Abstract
Recurrence of pressure ulcers remains common. We have employed resorbable antibiotic beads as a therapeutic strategy to deliver high local antibiotic concentrations to the debridement site. Our objective was to determine whether the use of resorbable antibiotic- beads would reduce pressure ulcer recurrence. We reviewed all stage IV pressure ulcers treated with excision, partial ostectomy and flap coverage over 16 years. Baseline patient factors (location of ulcer, presence of osteomyelitis, preoperative prealbumin), surgical factors (type of flap, use of antibiotic beads, bone culture results) and postoperative outcomes (ulcer recurrence at 1 year, dehiscence, seroma, cellulitis) were collected. Outcomes of patients who received antibiotic-impregnated beads were compared to those who did not. Eighty-six patients with 120 stage IV pressure ulcers underwent excision and flap coverage. This included 16 ulcers where antibiotic beads were used and 104 where they were not. The overall ulcer recurrence rate at 12 months was 35.8%. The recurrence rate in the group treated with antibiotic beads was significantly lower than the group without beads (12.5% vs. 39.4%, p = 0.03). Overall, complication rates between the two groups were similar (43.8% vs. 51.9%, p = 0.54). No systemic or local toxicity from antibiotic beads occurred. Scanning electron microscopy images of sacral bone from one case showed bacterial biofilm even after debridement. Pressure ulcer recurrence at 1 year after excision and flap coverage decreased significantly with the use of resorbable antibiotic beads.
Pressure ulcers are quite common in acutely and chronically ill patients.1 Surgical treatment of these ulcers consists of bone and soft tissue excision, and coverage with a flap. However, even with this radical approach, recurrence rates remain as high as 80%.2 We hypothesized that persistent infection such as underlying osteomyelitis is a major contributor to recurrence.3
Intraoperatively, any infected or necrotic bone should be debrided, and bone samples should be obtained to direct postoperative antibiotics.4 Despite adequate debridement and antibiotics, many patients still have persistent osteo-myelitis. Infected bone has poor blood supply, and even when intravenous antibiotics are appropriately dosed to reach therapeutic serum levels, the local concentration is often subsubtherapeutic.5 An exacerbating factor is bacterial encasement within biofilm, which limits effectiveness of intravenous antibiotics.6 Biofilm consists of extracellular polymeric substance (EPS) including extracellular DNA, polysaccharides and proteins. It represents a protected mode of bacterial growth. Bacteria within biofilm avoid eradication by targeting and disabling neutrophils, and increasing antibiotic recalcitrance.6 Importantly, bacteria within biofilm are not reliably detected by standard culture techniques7 and require scanning electron microscopy (SEM) to detect/diagnose. Biofilm readily recurs after debridement,8 but debridement can temporarily convert bacteria to a planktonic state making them susceptible to antibiotics.9
Biofilm could potentially decrease the success of flap reconstruction, because it disables skin-barrier function through down regulation of tight junction proteins, leading to increased transepidermal water loss and loss of skin barrier function.8 Biofilm is also is capable of inducing expression of ceramidases and matrix metalloproteinases that degrade overlying soft tissues10 theoretically leading to sinus tracts and ulcer recurrence.
Orthopedic surgeons have faced similar issues with open extremity fractures and infected arthroplasties. They have used antibiotic-impregnated beads placed into the operative site after debridement to provide much higher local concentrations of antibiotics at the bone surface than could be provided via the intravenous route5 without significantly raising systemic antibiotic levels.11 Those high local anti-biotic levels have been shown to lower bacterial counts significantly.12–14 In addition, those beads have a large bacterial zone of inhibition.15 Clinically, treatment of open fractures with antibiotic beads have lowered the rate of infectious complications and chronic osteomyelitis, compared to intravenous antibiotics alone in retrospective stud ies.16 Although there is compelling data from retrospective studies16,17 and the use of antibiotic beads has been widely adopted, there has not been a well-designed randomized controlled trial on the use of antibiotic beads in open fractures. The lack of level I evidence has led the Surgical Infection Society to state in a 2006 publication that the evidence is insufficient to endorse the use of antibiotic-impregnated beads.18
The vast majority of orthopedic literature on antibiotic beads used nonabsorbable-absorbable beads. Performing a second operation to retrieve antibiotic impregnated beads is not an option with pressure ulcer flap surgery. The use of absorbable antibiotic beads in humans is a very recent development. We sought to determine whether absorbable antibiotic beads are efficacious in decreasing the rates of flap failure and pressure ulcer recurrence. infection, was causing flap failure, and that the beads would deliver high levels of infection, was causing flap failure, and that the beads would deliver high levels of antibiotics directly to the bone that systemic antibiotics may not be able to achieve.19 Since 2013 the senior author has used resorbable antibiotic-impregnated beads as an adjunct to pressure ulcers excision and flap coverage. In this retrospective analysis we compare the outcomes of pressure ulcers that were treated with antibiotic-impregnated beads to those that were not.
MATERIALS AND METHODS
Patient management
All patients with stage IV pressure ulcers underwent a careful preoperative workup. This included investigation into the contributing factors of the ulcer (unrelieved pressure, poorly padded wheelchair cushion, shear, moisture, malnutrition, noncompliance, poor social support), and those factors were addressed preoperatively. Fecal and/or urinary diversion was performed if necessary. Nutrition was optimized preoperatively, with a goal prealbumin above 15 mg/dL. The presence and extent of osteomyelitis for all patients was determined preoperatively using C-reactive protein, erythrocyte sedimentation rate, pelvic radiographs, magnetic resonance imaging or white blood cell scans. Preoperative pressure mapping was obtained.
Operative management consisted of methylene blue-guided ulcer bursectomy, excision of heterotopic bone, and excision of infected bone defined as residual healthy cancellous bone with pinpoint bleeding and no sharp edges. Any necrotic or soft bone was removed. Bone cultures were obtained from the residual bone after debridement. In 2013, we started using resorbable antibiotic-impregnated calcium-sulfate beads (Stimulan, Biocomposites Ltd., Keele, UK) for patients with localized osteomyelitis. 10cc of standard Stimulan was mixed with 1g of vancomy-cin and 300mg of tobramycin, placed into a mold to form 4.8 mm beads, and allowed to cure for 8 minutes. The beads were then placed in the ulcer cavity after debridement and prior to flap inset. Wound coverage was achieved with primary closure or a flap, depending on the amount of dead space. Ischial pressure ulcers were covered with gluteal myocutaneous rotation flaps or posterior thigh/hamstring advancement flaps. Sacral pressure ulcers were covered with gluteal myocutaneous rotation flaps or gluteal fasciocutaneous V-Y advancement flaps. Trochanteric pressure ulcers were covered with tensor fascia lata myocutaneous flaps. For femoral head osteomyelitis, femoral head resection was performed, with a vastus lateralis muscle flap to obliterate any dead space.
Postoperatively, vancomycin and tobramycin serum levels were checked on postoperative day 1. All patients were kept on flat bedrest on an air-fluidized (Dolphin Bed, Wound Systems, Atlanta, GA) or alternating pressure mattress for 6 weeks. All patients with positive bone cultures were treated with culture-directed antibiotics with guidance from the infectious disease service, regardless of whether antibiotic beads had been used or not. Patients were kept in the hospital for one week then discharged to home or skilled nursing facility.
After 6 weeks, patients were allowed to advance their activity. In patients who underwent ostectomy, repeat pressure mapping was obtained. All patients were followed for at least one year postoperatively.
Scanning electron microscopy
Tissue samples obtained intraoperatively were processed and imaged as described previously.7,8 In brief, samples were collected and fixed in 2.5% glutaraldehyde solution in 0.2M phosphate buffer. Following graded ethanol dehydration, samples were treated with hexamethyldisilazane (HMDS, Ted Pella Inc., Redding, CA) and left to dry overnight. Before scanning, samples were mounted on pin stub and coated with gold- palladium. SEM was done using a FEI Nova nano SEM (FEI, Hillsboro, OR) equipped with field-emission gun electron source used for imaging.
Case series
After Institutional Review Board approval was obtained (The Ohio State University, Protocol# 2015E0443), a retrospective review of all patients who underwent pressure ulcer excision and flap coverage by the senior author between 1999 and 2014 was performed. Patients with at least one-year follow-up were included, other-wise none were excluded. Baseline patient factors, surgical factors and postoperative outcomes (ulcer recurrence, superficial dehiscence, seroma, cellulitis) were collected. Pressure ulcer recurrence was defined as a deep non-healing wound at the site of the flap occurring within 1 year of surgery. Superficial dehiscence was defined as a skin-only separation of the incision that healed with dressing changes. Outcomes of patients who received antibiotic-impregnated beads were compared to those who did not, using chi-squared analysis and the Fisher’s exact test, with p < 0.05 as a threshold for statistical significance.
RESULTS PATIENTS
Eighty-six patients with 120 stage IV pressure ulcers underwent excision and flap coverage between 1999 and 2014. These included 80 ischial ulcers, 22 sacral ulcers, 7 trochanteric ulcers, 11 femoral head osteomyelitides. Antibiotic-impregnated beads were used in 16 ulcers, and were not used in 104 ulcers (Table 1). At 12 months, 43 of 120 (35.8%) ulcers had recurred. This included two ulcers in the antibiotic bead group (12.5%), and 41 ulcers in the no-bead group (39.4%, p = 0.037). Patients in the bead group had a lower recurrence rate than patients in the no-bead group for all four types of pressure ulcers, although the differences were not statistically significant.
Table 1.
Baseline characteristics and outcomes of the patients included in the study
| Antibiotic beads | No beads | p-value | |
|---|---|---|---|
| Patients | 15 | 71 | |
| Ulcers | 16 | 104 | |
| Ischial | 56.3% | 68.3% | NS |
| Sacral | 6.3% | 20.2% | NS |
| Trochanteric | 0% | 6.7% | NS |
| Femoral head osteomyelitis | 37.5% | 4.8% | <0.05 |
| Procedure performed | |||
| Primary closure | 6.3% | 17.3% | NS |
| Gluteal rotation flap | 43.8% | 50% | NS |
| Gluteal V-Y advancement flap | 6.3% | 7.7% | NS |
| Posterior thigh/hamstring flap | 6.3% | 14.4% | NS |
| Tensor fascia lata flap | 0% | 5.8% | NS |
| Femoral head ostectomy and vastus lateralis | 37.5% | 4.8% | <0.05 |
| Intraoperative bone culture | |||
| No growth | 25% | 33.9% | NS |
| Gram-positive cocci | 25% | 26.8% | NS |
| Gram-positive bacilli | 18.8% | 8.9% | NS |
| Gram-negative bacilli | 25% | 25% | NS |
| Fungus | 6.3% | 5.4% | NS |
| Complications | 43.8% | 51.9% | NS |
| Recurrence at 1 year | 12.5% | 39.4% | 0.037 |
| Dehiscence | 18.8% | 10.6% | NS |
| Seroma | 12.5% | 0% | 0.001 |
| Cellulitis | 0% | 1.9% | NS |
| Recurrence by ulcer type | |||
| Ischial | 22.2% | 45.1% | NS |
| Sacral | 0% | 28.6% | NS |
| Trochanteric | – | 28.6% | – |
| Femoral head osteomyelitis | 0% | 20% | NS |
Two patients in the antibiotic bead group developed a postoperative seroma that required percutaneous drainage (Table 1). Results of intraoperative bone cultures demonstrated gram-positive cocci in 26.8% of cases, gram-positive bacilli in 8.9%, gram-negative bacilli in 25%, fungus in 5.4%, and no growth in 33.9%. There were no significant differences in bone culture results between patients in the antibiotic bead group and those in the no-bead group (Table 1). Patients with more than one pressure ulcer did not have a higher recurrence rate than patients with one pressure ulcer.
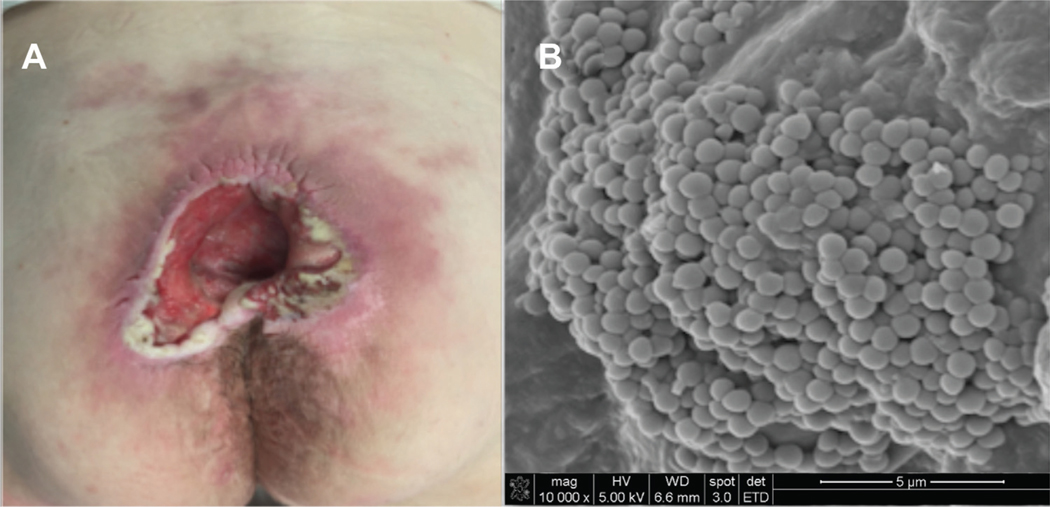
To determine whether occult biofilm infection may be present in ulcers after surgical debridement, SEM images of a stage IV sacral pressure ulcer from one patient that had been treated twice with intravenous antibiotics for methicillin-resistant Staphylococcus aureus osteomyelitis (MRSA) are shown in Figure 1. Half of each tissue biopsy collected for SEM was sent to hospital microbiology for culture. Figure 1A shows the pressure ulcer in the OR prior to debridement with no exposed bone. Figure 1B is the SEM from the first piece of granulation tissue removed from the wound. A cluster of classic descriptions of bacterial biofilm infection. Bacterial cocci over a robust biofilm is seen coating the wound surface, consistent with classic descriptions of bacterial biofilm infection.
Figure 1.
Predebridement biofilm detection in a stage 4 sacral pressure ulcer with history of MRSA osteomyelitis. (A) Sacral pressure ulcer prior to surgical debridement and flap coverage. (B) Scanning electron microscopy image of tissue biopsy taken before surgical debridement (10,000x magnification). Mushroom like projections of cocci emanate from the biofilm-coated surface of the wound.
Figure 2A shows the pressure ulcer from the patient after debridement. Figure 2B shows a biopsy of the soft tissue after debridement. Figure 2C and D show SEM of the bone after thorough debridement, demonstrating massive coating of bacterial cocci and rods on sacral bone covered with bacterial biofilm. It should be noted that cultures from these postdebridement bone and soft tissue biopsies were negative. Figure 3 shows the antibiotic-impregnated beads in the debrided pressure ulcer, followed by V-Y flap closure of the ulcer. The patient was not given any post-operative IV antibiotic therapy because the intraoperative wound tissue sent to microbiology was culture negative. The SEM images were not available at the time of discharge. The patient had a delayed postoperative dehiscence (28 days), and tissue biopsies at that time grew Pseudomonas aeruginosa.
Figure 2.
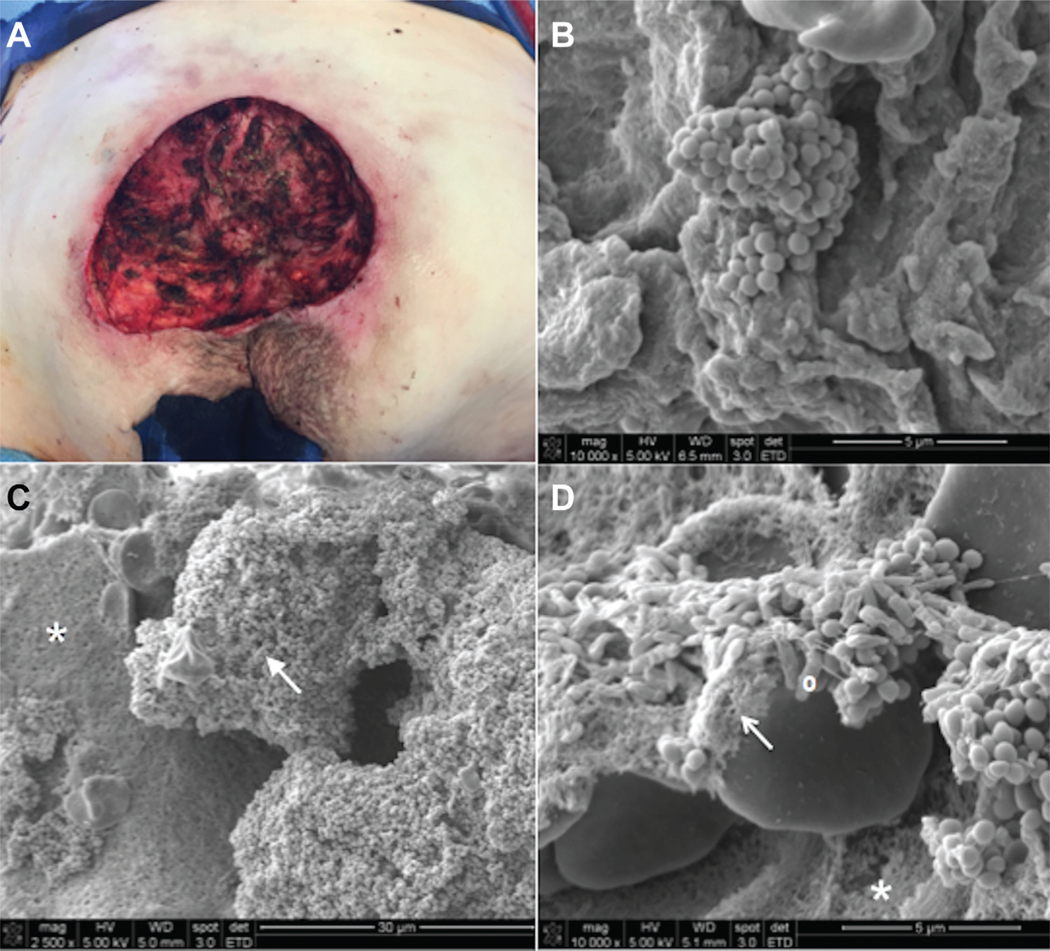
Postdebridement images showing persistent bacterial biofilm despite radical excision of pressure ulcer. (A) Appearance of sacral pressure ulcer after full, en bloc excision of ulcer bursa. The aggressive approach results in removal of all ulcer and periwound fibrotic tissue. (B) SEM of soft tissue biopsy (10,000x magnification) taken at completion of debridement shows mushroom like projection of cocci emanating from biofilm-coated surface of wound. (C) SEM of bone biopsy obtained at completion of debridement (2,500x magnification) shows that the majority of the bone is covered in cocci (arrow). The asterisk indicates an area of bone trabeculae without any bacteria. (D) SEM of bone biopsy taken at completion of debridement (10,000x magnification) reveals both cocci and rods adherent to the bone trabeculae (trabeculae indicated by the white asterisk). The white arrow indicates the presence of extracellular polymeric substance that creates the biofilm, and the white circle is next to a bacterial rod.
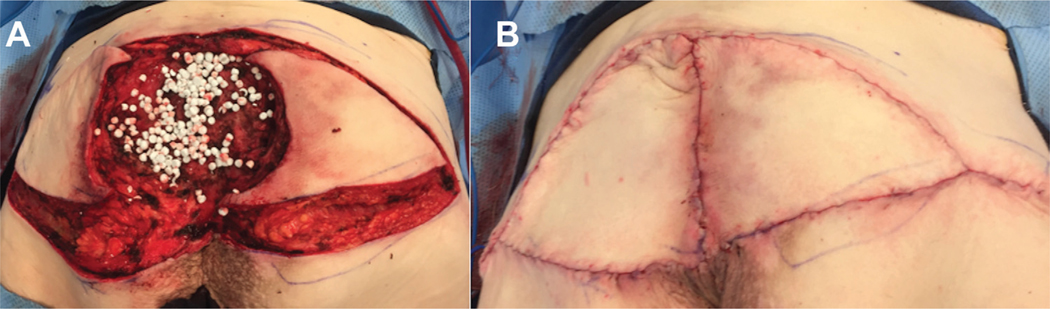
Figure 3.
(A) Placement of resorbable antibiotic-impregnated beads for treatment of sacral pressure ulcer. (B) V-Y fasciocutaneous flap closure of sacral pressure ulcer 340×99mm (72 × 72 DPI).
Bone culture results from our case series demonstrated a mixture of pathogens, with the most common species being Staphylococcus, Streptococcus, Pseudomonas, Acinetobacter and Escherichia, all of which were sensitive to vancomycin and/or tobramycin. We did not observe development of antibiotic resistance with the use of antibiotic beads: of the two patients who received antibiotic beads and had an ulcer recurrence, one grew Pseudomonas aeruginosa, which was sensitive to tobramycin, and the second grew Pseudomonas aeruginosa and MRSA, sensitive to tobramycin and vancomycin, respectively. We encountered two postoperative seromas among the patients who received antibiotic beads, despite placement of a closed-suction drain under the flap. Both seromas resolved after percutaneous drainage. Random levels of vancomycin and tobramycin were obtained on postoperative day 1 for all patients with beads. Elevated serum levels were not observed.
For ischial pressure ulcers, primary closure tended to have the highest recurrence rate at 1 year (60%), followed by posterior thigh/hamstring advancement flaps (50%) and gluteal myocutaneous rotation flaps (37%). However, none of the differences were statistically significant.
DISCUSSION
This is the first report detailing the outcomes of the use of dissolvable antibiotic beads as an adjunct in the treatment of pressure ulcers. Our retrospective study provides a long-term, single-surgeon experience with comprehensive treatment of pressure ulcers. The surgical technique remained consistent throughout the study period, except for the introduction of antibiotic-impregnated beads in 2013 in patients with a preoperative diagnosis of osteomyelitis. Our study may have confounders that are difficult to measure and control, such as patient compliance with pressure offloading. To address these differences, which would be most significant in the acute postoperative period, we used the rate of ulcer recurrence at 12 months as our primary outcome measure.
Among the patients who received antibiotics beads, there was a higher proportion of patients who underwent femoral head ostectomy and vastus lateralis flap, compared to the patients who did not receive antibiotic beads. This is unlikely to have affected our results, since antibiotic beads decreased pressure ulcer recurrence rates for all types of pressure ulcers (Table 1). The decreases in ulcer subgroups were not statistically significant, most likely due to small number of ulcers studied (n = 16). It is important to amenable to surgical resection. Patients with diffuse pelvic osteomyelitis that is not note that antibiotics beads were only used in patients with focal areas of osteomyelitis amenable to surgical resection. Patients with diffuse pelvic osteomyelitis that is not amenable to resection necessary to disrupt biofilm would be unlikely to benefit from beads or even IV antibiotics.9
This study illustrated, using SEM, the presence of bacterial biofilm infection in a pressure ulcer after aggressive intraoperative surgical debridement. This observation may have two very important implications: (1)) surgical excision alone, even when performed aggressively, may not eliminate biofilm and (2)) conventional bacterial culture techniques are inadequate to diagnose bacterial biofilm infection, since the patient from Figure 2 had negative post-debridement cultures. This is consistent with work that we have previously reported where 6 patients had SEM evidence of biofilm infection on sternal wires, but only 1 of those 6 patients had positive wound cultures.7 This suggests the need for better culture methods and more effective surgical techniques directed toward biofilm eradication. Advanced methods, such as scanning electron microscopy and metagenomic footprinting,20 would be much more sensitive for identifying non-culturable organisms. Sample sonication to recover bacteria in a planktonic state is used in research laboratories studying microbial biofilm, and may also be a useful adjunct for clinical microbiology laboratories.21 The dehiscence in the culture-negative pressure ulcer patient presented in Figures 2 and 3 indicates that beads alone may not be sufficient and that the addition of intravenous antibiotics is necessary to resolve infection. A case series of 1,085 consecutive limb fractures showed a decrease infection rate from 12% to 3.7% in patients treated with systemic antibiotics vs. antibiotics plus antibiotic impregnated nonabsorbable beads.17 Such studies were done with nonabsorbable beads but suggest that the best possible outcomes likely result from the combination of systemic and topical antibiotic therapy.
Pharmaceutical-grade calcium sulfate is a biocompatible powder and dissolves over a six-week period while releasing its antibiotic content. It does not require removal like nondissolvable polymethylmethacrylate (PMMA) beads, which have been used for many years. Unlike PMMA, pharmaceutical-grade calcium sulfate cures at low temperature, thus allowing heat-sensitive antibiotics to be mixed in. High local antibiotic concentrations from antibiotic beads have not been found to be toxic to fibroblasts,5 nor have they been found to result in bacterial resistance.12 If elevated serum levels were ever encountered, saline irrigation through the closed-suction drains would be used to dissolve the beads. There have been case reports of delayed hypersensitivity reactions to beads impregnated with piperacillin-tazobactam,22 but not to vancomycin or tobramycin. Vancomycin and tobramycin should be avoided in patients with renal impairment. Not all antibiotics, e.g., gentamicin, are compatible with the beads.
This study has several limitations. It is a retrospective study with historical controls, which introduces bias. Our study also has a small sample size in the beads group, and we only obtained SEM images from one patient. A future confirmation study would be a randomized-controlled trial where one group of patients would receive plain impregnated with antibiotics.
The change in clinical practice to incorporate antibiotic beads was inspired by the hypothesis that persistent infection contributed to the high rates of pressure ulcer recurrence rate. The lower recurrence rate with antibiotic beads and the SEM images of biofilm infection after aggressive surgical debridement are important evidence that support that hypothesis. It is important to keep in mind that the effectiveness of antibiotic beads in the treatment of persistent bacterial biofilm after debridement is simply a hypothesis. While the current findings are not definitive, they provide an important initial proof of concept and perhaps a new paradigm for understanding the role of occult infection, and especially biofilm infection, in the clinically vexing problem of pressure ulcer recurrence.
CONCLUSION
The rate of pressure ulcer recurrence at 1 year after excision and flap coverage decreased significantly with the use of resorbable antibiotic-impregnated beads. We observed persistent bacterial biofilm infection in a pressure ulcer even after operative debridement. Antibiotic beads in conjunction with debridement may have utility in the eradication of this persistent infection to reduce the risk of pressure ulcer recurrence.
This study was supported in part by NIH R01 awards NR013898 and NR015676 to GMG and CKS.
Footnotes
DISCLOSURES
None of the authors have any financial conflicts to disclose.
REFERENCES
- 1.Langemo DK, Olson B, Hunter S, Hanson D, Burd C, Cathcart-Silberberg T. Incidence and prediction of pressure ulcers in five patient care settings. Decubitus 1991; 4: 25. [PubMed] [Google Scholar]
- 2.Evans GR, Dufresne CR, Manson PN. Surgical correction of pressure ulcers in an urban center: is it efficacious? Adv Wound Care 1994; 7: 40–46. [PubMed] [Google Scholar]
- 3.Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects, part 2. N Engl J Med 1970; 282: 260. [DOI] [PubMed] [Google Scholar]
- 4.Han H, Lewis VL, Wiedrich TA, Patel PK. The value of Jamshidi core needle bone biopsy in predicting postoperative osteomyelitis in grade IV pressure ulcer patients. Plast Reconstr Surg 2002; 110: 118. [DOI] [PubMed] [Google Scholar]
- 5.Roeder B, Van Gils CC, Maling S. Antibiotic beads in the treatment of diabetic pedal osteomyelitis. J Foot Ankle Surg 2000; 39: 124–130. [DOI] [PubMed] [Google Scholar]
- 6.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999; 284: 1318–1322. [DOI] [PubMed] [Google Scholar]
- 7.Elgharably H, Mann E, Awad H, Ganesh K, Ghatak PD, Gordillo G, et al. First evidence of sternal wound biofilm following cardiac surgery. PLoS One 2013; 8: e70360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy S, Elgharably H, Sinha M, Ganesh K, Chaney S, Mann E, et al. Mixed-species biofilm compromises wound healing by disrupting epidermal barrier function. J Pathol 2014; 233: 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolcott RD, Runbaugh KP, Dowd SE, Schultz G, Phillips P, Yang Q, et al. Biofilm maturity studies indicate sharp debridement opens a time-dependent therapeutic window. J Wound Care 2010; 19: 320–328. [DOI] [PubMed] [Google Scholar]
- 10.McCarty SM, Cochrane CA, Clegg PD, Percival SL. The role of endogenous and exogenous enzymes in chronic wounds: a focus on the implications of aberrant levels of both host and bacterial proteases in wound healing. Wound Repair Regen 2012; 20: 125–136. [DOI] [PubMed] [Google Scholar]
- 11.Liu SJ, Ueng SWN, Lin SS, Chan EC. In Vivo release of vancomycin from biodegradable beads. J Biomed Mater Res (Appl Biomater) 2002; 63: 80781. [DOI] [PubMed] [Google Scholar]
- 12.Chen NT, Hong HZ, Hooper DC, May JW. The effect of systemic antibiotic and antibiotic-impregnated polymethyl-methacrylate beads on the bacterial clearance in wounds containing contaminated dead bone. Plast Reconstr Surg 1993;92:1305. [PubMed] [Google Scholar]
- 13.Hake ME, Young H, Mauffrey C, Stahel PF, Hammerberg EM, Mauffrey C, et al. Local antibiotic therapy strategies in orthopaedic trauma: practical tips and tricks and review of the literature. Injury 2015; 46: 1447–1456. [DOI] [PubMed] [Google Scholar]
- 14.Mounasamy V, Fulco P, Desai P, Adelaar R, Bearman G. The successful use of vancomycin-impregnated cement beads in a patient with vancomycin systemic toxicity: a case report with review of the literature. Eur J Orthop Surg Traumatol 2013; 23: 299–302. [DOI] [PubMed] [Google Scholar]
- 15.Karr JC, Lauretta J, Keriazes G. In vitro antimicrobial activity of calcium sulfate and hydroxyapatite (Cerament bone void filler) discs using heat-sensitive and non–heat-sensitive antibiotics against methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa. J Am Podiatr Med Assoc 2011; 101: 146–152. [DOI] [PubMed] [Google Scholar]
- 16.Ostermann PA, Henry SL, Seligson D. The role of local anti-biotic therapy in the management of compound fractures. Clin Orthop Relat Res 1993; 295: 102–111. [PubMed] [Google Scholar]
- 17.Ostermann PA, Seligson D, Henry SL. Local antibiotic therapy for severe open fractures: a review of 1985 consecutive cases. J Bone and Joint Surg Br 1995; 77: 93–97. [PubMed] [Google Scholar]
- 18.Hauser CJ, Adams CA. Surgical Infection Society guideline: prophylactic antibiotic use in open fractures: an evidence-based guideline. Surg Infect (Larchm) 2006; 7: 379–405. [DOI] [PubMed] [Google Scholar]
- 19.Howlin RP, Brayford MJ, Stoodley P, Webb JS, Cooper JJ, Aiken SS, et al. Antibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob Agents Chemother 2015; 59: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghurye JS, Cepeda-Espinoza V, Pop M. Metagenomic assembly: overview, challenges and applications. Yale J Biol Med 2016; 89: 353–362. [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi H, Oethinger M, Tuohy MJ, Procop GW, Bauer TW. Improved detection of biofilm-formative bacteria by vortexing and sonication: a pilot study. Clin Orthop Relat Res 2009; 467: 360–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song EK, Seon JK, Jeong MS. Delayed-type hypersensitivity reaction to piperacillin/tazobactam in a patient with an infected total knee replacement. J Bone Joint Surg 2010; 92B: 1596–1599. [DOI] [PubMed] [Google Scholar]