Abstract
Depression affects over 300 million individuals worldwide and is responsible for most of the 800,000 annual suicides. Clinical practice guidelines (CPGs) for treatment of depression, founded on scientific evidence, are essential to improve patient care. However, economic and sociocultural factors may influence CPG elaboration, potentially leading to divergences in their recommendations. Consequently, we analyzed pharmacological recommendations for the treatment of depression from the most relevant CPGs. We included four CPGs with scores ≥ 80% for Domain 3 (rigor of development) on the Appraisal of Guidelines for Research and Evaluation and two other commonly used CPGs. The recommendations, their strengths, and the level of evidence were extracted from each CPG by two independent researchers and grouped as follows: (1) general recommendations for the pharmacological treatment for depression (suicide risk, acute treatment, continuation and maintenance phases, and treatment discontinuation); (2) treatment of non-responsive or partially responsive patients; and (3) treatment for subtypes of depression (chronic, psychotic, catatonic, melancholic, seasonal, somatic, mixed, and atypical). Only 50% of CPGs included recommendations for the risk of suicide associated with pharmacotherapy. All CPGs included serotonin selective reuptake inhibitors (SSRIs) as first-line treatment; however, one CPG also included agomelatine, milnacipran, and mianserin as first-line alternatives. Recommendations for depression subtypes (catatonic, atypical, melancholic) were included in three CPGs. The strength of recommendation and level of evidence clearly differed among CPGs, especially regarding treatment augmentation strategies. We conclude that, although CPGs converged in some recommendations (e.g., SSRIs as first-line treatment), they diverged in cardinal topics including the absence of recommendations regarding the risk of suicide associated with pharmacotherapy. Consequently, the recommendations listed in a specific CPG should be followed with caution.
Introduction
Globally, mental illness affects approximately 22% of the population [1]. Depression is the most prevalent psychiatric disorder, which affects more than 300 million individuals [2]. It is an incapacitating disorder, responsible for most of the 800,000 annual suicides [2]. Along with population growth and aging, the number of individuals with depression has also increased considerably and led to overloaded healthcare systems, thereby generating the need for resource optimization [1,3]. A primary challenge in the field of mental health is the development of health interventions based on scientific evidence to combat depression [4].
Carefully developed clinical practice guidelines (CPGs) can improve patient healthcare by outlining practices recommended based on scientific research [5–7].
CPGs should ensure that potential biases are properly approached during the development process and established recommendations have the viability to be implemented [8]. The development process of original high-quality CPGs demands time, resources, and an experienced team [9]. Scarcity of resources, particularly in developing countries, restricts the development of CPGs, potentially compromising their quality and validity [9]. Additionally, potential biases might result from cultural issues, even in developed countries. Recently, there has been an increase in the number of CPG publications, and problems concerning their quality have been highlighted in various studies [10–12].
The current study aimed to analyze the most relevant CPGs for the pharmacological treatment of depression and clarify a matrix of recommendations including agreements and potential disagreements among CPGs. Such a matrix can contribute to the development of a critical view of CPGs for practitioners and possibly help the development and adaptation of CPGs.
Materials and methods
Identification of clinical practice guidelines
We recently reported CPGs for the pharmacological treatment of noncommunicable diseases that could be considered “high-quality” [12]. In that study, we conducted individual systematic reviews for each included disease; and using the second version of the Appraisal of Guidelines for Research and Evaluation (AGREE II), evaluated 421 CPGs to establish the quality of their protocol registered on PROSPERO (CRD42016043364) [12]. In this study, we focus specifically on the part of that systematic review about the pharmacological treatment of depression [12].
We conducted a comprehensive search in MEDLINE, Embase, and the Cochrane Library, as well as in 12 specific websites for CPGs, because all such databases are well-recognized guideline repositories that have been cited frequently in previous studies of systematic reviews [12, 13]. The CPG searched were published between 2011 and 2016 (details of search strategies are in S1 Appendix). In April 2019, we searched the literature to update the included CPGs. Two independent reviewers screened the records regarding the eligibility criteria and conducted the data extraction. Discrepancies were solved by consensus.
To be included in this study, a CPG for the treatment of depression should have recommendations concerning the pharmacological treatment of depression in adults. CPGs published in English, Portuguese, or Spanish with free or restricted access to updated versions were eligible. To be included in the analysis of recommendations the CPG should have been considered of high quality (see next paragraph or had to be one of the most well-known and widely accepted CPGs [14]. All CPGs included in the analysis of the recommendations was evaluated to verify the presence of their updated version until April 2019. CPGs were excluded if they were specific for inpatients; specific for local use; focused on specific treatment approaches, such as psychotherapy or neuromodulation; or were for specific groups including pregnant women and children. CPGs addressing comorbidities in depression were also excluded.
CPG quality was judged by three independent appraisers on the basis of the six domains of the AGREE-II, as described previously [12]. Of these six domains, domain 3 (rigor of development) is considered the most relevant for the reliability of the recommendations[15–17]. The AGREE II does not suggest a cutoff value denoting acceptable or high quality; instead, cutoff values were determined by groups assessing CPG quality [17]. The domain 3 cut-off of ≥80% was adopted for this study to indicate high quality, as proposed in prior studies [18–20]. Details of quality appraisal are shown in S3 Appendix.
Extraction and analysis of recommendations
All recommendations regarding pharmacological treatment and the classification of the level of evidence from the included CPGs (when this information was available) were independently extracted by two researchers. Disagreements between the researchers (FCG and NCLS) were resolved by consensus; in the absence of a consensus, a senior investigator (ER) was included to solve the disagreement.
To perform the analyses, recommendations were classified based on their type and organized into tables by main topics. One of the authors (FCG) developed the first version of the classification, which was discussed with professors of pharmacy (ER) and psychiatry (RF).
The final version of the comparative tables of recommendations were achieved after three rounds of discussion. The recommendations were grouped by the main topics: 1) general recommendations for pharmacological treatment of depression (acute suicide risk and treatment, continuation phase treatment, maintenance phase treatment, and treatment discontinuation), 2)recommendations for treatment for those who did not respond or partially responded to therapy, and 3) recommendations for the treatment of depression subtypes (chronic depression or dysthymia, psychotic depression, catatonic depression, atypical conditions, melancholic depression, seasonal depression, somatic depression, and mixed depression).
Results
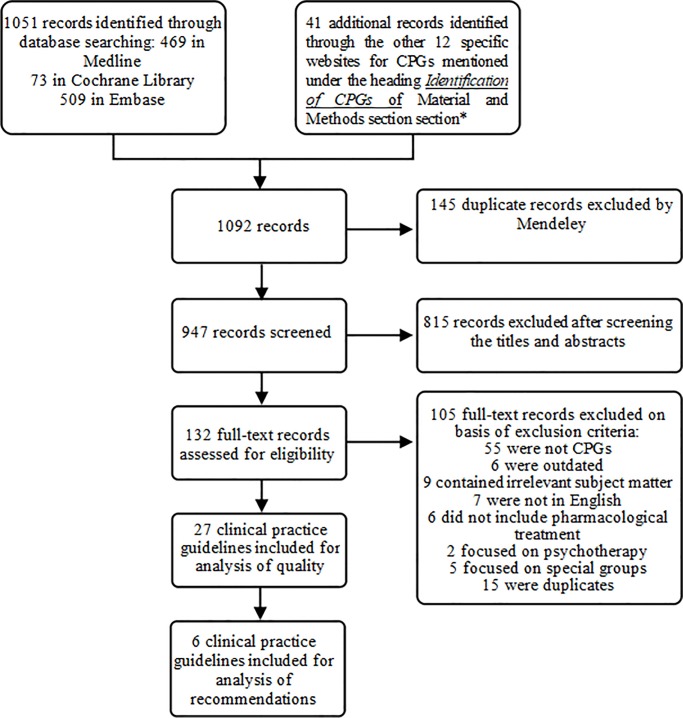
In our initial search, we identified 947 citations and abstracts after removing duplicates. Thereafter, by reading the full text and applying the eligibility criteria, we selected 27 CPGs for this study (Fig 1). (S2 Appendix includes the reason for excluding 105 full records).
Fig 1. Flowchart of clinical practice guidelines selection.
For the analysis of recommendations, 6 CPGs were included. Four CPGs presented a score ≥ 80% for Domain 3 and were considered high-quality [21–24]. In addition to these selected CPGs, two others were included based on their widespread acceptance [14]: the Canadian Network for Mood and Anxiety Treatments (CANMAT) and the American Psychiatric Association (APA) guidelines [25,26]. The six CPGs selected for analysis of their recommendation, based on their AGREE II Domain 3 score or on their acceptability, were as follows: Guía Clínica AUGE [21], score = 89%; Guía de Práctica Clínica [22], score = 86%; Depression in adults [23], score = 84%; Depression, adult in primary care [24], score = 81%; Practice guideline for the treatment of patients with major depressive disorder [26], score = 46%; and CANMAT [25], score = 54%. Table 1 briefly describes their characteristics.
Table 1. Clinical practice guidelines for the pharmacological treatment of depression included for analysis of recommendations.
| Clinical practice guideline/Country/ Development organization | Main characteristics |
|---|---|
| Guía Clínica AUGE3 [21] / Chile/ Ministry of Health) | This CPG1 was prepared in 2013, after two previous versions developed in 2007 and 2009. It has a very well-organized format and flowcharts that allow a clear understanding of recommendations. There are 11 recommendations for pharmacological treatment. Among them, some are intended for specific populations that were not included in this study (e.g., pregnant women and adolescents). This CPG has a methodological manual that recommends the use of a GRADE2 evidence classification system. However, it makes clear that a simplified system was used to classify the evidence, since the transition to the GRADE methodology is a gradual process that requires specific skills. |
| Guía de Práctica Clínica [22] / Colombia/ Ministry of Health | This CPG, prepared in 2013, used the adaptation of earlier versions of NICE4 and CANMAT5. It is quite extensive, and its recommendations are organized into clinical questions. |
| Depression in adults [23] / England / NICE | This CPG was first published in 2009 and was updated in 2016. The full version of the document is quite extensive. It uses GRADE but does not contain the level of evidence or the strength of recommendation explicitly in the recommendations. In 2018, NICE updated this CPG exclusively to make recommendations regarding the use of valproate in pregnancy. These were not included in the current study. |
| Depression, adult in primary care [24] / USA / ICSI | This CPG comprises many documents. One of them contains the main recommendations clearly highlighted according to their level of evidence and strength of recommendation. Some recommendations are scattered in the text, and do not contain the level of evidence or strength of recommendation. |
| Practice guideline for the treatment of patients with major depressive disorder [26] / USA / APA | This is the third version of this CPG. APA reaffirmed this guideline in October 2015. It contains several detailed recommendations mainly related to subtypes of depression comorbidities and other clinical conditions associated with depression. It has a simplified evidence classification scale and a manual containing information about its elaboration and development. |
| Canadian Network for Mood and Anxiety Treatments 2016 [25] / CANADA / CANMAT | This CPG was created in 2001, followed by a subsequent update in 2009. It is currently in its third version. It comprises 21 questions followed by their respective answers. Researchers chose not to follow AGREE II for its development. The developers understood the wide international use of GRADE but opted instead for a self-classification evidence scale. This CPG also contains a separate document in which its development methods are described. |
1CPG = clinical practice guideline;
2GRADE = The Grading of Recommendations Assessment, Development and Evaluation;
3AUGE = Aseguramiento Universal de las Garantías Explícitas;
4NICE = National Institute for Health and Care Excellence;
5CANMAT = Canadian Network for Mood and Anxiety Treatments;
6ICSI = Institute for Clinical Systems Improvement;
7APA = American Psychiatry Association.
General recommendations according to the main topic for pharmacological treatment of depression
Only three CPGs provided information regarding the evaluation and management of the risk of suicide with pharmacological treatment, and all recommendations about this subject were considered strong [22,23,26]. Four CPGs recommended non-pharmacological treatment as first-line treatment for mild depression [21–23,25]. From the CPGs that mentioned recommendations about the indication of pharmacological treatment for patients with moderate or severe depression and a combination of pharmacological and psychotherapy treatments [21–23,26], such recommendations were considered strong (Table 2).
Table 2. Clinical practice guidelines for the pharmacological treatment of depression: Recommendations.
| Recommendations/clinical practice guidelines | Chilean CPG1 [21] | Colombian CPG [22] | England CPG [23] | ICSI2 CPG [24] | APA3 CPG [26] | CANMAT4 CPG [25] |
|---|---|---|---|---|---|---|
| Risk of suicide (related to pharmacotherapy) | - | ● | ● | - | ● | - |
| Frequent monitoring of patients who take antidepressants | - | ● | ● | - | - | - |
| Risk of overdose toxicity | - | ● | ● | - | - | - |
| Treatment suggestions for patients at high risk for suicide | - | - | - | - | ● | - |
| Indication of pharmacological treatment | ● | ● | ● | ● | ● | ● |
| Preference for non-pharmacological treatment for mild depression | ● | ● | ● | - | - | ● |
| Combination of antidepressants and psychotherapy whenever possible | - | - | - | ● | - | - |
| Combination of antidepressants and psychotherapy | ||||||
| - in moderate depression | - | ● | ● | ● | - | |
| - in severe depression | ● | ● | ● | - | ● | - |
| First-line drug choice | ● | ● | ● | ● | ● | ● |
| Selective serotonin reuptake inhibitors | ● | ● | ● | ● | ● | ● |
| Contraindication of tricyclics | ● | - | - | ● | - | - |
| Amitriptyline | - | ● | - | - | - | - |
| Mirtazapine | - | ● | - | ● | ● | ● |
| Agomelatine, mianserin, and milnacipran | - | - | - | - | - | ● |
| Follow-up phase—duration of treatment after remission | ● | ● | ● | - | - | ● |
| 6 months | ● | ● | ● | - | - | ● |
| 4–9 months | - | - | - | - | - | ● |
| Maintenance phase—when deciding to prolong treatment (considerations) | ● | ● | ● | - | ● | ● |
| Number of previous episodes | ● | ● | ● | - | ● | ● |
| Residual symptoms and coexisting conditions | - | ● | ● | - | ● | ● |
| Severity of symptoms | - | ● | ● | - | ● | - |
| Age | - | - | ● | - | ● | - |
| Frequency and persistence of symptoms | - | - | - | - | ● | - |
| Risk factors (lead to treatment for 2 years or longer) | - | ● | ● | - | - | ● |
| Treatment discontinuation | - | ● | ● | - | ● | - |
| Gradual suspension of antidepressants | - | ● | ● | - | ● | - |
| Need for a more progressive suspension for specific drugs (paroxetine and venlafaxine) | - | ● | ● | - | - | - |
| Informing patients about discontinuation symptoms | - | - | ● | - | ● | - |
1CPG = clinical practice guideline;
2ICSI = Institute for Clinical Systems Improvement;
3APA = American Psychiatry Association;
4CANMAT = Canadian Network for Mood and Anxiety Treatments.
● = CPG does contain topic; - = CPG does not contain topic.
All CPGs indicated serotonin selective reuptake inhibitors (SSRIs) as an option for first-line treatment for depression, and the recommendations were based on high-quality studies; however, most CPGs did not cite specific drugs. Mirtazapine was considered as first-line treatment by four CPGs [22,24–26]. The tricyclic amitriptyline, fluoxetine, sertraline, and mirtazapine were considered first-line drugs by the Colombian CPG—amitriptyline for patients without contraindications and the others for patients with contraindications to tricyclics. The recommendations were based on pharmacoeconomic studies. Sertraline and mirtazapine were considered owing to cost per quality-adjusted life years [22]. The CANMAT CPG recommended agomelatine, mianserin, and milnacipran as first-line treatment [25]. More details are available in S1 Table.
Recommendations for treatment of depression in those who did not respond or partially responded
All CPGs included recommendations for the treatment of those who did not respond or partially responded to first-line therapy. Such recommendations are synthesized in Table 3 and details are presented in S2 Table. Almost all recommendations were considered strong regarding the adjustment of drug dosages when there was a lack of response to the initial pharmacological treatment. Moreover, antipsychotic agents were recommended as an augmentation strategy by five CPGs.
Table 3. Clinical practice guidelines for the pharmacological treatment of depression: Patients with a partial or no response.
| Recommendations/clinical practice guidelines | Chilean CPG1 [21] | Colombian CPG [22] | England CPG [23] | ICSI2 CPG [24] | APA3 CPG [26] | CANMAT4 CPG [25] |
|---|---|---|---|---|---|---|
| Strategies to improve the treatment for partial responders | ● | ● | ● | ● | ● | ● |
| Analysis of the factors contributing to an unsatisfactory response | - | - | ● | - | ● | - |
| Check compliance to treatment and drugs dosage | - | - | ● | - | ● | - |
| Check for adverse effects caused by the drugs | - | - | ● | - | ● | - |
| Adjusting the drugs dosage | ● | ● | ● | ● | ● | ● |
| Consider age, coexisting conditions, concomitant pharmacological treatment, and adverse effects caused by the drugs | - | - | ● | - | ● | - |
| Consider increasing the dosage | ● | ● | ● | ● | ● | ● |
| Replacing the drugs | ● | ● | ● | ● | ● | ● |
| Consider replacing the drugs | ● | ● | ● | ● | ● | ● |
| Replacement: | ||||||
| - at first, within the same antidepressant class | - | ● | ● | - | - | - |
| - a different class of antidepressants ( | - | ● | - | - | - | ● |
| Amitriptyline as a second-line strategy | - | ● | ● | - | - | ● |
| Combination or augmentation drugs | ● | ● | ● | ● | ● | ● |
| Antipsychotic agents | - | ● | ● | ● | ● | ● |
| Monoamine oxidase inhibitors | - | ● | - | - | ● | - |
| Olanzapine | - | - | ● | ● | - | ● |
| Mirtazapine | - | - | ● | ● | - | ● |
| Risperidone | - | ● | ● | - | - | ● |
| Lithium | ● | ● | ● | - | ● | ● |
| Thyroid hormones | ● | - | - | - | ● | - |
1CPG = clinical practice guideline;
2ICSI = Institute for Clinical Systems Improvement;
3APA = American Psychiatry Association;
4CANMAT = Canadian Network for Mood and Anxiety Treatments.
● = CPG does contain topic; - = CPG does not contain topic.
However, considering the differences found among the CPGs, the CANMAT was the only one that grouped the recommendations for adjuvant treatment (i.e., combination and augmentation strategies), in first-, second-, and third-line strategy. Moreover, the level of evidence and strength of recommendations for the combination and augmentation strategies varied among the CPGs. Although the same evidence scale was not precisely used to classify among the five CPGs, the evidence for recommendation of antipsychotic agents for augmentation was considered with substantial clinical safety by the APA CPG, weakly by the Colombian CPG. The remaining CPGs did not specify the level of the evidence or the strength of the recommendations.
Among the five CPGs that included lithium as an augmentation strategy, only three clearly indicated the strength of recommendation; however, discrepancies existed among them. While the Chilean CPG strongly recommended it, the Colombian CPG recommendation was weak, and the APA CPG recommended it, based on moderate clinical safety. Considering thyroid hormones as an augmentation strategy, two CPGs included it; however, they disagreed on the strength of the recommendation [21,26]. The Chilean CPG strongly recommended thyroid hormones, while the APA CPG indicated that there was moderate evidence of clinical safety.
Recommendations for treatment of depression considering its subtypes
Except for the Chilean CPG, all included recommendations per depression subtypes (Table 4). Moreover, most CPGs [22,23,25,26] provided recommendations for treating psychotic depression, except the ICSI and the Chilean CPG. The recommendations for treating depression with psychotic features was the use of antipsychotic and antidepressants agents. Regardless, the classification method applied to the significance of the recommendation or the quality of the evidence, using these drugs was considered a strong recommendation or was based on high-quality evidence.
Table 4. Clinical practice guidelines for the pharmacological treatment of depression: Subtypes of depression.
| Recommendations/clinical practice guidelines | Chilean CPG1 [21] | Colombian CPG [22] | England CPG [23] | ICSI2 CPG [24] | APA3 CPG [26] | CANMAT4 CPG [25] |
|---|---|---|---|---|---|---|
| Subtypes of depression | - | ● | ● | ● | ● | ● |
| Chronic or dysthymia | - | - | - | ● | - | - |
| Combination of antidepressants and psychotherapy | - | - | - | ● | - | - |
| Beginning of pharmacological treatment (pure dysthymia) | - | - | - | ● | - | - |
| Depression with psychotic features | - | ● | ● | - | ● | ● |
| Contraindication of isolated psychotherapy | - | - | - | - | - | - |
| Indication of antipsychotic agents in combination with antidepressants | - | ● | ● | - | ● | ● |
| Catatonic | - | - | - | - | ● | ● |
| Benzodiazepines in combination with antidepressants | - | - | - | - | ● | ● |
| Barbiturates in combination with antidepressants | - | - | - | - | ● | - |
| Depression with atypical features | - | - | - | ● | ● | ● |
| Monoamine oxidase inhibitors | - | - | - | ● | ● | - |
| Selective serotonin reuptake inhibitors and bupropion | - | - | - | - | ● | - |
| No specific antidepressants have demonstrated superiority | - | - | - | - | - | ● |
| Depression with melancholic features | - | - | - | - | ● | ● |
| Serotonin and noradrenaline reuptake inhibitors and tricyclic antidepressants | - | - | - | - | ● | - |
| No specific antidepressants have demonstrated superiority | - | - | - | - | - | ● |
| Depression with seasonal pattern | - | - | - | - | - | ● |
| No specific antidepressants have demonstrated superiority | - | - | - | - | - | ● |
| Depression with somatic symptoms (fatigue) | - | - | - | - | - | ● |
| Mixed depression disorder | - | - | - | - | - | ● |
1CPG = clinical practice guideline;
2ICSI = Institute for Clinical Systems Improvement;
3APA = American Psychiatry Association;
4CANMAT = Canadian Network for Mood and Anxiety Treatments.
● = CPG does contain topic; - = CPG does not contain topic.
No high-quality CPG mentioned the treatment of catatonic depression. On the other hand, the most employed guidelines in clinical practice—CANMAT and APA CPG—recommended using benzodiazepines for the treatment of catatonic depression; however, they disagreed on the classification of the quality of the evidence [25,26]. Moreover, depression with atypical characteristics was considered in the CPGs most used in clinical practice and in the ICSI CPG [24–26]. Depression with melancholic characteristics was contemplated only in the CPGs most used in clinical practice [25,26]. Seasonal, with somatic symptoms, and mixed depression disorders were only addressed in the CANMAT CPG [25]. More details are shown in S3 Table.
Discussion
First, it should be noted that pharmacological treatment of depression is one of the strategies that should be considered to ensure adequate patient care. Pharmacotherapy should be prescribed only after a careful evaluation of the patient, including risk of suicide, requirement of hospitalization, indication of psychotherapy, and existence of comorbidities among other clinical and psychosocial aspects.
Relevant findings of this study include the absence of specific recommendations regarding suicide risk associated with pharmacotherapy in some CPGs [21,24,25]; convergence on SSRIs as first-line antidepressants by all CPGs, aside with indication of some peculiar antidepressants as first-line by two CPGs [22,25]; specific strategies for treatment of a partial response are missing in various CPGs; and treatment specificities for depression subtypes being covered by all CPGs except one [21].
Recommendations for pharmacological treatment of depression.
Suicidalit
Depression is considered the principal risk factor for suicide, and suicide risk peaks in the first weeks of antidepressant treatment [27]. However, only three of the 6 CPGs included a topic concerning the risk of suicide associated with pharmacotherapy [22,23,26]. Thus, our findings highlight that future CPGs should consider the risk of suicide associated with pharmacological treatment.
First-line treatment
All CPGs considered SSRIs as a first-line antidepressant treatment. However, we identified two important discrepancies. Besides SSRIS, as options, the CANMAT CPG recommended the use of agomelatine, milnacipran, and mianserin [25]; and the Colombian CPG recommended the use of amitriptyline [22] as first-line treatment based on pharmacoeconomic studies.
Discrepancies among recommendations can negatively affect healthcare professionals’ confidence in CPGs [28]. We found such differences even among high-quality CPGs. CPGs may provide different recommendations according to cultural differences or based on the availability and infrastructure of a healthcare system [29]. Consequently, values shared by developers and patients, aside from cost issues, may influence the choice of a recommended treatment and reduce the reliability of the recommendation, particularly when scientific evidence is weak [30–32].
According to the CANMAT CPG [25], agomelatine demonstrated favorable efficacy and tolerability in a network meta-analysis of new-generation antidepressants conducted by Khoo et al (2015) [33]. The advantage of network meta-analysis is that multiple treatments can be subjected to both direct (i.e., among randomized controlled trials) and indirect (i.e., across trials based on a common variable) comparisons of interventions; the effects of different interventions that have not been investigated in trials can be compared, and the analysis of all interventions enables ranking of therapeutic alternatives with regard to a given outcome. However, other aspects must be taken into account: To assess the level of evidence or strength of the recommendation, it is important to consider not only the type of study but also its quality or risk of bias, or both, as recommended in the GRADE method, for example [29,34]. Network meta-analysis should be conducted under strict and specific methodology conditions (transitivity and consistency criteria met); the inclusion of studies at risk of bias may negatively compromise the validity of the findings [35]. In their network meta-analysis, Khoo et al did not describe the result of the risk of bias assessment for any of the eight clinical trials included in the meta-analysis, but in the general assessment, most of the primary studies included were judged as having high or unclear risk of bias in at least one domain of the Cochrane Collaboration risk of bias tool [33]. In a 2013 Cochrane review of 13 trials in a pairwise meta-analysis [36] and in a 2018 network meta-analysis conducted by Cipriani et al [3], agomelatine did not show robust advantages over the other antidepressants for effectiveness, but it appeared to be better tolerated. In both studies, the limitation was that the primary studies had biases ([3]). In addition, agomelatine was not approved by the U.S. Food and Drug Administration to treat depression, and its safety and efficacy have been questioned [36]. The Colombian CPG explicitly recommends not using agomelatine because of insufficient evidence on its effectiveness [22].
The CANMAT CPG recommendation of milnacipran as a first-line treatment is based more on its tolerability than on its efficacy [37], which does not follow the recommended sequence for the rational use of medicines: efficacy, availability, and safety [38]. Moreover, in Cipriani et al’s study [3], milnacipran did not stand out in terms of either effectiveness or safety [3]. Milnacipran is also not approved by the U.S. Food and Drug Administration to treat depression [39].
The recommendation of mianserin as a first-line option is controversial because the CANMAT CPG itself cites the results of a network meta-analysis that demonstrated few differences in response, although SSRIs and TCAs were superior to mianserin/mirtazapine and moclobemide [25,40]. Indeed, Linde et al [40] affirmed that physicians should be aware that SSRIs and TCAs have a somewhat more solid evidence base than do other pharmacological classes. Cipriani et al [3] did not include mianserin in network meta-analysis [3]. Arroll et al [41] conducted a meta-analysis of two trials in which mianserin was compared with placebo and concluded that mianserin was effective for continuous outcomes but did not affect rates of remission and response [41].
The Colombian CPG inclusion of amitriptyline (for patients without a contraindication to CTAs) as a first-line option was based on its good cost-effectiveness profile. In the pharmacoeconomic study conducted by the Colombian CPG, sertraline was considered more cost-effective than amitriptyline regarding outcome quality–adjusted life years. With regard to its efficacy, a meta-analysis supported the superiority of amitriptyline over other tricyclic/heterocyclic antidepressants and over SSRIs; however, the effect size was clinically not relevant [42]. Cipriani et al [3] confirmed the effectiveness of amitriptyline but revealed that it had one of the highest rates of treatment abandonment owing to adverse events [3]. In addition, evidence regarding the tolerability and risk of CTAs is plentiful and includes a narrow safety margin and considerable risk of death in cases of overdose [43]. With regard to individuals at risk for adverse events, such as older adults (i.e., the Beers criteria), the ICSI CPG stresses that tricyclics must not be used by older adults because of their anticholinergic effects and their capacity to induce orthostatic hypotension and stimulation of the central nervous system [44].
Despite recommending SSRIs as first-line treatments, most CPGs did not cite specific SSRIs. Only three CPGs specifically indicated the SSRIs used as the first-line treatment [22,24,25]. Although only a few controlled randomized trials have compared SSRIs head-to-head [3], it is difficult to explain why some CPGs recommend specific SSRIs while others recommend a group of SSRIs.
Partial and non-responders
The initial pharmacological treatment for depression had a response rate ranging from 40–60% [45], and only around 30% achieved remission [45,46]. Consequently, recommendations to non-responders should be an essential part of any CPG. In fact, all the present CPG recommendations addressed first-line therapy and non-responders. Adjustment of the dosage with strong evidence was a consensus among the CPGs.
It is worth noting that many CPGs describe the alternatives of replacing the antidepressant for those not responding to first line treatment without specifying which should be considered the second, the third or the fourth-line therapy. Additionally, among those specifying a sequence of strategies, the concept and support to define each line of therapy varies across the CPGs, leading to considerable discrepancies. For example, the Colombian CPG, instead of including other antidepressants, recommends using as second-line treatment, first-line alternatives such as fluoxetine, sertraline, amitriptyline or mirtazapine, that had not been prescribed. Regarding third-line treatment, the Colombian CPG recommends imipramine, clomipramine, paroxetine, escitalopram, citalopram, fluvoxamine, venlafaxine, duloxetine, desvenlafaxine, trazodone, and bupropion [22], while third line alternatives for the Canadian CPG (CANMAT) are Monoamine oxidase inhibitors (i.e. Phenelzine and tranylcypromine) and reboxetine [25]. On the other hand, for the APA CPG, MAOIs are considered fourth line therapy [26].
Also recommended by most CPGs was the augmentation with antipsychotics for partial responders. However, some CPGs did not report, while others disagreed, about the strength of recommendation or level of evidence for augmentation strategies. Furthermore, the CANMAT CPG was the only one recommending an order for the augmentation strategies.
Subtypes of depression
Depression with psychotic features was the subtype receiving most consistent recommendations across the CPGs. Four CPGs recommended antipsychotic agents in combination with antidepressants for the treatment of depression with psychotic features [22,23,25,26]. Various studies, including meta-analyses, have supported the advantage of associating antidepressants with antipsychotics for the treatment of depression with psychotic features [47]. The goal of the ICSI CPG was primary-care attention, which explains its absence of recommendations for depression with psychotic features. The Chilean CPG included only comments about the effectiveness of antidepressants and antipsychotics for psychotic depression; however, it is outside the topic of recommendations [21].
Data supporting distinct antidepressant response of atypical depression to monoamine oxidase inhibitors (MAOIs; i.e., tranylcypromine) have been reported since the 1960s [48]. The presence of melancholic or atypical features was considered respectively by two and three CPGs. The APA CPG was the only CPG that addressed specific treatment for melancholic depression—serotonin and noradrenaline reuptake inhibitors (SSRIs) and tricyclic antidepressants; the CANMAT CPG stated that no antidepressant has been proven to be superior for melancholic depression. For depression with atypical features, the APA CPG and the ICSI CPG recommended MAOIs, while the CANMAT CPG stated that no antidepressant has been proven to be superior. Such discrepancies are in line with controversies in the literature. Notwithstanding the traditional view of superiority of MAOIs for depression with atypical features, the iSPOT-D Trial compared the efficacy of venlafaxine, escitalopram, and sertraline and found similar symptom reduction trajectories among depression subtypes including melancholic, atypical, anxious depression, and subtype combinations [49].
Considering catatonic depression, benzodiazepines may lead to a rapid relive for some patients [50]; however, only the APA and the CANMAT CPGs included such recommendation. Although, it can be argued that catatonic depression is not frequent, it has a high morbidity and our view is that consideration to specificities of its treatment should be included in CPGs.
Strengths and limitations
We included CPGs published in English, Portuguese, and Spanish; thus, the results might not reflect relevant CPGs in other languages. The intrinsic subjectivity limitation of the AGREE II should also be considered; to minimize this, we included three evaluators and disagreements among them were solved by discussion until consensus was reached. We also included two additional CPGs based on their wild acceptability (CANMAT and APA CPG) [25,26] instead of basing the study only on the AGREE II evaluation. Another point to consider is that some recommendations might have not been considered because they were placed outside the topic of pharmacological treatment or it was not clear if they were recommendations or mere dissecting of evidence.
Among the strengths of this study, we cite the comprehensive search and the careful training of appraisers. In describing the recommendations, we offer a comparative view of distinct CPGs that provide physicians and patients a more comprehensive understanding of pharmacological approaches to the treatment of depression. Our findings could help in the elaboration/adaptation of a CPG because we identified important divergences among existing CPGs to which stakeholders (patients and professionals) should give special attention. Moreover, the identification of the points at which CPGs converge fully and that have been well addressed in certain CPGs may also be helpful for the elaboration/adaptation of a CPG for local contexts, as well as contribute to clinical decisions about treatment for this severe mental health problem.
Conclusion
We found that the various CPGs were typically consistent with each other; however, they presented some vital differences. The use of SSRIs as first-line pharmacological treatment and its dose adjustment for non-responsive patients were consistent among all the included CPGs. Largely consistent was the use of antipsychotics as augmentation (except for the ICSI CPG, which addressed primary care) for non-responders. Importantly, only 50% of the CPGs addressed the risk of suicide associated with pharmacotherapy. Considering the increased risk of suicide associated with the first few weeks of antidepressant treatment, recommendations regarding this topic should be mandatory in all CPGs. Moreover, specificities for some subtypes of depression (e.g., catatonic and atypical) were addressed by some but not all CPGs. Differences in the level of evidence or strength of recommendation were very frequent among the CPGs, and some of them presented unique recommendations. These findings support that, when using a specific guideline for the treatment of depression, caution is needed to provide the most appropriate treatment to each patient.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOC)
Acknowledgments
We would like to acknowledge everyone who contributed their time and knowledge to this study without any financial support. We would like to thank CHRONIDE for their support in the appraisal of CPGs. We also appreciate the assistance of Professor Carlota de Oliveira Rangel Yagui in manuscript preparation. We are indebted to Caroline Molino, Luciana Vasconcelos, and Sheila Kalb Wainberg for their invaluable assistance in helping us understand the method of appraisal of CPGs. We also recognize Andrea Fernandes Larruscain and Aliandra Fantinell de Oliveira for their help with manuscript formatting. Finally, we are grateful to Alfredo Jose Neto for his assistance in discussion of data synthesis and Carlos Eduardo Moscato Fuzaro for his support in data.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001 to FCG and NCL. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was provided for this study.
References
- 1.Murray CJL, Barber RM, Foreman KJ, Ozgoren AA, Abd-Allah F, Abera SF, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015. November 28;386(10009):2145–2191. 10.1016/S0140-6736(15)61340-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Depression and other common mental disorders: global health estimates. [cited 2017 December 8; Internet]. Geneva: World Health Organization; 2017. [21 p.] http://apps.who.int/iris/bitstream/10665/254610/1/WHO-MSD-MER-2017.2-eng.pdf. [Google Scholar]
- 3.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review. Lancet. 2018. April 7;391(10128):1357–1366. 10.1016/S0140-6736(17)32802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, et al. Grand challenges in global mental health. Nature. 2011. July 6;475(7354):27–30. 10.1038/475027a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Institute of Medicine (US). Clinical practice guidelines we can trust. Washington (DC): National Academies Press; 2011. 10.17226/13058 [DOI] [Google Scholar]
- 6.Shiffman RN, Shekelle P, Overhage JM, Slutsky J, Grimshaw J, Deshpande AM. Standardized reporting of clinical practice guidelines: a proposal from the Conference on Guideline Standardization. Ann Intern Med. 2003. September 16;139(6):493–498. 10.7326/0003-4819-139-6-200309160-00013 [DOI] [PubMed] [Google Scholar]
- 7.Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J. Potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999. February 20;318(7182):527–530. 10.1136/bmj.318.7182.527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AGREE Collaboration. Appraisal of guidelines for research and evaluation (AGREE) instrument [cited 2017 December 8; Internet]. London: St. Georges Hospital Medical School; 2001. https://www.paho.org/hq/dmdocuments/2012/AGREEworksheet-guideline-appraisal-sheet.pdf. [Google Scholar]
- 9.Raine R, Sanderson C, Black N. Developing clinical guidelines: a challenge to current methods. BMJ. 2005. September 17;331(7517):631–633. 10.1136/bmj.331.7517.631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonso-Coello P, Irfan A, Solà I, Gich I, Delgado-Noguera M, Rigau D, et al. The quality of clinical practice guidelines over the last two decades: a systematic review of guideline appraisal studies. Qual Saf Health Care. 2010. December;19(6):e58 10.1136/qshc.2010.042077 [DOI] [PubMed] [Google Scholar]
- 11.Armstrong JJ, Goldfarb AM, Instrum RS, MacDermid JC. Improvement evident but still necessary in clinical practice guideline quality: a systematic review. J Clin Epidemiol. 2017. January; 81:13–21. 10.1016/j.jclinepi.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 12.Molino CGRC, Leite-Santos NC, Gabriel FC, Wainberg SK, Vasconcelos LP, Silva RAM, et al. Factors associated with high-quality guidelines: a systematic review and appraisal of 421 guidelines for the pharmacological management of chronic diseases in primary care. JAMA Intern Med. 2019. February 18 10.1001/jamainternmed.2018.7529 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13.Johnston A, Kelly SE, Hsieh SC, Skidmore B, Wells GA. Systematic reviews of clinical practice guidelines: a methodological guide. J Clin Epidemiol. 2019;108: 64–76. 10.1016/j.jclinepi.2018.11.030 [DOI] [PubMed] [Google Scholar]
- 14.Saddichha S, Chaturvedi SK. Clinical practice guidelines in psychiatry: more confusion than clarity? A critical review and recommendation of a unified guideline. SRN Psychiatry. 2014. March 31; 2014:828917 10.1155/2014/828917 eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann-Eßer W, Siering U, Neugebauer EAM, Brockhaus AC, Lampert U, Eikermann M. Guideline appraisal with AGREE II: systematic review of the current evidence on how users handle the 2 overall assessments. PLoS One. 2017;12(3): e0174831 10.1371/journal.pone.0174831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann-Eßer W, Siering U, Neugebauer EAM, Brockhaus AC, McGauran N, Eikermann M. Guideline appraisal with AGREE II: online survey of the potential influence of AGREE II items on overall assessment of guideline quality and recommendation for use. BMC Health Serv Res. 2018. February 27;18(1):143 10.1186/s12913-018-2954-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann-Eßer W, Siering U, Neugebauer EAM, Lampert U, Eikermann M. Systematic review of current guideline appraisals performed with the Appraisal of Guidelines for Research & Evaluation II instrument—a third of AGREE II users apply a cut-off for guideline quality. J Clin Epidemiol. 2018. March; 95:120–127. 10.1016/j.jclinepi.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 18.Lee GY, Yamada J, Kyololo O, Shorkey A, Stevens B. Pediatric clinical practice guidelines for acute procedural pain: a systematic review. Pediatrics. 2014. March;133(3):500–515. 10.1542/peds.2013-2744 [DOI] [PubMed] [Google Scholar]
- 19.Piano V, Schalkwijk A, Burgers J, Verhagen S, Kress H, Hekster Y, et al. Guidelines for neuropathic pain management in patients with cancer: a European survey and comparison. Pain Pract. 2013. June;13(5):349–357. 10.1111/j.1533-2500.2012.00602.x [DOI] [PubMed] [Google Scholar]
- 20.Barriocanal AM, López A, Monreal M, Montané E. Quality assessment of peripheral artery disease clinical guidelines. J Vasc Surg. 2016. April;63(4):1091–8. 10.1016/j.jvs.2015.12.040 [DOI] [PubMed] [Google Scholar]
- 21.Chile, Ministerio de Salud (MINSAL). Guía clínica AUGE depresion en personas de 15 años y mas [Internet]. [Santiago: ]: MINSAL; 2013. [cited 2017 June 30]. [Serie Guías Clínicas]. http://supersalud.gob.cl/difusion/572/articles-652_recurso_1.pdf. [Google Scholar]
- 22.Colombia, Ministerio de Salud. Guía de Práctica clínica: detección temprana y diagnóstico del episodio depresivo y trastorno depresivo recurrente en adultos: atención integral de los adultos con diagnóstico de episodio depresivo o trastorno depresivo recurrente. c2013 [cited 2017 June 30; Internet]. http://gpc.minsalud.gov.co/gpc_sites/Repositorio/Conv_500/GPC_td/gpc_td.aspx. Spanish.
- 23.National Institute for Health and Care Excellence. Depression in adults: recognition and management. 2009. October [last updated 2018 April; cited 2017 June 30; Internet]. https://www.nice.org.uk/guidance/cg90/evidence. [PubMed] [Google Scholar]
- 24.Trangle M, Gursky J, Haight R, Hardwig J, Hinnenkamp T, Kessler D, et al. Adult depression in primary care. [Bloomington: ]: Institute for Clinical Systems Improvement; Updated March 2016. [Google Scholar]
- 25.Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016: clinical guidelines for the management of adults with major depressive disorder: Section 3. Pharmacological Treatments. Can J Psychiatry. 2016. September;61(9):540–560. 10.1177/0706743716659417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gelenberg AJ, Freeman MP, Markowitz JC, Rosenbaum JF, Thase ME, Trivedi MH, et al. Practice guideline for the treatment of patients with major depressive disorder, third edition [Washington, D.C.]: American Psychiatry Association; 2010. [Google Scholar]
- 27.World Health Organization. Suicide prevention: a resource for counsellors. Geneva: World Health Organization; 2006. [Google Scholar]
- 28.Burgers J S, Grol RP, Zaat JO, Spies TH, van der Bij AK, Mokkink HG. Characteristics of effective clinical guidelines for general practice. Br J Gen Pract. 2003. January;53(486):15–19. [PMC free article] [PubMed] [Google Scholar]
- 29.Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017. July; 87:4–13. 10.1016/j.jclinepi.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fervers B, Burgers JS, Voellinger R, Brouwers M, Browman GP, Graham ID, et al. Guideline adaptation: an approach to enhance efficiency in guideline development and improve utilisation. BMJ Qual Saf. 2011. March;20(3):228–236. 10.1136/bmjqs.2010.043257 [DOI] [PubMed] [Google Scholar]
- 31.Eisinger F, Geller G, Burke W, Holtzman NA. Cultural basis for differences between US and French clinical recommendations for women at increased risk of breast and ovarian cancer. Lancet. 1999. March 13;353(9156):919–920. 10.1016/s0140-6736(98)07516-3 [DOI] [PubMed] [Google Scholar]
- 32.Woolf S, Schünemann HJ, Eccles MP, Grimshaw JM, Shekelle P. Developing clinical practice guidelines: types of evidence and outcomes; values and economics, synthesis, grading, and presentation and deriving recommendations. Implement Sci. 2012. July 4; 7:61 10.1186/1748-5908-7-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khoo AL, Zhou HJ, Teng M, Lin L, Zhao YJ, Soh LB, et al. Network meta-analysis and cost-effectiveness analysis of new generation antidepressants. CNS Drugs. 2015. August 1; 29:695–712. 10.1007/s40263-015-0267-6 [DOI] [PubMed] [Google Scholar]
- 34.GRADE Work Group: Grading of Recommendations Assessment, Development, and Evaluation (GRADE) [Internet]. [n.p.]: GRADE Work Group; [cited 2007 Feb 21]. www.gradeworkinggroup.org. [Google Scholar]
- 35.Higgins JPT, Altman DG. Assessing risk of bias in included studies In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions Version 5.1.0 [Internet]. [Chichester (UK)]: Cochrane; 2011. [update 2011 Mar; cited 2012 Oct 3]. www.training.cochrane.org/handbook. [Google Scholar]
- 36.Guaiana G, Gupta S, Chiodo D, Davies SJC, Haederle K, Koesters M. Agomelatine versus other antidepressive agents for major depression. Cochrane Database Syst Rev. 2013. December 17;(12):CD008851 10.1002/14651858.CD008851.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Etten LA. CANMAT guidelines (2016) for the pharmacological treatment of unipolar depression (Episode #02). 13 October 2017. [cited 2018 July 18; Internet]. https://podtail.com/ podcast/pqu-podcast/episodio-02-diretrizes-do-canmat-2016-para-tratame/. [Google Scholar]
- 38.Brasil, Ministério da Saúde, Secretaria de Ciência, Tecnologia e Insumos Estratégicos. [Rational use of medicines: selected subject]. Brasília: MS, 2012. [Internet] http://bvsms.saude.gov.br/bvs/publicacoes/uso_racional_medicamentos_temas_selecionados.pdf.
- 39.Goldenberg MM. Pharmaceutical Approval Update. PT. 2009. February;34(2):86–94. [PMC free article] [PubMed] [Google Scholar]
- 40.Linde K, Kriston L, Rücker G, Jamil S, Schumann I, Meissner K, et al. Efficacy and acceptability of pharmacological treatments for depressive disorders in primary care: systematic review and network meta-analysis. Ann Fam Med. 2015; 13:69–79. 10.1370/afm.1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arroll B, Chin WY, Martis W, Goodyear-Smith F, Mount V, Kingsford D, et al. Antidepressants for treatment of depression in primary care: a systematic review and meta-analysis. J Prim Health Care. 2016. December;8(4): 325–334. 10.1071/HC16008 [DOI] [PubMed] [Google Scholar]
- 42.Guaiana G, Barbui C, Hotopf M. Amitriptyline for depression. Cochrane Database Syst Rev. 2007. CD004186. 10.1002/14651858.CD004186.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newton A. Tricyclic antidepressant overdose. BMJ Best Practice. [cited 2019 Mar 11; Internet]. Available from: https://bestpractice.bmj.com/topics/pt-br/342. [Google Scholar]
- 44.Montezano Lopez L, Figueiredo TP, Costa SC, Reis AMM. Use of potentially inappropriate medications by the elderly at home. Cien Saude Colet. 2016;21(11):3429–38. 10.1590/1413-812320152111.14302015 [DOI] [PubMed] [Google Scholar]
- 45.Carvalho AF, Cavalcante JL, Castelo MS, Lima MC. Augmentation strategies for treatment-resistant depression: a literature review. J Clin Pharm Ther. 2007. October;32(5):415–428. 10.1111/j.1365-2710.2007.00846.x [DOI] [PubMed] [Google Scholar]
- 46.Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, et al. Medication augmentation after the failure of SSRIS for depression. N Engl J Med. 2006. March 23;354(12):1243–52. 10.1056/NEJMoa052964 [DOI] [PubMed] [Google Scholar]
- 47.Wijkstra J, Lijmer J, Burger H, Cipriani A, Geddes J, Nolen WA. Pharmacological treatment for psychotic depression. Cochrane Database Syst Rev. 2015. July 30;(7):CD004044 10.1002/14651858.CD004044.pub4 [DOI] [PubMed] [Google Scholar]
- 48.Ulrich S, Ricken R, Adli M. Tranylcypromine in mind (Part I): Review of pharmacology. Eur Neuropsychopharmacol. 2017. August;27(8):697–713. 10.1016/j.euroneuro.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 49.Arnow BA, Blasey C, Williams LM, Palmer DM, Rekshan W, Schatzberg AF, et al. Depression subtypes in predicting antidepressant response: a report from the iSPOT-D Trial. Am J Psychiatry. 2015. August 1;172(8):743–750. 10.1176/appi.ajp.2015.14020181 [DOI] [PubMed] [Google Scholar]
- 50.Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry. 1990. September;51(9):357–362. [PubMed] [Google Scholar]