Abstract
Background
Papillary thyroid carcinoma (PTC) is the most common malignant thyroid neoplasm comprising 80–90% of all thyroid malignancies. Molecular changes in thyroid follicular cells are likely associated with the development of PTC. Mutations in serine/threonine-protein kinase (BRAF) and Rat sarcoma viral oncogene homolog (RAS) are commonly seen in PTC.
Methods
In total, 90 cases of PTC are randomly selected from archive paraffin blocks and 10μm sections were cut and processed for DNA extraction. BRAF V600E mutation and 8 types of KRAS mutations were investigated using Real Time PCR.
Results
BRAF V600E mutation was identified in 46% of PTC while KRAS mutations were seen in 11% of PTC. There was significant correlation between BRAF V600E mutation and PTC larger than 5cm in diameter, positive surgical margin and lymph node metastasis. BRAF V600E mutation was significantly higher in patients with less than 55-year of age than those more than 55-year of age. BRAF V600E mutation was significantly higher in patients with family history of thyroid cancer than those without. There was no significant difference in BRAFV600E mutation between males and females, PTC classic and follicular variants, unifocal and multifocal PTC. There was a significant higher percentage of BRAF V600E mutation in classic PTC than papillary microcarcinoma variant. There was no significant age, gender, histologic type, tumor size, lymph node metastasis, tumor focality, and surgical margin status differences between KRAS mutated and non-mutated PTC.
Conclusion
BRAF V600E and KRAS mutation are seen in a significant number of PTC in the UAE. BRAF mutation is significantly correlated with large tumor size, positive surgical margins and lymph node metastasis suggesting an association between BRAF V600E mutation and tumor growth and spread.
Introduction
Thyroid cancer is the most common endocrine malignancy and clinical thyroid cancer accounts for 1–2% of all cancers [1]. Papillary thyroid carcinoma (PTC) is the most common malignant thyroid neoplasm comprising 80–90% of all thyroid malignancies [2].
PTC has many histologic subtypes including, classical papillary, follicular, encapsulated, papillary microcarcinoma, columnar cell, diffuse sclerosing, tall cell, cribriform-morular, hobnail, PTC with fibromatosis, solid/trabecular, spindle cell, clear cell, warthin–like and oncocytic PTC [3].
The clinical behavior of PTC diverges widely, from non-aggressive microcarcinomas that grow very slowly and are usually associated with excellent prognosis to an aggressive widely invasive PTC with metastasis that can be fatal [4]. Molecular alterations in the sequence composition of cellular molecules such as DNA, RNA, and proteins usually precede the development of PTC. These alterations are frequently initiated by specific mutations in growth signal genes such as serine/threonine-protein kinase (BRAF) or Rat sarcoma viral oncogene homolog (KRAS) which will be translated into oncoproteins that lead to uncontrolled growth signals within affected follicular cells [5–7]. BRAF and KRAS mutations are commonly seen in PTC [5].
BRAF, which is one of the three RAF genes of serine/threonine kinases (ARAF, BRAF, and CRAF) is involved in growth signals transmission and it is the immediate downstream of RAS gene. BRAF is an important player of the mitogen-activated protein kinase (MAPK) pathway.
This pathway conveys the extracellular signals from various hormones, cytokines and growth factors to the nucleus through the activation of signal cascades. Normally, activation of receptor tyrosine kinase (RTK) leads to the dimerization of receptors and tyrosine residue phosphorylation, which activates RAS kinase. Then, RAS kinase activates the phosphorylation of RAF kinases, which in turn activate the dual-specificity protein kinases: MAP/extracellular signal-regulated kinase (MEK) 1 and 2. MEK1/2 phosphorylates and activates extracellular signal-regulated kinases (ERK) 1 and 2. ERK1/2 regulates various transcription factors involved in increased expression of genes involved in cell proliferation, differentiation and apoptosis [5].
Mutation in BRAF is seen in 29–69% of PTC, making BRAF mutations the most common demarcated molecular abnormality in PTC [8]. Carcinogenesis is a complex process involving complex interacting signaling pathways instead of a single linear stream of the MAPK pathway. The high frequency of BRAFV600E mutation in tall-cell variant, an aggressive variant of PTC, suggests that BRAF V600E mutations might be associated with an aggressive phenotype [9]. Studies have shown that induction of BRAFV600E expression in rat thyroid cells facilitated the acquisition of secondary genetic events through induction of genomic instability [10].
The RAS proteins are located on the cytoplasmic surface of the cell membrane. RAS proteins convey extracellular signals that promote the proliferation, differentiation, and survival of cells. [11]. The RAS-RAF-MEK-ERK pathway is activated in 30% of human cancer [12].
In PTC, functional mutation in RAS has been identified in 0–10% of Asian PTC [13]. RAS mutation can promote thyroid tumorigenesis through the RAS-RAF-MEK-ERK pathway or through its interaction with PI3K/AKT pathway [14].
Thyroid cancer is the 3rd most common cancer among UAE citizens and the 2nd most common cancer among females in the UAE [15]; hence identification of the molecular changes may have impact on the diagnosis and treatment of PTC. In this study, we evaluate the frequency of BRAF V600E and KRAS mutations in PTC and their correlation with clinical and pathological changes. This is the first study on BRAF V600E and KRAS mutations in PTC in the United Arab Emirates.
Materials and methods
Collection of specimens
In total, 90 formalin fixed paraffin-embedded (FFPE) tissue blocks from surgically removed thyroid specimens, during the period 2011–2016, were randomly collected from the Department of Pathology, Tawam Hospital, Al Ain, United Arab Emirates. Three-um sections were prepared from selected blocks and stained with hematoxylin and eosin (H&E) stain. All sections were examined microscopically by a pathologist who participates in this study to be sure that the sections contain significant area of PTC (>50%of neoplastic cells). One ten-um section containing tumor-rich areas was taken from each block and was put in a separate labeled Eppendorf tube. New blade was used in cutting each block to prevent tissue contamination from case to case.
The protocol of the present study conformed to the ethical guidelines of the World Medical Association, Declaration of Helsinki, and was approved by Al Ain Medical District Human Research Ethics Committee (THREC-438). Patients or their caregivers signed a written consent allowing using their anonymous material for research purposes.
Histopathological classification of selected cases
H&E stained sections of selected cases were reviewed and classified according to the 4th edition of WHO classification of tumors of thyroid gland [3] by a pathologist participated in this project. Tumors were called classic if they show predominant papillary growth with classic papillary nuclear features. Tumors were called papillary microcarcinoma if they show predominant papillary or follicular growth with classic papillary nuclear features and have a size of ≤1cm in greatest dimension. Tumors were called follicular variant if they show predominant follicular growth with classic papillary nuclear features. Follicular variant has two subtypes; the encapsulated with invasion, when the tumor is encapsulated and there is invasion of the capsule, while the infiltrative subtype when the tumor lack the capsule and shows infiltration of the stroma.
Collection of demographic data of selected cases
The demographic data and the clinical information were extracted from the electronic medical files of the subjects with the identified papillary thyroid carcinoma. The collected data include age at diagnosis, gender, body mass index (BMI), tumour size, thyroiditis, focality, family history of thyroid cancer, exposure to external radiation, smoking and post surgical TNM staging.
DNA isolation
Genomic DNA was isolated from each sampled tissue sections with the REPLI-g FFPE Kit (Qiagen, Hilden, Germany) for direct whole genome amplification of DNA from FFPE tissue according to the manufacturer’s instructions. Briefly, 1x FFPE lysis solution was prepared and 100 μl was added to the tissue section and mixed and centrifuge briefly. The samples were incubated at 95°C for 10 min to melt the paraffin followed by cooling down the sample to room temperature. Then 2μl of Proteinase K was added to each sample and mixed and centrifuge briefly. Each sample was then incubated for 60 min at 60°C and then for a further 10 min at 95°C. Then, each 10μl of the lysed tissue section was transferred into a new micro centrifuge tube. The FFPE master mix was prepared as per manufacturer instructions on ice and vortex and centrifuge briefly. Then, 10μl FFPE master mix was added to 10 μl DNA from the lysed tissue then mixed and centrifuged briefly. Then the samples were incubated at 24°C for 30 min. Then the reaction was stopped by incubation at 95°C for 5 min followed by cooling down to 4°C using a thermal cycler. Finally, the samples were incubated at 30°C for 8 h (high-yield reaction) then stopping the reaction by incubation at 95°C for 10 min. The amplified DNA was stored at -20°C until required for downstream applications.
Quantification of DNA
Quantifiler™ Trio DNA Quantification, Kit Catalog number: 4482910 was used to quantify the total amount of amplifiable human DNA in the sample. For the Quantifiler™ Trio DNA Quantification Kit: the Quantifiler™ Trio Primer Mix and Quantifiler™ THP PCR Reaction Mix was mixed as per manufacturer’s instructions. The PCR mix was vortexed and centrifuged briefly. The 2 μL of gDNA was added to the applicable wells. The reaction plate was sealed with the Optical Adhesive Cover and care was taken to remove bubbles. The plate was centrifuged at 3,000 rpm for about 20 seconds in a tabletop centrifuge with plate holders to remove any bubbles. A total of 90 samples were processed for DNA isolation. The concentration of gDNA was determined using Nanodrop instrument using nuclease free water as blank solvent.
Determination of KRAS/ BRAF mutation
GenoScreen KRAS/BRAF Real Time PCR Kit (DiagCor Bioscience Inc. Ltd, Hong Kong) was used for a qualitative assessment intended for the detection of eight KRAS mutations in codon 12 and 13, and one BRAF mutation in codon 600 using real-time PCR assay. The GenoScreen KRAS/BRAF Real Time PCR Kit is developed based on PCR amplification of mutant DNA with specific primers, detected by real time polymerase chain reaction (PCR) technology. Detection of target amplified product (amplicon) is achieved by the cleavage of dual fluorescent dye labeled oligonucleotide probes during the quantitation of mutant DNA.
Procedure
All the PCR reagents were pipetted mix and spin down before use. The final PCR reaction (volume 10 μL) was prepared according to the manual. The recommended reaction component volumes to amplify DNA for KRAS/BRAF PCR Master Mix: 5μL, Primer Mix: 3μL, Template (DNA/ H2O): 2μL. From the 8 μL of PCR mixture (containing KRAS/BRAF PCR Master Mix and Primer Mix) aliquoted into each PCR reaction and added the appropriate amount of DNA template suggested and finally the reaction volume was top up to 10 μL with DNase Free Water if necessary. The mixture was then spin down and placed in real time PCR thermal cycler, QuantStudio3 (QS3). The reporter was select “FAM”, “NFQ-MGB” for Quencher and “ROX” for passive reference. Amplification Profile was set at 95°C, 10 min/1 cycle and amplification at 95°C, 15 sec/ 50cycle finally 60°C, 1 min FAM channel.
Data analysis and interpretation
The data was analyzed after setting the threshold for FAM signals. The cycle threshold (Ct) value was set at 1/20 of each individual marker’s highest fluorescence point for the run (i.e. FAM). The FAM signal from the positive and negative assay was used to determine the validity of the real time run. The positive assay gave the Ct values between 24–38, and Negative Control assay was Ct value greater than 45. The results were determined using QS3 Real-Time PCR System. For each mutation assay the Ct value was used per the manufacturer’s instructions. For KRAS G12R< 35, KRAS G12S< 33, KRAS G12C < 34, KRAS S G12D < 35, KRAS G12A< 34, KRAS G12V < 32, KRAS G13C< 36, KRAS G13D< 35, BRAF V600E < 35, Positive Control 24–38, Negative Control> 45.
Statistical analysis
The statistical analysis was computer assisted using SPSS for windows version 20 (SPSS Inc, Chicago, USA). Student’s t-test was used to compare continuous variables. Quantitative variables were analyzed with the chi-squared test and correlations of ordinal variables using the Spearman rank correlation coefficient and Chi-square (Fisher’s exact) test. P value <0.05 were considered significant. Where appropriate numerical data were presented as the mean ±SD.
Results
Demographic data
In total, 90 cases of PTC were analyzed in this study. The mean age was 41.21 ± 13.94, the mean BMI was 29.18 ± 6.01, and the female to male ratio was 2.33. Family history of thyroid cancer was seen in 13% of cases, while family history for other neuroendocrine tumors was seen in 1% of cases. History of exposure to external radiation was seen in 3% of cases. Only 8% of cases were smokers (Table 1).
Table 1. Demographic data of 90 cases of PTC.
| Parameter | Mean ± SD (%) |
|---|---|
| Age | 41.21 ± 13.94 |
| BMI | 29.18 ± 6.01 |
| Male | 27 (30.0%) |
| Female | 63 (70.0%) |
| Family History of thyroid cancer | 12(13%) |
| Family History of other neuro-endocrine tumors | 1(1%) |
| History of Exposure to external radiation | 3(3%) |
| Smoker | 7(8%) |
Histologic types of PTC
Classic (conventional) PTC, which exhibits a predominant papillary pattern of growth with characteristic nuclear features of PTC, was the most common type comprising 46.6% (42) of the cases followed by microcarcinoma 30% (27) and follicular variant PTC comprising 23.4% (21).
Most of the papillary microcarcinomas 25 (93%) exhibit papillary pattern of growth similar to the classic PTC, and only 2 cases (7%) exhibit predominate follicular pattern of growth similar to follicular variant PTC. The follicular variant of PTC, which exhibit a predominant follicular pattern of growth with the characteristic nuclear features of PTC, has two subtypes. The infiltrative which comprises 45% (10) of the cases, and the encapsulated invasive subtype which comprises 55% (11) of the cases (Table 5).
Table 5. Correlation between BRAF and KRAS mutations and demographic and histological parameters.
| KRAS MUTATION | BRAF V600E MUTATION | |||||
|---|---|---|---|---|---|---|
| Negative | Positive | P-value | Negative | Positive | P-value | |
| N (%) | N (%) | N (%) | N (%) | |||
| Age | ||||||
| <55 (N = 74) | 66 (89.2) | 8 (10.8) | 0.385 | 38 (51.4) | 36 (48.6) | 0.014 |
| >55 (N = 16) | 13 (81.3) | 3(18.8) | 11(68.8) | 5 (31.3) | ||
| Gender | ||||||
| Male (N = 27) | 24(88.9) | 3(11.1) | 0.835 | 13(48.1) | 14(51.9) | 0.493 |
| Female (N = 63) | 55(87.3) | 8(12.7) | 36 (57.1) | 27 (42.9) | ||
| Family history of thyroid cancer | 12(100) | 0 (0) | 0.329 | 3 (25) | 9 (75) | 0.036 |
| History of smoking | 7 (100) | 0 (0) | 1.00 | 3 (42) | 4 (58) | 0.702 |
| Exposure to radiation | 3 (100) | 0 (0) | 1.00 | 1 (33) | 2 (66) | 0.598 |
| Histologic type | ||||||
| Classic (N = 42) | 38(90) | 4 (10) | 19 (45) | 23(55) | ||
| Microcarcinoma (N = 29) | 22(81) | 5(19) | 0.434 | 17 (63) | 10(37) | 0.01 |
| Follicular(N = 19) | 19 (90) | 2 (10) | 1.00 | 13 (58) | 8 (42) | 0.08 |
| Encapsulated with invasion(11) | 11 (100) | 0(0) | 9 (82) | 2(18) | ||
| Infiltrative (10) | 9 (90) | 1(10) | 1.00 | 6 (60) | 4(40) | 0.001 |
| Tumor size | ||||||
| T1(N = 49) | 43 (87.8) | 6(12.2) | 0.994 | 31(63.3) | 18(36.7) | 0.052 |
| T2(N = 22) | 20(90.9) | 2(9.1) | 0.611 | 12(54.5) | 10(45.5) | 0.594 |
| T3+T4(N = 19) | 16(84.2) | 3(15.8) | 0.598 | 6(31.6) | 13(68.4) | 0.023 |
| LN Metastasis | ||||||
| Yes (N = 23) | 20(87.0) | 3(13.0) | 0.572 | 6 (26) | 17(74) | 0.002 |
| Focality | ||||||
| Unifocal (N = 60) | 52(86.7) | 8(13.3) | 0.653 | 34(56.7 | 26(43.3) | 0.354 |
| Multifocal (N = 30) | 27(90.0) | 3(10.0) | 15(50.0) | 15(50.0) | ||
| Surgical margin | ||||||
| Positive (N = 22) | 19 (86.4) | 3(13.6) | 0.818 | 8(36.4) | 14(63.6) | 0.043 |
| Lymphovascular invasion | ||||||
| Yes (N = 13) | 11(84.6) | 2(15.4) | 0.710 | 5(38.5) | 8(61.5) | 0.171 |
Histopathological features
Site of involvement
Right lobe was the most common site of PTC comprising 48% (43) of cases, while left lobe and both lobes were involved in 28% (24) and 21.2% (22) of cases respectively (Table 2).
Table 2. Showing histopathologic features of 90 cases with PTC.
| Features | Frequency | |
|---|---|---|
| Lobes | Right | 43 (48%) |
| Left | 25 (28%) | |
| Both | 22 (24%) | |
| Focality | Unifocal | 58 (64%) |
| Multifocal | 32 (36%) | |
| Capsule | No capsule | 72 (80.0%) |
| Encapsulated | 18 (20%) | |
| Positive lymphovascular invasion | 13 (14%) | |
| Positive surgical margins | 22 (24%) | |
| Thyroiditis | 37 (41%) | |
Focality
Unifocal PTCs were seen in 64% (58) of the cases while multifocal PTC was seen in 36% (32) of the cases (Table 2).
Presence of capsule
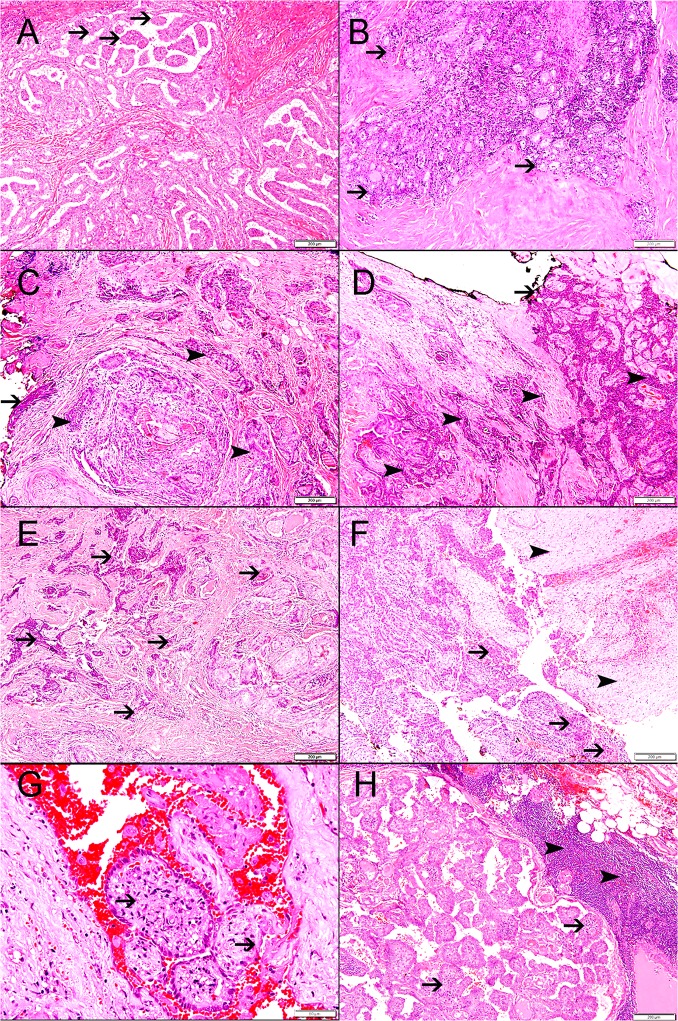
Most of PTC were non-encapsulated and comprising 80% (72) of cases while encapsulated PTCs were seen in 20% (18) of the cases (Table 2) (Fig 1).
Fig 1.
Lymphovascular Invasion (LVI)
Positive LVI was seen in 14% (13) of PTC, while unidentified LVI was seen in 86% (76) of the cases (Table 2) (Fig 1).
Surgical margin involvement
Positive surgical margin was seen in 24% (22) of PTC, while free surgical margin was seen in 76% (68) of the cases (Table 2), (Fig 1).
Lymphocytic thyroiditis
Lymphocytic thyroiditis was seen in 41% (37) of PTC (Table 2).
TNM staging
Tumor size
In total, 49 (54%) cases of PTC were diagnosed at T1, while 22 (24%), 17 (19%) and 2 (2%), were diagnosed at T2, T3, and T4 respectively (Table 3).
Table 3. Showing TNM staging system characteristics of 90 cases of PTC.
| Parameter | Frequency | Percent | |
|---|---|---|---|
| T1 | Tumor ≤2 in greatest dimension, limited to the thyroid | 49 | 54.4 |
| T2 | Tumor >2 cm, but ≤4 cm in greatest dimension, limited to thyroid | 22 | 24.5 |
| T3 | Tumor >4 cm limited to the thyroid, or gross extrathyroidal extension invading only strap muscles | 17 | 18.9 |
| T4 | Gross extrathyroidal extension beyond the strap muscles | 2 | 2.2 |
| N1 | Metastasis to regional nodes | 23 | 25.5 |
| M1 | Distant metastasis | 1 | 1.1 |
Lymph nodes involvements
In total 22 (24%) cases show N1, while 1 (1%), and 1 (1%) were N2 and N3 respectively. 66 (74%) of PTC have no lymph node metastasis (Table 3), (Fig 1).
Distant metastasis
Only one cases of PTC shows distant metastasis (Table 3).
Frequency of mutations
BRAF V600E mutation was seen in 46% (41) of PTC. KRAS mutations were seen in 12% (11) of PTC. In total, 8 different KRAS mutations at codon 12 and 13 0f KRAS were investigated. KRAS_G12V mutation was the most common KRAS mutation and was seen in 7% (6) of PTC, while KRAS_G12S, KRAS_G12D, KRAS_G13C and KRAS_G13D were seen in 2% (2), 1% (1), 1% (1), 1% (1) respectively. KRAS_G12A, KRAS_G12C and KRAS_G12R mutations were not identified in our samples (Table 4).
Table 4. Frequency of mutation.
| Type of mutation | Papillary carcinoma (n = 90) |
|---|---|
| BRAF_V600E | 41 (46%) |
| Any KRAS mutation | 11 (12. %) |
| KRAS_G12V | 6 (7%) |
| KRAS_G12S | 2 (2%) |
| KRAS_G12D | 1 (1%) |
| KRAS_G13C | 1 (1%) |
| KRAS_G13D | 1 (1%) |
| KRAS_G12A | Nil |
| KRAS_G12C | Nil |
| KRAS_G12R | Nil |
Correlation between BRAF V600E and KRAS mutations with demographic and histological parameters
Age and BRAF V600E and KRAS mutations
BRAF V600E mutation was significantly higher in patients with less than 55-year of age than those higher than 55-year of age (P = 0.014) (Table 5). There was no signification correlation between KRAS mutation and any age group (Table 5).
Gender and BRAF V600E and KRAS mutations
There was no signification correlation between BRAF V600E mutation and any gender group as well as there was no signification correlation between KRAS mutation and any gender group (Table 5).
Family history of thyroid cancer
There was a significant correlation between family history of thyroid cancer and BRAF V600E mutation (P = 0.036). There was no significant correlation between family history of thyroid cancer and KRAS mutation (Table 5).
History of smoking
There was no significant correlation between history of smoking and BRAF V600E and KRAS mutation (Table 5).
Exposure to radiation
There was no significant correlation between history of exposure to radiation and BRAF V600E and KRAS mutation (Table 5).
Histologic variant and BRAF V600E and KRAS mutations
The percentage of BRAF V600E mutation was higher in classic PTC than follicular variant PTC but it did not reach the statistical significant (P = 0.08). In addition, there was no significant difference in the percentage of KRAS mutation between the classic PTC and the follicular variant PTC. (Table 5). There was a significantly higher percentage of BRAF V600E mutation in classic PTC than papillary microcarcinoma (P = 0.0157), while there was no significant differences in the percentage of KRAS mutation between classic PTC and papillary microcarcinoma.
Tumor size and BRAF V600E and KRAS mutations
Tumor sizes above 4cm are significantly correlated with BRAF V600E mutation (P = 0.023). Any tumor size was not correlated with any KRAS mutation (Table 5).
Lymph node metastasis and BRAF V600E and KRAS mutations
There was a significant correlation between lymph node metastasis and BRAF V600E mutation (P = 0.002). There was no significant correlation between lymph node metastasis and KRAS mutation (Table 5) (Fig 1).
Tumor focality and BRAF V600E and KRAS mutations
There was no signification correlations between BRAF V600E mutation and focality as well as there was no signification correlation between KRAS mutation and focality (Table 5).
Surgical margin and BRAF V600E and KRAS mutations
There was a significant correlation between positive surgical margin and BRAF V600E mutation (P = 0.43). There was no signification correlation between KRAS mutation and positive surgical margin (Table 5) (Fig 1).
Lymphovascular invasion and BRAF V600E and KRAS mutations
There was no correlation between lymphovascular invasion and BRAF V600E or KRAS mutations (Table 5) (Fig 1).
Discussion
Papillary thyroid carcinoma is the most prevalent type of thyroid cancer worldwide [16]. A lot of works have been done to identify fundamental mechanisms involved in the development of PTC [13, 14, 16–21]. Many studies have shown a constant rise in the incidence of PTC in different countries all over the world over the last decades [17]. Jung et al. have shown the increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp Increase in RAS mutations [18]. Identifying molecular changes in PTC is an important step in understanding major mechanisms participate in its development as well as open new doors for early diagnosis and treatment.
The MAPK pathway is an important intracellular signal transduction pathway that is required for maintaining cell proliferation, differentiation, and programed cell death in response to tyrosine kinase receptor (RTK) stimulation [19]. Moreover, it is a crucial player in the pathogenesis of PTC, as somatic mutations in its various components constantly drive the oncogenic process [20].
In this study we have identified KRAS mutation in 12% of PTC. We have identified 5 out of 8 investigated mutations in KRAS gene. Those mutations were identified in codon 12 and 13, and include KRAS_12V, KRAS_G12S, KRAS_G12D, KRAS_G13C and KRAS_G13D were seen in 7%, 2% (2), 1% (1), 1% (1), 1% (1) of PTC, respectively. In fact, to the best of our knowledge this is the first report of these mutations in KRAS gene in PTC using Real Time PCR.
In addition, there was no significant difference in the percentage of KRAS mutation between the classic PTC and the follicular variant PTC. This finding might be related to the low number of selected cases in this study.
The increase of the RAS mutations is explainable with a decrease of classic PTC variant and a sharp increase of follicular PTC variant in the last decades [18]. In our study, one quarter of the cases were pure follicular variant of PTC. Besides, the other three quarters were classic PTC, and although predominantly show classic papillary pattern, there are foci of follicular pattern as well seen in many of these PTCs, as part of histologic spectrum of classic PTC. This observation may explain the high rate of KRAS mutations in our study.
There are variable frequencies of RAS mutations in PTC. Goutas et al. have identified KRAS mutation in 54.5% of PTC while, Di Cristofaro et al. [22], Siraj et al. [20], Jung et al. [18] and Naito et al. [23] have shown RAS mutation in 25%, 8%, 14%, and 50% respectively.
Variability in frequencies of RAS mutations reflects differences in samples, method of DNA extraction, detection method of mutations, and possible geographical difference in the pattern of RAS mutation in PTC.
RAS mutation is considered as an early molecular event in follicular cell oncogenesis that leads to a well-differentiated neoplasm and may progress to a de-differentiated tumor following the gaining of further mutations [23, 24]. Knauf et al. have shown active RAS mutation can accelerate progression of cell cycle and promotes DNA damage by interfering with different cell cycle check points [24].
We have also shown BRAFV600E mutation in 46% of PTC. Our finding has an intermediate position between Mediterranean countries and North American and some Far East countries [25–40] (Table 6). Some studies have close results to ours [28, 29, 30, 35, 36, 38, 39].
Table 6. Frequency BRAFV600E mutation in different studies worldwide.
| Study | BRAF V600E mutation % | Country |
|---|---|---|
| Rosenbaum et al. [23] | 65 | USA |
| Guan et al. [24] | 62 | USA |
| Wang et al. [25] | 50 | USA |
| Kebebew et al. [26] | 49 | USA |
| Frasca et al. [27] | 39 | ITALY |
| Lupi et al. [28] | 44 | ITALY |
| Elisei et al. [29] | 37 | ITALY |
| Fugazzola et al. [30] | 32 | ITALY |
| Costa et al. [31] | 55 | PORTUGAL |
| Zoghlami et al. [32] | 43 | FRANCE |
| Goutas et al. [19] | 27 | GREECE |
| Kim et al. [33] | 73 | KOREA |
| Ito et al. [34] | 38 | JAPAN |
| Langping et al. [35] | 63 | CHINA |
| Nelson etal. [36] | 51 | INDIA |
| TANG et al. [37] | 50 | TAIWAN |
| MARWA et al. [38] | 55 | EGYPT |
| Siraj et al. [18] | 59 | KINGDOM OF SAUDI ARABIA |
| Our study | 46 | UAE |
The percentage of BRAF mutation was higher in classic PTC than follicular variant PTC but it did not reach the statistical significant (P = 0.08). It is possible that a higher number of included cases will improve the results. We think that this is a limitation in our study. In addition, we have shown a significantly higher percentage of BRAFV600E in classic PTC than papillary microcarcinoma.
Moreover, we have also show a significantly higher percentage of BRAFV600E mutation in infiltrative follicular variant of PTC than the encapsulate with invasion subtype of follicular variant of PTC. These results suggest that BRAFV600E mutation is associated with tumor growth and spread [22, 33, 39].
Ugolini et al. have identified frequent BRAF V600E mutation in papillary thyroid microcarcinomas (PTMC) [41].
Knauf et al. have shown that BRAFV600E transgenic mice develops poorly differentiated PTC with aggressive behavior which confirms the oncogenic role of BRAFV600E mutation [42].
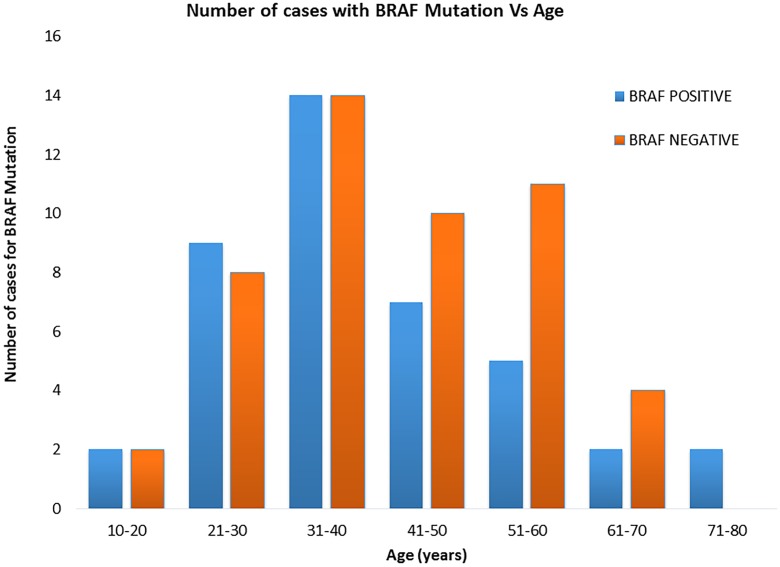
We also have shown a significantly higher frequency of BRAF V600E mutation in patients with ages younger than 55 years. Other studies [43–45] have shown a higher frequency BRAF V600E mutation among patients with ages higher than 55 years. We believe that this difference in the age pattern of BRAF V600E mutation is mainly due to the differences in patients samples between ours and these studies; as most of our PTC samples (70%) are from patients with ages younger than 55 years (Fig 2). In addition, a previous study in the UAE [16] have also shown more than three quarters of PTC cases were diagnosed below the age of 55 years. Geng et al. [46] have also shown a higher frequency of PTC with BRAF V600E mutation in pediatric age group of less than 10 years of age.
Fig 2.
Moreover, we have shown a significant correlation between BRAF V600E mutation and PTC larger than 5cm in diameter and positive surgical margin suggesting an association between BRAF V600E mutation and an aggressive phenotype. This was also seen in other studies [47–50].
The association of a large size PTC with BRAF V600E mutation suggesting a critical rule of this mutation with cell proliferation. On the other hand, the clear associations of BRAF V600E mutation with positive surgical margin in this study points towards a crucial role of this mutation in increasing invasiveness of proliferating neoplastic cells. This was reported by Mesa et al., whom have shown conditional expression of BRAF V600E in thyroid cells markedly increased the Matrigel™ invasion of the transformed thyroid cells, which is more invasive than RET/PTC expressed cells [51].
We have shown a significant correlation between lymph node metastasis and BRAF V600E mutation, this finding supported by Carol et al. study [52] Lymphovascular invasion (LVI) is an important prognostic factor in in PTC and significantly associated with BRAF V600E mutation suggesting the presence of LVI should be considered as an indicator of aggressive clinicopathological features and patients with positive LVI should be followed up carefully for possible recurrence or metastasis [53]. In addition, BRAFV600E mutation is an independent predictor of lymph node metastasis in PTC [54, 55].
Conclusion
BRAF V600E and KRAS mutation are seen in a significant number of PTC in the UAE. BRAF mutation is significantly correlated with large tumor size, positive surgical margins and lymph node metastasis suggesting an association between BRAF V600E mutation and tumor growth and spread.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the College of Medicine & Health Sciences, UAE University (Research Grant 31M176) to JAK and the Laboratory Medicine Department, Anatomic Pathology Division at Tawam Hospital, Al Ain City, UAE. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013; 965212 10.1155/2013/965212. 10.1155/2013/965212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abubaker J, Jehan Z, Bavi P, Sultana M, Al-Harbi S, Ibrahim M, et al. Clinico-pathological analysis of papillary thyroid cancer with PIK3CA alterations in a Middle Eastern population. J Clin Endocrinol Metab. 2008; 93:611–8. 10.1210/jc.2007-1717 [DOI] [PubMed] [Google Scholar]
- 3.Lloyd RV, Osamura RY, Klöppel G, Rosai J (editors) WHO Classification of Tumours of Endocrine Organs, 4th edn Lyon, France: IARC, 2017. [Google Scholar]
- 4.Shah JP. Thyroid carcinoma: epidemiology, histology, and diagnosis. Clin Adv Hematol Oncol. 2015; 13:3–6. [PMC free article] [PubMed] [Google Scholar]
- 5.Trovisco V, Soares P, Preto A, Castro P, Máximo V, Sobrinho-Simões M. Molecular genetics of papillary thyroid carcinoma: great expectations. Arq Bras Endocrinol Metabol. 2007; 51: 643–53. 10.1590/s0004-27302007000500002 [DOI] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014. October 23;159(3):676–90. 10.1016/j.cell.2014.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tavares C, Melo M, Cameselle-Teijeiro JM, Soares P, Sobrinho-Simões M. Endocrine tumours: Genetic predictors of thyroid cancer outcome. Eur J Endocrinol. 2016. April;174(4):R117–26 10.1530/EJE-15-0605 [DOI] [PubMed] [Google Scholar]
- 8.Tang KT, Lee CH. BRAF mutation in papillary thyroid carcinoma: pathogenic role and clinical implications. J Chin Med Assoc. 2010; 73:113–28. 10.1016/S1726-4901(10)70025-3 [DOI] [PubMed] [Google Scholar]
- 9.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endoc Rev. 2007; 28:742–62. [DOI] [PubMed] [Google Scholar]
- 10.Mitsutake N, Knauf JA, Mitsutake S, Mesa C Jr, Zhang L, Fagin JA. Conditional BRAFV600E expression induces DNA synthesis, apoptosis, dedifferentiation, and chromosomal instability in thyroid PCCL3 cells. Cancer Res. 2005. 65:2465–73. 10.1158/0008-5472.CAN-04-3314 [DOI] [PubMed] [Google Scholar]
- 11.Giordano TJ, Kuick R, Thomas DG, Misek DE, Vinco M, Sanders D, et al. Molecular classification of papillary thyroid carcinoma: distinct BRAF, RAS, and RET/PTC mutation specific gene expression profiles discovered by DNA microarray analysis. Oncogene 2005; 24:6646–56. 10.1038/sj.onc.1208822 [DOI] [PubMed] [Google Scholar]
- 12.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, et al. Roles of the Raf /MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007; 1773:1263–84. 10.1016/j.bbamcr.2006.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song YS, Lim JA, Park YJ. Mutation Profile of Well-Differentiated Thyroid Cancer in Asians. Endocrinol Metab. (Seoul) 2015; 30: 252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukushima T, Takenoshita S. Roles of RAS and BRAF mutations in thyroid carcinogenesis. Fukushima J Med Sci. 2005; 51:67–5. 10.5387/fms.51.67 [DOI] [PubMed] [Google Scholar]
- 15.Cancer registry report, Ministry of Health and Prevention, 2014. http://www.mohap.gov.ae/Files/MOH_OpenData/UAE%20Cancer%20Registry%20Report%202014_En.pdf.
- 16.Al-Zaher N, Al-Salam S, El Teraifi H. Thyroid carcinoma in the United Arab Emirates: perspectives and experience of a tertiary care hospital. Hematol Oncol Stem Cell Ther. 2008; 1:14–21. 10.1016/s1658-3876(08)50055-0 [DOI] [PubMed] [Google Scholar]
- 17.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006; 295:2164–7. 10.1001/jama.295.18.2164 [DOI] [PubMed] [Google Scholar]
- 18.Jung CK, Little MP, Lubin JH, Brenner AV, Wells SA Jr, Sigurdson AJ, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab. 2014; 99: 276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacCorkle RA, Tan TH. Mitogen-activated protein kinases in cell-cycle control. Cell Biochem Biophys. 2005; 43:451–61. 10.1385/CBB:43:3:451 [DOI] [PubMed] [Google Scholar]
- 20.Siraj AK, Masoodi T, Bu R, Beg S, Al-Sobhi SS, Al-Dayel F, et al. Genomic Profiling of Thyroid Cancer Reveals a Role for Thyroglobulin in Metastasis. Am J Hum Genet. 2016; 98:1170–80. 10.1016/j.ajhg.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goutas N, Vlachodimitropoulos D, Bouka M, Lazaris AC, Nasioulas G, Gazouli M. BRAF and K-RAS mutation in a Greek papillary and medullary thyroid carcinoma cohort. Anticancer Res. 2008; 28: 305–8. [PubMed] [Google Scholar]
- 22.Di Cristofaro J, Marcy M, Vasko V, Sebag F, Fakhry N, Wynford-Thomas D, et al. Molecular genetic study comparing follicular variant versus classic papillary thyroid carcinomas: association of N-ras mutation in codon 61 with follicular variant. Hum Pathol. 2006; 37:824–30. 10.1016/j.humpath.2006.01.030 [DOI] [PubMed] [Google Scholar]
- 23.Naito H, Pairojkul C, Kitahori Y, Yane K, Miyahara H, Konishi N. Different ras gene mutational frequencies in thyroid papillary carcinomas in Japan and Thailand. Cancer Lett. 1998; 131:171–5. 10.1016/s0304-3835(98)00149-9 [DOI] [PubMed] [Google Scholar]
- 24.Knauf JA, Ouyang B, Knudsen ES, Fukasawa K, Babcock G, Fagin JA. Oncogenic RAS induces accelerated transition through G2/M and promotes defects in the G2 DNA damage and mitotic spindle checkpoints. J Biol Chem. 2006; 281: 3800–9. 10.1074/jbc.M511690200 [DOI] [PubMed] [Google Scholar]
- 25.Rosenbaum E, Hosler G, Zahurak M, Cohen Y, Sidransky D, Westra WH. Mutational activation of BRAF is not a major event in sporadic childhood papillary thyroid carcinoma. Mod Pathol. 2005; 18:898–902. 10.1038/modpathol.3800252 [DOI] [PubMed] [Google Scholar]
- 26.Guan H, Ji M, Bao R, Yu H, Wang Y, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009; 94:1612–7. 10.1210/jc.2008-2390 [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Ji M, Wang W, Miao Z, Hou P, Chen X, et al. Association of the T1799A BRAF mutation with tumor extrathyroidal invasion, higher peripheral platelet counts, and over-expression of platelet-derived growth factor-B in papillary thyroid cancer. Endocr Relat Can. 2008; 15:183–90. [DOI] [PubMed] [Google Scholar]
- 28.Kebebew E, Weng J, Bauer J, Ranvier G, Clark OH. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg. 2007; 246:466–71 10.1097/SLA.0b013e318148563d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frasca F, Nucera C, Pellegriti G, Gangemi P, Attard M, et al. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr Relat Can. 2008; 15:191–205. [DOI] [PubMed] [Google Scholar]
- 30.Lupi C, Giannini R, Ugolini C, Proietti A, Berti P, et al. Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2007; 92:4085–90. 10.1210/jc.2007-1179 [DOI] [PubMed] [Google Scholar]
- 31.Elisei R, Ugolini C, Viola D, Lupi C, Biagini A, Giannini R, et al. BRAF (V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008; 93:3943–9. 10.1210/jc.2008-0607 [DOI] [PubMed] [Google Scholar]
- 32.Fugazzola L, Mannavola D, Cirello V, Vannucchi G, Muzza M, Vicentini L, et al. BRAF mutations in an Italian cohort of thyroid cancers. Clin Endocrinol. (Oxf) 2004; 61:239–43. [DOI] [PubMed] [Google Scholar]
- 33.Costa AM, Herrero A, Fresno MF, Heymann J, Alvarez JA, Cameselle-Teijeiro J, et al. BRAF mutation associated with other genetic events identifies a subset of aggressive papillary thyroid carcinoma. Clin Endocrino.l (Oxf) 2008; 68:618–34. [DOI] [PubMed] [Google Scholar]
- 34.Zoghlami A, Roussel F, Sabourin JC, Kuhn JM, Mariea JP, Dehesdin D., et al. BRAF mutation in papillary thyroid carcinoma: Predictive value for long-term prognosis and radioiodine sensitivity. Eur Annals Otorhinolaryngology, Head and Neck dis. 2014; 131: 7–13. [DOI] [PubMed] [Google Scholar]
- 35.Kim TY, Kim WB, Rhee YS, Song JY, Kim JM, Gong G, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol. (Oxf) 2006; 65:364–8. [DOI] [PubMed] [Google Scholar]
- 36.Ito Y, Yoshida H, Maruo R, Morita S, Takano T, Hirokawa M, et al. BRAF mutation in papillary thyroid carcinoma in a Japanese population: its lack of correlation with high-risk clinicopathological features and disease-free survival of patients. Endocr J. 2009; 56:89–97. 10.1507/endocrj.k08e-208 [DOI] [PubMed] [Google Scholar]
- 37.Jin Langping, Chen Endong, Dong Siyang, Cai Yefeng, Zhang Xiangjian, Zhou Yili, et al. BRAF and TERT promoter mutations in the aggressiveness of papillary thyroid carcinoma: a study of 653 patients. Oncotarget 2016; 7: 18346–55 10.18632/oncotarget.7811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.George Nelson, Agarwal Amit, Kumari Niraj, Agarwal Sarita, Krisnani Narendra, Gupta Sushil Kumar. Mutational Profile of Papillary Thyroid Carcinoma in an Endemic Goiter Region of North India. Indian J Endocrinol Metab. 2018; 22: 505–10. 10.4103/ijem.IJEM_441_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang KT, Lee CH. BRAF mutation in papillary thyroid carcinoma: pathogenic role and clinical implications. J Chin Med Assoc. 2010; 73:113–28. 10.1016/S1726-4901(10)70025-3 [DOI] [PubMed] [Google Scholar]
- 40.S Marwa, EL S Marwa, Hussein O, H Husam. Diagnostic Value of High Resolution Neckultrasongraghy, Fine Needle Aspiration Cytology and BRAFV600E Mutation in Diagnosis of Malignant Thyroid Nodules. J Endocrinol Thyroid Res. 2017; 2:555580. [Google Scholar]
- 41.Ugolini C, Giannini R, Lupi C, Salvatore G, Miccoli P, Proietti A, et al. Presence of BRAF V600E in veryearly stages of papillary thyroid carcinoma. Thyroid 2007; 17:381–8. 10.1089/thy.2006.0305 [DOI] [PubMed] [Google Scholar]
- 42.Knauf JA, Ma X, Smith EP, Zhang L, Mitsutake N, Liao XH, et al. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 2005; 65:4238–45. 10.1158/0008-5472.CAN-05-0047 [DOI] [PubMed] [Google Scholar]
- 43.Pessôa-Pereira D, Medeiros MFDS, Lima VMS, Silva JCD Jr, Cerqueira TLO, Silva ICD, et al. Association between BRAF (V600E) mutation and clinicopathological features of papillary thyroid carcinoma: a Brazilian single-centre case series. Arch Endocrinol Metab. 2019; 63(2):97–106. 10.20945/2359-3997000000120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015; 33:42–50. 10.1200/JCO.2014.56.8253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005; 90:6373–6379. 10.1210/jc.2005-0987 [DOI] [PubMed] [Google Scholar]
- 46.Geng J, Wang H, Liu Y, Tai J, Jin Y, Zhang J, et al. Correlation between BRAF V600E mutation and clinicopathological features in pediatric papillary thyroid carcinoma. Sci China Life Sci. 2017; 60(7):729–38. 10.1007/s11427-017-9083-8 [DOI] [PubMed] [Google Scholar]
- 47.Chunping L, Tianwen C, Zeming L. Associations between BRAFV600E and prognostic factors and poor outcomes in papillary thyroid carcinoma: a meta-analysis. World J Surg Oncol. 2016; 14: 241 10.1186/s12957-016-0979-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mingzhao X, Rengyun L, Xiaoli L, Avaniyapuram KM, Guangwu Z, Martha A, et al. BRAF V600E and TERT Promoter Mutations Cooperatively Identify the Most Aggressive Papillary Thyroid Cancer With Highest Recurrence. J Clin Oncol. 2014; 32: 2718–26. 10.1200/JCO.2014.55.5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li C, Aragon HP, Lee KC, Lee LC, Fox AC, Beninato T, et al. Does BRAF V600E mutation predict aggressive features in papillary thyroid cancer? Results from four endocrine surgery centers. J Clin Endocrinol Metab. 2013; 98:3702–12. 10.1210/jc.2013-1584 [DOI] [PubMed] [Google Scholar]
- 50.Yang LB, Sun LY, Jiang Y, Tang Y, Li ZH, Zhang HY, et al. , The Clinicopathological Features of BRAF Mutated Papillary Thyroid Cancers in Chinese Patients. Inter J of Endocr. 2015; 642046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mesa C Jr, Mirza M, Mitsutake N, Sartor M, Medvedovic M, Tomlinson C. Conditional activation of RET/PTC3 and BRAFV600E in thyroid cells is associated with gene expression profiles that predict a preferential role of BRAF in extracellular matrix remodeling. Cancer Res. 2006; 66: 6521–9. 10.1158/0008-5472.CAN-06-0739 [DOI] [PubMed] [Google Scholar]
- 52.Li Carol, Lee Kathleen C., Schneider Eric B., Zeiger Martha A. BRAF V600E mutation and Its Association with Clinicopathological Features of Papillary Thyroid Cancer: A Meta-Analysis J Clin Endocrinol Metab. 2012. December; 97(12): 4559–4570. 10.1210/jc.2012-2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sezer A, Celik M, Yilmaz BB, Can N, Tastekin E, Ayturk S, et al. Relationship between lymphovascular invasion and clinicopathological features of papillary thyroid carcinoma. Bosn J Basic Med Sci. 2017; 17:144–51. 10.17305/bjbms.2017.1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zou Minjing, Essa YB, Alzahrani AS, BinHumaid FS., Alkhafaji D, Al-Rijjal RA, et al. Concomitant RAS, RET/PTC, or BRAF Mutations in Advanced Stage of Papillary Thyroid Carcinoma. Thyroid 2014; 24:1256–66 10.1089/thy.2013.0610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melillo RM, Castellone MD, Guarino V, De Falco V, Cirafici AM, Salvatore G, et al. The RET/PTCRAS- BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J Clin Invest. 2005; 15:1068–81. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.