Abstract
Background
Ulcerative colitis (UC) is a chronic disease characterized by periods of activity and remission. The platelet, one of the main activators of neutrophils, contains Interleukin 8 (IL-8), a potent neutrophil chemo-attractant and P-selectin that induces excretion of superoxide in the neutrophils, forming platelet-neutrophil aggregates that are increased in individuals with active UC, hence an index of both cells could produce a monitoring tool. No previous studies have evaluated this ratio in UC.
Goal
To evaluate the clinical utility of the Neutrophil-Platelet (NeuPla) ratio in patients with UC.
Study
A total of 158 patients with a diagnosis of UC. This index was based on the ratio between platelets and the neutrophil differential in blood count. The activity was classified using Mayo endoscopic sub-score, histological (Riley score) and for clinical was used the Truelove-Witts, Montreal, Mayo and Yamamoto-Furusho scores.
Results
The correlation of the NeuPla ratio with activity scales were significant (P <0.05). An optimal cut-off point to classify patients with clinical activity was 14.94 with a sensitivity 87.95% and specificity 63.5 and endoscopy activity with a cut-off 14.64 with a sensibility of 70.5% and specificity of 61.8%.
Conclusions
The NeuPla ratio showed an adequate diagnostic utility to identify UC patients with clinical and endoscopy activity without the use of invasive studies like a colonoscopy or expensive fecal biomarkers such as calprotectin and have a better diagnostic performance in comparison to other serum biomarkers (C reactive protein, erythrocyte sedimentation rate and albumin).
Introduction
Ulcerative Colitis (UC) is a disease under the spectrum of Inflammatory Bowel Disease (IBD) characterized by chronic inflammation in the colonic mucosa and submucosa with exacerbation (chronic diarrhea with blood, abdominal pain, fatigue, weight loss) and remission periods.
Biomarkers like erythrocyte sedimentation rate (ESR) and C reactive protein (CRP) are used for monitoring the disease but present a low specificity and sensitivity in UC [1]. There are fecal biomarkers with better performance for detecting disease activity in UC like fecal calprotectin and lactoferrin [2], however, are very expensive.
Previous studies have shown a strong relationship between neutrophils and platelets in the pathophysiology of UC. For example, histopathology findings are characterized by the presence of neutrophils in the colonic mucosa in the UC patient [3,4] and there are serological markers such as anti-neutrophil cytoplasmic antibodies (ANCAs) present in most of the UC patients [5]. From a biochemical aspect, the neutrophils are the principal source of calprotectin [6].
Platelets present granules with Interleukin 8 (IL-8) which is a potent neutrophil chemoattractant [7] and its cytoplasm contains P-selectin that induces overproduction of superoxide and the formation of neutrophil-platelet aggregates, which are elevated in patients with UC and related with the activity also in Dextran Sulfate Sodium (DSS)-Induced Colitis Model the inhibition of this aggregates suppress the appearance of colon inflammation [8,9]. The mean platelet volume (MPV) is correlated to the UC activity [10] as also the increase of white blood count (WBC) mainly neutrophils [11]. The interaction of neutrophil-platelet is a possible explanation of the high prevalence of thrombosis in UC due to the formation of Neutrophil Extracellular Traps (NETs) through Toll-like receptor 4 (TLR-4) [10]. A neutrophil-platelet ratio (NeuPla ratio) could be used as a monitor tool for UC, this ratio has been already used in other pathologies [12,13] and only required a complete blood count (CBC) making it an accessible, fast, easy to use and low-cost tool.
The present study explores the clinical utility of NeuPla ratio for the evaluation of UC activity and was correlated with endoscopy findings (Mayo sub-score, Montreal), histological (Riley score) [14], biochemical and clinical scores (Truelove-Witts and Mayo) [15,16] and novel integral index such as Yamamoto-Furusho score [17].
Material and methods
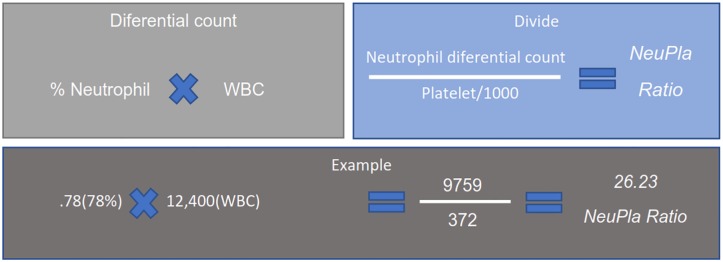
A total of 158 patients with UC were included belonging to the Inflammatory Bowel Disease Clinic at the National Institute of Medical Sciences and Nutrition Salvador Zubirán (INCMNSZ) between July 2016 and September of 2019. All patients were older than eighteen years old with a definitive diagnosis based on clinical criteria (chronic diarrhea with blood), biochemistry, endoscopic features and histopathology findings. The clinical and demographic data were gathered from the clinical records: sex, current age, age at diagnosis, disease duration, extra-intestinal manifestations (EIMs), current medical treatment and extent of disease. Biochemical parameters included were CBC, CRP, ESR and fecal calprotectin. The NeuPla ratio was calculated by the differential count of neutrophils and the division with the platelets divided by 1000 as shown in Fig 1.
Fig 1. Procedure to calculate the NeuPla ratio.
WBC; White Blood Count, NeuPla; Neutrophil-Platelet ratio.
The biochemical activity was evaluated by fecal calprotectin, CRP and ESR. The endoscopy activity by Mayo sub-score and Montreal. Histological activity by Riley score, and clinical activity by Truelove-Witts, full Mayo score and Yamamoto-Furusho index. The full Mayo score was used to assess disease activity. Remission was defined as a full Mayo score ≤2. Mild activity was defined as a full Mayo score 3 to 5. Moderate activity was defined as a full Mayo score 6 to 9. Severe activity was defined as a full Mayo score 10 to 12.
The exclusion criteria were infectious colitis, lack of recent laboratory results, laboratory results from another hospital.
Results were expressed as mean ± standard deviation (or standard error of the mean) or median and interquartile range, depending on sample distribution. Comparisons between groups were made by Student’s T-test or U Mann Whitney for independent samples, according to the sample distribution. Spearman’s correlation coefficient was used for correlation. A curve ROC (receiver operating characteristic curve) for the optimal cut determining the sensitivity, specificity, positive predictive value, and negative predictive value. A p-value < 0.05 was considered statistically significant. The analysis was performed with SPSS software v. 24 (SPSS Inc., Chicago, IL).
Ethical considerations
This work was accomplished according to the principles expressed in the Declaration of Helsinki. This study was approved by the research committee as well as the ethics of the National Institute of Medical Sciences and Nutrition Salvador Zubirán with reference number 3006 and also a written informed consent was obtained from all patients.
Results and discussion
One hundred and fifty-eight patients were studied, and the clinical and demographic data are shown in Table 1. Serological and fecal biomarkers between patients in remission and clinical activity are shown in Table 2.
Table 1. Demographic and clinical characteristics of patients with UC.
| Variables | |
|---|---|
| Sex (Male/Female) | 73(44.5) /87(54.4) |
| Current age (years) | 43.53±14.35 |
| Age at diagnosis (years) | 36.6±15.07 |
| Years from diagnosis | 11.27±8.136 |
| Activity | |
| • Active | 47(29.4) |
| • Remission | 113(70.6) |
| Extent of disease | |
| • Proctitis (E1) | 22(13.8) |
| • Left colon (E2) | 31(19.4) |
| • Pancolitis (E3) | 107 (66.8% |
| Extraintestinal Manifestations (%) | 55(34.81) |
| • Arthralgia | 28(17.1) |
| • Primary Sclerosing Cholangitis | 12(7.3) |
| • Spondylitis | 4(2.4) |
| Treatment (%) | |
| • Mesalazine | 154(93.9) |
| • Steroids | 43(26.2) |
| • Azathioprine | 26(15.9) |
Values are expressed as number (%), mean ± standard deviation
Table 2. Serological and fecal biomarkers in active and remission UC patients.
| Clinical Activity | Mean | Standard Deviation | P value | |
|---|---|---|---|---|
| Fecal Calprotectin | REMISSION | 397.2857 | 1092.37036 | < .001 |
| ACTIVE | 2163.2441 | 3316.19933 | ||
| CRP | REMISSION | .55419 | 1.037965 | < .001 |
| ACTIVE | 1.92998 | 3.380446 | ||
| Albumin | REMISSION | 4.4519 | .37880 | < .001 |
| ACTIVE | 4.0350 | .55233 | ||
| Platelet count | REMISSION | 267.39 | 71.619 | .194 |
| ACTIVE | 284.66 | 85.763 | ||
| MPV | REMISSION | 8.472 | .9252 | .833 |
| ACTIVE | 8.436 | 1.0969 | ||
| WBC | REMISSION | 6115.32 | 1652.887 | < .001 |
| ACTIVE | 8454.23 | 2895.326 | ||
| Leukocytes | REMISSION | 1833.78468 | 669.155945 | .459 |
| ACTIVE | 1748.09149 | 650.325310 | ||
| Neutrophils | REMISSION | 3646.10435 | 1250.753429 | < .001 |
| ACTIVE | 5838.14843 | 2478.353209 | ||
| NeuPla | REMISSION | 14.02382 | 4.529504 | < .001 |
| ACTIVE | 21.48726 | 8.950042 | ||
| NLR | REMISSION | 2.21584 | 1.075825 | < .001 |
| ACTIVE | 4.06170 | 2.717627 |
CRP; C Reactive Protein, MPV; Mean Platelet Volume, WBC; White blood count, ESR; Erythrocyte Sedimentation Rate, NeuPla; Neutrophil-Platelet ratio NLR; Neutrophil-Lymphocyte Ratio
Correlation between NeuPla ratio and UC index scores
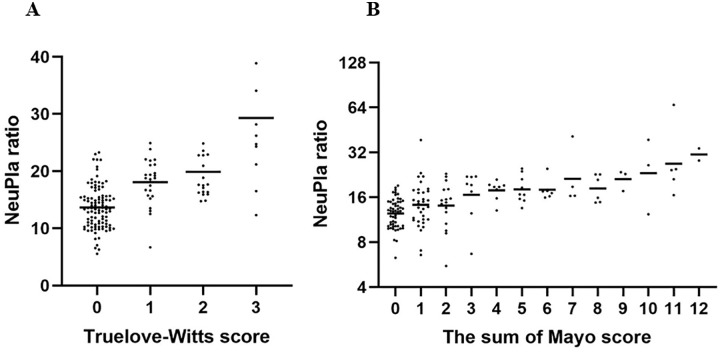
The mean of the NeuPla ratio was 14.02 ± 4.52 in UC patients with remission, significantly lower compared to mild (16.4 ± 6.90 P = 0.003), moderate (18.39 ± 9.52 P = 0.0003), and severe activity (21.44 ± 5.39 P = 0.00003), the distribution of NeuPla ratio is shown in Fig 2. The correlation between the NeuPla ratio with several indexes was statistically significant for Mayo endoscopic sub-score, histopathological index (Riley score), Truelove-Witts, Yamamoto-Furusho, Full Mayo score, and Montreal as you can see in Table 3.
Fig 2. Distribution of NeuPla ratio.
Scatter plot of NeuPla ratio and (A) Clinical activity (Truelove-Witts score); and (B) Clinical-endoscopic activity (Mayo full score).
Table 3. Correlations of serological and fecal biomarkers with endoscopy, histology and activity scales.
| CALPROTECTIN | ENDOSCOPY | PATHOLOGY | TRUELOVE-WITTS | MAYO | YAMAMOTO-FURUSHO | MONTREAL | ||
|---|---|---|---|---|---|---|---|---|
| NeuPla | Rho | .532** | .462** | .288** | .476** | .585** | .544** | .346** |
| P | .000 | .000 | .000 | .000 | .000 | .000 | .000 | |
| NLR | Rho | .347** | .310** | -.164** | .370** | .439** | .401** | .208** |
| P | .000 | .000 | .000 | .000 | .000 | .000 | .000 | |
| Calprotectin | Rho | 1.000 | .658** | .363** | .567** | .691** | .608** | .519** |
| P | .000 | .000 | .000 | .000 | .000 | .000 | ||
| Neutrophil Count | Rho | .453** | .482** | .285** | .469** | .573** | .607** | .377** |
| P | .000 | .000 | .000 | .000 | .000 | .000 | .000 | |
| Lymphocyte Count | Rho | .111 | .090 | .020 | .002 | .045 | .080 | .137 |
| P | .265 | .262 | .805 | .982 | .574 | .320 | .085 | |
| Platelet Count | Rho | .081 | .160* | .056 | .136 | .193* | .223 | .179* |
| P | .416 | .045 | .487 | .088 | .015 | .003 | .024 | |
| CRP | Rho | .330** | .355** | .205* | .294** | .348** | .613** | .295** |
| P | .001 | .000 | .013 | .000 | .000 | .000 | .000 | |
| ESR | Rho | .195 | .222** | .099 | .196* | .273** | .315** | .156 |
| P | .051 | .006 | .234 | .017 | .001 | .000 | .058 | |
CRP; C Reactive Protein, ESR; Erythrocyte Sedimentation Rate, NeuPla; Neutrophil-Platelet ratio NLR; Neutrophil-Lymphocyte Ratio
*<0.05
**<0.01
No association was found between the dose of corticosteroids more than 20 mg vs less than 20 mg (P = 0.51) and more than 20 mg vs non-users (P = 0.38). On the other hand, no association was found between standard dose of azathioprine vs low dose of azathioprine less than 125mg per day (P = 0.32) and vs non-users of azathioprine (P = .11).
Correlation between NeuPla ratio and biochemical markers
NeuPla ratio surpasses the serum biomarkers correlations with endoscopy. As also, correlates with some of these biomarkers like CRP (Rho = .308, P = 0.0001), serum albumin (Rho = .383, P = 0.0001) and fecal calprotectin (rho: 0.532, P = .0001).
Clinical utility of NeuPla Ratio
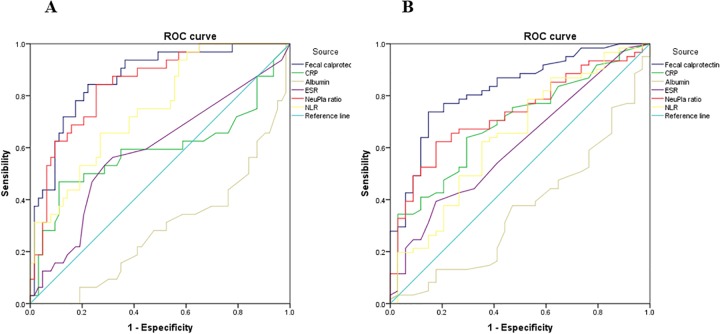
ROC curve was used to determine the cut-off level of NeuPla ratio according to clinical activity (optimal cut-off 14.94 with a sensibility of 87.95% and specificity of 63.5% and AUC of .853) as well as endoscopy activity (optimal cut-off 14.64 with a sensibility of 70.5%, specificity of 61.8% and AUC of .728) as you can see in Fig 3. The sensibility and specificity were made for other inflammatory biomarkers shown in Table 4.
Fig 3. ROC curve of serological and fecal biomarkers to evaluate endoscopy activity.
(A) ROC curve clinical activity; (B) ROC curve endoscopy activity. CPR; C Reactive Protein, ESR; Erythrocyte Sedimentation Rate, NeuPla; Neutrophil-Platelet ratio NLR; Neutrophil-Lymphocyte Ratio.
Table 4. Diagnostic performance of the NeuPla ratio with other inflammatory biomarkers for the evaluation of endoscopic activity in patients with UC.
Clinical and endoscopy activity.
| Activity | Biomarker | Sensibility | Specificity |
|---|---|---|---|
| Clinical | CRP (>.355mg/dL) | 59.4% | 63.6% |
| NLR (>2) | 75% | 52.4% | |
| NeuPla (>14.94) | 87.95% | 63.5% | |
| Fecal Calprotectin (>216) | 90.6% | 65.1% | |
| Endoscopy | CRP (>.355 mg/dL) | 52.5% | 70.6% |
| NLR (>2.09) | 63.9% | 58.8% | |
| NeuPla (14.64) | 70.5% | 61.8% | |
| Fecal Calprotectin (>366) | 73.8% | 85.3% |
CRP; C Reactive Protein, NeuPla; Neutrophil-Platelet ratio NLR; Neutrophil-Lymphocyte Ratio
Discussion
This is the first study to our best knowledge that evaluated the clinical utility of NeuPla ratio in patients with UC, the NeuPla ratio had a better diagnostic performance than traditional biomarkers such as CRP, ESR, and serum albumin with a sensibility and specificity similar to fecal calprotectin, however, NeuPla ratio has a lower cost, easy access and faster results because it can be calculated with a complete blood count. Also, is adaptable for hospitals that are lacking of fecal calprotectin or low access due to the high cost.
Many studies demonstrated a clinical performance of Neutrophil-to-Lymphocyte Ratio (NLR) in UC [18,19] though some of them showed a suboptimal function and low correlation as also with no association with disease extension [20,21]. It had been suggested in NLR that the prognostic value could be produced by the total count of neutrophils and lymphocytes count makes little contribution or neither [22]. NeuPla ratio had a higher correlation with disease activity a better sensibility and specificity as also correlated with extent of disease (rho = .346 P = < .05) making it a better tool for UC. An explain of the better performance could be seen in Table 3 as platelet count (one of the elements of NeuPla ratio) had a better correlation with endoscopy activity, Full Mayo and Montreal scores than lymphocyte count (one of the elements of NLR).
The functionality of NeuPla ratio in UC might be explained by neutrophil-platelet relation is important in the process for migration of neutrophils to the tissues and contributes to the inflammation process [23]. On the other hand, Crohn´s disease (CD) has a delay of the recruitment of neutrophils to sites of infection and trauma as also an abnormally low secretion of IL-8 (potent neutrophil chemo-attractant) [24,25]. Some studies had been taken the neutrophil-platelet approach. For example a study on colorectal cancer and neutrophil-platelet score (NPS) showed that highest NPS score present a worse outcome as also high correlation with CRP and serum albumin the authors hypothesized that this score could be an inflammatory systemic marker and that had also clinical utility in other types of cancer like breast, bladder, and prostate [22]. In the ischemic stroke, it is well taken as a survival marker to 90 days as it has been demonstrated that a lower NeuPla ratio translates as less inflammation and necrosis due to authors conclude again that the NeuPla ratio is a tool that could measure inflammation and necrosis [12].
The importance of this study recalls in showing the neutrophil and platelet could be also a piece of the pathogenesis in the inflammatory process in UC patients.
NeuPla ratio is a way to show the proportion between neutrophils and platelets a NeuPla ratio of 10 means that are 1 neutrophil per 100 platelets. This relation rises when a flare or activity of the disease appears, may be because as proportion more neutrophils are produced than platelets or because the platelets take 2 to 4 days to rise even 6 in their active form [26]. This UC population has no presented platelet count more than 450,000 cells per microliter defined as thrombocytosis and it could be possible that patients who present a severe activity, the NeuPla ratio is not useful because the clinical symptoms are obvious. However, NeuPla ratio could be very useful in those patients with mild to moderate activity in which the symptoms are not evident in order to optimize the medical treatment and avoiding complications or a bad outcome. Finally, NeuPla ratio could be used in hospitals where lack of fecal biomarkers like calprotectin or limited access for the cost and time for getting results.
In conclusion, the NeuPla ratio showed an adequate diagnostic utility to identify patients with clinical and endoscopy activity in UC patients without the use of invasive studies like a colonoscopy or expensive fecal biomarkers such as calprotectin. It is necessary to perform future studies to understand the nature of this Neutrophil-Platelet proportion in UC patients.
Supporting information
(XLSX)
Data Availability
All relevant data are within the manuscript and its Supporting Information file.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Iskandar HN, Ciorba MA. Biomarkers in inflammatory bowel disease: Current practices and recent advances. Transl Res. 2012;159(4):313–25. 10.1016/j.trsl.2012.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mumolo MG, Bertani L, Ceccarelli L, Laino G, Di Fluri G, Albano E, et al. From bench to bedside: Fecal calprotectin in inflammatory bowel diseases clinical setting. World J Gastroenterol. 2018;24(33):3681–94. 10.3748/wjg.v24.i33.3681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geboes K, Riddell R, Öst A, Jensfelt B, Persson T, Löfberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47(3):404–9. 10.1136/gut.47.3.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guardiola J, Lobatón T, Rodríguez-Alonso L, Ruiz-Cerulla A, Arajol C, Loayza C, et al. Fecal Level of Calprotectin Identifies Histologic Inflammation inPatients With Ulcerative Colitis in Clinical and Endoscopic Remission. Clin Gastroenterol Hepatol. 2014;12(11):1865–70. 10.1016/j.cgh.2014.06.020 [DOI] [PubMed] [Google Scholar]
- 5.Bennike T, Birkelund S, Stensballe A, Andersen V. Biomarkers in inflammatory bowel diseases: Current status and proteomics identification strategies. World J Gastroenterol. 2014;20(12):3231–44. 10.3748/wjg.v20.i12.3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanai H, Takeuchi K, Iida T, Kashiwagi N, Saniabadi AR, Matsushita I, et al. Relationship between fecal calprotectin, intestinal inflammation, and peripheral blood neutrophils in patients with active ulcerative colitis. Dig Dis Sci. 2004;49(9):1438–43. 10.1023/b:ddas.0000042243.47279.87 [DOI] [PubMed] [Google Scholar]
- 7.Danese S, De La Motte C, Fiocchi C. Platelets in inflammatory bowel disease: Clinical, pathogenic, and therapeutic implications. Am J Gastroenterol. 2004;99(5):938–45 [DOI] [PubMed] [Google Scholar]
- 8.Pamuk GE, Vural Ö, Turgut B, Demir M, Ümit H, Tezel A. Increased circulating platelet-neutrophil, platelet-monocyte complexes, and platelet activation in patients with ulcerative colitis: A comparative study. Am J Hematol. 2006;81(10):753–9. 10.1002/ajh.20655 [DOI] [PubMed] [Google Scholar]
- 9.Gironella M, Mollà M, Salas A, Soriano A, Sans M, Piqué JM, et al. The role of P-selectin in experimental colitis as determined by antibody immunoblockade and genetically deficient mice. J Leukoc Biol. 2002;72(1):56–64. [PubMed] [Google Scholar]
- 10.Voudoukis E, Karmiris K, Koutroubakis IE. Multipotent role of platelets in inflammatory bowel diseases: A clinical approach. World J Gastroenterol. 2014;20(12):3180–90. 10.3748/wjg.v20.i12.3180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thayer WR, Charland C, Field CE. The Subpopulations of Circulating White Blood Cells in Inflammatory Bowel Disease. Gastroenterology. 1976;71(3):379–84. [PubMed] [Google Scholar]
- 12.Jin PP, Li XM, Chen J, Zhang ZR, Hu WW, Chen LY, et al. Platelet-to-neutrophil ratio is a prognostic marker for 90-days outcome in acute ischemic stroke. J Clin Neurosci. 2019;63:110–5. 10.1016/j.jocn.2019.01.028 [DOI] [PubMed] [Google Scholar]
- 13.Mercier J, Voutsadakis IA. The platelets-neutrophils to lymphocytes ratio: A new prognostic marker in metastatic colorectal cancer. J Gastrointest Oncol. 2018;9(3):478–86. 10.21037/jgo.2018.03.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riley SA, Mani V, Goodman MJ, Dutt S, Herd ME. Microscopic activity in ulcerative colitis: What does it mean? Gut. 1991;32(2):174–8. 10.1136/gut.32.2.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Truelove SC. Cortisone in Ulcerative Colitis Final Report on a Therapeutic Trial. Br Med J. 1955;2(4947):1041–8. 10.1136/bmj.2.4947.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated Oral 5-Aminosalicylic Acid Therapy for Mildly to Moderately Active Ulcerative Colitis. N Engl J Med. 2010;317(26):1625–9. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto-Furusho JK, Bozada-Gutiérrez KE, Sánchez-Rodríguez A, Bojalil-Romano F, Barreto-Zuñiga R, Martínez-Benitez B. Validation of a novel integral disease index for evaluating the grade of activity in Mexican patients with ulcerative colitis: A prospective cohort study. Rev Gastroenterol Mex. 2019; [DOI] [PubMed] [Google Scholar]
- 18.Nishida Y, Hosomi S, Yamagami H, Yukawa T, Otani K, Nagami Y, et al. Neutrophil-to-lymphocyte ratio for predicting loss of response to infliximab in ulcerative colitis. PLoS One. 2017;12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishida Y, Hosomi S, Yamagami H, Sugita N, Itani S, Yukawa T, et al. Pretreatment neutrophil-to-lymphocyte ratio predicts clinical relapse of ulcerative colitis after tacrolimus induction. PLoS One. 2019;14(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Celikbilek M, Dogan S, Ozbakir O, Zararsiz G, Kücük H, Gürsoy S, et al. Neutrophil-Lymphocyte Ratio as a Predictor of Disease Severity in Ulcerative Colitis. J Clin Lab Anal. 2013;27(1):72–6. 10.1002/jcla.21564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akpinar MY, Ozin YO, Kaplan M, Ates I, Kalkan IH, Kilic ZMY, et al. Platelet-to-Lymphocyte Ratio and Neutrophil-to-Lymphocyte Ratio Predict Mucosal Disease Severity in Ulcerative Colitis. J Med Biochem. 2018;37(2):155–62. 10.1515/jomb-2017-0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watt DG, Proctor MJ, Park JH, Horgan PG, McMillan DC. The neutrophil-platelet score (NPS) predicts survival in primary operable colorectal cancer and a variety of common cancers. PLoS One. 2015;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sreeramkumar V, Adrover JM, Ballesteros I, Cuartero MI, Rossaint J, Bilbao I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science (80-). 2014;346(6214):1234–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segal AW. The role of neutrophils in the pathogenesis of Crohn’s disease. Eur J Clin Invest. 2018;48. [DOI] [PubMed] [Google Scholar]
- 25.Marks DJB, Harbord MWN, MacAllister R, Rahman FZ, Young J, Al-Lazikani B, et al. Defective acute inflammation in Crohn’s disease: A clinical investigation. Lancet. 2006;367(9511):668–78. 10.1016/S0140-6736(06)68265-2 [DOI] [PubMed] [Google Scholar]
- 26.Yan SLS, Russell J, Harris NR, Senchenkova EY, Yildirim A, Granger DN. Platelet abnormalities during colonic inflammation. Inflamm Bowel Dis. 2013;19(6):1245–53. 10.1097/MIB.0b013e318281f3df [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information file.