicaltrials.gov/ct2/show/NCT00922454?term=NCT00922454&rank=1 (accessed June 2015).
Additional references
Ashton 2002
- Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RA, et al. Multicentre Aneurysm Screening Study Group. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002;360(9345):1531‐9. [DOI] [PubMed] [Google Scholar]
Badger 2014
- Badger S, Bedenis R, Blair PH, Ellis P, Kee F, Harkin DW. Endovascular treatment for ruptured abdominal aortic aneurysm. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD005261.pub2] [DOI] [PubMed] [Google Scholar]
Blanchard 2000
- Blanchard JF, Armenian HK, Friesen PP. Risk factors for abdominal aortic aneurysm: results of a case‐control study. American Journal of Epidemiology 2000;151(6):575‐83. [DOI] [PubMed] [Google Scholar]
Brown 2012
Campbell 1991
- Campbell WB. Mortality statistics for elective aortic aneurysms. European Journal of Vascular Surgery 1991;5(2):111‐3. [DOI] [PubMed] [Google Scholar]
Dias 2009
- Dias NV, Riva L, Ivancev K, Resch T, Sonesson B, Malina M. Is there a benefit of frequent CT follow‐up after EVAR?. European Journal of Vascular and Endovascular Surgery 2009;37(4):425‐30. [DOI] [PubMed] [Google Scholar]
Greenhalgh 2004
- Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG, EVAR Trial Participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30‐day operative mortality results: randomised controlled trial. Lancet 2004;364(9437):843‐8. [DOI] [PubMed] [Google Scholar]
Harris 2000
- Harris PL, Vallabhaneni SR, Desgranges P, Becquemin JP, Marrewijk C, Laheij RJ. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. European Collaborators on stent/graft techniques for aortic aneurysm repair. Journal of Vascular Surgery 2000;32(4):739‐49. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Katzen 2005
- Katzen BT, MacLean AA. Past, present, and future endograft devices. Techniques in Vascular and Interventional Radiology 2005;8(1):16‐21. [DOI] [PubMed] [Google Scholar]
Kelso 2009
- Kelso RL, Lyden SP, Butler B, Greenberg RK, Eagleton MJ, Clair DG. Late conversion of aortic stent grafts. Journal of Vascular Surgery 2009;49(3):589‐95. [DOI] [PubMed] [Google Scholar]
Larsson 2009
- Larsson E, Granath F, Swedenborg J, Hultgren R. A population‐based case‐control study of the familial risk of abdominal aortic aneurysm. Journal of Vascular Surgery 2009;49(1):47‐50; discussion 51. [DOI] [PubMed] [Google Scholar]
Najibi 2001
- Najibi S, Steinberg J, Katzen BT, Zemel G, Lin PH, Weiss VJ, et al. Detection of isolated hook fractures 36 months after implantation of the Ancure endograft: a cautionary note. Journal of Vascular Surgery 2001;34(2):353‐6. [DOI] [PubMed] [Google Scholar]
Parodi 1991
- Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Annals of Vascular Surgery 1991;5(6):491‐9. [DOI] [PubMed] [Google Scholar]
Prinssen 2004
- Prinssen M, Verhoeven EL, Buth J, Cuypers PW, Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. New England Journal of Medicine 2004;351(16):1607‐18. [DOI] [PubMed] [Google Scholar]
Rolph 2015
- Rolph R, Duffy JMN, Waltham M. Stent graft types for endovascular repair of thoracic aortic aneurysms. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD008448.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sampram 2003
- Sampram ES, Karafa MT, Mascha EJ, Clair DG, Greenberg RK, Lyden SP, et al. Nature, frequency, and predictors of secondary procedures after endovascular repair of abdominal aortic aneurysm. Journal of Vascular Surgery 2003;37(5):930‐7. [DOI] [PubMed] [Google Scholar]
Tanski 2007
- Tanski W 3rd, Fillinger M. Outcomes of original and low‐permeability Gore Excluder endoprosthesis for endovascular abdominal aortic aneurysm repair. Journal of Vascular Surgery 2007;45(2):243‐9. [DOI] [PubMed] [Google Scholar]
UK EVAR Trial 2007
- The EVAR Trial Participants. Secondary interventions and mortality following endovascular aortic aneurysm repair: Device‐specific result from the UK EVAR trials. European Journal of Vascular and Endovascular Surgery 2007;34(3):281‐90. [DOI] [PubMed] [Google Scholar]
Verhagen 2009
- Verhagen HJ, Torsello G, Vries JP, Cuypers PH, Herwaarden JA, Florek HJ, et al. Endurant stent‐graft system: preliminary report on an innovative treatment for challenging abdominal aortic aneurysm. The Journal of Cardiovascular Surgery 2009;50(2):153‐8. [PubMed] [Google Scholar]
Visser 2005
- Visser P, Akkersdijk GJM, Blankensteijn JD. In‐hospital operative mortality of ruptured abdominal aortic aneurysm: a population‐based analysis of 5593 patients in the Netherlands over a 10‐year period. European Journal of Vascular and Endovascular Surgery 2005;30(4):359‐64. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Duffy 2010
- Duffy JMN, Rolph R, Clough R, Modarai B, Taylor P, Waltham M. Stent graft types for endovascular repair of abdominal aortic aneurysms. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD008447] [DOI] [PubMed] [Google Scholar]
Duffy 2013
- Duffy JMN, Rolph R, Clough RE, Modarai B, Taylor P, Waltham M. Stent graft types for endovascular repair of abdominal aortic aneurysms. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD008447.pub2] [DOI] [PubMed] [Google Scholar]


