Abstract
Gut microbiome plays an essential role in asthma development, and probiotic-based manipulation of the gut microbiome has been proposed to prevent asthma. Although the preventive effect of Lactobacillus supplementation against allergies has been reported, the precise Lactobacillus species beneficial for effective prevention of asthma remain unidentified and the underlying mechanisms remain unclear. Therefore, we aimed to investigate the efficacy of oral administration of six Lactobacillus species and the mechanism underlying asthma prevention via gut microbiome modulation. We investigated the effects of oral administration of L. rhamnosus, L. fermentum, L. casei, L. gasseri, L. salivarius, and L. reuteri (five strains of each species) on asthma and gut microbiome of house dust mite (HDM)-treated murine models of asthma. Of these, L. reuteri administration was the most effective: it alleviated airway inflammation, decreased total IgE and HDM-IgG1, and reduced Th2-associated pro-inflammatory cytokines. Moreover, modulation of specific microbial genera by L. reuteri was more effective in asthma prevention than the modulation of the overall microbiota composition. Lactobacillus and Enterococcus were enriched after L. reuteri supplementation and were closely associated with total IgE and IL-13 production. Furthermore, L. reuteri specifically altered the gut microbial function toward butyrate generation. Thus, L. reuteri may reduce the risk of asthma development by modulating specific gut microbiota to improve the lung immune environment. Our study suggests a novel option for gut microbiome manipulation via L. reuteri supplementation for suppression of asthma and other allergic diseases.
Introduction
Allergies are immune-mediated disorders primarily caused by an IgE-dependent immunological reaction to an allergen (an innocuous environmental antigen). Depending on the allergen contact site, different clinical manifestations, characterized by the presence of IL-4, IL-5, IL-13, and IL-17A and increased levels of IgE and IgG, may occur in the gastrointestinal tract, skin, or airways [1–3]. Asthma is one such allergic disease, and it is defined as an allergen-mediated airway inflammatory disease [4], and among the most common affliction in the world [5]. The currently available treatment options alleviate the symptoms of asthma and other allergic diseases but cannot provide complete cure.
Intestinal microbiota is an important stimulatory factor for immune system development and function. The microbiota composition and metabolites in individuals with allergies have been reported to be different from those in healthy individuals [6–8]. Moreover, asthma involves gut microbiome perturbation and is associated with metabolic dysfunction. In mice, manipulation of the gut microbiome using oral probiotics or high-fiber dietary supplementation (which increases the synthesis of short-chain fatty acids (SCFA)) facilitates pro-resolving local and remote mucosal immunity [9,10]. Therefore, targeting the gut microbiota with probiotics, prebiotics, and dietary alteration would be a rational therapeutic approach to prevent asthma and other allergic diseases.
Lactobacillus is one of the most widely known probiotics. Low relative abundance of Lactobacillus was reported to be associated with asthma development early in life [11]. Lactobacillus rhamnosus GG was shown to be effective in the prevention of asthma in children at high risk [7], whereas L. gasseri was suggested to provide clinical benefits in school children with asthma [12]. However, L. paracasei supplementation did not ameliorate asthma in infants [13]. Thus, several studies have focused on the effectiveness of Lactobacillus in asthma, and Lactobacillus supplementation has been reported as an effective preventive strategy for allergy development in experimental and clinical studies. However, the precise Lactobacillus species that provide the essential beneficial effect and the underlying gut microbiome-dependent mechanisms remain unclear.
Therefore, in this study, we investigated the asthma-preventive effects of six Lactobacillus species, each constituting five strains. We aimed to assess the effectiveness of Lactobacillus against asthma and explore the mechanisms involved to better understand the immunomodulatory and preventive effects of probiotic in allergies.
Materials and methods
Bacterial strains
The study included 30 Lactobacillus strains belonging to six species: L. rhamnosus, L. fermentum, L. casei, L. gasseri, L. salivarius, and L. reuteri. The strains were isolated from food and fecal samples collected from healthy humans from several Chinese cities (Table 1). The stool samples were collected from the participants after obtaining a written informed consent from them or their legal guardians. All the strains were cultured in MRS medium at 37°C under static conditions. The bacterial cells were collected by centrifugation (6000 g for 15 min at 4°C), washed twice with sterile saline, and stored at −80°C until further use. Each candidate strain was adjusted to 109 CFU. Five strains of the same species were mixed and administered orally to each mouse. The gradient dilution method was used to determine the number of bacterial cells.
Table 1. Strains used in animal experiments.
| Group | Strain | Regional origin | Source |
|---|---|---|---|
| L. rhamnosus | JS-WX-24-1 | Wuxi,Jiangsu Province,China | Infant feces |
| JS-WX-3-L-2 | Wuxi,Jiangsu Province,China | Infant feces | |
| TJ-DG-10-L10-6-1 | Dagang,Tianjin Province,China | Infant feces | |
| H28L-1 | Zhongxiang,Hubei Provice,China | Elder feces | |
| TJ-DG-9-L⑪10-5-1 | Dagang,Tianjin Province,China | Infant feces | |
| L. fermentum | HeNa-10-2-G- | Boai,Hennan Province,China | Elder feces |
| TJ-DG-8-L⑨10-5-1b | Dagang,Tianjin Province,China | Infant feces | |
| 13G-9 | Rouergai,Sichuan Province,China | Human feces | |
| B76 | Nantong,Jiangsu Province,China | Elder feces | |
| DL3-9 | Meisan,Sichuan Province,China | Fermented milk | |
| L. casei | M2-07-F01-L4-2-1 | Rouergai,Sichuan Province,China | Human feces |
| M2-06-F01-L4-2-3 | Rouergai,Sichuan Province,China | cow dung | |
| CCFM1073 (JS-WX-3-L-3) | Wuxi,Jiangsu Province,China | Infant feces | |
| RS8-5 | Meisan,Sichuan Province,China | Fermented milk | |
| FGDLZ41 | Lianzhou,Guangdong Province,China | Infant feces | |
| L.gasseri | lishouqian 3 | Chengmai,Hainan Province,China | Elder feces |
| C-1 A31 | Boai,Hennan Province,China | Elder feces | |
| JS-WX-9-L-5 | Wuxi,Jiangsu Province,China | Infant feces | |
| M2-C-F03-L-2 | Rouergai,Xizang Province,China | Human feces | |
| AH-WH-7-4 | Wuhu,Anhui Province,China | Elder feces | |
| L. salivarius | GuXi-8-2-GMM | Bama,Guangxi Province,China | Elder feces |
| GuXi-8-3-GMM | Bama,Guangxi Province,China | Elder feces | |
| GuXi-8-3-GMM | Bama,Guangxi Province,China | Elder feces | |
| GuXi-8-5-GMM | Bama,Guangxi Province,China | Elder feces | |
| 4L-4 | Enshi,Hubei Province,China | Elder feces | |
| L. reuteri | CCFM1072 (FSDLZ13M6) | Laizhou,Shandong Province,China | Elder feces |
| DYNDL2-16 | Yunnan,Dali Province,China | Fermented milk | |
| CCFM1040 (YN-DL-1-3) | Yunnan,Dali Province,China | milk | |
| GDLZ10-5 | Lianzhou,Guangdong Province,China | Child’s feces | |
| FZJTZ20M3 | Taizhou,Zhejiang Province,China | Human feces |
Animal experiments
All animal experiments were performed according to the protocols approved by the Institutional Animal Ethics Committee of Jiangnan University (JN. No: 20170915b1920115 [11]) and were in compliance with the recommendations of national and international guidelines for the Care and Use of Laboratory Animals. All procedures were performed under anesthesia to minimize suffering.
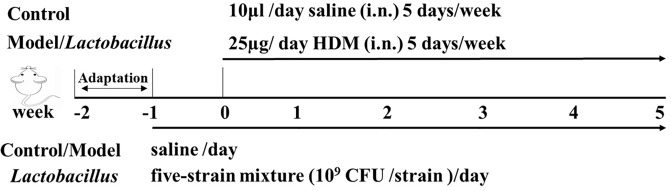
Female BALB/c mice (aged 4–5 weeks) were obtained from Shanghai Experimental Animal Center (Shanghai, China) and housed in specific pathogen-free conditions. A standard diet and sterile filtered drinking water were provided ad libitum. The mouse model was developed as described previously [14]. Briefly, mice were intranasally exposed (i.n.) to purified 25 μg house dust mite (HDM) extract (Greer Laboratories Inc., Lenoir, NC, USA) dissolved in 10 μL saline for 5 days/week for 5 consecutive weeks. Control animals received 10 μL saline. After 1 week of acclimatization, the HDM-treated mice were randomly divided into seven groups (n = 6–7): the model group and six experimental groups. The control and model mice received the same volume of sterile saline every day. The six experimental group mice were orally administered the five-strain mixture of six different Lactobacillus species, starting 1 week before the first sensitization and continued till the end of the experiment. Allergic airway inflammation was analyzed on week 5 after the last challenge (Fig 1).
Fig 1. House dust mite (HDM) sensitization and exposure protocols.
A timeline of HDM immunization and exposure and the administration of the six Lactobacillus species in the model has been provided.
Characterization of the allergic phenotype
To characterize the allergic phenotype, the following parameters were analyzed: (a) inflammation score in lung histology, (b) serum immunoglobulin, and (c) cytokines in BALF.
Serum immunoglobulin analysis
Mouse serum was collected and frozen at −20°C. Serum immunoglobulins were measured using commercial ELISA kits: Mouse IgE ELISA kits (SenBeiJia Biotechnology Co., Ltd.) for total IgE; Mouse Serum Anti-HDM IgE and IgG1 Antibody Assay ELISA kits for HDM-specific IgE and IgG1 respectively (Chondrex, Inc., Washington USA); and Mouse HDM-IgG2a ELISA kit for HDM-specific IgG2a (Mlbio, Shanghai, China). Measurements were performed according to the manufacturers’ instructions.
Lung cytokine analysis
Bronchoalveolar lavage was performed, and the bronchoalveolar lavage fluid (BALF) was collected and frozen at −20°C. Levels of IL-5, IL-13, and IL-17A in the BALF were measured using DuoSet ELISA kits (R&D Systems, Minneapolis, MN, USA) per the manufacturers’ recommendations.
Lung histology
After sacrifice, left lobes of the mouse lungs were fixed in 4% paraformaldehyde and embedded in paraffin. Then, 5 μm-thick sections were obtained and stained with hematoxylin and eosin (H&E) and periodic acid–Schiff (PAS). Histological damage scores were assessed using a previously described system [15]. Four mice were randomly selected from each group to determine the degree of histological damage to the lung. The qualitative airway inflammation score of each mouse was on a 0–4 scale.
Fecal sampling, DNA extraction, and amplicon sequencing
Feces from the colons of individual mice were collected in a sterile tube, snap frozen, and stored at –80°C until DNA extraction. DNA was extracted using a Fast DNA SPIN Kit for Feces (MP Biomedicals, Carlsbad, CA, USA). Then, 16S rDNA genes were PCR amplified using specific primers for the V3–V4 region of bacterial rDNA (341F and 806R) as described previously [16–18].
Bioinformatics analyses
A data set consisting of 1,551,894 high-quality, classifiable read counts was generated via MiSeq sequencing analysis of 48 samples. All the sequences were clustered with representative sequences; 97% sequence identity cutoff was used. All data could undergo total sum scaling (TSS) normalization. Bacterial community diversity was analyzed using the R phyloseq [19] and vegan packages [20]. Alpha diversity (Shannon index)of the groups was compared using the Mann-Whitney/Kruskal-Wallis test (non-parametric); the data used for alpha diversity analysis were not rarefied. Hierarchical clustering was performed using the hclust function in the package stat of R. Linear discriminant analysis (LDA) effect size (LEfSe) was performed [21], and the LDA score was computed for taxa differentially abundant between two groups. |Log10 [LDA]| ≥ 2.0 and p < 0.05 (Kruskal–Wallis test) was considered to indicate statistical significance. Spearman’s rank test was performed for correlation analysis.
SCFA analysis
SCFA levels in the cecal contents of mice were measured as described previously [22]. Briefly, cecal samples of approximately 50 mg were suspended with saturated NaCl and mixed thoroughly. SCFA were extracted using diethyl ether and quantified using a gas chromatograph equipped with a mass spectrometric detector (GCMS-QP2010 Ultra system, Shimadzu Corporation, Kyoto, Japan).
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 6 (La Jolla, CA, USA) and SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). Results are presented as means with SEM. One-way ANOVA with Duncan’s new multiple range method was used to compare multiple groups. Differences between groups were considered significant at *p < 0.05.
Results
L. reuteri protects against airway inflammation in HDM-induced asthma model
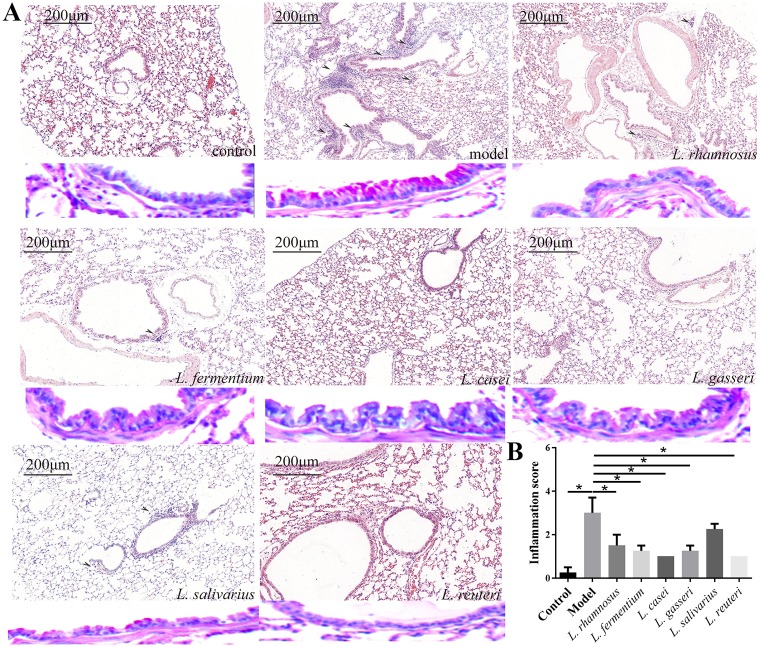
In order to prove the impact of different Lactobacillus species on the development of airway inflammation, airway inflammatory cell infiltrate and goblet cell hyperplasia were assessed. For light microscopy and morphometry, mouse lung sections were stained with H&E and PAS to assess the inflammatory cell infiltrate and identify airway goblet cell hyperplasia, respectively. Histological analysis of lung sections revealed that Lactobacillus administration reduced airway inflammation in HDM-treated mice (Fig 2A). Moreover, the inflammation score (p < 0.05), a hallmark of airway inflammation, the higher the score, the more severe the inflammation, in all the Lactobacillus groups, except the L. salivarius group, was significantly lower than that of the model group. Compared to the control, the L. fermentum, L. casei, L. gasseri, and L. reuteri groups showed no significant difference in the inflammation score (Fig 2B).
Fig 2. Ability of six Lactobacillus species to alleviate airway inflammation in house dust mite (HDM)-treated mice.
(A) H&E-stained mouse lung sections showing airway inflammatory cell infiltrate; PAS-stained mouse lung sections showing goblet cell hyperplasia (red colour). (B) Inflammation scores for lung tissues (n = 4). *p<0.05.
L. reuteri decreased Th2- and B cell-associated serum immunoglobulins
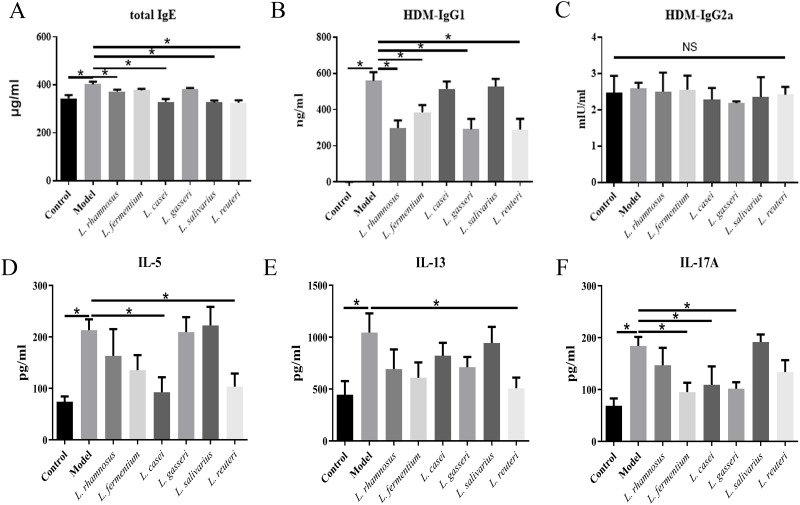
The total IgE serum levels in the L. rhamnosus, L. casei, L. salivarius, and L. reuteri groups were significantly lower than those in the model group. Furthermore, compared to the control group, the L. casei, L. salivarius, and L. reuteri groups showed no significant difference in total IgE serum level. HDM-IgG1 levels in the L. rhamnosus, L. fermentum, L. gasseri, and L. reuteri groups were significantly lower (by 46.6%, 31.0%, 47.8%, and 47.9% respectively) than that in the model group. However, the HDM- IgG2a levels of the different groups were not significantly different (Fig 3A–3C).
Fig 3. Ability of six Lactobacillus species to decrease asthma-associated phenotypes in house dust mite (HDM)-treated mice.
(A–C) Serum levels of total IgE, HDM-specific IgG1, and HDM-specific IgG2a. (D–F) Cytokine production in the bronchoalveolar lavage fluid (BALF). *p<0.05.
L. reuteri reduced Th2-based but not Th17-based inflammatory cytokine secretion in lungs
The secretory levels of IL-5, IL-13, and IL17A in the BALF from the HDM-treated model mice were significantly higher than those in the BALF from control mice. IL-5 levels were significantly reduced in the L. casei and L. reuteri groups, compared to the model group, but were not significantly different from those of the control group. The secretory levels of IL-13 were significantly decreased only in the L. reuteri group compared to the model group. Compared to the model group, the L. fermentum, L. casei, and L. gasseri groups showed reduced IL-17A levels (Fig 3D–3F).
Collectively, these results indicate that the different HDM-induced cytokine response in the lungs were significantly ameliorated in different Lactobacillus groups. L. reuteri administration ameliorated all the HDM-induced cytokine response in the lungs except IL-17A levels.
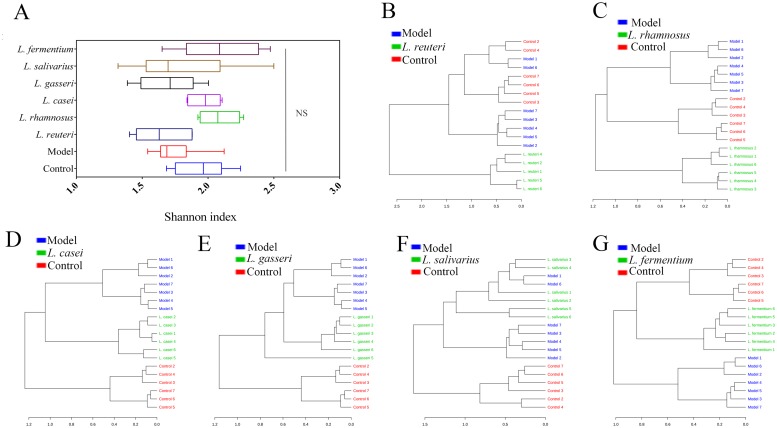
L. reuteri altered the overall microbiota diversity in the colon of HDM-treated mice
The overall microbiota diversity includes within-group and across-group microbial diversity. Within-group microbial diversity, Shannon index including the evenness and richness parameters, was not markedly different between the groups (Fig 4A). Furthermore, to assess across-group microbial diversity, hierarchical clustering was performed to obtain a dendrogram (Fig 4B–4G). Clustering analysis indicated that the bacterial profile in the model group was markedly different from that in groups subjected to Lactobacillus supplementation, except the L. salivarius group. The bacterial profiles in the L. rhamnosus and L. reuteri groups were considerably different from the microbiota profile of both control and model groups. Those in the L. casei and L. gasseri groups were different only from the model group. However, the bacterial profile in the L. fermentum group was considerably different from that in the model group and showed higher similarity with the bacterial profile of the control group.
Fig 4. Taxonomic summary of gut microbiota in different mouse groups.
(A) Boxplot for α-diversity measured using Shannon index at genus level. (B–G) Dendograms showing the results of cluster analysis. Distance was measured using Bray-Curtis distance and clustering algorithm using Ward’s method at the OTU level.
L. reuteri-treated increase in colonic Lactobacillus and Enterococcus was correlated with the improvement of the lung immune environment
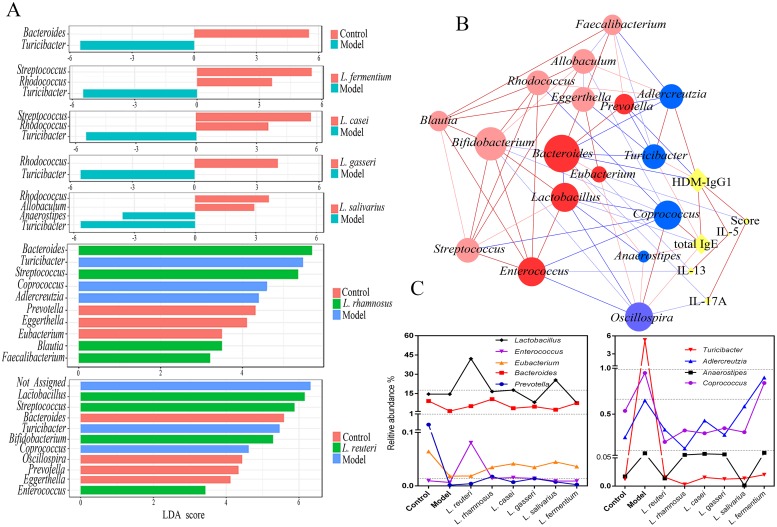
At the genus level, the control and model groups showed a marked difference in the abundance of two bacterial taxa. Compared to the controls, the models showed significantly reduced Bacteroides but considerably increased Turicibacter (Fig 5A).
Fig 5. Gut microbiota analysis of house dust mite (HDM)-treated mice.
(A) Linear discriminant analysis (LDA) score for gut microbiota from the control, model, Lactobacillus reuteri, L. rhamnosus, L. casei, L. gasseri, L. salivarius, and L. fermentum groups. (B) Network showing correlations between specific genera and asthma phenotype. Circle size is representative of the number of lines proportional to significant interactions. Red circles: ‘protective’ (dark color represents significant correlation with asthma phenotype); blue circles: ‘negative’; red lines: positive correlation; blue lines: negative correlation (light color represents significant correlation at the 0.05 level; dark color at the 0.01 level). (C) ‘protective’ and ‘negative’ relative abundance of altered bacterial genera in each Lactobacillus group.
Using the results from hierarchical clustering, we categorized the Lactobacillus groups into three classes for further LEfSe analysis. We found that 17 taxa sequences were significantly altered in the Lactobacillus groups. Rhodococcus, Streptococcus, Allobaculum, Blautia, Faecalibacterium, Lactobacillus, Bifidobacterium, and Enterococcus increased whereas Turicibacter, Anaerostipes, Coprococcus, and Adlercreutzia decreased in the Lactobacillus groups, compared with the model group. The abundance of Bacteroides, which decreased in the model group, increased only after L. rhamnosus administration. Prevotella, Eggerthella, Eubacterium, and Oscillospira were enriched in control (Fig 5A).
Global integration using Spearman correlation for all asthma phenotypes with the 17 microbial genera revealed that ten microbial genera were associated with the asthma phenotypes, except IL-5 levels (Fig 5B). The bacteria that were enriched in the model group and showed the highest value for positive correlation with asthma phenotypes included Turicibacter and total IgE (ρ = 0.429, P = 0.010) and HDM-IgG1 (ρ = 0.342, P = 0.039); Anaerostipes and total IgE (ρ = 0.328, P = 0.047); Coprococcus and total IgE (ρ = 0.485, P = 0.002); and Adlercreutzia and HDM-IgG1 (ρ = 0.517, P = 0.001). Significant negative correlations observed in the Lactobacillus groups included Lactobacillus and total IgE (ρ = -0.414, P = 0.011) and Enterococcus and IL-13 (ρ = -0.365, P = 0.012), and those in the controls included Bacteroides and IL-17A (ρ = -0.362, P = 0.012); HDM-IgG1 (ρ = -0.553, P = 0.001); Prevotella and HDM-IgG1 (ρ = -0.470, P = 0.004); Eubacterium and IL-13 (ρ = -0.349, P = 0.016); and Eubacterium and score (ρ = -0.391, P = 0.033). Interestingly, Oscillospira was positively correlated with total IgE (ρ = 0.385, P = 0.019) and negatively correlated with IL-17A (ρ = -0.362, P = 0.012).
Except Lactobacillus and Enterococcus, eight other microbial genera differed between the control and model groups. The abundance of Lactobacillus and Enterococcus increased simultaneously only after L. reuteri administration (Fig 5C).
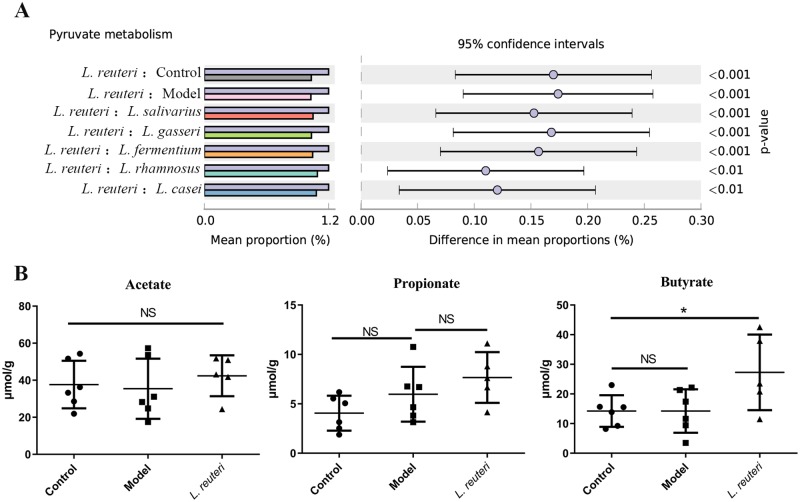
L. reuteri altered metabolic function and increases butyrate in mouse cecum
Microbial functions were predicted using PICRUSt. Compared with the model and control groups, the L. reuteri group showed significant changes in the levels of 153 metabolites. Pyruvate metabolism, the classic pathway for the SCFA formation, was the most significantly altered pathway in the L. reuteri group, compared to the other groups (Fig 6A). Cecal contents were analyzed to confirm whether L. reuteri regulated gut microbiota to increase SCFA production. Compared to the model group, the L. reuteri group showed significant increase in cecal butyrate levels but not in cecal acetate and propionate levels (Fig 6B).
Fig 6. Effect of Lactobacillus reuteri supplementation on short-chain fatty acid (SCFA) production.
(A) A significant occurrence of difference pyruvate metabolism between L. reuteri and other groups. (B) Acetate, propionate, and butyrate levels in cecum contents of mice.
Discussion
The aim of this study was to investigate the preventive effect of different Lactobacillus species on modulation of gut microbiota for asthma. Using an HDM-treated murine model for asthma, we demonstrate the preventive effects of edible Lactobacillus strains against the development of Th2 and Th17-driven allergic inflammation. Although the different Lactobacillus species showed varying degrees of attenuation of the HDM-induced allergic inflammation, our results reveal that continuous Lactobacillus supplementation could regulate immune function in mice and impart an anti-inflammatory effect in the lung. Of the six Lactobacillus species tested, L. reuteri showed better efficacy in ameliorating asthma phenotypes.
All Lactobacillus species investigated in this study could modulate the overall gut microbiota. L. fermentum supplementation showed recovery of the gut microbiota from that observed in the models to that observed in the controls; however, it did not show the best preventive effect against asthma. It has been speculated that specific microbes might be more important than overall microbial diversity in association with allergic diseases [8].
In this study, specific microbes modulated by the different Lactobacillus species were similar. This showed similar patterns of alteration in the microbiota composition, suggesting the same role of these Lactobacillus species in asthma pathogenesis. Therefore, we further speculate that these Lactobacillus species with different ability may occupy the same ecological niche in the gut. However, few studies have focused on the functional properties of specific microbial genera in allergic diseases, including asthma.
In this study, we performed Spearman correlation test to evaluate the relations among specific genera and asthma phenotypes. The specific genera enriched in the Lactobacillus groups showed positive correlation with the specific genera enriched in the control, and negative correlation in the model and with asthma phenotypes. They could be divided into ‘protective’ (more abundant in Lactobacillus groups and control group) and ‘negative’ (more abundant in the model group) genera.
Lactobacillus, Bifidobacterium, Enterococcus, and Streptococcus are widely considered as probiotics [23]. Lactobacillus and Bifidobacterium are beneficial in reducing allergic diseases [24,25]. Rhodococcus and Enterococcus exhibit anti-inflammatory effects in sensitized animal models [26,27]. Furthermore, Bacteroides were found to be more abundant in healthy adults than in asthmatic children [1,28], and Streptococcus abundance decreased in children with food sensitization in early life [29]. Moreover, inflammation has been suggested to provide a favorable environment for Turicibacter [30,31], thereby implicating the role of Turicibacter in disrupting the gut microbiota and influencing asthma phenotypes.
These results support the surmise that specific genera contribute to allergic sensitization and are ‘protective’ or ‘negative’ with respect to asthma initiation. This may be one reason underlying the preventive effect of Lactobacillus against asthma. The ‘protective’ bacteria can act against the ‘negative’ bacteria via microbial exclusion from the mucosa (an ecological effect) [32]. However, further studies are warranted to confirm these possible causal relations.
Of all the Lactobacillus species investigated in this study, L. reuteri showed the strongest effect against asthma. Increase in the abundance of Lactobacillus and Enterococcus by L. reuteri administration may reduce asthma risk. Peculiarly, the abundance of these two bacteria was not markedly different between other Lactobacillus groups and model mice. Lactobacillus was the only specific genera that was strongly negatively associated with the level of total IgE, the biomarker for allergy [33]. Moreover, it was the most abundant among the ‘protective’ bacteria and was significantly increased by three folds after L. reuteri supplementation. Because Lactobacillus colonizes the intestinal tract, it may be considered an effective probiotic with properties not restricted to the gut. Pathway analysis indicated that L. reuteri specifically treated a functional shift towards SCFA production in the gut. We observed an increase in butyrate content in the gut only after L. reuteri supplementation. The anti-inflammatory role of butyrate has been reported previously [34]. In fact, Enterococcus and Streptococcus are butyrate-producing bacteria. In addition, Lactobacillus and Bifidobacterium also play an important role in the increase of butyrate production: they are able to produce the acetate and lactate, which are used to synthesize butyrate. Therefore, the upregulation of butyrate in L. reuteri group observed in our study is important for the preventive effect against asthma and may be associated with the increase in Lactobacillus, Bifidobacterium, Enterococcus and Streptococcus abundance.
In this study, we demonstrated the effectiveness of Lactobacillus against asthma. In addition to modulating the common microbiome of Lactobacillus, L. reuteri also modulated specific gut microbial genera, thereby providing a better protective effect against asthma. L. reuteri may be considered as a potential candidate when screening for Lactobacilli with preventive effect against asthma. Because the reciprocal interaction of Lactobacillus supplementation with the gut microbiota is complex, further research involving different L. reuteri strains is essential to elucidate the mechanisms underlying the preventive role of Lactobacillus.
Conclusions
To our knowledge, this is the most large-scale report in which the preventive effects of different Lactobacillus species on allergies are investigated in a murine HDM-induced asthma. Our study showed that L. reuteri supplementation was more efficient than the five other species investigated in this study for asthma prevention. The modulation of specific gut microbes by L. reuteri altered the gut microbial function toward increased butyrate production, which alleviated airway inflammation and the Th2 response in lung tissues. Therefore, such modulation is more effective for asthma prevention than the modulation of overall gut microbiota composition. Thus, targeting the gut microbiota via L. reuteri supplementation may be considered as an ideal strategy for the prevention of asthma and other allergic diseases.
Acknowledgments
The work was supported by the Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
Data Availability
The datasets generated for this study can be found in the NCBI PRJNA573760
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant No. 31820103010, 31771953, 31871774), the Fundamental Research Funds for the Central Universities (JUSRP51903B), the National first class discipline program of Food Science and Technology (JUFSTR20180102) The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5(1): e8578 10.1371/journal.pone.0008578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ong YE, Menzies-Gow A, Barkans J, Benyahia F, Ou TT, Ying S, et al. Anti-IgE (omalizumab) inhibits late-phase reactions and inflammatory cells after repeat skin allergen challenge. J Allergy Clin Immunol. 2005;116(3): 558–564. 10.1016/j.jaci.2005.05.035 [DOI] [PubMed] [Google Scholar]
- 3.van der Heijden FL, van Neerven RJ, Kapsenberg ML. Relationship between facilitated allergen presentation and the presence of allergen-specific IgE in serum of atopic patients. Clin Exp Immunol. 1995;99(2): 289–293. 10.1111/j.1365-2249.1995.tb05547.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122): 783–800. 10.1016/S0140-6736(17)33311-1 [DOI] [PubMed] [Google Scholar]
- 5.Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368(9537): 733–743. 10.1016/S0140-6736(06)69283-0 [DOI] [PubMed] [Google Scholar]
- 6.Björkstén B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001;108(4): 516–520. 10.1067/mai.2001.118130 [DOI] [PubMed] [Google Scholar]
- 7.Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357(9262): 1076–1079. 10.1016/S0140-6736(00)04259-8 [DOI] [PubMed] [Google Scholar]
- 8.Zimmermann P, Messina N, Mohn WW, Finlay BB, Curtis N. Association between the intestinal microbiota and allergic sensitization, eczema, and asthma: A systematic review. J Allergy Clin Immunol. 2019;143(2): 467–485. 10.1016/j.jaci.2018.09.025 [DOI] [PubMed] [Google Scholar]
- 9.Fonseca W, Lucey K, Jang S, Fujimura KE, Rasky A, Ting HA, et al. Lactobacillus johnsonii supplementation attenuates respiratory viral infection via metabolic reprogramming and immune cell modulation. Mucosal Immunol. 2017;10(6): 1569–1580. 10.1038/mi.2017.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorburn Alison N., et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nature communications. 2015;6: 7320 10.1038/ncomms8320 [DOI] [PubMed] [Google Scholar]
- 11.Johansson MA, Sjögren YM, Persson JO, Nilsson C, Sverremark-Ekström E. Early colonization with a group of Lactobacilli decreases the risk for allergy at five years of age despite allergic heredity. PLoS One. 2011;6(8): e23031 10.1371/journal.pone.0023031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YS, Lin YL, Jan RL, Chen HH, Wang JY. Randomized placebo-controlled trial of lactobacillus on asthmatic children with allergic rhinitis. Pediatr Pulmonol. 2010;45(11): 1111–1120. 10.1002/ppul.21296 [DOI] [PubMed] [Google Scholar]
- 13.Mennini M, Dahdah L, Artesani MC, Fiocchi A, Martelli A. Probiotics in asthma and allergy prevention. Front Pediatr. 2017;5: 165 10.3389/fped.2017.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, et al. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med. 2004;169(3): 378–385. 10.1164/rccm.200308-1094OC [DOI] [PubMed] [Google Scholar]
- 15.Henderson WR, Lewis DB, Albert RK, Zhang Y, Lamm WJ, Chiang GK, et al. The importance of leukotrienes in airway inflammation in a mouse model of asthma. J Exp Med. 1996;184(4): 1483–1494. 10.1084/jem.184.4.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci. 2011;108(Suppl 1): 4516–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao B, Li D, Zhao J, Liu X, Gu Z, Chen YQ, et al. Metagenomic insights into the effects of fructo-oligosaccharides (FOS) on the composition of fecal microbiota in mice. J Agric Food Chem. 2015;63(3): 856–863. 10.1021/jf505156h [DOI] [PubMed] [Google Scholar]
- 18.Zhao L, Wang G, Siegel P, He C, Wang H, Zhao W, et al. Quantitative genetic background of the host influences gut microbiomes in chickens. Sci Rep. 2013;3: 1163 10.1038/srep01163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8(4): e61217 10.1371/journal.pone.0061217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon P. VEGAN, a package of R functions for community ecology. J Veg Sci. 2003;14(6): 927–930. [Google Scholar]
- 21.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6): R60 10.1186/gb-2011-12-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreau NM, Goupry SM, Antignac JP, Monteau FJ, Le Bizec BJ, Champ MM, et al. Simultaneous measurement of plasma concentrations and 13C-enrichment of short-chain fatty acids, lactic acid and ketone bodies by gas chromatography coupled to mass spectrometry. J Chromatogr B. 2003;784(2): 395–403. [DOI] [PubMed] [Google Scholar]
- 23.Kechagia M, Basoulis D, Konstantopoulou S, Dimitriadi D, Gyftopoulou K, Skarmoutsou N, et al. Health benefits of probiotics: a review. ISRN Nutr. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu L, Song J, Wang C, Fu S, Wang Y. Bifidobacterium infantis potentially alleviates shrimp tropomyosin-treated allergy by tolerogenic dendritic cell-dependent induction of regulatory T cells and alterations in gut microbiota. Front Immunol. 2017;8: 1536 10.3389/fimmu.2017.01536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schabussova I, Hufnagl K, Tang ML, Hoflehner E, Wagner A, Loupal G, et al. Perinatal maternal administration of Lactobacillus paracasei NCC 2461 prevents allergic inflammation in a mouse model of birch pollen allergy. PLoS One. 2012;7(7): e40271 10.1371/journal.pone.0040271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marro A, Pirles M, Schiaffino L, Bin L, Davila H, Bottasso OA, et al. Successful immunotherapy of canine flea allergy with injected Actinomycetales preparations. Immunotherapy. 2011;3(8): 971–978. 10.2217/imt.11.93 [DOI] [PubMed] [Google Scholar]
- 27.Zhang B, An J, Shimada T, Liu S, Maeyama K. Oral administration of Enterococcus faecalis FK-23 suppresses Th17 cell development and attenuates allergic airway responses in mice. Int J Mol Med. 2012; 30(2): 248–254. 10.3892/ijmm.2012.1010 [DOI] [PubMed] [Google Scholar]
- 28.Yee AL, Miller E, Dishaw LJ, Gordon JM, Ji M, Dutra S, et al. Longitudinal microbiome composition and stability correlate with increased weight and length of very-low-birth-weight infants. mSystems. 2019;4(1): e00229–18. 10.1128/mSystems.00229-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CC, Chen KJ, Kong MS, Chang HJ, Huang JL. Alterations in the gut microbiotas of children with food sensitization in early life. Pediatr Allergy Immunol. 2016;27(3): 254–262. 10.1111/pai.12522 [DOI] [PubMed] [Google Scholar]
- 30.Nguyen M, Sharma A, Wu W, Gomi R, Sung B, Hospodsky D, et al. The fermentation product 2,3-butanediol alters P. aeruginosa clearance, cytokine response and the lung microbiome. ISME J. 2016;10(12): 2978–2983. 10.1038/ismej.2016.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rausch P, Steck N, Suwandi A, Seidel JA, Kunzel S, Bhullar K, et al. Expression of the blood-group-related gene B4galnt2 alters susceptibility to Salmonella infection. PLoS pathog. 2015;11(7): e1005008 10.1371/journal.ppat.1005008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noverr MC, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends Microbiol. 2004;12(12): 562–568. 10.1016/j.tim.2004.10.008 [DOI] [PubMed] [Google Scholar]
- 33.Chaker AM, Zissler UM, Wagenmann M, Schmidt-Weber C. Biomarkers in allergic airway disease: simply complex. ORL. 2017;79(1–2): 72–77. 10.1159/000455725 [DOI] [PubMed] [Google Scholar]
- 34.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480): 446–450. 10.1038/nature12721 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study can be found in the NCBI PRJNA573760